Abstract
The present study aimed for the extraction of color dyes from different sources, such as Brassica oleracea, Brassica campestris, Citrus limon, Citrus limetta, Tagetes erecta, Spinacea oleracea, Beta vulgaris, Rosa indica and Curcuma longa. The leftovers of such plants were mainly used for color dye extraction and their confirmation using spectrophotometric analysis. The specific color pigments like carotenoids, anthocyanin, chlorophyll and betanin were found to be the main coloring agents that impart specific color to the samples. Among all these samples, the maximum yield was obtained from C. limetta aqueous peel extract, and among all the temperatures employed room temperature was found out to be the most suitable temperature for the stability of color extracts. The extracted colors were utilized in candy making and sugar syrup making and were also used for coloring various foods stuffs. Moreover, the extracted color dyes were applied for dyeing purposes on cotton cloth with alum showing better and more enhancing color fastness results than the lime.
1 Introduction
From primeval times, mankind has been fascinated by color and has left an impression on his environment in the form of painted images, which beautified his world and expressed his thoughts and feelings. In fact, color has always been a dominating element in the cultures of peoples all over the world, having been used throughout history for a great variety of purposes, not only, for instance, to produce artworks and adorn objects or individuals, but often to designate significance and hierarchical status as well. Early civilizations such as the Romans accepted that people “eat with their eyes”. Natural dyestuffs have been utilized for a long time. Natural dyes were found accidentally; they have a great influence on human life, and it is impossible to think of life without colors [1].
Color pigments that occur in plants are commonly referred to as natural colors. Ancient people employed color in their everyday life for coloring their body parts as they thought the colors provided an attractive look. They also dyed animal furs and skins to utilize them for clothing. Colors were also used for decorating the walls of their mud houses. For all these purposes the colors were extracted from different natural materials [2]. Four major types of color pigments are present as chlorophyll, anthocyanin, carotenoids and betalains. Natural colors have low stability when exposed to oxygen, metal, lignin, and oxidizing agents, as well as to extreme temperature and pH.
The beautiful colors that are created from natural dyes would initially appear stunning but would soon fade. Lack of color fastness, helped in promoting the discovery of mordants. Mordants are substances that aid in the absorption of dyes. Natural colors do not easily dye fabrics but require mordants to lock the dyes into the fabrics and prevent the color from vanishing. Mordants consist of chemical elements that lock the color onto the cloth stuff. It is a supplementary constituent involved in the response between the fiber and color dye which is normally utilized throughout natural dyeing procedure. Dyeing by using diverse mordants generate limited outcomes that resulted in unusual colors on the dyed fabrics [3].
Natural dyes work in combination with mordants to give a particularly good color range on different fabrics like cotton, silk, and linen [4]. Unfortunately, dyes that are extracted naturally are seldom used during dying. It has been generally perceived that naturally extracted colors give faint and less compatible colors compared to synthetic ones, but it has been observed that natural colors give better color shades compared to synthetic dyes [5].
The above-mentioned studies reveal that the use of natural color dyes is eco-friendly to the environment compared to synthetic dyes, which are composed of chemicals that might be carcinogenic and may cause serious health problems. The use of locally prepared natural dyes using cheap resources may reduce the importation of expensive chemicals which is at present a great burden to the Pakistani economy.
2 Materials and methods
The present investigation was commenced for color extraction from such sources that were mainly considered as waste, and then the experiment was performed to evaluate the utilization of the extracted colors in food and for dyeing purposes.
2.1 Plant material
For the extraction of color dyes for the various experimental analyses, plant materials (including leaves, leaf stalks, petals, and peels) were obtained from different markets and gardens around Lahore (Table 1).
Plant materials used for color extraction.
| Serial no. | Samples | Common name | Part used |
|---|---|---|---|
| 1 | Atriplex rubra (L.) Crantz | Red orchid | Leaves |
| 2 | Brassica oleracea L. | Red cabbage | Leaves |
| 3 | Brassica campestris L. | Mustard, sarson | Leaves and stem |
| 4 | Citrus limon L. | Lemon | Peel |
| 5 | Tagetes erecta L. | Marigold | Petals |
| 6 | Spinacea oleracea L. | Spinach | Leaves |
| 7 | Citrus limetta Risso | Sweet lemon, musambi | Peel |
| 8 | Curcuma longa L. | Turmeric, haldi | Rhizome |
For the selection of plant material, preferably cheap sources were chosen. Moreover, it was also taken into account that the samples should be free of contaminants.
2.2 Color extraction
Crude color extraction was done by following the methodology given by [6]. Briefly, the selected plant sample was washed properly with water and then dried at room temperature. When the sample was dried, the weighed amount of sample was blended with different solvents for 15–20 min, and the solvents that give highest color yield were selected. After that the blended material was filtered using cotton cloth and compressed, until all the solvent was squeezed out. The residue was again blended with a small volume of the solvent for complete color extraction.
A pinch of citric acid was added to the extracted color liquid, acting as a stabilizer. The filtrate was placed in the oven at 40–45°C for 24 h. The dried sample, in powder form or in paste, was saved in a dry sterilized jar.
The percentage (%) yield of the crude dye was determined using the equation given below:
where
Wdy=% yield of crude dye
Wbe=weight of plant material before extraction
Wae=weight of crude material after evaporation.
2.3 Thermo-stability of extracted color dye
The stability of extracted color dyes was determined at various temperatures by keeping the extracts at specific ranges of temperature. The samples were kept at the various temperatures for 15 days to ascertain the stability of the colors.
2.3.1 Procedure
The weighed amount of color sample was placed in a sterilized Petri plate, and these samples were then placed at the following different temperature ranges.
Color extract was kept at room temperature, 30–35°C.
Color extract was kept in incubator at temperature 45–55°C.
Color extract was kept in freezer at −15 to −20°C.
Color extract was kept at temperature 15–20°C.
The samples were re-weighed after 15 days, and the loss in weight was noted.
2.4 Determination of color pigments
The amount of different color pigments in crude extracts was determined using the spectrophotometry method.
2.4.1 Determination of percent curcumin
2.4.1.1 Sample preparation
For the preparation of sample, the methodology of [7] was used. For this, 0.1 g of sample was dissolved in 25 ml of ethanol. Then the solution was filtered, and the volume was increased to 100 ml using distilled water. Afterward, about 10 ml of this solution was placed in a separate flask, and the volume was increased to 100 ml with ethanol. Absorbance was recorded at 425 nm.
2.4.1.2 Calculation
The percentage (%) of curcumin and the strength of the color of a pigment was measured using the respective standards: a 425 nm, the standard value of curcumin is 0.42; the absorptive value for curcumin is A=16.8; the percentage (%) of curcumin in the samples was calculated using the following formula:
where
a=absorbance of sample at 425 nm
L=distance, that is 1 cm
A= absorption of curcumin
Color value=a×1000
2.4.2 Determination of anthocyanin
2.4.2.1 Preparation of chemicals
2.4.2.1.1 0.02 m Potassium chloride buffer
The following two solutions were required for the preparation of this buffer.
Solution A: This solution was prepared by dissolving 14.91 g KCl in 1 l of distilled water in a flask.
Solution B: This solution was prepared by dissolving 16.6 ml of HCl in 1 l of distilled water in a flask.
Then 50 ml of solution A and 50 ml of solution B were placed in a separate flask, and the final volume was increased to 200 ml with distilled water. Using HCl and NaOH, the pH of the buffer was maintained at 1.
2.4.2.1.2 0.4 m Sodium acetate buffer
For the preparation of 0.4 m sodium acetate buffer, 8.945 g of disodium hydrogen phosphate and 3.4023 g of potassium iodide were placed in a flask, and the volume was increased to 1 l using distilled water. The pH was maintained at 4.5 using concentrated solutions of HCl and NaOH.
Procedure: For the determination of anthocyanin, 1 g sample of the crude dye extract was placed in a flask, and it was dissolved in 10 ml of acidified methanol.
Solution A: 0.8 ml of the sample solution was placed in a separate flask, and 3 ml of potassium chloride buffer was added to it.
Solution B: 0.8 ml of the sample solution was placed again in a separate flask and 3 ml of sodium acetate buffer was added to it.
The absorbance of solution A was measured at 700 nm, and solution B was measured at 525 nm, whereas water was used as a blank.
The concentration of anthocyanin was determined using the formula given below.
where
MV=molecular weight=449.2 g/mol
E=absorption coefficient=26,900/mol/cm
Df=dilution factor=37.5
2.4.3 Determination of carotenoids
A stock solution was prepared by following the standard protocol given by [8]. For its preparation, 5 mg of crude dye extract was dissolved in 50 ml of n-hexane. The absorbance of stock solution was measured at 452 nm, while water was used as a blank.
The amount of β-carotene was calculated using the following formula.
where
A=absorbance at 452 nm
V=total volume of extract used
p=weight of sample
€=extinction coefficient on hexane=139,200 l/mol
2.4.4 Determination of betanin
2.4.4.1 Citric acid phosphate buffer
2.4.4.1.1 Solution A: 0.1 m solution of citric acid monohydrate
For preparation of this solution, 2.101 g of the citric acid was placed in a flask, and the volume was increased to 100 ml using distilled water.
2.4.4.1.2 Solution B: 0.2 m solution of Na2HPO4⋅12H2O
For preparation of 0.2 m solution of Na2HPO4⋅12H2O, 7.164 g of acid was placed in a flask and the volume was increased to 100 ml using distilled water.
The final solution was prepared by mixing 89.1 ml of solution A and 10.9 ml of solution B in a separate flask, and the solution was mixed to prepare a homogenized solution.
Procedure: The amount of betanin was determined following the methodology given by [9]. The 0.5123 mg crude dye extract was placed in a sterilized flask, and the volume was increased to 10 ml using citric acid phosphate buffer. The solution was allowed to stand for 10 min at room temperature. Absorption was observed at 530 nm. Total betanin content was calculated using the following formula:
where
A=absorption value at 535 nm
DF=dilution volume
L=path length of cuvette
MW=molecular weight of betalain (550 g/mol)
€=extinction coefficient for betalain 60,000 l/mol.
2.5 Application of extracted dye on fabric
2.5.1 Textile materials
Cotton fabric was selected for the current study. The size of the samples was 10 cm×10 cm.
Procedure: Pretreatment of cloth before dying: the cloth was heated in NaOH and detergent-containing solution for 30 min at 60°C to remove the starch. The cloth was washed thoroughly with distilled water and allowed to dry. The extracted solution of each dye was applied to cotton fabric with mordant and without mordant under the same conditions. The direct dyeing (without mordant) method was performed to find out whether the extracted dye showed affinity to cotton in the absence of mordant [10].
2.5.2 Mordants
Three different mordants as given in Table 2 were used singly or in combination to stabilize the natural dye on the cotton cloth. The mordants used were alum potassium, aluminum sulfate, and lemon.
Mordanting recipe for textile material.
| Mordants | Amount (g) | Water (ml) | Boiling time (min) | Mordanting time (min) | Mordanting temperature (°C) |
|---|---|---|---|---|---|
| Alum | 1.0 | 200 | 60 | 30 | 70–90 |
| Lemon | 1.0 | 200 | 60 | 30 | 70–90 |
Procedure: Firstly, the weighted mordants were put into a beaker, and then water was added. The procedure was carried out over the hotplate to dissolve the mordants. The mordants were dissolved in the water at 90°C. Mordants were stirred constantly. The cotton cloth was then immersed into the mordant solution for mordanting for 30 min.
The mordant-treated cloth was not washed and was directly dyed at temperature range of 90–100°C for an hour. After dying, the fabrics were washed properly, and washing was carried out using 2 g/l detergent. The dyed samples were dried to check the washing fastness properties.
3 Results and discussion
Research work was conducted for the extraction of color dyes from a variety of natural sources. While selecting such sources, the main focus was to select cheap and easily available sources. The extracted color dyes were evaluated by taking into consideration the economic and commercial aspects of the plant color extract. Different solvents were employed for the extraction process, but finally the solvent that gave the maximum yield was used.
3.1 Extraction of color dye extracts
The extraction of different color dyes was done using different plant parts, such as peels, leaves, petals, and rhizomes (Figures 1 and 2 ). The percentage (%) of extraction of crude dye showed a maximum yield of 30.67% obtained from Citrus limetta peel, whereas an intermediate yield of 16.22% was obtained from Citrus limon peel, and the comparatively lesser yield of 14.11% was obtained from Beta vulgaris extract. Among peels, maximum yield of 30.67% was obtained from C. limetta peel. However, Rosa indica petals gave a yield of 18.41%, while Spinacea oleracea leaves gave a yield of 28.01% as indicated in Figure 3.
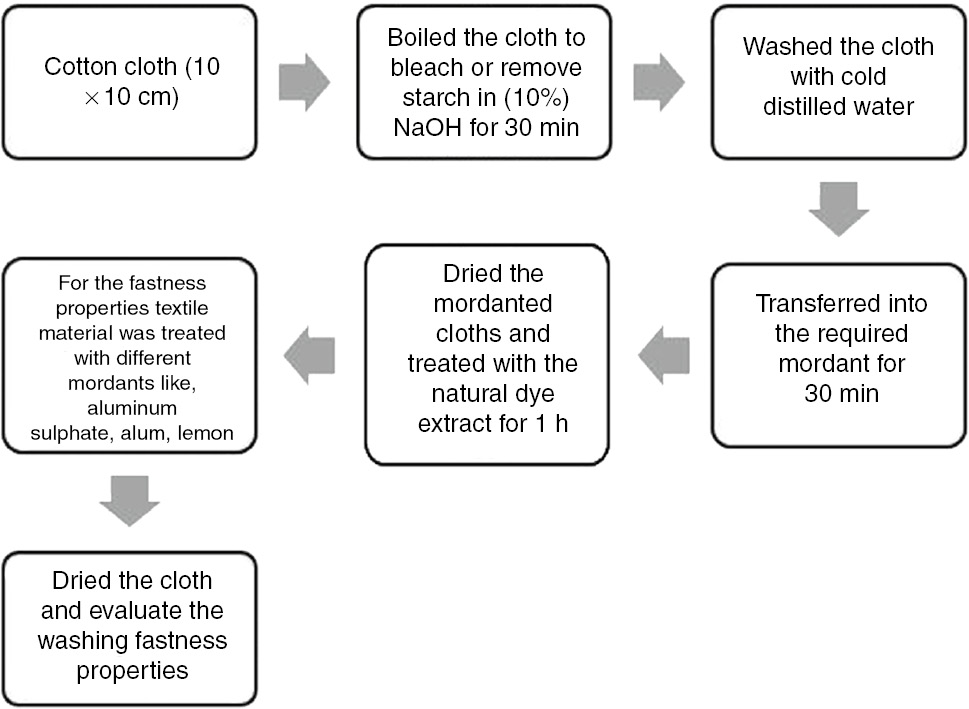
Flow chart showing the dying procedure.
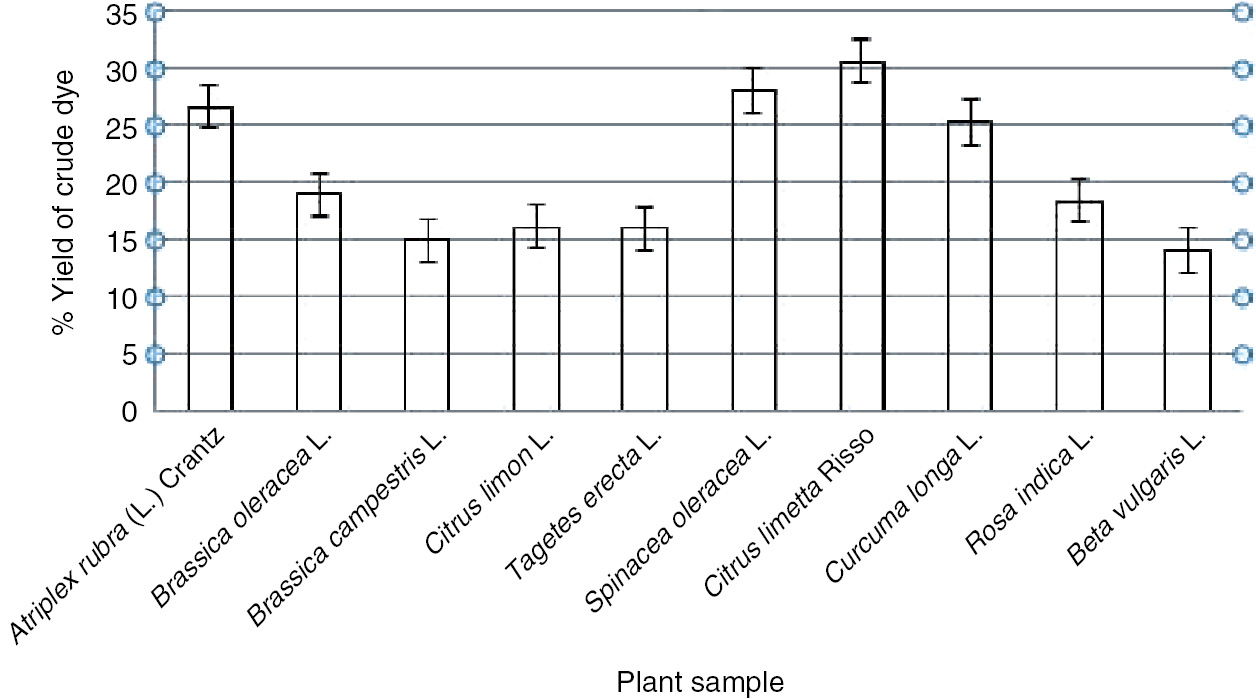
Determination of percent yield of crude dyes from different natural sources.

Extracted crude dyes from various sources.
3.2 Thermo-stability of color compound
The thermo-stability of color compounds was examined by subjecting the extracts to a variety of temperatures. The samples that were placed in the incubator showed maximum weight loss and a wide range in pH, and their physical appearances are shown in Figure 4. On the other hand, the samples that were placed in the freezer showed the highest gain in moisture and great variability in their pH. So the best and most suitable temperature for stability of samples was the room temperature, because at this temperature there was less variation in weight loss, pH, and color.
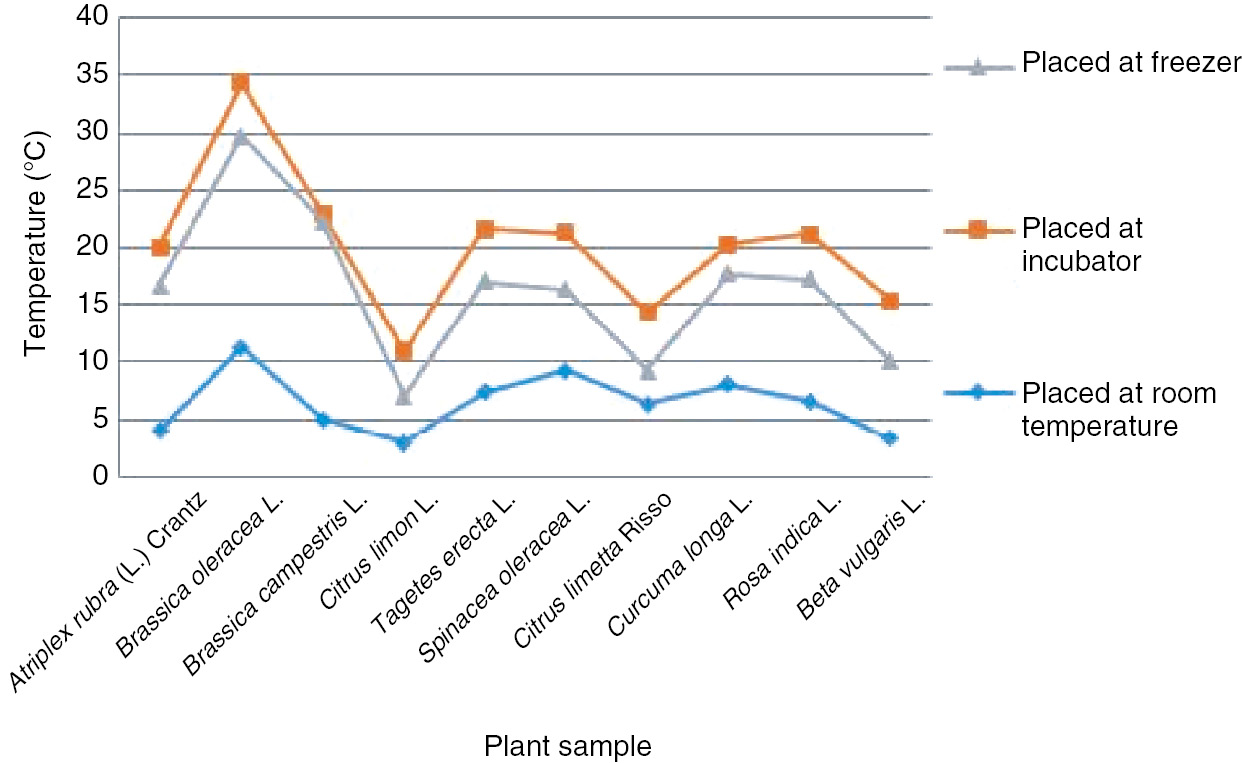
Stability of extracts at various temperatures.
3.3 Spectrophotometric analysis
Spectrophotometric analysis was performed to verify the presence of different color pigments. Plants mainly contain a number of light-absorbing pigments, most of which are commonly associated with photosynthesis. Organic substances in plants that are reliable in giving diverse colors to plant parts are called pigments. For this analysis Brassica oleracea, Brassica campestris, C. limon, Tagetes erecta, S. oleracea, C. limetta, B. vulgaris, R. indica, and Curcuma longa extracts were used. Different samples showed the presence of specific color pigment at different wavelengths; for example, betanin presence was recorded at 523 nm, anthocyanin pigment was detected at 525 and 700 nm, curcumin presence was recorded at 425 nm, and carotenoid presence was recorded at 452 nm. These wavelengths showed the presence of specific color pigments in all the extracted color samples. That gives particular color to the extracts (Table 3).
Spectrophotometric analysis of different color compounds.
| Extract | Absorbance (nm) | Fresh extract | Placed at room temperature | Placed in incubator | Placed in freezer |
|---|---|---|---|---|---|
| Betanin | |||||
| Beta vulgarius L. | 523 nm | 1.571±0.088 | 1.121±0.026 | 1.092±0.028 | 0.645±0.099 |
| Anthocyanin (mg/g) | |||||
| Brassica oleracea L. | 525 and 700 nm | 1.519±0.072 | 1.14±0.035 | 1.17±0.04 | 1.13±0.00 |
| Rosa indica L. | 0.16±0.01 | 0.17±0.01 | 0.10±0.00 | 0.04±0.01 | |
| Curcumin (%) | |||||
| Curcuma longa L | 425 nm | 60.4±6.38 | 52.2±10.2 | 37.23±6.42 | 9.74±2.25 |
| Carotenoid (mg/g) | |||||
| Brassica campestris L. | 452 nm | 0.028±0.004 | 0.031±0.002 | 0.030±0.003 | 0.03±0.003 |
| Citrus limon L. | 0.028±0.001 | 0.027±0.000 | 0.027±0.000 | 0.024±0.000 | |
| Tagetes erecta L. | 0.028±0.001 | 0.027±0.000 | 0.026±0.000 | 0.025±0.000 | |
| Citrus limetta L. | 0.031±0.002 | 0.027±0.001 | 0.026±0.000 | 0.026±0.000 | |
This methodology helps us to determine the existence of color pigments in the crude extracts. The crude extract is the mixture of more than two color pigments but by utilizing this methodology the presence of specific color pigments was analyzed and that is helpful in giving a particular color to the specific compound.
3.4 Application of different color compounds
Dyes provide a striking color to foods, that on the other hand looks unappealing or flavorless. It is supposed that satisfaction from food depends on “eye appeal” among many other factors and that the beautiful and attractive coloration of food are major contributing factors to the consumer’s appetite. For this analysis B. oleracea, Beta campestris, C. limon, T. erecta, S. oleracea, C. limetta, B. vulgaris, R. indica, and C. longa extracts were used in different food products to give striking colors to the food.
Along with the application of extracted color dyes in different food items (Figures 5 and 6), the extracted color dyes were also used on textile fibers for dying purposes. For this, the extracted colors were used alone or in combination with mordants. It was found that both mordants (alum and lemon) were helpful in enhancing the color strength of extracted colors. But alum was considered to be the better mordant because it gives a sharper color result compared to the lemon. The dyed cloths were permanently dyed, meaning that on washing the cloth with water the color did not lighten (Figures 7–9 ). These results indicate that these extracted colors can be utilized easily in the textile and food industries for the replacement of synthetic dyes that are the major cause of many health problems.

Application of yellow-orange color on boiled rice.

Application of yellow-orange color on boiled Spaghetti.
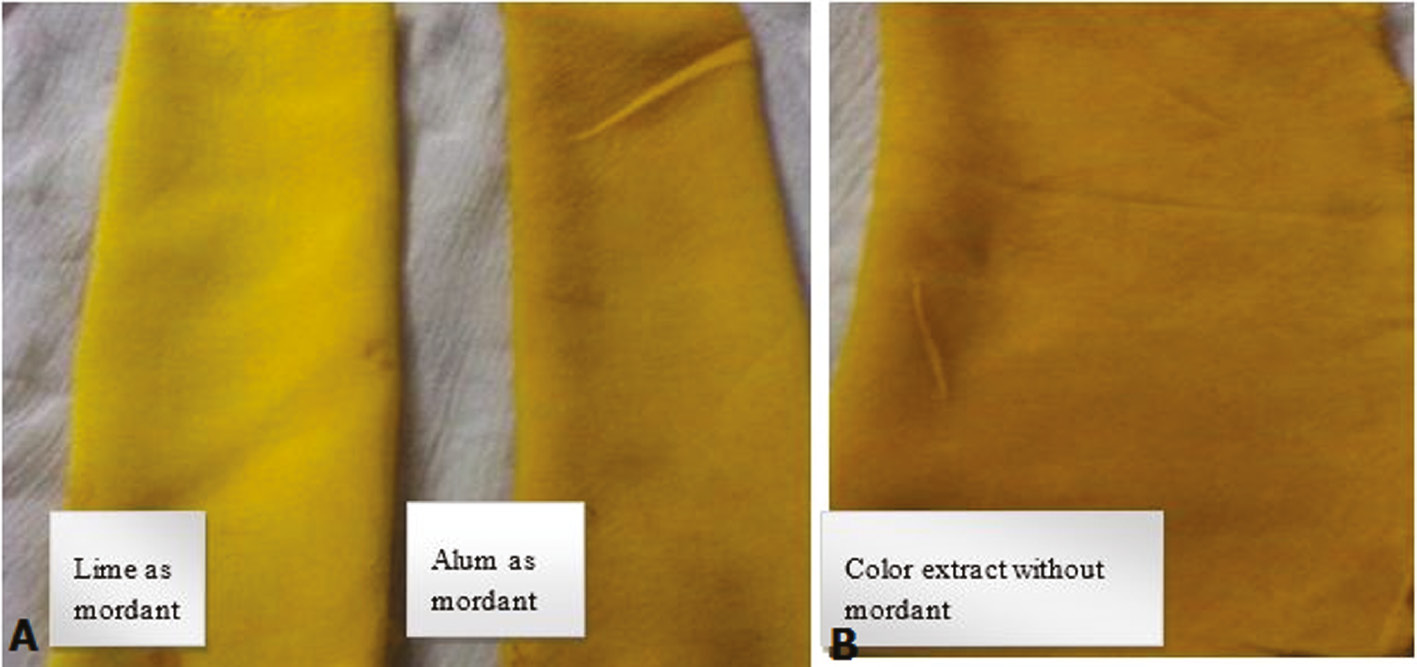
Application of color extracted from Citrus limon. (A) Change in colour when applied Lime and Alum as mordant (B) Colour effect without mordant.
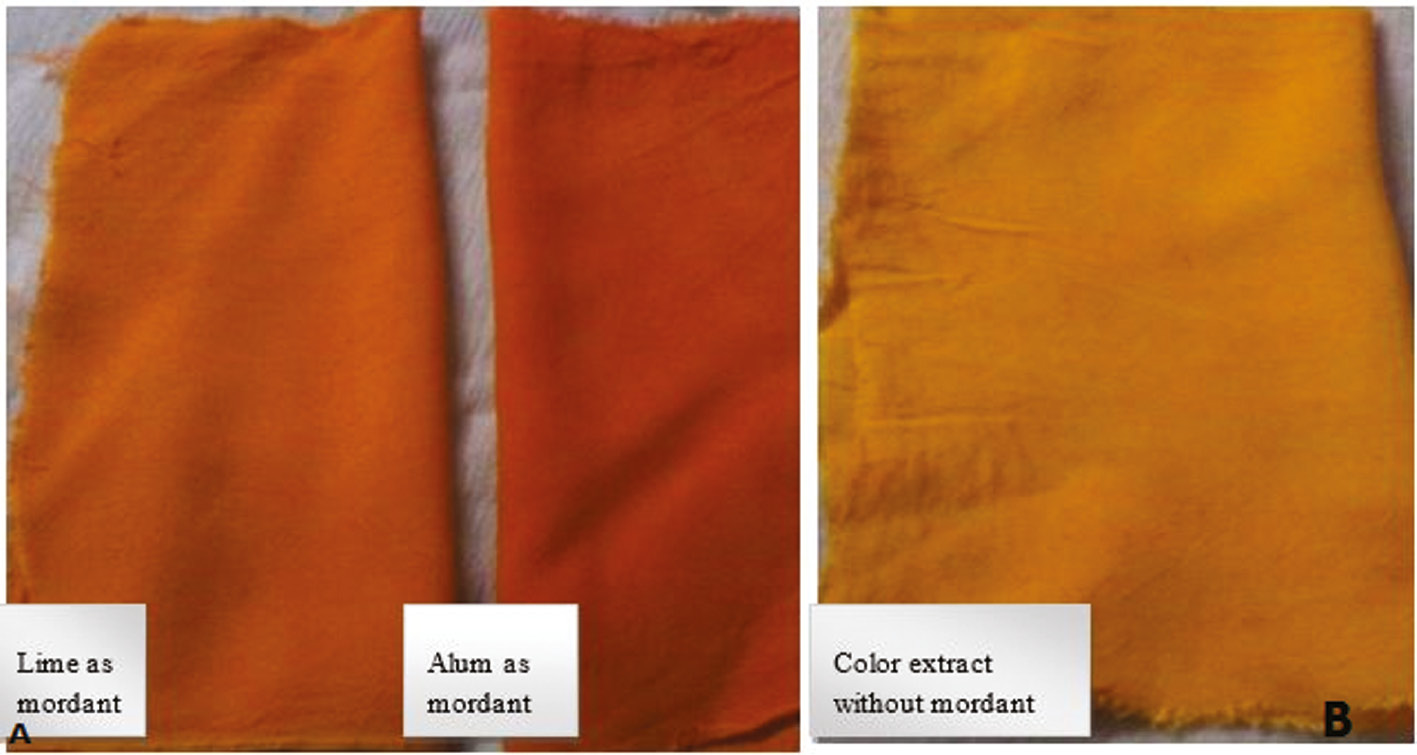
Application of color extracted from Curcuma longa. (A) Change in colour when applied Lime and Alum as mordant (B) Colour effect without mordant.
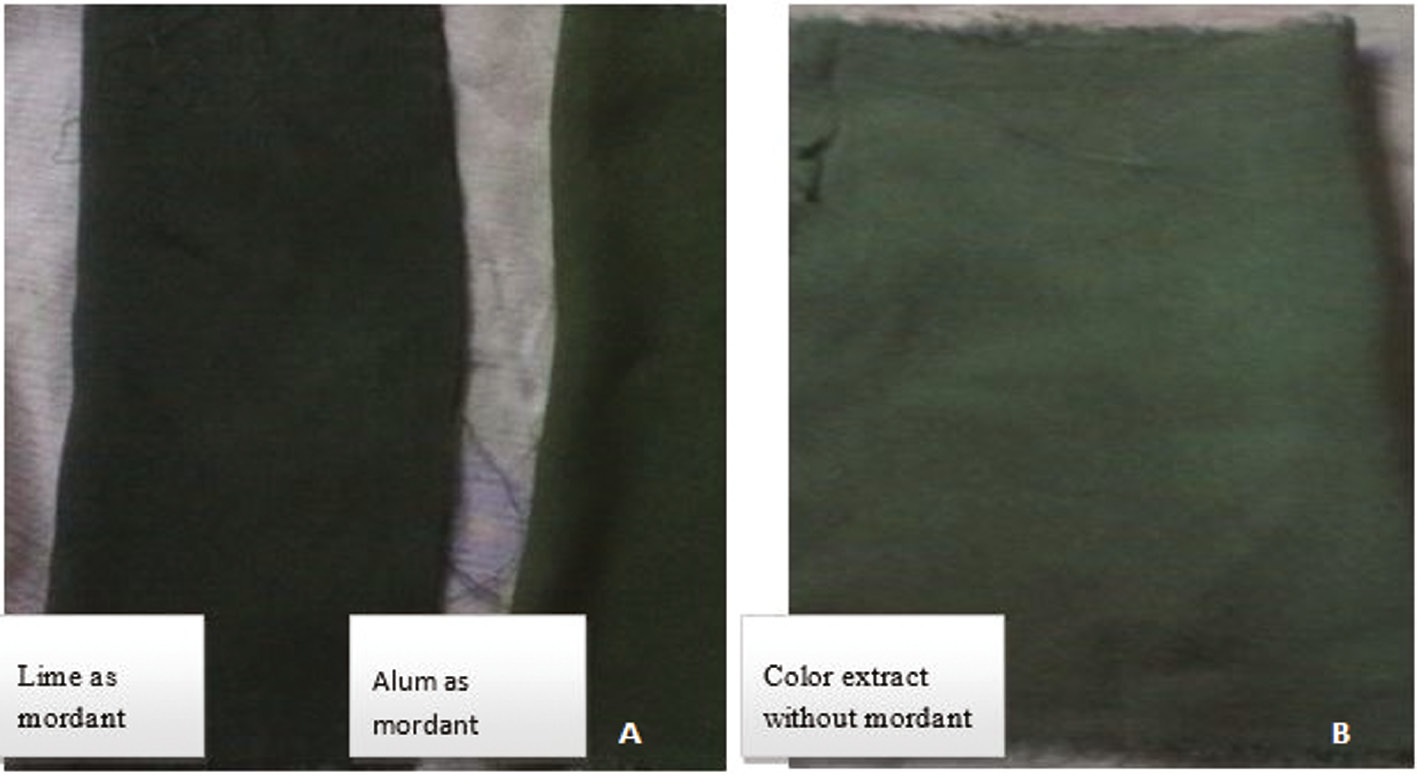
Application of color extracted from Atriplex rubra and Spinacea oleracea. (A) Change in colour when applied Lime and Alum as mordant (B) Colour effect without mordant.
3.5 Discussion
The maximum amount of color extract was obtained from different samples like Atriplex rubra, B. oleracea, Be. campestris, C. limon, T. erecta, S. oleracea, C. limetta, C. longa, R. indica, and B. vulgaris. These outcomes were almost the same as those obtained by [11, [12] from leaves of Cucurbita pepo and by McGovern et al. 13] in color extraction from Livistona speciosa. An appropriate quantity of extracts can be acquired using these strategies.
Colors were extracted from fruits peels, vegetables, and petals for utilization in the food and textile industries. The appearance of food was a major factor in determining its acceptance by the consumer, who has a number of built-in expectations regarding the “proper” color for a particular type of food, also color in this regard is often the primary consideration for purchasing clothes [14].
Furthermore, as dyes were developed and experimented with, people became more adventurous and would attempt different mediums in making dyes, hence finding synthetic dyes which helped the dyeing industry to develop fast. Though synthetic dyes are colorfast, they pose a danger to the health of people owing to the harmful chemicals in them. They could cause cancer and other diseases, and so their use should be minimized or eliminated altogether. Taking this into consideration, extraction of color from various natural sources is employed nowadays. Research into the extraction and application of dyes from local plants to serve as colorants for textiles and food is therefore very critical to assess the potency of the dyes and the various medicinal and food plants for their potential to color everyday clothes, fibers, yarns, and also food items to enhance their appeal to consumers while also promoting healthy lifestyles through the use of plant-based food colors as substitutes for the chemical ones sold on the open market.
The extracted color dyes were applied for dyeing purposes on cotton cloth with or without the help of mordants. Two different mordants were used: alum and lime. It was found that alum showed better and more enhancing color fastness results than lime. The shade and fastness effect of color increased when using mordants. The extraction of brilliant yellow and red colors from C. longa and Tagetus errecta was performed by [15]. The extracts were applied as direct dyes and in the presence of iron or alum mordant [16, [17]. The shade of color was enhanced when using different mordants. Among all the mordants applied, the experimentally used alum was found to be best mordant for enhancing the shade of color 13].
4 Conclusion
The food and beverage industry releases considerable amounts of wastes which contain natural dyes. Such wastes like lemon and orange peels could serve as sources for the extraction of natural dyes for food industry usage like the extracted colors used in candy making, sugar syrup making, and coloring various foods stuffs. The results prove the potential of such wastes as a source for natural dyestuff extraction to extract color from various natural sources using barks, berries and fruits, and these colors were employed for coloring different food items like butter, candies, cakes, bakery items, and sugar syrups. The extracted colors were found to be a better substitute for the synthetic chemical dyes that cause many health hazardous problems and that also needed to be imported. The colors extracted using natural sources including wastes were considered to be the best replacement for synthetic dyes in both food and textile industries.
Acknowledgments
This work was supported by the FBRC (Food Biotechnology Research Center) Department PCSIR (Pakistan Council of Scientific and Industrial Research) Lahore.
References
[1] Siva R, Senthil Kumar T, Krishnamurthy KV. Natural dye yielding plants of Shervaroy Hills of eastern Ghats. Environment Protection Training and Research Institute, 2002, pp. 151–153.Search in Google Scholar
[2] Christie RM. Colour Chemistry, Royal Society of Chemistry: Cambridge, 2001, p. 168.Search in Google Scholar
[3] Siva R. Curr. Sci. 2007, 92, 916–925.Search in Google Scholar
[4] Vankar PS. Resonance 2000, 5, 73–80.10.1007/BF02836844Search in Google Scholar
[5] Gilbert KG, Cooke DT. Plant Growth Regul 2001, 34, 57–69.10.1023/A:1013374618870Search in Google Scholar
[6] Kechi A, Chavan RB, Moeckel R. Text. Light Ind Sci Technol. 2013, 2, 137–145.Search in Google Scholar
[7] Himesh S, Patel SS, Govind N, Ak S. Int. Res. J. Pharm. 2011, 2, 180–184.Search in Google Scholar
[8] Britton G, Liaanen-Jensen S, Pfander H. Carotenoids Handbook, BirkhäuserVerlag: Basel, Boston, Berlin, 2004.10.1007/978-3-0348-7836-4Search in Google Scholar
[9] Castellar MR, Obón JM, Alacid M, Fernández-López JA. J. Agr. Food Chem. 2003, 51, 2772–2776.10.1021/jf021045hSearch in Google Scholar
[10] Kulkarni SS, Gokhale AV, Bodake UM, Pathade GR. Univers. J. Environ. Res. Technol. 2011, 1, 135–139.Search in Google Scholar
[11] Hasan B, Tahsin K. Turk. J. Agric. 2006, 30, 287–293.Search in Google Scholar
[12] Shanker R, Vankar PS. Dyes Pigments 2007, 74, 464–469.10.1016/j.dyepig.2006.03.007Search in Google Scholar
[13] McGovern PE, Lazar J, Michel HR. J. Soc. Dyers Colour. 1990, 1, 22.10.1111/j.1478-4408.1990.tb01225.xSearch in Google Scholar
[14] Jothi D. AUTEX Res. J. 2008, 8, 52–59.Search in Google Scholar
[15] Saxena S, Vigneshwaran A, Nachane ND, Mahangade RR, Narkar RS. Colourage 2013, 44, 23–24, 26–28.Search in Google Scholar
[16] Deveoglu O, Recep K, Treet K, Turkan B. J. Food Sci. Technol. 2013, 3, 45–57.Search in Google Scholar
[17] Smith J. Chem. Tech. Assess. Man 2006, 6, 1–21.10.1016/S1535-1513(08)70446-6Search in Google Scholar
©2019 Walter de Gruyter GmbH, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 Public License.
Articles in the same Issue
- Regular Articles
- Studies on the preparation and properties of biodegradable polyester from soybean oil
- Flow-mode biodiesel production from palm oil using a pressurized microwave reactor
- Reduction of free fatty acids in waste oil for biodiesel production by glycerolysis: investigation and optimization of process parameters
- Saccharin: a cheap and mild acidic agent for the synthesis of azo dyes via telescoped dediazotization
- Optimization of lipase-catalyzed synthesis of polyethylene glycol stearate in a solvent-free system
- Green synthesis of iron oxide nanoparticles using Platanus orientalis leaf extract for antifungal activity
- Ultrasound assisted chemical activation of peanut husk for copper removal
- Room temperature silanization of Fe3O4 for the preparation of phenyl functionalized magnetic adsorbent for dispersive solid phase extraction for the extraction of phthalates in water
- Evaluation of the saponin green extraction from Ziziphus spina-christi leaves using hydrothermal, microwave and Bain-Marie water bath heating methods
- Oxidation of dibenzothiophene using the heterogeneous catalyst of tungsten-based carbon nanotubes
- Calcined sodium silicate as an efficient and benign heterogeneous catalyst for the transesterification of natural lecithin to L-α-glycerophosphocholine
- Synergistic effect between CO2 and H2O2 on ethylbenzene oxidation catalyzed by carbon supported heteropolyanion catalysts
- Hydrocyanation of 2-arylmethyleneindan-1,3-diones using potassium hexacyanoferrate(II) as a nontoxic cyanating agent
- Green synthesis of hydratropic aldehyde from α-methylstyrene catalyzed by Al2O3-supported metal phthalocyanines
- Environmentally benign chemical recycling of polycarbonate wastes: comparison of micro- and nano-TiO2 solid support efficiencies
- Medicago polymorpha-mediated antibacterial silver nanoparticles in the reduction of methyl orange
- Production of value-added chemicals from esterification of waste glycerol over MCM-41 supported catalysts
- Green synthesis of zerovalent copper nanoparticles for efficient reduction of toxic azo dyes congo red and methyl orange
- Optimization of the biological synthesis of silver nanoparticles using Penicillium oxalicum GRS-1 and their antimicrobial effects against common food-borne pathogens
- Optimization of submerged fermentation conditions to overproduce bioethanol using two industrial and traditional Saccharomyces cerevisiae strains
- Extraction of In3+ and Fe3+ from sulfate solutions by using a 3D-printed “Y”-shaped microreactor
- Foliar-mediated Ag:ZnO nanophotocatalysts: green synthesis, characterization, pollutants degradation, and in vitro biocidal activity
- Green cyclic acetals production by glycerol etherification reaction with benzaldehyde using cationic acidic resin
- Biosynthesis, characterization and antimicrobial activities assessment of fabricated selenium nanoparticles using Pelargonium zonale leaf extract
- Synthesis of high surface area magnesia by using walnut shell as a template
- Controllable biosynthesis of silver nanoparticles using actinobacterial strains
- Green vegetation: a promising source of color dyes
- Mechano-chemical synthesis of ammonia and acetic acid from inorganic materials in water
- Green synthesis and structural characterization of novel N1-substituted 3,4-dihydropyrimidin-2(1H)-ones
- Biodiesel production from cotton oil using heterogeneous CaO catalysts from eggshells prepared at different calcination temperatures
- Regeneration of spent mercury catalyst for the treatment of dye wastewater by the microwave and ultrasonic spray-assisted method
- Green synthesis of the innovative super paramagnetic nanoparticles from the leaves extract of Fraxinus chinensis Roxb and their application for the decolourisation of toxic dyes
- Biogenic ZnO nanoparticles: a study of blueshift of optical band gap and photocatalytic degradation of reactive yellow 186 dye under direct sunlight
- Leached compounds from the extracts of pomegranate peel, green coconut shell, and karuvelam wood for the removal of hexavalent chromium
- Enhancement of molecular weight reduction of natural rubber in triphasic CO2/toluene/H2O systems with hydrogen peroxide for preparation of biobased polyurethanes
- An efficient green synthesis of novel 1H-imidazo[1,2-a]imidazole-3-amine and imidazo[2,1-c][1,2,4]triazole-5-amine derivatives via Strecker reaction under controlled microwave heating
- Evaluation of three different green fabrication methods for the synthesis of crystalline ZnO nanoparticles using Pelargonium zonale leaf extract
- A highly efficient and multifunctional biomass supporting Ag, Ni, and Cu nanoparticles through wetness impregnation for environmental remediation
- Simple one-pot green method for large-scale production of mesalamine, an anti-inflammatory agent
- Relationships between step and cumulative PMI and E-factors: implications on estimating material efficiency with respect to charting synthesis optimization strategies
- A comparative sorption study of Cr3+ and Cr6+ using mango peels: kinetic, equilibrium and thermodynamic
- Effects of acid hydrolysis waste liquid recycle on preparation of microcrystalline cellulose
- Use of deep eutectic solvents as catalyst: A mini-review
- Microwave-assisted synthesis of pyrrolidinone derivatives using 1,1’-butylenebis(3-sulfo-3H-imidazol-1-ium) chloride in ethylene glycol
- Green and eco-friendly synthesis of Co3O4 and Ag-Co3O4: Characterization and photo-catalytic activity
- Adsorption optimized of the coal-based material and application for cyanide wastewater treatment
- Aloe vera leaf extract mediated green synthesis of selenium nanoparticles and assessment of their In vitro antimicrobial activity against spoilage fungi and pathogenic bacteria strains
- Waste phenolic resin derived activated carbon by microwave-assisted KOH activation and application to dye wastewater treatment
- Direct ethanol production from cellulose by consortium of Trichoderma reesei and Candida molischiana
- Agricultural waste biomass-assisted nanostructures: Synthesis and application
- Biodiesel production from rubber seed oil using calcium oxide derived from eggshell as catalyst – optimization and modeling studies
- Study of fabrication of fully aqueous solution processed SnS quantum dot-sensitized solar cell
- Assessment of aqueous extract of Gypsophila aretioides for inhibitory effects on calcium carbonate formation
- An environmentally friendly acylation reaction of 2-methylnaphthalene in solvent-free condition in a micro-channel reactor
- Aegle marmelos phytochemical stabilized synthesis and characterization of ZnO nanoparticles and their role against agriculture and food pathogen
- A reactive coupling process for co-production of solketal and biodiesel
- Optimization of the asymmetric synthesis of (S)-1-phenylethanol using Ispir bean as whole-cell biocatalyst
- Synthesis of pyrazolopyridine and pyrazoloquinoline derivatives by one-pot, three-component reactions of arylglyoxals, 3-methyl-1-aryl-1H-pyrazol-5-amines and cyclic 1,3-dicarbonyl compounds in the presence of tetrapropylammonium bromide
- Preconcentration of morphine in urine sample using a green and solvent-free microextraction method
- Extraction of glycyrrhizic acid by aqueous two-phase system formed by PEG and two environmentally friendly organic acid salts - sodium citrate and sodium tartrate
- Green synthesis of copper oxide nanoparticles using Juglans regia leaf extract and assessment of their physico-chemical and biological properties
- Deep eutectic solvents (DESs) as powerful and recyclable catalysts and solvents for the synthesis of 3,4-dihydropyrimidin-2(1H)-ones/thiones
- Biosynthesis, characterization and anti-microbial activity of silver nanoparticle based gel hand wash
- Efficient and selective microwave-assisted O-methylation of phenolic compounds using tetramethylammonium hydroxide (TMAH)
- Anticoagulant, thrombolytic and antibacterial activities of Euphorbia acruensis latex-mediated bioengineered silver nanoparticles
- Volcanic ash as reusable catalyst in the green synthesis of 3H-1,5-benzodiazepines
- Green synthesis, anionic polymerization of 1,4-bis(methacryloyl)piperazine using Algerian clay as catalyst
- Selenium supplementation during fermentation with sugar beet molasses and Saccharomyces cerevisiae to increase bioethanol production
- Biosynthetic potential assessment of four food pathogenic bacteria in hydrothermally silver nanoparticles fabrication
- Investigating the effectiveness of classical and eco-friendly approaches for synthesis of dialdehydes from organic dihalides
- Pyrolysis of palm oil using zeolite catalyst and characterization of the boil-oil
- Azadirachta indica leaves extract assisted green synthesis of Ag-TiO2 for degradation of Methylene blue and Rhodamine B dyes in aqueous medium
- Synthesis of vitamin E succinate catalyzed by nano-SiO2 immobilized DMAP derivative in mixed solvent system
- Extraction of phytosterols from melon (Cucumis melo) seeds by supercritical CO2 as a clean technology
- Production of uronic acids by hydrothermolysis of pectin as a model substance for plant biomass waste
- Biofabrication of highly pure copper oxide nanoparticles using wheat seed extract and their catalytic activity: A mechanistic approach
- Intelligent modeling and optimization of emulsion aggregation method for producing green printing ink
- Improved removal of methylene blue on modified hierarchical zeolite Y: Achieved by a “destructive-constructive” method
- Two different facile and efficient approaches for the synthesis of various N-arylacetamides via N-acetylation of arylamines and straightforward one-pot reductive acetylation of nitroarenes promoted by recyclable CuFe2O4 nanoparticles in water
- Optimization of acid catalyzed esterification and mixed metal oxide catalyzed transesterification for biodiesel production from Moringa oleifera oil
- Kinetics and the fluidity of the stearic acid esters with different carbon backbones
- Aiming for a standardized protocol for preparing a process green synthesis report and for ranking multiple synthesis plans to a common target product
- Microstructure and luminescence of VO2 (B) nanoparticle synthesis by hydrothermal method
- Optimization of uranium removal from uranium plant wastewater by response surface methodology (RSM)
- Microwave drying of nickel-containing residue: dielectric properties, kinetics, and energy aspects
- Simple and convenient two step synthesis of 5-bromo-2,3-dimethoxy-6-methyl-1,4-benzoquinone
- Biodiesel production from waste cooking oil
- The effect of activation temperature on structure and properties of blue coke-based activated carbon by CO2 activation
- Optimization of reaction parameters for the green synthesis of zero valent iron nanoparticles using pine tree needles
- Microwave-assisted protocol for squalene isolation and conversion from oil-deodoriser distillates
- Denitrification performance of rare earth tailings-based catalysts
- Facile synthesis of silver nanoparticles using Averrhoa bilimbi L and Plum extracts and investigation on the synergistic bioactivity using in vitro models
- Green production of AgNPs and their phytostimulatory impact
- Photocatalytic activity of Ag/Ni bi-metallic nanoparticles on textile dye removal
- Topical Issue: Green Process Engineering / Guest Editors: Martine Poux, Patrick Cognet
- Modelling and optimisation of oxidative desulphurisation of tyre-derived oil via central composite design approach
- CO2 sequestration by carbonation of olivine: a new process for optimal separation of the solids produced
- Organic carbonates synthesis improved by pervaporation for CO2 utilisation
- Production of starch nanoparticles through solvent-antisolvent precipitation in a spinning disc reactor
- A kinetic study of Zn halide/TBAB-catalysed fixation of CO2 with styrene oxide in propylene carbonate
- Topical on Green Process Engineering
Articles in the same Issue
- Regular Articles
- Studies on the preparation and properties of biodegradable polyester from soybean oil
- Flow-mode biodiesel production from palm oil using a pressurized microwave reactor
- Reduction of free fatty acids in waste oil for biodiesel production by glycerolysis: investigation and optimization of process parameters
- Saccharin: a cheap and mild acidic agent for the synthesis of azo dyes via telescoped dediazotization
- Optimization of lipase-catalyzed synthesis of polyethylene glycol stearate in a solvent-free system
- Green synthesis of iron oxide nanoparticles using Platanus orientalis leaf extract for antifungal activity
- Ultrasound assisted chemical activation of peanut husk for copper removal
- Room temperature silanization of Fe3O4 for the preparation of phenyl functionalized magnetic adsorbent for dispersive solid phase extraction for the extraction of phthalates in water
- Evaluation of the saponin green extraction from Ziziphus spina-christi leaves using hydrothermal, microwave and Bain-Marie water bath heating methods
- Oxidation of dibenzothiophene using the heterogeneous catalyst of tungsten-based carbon nanotubes
- Calcined sodium silicate as an efficient and benign heterogeneous catalyst for the transesterification of natural lecithin to L-α-glycerophosphocholine
- Synergistic effect between CO2 and H2O2 on ethylbenzene oxidation catalyzed by carbon supported heteropolyanion catalysts
- Hydrocyanation of 2-arylmethyleneindan-1,3-diones using potassium hexacyanoferrate(II) as a nontoxic cyanating agent
- Green synthesis of hydratropic aldehyde from α-methylstyrene catalyzed by Al2O3-supported metal phthalocyanines
- Environmentally benign chemical recycling of polycarbonate wastes: comparison of micro- and nano-TiO2 solid support efficiencies
- Medicago polymorpha-mediated antibacterial silver nanoparticles in the reduction of methyl orange
- Production of value-added chemicals from esterification of waste glycerol over MCM-41 supported catalysts
- Green synthesis of zerovalent copper nanoparticles for efficient reduction of toxic azo dyes congo red and methyl orange
- Optimization of the biological synthesis of silver nanoparticles using Penicillium oxalicum GRS-1 and their antimicrobial effects against common food-borne pathogens
- Optimization of submerged fermentation conditions to overproduce bioethanol using two industrial and traditional Saccharomyces cerevisiae strains
- Extraction of In3+ and Fe3+ from sulfate solutions by using a 3D-printed “Y”-shaped microreactor
- Foliar-mediated Ag:ZnO nanophotocatalysts: green synthesis, characterization, pollutants degradation, and in vitro biocidal activity
- Green cyclic acetals production by glycerol etherification reaction with benzaldehyde using cationic acidic resin
- Biosynthesis, characterization and antimicrobial activities assessment of fabricated selenium nanoparticles using Pelargonium zonale leaf extract
- Synthesis of high surface area magnesia by using walnut shell as a template
- Controllable biosynthesis of silver nanoparticles using actinobacterial strains
- Green vegetation: a promising source of color dyes
- Mechano-chemical synthesis of ammonia and acetic acid from inorganic materials in water
- Green synthesis and structural characterization of novel N1-substituted 3,4-dihydropyrimidin-2(1H)-ones
- Biodiesel production from cotton oil using heterogeneous CaO catalysts from eggshells prepared at different calcination temperatures
- Regeneration of spent mercury catalyst for the treatment of dye wastewater by the microwave and ultrasonic spray-assisted method
- Green synthesis of the innovative super paramagnetic nanoparticles from the leaves extract of Fraxinus chinensis Roxb and their application for the decolourisation of toxic dyes
- Biogenic ZnO nanoparticles: a study of blueshift of optical band gap and photocatalytic degradation of reactive yellow 186 dye under direct sunlight
- Leached compounds from the extracts of pomegranate peel, green coconut shell, and karuvelam wood for the removal of hexavalent chromium
- Enhancement of molecular weight reduction of natural rubber in triphasic CO2/toluene/H2O systems with hydrogen peroxide for preparation of biobased polyurethanes
- An efficient green synthesis of novel 1H-imidazo[1,2-a]imidazole-3-amine and imidazo[2,1-c][1,2,4]triazole-5-amine derivatives via Strecker reaction under controlled microwave heating
- Evaluation of three different green fabrication methods for the synthesis of crystalline ZnO nanoparticles using Pelargonium zonale leaf extract
- A highly efficient and multifunctional biomass supporting Ag, Ni, and Cu nanoparticles through wetness impregnation for environmental remediation
- Simple one-pot green method for large-scale production of mesalamine, an anti-inflammatory agent
- Relationships between step and cumulative PMI and E-factors: implications on estimating material efficiency with respect to charting synthesis optimization strategies
- A comparative sorption study of Cr3+ and Cr6+ using mango peels: kinetic, equilibrium and thermodynamic
- Effects of acid hydrolysis waste liquid recycle on preparation of microcrystalline cellulose
- Use of deep eutectic solvents as catalyst: A mini-review
- Microwave-assisted synthesis of pyrrolidinone derivatives using 1,1’-butylenebis(3-sulfo-3H-imidazol-1-ium) chloride in ethylene glycol
- Green and eco-friendly synthesis of Co3O4 and Ag-Co3O4: Characterization and photo-catalytic activity
- Adsorption optimized of the coal-based material and application for cyanide wastewater treatment
- Aloe vera leaf extract mediated green synthesis of selenium nanoparticles and assessment of their In vitro antimicrobial activity against spoilage fungi and pathogenic bacteria strains
- Waste phenolic resin derived activated carbon by microwave-assisted KOH activation and application to dye wastewater treatment
- Direct ethanol production from cellulose by consortium of Trichoderma reesei and Candida molischiana
- Agricultural waste biomass-assisted nanostructures: Synthesis and application
- Biodiesel production from rubber seed oil using calcium oxide derived from eggshell as catalyst – optimization and modeling studies
- Study of fabrication of fully aqueous solution processed SnS quantum dot-sensitized solar cell
- Assessment of aqueous extract of Gypsophila aretioides for inhibitory effects on calcium carbonate formation
- An environmentally friendly acylation reaction of 2-methylnaphthalene in solvent-free condition in a micro-channel reactor
- Aegle marmelos phytochemical stabilized synthesis and characterization of ZnO nanoparticles and their role against agriculture and food pathogen
- A reactive coupling process for co-production of solketal and biodiesel
- Optimization of the asymmetric synthesis of (S)-1-phenylethanol using Ispir bean as whole-cell biocatalyst
- Synthesis of pyrazolopyridine and pyrazoloquinoline derivatives by one-pot, three-component reactions of arylglyoxals, 3-methyl-1-aryl-1H-pyrazol-5-amines and cyclic 1,3-dicarbonyl compounds in the presence of tetrapropylammonium bromide
- Preconcentration of morphine in urine sample using a green and solvent-free microextraction method
- Extraction of glycyrrhizic acid by aqueous two-phase system formed by PEG and two environmentally friendly organic acid salts - sodium citrate and sodium tartrate
- Green synthesis of copper oxide nanoparticles using Juglans regia leaf extract and assessment of their physico-chemical and biological properties
- Deep eutectic solvents (DESs) as powerful and recyclable catalysts and solvents for the synthesis of 3,4-dihydropyrimidin-2(1H)-ones/thiones
- Biosynthesis, characterization and anti-microbial activity of silver nanoparticle based gel hand wash
- Efficient and selective microwave-assisted O-methylation of phenolic compounds using tetramethylammonium hydroxide (TMAH)
- Anticoagulant, thrombolytic and antibacterial activities of Euphorbia acruensis latex-mediated bioengineered silver nanoparticles
- Volcanic ash as reusable catalyst in the green synthesis of 3H-1,5-benzodiazepines
- Green synthesis, anionic polymerization of 1,4-bis(methacryloyl)piperazine using Algerian clay as catalyst
- Selenium supplementation during fermentation with sugar beet molasses and Saccharomyces cerevisiae to increase bioethanol production
- Biosynthetic potential assessment of four food pathogenic bacteria in hydrothermally silver nanoparticles fabrication
- Investigating the effectiveness of classical and eco-friendly approaches for synthesis of dialdehydes from organic dihalides
- Pyrolysis of palm oil using zeolite catalyst and characterization of the boil-oil
- Azadirachta indica leaves extract assisted green synthesis of Ag-TiO2 for degradation of Methylene blue and Rhodamine B dyes in aqueous medium
- Synthesis of vitamin E succinate catalyzed by nano-SiO2 immobilized DMAP derivative in mixed solvent system
- Extraction of phytosterols from melon (Cucumis melo) seeds by supercritical CO2 as a clean technology
- Production of uronic acids by hydrothermolysis of pectin as a model substance for plant biomass waste
- Biofabrication of highly pure copper oxide nanoparticles using wheat seed extract and their catalytic activity: A mechanistic approach
- Intelligent modeling and optimization of emulsion aggregation method for producing green printing ink
- Improved removal of methylene blue on modified hierarchical zeolite Y: Achieved by a “destructive-constructive” method
- Two different facile and efficient approaches for the synthesis of various N-arylacetamides via N-acetylation of arylamines and straightforward one-pot reductive acetylation of nitroarenes promoted by recyclable CuFe2O4 nanoparticles in water
- Optimization of acid catalyzed esterification and mixed metal oxide catalyzed transesterification for biodiesel production from Moringa oleifera oil
- Kinetics and the fluidity of the stearic acid esters with different carbon backbones
- Aiming for a standardized protocol for preparing a process green synthesis report and for ranking multiple synthesis plans to a common target product
- Microstructure and luminescence of VO2 (B) nanoparticle synthesis by hydrothermal method
- Optimization of uranium removal from uranium plant wastewater by response surface methodology (RSM)
- Microwave drying of nickel-containing residue: dielectric properties, kinetics, and energy aspects
- Simple and convenient two step synthesis of 5-bromo-2,3-dimethoxy-6-methyl-1,4-benzoquinone
- Biodiesel production from waste cooking oil
- The effect of activation temperature on structure and properties of blue coke-based activated carbon by CO2 activation
- Optimization of reaction parameters for the green synthesis of zero valent iron nanoparticles using pine tree needles
- Microwave-assisted protocol for squalene isolation and conversion from oil-deodoriser distillates
- Denitrification performance of rare earth tailings-based catalysts
- Facile synthesis of silver nanoparticles using Averrhoa bilimbi L and Plum extracts and investigation on the synergistic bioactivity using in vitro models
- Green production of AgNPs and their phytostimulatory impact
- Photocatalytic activity of Ag/Ni bi-metallic nanoparticles on textile dye removal
- Topical Issue: Green Process Engineering / Guest Editors: Martine Poux, Patrick Cognet
- Modelling and optimisation of oxidative desulphurisation of tyre-derived oil via central composite design approach
- CO2 sequestration by carbonation of olivine: a new process for optimal separation of the solids produced
- Organic carbonates synthesis improved by pervaporation for CO2 utilisation
- Production of starch nanoparticles through solvent-antisolvent precipitation in a spinning disc reactor
- A kinetic study of Zn halide/TBAB-catalysed fixation of CO2 with styrene oxide in propylene carbonate
- Topical on Green Process Engineering

