Abstract
Skin wound healing is a continuous and complex process affected by many factors. Growth factors play an important role in the process of wound healing. Local application of growth factors can significantly promote wound healing. However, the degradation and time dependence of growth factors require appropriate delivery systems to help them play a role in wound healing. In recent years, wound dressing products with hydrogels as matrix materials or main components have shown obvious advantages in promoting wound healing. By modifying the hydrogel or combining it with other factors or materials that are beneficial to wound healing, the healing effect can be further enhanced. This review will introduce the research status of growth factors and hydrogels based on natural biological materials in skin wound repair and review the effects and research progress of the combination of growth factors and hydrogels in skin wound healing.
1 Introduction
Skin is the largest organ of the human body. It is wrapped on the surface of the body and is in direct contact with the external environment. It has the functions of protection, excretion, regulating body temperature, and feeling external stimulation [1]. The tight and intact skin structure is the decisive factor for the proper functioning of the skin [2]. However, under the action of a variety of adverse factors, the integrity of the skin is easily damaged to form wounds, which then lose its normal structure and function. The regenerative ability of skin makes the majority of skin injuries basically recover after a period of time, but the skin healing process is dynamic and continuous, and this process is easily affected by a variety of external factors [3]. Infection, burns, bedsores, diabetes, and other factors can cause the abnormal healing process of skin wounds, resulting in nonhealing or delayed healing of wounds and the formation of chronic wounds [4]. Therefore, it is very important to carry out appropriate intervention and protection measures in the process of wound recovery and provide a suitable environment and conditions for wound healing.
Wound dressings can cover the skin wound surface, protect the damaged skin, and play a certain role in protecting the wound [5]. Traditional wound dressings such as gauze, cotton balls, and other natural fibrous materials can keep the surface of the wound dry while insulating the wound from the outside environment and acting as a physical barrier [6]. However, traditional wound dressings have problems, including scabbling with exudate and wound adhesion. Therefore, it is of great significance to develop a more suitable dressing for wound healing [7]. The ideal wound dressing should have the following abilities [8]:
maintain a moist environment for the wound, preventing wound surface moisture from evaporating and drying,
excellent gas exchange ability, allowing oxygen to contact the wound through the dressing,
good water imbibition, and preventing the buildup of fluid seepage on the wound surface,
antimicrobial properties or sterilization ability, reducing the occurrence of wound infection opportunities,
hemostatic function,
good adhesion, close attachment to the wound,
certain mechanical strength, preventing physical secondary injury to the wound,
good biocompatibility, no allergic reactions, and
economic raw materials that are easy to obtain and inexpensive.
Under the background of the ideal wound dressing mentioned above, an increasing number of materials have been developed and applied for the preparation of wound dressings in recent years, such as fiber spinning, semipermeable membranes, fiber sponges, hydrogels, etc. Among these materials, hydrogels are the most competitive candidates for wound dressings due to their excellent hydrophilicity, adhesion, permeability, and biocompatibility [9]. Compared with synthetic hydrogels, natural biomaterial hydrogels from living organisms and the natural environment are easier to prepare and have better biocompatibility and degradability [10]. In addition, the matrix materials of natural hydrogels are rich in functional groups, which provide corresponding binding sites for stable binding of growth factors. The growth factor loaded into the hydrogel extends the time it is hydrolyzed, thus prolonging the duration of the growth factor’s action. The sustained-release ability of the hydrogel can make the growth factor release steadily and continuously, avoiding the early high concentration and aggregation of growth factor and the rapid attenuation of concentration in the later period when the growth factor is directly applied. Therefore, using natural hydrogels as carriers for carrying growth factors in skin wound healing has inherent advantages and good application prospects.
Although using hydrogels as wound dressings can protect wounds to a certain extent, hydrogels themselves lack the ability to regulate and promote the healing process of skin [11]. Growth factors are polypeptide substances secreted by cells that regulate cell growth and cell function by binding with specific cell membrane receptors [12]. In the process of skin wound repair, a variety of growth factors are secreted and released successively or simultaneously in the injured area, participating in the repair process of neovascularization, collagen synthesis, inflammatory regulation, epithelial regeneration, and so on [13]. Adding growth factors to dressings can cause growth factors to directly act on local wounds to regulate skin healing while releasing long-term effects, which has good application prospects and effects [14]. This review introduces the normal structure of skin and the local pathological changes and healing process of skin after wound formation. Next we introduced the role and application of various growth factors in skin wound repair. Finally, we describe the properties of natural biomaterial hydrogels and the effect of combining hydrogels and growth factors in skin wound repair and briefly summarize the advantages and disadvantages of this treatment combination. This review will systematically introduce the mechanisms and effects of the combined application of various natural hydrogels and growth factors in wound repair. In this article, we summarize the common natural hydrogels and growth factors corresponding to skin injury repair, and introduce the advanced experimental combinations of natural hydrogels and growth factors in recent years, so as to provide reference for the future research on natural hydrogels and growth factors as therapeutic combinations.
2 Characteristic of wound healing
2.1 The structure and function of normal skin tissue
Normal skin tissue consists of the epidermis and dermis. The epidermis layer is located on the outside of the skin. More than 95% of the cells in the epidermis are keratinocytes, with the rest made up of melanocytes, Merkel cells, and Langerhans cells [15]. As shown in Figure 1, the epidermis is divided into a corneous layer, transparent layer, granular layer, spinous layer, and basal layer from outside to inside [16]. The structural integrity and activity of the cells in each layer increased from the outside to the inside, and the cells in the inner layer gradually transformed to the outer layer over time [17]. The cells of the corneum have been completely keratinized, and the desmosomes between the cells are loose and shed to form dandruff [18]. The lucidum layer consists of 2–3 layers of flattened cells with fuzzy cell boundaries and the absence of nuclei and organelles [19]. The cells in the granular layer are mainly spindle cells, with nuclei and organelles that begin to degenerate, and there are many lamellar granules and keratohyalin granules in the cells [20]. There are a large number of spinous cells in the stratum spinosum, which have a strong protein synthesis function and can synthesize a large number of keratin filaments and lamellar granules [21]. At the same time, the desmosomes between the spinous cells are closely connected, which can prevent external substances from penetrating the epidermis and has the function of protection and isolation [22]. The basal layer is the most important part of the epidermis. The self-renewal and repair of the epidermis mainly depends on the basal cells attached to the basal membrane in the basal layer [23]. These basal cells are the stem cells in the epidermis with the ability to proliferate and differentiate [24]. In the process of proliferation, basal cells can separate from the basal membrane and differentiate into spinous cells, which can replenish the number of spinous layer cells and play a role in self-renewal [21]. After skin injury, the number and activity of basal cells have an important influence on the healing effect.
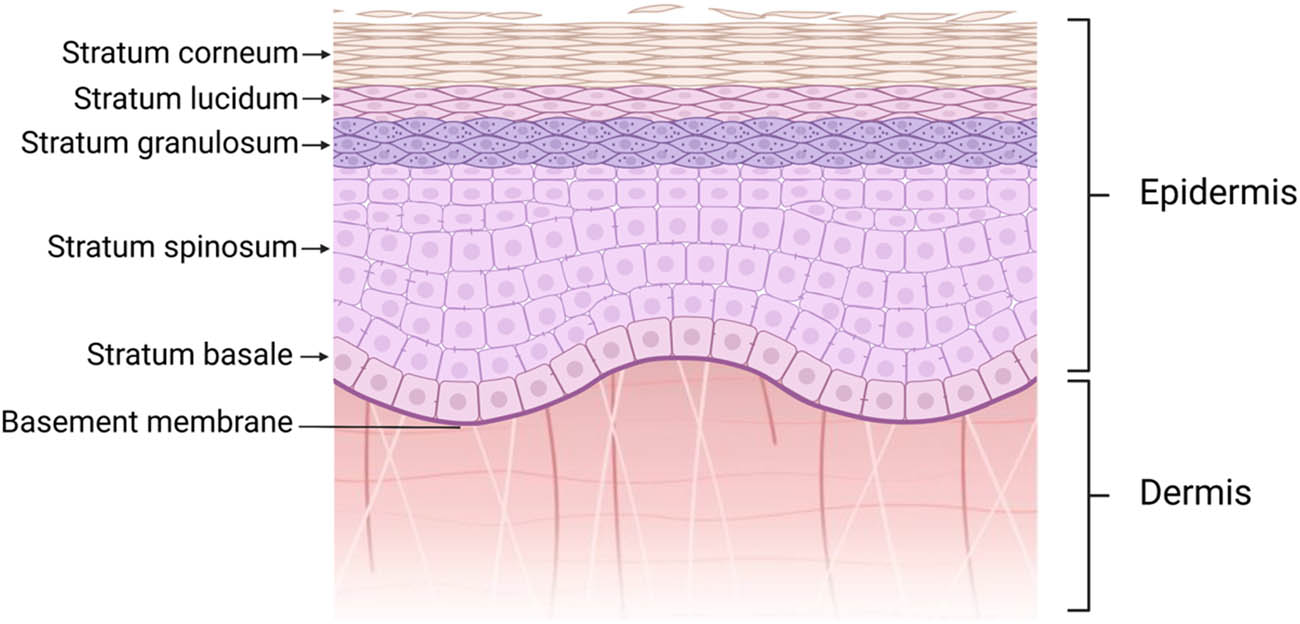
Normal structure of skin. Normal skin tissue consists of the epidermis and dermis. The epidermis is divided into a corneous layer, transparent layer, granular layer, spinous layer, and basal layer from outside to inside.
The dermis is a dense connective tissue located below the epidermis, which is divided into the papillary layer and the reticulated layer. There are abundant capillaries in the papillary layer, which protrude to the epidermis to form papillary structures, increasing the contact and connection between the epidermis and dermis and facilitating the epidermis to obtain nutrients from the dermis [25]. The reticulated layer contains a large number of collagen fibers, which interweave to form a network that provides skin with toughness and elasticity [26]. Under normal conditions, the basal cells in the basal layer of the epidermis obtain nutrients from the dermis, and while proliferating and renewing themselves, some of the basal cells differentiate into spinous cells to supplement the number of spinous cells and maintain normal epidermal functions [27]. As time passes, the cells in the lateral layers gradually shift to the more lateral layers until they fall off as keratinocytes.
2.2 Physiology of wound healing
The normal skin tissue structure can protect the internal tissues and organs of the body from damage by external harmful substances and is the first barrier to maintain homeostasis of the human body environment. When the skin is injured by external factors such as surgery, external forces, chemical reagents, heat, and electric current, the normal physiological structure of the skin is damaged, and the protective effect of the body weakens or disappears. The process of skin wound healing can be divided into four continuous stages, namely, hemostasis, inflammation, proliferation, and remodeling, as shown in Figure 2 [28]. When injury occurs, the blood vessels in the damaged area rupture, the subcutaneous matrix in the blood vessels is exposed, and the glycoproteins on the platelet membrane bind with the collagen of the basement membrane, making the platelets adhere to and be activated [29]. Activated platelets release endogenous ADP and TXA2, which in turn promote irreversible aggregation of platelets, resulting in platelet thrombosis [30]. In addition to hemostasis, platelets can also release cytokines and growth factors such as interleukin (IL)-1β, tumor necrosis factor (TNF)-α, fibroblast growth factor (FGF), and platelet derivation growth factor (PDGF) together with the cells in the injured area and promote the migration of neutrophils and macrophages in the wound area [31]. In the early stage of inflammation, neutrophils first accumulate in the wound area and phagocytose and release reactive oxygen species (ROS), antimicrobial peptides, proteolytic enzymes, and other substances to remove necrotic tissues and pathogens [3,32]. Mast cells and macrophages then arrive at the damaged area to remove remaining cell debris and neutrophils [33].
![Figure 2
The stages of wound repair and their major cellular components [28]. The process of skin wound healing can be divided into four continuous stages, namely, hemostasis, inflammation, proliferation, and remodeling.](/document/doi/10.1515/ntrev-2022-0122/asset/graphic/j_ntrev-2022-0122_fig_002.jpg)
The stages of wound repair and their major cellular components [28]. The process of skin wound healing can be divided into four continuous stages, namely, hemostasis, inflammation, proliferation, and remodeling.
At the later stage of inflammation, macrophages develop an anti-inflammatory, profibrotic phenotype that promotes the proliferation of fibroblasts, keratinocytes, and endothelial cells by secreting a variety of growth factors and cytokines [34,35]. Under the action of FGF, fibroblasts provide the skeleton structure for cell migration, proliferation, and growth to the wound area through the synthesis of type III collagen, proteoglycan, and fibronectin [36]. At the same time, endothelial cells at the wound site proliferate and migrate under the stimulation of factors such as VEGF and angiopoietin, forming a new capillary network, which is necessary for tissue regeneration and granulation tissue formation and transportation in the injured area [37]. Keratinocytes from near the injured area migrate to the injured area under the stimulation of growth factors and promote the epithelialization of the epidermal tissue through proliferation and differentiation [38]. When wound repair enters the remodeling stage, the remaining temporarily transplanted cells in the damaged area are apoptotic, and fibroblasts synthesize type I collagen, elastin, and other extracellular matrices [39]. Type III collagen in the extracellular matrix (ECM) of the injured area was gradually replaced by type I collagen, the collagen fibers rearranged into a lattice-like structure, and the skin tissue in the injured area regained strength and toughness similar to normal tissue [40].
2.3 Factors affecting wound healing
Wound healing involves many factors, and it takes a long time from the occurrence of injury to the completion of remodeling. Figure 3 illustrates common factors that affect skin healing. The factors affecting wound healing are mainly divided into endogenous factors and exogenous factors. Age is one of the endogenous factors affecting wound healing and repair. With increasing age, the thickness of the cuticle decreases, the activity of macrophages decreases, and the release of growth factors and cytokines decreases [41]. These factors lead to a prolonged proliferation period and an increase in the time required for wound healing. In addition, the body’s hormone level also has a certain effect on wound healing. For example, estrogen can promote the re-epithelialization of keratinocytes and the angiogenesis of endothelial cells in the process of wound healing, which has a certain promoting effect on wound healing [42]. Glucocorticoids and cortisol can inhibit the body’s own immune inflammatory response, which restricts the migration and aggregation of white blood cells to the injured area during inflammation [43]. The healing process can also be influenced by genetic factors. Studies have shown that keloids are a wound repair abnormality with a strong genetic tendency. In such people, excessive collagen deposition leads to the formation of a large number of fibrous scars on the wound surface, resulting in scar healing [44].
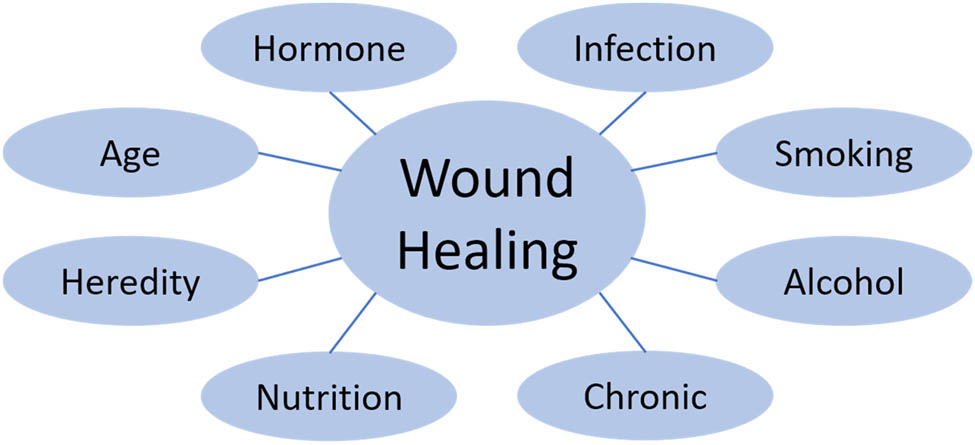
Factors affecting wound healing.
In addition to the influence of the body’s own factors on wound healing, exogenous factors are also the main factors affecting wound healing. Local infection after skin injury is an important factor affecting wound healing. When the wound is infected by S. aureus or other Streptococcus, a large number of white blood cells migrate and gather to the injured area, and the bacteria are removed by phagocytosis of white blood cells. The process of removing bacteria will cause the release of bacterial endotoxin, resulting in necrosis and inflammation of the local tissues [2]. In addition, due to the increase in proinflammatory cytokines, the number of released growth factors decreased, which inhibited the growth of cells in the proliferation stage, resulting in delayed wound healing or unhealing [45]. The nutritional status of the body is another important factor affecting wound healing. In the process of wound healing, a variety of trace elements, vitamins, fatty acids, and proteins are necessary, and the loss of any nutrients will cause the delay of wound healing [46,47,48]. In addition, many chronic diseases affect wound healing by interfering with platelet activation, the inflammatory response, epithelial formation, and matrix remodeling. Diabetes, for example, affects the migration and activation of white blood cells to the damaged area and increases the release of proinflammatory cytokines, leading to chronic inflammation [49]. Diabetes also causes abnormalities in microvascular formation that inhibit keratinocyte- and fibroblast-mediated epithelial reformation and ECM remodeling [50]. Drugs and unhealthy lifestyle habits such as smoking and drinking can also affect wound healing. Smoking can increase the aggregation and adhesion of platelets, raising the risk of blood clots [51]. Alcohol inhibits the body’s immune response and reduces collagen formation and matrix metalloproteinases (MMP) concentrations [52].
3 The role of growth factors in wound repair
Growth factors are polypeptide substances secreted by cells that regulate cell growth and cell function by binding with specific cell membrane receptors. According to growth factor source or the cell type that combines to play an effect, growth factors can be divided into platelet derivation growth factor (PDGF), vascular endothelial cell growth factor (VEGF), epidermal growth factor (EGF), fibroblast growth factor (FGF), nerve growth factor (NGF), transformation growth factor (TGF), etc. Growth factors are secreted by cells in an autocrine or paracrine manner in the synthesis and release to the environment through binding with specific receptor proteins on the surface of the cell membrane, regulating cell proliferation differentiation and the function of cells, including immune regulation, hematopoietic regulation, apoptosis, angiogenesis, and tumorigenesis. In the process of skin wound repair, a variety of growth factors are secreted and released successively or simultaneously in the injured area, participating in the repair process of neovascularization, collagen synthesis, inflammatory regulation, epithelial regeneration, and so on. Table 1 lists the growth factors involved in the process of skin wound healing. The specific roles of various growth factors in skin wound repair are as follows.
Effects of major growth factors on wound repair in different periods
| Growth factor | Hemostasis | Inflammation | Proliferation | Remodeling |
|---|---|---|---|---|
| PDGF | Promotes inflammatory cell migration and stimulates macrophage and TGF-β secretion | Promotes fibroblast proliferation, improves ECM synthesis, and promotes vascular endothelial cells migration and proliferation | Stimulates fibroblasts and differentiates into myofibroblasts, and upregulates MMP synthesis | |
| FGF | Chemotactic inflammatory cells | Promotes EMT of keratinocytes and promotes proliferation of fibroblasts and endothelial cells | Inhibits terminal differentiation of myofibroblasts, and reduce collagen synthesis and scar formation | |
| VEGF | Increases vascular permeability | Promotes vascular endothelial cell migration and proliferation and granulation formation | Promotes scar formation | |
| EGF | Promotes inflammatory response | Stimulates epithelial cell proliferation and division and promotes wound shrinkage | Inhibits inflammation and reduce scar formation | |
| TGF–β1/2 | Promotes inflammatory cell migration | Promotes the synthesis of collagen, elastic fibers, and fibronectin and promote granulation formation | ||
| Promotes EMT | Promotes scar formation | |||
| NGF | Promotes keratinocyte proliferation and angiogenesis |
3.1 Role of PDGF
PDGF is a basic protein stored in the platelet α particles. It is a dimer glycoprotein formed by two peptide chains linked by disulfide bonds [53]. According to the difference of two peptide chains, PDGF can be divided into 5 types: PDGF–AA, PDGF–BB, PDGF–CC, PDGF–DD, and PDGF–AB [54]. A variety of cells, such as macrophages, fibroblasts, and endothelial cells, can secrete and synthesize PDGF [55,56]. PDGF is involved in the whole process of wound healing and plays an important role in promoting wound healing. In the inflammatory stage, PDGF can promote the migration of neutrophils and macrophages to the injured site [57]. In addition, PDGF can stimulate macrophages to secrete synthetic TGF-β, further enhancing the chemotactic effect on inflammatory cells [55]. PDGF can also promote the proliferation and differentiation of fibroblasts and promote the synthesis of ECM of fibroblasts [53,58]. PDGF can promote the migration and proliferation of vascular endothelial cells at the injury site and promote the formation of new blood vessels [59]. During remodeling, PDGF can stimulate the differentiation of fibroblasts into myofibroblasts, upregulate the expression, synthesize MMP, and regulate the degradation and remodeling of ECM [60].
3.2 Role of FGF
There are 23 types of FGF families, among which FGF–2, FGF–7, and FGF–10 are related to wound repair. FGF is produced by keratinocytes, fibroblasts, endothelial cells, smooth muscle cells, chondrocytes, and mast cells. When injury occurs, the concentration of FGF increases sharply in the injury area and stimulates the migration of macrophages, white blood cells, and neutrophils to the injury area [61]. FGF can directly promote the proliferation and differentiation of fibroblasts, vascular endothelial cells, and smooth muscle cells, and promote the formation of granulation tissues [62,63]. In addition, FGF can also promote epithelial mesenchymal transformation by binding with TGF–β1 [64]. FGF can also promote the secretion of EGF, promote the proliferation of epidermal cells, regulate the differentiation of fibroblasts to myofibroblasts, and accelerate wound contraction activity, which is conducive to early wound coverage [62]. FGF can inhibit terminal differentiation of myofibroblasts and reduce glial scar hyperplasia during wound remodeling [65]. bFGF can also reduce collagen synthesis and scar formation by increasing procollagen mRNA degradation and inhibiting mRNA transcription [66].
3.3 Role of VEGF
VEGF is a family that includes VEGF–A, VEGF–B, VEGF–C, VEGF–D, VEGF–E, and placental growth factor (PGF). When injury occurs, activated platelets release VEGF to improve the lumen permeability of residual vessels, which is conducive to the migration and aggregation of inflammatory cells to the injured area [67–69]. VEGF promotes the proliferation, migration, and differentiation of vascular endothelial cells and the formation and functionalization of new capillaries during the proliferation phase [70,71]. In addition, VEGF can also promote lymphatic vessel formation by activating VEGFR–2 [72]. VEGF is also associated with scar tissue formation during remodeling. Wilgus T. A. found that injection of recombinant VEGF into wounds can increase scar formation [73].
3.4 Role of EGF
In the EGF family, EGF, heparin-binding EGF (HB–EGF), and TGF–α are mainly related to wound repair [74,75]. Platelet granules can release EGF during hemostasis, and epithelial cells migrate from the wound to the wound surface [76]. In the inflammatory stage, EGF can chemotaxis macrophages and fibroblasts to the wound surface and promote the inflammatory response [77]. EGF can stimulate the proliferation and division of endothelial cells and epithelial cells so that the epithelial cells migrate to the surface and promote wound shrinkage [78]. During remodeling, EGF inhibits the inflammatory response, reduces TGF–β1 expression, mediates collagen formation, and reduces scar tissue formation [79].
3.5 Role of TGF–β
TGF–β mainly includes three types: TGF–β1, TGF–β2, and TGF–β3, among which TGF–β1 plays a dominant role in wound healing [80]. TGF–β1 can promote the aggregation of inflammatory cells such as neutrophils and macrophages during inflammation [81,82]. TGF–β1 promotes the synthesis of collagen, elastic fibers, and fibronectin in fibroblasts and the formation of granulation tissue [83]. TGF–β1 regulates epithelial mesenchymal transformation and promotes cell migration, angiogenesis, and fibrous tissue proliferation [84]. TGF–β1 is involved in the synthesis of type I and III collagen during wound remodeling, inhibits the synthesis of MMP, reduces the degradation of collagen, and promotes the formation of scar tissue [85,86]. TGF–β1 and TGF–β2 had similar effects on wound healing, while TGF–β3 had opposite effects. TGF–β3 inhibits inflammation and protein synthesis, reducing scar formation by inhibiting collagen formation [87,88].
3.6 Role of NGF
In addition to playing a role in the nervous system, NGF also plays an important role in wound healing. NGF can promote the proliferation and migration of fibroblasts and keratinocytes and improve the synthesis level of ECM [89]. NGF also promotes angiogenesis and myofibroblast differentiation and promotes wound contraction by promoting collagen synthesis [90].
4 Characteristics of hydrogels based on natural biological materials and growth factors
In the process of wound healing, an appropriate local environment of the wound will be beneficial to wound healing and repair. The ideal wound dressing should have the following characteristics: good biocompatibility; suitable hydrophilicity and water absorption; maintenance of the moist wound environment; air permeability and heat insulation ability; antibacterial properties; suitable adhesion to the wound surface; and non-adhesive properties [91–93]. Traditional wound accessories, such as gauze, can isolate the wound from the outside world, but they cannot keep the moist environment of the wound and easily stick to the wound to form secondary damage during replacement, which has certain deficiencies in promoting wound repair [94]. Hydrogels are hydrophilic 3D gels that generally have good hydrophilicity, adhesion, permeability, and biocompatibility [95]. These characteristics make hydrogels very suitable for wound dressings. In addition, by modifying the hydrogel, the hydrogel can have more biological functions conducive to wound repair. Hydrogels can be divided into natural biological materials and synthetic materials according to the source of raw materials. Natural biological materials have been widely used in the preparation of wound excipients due to their good biocompatibility and degradability [10]. Common natural materials include chitosan, alginate, collagen, fibrin, gelatin, etc. The following part of this review will introduce the properties of various natural materials and their application with growth factors in wound healing.
4.1 Chitosan
Chitosan is the product of the natural polysaccharide chitin removing part of the acetyl group, which has good biocompatibility and degradability. Meanwhile, chitosan also has antibacterial and antitumor properties, which make it very suitable for the preparation of wound dressings [94]. Chitosan can also participate in wound healing. Chitosan can enhance the expression of GPIIB–IIIA on the platelet membrane and promote platelet adhesion to the vascular wall [96]. In addition, positively charged chitosan can also interact with a large number of negatively charged substances on the surface of activated platelets to promote platelet aggregation [97]. Through the above mechanisms, chitosan can promote wound hemostasis, induce RBC aggregation, and inhibit fibrinolysis. In addition, chitosan can increase the secretion of IL-8 in fibroblasts, accelerate the inflammatory process, and promote angiogenesis [98,99]. Chitosan with a low molecular weight can also promote the proliferation of fibroblasts, enhance the synthesis and secretion of collagen, and promote the growth of granulation tissue [98]. Chitosan also has an inhibitory effect on bacterial growth, which may be achieved by destroying the bacterial cell wall and membrane, chelating with metal cations, interacting with intracellular targets, and deposition on the bacterial surface [100–102]. Table 2 listed some combination of chitosan and growth factors in skin wound healing.
Summary of chitosan hydrogel loaded with growth factors in wound healing
| Other polymers used | Factors loaded | Outcomes | Ref. |
|---|---|---|---|
| PAA and pHEMA | EGF | Better wound healing rate | [103] |
| CNC | EGF | Accelerates granulation formation, promotes collagen deposition, and accelerates the healing process | [105] |
| Alginate | EGF | Promotes fibroblast proliferation and promotes wound healing | [106] |
| PDA and Cip | EGF | Enhances antibacterial effect and shortens the wound healing cycle. | [107] |
| HA | EGF and CNP | Promotes ECM remodeling and epithelial reformation and accelerates granulation formation | [108] |
| OHA | EGF and insulin | Promotes fibroblast proliferation and promote collagen deposition. | [109] |
| Pluronic | EGF | Promotes epidermal differentiation. | [104] |
| PEG | VEGF and PDGF | Promotes angiogenesis, accelerates wound reepithelialization and wound healing. | [110] |
| HA | VEGF Vancomycin | Reduces inflammation and promotes angiogenesis. | [111] |
| PLGA | PDGF | Promotes granulation formation and collagen synthesis and reduces autophagy | [112] |
| Azide and lactose | FGF | Promotes granulation formation, angiogenesis, and epithelial formation. | [113] |
| PEG | EGF and FGF | Promotes fibroblast proliferation and enhances wound healing. | [114] |
| Collagen | FGF | Promotes dermal cell proliferation and accelerates collagen production. | [115] |
The hydrogel form of chitosan applied in wound repair can retain the properties of chitosan itself and has better flexibility, moisture retention, adsorption capacity, and air permeability. On this basis, hydrogel-like chitosan can be further modified and combined with growth factors to construct a more suitable composite dressing for wound healing. Yao et al. prepared a chitosan-PAA-pHEMA hydrogel (CSPAH) by UV polymerization grafting poly(acrylic acid) (PAA) and poly(hydroxyethyl methacrylate) (pHEMA) and loaded EGF into the hydrogel to promote wound healing. The results showed that the hydrogel had good thrombosis ability and antibacterial activity. At the same time, CSPAH can effectively delay the release of EGF, which can be as long as 7 days or even longer, enhancing the activity of EGF in the wound site. Animal experimental results shown in Figure 4 illustrated that CSPAH loaded with EGF could significantly improve the wound healing rate. HE staining results showed that epithelial migration, inflammatory cell migration and neovascularization were more obvious in the EGF group [103]. Choi and Yoo prepared a Pluronic/chitosan hydrogel containing EGF and showed that chitooligosaccharide and rhEGF in the hydrogel significantly enhanced epidermal differentiation during wound healing [104]. Mariia et al. prepared a cellulose nanocrystal (CNC)-enhanced chitosan hydrogel (CS-U-CNC-EGF) loaded with EGF by the freeze-drying method. The results showed that granulation tissue formation in the wound area was significantly accelerated, collagen deposition was increased, and the wound healing rate was significantly improved in the treatment group [105].
![Figure 4
Representative images and HE staining results of wound surface healing over time in CSPAH, CSPAH-incorporating EGF, commercial dressings, and the control at days 10 and 20. HEMA: hydroxyethyl methacrylate; CSPAH: chitosan-PAA-pHEMA; EGF: epidermal growth factor; PAA: poly(acrylic acid) [103].](/document/doi/10.1515/ntrev-2022-0122/asset/graphic/j_ntrev-2022-0122_fig_004.jpg)
Representative images and HE staining results of wound surface healing over time in CSPAH, CSPAH-incorporating EGF, commercial dressings, and the control at days 10 and 20. HEMA: hydroxyethyl methacrylate; CSPAH: chitosan-PAA-pHEMA; EGF: epidermal growth factor; PAA: poly(acrylic acid) [103].
In addition to EGF, chitosan can also be combined with VEGF and PDGF to improve wound angiogenesis. Yang et al. prepared visible light curable GC hydrogels capable of continuously releasing VEGF and PDGF to promote angiogenesis in the injured area, accelerate wound reepithelialization, and promote wound healing by continuously releasing VEGF and PDGF [110]. Huang et al. prepared a tunable sequential drug delivery system based on chitosan/hyaluronic acid (HA) and PLGA microspheres, which promoted angiogenesis by carrying VEGF and vancomycin, reduced the inflammatory response caused by the bacterial growth, and promoted wound healing [111]. Xu et al. prepared a thermosensitive chitosan hydrogel containing PLGA microspheres and loaded it with PDGF for the treatment of skin injury. The results showed that hydrogels containing PDGF could promote the formation of granulation tissue and collagen, reduce the level of autophagy and promote wound healing. Fibroblasts play an important role in wound healing and proliferation [112]. Obara and others used ultraviolet radiation crosslinking chitosan (Az-CH-LA) in aqueous solution containing FGF, prepared flexible water insoluble gel, and applied water gel to diabetic mouse skin defects. The results showed that hydrogels containing FGF raised wound granulation tissue, blood capillaries, and epithelization levels, significantly induced wound contraction, and accelerated wound closure [113].
4.2 Alginate
Alginate is a kind of natural anionic polysaccharide compound extracted from the cell wall of brown algae and some bacterial strains, such as nitrogen-fixing bacteria and Pseudomonas. They are long chain polymers composed of 1,4-β-crosslinked d-mannuronic acid and 1,4-α-crosslinked guluronic acid [116]. Alginate from algae is insoluble in water, but its sodium salts are solvable. Adding Ca2+ to alginate solution can precipitate alginate in a gel state [117]. Alginate can be divided into the M region (mannuronic acid-rich region), G region (guluronic acid-rich region), and MG region (rich in both uronic acids) [118]. The proportion of MG in alginate from different sources is quite different, and the different proportion of MG determines the different properties of alginate [119]. For example, alginate gels with high G content have greater hardness, while alginate saltwater gels with high M content are softer and more elastic [120]. Alginate is nondegradable in mammals due to the absence of alginase [121]. Some studies believe that alginate has certain immunogenicity, which can cause toxicity or immune response in the body, but this is not caused by alginate itself. Heavy metals and impurities in the extraction and preparation of alginate are the causes of this problem [122,123]. Alginate has certain antibacterial and antiviral properties [124,125]. In addition, alginate can promote hemostasis by activating platelets and promoting thrombin clot formation [126]. Dry alginate is not suitable for wound surfaces because dry alginate is highly absorbent and can cause wound adhesion [127]. However, the alginate hydrogel showed good water retention, which is conducive to keeping the wound environment moist and promoting wound healing [128]. At the same time, alginate hydrogel can ensure good adhesion to the wound and will not adhere to the wound tissue, so the removal will not cause secondary injury to the wound [129]. These properties make alginate hydrogels have the potential to be used in wound dressings. Table 3 lists some combination of alginate and growth factors in skin wound healing.
Summary of alginate hydrogel loaded with growth factors in wound healing
| Other polymers used | Factors loaded | Outcomes | Ref. |
|---|---|---|---|
| Zn and SCS | EGF | Is antibacterial, and accelerates early wound healing, promotes fibroblast migration, promotes angiogenesis, and increase collagen deposition | [130] |
| HA | EGF | Promotes cell proliferation and adhesion | [131] |
| PNIPAM and silk fibroin | EGF and VANCO | Is antibacterial and promotes fibroblast proliferation | [132] |
| Nap–GFFKH | FGF and penicillin | Inhibits inflammation and accelerates wound healing | [133] |
| Collagen | BFGF and VEGF | Promotes angiogenesis | [134] |
| PF127 | VEGF | Accelerates granulation formation and has better wound healing rate | [135] |
| Methacrylate | PDGF–BB | Promotes angiogenesis and fibroblast proliferation | [136] |
| Dex | PDGF and BMSC | Promotes angiogenesis and enhances wound healing | [137] |
Since alginate brine gel itself cannot promote cell migration and cell adhesion, it has good application prospects to load growth factors into alginate hydrogels or modify hydrogels with other substances to obtain a better effect. As shown in Figure 5, Zhu et al. constructed a zinc-doped bioactive glass (ZBG)/succinyl chitosan (SCS)/oxidized alginate (OAL) composite hydrogel (GEL–ZBG) and embedded EGF into the hydrogel to improve cell proliferation and tissue remodeling in the wound bed. The results showed that the hydrogel had good antibacterial properties and that the addition of EGF improved the early healing speed of wounds. Histological results showed that GEL–ZBG loaded with EGF could promote the migration and enrichment of fibroblasts to the wound area, promote the growth of granulation tissue and neovascularization, and increase the deposition level of collagen and collagen fibers [130]. Liu et al. prepared a HAAL hydrogel composed of HA and sodium alginate by ion and covalent crosslinking, loaded it with EGF in the hydrogel, and found that the EGF-loaded HAAL hydrogel showed enhanced cell proliferation and adhesion [131]. Rezaei et al. constructed a dual drug delivery system in which EGF and pH-sensitive silk fibroin/alginate nanoparticles were embedded in PNIPAM hydrogel for the treatment of severe infectious burn wounds. The results showed that the system could significantly reduce the incidence of wound infection and promote the proliferation and growth of fibroblasts [132].
![Figure 5
(a and b) TEM image for BG and ZBG. SEM images of (c) Gel and (d) Gel–ZBG composite hydrogels. (e) Schematic representation of the composite hydrogels as wound dressing for wound closure of SD rats [128].](/document/doi/10.1515/ntrev-2022-0122/asset/graphic/j_ntrev-2022-0122_fig_005.jpg)
(a and b) TEM image for BG and ZBG. SEM images of (c) Gel and (d) Gel–ZBG composite hydrogels. (e) Schematic representation of the composite hydrogels as wound dressing for wound closure of SD rats [128].
To rapidly control inflammation and accelerate wound healing, Cui et al. developed a two-drug delivery system in which micron alginate fibers, FGF, and penicillin were encapsulated in instant self-assembling peptide hydrogels. The results showed that this two-drug delivery system could continuously release FGF and suddenly release antibiotics. Animal studies have shown that hydrogels can quickly reduce wound inflammation and speed up wound healing [133]. Azarpira et al. mixed alginate oxide containing VEGF and bFGF into acellular collagen-alginate complex hydrogels and found that it could significantly improve blood vessel generation and promote endothelial cell migration and growth [134]. Chou et al. constructed a thermosensitive and reversible IPN hydrogel by treating alginate with Pluronic F127 (PF127), which was equipped with VEGF to further promote tissue regeneration and wound healing rate [135]. In recent years, PDGF and alginate hydrogels have been combined to promote wound healing. Babavalian et al. prepared alginate sulfate hydrogels by photocrosslinking and applied them in the treatment of rat skin injury by loading PDGF. The results showed that hydrogels containing PDGF could significantly promote angiogenesis and fibroblast proliferation and promote wound recovery [136]. Zhang et al. synthesized an injectable hydrogel using sodium alginate and glucan as a delivery system to deliver PDGF and BMSCs simultaneously in the wound to promote wound healing. The results showed that the PDGF/SA/Dex hydrogel could promote angiogenesis by activating the PDGF-BB/PDGFR-β-mediated PI3K/Akt/eNOS pathway, thus accelerating BMSC-mediated skin wound healing [137].
4.3 Collagen
Collagen is the most abundant protein in mammals, accounting for 25–30% of the total protein. Collagen is widely found in various connective tissues. Approximately 29 kinds of collagen have been discovered thus far, among which type I collagen is the most important type used [120]. Collagen is a major component of the ECM and plays a dominant role in maintaining the biological and structural integrity of the ECM. Collagen is resistant to protease hydrolysis and has good biocompatibility as a protein produced from the organism itself [138]. Although the use of collagen between species may cause an immune response, this problem can be avoided to some extent by heterologous expression in animals or in E. coli [139,140]. Collagen stimulates cell migration and contributes to the development of new tissue. Collagen stimulates and recruits corresponding cell types, such as macrophages and fibroblasts, according to the healing cascade order, promoting cell aggregation in the wound area [141]. Due to the chemotactic effect of collagen on fibroblasts, exogenous collagen can stimulate the aggregation of fibroblasts in the wound and the deposition of endogenous collagen, forming a local healing environment. Collagen also has a good moisturizing effect and adhesion to the wound and is easy to replace without damaging the wound surface. Collagen can also form 3D structures with high tensile strength and stability through crosslinking and self-aggregation [142]. Such excellent mechanical properties make collagen suitable as a matrix material for wound dressings. Table 4 gives the summary of collagen loaded with growth factors in wound healing.
Summary of collagen loaded with growth factors in wound healing
| Other polymers used | Factors loaded | Outcomes | Ref. |
|---|---|---|---|
| HA and Sulfated HA | HB–EGF | Promotes keratinocyte migration, EGFR signal transduction, and promotes HGF expression | [143] |
| HA | EGF | Increases VEGF and HGF release, accelerates wound healing, and promotes granulation formation | [144] |
| HA | EGF and vitamin C | Promotes granulation formation, angiogenesis, and accelerates epithelial formation | [145] |
| — | EGF | Promotes migration of fibroblasts and collagen deposition, and accelerates wound reepithelialization | [146] |
| Chitosan | FGF | Accelerates collagen production and promotes TGF expression | [115] |
| — | FGF | Promotes angiogenesis and promotes epithelial formation | [147] |
| — | FGF and Ag | Promotes fibroblast proliferation | [148] |
| Chitosan | PDGF | Increases collagen synthesis and promotes granulation formation | [57] |
| — | PDGF | Promotes fibroblast proliferation | [58] |
| — | TGF–β1 | Promote chronic wound healings | [149] |
Note: “–” means no other polymers were used in the hydrogel synthesis.
Collagen is widely used in tissue regeneration and tissue repair due to its excellent biological properties. At present, commercial collagen products such as gelatin sponges have been used in tissue repair and other fields. Using other materials to modify collagen and load factors to make it more suitable for wound repair is a research hotspot.Thones et al. constructed a HA/collagen hydrogel containing HA sulfate to promote wound healing through continuous release of heparin binding EGF-like growth factor, as shown in Figure 6. In vitro experiments showed that hydrogels containing HB–EGF induced keratinocyte migration, EGFR signaling, and HGF expression in dermal fibroblasts. In a porcine skin organ culture model, it was found that the growth of the epithelial tip could be significantly improved [143]. Kondo et al. constructed a HA collagen sponge dressing made of HA and collagen of different molecular weights and added EGF to it. They found that EGF-loaded dressings significantly increased the release of VEGF and HGF in fibroblasts, promoting wound healing and granulation tissue formation [144]. Niiyama and Kuroyanagi constructed a new wound dressing composed of HA and collagen loaded with vitamin C and EGF and found that the new wound dressing promoted granulation tissue and angiogenesis and accelerated epithelial formation in SD rats and diabetic mice [145]. Cheng et al. prepared a hybrid lyophilized dressing composed of EGF and recombinant human collagen and found that this dressing significantly enhanced fibroblast proliferation adhesion and migration to the wound. Immunohistochemical results showed that the wound healing rate, epithelial regeneration, and orderly arrangement and deposition of collagen were significantly accelerated in rats [146].
![Figure 6
Schematic diagram of HE-EGF-combined hyaluronan/collagen hydrogels as wound dressings for wound healing [143].](/document/doi/10.1515/ntrev-2022-0122/asset/graphic/j_ntrev-2022-0122_fig_006.jpg)
Schematic diagram of HE-EGF-combined hyaluronan/collagen hydrogels as wound dressings for wound healing [143].
Wang et al. constructed a chitosan crosslinked collagen sponge containing FGF and applied it in diabetic rat wound healing. The results showed that the wound healing time was shortened, collagen generation was the fastest, and TGF expression was the earliest, which significantly promoted the wound healing of diabetes [115]. Pandit et al. treated full-thickness skin defects in rabbits by delivering FGF via collagen scaffolds, and the results showed that FGF collagen scaffolds increased angiogenesis and epithelial formation and promoted wound healing [147]. Chakrabarti et al. found that FGF-loaded collagen-AgSD hydrogels accelerated burn healing in animal models, alleviated burn inflammation by activating ERK and TRK pathways, and promoted fibroblast proliferation [148]. Juzith et al. developed a novel complex hydrogel that combines collagen, chitosan, and PDGF, which increased the levels of antioxidants and lipid peroxides, increased collagen synthesis and granulation tissue production, and significantly shortened the wound healing time in the treated group than in the control group [57]. Lin et al. constructed a collagen scaffold with PDGF targeted binding for wound repair and found that such collagen targeted binding PDGF had a longer release cycle, significantly increased fibroblast proliferation and accelerated wound granulation tissue formation [58]. Tateshita et al. applied a collagen matrix containing TGF-β1 to the surface of wounds, and the results showed that the application of TGF-β1-treated collagen matrix is effective for preventing contraction producing the so-called “neodermis” in treating a delayed healing type model and may be highly beneficial for treating chronic wounds [149].
4.4 Fibrin
Fibrin is a natural polymer formed during wound healing and is formed by cleavage of fibrinogen by serine protease thrombin during wound healing and hemostasis. Fibrin, along with platelets, is involved in wound hemostasis and provides the basis for subsequent repair. During the proliferative stage, fibrin and fibronectin can promote the proliferation and migration of fibroblasts to the wound site and promote the formation and deposition of collagen [150]. As the wound repair process progresses, fibrin clots eventually become deabsorbed by plasmin and participate in the formation of ECM. Fibrin can also serve as a reservoir of growth factors and cytokines during wound repair, and a variety of cytokines, such as EGF, FGF, and PDGF, can be found in fibrin clots [151]. Growth factors coated with fibrin can avoid rapid release and degradation in the body and prolong the action time of growth factors in wound repair. This property makes fibrin particularly suitable for the delivery of growth factors and cells during wound healing and has the potential to become a wound dressing [152]. However, the rapid degradation of fibrin has always been the main problem restricting its clinical transformation. Because fibrin can be easily degraded by plasmin in the body, its ability to maintain growth factors and cells is significantly reduced [153]. To solve this problem, there are a variety of modification methods for fibrin, such as adding heparin to fibrin and combining it with other biological matrix materials [154]. These methods prolong the degradation cycle of fibrin to a certain extent and increase the effect and feasibility of fibrin in clinical applications.
The schematic diagram made by Rahman et al. in Figure 7 constructed a platelet-rich and fibrin-rich hydrogel from expired platelet components that contained physiological concentrations of TGF–β1, PDGF, IGF–1, FGF, and EGF. Application of the hydrogel to skin wounds in SKH–1 mice significantly enhanced angiogenesis in the wound area. High levels of IL–6 and KC also lasted longer in treated wounds than in untreated wounds. The epithelialization and healing of the wound area were significantly accelerated [156]. Certelli et al. constructed a system capable of instantaneous delivery of engineered VEGF and PDGF–BB in fibrin hydrogels. On applying this system to a diabetic mouse model of skin injury, it was found that this combination of treatments could establish stable angiogenesis in the injured area and promote wound growth and healing [157]. Tan et al. constructed a 3D fibrin gel embedded with BMSCs and VEGF, and BMSCs in the gel differentiated into CD31 + and vWF + ECs induced by VEGF. Animal studies have shown that fibrin gel can stop bleeding quickly without secondary bleeding. Fibrin gels containing BMSCs have shown the potential to promote neovascularization and wound healing [155].
![Figure 7
Schematic diagram of biofunctionalized fibrin gel co-embedded with BMSCs and VEGF for accelerating skin injury repair [155].](/document/doi/10.1515/ntrev-2022-0122/asset/graphic/j_ntrev-2022-0122_fig_007.jpg)
Schematic diagram of biofunctionalized fibrin gel co-embedded with BMSCs and VEGF for accelerating skin injury repair [155].
4.5 Gelatin
Gelatin has homology with collagen and is the product of partial hydrolysis of collagen. The structure of gelatin is similar to that of the ECM, and it can simulate the structure and morphology of normal tissues well, which is conducive to cell migration and growth [158]. Gelatin has temperature reversibility. By adjusting the temperature, hydrogel materials with high tensile strength, dermal structure, and good permeability and water can be prepared [159]. Gelatin molecules also contain a large number of functional groups, which can be chemically modified or modified to carry different drugs or growth factors into the molecules to improve their performance or therapeutic effect. In addition, as a hydrolysate of collagen, gelatin has significantly lower immunogenicity and better histocompatibility than collagen [159]. However, gelatin itself is a hydrophilic protein that usually needs to be modified to increase its mechanical properties and stability when it is used in the preparation of wound dressings or hydrogels [6]. In addition, gelatin itself lacks certain antibacterial properties, and simple gelatin easily causes bacteria to grow on its surface, so in the application, it should be combined with antibacterial substances to enhance its antibacterial properties [160].
Augustine et al. constructed a poly(3-hydroxybutyrate-co-3-hydroxyvalerate)/gelatin methacryloyl hybrid patch using GelMA and PHBV, in which EGF was loaded for the treatment of diabetic wound healing. GelMA shows good moisture retention and water absorption, while the PHBV film provides the hydrogel with the right mechanical strength. EGF-loaded patches can promote cell migration, cell proliferation, and angiogenesis and thereby, the rapid healing of diabetic wounds [161] (Figure 8). Huang et al. constructed a functional bilayer skin substitute for wound repair using tissue-engineered ECM and gelatin hydrogel doped with microspheres. It was found that the bionic skin improved healing in the wound area, showing early epithelial regeneration in the wound area [162]. Jin et al. prepared a foam dressing for skin regeneration using gelatin, HA, and carboxymethyl chitosan containing FGF and found that wound healing was significantly accelerated in animal experiments [163]. Wan et al. constructed a 3D double-layer hydrogel scaffold loaded with PDGF and Ag, which can significantly reduce wound surface infection, results showed great potential for promoting diabetic wound healing and fighting bacterial infection [164].
![Figure 8
Schematic diagram of growth factor-loaded in situ photocrosslinkable poly(3-hydroxybutyrate-co-3-hydroxyvalerate)/gelatin methacryloyl hybrid patch for diabetic wound healing [159].](/document/doi/10.1515/ntrev-2022-0122/asset/graphic/j_ntrev-2022-0122_fig_008.jpg)
Schematic diagram of growth factor-loaded in situ photocrosslinkable poly(3-hydroxybutyrate-co-3-hydroxyvalerate)/gelatin methacryloyl hybrid patch for diabetic wound healing [159].
5 Advantages and disadvantages
Growth factors can be chemically grafted or directly mixed into hydrogels for skin wound healing. As shown in Figure 9, compared with traditional dressings, hydrogels have better properties, such as moisture retention, adhesion, air permeability, and biocompatibility [89]. At the same time, some natural biological materials, such as chitosan, also have hemostatic ability and certain antibacterial ability, which can participate in the pathological process of wound healing and play a role in promoting healing [98]. Growth factors play an important role in the process of wound healing, and the local wound itself can secrete growth factors to regulate the progress of the healing process. Growth factor loaded in hydrogel can maintain growth factor in the wound area for a longer time through the slow-release effect of hydrogel to have a more lasting effect of promoting proliferation and differentiation and accelerating wound healing [16]. However, there are still some shortcomings in this application (Figure 9). First, the mechanical properties of hydrogels based on natural biological materials are mostly deficient, and the application of hydrogels directly prepared by the materials themselves into wound dressings cannot provide sufficient mechanical strength and stability [165]. It is often necessary to combine with other nondegradable materials or polymer compounds to improve their mechanical strength. Second, the degradation rate of hydrogels based on biological materials needs to be regulated to reach the appropriate length of time. Some matrix materials, such as collagen, cannot sustain a sufficiently long degradation cycle and need to be modified to match the degradation time with the wound healing cycle [166]. In addition, some biological materials lack antibacterial properties, and their main components are proteins or polysaccharides, which can easily cause bacterial breeding, infection, or inflammation [167]. Finally, the high cost of preparation and preservation of some natural biomaterials also limits the large-scale clinical application of natural biomaterial-based hydrogels [9].
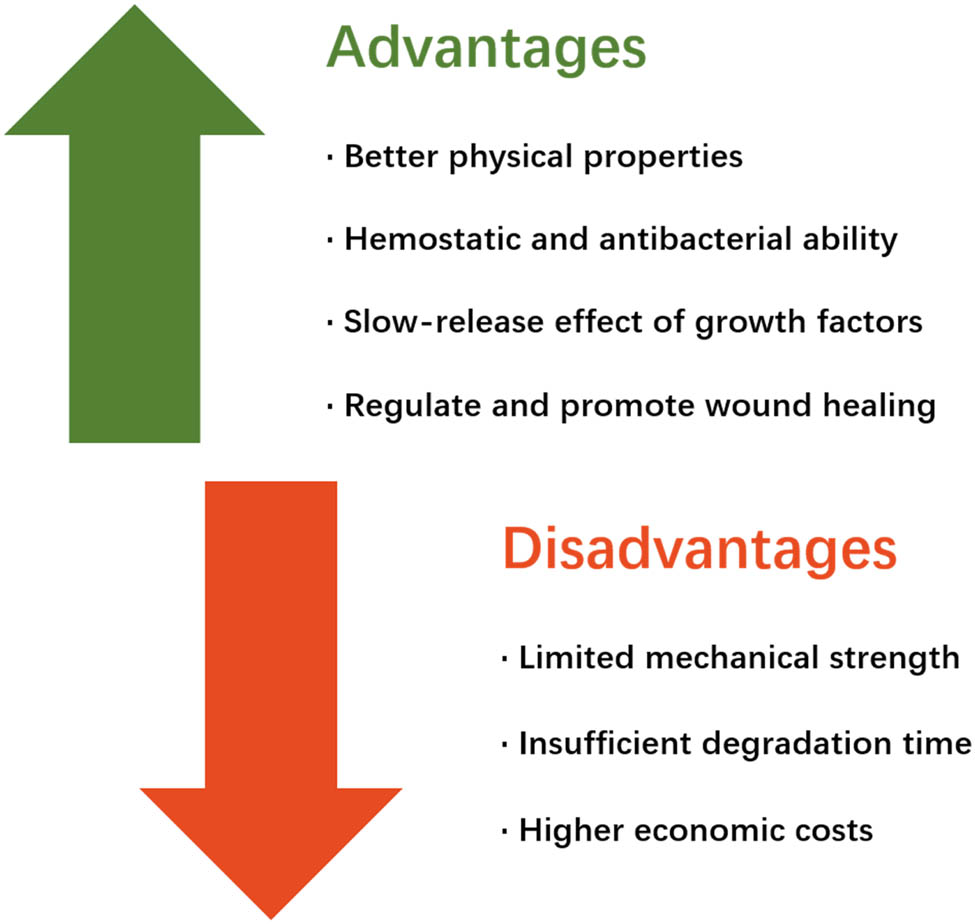
The advantages and disadvantages of the combination of natural-based biological hydrogels and growth factors for wound healing.
6 Conclusion and future perspectives
Skin wound healing is a continuous and complex process that is affected by many factors. Growth factors play an important role in skin wound healing and are an indispensable part of wound healing. Using hydrogels to provide an environment for local delivery and continuous release of growth factors can not only promote wound healing and growth but also provide a suitable environment for wound surfaces with excellent performance and play a protective role. Existing experimental studies have proven that hydrogels combined with growth factors based on natural biological materials have very precise therapeutic effects and advantages in the treatment of skin wound healing. This combination provides excellent moisture, adhesion, air permeability and biocompatibility as well as promotes proliferation and growth regulation of wound growth that traditional dressings cannot match.
This review focuses on hydrogels constructed from natural materials and summarizes the effect of the combination of natural hydrogels and growth factors on skin wound healing and the potential mechanism of the combination in the healing process. A large number of experimental results have proved to us that the wound dressing constructed with natural medical hydrogel as the matrix material has good biocompatibility and is very beneficial to wound protection and cell growth. Growth factor, as a kind of peptide substance with multiple functions secreted by body cells, plays an important role in the healing process of skin. However, the growth factor produced by the body itself is very limited in both quantity and duration, and cannot maintain the required concentration and dose in the wound healing process for a long time. Therefore, growth factors can be added to the wound dressing to compensate for this deficiency and maximize the healing process of the wound. However, due to the variety of natural hydrogels and growth factors, the combination of different natural hydrogels and growth factors may have different effects through different mechanisms in the process of wound healing. Therefore, this review will give a comprehensive overview of all the natural based hydrogels and various growth factors applied in skin wound repair, and systematically introduce the mechanism of action and application effect of various natural based hydrogels and growth factors combined in wound repair. It provides a systematic reference for the selection of matrix materials and growth factors in the subsequent design of wound healing dressings, enabling experimenters to more appropriately select the corresponding combination to achieve better therapeutic effects. Although hydrogels based on natural biological materials have some shortcomings, it can be predicted that these problems and shortcomings can be partially or completely remedied by modifying the matrix materials so that their performance is closer to that of ideal skin materials.
-
Funding information: This work was supported by the National Natural Science Foundation of China Grants: 21736002, 31961133015 to. F.W. and 31870791 to Z.M.
-
Author contributions: F.W. and Z.M. conceptualized the study. F.W. and Z.M. oversaw the literature review involved in all aspects of designing and writing the manuscript. F.W., Y.G., and Z.M. wrote the manuscript and designed the figures. H.L., L.Z., H.S., S.F., and J.C. provided input on the discussion of various sections. All authors contributed to the article and approved the submitted version. All authors have accepted responsibility for the entire content of this manuscript and approved its submission.
-
Conflict of interest: The authors state no conflict of interest.
-
Data availability statement: The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
[1] Dąbrowska AK, Spano F, Derler S, Adlhart C, Spencer ND, Rossi RM. The relationship between skin function, barrier properties, and body-dependent factors. Skin Res Technol. 2018;24(2):165–74.10.1111/srt.12424Search in Google Scholar PubMed
[2] Guo S, Dipietro LA. Factors affecting wound healing. J Dent Res. 2010;89(3):219–29.10.1177/0022034509359125Search in Google Scholar PubMed PubMed Central
[3] Reinke JM, Sorg H. Wound repair and regeneration. Eur Surg Res. 2012;49(1):35–43.10.1159/000339613Search in Google Scholar PubMed
[4] Gantwerker EA, Hom DB. Skin: histology and physiology of wound healing. Facial Plast Surg Clin North Am. 2011;19(3):441–53.10.1016/j.fsc.2011.06.009Search in Google Scholar PubMed
[5] Wang F, Zhang W, Li H, Chen X, Feng S, Mei Z. How effective are nano-based dressings in diabetic wound healing? a comprehensive review of literature. Int J Nanomed. 2022(17):2097–119.10.2147/IJN.S361282Search in Google Scholar PubMed PubMed Central
[6] Koehler J, Brandl FP, Goepferich AM. Hydrogel wound dressings for bioactive treatment of acute and chronic wounds. Eur Polym J. 2018;100:1–11.10.1016/j.eurpolymj.2017.12.046Search in Google Scholar
[7] Liang YP, He JH, Guo BL. Functional hydrogels as wound dressing to enhance wound healing. Acs Nano. 2021;15(8):12687–722.10.1021/acsnano.1c04206Search in Google Scholar PubMed
[8] Rivadeneira J, Di Virgilio AL, Audisio MC, Boccaccini AR, Gorustovich AA. Evaluation of antibacterial and cytotoxic effects of nano-sized bioactive glass/collagen composites releasing tetracycline hydrochloride. J Appl Microbiol. 2014;116(6):1438–46.10.1111/jam.12476Search in Google Scholar PubMed
[9] Ghomi ER, Khalili S, Khorasani SN, Neisiany RE, Ramakrishna S. Wound dressings: Current advances and future directions. J Appl Polym Sci. 2019;136(27):47338.10.1002/app.47738Search in Google Scholar
[10] Ullah F, Othman MBH, Javed F, Ahmad Z, Akil HM. Classification, processing and application of hydrogels: A review. Mat Sci Eng C-Mater. 2015;57:414–33.10.1016/j.msec.2015.07.053Search in Google Scholar PubMed
[11] Gillespie BM, Chaboyer W, Allen P, Morely N, Nieuwenhoven P. Wound care practices: a survey of acute care nurses. J Clin Nurs. 2014;23(17–18):2618–26.10.1111/jocn.12479Search in Google Scholar PubMed
[12] Zubair M, Ahmad J. Role of growth factors and cytokines in diabetic foot ulcer healing: A detailed review. Rev Endocr Metab Disord. 2019;20(2):207–17.10.1007/s11154-019-09492-1Search in Google Scholar PubMed
[13] Pikuła M, Langa P, Kosikowska P, Trzonkowski P. Stem cells and growth factors in wound healing. Postepy Hig Med Dosw (Online). 2015;69:874–85.10.5604/17322693.1162989Search in Google Scholar PubMed
[14] Zarei F, Soleimaninejad M. Role of growth factors and biomaterials in wound healing. Artif Cell Nanomed B. 2018;46(sup1):906–11.10.1080/21691401.2018.1439836Search in Google Scholar PubMed
[15] Hwa C, Bauer EA, Cohen DE. Skin biology. Dermatol Ther. 2011;24(5):464–70.10.1111/j.1529-8019.2012.01460.xSearch in Google Scholar PubMed
[16] Wang PH, Huang BS, Horng HC, Yeh CC, Chen YJ. Wound healing. J Chin Med Assoc. 2018;81(2):94–101.10.1016/j.jcma.2017.11.002Search in Google Scholar PubMed
[17] Proksch E, Brandner JM, Jensen JM. The skin: an indispensable barrier. Exp Dermatol. 2008;17(12):1063–72.10.1111/j.1600-0625.2008.00786.xSearch in Google Scholar PubMed
[18] Lee T, Friedman A. Skin Barrier health: regulation and repair of the stratum corneum and the role of over-the-counter skin care. J Drugs Dermatol. 2016;15(9):1047–51.Search in Google Scholar
[19] Freeman SC, Sonthalia S. Histology, keratohyalin granules. StatPearls. Treasure Island (FL): StatPearls Publishing Copyright © 2021, StatPearls Publishing LLC.; 2021.Search in Google Scholar
[20] Suzuki H, Kurosumi K. Lamellar granules and keratohyalin granules in the epidermal keratinocytes, with special reference to their origin, fate and function. J Electron Microsc (Tokyo). 1972;21(4):285–92.Search in Google Scholar
[21] Losquadro WD. Anatomy of the skin and the pathogenesis of nonmelanoma skin cancer. Facial Plast Surg Clin North Am. 2017;25(3):283–9.10.1016/j.fsc.2017.03.001Search in Google Scholar PubMed
[22] Jensen JM, Proksch E. The skin’s barrier. G Ital Dermatol Venereol. 2009;144(6):689–700.Search in Google Scholar
[23] McGovern JA, Heinemann JR, Burke LJ, Dawson R, Parker TJ, Upton Z, et al. Stratum basale keratinocyte expression of the cell-surface glycoprotein CDCP1 during epidermogenesis and its role in keratinocyte migration. Br J Dermatol. 2013;168(3):496–503.10.1111/bjd.12119Search in Google Scholar PubMed
[24] McLafferty E, Hendry C, Alistair F. The integumentary system: anatomy, physiology and function of skin. Nurs Stand. 2012;27(3):35–42.10.7748/ns2012.09.27.3.35.c9299Search in Google Scholar
[25] Woodley DT. Distinct fibroblasts in the papillary and reticular dermis: implications for wound healing. Dermatol Clin. 2017;35(1):95–100.10.1016/j.det.2016.07.004Search in Google Scholar PubMed
[26] Arda O, Göksügür N, Tüzün Y. Basic histological structure and functions of facial skin. Clin Dermatol. 2014;32(1):3–13.10.1016/j.clindermatol.2013.05.021Search in Google Scholar PubMed
[27] Usansky I, Jaworska P, Asti L, Kenny FN, Hobbs C, Sofra V, et al. A developmental basis for the anatomical diversity of dermis in homeostasis and wound repair. J Pathol. 2021;253(3):315–25.10.1002/path.5589Search in Google Scholar PubMed PubMed Central
[28] Gorain B, Pandey M, Leng NH, Yan CW, Nie KW, Kaur SJ, et al. Advanced drug delivery systems containing herbal components for wound healing. Int J Pharm. 2022;617:121617.10.1016/j.ijpharm.2022.121617Search in Google Scholar PubMed
[29] Velnar T, Bailey T, Smrkolj V. The wound healing process: an overview of the cellular and molecular mechanisms. J Int Med Res. 2009;37(5):1528–42.10.1177/147323000903700531Search in Google Scholar PubMed
[30] Golebiewska EM, Poole AW. Platelet secretion: From haemostasis to wound healing and beyond. Blood Rev. 2015;29(3):153–62.10.1016/j.blre.2014.10.003Search in Google Scholar PubMed PubMed Central
[31] Gurtner GC, Werner S, Barrandon Y, Longaker MT. Wound repair and regeneration. Nature 2008;453(7193):314–21.10.1038/nature07039Search in Google Scholar PubMed
[32] Segel GB, Halterman MW, Lichtman MA. The paradox of the neutrophil’s role in tissue injury. J Leukoc Biol. 2011;89(3):359–72.10.1189/jlb.0910538Search in Google Scholar PubMed PubMed Central
[33] Koh TJ, DiPietro LA. Inflammation and wound healing: the role of the macrophage. Expert Rev Mol Med. 2011;13:e23.10.1017/S1462399411001943Search in Google Scholar PubMed PubMed Central
[34] Singer AJ, Clark RA. Cutaneous wound healing. N Engl J Med. 1999;341(10):738–46.10.1056/NEJM199909023411006Search in Google Scholar PubMed
[35] Eming SA, Krieg T, Davidson JM. Inflammation in wound repair: molecular and cellular mechanisms. J Invest Dermatol. 2007;127(3):514–25.10.1038/sj.jid.5700701Search in Google Scholar PubMed
[36] Bainbridge P. Wound healing and the role of fibroblasts. J Wound Care. 2013;22(8):407–8.10.12968/jowc.2013.22.8.407Search in Google Scholar
[37] Veith AP, Henderson K, Spencer A, Sligar AD, Baker AB. Therapeutic strategies for enhancing angiogenesis in wound healing. Adv Drug Deliv Rev. 2019;146:97–125.10.1016/j.addr.2018.09.010Search in Google Scholar
[38] Tomic-Canic M, Wong LL, Smola H. The epithelialisation phase in wound healing: options to enhance wound closure. J Wound Care. 2018;27(10):646–58.10.12968/jowc.2018.27.10.646Search in Google Scholar
[39] Diegelmann RF, Evans MC. Wound healing: an overview of acute, fibrotic and delayed healing. Front Biosci. 2004;9:283–9.10.2741/1184Search in Google Scholar
[40] Witte MB, Barbul A. General principles of wound healing. Surg Clin North Am. 1997;77(3):509–28.10.1016/S0039-6109(05)70566-1Search in Google Scholar
[41] Bonifant H, Holloway S. A review of the effects of ageing on skin integrity and wound healing. Br J Community Nurs. 2019;24(Sup3):S28–33.10.12968/bjcn.2019.24.Sup3.S28Search in Google Scholar PubMed
[42] Wilkinson HN, Hardman MJ. The role of estrogen in cutaneous ageing and repair. Maturitas. 2017;103:60–4.10.1016/j.maturitas.2017.06.026Search in Google Scholar PubMed
[43] Slominski AT, Zmijewski MA. Glucocorticoids inhibit wound healing: novel mechanism of action. J Invest Dermatol. 2017;137(5):1012–4.10.1016/j.jid.2017.01.024Search in Google Scholar PubMed PubMed Central
[44] Amadeu TP, Braune AS, Porto LC, Desmoulière A, Costa AM. Fibrillin-1 and elastin are differentially expressed in hypertrophic scars and keloids. Wound Repair Regen. 2004;12(2):169–74.10.1111/j.1067-1927.2004.012209.xSearch in Google Scholar PubMed
[45] Edwards R, Harding KG. Bacteria and wound healing. Curr Opin Infect Dis. 2004;17(2):91–6.10.1097/00001432-200404000-00004Search in Google Scholar PubMed
[46] Lin P-H, Sermersheim M, Li H, Lee PHU, Steinberg SM, Ma J. Zinc in wound healing modulation. Nutrients 2018;10(1):16.10.3390/nu10010016Search in Google Scholar
[47] Stechmiller JK. Understanding the role of nutrition and wound healing. Nutr Clin Pract. 2010;25(1):61–8.10.1177/0884533609358997Search in Google Scholar
[48] Carr AC, Maggini S. Vitamin C and immune function. Nutrients. 2017;9(11):1211.10.3390/nu9111211Search in Google Scholar
[49] Beyene RT, Derryberry SL Jr, Barbul A. The effect of comorbidities on wound healing. Surg Clin North Am. 2020;100(4):695–705.10.1016/j.suc.2020.05.002Search in Google Scholar
[50] Okonkwo UA, DiPietro LA. Diabetes and wound angiogenesis. Int J Mol Sci. 2017;18(7):1419.10.3390/ijms18071419Search in Google Scholar
[51] Silverstein P. Smoking and wound healing. Am J Med. 1992;93(1a):s22–4.10.1016/0002-9343(92)90623-JSearch in Google Scholar
[52] Rosa DF, Sarandy MM, Novaes RD, Freitas MB, do Carmo Gouveia Pelúzio M, Gonçalves RV. High-fat diet and alcohol intake promotes inflammation and impairs skin wound healing in Wistar rats. Mediators Inflamm. 2018;2018:4658583.10.1155/2018/4658583Search in Google Scholar PubMed PubMed Central
[53] Pierce GF, Mustoe TA, Altrock BW, Deuel TF, Thomason A. Role of platelet-derived growth factor in wound healing. J Cell Biochem. 1991;45(4):319–26.10.1002/jcb.240450403Search in Google Scholar PubMed
[54] Gope R. The effect of epidermal growth factor & platelet-derived growth factors on wound healing process. Indian J Med Res. 2002;116:201–6.Search in Google Scholar
[55] Uutela M, Wirzenius M, Paavonen K, Rajantie I, He Y, Karpanen T, et al. PDGF-D induces macrophage recruitment, increased interstitial pressure, and blood vessel maturation during angiogenesis. Blood. 2004;104(10):3198–204.10.1182/blood-2004-04-1485Search in Google Scholar PubMed
[56] Niessen FB, Andriessen MP, Schalkwijk J, Visser L, Timens W. Keratinocyte-derived growth factors play a role in the formation of hypertrophic scars. J Pathol. 2001;194(2):207–16.10.1002/path.853Search in Google Scholar PubMed
[57] Judith R, Nithya M, Rose C, Mandal AB. Application of a PDGF-containing novel gel for cutaneous wound healing. Life Sci.2010;87(1–2):1–8.10.1016/j.lfs.2010.05.003Search in Google Scholar PubMed
[58] Lin H, Chen B, Sun W, Zhao W, Zhao Y, Dai J. The effect of collagen-targeting platelet-derived growth factor on cellularization and vascularization of collagen scaffolds. Biomaterials 2006;27(33):5708–14.10.1016/j.biomaterials.2006.07.023Search in Google Scholar PubMed
[59] Xie H, Cui Z, Wang L, Xia Z, Hu Y, Xian L, et al. PDGF-BB secreted by preosteoclasts induces angiogenesis during coupling with osteogenesis. Nat Med. 2014;20(11):1270–8.10.1038/nm.3668Search in Google Scholar PubMed PubMed Central
[60] Jinnin M, Ihn H, Mimura Y, Asano Y, Yamane K, Tamaki K. Regulation of fibrogenic/fibrolytic genes by platelet-derived growth factor C, a novel growth factor, in human dermal fibroblasts. J Cell Physiol. 2005;202(2):510–7.10.1002/jcp.20154Search in Google Scholar PubMed
[61] Grazul-Bilska AT, Johnson ML, Bilski JJ, Redmer DA, Reynolds LP, Abdullah A, et al. Wound healing: the role of growth factors. Drugs Today (Barc). 2003;39(10):787–800.10.1358/dot.2003.39.10.799472Search in Google Scholar PubMed
[62] Huang W, Shao M, Liu H, Chen J, Hu J, Zhu L, et al. Fibroblast growth factor 21 enhances angiogenesis and wound healing of human brain microvascular endothelial cells by activating PPARγ. J Pharmacol Sci. 2019;140(2):120–7.10.1016/j.jphs.2019.03.010Search in Google Scholar PubMed
[63] Li H, Wang F. Core-shell chitosan microsphere with antimicrobial and vascularized functions for promoting skin wound healing. Mater Des. 2021;204:204.10.1016/j.matdes.2021.109683Search in Google Scholar
[64] Koike Y, Yozaki M, Utani A, Murota H. Fibroblast growth factor 2 accelerates the epithelial-mesenchymal transition in keratinocytes during wound healing process. Sci Rep. 2020;10(1):18545.10.1038/s41598-020-75584-7Search in Google Scholar PubMed PubMed Central
[65] Abe M, Yokoyama Y, Ishikawa O. A possible mechanism of basic fibroblast growth factor-promoted scarless wound healing: the induction of myofibroblast apoptosis. Eur J Dermatol. 2012;22(1):46–53.10.1684/ejd.2011.1582Search in Google Scholar PubMed
[66] Akita S, Akino K, Hirano A. Basic fibroblast growth factor in scarless wound healing. Adv Wound Care. 2013;2(2):44–9.10.1089/wound.2011.0324Search in Google Scholar PubMed PubMed Central
[67] Shalaby F, Rossant J, Yamaguchi TP, Gertsenstein M, Wu XF, Breitman ML, et al. Failure of blood-island formation and vasculogenesis in Flk-1-deficient mice. Nature. 1995;376(6535):62–6.10.1038/376062a0Search in Google Scholar PubMed
[68] Murohara T, Horowitz JR, Silver M, Tsurumi Y, Chen D, Sullivan A, et al. Vascular endothelial growth factor/vascular permeability factor enhances vascular permeability via nitric oxide and prostacyclin. Circulation. 1998;97(1):99–107.10.1161/01.CIR.97.1.99Search in Google Scholar
[69] Bao P, Kodra A, Tomic-Canic M, Golinko MS, Ehrlich HP, Brem H. The role of vascular endothelial growth factor in wound healing. J Surg Res. 2009;153(2):347–58.10.1016/j.jss.2008.04.023Search in Google Scholar PubMed PubMed Central
[70] Detmar M, Yeo KT, Nagy JA, Van de Water L, Brown LF, Berse B, et al. Keratinocyte-derived vascular permeability factor (vascular endothelial growth factor) is a potent mitogen for dermal microvascular endothelial cells. J Invest Dermatol. 1995;105(1):44–50.10.1111/1523-1747.ep12312542Search in Google Scholar PubMed
[71] Galeano M, Deodato B, Altavilla D, Cucinotta D, Arsic N, Marini H, et al. Adeno-associated viral vector-mediated human vascular endothelial growth factor gene transfer stimulates angiogenesis and wound healing in the genetically diabetic mouse. Diabetologia. 2003;46(4):546–55.10.1007/s00125-003-1064-1Search in Google Scholar PubMed
[72] Hong YK, Lange-Asschenfeldt B, Velasco P, Hirakawa S, Kunstfeld R, Brown LF, et al. VEGF-A promotes tissue repair-associated lymphatic vessel formation via VEGFR-2 and the alpha1 beta1 and alpha2 beta1 integrins. FASEB J. 2004;18(10):1111–3.10.1096/fj.03-1179fjeSearch in Google Scholar PubMed
[73] Wilgus TA, Ferreira AM, Oberyszyn TM, Bergdall VK, Dipietro LA. Regulation of scar formation by vascular endothelial growth factor. Lab Invest. 2008;88(6):579–90.10.1038/labinvest.2008.36Search in Google Scholar PubMed PubMed Central
[74] Mine N, Iwamoto R, Mekada E. HB-EGF promotes epithelial cell migration in eyelid development. Development. 2005;132(19):4317–26.10.1242/dev.02030Search in Google Scholar PubMed
[75] Coffey RJ Jr, Derynck R, Wilcox JN, Bringman TS, Goustin AS, Moses HL, et al. Production and auto-induction of transforming growth factor-alpha in human keratinocytes. Nature. 1987;328(6133):817–20.10.1038/328817a0Search in Google Scholar PubMed
[76] Schultz G, Rotatori DS, Clark W. EGF and TGF-alpha in wound healing and repair. J Cell Biochem. 1991;45(4):346–52.10.1002/jcb.240450407Search in Google Scholar PubMed
[77] Hardwicke J, Schmaljohann D, Boyce D, Thomas D. Epidermal growth factor therapy and wound healing-past, present and future perspectives. Surgeon. 2008;6(3):172–7.10.1016/S1479-666X(08)80114-XSearch in Google Scholar
[78] Kwon YB, Kim HW, Roh DH, Yoon SY, Baek RM, Kim JY, et al. Topical application of epidermal growth factor accelerates wound healing by myofibroblast proliferation and collagen synthesis in rat. J Vet Sci. 2006;7(2):105–9.10.4142/jvs.2006.7.2.105Search in Google Scholar PubMed PubMed Central
[79] Kim YS, Lew DH, Tark KC, Rah DK, Hong JP. Effect of recombinant human epidermal growth factor against cutaneous scar formation in murine full-thickness wound healing. J Korean Med Sci. 2010;25(4):589–96.10.3346/jkms.2010.25.4.589Search in Google Scholar PubMed PubMed Central
[80] Rolfe KJ, Richardson J, Vigor C, Irvine LM, Grobbelaar AO, Linge C. A role for TGF-beta1-induced cellular responses during wound healing of the non-scarring early human fetus? J Invest Dermatol. 2007;127(11):2656–67.10.1038/sj.jid.5700951Search in Google Scholar PubMed
[81] Wan M, Li C, Zhen G, Jiao K, He W, Jia X, et al. Injury-activated transforming growth factor β controls mobilization of mesenchymal stem cells for tissue remodeling. Stem Cell. 2012;30(11):2498–511.10.1002/stem.1208Search in Google Scholar PubMed PubMed Central
[82] Wang XJ, Han G, Owens P, Siddiqui Y, Li AG. Role of TGF beta-mediated inflammation in cutaneous wound healing. J Investig Dermatol Symp Proc. 2006;11(1):112–7.10.1038/sj.jidsymp.5650004Search in Google Scholar PubMed
[83] Ramirez H, Patel SB, Pastar I. The role of TGFβ signaling in wound epithelialization. Adv Wound Care (N Rochelle). 2014;3(7):482–91.10.1089/wound.2013.0466Search in Google Scholar PubMed PubMed Central
[84] Miscianinov V, Martello A, Rose L, Parish E, Cathcart B, Mitić T, et al. MicroRNA-148b targets the TGF-β pathway to regulate angiogenesis and endothelial-to-mesenchymal transition during skin wound healing. Mol Ther. 2018;26(8):1996–2007.10.1016/j.ymthe.2018.05.002Search in Google Scholar PubMed PubMed Central
[85] Mauviel A, Chung KY, Agarwal A, Tamai K, Uitto J. Cell-specific induction of distinct oncogenes of the Jun family is responsible for differential regulation of collagenase gene expression by transforming growth factor-beta in fibroblasts and keratinocytes. J Biol Chem. 1996;271(18):10917–23.10.1074/jbc.271.18.10917Search in Google Scholar PubMed
[86] Zeng G, McCue HM, Mastrangelo L, Millis AJ. Endogenous TGF-beta activity is modified during cellular aging: effects on metalloproteinase and TIMP-1 expression. Exp Cell Res. 1996;228(2):271–6.10.1006/excr.1996.0326Search in Google Scholar PubMed
[87] Schrementi ME, Ferreira AM, Zender C, DiPietro LA. Site-specific production of TGF-beta in oral mucosal and cutaneous wounds. Wound Repair Regen. 2008;16(1):80–6.10.1111/j.1524-475X.2007.00320.xSearch in Google Scholar PubMed
[88] Shah M, Foreman DM, Ferguson MW. Neutralisation of TGF-beta 1 and TGF-beta 2 or exogenous addition of TGF-beta 3 to cutaneous rat wounds reduces scarring. J Cell Sci. 1995;108 (Pt 3):985–1002.10.1242/jcs.108.3.985Search in Google Scholar PubMed
[89] Liu Z, Wu H, Huang S. Role of NGF and its receptors in wound healing (Review). Exp Ther Med. 2021;21(6):599.10.3892/etm.2021.10031Search in Google Scholar PubMed PubMed Central
[90] Gostynska N, Pannella M, Rocco ML, Giardino L, Aloe L, Calzà L. The pleiotropic molecule NGF regulates the in vitro properties of fibroblasts, keratinocytes, and endothelial cells: implications for wound healing. Am J Physiol Cell Physiol. 2020;318(2):C360–c71.10.1152/ajpcell.00180.2019Search in Google Scholar PubMed
[91] Gyles DA, Castro LD, Silva JOC, Ribeiro-Costa RM. A review of the designs and prominent biomedical advances of natural and synthetic hydrogel formulations. Eur Polym J. 2017;88:373–92.10.1016/j.eurpolymj.2017.01.027Search in Google Scholar
[92] Bashir S, Hina M, Iqbal J, Rajpar AH, Mujtaba MA, Alghamdi NA, et al. Fundamental concepts of hydrogels: synthesis, properties, and their applications. Polymers. 2020;12(11):2702.10.3390/polym12112702Search in Google Scholar PubMed PubMed Central
[93] Su J, Li J, Liang J, Zhang K, Li J. Hydrogel preparation methods and biomaterials for wound dressing. Life. 2021;11(10):1016.10.3390/life11101016Search in Google Scholar PubMed PubMed Central
[94] Liu H, Wang C, Li C, Qin Y, Wang Z, Yang F, et al. A functional chitosan-based hydrogel as a wound dressing and drug delivery system in the treatment of wound healing. Rsc Adv. 2018;8(14):7533–49.10.1039/C7RA13510FSearch in Google Scholar PubMed PubMed Central
[95] Feng P, Luo Y, Ke C, Qiu H, Wang W, Zhu Y, et al. Chitosan-based functional materials for skin wound repair: mechanisms and applications. Front Bioeng Biotech. 2021;9:9.10.3389/fbioe.2021.650598Search in Google Scholar PubMed PubMed Central
[96] Jackson SP, Schoenwaelder SM, Goncalves I, Nesbitt WS, Yap CL, Wright CE, et al. PI 3-kinase p110beta: a new target for antithrombotic therapy. Nat Med. 2005;11(5):507–14.10.1038/nm1232Search in Google Scholar PubMed
[97] Wang YH, Liu CC, Cherng JH, Fan GY, Wang YW, Chang SJ, et al. Evaluation of chitosan-based dressings in a swine model of artery-injury-related shock. Sci Rep. 2019;9(1):14608.10.1038/s41598-019-51208-7Search in Google Scholar
[98] Howling GI, Dettmar PW, Goddard PA, Hampson FC, Dornish M, Wood EJ. The effect of chitin and chitosan on the proliferation of human skin fibroblasts and keratinocytes in vitro. Biomaterials 2001;22(22):2959–66.10.1016/S0142-9612(01)00042-4Search in Google Scholar
[99] Kibungu C, Kondiah PPD, Kumar P, Choonara YE. This review recent advances in chitosan and alginate-based hydrogels for wound healing application. Front Mater. 2021;8:8.10.3389/fmats.2021.681960Search in Google Scholar
[100] Clifton LA, Skoda MW, Le Brun AP, Ciesielski F, Kuzmenko I, Holt SA, et al. Effect of divalent cation removal on the structure of gram-negative bacterial outer membrane models. Langmuir 2015;31(1):404–12.10.1021/la504407vSearch in Google Scholar
[101] Helander IM, Nurmiaho-Lassila EL, Ahvenainen R, Rhoades J, Roller S. Chitosan disrupts the barrier properties of the outer membrane of gram-negative bacteria. Int J Food Microbiol. 2001;71(2–3):235–44.10.1016/S0168-1605(01)00609-2Search in Google Scholar
[102] Farhadihosseinabadi B, Zarebkohan A, Eftekhary M, Heiat M, Moosazadeh Moghaddam M, Gholipourmalekabadi M. Crosstalk between chitosan and cell signaling pathways. Cell Mol Life Sci. 2019;76(14):2697–718.10.1007/s00018-019-03107-3Search in Google Scholar PubMed
[103] Yao HY, Lin HR, Sue GP, Lin YJ. Chitosan-based hydrogels prepared by UV polymerization for wound dressing. Polym Polym Compos. 2019;27(3):155–67.10.1177/0967391118820477Search in Google Scholar
[104] Choi JS, Yoo HS. Pluronic/chitosan hydrogels containing epidermal growth factor with wound-adhesive and photo-crosslinkable properties. J Biomed Mater Res A. 2010;95A(2):564–73.10.1002/jbm.a.32848Search in Google Scholar PubMed
[105] Mariia K, Arif M, Shi J, Song FL, Chi Z, Liu CG. Novel chitosan-ulvan hydrogel reinforcement by cellulose nanocrystals with epidermal growth factor for enhanced wound healing: In vitro and in vivo analysis. Int J Biol Macromol. 2021;183:435–46.10.1016/j.ijbiomac.2021.04.156Search in Google Scholar PubMed
[106] Hu Y, Zhang Z, Li Y, Ding X, Li D, Shen C, Xu FJ. Dual-crosslinked amorphous polysaccharide hydrogels based on chitosan/alginate for wound healing applications. Macromol Rapid Comm. 2018;39(20):1800069.10.1002/marc.201800069Search in Google Scholar PubMed
[107] Liu S, Liu Z, Wu M, Xu X, Huang F, Zhang L, et al. NIR as a “trigger switch” for rapid phase change, on-demand release, and photothermal synergistic antibacterial treatment with chitosan-based temperature-sensitive hydrogel. Int J Biol Macromol. 2021;191:344–58.10.1016/j.ijbiomac.2021.09.093Search in Google Scholar
[108] Hu B, Gao M, Boakye-Yiadom KO, Ho W, Yu W, Xu X, et al. An intrinsically bioactive hydrogel with on-demand drug release behaviors for diabetic wound healing. Bioact Mater. 2021;6(12):4592–606.10.1016/j.bioactmat.2021.04.040Search in Google Scholar
[109] Zhu J, Jiang G, Hong W, Zhang Y, Xu B, Song G, et al. Rapid gelation of oxidized hyaluronic acid and succinyl chitosan for integration with insulin-loaded micelles and epidermal growth factor on diabetic wound healing. Mat Sci Eng C-Mater 2020;117:117.10.1016/j.msec.2020.111273Search in Google Scholar
[110] Yang DH, Seo DI, Lee DW, Bhang SH, Park K, Jang G, et al. Preparation and evaluation of visible-light cured glycol chitosan hydrogel dressing containing dual growth factors for accelerated wound healing. J Ind Eng Chem. 2017;53:360–70.10.1016/j.jiec.2017.05.007Search in Google Scholar
[111] Huang J, Ren J, Chen G, Li Z, Liu Y, Wang G, Wu X. Tunable sequential drug delivery system based on chitosan/hyaluronic acid hydrogels and PLGA microspheres for management of non-healing infected wounds. Mat Sci Eng C-Mater. 2018;89:213–22.10.1016/j.msec.2018.04.009Search in Google Scholar
[112] Xu K, An NC, Zhang HJ, Zhang QL, Zhang KL, Hu XY, et al. Sustained-release of PDGF from PLGA microsphere embedded thermosensitive hydrogel promoting wound healing by inhibiting autophagy. J Drug Deliv Sci Tec. 2020;55:101405.10.1016/j.jddst.2019.101405Search in Google Scholar
[113] Obara K, Ishihara M, Ishizuka T, Fujita M, Ozeki Y, Maehara T, et al. Photocrosslinkable chitosan hydrogel containing fibroblast growth factor-2 stimulates wound healing in healing-impaired db/db mice. Biomaterials. 2003;24(20):3437–44.10.1016/S0142-9612(03)00220-5Search in Google Scholar
[114] Yoo Y, Hyun H, Yoon SJ, Kim SY, Lee DW, Um S, et al. Visible light-cured glycol chitosan hydrogel dressing containing endothelial growth factor and basic fibroblast growth factor accelerates wound healing in vivo. J Ind Eng Chem. 2018;67:365–72.10.1016/j.jiec.2018.07.009Search in Google Scholar
[115] Wang W, Lin S, Xiao Y, Huang Y, Tan Y, Cai L, et al. Acceleration of diabetic wound healing with chitosan-crosslinked collagen sponge containing recombinant human acidic fibroblast growth factor in healing-impaired STZ diabetic rats. Life Sci. 2008;82(3–4):190–204.10.1016/j.lfs.2007.11.009Search in Google Scholar PubMed
[116] Raus RA, Nawawi W, Nasaruddin RR. Alginate and alginate composites for biomedical applications. Asian J Pharm Sci. 2021;16(3):280–306.10.1016/j.ajps.2020.10.001Search in Google Scholar PubMed PubMed Central
[117] Barbu A, Neamtu B, Zahan M, Iancu GM, Bacila C, Miresan V. Current trends in advanced alginate-based wound dressings for chronic wounds. J Pers Med. 2021;11(9):890.10.3390/jpm11090890Search in Google Scholar
[118] Sahoo DR, Biswal T. Alginate and its application to tissue engineering. Sn Appl Sci. 2021;3(1):30.10.1007/s42452-020-04096-wSearch in Google Scholar
[119] Orive G, Tam SK, Pedraz JL, Hallé J-P. Biocompatibility of alginate–poly-l-lysine microcapsules for cell therapy. Biomaterials. 2006;27(20):3691–700.10.1016/j.biomaterials.2006.02.048Search in Google Scholar
[120] Chaudhari AA, Vig K, Baganizi DR, Sahu R, Dixit S, Dennis V, et al. Future prospects for scaffolding methods and biomaterials in skin tissue engineering: a review. Int J Mol Sci. 2016;17(12):1974.10.3390/ijms17121974Search in Google Scholar
[121] Bouhadir KH, Lee KY, Alsberg E, Damm KL, Anderson KW, Mooney DJ. Degradation of partially oxidized alginate and its potential application for tissue engineering. Biotechnol Progr. 2001;17(5):945–50.10.1021/bp010070pSearch in Google Scholar
[122] Orive G, Ponce S, Hernández RM, Gascón AR, Igartua M, Pedraz JL. Biocompatibility of microcapsules for cell immobilization elaborated with different types of alginates. Biomaterials 2002;23(18):3825–31.10.1016/S0142-9612(02)00118-7Search in Google Scholar
[123] Fertah M, Belfkira A, Dahmane EM, Taourirte M, Brouillette F. Extraction and characterization of sodium alginate from Moroccan Laminaria digitata brown seaweed. Arab J Chem. 2017;10:S3707–14.10.1016/j.arabjc.2014.05.003Search in Google Scholar
[124] Barbu A, Neamtu MB, Zahan M, Miresan V. Trends in alginate-based films and membranes for wound healing. Rom Biotech Lett. 2020;25(4):1683–9.Search in Google Scholar
[125] Raposo MFD, de Morais AMB, de Morais R. Marine polysaccharides from algae with potential biomedical applications. Mar Drugs. 2015;13(5):2967–3028.10.3390/md13052967Search in Google Scholar PubMed PubMed Central
[126] Szekalska M, Pucilowska A, Szymanska E, Ciosek P, Winnicka K. Alginate: current use and future perspectives in pharmaceutical and biomedical applications. Int J Polym Sci. 2016;2016:1–17.10.1155/2016/7697031Search in Google Scholar
[127] Dabiri G, Damstetter E, Phillips T. Choosing a wound dressing based on common wound characteristics. Adv Wound Care. 2014;5(1):32–41.10.1089/wound.2014.0586Search in Google Scholar PubMed PubMed Central
[128] Piacquadio D, Nelson DB. Alginates: a “new” dressing alternative. J Dermatol Surg Oncol. 1992;18(11):992–5.10.1111/j.1524-4725.1992.tb02773.xSearch in Google Scholar PubMed
[129] Fonder MA, Lazarus GS, Cowan DA, Aronson-Cook B, Kohli AR, Mamelak AJ. Treating the chronic wound: A practical approach to the care of nonhealing wounds and wound care dressings. J Am Acad Dermatol. 2008;58(2):185–206.10.1016/j.jaad.2007.08.048Search in Google Scholar PubMed
[130] Zhu J, Jiang G, Song G, Liu T, Cao C, Yang Y, et al. Incorporation of ZnO/bioactive glass nanoparticles into alginate/chitosan composite hydrogels for wound closure. Acs Appl Bio Mater. 2019;2(11):5042–52.10.1021/acsabm.9b00727Search in Google Scholar PubMed
[131] Liu Y, Duan LJ, Kim MJ, Kim J-H, Chung DJ. In situ sodium alginate-hyaluronic acid hydrogel coating method for clinical applications. Macromol Res. 2014;22(3):240–7.10.1007/s13233-014-2001-5Search in Google Scholar
[132] Rezaei F, Damoogh S, Reis RL, Kundu SC, Mottaghitalab F, Farokhi M. Dual drug delivery system based on pH-sensitive silk fibroin/alginate nanoparticles entrapped in PNIPAM hydrogel for treating severe infected burn wound. Biofabrication. 2020;13(1):015005.10.1088/1758-5090/abbb82Search in Google Scholar PubMed
[133] Cui T, Li X, He S, Xu D, Yin L, Huang X, et al. Instant self-assembly peptide hydrogel encapsulation with fibrous alginate by microfluidics for infected wound healing. ACS Biomater Sci Eng. 2020;6(9):5001–11.10.1021/acsbiomaterials.0c00581Search in Google Scholar PubMed
[134] Azarpira N, Kaviani M, Sarvestani FS. Incorporation of VEGF-and bFGF-loaded alginate oxide particles in acellular collagen-alginate composite hydrogel to promote angiogenesis. Tissue Cell. 2021;72:72.10.1016/j.tice.2021.101539Search in Google Scholar PubMed
[135] Chou H-Y, Weng C-C, Lai J-Y, Lin S-Y, Tsai H-C. Design of an interpenetrating polymeric network hydrogel made of calcium-alginate from a thermos-sensitive pluronic template as a thermal-ionic reversible wound dressing. Polymers 2020;12(9):2138.10.3390/polym12092138Search in Google Scholar PubMed PubMed Central
[136] Babavalian H, Tebyanian H, Latifi AM, Shokrgozar MA, Bonakdar S, Shakeri F. The effect of synthetic alginate sulfate hydrogels with recombinant PDGF-BB on wound healing. Bratisl Med J. 2018;119(6):391–6.10.4149/BLL_2018_072Search in Google Scholar PubMed
[137] Zhu Y, Zhang Y. Clinical features of EGFR mutation negative in patients with non-small cell lung cancer and brain metastases. Zhongguo Fei Ai Za Zhi. 2021;24(1):43–8.Search in Google Scholar
[138] Chattopadhyay S, Raines RT. Review collagen-based biomaterials for wound healing. Biopolymers. 2014;101(8):821–33.10.1002/bip.22486Search in Google Scholar PubMed PubMed Central
[139] Pinkas DM, Ding S, Raines RT, Barron AE. Tunable, post-translational hydroxylation of collagen domains in Escherichia coli. ACS Chem Biol. 2011;6(4):320–4.10.1021/cb100298rSearch in Google Scholar
[140] Buechter DD, Paolella DN, Leslie BS, Brown MS, Mehos KA, Gruskin EA. Co-translational incorporation of trans-4-hydroxyproline into recombinant proteins in bacteria. J Biol Chem. 2003;278(1):645–50.10.1074/jbc.M209364200Search in Google Scholar
[141] Fleck CA, Simman R. Modern collagen wound dressings: function and purpose. J Am Col Certif Wound Spec. 2010;2(3):50–4.10.1016/j.jcws.2010.12.003Search in Google Scholar
[142] Maeda M, Tani S, Sano A, Fujioka K. Microstructure and release characteristics of the minipellet, a collagen-based drug delivery system for controlled release of protein drugs. J Control Rel. 1999;62(3):313–24.10.1016/S0168-3659(99)00156-XSearch in Google Scholar
[143] Thönes S, Rother S, Wippold T, Blaszkiewicz J, Balamurugan K, Moeller S, et al. Hyaluronan/collagen hydrogels containing sulfated hyaluronan improve wound healing by sustained release of heparin-binding EGF-like growth factor. Acta Biomater. 2019;86:135–47.10.1016/j.actbio.2019.01.029Search in Google Scholar PubMed
[144] Kondo S, Kuroyanagi Y. Development of a wound dressing composed of hyaluronic acid and collagen sponge with epidermal growth factor. J Biomat Sci-Polym E. 2012;23(5):629–43.10.1163/092050611X555687Search in Google Scholar PubMed
[145] Niiyama H, Kuroyanagi Y. Development of novel wound dressing composed of hyaluronic acid and collagen sponge containing epidermal growth factor and vitamin C derivative. J Artif Organs. 2014;17(1):81–7.10.1007/s10047-013-0737-xSearch in Google Scholar PubMed
[146] Cheng Y, Li Y, Huang S, Yu F, Bei Y, Zhang Y, et al. Hybrid freeze-dried dressings composed of epidermal growth factor and recombinant human-like collagen enhance cutaneous wound healing in rats. Front Bioeng Biotechnol. 2020;8:8.10.3389/fbioe.2020.00742Search in Google Scholar PubMed PubMed Central
[147] Pandit A, Ashar R, Feldman D, Thompson A. Investigation of acidic fibroblast growth factor delivered through a collagen scaffold for the treatment of full-thickness skin defects in a rabbit model. Plast Reconstr Surg. 1998;101(3):766–75.10.1097/00006534-199803000-00028Search in Google Scholar PubMed
[148] Chakrabarti S, Mazumder B, Rajkonwar J, Pathak MP, Patowary P, Chattopadhyay P. bFGF and collagen matrix hydrogel attenuates burn wound inflammation through activation of ERK and TRK pathway. Sci Rep. 2021;11(1):3357.10.1038/s41598-021-82888-9Search in Google Scholar PubMed PubMed Central
[149] Tateshita T, Ono I, Kaneko F. Effects of collagen matrix containing transforming growth factor (TGF)-beta 1 on wound contraction. J Dermatol Sci. 2001;27(2):104–13.10.1016/S0923-1811(01)00122-0Search in Google Scholar
[150] Heher P, Muhleder S, Mittermayr R, Redl H, Slezak P. Fibrin-based delivery strategies for acute and chronic wound healing. Adv Drug Deliv Rev. 2018;129:134–47.10.1016/j.addr.2017.12.007Search in Google Scholar
[151] Whelan D, Caplice NM, Clover AJP. Fibrin as a delivery system in wound healing tissue engineering applications. J Control Rel. 2014;196:1–8.10.1016/j.jconrel.2014.09.023Search in Google Scholar
[152] Ahmed TAE, Dare EV, Hincke M. Fibrin: A versatile scaffold for tissue engineering applications. Tissue Eng Part B-Re. 2008;14(2):199–215.10.1089/ten.teb.2007.0435Search in Google Scholar
[153] Sacchi V, Mittermayr R, Hartinger J, Martino MM, Lorentz KM, Wolbank S, et al. Long-lasting fibrin matrices ensure stable and functional angiogenesis by highly tunable, sustained delivery of recombinant VEGF164. P Natl Acad Sci USA. 2014;111(19):6952–7.10.1073/pnas.1404605111Search in Google Scholar
[154] Sakiyama-Elbert SE, Hubbell JA. Development of fibrin derivatives for controlled release of heparin-binding growth factors. J Controlled Rel. 2000;65(3):389–402.10.1016/S0168-3659(99)00221-7Search in Google Scholar
[155] Tan J, Li L, Wang H, Wei L, Gao X, Zeng Z, et al. Biofunctionalized fibrin gel co-embedded with BMSCs and VEGF for accelerating skin injury repair. Mat Sci Eng C-Mater. 2021;121:121.10.1016/j.msec.2020.111749Search in Google Scholar PubMed
[156] Rahman MM, Garcia N, Loh YS, Marks DC, Banakh I, Jagadeesan P, et al. A platelet-derived hydrogel improves neovascularisation in full thickness wounds. Acta Biomater. 2021;136:199–209.10.1016/j.actbio.2021.09.043Search in Google Scholar PubMed
[157] Certelli A, Valente P, Uccelli A, Grosso A, Di Maggio N, D’Amico R, et al. Robust angiogenesis and arteriogenesis in the skin of diabetic mice by transient delivery of engineered VEGF and PDGF-BB proteins in fibrin hydrogels. Front Bioeng Biotech. 2021;9:9.10.3389/fbioe.2021.688467Search in Google Scholar PubMed PubMed Central
[158] Mogosanu GD, Grumezescu AM. Natural and synthetic polymers for wounds and burns dressing. Int J Pharm. 2014;463(2):127–36.10.1016/j.ijpharm.2013.12.015Search in Google Scholar PubMed
[159] Ndlovu SP, Ngece K, Alven S, Aderibigbe BA. Gelatin-based hybrid scaffolds: promising wound dressings. Polymers. 2021;13(17):2959.10.3390/polym13172959Search in Google Scholar PubMed PubMed Central
[160] Salahuddin B, Wang S, Sangian D, Aziz S, Gu Q. Hybrid gelatin hydrogels in nanomedicine applications. ACS Appl Bio Mater. 2021;4(4):2886–906.10.1021/acsabm.0c01630Search in Google Scholar PubMed
[161] Augustine R, Hasan A, Dalvi YB, Rehman SRU, Varghese R, Unni RN, et al. Growth factor loaded in situ photocrosslinkable poly(3-hydroxybutyrate-co-3-hydroxyvalerate)/gelatin methacryloyl hybrid patch for diabetic wound healing. Mat Sci Eng C-Mater. 2021;118:118.10.1016/j.msec.2020.111519Search in Google Scholar PubMed
[162] Huang S, Zhang Y, Tang L, Deng Z, Lu W, Feng F, et al. Functional bilayered skin substitute constructed by tissue-engineered extracellular matrix and microsphere-incorporated gelatin hydrogel for wound repair. Tissue Eng Pt A. 2009;15(9):2617–24.10.1089/ten.tea.2008.0505Search in Google Scholar PubMed
[163] Jin L, Yoon SJ, Lee DH, Pyun YC, Kim WY, Lee JH, et al. Preparation of foam dressings based on gelatin, hyaluronic acid, and carboxymethyl chitosan containing fibroblast growth factor-7 for dermal regeneration. Polymers. 2021;13(19):3279.10.3390/polym13193279Search in Google Scholar PubMed PubMed Central
[164] Wan WB, Cai F, Huang JY, Chen SX, Liao Q. A skin-inspired 3D bilayer scaffold enhances granulation tissue formation and anti-infection for diabetic wound healing. J Mater Chem B 2019;7(18):2954–61.10.1039/C8TB03341BSearch in Google Scholar
[165] Echave MC, Saenz del Burgo L, Pedraz JL, Orive G. Gelatin as biomaterial for tissue engineering. Curr Pharm Des. 2017;23(24):3567–84.10.2174/0929867324666170511123101Search in Google Scholar PubMed
[166] Bunyaratavej P, Wang HL. Collagen membranes: a review. J Periodontol. 2001;72(2):215–29.10.1902/jop.2001.72.2.215Search in Google Scholar PubMed
[167] Liu T, Shi L, Gu Z, Dan W, Dan N. A novel combined polyphenol-aldehyde crosslinking of collagen film – Applications in biomedical materials. Int J Biol Macromol. 2017;101:889–95.10.1016/j.ijbiomac.2017.03.166Search in Google Scholar PubMed
© 2022 Feng Wang et al., published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Research Articles
- Theoretical and experimental investigation of MWCNT dispersion effect on the elastic modulus of flexible PDMS/MWCNT nanocomposites
- Mechanical, morphological, and fracture-deformation behavior of MWCNTs-reinforced (Al–Cu–Mg–T351) alloy cast nanocomposites fabricated by optimized mechanical milling and powder metallurgy techniques
- Flammability and physical stability of sugar palm crystalline nanocellulose reinforced thermoplastic sugar palm starch/poly(lactic acid) blend bionanocomposites
- Glutathione-loaded non-ionic surfactant niosomes: A new approach to improve oral bioavailability and hepatoprotective efficacy of glutathione
- Relationship between mechano-bactericidal activity and nanoblades density on chemically strengthened glass
- In situ regulation of microstructure and microwave-absorbing properties of FeSiAl through HNO3 oxidation
- Research on a mechanical model of magnetorheological fluid different diameter particles
- Nanomechanical and dynamic mechanical properties of rubber–wood–plastic composites
- Investigative properties of CeO2 doped with niobium: A combined characterization and DFT studies
- Miniaturized peptidomimetics and nano-vesiculation in endothelin types through probable nano-disk formation and structure property relationships of endothelins’ fragments
- N/S co-doped CoSe/C nanocubes as anode materials for Li-ion batteries
- Synergistic effects of halloysite nanotubes with metal and phosphorus additives on the optimal design of eco-friendly sandwich panels with maximum flame resistance and minimum weight
- Octreotide-conjugated silver nanoparticles for active targeting of somatostatin receptors and their application in a nebulized rat model
- Controllable morphology of Bi2S3 nanostructures formed via hydrothermal vulcanization of Bi2O3 thin-film layer and their photoelectrocatalytic performances
- Development of (−)-epigallocatechin-3-gallate-loaded folate receptor-targeted nanoparticles for prostate cancer treatment
- Enhancement of the mechanical properties of HDPE mineral nanocomposites by filler particles modulation of the matrix plastic/elastic behavior
- Effect of plasticizers on the properties of sugar palm nanocellulose/cinnamon essential oil reinforced starch bionanocomposite films
- Optimization of nano coating to reduce the thermal deformation of ball screws
- Preparation of efficient piezoelectric PVDF–HFP/Ni composite films by high electric field poling
- MHD dissipative Casson nanofluid liquid film flow due to an unsteady stretching sheet with radiation influence and slip velocity phenomenon
- Effects of nano-SiO2 modification on rubberised mortar and concrete with recycled coarse aggregates
- Mechanical and microscopic properties of fiber-reinforced coal gangue-based geopolymer concrete
- Effect of morphology and size on the thermodynamic stability of cerium oxide nanoparticles: Experiment and molecular dynamics calculation
- Mechanical performance of a CFRP composite reinforced via gelatin-CNTs: A study on fiber interfacial enhancement and matrix enhancement
- A practical review over surface modification, nanopatterns, emerging materials, drug delivery systems, and their biophysiochemical properties for dental implants: Recent progresses and advances
- HTR: An ultra-high speed algorithm for cage recognition of clathrate hydrates
- Effects of microalloying elements added by in situ synthesis on the microstructure of WCu composites
- A highly sensitive nanobiosensor based on aptamer-conjugated graphene-decorated rhodium nanoparticles for detection of HER2-positive circulating tumor cells
- Progressive collapse performance of shear strengthened RC frames by nano CFRP
- Core–shell heterostructured composites of carbon nanotubes and imine-linked hyperbranched polymers as metal-free Li-ion anodes
- A Galerkin strategy for tri-hybridized mixture in ethylene glycol comprising variable diffusion and thermal conductivity using non-Fourier’s theory
- Simple models for tensile modulus of shape memory polymer nanocomposites at ambient temperature
- Preparation and morphological studies of tin sulfide nanoparticles and use as efficient photocatalysts for the degradation of rhodamine B and phenol
- Polyethyleneimine-impregnated activated carbon nanofiber composited graphene-derived rice husk char for efficient post-combustion CO2 capture
- Electrospun nanofibers of Co3O4 nanocrystals encapsulated in cyclized-polyacrylonitrile for lithium storage
- Pitting corrosion induced on high-strength high carbon steel wire in high alkaline deaerated chloride electrolyte
- Formulation of polymeric nanoparticles loaded sorafenib; evaluation of cytotoxicity, molecular evaluation, and gene expression studies in lung and breast cancer cell lines
- Engineered nanocomposites in asphalt binders
- Influence of loading voltage, domain ratio, and additional load on the actuation of dielectric elastomer
- Thermally induced hex-graphene transitions in 2D carbon crystals
- The surface modification effect on the interfacial properties of glass fiber-reinforced epoxy: A molecular dynamics study
- Molecular dynamics study of deformation mechanism of interfacial microzone of Cu/Al2Cu/Al composites under tension
- Nanocolloid simulators of luminescent solar concentrator photovoltaic windows
- Compressive strength and anti-chloride ion penetration assessment of geopolymer mortar merging PVA fiber and nano-SiO2 using RBF–BP composite neural network
- Effect of 3-mercapto-1-propane sulfonate sulfonic acid and polyvinylpyrrolidone on the growth of cobalt pillar by electrodeposition
- Dynamics of convective slippery constraints on hybrid radiative Sutterby nanofluid flow by Galerkin finite element simulation
- Preparation of vanadium by the magnesiothermic self-propagating reduction and process control
- Microstructure-dependent photoelectrocatalytic activity of heterogeneous ZnO–ZnS nanosheets
- Cytotoxic and pro-inflammatory effects of molybdenum and tungsten disulphide on human bronchial cells
- Improving recycled aggregate concrete by compression casting and nano-silica
- Chemically reactive Maxwell nanoliquid flow by a stretching surface in the frames of Newtonian heating, nonlinear convection and radiative flux: Nanopolymer flow processing simulation
- Nonlinear dynamic and crack behaviors of carbon nanotubes-reinforced composites with various geometries
- Biosynthesis of copper oxide nanoparticles and its therapeutic efficacy against colon cancer
- Synthesis and characterization of smart stimuli-responsive herbal drug-encapsulated nanoniosome particles for efficient treatment of breast cancer
- Homotopic simulation for heat transport phenomenon of the Burgers nanofluids flow over a stretching cylinder with thermal convective and zero mass flux conditions
- Incorporation of copper and strontium ions in TiO2 nanotubes via dopamine to enhance hemocompatibility and cytocompatibility
- Mechanical, thermal, and barrier properties of starch films incorporated with chitosan nanoparticles
- Mechanical properties and microstructure of nano-strengthened recycled aggregate concrete
- Glucose-responsive nanogels efficiently maintain the stability and activity of therapeutic enzymes
- Tunning matrix rheology and mechanical performance of ultra-high performance concrete using cellulose nanofibers
- Flexible MXene/copper/cellulose nanofiber heat spreader films with enhanced thermal conductivity
- Promoted charge separation and specific surface area via interlacing of N-doped titanium dioxide nanotubes on carbon nitride nanosheets for photocatalytic degradation of Rhodamine B
- Elucidating the role of silicon dioxide and titanium dioxide nanoparticles in mitigating the disease of the eggplant caused by Phomopsis vexans, Ralstonia solanacearum, and root-knot nematode Meloidogyne incognita
- An implication of magnetic dipole in Carreau Yasuda liquid influenced by engine oil using ternary hybrid nanomaterial
- Robust synthesis of a composite phase of copper vanadium oxide with enhanced performance for durable aqueous Zn-ion batteries
- Tunning self-assembled phases of bovine serum albumin via hydrothermal process to synthesize novel functional hydrogel for skin protection against UVB
- A comparative experimental study on damping properties of epoxy nanocomposite beams reinforced with carbon nanotubes and graphene nanoplatelets
- Lightweight and hydrophobic Ni/GO/PVA composite aerogels for ultrahigh performance electromagnetic interference shielding
- Research on the auxetic behavior and mechanical properties of periodically rotating graphene nanostructures
- Repairing performances of novel cement mortar modified with graphene oxide and polyacrylate polymer
- Closed-loop recycling and fabrication of hydrophilic CNT films with high performance
- Design of thin-film configuration of SnO2–Ag2O composites for NO2 gas-sensing applications
- Study on stress distribution of SiC/Al composites based on microstructure models with microns and nanoparticles
- PVDF green nanofibers as potential carriers for improving self-healing and mechanical properties of carbon fiber/epoxy prepregs
- Osteogenesis capability of three-dimensionally printed poly(lactic acid)-halloysite nanotube scaffolds containing strontium ranelate
- Silver nanoparticles induce mitochondria-dependent apoptosis and late non-canonical autophagy in HT-29 colon cancer cells
- Preparation and bonding mechanisms of polymer/metal hybrid composite by nano molding technology
- Damage self-sensing and strain monitoring of glass-reinforced epoxy composite impregnated with graphene nanoplatelet and multiwalled carbon nanotubes
- Thermal analysis characterisation of solar-powered ship using Oldroyd hybrid nanofluids in parabolic trough solar collector: An optimal thermal application
- Pyrene-functionalized halloysite nanotubes for simultaneously detecting and separating Hg(ii) in aqueous media: A comprehensive comparison on interparticle and intraparticle excimers
- Fabrication of self-assembly CNT flexible film and its piezoresistive sensing behaviors
- Thermal valuation and entropy inspection of second-grade nanoscale fluid flow over a stretching surface by applying Koo–Kleinstreuer–Li relation
- Mechanical properties and microstructure of nano-SiO2 and basalt-fiber-reinforced recycled aggregate concrete
- Characterization and tribology performance of polyaniline-coated nanodiamond lubricant additives
- Combined impact of Marangoni convection and thermophoretic particle deposition on chemically reactive transport of nanofluid flow over a stretching surface
- Spark plasma extrusion of binder free hydroxyapatite powder
- An investigation on thermo-mechanical performance of graphene-oxide-reinforced shape memory polymer
- Effect of nanoadditives on the novel leather fiber/recycled poly(ethylene-vinyl-acetate) polymer composites for multifunctional applications: Fabrication, characterizations, and multiobjective optimization using central composite design
- Design selection for a hemispherical dimple core sandwich panel using hybrid multi-criteria decision-making methods
- Improving tensile strength and impact toughness of plasticized poly(lactic acid) biocomposites by incorporating nanofibrillated cellulose
- Green synthesis of spinel copper ferrite (CuFe2O4) nanoparticles and their toxicity
- The effect of TaC and NbC hybrid and mono-nanoparticles on AA2024 nanocomposites: Microstructure, strengthening, and artificial aging
- Excited-state geometry relaxation of pyrene-modified cellulose nanocrystals under UV-light excitation for detecting Fe3+
- Effect of CNTs and MEA on the creep of face-slab concrete at an early age
- Effect of deformation conditions on compression phase transformation of AZ31
- Application of MXene as a new generation of highly conductive coating materials for electromembrane-surrounded solid-phase microextraction
- A comparative study of the elasto-plastic properties for ceramic nanocomposites filled by graphene or graphene oxide nanoplates
- Encapsulation strategies for improving the biological behavior of CdS@ZIF-8 nanocomposites
- Biosynthesis of ZnO NPs from pumpkin seeds’ extract and elucidation of its anticancer potential against breast cancer
- Preliminary trials of the gold nanoparticles conjugated chrysin: An assessment of anti-oxidant, anti-microbial, and in vitro cytotoxic activities of a nanoformulated flavonoid
- Effect of micron-scale pores increased by nano-SiO2 sol modification on the strength of cement mortar
- Fractional simulations for thermal flow of hybrid nanofluid with aluminum oxide and titanium oxide nanoparticles with water and blood base fluids
- The effect of graphene nano-powder on the viscosity of water: An experimental study and artificial neural network modeling
- Development of a novel heat- and shear-resistant nano-silica gelling agent
- Characterization, biocompatibility and in vivo of nominal MnO2-containing wollastonite glass-ceramic
- Entropy production simulation of second-grade magnetic nanomaterials flowing across an expanding surface with viscidness dissipative flux
- Enhancement in structural, morphological, and optical properties of copper oxide for optoelectronic device applications
- Aptamer-functionalized chitosan-coated gold nanoparticle complex as a suitable targeted drug carrier for improved breast cancer treatment
- Performance and overall evaluation of nano-alumina-modified asphalt mixture
- Analysis of pure nanofluid (GO/engine oil) and hybrid nanofluid (GO–Fe3O4/engine oil): Novel thermal and magnetic features
- Synthesis of Ag@AgCl modified anatase/rutile/brookite mixed phase TiO2 and their photocatalytic property
- Mechanisms and influential variables on the abrasion resistance hydraulic concrete
- Synergistic reinforcement mechanism of basalt fiber/cellulose nanocrystals/polypropylene composites
- Achieving excellent oxidation resistance and mechanical properties of TiB2–B4C/carbon aerogel composites by quick-gelation and mechanical mixing
- Microwave-assisted sol–gel template-free synthesis and characterization of silica nanoparticles obtained from South African coal fly ash
- Pulsed laser-assisted synthesis of nano nickel(ii) oxide-anchored graphitic carbon nitride: Characterizations and their potential antibacterial/anti-biofilm applications
- Effects of nano-ZrSi2 on thermal stability of phenolic resin and thermal reusability of quartz–phenolic composites
- Benzaldehyde derivatives on tin electroplating as corrosion resistance for fabricating copper circuit
- Mechanical and heat transfer properties of 4D-printed shape memory graphene oxide/epoxy acrylate composites
- Coupling the vanadium-induced amorphous/crystalline NiFe2O4 with phosphide heterojunction toward active oxygen evolution reaction catalysts
- Graphene-oxide-reinforced cement composites mechanical and microstructural characteristics at elevated temperatures
- Gray correlation analysis of factors influencing compressive strength and durability of nano-SiO2 and PVA fiber reinforced geopolymer mortar
- Preparation of layered gradient Cu–Cr–Ti alloy with excellent mechanical properties, thermal stability, and electrical conductivity
- Recovery of Cr from chrome-containing leather wastes to develop aluminum-based composite material along with Al2O3 ceramic particles: An ingenious approach
- Mechanisms of the improved stiffness of flexible polymers under impact loading
- Anticancer potential of gold nanoparticles (AuNPs) using a battery of in vitro tests
- Review Articles
- Proposed approaches for coronaviruses elimination from wastewater: Membrane techniques and nanotechnology solutions
- Application of Pickering emulsion in oil drilling and production
- The contribution of microfluidics to the fight against tuberculosis
- Graphene-based biosensors for disease theranostics: Development, applications, and recent advancements
- Synthesis and encapsulation of iron oxide nanorods for application in magnetic hyperthermia and photothermal therapy
- Contemporary nano-architectured drugs and leads for ανβ3 integrin-based chemotherapy: Rationale and retrospect
- State-of-the-art review of fabrication, application, and mechanical properties of functionally graded porous nanocomposite materials
- Insights on magnetic spinel ferrites for targeted drug delivery and hyperthermia applications
- A review on heterogeneous oxidation of acetaminophen based on micro and nanoparticles catalyzed by different activators
- Early diagnosis of lung cancer using magnetic nanoparticles-integrated systems
- Advances in ZnO: Manipulation of defects for enhancing their technological potentials
- Efficacious nanomedicine track toward combating COVID-19
- A review of the design, processes, and properties of Mg-based composites
- Green synthesis of nanoparticles for varied applications: Green renewable resources and energy-efficient synthetic routes
- Two-dimensional nanomaterial-based polymer composites: Fundamentals and applications
- Recent progress and challenges in plasmonic nanomaterials
- Apoptotic cell-derived micro/nanosized extracellular vesicles in tissue regeneration
- Electronic noses based on metal oxide nanowires: A review
- Framework materials for supercapacitors
- An overview on the reproductive toxicity of graphene derivatives: Highlighting the importance
- Antibacterial nanomaterials: Upcoming hope to overcome antibiotic resistance crisis
- Research progress of carbon materials in the field of three-dimensional printing polymer nanocomposites
- A review of atomic layer deposition modelling and simulation methodologies: Density functional theory and molecular dynamics
- Recent advances in the preparation of PVDF-based piezoelectric materials
- Recent developments in tensile properties of friction welding of carbon fiber-reinforced composite: A review
- Comprehensive review of the properties of fly ash-based geopolymer with additive of nano-SiO2
- Perspectives in biopolymer/graphene-based composite application: Advances, challenges, and recommendations
- Graphene-based nanocomposite using new modeling molecular dynamic simulations for proposed neutralizing mechanism and real-time sensing of COVID-19
- Nanotechnology application on bamboo materials: A review
- Recent developments and future perspectives of biorenewable nanocomposites for advanced applications
- Nanostructured lipid carrier system: A compendium of their formulation development approaches, optimization strategies by quality by design, and recent applications in drug delivery
- 3D printing customized design of human bone tissue implant and its application
- Design, preparation, and functionalization of nanobiomaterials for enhanced efficacy in current and future biomedical applications
- A brief review of nanoparticles-doped PEDOT:PSS nanocomposite for OLED and OPV
- Nanotechnology interventions as a putative tool for the treatment of dental afflictions
- Recent advancements in metal–organic frameworks integrating quantum dots (QDs@MOF) and their potential applications
- A focused review of short electrospun nanofiber preparation techniques for composite reinforcement
- Microstructural characteristics and nano-modification of interfacial transition zone in concrete: A review
- Latest developments in the upconversion nanotechnology for the rapid detection of food safety: A review
- Strategic applications of nano-fertilizers for sustainable agriculture: Benefits and bottlenecks
- Molecular dynamics application of cocrystal energetic materials: A review
- Synthesis and application of nanometer hydroxyapatite in biomedicine
- Cutting-edge development in waste-recycled nanomaterials for energy storage and conversion applications
- Biological applications of ternary quantum dots: A review
- Nanotherapeutics for hydrogen sulfide-involved treatment: An emerging approach for cancer therapy
- Application of antibacterial nanoparticles in orthodontic materials
- Effect of natural-based biological hydrogels combined with growth factors on skin wound healing
- Nanozymes – A route to overcome microbial resistance: A viewpoint
- Recent developments and applications of smart nanoparticles in biomedicine
- Contemporary review on carbon nanotube (CNT) composites and their impact on multifarious applications
- Interfacial interactions and reinforcing mechanisms of cellulose and chitin nanomaterials and starch derivatives for cement and concrete strength and durability enhancement: A review
- Diamond-like carbon films for tribological modification of rubber
- Layered double hydroxides (LDHs) modified cement-based materials: A systematic review
- Recent research progress and advanced applications of silica/polymer nanocomposites
- Modeling of supramolecular biopolymers: Leading the in silico revolution of tissue engineering and nanomedicine
- Recent advances in perovskites-based optoelectronics
- Biogenic synthesis of palladium nanoparticles: New production methods and applications
- A comprehensive review of nanofluids with fractional derivatives: Modeling and application
- Electrospinning of marine polysaccharides: Processing and chemical aspects, challenges, and future prospects
- Electrohydrodynamic printing for demanding devices: A review of processing and applications
- Rapid Communications
- Structural material with designed thermal twist for a simple actuation
- Recent advances in photothermal materials for solar-driven crude oil adsorption
Articles in the same Issue
- Research Articles
- Theoretical and experimental investigation of MWCNT dispersion effect on the elastic modulus of flexible PDMS/MWCNT nanocomposites
- Mechanical, morphological, and fracture-deformation behavior of MWCNTs-reinforced (Al–Cu–Mg–T351) alloy cast nanocomposites fabricated by optimized mechanical milling and powder metallurgy techniques
- Flammability and physical stability of sugar palm crystalline nanocellulose reinforced thermoplastic sugar palm starch/poly(lactic acid) blend bionanocomposites
- Glutathione-loaded non-ionic surfactant niosomes: A new approach to improve oral bioavailability and hepatoprotective efficacy of glutathione
- Relationship between mechano-bactericidal activity and nanoblades density on chemically strengthened glass
- In situ regulation of microstructure and microwave-absorbing properties of FeSiAl through HNO3 oxidation
- Research on a mechanical model of magnetorheological fluid different diameter particles
- Nanomechanical and dynamic mechanical properties of rubber–wood–plastic composites
- Investigative properties of CeO2 doped with niobium: A combined characterization and DFT studies
- Miniaturized peptidomimetics and nano-vesiculation in endothelin types through probable nano-disk formation and structure property relationships of endothelins’ fragments
- N/S co-doped CoSe/C nanocubes as anode materials for Li-ion batteries
- Synergistic effects of halloysite nanotubes with metal and phosphorus additives on the optimal design of eco-friendly sandwich panels with maximum flame resistance and minimum weight
- Octreotide-conjugated silver nanoparticles for active targeting of somatostatin receptors and their application in a nebulized rat model
- Controllable morphology of Bi2S3 nanostructures formed via hydrothermal vulcanization of Bi2O3 thin-film layer and their photoelectrocatalytic performances
- Development of (−)-epigallocatechin-3-gallate-loaded folate receptor-targeted nanoparticles for prostate cancer treatment
- Enhancement of the mechanical properties of HDPE mineral nanocomposites by filler particles modulation of the matrix plastic/elastic behavior
- Effect of plasticizers on the properties of sugar palm nanocellulose/cinnamon essential oil reinforced starch bionanocomposite films
- Optimization of nano coating to reduce the thermal deformation of ball screws
- Preparation of efficient piezoelectric PVDF–HFP/Ni composite films by high electric field poling
- MHD dissipative Casson nanofluid liquid film flow due to an unsteady stretching sheet with radiation influence and slip velocity phenomenon
- Effects of nano-SiO2 modification on rubberised mortar and concrete with recycled coarse aggregates
- Mechanical and microscopic properties of fiber-reinforced coal gangue-based geopolymer concrete
- Effect of morphology and size on the thermodynamic stability of cerium oxide nanoparticles: Experiment and molecular dynamics calculation
- Mechanical performance of a CFRP composite reinforced via gelatin-CNTs: A study on fiber interfacial enhancement and matrix enhancement
- A practical review over surface modification, nanopatterns, emerging materials, drug delivery systems, and their biophysiochemical properties for dental implants: Recent progresses and advances
- HTR: An ultra-high speed algorithm for cage recognition of clathrate hydrates
- Effects of microalloying elements added by in situ synthesis on the microstructure of WCu composites
- A highly sensitive nanobiosensor based on aptamer-conjugated graphene-decorated rhodium nanoparticles for detection of HER2-positive circulating tumor cells
- Progressive collapse performance of shear strengthened RC frames by nano CFRP
- Core–shell heterostructured composites of carbon nanotubes and imine-linked hyperbranched polymers as metal-free Li-ion anodes
- A Galerkin strategy for tri-hybridized mixture in ethylene glycol comprising variable diffusion and thermal conductivity using non-Fourier’s theory
- Simple models for tensile modulus of shape memory polymer nanocomposites at ambient temperature
- Preparation and morphological studies of tin sulfide nanoparticles and use as efficient photocatalysts for the degradation of rhodamine B and phenol
- Polyethyleneimine-impregnated activated carbon nanofiber composited graphene-derived rice husk char for efficient post-combustion CO2 capture
- Electrospun nanofibers of Co3O4 nanocrystals encapsulated in cyclized-polyacrylonitrile for lithium storage
- Pitting corrosion induced on high-strength high carbon steel wire in high alkaline deaerated chloride electrolyte
- Formulation of polymeric nanoparticles loaded sorafenib; evaluation of cytotoxicity, molecular evaluation, and gene expression studies in lung and breast cancer cell lines
- Engineered nanocomposites in asphalt binders
- Influence of loading voltage, domain ratio, and additional load on the actuation of dielectric elastomer
- Thermally induced hex-graphene transitions in 2D carbon crystals
- The surface modification effect on the interfacial properties of glass fiber-reinforced epoxy: A molecular dynamics study
- Molecular dynamics study of deformation mechanism of interfacial microzone of Cu/Al2Cu/Al composites under tension
- Nanocolloid simulators of luminescent solar concentrator photovoltaic windows
- Compressive strength and anti-chloride ion penetration assessment of geopolymer mortar merging PVA fiber and nano-SiO2 using RBF–BP composite neural network
- Effect of 3-mercapto-1-propane sulfonate sulfonic acid and polyvinylpyrrolidone on the growth of cobalt pillar by electrodeposition
- Dynamics of convective slippery constraints on hybrid radiative Sutterby nanofluid flow by Galerkin finite element simulation
- Preparation of vanadium by the magnesiothermic self-propagating reduction and process control
- Microstructure-dependent photoelectrocatalytic activity of heterogeneous ZnO–ZnS nanosheets
- Cytotoxic and pro-inflammatory effects of molybdenum and tungsten disulphide on human bronchial cells
- Improving recycled aggregate concrete by compression casting and nano-silica
- Chemically reactive Maxwell nanoliquid flow by a stretching surface in the frames of Newtonian heating, nonlinear convection and radiative flux: Nanopolymer flow processing simulation
- Nonlinear dynamic and crack behaviors of carbon nanotubes-reinforced composites with various geometries
- Biosynthesis of copper oxide nanoparticles and its therapeutic efficacy against colon cancer
- Synthesis and characterization of smart stimuli-responsive herbal drug-encapsulated nanoniosome particles for efficient treatment of breast cancer
- Homotopic simulation for heat transport phenomenon of the Burgers nanofluids flow over a stretching cylinder with thermal convective and zero mass flux conditions
- Incorporation of copper and strontium ions in TiO2 nanotubes via dopamine to enhance hemocompatibility and cytocompatibility
- Mechanical, thermal, and barrier properties of starch films incorporated with chitosan nanoparticles
- Mechanical properties and microstructure of nano-strengthened recycled aggregate concrete
- Glucose-responsive nanogels efficiently maintain the stability and activity of therapeutic enzymes
- Tunning matrix rheology and mechanical performance of ultra-high performance concrete using cellulose nanofibers
- Flexible MXene/copper/cellulose nanofiber heat spreader films with enhanced thermal conductivity
- Promoted charge separation and specific surface area via interlacing of N-doped titanium dioxide nanotubes on carbon nitride nanosheets for photocatalytic degradation of Rhodamine B
- Elucidating the role of silicon dioxide and titanium dioxide nanoparticles in mitigating the disease of the eggplant caused by Phomopsis vexans, Ralstonia solanacearum, and root-knot nematode Meloidogyne incognita
- An implication of magnetic dipole in Carreau Yasuda liquid influenced by engine oil using ternary hybrid nanomaterial
- Robust synthesis of a composite phase of copper vanadium oxide with enhanced performance for durable aqueous Zn-ion batteries
- Tunning self-assembled phases of bovine serum albumin via hydrothermal process to synthesize novel functional hydrogel for skin protection against UVB
- A comparative experimental study on damping properties of epoxy nanocomposite beams reinforced with carbon nanotubes and graphene nanoplatelets
- Lightweight and hydrophobic Ni/GO/PVA composite aerogels for ultrahigh performance electromagnetic interference shielding
- Research on the auxetic behavior and mechanical properties of periodically rotating graphene nanostructures
- Repairing performances of novel cement mortar modified with graphene oxide and polyacrylate polymer
- Closed-loop recycling and fabrication of hydrophilic CNT films with high performance
- Design of thin-film configuration of SnO2–Ag2O composites for NO2 gas-sensing applications
- Study on stress distribution of SiC/Al composites based on microstructure models with microns and nanoparticles
- PVDF green nanofibers as potential carriers for improving self-healing and mechanical properties of carbon fiber/epoxy prepregs
- Osteogenesis capability of three-dimensionally printed poly(lactic acid)-halloysite nanotube scaffolds containing strontium ranelate
- Silver nanoparticles induce mitochondria-dependent apoptosis and late non-canonical autophagy in HT-29 colon cancer cells
- Preparation and bonding mechanisms of polymer/metal hybrid composite by nano molding technology
- Damage self-sensing and strain monitoring of glass-reinforced epoxy composite impregnated with graphene nanoplatelet and multiwalled carbon nanotubes
- Thermal analysis characterisation of solar-powered ship using Oldroyd hybrid nanofluids in parabolic trough solar collector: An optimal thermal application
- Pyrene-functionalized halloysite nanotubes for simultaneously detecting and separating Hg(ii) in aqueous media: A comprehensive comparison on interparticle and intraparticle excimers
- Fabrication of self-assembly CNT flexible film and its piezoresistive sensing behaviors
- Thermal valuation and entropy inspection of second-grade nanoscale fluid flow over a stretching surface by applying Koo–Kleinstreuer–Li relation
- Mechanical properties and microstructure of nano-SiO2 and basalt-fiber-reinforced recycled aggregate concrete
- Characterization and tribology performance of polyaniline-coated nanodiamond lubricant additives
- Combined impact of Marangoni convection and thermophoretic particle deposition on chemically reactive transport of nanofluid flow over a stretching surface
- Spark plasma extrusion of binder free hydroxyapatite powder
- An investigation on thermo-mechanical performance of graphene-oxide-reinforced shape memory polymer
- Effect of nanoadditives on the novel leather fiber/recycled poly(ethylene-vinyl-acetate) polymer composites for multifunctional applications: Fabrication, characterizations, and multiobjective optimization using central composite design
- Design selection for a hemispherical dimple core sandwich panel using hybrid multi-criteria decision-making methods
- Improving tensile strength and impact toughness of plasticized poly(lactic acid) biocomposites by incorporating nanofibrillated cellulose
- Green synthesis of spinel copper ferrite (CuFe2O4) nanoparticles and their toxicity
- The effect of TaC and NbC hybrid and mono-nanoparticles on AA2024 nanocomposites: Microstructure, strengthening, and artificial aging
- Excited-state geometry relaxation of pyrene-modified cellulose nanocrystals under UV-light excitation for detecting Fe3+
- Effect of CNTs and MEA on the creep of face-slab concrete at an early age
- Effect of deformation conditions on compression phase transformation of AZ31
- Application of MXene as a new generation of highly conductive coating materials for electromembrane-surrounded solid-phase microextraction
- A comparative study of the elasto-plastic properties for ceramic nanocomposites filled by graphene or graphene oxide nanoplates
- Encapsulation strategies for improving the biological behavior of CdS@ZIF-8 nanocomposites
- Biosynthesis of ZnO NPs from pumpkin seeds’ extract and elucidation of its anticancer potential against breast cancer
- Preliminary trials of the gold nanoparticles conjugated chrysin: An assessment of anti-oxidant, anti-microbial, and in vitro cytotoxic activities of a nanoformulated flavonoid
- Effect of micron-scale pores increased by nano-SiO2 sol modification on the strength of cement mortar
- Fractional simulations for thermal flow of hybrid nanofluid with aluminum oxide and titanium oxide nanoparticles with water and blood base fluids
- The effect of graphene nano-powder on the viscosity of water: An experimental study and artificial neural network modeling
- Development of a novel heat- and shear-resistant nano-silica gelling agent
- Characterization, biocompatibility and in vivo of nominal MnO2-containing wollastonite glass-ceramic
- Entropy production simulation of second-grade magnetic nanomaterials flowing across an expanding surface with viscidness dissipative flux
- Enhancement in structural, morphological, and optical properties of copper oxide for optoelectronic device applications
- Aptamer-functionalized chitosan-coated gold nanoparticle complex as a suitable targeted drug carrier for improved breast cancer treatment
- Performance and overall evaluation of nano-alumina-modified asphalt mixture
- Analysis of pure nanofluid (GO/engine oil) and hybrid nanofluid (GO–Fe3O4/engine oil): Novel thermal and magnetic features
- Synthesis of Ag@AgCl modified anatase/rutile/brookite mixed phase TiO2 and their photocatalytic property
- Mechanisms and influential variables on the abrasion resistance hydraulic concrete
- Synergistic reinforcement mechanism of basalt fiber/cellulose nanocrystals/polypropylene composites
- Achieving excellent oxidation resistance and mechanical properties of TiB2–B4C/carbon aerogel composites by quick-gelation and mechanical mixing
- Microwave-assisted sol–gel template-free synthesis and characterization of silica nanoparticles obtained from South African coal fly ash
- Pulsed laser-assisted synthesis of nano nickel(ii) oxide-anchored graphitic carbon nitride: Characterizations and their potential antibacterial/anti-biofilm applications
- Effects of nano-ZrSi2 on thermal stability of phenolic resin and thermal reusability of quartz–phenolic composites
- Benzaldehyde derivatives on tin electroplating as corrosion resistance for fabricating copper circuit
- Mechanical and heat transfer properties of 4D-printed shape memory graphene oxide/epoxy acrylate composites
- Coupling the vanadium-induced amorphous/crystalline NiFe2O4 with phosphide heterojunction toward active oxygen evolution reaction catalysts
- Graphene-oxide-reinforced cement composites mechanical and microstructural characteristics at elevated temperatures
- Gray correlation analysis of factors influencing compressive strength and durability of nano-SiO2 and PVA fiber reinforced geopolymer mortar
- Preparation of layered gradient Cu–Cr–Ti alloy with excellent mechanical properties, thermal stability, and electrical conductivity
- Recovery of Cr from chrome-containing leather wastes to develop aluminum-based composite material along with Al2O3 ceramic particles: An ingenious approach
- Mechanisms of the improved stiffness of flexible polymers under impact loading
- Anticancer potential of gold nanoparticles (AuNPs) using a battery of in vitro tests
- Review Articles
- Proposed approaches for coronaviruses elimination from wastewater: Membrane techniques and nanotechnology solutions
- Application of Pickering emulsion in oil drilling and production
- The contribution of microfluidics to the fight against tuberculosis
- Graphene-based biosensors for disease theranostics: Development, applications, and recent advancements
- Synthesis and encapsulation of iron oxide nanorods for application in magnetic hyperthermia and photothermal therapy
- Contemporary nano-architectured drugs and leads for ανβ3 integrin-based chemotherapy: Rationale and retrospect
- State-of-the-art review of fabrication, application, and mechanical properties of functionally graded porous nanocomposite materials
- Insights on magnetic spinel ferrites for targeted drug delivery and hyperthermia applications
- A review on heterogeneous oxidation of acetaminophen based on micro and nanoparticles catalyzed by different activators
- Early diagnosis of lung cancer using magnetic nanoparticles-integrated systems
- Advances in ZnO: Manipulation of defects for enhancing their technological potentials
- Efficacious nanomedicine track toward combating COVID-19
- A review of the design, processes, and properties of Mg-based composites
- Green synthesis of nanoparticles for varied applications: Green renewable resources and energy-efficient synthetic routes
- Two-dimensional nanomaterial-based polymer composites: Fundamentals and applications
- Recent progress and challenges in plasmonic nanomaterials
- Apoptotic cell-derived micro/nanosized extracellular vesicles in tissue regeneration
- Electronic noses based on metal oxide nanowires: A review
- Framework materials for supercapacitors
- An overview on the reproductive toxicity of graphene derivatives: Highlighting the importance
- Antibacterial nanomaterials: Upcoming hope to overcome antibiotic resistance crisis
- Research progress of carbon materials in the field of three-dimensional printing polymer nanocomposites
- A review of atomic layer deposition modelling and simulation methodologies: Density functional theory and molecular dynamics
- Recent advances in the preparation of PVDF-based piezoelectric materials
- Recent developments in tensile properties of friction welding of carbon fiber-reinforced composite: A review
- Comprehensive review of the properties of fly ash-based geopolymer with additive of nano-SiO2
- Perspectives in biopolymer/graphene-based composite application: Advances, challenges, and recommendations
- Graphene-based nanocomposite using new modeling molecular dynamic simulations for proposed neutralizing mechanism and real-time sensing of COVID-19
- Nanotechnology application on bamboo materials: A review
- Recent developments and future perspectives of biorenewable nanocomposites for advanced applications
- Nanostructured lipid carrier system: A compendium of their formulation development approaches, optimization strategies by quality by design, and recent applications in drug delivery
- 3D printing customized design of human bone tissue implant and its application
- Design, preparation, and functionalization of nanobiomaterials for enhanced efficacy in current and future biomedical applications
- A brief review of nanoparticles-doped PEDOT:PSS nanocomposite for OLED and OPV
- Nanotechnology interventions as a putative tool for the treatment of dental afflictions
- Recent advancements in metal–organic frameworks integrating quantum dots (QDs@MOF) and their potential applications
- A focused review of short electrospun nanofiber preparation techniques for composite reinforcement
- Microstructural characteristics and nano-modification of interfacial transition zone in concrete: A review
- Latest developments in the upconversion nanotechnology for the rapid detection of food safety: A review
- Strategic applications of nano-fertilizers for sustainable agriculture: Benefits and bottlenecks
- Molecular dynamics application of cocrystal energetic materials: A review
- Synthesis and application of nanometer hydroxyapatite in biomedicine
- Cutting-edge development in waste-recycled nanomaterials for energy storage and conversion applications
- Biological applications of ternary quantum dots: A review
- Nanotherapeutics for hydrogen sulfide-involved treatment: An emerging approach for cancer therapy
- Application of antibacterial nanoparticles in orthodontic materials
- Effect of natural-based biological hydrogels combined with growth factors on skin wound healing
- Nanozymes – A route to overcome microbial resistance: A viewpoint
- Recent developments and applications of smart nanoparticles in biomedicine
- Contemporary review on carbon nanotube (CNT) composites and their impact on multifarious applications
- Interfacial interactions and reinforcing mechanisms of cellulose and chitin nanomaterials and starch derivatives for cement and concrete strength and durability enhancement: A review
- Diamond-like carbon films for tribological modification of rubber
- Layered double hydroxides (LDHs) modified cement-based materials: A systematic review
- Recent research progress and advanced applications of silica/polymer nanocomposites
- Modeling of supramolecular biopolymers: Leading the in silico revolution of tissue engineering and nanomedicine
- Recent advances in perovskites-based optoelectronics
- Biogenic synthesis of palladium nanoparticles: New production methods and applications
- A comprehensive review of nanofluids with fractional derivatives: Modeling and application
- Electrospinning of marine polysaccharides: Processing and chemical aspects, challenges, and future prospects
- Electrohydrodynamic printing for demanding devices: A review of processing and applications
- Rapid Communications
- Structural material with designed thermal twist for a simple actuation
- Recent advances in photothermal materials for solar-driven crude oil adsorption

