Abstract
Crude dimer acid (DA) was prepared with soybean oil (SO) used as raw material and organic montmorillonite as a catalyst. Fourier transform infrared (FTIR) spectroscopy, hydrogen nuclear magnetic resonance (1H-NMR) and gel permeation chromatography (GPC) were used to characterize the structure of DA. It was demonstrated that the synthesis of crude DA using SO was feasible. A molecular weight of 995–1304 g/mol was obtained by GPC measurement. Then, a type of polyester was synthesized using the crude DA and polyethylene glycol. The effects of reaction temperatures and different catalysts on the conversion rate were explored. The results showed that the esterification conversion rate was improved to 83.13% when SnCl2 was used as the catalyst, with a reaction temperature of 180°C The FTIR, 1H-NMR, GPC and TGA were used to characterize the structure and performance of this polyester. The polyester had a molecular weight ranging from 8259 to 10892 g/mol. In addition, its biodegradable behavior was analyzed by the soil burial test and was compared with that of terephthalic acid. The results showed that the composites prepared from DA had a pronounced effect on weight loss during biodegradation.
1 Introduction
In today’s market, the requirements of renewable materials are increasing. First, lower costs for producing renewable fuels are needed compared with those incurred from using petroleum derivatives. Then, other reasons remained, such as the reduction of reserves, high oil prices and the desire for green or biodegradable materials [1]. In addition, social emphasis concerning waste disposal, the depletion of nonrenewable resources and the environment are considered the other main benefits from the application of bio-based products. For these reasons, vegetable oils are often chosen to produce fuels and polymeric materials [2], [3].
Waste vegetable oil can be used to produce biodiesel. Researchers focused on biodiesel production using different types of catalyzed transesterification reactions [4]. The conversion, yield and quality of biodiesel are commonly affected by certain parameters, such as membrane reactor, reactive absorption and ultrasonic irradiation. Guzatto et al. [5], [6] modified the double-step transesterification process, in which ethanol was used as a type of transesterification agent to produce biodiesel from vegetable and waste oils. The obtained biodiesels were analyzed by hydrogen nuclear magnetic resonance (1H-NMR), 13C-NMR and Fourier transform infrared (FTIR) spectroscopy as well as by standard physico-chemical techniques. These results indicated that the biodiesel products had high quality and purity. Istadi et al. [7] prepared a novel type of solid acid catalysts, sulphated zinc oxide, in the transesterification of soybean oil with methanol to produce biodiesel. From the testing results, this catalyst exhibited properties of being a good candidate catalyst for the transesterification of soybean oil to produce biodiesel. Zargar et al. [8] used waste vegetable oil to replace a natural agent for bitumen. The properties of the original, aged and rejuvenated bitumen were all evaluated. The results showed that the physical properties of aged bitumen group 40/50 was almost the same as the properties of the original bitumen (80/100) with the addition of 3–4% of waste vegetable oil.
In addition, because of their biodegradability, non-toxicity and low production cost, vegetable oils have also been used as raw materials for the preparation of novel polymers instead of traditional polymers. Liu and Erhan [9] synthesized biopolymers based on soybean oil (SO) using the cationic polymerization method. This process occurred in the supercritical carbon dioxide solvent and initiated by the boron trifluoride diethyl etherate. The molecular weight of the obtained polymers ranged from 21,842 to 118,300 g/mol. Guo et al. [10] investigated the preparation of the rigid polyurethane foams from polyols derived from soybean oil, and found that these foams possessed good mechanical and thermoinsulating properties together with better thermal degradation and thermal oxidation properties compared with that of foams synthesized from petrochemical origin. Sahoo Sushanta et al. [11] used the in situ method to synthesize a novel type of bioresin and epoxidize SO to blend with epoxy in different ratios. It may act as a kind of reactive diluent, thus improving the processibility and toughened nature of the epoxy. Their results showed that 20 wt% bioresin in the composite improved impact strength compared with the virgin epoxy. However, the above methodology only gave two ways for the utilization of vegetable oils. To date, the preparation and characterization of dimer acid (DA) and polyester using vegetable oils had not been systematically researched.
In the present work, crude DA and a type of biodegradation polyester was prepared using SO. The preparation, structure, morphology, thermal stability and biodegradation behavior of this polymer were investigated.
2 Materials and methods
2.1 Materials
The SO employed in this study was bought from Legou Supermarket (Shanghai, China). The stannous chloride, ammonia acid, phenolphthalein, concentrated sulfuric acid, acetic acid, acetone, acetate, ethanol, potassium hydroxide and terephthalic acid (TA) were chemically pure and obtained from the Shanghai Guoyao Agent Company (Shanghai, China). The polyethylene glycol (PEG) of chemical grade was obtained from Zhenjiang Huaxing New Materials Co., Ltd. (Jiangsu, China). The organic montmorillonite was homemade and of industrial grade [12].
2.2 Preparation of DA and polyester
About 10 g of SO was added into a three-necked flask with 5 ml of acetate buffer. Then, 20 ml of acetone and ethanol with a ratio of 1:1 was added to the flask. By titration with 0.1 n KOH aqueous solution, the total fatty acid generated in the mixture was determined.
Next, 1.2 g of organic montmorillonite was dissolved in 100 g of the fatty acid. The above mixture was then added into a three-necked flask, heated to 200°C and treated at this temperature for 6 h to allow the reaction to proceed completely. Crude DA was thus prepared. Then, 14 g of crude DA was poured into a three-necked flask, to which 25 g of PEG and 0.12 g of catalyst were added. This mixture was cured for 6 h at 190ºC. Thus, a type of polyester was obtained.
2.3 Characterization of the DA and polyester
The FTIR was performed on a Nicolet Avatar 370 spectrophotometer. The ranges were between 4000 and 700 cm−1 with a resolution of 2 cm−1. The pellets were formed by grinding samples with KBr. The spectra with good signal-to-noise ratios were obtained by 64 scans.
The 1H-NMR was conducted on a Bruker AV600 spectrometer. Deuterated chloroform (CDCl3, 99.8%) was used to prepare the sample solutions for testing. The tetrahydrofuran was used to dissolve the above liquids. An internal standard was referred to tetramethylsilane (TMS).
The molecular weight was measured by gel permeation chromatography (GPC) (PL-GPC50, the relative molecular mass range: 500~19,000, British). Tetrahydrofuran was used as the mobile phase with a flow rate of 1 ml/min.
Thermogravimetric analysis (TGA and DTG) was conducted on a Linseis PT-1000 microbalance. This test was conducted under a nitrogen atmosphere in an increasing rate of temperature, 20°C/min. In addition, 10 mg of the samples was used during the measurement.
The acid values of the polyester were measured according to the Chinese National Standard HG/T 2708-95. The following formula was used in the calculation of the values:
where AV is the acid value of the sample, V1 is the titration of the sample standard solution of potassium hydroxide (ml), V2 is the blank test of the titration of the potassium hydroxide standard solution consumed (ml), T is the standard equivalent concentration of potassium hydroxide solution and M is the specimen weight (g).
The soil burial test was conducted to characterize the biodegradable properties. The composites were cut into 60 mm×30 mm×2 mm portions. Their weights were measured, after which they were buried in soil with a depth of 15.24 cm. After a certain number of days, they were taken out from the soil. In addition, the soil was removed from their surface by washing with distilled water. The samples were dried in an oven for 6 h and kept at room temperature for 24 h. Finally, their weight loss was measured, and thus the biodegradability property was obtained.
3 Results and discussion
3.1 Preparation of the DA
3.1.1 Preparation mechanism of the DA from SO
The hydrolysis of SO under acid conditions is shown in Scheme 1. The products of this hydrolysis were glycerol and fatty acid. SO is considered a type of semidrying oil due to its lower double-bond density. Among others, it is the second cheapest vegetable oil [13]. The structure of SO consists of the tri-glyceride ester of fatty acids [8], [14]. The unsaturation in the fatty acid makes it an ideal monomer for preparing other polymers. However, its reactivity is rather low due to its multiple chain structure.
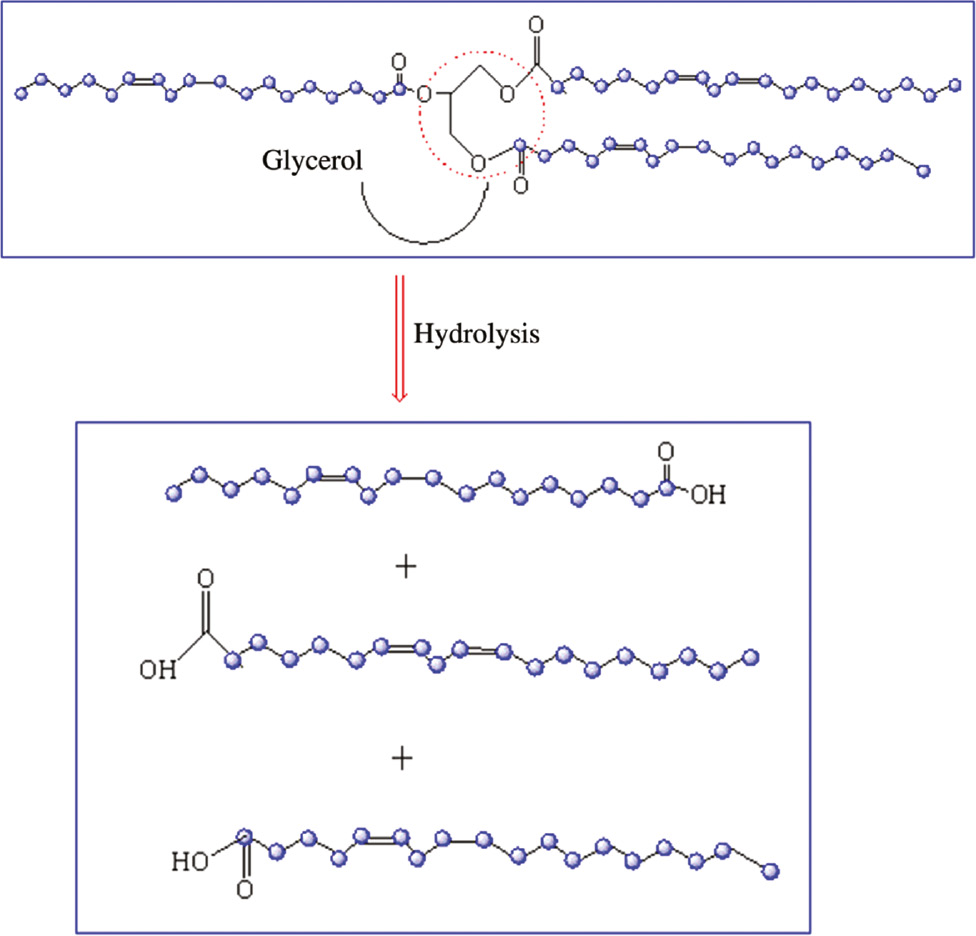
Hydrolysis of SO.
The preparation mechanism of DA from the hydrolysis product of SO was via the Diels-Alder reaction. In this reaction, a conjugated diene is produced from the non-conjugated diene. Then, it reacts with a double bond in another unsaturated fatty group. This reaction could be an intramolecular cycloaddition from which cyclic products could be formed [15]. Polymers with high molecular weight can be synthesized between dimers and trimers through polymerization and intermolecular Diels-Alder reactions.
3.1.2 Characterization of the DA
Photos of the DA are shown in Figure 1. As can be seen from the photo, SO is a kind of transparent liquid with a pale yellow color, whereas the crude DA is brown, viscous and can be crystallized at low temperatures.
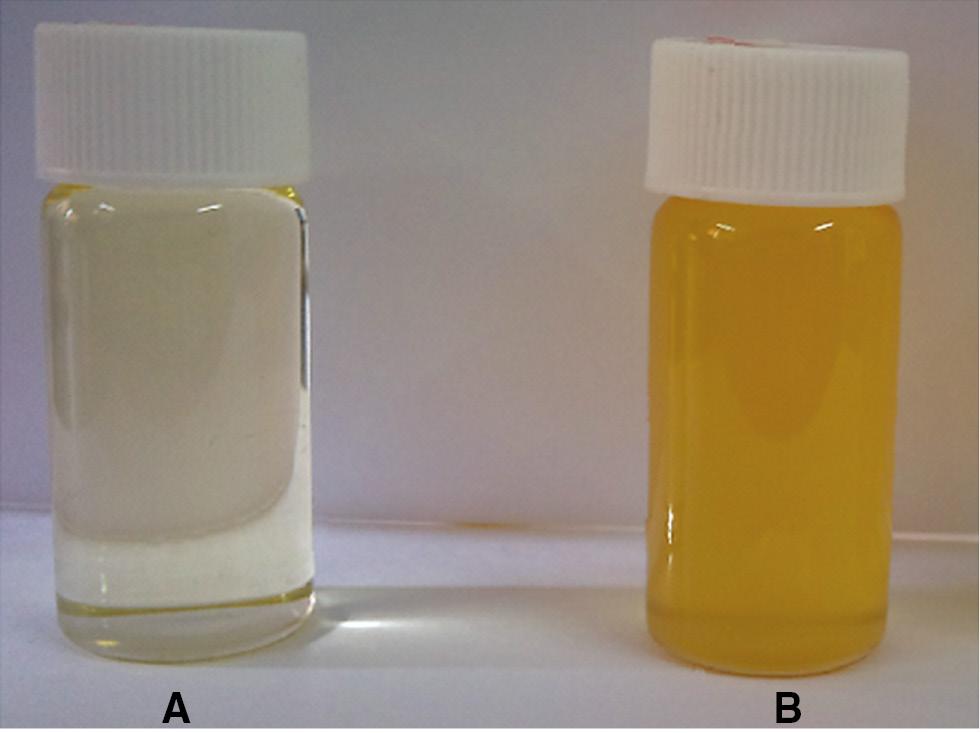
Digital photos of the raw material and the intermediate (A) SO, (B) DA. These photos were conducted at an ambient temperature.
From the FTIR spectrum of SO, Figure 2A, three obvious absorption peaks were observed at 3000, 2800 and 1550 cm−1, respectively. These peaks were related to the stretching and bending vibrations of C-H bonds in the compound. The peaks at 3000–3100 cm−1 and 950 cm−1 were ascribed to the stretching and bending vibrations of =CH. The very obvious absorption peak at 1750 cm−1 resulted from the stretching vibrations of the C=O groups. In addition, the peaks at 1180 cm−1 resulted from the C-O bonds, indicating that the -COO groups remained in the structure.
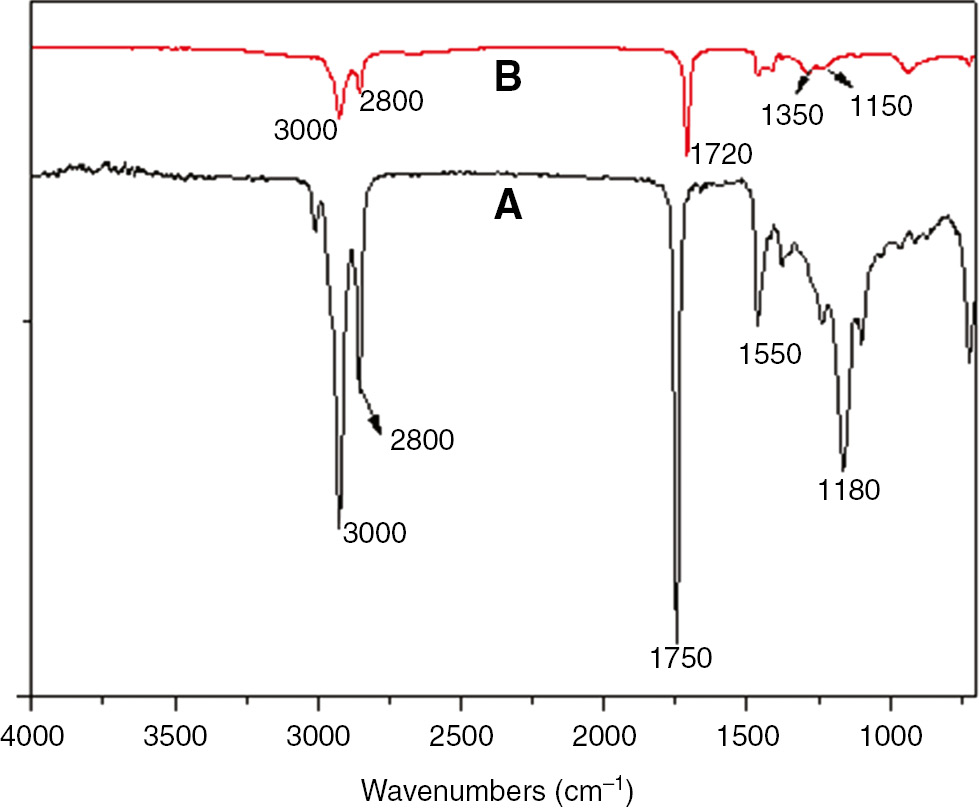
FTIR of the raw material and the intermediate (A) SO, (B) DA.
The scanning was performed in a range between 4000 and 700 cm−1 with a resolution of 2 cm−1.
In the spectrum of DA (Figure 2B), strong peaks appeared at 3000–2800 and 1400–1500 cm−1, which resulted from the stretching and bending vibrations of the C-H bonds in the structure. The absorption peaks at 3000–3100 cm−1 disappeared, illustrating that the double bond reactions and α-carbon reaction occurred in this olefin [16], [17]. The absorption peak at 1720 cm−1 resulted from the stretching vibrations of the C=O bonds, and those at 1180 cm−1 were ascribed to C-O. This demonstrated that the –COO groups also existed in this molecule. Wider peaks appeared at 1150–1350 cm−1, which resulted from the bending vibration of =CH caused by the cyclohexene. This proved that the cyclohexene structure existed in the molecular structure of DA [18]. In other words, the DA product had the typical structure of a dimer.
The 1H-NMR spectra of SO and DA are shown in Figure 3. the signals at 5.2–5.4 ppm, shown in Figure 3A, can be ascribed to the olefinic hydrogens, whereas those at 4.1–4.4 ppm were the methylene protons in the -CH2 adjacent to the C=C bond. The signals at 1.1–2.9 ppm also resulted from the methylene protons of -CH2–CH2–CH2– in the glycerin. In Figure 3B, the peaks at 1.2–1.5 and 2.1–2.4 ppm were greatly decreased compared with the proton in SO. The peaks at 5.2–5.4, 4.1–4.5 and 2.8 ppm also disappeared. This observation illustrated the reduced number of C=C bonds and the successful polymerization of DA [9], [15], [19].
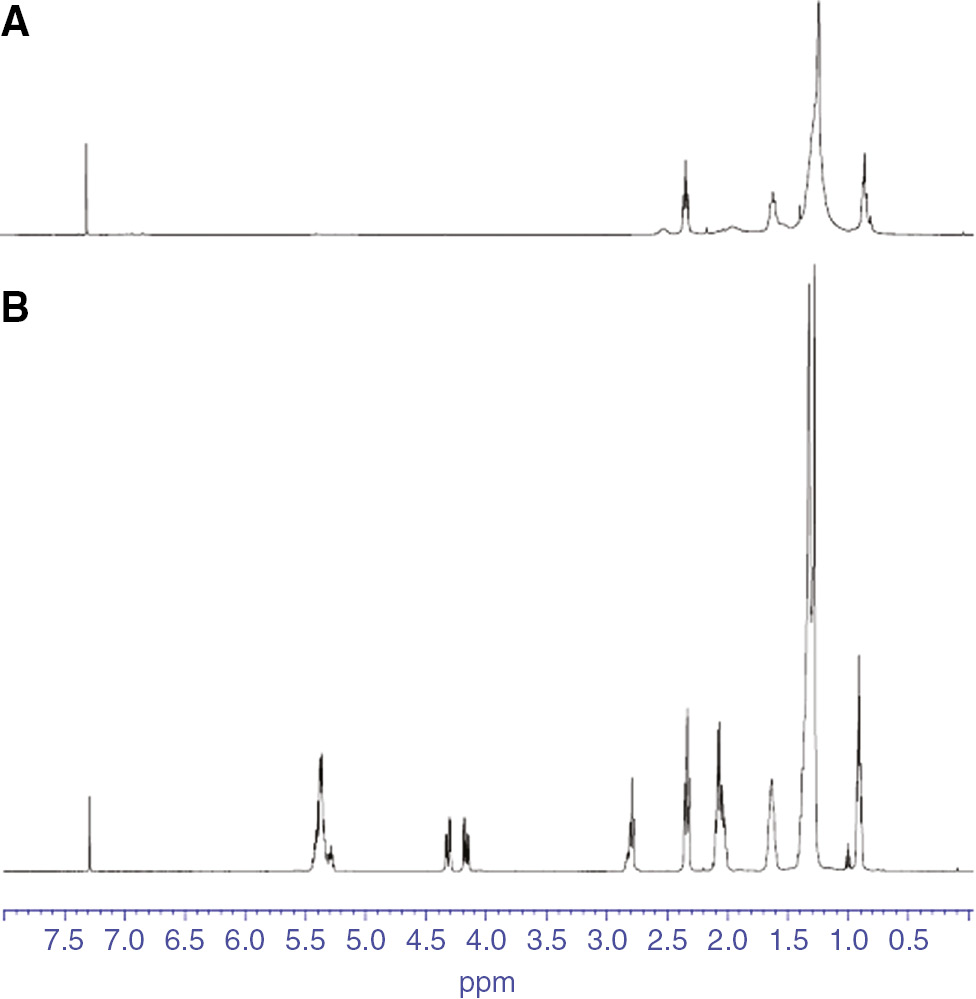
The 1H-NMR spectra of the raw material and the intermediate (A) SO, (B) DA. Deuterated chloroform (CDCl3, 99.8%) was used to prepare the sample solutions for testing.
The GPC results of the prepared DA are summarized in Table 1. As can be seen, about 87% of the average molecular weights ranged from 995 to 1304 g/mol, which were higher than the theoretical average molecular weight of 560 g/mol. This was due to the complex components in the edible SO, which contained different ratios of palmitic acid, arachidic acid and stearic acid. The unsaturated groups in these components may participate in the conjugation reactions, thus increasing the molecular weight of DA [20].
The GPC results of DA.
| DA | M̅n | M̅w | M̅z | Ratio (%) |
|---|---|---|---|---|
| 1 | 3402 | 4817 | 7668 | 13.05 |
| 2 | 1304 | 1314 | 1324 | 44.89 |
| 3 | 995 | 1012 | 1025 | 42.06 |
3.2 Preparation of the DA/PEG polyester
3.2.1 Preparation mechanism of the DA/PEG polyester
The preparation process of polyester from DA and PEG is shown in Scheme 2. The main factors that affected the esterification between DA and PEG were dosage, reaction time, reaction temperature and reactant ratios. The experiments were conducted to investigate these factors. Two reaction temperatures, 180°C and 190°C, were selected due to the limited experimental conditions. In this experiment, the ratios of the reactants and the reaction time were fixed, but the catalyst and reaction temperature were changed.
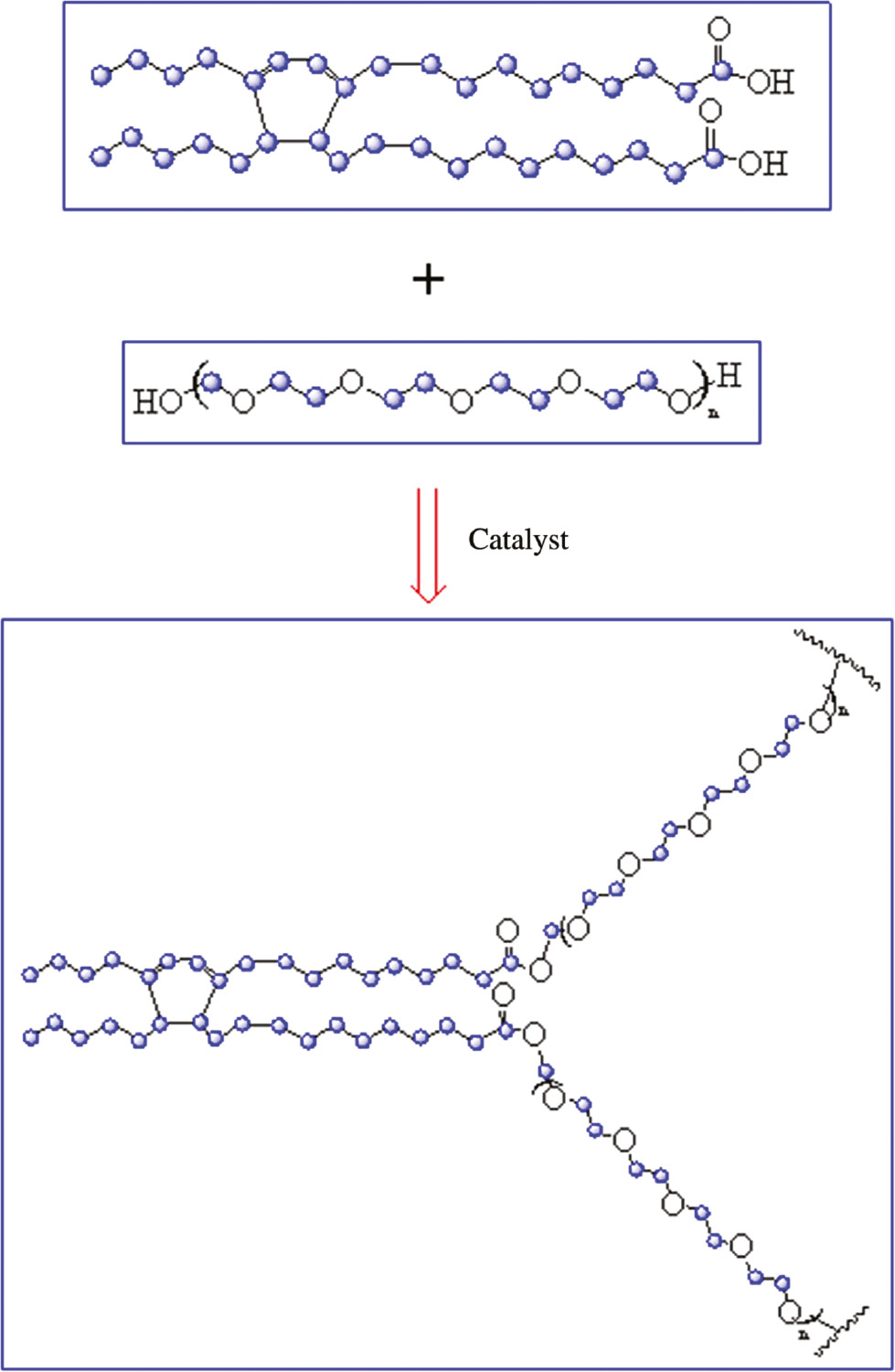
The preparation process of polyester from DA and PEG.
Table 2 shows the results of the acid values and ester conversion rates using different types of catalyst. It can be seen that the conversion rate was the highest when SnCl2 was used as the catalyst. The catalytic mechanism [21], [22], [23] of SnCl2 was illustrated as follows. First, SnCl2 had antioxidant abilities; when it was added to the reaction system, the incidence of side effects was effectively reduced and the esterification rate increased. Second, the catalyst SnCl2 provided a no-electron atomic orbit and combined with the lone pair electrons of the oxygen in the carbonyl. This enhanced the positive electrical charge of the carbon in carbonyl. Due to these reasons, the oxygen in the hydroxyl groups of PEG combined with the carbon atoms in the carbonyl. Thus, the appropriate amount of SnCl2 was suitable to accelerate this reaction.
The acid value and ester conversion rate of polyester.
| Catalyst | Temperature (°C) | Mass (g) | Consummation of KOH (ml) | Conversion rate (%) |
|---|---|---|---|---|
| No. | 180 | 2.2118 | 26.80 | 60.26 |
| Stannous chloride | 180 | 2.3411 | 16.45 | 83.13 |
| Stannous chloride | 190 | 2.1947 | 17.50 | 79.76 |
| Concentrated sulfuric acid | 180 | 2.0742 | 20.70 | 71.38 |
| Concentrated sulfuric acid | 190 | 2.2293 | 25.00 | 64.35 |
| Glacial acetic acid | 180 | 2.2163 | 24.60 | 64.98 |
| Glacial acetic acid | 190 | 2.2707 | 26.30 | 62.32 |
| Ammonia acid | 180 | 2.1545 | 25.90 | 61.16 |
| Ammonia acid | 190 | 2.6653 | 31.95 | 58.00 |
In Table 2, it was shown that the conversion rate under 180°C was 83.13%, which was higher than that under 190°C. The decarboxylation of the fatty acid can be caused by high temperatures. This increased the pressure in the flask, which resulted in an increase of the monomer content and a decrease of the acid value and viscosity [24]. Therefore, using SnCl2 as catalyst and setting the the reaction temperature to 180°C were the optimal conditions for the preparation of the polyester.
3.2.2 Characterization of the DA/PEG polyester
Figure 4 presents the FTIR spectrum of the DA/PEG polyester. The table shows two obvious absorption peaks at 3300–3700 and 3000–2800 cm−1, respectively. These corresponded to the stretching vibrations of the O-H and C-H bonds in the structure. The obvious peak at 1730 cm−1 resulted from the stretching vibration of the C=O group. Those at 1160 cm−1 were related to the C-O bond. This showed that the –COO groups existed in the polymer. There were no absorptions at 1800–2700 cm−1, indicating that the sample had a high purity [25]. In conclusion, the polyester was properly synthesized by the DA and PEG.
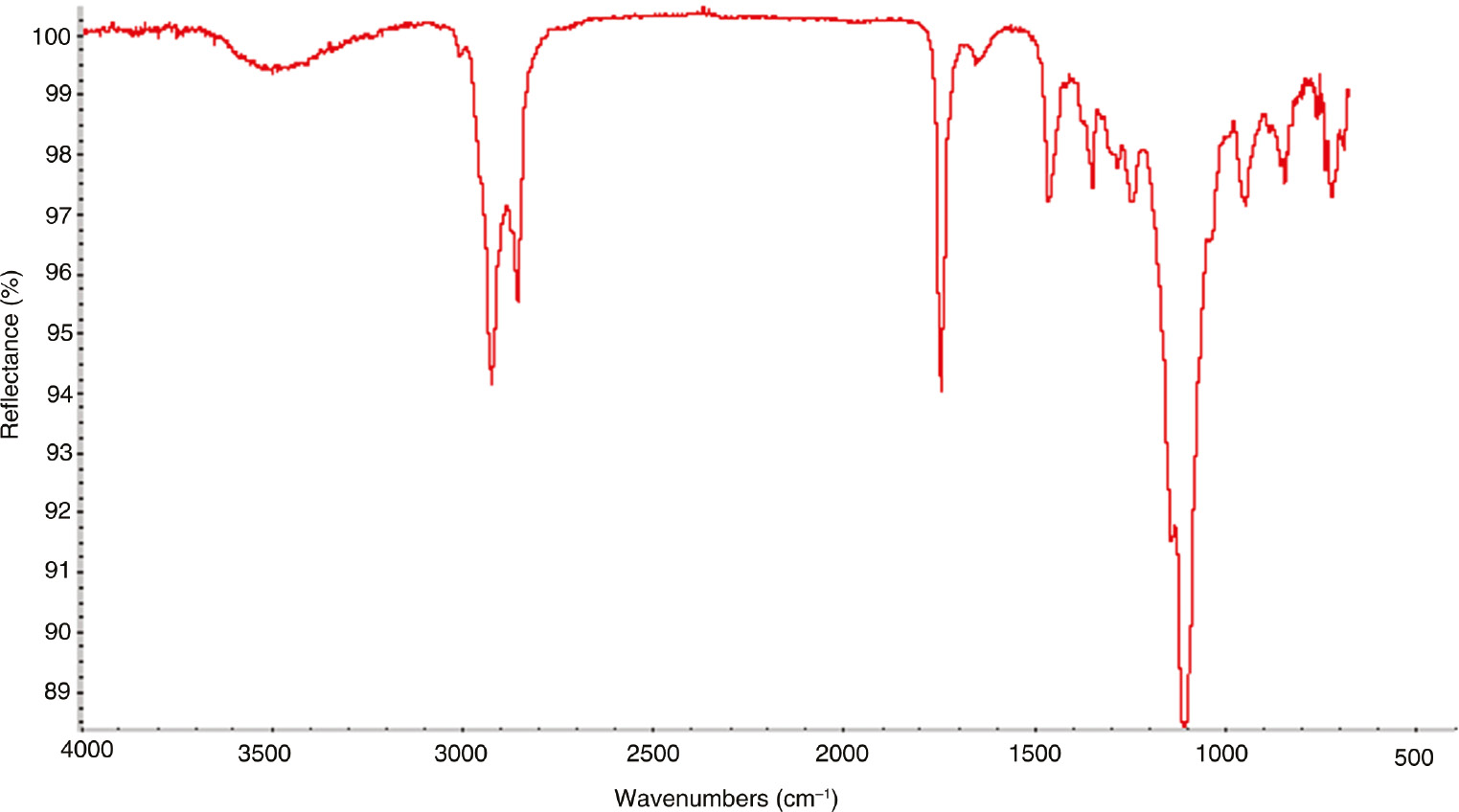
The FTIR of DA/PEG polyester.
The 1H-NMR spectrum of the polyester is illustrated in Figure 5. The chemical shifts between 3.5 and 4.1 ppm resulted from the CH2-O group. The bands appearing between 2.0 and 2.5 ppm resulted from the -COCH3 structure in the remaining monomers. Moreover, the multiple peaks at 0.8–1.7 ppm may be due to the complicated chemical environment of the H protons in the methyl groups among the macromolecular chains [26].
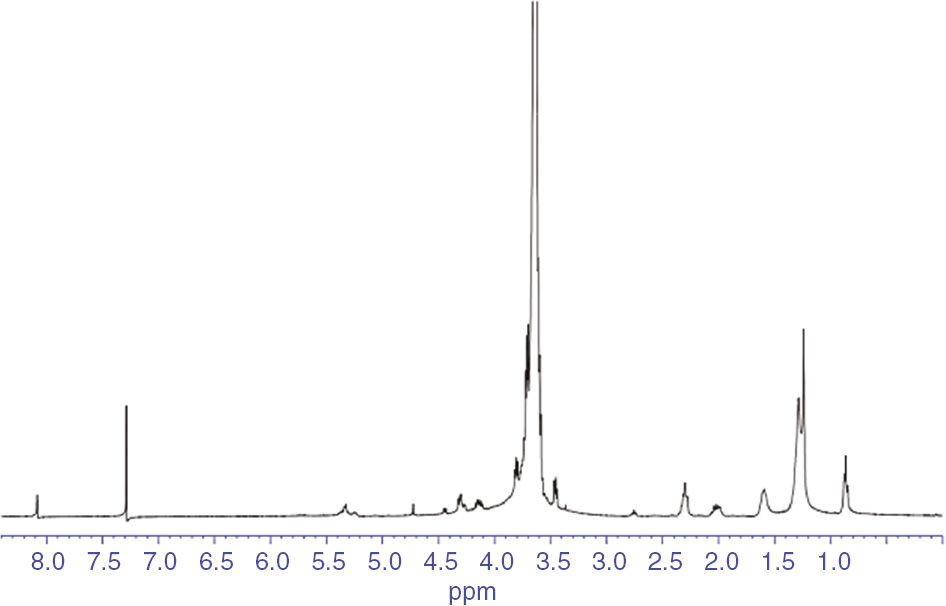
The 1H-NMR spectrum of DA/PEG polyester.
The TGA and DTG curves of the DA/PEG polyester are shown Figure 6. Its degradation under air occurred in three successive steps. Initial weight loss was first shown between 250°C and 320°C. This was due to the degradation of the synthetic monomer, such as DA and PEG, in the resin. The second step was observed between 320°C and 440°C. This was attributed to the degradation of the alkane hydrocarbon groups in the structure. The third step appeared between 440°C and 510°C, and showed the highest degradation rate at about 440°C. This had something to do with the degradation of the resin residue sin. This resin showed a characteristic thermal behavior of polymer resins [27]. In conclusion, the results above demonstrated that this polyester resin was successfully prepared.
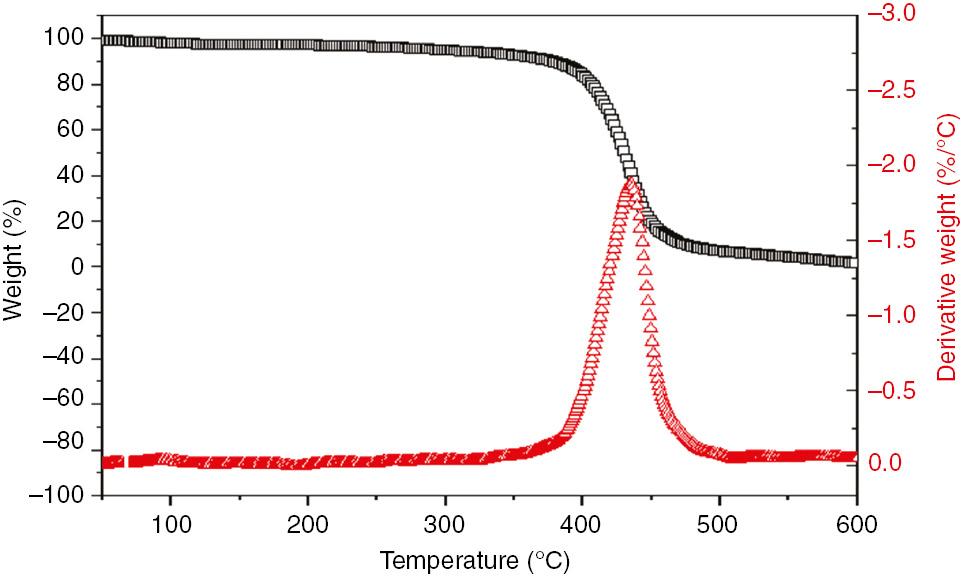
The TGA and DTG curves of DA/PEG polyester.
Table 3 records the GPC results of the DA/PEG polyester. About 55% of the molecular weight of the polyester was 10,892 g/mol, whereas 45% was 8259 g/mol. This showed that the condensation reaction was not sufficient. It can be inferred that the dehydration reaction of the crude DA occurred, and the product with a carboxyl group was reduced. Hence, the carboxyl functional groups were unable to form a double DA, leading to a capped reaction in PEG. This phenomenon was not beneficial for the possible formation of polyesters with high molecular weights. In addition, according to the principle of kinetics, the formation of the polyester may be blocked due to the presence of water [28].
GPC results of DA/PEG polyester.
| DA/PEG polyester | M̅n | M̅w | M̅z | Ratio (%) |
|---|---|---|---|---|
| 1 | 10,892 | 11,548 | 12,892 | 55.5 |
| 2 | 8259 | 9564 | 10,257 | 44.5 |
The biodegradability of the polyester was determined by measuring the weight loss of the composite during the soil burial test. Figure 7 describes the percentage weight loss of the biodegraded polymers after 35 days. In order to know its biodegradable property, the polyesters prepared from the crude DA and PEG were compared with those from TA and PEG.
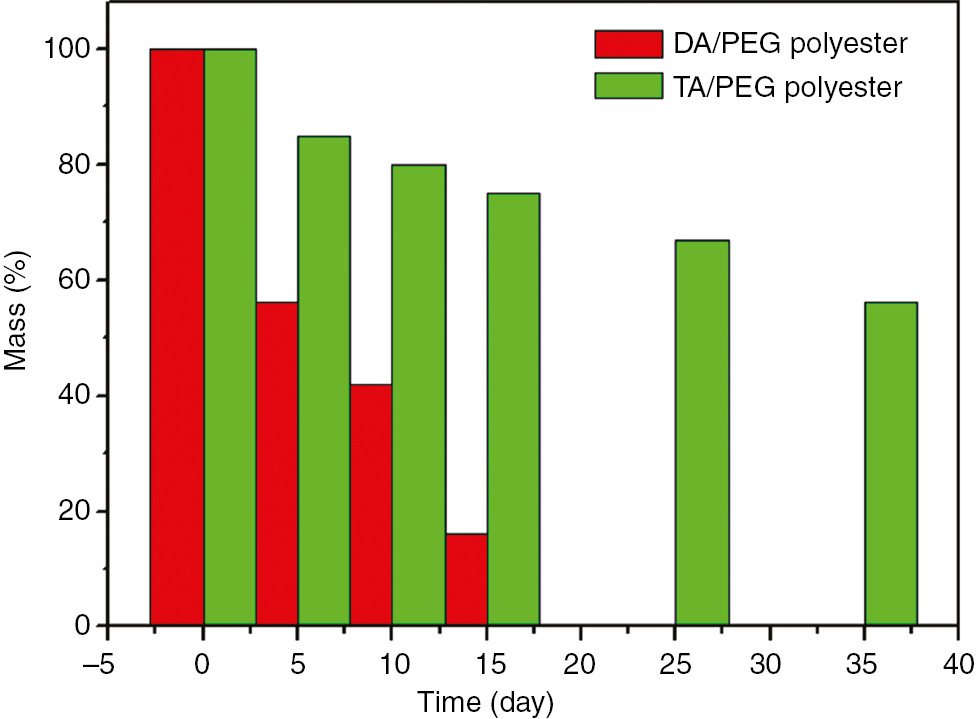
The weight losses of different polyesters under the soil burial test.
The weight loss showed an approximately linear relation with degradation time for both materials. The weight loss of the polyester prepared from TA was about 25% after biodegradation for 15 days. The average degradation rate was at a relatively lower rate of 1.6%. In comparison, the weight loss of polyester prepared from crude DA was at 60%. The average degradation rate was about 4% per day. These data indicated that the weight loss of the polyester prepared from the DA was relatively higher compared with that prepared from TA. In other words, the presence of DA prepared from SO had a pronounced effect on the weight loss during biodegradation.
4 Conclusions
The biodegradable polyester was prepared by a novel method using SO. The new method was based on the first preparation of the DA. The proposed method was feasible for the synthesis of crude DA by using SO. The experimental conditions were the combination of the following conditions: temperature of 200°C, organic montmorillonite as a catalyst, and the reaction time of 6 h. The structure and properties of DA were analyzed by FTIR, 1H-NMR and GPC. The DA had an average molecular weight of 995–1304 g/mol.
The optimal experimental conditions for the preparation of the biodegradation polyesters were determined: temperature of 180°C and SnCl2 as the catalyst. The structure and properties of the polyester were analyzed by FTIR, 1H-NMR, GPC and soil burial test. The polyester had a molecular weight of 8259–10,892 g/mol. Meanwhile, the polyester prepared from the crude DA and PEG showed a better biodegradation property than that obtained from TA and PEG.
Acknowledgments
This work was financially supported by the Capacity Building Project of Some Local Colleges and Universities in Shanghai (Grant No. 17030501200).
References
[1] Zahira Y, Masita M, Mohammad A, Zahangir A, Kamaruzaman S. Renew. Sust. Energ. Rev. 2013, 18, 184–193.10.1016/j.rser.2012.10.016Search in Google Scholar
[2] Wang Y, Ou S, Liu P, Zhang Z. Energ. Convers. Manage. 2007, 48, 184–188.10.1016/j.enconman.2006.04.016Search in Google Scholar
[3] Pinzi S, Garcia IL, Lopez-Gimenez FJ, Luque de Castro MD, Dorado G, Dorado MP. Energ Fuel 2009, 23, 2325–2341.10.1021/ef801098aSearch in Google Scholar
[4] Man KL, Keat TL, Abdul RM. Biotechnol. Adv. 2010, 28, 500–518.10.1016/j.biotechadv.2010.03.002Search in Google Scholar
[5] Guzatto R, Defferrari D, Reiznautt QB, Cadore IR, Samios D. Fuel 2012, 92, 197–203.10.1016/j.fuel.2011.08.010Search in Google Scholar
[6] Guzatto R, Luis de Martini T, Samios D. Fuel Process. Technol. 2011, 92, 2083–2088.10.1016/j.fuproc.2011.06.013Search in Google Scholar
[7] Istadi I, Anggoro DD, Buchori L, Rahmawati DA, Intaningrum D. Procedia Environ. Sci. 2015, 23, 385–393.10.1016/j.proenv.2015.01.055Search in Google Scholar
[8] Zargar M, Ahmadinia E, Asli H, Karim MR. J. Hazard. Mater. 2012, 233–234, 254.10.1016/j.jhazmat.2012.06.021Search in Google Scholar
[9] Liu ZS, Erhan SZ. J. Polym. Environ. 2010, 18, 243–249.10.1007/s10924-010-0179-ySearch in Google Scholar
[10] Guo A, Javni I, Petrovic Z. J. Appl. Polym. Sci. 2015, 77, 467–473.10.1002/(SICI)1097-4628(20000711)77:2<467::AID-APP25>3.0.CO;2-FSearch in Google Scholar
[11] Sahoo Sushanta K, Smita M, Nayak Sanjay K. Chin. J. Poly. Sci. 2015, 33, 137–152.10.1007/s10118-015-1568-4Search in Google Scholar
[12] Wang JC, Yang K. J. Appl. Polym. Sci. 2012, 123, 1293–1301.10.1002/app.34683Search in Google Scholar
[13] Gunstone FD, Padley FB. Lipid Technologies and Applications, Marcel Dekker, Inc.: New York, 1997, p. 714.Search in Google Scholar
[14] Wang CH, Erhan S. J. Am. Oil Chem. Soc. 1999, 76, 1211–1216.10.1007/s11746-999-0096-1Search in Google Scholar
[15] Mert A, Brajendra KS, Neil PJP, Joseph MP, Kenneth MD. J. Am. Oil Chem. Soc. 2012, 89, 987–994.10.1007/s11746-011-2002-xSearch in Google Scholar
[16] Sun LM, Feng SB. Chinese Grease 2006, 31, 58.10.1017/CBO9780511755019Search in Google Scholar
[17] Zhang SW. Novel Chem. Mater. 1995, 8, 25.Search in Google Scholar
[18] Feng GZ, Cui YD, Lu Q. Mach. Cereals Oil Food Proc. 2005, 11, 53.Search in Google Scholar
[19] Miyake Y, Yokomizo K, Matsuzaki N. J. Am. Oil Chem. Soc. 1998, 75, 1091–1094.10.1007/s11746-998-0118-4Search in Google Scholar
[20] Zhang SL. Petrochemicals 1995, 6, 71.Search in Google Scholar
[21] Niu MJ. Chem. Adhes. 1997, 1, 35.Search in Google Scholar
[22] Feng GZ, Li HP, Cui YD. Chem. Eng. 2007, 35, 75.Search in Google Scholar
[23] Feng GZ, Qu H, Cui YD, Li HP, Lu K. J. Polym. Res. 2007, 14, 115–119.10.1007/s10965-006-9090-6Search in Google Scholar
[24] Hwang HS, Singh M, Bakota EL, Winkler-Moser JK, Kim S, Liu SX. J. Am. Oil Chem. Soc. 2013, 90, 1705–1712.10.1007/s11746-013-2315-zSearch in Google Scholar
[25] Farhoosh R, Moosavi SMR. J. Agric. Sci. Technol. 2009, 11, 173–179.Search in Google Scholar
[26] Yang XH, Xia JL, Huang K. Forest Prod. Chem. Ind. 2010, 30, 31–34.Search in Google Scholar
[27] Wang JC, Chen SH, Xu, ZC. Food Sci. Technol. 2012, 7, 229.Search in Google Scholar
[28] Zhou B. Coat. Ind. 2007, 6, 58–60.Search in Google Scholar
©2019 Walter de Gruyter GmbH, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 Public License.
Articles in the same Issue
- Regular Articles
- Studies on the preparation and properties of biodegradable polyester from soybean oil
- Flow-mode biodiesel production from palm oil using a pressurized microwave reactor
- Reduction of free fatty acids in waste oil for biodiesel production by glycerolysis: investigation and optimization of process parameters
- Saccharin: a cheap and mild acidic agent for the synthesis of azo dyes via telescoped dediazotization
- Optimization of lipase-catalyzed synthesis of polyethylene glycol stearate in a solvent-free system
- Green synthesis of iron oxide nanoparticles using Platanus orientalis leaf extract for antifungal activity
- Ultrasound assisted chemical activation of peanut husk for copper removal
- Room temperature silanization of Fe3O4 for the preparation of phenyl functionalized magnetic adsorbent for dispersive solid phase extraction for the extraction of phthalates in water
- Evaluation of the saponin green extraction from Ziziphus spina-christi leaves using hydrothermal, microwave and Bain-Marie water bath heating methods
- Oxidation of dibenzothiophene using the heterogeneous catalyst of tungsten-based carbon nanotubes
- Calcined sodium silicate as an efficient and benign heterogeneous catalyst for the transesterification of natural lecithin to L-α-glycerophosphocholine
- Synergistic effect between CO2 and H2O2 on ethylbenzene oxidation catalyzed by carbon supported heteropolyanion catalysts
- Hydrocyanation of 2-arylmethyleneindan-1,3-diones using potassium hexacyanoferrate(II) as a nontoxic cyanating agent
- Green synthesis of hydratropic aldehyde from α-methylstyrene catalyzed by Al2O3-supported metal phthalocyanines
- Environmentally benign chemical recycling of polycarbonate wastes: comparison of micro- and nano-TiO2 solid support efficiencies
- Medicago polymorpha-mediated antibacterial silver nanoparticles in the reduction of methyl orange
- Production of value-added chemicals from esterification of waste glycerol over MCM-41 supported catalysts
- Green synthesis of zerovalent copper nanoparticles for efficient reduction of toxic azo dyes congo red and methyl orange
- Optimization of the biological synthesis of silver nanoparticles using Penicillium oxalicum GRS-1 and their antimicrobial effects against common food-borne pathogens
- Optimization of submerged fermentation conditions to overproduce bioethanol using two industrial and traditional Saccharomyces cerevisiae strains
- Extraction of In3+ and Fe3+ from sulfate solutions by using a 3D-printed “Y”-shaped microreactor
- Foliar-mediated Ag:ZnO nanophotocatalysts: green synthesis, characterization, pollutants degradation, and in vitro biocidal activity
- Green cyclic acetals production by glycerol etherification reaction with benzaldehyde using cationic acidic resin
- Biosynthesis, characterization and antimicrobial activities assessment of fabricated selenium nanoparticles using Pelargonium zonale leaf extract
- Synthesis of high surface area magnesia by using walnut shell as a template
- Controllable biosynthesis of silver nanoparticles using actinobacterial strains
- Green vegetation: a promising source of color dyes
- Mechano-chemical synthesis of ammonia and acetic acid from inorganic materials in water
- Green synthesis and structural characterization of novel N1-substituted 3,4-dihydropyrimidin-2(1H)-ones
- Biodiesel production from cotton oil using heterogeneous CaO catalysts from eggshells prepared at different calcination temperatures
- Regeneration of spent mercury catalyst for the treatment of dye wastewater by the microwave and ultrasonic spray-assisted method
- Green synthesis of the innovative super paramagnetic nanoparticles from the leaves extract of Fraxinus chinensis Roxb and their application for the decolourisation of toxic dyes
- Biogenic ZnO nanoparticles: a study of blueshift of optical band gap and photocatalytic degradation of reactive yellow 186 dye under direct sunlight
- Leached compounds from the extracts of pomegranate peel, green coconut shell, and karuvelam wood for the removal of hexavalent chromium
- Enhancement of molecular weight reduction of natural rubber in triphasic CO2/toluene/H2O systems with hydrogen peroxide for preparation of biobased polyurethanes
- An efficient green synthesis of novel 1H-imidazo[1,2-a]imidazole-3-amine and imidazo[2,1-c][1,2,4]triazole-5-amine derivatives via Strecker reaction under controlled microwave heating
- Evaluation of three different green fabrication methods for the synthesis of crystalline ZnO nanoparticles using Pelargonium zonale leaf extract
- A highly efficient and multifunctional biomass supporting Ag, Ni, and Cu nanoparticles through wetness impregnation for environmental remediation
- Simple one-pot green method for large-scale production of mesalamine, an anti-inflammatory agent
- Relationships between step and cumulative PMI and E-factors: implications on estimating material efficiency with respect to charting synthesis optimization strategies
- A comparative sorption study of Cr3+ and Cr6+ using mango peels: kinetic, equilibrium and thermodynamic
- Effects of acid hydrolysis waste liquid recycle on preparation of microcrystalline cellulose
- Use of deep eutectic solvents as catalyst: A mini-review
- Microwave-assisted synthesis of pyrrolidinone derivatives using 1,1’-butylenebis(3-sulfo-3H-imidazol-1-ium) chloride in ethylene glycol
- Green and eco-friendly synthesis of Co3O4 and Ag-Co3O4: Characterization and photo-catalytic activity
- Adsorption optimized of the coal-based material and application for cyanide wastewater treatment
- Aloe vera leaf extract mediated green synthesis of selenium nanoparticles and assessment of their In vitro antimicrobial activity against spoilage fungi and pathogenic bacteria strains
- Waste phenolic resin derived activated carbon by microwave-assisted KOH activation and application to dye wastewater treatment
- Direct ethanol production from cellulose by consortium of Trichoderma reesei and Candida molischiana
- Agricultural waste biomass-assisted nanostructures: Synthesis and application
- Biodiesel production from rubber seed oil using calcium oxide derived from eggshell as catalyst – optimization and modeling studies
- Study of fabrication of fully aqueous solution processed SnS quantum dot-sensitized solar cell
- Assessment of aqueous extract of Gypsophila aretioides for inhibitory effects on calcium carbonate formation
- An environmentally friendly acylation reaction of 2-methylnaphthalene in solvent-free condition in a micro-channel reactor
- Aegle marmelos phytochemical stabilized synthesis and characterization of ZnO nanoparticles and their role against agriculture and food pathogen
- A reactive coupling process for co-production of solketal and biodiesel
- Optimization of the asymmetric synthesis of (S)-1-phenylethanol using Ispir bean as whole-cell biocatalyst
- Synthesis of pyrazolopyridine and pyrazoloquinoline derivatives by one-pot, three-component reactions of arylglyoxals, 3-methyl-1-aryl-1H-pyrazol-5-amines and cyclic 1,3-dicarbonyl compounds in the presence of tetrapropylammonium bromide
- Preconcentration of morphine in urine sample using a green and solvent-free microextraction method
- Extraction of glycyrrhizic acid by aqueous two-phase system formed by PEG and two environmentally friendly organic acid salts - sodium citrate and sodium tartrate
- Green synthesis of copper oxide nanoparticles using Juglans regia leaf extract and assessment of their physico-chemical and biological properties
- Deep eutectic solvents (DESs) as powerful and recyclable catalysts and solvents for the synthesis of 3,4-dihydropyrimidin-2(1H)-ones/thiones
- Biosynthesis, characterization and anti-microbial activity of silver nanoparticle based gel hand wash
- Efficient and selective microwave-assisted O-methylation of phenolic compounds using tetramethylammonium hydroxide (TMAH)
- Anticoagulant, thrombolytic and antibacterial activities of Euphorbia acruensis latex-mediated bioengineered silver nanoparticles
- Volcanic ash as reusable catalyst in the green synthesis of 3H-1,5-benzodiazepines
- Green synthesis, anionic polymerization of 1,4-bis(methacryloyl)piperazine using Algerian clay as catalyst
- Selenium supplementation during fermentation with sugar beet molasses and Saccharomyces cerevisiae to increase bioethanol production
- Biosynthetic potential assessment of four food pathogenic bacteria in hydrothermally silver nanoparticles fabrication
- Investigating the effectiveness of classical and eco-friendly approaches for synthesis of dialdehydes from organic dihalides
- Pyrolysis of palm oil using zeolite catalyst and characterization of the boil-oil
- Azadirachta indica leaves extract assisted green synthesis of Ag-TiO2 for degradation of Methylene blue and Rhodamine B dyes in aqueous medium
- Synthesis of vitamin E succinate catalyzed by nano-SiO2 immobilized DMAP derivative in mixed solvent system
- Extraction of phytosterols from melon (Cucumis melo) seeds by supercritical CO2 as a clean technology
- Production of uronic acids by hydrothermolysis of pectin as a model substance for plant biomass waste
- Biofabrication of highly pure copper oxide nanoparticles using wheat seed extract and their catalytic activity: A mechanistic approach
- Intelligent modeling and optimization of emulsion aggregation method for producing green printing ink
- Improved removal of methylene blue on modified hierarchical zeolite Y: Achieved by a “destructive-constructive” method
- Two different facile and efficient approaches for the synthesis of various N-arylacetamides via N-acetylation of arylamines and straightforward one-pot reductive acetylation of nitroarenes promoted by recyclable CuFe2O4 nanoparticles in water
- Optimization of acid catalyzed esterification and mixed metal oxide catalyzed transesterification for biodiesel production from Moringa oleifera oil
- Kinetics and the fluidity of the stearic acid esters with different carbon backbones
- Aiming for a standardized protocol for preparing a process green synthesis report and for ranking multiple synthesis plans to a common target product
- Microstructure and luminescence of VO2 (B) nanoparticle synthesis by hydrothermal method
- Optimization of uranium removal from uranium plant wastewater by response surface methodology (RSM)
- Microwave drying of nickel-containing residue: dielectric properties, kinetics, and energy aspects
- Simple and convenient two step synthesis of 5-bromo-2,3-dimethoxy-6-methyl-1,4-benzoquinone
- Biodiesel production from waste cooking oil
- The effect of activation temperature on structure and properties of blue coke-based activated carbon by CO2 activation
- Optimization of reaction parameters for the green synthesis of zero valent iron nanoparticles using pine tree needles
- Microwave-assisted protocol for squalene isolation and conversion from oil-deodoriser distillates
- Denitrification performance of rare earth tailings-based catalysts
- Facile synthesis of silver nanoparticles using Averrhoa bilimbi L and Plum extracts and investigation on the synergistic bioactivity using in vitro models
- Green production of AgNPs and their phytostimulatory impact
- Photocatalytic activity of Ag/Ni bi-metallic nanoparticles on textile dye removal
- Topical Issue: Green Process Engineering / Guest Editors: Martine Poux, Patrick Cognet
- Modelling and optimisation of oxidative desulphurisation of tyre-derived oil via central composite design approach
- CO2 sequestration by carbonation of olivine: a new process for optimal separation of the solids produced
- Organic carbonates synthesis improved by pervaporation for CO2 utilisation
- Production of starch nanoparticles through solvent-antisolvent precipitation in a spinning disc reactor
- A kinetic study of Zn halide/TBAB-catalysed fixation of CO2 with styrene oxide in propylene carbonate
- Topical on Green Process Engineering
Articles in the same Issue
- Regular Articles
- Studies on the preparation and properties of biodegradable polyester from soybean oil
- Flow-mode biodiesel production from palm oil using a pressurized microwave reactor
- Reduction of free fatty acids in waste oil for biodiesel production by glycerolysis: investigation and optimization of process parameters
- Saccharin: a cheap and mild acidic agent for the synthesis of azo dyes via telescoped dediazotization
- Optimization of lipase-catalyzed synthesis of polyethylene glycol stearate in a solvent-free system
- Green synthesis of iron oxide nanoparticles using Platanus orientalis leaf extract for antifungal activity
- Ultrasound assisted chemical activation of peanut husk for copper removal
- Room temperature silanization of Fe3O4 for the preparation of phenyl functionalized magnetic adsorbent for dispersive solid phase extraction for the extraction of phthalates in water
- Evaluation of the saponin green extraction from Ziziphus spina-christi leaves using hydrothermal, microwave and Bain-Marie water bath heating methods
- Oxidation of dibenzothiophene using the heterogeneous catalyst of tungsten-based carbon nanotubes
- Calcined sodium silicate as an efficient and benign heterogeneous catalyst for the transesterification of natural lecithin to L-α-glycerophosphocholine
- Synergistic effect between CO2 and H2O2 on ethylbenzene oxidation catalyzed by carbon supported heteropolyanion catalysts
- Hydrocyanation of 2-arylmethyleneindan-1,3-diones using potassium hexacyanoferrate(II) as a nontoxic cyanating agent
- Green synthesis of hydratropic aldehyde from α-methylstyrene catalyzed by Al2O3-supported metal phthalocyanines
- Environmentally benign chemical recycling of polycarbonate wastes: comparison of micro- and nano-TiO2 solid support efficiencies
- Medicago polymorpha-mediated antibacterial silver nanoparticles in the reduction of methyl orange
- Production of value-added chemicals from esterification of waste glycerol over MCM-41 supported catalysts
- Green synthesis of zerovalent copper nanoparticles for efficient reduction of toxic azo dyes congo red and methyl orange
- Optimization of the biological synthesis of silver nanoparticles using Penicillium oxalicum GRS-1 and their antimicrobial effects against common food-borne pathogens
- Optimization of submerged fermentation conditions to overproduce bioethanol using two industrial and traditional Saccharomyces cerevisiae strains
- Extraction of In3+ and Fe3+ from sulfate solutions by using a 3D-printed “Y”-shaped microreactor
- Foliar-mediated Ag:ZnO nanophotocatalysts: green synthesis, characterization, pollutants degradation, and in vitro biocidal activity
- Green cyclic acetals production by glycerol etherification reaction with benzaldehyde using cationic acidic resin
- Biosynthesis, characterization and antimicrobial activities assessment of fabricated selenium nanoparticles using Pelargonium zonale leaf extract
- Synthesis of high surface area magnesia by using walnut shell as a template
- Controllable biosynthesis of silver nanoparticles using actinobacterial strains
- Green vegetation: a promising source of color dyes
- Mechano-chemical synthesis of ammonia and acetic acid from inorganic materials in water
- Green synthesis and structural characterization of novel N1-substituted 3,4-dihydropyrimidin-2(1H)-ones
- Biodiesel production from cotton oil using heterogeneous CaO catalysts from eggshells prepared at different calcination temperatures
- Regeneration of spent mercury catalyst for the treatment of dye wastewater by the microwave and ultrasonic spray-assisted method
- Green synthesis of the innovative super paramagnetic nanoparticles from the leaves extract of Fraxinus chinensis Roxb and their application for the decolourisation of toxic dyes
- Biogenic ZnO nanoparticles: a study of blueshift of optical band gap and photocatalytic degradation of reactive yellow 186 dye under direct sunlight
- Leached compounds from the extracts of pomegranate peel, green coconut shell, and karuvelam wood for the removal of hexavalent chromium
- Enhancement of molecular weight reduction of natural rubber in triphasic CO2/toluene/H2O systems with hydrogen peroxide for preparation of biobased polyurethanes
- An efficient green synthesis of novel 1H-imidazo[1,2-a]imidazole-3-amine and imidazo[2,1-c][1,2,4]triazole-5-amine derivatives via Strecker reaction under controlled microwave heating
- Evaluation of three different green fabrication methods for the synthesis of crystalline ZnO nanoparticles using Pelargonium zonale leaf extract
- A highly efficient and multifunctional biomass supporting Ag, Ni, and Cu nanoparticles through wetness impregnation for environmental remediation
- Simple one-pot green method for large-scale production of mesalamine, an anti-inflammatory agent
- Relationships between step and cumulative PMI and E-factors: implications on estimating material efficiency with respect to charting synthesis optimization strategies
- A comparative sorption study of Cr3+ and Cr6+ using mango peels: kinetic, equilibrium and thermodynamic
- Effects of acid hydrolysis waste liquid recycle on preparation of microcrystalline cellulose
- Use of deep eutectic solvents as catalyst: A mini-review
- Microwave-assisted synthesis of pyrrolidinone derivatives using 1,1’-butylenebis(3-sulfo-3H-imidazol-1-ium) chloride in ethylene glycol
- Green and eco-friendly synthesis of Co3O4 and Ag-Co3O4: Characterization and photo-catalytic activity
- Adsorption optimized of the coal-based material and application for cyanide wastewater treatment
- Aloe vera leaf extract mediated green synthesis of selenium nanoparticles and assessment of their In vitro antimicrobial activity against spoilage fungi and pathogenic bacteria strains
- Waste phenolic resin derived activated carbon by microwave-assisted KOH activation and application to dye wastewater treatment
- Direct ethanol production from cellulose by consortium of Trichoderma reesei and Candida molischiana
- Agricultural waste biomass-assisted nanostructures: Synthesis and application
- Biodiesel production from rubber seed oil using calcium oxide derived from eggshell as catalyst – optimization and modeling studies
- Study of fabrication of fully aqueous solution processed SnS quantum dot-sensitized solar cell
- Assessment of aqueous extract of Gypsophila aretioides for inhibitory effects on calcium carbonate formation
- An environmentally friendly acylation reaction of 2-methylnaphthalene in solvent-free condition in a micro-channel reactor
- Aegle marmelos phytochemical stabilized synthesis and characterization of ZnO nanoparticles and their role against agriculture and food pathogen
- A reactive coupling process for co-production of solketal and biodiesel
- Optimization of the asymmetric synthesis of (S)-1-phenylethanol using Ispir bean as whole-cell biocatalyst
- Synthesis of pyrazolopyridine and pyrazoloquinoline derivatives by one-pot, three-component reactions of arylglyoxals, 3-methyl-1-aryl-1H-pyrazol-5-amines and cyclic 1,3-dicarbonyl compounds in the presence of tetrapropylammonium bromide
- Preconcentration of morphine in urine sample using a green and solvent-free microextraction method
- Extraction of glycyrrhizic acid by aqueous two-phase system formed by PEG and two environmentally friendly organic acid salts - sodium citrate and sodium tartrate
- Green synthesis of copper oxide nanoparticles using Juglans regia leaf extract and assessment of their physico-chemical and biological properties
- Deep eutectic solvents (DESs) as powerful and recyclable catalysts and solvents for the synthesis of 3,4-dihydropyrimidin-2(1H)-ones/thiones
- Biosynthesis, characterization and anti-microbial activity of silver nanoparticle based gel hand wash
- Efficient and selective microwave-assisted O-methylation of phenolic compounds using tetramethylammonium hydroxide (TMAH)
- Anticoagulant, thrombolytic and antibacterial activities of Euphorbia acruensis latex-mediated bioengineered silver nanoparticles
- Volcanic ash as reusable catalyst in the green synthesis of 3H-1,5-benzodiazepines
- Green synthesis, anionic polymerization of 1,4-bis(methacryloyl)piperazine using Algerian clay as catalyst
- Selenium supplementation during fermentation with sugar beet molasses and Saccharomyces cerevisiae to increase bioethanol production
- Biosynthetic potential assessment of four food pathogenic bacteria in hydrothermally silver nanoparticles fabrication
- Investigating the effectiveness of classical and eco-friendly approaches for synthesis of dialdehydes from organic dihalides
- Pyrolysis of palm oil using zeolite catalyst and characterization of the boil-oil
- Azadirachta indica leaves extract assisted green synthesis of Ag-TiO2 for degradation of Methylene blue and Rhodamine B dyes in aqueous medium
- Synthesis of vitamin E succinate catalyzed by nano-SiO2 immobilized DMAP derivative in mixed solvent system
- Extraction of phytosterols from melon (Cucumis melo) seeds by supercritical CO2 as a clean technology
- Production of uronic acids by hydrothermolysis of pectin as a model substance for plant biomass waste
- Biofabrication of highly pure copper oxide nanoparticles using wheat seed extract and their catalytic activity: A mechanistic approach
- Intelligent modeling and optimization of emulsion aggregation method for producing green printing ink
- Improved removal of methylene blue on modified hierarchical zeolite Y: Achieved by a “destructive-constructive” method
- Two different facile and efficient approaches for the synthesis of various N-arylacetamides via N-acetylation of arylamines and straightforward one-pot reductive acetylation of nitroarenes promoted by recyclable CuFe2O4 nanoparticles in water
- Optimization of acid catalyzed esterification and mixed metal oxide catalyzed transesterification for biodiesel production from Moringa oleifera oil
- Kinetics and the fluidity of the stearic acid esters with different carbon backbones
- Aiming for a standardized protocol for preparing a process green synthesis report and for ranking multiple synthesis plans to a common target product
- Microstructure and luminescence of VO2 (B) nanoparticle synthesis by hydrothermal method
- Optimization of uranium removal from uranium plant wastewater by response surface methodology (RSM)
- Microwave drying of nickel-containing residue: dielectric properties, kinetics, and energy aspects
- Simple and convenient two step synthesis of 5-bromo-2,3-dimethoxy-6-methyl-1,4-benzoquinone
- Biodiesel production from waste cooking oil
- The effect of activation temperature on structure and properties of blue coke-based activated carbon by CO2 activation
- Optimization of reaction parameters for the green synthesis of zero valent iron nanoparticles using pine tree needles
- Microwave-assisted protocol for squalene isolation and conversion from oil-deodoriser distillates
- Denitrification performance of rare earth tailings-based catalysts
- Facile synthesis of silver nanoparticles using Averrhoa bilimbi L and Plum extracts and investigation on the synergistic bioactivity using in vitro models
- Green production of AgNPs and their phytostimulatory impact
- Photocatalytic activity of Ag/Ni bi-metallic nanoparticles on textile dye removal
- Topical Issue: Green Process Engineering / Guest Editors: Martine Poux, Patrick Cognet
- Modelling and optimisation of oxidative desulphurisation of tyre-derived oil via central composite design approach
- CO2 sequestration by carbonation of olivine: a new process for optimal separation of the solids produced
- Organic carbonates synthesis improved by pervaporation for CO2 utilisation
- Production of starch nanoparticles through solvent-antisolvent precipitation in a spinning disc reactor
- A kinetic study of Zn halide/TBAB-catalysed fixation of CO2 with styrene oxide in propylene carbonate
- Topical on Green Process Engineering

