Abstract
In this article, nano-ZrSi2-modified phenolic (Ph) resin and nano-ZrSi2-modified quartz–phenolic (Q–Ph) composites are, respectively, prepared by resin casting and compression molding. The effect of nano-ZrSi2 on the thermal stability of Ph resin and the role of nano-ZrSi2 on the thermal reusability of Q–Ph composites are investigated by multiple thermal gravimetric analyses and mechanical tests. The strengthening mechanism of nano-ZrSi2 modification is investigated by the evolution of microstructure. The results show that the addition of nano-ZrSi2 enhances the thermal stability of Ph resin under repeated heating at 1,200°C in air. The enhancement in thermal stability of resin exhibits a positive effect on improving the thermal reusability of composites. Within the range of 20 repeated heating times, the flexural strength of nano-ZrSi2-modified composites is above 16.01 MPa, which is 163.8% higher than that of unmodified composites. The strengthening mechanism of nano-ZrSi2 is mainly in the inhibition of thermal oxidation and the reduction of microstructural defects during the repeated thermal environment.
1 Introduction
With the development of aerospace technology, especially hypersonic vehicle technology, the requirements for advanced thermal protection materials are constantly increasing [1,2]. Hypersonic vehicle has the characteristics of high velocity, long range, and quick response [1]. These require thermal protection materials to have corresponding characteristics, such as high-temperature resistance, low density, high strength, and manufacturability [3]. In addition, thermal protection materials need to be reusable to meet the requirements, such as air-breathing hypersonic vehicle that can not only cruise in the atmosphere at high speed but also pass through the atmosphere as a space transportation vehicle [1]. This also requires thermal protection materials that are resistant to oxidation and reusable in high-temperature oxygen-containing environments.
Typical inorganic nonmetallic reusable thermal protection materials mainly include ceramic matrix composites, C/C, C/SiC, SiC/SiC, and other materials, which have high thermal stability and chemical inertness [4,5]. However, the disadvantage of these materials is the complex preparation process. Reusable thermal protection materials require innovation. Adding nanoparticles can effectively improve the properties of materials [6,7], and many scholars have done related research in this regard. Eslami et al. [8] investigated the thermal, mechanical, and ablation properties of carbon fiber/phenolic (Ph) composites filled with multiwall carbon nanotubes (MWCNTs). The thermal stability and flexural properties of the nanocomposites increased by increasing the content of MWCNTs (wt% ≤1). MWCNTs in the composite formed a strong network char layer without any cracks or openings. Park et al. [9] studied the mechanical and thermal properties of graphite oxide (GO)–Ph composites with different sizes of GO. They concluded that the composites with larger sizes of GO particles typically exhibited better mechanical properties. The incorporation of GO particles significantly accelerated the crystallization process of the Ph resin matrix, and the thermal stability of composites was improved. Srikanth et al. [10] manufactured the high ablation resistance rayon-based carbon-fabric/Ph composites using nano-SiO2 powder as a filler material. Nano-silica-filled composite exhibited higher ablation resistance when compared to the unfilled composite. Asaro et al. [11] used mesoporous SiO2 particles as reinforcement of a Ph resin to develop new ablative materials. They concluded that the composite with 20% silica particles achieved the best performance because the mesoporous structure was responsible for the improved ablation performance. Duan et al. [12] studied the effects of borosilicate glass and polycarbosilane on the oxidation and ablation behaviors of carbon fiber/Ph resin composites. They found that the fillers changed the oxidation mechanism of the composite at temperatures above 1,000°C. This modification improved the strength of the composite after oxidation. Wang et al. [13] evaluated the effects of zirconium carbide content on thermal stability and ablation properties of carbon/Ph composites. They showed that increasing ZrC content could lead to an evident increase in char yield but an observable reduction in linear ablation rates and back-face temperatures because of the formation of the ZrO2 layer on the ablation surface. Chen et al. [14] introduced ZrB2 particles into Ph resin and prepared modified carbon–phenolic (C–Ph) composites with this resin as a matrix. ZrB2 could improve the thermal stability of Ph resin. The formation of ZrO2 and B2O3 could notably improve the ablation resistance of C–Ph composites during the ablation process.
In our previous study, ZrSi2 particles were used as the filler material in Ph resin and C–Ph composites [15,16]. The thermal stability of ZrSi2 to Ph resin under inert gas and the ablation resistance of ZrSi2 to C–Ph composite under oxyacetylene flame have been deeply studied and discussed. The introduced ZrSi2 particles reacted with pyrolytic volatiles derived from Ph resin, which could increase the char yield of Ph resin. The increased char yield, as well as the formations of SiO2 and ZrO2, could contribute to the improvement of ablation resistance for C–Ph composites. In addition, TiB2 particles were used as the filler material in Ph resin and C–Ph composites [17]. The effect of TiB2 on the thermal stability of Ph and the role of TiB2 on the high-temperature mechanical property of C–Ph composites have been investigated. At high temperatures, TiB2 particles reacted with oxygen or oxygen-containing molecules released by Ph pyrolysis. The residue of Ph after pyrolysis coated with glassy B2O3 and ceramic particles could form a new compact matrix. The new compact matrix and well-bonded interface ensured the improved mechanical properties of TiB2-modified C–Ph composites at high temperatures.
All these studies showed that the non-oxide ceramic particle-modified C–Ph composites performed well in single-use thermal environments. However, for the thermal protection requirements of reusable hypersonic vehicles, the thermal stability and thermal reusability of such materials under repeated heating have not been investigated.
In this article, we prepare modified Ph resin-based composites by nano-ZrSi2 particles as additives and quartz fibers as reinforcements. The aims of this article are: (i) to study the effect of nano-ZrSi2 on the thermal stability of Ph under repeated heating; (ii) to investigate the thermal reusability of nano-ZrSi2-modified quartz–phenolic (Z/Q–Ph) composites; and (iii) to study the microstructure evolution to understand the strengthening mechanism of nano-ZrSi2 during repeated thermal environment.
2 Experimental
2.1 Materials
Boron phenolic resin (Ph) solid content ≥ 60%, Shanxi Taihang Fire Resistant Polymer Co., Ltd. Quartz fiber fabric, surface density 840 ± 42 g/m2, Wuhan Xin Youtai Optoelectronic Technology Co., Ltd. Nano-ZrSi2 particles, purity ≥95%, Shanghai Aladdin Biochemical Technology Co., Ltd, particle size distribution, and micromorphology as shown in Figures 1 and 2.
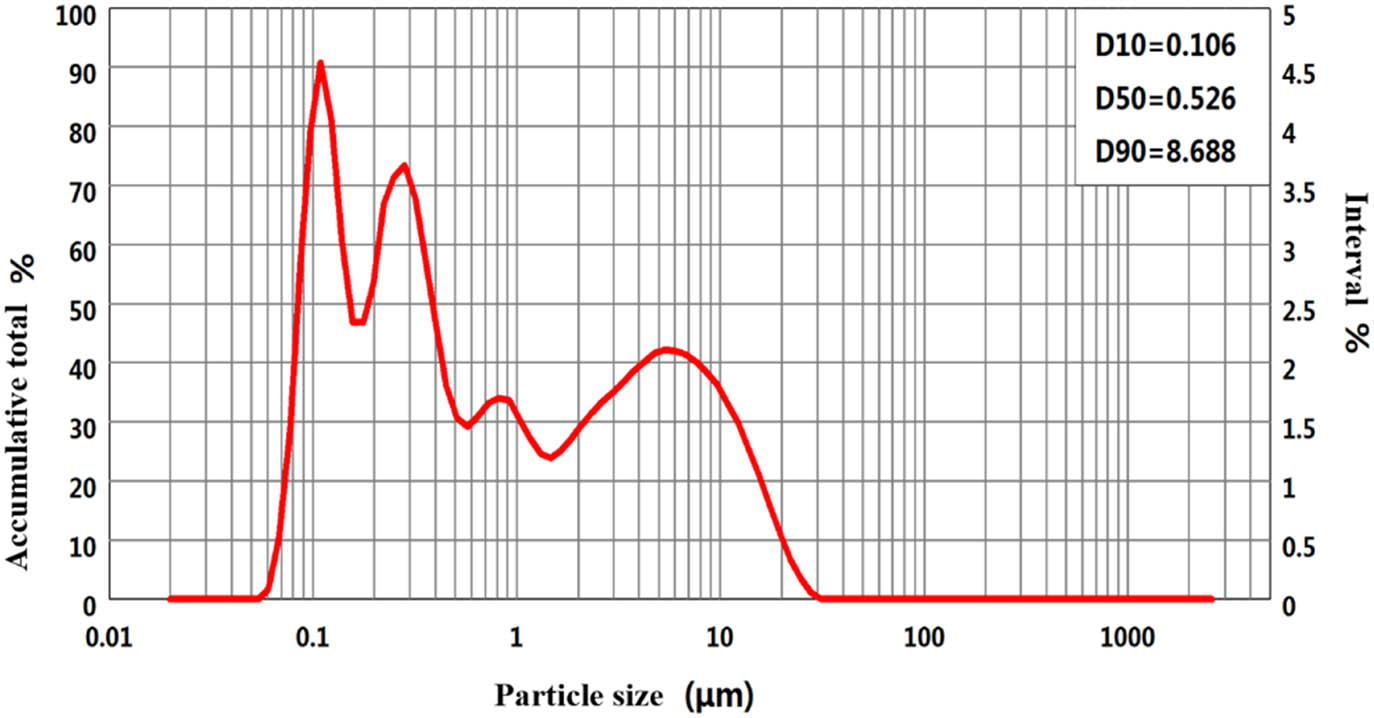
Particle size distribution of nano-ZrSi2 particles.
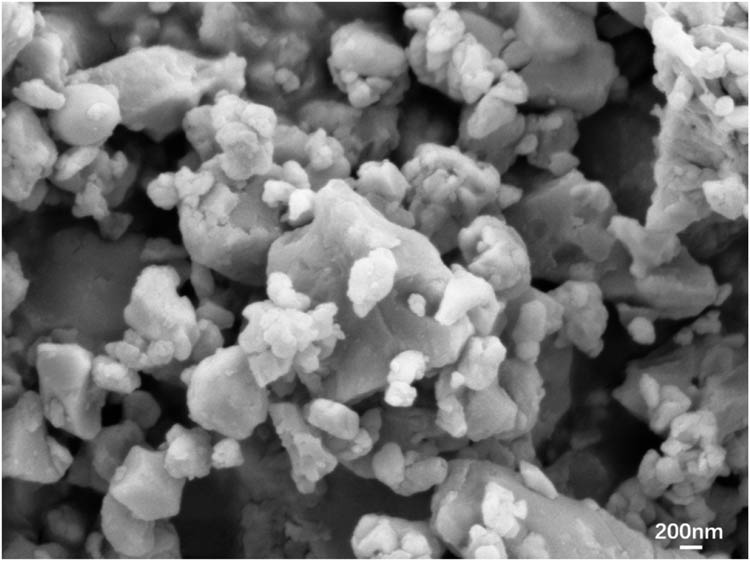
Scanning electron micrograph of nano-ZrSi2 particles.
2.2 Sample preparation
2.2.1 Fabrication process of Z/Ph resin
The preparation of nano-ZrSi2-modified phenolic (Z/Ph) resin was divided into two steps: glue preparation and resin casting process. First, nano-ZrSi2 particles were introduced into the Ph resin in the mass fraction of 60 wt% by mechanical mixing for 30 min. Then, the mixture was poured into a thin mold and cured in an oven at 180°C for 3 h to obtain nano-ZrSi2 modified Ph resin. The pure Ph resin was also fabricated into a sub-sample with the same fabrication process.
The two resin samples were subjected to repeated heat treatment at 1,200°C for 0–20 times. The repeated heat treatment process was as follows: put the sample into a muffle furnace at 1,200°C and the single heating time is 10 min. The whole process environment is an air atmosphere. The number of heat treatments is used as a suffix for the sample marking. For example, unheated modified Ph resin is marked as Z/Ph-0, unheated Ph resin is marked as Ph-0, modified Ph resin heated 20 times is marked as Z/Ph-20, and Ph resin heated 20 times is marked as Ph-20.
2.2.2 Fabrication process of Z/Q–Ph composite
The preparation of Z/Q–Ph composites was divided into three steps: glue preparation, prepreg preparation, and compression molding. First, the resin solution was prepared according to the proportion of Z/Ph resin. Then, the resin solution was painted on the quartz fiber fabric in a mass rate of 2:1, and the fabric was dried at room temperature to form a prepreg. Finally, the prepregs were laminated and compressed in a mold to form the Z/Q–Ph composite. The pure quartz–phenolic (Q–Ph) composite was also fabricated into a sub-sample with the same fabrication process. The curing process of all samples is shown in Figure 3.
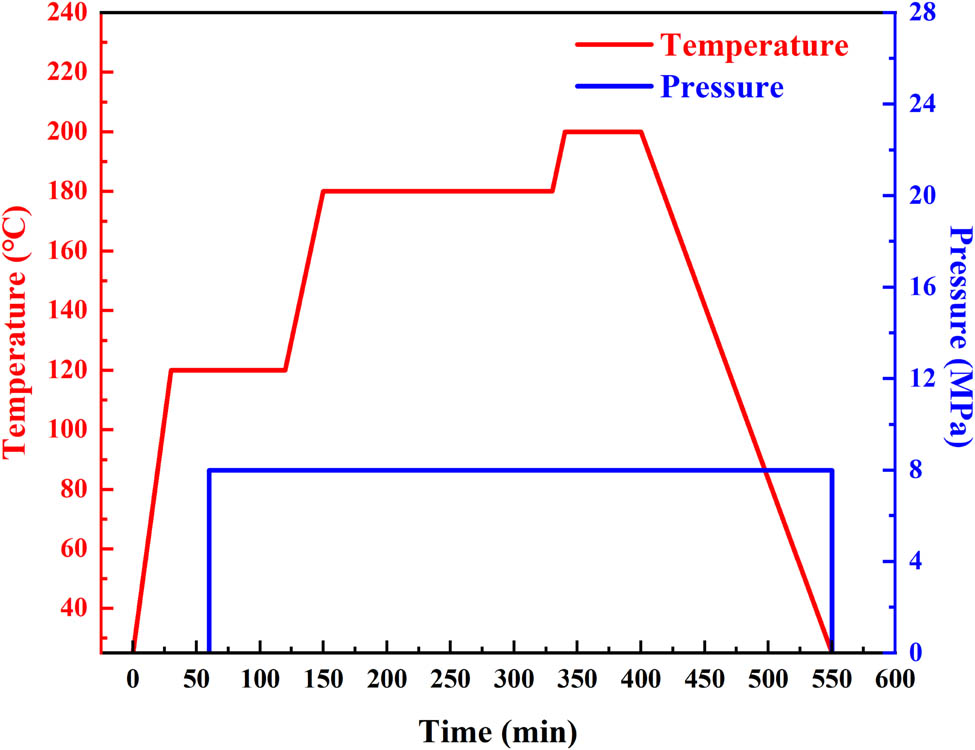
Curing curves of molding process.
The two composite samples were also subjected to repeated heat treatment at 1,200°C for 0–20 times. The repeated heat treatment process of composites is the same as that of resins. The number of heat treatments is used as a suffix for the sample marking. For example, unheated Z/Q–Ph composite material is marked as Z/Q–Ph-0, unheated Q–Ph composite material is marked as Q–Ph-0, modified Z/Q–Ph composite material heated 20 times is marked as Z/Ph-20, and Q–Ph composite material heated 20 times is marked as Q–Ph-20.
2.3 Characterization
The thermal stabilities of Ph resin and Z/Ph resin were characterized by thermal analyzer (model DSC8500). Thermal gravimetric tests were in an atmosphere of air with a heating rate of 20.0°C/min and a temperature range of 30–1,200°C. The mechanical properties of Q–Ph composites and Z/Q–Ph composites after different heating times were investigated by a universal machine (model Instron-1341). The flexural strength of the sample was tested concerning the standard GB/T1449-2005, and the loading rate was 2 mm/min. The microscopic morphologies of resins and composites after different heating times were tested using a scanning electron microscope (SEM) (model JSM-5610LV).
3 Results and discussion
3.1 Thermal stability of resin
Residual weight is an important indicator to measure the thermal stability of resin. Generally speaking, higher residual weight of resin means better thermal stability [18,19,20]. The thermal stabilities of pure Ph resin and Z/Ph resin in the air were characterized by thermogravimetric (TG) analysis. Figure 4 compares the TG curves of Ph resin and Z/Ph resin.
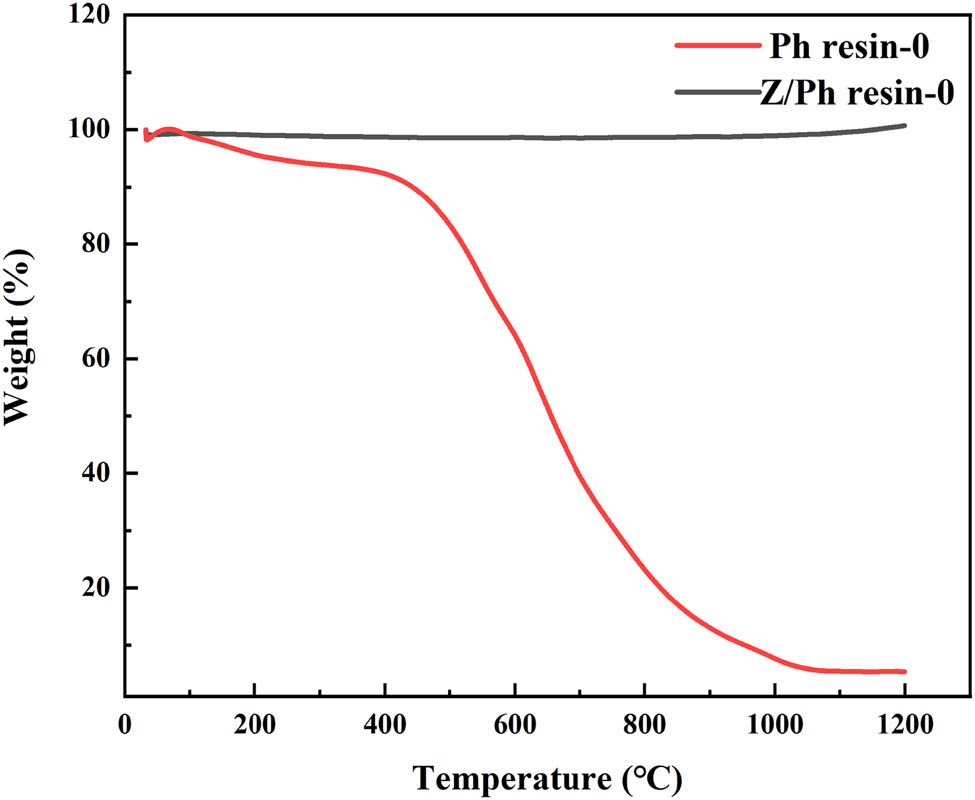
TG curves of Ph resin and Z/Ph resin.
As seen from the curve of Ph resin in Figure 4, the oxidative decomposition process of Ph resin is divided into three stages [21]. In the first stage of 30–350°C, the weight loss rate of Ph resin is 6.6%. It can be known that the weight loss of this part is not the decomposition of Ph, but the post-curing of Ph [22]. In the second stage of 350–850°C, the Ph resin shows a significant weight loss, reaching 76.3% by weight, which is due to the thermal oxidation of Ph resin [23]. In the third stage of 850–1,200°C, the weight loss rate of Ph resin is 11.8%, which is mainly caused by the further pyrolysis of the benzene ring [24]. At 1,200°C, the residual weight rate of Ph resin is 5.3%. Unlike Ph resin, the TG curve of Z/Ph resin remains almost horizontal. Moreover, the weight of Z/Ph resin even increases slightly above 1,000°C, and the residual weight rate of Z/Ph resin at 1,200°C is 100.7%. This is mainly due to the oxidation reaction of nano-ZrSi2 itself, which increases the residual weight of Z/Ph resin. In addition, oxidation products of nano-ZrSi2 have protective effects on the Ph resin, thereby inhibiting the thermal oxidation reaction of Ph resin during the heating process [25].
To further study the effect of nano-ZrSi2 on the thermal stability of Ph under repeated heating, the Z/Ph resin samples treated with different heating times are measured again by TG. Figure 5 shows the TG curves of Z/Ph resin after 0, 1, 4, and 8 heating times.
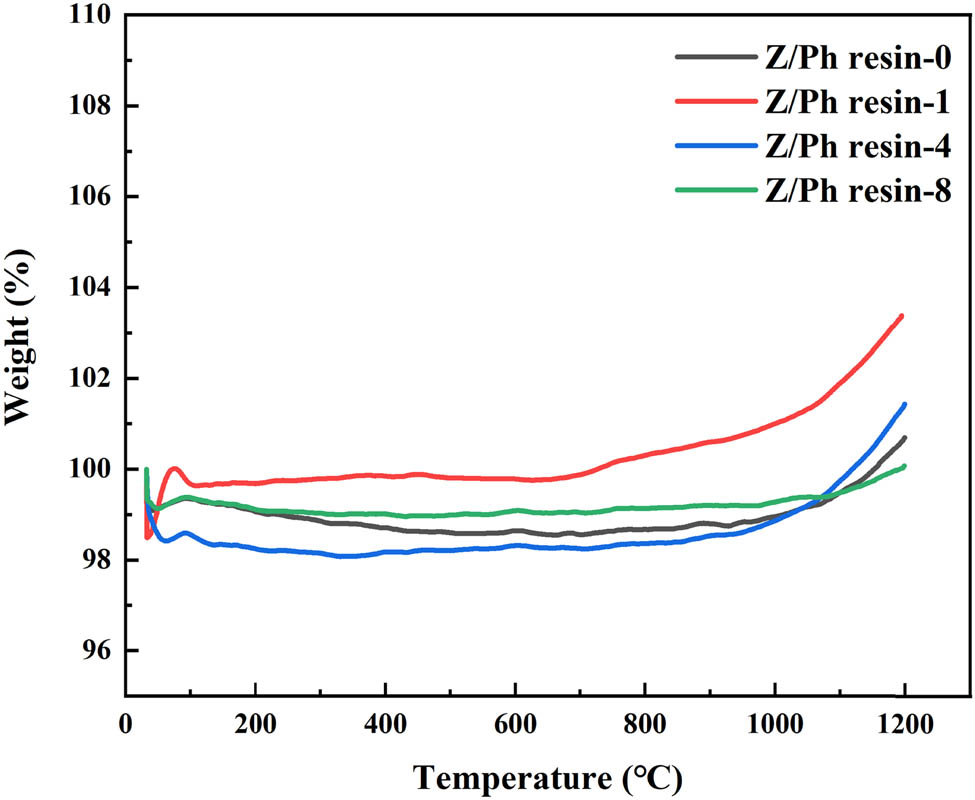
TG curves of Z/Ph resins after different heating times.
As shown in Figure 5, the TG curves of Z/Ph resin after different heating times tend to be stable gradually. The weight change process of Z/Ph resin is mainly divided into two stages. In the first stage of 30–1,000°C, the residual weights of Z/Ph-0, Z/Ph-1, Z/Ph-4, and Z/Ph-8 have various changes. At 1,000°C, the residual weight rates of above four samples are 99, 101, 98.9, and 99.3%, respectively. Notably, Z/Ph-1 shows no weight loss but weight gain. This is because one heating time is relatively short. Nano-ZrSi2 particles are not sufficiently oxidized, and thus, weight gain occurs. With the increase in heating times, nano-ZrSi2 particles are fully oxidized at high temperature [26,27,28], and the TG curves of samples gradually maintain a horizontal state. In the second stage of 1,000–1,200°C, the residual weights of four samples increase slightly, which is the result of the nanoparticles oxidation at elevated temperature. At 1,200°C, the residual weight rates of four samples are 100.7, 103.4, 101.4, and 100.1%. In both stages, the change range of residual weight rates is within 4%. This shows that the modified Ph resin exhibits excellent thermal reusability in the range below 1,200°C.
3.2 Microstructural evolution of resin
The microstructural evolution of resin under repeated thermal environment is investigated by SEM. Figure 6 shows the microstructures of Ph resin and Z/Ph resin after different heating times at 1,200°C.
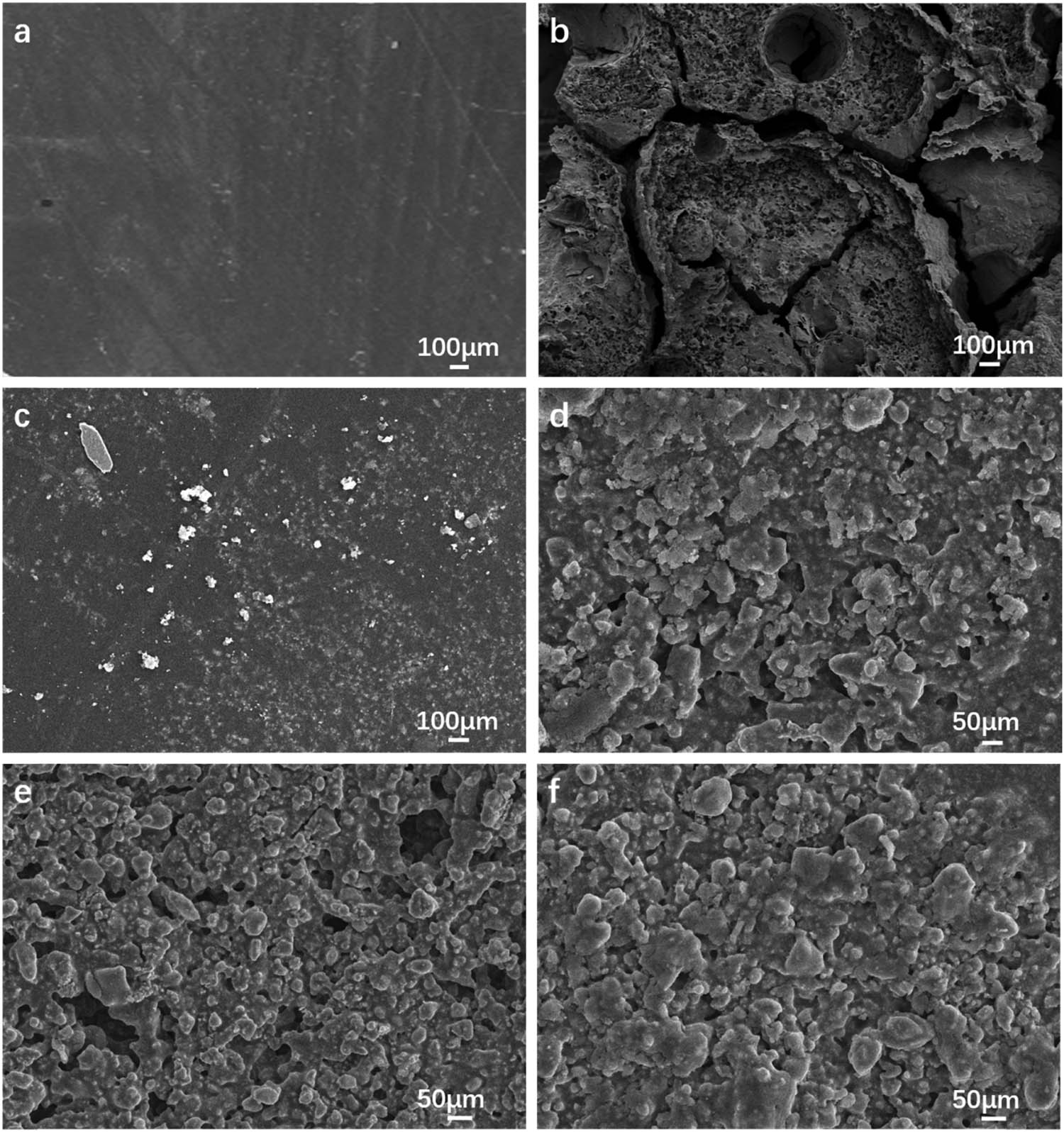
Micromorphology of Ph resin and Z/Ph resin after different heating times (a) Ph-0, (b) Ph-1, (c) Z/Ph-0, (d) Z/Ph-1, (e) Z/Ph-4, and (f) Z/Ph-8.
The microstructure of Ph resin is shown in Figure 6(a), and that after 1 heating at 1,200°C is shown in Figure 6(b). As seen in Figure 6(a) and (b), the resin surface has evolved from defect free to many voids, cracks, and other defects. It is well known that thermal oxidation of carbon materials starts at 450°C, and Ph resin is easily oxidized and destroyed in high-temperature air environment [29,30,31,32,33]. Therefore, the thermal degradation of Ph resin at 1,200°C is serious.
The microstructure of cured Z/Ph resin is shown in Figure 6(c), and those after 1, 4, and 8 heating times at 1,200°C are shown in Figure 6(d)–(f), respectively. As shown in Figure 6(c), nanoparticles are relatively uniformly dispersed in the modified resin, and the surface of modified resin has almost no defects. As shown in Figure 6(d), the nano-ZrSi2-modified resin is transformed into ceramics with layered structure after 1 heating. Due to the ceramization of modified resin, the surface defects of Z/Ph resin have been greatly reduced compared with those of heated Ph resin under the same condition. As shown in Figure 6(e), a few voids appear on the surface of Z/Ph resin after 4 heating times. However, there are no defects, such as cracking and large-area voids like heated Ph resin after heating. As shown in Figure 6(f), the compactness of sheet structure ceramic is improved, and the surface has few voids. The surface after 8 heating times has a healing phenomenon compared with the surface after 4 heating times.
With the increase in heating times, the surface of Z/Ph resin undergoes an evolution process from relatively dense to defective and then to relatively dense. The surfaces of all heated Z/Ph resin do not have defects, such as cracks and large-sized voids on the surface of heated Ph resin. Nano-ZrSi2 particles induce the ceramization of modified resin during heating, which plays a good role in the microstructure protection of Ph resin under high temperature and repeated high-temperature heating.
3.3 Thermal reusability of composites
To explore the thermal reusability of composites, the mechanical properties of samples after different heating times are tested. Composite samples are put into a furnace at 1,200°C for high-temperature treatment for 1, 2, 4, 6, 8, 10, 12, 16, 18, and 20 times. Each heating time is 10 min. After heat treatment, the flexural strength of each composite sample is tested, and the results are shown in Figure 7.
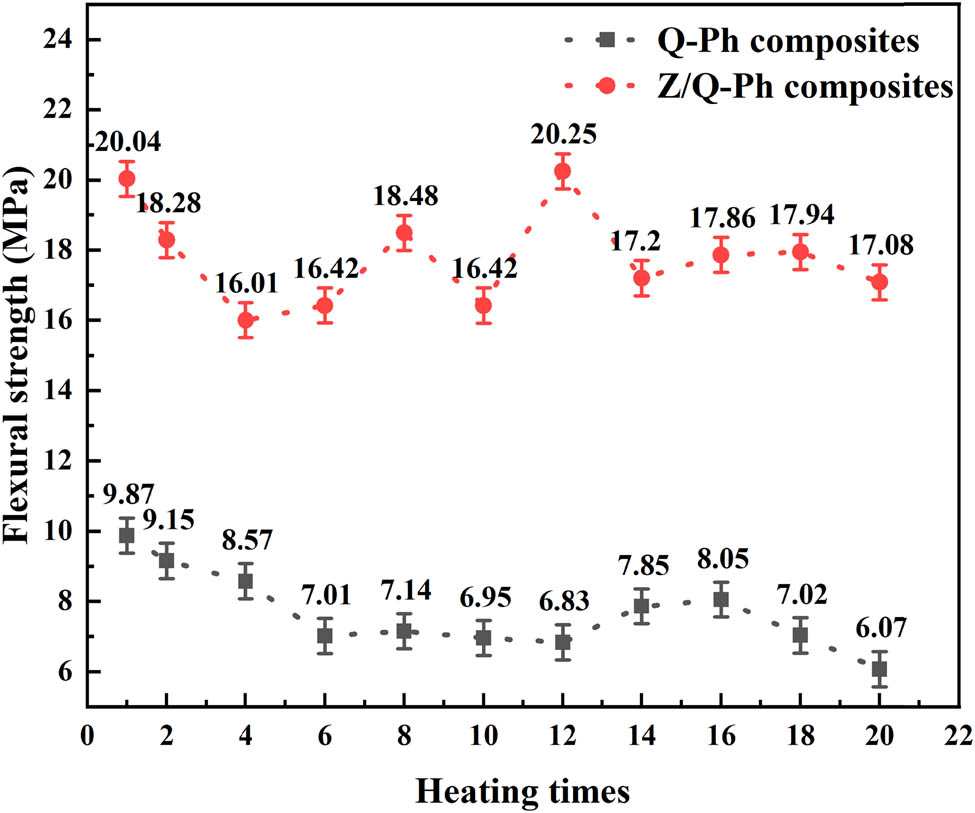
Mechanical properties of Q–Ph composites and Z/Q–Ph composites after different heating times.
After 1 heating, the flexural strength of Q–Ph composites is 9.87 MPa. With the increase in heating times, the flexural strength of Q–Ph composites decreases gradually. Although between 12 and 16 times, the flexural strength of Q–Ph composites recovers slightly. In the range of 1–20 times of heating, the flexural strength of Q–Ph composites shows a downward trend in general, and the lowest value of flexural strength appears after 20 times of heating, which is only 6.07 MPa.
The flexural strength of Q–Ph composites increases significantly after nano-ZrSi2 modification. After 1 heating, the flexural strength of the Z/Q–Ph composites is 20.04 MPa, which is 103.04% higher than that of Q–Ph composites under the same condition. With the increase in heating times, the flexural strength of Z/Q–Ph composites first decreases, then increases, and finally stabilizes overall. In the range of 1–20 times of heating, the lowest flexural strength of Z/Q–Ph composites appears after 4 times of heating, and its value is 16.01 MPa. After 20 times of heating, the flexural strength of Z/Q–Ph composites is 17.08 MPa.
In the range of 20 repeated heating, the flexural strength of Q–Ph composites is 6.08–9.87 MPa, while that of Z/Q–Ph composites is 16.01–20.04 MPa. Calculated at the lowest value, the flexural strength of Z/Q–Ph composites is 163.8% higher than that of pure Q–Ph composites. It can be concluded that the mechanical properties of Q–Ph composites after heating at 1,200°C are effectively improved with the addition of nano-ZrSi2. And the mechanical properties of Z/Q–Ph composites do not decrease significantly after repeated heating.
3.4 Enhancement mechanism of nano-ZrSi2 on composites
To explore the strengthening mechanism of nano-ZrSi2 on the heated composites, macromorphology, micromorphology, and element distribution of composites are studied. Photographs of Q–Ph samples and Z/Q–Ph samples after different heating times are shown in Figure 8.
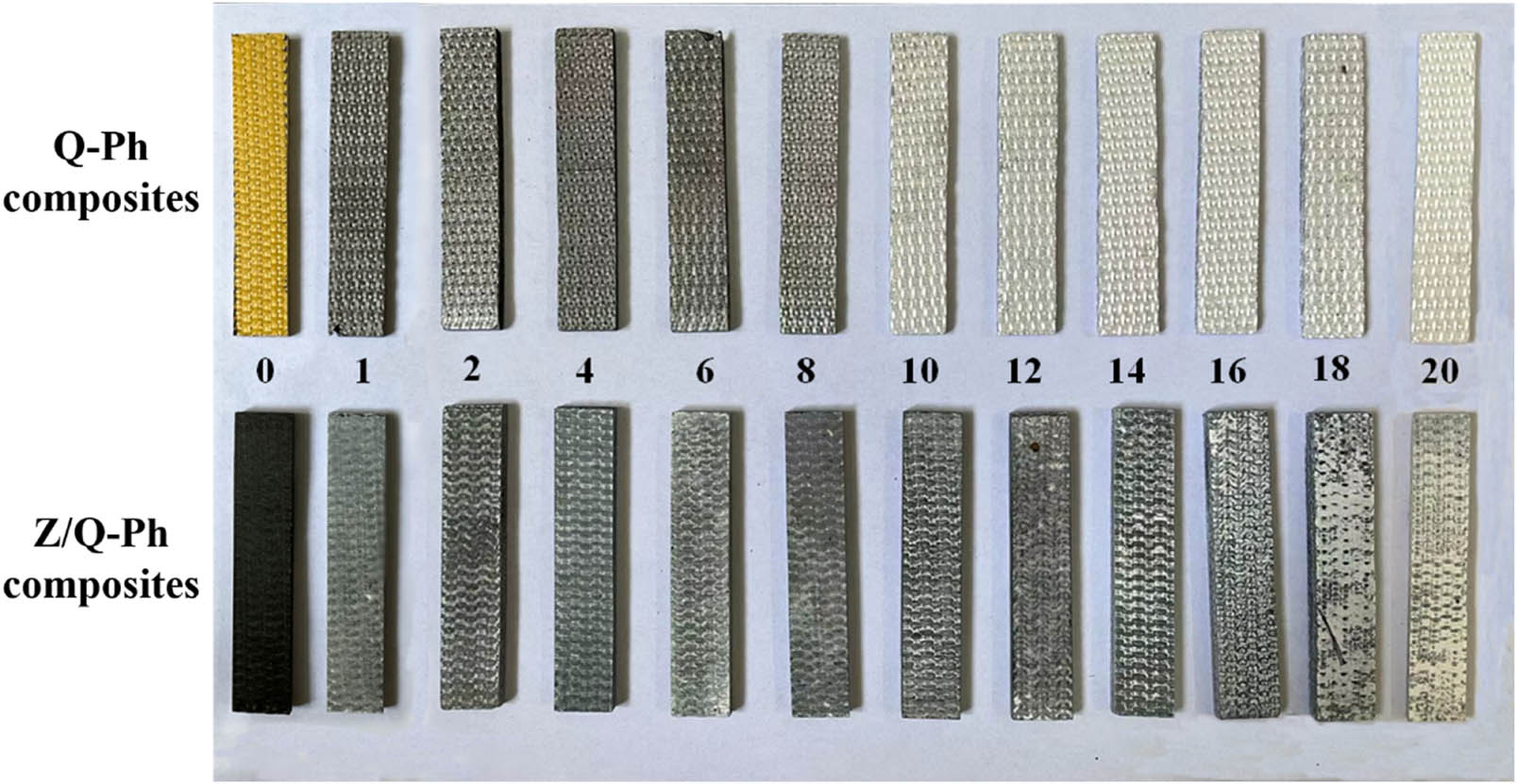
Photographs of Q–Ph composites and Z/Q–Ph composites after different heating times.
As shown in the photographs of Q–Ph composites, the surface of Q–Ph composites changes from the yellow of Ph resin to the white of quartz fibers with the increase of heating times. Due to the high-temperature oxidation of Ph resin, almost no residual Ph resin can be seen on the surface, and only the quartz fibers are exposed. As shown in the photographs of Z/Q–Ph composites, the surface of Z/Q–Ph composites changes slowly from the black of modified resin to a dark gray like a ceramic with the increase in heating times. Due to the protection of nano-ZrSi2, the surface of appears firm without exposed white fibers.
The cross-sectional microstructure of heated composites and the elemental distribution of typical area are shown in Figure 9.
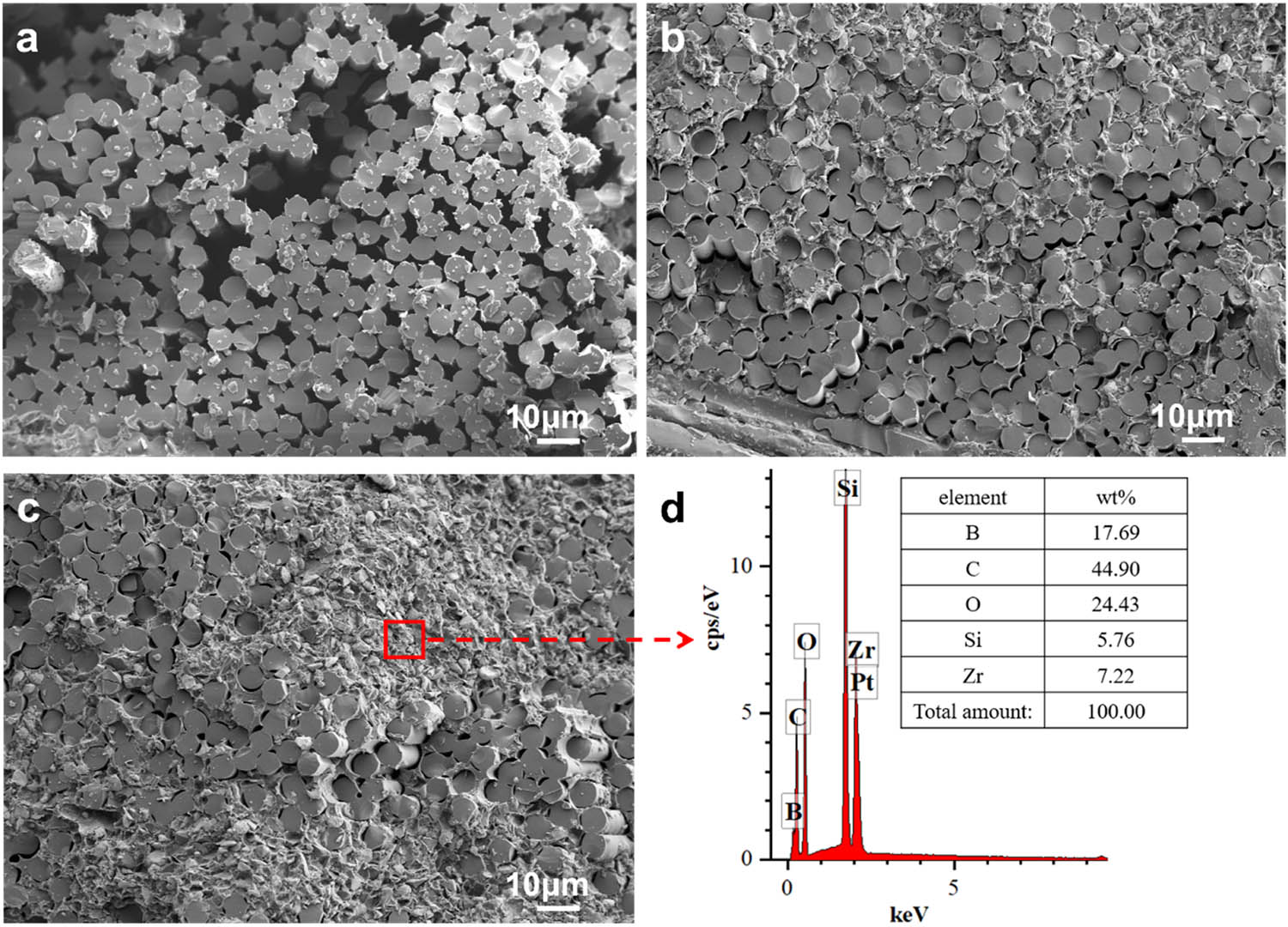
Cross-sectional micromorphology of heated composites. (a) Q–Ph-4, (b) Z/Q–Ph-4, (c) Z/Q–Ph-8, and (d) ceramic layer EDS of Z/Q–Ph-8.
As shown in Figure 9(a), large voids and loose fiber bundles appear on the cross-section of Q–Ph-4 composites. The generation of such defects is due to the thermal oxidation of Ph resin matrix. During the repeated thermal oxidation, the matrix has been exhausted, and the fibers on the cross-section are not bound together. As shown in Figure 9(b), the defects on the cross-section of Z/Q–Ph-4 composites are greatly reduced. No obvious large voids are seen in Figure 9(b), and the fibers are bound firmly with the generated ceramic matrix. As shown in Figure 9(c), the dense ceramic matrix can be clearly observed on the cross-section of Z/Q–Ph-8 composites. As shown in Figure 9(d), elements B, C, O, Si, and Zr exist in the EDS analysis of the ceramic matrix. Notably, the molar ratio of O to Si and Zr is approximately equal to 2. It can be considered that nano-ZrSi2 particles are oxidized to form SiO2 and ZrO2 with heating under air atmosphere, which has been proved in previous studies [15,16]. That is, the ceramic matrix mainly composed of SiO2, ZrO2, C, and B is formed in an air atmosphere at 1,200°C and is stable during the repeated heating process.
The strengthening mechanism of nano-ZrSi2 on the heated composites can be analyzed as follows: without the introduction of nano-ZrSi2, pure Ph resin matrix is easy to be oxidized and eroded during heating, which seriously damages the interface of heated Q–Ph composites. When the heated sample is under load, it can only be supported by the quartz fibers alone, without the assistance of the matrix and the effect of interfacial stress transfer. Thus, the heated Q–Ph composites have very low mechanical properties. With the introduction of nano-ZrSi2, nanoparticles inhibit the oxidation of Ph resin matrix through self-oxidation at high temperatures. After 1 heating, the modified Ph resin matrix is transformed into a ceramic matrix containing oxidation products of nanoparticles and residual products of resin, and the carbon in the resin is well preserved. The ceramic matrix holds the fibers firmly in place and binds the quartz fibers together. When the heated Z/Q–Ph composite material is under load, both the fibers and the matrix bear the stress, with the quartz fibers as the main and the ceramic matrix as the auxiliary. The tight interfacial bond ensures the transfer of stress between the fibers and the matrix. This results in the improved mechanical properties of heated Z/Q–Ph composites. Moreover, the ceramic matrix is stable at high temperatures, which ensures that the load-bearing state of modified composites is not affected with the increase in heating times. Therefore, Z/Q–Ph composites exhibit significant mechanical properties under repeated heating environments.
4 Conclusion
Z/Q–Ph composites are prepared with Z/Ph resin as matrix and quartz fiber as reinforcement. The thermal stability of resin is characterized by multiple TG analyses, and the thermal reusability of composites is characterized by multiple mechanical tests. Their microstructural evolution is characterized by SEM. The main conclusions are as follows:
The introduction of nano-ZrSi2 significantly improves the thermal stability of Ph resin in air. At 1,200°C, the residual weight rate of Z/Ph resin is 100.7%, while that of Ph resin is only 5.3%. And Z/Ph resin shows excellent thermal stability under repeated heating. In the range of eight repeated heating, Z/Ph resin shows a small change range of residual weight rates, all within 4%.
The introduction of nano-ZrSi2 significantly improves the flexural strength of Q–Ph composites under repeated heating environment. In the range of 20 repeated heating, the flexural strength of Z/Q–Ph composites is above 16.01 MPa. Calculated at the lowest value, the flexural strength of Z/Q–Ph composites is 163.8% higher than that of pure Q–Ph composites. Z/Q–Ph composites have excellent thermal reusability.
The introduction of nano-ZrSi2 significantly reduces the microstructural defects of all heated samples. In addition, Z/Ph resin matrix can be transformed into a new ceramic matrix after heating in air. The ceramic matrix is relatively stable during repeated heating, which provides the basis for the thermal stability of resin and the thermal reusability of composites under repeated thermal environment.
-
Funding information: Financial support was received from the Fundamental Research Funds for the Central Universities, China (WUT: 2021III003XZ).
-
Author contributions: All authors have accepted responsibility for the entire content of this article and approved its submission.
-
Conflict of interest: The authors state no conflict of interest.
References
[1] Ding YB, Yue XK, Chen GS, Si JS. Review of control and guidance technology on hypersonic vehicle. Chin J Aeronautics Forthcom. 2021;35:1–18.10.1016/j.cja.2021.10.037Search in Google Scholar
[2] Voland RT, Huebner LD, McClinton CR. X-43A Hypersonic vehicle technology development. Acta Astronautica. 2006;59(1–5):181–91.10.1016/j.actaastro.2006.02.021Search in Google Scholar
[3] Ahmad S, Ali S, Salman M, Baluch AH. A comparative study on the effect of carbon-based and ceramic additives on the properties of fiber reinforced polymer matrix composites for high temperature applications. Ceram Int. 2021;47(24):33956–71.10.1016/j.ceramint.2021.08.356Search in Google Scholar
[4] Uyanna O, Najafi H. Thermal protection systems for space vehicles: A review on technology development, current challenges and future prospects. Acta Astronautica. 2020;176:341–56.10.1016/j.actaastro.2020.06.047Search in Google Scholar
[5] Baiocco P. Overview of reusable space systems with a look to technology aspects. Acta Astronautica. 2021;189:10–25.10.1016/j.actaastro.2021.07.039Search in Google Scholar
[6] Bi W, Li H, Hui D, Gaff M, Lorenzo R, Corbi I, et al. Effects of chemical modification and nanotechnology on wood properties. Nanotechnol Rev. 2021;10(1):978–1008.10.1515/ntrev-2021-0065Search in Google Scholar
[7] Cui LN, Huang CY, Xia H, Qiu YP, Ni QQ. Transparent ultraviolet-shielding composite films made from dispersing pristine zinc oxide nanoparticles in low-density polyethylene. Nanotechnol Rev. 2020;9(1):1368–80.10.1515/ntrev-2020-0099Search in Google Scholar
[8] Eslami Z, Yazdani F, Mirzapour MA. Thermal and mechanical properties of phenolic-based composites reinforced by carbon fibres and multiwall carbon nanotubes. Compos Part A Appl Sci Manuf. 2015;72:22–31.10.1016/j.compositesa.2015.01.015Search in Google Scholar
[9] Park JM, Kwon DJ, Wang ZJ, Gu GY, DeVries KL. Interfacial, fire retardancy, and thermal stability evaluation of graphite oxide (GO)-phenolic composites with different GO particle sizes. Compos Part B Eng. 2013;53:290–6.10.1016/j.compositesb.2013.04.063Search in Google Scholar
[10] Srikanth I, Daniel A, Kumar S, Padmavathi N, Singh V, Ghosal P, et al. Nano silica modified carbon-phenolic composites for enhanced ablation resistance. Scr Mater. 2010;63(2):200–3.10.1016/j.scriptamat.2010.03.052Search in Google Scholar
[11] Asaro L, Manfredi LB, Pellice SA, Procaccini R, Rodriguez ES. Innovative ablative fire resistant composites based on phenolic resins modified with mesoporous silica particles. Polym Degrad Stab. 2017;144:7–16.10.1016/j.polymdegradstab.2017.07.023Search in Google Scholar
[12] Duan LY, Zhao X, Wang YG. Oxidation and ablation behaviors of carbon fiber/phenolic resin composites modified with borosilicate glass and polycarbosilane interface. J Alloy Compd. 2020;827:154277.10.1016/j.jallcom.2020.154277Search in Google Scholar
[13] Wang S, Huang HM, Tian Y. Effects of zirconium carbide content on thermal stability and ablation properties of carbon/phenolic composites. Ceram Int. 2019;46(4):4307–13.10.1016/j.ceramint.2019.10.152Search in Google Scholar
[14] Chen YX, Chen P, Hong CQ, Zhang BX, Hui D. Improved ablation resistance of carbon–phenolic composites by introducing zirconium diboride particles. Compos Part B Eng. 2013;47:320–5.10.1016/j.compositesb.2012.11.007Search in Google Scholar
[15] Ding J, Huang ZX, Qin Y, Shi MX, Huang C, Mao JW. Improved ablation resistance of carbon– phenolic composites by introducing zirconium silicide particles. Compos Part B Eng. 2015;82:100–7.10.1016/j.compositesb.2015.08.023Search in Google Scholar
[16] Ding J, Yang T, Huang ZX, Qin Y, Wang YB. Thermal stability and ablation resistance, and ablation mechanism of carbon–phenolic composites with different zirconium silicide particle loadings. Compos Part B Eng. 2018;154:313–20.10.1016/j.compositesb.2018.07.057Search in Google Scholar
[17] Ding J, Sun JM, Huang ZX, Wang YB. Improved high-temperature mechanical property of carbon-phenolic composites by introducing titanium diboride particles. Compos Part B Eng. 2019;157:289–94.10.1016/j.compositesb.2018.08.124Search in Google Scholar
[18] Mirzapour A, Asadollahi MA, Baghshaei S, Akbari M. Effect of nanosilica on the microstructure, thermal properties and bending strength of nanosilica modified carbon fiber/phenolic nanocomposite. Compos Part A Appl Sci Manuf. 2014;63:159–67.10.1016/j.compositesa.2014.04.009Search in Google Scholar
[19] Wang SJ, Xing XL, Wang YN, Wang W, Jing XL. Influence of poly (dihydroxybiphenyl borate) on the curing behaviour and thermal pyrolysis mechanism of phenolic resin. Polym Degrad Stab. 2017;144:378–91.10.1016/j.polymdegradstab.2017.08.034Search in Google Scholar
[20] Zhong YH, Jing XL, Wang SJ, Jia QX. Behavior investigation of phenolic hydroxyl groups during the pyrolysis of cured phenolic resin via molecular dynamics simulation. Polym Degrad Stab. 2016;125:97–104.10.1016/j.polymdegradstab.2015.11.017Search in Google Scholar
[21] Rao MPR, Rao BSM, Rajan CR, Ghadage RS. Thermal degradation kinetics of phenol – crotonaldehyde resins. Polym Degrad Stab. 1998;61(2):283–8.10.1016/S0141-3910(97)00210-3Search in Google Scholar
[22] Torres-Herrador F, Eschenbacher A, Coheur J, Blondeau J, Magin TE, Van Geem KM. Decomposition of carbon/phenolic composites for aerospace heatshields: Detailed speciation of phenolic resin pyrolysis products. Aerosp Sci Technol. 2021;119:107079.10.1016/j.ast.2021.107079Search in Google Scholar
[23] Brezinsky K, Pecullan M, Glassman I. Pyrolysis and oxidation of phenol. J Phys Chem A. 1998;102(44):8614–9.10.1021/jp982177+Search in Google Scholar
[24] Park BD, Kadla JF. Thermal degradation kinetics of resole phenol-formaldehyde resin/multi-walled carbon nanotube/cellulose nanocomposite. Thermochim Acta. 2012;540:107–15.10.1016/j.tca.2012.04.021Search in Google Scholar
[25] Ding J, Qin ZY, Luo HT, Yang W, Wang YB, Huang ZX. Nano-silica modified phenolic resin film: manufacturing and properties. Nanotechnol Rev. 2020;9(1):209–18.10.1515/ntrev-2020-0018Search in Google Scholar
[26] Yeom H, Maier B, Mariani R, Bai D, Sirdharan K. Evolution of multilayered scale structures during high temperature oxidation of ZrSi2. J Mater Res. 2016;31(21):3409–19.10.1557/jmr.2016.363Search in Google Scholar
[27] Perez P. Oxidation behavior of Al-Alloyed ZrSi2. Oxid Met. 2001;56(1–2):163–76.10.1023/A:1010317510122Search in Google Scholar
[28] Wang SZ, Zhou SF, Huang J, Zhao GZ, Liu YQ. Attaching ZrO2 nanoparticles onto the surface of graphene oxide via electrostatic self-assembly for enhanced mechanical and tribological performance of phenolic resin composites. J Mater Sci. 2019;54(11):8247–61.10.1007/s10853-019-03512-wSearch in Google Scholar
[29] Costa L, Montelera LRD, Camino G, Weil ED, Pearce EM. Structure-charring relationship in phenol-formaldehyde type resins. Polym Degrad Stab. 1997;56(1):23–35.10.1016/S0141-3910(96)00171-1Search in Google Scholar
[30] Wang JG, Jiang HY, Jiang N. Study on the pyrolysis of phenol-formaldehyde (PF) resin and modified PF resin. Thermochim Acta. 2009;496(1–2):136–42.10.1016/j.tca.2009.07.012Search in Google Scholar
[31] Zhang Y, Xu Y, Gao L, Zhang L, Cheng L. Microstructural evolution of phenolic resin-based carbon/carbon composites during pyrolysis. Acta Materiae Compositae Sin. 2006;23(1):37–43.Search in Google Scholar
[32] Ko TH, Kuo WS, Chang YH. Microstructural changes of phenolic resin during pyrolysis. J Appl Polym Sci. 2010;81(5):1084–9.10.1002/app.1530Search in Google Scholar
[33] Tzeng SS, Chr YG. Evolution of microstructure and properties of phenolic resin-based carbon/carbon composites during pyrolysis. Mater Chem & Phys. 2002;73(2–3):162–9.10.1016/S0254-0584(01)00358-3Search in Google Scholar
© 2022 Jie Ding et al., published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Research Articles
- Theoretical and experimental investigation of MWCNT dispersion effect on the elastic modulus of flexible PDMS/MWCNT nanocomposites
- Mechanical, morphological, and fracture-deformation behavior of MWCNTs-reinforced (Al–Cu–Mg–T351) alloy cast nanocomposites fabricated by optimized mechanical milling and powder metallurgy techniques
- Flammability and physical stability of sugar palm crystalline nanocellulose reinforced thermoplastic sugar palm starch/poly(lactic acid) blend bionanocomposites
- Glutathione-loaded non-ionic surfactant niosomes: A new approach to improve oral bioavailability and hepatoprotective efficacy of glutathione
- Relationship between mechano-bactericidal activity and nanoblades density on chemically strengthened glass
- In situ regulation of microstructure and microwave-absorbing properties of FeSiAl through HNO3 oxidation
- Research on a mechanical model of magnetorheological fluid different diameter particles
- Nanomechanical and dynamic mechanical properties of rubber–wood–plastic composites
- Investigative properties of CeO2 doped with niobium: A combined characterization and DFT studies
- Miniaturized peptidomimetics and nano-vesiculation in endothelin types through probable nano-disk formation and structure property relationships of endothelins’ fragments
- N/S co-doped CoSe/C nanocubes as anode materials for Li-ion batteries
- Synergistic effects of halloysite nanotubes with metal and phosphorus additives on the optimal design of eco-friendly sandwich panels with maximum flame resistance and minimum weight
- Octreotide-conjugated silver nanoparticles for active targeting of somatostatin receptors and their application in a nebulized rat model
- Controllable morphology of Bi2S3 nanostructures formed via hydrothermal vulcanization of Bi2O3 thin-film layer and their photoelectrocatalytic performances
- Development of (−)-epigallocatechin-3-gallate-loaded folate receptor-targeted nanoparticles for prostate cancer treatment
- Enhancement of the mechanical properties of HDPE mineral nanocomposites by filler particles modulation of the matrix plastic/elastic behavior
- Effect of plasticizers on the properties of sugar palm nanocellulose/cinnamon essential oil reinforced starch bionanocomposite films
- Optimization of nano coating to reduce the thermal deformation of ball screws
- Preparation of efficient piezoelectric PVDF–HFP/Ni composite films by high electric field poling
- MHD dissipative Casson nanofluid liquid film flow due to an unsteady stretching sheet with radiation influence and slip velocity phenomenon
- Effects of nano-SiO2 modification on rubberised mortar and concrete with recycled coarse aggregates
- Mechanical and microscopic properties of fiber-reinforced coal gangue-based geopolymer concrete
- Effect of morphology and size on the thermodynamic stability of cerium oxide nanoparticles: Experiment and molecular dynamics calculation
- Mechanical performance of a CFRP composite reinforced via gelatin-CNTs: A study on fiber interfacial enhancement and matrix enhancement
- A practical review over surface modification, nanopatterns, emerging materials, drug delivery systems, and their biophysiochemical properties for dental implants: Recent progresses and advances
- HTR: An ultra-high speed algorithm for cage recognition of clathrate hydrates
- Effects of microalloying elements added by in situ synthesis on the microstructure of WCu composites
- A highly sensitive nanobiosensor based on aptamer-conjugated graphene-decorated rhodium nanoparticles for detection of HER2-positive circulating tumor cells
- Progressive collapse performance of shear strengthened RC frames by nano CFRP
- Core–shell heterostructured composites of carbon nanotubes and imine-linked hyperbranched polymers as metal-free Li-ion anodes
- A Galerkin strategy for tri-hybridized mixture in ethylene glycol comprising variable diffusion and thermal conductivity using non-Fourier’s theory
- Simple models for tensile modulus of shape memory polymer nanocomposites at ambient temperature
- Preparation and morphological studies of tin sulfide nanoparticles and use as efficient photocatalysts for the degradation of rhodamine B and phenol
- Polyethyleneimine-impregnated activated carbon nanofiber composited graphene-derived rice husk char for efficient post-combustion CO2 capture
- Electrospun nanofibers of Co3O4 nanocrystals encapsulated in cyclized-polyacrylonitrile for lithium storage
- Pitting corrosion induced on high-strength high carbon steel wire in high alkaline deaerated chloride electrolyte
- Formulation of polymeric nanoparticles loaded sorafenib; evaluation of cytotoxicity, molecular evaluation, and gene expression studies in lung and breast cancer cell lines
- Engineered nanocomposites in asphalt binders
- Influence of loading voltage, domain ratio, and additional load on the actuation of dielectric elastomer
- Thermally induced hex-graphene transitions in 2D carbon crystals
- The surface modification effect on the interfacial properties of glass fiber-reinforced epoxy: A molecular dynamics study
- Molecular dynamics study of deformation mechanism of interfacial microzone of Cu/Al2Cu/Al composites under tension
- Nanocolloid simulators of luminescent solar concentrator photovoltaic windows
- Compressive strength and anti-chloride ion penetration assessment of geopolymer mortar merging PVA fiber and nano-SiO2 using RBF–BP composite neural network
- Effect of 3-mercapto-1-propane sulfonate sulfonic acid and polyvinylpyrrolidone on the growth of cobalt pillar by electrodeposition
- Dynamics of convective slippery constraints on hybrid radiative Sutterby nanofluid flow by Galerkin finite element simulation
- Preparation of vanadium by the magnesiothermic self-propagating reduction and process control
- Microstructure-dependent photoelectrocatalytic activity of heterogeneous ZnO–ZnS nanosheets
- Cytotoxic and pro-inflammatory effects of molybdenum and tungsten disulphide on human bronchial cells
- Improving recycled aggregate concrete by compression casting and nano-silica
- Chemically reactive Maxwell nanoliquid flow by a stretching surface in the frames of Newtonian heating, nonlinear convection and radiative flux: Nanopolymer flow processing simulation
- Nonlinear dynamic and crack behaviors of carbon nanotubes-reinforced composites with various geometries
- Biosynthesis of copper oxide nanoparticles and its therapeutic efficacy against colon cancer
- Synthesis and characterization of smart stimuli-responsive herbal drug-encapsulated nanoniosome particles for efficient treatment of breast cancer
- Homotopic simulation for heat transport phenomenon of the Burgers nanofluids flow over a stretching cylinder with thermal convective and zero mass flux conditions
- Incorporation of copper and strontium ions in TiO2 nanotubes via dopamine to enhance hemocompatibility and cytocompatibility
- Mechanical, thermal, and barrier properties of starch films incorporated with chitosan nanoparticles
- Mechanical properties and microstructure of nano-strengthened recycled aggregate concrete
- Glucose-responsive nanogels efficiently maintain the stability and activity of therapeutic enzymes
- Tunning matrix rheology and mechanical performance of ultra-high performance concrete using cellulose nanofibers
- Flexible MXene/copper/cellulose nanofiber heat spreader films with enhanced thermal conductivity
- Promoted charge separation and specific surface area via interlacing of N-doped titanium dioxide nanotubes on carbon nitride nanosheets for photocatalytic degradation of Rhodamine B
- Elucidating the role of silicon dioxide and titanium dioxide nanoparticles in mitigating the disease of the eggplant caused by Phomopsis vexans, Ralstonia solanacearum, and root-knot nematode Meloidogyne incognita
- An implication of magnetic dipole in Carreau Yasuda liquid influenced by engine oil using ternary hybrid nanomaterial
- Robust synthesis of a composite phase of copper vanadium oxide with enhanced performance for durable aqueous Zn-ion batteries
- Tunning self-assembled phases of bovine serum albumin via hydrothermal process to synthesize novel functional hydrogel for skin protection against UVB
- A comparative experimental study on damping properties of epoxy nanocomposite beams reinforced with carbon nanotubes and graphene nanoplatelets
- Lightweight and hydrophobic Ni/GO/PVA composite aerogels for ultrahigh performance electromagnetic interference shielding
- Research on the auxetic behavior and mechanical properties of periodically rotating graphene nanostructures
- Repairing performances of novel cement mortar modified with graphene oxide and polyacrylate polymer
- Closed-loop recycling and fabrication of hydrophilic CNT films with high performance
- Design of thin-film configuration of SnO2–Ag2O composites for NO2 gas-sensing applications
- Study on stress distribution of SiC/Al composites based on microstructure models with microns and nanoparticles
- PVDF green nanofibers as potential carriers for improving self-healing and mechanical properties of carbon fiber/epoxy prepregs
- Osteogenesis capability of three-dimensionally printed poly(lactic acid)-halloysite nanotube scaffolds containing strontium ranelate
- Silver nanoparticles induce mitochondria-dependent apoptosis and late non-canonical autophagy in HT-29 colon cancer cells
- Preparation and bonding mechanisms of polymer/metal hybrid composite by nano molding technology
- Damage self-sensing and strain monitoring of glass-reinforced epoxy composite impregnated with graphene nanoplatelet and multiwalled carbon nanotubes
- Thermal analysis characterisation of solar-powered ship using Oldroyd hybrid nanofluids in parabolic trough solar collector: An optimal thermal application
- Pyrene-functionalized halloysite nanotubes for simultaneously detecting and separating Hg(ii) in aqueous media: A comprehensive comparison on interparticle and intraparticle excimers
- Fabrication of self-assembly CNT flexible film and its piezoresistive sensing behaviors
- Thermal valuation and entropy inspection of second-grade nanoscale fluid flow over a stretching surface by applying Koo–Kleinstreuer–Li relation
- Mechanical properties and microstructure of nano-SiO2 and basalt-fiber-reinforced recycled aggregate concrete
- Characterization and tribology performance of polyaniline-coated nanodiamond lubricant additives
- Combined impact of Marangoni convection and thermophoretic particle deposition on chemically reactive transport of nanofluid flow over a stretching surface
- Spark plasma extrusion of binder free hydroxyapatite powder
- An investigation on thermo-mechanical performance of graphene-oxide-reinforced shape memory polymer
- Effect of nanoadditives on the novel leather fiber/recycled poly(ethylene-vinyl-acetate) polymer composites for multifunctional applications: Fabrication, characterizations, and multiobjective optimization using central composite design
- Design selection for a hemispherical dimple core sandwich panel using hybrid multi-criteria decision-making methods
- Improving tensile strength and impact toughness of plasticized poly(lactic acid) biocomposites by incorporating nanofibrillated cellulose
- Green synthesis of spinel copper ferrite (CuFe2O4) nanoparticles and their toxicity
- The effect of TaC and NbC hybrid and mono-nanoparticles on AA2024 nanocomposites: Microstructure, strengthening, and artificial aging
- Excited-state geometry relaxation of pyrene-modified cellulose nanocrystals under UV-light excitation for detecting Fe3+
- Effect of CNTs and MEA on the creep of face-slab concrete at an early age
- Effect of deformation conditions on compression phase transformation of AZ31
- Application of MXene as a new generation of highly conductive coating materials for electromembrane-surrounded solid-phase microextraction
- A comparative study of the elasto-plastic properties for ceramic nanocomposites filled by graphene or graphene oxide nanoplates
- Encapsulation strategies for improving the biological behavior of CdS@ZIF-8 nanocomposites
- Biosynthesis of ZnO NPs from pumpkin seeds’ extract and elucidation of its anticancer potential against breast cancer
- Preliminary trials of the gold nanoparticles conjugated chrysin: An assessment of anti-oxidant, anti-microbial, and in vitro cytotoxic activities of a nanoformulated flavonoid
- Effect of micron-scale pores increased by nano-SiO2 sol modification on the strength of cement mortar
- Fractional simulations for thermal flow of hybrid nanofluid with aluminum oxide and titanium oxide nanoparticles with water and blood base fluids
- The effect of graphene nano-powder on the viscosity of water: An experimental study and artificial neural network modeling
- Development of a novel heat- and shear-resistant nano-silica gelling agent
- Characterization, biocompatibility and in vivo of nominal MnO2-containing wollastonite glass-ceramic
- Entropy production simulation of second-grade magnetic nanomaterials flowing across an expanding surface with viscidness dissipative flux
- Enhancement in structural, morphological, and optical properties of copper oxide for optoelectronic device applications
- Aptamer-functionalized chitosan-coated gold nanoparticle complex as a suitable targeted drug carrier for improved breast cancer treatment
- Performance and overall evaluation of nano-alumina-modified asphalt mixture
- Analysis of pure nanofluid (GO/engine oil) and hybrid nanofluid (GO–Fe3O4/engine oil): Novel thermal and magnetic features
- Synthesis of Ag@AgCl modified anatase/rutile/brookite mixed phase TiO2 and their photocatalytic property
- Mechanisms and influential variables on the abrasion resistance hydraulic concrete
- Synergistic reinforcement mechanism of basalt fiber/cellulose nanocrystals/polypropylene composites
- Achieving excellent oxidation resistance and mechanical properties of TiB2–B4C/carbon aerogel composites by quick-gelation and mechanical mixing
- Microwave-assisted sol–gel template-free synthesis and characterization of silica nanoparticles obtained from South African coal fly ash
- Pulsed laser-assisted synthesis of nano nickel(ii) oxide-anchored graphitic carbon nitride: Characterizations and their potential antibacterial/anti-biofilm applications
- Effects of nano-ZrSi2 on thermal stability of phenolic resin and thermal reusability of quartz–phenolic composites
- Benzaldehyde derivatives on tin electroplating as corrosion resistance for fabricating copper circuit
- Mechanical and heat transfer properties of 4D-printed shape memory graphene oxide/epoxy acrylate composites
- Coupling the vanadium-induced amorphous/crystalline NiFe2O4 with phosphide heterojunction toward active oxygen evolution reaction catalysts
- Graphene-oxide-reinforced cement composites mechanical and microstructural characteristics at elevated temperatures
- Gray correlation analysis of factors influencing compressive strength and durability of nano-SiO2 and PVA fiber reinforced geopolymer mortar
- Preparation of layered gradient Cu–Cr–Ti alloy with excellent mechanical properties, thermal stability, and electrical conductivity
- Recovery of Cr from chrome-containing leather wastes to develop aluminum-based composite material along with Al2O3 ceramic particles: An ingenious approach
- Mechanisms of the improved stiffness of flexible polymers under impact loading
- Anticancer potential of gold nanoparticles (AuNPs) using a battery of in vitro tests
- Review Articles
- Proposed approaches for coronaviruses elimination from wastewater: Membrane techniques and nanotechnology solutions
- Application of Pickering emulsion in oil drilling and production
- The contribution of microfluidics to the fight against tuberculosis
- Graphene-based biosensors for disease theranostics: Development, applications, and recent advancements
- Synthesis and encapsulation of iron oxide nanorods for application in magnetic hyperthermia and photothermal therapy
- Contemporary nano-architectured drugs and leads for ανβ3 integrin-based chemotherapy: Rationale and retrospect
- State-of-the-art review of fabrication, application, and mechanical properties of functionally graded porous nanocomposite materials
- Insights on magnetic spinel ferrites for targeted drug delivery and hyperthermia applications
- A review on heterogeneous oxidation of acetaminophen based on micro and nanoparticles catalyzed by different activators
- Early diagnosis of lung cancer using magnetic nanoparticles-integrated systems
- Advances in ZnO: Manipulation of defects for enhancing their technological potentials
- Efficacious nanomedicine track toward combating COVID-19
- A review of the design, processes, and properties of Mg-based composites
- Green synthesis of nanoparticles for varied applications: Green renewable resources and energy-efficient synthetic routes
- Two-dimensional nanomaterial-based polymer composites: Fundamentals and applications
- Recent progress and challenges in plasmonic nanomaterials
- Apoptotic cell-derived micro/nanosized extracellular vesicles in tissue regeneration
- Electronic noses based on metal oxide nanowires: A review
- Framework materials for supercapacitors
- An overview on the reproductive toxicity of graphene derivatives: Highlighting the importance
- Antibacterial nanomaterials: Upcoming hope to overcome antibiotic resistance crisis
- Research progress of carbon materials in the field of three-dimensional printing polymer nanocomposites
- A review of atomic layer deposition modelling and simulation methodologies: Density functional theory and molecular dynamics
- Recent advances in the preparation of PVDF-based piezoelectric materials
- Recent developments in tensile properties of friction welding of carbon fiber-reinforced composite: A review
- Comprehensive review of the properties of fly ash-based geopolymer with additive of nano-SiO2
- Perspectives in biopolymer/graphene-based composite application: Advances, challenges, and recommendations
- Graphene-based nanocomposite using new modeling molecular dynamic simulations for proposed neutralizing mechanism and real-time sensing of COVID-19
- Nanotechnology application on bamboo materials: A review
- Recent developments and future perspectives of biorenewable nanocomposites for advanced applications
- Nanostructured lipid carrier system: A compendium of their formulation development approaches, optimization strategies by quality by design, and recent applications in drug delivery
- 3D printing customized design of human bone tissue implant and its application
- Design, preparation, and functionalization of nanobiomaterials for enhanced efficacy in current and future biomedical applications
- A brief review of nanoparticles-doped PEDOT:PSS nanocomposite for OLED and OPV
- Nanotechnology interventions as a putative tool for the treatment of dental afflictions
- Recent advancements in metal–organic frameworks integrating quantum dots (QDs@MOF) and their potential applications
- A focused review of short electrospun nanofiber preparation techniques for composite reinforcement
- Microstructural characteristics and nano-modification of interfacial transition zone in concrete: A review
- Latest developments in the upconversion nanotechnology for the rapid detection of food safety: A review
- Strategic applications of nano-fertilizers for sustainable agriculture: Benefits and bottlenecks
- Molecular dynamics application of cocrystal energetic materials: A review
- Synthesis and application of nanometer hydroxyapatite in biomedicine
- Cutting-edge development in waste-recycled nanomaterials for energy storage and conversion applications
- Biological applications of ternary quantum dots: A review
- Nanotherapeutics for hydrogen sulfide-involved treatment: An emerging approach for cancer therapy
- Application of antibacterial nanoparticles in orthodontic materials
- Effect of natural-based biological hydrogels combined with growth factors on skin wound healing
- Nanozymes – A route to overcome microbial resistance: A viewpoint
- Recent developments and applications of smart nanoparticles in biomedicine
- Contemporary review on carbon nanotube (CNT) composites and their impact on multifarious applications
- Interfacial interactions and reinforcing mechanisms of cellulose and chitin nanomaterials and starch derivatives for cement and concrete strength and durability enhancement: A review
- Diamond-like carbon films for tribological modification of rubber
- Layered double hydroxides (LDHs) modified cement-based materials: A systematic review
- Recent research progress and advanced applications of silica/polymer nanocomposites
- Modeling of supramolecular biopolymers: Leading the in silico revolution of tissue engineering and nanomedicine
- Recent advances in perovskites-based optoelectronics
- Biogenic synthesis of palladium nanoparticles: New production methods and applications
- A comprehensive review of nanofluids with fractional derivatives: Modeling and application
- Electrospinning of marine polysaccharides: Processing and chemical aspects, challenges, and future prospects
- Electrohydrodynamic printing for demanding devices: A review of processing and applications
- Rapid Communications
- Structural material with designed thermal twist for a simple actuation
- Recent advances in photothermal materials for solar-driven crude oil adsorption
Articles in the same Issue
- Research Articles
- Theoretical and experimental investigation of MWCNT dispersion effect on the elastic modulus of flexible PDMS/MWCNT nanocomposites
- Mechanical, morphological, and fracture-deformation behavior of MWCNTs-reinforced (Al–Cu–Mg–T351) alloy cast nanocomposites fabricated by optimized mechanical milling and powder metallurgy techniques
- Flammability and physical stability of sugar palm crystalline nanocellulose reinforced thermoplastic sugar palm starch/poly(lactic acid) blend bionanocomposites
- Glutathione-loaded non-ionic surfactant niosomes: A new approach to improve oral bioavailability and hepatoprotective efficacy of glutathione
- Relationship between mechano-bactericidal activity and nanoblades density on chemically strengthened glass
- In situ regulation of microstructure and microwave-absorbing properties of FeSiAl through HNO3 oxidation
- Research on a mechanical model of magnetorheological fluid different diameter particles
- Nanomechanical and dynamic mechanical properties of rubber–wood–plastic composites
- Investigative properties of CeO2 doped with niobium: A combined characterization and DFT studies
- Miniaturized peptidomimetics and nano-vesiculation in endothelin types through probable nano-disk formation and structure property relationships of endothelins’ fragments
- N/S co-doped CoSe/C nanocubes as anode materials for Li-ion batteries
- Synergistic effects of halloysite nanotubes with metal and phosphorus additives on the optimal design of eco-friendly sandwich panels with maximum flame resistance and minimum weight
- Octreotide-conjugated silver nanoparticles for active targeting of somatostatin receptors and their application in a nebulized rat model
- Controllable morphology of Bi2S3 nanostructures formed via hydrothermal vulcanization of Bi2O3 thin-film layer and their photoelectrocatalytic performances
- Development of (−)-epigallocatechin-3-gallate-loaded folate receptor-targeted nanoparticles for prostate cancer treatment
- Enhancement of the mechanical properties of HDPE mineral nanocomposites by filler particles modulation of the matrix plastic/elastic behavior
- Effect of plasticizers on the properties of sugar palm nanocellulose/cinnamon essential oil reinforced starch bionanocomposite films
- Optimization of nano coating to reduce the thermal deformation of ball screws
- Preparation of efficient piezoelectric PVDF–HFP/Ni composite films by high electric field poling
- MHD dissipative Casson nanofluid liquid film flow due to an unsteady stretching sheet with radiation influence and slip velocity phenomenon
- Effects of nano-SiO2 modification on rubberised mortar and concrete with recycled coarse aggregates
- Mechanical and microscopic properties of fiber-reinforced coal gangue-based geopolymer concrete
- Effect of morphology and size on the thermodynamic stability of cerium oxide nanoparticles: Experiment and molecular dynamics calculation
- Mechanical performance of a CFRP composite reinforced via gelatin-CNTs: A study on fiber interfacial enhancement and matrix enhancement
- A practical review over surface modification, nanopatterns, emerging materials, drug delivery systems, and their biophysiochemical properties for dental implants: Recent progresses and advances
- HTR: An ultra-high speed algorithm for cage recognition of clathrate hydrates
- Effects of microalloying elements added by in situ synthesis on the microstructure of WCu composites
- A highly sensitive nanobiosensor based on aptamer-conjugated graphene-decorated rhodium nanoparticles for detection of HER2-positive circulating tumor cells
- Progressive collapse performance of shear strengthened RC frames by nano CFRP
- Core–shell heterostructured composites of carbon nanotubes and imine-linked hyperbranched polymers as metal-free Li-ion anodes
- A Galerkin strategy for tri-hybridized mixture in ethylene glycol comprising variable diffusion and thermal conductivity using non-Fourier’s theory
- Simple models for tensile modulus of shape memory polymer nanocomposites at ambient temperature
- Preparation and morphological studies of tin sulfide nanoparticles and use as efficient photocatalysts for the degradation of rhodamine B and phenol
- Polyethyleneimine-impregnated activated carbon nanofiber composited graphene-derived rice husk char for efficient post-combustion CO2 capture
- Electrospun nanofibers of Co3O4 nanocrystals encapsulated in cyclized-polyacrylonitrile for lithium storage
- Pitting corrosion induced on high-strength high carbon steel wire in high alkaline deaerated chloride electrolyte
- Formulation of polymeric nanoparticles loaded sorafenib; evaluation of cytotoxicity, molecular evaluation, and gene expression studies in lung and breast cancer cell lines
- Engineered nanocomposites in asphalt binders
- Influence of loading voltage, domain ratio, and additional load on the actuation of dielectric elastomer
- Thermally induced hex-graphene transitions in 2D carbon crystals
- The surface modification effect on the interfacial properties of glass fiber-reinforced epoxy: A molecular dynamics study
- Molecular dynamics study of deformation mechanism of interfacial microzone of Cu/Al2Cu/Al composites under tension
- Nanocolloid simulators of luminescent solar concentrator photovoltaic windows
- Compressive strength and anti-chloride ion penetration assessment of geopolymer mortar merging PVA fiber and nano-SiO2 using RBF–BP composite neural network
- Effect of 3-mercapto-1-propane sulfonate sulfonic acid and polyvinylpyrrolidone on the growth of cobalt pillar by electrodeposition
- Dynamics of convective slippery constraints on hybrid radiative Sutterby nanofluid flow by Galerkin finite element simulation
- Preparation of vanadium by the magnesiothermic self-propagating reduction and process control
- Microstructure-dependent photoelectrocatalytic activity of heterogeneous ZnO–ZnS nanosheets
- Cytotoxic and pro-inflammatory effects of molybdenum and tungsten disulphide on human bronchial cells
- Improving recycled aggregate concrete by compression casting and nano-silica
- Chemically reactive Maxwell nanoliquid flow by a stretching surface in the frames of Newtonian heating, nonlinear convection and radiative flux: Nanopolymer flow processing simulation
- Nonlinear dynamic and crack behaviors of carbon nanotubes-reinforced composites with various geometries
- Biosynthesis of copper oxide nanoparticles and its therapeutic efficacy against colon cancer
- Synthesis and characterization of smart stimuli-responsive herbal drug-encapsulated nanoniosome particles for efficient treatment of breast cancer
- Homotopic simulation for heat transport phenomenon of the Burgers nanofluids flow over a stretching cylinder with thermal convective and zero mass flux conditions
- Incorporation of copper and strontium ions in TiO2 nanotubes via dopamine to enhance hemocompatibility and cytocompatibility
- Mechanical, thermal, and barrier properties of starch films incorporated with chitosan nanoparticles
- Mechanical properties and microstructure of nano-strengthened recycled aggregate concrete
- Glucose-responsive nanogels efficiently maintain the stability and activity of therapeutic enzymes
- Tunning matrix rheology and mechanical performance of ultra-high performance concrete using cellulose nanofibers
- Flexible MXene/copper/cellulose nanofiber heat spreader films with enhanced thermal conductivity
- Promoted charge separation and specific surface area via interlacing of N-doped titanium dioxide nanotubes on carbon nitride nanosheets for photocatalytic degradation of Rhodamine B
- Elucidating the role of silicon dioxide and titanium dioxide nanoparticles in mitigating the disease of the eggplant caused by Phomopsis vexans, Ralstonia solanacearum, and root-knot nematode Meloidogyne incognita
- An implication of magnetic dipole in Carreau Yasuda liquid influenced by engine oil using ternary hybrid nanomaterial
- Robust synthesis of a composite phase of copper vanadium oxide with enhanced performance for durable aqueous Zn-ion batteries
- Tunning self-assembled phases of bovine serum albumin via hydrothermal process to synthesize novel functional hydrogel for skin protection against UVB
- A comparative experimental study on damping properties of epoxy nanocomposite beams reinforced with carbon nanotubes and graphene nanoplatelets
- Lightweight and hydrophobic Ni/GO/PVA composite aerogels for ultrahigh performance electromagnetic interference shielding
- Research on the auxetic behavior and mechanical properties of periodically rotating graphene nanostructures
- Repairing performances of novel cement mortar modified with graphene oxide and polyacrylate polymer
- Closed-loop recycling and fabrication of hydrophilic CNT films with high performance
- Design of thin-film configuration of SnO2–Ag2O composites for NO2 gas-sensing applications
- Study on stress distribution of SiC/Al composites based on microstructure models with microns and nanoparticles
- PVDF green nanofibers as potential carriers for improving self-healing and mechanical properties of carbon fiber/epoxy prepregs
- Osteogenesis capability of three-dimensionally printed poly(lactic acid)-halloysite nanotube scaffolds containing strontium ranelate
- Silver nanoparticles induce mitochondria-dependent apoptosis and late non-canonical autophagy in HT-29 colon cancer cells
- Preparation and bonding mechanisms of polymer/metal hybrid composite by nano molding technology
- Damage self-sensing and strain monitoring of glass-reinforced epoxy composite impregnated with graphene nanoplatelet and multiwalled carbon nanotubes
- Thermal analysis characterisation of solar-powered ship using Oldroyd hybrid nanofluids in parabolic trough solar collector: An optimal thermal application
- Pyrene-functionalized halloysite nanotubes for simultaneously detecting and separating Hg(ii) in aqueous media: A comprehensive comparison on interparticle and intraparticle excimers
- Fabrication of self-assembly CNT flexible film and its piezoresistive sensing behaviors
- Thermal valuation and entropy inspection of second-grade nanoscale fluid flow over a stretching surface by applying Koo–Kleinstreuer–Li relation
- Mechanical properties and microstructure of nano-SiO2 and basalt-fiber-reinforced recycled aggregate concrete
- Characterization and tribology performance of polyaniline-coated nanodiamond lubricant additives
- Combined impact of Marangoni convection and thermophoretic particle deposition on chemically reactive transport of nanofluid flow over a stretching surface
- Spark plasma extrusion of binder free hydroxyapatite powder
- An investigation on thermo-mechanical performance of graphene-oxide-reinforced shape memory polymer
- Effect of nanoadditives on the novel leather fiber/recycled poly(ethylene-vinyl-acetate) polymer composites for multifunctional applications: Fabrication, characterizations, and multiobjective optimization using central composite design
- Design selection for a hemispherical dimple core sandwich panel using hybrid multi-criteria decision-making methods
- Improving tensile strength and impact toughness of plasticized poly(lactic acid) biocomposites by incorporating nanofibrillated cellulose
- Green synthesis of spinel copper ferrite (CuFe2O4) nanoparticles and their toxicity
- The effect of TaC and NbC hybrid and mono-nanoparticles on AA2024 nanocomposites: Microstructure, strengthening, and artificial aging
- Excited-state geometry relaxation of pyrene-modified cellulose nanocrystals under UV-light excitation for detecting Fe3+
- Effect of CNTs and MEA on the creep of face-slab concrete at an early age
- Effect of deformation conditions on compression phase transformation of AZ31
- Application of MXene as a new generation of highly conductive coating materials for electromembrane-surrounded solid-phase microextraction
- A comparative study of the elasto-plastic properties for ceramic nanocomposites filled by graphene or graphene oxide nanoplates
- Encapsulation strategies for improving the biological behavior of CdS@ZIF-8 nanocomposites
- Biosynthesis of ZnO NPs from pumpkin seeds’ extract and elucidation of its anticancer potential against breast cancer
- Preliminary trials of the gold nanoparticles conjugated chrysin: An assessment of anti-oxidant, anti-microbial, and in vitro cytotoxic activities of a nanoformulated flavonoid
- Effect of micron-scale pores increased by nano-SiO2 sol modification on the strength of cement mortar
- Fractional simulations for thermal flow of hybrid nanofluid with aluminum oxide and titanium oxide nanoparticles with water and blood base fluids
- The effect of graphene nano-powder on the viscosity of water: An experimental study and artificial neural network modeling
- Development of a novel heat- and shear-resistant nano-silica gelling agent
- Characterization, biocompatibility and in vivo of nominal MnO2-containing wollastonite glass-ceramic
- Entropy production simulation of second-grade magnetic nanomaterials flowing across an expanding surface with viscidness dissipative flux
- Enhancement in structural, morphological, and optical properties of copper oxide for optoelectronic device applications
- Aptamer-functionalized chitosan-coated gold nanoparticle complex as a suitable targeted drug carrier for improved breast cancer treatment
- Performance and overall evaluation of nano-alumina-modified asphalt mixture
- Analysis of pure nanofluid (GO/engine oil) and hybrid nanofluid (GO–Fe3O4/engine oil): Novel thermal and magnetic features
- Synthesis of Ag@AgCl modified anatase/rutile/brookite mixed phase TiO2 and their photocatalytic property
- Mechanisms and influential variables on the abrasion resistance hydraulic concrete
- Synergistic reinforcement mechanism of basalt fiber/cellulose nanocrystals/polypropylene composites
- Achieving excellent oxidation resistance and mechanical properties of TiB2–B4C/carbon aerogel composites by quick-gelation and mechanical mixing
- Microwave-assisted sol–gel template-free synthesis and characterization of silica nanoparticles obtained from South African coal fly ash
- Pulsed laser-assisted synthesis of nano nickel(ii) oxide-anchored graphitic carbon nitride: Characterizations and their potential antibacterial/anti-biofilm applications
- Effects of nano-ZrSi2 on thermal stability of phenolic resin and thermal reusability of quartz–phenolic composites
- Benzaldehyde derivatives on tin electroplating as corrosion resistance for fabricating copper circuit
- Mechanical and heat transfer properties of 4D-printed shape memory graphene oxide/epoxy acrylate composites
- Coupling the vanadium-induced amorphous/crystalline NiFe2O4 with phosphide heterojunction toward active oxygen evolution reaction catalysts
- Graphene-oxide-reinforced cement composites mechanical and microstructural characteristics at elevated temperatures
- Gray correlation analysis of factors influencing compressive strength and durability of nano-SiO2 and PVA fiber reinforced geopolymer mortar
- Preparation of layered gradient Cu–Cr–Ti alloy with excellent mechanical properties, thermal stability, and electrical conductivity
- Recovery of Cr from chrome-containing leather wastes to develop aluminum-based composite material along with Al2O3 ceramic particles: An ingenious approach
- Mechanisms of the improved stiffness of flexible polymers under impact loading
- Anticancer potential of gold nanoparticles (AuNPs) using a battery of in vitro tests
- Review Articles
- Proposed approaches for coronaviruses elimination from wastewater: Membrane techniques and nanotechnology solutions
- Application of Pickering emulsion in oil drilling and production
- The contribution of microfluidics to the fight against tuberculosis
- Graphene-based biosensors for disease theranostics: Development, applications, and recent advancements
- Synthesis and encapsulation of iron oxide nanorods for application in magnetic hyperthermia and photothermal therapy
- Contemporary nano-architectured drugs and leads for ανβ3 integrin-based chemotherapy: Rationale and retrospect
- State-of-the-art review of fabrication, application, and mechanical properties of functionally graded porous nanocomposite materials
- Insights on magnetic spinel ferrites for targeted drug delivery and hyperthermia applications
- A review on heterogeneous oxidation of acetaminophen based on micro and nanoparticles catalyzed by different activators
- Early diagnosis of lung cancer using magnetic nanoparticles-integrated systems
- Advances in ZnO: Manipulation of defects for enhancing their technological potentials
- Efficacious nanomedicine track toward combating COVID-19
- A review of the design, processes, and properties of Mg-based composites
- Green synthesis of nanoparticles for varied applications: Green renewable resources and energy-efficient synthetic routes
- Two-dimensional nanomaterial-based polymer composites: Fundamentals and applications
- Recent progress and challenges in plasmonic nanomaterials
- Apoptotic cell-derived micro/nanosized extracellular vesicles in tissue regeneration
- Electronic noses based on metal oxide nanowires: A review
- Framework materials for supercapacitors
- An overview on the reproductive toxicity of graphene derivatives: Highlighting the importance
- Antibacterial nanomaterials: Upcoming hope to overcome antibiotic resistance crisis
- Research progress of carbon materials in the field of three-dimensional printing polymer nanocomposites
- A review of atomic layer deposition modelling and simulation methodologies: Density functional theory and molecular dynamics
- Recent advances in the preparation of PVDF-based piezoelectric materials
- Recent developments in tensile properties of friction welding of carbon fiber-reinforced composite: A review
- Comprehensive review of the properties of fly ash-based geopolymer with additive of nano-SiO2
- Perspectives in biopolymer/graphene-based composite application: Advances, challenges, and recommendations
- Graphene-based nanocomposite using new modeling molecular dynamic simulations for proposed neutralizing mechanism and real-time sensing of COVID-19
- Nanotechnology application on bamboo materials: A review
- Recent developments and future perspectives of biorenewable nanocomposites for advanced applications
- Nanostructured lipid carrier system: A compendium of their formulation development approaches, optimization strategies by quality by design, and recent applications in drug delivery
- 3D printing customized design of human bone tissue implant and its application
- Design, preparation, and functionalization of nanobiomaterials for enhanced efficacy in current and future biomedical applications
- A brief review of nanoparticles-doped PEDOT:PSS nanocomposite for OLED and OPV
- Nanotechnology interventions as a putative tool for the treatment of dental afflictions
- Recent advancements in metal–organic frameworks integrating quantum dots (QDs@MOF) and their potential applications
- A focused review of short electrospun nanofiber preparation techniques for composite reinforcement
- Microstructural characteristics and nano-modification of interfacial transition zone in concrete: A review
- Latest developments in the upconversion nanotechnology for the rapid detection of food safety: A review
- Strategic applications of nano-fertilizers for sustainable agriculture: Benefits and bottlenecks
- Molecular dynamics application of cocrystal energetic materials: A review
- Synthesis and application of nanometer hydroxyapatite in biomedicine
- Cutting-edge development in waste-recycled nanomaterials for energy storage and conversion applications
- Biological applications of ternary quantum dots: A review
- Nanotherapeutics for hydrogen sulfide-involved treatment: An emerging approach for cancer therapy
- Application of antibacterial nanoparticles in orthodontic materials
- Effect of natural-based biological hydrogels combined with growth factors on skin wound healing
- Nanozymes – A route to overcome microbial resistance: A viewpoint
- Recent developments and applications of smart nanoparticles in biomedicine
- Contemporary review on carbon nanotube (CNT) composites and their impact on multifarious applications
- Interfacial interactions and reinforcing mechanisms of cellulose and chitin nanomaterials and starch derivatives for cement and concrete strength and durability enhancement: A review
- Diamond-like carbon films for tribological modification of rubber
- Layered double hydroxides (LDHs) modified cement-based materials: A systematic review
- Recent research progress and advanced applications of silica/polymer nanocomposites
- Modeling of supramolecular biopolymers: Leading the in silico revolution of tissue engineering and nanomedicine
- Recent advances in perovskites-based optoelectronics
- Biogenic synthesis of palladium nanoparticles: New production methods and applications
- A comprehensive review of nanofluids with fractional derivatives: Modeling and application
- Electrospinning of marine polysaccharides: Processing and chemical aspects, challenges, and future prospects
- Electrohydrodynamic printing for demanding devices: A review of processing and applications
- Rapid Communications
- Structural material with designed thermal twist for a simple actuation
- Recent advances in photothermal materials for solar-driven crude oil adsorption

