Abstract
Nanoparticles are smaller than nuclei having large surface area and more reactivity. Green production of metals and metal oxide nanoparticles is a benign and sustainable approach. Present work deals with the green production and optimization of nanoparticles by using indigenous plant growth promoting bacteria. Four different methods were used for silver nanoparticles (AgNPs) synthesis, i.e. extracellular, intracellular, oven assisted and extended incubation period. With extended incubation period AgNPs production improved. The nanoparticles synthesized were then applied to Triticum aestivum L. and their phytostimulatory impact was evaluated. The results revealed the growth promotional ability of nanoparticles. It is concluded that nanoparticles stimulate growth of Triticum aestivum L. with significant improvement in shoot length, root length, number of leaves and fresh weight. Thus in the present work we report environmentally benign nanoparticles exhibiting phytostimulatory potential. Biosynthesis of AgNPs by bacteria is a safe easy, less time consuming and economical approach which can be utilized as an alternative method to conventional chemical and physical approaches. Biosynthesized AgNPs can be effectively utilized as environment friendly nanofertilizers for plant growth promotion.
1 Introduction
One of the most dynamic areas of recent research is nanotechnology [1]. Nanoparticles are nanoscale particles range in size from 1-100 nm which have unique chemical and physical properties. Owing to their extraordinary properties, they are commonly used in science and technology. Various methods are used now-a-days for the synthesis of nanoparticles like physical, chemical and biological methods. Physical methods are highly exothermic reactions with lesser yields. Chemical methods have toxic effects because of production of large amount of by-products. Biological methods of nanoparticle synthesis are comparatively economical, ecofriendly and sustainable approach. Microbes have unique property of interacting and sequestering metal ions into their bodies due to the advent of metal binding proteins interaction. AgNPs are important metal particles which are commonly used in science and technology. In biological process of metal nanoparticles synthesis, the principles of green chemistry are utilized and microorganisms (bacteria, fungi and Actinomycetes) and plants are employed for the biosynthesis of AgNPs [2]. Bacteria sequester silver ions from the environment and reduced it into useful metal nanoparticles. Green synthesis of nanoparticles especially noble nanomaterial like silver nanoparticles (AgNPs) is more efficient, stable, fast and ecofriendly technique [3] Green chemistry reduces pollution risk and time required for the removal of toxic by-products formed as a result of chemical synthesis of nanoparticles (NPs) and is a safe alternative to chemical methods [4,5]. Current study deals with the biosynthesis of AgNPs by harnessing metal reduction potential of bacteria and evaluation of their phytostimulatory impact on plant growth. The bacteria utilized in the current study, have growth promotional potential and are also capable of synthesizing AgNPs which can also be utilized as better and effective phytostimulatory tool owing to their high surface area to volume ratio. Thus bacteria can be ecofriendly nano-factories for the application of nanofertilizers to improve plant growth organically.
2 Materials and methods
2.1 Screening and identification of bacterial isolates
Four bacterial isolates, i.e. Bacillus cereus (So3II), Brevundimonas diminuta (S5a), Serratia marcescens (S4c1) and Bacillus subtilis (Mt3b) were utilized in the current study. These bacterial strains were screened on the basis of their high auxin production potential following Ahmed and Hasnain [6] and identified through 16S rRNA sequencing following Ahmed and Hasnain [6]. Sequences were submitted to Genbank for accession numbers these bacterial strains were used in the current study for the synthesis of nanoparticles as a reducing and stabilizing agent.
2.2 Biosynthesis and characterization of bacterial AgNPs
In the present study, four different methods were utilized for the synthesis of bacterial AgNPs.
2.2.1 Extracellular and intracellular biosynthesis of bacterial AgNPs
Extracellular and intracellular synthesis of bacterial AgNPs (AgNPs) was carried out following Mahmoud et al. [7] with slight modification using cell free supernatant and bacterial cell pellet for extracellular and intracellular synthesis of AgNPs, respectively. The cell supernatant and cell pellet was allowed to react with AgNO3 and incubated for 24 h. Optical density of the reaction mixture was recorded to confirm the synthesis of silver nanoparticles (AgNPs) at 320, 400, 500, 600 and 700 nm after 24 h of incubation. UV-Visible spectroscopic analysis was also carried out to confirm the synthesis of AgNPs after 24 h of incubation.
2.2.2 Biosynthesis of bacterial AgNPs with extended incubation
Bacterial cultures (72 h old) were used for the formation of AgNPs following Jeevan et al. [8]. Synthesis of nanoparticles was observed by recording optical density at 320, 400, 500, 600 and 700 nm at equal intervals of time, i.e. 2, 4 and 6 h to study the rate at which nanoparticles were synthesized. Synthesis of nanoparticles was also observed by UV- Visible spectroscopic analysis to confirm the production of AgNPs.
2.2.3 Oven assisted biosynthesis of bacterial AgNPs
Heat method for the synthesis of AgNPs was carried out following Mahmoud et al. [7] with slight modifications. Bacterial cultures (24 h old) were used to inoculate L-broth and incubated at 37°C for 24 h. Cultures were centrifuged at 10,000 rpm for 10 min and supernatant was decanted off in another set of test tubes and its pH was adjusted at 10 then 1 mM AgNO3 solution was added and the mixture was incubated at 60°C for 24 h. Results were recorded silver nanoparticles were analyzed by recording optical density of reaction mixture at 320, 400, 500, 600 and 700 nm. Results were recorded after 1 h of incubation.
2.3 Transmission Electron Microscopy (TEM) of bacterial AgNPs
Bacterially synthesized AgNPs were examined through Transmission Electron Microscopy (TEM) to analyze their shape and size range following Oza et al. [9]. The Philips Tecnai F20 FEG-S/TEM was used for AgNPs analysis.
2.4 FTIR analysis of bacterial AgNPs
The bacterial AgNPs were examined through FTIR analysis (400-4000 cm-1) following Thamilselvi and Radha [3]. Agilent 630 FTIR machine was used for the FTIR analysis.
2.5 UV-Visible analysis of bacterial AgNPs
Production of AgNPs through all the four methods was analyzed by UV-Visible spectroscopic analysis at 400 nm.
2.6 Growth promoting potential of bacterial AgNPs
Growth experiment with Triticum aestivum was conducted under laboratory conditions to evaluate the phytostimulatory potential of bacterial AgNPs. Sterilized seeds of Triticum aestivum var. Fsd 2008 were procured from Punjab Seed Corporation, Lahore, Pakistan and surface sterilized using sodium hypochlorite solution following Ahmed and Hasnain [6] and sterilized seeds were primed with AgNPs of respective bacterial strains (Bacillus cereus (So3II), Brevundimonas diminuta (S5a), Serratia marcescens (S4c1) and Bacillus subtilis (Mt3b) obtained through extracellular method for half an hour. In order to study the overall effect of these bacterially produced nanoparticles, three sets of experiments were conducted simultaneously. One treatment in which seeds were primed with nanoparticles obtained from respective strains [Bacillus cereus (So3II), Brevundimonas diminuta (S5a), Serratia marcescens (S4c1) and Bacillus subtilis (Mt3b) is named as treatment T1 (bacterial AgNPs). Second treatment in which bacterial inoculum [Bacillus cereus (So3II), Brevundimonas diminuta (S5a), Serratia marcescens (S4c1) and Bacillus subtilis (Mt3b)] and bacterial AgNPs synthesized by the respective bacterial isolates was given simultaneously as a combination is named as treatment T2 (bacterial AgNPs+BI) and third in which only bacterial inoculum [Bacillus cereus (So3II), Brevundimonas diminuta (S5a), Serratia marcescens (S4c1) and Bacillus subtilis (Mt3b) was used for treatment is named as treatment T3 (BI). Equal number of bacteria and AgNPs were used in each treatment to prime the seeds and study their impact on plant growth. Six seeds were sown in each pot. Seeds treated with distilled water only were taken as control. Also the seeds treated with AgNO3 (1 mM) were taken as negative control. Experiment was conducted in triplicate and pots were kept in light (16 h duration and 10 K lux) at 25 ± 2°C and watering was done regularly. After 24-26 days of sowing, plants were harvested and various growth parameters of treated and control plants such as shoot length, root length, number of leaves and fresh weight were recorded and analysed.
2.7 Statistical analysis
The data was statistically analysed using SPSS V.16 software through DMR test.
3 Results
3.1 Screening and identification of bacterial isolates
Four identified rhizobacterial strains [Bacillus cereus (So3II), Brevundimonas diminuta (S5a), Serratia marcescens (S4c1), Bacillus subtilis (Mt3b)] screened on the basis of their high auxin production potential were selected for the current study. Isolates have shown auxin production potential with Bacillus cereus (So3II) showing 35.5 μg/mL, Brevundimonas diminuta (S5a) showing 47 μg/mL, Serratia marcescens (S4c1) showing 46 μg/mL and Bacillus subtilis (Mt3b) showing 36.6 μg/mL, respectively. The selected strains were identified and the sequences were submitted to GenBank. The accession numbers of the isolates were obtained [Serratia marcescens (S4c1) – KM43801.1, Bacillus cereus (So3II) – KM438011.1, Bacillus subtilis (Mt3b) – KT025250.1 and Brevundimonas diminuta (S5a) – KT025251.1]. Current study deals with the optimization of bacterial synthesis of AgNPs by using rhizospheric bacterial isolates collected from indigenous plants. Four identified bacterial strains (S4c1, S5a, So3II and Mt3b) were used in the present study. Four different methods were followed for the synthesis of AgNPs. AgNPs were successfully produced by all four methods; however, maximum quantity of AgNPs was obtained through extended incubation time period (Figure 1).
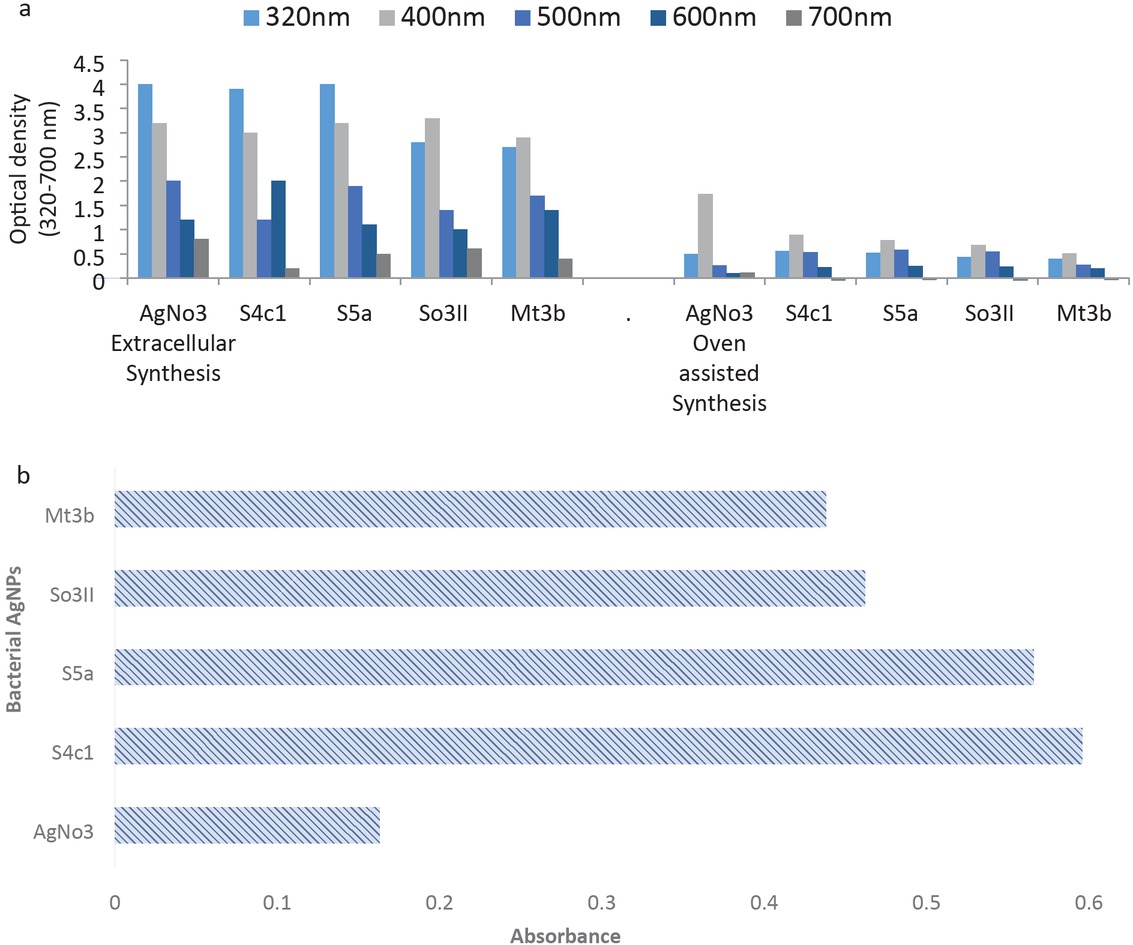
(a) Extra-cellular and oven assisted synthesis of bacterial AgNPs; (b) UV-Visible spectroscopic analysis of bacterial AgNPs.
3.2 Biosynthesis and characterization of bacterial AgNPs
In the current study, four different methods were followed for the synthesis of silver nanoparticles (AgNPs) from bacterial cultures.
3.2.1 Extracellular and intracellular synthesis of bacterial AgNPs
Significant production of AgNPs was recorded by following the method of Mahmoud et al. [7], i.e. extracellular synthesis of AgNPs. Change in color of reaction mixture (blackish brown) was the indication for the synthesis of bacterial AgNPs. The isolates Serratia marcescens (S4c1) and Brevundimonas diminuta (S5a) have shown maximum production of AgNPs (Figure 1). Synthesis of AgNPs via intracellular method of AgNPs did not exhibit significant production of AgNPs and only slight change in the color of the reaction mixture was observed which got intense with the passage of time. The isolate Serratia marcescens (S4c1) has shown maximum production of AgNPs (Figure 1).
3.2.2 Biosynthesis of AgNPs with extended incubation
This method showed higher production of AgNPs in shortest time so proved very effective. With increasing incubation time, the amount of nanoparticles in the reaction mixture was improved up to 24 h of incubation. But, after 24 h of incubation, gradual decrease in the production of AgNPs was observed. Results were monitored after every 2 h up to 6 h to record the time for the production of AgNPs. After 6 h of incubation, all bacterial strains showed production potential for AgNPs.
3.2.3 Oven-assisted AgNPs synthesis
AgNPs synthesis was also carried out by following oven assisted method for silver nanoparticle formation. This method proved very effective since only after 30 min of incubation at 60°C, intense color was produced which is indication of AgNPs formation. Maximum production of AgNPs was observed with Serratia marcescens (S4c1). The rate of reduction of metal ions at ambient temperature and pressure is quite fast by living organisms especially by bacteria as compared to synthetic method for AgNPs production (Figure 1).
3.3 Transmission Electron Microscopy (TEM) of bacterial AgNPs
Bacterially synthesized AgNPs were analyzed through Transmission Electron Microscopy (TEM). Most of the nanoparticles observed were monodispersed and polydispersed whereas some agglomerated AgNPs were also found to be present. Agglomeration of AgNPs generally results from the destabilizing action of silver ions [9]. The size range of AgNPs recorded was from 9.6 nm to 32 nm (Figure 2).
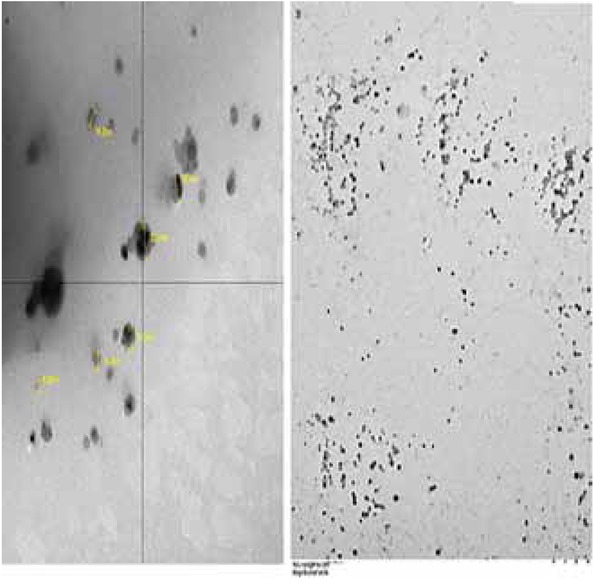
Transmission Electron Microscopic (TEM) analysis of bacterial synthesized silver nanoparticles.
3.4 FTIR analysis of bacterial AgNPs
FTIR spectrum of bacterial AgNPs was examined after FTIR analysis to identify the functional groups, possible composition and stability of AgNPs and their interaction with protein molecules. The IR spectrum of bacterial AgNPs showed intense bands at 3466.77 cm−1 which represent the stretching vibrations of amines while band at 1637.88 cm−1 represents phenyl ring substitution and its banding. Also band at 1637 cm−1 has shown carbonyl stretch in the amide linkages of protein. NO2 group exhibits vibrations at 1390 cm−1 (Figure 3). The possible mechanism for the reduction of Ag+ to AgNPs is that bacterial proteins react and bind with metals particles and caused its reduction to AgNPs (Figure 3).
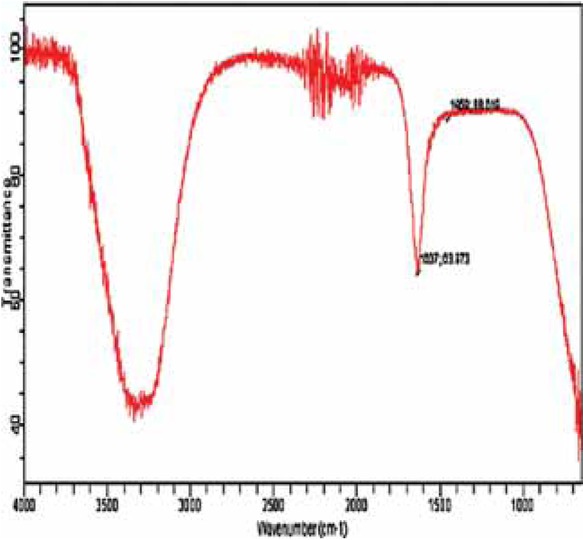
FTIR spectrum of bacterial AgNPs.
3.5 UV-Visible analysis of bacterial AgNPs
Production of AgNPs through all the four methods was analyzed by UV-Visible spectroscopic analysis at 400 nm. The isolates Serratia marcescens (S4c1) and Brevundimonas diminuta (S5a) have shown maximum production of AgNPs through extracellular synthesis method (Figure 1). The isolate Serratia marcescens (S4c1) has shown maximum production of AgNPs through intracellular method (0.512 a.u.). Bacterial AgNPs produced through oven assisted method have shown variable results. Maximum absorbance was recorded from bacterial strain Serratia marcescens (S4c1), i.e. 0.612 a.u. Extracellular production of AgNPs with extended incubation period was considered to be the most suitable method for AgNPs production (Figure 1).
3.6 Growth promoting potential of bacterial AgNPs
Growth promotional potential of bacterial AgNPs was evaluated using T. aestivum.
3.6.1 Germination percentage
Significant increase in germination was observed in treatment T1 (bacterial AgNPs) with AgNPs obtained from Bacillus cereus (So3II), up to 94% as compared to control plants. When treatment T2 (bacterial AgNPs+BI) was given to the T. aestivum seeds in which synergistic effect of both nanoparticles and bacterial isolates was recorded, significant improvement in germination percentage was observed in case of Bacillus cereus (So3II), Brevundimonas diminuta (S5a) and Serratia marcescens (S4c1) treatment up to 96% increase was recorded in comparison with control plants. While in only bacterial treatment which was treatment T3 (BI), Brevundimonas diminuta (S5a) treatment significantly improved germination percentage (11.1%) was observed in comparison with control plants.
3.6.2 Shoot length
In almost all the three sets of treatments, i.e. treatment T1 (bacterial AgNPs), bacterial and AgNPs treatment T2 (bacterial AgNPs+BI) and bacterial treatment T3 (BI), improvement in shoot length of T. aestivum was observed. AgNPs that were prepared using bacterial strain Brevundimonas diminuta S5a (Treatment T1) resulted in significant increase in shoot length up to 83% as compared to control plants that were only treated with distilled water (C1) and AgNO3 (C2), respectively. Synergistic effect of both bacterial AgNPs and bacterial inoculum showed significant improvement in shoot length of T. aestivum. In treatment T2, the isolates Bacillus cereus (So3II) and Serratia marcescens (S4c1) significantly improved shoot length by 81% and 82%, respectively, as compared to control plants. Bacterial inoculation in treatment T3 with Brevundimonas diminuta (S5a) showed 80% increment in shoot length over control plants (Figures 4 and 6)
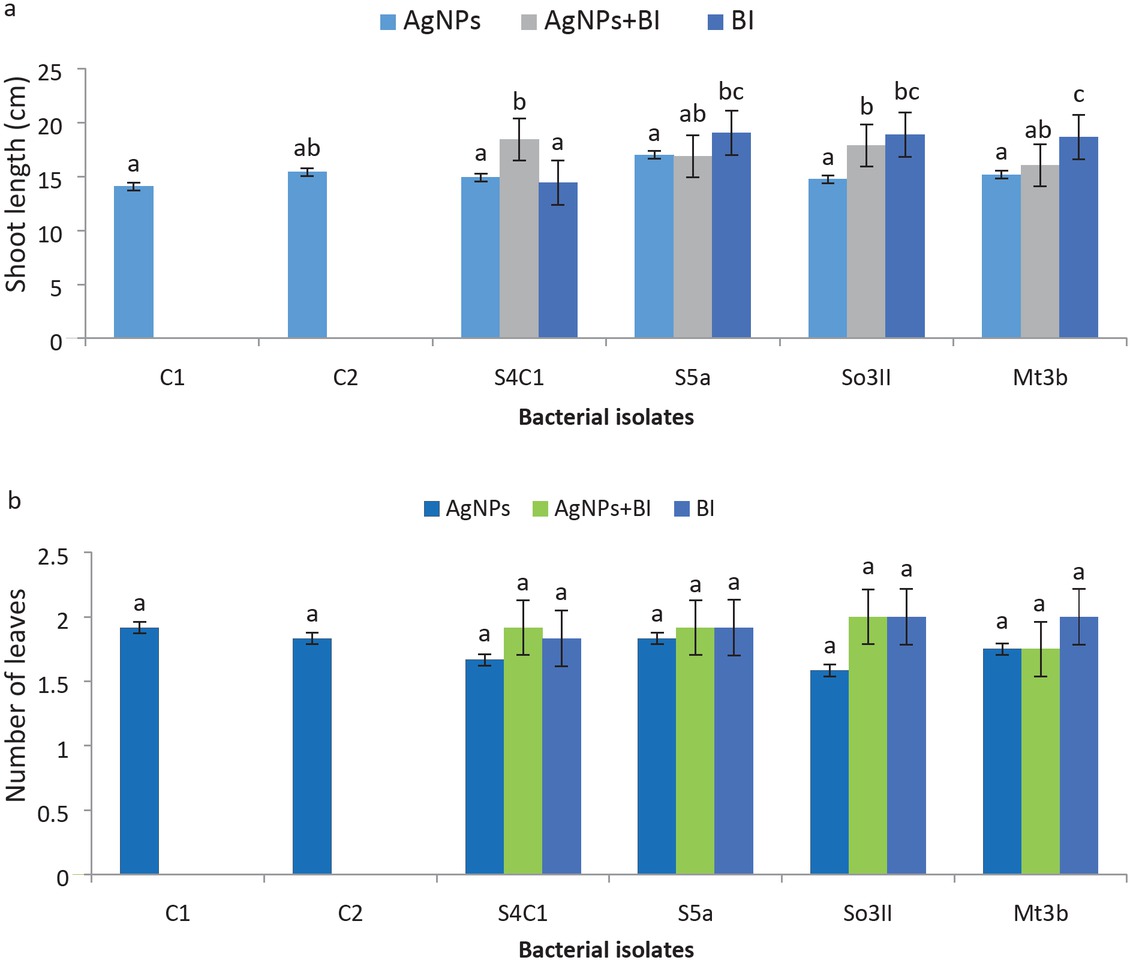
(a) Shoot length (cm); (b) Number of leaves after inoculation treatment T1 (bacterial AgNPs). T2 – bacterial AgNPs+BI; T3 – BI; C1 – Control treatment using water; C2 – Control treatment using AgNO3. Different letters indicate significant difference between treatments using Duncan’s multiple range test (P = 0.05).
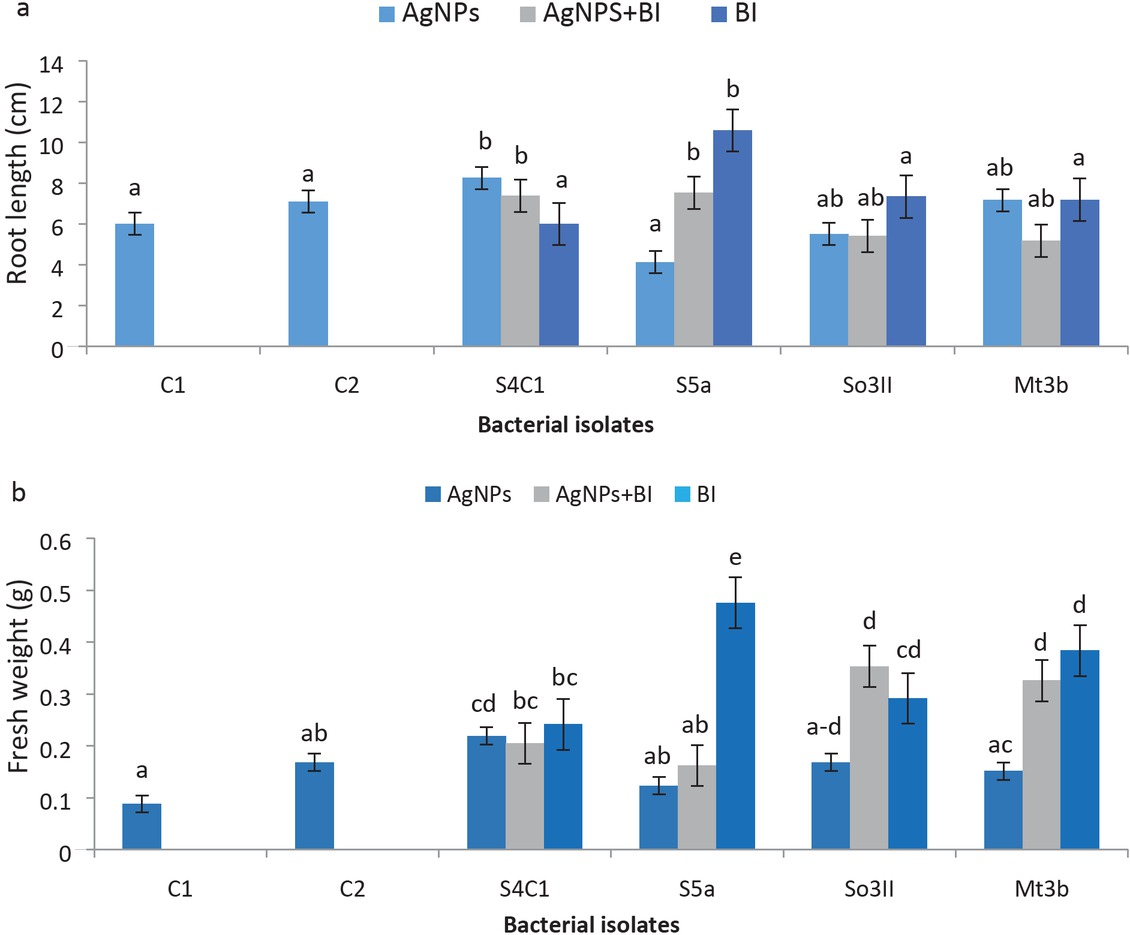
(a) Root length (cm); (b) Fresh weight (g) after inoculation treatment.
T1 – bacterial AgNPs; T2 – bacterial AgNPs+BI; T3 – BI; C1 – Control treatment using water, C2 – Control treatment using AgNO3. Different letters indicate significant difference between treatments using Duncan’s multiple range test (P = 0.05).

Shoot length (cm) of treated plants: A – control; B – T1 (bacterial AgNPs); C – T3 (BI); D – T2 (bacterial AgNPs+BI).
3.6.3 Root length
Maximum increment in root length was observed with treatment T1 (bacterial AgNPs). The bacterial isolate Bacillus cereus (So3II) treated T. aestivum plants produced significantly long roots with improvement up to 91% as compared to control plants. When treatment T2 (bacterial AgNPs+BI) was analysed, Bacillus cereus (So3II) has shown significant improvement in root length up to 92% as compared with control plants (plants treated with distilled water and silver nitrate solution separately). In treatment T3 (BI), Brevundimonas diminuta (S5a) treated plants also improved root length significantly up to 92% in comparison with control plants (Figure 5).
3.6.4 Number of leaves
In treatment T1 (bacterial AgNPs), Brevundimonas diminuta (S5a) increased number of leaves per plants up to 98% as compared to control plants while in treatment T2 (bacterial AgNPs+BI), Bacillus cereus (So3II), Brevundimonas diminuta (S5a) and Serratia marcescens (S4c1) improved number of leaves up to 98% in comparison to control plants (plants treated with distilled water and silver nitrate solution separately). In treatment T3 (BI), Brevundimonas diminuta (S5a), Serratia marcescens (S4c1) and Bacillus subtilis (Mt3b) treated plants improved number of leaves (98%) as compared to control plants (Figure 6).
3.6.5 Fresh weight
Nanoparticles synthesized from bacterial strain Bacillus cereus (So3II) in treatment T1 (bacterial AgNPs), significantly improved plant fresh weight up to 99% in comparison with control plants. In treatment T2 (bacterial AgNPs+BI), Bacillus cereus (So3II), Serratia marcescens (S4c1) and Bacillus subtilis (Mt3b) significantly enhanced plant biomass up to 99% as compared to control plants. In treatment T3 (BI), treatments with Brevundimonas diminuta (S5a) and Bacillus subtilis (Mt3b) significantly improved fresh weight as compared to control plants.
4 Discussion
Four identified bacterial strains Bacillus cereus (So3II), Brevundimonas diminuta (S5a), Serratia marcescens (S4c1) and Bacillus subtilis (Mt3b) were utilized in this study to evaluate their nanoparticles production potential and impact of these bacterial synthesized AgNPs on growth of T.aestivum was studied. The selection criteria for the bacterial isolates was their high auxin production potential. The isolates have shown auxin production potential with Bacillus cereus (So3II) showing 35.8 μg/mL, Brevundimonas diminuta (S5a) showing 47 μg/mL, Serratia marcescens (S4c1) showing 46 μg/mL and Bacillus subtilis (Mt3b) showing 36.6 μg/mL IAA production.
These selected bacterial isolates were used for the biosynthesis of bacterial AgNPs. All the strains have potential to reduce silver metal ions into AgNPs. These bacterial strains have the potential to sequester metals like silver from the soil and convert it into usable form such as AgNPs which can be used for plant growth improvement. All the four methods used for AgNPs synthesis produced AgNPs at varying rate and concentrations. The maximum quantity of AgNPs was obtained through extended incubation time method in which extracellular nanoparticles were incubated for extended time (Figure 1). Better production of AgNPs by this method might be due maximum activity level of bacterial isolates where most of them were at their log phase and thereby facilitating maximum nanoparticles production with the help of enzymes, e.g. nitrate reductase. This enzyme is responsible for the reduction of silver ions to AgNPs. Thamilselvi and Radha [3] also reported extended incubation method for the synthesis of AgNPs. Bacterial production of AgNPs is a defensive mechanism against metal toxicity. This defensive mechanism of bacteria is employed in the synthesis of AgNPs and is advantageous over conventional chemical method because this method is sustainable and ecofriendly. Bacterial cell wall play a pivotal role in the synthesis of AgNPs because bacterial cell wall is negatively charged and it interacts electrochemically with positively charged metal ions (Ag+) and hence, caused bioreduction of metal ions to metal nanoparticles (AgNPs) [3]. The chemical and physical methods of AgNPs production generate lots of heat and toxic chemicals whereas bacterial synthesis of NPs is environmentally safe and economic technique.
Maximum optical density was recorded at 320 and 400 nm wavelength. Das et al. [10] also reported strong absorption peaks at 450 nm. UV-Visible spectroscopic analysis of AgNPs was also performed for further confirmation. Maximum AgNPs synthesis was recorded at 400. Most of the findings indicated the production of AgNPs at 400 to 500 nm similar to our data.
Transmission Electron Microscopic (TEM) analysis of bacterial AgNPs revealed monodispersed and agglomerated AgNPs. Agglomeration of AgNPs generally results from the destabilizing action of silver ions [9]. TEM analysis of bacterial AgNPs showed of variable size ranging from 9.6 nm to 32 nm (Figure 2). The importance of variable nanoparticles size and shape is different in various fields of science and technology. The current study focuses on the phytostimulatory impact of AgNPs so all bacterial AgNPs synthesized were applied irrespective of their size to evaluate their impact on plant growth.
FTIR spectra confirmed the stabilization of nanoparticles with amines interaction (Figure 3). Bacterial proteins develop a coating on AgNPs which results in the stabilization of these particles. Surface bound proteins of bacteria play an important role in the production and stabilization of AgNPs. The proteins were observed to cap the nanoparticles using amino acids and amines, hence, caused reduction of Ag+ to AgNPs [11]. Silver ions (Ag+) bind with AgNPs which prevent disruption of secondary structure of proteins [11].
Phytostimulatory potential of bacterial AgNPs was evaluated using Triticum aestivum L. Treatment T1 (bacterial AgNPs) with AgNPs obtained from Bacillus cereus (So3II) and Treatment T2 (bacterial AgNPs+BI) significantly improved germination percentage up to 94 and 95% as compared to control plants. Significant improvement in shoot length was recorded in all the three sets of Treatments, i.e. Treatment T1 (bacterial AgNPs), Treatment T2 (bacterial AgNPs+BI) and Treatment T3 (BI) (Figures 4 and 6). AgNPs produced by bacterial strain Bacillus cereus (So3II) when used for treating T. aestivum resulted in significantly long roots with improvement up to 91% as compared to control plants. In Treatment T2 (bacterial AgNPs+BI) Bacillus cereus (So3II) has shown significant improvement in root length up to 92% as compared with control plants (plants treated with distilled water and silver nitrate solution separately). In Treatment T3 (BI), Brevundimonas diminuta (S5a) treated plants also improved root length significantly up to 92% in comparison with control plants (Figure 5). In all three sets of experiments (T1, T2, T3) improvement in the number of leaves was recorded as compared to control plants. In Treatment T2 (bacterial AgNPs+BI), Bacillus cereus (So3II), Serratia marcescens (S4c1) and Bacillus subtilis (Mt3b) significantly enhanced plant biomass up to 99% as compared to control plants. Pallavi et al. [12] also reported improvement in seed germination with application of nanoparticles. Treatment T2 (bacterial AgNPs+BI) and Treatment T3 (BI) improved shoot length but Treatment T1 (bacterial AgNPs) did not cause much increase in the height of plants. Treatment T1 using S5a caused insignificant improvement in shoot length over control. So according to our findings, Treatment T2 (bacterial AgNPs+BI) using bacterial strains Brevundimonas diminuta (S5a), Serratia marcescens (S4c1) and Bacillus subtilis (Mt3b) was more effective in improving plant height (shoot length) as compared to control plants (Figures 4 and 6). Treatment T1 using S4c1 significantly improved plant root length as compared to control plants. Treatment T2 (Bacillus cereus (So3II), Brevundimonas diminuta (S5a) significantly enhanced root length as compared to control plants (Figure 5). Improved root length was due to Jacalin related lactin (JAL) gene that improves production of precursor (nitrile) of auxin so multiple biochemical pathways are stimulated when AgNPs were applied and these ultimately improved plant growth [11]. So bacterial nanoparticles separately (Treatment T1) and in combination with bacterial inoculum (Treatment T2) produced almost similar results in case of Mt3b and S5a indicating that both exert similar effects in improving number of leaves. Significant improvement in fresh weight was observed when Treatment T2 was given in case of the isolate So3II while in case of Treatment T3 improvement was recorded in S5a and Mt3b so among the isolates used, AgNPs obtained from the isolate S4cI performed as the best sample used showing significant growth promotion in comparison with either the T2 or T3 or control treatments.
Nanoparticles interact with plant hormones and antioxidants, hence, affect plant growth patterns leading to plant growth promotion. Improvement in germination and biomass due to the application of nanoparticles was reported by Mehta et al. [14]. Application of AgNPs promotes root exudates production which may facilitate plant-microbes interactions and thus play its part to improve plant growth. Jasim et al. [13] also reported increment in growth parameters when AgNPs were applied on Fenugreek (Trigonellafoenum-graecum L.). The level of ABA and IAA was changed due to up-regulation of their genes. Gruyer et al. [15] also reported positive effect of AgNPs in plant growth of green-house grown radish and lettuce and the effect varies with the type of species. All the bacterial strains used in the present study exhibited potential for the synthesis of AgNPs. AgNPs exhibited antibacterial activity against pathogenic bacteria. So these bacterial AgNPs can act also as potential agents to be effectively used in pharmaceutical and medical fields. Bacterial AgNPs also exhibited potential to be used as biofertilizers. Ahmed et al. [16] also reported the environmentally benign production of AgNPs. The growth promotional activity of bacterial AgNPs as revealed in the current study indicates that these bacterial NPs can be employed commercially for the benefit of mankind in an economic and ecofriendly manner.
5 Conclusion
Nanoparticles produced in the current study by harnessing the potential of bacterial repositories as a sustainable green chemistry approach. The biosynthesized AgNPs have potential to improve T. aestivum growth both individually as well as synergistically along with bacterial inoculation. the bacterial AgNPs have potential to be used not only as bioremediators to extract silver ions but also improve plant growth. Hence PGPB can be exploited as for their dual mechanism of bioremediation and nanofertilization simultaneously through bacterial production of AgNPs as a successful alternative strategy for plant growth improvement.
List of Abbreviations
- FTIR –
Fourier Transform Infrared Spectroscopic analysis
- TEM –
Transmission Electron Microscopy
- AgNPs
– Silver nanoparticles
Acknowledgements
This work is supported by the financial grant from University of the Punjab, Lahore, Pakistan.
Conflict of interest:
The authors declare no conflicts of interest.
References
[1] Nikalje A.P., Nanotechnology and its applications in medicine. Med Chem., 2015, 5(2), 081-089.10.4172/2161-0444.1000247Search in Google Scholar
[2] Gomathi M., Rajkumar P.V., Prakasam A., Ravichandran K., Green synthesis of AgNPs using Datura stramonium leaf extract and assessment of their antibacterial activity. Resource-Efficient Technology, 2017, 3 (3), 280-284.10.1016/j.reffit.2016.12.005Search in Google Scholar
[3] Thamilselvi V., Radha K.V., Synthesis of AgNPs from Pseudomonas putida NCIM 2650 in silver nitrate supplemented growth medium and optimization using response surface methodology. Dig. J. Nanomater. Bios., 2013, 8 (3), 1101-1111.Search in Google Scholar
[4] Sabir S., Arshad M., Chaudhari S.K., Zinc oxide nanoparticles for revolutionizing agriculture; synthesis and application. The Sci. World J., 2014, 2014, 1-8, DOI: 10.1155/2014/925494.10.1155/2014/925494Search in Google Scholar PubMed PubMed Central
[5] Li J., Ma Q., Shao H., Zhou X., Xia H., Xie J., Biosynthesis, characterization and antibacterial activity of AgNPs produced from rice straw biomass. BioResources, 2017, 12(3), 4897-4911.10.15376/biores.12.3.4897-4911Search in Google Scholar
[6] Ahmed A., Hasnain S., Auxin producing Bacillus sp. Auxin quantification and effect on the growth of Solanum tuberosum Pure Appl. Chem., 2010, 82(1), 313-319.10.1351/PAC-CON-09-02-06Search in Google Scholar
[7] Mahmoud W.M., Abdelmoneim T.S., Elazzazy A.M., The impact of AgNPs produced by Bacillus pumillus as antimicrobial and nematicide. Front. Microbiol., 2016, 7, 1746, DOI:10.3389/fmicb.2016.01746.10.3389/fmicb.2016.01746Search in Google Scholar PubMed PubMed Central
[8] Jeevan P., Ramya K., Reena A.E., Extracellular biosynthesis of AgNPs by culture supernatant of Pseudomonas aeruginosa Indian. J. Biotechnol., 2012, 11, 42-46.Search in Google Scholar
[9] Oza G., Pandey S., Shah R., Sharon M., Extracelllar fabrication of AgNPs using Pseudomonas aeruginosa and its antimicrobial assay. Adv. Appl. Sci. Res., 2012, 3(3), 1776-1783.Search in Google Scholar
[10] Das V.L, Thomas R., Varghese R.T., Soniya Mathew E.V.J., Radhakrishnan E.K., Extracellular synthesis of AgNPs by the Bacillus strain CS 11 isolated from industrialized areas. 3 Biotech., 2014, 4(2), 121-126.10.1007/s13205-013-0130-8Search in Google Scholar PubMed PubMed Central
[11] Tran Q.H., Le A.T., AgNPs: synthesis, properties toxicology, applications and perspectives. Adv. Nat. Sci. Nanosci. Nanotechnol., 2013, 4(3), 033001, DOI:10.1088/2043-6262/4/3/033001.10.1088/2043-6262/4/3/033001Search in Google Scholar
[12] Pallavi Mehta C.M., Srivastava R., Arora S., Sharma A.K., Impact assessment of AgNPs on plant growth and soil bacterial diversity. 3 Biotechnol., 2016, 6(2), 254, DOI:10.1007/s13205-016-056707.10.1007/s13205-016-056707Search in Google Scholar
[13] Jasim B., Thomas R., Mathew J., Radhakrishnan E.K., Plant growth and diosgenin enhancement effect of AgNPs in Fenugreek Trigonella foenum graecum L.). Saudi Pharm. J., 2017, 25(3), 443-447.10.1016/j.jsps.2016.09.012Search in Google Scholar PubMed PubMed Central
[14] Mehta C.M., Srivastava R., Arora S., Sharma A.K., Impact assessment of AgNPs on plant growth and soil bacterial diversity. Biotechnol., 2016, 6(2), 254.10.1007/s13205-016-0567-7Search in Google Scholar PubMed PubMed Central
[15] Gruyer N., Dorais M., Bastien C., Dassylva N., Triffault-Bouchet G., Interaction between AgNPs and plant growth. In: International Symposium on New Technologies for Environment Control, Energy-Saving and Crop Production in Greenhouse and Plant. 2013, 1037, 795-800.Search in Google Scholar
[16] Ahmed S., Kaur G., Sharma P., Singh S., Ikram S., Evaluation of the antioxidant, antibacterial and anticancer (lung cancer cell line A549) activity of Punica granatum mediated AgNPs. Toxicol. Res.-UK, 2018, 7(5), 923-930.10.1039/C8TX00103KSearch in Google Scholar
© 2019 Wagi and Ahmed, published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 Public License.
Articles in the same Issue
- Regular Articles
- Studies on the preparation and properties of biodegradable polyester from soybean oil
- Flow-mode biodiesel production from palm oil using a pressurized microwave reactor
- Reduction of free fatty acids in waste oil for biodiesel production by glycerolysis: investigation and optimization of process parameters
- Saccharin: a cheap and mild acidic agent for the synthesis of azo dyes via telescoped dediazotization
- Optimization of lipase-catalyzed synthesis of polyethylene glycol stearate in a solvent-free system
- Green synthesis of iron oxide nanoparticles using Platanus orientalis leaf extract for antifungal activity
- Ultrasound assisted chemical activation of peanut husk for copper removal
- Room temperature silanization of Fe3O4 for the preparation of phenyl functionalized magnetic adsorbent for dispersive solid phase extraction for the extraction of phthalates in water
- Evaluation of the saponin green extraction from Ziziphus spina-christi leaves using hydrothermal, microwave and Bain-Marie water bath heating methods
- Oxidation of dibenzothiophene using the heterogeneous catalyst of tungsten-based carbon nanotubes
- Calcined sodium silicate as an efficient and benign heterogeneous catalyst for the transesterification of natural lecithin to L-α-glycerophosphocholine
- Synergistic effect between CO2 and H2O2 on ethylbenzene oxidation catalyzed by carbon supported heteropolyanion catalysts
- Hydrocyanation of 2-arylmethyleneindan-1,3-diones using potassium hexacyanoferrate(II) as a nontoxic cyanating agent
- Green synthesis of hydratropic aldehyde from α-methylstyrene catalyzed by Al2O3-supported metal phthalocyanines
- Environmentally benign chemical recycling of polycarbonate wastes: comparison of micro- and nano-TiO2 solid support efficiencies
- Medicago polymorpha-mediated antibacterial silver nanoparticles in the reduction of methyl orange
- Production of value-added chemicals from esterification of waste glycerol over MCM-41 supported catalysts
- Green synthesis of zerovalent copper nanoparticles for efficient reduction of toxic azo dyes congo red and methyl orange
- Optimization of the biological synthesis of silver nanoparticles using Penicillium oxalicum GRS-1 and their antimicrobial effects against common food-borne pathogens
- Optimization of submerged fermentation conditions to overproduce bioethanol using two industrial and traditional Saccharomyces cerevisiae strains
- Extraction of In3+ and Fe3+ from sulfate solutions by using a 3D-printed “Y”-shaped microreactor
- Foliar-mediated Ag:ZnO nanophotocatalysts: green synthesis, characterization, pollutants degradation, and in vitro biocidal activity
- Green cyclic acetals production by glycerol etherification reaction with benzaldehyde using cationic acidic resin
- Biosynthesis, characterization and antimicrobial activities assessment of fabricated selenium nanoparticles using Pelargonium zonale leaf extract
- Synthesis of high surface area magnesia by using walnut shell as a template
- Controllable biosynthesis of silver nanoparticles using actinobacterial strains
- Green vegetation: a promising source of color dyes
- Mechano-chemical synthesis of ammonia and acetic acid from inorganic materials in water
- Green synthesis and structural characterization of novel N1-substituted 3,4-dihydropyrimidin-2(1H)-ones
- Biodiesel production from cotton oil using heterogeneous CaO catalysts from eggshells prepared at different calcination temperatures
- Regeneration of spent mercury catalyst for the treatment of dye wastewater by the microwave and ultrasonic spray-assisted method
- Green synthesis of the innovative super paramagnetic nanoparticles from the leaves extract of Fraxinus chinensis Roxb and their application for the decolourisation of toxic dyes
- Biogenic ZnO nanoparticles: a study of blueshift of optical band gap and photocatalytic degradation of reactive yellow 186 dye under direct sunlight
- Leached compounds from the extracts of pomegranate peel, green coconut shell, and karuvelam wood for the removal of hexavalent chromium
- Enhancement of molecular weight reduction of natural rubber in triphasic CO2/toluene/H2O systems with hydrogen peroxide for preparation of biobased polyurethanes
- An efficient green synthesis of novel 1H-imidazo[1,2-a]imidazole-3-amine and imidazo[2,1-c][1,2,4]triazole-5-amine derivatives via Strecker reaction under controlled microwave heating
- Evaluation of three different green fabrication methods for the synthesis of crystalline ZnO nanoparticles using Pelargonium zonale leaf extract
- A highly efficient and multifunctional biomass supporting Ag, Ni, and Cu nanoparticles through wetness impregnation for environmental remediation
- Simple one-pot green method for large-scale production of mesalamine, an anti-inflammatory agent
- Relationships between step and cumulative PMI and E-factors: implications on estimating material efficiency with respect to charting synthesis optimization strategies
- A comparative sorption study of Cr3+ and Cr6+ using mango peels: kinetic, equilibrium and thermodynamic
- Effects of acid hydrolysis waste liquid recycle on preparation of microcrystalline cellulose
- Use of deep eutectic solvents as catalyst: A mini-review
- Microwave-assisted synthesis of pyrrolidinone derivatives using 1,1’-butylenebis(3-sulfo-3H-imidazol-1-ium) chloride in ethylene glycol
- Green and eco-friendly synthesis of Co3O4 and Ag-Co3O4: Characterization and photo-catalytic activity
- Adsorption optimized of the coal-based material and application for cyanide wastewater treatment
- Aloe vera leaf extract mediated green synthesis of selenium nanoparticles and assessment of their In vitro antimicrobial activity against spoilage fungi and pathogenic bacteria strains
- Waste phenolic resin derived activated carbon by microwave-assisted KOH activation and application to dye wastewater treatment
- Direct ethanol production from cellulose by consortium of Trichoderma reesei and Candida molischiana
- Agricultural waste biomass-assisted nanostructures: Synthesis and application
- Biodiesel production from rubber seed oil using calcium oxide derived from eggshell as catalyst – optimization and modeling studies
- Study of fabrication of fully aqueous solution processed SnS quantum dot-sensitized solar cell
- Assessment of aqueous extract of Gypsophila aretioides for inhibitory effects on calcium carbonate formation
- An environmentally friendly acylation reaction of 2-methylnaphthalene in solvent-free condition in a micro-channel reactor
- Aegle marmelos phytochemical stabilized synthesis and characterization of ZnO nanoparticles and their role against agriculture and food pathogen
- A reactive coupling process for co-production of solketal and biodiesel
- Optimization of the asymmetric synthesis of (S)-1-phenylethanol using Ispir bean as whole-cell biocatalyst
- Synthesis of pyrazolopyridine and pyrazoloquinoline derivatives by one-pot, three-component reactions of arylglyoxals, 3-methyl-1-aryl-1H-pyrazol-5-amines and cyclic 1,3-dicarbonyl compounds in the presence of tetrapropylammonium bromide
- Preconcentration of morphine in urine sample using a green and solvent-free microextraction method
- Extraction of glycyrrhizic acid by aqueous two-phase system formed by PEG and two environmentally friendly organic acid salts - sodium citrate and sodium tartrate
- Green synthesis of copper oxide nanoparticles using Juglans regia leaf extract and assessment of their physico-chemical and biological properties
- Deep eutectic solvents (DESs) as powerful and recyclable catalysts and solvents for the synthesis of 3,4-dihydropyrimidin-2(1H)-ones/thiones
- Biosynthesis, characterization and anti-microbial activity of silver nanoparticle based gel hand wash
- Efficient and selective microwave-assisted O-methylation of phenolic compounds using tetramethylammonium hydroxide (TMAH)
- Anticoagulant, thrombolytic and antibacterial activities of Euphorbia acruensis latex-mediated bioengineered silver nanoparticles
- Volcanic ash as reusable catalyst in the green synthesis of 3H-1,5-benzodiazepines
- Green synthesis, anionic polymerization of 1,4-bis(methacryloyl)piperazine using Algerian clay as catalyst
- Selenium supplementation during fermentation with sugar beet molasses and Saccharomyces cerevisiae to increase bioethanol production
- Biosynthetic potential assessment of four food pathogenic bacteria in hydrothermally silver nanoparticles fabrication
- Investigating the effectiveness of classical and eco-friendly approaches for synthesis of dialdehydes from organic dihalides
- Pyrolysis of palm oil using zeolite catalyst and characterization of the boil-oil
- Azadirachta indica leaves extract assisted green synthesis of Ag-TiO2 for degradation of Methylene blue and Rhodamine B dyes in aqueous medium
- Synthesis of vitamin E succinate catalyzed by nano-SiO2 immobilized DMAP derivative in mixed solvent system
- Extraction of phytosterols from melon (Cucumis melo) seeds by supercritical CO2 as a clean technology
- Production of uronic acids by hydrothermolysis of pectin as a model substance for plant biomass waste
- Biofabrication of highly pure copper oxide nanoparticles using wheat seed extract and their catalytic activity: A mechanistic approach
- Intelligent modeling and optimization of emulsion aggregation method for producing green printing ink
- Improved removal of methylene blue on modified hierarchical zeolite Y: Achieved by a “destructive-constructive” method
- Two different facile and efficient approaches for the synthesis of various N-arylacetamides via N-acetylation of arylamines and straightforward one-pot reductive acetylation of nitroarenes promoted by recyclable CuFe2O4 nanoparticles in water
- Optimization of acid catalyzed esterification and mixed metal oxide catalyzed transesterification for biodiesel production from Moringa oleifera oil
- Kinetics and the fluidity of the stearic acid esters with different carbon backbones
- Aiming for a standardized protocol for preparing a process green synthesis report and for ranking multiple synthesis plans to a common target product
- Microstructure and luminescence of VO2 (B) nanoparticle synthesis by hydrothermal method
- Optimization of uranium removal from uranium plant wastewater by response surface methodology (RSM)
- Microwave drying of nickel-containing residue: dielectric properties, kinetics, and energy aspects
- Simple and convenient two step synthesis of 5-bromo-2,3-dimethoxy-6-methyl-1,4-benzoquinone
- Biodiesel production from waste cooking oil
- The effect of activation temperature on structure and properties of blue coke-based activated carbon by CO2 activation
- Optimization of reaction parameters for the green synthesis of zero valent iron nanoparticles using pine tree needles
- Microwave-assisted protocol for squalene isolation and conversion from oil-deodoriser distillates
- Denitrification performance of rare earth tailings-based catalysts
- Facile synthesis of silver nanoparticles using Averrhoa bilimbi L and Plum extracts and investigation on the synergistic bioactivity using in vitro models
- Green production of AgNPs and their phytostimulatory impact
- Photocatalytic activity of Ag/Ni bi-metallic nanoparticles on textile dye removal
- Topical Issue: Green Process Engineering / Guest Editors: Martine Poux, Patrick Cognet
- Modelling and optimisation of oxidative desulphurisation of tyre-derived oil via central composite design approach
- CO2 sequestration by carbonation of olivine: a new process for optimal separation of the solids produced
- Organic carbonates synthesis improved by pervaporation for CO2 utilisation
- Production of starch nanoparticles through solvent-antisolvent precipitation in a spinning disc reactor
- A kinetic study of Zn halide/TBAB-catalysed fixation of CO2 with styrene oxide in propylene carbonate
- Topical on Green Process Engineering
Articles in the same Issue
- Regular Articles
- Studies on the preparation and properties of biodegradable polyester from soybean oil
- Flow-mode biodiesel production from palm oil using a pressurized microwave reactor
- Reduction of free fatty acids in waste oil for biodiesel production by glycerolysis: investigation and optimization of process parameters
- Saccharin: a cheap and mild acidic agent for the synthesis of azo dyes via telescoped dediazotization
- Optimization of lipase-catalyzed synthesis of polyethylene glycol stearate in a solvent-free system
- Green synthesis of iron oxide nanoparticles using Platanus orientalis leaf extract for antifungal activity
- Ultrasound assisted chemical activation of peanut husk for copper removal
- Room temperature silanization of Fe3O4 for the preparation of phenyl functionalized magnetic adsorbent for dispersive solid phase extraction for the extraction of phthalates in water
- Evaluation of the saponin green extraction from Ziziphus spina-christi leaves using hydrothermal, microwave and Bain-Marie water bath heating methods
- Oxidation of dibenzothiophene using the heterogeneous catalyst of tungsten-based carbon nanotubes
- Calcined sodium silicate as an efficient and benign heterogeneous catalyst for the transesterification of natural lecithin to L-α-glycerophosphocholine
- Synergistic effect between CO2 and H2O2 on ethylbenzene oxidation catalyzed by carbon supported heteropolyanion catalysts
- Hydrocyanation of 2-arylmethyleneindan-1,3-diones using potassium hexacyanoferrate(II) as a nontoxic cyanating agent
- Green synthesis of hydratropic aldehyde from α-methylstyrene catalyzed by Al2O3-supported metal phthalocyanines
- Environmentally benign chemical recycling of polycarbonate wastes: comparison of micro- and nano-TiO2 solid support efficiencies
- Medicago polymorpha-mediated antibacterial silver nanoparticles in the reduction of methyl orange
- Production of value-added chemicals from esterification of waste glycerol over MCM-41 supported catalysts
- Green synthesis of zerovalent copper nanoparticles for efficient reduction of toxic azo dyes congo red and methyl orange
- Optimization of the biological synthesis of silver nanoparticles using Penicillium oxalicum GRS-1 and their antimicrobial effects against common food-borne pathogens
- Optimization of submerged fermentation conditions to overproduce bioethanol using two industrial and traditional Saccharomyces cerevisiae strains
- Extraction of In3+ and Fe3+ from sulfate solutions by using a 3D-printed “Y”-shaped microreactor
- Foliar-mediated Ag:ZnO nanophotocatalysts: green synthesis, characterization, pollutants degradation, and in vitro biocidal activity
- Green cyclic acetals production by glycerol etherification reaction with benzaldehyde using cationic acidic resin
- Biosynthesis, characterization and antimicrobial activities assessment of fabricated selenium nanoparticles using Pelargonium zonale leaf extract
- Synthesis of high surface area magnesia by using walnut shell as a template
- Controllable biosynthesis of silver nanoparticles using actinobacterial strains
- Green vegetation: a promising source of color dyes
- Mechano-chemical synthesis of ammonia and acetic acid from inorganic materials in water
- Green synthesis and structural characterization of novel N1-substituted 3,4-dihydropyrimidin-2(1H)-ones
- Biodiesel production from cotton oil using heterogeneous CaO catalysts from eggshells prepared at different calcination temperatures
- Regeneration of spent mercury catalyst for the treatment of dye wastewater by the microwave and ultrasonic spray-assisted method
- Green synthesis of the innovative super paramagnetic nanoparticles from the leaves extract of Fraxinus chinensis Roxb and their application for the decolourisation of toxic dyes
- Biogenic ZnO nanoparticles: a study of blueshift of optical band gap and photocatalytic degradation of reactive yellow 186 dye under direct sunlight
- Leached compounds from the extracts of pomegranate peel, green coconut shell, and karuvelam wood for the removal of hexavalent chromium
- Enhancement of molecular weight reduction of natural rubber in triphasic CO2/toluene/H2O systems with hydrogen peroxide for preparation of biobased polyurethanes
- An efficient green synthesis of novel 1H-imidazo[1,2-a]imidazole-3-amine and imidazo[2,1-c][1,2,4]triazole-5-amine derivatives via Strecker reaction under controlled microwave heating
- Evaluation of three different green fabrication methods for the synthesis of crystalline ZnO nanoparticles using Pelargonium zonale leaf extract
- A highly efficient and multifunctional biomass supporting Ag, Ni, and Cu nanoparticles through wetness impregnation for environmental remediation
- Simple one-pot green method for large-scale production of mesalamine, an anti-inflammatory agent
- Relationships between step and cumulative PMI and E-factors: implications on estimating material efficiency with respect to charting synthesis optimization strategies
- A comparative sorption study of Cr3+ and Cr6+ using mango peels: kinetic, equilibrium and thermodynamic
- Effects of acid hydrolysis waste liquid recycle on preparation of microcrystalline cellulose
- Use of deep eutectic solvents as catalyst: A mini-review
- Microwave-assisted synthesis of pyrrolidinone derivatives using 1,1’-butylenebis(3-sulfo-3H-imidazol-1-ium) chloride in ethylene glycol
- Green and eco-friendly synthesis of Co3O4 and Ag-Co3O4: Characterization and photo-catalytic activity
- Adsorption optimized of the coal-based material and application for cyanide wastewater treatment
- Aloe vera leaf extract mediated green synthesis of selenium nanoparticles and assessment of their In vitro antimicrobial activity against spoilage fungi and pathogenic bacteria strains
- Waste phenolic resin derived activated carbon by microwave-assisted KOH activation and application to dye wastewater treatment
- Direct ethanol production from cellulose by consortium of Trichoderma reesei and Candida molischiana
- Agricultural waste biomass-assisted nanostructures: Synthesis and application
- Biodiesel production from rubber seed oil using calcium oxide derived from eggshell as catalyst – optimization and modeling studies
- Study of fabrication of fully aqueous solution processed SnS quantum dot-sensitized solar cell
- Assessment of aqueous extract of Gypsophila aretioides for inhibitory effects on calcium carbonate formation
- An environmentally friendly acylation reaction of 2-methylnaphthalene in solvent-free condition in a micro-channel reactor
- Aegle marmelos phytochemical stabilized synthesis and characterization of ZnO nanoparticles and their role against agriculture and food pathogen
- A reactive coupling process for co-production of solketal and biodiesel
- Optimization of the asymmetric synthesis of (S)-1-phenylethanol using Ispir bean as whole-cell biocatalyst
- Synthesis of pyrazolopyridine and pyrazoloquinoline derivatives by one-pot, three-component reactions of arylglyoxals, 3-methyl-1-aryl-1H-pyrazol-5-amines and cyclic 1,3-dicarbonyl compounds in the presence of tetrapropylammonium bromide
- Preconcentration of morphine in urine sample using a green and solvent-free microextraction method
- Extraction of glycyrrhizic acid by aqueous two-phase system formed by PEG and two environmentally friendly organic acid salts - sodium citrate and sodium tartrate
- Green synthesis of copper oxide nanoparticles using Juglans regia leaf extract and assessment of their physico-chemical and biological properties
- Deep eutectic solvents (DESs) as powerful and recyclable catalysts and solvents for the synthesis of 3,4-dihydropyrimidin-2(1H)-ones/thiones
- Biosynthesis, characterization and anti-microbial activity of silver nanoparticle based gel hand wash
- Efficient and selective microwave-assisted O-methylation of phenolic compounds using tetramethylammonium hydroxide (TMAH)
- Anticoagulant, thrombolytic and antibacterial activities of Euphorbia acruensis latex-mediated bioengineered silver nanoparticles
- Volcanic ash as reusable catalyst in the green synthesis of 3H-1,5-benzodiazepines
- Green synthesis, anionic polymerization of 1,4-bis(methacryloyl)piperazine using Algerian clay as catalyst
- Selenium supplementation during fermentation with sugar beet molasses and Saccharomyces cerevisiae to increase bioethanol production
- Biosynthetic potential assessment of four food pathogenic bacteria in hydrothermally silver nanoparticles fabrication
- Investigating the effectiveness of classical and eco-friendly approaches for synthesis of dialdehydes from organic dihalides
- Pyrolysis of palm oil using zeolite catalyst and characterization of the boil-oil
- Azadirachta indica leaves extract assisted green synthesis of Ag-TiO2 for degradation of Methylene blue and Rhodamine B dyes in aqueous medium
- Synthesis of vitamin E succinate catalyzed by nano-SiO2 immobilized DMAP derivative in mixed solvent system
- Extraction of phytosterols from melon (Cucumis melo) seeds by supercritical CO2 as a clean technology
- Production of uronic acids by hydrothermolysis of pectin as a model substance for plant biomass waste
- Biofabrication of highly pure copper oxide nanoparticles using wheat seed extract and their catalytic activity: A mechanistic approach
- Intelligent modeling and optimization of emulsion aggregation method for producing green printing ink
- Improved removal of methylene blue on modified hierarchical zeolite Y: Achieved by a “destructive-constructive” method
- Two different facile and efficient approaches for the synthesis of various N-arylacetamides via N-acetylation of arylamines and straightforward one-pot reductive acetylation of nitroarenes promoted by recyclable CuFe2O4 nanoparticles in water
- Optimization of acid catalyzed esterification and mixed metal oxide catalyzed transesterification for biodiesel production from Moringa oleifera oil
- Kinetics and the fluidity of the stearic acid esters with different carbon backbones
- Aiming for a standardized protocol for preparing a process green synthesis report and for ranking multiple synthesis plans to a common target product
- Microstructure and luminescence of VO2 (B) nanoparticle synthesis by hydrothermal method
- Optimization of uranium removal from uranium plant wastewater by response surface methodology (RSM)
- Microwave drying of nickel-containing residue: dielectric properties, kinetics, and energy aspects
- Simple and convenient two step synthesis of 5-bromo-2,3-dimethoxy-6-methyl-1,4-benzoquinone
- Biodiesel production from waste cooking oil
- The effect of activation temperature on structure and properties of blue coke-based activated carbon by CO2 activation
- Optimization of reaction parameters for the green synthesis of zero valent iron nanoparticles using pine tree needles
- Microwave-assisted protocol for squalene isolation and conversion from oil-deodoriser distillates
- Denitrification performance of rare earth tailings-based catalysts
- Facile synthesis of silver nanoparticles using Averrhoa bilimbi L and Plum extracts and investigation on the synergistic bioactivity using in vitro models
- Green production of AgNPs and their phytostimulatory impact
- Photocatalytic activity of Ag/Ni bi-metallic nanoparticles on textile dye removal
- Topical Issue: Green Process Engineering / Guest Editors: Martine Poux, Patrick Cognet
- Modelling and optimisation of oxidative desulphurisation of tyre-derived oil via central composite design approach
- CO2 sequestration by carbonation of olivine: a new process for optimal separation of the solids produced
- Organic carbonates synthesis improved by pervaporation for CO2 utilisation
- Production of starch nanoparticles through solvent-antisolvent precipitation in a spinning disc reactor
- A kinetic study of Zn halide/TBAB-catalysed fixation of CO2 with styrene oxide in propylene carbonate
- Topical on Green Process Engineering

