Abstract
The aim of this study was to apply the central composite design technique to study the interaction of the amount of formic acid (6-12 mL), amount of hydrogen peroxide (6-10 mL), temperature (54-58°C) and reaction time (40-60 min) during the oxidative desulphurisation (ODS) of tyre-derived oil (TDO). The TDO was oxidised at various parametric interactions before being subjected to solvent extraction using acetonitrile. The acetonitrile to oil ratios used during the extraction were 1:1 and 1:2. The content of sulphur before and after desulphurisation was analysed using ICP-AES. The maximum sulphur removal achieved using a 1:1 acetonitrile to oxidised oil ratio was 86.05%, and this was achieved at formic acid amount, hydrogen peroxide amount, temperature and a reaction time of 9 mL, 8 mL, 54°C and 50 min respectively. Analysis of variance (ANOVA) indicated that the reduced cubic model could best predict the sulphur removal for the ODS process. Coefficient of determination (R2 = 0.9776), adjusted R2 = 0.9254, predicted R2 = 0.8356 all indicated that the model was significant. In addition, the p-value of lack of fit (LOF) was 0.8926, an indication of its insignificance relative to pure error.
1 Introduction
With the increasing population size, the global consumption of tyres has increased over the years in order to meet both personal and commercial transport needs. The amount of waste tyres dumped all over the world is approximately 1.5 billion [1, 2], of which the percentage taken for reuse is only 15-20% [3] while the remainder is simply dumped into the earth. As waste tyres are increasingly dumped, it is necessary that a suitable way of utilising them is explored. Despite the fact that part of these wastes is recycled, the proportion recycled is small given the continuous accumulation of these scraps [4]. At the same time, owing to the rising concerns of crude oil prices and environmental impacts governments have been forced to pay particular attention to the development of alternative fuels [5].
Tyres contain over 100 different substances such as steel, rubber, silica gel, carbon black, zinc oxide, sulphur among other additives. During the manufacture of tyres, the rubbers commonly used are butadiene rubber, natural rubber and styrene-butadiene rubber or the blends of these three types. When rubber is subjected to prolonged effort or mild temperatures, plastic deformation occurs due to the sliding of polymer chains [6]. These components decompose at various temperature ranges. The behaviour of thermal decomposition of waste tyres is dependent upon the kind of rubber as well as its contents [7, 8, 9].
The valorisation of waste plastics and tyres contributes to a reduction in fossil fuel consumption and a deceleration of climate change [10]. Energy recovery is considered an attractive option for recycling since scrap tyres have high gross calorific value (33-35 MJ kg-1) and volatile compound content [11]. Tyres also have higher heating values in comparison with that of materials such as coal [12, 13]. Pyrolysis can potentially be involved in the recovery of energy from waste tyres. By definition, pyrolysis is simply a thermal degradation process (in an oxygen-free environment), in which the organic volatile matter in the tyres is transformed into products with low molecular weight, while the inorganic constituents, majorly from carbon black and steel are retained as solid residue [14]. The main products of the waste tyre pyrolysis process are tyre-derived oil, the non-condensable gases and char.
The major limitation of the utilisation of tyre-derived oil in a wider range of application in real combustion processes is its high sulphur content of between 10000 to 14000 ppm [15]. Pilusa [16] recently used crude tyre-derived oil with a sulphur content of 11450 mg/kg in a study to test its performance in internal combustion applications. However, it is important to note that the content of sulphur in the tyre-derived oil could be lower than the aforementioned ranges depending on the original composition of the waste tyre. The method commonly used for the removal of organosulphur compounds in the oil refinery industry is hydrodesulphurisation (HDS). This process can remove large sulphur amounts in fuels, but its shortcoming lies in the need for high temperature for the reaction (300-400°C) and high pressure of hydrogen (30-130 atm) with availability of active catalysts contained in huge reactor volume in order to facilitate the conversion of sulphur compounds to hydrogen sulphide [17, 18, 19]. Tyre-derived oil contains Dibenzothiophene (DBT), and as such HDS may not be effective in their treatment due to the steric sulphur atom hindrance [20, 21]. From studies on hydrodesulphurisation of diesel fuels, there has been an indication that alkyl-Dibenzothiophene consisting of alkyl groups at 6- and/or 4-position are the organosulphur compounds that remain at levels below 0.1 wt%. These compounds, which are categorised as extremely refractory, have a low reactivity during hydrodesulphurisation [22, 23].
Since tyre-derived oil is produced mainly on small scale basis, the application of HDS for sulphur content reduction is limited. Due to the high operating conditions and the need for expensive catalysts in the HDS system, oxidative desulphurisation has in the recent past been considered a complementary technology for deep desulphurisation [24]. The ODS process utilises milder operating conditions. The oxidative desulphurisation process occurs in two stages. First, the organic sulphur compounds are oxidised to their corresponding polar sulphoxides and sulphones using oxidising agents. This is followed by the removal step, in which the sulphoxides and sulphones are extracted using polar solvents [17, 24, 25]. Figure 1 shows the chemical reaction that takes place during the conversion of the sulphur-containing compounds into polar sulphoxides and sulphones. In an ODS process, hydrogen peroxide or its mixture with a strong or organic acid is used to boost the efficiency of sulphur removal [26, 27]. As opposed to the cases in conventional liquid fossil fuels, the sulphur compounds contained in the TDO present themselves in the forms of polycyclic aromatic sulphur heterocycles such as Dibenzothiophene and its derivatives [28, 29].
![Figure 1 Mechanism of oxidation of sulphur-containing compounds [24].](/document/doi/10.1515/gps-2019-0013/asset/graphic/j_gps-2019-0013_fig_001.jpg)
Mechanism of oxidation of sulphur-containing compounds [24].
Peroxyacids, as oxidants, are very effective [30]. In a study carried out by Zannikos et al. [31] on desulphurisation of petroleum fractions at mild conditions using combined oxidation and liquid-liquid extraction, 90% sulphur removal was achieved. A number of ODS studies involving the use of hydrogen peroxide and acetic acid, hydrogen peroxide and formic acid and sulphuric acid have been carried out by different researchers [24, 32, 33, 34, 35]. The use of hydrogen peroxide during ODS has been widespread since its conversion leads to complete decomposition into H2O and oxygen, and therefore no pollutants are produced in the process [36]. Moreover, the use of H2O2 with an acid during the ODS can speed up the desulphurisation process.
In a study carried out by Ali et al. [37] with incorporation of hydrogen peroxide and formic acid, a reduction in the fuel sulphur content from 1044 mg/L to 100 mg/L was achieved. In another study by Ali et al. [38] using hydrogen peroxide and acetic acid as part of the oxidation system in presence of sulphuric acid catalyst, a 90% reduction in the content of sulphur in petroleum products was reported. Recently the ability of sulphuric acid to act as a desulphurisation agent was investigated by Nabi et al. [39]. In the study, 8% concentric hydrosulphuric acid together with TDO was subjected to stirring after which the mixture was left to settle for forty hours, forming two distinct layers. It was found that the raw oil was dark reddish in colour while the desulphurised oil was yellowish.
Solvent extraction, also commonly termed liquid-liquid extraction is a compounds’ separation technique that utilises the differences in the relative solubilities of two immiscible liquids, commonly an organic solvent and water. It refers to solute distribution between two immiscible liquid phases that are in contact with each other [40]. The separation of extremely polar sulphoxides or sulphones from the non-polar compounds in the oxidised oil can be achieved through solvent extraction
by making use of a polar solvent. Acetonitrile, owing to its relatively low boiling point of 355 K, is considered an optimal solvent for use during extraction. In addition, its separation from sulphone solutes via distillation at a boiling point range of 550-950 K is much easier [23]. Sulphur compounds in the light fraction of oil, by virtue of being highly polar, are spread across the acetonitrile phase [41, 42]. In a study carried out by Murata et al. [43] using acetonitrile as the extraction solvent, the sulphur content in the oil was reduced by 80%. Similar results were obtained in another study conducted by Shiraishi et al. [44].
Response surface methodology (RSM) is among the best techniques used to empirically study the relationship between one or more estimated response functions [45]. RSM makes use of statistical and mathematical methods to depict the domain of all practicable solutions for a process model, and once the model development is completed, process optimisation can be conducted devoid of a trial and error procedure [46]. RSM constitutes selected statistical and mathematical methods used for the analysis of problems in which the input variables influence the responses [47]. Regression analysis, with basis on polynomials, is used to define the relationship between the input variables and the responses.
Studies on the application of oxidative desulphurisation for sulphur reduction in both model diesel fuels [37, 48, 49] and tyre-derived oil [23, 24, 32, 35] have previously been carried out. Most of these studies, however, have been conducted at constant temperatures and reaction time while varying only the ratio of the oil to the acid and/or H2O2 during the oxidation stage of the ODS process. Conventionally, during the optimisation of process parameters, the approach used is the one in which one variable is changed at a time whereas the other factors are kept constant. The drawback of this classical approach is that a lot of time is consumed and there is need for many experiments to be carried out. In addition, the one-factor-at-a-time method does not provide information on the interactive effects of process variables at a single time. The RSM is one of the statistical optimisation methods that can be used to eliminate the shortcomings associated with the one-factor-at-a-time approach [50]. As far as we know, there is no trace of studies that have involved the investigation of parametric interactions during the study of the oxidative desulphurisation of tyre-derived oil. Due to the limited data and information in the previously conducted studies, in terms of parametric interactions, the present study is therefore justified.
The purpose of this work was to study the oxidative desulphurisation of tyre-derived oil with the incorporation of parametric interaction option by applying the “small” central composite design (CCD) technique of the response surface methodology. The parameters whose interaction was invistigated were the temperature, reaction time, amount of formic acid and the amount of hydrogen peroxide. Model-fitting and analysis of variance (ANOVA) yielded the reduced cubic model (RCM) for the prediction of sulphur removal. The optimisation of the process was also performed by setting the goals of the parameters to be within the range of the design space while the goal of the respones was set to “maximise”.
2 Materials and methods
2.1 Materials used in the study
The tyre-derived oil (with a sulphur content of 8689 mg/L) used for the oxidative desulphurisation experiments was obtained from FFS Refiners, Durban, South Africa. Hydrogen peroxide (50 wt%) and formic acid with a concentration of 95 wt% were purchased from Shalom Laboratory supplies, South Africa. The hydrogen peroxide was used as received whereas the formic acid was further subjected to dilution to a concentration of 85 wt%. Acetonitrile with a concentration of 99.8% was supplied by Macron Fine ChemicalsTM, and was also used as received. The equipment utilised for the oxidation stage of the oxidative desulphurisation experiments consisted of a water bath (temperature-controlled), a 250 mL round-bottom flask, a reflux condenser and a mechanical stirrer with customised blades and shaft. The liquid-liquid extraction was carried out using a 60 mL separatory funnel.
2.2 Procedure for the oxidative desulphurisation of the oil
The oxidative desulphurisation experiments were conducted using a water bath, placed in a fume cupboard. The stirring speed and the amount of TDO in all runs were kept constant at 300 rpm and 100 mL respectively. During the experiments, the temperature needed for a given run was first set on the water bath. This was followed by the transfer of the TDO amount (100 mL in all experiments) into the round-bottom flask. The required volumes of hydrogen peroxide and formic acid were then transferred into the round-bottom flask after which the reflux condenser was connected. The flask was then clamped and placed in the water bath, while setting and monitoring the reaction time simultaneously. The water bath was switched off at the end of the set reaction time. The round-bottom flask was then removed from the water bath, and the oxidised TDO was transferred to a beaker ready for solvent extraction.
Acetonitrile was used for solvent extraction. The acetonitrile to oxidised oil ratios used were 1:1 and 1:2. During the 1:1 solvent to oil ratio extraction process, 10 mL of the solvent and 10 mL of the oxidised TDO were transferred into a separatory funnel, whereas for the 1:2 solvent to oil ratio case, 5 mL of the solvent (acetonitrile) and 10 mL of the oxidised TDO were transferred into a separatory funnel. This was followed by vigorous manual shaking of the mixture (with repeated venting for pressure release) for approximately three minutes. The mixture was then charged with deionised water (10 mL) and left to settle for five minutes, where the aqueous layer was formed at the bottom while the oil layer was formed at the top. In order to separate the two phases, the stopcock was opened to allow lower aqueous phase to be collected at the bottom, while the oil phase was collected from the top. The two layers were collected at different sections of the separatory funnel to ensure the two phases did not mix.
Inductively coupled plasma atomic emission spectroscopy (ICP-AES) was used to analyse the content of sulphur in both the original and the desulphurised tyre oils. The percentage reduction in sulphur content of the TDO was calculated using Eq. 1.
where S 0 (mg/L) is the content of sulphur in the TDO before desulphurisation while Sf (mg/L) is the content of sulphur in the oil after desulphurisation.
2.3 ICP-AES analysis procedure
The ICP-AES analysis for sulphur content determination in the original and desulphurised tyre oils was conducted using Thermo Scientific iCAP 6000 ICP Spectrometer with a radio frequency (RF) power of 1.35 kW. Argon was used as both the carrier gas and auxiliary gas. The flow of the carrier and auxiliary gases was maintained at 0.65 and 1 L/min respectively. On the other hand, the rate of flow in the MicroMist nebuliser was kept at 2 mL/min. The standard used internally for sulphur was 1 μg/mL Yttrium. MARS microwave digester (CEM Corporation) was used for microwave digestion of the oil samples before the ICP-AES analysis. One millilitre of hydrogen peroxide and 6 mL of 2% HNO3 were added to the TDO samples during the microwave digestion. In the process of microwave digestion, the level of power was set at 100% of 1600 W. The hold time, pressure and ramp time for the digestion process were 10 min, 800 psi and 25 min respectively.
The introduction of the digested oil samples into the spectrometer was done with the aid of a peristatic pump through an auto sampler. The samples were then passed through a nebuliser, producing fine aerosols. The large droplets were removed using a spray chamber whereas the smaller ones were passed to the plasma. In the plasma, the solvent vaporised while the residual sample got atomised and ionised. The ions (in the plasma) were excited, leading to emission of characteristic light. The light was measured by Echelle optical design with charge injection device solid-state detector. The accuracy (recovery) of the of the control standard was 100.1%. In order to process the data from the spectrometer, the iTEVA software was used.
2.4 Design of experiments
The design and analysis of experiments was carried out with the aid of the Design-Expert® Software Version 10-Stat-Ease. The central composite design of the response surface methodology was applied in the investigation of the interaction of the parameters involved in the ODS of the tyre-derived oil. There are two available options for the CCD in Design-Expert. The first option is the full design while the second option is the “small” design. The “small” CCD is only applicable if the factors involved are more than two. In the present study, the “small” CCD, which is the minimal-point designs required for the estimation of the terms in a second order model, was used to generate the experimental runs and analyse the sulphur removal in the tyre-derived oil.
Response surface methodology is a set of statistical and mathematical techniques applied in the analysis of the effects of independent variables and the determination of the optimum conditions of a process [51]. The most common response surface methodology design matrices are the CCD, Box-Behnken design, small CCD and orthogonal design, which incorporates the fractional and 2k factorial design points [52]. The central composite design is the most widely used technique in application [50], and it can be efficiently used to fit the data from experimental runs in the second-order model [53]. Moreover, RSM can accurately predict the optimised condition using a minimum number of experimental runs [54].
The design points for a CCD are the star points (also called axial points), centre points and two-level factorial/fractional factorial design points. All possible combinations of +1 and -1 are contained in the two-level factorial component of the design. For instance, in the two factors scenario, four design points exist i.e. (-1, -1), (+1, -1), (-1, +1) and (+1, +1). On the other hand, for axial points, all of the factors apart from a single factor (with the value of +/- α) are set to 0. The axial points for a two factor scenario are (-α, 0) (+α, 0) (0, - α) (0, + α). As the name suggests, the centre points are points in which all levels are set to coded level 0 i.e. the middle point (0, 0) of the range of each factor. To obtain a good estimate of pure error (experimental error), the centre points are repeated between 4-6 times. Eq. 2 can be used to determine the total number of experiments (N) for a central composite design [55]. On the other hand, Eq. 3 can be used to calculate the total number of experimental runs for a “small” CCD [47, 56].
where k is the number of factors, 2k the axial points, N0 the centre points and 2K the factorial points [57]. The number of experiments for complete replication of the design increases sharply as the number of factors, k increase [58]. The centre point in the CCD aids to determine the experimental error. The distance from the centre points to the axial points depends on the number of factors [59]. In a central composite design, five levels of each factor are required. These levels are -α, -1, 0, 1, and α. One of the exceptional features of a CCD is the capability of its structure to lend itself to sequential experimentation. The coded and uncoded forms of the factors are correlated according to equation:
where xi represents the actual value of the ith variable in uncoded terms, xo is the mid-level value of xi, while Δx denotes the step change [53].
Table 1 shows the design ranges for the 4 factors investigated during the oxidative desulphurisation of the TDO in the present study. These ranges were chosen, partly, based on the ODS studies carried out previously [23, 24, 32]. Based on the design criteria (“small” CCD option), the total number of runs obtained for each of the solvent extraction scenarios was 21. The runs consisted of eight, five and eight axial points, centre points and factorial points respectively.
The levels of factors for the central composite design (small).
| Parameter | Levels | ||||
|---|---|---|---|---|---|
| –α(–1.682) | –1 | 0 | +1 | +α(+1.682) | |
| A:HCOOH (mL) | 3.95 | 6 | 9 | 12 | 14.05 |
| B:H2O2 (mL) | 4.64 | 6 | 8 | 10 | 11.36 |
| C:Temperature (°C) | 47.27 | 50 | 54 | 58 | 60.73 |
| D:Reaction time (min) | 33.18 | 40 | 50 | 60 | 66.82 |
The first order and second order models are the most common polynomial functions for approximation [60]. Eq. 5 shows the first order polynomial function while Eq. 6 presents the second order polynomial. Eq. 6 can aid in the determination of the correlation between the response and the variables involved in a process [50, 55, 61, 62].
where ε is the error, Xi and Xj are independent variables, β0 is a constant coefficient, βi the coefficient for linear interaction effect while βii and βij are the coefficients for quadratic interaction effect.
3 Results and discussion
3.1 Results of sulphur removal in the tyre-derived oil
Table 2 presents the sulphur removal percentages for various parametric interactions as obtained using the CCD. The sulphur removal was in the range of 34.02 to 86.05% for the various parametric interactions using a 1:1 acetonitrile to oxidised TDO ratio for the solvent extraction while sulphur removal values ranging from 27.91 to 52.77% were achieved when a 1:2 acetonitrile to oxidised TDO ratio was used. It can be seen that the highest sulphur removal was achieved in the case when a 1:1 acetonitrile to oxidised TDO ratio was used during the solvent extraction step. The results from this case were therefore used further to model the ODS process of the tyre-derived oil.
CCD of sulphur removal for various parametric interactions.
| Run | A: | B: | C: | D: | Sulphur removal | |
|---|---|---|---|---|---|---|
| order | HCOOH | H2O2 | Temperature | Reaction time | S1 | S2 |
| (mL) | (mL) | (°C) | (min) | (%) | (%) | |
| 1 | –1 | 1 | 1 | 1 | 66.08 | 33.78 |
| 2 | 1 | 1 | 1 | –1 | 69.86 | 34.23 |
| 3 | –1.682 | 0 | 0 | 0 | 55.04 | 27.91 |
| 4 | 1 | 1 | –1 | –1 | 84.41 | 42.03 |
| 5 | 1.682 | 0 | 0 | 0 | 72.78 | 34.95 |
| 6 | 0 | 0 | 1.682 | 0 | 84.49 | 45.87 |
| 7 | –1 | –1 | –1 | –1 | 52.39 | 47.74 |
| 8 | 1 | –1 | –1 | 1 | 70.22 | 39.98 |
| 9 | –1 | –1 | 1 | –1 | 34.02 | 32.05 |
| 10 | 0 | 0 | 0 | 0 | 82.94 | 43.54 |
| 11 | 0 | 0 | 0 | 0 | 86.05 | 36.82 |
| 12 | 0 | 0 | 0 | –1.682 | 79.85 | 45.61 |
| 13 | 0 | 0 | 0 | 0 | 74.61 | 43.55 |
| 14 | 0 | –1.682 | 0 | 0 | 60.05 | 32.12 |
| 15 | 0 | 1.682 | 0 | 0 | 64.97 | 52.77 |
| 16 | 0 | 0 | 0 | 0 | 81.42 | 43.15 |
| 17 | 0 | 0 | 0 | 1.682 | 66.21 | 41.74 |
| 18 | 0 | 0 | 0 | 0 | 83.57 | 42.2 |
| 19 | 0 | 0 | –1.682 | 0 | 68.05 | 45.15 |
| 20 | 1 | –1 | 1 | 1 | 82.15 | 44.04 |
| 21 | –1 | 1 | –1 | 1 | 59.97 | 32.48 |
S1: Sulphur removal using a 1:1 acetonitrile to TDO ratio
S2: Sulphur removal using a 1:2 acetonitrile to TDO ratio
3.2 Model fitting and diagnosis
The model results presented further are those for the sulphur removal in which a 1:1 acetonitrile to oxidised TDO ratio was used during the solvent extraction. Preliminary results of model fitting suggested that the quadratic model is a better predictor of sulphur removal during ODS. However, a negative predicted R2 value indicated that, in fact, the overall mean is better predictor of the sulphur removal than the suggested model. The summary of model statistics is presented in Table 3.
Summary of model Statistics.
| Source | SD | R2 | Adjusted | Predicted | PRESS | |
|---|---|---|---|---|---|---|
| R2 | R2 | |||||
| Linear | 11.67 | 0.3802 | 0.2252 | –0.0923 | 3838.3 | |
| 2FI | 11.83 | 0.6015 | 0.2030 | –4.8809 | 20664.7 | |
| Quadratic | 6.18 | 0.9348 | 0.7827 | –1.5430 | 8935.6 | Suggested |
| Cubic | 4.31 | 0.9789 | 0.8943 | + | Aliased |
Due to the negative value of the predicted R2, model modification was performed in order to improve the model’s sulphur removal predictability. In the model reduction procedures, the backward selection was used for p-value criterion, Akaike information criterion (AICc) and Bayesian information criterion (BIC). On the other hand, ‘all hierarchical’ selection was applied when the adjusted R2 was used as the reduction criterion. The summary of model reduction tests based on various criteria is presented in Table 4. The reduced cubic model (RCM) obtained using BIC as the reduction criterion had the highest predictability compared to the suggested quadratic model and the other reduced cubic models and reduced quadratic models (RQM). The selected RCM was used in subsequent analysis and presentation of model results for sulphur removal in the tyre-derived oil.
Model reduction tests.
| Model | Criterion | Model statistics | |||||
|---|---|---|---|---|---|---|---|
| p | LOF | R2 | Adj. R2 | Pred. R2 | Adequate Precision | ||
| Quadratic | suggested | 0.0154 | 0.1053 | 0.9348 | 0.7827 | –1.5430 | 9.218 |
| RCM | p value | 0.0005 | 0.4250 | 0.9530 | 0.8825 | 0.7139 | 14.764 |
| RCM | AICc | 0.0010 | 0.1277 | 0.8362 | 0.7270 | 0.3694 | 9.192 |
| RCM | BIC | 0.0009 | 0.8926 | 0.9776 | 0.9254 | 0.8356 | 16.878 |
| RCM | Adj. R2 | 0.0007 | 0.5536 | 0.9661 | 0.9032 | 0.8548 | 16.132 |
| RQM | p value | 0.0008 | 0.1701 | 0.8760 | 0.7746 | 0.4114 | 10.528 |
| RQM | AICc | 0.0010 | 0.1277 | 0.8362 | 0.7270 | 0.3694 | 9.192 |
| RQM | BIC | 0.0011 | 0.2284 | 0.9199 | 0.8219 | 0.6811 | 10.813 |
| RQM | Adj. R2 | 0.0011 | 0.2284 | 0.9199 | 0.8219 | 0.6811 | 10.813 |
3.3 Model analysis
The obtained reduced cubic model (RCM) in terms of coded factors for the sulphur removal in the tyre-derived oil is presented in Eq. 7. This model shows the relationship between the dependent variable (sulphur removal) and the independent variables. In Eq. 7, the synergetic and antagonistic effects are represented by the positive and negative signs that appear before the linear terms respectively. Eq. 7 can predict the sulphur removal for particular levels of each factor, in which case the factors’ low and high levels are coded as −1 and +1 respectively. Moreover, this equation can aid to identify the relative impact of the factors through the comparison of factor coefficients.
The ANOVA results for the sulphur removal in the tyre-derived oil are presented in Table 5. These results are based on the reduced cubic model obtained after the model modification. The F-value of the model is 18.72 which means that the model is significant, in the sense that there is only a 0.09% chance that an F-value of this magnitude could be due to noise. Table 5 shows that the model terms A, C, D, AB, BD, CD, A2, B2, D2 and A2C are significant since the p-values for each of these terms is less than 0.05. On the other hand, B, AC, AD and C2 model terms are not significant as the p-values in these cases are greater than 0.1. It is important, however, to note that the hierarchical factor B (H2O2), which is among the insignificant model terms is involved in more than one significant interactions.
ANOVA for the CCD of the reduced cubic model for sulphur removal.
| Source | SS | DF | MS | F-value | p-value | Remarks |
|---|---|---|---|---|---|---|
| Model | 3435.2 | 14 | 245.37 | 18.72 | 0.0009 | significant |
| A-HCOOH | 157.35 | 1 | 157.35 | 12 | 0.0134 | |
| B-H2O2 | 12.1 | 1 | 12.1 | 0.92 | 0.3737 | |
| C-Temperature | 135.14 | 1 | 135.14 | 10.31 | 0.0183 | |
| D-Reaction time | 93.02 | 1 | 93.02 | 7.1 | 0.0373 | |
| AB | 255.02 | 1 | 255.02 | 19.46 | 0.0045 | |
| AC | 11.62 | 1 | 11.62 | 0.89 | 0.3828 | |
| AD | 46.1 | 1 | 46.1 | 3.52 | 0.1099 | |
| BD | 139.93 | 1 | 139.93 | 10.68 | 0.0171 | |
| CD | 324.62 | 1 | 324.62 | 24.77 | 0.0025 | |
| A2 | 540.64 | 1 | 540.64 | 41.25 | 0.0007 | |
| B2 | 633.28 | 1 | 633.28 | 48.31 | 0.0004 | |
| C2 | 40.43 | 1 | 40.43 | 3.08 | 0.1296 | |
| D2 | 116.36 | 1 | 116.36 | 8.88 | 0.0247 | |
| A2C | 150.88 | 1 | 150.88 | 11.51 | 0.0146 | |
| Residual | 78.65 | 6 | 13.11 | |||
| Lack of Fit | 4.34 | 2 | 2.17 | 0.12 | 0.8926 | not significant |
| Pure Error | 74.3 | 4 | 18.58 | |||
| Correction total | 3513.85 | 20 |
R2 = 0.9776, adjusted R2 = 0.9254, predicted R2 = 0.8356, adequate precision = 16.878
The number of significant model terms outweighs the non-hierarchical insignificant terms, which implies that the model fits well the experimental data. Moreover, the lack of fit (LOF) F-value of 0.12 shows its insignificance in comparison with pure error i.e. there is an 89.26% possibility that such a large F-value for the LOF could be because of noise. The insignificant LOF implies that the model fits well. The coefficient of determination, R2 value of 0.9776 implies that the empirical model could explain the over 97.76% of the data deviation, an indication of statistical significance of the regression model. The obtained adjusted R2 value of 0.9254 indicates that the correlation between the experimental and predicted responses is high. In addition, the difference of less than 0.2 between the adjusted R2 = 0.9254 and predicted R2 = 0.8356 indicates that the two values of the coefficient of determination were in reasonable agreement. The adequate precision, which is the measure of the signal to noise ratio was 16.878 (>4), a desirable value with an adequate signal. This, therefore, implies that the model can be applied for design space navigation.
3.4 Diagnostics plots
The plot of predicted sulphur removal versus experimental values is presented in Figure 2. It can be seen that there is a good agreement (R2 = 0.9776) between the predicted and actual sulphur removal, an indication that the model is adequate and significant, and thus can perfectly be used to reproduce the experimental data in the range studied.
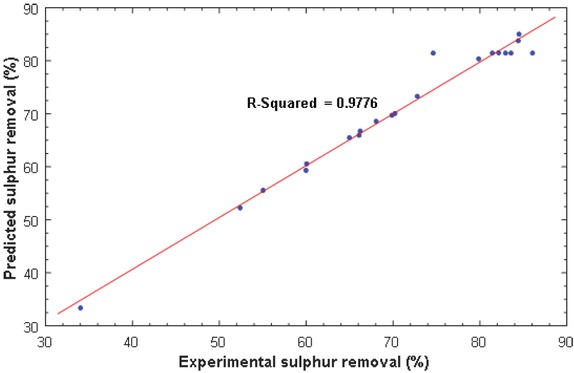
Plot of predicted versus experimental sulphur removal.
Figure 3 shows the plot of externally studentized residuals against the run number. There is a random dispersal of residuals around the line, which is also an indication of the adequacy of the reduced cubic model. Figure 4 presents the normal probability plot of the externally studentized residuals. There is a consistent appearance of the data points obtained on a linear trendline (Figure 4), which signifies the absence of obvious dispersal, and that the residuals are normally distributed.

Plot of externally studentized residuals versus run number.
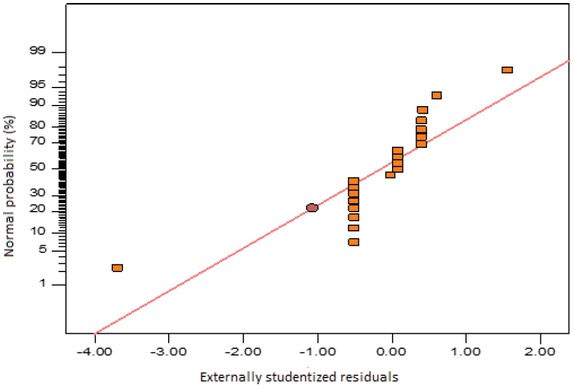
Normal probability plot of externally studentized residuals.
3.5 Perturbation plot
The perturbation plot showing the sulphur removal as a function of all factors on one graph is presented in
Figure 5. This plot indicates the behaviour of the change of the response as each factor deviates from the selected point of reference, with the other remaining factors being constant at the reference value. The reference point in this case is set at the mid-point of the design space i.e. the coded zero level of each factor. The perturbation, therefore, shows how the factorial level deviates from the modified reference point of all the factors. It can be seen that, formic acid amount (A), hydrogen peroxide amount (B), Temperature (C) and reaction time (D) are the parameters involved in the control of sulphur removal in the tyre-derived oil (Figure 5). The sharpness of the formic acid and temperature curvatures is an indication of high sensitivity of sulphur removal to these two factors. The reaction time showed a slightly sharper curvature than the hydrogen peroxide amount (flattest of all factors), which indicates that it had more influence on sulphur removal than the amount of hydrogen peroxide. However, the influence of the reaction time on sulphur removal was lower compared to that of either the amount of formic acid or temperature.
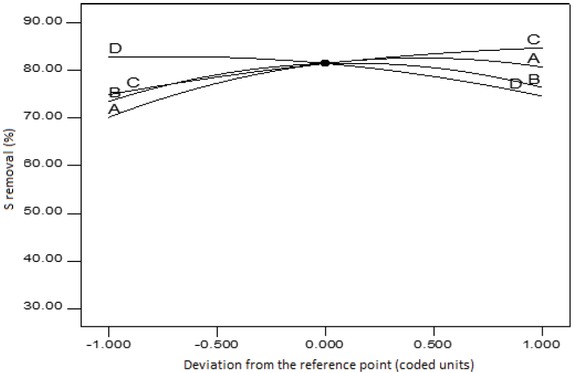
Plot of perturbation of the four parameters.
3.6 Interaction graphs
The two-factor interaction plots for AB (formic acid and hydrogen peroxide amounts), AC (formic acid amount and temperature), AD (formic acid amount and reaction time), BD (hydrogen peroxide amount and reaction time) and CD (temperature and reaction time) at mid-level points of the other two factors are presented in Figures 6-10, respectively. Least-significant-difference (LSD) bars in the interaction graphs are used to determine if a difference between two means exists. The height of the bar is dependent upon the design, confidence level and the model. The LSD can aid in testing for a significant difference in predictions if a significant overall model result is obtained from ANOVA. The interaction plots show the average LSD I-beams around the predictions. There is a high likelihood of significant differences in predictions if there is no overlapping of the I-beams [56]. If the lines are not parallel (i.e. do not cross each other) in the interaction plots, then there is an indication that the effect of one factor is dependent upon the level of the other [63].
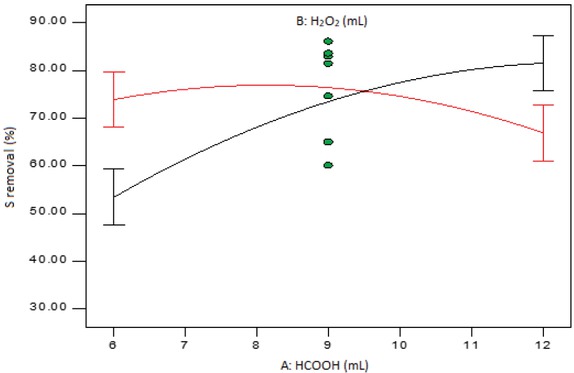
Interaction plot of formic acid and hydrogen peroxide amounts at mid-level temperature and reaction time.
Figure 6 shows the interaction plot of AB at mid-levels of factors C and D. The black line indicates what happens to the sulphur removal as the amount of formic acid changes from low to high level while the amount of hydrogen peroxide is held at low level (6 mL). The red line, on the other hand, shows the variation of sulphur removal with amount of formic acid when the amount of hydrogen peroxide is held at high level. It can be seen that there is a significant difference between the two levels of the amount of hydrogen peroxide when the formic acid amount is set at either low (6 mL) or high (12 mL) level. This is because the LSD bars do not overlap in either case.
Figure 7 presents the interaction graph of factor A (amount of formic acid) and factor C (temperature) when factor B (amount of hydrogen peroxide) and factor D (reaction time) are set at mid-levels. It can be seen that there is not a significant difference in the two levels of temperature when the amount of formic acid is set at either low or high level. This is because the LSD bars overlap at both ends. It can also be noted that the plot exhibits an insignificant difference between the low and high level of the amount of formic acid when low temperature is used. However, the difference is more significant at high level of temperature.
The interaction graph of the amount of formic acid and the reaction time at mid-levels of the amount of hydrogen peroxide and temperature is presented in Figure 8. It can be seen that there is not a significant difference in the two levels of the reaction time when the amount of formic acid is set at low level since the LSD bars overlap. However, when the amount of formic acid is set at high level, the difference in the two levels of the reaction time is significant.
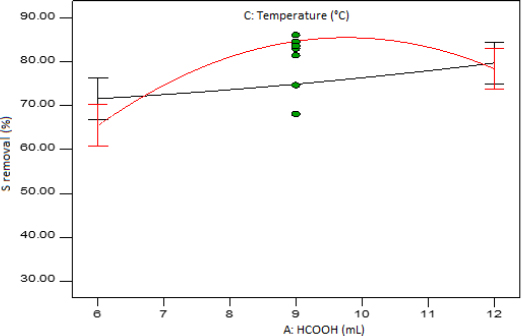
Interaction plot of formic acid amount and temperature at mid-level reaction time and hydrogen peroxide amount.
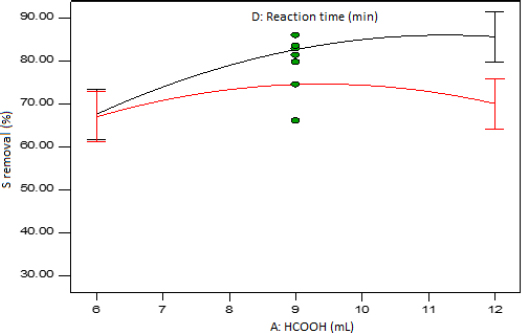
Interaction plot of formic acid amount and reaction time at mid-level temperature and hydrogen peroxide amount.
Figure 9 presents the interaction graph of the amount of hydrogen peroxide and the reaction time at middle levels of the temperature and the amount of formic acid. It can be deduced that at high level of the amount of hydrogen peroxide, there is a significant difference in the two levels of the reaction time. However, at low level of hydrogen peroxide, the difference in the two levels of the reaction time is insignificant.
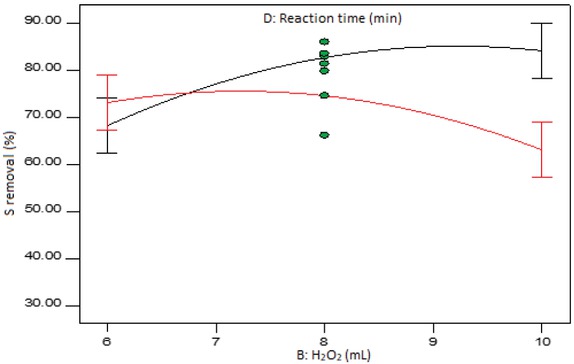
Interaction plot of hydrogen peroxide amount and reaction time at mid-level temperature and formic acid amount.
Figure 10 shows the interaction plot of the temperature and the reaction time at mid-levels of the amount of formic acid and the amount of hydrogen peroxide. It can be seen that at low level of the temperature (50°C), there is a significant difference in the low and high levels of the reaction time. The case is different when the temperature is set at high (58°C) level, where, as evidenced by the overlapping LSD bars, the difference in the two levels of the reaction time is insignificant. The sulphur removal is enhanced as the temperature increases from low to high level when the reaction time is kept at high level. It can also be seen that at higher level of temperature the reaction time did not have any influence on the sulphur removal. This could be attributed to the fact that at higher temperatures, the sulphur-containing compounds in the tyre oil are converted to sulphones and sulphoxides much faster and therefore prolonging the reaction time does not lead to any further conversion. Similar observations were made in a previous study conducted by Al-Lal et al. [24], where prolonged reaction times did not significantly affect the sulphur removal.
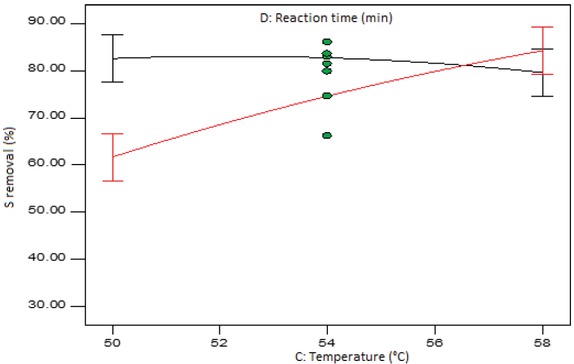
Interaction plot of temperature and reaction time at mid-level formic acid and hydrogen peroxide amount.
3.7 Optimisation of the ODS process
Numerical optimisation was used to determine the optimal conditions for the sulphur removal in the TDO. The optimisation method used by Design-Expert® Software is based on a technique developed by Derringer and Suich [64], and its description can be found in [65]. The parameters’ goals were set to be within the range of the design space while the goal of the response was set to ‘maximise’, with lower and upper limits of 85 and 90 respectively. Under the aforementioned optimisation criteria, the ramps results (with a response desirability of one) shown in Figure 11 were obtained. The amount of formic acid of 11.96 mL, amount of hydrogen peroxide of 7.56 mL, reaction temperature of 50.1°C and reaction time of 40.7 min could be used to achieve a 90.18% sulphur removal in the tyre-derived oil. In addition, a desirability value of one is an indication of an ideal case for the sulphur removal. The optimisation criteria is presented in Table 6.

Predicted numerical optimisation ramps for targeted sulphur removal.
Optimisation criteria.
| Name | Goal | Lower Limit | Upper Limit | Lower Weight | Upper Weight | Importance |
|---|---|---|---|---|---|---|
| A:HCOOH | in range | 6 | 12 | 1 | 1 | 3 |
| B:H2O2 | in range | 6 | 10 | 1 | 1 | 3 |
| C:Temperature | in range | 50 | 58 | 1 | 1 | 3 |
| D:Reaction time | in range | 40 | 60 | 1 | 1 | 3 |
| S Removal | maximize | 85 | 90 | 1 | 1 | 5 |
4 Conclusion
This study focused on the understanding of the interaction of different factors involved in the oxidative desulphurisation of tyre-derived oil. This was done using the central composite design. The amount of formic acid, amount of hydrogen peroxide, reaction time and temperature were the four factors whose interactive effect on sulphur removal was investigated. The TDO was oxidised at various parametric interactions before being subjected to solvent extraction using acetonitrile. The acetonitrile to oxidised TDO ratios used during the extraction were 1:1 and 1:2. The maximum sulphur removal obtained using a 1:1 acetonitrile to oxidised TDO ratio during the solvent extraction step was 86.05%, and this was achieved at the formic acid amount, hydrogen peroxide amount, temperature and reaction time of 9 mL, 8 mL, 54°C and 50 min respectively. ANOVA results (as backed by statistical
parameters) indicated that the reduced cubic model was the best predictor of sulphur removal for the ODS process. This is a novel study on the interaction of temperature, reaction time and the oxidation system associated with the oxidative desulphurisation of tyre-derived oil.
Acknowledgements
The first author is grateful to South Africa’s National Research Fund (NRF) for the financial support in form of Doctoral Fellowship.
References
[1] Su Y., Zhao B., Pyrolysis of waste tire powder and its comparison with Shenhua coal. In: Energy and Environment Technology International Conference, 2009, 262-265.10.1109/ICEET.2009.69Search in Google Scholar
[2] Uçar S., Karagöz S., Co-pyrolysis of pine nut shells with scrap tires. Fuel, 2014, 137, 85-93.10.1016/j.fuel.2014.07.082Search in Google Scholar
[3] Su Y., Deng W.A., A thermogravimetric study of waste tire powder. In: E-Product E-Service and E-Entertainment (ICEEE) International Conference, 2010, 1-4.10.1109/ICEEE.2010.5660888Search in Google Scholar
[4] Parthasarathy P., Choi H.S., Park H.C., Hwang J.G., Yoo H.S., Lee B.K., et al., Influence of process conditions on product yield of waste tyre pyrolysis-A review. Korean J. Chem. Eng., 2016, 33, 2268-2286.10.1007/s11814-016-0126-2Search in Google Scholar
[5] Lin J., Gaustad G., Trabold T.A., Profit and policy implications of producing biodiesel–ethanol–diesel fuel blends to specification. Appl. Energy, 2013, 104, 936-944.10.1016/j.apenergy.2012.11.049Search in Google Scholar
[6] Nieto-Márquez A., Atanes E., Morena J., Fernández-Martínez F., Valverde J.L., Upgrading waste tires by chemical activation for the capture of SO2. Fuel Process. Technol., 2016, 144, 274-281.10.1016/j.fuproc.2016.01.009Search in Google Scholar
[7] Islam M.R., Haniu H., Fardoushi J., Pyrolysis kinetics behavior of solid tire wastes available in Bangladesh. Waste Manag., 2009, 29, 668-677.10.1016/j.wasman.2008.04.009Search in Google Scholar
[8] Leung D.Y.C., Wang C.L., Kinetic study of scrap tyre pyrolysis and combustion. J. Anal. Appl. Pyrolysis, 1998, 45, 153-169.10.1016/S0165-2370(98)00065-5Search in Google Scholar
[9] Seidelt S., Müller-Hagedorn M., Bockhorn H.J., Description of tire pyrolysis by thermal degradation behaviour of main components. Anal. Appl. Pyrolysis, 2006, 75, 11-18.10.1016/j.jaap.2005.03.002Search in Google Scholar
[10] Lopez G., Aguado R., Olazar M., Arabiourrutia M., Bilbao J., Kinetics of scrap tyre pyrolysis under vacuum conditions. Waste Manag., 2009, 29, 2649-2655.10.1016/j.wasman.2009.06.005Search in Google Scholar PubMed
[11] López F.A., Centeno T.A., Alguacil F.J., Lobato B.J., Distillation of granulated scrap tires in a pilot plant. Hazard. Mater., 2011, 190, 285-292.10.1016/j.jhazmat.2011.03.039Search in Google Scholar
[12] Miranda M., Pinto F., Gulyurtlu I., Cabrita I., Pyrolysis of rubber tyre wastes: A kinetic study. Fuel, 2013, 103, 542-552.10.1016/j.fuel.2012.06.114Search in Google Scholar
[13] Leung D., Wang C., Kinetic modeling of scrap tire pyrolysis. Energy Fuels, 1999, 13, 421-427.10.1021/ef980124lSearch in Google Scholar
[14] de Marco Rodriguez I., Laresgoiti M.F., Cabrero M.A., Torres A., Chomón M.J., Caballero B., Pyrolysis of scrap tyres. Fuel Process. Technol., 2001, 72, 9-22.10.1016/S0378-3820(01)00174-6Search in Google Scholar
[15] Laresgoiti M.F., Caballero B.M., de Marco I., Torres A., Cabrero M.A., Chomón M.J., Characterization of the liquid products obtained in tyre pyrolysis. J. Anal. Appl. Pyrolysis, 2004, 71, 917-934.10.1016/j.jaap.2003.12.003Search in Google Scholar
[16] Pilusa T., The use of modified tyre derived fuel for compression ignition engines. Waste Manag., 2017, 60, 451-459.10.1016/j.wasman.2016.06.020Search in Google Scholar
[17] Te M., Fairbridge C., Ring Z., Oxidation reactivities of dibenzothiophenes in polyoxometalate/H2O2 and formic acid/H2O2 systems. Appl. Catal. A: Gen., 2001, 219, 267-280.10.1016/S0926-860X(01)00699-8Search in Google Scholar
[18] Murti S.D.S., Choi K.H., Sakanishi K., Okuma O., Korai Y., Mochida I., Analysis and removal of heteroatom containing species in coal liquid distillate over NiMo catalysts Fuel, 2005, 84, 135-142.10.1016/j.fuel.2004.08.014Search in Google Scholar
[19] Ahmad S., Ahmad M. I., Desulfurization of Oils; Produced from Pyrolysis of Scrap Tires. NUST J. Eng. Sci., 2013, 6, 27-32.Search in Google Scholar
[20] Dharaskar S.A., Varma M.N., Shende D.Z., Yoo C.K., Wasewar K.L., Synthesis, characterization and application of 1-butyl-3 methylimidazolium chloride as green material for extractive desulfurization of liquid fuel. Sci. World J., 2013.10.1155/2013/395274Search in Google Scholar PubMed PubMed Central
[21] Huang C., Chen B., Zhang J., Liu Z., Li Y., Desulfurization of gasoline by extraction with new ionic liquids. Energy Fuels, 2004, 18, 1862-1864.10.1021/ef049879kSearch in Google Scholar
[22] Wan M.W., Yen T.F., Enhance efficiency of tetraoctylammonium fluoride applied to ultrasound-assisted oxidative desulfurization (UAOD) process. Appl. Catal. A: Gen., 2007, 319, 237-245.10.1016/j.apcata.2006.12.008Search in Google Scholar
[23] Chen T.C., Shen Y.H., Lee W.J., Lin C.C., Wan M.W., The study of ultrasound-assisted oxidative desulfurization process applied to the utilization of pyrolysis oil from waste tires. J. Clean. Prod., 2010, 18, 1850-1858.10.1016/j.jclepro.2010.07.019Search in Google Scholar
[24] Al-Lal A.M., Bolonio D., Llamas A., Lapuerta M., Canoira L., Desulfurization of pyrolysis fuels obtained from waste: Lube oils, tires and plastics. Fuel, 2015, 150, 208-216.10.1016/j.fuel.2015.02.034Search in Google Scholar
[25] Song C., Ma X., New design approaches to ultra-clean diesel fuels by deep desulfurization and deep dearomatization. Appl. Catal. B: Environ., 2003, 41, 207-238.10.1016/S0926-3373(02)00212-6Search in Google Scholar
[26] García-Gutiérrez J.L., Fuentes G.A., Hernández-Terán M.E., García P., Murrieta-Guevara.F., Jiménez-Cruz F., Ultra-deep oxidative desulfurization of diesel fuel by the Mo/Al2O3-H2O2 system: The effect of system parameters on catalytic activity. Appl. Catal. A: Gen., 2008, 334, 366-373.10.1016/j.apcata.2007.10.024Search in Google Scholar
[27] Haw K.G., Bakar W.A.W.A., Ali R., Chong J.F., Kadir A.A.A., Catalytic oxidative desulfurization of diesel utilizing hydrogen peroxide and functionalized-activated carbon in a biphasic diesel–acetonitrile system. Fuel Process. Technol., 2010, 91, 1105-1112.10.1016/j.fuproc.2010.03.021Search in Google Scholar
[28] Pakdel H., Roy C., Simultaneous gas chromatographic—Fourier transform infrared spectroscopic—mass spectrometric analysis of synthetic fuel derived from used tire vacuum pyrolysis oil, naphtha fraction. J. Chromatogr. A., 1994, 683, 203-214.10.1016/S0021-9673(94)89117-6Search in Google Scholar
[29] Williams P.T., Bottrill R.P., Sulfur-polycyclic aromatic hydrocarbons in tyre pyrolysis oil. Fuel, 1995, 74, 736-742.10.1016/0016-2361(94)00005-CSearch in Google Scholar
[30] Aida T., Yamamoto D., Annual Book of ASTM Standards, 2005. Standard test method for determination of sulfur compounds in natural gas and gaseous fuels by gas chromatography and chemilumi-nescence. Prepr. Pap. Am. Chem. Soc., Div. Fuel Chem., 1994, 39, 623.Search in Google Scholar
[31] Zannikos F., Lois E., Stournas S., Desulfurization of petroleum fractions by oxidation and solvent extraction. Fuel Process. Technol., 1995, 42, 35-45.10.1016/0378-3820(94)00104-2Search in Google Scholar
[32] Aydın H., İlkılıç C., Optimization of fuel production from waste vehicle tires by pyrolysis and resembling to diesel fuel by various desulfurization methods. Fuel, 2012, 102, 605-612.10.1016/j.fuel.2012.06.067Search in Google Scholar
[33] Dehkordi A., Sobati M., Nazem M., An experimental investigation on the oxidative desulfurization of kerosene feedstock. Energy Sources, Part A: Recover. Utilization Environ. Eff., 2013, 35, 226-234.10.1080/15567036.2010.509087Search in Google Scholar
[34] Murugan S., Ramaswamy M.C., Nagarajan G., A comparative study on the performance, emission and combustion studies of a DI diesel engine using distilled tyre pyrolysis oil–diesel blends. Fuel, 2008, 87, 2111-2121.10.1016/j.fuel.2008.01.008Search in Google Scholar
[35] Bunthid D., Prasassarakich P., Hinchiranan N., Oxidative desulfurization of tire pyrolysis naphtha in formic acid/H2O2/ pyrolysis char system. Fuel, 2010, 89, 2617-2622.10.1016/j.fuel.2010.04.026Search in Google Scholar
[36] Zhang G., Yu F., Wang R., Research advances in oxidative desulfurization technologies for the production of low sulfur fuel oils. Pet. Coal, 2009, 51, 196-207.Search in Google Scholar
[37] Ali M.F., Al-Malki A., El-Ali B., Martinie G., Siddiqui M.N., Deep desulphurization of gasoline and diesel fuels using non-hydrogen consuming techniques. Fuel, 2006, 85, 1354-1363.10.1016/j.fuel.2005.12.006Search in Google Scholar
[38] Ali M.F., Al-Malki A., Ahmed S., Chemical desulfurization of petroleum fractions for ultra-low sulfur fuels. Fuel Process. Technol., 2009, 90, 536-544.10.1016/j.fuproc.2009.01.005Search in Google Scholar
[39] Nabi A.R., Masud M.H., Alam Q.I., Purification of TPO (Tire Pyrolytic Oil) and its use in diesel engine. IOSR, J. Eng., 2014, 4, 1.10.9790/3021-04320108Search in Google Scholar
[40] Rydberg J., Solvent extraction principles and practice, revised and expanded. CRC Press, 2004.10.1201/9780203021460Search in Google Scholar
[41] Shiraishi Y., Hirai T., Komasawa I., A deep desulfurization process for light oil by photochemical reaction in an organic two-phase liquid− liquid extraction system. Ind. Eng. Chem. Res., 1998, 37, 203-211.10.1021/ie970388fSearch in Google Scholar
[42] Shiraishi Y., Hirai T., Komasawa I., Oxidative desulfurization process for light oil using titanium silicate molecular sieve catalysts. J. Chem. Eng. Jpn., 2002, 35, 305-1311.10.1252/jcej.35.1305Search in Google Scholar
[43] Murata S., Murata K., Kidena K., Nomura M., A novel oxidative desulfurization system for diesel fuels with molecular oxygen in the presence of cobalt catalysts and aldehydes. Energy Fuels, 2004, 18, 116-121.10.1021/ef034001zSearch in Google Scholar
[44] Shiraishi Y., Naito T., Hirai T., Vanadosilicate molecular sieve as a catalyst for oxidative desulfurization of light oil. Ind. Eng. Chem. Res., 2003, 42, 6034-6039.10.1021/ie030328bSearch in Google Scholar
[45] Voznesensky V., Experiment Planning Statistical Methods for Technical and Economical Investigations. Statistics, Moscow, 1974 (in Russian).Search in Google Scholar
[46] Box G.E., Hunter W.G., Hunter J.S., Statistics for Experimenters: An Introduction to Design, Data Analysis and Model Building, 1978.Search in Google Scholar
[47] Montgomery D.C., Design and Analysis of Experiments with Design Expert Software (6th Ed.). John Wiley & Sons, 2004.Search in Google Scholar
[48] Yu G., Lu S., Chen H., Zhu Z., Diesel fuel desulfurization with hydrogen peroxide promoted by formic acid and catalyzed by activated carbon. Carbon, 2005, 43, 2285-2294.10.1016/j.carbon.2005.04.008Search in Google Scholar
[49] Mokhtar W.N.A.W., Bakar W.A.W.A., Ali R., Kadir A.A.A., Optimization of oxidative desulfurization of Malaysian Euro II diesel fuel utilizing tert-butyl hydroperoxide– dimethylformamide system. Fuel, 2015, 161, 26-33.10.1016/j.fuel.2015.08.031Search in Google Scholar
[50] Dutta S.K., Halder G., Mandal M.K., Modeling and optimization of bi-directional delignification of rice straw for production of bio-fuel feedstock using central composite design approach. Energy, 2014, 71, 579-587.10.1016/j.energy.2014.04.108Search in Google Scholar
[51] Mukherjee S., Halder G., Assessment of fluoride uptake performance of raw biomass and activated biochar of Colocasia esculenta stem: optimization through response surface methodology. Environ. Progr. Sustain. Energy, 2016, 35, 1305-1316.10.1002/ep.12346Search in Google Scholar
[52] Box G.E., Behnken D.W., Some new three level designs for the study of quantitative variables. Technometrics, 1960, 2, 455-475.10.1080/00401706.1960.10489912Search in Google Scholar
[53] Dhawane S.H., Kumar T., Halder G., Central composite design approach towards optimization of flamboyant pods derived steam activated carbon for its use as heterogeneous catalyst in transesterification of Hevea brasiliensis oil. Energy Convers. Manag., 2015, 100, 277-287.10.1016/j.enconman.2015.04.083Search in Google Scholar
[54] Dhawane S.H., Khan A.A., Singh K., Tripathi A., Hasda R., Halder G., Insight into Optimization, isotherm, kinetics, and thermodynamics of fluoride adsorption onto activated alumina. Environ. Progr. Sustain. Energy, 2018, 37, 766-776.10.1002/ep.12814Search in Google Scholar
[55] Halder S., Dhawane S.H., Kumar T., Halder G., Acid-catalyzed esterification of castor Ricinus communis oil: optimization through a central composite design approach. Biofuels, 2015, 6, 191-201.10.1080/17597269.2015.1078559Search in Google Scholar
[56] Stat-Ease, Stat-Ease Design Expert Package, Version 7.0. 0, ed. Stat-Ease Minneapolis, 2005.Search in Google Scholar
[57] Halder G., Dhawane S., Barai P.K., Das A., Optimizing chromium (VI) adsorption onto superheated steam activated granular carbon through response surface methodology and artificial neural network. Environ. Progr. Sustain. Energy, 2015, 34, 638-647.10.1002/ep.12028Search in Google Scholar
[58] Halder G., Sinha K., Dhawane S., Defluoridation of wastewater using powdered activated carbon developed from Eichhornia crassipes stem: optimization by response surface methodology. Desalination Water Treat., 2015, 56, 953-966.10.1080/19443994.2014.942375Search in Google Scholar
[59] Montgomery D.C., Design and Analysis of Experiments. John Wiley & Sons, New York, 2001, 64-65.Search in Google Scholar
[60] Montgomery D.C., Design and analysis of experiments (7th ed.). John Wiley & Sons, New York, 2009.Search in Google Scholar
[61] Mondal S., Aikat K., Siddharth K., Sarkar K., DasChaudhury R., Mandal G., et al., Optimizing ranitidine hydrochloride uptake of Parthenium hysterophorus derived N-biochar through response surface methodology and artificial neural network. Process Saf. Environ. Prot., 2017, 107, 388-401.10.1016/j.psep.2017.03.011Search in Google Scholar
[62] Mondal S., Aikat K., Halder G., Optimization of ranitidine hydrochloride removal from simulated pharmaceutical waste by activated charcoal from mung bean husk using response surface methodology and artificial neural network. Desalination Water Treat., 2016, 57, 18366-18378.10.1080/19443994.2015.1088899Search in Google Scholar
[63] Abdul-Wahab S., Abdo J., Optimization of multistage flash desalination process by using a two-level factorial design. Appl. Therm. Eng., 2007, 27, 413-421.10.1016/j.applthermaleng.2006.07.010Search in Google Scholar
[64] Derringer G., Suich R., Simultaneous optimization of several response variables. J. Qual. Technol., 1980, 12, 214-219.10.1080/00224065.1980.11980968Search in Google Scholar
[65] Myers R.H., Montgomery D.C., Anderson-Cook C., Response surface methodology. John Wiley & Sons, Inc., New Jersey, 2009, 20, 38-44.Search in Google Scholar
© 2019 Cherop et al., published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 Public License.
Articles in the same Issue
- Regular Articles
- Studies on the preparation and properties of biodegradable polyester from soybean oil
- Flow-mode biodiesel production from palm oil using a pressurized microwave reactor
- Reduction of free fatty acids in waste oil for biodiesel production by glycerolysis: investigation and optimization of process parameters
- Saccharin: a cheap and mild acidic agent for the synthesis of azo dyes via telescoped dediazotization
- Optimization of lipase-catalyzed synthesis of polyethylene glycol stearate in a solvent-free system
- Green synthesis of iron oxide nanoparticles using Platanus orientalis leaf extract for antifungal activity
- Ultrasound assisted chemical activation of peanut husk for copper removal
- Room temperature silanization of Fe3O4 for the preparation of phenyl functionalized magnetic adsorbent for dispersive solid phase extraction for the extraction of phthalates in water
- Evaluation of the saponin green extraction from Ziziphus spina-christi leaves using hydrothermal, microwave and Bain-Marie water bath heating methods
- Oxidation of dibenzothiophene using the heterogeneous catalyst of tungsten-based carbon nanotubes
- Calcined sodium silicate as an efficient and benign heterogeneous catalyst for the transesterification of natural lecithin to L-α-glycerophosphocholine
- Synergistic effect between CO2 and H2O2 on ethylbenzene oxidation catalyzed by carbon supported heteropolyanion catalysts
- Hydrocyanation of 2-arylmethyleneindan-1,3-diones using potassium hexacyanoferrate(II) as a nontoxic cyanating agent
- Green synthesis of hydratropic aldehyde from α-methylstyrene catalyzed by Al2O3-supported metal phthalocyanines
- Environmentally benign chemical recycling of polycarbonate wastes: comparison of micro- and nano-TiO2 solid support efficiencies
- Medicago polymorpha-mediated antibacterial silver nanoparticles in the reduction of methyl orange
- Production of value-added chemicals from esterification of waste glycerol over MCM-41 supported catalysts
- Green synthesis of zerovalent copper nanoparticles for efficient reduction of toxic azo dyes congo red and methyl orange
- Optimization of the biological synthesis of silver nanoparticles using Penicillium oxalicum GRS-1 and their antimicrobial effects against common food-borne pathogens
- Optimization of submerged fermentation conditions to overproduce bioethanol using two industrial and traditional Saccharomyces cerevisiae strains
- Extraction of In3+ and Fe3+ from sulfate solutions by using a 3D-printed “Y”-shaped microreactor
- Foliar-mediated Ag:ZnO nanophotocatalysts: green synthesis, characterization, pollutants degradation, and in vitro biocidal activity
- Green cyclic acetals production by glycerol etherification reaction with benzaldehyde using cationic acidic resin
- Biosynthesis, characterization and antimicrobial activities assessment of fabricated selenium nanoparticles using Pelargonium zonale leaf extract
- Synthesis of high surface area magnesia by using walnut shell as a template
- Controllable biosynthesis of silver nanoparticles using actinobacterial strains
- Green vegetation: a promising source of color dyes
- Mechano-chemical synthesis of ammonia and acetic acid from inorganic materials in water
- Green synthesis and structural characterization of novel N1-substituted 3,4-dihydropyrimidin-2(1H)-ones
- Biodiesel production from cotton oil using heterogeneous CaO catalysts from eggshells prepared at different calcination temperatures
- Regeneration of spent mercury catalyst for the treatment of dye wastewater by the microwave and ultrasonic spray-assisted method
- Green synthesis of the innovative super paramagnetic nanoparticles from the leaves extract of Fraxinus chinensis Roxb and their application for the decolourisation of toxic dyes
- Biogenic ZnO nanoparticles: a study of blueshift of optical band gap and photocatalytic degradation of reactive yellow 186 dye under direct sunlight
- Leached compounds from the extracts of pomegranate peel, green coconut shell, and karuvelam wood for the removal of hexavalent chromium
- Enhancement of molecular weight reduction of natural rubber in triphasic CO2/toluene/H2O systems with hydrogen peroxide for preparation of biobased polyurethanes
- An efficient green synthesis of novel 1H-imidazo[1,2-a]imidazole-3-amine and imidazo[2,1-c][1,2,4]triazole-5-amine derivatives via Strecker reaction under controlled microwave heating
- Evaluation of three different green fabrication methods for the synthesis of crystalline ZnO nanoparticles using Pelargonium zonale leaf extract
- A highly efficient and multifunctional biomass supporting Ag, Ni, and Cu nanoparticles through wetness impregnation for environmental remediation
- Simple one-pot green method for large-scale production of mesalamine, an anti-inflammatory agent
- Relationships between step and cumulative PMI and E-factors: implications on estimating material efficiency with respect to charting synthesis optimization strategies
- A comparative sorption study of Cr3+ and Cr6+ using mango peels: kinetic, equilibrium and thermodynamic
- Effects of acid hydrolysis waste liquid recycle on preparation of microcrystalline cellulose
- Use of deep eutectic solvents as catalyst: A mini-review
- Microwave-assisted synthesis of pyrrolidinone derivatives using 1,1’-butylenebis(3-sulfo-3H-imidazol-1-ium) chloride in ethylene glycol
- Green and eco-friendly synthesis of Co3O4 and Ag-Co3O4: Characterization and photo-catalytic activity
- Adsorption optimized of the coal-based material and application for cyanide wastewater treatment
- Aloe vera leaf extract mediated green synthesis of selenium nanoparticles and assessment of their In vitro antimicrobial activity against spoilage fungi and pathogenic bacteria strains
- Waste phenolic resin derived activated carbon by microwave-assisted KOH activation and application to dye wastewater treatment
- Direct ethanol production from cellulose by consortium of Trichoderma reesei and Candida molischiana
- Agricultural waste biomass-assisted nanostructures: Synthesis and application
- Biodiesel production from rubber seed oil using calcium oxide derived from eggshell as catalyst – optimization and modeling studies
- Study of fabrication of fully aqueous solution processed SnS quantum dot-sensitized solar cell
- Assessment of aqueous extract of Gypsophila aretioides for inhibitory effects on calcium carbonate formation
- An environmentally friendly acylation reaction of 2-methylnaphthalene in solvent-free condition in a micro-channel reactor
- Aegle marmelos phytochemical stabilized synthesis and characterization of ZnO nanoparticles and their role against agriculture and food pathogen
- A reactive coupling process for co-production of solketal and biodiesel
- Optimization of the asymmetric synthesis of (S)-1-phenylethanol using Ispir bean as whole-cell biocatalyst
- Synthesis of pyrazolopyridine and pyrazoloquinoline derivatives by one-pot, three-component reactions of arylglyoxals, 3-methyl-1-aryl-1H-pyrazol-5-amines and cyclic 1,3-dicarbonyl compounds in the presence of tetrapropylammonium bromide
- Preconcentration of morphine in urine sample using a green and solvent-free microextraction method
- Extraction of glycyrrhizic acid by aqueous two-phase system formed by PEG and two environmentally friendly organic acid salts - sodium citrate and sodium tartrate
- Green synthesis of copper oxide nanoparticles using Juglans regia leaf extract and assessment of their physico-chemical and biological properties
- Deep eutectic solvents (DESs) as powerful and recyclable catalysts and solvents for the synthesis of 3,4-dihydropyrimidin-2(1H)-ones/thiones
- Biosynthesis, characterization and anti-microbial activity of silver nanoparticle based gel hand wash
- Efficient and selective microwave-assisted O-methylation of phenolic compounds using tetramethylammonium hydroxide (TMAH)
- Anticoagulant, thrombolytic and antibacterial activities of Euphorbia acruensis latex-mediated bioengineered silver nanoparticles
- Volcanic ash as reusable catalyst in the green synthesis of 3H-1,5-benzodiazepines
- Green synthesis, anionic polymerization of 1,4-bis(methacryloyl)piperazine using Algerian clay as catalyst
- Selenium supplementation during fermentation with sugar beet molasses and Saccharomyces cerevisiae to increase bioethanol production
- Biosynthetic potential assessment of four food pathogenic bacteria in hydrothermally silver nanoparticles fabrication
- Investigating the effectiveness of classical and eco-friendly approaches for synthesis of dialdehydes from organic dihalides
- Pyrolysis of palm oil using zeolite catalyst and characterization of the boil-oil
- Azadirachta indica leaves extract assisted green synthesis of Ag-TiO2 for degradation of Methylene blue and Rhodamine B dyes in aqueous medium
- Synthesis of vitamin E succinate catalyzed by nano-SiO2 immobilized DMAP derivative in mixed solvent system
- Extraction of phytosterols from melon (Cucumis melo) seeds by supercritical CO2 as a clean technology
- Production of uronic acids by hydrothermolysis of pectin as a model substance for plant biomass waste
- Biofabrication of highly pure copper oxide nanoparticles using wheat seed extract and their catalytic activity: A mechanistic approach
- Intelligent modeling and optimization of emulsion aggregation method for producing green printing ink
- Improved removal of methylene blue on modified hierarchical zeolite Y: Achieved by a “destructive-constructive” method
- Two different facile and efficient approaches for the synthesis of various N-arylacetamides via N-acetylation of arylamines and straightforward one-pot reductive acetylation of nitroarenes promoted by recyclable CuFe2O4 nanoparticles in water
- Optimization of acid catalyzed esterification and mixed metal oxide catalyzed transesterification for biodiesel production from Moringa oleifera oil
- Kinetics and the fluidity of the stearic acid esters with different carbon backbones
- Aiming for a standardized protocol for preparing a process green synthesis report and for ranking multiple synthesis plans to a common target product
- Microstructure and luminescence of VO2 (B) nanoparticle synthesis by hydrothermal method
- Optimization of uranium removal from uranium plant wastewater by response surface methodology (RSM)
- Microwave drying of nickel-containing residue: dielectric properties, kinetics, and energy aspects
- Simple and convenient two step synthesis of 5-bromo-2,3-dimethoxy-6-methyl-1,4-benzoquinone
- Biodiesel production from waste cooking oil
- The effect of activation temperature on structure and properties of blue coke-based activated carbon by CO2 activation
- Optimization of reaction parameters for the green synthesis of zero valent iron nanoparticles using pine tree needles
- Microwave-assisted protocol for squalene isolation and conversion from oil-deodoriser distillates
- Denitrification performance of rare earth tailings-based catalysts
- Facile synthesis of silver nanoparticles using Averrhoa bilimbi L and Plum extracts and investigation on the synergistic bioactivity using in vitro models
- Green production of AgNPs and their phytostimulatory impact
- Photocatalytic activity of Ag/Ni bi-metallic nanoparticles on textile dye removal
- Topical Issue: Green Process Engineering / Guest Editors: Martine Poux, Patrick Cognet
- Modelling and optimisation of oxidative desulphurisation of tyre-derived oil via central composite design approach
- CO2 sequestration by carbonation of olivine: a new process for optimal separation of the solids produced
- Organic carbonates synthesis improved by pervaporation for CO2 utilisation
- Production of starch nanoparticles through solvent-antisolvent precipitation in a spinning disc reactor
- A kinetic study of Zn halide/TBAB-catalysed fixation of CO2 with styrene oxide in propylene carbonate
- Topical on Green Process Engineering
Articles in the same Issue
- Regular Articles
- Studies on the preparation and properties of biodegradable polyester from soybean oil
- Flow-mode biodiesel production from palm oil using a pressurized microwave reactor
- Reduction of free fatty acids in waste oil for biodiesel production by glycerolysis: investigation and optimization of process parameters
- Saccharin: a cheap and mild acidic agent for the synthesis of azo dyes via telescoped dediazotization
- Optimization of lipase-catalyzed synthesis of polyethylene glycol stearate in a solvent-free system
- Green synthesis of iron oxide nanoparticles using Platanus orientalis leaf extract for antifungal activity
- Ultrasound assisted chemical activation of peanut husk for copper removal
- Room temperature silanization of Fe3O4 for the preparation of phenyl functionalized magnetic adsorbent for dispersive solid phase extraction for the extraction of phthalates in water
- Evaluation of the saponin green extraction from Ziziphus spina-christi leaves using hydrothermal, microwave and Bain-Marie water bath heating methods
- Oxidation of dibenzothiophene using the heterogeneous catalyst of tungsten-based carbon nanotubes
- Calcined sodium silicate as an efficient and benign heterogeneous catalyst for the transesterification of natural lecithin to L-α-glycerophosphocholine
- Synergistic effect between CO2 and H2O2 on ethylbenzene oxidation catalyzed by carbon supported heteropolyanion catalysts
- Hydrocyanation of 2-arylmethyleneindan-1,3-diones using potassium hexacyanoferrate(II) as a nontoxic cyanating agent
- Green synthesis of hydratropic aldehyde from α-methylstyrene catalyzed by Al2O3-supported metal phthalocyanines
- Environmentally benign chemical recycling of polycarbonate wastes: comparison of micro- and nano-TiO2 solid support efficiencies
- Medicago polymorpha-mediated antibacterial silver nanoparticles in the reduction of methyl orange
- Production of value-added chemicals from esterification of waste glycerol over MCM-41 supported catalysts
- Green synthesis of zerovalent copper nanoparticles for efficient reduction of toxic azo dyes congo red and methyl orange
- Optimization of the biological synthesis of silver nanoparticles using Penicillium oxalicum GRS-1 and their antimicrobial effects against common food-borne pathogens
- Optimization of submerged fermentation conditions to overproduce bioethanol using two industrial and traditional Saccharomyces cerevisiae strains
- Extraction of In3+ and Fe3+ from sulfate solutions by using a 3D-printed “Y”-shaped microreactor
- Foliar-mediated Ag:ZnO nanophotocatalysts: green synthesis, characterization, pollutants degradation, and in vitro biocidal activity
- Green cyclic acetals production by glycerol etherification reaction with benzaldehyde using cationic acidic resin
- Biosynthesis, characterization and antimicrobial activities assessment of fabricated selenium nanoparticles using Pelargonium zonale leaf extract
- Synthesis of high surface area magnesia by using walnut shell as a template
- Controllable biosynthesis of silver nanoparticles using actinobacterial strains
- Green vegetation: a promising source of color dyes
- Mechano-chemical synthesis of ammonia and acetic acid from inorganic materials in water
- Green synthesis and structural characterization of novel N1-substituted 3,4-dihydropyrimidin-2(1H)-ones
- Biodiesel production from cotton oil using heterogeneous CaO catalysts from eggshells prepared at different calcination temperatures
- Regeneration of spent mercury catalyst for the treatment of dye wastewater by the microwave and ultrasonic spray-assisted method
- Green synthesis of the innovative super paramagnetic nanoparticles from the leaves extract of Fraxinus chinensis Roxb and their application for the decolourisation of toxic dyes
- Biogenic ZnO nanoparticles: a study of blueshift of optical band gap and photocatalytic degradation of reactive yellow 186 dye under direct sunlight
- Leached compounds from the extracts of pomegranate peel, green coconut shell, and karuvelam wood for the removal of hexavalent chromium
- Enhancement of molecular weight reduction of natural rubber in triphasic CO2/toluene/H2O systems with hydrogen peroxide for preparation of biobased polyurethanes
- An efficient green synthesis of novel 1H-imidazo[1,2-a]imidazole-3-amine and imidazo[2,1-c][1,2,4]triazole-5-amine derivatives via Strecker reaction under controlled microwave heating
- Evaluation of three different green fabrication methods for the synthesis of crystalline ZnO nanoparticles using Pelargonium zonale leaf extract
- A highly efficient and multifunctional biomass supporting Ag, Ni, and Cu nanoparticles through wetness impregnation for environmental remediation
- Simple one-pot green method for large-scale production of mesalamine, an anti-inflammatory agent
- Relationships between step and cumulative PMI and E-factors: implications on estimating material efficiency with respect to charting synthesis optimization strategies
- A comparative sorption study of Cr3+ and Cr6+ using mango peels: kinetic, equilibrium and thermodynamic
- Effects of acid hydrolysis waste liquid recycle on preparation of microcrystalline cellulose
- Use of deep eutectic solvents as catalyst: A mini-review
- Microwave-assisted synthesis of pyrrolidinone derivatives using 1,1’-butylenebis(3-sulfo-3H-imidazol-1-ium) chloride in ethylene glycol
- Green and eco-friendly synthesis of Co3O4 and Ag-Co3O4: Characterization and photo-catalytic activity
- Adsorption optimized of the coal-based material and application for cyanide wastewater treatment
- Aloe vera leaf extract mediated green synthesis of selenium nanoparticles and assessment of their In vitro antimicrobial activity against spoilage fungi and pathogenic bacteria strains
- Waste phenolic resin derived activated carbon by microwave-assisted KOH activation and application to dye wastewater treatment
- Direct ethanol production from cellulose by consortium of Trichoderma reesei and Candida molischiana
- Agricultural waste biomass-assisted nanostructures: Synthesis and application
- Biodiesel production from rubber seed oil using calcium oxide derived from eggshell as catalyst – optimization and modeling studies
- Study of fabrication of fully aqueous solution processed SnS quantum dot-sensitized solar cell
- Assessment of aqueous extract of Gypsophila aretioides for inhibitory effects on calcium carbonate formation
- An environmentally friendly acylation reaction of 2-methylnaphthalene in solvent-free condition in a micro-channel reactor
- Aegle marmelos phytochemical stabilized synthesis and characterization of ZnO nanoparticles and their role against agriculture and food pathogen
- A reactive coupling process for co-production of solketal and biodiesel
- Optimization of the asymmetric synthesis of (S)-1-phenylethanol using Ispir bean as whole-cell biocatalyst
- Synthesis of pyrazolopyridine and pyrazoloquinoline derivatives by one-pot, three-component reactions of arylglyoxals, 3-methyl-1-aryl-1H-pyrazol-5-amines and cyclic 1,3-dicarbonyl compounds in the presence of tetrapropylammonium bromide
- Preconcentration of morphine in urine sample using a green and solvent-free microextraction method
- Extraction of glycyrrhizic acid by aqueous two-phase system formed by PEG and two environmentally friendly organic acid salts - sodium citrate and sodium tartrate
- Green synthesis of copper oxide nanoparticles using Juglans regia leaf extract and assessment of their physico-chemical and biological properties
- Deep eutectic solvents (DESs) as powerful and recyclable catalysts and solvents for the synthesis of 3,4-dihydropyrimidin-2(1H)-ones/thiones
- Biosynthesis, characterization and anti-microbial activity of silver nanoparticle based gel hand wash
- Efficient and selective microwave-assisted O-methylation of phenolic compounds using tetramethylammonium hydroxide (TMAH)
- Anticoagulant, thrombolytic and antibacterial activities of Euphorbia acruensis latex-mediated bioengineered silver nanoparticles
- Volcanic ash as reusable catalyst in the green synthesis of 3H-1,5-benzodiazepines
- Green synthesis, anionic polymerization of 1,4-bis(methacryloyl)piperazine using Algerian clay as catalyst
- Selenium supplementation during fermentation with sugar beet molasses and Saccharomyces cerevisiae to increase bioethanol production
- Biosynthetic potential assessment of four food pathogenic bacteria in hydrothermally silver nanoparticles fabrication
- Investigating the effectiveness of classical and eco-friendly approaches for synthesis of dialdehydes from organic dihalides
- Pyrolysis of palm oil using zeolite catalyst and characterization of the boil-oil
- Azadirachta indica leaves extract assisted green synthesis of Ag-TiO2 for degradation of Methylene blue and Rhodamine B dyes in aqueous medium
- Synthesis of vitamin E succinate catalyzed by nano-SiO2 immobilized DMAP derivative in mixed solvent system
- Extraction of phytosterols from melon (Cucumis melo) seeds by supercritical CO2 as a clean technology
- Production of uronic acids by hydrothermolysis of pectin as a model substance for plant biomass waste
- Biofabrication of highly pure copper oxide nanoparticles using wheat seed extract and their catalytic activity: A mechanistic approach
- Intelligent modeling and optimization of emulsion aggregation method for producing green printing ink
- Improved removal of methylene blue on modified hierarchical zeolite Y: Achieved by a “destructive-constructive” method
- Two different facile and efficient approaches for the synthesis of various N-arylacetamides via N-acetylation of arylamines and straightforward one-pot reductive acetylation of nitroarenes promoted by recyclable CuFe2O4 nanoparticles in water
- Optimization of acid catalyzed esterification and mixed metal oxide catalyzed transesterification for biodiesel production from Moringa oleifera oil
- Kinetics and the fluidity of the stearic acid esters with different carbon backbones
- Aiming for a standardized protocol for preparing a process green synthesis report and for ranking multiple synthesis plans to a common target product
- Microstructure and luminescence of VO2 (B) nanoparticle synthesis by hydrothermal method
- Optimization of uranium removal from uranium plant wastewater by response surface methodology (RSM)
- Microwave drying of nickel-containing residue: dielectric properties, kinetics, and energy aspects
- Simple and convenient two step synthesis of 5-bromo-2,3-dimethoxy-6-methyl-1,4-benzoquinone
- Biodiesel production from waste cooking oil
- The effect of activation temperature on structure and properties of blue coke-based activated carbon by CO2 activation
- Optimization of reaction parameters for the green synthesis of zero valent iron nanoparticles using pine tree needles
- Microwave-assisted protocol for squalene isolation and conversion from oil-deodoriser distillates
- Denitrification performance of rare earth tailings-based catalysts
- Facile synthesis of silver nanoparticles using Averrhoa bilimbi L and Plum extracts and investigation on the synergistic bioactivity using in vitro models
- Green production of AgNPs and their phytostimulatory impact
- Photocatalytic activity of Ag/Ni bi-metallic nanoparticles on textile dye removal
- Topical Issue: Green Process Engineering / Guest Editors: Martine Poux, Patrick Cognet
- Modelling and optimisation of oxidative desulphurisation of tyre-derived oil via central composite design approach
- CO2 sequestration by carbonation of olivine: a new process for optimal separation of the solids produced
- Organic carbonates synthesis improved by pervaporation for CO2 utilisation
- Production of starch nanoparticles through solvent-antisolvent precipitation in a spinning disc reactor
- A kinetic study of Zn halide/TBAB-catalysed fixation of CO2 with styrene oxide in propylene carbonate
- Topical on Green Process Engineering

