Abstract
In the present study, high surface area amorphous magnesia was synthesized using walnut shell as a template. This green, simple and useful synthetic protocol was based on the precipitation of magnesium nitrate on biomass in an aqueous phase, followed by calcination. Materials were characterized using X-ray diffraction, scanning electron microscopy (SEM) and N2 adsorption/desorption porosimetry, and the results exhibited high surface area for magnesium oxide. Furthermore, the pore size and surface area of these mesoporous materials can be adjusted by varying the biomass/magnesium nitrate ratio. In addition, magnesium oxide was studied as the support of palladium nanoparticles for the aerobic oxidation of alcohols. We have found out that the resulting Pd-loaded material acts as an effective catalytic system for the aerobic oxidation of benzylic and aliphatic alcohols. The catalyst can be recovered and reused three times without loss of activity. Also, to test the catalytic activity of magnesium oxides as a solid catalyst, we selected Meerwein-Ponndorf-Verley reduction of cyclohexanone with 2-propanol over different magnesium oxides.
1 Introduction
Material chemists are interested in magnesium oxide or magnesia (MgO) because of its outstanding properties, such as lowest solubility among the alkaline earth oxides, easy obtainability and cheapness, as well as its textural properties, such as high surface area. Nowadays, magnesia nanomaterials are widely employed in various areas, such as paint, adsorption, superconductivity, catalysis and toxic waste elimination [1], [2], [3].
Several preparative procedures for the synthesis of high surface area magnesia have been reported. While one of the simplest methods to prepare nanoporous MgO with relatively low surface area (97–161 m2g−1) is through hydrothermal treatment of commercial bulk MgO crystals in water [4] or magnesium salts in mixed solvents of ethylenediamine and water [5] followed by calcination, some chemists have synthesized magnesium oxide with higher surface area (72–361.5 m2g−1) using co-precipitation method by magnesium nitrate and ammonia or urea [6], [7], [8]. Another strategy focuses on the synthesis of magnesium bicarbonate Mg(HCO3)2 by bubbling CO2 into aqueous suspension of commercial magnesium oxide. By calcination of magnesium carbonate prepared from Mg(HCO3)2, porous MgO was synthesized [9], [10]. Magnesium oxide nanoparticles can be synthesized by hydrolysis of magnesium alkoxide Mg(OCH3)2 in a toluene-CH3OH solvent [11], [12]. Calcination of the synthesized gel resulted in the formation of magnesia with surface area of 201–503 m2g−1. Evaporation and oxidation of Mg pieces at 913 K and in high vacuum in quartz glass tube resulted in MgO nanocubes with particle size distribution in the range of 10–1000 nm and surface area up to 300 m2g−1 [13].
In separate studies, Li et al. and Purwajanti et al. described mesoporous magnesia preparation using hard-templating method by mesoporous carbon prepared by pyrolization of resorcinol/formaldehyde polymer as a template [14], [15]. Nanoporous MgO was also obtained by the mesoporous carbon hard-templating method, and the corresponding surface area can reach up to 175 m2g−1.
Three-dimensionally ordered macroporous magnesium oxide has been prepared by using triblock copolymer EO106PO70EO106 (Pluronic F127) as a surfactant and monodispersive polymethyl methacrylate (PMMA) microspheres as a hard template, which was removed by high-temperature calcination [16]. The magnesia samples prepared with this method possessed high surface areas (125–243 m2g−1). Nanoporous magnesium oxide with maximum BET surface area of 66.25 m2g−1 was prepared by simple hydrothermal method using urea as precipitating agent in the presence of polyethylene glycol (PEG400) as template [17]. Yang et al. reported the synthesis of flowerlike magnesia with high surface area of 343 m2g−1 by solvothermal route using ammonium hydroxide as precipitating agent and ethylene glycol as solvent in the presence of povidone with an average molecular weight of 40,000 [18].
Although various methods for the synthesis of high surface area magnesia with controllable textural properties have been reported, these methods usually use non-aqueous solvent (e.g. ethylene glycol), precipitating agents (e.g. ammonia and urea) and synthetic template (e.g. polymers and mesoporous carbon). These conditions encounter environmental and economical challenges in large-scale application. For these reasons, synthesis of mesoporous MgO in aqueous phase without any unsafe chemicals has attracted intensive attention. From a green chemistry point of view, agricultural waste biomass can also be recognized as valuable resource in the chemical synthesis and production of fuels [19]. Attention in biomass transformation to fuel and other chemical compounds has dramatically increased in the last decade within academic and industrial fields, with the support of NGOs and governments, because of their bio-based and renewable chemicals, which have many benefits, such as reduction of greenhouse gases and reduction of chemical contamination [20], [21], [22].
Herein, magnesia was prepared by impregnation and hydrothermal process in the presence of walnut shell as a template and magnesium nitrate as a magnesium source, and its catalytic activity in the aerobic oxidation of alcohols and Meerwein-Ponndorf-Verley (MPV) reduction was also studied.
2 Materials and methods
2.1 Materials
Magnesium nitrate hexahydrate (Mg(NO) · 6HO), palladium(II) chloride, toluene and other chemicals were purchased from Merck (Merck KGaA, Darmstadt, Germany) and used without further purification. The walnut shell from a local walnut tree in Urmia, Iran, was crushed using with a high-speed rotary cutting mill and screened to collect the particles with a size smaller than 0.45 mm. All chemicals were used without further purification.
2.2 Magnesia preparation
Magnesia was prepared by magnesium nitrate hexahydrate in the presence of 10-g walnut shell powder using various different magnesium/biomass ratios: 4:96, 8:92 and 12:88 in 100 ml of deionized water (Millipore, Milli-Q grade) at room temperature. After 5-h stirring, water from resulting mixture was evaporated by rotary evaporator under low pressure to give a brown solid. This solid was subsequently calcined in an electrical furnace in air (4:96, MgO-1; 8:92, MgO-2; and 12:88, MgO-3) at 500°C for 4 h (at a heating rate of 10°C/min). Resulting solids (materials A) were stirred in 50 ml of deionized water for 5 h under refluxing conditions followed by drying and calcination at 500°C for 4 h to give white powder (materials B: re-MgO-1, re-MgO-2 and re-MgO-3). For comparison, reference samples MgO-0 and re-MgO-0 were produced by the same way but without the use of biomass.
2.3 Catalyst preparation
MgO-supported palladium nanoparticle (PdNP) catalyst was synthesized by impregnation method. Na2PdCl4 was prepared from PdCl2 (0.04 g) and NaCl (0.027 g) in water (50 ml). Two grams of re-MgO-2 was mixed with the abovementioned Na2PdCl4 solution at room temperature for 12 h. Water from the resulting mixture was evaporated by rotary evaporator under low pressure. The resulting solid was subsequently calcined at 250°C in air for 2 h. Finally, this solid was refluxed in ethanol for 1 h, followed by filtration and drying in 80°C (PdNP@re-MgO-2). The loading of palladium was determined using atomic absorption spectroscopy and showed loading at 0.1 mmolg−1.
2.4 Characterization
The textural parameters were analyzed by N2 adsorption experiments using Bel Japan Belsorp-mini II, analyzer at 77 K. Before measurement, the materials were degassed at 473 K for 5 h. The total surface areas were calculated with the multipoint Brunauer-Emmett-Teller (BET) method. X-ray diffraction patterns (XRD) of the obtained materials were recorded at room temperature on Shimadzu XRD-6000 diffractometer (Kyoto, Japan) with Cu-Kα radiation. The morphology of materials was observed by TSCAN VEGA II XMU SEM Instrument (Czech Republic).
2.5 Oxidation reaction
Typically, the aerobic oxidation of alcohols was carried out in 25-ml flask equipped with a reflux condenser in undried toluene as solvent. A mixture of the alcohol (1 mmol) and PdNP@re-MgO-2 in undried toluene (5 ml) was prepared in a two-necked flask. This mixture was stirred at 80°C under the atmospheric pressure of O2 or air (balloon filled). The oxidation reaction progression was checked by silica gel TLC. At the end of the reaction, the catalyst was filtered, and gas chromatograph (Shimadzu, GC-2010) showed product yields.
2.6 MPV reduction
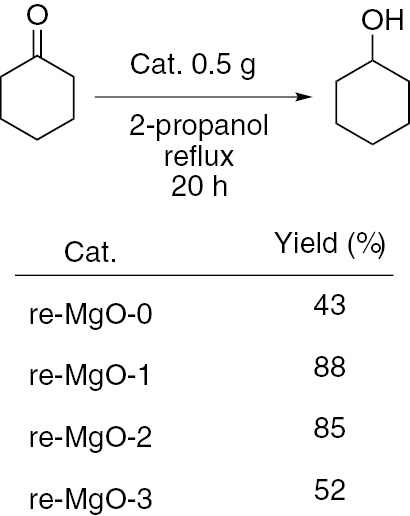
Typically, to a solution of cyclohexanone (3 mmol), in 5 ml of 2-propanol was added 0.5 g of magnesium oxide. The reaction mixture was heated to reflux temperature under magnetic stirring. Product yield was analyzed by gas chromatography (Shimadzu, GC-2010).
3 Results and discussion
In a typical experiment, walnut shell powder was dispersed into magnesium nitrate solution in deionized water. After stirring followed by evaporation of water, the solid obtained was transferred to a furnace and heated at 500°C for 5 h. XRD pattern of the prepared products (Figure 1) showed that magnesium nitrate precursor transforms MgO (JCPDS 75-0447) crystalline phase. N2 adsorption/desorption isotherms for magnesium oxide samples (materials A) studied are shown in Figure 2. Textural parameters of these materials are also indicated in Table 1, entries 1–4.
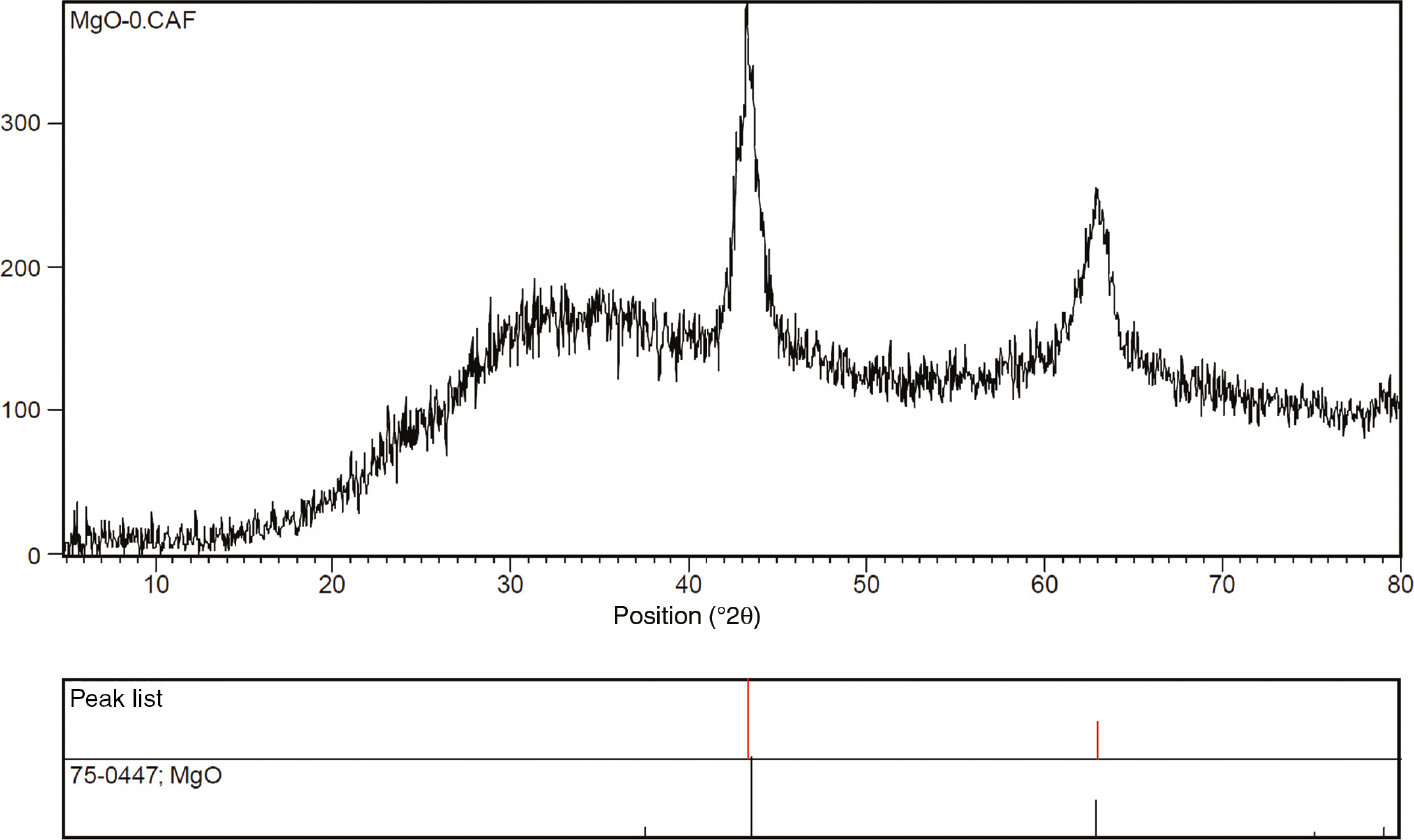
XRD patterns of MgO-2.

N2 adsorptione/desorption isotherms of materials A.
Textural properties of magnesia samples.
| Entry | Sample | SBET (m2/g) | VBJH (cm3/g) | DBJH (nm) |
|---|---|---|---|---|
| 1 | MgO-0 | 44 | 0.11 | 2.6 |
| 2 | MgO-1 | 62 | 0.27 | 7.2 |
| 3 | MgO-2 | 79 | 0.26 | 7.2 |
| 4 | MgO-3 | 63 | 0.15 | 7.2 |
| 5 | re-MgO-0 | 78 | 0.53 | 13.9 |
| 6 | re-MgO-1 | 195 | 0.70 | 10.6 |
| 7 | re-MgO-2 | 212 | 0.62 | 10.6 |
| 8 | re-MgO-3 | 95 | 0.61 | 10.6 |
DBJH, BJH average pore diameter; SBET, BET surface area; VBJH, BJH total pore volume.
As seen in Figure 2, according to the IUPAC classification [23], MgO-1 and MgO-2 exhibited type II isotherm and H3-shaped hysteresis loop in the relative pressure (p/p0) range of 0.8–1.0, which are characteristic properties of a mesoporous material with slit-shaped pores. Materials A has BET surface area and pore volume up to 79 m2/g and 0.26 cm3/g (MgO-2, Table 1, entry 3). Magnesium oxide prepared in the absence of walnut shell (MgO-0) has the lowest surface area and pore volume (44 m2/g, 0.11 cm3/g), which indicated that the presence of walnut shell is critical for the synthesis of mesoporous alumina (Table 1, entry 1). Figure 3A–D shows the BJH pore-size distribution of materials A. Most pore diameters in MgO-1 and MgO-2 samples are in the ranges of 4–14 nm and 4–12 nm, respectively (Figure 3B and C). From Figure 3A and D, one can observe that the MgO-0 and MgO-3 samples exhibited relatively broader pore-size distribution.
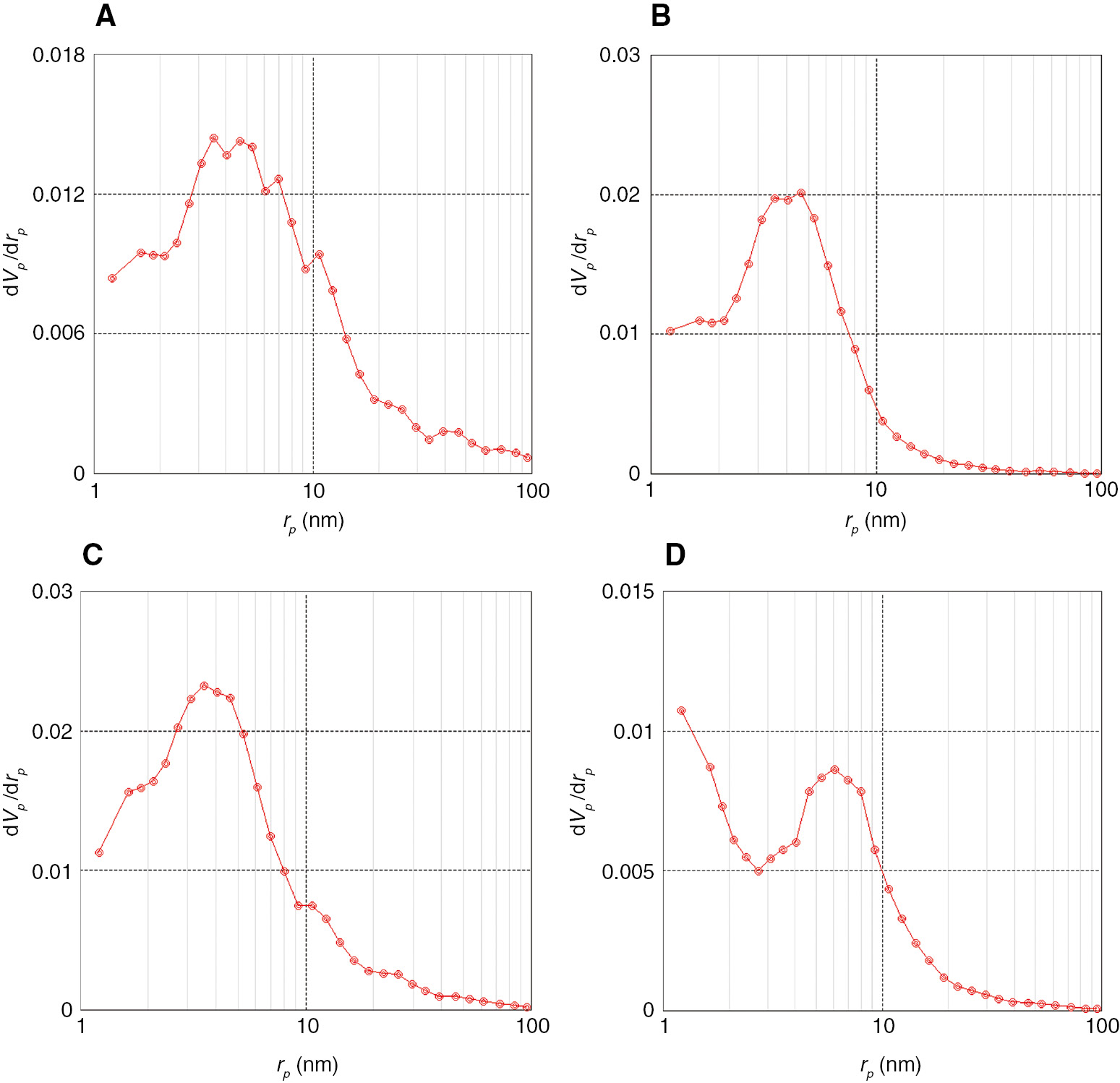
Pore-size distribution of materials A: (A) MgO-0, (B) MgO-1, (C) MgO-2, (D) MgO-3.
Figures 4 and 5 show the XRD pattern and N2 sorption isotherms, respectively, for synthesized materials B by hydrothermal treatment of materials A under reflux conditions, followed by calcination in air. Surprisingly, the specific surface area of re-MgO-1 and re-MgO-2 samples increases from the range of 62 and 79 m2/g in MgO-1 and MgO-2 to 195 and 212 m2/g, respectively (Table 1, entries 6 and 7). Also similar behavior is found in re-MgO-0 and re-MgO-3. As seen in Figure 5, re-MgO-1 and re-MgO-2 exhibited type IV isotherm and the hysteresis loop can be classified as an intermediate between H1 and H3 types. However, N2 adsorption/desorption isotherm measured for re-MgO-0 and re-MgO-3 is distinctive, being a type II isotherm, which is generally observed for macroporous materials (Figure 5). Figure 6A–D shows the BJH pore-size distribution of materials B. Most pore diameters in MgO-1 and MgO-2 samples are between 3 and 18 nm (Figure 6B and C). From Figure 6A and D, it can be detected that the MgO-0 and MgO-3 samples exhibited relatively broader pore-size distribution. SEM images of MgO-2 and re-MgO-2 reveal the nonrigid aggregates of plate-like particles forming slit-shaped pores (Figure 7).
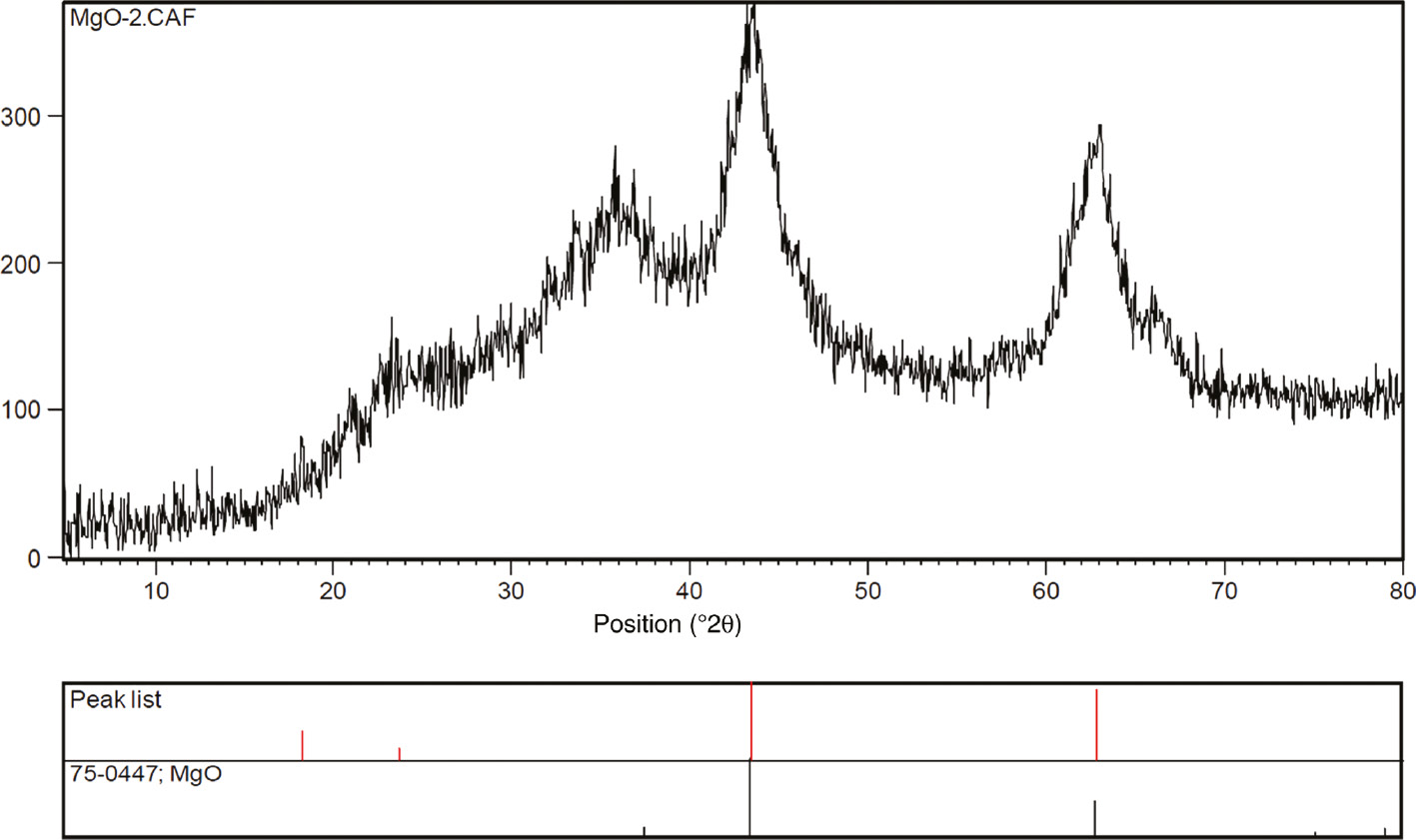
XRD patterns of re-MgO-2.
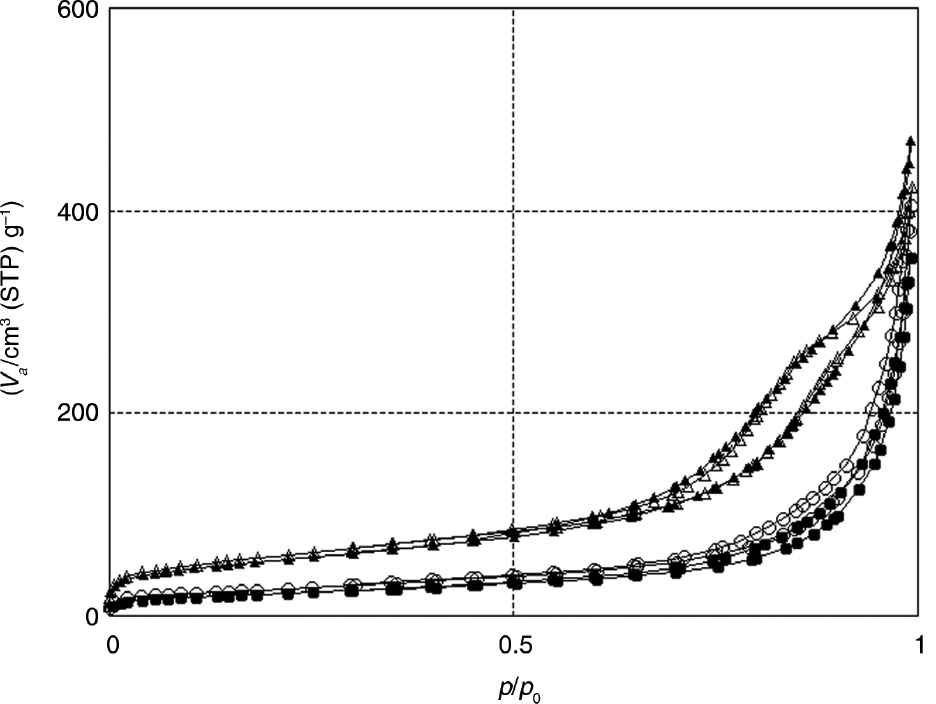
N2 adsorptione/desorption isotherms of materials B.
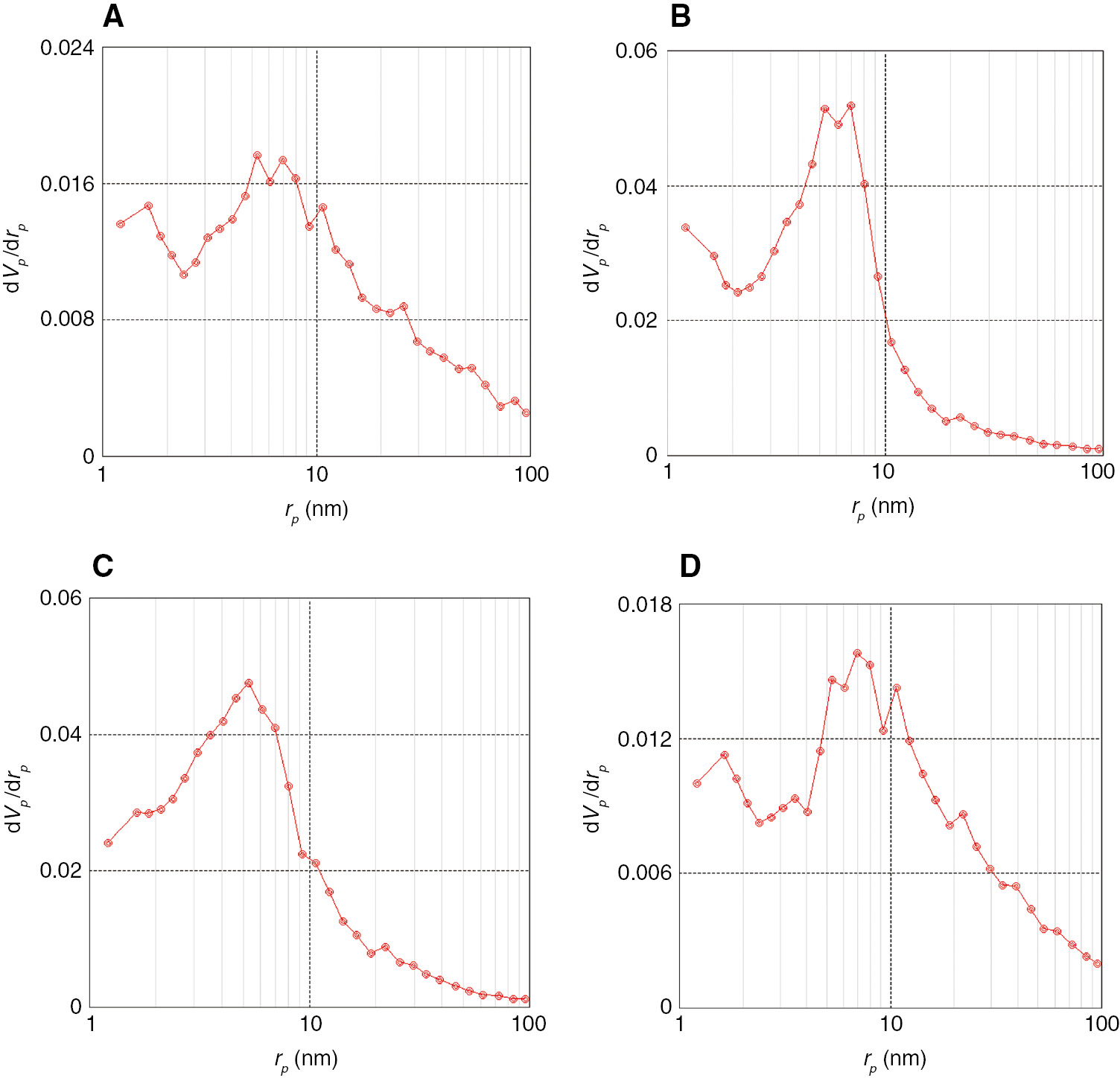
Pore size distribution of materials B: (A) re-MgO-0, (B) re-MgO-1, (C) re-MgO-2, (D) re-MgO-3.

SEM of (A) MgO-2 and (B) re-MgO-2.
A mechanism for the synthesis of the high porous magnesia can be suggested by which magnesium cations were dispersed on lignocellulosic biomass via coordination with their hydroxyl groups. Therefore, lignocellulosic component acts as a template for the magnesia precursors. After dispersion of the Mg(II) on the template and increasing the temperature, the template was removed by transformation of CO, CO2 and H2O. Simultaneously with gas release from the template, magnesia is formed due to temperature increase. Magnesia should be highly porous due to two reasons, including (1) use of the template and (2) release of gases, which increases porosity.
Magnesia with the highest surface area, re-MgO-2, prepared by the walnut shell-templating technique was tested as catalyst support in the palladium nanoparticle (PdNP@re-MgO-2) aerobic oxidation of benzylic and aliphatic alcohols. TEM image of PdNP@re-MgO-2 in Figure 8 demonstrates that most sizes of Pd nanoparticles are between 8 and 17 nm. Table 2 demonstrates the catalytic oxidation reaction results of PdNP@re-MgO-2. Thus, benzyl alcohol was oxidized in the presence of PdNP@re-MgO-2 (0.25 mol%) in toluene at 80°C for 2 h (Table 2, entry 1) without the use of exogenous baseor hydrogen acceptor additives and without additional purification and drying of solvent. Also, air has been used instead of molecular oxygen without any significant reduction in activity. After filtration of the reaction mixture, the catalyst was washed twice with CH2Cl2 and recycled. PdNP@re-MgO-2 also efficiently catalyzes the aerobic oxidation of 1-phenyl ethanol, 1-octanol and 2-octanol (Table 2, entries 5–7). This solid catalyst has been recovered and reused up to three times without significant loss of its activity (Table 2, entries 2–4).
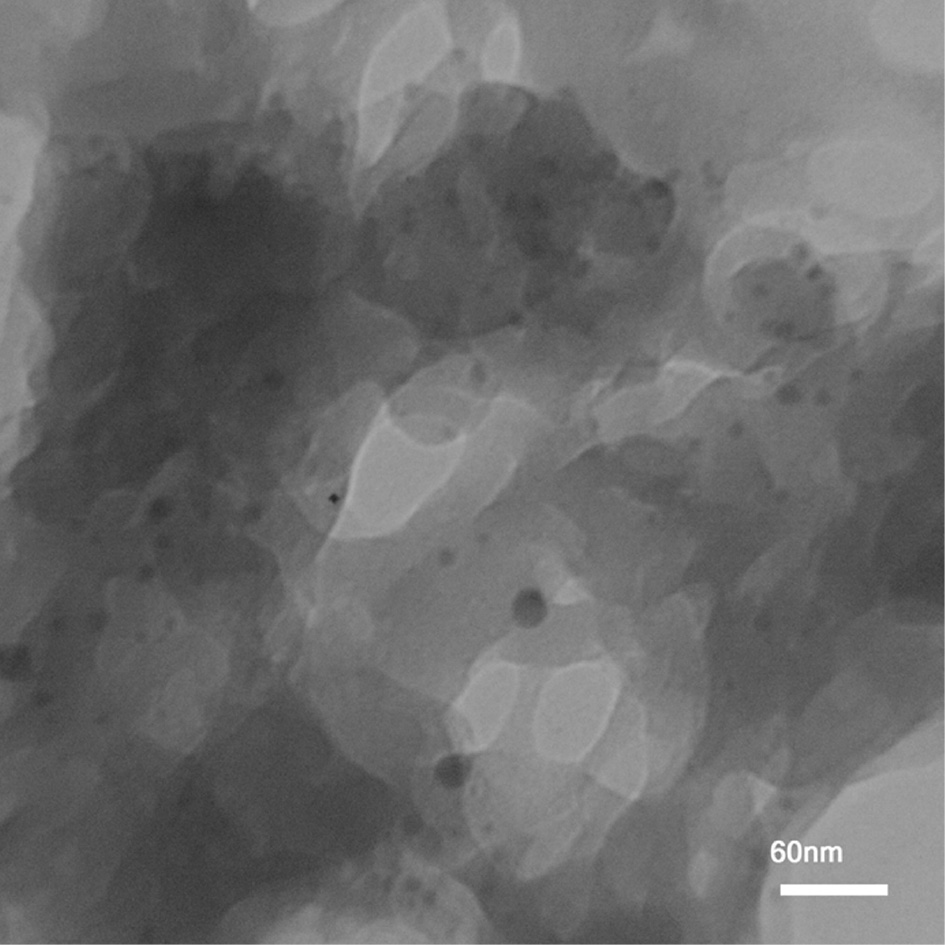
TEM of PdNP@re-MgO-2.
PdNP@re-MgO-2 catalyzed aerobic oxidation of alcohols.a
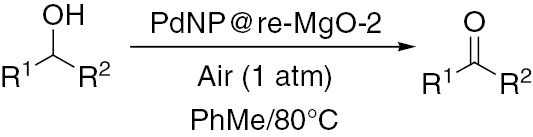
| Entry | R1 | R2 | Cat. (mol%) | Time (h) | Yield (%)b |
|---|---|---|---|---|---|
| 1 | Ph | H | 0.25 | 2 | 97 |
| 2 | Ph | H | 0.25 | 2 | 90c |
| 3 | Ph | H | 0.25 | 2 | 91d |
| 4 | Ph | H | 0.25 | 2 | 85e |
| 5 | Ph | Me | 0.25 | 3 | 88 |
| 6 | n-C6H13 | Me | 0.5 | 3 | 90 |
| 7 | n-C8H17 | H | 0.5 | 3 | 87 |
aReaction conditions: alcohol (1 mmol), PdNP@re-MgO-2, Toluene (5 ml) stirred under air at 80°C for the appropriate time.
bGC yield.
cFirst reuse.
dSecond reuse.
eThird reuse.
In order to illustrate the merit of our system for the aerobic oxidation of alcohols, we have shown the benefits of present technique by comparing our outcomes with those previously stated in the literature for the magnesia-supported palladium nanoparticle catalyzed aerobic oxidation of 1-octanol. Based on previous reports (Table 3), this is one of the efficient catalytic systems in the aerobic oxidation of alcohols at the least time possible and without the presence of any added base in good yields (Table 3, entry 3). The yield of aerobic oxidation of 1-octanol catalyzed by PdNPs supported on MgO from calcination of magnesium nitrate is very low in an expansive solvent after 20 h (Table 3, entry 1). On the other hand, PdNPs supported on nanocrystalline magnesia afforded moderate yield at this time in the presence of an exogenous base.
Comparison of magnesia supported palladium nanoparticle catalysts for the aerobic oxidation of 1-octanol.
| Entry | Magnesia (Pd mol%) | Solvent/Temp. (°C) | Time (h) | Yield (%) | Ref. |
|---|---|---|---|---|---|
| 1 | From calcination of magnesium nitrate, SA: 39 m2g−1 (1) | PhCF3/80 | 20 | 20a | [24] |
| 2 | Nanocrystalline magnesia, commercial, SA: 590 m2g−1 (0.14) | PhMe/r.t. | 20 | 68b | [25] |
| 3 | Our protocol (0.5) | PhMe/80 | 3 | 87a |
aWithout exogenous base.
bWith 1.2 equivalent K2CO3.
To test the catalytic activity of magnesium oxides as a solid catalyst, we selected MPV reduction of cyclohexanone with 2-propanol over different magnesium oxides. This catalyst was found to be effective for this MPV reaction, and recovering and reusing the catalyst happened to be easy. We have investigated the effect of surface area of MgO in this reaction (Scheme 1). It is of interest that different surface areas of MgO exhibit different catalytic activities. re-MgO-1 and re-MgO-2 with the highest surface area NPs showed maximum activity.
Effect of surface area in MgO-catalyzed MPV reaction.
4 Conclusion
In summary, a green, simple and useful synthetic protocol to produce high surface area mesoporous amorphous magnesia using walnut shell as a template in aqueous phase was presented above. Most importantly, the textural properties of materials were simply characterized in our studies using N2 adsorption/desorption porosimetry. SEM analysis shows the mesoporosity nature of the materials. On the other hand, in the aerobic oxidation of alcohols, the walnut-shell-templated mesoporous magnesia as catalyst support presented considerable activity, which suggests great potential uses in oxidative catalytic processes. The resulting Pd-loaded material acts as an effective catalytic system for the oxidation of a wide variety of alcohols in toluene. The catalyst can be recovered and reused without loss of activity.
Acknowledgment
The authors gratefully acknowledge the financial support for this work by the research council of Urmia University.
References
[1] Adachi S. Optical Constants of Crystalline and Amorphous Semiconductors, Springer: Boston, 1999.10.1007/978-1-4615-5247-5Search in Google Scholar
[2] Seeger M, Otto W, Flick W, Bickelhaupt F, Akkerman OS. Magnesium compounds. Ullmann’s Encyclopedia of Industrial Chemistry, Wiley-VCH: Weinheim, 2005, Vol. 22, p 41.Search in Google Scholar
[3] Shand MA. The Chemistry and Technology of Magnesia, John Wiley & Sons: New Jersey, 2006.10.1002/0471980579Search in Google Scholar
[4] Yu JC, Xu A, Zhang L, Song R, Wu L. J. Phys. Chem. B 2004, 108, 64–70.10.1021/jp035340wSearch in Google Scholar
[5] Ding Y, Zhang G, Wu H, Hai B, Wang L, Qian Y. Chem. Mater. 2001, 13, 435–440.10.1021/cm000607eSearch in Google Scholar
[6] Hanif A, Dasgupta S, Nanoti A. Ind. Eng. Chem. Res. 2016, 55, 8070–8078.10.1021/acs.iecr.6b00647Search in Google Scholar
[7] Cao C-Y, Qu J, Wei F, Liu H, Song W-G. ACS Appl. Mater. Interfaces 2012, 4, 4283–4287.10.1021/am300972zSearch in Google Scholar PubMed
[8] Liu Y, Li Q, Gao S, Shang JK. J. Am. Ceram. Soc. 2011, 94, 217–223.10.1111/j.1551-2916.2010.04043.xSearch in Google Scholar
[9] Zhang L, Fu W, Xiang M, Wang W, He M, Tang T. Ind. Eng. Chem. Res. 2015, 54, 5580–5588.10.1021/acs.iecr.5b00452Search in Google Scholar
[10] Tian P, Han X, Ning G, Fang H, Ye J, Gong W, Lin Y. ACS Appl. Mater. Interfaces 2013, 5, 12411–12418.10.1021/am403352ySearch in Google Scholar PubMed
[11] Vu A-T, Jiang S, Kim Y-H, Lee C-H. Ind. Eng. Chem. Res. 2014, 53, 13228–13235.10.1021/ie5018546Search in Google Scholar
[12] Klabunde KJ, Stark J, Koper O, Mohs C, Park DG, Decker S, Jiang Y, Lagadic I, Zhang D. J. Phys. Chem. 1996, 100, 12142–12153.10.1021/jp960224xSearch in Google Scholar
[13] Baumann SO, Schneider J, Sternig A, Thomele D, Stankic S, Berger T, Grönbeck H, Diwald O. Langmuir. 2015, 31, 2770–2776.10.1021/la504651vSearch in Google Scholar PubMed
[14] Li W-C, Lu A-H, Weidenthaler C, Schüth F. Chem. Mater. 2004, 16, 5676–5681.10.1021/cm048759nSearch in Google Scholar
[15] Purwajanti S, Zhang H, Huang X, Song H, Yang Y, Zhang J, Niu Y, Meka AK, Noonan O, Yu C. ACS Appl. Mater. Interfaces 2016, 8, 25306–25312.10.1021/acsami.6b08322Search in Google Scholar PubMed
[16] Li H, Zhang L, Dai H, He H. Inorg. Chem. 2009, 48, 4421–4434.10.1021/ic900132kSearch in Google Scholar PubMed
[17] Zhou J, Wang W, Cheng Y, Zhang Z. Integr. Ferroelectr. 2012, 137, 18–29.10.1080/10584587.2012.687251Search in Google Scholar
[18] Yang S, Huang P, Peng L, Cao C, Zhu Y-N, Wei F, Sun Y, Song W. J. Mater. Chem. A 2016, 4, 400–406.10.1039/C5TA08542JSearch in Google Scholar
[19] Nelson WM. ACS Symposium Series 2004, 887, 7–2110.1021/bk-2004-0887.ch002Search in Google Scholar
[20] Chheda JN, Huber GW, Dumesic JA. Angew. Chem. Int. Ed. 2007, 46, 7164–7183.10.1002/anie.200604274Search in Google Scholar PubMed
[21] Höfer R, Bigorr J. Green Chem. Lett. Rev. 2008, 1, 79–97.10.1080/17518250802342519Search in Google Scholar
[22] Zhou C-H, Xia X, Lin C-X, Tong D-S, Beltramini J. Chem. Soc. Rev. 2011, 40, 5588–5617.10.1039/c1cs15124jSearch in Google Scholar PubMed
[23] Rouquerol F, Rouquerol J, Sing K. Adsorption by Powders and Porous Solids, Academic Press: San Diego, 1999, pp 18–20.Search in Google Scholar
[24] Pillai UR, Sahle-Demessie E. Green Chem. 2004, 6, 161–165.10.1039/b316414bSearch in Google Scholar
[25] Layek K, Maheswaran H, Arundhathi R, Kantam ML, Bhargava SK. Adv. Synth. Catal. 2011, 353, 606–616.10.1002/adsc.201000591Search in Google Scholar
©2019 Walter de Gruyter GmbH, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 Public License.
Articles in the same Issue
- Regular Articles
- Studies on the preparation and properties of biodegradable polyester from soybean oil
- Flow-mode biodiesel production from palm oil using a pressurized microwave reactor
- Reduction of free fatty acids in waste oil for biodiesel production by glycerolysis: investigation and optimization of process parameters
- Saccharin: a cheap and mild acidic agent for the synthesis of azo dyes via telescoped dediazotization
- Optimization of lipase-catalyzed synthesis of polyethylene glycol stearate in a solvent-free system
- Green synthesis of iron oxide nanoparticles using Platanus orientalis leaf extract for antifungal activity
- Ultrasound assisted chemical activation of peanut husk for copper removal
- Room temperature silanization of Fe3O4 for the preparation of phenyl functionalized magnetic adsorbent for dispersive solid phase extraction for the extraction of phthalates in water
- Evaluation of the saponin green extraction from Ziziphus spina-christi leaves using hydrothermal, microwave and Bain-Marie water bath heating methods
- Oxidation of dibenzothiophene using the heterogeneous catalyst of tungsten-based carbon nanotubes
- Calcined sodium silicate as an efficient and benign heterogeneous catalyst for the transesterification of natural lecithin to L-α-glycerophosphocholine
- Synergistic effect between CO2 and H2O2 on ethylbenzene oxidation catalyzed by carbon supported heteropolyanion catalysts
- Hydrocyanation of 2-arylmethyleneindan-1,3-diones using potassium hexacyanoferrate(II) as a nontoxic cyanating agent
- Green synthesis of hydratropic aldehyde from α-methylstyrene catalyzed by Al2O3-supported metal phthalocyanines
- Environmentally benign chemical recycling of polycarbonate wastes: comparison of micro- and nano-TiO2 solid support efficiencies
- Medicago polymorpha-mediated antibacterial silver nanoparticles in the reduction of methyl orange
- Production of value-added chemicals from esterification of waste glycerol over MCM-41 supported catalysts
- Green synthesis of zerovalent copper nanoparticles for efficient reduction of toxic azo dyes congo red and methyl orange
- Optimization of the biological synthesis of silver nanoparticles using Penicillium oxalicum GRS-1 and their antimicrobial effects against common food-borne pathogens
- Optimization of submerged fermentation conditions to overproduce bioethanol using two industrial and traditional Saccharomyces cerevisiae strains
- Extraction of In3+ and Fe3+ from sulfate solutions by using a 3D-printed “Y”-shaped microreactor
- Foliar-mediated Ag:ZnO nanophotocatalysts: green synthesis, characterization, pollutants degradation, and in vitro biocidal activity
- Green cyclic acetals production by glycerol etherification reaction with benzaldehyde using cationic acidic resin
- Biosynthesis, characterization and antimicrobial activities assessment of fabricated selenium nanoparticles using Pelargonium zonale leaf extract
- Synthesis of high surface area magnesia by using walnut shell as a template
- Controllable biosynthesis of silver nanoparticles using actinobacterial strains
- Green vegetation: a promising source of color dyes
- Mechano-chemical synthesis of ammonia and acetic acid from inorganic materials in water
- Green synthesis and structural characterization of novel N1-substituted 3,4-dihydropyrimidin-2(1H)-ones
- Biodiesel production from cotton oil using heterogeneous CaO catalysts from eggshells prepared at different calcination temperatures
- Regeneration of spent mercury catalyst for the treatment of dye wastewater by the microwave and ultrasonic spray-assisted method
- Green synthesis of the innovative super paramagnetic nanoparticles from the leaves extract of Fraxinus chinensis Roxb and their application for the decolourisation of toxic dyes
- Biogenic ZnO nanoparticles: a study of blueshift of optical band gap and photocatalytic degradation of reactive yellow 186 dye under direct sunlight
- Leached compounds from the extracts of pomegranate peel, green coconut shell, and karuvelam wood for the removal of hexavalent chromium
- Enhancement of molecular weight reduction of natural rubber in triphasic CO2/toluene/H2O systems with hydrogen peroxide for preparation of biobased polyurethanes
- An efficient green synthesis of novel 1H-imidazo[1,2-a]imidazole-3-amine and imidazo[2,1-c][1,2,4]triazole-5-amine derivatives via Strecker reaction under controlled microwave heating
- Evaluation of three different green fabrication methods for the synthesis of crystalline ZnO nanoparticles using Pelargonium zonale leaf extract
- A highly efficient and multifunctional biomass supporting Ag, Ni, and Cu nanoparticles through wetness impregnation for environmental remediation
- Simple one-pot green method for large-scale production of mesalamine, an anti-inflammatory agent
- Relationships between step and cumulative PMI and E-factors: implications on estimating material efficiency with respect to charting synthesis optimization strategies
- A comparative sorption study of Cr3+ and Cr6+ using mango peels: kinetic, equilibrium and thermodynamic
- Effects of acid hydrolysis waste liquid recycle on preparation of microcrystalline cellulose
- Use of deep eutectic solvents as catalyst: A mini-review
- Microwave-assisted synthesis of pyrrolidinone derivatives using 1,1’-butylenebis(3-sulfo-3H-imidazol-1-ium) chloride in ethylene glycol
- Green and eco-friendly synthesis of Co3O4 and Ag-Co3O4: Characterization and photo-catalytic activity
- Adsorption optimized of the coal-based material and application for cyanide wastewater treatment
- Aloe vera leaf extract mediated green synthesis of selenium nanoparticles and assessment of their In vitro antimicrobial activity against spoilage fungi and pathogenic bacteria strains
- Waste phenolic resin derived activated carbon by microwave-assisted KOH activation and application to dye wastewater treatment
- Direct ethanol production from cellulose by consortium of Trichoderma reesei and Candida molischiana
- Agricultural waste biomass-assisted nanostructures: Synthesis and application
- Biodiesel production from rubber seed oil using calcium oxide derived from eggshell as catalyst – optimization and modeling studies
- Study of fabrication of fully aqueous solution processed SnS quantum dot-sensitized solar cell
- Assessment of aqueous extract of Gypsophila aretioides for inhibitory effects on calcium carbonate formation
- An environmentally friendly acylation reaction of 2-methylnaphthalene in solvent-free condition in a micro-channel reactor
- Aegle marmelos phytochemical stabilized synthesis and characterization of ZnO nanoparticles and their role against agriculture and food pathogen
- A reactive coupling process for co-production of solketal and biodiesel
- Optimization of the asymmetric synthesis of (S)-1-phenylethanol using Ispir bean as whole-cell biocatalyst
- Synthesis of pyrazolopyridine and pyrazoloquinoline derivatives by one-pot, three-component reactions of arylglyoxals, 3-methyl-1-aryl-1H-pyrazol-5-amines and cyclic 1,3-dicarbonyl compounds in the presence of tetrapropylammonium bromide
- Preconcentration of morphine in urine sample using a green and solvent-free microextraction method
- Extraction of glycyrrhizic acid by aqueous two-phase system formed by PEG and two environmentally friendly organic acid salts - sodium citrate and sodium tartrate
- Green synthesis of copper oxide nanoparticles using Juglans regia leaf extract and assessment of their physico-chemical and biological properties
- Deep eutectic solvents (DESs) as powerful and recyclable catalysts and solvents for the synthesis of 3,4-dihydropyrimidin-2(1H)-ones/thiones
- Biosynthesis, characterization and anti-microbial activity of silver nanoparticle based gel hand wash
- Efficient and selective microwave-assisted O-methylation of phenolic compounds using tetramethylammonium hydroxide (TMAH)
- Anticoagulant, thrombolytic and antibacterial activities of Euphorbia acruensis latex-mediated bioengineered silver nanoparticles
- Volcanic ash as reusable catalyst in the green synthesis of 3H-1,5-benzodiazepines
- Green synthesis, anionic polymerization of 1,4-bis(methacryloyl)piperazine using Algerian clay as catalyst
- Selenium supplementation during fermentation with sugar beet molasses and Saccharomyces cerevisiae to increase bioethanol production
- Biosynthetic potential assessment of four food pathogenic bacteria in hydrothermally silver nanoparticles fabrication
- Investigating the effectiveness of classical and eco-friendly approaches for synthesis of dialdehydes from organic dihalides
- Pyrolysis of palm oil using zeolite catalyst and characterization of the boil-oil
- Azadirachta indica leaves extract assisted green synthesis of Ag-TiO2 for degradation of Methylene blue and Rhodamine B dyes in aqueous medium
- Synthesis of vitamin E succinate catalyzed by nano-SiO2 immobilized DMAP derivative in mixed solvent system
- Extraction of phytosterols from melon (Cucumis melo) seeds by supercritical CO2 as a clean technology
- Production of uronic acids by hydrothermolysis of pectin as a model substance for plant biomass waste
- Biofabrication of highly pure copper oxide nanoparticles using wheat seed extract and their catalytic activity: A mechanistic approach
- Intelligent modeling and optimization of emulsion aggregation method for producing green printing ink
- Improved removal of methylene blue on modified hierarchical zeolite Y: Achieved by a “destructive-constructive” method
- Two different facile and efficient approaches for the synthesis of various N-arylacetamides via N-acetylation of arylamines and straightforward one-pot reductive acetylation of nitroarenes promoted by recyclable CuFe2O4 nanoparticles in water
- Optimization of acid catalyzed esterification and mixed metal oxide catalyzed transesterification for biodiesel production from Moringa oleifera oil
- Kinetics and the fluidity of the stearic acid esters with different carbon backbones
- Aiming for a standardized protocol for preparing a process green synthesis report and for ranking multiple synthesis plans to a common target product
- Microstructure and luminescence of VO2 (B) nanoparticle synthesis by hydrothermal method
- Optimization of uranium removal from uranium plant wastewater by response surface methodology (RSM)
- Microwave drying of nickel-containing residue: dielectric properties, kinetics, and energy aspects
- Simple and convenient two step synthesis of 5-bromo-2,3-dimethoxy-6-methyl-1,4-benzoquinone
- Biodiesel production from waste cooking oil
- The effect of activation temperature on structure and properties of blue coke-based activated carbon by CO2 activation
- Optimization of reaction parameters for the green synthesis of zero valent iron nanoparticles using pine tree needles
- Microwave-assisted protocol for squalene isolation and conversion from oil-deodoriser distillates
- Denitrification performance of rare earth tailings-based catalysts
- Facile synthesis of silver nanoparticles using Averrhoa bilimbi L and Plum extracts and investigation on the synergistic bioactivity using in vitro models
- Green production of AgNPs and their phytostimulatory impact
- Photocatalytic activity of Ag/Ni bi-metallic nanoparticles on textile dye removal
- Topical Issue: Green Process Engineering / Guest Editors: Martine Poux, Patrick Cognet
- Modelling and optimisation of oxidative desulphurisation of tyre-derived oil via central composite design approach
- CO2 sequestration by carbonation of olivine: a new process for optimal separation of the solids produced
- Organic carbonates synthesis improved by pervaporation for CO2 utilisation
- Production of starch nanoparticles through solvent-antisolvent precipitation in a spinning disc reactor
- A kinetic study of Zn halide/TBAB-catalysed fixation of CO2 with styrene oxide in propylene carbonate
- Topical on Green Process Engineering
Articles in the same Issue
- Regular Articles
- Studies on the preparation and properties of biodegradable polyester from soybean oil
- Flow-mode biodiesel production from palm oil using a pressurized microwave reactor
- Reduction of free fatty acids in waste oil for biodiesel production by glycerolysis: investigation and optimization of process parameters
- Saccharin: a cheap and mild acidic agent for the synthesis of azo dyes via telescoped dediazotization
- Optimization of lipase-catalyzed synthesis of polyethylene glycol stearate in a solvent-free system
- Green synthesis of iron oxide nanoparticles using Platanus orientalis leaf extract for antifungal activity
- Ultrasound assisted chemical activation of peanut husk for copper removal
- Room temperature silanization of Fe3O4 for the preparation of phenyl functionalized magnetic adsorbent for dispersive solid phase extraction for the extraction of phthalates in water
- Evaluation of the saponin green extraction from Ziziphus spina-christi leaves using hydrothermal, microwave and Bain-Marie water bath heating methods
- Oxidation of dibenzothiophene using the heterogeneous catalyst of tungsten-based carbon nanotubes
- Calcined sodium silicate as an efficient and benign heterogeneous catalyst for the transesterification of natural lecithin to L-α-glycerophosphocholine
- Synergistic effect between CO2 and H2O2 on ethylbenzene oxidation catalyzed by carbon supported heteropolyanion catalysts
- Hydrocyanation of 2-arylmethyleneindan-1,3-diones using potassium hexacyanoferrate(II) as a nontoxic cyanating agent
- Green synthesis of hydratropic aldehyde from α-methylstyrene catalyzed by Al2O3-supported metal phthalocyanines
- Environmentally benign chemical recycling of polycarbonate wastes: comparison of micro- and nano-TiO2 solid support efficiencies
- Medicago polymorpha-mediated antibacterial silver nanoparticles in the reduction of methyl orange
- Production of value-added chemicals from esterification of waste glycerol over MCM-41 supported catalysts
- Green synthesis of zerovalent copper nanoparticles for efficient reduction of toxic azo dyes congo red and methyl orange
- Optimization of the biological synthesis of silver nanoparticles using Penicillium oxalicum GRS-1 and their antimicrobial effects against common food-borne pathogens
- Optimization of submerged fermentation conditions to overproduce bioethanol using two industrial and traditional Saccharomyces cerevisiae strains
- Extraction of In3+ and Fe3+ from sulfate solutions by using a 3D-printed “Y”-shaped microreactor
- Foliar-mediated Ag:ZnO nanophotocatalysts: green synthesis, characterization, pollutants degradation, and in vitro biocidal activity
- Green cyclic acetals production by glycerol etherification reaction with benzaldehyde using cationic acidic resin
- Biosynthesis, characterization and antimicrobial activities assessment of fabricated selenium nanoparticles using Pelargonium zonale leaf extract
- Synthesis of high surface area magnesia by using walnut shell as a template
- Controllable biosynthesis of silver nanoparticles using actinobacterial strains
- Green vegetation: a promising source of color dyes
- Mechano-chemical synthesis of ammonia and acetic acid from inorganic materials in water
- Green synthesis and structural characterization of novel N1-substituted 3,4-dihydropyrimidin-2(1H)-ones
- Biodiesel production from cotton oil using heterogeneous CaO catalysts from eggshells prepared at different calcination temperatures
- Regeneration of spent mercury catalyst for the treatment of dye wastewater by the microwave and ultrasonic spray-assisted method
- Green synthesis of the innovative super paramagnetic nanoparticles from the leaves extract of Fraxinus chinensis Roxb and their application for the decolourisation of toxic dyes
- Biogenic ZnO nanoparticles: a study of blueshift of optical band gap and photocatalytic degradation of reactive yellow 186 dye under direct sunlight
- Leached compounds from the extracts of pomegranate peel, green coconut shell, and karuvelam wood for the removal of hexavalent chromium
- Enhancement of molecular weight reduction of natural rubber in triphasic CO2/toluene/H2O systems with hydrogen peroxide for preparation of biobased polyurethanes
- An efficient green synthesis of novel 1H-imidazo[1,2-a]imidazole-3-amine and imidazo[2,1-c][1,2,4]triazole-5-amine derivatives via Strecker reaction under controlled microwave heating
- Evaluation of three different green fabrication methods for the synthesis of crystalline ZnO nanoparticles using Pelargonium zonale leaf extract
- A highly efficient and multifunctional biomass supporting Ag, Ni, and Cu nanoparticles through wetness impregnation for environmental remediation
- Simple one-pot green method for large-scale production of mesalamine, an anti-inflammatory agent
- Relationships between step and cumulative PMI and E-factors: implications on estimating material efficiency with respect to charting synthesis optimization strategies
- A comparative sorption study of Cr3+ and Cr6+ using mango peels: kinetic, equilibrium and thermodynamic
- Effects of acid hydrolysis waste liquid recycle on preparation of microcrystalline cellulose
- Use of deep eutectic solvents as catalyst: A mini-review
- Microwave-assisted synthesis of pyrrolidinone derivatives using 1,1’-butylenebis(3-sulfo-3H-imidazol-1-ium) chloride in ethylene glycol
- Green and eco-friendly synthesis of Co3O4 and Ag-Co3O4: Characterization and photo-catalytic activity
- Adsorption optimized of the coal-based material and application for cyanide wastewater treatment
- Aloe vera leaf extract mediated green synthesis of selenium nanoparticles and assessment of their In vitro antimicrobial activity against spoilage fungi and pathogenic bacteria strains
- Waste phenolic resin derived activated carbon by microwave-assisted KOH activation and application to dye wastewater treatment
- Direct ethanol production from cellulose by consortium of Trichoderma reesei and Candida molischiana
- Agricultural waste biomass-assisted nanostructures: Synthesis and application
- Biodiesel production from rubber seed oil using calcium oxide derived from eggshell as catalyst – optimization and modeling studies
- Study of fabrication of fully aqueous solution processed SnS quantum dot-sensitized solar cell
- Assessment of aqueous extract of Gypsophila aretioides for inhibitory effects on calcium carbonate formation
- An environmentally friendly acylation reaction of 2-methylnaphthalene in solvent-free condition in a micro-channel reactor
- Aegle marmelos phytochemical stabilized synthesis and characterization of ZnO nanoparticles and their role against agriculture and food pathogen
- A reactive coupling process for co-production of solketal and biodiesel
- Optimization of the asymmetric synthesis of (S)-1-phenylethanol using Ispir bean as whole-cell biocatalyst
- Synthesis of pyrazolopyridine and pyrazoloquinoline derivatives by one-pot, three-component reactions of arylglyoxals, 3-methyl-1-aryl-1H-pyrazol-5-amines and cyclic 1,3-dicarbonyl compounds in the presence of tetrapropylammonium bromide
- Preconcentration of morphine in urine sample using a green and solvent-free microextraction method
- Extraction of glycyrrhizic acid by aqueous two-phase system formed by PEG and two environmentally friendly organic acid salts - sodium citrate and sodium tartrate
- Green synthesis of copper oxide nanoparticles using Juglans regia leaf extract and assessment of their physico-chemical and biological properties
- Deep eutectic solvents (DESs) as powerful and recyclable catalysts and solvents for the synthesis of 3,4-dihydropyrimidin-2(1H)-ones/thiones
- Biosynthesis, characterization and anti-microbial activity of silver nanoparticle based gel hand wash
- Efficient and selective microwave-assisted O-methylation of phenolic compounds using tetramethylammonium hydroxide (TMAH)
- Anticoagulant, thrombolytic and antibacterial activities of Euphorbia acruensis latex-mediated bioengineered silver nanoparticles
- Volcanic ash as reusable catalyst in the green synthesis of 3H-1,5-benzodiazepines
- Green synthesis, anionic polymerization of 1,4-bis(methacryloyl)piperazine using Algerian clay as catalyst
- Selenium supplementation during fermentation with sugar beet molasses and Saccharomyces cerevisiae to increase bioethanol production
- Biosynthetic potential assessment of four food pathogenic bacteria in hydrothermally silver nanoparticles fabrication
- Investigating the effectiveness of classical and eco-friendly approaches for synthesis of dialdehydes from organic dihalides
- Pyrolysis of palm oil using zeolite catalyst and characterization of the boil-oil
- Azadirachta indica leaves extract assisted green synthesis of Ag-TiO2 for degradation of Methylene blue and Rhodamine B dyes in aqueous medium
- Synthesis of vitamin E succinate catalyzed by nano-SiO2 immobilized DMAP derivative in mixed solvent system
- Extraction of phytosterols from melon (Cucumis melo) seeds by supercritical CO2 as a clean technology
- Production of uronic acids by hydrothermolysis of pectin as a model substance for plant biomass waste
- Biofabrication of highly pure copper oxide nanoparticles using wheat seed extract and their catalytic activity: A mechanistic approach
- Intelligent modeling and optimization of emulsion aggregation method for producing green printing ink
- Improved removal of methylene blue on modified hierarchical zeolite Y: Achieved by a “destructive-constructive” method
- Two different facile and efficient approaches for the synthesis of various N-arylacetamides via N-acetylation of arylamines and straightforward one-pot reductive acetylation of nitroarenes promoted by recyclable CuFe2O4 nanoparticles in water
- Optimization of acid catalyzed esterification and mixed metal oxide catalyzed transesterification for biodiesel production from Moringa oleifera oil
- Kinetics and the fluidity of the stearic acid esters with different carbon backbones
- Aiming for a standardized protocol for preparing a process green synthesis report and for ranking multiple synthesis plans to a common target product
- Microstructure and luminescence of VO2 (B) nanoparticle synthesis by hydrothermal method
- Optimization of uranium removal from uranium plant wastewater by response surface methodology (RSM)
- Microwave drying of nickel-containing residue: dielectric properties, kinetics, and energy aspects
- Simple and convenient two step synthesis of 5-bromo-2,3-dimethoxy-6-methyl-1,4-benzoquinone
- Biodiesel production from waste cooking oil
- The effect of activation temperature on structure and properties of blue coke-based activated carbon by CO2 activation
- Optimization of reaction parameters for the green synthesis of zero valent iron nanoparticles using pine tree needles
- Microwave-assisted protocol for squalene isolation and conversion from oil-deodoriser distillates
- Denitrification performance of rare earth tailings-based catalysts
- Facile synthesis of silver nanoparticles using Averrhoa bilimbi L and Plum extracts and investigation on the synergistic bioactivity using in vitro models
- Green production of AgNPs and their phytostimulatory impact
- Photocatalytic activity of Ag/Ni bi-metallic nanoparticles on textile dye removal
- Topical Issue: Green Process Engineering / Guest Editors: Martine Poux, Patrick Cognet
- Modelling and optimisation of oxidative desulphurisation of tyre-derived oil via central composite design approach
- CO2 sequestration by carbonation of olivine: a new process for optimal separation of the solids produced
- Organic carbonates synthesis improved by pervaporation for CO2 utilisation
- Production of starch nanoparticles through solvent-antisolvent precipitation in a spinning disc reactor
- A kinetic study of Zn halide/TBAB-catalysed fixation of CO2 with styrene oxide in propylene carbonate
- Topical on Green Process Engineering


