Abstract
In this report, we present a simple and unexplored procedure for green synthesis of silver nanoparticles featuring exudation of Euphorbia acruensis along with the study of its antibacterial and anticoagulant properties. Analytical techniques like ultraviolet visible spectroscopy (UV-Vis), X-ray diffraction (XRD) and high resolution transmission electron microscopy (HRTEM) were used to analyse the production, crystallinity and morphology of bio-reduced silver nanoparticles. The antibacterial study was performed by following standard disc diffusion method. Most importantly, the anticoagulant and thrombolytic activities of biogenic silver nanoparticles were evaluated by addition of nanoparticles to human blood samples under practical conditions. These green synthesized silver nanoparticles were found to have potent antibacterial, anticoagulant and thrombolytic properties which make them an attractive choice for future medical applications.
1 Introduction
Research in the field of green synthesis of nanoparticles continues to expand because of the abundance of biomaterials in nature, which are potent source of organic reductants for eco-benign production of nanoparticles. The green procedures avoid the use of harmful and expensive chemicals for production of nanomaterials unlike chemical routes and the biogenic nanoparticles have shown considerable degree of biocompatibility with low toxicity [1,2]. In this context, several natural bio-products extracted from plants, micro-organisms and others have already been used for green production of nanomaterials [3, 4, 5]. Among different metallic nanoparticles developed so far, nanoparticles of silver have been the most anticipated one due to its diverse applications in the fields of optoelectronics, environmental remediation, electrochemistry and biomedicine as well [6, 7, 8]. Antibacterial and antifungal activities are the most investigated biological properties of silver nanoparticles till date [9,10]. But other biochemical properties of silver nanoparticles like anticoagulant and thrombolytic activities can also be explored to assess their potentiality in the field of haematology.
Euphorbia acruensis is a succulent plant (Figure 1) of Euphorbiaceae family and found in Africa, America and parts of South East Asia. It exudes milky white latex that contains active bio-reductants and capping agents like aldehydes, amines and aromatics [11]. This study focused on the use of E. acruensis latex for bio-reduction of silver ions in aqueous medium leading to the formation of silver nanoparticles. After green production, the nanoparticles were analysed using standard characterization tools like UV-Vis spectrometry, X-ray diffraction (XRD), transmission electron miscroscopy (TEM), Fourier transform infra-red spectroscopy (FTIR), etc. Finally, we demonstrated the antibacterial, anticoagulant and thrombolytic properties of these green synthesized silver nanoparticles following standard protocols [12]. This is a novel report on the use of Euphorbia acruensis latex for biosynthesis of silver nanoparticles along with the study of their diverse biological properties stated earlier.

Euphorbia acruensis plant.
2 Materials and methods
Euphorbia acruensis was collected from university garden (at Jadavpur University, India) and authenticated before the experiment. Analytical grade silver nitrate was purchased from Merck India Ltd. (Mumbai, India). Nutrient agar required for antibacterial assay was procured from Himedia (India). De-ionized (DI) water was required to perform all experimental work. The glasswares were cleaned with de-ionized water and dried prior to the experiments.
2.1 Preparation of silver nanoparticles
Euphorbia acruensis is a succulent plant that exudes milky white latex unlike cacti. At first, the Euphorbia acruensis was cleaned with DI water, chopped and the released milky-white latex was collected for experimental use. Silver nanoparticles were biologically prepared by addition of 50 mL plant extract to the equal amount of 20 mM silver nitrate solution. The reacting mixture was observed at room temperature (30°C) for next 12 h. The reacting temperature was kept at 30°C to avoid any change in the composition of milky latex due to heating. After 15 min of addition, drastic change in the solution color (from colorless to light brown) was noticed indicating formation of metallic silver nanoparticles in the solution.
2.2 Characterization of silver nanoparticles
Generation of silver nanoparticles during reaction in the mixture was tracked at periodic intervals by scanning the mixture under double-beam UV-Vis spectroscope (Perkin Elmer, USA) and recording the UV-Vis spectra between 300 to 800 nm wavelengths. The produced nanoparticles were separated from the reacting solution by centrifuging it at 10,000 rpm for 12 min. The obtained soup after centrifugation was discarded and the pellet formed at the bottom (of centrifuge tube) was re-suspended in de-ionized water and centrifuged again to get rid of biomass residue completely. After repeated centrifugation, the precipitate was carefully collected and dried in a vacuum dryer overnight to obtain dry powder of silver nanoparticles. The XRD pattern of dry nanoparticles was recorded with the help of Rigaku Ultima-III X-ray diffractometer (CuKα radiation λ = 0.154 nm; operating volt.- 40kV; 2θ = 20°-80°). Fourier transform infra-red (FTIR) spectroscopy of these dry nanoparticles was performed on KBr pellet by using IR-Prestige FTIR spectroscope (Shimadzu, Japan) to identify the biomolecules associated with the stabilization of nanoparticles. Sample for high resolution TEM was carefully prepared by suspending dry silver nanoparticles in DI water keeping around 50 μg/mL concentration, i.e. a standard concentration for scanning nanoparticles under high resolution TEM. The suspension was further sonicated for 10 min and 2-3 drops of it were placed on carbon coated copper grid before drying it inside a desiccator. The grid was eventually scanned under high resolution TEM (Model name - JEOL-2010; operating volt.- 200 kV) to study the shape and morphology of biogenic silver nanoparticles. Energy dispersive X-ray (EDX) spectroscopy was performed by spreading 3-4 drops of this suspension on EDX sample holder and air-drying it prior to scanning under Inca X-stream EDX spectroscope (Oxford Instruments, UK).
2.3 Antibacterial assay
Antibacterial property of green synthesized silver nanoparticles was evaluated following agar disc diffusion method. Inoculates of two bacterial species- Pseudomonas putida and Staphylococcus aureus were prepared by growing single bacterial colony in the favourable nutrient broth medium overnight. The bacterial species were evenly spread on agar discs before creation of cups or wells on the discs. The suspension of biogenic silver nanoparticles prepared for TEM study (with concentration 50 μg/mL) was used as sample A while the half-diluted part (i.e. concentration 25 μg/mL) of it was taken as sample B for performing quantitative antibacterial test. Pure exudation of E. acruensis was taken as a negative control and labelled as sample C in this study. The three samples – A, B and C – were added in three different wells created on each agar disc seeded with a specific bacterial species. These discs were then incubated overnight inside an incubator at 37°C and the zone of inhibition formed around wells after 24 h were measured to assess the antibacterial efficacy of these nanoparticles. The agar discs were maintained in triplicates for data acquisition of antibacterial assay with more accuracy.
2.4 Study of anticoagulant and thrombolytic property
The anticoagulant activity of the biogenic silver nanoparticles was evaluated on freshly collected human blood at room temperature. In this study, 1 mL suspension of silver nanoparticles (concentration 50 μg/mL) was added to 10 mL fresh human blood (vial B). Another 10 mL of blood without addition was taken as a control (vial A).The two blood samples were then observed for next 1 h at room temperature for any noticeable changes.
Thrombolytic property of these nanoparticles was tested by dissolving fresh human blood clots in a clinical setup. Initially, 2-3 drops of fresh blood were spread on clean glass slide and allowed to form clot. 0.5 mL suspension of silver nanoparticles (concentration 50 μg/mL) was then added to it. The blood clot sample was then observed visually as well as microscopically at room temperature for next 60 min to have an insight into various stages of thrombolysis.
3 Results and discussion
3.1 Green synthesis of silver nanoparticles
In order to utilize the reducing abilities of the functional organic molecules present in the latex, the milky latex of E. acruensis was incubated with silver nitrate solution as described above and the UV-Vis spectra at regular intervals were obtained [13]. As expected, silver nanoparticles were generated as the components of the exudation targeted silver ions present in the solution and reduced them to nanoscale metallic silver. The change of solution color from colorless to light brown after 15 min of incubation was a firm indication of the production of silver nanoparticles (Figure 2). The solution color intensified to dark brown and after 24 h, no further change was visually observed indicating saturation of nanoparticle formation. The spectrometric data corroborated the observations as the absorbance reached its peak value at 420 nm which can be attributed to the wavelength of the surface plasmonic vibrations of metallic nano-silver (Figure 3) [14]. Figure 3 also demonstrates that the peak absorption value rose with incubation period and finally saturated after 24 h according to visual observance. The green synthesis was performed at different pH levels of the medium and no significant change was noticed for different pH values. This peak value of absorbance of the reacting medium increased with incubation time possibly due to production of more number of nanoparticles in the solution till arriving the state of equilibrium [15].

Colour change of reacting solution with time.

UV-Vis spectra of reacting medium at specific intervals.
3.2 Characterization analysis
X-ray diffraction pattern of dry nanoparticles was obtained to study the phase and crystallographic structures of the nanoparticles. The pattern consists of seven peaks (Figure 4) at 2θ values of 27.8°, 32.2°, 46.25°, 54.75°, 57.4°, 67.4° and 76.76° corresponding to (220), (122), (231), (331), (241), (104) and (311) planes of silver respectively as observed and correlated to the powder diffraction card of JCPDS (file no. 4-783) [16]. These findings confirm that the nanoparticles have face centred cubic (fcc) crystal structure. The crystalline size was estimated following Debye-Scherrer’s equation and it was found to be around 35 nm.

XRD pattern of silver nanoparticles.
Figure 5 shows the TEM images of green synthesized silver nanoparticles where the particles were observed to be closely spherical-shaped. The size distribution profile was obtained by counting at least 200 nanoparticles and the histogram is given in Figure 5d From histogram, it is evident that most of the particles belong to the size range of 10 to 40 nm which is in accordance with the result of XRD. From Figure 5c the interplanar spacings were manipulated to be 0.27 and 0.32 nm that may correspond to (122) and (220) crystal planes of silver nanoparticles respectively.

HRTEM images and histogram of silver nanoparticles.
FTIR spectroscopy was performed to have an insight into the capping and stabilization mechanism of nanoparticles during interaction with Euphorbia acruensis latex. The recorded FTIR spectra of E. acruensis latex and biogenic silver nanoparticles are shown in Figure 6. The spectrum of plant latex consists of eight noticeable peaks which can be correlated to the standard IR database to identify the organic molecules responsible for reduction and capping. The peaks at 1649 (peak 6) and 3397 cm-1 (peak 8) indicate stretching of C=O and O-H bonds present in amides and alcohols respectively [17]. A noticeable band present at 1436 cm-1 (peak 5) may be attributed to stretching of benzene ring present in aromatic compounds like ascorbic acid, phenol etc. [18]. Two vibrational bands at 2941 (peak 7) and 887 cm-1 (peak 3) may correspond to stretching and bending of C-H bonds present in alkanes and alkenes respectively [19]. The rest of the peaks (peak 1, 2 and 4) denote the stretching of carbon-halogen bonds present in alkyl halides. On the other hand, the spectrum of green synthesized silver nanoparticles features no significant absorbance peak indicating thorough removal of biomass residue from particle surface after production and eventually high level of purity of the biogenic nanoparticles.

FTIR spectra of plant latex and biogenic silver nanoparticles.
The presence of aromatic compounds (like ascorbic acid, phenol etc.) along with amides and aldehydes in the plant latex probably indicates that these organic molecules played the key role of capping and stabilizing nanoparticles during interaction of plant exudation with metal ions in the reacting solution [19,20].
EDX spectroscopy was employed to analyse the purity and weight percentage of metallic silver in the produced nanoparticles. The result of EDX (Figure 7) shows a couple of silver peaks and the weight percentage of Ag was found to be 74%. This data further confirms the production and purity of metallic silver. Small peaks of carbon, oxygen and chlorine were observed probably due to the presence of organic capping agents on the surface of particles [21].
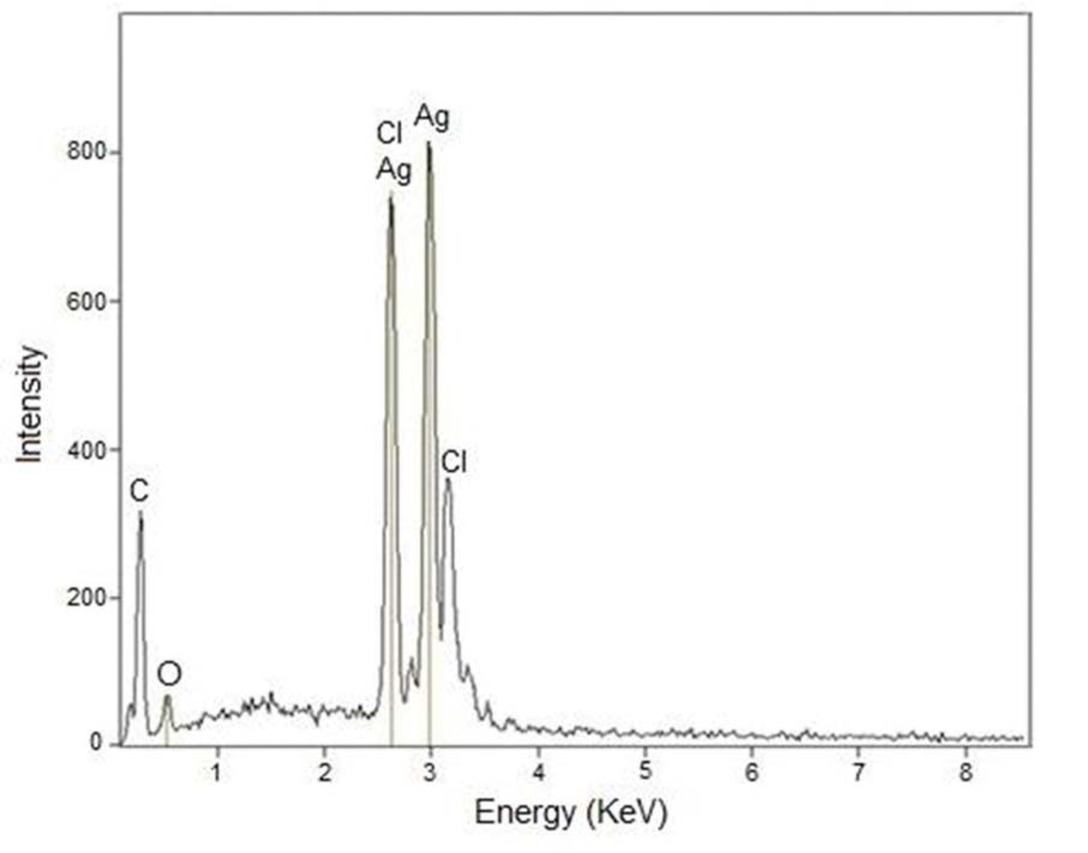
EDX spectrum of green synthesized nanoparticles.
3.3 Study of antibacterial property
Antibacterial property of green synthesized silver nanoparticles was tested towards a couple of pathogenic bacteria – P. putida and S. aureus. Pseudomonas putida is a gram-negative bacteria whereas Staphylococcus aureus is a gram-positive type. As seen from Figure 8, the larger inhibition zone was noticed against P. putida irrespective of the concentration of the nanoparticles added. S. aureus showed comparatively lower inhibition levels. Quantitative analysis of inhibitory effect against the tested bacterial species indicated a sharp rise in the inhibitory effect depending on higher levels of concentrations of biogenic nanoparticles (Table 1). The plant latexexhibited zero inhibition denoting no involvement in the antibacterial process. The better antibacterial activity of silver nanoparticles towards gram-negative P. putida may be due to the difference in cell wall compositions of gram-positive and gram-negative bacteria [22]. Cell wall of gram positive bacteria is composed of multiple thicker layers of bio-polymer called peptidoglycan whereas the cell wall of gram negative bacteria (like P. putida) consists of only single or double layers of it [23]. Therefore, the penetration of colloidal particles into the cellular system was easier in case of gram negative bacteria than gram positive.

Result of antibacterial disc diffusion assay.
Result of antibacterial assay.
| Tested bacterial species | Concentration of silver nanoparticles in suspension (μg/mL) | Inhibition zone diameter (mean of triplicates) (mm) |
|---|---|---|
| Pseudomonas | 0 (control) | – |
| putida | 25 | 6.87 mm |
| 50 | 14.05 mm | |
| Staphylococcus | 0 (control) | – |
| aureus | 25 | 6.08 mm |
| 50 | 12.85 mm |
Although the entire antibacterial mechanism of silver nanoparticles is not elucidated yet, but a few proposed theories are available in the literature to comprehend the action of metal nanoparticles on microbial cells. A few reports claim that the gold or silver nanoparticles have the ability to be attached on the bacterial cell wall due to electrostatic attraction and then penetrate it forming holes or ‘pits’ [24]. The formation of ‘pits’ on cell membrane alters the membrane permeability causing leakage of cellular fluid. Due to leakage of fluid, cellular transport degrades and the cells fail to survive for too long [25]. Another possible factor for cell death may be the metal ions which are released from nanoparticles during encounter with bacterial cells. The metal ions inhibit respiratory enzymes and break the cellular respiratory chain resulting into reactive oxygen species (ROS) generation [9,26]. ROS can impart oxidative stress to bacterial cells and eventually the cells cease all regular functions and expire [27].
3.4 Anticoagulant and thrombolytic property
Anticoagulant and thrombolytic activity of green synthesized nanoparticles was tested by addition of nanoparticles to human blood samples and further observation. The control in vial A began to coagulate within 10 min of incubation. The blood sample thickened over time and finally formed thick blood clot after 60 min (Figure 9). On the other hand, the blood sample with addition of nanoparticles (in vial B) underwent no significant changes and eventually no mark of coagulation was observed after 60 min of incubation (Figure 9).

Anticoagulant activity of silver nanoparticles.
During the assessment of thrombolytic property, the suspension of biogenic nanoparticles was added to a preformed blood clot for further observation. Immediately after addition, the blood clot on the glass slide began to be liquefied gradually and was dissolved completely after 30 min as shown in Figure 10.

Thrombolytic activity of silver nanoparticles.
The mechanisms for anticoagulant and thrombolytic activity of silver nanostructures are not theoretically decoded yet, but probable biochemical mechanism can be explained in the light of thrombolysis process. Biogenic silver nanoparticles may involve into the inhibition of enzymes which are responsible for generating blood clotting proteins [28]. Probably nano-silver inhibits the conversion of prothrombin into thrombin which is the key factor for producing insoluble strands of fibrin and catalysing other coagulating factors [29]. In addition, silver nanoparticles may be involved in activating enzymes that produce plasmin which can break cross-links of fibrin molecules and dissolve blood clots [30].
The result obtained here is in accordance with the thrombolytic activity of biochemical mediated silver nanoparticles reported by Harish et al. [31]. The images clearly indicated full dispersion of blood clot by green synthesized silver nanoparticles. While blood coagulation is an essential process to prevent excessive bleeding, timely dissolution of clots is equally necessary to curb thrombosis [32]. Conventional antithrombic treatments like streptokinase have limited scope of application due to short half-life, foreign agent neutralization and possibility of excessive bleeding [33]. Although very limited information is available in the literature on using nano-silver as thrombolytic agent, this report claims to have proved the potency of silver nanoparticles as anticoagulant and thrombolytic agent in the management of thrombosis. The potentiality shown by the biogenic silver nanoparticles in this study may have some useful applications in clinical domain for prevention of thrombosis and other disorders associated with it.
4 Conclusion
Plant extract-mediated green synthetic protocols of nanoparticles have drawn the attention of researchers for their fascinating, low cost and eco-benign features. In this report, we introduced a novel green platform where the milky white exudation of the plant- E. acruensis effectively reduced silver salt and produced silver nanoparticles in aqueous medium. Standard characterization tools were used to reveal the crystal phases and microstructure of the produced nanoparticles also to detect the role of biomolecules as stabilizing agents during interaction. The biosynthesized silver nanoparticles were proved to have obtrusive antibacterial efficacy towards gram positive and as well as gram negative bacterial strains. Most importantly, these nanoparticles were found to possess prominent anticoagulant and thrombolytic properties. These results extend the knowledge of bio-applications of silver nanoparticles and open up new possibilities in the domain of biomedical research.
References
[1] Irimia-Vladu M., “Green” electronics: biodegradable and biocompatible materials and devices for sustainable future. Chem. Soc. Rev., 2014, 43, 588-610.10.1039/C3CS60235DSuche in Google Scholar
[2] Saif S., Tahir A., Chen Y., Green synthesis of iron nanoparticles and their environmental applications and implications. Nanomater., 2016, 6 (11), 209.10.3390/nano6110209Suche in Google Scholar PubMed PubMed Central
[3] Ashraf J.M., Ansari M.A., Khan H.M., Alzohairy M.A., Choi I., Green synthesis of silver nanoparticles and characterization of their inhibitory effects on AGEs formation using biophysical techniques. Sci. Rep., 2016, 6, 20414.10.1038/srep20414Suche in Google Scholar PubMed PubMed Central
[4] Roy K., Ghosh C.K., Sarkar C.K., Selective amino acid detection by green synthesized copper nanoparticles prepared using basil Ocimum tenuiflorum flower extract. Microsyst. Technol., 2018, DOI:10.1007/s00542-018-4205-7.10.1007/s00542-018-4205-7Suche in Google Scholar
[5] Ahmed S., Ahmad S.M., Swami B.L., Ikram S., Green synthesis of silver nanoparticles using Azadirachta indica aqueous leaf extract. J. Radiat. Res. 2016, 9(1), 1-7.10.1016/j.jrras.2015.06.006Suche in Google Scholar
[6] Ismail R.A., Almashhadani N.J., Sadik R.H., Preparation and properties of polystyrene incorporated with gold and silver nanoparticles for optoelectronic applications. Appl. Nanosci., 2017, 7(3), 109-116.10.1007/s13204-017-0550-6Suche in Google Scholar
[7] Roy K., Ghosh C.K., Measurement of electrical conductivity of thin film composed of green synthesized copper nanoparticles. 1st Intern. Conf. Emerg. Trend. Electron. Dev. Comput. Tech. (EDCT), 2018, DOI:10.1109/EDCT.2018.8405073.10.1109/EDCT.2018.8405073Suche in Google Scholar
[8] Giner-Casares J.J., Henriksen-Lacey M., Coronado-Puchau M., Liz-Marzan L.M., Plasmonic surfaces for cell growth and retrieval triggered by near‐infrared light. Mater. Today, 2016, 19(1), 19-28.10.1016/j.mattod.2015.07.004Suche in Google Scholar
[9] Prabhu S., Poulose E.K., Silver nanoparticles: mechanism of antimicrobial action, synthesis, medical applications, and toxicity effects. Int. Nano Lett., 2012, 2(32), 1-10.10.1186/2228-5326-2-32Suche in Google Scholar
[10] Roy K., Ghosh C.K., Biological synthesis of metallic nanoparticles: A green alternative (Chapter 7), Nanotechnology: Synthesis to Applications. CRC Press, 2017, ISBN: 978-1-138-03273-6, 153-168.Suche in Google Scholar
[11] Spano D., Pintus F., Mascia C., Scorciapino M.A., Casu M., Floris G., et al., Extraction and characterization of a natural rubber from Euphorbia characias latex. Biopolymers, 2012, 97(8), 589-594.10.1002/bip.22044Suche in Google Scholar PubMed
[12] Ghatage S.L., Navale S.S., Mujawar N.K., Patil S., Patil V., Antimicrobial screening. Ind. J. Drug., 2014, 2(3), 84-88.Suche in Google Scholar
[13] Ioannidis A.S., Papageorgiou K.I., Andreou P.S., Exposure to Euphorbia lathyris latex resulting in alkaline chemical injury: a case report. J. Med. Case Rep., 2009, 3, 115.10.1186/1752-1947-3-115Suche in Google Scholar PubMed PubMed Central
[14] Roy K., Ghosh C.K., Sarkar C.K., Rapid colorimetric detection of Hg2+ ion by green silver nanoparticles synthesized using Dahlia pinnata leaf extract. Green Process. Synth., 2015, 4(6) 455-461.10.1515/gps-2015-0052Suche in Google Scholar
[15] Sasikala D., Govindaraju K., Tamilselvan S., Singaravelu G., Soybean protein: A natural source for the production of green silver nanoparticles. Biotechnol. Bioprocess. Eng., 2012, 17(6), 1176-1181.10.1007/s12257-012-0021-6Suche in Google Scholar
[16] Li H.J., Zhang A.Q., Hu Y., Sui L., Qian D.J., Chen M., Large-scale synthesis and self-organization of silver nanoparticles with Tween 80 as a reductant and stabilizer. Nanoscale Res. Lett., 2012, 7, 612.10.1186/1556-276X-7-612Suche in Google Scholar PubMed PubMed Central
[17] Umer A., Naveed S., Ramzan N., Rafique M.S., Imran M., A green method for the synthesis of Copper Nanoparticles using L-ascorbic acid. Matéria (Rio J.), 2014, 19(3), 197-203.10.1590/S1517-70762014000300002Suche in Google Scholar
[18] Ahmad S.I., Syed I.A., Prasad P.R., Ahmad A., Quantitation of urea in urine by Fourier transforms infrared spectroscopy, Der Pharma Chemica, 2014, 6(1), 90-96.Suche in Google Scholar
[19] Ahmmad S.K., Samee M.A., Taqiullah S.M., Rahman S., FT-IR and Raman spectroscopic studies of ZnF2–ZnO–As2O3–TeO2 glasses. J. Taibah Univ. Sci., 2016, 10(3), 329-339.10.1016/j.jtusci.2014.12.008Suche in Google Scholar
[20] Shetgiri N.P., Nayak B.K., Synthesis and antimicrobial activity of some succinimides. Ind. J. Chem., 2005, 44B, 1933-1936.10.1002/chin.200601062Suche in Google Scholar
[21] Anandalakhsmi K., Venugobal J., Ramasamy V., Characterization of silver nanoparticles by green synthesis method using Pedalium murex leaf extract and their antibacterial activity. Appl. Nanosci., 2016, 6(3), 399-408.10.1007/s13204-015-0449-zSuche in Google Scholar
[22] Kruk T., Szczepanowicz K., Stefanska J., Socha R.P., Warszynski P., Synthesis and antimicrobial activity of monodisperse copper nanoparticles. Colloid. Surface B, 2015, 128, 17-22.10.1016/j.colsurfb.2015.02.009Suche in Google Scholar PubMed
[23] Aziz N., Faraz M., Pandey R., Shakir M., Fatma T., Varma A., et al., Facile algae-derived route to biogenic silver nanoparticles: synthesis, antibacterial, and photocatalytic properties. Langmuir, 2015, 31, 11605-11612.10.1021/acs.langmuir.5b03081Suche in Google Scholar PubMed
[24] Shi T., Sun X., He Q., Cytotoxicity of silver nanoparticles against bacteria and tumor cells. Curr. Protein Pept. Sci., 2018, 19(6), 525-536.10.2174/1389203718666161108092149Suche in Google Scholar PubMed
[25] Roy K., Ghosh C.K., Environmental and biological applications of nanoparticles (Chapter 8), Nanotechnology: Synthesis to Applications. CRC Press, 2017, ISBN: 978-1-138-03273-6, 169-192.10.1201/9781315116730Suche in Google Scholar
[26] Chakrapani V., Ahmed K.B.A., Kumar V.V., Ganapathy V., Anthony S.P., Anbazhagan V., A facile route to synthesize casein capped copper nanoparticles: an effective antibacterial agent and selective colorimetric sensor for mercury and tryptophan. RSC Adv., 2014, 4, 33215-33221.10.1039/C4RA03086ASuche in Google Scholar
[27] Dobrucka R., Dlugaszewska J., Biosynthesis and antibacterial activity of ZnO nanoparticles using Trifolium pratense flower extract. Saudi J. Biol. Sci., 2016, 23(4), 517-523.10.1016/j.sjbs.2015.05.016Suche in Google Scholar PubMed PubMed Central
[28] Huang H., Lai W., Cui M., Liang L., Lin Y., Fang Q., et al., An evaluation of blood compatibility of silver nanoparticles. Sci. Rep., 2016, 6, 25518.10.1038/srep25518Suche in Google Scholar PubMed PubMed Central
[29] Lateef A., Akande M.A., Ojo S.A., Folarin B.I., Gueguim-Kana E.B., Beukes L.S., Paper wasp nest-mediated biosynthesis of silver nanoparticles for antimicrobial, catalytic, anticoagulant, and thrombolytic applications. 3 Biotech., 2016, 6, 140.10.1007/s13205-016-0459-xSuche in Google Scholar PubMed PubMed Central
[30] Cheng R., Huang W., Huang L., Yang B., Mao L., Jin K., et al., Acceleration of tissue plasminogen activator-mediated thrombolysis by magnetically powered nanomotors. ACS Nano, 2014, 8(8), 7746-7754.10.1021/nn5029955Suche in Google Scholar PubMed PubMed Central
[31] Harish B.S., Uppuluri K.B., Anbazhagan V., Synthesis of fibrinolytic active silver nanoparticle using wheat bran xylan as a reducing and stabilizing agent. Carbohyd. Polym., 2015, 132, 104-110.10.1016/j.carbpol.2015.06.069Suche in Google Scholar PubMed
[32] Chapin J.C., Hajjar K.A., Fibrinolysis and the control of blood coagulation. Blood Rev., 2015, 29(1), 17-24.10.1016/j.blre.2014.09.003Suche in Google Scholar PubMed PubMed Central
[33] Banerjee A., Chisti Y., Banerjee U.C., Streptokinase--a clinically useful thrombolytic agent. Biotech. Adv., 2004, 22, 287-307.10.1016/j.biotechadv.2003.09.004Suche in Google Scholar PubMed
© 2019 Roy et al., published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 Public License.
Artikel in diesem Heft
- Regular Articles
- Studies on the preparation and properties of biodegradable polyester from soybean oil
- Flow-mode biodiesel production from palm oil using a pressurized microwave reactor
- Reduction of free fatty acids in waste oil for biodiesel production by glycerolysis: investigation and optimization of process parameters
- Saccharin: a cheap and mild acidic agent for the synthesis of azo dyes via telescoped dediazotization
- Optimization of lipase-catalyzed synthesis of polyethylene glycol stearate in a solvent-free system
- Green synthesis of iron oxide nanoparticles using Platanus orientalis leaf extract for antifungal activity
- Ultrasound assisted chemical activation of peanut husk for copper removal
- Room temperature silanization of Fe3O4 for the preparation of phenyl functionalized magnetic adsorbent for dispersive solid phase extraction for the extraction of phthalates in water
- Evaluation of the saponin green extraction from Ziziphus spina-christi leaves using hydrothermal, microwave and Bain-Marie water bath heating methods
- Oxidation of dibenzothiophene using the heterogeneous catalyst of tungsten-based carbon nanotubes
- Calcined sodium silicate as an efficient and benign heterogeneous catalyst for the transesterification of natural lecithin to L-α-glycerophosphocholine
- Synergistic effect between CO2 and H2O2 on ethylbenzene oxidation catalyzed by carbon supported heteropolyanion catalysts
- Hydrocyanation of 2-arylmethyleneindan-1,3-diones using potassium hexacyanoferrate(II) as a nontoxic cyanating agent
- Green synthesis of hydratropic aldehyde from α-methylstyrene catalyzed by Al2O3-supported metal phthalocyanines
- Environmentally benign chemical recycling of polycarbonate wastes: comparison of micro- and nano-TiO2 solid support efficiencies
- Medicago polymorpha-mediated antibacterial silver nanoparticles in the reduction of methyl orange
- Production of value-added chemicals from esterification of waste glycerol over MCM-41 supported catalysts
- Green synthesis of zerovalent copper nanoparticles for efficient reduction of toxic azo dyes congo red and methyl orange
- Optimization of the biological synthesis of silver nanoparticles using Penicillium oxalicum GRS-1 and their antimicrobial effects against common food-borne pathogens
- Optimization of submerged fermentation conditions to overproduce bioethanol using two industrial and traditional Saccharomyces cerevisiae strains
- Extraction of In3+ and Fe3+ from sulfate solutions by using a 3D-printed “Y”-shaped microreactor
- Foliar-mediated Ag:ZnO nanophotocatalysts: green synthesis, characterization, pollutants degradation, and in vitro biocidal activity
- Green cyclic acetals production by glycerol etherification reaction with benzaldehyde using cationic acidic resin
- Biosynthesis, characterization and antimicrobial activities assessment of fabricated selenium nanoparticles using Pelargonium zonale leaf extract
- Synthesis of high surface area magnesia by using walnut shell as a template
- Controllable biosynthesis of silver nanoparticles using actinobacterial strains
- Green vegetation: a promising source of color dyes
- Mechano-chemical synthesis of ammonia and acetic acid from inorganic materials in water
- Green synthesis and structural characterization of novel N1-substituted 3,4-dihydropyrimidin-2(1H)-ones
- Biodiesel production from cotton oil using heterogeneous CaO catalysts from eggshells prepared at different calcination temperatures
- Regeneration of spent mercury catalyst for the treatment of dye wastewater by the microwave and ultrasonic spray-assisted method
- Green synthesis of the innovative super paramagnetic nanoparticles from the leaves extract of Fraxinus chinensis Roxb and their application for the decolourisation of toxic dyes
- Biogenic ZnO nanoparticles: a study of blueshift of optical band gap and photocatalytic degradation of reactive yellow 186 dye under direct sunlight
- Leached compounds from the extracts of pomegranate peel, green coconut shell, and karuvelam wood for the removal of hexavalent chromium
- Enhancement of molecular weight reduction of natural rubber in triphasic CO2/toluene/H2O systems with hydrogen peroxide for preparation of biobased polyurethanes
- An efficient green synthesis of novel 1H-imidazo[1,2-a]imidazole-3-amine and imidazo[2,1-c][1,2,4]triazole-5-amine derivatives via Strecker reaction under controlled microwave heating
- Evaluation of three different green fabrication methods for the synthesis of crystalline ZnO nanoparticles using Pelargonium zonale leaf extract
- A highly efficient and multifunctional biomass supporting Ag, Ni, and Cu nanoparticles through wetness impregnation for environmental remediation
- Simple one-pot green method for large-scale production of mesalamine, an anti-inflammatory agent
- Relationships between step and cumulative PMI and E-factors: implications on estimating material efficiency with respect to charting synthesis optimization strategies
- A comparative sorption study of Cr3+ and Cr6+ using mango peels: kinetic, equilibrium and thermodynamic
- Effects of acid hydrolysis waste liquid recycle on preparation of microcrystalline cellulose
- Use of deep eutectic solvents as catalyst: A mini-review
- Microwave-assisted synthesis of pyrrolidinone derivatives using 1,1’-butylenebis(3-sulfo-3H-imidazol-1-ium) chloride in ethylene glycol
- Green and eco-friendly synthesis of Co3O4 and Ag-Co3O4: Characterization and photo-catalytic activity
- Adsorption optimized of the coal-based material and application for cyanide wastewater treatment
- Aloe vera leaf extract mediated green synthesis of selenium nanoparticles and assessment of their In vitro antimicrobial activity against spoilage fungi and pathogenic bacteria strains
- Waste phenolic resin derived activated carbon by microwave-assisted KOH activation and application to dye wastewater treatment
- Direct ethanol production from cellulose by consortium of Trichoderma reesei and Candida molischiana
- Agricultural waste biomass-assisted nanostructures: Synthesis and application
- Biodiesel production from rubber seed oil using calcium oxide derived from eggshell as catalyst – optimization and modeling studies
- Study of fabrication of fully aqueous solution processed SnS quantum dot-sensitized solar cell
- Assessment of aqueous extract of Gypsophila aretioides for inhibitory effects on calcium carbonate formation
- An environmentally friendly acylation reaction of 2-methylnaphthalene in solvent-free condition in a micro-channel reactor
- Aegle marmelos phytochemical stabilized synthesis and characterization of ZnO nanoparticles and their role against agriculture and food pathogen
- A reactive coupling process for co-production of solketal and biodiesel
- Optimization of the asymmetric synthesis of (S)-1-phenylethanol using Ispir bean as whole-cell biocatalyst
- Synthesis of pyrazolopyridine and pyrazoloquinoline derivatives by one-pot, three-component reactions of arylglyoxals, 3-methyl-1-aryl-1H-pyrazol-5-amines and cyclic 1,3-dicarbonyl compounds in the presence of tetrapropylammonium bromide
- Preconcentration of morphine in urine sample using a green and solvent-free microextraction method
- Extraction of glycyrrhizic acid by aqueous two-phase system formed by PEG and two environmentally friendly organic acid salts - sodium citrate and sodium tartrate
- Green synthesis of copper oxide nanoparticles using Juglans regia leaf extract and assessment of their physico-chemical and biological properties
- Deep eutectic solvents (DESs) as powerful and recyclable catalysts and solvents for the synthesis of 3,4-dihydropyrimidin-2(1H)-ones/thiones
- Biosynthesis, characterization and anti-microbial activity of silver nanoparticle based gel hand wash
- Efficient and selective microwave-assisted O-methylation of phenolic compounds using tetramethylammonium hydroxide (TMAH)
- Anticoagulant, thrombolytic and antibacterial activities of Euphorbia acruensis latex-mediated bioengineered silver nanoparticles
- Volcanic ash as reusable catalyst in the green synthesis of 3H-1,5-benzodiazepines
- Green synthesis, anionic polymerization of 1,4-bis(methacryloyl)piperazine using Algerian clay as catalyst
- Selenium supplementation during fermentation with sugar beet molasses and Saccharomyces cerevisiae to increase bioethanol production
- Biosynthetic potential assessment of four food pathogenic bacteria in hydrothermally silver nanoparticles fabrication
- Investigating the effectiveness of classical and eco-friendly approaches for synthesis of dialdehydes from organic dihalides
- Pyrolysis of palm oil using zeolite catalyst and characterization of the boil-oil
- Azadirachta indica leaves extract assisted green synthesis of Ag-TiO2 for degradation of Methylene blue and Rhodamine B dyes in aqueous medium
- Synthesis of vitamin E succinate catalyzed by nano-SiO2 immobilized DMAP derivative in mixed solvent system
- Extraction of phytosterols from melon (Cucumis melo) seeds by supercritical CO2 as a clean technology
- Production of uronic acids by hydrothermolysis of pectin as a model substance for plant biomass waste
- Biofabrication of highly pure copper oxide nanoparticles using wheat seed extract and their catalytic activity: A mechanistic approach
- Intelligent modeling and optimization of emulsion aggregation method for producing green printing ink
- Improved removal of methylene blue on modified hierarchical zeolite Y: Achieved by a “destructive-constructive” method
- Two different facile and efficient approaches for the synthesis of various N-arylacetamides via N-acetylation of arylamines and straightforward one-pot reductive acetylation of nitroarenes promoted by recyclable CuFe2O4 nanoparticles in water
- Optimization of acid catalyzed esterification and mixed metal oxide catalyzed transesterification for biodiesel production from Moringa oleifera oil
- Kinetics and the fluidity of the stearic acid esters with different carbon backbones
- Aiming for a standardized protocol for preparing a process green synthesis report and for ranking multiple synthesis plans to a common target product
- Microstructure and luminescence of VO2 (B) nanoparticle synthesis by hydrothermal method
- Optimization of uranium removal from uranium plant wastewater by response surface methodology (RSM)
- Microwave drying of nickel-containing residue: dielectric properties, kinetics, and energy aspects
- Simple and convenient two step synthesis of 5-bromo-2,3-dimethoxy-6-methyl-1,4-benzoquinone
- Biodiesel production from waste cooking oil
- The effect of activation temperature on structure and properties of blue coke-based activated carbon by CO2 activation
- Optimization of reaction parameters for the green synthesis of zero valent iron nanoparticles using pine tree needles
- Microwave-assisted protocol for squalene isolation and conversion from oil-deodoriser distillates
- Denitrification performance of rare earth tailings-based catalysts
- Facile synthesis of silver nanoparticles using Averrhoa bilimbi L and Plum extracts and investigation on the synergistic bioactivity using in vitro models
- Green production of AgNPs and their phytostimulatory impact
- Photocatalytic activity of Ag/Ni bi-metallic nanoparticles on textile dye removal
- Topical Issue: Green Process Engineering / Guest Editors: Martine Poux, Patrick Cognet
- Modelling and optimisation of oxidative desulphurisation of tyre-derived oil via central composite design approach
- CO2 sequestration by carbonation of olivine: a new process for optimal separation of the solids produced
- Organic carbonates synthesis improved by pervaporation for CO2 utilisation
- Production of starch nanoparticles through solvent-antisolvent precipitation in a spinning disc reactor
- A kinetic study of Zn halide/TBAB-catalysed fixation of CO2 with styrene oxide in propylene carbonate
- Topical on Green Process Engineering
Artikel in diesem Heft
- Regular Articles
- Studies on the preparation and properties of biodegradable polyester from soybean oil
- Flow-mode biodiesel production from palm oil using a pressurized microwave reactor
- Reduction of free fatty acids in waste oil for biodiesel production by glycerolysis: investigation and optimization of process parameters
- Saccharin: a cheap and mild acidic agent for the synthesis of azo dyes via telescoped dediazotization
- Optimization of lipase-catalyzed synthesis of polyethylene glycol stearate in a solvent-free system
- Green synthesis of iron oxide nanoparticles using Platanus orientalis leaf extract for antifungal activity
- Ultrasound assisted chemical activation of peanut husk for copper removal
- Room temperature silanization of Fe3O4 for the preparation of phenyl functionalized magnetic adsorbent for dispersive solid phase extraction for the extraction of phthalates in water
- Evaluation of the saponin green extraction from Ziziphus spina-christi leaves using hydrothermal, microwave and Bain-Marie water bath heating methods
- Oxidation of dibenzothiophene using the heterogeneous catalyst of tungsten-based carbon nanotubes
- Calcined sodium silicate as an efficient and benign heterogeneous catalyst for the transesterification of natural lecithin to L-α-glycerophosphocholine
- Synergistic effect between CO2 and H2O2 on ethylbenzene oxidation catalyzed by carbon supported heteropolyanion catalysts
- Hydrocyanation of 2-arylmethyleneindan-1,3-diones using potassium hexacyanoferrate(II) as a nontoxic cyanating agent
- Green synthesis of hydratropic aldehyde from α-methylstyrene catalyzed by Al2O3-supported metal phthalocyanines
- Environmentally benign chemical recycling of polycarbonate wastes: comparison of micro- and nano-TiO2 solid support efficiencies
- Medicago polymorpha-mediated antibacterial silver nanoparticles in the reduction of methyl orange
- Production of value-added chemicals from esterification of waste glycerol over MCM-41 supported catalysts
- Green synthesis of zerovalent copper nanoparticles for efficient reduction of toxic azo dyes congo red and methyl orange
- Optimization of the biological synthesis of silver nanoparticles using Penicillium oxalicum GRS-1 and their antimicrobial effects against common food-borne pathogens
- Optimization of submerged fermentation conditions to overproduce bioethanol using two industrial and traditional Saccharomyces cerevisiae strains
- Extraction of In3+ and Fe3+ from sulfate solutions by using a 3D-printed “Y”-shaped microreactor
- Foliar-mediated Ag:ZnO nanophotocatalysts: green synthesis, characterization, pollutants degradation, and in vitro biocidal activity
- Green cyclic acetals production by glycerol etherification reaction with benzaldehyde using cationic acidic resin
- Biosynthesis, characterization and antimicrobial activities assessment of fabricated selenium nanoparticles using Pelargonium zonale leaf extract
- Synthesis of high surface area magnesia by using walnut shell as a template
- Controllable biosynthesis of silver nanoparticles using actinobacterial strains
- Green vegetation: a promising source of color dyes
- Mechano-chemical synthesis of ammonia and acetic acid from inorganic materials in water
- Green synthesis and structural characterization of novel N1-substituted 3,4-dihydropyrimidin-2(1H)-ones
- Biodiesel production from cotton oil using heterogeneous CaO catalysts from eggshells prepared at different calcination temperatures
- Regeneration of spent mercury catalyst for the treatment of dye wastewater by the microwave and ultrasonic spray-assisted method
- Green synthesis of the innovative super paramagnetic nanoparticles from the leaves extract of Fraxinus chinensis Roxb and their application for the decolourisation of toxic dyes
- Biogenic ZnO nanoparticles: a study of blueshift of optical band gap and photocatalytic degradation of reactive yellow 186 dye under direct sunlight
- Leached compounds from the extracts of pomegranate peel, green coconut shell, and karuvelam wood for the removal of hexavalent chromium
- Enhancement of molecular weight reduction of natural rubber in triphasic CO2/toluene/H2O systems with hydrogen peroxide for preparation of biobased polyurethanes
- An efficient green synthesis of novel 1H-imidazo[1,2-a]imidazole-3-amine and imidazo[2,1-c][1,2,4]triazole-5-amine derivatives via Strecker reaction under controlled microwave heating
- Evaluation of three different green fabrication methods for the synthesis of crystalline ZnO nanoparticles using Pelargonium zonale leaf extract
- A highly efficient and multifunctional biomass supporting Ag, Ni, and Cu nanoparticles through wetness impregnation for environmental remediation
- Simple one-pot green method for large-scale production of mesalamine, an anti-inflammatory agent
- Relationships between step and cumulative PMI and E-factors: implications on estimating material efficiency with respect to charting synthesis optimization strategies
- A comparative sorption study of Cr3+ and Cr6+ using mango peels: kinetic, equilibrium and thermodynamic
- Effects of acid hydrolysis waste liquid recycle on preparation of microcrystalline cellulose
- Use of deep eutectic solvents as catalyst: A mini-review
- Microwave-assisted synthesis of pyrrolidinone derivatives using 1,1’-butylenebis(3-sulfo-3H-imidazol-1-ium) chloride in ethylene glycol
- Green and eco-friendly synthesis of Co3O4 and Ag-Co3O4: Characterization and photo-catalytic activity
- Adsorption optimized of the coal-based material and application for cyanide wastewater treatment
- Aloe vera leaf extract mediated green synthesis of selenium nanoparticles and assessment of their In vitro antimicrobial activity against spoilage fungi and pathogenic bacteria strains
- Waste phenolic resin derived activated carbon by microwave-assisted KOH activation and application to dye wastewater treatment
- Direct ethanol production from cellulose by consortium of Trichoderma reesei and Candida molischiana
- Agricultural waste biomass-assisted nanostructures: Synthesis and application
- Biodiesel production from rubber seed oil using calcium oxide derived from eggshell as catalyst – optimization and modeling studies
- Study of fabrication of fully aqueous solution processed SnS quantum dot-sensitized solar cell
- Assessment of aqueous extract of Gypsophila aretioides for inhibitory effects on calcium carbonate formation
- An environmentally friendly acylation reaction of 2-methylnaphthalene in solvent-free condition in a micro-channel reactor
- Aegle marmelos phytochemical stabilized synthesis and characterization of ZnO nanoparticles and their role against agriculture and food pathogen
- A reactive coupling process for co-production of solketal and biodiesel
- Optimization of the asymmetric synthesis of (S)-1-phenylethanol using Ispir bean as whole-cell biocatalyst
- Synthesis of pyrazolopyridine and pyrazoloquinoline derivatives by one-pot, three-component reactions of arylglyoxals, 3-methyl-1-aryl-1H-pyrazol-5-amines and cyclic 1,3-dicarbonyl compounds in the presence of tetrapropylammonium bromide
- Preconcentration of morphine in urine sample using a green and solvent-free microextraction method
- Extraction of glycyrrhizic acid by aqueous two-phase system formed by PEG and two environmentally friendly organic acid salts - sodium citrate and sodium tartrate
- Green synthesis of copper oxide nanoparticles using Juglans regia leaf extract and assessment of their physico-chemical and biological properties
- Deep eutectic solvents (DESs) as powerful and recyclable catalysts and solvents for the synthesis of 3,4-dihydropyrimidin-2(1H)-ones/thiones
- Biosynthesis, characterization and anti-microbial activity of silver nanoparticle based gel hand wash
- Efficient and selective microwave-assisted O-methylation of phenolic compounds using tetramethylammonium hydroxide (TMAH)
- Anticoagulant, thrombolytic and antibacterial activities of Euphorbia acruensis latex-mediated bioengineered silver nanoparticles
- Volcanic ash as reusable catalyst in the green synthesis of 3H-1,5-benzodiazepines
- Green synthesis, anionic polymerization of 1,4-bis(methacryloyl)piperazine using Algerian clay as catalyst
- Selenium supplementation during fermentation with sugar beet molasses and Saccharomyces cerevisiae to increase bioethanol production
- Biosynthetic potential assessment of four food pathogenic bacteria in hydrothermally silver nanoparticles fabrication
- Investigating the effectiveness of classical and eco-friendly approaches for synthesis of dialdehydes from organic dihalides
- Pyrolysis of palm oil using zeolite catalyst and characterization of the boil-oil
- Azadirachta indica leaves extract assisted green synthesis of Ag-TiO2 for degradation of Methylene blue and Rhodamine B dyes in aqueous medium
- Synthesis of vitamin E succinate catalyzed by nano-SiO2 immobilized DMAP derivative in mixed solvent system
- Extraction of phytosterols from melon (Cucumis melo) seeds by supercritical CO2 as a clean technology
- Production of uronic acids by hydrothermolysis of pectin as a model substance for plant biomass waste
- Biofabrication of highly pure copper oxide nanoparticles using wheat seed extract and their catalytic activity: A mechanistic approach
- Intelligent modeling and optimization of emulsion aggregation method for producing green printing ink
- Improved removal of methylene blue on modified hierarchical zeolite Y: Achieved by a “destructive-constructive” method
- Two different facile and efficient approaches for the synthesis of various N-arylacetamides via N-acetylation of arylamines and straightforward one-pot reductive acetylation of nitroarenes promoted by recyclable CuFe2O4 nanoparticles in water
- Optimization of acid catalyzed esterification and mixed metal oxide catalyzed transesterification for biodiesel production from Moringa oleifera oil
- Kinetics and the fluidity of the stearic acid esters with different carbon backbones
- Aiming for a standardized protocol for preparing a process green synthesis report and for ranking multiple synthesis plans to a common target product
- Microstructure and luminescence of VO2 (B) nanoparticle synthesis by hydrothermal method
- Optimization of uranium removal from uranium plant wastewater by response surface methodology (RSM)
- Microwave drying of nickel-containing residue: dielectric properties, kinetics, and energy aspects
- Simple and convenient two step synthesis of 5-bromo-2,3-dimethoxy-6-methyl-1,4-benzoquinone
- Biodiesel production from waste cooking oil
- The effect of activation temperature on structure and properties of blue coke-based activated carbon by CO2 activation
- Optimization of reaction parameters for the green synthesis of zero valent iron nanoparticles using pine tree needles
- Microwave-assisted protocol for squalene isolation and conversion from oil-deodoriser distillates
- Denitrification performance of rare earth tailings-based catalysts
- Facile synthesis of silver nanoparticles using Averrhoa bilimbi L and Plum extracts and investigation on the synergistic bioactivity using in vitro models
- Green production of AgNPs and their phytostimulatory impact
- Photocatalytic activity of Ag/Ni bi-metallic nanoparticles on textile dye removal
- Topical Issue: Green Process Engineering / Guest Editors: Martine Poux, Patrick Cognet
- Modelling and optimisation of oxidative desulphurisation of tyre-derived oil via central composite design approach
- CO2 sequestration by carbonation of olivine: a new process for optimal separation of the solids produced
- Organic carbonates synthesis improved by pervaporation for CO2 utilisation
- Production of starch nanoparticles through solvent-antisolvent precipitation in a spinning disc reactor
- A kinetic study of Zn halide/TBAB-catalysed fixation of CO2 with styrene oxide in propylene carbonate
- Topical on Green Process Engineering

