Abstract
Influence of the process parameters for the industrially relevant reaction of free fatty acid (FFA) with glycerol is investigated. Furthermore, several drying techniques are investigated and a novel method is suggested that can provide more realistic experimental conditions. Silica as an absorbent is found to be a more suitable method for water removal than distillation or carrier gas. Using response surface methodology, important parameters are identified and optimal conditions found. Empirical correlation is developed to account for the most important parameters. Both oil:glycerol ratio and temperature have optimal values for which the highest conversion can be achieved. Interestingly, the highest conversion can be obtained at 220°C; above this temperature the conversion decreases. It is found that the influence of oil:glycerol ratio also exhibits anomalous behavior, where conversion is constant and decreases above a certain value. At optimal conditions, the FFA is reduced to 1.6% from the initial 8.6%.
1 Introduction
Diminishing reserves of crude oil, increasing cost and environmental impact of fossil fuels encourage research to find suitable substitutes that replace or at least reduce their use. The usage of biodiesel, as one of the alternatives to fossil diesel, is still marginal due to its relatively high production costs and limited feedstock availability [1], [2]. Considering that the price of oil feedstock has the highest impact on the production cost of biodiesel [3], there are several proposals to find an alternative feedstock which would lower production costs (e.g. used cooking oil, oil from algae) [4]. Using waste oil as a raw material can lower production costs and environmental footprint and increase the sustainability of biodiesel as a fuel [5]. However, used cooking oil contains various amounts of water, free fatty acids (FFA) and food residues which lower its value as a biodiesel feedstock. Based on its FFA content, waste oil can be roughly divided into two categories: yellow grease, which has <15% FFA, and brown grease, which contains more than 15% FFAs [6], [7]. There is a drastic difference between the price and environmental impact of yellow and brown grease. The price of yellow grease is high, around 0.2–0.3 dollars per kg [8], whereas brown grease is usually collected for a small fee or free from households, restaurants and wastewater treatment plants [7]. Therefore, it is reasonable to assume that using brown grease as a biodiesel feedstock can make the process more cost effective. Furthermore, by using used oil, potentially hazardous waste is utilized in an efficient and environmentally friendly way.
Homogeneous basic catalysis is commonly used for biodiesel production in industrial processes. However, it is impossible to use waste oil with high FFA content in this process, due to saponification reactions. Soaps are formed from the reaction of FFAs with sodium or potassium ions from the catalyst. Soaps are difficult to separate from biodiesel and act as emulsifier forming stable glycerol/biodiesel emulsions [9], [10], [11]. A commonly applied solution for transesterification of oil with high FFA content is to use homogeneous acid catalysis esterification as a pretreatment step to reduce the FFA content in the feedstock. However, this process requires high temperatures (above 100°C) and high pressures (usually 1.7–2 bar), while the methanol:oil ratio is usually above 40:1 [12]. Because of the slow reaction, the process requires long reaction times and strong acids for oils with high FFA content, and it is necessary to use large and expensive specialized process equipment to avoid corrosion. Furthermore, in the homogenous acid catalyzed process, the oil has to be periodically dehydrated, since water is formed during the esterification. The presence of water slows down the reaction and promotes the formation of carboxylic acids. After the pretreatment step the acid has to be neutralized with base before the basic homogeneous transesterification is applied. Consequently, relatively large amounts of waste salts and wastewater are produced from washing.
There are some novel solutions for processing waste oil with high FFA and water content, most notably the enzyme catalyzed and the supercritical transesterification. Supercritical transesterification is virtually insensitive to FFA and water, since no catalyst is used. The process is characterized by a fast reaction; however, it requires high temperatures (above 300°C), pressures (above 100 bar) and methanol to oil molar ratio (approximately 42:1). Furthermore, relatively low yields are achieved compared to other processes, with maximum yield around 95% [11], [13].
Enzyme catalyzed transesterification has many disadvantages. Lipases are very sensitive to the type of alcohol; glycerin can inhibit the reaction, and require very specific operation parameters. Furthermore, they are expensive and their immobilization on carriers and effective reuse are yet to be solved [4].
Glycerol is a co-product of biodiesel production. As a consequence of increased biodiesel production, large quantities of glycerol are produced globally. This has led to the chronic oversaturation of the market and low prices of glycerol [14]. Several novel green products have been suggested recently, with the aim to integrate glycerol in biorefinery processes and obtain value added products [15], [16]. For this reason, glycerolysis is investigated in this paper as a method to valorize glycerol. Glycerolysis may refer to the esterification reaction of glycerol with FFAs to obtain glycerides [17], [18] in the presence of a catalyst or transesterification of fatty acid methyl esters with glycerol [19]. Glycerolysis reaction can be used for the selective production of monoglycerides, as alternative green and less toxic surfactant and to lower the FFA content in waste oil, which can be further processed into biodiesel using conventional processes [17], [20]. Replacing acid catalyzed pretreatment with glycerolysis eliminates the need for high methanol:oil ratio, strong acids, corrosion resistant equipment and long reaction times [10], [21]. Nevertheless, glycerolysis still requires relatively high temperatures of above 200°C. This can be remedied by optimizing process parameters, to use the lowest possible amount of glycerol and temperature for the highest conversion.
When using glycerolysis in a two-step biodiesel production process, in the first step, the oil is treated with glycerol to lower the FFA content, while in the second step, conventional basic homogeneous transesterification is performed. The main advantage of this concept is that the currently existing production facilities can be used to process waste oil, with only little modifications. There are only a few studies which have investigated the possibility of FFA reduction in oil by glycerolysis. For example Felizardo et al. [20] obtained approximately 90% FFA reduction from an oil feedstock initially containing 20–50% FFA in <3 h at 200°C and an oil:glycerol ratio 2:1.
In the first step of the glycerolysis reaction, the FFA reacts with glycerol to form the corresponding monoglyceride; afterwards, diglycerides and triglycerides are also formed according to reactions 2–6:
Many more complex reactions are possible if we take into consideration that there are several types of fatty acids in the oil (R groups). This system has several hundred reversible reactions, making detailed kinetic modeling a difficult task [19], [22], [23].
2 Materials and methods
2.1 Materials
For the experiments, aged sunflower seed oil was used. The oil was exposed to humidity and high temperature for a year. This resulted in its degradation and the acid number increased to 17.2 mg KOH g−1. Oil with such a high acid number is not suitable for human or animal consumption. The oil was analyzed with Clarus 500 gas chromatograph according to standard ISO 5509:2007 and ISO 5508:2009. The oil used in the experiments contained 9.36% saturated, 28.42% unsaturated and 60.18% polyunsaturated fatty acids. The calculated molar mass is 878.55 g mol−1, whereas the iodine number is 131.827 g iodine·100 g−1. Some physical and chemical properties of the oil used in the experiment are given in Table 1.
Physical and chemical properties of the oil used in the experiment.
| Characteristic | Units | Value | Method |
|---|---|---|---|
| Density (20°C) | g cm−3 | 0.9075 | EN ISO 3675 |
| Viscosity (40°C) | mm2 s−1 | 15.01 | EN ISO 3104 |
| Water content | % | 0.01 | ISO 665 and 662 |
| Acid value | mg KOH g−1 | 17.2 | ISO 660 |
| Solid content | % | 0.29 | ISO 663 |
| Iodine value | g I·100 g−1 | 131.827 | EN 14214 |
| Fatty acid composition | |||
| Myristic (C14:0) | wt% | 0.075 | |
| Palmitic (C16:0) | wt% | 5.953 | |
| Stearic (C18:0) | wt% | 2.415 | |
| Oleic (C18:1) | wt% | 28.28 | |
| Linoleic (C18:2n6c) | wt% | 61.851 | |
| Linolenic (C18:3n3) | wt% | 0.056 | |
| Arachidic (C20:0) | wt% | 0.201 | |
| Eicosenoic (C20:1) | wt% | 0.186 | |
| Behenic (C22:0) | wt% | 0.611 | |
| Erucic (C22:1n9) | wt% | 0.122 | |
| Lignoceric (C24:0) | wt% | 0.243 | |
The glycerolysis reaction was performed using pure glycerol (obtained from Mina-Cosmico, Valjevo, Serbia) in the presence of anhydrous ZnCl2 as the catalyst (obtained from Centrohem doo, Stara Pazova, Serbia) and silica gel with bead size 2–5 mm (obtained from Centrohem doo, Stara Pazova, Serbia) to remove the water formed during the reaction.
2.2 Experimental
The experiments were performed in a 2 l batch reactor Anton Parr 4520. Firstly, the oil had been added to the reactor and heated to the desired temperature. Then, the required amount of pure glycerin and catalyst (catalyst loading 0.15%) were added and this moment was considered as the start of the reaction. During the reaction, the reaction mixture was kept at constant temperature and pressure with intensive mixing (670 rpm). It is found that changing the mixing speed did not influence the conversion profiles, i.e. the reaction is not limited by mass transfer. Acid number is measured every 15 min and the end of the reaction is considered when there is no change in the acid number. After the reaction time, the samples and the reactor content is transferred to a gravity separator for 8 h where the oil is separated from the unreacted glycerol. The obtained oil phase is centrifuged and the acid number (Ano) is measured by titration. FFA concentration is calculated using the following formula:
According to reactions 1–3, for every mole of FFA consumed in the reaction, a mole of water is formed. A suitable method had to be found to selectively remove water from the reaction, since the presence of water favors hydrolysis, which is the reverse of the glycerolysis reaction. Exploratory experiments were performed at 170°C, oil:glycerol mass ratio 6:1 and 0.15% ZnCl2 catalyst to test and compare the effect of water and its removal methods. The following possible approaches were tested and compared:
Silica gel is introduced in the reaction mixture at the start of the reaction to in situ absorb the water (Figure 1D). This method is for the first time investigated here.
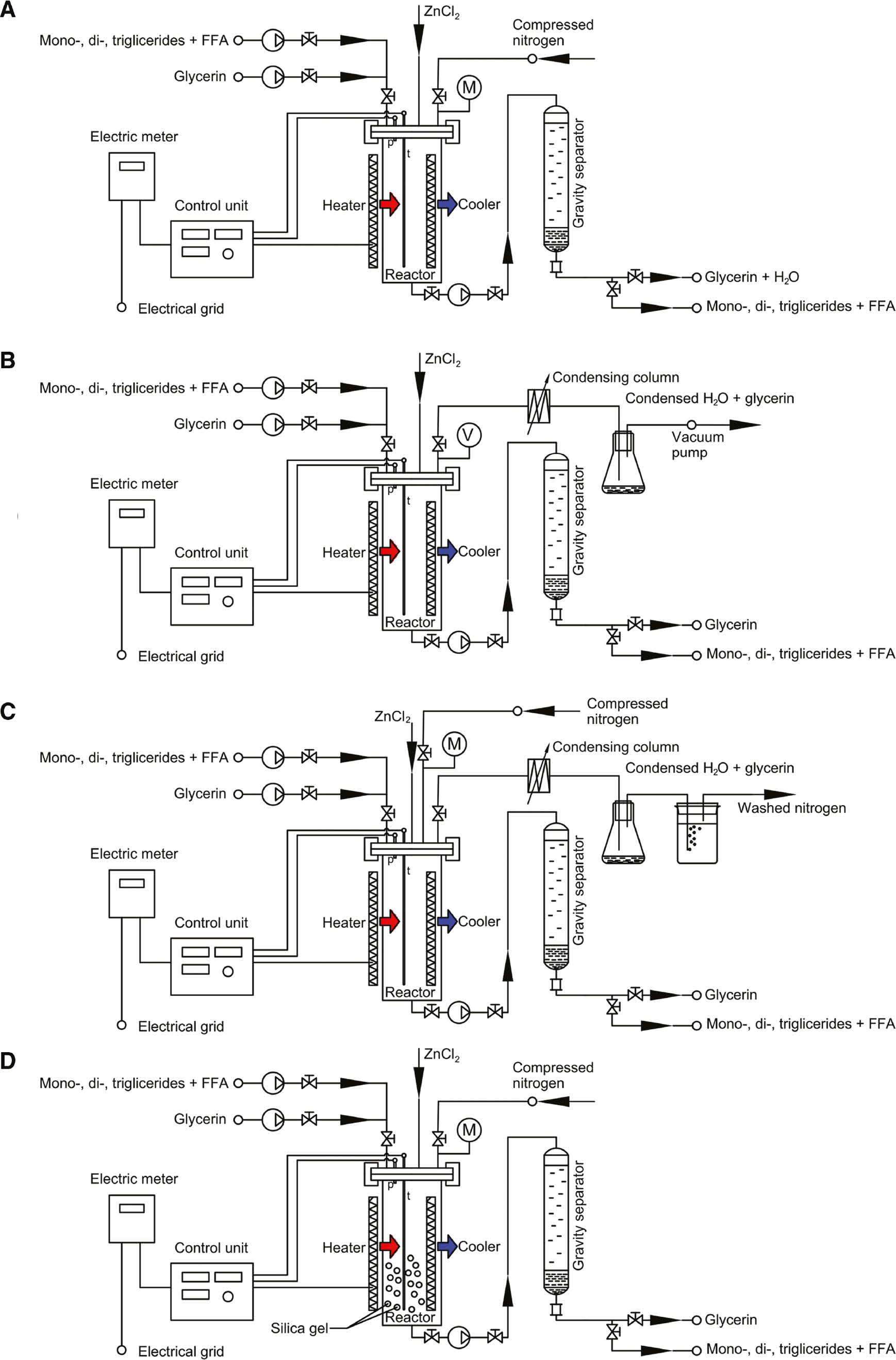
Different experimental set-up and water removal strategies: (A) without removing water, (B) vacuum distillation, (C) drying with nitrogen as sweep gas and (D) using silica gel as absorbent.
To investigate the effect of FFA reduction on biodiesel conversion, standard basic transesterification was carried out using 500 g of the obtained oil, 100 ml of methanol and 3 g of KOH at 65°C for 45 min at different FFA concentrations. The biodiesel obtained this way was separated and washed before measuring the conversion (wt%).
2.3 Statistical analysis
To investigate the effects of temperature, oil to glycerol mass ratio and pressure, a semi-factorial experimental design was performed with temperature having three levels (195°C, 220°C and 240°C), oil to glycerol mass ratio also having three levels (4.5:1, 6:1, 9:1) which roughly correspond to molar ratio (1:1, 1:1.5 and 1:2), and pressure having two levels (1.4 bar and 3 bar). Data also included several exploratory runs to test possible interferences of several factors. Data processing, analysis and optimization were done using programming language R v.3.2.5. and the necessary packages. To determine the relationship between the variables and predict the response, the data is regressed by response surface methodology that accounts for the first and second order and interaction terms using equation:
where β0, βi, βii and βij are the regression coefficients for the intercept, linear, quadratic and interaction terms, respectively, Xi stands for the process variables and ŷ is the predicted response. The regression coefficients are fitted to produce a model with the lowest difference between the experimental and the predicted response [25].
3 Results and discussion
3.1 Water removal strategies
During the esterification of FFA with glycerol, water is formed which significantly slows down and eventually stops the reaction, due to unfavorable chemical equilibrium and favoring the reverse reaction. For this reason to investigate this reaction a continuous method which selectively removes water has to be used. The results of the four different water removal strategies are shown in Figure 2, with all the experiments conducted at identical conditions.
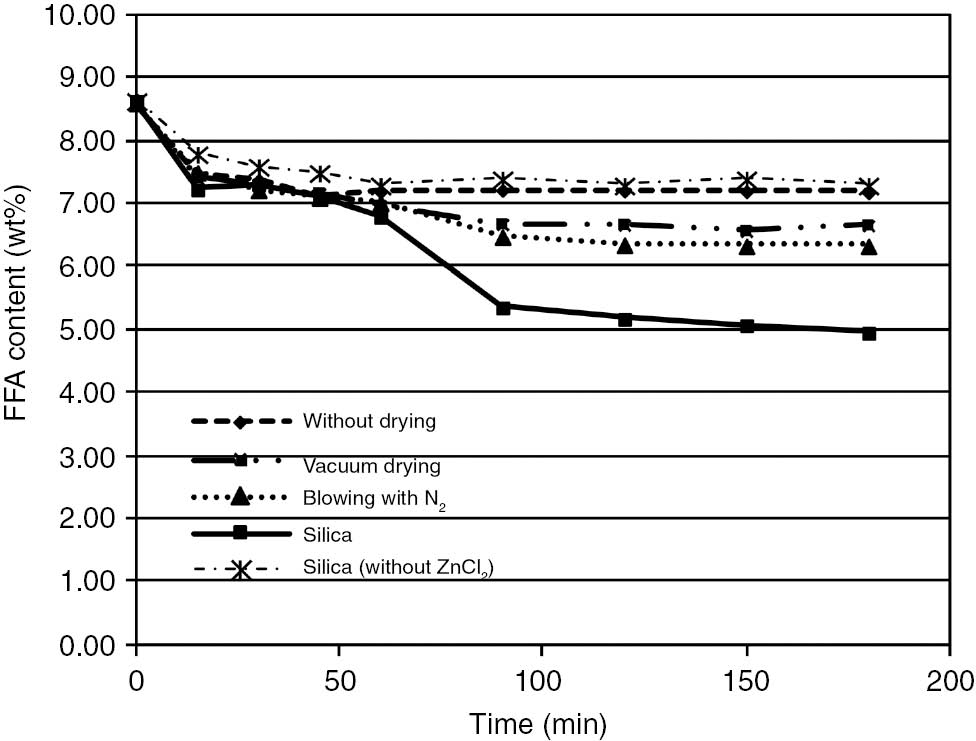
Influence of water removal methods on free fatty acid (FFA) reduction (T=170°C, p=3 bar, oil:glycerol mass ratio=6:1).
As can be seen from Figure 2, in the first 45 min the reaction rates and conversions are identical. As the reaction advances, water is formed and if it is not removed equilibrium is reached and the FFA concentration remains constant. Without removing water, FFA concentration is lowered only by 1.4%, i.e. 14.5% conversion is achieved and after 1 h, FFA concentration slightly rises due to reverse hydrolysis.
Removing water by drying with vacuum distillation or nitrogen drying slightly improves results, achieving reduction of FFA by 22.8% and 26.7%, respectively. Distillation is a highly inefficient method for removing water from this system. Due to the low amount of water present in the system, the vapor phase is mostly glycerol (approximately 90%) and multistage vacuum distillation would be required to separate water from glycerol. Furthermore, high vacuum is required since the boiling point of the reaction mixture is higher than the optimal reaction temperature. Even at the highest conversion, the maximum water content is 4% and the boiling point of this mixture is 250°C at atmospheric pressure [26]. In the case of vacuum distillation, the reaction stops due to the gradual removal of glycerol.
In the third approach, i.e. by drying with nitrogen, a similar problem as in distillation is encountered. From the reaction mixture not only water is carried away with nitrogen, but also glycerin, even at low flow rates. After blowing just 0.05 Nm3 of nitrogen through the system, more than half of the glycerol (17 g) is carried away in the gas phase. At high nitrogen flow rate, glycerin is quickly carried away in the form of mist and the reaction stops. This was also observed by Singh et al. [22], where they misinterpreted that the reaction mixture is boiling when in fact only glycerin is carried away. Low water concentration makes drying with nitrogen or distillation not practical for water removal during the glycerolysis, since the reactants are carried away from the system. It was further observed in [23] that FFA increased and the oil took a brownish color upon increasing the N2 flow.
After testing several absorbents (anhydrous CaO and Na2SO4), the best FFA reduction effect was achieved with silica. Other absorbents reacted with the ZnCl2 and interfered with the analysis. At the same conditions as before, 35.7% FFA conversion is achieved with silica absorbent. During this time, the system did not reach equilibrium and further FFA reduction could be expected from the trend in Figure 2.
To investigate if the lowering of FFA concentration was not simply due to absorption of FFA on silica, a test was carried out without glycerol in the reaction system. In this case, the FFA concentration slightly increased over time, eliminating the possibility of this phenomenon (Figure 4). Since silica might contain functional groups (e.g. -OH) the reaction was also carried out without ZnCl2 in the system to verify that the silica used does not act also as a catalyst. In the absence of ZnCl2, the conversion remains very low in the observed time period excluding the possibility of catalytic influence (Figure 2). For these reasons, silica was chosen as the absorbent for water removal from the reaction system in further experiments.
3.2 Influence of reaction parameters on FFA reduction
To determine the influence of experimental parameters on conversion and reaction speed, further experiments were conducted using the set-up in Figure 1D and silica as absorbent. The experimental design included temperature, oil:glycerol mass ratio and pressure.
3.2.1 Influence of temperature on FFA reduction
The influence of different reaction temperatures on FFA reduction was investigated at three levels, 195°C, 220°C and 240°C, and data from the exploratory experiments at 170°C were also included in the analysis. The influence of temperature on FFA reduction is shown in Figure 3 for constant oil:glycerol mass ratio (6:1) and pressure (3 bar). It can be seen that equilibrium conversion exhibits a typical volcano shape. FFA conversion increases with temperature, however, above a certain value it decreases with increasing temperature. This is observed considering the whole reaction mixture. However, due to the highly complex nature of the reaction system with several parallel and consecutive reactions, it is difficult to determine the activation energies and kinetic parameters of individual reaction steps (similar observations were made by, e.g. [19]).
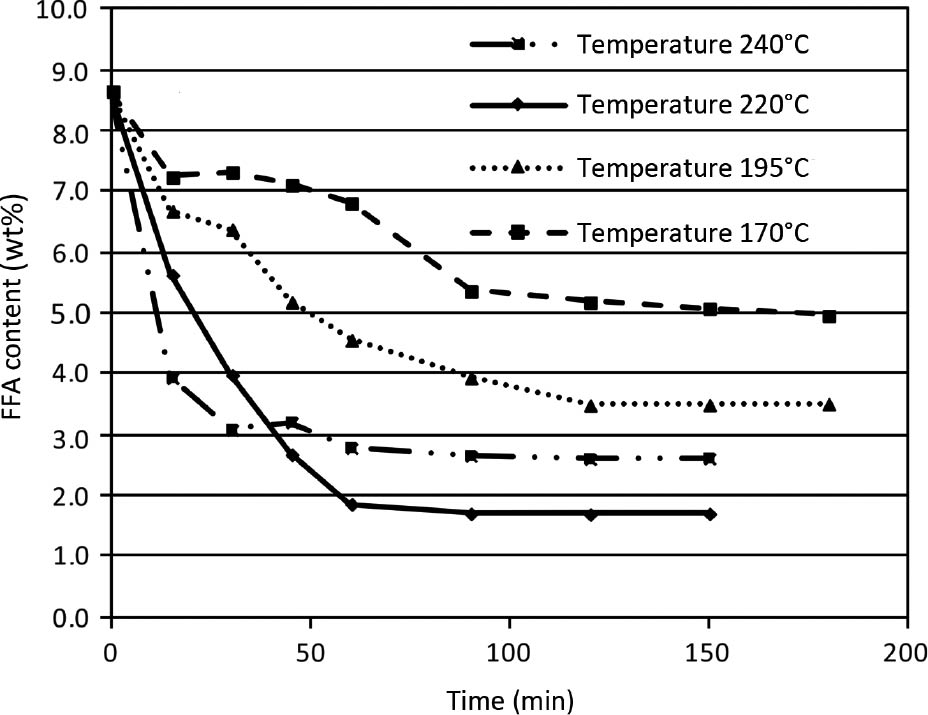
Influence of temperature on reaction speed and equilibrium conversion of free fatty acid (FFA) (oil:glycerol mass ratio=6:1 and p=3 bar).
3.2.2 Influence of oil:glycerol mass ratio on FFA reduction
The influence of different oil:glycerol mass ratios (4.5:1, 6:1 and 9:1) on FFA reduction is depicted in Figure 4. The excess glycerol also ensures that potential side reactions do not interfere with the main reaction. Indeed, the obtained oil from glycerolysis exhibited similar physical characteristics as pure oil: the color changed from turbid brown to clear yellow and viscosity decreased notably. Since all the reactions (1–5) are reversible, excess glycerol is required to drive the reaction to the product side. Increasing the glycerol concentration has a positive effect on equilibrium conversion, however, after a certain point equilibrium conversion slightly decreases, which is in line with observations by [18], [20], [22], [27]. It is suggested that this is because of reactions 4 and 5 where monoglycerides and diglycerides are produced from triglycerides. This means that the solubility of glycerol in the ester phase is not constant during the reaction, but increases, as mono and diglycerides are formed. After all the triglycerides are consumed the solubility of glycerol remains constant. This is further supported by the fact that the optimal oil:glycerol ratio (in this work and see, e.g. [18], [20], [22], [27]) is where the triglyceride conversion reaches equilibrium [23] and it is influenced only slightly by the initial FFA concentration.
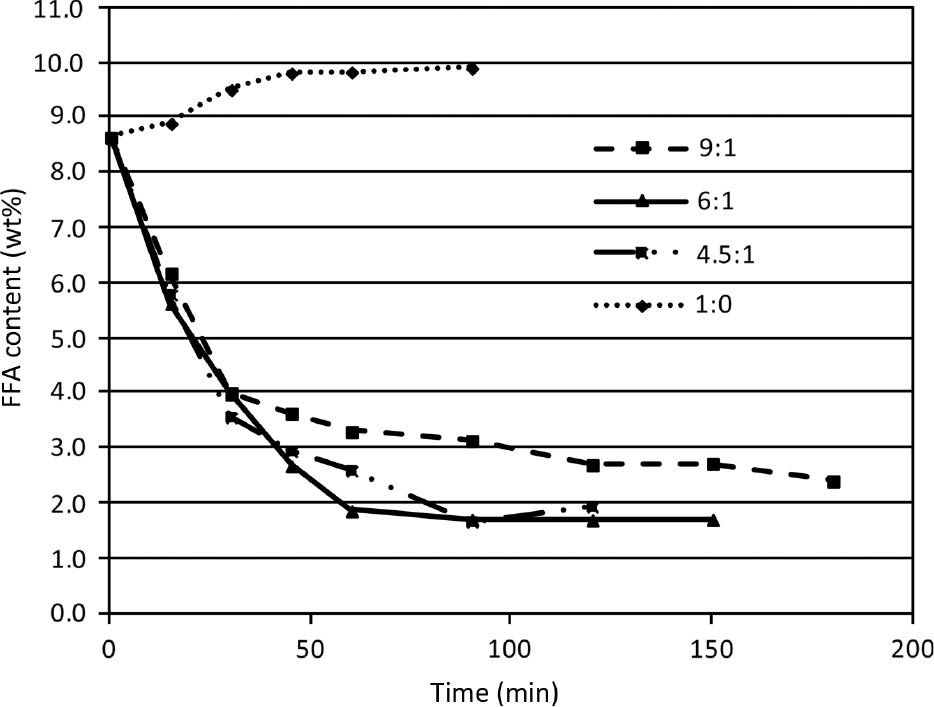
Influence of oil:glycerol mass ratio on free fatty acid (FFA) reduction (T=220°C, p=3 bar).
The reaction takes place in the glycerol-oil contact phase [19] and increasing the liquid-liquid surface increases the phase transfer of the reactants and the reaction rate, however, not the equilibrium conversion. This is not only due to better reactant contact, but increasing the hydrophilic glycerol phase enhances the water extraction and removal from the oil phase, shifting the reaction to the desired products in reactions 1–3.
It is desirable to use the lowest possible amount of glycerol with the highest conversion, since glycerol has to be separated from the oil before transesterification. When there is no glycerol in the reaction mixture, FFA concentration increases (Figure 4) eliminating the possibility of FFA absorption on silica.
3.2.3 Influence of pressure on FFA reduction
Pressure has no influence on FFA conversion in the observed range as can be seen in Figure 5. This is because the investigated temperature range is well below the boiling point of the whole reaction mixture, which is 250°C at 1 bar. Pressure would have an influence only if is too low or higher than the critical pressure. When low pressure is applied, glycerol starts to boil and if the system is not closed, it is carried away and the reaction comes to a halt.
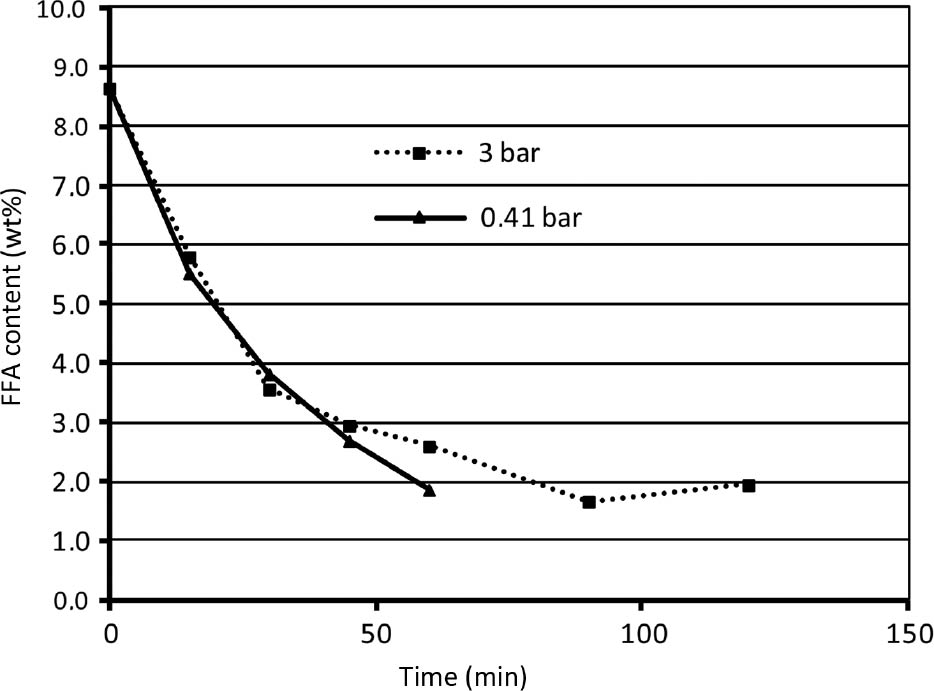
Influence of pressure on free fatty acid (FFA) reduction (T=220°C, oil:glycerol mass ratio 6:1).
3.2.4 Influence of FFA reduction on biodiesel yield
There is a clear relationship between the FFA reduction and biodiesel yield. With the untreated oil containing 10.5% FFA the biodiesel yield was just 40%, while for the treated oil that had 2% FFA content, the biodiesel yield increased to 76%. This relation with several other data points can be seen in Figure 6. Furthermore, it should be noted that with oils with high FFA concentration, soap formed during the reaction and the resulting mixture was a stable emulsion. It was more difficult to separate biodiesel from the product mixture and took a longer time to decant. Soap formation was very fast at higher KOH concentration.
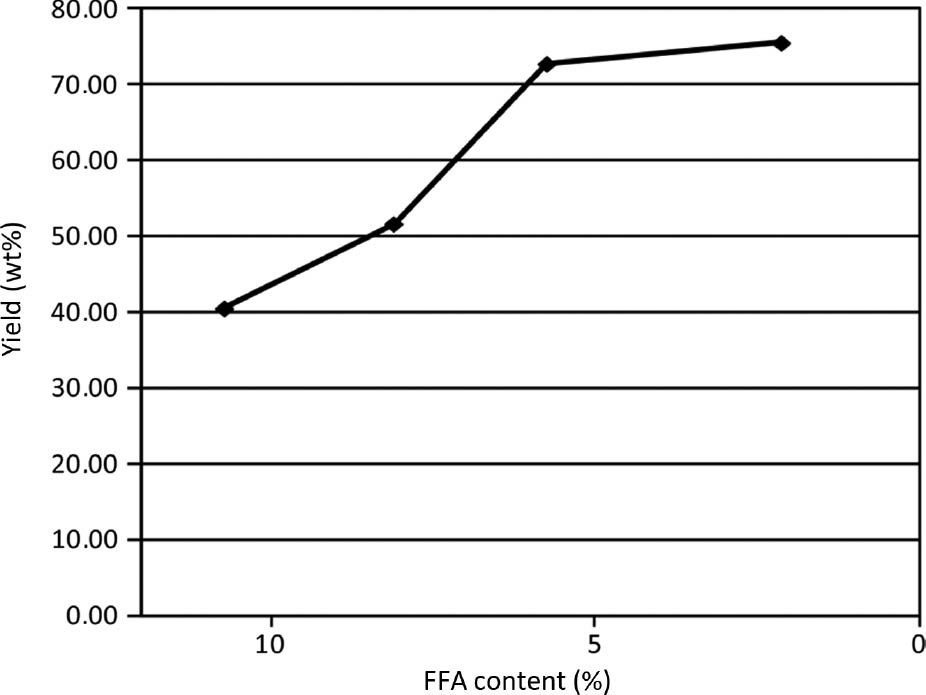
Influence of free fatty acid (FFA) concentration on biodiesel yield (T=65°C, t=45 min, 0.5 wt% KOH).
3.3 Optimization of process parameters
Since in the observed range pressure has negligible influence on conversion, only the effects of temperature and oil:glycerol molar ratio are modeled with the response surface method. A second order polynomial fitted the data well, producing a predictive equation with coefficient of regression R2=0.9874 and p-value <0.01. The regression coefficients and corresponding p-values are shown in Table 2, and the plots are visualized in Figure 7.
Regressional coefficients of response surface methodology.
| Coefficient (β) | p-Value | |
|---|---|---|
| Intercept | 132.31 | 5.620×10−7 |
| Temperature, °C (X1) | −1.1443 | 5.581×10−7 |
| Oil:glycerol mass ratio (X2) | −1.1835 | 0.0159367 |
| X1:X2 | 6.2016×10−4 | 0.6278871 |
| X12 | 2.5662×10−3 | 4.787×10−7 |
| X22 | 8.9233×10−2 | 8.848×10−4 |
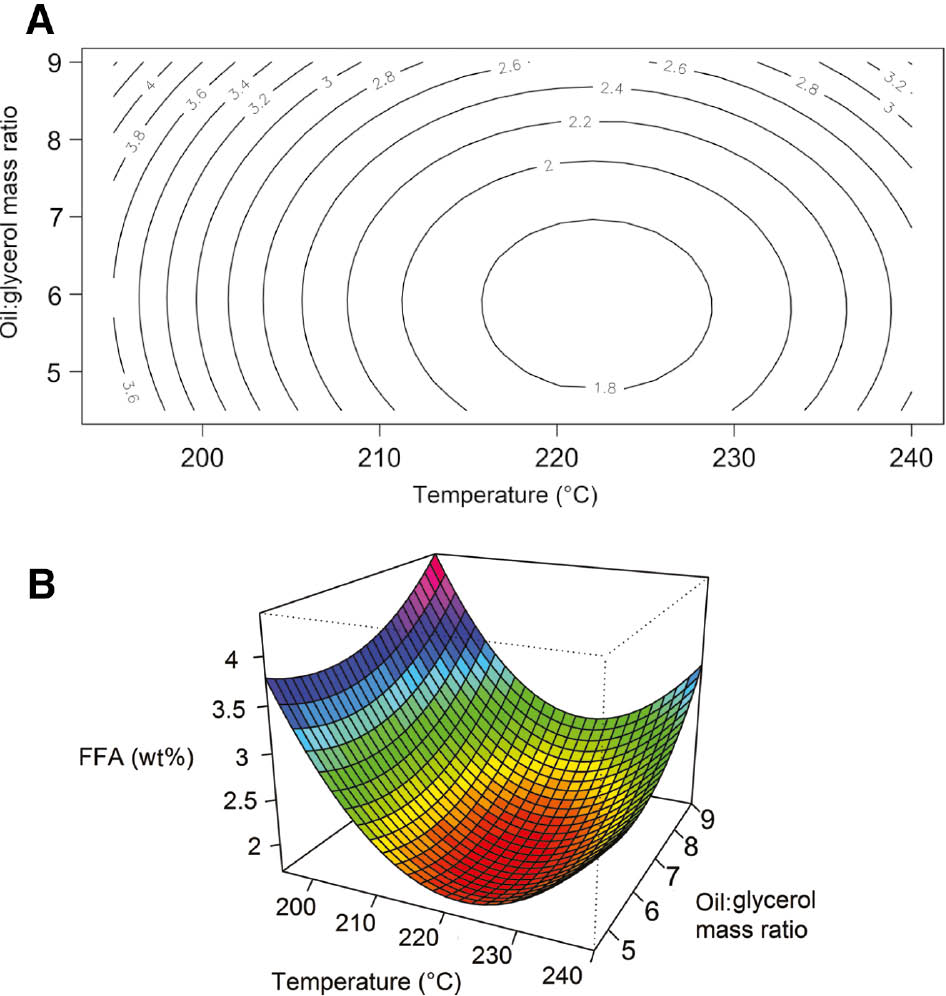
Influence of temperature and oil:glycerol ratio on free fatty acid (FFA) reduction: (A) the contour lines are of constant FFA concentration (%) and (B) perspective plot of the modeled system.
While this regression model is purely empirical, useful conclusions can be drawn from it. We can see from the coefficient and high p-value of the interaction term that the interaction of temperature and oil:glycerol mass ratio has negligible influence on FFA reduction. The linear and quadratic terms of the two main variables are highly significant, with temperature having a much larger influence on conversion than glycerol concentration in the observed region.
As mentioned in the previous section, the influence of temperature has a volcano shape and can be clearly seen in Figure 6. Equilibrium rules dictate that up to this point the overall reaction was endothermic and conversion increases with a higher temperature. However, at a higher temperature, the reaction is exothermic and with further increase in the temperature the reversible reaction prevails and FFA conversion decreases. The highest FFA reduction can be determined from the stationary point in Figure 6 with optimal temperature for this system 222°C and oil:glycerol mass ratio 5.86, where FFA concentration is reduced to 1.6% from the initial 8.6%, i.e. 81% conversion is achieved.
4 Conclusions
In the present paper, the effects of the most important process parameters, temperature, oil:glycerol ratio, pressure and water removal strategies, on the esterification reaction of FFA with glycerol were investigated. Interesting behavior was observed for temperature, whereby at 222°C the largest conversion of FFA was achieved and it decreased above this point. Oil:glycerol mass ratio also exhibited somewhat unexpected behavior, where the conversion rises until the ratio is 5.86 and stays constant (and even slightly decreases above). The quadratic response surface method provided a reliable method for the qualitative and quantitative analysis of the reaction system, further suggesting a higher importance of temperature on conversion and interestingly the lack of interaction between the process parameters. Further investigation is necessary to account for these phenomena, i.e. descriptions of kinetics, thermodynamics, and liquid-liquid equilibriums of this multiphase reacting system.
Water produced during the reaction is observed to strongly inhibit the desired reaction. Vacuum distillation and nitrogen purging are found to be unsuitable methods to remove water, since glycerol is also carried away from the reaction mixture. Using silica as neutral absorbent, a much higher conversion is achieved and water is quickly removed. It is important to note that FFA is not absorbed on silica. Glycerolysis is found to be a viable and economically justified pretreatment step of waste oil with high FFA content.
Acknowledgements
This paper was realized as a part of projects OI-172059 and TR-31046 financed by the Ministry of Education, Science and Technological Development of the Republic of Serbia.
References
[1] EIA. International Energy Outlook 2016. https://www.eia.gov/outlooks/ieo/pdf/0484 (2016).pdf. Accessed Jul 29, 2017.Search in Google Scholar
[2] EIA. Monthly Biodiesel Production Report–With data for April 2017. https://www.eia.gov/biofuels/biodiesel/production/biodiesel.pdf. Accessed Jul 29, 2017.Search in Google Scholar
[3] Kiss FE, Jovanovic M, Boskovic GC. Fuel Process. Technol. 2010, 91, 1316–1320.10.1016/j.fuproc.2010.05.001Search in Google Scholar
[4] Lam MK, Lee KT, Mohamed AR. Biotechnol. Adv. 2010, 28, 500–518.10.1016/j.biotechadv.2010.03.002Search in Google Scholar PubMed
[5] Tomic M, Micic R, Kiss F, Dedovic N, Simikic M. Energy Convers. Manage. 2015, 99, 8–19.10.1016/j.enconman.2015.04.010Search in Google Scholar
[6] Hums ME, Cairncross RA, Spatari S. Environ. Sci. Technol. 2016, 50, 2718–2726.10.1021/acs.est.5b02667Search in Google Scholar PubMed
[7] URS Corporation, San Francisco Public Utilities Commission. Brown grease recovery and biofuel demonstration project. https://sfwater.org/modules/showdocument.aspx?documentid=4187. Accessed Jul 29, 2017.Search in Google Scholar
[8] United States Department of Agriculture Agricultural Marketing Service. National Weekly Ag Energy Round-Up. https://www.ams.usda.gov/mnreports/lswagenergy.pdf. Accessed Jul 29, 2017.Search in Google Scholar
[9] Ma F, Clements L, Hanna M. Trans. ASAE 1998, 41, 1261–1264.10.13031/2013.17292Search in Google Scholar
[10] Canakci M, Van Gerpen J. Trans. ASAE 2001, 44, 1429–1436.Search in Google Scholar
[11] Micic RD, Tomic MD, Kiss FE, Martinovic FL, Simikic M, Molnar TT. Energy Convers. Manage. 2016, 124, 377–388.10.1016/j.enconman.2016.07.043Search in Google Scholar
[12] Chai M, Tu Q, Lu M, Yang YJ. Fuel Process. Technol. 2014, 125, 106–113.10.1016/j.fuproc.2014.03.025Search in Google Scholar
[13] Hoang D, Bensaid S, Saracco G. Green Process. Synth. 2013, 2, 407–425.Search in Google Scholar
[14] Ciriminna R, Della Pina C, Rossi M, Pagliaro M. Eur. J. Lipid Sci. Technol. 2014, 116, 1432–1439.10.1002/ejlt.201400229Search in Google Scholar
[15] Luo X, Ge X, Cui S, Li Y. Bioresour. Technol. 2016, 215, 144–154.10.1016/j.biortech.2016.03.042Search in Google Scholar PubMed
[16] Yang F, Hanna MA, Sun R. Biotechnol. Biofuels 2012, 5, 13.10.1186/1754-6834-5-13Search in Google Scholar PubMed PubMed Central
[17] Islam A, Masoumi HRF, Teo SH, Abdollahi Y, Janaun J, Taufiq-Yap YH. Fuel 2016, 174, 133–139.10.1016/j.fuel.2016.01.088Search in Google Scholar
[18] Kombe GG, Temu AK, Rajabu HM, Mrema GD, Kansedo J, Lee KT. Adv. Chem. Eng. Sci. 2013, 3, 242–247.10.4236/aces.2013.34031Search in Google Scholar
[19] Negi DS, Sobotka F, Kimmel T, Wozny G, Schomäcker R. J. Am. Oil Chem. Soc. 2007, 84, 83–90.10.1007/s11746-006-1009-1Search in Google Scholar
[20] Felizardo P, MacHado J, Vergueiro D, Correia MJN, Gomes JP, Bordado JM. Fuel Process. Technol. 2011, 92, 1225–1229.10.1016/j.fuproc.2011.01.020Search in Google Scholar
[21] Leung DYC, Wu X, Leung MKH. Appl. Energy 2010, 87, 1083–1095.10.1016/j.apenergy.2009.10.006Search in Google Scholar
[22] Singh D, Patidar P, Ganesh A, Mahajani S. Ind. Eng. Chem. Res. 2013, 52, 14776–14786.10.1021/ie401636vSearch in Google Scholar
[23] Ferretti CA, Spotti ML, Di Cosimo JI. Catal. Today 2017. doi.org/10.1016/j.cattod.2017.04.008.doi.org/10.1016/j.cattod.2017.04.008Search in Google Scholar
[24] Gole VL, Gogate PR. Fuel Process. Technol. 2014, 118, 110–116.10.1016/j.fuproc.2013.08.018Search in Google Scholar
[25] Lenth RV. J. Stat. Software 2012, 32, 1–17.10.1007/BF03323511Search in Google Scholar
[26] Oliveira MB, Teles ARR, Queimada AJ, Coutinho JAP. Fluid Phase Equilib. 2009, 280, 22–29.10.1016/j.fluid.2009.03.011Search in Google Scholar
[27] Tu Q, Lu M, Knothe G. J. Cleaner Prod. 2017, 162, 504–511.10.1016/j.jclepro.2017.06.064Search in Google Scholar
©2019 Walter de Gruyter GmbH, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 Public License.
Articles in the same Issue
- Regular Articles
- Studies on the preparation and properties of biodegradable polyester from soybean oil
- Flow-mode biodiesel production from palm oil using a pressurized microwave reactor
- Reduction of free fatty acids in waste oil for biodiesel production by glycerolysis: investigation and optimization of process parameters
- Saccharin: a cheap and mild acidic agent for the synthesis of azo dyes via telescoped dediazotization
- Optimization of lipase-catalyzed synthesis of polyethylene glycol stearate in a solvent-free system
- Green synthesis of iron oxide nanoparticles using Platanus orientalis leaf extract for antifungal activity
- Ultrasound assisted chemical activation of peanut husk for copper removal
- Room temperature silanization of Fe3O4 for the preparation of phenyl functionalized magnetic adsorbent for dispersive solid phase extraction for the extraction of phthalates in water
- Evaluation of the saponin green extraction from Ziziphus spina-christi leaves using hydrothermal, microwave and Bain-Marie water bath heating methods
- Oxidation of dibenzothiophene using the heterogeneous catalyst of tungsten-based carbon nanotubes
- Calcined sodium silicate as an efficient and benign heterogeneous catalyst for the transesterification of natural lecithin to L-α-glycerophosphocholine
- Synergistic effect between CO2 and H2O2 on ethylbenzene oxidation catalyzed by carbon supported heteropolyanion catalysts
- Hydrocyanation of 2-arylmethyleneindan-1,3-diones using potassium hexacyanoferrate(II) as a nontoxic cyanating agent
- Green synthesis of hydratropic aldehyde from α-methylstyrene catalyzed by Al2O3-supported metal phthalocyanines
- Environmentally benign chemical recycling of polycarbonate wastes: comparison of micro- and nano-TiO2 solid support efficiencies
- Medicago polymorpha-mediated antibacterial silver nanoparticles in the reduction of methyl orange
- Production of value-added chemicals from esterification of waste glycerol over MCM-41 supported catalysts
- Green synthesis of zerovalent copper nanoparticles for efficient reduction of toxic azo dyes congo red and methyl orange
- Optimization of the biological synthesis of silver nanoparticles using Penicillium oxalicum GRS-1 and their antimicrobial effects against common food-borne pathogens
- Optimization of submerged fermentation conditions to overproduce bioethanol using two industrial and traditional Saccharomyces cerevisiae strains
- Extraction of In3+ and Fe3+ from sulfate solutions by using a 3D-printed “Y”-shaped microreactor
- Foliar-mediated Ag:ZnO nanophotocatalysts: green synthesis, characterization, pollutants degradation, and in vitro biocidal activity
- Green cyclic acetals production by glycerol etherification reaction with benzaldehyde using cationic acidic resin
- Biosynthesis, characterization and antimicrobial activities assessment of fabricated selenium nanoparticles using Pelargonium zonale leaf extract
- Synthesis of high surface area magnesia by using walnut shell as a template
- Controllable biosynthesis of silver nanoparticles using actinobacterial strains
- Green vegetation: a promising source of color dyes
- Mechano-chemical synthesis of ammonia and acetic acid from inorganic materials in water
- Green synthesis and structural characterization of novel N1-substituted 3,4-dihydropyrimidin-2(1H)-ones
- Biodiesel production from cotton oil using heterogeneous CaO catalysts from eggshells prepared at different calcination temperatures
- Regeneration of spent mercury catalyst for the treatment of dye wastewater by the microwave and ultrasonic spray-assisted method
- Green synthesis of the innovative super paramagnetic nanoparticles from the leaves extract of Fraxinus chinensis Roxb and their application for the decolourisation of toxic dyes
- Biogenic ZnO nanoparticles: a study of blueshift of optical band gap and photocatalytic degradation of reactive yellow 186 dye under direct sunlight
- Leached compounds from the extracts of pomegranate peel, green coconut shell, and karuvelam wood for the removal of hexavalent chromium
- Enhancement of molecular weight reduction of natural rubber in triphasic CO2/toluene/H2O systems with hydrogen peroxide for preparation of biobased polyurethanes
- An efficient green synthesis of novel 1H-imidazo[1,2-a]imidazole-3-amine and imidazo[2,1-c][1,2,4]triazole-5-amine derivatives via Strecker reaction under controlled microwave heating
- Evaluation of three different green fabrication methods for the synthesis of crystalline ZnO nanoparticles using Pelargonium zonale leaf extract
- A highly efficient and multifunctional biomass supporting Ag, Ni, and Cu nanoparticles through wetness impregnation for environmental remediation
- Simple one-pot green method for large-scale production of mesalamine, an anti-inflammatory agent
- Relationships between step and cumulative PMI and E-factors: implications on estimating material efficiency with respect to charting synthesis optimization strategies
- A comparative sorption study of Cr3+ and Cr6+ using mango peels: kinetic, equilibrium and thermodynamic
- Effects of acid hydrolysis waste liquid recycle on preparation of microcrystalline cellulose
- Use of deep eutectic solvents as catalyst: A mini-review
- Microwave-assisted synthesis of pyrrolidinone derivatives using 1,1’-butylenebis(3-sulfo-3H-imidazol-1-ium) chloride in ethylene glycol
- Green and eco-friendly synthesis of Co3O4 and Ag-Co3O4: Characterization and photo-catalytic activity
- Adsorption optimized of the coal-based material and application for cyanide wastewater treatment
- Aloe vera leaf extract mediated green synthesis of selenium nanoparticles and assessment of their In vitro antimicrobial activity against spoilage fungi and pathogenic bacteria strains
- Waste phenolic resin derived activated carbon by microwave-assisted KOH activation and application to dye wastewater treatment
- Direct ethanol production from cellulose by consortium of Trichoderma reesei and Candida molischiana
- Agricultural waste biomass-assisted nanostructures: Synthesis and application
- Biodiesel production from rubber seed oil using calcium oxide derived from eggshell as catalyst – optimization and modeling studies
- Study of fabrication of fully aqueous solution processed SnS quantum dot-sensitized solar cell
- Assessment of aqueous extract of Gypsophila aretioides for inhibitory effects on calcium carbonate formation
- An environmentally friendly acylation reaction of 2-methylnaphthalene in solvent-free condition in a micro-channel reactor
- Aegle marmelos phytochemical stabilized synthesis and characterization of ZnO nanoparticles and their role against agriculture and food pathogen
- A reactive coupling process for co-production of solketal and biodiesel
- Optimization of the asymmetric synthesis of (S)-1-phenylethanol using Ispir bean as whole-cell biocatalyst
- Synthesis of pyrazolopyridine and pyrazoloquinoline derivatives by one-pot, three-component reactions of arylglyoxals, 3-methyl-1-aryl-1H-pyrazol-5-amines and cyclic 1,3-dicarbonyl compounds in the presence of tetrapropylammonium bromide
- Preconcentration of morphine in urine sample using a green and solvent-free microextraction method
- Extraction of glycyrrhizic acid by aqueous two-phase system formed by PEG and two environmentally friendly organic acid salts - sodium citrate and sodium tartrate
- Green synthesis of copper oxide nanoparticles using Juglans regia leaf extract and assessment of their physico-chemical and biological properties
- Deep eutectic solvents (DESs) as powerful and recyclable catalysts and solvents for the synthesis of 3,4-dihydropyrimidin-2(1H)-ones/thiones
- Biosynthesis, characterization and anti-microbial activity of silver nanoparticle based gel hand wash
- Efficient and selective microwave-assisted O-methylation of phenolic compounds using tetramethylammonium hydroxide (TMAH)
- Anticoagulant, thrombolytic and antibacterial activities of Euphorbia acruensis latex-mediated bioengineered silver nanoparticles
- Volcanic ash as reusable catalyst in the green synthesis of 3H-1,5-benzodiazepines
- Green synthesis, anionic polymerization of 1,4-bis(methacryloyl)piperazine using Algerian clay as catalyst
- Selenium supplementation during fermentation with sugar beet molasses and Saccharomyces cerevisiae to increase bioethanol production
- Biosynthetic potential assessment of four food pathogenic bacteria in hydrothermally silver nanoparticles fabrication
- Investigating the effectiveness of classical and eco-friendly approaches for synthesis of dialdehydes from organic dihalides
- Pyrolysis of palm oil using zeolite catalyst and characterization of the boil-oil
- Azadirachta indica leaves extract assisted green synthesis of Ag-TiO2 for degradation of Methylene blue and Rhodamine B dyes in aqueous medium
- Synthesis of vitamin E succinate catalyzed by nano-SiO2 immobilized DMAP derivative in mixed solvent system
- Extraction of phytosterols from melon (Cucumis melo) seeds by supercritical CO2 as a clean technology
- Production of uronic acids by hydrothermolysis of pectin as a model substance for plant biomass waste
- Biofabrication of highly pure copper oxide nanoparticles using wheat seed extract and their catalytic activity: A mechanistic approach
- Intelligent modeling and optimization of emulsion aggregation method for producing green printing ink
- Improved removal of methylene blue on modified hierarchical zeolite Y: Achieved by a “destructive-constructive” method
- Two different facile and efficient approaches for the synthesis of various N-arylacetamides via N-acetylation of arylamines and straightforward one-pot reductive acetylation of nitroarenes promoted by recyclable CuFe2O4 nanoparticles in water
- Optimization of acid catalyzed esterification and mixed metal oxide catalyzed transesterification for biodiesel production from Moringa oleifera oil
- Kinetics and the fluidity of the stearic acid esters with different carbon backbones
- Aiming for a standardized protocol for preparing a process green synthesis report and for ranking multiple synthesis plans to a common target product
- Microstructure and luminescence of VO2 (B) nanoparticle synthesis by hydrothermal method
- Optimization of uranium removal from uranium plant wastewater by response surface methodology (RSM)
- Microwave drying of nickel-containing residue: dielectric properties, kinetics, and energy aspects
- Simple and convenient two step synthesis of 5-bromo-2,3-dimethoxy-6-methyl-1,4-benzoquinone
- Biodiesel production from waste cooking oil
- The effect of activation temperature on structure and properties of blue coke-based activated carbon by CO2 activation
- Optimization of reaction parameters for the green synthesis of zero valent iron nanoparticles using pine tree needles
- Microwave-assisted protocol for squalene isolation and conversion from oil-deodoriser distillates
- Denitrification performance of rare earth tailings-based catalysts
- Facile synthesis of silver nanoparticles using Averrhoa bilimbi L and Plum extracts and investigation on the synergistic bioactivity using in vitro models
- Green production of AgNPs and their phytostimulatory impact
- Photocatalytic activity of Ag/Ni bi-metallic nanoparticles on textile dye removal
- Topical Issue: Green Process Engineering / Guest Editors: Martine Poux, Patrick Cognet
- Modelling and optimisation of oxidative desulphurisation of tyre-derived oil via central composite design approach
- CO2 sequestration by carbonation of olivine: a new process for optimal separation of the solids produced
- Organic carbonates synthesis improved by pervaporation for CO2 utilisation
- Production of starch nanoparticles through solvent-antisolvent precipitation in a spinning disc reactor
- A kinetic study of Zn halide/TBAB-catalysed fixation of CO2 with styrene oxide in propylene carbonate
- Topical on Green Process Engineering
Articles in the same Issue
- Regular Articles
- Studies on the preparation and properties of biodegradable polyester from soybean oil
- Flow-mode biodiesel production from palm oil using a pressurized microwave reactor
- Reduction of free fatty acids in waste oil for biodiesel production by glycerolysis: investigation and optimization of process parameters
- Saccharin: a cheap and mild acidic agent for the synthesis of azo dyes via telescoped dediazotization
- Optimization of lipase-catalyzed synthesis of polyethylene glycol stearate in a solvent-free system
- Green synthesis of iron oxide nanoparticles using Platanus orientalis leaf extract for antifungal activity
- Ultrasound assisted chemical activation of peanut husk for copper removal
- Room temperature silanization of Fe3O4 for the preparation of phenyl functionalized magnetic adsorbent for dispersive solid phase extraction for the extraction of phthalates in water
- Evaluation of the saponin green extraction from Ziziphus spina-christi leaves using hydrothermal, microwave and Bain-Marie water bath heating methods
- Oxidation of dibenzothiophene using the heterogeneous catalyst of tungsten-based carbon nanotubes
- Calcined sodium silicate as an efficient and benign heterogeneous catalyst for the transesterification of natural lecithin to L-α-glycerophosphocholine
- Synergistic effect between CO2 and H2O2 on ethylbenzene oxidation catalyzed by carbon supported heteropolyanion catalysts
- Hydrocyanation of 2-arylmethyleneindan-1,3-diones using potassium hexacyanoferrate(II) as a nontoxic cyanating agent
- Green synthesis of hydratropic aldehyde from α-methylstyrene catalyzed by Al2O3-supported metal phthalocyanines
- Environmentally benign chemical recycling of polycarbonate wastes: comparison of micro- and nano-TiO2 solid support efficiencies
- Medicago polymorpha-mediated antibacterial silver nanoparticles in the reduction of methyl orange
- Production of value-added chemicals from esterification of waste glycerol over MCM-41 supported catalysts
- Green synthesis of zerovalent copper nanoparticles for efficient reduction of toxic azo dyes congo red and methyl orange
- Optimization of the biological synthesis of silver nanoparticles using Penicillium oxalicum GRS-1 and their antimicrobial effects against common food-borne pathogens
- Optimization of submerged fermentation conditions to overproduce bioethanol using two industrial and traditional Saccharomyces cerevisiae strains
- Extraction of In3+ and Fe3+ from sulfate solutions by using a 3D-printed “Y”-shaped microreactor
- Foliar-mediated Ag:ZnO nanophotocatalysts: green synthesis, characterization, pollutants degradation, and in vitro biocidal activity
- Green cyclic acetals production by glycerol etherification reaction with benzaldehyde using cationic acidic resin
- Biosynthesis, characterization and antimicrobial activities assessment of fabricated selenium nanoparticles using Pelargonium zonale leaf extract
- Synthesis of high surface area magnesia by using walnut shell as a template
- Controllable biosynthesis of silver nanoparticles using actinobacterial strains
- Green vegetation: a promising source of color dyes
- Mechano-chemical synthesis of ammonia and acetic acid from inorganic materials in water
- Green synthesis and structural characterization of novel N1-substituted 3,4-dihydropyrimidin-2(1H)-ones
- Biodiesel production from cotton oil using heterogeneous CaO catalysts from eggshells prepared at different calcination temperatures
- Regeneration of spent mercury catalyst for the treatment of dye wastewater by the microwave and ultrasonic spray-assisted method
- Green synthesis of the innovative super paramagnetic nanoparticles from the leaves extract of Fraxinus chinensis Roxb and their application for the decolourisation of toxic dyes
- Biogenic ZnO nanoparticles: a study of blueshift of optical band gap and photocatalytic degradation of reactive yellow 186 dye under direct sunlight
- Leached compounds from the extracts of pomegranate peel, green coconut shell, and karuvelam wood for the removal of hexavalent chromium
- Enhancement of molecular weight reduction of natural rubber in triphasic CO2/toluene/H2O systems with hydrogen peroxide for preparation of biobased polyurethanes
- An efficient green synthesis of novel 1H-imidazo[1,2-a]imidazole-3-amine and imidazo[2,1-c][1,2,4]triazole-5-amine derivatives via Strecker reaction under controlled microwave heating
- Evaluation of three different green fabrication methods for the synthesis of crystalline ZnO nanoparticles using Pelargonium zonale leaf extract
- A highly efficient and multifunctional biomass supporting Ag, Ni, and Cu nanoparticles through wetness impregnation for environmental remediation
- Simple one-pot green method for large-scale production of mesalamine, an anti-inflammatory agent
- Relationships between step and cumulative PMI and E-factors: implications on estimating material efficiency with respect to charting synthesis optimization strategies
- A comparative sorption study of Cr3+ and Cr6+ using mango peels: kinetic, equilibrium and thermodynamic
- Effects of acid hydrolysis waste liquid recycle on preparation of microcrystalline cellulose
- Use of deep eutectic solvents as catalyst: A mini-review
- Microwave-assisted synthesis of pyrrolidinone derivatives using 1,1’-butylenebis(3-sulfo-3H-imidazol-1-ium) chloride in ethylene glycol
- Green and eco-friendly synthesis of Co3O4 and Ag-Co3O4: Characterization and photo-catalytic activity
- Adsorption optimized of the coal-based material and application for cyanide wastewater treatment
- Aloe vera leaf extract mediated green synthesis of selenium nanoparticles and assessment of their In vitro antimicrobial activity against spoilage fungi and pathogenic bacteria strains
- Waste phenolic resin derived activated carbon by microwave-assisted KOH activation and application to dye wastewater treatment
- Direct ethanol production from cellulose by consortium of Trichoderma reesei and Candida molischiana
- Agricultural waste biomass-assisted nanostructures: Synthesis and application
- Biodiesel production from rubber seed oil using calcium oxide derived from eggshell as catalyst – optimization and modeling studies
- Study of fabrication of fully aqueous solution processed SnS quantum dot-sensitized solar cell
- Assessment of aqueous extract of Gypsophila aretioides for inhibitory effects on calcium carbonate formation
- An environmentally friendly acylation reaction of 2-methylnaphthalene in solvent-free condition in a micro-channel reactor
- Aegle marmelos phytochemical stabilized synthesis and characterization of ZnO nanoparticles and their role against agriculture and food pathogen
- A reactive coupling process for co-production of solketal and biodiesel
- Optimization of the asymmetric synthesis of (S)-1-phenylethanol using Ispir bean as whole-cell biocatalyst
- Synthesis of pyrazolopyridine and pyrazoloquinoline derivatives by one-pot, three-component reactions of arylglyoxals, 3-methyl-1-aryl-1H-pyrazol-5-amines and cyclic 1,3-dicarbonyl compounds in the presence of tetrapropylammonium bromide
- Preconcentration of morphine in urine sample using a green and solvent-free microextraction method
- Extraction of glycyrrhizic acid by aqueous two-phase system formed by PEG and two environmentally friendly organic acid salts - sodium citrate and sodium tartrate
- Green synthesis of copper oxide nanoparticles using Juglans regia leaf extract and assessment of their physico-chemical and biological properties
- Deep eutectic solvents (DESs) as powerful and recyclable catalysts and solvents for the synthesis of 3,4-dihydropyrimidin-2(1H)-ones/thiones
- Biosynthesis, characterization and anti-microbial activity of silver nanoparticle based gel hand wash
- Efficient and selective microwave-assisted O-methylation of phenolic compounds using tetramethylammonium hydroxide (TMAH)
- Anticoagulant, thrombolytic and antibacterial activities of Euphorbia acruensis latex-mediated bioengineered silver nanoparticles
- Volcanic ash as reusable catalyst in the green synthesis of 3H-1,5-benzodiazepines
- Green synthesis, anionic polymerization of 1,4-bis(methacryloyl)piperazine using Algerian clay as catalyst
- Selenium supplementation during fermentation with sugar beet molasses and Saccharomyces cerevisiae to increase bioethanol production
- Biosynthetic potential assessment of four food pathogenic bacteria in hydrothermally silver nanoparticles fabrication
- Investigating the effectiveness of classical and eco-friendly approaches for synthesis of dialdehydes from organic dihalides
- Pyrolysis of palm oil using zeolite catalyst and characterization of the boil-oil
- Azadirachta indica leaves extract assisted green synthesis of Ag-TiO2 for degradation of Methylene blue and Rhodamine B dyes in aqueous medium
- Synthesis of vitamin E succinate catalyzed by nano-SiO2 immobilized DMAP derivative in mixed solvent system
- Extraction of phytosterols from melon (Cucumis melo) seeds by supercritical CO2 as a clean technology
- Production of uronic acids by hydrothermolysis of pectin as a model substance for plant biomass waste
- Biofabrication of highly pure copper oxide nanoparticles using wheat seed extract and their catalytic activity: A mechanistic approach
- Intelligent modeling and optimization of emulsion aggregation method for producing green printing ink
- Improved removal of methylene blue on modified hierarchical zeolite Y: Achieved by a “destructive-constructive” method
- Two different facile and efficient approaches for the synthesis of various N-arylacetamides via N-acetylation of arylamines and straightforward one-pot reductive acetylation of nitroarenes promoted by recyclable CuFe2O4 nanoparticles in water
- Optimization of acid catalyzed esterification and mixed metal oxide catalyzed transesterification for biodiesel production from Moringa oleifera oil
- Kinetics and the fluidity of the stearic acid esters with different carbon backbones
- Aiming for a standardized protocol for preparing a process green synthesis report and for ranking multiple synthesis plans to a common target product
- Microstructure and luminescence of VO2 (B) nanoparticle synthesis by hydrothermal method
- Optimization of uranium removal from uranium plant wastewater by response surface methodology (RSM)
- Microwave drying of nickel-containing residue: dielectric properties, kinetics, and energy aspects
- Simple and convenient two step synthesis of 5-bromo-2,3-dimethoxy-6-methyl-1,4-benzoquinone
- Biodiesel production from waste cooking oil
- The effect of activation temperature on structure and properties of blue coke-based activated carbon by CO2 activation
- Optimization of reaction parameters for the green synthesis of zero valent iron nanoparticles using pine tree needles
- Microwave-assisted protocol for squalene isolation and conversion from oil-deodoriser distillates
- Denitrification performance of rare earth tailings-based catalysts
- Facile synthesis of silver nanoparticles using Averrhoa bilimbi L and Plum extracts and investigation on the synergistic bioactivity using in vitro models
- Green production of AgNPs and their phytostimulatory impact
- Photocatalytic activity of Ag/Ni bi-metallic nanoparticles on textile dye removal
- Topical Issue: Green Process Engineering / Guest Editors: Martine Poux, Patrick Cognet
- Modelling and optimisation of oxidative desulphurisation of tyre-derived oil via central composite design approach
- CO2 sequestration by carbonation of olivine: a new process for optimal separation of the solids produced
- Organic carbonates synthesis improved by pervaporation for CO2 utilisation
- Production of starch nanoparticles through solvent-antisolvent precipitation in a spinning disc reactor
- A kinetic study of Zn halide/TBAB-catalysed fixation of CO2 with styrene oxide in propylene carbonate
- Topical on Green Process Engineering

