Abstract
In this study, enantiomerically pure (S)-1-phenylethanol was produced via asymmetric bioreduction of acetophenone. Ispir bean (Phaseolus vulgaris) was used as an alcohol dehydrogenase (ADH) source since whole cells are cheaper than isolated enzymes. Acetone powder methodology was applied for biocatalyst. Glucose was used as a cosubstrate in-order to regenerate cofactor (NADPH). The reactions were carried out in an orbital shaker whose temperature and agitation rate can be controlled. (S)-1-phenylethanol concentration was analyzed by HPLC using a Chiralcel OB column. Effects of the reaction time, substrate concentration, cosubstrate concentration and biocatalyst concentration on the (S)-1-phenylethanol production were investigated using Response Surface Methodology (RSM). 36 h bioreduction time, 6 mM acetophenone concentration, 25.15 mM glucose concentration, and 175 mg/mL biocatalyst concentration were determined as optimum values. In these conditions, 2.4 mM (S)-1-phenylethanol was obtained in phosphate buffer (pH=7.0) at 30°C with >99% enantiomeric excess.
1 Introduction
The mechanism of drug interactions as well as the significance of chirality is well understood. Often only one stereoisomer exhibits therapeutic biological activity, the other isomer does not have such an effect. Therefore, enantiopurity has the foremost significance of the production [1]. Chiral alcohols are valuable and helpful starting materials for the synthesis of several modern pharmaceuticals and agrochemicals. Thus it is important to provide enantiopure compounds in biotechnology [2, 3, 4, 5, 6]. In a kinetic resolution, the theoretical yield is 50% of the starting material [6]. Therefore, asymmetric reduction of prochiral ketones into chiral alcohols is a widely used process [7, 8]. Since, it can give 100% theoretical yield [9].
(R)- and (S)- phenylethanols are useful building blocks for the synthesis of complex molecules and are attractive compounds for a wide range of potential application in drug industry [10, 11, 12].
Biocatalysts have many advantages over all other catalysts. Enzymes are one kind of biocatalyst and they specifically catalyze chemical reactions. Because they can actively work under moderate condition, enzymes are an important alternative in terms of environmental factors in chemical processes industry [6]. Instead of using an isolated enzyme as a biocatalyst in asymmetric reduction reactions, whole-cell is a preferable alternative with the main advantage of the elimination of costly enzyme purification process. At the same time, enzymes are most stable due to the presence of their natural environment inside the cells [13, 14]. Also with the use of whole cells, may not necessitate the addition of expensive cofactors [13, 14, 15]. When whole-cell biocatalysts were used, internal cofactor regeneration is possible by adding cosubstrate or glucose [14]. Plant cells are a potential enzyme source for the asymmetric reduction reactions. The use of locally accessible plant cells may offer an alternative to isolated enzymes in recent years [5, 9, 13, 15, 16, 17].
There are many asymmetric reduction reactions with various fruits and vegetables, for example, carrot, potato, sweet potato, apple, cucumber, onion, radish, grape, garlic, tomato, peach, orange, apple, bean, turnip are reported in the literature [5, 7, 8, 9,13,15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25]. In these studies, the conversion and enantiomeric excess values range from 30% to 100% and 33% to >99% respectively.
Also, different biological whole-cell catalyst can be used as an enzyme source for the asymmetric reduction reactions of different substrates. For example, Lactobacillus kefir reduced the acetophenone to (R)-1-phenylethanol with >%99 enantiomeric excess and 79% conversion [26], Kluyveromyces marxianus reduced different aryl ketones to (S)-alcohols in the under mild reaction conditions with nearly 96% enantiomeric excess [27], Lactobacillus reuteri whole cells could reduce various aryl ketones with high conversion and enantiomeric excess [28], and baker’s yeast reduced the different aryl-containing ketones generally up to 90% enantiomeric excess and conversion [29].
The acetone powder methodology, which is a preparation process applied to biocatalyst, has become a preferred method in recent years. Under favor of this pretreatment, biocatalyst size can be minimized, mass transfer limitations are reduced and enzyme-substrate interaction is increased. Asymmetric reduction reactions are catalyzed by ADH. ADH catalyze the enantioselective reduction of ketones with the help of nicotinamide cofactors (NADH or NADPH). However, cofactors are highly expensive and at the end of the reaction nicotinamide cofactors run out. With ADH-GDH enzyme couple, cofactor regeneration was carried out successfully.
Only a few acetone powder biocatalyst studies have been reported in the literature. Nakamura et al. [30] reported acetone powder of Geotrichum candidum to catalyze the reduction of ketones. They compared resting cell and acetone powder on the reduction of Methyl 3-Oxobutanoate. For 30 mM substrate concentration, resting cell and acetone powder showed that 97% yield, 39% enantiomeric excess and 99% yield and 99% enantiomeric excess. They performed the reduction reaction presence of 2-hexanol and a small amount of a coenzyme, NAD+ and as a result the enantiomeric excess increased from 39% to 99%. On the other hand, they showed that acetone powder of G. candidum reduced acetophenone with 89% yield and 99% enantiomeric excess. Hamada et al. [31] reported acetone powder of G. candidum reduced (R)-2-chloro-1-(m-chlorophenyl) ethanol with 94% yield and 98% enantiomeric excess for 8.3 mM initial substrate concentration. They used NAD+ for coenzyme and 2-propanol for reducing agent. Xie et al. [9] reported that acetone powder of adzuki bean could reduce various aromatic ketones at high concentrations. Their study showed that the adzuki bean could reduce 100 mM acetophenone, exhibiting 98.6% enantiomeric excess and 90.5% conversion. Nakamura et al. [32] reported that when biocatalyst changed from wet whole-cell to powdered, no reduction was observed because of the loss of the necessary co-enzyme(s) and/or co-enzyme regeneration systems during treatment the cells with acetone. They recommended to the addition of cosubstrate for improvement the enantioselectivity. Moreover, they mentioned that the addition of both cosubstrate and NAD+ enormously advanced both chemical yield and enantiomeric excess.
In this study, (S)-1-phenylethanol which is the precursor of many pharmacological products was produced by asymmetric bioreduction of acetophenone. Aceton powder of Ispir beans have been used as ADH source. Optimum conditions for reaction time, initial substrate concentration, cosubstrate concentration and, biocatalyst concentration to produce (S)-1-phenylethanol was defined using Response Surface Methodology.
2 Materials and methods
2.1 Chemicals
Acetophenone, (R)-1-phenylethanol, (S)-1-phenylethanol, and other chemicals were purchased from Sigma Aldrich (Sigma-Aldrich Corporate, St. Louis, MO USA).
2.2 Biocatalyst
Ispir beans (Phaseolus vulgaris) were obtained from a local producer in Ispir-Erzurum-Turkey. A metal mortar was used to grind the Ispir beans.
2.3 Biocatalyst preparation: Acetone powder
Ispir bean particulates were kept in water (the mass ratio of particulate to water was 1/5) for 5-6 h at room temperature with 100 rpm stirring rate. The supernatant after steeping was centrifuged at 7500 rpm for 20 min and then filtrated. The supernatant was slowly mixed with chilled acetone (−20°C, v/v = 1/1). The suspension was centrifuged again at 7500 rpm for 20 min and the precipitate was collected and stored at 4°C [9].
2.4 Bioreduction experiments in a batch bioreactor
Biotransformation of acetophenone (Figure 1) by asymmetric reduction was carried out in a batch bioreactor. Experiments performed in the batch system were carried out in 50 mL mouth-capped bottles with 10 mL working volume. Desired amount of biocatalyst was added to the buffer medium. Acetophenone dissolved in the dimethyl sulfoxide solvent and glucose used for the cofactor regeneration was added to the buffer medium (pH=7.0) to initiate the reaction. The reaction was carried out 30°C and 150 rpm on an orbital shaker.
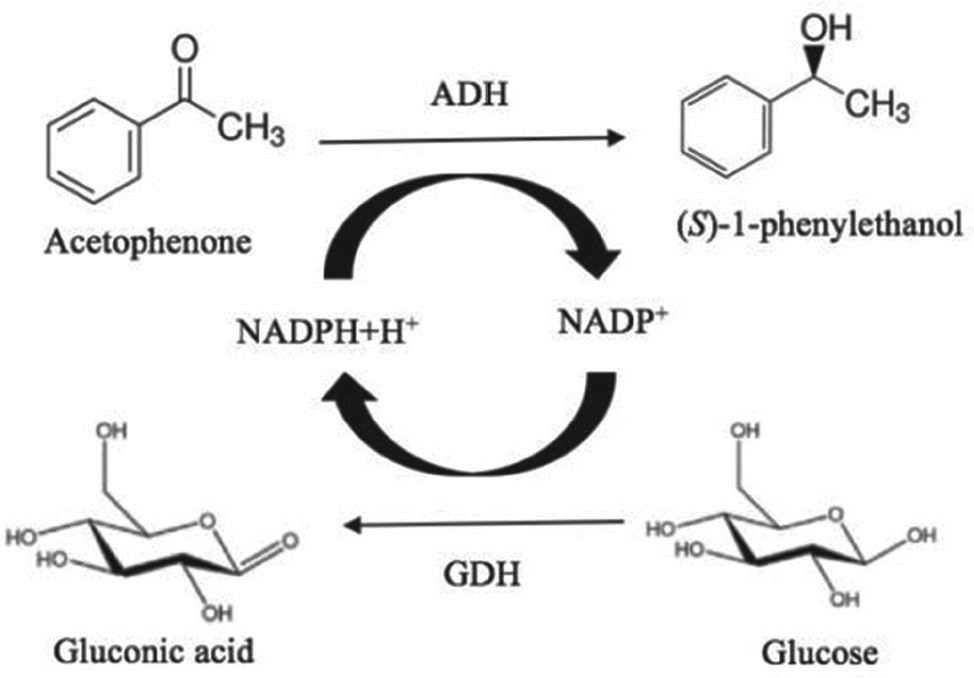
Asymmetric bioreduction of acetophenone catalyzed by Ispir bean.
All of the reactions were carried out as double-repetitive. In bioreduction reactions, reaction time, substrate concentration, cosubstrate concentration and, biocatalyst concentration were optimized to maximize the produced (S)-1-phenylethanol using Response Surface Methodology (RSM).
2.5 Analytical methods
At the end of the reaction, the organic and aqueous phases were separated and the organic phase was analyzed by HPLC. (R)- and (S)- phenylethanols analysis was performed Chiralcel OB column (4.6 mm × 50 mm, Daicel Chemical Ind. Ltd. France) at 30°C, 10 microliters of injection volume, 0.90 mL/min flow rate of hexane/2-propanol (95/05) eluent, with a 254 nm UV detector [26]. The enantiomeric excess (ee) and conversion rate (C) were calculated using Eq. 1 and Eq. 2, respectively.
2.6 Response surface methodology
RSM is a mathematical and statistical method used to optimize the operation conditions. It is also used to define the optimum operating conditions. In RSM, the quadratic model is widely used. Since the it is more flexible and in it is easy to estimate the parameters. Also, quadratic models are good at solving real-response surface problems [33].
Quadratic model can be shown as:
where x1 − xn are the input variables, Y is a response, Bi and Bij are unknown parameters and λ is an error [34].
For the quadratic model used in this study, the range and levels of independent variables and the numerical values of quantities are displayed in Table 1.
Experimental range and levels of independent variables.
| Independent variables | Range and levels | ||||
|---|---|---|---|---|---|
| –2 | –1 | 0 | 1 | 2 | |
| Reaction time (h), X1 | 24 | 36 | 48 | 60 | 72 |
| Substrate concentration (mM), X2 | 2 | 6 | 10 | 14 | 18 |
| Glucose concentration (mM), X3 | 20 | 25 | 30 | 35 | 40 |
| Biocatalyst concentration (mg/mL), X4 | 100 | 125 | 150 | 175 | 200 |
In the regression equation, the test variables were coded according to the following equation:
where xi is the coded value of the i-th independent variable, Xi is the uncoded value of the i-th independent variable, X* iis the uncoded i-th independent variable at the center point and ΔXi is the step change value [34]. 24 full factorial center composite design for four independent variables was used in this study. The full factorial composite design consists of a complete 2k factorial design, where k is the number of test variables, n0 center points (n0≥1), and two axial points on the axis of each design variable at a distance of α (=2k/4, (=2 for k=4) from the design center. The total number of design points is N=2k+2k+n0; thus for this procedure, 30 experiments were required for 4 independent variables [35].
Pre-experimental studies on asymmetric reduction of acetophenone show that the input minimum and maximum values of substrate concentration, cosubstrate concentration, and biocatalyst concentration will be as listed in Table 1.
Reaction time, substrate concentration, glucose concentration, and biocatalyst concentration were chosen as the independent output variables and response variable is determined as (S)-1-phenylethanol concentration obtained as the product. The ‘Design Expert’ software (Version 6.01, Stat-Ease, Inc., Minneapolis, USA) was used for regression and graphical analysis of the data obtained.
The statistical importance of the quadratic model was determined by the analysis of variance (ANOVA). The effect of each parameter was evaluated by the F-value and the p-value. The characteristic of the fit of the quadratic model was identified by the R2 value.
3 Results
In this study, enantiopure (S)-1-phenylethanol with high enantioselectivity and chemical yield was produced with asymmetric bioreduction of acetophenone. The asymmetric synthesis of alcohol is significantly influenced by the initial substrate concentration, glucose concentration, reaction time and biocatalyst concentration [36].
The RSM experiments performed and the results obtained under the operational conditions are listed in Table 2. The quadratic model equation is given below for the actual value (Eq. 5).
The actual values of the experimental conditions and response variable.
| Run | t (h) | CSo(mM) | Cg(mM) | Cb(mg/mL) | C(s)-1-PE(mM) |
|---|---|---|---|---|---|
| 1 | 36 | 6 | 25 | 125 | 1.30 |
| 2 | 60 | 6 | 25 | 125 | 1.70 |
| 3 | 36 | 14 | 25 | 125 | 2.76 |
| 4 | 60 | 14 | 25 | 125 | 2.73 |
| 5 | 36 | 6 | 35 | 125 | 1.48 |
| 6 | 60 | 6 | 35 | 125 | 1.42 |
| 7 | 36 | 14 | 35 | 125 | 1.93 |
| 8 | 60 | 14 | 35 | 125 | 2.52 |
| 9 | 36 | 6 | 25 | 175 | 2.37 |
| 10 | 60 | 6 | 25 | 175 | 1.95 |
| 11 | 36 | 14 | 25 | 175 | 2.98 |
| 12 | 60 | 14 | 25 | 175 | 2.65 |
| 13 | 36 | 6 | 35 | 175 | 2.53 |
| 14 | 60 | 6 | 35 | 175 | 0.39 |
| 15 | 36 | 14 | 35 | 175 | 0.41 |
| 16 | 60 | 14 | 35 | 175 | 0.69 |
| 17 | 24 | 10 | 30 | 150 | 1.67 |
| 18 | 72 | 10 | 30 | 150 | 0.27 |
| 19 | 48 | 2 | 30 | 150 | 1.44 |
| 20 | 48 | 18 | 30 | 150 | 2.07 |
| 21 | 48 | 10 | 20 | 150 | 1.86 |
| 22 | 48 | 10 | 40 | 150 | 0.42 |
| 23 | 48 | 10 | 30 | 100 | 0.26 |
| 24 | 48 | 10 | 30 | 200 | 0.33 |
| 25 | 48 | 10 | 30 | 150 | 0.50 |
| 26 | 48 | 10 | 30 | 150 | 0.50 |
| 27 | 48 | 10 | 30 | 150 | 0.46 |
| 28 | 48 | 10 | 30 | 150 | 0.59 |
| 29 | 48 | 10 | 30 | 150 | 0.27 |
| 30 | 48 | 10 | 30 | 150 | 0.38 |
where Y is the concentration of (S)-1-phenylethanol (C(s)-1-PE). Enantiomeric excess values were obtained at > 99% in all the experiments.
In Table 3, the model F-value of 3.67 implies the model is significant. Probe values indicate the importance of each coefficient. These design variables are important parameters when the probe> F value is less than 0.05. For the model, Probe> F (<0.0088) less than 0.05 indicates that the model represents the system well. The “Lack of Fit F-value” of 45.44 implies the Lack of Fit is significant. Adeq Precision measures the signal to noise ratio and a ratio greater than 4 is desirable. For the model, the ratio of 5.986 indicates an adequate signal. This model can be used to navigate the design space.
ANOVA for quadratic model.
| Source | Sum of Squares | DF | Mean Square | F-value | p-value Prob > F |
|---|---|---|---|---|---|
| Model | 19.50 | 14 | 1.39 | 3.67 | 0.0088 |
| x1 | 0.85 | 1 | 0.85 | 2.23 | 0.1560 |
| x2 | 0.95 | 1 | 0.96 | 2.52 | 0.1335 |
| x3 | 4.13 | 1 | 4.12 | 10.86 | 0.0049 |
| x4 | 0.12 | 1 | 0.12 | 0.32 | 0.5751 |
| x12 | 1.96 | 1 | 1.96 | 5.15 | 0.0383 |
| x22 | 5.89 | 1 | 5.89 | 15.51 | 0.0013 |
| x32 | 2.63 | 1 | 2.63 | 6.92 | 0.0189 |
| x42 | 0.26 | 1 | 0.26 | 0.69 | 0.4160 |
| x1 x2 | 0.46 | 1 | 0.46 | 1.23 | 0.2855 |
| x1 x3 | 0.06 | 1 | 0.06 | 0.15 | 0.7054 |
| x1 x4 | 0.77 | 1 | 0.77 | 2.03 | 0.1749 |
| x2 x3 | 1.04 | 1 | 1.04 | 2.73 | 0.1195 |
| x2 x4 | 1.29 | 1 | 1.29 | 3.41 | 0.0848 |
| x3 x4 | 1.43 | 1 | 1.43 | 3.78 | 0.0710 |
| Residual | 5.69 | 15 | 0.38 | ||
| Lack of Fit | 5.63 | 10 | 0.56 | 45.44 | 0.0003 |
| Pure Error | 0.062 | 5 | 0.01 | ||
| Cor Total | 25.19 | 29 |
R2=0.77
Adeq Precision=5.986
As shown in Table 3, X3 (cosubstrate, glucose concentration) and X22 (second order effect of substrate concentration) are the most important model parameters because the Prob> F value is 0.0049 for X3 and 0.0013 for X22. Model terms with a prob> F value greater than 0.1 are not significant. These results show that the second order effect of substrate concentration and glucose concentration have a direct effect on (S)-1-phenylethanol concentration. The response surface contour-plots (Figures 2-7) were drawn to predict the effects of the independent variables on the (S)-1-phenylethanol concentration. Each contour curve represents an infinite number of combinations of two test variables with the other two maintained at their respective zero level. (S)-1-phenylethanol concentration increases with increasing initial substrate concentration (Figures 2, 5 and 6) and biocatalyst concentration (Figures 4, 6 and 7). On the other hand, (S)-1-phenylethanol concentration decreases as the reaction time increases (Figures 2-4). This is thought to be due to the presence of a lot of a number of enzymes in the structure of the plant cells and the product, (S) -1-phenylethanol, to be converted into unknown products during the reaction. Increasing glucose concentration can cause cosubstrate inhibition and because of this inhibition, (S)-1-phenylethanol concentration decreases with increasing of the glucose concentration (Figures 3, 5 and 7). In order to maximize the concentration of (S)-1-phenylethanol, the optimum conditions were obtained by the response surface methodology with the Design expert (6.01) program are given in Table 4.
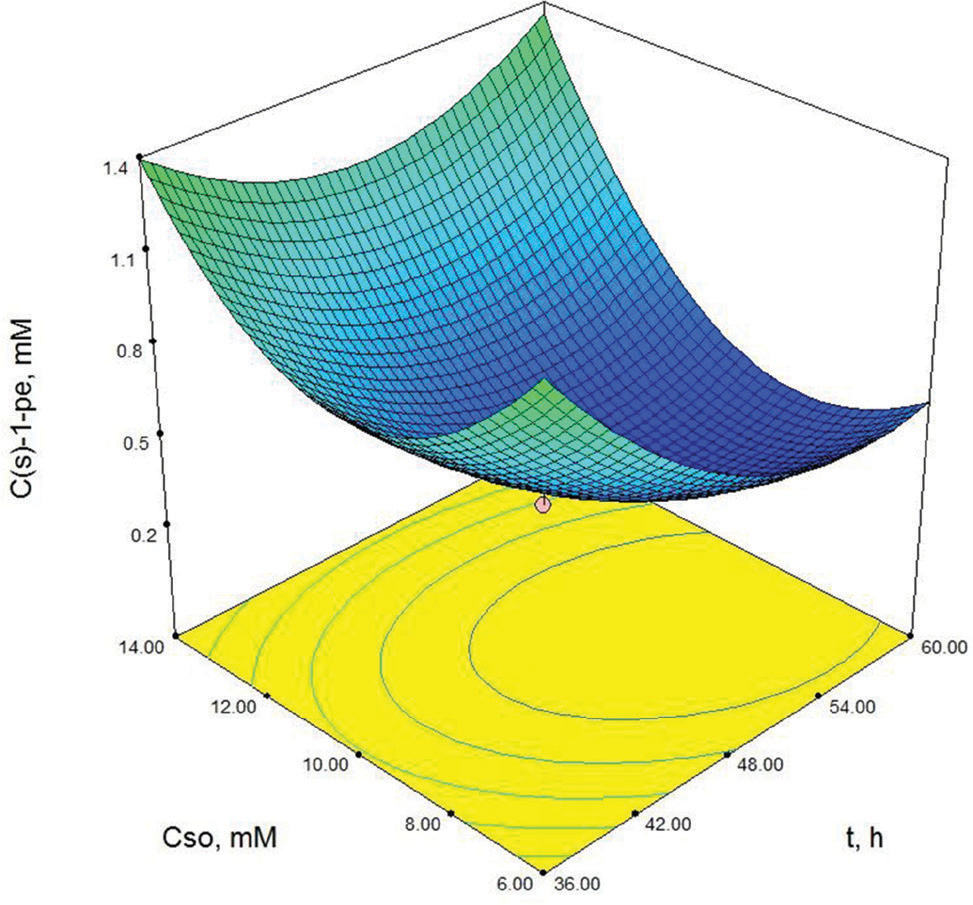
Contour-plot of (S)-1-phenylethanol concentration: The effect of time and initial substrate concentration. Other variable is held at zero level. Cg=30 mM, Cb=150 mg/mL.
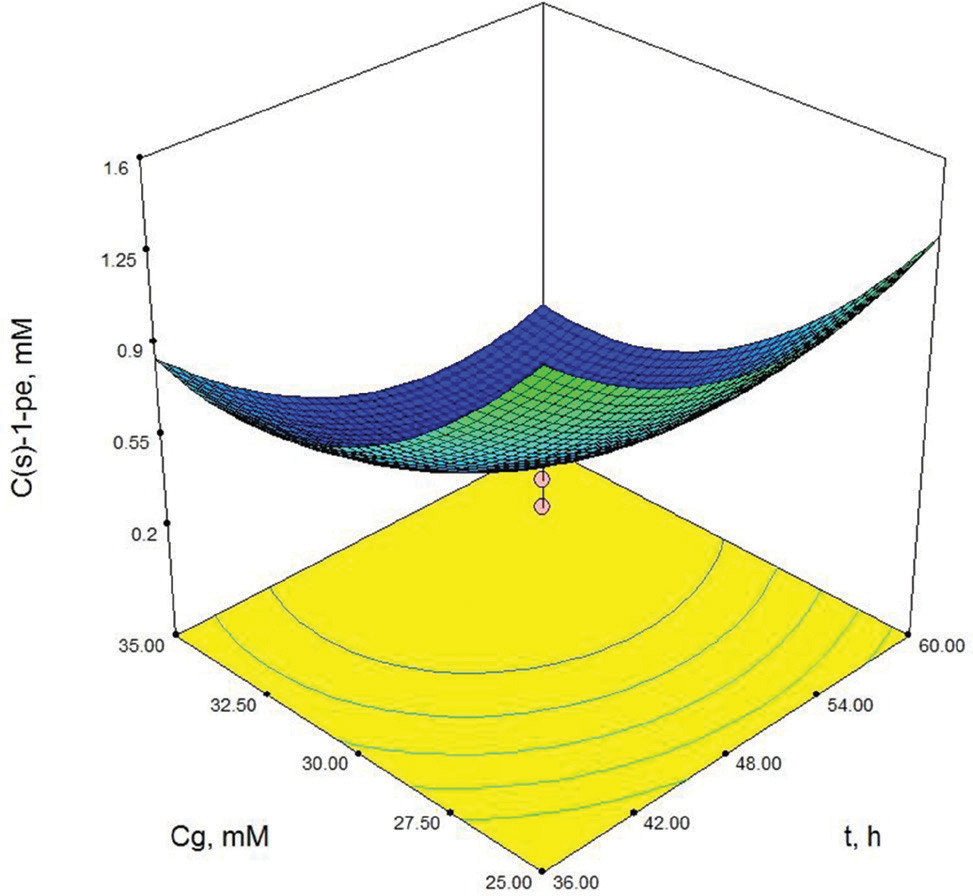
Contour-plot of (S)-1-phenylethanol concentration: The effect of time and glucose concentration. Other variable is held at zero level. Cso=10 mM, Cb=150 mg/mL.
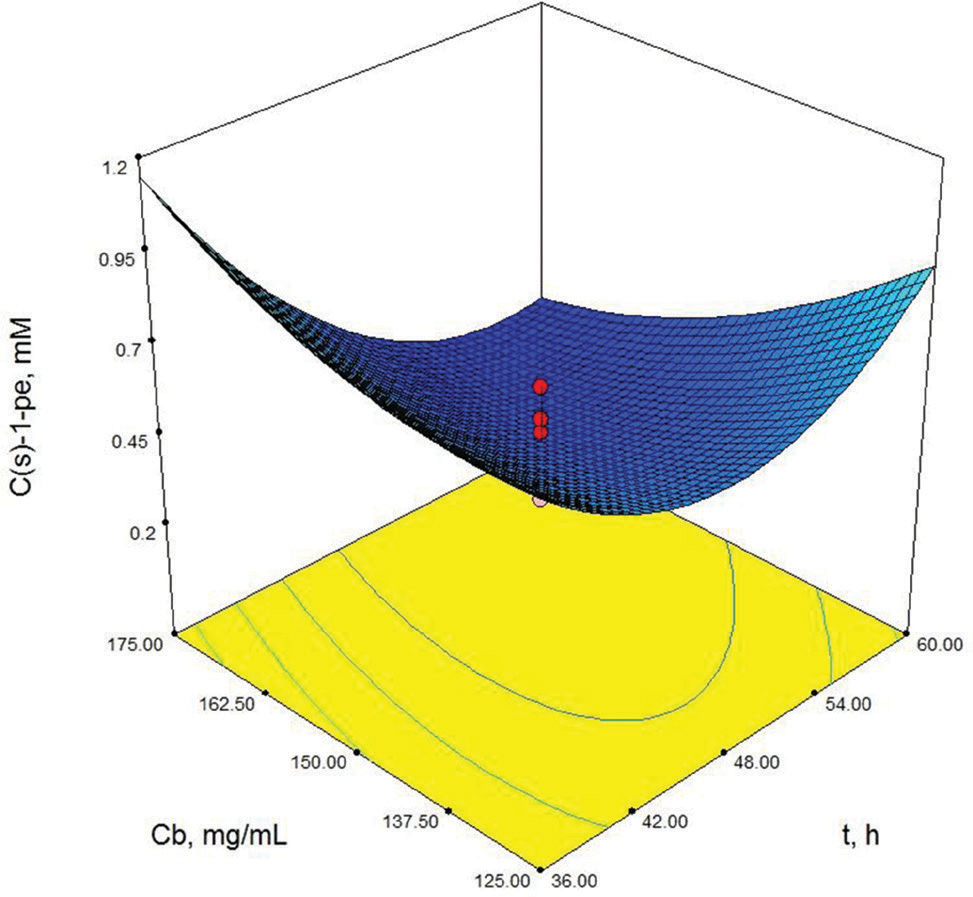
Contour-plot of (S)-1-phenylethanol concentration: The effect of time and biocatalyst concentration. Other variable is held at zero level. Cso=10 mM, Cg=30 mM.
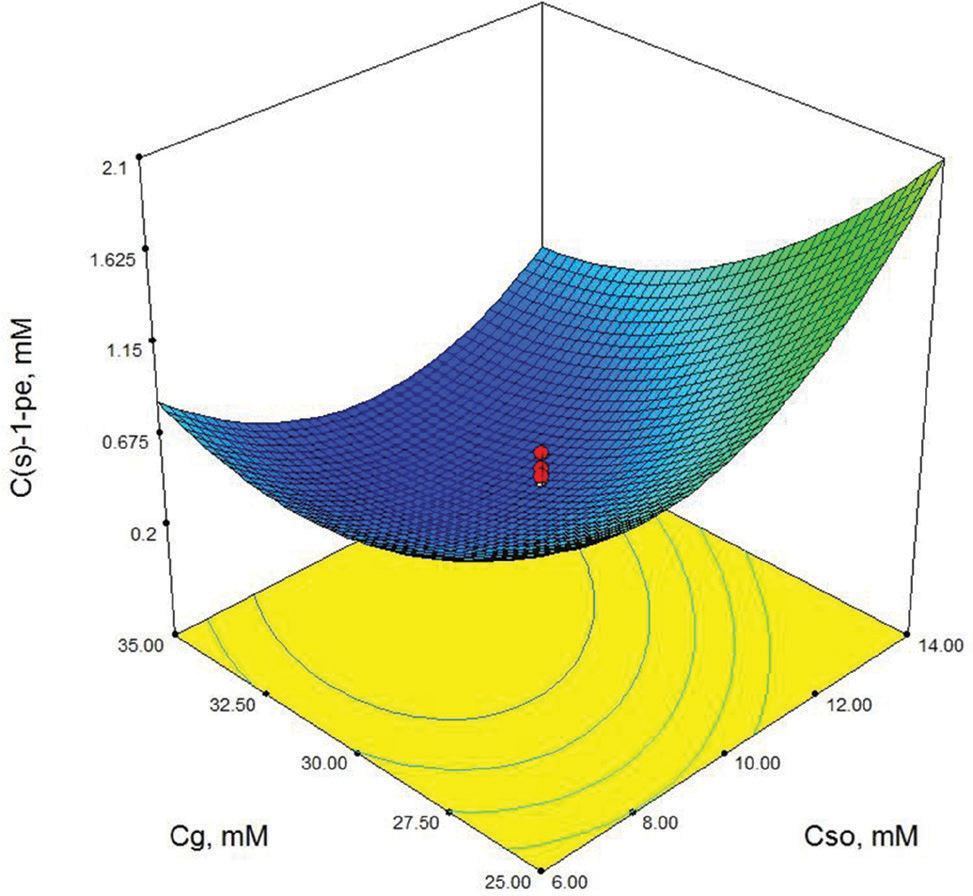
Contour-plot of (S)-1-phenylethanol concentration: The effect of initial substrate concentration and glucose concentration. Other variable is held at zero level. t=48 h, Cb=150 mg/mL.
Optimum values of independent variables obtained with RSM.
| Independent variables | Optimum value |
|---|---|
| t, h | 36 |
| CSo, mM | 6 |
| Cg, mM | 25.15 |
| Cb, mg/mL | 175 |
With these optimum values, (S)-1-phenylethanol concentration was obtained at 2.58 mM. Enantiomeric excess and conversion values were >99% and 40%, respectively. In order to verify these results, an experimental study was carried out it these conditions and (S)-1-phenylethanol concentration was obtained at 2.40 mM. The relative deviation between the computer solution and the experimental results was calculated as 6.9%.
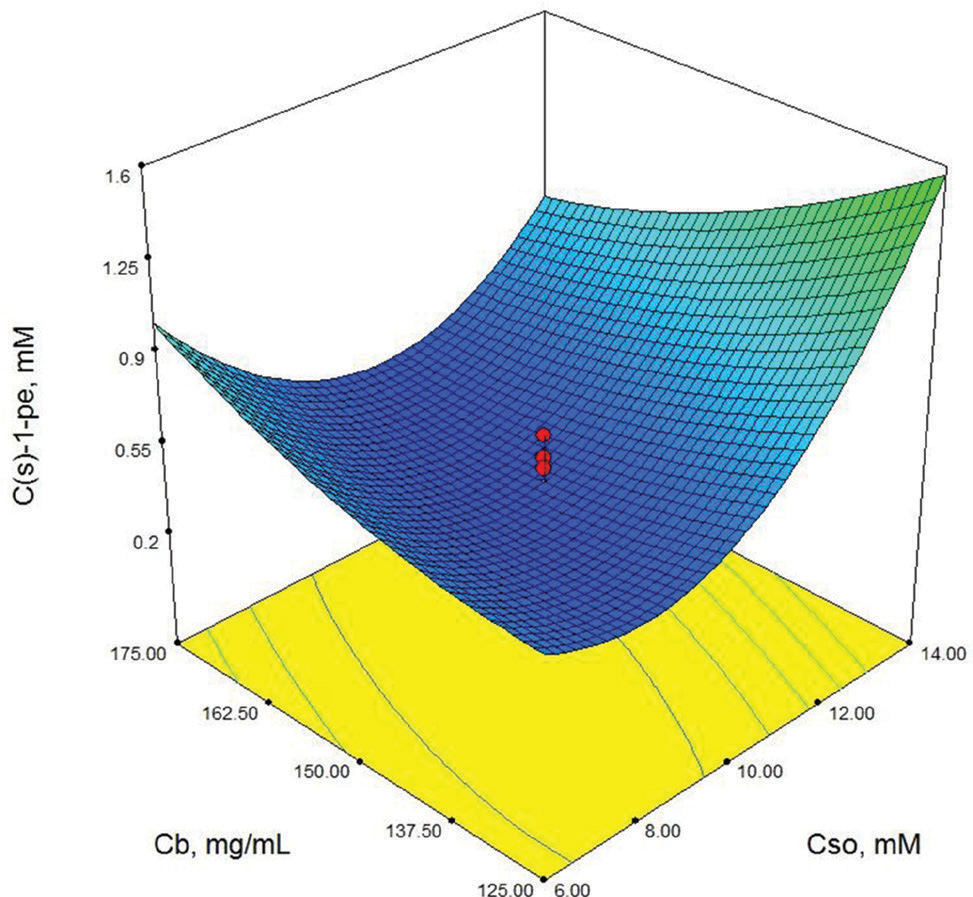
Contour-plot of (S)-1-phenylethanol concentration: The effect of initial substrate concentration and biocatalyst concentration. Other variable is held at zero level. t=48 h, Cg=30 mM.
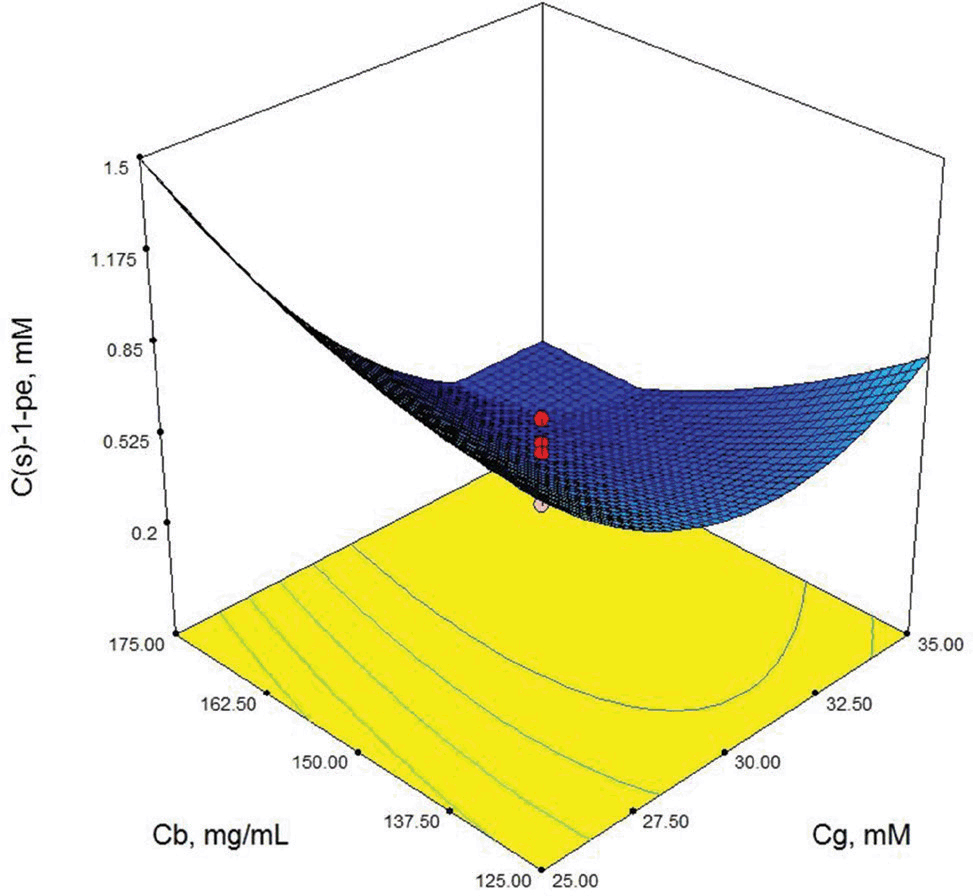
Contour-plot of (S)-1-phenylethanol concentration: The effect of glucose concentration and biocatalyst concentration. Other variable is held at zero level. t=48 h, Cso=10 Mm.
In the literature, for (S)-1-pheylethanol production, Chang et al. [16] reported 96% enantiomeric excess value with carrot as biocatalyst, Xie et al. [9] reported 98% enantiomeric excess value with acetone powder of adzuki bean as biocatalyst, Yang et al. [13] reported 96.4%, 75.8%, 73.8% and 72.8% enantiomeric excess values with carrot, cucumber, onion and radish as biocatalyst, respectively. In our previous study, enantiomeric excess, conversion and (S)-1-pheylethanol concentration were obtained as >99%, 58% and 0.6 mM, respectively using carrot as biocatalyst [23].
When compared with the literature, our results which show that the enantiomeric excess value is above 99% are encouraging.
4 Discussion
In this study, asymmetric reduction of acetophenone to (S)-1-phenylethanol resulted in >99% enantiomeric excess and 40% conversion, using Ispir bean which was pretreated with acetone powder methodology. Response surface methodology was used to optimize the production of (S)-1-phenylethanol. It was found that the most effective parameters were glucose concentration and second order effect of acetophenone concentration. Our results showed that, Ispir bean is a good biocatalyst for asymmetric reduction of acetophenone with its enantioselective reaction capability. In commercial products, it is desirable that the enantiomeric excess is >99%. As a result, the main goal of this study was to reach high enantiomeric excess and this was achieved. In order to increase the conversion of asymmetric reduction of acetophenone, our studies are going on.
List of abbreviations
- ADH
alcohol dehydrogenase
- GDH
glucose dehydrogenase
- NADPH
nicotinamide adenine dinucleotide
- Cb
biocatalyst concentration, mg/mL
- C(s)-1-pe
(S)-1-phenylethanol concentration, mM
- Cg
glucose concentration, mM
- CR
(R)-enantiomer concentration, mM
- CS
(S)-enantiomer concentration, mM
- CSo
initial substrate concentration, mM
- C
conversion
- ee
enantiomeric excess
- N
agitation rate, rpm
- T
temperature, oC
- t
reaction time, h
- HPLC
high pressure liquid chromotography
- RSM
response surface methodolgy
Acknowledgement
This work was financially supported by Ankara University, Research Projects Unit (Project Number: 12B4343010).
References
[1] Patel R.N., Biocatalysis for synthesis of pharmaceuticals. Bioorgan. Med. Chem., 2018, 26, 1252-1274.10.1016/j.bmc.2017.05.023Search in Google Scholar
[2] Patel R.N., Hanson R.L., Banerjee A., Szarka L.J., Biocatalytic synthesis of some chiral drug intermediates by oxidoreductases. J. Am. Oil Chem. Soc., 1997, 74, 1345-1360.10.1007/s11746-997-0237-3Search in Google Scholar
[3] Nakamura K., Matsuda T., Asymmetric reduction of ketones by the acetone powder of Geotrichum candidum J. Org. Chem., 1998, 63, 8957-8964.10.1021/jo9812779Search in Google Scholar
[4] Hasegawa Y., Adachi S., Matsuno R., Asymmetric reduction of acetophenone by immobilized Hansenula capsulata cells. J. Ferment. Bioeng., 1998, 85, 322-327.10.1016/S0922-338X(97)85683-8Search in Google Scholar
[5] Yadav J.S., Nanda S., Reddy P.T., Rao A.B., Efficient enantioselective reduction of ketones with Daucus carota root. J. Org. Chem., 2002, 67, 3900-3903.10.1021/jo010399pSearch in Google Scholar
[6] Kataoka M., Kita K., Wada M., Yasohara Y., Hasegawa J., Shimizu S., Novel bioreduction system for the production of chiral alcohols. Appl. Microbiol. Biot., 2003, 62, 437-445.10.1007/s00253-003-1347-ySearch in Google Scholar
[7] Utsukihara T., Watanabe S., Tomiyama A., Chai W., Horiuchi C.A., Stereoselective reduction of ketones by various vegetables. J. Mol. Catal. B: Enzym., 2006, 41, 103-109.10.1016/j.molcatb.2006.05.001Search in Google Scholar
[8] Yadav J.S., Reddy G.S., Sabitha G., Krishna A.D., Prasad A.R., Rao K.V., et al., Daucus carota and baker’s yeast mediated bio-reduction of prochiral ketones. Tetrahedron-Asymmetr., 2007, 18, 717-723.10.1016/j.tetasy.2007.03.009Search in Google Scholar
[9] Xie Y., Xu J.H., Lu W.Y., Lin G.Q., Adzuki bean: a new resource of biocatalyst for asymmetric reduction of aromatic ketones with high stereoselectivity and substrate tolerance. Bioresource Technol., 2009, 100, 2463-2468.10.1016/j.biortech.2008.11.054Search in Google Scholar
[10] Shimizu S., Kataoka M., Kita K., Chiral alcohol synthesis with yeast carbonyl reductases. J. Mol. Catal. B: Enzym., 1998, 5, 321-325.10.1016/S1381-1177(98)00064-2Search in Google Scholar
[11] Pollard D., Truppo M., Pollard J., Chen C.Y., Moore J., Effective synthesis of S-3, 5-bistrifluoromethylphenyl ethanol by asymmetric enzymatic reduction. Tetrahedron-Asymmetr., 2006, 17, 554-559.10.1016/j.tetasy.2006.01.039Search in Google Scholar
[12] Kurbanoglu E.B., Zilbeyaz K., Ozdal M., Taskin M., Kurbanoglu N.I., Asymmetric reduction of substituted acetophenones using once immobilized Rhodotorula glutinis cells. Bioresour. Technol., 2010, 101, 3825-3829.10.1016/j.biortech.2010.01.016Search in Google Scholar
[13] Yang Z.H., Zeng R., Yang G., Wang Y., Li L.Z., Lv Z.S., et al., Asymmetric reduction of prochiral ketones to chiral alcohols catalyzed by plants tissue. J. Ind. Microbiol. Biot., 2008, 35, 1047-1051.10.1007/s10295-008-0381-2Search in Google Scholar
[14] Goldberg K., Schroer K., Lütz S., Liese A., Biocatalytic ketone reduction-a powerful tool for the production of chiral alcohols-part II: whole-cell reductions. Appl. Microbiol. Biot., 2007, 76, 249-255.10.1007/s00253-007-1005-xSearch in Google Scholar
[15] Orden A.A., Bisogno F.R., Giordano O.S., Sanz M.K., Comparative study in the asymmetric bioreduction of ketones by plant organs and undifferentiated cells. J. Mol. Catal. B: Enzym., 2008, 51, 49-55.10.1016/j.molcatb.2007.10.003Search in Google Scholar
[16] Chang X., Zhonghua Y.A.N.G., Rong Z.E.N.G., Gai Y.A.N.G., Jiabao Y.A.N., Production of chiral aromatic alcohol by asymmetric reduction with vegetable catalyst. Chinese J. Chem. Eng., 2010, 18, 1029-1033.10.1016/S1004-9541(09)60164-6Search in Google Scholar
[17] Ou Z., Chen Q., Yang G., Xu L., Asymmetric reduction of 3-oxo-3-phenylpropionic acid ethyl ester by undifferentiated cells of white turnip in phosphate buffer/organic solvent. Korean J. Chem. Eng., 2011, 28, 378-382.10.1007/s11814-010-0353-xSearch in Google Scholar
[18] Baskar B., Ganesh S., Lokeswari T.S., Chadha A., Highly stereoselective reduction of 4-Aryl-2-oxo but-3-enoic carboxylic esters by plant cell culture of Daucus carota J. Mol. Catal. B: Enzym., 2004, 27, 13-17.10.1016/j.molcatb.2003.09.004Search in Google Scholar
[19] Tong L.P., Cui J.N., Ren W.M., Wang X.Y., Qian X.H., Asymmetric bioreduction of substituted acenaphthenequinones using plant enzymatic systems: A novel strategy for the preparation of (+)and (−)-mono hydroxyacenaphthenones. Chinese Chem. Lett., 2008, 19, 1179-1182.10.1016/j.cclet.2008.06.037Search in Google Scholar
[20] Xie B., Yang J., Yang Q., Yuan W., Enantioselective reduction of fluorenones in surfactant-aqueous solution by fruits and vegetables. J. Mol. Catal. B: Enzym., 2009, 61, 284-288.10.1016/j.molcatb.2009.08.007Search in Google Scholar
[21] Bordón D.L., Villalba L.D., Aimar M.L., Cantero J.J., Vázquez A.M., Formica S.M., et al., Weeds as biocatalysts in the stereoselective synthesis of chiral phenylethanols used as key intermediates for pharmaceuticals. Biocatal. Agric. Biotech., 2015, 4, 493-499.10.1016/j.bcab.2015.08.001Search in Google Scholar
[22] Maia da Silva F.F., Ferreira D.A., Monte F.J.Q., Carlos de Mattos M., Gomes de Lemos T.L., The orange peel as biocatalyst for the hydrolysis of esters. Ind. Crop. Prod., 2016, 84, 22-27.10.1016/j.indcrop.2016.01.017Search in Google Scholar
[23] Celik Kazici H., Bayraktar E., Mehmetoglu Ü., Optimization of the asymmetric synthesis of chiral aromatic alcohol using freezedried carrots as whole-cell biocatalysts. Green Process. Synth., 2016, 5, 131-137.10.1515/gps-2015-0118Search in Google Scholar
[24] Celik Kazici H., Bayraktar E., Mehmetoglu Ü., Production of precursors for anti-Alzheimer drugs: Asymmetric bioreduction in a packed-bed bioreactor using immobilized D. carota cells. Prep. Biochem. Biotech., 2017, 47, 67-73.10.1080/10826068.2016.1168840Search in Google Scholar
[25] Pavoković D., Buđa R., Andrašec F., Roje M., Bubalo M.C., Redovniković I.R., Plant-mediated asymmetric reduction of 1-(3,4-dimethylphenyl) ethanone. Tetrahedron-Asymmetr., 2017, 28, 730-733.10.1016/j.tetasy.2017.04.003Search in Google Scholar
[26] Aydogan Ö., Bayraktar E., Mehmetoglu Ü., Determination of effective diffusion coefficient of acetophenone in κ-carrageenan and asymmetric bioreduction in packed bed reactor. J. Mol. Catal. B: Enzym., 2011, 72, 46-52.10.1016/j.molcatb.2011.04.023Search in Google Scholar
[27] Vitale P., D’Introno C., Perna F.M., Perrone M.G., Scilimati A., Kluyveromyces marxianus CBS 6556 growing cells as a new biocatalyst in the asymmetric reduction of substituted acetophenones. Tetrahedron-Asymmetr., 2013, 24, 389-394.10.1016/j.tetasy.2013.02.001Search in Google Scholar
[28] Perna F.M., Ricci M.A., Scilimati A., Mena M.C., Pisano I., Palmieri L., et al., Cheap and environmentally sustainable stereoselective arylketones reduction by Lactobacillus reuteri whole cells. J. Mol. Catal. B: Enzym., 2016, 124, 29-37.10.1016/j.molcatb.2015.11.025Search in Google Scholar
[29] Vitale P., Abbinante V.M., Perna F.M., Salomone A., Cardellicchio C., Capriati V., Unveiling the hidden performance of whole cells in the asymmetric bioreduction of aryl-containing ketones in aqueous deep eutectic solvents. Adv. Synth. Catal., 2017, 359, 1049-1057.10.1002/adsc.201601064Search in Google Scholar
[30] Nakamura K., Kitano K., Matsuda T., Ohno A., Asymmetric reduction of ketones by the acetone powder of Geotrichum candidum Tetrahedron Lett., 1996, 37, 1629-1632.10.1016/0040-4039(96)00073-1Search in Google Scholar
[31] Hamada H., Miura T., Kumobayashi H., Matsuda T., Harada T., Nakamura K., Asymmetric synthesis of (R)-2-chloro-1-(m-chlorophenyl)ethanol using acetone powder of Geotrichum candidum Biotechnol. Letters., 2001, 23, 1603-1606.10.1023/A:1011922823367Search in Google Scholar
[32] Nakamura K., Yamanaka R., Matsuda T., Harada T., Recent developments in asymmetric reduction of ketones with biocatalysts. Tetrahedron-Asymmetr., 2003, 14, 2659-2681.10.1016/S0957-4166(03)00526-3Search in Google Scholar
[33] Myers R.H., Montgomery D.C., Anderson-Cook C.M., Responce surface methodolgy (4th ed.). John Wiley&Sons, New Jersey, 2016.Search in Google Scholar
[34] Bayraktar E., Response surface optimization of the separation of DL-tryptophan using an emulsion liquid membrane. Process Biochem., 2001, 37, 169-175.10.1016/S0032-9592(01)00192-3Search in Google Scholar
[35] Murthy M.S.R.C., Swaminathan T., Rakshit S.K., Kosugi Y., Statistical optimization of lipase catalyzed hydrolysis of methyloleate by response surface methodology. Bioprocess Eng., 2000, 22, 35-39.10.1007/PL00009097Search in Google Scholar
[36] Baydar G., Investigation of asymmetric reduction reactions via plant biocatalyst. MSc Thesis, Ankara University, Ankara, Turkey, 2014.Search in Google Scholar
© 2019 Baydar Atak et al., published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 Public License.
Articles in the same Issue
- Regular Articles
- Studies on the preparation and properties of biodegradable polyester from soybean oil
- Flow-mode biodiesel production from palm oil using a pressurized microwave reactor
- Reduction of free fatty acids in waste oil for biodiesel production by glycerolysis: investigation and optimization of process parameters
- Saccharin: a cheap and mild acidic agent for the synthesis of azo dyes via telescoped dediazotization
- Optimization of lipase-catalyzed synthesis of polyethylene glycol stearate in a solvent-free system
- Green synthesis of iron oxide nanoparticles using Platanus orientalis leaf extract for antifungal activity
- Ultrasound assisted chemical activation of peanut husk for copper removal
- Room temperature silanization of Fe3O4 for the preparation of phenyl functionalized magnetic adsorbent for dispersive solid phase extraction for the extraction of phthalates in water
- Evaluation of the saponin green extraction from Ziziphus spina-christi leaves using hydrothermal, microwave and Bain-Marie water bath heating methods
- Oxidation of dibenzothiophene using the heterogeneous catalyst of tungsten-based carbon nanotubes
- Calcined sodium silicate as an efficient and benign heterogeneous catalyst for the transesterification of natural lecithin to L-α-glycerophosphocholine
- Synergistic effect between CO2 and H2O2 on ethylbenzene oxidation catalyzed by carbon supported heteropolyanion catalysts
- Hydrocyanation of 2-arylmethyleneindan-1,3-diones using potassium hexacyanoferrate(II) as a nontoxic cyanating agent
- Green synthesis of hydratropic aldehyde from α-methylstyrene catalyzed by Al2O3-supported metal phthalocyanines
- Environmentally benign chemical recycling of polycarbonate wastes: comparison of micro- and nano-TiO2 solid support efficiencies
- Medicago polymorpha-mediated antibacterial silver nanoparticles in the reduction of methyl orange
- Production of value-added chemicals from esterification of waste glycerol over MCM-41 supported catalysts
- Green synthesis of zerovalent copper nanoparticles for efficient reduction of toxic azo dyes congo red and methyl orange
- Optimization of the biological synthesis of silver nanoparticles using Penicillium oxalicum GRS-1 and their antimicrobial effects against common food-borne pathogens
- Optimization of submerged fermentation conditions to overproduce bioethanol using two industrial and traditional Saccharomyces cerevisiae strains
- Extraction of In3+ and Fe3+ from sulfate solutions by using a 3D-printed “Y”-shaped microreactor
- Foliar-mediated Ag:ZnO nanophotocatalysts: green synthesis, characterization, pollutants degradation, and in vitro biocidal activity
- Green cyclic acetals production by glycerol etherification reaction with benzaldehyde using cationic acidic resin
- Biosynthesis, characterization and antimicrobial activities assessment of fabricated selenium nanoparticles using Pelargonium zonale leaf extract
- Synthesis of high surface area magnesia by using walnut shell as a template
- Controllable biosynthesis of silver nanoparticles using actinobacterial strains
- Green vegetation: a promising source of color dyes
- Mechano-chemical synthesis of ammonia and acetic acid from inorganic materials in water
- Green synthesis and structural characterization of novel N1-substituted 3,4-dihydropyrimidin-2(1H)-ones
- Biodiesel production from cotton oil using heterogeneous CaO catalysts from eggshells prepared at different calcination temperatures
- Regeneration of spent mercury catalyst for the treatment of dye wastewater by the microwave and ultrasonic spray-assisted method
- Green synthesis of the innovative super paramagnetic nanoparticles from the leaves extract of Fraxinus chinensis Roxb and their application for the decolourisation of toxic dyes
- Biogenic ZnO nanoparticles: a study of blueshift of optical band gap and photocatalytic degradation of reactive yellow 186 dye under direct sunlight
- Leached compounds from the extracts of pomegranate peel, green coconut shell, and karuvelam wood for the removal of hexavalent chromium
- Enhancement of molecular weight reduction of natural rubber in triphasic CO2/toluene/H2O systems with hydrogen peroxide for preparation of biobased polyurethanes
- An efficient green synthesis of novel 1H-imidazo[1,2-a]imidazole-3-amine and imidazo[2,1-c][1,2,4]triazole-5-amine derivatives via Strecker reaction under controlled microwave heating
- Evaluation of three different green fabrication methods for the synthesis of crystalline ZnO nanoparticles using Pelargonium zonale leaf extract
- A highly efficient and multifunctional biomass supporting Ag, Ni, and Cu nanoparticles through wetness impregnation for environmental remediation
- Simple one-pot green method for large-scale production of mesalamine, an anti-inflammatory agent
- Relationships between step and cumulative PMI and E-factors: implications on estimating material efficiency with respect to charting synthesis optimization strategies
- A comparative sorption study of Cr3+ and Cr6+ using mango peels: kinetic, equilibrium and thermodynamic
- Effects of acid hydrolysis waste liquid recycle on preparation of microcrystalline cellulose
- Use of deep eutectic solvents as catalyst: A mini-review
- Microwave-assisted synthesis of pyrrolidinone derivatives using 1,1’-butylenebis(3-sulfo-3H-imidazol-1-ium) chloride in ethylene glycol
- Green and eco-friendly synthesis of Co3O4 and Ag-Co3O4: Characterization and photo-catalytic activity
- Adsorption optimized of the coal-based material and application for cyanide wastewater treatment
- Aloe vera leaf extract mediated green synthesis of selenium nanoparticles and assessment of their In vitro antimicrobial activity against spoilage fungi and pathogenic bacteria strains
- Waste phenolic resin derived activated carbon by microwave-assisted KOH activation and application to dye wastewater treatment
- Direct ethanol production from cellulose by consortium of Trichoderma reesei and Candida molischiana
- Agricultural waste biomass-assisted nanostructures: Synthesis and application
- Biodiesel production from rubber seed oil using calcium oxide derived from eggshell as catalyst – optimization and modeling studies
- Study of fabrication of fully aqueous solution processed SnS quantum dot-sensitized solar cell
- Assessment of aqueous extract of Gypsophila aretioides for inhibitory effects on calcium carbonate formation
- An environmentally friendly acylation reaction of 2-methylnaphthalene in solvent-free condition in a micro-channel reactor
- Aegle marmelos phytochemical stabilized synthesis and characterization of ZnO nanoparticles and their role against agriculture and food pathogen
- A reactive coupling process for co-production of solketal and biodiesel
- Optimization of the asymmetric synthesis of (S)-1-phenylethanol using Ispir bean as whole-cell biocatalyst
- Synthesis of pyrazolopyridine and pyrazoloquinoline derivatives by one-pot, three-component reactions of arylglyoxals, 3-methyl-1-aryl-1H-pyrazol-5-amines and cyclic 1,3-dicarbonyl compounds in the presence of tetrapropylammonium bromide
- Preconcentration of morphine in urine sample using a green and solvent-free microextraction method
- Extraction of glycyrrhizic acid by aqueous two-phase system formed by PEG and two environmentally friendly organic acid salts - sodium citrate and sodium tartrate
- Green synthesis of copper oxide nanoparticles using Juglans regia leaf extract and assessment of their physico-chemical and biological properties
- Deep eutectic solvents (DESs) as powerful and recyclable catalysts and solvents for the synthesis of 3,4-dihydropyrimidin-2(1H)-ones/thiones
- Biosynthesis, characterization and anti-microbial activity of silver nanoparticle based gel hand wash
- Efficient and selective microwave-assisted O-methylation of phenolic compounds using tetramethylammonium hydroxide (TMAH)
- Anticoagulant, thrombolytic and antibacterial activities of Euphorbia acruensis latex-mediated bioengineered silver nanoparticles
- Volcanic ash as reusable catalyst in the green synthesis of 3H-1,5-benzodiazepines
- Green synthesis, anionic polymerization of 1,4-bis(methacryloyl)piperazine using Algerian clay as catalyst
- Selenium supplementation during fermentation with sugar beet molasses and Saccharomyces cerevisiae to increase bioethanol production
- Biosynthetic potential assessment of four food pathogenic bacteria in hydrothermally silver nanoparticles fabrication
- Investigating the effectiveness of classical and eco-friendly approaches for synthesis of dialdehydes from organic dihalides
- Pyrolysis of palm oil using zeolite catalyst and characterization of the boil-oil
- Azadirachta indica leaves extract assisted green synthesis of Ag-TiO2 for degradation of Methylene blue and Rhodamine B dyes in aqueous medium
- Synthesis of vitamin E succinate catalyzed by nano-SiO2 immobilized DMAP derivative in mixed solvent system
- Extraction of phytosterols from melon (Cucumis melo) seeds by supercritical CO2 as a clean technology
- Production of uronic acids by hydrothermolysis of pectin as a model substance for plant biomass waste
- Biofabrication of highly pure copper oxide nanoparticles using wheat seed extract and their catalytic activity: A mechanistic approach
- Intelligent modeling and optimization of emulsion aggregation method for producing green printing ink
- Improved removal of methylene blue on modified hierarchical zeolite Y: Achieved by a “destructive-constructive” method
- Two different facile and efficient approaches for the synthesis of various N-arylacetamides via N-acetylation of arylamines and straightforward one-pot reductive acetylation of nitroarenes promoted by recyclable CuFe2O4 nanoparticles in water
- Optimization of acid catalyzed esterification and mixed metal oxide catalyzed transesterification for biodiesel production from Moringa oleifera oil
- Kinetics and the fluidity of the stearic acid esters with different carbon backbones
- Aiming for a standardized protocol for preparing a process green synthesis report and for ranking multiple synthesis plans to a common target product
- Microstructure and luminescence of VO2 (B) nanoparticle synthesis by hydrothermal method
- Optimization of uranium removal from uranium plant wastewater by response surface methodology (RSM)
- Microwave drying of nickel-containing residue: dielectric properties, kinetics, and energy aspects
- Simple and convenient two step synthesis of 5-bromo-2,3-dimethoxy-6-methyl-1,4-benzoquinone
- Biodiesel production from waste cooking oil
- The effect of activation temperature on structure and properties of blue coke-based activated carbon by CO2 activation
- Optimization of reaction parameters for the green synthesis of zero valent iron nanoparticles using pine tree needles
- Microwave-assisted protocol for squalene isolation and conversion from oil-deodoriser distillates
- Denitrification performance of rare earth tailings-based catalysts
- Facile synthesis of silver nanoparticles using Averrhoa bilimbi L and Plum extracts and investigation on the synergistic bioactivity using in vitro models
- Green production of AgNPs and their phytostimulatory impact
- Photocatalytic activity of Ag/Ni bi-metallic nanoparticles on textile dye removal
- Topical Issue: Green Process Engineering / Guest Editors: Martine Poux, Patrick Cognet
- Modelling and optimisation of oxidative desulphurisation of tyre-derived oil via central composite design approach
- CO2 sequestration by carbonation of olivine: a new process for optimal separation of the solids produced
- Organic carbonates synthesis improved by pervaporation for CO2 utilisation
- Production of starch nanoparticles through solvent-antisolvent precipitation in a spinning disc reactor
- A kinetic study of Zn halide/TBAB-catalysed fixation of CO2 with styrene oxide in propylene carbonate
- Topical on Green Process Engineering
Articles in the same Issue
- Regular Articles
- Studies on the preparation and properties of biodegradable polyester from soybean oil
- Flow-mode biodiesel production from palm oil using a pressurized microwave reactor
- Reduction of free fatty acids in waste oil for biodiesel production by glycerolysis: investigation and optimization of process parameters
- Saccharin: a cheap and mild acidic agent for the synthesis of azo dyes via telescoped dediazotization
- Optimization of lipase-catalyzed synthesis of polyethylene glycol stearate in a solvent-free system
- Green synthesis of iron oxide nanoparticles using Platanus orientalis leaf extract for antifungal activity
- Ultrasound assisted chemical activation of peanut husk for copper removal
- Room temperature silanization of Fe3O4 for the preparation of phenyl functionalized magnetic adsorbent for dispersive solid phase extraction for the extraction of phthalates in water
- Evaluation of the saponin green extraction from Ziziphus spina-christi leaves using hydrothermal, microwave and Bain-Marie water bath heating methods
- Oxidation of dibenzothiophene using the heterogeneous catalyst of tungsten-based carbon nanotubes
- Calcined sodium silicate as an efficient and benign heterogeneous catalyst for the transesterification of natural lecithin to L-α-glycerophosphocholine
- Synergistic effect between CO2 and H2O2 on ethylbenzene oxidation catalyzed by carbon supported heteropolyanion catalysts
- Hydrocyanation of 2-arylmethyleneindan-1,3-diones using potassium hexacyanoferrate(II) as a nontoxic cyanating agent
- Green synthesis of hydratropic aldehyde from α-methylstyrene catalyzed by Al2O3-supported metal phthalocyanines
- Environmentally benign chemical recycling of polycarbonate wastes: comparison of micro- and nano-TiO2 solid support efficiencies
- Medicago polymorpha-mediated antibacterial silver nanoparticles in the reduction of methyl orange
- Production of value-added chemicals from esterification of waste glycerol over MCM-41 supported catalysts
- Green synthesis of zerovalent copper nanoparticles for efficient reduction of toxic azo dyes congo red and methyl orange
- Optimization of the biological synthesis of silver nanoparticles using Penicillium oxalicum GRS-1 and their antimicrobial effects against common food-borne pathogens
- Optimization of submerged fermentation conditions to overproduce bioethanol using two industrial and traditional Saccharomyces cerevisiae strains
- Extraction of In3+ and Fe3+ from sulfate solutions by using a 3D-printed “Y”-shaped microreactor
- Foliar-mediated Ag:ZnO nanophotocatalysts: green synthesis, characterization, pollutants degradation, and in vitro biocidal activity
- Green cyclic acetals production by glycerol etherification reaction with benzaldehyde using cationic acidic resin
- Biosynthesis, characterization and antimicrobial activities assessment of fabricated selenium nanoparticles using Pelargonium zonale leaf extract
- Synthesis of high surface area magnesia by using walnut shell as a template
- Controllable biosynthesis of silver nanoparticles using actinobacterial strains
- Green vegetation: a promising source of color dyes
- Mechano-chemical synthesis of ammonia and acetic acid from inorganic materials in water
- Green synthesis and structural characterization of novel N1-substituted 3,4-dihydropyrimidin-2(1H)-ones
- Biodiesel production from cotton oil using heterogeneous CaO catalysts from eggshells prepared at different calcination temperatures
- Regeneration of spent mercury catalyst for the treatment of dye wastewater by the microwave and ultrasonic spray-assisted method
- Green synthesis of the innovative super paramagnetic nanoparticles from the leaves extract of Fraxinus chinensis Roxb and their application for the decolourisation of toxic dyes
- Biogenic ZnO nanoparticles: a study of blueshift of optical band gap and photocatalytic degradation of reactive yellow 186 dye under direct sunlight
- Leached compounds from the extracts of pomegranate peel, green coconut shell, and karuvelam wood for the removal of hexavalent chromium
- Enhancement of molecular weight reduction of natural rubber in triphasic CO2/toluene/H2O systems with hydrogen peroxide for preparation of biobased polyurethanes
- An efficient green synthesis of novel 1H-imidazo[1,2-a]imidazole-3-amine and imidazo[2,1-c][1,2,4]triazole-5-amine derivatives via Strecker reaction under controlled microwave heating
- Evaluation of three different green fabrication methods for the synthesis of crystalline ZnO nanoparticles using Pelargonium zonale leaf extract
- A highly efficient and multifunctional biomass supporting Ag, Ni, and Cu nanoparticles through wetness impregnation for environmental remediation
- Simple one-pot green method for large-scale production of mesalamine, an anti-inflammatory agent
- Relationships between step and cumulative PMI and E-factors: implications on estimating material efficiency with respect to charting synthesis optimization strategies
- A comparative sorption study of Cr3+ and Cr6+ using mango peels: kinetic, equilibrium and thermodynamic
- Effects of acid hydrolysis waste liquid recycle on preparation of microcrystalline cellulose
- Use of deep eutectic solvents as catalyst: A mini-review
- Microwave-assisted synthesis of pyrrolidinone derivatives using 1,1’-butylenebis(3-sulfo-3H-imidazol-1-ium) chloride in ethylene glycol
- Green and eco-friendly synthesis of Co3O4 and Ag-Co3O4: Characterization and photo-catalytic activity
- Adsorption optimized of the coal-based material and application for cyanide wastewater treatment
- Aloe vera leaf extract mediated green synthesis of selenium nanoparticles and assessment of their In vitro antimicrobial activity against spoilage fungi and pathogenic bacteria strains
- Waste phenolic resin derived activated carbon by microwave-assisted KOH activation and application to dye wastewater treatment
- Direct ethanol production from cellulose by consortium of Trichoderma reesei and Candida molischiana
- Agricultural waste biomass-assisted nanostructures: Synthesis and application
- Biodiesel production from rubber seed oil using calcium oxide derived from eggshell as catalyst – optimization and modeling studies
- Study of fabrication of fully aqueous solution processed SnS quantum dot-sensitized solar cell
- Assessment of aqueous extract of Gypsophila aretioides for inhibitory effects on calcium carbonate formation
- An environmentally friendly acylation reaction of 2-methylnaphthalene in solvent-free condition in a micro-channel reactor
- Aegle marmelos phytochemical stabilized synthesis and characterization of ZnO nanoparticles and their role against agriculture and food pathogen
- A reactive coupling process for co-production of solketal and biodiesel
- Optimization of the asymmetric synthesis of (S)-1-phenylethanol using Ispir bean as whole-cell biocatalyst
- Synthesis of pyrazolopyridine and pyrazoloquinoline derivatives by one-pot, three-component reactions of arylglyoxals, 3-methyl-1-aryl-1H-pyrazol-5-amines and cyclic 1,3-dicarbonyl compounds in the presence of tetrapropylammonium bromide
- Preconcentration of morphine in urine sample using a green and solvent-free microextraction method
- Extraction of glycyrrhizic acid by aqueous two-phase system formed by PEG and two environmentally friendly organic acid salts - sodium citrate and sodium tartrate
- Green synthesis of copper oxide nanoparticles using Juglans regia leaf extract and assessment of their physico-chemical and biological properties
- Deep eutectic solvents (DESs) as powerful and recyclable catalysts and solvents for the synthesis of 3,4-dihydropyrimidin-2(1H)-ones/thiones
- Biosynthesis, characterization and anti-microbial activity of silver nanoparticle based gel hand wash
- Efficient and selective microwave-assisted O-methylation of phenolic compounds using tetramethylammonium hydroxide (TMAH)
- Anticoagulant, thrombolytic and antibacterial activities of Euphorbia acruensis latex-mediated bioengineered silver nanoparticles
- Volcanic ash as reusable catalyst in the green synthesis of 3H-1,5-benzodiazepines
- Green synthesis, anionic polymerization of 1,4-bis(methacryloyl)piperazine using Algerian clay as catalyst
- Selenium supplementation during fermentation with sugar beet molasses and Saccharomyces cerevisiae to increase bioethanol production
- Biosynthetic potential assessment of four food pathogenic bacteria in hydrothermally silver nanoparticles fabrication
- Investigating the effectiveness of classical and eco-friendly approaches for synthesis of dialdehydes from organic dihalides
- Pyrolysis of palm oil using zeolite catalyst and characterization of the boil-oil
- Azadirachta indica leaves extract assisted green synthesis of Ag-TiO2 for degradation of Methylene blue and Rhodamine B dyes in aqueous medium
- Synthesis of vitamin E succinate catalyzed by nano-SiO2 immobilized DMAP derivative in mixed solvent system
- Extraction of phytosterols from melon (Cucumis melo) seeds by supercritical CO2 as a clean technology
- Production of uronic acids by hydrothermolysis of pectin as a model substance for plant biomass waste
- Biofabrication of highly pure copper oxide nanoparticles using wheat seed extract and their catalytic activity: A mechanistic approach
- Intelligent modeling and optimization of emulsion aggregation method for producing green printing ink
- Improved removal of methylene blue on modified hierarchical zeolite Y: Achieved by a “destructive-constructive” method
- Two different facile and efficient approaches for the synthesis of various N-arylacetamides via N-acetylation of arylamines and straightforward one-pot reductive acetylation of nitroarenes promoted by recyclable CuFe2O4 nanoparticles in water
- Optimization of acid catalyzed esterification and mixed metal oxide catalyzed transesterification for biodiesel production from Moringa oleifera oil
- Kinetics and the fluidity of the stearic acid esters with different carbon backbones
- Aiming for a standardized protocol for preparing a process green synthesis report and for ranking multiple synthesis plans to a common target product
- Microstructure and luminescence of VO2 (B) nanoparticle synthesis by hydrothermal method
- Optimization of uranium removal from uranium plant wastewater by response surface methodology (RSM)
- Microwave drying of nickel-containing residue: dielectric properties, kinetics, and energy aspects
- Simple and convenient two step synthesis of 5-bromo-2,3-dimethoxy-6-methyl-1,4-benzoquinone
- Biodiesel production from waste cooking oil
- The effect of activation temperature on structure and properties of blue coke-based activated carbon by CO2 activation
- Optimization of reaction parameters for the green synthesis of zero valent iron nanoparticles using pine tree needles
- Microwave-assisted protocol for squalene isolation and conversion from oil-deodoriser distillates
- Denitrification performance of rare earth tailings-based catalysts
- Facile synthesis of silver nanoparticles using Averrhoa bilimbi L and Plum extracts and investigation on the synergistic bioactivity using in vitro models
- Green production of AgNPs and their phytostimulatory impact
- Photocatalytic activity of Ag/Ni bi-metallic nanoparticles on textile dye removal
- Topical Issue: Green Process Engineering / Guest Editors: Martine Poux, Patrick Cognet
- Modelling and optimisation of oxidative desulphurisation of tyre-derived oil via central composite design approach
- CO2 sequestration by carbonation of olivine: a new process for optimal separation of the solids produced
- Organic carbonates synthesis improved by pervaporation for CO2 utilisation
- Production of starch nanoparticles through solvent-antisolvent precipitation in a spinning disc reactor
- A kinetic study of Zn halide/TBAB-catalysed fixation of CO2 with styrene oxide in propylene carbonate
- Topical on Green Process Engineering

