Abstract
A bench scale submerged fermentation process was used to bioethanol produce using sugar beet molasses and Saccharomyces cerevisiae, as substrate and microbial strain, respectively. Effects of selenium amount on growth of S. cerevisiae and bioethanol production were evaluated. The obtained results indicated that growth of S. cerevisiae (manifested as turbidity intensity) in the samples containing 0, 5, 10, 15, 20 and 25 μg sodium selenite, during aerobic process, was 0.1707, 0.1678, 0.1679, 0.1664, 0.1627 and 0.160% a.u./h (after 14 h incubation), respectively. Statistical analysis based on compression test indicated that there were insignificant (p > 0.05) differences between growth rate of the yeast in the fermented samples containing S. cerevisiae and 5 to 25 μg selenium salt. Response surface methodology was utilized to evaluate effects of two fermentation parameters namely, amount of selenium (5-25 μg) and substrate brix (10-25°Bx) on the concentration (g/L) of produced bioethanol. Obtained results revealed that maximum bioethanol concentration (55 g/L) was achieved using 15 μg selenium and molasses with 25°Bx. Furthermore, results have also indicated that, without using selenium and using molasses with 25°Bx, bioethanol with concentration of 29 g/L was produced.
1 Introduction
Food wastes contain main carbohydrates such as pectic, starchy and sugary compounds which those are accumulated every year and caused ecological problems. Incorporation of biotechnology methods and approaches into chemical and environmental engineering aspects, is of great interest to overcome the environmentally concerns resulted by the wastes. Recently, food and agro-wastes have gained more attention to be utilized in biotechnologically processes as an enriched and suitable substrate to produce valuable products such as food additives, enzymes, antibiotics, organic acids, biofuel and biogas [1].
Bioethanol is known as clean, cost-effective and eco-friendly fuel and has been widely utilized in developed countries as alternative and replacement of the fossil fuels [2,3]. In fact, bioethanol can also be used as gasoline improver or octane enhancer to increase flames speed and heats of vaporization with minimum toxicity and airborne pollutants [4]. Therefore, its production through submerged fermentation and using agro-industrial by-products, especially sugar beet molasses, is an attractive and eco-friendly topic these days.
Several studies have been indicated that the yield of bioethanol production through fermentation, influences by numerous parameters such as type and volume of inoculum (microorganisms strain), composition and concentration of substrate (growth media), pH, temperature, osmotic pressure of the culture media, and presence of nutrients, minerals and precursors [5,6]. Selenium as an essential trace element has crucial role in animal lives, human health and micro/macro flora. But, due to its low concentration in vegetables (as human diets) and its short bioavailability, many people are poor because of this vital element [7,8]. Conversion of selenium to organic selenium compounds such as seleno-proteins (especially seleno-methionine and seleno-cysteine) and seleno-enzymes, improves selenium deficiency in the human body and shows numerous biological activities such as antioxidant and anti-inflammatory activities [8,9]. Several studies indicated that yeast cells are capable to absorb selenium and bio-transform it into seleno-methionine and seleno-cysteine [10, 11, 12, 13]. Selenium-enriched yeasts, which are known as selenised yeasts, are the base of many consumed supplements and most popular. For examples, enriched yeasts with selenium, such as Saccharomyces cerevisiae, S. bayanus and S. boulardii have been utilized to produce bread, probiotic products and alcoholic beverages [12,14,15]. Several studies indicated that, glutamate in the yeast, can decrease the energy generation and fermentation rate, by altering the mitochondrial structure and dynamics, which those negatively impacts can be prevented by organic selenium such as seleno-cysteine which that is resulted during bio-transformation of inorganic selenium using yeasts such as S. cerevisiae [12,14]. Other study indicated that contents of organic selenium in the S. cerevisiae during fermentation process to produce bioethanol, increased with the increase in selenium concentration up to 2 μg/mL followed by a gradual decrease after 24 h of incubation. It reveals that organic selenium has low bioavailability [16]. Furthermore, some species of Saccharomyces, such as Yarrowia lipolytica, has selenium tolerance and selenium in lower amounts, cannot significantly inhibit its growth [15]. To the best of our knowledge, there is not any comprehensive study to evaluate effects of selenium salt and culture media concentrations on production of bioethanol through submerged fermentation process using S. cerevisiae and sugar beet molasses. Furthermore, the mechanism of the selenium on production of bioethanol using S. cerevisiae is unknown.
Therefore, the main objectives of the present study were to i) evaluate the effect of selenium on the concentration of produced bioethanol, ii) optimize submerged fermentation parameters namely substrate and selenium concentrations to achieve bioethanol with highest concentration, and iii) compare concentration of produced bioethanol, between cultivation made with and without selenium.
2 Material and methods
2.1 Materials
Sugar beet molasses, as substrate, was provided from Sahand Company (Khoy, Iran). It has °Brix, total reduced sugar amount, pH, ash content and density values of 74.07 (°Bx), 48.65%, 6.16, 9.6 (% v/v) and 1.385×103 (kg/m3), respectively. Commercial S. cerevisiae strain, SFO6, was obtained from Iran Mayeh Company (Tehran, Iran). 30% v/v sulfuric acid (as pH adjuster), Diammonium hydrogen phosphate (as phosphorus source) and urea (as nitrogen supplement) were purchased from the Dr. Mojallali Company (Tehran, Iran). Sodium selenite (Na2SeO3) with purity of higher than 99% (as selenium precursor) was provided from Merck Company (Merck Co., Darmstadt, Germany).
2.2 Inoculum preparation through aerobic process
Provided molasses was sterilized using a laboratory autoclave (RT-1, Reyhan Teb, Tehran, Iran), adjusted at temperature of 121°C and pressure of 1.5 bar for 15 min, diluted using sterilized distilled water to prepared aqueous molasses with brix value of 11 (°Bx) using a refractometer (Index instrument Ltd., Kissimmee, FL, USA), enriched with 250 mg/L urea and 500 mg/L urea and diamonium hidrogen phosphate, and adjusted its pH to 4.2. After that, prepared substrate containing 0.3 g/L provided industrial yeast, was aerated using shaker incubator (S1-300, Jeio Tech, Daejeon, Korea,) 120 rpm, 1 (VVM) at 32°C for 24 h.
2.3 Growth of S. cerevisiae in the prepared molasses
Optical density measurement was used to monitore the growth of S. cerevisiae in the provided molasses with °Bx of 11, as mentioned in inoculum preparation through aerobic process. In order to evaluate of the effect of selenium amounts (0, 5, 10, 15, 20 and 25 μg) on the growth of the S. cerevisiae, after preparation of molasses containing 0.3 g/L of the yeast, defined amounts of selenium were added into that and the absorbance of the prepared samples, as an optical density, was measured every 1 h, using UV-Vis spectrophotometry (Jenway UV-Vis spectrophotometer 6705, Staffordshire, UK) adjusted at wavelength of 625 nm [5,6]. For this reason, samples were diluted 4 times with distilled water to decrease their colour intensity and after that, those were subjected to the spectrophotometer. The recorded values were then multiplied in 4 to obtain exact value of optical density for the samples.
2.4 Bioethanol production through anaerobic submerged fermentation and its concentration measurment
Bioethanol was produced using anaerobic batch submerged fermentation when, 60 mL of the prepared inoculum with 11°Bx and different amount of sodium selenite (5-25 μg) were added into the 140 mL of the diluted and sterilized molasses with different brix value ranging 10 to 25°Bx. The mixture solutions were then filled into the 250 mL rubber sealed glass jars and incubated (at 32°C for 32 h). Finaly, the concentration of the produced bioethanol was calculated using technique based on distillation. In this manner, after bioethanol distillation and true brix measurement in distilled fermentation broth by a refractometer (Index instrument Ltd., Kissimmee, FL, USA), bioethanol concentration was measured using a hydrometer [5].
2.5 Experimental design and statistical analysis
According to the literature studies, two independent variables namely, amount of sodium selenite (μg, X1) and substrate brix (°Bx, X2) were selected to evaluate their effects on bioethanol concentration (g/L, Y), as response variable, using response surface methodology (RSM) [6,8,9,14]. Central composite design (CCD) was utilized to design of experiments, including 13 experiment runs (Table 1), based on axial point system and 1 block [17,18]. In order to model the bioethanol concentration (g/L, Y) as function of two selected independent variables, a second order polynomial equation was selected [19,20]. Suitability of the generated model was studied based on the coefficient of determination (R2) and lack-of-fit p-value [21,22]. In order to significance determination of the resulted model, analysis of variance (ANOVA) was used based on p-value term (p < 0.05) [23,24]. Minitab software (v.16 statistical package, Minitab Inc., PA, USA) was used to design of experiments and statistical analysis.
Central composite design (CCD) for the bioethanol production using S. cerevisiae.
| Run | Selenium amount (μg | Substrate brix (°Bx | Experimental bioethanol concentration (g/L) | Predicted bioethanol concentration (g/L) |
|---|---|---|---|---|
| 1 | 15 | 10 | 15 | 15.04 |
| 2 | 15 | 25 | 55 | 55.20 |
| 3 | 5 | 17.5 | 27 | 27.38 |
| 4 | 8 | 12.2 | 17 | 16.74 |
| 5 | 15 | 17.5 | 29 | 29.00 |
| 6 | 15 | 17.5 | 29 | 29.00 |
| 7 | 15 | 17.5 | 29 | 29.00 |
| 8 | 25 | 17.5 | 33 | 32.86 |
| 9 | 22 | 22.8 | * | * |
| 10 | 8 | 22.8 | 45 | 44.63 |
| 11 | 15 | 17.5 | 29 | 29.00 |
| 12 | 15 | 17.5 | 29 | 29.00 |
| 13 | 22 | 12.2 | 20 | 20.11 |
* Out of range
2.6 Optimization of the bioethanol fermentation conditions
To find optimum area with in defined ranges for the fermentation variables, contour plot was establish [25]. Furthermore, to obtain the exact values of the optimized fermentation conditions which in that bioethanol with highest concentration were produced, numerical optimization was used [6]. Three additional approval tests were performed at obtained optimum conditions to verify the validity of the statistical experimental method [26]. For this reason, Tukey’s comparison test was performed between the values of the predicted and experimental bioethanol concentration at obtained optimum fermentation conditions. Minitab software (v.16 statistical package, Minitab Inc., PA, USA) was used to optimization and validation procedures.
3 Results and discussion
3.1 Effects of selenium on the growth of S. cerevisiae
Effects of different amounts of selenium on the growth (manifested as turbidity intensity) of S. cerevisiae, show in Figure 1. As clearly observed in this figure, the growth of the yeast in the provided molasses without selenium was significantly (p < 0.05) higher than that of those which were included with different amounts of selenium. According to the Figure 1, the slope of each curve, as growth rate, was calculated from beginning of the experiments up to 14 h after incubation. The obtained results indicated that growth rate (manifested as turbidity intensity (% a.u.)) of the S. cerevisiae in the samples including, 0, 5, 10, 15, 20 and 25 μg selenium was 0.1707, 0.1678, 0.1679, 0.1664, 0.1627 and 0.160% a.u./h, respectively. Tukey’s comparison test was indicated that there was insignificant (p > 0.05) differences between growth rate (manifested as turbidity intensity) of the S. cerevisiae in the samples containing S. cerevisiae and 5 to 25 μg selenium. It can be related to the lower bioavailability of the organic selenium in S. cerevisiae [16]. The obtained result was also in agreement with findings of Hamza et al. [15]. They also found that some species of Saccharomyces, had selenium tolerance and selenium in lower amounts, could not be significantly inhibit its growth.
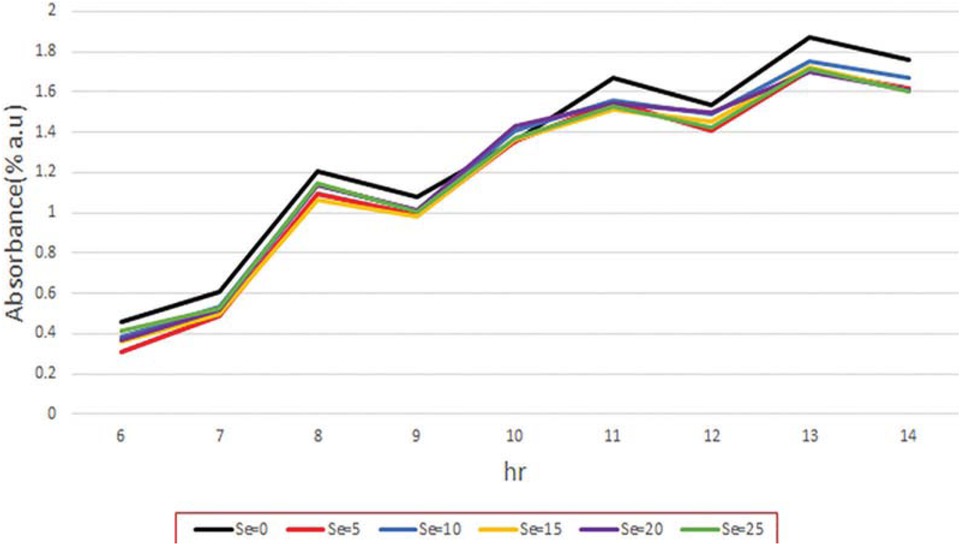
Effects of different amounts of selenium on the growth of S. cerevisiae.
3.2 Effects of substrate brix and selenium amount on the concentration of produced bioethanol
According to the experiment runs and obtained values for the concentration of produced bioethanol through submerged fermentation (Table 1), the second order model fitted to correlate bioethanol concentration to the fermentation parameters, namely selenium amount and inoculum brix. Estimated regression coefficients and P-values of the main, quadratic and interaction terms of the generated polynomial model are presented in Table 2. As clearly observed in Table 2, the main term of the selenium amount and its interaction with medium brix had insignificant (p > 0.05) effects on the concentration of bioethanol. But, the main term of medium brix and quadratic terms of both selected fermentation parameters had significant (p <> 0.05) effects on the produced bioethanol concentration. It means that with in the defined ranges for the independent variables, concentration of the produced bioethanol affected by lower and higher brix of the medium, and only higher amounts of selenium salt. Statistical analysis had also shown high values
P values and regression coefficients for the generated model using S. cerevisiae.
| P-value | Regression coefficient | |||
|---|---|---|---|---|
| Parameters | Independent variables | P-value | β | Coefficient |
| Constant | 0.000 | β0 | 15.71 | |
| (Constant) | ||||
| Main term | X1 | 0.135 | β1 | - 0.18 |
| X2 | 0.000 | β2 | - 1.23 | |
| Quadratic | X21 | 0.002 | β11 | 0.01 |
| term | X22 | 0.000 | β22 | 0.1 |
| Interaction term | X1X2 | 0.189 | β12 | 0.00 |
| R2 | 0.9997 | |||
| Lack-of-fit (p-value) | 0.420 | |||
1: Amount of selenium (µg)
2: Substrate brix (·Bx)
for the R2 (0.9997) and lack-of-fit (p-value of 0.420) of the generated model which those indicated suitability and accuracy of the resulted model for predicting of bioethanol concentration with in the defined ranges for the fermentation parameters [19,22].
As can be seen in Table 1, the concentration of the produced bioethanol was varied from 15 to 55 g/L. Figure 2, indicates the effects of selenium amount and substrate brix on the concentration of produced bioethanol. As can be seen in Figure 2, at any constant amount of selenium, by increasing the brix of substrate, bioethanol concentration, increased. This result was in agreement with finding of Shaghaghi-Moghadam et al. [6]. They indicated by increasing the substrate brix, the concentration of the fermentable sugar increased which in turn, increased the concentration of the produced bioethanol. One of the factors which can highly affect the fermentation performance is the medium osmotic pressure which that increases by increasing the medium brix and may negatively affect the yeast growth and bioethanol production [6]. As same as this pattern, at any constant substrate brix, by increasing the amount of selenium, the concentration of the produced bioethanol was increased. However, the effect of substrate brix on the increasing of the bioethanol was higher than the selenium amount, due to its lower p-value (0.000). The presence of no curvature in the Figure 2 also demonstrated that the interaction between substrate brix and amount of selenium did not has significant (p < 0.05) effect on bioethanol concentration. Obtained result was reconfirmed by achieved high p-value (p > 0.05) of the interaction term (0.189) as can be observed clearly in Table 2.
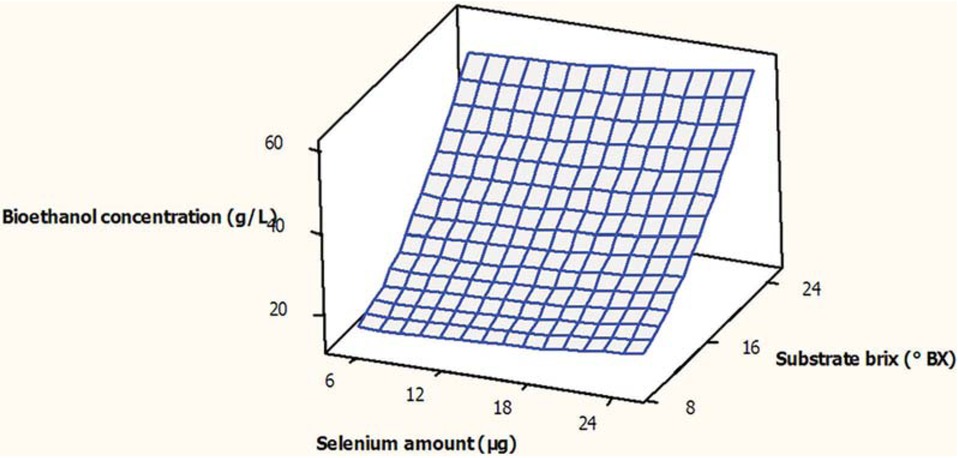
Surface plot for concentration of the produced bioethanol (g/L) as function of the substrate brix (°Bx) and amount of selenium (μg), during submerged fermentation.
3.3 Optimization of the fermentation process
In order to achieve bioethanol with highest concentration through submerged fermentation, the obtained numerical optimization result revealed that fermentation using 15 μg sodium selenite and substrate with 25°Bx attained to produce bioethanol with highest concentration value of 55.2 g/L. Graphical optimization shows in Figure 3. As can be seen in this figure, at any constant substrate brix value, for lower amount of selenium, by increasing the amount of selenium, the concentration of produced bioethanol was constant and at higher amounts of selenium, increasing its amount had significant (p < 0.05) effect on the concentration of bioethanol. This result was reconfirmed by the obtained statistical data for the insignificant effect of lower amounts of selenium (p-value = 0.135) on the concentration produced bioethanol (Table 2). Graphical optimization plot was illustrated that, with in the defined ranges for the independent variables, maximum bioethanol was achieved using highest substrate brix values. Experimental data for the obtained bioethanol concentration (55 ± 2 g/L) using the optimum fermentation parameters revealed that there was insignificant (p > 0.05) difference between the values of the experimental and predicted concentration of produced bioethanol and indicated the adequacy of the fitted model.
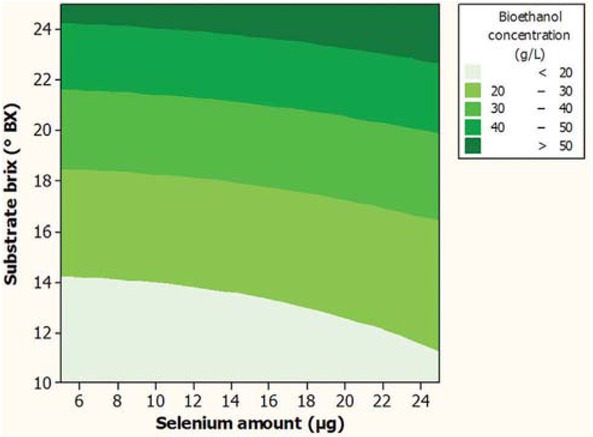
Graphical optimization plot for concentration of the produced bioethanol (g/L) as function of the substrate brix (°Bx) and amount of selenium (μg), during submerged fermentation.
In this work, selenium was applied in a submerged fermentation to study its effects on production of bioethanol. For this reason, an anaerobic fermentation process was run at same conditions as obtained optimum conditions without selenium. In fact, at this fermentation process, the substrate brix was chosen at 25°Bx and the amount of sodium selenite was zero. Obtained result indicated that the concentration of produced bioethanol was 29 g/L which was 52.3% lower than that of obtained using 15 μg selenium (55 ± 2 g/L). Obtained result can be explained by the fact that existed glutamate in the yeast, negatively impacts the mitochondria by altering the mitochondrial structure and dysregulation of mitochondria dynamics which is decreased the energy generation and fermentation rate. S. cerevisiae by converting inorganic selenium to seleno-cysteine, utilized this seleno-amino acid to prevent glutamate-induced effect [27]. It seems that S. cerevisiae has high potential to bio-transform selenium to organic selenium compounds such as seleno-proteins which was confirmed by findings of Pérez-Corona et al. [14] and Porto et al. [12].
4 Conclusions
Present study indicated that production of bioethanol with high concentration, through submerged fermentation process, could be resulted by increasing the concentration of molasses (substrate) and utilizing high amounts of selenium, as regulator of the yeast mitochondria dynamics function. The obtained results indicated that lower amounts of selenium had insignificant effect on the concentration of the produced bioethanol. Results also revealed that the selected industrial S. cerevisiae strain had high resistance against osmotic pressure of the fermented broth which it makes possible to achieve bioethanol with twice concentration when comparison was made between cultivation made with and without selenium using highest substrate brix. Finally, RSM could be successfully used to generate model, optimize the process and predict the bioethanol concentration with in the defined ranges for the selenium amount and substrate brix.
Acknowledgment
The authors would like to appreciate the Bidestan Company (Qazvin, Iran) for the analysis and material supports.
Conflicts of interest: All authors declare no conflict of interest.
References
[1] Jafari N., Jafarizadeh-Malmiri H., Hamzeh-Mivehroud M., Adibpour M., Optimization of UV irradiation mutation conditions for cellulase production by mutant fungal strains of Aspergillus niger through solid state fermentation. Green Process. Synth., 2017, 6, 333-340.10.1515/gps-2016-0145Search in Google Scholar
[2] Hill J., Nelson E., Tilman D., Polasky S., Tiffany D., Environmental, economic, and energetic costs and benefits of biodiesel and ethanol biofuels. Proc. Nat. Acad. Sci., 2006, 103, 11206-11210.10.1073/pnas.0604600103Search in Google Scholar PubMed PubMed Central
[3] Ko J.K., Lee S.M., Advances in cellulosic conversion to fuels: engineering yeasts for cellulosic bioethanol and biodiesel production. Curr. Opin. Biotechnol., 2018, 50, 72-80.10.1016/j.copbio.2017.11.007Search in Google Scholar PubMed
[4] Mohd Azhar S.H., Abdulla R., Jambo S.A., Marbawi H., Gansau J.A., Mohd Faik A.A., et al., Yeasts in sustainable bioethanol production: A review. Biochem. Biophys. Rep., 2017, 10, 52-61.10.1016/j.bbrep.2017.03.003Search in Google Scholar PubMed PubMed Central
[5] Shaghaghi-Moghadam R., Jafarizadeh-Malmiri H., Mehdikhani P., Jalalian S., Alijanianzadeh R., Screening of the five different wild, traditional and industrial Saccharomyces cerevisiae strains to overproduce bioethanol in the batch submerged fermentation. Z. Naturforsch., 2018, 73, 361-366.10.1515/znc-2017-0180Search in Google Scholar PubMed
[6] Shaghaghi-Moghadam R., Jafarizadeh-Malmiri H., Mehdikhani P., Alijanianzadeh R., Jalalian S., Optimization of submerged fermentation conditions to overproduce bioethanol using two industrial and traditional Saccharomyces cerevisiae strains. Green Process. Synth., 2019, 8, 157-162.10.1515/gps-2018-0044Search in Google Scholar
[7] Izquierdo A., Casas C., Herrero E., Selenite-induced cell death in Saccharomyces cerevisiae protective role of glutaredoxins. Microbiol., 2010, 156, 2608-2620.10.1099/mic.0.039719-0Search in Google Scholar PubMed
[8] Oraby M.M., Allababidy T., Ramadan E.M., The bioavailability of selenium in Saccharomyces cerevisiae Ann. Agric. Sci., 2015, 60, 307-315.10.1016/j.aoas.2015.10.006Search in Google Scholar
[9] Ponce de Leon C.A., Bayon M.M., Paquin C., Caruso J.A., Selenium incorporation into Saccharomyces cerevisiae cells: a study of different incorporation methods. J. Appl. Microbiol., 2002, 92, 602-610.10.1046/j.1365-2672.2002.01562.xSearch in Google Scholar PubMed
[10] Bronzetti G., Cini M., Andreoli E., Caltavuturo L., Panunzio M., Croce C.D., Protective effects of vitamins and selenium compounds in yeast. Mutat. Res., 2001, 496, 105-115.10.1016/S1383-5718(01)00213-3Search in Google Scholar PubMed
[11] Fagan S., Owens R., Ward P., Connolly C., Doyel S., Murphy R., Biochemical comparison of commercial selenium yeast preparations. Biol. Trace Elem. Res., 2015, 166, 245-259.10.1007/s12011-015-0242-6Search in Google Scholar PubMed
[12] Porto B.A.A., Mangiapane E., Pessione A., Neves M.J., Pessione E., Martins F., Evaluation of sodium selenite effects on the potential probiotic Saccharomyces cerevisiae UFMG A-905: A physiological and proteomic analysis. J. Funct. Foods, 2015, 17, 828-836.10.1016/j.jff.2015.06.048Search in Google Scholar
[13] Jiménez-Lamana J., Abed-Alvaro I., Bierla K., Laborda F., Szpunar J., Lobinski R., Detection and characterization of biogenic selenium nanoparticles in selenium-rich yeast by single particle ICPMS. J. Anal. At. Spectrom., 2018, 33, 452-460.10.1039/C7JA00378ASearch in Google Scholar
[14] Pérez-Corona M.T., Sánchez-Martínez M., Valderrama M.J., Rodríguez M.E., Cámara C., Madrid Y., Selenium biotransformation by Saccharomyces cerevisiae and Saccharomyces bayanus during white wine manufacture: laboratory-scale experiments. Food Chem., 2011, 124, 1050-1055.10.1016/j.foodchem.2010.07.073Search in Google Scholar
[15] Hamza F., Vaidya A., Apte M., Kumar A.R., Zinjarde S., Selenium nanoparticle-enriched biomass of Yarrowia lipolytica enhances growth and survival of Artemia salina Enzyme Microb. Technol., 2017, 106, 48-56.10.1016/j.enzmictec.2017.07.002Search in Google Scholar PubMed
[16] Oraby M.M., Allababidy T., Ramadan E.M., The bioavailability of selenium in Saccharomyces cerevisiae Ann. Agric. Sci., 2015, 60, 307-315.10.1016/j.aoas.2015.10.006Search in Google Scholar
[17] Amirkhani L., Moghaddas J., Jafarizadeh-Malmiri H., Candida rugosa lipase immobilization on magnetic silica aerogel nanodispersion. RSC Adv., 2016, 6, 12676-12687.10.1039/C5RA24441BSearch in Google Scholar
[18] Eskandari-Nojedehi M., Jafarizadeh-Malmiri H., Rahbar-Shahrouzi J., Hydrothermal green synthesis of gold nanoparticles using mushroom Agaricus bisporus extract: physico-chemical characteristics and antifungal activity studies. Green Process. Synth., 2018, 7, 38-47.10.1515/gps-2017-0004Search in Google Scholar
[19] Ahdno H., Jafarizadeh-Malmiri H., Development of a sequenced enzymatically pre-treatment and filter pre-coating process to clarify date syrup. Food Bioprod. Process., 2017, 101, 193-204.10.1016/j.fbp.2016.11.008Search in Google Scholar
[20] Mohammadlou M., Jafarizadeh-Malmiri H., Maghsoudi H., Hydrothermal green synthesis of silver nanoparticles using Pelargonium/Geranium leaf extract and evaluation of their antifungal activity. Green Process. Synth., 2017, 6, 31-42.10.1515/gps-2016-0075Search in Google Scholar
[21] Ahmadi O., Jafarizadeh-Malmiri H., Jodeiri N., Eco-friendly microwave-enhanced green synthesis of silver nanoparticles using Aloe vera leaf extract and their physico-chemical and antibacterial studies. Green Process. Synth., 2018, 7, 231-140.10.1515/gps-2017-0039Search in Google Scholar
[22] Ghanbari S., Vaghari H., Sayyar Z., Adibpour M., Jafarizadeh-Malmiri H., Autoclave-assisted green synthesis of silver nanoparticles using A. fumigatus mycelia extract and the evaluation of their physico-chemical properties and antibacterial activity. Green Process. Synth., 2018, 7, 217-224.10.1515/gps-2017-0062Search in Google Scholar
[23] Eskandari-Nojedehi M., Jafarizadeh-Malmiri H., Jafarizad A., Microwave accelerated green synthesis of gold nanoparticles using gum Arabic and their physico-chemical properties assessments. Z. Phys. Chem., 2018, 232, 325-343.10.1515/zpch-2017-1001Search in Google Scholar
[24] Nottagh S., Hesari J., Peighambardoust S.H., Rezaei-Mokarram R., Jafarizadeh-Malmiri H., Development of a biodegradable coating formulation based on the biological characteristics of the Iranian Ultra-filtrated cheese. Biologia, 2018, 73, 403-410.10.2478/s11756-018-0039-0Search in Google Scholar
[25] Anarjan N., Jaberi N., Yeganeh-Zare S., Banafshehchin E., Rahimirad A., Jafarizadeh- Malmiri H., Optimization of mixing parameters for α-Tocopherol nanodispersions prepared using solvent displacement method. J. Am. Oil Chem. Soc., 2014, 91, 1397-1405.10.1007/s11746-014-2482-6Search in Google Scholar
[26] Jafarizadeh-Malmiri H., Osman A., Tan C.P., Abdul Rahman R., Effects of edible surface coatings (sodium carboxymethyl cellulose, sodium caseinate and glycerol) on storage quality of berangan banana Musa sapientum cv. Berangan) using response surface methodology. J. Food Process. Preserv., 2012, 36, 252-261.10.1111/j.1745-4549.2011.00583.xSearch in Google Scholar
[27] Ma M.Y., Guo Y.Z., Ibeanu G., Wang L.Y., Dong J.D., Wang J., et al., Overexpression of selenoprotein H prevents mitochondrial dynamic imbalance induced by glutamate exposure. Int. J. Biol. Sci., 2017, 13, 1458-1469.10.7150/ijbs.21300Search in Google Scholar PubMed PubMed Central
© 2019 Faramarzi et al., published by De Gruyter.
This work is licensed under the Creative Commons Attribution 4.0 Public License.
Articles in the same Issue
- Regular Articles
- Studies on the preparation and properties of biodegradable polyester from soybean oil
- Flow-mode biodiesel production from palm oil using a pressurized microwave reactor
- Reduction of free fatty acids in waste oil for biodiesel production by glycerolysis: investigation and optimization of process parameters
- Saccharin: a cheap and mild acidic agent for the synthesis of azo dyes via telescoped dediazotization
- Optimization of lipase-catalyzed synthesis of polyethylene glycol stearate in a solvent-free system
- Green synthesis of iron oxide nanoparticles using Platanus orientalis leaf extract for antifungal activity
- Ultrasound assisted chemical activation of peanut husk for copper removal
- Room temperature silanization of Fe3O4 for the preparation of phenyl functionalized magnetic adsorbent for dispersive solid phase extraction for the extraction of phthalates in water
- Evaluation of the saponin green extraction from Ziziphus spina-christi leaves using hydrothermal, microwave and Bain-Marie water bath heating methods
- Oxidation of dibenzothiophene using the heterogeneous catalyst of tungsten-based carbon nanotubes
- Calcined sodium silicate as an efficient and benign heterogeneous catalyst for the transesterification of natural lecithin to L-α-glycerophosphocholine
- Synergistic effect between CO2 and H2O2 on ethylbenzene oxidation catalyzed by carbon supported heteropolyanion catalysts
- Hydrocyanation of 2-arylmethyleneindan-1,3-diones using potassium hexacyanoferrate(II) as a nontoxic cyanating agent
- Green synthesis of hydratropic aldehyde from α-methylstyrene catalyzed by Al2O3-supported metal phthalocyanines
- Environmentally benign chemical recycling of polycarbonate wastes: comparison of micro- and nano-TiO2 solid support efficiencies
- Medicago polymorpha-mediated antibacterial silver nanoparticles in the reduction of methyl orange
- Production of value-added chemicals from esterification of waste glycerol over MCM-41 supported catalysts
- Green synthesis of zerovalent copper nanoparticles for efficient reduction of toxic azo dyes congo red and methyl orange
- Optimization of the biological synthesis of silver nanoparticles using Penicillium oxalicum GRS-1 and their antimicrobial effects against common food-borne pathogens
- Optimization of submerged fermentation conditions to overproduce bioethanol using two industrial and traditional Saccharomyces cerevisiae strains
- Extraction of In3+ and Fe3+ from sulfate solutions by using a 3D-printed “Y”-shaped microreactor
- Foliar-mediated Ag:ZnO nanophotocatalysts: green synthesis, characterization, pollutants degradation, and in vitro biocidal activity
- Green cyclic acetals production by glycerol etherification reaction with benzaldehyde using cationic acidic resin
- Biosynthesis, characterization and antimicrobial activities assessment of fabricated selenium nanoparticles using Pelargonium zonale leaf extract
- Synthesis of high surface area magnesia by using walnut shell as a template
- Controllable biosynthesis of silver nanoparticles using actinobacterial strains
- Green vegetation: a promising source of color dyes
- Mechano-chemical synthesis of ammonia and acetic acid from inorganic materials in water
- Green synthesis and structural characterization of novel N1-substituted 3,4-dihydropyrimidin-2(1H)-ones
- Biodiesel production from cotton oil using heterogeneous CaO catalysts from eggshells prepared at different calcination temperatures
- Regeneration of spent mercury catalyst for the treatment of dye wastewater by the microwave and ultrasonic spray-assisted method
- Green synthesis of the innovative super paramagnetic nanoparticles from the leaves extract of Fraxinus chinensis Roxb and their application for the decolourisation of toxic dyes
- Biogenic ZnO nanoparticles: a study of blueshift of optical band gap and photocatalytic degradation of reactive yellow 186 dye under direct sunlight
- Leached compounds from the extracts of pomegranate peel, green coconut shell, and karuvelam wood for the removal of hexavalent chromium
- Enhancement of molecular weight reduction of natural rubber in triphasic CO2/toluene/H2O systems with hydrogen peroxide for preparation of biobased polyurethanes
- An efficient green synthesis of novel 1H-imidazo[1,2-a]imidazole-3-amine and imidazo[2,1-c][1,2,4]triazole-5-amine derivatives via Strecker reaction under controlled microwave heating
- Evaluation of three different green fabrication methods for the synthesis of crystalline ZnO nanoparticles using Pelargonium zonale leaf extract
- A highly efficient and multifunctional biomass supporting Ag, Ni, and Cu nanoparticles through wetness impregnation for environmental remediation
- Simple one-pot green method for large-scale production of mesalamine, an anti-inflammatory agent
- Relationships between step and cumulative PMI and E-factors: implications on estimating material efficiency with respect to charting synthesis optimization strategies
- A comparative sorption study of Cr3+ and Cr6+ using mango peels: kinetic, equilibrium and thermodynamic
- Effects of acid hydrolysis waste liquid recycle on preparation of microcrystalline cellulose
- Use of deep eutectic solvents as catalyst: A mini-review
- Microwave-assisted synthesis of pyrrolidinone derivatives using 1,1’-butylenebis(3-sulfo-3H-imidazol-1-ium) chloride in ethylene glycol
- Green and eco-friendly synthesis of Co3O4 and Ag-Co3O4: Characterization and photo-catalytic activity
- Adsorption optimized of the coal-based material and application for cyanide wastewater treatment
- Aloe vera leaf extract mediated green synthesis of selenium nanoparticles and assessment of their In vitro antimicrobial activity against spoilage fungi and pathogenic bacteria strains
- Waste phenolic resin derived activated carbon by microwave-assisted KOH activation and application to dye wastewater treatment
- Direct ethanol production from cellulose by consortium of Trichoderma reesei and Candida molischiana
- Agricultural waste biomass-assisted nanostructures: Synthesis and application
- Biodiesel production from rubber seed oil using calcium oxide derived from eggshell as catalyst – optimization and modeling studies
- Study of fabrication of fully aqueous solution processed SnS quantum dot-sensitized solar cell
- Assessment of aqueous extract of Gypsophila aretioides for inhibitory effects on calcium carbonate formation
- An environmentally friendly acylation reaction of 2-methylnaphthalene in solvent-free condition in a micro-channel reactor
- Aegle marmelos phytochemical stabilized synthesis and characterization of ZnO nanoparticles and their role against agriculture and food pathogen
- A reactive coupling process for co-production of solketal and biodiesel
- Optimization of the asymmetric synthesis of (S)-1-phenylethanol using Ispir bean as whole-cell biocatalyst
- Synthesis of pyrazolopyridine and pyrazoloquinoline derivatives by one-pot, three-component reactions of arylglyoxals, 3-methyl-1-aryl-1H-pyrazol-5-amines and cyclic 1,3-dicarbonyl compounds in the presence of tetrapropylammonium bromide
- Preconcentration of morphine in urine sample using a green and solvent-free microextraction method
- Extraction of glycyrrhizic acid by aqueous two-phase system formed by PEG and two environmentally friendly organic acid salts - sodium citrate and sodium tartrate
- Green synthesis of copper oxide nanoparticles using Juglans regia leaf extract and assessment of their physico-chemical and biological properties
- Deep eutectic solvents (DESs) as powerful and recyclable catalysts and solvents for the synthesis of 3,4-dihydropyrimidin-2(1H)-ones/thiones
- Biosynthesis, characterization and anti-microbial activity of silver nanoparticle based gel hand wash
- Efficient and selective microwave-assisted O-methylation of phenolic compounds using tetramethylammonium hydroxide (TMAH)
- Anticoagulant, thrombolytic and antibacterial activities of Euphorbia acruensis latex-mediated bioengineered silver nanoparticles
- Volcanic ash as reusable catalyst in the green synthesis of 3H-1,5-benzodiazepines
- Green synthesis, anionic polymerization of 1,4-bis(methacryloyl)piperazine using Algerian clay as catalyst
- Selenium supplementation during fermentation with sugar beet molasses and Saccharomyces cerevisiae to increase bioethanol production
- Biosynthetic potential assessment of four food pathogenic bacteria in hydrothermally silver nanoparticles fabrication
- Investigating the effectiveness of classical and eco-friendly approaches for synthesis of dialdehydes from organic dihalides
- Pyrolysis of palm oil using zeolite catalyst and characterization of the boil-oil
- Azadirachta indica leaves extract assisted green synthesis of Ag-TiO2 for degradation of Methylene blue and Rhodamine B dyes in aqueous medium
- Synthesis of vitamin E succinate catalyzed by nano-SiO2 immobilized DMAP derivative in mixed solvent system
- Extraction of phytosterols from melon (Cucumis melo) seeds by supercritical CO2 as a clean technology
- Production of uronic acids by hydrothermolysis of pectin as a model substance for plant biomass waste
- Biofabrication of highly pure copper oxide nanoparticles using wheat seed extract and their catalytic activity: A mechanistic approach
- Intelligent modeling and optimization of emulsion aggregation method for producing green printing ink
- Improved removal of methylene blue on modified hierarchical zeolite Y: Achieved by a “destructive-constructive” method
- Two different facile and efficient approaches for the synthesis of various N-arylacetamides via N-acetylation of arylamines and straightforward one-pot reductive acetylation of nitroarenes promoted by recyclable CuFe2O4 nanoparticles in water
- Optimization of acid catalyzed esterification and mixed metal oxide catalyzed transesterification for biodiesel production from Moringa oleifera oil
- Kinetics and the fluidity of the stearic acid esters with different carbon backbones
- Aiming for a standardized protocol for preparing a process green synthesis report and for ranking multiple synthesis plans to a common target product
- Microstructure and luminescence of VO2 (B) nanoparticle synthesis by hydrothermal method
- Optimization of uranium removal from uranium plant wastewater by response surface methodology (RSM)
- Microwave drying of nickel-containing residue: dielectric properties, kinetics, and energy aspects
- Simple and convenient two step synthesis of 5-bromo-2,3-dimethoxy-6-methyl-1,4-benzoquinone
- Biodiesel production from waste cooking oil
- The effect of activation temperature on structure and properties of blue coke-based activated carbon by CO2 activation
- Optimization of reaction parameters for the green synthesis of zero valent iron nanoparticles using pine tree needles
- Microwave-assisted protocol for squalene isolation and conversion from oil-deodoriser distillates
- Denitrification performance of rare earth tailings-based catalysts
- Facile synthesis of silver nanoparticles using Averrhoa bilimbi L and Plum extracts and investigation on the synergistic bioactivity using in vitro models
- Green production of AgNPs and their phytostimulatory impact
- Photocatalytic activity of Ag/Ni bi-metallic nanoparticles on textile dye removal
- Topical Issue: Green Process Engineering / Guest Editors: Martine Poux, Patrick Cognet
- Modelling and optimisation of oxidative desulphurisation of tyre-derived oil via central composite design approach
- CO2 sequestration by carbonation of olivine: a new process for optimal separation of the solids produced
- Organic carbonates synthesis improved by pervaporation for CO2 utilisation
- Production of starch nanoparticles through solvent-antisolvent precipitation in a spinning disc reactor
- A kinetic study of Zn halide/TBAB-catalysed fixation of CO2 with styrene oxide in propylene carbonate
- Topical on Green Process Engineering
Articles in the same Issue
- Regular Articles
- Studies on the preparation and properties of biodegradable polyester from soybean oil
- Flow-mode biodiesel production from palm oil using a pressurized microwave reactor
- Reduction of free fatty acids in waste oil for biodiesel production by glycerolysis: investigation and optimization of process parameters
- Saccharin: a cheap and mild acidic agent for the synthesis of azo dyes via telescoped dediazotization
- Optimization of lipase-catalyzed synthesis of polyethylene glycol stearate in a solvent-free system
- Green synthesis of iron oxide nanoparticles using Platanus orientalis leaf extract for antifungal activity
- Ultrasound assisted chemical activation of peanut husk for copper removal
- Room temperature silanization of Fe3O4 for the preparation of phenyl functionalized magnetic adsorbent for dispersive solid phase extraction for the extraction of phthalates in water
- Evaluation of the saponin green extraction from Ziziphus spina-christi leaves using hydrothermal, microwave and Bain-Marie water bath heating methods
- Oxidation of dibenzothiophene using the heterogeneous catalyst of tungsten-based carbon nanotubes
- Calcined sodium silicate as an efficient and benign heterogeneous catalyst for the transesterification of natural lecithin to L-α-glycerophosphocholine
- Synergistic effect between CO2 and H2O2 on ethylbenzene oxidation catalyzed by carbon supported heteropolyanion catalysts
- Hydrocyanation of 2-arylmethyleneindan-1,3-diones using potassium hexacyanoferrate(II) as a nontoxic cyanating agent
- Green synthesis of hydratropic aldehyde from α-methylstyrene catalyzed by Al2O3-supported metal phthalocyanines
- Environmentally benign chemical recycling of polycarbonate wastes: comparison of micro- and nano-TiO2 solid support efficiencies
- Medicago polymorpha-mediated antibacterial silver nanoparticles in the reduction of methyl orange
- Production of value-added chemicals from esterification of waste glycerol over MCM-41 supported catalysts
- Green synthesis of zerovalent copper nanoparticles for efficient reduction of toxic azo dyes congo red and methyl orange
- Optimization of the biological synthesis of silver nanoparticles using Penicillium oxalicum GRS-1 and their antimicrobial effects against common food-borne pathogens
- Optimization of submerged fermentation conditions to overproduce bioethanol using two industrial and traditional Saccharomyces cerevisiae strains
- Extraction of In3+ and Fe3+ from sulfate solutions by using a 3D-printed “Y”-shaped microreactor
- Foliar-mediated Ag:ZnO nanophotocatalysts: green synthesis, characterization, pollutants degradation, and in vitro biocidal activity
- Green cyclic acetals production by glycerol etherification reaction with benzaldehyde using cationic acidic resin
- Biosynthesis, characterization and antimicrobial activities assessment of fabricated selenium nanoparticles using Pelargonium zonale leaf extract
- Synthesis of high surface area magnesia by using walnut shell as a template
- Controllable biosynthesis of silver nanoparticles using actinobacterial strains
- Green vegetation: a promising source of color dyes
- Mechano-chemical synthesis of ammonia and acetic acid from inorganic materials in water
- Green synthesis and structural characterization of novel N1-substituted 3,4-dihydropyrimidin-2(1H)-ones
- Biodiesel production from cotton oil using heterogeneous CaO catalysts from eggshells prepared at different calcination temperatures
- Regeneration of spent mercury catalyst for the treatment of dye wastewater by the microwave and ultrasonic spray-assisted method
- Green synthesis of the innovative super paramagnetic nanoparticles from the leaves extract of Fraxinus chinensis Roxb and their application for the decolourisation of toxic dyes
- Biogenic ZnO nanoparticles: a study of blueshift of optical band gap and photocatalytic degradation of reactive yellow 186 dye under direct sunlight
- Leached compounds from the extracts of pomegranate peel, green coconut shell, and karuvelam wood for the removal of hexavalent chromium
- Enhancement of molecular weight reduction of natural rubber in triphasic CO2/toluene/H2O systems with hydrogen peroxide for preparation of biobased polyurethanes
- An efficient green synthesis of novel 1H-imidazo[1,2-a]imidazole-3-amine and imidazo[2,1-c][1,2,4]triazole-5-amine derivatives via Strecker reaction under controlled microwave heating
- Evaluation of three different green fabrication methods for the synthesis of crystalline ZnO nanoparticles using Pelargonium zonale leaf extract
- A highly efficient and multifunctional biomass supporting Ag, Ni, and Cu nanoparticles through wetness impregnation for environmental remediation
- Simple one-pot green method for large-scale production of mesalamine, an anti-inflammatory agent
- Relationships between step and cumulative PMI and E-factors: implications on estimating material efficiency with respect to charting synthesis optimization strategies
- A comparative sorption study of Cr3+ and Cr6+ using mango peels: kinetic, equilibrium and thermodynamic
- Effects of acid hydrolysis waste liquid recycle on preparation of microcrystalline cellulose
- Use of deep eutectic solvents as catalyst: A mini-review
- Microwave-assisted synthesis of pyrrolidinone derivatives using 1,1’-butylenebis(3-sulfo-3H-imidazol-1-ium) chloride in ethylene glycol
- Green and eco-friendly synthesis of Co3O4 and Ag-Co3O4: Characterization and photo-catalytic activity
- Adsorption optimized of the coal-based material and application for cyanide wastewater treatment
- Aloe vera leaf extract mediated green synthesis of selenium nanoparticles and assessment of their In vitro antimicrobial activity against spoilage fungi and pathogenic bacteria strains
- Waste phenolic resin derived activated carbon by microwave-assisted KOH activation and application to dye wastewater treatment
- Direct ethanol production from cellulose by consortium of Trichoderma reesei and Candida molischiana
- Agricultural waste biomass-assisted nanostructures: Synthesis and application
- Biodiesel production from rubber seed oil using calcium oxide derived from eggshell as catalyst – optimization and modeling studies
- Study of fabrication of fully aqueous solution processed SnS quantum dot-sensitized solar cell
- Assessment of aqueous extract of Gypsophila aretioides for inhibitory effects on calcium carbonate formation
- An environmentally friendly acylation reaction of 2-methylnaphthalene in solvent-free condition in a micro-channel reactor
- Aegle marmelos phytochemical stabilized synthesis and characterization of ZnO nanoparticles and their role against agriculture and food pathogen
- A reactive coupling process for co-production of solketal and biodiesel
- Optimization of the asymmetric synthesis of (S)-1-phenylethanol using Ispir bean as whole-cell biocatalyst
- Synthesis of pyrazolopyridine and pyrazoloquinoline derivatives by one-pot, three-component reactions of arylglyoxals, 3-methyl-1-aryl-1H-pyrazol-5-amines and cyclic 1,3-dicarbonyl compounds in the presence of tetrapropylammonium bromide
- Preconcentration of morphine in urine sample using a green and solvent-free microextraction method
- Extraction of glycyrrhizic acid by aqueous two-phase system formed by PEG and two environmentally friendly organic acid salts - sodium citrate and sodium tartrate
- Green synthesis of copper oxide nanoparticles using Juglans regia leaf extract and assessment of their physico-chemical and biological properties
- Deep eutectic solvents (DESs) as powerful and recyclable catalysts and solvents for the synthesis of 3,4-dihydropyrimidin-2(1H)-ones/thiones
- Biosynthesis, characterization and anti-microbial activity of silver nanoparticle based gel hand wash
- Efficient and selective microwave-assisted O-methylation of phenolic compounds using tetramethylammonium hydroxide (TMAH)
- Anticoagulant, thrombolytic and antibacterial activities of Euphorbia acruensis latex-mediated bioengineered silver nanoparticles
- Volcanic ash as reusable catalyst in the green synthesis of 3H-1,5-benzodiazepines
- Green synthesis, anionic polymerization of 1,4-bis(methacryloyl)piperazine using Algerian clay as catalyst
- Selenium supplementation during fermentation with sugar beet molasses and Saccharomyces cerevisiae to increase bioethanol production
- Biosynthetic potential assessment of four food pathogenic bacteria in hydrothermally silver nanoparticles fabrication
- Investigating the effectiveness of classical and eco-friendly approaches for synthesis of dialdehydes from organic dihalides
- Pyrolysis of palm oil using zeolite catalyst and characterization of the boil-oil
- Azadirachta indica leaves extract assisted green synthesis of Ag-TiO2 for degradation of Methylene blue and Rhodamine B dyes in aqueous medium
- Synthesis of vitamin E succinate catalyzed by nano-SiO2 immobilized DMAP derivative in mixed solvent system
- Extraction of phytosterols from melon (Cucumis melo) seeds by supercritical CO2 as a clean technology
- Production of uronic acids by hydrothermolysis of pectin as a model substance for plant biomass waste
- Biofabrication of highly pure copper oxide nanoparticles using wheat seed extract and their catalytic activity: A mechanistic approach
- Intelligent modeling and optimization of emulsion aggregation method for producing green printing ink
- Improved removal of methylene blue on modified hierarchical zeolite Y: Achieved by a “destructive-constructive” method
- Two different facile and efficient approaches for the synthesis of various N-arylacetamides via N-acetylation of arylamines and straightforward one-pot reductive acetylation of nitroarenes promoted by recyclable CuFe2O4 nanoparticles in water
- Optimization of acid catalyzed esterification and mixed metal oxide catalyzed transesterification for biodiesel production from Moringa oleifera oil
- Kinetics and the fluidity of the stearic acid esters with different carbon backbones
- Aiming for a standardized protocol for preparing a process green synthesis report and for ranking multiple synthesis plans to a common target product
- Microstructure and luminescence of VO2 (B) nanoparticle synthesis by hydrothermal method
- Optimization of uranium removal from uranium plant wastewater by response surface methodology (RSM)
- Microwave drying of nickel-containing residue: dielectric properties, kinetics, and energy aspects
- Simple and convenient two step synthesis of 5-bromo-2,3-dimethoxy-6-methyl-1,4-benzoquinone
- Biodiesel production from waste cooking oil
- The effect of activation temperature on structure and properties of blue coke-based activated carbon by CO2 activation
- Optimization of reaction parameters for the green synthesis of zero valent iron nanoparticles using pine tree needles
- Microwave-assisted protocol for squalene isolation and conversion from oil-deodoriser distillates
- Denitrification performance of rare earth tailings-based catalysts
- Facile synthesis of silver nanoparticles using Averrhoa bilimbi L and Plum extracts and investigation on the synergistic bioactivity using in vitro models
- Green production of AgNPs and their phytostimulatory impact
- Photocatalytic activity of Ag/Ni bi-metallic nanoparticles on textile dye removal
- Topical Issue: Green Process Engineering / Guest Editors: Martine Poux, Patrick Cognet
- Modelling and optimisation of oxidative desulphurisation of tyre-derived oil via central composite design approach
- CO2 sequestration by carbonation of olivine: a new process for optimal separation of the solids produced
- Organic carbonates synthesis improved by pervaporation for CO2 utilisation
- Production of starch nanoparticles through solvent-antisolvent precipitation in a spinning disc reactor
- A kinetic study of Zn halide/TBAB-catalysed fixation of CO2 with styrene oxide in propylene carbonate
- Topical on Green Process Engineering

