Abstract
As the lightest metal structural materials, magnesium (Mg) alloys offer extensive application potential. Gadolinium (Gd), as the primary alloying element in Mg alloys and recognized for its notable thermal neutron capture cross-section, is considered one of the most efficient neutron absorbers. Thus, the Mg–Gd alloy is highly expected to emerge as a material with remarkable neutron absorption capacity. Hence, in this study, the thermal neutron-shielding capabilities of Mg–Gd alloys were comprehensively examined by fabricating four as-cast Mg–xGd alloys with varying compositions (x = 0, 5, 10, and 15 wt%). The obtained results were further corroborated by sophisticated modeling and calculations using SuperMC. The results revealed a direct correlation between the thermal neutron absorption capacity of the Mg–Gd alloys and the increase in Gd content, with a noteworthy neutron attenuation factor of 22.33. Moreover, in an Au ion irradiation experiment conducted at 200°C, the Mg–15Gd alloy exhibited exceptional radiation resistance, with a displacement per atom (dpa) of 10. The matrix and second-phase regions were devoid of any cavity formation. Instead, a finite number of dislocation rings were observed, forming both leaf-like and granular Gd-rich nanoscale precipitates. This study underscores the versatility of Mg–Gd alloys as efficient neutron shielding materials and structural materials tailored for applications demanding radiation resistance in diverse environments.
1 Introduction
Nuclear energy has immense potential and significance as a clean and efficient energy source, with its capabilities extending beyond meeting escalating global energy demands, as it also contributes to mitigating greenhouse gas emissions and combating challenges posed by climate change [1]. The progressive development of nuclear energy has been instrumental in driving scientific and technological advancements and facilitating industrial upgrades, thereby providing critical support for economic growth and societal progress. However, with the continuous evolution of nuclear technology, the requirements for reactor materials have increased [2,3]. Specifically, concerning shielding materials, thermal neutron shielding materials are expected to exhibit characteristics such as low density, high-temperature tolerance, and resistance to aging, as well as the capability to seamlessly integrate neutron moderation and absorption [4,5].
Diverse neutron-shielding materials are currently in use [6]. Boron steel, known for its neutron-weakening function and excellent mechanical properties, is a commonly employed shielding material in radiation protection designs [7]. However, the low solubility of boron in steel poses challenges, with trace amounts (0.001–0.007%) adversely affecting the hardenability of steel. High boron content also compromises the ductility and impact resistance of boron steel, restricting its predominant use in applications such as control rods and spent fuel storage materials [8,9].
Lead boron polyethylene, while offering neutron shielding capabilities, exhibits slightly insufficient mechanical structural performance and limited resistance to high temperatures, radiation, and corrosion. Consequently, they are primarily used as single functional material [10]. Recent studies have demonstrated that epoxy resin composites incorporating B or W exhibit exceptional thermal stability and long-term radiation resistance at a low cost. As a result, these composites have extensive applications in radiation shielding; however, they are unsuitable for use as structural materials [11,12]. Additionally, the neutron absorption cross-section of B11, a transmutation product of boron after neutron absorption, sharply decreases over time, rendering materials containing boron less effective in high-dose neutron environments [13]. Concrete, characterized by its adjustable composition, cost-effectiveness, and local availability, is an attractive option for shielding against radiation across various energy segments. However, ordinary concrete has a relatively low density (2.2–2.4 g/cm3) and limited strength. To enhance the mechanical strength of concrete and its neutron and gamma-ray shielding performance, high-performance or heavy concrete is often formulated by incorporating mineral admixtures or natural/artificial functional coarse aggregates. Despite performance improvements, these formulations result in larger volumes and heavier masses (density 3.0–6.0 g/cm3) [14,15].
Metal-based materials are an alternative to address these issues. Tungsten, with its effective moderating effect on fast neutrons, achieves 93% shielding of fast neutrons when passing through a 10 cm-thick tungsten plate [16]. As such, tungsten can be used as a shield against secondary high-energy gamma rays produced by neutrons and is an excellent substitute for lead. Some tungsten-containing metal–matrix composites have shielding efficiencies several times higher than that of lead composites [17]. However, the relatively high cost of tungsten has led to the development of tungsten alloys containing Ni, Cu, Co, Mo, and Cr [18]. These alloys, known as high specific gravity alloys (with tungsten mass fractions ranging from 70 to 99%), offer advantages such as a high specific weight, high strength, strong ray absorption capacity, substantial thermal conductivity, good weldability, and processability. Other metals, including hafnium, samarium, and cadmium, have been combined to create neutron and gamma-ray shielding materials [19,20,21]. However, owing to issues such as source scarcity, high costs, significant secondary radiation yield, and toxicity concerns, these materials are seldom used in practical applications and are gradually being replaced with alternative materials.
Currently, traditional materials such as water, lead, and concrete remain prevalent in the realm of reactor radiation protection. However, these materials exhibit notable limitations and are primarily suited for stationary nuclear power reactors and accelerators where stringent requirements for the weight and volume of shielding materials are typically absent, and cost is the primary consideration. Consequently, researchers are focusing on developing new materials that address these shortcomings by offering high-temperature resistance, excellent mechanical properties, environmental friendliness, non-toxicity, and enhanced neutron absorption capabilities.
The neutron capture cross-section of Mg is widely acknowledged for its moderating properties, while magnesium (Mg) alloys demonstrate exceptional compatibility with both fuel and coolant materials. It possesses several advantages such as low induced radioactivity, high thermal conductivity, and ease of welding. Additionally, it demonstrated strong adaptability to dimensional changes resulting from stress variations caused by thermal cycling and irradiation. Consequently, the neutron capture cross-section of Mg is frequently applied in carbon dioxide gas-cooled reactors and as a shielding material for natural uranium cladding [22]. The combination of Mg alloys with specific rare earth elements can have a unique impact on the field of nuclear energy. Gadolinium (Gd) has a significant thermal neutron capture cross-section, and the transmutation products of Gd have a significantly large capture cross-section, making it an excellent neutron-absorbing material. The transmutation product of Gd is (
2 Materials and methods
2.1 Materials
Three compositions of binary as-cast Mg–Gd alloys with Gd contents of 5, 10, and 15% were designed, along with a control group of pure Mg alloy. The experimental materials consisted of a pure Mg ingot (99.95%) and a Mg–30Gd master alloy. Melting was performed in a resistance furnace within a heat-resistant mild steel crucible under a protective gas environment composed of 99% CO2 and 1% SF6. Subsequently, the molten mixture was poured into a preheated steel mold at a temperature of 500°C, following an isothermal holding time of 15 min. The chemical composition of the designed alloys was detected by the X-ray fluorescence spectrometer. The related results are listed in Table 1. The contents of alloying elements were consisted with the design.
Chemical composition of Mg–xGd
| Sample | Mg (wt%) | Gd (wt%) |
|---|---|---|
| Pure Mg | 99.71 | — |
| Mg–5Gd | 95.31 | 4.11 |
| Mg–10Gd | 91.09 | 8.75 |
| Mg–15Gd | 84.47 | 14.12 |
2.2 Irradiation experiment
Slabs measuring 150 mm × 150 mm × 4 mm were obtained from the pure Mg, Mg–5Gd, Mg–10Gd, and Mg–15Gd ingots for conducting neutron shielding performance tests at the Beijing Research Center for Radiation Application. The experiment utilized a 252Cf neutron source with an emission rate of 8.2 × 107 s−1 installed within a collimator made of polyethylene and featured a collimation aperture diameter of φ10 cm. The fast neutrons emitted by the 252Cf source were moderated effectively to form a thermal neutron field. The neutrons were detected using a 3He proportional counter.
Irradiation experiments were conducted at the Institute of Nuclear Science and Technology, Sichuan University, Chengdu, China, using a 2 × 3 MV tandem accelerator [9]. The dimensions of the samples used for the irradiation were 5 mm × 5 mm × 0.5 mm. A focused ion beam (FIB) technique was employed to prepare cross-sectional transmission electron microscopy (TEM) specimens for Au-ion irradiation. The Au-ion irradiation was conducted at a temperature of 200°C, reaching a fluence of approximately 1.25 × 1016 Au/cm2, with the energy of Au+ set at 6 MeV.
2.3 SuperMC (Monte Carlo N particle transport code)
SuperMC is a software program that employs the Monte Carlo method to compute the transport phenomena associated with neutrons, photons, and electrons within intricate three-dimensional geometrical structures [27]. Figure 1(a) illustrates the SuperMC model, which accurately represents the experimental conditions for evaluating neutron shielding performance [28]. Figure 1(b) illustrates the neutron source utilized for both simulation and experimentation. Using a252Cf source, neutrons were guided through a collimator with a cross-sectional diameter of 100 mm to generate a neutron field. This collimated field was further modified by incorporating paraffin neutron moderators within the fillings of the collimation apertures, which induced the formation of a thermal neutron field. The Mg–xGd sample was placed on the other side of the collimator to simulate the actual experimental setup. The radiation field was established along the central axis and positioned 1,580 mm from the radiation source. A counting sphere was set up on the center axis of the radiation field, 1,580 mm from the source, with the neutron flux through the counting surface recorded to obtain information on the neutron flux with and without the added sample.

(a) SuperMC model; and (b) neutron sources for simulation.
2.4 Characterizations
After polishing and etching, the microstructure of the alloy was examined using optical metallography. Scanning electron microscopy (SEM) was used to investigate the distribution and size of the alloys. Tensile testing was conducted at room temperature (RT) using a standard tensile testing machine with a strain rate of 1.7 × 10−3 s−1 to obtain the yield strength (YS), tensile strength (UTS), and elongation (FE) of the alloy. TEM was used to analyze the phase structure and morphology of the post-irradiation material. Sub-100-nm thin specimens were prepared using an FIB lift-out technique with an FIB/SEM instrument. During the TEM sample preparation, thin Pt layers were deposited to protect against FIB gallium contamination. An aberration-corrected JEOL2100F TEM/STEM operating at 200 keV was used to characterize the FIB foils through conventional bright-field and dark-field techniques.
3 Results
3.1 Microstructure
The microstructures of the as-cast Mg–Gd alloys are shown in Figures 2 and 3. According to existing research, the alloy structure of as-cast Mg–Gd alloys with different Gd content is primarily composed of α-Mg and eutectic phases (α-Mg + Mg5Gd), along with some non-equilibrium solidified Mg3Gd phases [29,30]. As the Gd content increases, the number of precipitated Mg5Gd phases gradually increases, and the morphology changes. Upon increasing the Gd content from 5 to 10%, a notable transition in the morphology of the Mg5Gd phase was observed, shifting from a granular structure (Figures 2(b) and 3(a)) to a skeletal morphology (Figures 2(c) and 3(b)), accompanied by a reduction in grain size compared to pure Mg. In the context of non-equilibrium solidification, the Mg–15Gd alloy displayed a dendritic morphology attributed to Gd segregation in the as-cast state (Figures 2(d) and 3(c)), with the Mg5Gd phase distributed in a discontinuous reticular pattern along the interdendritic regions. The average grain sizes of Mg–5Gd, Mg–10Gd, and Mg–15Gd alloys are, respectively, 270, 170, and 140 μm.
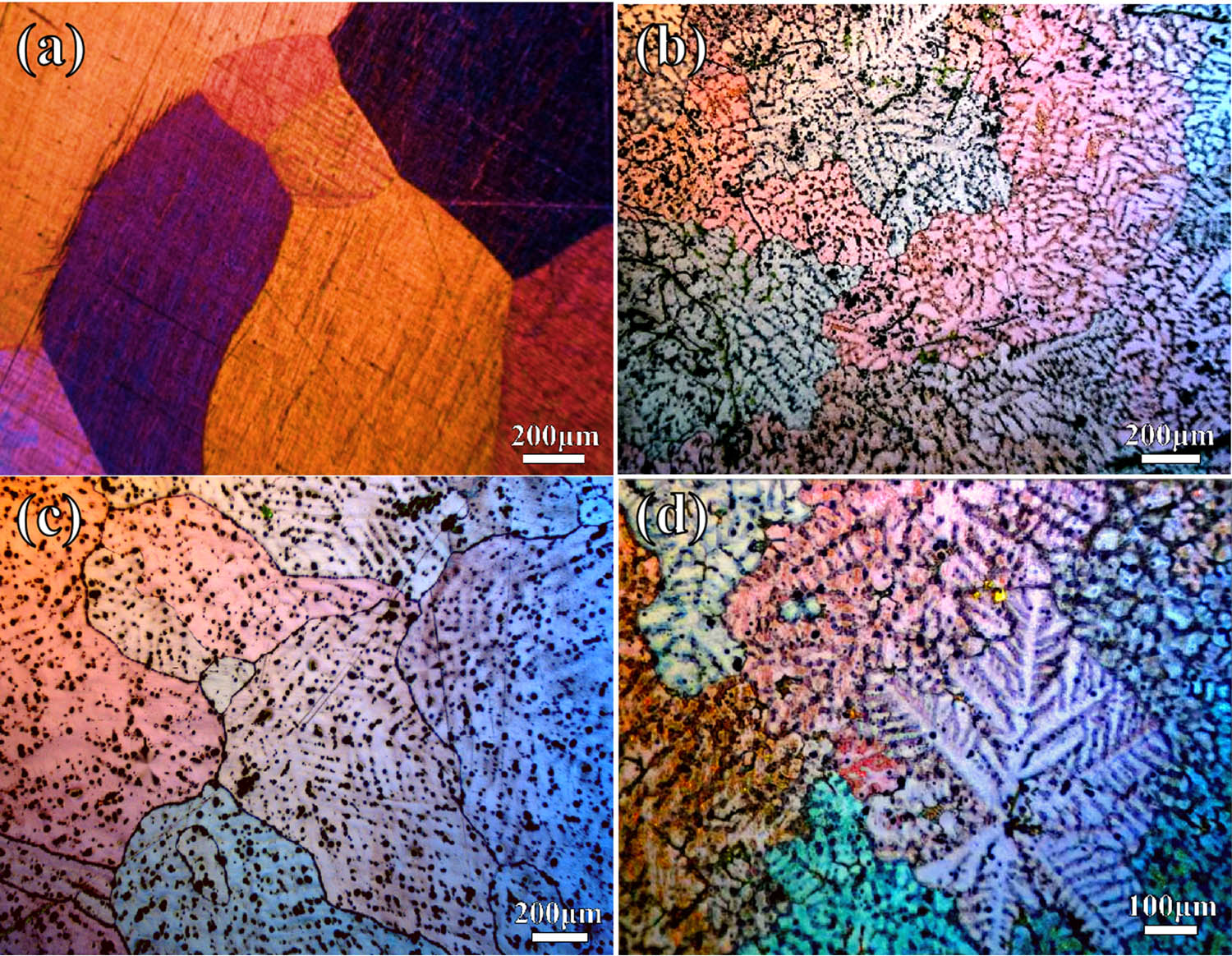
OM of Mg alloy: (a) pure Mg, (b) Mg–5Gd, (c) Mg–10Gd, and (d) Mg–15Gd.
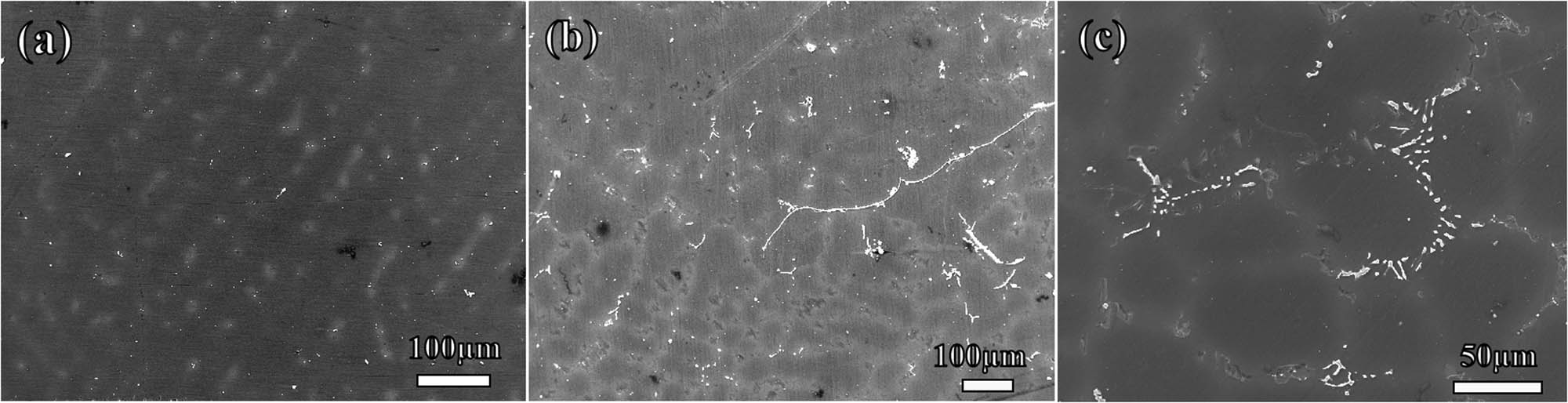
SEM of Mg alloy: (a) Mg–5Gd, (b) Mg–10Gd, and (c) Mg–15Gd.
3.2 Mechanical properties
Table 2 presents the YS, UTS, and FE of pure Mg and the as-cast Mg–Gd alloys at RT. When comparing the Mg–xGd (x = 0, 5, 10, and 15 wt%) alloys, we observe a significant increase in both YS and UTS as the percentage of Gd in the alloys increased from 5 to 15%. The Mg–15Gd alloy exhibited a UTS of 142.2 MPa, a substantial improvement over the 67.6 MPa of the Mg–5Gd alloy. Similarly, the YS of the Mg–15Gd alloy matches that of the Mg–5Gd alloy, both at 91.7 MPa, reflecting a notable increase from the initial 33.4 MPa in the Mg–5Gd alloy. However, the plasticity of the alloys follows a different change rule. Initially, as the Gd content increased, the elongation decreased from 2.9% for the Mg–5Gd alloy to 1.3% for the Mg–10Gd alloy. However, with a further increase in the Gd content of the Mg–15Gd alloy, the elongation partially recovered, reaching 1.8%.
Tensile mechanical properties of as-cast alloys
| Sample | YS (MPa) | UTS (MPa) | FE (%) |
|---|---|---|---|
| Pure Mg | 10.2 | 65.5 | 6.1 |
| Mg–5Gd | 33.4 | 67.6 | 2.9 |
| Mg–10Gd | 59.3 | 83.8 | 1.3 |
| Mg–15Gd | 91.7 | 142.2 | 1.8 |
3.3 Neutron shielding properties
The neutron shielding efficacy of the alloy was assessed by the neutron decay ratio, which is defined as the ratio of I 0 to I; I 0 represents the count obtained without any shielding material, and I represents the count after the shielding material is introduced. Table 3 presents the comparative analysis of the experimental and simulation results. The results obtained under the same conditions demonstrated remarkable consistency between the neutron decay ratios obtained through experimental observation and SuperMC simulation. The neutron decay rate obtained by these two methods was consistent with the variation trend of the Gd content. Notably, compared with pure Mg, introducing Gd significantly alters the thermal neutron attenuation characteristics of the alloy and enhances its shielding properties.
Experimental and SuperMC simulated thermal neutron attenuation ratio
| Mg | Mg–5Gd | Mg–10Gd | Mg–15Gd | |
|---|---|---|---|---|
| Experiment | 1.03 | 8.44 | 12.97 | 22.33 |
| SuperMC | 1.04 | 7.60 | 13.48 | 20.18 |
The deviations between the experimental tests and simulations were less than 10%, with the thermal neutron calculation results generally smaller than the experimental results, attributed to the following reasons: the simulation calculation was performed under ideal conditions, ignoring the influence of neutron reflection and scattering in the surrounding environment. In the theoretical calculation model, the elements in the material are evenly distributed in space. However, in the as-cast Mg–Gd alloy, the microscopic distribution is not uniform. Specifically, as the thermal neutron effective absorption element Gd increased, it accumulated more at the grain boundaries and formed a second phase. This uneven distribution may lead to changes in the shielding properties of the alloy in different regions.
3.4 Ion irradiation
Figure 4 shows the TEM micrographs near the [01
![Figure 4
TEM micrographs, under different diffraction vectors, near the [01
1
̅
\bar{1}
0] zone axis, depicting the microstructure of Mg–15Gd alloy after Au ion irradiation. (a) Target area within the red box on the FIB sample. (b) Along the [01
1
̅
\bar{1}
0] zone axis. (c) g = [0002]. (d) g = [
2
̅
\bar{2}
110]. Yellow and blue circles represent 〈c + a〉 and 〈c〉 dislocation loops; the red dashed line indicates some uncollected point defects.](/document/doi/10.1515/ntrev-2024-0007/asset/graphic/j_ntrev-2024-0007_fig_004.jpg)
TEM micrographs, under different diffraction vectors, near the [01
Figure 5 shows the nanoscale Gd-rich precipitates induced by irradiation in the Mg matrix and secondary phases. The precise composition of these precipitates is listed in Table 4, which suggests that the precipitate could be the Mg45.9Gd9.08 phase. An in-depth examination of the intricate phase relationships between the second phase and granular precipitates is presented in Figure 6(b). A superlattice structure is observed in the electron diffraction pattern, with (111)Mg3Gd parallel to (111)Mg45.9Gd9.08 and (220)Mg3Gd parallel to (220)Mg45.9Gd9.08. Additionally, Figure 6(a) shows the irradiation-induced defects in the second phase, with a small number of dislocation loops observed at the edges. These defects were formed along the (001) plane, demonstrating a distinct regularity.
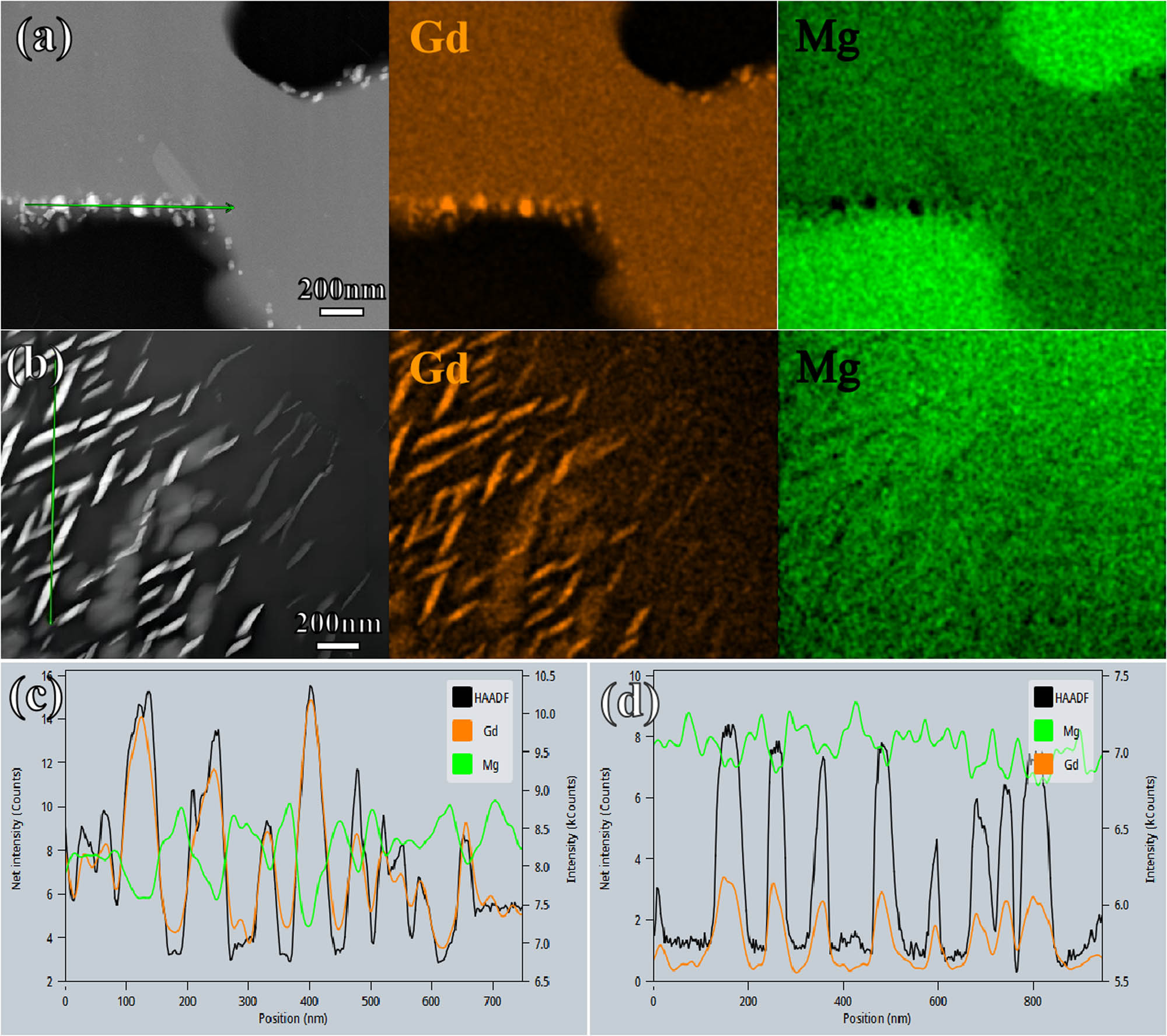
(a) Granular Gd-rich phase precipitated in the second phase. (b) Leaf-like Gd-rich phase precipitated in the matrix. EDS line scan of the (c) granular and (d) leaf-like phases.
EDS of (a) granular phase and (b) leaf-like phase in Figure 4
| Phases | Mg (at.%) | Gd (at.%) |
|---|---|---|
| Granular phase | 89.73 | 10.27 |
| Willow leaf phase | 84.25 | 15.75 |
![Figure 6
(a) Observation of dislocations in the second phase along the [001] axis. (b) Electron diffraction pattern of the yellow area in the second phase containing granular precipitates.](/document/doi/10.1515/ntrev-2024-0007/asset/graphic/j_ntrev-2024-0007_fig_006.jpg)
(a) Observation of dislocations in the second phase along the [001] axis. (b) Electron diffraction pattern of the yellow area in the second phase containing granular precipitates.
In the FCC structure, close-packed planes exhibited the lowest surface energies. Hence, during irradiation, oversaturated vacancies preferentially aggregate along the {111} planes, forming vacancy clusters [38]. When these clusters attain sufficient size, the unstable crystal planes on both sides collapse, forming dislocation loops. Concurrently, the dislocation loops in the matrix may originate from the aggregation of point defects generated by cascade collisions during irradiation.
Remarkably, even at a high dose of 10 dpa, the alloy did not exhibit a significant number of voids, highlighting its excellent radiation resistance attributed to the substantial amount of FCC phases present in the alloy [39]. When ions interact with these Gd-containing phases within the FCC structure, the displaced atoms move along interstitial paths in the close-packed crystal directions 〈110〉 increasing the cascade collision distances. This phenomenon, known as the “channeling effect,” inhibits the generation of point defects, thereby enhancing the overall stability of the alloy under irradiation.
In contrast, atoms traveling along other FCC crystallographic directions within the Mg matrix undergo “focusing collisions” following the initial collision [40,41]. These focusing collisions progressively diminished the scattering angles for each displaced atom in a specific crystal direction, thereby facilitating energy transfer. Notably, these collisions occurred predominantly in the close-packed directions, and the hexagonal close-packed structure of the Mg alloy augmented these interactions. Consequently, the displaced atoms experience increased energy losses, leading to a reduction in defect generation.
Furthermore, void formation is intricately linked to temperature variations. The aggregation of vacancies and self-interstitial atoms during cascade collisions within the FCC crystal structure influences the emergence of voids in the Mg5Gd phase, which become discernible via TEM only at temperatures surpassing approximately 0.35 times the material’s melting point (T m) [42]. Additionally, given the higher melting point of the Mg5Gd phase, no void formation was observed during irradiation at 200℃. These temperature-dependent void-formation characteristics highlight the unique behavioral responses of the alloy under varying conditions.
Finally, the emerging dislocations served as conduits for atomic diffusion, providing an auxiliary driving force that propelled the precipitation of Gd-containing phases with particle or leaf-like morphologies.
4 Discussion
4.1 Strengthening mechanism of mechanical properties
The strengthening mechanism of Mg alloys at room temperature involves solid-solution strengthening, grain refinement strengthening, and second-phase strengthening. The improvement in the strength of the as-cast Mg–Gd alloy was mainly attributed to second-phase strengthening and grain refinement. The eutectic phase β-Mg5Gd typically precipitates parallel to the {10
The grain refinement by Gd partially originates from the influence of Mg5Gd on heterogeneous nucleation. The interface energy between the nucleation substrate and crystalline phase determines the heterogeneous nucleation ability, with the mismatch between the substrate and crystalline phase being a key factor in determining the interface energy. The mismatch between the Mg5Gd phase (111) plane and the α-Mg phase (10
However, as the Gd content increased, several dendritic structures were internally generated in the alloy, leading to stress concentration. This stress concentration results in a distinctly brittle fracture behavior, which exerts a certain embrittling effect on the matrix and reduces the plasticity of the alloy. Consequently, the plasticity of the alloy was lower than that of pure Mg.
4.2 Effect of Gd on thermal neutron shielding performance
The enhancement of neutron shielding performance of the alloy with increasing Gd content can be attributed to two main factors, as inferred from the simulation and experimental results: (1) Adding effective absorptive elements alters the macroscopic absorption cross-section of the material. Gd possesses a significantly larger thermal neutron absorption cross-section (approximately 49,000 b) compared to that of Mg (0.04 b) [45]. (2) The microstructure of Mg alloys affects their neutron-shielding efficiency. The alloy grains were noticeably refined, and the number of Gd-containing phases increased in both the grain interiors and grain boundaries. Generally, the neutron absorption efficiency of a material increases as the mean free path of neutrons decreases. A reduced neutron mean free path signifies a higher probability of collisions between neutrons and atoms within the same material thickness; in other words, neutrons can be effectively absorbed by atoms over a shorter distance. The refinement of the alloy grains and the increase in precipitated phases reduced the distance between the Gd-containing phases, leading to a more uniform distribution, an increased probability of collisions between neutrons and Gd, and a decrease in the mean free path.
5 Conclusion
In summary, incorporating Gd in the 0–15 wt% range significantly enhances the thermal neutron absorption capability, along with notable increases in tensile and YS of Mg alloys. The as-cast Mg–15Gd alloy exhibited good radiation tolerance under Au ion irradiation at 200℃ and 10 dpa. The presence of 1/6 〈20
Acknowledgments
The authors are grateful to Dr. Zhu Changda from Sichuan University for his support techniques and guidance in radiation experiments. The authors thank the Joint Lab for Electron Microscopy of Chongqing University and the Analytical and Testing Center of Chongqing University.
-
Funding information: This work was funded by Key Research and Development Plan of Sichuan Province (24QYCX0537).
-
Author contributions: All authors have accepted responsibility for the entire content of this manuscript and approved its submission.
-
Conflict of interest: The authors state no conflict of interest.
-
Data availability statement: The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
[1] Dittmar MJE. Nuclear energy: Status and future limitations. Energy. 2012;37(1):35–40.10.1016/j.energy.2011.05.040Search in Google Scholar
[2] Uğur FA. New applications and developments in the neutron shielding. EPJ Web of Conferences. EDP Sciences; 2017. p. 0102210.1051/epjconf/201715401022Search in Google Scholar
[3] Hoffelner W. Mechanical properties of nuclear materials. In: Hoffelner W, editor. Materials for Nuclear Plants: From Safe Design to Residual Life Assessments. London: Springer; 2013. p. 197–254.10.1007/978-1-4471-2915-8_4Search in Google Scholar
[4] Zinkle SJ, Was GS. Materials challenges in nuclear energy. Acta Mater. 2013;61(3):735–58.10.1016/j.actamat.2012.11.004Search in Google Scholar
[5] Zinkle SJ, Busby JT. Structural materials for fission & fusion energy. Mater Today. 2009;12(11):12–9.10.1016/S1369-7021(09)70294-9Search in Google Scholar
[6] Halliwell E, Couch C, Begum R. Increase in linear attenuation coefficient by changing crystal structure of materials for radiation shielding and biomedical devices safety. Colloid Surface A. 2021;622:126646.10.1016/j.colsurfa.2021.126646Search in Google Scholar
[7] Saeed A. Developed borated austenitic stainless steel alloys as nuclear reactor control rods. Nucl Eng Des. 2023;413:112515.10.1016/j.nucengdes.2023.112515Search in Google Scholar
[8] Xu ZG, Jiang LT, Zhang Q. The design of a novel neutron shielding B4C/Al composite containing Gd. Mater Design. 2016;111:375–81.10.1016/j.matdes.2016.07.140Search in Google Scholar
[9] Sun WQ, Yu XH. Study on a high-boron-content stainless steel composite for nuclear radiation. Materials. 2021;14:7004–8.10.3390/ma14227004Search in Google Scholar PubMed PubMed Central
[10] Merklein M, Wieland M, Lechner M. Hot stamping of boron steel sheets with tailored properties: A review. J Mater Process Tech. 2016;228:11–24.10.1016/j.jmatprotec.2015.09.023Search in Google Scholar
[11] Rotkovich AA, Tishkevich DI, Bondaruk AA, German SA, Razanau IU, Zubar TI, et al. Epoxy-w composite materials: microstructure, structure, and radiation efficiency. Adv Phys Res. 2023;5(3):133–45.Search in Google Scholar
[12] Dong M, Zhou S, Xue X, Feng X, Yang H, Sayyed MI, et al. Upcycling of boron bearing blast furnace slag as highly cost-effective shield for protection of neutron radiation hazard: An innovative way and proposal of shielding mechanism. J Clean Prod. 2022;355:131817.10.1016/j.jclepro.2022.131817Search in Google Scholar
[13] Jian S, Zhu HY, Wang WS, Duan YQ. Effect of boron segregation on the surface crack of low carbon boron-bearing steel. Results Phys. 2012;2:089–101.Search in Google Scholar
[14] Shang Y, Yang G, Su FM. Multilayer polyethylene/hexagonal boron nitride composites showing high neutron shielding efficiency and thermal conductivity. Compos Commun. 2020;3:7–12.10.1016/j.coco.2020.03.007Search in Google Scholar
[15] Liu XC, Li XJ, Li ZX, Wang XH. Influence of heavy concrete on seismic response of transfer and purging rooms of nuclear power plant. Structures. 2023;51:1372–83.10.1016/j.istruc.2023.03.119Search in Google Scholar
[16] García Gallardo JA, Giménez MAN, Gervasoni JL. Nuclear properties of Tungsten under 14 MeV neutron irradiation for fusion-fission hybrid reactors. Ann Nucl Energy. 2020;147:107739.10.1016/j.anucene.2020.107739Search in Google Scholar
[17] Tishkevich DI, Rotkovich AA, German SA, Zhaludkevich AL, Vershinina TN, Bondaruk AA, et al. Heavy alloy based on tungsten and bismuth: fabrication, crystal structure, morphology, and shielding efficiency against gamma-radiation. Rsc Adv. 2023;13(35):24491–98.10.1039/D3RA04509ASearch in Google Scholar
[18] Al-Shelkamy SA, Vega-Carrillo HR, Xie Z, El-Hossary FM, Mosa ES, Mahdy AA, et al. Mechanical and radiation shielding characterization of W-based alloys for advanced nuclear unit. Appl Radiat Isotopes. 2023;201:110995.10.1016/j.apradiso.2023.110995Search in Google Scholar PubMed
[19] Wang JQ, Qin XZ, Cheng SH. The microstructure and mechanical performance optimization of a new Fe-Ni-based super alloy for Gen IV nuclear reactor: The critical role of Nb alloying strategy. Mater Charact. 2023;205:113240.10.1016/j.matchar.2023.113240Search in Google Scholar
[20] Wharry JP, Clement CD, Zhao Y, Baird K, Frazer D, Burns J, et al. Mechanical testing data from neutron irradiations of PM-HIP and conventionally manufactured nuclear structural alloys. Data in Brief. 2023;48:109092.10.1016/j.dib.2023.109092Search in Google Scholar PubMed PubMed Central
[21] Zhang P, Li J, Wang WX, Tan XY, Xie L, Chen XP. Design, shielding mechanism and tensile property of a novel (B4C + 6061Al)/Cf/6061Al laminar neutron-shielding composite. Vacuum. 2020;177:109383.10.1016/j.vacuum.2020.109383Search in Google Scholar
[22] Sturcken EF. Irradiation effects in magnesium and aluminum alloys. J Nucl Mater. 1979;82(1):39–53.10.1016/0022-3115(79)90037-0Search in Google Scholar
[23] Zheng J, Yan ZM, Ji JS. Effect of heat treatment on mechanical properties and microstructure evolution of Mg-9.5Gd-4Y-2.2Zn-0.5Zr alloy. J Magnes Alloys. 2022;10(4):1124–32.10.1016/j.jma.2021.05.018Search in Google Scholar
[24] Wan S. 155/157Gd areal density: a model for design and fabrication of Gd2O3/316L novel neutron shielding composites. Vacuum. 2020;89:102–9.10.1016/j.vacuum.2020.109304Search in Google Scholar
[25] Liu K, Hu DL, Lou F. Effects of deformation temperatures on microstructures, aging behaviors and mechanical properties of Mg-Gd-Er-Zr alloys fabricated by hard-plate rolling. J Magnes Alloys. 2022;10(2):049–53.10.1016/j.jma.2022.10.009Search in Google Scholar
[26] Liu LZ, Chen XH, Pan FS. A review on electromagnetic shielding magnesium alloys. J Magnes Alloys. 2021;9(6):1906–21.10.1016/j.jma.2021.10.001Search in Google Scholar
[27] Wu YC, Song J, Hu LQ. Super Monte Carlo Simulation Program for Nuclear and Radiation Process: SuperMC. Nucl Sci Eng. 2016;36(1):62–71.Search in Google Scholar
[28] Qi ZD, Yang Z, Meng XF, Yang XG, Liang MX, Li CY, et al. Microstructure, thermophysical properties and neutron shielding properties of Gd/316L composites for spent nuclear fuel transportation and storage. Mater Today Commun. 2023;37:107315.10.1016/j.mtcomm.2023.107315Search in Google Scholar
[29] Verbovytskyy Y, Gonçalves AP. On the ternary RExMg1-xAl2 (RE = Gd-Tm), RE3Ag5 ± xMg11 ± x, REAg4 + xMg2-x, RE4Ag10. 3Mg12 and RE4Ag10 + xMg3-x (RE = Ce-Nd, Sm) phases. Solid State Sci. 2015;40(8):4–91.10.1016/j.solidstatesciences.2015.01.006Search in Google Scholar
[30] Tong X, Zai L, You G, Wu H, Wen H, Long S. Effects of bonding temperature on microstructure and mechanical properties of diffusion-bonded joints of as-cast Mg–Gd alloy. Mat Sci Eng A-Struct. 2019;767:138408.10.1016/j.msea.2019.138408Search in Google Scholar
[31] Xu WZ, Zhang YF, Cheng GM, Jian WW, Millett PC, Koch CC, et al. Dynamic void growth and shrinkage in mg under electron irradiation. Mater Res Lett. 2014;2(3):176–83.10.1080/21663831.2014.904826Search in Google Scholar
[32] Khan AK, Yao Z, Daymond MR. Effect of foil orientation on damage accumulation during irradiation in magnesium and annealing response of dislocation loops. J Nucl Mater. 2012;423(1-3):132–41.10.1016/j.jnucmat.2012.01.003Search in Google Scholar
[33] Wu S, Cao C, Bo H. In-situ atomic-scale observation the escape of irradiation-induced dislocation loops in magnesium. J Alloy Compd. 2022;895:162708.10.1016/j.jallcom.2021.162708Search in Google Scholar
[34] Xu W, Zhang Y, Cheng G. On the origin and behavior of irradiation-induced c-component dislocation loops in magnesium. Acta Mater. 2017;131:457–66.10.1016/j.actamat.2017.04.015Search in Google Scholar
[35] Khan AK, Yao Z, Daymond MR, Holt RA. Irradiation damage in commercial purity magnesium. Nucl Instrum Meth B. 2012;272:231–5.10.1016/j.nimb.2011.01.072Search in Google Scholar
[36] Griffiths M. Evolution of microstructure in hcp metals during irradiation. J Nucl Mater. 1993;205:225–41.10.1016/0022-3115(93)90085-DSearch in Google Scholar
[37] Geng J, Chisholm MF, Mishra RK, Kumar KS. The structure of 〈c + a〉 type dislocation loops in magnesium. Phil Mag Lett. 2014;94(6):377–86.10.1080/09500839.2014.916423Search in Google Scholar
[38] Osetsky YN, Bacon DJ, Serra A, Singh BN, Golubov SI. Stability and mobility of defect clusters and dislocation loops in metals. J Nucl Mater. 2000;276(1–3):53–77.10.1016/S0022-3115(99)00170-1Search in Google Scholar
[39] Bacon DJ, Gao F, Osetsky YN. The primary damage state in fcc, bcc and hcp metals as seen in molecular dynamics simulations. J Nucl Mater. 2000;276(1–3):1–12.10.1016/S0022-3115(99)00165-8Search in Google Scholar
[40] Silsbee RH. Focusing in collision problems in solids. J Appl Phys. 1957;28(11):1246–50.10.1063/1.1722626Search in Google Scholar
[41] Leibfried G. Defects in dislocations produced by focusing collisions in fcc lattices. J Appl Phys. 1960;31(1):117–21.10.1063/1.1735384Search in Google Scholar
[42] Singh BN, Evans JH. Significant differences in defect accumulation behaviour between fcc and bcc crystals under cascade damage conditions. J Nucl Mater. 1995;226(3):277–85.10.1016/0022-3115(95)00121-2Search in Google Scholar
[43] Gao X, He SM, Zeng XQ, Peng LM, Ding WJ, Nie JF. Microstructure evolution in a Mg–15Gd–0.5 Zr (wt.%) alloy during isothermal aging at 250°C. Mat Sci Eng A-Struct. 2006;431(1–2):322–7.10.1016/j.msea.2006.06.018Search in Google Scholar
[44] Guan K, Egusa D, Abe E, Zhang J, Qiu X, Yang Q, et al. Microstructures and mechanical properties of as-cast Mg-Sm-Zn-Zr alloys with varying Gd contents. J Magnes Alloys. 2022;10(5):1220–34.10.1016/j.jma.2021.09.013Search in Google Scholar
[45] Zhang P, Li J, Wang WX, Tan XY, Xie L, Guo FY. The design, microstructure and mechanical properties of a novel Gd2O3/6061Al neutron shielding composite. Vacuum. 2019;162:92–100.10.1016/j.vacuum.2019.01.004Search in Google Scholar
© 2024 the author(s), published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Research Articles
- Tension buckling and postbuckling of nanocomposite laminated plates with in-plane negative Poisson’s ratio
- Polyvinylpyrrolidone-stabilised gold nanoparticle coatings inhibit blood protein adsorption
- Energy and mass transmission through hybrid nanofluid flow passing over a spinning sphere with magnetic effect and heat source/sink
- Surface treatment with nano-silica and magnesium potassium phosphate cement co-action for enhancing recycled aggregate concrete
- Numerical investigation of thermal radiation with entropy generation effects in hybrid nanofluid flow over a shrinking/stretching sheet
- Enhancing the performance of thermal energy storage by adding nano-particles with paraffin phase change materials
- Using nano-CaCO3 and ceramic tile waste to design low-carbon ultra high performance concrete
- Numerical analysis of thermophoretic particle deposition in a magneto-Marangoni convective dusty tangent hyperbolic nanofluid flow – Thermal and magnetic features
- Dual numerical solutions of Casson SA–hybrid nanofluid toward a stagnation point flow over stretching/shrinking cylinder
- Single flake homo p–n diode of MoTe2 enabled by oxygen plasma doping
- Electrostatic self-assembly effect of Fe3O4 nanoparticles on performance of carbon nanotubes in cement-based materials
- Multi-scale alignment to buried atom-scale devices using Kelvin probe force microscopy
- Antibacterial, mechanical, and dielectric properties of hydroxyapatite cordierite/zirconia porous nanocomposites for use in bone tissue engineering applications
- Time-dependent Darcy–Forchheimer flow of Casson hybrid nanofluid comprising the CNTs through a Riga plate with nonlinear thermal radiation and viscous dissipation
- Durability prediction of geopolymer mortar reinforced with nanoparticles and PVA fiber using particle swarm optimized BP neural network
- Utilization of zein nano-based system for promoting antibiofilm and anti-virulence activities of curcumin against Pseudomonas aeruginosa
- Antibacterial effect of novel dental resin composites containing rod-like zinc oxide
- An extended model to assess Jeffery–Hamel blood flow through arteries with iron-oxide (Fe2O3) nanoparticles and melting effects: Entropy optimization analysis
- Comparative study of copper nanoparticles over radially stretching sheet with water and silicone oil
- Cementitious composites modified by nanocarbon fillers with cooperation effect possessing excellent self-sensing properties
- Confinement size effect on dielectric properties, antimicrobial activity, and recycling of TiO2 quantum dots via photodegradation processes of Congo red dye and real industrial textile wastewater
- Biogenic silver nanoparticles of Moringa oleifera leaf extract: Characterization and photocatalytic application
- Novel integrated structure and function of Mg–Gd neutron shielding materials
- Impact of multiple slips on thermally radiative peristaltic transport of Sisko nanofluid with double diffusion convection, viscous dissipation, and induced magnetic field
- Magnetized water-based hybrid nanofluid flow over an exponentially stretching sheet with thermal convective and mass flux conditions: HAM solution
- A numerical investigation of the two-dimensional magnetohydrodynamic water-based hybrid nanofluid flow composed of Fe3O4 and Au nanoparticles over a heated surface
- Development and modeling of an ultra-robust TPU-MWCNT foam with high flexibility and compressibility
- Effects of nanofillers on the physical, mechanical, and tribological behavior of carbon/kenaf fiber–reinforced phenolic composites
- Polymer nanocomposite for protecting photovoltaic cells from solar ultraviolet in space
- Study on the mechanical properties and microstructure of recycled concrete reinforced with basalt fibers and nano-silica in early low-temperature environments
- Synergistic effect of carbon nanotubes and polyvinyl alcohol on the mechanical performance and microstructure of cement mortar
- CFD analysis of paraffin-based hybrid (Co–Au) and trihybrid (Co–Au–ZrO2) nanofluid flow through a porous medium
- Forced convective tangent hyperbolic nanofluid flow subject to heat source/sink and Lorentz force over a permeable wedge: Numerical exploration
- Physiochemical and electrical activities of nano copper oxides synthesised via hydrothermal method utilising natural reduction agents for solar cell application
- A homotopic analysis of the blood-based bioconvection Carreau–Yasuda hybrid nanofluid flow over a stretching sheet with convective conditions
- In situ synthesis of reduced graphene oxide/SnIn4S8 nanocomposites with enhanced photocatalytic performance for pollutant degradation
- A coarse-grained Poisson–Nernst–Planck model for polyelectrolyte-modified nanofluidic diodes
- A numerical investigation of the magnetized water-based hybrid nanofluid flow over an extending sheet with a convective condition: Active and passive controls of nanoparticles
- The LyP-1 cyclic peptide modified mesoporous polydopamine nanospheres for targeted delivery of triptolide regulate the macrophage repolarization in atherosclerosis
- Synergistic effect of hydroxyapatite-magnetite nanocomposites in magnetic hyperthermia for bone cancer treatment
- The significance of quadratic thermal radiative scrutinization of a nanofluid flow across a microchannel with thermophoretic particle deposition effects
- Ferromagnetic effect on Casson nanofluid flow and transport phenomena across a bi-directional Riga sensor device: Darcy–Forchheimer model
- Performance of carbon nanomaterials incorporated with concrete exposed to high temperature
- Multicriteria-based optimization of roller compacted concrete pavement containing crumb rubber and nano-silica
- Revisiting hydrotalcite synthesis: Efficient combined mechanochemical/coprecipitation synthesis to design advanced tunable basic catalysts
- Exploration of irreversibility process and thermal energy of a tetra hybrid radiative binary nanofluid focusing on solar implementations
- Effect of graphene oxide on the properties of ternary limestone clay cement paste
- Improved mechanical properties of graphene-modified basalt fibre–epoxy composites
- Sodium titanate nanostructured modified by green synthesis of iron oxide for highly efficient photodegradation of dye contaminants
- Green synthesis of Vitis vinifera extract-appended magnesium oxide NPs for biomedical applications
- Differential study on the thermal–physical properties of metal and its oxide nanoparticle-formed nanofluids: Molecular dynamics simulation investigation of argon-based nanofluids
- Heat convection and irreversibility of magneto-micropolar hybrid nanofluids within a porous hexagonal-shaped enclosure having heated obstacle
- Numerical simulation and optimization of biological nanocomposite system for enhanced oil recovery
- Laser ablation and chemical vapor deposition to prepare a nanostructured PPy layer on the Ti surface
- Cilostazol niosomes-loaded transdermal gels: An in vitro and in vivo anti-aggregant and skin permeation activity investigations towards preparing an efficient nanoscale formulation
- Linear and nonlinear optical studies on successfully mixed vanadium oxide and zinc oxide nanoparticles synthesized by sol–gel technique
- Analytical investigation of convective phenomena with nonlinearity characteristics in nanostratified liquid film above an inclined extended sheet
- Optimization method for low-velocity impact identification in nanocomposite using genetic algorithm
- Analyzing the 3D-MHD flow of a sodium alginate-based nanofluid flow containing alumina nanoparticles over a bi-directional extending sheet using variable porous medium and slip conditions
- A comprehensive study of laser irradiated hydrothermally synthesized 2D layered heterostructure V2O5(1−x)MoS2(x) (X = 1–5%) nanocomposites for photocatalytic application
- Computational analysis of water-based silver, copper, and alumina hybrid nanoparticles over a stretchable sheet embedded in a porous medium with thermophoretic particle deposition effects
- A deep dive into AI integration and advanced nanobiosensor technologies for enhanced bacterial infection monitoring
- Effects of normal strain on pyramidal I and II 〈c + a〉 screw dislocation mobility and structure in single-crystal magnesium
- Computational study of cross-flow in entropy-optimized nanofluids
- Significance of nanoparticle aggregation for thermal transport over magnetized sensor surface
- A green and facile synthesis route of nanosize cupric oxide at room temperature
- Effect of annealing time on bending performance and microstructure of C19400 alloy strip
- Chitosan-based Mupirocin and Alkanna tinctoria extract nanoparticles for the management of burn wound: In vitro and in vivo characterization
- Electrospinning of MNZ/PLGA/SF nanofibers for periodontitis
- Photocatalytic degradation of methylene blue by Nd-doped titanium dioxide thin films
- Shell-core-structured electrospinning film with sequential anti-inflammatory and pro-neurogenic effects for peripheral nerve repairment
- Flow and heat transfer insights into a chemically reactive micropolar Williamson ternary hybrid nanofluid with cross-diffusion theory
- One-pot fabrication of open-spherical shapes based on the decoration of copper sulfide/poly-O-amino benzenethiol on copper oxide as a promising photocathode for hydrogen generation from the natural source of Red Sea water
- A penta-hybrid approach for modeling the nanofluid flow in a spatially dependent magnetic field
- Advancing sustainable agriculture: Metal-doped urea–hydroxyapatite hybrid nanofertilizer for agro-industry
- Utilizing Ziziphus spina-christi for eco-friendly synthesis of silver nanoparticles: Antimicrobial activity and promising application in wound healing
- Plant-mediated synthesis, characterization, and evaluation of a copper oxide/silicon dioxide nanocomposite by an antimicrobial study
- Effects of PVA fibers and nano-SiO2 on rheological properties of geopolymer mortar
- Investigating silver and alumina nanoparticles’ impact on fluid behavior over porous stretching surface
- Potential pharmaceutical applications and molecular docking study for green fabricated ZnO nanoparticles mediated Raphanus sativus: In vitro and in vivo study
- Effect of temperature and nanoparticle size on the interfacial layer thickness of TiO2–water nanofluids using molecular dynamics
- Characteristics of induced magnetic field on the time-dependent MHD nanofluid flow through parallel plates
- Flexural and vibration behaviours of novel covered CFRP composite joints with an MWCNT-modified adhesive
- Experimental research on mechanically and thermally activation of nano-kaolin to improve the properties of ultra-high-performance fiber-reinforced concrete
- Analysis of variable fluid properties for three-dimensional flow of ternary hybrid nanofluid on a stretching sheet with MHD effects
- Biodegradability of corn starch films containing nanocellulose fiber and thymol
- Toxicity assessment of copper oxide nanoparticles: In vivo study
- Some measures to enhance the energy output performances of triboelectric nanogenerators
- Reinforcement of graphene nanoplatelets on water uptake and thermomechanical behaviour of epoxy adhesive subjected to water ageing conditions
- Optimization of preparation parameters and testing verification of carbon nanotube suspensions used in concrete
- Max-phase Ti3SiC2 and diverse nanoparticle reinforcements for enhancement of the mechanical, dynamic, and microstructural properties of AA5083 aluminum alloy via FSP
- Advancing drug delivery: Neural network perspectives on nanoparticle-mediated treatments for cancerous tissues
- PEG-PLGA core–shell nanoparticles for the controlled delivery of picoplatin–hydroxypropyl β-cyclodextrin inclusion complex in triple-negative breast cancer: In vitro and in vivo study
- Conduction transportation from graphene to an insulative polymer medium: A novel approach for the conductivity of nanocomposites
- Review Articles
- Developments of terahertz metasurface biosensors: A literature review
- Overview of amorphous carbon memristor device, modeling, and applications for neuromorphic computing
- Advances in the synthesis of gold nanoclusters (AuNCs) of proteins extracted from nature
- A review of ternary polymer nanocomposites containing clay and calcium carbonate and their biomedical applications
- Recent advancements in polyoxometalate-functionalized fiber materials: A review
- Special contribution of atomic force microscopy in cell death research
- A comprehensive review of oral chitosan drug delivery systems: Applications for oral insulin delivery
- Cellular senescence and nanoparticle-based therapies: Current developments and perspectives
- Cyclodextrins-block copolymer drug delivery systems: From design and development to preclinical studies
- Micelle-based nanoparticles with stimuli-responsive properties for drug delivery
- Critical assessment of the thermal stability and degradation of chemically functionalized nanocellulose-based polymer nanocomposites
- Research progress in preparation technology of micro and nano titanium alloy powder
- Nanoformulations for lysozyme-based additives in animal feed: An alternative to fight antibiotic resistance spread
- Incorporation of organic photochromic molecules in mesoporous silica materials: Synthesis and applications
- A review on modeling of graphene and associated nanostructures reinforced concrete
- A review on strengthening mechanisms of carbon quantum dots-reinforced Cu-matrix nanocomposites
- Review on nanocellulose composites and CNFs assembled microfiber toward automotive applications
- Nanomaterial coating for layered lithium rich transition metal oxide cathode for lithium-ion battery
- Application of AgNPs in biomedicine: An overview and current trends
- Nanobiotechnology and microbial influence on cold adaptation in plants
- Hepatotoxicity of nanomaterials: From mechanism to therapeutic strategy
- Applications of micro-nanobubble and its influence on concrete properties: An in-depth review
- A comprehensive systematic literature review of ML in nanotechnology for sustainable development
- Exploiting the nanotechnological approaches for traditional Chinese medicine in childhood rhinitis: A review of future perspectives
- Twisto-photonics in two-dimensional materials: A comprehensive review
- Current advances of anticancer drugs based on solubilization technology
- Recent process of using nanoparticles in the T cell-based immunometabolic therapy
- Future prospects of gold nanoclusters in hydrogen storage systems and sustainable environmental treatment applications
- Preparation, types, and applications of one- and two-dimensional nanochannels and their transport properties for water and ions
- Microstructural, mechanical, and corrosion characteristics of Mg–Gd–x systems: A review of recent advancements
- Functionalized nanostructures and targeted delivery systems with a focus on plant-derived natural agents for COVID-19 therapy: A review and outlook
- Mapping evolution and trends of cell membrane-coated nanoparticles: A bibliometric analysis and scoping review
- Nanoparticles and their application in the diagnosis of hepatocellular carcinoma
- In situ growth of carbon nanotubes on fly ash substrates
- Structural performance of boards through nanoparticle reinforcement: An advance review
- Reinforcing mechanisms review of the graphene oxide on cement composites
- Seed regeneration aided by nanomaterials in a climate change scenario: A comprehensive review
- Surface-engineered quantum dot nanocomposites for neurodegenerative disorder remediation and avenue for neuroimaging
- Graphitic carbon nitride hybrid thin films for energy conversion: A mini-review on defect activation with different materials
- Nanoparticles and the treatment of hepatocellular carcinoma
- Special Issue on Advanced Nanomaterials and Composites for Energy Conversion and Storage - Part II
- Highly safe lithium vanadium oxide anode for fast-charging dendrite-free lithium-ion batteries
- Recent progress in nanomaterials of battery energy storage: A patent landscape analysis, technology updates, and future prospects
- Special Issue on Advanced Nanomaterials for Carbon Capture, Environment and Utilization for Energy Sustainability - Part II
- Calcium-, magnesium-, and yttrium-doped lithium nickel phosphate nanomaterials as high-performance catalysts for electrochemical water oxidation reaction
- Low alkaline vegetation concrete with silica fume and nano-fly ash composites to improve the planting properties and soil ecology
- Mesoporous silica-grafted deep eutectic solvent-based mixed matrix membranes for wastewater treatment: Synthesis and emerging pollutant removal performance
- Electrochemically prepared ultrathin two-dimensional graphitic nanosheets as cathodes for advanced Zn-based energy storage devices
- Enhanced catalytic degradation of amoxicillin by phyto-mediated synthesised ZnO NPs and ZnO-rGO hybrid nanocomposite: Assessment of antioxidant activity, adsorption, and thermodynamic analysis
- Incorporating GO in PI matrix to advance nanocomposite coating: An enhancing strategy to prevent corrosion
- Synthesis, characterization, thermal stability, and application of microporous hyper cross-linked polyphosphazenes with naphthylamine group for CO2 uptake
- Engineering in ceramic albite morphology by the addition of additives: Carbon nanotubes and graphene oxide for energy applications
- Nanoscale synergy: Optimizing energy storage with SnO2 quantum dots on ZnO hexagonal prisms for advanced supercapacitors
- Aging assessment of silicone rubber materials under corona discharge accompanied by humidity and UV radiation
- Tuning structural and electrical properties of Co-precipitated and Cu-incorporated nickel ferrite for energy applications
- Sodium alginate-supported AgSr nanoparticles for catalytic degradation of malachite green and methyl orange in aqueous medium
- An environmentally greener and reusability approach for bioenergy production using Mallotus philippensis (Kamala) seed oil feedstock via phytonanotechnology
- Micro-/nano-alumina trihydrate and -magnesium hydroxide fillers in RTV-SR composites under electrical and environmental stresses
- Mechanism exploration of ion-implanted epoxy on surface trap distribution: An approach to augment the vacuum flashover voltages
- Nanoscale engineering of semiconductor photocatalysts boosting charge separation for solar-driven H2 production: Recent advances and future perspective
- Excellent catalytic performance over reduced graphene-boosted novel nanoparticles for oxidative desulfurization of fuel oil
- Special Issue on Advances in Nanotechnology for Agriculture
- Deciphering the synergistic potential of mycogenic zinc oxide nanoparticles and bio-slurry formulation on phenology and physiology of Vigna radiata
- Nanomaterials: Cross-disciplinary applications in ornamental plants
- Special Issue on Catechol Based Nano and Microstructures
- Polydopamine films: Versatile but interface-dependent coatings
- In vitro anticancer activity of melanin-like nanoparticles for multimodal therapy of glioblastoma
- Poly-3,4-dihydroxybenzylidenhydrazine, a different analogue of polydopamine
- Chirality and self-assembly of structures derived from optically active 1,2-diaminocyclohexane and catecholamines
- Advancing resource sustainability with green photothermal materials: Insights from organic waste-derived and bioderived sources
- Bioinspired neuromelanin-like Pt(iv) polymeric nanoparticles for cancer treatment
- Special Issue on Implementing Nanotechnology for Smart Healthcare System
- Intelligent explainable optical sensing on Internet of nanorobots for disease detection
- Special Issue on Green Mono, Bi and Tri Metallic Nanoparticles for Biological and Environmental Applications
- Tracking success of interaction of green-synthesized Carbopol nanoemulgel (neomycin-decorated Ag/ZnO nanocomposite) with wound-based MDR bacteria
- Green synthesis of copper oxide nanoparticles using genus Inula and evaluation of biological therapeutics and environmental applications
- Biogenic fabrication and multifunctional therapeutic applications of silver nanoparticles synthesized from rose petal extract
- Metal oxides on the frontlines: Antimicrobial activity in plant-derived biometallic nanoparticles
- Controlling pore size during the synthesis of hydroxyapatite nanoparticles using CTAB by the sol–gel hydrothermal method and their biological activities
- Special Issue on State-of-Art Advanced Nanotechnology for Healthcare
- Applications of nanomedicine-integrated phototherapeutic agents in cancer theranostics: A comprehensive review of the current state of research
- Smart bionanomaterials for treatment and diagnosis of inflammatory bowel disease
- Beyond conventional therapy: Synthesis of multifunctional nanoparticles for rheumatoid arthritis therapy
Articles in the same Issue
- Research Articles
- Tension buckling and postbuckling of nanocomposite laminated plates with in-plane negative Poisson’s ratio
- Polyvinylpyrrolidone-stabilised gold nanoparticle coatings inhibit blood protein adsorption
- Energy and mass transmission through hybrid nanofluid flow passing over a spinning sphere with magnetic effect and heat source/sink
- Surface treatment with nano-silica and magnesium potassium phosphate cement co-action for enhancing recycled aggregate concrete
- Numerical investigation of thermal radiation with entropy generation effects in hybrid nanofluid flow over a shrinking/stretching sheet
- Enhancing the performance of thermal energy storage by adding nano-particles with paraffin phase change materials
- Using nano-CaCO3 and ceramic tile waste to design low-carbon ultra high performance concrete
- Numerical analysis of thermophoretic particle deposition in a magneto-Marangoni convective dusty tangent hyperbolic nanofluid flow – Thermal and magnetic features
- Dual numerical solutions of Casson SA–hybrid nanofluid toward a stagnation point flow over stretching/shrinking cylinder
- Single flake homo p–n diode of MoTe2 enabled by oxygen plasma doping
- Electrostatic self-assembly effect of Fe3O4 nanoparticles on performance of carbon nanotubes in cement-based materials
- Multi-scale alignment to buried atom-scale devices using Kelvin probe force microscopy
- Antibacterial, mechanical, and dielectric properties of hydroxyapatite cordierite/zirconia porous nanocomposites for use in bone tissue engineering applications
- Time-dependent Darcy–Forchheimer flow of Casson hybrid nanofluid comprising the CNTs through a Riga plate with nonlinear thermal radiation and viscous dissipation
- Durability prediction of geopolymer mortar reinforced with nanoparticles and PVA fiber using particle swarm optimized BP neural network
- Utilization of zein nano-based system for promoting antibiofilm and anti-virulence activities of curcumin against Pseudomonas aeruginosa
- Antibacterial effect of novel dental resin composites containing rod-like zinc oxide
- An extended model to assess Jeffery–Hamel blood flow through arteries with iron-oxide (Fe2O3) nanoparticles and melting effects: Entropy optimization analysis
- Comparative study of copper nanoparticles over radially stretching sheet with water and silicone oil
- Cementitious composites modified by nanocarbon fillers with cooperation effect possessing excellent self-sensing properties
- Confinement size effect on dielectric properties, antimicrobial activity, and recycling of TiO2 quantum dots via photodegradation processes of Congo red dye and real industrial textile wastewater
- Biogenic silver nanoparticles of Moringa oleifera leaf extract: Characterization and photocatalytic application
- Novel integrated structure and function of Mg–Gd neutron shielding materials
- Impact of multiple slips on thermally radiative peristaltic transport of Sisko nanofluid with double diffusion convection, viscous dissipation, and induced magnetic field
- Magnetized water-based hybrid nanofluid flow over an exponentially stretching sheet with thermal convective and mass flux conditions: HAM solution
- A numerical investigation of the two-dimensional magnetohydrodynamic water-based hybrid nanofluid flow composed of Fe3O4 and Au nanoparticles over a heated surface
- Development and modeling of an ultra-robust TPU-MWCNT foam with high flexibility and compressibility
- Effects of nanofillers on the physical, mechanical, and tribological behavior of carbon/kenaf fiber–reinforced phenolic composites
- Polymer nanocomposite for protecting photovoltaic cells from solar ultraviolet in space
- Study on the mechanical properties and microstructure of recycled concrete reinforced with basalt fibers and nano-silica in early low-temperature environments
- Synergistic effect of carbon nanotubes and polyvinyl alcohol on the mechanical performance and microstructure of cement mortar
- CFD analysis of paraffin-based hybrid (Co–Au) and trihybrid (Co–Au–ZrO2) nanofluid flow through a porous medium
- Forced convective tangent hyperbolic nanofluid flow subject to heat source/sink and Lorentz force over a permeable wedge: Numerical exploration
- Physiochemical and electrical activities of nano copper oxides synthesised via hydrothermal method utilising natural reduction agents for solar cell application
- A homotopic analysis of the blood-based bioconvection Carreau–Yasuda hybrid nanofluid flow over a stretching sheet with convective conditions
- In situ synthesis of reduced graphene oxide/SnIn4S8 nanocomposites with enhanced photocatalytic performance for pollutant degradation
- A coarse-grained Poisson–Nernst–Planck model for polyelectrolyte-modified nanofluidic diodes
- A numerical investigation of the magnetized water-based hybrid nanofluid flow over an extending sheet with a convective condition: Active and passive controls of nanoparticles
- The LyP-1 cyclic peptide modified mesoporous polydopamine nanospheres for targeted delivery of triptolide regulate the macrophage repolarization in atherosclerosis
- Synergistic effect of hydroxyapatite-magnetite nanocomposites in magnetic hyperthermia for bone cancer treatment
- The significance of quadratic thermal radiative scrutinization of a nanofluid flow across a microchannel with thermophoretic particle deposition effects
- Ferromagnetic effect on Casson nanofluid flow and transport phenomena across a bi-directional Riga sensor device: Darcy–Forchheimer model
- Performance of carbon nanomaterials incorporated with concrete exposed to high temperature
- Multicriteria-based optimization of roller compacted concrete pavement containing crumb rubber and nano-silica
- Revisiting hydrotalcite synthesis: Efficient combined mechanochemical/coprecipitation synthesis to design advanced tunable basic catalysts
- Exploration of irreversibility process and thermal energy of a tetra hybrid radiative binary nanofluid focusing on solar implementations
- Effect of graphene oxide on the properties of ternary limestone clay cement paste
- Improved mechanical properties of graphene-modified basalt fibre–epoxy composites
- Sodium titanate nanostructured modified by green synthesis of iron oxide for highly efficient photodegradation of dye contaminants
- Green synthesis of Vitis vinifera extract-appended magnesium oxide NPs for biomedical applications
- Differential study on the thermal–physical properties of metal and its oxide nanoparticle-formed nanofluids: Molecular dynamics simulation investigation of argon-based nanofluids
- Heat convection and irreversibility of magneto-micropolar hybrid nanofluids within a porous hexagonal-shaped enclosure having heated obstacle
- Numerical simulation and optimization of biological nanocomposite system for enhanced oil recovery
- Laser ablation and chemical vapor deposition to prepare a nanostructured PPy layer on the Ti surface
- Cilostazol niosomes-loaded transdermal gels: An in vitro and in vivo anti-aggregant and skin permeation activity investigations towards preparing an efficient nanoscale formulation
- Linear and nonlinear optical studies on successfully mixed vanadium oxide and zinc oxide nanoparticles synthesized by sol–gel technique
- Analytical investigation of convective phenomena with nonlinearity characteristics in nanostratified liquid film above an inclined extended sheet
- Optimization method for low-velocity impact identification in nanocomposite using genetic algorithm
- Analyzing the 3D-MHD flow of a sodium alginate-based nanofluid flow containing alumina nanoparticles over a bi-directional extending sheet using variable porous medium and slip conditions
- A comprehensive study of laser irradiated hydrothermally synthesized 2D layered heterostructure V2O5(1−x)MoS2(x) (X = 1–5%) nanocomposites for photocatalytic application
- Computational analysis of water-based silver, copper, and alumina hybrid nanoparticles over a stretchable sheet embedded in a porous medium with thermophoretic particle deposition effects
- A deep dive into AI integration and advanced nanobiosensor technologies for enhanced bacterial infection monitoring
- Effects of normal strain on pyramidal I and II 〈c + a〉 screw dislocation mobility and structure in single-crystal magnesium
- Computational study of cross-flow in entropy-optimized nanofluids
- Significance of nanoparticle aggregation for thermal transport over magnetized sensor surface
- A green and facile synthesis route of nanosize cupric oxide at room temperature
- Effect of annealing time on bending performance and microstructure of C19400 alloy strip
- Chitosan-based Mupirocin and Alkanna tinctoria extract nanoparticles for the management of burn wound: In vitro and in vivo characterization
- Electrospinning of MNZ/PLGA/SF nanofibers for periodontitis
- Photocatalytic degradation of methylene blue by Nd-doped titanium dioxide thin films
- Shell-core-structured electrospinning film with sequential anti-inflammatory and pro-neurogenic effects for peripheral nerve repairment
- Flow and heat transfer insights into a chemically reactive micropolar Williamson ternary hybrid nanofluid with cross-diffusion theory
- One-pot fabrication of open-spherical shapes based on the decoration of copper sulfide/poly-O-amino benzenethiol on copper oxide as a promising photocathode for hydrogen generation from the natural source of Red Sea water
- A penta-hybrid approach for modeling the nanofluid flow in a spatially dependent magnetic field
- Advancing sustainable agriculture: Metal-doped urea–hydroxyapatite hybrid nanofertilizer for agro-industry
- Utilizing Ziziphus spina-christi for eco-friendly synthesis of silver nanoparticles: Antimicrobial activity and promising application in wound healing
- Plant-mediated synthesis, characterization, and evaluation of a copper oxide/silicon dioxide nanocomposite by an antimicrobial study
- Effects of PVA fibers and nano-SiO2 on rheological properties of geopolymer mortar
- Investigating silver and alumina nanoparticles’ impact on fluid behavior over porous stretching surface
- Potential pharmaceutical applications and molecular docking study for green fabricated ZnO nanoparticles mediated Raphanus sativus: In vitro and in vivo study
- Effect of temperature and nanoparticle size on the interfacial layer thickness of TiO2–water nanofluids using molecular dynamics
- Characteristics of induced magnetic field on the time-dependent MHD nanofluid flow through parallel plates
- Flexural and vibration behaviours of novel covered CFRP composite joints with an MWCNT-modified adhesive
- Experimental research on mechanically and thermally activation of nano-kaolin to improve the properties of ultra-high-performance fiber-reinforced concrete
- Analysis of variable fluid properties for three-dimensional flow of ternary hybrid nanofluid on a stretching sheet with MHD effects
- Biodegradability of corn starch films containing nanocellulose fiber and thymol
- Toxicity assessment of copper oxide nanoparticles: In vivo study
- Some measures to enhance the energy output performances of triboelectric nanogenerators
- Reinforcement of graphene nanoplatelets on water uptake and thermomechanical behaviour of epoxy adhesive subjected to water ageing conditions
- Optimization of preparation parameters and testing verification of carbon nanotube suspensions used in concrete
- Max-phase Ti3SiC2 and diverse nanoparticle reinforcements for enhancement of the mechanical, dynamic, and microstructural properties of AA5083 aluminum alloy via FSP
- Advancing drug delivery: Neural network perspectives on nanoparticle-mediated treatments for cancerous tissues
- PEG-PLGA core–shell nanoparticles for the controlled delivery of picoplatin–hydroxypropyl β-cyclodextrin inclusion complex in triple-negative breast cancer: In vitro and in vivo study
- Conduction transportation from graphene to an insulative polymer medium: A novel approach for the conductivity of nanocomposites
- Review Articles
- Developments of terahertz metasurface biosensors: A literature review
- Overview of amorphous carbon memristor device, modeling, and applications for neuromorphic computing
- Advances in the synthesis of gold nanoclusters (AuNCs) of proteins extracted from nature
- A review of ternary polymer nanocomposites containing clay and calcium carbonate and their biomedical applications
- Recent advancements in polyoxometalate-functionalized fiber materials: A review
- Special contribution of atomic force microscopy in cell death research
- A comprehensive review of oral chitosan drug delivery systems: Applications for oral insulin delivery
- Cellular senescence and nanoparticle-based therapies: Current developments and perspectives
- Cyclodextrins-block copolymer drug delivery systems: From design and development to preclinical studies
- Micelle-based nanoparticles with stimuli-responsive properties for drug delivery
- Critical assessment of the thermal stability and degradation of chemically functionalized nanocellulose-based polymer nanocomposites
- Research progress in preparation technology of micro and nano titanium alloy powder
- Nanoformulations for lysozyme-based additives in animal feed: An alternative to fight antibiotic resistance spread
- Incorporation of organic photochromic molecules in mesoporous silica materials: Synthesis and applications
- A review on modeling of graphene and associated nanostructures reinforced concrete
- A review on strengthening mechanisms of carbon quantum dots-reinforced Cu-matrix nanocomposites
- Review on nanocellulose composites and CNFs assembled microfiber toward automotive applications
- Nanomaterial coating for layered lithium rich transition metal oxide cathode for lithium-ion battery
- Application of AgNPs in biomedicine: An overview and current trends
- Nanobiotechnology and microbial influence on cold adaptation in plants
- Hepatotoxicity of nanomaterials: From mechanism to therapeutic strategy
- Applications of micro-nanobubble and its influence on concrete properties: An in-depth review
- A comprehensive systematic literature review of ML in nanotechnology for sustainable development
- Exploiting the nanotechnological approaches for traditional Chinese medicine in childhood rhinitis: A review of future perspectives
- Twisto-photonics in two-dimensional materials: A comprehensive review
- Current advances of anticancer drugs based on solubilization technology
- Recent process of using nanoparticles in the T cell-based immunometabolic therapy
- Future prospects of gold nanoclusters in hydrogen storage systems and sustainable environmental treatment applications
- Preparation, types, and applications of one- and two-dimensional nanochannels and their transport properties for water and ions
- Microstructural, mechanical, and corrosion characteristics of Mg–Gd–x systems: A review of recent advancements
- Functionalized nanostructures and targeted delivery systems with a focus on plant-derived natural agents for COVID-19 therapy: A review and outlook
- Mapping evolution and trends of cell membrane-coated nanoparticles: A bibliometric analysis and scoping review
- Nanoparticles and their application in the diagnosis of hepatocellular carcinoma
- In situ growth of carbon nanotubes on fly ash substrates
- Structural performance of boards through nanoparticle reinforcement: An advance review
- Reinforcing mechanisms review of the graphene oxide on cement composites
- Seed regeneration aided by nanomaterials in a climate change scenario: A comprehensive review
- Surface-engineered quantum dot nanocomposites for neurodegenerative disorder remediation and avenue for neuroimaging
- Graphitic carbon nitride hybrid thin films for energy conversion: A mini-review on defect activation with different materials
- Nanoparticles and the treatment of hepatocellular carcinoma
- Special Issue on Advanced Nanomaterials and Composites for Energy Conversion and Storage - Part II
- Highly safe lithium vanadium oxide anode for fast-charging dendrite-free lithium-ion batteries
- Recent progress in nanomaterials of battery energy storage: A patent landscape analysis, technology updates, and future prospects
- Special Issue on Advanced Nanomaterials for Carbon Capture, Environment and Utilization for Energy Sustainability - Part II
- Calcium-, magnesium-, and yttrium-doped lithium nickel phosphate nanomaterials as high-performance catalysts for electrochemical water oxidation reaction
- Low alkaline vegetation concrete with silica fume and nano-fly ash composites to improve the planting properties and soil ecology
- Mesoporous silica-grafted deep eutectic solvent-based mixed matrix membranes for wastewater treatment: Synthesis and emerging pollutant removal performance
- Electrochemically prepared ultrathin two-dimensional graphitic nanosheets as cathodes for advanced Zn-based energy storage devices
- Enhanced catalytic degradation of amoxicillin by phyto-mediated synthesised ZnO NPs and ZnO-rGO hybrid nanocomposite: Assessment of antioxidant activity, adsorption, and thermodynamic analysis
- Incorporating GO in PI matrix to advance nanocomposite coating: An enhancing strategy to prevent corrosion
- Synthesis, characterization, thermal stability, and application of microporous hyper cross-linked polyphosphazenes with naphthylamine group for CO2 uptake
- Engineering in ceramic albite morphology by the addition of additives: Carbon nanotubes and graphene oxide for energy applications
- Nanoscale synergy: Optimizing energy storage with SnO2 quantum dots on ZnO hexagonal prisms for advanced supercapacitors
- Aging assessment of silicone rubber materials under corona discharge accompanied by humidity and UV radiation
- Tuning structural and electrical properties of Co-precipitated and Cu-incorporated nickel ferrite for energy applications
- Sodium alginate-supported AgSr nanoparticles for catalytic degradation of malachite green and methyl orange in aqueous medium
- An environmentally greener and reusability approach for bioenergy production using Mallotus philippensis (Kamala) seed oil feedstock via phytonanotechnology
- Micro-/nano-alumina trihydrate and -magnesium hydroxide fillers in RTV-SR composites under electrical and environmental stresses
- Mechanism exploration of ion-implanted epoxy on surface trap distribution: An approach to augment the vacuum flashover voltages
- Nanoscale engineering of semiconductor photocatalysts boosting charge separation for solar-driven H2 production: Recent advances and future perspective
- Excellent catalytic performance over reduced graphene-boosted novel nanoparticles for oxidative desulfurization of fuel oil
- Special Issue on Advances in Nanotechnology for Agriculture
- Deciphering the synergistic potential of mycogenic zinc oxide nanoparticles and bio-slurry formulation on phenology and physiology of Vigna radiata
- Nanomaterials: Cross-disciplinary applications in ornamental plants
- Special Issue on Catechol Based Nano and Microstructures
- Polydopamine films: Versatile but interface-dependent coatings
- In vitro anticancer activity of melanin-like nanoparticles for multimodal therapy of glioblastoma
- Poly-3,4-dihydroxybenzylidenhydrazine, a different analogue of polydopamine
- Chirality and self-assembly of structures derived from optically active 1,2-diaminocyclohexane and catecholamines
- Advancing resource sustainability with green photothermal materials: Insights from organic waste-derived and bioderived sources
- Bioinspired neuromelanin-like Pt(iv) polymeric nanoparticles for cancer treatment
- Special Issue on Implementing Nanotechnology for Smart Healthcare System
- Intelligent explainable optical sensing on Internet of nanorobots for disease detection
- Special Issue on Green Mono, Bi and Tri Metallic Nanoparticles for Biological and Environmental Applications
- Tracking success of interaction of green-synthesized Carbopol nanoemulgel (neomycin-decorated Ag/ZnO nanocomposite) with wound-based MDR bacteria
- Green synthesis of copper oxide nanoparticles using genus Inula and evaluation of biological therapeutics and environmental applications
- Biogenic fabrication and multifunctional therapeutic applications of silver nanoparticles synthesized from rose petal extract
- Metal oxides on the frontlines: Antimicrobial activity in plant-derived biometallic nanoparticles
- Controlling pore size during the synthesis of hydroxyapatite nanoparticles using CTAB by the sol–gel hydrothermal method and their biological activities
- Special Issue on State-of-Art Advanced Nanotechnology for Healthcare
- Applications of nanomedicine-integrated phototherapeutic agents in cancer theranostics: A comprehensive review of the current state of research
- Smart bionanomaterials for treatment and diagnosis of inflammatory bowel disease
- Beyond conventional therapy: Synthesis of multifunctional nanoparticles for rheumatoid arthritis therapy

