Abstract
A mechano-chemical reaction formed by a simple high-energy ball milling was applied to simulate the synthesis of ammonia and organic precursors from common inorganic materials that occurred on early Earth. By milling for 0.5–64 h at centrifugal accelerations of 20–150 G, ammonia and acetic acid were produced from inorganic materials, such as iron nitride, nitrate, carbide, and carbonates, in water. The experimental results can offer waste processing of metallic acids by the mechanical method without using any alkaline sources and suggest that the high-energy milling technology provides a new synthesis mode to form wide ranges of organic materials and scope for broader applications. This study also offers a new route to the formation of the precursors on early Earth and proposes that tremors and friction initiate micro-impacts between rocks and sand in the terrestrial crust, resulting in the formation of ammonia and organic materials from inorganic materials.
1 Introduction
Ammonia is indispensable for use in numerous chemicals such as fertilizers and is industrially produced from nitrogen and hydrogen by the Haber-Bosch process. Ammonia is assumed to have been one of the essential amino acid precursors and thus an important contributor to the origin of life on early Earth 4.6 billion years ago. To reproduce the synthesis of ammonia on early Earth, a shock wave method using a single-stage propellant gun has been reported [1], which reproduces a meteorite plume consisting of nitrogen, water, and iron as one of the extraterrestrial materials vaporized by meteorite impacts [2, [3]. The shock wave parameters delivered to the specimens by the single-stage propellant gun were estimated to be about 6 GPa and 2900 K, with a much higher shock pressure of about 20 GPa applied to the container 1, [4]. A rate of up to 8% was obtained for the conversion of nitrogen to ammonia. Under similar conditions for forming organic precursors, shock pressure of 6 GPa, temperature of 5000 K, and duration of 0.7 μs were reported 5]. These shock wave conditions were much more severe compared to the mild conditions of the Haber-Bosch process (~700 K and ~30 MPa) containing catalysts such as iron and were unsuitable for the production of a wide range of organic materials.
As ceramists well know, mild conditions for forming ammonia and organic materials can also be obtained using the simple and traditional method of ball milling. As illustrated in Figure 1, collisions between small milling balls (ϕ2–5 mm) simulate meteorites colliding with Earth, with the micro-impacts from the balls delivering a high temperature (>1000 K) and pressure [6, [7]. In particular, high-energy milling methods such as the nanopowder process are assumed to be most suitable not only for producing local high-temperature and high-pressure conditions but also for delivering higher ball impact frequencies, yielding higher reaction rates 8]. Mechano-chemical (MeChem) reactions, which utilize a characteristic field of micro-impacts, are widely used in the synthesis of ceramic powders [9]. Additionally, MeChem reactions have been used in the decomposition of organic materials, in hydrogen production [10], and in the formation of metal nitrides in ammonia gas [11].
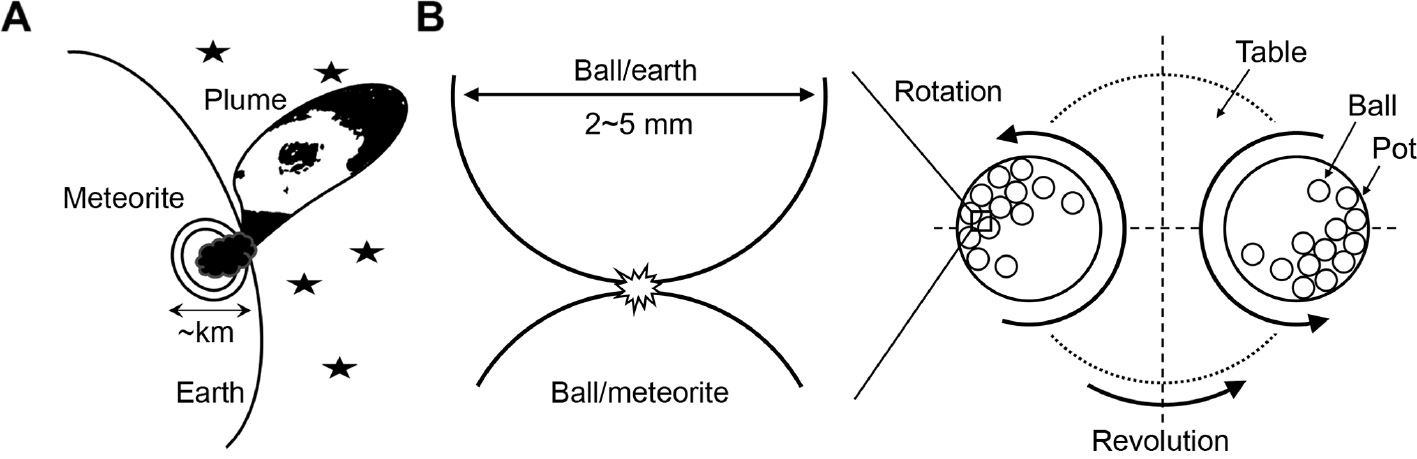
Schematic illustrations of (A) a meteorite impact and (B) a high-energy ball mill showing micro-impact fields.
Herein we report the reverse reaction under extremely high MeChem impact fields: the formation of ammonia from nitrides and nitrates and the formation of simple organic precursors from inorganic materials such as iron carbide and metal carbonates. A new route to the organic precursors of amino acids is proposed regarding the origin of life on early Earth, and extended to the formation of precursors in the deep terrestrial crusts caused by diastrophisms such as seismic tremors.
2 Materials and methods
Planetary ball mills (High-G BX254E and X382, Kurimoto, Osaka, Japan), a type of high-energy milling machine, were used as they can accommodate two pots at a time and create a continuous centrifugal acceleration of up to 150 G. Fe4N (99.9% purity, Kojundo Chemical Laboratory, Saitama, Japan), Fe3C (99.9%, Rare Metallic, Tokyo, Japan), Fe(NO3)3·9H2O (98.5%), CaCO3 (99.5%), MnCO3 (99.9%), FeS (>70%) (Kanto Chemical, Tokyo, Japan), and montmorillonite (naturally occurring mineral, Alfa Aesar, Lancashire, UK) were used as the starting materials. The purity of these chemicals was reported by each manufacturer. The clay montmorillonite was added to reduce excessive impact forces and concentrate the reactants by adsorption. The experimental conditions are summarized in Table 1. It is noted that all the reagents were inorganic materials and the total amount used in each experiment was much greater than that used in the gunshot experiment [1, [5]. A stainless steel (SUS) vessel was used as the milling pot. Zirconia balls of different diameters (ϕ2–5 mm) were used, except for specimens 2a and 2b, for which SUS balls were used. The milling operations were carried out in the range 20–150 G for 0.5–64 h. The centrifugal acceleration was calculated using technical data such as revolution diameter and frequency of the motor (F). The detailed calculation process has been described by Hashishin et al. 12], wherein they used the same planetary ball mill as that used in this study. Basically, the centrifugal acceleration (g*) was controlled from the equation g*=0.0441·F2. After milling, the resulting muddy products were collected and separated into solids and liquids by centrifugation or filtration.
Experimental conditions and starting materials.
| No. | Milling machine and conditions | Starting materials (g) | |||||
|---|---|---|---|---|---|---|---|
| Fe4N | Fe nitrate | Clay | C sources | H2O | Others | ||
| 1 | BX, 150 G, 64 h, 5ϕ (ZrO2) | 4.01 | 1.05 | 50 | |||
| 2a | X, 20 G, 0.5 h, 4ϕ (SUS) | 7.00 | 170 | ||||
| 2b | X, 20 G, 1 h, 4ϕ (SUS) | 7.00 | 170 | ||||
| 3a | X, 40 G, 39 h, 2ϕ (ZrO2) | 12.03 | 3.00 | Fe3C 18.21 | 150 | ||
| 3b | X, 40 G, 39 h, 2ϕ (ZrO2) | 6.80 | 3.00 | MnCO3 11.60 | 150 | ||
| 4 | BX, 50 G, 16 h, 2ϕ (ZrO2) | 3.52 | CaCO3 8.76 | 50 | FeS 7.69 | ||
| 5 | BX, 40 G, 30 h, 2ϕ (ZrO2) | 2.03 | 1.16 | 0.50 | Fe3C 6.14 | 60 | |
The crystalline phases of the products were identified by powder X-ray diffraction (XRD; Bruker AXS, D2 PHASER, Karlsruhe, Germany) using Cu Kα radiation. The total C/N contents in the solutions were evaluated using a total organic carbon (TOC) analyzer (Shimadzu, TOC-L CPH, Kyoto, Japan). Some specimens were examined at analytical institutes (ITES, Shiga, Japan, and Hitachi High-Tech Science Corporation, Tokyo, Japan) by standard procedures using a gas chromatograph mass spectrometer (GCMS; Perkin Elmer XL/TurboMass, column: DB-5 at a temperature of 50–320°C, MS scan: 35–600, MS sim: 45, 57, 60, 73, 74, 88, and 102), an ion chromatography (IC) system (Thermo Fisher Scientific/ICS-1100, column: CS12A at 35°C, intake: 25 μl), and an amino acid analyzer (Hitachi LA8080 AminoSAAYA, column: 2622 PH at 135°C, intake: 20 μl).
3 Results and discussion
Upon opening the pots after milling, a strong ammonia or sewage-like odor was detected, confirming the formation of ammonia. Furthermore, an increase in pH for all conditions, as shown in Table 2, suggested the formation of a strong alkaline material. Ammonia was identified as the only possible compound that could have formed from the starting materials, based on the experimental conditions (nitrogen – from the air, ZrO2 balls, and water). These results indicate that waste processing of metal acids can be done by MeChem reactions without using any alkaline sources.
Summary of experimental results.
| No. | Starting materials | Main phases from XRD | Smella | pH change | Inner pressure |
|---|---|---|---|---|---|
| 1 | Fe4N, clay | Fe3O4 | SN | 7→11 | High |
| 2a | Fe nitrate | Fe3O4 | SO | 2→8 | Low |
| 2b | Fe nitrate | Fe3O4 | SN | 2→9 | Low |
| 3a | Fe4N, Fe3C, clay | Fe3O4 | SO | 7→11 | High |
| 3b | Fe nitrate, MnCO3, clay | MnCO3, Fe3O4 | SO | 2→10 | Low |
| 4 | Fe nitrate, CaCO3, FeS | CaCO3, Fe3O4 | SO | 2→9 | Low |
| 5 | Fe nitrate, Fe4N, Fe3C, clay | Fe3O4 | SO | 2→11 | Low |
aSN, Strong ammonia smell; SO, sewage-like odor.
For specimen 1, the resulting black muddy paste bubbled for some time and produced a strong ammonia smell. This paste had a high pH of 11 as shown in Table 2. The XRD pattern of the dried product for specimen 1 indicated that Fe4N was completely decomposed into Fe3O4 (Figure 2). Elemental analysis of the mud by energy-dispersive X-ray spectrometry detected the presence of Zr, Cr, and Ni, besides Fe, which revealed some erosion of the SUS pot and ZrO2 balls. The SUS pot was eroded by about 1.8 g, which was non-negligible compared to the amount of starting materials. A shorter milling time and reduced centrifugal acceleration are expected to effectively reduce erosion.
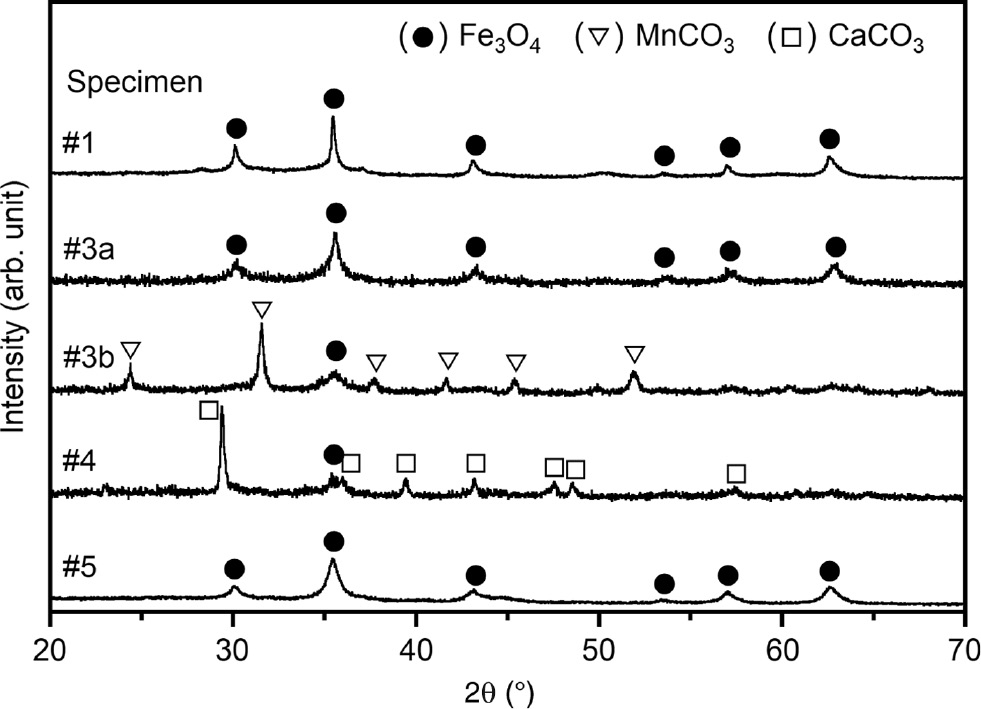
XRD patterns of the products after high-energy ball milling.
Unlike the gunshot impacts, the micro-impact fields generated by small balls (Figure 1) are not so intense as to disintegrate molecules into atomic elements, but still sufficient to activate the particle surfaces. An intermediate such as HO-Fe-NH is formed on the surface of Fe4N particles which reacts with H2O molecules and decomposes into Fe3O4 and NH3. Based on the resulting pH of 11 and water content of 50 g, the conversion rate from Fe4N to ammonia was calculated to be about 30%. Most of the remaining nitrogen was likely released from the pot as a gas. This conversion rate is much higher than that observed in the gun shock wave experiment (8%) [1]. The reactions may take place similar to a hydrothermal decomposition and are given by the following equations:
The side reaction of the eroded metal also produces hydrogen:
The excessive formation of nitrogen and hydrogen can produce a high pressure inside the pot. The observed bubbles were thus due to the release of H2 and NH3 from the clay.
The formation of Fe3O4 instead of the FeO phase is expected even under high hydrogen pressure if adequate moisture is present. When the ratio of the partial pressure of H2O to that of H2 is about 10 or above at the presumed temperature of 1000 K, the Fe3O4 phase is stable [13, [14]. When the partial pressure ratio is less than 1 or hydrogen pressure is much higher, FeO becomes more stable. This oxide transformation corresponds well to the surface topology change of crystalline MnZnFe spinels at ~1000 K under a N2 atmosphere 15]. By conducting similar experiments on Ni, Co, and Mn, the pressure and temperature at the impact fields can be determined more precisely. This will aid in understanding the mechanism of the MeChem reaction and improving the transformation yield.
For specimens 2a and 2b, mild conditions (short milling times up to 1 h and a low centrifugal acceleration of 20 G) were used to confirm ammonia formation from Fe nitrate. The change in pH from 2 to 8 after milling in half an hour showed almost half of the nitrate was transformed to ammonia, most likely in the form of ammonium nitrate. A slight sewage-like odor was detected, but there was no ammonia smell. However, with an increase in milling time, the pH increased to 9 and the characteristic ammonia smell was increasingly observed (specimen 2b). This result indicated that even shorter milling times and lower centrifugal accelerations were sufficient for ammonia formation.
Based on the formation of Fe3O4 and evolution of NH3 and NO2 (as discussed later), the decomposition reactions of nitrate are assumed to be as follows:
When these reactions are combined in a one-to-one ratio, the reaction becomes:
It is also expected that the well-known selective catalytic reduction of NOx by NH3 may take place at the same time, producing nitrogen. A typical reaction is expressed as follows:
A part of the oxygen is consumed by reaction with the eroded metal. Iron nitrate is stable in the hydrothermal condition, and the decomposition in water is not possible thermodynamically. The decomposition is assumed to take place by forming iron oxide through a donated oxygen from water or nitrate in non-equilibrium conditions under the MeChem field and segregation of the oxide in quenching. Segregated hydrogen and NOx may form ammonia and others during quenching. However, if Fe(II) is present, the reduction of nitrite to ammonia by Fe(II) is observed when the pH is higher than 7.3 [16]. The reduction of nitrate is also implied under comparable conditions [16]. In the case of Fe nitrate, no reaction was expected to occur as the initial pH was about 2 (Table 2), even though the eroded metal, Fe, was present at the beginning of milling. This implies that as the MeChem reaction progresses, the increasing pH leads to an acceleration in the reduction reaction.
More complex combinations of starting materials, including carbides and carbonates, were carried out in specimens 3–5. The results of the IC and GCMS analyses of the products are shown in Figures 3 and 4 , respectively, and summarized in Table 3. The total amounts of nitrogen initially charged in each specimen were in the range 1390–4180 mg/l. After milling, the conversion yields of nitrogen to ammonia in specimens 3a, 4, and 5 were about 15%, 30%, and 50%, respectively. These conversion ratios were much higher than those reported from a previous work [1]. In specimen 4, NO2 and S8 were detected (Figure 4). The total N content measured by TOC (2088 mg/l) was much larger than the NH3 content (800 ppm). The low NH3 content was likely due to the formation of NO2, although further studies are needed to confirm this.
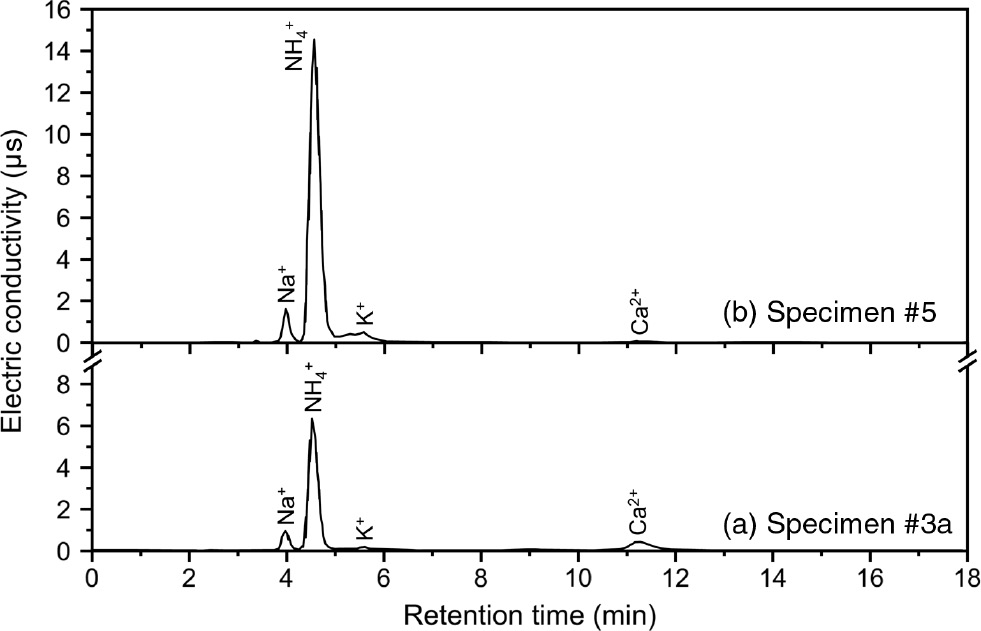
IC spectra of specimens 3a and 5.
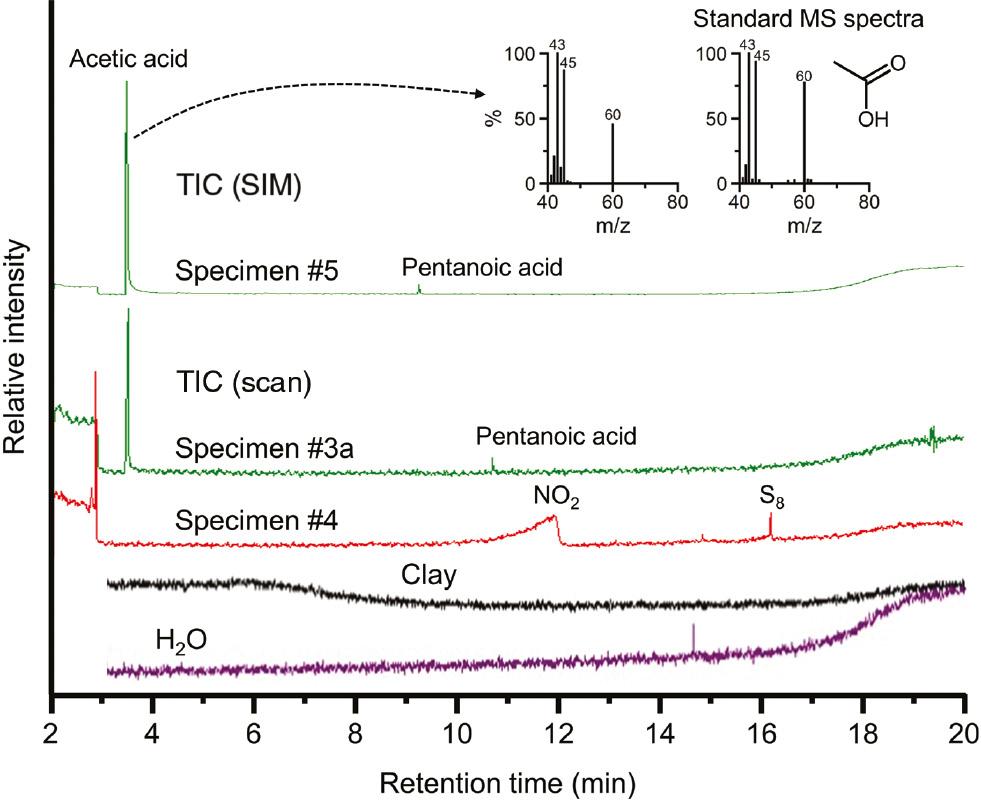
GCMS spectra of specimens 3a, 4, and 5. The spectra of clay and H2O are also shown. The insets show MS spectra of the peak observed at about 3.5 min, as well as an acetic acid standard.
Analytical results of the TOC, IC, and GCMS measurements.
| No. | Starting materials | Total N (mg/l) | NH3 content (ppm) | Total C (mg/l) | Acetic acid (ppm) | Initial content N/C (mg/l) |
|---|---|---|---|---|---|---|
| 3a | Fe4N, Fe3C, clay | 910 | 600 | 330 | 50 | 4180/7160 |
| 3b | Fe nitrate, MnCO3, clay | 990 | – | 90 | – | 1390/7130 |
| 4 | Fe nitrate, CaCO3, FeS | 2090 | 800 | 30 | 0 | 2440/21,000 |
| 5 | Fe nitrate, Fe4N, Fe3C, clay | 1250 | 1500 | 280 | 100 | 2670/6840 |
Total C/N content determined by TOC, NH3 content by IC, and acetic acid content by GCMS.
The TOC results (Table 3) indicate some organic formation, while the MS spectra obtained by GCMS (Figure 4) clearly show the presence of a small amount of acetic acid. From the results of the Fe3O4 formation (Figure 2), the hydrolysis of Fe3C, similar to Fe4N hydrolysis, can be assumed to be as follows:
The formation reaction of acetic acid is expected to be as follows:
The organic carbon yields observed by the TOC analyses were slightly above 4% at most, much less than the ammonia yields of 15%–50%. These poor yields were due to the loss of the main organic product CH4 from the pots upon opening (whereas NH3 was contained in the aqueous specimen) and the increase in hydrogen gas pressure that inhibited the reaction. The noteworthy TOC values of 90 and 30 mg/l (specimens 3b and 4, respectively) suggested the transformation of stable carbonates such as MnCO3 and CaCO3 to organic molecules, most likely CH4. The measured TOC values were comparable to the solubility limit in water of 35 ppm at room temperature, thus ruling out the formation of other organic molecules.
As shown in Figure 4, acetic acid and a small amount of pentanoic acid were detected in specimens 3a and 5. Small organic molecules other than acetic acid (70%–85% against the detected TOC values) were present, such as methyl alcohol and formaldehyde. Although the conversion yields of acetic acid from the carbon amounts initially loaded were only about 1.5%, the yields were much greater than those reported by Furukawa et al. [5]. According to the above reactions, the yield of acetic acid can be improved by the removal of excess hydrogen. The excess hydrogen tends to bond with a terminal carbon, which is undesirable for producing more complex molecules. As indicated in Eqs. 4–6, a combination with Fe(NO3)3 is preferable as it produces excess oxygen, leading to hydrogen consumption. Therefore, the high conversion yield of specimen 5 compared to that of specimen 3 was due to the addition of Fe(NO3)3.
The preliminary measurements by an amino acid analyzer revealed that amino acids such as glycine and alanine might be produced in specimen 5, whereas no organic materials were present in the starting materials (Figure 5). However, the amount of amino acids detected (0.5–1.5 ppm) was too small to eliminate biological contamination and thus confirm their formation during milling. Conversely, the amount of ammonia detected was over 1000 ppm, corresponding to the value given by IC analysis (1400 ppm), with only a small amount of ammonia (10 ppm) detected in the starting materials. This result strongly suggests the formation of ammonia from the nitride and nitrate by the MeChem reaction.
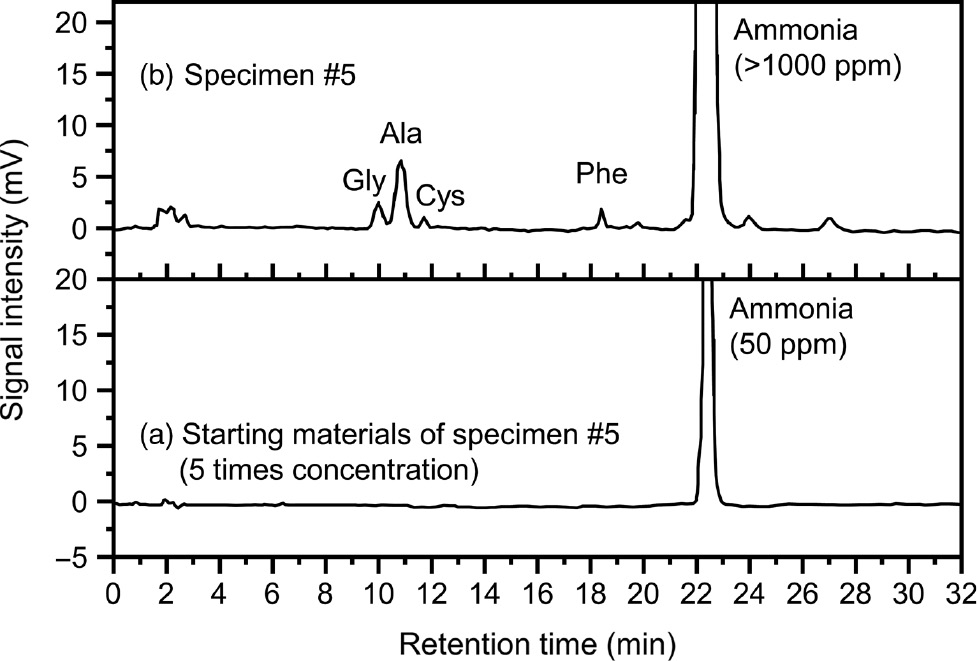
Analysis of amino acids in specimen 5 and its starting materials.
The results from the TOC, GCMS, IC, and amino acid analyses confirmed the formation of ammonia and acetic acid from inorganic starting materials such as nitrides, carbides, carbonates, and water by the micro-impacts of ZrO2 balls during the MeChem reaction. Since rocks and sand can act as ZrO2 balls, these results lead to the following assumption. On the early Earth some billions of years ago, extraterrestrial impacts by meteorites generated gigantic tremors and internal friction in terrestrial crusts that resulted in micro-impacts between rocks and sand, triggering the formation of organic precursors from the inorganic materials in the crusts. This formation route should be considered alongside the route to precursors in the hot plumes produced by meteorites [1, 5]. Since the temperature and pressure increase further in a deep terrestrial crust, even small tremors spread over long distances would effectively promote the formation of precursors. The MeChem reaction in the crusts thus offers a new route for precursor formation.
The results obtained for mild milling at 20 G for 0.5 h (specimen 2a) suggested that even a weak impact or short milling time is sufficient to produce ammonia. Such impacts are commonly observed around the world today. For instance, huge earthquakes cause tremors or impacts near 1 G, and the tremors may reach well over 20 G in the vicinity of the hypocenters. These tremors are expected to produce precursors through MeChem reactions in crusts or subducted ocean floors rich in nitrate and carbonate sediments [17]. As mentioned above, since the temperature and pressure are high in deep crusts, even slight tremors are effective. The presence of an effective catalyst can boost the production of precursors even further. Such arguments lead to the hypothesis that precursors were formed by crust events such as earthquakes and tidal movements, and accumulate in huge reaction volumes over the years and greatly surpass the amounts of precursors produced by much rarer meteorite impacts. This hypothesis may also give a basis for the abiotic petroleum formation theory.
4 Conclusions
Micro-impacts achieved by a high-energy ball mill enabled the formation of ammonia and acetic acid from iron nitride, nitrate, carbide, and water in high yields. Although the MeChem reaction due to each micro-impact between the balls results in only a tiny amount of products, frequent collisions between numerous balls during milling led to a satisfactory yield. The observed experimental results were as follows:
Nitrogen contained in the starting iron nitride was converted to ammonia with a yield of over 30% in the MeChem reaction with water at 150 G for 64 h.
The decomposition of iron nitrate to iron oxide was observed at 20 G in half an hour, even though this reaction is not possible thermodynamically. This decomposition reaction can offer a metallic acid waste processing by the simple mechanical process without any alkaline sources.
The organic carbon yields from iron carbide and nitrate were estimated to be 4% by TOC analysis and acetic acid yields to be 100 ppm by GCMS measurements after the MeChem reaction for over 30 h at 40 G. A small amount of pentanoic acid was also detected.
This study demonstrated that not only a grinding of particles but also a reaction with solvents, a formation of stable oxides, and even a formation of organic materials can occur in a milling process. The MeChem reaction with micro-impacts is proposed as a new route to precursors: extraterrestrial impacts generate gigantic tremors, resulting in micro-impacts between the rocks and sand in terrestrial crusts. Large amounts of precursors are thus formed in the crusts, which act as MeChem cradles. Considering the complex mechanism of the MeChem reaction, many kinds of precursors including amino acids may be produced through various combinations of inorganic reactants and processing conditions. Therefore, the MeChem reaction utilizing a high-energy ball mill will present the formation and evolution of inorganic and organic materials on early Earth.
Acknowledgements
The authors wish to thank Mr. K. Itakura (Kurimoto) and Mr. Y. Ochiai (Sankei High-Precision) for the cooperation in the High G milling experiments, Mr. T. Kameda (ITES) for his elaborated work with the GCMS and IC measurements, and Mr. H. Suzuki (Hitachi High-Tech Science Corporation) for the amino acid analysis.
Conflict of interest statement: The authors declare to have no conflicts of interest regarding this article.
References
[1] Nakazawa H, Sekine T, Kakegawa T, Nakazawa S. Earth Planet. Sci. Lett. 2005, 235, 356–360.10.1016/j.epsl.2005.03.024Search in Google Scholar
[2] Brandes JA, Boctor NZ, Cody GD, Cooper BA, Hazen RM, Yoder Jr HS. Nature 1998, 395, 365–367.10.1038/26450Search in Google Scholar
[3] Kasting JF, Howard MT. Phil. Trans. R. Soc. Lond. B 2006, 361, 1733–1742.10.1098/rstb.2006.1902Search in Google Scholar
[4] Sekine T. Eur. J. Solid State Inorg. Chem. 1997, 34, 823–833.Search in Google Scholar
[5] Furukawa Y, Sekine T, Oba M, Kakegawa T, Nakazawa H. Nat. Geosci. 2009, 2, 62–66.10.1038/ngeo383Search in Google Scholar
[6] Dachille F, Roy R. Nature 1960, 186, 34, 71.10.1038/186034a0Search in Google Scholar
[7] Nogi K, Naito M, Kondo A, Nakahira A, Niihara K, Yokoyama T. Funtai oyobi Funmatsu Yakin 1996, 43, 396–401.10.2497/jjspm.43.396Search in Google Scholar
[8] Kugimiya, K. In Ceramic Innovations in the 20th Century, Wachtman Jr, JB, Ed., American Ceramic Society: Westerville, 1999, p. 131.Search in Google Scholar
[9] Šepelák V, Düvel A, Wilkening M, Becker KD, Heitjans P. Chem. Soc. Rev. 2013, 42, 7507–7520.10.1039/c2cs35462dSearch in Google Scholar
[10] Uno M, Nishimoto S, Kameshima Y, Miyake M. Int. J. Hydrogen. Energy 2013, 38, 15049–15054.10.1016/j.ijhydene.2013.09.118Search in Google Scholar
[11] Chen Y, Calka A, Williams JS, Ninham BW. Mater. Sci. Eng. A 1994, 187, 51–55.10.1016/0921-5093(94)90330-1Search in Google Scholar
[12] Hashishin T, Tan Z, Yamamoto K, Qiu N, Kim J, Numako C, Naka T, Valmalette JC, Ohara S. Sci. Rep. 2014, 4, 4700.10.1038/srep04700Search in Google Scholar PubMed PubMed Central
[13] Blank JM. US patent No. 3027327, 1962.Search in Google Scholar
[14] Reed, TB. In Ferrites: Proceeding of the International Conference, Hoshino, Y, Iida, S, Sugimoto, M, Eds., University of Tokyo Press: Tokyo, 1970, 289–295.Search in Google Scholar
[15] Hirota E, Kugimiya K. Nat. Tech. Rep. 1976, 6, 753–773.Search in Google Scholar
[16] Summers DP, Chang S. Nature 1993, 365, 630–633.10.1038/365630a0Search in Google Scholar PubMed
[17] Hatakeyama K, Katayama I, Hirauchi K, Michibayashi K. Sci. Rep. 2017, 7, 13870.10.1038/s41598-017-14309-9Search in Google Scholar PubMed PubMed Central
©2019 Walter de Gruyter GmbH, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 Public License.
Articles in the same Issue
- Regular Articles
- Studies on the preparation and properties of biodegradable polyester from soybean oil
- Flow-mode biodiesel production from palm oil using a pressurized microwave reactor
- Reduction of free fatty acids in waste oil for biodiesel production by glycerolysis: investigation and optimization of process parameters
- Saccharin: a cheap and mild acidic agent for the synthesis of azo dyes via telescoped dediazotization
- Optimization of lipase-catalyzed synthesis of polyethylene glycol stearate in a solvent-free system
- Green synthesis of iron oxide nanoparticles using Platanus orientalis leaf extract for antifungal activity
- Ultrasound assisted chemical activation of peanut husk for copper removal
- Room temperature silanization of Fe3O4 for the preparation of phenyl functionalized magnetic adsorbent for dispersive solid phase extraction for the extraction of phthalates in water
- Evaluation of the saponin green extraction from Ziziphus spina-christi leaves using hydrothermal, microwave and Bain-Marie water bath heating methods
- Oxidation of dibenzothiophene using the heterogeneous catalyst of tungsten-based carbon nanotubes
- Calcined sodium silicate as an efficient and benign heterogeneous catalyst for the transesterification of natural lecithin to L-α-glycerophosphocholine
- Synergistic effect between CO2 and H2O2 on ethylbenzene oxidation catalyzed by carbon supported heteropolyanion catalysts
- Hydrocyanation of 2-arylmethyleneindan-1,3-diones using potassium hexacyanoferrate(II) as a nontoxic cyanating agent
- Green synthesis of hydratropic aldehyde from α-methylstyrene catalyzed by Al2O3-supported metal phthalocyanines
- Environmentally benign chemical recycling of polycarbonate wastes: comparison of micro- and nano-TiO2 solid support efficiencies
- Medicago polymorpha-mediated antibacterial silver nanoparticles in the reduction of methyl orange
- Production of value-added chemicals from esterification of waste glycerol over MCM-41 supported catalysts
- Green synthesis of zerovalent copper nanoparticles for efficient reduction of toxic azo dyes congo red and methyl orange
- Optimization of the biological synthesis of silver nanoparticles using Penicillium oxalicum GRS-1 and their antimicrobial effects against common food-borne pathogens
- Optimization of submerged fermentation conditions to overproduce bioethanol using two industrial and traditional Saccharomyces cerevisiae strains
- Extraction of In3+ and Fe3+ from sulfate solutions by using a 3D-printed “Y”-shaped microreactor
- Foliar-mediated Ag:ZnO nanophotocatalysts: green synthesis, characterization, pollutants degradation, and in vitro biocidal activity
- Green cyclic acetals production by glycerol etherification reaction with benzaldehyde using cationic acidic resin
- Biosynthesis, characterization and antimicrobial activities assessment of fabricated selenium nanoparticles using Pelargonium zonale leaf extract
- Synthesis of high surface area magnesia by using walnut shell as a template
- Controllable biosynthesis of silver nanoparticles using actinobacterial strains
- Green vegetation: a promising source of color dyes
- Mechano-chemical synthesis of ammonia and acetic acid from inorganic materials in water
- Green synthesis and structural characterization of novel N1-substituted 3,4-dihydropyrimidin-2(1H)-ones
- Biodiesel production from cotton oil using heterogeneous CaO catalysts from eggshells prepared at different calcination temperatures
- Regeneration of spent mercury catalyst for the treatment of dye wastewater by the microwave and ultrasonic spray-assisted method
- Green synthesis of the innovative super paramagnetic nanoparticles from the leaves extract of Fraxinus chinensis Roxb and their application for the decolourisation of toxic dyes
- Biogenic ZnO nanoparticles: a study of blueshift of optical band gap and photocatalytic degradation of reactive yellow 186 dye under direct sunlight
- Leached compounds from the extracts of pomegranate peel, green coconut shell, and karuvelam wood for the removal of hexavalent chromium
- Enhancement of molecular weight reduction of natural rubber in triphasic CO2/toluene/H2O systems with hydrogen peroxide for preparation of biobased polyurethanes
- An efficient green synthesis of novel 1H-imidazo[1,2-a]imidazole-3-amine and imidazo[2,1-c][1,2,4]triazole-5-amine derivatives via Strecker reaction under controlled microwave heating
- Evaluation of three different green fabrication methods for the synthesis of crystalline ZnO nanoparticles using Pelargonium zonale leaf extract
- A highly efficient and multifunctional biomass supporting Ag, Ni, and Cu nanoparticles through wetness impregnation for environmental remediation
- Simple one-pot green method for large-scale production of mesalamine, an anti-inflammatory agent
- Relationships between step and cumulative PMI and E-factors: implications on estimating material efficiency with respect to charting synthesis optimization strategies
- A comparative sorption study of Cr3+ and Cr6+ using mango peels: kinetic, equilibrium and thermodynamic
- Effects of acid hydrolysis waste liquid recycle on preparation of microcrystalline cellulose
- Use of deep eutectic solvents as catalyst: A mini-review
- Microwave-assisted synthesis of pyrrolidinone derivatives using 1,1’-butylenebis(3-sulfo-3H-imidazol-1-ium) chloride in ethylene glycol
- Green and eco-friendly synthesis of Co3O4 and Ag-Co3O4: Characterization and photo-catalytic activity
- Adsorption optimized of the coal-based material and application for cyanide wastewater treatment
- Aloe vera leaf extract mediated green synthesis of selenium nanoparticles and assessment of their In vitro antimicrobial activity against spoilage fungi and pathogenic bacteria strains
- Waste phenolic resin derived activated carbon by microwave-assisted KOH activation and application to dye wastewater treatment
- Direct ethanol production from cellulose by consortium of Trichoderma reesei and Candida molischiana
- Agricultural waste biomass-assisted nanostructures: Synthesis and application
- Biodiesel production from rubber seed oil using calcium oxide derived from eggshell as catalyst – optimization and modeling studies
- Study of fabrication of fully aqueous solution processed SnS quantum dot-sensitized solar cell
- Assessment of aqueous extract of Gypsophila aretioides for inhibitory effects on calcium carbonate formation
- An environmentally friendly acylation reaction of 2-methylnaphthalene in solvent-free condition in a micro-channel reactor
- Aegle marmelos phytochemical stabilized synthesis and characterization of ZnO nanoparticles and their role against agriculture and food pathogen
- A reactive coupling process for co-production of solketal and biodiesel
- Optimization of the asymmetric synthesis of (S)-1-phenylethanol using Ispir bean as whole-cell biocatalyst
- Synthesis of pyrazolopyridine and pyrazoloquinoline derivatives by one-pot, three-component reactions of arylglyoxals, 3-methyl-1-aryl-1H-pyrazol-5-amines and cyclic 1,3-dicarbonyl compounds in the presence of tetrapropylammonium bromide
- Preconcentration of morphine in urine sample using a green and solvent-free microextraction method
- Extraction of glycyrrhizic acid by aqueous two-phase system formed by PEG and two environmentally friendly organic acid salts - sodium citrate and sodium tartrate
- Green synthesis of copper oxide nanoparticles using Juglans regia leaf extract and assessment of their physico-chemical and biological properties
- Deep eutectic solvents (DESs) as powerful and recyclable catalysts and solvents for the synthesis of 3,4-dihydropyrimidin-2(1H)-ones/thiones
- Biosynthesis, characterization and anti-microbial activity of silver nanoparticle based gel hand wash
- Efficient and selective microwave-assisted O-methylation of phenolic compounds using tetramethylammonium hydroxide (TMAH)
- Anticoagulant, thrombolytic and antibacterial activities of Euphorbia acruensis latex-mediated bioengineered silver nanoparticles
- Volcanic ash as reusable catalyst in the green synthesis of 3H-1,5-benzodiazepines
- Green synthesis, anionic polymerization of 1,4-bis(methacryloyl)piperazine using Algerian clay as catalyst
- Selenium supplementation during fermentation with sugar beet molasses and Saccharomyces cerevisiae to increase bioethanol production
- Biosynthetic potential assessment of four food pathogenic bacteria in hydrothermally silver nanoparticles fabrication
- Investigating the effectiveness of classical and eco-friendly approaches for synthesis of dialdehydes from organic dihalides
- Pyrolysis of palm oil using zeolite catalyst and characterization of the boil-oil
- Azadirachta indica leaves extract assisted green synthesis of Ag-TiO2 for degradation of Methylene blue and Rhodamine B dyes in aqueous medium
- Synthesis of vitamin E succinate catalyzed by nano-SiO2 immobilized DMAP derivative in mixed solvent system
- Extraction of phytosterols from melon (Cucumis melo) seeds by supercritical CO2 as a clean technology
- Production of uronic acids by hydrothermolysis of pectin as a model substance for plant biomass waste
- Biofabrication of highly pure copper oxide nanoparticles using wheat seed extract and their catalytic activity: A mechanistic approach
- Intelligent modeling and optimization of emulsion aggregation method for producing green printing ink
- Improved removal of methylene blue on modified hierarchical zeolite Y: Achieved by a “destructive-constructive” method
- Two different facile and efficient approaches for the synthesis of various N-arylacetamides via N-acetylation of arylamines and straightforward one-pot reductive acetylation of nitroarenes promoted by recyclable CuFe2O4 nanoparticles in water
- Optimization of acid catalyzed esterification and mixed metal oxide catalyzed transesterification for biodiesel production from Moringa oleifera oil
- Kinetics and the fluidity of the stearic acid esters with different carbon backbones
- Aiming for a standardized protocol for preparing a process green synthesis report and for ranking multiple synthesis plans to a common target product
- Microstructure and luminescence of VO2 (B) nanoparticle synthesis by hydrothermal method
- Optimization of uranium removal from uranium plant wastewater by response surface methodology (RSM)
- Microwave drying of nickel-containing residue: dielectric properties, kinetics, and energy aspects
- Simple and convenient two step synthesis of 5-bromo-2,3-dimethoxy-6-methyl-1,4-benzoquinone
- Biodiesel production from waste cooking oil
- The effect of activation temperature on structure and properties of blue coke-based activated carbon by CO2 activation
- Optimization of reaction parameters for the green synthesis of zero valent iron nanoparticles using pine tree needles
- Microwave-assisted protocol for squalene isolation and conversion from oil-deodoriser distillates
- Denitrification performance of rare earth tailings-based catalysts
- Facile synthesis of silver nanoparticles using Averrhoa bilimbi L and Plum extracts and investigation on the synergistic bioactivity using in vitro models
- Green production of AgNPs and their phytostimulatory impact
- Photocatalytic activity of Ag/Ni bi-metallic nanoparticles on textile dye removal
- Topical Issue: Green Process Engineering / Guest Editors: Martine Poux, Patrick Cognet
- Modelling and optimisation of oxidative desulphurisation of tyre-derived oil via central composite design approach
- CO2 sequestration by carbonation of olivine: a new process for optimal separation of the solids produced
- Organic carbonates synthesis improved by pervaporation for CO2 utilisation
- Production of starch nanoparticles through solvent-antisolvent precipitation in a spinning disc reactor
- A kinetic study of Zn halide/TBAB-catalysed fixation of CO2 with styrene oxide in propylene carbonate
- Topical on Green Process Engineering
Articles in the same Issue
- Regular Articles
- Studies on the preparation and properties of biodegradable polyester from soybean oil
- Flow-mode biodiesel production from palm oil using a pressurized microwave reactor
- Reduction of free fatty acids in waste oil for biodiesel production by glycerolysis: investigation and optimization of process parameters
- Saccharin: a cheap and mild acidic agent for the synthesis of azo dyes via telescoped dediazotization
- Optimization of lipase-catalyzed synthesis of polyethylene glycol stearate in a solvent-free system
- Green synthesis of iron oxide nanoparticles using Platanus orientalis leaf extract for antifungal activity
- Ultrasound assisted chemical activation of peanut husk for copper removal
- Room temperature silanization of Fe3O4 for the preparation of phenyl functionalized magnetic adsorbent for dispersive solid phase extraction for the extraction of phthalates in water
- Evaluation of the saponin green extraction from Ziziphus spina-christi leaves using hydrothermal, microwave and Bain-Marie water bath heating methods
- Oxidation of dibenzothiophene using the heterogeneous catalyst of tungsten-based carbon nanotubes
- Calcined sodium silicate as an efficient and benign heterogeneous catalyst for the transesterification of natural lecithin to L-α-glycerophosphocholine
- Synergistic effect between CO2 and H2O2 on ethylbenzene oxidation catalyzed by carbon supported heteropolyanion catalysts
- Hydrocyanation of 2-arylmethyleneindan-1,3-diones using potassium hexacyanoferrate(II) as a nontoxic cyanating agent
- Green synthesis of hydratropic aldehyde from α-methylstyrene catalyzed by Al2O3-supported metal phthalocyanines
- Environmentally benign chemical recycling of polycarbonate wastes: comparison of micro- and nano-TiO2 solid support efficiencies
- Medicago polymorpha-mediated antibacterial silver nanoparticles in the reduction of methyl orange
- Production of value-added chemicals from esterification of waste glycerol over MCM-41 supported catalysts
- Green synthesis of zerovalent copper nanoparticles for efficient reduction of toxic azo dyes congo red and methyl orange
- Optimization of the biological synthesis of silver nanoparticles using Penicillium oxalicum GRS-1 and their antimicrobial effects against common food-borne pathogens
- Optimization of submerged fermentation conditions to overproduce bioethanol using two industrial and traditional Saccharomyces cerevisiae strains
- Extraction of In3+ and Fe3+ from sulfate solutions by using a 3D-printed “Y”-shaped microreactor
- Foliar-mediated Ag:ZnO nanophotocatalysts: green synthesis, characterization, pollutants degradation, and in vitro biocidal activity
- Green cyclic acetals production by glycerol etherification reaction with benzaldehyde using cationic acidic resin
- Biosynthesis, characterization and antimicrobial activities assessment of fabricated selenium nanoparticles using Pelargonium zonale leaf extract
- Synthesis of high surface area magnesia by using walnut shell as a template
- Controllable biosynthesis of silver nanoparticles using actinobacterial strains
- Green vegetation: a promising source of color dyes
- Mechano-chemical synthesis of ammonia and acetic acid from inorganic materials in water
- Green synthesis and structural characterization of novel N1-substituted 3,4-dihydropyrimidin-2(1H)-ones
- Biodiesel production from cotton oil using heterogeneous CaO catalysts from eggshells prepared at different calcination temperatures
- Regeneration of spent mercury catalyst for the treatment of dye wastewater by the microwave and ultrasonic spray-assisted method
- Green synthesis of the innovative super paramagnetic nanoparticles from the leaves extract of Fraxinus chinensis Roxb and their application for the decolourisation of toxic dyes
- Biogenic ZnO nanoparticles: a study of blueshift of optical band gap and photocatalytic degradation of reactive yellow 186 dye under direct sunlight
- Leached compounds from the extracts of pomegranate peel, green coconut shell, and karuvelam wood for the removal of hexavalent chromium
- Enhancement of molecular weight reduction of natural rubber in triphasic CO2/toluene/H2O systems with hydrogen peroxide for preparation of biobased polyurethanes
- An efficient green synthesis of novel 1H-imidazo[1,2-a]imidazole-3-amine and imidazo[2,1-c][1,2,4]triazole-5-amine derivatives via Strecker reaction under controlled microwave heating
- Evaluation of three different green fabrication methods for the synthesis of crystalline ZnO nanoparticles using Pelargonium zonale leaf extract
- A highly efficient and multifunctional biomass supporting Ag, Ni, and Cu nanoparticles through wetness impregnation for environmental remediation
- Simple one-pot green method for large-scale production of mesalamine, an anti-inflammatory agent
- Relationships between step and cumulative PMI and E-factors: implications on estimating material efficiency with respect to charting synthesis optimization strategies
- A comparative sorption study of Cr3+ and Cr6+ using mango peels: kinetic, equilibrium and thermodynamic
- Effects of acid hydrolysis waste liquid recycle on preparation of microcrystalline cellulose
- Use of deep eutectic solvents as catalyst: A mini-review
- Microwave-assisted synthesis of pyrrolidinone derivatives using 1,1’-butylenebis(3-sulfo-3H-imidazol-1-ium) chloride in ethylene glycol
- Green and eco-friendly synthesis of Co3O4 and Ag-Co3O4: Characterization and photo-catalytic activity
- Adsorption optimized of the coal-based material and application for cyanide wastewater treatment
- Aloe vera leaf extract mediated green synthesis of selenium nanoparticles and assessment of their In vitro antimicrobial activity against spoilage fungi and pathogenic bacteria strains
- Waste phenolic resin derived activated carbon by microwave-assisted KOH activation and application to dye wastewater treatment
- Direct ethanol production from cellulose by consortium of Trichoderma reesei and Candida molischiana
- Agricultural waste biomass-assisted nanostructures: Synthesis and application
- Biodiesel production from rubber seed oil using calcium oxide derived from eggshell as catalyst – optimization and modeling studies
- Study of fabrication of fully aqueous solution processed SnS quantum dot-sensitized solar cell
- Assessment of aqueous extract of Gypsophila aretioides for inhibitory effects on calcium carbonate formation
- An environmentally friendly acylation reaction of 2-methylnaphthalene in solvent-free condition in a micro-channel reactor
- Aegle marmelos phytochemical stabilized synthesis and characterization of ZnO nanoparticles and their role against agriculture and food pathogen
- A reactive coupling process for co-production of solketal and biodiesel
- Optimization of the asymmetric synthesis of (S)-1-phenylethanol using Ispir bean as whole-cell biocatalyst
- Synthesis of pyrazolopyridine and pyrazoloquinoline derivatives by one-pot, three-component reactions of arylglyoxals, 3-methyl-1-aryl-1H-pyrazol-5-amines and cyclic 1,3-dicarbonyl compounds in the presence of tetrapropylammonium bromide
- Preconcentration of morphine in urine sample using a green and solvent-free microextraction method
- Extraction of glycyrrhizic acid by aqueous two-phase system formed by PEG and two environmentally friendly organic acid salts - sodium citrate and sodium tartrate
- Green synthesis of copper oxide nanoparticles using Juglans regia leaf extract and assessment of their physico-chemical and biological properties
- Deep eutectic solvents (DESs) as powerful and recyclable catalysts and solvents for the synthesis of 3,4-dihydropyrimidin-2(1H)-ones/thiones
- Biosynthesis, characterization and anti-microbial activity of silver nanoparticle based gel hand wash
- Efficient and selective microwave-assisted O-methylation of phenolic compounds using tetramethylammonium hydroxide (TMAH)
- Anticoagulant, thrombolytic and antibacterial activities of Euphorbia acruensis latex-mediated bioengineered silver nanoparticles
- Volcanic ash as reusable catalyst in the green synthesis of 3H-1,5-benzodiazepines
- Green synthesis, anionic polymerization of 1,4-bis(methacryloyl)piperazine using Algerian clay as catalyst
- Selenium supplementation during fermentation with sugar beet molasses and Saccharomyces cerevisiae to increase bioethanol production
- Biosynthetic potential assessment of four food pathogenic bacteria in hydrothermally silver nanoparticles fabrication
- Investigating the effectiveness of classical and eco-friendly approaches for synthesis of dialdehydes from organic dihalides
- Pyrolysis of palm oil using zeolite catalyst and characterization of the boil-oil
- Azadirachta indica leaves extract assisted green synthesis of Ag-TiO2 for degradation of Methylene blue and Rhodamine B dyes in aqueous medium
- Synthesis of vitamin E succinate catalyzed by nano-SiO2 immobilized DMAP derivative in mixed solvent system
- Extraction of phytosterols from melon (Cucumis melo) seeds by supercritical CO2 as a clean technology
- Production of uronic acids by hydrothermolysis of pectin as a model substance for plant biomass waste
- Biofabrication of highly pure copper oxide nanoparticles using wheat seed extract and their catalytic activity: A mechanistic approach
- Intelligent modeling and optimization of emulsion aggregation method for producing green printing ink
- Improved removal of methylene blue on modified hierarchical zeolite Y: Achieved by a “destructive-constructive” method
- Two different facile and efficient approaches for the synthesis of various N-arylacetamides via N-acetylation of arylamines and straightforward one-pot reductive acetylation of nitroarenes promoted by recyclable CuFe2O4 nanoparticles in water
- Optimization of acid catalyzed esterification and mixed metal oxide catalyzed transesterification for biodiesel production from Moringa oleifera oil
- Kinetics and the fluidity of the stearic acid esters with different carbon backbones
- Aiming for a standardized protocol for preparing a process green synthesis report and for ranking multiple synthesis plans to a common target product
- Microstructure and luminescence of VO2 (B) nanoparticle synthesis by hydrothermal method
- Optimization of uranium removal from uranium plant wastewater by response surface methodology (RSM)
- Microwave drying of nickel-containing residue: dielectric properties, kinetics, and energy aspects
- Simple and convenient two step synthesis of 5-bromo-2,3-dimethoxy-6-methyl-1,4-benzoquinone
- Biodiesel production from waste cooking oil
- The effect of activation temperature on structure and properties of blue coke-based activated carbon by CO2 activation
- Optimization of reaction parameters for the green synthesis of zero valent iron nanoparticles using pine tree needles
- Microwave-assisted protocol for squalene isolation and conversion from oil-deodoriser distillates
- Denitrification performance of rare earth tailings-based catalysts
- Facile synthesis of silver nanoparticles using Averrhoa bilimbi L and Plum extracts and investigation on the synergistic bioactivity using in vitro models
- Green production of AgNPs and their phytostimulatory impact
- Photocatalytic activity of Ag/Ni bi-metallic nanoparticles on textile dye removal
- Topical Issue: Green Process Engineering / Guest Editors: Martine Poux, Patrick Cognet
- Modelling and optimisation of oxidative desulphurisation of tyre-derived oil via central composite design approach
- CO2 sequestration by carbonation of olivine: a new process for optimal separation of the solids produced
- Organic carbonates synthesis improved by pervaporation for CO2 utilisation
- Production of starch nanoparticles through solvent-antisolvent precipitation in a spinning disc reactor
- A kinetic study of Zn halide/TBAB-catalysed fixation of CO2 with styrene oxide in propylene carbonate
- Topical on Green Process Engineering


