Green cyclic acetals production by glycerol etherification reaction with benzaldehyde using cationic acidic resin
-
Kariyn Yamamoto
Abstract
In this paper, we investigated the effect of temperature, glycerol etherification concentration with benzaldehyde, organic solvent and catalyst reuse effects using a cationic acidic resin as catalyst for production of green cyclic acetals of high commercial value. The best reaction conditions show a conversion above 93% of glycerol and yield to cyclic acetals above 61%. The highest selectivity elements observed were 2-phenyl-1,3-dioxan-5-ol, in cis and trans isomer forms reaching 80%. The temperature had a positive effect increasing on glycerol conversion, though it also favored the formation of undesired compounds. A high concentration of benzaldehyde reactant kept the selectivity values constant but increased glycerol conversion resulting in higher yields, mainly when organic solvents were used. Reuse of the catalyst resulted in a slight decrease in yield values, which demonstrated stability and durability of the catalyst used.
1 Introduction
Considering all the biodiesel production chain, it is necessary to observe the glycerin formation, generated as a byproduct. The economic viability of glycerin has become an obstacle to its production. Thus, it was estimated that approximately 1 kg of crude glycerol is generated for every 10 kg of biodiesel produced [1], [2].
Glycerol (or glycerin) has great application in the cosmetics, personal hygiene, food, medicine and tobacco sectors. In terms of chemical transformation, it still presents limited applications, the main one being in explosives production, like nitroglycerin, and in the formation of alkyd resins [3].
In Brazil, the policy of using biodiesel as an alternative fuel to petrodiesel is conditioned by the blend rate between the two fuels, which is regulated by Ministry of Mines and Energy laws and supervised by its National Agency of Petroleum, Natural Gas and Biofuels. Actually, the blend value is 8% of biodiesel in petrodiesel (B8) and will increase to 10% in 2019. According to the Brazilian Ministry of Development, Industry and Foreign Trade, Brazil produced approximately 380,000 tons of glycerin and exported 244,000 tons of crude glycerin and 60,000 tons of refined glycerin (glycerol), indicating internal consumption of glycerol of 76,000 tons [4]. This large production of glycerol must be destined to develop new chemical products to take advantage of its potential and to add economic production values.
One of the main transformation patterns of glycerol is the etherification reaction with alcohols, ketones or aldehydes, to promote formation of chemical compounds that present better commercial and economic value such as renewable fuels additives, surfactants, flavorings and solvents for use in medicine as well as anti-bacterial agents [5], [6].
Condensation reactions of glycerol with other alcohols produce glycerol ethers that may increase the burning properties of gasoline. Therefore, it may be considered a green additive to fuels. Researches show that glycerol acetals also aid improving blends with biodiesel [7].
Natural benzaldehyde has gained a space in industrial production as one of the most produced fragrances in the world and is obtained from natural compounds like cinnamon oil [8], [9]. Glycerol acetalization with benzaldehyde is capable of producing green cyclic acetals such as 2-phenyl-1, 3-dioxan-5-ol or 2-phenyl-1,3-dioxolane-4-methanol (six- or five-ring atoms) which are seen as important reaction intermediates of the green platform for the production of dihydroxyacetone and 1,2-propanediol, of great commercial value [10], [11].
Scientific research indicates that acid catalysis promotes etherification reactions of glycerol, and the use of heterogeneous catalysis has been developed for chemical processes to have better separation stages and the possibility of reuse/catalysts regeneration [5]. Some research groups are using as catalysts ion exchange resins, zeolites, aluminosilicates, molecular sieves, acidic clays and mesoporous carbon, with modifications in their chemical structures being responsible for increasing the number of acidic sites [12], [13], [14], [15], [16].
In this work, glycerol etherification reactions with benzaldehyde were performed in order to form important cyclic acetals for the chemical industry, investigating the acidic cation exchange resin as a reaction catalyst. Parameters such as reaction temperature, reagent ratio, organic solvents and catalyst reuse were investigated to obtain valuable information on reaction conversion, selectivity and reaction yield.
2 Materials and methods
2.1 Materials
Glycerol (99%, Synth Inc., Brazil), benzaldehyde (99%, Vetec Chemical Inc., Brazil), methanol (99.5%, Dinâmica Chemical Inc., Brazil), sodium chloride and sodium hydroxide (Vetec Chemical Inc., Brazil) are from an analytical standard used without prior treatment. Cationic acidic resin (IRA 120 Sigma-Aldrich, US) was pretreated in a forced circulation oven at 90°C for 2 h before being used as a reaction catalyst. Monoethylene glycol (99%, Vetec Chemical Inc., Brazil) was used as an internal standard for gas chromatography (GC) analyses. Magnetic stirrer with heating control, pH meter and various glassware were also used.
2.2 Products analysis
Analysis of the reaction products utilized the Shimadzu QP-2010 Ultra gas chromatography with mass detector, using Rtx-Wax (Restek) column of 30 m×0.25 mm×0.25 μm. The temperature of the injector chamber was 250°C, varying oven temperature from 40°C to 200°C with a rate of 10°C·min−1 with hold for 9 min in the final temperature. Split dilution was used at 1:60. External standards for GC analysis were used for quantification of glycerol and benzaldehyde. The monoethylene glycol was used as an internal standard diluted on methanol in the concentration of 100 mg·ml−1, for quantification of the acetals products.
Glycerol conversion equation, as well as the selectivity for desired products and reaction yield, are defined by Equations (1) to (3) [17].
2.3 Reactional experiments
The experiment was conducted through etherification of glycerol with benzaldehyde in 50 ml borosilicate glass batch reactors, with and without use of organic solvents. Moreover, to control the reaction solution, external-controlled hotplates with external thermostat and a magnetic stirring system were used. Besides that, the reactor was not attached to the reflux condenser in order to remove excess water from the reaction solution. Reactions with organic solvents needed to use reflux condenser.
2.3.1 Reactions without any solvent
No organic solvent was used in the first-stage etherification reactions. The reagents were added, and their quantities are specified in Table 1. The use of 500 mg of resin per test and the reaction time of 2 h was also fixed. We investigated three temperatures: 90°C, 110°C and 120°C, and the ratio of glycerol to benzaldehyde varied at 1:1, 1:2 and 2:1. At the end of the reactions, an amount of material was filtered on qualitative filter papers (grade 4) with cotton, collected in 2 ml vials and stored in a freezer. The experiments were performed in duplicate form. Catalyst amount used was chosen according to literature works [6], [10].
Performed experiments without solvent use.
| Experimentsa | Temperature (°C) | Ratio Gly/Bz | Quantity (mmol) | |
|---|---|---|---|---|
| Glycerol | Benzaldehyde | |||
| 1 | 90 | 1:1 | 55 | 55 |
| 2 | 1:2 | 55 | 110 | |
| 3 | 2:1 | 110 | 55 | |
| 4 | 110 | 1:1 | 55 | 55 |
| 5 | 1:2 | 55 | 110 | |
| 6 | 2:1 | 110 | 55 | |
| 7 | 120 | 1:1 | 55 | 55 |
| 8 | 1:2 | 55 | 110 | |
| 9 | 2:1 | 110 | 55 | |
aAll experiments are in duplicate samples.
2.3.2 Reactions with organic solvent and catalyst reuse
Acetonitrile and dimethylsulfoxide (DMSO) were used in the etherification reactions aiming to verify some possible improvements in the reaction yield and behavior. Reuse of the acid resin was also tested. The temperature was fixed at 120°C, time reaction was 2 h, the quantities of all organic solvents were defined with 200 mmol used for each reaction sample, and 270 mg of resin was also used in proportional quantity with reagents and the reactions without solvent stage. Table 2 presents the quantities of the reagents used in the experiments of this section. In the reuse experiments, catalysts were separated by simple filtration with qualitative paper (grade 4) and cotton, washed with distilled water, dried in forced circulation oven at 90°C for 2 h and then collected.
Performed experiments with organic solvents and catalyst reuse.
| Experimentsa | Ratio Gly/Bz | Quantity (mmol) | |
|---|---|---|---|
| Glycerol | Benzaldehyde | ||
| 10 | 1:1 | 30 | 30 |
| 11 | 1:2 | 30 | 60 |
| 12 | 2:1 | 60 | 30 |
| 13 | 1:1 | 30 | 30 |
| 14 | 1:2 | 30 | 60 |
| 15 | 2:1 | 60 | 30 |
| 16 | 1:1 | 30 | 30 |
| 17 | 1:2 | 30 | 60 |
| 18 | 2:1 | 60 | 30 |
| Reuse experiments | |||
| 19 | 1:1 | 30 | 30 |
| 20 | 1:2 | 30 | 60 |
| 21 | 2:1 | 60 | 30 |
aAll experiments are in duplicate samples.
Scheme 1 presents the glycerol etherification reaction with benzaldehyde to obtain the main products according to the present work.
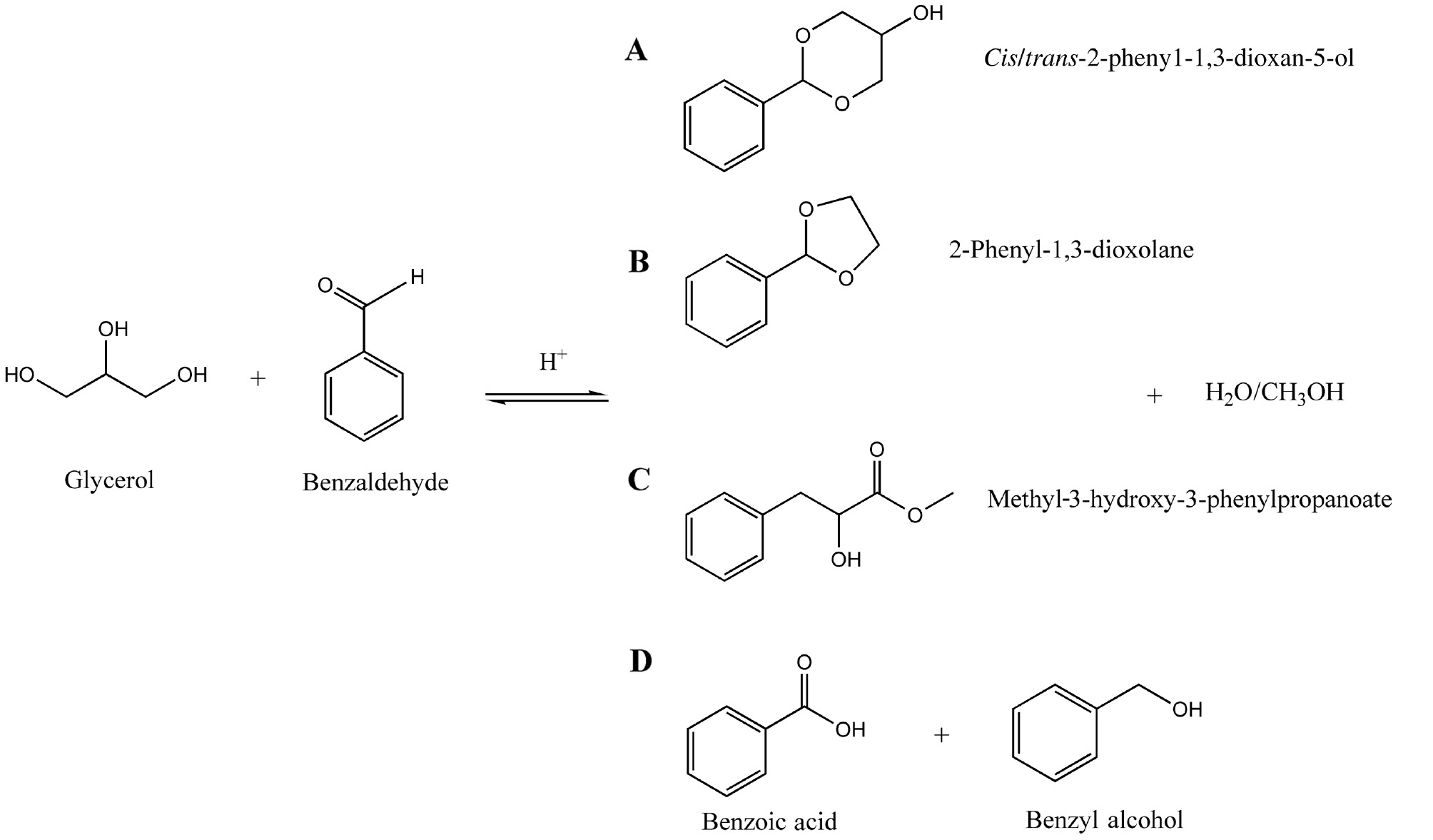
Main products observed in the glycerol etherification reaction with benzaldehyde.
2.4 Acid exchange resin
The amount of Brönsted acid sites of the catalysts was estimated by an acid-base titration technique. About 0.2 g of catalyst was weighed and immersed in 20 ml of 3 m sodium chloride (NaCl) solution under stirring for 30 h. During this period, ion exchange occurred between the H+ of the catalyst surface and the Na+ in the solution. Afterward the mixture was filtered, and the titrated solution was treated with 1 m sodium hydroxide solution and monitored by a pH meter. Phenolphthalein indicator was used. Titration endpoint was determined when the pH reached neutrality [13], [18].
The cation exchange resin employed had a density of acidic sites of 1671±26 μmol/g. It means that it has important acid characteristics for application as the catalyst in etherification reactions.
3 Results and discussion
Glycerol etherification with benzaldehyde may form desired cyclic acetals as well as other open-chain acetals. We considered the formation of cis/trans-2-phenyl-1,3- dioxan-5-ol (cyclic six-membered atoms) and 2-phenyl-1,3-dioxolane (cyclic five-membered atoms) as desired major products (cyclic acetals). Besides that, the formation of methyl-3-hydroxy-3-phenylpropanoate (non-cyclic acetal), benzoic acid and other minor products was also observed.
From Figures 1 and 2, glycerol conversion and reaction yield in the formation of the desired cyclic products were observed. At this stage, the time and quantity of catalyst used was fixed at 2 h and 500 mg, varying the temperature and the stoichiometry of the reactants. Reactions were performed without use of organic solvent.
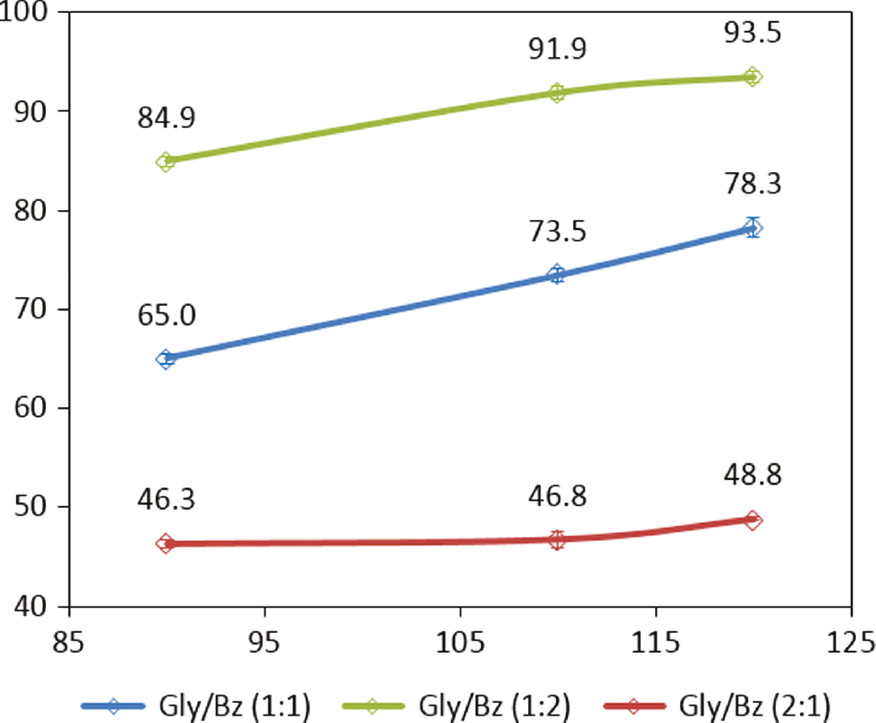
Glycerol conversion (%) versus different temperatures (°C) and reagents ratios.
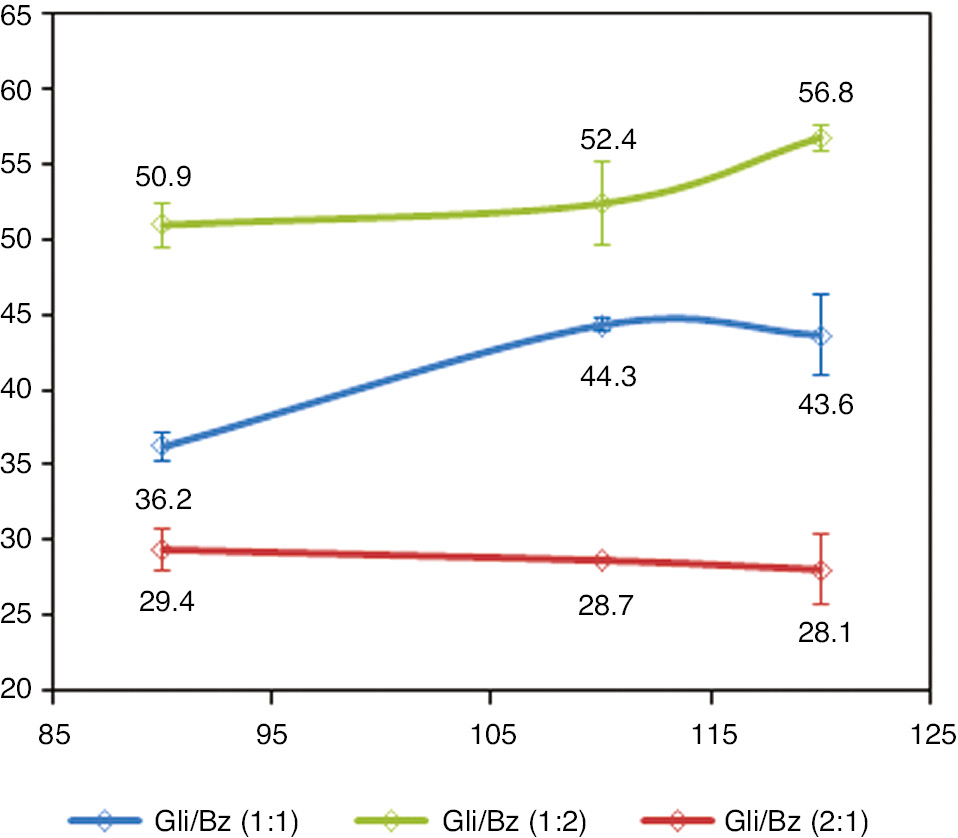
Reaction yields (%) versus different temperatures (°C) and reagents ratios.
Etherification reactions of glycerol with benzaldehyde had temperature-dependent behavior. The increase in temperature favored gain in glycerol conversion, both in the limiting and in excess reagent condition. Comparing the glycerol conversion results in the stoichiometric ratio and in the situation containing excess benzaldehyde, it was observed that the reaction with excess benzaldehyde favored the conversion of glycerol (Gly/Bz ratio 1:2), probably altering the equilibrium of reaction in the formation of their products. These results were reached at all observed temperatures. However, the difference between the values obtained is more evident at the temperature of 90°C. At 120°C, the maximum glycerol conversion reached 93.5% with reaction yield up to 56.8%. At this point, glycerol conversion reached 93.5%.
In a similar work, Silva and co-workers [19] investigated etherification of glycerol with several C4 to C10 open-chain aldehydes and observed conversions of 17–75% with the use of butanal in a 2-h reaction with 1:1.05 ratio of glycerol/aldehyde at temperature of 70°C, using DMSO as the reaction solvent. The results presented are close to the values contained in Figure 2, indicating that the use of benzaldehyde is also capable of forming acetals and, in our work, cyclic acetals.
Deutsch and co-workers [10] used several catalysts such as acid zeolites, cation exchange resins and acidic clays obtaining yields of up to 94% in cyclic acetals by etherification of glycerol with benzaldehyde, removing the water from the reaction medium during the reactions. According to the authors, the presence of water (reaction product) contributes greatly to the reduction of reaction yield. In our work, it was not possible to remove the water completely, indicating that the reactions could have higher yields than those observed.
Rodrigues and co-workers [20], [21], [22], [23] investigated the role of mass transfer and the adsorption of the reactant molecules on the reaction of ethanol with acetaldehyde and glycerol with acetaldehyde for the formation of ethylacetals or glycerol ethyl acetals. According to the authors, the effects of mass transfer represented by the reactants’ solubility and diffusivity of the elements on the catalytic surface are important factors for the reaction yield increase and, consequently, the reaction kinetics.
In our work, high values of glycerol conversion and yield in the desired products may indicate that the diffusive aspect of the glycerol in the reaction medium as well as the surface of the cation resin contribute to the dynamics of the reaction. However, this fact did not present a behavior that has great influence in the experimental conditions observed due to the high results obtained in the conversion of glycerol. Certainly, this fact needs further investigation.
In Figure 3A–C we observe that there was a higher selectivity of products besides cyclic acetals for most temperatures and reagent concentration conditions analyzed, indicating that the acid cationic resin used demonstrated efficient production of acetals 2-phenyl-1,3-dioxan-5-ol in cis and trans isomers and 2-phenyl-1,3-dioxolane. Selectivity values reached 57.0–63.5% for cyclic acetals.
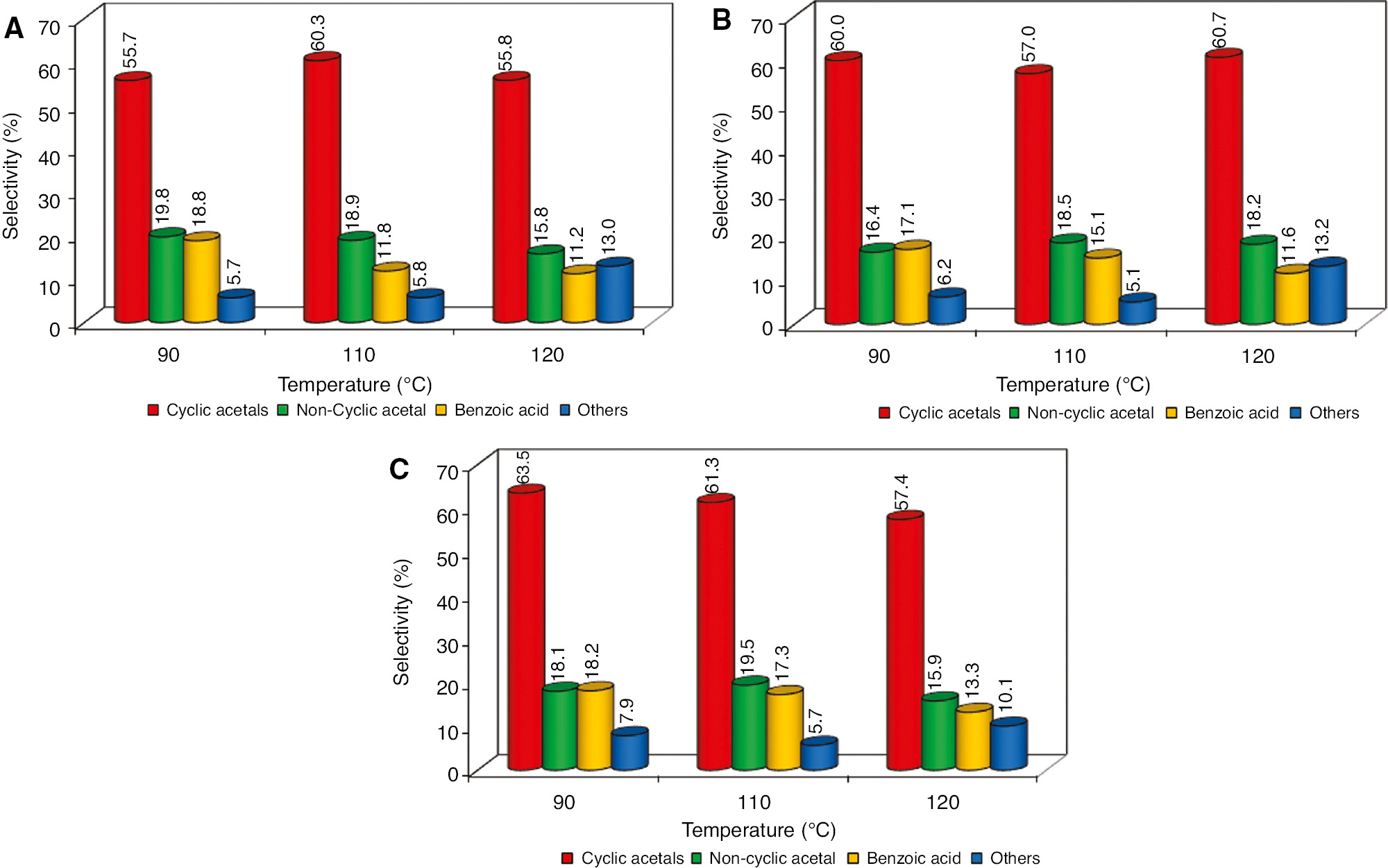
Selectivity (%) comparison ratios versus temperature (°C) about the main products of the glycerol etherification with benzaldehyde. (A) Gly/Bz ratio (1:1), (B) Gly/Bz ratio (1:2), and (C) Gly/Bz ratio (2:1). Errors deviation in 0.5–1.0%.
All concentration ratios observed between glycerol and benzaldehyde had no significant differences in selectivity behavior to cyclic and non-cyclic acetals products. The increase in temperature favored the formation the other non-desired products, but this increase was small, reaching only 13%. However, the increase in temperature resulted in a significant decrease in the formation of benzoic acid (from 18.8% to 11.2%), a behavior contrary to that observed in the formation of other non-desired reaction products. It is possible that the benzoic acid has been transformed into subsequent reaction products with the reaction temperature gain.
3.1 Use of organic solvent effects
Acetonitrile and DMSO were applied in the etherification reactions to investigate the behavior with the use of these solvents in the reaction medium. The quantities of reagents are shown in Table 2. Reactions without the use of resin catalyst were also performed and named “Blank”.
In Figure 4, in general terms, the use of acetonitrile showed greater gains in the conversion of glycerol compared to the use of DMSO, considering all the glycerol/benzaldehyde ratios. The best values reached 82.4% of conversion. However, these results are less expressive when compared to reaction without use of organic solvents (Figure 2). It can be deduced that the possible difficulties of diffusion and mass transfer reactions without solvents could have been minimized due to the temperature, stirring and solubility conditions between the reactants. Dilution did not demonstrate a different effect to improve glycerol conversion values. Regarding glycerol/benzaldehyde ratios, excess of benzaldehyde boosted glycerol conversion.
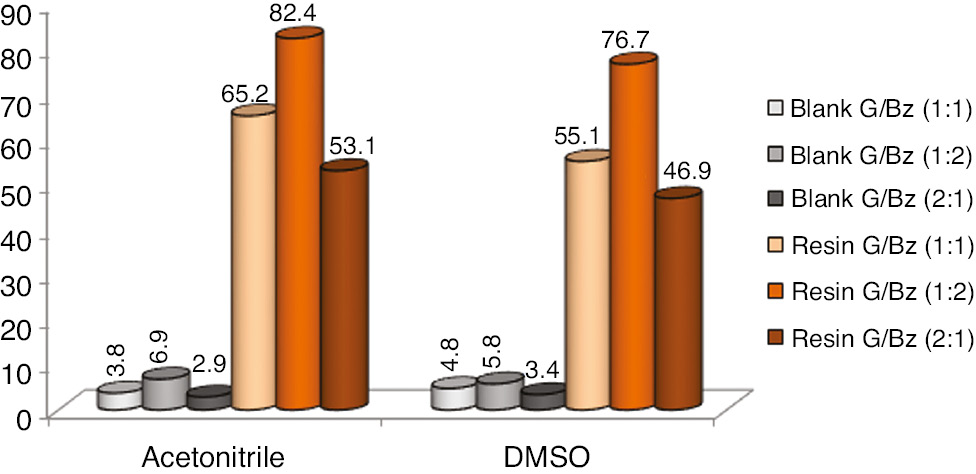
Glycerol conversion (%) comparison ratios versus organic solvents. Errors deviation in 1.0–1.8%.
Figures 5 and 6 A–C present profiles of glycerol yield and selectivity for the desired products. The use of organic solvents increased the selectivity for cyclic acetals reaching up to 80.9%. DMSO showed better results compared to acetonitrile with a difference of up to 10%. This selectivity behavior is responsible for the reaction yield results where both solvents used provide similar results in all conditions analyzed. Again, the reactions containing an excess of reagent benzaldehyde present higher reaction yield. Reactions with the use of acetonitrile promoted more activity reaction medium than the DMSO solvent did; nevertheless, DMSO promoted more selectivity medium for the desired products resulting in a compensation effect of the reaction yield.
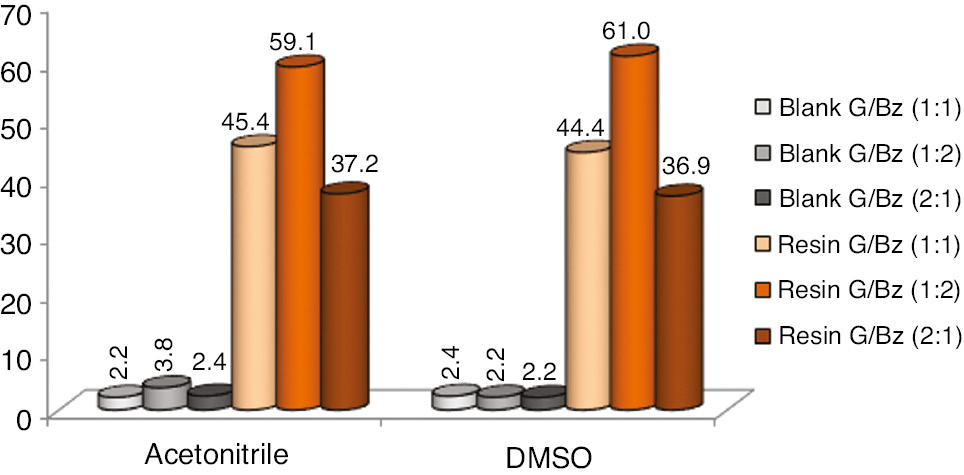
Glycerol yield (%) comparison ratios versus organic solvents. Errors deviation in 1.0–2.0%.
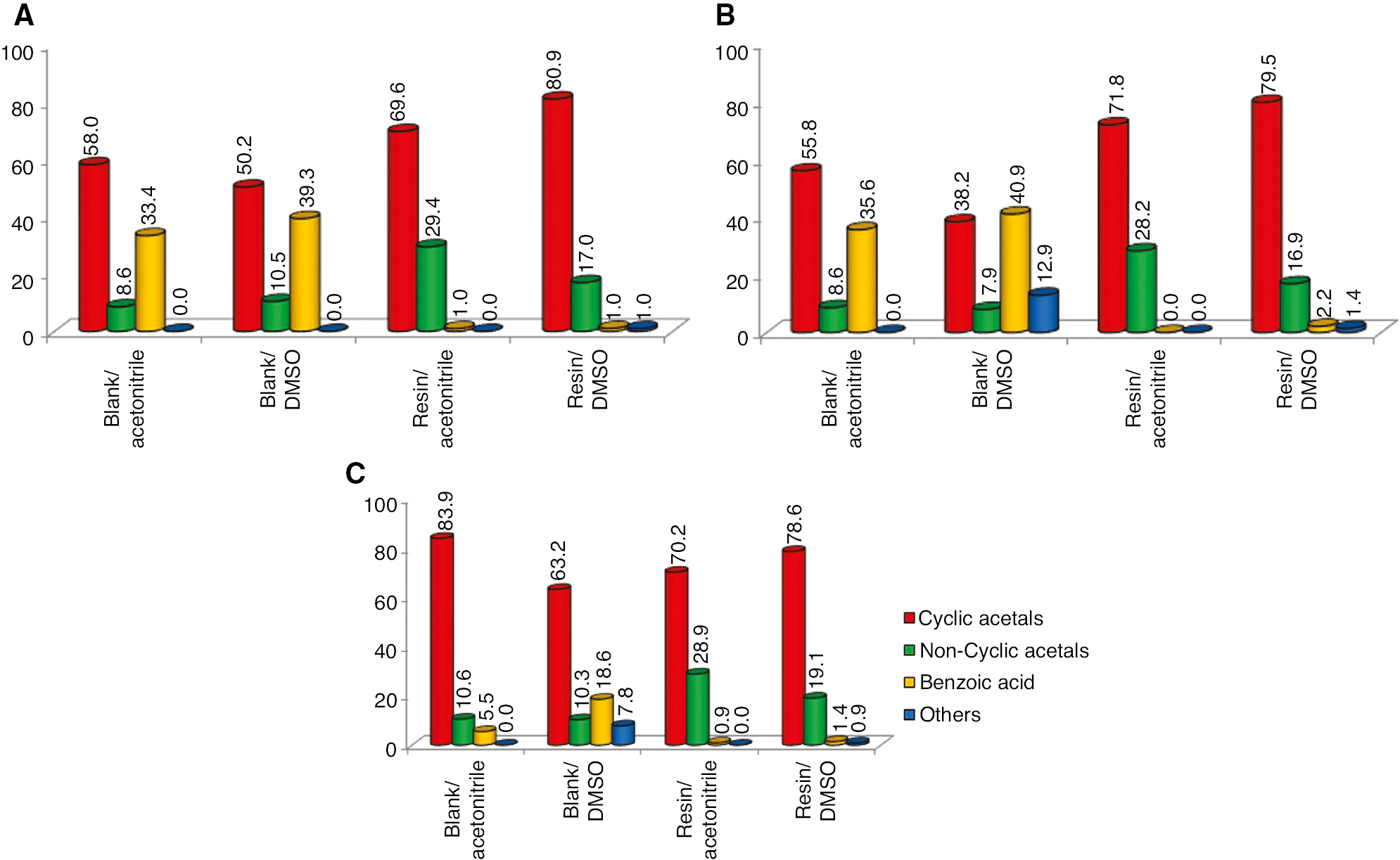
Selectivity (%) comparison ratios versus organic solvents about the main products of the glycerol etherification with benzaldehyde. (A) Gly/Bz ratio (1:1), (B) Gly/Bz ratio (1:2), and (C) Gly/Bz ratio (2:1). Errors deviation in 0.7–2.5%.
3.2 Effects of catalyst reuse
Reuse of the acidic resin was conducted in the experiments to identify characteristics of catalyst durability in the reaction and is presented in Figure 7.
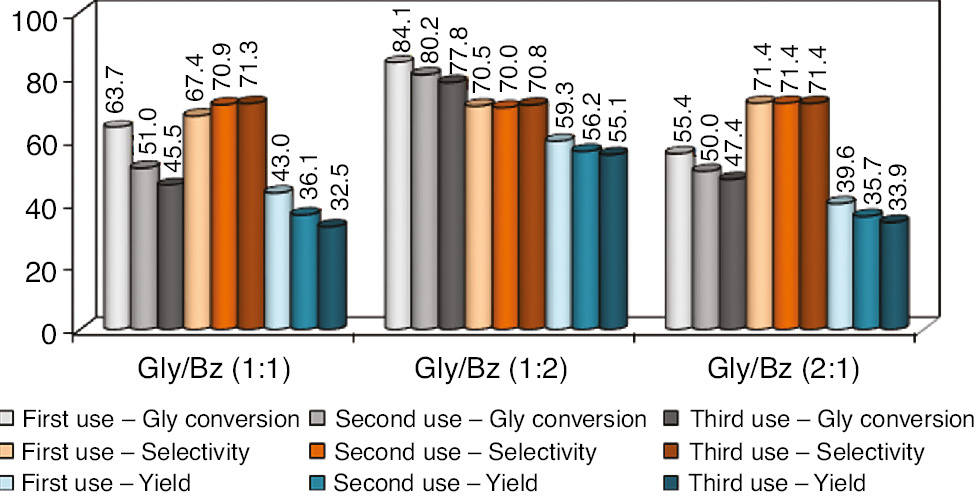
Catalyst reuse experiments stats (%) versus glycerol/benzaldehyde comparison ratios. Glycerol conversion in shades of gray, cyclic acetals selectivity in shades of red and reaction yield in shades of blue. Errors deviation in 0.5–1.5%.
After three reaction times, glycerol conversion had a slight decrease in all glycerol/benzaldehyde ratios which shows good catalytic stability, especially in benzaldehyde excess concentration, ranging 84.1–77.8%. No significant differences should be observed with respect to selectivity values about formation of cyclic acetals, and values obtained were close to 70%. Consequently, yield behavior is the same as conversion behavior, and best values ranged between 59.1% and 55.1%.
After three-times catalyst reuse, acid-base titration was conducted again to determine new acidic site density, and values of 1432±37 μmol/g were observed. This reduction represents 14.3% compared to the fresh acidic values and is expected; however, it is consistent with the decrease in values of conversion and yield observed. This behavior brings safety and durability to the resin application in glycerol etherification reactions for industrial applicability.
3.3 Reaction pathway
Sudarsanam and co-workers [11] also studied etherification reactions between glycerol and benzaldehyde and presented a possible reaction pathway. The formation of a positively charged carbonyl carbon of the benzaldehyde added with a strong interaction between the oxygen atoms of the carbonyl group and the solid acid catalyst occurs in the first steps of the surface reaction. Afterward, the oxygen atom of the glycerol attacks the positively charged carbonyl carbon forming the benzyl cation, an intermediate product. In the follow step, two isomeric cyclic acetals, namely, [A] 2-phenyl-1,3-dioxan-5-ol and 2-phenyl-1,3-dioxolane-4-methanol appears through different routes, respectively. The formation of the cyclic compound [B] 2-phenyl-1,3-dioxolane is more complex, which may indicate its less formation in the reaction medium. Following the formation of a reaction intermediate, 2-phenyl-1,3-dioxolane-4-methanol is formed which, in the presence of water and benzaldehyde, could form 2-phenyl-1,3-dioxolane as well as benzoic acid and methanol.
In addition to that, a non-cyclic acetal, namely, [C] methyl-3-hydroxy-3-phenylpropanoate, was formed with another glycerol oxygen attack with water formation [14]. Formation of [D] benzoic acid could occur with hydrolysis reaction or a redox disproportionation of benzaldehyde to carboxylic acids and alcohols called the Cannizzaro reaction [24]. Scheme 2 presents the reaction pathway observed.
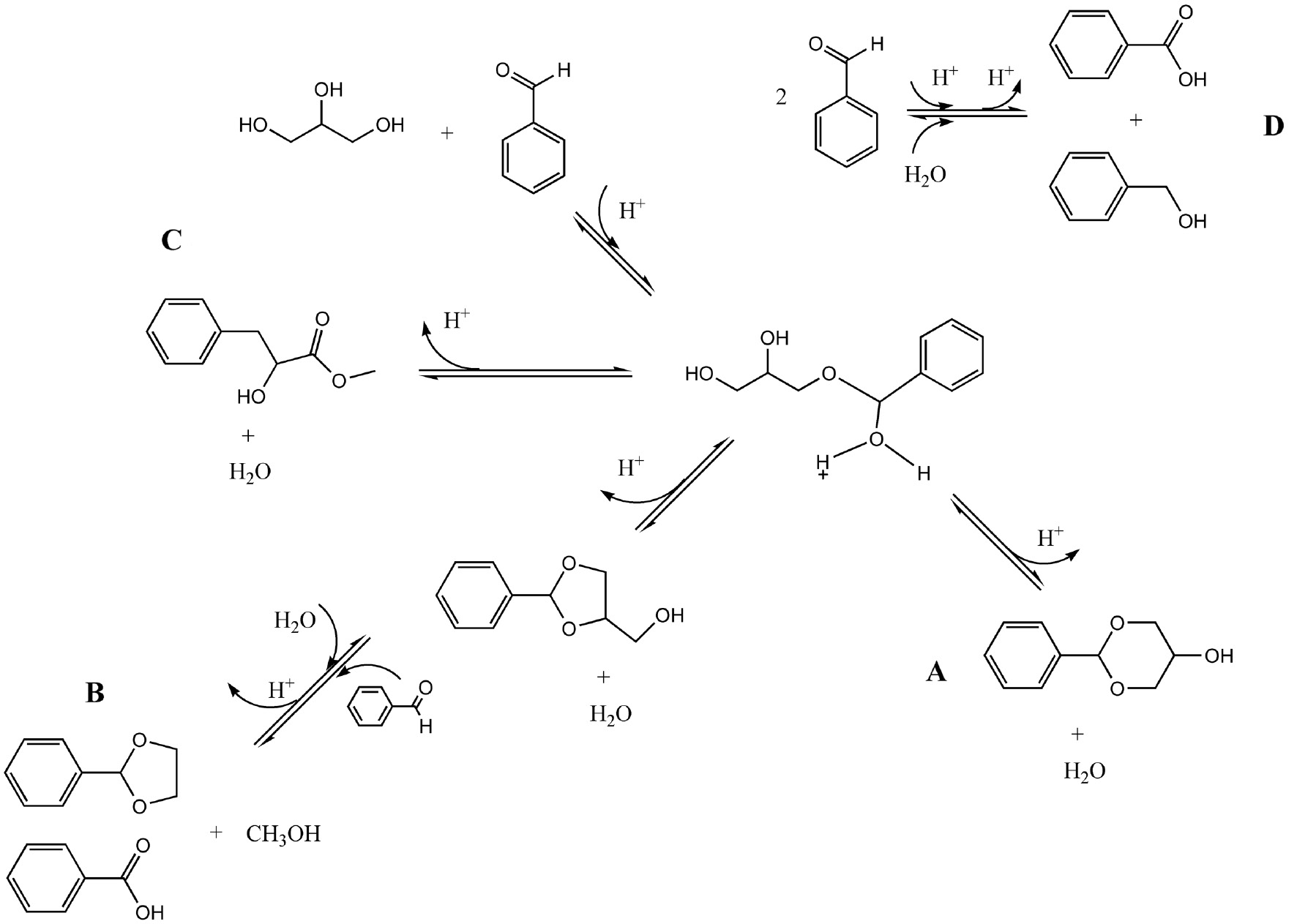
Possible reaction mechanism for the glycerol etherification with benzaldehyde. (A) Cis/trans-2-phenyl-1.3-dioxan-5-ol, (B) 2-phenyl-1.3-dioxolane, (C) methyl-3-hidroxy-3-phenylpropanoate, and (D) benzoic acid and benzyl alcohol.
4 Conclusions
Cyclic acetals of high commercial value were produced through glycerol etherification with benzaldehyde using a cationic acidic resin, with and without the use of organic solvents as reaction medium. 2-Phenyl-1,3-dioxan-5-ol compounds in cis and trans isomers were the major cyclic acetals formed in the reaction, achieving conversions of 93%, selectivity above 80% and better yields around 61%.
Temperature had a positive effect on glycerol conversion; however, it also favored the formation of undesired compounds, mainly caused by high reactivity of benzaldehyde with glycerol, forming benzoic acid and other second undesired products. On the other hand, the ratios of reagents, richer in glycerol or benzaldehyde, were important to verify the shift in selectivity behavior of desired compounds. Benzaldehyde-rich configuration forced a greater glycerol conversion above 93% with no significant selective decrease equalizing the selectivity effect and giving a final yield above 56.8% to 2-phenyl-1,3-dioxan-5-ol.
Reactions with the use of organic solvents increase the selectivity for cyclic acetals reaching 80.9% responsible for increasing reaction yield up to 59–61%. Reactions in three-time catalyst reuse presented a slight decrease on glycerol conversion with no significant effect in selectivity values, which resulted in a small decrease; values of reaction yield ranged between 59% and 55%.
Acknowledgements
We are grateful to UTFPR – Campus Apucarana, DEQ/UEM and Fundação Araucária for financial support.
References
[1] Zheng Y, Chen X, Shen Y. Chem. Rev. 2008, 108, 5253–5277.Search in Google Scholar
[2] Okoye PU, Hammed BH. Renew. Sust. Energ. Rev. 2016, 53, 558–574.10.1016/j.rser.2015.08.064Search in Google Scholar
[3] Mota CJA, Silva CXA, Gonçalves VLC. Quím. Nova 2009, 32, 639–648.10.1590/S0100-40422009000300008Search in Google Scholar
[4] EPE, Empresa de Pesquisa Energética. Análise de conjuntura dos biocombustíveis. Available at http://epe.gov.br/sites-pt/publicacoes-dados-abertos/publicacoes/PublicacoesArquivos/publicacao-167/Analise_de_Conjuntura_dos_Biocombustiveis-Ano_2017.pdf. Accessed in May 2018.Search in Google Scholar
[5] Gu Y, Azzouri A, Pouilloux Y, Jérôme F, Barrault J. Green Chem. 2008, 10, 164–167.10.1039/B715802ESearch in Google Scholar
[6] Silva CRB, Gonçalves VLC, Lachter ER, Mota CJA. J. Braz. Chem. Soc. 2009, 20, 201–204.10.1590/S0103-50532009000200002Search in Google Scholar
[7] Garcia R, Besson M, Gallezot P. Appl. Catal. A: Gen. 1995, 127, 165–176.10.1016/0926-860X(95)00048-8Search in Google Scholar
[8] Chen H, Ji H, Zhou X, Wang L. Tetrahedron 2010, 66, 9888–9893.10.1016/j.tet.2010.10.063Search in Google Scholar
[9] Passos ML, Ribeiro CP. Innovation in Food Engineering: New Techniques and Products, CRC Press: Boca Raton, 2010.Search in Google Scholar
[10] Deutsch J, Martin A, Lieske H. J. Catal. 2007, 245, 428–435.10.1016/j.jcat.2006.11.006Search in Google Scholar
[11] Sudarsanam P, Mallesham B, Prasad AN, Reddy PS, Reddy BM. Fuel Process. Technol. 2013, 106, 539–545.10.1016/j.fuproc.2012.09.025Search in Google Scholar
[12] Melero JA, Vicente G, Paniagua M, Morales G, Muñoz P. Bioresour. Technol. 2012, 103, 142–151.10.1016/j.biortech.2011.09.105Search in Google Scholar PubMed
[13] Pico MP, Rodríguez S, Santos A, Romero A. Ind. Eng. Chem. Res. 2013, 52, 14545–14555.10.1021/ie402026tSearch in Google Scholar
[14] Silva CXA, Gonçalves VLC, Mota CJA. Green Chem. 2009, 11, 38–41.10.1039/B813564ASearch in Google Scholar
[15] Khayoon MS, Hameed BH. Appl. Catal. A: Gen. 2013, 464–465, 191–199.10.1016/j.apcata.2013.05.035Search in Google Scholar
[16] Khayoon MS, Hameed BH. Bioresour. Technol. 2010, 101, 6225–6229.10.1016/j.biortech.2010.02.101Search in Google Scholar
[17] Missen RW, Mins CA, Saville BA. Introduction to Chemical Reaction Engineering and Kinetics, John Wiley & Sons: New York, 1999.Search in Google Scholar
[18] López DE, Goodwin JG, Jr., Bruce DA. J. Catal. 2007, 245, 381–391.10.1016/j.jcat.2006.10.027Search in Google Scholar
[19] Silva PHR, Gonçalves VLC, Mota CJA. Bioresour. Technol. 2010, 101, 6225–6229.10.1016/j.biortech.2010.02.101Search in Google Scholar
[20] Silva VMTM, Rodrigues AE. Chem. Eng. Sci. 2006, 61, 316–331.10.1016/j.ces.2005.07.017Search in Google Scholar
[21] Silva VMTM, Rodrigues AE. Chem. Eng. Sci. 2001, 56, 1255–1263.10.1016/S0009-2509(00)00347-XSearch in Google Scholar
[22] Faria RPV, Pereira CSM, Silva VMTM, Loureiro JM, Rodrigues AE. Chem. Eng. J. 2014, 258, 229–239.10.1016/j.cej.2014.07.073Search in Google Scholar
[23] Faria RPV, Pereira CSM, Silva VMTM, Loureiro JM, Rodrigues AE. Chem. Eng. J. 2013, 233, 159–167.10.1016/j.cej.2013.08.035Search in Google Scholar
[24] Kikhtyanin O, Lesnik E, Kubička D. Appl. Catal. A: Gen. 2016, 525, 215–225.10.1016/j.apcata.2016.08.007Search in Google Scholar
©2019 Walter de Gruyter GmbH, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 Public License.
Articles in the same Issue
- Regular Articles
- Studies on the preparation and properties of biodegradable polyester from soybean oil
- Flow-mode biodiesel production from palm oil using a pressurized microwave reactor
- Reduction of free fatty acids in waste oil for biodiesel production by glycerolysis: investigation and optimization of process parameters
- Saccharin: a cheap and mild acidic agent for the synthesis of azo dyes via telescoped dediazotization
- Optimization of lipase-catalyzed synthesis of polyethylene glycol stearate in a solvent-free system
- Green synthesis of iron oxide nanoparticles using Platanus orientalis leaf extract for antifungal activity
- Ultrasound assisted chemical activation of peanut husk for copper removal
- Room temperature silanization of Fe3O4 for the preparation of phenyl functionalized magnetic adsorbent for dispersive solid phase extraction for the extraction of phthalates in water
- Evaluation of the saponin green extraction from Ziziphus spina-christi leaves using hydrothermal, microwave and Bain-Marie water bath heating methods
- Oxidation of dibenzothiophene using the heterogeneous catalyst of tungsten-based carbon nanotubes
- Calcined sodium silicate as an efficient and benign heterogeneous catalyst for the transesterification of natural lecithin to L-α-glycerophosphocholine
- Synergistic effect between CO2 and H2O2 on ethylbenzene oxidation catalyzed by carbon supported heteropolyanion catalysts
- Hydrocyanation of 2-arylmethyleneindan-1,3-diones using potassium hexacyanoferrate(II) as a nontoxic cyanating agent
- Green synthesis of hydratropic aldehyde from α-methylstyrene catalyzed by Al2O3-supported metal phthalocyanines
- Environmentally benign chemical recycling of polycarbonate wastes: comparison of micro- and nano-TiO2 solid support efficiencies
- Medicago polymorpha-mediated antibacterial silver nanoparticles in the reduction of methyl orange
- Production of value-added chemicals from esterification of waste glycerol over MCM-41 supported catalysts
- Green synthesis of zerovalent copper nanoparticles for efficient reduction of toxic azo dyes congo red and methyl orange
- Optimization of the biological synthesis of silver nanoparticles using Penicillium oxalicum GRS-1 and their antimicrobial effects against common food-borne pathogens
- Optimization of submerged fermentation conditions to overproduce bioethanol using two industrial and traditional Saccharomyces cerevisiae strains
- Extraction of In3+ and Fe3+ from sulfate solutions by using a 3D-printed “Y”-shaped microreactor
- Foliar-mediated Ag:ZnO nanophotocatalysts: green synthesis, characterization, pollutants degradation, and in vitro biocidal activity
- Green cyclic acetals production by glycerol etherification reaction with benzaldehyde using cationic acidic resin
- Biosynthesis, characterization and antimicrobial activities assessment of fabricated selenium nanoparticles using Pelargonium zonale leaf extract
- Synthesis of high surface area magnesia by using walnut shell as a template
- Controllable biosynthesis of silver nanoparticles using actinobacterial strains
- Green vegetation: a promising source of color dyes
- Mechano-chemical synthesis of ammonia and acetic acid from inorganic materials in water
- Green synthesis and structural characterization of novel N1-substituted 3,4-dihydropyrimidin-2(1H)-ones
- Biodiesel production from cotton oil using heterogeneous CaO catalysts from eggshells prepared at different calcination temperatures
- Regeneration of spent mercury catalyst for the treatment of dye wastewater by the microwave and ultrasonic spray-assisted method
- Green synthesis of the innovative super paramagnetic nanoparticles from the leaves extract of Fraxinus chinensis Roxb and their application for the decolourisation of toxic dyes
- Biogenic ZnO nanoparticles: a study of blueshift of optical band gap and photocatalytic degradation of reactive yellow 186 dye under direct sunlight
- Leached compounds from the extracts of pomegranate peel, green coconut shell, and karuvelam wood for the removal of hexavalent chromium
- Enhancement of molecular weight reduction of natural rubber in triphasic CO2/toluene/H2O systems with hydrogen peroxide for preparation of biobased polyurethanes
- An efficient green synthesis of novel 1H-imidazo[1,2-a]imidazole-3-amine and imidazo[2,1-c][1,2,4]triazole-5-amine derivatives via Strecker reaction under controlled microwave heating
- Evaluation of three different green fabrication methods for the synthesis of crystalline ZnO nanoparticles using Pelargonium zonale leaf extract
- A highly efficient and multifunctional biomass supporting Ag, Ni, and Cu nanoparticles through wetness impregnation for environmental remediation
- Simple one-pot green method for large-scale production of mesalamine, an anti-inflammatory agent
- Relationships between step and cumulative PMI and E-factors: implications on estimating material efficiency with respect to charting synthesis optimization strategies
- A comparative sorption study of Cr3+ and Cr6+ using mango peels: kinetic, equilibrium and thermodynamic
- Effects of acid hydrolysis waste liquid recycle on preparation of microcrystalline cellulose
- Use of deep eutectic solvents as catalyst: A mini-review
- Microwave-assisted synthesis of pyrrolidinone derivatives using 1,1’-butylenebis(3-sulfo-3H-imidazol-1-ium) chloride in ethylene glycol
- Green and eco-friendly synthesis of Co3O4 and Ag-Co3O4: Characterization and photo-catalytic activity
- Adsorption optimized of the coal-based material and application for cyanide wastewater treatment
- Aloe vera leaf extract mediated green synthesis of selenium nanoparticles and assessment of their In vitro antimicrobial activity against spoilage fungi and pathogenic bacteria strains
- Waste phenolic resin derived activated carbon by microwave-assisted KOH activation and application to dye wastewater treatment
- Direct ethanol production from cellulose by consortium of Trichoderma reesei and Candida molischiana
- Agricultural waste biomass-assisted nanostructures: Synthesis and application
- Biodiesel production from rubber seed oil using calcium oxide derived from eggshell as catalyst – optimization and modeling studies
- Study of fabrication of fully aqueous solution processed SnS quantum dot-sensitized solar cell
- Assessment of aqueous extract of Gypsophila aretioides for inhibitory effects on calcium carbonate formation
- An environmentally friendly acylation reaction of 2-methylnaphthalene in solvent-free condition in a micro-channel reactor
- Aegle marmelos phytochemical stabilized synthesis and characterization of ZnO nanoparticles and their role against agriculture and food pathogen
- A reactive coupling process for co-production of solketal and biodiesel
- Optimization of the asymmetric synthesis of (S)-1-phenylethanol using Ispir bean as whole-cell biocatalyst
- Synthesis of pyrazolopyridine and pyrazoloquinoline derivatives by one-pot, three-component reactions of arylglyoxals, 3-methyl-1-aryl-1H-pyrazol-5-amines and cyclic 1,3-dicarbonyl compounds in the presence of tetrapropylammonium bromide
- Preconcentration of morphine in urine sample using a green and solvent-free microextraction method
- Extraction of glycyrrhizic acid by aqueous two-phase system formed by PEG and two environmentally friendly organic acid salts - sodium citrate and sodium tartrate
- Green synthesis of copper oxide nanoparticles using Juglans regia leaf extract and assessment of their physico-chemical and biological properties
- Deep eutectic solvents (DESs) as powerful and recyclable catalysts and solvents for the synthesis of 3,4-dihydropyrimidin-2(1H)-ones/thiones
- Biosynthesis, characterization and anti-microbial activity of silver nanoparticle based gel hand wash
- Efficient and selective microwave-assisted O-methylation of phenolic compounds using tetramethylammonium hydroxide (TMAH)
- Anticoagulant, thrombolytic and antibacterial activities of Euphorbia acruensis latex-mediated bioengineered silver nanoparticles
- Volcanic ash as reusable catalyst in the green synthesis of 3H-1,5-benzodiazepines
- Green synthesis, anionic polymerization of 1,4-bis(methacryloyl)piperazine using Algerian clay as catalyst
- Selenium supplementation during fermentation with sugar beet molasses and Saccharomyces cerevisiae to increase bioethanol production
- Biosynthetic potential assessment of four food pathogenic bacteria in hydrothermally silver nanoparticles fabrication
- Investigating the effectiveness of classical and eco-friendly approaches for synthesis of dialdehydes from organic dihalides
- Pyrolysis of palm oil using zeolite catalyst and characterization of the boil-oil
- Azadirachta indica leaves extract assisted green synthesis of Ag-TiO2 for degradation of Methylene blue and Rhodamine B dyes in aqueous medium
- Synthesis of vitamin E succinate catalyzed by nano-SiO2 immobilized DMAP derivative in mixed solvent system
- Extraction of phytosterols from melon (Cucumis melo) seeds by supercritical CO2 as a clean technology
- Production of uronic acids by hydrothermolysis of pectin as a model substance for plant biomass waste
- Biofabrication of highly pure copper oxide nanoparticles using wheat seed extract and their catalytic activity: A mechanistic approach
- Intelligent modeling and optimization of emulsion aggregation method for producing green printing ink
- Improved removal of methylene blue on modified hierarchical zeolite Y: Achieved by a “destructive-constructive” method
- Two different facile and efficient approaches for the synthesis of various N-arylacetamides via N-acetylation of arylamines and straightforward one-pot reductive acetylation of nitroarenes promoted by recyclable CuFe2O4 nanoparticles in water
- Optimization of acid catalyzed esterification and mixed metal oxide catalyzed transesterification for biodiesel production from Moringa oleifera oil
- Kinetics and the fluidity of the stearic acid esters with different carbon backbones
- Aiming for a standardized protocol for preparing a process green synthesis report and for ranking multiple synthesis plans to a common target product
- Microstructure and luminescence of VO2 (B) nanoparticle synthesis by hydrothermal method
- Optimization of uranium removal from uranium plant wastewater by response surface methodology (RSM)
- Microwave drying of nickel-containing residue: dielectric properties, kinetics, and energy aspects
- Simple and convenient two step synthesis of 5-bromo-2,3-dimethoxy-6-methyl-1,4-benzoquinone
- Biodiesel production from waste cooking oil
- The effect of activation temperature on structure and properties of blue coke-based activated carbon by CO2 activation
- Optimization of reaction parameters for the green synthesis of zero valent iron nanoparticles using pine tree needles
- Microwave-assisted protocol for squalene isolation and conversion from oil-deodoriser distillates
- Denitrification performance of rare earth tailings-based catalysts
- Facile synthesis of silver nanoparticles using Averrhoa bilimbi L and Plum extracts and investigation on the synergistic bioactivity using in vitro models
- Green production of AgNPs and their phytostimulatory impact
- Photocatalytic activity of Ag/Ni bi-metallic nanoparticles on textile dye removal
- Topical Issue: Green Process Engineering / Guest Editors: Martine Poux, Patrick Cognet
- Modelling and optimisation of oxidative desulphurisation of tyre-derived oil via central composite design approach
- CO2 sequestration by carbonation of olivine: a new process for optimal separation of the solids produced
- Organic carbonates synthesis improved by pervaporation for CO2 utilisation
- Production of starch nanoparticles through solvent-antisolvent precipitation in a spinning disc reactor
- A kinetic study of Zn halide/TBAB-catalysed fixation of CO2 with styrene oxide in propylene carbonate
- Topical on Green Process Engineering
Articles in the same Issue
- Regular Articles
- Studies on the preparation and properties of biodegradable polyester from soybean oil
- Flow-mode biodiesel production from palm oil using a pressurized microwave reactor
- Reduction of free fatty acids in waste oil for biodiesel production by glycerolysis: investigation and optimization of process parameters
- Saccharin: a cheap and mild acidic agent for the synthesis of azo dyes via telescoped dediazotization
- Optimization of lipase-catalyzed synthesis of polyethylene glycol stearate in a solvent-free system
- Green synthesis of iron oxide nanoparticles using Platanus orientalis leaf extract for antifungal activity
- Ultrasound assisted chemical activation of peanut husk for copper removal
- Room temperature silanization of Fe3O4 for the preparation of phenyl functionalized magnetic adsorbent for dispersive solid phase extraction for the extraction of phthalates in water
- Evaluation of the saponin green extraction from Ziziphus spina-christi leaves using hydrothermal, microwave and Bain-Marie water bath heating methods
- Oxidation of dibenzothiophene using the heterogeneous catalyst of tungsten-based carbon nanotubes
- Calcined sodium silicate as an efficient and benign heterogeneous catalyst for the transesterification of natural lecithin to L-α-glycerophosphocholine
- Synergistic effect between CO2 and H2O2 on ethylbenzene oxidation catalyzed by carbon supported heteropolyanion catalysts
- Hydrocyanation of 2-arylmethyleneindan-1,3-diones using potassium hexacyanoferrate(II) as a nontoxic cyanating agent
- Green synthesis of hydratropic aldehyde from α-methylstyrene catalyzed by Al2O3-supported metal phthalocyanines
- Environmentally benign chemical recycling of polycarbonate wastes: comparison of micro- and nano-TiO2 solid support efficiencies
- Medicago polymorpha-mediated antibacterial silver nanoparticles in the reduction of methyl orange
- Production of value-added chemicals from esterification of waste glycerol over MCM-41 supported catalysts
- Green synthesis of zerovalent copper nanoparticles for efficient reduction of toxic azo dyes congo red and methyl orange
- Optimization of the biological synthesis of silver nanoparticles using Penicillium oxalicum GRS-1 and their antimicrobial effects against common food-borne pathogens
- Optimization of submerged fermentation conditions to overproduce bioethanol using two industrial and traditional Saccharomyces cerevisiae strains
- Extraction of In3+ and Fe3+ from sulfate solutions by using a 3D-printed “Y”-shaped microreactor
- Foliar-mediated Ag:ZnO nanophotocatalysts: green synthesis, characterization, pollutants degradation, and in vitro biocidal activity
- Green cyclic acetals production by glycerol etherification reaction with benzaldehyde using cationic acidic resin
- Biosynthesis, characterization and antimicrobial activities assessment of fabricated selenium nanoparticles using Pelargonium zonale leaf extract
- Synthesis of high surface area magnesia by using walnut shell as a template
- Controllable biosynthesis of silver nanoparticles using actinobacterial strains
- Green vegetation: a promising source of color dyes
- Mechano-chemical synthesis of ammonia and acetic acid from inorganic materials in water
- Green synthesis and structural characterization of novel N1-substituted 3,4-dihydropyrimidin-2(1H)-ones
- Biodiesel production from cotton oil using heterogeneous CaO catalysts from eggshells prepared at different calcination temperatures
- Regeneration of spent mercury catalyst for the treatment of dye wastewater by the microwave and ultrasonic spray-assisted method
- Green synthesis of the innovative super paramagnetic nanoparticles from the leaves extract of Fraxinus chinensis Roxb and their application for the decolourisation of toxic dyes
- Biogenic ZnO nanoparticles: a study of blueshift of optical band gap and photocatalytic degradation of reactive yellow 186 dye under direct sunlight
- Leached compounds from the extracts of pomegranate peel, green coconut shell, and karuvelam wood for the removal of hexavalent chromium
- Enhancement of molecular weight reduction of natural rubber in triphasic CO2/toluene/H2O systems with hydrogen peroxide for preparation of biobased polyurethanes
- An efficient green synthesis of novel 1H-imidazo[1,2-a]imidazole-3-amine and imidazo[2,1-c][1,2,4]triazole-5-amine derivatives via Strecker reaction under controlled microwave heating
- Evaluation of three different green fabrication methods for the synthesis of crystalline ZnO nanoparticles using Pelargonium zonale leaf extract
- A highly efficient and multifunctional biomass supporting Ag, Ni, and Cu nanoparticles through wetness impregnation for environmental remediation
- Simple one-pot green method for large-scale production of mesalamine, an anti-inflammatory agent
- Relationships between step and cumulative PMI and E-factors: implications on estimating material efficiency with respect to charting synthesis optimization strategies
- A comparative sorption study of Cr3+ and Cr6+ using mango peels: kinetic, equilibrium and thermodynamic
- Effects of acid hydrolysis waste liquid recycle on preparation of microcrystalline cellulose
- Use of deep eutectic solvents as catalyst: A mini-review
- Microwave-assisted synthesis of pyrrolidinone derivatives using 1,1’-butylenebis(3-sulfo-3H-imidazol-1-ium) chloride in ethylene glycol
- Green and eco-friendly synthesis of Co3O4 and Ag-Co3O4: Characterization and photo-catalytic activity
- Adsorption optimized of the coal-based material and application for cyanide wastewater treatment
- Aloe vera leaf extract mediated green synthesis of selenium nanoparticles and assessment of their In vitro antimicrobial activity against spoilage fungi and pathogenic bacteria strains
- Waste phenolic resin derived activated carbon by microwave-assisted KOH activation and application to dye wastewater treatment
- Direct ethanol production from cellulose by consortium of Trichoderma reesei and Candida molischiana
- Agricultural waste biomass-assisted nanostructures: Synthesis and application
- Biodiesel production from rubber seed oil using calcium oxide derived from eggshell as catalyst – optimization and modeling studies
- Study of fabrication of fully aqueous solution processed SnS quantum dot-sensitized solar cell
- Assessment of aqueous extract of Gypsophila aretioides for inhibitory effects on calcium carbonate formation
- An environmentally friendly acylation reaction of 2-methylnaphthalene in solvent-free condition in a micro-channel reactor
- Aegle marmelos phytochemical stabilized synthesis and characterization of ZnO nanoparticles and their role against agriculture and food pathogen
- A reactive coupling process for co-production of solketal and biodiesel
- Optimization of the asymmetric synthesis of (S)-1-phenylethanol using Ispir bean as whole-cell biocatalyst
- Synthesis of pyrazolopyridine and pyrazoloquinoline derivatives by one-pot, three-component reactions of arylglyoxals, 3-methyl-1-aryl-1H-pyrazol-5-amines and cyclic 1,3-dicarbonyl compounds in the presence of tetrapropylammonium bromide
- Preconcentration of morphine in urine sample using a green and solvent-free microextraction method
- Extraction of glycyrrhizic acid by aqueous two-phase system formed by PEG and two environmentally friendly organic acid salts - sodium citrate and sodium tartrate
- Green synthesis of copper oxide nanoparticles using Juglans regia leaf extract and assessment of their physico-chemical and biological properties
- Deep eutectic solvents (DESs) as powerful and recyclable catalysts and solvents for the synthesis of 3,4-dihydropyrimidin-2(1H)-ones/thiones
- Biosynthesis, characterization and anti-microbial activity of silver nanoparticle based gel hand wash
- Efficient and selective microwave-assisted O-methylation of phenolic compounds using tetramethylammonium hydroxide (TMAH)
- Anticoagulant, thrombolytic and antibacterial activities of Euphorbia acruensis latex-mediated bioengineered silver nanoparticles
- Volcanic ash as reusable catalyst in the green synthesis of 3H-1,5-benzodiazepines
- Green synthesis, anionic polymerization of 1,4-bis(methacryloyl)piperazine using Algerian clay as catalyst
- Selenium supplementation during fermentation with sugar beet molasses and Saccharomyces cerevisiae to increase bioethanol production
- Biosynthetic potential assessment of four food pathogenic bacteria in hydrothermally silver nanoparticles fabrication
- Investigating the effectiveness of classical and eco-friendly approaches for synthesis of dialdehydes from organic dihalides
- Pyrolysis of palm oil using zeolite catalyst and characterization of the boil-oil
- Azadirachta indica leaves extract assisted green synthesis of Ag-TiO2 for degradation of Methylene blue and Rhodamine B dyes in aqueous medium
- Synthesis of vitamin E succinate catalyzed by nano-SiO2 immobilized DMAP derivative in mixed solvent system
- Extraction of phytosterols from melon (Cucumis melo) seeds by supercritical CO2 as a clean technology
- Production of uronic acids by hydrothermolysis of pectin as a model substance for plant biomass waste
- Biofabrication of highly pure copper oxide nanoparticles using wheat seed extract and their catalytic activity: A mechanistic approach
- Intelligent modeling and optimization of emulsion aggregation method for producing green printing ink
- Improved removal of methylene blue on modified hierarchical zeolite Y: Achieved by a “destructive-constructive” method
- Two different facile and efficient approaches for the synthesis of various N-arylacetamides via N-acetylation of arylamines and straightforward one-pot reductive acetylation of nitroarenes promoted by recyclable CuFe2O4 nanoparticles in water
- Optimization of acid catalyzed esterification and mixed metal oxide catalyzed transesterification for biodiesel production from Moringa oleifera oil
- Kinetics and the fluidity of the stearic acid esters with different carbon backbones
- Aiming for a standardized protocol for preparing a process green synthesis report and for ranking multiple synthesis plans to a common target product
- Microstructure and luminescence of VO2 (B) nanoparticle synthesis by hydrothermal method
- Optimization of uranium removal from uranium plant wastewater by response surface methodology (RSM)
- Microwave drying of nickel-containing residue: dielectric properties, kinetics, and energy aspects
- Simple and convenient two step synthesis of 5-bromo-2,3-dimethoxy-6-methyl-1,4-benzoquinone
- Biodiesel production from waste cooking oil
- The effect of activation temperature on structure and properties of blue coke-based activated carbon by CO2 activation
- Optimization of reaction parameters for the green synthesis of zero valent iron nanoparticles using pine tree needles
- Microwave-assisted protocol for squalene isolation and conversion from oil-deodoriser distillates
- Denitrification performance of rare earth tailings-based catalysts
- Facile synthesis of silver nanoparticles using Averrhoa bilimbi L and Plum extracts and investigation on the synergistic bioactivity using in vitro models
- Green production of AgNPs and their phytostimulatory impact
- Photocatalytic activity of Ag/Ni bi-metallic nanoparticles on textile dye removal
- Topical Issue: Green Process Engineering / Guest Editors: Martine Poux, Patrick Cognet
- Modelling and optimisation of oxidative desulphurisation of tyre-derived oil via central composite design approach
- CO2 sequestration by carbonation of olivine: a new process for optimal separation of the solids produced
- Organic carbonates synthesis improved by pervaporation for CO2 utilisation
- Production of starch nanoparticles through solvent-antisolvent precipitation in a spinning disc reactor
- A kinetic study of Zn halide/TBAB-catalysed fixation of CO2 with styrene oxide in propylene carbonate
- Topical on Green Process Engineering

