Abstract
An efficient and solvent-free acylation of 2-methylnaphthalene (2-MN) is presented using acid chloride as both the acylating agent and solvent in a micro channel reactor. The effect of the catalyst, reactant ratio, mixing temperature, reaction temperature and reaction time on the product yield and selectivity was investigated. At room temperature with a reaction time of only 15 min, the target product, 2-methyl-6-propionylnaphthalene (2,6-MPN), was obtained in 72.3% yield with 73.8% selectivity, and 2-methyl-6-acetylnaphthalene (2,6-MAN) was obtained in 54.1% yield with 55.4% selectivity. The route of synthesis provides a more environmentally friendly and efficient method to prepare 2,6-MPN with no other toxic solvents and efficient mass transfer and heat transfer.
1 Introduction
The Friedel-Crafts acylation of aromatic and heterocyclic compounds is an important reaction process used for the preparation of aromatic ketones [1, 2, 3]. In traditional organic synthesis, an organic solvent is the most commonly used reaction medium because it can dissolve organic matter and ensure uniform material distribution and heat exchange stability [4, 5]. In addition, reaction processes conventionally used homogeneous catalysts, including Lewis acids (such as metal halides) and Brønsted acids (H2SO4, HCl, HF) [6, 7]. However, in practical applications, the toxicity and difficulty of organic solvents and homogeneous catalysts are the main factors that lead to many problems concerning waste discharge, poor security, energy consumption, serious pollution and other shortcomings. Therefore, synthetic chemistry continues to develop various techniques to produce more green and safe chemicals.
Currently, the development of solvent-free organic synthetic methods has become an important and popular research area [8, 9]. Researchers have begun to study organic reactions under solvent-free conditions, which has opened up a new field for the study of organic synthesis methods. There are many references on acylation reactions under solvent-free conditions, which can be attributed to two cases, those including the use of an ionic liquid as both the catalyst and solvent, and those using the acylating agent or raw material itself as the solvent. For instance, the acylation of maltodextrin using vinyl stearate or stearic acid can be carried out in 1-butyl-3-methylimidazolium dicyanamide. This work demonstrated that an ionic liquid can simultaneously act as the solvent and catalyst during the acylation reaction [10]. The acylation of salicylamide to 5-acetylsalicylamide can be carried in 1-butyl-3-methylimidazolium chloroaluminate or N-butylpyridinium chloroaluminate at low reaction temperatures, and the yield of the desired product can reach 81.3% and 89.2%, respectively. The Lewis acidic ionic liquid catalyst was not only used as the catalyst, but also as the reaction solvent to replace nitrobenzene in the reaction [11]. Shrinivas et al. studied the Friedel-Crafts acylation reaction under solvent-free conditions wherein acetyl chloride (AC) was used during the acylation of anisole or veratrole. Anisole and veratrole were used as both the substrate and solvent during this work [12]. 2-naphthol can be reacted with acetic anhydride in the presence of a sulphated zirconia catalyst with acetic anhydride acting as both the acylating agent and solvent [13]. In the acylation reaction of aromatic compounds using carboxylic acids, a mixture of graphite and p-toluenesulfonic acid was shown to be the best catalyst for the reaction. Carboxylic acids are not only used as a green acylating agent, but also as a solvent in this reaction [14]. The acylation of anisole with PC using MoO4(AlCl2)2 as the catalyst, in this reaction PC is the main reaction solvent [15].
In addition, the reaction rate observed in the absence of a solvent leads to more favorable reaction kinetics than those found in a solution, and the stereoselectivity and yield of the reaction may be improved because of the high concentration of the reactants. Now, traditional stirred reactors are commonly used as the reactor for most solvent-free reactions, which have low reaction efficiency, low selectivity, strong operation intensity and waste discharge [16, 17, 18]. Therefore, it is very important to find the suitable way to perform these reactions toward the development of green chemical processes for environmental protection and to reduce energy consumption. For example, the acylation reaction is one type of very fast exothermic reaction, and some acylation reactions can be carried out in a microchannel reactor [19, 20, 21]. Microchannel reactors can precisely control the reaction conditions, which offers the possibility for some solvent-free reactions [22]. A perfluoroalkoxy reactor was used to produce O-isopropyl propionylbenzene [23]. In this reaction, the main raw materials, isopropylbenzene and propionyl chloride (PC), were reacted under solvent-free conditions.
The possibility of using PC itself as the solvent was studied in our group. The complex formed between AlCl3 and PC was found to be stable for at least three days. Meanwhile, the acylation of 2-MN in different solvent was investigated [24]. The effects of the different reaction solvents on the selectivity and yield of the target product were discussed, with nitrobenzene chosen as the optimal solvent. Under the optimal conditions, the target product, 2,6-MPN, was obtained in 85.8% yield with 87.5% selectivity. After the acylation of 2-MN using PC or AC, the target products, 2,6-MPN or 2,6-MAN, need to be separated from the nitrobenzene solvent, which consumes a lot energy. In addition, the nitrobenzene solvent is toxic.
Therefore, guided by the concept of green chemistry, we continue to study the acylation of 2-MN in a continuous micro-channel reactor with no solvent. The effects of the catalyst, and reaction processes and conditions on the results of the reaction are discussed, and the existing synthetic route to achieve green synthesis is optimized in this study.
2 Experimental
2.1 Materials
2-Methylnaphthalene, propionyl chloride (98%), acetyl chloride (98%), AlCl3 (99%), ZnCl2 (99%) and FeCl3 (99%) were purchased from Shanghai Macklin Biochemical Co.
2.2 Methods and data treatment
The experimental set-up used for the present investigation is shown schematically in Figure 1, and the detailed experimental steps are as follows.

The experimental set-up.
(1 - Glass syringe, 2 - T-micromixer, 3 - Microchannel reactor, 4 - Thermocouple, 5- Thermostatic bath)
Preparation of the two acylating solutions: PC (AC) and AlCl3 were added to a glass-jacketed reactor with stirring and cooling in an ice bath, PC (AC) and 2-MN were also added to another glass-jacketed reactor with stirring and cooling in an ice bath. Stop stirring when the AlCl3 or 2-MN were completely dissolved in PC (AC).
Mixing and Reaction: The two solutions were loaded into two glass syringes (20 mL, Bolivian Pigeons, China), which were attached to two syringe pumps, and mixed in a static T-micro mixer (inner diameter = 0.5 mm) with cooling in an ice bath. After mixing, the obtained solution entered the micro channel (inner diameter = 1 mm, length = 2 m) to react at a constant temperature.
Distillation: After the reaction, the product was dissolved in the excess PC or AC, which can be separated by distillation and the distilled product washed in a beaker with stirring.
A sample of the reaction mixture was dissolved in acetone and analyzed using gas chromatography on a Shimadzu GC-2014 gas chromatograph equipped with a flame ionization detector and an HP-5 capillary column (50 m × 0.2 mm × 0.33 μm film thickness). The yield (Y) and selectivity (X) of the target product were calculated as follows.
where Y is the yield (% by mass) of 2,6-MPN, X is the selectivity (% by mass) of 2,6-MPN, W1 is the mass of 2,6-MPN, W2 is the mass of all the acylation products, W3 is the actual mass of 2,6-MPN and W4 is the theoretical mass of 2,6-MPN.
3 Results and discussion
3.1 The effect of the catalyst
Solid metal halides are commonly used a Lewis acid catalyst to catalyze an acylation reaction [25]. The acylation of 2-MN is generally performed using metal chlorides and acid chlorides as catalysts and acylating agents, respectively, via the mechanism shown in Figure 2.

Mechanism of the acylation reaction of 2-MN.
RCOCl: acetyl chloride (AC) or propionyl chloride (PC); Product: 2-methyl-6-acetylnaphthalene (2,6-MAN) or 2,6-MPN
In this study, the relationship between different catalysts (AlCl3, FeCl3 and ZnCl2) and the result of the acylation reaction of 2-MN was investigated as shown in Table 1. AlCl3 was found to be the optimal catalyst for the acylation of 2-MN using PC as the acylating agent. This was attributed to the electrophilicity of Al3+ being better than that of Fe3+ and Zn2+. Thus, AlCl3 was found to be the optimal catalyst and was used in the subsequent experiments.
The relationship between different catalysts and the results of the acylation reaction of 2-MN.
| No. | Catalyst | Reaction | Selectivity | Yield |
|---|---|---|---|---|
| Temperature (°C) | (wt%) | (wt%) | ||
| 1 | AlCl 3 | 25 | 73.4 | 72.3 |
| 2 | AlCl 3 | 40 | 70.0 | 68.8 |
| 3 | FeCl 3 | 25 | 68.2 | 67.1 |
| 4 | FeCl 3 | 40 | 67.3 | 66.2 |
| 5 | ZnCl 2 | 25 | 30.2 | 29.3 |
| 6 | ZnCl 2 | 40 | 29.8 | 29.1 |
Note: The molar ratio of 2-MN:AlCl3:PC = 0.15:0.17:1, mixing temperature = 15°C and reaction time = 15 min.
3.2 The effect of the molar ratio of reactants
The different molar ratios of the reactants have a significant effect on the acylation reaction when using nitrobenzene as the reaction solvent [26]. Thus, the molar ratio of reactants had a significant effect on the product yield and selectivity under solvent-free conditions, and the best result was obtained using a molar ratio of 2-MN:AlCl3:PC = 0.15:0.17:1 (Table 2). The higher molar ratio of reactants used in this reaction system has a significant effect on the activity of the reaction, which will release a large amount of heat and affect the selectivity of the target product and the stability of the reaction. Therefore, at the higher molar ratios studied, the results of the reaction were poor and at the lower molar ratios, the output coefficient of PC was low.
The relationship between the molar ratio of the reactants and the results of the acylation reaction of 2-MN.
| No. | n(2-MN)/n(AlCl3)/n(PC) | Selectivity (wt%) | Yield (wt%) |
|---|---|---|---|
| 1 | 0.30:0.32:1 | 60.0 | 58.8 |
| 2 | 0.25:0.30:1 | 66.6 | 65.3 |
| 3 | 0.20:0.22:1 | 71.3 | 69.9 |
| 4 | 0.15:0.17:1 | 73.8 | 72.3 |
| 5 | 0.12:0.15:1 | 73.3 | 71.8 |
| 6 | 0.10:0.12:1 | 72.9 | 71.4 |
| 7 | 0.08:0.10:1 | 73.1 | 71.6 |
Note: The mixing temperature = 15°C, reaction temperature = 25°C and reaction time = 15 min.
3.3 The effect of the mixing temperature
When two different acylating solutions are mixed in the T-micro mixer, they quickly release a lot of heat and the mixing temperature increases. Therefore, the relationship between the mixing temperature and the results of the acylation reaction was investigated, and given in Table 3.
The relationship between temperature and the results of the acylation reaction of 2-MN.
| NO. | Mixing | Reaction | Selectivity | Yield |
|---|---|---|---|---|
| Temperature (°C) | Temperature (°C) | (wt%) | (wt%) | |
| 1 | –5 | 25 | 73.5 | 72.0 |
| 2 | –5 | 15 | 71.2 | 69.8 |
| 3 | 5 | 25 | 73.6 | 72.1 |
| 4 | 5 | 15 | 70.9 | 69.5 |
| 5 | 15 | 25 | 73.8 | 72.3 |
| 6 | 15 | 15 | 71.6 | 70.2 |
| 7 | 25 | 25 | 72.1 | 70.7 |
Note: The molar of 2-MN:AlCl3:PC = 0.15:0.17:1, catalyst = AlCl3, acylating agent = PC and reaction time = 15 min.
The mixing temperature has a slight effect on the reaction. In order to better control the reaction temperature, the mixing temperature was lower than 25°C. However, the mixing temperature has a significant effect on the results of the acylation reaction using nitrobenzene as the reaction solvent [26]. The concentration of PC under solvent-free conditions is higher than that of PC dissolved in nitrobenzene. [C2H5CO]+[AlCl4]- is easily converted to [(C2H5CO)3CHCH3CO]+[AlCl4]- or other complexes, so the mixing temperature has a slight effect on the results [27].
3.4 The effect of the reaction temperature
The relationship between the reaction temperature and product selectivity is shown in Figure 3. With increasing the reaction temperature, the selectivity of 2,6-MPN and 2,6-MAN increases slowly. But they decrease in the reaction temperature over 25°C. The acylation reaction of 2-MN under solvent-free conditions was a very fast reaction.
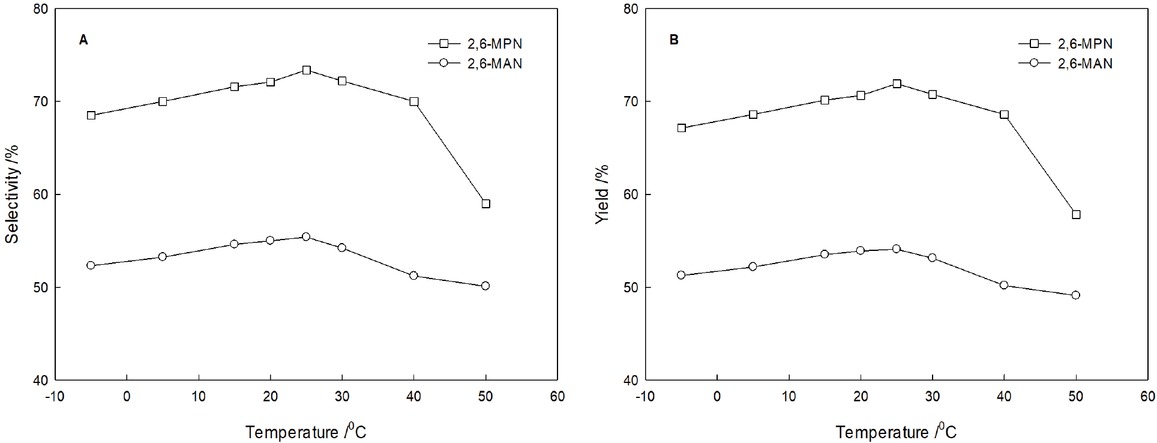
The relationship between the reaction temperature and the results of the acylation reaction of 2-MN. (2-MN:AlCl3:PC or AC = 0.15:0.17:1, AlCl3, PC or AC, 15 min)
In addition, increasing the temperature is beneficial to increasing the reaction rate and increasing the yield. On the other hand, increasing the temperature also accelerates the process of side reactions and increases the amount of additional monoacylated methylnaphthalene isomers in the product, which can reduce the purity of product. At 25°C, the selectivity and yield of the target product, 2,6-MPN, were 73.8% and 72.3%, respectively, and the selectivity and yield of the target product, 2,6-MAN, were 55.4% and 54.1%, respectively.
3.5 The effect of the reaction time
The relationship between the reaction time and the results of the acylation reaction is shown in Figure 4. In this reaction, the flow rate of the two syringe pumps was changed to control the reaction time. The conversion of 2-MN is 100% in 5 min. The selectivity of 2,6-MPN increased with time up to 15 min and that of 2,6-MAN will also increase with time up to 10 min. At the beginning, some 2-MN can generate unstable product isomers and some of these isomers converted into the target product. This acylation reaction under solvent-free conditions is a very fast reaction, and the selectivity has a significant effect on the yield. At 15 min, 2,6-MPN can be obtained in 72.3% yield and 73.8% selectivity, and 2,6-MAN can be obtained in 54.1% yield and 55.4% selectivity.

The relationship between the reaction time and the results of the acylation reaction of 2-MN. (2-MN:AlCl3:PC or AC = 0.15:0.17:1, AlCl3, PC or AC, 25°C)
3.6 The effect of the acylating agent
In order to examine the effect of the different acylating agents, propanoic anhydride (PA) was used as the acylating agent for the acylation reaction of 2-MN. AlCl3 was the optimal catalyst for the acylation of 2-MN with PC, but a mixture of PA and AlCl3 will not be stable and produce a pale yellow gum. Therefore, FeCl3 was selected as the catalyst for the acylation of 2-MN with PA. The effects of the molar ratio of the reactants, mixing temperature, reaction temperature and reaction time on the acylation reaction were studied. When the mixing temperature was 30°C, the reaction temperature is 40°C, the molar ratio of 2-MN:FeCl3:PC was 0.28:0.3:1 and the reactants reacted for 30 min, the selectivity and yield of the target product were 69.5% and 43%, respectively.
4 Conclusions
Nitrobenzene is toxic and needs to be separated from the target product by vacuum distillation, which consumes a lot of energy. The acylation of 2-MN can be carried out under solvent-free conditions, which can result in a more efficient and environmentally friendly way to produce 2,6-MPN when compared with the acylation reaction conducted using nitrobenzene as the reaction solvent. In this acylation reaction, the reactant concentration is higher than the acylation carried out in the presence of a solvent, so the heat and mass transfer efficiency are high. When the mixing temperature is lower than 25°C, the reaction temperature is 25°C, the molar ratio of 2-MN:FeCl3:PC is 0.15:0.17:1 and the reactants reacted for 15 min, the selectivity and yield of target product were 73.8% and 72.3%, respectively.
A comparison of PA and PC shows that although PA can dissolve more 2-MN and catalyst, the yield of the product is lower because the catalytic activity of FeCl3 is minor than
that of AlCl3, and the 2-MN conversion rate is less than 100%. Therefore, PC is the best acylating agent for the acylation of 2-MN conducted under solvent-free conditions.
Acknowledgement
Financial supported by the National Natural Science Foundation of China (91634101) and The Project of Construction of Innovative Teams and Teacher Career Development for Universities and Colleges under Beijing Municipality (IDHT20180508).
References
[1] Witjens P.H., Wepster B.M., Verkade P.E., Deacylation of aromatic acylamino compounds having a nitro group in the ortho or para position. Steric hindrance of resonance. Recueil des Travaux Chimiques des Pays-Bas, 1943, 62, 523-530.10.1002/recl.19430620806Search in Google Scholar
[2] Franz E., Gerhard E., Trifluoromethanesulfonic-carboxylic anhydrides, highly active acylating agents. Angew. Chem. Int. Ed., 2010, 11, 299-301.10.1002/anie.197202991Search in Google Scholar
[3] Dar B.A., Mohsin M., Basit A., Sand M.F., Sand: A natural and potential catalyst in renowned Friedel-Craft’s acylation of aromatic compounds. J. Saudi Chem. Soc., 2013, 17, 177-180.10.1016/j.jscs.2011.03.004Search in Google Scholar
[4] Forero J.S.B., Muñoz J.A.H., Junior J.J., Silva F.M.D., Propylene carbonate in organic synthesis: exploring its potential as a green solvent. Curr. Org. Synth., 2016, 13(6), 834-846.10.2174/1570179413999160211094705Search in Google Scholar
[5] Yamamoto H, Sasaki I., Mitsutake M., Karasudani A., Imagawa H., et al., An efficient pyrrole synthesis via silaphenylmercuric triflate catalyzed cyclization of homopropargyl azides. Synlett, 2009, 16, 2685-2687.10.1055/s-0031-1289566Search in Google Scholar
[6] Kangani C.O., Day B.W.. Mild, efficient Friedel-Crafts acylations from carboxylic acids using cyanuric chloride and AlCl3 Org. Lett., 2008, 10, 2645-2648.10.1021/ol800752vSearch in Google Scholar PubMed
[7] Michigami K., Yoshimoto K., Hayashi M., HF–Pyridine: A Versatile promoter for monoacylation/ sulfonylation of phenolic diols and for direct conversion of t-butyldimethylsilyl ethers to the corresponding acetates. Chem. Lett., 2012, 41, 138-139.10.1246/cl.2012.138Search in Google Scholar
[8] Tang L., Feng B., Promotion method and prospect of solvent- free organic synthesis. Adv. Fine Petrochem., 2008, 9(3), 19-22 (in Chinese).Search in Google Scholar
[9] Loupy A., Solvent‐free microwave organic synthesis as an efficient procedure for green chemistry. C. R. Chimie, 2004, 7, 103-112.10.1016/j.crci.2003.10.015Search in Google Scholar
[10] Biswas A., Shogren R.L., Willett J.L., Ionic liquid as a solvent and catalyst for acylation of maltodextrin. Ind. Crop. Prod., 2009, 30, 172-175.10.1016/j.indcrop.2009.02.003Search in Google Scholar
[11] Chen W., Yin H., Zhang Y., Lu Z., Wang A., et al., Acylation of salicylamide to 5-acetylsalicylamide using ionic liquids as dual catalyst and solvent. J. Ind. Eng. Chem., 2010, 16, 800-804.10.1016/j.jiec.2010.05.009Search in Google Scholar
[12] Sarvari M.H., Sharghi H., Solvent‐free catalytic Friedel–Crafts acylation of aromatic compounds with carboxylic acids by using a novel heterogeneous catalyst system: p-Toluenesulfonic acid/graphite. Helv. Chim., Acta, 2005, 88, 2282-2287.10.1002/hlca.200590162Search in Google Scholar
[13] Ratnam K.J., Reddy R.S., Sekhar N.S., Kantam M.L., Figueras F., Sulphated zirconia catalyzed acylation of phenols, alcohols and amines under solvent free conditions. J. Mol. Catal. A-Chem., 2007, 276, 230-234.10.1016/j.molcata.2007.07.008Search in Google Scholar
[14] Schultz P., Lüning U., Acylation of 6‐O‐Functionalized cellulose ethers with diphenylketene. Macromol. Chem. Phys., 2002, 203, 961-967.10.1002/1521-3935(20020401)203:7<961::AID-MACP961>3.0.CO;2-OSearch in Google Scholar
[15] Jadhav A.H., Chinnappan A., Hiremath V., Seo J.G., Synthesis and characterization of AlCl3 impregnated molybdenum oxide as heterogeneous nano-catalyst for the Friedel-Crafts acylation reaction in ambient condition. J. Nanosci. Nanotechno., 2015, 15, 8243-8250.10.1166/jnn.2015.11253Search in Google Scholar
[16] Wei W., Feng Y.F., Zhang X., Cao X., Zhang J., Synthesis of structured lipid 1,3-dioleoyl-2- palmitoylglycerol in both solvent and solvent-free system. LWT-Food Sci. Technol., 2015, 60, 1187-1194.10.1016/j.lwt.2014.09.013Search in Google Scholar
[17] Li P., Zheng Y., Shi T., Wang Y., Li M., et al., A solvent-free graphene oxide nanoribbon colloid as filler phase for epoxy-matrix composites with enhanced mechanical, thermal and tribological performance. Carbon, 2016, 96, 40-48.10.1016/j.carbon.2015.09.035Search in Google Scholar
[18] Tregnago G., Wykes M., Paternò G.M., Beljonne D., Cacialli F., Low-temperature photoluminescence spectroscopy of solvent-free PCBM single-crystals. J. Phys. Chem. C, 2015, 119, 11846-11851.10.1021/acs.jpcc.5b02345Search in Google Scholar
[19] Lenden P., Ylioja P.M., Gonzalez-Rodriguez C., Entwistle D.A., Willis M.C., Replacing dichloroethane as a solvent for rhodium‐ catalyzed intermolecular alkyne hydroacylation reactions: the utility of propylene carbonate. Cheminform., 2011, 42, 1980-1982.10.1002/chin.201152076Search in Google Scholar
[20] Hagen G.P., Chicago. W., 2-Acetyl-6-Methyl Naphthalene Preparation. USP 5138098, 1992.Search in Google Scholar
[21] Skácel J., Budka J., Eigner V., Lhotak P., Regioselective Friedel–Crafts acylation of calix[4]arenes. Tetrahedron, 2015, 71, 1959-1965.10.1016/j.tet.2015.02.021Search in Google Scholar
[22] Chang C.C., Kuo C.Y., Wang C.Y., Unsteady electroosmosis in a microchannel with Poisson–Boltzmann charge distribution. Electrophoresis, 2011, 32, 3341-3347.10.1002/elps.201100181Search in Google Scholar
[23] Snead D.R., Jamison T.F., A Three‐minute synthesis and purification of ibuprofen: pushing the limits of continuous‐flow processing. Angew. Chem., 2015, 54, 983-987.10.1002/anie.201409093Search in Google Scholar PubMed
[24] Li W., Yang S., Guo X., He G., Jin H., The effect of operating conditions on acylation of 2-methylnaphthalene in a microchannel reactor. Chin. J. Chem. Eng., 2018, 26, 1307-1311.10.1016/j.cjche.2018.01.004Search in Google Scholar
[25] Desmurs J.R., Ratton S., Industrial chemistry library. Elsevier, Amsterdam, 1996, 8, 3-14.Search in Google Scholar
[26] Grob R.L., Modern practice in gas chromatography. John Wiley and Sons, New York, 1977, 181-184.Search in Google Scholar
[27] Csihony S., Mehdi H., Homonnay Z., Vertes A., Farkas O., et al., In situ spectroscopic studies related to the mechanism of the Friedel–Crafts acetylation of benzene in ionic liquids using AlCl3 and FeCl3 J. Chem. Soc. Dalton, 2002, 5, 680-685.10.1039/b109303gSearch in Google Scholar
© 2019 Li et al., published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 Public License.
Articles in the same Issue
- Regular Articles
- Studies on the preparation and properties of biodegradable polyester from soybean oil
- Flow-mode biodiesel production from palm oil using a pressurized microwave reactor
- Reduction of free fatty acids in waste oil for biodiesel production by glycerolysis: investigation and optimization of process parameters
- Saccharin: a cheap and mild acidic agent for the synthesis of azo dyes via telescoped dediazotization
- Optimization of lipase-catalyzed synthesis of polyethylene glycol stearate in a solvent-free system
- Green synthesis of iron oxide nanoparticles using Platanus orientalis leaf extract for antifungal activity
- Ultrasound assisted chemical activation of peanut husk for copper removal
- Room temperature silanization of Fe3O4 for the preparation of phenyl functionalized magnetic adsorbent for dispersive solid phase extraction for the extraction of phthalates in water
- Evaluation of the saponin green extraction from Ziziphus spina-christi leaves using hydrothermal, microwave and Bain-Marie water bath heating methods
- Oxidation of dibenzothiophene using the heterogeneous catalyst of tungsten-based carbon nanotubes
- Calcined sodium silicate as an efficient and benign heterogeneous catalyst for the transesterification of natural lecithin to L-α-glycerophosphocholine
- Synergistic effect between CO2 and H2O2 on ethylbenzene oxidation catalyzed by carbon supported heteropolyanion catalysts
- Hydrocyanation of 2-arylmethyleneindan-1,3-diones using potassium hexacyanoferrate(II) as a nontoxic cyanating agent
- Green synthesis of hydratropic aldehyde from α-methylstyrene catalyzed by Al2O3-supported metal phthalocyanines
- Environmentally benign chemical recycling of polycarbonate wastes: comparison of micro- and nano-TiO2 solid support efficiencies
- Medicago polymorpha-mediated antibacterial silver nanoparticles in the reduction of methyl orange
- Production of value-added chemicals from esterification of waste glycerol over MCM-41 supported catalysts
- Green synthesis of zerovalent copper nanoparticles for efficient reduction of toxic azo dyes congo red and methyl orange
- Optimization of the biological synthesis of silver nanoparticles using Penicillium oxalicum GRS-1 and their antimicrobial effects against common food-borne pathogens
- Optimization of submerged fermentation conditions to overproduce bioethanol using two industrial and traditional Saccharomyces cerevisiae strains
- Extraction of In3+ and Fe3+ from sulfate solutions by using a 3D-printed “Y”-shaped microreactor
- Foliar-mediated Ag:ZnO nanophotocatalysts: green synthesis, characterization, pollutants degradation, and in vitro biocidal activity
- Green cyclic acetals production by glycerol etherification reaction with benzaldehyde using cationic acidic resin
- Biosynthesis, characterization and antimicrobial activities assessment of fabricated selenium nanoparticles using Pelargonium zonale leaf extract
- Synthesis of high surface area magnesia by using walnut shell as a template
- Controllable biosynthesis of silver nanoparticles using actinobacterial strains
- Green vegetation: a promising source of color dyes
- Mechano-chemical synthesis of ammonia and acetic acid from inorganic materials in water
- Green synthesis and structural characterization of novel N1-substituted 3,4-dihydropyrimidin-2(1H)-ones
- Biodiesel production from cotton oil using heterogeneous CaO catalysts from eggshells prepared at different calcination temperatures
- Regeneration of spent mercury catalyst for the treatment of dye wastewater by the microwave and ultrasonic spray-assisted method
- Green synthesis of the innovative super paramagnetic nanoparticles from the leaves extract of Fraxinus chinensis Roxb and their application for the decolourisation of toxic dyes
- Biogenic ZnO nanoparticles: a study of blueshift of optical band gap and photocatalytic degradation of reactive yellow 186 dye under direct sunlight
- Leached compounds from the extracts of pomegranate peel, green coconut shell, and karuvelam wood for the removal of hexavalent chromium
- Enhancement of molecular weight reduction of natural rubber in triphasic CO2/toluene/H2O systems with hydrogen peroxide for preparation of biobased polyurethanes
- An efficient green synthesis of novel 1H-imidazo[1,2-a]imidazole-3-amine and imidazo[2,1-c][1,2,4]triazole-5-amine derivatives via Strecker reaction under controlled microwave heating
- Evaluation of three different green fabrication methods for the synthesis of crystalline ZnO nanoparticles using Pelargonium zonale leaf extract
- A highly efficient and multifunctional biomass supporting Ag, Ni, and Cu nanoparticles through wetness impregnation for environmental remediation
- Simple one-pot green method for large-scale production of mesalamine, an anti-inflammatory agent
- Relationships between step and cumulative PMI and E-factors: implications on estimating material efficiency with respect to charting synthesis optimization strategies
- A comparative sorption study of Cr3+ and Cr6+ using mango peels: kinetic, equilibrium and thermodynamic
- Effects of acid hydrolysis waste liquid recycle on preparation of microcrystalline cellulose
- Use of deep eutectic solvents as catalyst: A mini-review
- Microwave-assisted synthesis of pyrrolidinone derivatives using 1,1’-butylenebis(3-sulfo-3H-imidazol-1-ium) chloride in ethylene glycol
- Green and eco-friendly synthesis of Co3O4 and Ag-Co3O4: Characterization and photo-catalytic activity
- Adsorption optimized of the coal-based material and application for cyanide wastewater treatment
- Aloe vera leaf extract mediated green synthesis of selenium nanoparticles and assessment of their In vitro antimicrobial activity against spoilage fungi and pathogenic bacteria strains
- Waste phenolic resin derived activated carbon by microwave-assisted KOH activation and application to dye wastewater treatment
- Direct ethanol production from cellulose by consortium of Trichoderma reesei and Candida molischiana
- Agricultural waste biomass-assisted nanostructures: Synthesis and application
- Biodiesel production from rubber seed oil using calcium oxide derived from eggshell as catalyst – optimization and modeling studies
- Study of fabrication of fully aqueous solution processed SnS quantum dot-sensitized solar cell
- Assessment of aqueous extract of Gypsophila aretioides for inhibitory effects on calcium carbonate formation
- An environmentally friendly acylation reaction of 2-methylnaphthalene in solvent-free condition in a micro-channel reactor
- Aegle marmelos phytochemical stabilized synthesis and characterization of ZnO nanoparticles and their role against agriculture and food pathogen
- A reactive coupling process for co-production of solketal and biodiesel
- Optimization of the asymmetric synthesis of (S)-1-phenylethanol using Ispir bean as whole-cell biocatalyst
- Synthesis of pyrazolopyridine and pyrazoloquinoline derivatives by one-pot, three-component reactions of arylglyoxals, 3-methyl-1-aryl-1H-pyrazol-5-amines and cyclic 1,3-dicarbonyl compounds in the presence of tetrapropylammonium bromide
- Preconcentration of morphine in urine sample using a green and solvent-free microextraction method
- Extraction of glycyrrhizic acid by aqueous two-phase system formed by PEG and two environmentally friendly organic acid salts - sodium citrate and sodium tartrate
- Green synthesis of copper oxide nanoparticles using Juglans regia leaf extract and assessment of their physico-chemical and biological properties
- Deep eutectic solvents (DESs) as powerful and recyclable catalysts and solvents for the synthesis of 3,4-dihydropyrimidin-2(1H)-ones/thiones
- Biosynthesis, characterization and anti-microbial activity of silver nanoparticle based gel hand wash
- Efficient and selective microwave-assisted O-methylation of phenolic compounds using tetramethylammonium hydroxide (TMAH)
- Anticoagulant, thrombolytic and antibacterial activities of Euphorbia acruensis latex-mediated bioengineered silver nanoparticles
- Volcanic ash as reusable catalyst in the green synthesis of 3H-1,5-benzodiazepines
- Green synthesis, anionic polymerization of 1,4-bis(methacryloyl)piperazine using Algerian clay as catalyst
- Selenium supplementation during fermentation with sugar beet molasses and Saccharomyces cerevisiae to increase bioethanol production
- Biosynthetic potential assessment of four food pathogenic bacteria in hydrothermally silver nanoparticles fabrication
- Investigating the effectiveness of classical and eco-friendly approaches for synthesis of dialdehydes from organic dihalides
- Pyrolysis of palm oil using zeolite catalyst and characterization of the boil-oil
- Azadirachta indica leaves extract assisted green synthesis of Ag-TiO2 for degradation of Methylene blue and Rhodamine B dyes in aqueous medium
- Synthesis of vitamin E succinate catalyzed by nano-SiO2 immobilized DMAP derivative in mixed solvent system
- Extraction of phytosterols from melon (Cucumis melo) seeds by supercritical CO2 as a clean technology
- Production of uronic acids by hydrothermolysis of pectin as a model substance for plant biomass waste
- Biofabrication of highly pure copper oxide nanoparticles using wheat seed extract and their catalytic activity: A mechanistic approach
- Intelligent modeling and optimization of emulsion aggregation method for producing green printing ink
- Improved removal of methylene blue on modified hierarchical zeolite Y: Achieved by a “destructive-constructive” method
- Two different facile and efficient approaches for the synthesis of various N-arylacetamides via N-acetylation of arylamines and straightforward one-pot reductive acetylation of nitroarenes promoted by recyclable CuFe2O4 nanoparticles in water
- Optimization of acid catalyzed esterification and mixed metal oxide catalyzed transesterification for biodiesel production from Moringa oleifera oil
- Kinetics and the fluidity of the stearic acid esters with different carbon backbones
- Aiming for a standardized protocol for preparing a process green synthesis report and for ranking multiple synthesis plans to a common target product
- Microstructure and luminescence of VO2 (B) nanoparticle synthesis by hydrothermal method
- Optimization of uranium removal from uranium plant wastewater by response surface methodology (RSM)
- Microwave drying of nickel-containing residue: dielectric properties, kinetics, and energy aspects
- Simple and convenient two step synthesis of 5-bromo-2,3-dimethoxy-6-methyl-1,4-benzoquinone
- Biodiesel production from waste cooking oil
- The effect of activation temperature on structure and properties of blue coke-based activated carbon by CO2 activation
- Optimization of reaction parameters for the green synthesis of zero valent iron nanoparticles using pine tree needles
- Microwave-assisted protocol for squalene isolation and conversion from oil-deodoriser distillates
- Denitrification performance of rare earth tailings-based catalysts
- Facile synthesis of silver nanoparticles using Averrhoa bilimbi L and Plum extracts and investigation on the synergistic bioactivity using in vitro models
- Green production of AgNPs and their phytostimulatory impact
- Photocatalytic activity of Ag/Ni bi-metallic nanoparticles on textile dye removal
- Topical Issue: Green Process Engineering / Guest Editors: Martine Poux, Patrick Cognet
- Modelling and optimisation of oxidative desulphurisation of tyre-derived oil via central composite design approach
- CO2 sequestration by carbonation of olivine: a new process for optimal separation of the solids produced
- Organic carbonates synthesis improved by pervaporation for CO2 utilisation
- Production of starch nanoparticles through solvent-antisolvent precipitation in a spinning disc reactor
- A kinetic study of Zn halide/TBAB-catalysed fixation of CO2 with styrene oxide in propylene carbonate
- Topical on Green Process Engineering
Articles in the same Issue
- Regular Articles
- Studies on the preparation and properties of biodegradable polyester from soybean oil
- Flow-mode biodiesel production from palm oil using a pressurized microwave reactor
- Reduction of free fatty acids in waste oil for biodiesel production by glycerolysis: investigation and optimization of process parameters
- Saccharin: a cheap and mild acidic agent for the synthesis of azo dyes via telescoped dediazotization
- Optimization of lipase-catalyzed synthesis of polyethylene glycol stearate in a solvent-free system
- Green synthesis of iron oxide nanoparticles using Platanus orientalis leaf extract for antifungal activity
- Ultrasound assisted chemical activation of peanut husk for copper removal
- Room temperature silanization of Fe3O4 for the preparation of phenyl functionalized magnetic adsorbent for dispersive solid phase extraction for the extraction of phthalates in water
- Evaluation of the saponin green extraction from Ziziphus spina-christi leaves using hydrothermal, microwave and Bain-Marie water bath heating methods
- Oxidation of dibenzothiophene using the heterogeneous catalyst of tungsten-based carbon nanotubes
- Calcined sodium silicate as an efficient and benign heterogeneous catalyst for the transesterification of natural lecithin to L-α-glycerophosphocholine
- Synergistic effect between CO2 and H2O2 on ethylbenzene oxidation catalyzed by carbon supported heteropolyanion catalysts
- Hydrocyanation of 2-arylmethyleneindan-1,3-diones using potassium hexacyanoferrate(II) as a nontoxic cyanating agent
- Green synthesis of hydratropic aldehyde from α-methylstyrene catalyzed by Al2O3-supported metal phthalocyanines
- Environmentally benign chemical recycling of polycarbonate wastes: comparison of micro- and nano-TiO2 solid support efficiencies
- Medicago polymorpha-mediated antibacterial silver nanoparticles in the reduction of methyl orange
- Production of value-added chemicals from esterification of waste glycerol over MCM-41 supported catalysts
- Green synthesis of zerovalent copper nanoparticles for efficient reduction of toxic azo dyes congo red and methyl orange
- Optimization of the biological synthesis of silver nanoparticles using Penicillium oxalicum GRS-1 and their antimicrobial effects against common food-borne pathogens
- Optimization of submerged fermentation conditions to overproduce bioethanol using two industrial and traditional Saccharomyces cerevisiae strains
- Extraction of In3+ and Fe3+ from sulfate solutions by using a 3D-printed “Y”-shaped microreactor
- Foliar-mediated Ag:ZnO nanophotocatalysts: green synthesis, characterization, pollutants degradation, and in vitro biocidal activity
- Green cyclic acetals production by glycerol etherification reaction with benzaldehyde using cationic acidic resin
- Biosynthesis, characterization and antimicrobial activities assessment of fabricated selenium nanoparticles using Pelargonium zonale leaf extract
- Synthesis of high surface area magnesia by using walnut shell as a template
- Controllable biosynthesis of silver nanoparticles using actinobacterial strains
- Green vegetation: a promising source of color dyes
- Mechano-chemical synthesis of ammonia and acetic acid from inorganic materials in water
- Green synthesis and structural characterization of novel N1-substituted 3,4-dihydropyrimidin-2(1H)-ones
- Biodiesel production from cotton oil using heterogeneous CaO catalysts from eggshells prepared at different calcination temperatures
- Regeneration of spent mercury catalyst for the treatment of dye wastewater by the microwave and ultrasonic spray-assisted method
- Green synthesis of the innovative super paramagnetic nanoparticles from the leaves extract of Fraxinus chinensis Roxb and their application for the decolourisation of toxic dyes
- Biogenic ZnO nanoparticles: a study of blueshift of optical band gap and photocatalytic degradation of reactive yellow 186 dye under direct sunlight
- Leached compounds from the extracts of pomegranate peel, green coconut shell, and karuvelam wood for the removal of hexavalent chromium
- Enhancement of molecular weight reduction of natural rubber in triphasic CO2/toluene/H2O systems with hydrogen peroxide for preparation of biobased polyurethanes
- An efficient green synthesis of novel 1H-imidazo[1,2-a]imidazole-3-amine and imidazo[2,1-c][1,2,4]triazole-5-amine derivatives via Strecker reaction under controlled microwave heating
- Evaluation of three different green fabrication methods for the synthesis of crystalline ZnO nanoparticles using Pelargonium zonale leaf extract
- A highly efficient and multifunctional biomass supporting Ag, Ni, and Cu nanoparticles through wetness impregnation for environmental remediation
- Simple one-pot green method for large-scale production of mesalamine, an anti-inflammatory agent
- Relationships between step and cumulative PMI and E-factors: implications on estimating material efficiency with respect to charting synthesis optimization strategies
- A comparative sorption study of Cr3+ and Cr6+ using mango peels: kinetic, equilibrium and thermodynamic
- Effects of acid hydrolysis waste liquid recycle on preparation of microcrystalline cellulose
- Use of deep eutectic solvents as catalyst: A mini-review
- Microwave-assisted synthesis of pyrrolidinone derivatives using 1,1’-butylenebis(3-sulfo-3H-imidazol-1-ium) chloride in ethylene glycol
- Green and eco-friendly synthesis of Co3O4 and Ag-Co3O4: Characterization and photo-catalytic activity
- Adsorption optimized of the coal-based material and application for cyanide wastewater treatment
- Aloe vera leaf extract mediated green synthesis of selenium nanoparticles and assessment of their In vitro antimicrobial activity against spoilage fungi and pathogenic bacteria strains
- Waste phenolic resin derived activated carbon by microwave-assisted KOH activation and application to dye wastewater treatment
- Direct ethanol production from cellulose by consortium of Trichoderma reesei and Candida molischiana
- Agricultural waste biomass-assisted nanostructures: Synthesis and application
- Biodiesel production from rubber seed oil using calcium oxide derived from eggshell as catalyst – optimization and modeling studies
- Study of fabrication of fully aqueous solution processed SnS quantum dot-sensitized solar cell
- Assessment of aqueous extract of Gypsophila aretioides for inhibitory effects on calcium carbonate formation
- An environmentally friendly acylation reaction of 2-methylnaphthalene in solvent-free condition in a micro-channel reactor
- Aegle marmelos phytochemical stabilized synthesis and characterization of ZnO nanoparticles and their role against agriculture and food pathogen
- A reactive coupling process for co-production of solketal and biodiesel
- Optimization of the asymmetric synthesis of (S)-1-phenylethanol using Ispir bean as whole-cell biocatalyst
- Synthesis of pyrazolopyridine and pyrazoloquinoline derivatives by one-pot, three-component reactions of arylglyoxals, 3-methyl-1-aryl-1H-pyrazol-5-amines and cyclic 1,3-dicarbonyl compounds in the presence of tetrapropylammonium bromide
- Preconcentration of morphine in urine sample using a green and solvent-free microextraction method
- Extraction of glycyrrhizic acid by aqueous two-phase system formed by PEG and two environmentally friendly organic acid salts - sodium citrate and sodium tartrate
- Green synthesis of copper oxide nanoparticles using Juglans regia leaf extract and assessment of their physico-chemical and biological properties
- Deep eutectic solvents (DESs) as powerful and recyclable catalysts and solvents for the synthesis of 3,4-dihydropyrimidin-2(1H)-ones/thiones
- Biosynthesis, characterization and anti-microbial activity of silver nanoparticle based gel hand wash
- Efficient and selective microwave-assisted O-methylation of phenolic compounds using tetramethylammonium hydroxide (TMAH)
- Anticoagulant, thrombolytic and antibacterial activities of Euphorbia acruensis latex-mediated bioengineered silver nanoparticles
- Volcanic ash as reusable catalyst in the green synthesis of 3H-1,5-benzodiazepines
- Green synthesis, anionic polymerization of 1,4-bis(methacryloyl)piperazine using Algerian clay as catalyst
- Selenium supplementation during fermentation with sugar beet molasses and Saccharomyces cerevisiae to increase bioethanol production
- Biosynthetic potential assessment of four food pathogenic bacteria in hydrothermally silver nanoparticles fabrication
- Investigating the effectiveness of classical and eco-friendly approaches for synthesis of dialdehydes from organic dihalides
- Pyrolysis of palm oil using zeolite catalyst and characterization of the boil-oil
- Azadirachta indica leaves extract assisted green synthesis of Ag-TiO2 for degradation of Methylene blue and Rhodamine B dyes in aqueous medium
- Synthesis of vitamin E succinate catalyzed by nano-SiO2 immobilized DMAP derivative in mixed solvent system
- Extraction of phytosterols from melon (Cucumis melo) seeds by supercritical CO2 as a clean technology
- Production of uronic acids by hydrothermolysis of pectin as a model substance for plant biomass waste
- Biofabrication of highly pure copper oxide nanoparticles using wheat seed extract and their catalytic activity: A mechanistic approach
- Intelligent modeling and optimization of emulsion aggregation method for producing green printing ink
- Improved removal of methylene blue on modified hierarchical zeolite Y: Achieved by a “destructive-constructive” method
- Two different facile and efficient approaches for the synthesis of various N-arylacetamides via N-acetylation of arylamines and straightforward one-pot reductive acetylation of nitroarenes promoted by recyclable CuFe2O4 nanoparticles in water
- Optimization of acid catalyzed esterification and mixed metal oxide catalyzed transesterification for biodiesel production from Moringa oleifera oil
- Kinetics and the fluidity of the stearic acid esters with different carbon backbones
- Aiming for a standardized protocol for preparing a process green synthesis report and for ranking multiple synthesis plans to a common target product
- Microstructure and luminescence of VO2 (B) nanoparticle synthesis by hydrothermal method
- Optimization of uranium removal from uranium plant wastewater by response surface methodology (RSM)
- Microwave drying of nickel-containing residue: dielectric properties, kinetics, and energy aspects
- Simple and convenient two step synthesis of 5-bromo-2,3-dimethoxy-6-methyl-1,4-benzoquinone
- Biodiesel production from waste cooking oil
- The effect of activation temperature on structure and properties of blue coke-based activated carbon by CO2 activation
- Optimization of reaction parameters for the green synthesis of zero valent iron nanoparticles using pine tree needles
- Microwave-assisted protocol for squalene isolation and conversion from oil-deodoriser distillates
- Denitrification performance of rare earth tailings-based catalysts
- Facile synthesis of silver nanoparticles using Averrhoa bilimbi L and Plum extracts and investigation on the synergistic bioactivity using in vitro models
- Green production of AgNPs and their phytostimulatory impact
- Photocatalytic activity of Ag/Ni bi-metallic nanoparticles on textile dye removal
- Topical Issue: Green Process Engineering / Guest Editors: Martine Poux, Patrick Cognet
- Modelling and optimisation of oxidative desulphurisation of tyre-derived oil via central composite design approach
- CO2 sequestration by carbonation of olivine: a new process for optimal separation of the solids produced
- Organic carbonates synthesis improved by pervaporation for CO2 utilisation
- Production of starch nanoparticles through solvent-antisolvent precipitation in a spinning disc reactor
- A kinetic study of Zn halide/TBAB-catalysed fixation of CO2 with styrene oxide in propylene carbonate
- Topical on Green Process Engineering

