Abstract
A facile novel green methodology is presented for the synthesis of highly stable and well-dispersed copper oxide nanoparticles using aqueous wheat seed extract. Under optimal reaction conditions, the wheat seed extract-derived electron-rich biomolecules were functioned as a reducing and capping/ stabilizing agent. The ultraviolet-visible absorption peak at 300 nm was confirmed the formation of copper oxide nanoparticles. Fourier-transform infrared spectroscopy analysis determined Cu–O bonds in nanosample, indicating the active role of functional groups in the wheat seed extract in bio-reduction of Cu cations. X-ray diffraction pattern results demonstrated the monoclinic structure of highly pure biosynthesized copper oxide nanoparticles with a crystallite size of 20.76 nm. The stability of copper oxide nanoparticles was confirmed after 3 months’ storage of product with no sedimentation or suspension. Transmission electron microscopy results showed the spherical shape of nano-particle with an average size of 22 ± 1.5 nm. X-ray photo-electron spectroscopy analyses revealed only copper and oxygen elements in the sample, confirming the purity of copper oxide nanoparticles. Bio-assisted copper oxide nanoparticles demonstrated significant catalytic efficiency and reusability toward 4-nitrophenol removal by an average of 97.6% from aqueous solutions after successive 5 days’ exposure to UV irradiation.
1 Introduction
Nanoparticles owing to high surface area and a tiny particle size show inimitable material characteristics in comparison with bulk materials, which make them high in demand and widely applied area for miscellaneous applications in recent years. Presently, a considerable number of physical and chemical procedures were presented to fabricate the various types of nanoscale particles with different shape and size. Despite their popularity, they are high cost and often contain toxic materials (e.g. solvent, the reducing agent, acid and base reagents) which potentially expose the environment to serious health hazards; so that restricts their advantages. Whereas biological procedures are non-toxic, high atom economy, straightforward methodology and most importantly they are environment-friendly [1]. Hence, recent researches have been attempted to employ nature-based sources such as plants extracts [2,3], fungi [4], bacteria [5], and marine organisms [6], as sustainable alternative methods toward green nanoparticles fabrication [7]. Owing to presence highly active bio-organic molecules in plants, eco-friendly plant-based methodologies such as roots, flowers, seeds, leaves, bark, and fruits, have emerged as extremely promising biological factories for the formation of the diverse range of inorganic
nanoparticles [8]. Apparently, their active biomolecules most likely comprise amino, hydroxyl, and carboxyl biofunctional groups which would function a dual role as a reductant of metal salts and as protective agents to form a stabilizing layer on the biosynthesized nanoparticles [9].
Copper oxide nanoparticles (CuO NPs) and their derivatives as a type of metal oxide nanoparticles due to fair stability, cost-effectiveness and readily available compared to other expensive noble metals like Au, Pt, and Ag are broadly used in many applications such as a colorant in many ceramics applications, including the preparation of slips and glazes [10], batteries, catalyst for chemical reactions [11], solar cell [12], chemical sensor [13,14], absorbent [15], thermal conductivity enhancer [16], antifouling properties [17], wastewater treatment [18,19], thermal conductivity and anti-oxidation properties [20], bio-control agent [21], drug delivery [22], anticancer activity [23], and efficient anti-microbacterial agent [24,25].
There are a wide variety of traditional chemical and physical methods for CuO NPs fabrication including electrochemical methods [26], sonochemical [27], alcohothermal [28], hydrothermal decomposition [29], precipitation [30], polyol [31], and gamma radiation [32]. To avoid serious environmental hazards and health effects of chemical synthesis methods, several reports on phyto-assisted synthesis of CuO NPs by different parts of plant extracts have been published including abutilon indicum leaf [33], Saraca indica leaves [34], Rheum palmatum L. root [35], Aglaia elaeagnoidea flower [36], Malus domestica leaf [37], and green pea [38].
Common wheat (Triticum aestivum), bread wheat, is an annual cereal grain cultivated in greater quantities for its seed. It considers a global staple food with the highest monetary yield [39]. Wheat is the major source of carbohydrate biomolecules such as starch and simple electron-rich reducing sugars (e.g. glucose, and fructose) [40]. Therefore, the goal of this study is to present an alternative new abundant natural source based on 12 principles of green chemistry for the rapid one-step fabrication of highly pure and safer CuO NPs by aqueous extract of wheat seed. Size, purity, shape and surface chemistry of as-prepared CuO NPs were scrutinized through Visible and Ultraviolet spectroscopy (UV-Vis), Fourier-transform infrared spectroscopy (FTIR), X-ray photoelectron spectroscopy (XPS), X-ray powder diffraction (XRD), scanning electron microscope (SEM) and transmission electron microscope (TEM).
2 Experimental
2.1 Materials
Copper (II) sulfate pentahydrate (CuSO4‧5H2O) was acquired from Sigma-Aldrich supplier (USA) and utilized without further purification. Deionized (DI) water was used in all experiment steps for making molar solutions, washing, and dilution.
2.2 Preparation of the aqueous wheat seed extract
The aqueous extract of wheat seeds was acquired utilizing a facile soaking extraction method [41]. Generally, healthy wheat grains Triticum aestivum (T. aestivum) were collected at their harvest time from the local wheat field in Ahwaz (31°19’8.44”N, 48°41’3.12”E), Iran. To obtain the unblemished powder, the surfaces of T. aestivum seeds were thoroughly rinsed several times with distilled water to eliminate possible extraneous impurities, for instance, plant detritus, waste or debris of any kind. To reduce seeds moisture content, harvested wheat grain specimens were dehydrated at 60°C for 2 days and grounded to the fine powder using mini flour mill. Afterward, 5 g of sterilized seed powder was soaked in 300 mL of sterile distilled water to prepare an appropriate liquid extract. At a moderate temperature of 80°C, the solution was gently heated for 10 min. Finally, the product was centrifuged at 5000 rpm, filtered, and the obtained supernatant liquid was stored in the fridge at 5°C for more experimental tests.
2.3 Green synthesis of CuO NPs
In the characteristic recipe of wheat seed-mediated biological synthesis of CuO NPs, in an Erlenmeyer flask, 10 mL of liquid T. aestivum extract was added into 90 mL of 0.01 M aqueous solution of copper (II) sulfate. The resultant was continually stirred for 1 h at room temperature of 25°C to obtain a homogenous mixture in composition. Then, the solution was sonicated for 10 min and gently heated to 70°C for 20 min. After 10 min of the last heating process, the steady variation in color from dark blue to dark brown into solution was witnessed as an initial visual inspection of bioreduction copper ions to CuO NPs. The resultant was centrifuged at 12000 rpm for 10 min, dried in the furnace at 95°C for 120 min and the precipitate of CuO nanopowder was collected (Scheme 1). In so doing, the reaction was optimized by changing wheat seed extract concentrations, pH, salt precursor concentration, and contact time (see Supplementary material).
2.4 The characterization of CuO NPs
Crystal structure of the bioprepared CuO NPs X-ray was probed using XRD pattern (MPD from analytical). TEM (Zeiss-EM10C-100KV-Germany) and SEM (Zeiss-Sigma-Germany) techniques were used to examine the morphology and particle size of the sample. The surface chemistry of the CuO NPs was investigated using FTIR (VEATOR22-Burker Germany) by a KBr pellet. The formation of CuO NPs was recorded as a function of reaction process time via UV-Vis (Analytic Jena-Germany) in the range of 200-700 nm.
2.5 Catalytic elimination of 4-nitrophenol
To examine the catalytic activity of the biofabricated CuO NPs, in a 100 mL beaker, a 25 mL of 2 mM 4-nitrophenol (4-NP) as a modal pollutant was mixed with 0.2 g NaBH4 under gently stirring for 5 min. Then, 10 mg of CuO NPs were added as catalysts to the reaction mixture under constant stirring at room temperature of 25°C until the yellow color of solution turns to colorless. Next, 2.0 mL sample of the solution was taken out and diluted to 20 mL for further UV-Vis absorption analysis at certain intervals in the recorded scanning range of 200-700 nm. The elimination process of 4-NP was probed via monitoring the alterations in intensity of the major peak at 315 nm as a function of time.
3 Result and discussion
3.1 UV-visible spectrum of CuO NPs
UV-visible spectra of CuO NPs formation via the supernatant of the T. aestivum extract and crude T. aestivum extract were shown in Figure 1. CuO NPs has illustrated a relatively broad absorption peak at 305 nm most likely attributed to surface plasmon resonance (SPR) of CuO semiconductor excitation [42]. Using Tauc relation [43], the optical band gap energy (Eg, eV) of green CuO NPs achieved 4.13 eV (Figure 1c), which was higher value than the corresponding bulk CuO (~2.1 eV) [44]. The increasing Eg is attributed to decreasing particle size, thereby, CuO NPs might be
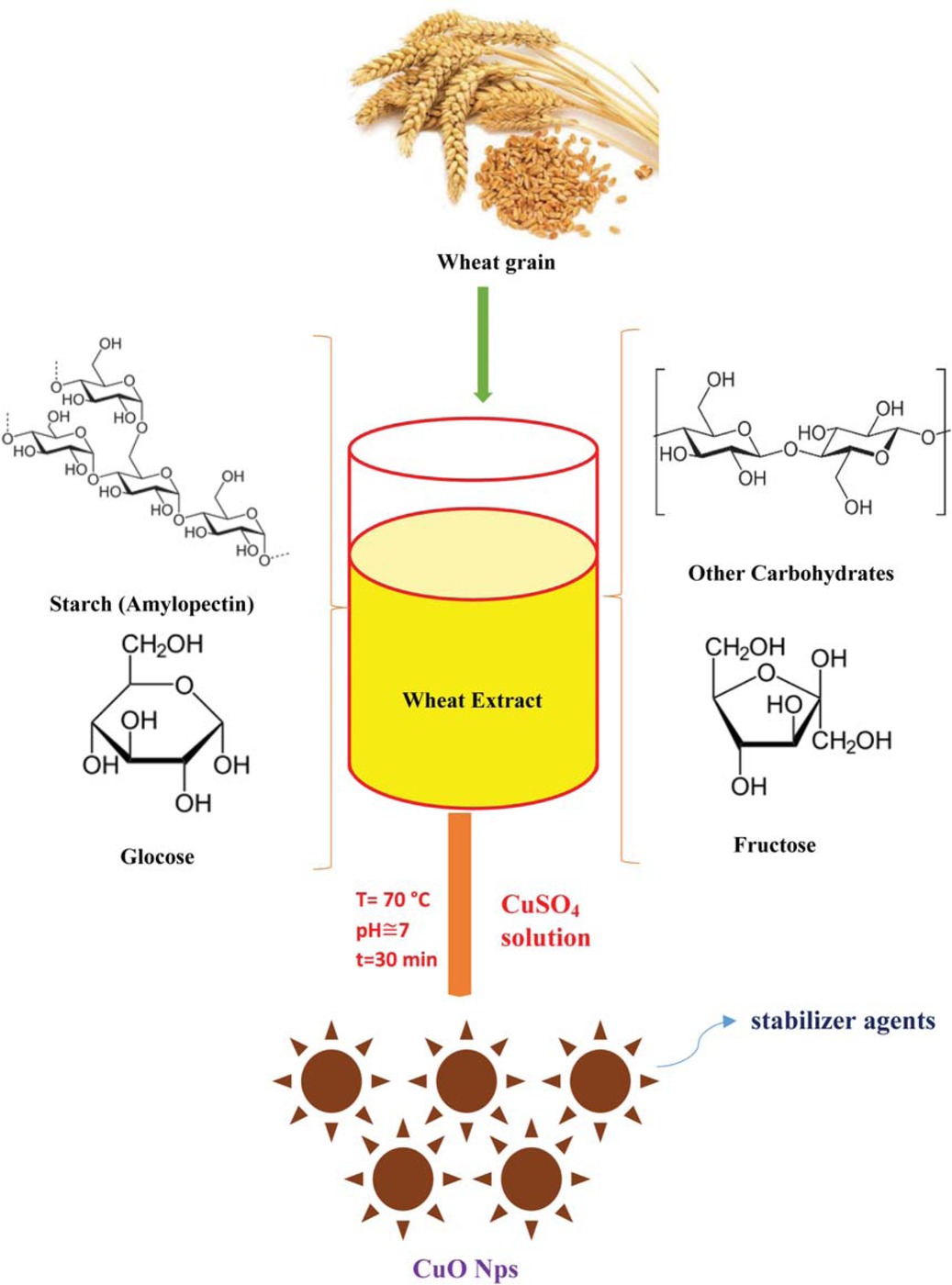
One-pot green synthesis of CuO nanoparticles using aqueous wheat extract.

UV–vis spectra of (a) aqueous wheat extract, (b) biofabricated CuO NPs, and (c) Tauc plot of CuO NPs.
used as an ideal harvester for solar irradiations and an effective photocatalyst for environmental remediation [45]. Moreover, UV-vis instrument displays no absorbance peak for pristine wheat seed extract as a blank sample (Figure 1). These observations obviously induce that active organic biomolecules in aqueous extract of wheat seeds were significantly engaged in the reduction of copper cations, Cu2+, to CuO NPs.
3.2 FTIR results
FTIR is a valuable technique to determine active biofunctional groups of wheat seed extract engaged in the copper ions bioreduction and their possible changes after CuO NPs synthesis. CuO NPs and aqueous wheat seed extract reveal a number of bands in different areas of FTIR spectrum, hence demonstrating the tangible dual role of abundant hydroxyls functional groups for synchronous bioreduction of Cu cations and stabilization of as-formed CuO NPs (Figure 2) [46]. In aqueous wheat seed extract (Figure 2a), The 3420, 1640, and 811 cm-1 bands were ascribed to O–H (hydroxyl), C=O (carbonyl), and out of plane C–H stretching, respectively. While FTIR spectrum of CuO NPs shows reasonable changes in intensity and position of above peaks along with the presence of new bands in the sample. Bands of 3328, 1552, 1433 and 1032 cm-1, were attributed to O–H (polyol), N–H (amino acid), C–O–(glycosidic linkage), and aromatic or aliphatic C–O stretching, respectively (Figure 4b). In the FTIR spectrum, the characteristic frequencies of CuO bonds of CuO NPs were appeared in 483 (Cu–O symmetric stretching), 541 (asymmetric stretching), and 672 cm-1 (wagging) bands, respectively (Figure 2b) [47]. These results evidently indicate an electrostatic interaction between electron-rich amino, ketonic C=O and alcoholic OH groups present in wheat seed extract and Cu ions which in turn leads to biosynthesize of CuO NPs [2].
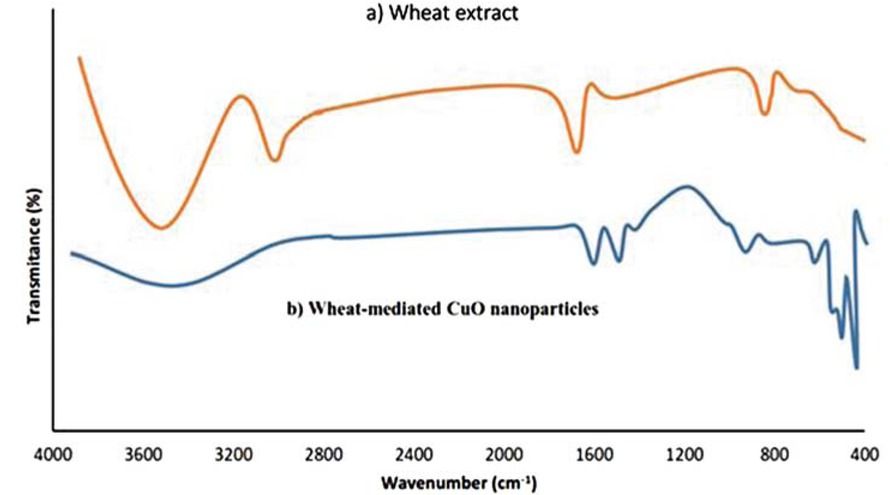
(a) FTIR spectra of wheat extract and (b) wheat-mediated CuO NPs.
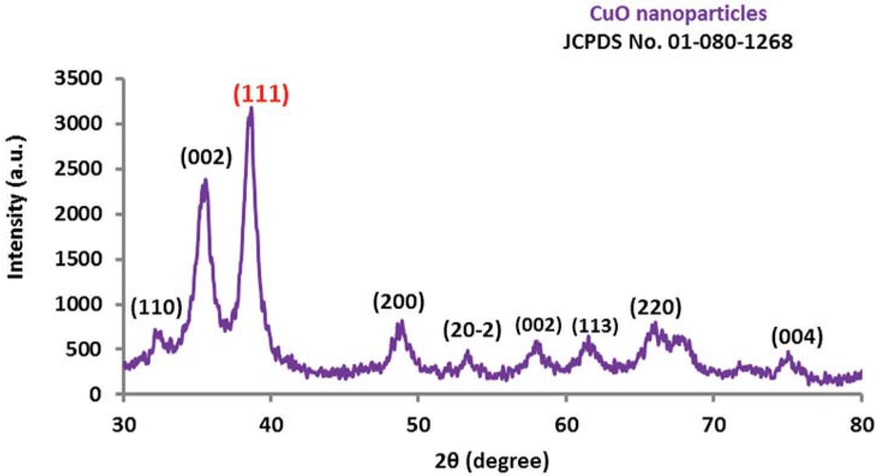
XRD patterns of CuO NPs synthesized by wheat extract.
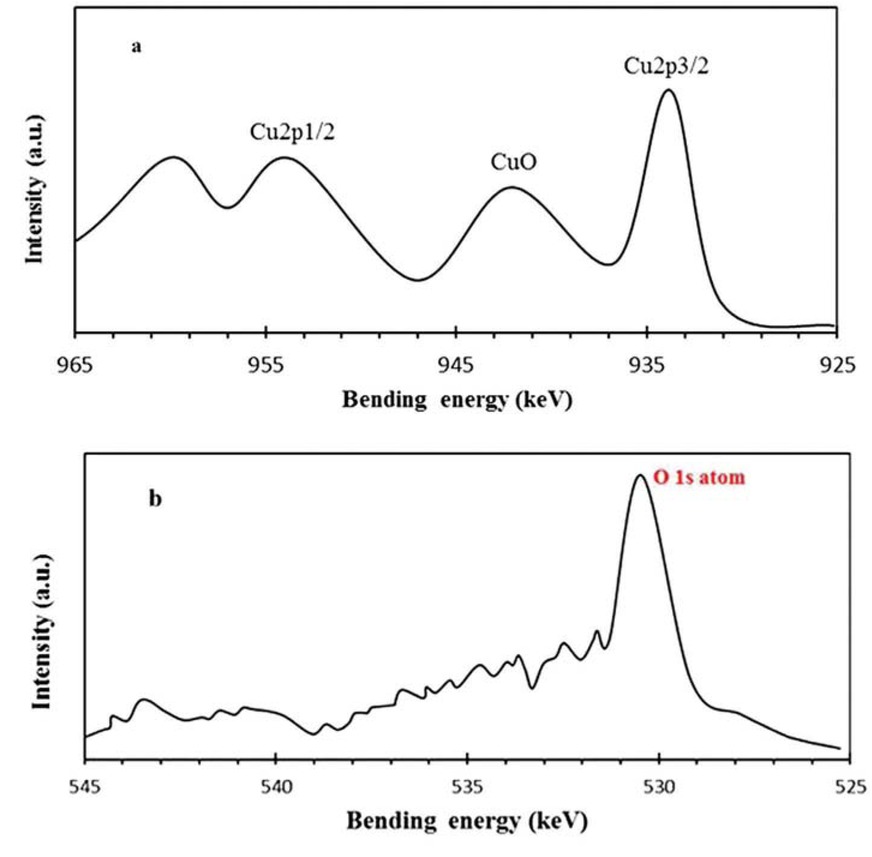
XPS spectra of wheat-mediated CuO NPs, (a) Cu 2P and (b) O 1s.
3.3 XRD examination of CuO NPs
The XRD pattern of biogenic CuO NPs is depicted in Figure 3. The presence of the quite clear and fairly sharp peaks with different 2θ values clearly demonstrated the highly crystalline structure of bio-assisted CuO nanopowder [34]. The analysis of assigned crystal planes (Bragg reflection) of rather strong peaks can be indexed to the monoclinic phase of CuO (JCPDS01-080-1268) [48]. Similarly, X-ray diffraction of biosynthesized CuO NPs via an aqueous black bean [23], and Aglaia elaeagnoidea flowers extracts revealed that the particles are monoclinic in nature [36]. XRD pattern exhibited no extra peaks, indicating the green CuO NPs were highly purified. Using the Debye-Scherrer formula, the crystal size of CuO NPs of the highest peak at 2 theta = 38.66° (111) was found to be 20.76 nm which fairly smaller than CuO NPs (90.0, 52.0, and 59.8 nm) synthesized by wet chemical precipitation [49], starch extract [50], and flower extract methods, respectively [36].
3.4 XPS analysis of CuO NPs
The purity of the bio-processed CuO nanoproduct was further scrutinized by means of XPS technique as shown in Figure 4. The XPS binding energy at 933.7 and 950.2 eV would assign respectively to Cu 2p3/2 and Cu 2p1/2 (Figure 4a). The sharp peak at 530.6 eV is anticipated to O atom bond to copper (II), producing CuO NPs (Figure 4b). These findings are consistent with literature reports [51], confirming the purity of bioproduced CuO NPs. Likewise, Rovani et al. have reported the green synthesis of highly pure silica nanoparticles with high adsorption capacity using sugarcane waste ash [52]. Moreover, according to the XPS results, no Cu2O by product was detected in nanoscale CuO specimen (Figure 4).
3.5 SEM and TEM characterizations of CuO NPs
The typical SEM and TEM images of phytosynthesized CuO NPs was depicted in Figure 5. The SEM results revealed the regular spherical shape with the range size approximately from 21 to 42 nm, confirming the formation of CuO NPs by aqueous wheat seed extract (Figure 5a). TEM micrograph clearly indicates the biofabrication of CuO NPs with relatively monodispersed spherical shaped particles and diameter of 5-40 nm measured by 300 particles (Figure 5b). The calculated average particle diameter of CuO NPs was 22 ± 1.5 nm, supporting XRD results (Figure 3). However, due to sample preparation, slight aggregation of particles has arbitrarily arisen in discrete spots.
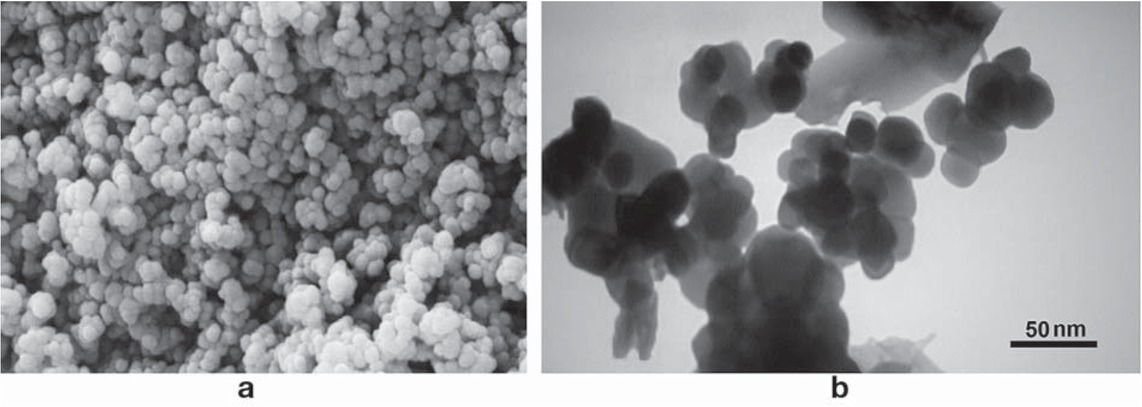
(a) SEM image at high magnification (150x) and (b) TEM micrograph of bio-assisted CuO NPs.
3.6 The mechanism for the formation of CuO nanoparticle
Scheme 1 is depicted as the tentative mechanism for the preparation of CuO NPs. Generally, three stages including activation, growth and termination phase could involve in wheat seed-assisted CuO NPs biosynthesis process (Scheme 2) [53]. In the bioreduction of copper procurer phytochemical compounds presence in wheat seed extract probably function dual role as reducing and capping agents.
In an initial activation step, Cu (II) cations would extract from CuSO4 salt precursor dissolved in DI water. During mixing progression process, the copper ions react with wheat seed-derived bioorganic compounds such as soluble saccharides via an oxidation-reduction mechanism (Scheme 3). For instance, the abundant electron-rich natural biomolecules containing hydroxyl groups with considerable reduction capabilities would reduce copper cations from divalent oxidation state to metallic form which immediately convert to CuO NPs as result of the superior chemical reactivity of bare nanoscale copper metal surface. Evidently, the broad peak of OH of wheat seed extract significantly becomes narrow in FTIR spectrum of CuO NPs indicating an efficient involvement of the alcoholic group in the bioreduction process (Figure 2). In the growth phase, the segregated copper atoms gradually combined to produce CuO NPs. Finally, in the termination step, stabilization of CuO NPs is developed. Wheat seed-derived natural biomacromolecules such as starch with linear and branched structures would surround the nucleated nanoparticles, creating a protective shield and restricting CuO NPs from growth [54]. In addition, the steric forces as a result of those biological macromolecules maintain the capped nanoparticles separated from each other, thus prevents them from agglomeration. This finding is in accordance with preceding reports relating to ZnO and alumina NPs biosynthesis using potato and algal extracts [55,56]. Likewise, Poinern et al. indicated that various bioactive compounds present in food waste are key responsible materials for bioreduction metal ions and stabilization of metallic NPs [57].
3.7 Catalytic activity and reusability of CuO NPs
The catalytic performance of the biofabricated CuO NPs was examined through measuring the degradation of the organic para-nitrophenol pollutant which is documented as a carcinogenic agent to the human being. It is utilized as a precursor for the manufacture of medical products, indicators, and a wide variety of phenetidine and acetophenetidine [58]. Upon the addition of CuO NPs, the intensity of UV-vis absorption peak of 4-nitrophenol at 315 nm was completely disappeared within 20 min, thus indicating the rapid adsorptive removal of an organic 4-nitrophenol pollutant from its aqueous solution (Figure 6). Meanwhile, recyclability of CuO NPs performance toward 4-NP degradation was examined in a UV chamber for five days in a raw. It is found that catalytic activity of the CuO NPs was remarkable (slight changes in removal%), indicating robustness and reusability of biogenic CuO NPs for 4-NP elimination (Figure 7).

Illustration of the possible formation mechanism strategy toward biological synthesis of CuO NPs using aqueous extract of wheat seeds.
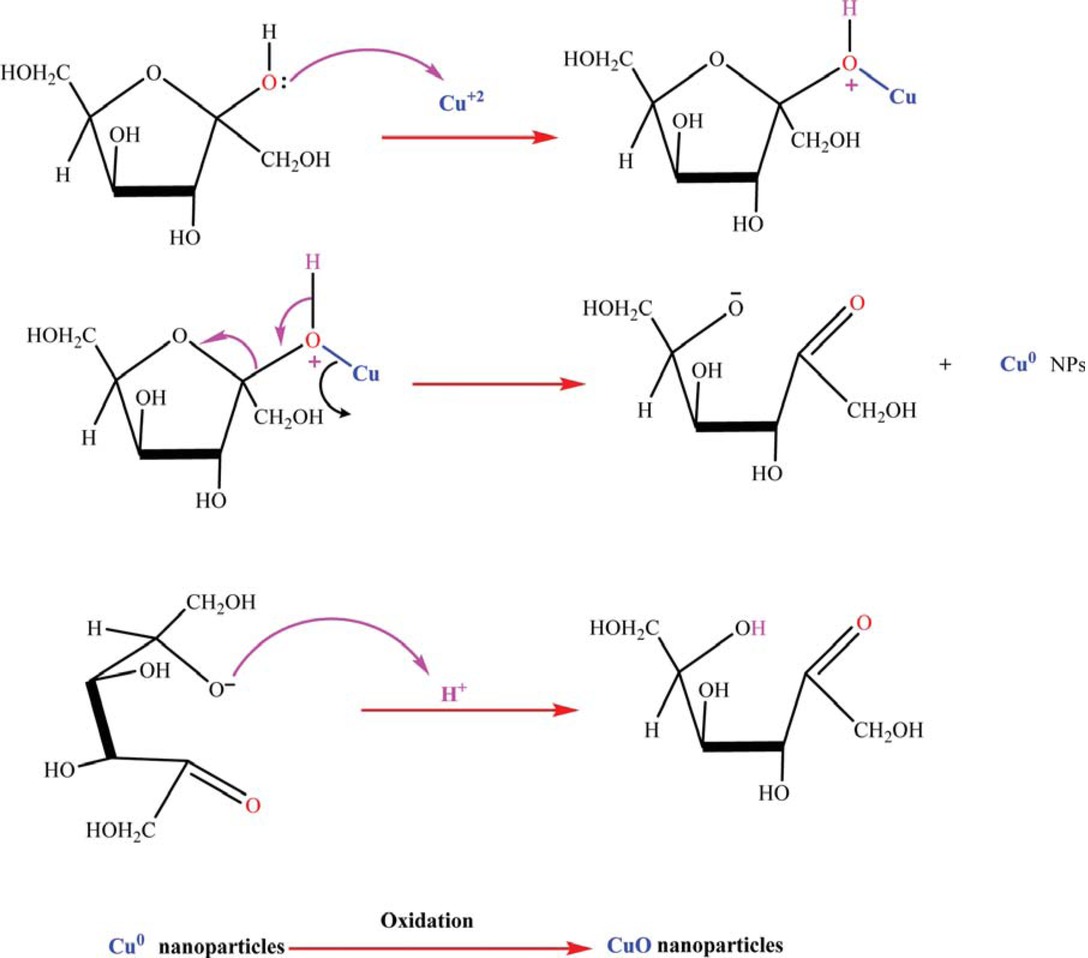
Schematic illustration of synthetic steps for bioreduction of copper cations using aqueous wheat extract (wheat-derived fructose as bioreducing agent model).
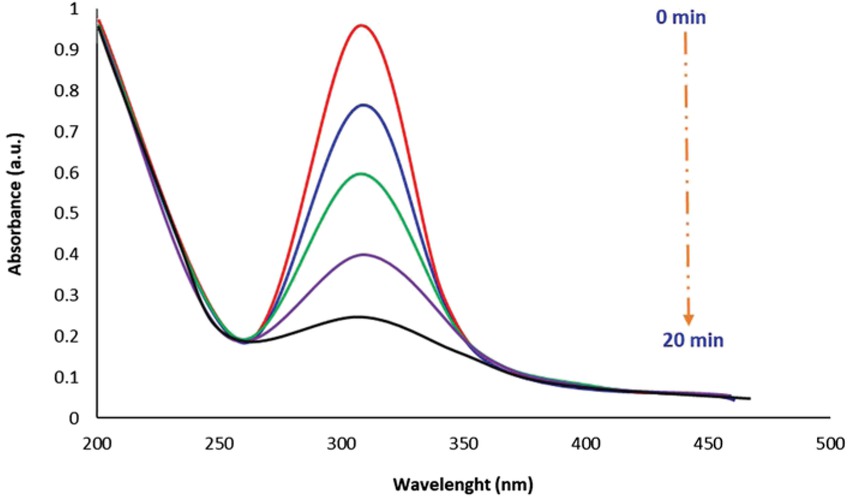
UV-Vis absorption spectral changes during absorption of 4-nitrophenol solution at different times using green CuO NPs.
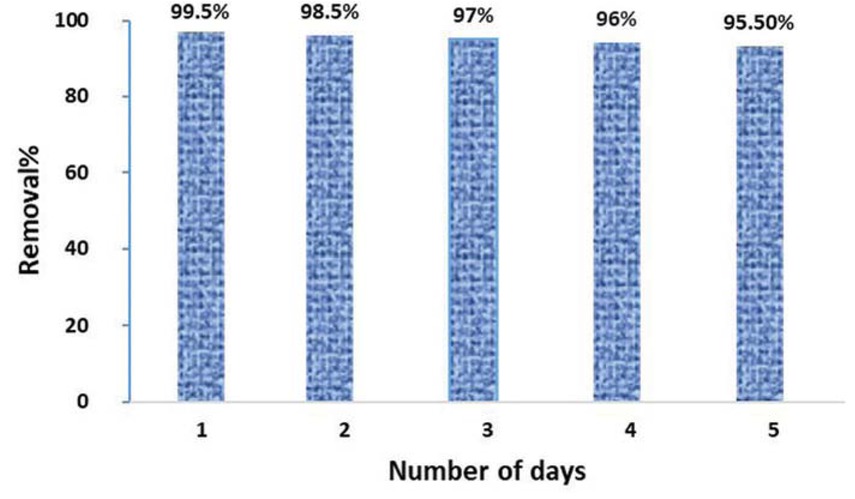
Reusability test of wheat-mediated CuO NPs catalytic efficiency toward 4-nitrophenol pollutant elimination.
3.8 Optimization of CuO NPs synthesis
Evidently, synthesis optimization of reaction is of paramount importance in obtaining desirable shape and size of bioproduced nanoparticles. Accordingly, the optimization process of crucial criteria including pH, salt concentration, contact time and extract concentration was investigated using UV-vis spectrophotometer. In so doing, different pH of 3, 5, 7 and 9 was adjusted by 0.1 N of HCl and NaOH. The absorbance of the resulting solution exhibit that the rate of CuO NPs formation accelerates with increasing pH up to neutral pH = 7 and then gradually reduce (Figure S1). The ionization of functional groups highly likely beyond the reduction of nanoparticle production. The concentration of precursor copper salt was optimized by varying copper sulfate solution (10, 30, 50 and 80 mM) in a constant volume of wheat seed extract (10 mL). Figure S2 has clearly demonstrated that at 50 mM induces the highest amount of CuO NPs on account of readily availability of Cu+2 cations. In addition, the concentration of the wheat extract was optimized with the increase in the concentration of the wheat extract solution (10, 15, 20, 25 and 30 mL). The highest absorption is observed at the slightest amount of aqueous extract of wheat seed (10 mL), indicating an efficient activity of bioactive molecules present in extract solution (Figure S3). Time variation of green chemical reaction led to the best quality of shape and size of biofabricated CuO NPs which optimized at a contact time of 25 min (Figure S4).
4 Conclusion
A simple atom-economy green route for biosynthesize highly stable copper nanoparticles through aqueous wheat seed extract was successfully developed. In this assay, the aqueous wheat seed extract of Triticum aestivum showed dual function as reducing and capping/ stabilizing agent for the biofabrication of fresh CuO nanoparticles. Copper oxide nanoparticles were obtained under optimal reaction conditions of neutral pH and short time of 25 min at a temperature of 70°C. All standard techniques including UV-vis, FTIR, XRD, SEM, and TEM confirmed the formation of well dispersed CuO nanoparticle. The XRD pattern showed CuO NPs has a monoclinic phase with a crystal size of 20.76 nm. The TEM results indicated the formation of rather spherical NPs with an average diameter of 22 ± 1.5 nm. Visual observations of CuO nanoproduct after three months’ storage at room temperature show no dispersion, color change or sedimentation, indicating high steric/ electrostatic stabilization of wheat seed extract biomolecules. Optimum conditions for the biosynthesis of CuO NPs are pH = 7, the concentration of CuSO4, 50 mM; wheat extract concentration, 10 mL; and contact time of 25 min. It is found that biogenic CuO NPs exhibited great catalytic efficiency in removal of a toxic 4-nitrophenol pollutant from the contaminated water. Biocompatibility, facile preparation, mild reaction condition, and cost-effectiveness of this approach would open a new window to the biogenic synthesis of a wide range of other inorganic nanoparticles. However, large-scale production of nanoparticles would require more research improving the industrial know-how of plant-based synthesis method.
Acknowledgement
This study has been funded by Ghadir Khuzestan Water Company, Ahwaz, Iran (research contract No. 96/001/118).
96/001/118).
Conflict of interest
Conflict of interest statement: The author declares no conflicts of interest regarding this article.
Abbreviations
- CuO
Copper Oxide
- FTIR
Fourier-transform infrared spectroscopy
- NPs
Nanoparticles
- 4-NP
4-Nitrophenol
- SEM
Scanning Electron Microscope
- TEM
Transmission Electron Microscope
- T.
Triticum
- XPS
X-Ray Photoelectron Spectroscopy
- XRD
X-Ray Powder Diffraction
- UV-Vis
Visible and Ultraviolet Spectroscopy
References
[1] Duan H., Wang D., Li Y., Green chemistry for nanoparticle synthesis. Chem. Soc. Rev., 2015, 44(16), 5778-5792.10.1039/C4CS00363BSearch in Google Scholar
[2] Iravani S., Green synthesis of metal nanoparticles using plants. Green. Chem., 2011, 13(10), 2638-2650.10.1039/c1gc15386bSearch in Google Scholar
[3] Khatami M., Alijani H.Q., Nejad M.S., Varma R.S., Core@shell Nanoparticles: Greener Synthesis Using Natural Plant Products. Appl. Sci., 2018, 8(3), 411.10.3390/app8030411Search in Google Scholar
[4] Uddandarao P., Balakrishnan R.M., Thermal and optical characterization of biologically synthesized ZnS nanoparticles synthesized from an endophytic fungus Aspergillus flavus A colorimetric probe in metal detection. Spectrochim. Acta A, 2017, 175, 200-207.10.1016/j.saa.2016.12.021Search in Google Scholar PubMed
[5] Santos A., Troncoso C., Lamilla C., Llanquinao V., Pavez M., Barrientos L., Nanoparticles Synthesized by Antarctic Bacteria and their Possible Mechanisms of Synthesis. Int. J. Morphol., 2017, 35(1), 26-33.10.4067/S0717-95022017000100005Search in Google Scholar
[6] Asmathunisha N., Kathiresan K., A review on biosynthesis of nanoparticles by marine organisms. Colloid. Surface. B, 2013, 103, 283-287.10.1016/j.colsurfb.2012.10.030Search in Google Scholar PubMed
[7] Singh P., Kim Y.-J., Zhang D., Yang D.-C., Biological synthesis of nanoparticles from plants and microorganisms. Trends Biotechnol., 2016, 34(7), 588-599.10.1016/j.tibtech.2016.02.006Search in Google Scholar PubMed
[8] Gurunathan S., Han J., Park J.H., Kim J.-H., A green chemistry approach for synthesizing biocompatible gold nanoparticles. Nanoscale Res. Lett., 2014, 9(1), 248.10.1186/1556-276X-9-248Search in Google Scholar PubMed PubMed Central
[9] Akhtar M.S., Panwar J., Yun Y.-S., Biogenic synthesis of metallic nanoparticles by plant extracts. ACS Sustain. Chem. Eng., 2013, 1(6), 591-602.10.1021/sc300118uSearch in Google Scholar
[10] Zhu C., Wang Y., Lu Q., Zhao H., Zhu X., Fa W., et al., Reproduction of Jun‐red glazes with nano‐sized copper oxide. J. Am. Ceram. Soc., DOI:10.1111/jace.14989.10.1111/jace.14989Search in Google Scholar
[11] Gawande M.B., Goswami A., Felpin F.-X., Asefa T., Huang X., Silva R., et al., Cu and Cu-based nanoparticles: synthesis and applications in catalysis. Chem. Rev., 2016, 116(6), 3722-3811.10.1021/acs.chemrev.5b00482Search in Google Scholar PubMed
[12] Wijesundera R., Fabrication of the CuO/Cu2O heterojunction using an electrodeposition technique for solar cell applications. Semicond. Sci. Technol., 2010, 25(4), 45015.10.1088/0268-1242/25/4/045015Search in Google Scholar
[13] Zhang J., Liu J., Peng Q., Wang X., Li Y., Nearly monodisperse Cu2O and CuO nanospheres: preparation and applications for sensitive gas sensors. Chem. Mater., 2006, 18(4), 867-871.10.1021/cm052256fSearch in Google Scholar
[14] Gou X., Wang G., Yang J., Park J., Wexler D., Chemical synthesis, characterisation and gas sensing performance of copper oxide nanoribbons. J. Mater. Chem., 2008, 18(9), 965-969.10.1039/b716745hSearch in Google Scholar
[15] Dubey S., Sharma Y.C., Calotropis procera mediated one pot green synthesis of Cupric oxide nanoparticles (CuO‐NPs) for adsorptive removal of Cr(VI) from aqueous solutions. Appl. Organomet. Chem., DOI:10.1002/aoc.3849.10.1002/aoc.3849Search in Google Scholar
[16] Zhu H.T., Zhang C.Y., Tang Y.M., Wang J.X., Novel synthesis and thermal conductivity of CuO nanofluid, J. Phys. Chem. C, 2007, 111(4), 1646-1650.10.1021/jp065926tSearch in Google Scholar
[17] Cioffi N., Torsi L., Ditaranto N., Tantillo G., Ghibelli L., Sabbatini L., et al., Copper nanoparticle/polymer composites with antifungal and bacteriostatic properties. Chem. Mater., 2005, 17(21), 5255-5262.10.1021/cm0505244Search in Google Scholar
[18] Ben-Moshe T., Dror I., Berkowitz B., Oxidation of organic pollutants in aqueous solutions by nanosized copper oxide catalysts. Appl. Catal. B, 2009, 85(3), 207-211.10.1016/j.apcatb.2008.07.020Search in Google Scholar
[19] Lu F., Astruc D., Nanomaterials for removal of toxic elements from water. Coord. Chem. Rev., 2018, 356, 147-164.10.1016/j.ccr.2017.11.003Search in Google Scholar
[20] Fan D., Zhou Q., Lv X., Jing J., Ye Z., Shao S., et al., Synthesis, thermal conductivity and anti-oxidation properties of copper nanoparticles encapsulated within few-layer h-BN. Ceram. Int., 2018, 44(1), 1205-1208.10.1016/j.ceramint.2017.10.018Search in Google Scholar
[21] Kuppusamy P., Ilavenil S., Srigopalram S., Maniam G.P., Yusoff M.M., Govindan N., et al., Treating of palm oil mill effluent using Commelina nudiflora mediated copper nanoparticles as a novel bio-control agent. J. Clean. Prod., 2017, 141, 1023-1029.10.1016/j.jclepro.2016.09.176Search in Google Scholar
[22] Assadi Z., Emtiazi G., Zarrabi A., Novel synergistic activities of tetracycline copper oxide nanoparticles integrated into chitosan micro particles for delivery against multiple drug resistant strains: Generation of reactive oxygen species (ROS) and cell death. J. Drug. Deliv. Sci. Tec., 2018, 44, 65-70.10.1016/j.jddst.2017.11.017Search in Google Scholar
[23] Nagajyothi P., Muthuraman P., Sreekanth T., Kim D.H., Shim J., Green synthesis: In-vitro anticancer activity of copper oxide nanoparticles against human cervical carcinoma cells. Arab. J. Chem., 2017, 10(2), 215-225.10.1016/j.arabjc.2016.01.011Search in Google Scholar
[24] Hans M., Erbe A., Mathews S., Chen Y., Solioz M., Mücklich F., Role of copper oxides in contact killing of bacteria. Langmuir, 2013, 29(52), 16160-16166.10.1021/la404091zSearch in Google Scholar
[25] Bala N., Sarkar M., Maiti M., Nandy P., Basu R., Das S., Phenolic compound-mediated single-step fabrication of copper oxide nanoparticles for elucidating their influence on antibacterial and catalytic activity. New J. Chem., 2017, 41(11), 4458-4467.10.1039/C6NJ04008JSearch in Google Scholar
[26] Yang H., Ouyang J., Tang A., Xiao Y., Li X., Dong X., et al., Electrochemical synthesis and photocatalytic property of cuprous oxide nanoparticles. Mater. Res. Bull., 2006, 41(7), 1310-1318.10.1016/j.materresbull.2006.01.004Search in Google Scholar
[27] Safarifard V., Morsali A., Sonochemical syntheses of a nano-sized copper(II) supramolecule as a precursor for the synthesis of copper(II) oxide nanoparticles. Ultrason. Sonochem., 2012, 19(4), 823-829.10.1016/j.ultsonch.2011.12.013Search in Google Scholar
[28] Hong Z.-S., Cao Y., Deng J.-F., A convenient alcohothermal approach for low temperature synthesis of CuO nanoparticles. Mater. Lett., 2002, 52(1), 34-38.10.1016/S0167-577X(01)00361-5Search in Google Scholar
[29] Chen D., Shen G., Tang K., Qian Y., Large-scale synthesis of CuO shuttle-like crystals via a convenient hydrothermal decomposition route. J. Cryst. Growth, 2003, 254(1), 225-228.10.1016/S0022-0248(03)01170-9Search in Google Scholar
[30] Lanje A.S., Sharma S.J., Pode R.B., Ningthoujam R.S., Synthesis and optical characterization of copper oxide nanoparticles. Adv. Appl. Sci. Res., 2010, 1(2), 36-40Search in Google Scholar
[31] Zhao Y., Zhu J.J., Hong J.M., Bian N., Chen H.Y., Microwave‐ Induced Polyol‐Process Synthesis of Copper and Copper Oxide Nanocrystals with Controllable Morphology. Eur. J. Inorg. Chem., 2004, 2004(20), 4072-4080.10.1002/ejic.200400258Search in Google Scholar
[32] El-Batal AI., El-Sayyad G.S., El-Ghamery A., Gobara M., Response surface methodology optimization of melanin production by Streptomyces cyaneus and synthesis of copper oxide nanoparticles using gamma radiation. J. Clust. Sci., 2017, 28(3), 1083-1112.10.1007/s10876-016-1101-0Search in Google Scholar
[33] Ijaz F., Shahid S., Khan S.A., Ahmad W., Zaman S., Green synthesis of copper oxide nanoparticles using Abutilon indicum leaf extract: Antimicrobial, antioxidant and photocatalytic dye degradation activities. Trop. J. Pharm. Res., 2017, 16, 743-753.10.4314/tjpr.v16i4.2Search in Google Scholar
[34] Prasad K.S., Patra A., Shruthi G., Chandan S., Aqueous Extract of Saraca indica Leaves in the Synthesis of Copper Oxide Nanoparticles: Finding a Way towards Going Green. J. Nanotech., DOI:10.1155/2017/7502610.10.1155/2017/7502610Search in Google Scholar
[35] Bordbar M., Sharifi-Zarchi Z., Khodadadi B., Green synthesis of copper oxide nanoparticles/clinoptilolite using Rheum palmatum L. root extract: high catalytic activity for reduction of 4-nitro phenol, rhodamine B, and methylene blue. J. Solgel. Sci. Technol., 2017, 81(3), 724-733.10.1007/s10971-016-4239-1Search in Google Scholar
[36] Manjari G., Saran S., Arun T., Rao A.V.B., Devipriya S.P., Catalytic and recyclability properties of phytogenic copper oxide nanoparticles derived from Aglaia elaeagnoidea flower extract. J. Saudi Chem. Soc., 2017, 21(5), 610-618.10.1016/j.jscs.2017.02.004Search in Google Scholar
[37] Jadhav M.S., Kulkarni S., Raikar P., Barretto D.A., Vootla S.K., Raikar U., Green biosynthesis of CuO & Ag–CuO nanoparticles from Malus domestica leaf extract and evaluation of antibacterial, antioxidant and DNA cleavage activities. New J. Chem., 2018, 42(1), 204-213.10.1039/C7NJ02977BSearch in Google Scholar
[38] Ochoa L., Medina-Velo I.A., Barrios A.C., Bonilla-Bird N.J., Hernandez-Viezcas J.A., Peralta-Videa J.R., et al., Modulation of CuO nanoparticles toxicity to green pea Pisum sativum Fabaceae by the phytohormone indole-3-acetic acid. Sci. Total. Environ., 2017, 598, 513-524.10.1016/j.scitotenv.2017.04.063Search in Google Scholar PubMed
[39] Xu T., Bian N., Wen M., Xiao J., Yuan C., Cao A., et al., Characterization of a common wheat Triticum aestivum L.) high-tillering dwarf mutant. Theor. Appl. Genet., 2017, 130(3), 483-494.10.1007/s00122-016-2828-6Search in Google Scholar PubMed
[40] D’appolonia B., Rayas-Duarte P., Wheat carbohydrates: structure and functionality. Springer, 1994, 107-127.10.1007/978-1-4615-2672-8_8Search in Google Scholar
[41] Rój E., Dobrzyńska‐Inger A., Dębczak A., Kostrzewa D., Stępnik K., Marine Algae Extracts: Processes, Products, and Applications. John Wiley & Sons, 2015, 101-120.10.1002/9783527679577.ch6Search in Google Scholar
[42] Felix S., Chakkravarthy R.B.P., Grace A.N., IOP Conference Series: Materials Science and Engineering. IOP Publishing, 2015, 012115.10.1088/1757-899X/73/1/012115Search in Google Scholar
[43] Abelès F (Ed.), Tauc J., Optical properties of solids. North-Holland. Publ. Co., 1972, 277, 153.Search in Google Scholar
[44] Bhaumik A., Shearin A.M., Patel R., Ghosh K., Significant enhancement of optical absorption through nano-structuring of copper based oxide semiconductors: possible future materials for solar energy applications. Phys. Chem. Chem. Phys., 2014, 16(22), 11054-11066.10.1039/C4CP00827HSearch in Google Scholar
[45] Varughese G., Rini V., Suraj S., Usha K., Characterisation and optical studies of copper oxide nanostructures doped with lanthanum ions. Adv. Mater. Sci. Eng. 2014, 14(4), 49-60.10.2478/adms-2014-0021Search in Google Scholar
[46] Kim S.-K., Chojnacka K., Marine Algae Extracts: Processes, Products, and Applications. John Wiley & Sons, 2015.10.1002/9783527679577Search in Google Scholar
[47] Arun K., Batra A., Krishna A., Bhat K., Aggarwal M., Francis J., Surfactant free hydrothermal synthesis of copper oxide nanoparticles. Am. J. Mater. Sci., 2015, 5(3A), 36-38.Search in Google Scholar
[48] Wang H., Xu J.-Z., Zhu J.-J., Chen H.-Y., Preparation of CuO nanoparticles by microwave irradiation. J. Cryst. Growth, 2002, 244(1), 88-94.10.1016/S0022-0248(02)01571-3Search in Google Scholar
[49] Ethiraj A.S., Kang D.J., Synthesis and characterization of CuO nanowires by a simple wet chemical method. Nanoscale Res. Lett., 2012, 7(1), 70.10.1186/1556-276X-7-70Search in Google Scholar PubMed PubMed Central
[50] Alishah H., Pourseyedi S., Ebrahimipour S.Y., Mahani S.E., Rafiei N., Green synthesis of starch-mediated CuO nanoparticles: preparation, characterization, antimicrobial activities and in vitro MTT assay against MCF-7 cell line. Rend. Lincei-Sci. Fis., 2017, 28(1), 65-71.10.1007/s12210-016-0574-ySearch in Google Scholar
[51] Tamuly C., Saikia I., Hazarika M., Das M.R., Reduction of aromatic nitro compounds catalyzed by biogenic CuO nanoparticles. RSC Adv., 2014, 4(95), 53229-53236.10.1039/C4RA10397ASearch in Google Scholar
[52] Rovani S., Santos J.J., Corio P., Fungaro D.A., Highly Pure Silica Nanoparticles with High Adsorption Capacity Obtained from Sugarcane Waste Ash. ACS Omega, 2018, 3(3), 2618-2627.10.1021/acsomega.8b00092Search in Google Scholar PubMed PubMed Central
[53] Shamaila S., Sajjad A.K.L., Farooqi S.A., Jabeen N., Majeed S., Farooq I., Advancements in nanoparticle fabrication by hazard free eco-friendly green routes. Appl. Mater. Today, 2016, 5, 150-199.10.1016/j.apmt.2016.09.009Search in Google Scholar
[54] Raveendran P., Fu J., Wallen S.L., Completely “green” synthesis and stabilization of metal nanoparticles. J. Am. Chem. Soc., 2003, 125(46), 13940-13941.10.1021/ja029267jSearch in Google Scholar PubMed
[55] Buazar F., Bavi M., Kroushawi F., Halvani M., Khaledi-Nasab A., Hossieni S., Potato extract as reducing agent and stabiliser in a facile green one-step synthesis of ZnO nanoparticles. J. Exp. Nanosci., 2016, 11(3), 175-184.10.1080/17458080.2015.1039610Search in Google Scholar
[56] Koopi H., Buazar F., A novel one-pot biosynthesis of pure alpha aluminum oxide nanoparticles using the macroalgae Sargassum ilicifolium A green marine approach. Ceram. Int., 2018, 44(8), 8940-8945.10.1016/j.ceramint.2018.02.091Search in Google Scholar
[57] Ghosh P.R., Fawcett D., Sharma S.B., Poinern G.E., Production of high-value nanoparticles via biogenic processes using aquacultural and horticultural food waste. Materials, 2017, 10(8), 852.10.3390/ma10080852Search in Google Scholar PubMed PubMed Central
[58] Alarcon P., Bustos A., Cañas B., Andres M., Polo L., Determination of priority pollutant phenols by isocratic HPLC. Chromatographia, 1987, 24(1), 613-616.10.1007/BF02688553Search in Google Scholar
© 2019 Buazar et al., published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 Public License.
Articles in the same Issue
- Regular Articles
- Studies on the preparation and properties of biodegradable polyester from soybean oil
- Flow-mode biodiesel production from palm oil using a pressurized microwave reactor
- Reduction of free fatty acids in waste oil for biodiesel production by glycerolysis: investigation and optimization of process parameters
- Saccharin: a cheap and mild acidic agent for the synthesis of azo dyes via telescoped dediazotization
- Optimization of lipase-catalyzed synthesis of polyethylene glycol stearate in a solvent-free system
- Green synthesis of iron oxide nanoparticles using Platanus orientalis leaf extract for antifungal activity
- Ultrasound assisted chemical activation of peanut husk for copper removal
- Room temperature silanization of Fe3O4 for the preparation of phenyl functionalized magnetic adsorbent for dispersive solid phase extraction for the extraction of phthalates in water
- Evaluation of the saponin green extraction from Ziziphus spina-christi leaves using hydrothermal, microwave and Bain-Marie water bath heating methods
- Oxidation of dibenzothiophene using the heterogeneous catalyst of tungsten-based carbon nanotubes
- Calcined sodium silicate as an efficient and benign heterogeneous catalyst for the transesterification of natural lecithin to L-α-glycerophosphocholine
- Synergistic effect between CO2 and H2O2 on ethylbenzene oxidation catalyzed by carbon supported heteropolyanion catalysts
- Hydrocyanation of 2-arylmethyleneindan-1,3-diones using potassium hexacyanoferrate(II) as a nontoxic cyanating agent
- Green synthesis of hydratropic aldehyde from α-methylstyrene catalyzed by Al2O3-supported metal phthalocyanines
- Environmentally benign chemical recycling of polycarbonate wastes: comparison of micro- and nano-TiO2 solid support efficiencies
- Medicago polymorpha-mediated antibacterial silver nanoparticles in the reduction of methyl orange
- Production of value-added chemicals from esterification of waste glycerol over MCM-41 supported catalysts
- Green synthesis of zerovalent copper nanoparticles for efficient reduction of toxic azo dyes congo red and methyl orange
- Optimization of the biological synthesis of silver nanoparticles using Penicillium oxalicum GRS-1 and their antimicrobial effects against common food-borne pathogens
- Optimization of submerged fermentation conditions to overproduce bioethanol using two industrial and traditional Saccharomyces cerevisiae strains
- Extraction of In3+ and Fe3+ from sulfate solutions by using a 3D-printed “Y”-shaped microreactor
- Foliar-mediated Ag:ZnO nanophotocatalysts: green synthesis, characterization, pollutants degradation, and in vitro biocidal activity
- Green cyclic acetals production by glycerol etherification reaction with benzaldehyde using cationic acidic resin
- Biosynthesis, characterization and antimicrobial activities assessment of fabricated selenium nanoparticles using Pelargonium zonale leaf extract
- Synthesis of high surface area magnesia by using walnut shell as a template
- Controllable biosynthesis of silver nanoparticles using actinobacterial strains
- Green vegetation: a promising source of color dyes
- Mechano-chemical synthesis of ammonia and acetic acid from inorganic materials in water
- Green synthesis and structural characterization of novel N1-substituted 3,4-dihydropyrimidin-2(1H)-ones
- Biodiesel production from cotton oil using heterogeneous CaO catalysts from eggshells prepared at different calcination temperatures
- Regeneration of spent mercury catalyst for the treatment of dye wastewater by the microwave and ultrasonic spray-assisted method
- Green synthesis of the innovative super paramagnetic nanoparticles from the leaves extract of Fraxinus chinensis Roxb and their application for the decolourisation of toxic dyes
- Biogenic ZnO nanoparticles: a study of blueshift of optical band gap and photocatalytic degradation of reactive yellow 186 dye under direct sunlight
- Leached compounds from the extracts of pomegranate peel, green coconut shell, and karuvelam wood for the removal of hexavalent chromium
- Enhancement of molecular weight reduction of natural rubber in triphasic CO2/toluene/H2O systems with hydrogen peroxide for preparation of biobased polyurethanes
- An efficient green synthesis of novel 1H-imidazo[1,2-a]imidazole-3-amine and imidazo[2,1-c][1,2,4]triazole-5-amine derivatives via Strecker reaction under controlled microwave heating
- Evaluation of three different green fabrication methods for the synthesis of crystalline ZnO nanoparticles using Pelargonium zonale leaf extract
- A highly efficient and multifunctional biomass supporting Ag, Ni, and Cu nanoparticles through wetness impregnation for environmental remediation
- Simple one-pot green method for large-scale production of mesalamine, an anti-inflammatory agent
- Relationships between step and cumulative PMI and E-factors: implications on estimating material efficiency with respect to charting synthesis optimization strategies
- A comparative sorption study of Cr3+ and Cr6+ using mango peels: kinetic, equilibrium and thermodynamic
- Effects of acid hydrolysis waste liquid recycle on preparation of microcrystalline cellulose
- Use of deep eutectic solvents as catalyst: A mini-review
- Microwave-assisted synthesis of pyrrolidinone derivatives using 1,1’-butylenebis(3-sulfo-3H-imidazol-1-ium) chloride in ethylene glycol
- Green and eco-friendly synthesis of Co3O4 and Ag-Co3O4: Characterization and photo-catalytic activity
- Adsorption optimized of the coal-based material and application for cyanide wastewater treatment
- Aloe vera leaf extract mediated green synthesis of selenium nanoparticles and assessment of their In vitro antimicrobial activity against spoilage fungi and pathogenic bacteria strains
- Waste phenolic resin derived activated carbon by microwave-assisted KOH activation and application to dye wastewater treatment
- Direct ethanol production from cellulose by consortium of Trichoderma reesei and Candida molischiana
- Agricultural waste biomass-assisted nanostructures: Synthesis and application
- Biodiesel production from rubber seed oil using calcium oxide derived from eggshell as catalyst – optimization and modeling studies
- Study of fabrication of fully aqueous solution processed SnS quantum dot-sensitized solar cell
- Assessment of aqueous extract of Gypsophila aretioides for inhibitory effects on calcium carbonate formation
- An environmentally friendly acylation reaction of 2-methylnaphthalene in solvent-free condition in a micro-channel reactor
- Aegle marmelos phytochemical stabilized synthesis and characterization of ZnO nanoparticles and their role against agriculture and food pathogen
- A reactive coupling process for co-production of solketal and biodiesel
- Optimization of the asymmetric synthesis of (S)-1-phenylethanol using Ispir bean as whole-cell biocatalyst
- Synthesis of pyrazolopyridine and pyrazoloquinoline derivatives by one-pot, three-component reactions of arylglyoxals, 3-methyl-1-aryl-1H-pyrazol-5-amines and cyclic 1,3-dicarbonyl compounds in the presence of tetrapropylammonium bromide
- Preconcentration of morphine in urine sample using a green and solvent-free microextraction method
- Extraction of glycyrrhizic acid by aqueous two-phase system formed by PEG and two environmentally friendly organic acid salts - sodium citrate and sodium tartrate
- Green synthesis of copper oxide nanoparticles using Juglans regia leaf extract and assessment of their physico-chemical and biological properties
- Deep eutectic solvents (DESs) as powerful and recyclable catalysts and solvents for the synthesis of 3,4-dihydropyrimidin-2(1H)-ones/thiones
- Biosynthesis, characterization and anti-microbial activity of silver nanoparticle based gel hand wash
- Efficient and selective microwave-assisted O-methylation of phenolic compounds using tetramethylammonium hydroxide (TMAH)
- Anticoagulant, thrombolytic and antibacterial activities of Euphorbia acruensis latex-mediated bioengineered silver nanoparticles
- Volcanic ash as reusable catalyst in the green synthesis of 3H-1,5-benzodiazepines
- Green synthesis, anionic polymerization of 1,4-bis(methacryloyl)piperazine using Algerian clay as catalyst
- Selenium supplementation during fermentation with sugar beet molasses and Saccharomyces cerevisiae to increase bioethanol production
- Biosynthetic potential assessment of four food pathogenic bacteria in hydrothermally silver nanoparticles fabrication
- Investigating the effectiveness of classical and eco-friendly approaches for synthesis of dialdehydes from organic dihalides
- Pyrolysis of palm oil using zeolite catalyst and characterization of the boil-oil
- Azadirachta indica leaves extract assisted green synthesis of Ag-TiO2 for degradation of Methylene blue and Rhodamine B dyes in aqueous medium
- Synthesis of vitamin E succinate catalyzed by nano-SiO2 immobilized DMAP derivative in mixed solvent system
- Extraction of phytosterols from melon (Cucumis melo) seeds by supercritical CO2 as a clean technology
- Production of uronic acids by hydrothermolysis of pectin as a model substance for plant biomass waste
- Biofabrication of highly pure copper oxide nanoparticles using wheat seed extract and their catalytic activity: A mechanistic approach
- Intelligent modeling and optimization of emulsion aggregation method for producing green printing ink
- Improved removal of methylene blue on modified hierarchical zeolite Y: Achieved by a “destructive-constructive” method
- Two different facile and efficient approaches for the synthesis of various N-arylacetamides via N-acetylation of arylamines and straightforward one-pot reductive acetylation of nitroarenes promoted by recyclable CuFe2O4 nanoparticles in water
- Optimization of acid catalyzed esterification and mixed metal oxide catalyzed transesterification for biodiesel production from Moringa oleifera oil
- Kinetics and the fluidity of the stearic acid esters with different carbon backbones
- Aiming for a standardized protocol for preparing a process green synthesis report and for ranking multiple synthesis plans to a common target product
- Microstructure and luminescence of VO2 (B) nanoparticle synthesis by hydrothermal method
- Optimization of uranium removal from uranium plant wastewater by response surface methodology (RSM)
- Microwave drying of nickel-containing residue: dielectric properties, kinetics, and energy aspects
- Simple and convenient two step synthesis of 5-bromo-2,3-dimethoxy-6-methyl-1,4-benzoquinone
- Biodiesel production from waste cooking oil
- The effect of activation temperature on structure and properties of blue coke-based activated carbon by CO2 activation
- Optimization of reaction parameters for the green synthesis of zero valent iron nanoparticles using pine tree needles
- Microwave-assisted protocol for squalene isolation and conversion from oil-deodoriser distillates
- Denitrification performance of rare earth tailings-based catalysts
- Facile synthesis of silver nanoparticles using Averrhoa bilimbi L and Plum extracts and investigation on the synergistic bioactivity using in vitro models
- Green production of AgNPs and their phytostimulatory impact
- Photocatalytic activity of Ag/Ni bi-metallic nanoparticles on textile dye removal
- Topical Issue: Green Process Engineering / Guest Editors: Martine Poux, Patrick Cognet
- Modelling and optimisation of oxidative desulphurisation of tyre-derived oil via central composite design approach
- CO2 sequestration by carbonation of olivine: a new process for optimal separation of the solids produced
- Organic carbonates synthesis improved by pervaporation for CO2 utilisation
- Production of starch nanoparticles through solvent-antisolvent precipitation in a spinning disc reactor
- A kinetic study of Zn halide/TBAB-catalysed fixation of CO2 with styrene oxide in propylene carbonate
- Topical on Green Process Engineering
Articles in the same Issue
- Regular Articles
- Studies on the preparation and properties of biodegradable polyester from soybean oil
- Flow-mode biodiesel production from palm oil using a pressurized microwave reactor
- Reduction of free fatty acids in waste oil for biodiesel production by glycerolysis: investigation and optimization of process parameters
- Saccharin: a cheap and mild acidic agent for the synthesis of azo dyes via telescoped dediazotization
- Optimization of lipase-catalyzed synthesis of polyethylene glycol stearate in a solvent-free system
- Green synthesis of iron oxide nanoparticles using Platanus orientalis leaf extract for antifungal activity
- Ultrasound assisted chemical activation of peanut husk for copper removal
- Room temperature silanization of Fe3O4 for the preparation of phenyl functionalized magnetic adsorbent for dispersive solid phase extraction for the extraction of phthalates in water
- Evaluation of the saponin green extraction from Ziziphus spina-christi leaves using hydrothermal, microwave and Bain-Marie water bath heating methods
- Oxidation of dibenzothiophene using the heterogeneous catalyst of tungsten-based carbon nanotubes
- Calcined sodium silicate as an efficient and benign heterogeneous catalyst for the transesterification of natural lecithin to L-α-glycerophosphocholine
- Synergistic effect between CO2 and H2O2 on ethylbenzene oxidation catalyzed by carbon supported heteropolyanion catalysts
- Hydrocyanation of 2-arylmethyleneindan-1,3-diones using potassium hexacyanoferrate(II) as a nontoxic cyanating agent
- Green synthesis of hydratropic aldehyde from α-methylstyrene catalyzed by Al2O3-supported metal phthalocyanines
- Environmentally benign chemical recycling of polycarbonate wastes: comparison of micro- and nano-TiO2 solid support efficiencies
- Medicago polymorpha-mediated antibacterial silver nanoparticles in the reduction of methyl orange
- Production of value-added chemicals from esterification of waste glycerol over MCM-41 supported catalysts
- Green synthesis of zerovalent copper nanoparticles for efficient reduction of toxic azo dyes congo red and methyl orange
- Optimization of the biological synthesis of silver nanoparticles using Penicillium oxalicum GRS-1 and their antimicrobial effects against common food-borne pathogens
- Optimization of submerged fermentation conditions to overproduce bioethanol using two industrial and traditional Saccharomyces cerevisiae strains
- Extraction of In3+ and Fe3+ from sulfate solutions by using a 3D-printed “Y”-shaped microreactor
- Foliar-mediated Ag:ZnO nanophotocatalysts: green synthesis, characterization, pollutants degradation, and in vitro biocidal activity
- Green cyclic acetals production by glycerol etherification reaction with benzaldehyde using cationic acidic resin
- Biosynthesis, characterization and antimicrobial activities assessment of fabricated selenium nanoparticles using Pelargonium zonale leaf extract
- Synthesis of high surface area magnesia by using walnut shell as a template
- Controllable biosynthesis of silver nanoparticles using actinobacterial strains
- Green vegetation: a promising source of color dyes
- Mechano-chemical synthesis of ammonia and acetic acid from inorganic materials in water
- Green synthesis and structural characterization of novel N1-substituted 3,4-dihydropyrimidin-2(1H)-ones
- Biodiesel production from cotton oil using heterogeneous CaO catalysts from eggshells prepared at different calcination temperatures
- Regeneration of spent mercury catalyst for the treatment of dye wastewater by the microwave and ultrasonic spray-assisted method
- Green synthesis of the innovative super paramagnetic nanoparticles from the leaves extract of Fraxinus chinensis Roxb and their application for the decolourisation of toxic dyes
- Biogenic ZnO nanoparticles: a study of blueshift of optical band gap and photocatalytic degradation of reactive yellow 186 dye under direct sunlight
- Leached compounds from the extracts of pomegranate peel, green coconut shell, and karuvelam wood for the removal of hexavalent chromium
- Enhancement of molecular weight reduction of natural rubber in triphasic CO2/toluene/H2O systems with hydrogen peroxide for preparation of biobased polyurethanes
- An efficient green synthesis of novel 1H-imidazo[1,2-a]imidazole-3-amine and imidazo[2,1-c][1,2,4]triazole-5-amine derivatives via Strecker reaction under controlled microwave heating
- Evaluation of three different green fabrication methods for the synthesis of crystalline ZnO nanoparticles using Pelargonium zonale leaf extract
- A highly efficient and multifunctional biomass supporting Ag, Ni, and Cu nanoparticles through wetness impregnation for environmental remediation
- Simple one-pot green method for large-scale production of mesalamine, an anti-inflammatory agent
- Relationships between step and cumulative PMI and E-factors: implications on estimating material efficiency with respect to charting synthesis optimization strategies
- A comparative sorption study of Cr3+ and Cr6+ using mango peels: kinetic, equilibrium and thermodynamic
- Effects of acid hydrolysis waste liquid recycle on preparation of microcrystalline cellulose
- Use of deep eutectic solvents as catalyst: A mini-review
- Microwave-assisted synthesis of pyrrolidinone derivatives using 1,1’-butylenebis(3-sulfo-3H-imidazol-1-ium) chloride in ethylene glycol
- Green and eco-friendly synthesis of Co3O4 and Ag-Co3O4: Characterization and photo-catalytic activity
- Adsorption optimized of the coal-based material and application for cyanide wastewater treatment
- Aloe vera leaf extract mediated green synthesis of selenium nanoparticles and assessment of their In vitro antimicrobial activity against spoilage fungi and pathogenic bacteria strains
- Waste phenolic resin derived activated carbon by microwave-assisted KOH activation and application to dye wastewater treatment
- Direct ethanol production from cellulose by consortium of Trichoderma reesei and Candida molischiana
- Agricultural waste biomass-assisted nanostructures: Synthesis and application
- Biodiesel production from rubber seed oil using calcium oxide derived from eggshell as catalyst – optimization and modeling studies
- Study of fabrication of fully aqueous solution processed SnS quantum dot-sensitized solar cell
- Assessment of aqueous extract of Gypsophila aretioides for inhibitory effects on calcium carbonate formation
- An environmentally friendly acylation reaction of 2-methylnaphthalene in solvent-free condition in a micro-channel reactor
- Aegle marmelos phytochemical stabilized synthesis and characterization of ZnO nanoparticles and their role against agriculture and food pathogen
- A reactive coupling process for co-production of solketal and biodiesel
- Optimization of the asymmetric synthesis of (S)-1-phenylethanol using Ispir bean as whole-cell biocatalyst
- Synthesis of pyrazolopyridine and pyrazoloquinoline derivatives by one-pot, three-component reactions of arylglyoxals, 3-methyl-1-aryl-1H-pyrazol-5-amines and cyclic 1,3-dicarbonyl compounds in the presence of tetrapropylammonium bromide
- Preconcentration of morphine in urine sample using a green and solvent-free microextraction method
- Extraction of glycyrrhizic acid by aqueous two-phase system formed by PEG and two environmentally friendly organic acid salts - sodium citrate and sodium tartrate
- Green synthesis of copper oxide nanoparticles using Juglans regia leaf extract and assessment of their physico-chemical and biological properties
- Deep eutectic solvents (DESs) as powerful and recyclable catalysts and solvents for the synthesis of 3,4-dihydropyrimidin-2(1H)-ones/thiones
- Biosynthesis, characterization and anti-microbial activity of silver nanoparticle based gel hand wash
- Efficient and selective microwave-assisted O-methylation of phenolic compounds using tetramethylammonium hydroxide (TMAH)
- Anticoagulant, thrombolytic and antibacterial activities of Euphorbia acruensis latex-mediated bioengineered silver nanoparticles
- Volcanic ash as reusable catalyst in the green synthesis of 3H-1,5-benzodiazepines
- Green synthesis, anionic polymerization of 1,4-bis(methacryloyl)piperazine using Algerian clay as catalyst
- Selenium supplementation during fermentation with sugar beet molasses and Saccharomyces cerevisiae to increase bioethanol production
- Biosynthetic potential assessment of four food pathogenic bacteria in hydrothermally silver nanoparticles fabrication
- Investigating the effectiveness of classical and eco-friendly approaches for synthesis of dialdehydes from organic dihalides
- Pyrolysis of palm oil using zeolite catalyst and characterization of the boil-oil
- Azadirachta indica leaves extract assisted green synthesis of Ag-TiO2 for degradation of Methylene blue and Rhodamine B dyes in aqueous medium
- Synthesis of vitamin E succinate catalyzed by nano-SiO2 immobilized DMAP derivative in mixed solvent system
- Extraction of phytosterols from melon (Cucumis melo) seeds by supercritical CO2 as a clean technology
- Production of uronic acids by hydrothermolysis of pectin as a model substance for plant biomass waste
- Biofabrication of highly pure copper oxide nanoparticles using wheat seed extract and their catalytic activity: A mechanistic approach
- Intelligent modeling and optimization of emulsion aggregation method for producing green printing ink
- Improved removal of methylene blue on modified hierarchical zeolite Y: Achieved by a “destructive-constructive” method
- Two different facile and efficient approaches for the synthesis of various N-arylacetamides via N-acetylation of arylamines and straightforward one-pot reductive acetylation of nitroarenes promoted by recyclable CuFe2O4 nanoparticles in water
- Optimization of acid catalyzed esterification and mixed metal oxide catalyzed transesterification for biodiesel production from Moringa oleifera oil
- Kinetics and the fluidity of the stearic acid esters with different carbon backbones
- Aiming for a standardized protocol for preparing a process green synthesis report and for ranking multiple synthesis plans to a common target product
- Microstructure and luminescence of VO2 (B) nanoparticle synthesis by hydrothermal method
- Optimization of uranium removal from uranium plant wastewater by response surface methodology (RSM)
- Microwave drying of nickel-containing residue: dielectric properties, kinetics, and energy aspects
- Simple and convenient two step synthesis of 5-bromo-2,3-dimethoxy-6-methyl-1,4-benzoquinone
- Biodiesel production from waste cooking oil
- The effect of activation temperature on structure and properties of blue coke-based activated carbon by CO2 activation
- Optimization of reaction parameters for the green synthesis of zero valent iron nanoparticles using pine tree needles
- Microwave-assisted protocol for squalene isolation and conversion from oil-deodoriser distillates
- Denitrification performance of rare earth tailings-based catalysts
- Facile synthesis of silver nanoparticles using Averrhoa bilimbi L and Plum extracts and investigation on the synergistic bioactivity using in vitro models
- Green production of AgNPs and their phytostimulatory impact
- Photocatalytic activity of Ag/Ni bi-metallic nanoparticles on textile dye removal
- Topical Issue: Green Process Engineering / Guest Editors: Martine Poux, Patrick Cognet
- Modelling and optimisation of oxidative desulphurisation of tyre-derived oil via central composite design approach
- CO2 sequestration by carbonation of olivine: a new process for optimal separation of the solids produced
- Organic carbonates synthesis improved by pervaporation for CO2 utilisation
- Production of starch nanoparticles through solvent-antisolvent precipitation in a spinning disc reactor
- A kinetic study of Zn halide/TBAB-catalysed fixation of CO2 with styrene oxide in propylene carbonate
- Topical on Green Process Engineering

