Abstract
Blue coke-based activated carbon (BAC) was prepared via CO2 activation with disused blue coke powder as raw materials at high temperature. The factor of activation temperature was intensively studied. The properties of sample were characterized by N2 adsorption-desorption techniques, scanning electron microscopy (SEM) and Fourier transform infrared (FTIR), and the activation mechanism was also proposed. The results showed that, with the increase of temperature, the yield of BAC decreased, while the iodine adsorption increased first and then decreased. The N2 adsorption-desorption isotherms revealed that the BAC had both micropores and mesopores, and the optimal temperature of porosity development was at 900-1000°C. When the activation temperature reached 1000°C, the maximum Brunauer-Emmett-Teller (BET) specific surface area and pore volume of BAC were 636.91 m2·g-1 and 0.3627 cm3·g-1, respectively. The FTIR results indicated that BAC surface contained large amounts of surface functional groups such as hydroxyl, ester, carboxyl, and so on. The content of them decreased with the increase of temperature. Mechanism analysis shows that radial hole-making function happen first then transverd hole-enlarging function as the temperature increases, for the formation of large amount of microporous, the radial activation was the main controlling process.
1 Introduction
The solid carbon material blue coke is prepared by medium and low temperature dry distillation with Jurassic nonstick coal and high volatile bituminous coal. These raw materials are widespread in the Shaan-Gan-Ning-Meng-Jin Region of Northwestern China [1]. In these areas, blue coke plays an important role in the coal chemical industry, because of its high fixed-carbon content, specific resistance, chemical activity, low ash content, sulfur content, phosphorus content, and volatility. Blue coke is commonly used in industries like calcium carbide, ferroalloy, ferrosilicon and silicon carbide, even as a substitute of metallurgical coke in some field [2].
During blue coke production, transportation, and storage, the powder byproduct with the grain size < 6 mm is inevitably generated, which has very low utilization and causes serious environmental challenges [1]. Statistics show that nearly one million tons of blue coke powder is being produced every year in Yulin Area, which seriously affects the sustainable development of the blue coke industry [3]. Finding clean and efficient processing and utilization technology of blue coke powder has great significance for the sustainable development of the blue coke industry.
One of the effective strategies to treat this challenge is producing the blue coke-based activated carbon (BAC), where the blue coke powder is transformed into the activated carbon using certain techniques. As the blue coke is easy to obtain, and activated carbon is widely used in daily life and industrial fields, this strategy shows promising prospect for application. Although the BAC was successfully obtained by activation with chemical agent and steam from our previous study, the chemical activation method has some drawbacks including high pollution, strong corrosiveness, the remainder activating reagent on the surfaces of BAC cannot be removed completely [4], the main problem of steam activation method is high energy consumption and low microporosity. So the industrialization promotion of BAC preparation technology needs more efforts.
Carbon dioxide is a commonly used and an effective activator for the activation techniques of activated carbon (AC), since it is clean, cheap, easy to obtain, and facile to control. A type of microporous AC was prepared from Pinang frond using CO2 as the activation agent [5]. The specific surface area and the pore volume of the products is 958.23 m2/g and 0.5469 mL/g, which has 87.6% adsorption rate of Remazol Brilliant Blue R (RBBR). Carbon dioxide activation for the super-activated carbon [6] yields a product with a 3500 m2/g specific surface area and a 1.84 cm3/g pore volume at 1100°C. Ruidong Zhao et al. [7] studied the effect that the marketed coconut shell activated carbon reactivation by CO2 had on pore structures. The micropore volume of product increases from 0.21 cm3/g to 0.27 cm3/g, the specific surface area increases from 627.22 m2/g to 822.71 m2/g, and there is a 23.77% increase in absorption capacity for the phenol after reactivation. By comparison with other activating reagent, the CO2 activation results in a better microporous structure, a larger micropore volume, and a narrower pore size distribution. Jerzy Choma et al. [8] studied the effects that CO2 and KOH activation had on the pore structure of AC made from Kevlar-derived carbon fibers. The results show that AC activated by CO2 has a more abundant microporous structure and has a better adsorption effect on CO2. The specific surface area and the pore volume is 1240 m2/g and 0.61 cm3/g, when the reaction is performed at 750°C for 3 h. Min She et al. [9] investigated the micropore morphology of AC via CO2 and H3PO4 activation with rice husk as the raw material. The results indicate that the AC activated by CO2 has flask shaped micropores and the specific surface area is 563.06 m2/g, which is greater than when activated by H3PO4. The microporous activated carbon that was prepared by K. Suresh Kumar Reddy et al. [10] through CO2 activation with date palm pits as the raw material had an average pore size of 1.51 nm. However, another AC activated by H3PO4 has additional mesopores with an average pore size of 2.91 nm. Xiangkun Jian et al. [11] found that the micropore content between 0.5-1.0 nm obtained by CO2 activation are 10% greater than when obtained by steam activation in the same experiment conditions. Similar conclusions have been drawn by Arenas [12] and Alcaniz [13]. Many scholars have studied the main controlling factors of CO2 activation. Mohammad Mazlan et al. [14] suggest that temperature is the main factor of rubber wood sawdust activation via CO2. With the increase of activation temperature, the specific surface area, the pore volume, and the microporosity initially increase and then decrease. The process of CO2 physical activation with a Hawaii nut shell raw material was analyzed using response surface methodology by Song Cheng et al. [15], showing that the activation temperature and the time have the greatest effect on the development of AC micropores. The temperature is shown to be the main control indicator of the activation process [16, 17, 18].
Although CO2 is a mature and high quality activation agent, few studies have focused on the activation of disused blue coke powder. In this paper, disused blue coke powder was employed to prepare the BAC with CO2 activation at high temperatures. The activation mechanism was explored by investigating the effect of activation temperature on the pore structure and the surface properties. The obtained results provide fundamental insights into the preparation process of BAC. Our results will also offer guidance in utilizing disused blue coke powder to prepare BAC.
2 Materials and methods
2.1 Materials
The blue coke powder was provided by Shenmu Sanjiang Coal Chemical Co., Ltd. The industrial and the elemental analyses of the blue coke powder are shown in Table 1.
Industrial analysis and elemental analysis of the blue coke powder.
| Industrial analysis | Elemental analysis | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Sample | Mad | Aad | Vad | FCad | Cad | Oad | Had | Nad | St,ad |
| Blue coke powder | 2.15 | 16.77 | 12.07 | 69.01 | 72.88 | 0.32 | 1.06 | 0.88 | 0.61 |
2.2 Methods
2.2.1 Raw material pre-treatment
The blue coke powder with granularities ranging between 2 mm to 3.2 mm were immersed in 1.0 mol/L hydrochloric acid and sodium hydroxide solution for 12 h respectively. The solid sample was washed with distilled water until the pH was neutral. The material was stored in an airtight package after it was dried at 110°C in a vacuum oven (DZF–6053) for 12 h.
2.2.2 Preparation of BAC
BAC preparation was conducted as follows: 5.0 g of blue coke powder after pre-treatment was placed into a self-made quartz reactor, which was placed into tubular heaters (TCXT–1700) and heated to 600°C, 700°C, 800°C, 900°C, 1000°C, and 1100°C under the protection of nitrogen. When heating to the activation temperature, CO2 was added, instead of N2, under a 200 L/h gas flow rate. After 120 min of activation, adding CO2 was stopped, and the BAC was cooled to room temperature under N2. The resulting materials were termed BAC–600, BAC–700, BAC–800, BAC–900, BAC–1000, and BAC–1100. The experimental device is shown in Figure 1.

CO2 activation device.
1 – CO2 Steel cylinder, 2 – N2 Steel cylinder, 3 – Valve, 4 – Flowmeter, 5 – Thermocouple, 6 – Tubular furnace, 7 – Quartz reactor, 8 – Raw material, 9 – Temperature controller, 10 – NaOH solution.
2.2.3 Characterization of prepared BAC
The yield was calculated from the mass ratio of product to raw material. The iodine number was determined following GB/T 7702.7–2008 (China). The nitrogen adsorption-desorption isotherm was obtained using a fully automatic physical adsorption apparatus (ASAP2420) at 77.35 K. The specific surface area of the BAC was calculated based on the Brunauer-Emmett-Teller (BET) model. The total pore volume and the pore size distribution of the BAC were analyzed via the Barrett-Joyner-Halenda (BJH) method. The micropore volume of the BAC was calculated with the t-plot method. The mesopore volume of the BAC was the difference between the total pore volume and the micropore volume. The microporosity of the BAC was the ratio of micropore volume to the total pore volume. The morphology of the BAC was examined via JSM-6700F scanning electron microscopy (SEM). The surface functional groups of the BAC were identified by IR-Prestige-21 Fourier transform infrared (FTIR) spectroscopy.
3 Results and discussion
3.1 The iodine adsorption value and the yield of BACs
The effect of the activation temperature on the iodine adsorption value and the yield of BACs are shown in Figure 2. The iodine adsorption value was regarded as an important index for measuring the level of activation, the microporous structure, and the adsorption capacity. The iodine adsorption value of the BACs initially increased between 600-1000°C, and then decreased as the activation temperature increased (Figure 2). When the temperature was higher than 700°C, particularly between 900°C to 1000°C, the iodine adsorption value increased drastically. At 1000°C, the iodine adsorption value reached to maximum, 856.19 mg/g.
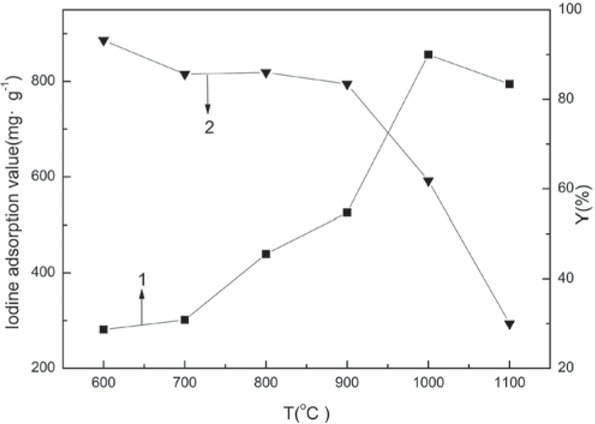
The effect that the activation temperature had on the iodine adsorption value and the BAC yield.
1 – Iodine adsorption value and 2 – Yield.
The yield represented the loss of raw material in the process of activation, which could reflect the changes of the pores inside the material to some extent. Figure 2 shows that the yield of the BAC decreased gradually as the temperature increased. When the temperature rose from 900°C to 1000°C, and then to 1100°C, the yield decreased from 83.4% to 61.8% and then to 30.0%, respectively. This illustrated that the carbon inside the raw material was continuously lost. The new pores during CO2 activation were constantly generated.
The results of the analysis showed that activation reaction rate of blue coke powder with CO2 was increased dramatically when the temperature was higher than 700°C. The reaction was even more severe when the temperature reached 900°C. At this point, the large amount of pores that were produced caused a sudden drop in yield, which was beneficial for the adsorption. The reduction of the iodine adsorption value when the temperature continued to rise to 1100°C can be attributed the over-activation of the raw materials, which led to a large number of carbon being overreacted and consumed. The entire structure of BAC loosened and a large number of micropores evolved into mesopores or macropores, so the adsorption capacity was weakened.
3.2 The pore structures of BACs
The nitrogen adsorption-desorption isotherm of BAC was studied to characterize the porosity texture of carbonaceous adsorbents, and analyzed in to further investigate the effects of the temperature on the specific surface area and the pore structure. The isotherm could provide structure parameters of the adsorbent, which were an important index for measuring the adsorption properties. The nitrogen adsorption-desorption isotherms, the pore size distribution, and the pore structure parameters of the BACs are shown in Figures 3 and 4, and Table 2.

N2 adsorption-desorption isotherm of the BACs.
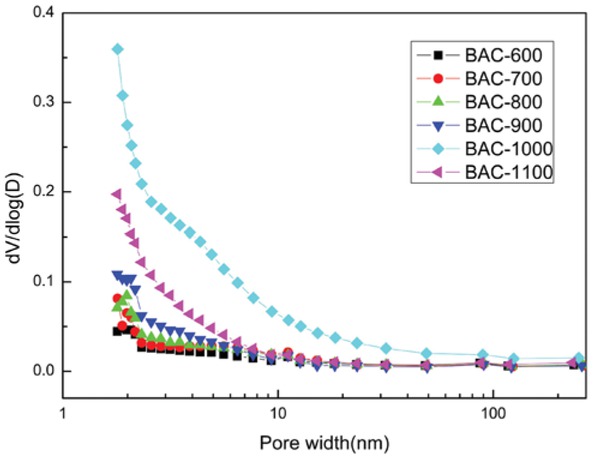
Pore size distribution of the BACs.
Pore structure parameters of the BACs.
| SBET | Smicro | Vtotal | Vmicro | Vmicro | dave | |
|---|---|---|---|---|---|---|
| Sample | (m2·g-1) | (m2·g-1) | (cm3·g-1) | (cm3·g-1) | /Vtotal(%) | (nm) |
| Blue coke powder | 7.77 | - | 0.025 | 0.002 | 7.62 | 13.05 |
| BAC–600 | 75.50 | 48.22 | 0.059 | 0.029 | 49.38 | 3.119 |
| BAC–700 | 93.59 | 62.30 | 0.076 | 0.039 | 51.52 | 1.469 |
| BAC–800 | 126.60 | 84.99 | 0.089 | 0.049 | 55.06 | 2.822 |
| BAC–900 | 247.44 | 190.50 | 0.130 | 0.084 | 64.52 | 2.104 |
| BAC–1000 | 636.91 | 457.40 | 0.363 | 0.201 | 68.87 | 2.28 |
| BAC–1100 | 503.40 | 407.50 | 0.238 | 0.164 | 55.50 | 1.89 |
As seen in Figure 3, the isotherms of BACs had a similar tendency, where it shifted upward gradually initially and then downward as the temperature rose, which indicated that the BAC adsorption capacity of N2 increased first and then decreased. The isotherm moved up obviously when the temperature rose from 900°C to 1000°C, which was the optimal temperature range. The adsorption capacity of N2 increased from 83.22 cm3/g to 234.49 cm3/g. When the temperature increased to 1100°C, the isotherm moved down and the adsorption capacity of N2 dropped to 153.74 cm3/g, which were in good agreement with those obtained by the iodine adsorption value analysis.
The results of nitrogen adsorption-desorption isotherms were used to calculate pore structure parameters, which is shown in Figures 2. The BET specific surface area, the microporous specific surface area, and the micropore volume of the BACs initially increased and
then decreased as the activation temperature increased. The BAC with maximum BET specific surface area and pore volume were obtained when the temperature was 1000°C, and the values were 636.91 m2/g and 0.363 cm3/g respectively. The pore structure parameters decreased when the temperature rose to 1100°C, indicating that the optimal activation temperature was 1000°C.
As shown in Figure 3, the isotherms belong to combination of type I and IV according to the International Union of Pure and Applied Chemistry (IUPAC) classification. The adsorption capacity increased sharply as P/P0 < 0.1, owing to the greatest adsorption rate at this time. When P/P0 > 0.1, the adsorption capacity remained unchanged, the shape of isotherm change to a horizontal platform, this proofs that the isotherms belongs to the type I according to the IUPAC classification. The van der Waals force caused a significant increase of adsorption potential inside the micropores, due to the adsorption force of pore with a short distance superimposed on each other. The absorption of N2 on BACs surface was a rapid process, which was completed at low relative pressure during a surprisingly short time. The characteristic of the microporous adsorption was present. Meanwhile, when the relative pressure was close to 1, the adsorption capacity of BACs increased slightly, and the isotherm showed a tailing and hysteresis phenomenon, which indicating that the isotherms belongs to the type IV also, and there were some mesopores and macropores inside of the BACs in addition to the micropores. As seen in Table 2, the microporosity of the BACs remained between 49.38% and 68.87%.
The pore size distributions of the BACs calculated by the BJH method is illustrated in Figure 4, where they had different shapes, but similar trends. The maximum peak occurred at BAC–1000, which had the most well-developed pore structure. The pore size peaks of the products were obtained between 1.5 mm to 2.5 mm, which indicated that the vast majority of the pores fell within the micropore and mesopore range. This conclusion confirmed the pore structure parameters and the results from the nitrogen adsorption-desorption isotherms.
3.3 The SEM images of BACs
SEM was used to observe the surface physical morphology of materials. The SEM images of BACs are shown in Figure 5. With rising of activation temperature, the morphology of BACs in the front side (Figures 5a-f) and the lateral sides (Figures 5g-l) had obvious differences. The morphologies of front side is observed from axial direction of pores, and the lateral side is observed from radial direction, which perpendicular to the direction of front side. When the temperature reached 600°C, the BAC–600 had very few pores, as seen from both front side (Figure 5a) and lateral sides (Figure 5g) which showed that the activation rate was relatively low at this temperature. As the activation temperature increased, the roughness and the defects gradually began to emerge in the surface of the material, which caused slit-shaped pores. The development of the fiber’s structure in the BACs was shown from the lateral sides of the products. The formation of pores and the rising of temperatures would help the CO2 diffuse into the inner the material, which further accelerated the process of CO2 activation and the pore formation.
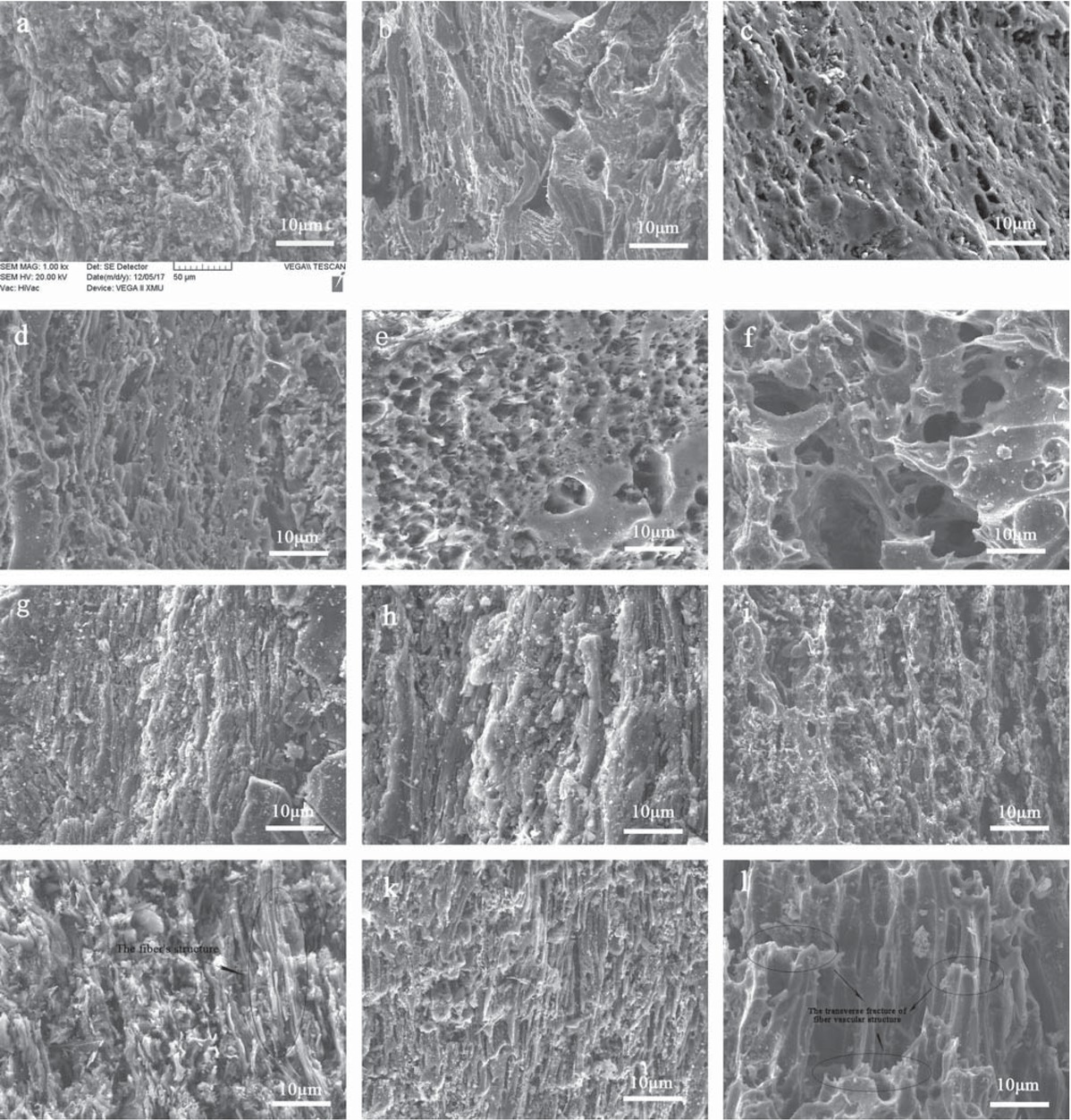
SEM images of BACs: (a,g) BAC-600, (b,h) BAC-700, (c,i) BAC-800, (d,g) BAC-900, (e,k) BAC-1000, (f,l) BAC-1100.
When the activation temperature rose to 1000°C, the front of BAC–1000 morphology (Figure 5e) showed many circular pores with different apertures in material surface, which formed into a honeycomb cavernous structure. The fiber vascular structure of BAC–1000 also advanced in lateral the sides of the morphology (Figure 5k) which signified that the well-developed pore structure had been formed.
The pore wall of the BAC–1100 had been destroyed as the temperature increased, and the additional macropores are formed from the collapse of mesopores and micropores. As seen from the lateral sides of the morphology (Figure 5l) the pores of BAC–1100 had a transverse fracture, the original fiber vascular structure was destroyed. These changes of pore morphology was proof that the BAC–1100 was over-activated, which was in good agreement with the experimental result of the adsorption isotherms and the pore structure parameters that were analyzed previously. There were some irregular solid particles that adhered to the surface of the BACs, these particles were the ash of the raw material that did not react with the CO2 in the high temperature.
3.4 The surface functional group of BACs
The surface functional groups on the BACs were analyzed by FTIR, and the results are shown in Figure 6. The broad absorption bands at 3650-3250 cm-1 were due to the stretching vibration of the hydroxyl (O–H) [19] or the water molecule adsorbed by the BACs [20]. The weak absorption peaks at 2920-2840 cm-1 were attributable to the C–H stretching vibration of the asymmetric aliphatic hydrocarbons, which showed that the BACs contained some –CH2 and –CH3 groups. The weak absorption bands at 1600-1400 cm-1 were indexed to the stretching vibration of C=O and C=C, or by the in-plane bending vibration of N–H, which indicated that the BACs contained some carboxylic acid, fatty ketone, or amino groups functional groups. The absorption peaks at 1300-1000 cm-1 were attributed to the C–O stretching vibrations, which are resulted from the hydroxyl, ester, and ether functional groups on the surface of the BACs [21]. The characteristic absorption peaks of the BACs were similar, although the intensity of absorption peak gradually decreased as the temperature increased. When the activation temperature exceeded 1000°C the absorption bands at 2920-2840 cm-1 and 1600-1400 cm-1 were no longer observed. This was because the side chains of partial functional groups in the surface of the raw material broke and separated from the carbon skeleton by the CO2 activation at high treatment temperatures, which led to the decreased content of the surface functional groups. The activation temperature had an obvious influence on the content of the surface functional groups.

FTIR spectra of the BACs.
3.5 Activation mechanism analysis
The essence of the CO2 activation was a process of partial gasification reactions between C and CO2, which exhibited an endothermic reaction. The reaction formula is described as follows (Reaction 1):
The reaction mechanism is described in Reactions 2-5:
where C* is the active point in the blue coke crystallites and ( ) means the atom or molecule in brackets is the adsorption state.
From a microscopic perspective, the activation reaction starts from the activity sites in the surface of raw material, which exhibited a high affinity. These activate sites were primarily distributed in the end, the section, and the lattice defect of the blue coke microcrystallites [22]. The heat transfer from the outside to the inside as the temperature rise. The detailed activation reaction is described as follows: the CO2 diffused to the active sites and is adsorbed on them at the beginning of the activation reaction. As the temperatures increased, reactions seen in Reactions 3 and 4 occurred. The CO was produced and released because of these two reactions. Partial oxygen atoms were attached to the surface of the blue coke [23], which produced CO with blue coke, or formed CO2 with CO as Reaction 5 illustrated. Consumption of CO as seen in Reaction 5 effectively accelerated Reactions 3 and 4, which promoted the activation reaction, resulted in continuous consumption of blue coke.
The macro level analysis revealed that the CO2 activation went through three procedures: hole-throughing, hole-making, and hole-enlargement [24]. The activation process was divided into three stages in different temperature ranges: the initial activation stage (600-900°C), the rapid activation stage (900-1000°C), and the over-activation stage (1000-1100°C). There were two main types of CO2 activation regarding to the pore development at different temperatures: the radial activation and the transverse activation. The variation of pore structure are shown in Figure 7.

The variation of pore structure in CO2 activation.
When the temperature reached 600°C, the activity sites in the surface of raw material were present only on a small percentage of the specific surface area, so the reaction was slow. With the rise of the activation temperature, the reactivity of the carbon atom on the surface of the blue coke increased, and the diffusion rate of CO2 molecule transport into the blue coke interior sped up. This resulted in the increased reaction rate as the reactive probabilities of CO2 with the carbon atom increased. The activated reaction was mainly in the radial direction rather than transverse direction [25], which resulted in new micropores forming continuously in the materials. The pore structure parameters also increased at the same time. The radial activation had a lower activation reaction rate, and was a progressive process. In addition, a small number of molten coal tar existed in the blue coke, which was decomposed into gaseous hydrocarbons escaping from the material to remove the blockage in pores.
The reaction rate accelerated rapidly due to the raised activity of the carbon atoms on surface of the raw material when the temperature increased to 1000°C. The carbon atom on the edges and the dislocations of the blue coke powder were reacted and eroded continuously. The activation reaction remained running from outside to inside and the yield dropped significantly. The hole-making function was a result of the radial activation and was dominant in this stage. The through-hole function occurred constantly, which induced the formation of a large number of new micropores and the pore structure parameters of the BACs increased.
The activation reaction was further aggravated when the temperature reached 1100°C, but the radial activation was not obvious, due to the decreased temperature gradients from inside to outside, so the reaction between C and CO2 occurred in the transverse direction. The transverse activation was stronger than the radial activation, which resulted in significant pore wall thinning and increased average pore sizes, suggesting the hole-enlargement function took place. The fibrous vascular structure of the BAC–1100 was burnt out and led to a collapse, where the number of mesopores and macropore rose and the yield decreased sharply. The collapsed carbon in the BAC–1100 would block the pores. This was caused the decline of the pore structure parameters and the iodine adsorption value. Similar conclusions were obtained by Roman et al. [26], who prepared AC from olive stone via CO2 activation.
By mechanism analysis, with the increase of temperature, a large number of micropores were initially produced because of radial activation. The transverse activation then took place, the micropores collapse caused the production of mesopores and macropores. In order to obtain the activated carbon with well-developed microporous structure and a high specific surface area, the radial activation via the control of activation temperature must be paid more attention.
4 Conclusions
The activation temperature had a remarkable effect on the pore structure and the adsorption properties of the BACs. As the activation temperature increased, the iodine adsorption value increased first then decreased, and the yield declined continuously.
The activation temperatures between 900°C and 1000°C were the best stages of the activation process. The highest specific surface area and the pore volume were 636.91 m2·g-1 and 0.363 cm3·g-1. The N2 adsorption isotherm belonged to the combination of type I and IV according to the IUPAC classification, which indicated that the sample had both a microporous and a mesoporous structure.
The FTIR results indicated that the BACs contained surface functional groups of hydroxyl, ester, ether, and carboxylic acid. The surface functional group content of the BACs decreased gradually as the activation temperature increased, but there were few significant effects on the type of surface functional groups.
The CO2 activation was essentially the partial gasification between C and CO2, which began at the activity sites in the surface of the raw material that exhibited a high affinity. The activation of the radial hole-making function from outside to inside initially occurred, and then the transverse hole-enlargement function took place. The radial activation was controlled by the activation temperature, which was beneficial for the preparation of the BAC with well-developed micropores and a high specific surface area.
Acknowledgements
This project was financially supported by the National Natural Science Foundation of China (51774227) and the Scientific Research Plan Projects of Shaanxi Education Department (No. 17JK1170).
List of Abbreviations
- ad –
air-dry basis
- Mad –
moisture
- Aad –
ash content
- Vad –
volatiles
- FCad –
fixed carbon
- Cad –
carbon
- Oad –
oxygen (by difference)
- Had –
hydrogen
- Nad –
nitrogen
- St,ad –
total sulfur
- *FCad =
100%-(Vad+Aad+Mad)
- *Oad =
100%-(Cad+Had+Nad+Sad+Aad+Mad)
- Y –
yield
- SBET –
specific surface area
- Smicro –
microporous specific surface area
- Vtotal –
pore volume
- V micro –
microporous pore volume
- d ave –
average pore diameter
References
[1] Song Y.H., Ma Q.N., Li X., Zhou J., Tian Y.H., The influence of activation temperature on structure and properties of semi-coke-based activated carbon. Mater. Rev., 2016, 30(1), 34-37.Search in Google Scholar
[2] Tian Y.H., Lan X.Z., Song Y.H., Liu C.B., Zhou J., Preparation and characterization of formed activated carbon from fine blue-coke. Int. J. Energy. Res., 2015, 39, 1800-1806.10.1002/er.3327Search in Google Scholar
[3] Tian Y.H., Lan X.Z., Li L.B., Chen X.Y., Hu T.H., Preparation of activated carbon from blue coke powder and its adsorption properties of Cr(VI). Adv. Mater. Res., 2010, 10, 1347-1351.10.4028/www.scientific.net/AMR.156-157.1347Search in Google Scholar
[4] Tian Y.H., Lan X.Z., Zhou J., Chen X.Y., Li L.B., Reparation of activated carbon from blue coke powder by microwave radiation and KOH activation. Chem. Eng., 2010, 38(10), 225-228.Search in Google Scholar
[5] Ahmad M.A., Herawan S.G., Yusof A.A., Effect of activation time on the pinang frond based activated carbon for remazol brilliant blue R removal. J. Mech. Eng. Sci., 2014, 12(7), 1085-1093.10.15282/jmes.7.2014.7.0105Search in Google Scholar
[6] Plaza-Recobert M., Trautwein G., Pérez-Cadenas M., Alcañiz-Monge J., Superactivated carbons by CO2 activation of loquat stones. Fuel Process. Technol., 2017, 159, 345-352.10.1016/j.fuproc.2017.02.006Search in Google Scholar
[7] Zhao R.D., Liu F.L., Zheng S.R., Wan H.Q., Xu Z.Y., Adsorption of phenol on CO2-treated activated carbon. Environ. Pollut. Control, 2010, 32(10), 33-36.Search in Google Scholar
[8] Choma J., Osuchowski L., Marszewski M., Dziura A., Jaroniec M., Developing microporosity in kevlar-derived carbon fibers by CO2 activation for CO2 adsorption. J. CO2 Util., 2016, 16, 7-22.10.1016/j.jcou.2016.05.004Search in Google Scholar
[9] She M., Duan Y.F., Zhu C., Hong Y.G., Zhou Q., Wang S.Q., Experiment study on mercury adsorption performances of rice husk chars activated by CO2H3PO4 and modified by NH4Br. J. Southeast Univ.: Nat. Sci. Ed., 2014, 44(2), 321-327.Search in Google Scholar
[10] Reddy K.S.K., Shoaibi A.A., Srinivasakannan C., A comparison of microstructure and adsorption characteristics of activated carbons by CO2 and H3PO4 activation from date palm pits. New Carbon Mater., 2012, 27(5), 344-351.10.1016/S1872-5805(12)60020-1Search in Google Scholar
[11] Jian X.K., Liu S.C., Bian Y., Effect of activation medium on microstructure and CO2 adsorption performance of activated carbon. J. Funct. Mater., 2014, 45(1), 1095-1098.Search in Google Scholar
[12] Arenas E., Chejne F., The effect of the activating agent and temperature on the porosity development of physically activated coal chars. Carbon, 2004, 42(12-13), 2451-2455.10.1016/j.carbon.2004.04.041Search in Google Scholar
[13] Alcañiz-Monge J., Cazorla-Amorós D., Linares-Solano A., Yoshida S., Oya A., Effect of the activating gas on tensile strength and pore structure of pitch-based carbon fibres. Carbon, 1994, 32 (7), 1277-1283.10.1016/0008-6223(94)90113-9Search in Google Scholar
[14] Mazlana M.A.F., Uemuraa Y., Yusupa S., Elhassana F., Uddin A., Hiwada A., el al., Activated carbon from rubber wood sawdust by carbon dioxide activation. Procedia Eng., 2016, 148, 530-537.10.1016/j.proeng.2016.06.549Search in Google Scholar
[15] Cheng S., Zhang L.B., Xia H.Y., Peng J.H., Zhang S.Z., Zhou C.J., Preparation of activated carbon from Hawaii nut shell via CO2 activation using response surface methodology. Chin. J. Environ. Eng., 2015, 9(9), 4495-4502.Search in Google Scholar
[16] Luo H.M., Liu J., Feng H.X., Zhang D.Y., Zhang J.Q., Study on activated carbon preparation with abandoned coke fines activated with KOH-K2CO3 Fuel. Chem. Proc., 2008, 39(4), 42- 45.Search in Google Scholar
[17] Muniandy L., Adam F., Mohamed A.R., Eng-Poh N., The synthesis and characterization of high purity mixed microporous/mesoporous activated carbon from rice husk using chemical activation with NaOH and KOH. Microp. Mesop. Mater., 2014, 197, 316-323.10.1016/j.micromeso.2014.06.020Search in Google Scholar
[18] Xu B., Wu F., Cao G.P., Yang Y.S., Effect of carbonization temperature on microstructure of PAN-based activated carbon fibers prepared by CO2 activation. New Carbon Mater., 2006, 21(1), 14-18.Search in Google Scholar
[19] Huang Y., Huang Y., Wang W.Q., Feng Q.M., Hu S.L., Synthesis and characterization of hydrohar adsorbent from walnut shell. J. Xinjiang Agr. Univ., 2016, 39(2), 149-154.Search in Google Scholar
[20] Liu C.B., Lan X.Z., Tian Y.H., Song Y.H., Influence of carbonization on temperature on the performance of formed activated carbon based on blue-coke. Coal Convers., 2012, 35(2), 69-72.Search in Google Scholar
[21] Jian X.K., Liu S.C., Bian Y., Technique research on preparation of activated carbon from corncob with boric acid catalytic. J. Cent. South. Univ. Forest. Technol., 2012, 32(10), 198-202.Search in Google Scholar
[22] Zhang L.B., Peng J.H., Yang K.B., Xia H.Y., Guo S.H., Zhang S.M., Preparation and characterization of activated carbons from tobacco stems with CO2 activation by microwave irradiation. Chem. Eng., 2007, 35(5), 67-70.Search in Google Scholar
[23] Nabais J.V., Carrott P., Carrott M.R., Luz V., Ortiz A.L., Influence of preparation conditions in the textural and chemical properties of activated carbons from a novel biomass precursor: The coffee endocarp. Bioresource Technol., 2008, 99(15), 7224-7231.10.1016/j.biortech.2007.12.068Search in Google Scholar
[24] Rodriguez-Reinoso F., Molina-Sabio M., Gonzlez M.T., The use of steam and CO2 as activating agents in the preparation of activated carbons. Carbon, 1995, 33(1), 15-23.10.1016/0008-6223(94)00100-ESearch in Google Scholar
[25] Xing W., Zhang M.J., Yan Z.F., Synthesis and activation mechanism of coke based super activated carbons. Acta. Phys-chim. Sin., 2012, 18(4), 340-345.10.3866/PKU.WHXB20020411Search in Google Scholar
[26] Roman S., Gonzalez J.F., Gonzalez C.M., Zamora F., Control of pore development during CO2 and steam activation of olive stone. Fuel Process. Technol., 2008, 89(8), 715-720.10.1016/j.fuproc.2007.12.015Search in Google Scholar
© 2019 Lan et al., published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 Public License.
Articles in the same Issue
- Regular Articles
- Studies on the preparation and properties of biodegradable polyester from soybean oil
- Flow-mode biodiesel production from palm oil using a pressurized microwave reactor
- Reduction of free fatty acids in waste oil for biodiesel production by glycerolysis: investigation and optimization of process parameters
- Saccharin: a cheap and mild acidic agent for the synthesis of azo dyes via telescoped dediazotization
- Optimization of lipase-catalyzed synthesis of polyethylene glycol stearate in a solvent-free system
- Green synthesis of iron oxide nanoparticles using Platanus orientalis leaf extract for antifungal activity
- Ultrasound assisted chemical activation of peanut husk for copper removal
- Room temperature silanization of Fe3O4 for the preparation of phenyl functionalized magnetic adsorbent for dispersive solid phase extraction for the extraction of phthalates in water
- Evaluation of the saponin green extraction from Ziziphus spina-christi leaves using hydrothermal, microwave and Bain-Marie water bath heating methods
- Oxidation of dibenzothiophene using the heterogeneous catalyst of tungsten-based carbon nanotubes
- Calcined sodium silicate as an efficient and benign heterogeneous catalyst for the transesterification of natural lecithin to L-α-glycerophosphocholine
- Synergistic effect between CO2 and H2O2 on ethylbenzene oxidation catalyzed by carbon supported heteropolyanion catalysts
- Hydrocyanation of 2-arylmethyleneindan-1,3-diones using potassium hexacyanoferrate(II) as a nontoxic cyanating agent
- Green synthesis of hydratropic aldehyde from α-methylstyrene catalyzed by Al2O3-supported metal phthalocyanines
- Environmentally benign chemical recycling of polycarbonate wastes: comparison of micro- and nano-TiO2 solid support efficiencies
- Medicago polymorpha-mediated antibacterial silver nanoparticles in the reduction of methyl orange
- Production of value-added chemicals from esterification of waste glycerol over MCM-41 supported catalysts
- Green synthesis of zerovalent copper nanoparticles for efficient reduction of toxic azo dyes congo red and methyl orange
- Optimization of the biological synthesis of silver nanoparticles using Penicillium oxalicum GRS-1 and their antimicrobial effects against common food-borne pathogens
- Optimization of submerged fermentation conditions to overproduce bioethanol using two industrial and traditional Saccharomyces cerevisiae strains
- Extraction of In3+ and Fe3+ from sulfate solutions by using a 3D-printed “Y”-shaped microreactor
- Foliar-mediated Ag:ZnO nanophotocatalysts: green synthesis, characterization, pollutants degradation, and in vitro biocidal activity
- Green cyclic acetals production by glycerol etherification reaction with benzaldehyde using cationic acidic resin
- Biosynthesis, characterization and antimicrobial activities assessment of fabricated selenium nanoparticles using Pelargonium zonale leaf extract
- Synthesis of high surface area magnesia by using walnut shell as a template
- Controllable biosynthesis of silver nanoparticles using actinobacterial strains
- Green vegetation: a promising source of color dyes
- Mechano-chemical synthesis of ammonia and acetic acid from inorganic materials in water
- Green synthesis and structural characterization of novel N1-substituted 3,4-dihydropyrimidin-2(1H)-ones
- Biodiesel production from cotton oil using heterogeneous CaO catalysts from eggshells prepared at different calcination temperatures
- Regeneration of spent mercury catalyst for the treatment of dye wastewater by the microwave and ultrasonic spray-assisted method
- Green synthesis of the innovative super paramagnetic nanoparticles from the leaves extract of Fraxinus chinensis Roxb and their application for the decolourisation of toxic dyes
- Biogenic ZnO nanoparticles: a study of blueshift of optical band gap and photocatalytic degradation of reactive yellow 186 dye under direct sunlight
- Leached compounds from the extracts of pomegranate peel, green coconut shell, and karuvelam wood for the removal of hexavalent chromium
- Enhancement of molecular weight reduction of natural rubber in triphasic CO2/toluene/H2O systems with hydrogen peroxide for preparation of biobased polyurethanes
- An efficient green synthesis of novel 1H-imidazo[1,2-a]imidazole-3-amine and imidazo[2,1-c][1,2,4]triazole-5-amine derivatives via Strecker reaction under controlled microwave heating
- Evaluation of three different green fabrication methods for the synthesis of crystalline ZnO nanoparticles using Pelargonium zonale leaf extract
- A highly efficient and multifunctional biomass supporting Ag, Ni, and Cu nanoparticles through wetness impregnation for environmental remediation
- Simple one-pot green method for large-scale production of mesalamine, an anti-inflammatory agent
- Relationships between step and cumulative PMI and E-factors: implications on estimating material efficiency with respect to charting synthesis optimization strategies
- A comparative sorption study of Cr3+ and Cr6+ using mango peels: kinetic, equilibrium and thermodynamic
- Effects of acid hydrolysis waste liquid recycle on preparation of microcrystalline cellulose
- Use of deep eutectic solvents as catalyst: A mini-review
- Microwave-assisted synthesis of pyrrolidinone derivatives using 1,1’-butylenebis(3-sulfo-3H-imidazol-1-ium) chloride in ethylene glycol
- Green and eco-friendly synthesis of Co3O4 and Ag-Co3O4: Characterization and photo-catalytic activity
- Adsorption optimized of the coal-based material and application for cyanide wastewater treatment
- Aloe vera leaf extract mediated green synthesis of selenium nanoparticles and assessment of their In vitro antimicrobial activity against spoilage fungi and pathogenic bacteria strains
- Waste phenolic resin derived activated carbon by microwave-assisted KOH activation and application to dye wastewater treatment
- Direct ethanol production from cellulose by consortium of Trichoderma reesei and Candida molischiana
- Agricultural waste biomass-assisted nanostructures: Synthesis and application
- Biodiesel production from rubber seed oil using calcium oxide derived from eggshell as catalyst – optimization and modeling studies
- Study of fabrication of fully aqueous solution processed SnS quantum dot-sensitized solar cell
- Assessment of aqueous extract of Gypsophila aretioides for inhibitory effects on calcium carbonate formation
- An environmentally friendly acylation reaction of 2-methylnaphthalene in solvent-free condition in a micro-channel reactor
- Aegle marmelos phytochemical stabilized synthesis and characterization of ZnO nanoparticles and their role against agriculture and food pathogen
- A reactive coupling process for co-production of solketal and biodiesel
- Optimization of the asymmetric synthesis of (S)-1-phenylethanol using Ispir bean as whole-cell biocatalyst
- Synthesis of pyrazolopyridine and pyrazoloquinoline derivatives by one-pot, three-component reactions of arylglyoxals, 3-methyl-1-aryl-1H-pyrazol-5-amines and cyclic 1,3-dicarbonyl compounds in the presence of tetrapropylammonium bromide
- Preconcentration of morphine in urine sample using a green and solvent-free microextraction method
- Extraction of glycyrrhizic acid by aqueous two-phase system formed by PEG and two environmentally friendly organic acid salts - sodium citrate and sodium tartrate
- Green synthesis of copper oxide nanoparticles using Juglans regia leaf extract and assessment of their physico-chemical and biological properties
- Deep eutectic solvents (DESs) as powerful and recyclable catalysts and solvents for the synthesis of 3,4-dihydropyrimidin-2(1H)-ones/thiones
- Biosynthesis, characterization and anti-microbial activity of silver nanoparticle based gel hand wash
- Efficient and selective microwave-assisted O-methylation of phenolic compounds using tetramethylammonium hydroxide (TMAH)
- Anticoagulant, thrombolytic and antibacterial activities of Euphorbia acruensis latex-mediated bioengineered silver nanoparticles
- Volcanic ash as reusable catalyst in the green synthesis of 3H-1,5-benzodiazepines
- Green synthesis, anionic polymerization of 1,4-bis(methacryloyl)piperazine using Algerian clay as catalyst
- Selenium supplementation during fermentation with sugar beet molasses and Saccharomyces cerevisiae to increase bioethanol production
- Biosynthetic potential assessment of four food pathogenic bacteria in hydrothermally silver nanoparticles fabrication
- Investigating the effectiveness of classical and eco-friendly approaches for synthesis of dialdehydes from organic dihalides
- Pyrolysis of palm oil using zeolite catalyst and characterization of the boil-oil
- Azadirachta indica leaves extract assisted green synthesis of Ag-TiO2 for degradation of Methylene blue and Rhodamine B dyes in aqueous medium
- Synthesis of vitamin E succinate catalyzed by nano-SiO2 immobilized DMAP derivative in mixed solvent system
- Extraction of phytosterols from melon (Cucumis melo) seeds by supercritical CO2 as a clean technology
- Production of uronic acids by hydrothermolysis of pectin as a model substance for plant biomass waste
- Biofabrication of highly pure copper oxide nanoparticles using wheat seed extract and their catalytic activity: A mechanistic approach
- Intelligent modeling and optimization of emulsion aggregation method for producing green printing ink
- Improved removal of methylene blue on modified hierarchical zeolite Y: Achieved by a “destructive-constructive” method
- Two different facile and efficient approaches for the synthesis of various N-arylacetamides via N-acetylation of arylamines and straightforward one-pot reductive acetylation of nitroarenes promoted by recyclable CuFe2O4 nanoparticles in water
- Optimization of acid catalyzed esterification and mixed metal oxide catalyzed transesterification for biodiesel production from Moringa oleifera oil
- Kinetics and the fluidity of the stearic acid esters with different carbon backbones
- Aiming for a standardized protocol for preparing a process green synthesis report and for ranking multiple synthesis plans to a common target product
- Microstructure and luminescence of VO2 (B) nanoparticle synthesis by hydrothermal method
- Optimization of uranium removal from uranium plant wastewater by response surface methodology (RSM)
- Microwave drying of nickel-containing residue: dielectric properties, kinetics, and energy aspects
- Simple and convenient two step synthesis of 5-bromo-2,3-dimethoxy-6-methyl-1,4-benzoquinone
- Biodiesel production from waste cooking oil
- The effect of activation temperature on structure and properties of blue coke-based activated carbon by CO2 activation
- Optimization of reaction parameters for the green synthesis of zero valent iron nanoparticles using pine tree needles
- Microwave-assisted protocol for squalene isolation and conversion from oil-deodoriser distillates
- Denitrification performance of rare earth tailings-based catalysts
- Facile synthesis of silver nanoparticles using Averrhoa bilimbi L and Plum extracts and investigation on the synergistic bioactivity using in vitro models
- Green production of AgNPs and their phytostimulatory impact
- Photocatalytic activity of Ag/Ni bi-metallic nanoparticles on textile dye removal
- Topical Issue: Green Process Engineering / Guest Editors: Martine Poux, Patrick Cognet
- Modelling and optimisation of oxidative desulphurisation of tyre-derived oil via central composite design approach
- CO2 sequestration by carbonation of olivine: a new process for optimal separation of the solids produced
- Organic carbonates synthesis improved by pervaporation for CO2 utilisation
- Production of starch nanoparticles through solvent-antisolvent precipitation in a spinning disc reactor
- A kinetic study of Zn halide/TBAB-catalysed fixation of CO2 with styrene oxide in propylene carbonate
- Topical on Green Process Engineering
Articles in the same Issue
- Regular Articles
- Studies on the preparation and properties of biodegradable polyester from soybean oil
- Flow-mode biodiesel production from palm oil using a pressurized microwave reactor
- Reduction of free fatty acids in waste oil for biodiesel production by glycerolysis: investigation and optimization of process parameters
- Saccharin: a cheap and mild acidic agent for the synthesis of azo dyes via telescoped dediazotization
- Optimization of lipase-catalyzed synthesis of polyethylene glycol stearate in a solvent-free system
- Green synthesis of iron oxide nanoparticles using Platanus orientalis leaf extract for antifungal activity
- Ultrasound assisted chemical activation of peanut husk for copper removal
- Room temperature silanization of Fe3O4 for the preparation of phenyl functionalized magnetic adsorbent for dispersive solid phase extraction for the extraction of phthalates in water
- Evaluation of the saponin green extraction from Ziziphus spina-christi leaves using hydrothermal, microwave and Bain-Marie water bath heating methods
- Oxidation of dibenzothiophene using the heterogeneous catalyst of tungsten-based carbon nanotubes
- Calcined sodium silicate as an efficient and benign heterogeneous catalyst for the transesterification of natural lecithin to L-α-glycerophosphocholine
- Synergistic effect between CO2 and H2O2 on ethylbenzene oxidation catalyzed by carbon supported heteropolyanion catalysts
- Hydrocyanation of 2-arylmethyleneindan-1,3-diones using potassium hexacyanoferrate(II) as a nontoxic cyanating agent
- Green synthesis of hydratropic aldehyde from α-methylstyrene catalyzed by Al2O3-supported metal phthalocyanines
- Environmentally benign chemical recycling of polycarbonate wastes: comparison of micro- and nano-TiO2 solid support efficiencies
- Medicago polymorpha-mediated antibacterial silver nanoparticles in the reduction of methyl orange
- Production of value-added chemicals from esterification of waste glycerol over MCM-41 supported catalysts
- Green synthesis of zerovalent copper nanoparticles for efficient reduction of toxic azo dyes congo red and methyl orange
- Optimization of the biological synthesis of silver nanoparticles using Penicillium oxalicum GRS-1 and their antimicrobial effects against common food-borne pathogens
- Optimization of submerged fermentation conditions to overproduce bioethanol using two industrial and traditional Saccharomyces cerevisiae strains
- Extraction of In3+ and Fe3+ from sulfate solutions by using a 3D-printed “Y”-shaped microreactor
- Foliar-mediated Ag:ZnO nanophotocatalysts: green synthesis, characterization, pollutants degradation, and in vitro biocidal activity
- Green cyclic acetals production by glycerol etherification reaction with benzaldehyde using cationic acidic resin
- Biosynthesis, characterization and antimicrobial activities assessment of fabricated selenium nanoparticles using Pelargonium zonale leaf extract
- Synthesis of high surface area magnesia by using walnut shell as a template
- Controllable biosynthesis of silver nanoparticles using actinobacterial strains
- Green vegetation: a promising source of color dyes
- Mechano-chemical synthesis of ammonia and acetic acid from inorganic materials in water
- Green synthesis and structural characterization of novel N1-substituted 3,4-dihydropyrimidin-2(1H)-ones
- Biodiesel production from cotton oil using heterogeneous CaO catalysts from eggshells prepared at different calcination temperatures
- Regeneration of spent mercury catalyst for the treatment of dye wastewater by the microwave and ultrasonic spray-assisted method
- Green synthesis of the innovative super paramagnetic nanoparticles from the leaves extract of Fraxinus chinensis Roxb and their application for the decolourisation of toxic dyes
- Biogenic ZnO nanoparticles: a study of blueshift of optical band gap and photocatalytic degradation of reactive yellow 186 dye under direct sunlight
- Leached compounds from the extracts of pomegranate peel, green coconut shell, and karuvelam wood for the removal of hexavalent chromium
- Enhancement of molecular weight reduction of natural rubber in triphasic CO2/toluene/H2O systems with hydrogen peroxide for preparation of biobased polyurethanes
- An efficient green synthesis of novel 1H-imidazo[1,2-a]imidazole-3-amine and imidazo[2,1-c][1,2,4]triazole-5-amine derivatives via Strecker reaction under controlled microwave heating
- Evaluation of three different green fabrication methods for the synthesis of crystalline ZnO nanoparticles using Pelargonium zonale leaf extract
- A highly efficient and multifunctional biomass supporting Ag, Ni, and Cu nanoparticles through wetness impregnation for environmental remediation
- Simple one-pot green method for large-scale production of mesalamine, an anti-inflammatory agent
- Relationships between step and cumulative PMI and E-factors: implications on estimating material efficiency with respect to charting synthesis optimization strategies
- A comparative sorption study of Cr3+ and Cr6+ using mango peels: kinetic, equilibrium and thermodynamic
- Effects of acid hydrolysis waste liquid recycle on preparation of microcrystalline cellulose
- Use of deep eutectic solvents as catalyst: A mini-review
- Microwave-assisted synthesis of pyrrolidinone derivatives using 1,1’-butylenebis(3-sulfo-3H-imidazol-1-ium) chloride in ethylene glycol
- Green and eco-friendly synthesis of Co3O4 and Ag-Co3O4: Characterization and photo-catalytic activity
- Adsorption optimized of the coal-based material and application for cyanide wastewater treatment
- Aloe vera leaf extract mediated green synthesis of selenium nanoparticles and assessment of their In vitro antimicrobial activity against spoilage fungi and pathogenic bacteria strains
- Waste phenolic resin derived activated carbon by microwave-assisted KOH activation and application to dye wastewater treatment
- Direct ethanol production from cellulose by consortium of Trichoderma reesei and Candida molischiana
- Agricultural waste biomass-assisted nanostructures: Synthesis and application
- Biodiesel production from rubber seed oil using calcium oxide derived from eggshell as catalyst – optimization and modeling studies
- Study of fabrication of fully aqueous solution processed SnS quantum dot-sensitized solar cell
- Assessment of aqueous extract of Gypsophila aretioides for inhibitory effects on calcium carbonate formation
- An environmentally friendly acylation reaction of 2-methylnaphthalene in solvent-free condition in a micro-channel reactor
- Aegle marmelos phytochemical stabilized synthesis and characterization of ZnO nanoparticles and their role against agriculture and food pathogen
- A reactive coupling process for co-production of solketal and biodiesel
- Optimization of the asymmetric synthesis of (S)-1-phenylethanol using Ispir bean as whole-cell biocatalyst
- Synthesis of pyrazolopyridine and pyrazoloquinoline derivatives by one-pot, three-component reactions of arylglyoxals, 3-methyl-1-aryl-1H-pyrazol-5-amines and cyclic 1,3-dicarbonyl compounds in the presence of tetrapropylammonium bromide
- Preconcentration of morphine in urine sample using a green and solvent-free microextraction method
- Extraction of glycyrrhizic acid by aqueous two-phase system formed by PEG and two environmentally friendly organic acid salts - sodium citrate and sodium tartrate
- Green synthesis of copper oxide nanoparticles using Juglans regia leaf extract and assessment of their physico-chemical and biological properties
- Deep eutectic solvents (DESs) as powerful and recyclable catalysts and solvents for the synthesis of 3,4-dihydropyrimidin-2(1H)-ones/thiones
- Biosynthesis, characterization and anti-microbial activity of silver nanoparticle based gel hand wash
- Efficient and selective microwave-assisted O-methylation of phenolic compounds using tetramethylammonium hydroxide (TMAH)
- Anticoagulant, thrombolytic and antibacterial activities of Euphorbia acruensis latex-mediated bioengineered silver nanoparticles
- Volcanic ash as reusable catalyst in the green synthesis of 3H-1,5-benzodiazepines
- Green synthesis, anionic polymerization of 1,4-bis(methacryloyl)piperazine using Algerian clay as catalyst
- Selenium supplementation during fermentation with sugar beet molasses and Saccharomyces cerevisiae to increase bioethanol production
- Biosynthetic potential assessment of four food pathogenic bacteria in hydrothermally silver nanoparticles fabrication
- Investigating the effectiveness of classical and eco-friendly approaches for synthesis of dialdehydes from organic dihalides
- Pyrolysis of palm oil using zeolite catalyst and characterization of the boil-oil
- Azadirachta indica leaves extract assisted green synthesis of Ag-TiO2 for degradation of Methylene blue and Rhodamine B dyes in aqueous medium
- Synthesis of vitamin E succinate catalyzed by nano-SiO2 immobilized DMAP derivative in mixed solvent system
- Extraction of phytosterols from melon (Cucumis melo) seeds by supercritical CO2 as a clean technology
- Production of uronic acids by hydrothermolysis of pectin as a model substance for plant biomass waste
- Biofabrication of highly pure copper oxide nanoparticles using wheat seed extract and their catalytic activity: A mechanistic approach
- Intelligent modeling and optimization of emulsion aggregation method for producing green printing ink
- Improved removal of methylene blue on modified hierarchical zeolite Y: Achieved by a “destructive-constructive” method
- Two different facile and efficient approaches for the synthesis of various N-arylacetamides via N-acetylation of arylamines and straightforward one-pot reductive acetylation of nitroarenes promoted by recyclable CuFe2O4 nanoparticles in water
- Optimization of acid catalyzed esterification and mixed metal oxide catalyzed transesterification for biodiesel production from Moringa oleifera oil
- Kinetics and the fluidity of the stearic acid esters with different carbon backbones
- Aiming for a standardized protocol for preparing a process green synthesis report and for ranking multiple synthesis plans to a common target product
- Microstructure and luminescence of VO2 (B) nanoparticle synthesis by hydrothermal method
- Optimization of uranium removal from uranium plant wastewater by response surface methodology (RSM)
- Microwave drying of nickel-containing residue: dielectric properties, kinetics, and energy aspects
- Simple and convenient two step synthesis of 5-bromo-2,3-dimethoxy-6-methyl-1,4-benzoquinone
- Biodiesel production from waste cooking oil
- The effect of activation temperature on structure and properties of blue coke-based activated carbon by CO2 activation
- Optimization of reaction parameters for the green synthesis of zero valent iron nanoparticles using pine tree needles
- Microwave-assisted protocol for squalene isolation and conversion from oil-deodoriser distillates
- Denitrification performance of rare earth tailings-based catalysts
- Facile synthesis of silver nanoparticles using Averrhoa bilimbi L and Plum extracts and investigation on the synergistic bioactivity using in vitro models
- Green production of AgNPs and their phytostimulatory impact
- Photocatalytic activity of Ag/Ni bi-metallic nanoparticles on textile dye removal
- Topical Issue: Green Process Engineering / Guest Editors: Martine Poux, Patrick Cognet
- Modelling and optimisation of oxidative desulphurisation of tyre-derived oil via central composite design approach
- CO2 sequestration by carbonation of olivine: a new process for optimal separation of the solids produced
- Organic carbonates synthesis improved by pervaporation for CO2 utilisation
- Production of starch nanoparticles through solvent-antisolvent precipitation in a spinning disc reactor
- A kinetic study of Zn halide/TBAB-catalysed fixation of CO2 with styrene oxide in propylene carbonate
- Topical on Green Process Engineering


