Abstract
The waste phenolic resin was utilized as the raw material to prepare activated carbon (AC) used KOH as the activating agent via microwave heating. The phenolic resin was carbonized at 500°C and then performed with a KOH/Char ratio of 4 and microwave power of 700 W for a duration of 15 min. The physic-chemical characteristics of the AC were characterized by N2 adsorption instrument, FTIR, SEM and TEM. The BET surface area and pore volume of AC were found to be 4269 m2/g and 2.396 ml/g, respectively. The activation process to generate such a phenomenally high surface area of the AC has little reported in open literatures and could pave way for preparation adsorbents that are far superior to the currently marketed adsorbents. The methylene blue (MB) was used as the model to assess its suitability to dye wastewater treatment. Towards this, the MB adsorption isotherms were conducted at three different temperatures and tested with different adsorption isotherm models. The adsorption isotherms could be modeled using Langmuir isotherm. While the kinetics could be used the pseudo-second order kinetics to describe. Thermodynamic results demonstrated that the adsorption process was a spontaneous, as well as an endothermic.
1 Introduction
Organic pollutants have received significant attention from the scientific community in the in recent years owe to the harmful impacts on human health and ecological environment [1]. Organic wastewater is produced from lots of industrial which include cottonocracy, plastics, and pulp manufacture [2]. The organic dyes in the wastewater can lead to considerable negative impacts on the environment which includes reducing light transmittance, cancerogenic substance and mutations in genes, as well as increase in the COD and BOD content of wastewater [3]. Hence it is imperative to treat the wastewater before being discharged into natural environment to reduce the impact on the natural environment. The dyes are harder to be biodegraded owing to their complex aromatic molecular structures as compared to other contaminant. And its removal utilizing conventional biological wastewater systems has been a challenge. Hence it is imperative to develop treatment systems that coulis effectively treat dyes economically.
The conventional techniques include coagulation [4], hyperfiltration [5], chemical oxidation [6], filter binding assay [7], biosorption [8], and ozonation [9]. However, these approaches lack the expected degree of effectiveness technologically as well as economically. Adsorption technology has attracted our attention because of its advantages among these methods. For instance, it has higher efficiency, lower cost, and relatively easy-to-adopt [10]. AC has abundant pore structure and powerful adsorption affinity, widely being used in dyes wastewater treatment [11]. The spent phenolic resin is obtained from industrial productions. Because of its low cost and higher carbon content, phenolic resin would be used as a potential raw material to prepare the phenolic resin-based carbon materials [12].
In general, AC can be prepared either using physical or chemical activation methods. Physical activation method requires the activation gas such as steam, CO2, or their compound. However, the process needs a high activation temperature for a long time, leading to low production yield and high costs [13]. Chemical activation method has been widely employed in improving AC performance such as pore volume and specific surface area. And the activation agents usually include H2SO4, HCl, KOH, NaOH, ZnCl2, K2CO3, Na2CO3 and H3PO4 [14, 15, 16, 17]. As one of the activation agents, KOH can make AC with abundant pore parameter [18, 19, 20]. Chemical activation has many advantages, which include lower activation temperatures, lower time, more abundant pore structure and higher production over physical activation [21].
Recently, microwave heating is a green heat method which has being widely applied for preparing of AC [14, 15]. The transfer of heat is not by either conduction or convection, from exterior to interior having a temperature gradient from exterior to interior being nonuniform [22]. The heating utilizing of the microwave energy is uniform as the material converting the absorbed microwave energy and heats up from within itself. The main advantage of the microwave heating are lower heating time, higher heating rate and the non-contact heating way [22, 23]. Therefore, microwave heating is applied in various manufacturing industries and considered to be a mature and promising processing technology. The present work utilizes the advantages of the microwave heating for conversion of waste phenolic resin into AC with KOH as the activating agent. The prepared AC is characterized by nitrogen adsorption isotherms, FTIR, SEM and TEM. And then the prepared AC would be used to treat the wastewater to study its adsorption performance.
2 Experimental material and method
2.1 Material
The raw material is collected from a company in China. The KOH and MB are both chemical reagent that purchased from China.
2.2 Experimental methods
Firstly, the raw material was heated via conventional coking furnace at the temperature of 500°C for 2 h under inert gas to obtain the char. 8 g char and 32 g KOH were mixed with the mass ratio of 1:4. And then the impregnated mixture was putted into microwave oven with microwave power of 700 W for 15 min. After cooling, AC was washed by HCl solution and then washed by distilled water. Finally, it filtered and dried, and stored in dry environment for characterization.
2.3 Characterization of AC
The pore structural parameters of the AC were tested by the nitrogen adsorption instrument (Autosorb-1-C, USA) at 77 K. Scanning electron microscope (SEM, Philips XL30ESEM-TMP) and transmission electron microscope (TEM, JEM-2100, Japan) was used to analyze the surface structure of the AC. The surface functional groups of AC and char were used FTIR (Thermo Nicolet Co., USA) to observe.
2.4 Adsorption isotherms
The adsorption performance of the AC was evaluated by the MB. Series of the adsorption experiments were did in the conical flasks. 0.1 g AC was mixed with the 100 ml MB solution with concentration of 200-400 mg/L, and then put into the gas bath thermostatic at the temperature of 30-50°C with the speed of 300 r/min until the equilibrium. The residual MB amount was calculated by UV-vis spectrophotometer. And then the absorbed amount of MB on AC, qe (mg/g), was calculated using the following expression:
where C0 (mg/L) is the initial concentrations of MB solutions, and Ce (mg/L) is the equilibrium concentrations of MB solutions. V and M are the volume of solution (L) and the weight of the adsorbent (g), respectively.
2.5 Adsorption kinetics
The kinetics experiments were also did in the conical flasks. And it was similar with the adsorption isotherms experiment. The mixed solution was sampled at regular time. The MB concentration adsorbed per time could be calculated via the following equation:
where Ct is MB liquid-phase concentration.
3 Result and discussion
3.1 Pore structure analysis
Figure 1 shows the N2 adsorption isotherms of AC and char [24]. This kind of adsorption isotherms is belonged to a type I isotherms on the basis of IUPAC classification. As Figure 1 shown, it manifests that absorption volume of AC is much greater than that char, demonstrating tremendous increase in the pore volume of the AC. The pore size distribution of AC and char is shown in Figure 2. As seen in Figure 2, the pore volume of the AC is obviously large in the micropore region, evidencing the reaction of KOH-C producing large quantities of micropores.
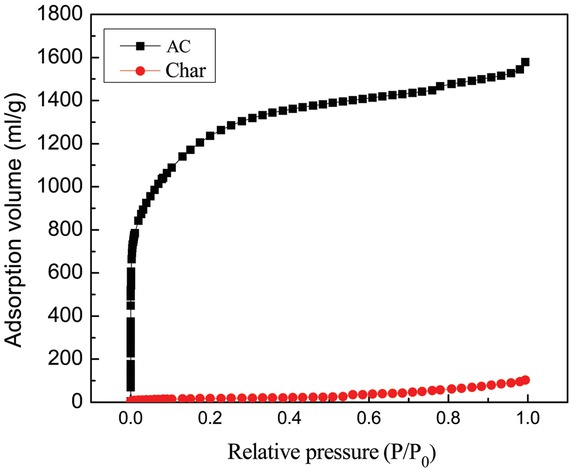
Nitrogen adsorption isotherm pore size distribution of the AC and char.
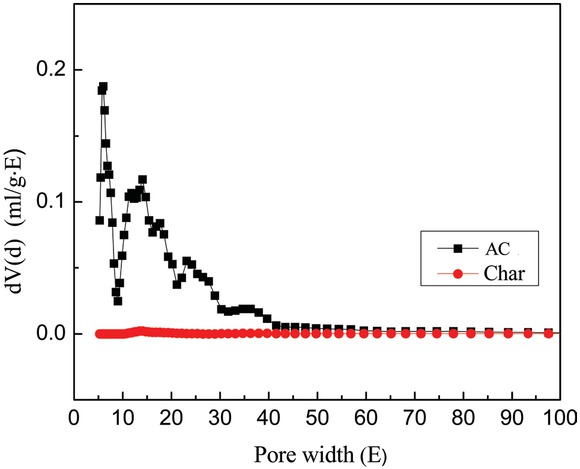
Pore size distribution (c) of the AC and char.
Table 1 shows the pore parameter of the AC and char [24]. Compared with the char, the total pore volume and the surface area tremendously increase for AC. Such high surface area has little reported in open literatures, which could possibly be attributed to the effectiveness of KOH activation in a microwave heating mode.
Pore structural parameters of the char and AC.
| SBET | Vtot | Da | Vmic | Smic | Sexternal Vmes | Vmic/Vtot | |
|---|---|---|---|---|---|---|---|
| (m2/g) | (ml/g) | (Å) | (ml/g) | (m2/g) | (m2/g) (ml/g) | (%) | |
| Char | 33 | 0.041 | 50.0 | 0.001 | 3 | 30 0.040 | 2.46 |
| AC | 4269 | 2.396 | 22.5 | 1.690 | 3561 | 708 0.706 | 70.53 |
3.2 FTIR analysis of AC and char
Figure 3 shows FTIR spectra of AC and char. It can be observed that there is little different from AC and char, based on Figure 3. The spectrum of the AC has peaks at 3435, 2925, 1630 and 1120 cm−1. While the spectrum of the char has peaks at 3443, 2914, 1697, 875 and 750 cm−1. The wavebands in the region of 3440–3420 cm−1 are attributed to O-H stretching vibration [25]. The intense band at about 2925 cm-1 and its shoulder at 2914 cm−1 are attributed to C-H stretching vibration [26]. The peak at the 1697 cm-1 indicates C-C symmetrical stretching of pyrone, while the low peak at 1630 cm−1 is attributed to vibration of H-O-H from absorbed water [27, 28]. Strong peaks at 875 cm−1 belongs to poly substituted aromatic ring, while the one at 750 cm−1 corresponds to 1, 2 C=C stretching in the aromatic rings, respectively [16, 22]. Finally, the peak at 1120 cm−1 is phenol C-O stretching [29]. Compared the FTIR spectrum of char with AC, the bands of char ranging from 1679 to 1120 cm-1 disappears.
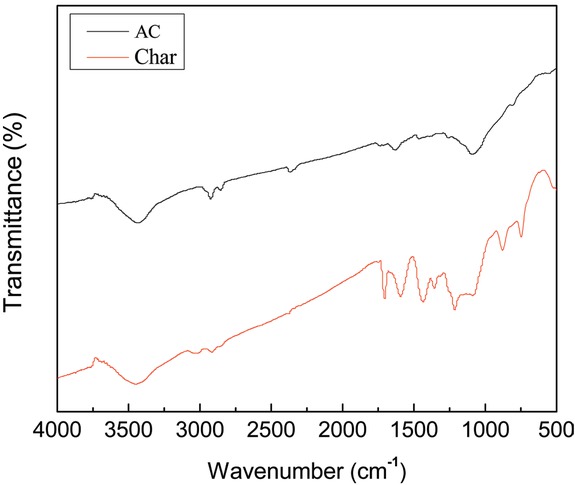
Fourier transform infrared spectroscopy (FTIR) spectra of the AC and char.
3.3 Adsorption isotherms
Adsorption isotherms are employed to study adsorption process, which helps to understand how the molecules are adsorbed onto the surface of AC. Four different popular adsorption isotherm models are used to analyze experiment data to choose the best model. The models attempted in this work are compiled in Table 2, which provides the details of the model equations. Table 3 lists the calculated fitting parameters of these models. The correlation coefficient (R2) of the Langmuir isotherm is the largest based on the Table 3. The adsorption amount of the MB is increase with the temperature increasing. The monolayer adsorption capacity was in the range of 460.8 to 485.4 mg/g which is far higher than the adsorption capacity of most adsorbents that has been reported in literatures.
Adsorption isotherm models adopted in this work and their parameters.
| Isotherm | Equation | Parameters |
|---|---|---|
| Langmuir | Ce is the equilibrium concentration (mg/L) | |
| Q0 (mg/g) is adsorption constant related to adsorption capacity | ||
| kL (L/g) is adsorption constant related to energy of adsorption | ||
| Freundlich | kF is adsorption constant related to adsorption capacity (mg/g).(L/mg)1/n | |
| n is adsorption constant measuring the adsorption intensity | ||
| Dubinin-Radushkevich | α is the adsorption capacity (mg g-1) | |
| β is the constant related to the adsorption energy (mol2kJ-2) | ||
| Temkin | A and B are constants |
Adsorption isotherm parameters at different temperatures.
| Isotherms | Parameters | Temperature (K) | ||
|---|---|---|---|---|
| 303 | 313 | 323 | ||
| Langmuir | Q0(mg/g) | 460.8 | 473.9 | 485.4 |
| KL(L/mg) | 1.1667 | 1.2712 | 1.5489 | |
| R2 | 0.99 | 0.99 | 0.99 | |
| Freundlich | 1/n | 2.6785 | 2.6106 | 2.8255 |
| KF((mg/g).(L/ | 240.36 | 255.13 | 281.14 | |
| mg)1/n) | ||||
| R2 | 0.99 | 0.98 | 0.94 | |
| Dubinin- | α (mg g-1) | 374.95 | 384.58 | 397.64 |
| Radushkevich | β(mol2J-2)10-8 | 11.96 | 9.96 | 7.37 |
| E(KJ/mol) | 2.04 | 2.24 | 2.60 | |
| R2 | 0.90 | 0.94 | 0.98 | |
| Temkin | R2 | 0.99 | 0.98 | 0.98 |
Table 4 summarizes the comparison of the MB adsorption capacity, BET and the Vmic/Vtot of various of AC. The MB adsorption capacity in this study is larger than those reported [30, 31, 32, 33], suggesting that the AC have great potential application in MB removal. As shown in Table 4, the BET of the AC that prepared from phenolic resin (phenolic resin-AC) is the biggest than other kinds of AC. But, the adsorption capability of the MB is only 20% more large than that AC prepared from biodiesel industry solid reside (The BET of the phenolic resin-AC is three times more than that the AC prepared from biodiesel industry solid reside). The reason may be that the micropore volume of the phenolic resin-AC has the 70% of the total volume indicating having lots of the micropore. The pore size of these micropore isn’t big enough to adsorb the MB molecule. So, the MB adsorption capability of the AC is only 20% more large than that AC prepared from biodiesel industry solid reside. Kasaoka et al. reported that adsorption occurred when the pore diameter of adsorbent at least 1.7 times as much as of the adsorbate [34]. The minimum molecular size of MB is about 0.8 nm, while the average pore sizes of phenolic resin-AC is 2.25 nm. Therefore, it is accessible adsorption MB molecule. If the pore size is not big enough, it cannot adsorb the big MB molecule. As shown in Table 4, the BET of the cashew nut-AC-1 is larger than that cashew nut-AC-2. But the MB adsorption capability of the cashew nut-AC-1 is lower than that cashew nut-AC-2. The reason is that the cashew nut-AC-1 has the bigger Vmic/Vtot. But if the BET is bigger enough, we can neglect the impact of the Vmic/Vtot on MB adsorption capability. (See the different between cashew nut-AC -0.5 and cashew nut-AC -1). Although the cashew nut-AC -0.5 has the bigger Vmic/Vtot, it also has the larger MB adsorption capability.
The MB adsorption capability of the different kinds AC.
| Adsorbents | BET | Vmic/Vtot (%) | Adsorption capacity (mg/g) | References |
|---|---|---|---|---|
| AC | 4269 | 70% | 485.4 | This study |
| Biodiesel industry solid reside-AC | 1372 | 42.11% | 395.3 | [30] |
| Regeneration Durian shell-AC | 621.51 | 47.45% | 410.85 | [31] |
| Spent coal-AC | 1233 | 64.63% | 375.93 | [32] |
| Cashew nut-AC –0.5 | 1100 | 70% | 277 | [33] |
| Cashew nut-AC | 1478 | 37% | 352 | [33] |
| –1.5 | ||||
| Cashew nut-AC –2 | 859 | 22% | 263 | [33] |
| Cashew nut-AC –1 | 875 | 58% | 215 | [33] |
Langmuir isotherm most suitably represents the adsorption data. Another characteristic parameter that can be used to evaluate adsorbents on Langmuir isotherm is dimensionless factor RL, which is calculated by:
In the above formula C0 is MB initial concentration and KL is Langmuir constant. The value of RL is depends on the type of isotherm: unfavorable (RL > 1), linear (RL = 1), favorable (0 < RL < 1), irreversible (RL = 0). The calculation RL value is 0.1717-0.5831, demonstrating the favorable of the MB adsorption onto AC. Langmuir isotherms are shown in Figure 4 at 30-50°C.
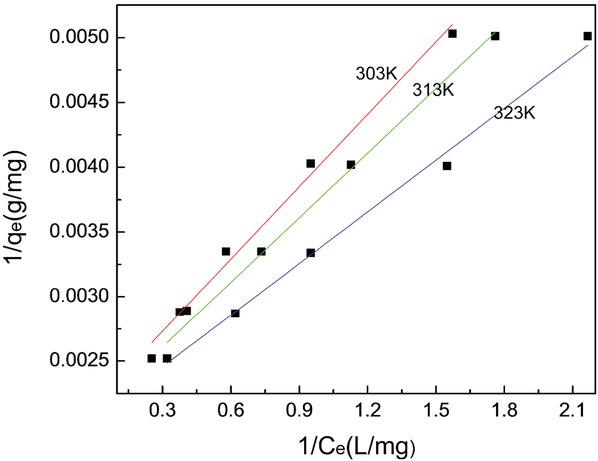
Langmuir isotherms for methylene blue adsorption onto AC at different temperatures.
In addition, the adsorption process was also analyzed using Dubinin-Radushkevich model. The E
3.4 Adsorption kinetics
Adsorption kinetics plays a formidable role in the design and sizing of the separation process equipment. Kinetics models such as Pseudo-first order, pseudo-second order, intraparticle diffusion and Elovich are employed to test experiment result so as to choose the most suitable model. Table 5 lists related model parameters. The fitting parameters of these models show in Table 6. The experimental result is agreement in pseudo-second-order model and R2 approaches to 1. Compared with the experimental data (qe,exp), Table 6 also provides the calculation qe,cal using the pseudo-second-order model (qe,cal), manifesting that there are very consistent. This finding is also consistent with previous studies on the adsorption of MB on the biodiesel industry solid reside [35] and Siris seed pods [21] based-AC. Figure 5 presents Pseudo-second-order model of the MB adsorption at different initial concentration.
Adsorption Kinetic models models adopted in this work and their parameters.
| Kinetic models | Equation | Parameters |
|---|---|---|
| Pseudo-first order | qe is the uptake of methylene blue at equilibrium (mg/g).K1 (1/min) is the adsorption rate constant, | |
| Pseudo-second order | K2 (g/mg min) is the rate constant of second-order equation | |
| Intraparticle diffusion | K3 (mg/g min1/2) is the intraparticle diffusion rate constant | |
| Elovich | C is a constanta (mg/g min) is the initial adsorption rat | |
| b (g/mg) is related to the extent of surface coverage and activation energy. |
Adsorption kinetics parameters at 30°C.
| Pseudo-first-order model | ||||
|---|---|---|---|---|
| C0 (mg/L) | qe.exp (mg/L) | qe.cal (mg/L) | K(1/min) 1 | R2 |
| 200 | 198.95 | 1.98 | 0.009 | 0.97 |
| 250 | 248.71 | 3.55 | 0.016 | 0.92 |
| 300 | 298.66 | 5.33 | 0.015 | 0.89 |
| 350 | 348.133 | 4.47 | 0.011 | 0.91 |
| 400 | 397.45 | 7.55 | 0.012 | 0.88 |
| Pseudo-second-order model | ||||
|---|---|---|---|---|
| C0 (mg/L) | ||||
| qe.exp (mg/L) | qe.cal (mg/L) | K(g/mg min) | R2 | |
| 2 | ||||
| 200 | 198.95 | 188.68 | 0.018 | 1 |
| 250 | 248.71 | 248.76 | 0.011 | 1 |
| 300 | 298.66 | 299.40 | 0.007 | 0.99 |
| 350 | 348.13 | 348.43 | 0.008 | 0.99 |
| 400 | 397.45 | 398.41 | 0.004 | 0.99 |
| C(mg/L) | Intraparticle diffusion model | |||
|---|---|---|---|---|
| 0 | qe.exp (mg/L) | C (mg/g) | K3 (mg/gmin | 1/2) R2 |
| 200 | 198.95 | 196.73 | 0.139 | 0.96 |
| 250 | 248.71 | 245.99 | 0.183 | 0.96 |
| 300 | 298.66 | 294.35 | 0.291 | 0.95 |
| 350 | 348.133 | 343.31 | 0.317 | 0.85 |
| 400 | 397.45 | 387.65 | 0.686 | 0.79 |
| Elovich kinetic models | ||||
|---|---|---|---|---|
| C(mg/L) 0 | qe.exp (mg/L) | 1/blnab (mg/g) | 1/b (mg/g) | R2 |
| 200 | 198.95 | 195.19 | 0.650 | 0.96 |
| 250 | 248.71 | 249.01 | 0.849 | 0.94 |
| 300 | 298.66 | 291.09 | 1.368 | 0.97 |
| 350 | 348.133 | 339.59 | 1.528 | 0.92 |
| 400 | 397.45 | 379.25 | 3.385 | 0.89 |
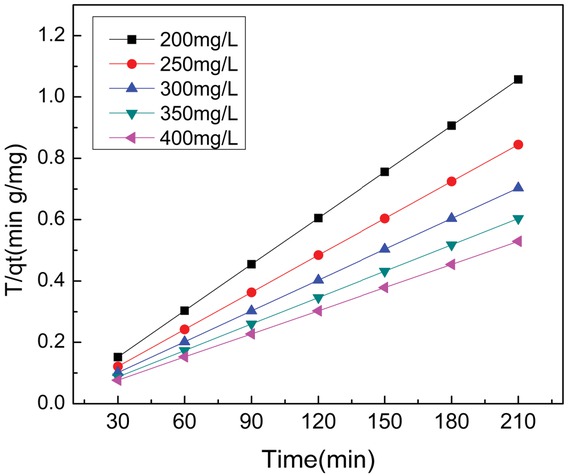
Pseudo-first-order kinetics for adsorption of methylene blue onto AC at 298 K.
3.5 Adsorption thermodynamics
The following equations are used to estimate the thermodynamic parameters of the ΔS, ΔH and ΔG:
With regard to MB, parameters such as ΔH and ΔS could be got via the slope and intercept of the Van’t Hoff graph of ln Kd and 1/t (Figure 6). The ΔG could be obtained by Eq. 5. Table 7 shows the activation energy and adsorption thermodynamics. The values of ΔG (-12.97, -14.03, -15.17 KJ/mol) is negative at 30-50°C, which manifest that adsorption process is spontaneous and feasible [25]. The results were similar the Moniruzzaman et al. [18]. As the temperature increases, the negative value of G decreases, indicating that MB adsorption process is easier as temperature increasing. The positive values of ΔH (19.77KJ/mol) indicates that the adsorption process has confirmed the physical adsorption. The adsorption reaction is the heat absorption property of AC based on the adsorption of MB with ΔH less than 80 kJ/mol. At the same time, positive ΔS (107.99J/mol) indicates that the affinity of AC to MB and randomness of solid interface increase [26].
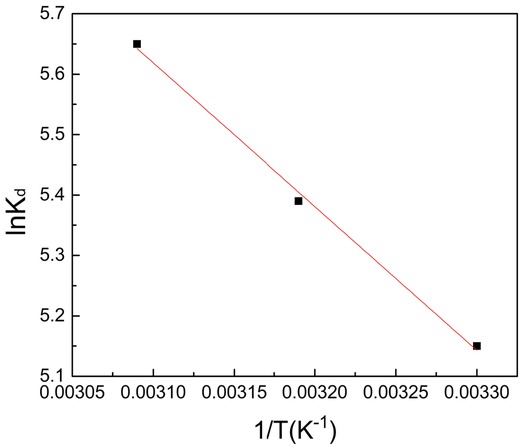
Van’t Hoff plot lnKd versus 1/T for methylene blue adsorption on HSAAC.
Activation energy and thermodynamic parameters.
| ΔH (KJ/mol) | ΔS (J/mol) | ΔG (KJ/mol) | ||
|---|---|---|---|---|
| 303 K | 313 K | 323 K | ||
| 19.77 | 107.99 | –12.97 | –14.03 | –15.17 |
4 Conclusion
The waste phenolic resin is a kind of solid waste, which could be used to prepare the AC by microwave heat and KOH as agent. The prepared AC is found to possess BET surface area of 4269 m2/g, average pore diameter 2.25 nm and pore volume of 2.396 cm3/g. The utility of the AC for dye wastewater applications is tested by the organic dye model of the MB, which had a monolayer adsorption capacity in the range of 460.8 to 485.4 mg/g far higher than the popular adsorbents commonly reported in literature. The adsorption isotherms were found to match with Langmuir isotherm model while the adsorption kinetics could be modelled using a pseudo-second-order kinetic model. The adsorption is endothermic and spontaneously in nature based on thermodynamic result.
Acknowledgements
The authors would like to express their gratitude to the Specialized Research Fund for the National Natural Science Foundation of China (21567013).
List of abbreviations
- Ac
Activated carbon
- FTIR
Fourier Transform infrared spectroscopy
- SEM
Scanning electron microscope
- TEM
Transmission electron microscope
- MB
Methylene blue
- COD
Chemical Oxygen Demand
- BOD
Biochemical Oxygen Demand
References
[1] Ahmed M.J., Theydan S.K., Microporous activated carbon from Siris seed pods by microwave-induced KOH activation for metronidazole adsorption. J. Anal. Appl. Pyrol., 2013, 99, 101-109.10.1016/j.jaap.2012.10.019Search in Google Scholar
[2] Alventosa D., Barredo D., Alcaina M., Iborra C., Ultrafiltration technology with a ceramic membrane for reactive dye removal: optimization of membrane performance. J. Hazard. Mater., 2012, 209–210, 492-500.10.1016/j.jhazmat.2012.01.065Search in Google Scholar PubMed
[3] Anirudhan T.S., Radhakrishnan P.G., Thermodynamics and kinetics of adsorption of Cu(II) from aqueous solutions onto a new cation exchanger derived from tamarind fruit shell. J. Chem. Thermodyn., 2008, 40, 702-709.10.1016/j.jct.2007.10.005Search in Google Scholar
[4] Auta M., Hameed B.H., Chitosan–clay composite as highly effective and low-cost adsorbent for batch and fixed-bed adsorption of methylene blue. Chem. Eng. J., 2014, 237, 352-361.10.1016/j.cej.2013.09.066Search in Google Scholar
[5] Autaa M., Hameed B.H., Preparation of waste tea activated carbon using potassium acetate as an activating agent for adsorption of Acid Blue 25 dye. Chem. Eng. J., 2011, 171, 502-509.10.1016/j.cej.2011.04.017Search in Google Scholar
[6] Bai Y., Huang Z.H., Kang F., Electrospun preparation of microporous carbon ultrafine fibers with tuned diameter, pore structure and hydrophobicity from phenolic resin. Carbon, 2014, 66, 705-712.10.1016/j.carbon.2013.09.074Search in Google Scholar
[7] Cheng S., Zhang L., Xia H., Peng J., Shu J., Ultrasound and microwave-assisted preparation of Fe-activated carbon as an effective low-cost adsorbent for dyes wastewater treatment. RSC Adv., 6/82, 2016.10.1039/C6RA14082CSearch in Google Scholar
[8] Cheng S., Zhang, L., Xia, H., Peng, J., Shu, J., Zhang Q., et al., Adsorption behavior of methylene blue onto waste-derived adsorbent and exhaust gases recycling. RSC Adv., 2017, 7, 27331-27341.10.1039/C7RA01482ASearch in Google Scholar
[9] Foo K.Y., Hameed B.H., Preparation and characterization of activated carbon from pistachio nut shells via microwave-induced chemical activation. Biomass Bioenerg., 2011, 35, 3257-3261.10.1016/j.biombioe.2011.04.023Search in Google Scholar
[10] Foo K.Y., Hameed B.H., Microwave-assisted preparation and adsorption performance of activated carbon from biodiesel industry solid reside: Influence of operational parameters. Bioresource Technol., 2012, 103, 398-404.10.1016/j.biortech.2011.09.116Search in Google Scholar PubMed
[11] Ghaedi M., Hajati S., Barazesh B., Karimi F., Ghezelbash G., Equilibrium, kinetic and isotherm of some metal ion biosorption. J. Ind. Eng. Chem., 2013, 19, 227-233.10.1016/j.jiec.2012.11.021Search in Google Scholar
[12] Gregg S.J., Ramsay J.D, Adsorption of carbon dioxide by magnesia studied by use of infrared and isotherm measurements. J. Chem. Soci. A Inorg. Phys. Theor., 1970, 2784-2787.10.1039/j19700002784Search in Google Scholar
[13] Huang, Y., Ma E., Zhao G., Thermal and structure analysis on reaction mechanisms during the preparation of activated carbon fibers by KOH activation from liquefied wood-based fibers. Ind. Crop. Prod., 2015, 69, 447-455.10.1016/j.indcrop.2015.03.002Search in Google Scholar
[14] Hui D., Li G., Yang H., Tang J., Tang J., Preparation of activated carbons from cotton stalk by microwave assisted KOH and K2CO3 activation. Chem. Eng. J., 2010, 163, 373-381.10.1016/j.cej.2010.08.019Search in Google Scholar
[15] Ji Y., Li T., Li Z., Wang X., Lin Q., Preparation of activated carbons by microwave heating KOH activation. App. Surf. Sci., 2007, 254, 506-512.10.1016/j.apsusc.2007.06.034Search in Google Scholar
[16] Kubota M., Hata A., Matsuda H., Preparation of activated carbon from phenolic resin by KOH chemical activation under microwave heating. Carbon, 2009, 47, 2805-2811.10.1016/j.carbon.2009.06.024Search in Google Scholar
[17] Mishra S., Mukul A., Sen G., Jha U., Microwave assisted synthesis of polyacrylamide grafted starch (St-g-PAM) and its applicability as flocculant for water treatment. Int. J. Biol. Macromol., 2011, 48, 106-111.10.1016/j.ijbiomac.2010.10.004Search in Google Scholar PubMed
[18] Moniruzzaman M., Ono T., Separation and characterization of cellulose fibers from cypress wood treated with ionic liquid prior to laccase treatment. Bioresource Technol., 2013, 127, 132.10.1016/j.biortech.2012.09.113Search in Google Scholar PubMed
[19] Muniandy L., Adam F., Mohamed A.R., Ng E.P., The synthesis and characterization of high purity mixed microporous/mesoporous activated carbon from rice husk using chemical activation with NaOH and KOH. Micropor. Mesopor. Mater., 2014, 197, 316-323.10.1016/j.micromeso.2014.06.020Search in Google Scholar
[20] Nabais J.M.V., Carrott P.J.M., Carrott M.M.L.R., Menéndez J.A., Preparation and modification of activated carbon fibres by microwave heating. Carbon, 2004, 42, 1315-1320.10.1016/j.carbon.2004.01.033Search in Google Scholar
[21] Shuang-Chen MA., Mao X.Y., Guo T.X., Zhao Y., Experimental study on desulfurization and denitrification from flue gas over modified activated carbon using microwave irradiation. J. Fuel Chem. Technol., 2010, 38, 739-744.Search in Google Scholar
[22] Tünay O., Kabdasl I., Eremektar G., Orhon D., Color removal from textile wastewaters. Water Sci. Technol., 1996, 34, 9-16.10.2166/wst.1996.0257Search in Google Scholar
[23] Tehranibagha A.R., Mahmoodi N.M., Menger F.M., Degradation of a persistent organic dye from colored textile wastewater by ozonation. Desalination, 2010, 260, 34-38.10.1016/j.desal.2010.05.004Search in Google Scholar
[24] Cheng S., Zhang L.B., Zhang S.Z., Xia H.Y., Peng J.H., Preparation of high surface area activated carbon from spent phenolic resin by microwave heating and KOH activation. High Temp. Mater. Proc., 2018, 37, 59-68.10.1515/htmp-2016-0042Search in Google Scholar
[25] Wang J., Kaskel S., KOH activation of carbon-based materials for energy storage. J. Mater. Chem., 2012, 22, 23710-23725.10.1039/c2jm34066fSearch in Google Scholar
[26] Yang J.B., Ling L.C., Liu L., Kang F.Y., Huang Z.H., Wu H., Preparation and properties of phenolic resin-based activated carbon spheres with controlled pore size distribution. Carbon, 2002, 40, 911-916.10.1016/S0008-6223(01)00222-6Search in Google Scholar
[27] Ji Y., Li T., Li Z., Wang X., Lin Q. Preparation of activated carbons by microwave heating KOH activation. Appl. Surf. Sci., 2007, 254, 506-512.10.1016/j.apsusc.2007.06.034Search in Google Scholar
[28] Moniruzzaman M., Ono T., Separation and characterization of cellulose fibers from cypress wood treated with ionic liquid prior to laccase treatment. Bioresource Technol., 2013, 127, 132-137.10.1016/j.biortech.2012.09.113Search in Google Scholar PubMed
[29] Zhao Y., Yan N., Feng M.W., Thermal degradation characteristics of phenol–formaldehyde resins derived from beetle infested pine barks. Thermochim. Acta, 2013, 555, 46-52.10.1016/j.tca.2012.12.002Search in Google Scholar
[30] Foo K.Y., Hameed B.H., Microwave-assisted preparation and adsorption performance of activated carbon from biodiesel industry solid reside: Influence of operational parameters. Bioresource Technol., 2012, 103, 398-404.10.1016/j.biortech.2011.09.116Search in Google Scholar PubMed
[31] Foo K.Y., Hameed B.H., A cost effective method for regeneration of durian shell and jackfruit peel activated carbons by microwave irradiation. Chem. Eng. J., 2012, 193-194.10.1016/j.cej.2012.04.055Search in Google Scholar
[32] Duan X.H., Srinivasakannan C., Liang J.S., Process optimization of thermal regeneration of spent coal based activated carbon using steam and application to methylene blue dye adsorption. J. Taiwan Inst. Chem. E., 2014, 45, 1618-1627.10.1016/j.jtice.2013.10.019Search in Google Scholar
[33] Spagnoli A.A., Dimitrios A.G., Svetlana B., Adsorption of methylene blue on cashew nut shell based carbons activated with zinc chloride: The role of surface and structural parameters. J. Mol. Liq., 2017, 229, 465-229, 471.10.1016/j.molliq.2016.12.106Search in Google Scholar
[34] Kasaoka S., Sakata Y., Tanaka E.R., Design of molecular sieving carbon-studies on adsorption of various dyes in liquid phase. Int. Chem. Eng. 1989, 29, 734-742.Search in Google Scholar
[35] Gang X., Rongbing W., Huilong Z., Mingjiang N., Xiang G., Kefa C., Preparation and characterization of activated carbons based alkali lignin by KOH chemical activation. J. Combustion Sci. Technol., 2014, 20, 14-20.Search in Google Scholar
© 2019 Hu et al., published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 Public License.
Articles in the same Issue
- Regular Articles
- Studies on the preparation and properties of biodegradable polyester from soybean oil
- Flow-mode biodiesel production from palm oil using a pressurized microwave reactor
- Reduction of free fatty acids in waste oil for biodiesel production by glycerolysis: investigation and optimization of process parameters
- Saccharin: a cheap and mild acidic agent for the synthesis of azo dyes via telescoped dediazotization
- Optimization of lipase-catalyzed synthesis of polyethylene glycol stearate in a solvent-free system
- Green synthesis of iron oxide nanoparticles using Platanus orientalis leaf extract for antifungal activity
- Ultrasound assisted chemical activation of peanut husk for copper removal
- Room temperature silanization of Fe3O4 for the preparation of phenyl functionalized magnetic adsorbent for dispersive solid phase extraction for the extraction of phthalates in water
- Evaluation of the saponin green extraction from Ziziphus spina-christi leaves using hydrothermal, microwave and Bain-Marie water bath heating methods
- Oxidation of dibenzothiophene using the heterogeneous catalyst of tungsten-based carbon nanotubes
- Calcined sodium silicate as an efficient and benign heterogeneous catalyst for the transesterification of natural lecithin to L-α-glycerophosphocholine
- Synergistic effect between CO2 and H2O2 on ethylbenzene oxidation catalyzed by carbon supported heteropolyanion catalysts
- Hydrocyanation of 2-arylmethyleneindan-1,3-diones using potassium hexacyanoferrate(II) as a nontoxic cyanating agent
- Green synthesis of hydratropic aldehyde from α-methylstyrene catalyzed by Al2O3-supported metal phthalocyanines
- Environmentally benign chemical recycling of polycarbonate wastes: comparison of micro- and nano-TiO2 solid support efficiencies
- Medicago polymorpha-mediated antibacterial silver nanoparticles in the reduction of methyl orange
- Production of value-added chemicals from esterification of waste glycerol over MCM-41 supported catalysts
- Green synthesis of zerovalent copper nanoparticles for efficient reduction of toxic azo dyes congo red and methyl orange
- Optimization of the biological synthesis of silver nanoparticles using Penicillium oxalicum GRS-1 and their antimicrobial effects against common food-borne pathogens
- Optimization of submerged fermentation conditions to overproduce bioethanol using two industrial and traditional Saccharomyces cerevisiae strains
- Extraction of In3+ and Fe3+ from sulfate solutions by using a 3D-printed “Y”-shaped microreactor
- Foliar-mediated Ag:ZnO nanophotocatalysts: green synthesis, characterization, pollutants degradation, and in vitro biocidal activity
- Green cyclic acetals production by glycerol etherification reaction with benzaldehyde using cationic acidic resin
- Biosynthesis, characterization and antimicrobial activities assessment of fabricated selenium nanoparticles using Pelargonium zonale leaf extract
- Synthesis of high surface area magnesia by using walnut shell as a template
- Controllable biosynthesis of silver nanoparticles using actinobacterial strains
- Green vegetation: a promising source of color dyes
- Mechano-chemical synthesis of ammonia and acetic acid from inorganic materials in water
- Green synthesis and structural characterization of novel N1-substituted 3,4-dihydropyrimidin-2(1H)-ones
- Biodiesel production from cotton oil using heterogeneous CaO catalysts from eggshells prepared at different calcination temperatures
- Regeneration of spent mercury catalyst for the treatment of dye wastewater by the microwave and ultrasonic spray-assisted method
- Green synthesis of the innovative super paramagnetic nanoparticles from the leaves extract of Fraxinus chinensis Roxb and their application for the decolourisation of toxic dyes
- Biogenic ZnO nanoparticles: a study of blueshift of optical band gap and photocatalytic degradation of reactive yellow 186 dye under direct sunlight
- Leached compounds from the extracts of pomegranate peel, green coconut shell, and karuvelam wood for the removal of hexavalent chromium
- Enhancement of molecular weight reduction of natural rubber in triphasic CO2/toluene/H2O systems with hydrogen peroxide for preparation of biobased polyurethanes
- An efficient green synthesis of novel 1H-imidazo[1,2-a]imidazole-3-amine and imidazo[2,1-c][1,2,4]triazole-5-amine derivatives via Strecker reaction under controlled microwave heating
- Evaluation of three different green fabrication methods for the synthesis of crystalline ZnO nanoparticles using Pelargonium zonale leaf extract
- A highly efficient and multifunctional biomass supporting Ag, Ni, and Cu nanoparticles through wetness impregnation for environmental remediation
- Simple one-pot green method for large-scale production of mesalamine, an anti-inflammatory agent
- Relationships between step and cumulative PMI and E-factors: implications on estimating material efficiency with respect to charting synthesis optimization strategies
- A comparative sorption study of Cr3+ and Cr6+ using mango peels: kinetic, equilibrium and thermodynamic
- Effects of acid hydrolysis waste liquid recycle on preparation of microcrystalline cellulose
- Use of deep eutectic solvents as catalyst: A mini-review
- Microwave-assisted synthesis of pyrrolidinone derivatives using 1,1’-butylenebis(3-sulfo-3H-imidazol-1-ium) chloride in ethylene glycol
- Green and eco-friendly synthesis of Co3O4 and Ag-Co3O4: Characterization and photo-catalytic activity
- Adsorption optimized of the coal-based material and application for cyanide wastewater treatment
- Aloe vera leaf extract mediated green synthesis of selenium nanoparticles and assessment of their In vitro antimicrobial activity against spoilage fungi and pathogenic bacteria strains
- Waste phenolic resin derived activated carbon by microwave-assisted KOH activation and application to dye wastewater treatment
- Direct ethanol production from cellulose by consortium of Trichoderma reesei and Candida molischiana
- Agricultural waste biomass-assisted nanostructures: Synthesis and application
- Biodiesel production from rubber seed oil using calcium oxide derived from eggshell as catalyst – optimization and modeling studies
- Study of fabrication of fully aqueous solution processed SnS quantum dot-sensitized solar cell
- Assessment of aqueous extract of Gypsophila aretioides for inhibitory effects on calcium carbonate formation
- An environmentally friendly acylation reaction of 2-methylnaphthalene in solvent-free condition in a micro-channel reactor
- Aegle marmelos phytochemical stabilized synthesis and characterization of ZnO nanoparticles and their role against agriculture and food pathogen
- A reactive coupling process for co-production of solketal and biodiesel
- Optimization of the asymmetric synthesis of (S)-1-phenylethanol using Ispir bean as whole-cell biocatalyst
- Synthesis of pyrazolopyridine and pyrazoloquinoline derivatives by one-pot, three-component reactions of arylglyoxals, 3-methyl-1-aryl-1H-pyrazol-5-amines and cyclic 1,3-dicarbonyl compounds in the presence of tetrapropylammonium bromide
- Preconcentration of morphine in urine sample using a green and solvent-free microextraction method
- Extraction of glycyrrhizic acid by aqueous two-phase system formed by PEG and two environmentally friendly organic acid salts - sodium citrate and sodium tartrate
- Green synthesis of copper oxide nanoparticles using Juglans regia leaf extract and assessment of their physico-chemical and biological properties
- Deep eutectic solvents (DESs) as powerful and recyclable catalysts and solvents for the synthesis of 3,4-dihydropyrimidin-2(1H)-ones/thiones
- Biosynthesis, characterization and anti-microbial activity of silver nanoparticle based gel hand wash
- Efficient and selective microwave-assisted O-methylation of phenolic compounds using tetramethylammonium hydroxide (TMAH)
- Anticoagulant, thrombolytic and antibacterial activities of Euphorbia acruensis latex-mediated bioengineered silver nanoparticles
- Volcanic ash as reusable catalyst in the green synthesis of 3H-1,5-benzodiazepines
- Green synthesis, anionic polymerization of 1,4-bis(methacryloyl)piperazine using Algerian clay as catalyst
- Selenium supplementation during fermentation with sugar beet molasses and Saccharomyces cerevisiae to increase bioethanol production
- Biosynthetic potential assessment of four food pathogenic bacteria in hydrothermally silver nanoparticles fabrication
- Investigating the effectiveness of classical and eco-friendly approaches for synthesis of dialdehydes from organic dihalides
- Pyrolysis of palm oil using zeolite catalyst and characterization of the boil-oil
- Azadirachta indica leaves extract assisted green synthesis of Ag-TiO2 for degradation of Methylene blue and Rhodamine B dyes in aqueous medium
- Synthesis of vitamin E succinate catalyzed by nano-SiO2 immobilized DMAP derivative in mixed solvent system
- Extraction of phytosterols from melon (Cucumis melo) seeds by supercritical CO2 as a clean technology
- Production of uronic acids by hydrothermolysis of pectin as a model substance for plant biomass waste
- Biofabrication of highly pure copper oxide nanoparticles using wheat seed extract and their catalytic activity: A mechanistic approach
- Intelligent modeling and optimization of emulsion aggregation method for producing green printing ink
- Improved removal of methylene blue on modified hierarchical zeolite Y: Achieved by a “destructive-constructive” method
- Two different facile and efficient approaches for the synthesis of various N-arylacetamides via N-acetylation of arylamines and straightforward one-pot reductive acetylation of nitroarenes promoted by recyclable CuFe2O4 nanoparticles in water
- Optimization of acid catalyzed esterification and mixed metal oxide catalyzed transesterification for biodiesel production from Moringa oleifera oil
- Kinetics and the fluidity of the stearic acid esters with different carbon backbones
- Aiming for a standardized protocol for preparing a process green synthesis report and for ranking multiple synthesis plans to a common target product
- Microstructure and luminescence of VO2 (B) nanoparticle synthesis by hydrothermal method
- Optimization of uranium removal from uranium plant wastewater by response surface methodology (RSM)
- Microwave drying of nickel-containing residue: dielectric properties, kinetics, and energy aspects
- Simple and convenient two step synthesis of 5-bromo-2,3-dimethoxy-6-methyl-1,4-benzoquinone
- Biodiesel production from waste cooking oil
- The effect of activation temperature on structure and properties of blue coke-based activated carbon by CO2 activation
- Optimization of reaction parameters for the green synthesis of zero valent iron nanoparticles using pine tree needles
- Microwave-assisted protocol for squalene isolation and conversion from oil-deodoriser distillates
- Denitrification performance of rare earth tailings-based catalysts
- Facile synthesis of silver nanoparticles using Averrhoa bilimbi L and Plum extracts and investigation on the synergistic bioactivity using in vitro models
- Green production of AgNPs and their phytostimulatory impact
- Photocatalytic activity of Ag/Ni bi-metallic nanoparticles on textile dye removal
- Topical Issue: Green Process Engineering / Guest Editors: Martine Poux, Patrick Cognet
- Modelling and optimisation of oxidative desulphurisation of tyre-derived oil via central composite design approach
- CO2 sequestration by carbonation of olivine: a new process for optimal separation of the solids produced
- Organic carbonates synthesis improved by pervaporation for CO2 utilisation
- Production of starch nanoparticles through solvent-antisolvent precipitation in a spinning disc reactor
- A kinetic study of Zn halide/TBAB-catalysed fixation of CO2 with styrene oxide in propylene carbonate
- Topical on Green Process Engineering
Articles in the same Issue
- Regular Articles
- Studies on the preparation and properties of biodegradable polyester from soybean oil
- Flow-mode biodiesel production from palm oil using a pressurized microwave reactor
- Reduction of free fatty acids in waste oil for biodiesel production by glycerolysis: investigation and optimization of process parameters
- Saccharin: a cheap and mild acidic agent for the synthesis of azo dyes via telescoped dediazotization
- Optimization of lipase-catalyzed synthesis of polyethylene glycol stearate in a solvent-free system
- Green synthesis of iron oxide nanoparticles using Platanus orientalis leaf extract for antifungal activity
- Ultrasound assisted chemical activation of peanut husk for copper removal
- Room temperature silanization of Fe3O4 for the preparation of phenyl functionalized magnetic adsorbent for dispersive solid phase extraction for the extraction of phthalates in water
- Evaluation of the saponin green extraction from Ziziphus spina-christi leaves using hydrothermal, microwave and Bain-Marie water bath heating methods
- Oxidation of dibenzothiophene using the heterogeneous catalyst of tungsten-based carbon nanotubes
- Calcined sodium silicate as an efficient and benign heterogeneous catalyst for the transesterification of natural lecithin to L-α-glycerophosphocholine
- Synergistic effect between CO2 and H2O2 on ethylbenzene oxidation catalyzed by carbon supported heteropolyanion catalysts
- Hydrocyanation of 2-arylmethyleneindan-1,3-diones using potassium hexacyanoferrate(II) as a nontoxic cyanating agent
- Green synthesis of hydratropic aldehyde from α-methylstyrene catalyzed by Al2O3-supported metal phthalocyanines
- Environmentally benign chemical recycling of polycarbonate wastes: comparison of micro- and nano-TiO2 solid support efficiencies
- Medicago polymorpha-mediated antibacterial silver nanoparticles in the reduction of methyl orange
- Production of value-added chemicals from esterification of waste glycerol over MCM-41 supported catalysts
- Green synthesis of zerovalent copper nanoparticles for efficient reduction of toxic azo dyes congo red and methyl orange
- Optimization of the biological synthesis of silver nanoparticles using Penicillium oxalicum GRS-1 and their antimicrobial effects against common food-borne pathogens
- Optimization of submerged fermentation conditions to overproduce bioethanol using two industrial and traditional Saccharomyces cerevisiae strains
- Extraction of In3+ and Fe3+ from sulfate solutions by using a 3D-printed “Y”-shaped microreactor
- Foliar-mediated Ag:ZnO nanophotocatalysts: green synthesis, characterization, pollutants degradation, and in vitro biocidal activity
- Green cyclic acetals production by glycerol etherification reaction with benzaldehyde using cationic acidic resin
- Biosynthesis, characterization and antimicrobial activities assessment of fabricated selenium nanoparticles using Pelargonium zonale leaf extract
- Synthesis of high surface area magnesia by using walnut shell as a template
- Controllable biosynthesis of silver nanoparticles using actinobacterial strains
- Green vegetation: a promising source of color dyes
- Mechano-chemical synthesis of ammonia and acetic acid from inorganic materials in water
- Green synthesis and structural characterization of novel N1-substituted 3,4-dihydropyrimidin-2(1H)-ones
- Biodiesel production from cotton oil using heterogeneous CaO catalysts from eggshells prepared at different calcination temperatures
- Regeneration of spent mercury catalyst for the treatment of dye wastewater by the microwave and ultrasonic spray-assisted method
- Green synthesis of the innovative super paramagnetic nanoparticles from the leaves extract of Fraxinus chinensis Roxb and their application for the decolourisation of toxic dyes
- Biogenic ZnO nanoparticles: a study of blueshift of optical band gap and photocatalytic degradation of reactive yellow 186 dye under direct sunlight
- Leached compounds from the extracts of pomegranate peel, green coconut shell, and karuvelam wood for the removal of hexavalent chromium
- Enhancement of molecular weight reduction of natural rubber in triphasic CO2/toluene/H2O systems with hydrogen peroxide for preparation of biobased polyurethanes
- An efficient green synthesis of novel 1H-imidazo[1,2-a]imidazole-3-amine and imidazo[2,1-c][1,2,4]triazole-5-amine derivatives via Strecker reaction under controlled microwave heating
- Evaluation of three different green fabrication methods for the synthesis of crystalline ZnO nanoparticles using Pelargonium zonale leaf extract
- A highly efficient and multifunctional biomass supporting Ag, Ni, and Cu nanoparticles through wetness impregnation for environmental remediation
- Simple one-pot green method for large-scale production of mesalamine, an anti-inflammatory agent
- Relationships between step and cumulative PMI and E-factors: implications on estimating material efficiency with respect to charting synthesis optimization strategies
- A comparative sorption study of Cr3+ and Cr6+ using mango peels: kinetic, equilibrium and thermodynamic
- Effects of acid hydrolysis waste liquid recycle on preparation of microcrystalline cellulose
- Use of deep eutectic solvents as catalyst: A mini-review
- Microwave-assisted synthesis of pyrrolidinone derivatives using 1,1’-butylenebis(3-sulfo-3H-imidazol-1-ium) chloride in ethylene glycol
- Green and eco-friendly synthesis of Co3O4 and Ag-Co3O4: Characterization and photo-catalytic activity
- Adsorption optimized of the coal-based material and application for cyanide wastewater treatment
- Aloe vera leaf extract mediated green synthesis of selenium nanoparticles and assessment of their In vitro antimicrobial activity against spoilage fungi and pathogenic bacteria strains
- Waste phenolic resin derived activated carbon by microwave-assisted KOH activation and application to dye wastewater treatment
- Direct ethanol production from cellulose by consortium of Trichoderma reesei and Candida molischiana
- Agricultural waste biomass-assisted nanostructures: Synthesis and application
- Biodiesel production from rubber seed oil using calcium oxide derived from eggshell as catalyst – optimization and modeling studies
- Study of fabrication of fully aqueous solution processed SnS quantum dot-sensitized solar cell
- Assessment of aqueous extract of Gypsophila aretioides for inhibitory effects on calcium carbonate formation
- An environmentally friendly acylation reaction of 2-methylnaphthalene in solvent-free condition in a micro-channel reactor
- Aegle marmelos phytochemical stabilized synthesis and characterization of ZnO nanoparticles and their role against agriculture and food pathogen
- A reactive coupling process for co-production of solketal and biodiesel
- Optimization of the asymmetric synthesis of (S)-1-phenylethanol using Ispir bean as whole-cell biocatalyst
- Synthesis of pyrazolopyridine and pyrazoloquinoline derivatives by one-pot, three-component reactions of arylglyoxals, 3-methyl-1-aryl-1H-pyrazol-5-amines and cyclic 1,3-dicarbonyl compounds in the presence of tetrapropylammonium bromide
- Preconcentration of morphine in urine sample using a green and solvent-free microextraction method
- Extraction of glycyrrhizic acid by aqueous two-phase system formed by PEG and two environmentally friendly organic acid salts - sodium citrate and sodium tartrate
- Green synthesis of copper oxide nanoparticles using Juglans regia leaf extract and assessment of their physico-chemical and biological properties
- Deep eutectic solvents (DESs) as powerful and recyclable catalysts and solvents for the synthesis of 3,4-dihydropyrimidin-2(1H)-ones/thiones
- Biosynthesis, characterization and anti-microbial activity of silver nanoparticle based gel hand wash
- Efficient and selective microwave-assisted O-methylation of phenolic compounds using tetramethylammonium hydroxide (TMAH)
- Anticoagulant, thrombolytic and antibacterial activities of Euphorbia acruensis latex-mediated bioengineered silver nanoparticles
- Volcanic ash as reusable catalyst in the green synthesis of 3H-1,5-benzodiazepines
- Green synthesis, anionic polymerization of 1,4-bis(methacryloyl)piperazine using Algerian clay as catalyst
- Selenium supplementation during fermentation with sugar beet molasses and Saccharomyces cerevisiae to increase bioethanol production
- Biosynthetic potential assessment of four food pathogenic bacteria in hydrothermally silver nanoparticles fabrication
- Investigating the effectiveness of classical and eco-friendly approaches for synthesis of dialdehydes from organic dihalides
- Pyrolysis of palm oil using zeolite catalyst and characterization of the boil-oil
- Azadirachta indica leaves extract assisted green synthesis of Ag-TiO2 for degradation of Methylene blue and Rhodamine B dyes in aqueous medium
- Synthesis of vitamin E succinate catalyzed by nano-SiO2 immobilized DMAP derivative in mixed solvent system
- Extraction of phytosterols from melon (Cucumis melo) seeds by supercritical CO2 as a clean technology
- Production of uronic acids by hydrothermolysis of pectin as a model substance for plant biomass waste
- Biofabrication of highly pure copper oxide nanoparticles using wheat seed extract and their catalytic activity: A mechanistic approach
- Intelligent modeling and optimization of emulsion aggregation method for producing green printing ink
- Improved removal of methylene blue on modified hierarchical zeolite Y: Achieved by a “destructive-constructive” method
- Two different facile and efficient approaches for the synthesis of various N-arylacetamides via N-acetylation of arylamines and straightforward one-pot reductive acetylation of nitroarenes promoted by recyclable CuFe2O4 nanoparticles in water
- Optimization of acid catalyzed esterification and mixed metal oxide catalyzed transesterification for biodiesel production from Moringa oleifera oil
- Kinetics and the fluidity of the stearic acid esters with different carbon backbones
- Aiming for a standardized protocol for preparing a process green synthesis report and for ranking multiple synthesis plans to a common target product
- Microstructure and luminescence of VO2 (B) nanoparticle synthesis by hydrothermal method
- Optimization of uranium removal from uranium plant wastewater by response surface methodology (RSM)
- Microwave drying of nickel-containing residue: dielectric properties, kinetics, and energy aspects
- Simple and convenient two step synthesis of 5-bromo-2,3-dimethoxy-6-methyl-1,4-benzoquinone
- Biodiesel production from waste cooking oil
- The effect of activation temperature on structure and properties of blue coke-based activated carbon by CO2 activation
- Optimization of reaction parameters for the green synthesis of zero valent iron nanoparticles using pine tree needles
- Microwave-assisted protocol for squalene isolation and conversion from oil-deodoriser distillates
- Denitrification performance of rare earth tailings-based catalysts
- Facile synthesis of silver nanoparticles using Averrhoa bilimbi L and Plum extracts and investigation on the synergistic bioactivity using in vitro models
- Green production of AgNPs and their phytostimulatory impact
- Photocatalytic activity of Ag/Ni bi-metallic nanoparticles on textile dye removal
- Topical Issue: Green Process Engineering / Guest Editors: Martine Poux, Patrick Cognet
- Modelling and optimisation of oxidative desulphurisation of tyre-derived oil via central composite design approach
- CO2 sequestration by carbonation of olivine: a new process for optimal separation of the solids produced
- Organic carbonates synthesis improved by pervaporation for CO2 utilisation
- Production of starch nanoparticles through solvent-antisolvent precipitation in a spinning disc reactor
- A kinetic study of Zn halide/TBAB-catalysed fixation of CO2 with styrene oxide in propylene carbonate
- Topical on Green Process Engineering


