Abstract
We report the effect of various parameters, namely substrate concentration, time, pH and temperature, on the biosynthesis of silver nanoparticles (AgNPs) by using the extract of actinobacterial strains, which were isolated from the sediments of Lonar Crater Lake in India. It was found that the formation of AgNPs and its morphology depended on synthesis conditions. Visual observation of the reaction mixture, ultraviolet-vis spectroscopic analysis and mass of synthesized AgNPs indicated that 25°C, pH 7 and 3 days of incubation time were optimal for its efficient synthesis. The transmission electron microscopy (TEM) analysis revealed aggregation and irregular shape of AgNPs both at acidic pH and below 25°C. It was found that alkaline pH and temperature higher than optimal fostered the formation of nanoparticle aggregates. Based on the obtained results, it was concluded that the efficiency of biological synthesis by using actinobacteria as well as the size and shape of fabricated nanoparticles can be manipulated by controlled conditions of synthesis process. The use of desired nanoparticles increases its potential for medical applications.
- Abbreviations
- AgNPs
silver nanoparticles
- ISP2
yeast extract-malt extract medium
- NPs
nanoparticles
- SCA
starch casein agar
- SPR
surface plasmon resonance
1 Introduction
Nanotechnology is a fast-growing branch of science that deals with the synthesis and development of different nanomaterials [1]. Silver nanoparticles (AgNPs) are the main focus of intensive study because of their wide applications, such as catalysts, optics, antimicrobials, anticancer agents, and for biomaterial production [[1], [2], [3], [4]. There is enormous interest in metal nanoparticles (NPs) because of their unique physical and chemical properties revealed at the nanoscale level 5].
The microbial recovery of noble metals with the formation of their NPs is a green alternative to physical and chemical methods, which use high amounts of energy or toxic solvents and reagents. A biological synthesis process provides a wide range of environmentally acceptable methodology, low-cost production and minimum time required [6].
The biosynthesis of AgNPs using various bacteria, fungi and plants is already well documented [[7], [8], [9], [10], [11]. However, the exploration of actinomycetes has recently gained interest for efficient biological synthesis of metallic NPs 6].
The bio-fabrication of NPs, which is a “bottom-up” approach, as well as the shape, size, distribution and composition of NPs, depends upon physicochemical properties such as temperature, time, pH, concentration of the substrate and the presence of biomolecules acting as reducing agents (amino acids, proteins, vitamins) and enzyme sources [12], [13], [14], [15], [16], [17], [18], [19]. NPs of desired shape and size can be obtained by controlling the above physical and chemical parameters, which influence the rate of nucleation, growth and atomic/molecular precursor [15], [20]. Hence, the demand for the controllable synthesis of NPs is growing day by day.
In the present study, the effect of substrate concentration, time, temperature and pH on the synthesis, morphology, size distribution and surface charge of AgNPs synthesized from actinobacterial strains was evaluated.
2 Materials and methods
2.1 Actinobacterial strains
Four actinobacterial strains (OT1, OF1, OF2 and IF19) were isolated from the sediments of Lonar Crater located in Buldhana district, Maharashtra, India, by the dilution plate procedure described by Rathod et al. [21] on the starch casein agar [22] supplemented with 5% (w/v) sodium chloride (NaCl), at pH 8.5. The identification of actinobacterial strains to the closest phylogenetic neighbors based on the nucleotide sequence analysis of 16S rRNA gene was made according to method described previously by Golińska et al. [23] and Rathod et al. [21].
In the preliminary studies, the growth of actinobacterial strains for the synthesis of AgNPs was previously estimated by using different media, namely starch casein broth [22], yeast extract-malt extract broth (ISP2) [24] and halophilic nutrient broth [25], all with 5% NaCl (w/v) and pH 8.5. The most abundant growth of actinobacterial strains was recovered in halophilic nutrient broth, which was further used for the cultivation of actinobacteria.
2.2 Effect of temperature and time on the formation of AgNPs using actinobacterial strains
The biosynthesis protocol of AgNPs was described in detail by Golińska et al. [23], Rathod et al. [21] and Wypij et al. [26]. Briefly, actinobacterial strains grew in halophilic nutrient broth [25] for 7 days at 26°C. The bacterial biomass was then collected by centrifugation (6000×g for 10 min) and washed thrice with sterile, distilled water. Cell pellet was then resuspended in sterile, distilled water for 3-day autolysis at 25°C. The supernatant, after centrifugation at 6000×g for 15 min, was combined with silver nitrate (AgNO3; final concentration 0.001 mol l−1) and incubated for 3 days at different temperatures of 10, 15, 20, 25 and 35°C (±2°C) for 3 days in the darkness. The formation of AgNPs was monitored visually at the interval of 24 h by color change of the reaction mixture from colorless to yellowish-brown and ultraviolet (UV)-visible spectroscopy (Nano Drop ND2000, Thermo Scientific, USA) in a wavelength range of 200–800 nm. The efficiency of AgNP synthesis was evaluated based on their dry mass (mg 100 ml−1). Meanwhile, the effect of time on the synthesis of AgNPs synthesized from actinobacterial strains was recorded at the interval of 24 h up to 7 days.
A final concentration of AgNO3 of 0.003 mol l−1 was also used for the synthesis of biogenic AgNPs. However, the use of higher concentration of AgNO3 demonstrated an inhibitory effect on the synthesis of AgNPs.
2.3 Effect of pH on the formation of AgNPs
The actinobacterial strain was cultivated as described above. An autolysis of cell biomass was made by suspending the cells in sterile, distilled water of different pH (4, 5, 6, 7 and 8) and incubated at 25°C for 3 days. The pH of distilled water was set up at 4, 5, 6, 7 and 8 with sterile 0.2 mol l−1 HCl and 0.2 mol l−1 NaOH using a pH meter (Hanna), sterilized by filtration and used for testing. After autolysis, the cell biomass was centrifuged (6000×g, 10 min) and the supernatant was combined with silver nitrate (final concentration of 0.001 mol l−1). The reaction mixture was then incubated for 3 days in the darkness. Visual observations and UV-vis spectroscopy analysis of the biosynthesized AgNPs were performed.
The autolysis of actinobacteria was also performed in the buffers, namely citrate and phosphate, to maintain appropriate pH value (4, 5, 6, 7, and 8). However, the use of buffers had an inhibitory effect on AgNP synthesis.
The physical properties of AgNPs biosynthesized under various conditions of pH and temperature were estimated for AgNPs from the OT1 strain.
2.4 Transmission electron microscopy analysis
The size and morphology of the AgNPs from Nocardiopsis valliformis OT1 actinobacterial strain, synthesized in different pH and temperatures, were analyzed by using transmission electron microscopy (TEM) (FEI Tecnai F20 X-Twintool, Fei, Hillsboro, OR, USA) operating at an acceleration voltage of 100 kV. The samples for analysis were prepared by dropping a small amount of solution of AgNPs on a carbon-coated copper grid (400 μm mesh size). The samples were then allowed to dry at room temperature prior to measurements. The obtained data were assessed by Statistica Software (StatSoft, USA).
2.5 Nanotracking analysis
The average particle size and size distribution of AgNPs synthesized from N. valliformis OT1 strain were analyzed by using a nanotracking analysis (NTA) system LM20 (NanoSight Ltd., UK) after AgNP dilution with the nuclease-free water. 0.5 ml of the sample was injected into the sample chamber and observed through LM20.
2.6 Zeta potential analysis
The surface charge of AgNPs synthesized from N. valliformis OT1 was evaluated in a colloidal suspension of AgNPs by zeta potential measurement. The NP liquid sample was diluted 10 times with water and sonicated at 20 Hz for 15 min (Sonic Ruptor 250, Omni International, USA) to break down NP aggregates. The mixture was then filtered through a 0.22-μm millipore filter and analyzed by using Malvern Zetasizer 90 (ZS 90; Malvern Instruments Ltd., Malvern, UK).
3 Results
3.1 Molecular identification of actinobacterial strains
Nearly complete 16S rRNA gene sequences (1412, 1418, 1413 and 1402 nt) of OT1, OF1, OF2 and IF19 strains, respectively, were determined. Based on the EzBioCloud analysis [27], OT1 strain was most closely related to N. valliformis DSM 45023T (99.4%) as previously described by Rathod et al. [21]. Isolates OF1 and OF2 were found to be most closely related to Streptomyces palmae CMU-AB204T (98.23 and 98.17%, respectively), while strain IF19, to Streptomyces alkaliphilus DSM 42118T (99.71%).
3.2 Effect of time, temperature and pH on the formation of AgNPs
The absorption spectra of AgNPs biosynthesized after various incubation times are presented in Figure 1, those biosynthesized at various temperatures (10, 15, 20, 25, 35), in Figure 2, while those synthesized at different pH (4, 5, 6, 7, 8), in Figure 3.
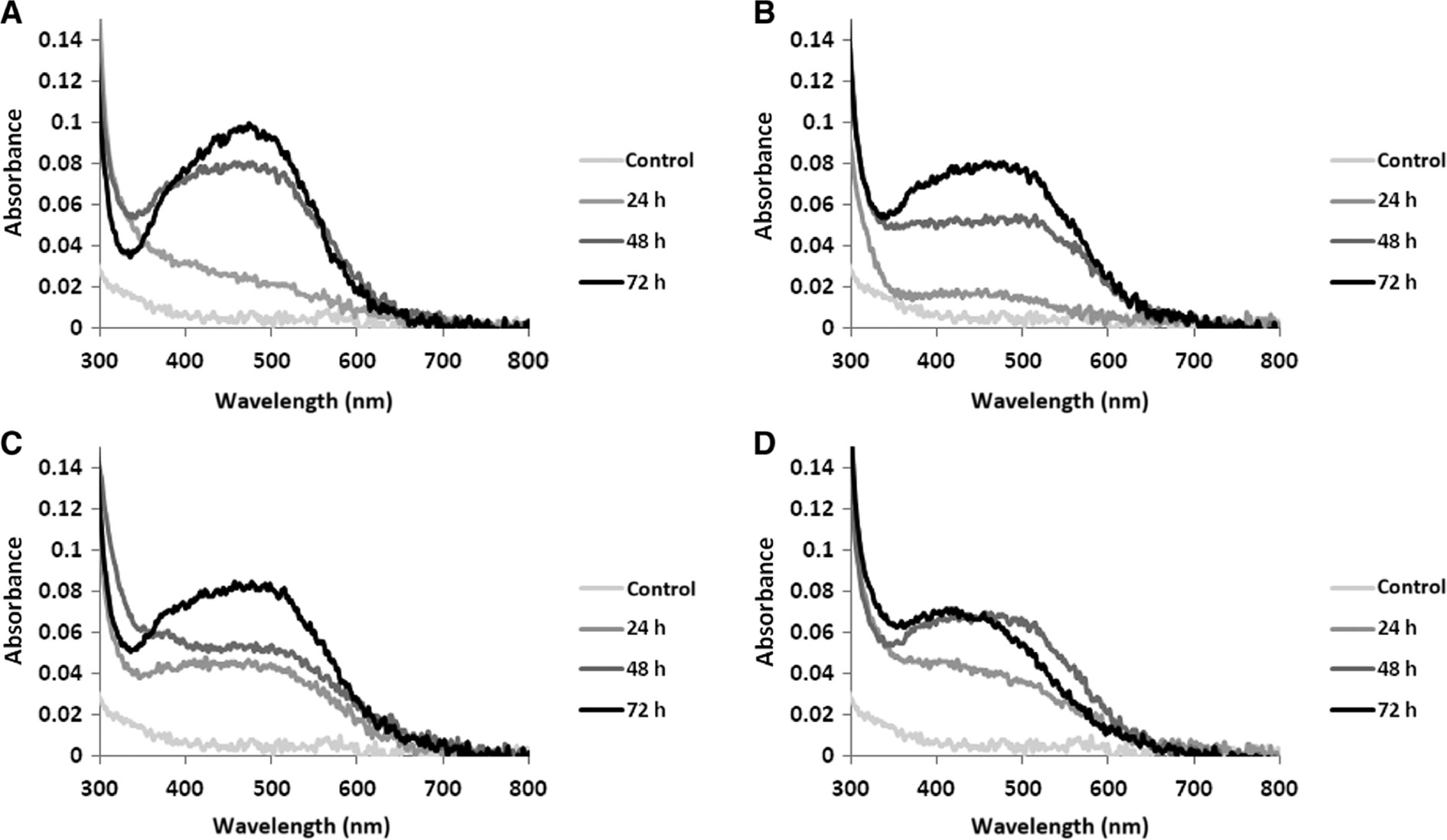
The absorption spectra of AgNPs biosynthesized after incubation time (24, 48 and 72 h) by N. valliformis OT1, S. palmae OF1, OF2 and also S. alkaliphilus IF19 strains. N. valliformis OT1 strain (A); S. palmae OF1 strain (B); S. palmae OF2 strain (C); S. alkaliphilus IF19 strain (D).
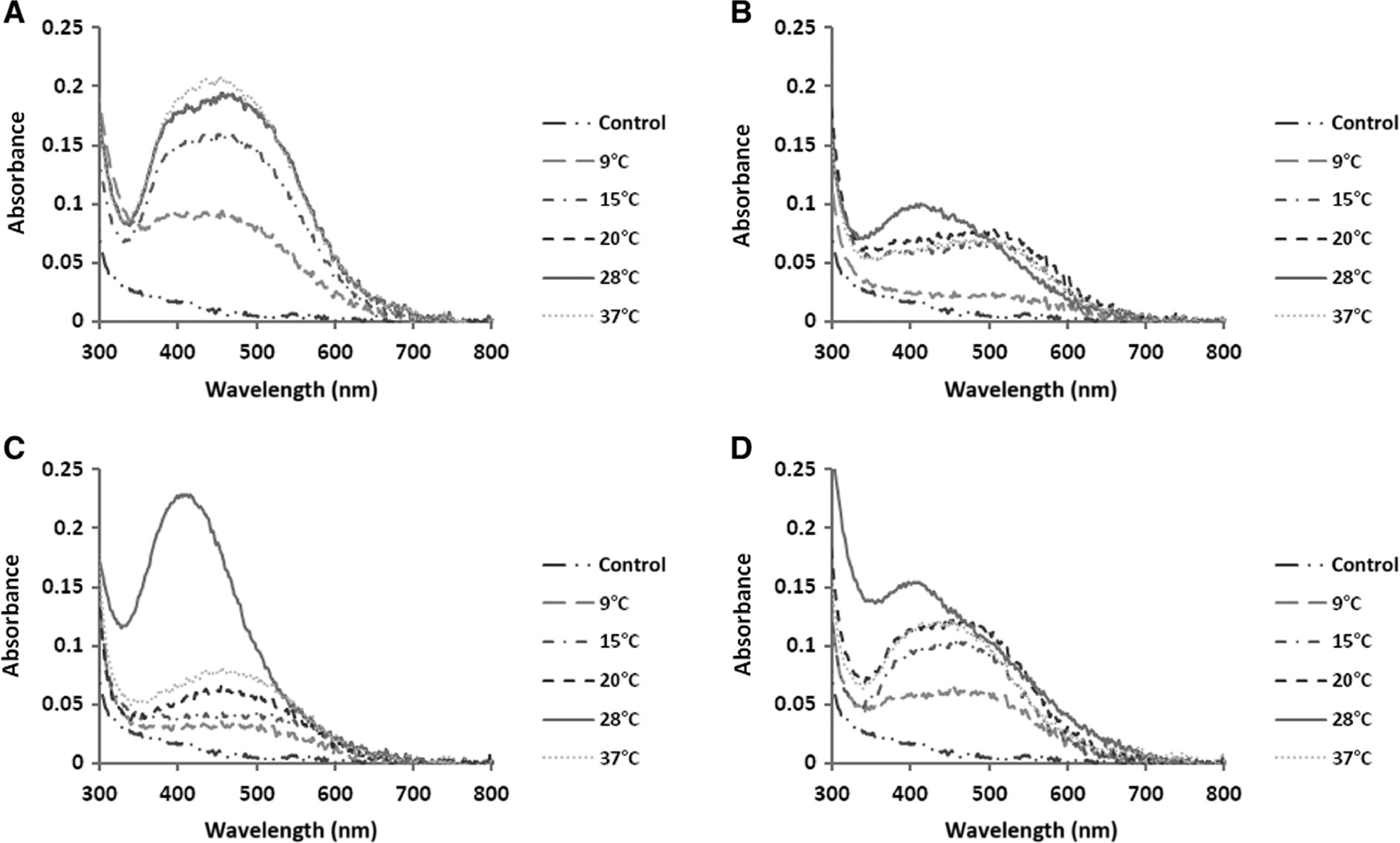
The absorption spectra of AgNPs biosynthesized under various conditions of temperature. N. valliformis OT1 strain (A); S. palmae OF1 strain (B); S. palmae OF2 strain (C); S. alkaliphilus IF19 strain (D).
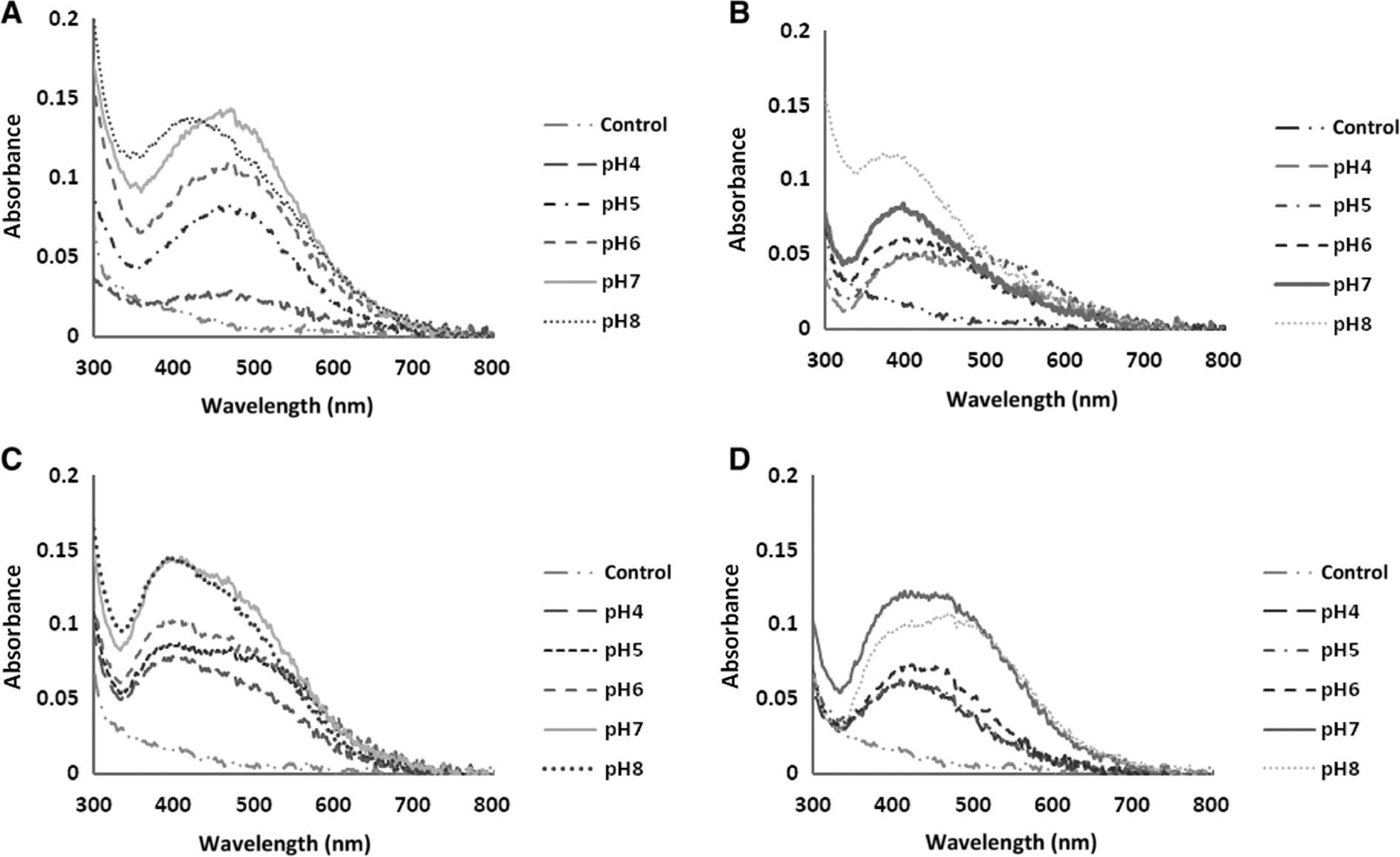
The absorption spectra of AgNPs biosynthesized under various conditions of pH. N. valliformis OT1 strain (A); S. palmae OF1 strain (B); S. palmae OF2 strain (C); S. alkaliphilus IF19 strain (D).
Time, pH and temperature were found to influence AgNP synthesis significantly. With the increase in duration of synthesis, temperature and pH, the reduction of silver salt was enhanced, as indicated by a change in the color intensity of the experimental samples (Figures 1–3).
The effects of time on the synthesis of AgNPs using N. valliformis OT1, S. palmae OF1 and OF2 and also S. alkaliphilus IF19 strains were studied visually and by UV-vis spectroscopy at an interval of 24 h. The color change in the reaction mixture and specific absorbance peaks in the range of 380–450 nm were observed after 48 and 72 h of incubation at 25°C. Moreover, absorption peak intensity increased with the increase in duration of biosynthesis process as a result of the continuous formation of AgNPs in the reaction system. The optimum time required for the completion of synthesis was found to be 72 h (Figure 1). Further extension of incubation time (up to 7 days) did not affect the synthesis process.
Generally, a broad peak of less intensity was observed after analysis of AgNPs synthesized at lower temperatures (10, 15 and 20°C). With an increase in temperature value to 25°C, the surface plasmon resonance (SPR) peaks (at wavelength about 400 nm) became sharper, which suggests that this temperature is optimal for the biosynthesis of AgNPs. Biosynthesis at a higher temperature (35°C) affected lower intensity peaks for AgNPs from OF1, OF2 and IF19 strains but not from OT1, which was similar to that recorded at 25°C (Figure 2).
The highest intensity of absorbance peaks was observed for AgNPs synthesized at alkaline pH, namely 7 and/or 8 (Figure 3).
The bands were observed in the wavelength range of 380–450 nm, which is typical for AgNPs (Figures 1–3).
Moreover, the efficiency of AgNP synthesis from N. valliformis OT1 strain after various incubation periods (24, 48 and 72 h) and under different temperatures (10, 15, 20, 25 and 35°C) and pH (4, 5, 6, 7 and 8) was evaluated based on their dry mass. The dry mass of AgNPs collected from 100 ml of experimental sample was found to be 0.0, 3.2, 6.6 and 1.0, 2.5, 3.2, 7.0, 6.0 and 2.0, 2.3, 4.0, 8.0, 8.7 mg, respectively.
3.3 Physical properties of AgNPs synthesized from N. valliformis OT1 strain under various synthesis conditions
The results of TEM measurements of NPs synthesized from N. valliformis OT1 strain under various conditions of temperature are presented in Figure 4. It was found that at temperatures below 25°C, NPs of irregular shape and bigger size were formed and showed a tendency to form aggregates. The higher aggregation of biosynthesized AgNPs was also observed after synthesis at 35°C. The spherical and smaller NPs were formed when synthesis process was performed at 25°C. The average size of AgNPs biosynthesized under different temperatures is presented in Table 1.
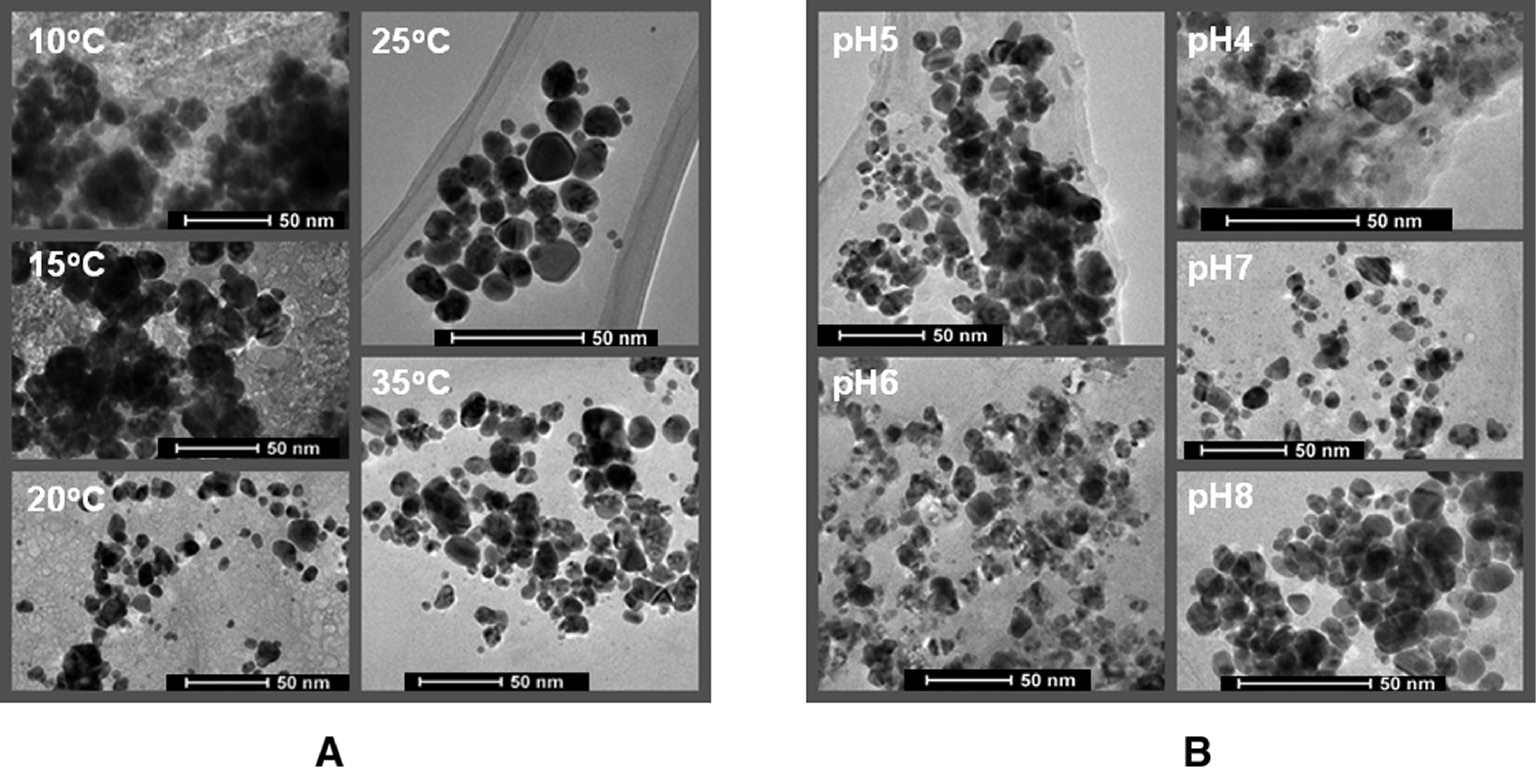
TEM analyses of AgNPs synthesized at different pH and temperature by using N. valliformis OT1 strain. Synthesis at different temperatures (A), synthesis at different pH (B).
Effect of different pH and temperatures on size of AgNPs synthesized from N. valliformis OT1 strain.
| Influent factor | Mean size of AgNPs by TEM (nm) | Mean size of AgNPs by NTA (nm) | Zeta potential (mV) |
|---|---|---|---|
| pH | |||
| 4 | 23.2 | 194.4±48.6 | −26.6 |
| 5 | 20.3 | 157.7±54.1 | −14.6 |
| 6 | 18.7 | 83.3±39.0 | −21.4 |
| 7 | 13.9 | 62.0±51.0 | −26.2 |
| 8 | 25.4 | 168.8±66.5 | −28.7 |
| Temperature (°C) | |||
| 10 | 32.6 | 153.3±39.3 | −21.4 |
| 15 | 35.1 | 143.8±73.0 | −24.6 |
| 20 | 17.1 | 145.4±136.4 | −15.0 |
| 25 | 16.5 | 62.0±51.0 | −17.1 |
| 35 | 29.8 | 179.3±57.8 | −25.0 |
The TEM analyses of AgNPs synthesized at different pH revealed that an increase in pH value resulted in the fabrication of smaller and more spherical NPs. The results of the TEM imaging confirmed those obtained from the absorption spectra. AgNPs synthesized at pH below and above neutral were irregular in shape and able to form numerous aggregates (Figure 4, Table 1). The formation of aggregates was also confirmed by NTA analysis when the biosynthesis process was performed at a pH value other than 7 (Table 1). The average size of AgNPs fabricated at different pH is presented in Table 1.
The zeta potential values of AgNPs synthesized from N. valliformis OT1 strain showed their varied stability and are presented in Table 1.
4 Discussion
The biological synthesis of nanomaterials offers better manipulation and control over crystal growth and their stabilization. This has led to an upsurge in research on the synthesis routes that allow better control of size and shape for a wide variety of nanotechnological applications [13], [28], [29].
In the present study, the effect of temperature, time, pH, incubation time and substrate concentration on the formation of NPs with desired properties such as the shape, size and distribution was considered.
The solution of experimental samples, in contrast to the control samples, was found to be intensified along with the increase in temperature, pH and incubation time that was associated with an increase in the yield of the synthesized NPs. The change in color occurs due to the excitation of free electrons in NPs, which generates the SPR. Due to SPR, a strong absorption of electromagnetic waves is exhibited by metal NPs in the visible range [[14], [30]. The SPR phenomenon arises when NPs are irradiated with visible light, because of the collective oscillations of the conduction electrons 31]. It is well known that AgNPs exhibit a yellowish-brown color in aqueous solutions due to the excitation of the SPR band in the region [32]. An increase in the intensity of the absorbance peak with time indicates the continued reduction of the silver ions and an increase in the concentration of AgNPs [[1], [33]. In the present study, an effect of duration on the synthesis of AgNPs was noticed. The longer duration of time for the synthesis resulted in a higher yield of fabricated NPs. Similar observations were reported by Amin et al. 34] and Darroudi et al. [35], who studied the synthesis of AgNPs by green approach. These authors showed that the intensity of an absorption band increased along with the duration of synthesis.
One of the interesting criteria is increasing bandwidth of resonance with the decrease in the dimensions of the particles as a result of electron scattering induction at the surface. Resonance shifting and the variation of its bandwidth are important information for NP characterization [1]. The position of the absorption peak in the visible absorption spectrum of colloidal solutions can be related to the particle size and shape due to the resonance through SPR [[36], [37]. It is claimed that the sharp narrow absorption peaks at lower wavelength region indicates the formation of smaller NPs 37].
Temperature is the basic physical factor that affects the formation of NPs as well as their shape and size [13]. In the present study, the intensity of peak maximum increases with increasing temperature, along with the shift in peak wavelength. The temperature of 25°C was the optimal temperature for the biosynthesis of AgNPs by test strain, which was confirmed by estimation of dry mass of AgNPs. Along with the decline in temperature, the absorption peaks decreased. It suggests the low concentration of biosynthesized NPs in solutions. The smallest particles were formed at optimal temperature (25°C), while below and above this value, a tendency to form bigger NPS and aggregates was observed. The increase in the average size of gold and iron oxide NPs, along with the increase in temperature, was reported by other researchers [[14], [38]. They suggested that it can be due to the higher activity of reducer enzymes at high temperature [14], [38]. In contrast, Verma and Mehata 39] reported that the size of the synthesized NPs decreases with an increase in temperature value. These authors claimed that it may be due to the faster reaction rate at higher temperatures. At high temperatures, the kinetic energy of the molecules increases and silver ions are consumed faster; thus, there is a possibility of decreased particle size growth. Quester et al. [40] studied the biosynthesis of AgNPs using the fungal extract of Neurospora crassa and observed the formation of smaller NPs (1–6 nm) when both temperature and pH values were low (4°C and pH 3), while at 25°C and pH 6 or 10, bigger NPs were formed (1–10 and 1–13 nm, respectively).
The important parameter that affects the synthesis, shape and size of NPs is the pH of the reaction solution. This factor has the ability to alter the charge of biomolecules, which may have effect on their capping as well as stabilizing properties [39].
It was found that as the pH value increased, the surface plasmon peak shifted to the left, indicating a decrease in the size of the prepared NPs. Moreover, this shift in the peak accompanied the decrease in the width of the peak, indicating size uniformity [12]. However, Verma and Mehata [39] showed that the shift in the peak wavelength indicates that the size of the particles increases with increasing pH of the solution. As the diameter of the particles get larger, the energy required for excitation of surface plasmon electrons decreases, as a result the absorption maximum shifts toward the longer wavelength region.
The present study demonstrated that the absorption intensity and amount of biosynthesized AgNPs increase along with the increase in pH value. Similar observations were reported by Sathishkumar and colleagues [41], who observed that a higher amount of AgNPs from Cinnamon zeylanicum bark extract was produced when the pH value of plant extract was higher than pH 5. Verma and Mehata [39] observed that pH 13 was the most favorable for the synthesis of AgNPs by extract of neem (Azadirachta indica) leaves. However, at such high pH, the NPs became unstable and agglomerated. In the present study, the AgNPs were slightly bigger in size and irregular in shape and exhibited greater tendency to form aggregates when the pH of the solution was lower than neutral. The tendency of AgNP agglomeration at pH > 7 was also recorded in our research. Alqadi and coauthors [12] who studied the pH effect on the aggregation of AgNPs synthesized by chemical reduction observed that NPs prepared under lower pH (7, 8 and 8.5) were mostly irregular in shape, while those formed at higher pH (10 and 11) were more regular and smaller. It was suggested that the irregularity in the shape of particles could be attributed to the slow reduction rate of the silver ions. Similar observations were also noted by other authors, for example Barabadi et al. [14] and Honary et al. [38], who studied fungal mediated preparation of gold and iron oxide NPs by using Penicillium crustosum and Penicillium waksmanii, respectively, and optimization of synthesis process by using mathematical methodology. The authors reported that the increase in the pH value of the solution significantly affected the average size of iron oxide and gold NPs. With the increase in the pH of the solution, the average size of NPs decreased [14], [38].
Moreover, the reducing and stabilizing agents such as diastase [16], tyrosine [15] and polyphenols of plant extracts [18] may affect the synthesis and size of the NPs of noble metals such as silver and gold. The biosynthesis process and size of NPs can be controllable by the change in pH of the enzyme or amino acid. It is well known that alkaline pH favors to improve the reduction potential of molecules containing several carboxylic groups [42]. Thus, the use of varied pH of diastase and tyrosine affects the synthesis and size of NPs. The authors showed that the smallest NPs of silver and gold were formed at pH 12, and the use of pH of diastase below 10 influences the aggregation of AgNPs with complete precipitation at pH 8 [17]. Similar results were observed when tyrosine mediated synthesis of AgNPs was performed [15].
The concentration of salt used for biological synthesis of NPs is an important parameter for the optimization of NP formation [19]. The authors found that out of the different concentrations of AgNO3 that they tested (0.0004, 0.0008, 0.0012, 0.0016 and 0.0024 mol l−1), 0.0016 mol l−1 AgNO3 was the optimum concentration required for the synthesis of AgNPs. In the present study, the use of 0.001 mol l−1 concentration of AgNO3 was found to be more efficient than 0.003 mol l−1 for biological synthesis of AgNPs. Other authors also found that on increasing concentration of salts used for the synthesis of gold (AuCl4) and iron oxide (FeCl3) NPs, the average size decreased but began to increase at higher concentrations. The authors suggested that the increased concentration of salt level allowed particle growth at a faster rate and that particles may aggregate in higher salt concentrations [14], [[38]. Maddinedi et al. 16] also found that a reduction in the size of gold NPs (AuNPs) was obtained with an increase in the quantity of diastase as a reducing agent. The authors explained that at a low quantity of diastase, the rate of nucleation was much higher, while the rate of coating of AuNPs with diastase was less, which resulted in the formation of bigger NPs. As the quantity of diastase increased, the rate of coating also increases when compared with nucleation rate, resulting in the formation of smaller NPs [16]. Maddinedi et al. [18] found that a higher volume of water extract of Cinnamonium tsoi leaf rich in polyphenols as a reducing agent for the preparation of AgNPs resulted in a higher reduction rate of AgNO3 to AgNPs formation.
It is well known that the stability and size of NPs are the main properties that affect their antimicrobial efficacy. Non-agglomerated and smaller NPs are desired for antimicrobial applications as they interact better with pathogenic bacteria and fungi [43].
5 Conclusions
The present study demonstrated the effect of different parameters on the biosynthesis of NPs synthesized from acinobacterial strains. The formation of AgNPs as well as their physical properties depended on reaction time, temperature pH and reactant concentration. It was found that the size and shape of the synthesized NPs can be altered by changing these parameters. Moreover, optimal temperature (25°C) and pH (7.0) required for synthesis of AgNPs were confluent with the optimal growth conditions of actinobacteria used for the synthesis of NPs, suggesting that bacterial enzymes could be involved in this process.
Acknowledgments
This study was funded by grants 2016/23/N/NZ9/00247 and 2017/01/X/NZ8/00140, Funder Id: 10.13039/501100004281 from the National Science Centre (NCN) as well as by grant no. 2844-B from Nicolaus Copernicus University.
Conflict of interest statement: The authors have no commercial or financial conflict of interest to declare.
References
[1] AbdelRahim K, Mahmoud SY, Ali AM, Almaary KS, Mustafa AE, Husseiny SM. Saudi J. Biol. Sci. 2017, 24, 208–216.10.1016/j.sjbs.2016.02.025Search in Google Scholar PubMed PubMed Central
[2] Qin X, Lu W, Luo Y, Chang G, Sun X. Electrochem. Commun. 2011, 13, 785–787.10.1016/j.elecom.2011.05.002Search in Google Scholar
[3] Zhang Y, Wang L, Tian J, Li H, Luo Y, Sun X. Langmuir 2011, 27, 2170–2175.10.1021/la105092fSearch in Google Scholar PubMed
[4] Barabadi H, Ovais M, Shinwari ZK, Saravanan M. Green. Chem. Lett. 2017, 10, 285–314.10.1080/17518253.2017.1385856Search in Google Scholar
[5] Khan I, Saeed K, Khan I. Arabian J. Chem. 2017, doi.org/10.1016/j.arabjc.2017.05.011.org/10.1016/j.arabjc.2017.05.011Search in Google Scholar
[6] Gandhi H, Khan S. J. Nanomed. Nanotechnol. 2016, 7, 1–3.Search in Google Scholar
[7] Golińska P, Wypij M, Ingle AP, Gupta I, Dahm H, Rai M. Appl. Microbiol. Biotechnol. 2014, 98, 8083–8097.10.1007/s00253-014-5953-7Search in Google Scholar PubMed
[8] Khan AU, Malik N, Khan M, Cho MH, Khan MM. Bioprocess Biosyst. Eng. 2018, 41, 1–20.10.1007/s00449-017-1846-3Search in Google Scholar PubMed
[9] Neihaya HZ, Zaman HH. Microb. Pathog. 2018, 16, 200–208.10.1016/j.micpath.2018.01.024Search in Google Scholar PubMed
[10] Wypij M, Czarnecka J, Świecimska M, Dahm H, Rai M, Golinska P. World J. Microbiol. Biotechnol. 2018, 34, 23–35.10.1007/s11274-017-2406-3Search in Google Scholar PubMed PubMed Central
[11] Yasir M, Singh J, Tripathi MK, Singh P, Shrivastava R. Pharmacogn. Mag. 2018, 4, 840–844.Search in Google Scholar
[12] Alqadi MK, Abo Noqtah OA, Alzoubi FY, Alzouby J, Aljarrah K. Mater. Sci. Poland 2014, 32, 107–111.10.2478/s13536-013-0166-9Search in Google Scholar
[13] Prasad R. J. Nanoparticles 2014, 2014, 1–8.10.1155/2014/963961Search in Google Scholar
[14] Barabadi H, Honary S, Ebrahimi P, Mahammadi MA, Alizadeh A, Naghibi F. Brazilian J. Microbiol. 2014, 45, 1493–1501.10.1590/S1517-83822014000400046Search in Google Scholar
[15] Maddinedi SB, Mandal BK, Anna KK. Environ. Toxicol. Pharmacol. 2017, 51, 23–29.10.1016/j.etap.2017.02.020Search in Google Scholar PubMed
[16] Maddinedi SB, Mandal BK, Ranjan S, Dasgupta N. RCS. Adv. 2015, 5, 26727–26733.10.1039/C5RA03117FSearch in Google Scholar
[17] Maddinedi SB, Mandal BK, Anna KK. Environ. Toxicol. Pharmacol. 2017, 49, 131–136.10.1016/j.etap.2016.11.019Search in Google Scholar PubMed
[18] Maddinedi SB, Mandal BK, Maddili SK. J. Photochem. Photobiol. B: Biology 2017, 167, 236–241.10.1016/j.jphotobiol.2017.01.003Search in Google Scholar PubMed
[19] Subbaiya R, Saravanan M, Priya AR, Shankar KR, Selvam M, Ovais M, Balajee R, Barabadi H. IET Nanobiotechnol. 2017, 11, 965–972.10.1049/iet-nbt.2016.0222Search in Google Scholar PubMed PubMed Central
[20] Xie J, Zheng Y, Ying JY. J. Am. Chem. Soc. 2009, 131, 888–889.10.1021/ja806804uSearch in Google Scholar PubMed
[21] Rathod D, Golinska P, Wypij M, Dahm H, Rai M. Med. Microbiol. Immunol. 2016, 205, 435–447.10.1007/s00430-016-0462-1Search in Google Scholar PubMed PubMed Central
[22] Kűster E, Williams ST. Nature 1964, 202, 928–929.10.1038/202928a0Search in Google Scholar PubMed
[23] Golińska P, Wypij M, Rathod D, Dahm H, Rai M. J. Basic Microbiol. 2015, 55, 1–16.10.1002/jobm.201470403Search in Google Scholar PubMed
[24] Shirling EB, Gottlieb D. Int. J. Syst. Bacteriol. 1966, 16, 313–340.10.1099/00207713-16-3-313Search in Google Scholar
[25] Atlas, RM, Ed., Handbook of Microbiological Media, 4th ed., CRC Press: Boston, 2010.10.1201/EBK1439804063Search in Google Scholar
[26] Wypij M, Golinska P, Dahm H, Rai M. IET Nanobiotechnol. 2017, 11, 336–342.10.1049/iet-nbt.2016.0112Search in Google Scholar PubMed PubMed Central
[27] Yoon SH, Ha SM, Kwon S, Lim J, Kim Y, Seo H, Chun J. Int. J. Syst. Evol. Microbiol. 2017, 67, 1613–1617.10.1099/ijsem.0.001755Search in Google Scholar PubMed PubMed Central
[28] Saifuddin N, Wong CW, Yasumira AAN. E-J Chem. 2009, 6, 61–70.10.1155/2009/734264Search in Google Scholar
[29] Verma VC, Kharwar RN, Gange AC. Nanomedicine 2010, 5, 33–40.10.2217/nnm.09.77Search in Google Scholar PubMed
[30] Roy K, Biswas S, Banaejee PC. Res. J. Phar. Bio. Chem. Sci. 2013, 4, 1271–1278.Search in Google Scholar
[31] Samanta S, Sarkar P, Pyne S, Prasad Sahoo G, Misra A. J. Mol. Liq. 2012, 165, 21–26.10.1016/j.molliq.2011.10.002Search in Google Scholar
[32] Parameshwaran R, Kalaiselvam S, Jayavel R. Mat. Chem. Phys. 2013, 140, 135–147.10.1016/j.matchemphys.2013.03.012Search in Google Scholar
[33] Birla S, Gaikwad S, Gade A, Rai M. Sci. World J. 2013, 2013, 1–12.10.1155/2013/796018Search in Google Scholar
[34] Amin M, Anwar F, Janjua MRSA, Iqbal MA, Rashid U. Int. J. Mol. Sci. 2012, 13, 9923–9941.10.3390/ijms13089923Search in Google Scholar PubMed PubMed Central
[35] Darroudi M, Ahmad MB, Zamiri R, Zak AK, Abdullah AH, Ibrahim NA. Int. J. Nanomedicine 2011, 6, 677–681.10.2147/IJN.S17669Search in Google Scholar PubMed PubMed Central
[36] Durán N, Marcato PD, Alves OL, Souza GI, Esposito E. J. Nanobiotechnol. 2005, 3, 8.10.1186/1477-3155-3-8Search in Google Scholar PubMed PubMed Central
[37] Ibrahim HM. J. Radiat. Res. Appl. Sci. 2015, 8, 265–275.10.1016/j.jrras.2015.01.007Search in Google Scholar
[38] Honary S, Barabadi H, Ebrahimi P, Naghibi F, Alizadeh A. J. Nano. Res. 2015, 30, 106–115.10.4028/www.scientific.net/JNanoR.30.106Search in Google Scholar
[39] Verma A, Mehata MS. J. Radiat. Res. Appl. Sci. 2016, 9, 109–115.10.1016/j.jrras.2015.11.001Search in Google Scholar
[40] Quester K, Avalos-Borja M, Castro-Longoria E. J. Biomater. Nanobiotechnol. 2016, 7, 118–125.10.4236/jbnb.2016.72013Search in Google Scholar
[41] Sathishkumar M, Sneha K, Won SW, Cho CW, Kim S, Yun S. Coll. Surf. B: Biointerfaces 2009, 73, 332–338.10.1016/j.colsurfb.2009.06.005Search in Google Scholar PubMed
[42] Ashraf S, Abbasi AZ, Pfeiffer C, Hussain SZ, Khalid ZM, Gil PR, Parak WJ, Hussain I. Coll. Surf. B: Biointerfaces 2013, 102, 511–518.10.1016/j.colsurfb.2012.09.032Search in Google Scholar PubMed
[43] Morones JR, Elechiguerra JL, Camacho A, Holt K. Nanotechnology 2005, 16, 2346–2353.10.1088/0957-4484/16/10/059Search in Google Scholar PubMed
©2019 Walter de Gruyter GmbH, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 Public License.
Articles in the same Issue
- Regular Articles
- Studies on the preparation and properties of biodegradable polyester from soybean oil
- Flow-mode biodiesel production from palm oil using a pressurized microwave reactor
- Reduction of free fatty acids in waste oil for biodiesel production by glycerolysis: investigation and optimization of process parameters
- Saccharin: a cheap and mild acidic agent for the synthesis of azo dyes via telescoped dediazotization
- Optimization of lipase-catalyzed synthesis of polyethylene glycol stearate in a solvent-free system
- Green synthesis of iron oxide nanoparticles using Platanus orientalis leaf extract for antifungal activity
- Ultrasound assisted chemical activation of peanut husk for copper removal
- Room temperature silanization of Fe3O4 for the preparation of phenyl functionalized magnetic adsorbent for dispersive solid phase extraction for the extraction of phthalates in water
- Evaluation of the saponin green extraction from Ziziphus spina-christi leaves using hydrothermal, microwave and Bain-Marie water bath heating methods
- Oxidation of dibenzothiophene using the heterogeneous catalyst of tungsten-based carbon nanotubes
- Calcined sodium silicate as an efficient and benign heterogeneous catalyst for the transesterification of natural lecithin to L-α-glycerophosphocholine
- Synergistic effect between CO2 and H2O2 on ethylbenzene oxidation catalyzed by carbon supported heteropolyanion catalysts
- Hydrocyanation of 2-arylmethyleneindan-1,3-diones using potassium hexacyanoferrate(II) as a nontoxic cyanating agent
- Green synthesis of hydratropic aldehyde from α-methylstyrene catalyzed by Al2O3-supported metal phthalocyanines
- Environmentally benign chemical recycling of polycarbonate wastes: comparison of micro- and nano-TiO2 solid support efficiencies
- Medicago polymorpha-mediated antibacterial silver nanoparticles in the reduction of methyl orange
- Production of value-added chemicals from esterification of waste glycerol over MCM-41 supported catalysts
- Green synthesis of zerovalent copper nanoparticles for efficient reduction of toxic azo dyes congo red and methyl orange
- Optimization of the biological synthesis of silver nanoparticles using Penicillium oxalicum GRS-1 and their antimicrobial effects against common food-borne pathogens
- Optimization of submerged fermentation conditions to overproduce bioethanol using two industrial and traditional Saccharomyces cerevisiae strains
- Extraction of In3+ and Fe3+ from sulfate solutions by using a 3D-printed “Y”-shaped microreactor
- Foliar-mediated Ag:ZnO nanophotocatalysts: green synthesis, characterization, pollutants degradation, and in vitro biocidal activity
- Green cyclic acetals production by glycerol etherification reaction with benzaldehyde using cationic acidic resin
- Biosynthesis, characterization and antimicrobial activities assessment of fabricated selenium nanoparticles using Pelargonium zonale leaf extract
- Synthesis of high surface area magnesia by using walnut shell as a template
- Controllable biosynthesis of silver nanoparticles using actinobacterial strains
- Green vegetation: a promising source of color dyes
- Mechano-chemical synthesis of ammonia and acetic acid from inorganic materials in water
- Green synthesis and structural characterization of novel N1-substituted 3,4-dihydropyrimidin-2(1H)-ones
- Biodiesel production from cotton oil using heterogeneous CaO catalysts from eggshells prepared at different calcination temperatures
- Regeneration of spent mercury catalyst for the treatment of dye wastewater by the microwave and ultrasonic spray-assisted method
- Green synthesis of the innovative super paramagnetic nanoparticles from the leaves extract of Fraxinus chinensis Roxb and their application for the decolourisation of toxic dyes
- Biogenic ZnO nanoparticles: a study of blueshift of optical band gap and photocatalytic degradation of reactive yellow 186 dye under direct sunlight
- Leached compounds from the extracts of pomegranate peel, green coconut shell, and karuvelam wood for the removal of hexavalent chromium
- Enhancement of molecular weight reduction of natural rubber in triphasic CO2/toluene/H2O systems with hydrogen peroxide for preparation of biobased polyurethanes
- An efficient green synthesis of novel 1H-imidazo[1,2-a]imidazole-3-amine and imidazo[2,1-c][1,2,4]triazole-5-amine derivatives via Strecker reaction under controlled microwave heating
- Evaluation of three different green fabrication methods for the synthesis of crystalline ZnO nanoparticles using Pelargonium zonale leaf extract
- A highly efficient and multifunctional biomass supporting Ag, Ni, and Cu nanoparticles through wetness impregnation for environmental remediation
- Simple one-pot green method for large-scale production of mesalamine, an anti-inflammatory agent
- Relationships between step and cumulative PMI and E-factors: implications on estimating material efficiency with respect to charting synthesis optimization strategies
- A comparative sorption study of Cr3+ and Cr6+ using mango peels: kinetic, equilibrium and thermodynamic
- Effects of acid hydrolysis waste liquid recycle on preparation of microcrystalline cellulose
- Use of deep eutectic solvents as catalyst: A mini-review
- Microwave-assisted synthesis of pyrrolidinone derivatives using 1,1’-butylenebis(3-sulfo-3H-imidazol-1-ium) chloride in ethylene glycol
- Green and eco-friendly synthesis of Co3O4 and Ag-Co3O4: Characterization and photo-catalytic activity
- Adsorption optimized of the coal-based material and application for cyanide wastewater treatment
- Aloe vera leaf extract mediated green synthesis of selenium nanoparticles and assessment of their In vitro antimicrobial activity against spoilage fungi and pathogenic bacteria strains
- Waste phenolic resin derived activated carbon by microwave-assisted KOH activation and application to dye wastewater treatment
- Direct ethanol production from cellulose by consortium of Trichoderma reesei and Candida molischiana
- Agricultural waste biomass-assisted nanostructures: Synthesis and application
- Biodiesel production from rubber seed oil using calcium oxide derived from eggshell as catalyst – optimization and modeling studies
- Study of fabrication of fully aqueous solution processed SnS quantum dot-sensitized solar cell
- Assessment of aqueous extract of Gypsophila aretioides for inhibitory effects on calcium carbonate formation
- An environmentally friendly acylation reaction of 2-methylnaphthalene in solvent-free condition in a micro-channel reactor
- Aegle marmelos phytochemical stabilized synthesis and characterization of ZnO nanoparticles and their role against agriculture and food pathogen
- A reactive coupling process for co-production of solketal and biodiesel
- Optimization of the asymmetric synthesis of (S)-1-phenylethanol using Ispir bean as whole-cell biocatalyst
- Synthesis of pyrazolopyridine and pyrazoloquinoline derivatives by one-pot, three-component reactions of arylglyoxals, 3-methyl-1-aryl-1H-pyrazol-5-amines and cyclic 1,3-dicarbonyl compounds in the presence of tetrapropylammonium bromide
- Preconcentration of morphine in urine sample using a green and solvent-free microextraction method
- Extraction of glycyrrhizic acid by aqueous two-phase system formed by PEG and two environmentally friendly organic acid salts - sodium citrate and sodium tartrate
- Green synthesis of copper oxide nanoparticles using Juglans regia leaf extract and assessment of their physico-chemical and biological properties
- Deep eutectic solvents (DESs) as powerful and recyclable catalysts and solvents for the synthesis of 3,4-dihydropyrimidin-2(1H)-ones/thiones
- Biosynthesis, characterization and anti-microbial activity of silver nanoparticle based gel hand wash
- Efficient and selective microwave-assisted O-methylation of phenolic compounds using tetramethylammonium hydroxide (TMAH)
- Anticoagulant, thrombolytic and antibacterial activities of Euphorbia acruensis latex-mediated bioengineered silver nanoparticles
- Volcanic ash as reusable catalyst in the green synthesis of 3H-1,5-benzodiazepines
- Green synthesis, anionic polymerization of 1,4-bis(methacryloyl)piperazine using Algerian clay as catalyst
- Selenium supplementation during fermentation with sugar beet molasses and Saccharomyces cerevisiae to increase bioethanol production
- Biosynthetic potential assessment of four food pathogenic bacteria in hydrothermally silver nanoparticles fabrication
- Investigating the effectiveness of classical and eco-friendly approaches for synthesis of dialdehydes from organic dihalides
- Pyrolysis of palm oil using zeolite catalyst and characterization of the boil-oil
- Azadirachta indica leaves extract assisted green synthesis of Ag-TiO2 for degradation of Methylene blue and Rhodamine B dyes in aqueous medium
- Synthesis of vitamin E succinate catalyzed by nano-SiO2 immobilized DMAP derivative in mixed solvent system
- Extraction of phytosterols from melon (Cucumis melo) seeds by supercritical CO2 as a clean technology
- Production of uronic acids by hydrothermolysis of pectin as a model substance for plant biomass waste
- Biofabrication of highly pure copper oxide nanoparticles using wheat seed extract and their catalytic activity: A mechanistic approach
- Intelligent modeling and optimization of emulsion aggregation method for producing green printing ink
- Improved removal of methylene blue on modified hierarchical zeolite Y: Achieved by a “destructive-constructive” method
- Two different facile and efficient approaches for the synthesis of various N-arylacetamides via N-acetylation of arylamines and straightforward one-pot reductive acetylation of nitroarenes promoted by recyclable CuFe2O4 nanoparticles in water
- Optimization of acid catalyzed esterification and mixed metal oxide catalyzed transesterification for biodiesel production from Moringa oleifera oil
- Kinetics and the fluidity of the stearic acid esters with different carbon backbones
- Aiming for a standardized protocol for preparing a process green synthesis report and for ranking multiple synthesis plans to a common target product
- Microstructure and luminescence of VO2 (B) nanoparticle synthesis by hydrothermal method
- Optimization of uranium removal from uranium plant wastewater by response surface methodology (RSM)
- Microwave drying of nickel-containing residue: dielectric properties, kinetics, and energy aspects
- Simple and convenient two step synthesis of 5-bromo-2,3-dimethoxy-6-methyl-1,4-benzoquinone
- Biodiesel production from waste cooking oil
- The effect of activation temperature on structure and properties of blue coke-based activated carbon by CO2 activation
- Optimization of reaction parameters for the green synthesis of zero valent iron nanoparticles using pine tree needles
- Microwave-assisted protocol for squalene isolation and conversion from oil-deodoriser distillates
- Denitrification performance of rare earth tailings-based catalysts
- Facile synthesis of silver nanoparticles using Averrhoa bilimbi L and Plum extracts and investigation on the synergistic bioactivity using in vitro models
- Green production of AgNPs and their phytostimulatory impact
- Photocatalytic activity of Ag/Ni bi-metallic nanoparticles on textile dye removal
- Topical Issue: Green Process Engineering / Guest Editors: Martine Poux, Patrick Cognet
- Modelling and optimisation of oxidative desulphurisation of tyre-derived oil via central composite design approach
- CO2 sequestration by carbonation of olivine: a new process for optimal separation of the solids produced
- Organic carbonates synthesis improved by pervaporation for CO2 utilisation
- Production of starch nanoparticles through solvent-antisolvent precipitation in a spinning disc reactor
- A kinetic study of Zn halide/TBAB-catalysed fixation of CO2 with styrene oxide in propylene carbonate
- Topical on Green Process Engineering
Articles in the same Issue
- Regular Articles
- Studies on the preparation and properties of biodegradable polyester from soybean oil
- Flow-mode biodiesel production from palm oil using a pressurized microwave reactor
- Reduction of free fatty acids in waste oil for biodiesel production by glycerolysis: investigation and optimization of process parameters
- Saccharin: a cheap and mild acidic agent for the synthesis of azo dyes via telescoped dediazotization
- Optimization of lipase-catalyzed synthesis of polyethylene glycol stearate in a solvent-free system
- Green synthesis of iron oxide nanoparticles using Platanus orientalis leaf extract for antifungal activity
- Ultrasound assisted chemical activation of peanut husk for copper removal
- Room temperature silanization of Fe3O4 for the preparation of phenyl functionalized magnetic adsorbent for dispersive solid phase extraction for the extraction of phthalates in water
- Evaluation of the saponin green extraction from Ziziphus spina-christi leaves using hydrothermal, microwave and Bain-Marie water bath heating methods
- Oxidation of dibenzothiophene using the heterogeneous catalyst of tungsten-based carbon nanotubes
- Calcined sodium silicate as an efficient and benign heterogeneous catalyst for the transesterification of natural lecithin to L-α-glycerophosphocholine
- Synergistic effect between CO2 and H2O2 on ethylbenzene oxidation catalyzed by carbon supported heteropolyanion catalysts
- Hydrocyanation of 2-arylmethyleneindan-1,3-diones using potassium hexacyanoferrate(II) as a nontoxic cyanating agent
- Green synthesis of hydratropic aldehyde from α-methylstyrene catalyzed by Al2O3-supported metal phthalocyanines
- Environmentally benign chemical recycling of polycarbonate wastes: comparison of micro- and nano-TiO2 solid support efficiencies
- Medicago polymorpha-mediated antibacterial silver nanoparticles in the reduction of methyl orange
- Production of value-added chemicals from esterification of waste glycerol over MCM-41 supported catalysts
- Green synthesis of zerovalent copper nanoparticles for efficient reduction of toxic azo dyes congo red and methyl orange
- Optimization of the biological synthesis of silver nanoparticles using Penicillium oxalicum GRS-1 and their antimicrobial effects against common food-borne pathogens
- Optimization of submerged fermentation conditions to overproduce bioethanol using two industrial and traditional Saccharomyces cerevisiae strains
- Extraction of In3+ and Fe3+ from sulfate solutions by using a 3D-printed “Y”-shaped microreactor
- Foliar-mediated Ag:ZnO nanophotocatalysts: green synthesis, characterization, pollutants degradation, and in vitro biocidal activity
- Green cyclic acetals production by glycerol etherification reaction with benzaldehyde using cationic acidic resin
- Biosynthesis, characterization and antimicrobial activities assessment of fabricated selenium nanoparticles using Pelargonium zonale leaf extract
- Synthesis of high surface area magnesia by using walnut shell as a template
- Controllable biosynthesis of silver nanoparticles using actinobacterial strains
- Green vegetation: a promising source of color dyes
- Mechano-chemical synthesis of ammonia and acetic acid from inorganic materials in water
- Green synthesis and structural characterization of novel N1-substituted 3,4-dihydropyrimidin-2(1H)-ones
- Biodiesel production from cotton oil using heterogeneous CaO catalysts from eggshells prepared at different calcination temperatures
- Regeneration of spent mercury catalyst for the treatment of dye wastewater by the microwave and ultrasonic spray-assisted method
- Green synthesis of the innovative super paramagnetic nanoparticles from the leaves extract of Fraxinus chinensis Roxb and their application for the decolourisation of toxic dyes
- Biogenic ZnO nanoparticles: a study of blueshift of optical band gap and photocatalytic degradation of reactive yellow 186 dye under direct sunlight
- Leached compounds from the extracts of pomegranate peel, green coconut shell, and karuvelam wood for the removal of hexavalent chromium
- Enhancement of molecular weight reduction of natural rubber in triphasic CO2/toluene/H2O systems with hydrogen peroxide for preparation of biobased polyurethanes
- An efficient green synthesis of novel 1H-imidazo[1,2-a]imidazole-3-amine and imidazo[2,1-c][1,2,4]triazole-5-amine derivatives via Strecker reaction under controlled microwave heating
- Evaluation of three different green fabrication methods for the synthesis of crystalline ZnO nanoparticles using Pelargonium zonale leaf extract
- A highly efficient and multifunctional biomass supporting Ag, Ni, and Cu nanoparticles through wetness impregnation for environmental remediation
- Simple one-pot green method for large-scale production of mesalamine, an anti-inflammatory agent
- Relationships between step and cumulative PMI and E-factors: implications on estimating material efficiency with respect to charting synthesis optimization strategies
- A comparative sorption study of Cr3+ and Cr6+ using mango peels: kinetic, equilibrium and thermodynamic
- Effects of acid hydrolysis waste liquid recycle on preparation of microcrystalline cellulose
- Use of deep eutectic solvents as catalyst: A mini-review
- Microwave-assisted synthesis of pyrrolidinone derivatives using 1,1’-butylenebis(3-sulfo-3H-imidazol-1-ium) chloride in ethylene glycol
- Green and eco-friendly synthesis of Co3O4 and Ag-Co3O4: Characterization and photo-catalytic activity
- Adsorption optimized of the coal-based material and application for cyanide wastewater treatment
- Aloe vera leaf extract mediated green synthesis of selenium nanoparticles and assessment of their In vitro antimicrobial activity against spoilage fungi and pathogenic bacteria strains
- Waste phenolic resin derived activated carbon by microwave-assisted KOH activation and application to dye wastewater treatment
- Direct ethanol production from cellulose by consortium of Trichoderma reesei and Candida molischiana
- Agricultural waste biomass-assisted nanostructures: Synthesis and application
- Biodiesel production from rubber seed oil using calcium oxide derived from eggshell as catalyst – optimization and modeling studies
- Study of fabrication of fully aqueous solution processed SnS quantum dot-sensitized solar cell
- Assessment of aqueous extract of Gypsophila aretioides for inhibitory effects on calcium carbonate formation
- An environmentally friendly acylation reaction of 2-methylnaphthalene in solvent-free condition in a micro-channel reactor
- Aegle marmelos phytochemical stabilized synthesis and characterization of ZnO nanoparticles and their role against agriculture and food pathogen
- A reactive coupling process for co-production of solketal and biodiesel
- Optimization of the asymmetric synthesis of (S)-1-phenylethanol using Ispir bean as whole-cell biocatalyst
- Synthesis of pyrazolopyridine and pyrazoloquinoline derivatives by one-pot, three-component reactions of arylglyoxals, 3-methyl-1-aryl-1H-pyrazol-5-amines and cyclic 1,3-dicarbonyl compounds in the presence of tetrapropylammonium bromide
- Preconcentration of morphine in urine sample using a green and solvent-free microextraction method
- Extraction of glycyrrhizic acid by aqueous two-phase system formed by PEG and two environmentally friendly organic acid salts - sodium citrate and sodium tartrate
- Green synthesis of copper oxide nanoparticles using Juglans regia leaf extract and assessment of their physico-chemical and biological properties
- Deep eutectic solvents (DESs) as powerful and recyclable catalysts and solvents for the synthesis of 3,4-dihydropyrimidin-2(1H)-ones/thiones
- Biosynthesis, characterization and anti-microbial activity of silver nanoparticle based gel hand wash
- Efficient and selective microwave-assisted O-methylation of phenolic compounds using tetramethylammonium hydroxide (TMAH)
- Anticoagulant, thrombolytic and antibacterial activities of Euphorbia acruensis latex-mediated bioengineered silver nanoparticles
- Volcanic ash as reusable catalyst in the green synthesis of 3H-1,5-benzodiazepines
- Green synthesis, anionic polymerization of 1,4-bis(methacryloyl)piperazine using Algerian clay as catalyst
- Selenium supplementation during fermentation with sugar beet molasses and Saccharomyces cerevisiae to increase bioethanol production
- Biosynthetic potential assessment of four food pathogenic bacteria in hydrothermally silver nanoparticles fabrication
- Investigating the effectiveness of classical and eco-friendly approaches for synthesis of dialdehydes from organic dihalides
- Pyrolysis of palm oil using zeolite catalyst and characterization of the boil-oil
- Azadirachta indica leaves extract assisted green synthesis of Ag-TiO2 for degradation of Methylene blue and Rhodamine B dyes in aqueous medium
- Synthesis of vitamin E succinate catalyzed by nano-SiO2 immobilized DMAP derivative in mixed solvent system
- Extraction of phytosterols from melon (Cucumis melo) seeds by supercritical CO2 as a clean technology
- Production of uronic acids by hydrothermolysis of pectin as a model substance for plant biomass waste
- Biofabrication of highly pure copper oxide nanoparticles using wheat seed extract and their catalytic activity: A mechanistic approach
- Intelligent modeling and optimization of emulsion aggregation method for producing green printing ink
- Improved removal of methylene blue on modified hierarchical zeolite Y: Achieved by a “destructive-constructive” method
- Two different facile and efficient approaches for the synthesis of various N-arylacetamides via N-acetylation of arylamines and straightforward one-pot reductive acetylation of nitroarenes promoted by recyclable CuFe2O4 nanoparticles in water
- Optimization of acid catalyzed esterification and mixed metal oxide catalyzed transesterification for biodiesel production from Moringa oleifera oil
- Kinetics and the fluidity of the stearic acid esters with different carbon backbones
- Aiming for a standardized protocol for preparing a process green synthesis report and for ranking multiple synthesis plans to a common target product
- Microstructure and luminescence of VO2 (B) nanoparticle synthesis by hydrothermal method
- Optimization of uranium removal from uranium plant wastewater by response surface methodology (RSM)
- Microwave drying of nickel-containing residue: dielectric properties, kinetics, and energy aspects
- Simple and convenient two step synthesis of 5-bromo-2,3-dimethoxy-6-methyl-1,4-benzoquinone
- Biodiesel production from waste cooking oil
- The effect of activation temperature on structure and properties of blue coke-based activated carbon by CO2 activation
- Optimization of reaction parameters for the green synthesis of zero valent iron nanoparticles using pine tree needles
- Microwave-assisted protocol for squalene isolation and conversion from oil-deodoriser distillates
- Denitrification performance of rare earth tailings-based catalysts
- Facile synthesis of silver nanoparticles using Averrhoa bilimbi L and Plum extracts and investigation on the synergistic bioactivity using in vitro models
- Green production of AgNPs and their phytostimulatory impact
- Photocatalytic activity of Ag/Ni bi-metallic nanoparticles on textile dye removal
- Topical Issue: Green Process Engineering / Guest Editors: Martine Poux, Patrick Cognet
- Modelling and optimisation of oxidative desulphurisation of tyre-derived oil via central composite design approach
- CO2 sequestration by carbonation of olivine: a new process for optimal separation of the solids produced
- Organic carbonates synthesis improved by pervaporation for CO2 utilisation
- Production of starch nanoparticles through solvent-antisolvent precipitation in a spinning disc reactor
- A kinetic study of Zn halide/TBAB-catalysed fixation of CO2 with styrene oxide in propylene carbonate
- Topical on Green Process Engineering

