Abstract
Rare earth tailings from the Bayan Obo mine are rich in rare earth, iron, and other catalytically active substances. In this study, Na2CO3 and Ca(OH)2 were mixed with rare earth tailings, roasted, and the tailings modified by HCl-citric acid leaching and pickling to prepare high-performance rare earth tailings-based denitrification catalysts. Denitrification performance tests show that, in the temperature range 700°C~900°C, the alkali and acid co-processed modified tailings sample gave the best catalytic denitrification performance. XRD, SEM, and H2-TPR analyses show that, compared with raw ore samples, Fe activity sites increased after alkali and acid co-treatment. Cracks and holes appeared on the surface of the sample, and the reduction temperature range was broadened. XPS analysis showed that Fe coexisted in the forms Fe2+ and Fe3+, and Ce in the forms Ce3+ and Ce4+. At a rare earth tailings microwave roasting temperature of 500°C, NO concentration of 500 ppm, CO/NO ratio 4:1, and reaction temperature of 900°C, the denitrification efficiency of the catalyst was optimal, at up to 96.2%. In this study, a relatively green and pollution-free method was used to prepare catalysts, which can provide reference for solving the problem of rare earth tailings accumulation.
1 Introduction
NOx is the main atmospheric pollutant produced during the combustion of fossil fuels. It is harmful to the environment and human health, so effective removal of NOx has always been a concern of industry and academia. At present, the catalysts commonly used for catalytic denitration mainly include alkalis and alkali metal oxides [1], metal oxides [2], and rare earth oxides [3]. These types of catalysts have effective denitrification properties. However, they also have high preparation costs, poor anti-toxicity qualities, and other problems. Therefore, the use of natural minerals [4,5] and metallurgical waste residues [6,7] as denitration catalysts have become a research hotspot.
Chinese rare earth reserves account for 23% of the Earth’s total, ranking them first in the world. The Baotou Bayan Obo mine accounts for 83% of China’s rare earth reserves. This mining area is rich in iron, rare earth, and niobium symbiotic deposits; rare earths are rich in light rare earth elements such as La, Ce, Sm, and Eu [8]. Rare earth tailings left after beneficiation of rare earth ores contain a variety of rare earth elements and transition metal elements [9]. Rare earth oxides and iron oxides [10] in tailings are common raw materials for the preparation of catalysts. After years of accumulation, the reserves of rare earth tailings have reached 200 million tons. However, due to the complex components of tailings and for technical reasons, these reserves have not been fully utilized, leading to serious waste of resources and environmental pollution [11,12]. Under the dual constraints of continuous utilization of energy and environmental governance, the secondary utilization of rare earth tailings is “extremely urgent”, and the aim is to address this issue by “treating waste by waste”.
Many scholars have studied various rare earth metal and transition metal denitrification catalysts. However, few studies have focused on the catalytic denitrification of rare earth-associated ores such as rare earth concentrate and rare earth tailings. The purpose of this study is to explore the preparation of rare earth tailings-based low-cost, high-efficiency denitration catalysts by using a relatively green and non-polluting method. Solutions will also be suggested for the tailings accumulation problem caused by the annual production of 8 million tons of tailings [13].
2 Experimental
2.1 Experiment material
The raw materials used in this experiment were rare earth tailings from the Bayan Obo mining area, Baotou, China. A quantity of rare earth tailings were crushed, ground, sieved, and then selected using a 200 mesh and reserved. The main components and content of rare earth tailings are shown in Table 1.
The main components analysis of rare earth tailings.
| Component | Fe2O3 | CeO2 | MgO | Al2O3 | SiO2 | La2O3 |
| Content (%) | 27.67 | 3.01 | 3.31 | 1.46 | 11.86 | 1.44 |
| Component | CaO | MnO | Nd2O3 | TiO2 | F | Others |
| Content (%) | 27.20 | 1.96 | 1.10 | 1.00 | 8.92 | 11.07 |
2.2 Rare earth tailings and modification
The modification treatments of rare earth tailings included microwave roasting, alkali treatment, acid treatment, and co-treatment with alkali and acid, as shown in Table 2.
Preparation of rare earth tailing sample.
| Sample number | Raw material | Solid-liquid ratio | Drying temperature | Calcination temperature | Roasting time | Test Methods |
|---|---|---|---|---|---|---|
| 1 | rare earth tailings | —— | —— | —— | —— | XRD/XPS/H2-TPR / SEM/ Activity detection |
| 2 | rare earth tailings | —— | —— | 500°C | 15 min | XRD /H2-TPR /SEM/ Activity detection |
| 3 | 0.1 mol/L HCl + 0.01 mol/L C6H8O7 + 5 g rare earth tailings + H2O | 1:10 | 100°C | 500°C | 15 min | BET/ XRD/ H2-TPR / Activity detection |
| 4 | 0.2 g Na2CO3 + 0.3 g Ca(OH)2 + 5 g rare earth tailings | —— | 100°C | 500°C | 15 min | XRD/ H2-TPR / Activity detection |
| 5 | 0.2 g Na2CO3 + 0.3g Ca(OH)2 + 0.1 mol/L HCl + 0.01 mol/L C6H8O7 + 5 g rare earth tailings + H2O | 1:10 | 100°C | 500°C | 15 min | XRD/XPS/H2-TPR / SEM/ Activity detection |
Sample 1 was rare earth tailings. Sample 2 was rare earth tailings microwave roasted at 500°C. Sample 3 was rare earth tailings mixed with 0.1 mol/L HCl and 0.01 mol/L citric acid solution, then magnetically stirred for 1 h, filtered, and washed with water; solid samples were obtained after drying at 100°C. Sample 4 was obtained by mixing rare earth tailings with Na2CO3 and Ca(OH)2, then microwave baking, washing, and drying. Sample 5 was rare earth tailings co-treated with alkali and acid; that is, the tailings were first mixed with alkali, ground, calcined, washed with water, and then subjected to pickling and filtration; they were then filtered by water washing, and solid samples obtained after drying.
2.3 Catalyst activity detection
The catalyst activity detection system (Figure 1) comprised three parts: a mixed gas system, a reaction system, and an online gas measurement system. The gas distribution system was a gas mixing box produced by Nanjing Boyuntong Instrument Technology Co., Ltd. (model GXD 08-4E), with a measuring range from 0 to 500 SCCM and an accuracy of ±1.5%. The flowmeter utilized a mass flow meter. The reaction system was also produced by Nanjing Boyuntong Instrument Technology Co., Ltd. The riser furnace used in the experiment was a VTL 1600, and the basic parameters of the riser furnace were as follows: the heating rate was less than 10 K/min, the maximum rated temperature was 1600°C, and the rated power was 5.5 KW. The online gas measurement system utilized the Fourier infrared spectroscopy flue gas analyzer, GASMET-DX 4000, produced in Finland, to conduct online measurement of the flue gas composition, and used a computer data acquisition system to record and save the information. The experimental principle of its activity is:
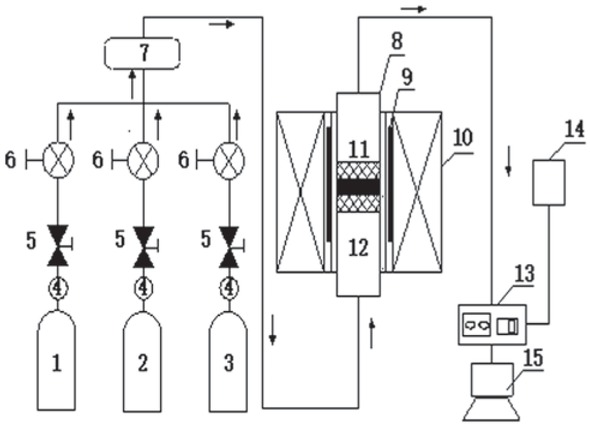
Reaction device schematic. 1. CO bottle; 2. NO bottle; 3. N2 bottle; 4. Pressure gauge; 5. Pressure-reducing valve; 6. Mass flowmeter; 7. Gas mixing box; 8. Quartz tube; 9. Thermocouple; 10. Protective layer and furnace wall; 11. Catalyst; 12. Quartz cotton; 13. Fourier infrared spectrum flue gas analyzer; 14. Tail gas treatment device; 15. Computer acquisition system.
The total experimental gas flow rate was 500 mL/ min, the concentration of NO was 500 ppm, and the concentration of CO was 2000 ppm. N2 was the equilibrium gas. The vertical tube furnace was heated from room temperature at 10°C/min to 700°C, 750°C, 800°C, 850°C, and 900°C, and a Fourier infrared spectroscopy flue gas analyzer was used for online monitoring. Before the experiment, a certain amount of quartz cotton was weighed and placed in the heating section of the quartz tube to support the catalyst. Each test was conducted using samples of 0.5 g. When the temperature and atmosphere were stable, the NO value was recorded as (NO)in. At this point, the quartz tube containing the sample was quickly placed in the furnace and sealed. The changes in CO and NO were measured online using Fourier infrared spectrometry and a computer data acquisition system, and the reaction time of each sample was not less than 20 min. The NO value was recorded as (NO)out when the gas concentration after the reaction tended to be stable. The denitrification rate was calculated using Eq. 4:
where η is the conversion rate of NO, (NO)in is the concentration of NO detected after the NO catalyst was added to the reactor, and (NO)out is the concentration of NO detected after catalyst addition and after stabilization.
3 Results and discussion
3.1 Denitrification activity of rare earth tailings-based catalyst
The denitrification performance of CO in terms of reduction of NOx was tested on rare earth tailings before and after modification. The temperature range was 700°C~900°C, NO concentration was 500 ppm, and CO/NO ratio was 4:1. As shown in the graph (Figure 2), when the temperature was 700°C and 750°C, the denitrification rate was sample 5 > sample 3 > sample 2 > sample 1 > sample 4; at 800°C, the denitrification rate was sample 5 > sample 2 > sample 1 > sample 3 > sample 4; at 850°C, the denitrification rate was sample 1 > sample 5 > sample 2 > sample 4 > sample 3; and, at 900°C, the denitrification rate was sample 5 > sample 1 > sample 3 > sample 2 > sample 4. The denitrification rate was highest for the original tailings (sample 1) at 850°C, reaching 90.3%; however, the acid-base co-treated rare earth tailings had higher denitrification rates at the other four temperature points than the other four samples, and exhibited the best catalytic denitrification performance in the temperature range 700°C~900°C. Furthermore, at 900°C, the denitrification rate of acid-base co-processed tailings reached 96.2%. The results show that the original tailings had a catalytic denitrification effect. And, after acid and alkali modification, the rare earth tailings catalyzed CO to reduce NOx to a greater extent.
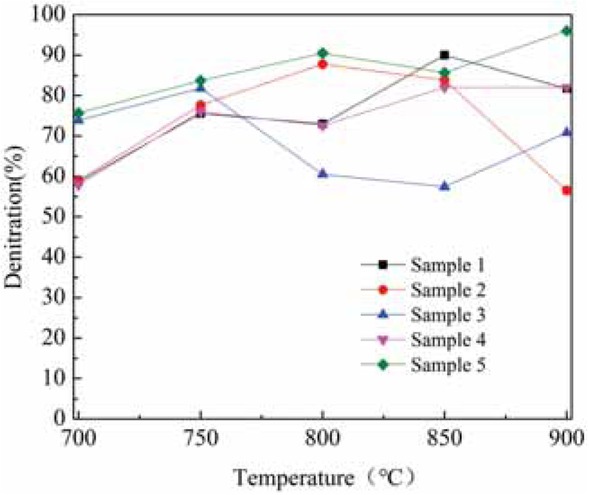
Denitrification efficiency before and after modification of rare earth tailings.
3.2 XRD characterization of rare earth tailings-based catalysts
As can be seen from Figure 3, the main components of rare earth tailings are Fe2O3 and CaF2. Among them, CaF2 has the strongest diffraction peak intensity. This indicates that there is a certain proportion of CaF2 in the rare earth tailings. Figure 4 shows that, after microwave roasting and acid-base treatment, the Fe2O3 of rare earth tailings showed new peaks, and the intensity of the diffraction peak of most Fe2O3 was greatly enhanced; however, the peak strength for CaF2 and SiO2 was greatly weakened. At the same time, rare earth tailings contain 71 different elements and 172 different minerals [14], and the components are relatively complex. XRD analysis can only analyze some of the main components of the main mineral phase, and lower content components cannot be displayed. Fe2O3 is an important active substance in catalytic reduction denitrification catalysts. The Fe2O3 content in the catalyst was increased, which is in line with the experimental design of the modified rare earth tailings as a denitrification catalyst.
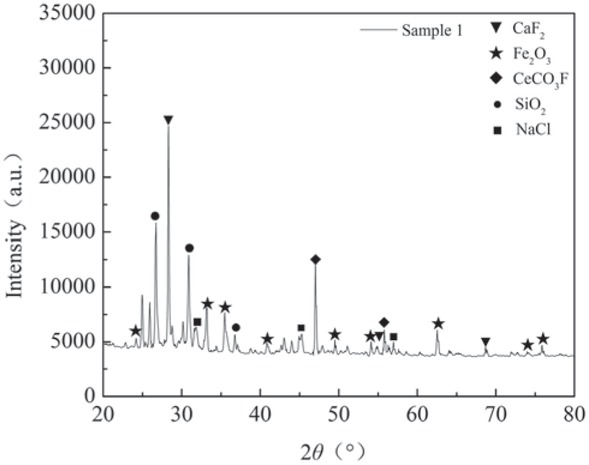
XRD characterization of rare earth tailings.
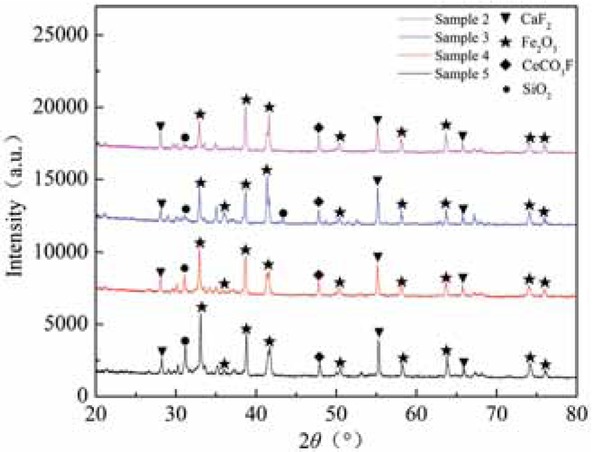
XRD characterization of modified rare earth tailings.
3.3 SEM characterization of rare earth tailings-based catalysts
As shown in the SEM diagram in Figure 5a, the original rare earth tailings displayed irregular blocks. The surface was smooth, and the particle size on the surface was between 4 and 45 μm. It can also be seen from the SEM diagram in Figure 5b that, after the rare earth tailings were microwave roasted at 500°C, a small number of cracks appeared on the surface, and the particle size changed slightly to between 3 and 45 μm. It can further be seen from the SEM diagram in Figure 5c that, after modification by alkali and acid, the surface was uneven, with cracks, many scrapes, and holes appearing, thus increasing the pore structure of the tailings. Microwave heating is a heating method that relies on an object absorbing microwave energy and converting it into heat energy, so heating the entire body at the same time. Materials generally absorb microwave energy to varying degrees, and the microwave energy absorbed by a material is a result of interaction between polar molecules and microwave electromagnetic fields in the material, under the action of an external alternating electromagnetic field. The polarization of polar molecules within a material is mutually frictional, thereby causing cracks in the material and increasing the pore volume. This action greatly improved the specific surface area of the tailings and, thereby, the denitrification activity of the tailings was improved.
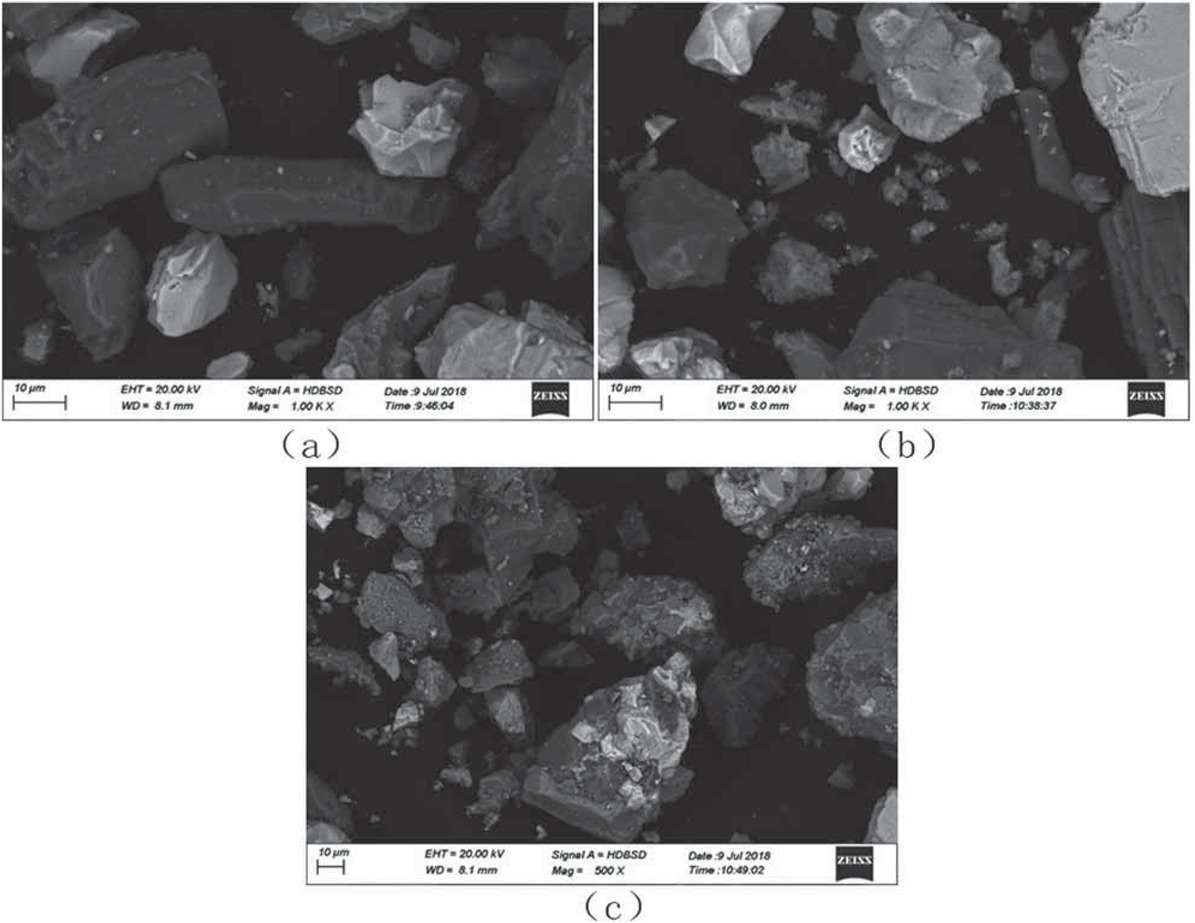
SEM diagram of rare earth tailings. (a) Rare earth tailings; (b) Microwave-roasted tailings; (c) Alkali and acid co-treated tailings.
3.4 H2-TPR analysis of rare earth tailings before and after modification
H2-TPR experiments were carried out to investigate the ability of metal ions on the surface of modified rare earth concentrates to be reduced to low-valency metal ions, as well as the ability to absorb and release oxygen. The experimental conditions are: Sample of the constant temperature under 200°C for 30 min dehydration processing, and then cooled to room temperature, then, the temperature was raised from room temperature to 900°C at a heating rate of 10°C/min, and the temperature was maintained for 10 min. The results are shown in Figure 6.
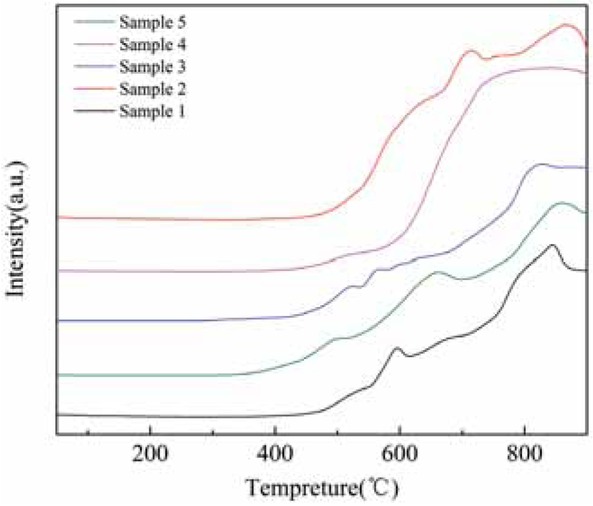
H2-TPR diagram before and after modification of rare earth tailings.
As shown in Figure 6, sample 1 has two hydrogen consumption peaks at around 596°C and 859°C. For sample 2, three hydrogen consumption peaks appeared at about 714°C, 767°C and 866°C. Sample 3 showed two hydrogen consumption peaks between 500°C and 600°C, and a broad hydrogen consumption peak appeared at 856°C. Sample 4 exhibited a broad hydrogen consumption peak between 600°C and 900°C. Sample 5 showed three hydrogen consumption peaks at around 504°C, 660°C, and 859°C. It can be seen that, for the acid-base co-treated tailings, the reduction temperature range was larger than for the original rare earth tailings, the roasted tailings, the acid-treated tailings, and the alkali-treated tailings. For pure CeO2, the reduction peak appeared at 300°C~600°C, corresponding to the reduction of CeO2 surface oxygen [15]. In summary, compared with the original tailings, after modification treatments the reduction temperature range of tailings was widened, and the reduction peak was increased. It was found that more substances in the modified tailings showed reducing ability, and the reduction temperature range of acid-base-treated tailings was the widest. This shows that modification treatment can enhance the redox capacity and oxygen storage capacity of rare earth tailings.
3.5 XPS characterization of rare earth tailings-based catalysts
XPS analysis was carried out for the elements Ce, Fe, and O on the surface of rare earth tailings and alkali and acid co-processed tailings.
Figure 7 shows the Ce 3d XPS diagram for sample 1, and Figure 8 shows the Ce 3d XPS diagram for sample 5. The Ce 3d peak was fitted according to previous reports [16]. There are two main valence states of Ce in compounds. Where v0 (B.E. ≈ 882.2 eV), v1 (B.E. ≈ 888.6 eV), v2 (B.E. ≈ 898 eV), v3 (B.E. ≈ 900.7 eV), v4 (B.E. ≈ 907.2 eV), and v5 (B.E. ≈ 916.15 eV), it can be seen from the standard map of Ce 3d that these six peaks correspond to the characteristic peaks of Ce4+. Similarly, the four peaks of u0 (B.E. ≈ 884.4 eV), u1 (B.E. ≈ 880.6 eV), u2 (B.E. ≈ 903.9 eV), and u3 (B.E. ≈ 899.3 eV) correspond to the characteristic peaks of Ce3+. The figure shows that the rare earth tailings have characteristic peaks of Ce3+ and Ce4+, while the tailings treated with alkali and acid also have characteristic peaks of Ce3+ and Ce4+. There is a certain number of Ce4+/Ce3+ redox electron pairs, which are beneficial to surface oxygen storage and release, enhance the surface oxidation of the catalyst, and increase the probability of NOx adsorption on the active site.
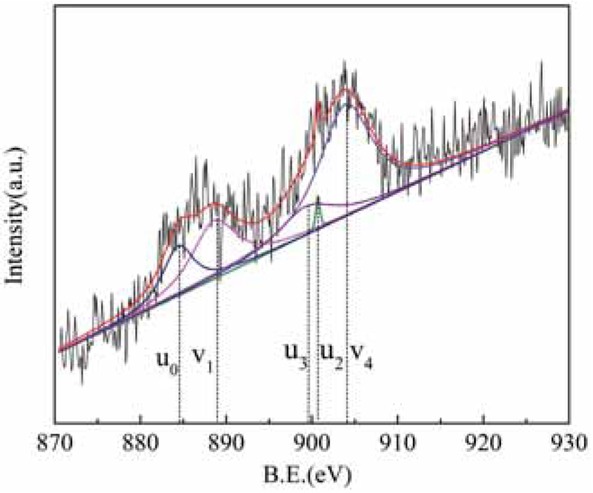
Ce3d XPS energy spectrum of rare earth tailings.
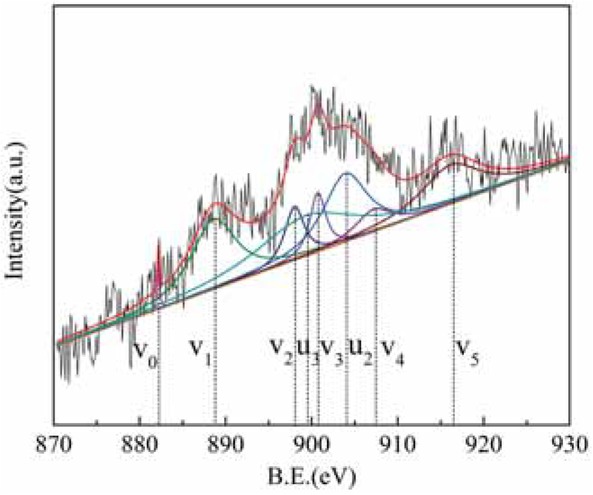
Ce3d XPS energy spectrum after modification of rare earth tailings.
Figure 9 shows the O 1s XPS diagram for rare earth tailings (sample 1) and the tailings treated by alkali and acid (sample 5). It can be seen from the XPS map that both the rare earth tailings and the alkali and acid-treated rare earth tailings have two peaks. One is the peak of adsorbed oxygen at 531.5~533.0 eV, and the other is the peak of lattice oxygen appearing at 529.5~530.5 eV. The lattice oxygen content of the alkali and acid-treated rare earth tailings was significantly higher than that of the original rare earth tailings. We speculated that CeO2 releases lattice oxygen under anoxic conditions, and the lattice oxygen is consumed to become surface-adsorbed oxygen; therefore, the Ce4+ portion of the CeO2 crystal will change to Ce3+, forming an O vacancy. Under oxygen-rich conditions, the oxygen adsorption becomes lattice oxygen and is stored, and Ce3+ once again becomes Ce4+. This transformation is beneficial to surface oxygen storage and release, enhances surface oxidation of the catalyst, and increases the NOx conversion rate at the active site. This increases the probability of NOx adsorption at the active site and, therefore, the denitrification efficiency of rare earth tailings treated with alkali acid is significantly higher than that of the original rare earth tailings.
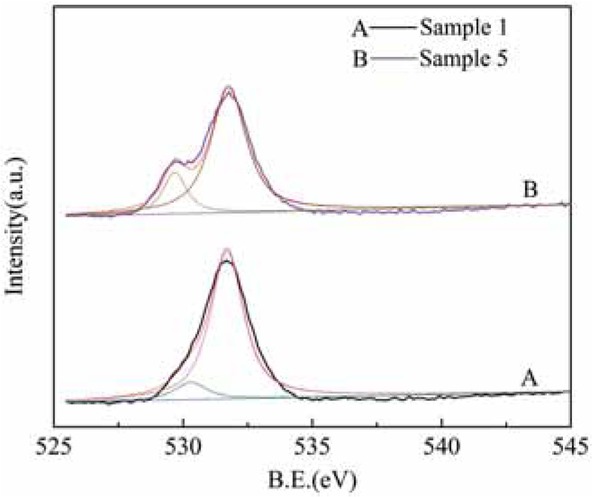
O 1s XPS energy spectrum before and after modification of rare earth tailings.
Figure 10 shows the Fe 2p XPS diagram for sample 1, and Figure 11 shows the Fe 2p XPS diagram for sample 5. For raw rare earth tailings (sample 1) and for alkali and acid-treated tailings (sample 5), two peaks appeared at binding energies of about W1 (B.E. ≈ 711 eV) and W2 (B.E. ≈ 725 eV); all of these peaks showed the characteristic peaks of Fe3+. Sample 1 and sample 5 showed characteristic peaks at a binding energy of U (B.E. ≈ 718~721 eV); this peak represented the characteristic peak of Fe2+ [17]. The above results show that Fe on the surface of the catalyst coexists in the forms Fe2+ and Fe3+, but mainly as Fe3+.
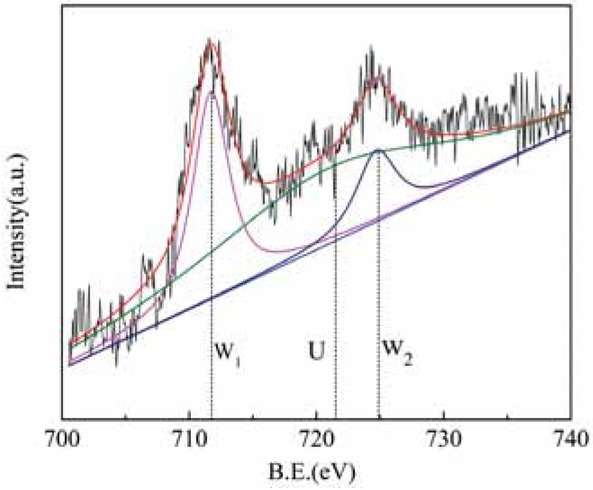
Fe2p XPS energy spectrum of rare earth tailings.
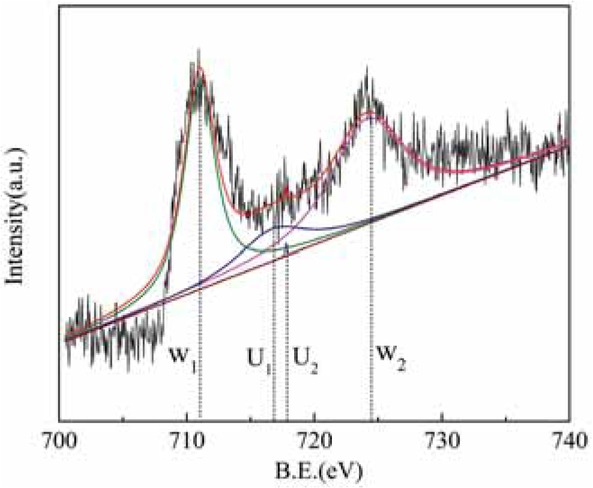
Fe2p XPS energy spectrum after modification of rare earth tailings.
3.6 Analysis of catalytic process of rare earth tailings-based catalysts
As shown in Figure 12, the cyclic conversions of Fe3+/Fe2+ and Ce4+/Ce3+ ensure the progress of the catalytic denitrification process. Fe2O3 adsorbs CO molecules and reduces them to FeO. Then NO molecule then oxidizes FeO and converts it to Fe2O3. Meanwhile, NO is reduced to N2. Similarly, the catalytic denitration process of cerium oxide is as follows: CeO2 adsorbs CO molecules and reduces them to Ce2O3. And then, Ce2O3 adsorbs NO molecules and is oxidized back into CeO2. Meanwhile, NO is reduced to N2. Therefore, the catalytic denitration reaction can be carried out continuously.
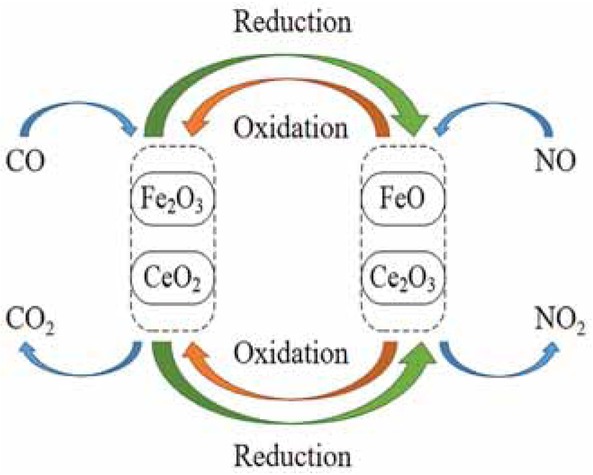
Schematic diagram of catalytic denitration.
4 Conclusion
In this study, the catalytic CO reduction of NO by modified rare earth tailings was investigated systematically. We can observe that the rare earth tailings have catalytic denitrification, and the rare earth tailings after alkali and acid treatment can improve the removal rate of NO to a greater extent. And the main conclusions are as follows:
After alkali and acid co-modification of the tailings, when the NO concentration was 500 ppm, CO/NO ratio was 4:1, and reaction temperature was 900°C, the optimal denitrification efficiency of the catalyst treated with acid-base was 96.2%.
After modification, the diffraction peak intensity of most Fe2O3 rare-earth tailings was enhanced, while CaF2 and SiO2 peak intensities were greatly weakened, and cracks and holes appeared on the surface.
XPS analysis indicated that Fe content on the catalyst surface increased. In the active component, Fe coexists in the forms Fe2+ and Fe3+, and Fe3+ content is higher; Ce coexists in the forms Ce3+ and Ce4+; and lattice oxygen appeared in rare earth tailings modified by alkali and acid. Thus, oxygen vacancies carry out oxygen transfer.
Modification treatment of rare earth tailings has a great influence on the redox properties of the catalyst. In general, catalysts obtained by acid-base co-treatment have the widest reduction temperature range. Furthermore, obvious reduction peaks appeared in the CeO2 catalyst at 300°C~600°C.
Acknowledgements
The investigation was financially supported by the National Natural Science Foundation of China (51866013) and by the Natural Science Foundation of the Inner Mongolia Autonomous Region (2017MS(LH)0529).
References
[1] Di M., Junlin X., De F., Zhe Z., Panpan D., He F., Research Progress on Alkali and Alkaline Earth Metal Poison of SCR Catalyst. Bull. Chin. Ceram. Soc., 2014, 33, 1398-1402, 1407.Search in Google Scholar
[2] Xin C., Yuxin D., Hailong L., Jie T., Rong S., Chongqing W., Research Progresses on Metal Oxide Catalysts for Low-Temperature Selective Catalytic Reduction in Flue Gas Denitrification. Environmental Protection of Chemical Industry, 2015, 35(4), 370-375.Search in Google Scholar
[3] Hui H., Shuxia W., Xiaoling Z., Quanzhong Z., Jin L., Study on Simultaneous Catalytic Reduction of Sulfur Dioxide and Nitric Oxide on Rare Earth Mixed Compounds. J. Rare Earth, 2006, 24(6), 695-698.10.1016/S1002-0721(07)60011-8Search in Google Scholar
[4] Tao W., Chengzhu Z., Haibo L., Yongpeng X., Xuehua Z., Bin X., et al., Performance of selective catalytic reduction of NO with NH3 over natural manganese ore catalysts at low temperature. Environ. Technol., 2017, 39(3), 1-10.10.1080/09593330.2017.1300190Search in Google Scholar PubMed
[5] Bin X., Tianhu C., Haibo L., Chengzhu Z., Dong C., Xuehua Z., et al., Preparation of γ-Fe2O3 catalyst by heat treatment of natural limonite for selective catalytic reduction of NO by NH3 Huan Jing Ke Xue, 2016, 37(7), 2807-2814.Search in Google Scholar
[6] Rui L., Juan Y., Chaoqun Y., Mei Y., Study on the properties of denitration catalysts based on metallurgical waste residue. Chem. Eng. Equip., 2016, 3, 7-11.Search in Google Scholar
[7] Shitian C., Shijie W., Fang W., Gu Z., Zhiyong W., Xianyu L., et al., Effect of metallurgical industry waste on denitrification and coal combustion. Coal Conversion, 2015, 38(2), 83-87.Search in Google Scholar
[8] Jianzhong C., Yunbing H., Liping C., Making Rational Multipurpose Use of Resources of RE in Baiyunebo Deposit. Chinese Rare Earths, 2007, 28(1), 70-74.Search in Google Scholar
[9] Yaxin S., Along S., Hao C., Experimental Study of NO Reduction by Iron in CO Atmosphere. Adv. Mat. Res., 2012, 518-523.10.4028/www.scientific.net/AMR.518-523.2138Search in Google Scholar
[10] Xingxing C., Xingyu Z., Dexin S., Zhiqiang W., Jingcai C., Chunyuan M., NO reduction by CO over copper catalyst supported on mixed CeO2 and Fe2O3 Catalyst design and activity test. Appl. Catal. B-Environ., 2018, 239, 485-501.10.1016/j.apcatb.2018.08.054Search in Google Scholar
[11] Wei G., Ruiying F., Renxin Z., Wenjing Z., Jiangyuan G., Jun Z., Distribution characteristic and current situation of soil rare earth contamination in the Bayan Obo mining area and Baotou tailing reservoir in Inner Mongolia. Environm. Sci., 2013, 34(5), 1895-1900.Search in Google Scholar
[12] Qifan W., Hua L., Chenghui M., Shunping Z., Xinhua Z., Shengqing X., et al., Investigation on the impact of the development and utilization of Bayan Obo associated mineral resources on regional environmental radioactive pollution. Radiat. Prot., 2011, 31(6), 364-370.Search in Google Scholar
[13] Xiaofang L., Xiaoe Z., Research Status of Soil Pollution of Bayan Obo Tailings Dam and Rare Earth Elements Toxicity. World Latest Medicine Information, 2017, 52.Search in Google Scholar
[14] DongLu L., ChunLong L., HuLin W., Mining, dressing and smelting technology research and technological progress of bayan obo special ore. BeiJing: Metallurgical Industry Press, 2007, 103-106.Search in Google Scholar
[15] Guillén-Hurtado N., Atribak I., Bueno-López A., García-García A., Influence of the cerium precursor on the physico-chemical features and NO to NO2 oxidation activity of ceria and ceria-zirconia catalysts. J. Mol. Catal. A-Chem., 2010, 323(1-2), 52-58.10.1016/j.molcata.2010.03.010Search in Google Scholar
[16] Trudeau M.L., Tschöpe A., Ying J.Y., XPS investigation of surface oxidation and reduction in nanocrystalline CexLa1−xO2−y. Surf. Interface Anal., 1995, 23(1), 11.10.1002/sia.740230405Search in Google Scholar
[17] Fudong L., Hong H., Structure-activity relationship of iron titanate catalysts in the selective catalytic; reduction of NOX with NH3 J. Phys. Chem. C, 2010, 114(40), 16929-16936.10.1021/jp912163kSearch in Google Scholar
© 2019 Wang et al., published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 Public License.
Articles in the same Issue
- Regular Articles
- Studies on the preparation and properties of biodegradable polyester from soybean oil
- Flow-mode biodiesel production from palm oil using a pressurized microwave reactor
- Reduction of free fatty acids in waste oil for biodiesel production by glycerolysis: investigation and optimization of process parameters
- Saccharin: a cheap and mild acidic agent for the synthesis of azo dyes via telescoped dediazotization
- Optimization of lipase-catalyzed synthesis of polyethylene glycol stearate in a solvent-free system
- Green synthesis of iron oxide nanoparticles using Platanus orientalis leaf extract for antifungal activity
- Ultrasound assisted chemical activation of peanut husk for copper removal
- Room temperature silanization of Fe3O4 for the preparation of phenyl functionalized magnetic adsorbent for dispersive solid phase extraction for the extraction of phthalates in water
- Evaluation of the saponin green extraction from Ziziphus spina-christi leaves using hydrothermal, microwave and Bain-Marie water bath heating methods
- Oxidation of dibenzothiophene using the heterogeneous catalyst of tungsten-based carbon nanotubes
- Calcined sodium silicate as an efficient and benign heterogeneous catalyst for the transesterification of natural lecithin to L-α-glycerophosphocholine
- Synergistic effect between CO2 and H2O2 on ethylbenzene oxidation catalyzed by carbon supported heteropolyanion catalysts
- Hydrocyanation of 2-arylmethyleneindan-1,3-diones using potassium hexacyanoferrate(II) as a nontoxic cyanating agent
- Green synthesis of hydratropic aldehyde from α-methylstyrene catalyzed by Al2O3-supported metal phthalocyanines
- Environmentally benign chemical recycling of polycarbonate wastes: comparison of micro- and nano-TiO2 solid support efficiencies
- Medicago polymorpha-mediated antibacterial silver nanoparticles in the reduction of methyl orange
- Production of value-added chemicals from esterification of waste glycerol over MCM-41 supported catalysts
- Green synthesis of zerovalent copper nanoparticles for efficient reduction of toxic azo dyes congo red and methyl orange
- Optimization of the biological synthesis of silver nanoparticles using Penicillium oxalicum GRS-1 and their antimicrobial effects against common food-borne pathogens
- Optimization of submerged fermentation conditions to overproduce bioethanol using two industrial and traditional Saccharomyces cerevisiae strains
- Extraction of In3+ and Fe3+ from sulfate solutions by using a 3D-printed “Y”-shaped microreactor
- Foliar-mediated Ag:ZnO nanophotocatalysts: green synthesis, characterization, pollutants degradation, and in vitro biocidal activity
- Green cyclic acetals production by glycerol etherification reaction with benzaldehyde using cationic acidic resin
- Biosynthesis, characterization and antimicrobial activities assessment of fabricated selenium nanoparticles using Pelargonium zonale leaf extract
- Synthesis of high surface area magnesia by using walnut shell as a template
- Controllable biosynthesis of silver nanoparticles using actinobacterial strains
- Green vegetation: a promising source of color dyes
- Mechano-chemical synthesis of ammonia and acetic acid from inorganic materials in water
- Green synthesis and structural characterization of novel N1-substituted 3,4-dihydropyrimidin-2(1H)-ones
- Biodiesel production from cotton oil using heterogeneous CaO catalysts from eggshells prepared at different calcination temperatures
- Regeneration of spent mercury catalyst for the treatment of dye wastewater by the microwave and ultrasonic spray-assisted method
- Green synthesis of the innovative super paramagnetic nanoparticles from the leaves extract of Fraxinus chinensis Roxb and their application for the decolourisation of toxic dyes
- Biogenic ZnO nanoparticles: a study of blueshift of optical band gap and photocatalytic degradation of reactive yellow 186 dye under direct sunlight
- Leached compounds from the extracts of pomegranate peel, green coconut shell, and karuvelam wood for the removal of hexavalent chromium
- Enhancement of molecular weight reduction of natural rubber in triphasic CO2/toluene/H2O systems with hydrogen peroxide for preparation of biobased polyurethanes
- An efficient green synthesis of novel 1H-imidazo[1,2-a]imidazole-3-amine and imidazo[2,1-c][1,2,4]triazole-5-amine derivatives via Strecker reaction under controlled microwave heating
- Evaluation of three different green fabrication methods for the synthesis of crystalline ZnO nanoparticles using Pelargonium zonale leaf extract
- A highly efficient and multifunctional biomass supporting Ag, Ni, and Cu nanoparticles through wetness impregnation for environmental remediation
- Simple one-pot green method for large-scale production of mesalamine, an anti-inflammatory agent
- Relationships between step and cumulative PMI and E-factors: implications on estimating material efficiency with respect to charting synthesis optimization strategies
- A comparative sorption study of Cr3+ and Cr6+ using mango peels: kinetic, equilibrium and thermodynamic
- Effects of acid hydrolysis waste liquid recycle on preparation of microcrystalline cellulose
- Use of deep eutectic solvents as catalyst: A mini-review
- Microwave-assisted synthesis of pyrrolidinone derivatives using 1,1’-butylenebis(3-sulfo-3H-imidazol-1-ium) chloride in ethylene glycol
- Green and eco-friendly synthesis of Co3O4 and Ag-Co3O4: Characterization and photo-catalytic activity
- Adsorption optimized of the coal-based material and application for cyanide wastewater treatment
- Aloe vera leaf extract mediated green synthesis of selenium nanoparticles and assessment of their In vitro antimicrobial activity against spoilage fungi and pathogenic bacteria strains
- Waste phenolic resin derived activated carbon by microwave-assisted KOH activation and application to dye wastewater treatment
- Direct ethanol production from cellulose by consortium of Trichoderma reesei and Candida molischiana
- Agricultural waste biomass-assisted nanostructures: Synthesis and application
- Biodiesel production from rubber seed oil using calcium oxide derived from eggshell as catalyst – optimization and modeling studies
- Study of fabrication of fully aqueous solution processed SnS quantum dot-sensitized solar cell
- Assessment of aqueous extract of Gypsophila aretioides for inhibitory effects on calcium carbonate formation
- An environmentally friendly acylation reaction of 2-methylnaphthalene in solvent-free condition in a micro-channel reactor
- Aegle marmelos phytochemical stabilized synthesis and characterization of ZnO nanoparticles and their role against agriculture and food pathogen
- A reactive coupling process for co-production of solketal and biodiesel
- Optimization of the asymmetric synthesis of (S)-1-phenylethanol using Ispir bean as whole-cell biocatalyst
- Synthesis of pyrazolopyridine and pyrazoloquinoline derivatives by one-pot, three-component reactions of arylglyoxals, 3-methyl-1-aryl-1H-pyrazol-5-amines and cyclic 1,3-dicarbonyl compounds in the presence of tetrapropylammonium bromide
- Preconcentration of morphine in urine sample using a green and solvent-free microextraction method
- Extraction of glycyrrhizic acid by aqueous two-phase system formed by PEG and two environmentally friendly organic acid salts - sodium citrate and sodium tartrate
- Green synthesis of copper oxide nanoparticles using Juglans regia leaf extract and assessment of their physico-chemical and biological properties
- Deep eutectic solvents (DESs) as powerful and recyclable catalysts and solvents for the synthesis of 3,4-dihydropyrimidin-2(1H)-ones/thiones
- Biosynthesis, characterization and anti-microbial activity of silver nanoparticle based gel hand wash
- Efficient and selective microwave-assisted O-methylation of phenolic compounds using tetramethylammonium hydroxide (TMAH)
- Anticoagulant, thrombolytic and antibacterial activities of Euphorbia acruensis latex-mediated bioengineered silver nanoparticles
- Volcanic ash as reusable catalyst in the green synthesis of 3H-1,5-benzodiazepines
- Green synthesis, anionic polymerization of 1,4-bis(methacryloyl)piperazine using Algerian clay as catalyst
- Selenium supplementation during fermentation with sugar beet molasses and Saccharomyces cerevisiae to increase bioethanol production
- Biosynthetic potential assessment of four food pathogenic bacteria in hydrothermally silver nanoparticles fabrication
- Investigating the effectiveness of classical and eco-friendly approaches for synthesis of dialdehydes from organic dihalides
- Pyrolysis of palm oil using zeolite catalyst and characterization of the boil-oil
- Azadirachta indica leaves extract assisted green synthesis of Ag-TiO2 for degradation of Methylene blue and Rhodamine B dyes in aqueous medium
- Synthesis of vitamin E succinate catalyzed by nano-SiO2 immobilized DMAP derivative in mixed solvent system
- Extraction of phytosterols from melon (Cucumis melo) seeds by supercritical CO2 as a clean technology
- Production of uronic acids by hydrothermolysis of pectin as a model substance for plant biomass waste
- Biofabrication of highly pure copper oxide nanoparticles using wheat seed extract and their catalytic activity: A mechanistic approach
- Intelligent modeling and optimization of emulsion aggregation method for producing green printing ink
- Improved removal of methylene blue on modified hierarchical zeolite Y: Achieved by a “destructive-constructive” method
- Two different facile and efficient approaches for the synthesis of various N-arylacetamides via N-acetylation of arylamines and straightforward one-pot reductive acetylation of nitroarenes promoted by recyclable CuFe2O4 nanoparticles in water
- Optimization of acid catalyzed esterification and mixed metal oxide catalyzed transesterification for biodiesel production from Moringa oleifera oil
- Kinetics and the fluidity of the stearic acid esters with different carbon backbones
- Aiming for a standardized protocol for preparing a process green synthesis report and for ranking multiple synthesis plans to a common target product
- Microstructure and luminescence of VO2 (B) nanoparticle synthesis by hydrothermal method
- Optimization of uranium removal from uranium plant wastewater by response surface methodology (RSM)
- Microwave drying of nickel-containing residue: dielectric properties, kinetics, and energy aspects
- Simple and convenient two step synthesis of 5-bromo-2,3-dimethoxy-6-methyl-1,4-benzoquinone
- Biodiesel production from waste cooking oil
- The effect of activation temperature on structure and properties of blue coke-based activated carbon by CO2 activation
- Optimization of reaction parameters for the green synthesis of zero valent iron nanoparticles using pine tree needles
- Microwave-assisted protocol for squalene isolation and conversion from oil-deodoriser distillates
- Denitrification performance of rare earth tailings-based catalysts
- Facile synthesis of silver nanoparticles using Averrhoa bilimbi L and Plum extracts and investigation on the synergistic bioactivity using in vitro models
- Green production of AgNPs and their phytostimulatory impact
- Photocatalytic activity of Ag/Ni bi-metallic nanoparticles on textile dye removal
- Topical Issue: Green Process Engineering / Guest Editors: Martine Poux, Patrick Cognet
- Modelling and optimisation of oxidative desulphurisation of tyre-derived oil via central composite design approach
- CO2 sequestration by carbonation of olivine: a new process for optimal separation of the solids produced
- Organic carbonates synthesis improved by pervaporation for CO2 utilisation
- Production of starch nanoparticles through solvent-antisolvent precipitation in a spinning disc reactor
- A kinetic study of Zn halide/TBAB-catalysed fixation of CO2 with styrene oxide in propylene carbonate
- Topical on Green Process Engineering
Articles in the same Issue
- Regular Articles
- Studies on the preparation and properties of biodegradable polyester from soybean oil
- Flow-mode biodiesel production from palm oil using a pressurized microwave reactor
- Reduction of free fatty acids in waste oil for biodiesel production by glycerolysis: investigation and optimization of process parameters
- Saccharin: a cheap and mild acidic agent for the synthesis of azo dyes via telescoped dediazotization
- Optimization of lipase-catalyzed synthesis of polyethylene glycol stearate in a solvent-free system
- Green synthesis of iron oxide nanoparticles using Platanus orientalis leaf extract for antifungal activity
- Ultrasound assisted chemical activation of peanut husk for copper removal
- Room temperature silanization of Fe3O4 for the preparation of phenyl functionalized magnetic adsorbent for dispersive solid phase extraction for the extraction of phthalates in water
- Evaluation of the saponin green extraction from Ziziphus spina-christi leaves using hydrothermal, microwave and Bain-Marie water bath heating methods
- Oxidation of dibenzothiophene using the heterogeneous catalyst of tungsten-based carbon nanotubes
- Calcined sodium silicate as an efficient and benign heterogeneous catalyst for the transesterification of natural lecithin to L-α-glycerophosphocholine
- Synergistic effect between CO2 and H2O2 on ethylbenzene oxidation catalyzed by carbon supported heteropolyanion catalysts
- Hydrocyanation of 2-arylmethyleneindan-1,3-diones using potassium hexacyanoferrate(II) as a nontoxic cyanating agent
- Green synthesis of hydratropic aldehyde from α-methylstyrene catalyzed by Al2O3-supported metal phthalocyanines
- Environmentally benign chemical recycling of polycarbonate wastes: comparison of micro- and nano-TiO2 solid support efficiencies
- Medicago polymorpha-mediated antibacterial silver nanoparticles in the reduction of methyl orange
- Production of value-added chemicals from esterification of waste glycerol over MCM-41 supported catalysts
- Green synthesis of zerovalent copper nanoparticles for efficient reduction of toxic azo dyes congo red and methyl orange
- Optimization of the biological synthesis of silver nanoparticles using Penicillium oxalicum GRS-1 and their antimicrobial effects against common food-borne pathogens
- Optimization of submerged fermentation conditions to overproduce bioethanol using two industrial and traditional Saccharomyces cerevisiae strains
- Extraction of In3+ and Fe3+ from sulfate solutions by using a 3D-printed “Y”-shaped microreactor
- Foliar-mediated Ag:ZnO nanophotocatalysts: green synthesis, characterization, pollutants degradation, and in vitro biocidal activity
- Green cyclic acetals production by glycerol etherification reaction with benzaldehyde using cationic acidic resin
- Biosynthesis, characterization and antimicrobial activities assessment of fabricated selenium nanoparticles using Pelargonium zonale leaf extract
- Synthesis of high surface area magnesia by using walnut shell as a template
- Controllable biosynthesis of silver nanoparticles using actinobacterial strains
- Green vegetation: a promising source of color dyes
- Mechano-chemical synthesis of ammonia and acetic acid from inorganic materials in water
- Green synthesis and structural characterization of novel N1-substituted 3,4-dihydropyrimidin-2(1H)-ones
- Biodiesel production from cotton oil using heterogeneous CaO catalysts from eggshells prepared at different calcination temperatures
- Regeneration of spent mercury catalyst for the treatment of dye wastewater by the microwave and ultrasonic spray-assisted method
- Green synthesis of the innovative super paramagnetic nanoparticles from the leaves extract of Fraxinus chinensis Roxb and their application for the decolourisation of toxic dyes
- Biogenic ZnO nanoparticles: a study of blueshift of optical band gap and photocatalytic degradation of reactive yellow 186 dye under direct sunlight
- Leached compounds from the extracts of pomegranate peel, green coconut shell, and karuvelam wood for the removal of hexavalent chromium
- Enhancement of molecular weight reduction of natural rubber in triphasic CO2/toluene/H2O systems with hydrogen peroxide for preparation of biobased polyurethanes
- An efficient green synthesis of novel 1H-imidazo[1,2-a]imidazole-3-amine and imidazo[2,1-c][1,2,4]triazole-5-amine derivatives via Strecker reaction under controlled microwave heating
- Evaluation of three different green fabrication methods for the synthesis of crystalline ZnO nanoparticles using Pelargonium zonale leaf extract
- A highly efficient and multifunctional biomass supporting Ag, Ni, and Cu nanoparticles through wetness impregnation for environmental remediation
- Simple one-pot green method for large-scale production of mesalamine, an anti-inflammatory agent
- Relationships between step and cumulative PMI and E-factors: implications on estimating material efficiency with respect to charting synthesis optimization strategies
- A comparative sorption study of Cr3+ and Cr6+ using mango peels: kinetic, equilibrium and thermodynamic
- Effects of acid hydrolysis waste liquid recycle on preparation of microcrystalline cellulose
- Use of deep eutectic solvents as catalyst: A mini-review
- Microwave-assisted synthesis of pyrrolidinone derivatives using 1,1’-butylenebis(3-sulfo-3H-imidazol-1-ium) chloride in ethylene glycol
- Green and eco-friendly synthesis of Co3O4 and Ag-Co3O4: Characterization and photo-catalytic activity
- Adsorption optimized of the coal-based material and application for cyanide wastewater treatment
- Aloe vera leaf extract mediated green synthesis of selenium nanoparticles and assessment of their In vitro antimicrobial activity against spoilage fungi and pathogenic bacteria strains
- Waste phenolic resin derived activated carbon by microwave-assisted KOH activation and application to dye wastewater treatment
- Direct ethanol production from cellulose by consortium of Trichoderma reesei and Candida molischiana
- Agricultural waste biomass-assisted nanostructures: Synthesis and application
- Biodiesel production from rubber seed oil using calcium oxide derived from eggshell as catalyst – optimization and modeling studies
- Study of fabrication of fully aqueous solution processed SnS quantum dot-sensitized solar cell
- Assessment of aqueous extract of Gypsophila aretioides for inhibitory effects on calcium carbonate formation
- An environmentally friendly acylation reaction of 2-methylnaphthalene in solvent-free condition in a micro-channel reactor
- Aegle marmelos phytochemical stabilized synthesis and characterization of ZnO nanoparticles and their role against agriculture and food pathogen
- A reactive coupling process for co-production of solketal and biodiesel
- Optimization of the asymmetric synthesis of (S)-1-phenylethanol using Ispir bean as whole-cell biocatalyst
- Synthesis of pyrazolopyridine and pyrazoloquinoline derivatives by one-pot, three-component reactions of arylglyoxals, 3-methyl-1-aryl-1H-pyrazol-5-amines and cyclic 1,3-dicarbonyl compounds in the presence of tetrapropylammonium bromide
- Preconcentration of morphine in urine sample using a green and solvent-free microextraction method
- Extraction of glycyrrhizic acid by aqueous two-phase system formed by PEG and two environmentally friendly organic acid salts - sodium citrate and sodium tartrate
- Green synthesis of copper oxide nanoparticles using Juglans regia leaf extract and assessment of their physico-chemical and biological properties
- Deep eutectic solvents (DESs) as powerful and recyclable catalysts and solvents for the synthesis of 3,4-dihydropyrimidin-2(1H)-ones/thiones
- Biosynthesis, characterization and anti-microbial activity of silver nanoparticle based gel hand wash
- Efficient and selective microwave-assisted O-methylation of phenolic compounds using tetramethylammonium hydroxide (TMAH)
- Anticoagulant, thrombolytic and antibacterial activities of Euphorbia acruensis latex-mediated bioengineered silver nanoparticles
- Volcanic ash as reusable catalyst in the green synthesis of 3H-1,5-benzodiazepines
- Green synthesis, anionic polymerization of 1,4-bis(methacryloyl)piperazine using Algerian clay as catalyst
- Selenium supplementation during fermentation with sugar beet molasses and Saccharomyces cerevisiae to increase bioethanol production
- Biosynthetic potential assessment of four food pathogenic bacteria in hydrothermally silver nanoparticles fabrication
- Investigating the effectiveness of classical and eco-friendly approaches for synthesis of dialdehydes from organic dihalides
- Pyrolysis of palm oil using zeolite catalyst and characterization of the boil-oil
- Azadirachta indica leaves extract assisted green synthesis of Ag-TiO2 for degradation of Methylene blue and Rhodamine B dyes in aqueous medium
- Synthesis of vitamin E succinate catalyzed by nano-SiO2 immobilized DMAP derivative in mixed solvent system
- Extraction of phytosterols from melon (Cucumis melo) seeds by supercritical CO2 as a clean technology
- Production of uronic acids by hydrothermolysis of pectin as a model substance for plant biomass waste
- Biofabrication of highly pure copper oxide nanoparticles using wheat seed extract and their catalytic activity: A mechanistic approach
- Intelligent modeling and optimization of emulsion aggregation method for producing green printing ink
- Improved removal of methylene blue on modified hierarchical zeolite Y: Achieved by a “destructive-constructive” method
- Two different facile and efficient approaches for the synthesis of various N-arylacetamides via N-acetylation of arylamines and straightforward one-pot reductive acetylation of nitroarenes promoted by recyclable CuFe2O4 nanoparticles in water
- Optimization of acid catalyzed esterification and mixed metal oxide catalyzed transesterification for biodiesel production from Moringa oleifera oil
- Kinetics and the fluidity of the stearic acid esters with different carbon backbones
- Aiming for a standardized protocol for preparing a process green synthesis report and for ranking multiple synthesis plans to a common target product
- Microstructure and luminescence of VO2 (B) nanoparticle synthesis by hydrothermal method
- Optimization of uranium removal from uranium plant wastewater by response surface methodology (RSM)
- Microwave drying of nickel-containing residue: dielectric properties, kinetics, and energy aspects
- Simple and convenient two step synthesis of 5-bromo-2,3-dimethoxy-6-methyl-1,4-benzoquinone
- Biodiesel production from waste cooking oil
- The effect of activation temperature on structure and properties of blue coke-based activated carbon by CO2 activation
- Optimization of reaction parameters for the green synthesis of zero valent iron nanoparticles using pine tree needles
- Microwave-assisted protocol for squalene isolation and conversion from oil-deodoriser distillates
- Denitrification performance of rare earth tailings-based catalysts
- Facile synthesis of silver nanoparticles using Averrhoa bilimbi L and Plum extracts and investigation on the synergistic bioactivity using in vitro models
- Green production of AgNPs and their phytostimulatory impact
- Photocatalytic activity of Ag/Ni bi-metallic nanoparticles on textile dye removal
- Topical Issue: Green Process Engineering / Guest Editors: Martine Poux, Patrick Cognet
- Modelling and optimisation of oxidative desulphurisation of tyre-derived oil via central composite design approach
- CO2 sequestration by carbonation of olivine: a new process for optimal separation of the solids produced
- Organic carbonates synthesis improved by pervaporation for CO2 utilisation
- Production of starch nanoparticles through solvent-antisolvent precipitation in a spinning disc reactor
- A kinetic study of Zn halide/TBAB-catalysed fixation of CO2 with styrene oxide in propylene carbonate
- Topical on Green Process Engineering

