Evaluation of three different green fabrication methods for the synthesis of crystalline ZnO nanoparticles using Pelargonium zonale leaf extract
-
Afsaneh Vahidi
Abstract
Zinc oxide nanoparticles (ZnO NPs) were green synthesized using Pelargonum zonale leaf extract under three different heating methods, and their characteristics were evaluated using X-ray diffractometry (XRD), Fourier-transform infrared spectroscopy (FT-IR), scanning electron microscopy (SEM), 2,2-diphenyl-2-picrylhydrazyl (DPPH) assay and antibacterial well diffusion method. The FT-IR analysis indicated that the Pelargonium leaf extract contained hydroxyl and amide I groups which were related to the proteins, carbohydrate, tannins and phenolic compounds of the extract and had an essential role in the reduction of the zinc ions and synthesis of the ZnO NPs. The obtained results revealed that the synthesized spherical individual ZnO NPs as well as the number of aggregates using microwave irradiation, autoclave and conventional heating (heater-stirrer) methods had average crystalline size of 51, 60 and 61 nm. Furthermore, antioxidant activities of the fabricated ZnO NPs were 7.8, 4.1 and 5.5% by using conventional heating, autoclave and microwave irradiation, respectively. The obtained results indicated that all the formed ZnO NPs had bactericidal effects against to the both Gram negative and Gram positive bacteria strains. However, the synthesized ZnO NPs using conventional heating method had the highest antibacterial activities toward both studied bacteria strains.
1 Introduction
Metal oxide nanoparticles (NPs) have been extensively synthesized and utilized in different areas because of their distinctive optical, physico-chemical, thermal and biological attributes [1]. In fact, as compared to the organic NPs, metal oxide NPs indicate unique stability, lower toxicity and higher heat resistance and selectivity [2]. Among the metal NPs, zinc oxide nanoparticles (ZnO NPs) are highlighted due to their numerous applications in the plastic, ceramics, cement, lubricants, paints, adhesives, pigments, packaging, cosmetics and textile industries [3], [[4]. Furthermore, ZnO NPs have been known as strong antibacterial and antifungal agents [4], [5], [6]. In fact, ZnO NPs, by the generation of the reactive oxygen on their surfaces, can effectively influence the permeability of the microorganisms’ cytoplasmic membrane and inhibit their normal activities 1], [2].
There are three different ZnO NPs synthesis methods, namely, physical (i.e. thermal evaporation and pulsed laser deposition), chemical (i.e. sol-gel, sonochemical and spray pyrolysis) and biological techniques based on using the microorganisms, polysaccharides, plants and enzymes [[7]. The physical synthesis methods require high vacuum and are energy consuming [8]. The chemical fabrication approaches are based on consuming chemical solvents and reagents in which their residuals in the final products can limit the application of the formed NPs and is not environmentally friendly. To overcome the mentioned physical and chemical NP synthesis methods, the development and achievement of alternative NP synthesis methods which are fast, clean, cost-effective and eco-friendly and also require mild reaction conditions are necessary these days [9]. Recently, the biological synthesis processes for the metal oxide NPs based on utilizing the natural compounds as reducing and stabilizing agents have attracted scientific interest. Numerous researches have been completed on the green synthesis of the ZnO NPs using plants and their derivatives such as leaf, root, flower and fruit 10], [[11]. As compared to the biosynthesis of the NPs using the microorganisms, NP green synthesis based on plant extract has several advantages including the availability of the resources, not needing the culture and downstream processes, rapid and one-step synthesis process, and easy to scale-up fabricated process 12]. Pelargonium belongs to the family Geraniaceae and has high potential applications in cosmetics, medicines and food products. Several studies indicated that Pelargonium leaves extract contains flavonoids, phenolic compounds, organic acids, proteins, enzymes and polysaccharides which have key roles in the reduction and stabilization of the metal NPs such as silver NPs [13].
Synthesis of the metal oxide NPs using plant extract is time-consuming as compared to the traditional synthesis techniques due to the lower concentration of the existing bioactive components. It decreases the yield and production rate of the synthesized NPs [14]. To increase the rate of the NP production rate, green synthesis of the NPs using plant extract can be coupled with three different heating methods, namely, reflux conditions, autoclave heating and microwave irradiation [15]. These methods can drastically accelerate the rate of the metal oxide NP formation in the aqueous solutions [16].
As a result, the main aims of this study were to (i) evaluate the synthesis potential of the Pelargonium leaves extract to fabricate ZnO NPs, (ii) synthesis of the ZnO NPs using Pelargonium leaves extract utilizing three heating methods, namely, microwave (at 250°C for 3 min), autoclave (at 121°C and 1.5 bar for 15 min) and conventional heating (at 150°C for 2 h), and (iii) study physico-chemical properties and antimicrobial activities of the fabricated ZnO NPs.
2 Materials and methods
2.1 Materials
Pelargonium zonale was provided from a greenhouse (Tabriz, Iran). Zn(NO3)3·6H2O was used as ZnO salt that was purchased from Sigma-Aldrich. Deionized double distilled water (Mojalali Co., Tehran, Iran) was used for plant extraction and preparation of all aqueous solutions. Staphylococcus aureus (PTCC 1112) and Escherichia coli (PTCC 1270) were obtained from microbial Persian type culture collection (PTCC, Tehran, Iran). Nutrient agar (NA) was provided from Biolife (Biolife Co., Milan, Italy).
2.2 Pelargonium leaf extract preparation
Pelargonium leaves were cut and washed to remove their dusts using double-distilled water and were dried at ambient conditions (at 20°C and 45% relative humidity) during 7 days. After that 1 g of the dried powder which was obtained using a domestic miller (MX-GX1521; Panasonic, Tokyo, Japan) was added into the boiling 100ml deionized water for 5 min and then filtered using filter paper (Whatman no. 1). The provided extract was kept at a cold temperature (4°C) throughout the experiments.
2.3 Synthesis of ZnO NPs
For preparation of the ZnO NPs, 2 g of the provided zinc nitrate salt was added into 20 ml of provided Pelargonium leaf extract and the mixture solutions were exposed to the three different heating methods namely autoclave, microwave irradiation and conventional heating (heater-stirrer) which were described below.
2.3.1 Conventional heating synthesis
In this method, the mixture solution was heated at 150°C for 2 h using a magnetic heater-stirrer adjusted at 700 rpm. Then, the prepared solution was placed into a ceramic crucible cup and kept in furnace (400°C for 2 h). The resulted yellow coloured powder, as fabricated ZnO NPs, was then studied.
2.3.2 Autoclave synthesis
In this method, the mixture solution was put in autoclave (15 psi and 121°C) for 15 min. Then, the provided solution was put into a ceramic crucible cup and transferred to the furnace set at mentioned conditions to form ZnO NPs. The resulted yellow coloured powder was then studied.
2.3.3 Microwave synthesis
In this method, the provided mixture solution was put into a domestic microwave oven (MG-2312W, LG Co., Seoul, South Korea) with the power of 800 W and a maximum oven temperature of 250°C for 3 min. As the same is mentioned in the previous methods, the solution was put into the furnace and the produced yellow coloured ZnO NPs powder was used to more physico-chemical and antimicrobial analysis.
2.4 Physico-chemical characteristics of the fabricated ZnO NPs
In order to find the main existing functional groups in the prepared Pelargonium leaf extract, Fourier-transform infrared spectroscopy (FT-IR) was utilized by using a Bruker Tensor 27 spectrometer (Bruker, Karlsruhe, Germany) and KBr pellets ranging from 4000 to 400 cm−1. By X-ray diffractometry (XRD: D5000, Siemens Co., Karlsruhe, Germany) using Cu Kα radiation and scanning electron microscopy (SEM, CamScan MV 2300, Tescan, Czech Republic), the structural properties and morphology of the fabricated ZnO NPs were evaluated. The average crystalline size of the synthesized ZnO NPs was obtained using Debye-Scherrer formula (Equation 1).
where, D, λ, β and θ are the crystalline size, X-ray wavelength, full line width at the half-maximum elevation of the main intensity peak, and Bragg angle, respectively [17].
For antioxidant activity evaluation of the formed ZnO NPs, the described method by Anzabi, based on the scavenging ability on 2,2-diphenyl-2-picrylhydrazyl (DPPH) was used. The scavenging ability was obtained by Equation 2 [14]:
where, I% is the inhibition percent and Acontrol and Asample are the absorbance of the control solution (without the main component) and the solution containing main component, respectively, which were measured using a UV-visible spectrophotometer (250–800 nm, Perkin Elmer, Überlingen, Germany).
2.5 Antibacterial activities of the fabricated ZnO NPs
For monitoring of the bactericidal effects of the fabricated ZnO NPs to the Staphylococcus aureus and Escherichia coli, well diffusion method was used according to the described procedures by Torabfam and Jafarizadeh-Malmiri [18]. The antibacterial activities of the fabricated ZnO NPs with three different methods could be related to the diameter of the created clear zone.
2.6 Statistical analysis
Physico-chemical analysis of the prepared extracts was carried out in three replications. Data were interpreted by analysis of variance (ANOVA) using Minitab v.16 statistical package (Minitab Inc., PA, USA). Tukey’s comparison test was used to compare the mean values. All comparison was made at 5% level of significance.
3 Results and discussion
3.1 FT-IR analysis of the Pelargonium leaf extract
To identify and study the existing functional groups in the Pelargonium leaf extract, FT-IR spectra of Pelargonium leaf extract was obtained (Figure 1). This figure indicates that four absorption peaks were centered at 669.44, 1636.59, 2068.15 and 3451.04 cm−1. The widest spectrum absorption peak (3451.04 cm−1) was related to the stretching vibrations of OH (hydroxyl groups) which was mainly obtained in the carbohydrate, tannins and phenolic compound structures, and the highlighted peak centered at 1636.59 cm−1 was attributed to the amide І group, which was related to the proteins [[13]. The presented OH groups in the leaf extract had a key role in the green fabrication of the ZnO NPs. In fact, hydroxyl groups of glucuronic acids present in hydrolysable tannins, reacted with zinc ions and resulted intermediate complexes which were oxidized and converted into the quinine forms. Quinine acted as reducing and capping agents and finally reduced zinc to the ZnO NPs [5]. It seems that the phenolic compounds of the leaf extract had the main potential of the reduction of zinc ions to form ZnO NPs. On the other hand, the opportunities of the nucleation and growth of the formed NPs increased by increasing the heating temperature due to the increasing collision rate of the fast reduced zinc ions and formation of the zinc elements in the synthesis solution 18].

FT-IR spectrums of Pelargonium zonae leaf extract.
3.2 XRD analysis of fabricated ZnO NPs
XRD patterns of the fabricated ZnO NPs using Pelargonium leaf extract and three different methods, namely, conventional heating (heater-stirrer), autoclave and microwave irradiation are indicated in Figure 2A–C, respectively. As clearly observed in Figure 2A, the peak position with 2θ values of 32.0069°, 34.7027°, 36.4775°, 47.7821°, 56.7634°, 63.0365°, 66.5725°, 68.0871°, 69.2823°, 72.7665°, 77.1089° and 81.6504° were indexed as (100), (002), (101), (102), (110), (103), (200), (112), (201), (004), (202) and (104) planes, which were in line with the International Center of Diffraction Data card (JCPDS-36-1451) and verified the formation of a crystalline hexagonal structure for the formed ZnO NPs [19]. The average crystalline size of the fabricated ZnO NPs using Pelargonium leaf extract and conventional heating method was 61 nm.
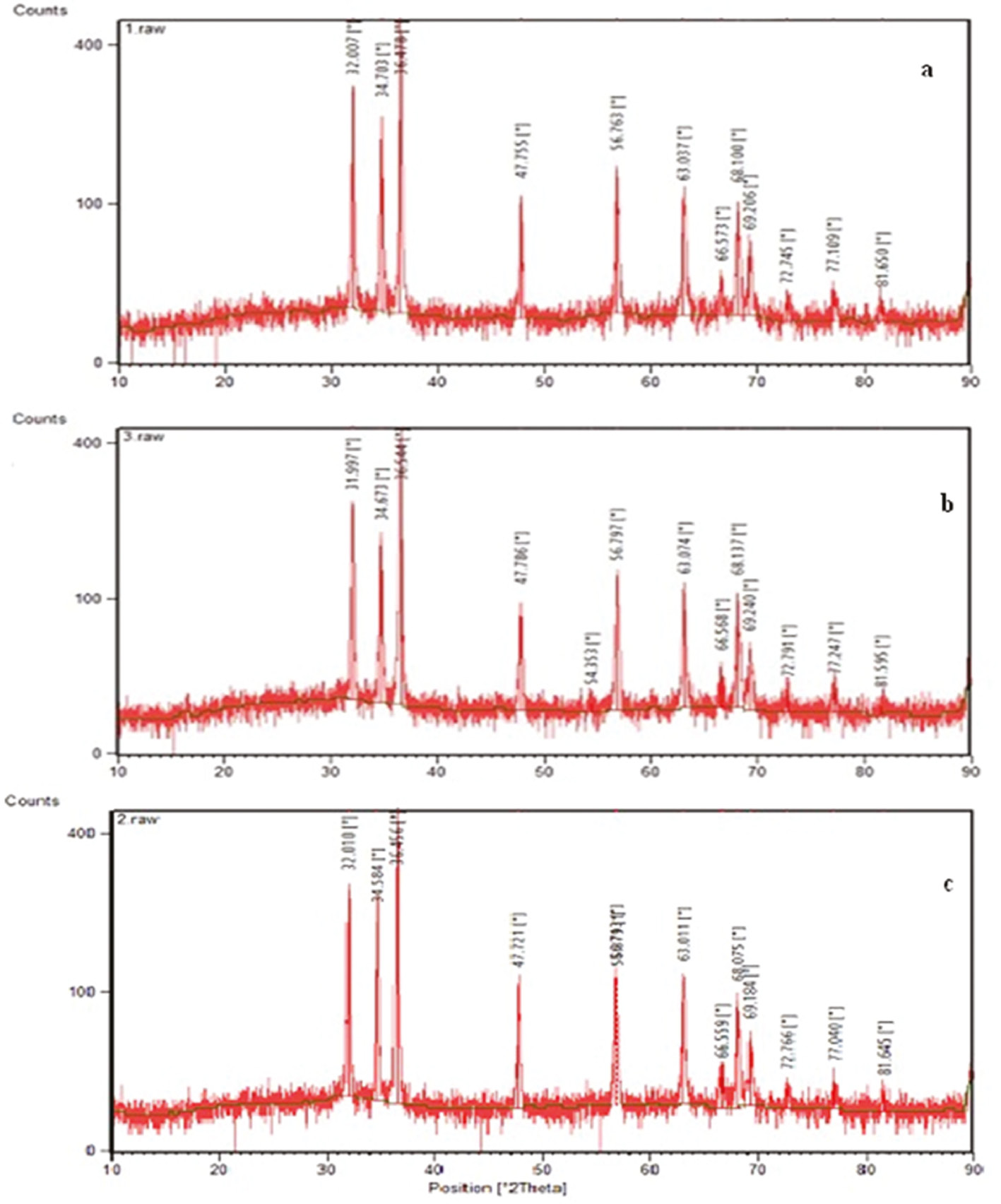
XRD pattern of the fabricated ZnO NPs using Pelargonium leaf extract and three different methods namely conventional heating (heater-stirrer) (A), autoclave (B), and microwave irradiation (C).
The XRD pattern of the synthesis ZnO NPs using autoclave heating and Pelargonium leaf extract, which is indicated in Figure 2B, showed the diffraction peaks centered at 2θ values of the 31.9974°, 34.6735°, 36.5438°, 47.7855°, 54.3528°, 56.7967°, 63.0736°, 66.5680°, 68.1375°, 69.2402°, 72.7907°, 77.2468°, and 81.5953° which were indexed as (100), (002), (101), (102), (110), (103), (200), (112), (201), (004), (202), (104) and (203) planes. The average crystalline size of the formed ZnO NPs during this procedure was calculated to be 60nm.
The XRD pattern of the synthesis ZnO NPs using microwave irradiation (Figure 2C) indicated various peaks placed at 2θ values of 32.0101°, 34.5839°, 36.4560°, 47.7213°, 56.7126°, 56.8791°, 63.0107°, 66.5589°, 68.0753°, 69.1841°, 72.7673°, 77.0398° and 81.6445°, which were indexed as (100), (002), (101), (102), (110), (103), (200), (112), (201), (004), (202), (104) and (203) planes. The average crystalline size of the synthesized ZnO NPs was calculated to be 51nm. The same XRD patterns obtained in the present study using three different heating methods verified the formation of ZnO NPs using Pelargonium leaf extract. The minimum crystaline size of the synthesized ZnO NPs under microwave irradiation (51nm), as compared to that of ZnO NPs obtained using another two heating methods, can be explained by the fact that in the microwave heating fabrication technique for formation of ZnO NPs to achieve high temperatures within a limited time, uniform heat distribution was formed through the mixture solution containing zinc salt and Pelargonium leaf extract and increased movement of the formed ZnO NPs, which in turn, inhibited growth of particles by coagulation.
The obtained results were close to the findings of Çolak and Karaköse [20]. They synthesized ZnO NPs using aqueous extract of Thyme and deionized water with average crystallite sizes of 42 and 85 nm, respectively.
3.3 Morphology
The typical SEM images for the fabricated ZnO NPs using Pelargonium leaf extract and three heating methods, namely, conventional heating (heater-stirrer), autoclave and microwave irradiation are shown in the Figure 3A–C, respectively. Figure 3 indicates that the individual and aggregate ZnO NPs were formed and the formed particles had spherical shape.

SEM images for the fabricated ZnO NPs using Pelargonium leaf extract and three heating methods, namely, conventional heating (heater-stirrer) (A), autoclave (B), and microwave irradiation (C).
3.4 Antibacterial
The antibacterial activities of the fabricated ZnO NPs using Pelargonium leaf extract and three heating methods, namely, conventional heating (heater-stirrer), autoclave and microwave irradiation is indicated in Table 1. The obtained results revealed that all the formed ZnO NPs had bactericidal effects against both the Gram negative and Gram positive bacteria strains. However, this effect was higher on Staphylococcus aureus (Figure 4A) as compared to the Escherichia coli (Figure 4B). Furthermore, the synthesized ZnO NPs using conventional heating method had highest antibacterial activities toward both the studied bacteria strains as compared to those fabricated using another two different synthesis methods. The main mechanism of the bactericidal activities of ZnO NPs can be related to their photochemical property. It seems that the adsorbed oxygen and H2O on the surface of ZnO NPs interact with photoinduced charge carriers and reactive oxygen forms such as singlet oxygen and hydroxyl radical which these compounds cause membrane lipid peroxidation and show bactericidal effect [[21], [22], [23]. The obtained results were in line with the findings of Shakeel et al. 24]. They also indicated that the synthesized ZnO NPs using Abutilon indicum, Clerodendrum infortunatum and Clerodendrum inerme had high antibacterial activity against both Gram negative and Gram positive bacteria strains.
Diameter of clear zones (mm) around the synthesized ZnO NPs using Pelargonium leaf extract and three heating methods, namely, conventional heating (heater-stirrer), autoclave, and microwave irradiation, is indicated.
| Synthesized methods | Staphylococcus aureus | Escherichia coli |
|---|---|---|
| Conventional heating | 20 ml | 14 ml |
| Autoclave heating | 13 ml | 10 ml |
| Microwave irradiation | 15 ml | 12 ml |
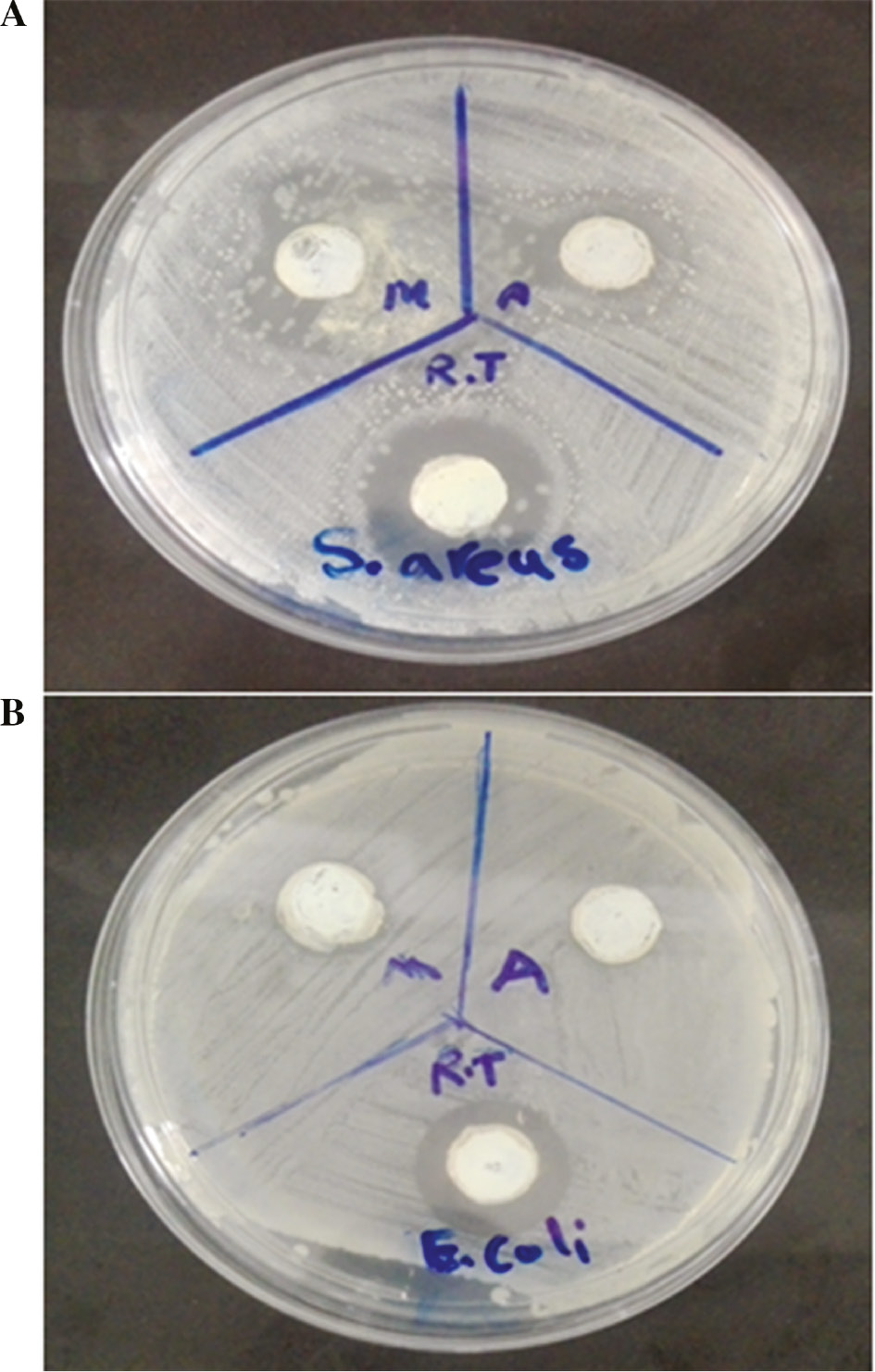
Antibacterial activities of the fabricated ZnO NPs using Pelargonium leaf extract and three heating methods to Staphylococcus aureus (A) and Escherichia coli (B).
3.5 Antioxidant
The obtained results indicated that antioxidant activities of the synthesized ZnO NPs using Pelargonium leaf extract and three different synthesis methods, namely, conventional heating, autoclave and microwave irradiation were 7.8, 4.1 and 5.5%, respectively. In fact, the highest antioxidant activity was achieved using conventional heating at 150°C and 2 h. The DPPH radical is purple in colour and upon reaction with hydrogen donors, changes to yellow in colour [25]. It seems that at the highest temperature and reaction time, the reaction between DPPH radical and hydrogen donors existing in the leaf extract was completed and the antioxidant value increased.
4 Conclusions
Clean, rapid and one-step fabrication method was developed using Pelargonium zonale leaf extract to the synthesis of the ZnO NPs. For this reason, three different heating methods, namely, conventional heating, autoclave and microwave irradiation, were utilized to accelerate the rate of the NP formation. The obtained results demonstrated the high potential application of the Pelargonium zonale in the fabrication of the ZnO NPs. Furthermore, the ZnO NPs with the highest antioxidant and antibacterial activities were fabricated using conventional heating method and the ZnO NPs with the lowest particle size was synthesized using microwave irradiation. Such developed green synthesis method can be utilized to make other metal and metal oxide NPs.
Acknowledgments
The authors would like to acknowledge the Iran Nanotechnology Initiatives Council (INIC) for its financial support (grant no. 140560).
Conflict of interest statement: The authors declare that they have no conflict of interest.
References
[1] Ramimoghadam D, Bin Hussein MZ, Taufiq-Yap YH. Chem. Cent. J. 2013, 7, 136–146.10.1186/1752-153X-7-136Search in Google Scholar PubMed PubMed Central
[2] Padmavathy N, Vijayaraghavan R. Sci. Technol. Adv. Mater. 2008, 9, 035004–035011.10.1088/1468-6996/9/3/035004Search in Google Scholar PubMed PubMed Central
[3] Ehsan S, Sajjad M. J. Biomater. Nanobiotechnol. 2017, 8, 159–175.10.4236/jbnb.2017.82011Search in Google Scholar
[4] Subramani K, Palanisamy S, Kolathupalayam SB, Rangaraj S, Malik M. Adv. Powder Technol. 2017, 28, 3184–3194.10.1016/j.apt.2017.09.033Search in Google Scholar
[5] Elumalai K, Velmurugan S, Ravi S, Kathiravan V, Ashokkumar S. Spectrochim. Acta A 2015, 143, 158–164.10.1016/j.saa.2015.02.011Search in Google Scholar PubMed
[6] Zare E, Pourseyedi S, Khatami M, Darezereshki E. J. Mol. Struct. 2017, 1146, 96–103.10.1016/j.molstruc.2017.05.118Search in Google Scholar
[7] Agarwal H, Venkat Kumar S, Rajeshkumar S. Resour. Technol. 2017, 3, 406–413.Search in Google Scholar
[8] Thema FT, Manikandan E, Dhlamini MS, Maaza M. Mater. Lett. 2015, 161, 124–127.10.1016/j.matlet.2015.08.052Search in Google Scholar
[9] Sabir S, Arshad M, Khalil Chaudhari S. Sci. World J. doi: 10.1155/2014/925494.10.1155/2014/925494Search in Google Scholar PubMed PubMed Central
[10] Çolak H, Karaköse E, Duman F. Environ. Chem. Lett. 2017, 15, 547–552.10.1007/s10311-017-0629-zSearch in Google Scholar
[11] Çolak H, Karaköse E, Duman F. Green Process. Synth. 2017, 6, 317–323.Search in Google Scholar
[12] Parveen K, Banse V, Ledwani L. AIP Conf. Proc. 2016, 1724, 020048-1–020048-7.Search in Google Scholar
[13] Mohammadlou M, Jafarizadeh-Malmiri H, Maghsoudi H. Green Process. Synth. 2017, 6, 31–42.Search in Google Scholar
[14] Anzabi Y. Green Process. Synth. 2018, 7, 114–121.10.1515/gps-2017-0014Search in Google Scholar
[15] Mikrovalov V. Mater. Technol. 2011, 45, 173–177.Search in Google Scholar
[16] Chandore V, Carpenter G, Sen R, Gupta N. Int. J. Env. Sci. 2013, 4, 45–47.Search in Google Scholar
[17] Rajakumar G, Thiruvengadam M, Mydhili G, Gomathi T, Chung IM. Bioprocess Biosys. Eng. 2018, 41, 21–30.10.1007/s00449-017-1840-9Search in Google Scholar PubMed
[18] Torabfam M, Jafarizadeh-Malmiri H. Green Process. Synth. 2018, 7, 530–537.10.1515/gps-2017-0139Search in Google Scholar
[19] Kawano T, Imai H. Cryst. Growth Des. 2006, 6, 1054–1056.10.1021/cg050338aSearch in Google Scholar
[20] Çolak H, Karaköse E. J. Mater. Sci: Mater. Electron. 2017, 28, 12184–12190.Search in Google Scholar
[21] Lipovsky A, Tzitrinovich Z, Friedmann H, Applerot G, Gedanken A, Lubart R. J. Phys. Chem. 2009, 113, 15997–16001.Search in Google Scholar
[22] Li Y, Zhang W, Niu J, Chen Y. ACS Nano 2012, 6, 5164–5173.10.1021/nn300934kSearch in Google Scholar PubMed
[23] Dutta RK, Nenavathu BP, Gangishetty MK, Reddy AVR. Colloids Surf. B Biointerfaces 2012, 94, 143–150.10.1016/j.colsurfb.2012.01.046Search in Google Scholar PubMed
[24] Shakeel AK, Farah N, Sadia K, Ahsan I, Ghulam H. Mater. Sci. Eng. 2018, 82, 46–59.10.1016/j.msec.2017.08.071Search in Google Scholar PubMed
[25] Mahendiran D, Subash G, Arumai Selvan D, Rehana D, Senthil Kumar R, Kalilur Rahiman A. BioNanoSci. 2017, 7, 530–545.10.1007/s12668-017-0418-ySearch in Google Scholar
©2019 Walter de Gruyter GmbH, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 Public License.
Articles in the same Issue
- Regular Articles
- Studies on the preparation and properties of biodegradable polyester from soybean oil
- Flow-mode biodiesel production from palm oil using a pressurized microwave reactor
- Reduction of free fatty acids in waste oil for biodiesel production by glycerolysis: investigation and optimization of process parameters
- Saccharin: a cheap and mild acidic agent for the synthesis of azo dyes via telescoped dediazotization
- Optimization of lipase-catalyzed synthesis of polyethylene glycol stearate in a solvent-free system
- Green synthesis of iron oxide nanoparticles using Platanus orientalis leaf extract for antifungal activity
- Ultrasound assisted chemical activation of peanut husk for copper removal
- Room temperature silanization of Fe3O4 for the preparation of phenyl functionalized magnetic adsorbent for dispersive solid phase extraction for the extraction of phthalates in water
- Evaluation of the saponin green extraction from Ziziphus spina-christi leaves using hydrothermal, microwave and Bain-Marie water bath heating methods
- Oxidation of dibenzothiophene using the heterogeneous catalyst of tungsten-based carbon nanotubes
- Calcined sodium silicate as an efficient and benign heterogeneous catalyst for the transesterification of natural lecithin to L-α-glycerophosphocholine
- Synergistic effect between CO2 and H2O2 on ethylbenzene oxidation catalyzed by carbon supported heteropolyanion catalysts
- Hydrocyanation of 2-arylmethyleneindan-1,3-diones using potassium hexacyanoferrate(II) as a nontoxic cyanating agent
- Green synthesis of hydratropic aldehyde from α-methylstyrene catalyzed by Al2O3-supported metal phthalocyanines
- Environmentally benign chemical recycling of polycarbonate wastes: comparison of micro- and nano-TiO2 solid support efficiencies
- Medicago polymorpha-mediated antibacterial silver nanoparticles in the reduction of methyl orange
- Production of value-added chemicals from esterification of waste glycerol over MCM-41 supported catalysts
- Green synthesis of zerovalent copper nanoparticles for efficient reduction of toxic azo dyes congo red and methyl orange
- Optimization of the biological synthesis of silver nanoparticles using Penicillium oxalicum GRS-1 and their antimicrobial effects against common food-borne pathogens
- Optimization of submerged fermentation conditions to overproduce bioethanol using two industrial and traditional Saccharomyces cerevisiae strains
- Extraction of In3+ and Fe3+ from sulfate solutions by using a 3D-printed “Y”-shaped microreactor
- Foliar-mediated Ag:ZnO nanophotocatalysts: green synthesis, characterization, pollutants degradation, and in vitro biocidal activity
- Green cyclic acetals production by glycerol etherification reaction with benzaldehyde using cationic acidic resin
- Biosynthesis, characterization and antimicrobial activities assessment of fabricated selenium nanoparticles using Pelargonium zonale leaf extract
- Synthesis of high surface area magnesia by using walnut shell as a template
- Controllable biosynthesis of silver nanoparticles using actinobacterial strains
- Green vegetation: a promising source of color dyes
- Mechano-chemical synthesis of ammonia and acetic acid from inorganic materials in water
- Green synthesis and structural characterization of novel N1-substituted 3,4-dihydropyrimidin-2(1H)-ones
- Biodiesel production from cotton oil using heterogeneous CaO catalysts from eggshells prepared at different calcination temperatures
- Regeneration of spent mercury catalyst for the treatment of dye wastewater by the microwave and ultrasonic spray-assisted method
- Green synthesis of the innovative super paramagnetic nanoparticles from the leaves extract of Fraxinus chinensis Roxb and their application for the decolourisation of toxic dyes
- Biogenic ZnO nanoparticles: a study of blueshift of optical band gap and photocatalytic degradation of reactive yellow 186 dye under direct sunlight
- Leached compounds from the extracts of pomegranate peel, green coconut shell, and karuvelam wood for the removal of hexavalent chromium
- Enhancement of molecular weight reduction of natural rubber in triphasic CO2/toluene/H2O systems with hydrogen peroxide for preparation of biobased polyurethanes
- An efficient green synthesis of novel 1H-imidazo[1,2-a]imidazole-3-amine and imidazo[2,1-c][1,2,4]triazole-5-amine derivatives via Strecker reaction under controlled microwave heating
- Evaluation of three different green fabrication methods for the synthesis of crystalline ZnO nanoparticles using Pelargonium zonale leaf extract
- A highly efficient and multifunctional biomass supporting Ag, Ni, and Cu nanoparticles through wetness impregnation for environmental remediation
- Simple one-pot green method for large-scale production of mesalamine, an anti-inflammatory agent
- Relationships between step and cumulative PMI and E-factors: implications on estimating material efficiency with respect to charting synthesis optimization strategies
- A comparative sorption study of Cr3+ and Cr6+ using mango peels: kinetic, equilibrium and thermodynamic
- Effects of acid hydrolysis waste liquid recycle on preparation of microcrystalline cellulose
- Use of deep eutectic solvents as catalyst: A mini-review
- Microwave-assisted synthesis of pyrrolidinone derivatives using 1,1’-butylenebis(3-sulfo-3H-imidazol-1-ium) chloride in ethylene glycol
- Green and eco-friendly synthesis of Co3O4 and Ag-Co3O4: Characterization and photo-catalytic activity
- Adsorption optimized of the coal-based material and application for cyanide wastewater treatment
- Aloe vera leaf extract mediated green synthesis of selenium nanoparticles and assessment of their In vitro antimicrobial activity against spoilage fungi and pathogenic bacteria strains
- Waste phenolic resin derived activated carbon by microwave-assisted KOH activation and application to dye wastewater treatment
- Direct ethanol production from cellulose by consortium of Trichoderma reesei and Candida molischiana
- Agricultural waste biomass-assisted nanostructures: Synthesis and application
- Biodiesel production from rubber seed oil using calcium oxide derived from eggshell as catalyst – optimization and modeling studies
- Study of fabrication of fully aqueous solution processed SnS quantum dot-sensitized solar cell
- Assessment of aqueous extract of Gypsophila aretioides for inhibitory effects on calcium carbonate formation
- An environmentally friendly acylation reaction of 2-methylnaphthalene in solvent-free condition in a micro-channel reactor
- Aegle marmelos phytochemical stabilized synthesis and characterization of ZnO nanoparticles and their role against agriculture and food pathogen
- A reactive coupling process for co-production of solketal and biodiesel
- Optimization of the asymmetric synthesis of (S)-1-phenylethanol using Ispir bean as whole-cell biocatalyst
- Synthesis of pyrazolopyridine and pyrazoloquinoline derivatives by one-pot, three-component reactions of arylglyoxals, 3-methyl-1-aryl-1H-pyrazol-5-amines and cyclic 1,3-dicarbonyl compounds in the presence of tetrapropylammonium bromide
- Preconcentration of morphine in urine sample using a green and solvent-free microextraction method
- Extraction of glycyrrhizic acid by aqueous two-phase system formed by PEG and two environmentally friendly organic acid salts - sodium citrate and sodium tartrate
- Green synthesis of copper oxide nanoparticles using Juglans regia leaf extract and assessment of their physico-chemical and biological properties
- Deep eutectic solvents (DESs) as powerful and recyclable catalysts and solvents for the synthesis of 3,4-dihydropyrimidin-2(1H)-ones/thiones
- Biosynthesis, characterization and anti-microbial activity of silver nanoparticle based gel hand wash
- Efficient and selective microwave-assisted O-methylation of phenolic compounds using tetramethylammonium hydroxide (TMAH)
- Anticoagulant, thrombolytic and antibacterial activities of Euphorbia acruensis latex-mediated bioengineered silver nanoparticles
- Volcanic ash as reusable catalyst in the green synthesis of 3H-1,5-benzodiazepines
- Green synthesis, anionic polymerization of 1,4-bis(methacryloyl)piperazine using Algerian clay as catalyst
- Selenium supplementation during fermentation with sugar beet molasses and Saccharomyces cerevisiae to increase bioethanol production
- Biosynthetic potential assessment of four food pathogenic bacteria in hydrothermally silver nanoparticles fabrication
- Investigating the effectiveness of classical and eco-friendly approaches for synthesis of dialdehydes from organic dihalides
- Pyrolysis of palm oil using zeolite catalyst and characterization of the boil-oil
- Azadirachta indica leaves extract assisted green synthesis of Ag-TiO2 for degradation of Methylene blue and Rhodamine B dyes in aqueous medium
- Synthesis of vitamin E succinate catalyzed by nano-SiO2 immobilized DMAP derivative in mixed solvent system
- Extraction of phytosterols from melon (Cucumis melo) seeds by supercritical CO2 as a clean technology
- Production of uronic acids by hydrothermolysis of pectin as a model substance for plant biomass waste
- Biofabrication of highly pure copper oxide nanoparticles using wheat seed extract and their catalytic activity: A mechanistic approach
- Intelligent modeling and optimization of emulsion aggregation method for producing green printing ink
- Improved removal of methylene blue on modified hierarchical zeolite Y: Achieved by a “destructive-constructive” method
- Two different facile and efficient approaches for the synthesis of various N-arylacetamides via N-acetylation of arylamines and straightforward one-pot reductive acetylation of nitroarenes promoted by recyclable CuFe2O4 nanoparticles in water
- Optimization of acid catalyzed esterification and mixed metal oxide catalyzed transesterification for biodiesel production from Moringa oleifera oil
- Kinetics and the fluidity of the stearic acid esters with different carbon backbones
- Aiming for a standardized protocol for preparing a process green synthesis report and for ranking multiple synthesis plans to a common target product
- Microstructure and luminescence of VO2 (B) nanoparticle synthesis by hydrothermal method
- Optimization of uranium removal from uranium plant wastewater by response surface methodology (RSM)
- Microwave drying of nickel-containing residue: dielectric properties, kinetics, and energy aspects
- Simple and convenient two step synthesis of 5-bromo-2,3-dimethoxy-6-methyl-1,4-benzoquinone
- Biodiesel production from waste cooking oil
- The effect of activation temperature on structure and properties of blue coke-based activated carbon by CO2 activation
- Optimization of reaction parameters for the green synthesis of zero valent iron nanoparticles using pine tree needles
- Microwave-assisted protocol for squalene isolation and conversion from oil-deodoriser distillates
- Denitrification performance of rare earth tailings-based catalysts
- Facile synthesis of silver nanoparticles using Averrhoa bilimbi L and Plum extracts and investigation on the synergistic bioactivity using in vitro models
- Green production of AgNPs and their phytostimulatory impact
- Photocatalytic activity of Ag/Ni bi-metallic nanoparticles on textile dye removal
- Topical Issue: Green Process Engineering / Guest Editors: Martine Poux, Patrick Cognet
- Modelling and optimisation of oxidative desulphurisation of tyre-derived oil via central composite design approach
- CO2 sequestration by carbonation of olivine: a new process for optimal separation of the solids produced
- Organic carbonates synthesis improved by pervaporation for CO2 utilisation
- Production of starch nanoparticles through solvent-antisolvent precipitation in a spinning disc reactor
- A kinetic study of Zn halide/TBAB-catalysed fixation of CO2 with styrene oxide in propylene carbonate
- Topical on Green Process Engineering
Articles in the same Issue
- Regular Articles
- Studies on the preparation and properties of biodegradable polyester from soybean oil
- Flow-mode biodiesel production from palm oil using a pressurized microwave reactor
- Reduction of free fatty acids in waste oil for biodiesel production by glycerolysis: investigation and optimization of process parameters
- Saccharin: a cheap and mild acidic agent for the synthesis of azo dyes via telescoped dediazotization
- Optimization of lipase-catalyzed synthesis of polyethylene glycol stearate in a solvent-free system
- Green synthesis of iron oxide nanoparticles using Platanus orientalis leaf extract for antifungal activity
- Ultrasound assisted chemical activation of peanut husk for copper removal
- Room temperature silanization of Fe3O4 for the preparation of phenyl functionalized magnetic adsorbent for dispersive solid phase extraction for the extraction of phthalates in water
- Evaluation of the saponin green extraction from Ziziphus spina-christi leaves using hydrothermal, microwave and Bain-Marie water bath heating methods
- Oxidation of dibenzothiophene using the heterogeneous catalyst of tungsten-based carbon nanotubes
- Calcined sodium silicate as an efficient and benign heterogeneous catalyst for the transesterification of natural lecithin to L-α-glycerophosphocholine
- Synergistic effect between CO2 and H2O2 on ethylbenzene oxidation catalyzed by carbon supported heteropolyanion catalysts
- Hydrocyanation of 2-arylmethyleneindan-1,3-diones using potassium hexacyanoferrate(II) as a nontoxic cyanating agent
- Green synthesis of hydratropic aldehyde from α-methylstyrene catalyzed by Al2O3-supported metal phthalocyanines
- Environmentally benign chemical recycling of polycarbonate wastes: comparison of micro- and nano-TiO2 solid support efficiencies
- Medicago polymorpha-mediated antibacterial silver nanoparticles in the reduction of methyl orange
- Production of value-added chemicals from esterification of waste glycerol over MCM-41 supported catalysts
- Green synthesis of zerovalent copper nanoparticles for efficient reduction of toxic azo dyes congo red and methyl orange
- Optimization of the biological synthesis of silver nanoparticles using Penicillium oxalicum GRS-1 and their antimicrobial effects against common food-borne pathogens
- Optimization of submerged fermentation conditions to overproduce bioethanol using two industrial and traditional Saccharomyces cerevisiae strains
- Extraction of In3+ and Fe3+ from sulfate solutions by using a 3D-printed “Y”-shaped microreactor
- Foliar-mediated Ag:ZnO nanophotocatalysts: green synthesis, characterization, pollutants degradation, and in vitro biocidal activity
- Green cyclic acetals production by glycerol etherification reaction with benzaldehyde using cationic acidic resin
- Biosynthesis, characterization and antimicrobial activities assessment of fabricated selenium nanoparticles using Pelargonium zonale leaf extract
- Synthesis of high surface area magnesia by using walnut shell as a template
- Controllable biosynthesis of silver nanoparticles using actinobacterial strains
- Green vegetation: a promising source of color dyes
- Mechano-chemical synthesis of ammonia and acetic acid from inorganic materials in water
- Green synthesis and structural characterization of novel N1-substituted 3,4-dihydropyrimidin-2(1H)-ones
- Biodiesel production from cotton oil using heterogeneous CaO catalysts from eggshells prepared at different calcination temperatures
- Regeneration of spent mercury catalyst for the treatment of dye wastewater by the microwave and ultrasonic spray-assisted method
- Green synthesis of the innovative super paramagnetic nanoparticles from the leaves extract of Fraxinus chinensis Roxb and their application for the decolourisation of toxic dyes
- Biogenic ZnO nanoparticles: a study of blueshift of optical band gap and photocatalytic degradation of reactive yellow 186 dye under direct sunlight
- Leached compounds from the extracts of pomegranate peel, green coconut shell, and karuvelam wood for the removal of hexavalent chromium
- Enhancement of molecular weight reduction of natural rubber in triphasic CO2/toluene/H2O systems with hydrogen peroxide for preparation of biobased polyurethanes
- An efficient green synthesis of novel 1H-imidazo[1,2-a]imidazole-3-amine and imidazo[2,1-c][1,2,4]triazole-5-amine derivatives via Strecker reaction under controlled microwave heating
- Evaluation of three different green fabrication methods for the synthesis of crystalline ZnO nanoparticles using Pelargonium zonale leaf extract
- A highly efficient and multifunctional biomass supporting Ag, Ni, and Cu nanoparticles through wetness impregnation for environmental remediation
- Simple one-pot green method for large-scale production of mesalamine, an anti-inflammatory agent
- Relationships between step and cumulative PMI and E-factors: implications on estimating material efficiency with respect to charting synthesis optimization strategies
- A comparative sorption study of Cr3+ and Cr6+ using mango peels: kinetic, equilibrium and thermodynamic
- Effects of acid hydrolysis waste liquid recycle on preparation of microcrystalline cellulose
- Use of deep eutectic solvents as catalyst: A mini-review
- Microwave-assisted synthesis of pyrrolidinone derivatives using 1,1’-butylenebis(3-sulfo-3H-imidazol-1-ium) chloride in ethylene glycol
- Green and eco-friendly synthesis of Co3O4 and Ag-Co3O4: Characterization and photo-catalytic activity
- Adsorption optimized of the coal-based material and application for cyanide wastewater treatment
- Aloe vera leaf extract mediated green synthesis of selenium nanoparticles and assessment of their In vitro antimicrobial activity against spoilage fungi and pathogenic bacteria strains
- Waste phenolic resin derived activated carbon by microwave-assisted KOH activation and application to dye wastewater treatment
- Direct ethanol production from cellulose by consortium of Trichoderma reesei and Candida molischiana
- Agricultural waste biomass-assisted nanostructures: Synthesis and application
- Biodiesel production from rubber seed oil using calcium oxide derived from eggshell as catalyst – optimization and modeling studies
- Study of fabrication of fully aqueous solution processed SnS quantum dot-sensitized solar cell
- Assessment of aqueous extract of Gypsophila aretioides for inhibitory effects on calcium carbonate formation
- An environmentally friendly acylation reaction of 2-methylnaphthalene in solvent-free condition in a micro-channel reactor
- Aegle marmelos phytochemical stabilized synthesis and characterization of ZnO nanoparticles and their role against agriculture and food pathogen
- A reactive coupling process for co-production of solketal and biodiesel
- Optimization of the asymmetric synthesis of (S)-1-phenylethanol using Ispir bean as whole-cell biocatalyst
- Synthesis of pyrazolopyridine and pyrazoloquinoline derivatives by one-pot, three-component reactions of arylglyoxals, 3-methyl-1-aryl-1H-pyrazol-5-amines and cyclic 1,3-dicarbonyl compounds in the presence of tetrapropylammonium bromide
- Preconcentration of morphine in urine sample using a green and solvent-free microextraction method
- Extraction of glycyrrhizic acid by aqueous two-phase system formed by PEG and two environmentally friendly organic acid salts - sodium citrate and sodium tartrate
- Green synthesis of copper oxide nanoparticles using Juglans regia leaf extract and assessment of their physico-chemical and biological properties
- Deep eutectic solvents (DESs) as powerful and recyclable catalysts and solvents for the synthesis of 3,4-dihydropyrimidin-2(1H)-ones/thiones
- Biosynthesis, characterization and anti-microbial activity of silver nanoparticle based gel hand wash
- Efficient and selective microwave-assisted O-methylation of phenolic compounds using tetramethylammonium hydroxide (TMAH)
- Anticoagulant, thrombolytic and antibacterial activities of Euphorbia acruensis latex-mediated bioengineered silver nanoparticles
- Volcanic ash as reusable catalyst in the green synthesis of 3H-1,5-benzodiazepines
- Green synthesis, anionic polymerization of 1,4-bis(methacryloyl)piperazine using Algerian clay as catalyst
- Selenium supplementation during fermentation with sugar beet molasses and Saccharomyces cerevisiae to increase bioethanol production
- Biosynthetic potential assessment of four food pathogenic bacteria in hydrothermally silver nanoparticles fabrication
- Investigating the effectiveness of classical and eco-friendly approaches for synthesis of dialdehydes from organic dihalides
- Pyrolysis of palm oil using zeolite catalyst and characterization of the boil-oil
- Azadirachta indica leaves extract assisted green synthesis of Ag-TiO2 for degradation of Methylene blue and Rhodamine B dyes in aqueous medium
- Synthesis of vitamin E succinate catalyzed by nano-SiO2 immobilized DMAP derivative in mixed solvent system
- Extraction of phytosterols from melon (Cucumis melo) seeds by supercritical CO2 as a clean technology
- Production of uronic acids by hydrothermolysis of pectin as a model substance for plant biomass waste
- Biofabrication of highly pure copper oxide nanoparticles using wheat seed extract and their catalytic activity: A mechanistic approach
- Intelligent modeling and optimization of emulsion aggregation method for producing green printing ink
- Improved removal of methylene blue on modified hierarchical zeolite Y: Achieved by a “destructive-constructive” method
- Two different facile and efficient approaches for the synthesis of various N-arylacetamides via N-acetylation of arylamines and straightforward one-pot reductive acetylation of nitroarenes promoted by recyclable CuFe2O4 nanoparticles in water
- Optimization of acid catalyzed esterification and mixed metal oxide catalyzed transesterification for biodiesel production from Moringa oleifera oil
- Kinetics and the fluidity of the stearic acid esters with different carbon backbones
- Aiming for a standardized protocol for preparing a process green synthesis report and for ranking multiple synthesis plans to a common target product
- Microstructure and luminescence of VO2 (B) nanoparticle synthesis by hydrothermal method
- Optimization of uranium removal from uranium plant wastewater by response surface methodology (RSM)
- Microwave drying of nickel-containing residue: dielectric properties, kinetics, and energy aspects
- Simple and convenient two step synthesis of 5-bromo-2,3-dimethoxy-6-methyl-1,4-benzoquinone
- Biodiesel production from waste cooking oil
- The effect of activation temperature on structure and properties of blue coke-based activated carbon by CO2 activation
- Optimization of reaction parameters for the green synthesis of zero valent iron nanoparticles using pine tree needles
- Microwave-assisted protocol for squalene isolation and conversion from oil-deodoriser distillates
- Denitrification performance of rare earth tailings-based catalysts
- Facile synthesis of silver nanoparticles using Averrhoa bilimbi L and Plum extracts and investigation on the synergistic bioactivity using in vitro models
- Green production of AgNPs and their phytostimulatory impact
- Photocatalytic activity of Ag/Ni bi-metallic nanoparticles on textile dye removal
- Topical Issue: Green Process Engineering / Guest Editors: Martine Poux, Patrick Cognet
- Modelling and optimisation of oxidative desulphurisation of tyre-derived oil via central composite design approach
- CO2 sequestration by carbonation of olivine: a new process for optimal separation of the solids produced
- Organic carbonates synthesis improved by pervaporation for CO2 utilisation
- Production of starch nanoparticles through solvent-antisolvent precipitation in a spinning disc reactor
- A kinetic study of Zn halide/TBAB-catalysed fixation of CO2 with styrene oxide in propylene carbonate
- Topical on Green Process Engineering

