Calcined sodium silicate as an efficient and benign heterogeneous catalyst for the transesterification of natural lecithin to L-α-glycerophosphocholine
-
Bin Li
, Pengjuan Fan
Abstract
Calcined sodium silicate could serve as an efficient and benign heterogeneous catalyst for the production of L-α-glycerophosphocholine from natural lecithin. The catalytic transesterification of natural lecithin by calcined sodium silicate proceeded to almost 100% conversion under the following conditions: sodium silicate amount of 6 wt%, reaction temperature of 65°C, lecithin concentration of 10.5 mmol/l, stirring intensity of 300 rpm, reaction time of 120 min. In addition, this catalyst could be separated completely by simple centrifugation and retained high activity after three re-uses. Compared to the previously reported catalysts, calcined sodium silicate, with the significantly important characteristics of non-toxicity and easy excretion from the human body, was beneficial to the benign production of L-α-glycerophosphocholine and its security as well as application in food and medicine fields.
1 Introduction
L-α-glycerophosphocholine (GPC) as a phospholipid metabolite can be used in pharmaceuticals field [1], [2]. In addition, some researches have demonstrated that GPC has positive effects on attention, memory, cognition, brain or mental disease [3], [4], [5]. As a functional food and medicine, the most suitable preparation method is the transesterification of natural lecithin [6], [7], [8], [9], [10]. However, only limited literatures have reported the transesterification of natural lecithin. In the early stage, homogeneous catalysts such as mercuric chloride [11], tetrabutylammonium hydroxide [12], and sodium methoxide [13] have been used to catalyze the transesterification of lecithin to prepare GPC. The serious drawback of this process is the catalyst residue in the prepared GPC, which can affect its security and usage [14].
Therefore, many investigations have paid attention to developing the novel process, in which there is no residue of toxic components, or the catalyst can be easily separated and recycled. For example, the low boiling point organic amines, quaternary ammonium resin has been used to catalyze the transesterification of lecithin, respectively. The main benefit of this process is that the catalyst can be easily separated by distillation [10] or simple centrifugation [15]. In spite of the remarkable advance, the resulting GPC may be contaminated by the toxic catalyst. Hence, there is still much scope for the development of a benign heterogeneous catalyst to achieve the aim of preparing GPC with a benign process.
Sodium silicate as an organic salt is non-toxic [16]. In addition, sodium silicate taken orally can be easily absorbed from the alimentary canal and excreted in urine [16]. These significant characteristics indicate that sodium silicate can serve as a suitable catalyst for the production of GPC. Therefore, we have reported herein the production of GPC from natural lecithin with the friendly calcined sodium silicate. The effects of reaction temperature, stirring intensity, lecithin concentration, and catalyst amount were investigated in detail. In addition, the recyclability of sodium silicate and the catalyst residue in reaction solution were also tested.
2 Materials and methods
2.1 Materials
Sodium metasilicate (Na2SiO3·9H2O) used in this study was purchased from Kermel Chemistry Co. Ltd. (Tianjin, China). Soybean lecithin (90% phospholipids) was obtained from Sigma-Aldrich Corp. (St. Louis, MO, USA). All other reagents were of standard laboratory grade.
2.2 Catalyst preparation and characterization
In order to determine the calcination temperature region, Na2SiO3·9H2O was analyzed by thermogravimetric analysis-derivative thermogravimetric (TG-DTG), which was performed on a thermogravimetric analyzer (STA449F3, NETZSCH, Germany) under nitrogen flow with a heating rate of 10 K/min from room temperature to 800°C. To prepare the catalyst, Na2SiO3·9H2O was calcined in an oven at the optimal temperature for 2 h. Then, the resulting catalyst was triturated and passed through a 120-mesh sieve.
The base strength and basicity of the catalysts calcined at different temperatures were determined by the Hammett indicator and Hammett indicator-benzene carboxylic acid titration methods [17], [18]. First, the base strength distribution was measured by the color changes of indicators adsorbed on sodium silicate. Then, the basicity of different alkali strengths was measured by the titration of methanol solution of benzene carboxylic acid (Kermel Chemistry Co. Ltd. Tianjin, China). In addition, the total basicity was the sum of all different base strength contents. X-ray diffraction (XRD) spectra were recorded on a powder XRD instrument (D8 ADVANCE, Bruker, Germany). Scanning electron microscopy images were detected on a scanning electron microscope (Sigma 500, Carl Zeiss, Germany). Fourier transform infrared spectroscopy (FT-IR) was performed using a Fourier transform infrared spectrometer (Frontier, PerkinElmer, USA). The fresh, recycled catalyst was dissolved in hydrofluoric acid and analyzed by an inductively coupled plasma (ICP) spectrometer (Optima 7000 DV, PerkinElmer, USA).
2.3 Transesterification process
Phospholipid (PC) was dissolved in methanol and the solution was introduced to a 150 ml three-necked flask equipped with a reflux condenser. The calcined sodium silicate was added to react for 120 min. The reaction mixture (20 μl, approximately 15 mg) was periodically withdrawn and centrifuged at 4000 rpm for 1 min. Some 10 μl of the upper liquid were taken to analyze the amount of PC reacted.
2.4 High performance liquid chromatography analysis
The PC in the reaction mixture were analyzed by high performance liquid chromatography (LC-20A, Shimadzu, Japan) equipped with an evaporative light-scattering detector (Chromachem, ESA, USA) under the reported testing condition [19]. The conversion of PC was calculated as the ratio of the difference between the initial mass of PC and that after reaction to the initial mass of PC.
3 Results and discussion
3.1 Catalyst characterization
As seen in Figure 1, the weight loss of sodium metasilicate was divided into two stages. The main weight loss (about 50%) range of room temperature to 170°C was ascribed to the desorption of adsorbed water [20] and the loss of bound water [21]. Less than 10% weight loss was observed in the 170–800°C region, which was likely attributed to the loss of water trapped in the framework [20]. By contrast, no DTG peak appeared at the second weight loss region, which showed that the high temperature calcination would not result in decomposition, crystallization and melting of sodium silicate [20]. In view of these results, the bound water was removed in the low temperature region; therefore, the range of 170–800°C was chosen to investigate the optimal calcination temperature. In addition, the sodium metasilicate was calcined at 200°C, 300°C, 400°C and 500°C.
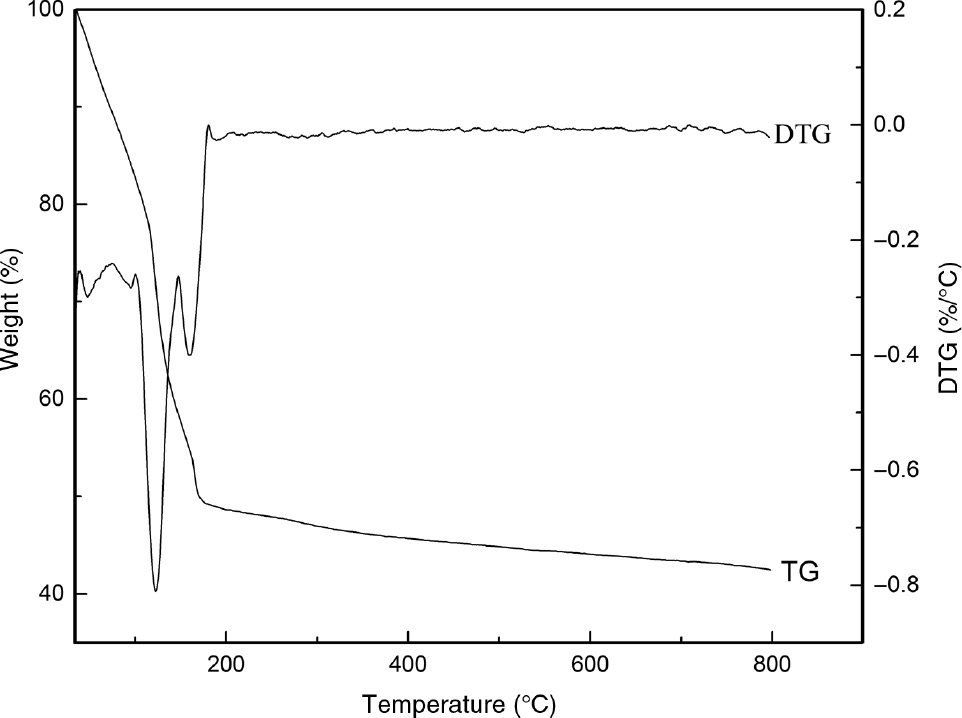
TG/DTG curves of sodium metasilicate.
The base strength distribution and basicity of sodium silicate calcined at different temperatures are summarized in Table 1. The total basicity first increased and then decreased with increasing calcination temperature. The result was similar to that obtained by Xie et al. [17]. With the calcination temperature of 300°C, the highest total basicity of 12.96 mmol/g was obtained. However, the optimum catalyst was obtained at 400°C, which possessed the highest strong base content (H0=15.0–18.4). Correspondingly, the highest PC conversion of 98.7% was obtained in the presence of this catalyst. This result was in agreement with that obtained from other reactions catalyzed by calcined sodium silicate [21], [22]. Therefore, the optimal calcination temperature was 400°C.
Basicity distribution of sodium silicate calcined at different temperature.
| Temperature | Basicity of different basic strength ranges (mmol/g) | Conversion (%) | |||
|---|---|---|---|---|---|
| H0=7.2–9.8 | H0=9.8–15.0 | H0=15.0–18.4 | Total basicity | ||
| 200°C | 1.95 | 8.12 | 0.43 | 10.51 | 69.4 |
| 300°C | 2.84 | 9.22 | 1.04 | 12.96 | 79.6 |
| 400°C | 4.19 | 6.58 | 1.59 | 12.34 | 98.7 |
| 500°C | 4.00 | 6.40 | 1.20 | 11.61 | 94.4 |
The XRD diffraction patterns of Na2SiO3·9H2O and calcined sodium silicate are shown in Figure 2. The non-calcined sample was assigned to Na2SiO3 with nine molecules of bound water (JCPDS No. 16-0815) and the crystalline phase was the orthorhombic phase [22]. After calcination treatment, the intensity of some diffraction peaks at 2θ angles of 16.79°, 25.05°, 34.93°, 37.22°, 48.16°, 52° and 65.65° (JCPDS No. 16-0818) increased, which suggested that the crystalline phase of Na2SiO3 was the hexagonal phase [22]. By contrast, no change in crystalline phases of the catalysts calcined at different temperatures was observed. However, the crystallite size of the catalyst calcined at 400°C (D=58.14 nm, calculated by Jade 6.0) was larger than that at 200°C (D=20.28 nm) and 500°C (D=30.61 nm). Correspondingly, the catalyst calcined at 400°C possessed the highest strong basic center content. Therefore, we speculated that the large crystallite size crystallization had a positive effect on the formation of strong alkali centers.
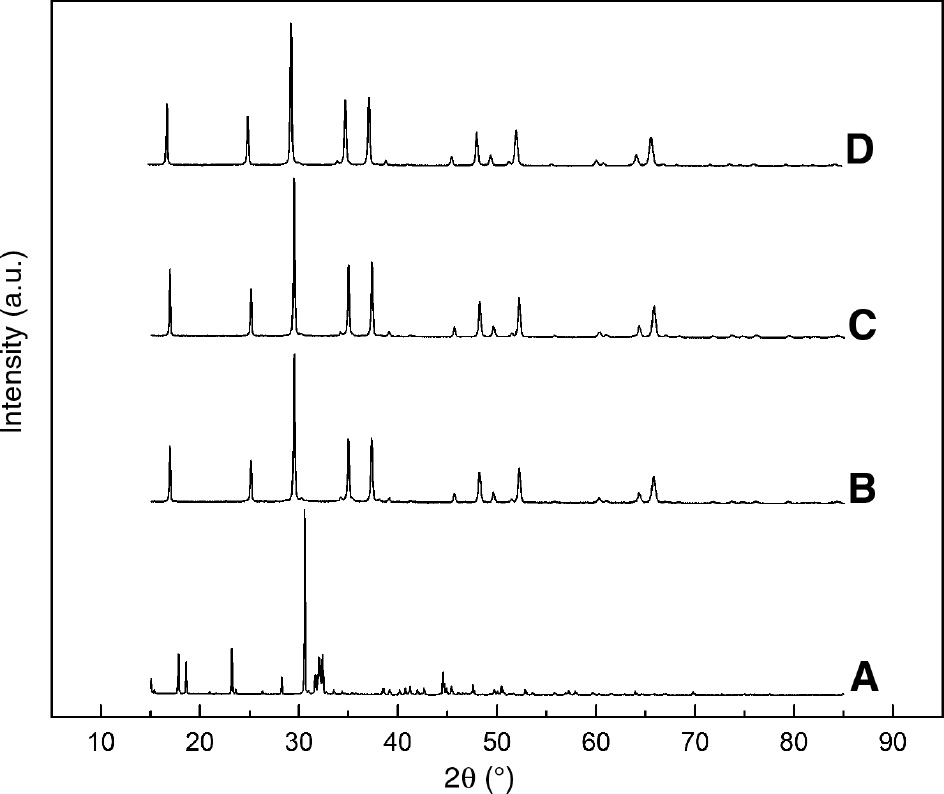
X-ray diffraction (XRD) patterns of (A) sodium metasilicate, (B) 200°C, (C) 400°C, and (D) 500°C.
The morphologies of the samples before and after calcination are shown in Figure 3. The sodium metasilicate surface was smooth and continuous. After being calcined at 400°C, a large number of particles loosely aggregated at the catalyst surface forming many interstices. These interstices could act as channels to readily facilitate the diffusion of PC/GPC molecules to/from the active base site in the internal surface. Therefore, the sodium silicate performed with higher activity after calcination.
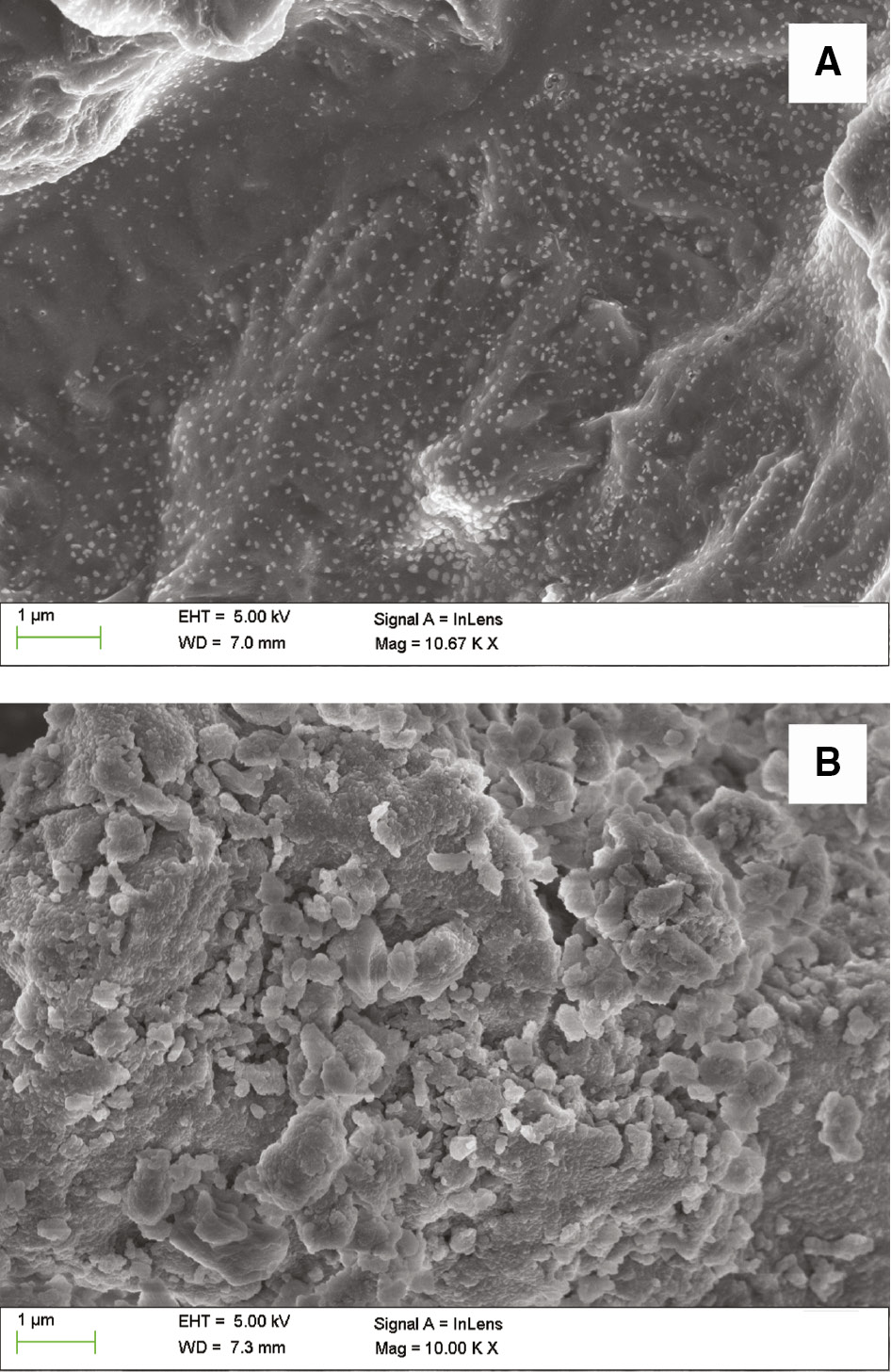
Scanning electron microscopy (SEM) images of (A) sodium metasilicate, (B) sodium silicate calcined at 400°C.
The FT-IR spectra of Na2SiO3·9H2O and calcined sodium silicate are shown in Figure 4. IR bands at about 3300 cm−1 and 1600 cm−1 were observed, which were assigned to the hydroxyl groups of adsorbed and bound water [23]. The bands at 849 cm−1 and 880 cm−1 were ascribed to the different forms of the stretching vibration of O-Si-O [21], [24]. After calcination, the bands at about 3300 cm−1 and 1600 cm−1 disappeared, demonstrating that the adsorbed and bound water were removed. The disappearance of the peaks at about 1100 cm−1 and 630 cm−1 was likely due to the change in tetrahedral SiO44−. New Si-O-Si stretching bands appeared at about 710 cm−1 and 880 cm−1, which were attributed to the aggregation of tetrahedral SiO44− and hence formed Si-O-Si [22]. The formed Si-O-Si was beneficial in increasing the mechanical strength [25]. In addition, the depolymerization of formed Si-O-Si (water >3%) and the gelation of sodium silicate (water >20%) [26] could be circumvented under anhydrous conditions. Therefore, the calcined sodium silicate could maintain high stability. Meanwhile, the intensity of Si-O-Na stretching vibration at about 1000 cm−1 [21] became weak. An explanation for this result was that the part of sodium ion was wrapped in the crystal interior [22].
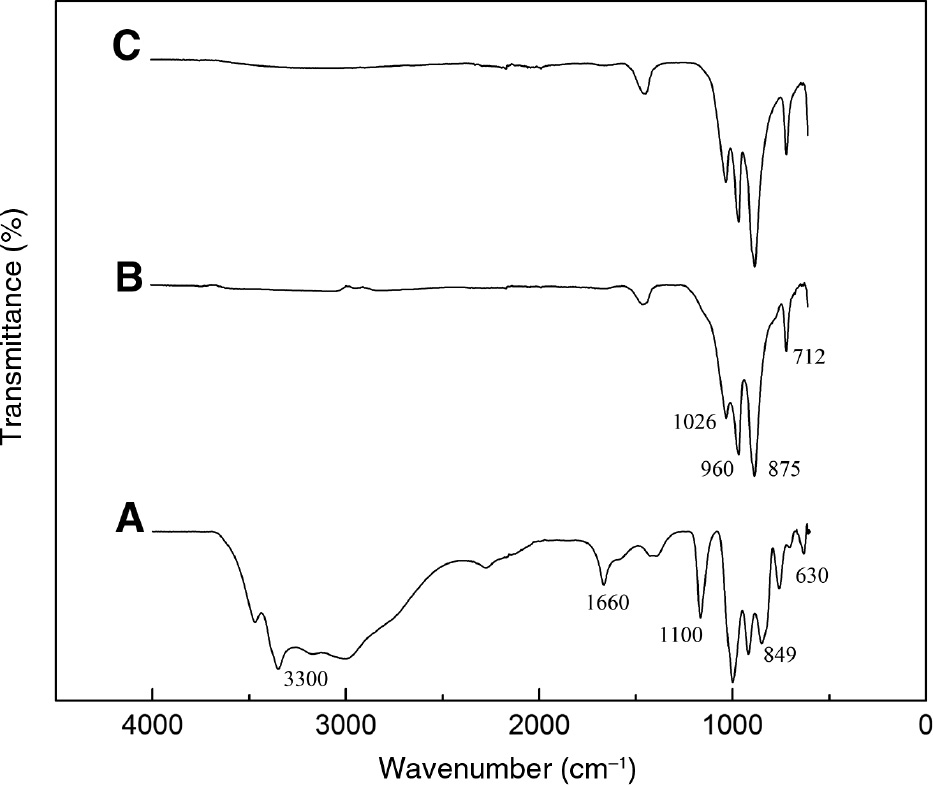
Fourier transform infrared spectroscopy (FT-IR) spectra of (A) sodium metasilicate, (B) sodium silicate calcined at 400°C, and (C) recycled sodium silicate.
3.2 Effects of transesterification parameters
The effect of stirring intensity on the transesterification of PC is shown in Figure 5. The PC conversion increased from 55% to 82% after 60 min with increase in the stirring intensity from 100 rpm to 300 rpm. The increase in reaction rate was probably due to the acceleration of mass transport of PC molecules from solution to particle surface. However, with a further increase in stirring intensity, no appreciable enhancement was observed in the conversion of PC. This phenomenon likely illustrates that the acceleration of diffusion came up to a maximum. Considering the PC conversion and economy aspect, the optimum stirring intensity was chosen as 300 rpm.
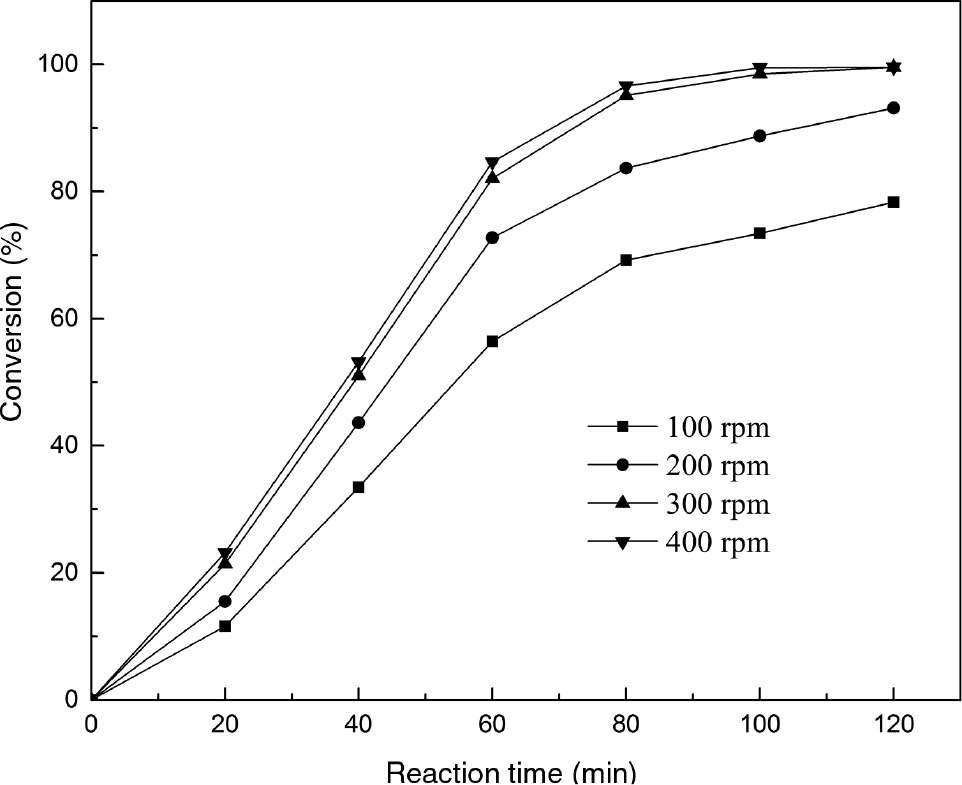
The effect of stirring intensity on catalytic transesterification of phospholipid (PC) by calcined sodium silicate.
The effect of different temperatures on the reaction was investigated. As seen in Figure 6, reaction temperature had a positive effect on the transesterification process. Raising the temperature from 55°C to 65°C resulted in the increase in PC conversion from 89% to about 100%. This was probably because elevating the temperature contributed to the miscibility and reactivity between the reactants [27]. When temperature was raised to 70°C, only slight enhancement was observed in the PC conversion compared to that at 65°C. This result was similar to that obtained by other researchers [28], [29], [30]. An explanation for this phenomenon was that the gasification of methanol could reduce the reactants concentration and hinder the transesterification [31], [32]. In view of these results, a temperature of 65°C was selected as the optimal reaction temperature.
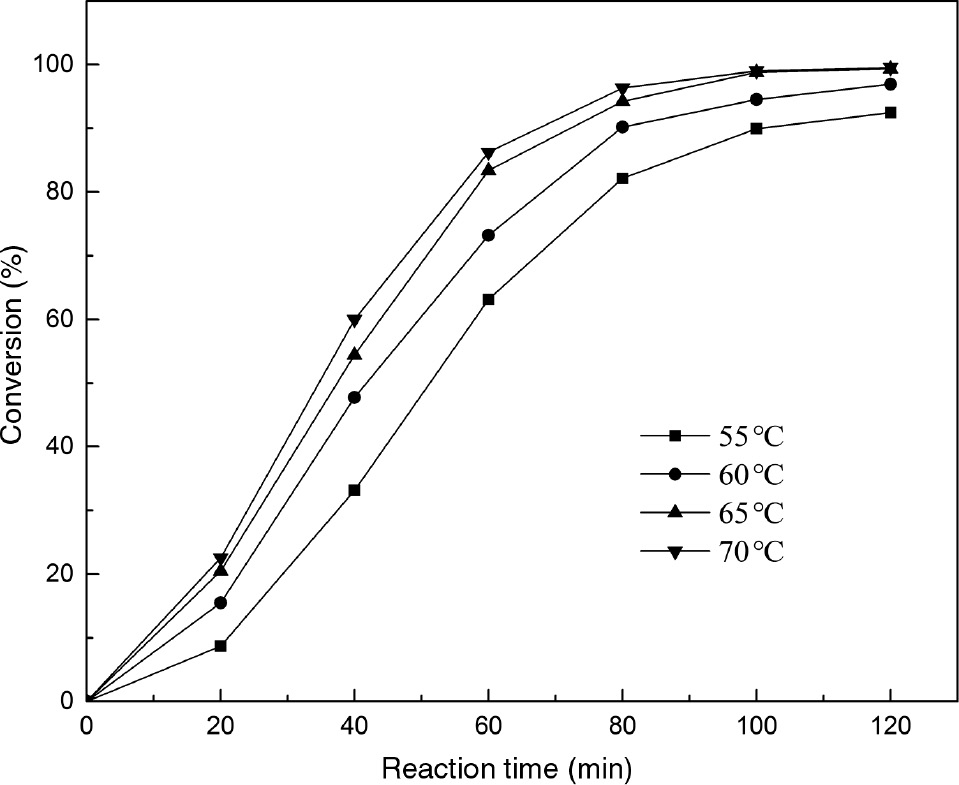
The effect of reaction temperature on transesterification of phospholipid (PC).
The effect of PC concentration on reaction was investigated, which is shown in Figure 7. It can be observed that increasing the PC concentration from 5.25 mmol/l to 10.5 mmol/l led to the enhancement in PC conversion. However, a sharp decrease in PC conversion was observed with a further increase in the PC concentration to 21 mmol/l. The single PC molecules could interact with each other to form large-size aggregates with increase in the concentration [33] and, as a result, the interfacial area between PC and sodium silicate could reduce, resulting in the decrease in reaction rate [34]. Considering the PC conversion and reaction rate, 10.5 mmol/l PC concentration was chosen as the optimum concentration.
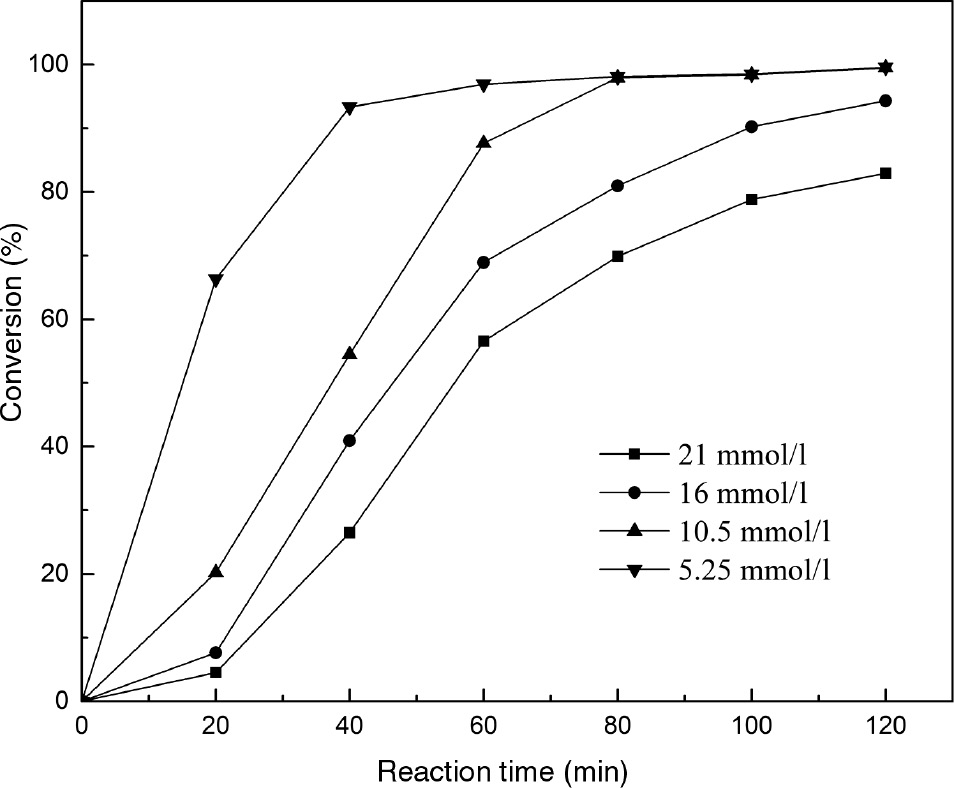
The effect of phospholipid (PC) concentration on transesterification of PC catalyzed by calcined sodium silicate.
The effect of different catalyst amounts from 4 wt% to 7 wt% on the transesterification is shown in Figure 8. The reaction rate was observed to increase with the increase in catalyst amount. As expected, PC conversion clearly increased from 62% to 93% after 80 min with increasing the catalyst amount from 4 wt% to 6 wt%. The easier availability of the catalytic active centers was largely responsible for the enhancement in reaction rate and this result was in accordance with the fact that the transesterification rate increased with increasing the number of active sites [35]. However, with a further addition of the catalyst, only slight acceleration of reaction rate was observed compared to that using 6 wt%. This was likely due to the shielding effect of catalyst particles on stirring [36]. In this context, the catalyst amount of 6 wt% was chosen as the optimization of transesterification.
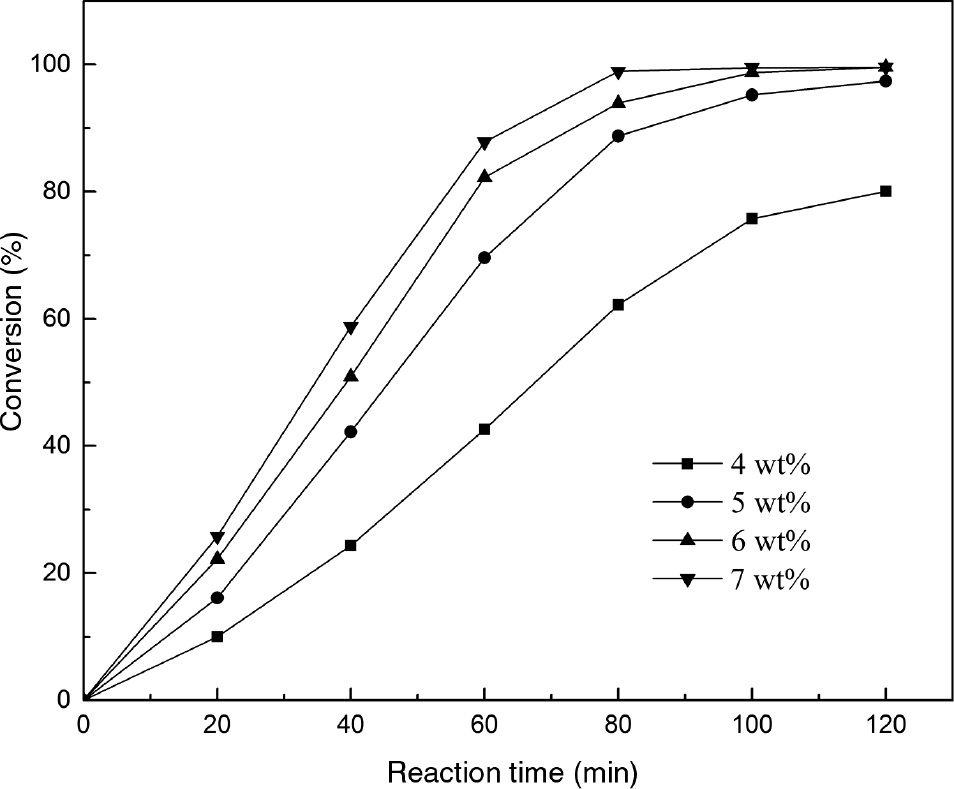
The effect of catalyst amount on transesterification of phospholipid (PC).
3.3 Recyclability of catalyst
The catalyst was reused in 10 consecutive cycles under optimal conditions, which included separation by filtration, washing with ethanol and drying under vacuum conditions. As seen in Figure 9, only 3% decrease in PC conversion was observed after recycling three times. However, PC conversion dropped dramatically with increasin in the recycling. The structure change in recycled sodium silicate is shown in Figure 4. After recycling, the intensity of Si-O-Si stretching at 710 cm−1 decreased and the intensity of Si-O bending vibration at 1022 cm−1 and Si-O stretching vibration at 958 cm−1 increased, which suggested that the Si-O-Si bond was broken. This characterization result was in agreement with that reported in previous literature [21]. By contrast, the fresh, recycled catalyst and catalyst residue in reaction solution were analyzed by an ICP spectrometer. It can be seen in Table 2 that the sodium ion content decreased from 23.86% to 10.89% after recycling 10 times, which was due to the ion-exchange between calcined sodium silicate and methanol [37]. In addition, the strong base content correspondingly decreased from 1.61 mmol/g to 0.35 mmol/g. The decrease in catalyst activity was essentially attributed to the dissolution of sodium ion [38] and the decrease in strong base content. In addition, the ICP result also confirmed that the calcined sodium silicate could be separated completely by filtration, which was beneficial to the purification of GPC.
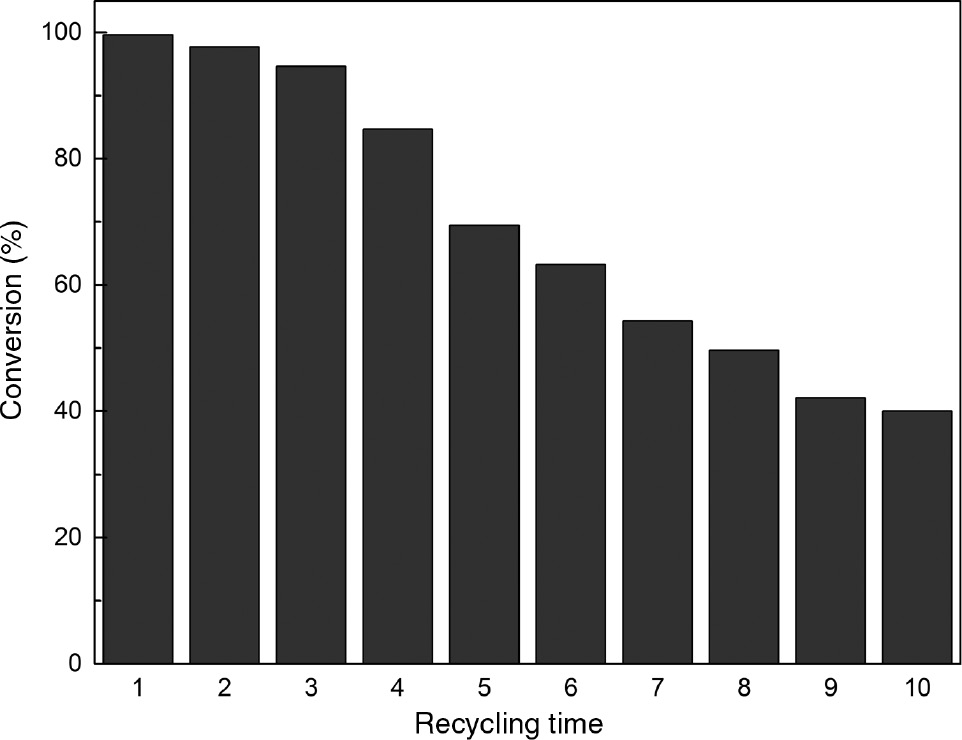
Recyclability of the calcined sodium silicate.
The sodium ion, strong base content of recycled catalyst and catalyst residue in filtrate.
| Recycling | Na+ content (%) | Catalyst residue in filtrate | Strong base strength content |
|---|---|---|---|
| 0 | 23.86 | A | 1.61 |
| 1 | 22.97 | A | 1.54 |
| 2 | 20.94 | A | 1.42 |
| 3 | 19.21 | A | 1.34 |
| 4 | 18.55 | A | 1.25 |
| 5 | 17.70 | A | 1.14 |
| 6 | 16.33 | A | 1.03 |
| 7 | 15.01 | A | 0.88 |
| 8 | 13.87 | A | 0.71 |
| 9 | 12.03 | A | 0.54 |
| 10 | 10.89 | A | 0.35 |
A: no catalyst residue.
In view of all of the above results, the transesterification of PC proceeded rapidly with 6 wt% sodium silicate, 10.5 mmol/l PC concentration and reaction temperature of 65°C for 120 min with stirring at 300 rpm. In addition, calcined sodium silicate could be completely separated by simple filtration and retained relatively high activity. Compared with the recently reported catalysts [10], [15], calcined sodium silicate had better catalytic activity in transesterification. In addition, the security of GPC and its application in food and medicine fields were not affected by calcined sodium silicate because of its non-toxicity and easy excretion from the human body. Although sodium ion dissolution was not avoided during the transesterification process, using sodium silicate as a catalyst could circumvent the drawback of toxic components or catalyst residue compared to the previously reported catalysts such as mercuric chloride [11], tetrabutylammonium hydroxide [12], and sodium methoxide [13]. Therefore, we believe that calcined sodium silicate has the potential to produce GPC in industry with a benign process.
4 Conclusion
Sodium silicate as an efficient and benign heterogeneous catalyst was successfully used in the production of GPC from natural lecithin. The maximum PC conversion of 99.5% could be obtained with 6 wt% sodium silicate, 10.5 mmol/l PC concentration and reaction temperature of 65°C for 120 min with stirring at 300 rpm. Because of the characteristics of non-toxicity, easy excretion from the human body and complete separation from reaction mixture, using calcined sodium silicate as a catalyst did not affect the security and usage of GPC. Therefore, calcined sodium silicate could meet the demand of producing GPC with a benign process.
References
[1] Lorenzo DF, Fausto B, Guido C, Pietro M, Oreste P. EP. 0575717 1998.Search in Google Scholar
[2] Yaqoob M, Nabi A, Hafeez I, Atif Ali S, Masoom-Yasinzai M. Anal. Lett. 1997, 30, 61–68.10.1080/00032719708002290Search in Google Scholar
[3] Dawsom RM, Mann T, White IG. Biochem. J. 1957, 65, 627–634.10.1042/bj0650627Search in Google Scholar
[4] Amenta F, Franch F, Ricci A, Vega JA. Ann. N. Y. Acad. Sci. 1993, 695, 311–313.10.1111/j.1749-6632.1993.tb23073.xSearch in Google Scholar
[5] Muratorio A, Bonuccelli U, Nuti A. Curr. Ther. Res. 1992, 52, 741–752.10.1016/S0011-393X(05)80518-1Search in Google Scholar
[6] Schmidt G, Thannhauser SJ. J. Biol. Chem. 1945, 161, 83–89.10.1016/S0021-9258(17)41524-9Search in Google Scholar
[7] Puricelli L. EP. 0486100 1992.Search in Google Scholar
[8] Puricelli L. EP. 0502357 1992.Search in Google Scholar
[9] Song YS, Song ES, Kang DS, Song IW, Kang PG, Moon SC, Lee BG. WO. 145476 2007.Search in Google Scholar
[10] Li HY, Zhang XL, Bai WL, Zhang JB, Zhang TT, Zhao BX. React. Kinet. Mech. Catal. 2013, 108, 305–316.10.1007/s11144-012-0509-2Search in Google Scholar
[11] Mcarthur CS. US. 2864848 1958.Search in Google Scholar
[12] Brockerhoff H, Yurkowski M. Can. J. Biochem. 1965, 43, 1777–1783.10.1139/o65-197Search in Google Scholar PubMed
[13] Giovanni T. EP. 0217765. 1990.Search in Google Scholar
[14] Zhang TT, Zhang XL, Li HY, Bai WL, Zhao BX. React. Kinet. Mech. Catal. 2013, 110, 31–39.10.1007/s11144-013-0579-9Search in Google Scholar
[15] Li HY, Zhang XL, Zhang TT, Dou KK, Zhao BX. React. Kinet. Mech. Catal. 2012, 107, 345–354.10.1007/s11144-012-0471-zSearch in Google Scholar
[16] Elmore AR. Int. J. Toxicol. 2005, 1, 103–117.Search in Google Scholar
[17] Xie WL, Peng H, Chen LG. J. Mol. Catal. A Chem. 2006, 246, 24–32.10.1016/j.molcata.2005.10.008Search in Google Scholar
[18] Take JI, Kikuchi N, Yoneda Y. J. Catal. 1971, 21, 164–170.10.1016/0021-9517(71)90134-5Search in Google Scholar
[19] Li BL, Wang J, Zhang XL, Zhao BX, Niu L. J. Agric. Food Chem. 2016, 64, 7555–7560.10.1021/acs.jafc.6b03448Search in Google Scholar PubMed
[20] Fan FL, Jia LH, Guo XF, Lu XP, Chen J. Energy Fuels 2013, 17, 5215–5221.10.1021/ef401514eSearch in Google Scholar
[21] Guo F, Peng ZG, Dai JY, Xiu ZL. Fuel Process. Technol. 2010, 91, 322–328.10.1016/j.fuproc.2009.11.003Search in Google Scholar
[22] Wang S, Hao PF, Li SX, Zhang AL, Guan YY, Zhang LN. Appl. Catal. A 2017, 542, 174–181.10.1016/j.apcata.2017.05.021Search in Google Scholar
[23] Tantirungrotechai J, Chotmongkolsap P, Pohmakotr M. Microporous Mesoporous Mater. 2010, 128, 41–47.10.1016/j.micromeso.2009.08.001Search in Google Scholar
[24] Halasz I, Agarwal M, Li R, Miller N. Catal. Lett. 2007, 117, 34–42.10.1007/s10562-007-9141-6Search in Google Scholar
[25] Ferraro JR, Manghnani MH. J. Appl. Phys. 1972, 44, 4595–4598.10.1063/1.1660971Search in Google Scholar
[26] Masui H, Chen DP, Akai T, Yazawa T. Z. Naturforsch. 2002, 57, 473–478.10.1515/zna-2002-6-734Search in Google Scholar
[27] Avhad MR, Sánchez M., Peńa E, Bouaid A, Martínez M, Aracil J, Marchetti JM. Fuel 2016, 179, 332–338.10.1016/j.fuel.2016.03.107Search in Google Scholar
[28] Liu XJ, Piao XL, Wang YJ, Zhu SL. Energy Fuels 2008, 22, 1313–1317.10.1021/ef700518hSearch in Google Scholar
[29] Li H, Niu SL, Lu CM, Li J. Fuel 2016, 176, 63–71.10.1016/j.fuel.2016.02.067Search in Google Scholar
[30] Meher LC, Dharmagadda VSS, Naik SN. Bioresour. Technol. 2006, 97, 1392–1397.10.1016/j.biortech.2005.07.003Search in Google Scholar
[31] Balat M, Balat H. Appl. Energy 2010, 87, 1815–1835.10.1016/j.apenergy.2010.01.012Search in Google Scholar
[32] Madhuvilakku R, Piraman S. Bioresour. Technol. 2013, 150, 55–59.10.1016/j.biortech.2013.09.087Search in Google Scholar
[33] Kodali DR, Tercyak A, Fahey DA, Small DM. Chem. Phys. Lipids 1990, 52, 163–170.10.1016/0009-3084(90)90111-4Search in Google Scholar
[34] Kalva A, Sivasankar T, Moholkar VS. Ind. Eng. Chem. Res. 2009, 48, 534544.Search in Google Scholar
[35] Gole VL, Gogate PR. Chem. Eng. Process. 2012, 53, 1–9.10.1016/j.cep.2011.12.008Search in Google Scholar
[36] Korkut I, Bayramoglu M. Fuel 2016, 180, 624–629.10.1016/j.fuel.2016.04.101Search in Google Scholar
[37] Guo F, Wei NN, Xiu ZL, Fang Z. Fuel 2012, 93, 468–472.10.1016/j.fuel.2011.08.064Search in Google Scholar
[38] Long YD, Guo F, Fang Z, Tian XF, Jiang LQ, Zhang F. Bioresour. Technol. 2011, 102, 6884–6886.10.1016/j.biortech.2011.04.007Search in Google Scholar PubMed
©2019 Walter de Gruyter GmbH, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 Public License.
Articles in the same Issue
- Regular Articles
- Studies on the preparation and properties of biodegradable polyester from soybean oil
- Flow-mode biodiesel production from palm oil using a pressurized microwave reactor
- Reduction of free fatty acids in waste oil for biodiesel production by glycerolysis: investigation and optimization of process parameters
- Saccharin: a cheap and mild acidic agent for the synthesis of azo dyes via telescoped dediazotization
- Optimization of lipase-catalyzed synthesis of polyethylene glycol stearate in a solvent-free system
- Green synthesis of iron oxide nanoparticles using Platanus orientalis leaf extract for antifungal activity
- Ultrasound assisted chemical activation of peanut husk for copper removal
- Room temperature silanization of Fe3O4 for the preparation of phenyl functionalized magnetic adsorbent for dispersive solid phase extraction for the extraction of phthalates in water
- Evaluation of the saponin green extraction from Ziziphus spina-christi leaves using hydrothermal, microwave and Bain-Marie water bath heating methods
- Oxidation of dibenzothiophene using the heterogeneous catalyst of tungsten-based carbon nanotubes
- Calcined sodium silicate as an efficient and benign heterogeneous catalyst for the transesterification of natural lecithin to L-α-glycerophosphocholine
- Synergistic effect between CO2 and H2O2 on ethylbenzene oxidation catalyzed by carbon supported heteropolyanion catalysts
- Hydrocyanation of 2-arylmethyleneindan-1,3-diones using potassium hexacyanoferrate(II) as a nontoxic cyanating agent
- Green synthesis of hydratropic aldehyde from α-methylstyrene catalyzed by Al2O3-supported metal phthalocyanines
- Environmentally benign chemical recycling of polycarbonate wastes: comparison of micro- and nano-TiO2 solid support efficiencies
- Medicago polymorpha-mediated antibacterial silver nanoparticles in the reduction of methyl orange
- Production of value-added chemicals from esterification of waste glycerol over MCM-41 supported catalysts
- Green synthesis of zerovalent copper nanoparticles for efficient reduction of toxic azo dyes congo red and methyl orange
- Optimization of the biological synthesis of silver nanoparticles using Penicillium oxalicum GRS-1 and their antimicrobial effects against common food-borne pathogens
- Optimization of submerged fermentation conditions to overproduce bioethanol using two industrial and traditional Saccharomyces cerevisiae strains
- Extraction of In3+ and Fe3+ from sulfate solutions by using a 3D-printed “Y”-shaped microreactor
- Foliar-mediated Ag:ZnO nanophotocatalysts: green synthesis, characterization, pollutants degradation, and in vitro biocidal activity
- Green cyclic acetals production by glycerol etherification reaction with benzaldehyde using cationic acidic resin
- Biosynthesis, characterization and antimicrobial activities assessment of fabricated selenium nanoparticles using Pelargonium zonale leaf extract
- Synthesis of high surface area magnesia by using walnut shell as a template
- Controllable biosynthesis of silver nanoparticles using actinobacterial strains
- Green vegetation: a promising source of color dyes
- Mechano-chemical synthesis of ammonia and acetic acid from inorganic materials in water
- Green synthesis and structural characterization of novel N1-substituted 3,4-dihydropyrimidin-2(1H)-ones
- Biodiesel production from cotton oil using heterogeneous CaO catalysts from eggshells prepared at different calcination temperatures
- Regeneration of spent mercury catalyst for the treatment of dye wastewater by the microwave and ultrasonic spray-assisted method
- Green synthesis of the innovative super paramagnetic nanoparticles from the leaves extract of Fraxinus chinensis Roxb and their application for the decolourisation of toxic dyes
- Biogenic ZnO nanoparticles: a study of blueshift of optical band gap and photocatalytic degradation of reactive yellow 186 dye under direct sunlight
- Leached compounds from the extracts of pomegranate peel, green coconut shell, and karuvelam wood for the removal of hexavalent chromium
- Enhancement of molecular weight reduction of natural rubber in triphasic CO2/toluene/H2O systems with hydrogen peroxide for preparation of biobased polyurethanes
- An efficient green synthesis of novel 1H-imidazo[1,2-a]imidazole-3-amine and imidazo[2,1-c][1,2,4]triazole-5-amine derivatives via Strecker reaction under controlled microwave heating
- Evaluation of three different green fabrication methods for the synthesis of crystalline ZnO nanoparticles using Pelargonium zonale leaf extract
- A highly efficient and multifunctional biomass supporting Ag, Ni, and Cu nanoparticles through wetness impregnation for environmental remediation
- Simple one-pot green method for large-scale production of mesalamine, an anti-inflammatory agent
- Relationships between step and cumulative PMI and E-factors: implications on estimating material efficiency with respect to charting synthesis optimization strategies
- A comparative sorption study of Cr3+ and Cr6+ using mango peels: kinetic, equilibrium and thermodynamic
- Effects of acid hydrolysis waste liquid recycle on preparation of microcrystalline cellulose
- Use of deep eutectic solvents as catalyst: A mini-review
- Microwave-assisted synthesis of pyrrolidinone derivatives using 1,1’-butylenebis(3-sulfo-3H-imidazol-1-ium) chloride in ethylene glycol
- Green and eco-friendly synthesis of Co3O4 and Ag-Co3O4: Characterization and photo-catalytic activity
- Adsorption optimized of the coal-based material and application for cyanide wastewater treatment
- Aloe vera leaf extract mediated green synthesis of selenium nanoparticles and assessment of their In vitro antimicrobial activity against spoilage fungi and pathogenic bacteria strains
- Waste phenolic resin derived activated carbon by microwave-assisted KOH activation and application to dye wastewater treatment
- Direct ethanol production from cellulose by consortium of Trichoderma reesei and Candida molischiana
- Agricultural waste biomass-assisted nanostructures: Synthesis and application
- Biodiesel production from rubber seed oil using calcium oxide derived from eggshell as catalyst – optimization and modeling studies
- Study of fabrication of fully aqueous solution processed SnS quantum dot-sensitized solar cell
- Assessment of aqueous extract of Gypsophila aretioides for inhibitory effects on calcium carbonate formation
- An environmentally friendly acylation reaction of 2-methylnaphthalene in solvent-free condition in a micro-channel reactor
- Aegle marmelos phytochemical stabilized synthesis and characterization of ZnO nanoparticles and their role against agriculture and food pathogen
- A reactive coupling process for co-production of solketal and biodiesel
- Optimization of the asymmetric synthesis of (S)-1-phenylethanol using Ispir bean as whole-cell biocatalyst
- Synthesis of pyrazolopyridine and pyrazoloquinoline derivatives by one-pot, three-component reactions of arylglyoxals, 3-methyl-1-aryl-1H-pyrazol-5-amines and cyclic 1,3-dicarbonyl compounds in the presence of tetrapropylammonium bromide
- Preconcentration of morphine in urine sample using a green and solvent-free microextraction method
- Extraction of glycyrrhizic acid by aqueous two-phase system formed by PEG and two environmentally friendly organic acid salts - sodium citrate and sodium tartrate
- Green synthesis of copper oxide nanoparticles using Juglans regia leaf extract and assessment of their physico-chemical and biological properties
- Deep eutectic solvents (DESs) as powerful and recyclable catalysts and solvents for the synthesis of 3,4-dihydropyrimidin-2(1H)-ones/thiones
- Biosynthesis, characterization and anti-microbial activity of silver nanoparticle based gel hand wash
- Efficient and selective microwave-assisted O-methylation of phenolic compounds using tetramethylammonium hydroxide (TMAH)
- Anticoagulant, thrombolytic and antibacterial activities of Euphorbia acruensis latex-mediated bioengineered silver nanoparticles
- Volcanic ash as reusable catalyst in the green synthesis of 3H-1,5-benzodiazepines
- Green synthesis, anionic polymerization of 1,4-bis(methacryloyl)piperazine using Algerian clay as catalyst
- Selenium supplementation during fermentation with sugar beet molasses and Saccharomyces cerevisiae to increase bioethanol production
- Biosynthetic potential assessment of four food pathogenic bacteria in hydrothermally silver nanoparticles fabrication
- Investigating the effectiveness of classical and eco-friendly approaches for synthesis of dialdehydes from organic dihalides
- Pyrolysis of palm oil using zeolite catalyst and characterization of the boil-oil
- Azadirachta indica leaves extract assisted green synthesis of Ag-TiO2 for degradation of Methylene blue and Rhodamine B dyes in aqueous medium
- Synthesis of vitamin E succinate catalyzed by nano-SiO2 immobilized DMAP derivative in mixed solvent system
- Extraction of phytosterols from melon (Cucumis melo) seeds by supercritical CO2 as a clean technology
- Production of uronic acids by hydrothermolysis of pectin as a model substance for plant biomass waste
- Biofabrication of highly pure copper oxide nanoparticles using wheat seed extract and their catalytic activity: A mechanistic approach
- Intelligent modeling and optimization of emulsion aggregation method for producing green printing ink
- Improved removal of methylene blue on modified hierarchical zeolite Y: Achieved by a “destructive-constructive” method
- Two different facile and efficient approaches for the synthesis of various N-arylacetamides via N-acetylation of arylamines and straightforward one-pot reductive acetylation of nitroarenes promoted by recyclable CuFe2O4 nanoparticles in water
- Optimization of acid catalyzed esterification and mixed metal oxide catalyzed transesterification for biodiesel production from Moringa oleifera oil
- Kinetics and the fluidity of the stearic acid esters with different carbon backbones
- Aiming for a standardized protocol for preparing a process green synthesis report and for ranking multiple synthesis plans to a common target product
- Microstructure and luminescence of VO2 (B) nanoparticle synthesis by hydrothermal method
- Optimization of uranium removal from uranium plant wastewater by response surface methodology (RSM)
- Microwave drying of nickel-containing residue: dielectric properties, kinetics, and energy aspects
- Simple and convenient two step synthesis of 5-bromo-2,3-dimethoxy-6-methyl-1,4-benzoquinone
- Biodiesel production from waste cooking oil
- The effect of activation temperature on structure and properties of blue coke-based activated carbon by CO2 activation
- Optimization of reaction parameters for the green synthesis of zero valent iron nanoparticles using pine tree needles
- Microwave-assisted protocol for squalene isolation and conversion from oil-deodoriser distillates
- Denitrification performance of rare earth tailings-based catalysts
- Facile synthesis of silver nanoparticles using Averrhoa bilimbi L and Plum extracts and investigation on the synergistic bioactivity using in vitro models
- Green production of AgNPs and their phytostimulatory impact
- Photocatalytic activity of Ag/Ni bi-metallic nanoparticles on textile dye removal
- Topical Issue: Green Process Engineering / Guest Editors: Martine Poux, Patrick Cognet
- Modelling and optimisation of oxidative desulphurisation of tyre-derived oil via central composite design approach
- CO2 sequestration by carbonation of olivine: a new process for optimal separation of the solids produced
- Organic carbonates synthesis improved by pervaporation for CO2 utilisation
- Production of starch nanoparticles through solvent-antisolvent precipitation in a spinning disc reactor
- A kinetic study of Zn halide/TBAB-catalysed fixation of CO2 with styrene oxide in propylene carbonate
- Topical on Green Process Engineering
Articles in the same Issue
- Regular Articles
- Studies on the preparation and properties of biodegradable polyester from soybean oil
- Flow-mode biodiesel production from palm oil using a pressurized microwave reactor
- Reduction of free fatty acids in waste oil for biodiesel production by glycerolysis: investigation and optimization of process parameters
- Saccharin: a cheap and mild acidic agent for the synthesis of azo dyes via telescoped dediazotization
- Optimization of lipase-catalyzed synthesis of polyethylene glycol stearate in a solvent-free system
- Green synthesis of iron oxide nanoparticles using Platanus orientalis leaf extract for antifungal activity
- Ultrasound assisted chemical activation of peanut husk for copper removal
- Room temperature silanization of Fe3O4 for the preparation of phenyl functionalized magnetic adsorbent for dispersive solid phase extraction for the extraction of phthalates in water
- Evaluation of the saponin green extraction from Ziziphus spina-christi leaves using hydrothermal, microwave and Bain-Marie water bath heating methods
- Oxidation of dibenzothiophene using the heterogeneous catalyst of tungsten-based carbon nanotubes
- Calcined sodium silicate as an efficient and benign heterogeneous catalyst for the transesterification of natural lecithin to L-α-glycerophosphocholine
- Synergistic effect between CO2 and H2O2 on ethylbenzene oxidation catalyzed by carbon supported heteropolyanion catalysts
- Hydrocyanation of 2-arylmethyleneindan-1,3-diones using potassium hexacyanoferrate(II) as a nontoxic cyanating agent
- Green synthesis of hydratropic aldehyde from α-methylstyrene catalyzed by Al2O3-supported metal phthalocyanines
- Environmentally benign chemical recycling of polycarbonate wastes: comparison of micro- and nano-TiO2 solid support efficiencies
- Medicago polymorpha-mediated antibacterial silver nanoparticles in the reduction of methyl orange
- Production of value-added chemicals from esterification of waste glycerol over MCM-41 supported catalysts
- Green synthesis of zerovalent copper nanoparticles for efficient reduction of toxic azo dyes congo red and methyl orange
- Optimization of the biological synthesis of silver nanoparticles using Penicillium oxalicum GRS-1 and their antimicrobial effects against common food-borne pathogens
- Optimization of submerged fermentation conditions to overproduce bioethanol using two industrial and traditional Saccharomyces cerevisiae strains
- Extraction of In3+ and Fe3+ from sulfate solutions by using a 3D-printed “Y”-shaped microreactor
- Foliar-mediated Ag:ZnO nanophotocatalysts: green synthesis, characterization, pollutants degradation, and in vitro biocidal activity
- Green cyclic acetals production by glycerol etherification reaction with benzaldehyde using cationic acidic resin
- Biosynthesis, characterization and antimicrobial activities assessment of fabricated selenium nanoparticles using Pelargonium zonale leaf extract
- Synthesis of high surface area magnesia by using walnut shell as a template
- Controllable biosynthesis of silver nanoparticles using actinobacterial strains
- Green vegetation: a promising source of color dyes
- Mechano-chemical synthesis of ammonia and acetic acid from inorganic materials in water
- Green synthesis and structural characterization of novel N1-substituted 3,4-dihydropyrimidin-2(1H)-ones
- Biodiesel production from cotton oil using heterogeneous CaO catalysts from eggshells prepared at different calcination temperatures
- Regeneration of spent mercury catalyst for the treatment of dye wastewater by the microwave and ultrasonic spray-assisted method
- Green synthesis of the innovative super paramagnetic nanoparticles from the leaves extract of Fraxinus chinensis Roxb and their application for the decolourisation of toxic dyes
- Biogenic ZnO nanoparticles: a study of blueshift of optical band gap and photocatalytic degradation of reactive yellow 186 dye under direct sunlight
- Leached compounds from the extracts of pomegranate peel, green coconut shell, and karuvelam wood for the removal of hexavalent chromium
- Enhancement of molecular weight reduction of natural rubber in triphasic CO2/toluene/H2O systems with hydrogen peroxide for preparation of biobased polyurethanes
- An efficient green synthesis of novel 1H-imidazo[1,2-a]imidazole-3-amine and imidazo[2,1-c][1,2,4]triazole-5-amine derivatives via Strecker reaction under controlled microwave heating
- Evaluation of three different green fabrication methods for the synthesis of crystalline ZnO nanoparticles using Pelargonium zonale leaf extract
- A highly efficient and multifunctional biomass supporting Ag, Ni, and Cu nanoparticles through wetness impregnation for environmental remediation
- Simple one-pot green method for large-scale production of mesalamine, an anti-inflammatory agent
- Relationships between step and cumulative PMI and E-factors: implications on estimating material efficiency with respect to charting synthesis optimization strategies
- A comparative sorption study of Cr3+ and Cr6+ using mango peels: kinetic, equilibrium and thermodynamic
- Effects of acid hydrolysis waste liquid recycle on preparation of microcrystalline cellulose
- Use of deep eutectic solvents as catalyst: A mini-review
- Microwave-assisted synthesis of pyrrolidinone derivatives using 1,1’-butylenebis(3-sulfo-3H-imidazol-1-ium) chloride in ethylene glycol
- Green and eco-friendly synthesis of Co3O4 and Ag-Co3O4: Characterization and photo-catalytic activity
- Adsorption optimized of the coal-based material and application for cyanide wastewater treatment
- Aloe vera leaf extract mediated green synthesis of selenium nanoparticles and assessment of their In vitro antimicrobial activity against spoilage fungi and pathogenic bacteria strains
- Waste phenolic resin derived activated carbon by microwave-assisted KOH activation and application to dye wastewater treatment
- Direct ethanol production from cellulose by consortium of Trichoderma reesei and Candida molischiana
- Agricultural waste biomass-assisted nanostructures: Synthesis and application
- Biodiesel production from rubber seed oil using calcium oxide derived from eggshell as catalyst – optimization and modeling studies
- Study of fabrication of fully aqueous solution processed SnS quantum dot-sensitized solar cell
- Assessment of aqueous extract of Gypsophila aretioides for inhibitory effects on calcium carbonate formation
- An environmentally friendly acylation reaction of 2-methylnaphthalene in solvent-free condition in a micro-channel reactor
- Aegle marmelos phytochemical stabilized synthesis and characterization of ZnO nanoparticles and their role against agriculture and food pathogen
- A reactive coupling process for co-production of solketal and biodiesel
- Optimization of the asymmetric synthesis of (S)-1-phenylethanol using Ispir bean as whole-cell biocatalyst
- Synthesis of pyrazolopyridine and pyrazoloquinoline derivatives by one-pot, three-component reactions of arylglyoxals, 3-methyl-1-aryl-1H-pyrazol-5-amines and cyclic 1,3-dicarbonyl compounds in the presence of tetrapropylammonium bromide
- Preconcentration of morphine in urine sample using a green and solvent-free microextraction method
- Extraction of glycyrrhizic acid by aqueous two-phase system formed by PEG and two environmentally friendly organic acid salts - sodium citrate and sodium tartrate
- Green synthesis of copper oxide nanoparticles using Juglans regia leaf extract and assessment of their physico-chemical and biological properties
- Deep eutectic solvents (DESs) as powerful and recyclable catalysts and solvents for the synthesis of 3,4-dihydropyrimidin-2(1H)-ones/thiones
- Biosynthesis, characterization and anti-microbial activity of silver nanoparticle based gel hand wash
- Efficient and selective microwave-assisted O-methylation of phenolic compounds using tetramethylammonium hydroxide (TMAH)
- Anticoagulant, thrombolytic and antibacterial activities of Euphorbia acruensis latex-mediated bioengineered silver nanoparticles
- Volcanic ash as reusable catalyst in the green synthesis of 3H-1,5-benzodiazepines
- Green synthesis, anionic polymerization of 1,4-bis(methacryloyl)piperazine using Algerian clay as catalyst
- Selenium supplementation during fermentation with sugar beet molasses and Saccharomyces cerevisiae to increase bioethanol production
- Biosynthetic potential assessment of four food pathogenic bacteria in hydrothermally silver nanoparticles fabrication
- Investigating the effectiveness of classical and eco-friendly approaches for synthesis of dialdehydes from organic dihalides
- Pyrolysis of palm oil using zeolite catalyst and characterization of the boil-oil
- Azadirachta indica leaves extract assisted green synthesis of Ag-TiO2 for degradation of Methylene blue and Rhodamine B dyes in aqueous medium
- Synthesis of vitamin E succinate catalyzed by nano-SiO2 immobilized DMAP derivative in mixed solvent system
- Extraction of phytosterols from melon (Cucumis melo) seeds by supercritical CO2 as a clean technology
- Production of uronic acids by hydrothermolysis of pectin as a model substance for plant biomass waste
- Biofabrication of highly pure copper oxide nanoparticles using wheat seed extract and their catalytic activity: A mechanistic approach
- Intelligent modeling and optimization of emulsion aggregation method for producing green printing ink
- Improved removal of methylene blue on modified hierarchical zeolite Y: Achieved by a “destructive-constructive” method
- Two different facile and efficient approaches for the synthesis of various N-arylacetamides via N-acetylation of arylamines and straightforward one-pot reductive acetylation of nitroarenes promoted by recyclable CuFe2O4 nanoparticles in water
- Optimization of acid catalyzed esterification and mixed metal oxide catalyzed transesterification for biodiesel production from Moringa oleifera oil
- Kinetics and the fluidity of the stearic acid esters with different carbon backbones
- Aiming for a standardized protocol for preparing a process green synthesis report and for ranking multiple synthesis plans to a common target product
- Microstructure and luminescence of VO2 (B) nanoparticle synthesis by hydrothermal method
- Optimization of uranium removal from uranium plant wastewater by response surface methodology (RSM)
- Microwave drying of nickel-containing residue: dielectric properties, kinetics, and energy aspects
- Simple and convenient two step synthesis of 5-bromo-2,3-dimethoxy-6-methyl-1,4-benzoquinone
- Biodiesel production from waste cooking oil
- The effect of activation temperature on structure and properties of blue coke-based activated carbon by CO2 activation
- Optimization of reaction parameters for the green synthesis of zero valent iron nanoparticles using pine tree needles
- Microwave-assisted protocol for squalene isolation and conversion from oil-deodoriser distillates
- Denitrification performance of rare earth tailings-based catalysts
- Facile synthesis of silver nanoparticles using Averrhoa bilimbi L and Plum extracts and investigation on the synergistic bioactivity using in vitro models
- Green production of AgNPs and their phytostimulatory impact
- Photocatalytic activity of Ag/Ni bi-metallic nanoparticles on textile dye removal
- Topical Issue: Green Process Engineering / Guest Editors: Martine Poux, Patrick Cognet
- Modelling and optimisation of oxidative desulphurisation of tyre-derived oil via central composite design approach
- CO2 sequestration by carbonation of olivine: a new process for optimal separation of the solids produced
- Organic carbonates synthesis improved by pervaporation for CO2 utilisation
- Production of starch nanoparticles through solvent-antisolvent precipitation in a spinning disc reactor
- A kinetic study of Zn halide/TBAB-catalysed fixation of CO2 with styrene oxide in propylene carbonate
- Topical on Green Process Engineering

