Abstract
Metal oxide nanoporous materials and nanoparticles have main potential uses in several different fields such as nanoelectronics, biomedical science, renewable solar energy, drug-gene delivery, thermal insulation, and so on. On the other hand, it is vital for scientists to understand that agricultural waste biomass-assisted synthesis is less costly, environmentally friendly and renewable strategy, and therefore, agricultural wastes are ideal renewable resources for production of nanostructures as a substitute for toxic chemicals. This present review includes significant recent improvements concerning the synthesis of agricultural waste biomass-assisted metal oxide nanostructures and their application. The goal is to provide a vision for the use of non-extracted agricultural waste, especially lignocellulosic biomass an inexpensive, green, differentiated resource and policy for the synthesis of valuable nanoporous materials and nanoparticles.
1 Introduction
Synthesis of nanostructures is often a key step in preparation of many important targets including nanocatalysts, nanomedicines, nanosensors, nanodevices and nanosystems [1]. There are various types of methods for the synthesis of a large number of nanostructures in the form of nanoparticles, nanorods and nanotubes, thin films and nanoporous materials. Some of the already existing classical procedures to synthesis of diverse types of nanomaterials are improved to acquire novel nanostructures and some new methods are developed. Nanoscience is an interdisciplinary field, so there are numerous bottom-up, top-down and hybrid procedures available to produce nanostructures. The procedure to be applied depends upon the morphology of material, textural properties (pore size, particle size, and surface area) chemical and thermal stability, and type of nanomaterial.
Due to semiconductor, or insulator character of metal oxide nanomaterials these nanostructures play a very important role in the production of micro and nanoelectronic circuits, nanosensors, piezoelectric devices, fuel cells, and catalysts [2]. Although various methods for the synthesis of metal oxide nanostructures including sol-gel route [3], reverse micelle technique [4] and coprecipitation process [5] have been reported, these methods usually use the expensive, moisture sensitive, and toxic chemicals which encounter the environmental and economical challenges in large scale application.
In the recent years, bio-based economy and use of renewable biomass as the raw material have been considered as sustainable options to tackle the problems associated with local and global pollutions. Agricultural waste biomass especially lignocellulosic ones (e.g. rice husk and walnut shell) has progressively attracted some attention as a low cost renewable resource for the production of fuels [6] and more recently chemicals and materials [7].
The aim of this review is description of recent advancements in the synthesis of agricultural waste biomass-assisted metal oxide nanomaterials and their significant uses in recent five years. The advantages of using low cost renewable agricultural residues as feedstock for nanomaterial synthesis are also highlighted. The main focus of this article is on the vital role of agricultural waste in nanoparticle and nanoporous material synthesis. Moreover, the various uses of agricultural residues-derived nanomaterial especially in catalysis are discussed in this review.
1.1 Rice husk and straw
Rice husks (or rice hulls) are the hard protecting pods of rice seeds that are 20-22% of total produce of rice, and large amounts of rice husk are currently prepared by rice mill industry as a one of the most abundant agricultural waste which contains cellulose 28.7-35.6%, hemicellulose 12.0-29.3%, and lignin 15.4-20.0% [8]. Interestingly, the high silica content (8.7-12.1%) is observed in its husk, [9]. Mostly in form amorphous hydrated SiO2 similar to that found in most of the other objects in the biosphere [10]. This section reviewed recent reports in syntheses of rice husks-derived SiO2 nanomaterials and their various applications, especially in catalysis.
In 2013, Thuc et al. used acid-washed Vietnamese rice husk for the synthesis of silica nanoparticles [11]. Acid treatment removes the small quantities of cations causing the increase of SiO2 extracted from rice husk by calcination followed by preparation of sodium silicate solution by addition of sodium hydroxide. Subsequently, this sodium silicate solution was slowly added into the cetyltrimethylammonium bromide (CTAB) solution in water/butanol mixture. The precipitation of silica nanoparticles (SiO2NPs) was performed at pH=4 by adding H2SO4. Controlling of the size of nanoparticles was done by surfactant concentration, aging time and aging temperature.
In a separate study, Wang et al. described the use of ionic liquid to extract lignocellulose from rice husk followed by calcination of separated rice husk residue at 700°C for 2 h to prepare 70 nm-sized SiO2NPs with 241.1 m2/g surface area [12]. Use of HCl instead of ionic liquid 50 nm-sized SiO2NPs with 283.3 m2/g surface area. Amorphous organo-functionalized SiO2NPs can be obtained from calcination of nitric acid treated rice husk followed by NaOH treatment, addition of 3-(chloropropyl) trimethoxysilane and pH reduction of resulted solution [13]. Along with this line, it was shown that silica-supported imidazole could be synthesized by these organo-functionalized SiO2NPs [14]. Mesoporous silicas have been fabricated from sodium silicate solution using rice husk ash, NaOH solution and CTAB as the structure-directing agent where fusion temperature of rice husk plays a crucial role in mesopore sizes and pore volumes [15]. It is proved that the silica materials possessed mesoporous structure with specific surface area, pore volume and average pore diameter in the range of 211-518 m2/g, 1.36-1.92 cm3/g and 11.8-25.8 nm, respectively. In addition, these silica materials can be used as supports for CO2 adsorption by dispersion of tetraethylenepentamine on the support.
In 2014, Andas et al. prepared sodium silicate solution by stirring the acid-treated and washed rice husk in NaOH solution to yield dark brown solution [16]. Addition of an acidic solution of Cobalt (II) to sodium silicate solution until pH=3 followed by aging resulted in high surface area mesoporous silica-supported cobalt catalysts (Co@SiO2). This catalyst was studied in the oxidation of phenol with hydrogen peroxide (Scheme 1). A recyclability study of this catalyst has also been successfully performed for five runs.
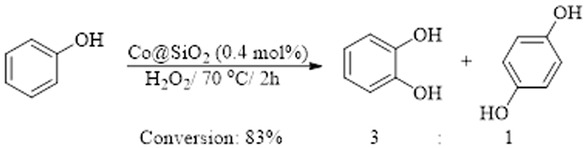
Co@SiO2-catalyzed oxidation of phenol.
Andas, and Adam reported the preparation and characterization of immobilized AgNPs (25 nm) on rice husk-derived silica with 514 m2g-1 BET surface area [17]. Synthesis of layered sodium silicates from rice husk developed by Lin et al. [18]. Phase transformation from β through δ to α was observed as the temperature and time of synthesis was enlarged. Produced silica with a higher content of the δ phase exhibited greater Mg2+ and Ca2+ binding capacities providing an appropriate substitute for polluting phosphorus-based detergents. Mukti and coworkers conducted the preparation of hierarchical ZSM-5 below 100oC by silica from rice husks and sodium aluminate in the presence of tetrapropylammonium bromide as structure-directing agent [19]. The synthesized zeolite exhibits hierarchical microporosity (0.55 nm) and the additional intercrystallite mesoporosity (3.5 nm) originated from the spherical morphology of zeolite, which was composed of nanocrystallites. In 2017, Mor and coworkers described the preparation of 10-15 nm rice husk-derived porous silica particles acidification of sodium silicate solution [20].
Hsu and co-workers prepared carbon–silica nanocomposite with 349 m2g-1 BET surface area by calcination of acid-washed rice husk agricultural waste under an inert atmosphere followed by hydrothermal treatment to decrease inorganic impurities [21]. Carbon–silica composite was mixed with the epoxy resin, curing agent and catalyst which resulted in epoxy/carbon– silica composite. The thermal conductivity and thermal-mechanical properties of CTE and storage modulus of epoxy/carbon-silica composite have been studied and improvement of this properties compared with pure epoxy polymer has been proved. In a separate study, Rangari and co-workers described the preparation of 14 nm sized silica/carbon hybrid nanoparticles from using pyrolysis of rice husk powder in high-pressure/temperature reactor [22]. This nanomaterial has been used to synthesis of nanocomposite from Ecoflex, a biodegradable compostable polymer, using 3D printing technique. This 3D printed biocomposite was further experienced for their mechanical and thermal properties. TGA and Tensile tests showed sensible improvement in thermal stability, due to the addition of silica based nanomaterial.
Silica-coated magnetic nanoparticles (Fe3O4NP@SiO2) have been prepared from dispersion of Fe3O4 nanoparticles and rice husk-derived silica in ammonia solution and used for supporting palladium (Fe3O4NP@SiO2-Pd) [23]. This nanomaterial was then studied as catalyst in the Suzuki coupling of electron-rich and electron-poor aryl iodides and bromides in the presence of waste eggshell as inexpensive and green solid base. with phenyl boronic acid (Scheme 2) with Pd loadings of 0.03 mol% (yields: 85-93%). The recovered catalyst could be reused at least four times without loss of activity.

Fe3O4NP@SiO2-Pd catalyzed Suzuki reaction.
In 2017, carbon quantum dot grafted silica nanoparticles have been prepared by calcination of under a nitrogen atmosphere followed by dispersion in H2SO4 and oxidation treatment in HNO3 [24]. Since the photoluminescence property of this nanomaterial can easily be detected under UV light at a pH=7–8, carbon quantum dot grafted silica nanoparticles can be used in biomedical fields. 34 nm sized SiO2NPs prepared from rice husk-extracted sodium silicate solution was used for the preparation of nanocomposite with styrene and wood and presented the best improvement in all physical and mechanical properties [25]. Also this nanocomposite considerably growths the decay resistance against white rot fungi.
It is noteworthy that, in addition to silica based-nanomaterials, nanoporous MnO2 have been prepared using rice husk by Lin and co-workers [26]. Washed and dried rice husk was calcined under an inert atmosphere and treated with NaOH solution to remove to remove of silica impurity. The resulted carbon powder after treatment with KOH solution and calcination was neutralized by HCl. Nanoporous MnO2 was synthesized by dispersion of MnSO4 and rice husk-derived carbon in propyl alcohol/water solution followed by addition of KMnO4. The product is nanocomposite of carbon and MnO2 with 1645 m2/g surface area. 52% of total pore volume in this nanocomposite is volume of mesopores. Based on cyclic voltammetric data MnO2 nanocomposite can be used as electrode material for high-performance supercapacitors. Raman et al. reported the application of rice husk-extracted silica nanoparticles as a green additive for the production of sustainable concrete [27].
Burri and co-workers prepared PdNPs of 3.5-4.5 nm in size supported on high surface area nanoporous silica-carbon (PdNP@Si-C) by calcination of acid wash husk rice under inert atmosphere [28]. This catalyst was studied in the carbonylative Suzuki coupling reaction of various types of aryl iodides in 1,4-dioxane at 120°C under carbon monoxide atmosphere (Scheme 3).

PdNP@Si-C catalyzed carbonylative Suzuki reaction.
In 2018, in a study reported by Mathur et al., the synthesis of 80-85 nm forsterite (Mg2SiO4) nanoparticle by rice-husk extracted silica and magnesium oxide through solid-state method has been described [29]. A polymeric membrane nanocomposite was synthesized using rice husk-derived silica nanoparticle as filler and polyether-polyamide block co-polymer for separation of CO2 from other gases [30]. Chauhan and co-workers prepared silica nanowires diameter in the range of 15-35 nm and length about 0.5 μm using rice husk ash [31]. In addition to the rice husk, 15-20 nm sized SiO2NPs has been synthesized using rice straw by Yadav and Kauldhar [32].
1.2 Sugar cane bagasse and leaves
Sugar cane bagasse (SCB) is the agricultural waste biomass of sugar and ethanol industries that is abundantly available. Pereira and co-workers in 2015 reported a simple and low-cost technique for modifying the textural properties and stability of mesoporous gamma-alumina using bayerite as aluminum source and SCB as sacrificial template [33]. The effect of waste biomass was evaluated by changing the template/bayerite ratio. The presence of biomass as a template improved surface area and pore volume up to 209 m2/g and 0.44 cm3/g, respectively. In addition, mean pore diameters are adjusted from 5.2 to 7.9 nm by varying the template/bayerite ratio.
Due to high content of silica in SCB, Norhasyimi and co-workers prepared SBA-15 using acid washed SCB ash as silica source and P123 as neutral template [34]. The BET surface area and total pore volume of synthesized SBA-15 were 466 m2/g and 0.14 cm3/g, respectively. In a separate study, SCB has been used for tuning the size of titanium oxide nanoparticles (TiO2NPs) where the amount of biomass template played an important role in size controlling [35]. TiO2 sol from titanium tetraisopropoxide in pH=4 was calcined under 200°C for 5 h resulted in TiO2 powder gel. Various amounts of SCB was added to this gel and stirred for 2 h at room temperature. SCB-supported TiO2NPs are in the range from 5–20 nm. The photocatalytic activity of SCB-supported TiO2NPs was tested in the degradation of azo dye methyl orange under visible light.
In 2018, Jabasingh and co-workers prepared 50-200 nm magnetic iron oxide nanoparticles by coprecipitation of Fe(III)/Fe(II) in the presence of ammonia and sugar cane bagasse under inert atmosphere followed by calcination in furnace [36]. This magnetic material can be used as supports for adsorption of Cr6+. Also, MgO hybrid spongelike carbonaceous composite has been synthesized using magnesium acetate and sugarcane leafy trash as a sacrificial template by thermal decomposition method under N2 flow [37]. This nanomaterial was comprised of nano-size magnesium oxide flakes and nanotube-like carbon sponge with surface area and pore volume up to 40.61 m2/g and 0.323 cm3/g, respectively.
1.3 Bamboo leaf
From ancient times bamboo was considered to have many applications such as food source and building material. Bamboo species are native to warm and humid climates especially areas in China, Japan, Korea, India, and Australia. Because of silica content of acid washed bamboo leaf ash, it has also been used successfully as silica source in synthesizing of 13.8 nm sized SiO2NPs by Venkatachalam and Rangaraj [38]. Based on nontoxicity nature of bamboo leaf-derived SiO2NPs, it has been proposed that these nanoparticles can be considered as a potential candidate for drug delivery and other medical applications. In 2017, aluminosilicate zeolite A has been synthesized from bamboo leaf ash by Ng and co-workers [39]. The catalytic activity of biomass-derived zeolite A was tested in cyanoethylation of methanol with 82% conversion and 100% product selectivity (Scheme 4). The recovered catalyst could be reused at least nine times without significant loss in activity.

Zeolite A catalyzed cyanoethylation of methanol.
1.4 Egg shell
The eggshell is 95-97% calcium carbonate crystals, stabilized by a protein matrix and may be considered as calcium source [40, 41]. In this regard, Jayasankar and co-workers prepared Dy3+-doped calcium silicate (Ca2SiO4) nanoparticles by calcined waste egg shell and rice husk through solid-state reaction technique at 1250oC [42]. Dysprosium (III) ions-doped materials are used in making laser materials, optical sensors and solar energy harvesting [43, 44]. Luminescence and excitation spectra of Dy3+-doped calcium silicate were investigated and the results indicate that the Dy3+-doped calcium silicate phosphors are appropriate for production of low cost white light emitting devices. Hen egg shell has also been used successfully to synthesize calcium oxide nanoparticles by Fulekar and Pandit [45]. Crushed egg shell was decomposed to CaO above 800°C. Resulted calcium oxide was refluxed in water and again calcined in furnace. The average size of calcium oxide nanoparticles were found 75 nm based on XRD pattern. This nanomaterial was then studied as catalyst in the transesterification of dry microalgae biomass (A. Obliquus) into biodiesel.
1.5 Walnut shell
In 2017, we synthesized 11 nm sized walnut shell-supported Cu/Cu2O nanoparticles (Cu/Cu2ONP@WS) [46]. Abundance of walnut shell as worthless lignocellulosic waste [47] in nature is main benefit of this protocol. Copper acetate solution in water was mixed with an aqueous mixture of walnut shell powder followed by addition of NaBH4 resulted in Cu/Cu2O NPs. This copper nanocomposite was employed in the synthesis of propargylamines via the three-component reaction between benzaldehydes, secondary cyclic amines and phenyl acetylene (Scheme 5). Also, walnut shell-stabilized copper nanoparticles were found to be an efficient, inexpensive, easy to prepare, green and reusable catalyst in the reduction of aromatic nitro and nitrile compounds to their corresponding amines with NaBH4 at 35°C in an aqueous medium (Scheme 6) [48]. We continued our studies on the application of this nanocomposite in the classic Ullman reaction to synthesize biaryl (Scheme 7) [48]. Also a size-dependence study showed that the catalytic efficiency of larger Cu nanoparticles was much lower than that of smaller ones under similar reaction conditions.

Cu/Cu2ONP@WS catalyzed synthesis of propargylamines.

Cu/Cu2ONP@WS catalyzed reduction of aromatic nitro and nitrile compounds.

Cu/Cu2ONP@WS catalyzed Ullman reaction.
Quite recently, we synthesized high surface area magnesia (MgO) [49], high surface area alumina (Al2O3) [50] and ceria (CeO2) nanoparticles [51] by walnut shell as sacrificial template. Walnut shell powder was mixed with an aqueous solution of magnesium nitrate hexahydrate in different weight ratios [49]. After stirring followed by evaporation of water, the solid was calcined at 500°C. Produced magnesium oxides have BET surface area up to 79 m2/g. Hydrothermal treatment of this magnesium oxide followed by annealing in air surprisingly increase surface area up to 212 m2/g (MgO-212). To test the catalytic activity of magnesia materials as heterogeneous catalysts we selected Meerwein-Ponndorf-Verley reduction of cyclohexanone with 2-propanol (Scheme 8). It is of interest that different surface areas of magnesia materials showed different catalytic activities. Magnesium oxides with the highest surface area exhibited maximum yields. Also, MgO-212 was tested as the support of palladium nanoparticles (PdNP@MgO-212) for the aerobic oxidation of alcohols (Scheme 9). Satisfying results were observed for the PdNP@MgO-212 catalyzed aerobic oxidation of benzylic and aliphatic alcohols under air without the use of exogenous base.

MgO-catalyzed Meerwein-Ponndorf-Verley reduction.

PdNP@MgO-212 catalyzed aerobic oxidation of alcohols.
We continued our studies on the synthesis of walnut shell-templated high surface area alumina and boehmite by the same way [50]. Produced aluminum oxides and boehmite has BET surface area up to 218 and 304 m2/g, respectively. Additionally, boehmite was studied as the support of vanadium catalyst for the oxidation of alcohols by hydrogen peroxide. We have found that resulting V-loaded material act as an effective catalytic system for the oxidation of a wide range of alcohols in 1,4-dioxane by hydrogen peroxide. The catalyst can be recovered and reused four times without loss of activity.
Along this line, it was shown that 9-21 nm sized cerium oxide nanoparticles (CeO2NPs) have been synthesized using cerium nitrate and walnut shell as a sacrificial template by thermal decomposition method [51]. Particle sizes can be adjusted by changing cerium nitrate/walnut shell ratio. To test the catalytic activity of ceria nanoparticles as a solid catalyst, we selected three-component synthesis of 3,4-dihydroquinoxalin-2-amine by tert-butyl isocyanide, acetone, and o-phenylenediamine in an aqueous medium (Scheme 10). We have studied the effect of ceria particle size in this process. It is of interest that smaller ceria NPs showed the highest activity.

CeO2NPs-catalyzed synthesis of 3,4-dihydroquinoxalin-2-amine.
1.6 Wheat straw
Recently Patel and co-workers prepared 100-200 nm silica nanoparticles by calcination of acid-washed wheat straw followed by treatment with NaOH solution and neutralization by HCl [52]. This catalyst was studied in the synthesis of pyrano[2, 3-c]pyrazole derivatives by one-pot, four-component process of benzaldehydes, hydrazine hydrate, ethyl acetoacetate, and malononitrile in an aqueous medium (Scheme 11). In a separate study, Chen et al. described the use wheat straw and Fe(NO3)3·9H2O to preparation of porous carbon-supported Fe2O3 ultrathin film by calcination of KOH-containing wheat straw under inert atmosphere followed by addition of Fe(II) solution to aqueous mixture of calcined powder and annealing at 200°C [53].
![Scheme 11 SiO2NPs-catalyzed synthesis of pyrano[2, 3-c]pyrazole derivatives.](/document/doi/10.1515/gps-2019-0010/asset/graphic/j_gps-2019-0010_fig_011.jpg)
SiO2NPs-catalyzed synthesis of pyrano[2, 3-c]pyrazole derivatives.
1.7 Coconut shell
In 2016, Asefa and co-workers prepared magnetic activated carbon by carbonization of coconut shell in the presence of ferric chloride (FeCl3⋅6H2O) under an inert atmosphere with different FeCl3/coconut shell ratios [54]. The BET surface area, total pore volume and average pore size of the resulted porous materials were 238-372 m2/g, 0.118-0.210 cm3/g, and 1.98-2.26 nm, respectively. Authors proposed that Fe3O4 was formed via formation of Fe2O3 and reduction of Fe (III) by carbon. It was shown that these nanomaterials are efficient adsorbents for toxic dyes such as Sunset yellow. 10-100 nm sized Iron oxide nanoparticles synthesized from coconut husk extract and ferric chloride by Sebastian and co-workers have been used to remove Ca and Cd from aqueous media [55].
1.8 Banana peel
In 2014, 20-50 nm Mn3O4 nanoparticles have been synthesized using banana peel extract by Yan and co-workers [56]. It is believed that banana peel extract plays a dual role, reducing KMnO4 to form Mn3O4 and preventing the agglomeration of nanoparticles during preparation. Also 20 nm SiO2NPs have been synthesized by addition of banana peel extract to an alkaline solution of tetraethylorthosilicate in ethanol followed by calcination of precipitate [57]. These nanoparticles can be employed for the adsorption of methylene blue.
1.9 Miscellaneous agricultural waste
Ahmaruzzaman et al. synthesized Fe3O4 nanocomposites (Fe3O4-NC) using papaya leaves as lignocellulosic agricultural waste by a simple thermal decomposition method [58]. Fe(II) and Fe(III) solution were mixed with washed and dried powder of papaya leaves followed by addition of NaOH solution. Resultant black precipitate was dried at 353 K (Fe3O4-NC353) and calcined in a furnace under air atmosphere at 573 K or 773 K (Fe3O4-NC573 and Fe3O4-NC773) to make nanoparticle with size 18-46 nm. Fe3O4-NC773 was found to be the most efficient nanocomposite for the removal of chlorazol black E, with an efficiency of 96%.
In 2015, the use of tea waste for the synthesis of supported hydrous aluminium oxide nanoparticles was reported by Wan et al. [59]. This porous nanomaterial has been synthesized by co-precipitation between aluminium sulfate and NaOH in the presence of tea waste and anionic polyacrylamide. Supported porous alumina was employed for defluoridation of drinking water by anion exchange of fluoride with sulfate ions.
In 2017, the use of pine needles for the synthesis of nanocomposites of nanocellulose and NiFe2O4 nanoparticles was reported by Singhal et al. [60]. These nanocomposites were fabricated by synthesis of carbon nanofibers (CNF) from pine needles followed by Silanization of these nanofibers (SCNF) by tetraethyl orthosilicate. Ni(II) and Fe(III) solutions were added to the aqueous mixture of CNF or SCNF. After adjusting pH of this mixture to 7.5 and stirring for 2 h, mixture hydrothermally treated at 160°C to make of nanocellulose/NiFe2O4 nanocomposites. Authors found that SNCF-supported NiFe2O4 (NiFe2O4@SCNF) act as an effective catalytic system for the oxidative degradation of Remazol Black 5 (RB5) and reduction of nitrophenols (Scheme 12). The catalyst can be used three times without loss of activity.
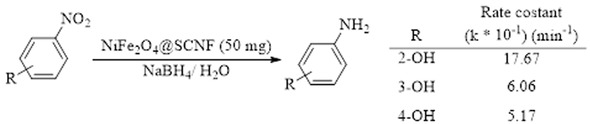
NiFe2O4@SCNF-catalyzed reduction of nitrophenols.
Recently Sınağ and co-workers prepared 10 nm titanium oxide nanoparticles supported on hazelnut shell or olive residue activated carbon and employed as a photocatalyst in photodegradation of methylene blue [61]. Tan et al. synthesized NiCo2O4 nanocomposites using honey pomelo peel derived porous carbon [62]. Ni(II), Co(II) and urea solution was mixed with porous carbon followed by treatment in 120°C resulted in carbon-supported NiCo2O4 nanosheets.
2 Conclusion
The use of biomass and phytochemicals in the design and synthesis of nanomaterials creates a significant connection between plant sciences and nanoscience that referred to as “green nanotechnology”. Green nanotechnology emphasizes use of green chemistry principles to the design and synthesis of nanostructure products and the application of these nanomaterials. Many synthesis methods of namomaterials include toxic chemicals, low conversions, high energy necessities, and difficult, wasteful purifications; thus, there are multiple opportunities to improve greener procedures for the synthesis of nanomaterials. Many of the green chemistry principles apply readily to the synthesis of nanostructure product.
Advancement of clean and bio-based approaches to nanomaterials is one of the most attractive fields of scientific and industrial researches in the recent years for the growth of green technologies, namely bio-derived nanostructures that are compatible with environmental necessities. Biomass derived materials with related uses (e.g., separation, adsorption, catalysis, sensing, semiconductivity and thermal insulation), will be absolutely necessary for research and development for renewable resources based fabrication especially non-extracted agricultural waste derived material synthesis. However, recent developments in the environmentally friendly nano synthesis opened a novel window to innovate new routes in large scale synthesis of thoroughly purified, morphologically well-defined, and metal or organo-functionalized nanostructures. In this article, an overview of the synthesis of agricultural waste biomass-assisted metal oxide nanostructures, and their potential uses in different fields were presented. Despite the growth over recent decades, substantial challenges exist that must be addressed to achieve optimal efficiency and deliver maximum benefits from the complete and comprehensive application of these agricultural waste biomass-assisted metal oxide nanostructures.
Acknowledgment
The authors gratefully acknowledge the financial support for this work by the research council of Urmia University.
References
[1] Kulkarni S.K., Nanotechnology: principles and practices (3rd ed.). Springer, New Delhi, 2015.10.1007/978-3-319-09171-6Suche in Google Scholar
[2] Rodríguez J.A., Fernández-García M. (Eds.), Synthesis, properties, and applications of oxide nanomaterials. John Wiley & Sons, New Jersey, 2007.10.1002/0470108975Suche in Google Scholar
[3] Mota T.L.R., de Oliveira A.P.M., Nunes E.H.M., Houmard M., Simple process for preparing mesoporous sol-gel silica adsorbents with high water adsorption capacities. Microporous Mesoporous Mater., 2017, 253, 177-182.10.1016/j.micromeso.2017.07.010Suche in Google Scholar
[4] Iqbal Y., Bae H., Rhee I., Hong S., Magnetic heating of silica-coated manganese ferrite nanoparticles. J. Magn. Magn. Mater., 2016, 409, 80-86.10.1016/j.jmmm.2016.02.078Suche in Google Scholar
[5] Fang L., Hou L., Zhang Y., Wang Y., Yan G., Synthesis of highly hydrophobic rutile titania-silica nanocomposites by an improved hydrolysis co-precipitation method. Ceram. Int., 2017, 43, 5592-5598.10.1016/j.ceramint.2017.01.091Suche in Google Scholar
[6] Lin C.S.K., Pfaltzgraff L.A., Herrero-Davila L., Mubofu E.B., Abderrahim S., Clark J.H., et al., Food waste as a valuable resource for the production of chemicals, materials and fuels. Current situation and global perspective. Energy Environ. Sci., 2013, 6, 426-464.10.1039/c2ee23440hSuche in Google Scholar
[7] Brun N., Hesemann P., Esposito D., Expanding the biomass derived chemical space. Chem. Sci., 2017, 8, 4724-4738.10.1039/C7SC00936DSuche in Google Scholar
[8] Isikgor F.H., Becer C.R., Lignocellulosic biomass: a sustainable platform for the production of bio-based chemicals and polymers. Polym. Chem., 2015, 6, 4497-4559.10.1039/C5PY00263JSuche in Google Scholar
[9] Ding T.P., Ma G.R., Shui M.X., Wan D.F., Li R.H., Silicon isotope study on rice plants from the Zhejiang province, China. Chem. Geol., 2005, 218, 41-50.10.1016/j.chemgeo.2005.01.018Suche in Google Scholar
[10] Asuncion M.Z., Hasegawa I., Kampf J.W., Laine R.M., The selective dissolution of rice hull ash to form [OSiO1.5]8[R4N]8 (R = Me, CH2CH2OH) octasilicates. Basic nanobuilding blocks and possible models of intermediates formed during biosilicification processes. J. Mater. Chem., 2005, 15, 2114-2121.10.1039/b502178bSuche in Google Scholar
[11] Le V.H., Thuc C.N.H., Thuc H.H., Synthesis of silica nanoparticles from Vietnamese rice husk by sol–gel method. Nanoscale Res. Lett., 2013, 8, 58-67.10.1186/1556-276X-8-58Suche in Google Scholar PubMed PubMed Central
[12] Chen H., Wang W., Martin J.C., Oliphant A.J., Doerr P.A., Xu J.F., et al., Extraction of lignocellulose and synthesis of porous silica nanoparticles from rice husks: a comprehensive utilization of rice husk biomass. Sustainable Chem. Eng., 2013, 1, 254-259.10.1021/sc300115rSuche in Google Scholar
[13] Adam F., Osman H., Hello K.M., The immobilization of 3-(chloropropyl)triethoxysilane onto silica by a simple one-pot synthesis. J. Colloid Interface Sci., 2009, 331, 143-147.10.1016/j.jcis.2008.11.048Suche in Google Scholar PubMed
[14] Adam F., Chew T.-S., Mannyarasai H., Appaturi J.N., Hello K.M., Synthesis and characterization of silica–imidazole mesostructured composite from agricultural biomass. Micropor. Mesopor. Mater., 2013, 167, 245-248.10.1016/j.micromeso.2012.09.007Suche in Google Scholar
[15] Zeng W., Bai H., Swelling-agent-free synthesis of rice husk derived silica materials with large mesopores for efficient CO2 capture. Chem. Eng. J., 2014, 251, 1-9.10.1016/j.cej.2014.04.041Suche in Google Scholar
[16] Andas J., Adam F., Rahman I.A., Sol-gel derived mesoporous cobalt silica catalyst: Synthesis, characterization and its activity in the oxidation of phenol. Appl. Surf. Sci., 2014, 315, 154-162.10.1016/j.apsusc.2014.07.118Suche in Google Scholar
[17] Andas J., Adam F., One-pot synthesis of nanoscale silver supported biomass-derived silica. Mater. Today: Proceedings, 2016, 3, 1345-1350.10.1016/j.matpr.2016.04.013Suche in Google Scholar
[18] Deng M., Zhang G., Zeng Y., Pei X., Huang R., Lin J., Simple process for synthesis of layered sodium silicates using rice husk ash as silica source. J. Alloys Compd., 2016, 683, 412-417.10.1016/j.jallcom.2016.05.115Suche in Google Scholar
[19] Kadja G.T.M., Fabiani V.A., Aziz M.H., Fajar A.T.N., Prasetyo A., Suendo V., et al., The effect of structural properties of natural silica precursors in the mesoporogen-free synthesis of hierarchical ZSM-5 below 100°C. Adv. Powder Technol., 2017, 28, 443-452.10.1016/j.apt.2016.10.017Suche in Google Scholar
[20] Mor S., Manchanda C.K., Kansal S.K., Ravindra K., Nanosilica extraction from processed agricultural residue using green technology. J. Clean Prod. 2017, 143, 1284-1290.10.1016/j.jclepro.2016.11.142Suche in Google Scholar
[21] Hsieh Y.-Y., Tsai Y.-C., He J.-R., Yang P.-F., Lin H.-P., Hsu C.-H., et al., Rice husk agricultural waste-derived low ionic content carbon–silica nanocomposite for green reinforced epoxy resin electronic packaging material. J. Taiwan Inst. Chem. Eng., 2017, 78, 493-499.10.1016/j.jtice.2017.06.010Suche in Google Scholar
[22] Biswas M.C., Jeelani S., Rangari V., Influence of biobased silica/carbon hybrid nanoparticles on thermal and mechanical properties of biodegradable polymer films. Compos. Commun., 2017, 4, 43-53.10.1016/j.coco.2017.04.005Suche in Google Scholar
[23] Khazaei A., Khazaei M., Nasrollahzadeh M., Nano-Fe3O4@SiO2 supported Pd(0) as a magnetically recoverable nanocatalyst for Suzuki coupling reaction in the presence of waste eggshell as low-cost natural base. Tetrahedron, 2017, 73, 5624-5633.10.1016/j.tet.2017.05.054Suche in Google Scholar
[24] Wang Z., Liu J., Wang W., Wei Z., Wang F., Gong P., et al., Photoluminescent carbon quantum dot grafted silica nanoparticles directly synthesized from rice husk biomass. J. Mater. Chem. B., 2017, 5, 4679-4689.10.1039/C7TB00811BSuche in Google Scholar PubMed
[25] Ghorbani M., Biparva P., Hosseinzadeh S., Effect of colloidal silica nanoparticles extracted from agricultural waste on physical, mechanical and antifungal properties of wood polymer composite. Eur. J. Wood Prod., 2018, 76, 749-757.10.1007/s00107-017-1157-zSuche in Google Scholar
[26] Yuan C., Lin H., Lu H., Xing E., Zhang Y., Xie B., Synthesis of hierarchically porous MnO2rice husks derived carbon composite as high-performance electrode material for supercapacitors. Appl. Energy., 2016, 178, 260-268.10.1016/j.apenergy.2016.06.057Suche in Google Scholar
[27] Lim J.L.G., Raman S.N., Lai F.C., Zain M.F.M., Hamid R., Synthesis of nano cementitious additives from agricultural wastes for the production of sustainable concrete. J. Clean Prod., 2018, 171, 1150-1160.10.1016/j.jclepro.2017.09.143Suche in Google Scholar
[28] Ketike T., Velpula V.R.K., Madduluri V.R., Kamaraju S.R.R., Burri D.R., Carbonylative Suzuki‐Miyaura cross‐coupling over Pd NPs/Rice‐Husk carbon‐silica solid catalyst: Effect of 1, 4‐dioxane solvent. Chem. Select., 2018, 3, 7164-7169.10.1002/slct.201801100Suche in Google Scholar
[29] Mathur L., Hossain S.K.S., Majhi M.R., Roy P.K., Synthesis of nano-crystalline forsterite (Mg2SiO4) powder from biomass rice husk silica by solid-state route. Bol. Soc. Esp. Ceram. V., 2018, 57, 112-118.10.1016/j.bsecv.2017.10.004Suche in Google Scholar
[30] Bhattacharya M., Mandal M.K., Synthesis of rice straw extracted nano-silica-composite membrane for CO2 separation. J. Clean Prod., 2018, 186, 241-252.10.1016/j.jclepro.2018.03.099Suche in Google Scholar
[31] Bathla A., Narula C., Chauhan R.P., Hydrothermal synthesis and characterization of silica nanowires using rice husk ash: an agricultural waste. J. Mater. Sci. Mater. El., 2018, 29, 6225-6231.10.1007/s10854-018-8598-ySuche in Google Scholar
[32] Kauldhar B.S,. Yadav S.K., Turning waste to wealth: A direct process for recovery of nano-silica and lignin from paddy straw agro-waste. J. Clean Prod., 2018, 194, 158-166.10.1016/j.jclepro.2018.05.136Suche in Google Scholar
[33] Cardoso C.S., Licea Y.E., Huang X., Willinger M., Louis B., Pereira M.M., Improving textural properties of γ-alumina by using second generation biomass in conventional hydrothermal method. Micropor. Mesopor. Mater., 2015, 207, 134-141.10.1016/j.micromeso.2015.01.015Suche in Google Scholar
[34] Norsuraya S., Fazlena H., Norhasyimi R., Sugarcane Bagasse as a renewable source of silica to synthesize Santa Barbara Amorphous-15 (SBA-15). Procedia Eng., 2016, 148, 839-846.10.1016/j.proeng.2016.06.627Suche in Google Scholar
[35] Xue H., Chen Y., Liu X., Qian Q., Luo Y., Cui M., et al., Visible light-assisted efficient degradation of dye pollutants with biomass-supported TiO2 hybrids. Mater. Sci. Eng, C., 2018, 82, 197-203.10.1016/j.msec.2017.08.060Suche in Google Scholar PubMed
[36] Jabasingh S.A., Belachew H., Yimam A., Iron oxide induced bagasse nanoparticles for the sequestration of Cr6+ ions from tannery effluent using a modified batch reactor. J. Appl. Polym. Sci., 2018, 135, 46683.10.1002/app.46683Suche in Google Scholar
[37] Li R., Liang W., Wang J.J., Gaston L.A., Huang D., Huang H., et al., Facilitative capture of As(V), Pb(II) and methylene blue from aqueous solutions with MgO hybrid sponge-like carbonaceous composite derived from sugarcane leafy trash. J. Environ. Manage., 2018, 212, 77-78.10.1016/j.jenvman.2017.12.034Suche in Google Scholar PubMed
[38] Rangaraj S., Venkatachalam R., A lucrative chemical processing of bamboo leaf biomass to synthesize biocompatible amorphous silica nanoparticles of biomedical importance. Appl. Nanosci., 2017, 7, 145-153.10.1007/s13204-017-0557-zSuche in Google Scholar
[39] Ng E.-P., Chow J.-H., Mukti R.R., Muraza O., Chuan Ling T., Wong K.-L., Hydrothermal synthesis of zeolite from bamboo leaf biomass and its catalytic activity in cyanoethylation of methanol under autogenic pressure and air conditions. Mater. Chem. Phys., 2017, 201, 78-85.10.1016/j.matchemphys.2017.08.044Suche in Google Scholar
[40] Hunton P., Research on eggshell structure and quality: An historical overview. Rev. Bras. Cienc. Avic., 2005, 7, 67-71.10.1590/S1516-635X2005000200001Suche in Google Scholar
[41] Nys Y., Gautron J., Garcia-Ruiz J.M., Hincke M.T., Avian eggshell mineralization: Biochemical and functional characterization of matrix proteins. C. R. Palevol., 2004, 3, 549-562.10.1016/j.crpv.2004.08.002Suche in Google Scholar
[42] Devi L.L., Basavapoornima Ch., Venkatramu V., Babu P., Jayasankar C.K., Synthesis of Ca2SiO4Dy3+ phosphors from agricultural waste for solid state lighting applications. Ceram. Int., 2017, 43, 16622-16627.10.1016/j.ceramint.2017.09.052Suche in Google Scholar
[43] Haritha P., Martín I.R., Dwaraka Viswanath C.S., Vijaya N., Krishnaiah K.V., Jayasankar C.K., et al., Structure, morphology andoptical characterization of Dy3+-doped BaYF5 nanocrystals for warm white lightemitting devices. Opt. Mat., 2017, 70, 16-24.10.1016/j.optmat.2017.05.002Suche in Google Scholar
[44] Rajeswari R., Jayasankar C.K., Ramachari D., Babu S.S., Synthesis, structural and luminescence properties of near white light emitting Dy3+-doped Y2CaZnO5 nanophosphor for solid state lighting. Cer. Inter., 2013, 39, 7523-7529.10.1016/j.ceramint.2013.03.003Suche in Google Scholar
[45] Pandit P.R., Fulekar M.H., Egg shell waste as heterogeneous nanocatalyst for biodiesel production: Optimized by response surface methodology. J. Environ. Manage., 2017, 198, 319-329.10.1016/j.jenvman.2017.04.100Suche in Google Scholar PubMed
[46] Saadati F., Leghaei V., Zamani A., Environmentally benign copper nanoparticles supported on walnut shell as a highly durable nanocatalyst for the synthesis of propargylamines. J. Serb. Chem. Soc., 2017, 82, 1-12.10.2298/JSC161221081SSuche in Google Scholar
[47] Kambarova G.B., Sarymsakov Sh., Preparation of activated charcoal from walnut shells. Solid Fuel Chem., 2008, 42, 183-186.10.3103/S0361521908030129Suche in Google Scholar
[48] Zamani A., Poursattar Marjani A., Nikoo A., Heidarpour M., Dehghan A., Synthesis and characterization of copper nanoparticles on walnut shell for catalytic reduction and CC coupling reaction. Inorg. Nano-Metal Chem., 2018, 48, 176-181.10.1080/24701556.2018.1503676Suche in Google Scholar
[49] Zamani A., Poursattar Marjani A., Abedi Mehmandar M., Synthesis of high surface area magnesia by using walnut shell as a template. Green Process. Synth., 2019, 8, 199-206.10.1515/gps-2018-0066Suche in Google Scholar
[50] Zamani A., Poursattar Marjani A., Abdollahpour N., Synthesis of high surface area boehmite and alumina by using walnut shell as template. Int. J. Nano Biomaterials, 2019, 8, 1-14.10.1504/IJNBM.2019.097588Suche in Google Scholar
[51] Zamani A., Poursattar Marjani A., Alimoradlu K., Walnut shell-templated ceria nanoparticles: green synthesis, characterization and catalytic application. Int. J. Nanosci., 2018, 17, 1850008.10.1142/S0219581X18500084Suche in Google Scholar
[52] Patel K.G., Misra N.M., Vekariya R.H., Shettigar R.R., One-pot multicomponent synthesis in aqueous medium of 1,4-dihydropyrano[2,3-c]pyrazole-5-carbonitrile and derivatives using a green and reusable nano-SiO2 catalyst from agricultural waste. Res. Chem. Intermed., 2018, 44, 289-304.10.1007/s11164-017-3104-3Suche in Google Scholar
[53] Fang K., Chen J., Zhou X., Mei C., Tian Q., Xu J., et al., Decorating biomass-derived porous carbon with Fe2O3 ultrathin film for high-performance supercapacitors. Electrochim. Acta., 2018, 261, 198-205.10.1016/j.electacta.2017.12.140Suche in Google Scholar
[54] Cazetta A.L., Pezoti O., Bedin K.C., Silva T.L., Junior A.P., Asefa T., et al., Magnetic activated carbon derived from biomass waste by concurrent synthesis: efficient adsorbent for toxic dyes. ACS Sustain. Chem. Eng., 2016, 4, 1058-1068.10.1021/acssuschemeng.5b01141Suche in Google Scholar
[55] Sebastian A., Nangia A., Prasad M.N.V., A green synthetic route to phenolics fabricated magnetite nanoparticles from coconut husk extract: Implications to treat metal contaminated water and heavy metal stress in Oryza sativa L. J. Clean Prod., 2018, 174, 355-366.10.1016/j.jclepro.2017.10.343Suche in Google Scholar
[56] Yan D., Zhang H., Chen L., Zhu G., Wang Z., Xu H., et al., Supercapacitive properties of Mn3O4 nanoparticles biosynthesized from banana peel extract. RSC Adv., 2014, 4, 23649-23652.10.1039/c4ra02603aSuche in Google Scholar
[57] Ali S.M., Fabrication of a nanocomposite from an agricultural waste and its application as a biosorbent for organic pollutants. J. Environ. Sci. Technol., 2018, 15, 1169-1178.10.1007/s13762-017-1477-xSuche in Google Scholar
[58] Ahmed M.J.K., Ahmaruzzaman M., Fabrication and characterization of novel lignocellulosic biomass tailored Fe3O4 nanocomposites: influence of annealing temperature and chlorazol black E sequestration. RSC Adv., 2015, 5, 107466-107473.10.1039/C5RA20605GSuche in Google Scholar
[59] Cai H., Chen G., Peng C., Xu L., Zhu X., Zhang Z., et al., Enhanced removal of fluoride by tea waste supported hydrous aluminium oxide nanoparticles: anionic polyacrylamide mediated aluminium assembly and adsorption mechanism. RSC Adv., 2015, 5, 29266-29275.10.1039/C5RA01560JSuche in Google Scholar
[60] Gupta K., Kaushik A., Tikoo K.B., Kumar V., Singhal S., Enhanced catalytic activity of composites of NiFe2O4 and nano cellulose derived from waste biomass for the mitigation of organic pollutants. Arab. J. Chem., 2017, in press, DOI: 10.1016/j. arabjc.2017.07.016.10.1016/j.arabjc.2017.07.016Suche in Google Scholar
[61] Donar Y.O., Bilge S., Sınağ A., Pliekhov O., TiO2Carbon materials derived from hydrothermal carbonization of waste biomass: a highly efficient, low‐cost visible‐light‐driven photocatalyst. Chem. Cat. Chem., 2018, 10, 1134-1139.10.1002/cctc.201701405Suche in Google Scholar
[62] Guo D., Zhang L., Song X., Tan L., Ma H., Jiao J., et al., NiCo2O4 nanosheets grown on interconnected honeycomb-like porous biomass carbon for high performance asymmetric supercapacitors. New J. Chem., 2018, 42, 8478-8484.10.1039/C8NJ00515JSuche in Google Scholar
© 2019 Zamani et al., published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 Public License.
Artikel in diesem Heft
- Regular Articles
- Studies on the preparation and properties of biodegradable polyester from soybean oil
- Flow-mode biodiesel production from palm oil using a pressurized microwave reactor
- Reduction of free fatty acids in waste oil for biodiesel production by glycerolysis: investigation and optimization of process parameters
- Saccharin: a cheap and mild acidic agent for the synthesis of azo dyes via telescoped dediazotization
- Optimization of lipase-catalyzed synthesis of polyethylene glycol stearate in a solvent-free system
- Green synthesis of iron oxide nanoparticles using Platanus orientalis leaf extract for antifungal activity
- Ultrasound assisted chemical activation of peanut husk for copper removal
- Room temperature silanization of Fe3O4 for the preparation of phenyl functionalized magnetic adsorbent for dispersive solid phase extraction for the extraction of phthalates in water
- Evaluation of the saponin green extraction from Ziziphus spina-christi leaves using hydrothermal, microwave and Bain-Marie water bath heating methods
- Oxidation of dibenzothiophene using the heterogeneous catalyst of tungsten-based carbon nanotubes
- Calcined sodium silicate as an efficient and benign heterogeneous catalyst for the transesterification of natural lecithin to L-α-glycerophosphocholine
- Synergistic effect between CO2 and H2O2 on ethylbenzene oxidation catalyzed by carbon supported heteropolyanion catalysts
- Hydrocyanation of 2-arylmethyleneindan-1,3-diones using potassium hexacyanoferrate(II) as a nontoxic cyanating agent
- Green synthesis of hydratropic aldehyde from α-methylstyrene catalyzed by Al2O3-supported metal phthalocyanines
- Environmentally benign chemical recycling of polycarbonate wastes: comparison of micro- and nano-TiO2 solid support efficiencies
- Medicago polymorpha-mediated antibacterial silver nanoparticles in the reduction of methyl orange
- Production of value-added chemicals from esterification of waste glycerol over MCM-41 supported catalysts
- Green synthesis of zerovalent copper nanoparticles for efficient reduction of toxic azo dyes congo red and methyl orange
- Optimization of the biological synthesis of silver nanoparticles using Penicillium oxalicum GRS-1 and their antimicrobial effects against common food-borne pathogens
- Optimization of submerged fermentation conditions to overproduce bioethanol using two industrial and traditional Saccharomyces cerevisiae strains
- Extraction of In3+ and Fe3+ from sulfate solutions by using a 3D-printed “Y”-shaped microreactor
- Foliar-mediated Ag:ZnO nanophotocatalysts: green synthesis, characterization, pollutants degradation, and in vitro biocidal activity
- Green cyclic acetals production by glycerol etherification reaction with benzaldehyde using cationic acidic resin
- Biosynthesis, characterization and antimicrobial activities assessment of fabricated selenium nanoparticles using Pelargonium zonale leaf extract
- Synthesis of high surface area magnesia by using walnut shell as a template
- Controllable biosynthesis of silver nanoparticles using actinobacterial strains
- Green vegetation: a promising source of color dyes
- Mechano-chemical synthesis of ammonia and acetic acid from inorganic materials in water
- Green synthesis and structural characterization of novel N1-substituted 3,4-dihydropyrimidin-2(1H)-ones
- Biodiesel production from cotton oil using heterogeneous CaO catalysts from eggshells prepared at different calcination temperatures
- Regeneration of spent mercury catalyst for the treatment of dye wastewater by the microwave and ultrasonic spray-assisted method
- Green synthesis of the innovative super paramagnetic nanoparticles from the leaves extract of Fraxinus chinensis Roxb and their application for the decolourisation of toxic dyes
- Biogenic ZnO nanoparticles: a study of blueshift of optical band gap and photocatalytic degradation of reactive yellow 186 dye under direct sunlight
- Leached compounds from the extracts of pomegranate peel, green coconut shell, and karuvelam wood for the removal of hexavalent chromium
- Enhancement of molecular weight reduction of natural rubber in triphasic CO2/toluene/H2O systems with hydrogen peroxide for preparation of biobased polyurethanes
- An efficient green synthesis of novel 1H-imidazo[1,2-a]imidazole-3-amine and imidazo[2,1-c][1,2,4]triazole-5-amine derivatives via Strecker reaction under controlled microwave heating
- Evaluation of three different green fabrication methods for the synthesis of crystalline ZnO nanoparticles using Pelargonium zonale leaf extract
- A highly efficient and multifunctional biomass supporting Ag, Ni, and Cu nanoparticles through wetness impregnation for environmental remediation
- Simple one-pot green method for large-scale production of mesalamine, an anti-inflammatory agent
- Relationships between step and cumulative PMI and E-factors: implications on estimating material efficiency with respect to charting synthesis optimization strategies
- A comparative sorption study of Cr3+ and Cr6+ using mango peels: kinetic, equilibrium and thermodynamic
- Effects of acid hydrolysis waste liquid recycle on preparation of microcrystalline cellulose
- Use of deep eutectic solvents as catalyst: A mini-review
- Microwave-assisted synthesis of pyrrolidinone derivatives using 1,1’-butylenebis(3-sulfo-3H-imidazol-1-ium) chloride in ethylene glycol
- Green and eco-friendly synthesis of Co3O4 and Ag-Co3O4: Characterization and photo-catalytic activity
- Adsorption optimized of the coal-based material and application for cyanide wastewater treatment
- Aloe vera leaf extract mediated green synthesis of selenium nanoparticles and assessment of their In vitro antimicrobial activity against spoilage fungi and pathogenic bacteria strains
- Waste phenolic resin derived activated carbon by microwave-assisted KOH activation and application to dye wastewater treatment
- Direct ethanol production from cellulose by consortium of Trichoderma reesei and Candida molischiana
- Agricultural waste biomass-assisted nanostructures: Synthesis and application
- Biodiesel production from rubber seed oil using calcium oxide derived from eggshell as catalyst – optimization and modeling studies
- Study of fabrication of fully aqueous solution processed SnS quantum dot-sensitized solar cell
- Assessment of aqueous extract of Gypsophila aretioides for inhibitory effects on calcium carbonate formation
- An environmentally friendly acylation reaction of 2-methylnaphthalene in solvent-free condition in a micro-channel reactor
- Aegle marmelos phytochemical stabilized synthesis and characterization of ZnO nanoparticles and their role against agriculture and food pathogen
- A reactive coupling process for co-production of solketal and biodiesel
- Optimization of the asymmetric synthesis of (S)-1-phenylethanol using Ispir bean as whole-cell biocatalyst
- Synthesis of pyrazolopyridine and pyrazoloquinoline derivatives by one-pot, three-component reactions of arylglyoxals, 3-methyl-1-aryl-1H-pyrazol-5-amines and cyclic 1,3-dicarbonyl compounds in the presence of tetrapropylammonium bromide
- Preconcentration of morphine in urine sample using a green and solvent-free microextraction method
- Extraction of glycyrrhizic acid by aqueous two-phase system formed by PEG and two environmentally friendly organic acid salts - sodium citrate and sodium tartrate
- Green synthesis of copper oxide nanoparticles using Juglans regia leaf extract and assessment of their physico-chemical and biological properties
- Deep eutectic solvents (DESs) as powerful and recyclable catalysts and solvents for the synthesis of 3,4-dihydropyrimidin-2(1H)-ones/thiones
- Biosynthesis, characterization and anti-microbial activity of silver nanoparticle based gel hand wash
- Efficient and selective microwave-assisted O-methylation of phenolic compounds using tetramethylammonium hydroxide (TMAH)
- Anticoagulant, thrombolytic and antibacterial activities of Euphorbia acruensis latex-mediated bioengineered silver nanoparticles
- Volcanic ash as reusable catalyst in the green synthesis of 3H-1,5-benzodiazepines
- Green synthesis, anionic polymerization of 1,4-bis(methacryloyl)piperazine using Algerian clay as catalyst
- Selenium supplementation during fermentation with sugar beet molasses and Saccharomyces cerevisiae to increase bioethanol production
- Biosynthetic potential assessment of four food pathogenic bacteria in hydrothermally silver nanoparticles fabrication
- Investigating the effectiveness of classical and eco-friendly approaches for synthesis of dialdehydes from organic dihalides
- Pyrolysis of palm oil using zeolite catalyst and characterization of the boil-oil
- Azadirachta indica leaves extract assisted green synthesis of Ag-TiO2 for degradation of Methylene blue and Rhodamine B dyes in aqueous medium
- Synthesis of vitamin E succinate catalyzed by nano-SiO2 immobilized DMAP derivative in mixed solvent system
- Extraction of phytosterols from melon (Cucumis melo) seeds by supercritical CO2 as a clean technology
- Production of uronic acids by hydrothermolysis of pectin as a model substance for plant biomass waste
- Biofabrication of highly pure copper oxide nanoparticles using wheat seed extract and their catalytic activity: A mechanistic approach
- Intelligent modeling and optimization of emulsion aggregation method for producing green printing ink
- Improved removal of methylene blue on modified hierarchical zeolite Y: Achieved by a “destructive-constructive” method
- Two different facile and efficient approaches for the synthesis of various N-arylacetamides via N-acetylation of arylamines and straightforward one-pot reductive acetylation of nitroarenes promoted by recyclable CuFe2O4 nanoparticles in water
- Optimization of acid catalyzed esterification and mixed metal oxide catalyzed transesterification for biodiesel production from Moringa oleifera oil
- Kinetics and the fluidity of the stearic acid esters with different carbon backbones
- Aiming for a standardized protocol for preparing a process green synthesis report and for ranking multiple synthesis plans to a common target product
- Microstructure and luminescence of VO2 (B) nanoparticle synthesis by hydrothermal method
- Optimization of uranium removal from uranium plant wastewater by response surface methodology (RSM)
- Microwave drying of nickel-containing residue: dielectric properties, kinetics, and energy aspects
- Simple and convenient two step synthesis of 5-bromo-2,3-dimethoxy-6-methyl-1,4-benzoquinone
- Biodiesel production from waste cooking oil
- The effect of activation temperature on structure and properties of blue coke-based activated carbon by CO2 activation
- Optimization of reaction parameters for the green synthesis of zero valent iron nanoparticles using pine tree needles
- Microwave-assisted protocol for squalene isolation and conversion from oil-deodoriser distillates
- Denitrification performance of rare earth tailings-based catalysts
- Facile synthesis of silver nanoparticles using Averrhoa bilimbi L and Plum extracts and investigation on the synergistic bioactivity using in vitro models
- Green production of AgNPs and their phytostimulatory impact
- Photocatalytic activity of Ag/Ni bi-metallic nanoparticles on textile dye removal
- Topical Issue: Green Process Engineering / Guest Editors: Martine Poux, Patrick Cognet
- Modelling and optimisation of oxidative desulphurisation of tyre-derived oil via central composite design approach
- CO2 sequestration by carbonation of olivine: a new process for optimal separation of the solids produced
- Organic carbonates synthesis improved by pervaporation for CO2 utilisation
- Production of starch nanoparticles through solvent-antisolvent precipitation in a spinning disc reactor
- A kinetic study of Zn halide/TBAB-catalysed fixation of CO2 with styrene oxide in propylene carbonate
- Topical on Green Process Engineering
Artikel in diesem Heft
- Regular Articles
- Studies on the preparation and properties of biodegradable polyester from soybean oil
- Flow-mode biodiesel production from palm oil using a pressurized microwave reactor
- Reduction of free fatty acids in waste oil for biodiesel production by glycerolysis: investigation and optimization of process parameters
- Saccharin: a cheap and mild acidic agent for the synthesis of azo dyes via telescoped dediazotization
- Optimization of lipase-catalyzed synthesis of polyethylene glycol stearate in a solvent-free system
- Green synthesis of iron oxide nanoparticles using Platanus orientalis leaf extract for antifungal activity
- Ultrasound assisted chemical activation of peanut husk for copper removal
- Room temperature silanization of Fe3O4 for the preparation of phenyl functionalized magnetic adsorbent for dispersive solid phase extraction for the extraction of phthalates in water
- Evaluation of the saponin green extraction from Ziziphus spina-christi leaves using hydrothermal, microwave and Bain-Marie water bath heating methods
- Oxidation of dibenzothiophene using the heterogeneous catalyst of tungsten-based carbon nanotubes
- Calcined sodium silicate as an efficient and benign heterogeneous catalyst for the transesterification of natural lecithin to L-α-glycerophosphocholine
- Synergistic effect between CO2 and H2O2 on ethylbenzene oxidation catalyzed by carbon supported heteropolyanion catalysts
- Hydrocyanation of 2-arylmethyleneindan-1,3-diones using potassium hexacyanoferrate(II) as a nontoxic cyanating agent
- Green synthesis of hydratropic aldehyde from α-methylstyrene catalyzed by Al2O3-supported metal phthalocyanines
- Environmentally benign chemical recycling of polycarbonate wastes: comparison of micro- and nano-TiO2 solid support efficiencies
- Medicago polymorpha-mediated antibacterial silver nanoparticles in the reduction of methyl orange
- Production of value-added chemicals from esterification of waste glycerol over MCM-41 supported catalysts
- Green synthesis of zerovalent copper nanoparticles for efficient reduction of toxic azo dyes congo red and methyl orange
- Optimization of the biological synthesis of silver nanoparticles using Penicillium oxalicum GRS-1 and their antimicrobial effects against common food-borne pathogens
- Optimization of submerged fermentation conditions to overproduce bioethanol using two industrial and traditional Saccharomyces cerevisiae strains
- Extraction of In3+ and Fe3+ from sulfate solutions by using a 3D-printed “Y”-shaped microreactor
- Foliar-mediated Ag:ZnO nanophotocatalysts: green synthesis, characterization, pollutants degradation, and in vitro biocidal activity
- Green cyclic acetals production by glycerol etherification reaction with benzaldehyde using cationic acidic resin
- Biosynthesis, characterization and antimicrobial activities assessment of fabricated selenium nanoparticles using Pelargonium zonale leaf extract
- Synthesis of high surface area magnesia by using walnut shell as a template
- Controllable biosynthesis of silver nanoparticles using actinobacterial strains
- Green vegetation: a promising source of color dyes
- Mechano-chemical synthesis of ammonia and acetic acid from inorganic materials in water
- Green synthesis and structural characterization of novel N1-substituted 3,4-dihydropyrimidin-2(1H)-ones
- Biodiesel production from cotton oil using heterogeneous CaO catalysts from eggshells prepared at different calcination temperatures
- Regeneration of spent mercury catalyst for the treatment of dye wastewater by the microwave and ultrasonic spray-assisted method
- Green synthesis of the innovative super paramagnetic nanoparticles from the leaves extract of Fraxinus chinensis Roxb and their application for the decolourisation of toxic dyes
- Biogenic ZnO nanoparticles: a study of blueshift of optical band gap and photocatalytic degradation of reactive yellow 186 dye under direct sunlight
- Leached compounds from the extracts of pomegranate peel, green coconut shell, and karuvelam wood for the removal of hexavalent chromium
- Enhancement of molecular weight reduction of natural rubber in triphasic CO2/toluene/H2O systems with hydrogen peroxide for preparation of biobased polyurethanes
- An efficient green synthesis of novel 1H-imidazo[1,2-a]imidazole-3-amine and imidazo[2,1-c][1,2,4]triazole-5-amine derivatives via Strecker reaction under controlled microwave heating
- Evaluation of three different green fabrication methods for the synthesis of crystalline ZnO nanoparticles using Pelargonium zonale leaf extract
- A highly efficient and multifunctional biomass supporting Ag, Ni, and Cu nanoparticles through wetness impregnation for environmental remediation
- Simple one-pot green method for large-scale production of mesalamine, an anti-inflammatory agent
- Relationships between step and cumulative PMI and E-factors: implications on estimating material efficiency with respect to charting synthesis optimization strategies
- A comparative sorption study of Cr3+ and Cr6+ using mango peels: kinetic, equilibrium and thermodynamic
- Effects of acid hydrolysis waste liquid recycle on preparation of microcrystalline cellulose
- Use of deep eutectic solvents as catalyst: A mini-review
- Microwave-assisted synthesis of pyrrolidinone derivatives using 1,1’-butylenebis(3-sulfo-3H-imidazol-1-ium) chloride in ethylene glycol
- Green and eco-friendly synthesis of Co3O4 and Ag-Co3O4: Characterization and photo-catalytic activity
- Adsorption optimized of the coal-based material and application for cyanide wastewater treatment
- Aloe vera leaf extract mediated green synthesis of selenium nanoparticles and assessment of their In vitro antimicrobial activity against spoilage fungi and pathogenic bacteria strains
- Waste phenolic resin derived activated carbon by microwave-assisted KOH activation and application to dye wastewater treatment
- Direct ethanol production from cellulose by consortium of Trichoderma reesei and Candida molischiana
- Agricultural waste biomass-assisted nanostructures: Synthesis and application
- Biodiesel production from rubber seed oil using calcium oxide derived from eggshell as catalyst – optimization and modeling studies
- Study of fabrication of fully aqueous solution processed SnS quantum dot-sensitized solar cell
- Assessment of aqueous extract of Gypsophila aretioides for inhibitory effects on calcium carbonate formation
- An environmentally friendly acylation reaction of 2-methylnaphthalene in solvent-free condition in a micro-channel reactor
- Aegle marmelos phytochemical stabilized synthesis and characterization of ZnO nanoparticles and their role against agriculture and food pathogen
- A reactive coupling process for co-production of solketal and biodiesel
- Optimization of the asymmetric synthesis of (S)-1-phenylethanol using Ispir bean as whole-cell biocatalyst
- Synthesis of pyrazolopyridine and pyrazoloquinoline derivatives by one-pot, three-component reactions of arylglyoxals, 3-methyl-1-aryl-1H-pyrazol-5-amines and cyclic 1,3-dicarbonyl compounds in the presence of tetrapropylammonium bromide
- Preconcentration of morphine in urine sample using a green and solvent-free microextraction method
- Extraction of glycyrrhizic acid by aqueous two-phase system formed by PEG and two environmentally friendly organic acid salts - sodium citrate and sodium tartrate
- Green synthesis of copper oxide nanoparticles using Juglans regia leaf extract and assessment of their physico-chemical and biological properties
- Deep eutectic solvents (DESs) as powerful and recyclable catalysts and solvents for the synthesis of 3,4-dihydropyrimidin-2(1H)-ones/thiones
- Biosynthesis, characterization and anti-microbial activity of silver nanoparticle based gel hand wash
- Efficient and selective microwave-assisted O-methylation of phenolic compounds using tetramethylammonium hydroxide (TMAH)
- Anticoagulant, thrombolytic and antibacterial activities of Euphorbia acruensis latex-mediated bioengineered silver nanoparticles
- Volcanic ash as reusable catalyst in the green synthesis of 3H-1,5-benzodiazepines
- Green synthesis, anionic polymerization of 1,4-bis(methacryloyl)piperazine using Algerian clay as catalyst
- Selenium supplementation during fermentation with sugar beet molasses and Saccharomyces cerevisiae to increase bioethanol production
- Biosynthetic potential assessment of four food pathogenic bacteria in hydrothermally silver nanoparticles fabrication
- Investigating the effectiveness of classical and eco-friendly approaches for synthesis of dialdehydes from organic dihalides
- Pyrolysis of palm oil using zeolite catalyst and characterization of the boil-oil
- Azadirachta indica leaves extract assisted green synthesis of Ag-TiO2 for degradation of Methylene blue and Rhodamine B dyes in aqueous medium
- Synthesis of vitamin E succinate catalyzed by nano-SiO2 immobilized DMAP derivative in mixed solvent system
- Extraction of phytosterols from melon (Cucumis melo) seeds by supercritical CO2 as a clean technology
- Production of uronic acids by hydrothermolysis of pectin as a model substance for plant biomass waste
- Biofabrication of highly pure copper oxide nanoparticles using wheat seed extract and their catalytic activity: A mechanistic approach
- Intelligent modeling and optimization of emulsion aggregation method for producing green printing ink
- Improved removal of methylene blue on modified hierarchical zeolite Y: Achieved by a “destructive-constructive” method
- Two different facile and efficient approaches for the synthesis of various N-arylacetamides via N-acetylation of arylamines and straightforward one-pot reductive acetylation of nitroarenes promoted by recyclable CuFe2O4 nanoparticles in water
- Optimization of acid catalyzed esterification and mixed metal oxide catalyzed transesterification for biodiesel production from Moringa oleifera oil
- Kinetics and the fluidity of the stearic acid esters with different carbon backbones
- Aiming for a standardized protocol for preparing a process green synthesis report and for ranking multiple synthesis plans to a common target product
- Microstructure and luminescence of VO2 (B) nanoparticle synthesis by hydrothermal method
- Optimization of uranium removal from uranium plant wastewater by response surface methodology (RSM)
- Microwave drying of nickel-containing residue: dielectric properties, kinetics, and energy aspects
- Simple and convenient two step synthesis of 5-bromo-2,3-dimethoxy-6-methyl-1,4-benzoquinone
- Biodiesel production from waste cooking oil
- The effect of activation temperature on structure and properties of blue coke-based activated carbon by CO2 activation
- Optimization of reaction parameters for the green synthesis of zero valent iron nanoparticles using pine tree needles
- Microwave-assisted protocol for squalene isolation and conversion from oil-deodoriser distillates
- Denitrification performance of rare earth tailings-based catalysts
- Facile synthesis of silver nanoparticles using Averrhoa bilimbi L and Plum extracts and investigation on the synergistic bioactivity using in vitro models
- Green production of AgNPs and their phytostimulatory impact
- Photocatalytic activity of Ag/Ni bi-metallic nanoparticles on textile dye removal
- Topical Issue: Green Process Engineering / Guest Editors: Martine Poux, Patrick Cognet
- Modelling and optimisation of oxidative desulphurisation of tyre-derived oil via central composite design approach
- CO2 sequestration by carbonation of olivine: a new process for optimal separation of the solids produced
- Organic carbonates synthesis improved by pervaporation for CO2 utilisation
- Production of starch nanoparticles through solvent-antisolvent precipitation in a spinning disc reactor
- A kinetic study of Zn halide/TBAB-catalysed fixation of CO2 with styrene oxide in propylene carbonate
- Topical on Green Process Engineering

