Abstract
Advancement in nanotechnology and its intervention into the medical field has led to significant development in the field of oral health. Also, the combination of nanomaterial science and biotechnology in dental nanorobotics has enthralled us by adding momentum to contemporary dental practices. The progressive nature of dental afflictions often requires an umbrella approach for their prevention, diagnosis, and complete treatment. Furthermore, the complex nature of dental diseases entails customized treatment modalities, which provides the development of various nanotechnology armamentariums. Furthermore, with the objective of controlled drug delivery, researchers have done a plethora of work to apply nanomaterials such as nanospheres, nanotubes, and nanocomposites for dental infections. However, the fundamental concern with nanotechnology is cost involvement and scaleup hurdles which limits its commercialization. Nevertheless, we hope that optimal utilization of the available nanotechnological interventions for modern dental practice will shortly improve oral health. Hence, this review primarily focuses on the types of nanotechnological interventions explored for various dental afflictions. Also, the authors have attempted to enlighten the readers about the practical aspects of nanotherapeutics for dental disease, that is, a journey from laboratory to product commercialization.
1 Introduction
The past few years have witnessed a remarkable development in the clinical applications of nanobiomaterials in health care and dentistry. Nanotechnology appears as a valuable tool to the health care industry, and its applications have led to a significant improvement in modern medicine and dental practices. Currently, nanotechnology is driving the dental material industry at a high pace [1]. Applications of nanotechnology offer an impeccable and suitable solution in dentistry and seem to have answers to the problems of conventional dental practices. These novel nanobiomaterials can mimic the surface and interface properties of dental tissues [2]. In the past few decades, biotechnology and regenerative medicine have significantly impacted human lives. However, an advanced level of research is still required to overcome the drawbacks of conventional biomaterials. Although nanodentistry is still in its infancy, it has enormous potential to give innovative solutions for operative and preventive dentistry, tooth restoration, and periodontics (Figure 1). The use of nanoparticles in root end sealants and fillers provides more strength and luster.
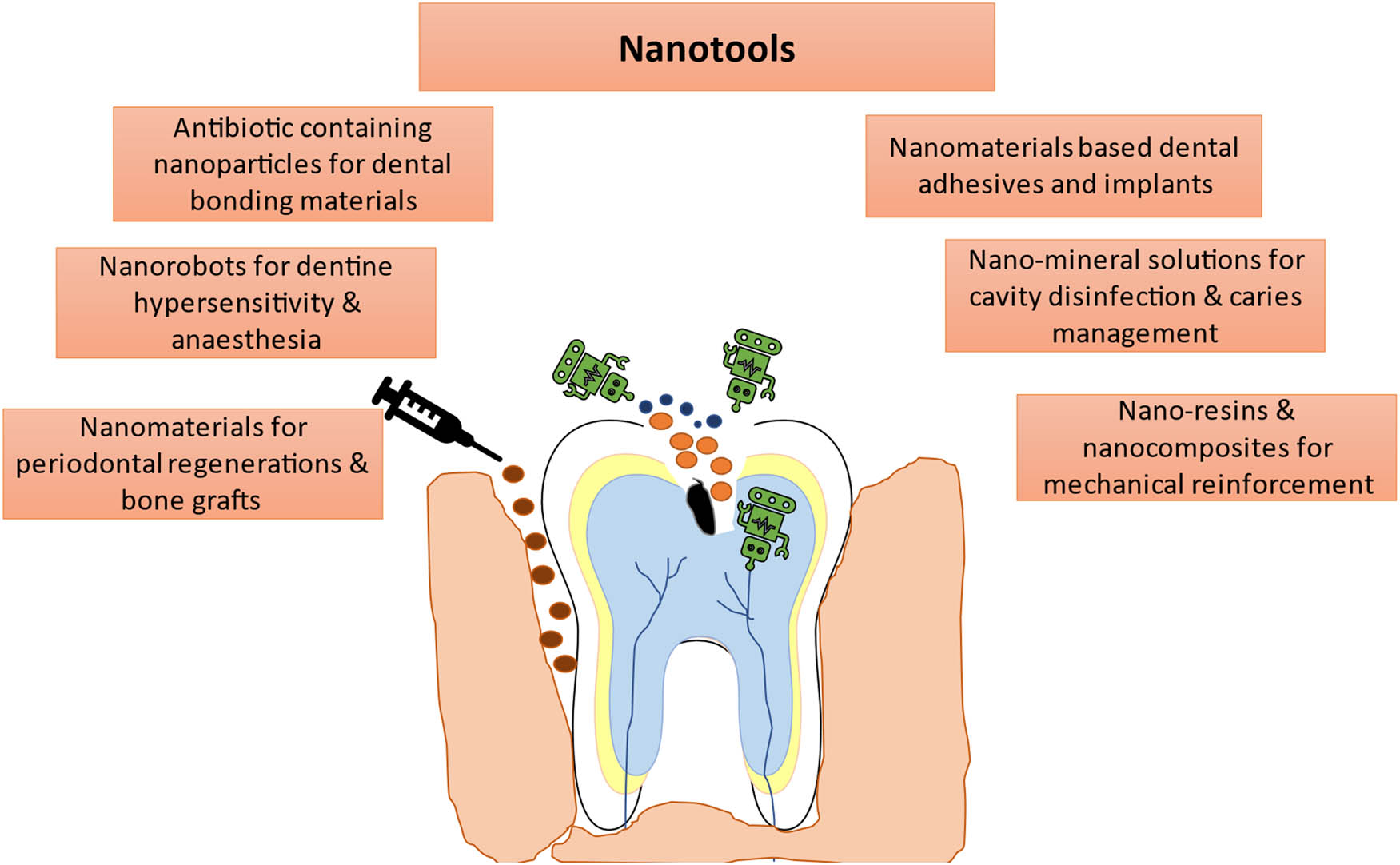
Various nanotools for dental afflictions.
Similarly, the incorporation of antimicrobial nanoparticles in restorative materials assures protection against caries forming bacteria and maintains the health of the oral environment. Also, a nanoparticle-based system is an attractive approach for localized drug delivery in periodontitis and oral squamous cell carcinomas [3,4]. Nanotechnology-based hydroxyapatite (HA) is well sufficient to treat osseous defects [5]. Neocis’ Yomi is the only Food and Drug Administration (FDA)-approved robotic navigation system used in US dentistry and has performed more than 1,000 implants in 2019 [6]. Altogether, it is believed that, in the future, nanotechnology will yield precise and customized solutions in dentistry [7,8].
However, due to the submicron size, physical, chemical, and mechanical properties of any material change, it is a matter of investigation. And as FDA regulates pre-market authorization of drugs and biologics, nanomedicines are also pre-clinically and clinically validated by FDA. Therefore, the safety of nanomaterials is a paramount concern [9].
Henceforth, this review attempts to appraise the readers about the various available nanotherapeutic tools for operative dentistry, preventive dentistry, and periodontotherapy. Also, information on the granted patents, clinical trials, available products in the market, and the regulatory aspects of nanobiomaterials have been collected. It is hoped that this review will enlighten the readers about the practical aspects of nanotools for dental disease, that is, a journey from laboratory to product commercialization.
2 Nanotherapeutic tools for dental afflictions
Oral health has been majorly affected by the emergence of advanced nanomaterials, tissue engineering techniques, and nanorobotics. The techniques, as mentioned earlier, aim to improvize dental therapy and reduce surgery-associated pain and phobia.
Nowadays, nano-anaesthesia during dental surgery is preferred to reduce pain. Oral nano-anaesthesia is the colloidal suspension of nanorobotic particles, which are analgesic in nature. When the anaesthesia is injected into the gingiva, it travels to the dentinal tubules, as directed by the dentist through the computerized navigation and desensitized the nerves, ensuring the analgesic effect. Once the surgical procedure is completed, nanorobots can be easily removed by the dentist. Excellent patient comfort, selectivity, and controllability are the significant advantages of nano-anaesthesia over conventional anaesthetics [10,11].
These days, caries is the most common teeth problem, which adversely affects an individual’s daily life. Their progressive and infectious nature requires mechanical excavation and further filling with the resins or the restorative materials. Conventional filling materials such as HA, metals, and inorganic glues have the problem of microleakage: the discrepancy in physicochemical properties exists between them and the tooth [12]. Clinical studies showed that conventional filling materials lack anticaries properties, which could result in a high incidence of secondary caries. There is approximately 50% failure in filling restorations, thereby leading to wastage of public health resources. In order to resolve the above issues, nanomaterial-based dental filling materials were introduced and they served as the major breakthrough in caries management. Various anticaries agents such as silver nanoparticles, nano-zinc and nano-zincoxide, other metal nanoparticles, remineralized nano-anticaries materials, and biomimetic nanocatalysts were explored by the researchers to challenge the current problems of caries management [13]. In nutshell, we can say that with the availability of nanomaterial-based tooth repair materials, diagnosis and treatment of dental caries can be improved. Furthermore, applications of nanotools in various segments of dentistry are explained as follows.
2.1 Nanotools in preventive dentistry
Nanotools are gaining much attention in modern dentistry to prevent disease progression, where tooth decay prevention and treatment of carious lesions are of prime concern. Controlling the dentine hypersensitivity with the nanotools is an emerging field [14].
Dental enamel being a calcified tissue is mainly consisting of calcium-deficient carbonate hydroxyapatite. On the nanoscale, they appeared as a crystalline nanorod-like structure where the calcium hydroxyapatite crystallites are arranged roughly parallel to each other. However, the dentine is a hydrated tissue and comprises minerals, collagenous and non-collagenous proteins, and fluids. And the dentinal matrix is mainly made up of type I collagen fibrils which form a three-dimensional scaffold and are supported by hydroxyapatite crystallites [15]. As biofilm deposition on the enamel surface leads to caries lesion due to acids from bacterial metabolism, frequent consumption of acidic foods and beverages may also cause demineralization and induce enamel erosion [16]. Various approaches for remineralization include fluoride treatment, casein phosphopeptide (CPP)-stabilized amorphous calcium phosphate (ACP) treatment, and biomimetic materials. Fluoride is a widely accepted agent for enamel remineralization [17]. CPP stabilizes calcium and phosphate ions by forming the amorphous nanocomplexes and ensures continuous availability of ions for biomineralization [18]. MI Paste, Recaldent, and GC Tooth Mousse are the marketed products containing CPP-ACP [19]. Amongst the biomimetic approaches to remineralization, biomimetic carbonate hydroxyapatite nanoparticles were used to repair the micrometre-sized tooth surface defects. These crystals have been incorporated into toothpastes or mouth rinses to promote enamel remineralization, and based upon their size, they are deposited onto the dentinal surface (20 nm) or enamel surface (100 nm) [20]. BioRepair from Coswell Laboratory is a commercially available product that contains carbonate hydroxyapatite nanoparticles for enamel remineralization which have been proved to be effective in in vitro conditions after 10 min application [21]. Also, it has been shown that the nano-hydroxyapatite toothpaste with either spheroidal or needle-like particles was comparatively more effective than the sodium fluoride solution for remineralization of etched enamel [22]. However, due to the complex organic and inorganic structure of the dentine, remineralizing dentine into a functional state remains one of the most difficult challenges of dentistry.
If caries or enamel defects enlarge, they may lead to tissue damage which cannot be repaired by remineralization techniques. But with the help of the tissue engineering approach, treatment of damaged tissue is possible. Cells or drugs can be easily loaded into the nanoparticles or scaffolds and selectively targeted to particular tissue, thereby ensuring the sustained and controlled release. Scaffolds are designed in such a way that they can carry both signalling molecules for homing and therapeutic molecules for targeted delivery. Furthermore, for tissue engineering, three components are necessary: cell (mainly stem cell), bioactive signalling molecule (to assist tissue regeneration), and a polymeric scaffold. Researchers have exploited numerous nanomaterials to fabricate tissue engineering scaffolds. Differentiation and functionality are the prerequisite of scaffold material to act as an extracellular matrix for supporting tissue regeneration. Different techniques exist for nanofibrous polymeric scaffolds, and electrospinning is the most commonly used one [14,23].
2.2 Nanotools in operative dentistry
Operative dentistry is based on the diagnosis, treatment, and prognosis of complex tissue defects of the tooth with a prime focus on restoring the form, function, and aesthetic [24]. Superior alternatives were developed by incorporating nanoparticles, nanofibres, and nanoclusters in traditional composites. The ideal size of a nanocomposite should be 1–100 nm. Nanohybrid composites are resin matrices made up of nanoparticles and large filler particles, and their size may vary from 0.4 to 5.0 µm [25]. The incorporation of nanostructures assures significantly enhanced properties of dental composites due to the availability of larger surface areas and binding sites. These dental composites also elicit improved smoothness, high gloss finish, better translucency, and aesthetic look fortified with excellent wear resistance [7,26]. InfinixTM Universal composite, approved by FDA in 2019, is based on Nobio’s QASi composite technology and consists of quaternary ammonium silica dioxide [27,28]. Another composite 3M™ (Filtek™ Supreme Flowable) contains loosely bound 5–20 nm zirconia/silica particles [29]. Other nanomaterial-based marketed products are listed in Table 1.
Nanomaterial-based dental products
| Product name | Product category | Product component | Application of product | Ref. |
|---|---|---|---|---|
| Nanohybrid composite (brand: NHC SR Phonares®) | Prosthodontics | Silicon oxide | Denture teeth | [30] |
| Nanoresin-modified glass ionomer cement (GIC) (brand: KetacTM Nano 3M ESPE) | Restorations | Zirconia/silica | High shear (enamel) nanofillers and nanoclusters | [31] |
| Nanocomposite resins (brand: Ceram.x®, MonoTM, Ceram.x®, DuoTM, Dentsply) | Conservative | Ceramic/polysiloxane | Organically modified compressive nanofillers | [32,33] |
| Nano-GIC (brand: GCP Glass Fill TM GCP Dental) | Conservative | Carbonized fluorapatite/HA nanoparticles | Lower hardness and bond strength dentine | [34,35] |
| Mineral solution (brand: NanoCare gold®, DNTTM) | Cavity disinfectant | Silver nanoparticles | Antibacterial nanoparticles | [36] |
| Silicon-based sealer (brand: GuttaFlowTM) | Endodontics | Nanosilver particles | Nanosized sealing agent | [37] |
| Bone grafts (brand: NanoBone®, Artoss GmbH®) | Periodontics | Nanocrystalline HA | Low cytotoxic and high biocompatible graft | [38] |
| Nano-implant coating (brand: NonoTite BIOMET 3i) | Implantology | Nano-HA | Biocompatible implant coating | [39] |
The difference in the size of filler particles and HA crystals of tooth enamel results in poor bonding of the material with the tooth, thereby making it a little unstable. This issue was resolved by adding the nanoparticles to the composites, and promising results in smooth surface transition and interaction with tooth tissues were observed. Therefore, incorporated nanostructures improve the physicomechanical and optical properties of the resin composites [40,41].
Longevity is the most desirable aspect of any dental restorative material; however, most of the restoration cannot meet the same due to the enzymatic activity of dental caries forming oral bacteria [42]. Orthodontic materials also suffer from a similar problem, that is to harbour the multiplication of caries-causing bacteria. These bacteria demineralize the enamel, which produces a white spot lesion in 50% of patients undergoing orthodontic treatments [43]. Recently, nanotechnology has become an important area of research with a prime focus on increasing the antimicrobial properties of dental restorative and orthodontic materials [44]. Among the various nanoparticles, silver nanoparticles were the most commonly exploited by researchers either alone [45] or in combination with others, such as nanoparticles of zinc oxide [45], HA nanowires [46], HA [47], silica nanoparticles [48], and quaternary ammonium dimethacrylate [49]. Titanium oxide nanoparticles are suitable candidates for dental bonding materials with additional antibacterial activity [44]. So, it can be concluded that nanoparticle-modified dental bonding materials offer a more significant antibacterial activity than conventional dental bonding materials.
In cases, when non-surgical approaches are contraindicated or fail, a surgical endodontic procedure is required to save the tooth. The basic steps are exposure of carious tooth, root-end resection, preparation, and filling of a root end sealing material [50]. Various nanomaterials have been explored for endodontic purposes such as sealants and irrigators for disinfection purposes.
EndoSequence BC sealer is a nanoparticle-based sealant having excellent dimensional stability and antimicrobial property with a setting time of 3–4 h. It consists of calcium hydroxide, calcium silicate, zirconia, thickening agent, and bioactive nanoparticles. After getting hydrated in the apical area of the tooth, it formed a nano-HA and calcium silicate and settled down over there [51].
Bioactive nanoparticles significantly improved the physical properties of the materials [52].
GuttaFlow sealer is another excellent root canal sealant with in-built resistance to bacterial penetration. It is made up of silicon-based material, silver nanoparticles, and dust of gutta-percha. This sealant is also dimensionally stable and has a setting time of 30 min [53].
Another sealant, nano-HA-modified gutta-percha, consists of nano-HA, bismuth oxide, hexamethylenetetramine, and bisphenol-A-diglycidylether as a liquid component [54].
The bioactive glass nanoparticles have proven their potential for root canal disinfection. Nanoparticles (20–90 nm) of bioactive glasses containing SiO2CaOP2O5 were prepared, assessed for antimicrobial activity, and observed promising results [55]. With time-lapse, they release alkaline species in the biological environment, which are antibacterial [56]. Bioceramics are bioactive and biocompatible, and further release of alkaline antimicrobial species increases their suitability for root canal disinfection [57].
2.3 Nanotools in periodontal therapy
Periodontitis is a complex disease that destroys collagen and tooth-supporting materials. Its treatment is based on the delivery of antimicrobials along with the host modulatory agents [58,59]. From high-dose systemic antibiotics to localized drug delivery devices, nanotechnology has tremendously improved the treatment strategy.
In periodontics, nanoparticles such as nanospheres and nanocapsules have been extensively used as drug delivery carriers. Clinical data suggest that Atridox® (Collagenex Pharmaceuticals, Newtown, PA, USA), a biodegradable sustained release device containing doxycycline hyclate, is capable of reducing probing periodontal pocket depths after initial treatment of peri-implant lesions [60].
Also, promising results have been observed in a controlled phase 3 clinical trial wherein Arestin® (microspheres of minocycline) was administered as an adjunct to scaling and root planning (SRP) for the treatment of periodontitis. Adjunctive treatment reduces probing depth significantly more than the SRP alone [61].
Since periodontitis is a classic example of biofilm-based disease, and similar to other biofilm-mediated infections, it is unmanageable with alone antibiotics and host modulatory agents. Also, for complete amelioration, multiple placements of localized drug delivery systems are required, and this ultimately mounts the cost. The non-invasive perio-protect method tray was proposed as a solution for this problem. A period-tray with a customized sealing system delivers medication deep inside the periodontal pockets and is the one and only FDA-approved medication delivery system for periodontitis. In clinical settings as well, promising results were observed with this system [62].
Apart from the killing of periodontopathic bacteria, regeneration of periodontium and bone is the prerequisite for the complete treatment of periodontal diseases. Localized delivery of growth factors (GFs) to the periodontal cavity is a new approach to the regeneration of periodontium. Various strategies such as micro-particles, nanoparticles, scaffolds, injectable gels, and composites are being explored to preserve the bioactivity of GF and control its release [51].
Bioactive glasses also promote bone formation by osteoinduction and osteoconduction, which has led to the use of bioactive glasses that have been widely applied in dentistry. It has been observed that the periodontal ligament cells, when placed adjacent to the bioactive glass nanoparticles, showed high growth and enhanced viability along with elevated alkaline phosphatase activity [63].
When multiwalled carbon nanotubes were immersed in calcium phosphate solution at 37°C for 2 weeks, they resulted in the formation of nanoscale HA, thus indicating the potential of carbon nanotubes for periodontal tissue regeneration [64]. Ostim (Heraeus Kutzer, Hanau, Germany), a ready-to-use paste consisting of 35% of nanocrystalline particles of HA and 65% the water, has been widely used for the treatment of osseous defects. Clinically, its application results in a marked reduction in periodontal pocket depth and, after therapy, attains clinical attachment in 6 months [65,66].
3 Patents and clinical trials
Based on the acceptable performance of nanomaterials for dental procedures, patents have been granted in various countries, and a few of the granted patents are listed in Table 2.
Patents on nanotherapeutic tools for dental intervention
| S.no | Grant no. | Study objective | Description |
|---|---|---|---|
| 1 | US20070043142A1 | Dental compositions based on nanofibre reinforcement | The present invention describes the dental composition based on nanofibres |
| 2 | US9545295B2 | Nanobubble generator for cleaning root canal of tooth and dental apparatus comprising the same | The present invention describes a nanobubble generator for root canal irrigation purposes |
| 3 | WO2001030307A1 | Dental materials with nanosized silica particles | The present invention describes the composition of dental materials for sealant, prosthesis, and filler purpose |
| 4 | US8298329B2 | Nanocrystalline dental ceramics | The present invention describes the composition of nanocrystalline dental ceramic |
| 5 | EP2495356A1 | Dental implant with nanostructured surface and process for obtaining it | The present invention describes the method of production of nanotubes of titanium dioxide as a coating for dental implants |
| 6 | WO2014087412A1 | Nanosurface-modified metallic titanium implants for orthopaedic or dental applications and methods of manufacturing thereof | The present invention describes the method of production of metallic implant |
| 7 | EP2409682A1 | HA-binding nano- and microparticles for caries prophylaxis and reduction of dental hypersensitivity | The present invention describes the application of oligopeptide functionalized nanoparticles or microparticles for caries prevention |
| 8 | US8357732B2 | Method for production of biocompatible nanoparticles containing dental adhesive | The present invention describes the process of production of HA nanorods containing dental adhesive |
| 9 | WO2017149474A1 | Process for the production of antimicrobial dental adhesives including graphene and relative product thereof | The present invention describes the process of production of dental adhesive with antibacterial and antibiofilm activity |
| 10 | US20130108708A1 | Dental composites comprising nanoparticles of amorphous calcium phosphate | The present invention describes the antibacterial agent containing dental composite |
| 11 | US20130017236A1 | Toothpaste or tooth gel containing silver nanoparticles coated with silver oxide | The present invention describes the composition of silver nanoparticle-containing toothpaste |
| 12 | US9795543B1 | Nanocomplexes for enamel remineralization | The present invention describes the preparation and composition of nanocomplexes |
| 13 | US20070213460A1 | Antimicrobial nano-silver additive for polymerizable dental materials | The present invention describes the polymerizable dental material that can be used as dental filling material |
| 14 | US8641418B2 | Titanium nanoscale etching on an implant surface | The present invention describes the process of roughening of implant surface for fixing dental prostheses thereon |
| 15 | CN103690376B | Dental pulp root canal filling paste | The present invention describes the preparation of paste for filling into the root pulp |
| 16 | US20100035214A1 | Radio-opaque dental prosthetic member | The present invention describes the nanoparticle-containing radio-opaque material for dental prosthetics |
| 17 | US6890968B2 | Pre-polymerized filler in dental restorative composite | The present invention describes the filler for a dental composite that can be used in stress-bearing restorations and cosmetic restorations |
| 18 | US9192545B2 | Dental root canal filling material having improved thermal conductive characteristics | The present invention describes the dental root canal filling material with improved thermal property dental composite of high strength |
| 19 | WO2002092022A2 | The dental composite containing discrete nanoparticles | The present invention describes the dental composite of high strength |
| 20 | US20100047224A1 | Biosilica-adhesive protein nanocomposite materials: synthesis and application in dentistry | The present invention describes the application of silicate in-silk fibroin fusion proteins in dentistry to formulate silica-containing nanocomposite materials for nanocomposite as filling material |
Although the nanomaterial-based dental products showed promising results in in vitro conditions, safety, and efficacy still need to be evaluated by clinical trials, few completed trials are listed in Table 3.
Clinical trials on nanotherapeutic tools for dental intervention
| S. no | Clinical trial no. | Study title | Condition | Intervention |
|---|---|---|---|---|
| 1 | NCT03186261 | Antibacterial effect of nano-silver fluoride versus chlorhexidine on occlusal carious molars treated with partial caries removal technique | Dental caries | Nanosilver fluoride solution was compared with cavity cleanser |
| 2 | NCT02093091 | Clinical evaluation of nano-ionomer filling in primary teeth | Dental caries | Ketac Nano was compared with a conventional filling material; vitremer |
| 3 | NCT02936830 | Effectiveness of nano-hydroxyapetite paste on reducing dentin hypersensitivity | Dentin hypersensitivity | 15% Nanohydroxyapetite paste was compared with Glycerol and 5% sodium fluoride varnish |
| 4 | NCT03980847 | Evaluation and the histomorphometric study of nanocrystalline HA (nanobone) with alendronate in the preservation of the tooth socket | Bone resorption | Alendronate 20 mg was combined with nanocrystal HA |
| 5 | NCT04213716 | Comparison of the efficacy of calcium hydroxide with silver nanoparticle and conventional calcium hydroxide intra-canal medications on post-operative pain in symptomatic root canal treatment failure cases | Retreatment | Combination product of silver nano-particulate solution mixed with calcium hydroxide powder was compared with alone combination product and alone conventional calcium hydroxide |
| Root canal retreatment | ||||
| Non-surgical retreatment | ||||
| 6 | NCT03792178 | Evaluation of postoperative sensitivity of bulk-fill resin composite versus the nano-resin composite | Sensitivity | Bulk fill composite was compared with nano-resin composite |
| 7 | NCT02895321 | Nano-HA with potassium nitrate in the therapy of the dental sensitivity | Dentin sensitivity | Cavex bite and white ExSense was compared with Colgate protection caries and placebo gel |
| 8 | NCT03193606 | Radiographic assessment of glass ionomer restorations with and without prior application of nano-silver fluoride in occlusal carious molars treated with partial caries removal technique | Partial dentin caries removal | Nanosilver fluoride solution was applied to carious dentin |
| 9 | NCT02918617 | Clinical efficacy in relieving dentin hypersensitivity of nano-HA-containing toothpastes and cream | Dentin sensitivity | Control toothpaste containing Novamin technology compared with control toothpaste containing 1500 ppm fluoride as sodium monofluorophosphate and test toothpaste containing a high concentration of nano-hydroxyapatite |
| 10 | NCT02893735 | Clinical comparison of two resin composites on diastema closure and reshaping at four years | Diastema | Charisma-Diamond was compared with Filtek-Z550 |
| 11 | NCT01464996 | Clinical evaluation of a new two-component self-etch universal adhesive | Composite restorations of tooth lesions | Adhesive: OptiBond XTR; composite: Herculite Ultra in Arm 1 was compared with |
| Adhesive: OptiBond FL; Composite: Herculite Ultra in Arm 2 | ||||
| 12 | NCT04643288 | Nanocrystalline HA bone substitute for treating periodontal intrabony defects | Chronic periodontitis | Open flap debridement procedure was performed in control group while nano-HA bone graft along with open flap debridement was given to intervention group |
| 13 | NCT02018783 | Single application of desensitizing pastes as dentin sensitivity treatment | Dentine hypersensitivity | Colgate |
| Sensodyne | ||||
| Nano P; and | ||||
| Cocorico were compared among themselves | ||||
| 14 | NCT04059250 | Nobio clinical study – demineralization prevention with a new antibacterial restorative composite | Dental caries, denture, partial, removable | Nobio composite was compared with traditional composite |
4 Regulatory aspect of nanotools or nano-based products for dentistry
The regulatory guidelines/aspects are defined as a range of scientific disciplines encompassing the quality, safety, and efficacy assessments of health products (medicinal products and medical devices). These guidelines provide informed regulatory decision-making throughout the lifecycle of a health product ranging from drug development, licensing, registration, manufacturing, and marketing. They are cumulatively derived from the diverse fields of basic medical science, applied medicinal science, and social sciences [67]. The regulatory standards and tools often vary in different countries and for different products as well. Examples of major regulatory authorities are FDA, USA; Therapeutic Goods Administration, Australia; and Central Drug Standard Control Organization, India and European Medicines Agency. The international organizations to mandate such rules and regulations are World Health Organization, Pan American Health Organization, World Trade Organization, International Conference on Harmonization, and World Intellectual Property Organization [68]. The category of health products is broadly divided into two main heads, namely, medicinal products and medical devices [69]. The medical devices are subdivided into the categories of invasive (either surgical or not) and non-invasive (coming into direct contact with the skin) medical devices. Examples of invasive medical devices in dentistry are dental fillers, composites, and crowns [70]. Nanomaterials are classified as natural or formulated materials containing unbound/aggregate (strongly bound)/agglomerate (weekly bound) particles in a size range of 1–100 nm. The health and medicinal products encompassing nanomaterials can be termed nanotools and nanomedicines or nanoproducts, respectively. The medical devices fortified with nanotechnology are known as nanomedical devices [71]. The outline of a regulatory guideline for nanotechnology-based health products is all risks (physical/chemical/environmental) must be evaluated and reduced as far as possible; material toxicity, compatibility, contaminants, residues, and leachates must be checked before processing and minimized risk of injury in context with physical features and external dimensions [72]. To determine the possible health effects of nanomaterials (free/fixed/embedded) used in medical devices, the guidelines have two norms, one is for the cases where the nanomaterial might inadvertently be released into the human body, and second, are the cases where the nanomaterial is intended to be released into the human body. The assessment of nanomaterials used in medical devices is necessary to ensure consumer safety, to examine the emerging/newly identified health and environmental risks [73]. The regulation norms for nanomaterials involve material characterization, that is, either natural based or synthetic or a combination. Physicochemical characterizations include an evaluation of various parameters such as chemical composition; particle size; particle/mass concentration; specific surface area; surface chemistry; surface charge; redox potential; solubility and partition properties; pH; viscosity; density and pore density; dustiness; chemical reactivity, catalytic and photocatalytic activities [74]. The nanomaterials used in dentistry tools and other medical devices are categorized in terms of low, medium, and high exposures and include various testing parameters such as toxicokinetic, cytotoxicity, acute toxicity, irritation, delayed-type hypersensitivity, genotoxicity, haemocompatibility, repeated-dose toxicity, implantation, chronic toxicity/carcinogenicity, reproductive, and developmental toxicity. For invasive dental products, a few additional studies include immunotoxicity, persistence, accumulation, and adsorption, distribution, metabolism, excretion (ADME) [75,76]. The risk evaluation of nanomaterials is based on release potential/kinetics; distribution and maintenance at the location site; and toxicity tests. The various aspects for evaluating the biocompatibility of nanomaterials used in dental and other medical devices are harmonized standards; assessment and testing in the risk management process; animal welfare requirements; toxicity studies; interactions with blood; implantation; irritation; and skin sensitization [77]. International Organization for Standardization (ISO) 10993 series describes considerations for the biocompatibility assessment or biological evaluation of nanomaterials based on dental and other medical devices. The additional considerations of the above series are surface nanostructures; nanomaterials incorporated within a medical device without intention to be released; nanomaterials bound on the surface of or within a medical device to be released; nanomaterials released from a medical device as degradation product, wear, or from mechanical treatment processes [78]. The general considerations of ISO 10993 include the assessment of release kinetics (rate and quantity), contract duration, potential cellular or tissue effects (beneficial or adverse), physicochemical characterization, and toxicokinetic (ADME)/tissue distribution of the nanomaterials [79]. The three prerequisites for the biological evaluation of dental nanomaterials are physical morphology, chemical composition, and extrinsic properties (interaction ability with the surrounding environment) [80]. The extrinsic properties cumulate protein–cellular interaction, cellular uptake (cross cellular and intracellular), interruption activity (DNA synthesis, oxidative stress, and other cellular functions), and translocation at the site of administration. Additionally, other studies such as evaluation of dose metrics, different properties to bulk form, mass/number concentration, surface area, aggregation, electric charge, and optical properties are also taken into consideration [81,82,83]. The significant toxicity studies include genotoxicity, carcinogenicity, reproductive toxicity, immunotoxicity, and systemic toxicity. The in vitro toxicity analysis demonstrates exposure to the cell nucleus, and in vivo analysis ensures nanomaterials reach the target organ. The ability of nanoparticles to initiate an immune response or immunotoxicity results in their irritation and sensitization potential. The additional toxicity study includes haemocompatibility which is based on nanomaterial’s ability to translocate from device to systemic and to induce prothrombotic effects, platelet activation, and inflammatory and hypersensitivity reactions [59,61,67].
5 Conclusion and future perspective
This up-to-date snapshot clearly explains the impact of nanotechnology on dentistry and how it has revolutionized the dental practice worldwide. Various nanotechnology-based products such as nanoresin modified-GIC, nanohybrid composite, nanocomposite resins, and nano-GIC are there on the market to restore the size, shape, and aesthetic of teeth. Also, nanotechnology-driven approaches have now improvized the diagnosis and treatment of dental caries too. Nano-based products such as Atridox and Arestin have gained much attention from clinicians to reduce the bacterial load of the periodontal cavity. However, due to the unpredictable nature of existing tissue engineering techniques, regeneration of the periodontal tissue still remains a challenge to clinicians across the world. But, delivery of GFs to the periodontal cavity along with nanocarriers and scaffolds can be considered for this purpose. Also, bone grafts and implants have shown promising results in periodontology. With the help of nano-anaesthesia and nanorobotics, dental surgeries are no more dreadful for patients. Although the communion of nanotechnology and dentistry is still in its infancy yet, its continuous progression has shown a greater impact on overall research and commercial translation. Due to their promising results in in vitro conditions, a large number of patents have been granted to dental nanoproducts across the world. However, to assure safety and efficacy, several clinical trials have been conducted, and many of them are now completed. The small size of these nanoproducts associates itself with nanotoxicity outcomes and hence is particularly subjected to FDA approval before marketing and patient usage and needs to pass the set criteria and pre-defined standards. In conclusion, it can be said that the nanotechnology-driven approaches have imparted an edge to the various dental procedures and serve as a valuable tool for dental science. However, concerted efforts are required to address the various issues which could be pertinent to bridge the gap between its translation from the bench side to the clinical settings and also to have a substantial effect on major tooth repair using nanodentistry.
Acknowledgments
Author Pooja Jain is thankful to CSIR for providing financial assistance in the form of SRF [09/0591(11905)/2021-EMR-I].
-
Funding information: The authors state no funding involved.
-
Author contributions: All authors have accepted responsibility for the entire content of this manuscript and approved its submission.
-
Conflict of interest: The authors state no conflict of interest.
References
[1] Padovani GC, Feitosa VP, Sauro S, Tay FR, Durán G, Paula AJ, et al. Advances in dental materials through nanotechnology: facts, perspectives and toxicological aspects. Trends Biotechnol. 2015;33:621–36.10.1016/j.tibtech.2015.09.005Search in Google Scholar PubMed
[2] Qasim SB, Rehman IU. Application of nanomaterials in dentistry. Micro and nanomanufacturing. Vol. II. New York City, US: Springer; 2018. p. 319–36.10.1007/978-3-319-67132-1_12Search in Google Scholar
[3] Fan L, Wang J, Xia C, Zhang Q, Pu Y, Chen L, et al. Glutathione-sensitive and folate-targeted nanoparticles loaded with paclitaxel to enhance oral squamous cell carcinoma therapy. J Mater Chem B. 2020;8:3113–22.10.1039/C9TB02818HSearch in Google Scholar
[4] Gharat SA, Momin M, Bhavsar C. Oral squamous cell carcinoma: current treatment strategies and nanotechnology-based approaches for prevention and therapy. Crit Rev Ther Drug Carr Syst. 2016;33(4):363–400.10.1615/CritRevTherDrugCarrierSyst.2016016272Search in Google Scholar PubMed
[5] Bordea IR, Candrea S, Alexescu GT, Bran S, Băciuț M, Băciuț G, et al. Nano-hydroxyapatite use in dentistry: a systematic review. Drug Metab Rev. 2020;52:319–32.10.1080/03602532.2020.1758713Search in Google Scholar PubMed
[6] Coutre L. Drilling into the future of robot-assisted dentistry. Mod Healthc Sep. 2019;25.Search in Google Scholar
[7] Jain P, Dilnawaz F, Iqbal Z. Insights into nanotools for dental interventions. In: Yata VK, Ranjan S, Dasgupta N, Lichtfouse E, editors. Nanopharmaceuticals: principles and applications. Vol. 3. Cham: Springer International Publishing; 2020. p. 53–79. 10.1007/978-3-030-47120-0_3.Search in Google Scholar
[8] Sharan J, Singh S, Lale SV, Mishra M, Koul V, Kharbanda OP. Applications of nanomaterials in dental science: a review. J Nanosci Nanotechnol. 2017;17:2235–55.10.1166/jnn.2017.13885Search in Google Scholar PubMed
[9] Bobo D, Robinson KJ, Islam J, Thurecht KJ, Corrie SR. Nanoparticle-based medicines: a review of FDA-approved materials and clinical trials to date. Pharm Res. 2016;33:2373–87.10.1201/9780429027819-7Search in Google Scholar
[10] Shetty NJ, Swati P, David K. Nanorobots: future in dentistry. Saudi Dental J. 2013;25:49–52. 10.1016/j.sdentj.2012.12.002.Search in Google Scholar PubMed PubMed Central
[11] Adeola HA, Sabiu S, Adekiya TA, Aruleba RT, Aruwa CE, Oyinloye BE. Prospects of nanodentistry for the diagnosis and treatment of maxillofacial pathologies and cancers. Heliyon. 2020;6:e04890. 10.1016/j.heliyon.2020.e04890.Search in Google Scholar PubMed PubMed Central
[12] Zhou B, Liu Y, Wei W, Mao J. GEPIs-HA hybrid: a novel biomaterial for tooth repair. Med Hypotheses. 2008;71:591–3.10.1016/j.mehy.2008.04.024Search in Google Scholar
[13] Chen H, Gu L, Liao B, Zhou X, Cheng L, Ren B. Advances of anti-caries nanomaterials. Molecules. 2020;25:5047. 10.3390/molecules25215047.Search in Google Scholar
[14] Uskoković V, Bertassoni LE. Nanotechnology in dental sciences: moving towards a finer way of doing dentistry. Materials. 2010;3:1674–91.10.3390/ma3031674Search in Google Scholar
[15] Hannig M, Hannig C. Nanomaterials in preventive dentistry. Nat Nanotech. 2010;5:565–9. 10.1038/nnano.2010.83.Search in Google Scholar
[16] Hannig C, Berndt D, Hoth-Hannig W, Hannig M. The effect of acidic beverages on the ultrastructure of the acquired pellicle – an in situ study. Arch Oral Biol. 2009;54:518–26.10.1016/j.archoralbio.2009.02.009Search in Google Scholar
[17] Selwitz RH, Ismail AI, Pitts NB. Dental caries. Lancet. 2007;369:51–9.10.1016/S0140-6736(07)60031-2Search in Google Scholar
[18] Cross K, Huq N, Reynolds E. Casein phosphopeptides in oral health-chemistry and clinical applications. Curr Pharm Des. 2007;13:793–800.10.2174/138161207780363086Search in Google Scholar PubMed
[19] Reynolds E, Cai F, Cochrane N, Shen P, Walker G, Morgan M, et al. Fluoride and casein phosphopeptide-amorphous calcium phosphate. J Dental Res. 2008;87:344–8.10.1177/154405910808700420Search in Google Scholar PubMed
[20] Li L, Pan H, Tao J, Xu X, Mao C, Gu X, et al. Repair of enamel by using hydroxyapatite nanoparticles as the building blocks. J Mater Chem. 2008;18:4079–84.10.1039/b806090hSearch in Google Scholar
[21] Roveri N, Battistella E, Bianchi CL, Foltran I, Foresti E, Iafisco M, et al. Surface enamel remineralization: biomimetic apatite nanocrystals and fluoride ions different effects. J Nanomaterials. 2009:9.10.1155/2009/746383Search in Google Scholar
[22] Lv KL, Zhang JX, Meng XC, Li XY. Remineralization effect of the nano-HA toothpaste on artificial caries. Trans Tech Publ. 2007;330:267–70.10.4028/0-87849-422-7.267Search in Google Scholar
[23] Zafar MS, Alnazzawi AA, Alrahabi M, Fareed MA, Najeeb S, Khurshid Z. 18 – Nanotechnology and nanomaterials in dentistry. In: Khurshid Z, Najeeb S, Zafar MS, Sefat F, editors. Advanced dental biomaterials. New Delhi, India: Woodhead Publishing; 2019. p. 477–505. 10.1016/B978-0-08-102476-8.00018-9.Search in Google Scholar
[24] Mount GJ, Ngo H. Minimal intervention: a new concept for operative dentistry. Quintessence International; 2000;31(8):1.Search in Google Scholar
[25] Van Dijken J, Pallesen U. A randomized 10-year prospective follow-up of Class II nanohybrid and conventional hybrid resin composite restorations. J Adhes Dent. 2014;16:585–92.Search in Google Scholar
[26] Ferracane JL. Current trends in dental composites. Crit Rev Oral Biol Med. 1995;6:302–18. 10.1177/10454411950060040301.Search in Google Scholar PubMed
[27] Lauren Burns. How antimicrobial nanotechnology is set to empower composites. Dental Products Repor. 2019;53(8).Search in Google Scholar
[28] Marley-320, FDA clears infinixTM antibacterial universal bond. Oral health group; 2019 https://www.oralhealthgroup.com/news/fda-clears-infinix-antibacterial-universal-bond-1003945401/ (accessed Aug 24, 2020).Search in Google Scholar
[29] Ilie N, Hickel R. Investigations on a methacrylate-based flowable composite based on the SDRTM technology. Dental Materials. 2011;27(4):348–55.10.1016/j.dental.2010.11.014Search in Google Scholar PubMed
[30] Munshi N, Rosenblum M, Jiang S, Flinton R. In vitro wear resistance of nano-hybrid composite denture teeth. J Prosthodont. 2017;26:224–9.10.1111/jopr.12412Search in Google Scholar PubMed
[31] Shebl EA, Etman WM, Genaid TM, Shalaby ME. Durability of bond strength of glass-ionomers to enamel. Tanta Dent J. 2015;12:16–27.10.1016/j.tdj.2014.08.001Search in Google Scholar
[32] Hegde MN, Hegde P, Bhandary S, Deepika K. An evalution of compressive strength of newer nanocomposite: an in vitro study. J Conserv Dent. 2011;14:36.10.4103/0972-0707.80734Search in Google Scholar PubMed PubMed Central
[33] Mitra SB, Wu D, Holmes BN. An application of nanotechnology in advanced dental materials. J Am Dental Assoc. 2003;134:1382–90.10.14219/jada.archive.2003.0054Search in Google Scholar PubMed
[34] Olegário IC, Malagrana APVFP, Kim SSH, Hesse D, Tedesco TK, Calvo AFB, et al. Mechanical properties of high-viscosity glass ionomer cement and nanoparticle glass carbomer. J Nanomater. 2015;16(1):37.10.1155/2015/472401Search in Google Scholar
[35] Arslanoglu Z, Altan H, Sahin O, Tekin MG, Adigüzel M. Evaluation of surface properties of four tooth-colored restorative materials. Acta Phys Polonica A. 2015;128:B-310.10.12693/APhysPolA.128.B-310Search in Google Scholar
[36] Mackiewicz A, Grzeczkowicz A, Granicka L, Antosiak-Iwańska M, Godlewska E, Gozdowski D, et al. Cytotoxicity of nanocare gold® in in vitro assay – pilot study. Dental Med Probl. 2015;52:167–74.Search in Google Scholar
[37] Patil P, Rathore VP, Hotkar C, Savgave SS, Raghavendra K, Ingale P. A comparison of apical sealing ability between GuttaFlow and AH plus: An in vitro study. J Int Soc Prev Commun Dent. 2016;6:377.10.4103/2231-0762.186794Search in Google Scholar PubMed PubMed Central
[38] Liu Q, Douglas T, Zamponi C, Becker ST, Sherry E, Sivananthan S, et al. Comparison of in vitro biocompatibility of NanoBone® and BioOss® for human osteoblasts. Clin Oral Implant Res. 2011;22:1259–64.10.1111/j.1600-0501.2010.02100.xSearch in Google Scholar PubMed
[39] Smeets R, Stadlinger B, Schwarz F, Beck-Broichsitter B, Jung O, Precht C, et al. Impact of dental implant surface modifications on osseointegration. BioMed Res Int. 2016:16.10.1155/2016/6285620Search in Google Scholar PubMed PubMed Central
[40] Tian M, Gao Y, Liu Y, Liao Y, Xu R, Hedin NE, et al. Bis-GMA/TEGDMA dental composites reinforced with electrospun nylon 6 nanocomposite nanofibers containing highly aligned fibrillar silicate single crystals. Polymer. 2007;48:2720–8.10.1016/j.polymer.2007.03.032Search in Google Scholar PubMed PubMed Central
[41] Xia Y, Zhang F, Xie H, Gu N. Nanoparticle-reinforced resin-based dental composites. J Dent. 2008;36:450–5.10.1016/j.jdent.2008.03.001Search in Google Scholar PubMed
[42] Chen L, Suh BI, Yang J. Antibacterial dental restorative materials: a review. Am J Dent. 2018;31:7.Search in Google Scholar
[43] Welch K, Cai Y, Engqvist H, Strømme M. Dental adhesives with bioactive and on-demand bactericidal properties. Dent Mater. 2010;26:491–9.10.1016/j.dental.2010.01.008Search in Google Scholar PubMed
[44] Ferrando-Magraner E, Bellot-Arcís C, Paredes-Gallardo V, Almerich-Silla JM, García-Sanz V, Fernández-Alonso M, et al. Antibacterial properties of nanoparticles in dental restorative materials. a systematic review and meta-analysis. Medicina. 2020;56:55. 10.3390/medicina56020055.Search in Google Scholar PubMed PubMed Central
[45] Kasraei S, Sami L, Hendi S, AliKhani M-Y, Rezaei-Soufi L, Khamverdi Z. Antibacterial properties of composite resins incorporating silver and zinc oxide nanoparticles on Streptococcus mutans and Lactobacillus. Restor Dent Endod. 2014;39:109–14.10.5395/rde.2014.39.2.109Search in Google Scholar PubMed PubMed Central
[46] Ai M, Du Z, Zhu S, Geng H, Zhang X, Cai Q, et al. Composite resin reinforced with silver nanoparticles–laden hydroxyapatite nanowires for dental application. Dent Mater. 2017;33:12–22.10.1016/j.dental.2016.09.038Search in Google Scholar PubMed
[47] Sodagar A, Akhavan A, Hashemi E, Arab S, Pourhajibagher M, Sodagar K, et al. Evaluation of the antibacterial activity of a conventional orthodontic composite containing silver/hydroxyapatite nanoparticles. Prog Orthod. 2016;17:1–7.10.1186/s40510-016-0153-xSearch in Google Scholar PubMed PubMed Central
[48] Ahn S-J, Lee S-J, Kook J-K, Lim B-S. Experimental antimicrobial orthodontic adhesives using nanofillers and silver nanoparticles. Dent Mater. 2009;25:206–13.10.1016/j.dental.2008.06.002Search in Google Scholar PubMed
[49] Li F, Weir MD, Chen J, Xu HH. Comparison of quaternary ammonium-containing with nano-silver-containing adhesive in antibacterial properties and cytotoxicity. Dent Mater. 2013;29:450–61.10.1016/j.dental.2013.01.012Search in Google Scholar PubMed PubMed Central
[50] Torabinejad M, Ford TRP. Root end filling materials: a review. Dent Traumatol. 1996;12:161–78. 10.1111/j.1600-9657.1996.tb00510.x.Search in Google Scholar PubMed
[51] Jasrotia A, Bhagat K, Sharma N. Comparative evaluation of sealing ability of bioceramic sealer, AH plus and epiphany sealer: an in vitro study. J Adv Med Dent Sci Res. 2021;9(4):121–4.Search in Google Scholar
[52] Koch K, Brave D. The increased use of bioceramics in endodontics. Dentaltown. 2009;10:39–43.Search in Google Scholar
[53] Zoufan K, Jiang J, Komabayashi T, Wang Y-H, Safavi KE, Zhu Q. Cytotoxicity evaluation of Gutta flow and endo sequence BC sealers. Oral Surg Oral Med Oral Pathol Oral Radiol Endodontol. 2011;112:657–61.10.1016/j.tripleo.2011.03.050Search in Google Scholar PubMed
[54] Abdo SB, Al Darrat A, Luddin N, Husien A. Sealing ability of gutta-percha/nano HA versus resilon/epiphany after 20 months using an electrochemical model – an in vitro study. Braz J Oral Sci. 2016;11(3):387–91.Search in Google Scholar
[55] Mortazavi V, Nahrkhalaji MM, Fathi M, Mousavi S, Esfahani BN. Antibacterial effects of sol‐gel‐derived bioactive glass nanoparticle on aerobic bacteria. J Biomed Mater Res Part A. 2010;94:160–8.10.1002/jbm.a.32678Search in Google Scholar PubMed
[56] Waltimo T, Brunner T, Vollenweider M, Stark WJ, Zehnder M. Antimicrobial effect of nanometric bioactive glass 45S5. J Dent Res. 2007;86:754–7.10.1177/154405910708600813Search in Google Scholar PubMed
[57] Haapasalo M, Parhar M, Huang X, Wei X, Lin J, Shen Y. Clinical use of bioceramic materials. Endod Top. 2015;32:97–117.10.1111/etp.12078Search in Google Scholar
[58] Jain P, Mirza MA, Iqbal Z. A 4-D approach for amelioration of periodontitis. Med Hypotheses. 2019;133:109392. 10.1016/j.mehy.2019.109392.Search in Google Scholar PubMed
[59] Jain P, Aamir Mirza M, Talegaonkar S, Nandy S, Dudeja M, Sharma N, et al. Design and in vitro/in vivo evaluations of a multiple-drug-containing gingiva disc for periodontotherapy. RSC Adv. 2020;10:8530–8. 10.1039/C9RA09569A.Search in Google Scholar PubMed PubMed Central
[60] Büchter A, Meyer U, Kruse-Lösler B, Joos U, Kleinheinz J. Sustained release of doxycycline for the treatment of peri-implantitis: randomised controlled trial. Br J Oral Maxillofac Surg. 2004;42:439–44.10.1016/j.bjoms.2004.06.005Search in Google Scholar PubMed
[61] Williams RC, Paquette DW, Offenbacher S, Adams DF, Armitage GC, Bray K, et al. Treatment of periodontitis by local administration of minocycline microspheres: a controlled trial. J Periodontol. 2001;72:1535–44.10.1902/jop.2001.72.11.1535Search in Google Scholar PubMed
[62] Dunlap T. Prescribing Hydrogen Peroxide in the Treatment of Periodontal Disease. Canada: Oral Health. 2016:64–8.Search in Google Scholar
[63] Carvalho SM, Moreira CD, Oliveira ACX, Oliveira AA, Lemos EM, Pereira MM. Bioactive glass nanoparticles for periodontal regeneration and applications in dentistry. Nanobiomaterials in clinical dentistry. Amsterdam, Netherlands: Elsevier; 2019. p. 351–83.10.1016/B978-0-12-815886-9.00015-2Search in Google Scholar
[64] Mei F, Zhong J, Yang X, Ouyang X, Zhang S, Hu X, et al. Improved biological characteristics of poly (L-lactic acid) electrospun membrane by incorporation of multiwalled carbon nanotubes/hydroxyapatite nanoparticles. Biomacromolecules. 2007;8:3729–35.10.1021/bm7006295Search in Google Scholar PubMed
[65] Thorwarth M, Schultze-Mosgau S, Kessler P, Wiltfang J, Schlegel KA. Bone regeneration in osseous defects using a resorbable nanoparticular hydroxyapatite. J Oral Maxillofac Surg. 2005;63:1626–33.10.1016/j.joms.2005.06.010Search in Google Scholar PubMed
[66] Schwarz F, Bieling K, Latz T, Nuesry E, Becker J. Healing of intrabony peri‐implantitis defects following application of a nanocrystalline hydroxyapatite (OstimTM) or a bovine‐derived xenograft (Bio‐OssTM) in combination with a collagen membrane (Bio‐GideTM). A case series. J Clin Periodontol. 2006;33:491–9.10.1111/j.1600-051X.2006.00936.xSearch in Google Scholar PubMed
[67] Sainz V, Conniot J, Matos AI, Peres C, Zupancic E, Moura L, et al. Regulatory aspects on nanomedicines. Biochem Biophys Res Commun. 2015;468:504–10. 10.1016/j.bbrc.2015.08.023.Search in Google Scholar PubMed
[68] North JJ. Full list of Clinical Research regulatory authorities across the world. IAOCR; n.d. https://iaocr.com/clinical-research-regulations/regulatory-authority-links/ (accessed Jan 25, 2021).Search in Google Scholar
[69] Halamoda-Kenzaoui B, Box H, van Elk M, Gaitan S, Geertsma RE, Lafuente EG, et al. Anticipation of regulatory needs for nanotechnology – enabled health products. LUXEMBOURG: Publications Office of the European Union; 2019.10.33218/001c.13521Search in Google Scholar
[70] WHO. Medical device – full definition. WHO; n.d. http://www.who.int/medical_devices/full_deffinition/en/ (accessed Jan 25, 2021).Search in Google Scholar
[71] Aguilar Z. Nanomaterials for medical applications. Oxford and Boston: Newnes; 2012.Search in Google Scholar
[72] Foulkes R, Man E, Thind J, Yeung S, Joy A, Hoskins C. The regulation of nanomaterials and nanomedicines for clinical application: current and future perspectives. Biomater Sci. 2020;8:4653–64.10.1039/D0BM00558DSearch in Google Scholar
[73] Miernicki M, Hofmann T, Eisenberger I, von der Kammer F, Praetorius A. Legal and practical challenges in classifying nanomaterials according to regulatory definitions. Nat Nanotechnol. 2019;14:208–16.10.1038/s41565-019-0396-zSearch in Google Scholar PubMed
[74] Rauscher H, Rasmussen K, Sokull-Klüttgen B. Regulatory aspects of nanomaterials in the EU. Chem Ing Technik. 2017;89:224–31.10.1002/cite.201600076Search in Google Scholar
[75] Limaye V, Fortwengel G, Limaye D. Regulatory roadmap for nanotechnology based medicines. Int J Drug Reg Aff. 2018;2:33–41. 10.22270/ijdra.v2i4.151.Search in Google Scholar
[76] Jones A-AD III, Mi G, Webster TJ. A status report on FDA approval of medical devices containing nanostructured materials. Trends Biotechnol. 2019;37:117–20.10.1016/j.tibtech.2018.06.003Search in Google Scholar PubMed
[77] Moore J. Global regulation of nanotechnologies and their products in medicine. In: Beran RG, editor. Legal and forensic medicine. Berlin, Heidelberg: Springer; 2013. p. 1755–81. 10.1007/978-3-642-32338-6_119.Search in Google Scholar
[78] 11.100.20 Biological evaluation of medical devices. 2018;5:41.Search in Google Scholar
[79] Use of International Standard ISO 10993-1. “Biological evaluation of medical devices – Part 1: evaluation and testing within a risk management process” – Guidance for industry and food and drug administration staff; 2020. p. 68.Search in Google Scholar
[80] Murray PE, Godoy CG, Godoy FG. How is the biocompatibilty of dental biomaterials evaluated? Med Oral Patol Oral Cir Bucal. 2007;12(3):258–66.Search in Google Scholar
[81] Pilownic KJ, Gomes APN, Wang ZJ, Almeida LHS, Romano AR, Shen Y, et al. Physicochemical and biological evaluation of endodontic filling materials for primary teeth. Braz Dent J. 2017;28:578–86. 10.1590/0103-6440201701573.Search in Google Scholar PubMed
[82] Dental products: standards, technical specifications and technical reports; n.d. https://www.ada.org/en/science-research/dental-standards/dental-products/products-standards-technical-specifications-and-technical-reports (accessed Jan 25, 2021).Search in Google Scholar
[83] Wennberg A, Mjör IA, Hensten‐Pettersen A. Biological evaluation of dental restorative materials – a comparison of different test methods. J Biomed Mater Res. 1983;17:23–36. 10.1002/jbm.820170103.Search in Google Scholar PubMed
© 2022 Pooja Jain et al., published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Research Articles
- Theoretical and experimental investigation of MWCNT dispersion effect on the elastic modulus of flexible PDMS/MWCNT nanocomposites
- Mechanical, morphological, and fracture-deformation behavior of MWCNTs-reinforced (Al–Cu–Mg–T351) alloy cast nanocomposites fabricated by optimized mechanical milling and powder metallurgy techniques
- Flammability and physical stability of sugar palm crystalline nanocellulose reinforced thermoplastic sugar palm starch/poly(lactic acid) blend bionanocomposites
- Glutathione-loaded non-ionic surfactant niosomes: A new approach to improve oral bioavailability and hepatoprotective efficacy of glutathione
- Relationship between mechano-bactericidal activity and nanoblades density on chemically strengthened glass
- In situ regulation of microstructure and microwave-absorbing properties of FeSiAl through HNO3 oxidation
- Research on a mechanical model of magnetorheological fluid different diameter particles
- Nanomechanical and dynamic mechanical properties of rubber–wood–plastic composites
- Investigative properties of CeO2 doped with niobium: A combined characterization and DFT studies
- Miniaturized peptidomimetics and nano-vesiculation in endothelin types through probable nano-disk formation and structure property relationships of endothelins’ fragments
- N/S co-doped CoSe/C nanocubes as anode materials for Li-ion batteries
- Synergistic effects of halloysite nanotubes with metal and phosphorus additives on the optimal design of eco-friendly sandwich panels with maximum flame resistance and minimum weight
- Octreotide-conjugated silver nanoparticles for active targeting of somatostatin receptors and their application in a nebulized rat model
- Controllable morphology of Bi2S3 nanostructures formed via hydrothermal vulcanization of Bi2O3 thin-film layer and their photoelectrocatalytic performances
- Development of (−)-epigallocatechin-3-gallate-loaded folate receptor-targeted nanoparticles for prostate cancer treatment
- Enhancement of the mechanical properties of HDPE mineral nanocomposites by filler particles modulation of the matrix plastic/elastic behavior
- Effect of plasticizers on the properties of sugar palm nanocellulose/cinnamon essential oil reinforced starch bionanocomposite films
- Optimization of nano coating to reduce the thermal deformation of ball screws
- Preparation of efficient piezoelectric PVDF–HFP/Ni composite films by high electric field poling
- MHD dissipative Casson nanofluid liquid film flow due to an unsteady stretching sheet with radiation influence and slip velocity phenomenon
- Effects of nano-SiO2 modification on rubberised mortar and concrete with recycled coarse aggregates
- Mechanical and microscopic properties of fiber-reinforced coal gangue-based geopolymer concrete
- Effect of morphology and size on the thermodynamic stability of cerium oxide nanoparticles: Experiment and molecular dynamics calculation
- Mechanical performance of a CFRP composite reinforced via gelatin-CNTs: A study on fiber interfacial enhancement and matrix enhancement
- A practical review over surface modification, nanopatterns, emerging materials, drug delivery systems, and their biophysiochemical properties for dental implants: Recent progresses and advances
- HTR: An ultra-high speed algorithm for cage recognition of clathrate hydrates
- Effects of microalloying elements added by in situ synthesis on the microstructure of WCu composites
- A highly sensitive nanobiosensor based on aptamer-conjugated graphene-decorated rhodium nanoparticles for detection of HER2-positive circulating tumor cells
- Progressive collapse performance of shear strengthened RC frames by nano CFRP
- Core–shell heterostructured composites of carbon nanotubes and imine-linked hyperbranched polymers as metal-free Li-ion anodes
- A Galerkin strategy for tri-hybridized mixture in ethylene glycol comprising variable diffusion and thermal conductivity using non-Fourier’s theory
- Simple models for tensile modulus of shape memory polymer nanocomposites at ambient temperature
- Preparation and morphological studies of tin sulfide nanoparticles and use as efficient photocatalysts for the degradation of rhodamine B and phenol
- Polyethyleneimine-impregnated activated carbon nanofiber composited graphene-derived rice husk char for efficient post-combustion CO2 capture
- Electrospun nanofibers of Co3O4 nanocrystals encapsulated in cyclized-polyacrylonitrile for lithium storage
- Pitting corrosion induced on high-strength high carbon steel wire in high alkaline deaerated chloride electrolyte
- Formulation of polymeric nanoparticles loaded sorafenib; evaluation of cytotoxicity, molecular evaluation, and gene expression studies in lung and breast cancer cell lines
- Engineered nanocomposites in asphalt binders
- Influence of loading voltage, domain ratio, and additional load on the actuation of dielectric elastomer
- Thermally induced hex-graphene transitions in 2D carbon crystals
- The surface modification effect on the interfacial properties of glass fiber-reinforced epoxy: A molecular dynamics study
- Molecular dynamics study of deformation mechanism of interfacial microzone of Cu/Al2Cu/Al composites under tension
- Nanocolloid simulators of luminescent solar concentrator photovoltaic windows
- Compressive strength and anti-chloride ion penetration assessment of geopolymer mortar merging PVA fiber and nano-SiO2 using RBF–BP composite neural network
- Effect of 3-mercapto-1-propane sulfonate sulfonic acid and polyvinylpyrrolidone on the growth of cobalt pillar by electrodeposition
- Dynamics of convective slippery constraints on hybrid radiative Sutterby nanofluid flow by Galerkin finite element simulation
- Preparation of vanadium by the magnesiothermic self-propagating reduction and process control
- Microstructure-dependent photoelectrocatalytic activity of heterogeneous ZnO–ZnS nanosheets
- Cytotoxic and pro-inflammatory effects of molybdenum and tungsten disulphide on human bronchial cells
- Improving recycled aggregate concrete by compression casting and nano-silica
- Chemically reactive Maxwell nanoliquid flow by a stretching surface in the frames of Newtonian heating, nonlinear convection and radiative flux: Nanopolymer flow processing simulation
- Nonlinear dynamic and crack behaviors of carbon nanotubes-reinforced composites with various geometries
- Biosynthesis of copper oxide nanoparticles and its therapeutic efficacy against colon cancer
- Synthesis and characterization of smart stimuli-responsive herbal drug-encapsulated nanoniosome particles for efficient treatment of breast cancer
- Homotopic simulation for heat transport phenomenon of the Burgers nanofluids flow over a stretching cylinder with thermal convective and zero mass flux conditions
- Incorporation of copper and strontium ions in TiO2 nanotubes via dopamine to enhance hemocompatibility and cytocompatibility
- Mechanical, thermal, and barrier properties of starch films incorporated with chitosan nanoparticles
- Mechanical properties and microstructure of nano-strengthened recycled aggregate concrete
- Glucose-responsive nanogels efficiently maintain the stability and activity of therapeutic enzymes
- Tunning matrix rheology and mechanical performance of ultra-high performance concrete using cellulose nanofibers
- Flexible MXene/copper/cellulose nanofiber heat spreader films with enhanced thermal conductivity
- Promoted charge separation and specific surface area via interlacing of N-doped titanium dioxide nanotubes on carbon nitride nanosheets for photocatalytic degradation of Rhodamine B
- Elucidating the role of silicon dioxide and titanium dioxide nanoparticles in mitigating the disease of the eggplant caused by Phomopsis vexans, Ralstonia solanacearum, and root-knot nematode Meloidogyne incognita
- An implication of magnetic dipole in Carreau Yasuda liquid influenced by engine oil using ternary hybrid nanomaterial
- Robust synthesis of a composite phase of copper vanadium oxide with enhanced performance for durable aqueous Zn-ion batteries
- Tunning self-assembled phases of bovine serum albumin via hydrothermal process to synthesize novel functional hydrogel for skin protection against UVB
- A comparative experimental study on damping properties of epoxy nanocomposite beams reinforced with carbon nanotubes and graphene nanoplatelets
- Lightweight and hydrophobic Ni/GO/PVA composite aerogels for ultrahigh performance electromagnetic interference shielding
- Research on the auxetic behavior and mechanical properties of periodically rotating graphene nanostructures
- Repairing performances of novel cement mortar modified with graphene oxide and polyacrylate polymer
- Closed-loop recycling and fabrication of hydrophilic CNT films with high performance
- Design of thin-film configuration of SnO2–Ag2O composites for NO2 gas-sensing applications
- Study on stress distribution of SiC/Al composites based on microstructure models with microns and nanoparticles
- PVDF green nanofibers as potential carriers for improving self-healing and mechanical properties of carbon fiber/epoxy prepregs
- Osteogenesis capability of three-dimensionally printed poly(lactic acid)-halloysite nanotube scaffolds containing strontium ranelate
- Silver nanoparticles induce mitochondria-dependent apoptosis and late non-canonical autophagy in HT-29 colon cancer cells
- Preparation and bonding mechanisms of polymer/metal hybrid composite by nano molding technology
- Damage self-sensing and strain monitoring of glass-reinforced epoxy composite impregnated with graphene nanoplatelet and multiwalled carbon nanotubes
- Thermal analysis characterisation of solar-powered ship using Oldroyd hybrid nanofluids in parabolic trough solar collector: An optimal thermal application
- Pyrene-functionalized halloysite nanotubes for simultaneously detecting and separating Hg(ii) in aqueous media: A comprehensive comparison on interparticle and intraparticle excimers
- Fabrication of self-assembly CNT flexible film and its piezoresistive sensing behaviors
- Thermal valuation and entropy inspection of second-grade nanoscale fluid flow over a stretching surface by applying Koo–Kleinstreuer–Li relation
- Mechanical properties and microstructure of nano-SiO2 and basalt-fiber-reinforced recycled aggregate concrete
- Characterization and tribology performance of polyaniline-coated nanodiamond lubricant additives
- Combined impact of Marangoni convection and thermophoretic particle deposition on chemically reactive transport of nanofluid flow over a stretching surface
- Spark plasma extrusion of binder free hydroxyapatite powder
- An investigation on thermo-mechanical performance of graphene-oxide-reinforced shape memory polymer
- Effect of nanoadditives on the novel leather fiber/recycled poly(ethylene-vinyl-acetate) polymer composites for multifunctional applications: Fabrication, characterizations, and multiobjective optimization using central composite design
- Design selection for a hemispherical dimple core sandwich panel using hybrid multi-criteria decision-making methods
- Improving tensile strength and impact toughness of plasticized poly(lactic acid) biocomposites by incorporating nanofibrillated cellulose
- Green synthesis of spinel copper ferrite (CuFe2O4) nanoparticles and their toxicity
- The effect of TaC and NbC hybrid and mono-nanoparticles on AA2024 nanocomposites: Microstructure, strengthening, and artificial aging
- Excited-state geometry relaxation of pyrene-modified cellulose nanocrystals under UV-light excitation for detecting Fe3+
- Effect of CNTs and MEA on the creep of face-slab concrete at an early age
- Effect of deformation conditions on compression phase transformation of AZ31
- Application of MXene as a new generation of highly conductive coating materials for electromembrane-surrounded solid-phase microextraction
- A comparative study of the elasto-plastic properties for ceramic nanocomposites filled by graphene or graphene oxide nanoplates
- Encapsulation strategies for improving the biological behavior of CdS@ZIF-8 nanocomposites
- Biosynthesis of ZnO NPs from pumpkin seeds’ extract and elucidation of its anticancer potential against breast cancer
- Preliminary trials of the gold nanoparticles conjugated chrysin: An assessment of anti-oxidant, anti-microbial, and in vitro cytotoxic activities of a nanoformulated flavonoid
- Effect of micron-scale pores increased by nano-SiO2 sol modification on the strength of cement mortar
- Fractional simulations for thermal flow of hybrid nanofluid with aluminum oxide and titanium oxide nanoparticles with water and blood base fluids
- The effect of graphene nano-powder on the viscosity of water: An experimental study and artificial neural network modeling
- Development of a novel heat- and shear-resistant nano-silica gelling agent
- Characterization, biocompatibility and in vivo of nominal MnO2-containing wollastonite glass-ceramic
- Entropy production simulation of second-grade magnetic nanomaterials flowing across an expanding surface with viscidness dissipative flux
- Enhancement in structural, morphological, and optical properties of copper oxide for optoelectronic device applications
- Aptamer-functionalized chitosan-coated gold nanoparticle complex as a suitable targeted drug carrier for improved breast cancer treatment
- Performance and overall evaluation of nano-alumina-modified asphalt mixture
- Analysis of pure nanofluid (GO/engine oil) and hybrid nanofluid (GO–Fe3O4/engine oil): Novel thermal and magnetic features
- Synthesis of Ag@AgCl modified anatase/rutile/brookite mixed phase TiO2 and their photocatalytic property
- Mechanisms and influential variables on the abrasion resistance hydraulic concrete
- Synergistic reinforcement mechanism of basalt fiber/cellulose nanocrystals/polypropylene composites
- Achieving excellent oxidation resistance and mechanical properties of TiB2–B4C/carbon aerogel composites by quick-gelation and mechanical mixing
- Microwave-assisted sol–gel template-free synthesis and characterization of silica nanoparticles obtained from South African coal fly ash
- Pulsed laser-assisted synthesis of nano nickel(ii) oxide-anchored graphitic carbon nitride: Characterizations and their potential antibacterial/anti-biofilm applications
- Effects of nano-ZrSi2 on thermal stability of phenolic resin and thermal reusability of quartz–phenolic composites
- Benzaldehyde derivatives on tin electroplating as corrosion resistance for fabricating copper circuit
- Mechanical and heat transfer properties of 4D-printed shape memory graphene oxide/epoxy acrylate composites
- Coupling the vanadium-induced amorphous/crystalline NiFe2O4 with phosphide heterojunction toward active oxygen evolution reaction catalysts
- Graphene-oxide-reinforced cement composites mechanical and microstructural characteristics at elevated temperatures
- Gray correlation analysis of factors influencing compressive strength and durability of nano-SiO2 and PVA fiber reinforced geopolymer mortar
- Preparation of layered gradient Cu–Cr–Ti alloy with excellent mechanical properties, thermal stability, and electrical conductivity
- Recovery of Cr from chrome-containing leather wastes to develop aluminum-based composite material along with Al2O3 ceramic particles: An ingenious approach
- Mechanisms of the improved stiffness of flexible polymers under impact loading
- Anticancer potential of gold nanoparticles (AuNPs) using a battery of in vitro tests
- Review Articles
- Proposed approaches for coronaviruses elimination from wastewater: Membrane techniques and nanotechnology solutions
- Application of Pickering emulsion in oil drilling and production
- The contribution of microfluidics to the fight against tuberculosis
- Graphene-based biosensors for disease theranostics: Development, applications, and recent advancements
- Synthesis and encapsulation of iron oxide nanorods for application in magnetic hyperthermia and photothermal therapy
- Contemporary nano-architectured drugs and leads for ανβ3 integrin-based chemotherapy: Rationale and retrospect
- State-of-the-art review of fabrication, application, and mechanical properties of functionally graded porous nanocomposite materials
- Insights on magnetic spinel ferrites for targeted drug delivery and hyperthermia applications
- A review on heterogeneous oxidation of acetaminophen based on micro and nanoparticles catalyzed by different activators
- Early diagnosis of lung cancer using magnetic nanoparticles-integrated systems
- Advances in ZnO: Manipulation of defects for enhancing their technological potentials
- Efficacious nanomedicine track toward combating COVID-19
- A review of the design, processes, and properties of Mg-based composites
- Green synthesis of nanoparticles for varied applications: Green renewable resources and energy-efficient synthetic routes
- Two-dimensional nanomaterial-based polymer composites: Fundamentals and applications
- Recent progress and challenges in plasmonic nanomaterials
- Apoptotic cell-derived micro/nanosized extracellular vesicles in tissue regeneration
- Electronic noses based on metal oxide nanowires: A review
- Framework materials for supercapacitors
- An overview on the reproductive toxicity of graphene derivatives: Highlighting the importance
- Antibacterial nanomaterials: Upcoming hope to overcome antibiotic resistance crisis
- Research progress of carbon materials in the field of three-dimensional printing polymer nanocomposites
- A review of atomic layer deposition modelling and simulation methodologies: Density functional theory and molecular dynamics
- Recent advances in the preparation of PVDF-based piezoelectric materials
- Recent developments in tensile properties of friction welding of carbon fiber-reinforced composite: A review
- Comprehensive review of the properties of fly ash-based geopolymer with additive of nano-SiO2
- Perspectives in biopolymer/graphene-based composite application: Advances, challenges, and recommendations
- Graphene-based nanocomposite using new modeling molecular dynamic simulations for proposed neutralizing mechanism and real-time sensing of COVID-19
- Nanotechnology application on bamboo materials: A review
- Recent developments and future perspectives of biorenewable nanocomposites for advanced applications
- Nanostructured lipid carrier system: A compendium of their formulation development approaches, optimization strategies by quality by design, and recent applications in drug delivery
- 3D printing customized design of human bone tissue implant and its application
- Design, preparation, and functionalization of nanobiomaterials for enhanced efficacy in current and future biomedical applications
- A brief review of nanoparticles-doped PEDOT:PSS nanocomposite for OLED and OPV
- Nanotechnology interventions as a putative tool for the treatment of dental afflictions
- Recent advancements in metal–organic frameworks integrating quantum dots (QDs@MOF) and their potential applications
- A focused review of short electrospun nanofiber preparation techniques for composite reinforcement
- Microstructural characteristics and nano-modification of interfacial transition zone in concrete: A review
- Latest developments in the upconversion nanotechnology for the rapid detection of food safety: A review
- Strategic applications of nano-fertilizers for sustainable agriculture: Benefits and bottlenecks
- Molecular dynamics application of cocrystal energetic materials: A review
- Synthesis and application of nanometer hydroxyapatite in biomedicine
- Cutting-edge development in waste-recycled nanomaterials for energy storage and conversion applications
- Biological applications of ternary quantum dots: A review
- Nanotherapeutics for hydrogen sulfide-involved treatment: An emerging approach for cancer therapy
- Application of antibacterial nanoparticles in orthodontic materials
- Effect of natural-based biological hydrogels combined with growth factors on skin wound healing
- Nanozymes – A route to overcome microbial resistance: A viewpoint
- Recent developments and applications of smart nanoparticles in biomedicine
- Contemporary review on carbon nanotube (CNT) composites and their impact on multifarious applications
- Interfacial interactions and reinforcing mechanisms of cellulose and chitin nanomaterials and starch derivatives for cement and concrete strength and durability enhancement: A review
- Diamond-like carbon films for tribological modification of rubber
- Layered double hydroxides (LDHs) modified cement-based materials: A systematic review
- Recent research progress and advanced applications of silica/polymer nanocomposites
- Modeling of supramolecular biopolymers: Leading the in silico revolution of tissue engineering and nanomedicine
- Recent advances in perovskites-based optoelectronics
- Biogenic synthesis of palladium nanoparticles: New production methods and applications
- A comprehensive review of nanofluids with fractional derivatives: Modeling and application
- Electrospinning of marine polysaccharides: Processing and chemical aspects, challenges, and future prospects
- Electrohydrodynamic printing for demanding devices: A review of processing and applications
- Rapid Communications
- Structural material with designed thermal twist for a simple actuation
- Recent advances in photothermal materials for solar-driven crude oil adsorption
Articles in the same Issue
- Research Articles
- Theoretical and experimental investigation of MWCNT dispersion effect on the elastic modulus of flexible PDMS/MWCNT nanocomposites
- Mechanical, morphological, and fracture-deformation behavior of MWCNTs-reinforced (Al–Cu–Mg–T351) alloy cast nanocomposites fabricated by optimized mechanical milling and powder metallurgy techniques
- Flammability and physical stability of sugar palm crystalline nanocellulose reinforced thermoplastic sugar palm starch/poly(lactic acid) blend bionanocomposites
- Glutathione-loaded non-ionic surfactant niosomes: A new approach to improve oral bioavailability and hepatoprotective efficacy of glutathione
- Relationship between mechano-bactericidal activity and nanoblades density on chemically strengthened glass
- In situ regulation of microstructure and microwave-absorbing properties of FeSiAl through HNO3 oxidation
- Research on a mechanical model of magnetorheological fluid different diameter particles
- Nanomechanical and dynamic mechanical properties of rubber–wood–plastic composites
- Investigative properties of CeO2 doped with niobium: A combined characterization and DFT studies
- Miniaturized peptidomimetics and nano-vesiculation in endothelin types through probable nano-disk formation and structure property relationships of endothelins’ fragments
- N/S co-doped CoSe/C nanocubes as anode materials for Li-ion batteries
- Synergistic effects of halloysite nanotubes with metal and phosphorus additives on the optimal design of eco-friendly sandwich panels with maximum flame resistance and minimum weight
- Octreotide-conjugated silver nanoparticles for active targeting of somatostatin receptors and their application in a nebulized rat model
- Controllable morphology of Bi2S3 nanostructures formed via hydrothermal vulcanization of Bi2O3 thin-film layer and their photoelectrocatalytic performances
- Development of (−)-epigallocatechin-3-gallate-loaded folate receptor-targeted nanoparticles for prostate cancer treatment
- Enhancement of the mechanical properties of HDPE mineral nanocomposites by filler particles modulation of the matrix plastic/elastic behavior
- Effect of plasticizers on the properties of sugar palm nanocellulose/cinnamon essential oil reinforced starch bionanocomposite films
- Optimization of nano coating to reduce the thermal deformation of ball screws
- Preparation of efficient piezoelectric PVDF–HFP/Ni composite films by high electric field poling
- MHD dissipative Casson nanofluid liquid film flow due to an unsteady stretching sheet with radiation influence and slip velocity phenomenon
- Effects of nano-SiO2 modification on rubberised mortar and concrete with recycled coarse aggregates
- Mechanical and microscopic properties of fiber-reinforced coal gangue-based geopolymer concrete
- Effect of morphology and size on the thermodynamic stability of cerium oxide nanoparticles: Experiment and molecular dynamics calculation
- Mechanical performance of a CFRP composite reinforced via gelatin-CNTs: A study on fiber interfacial enhancement and matrix enhancement
- A practical review over surface modification, nanopatterns, emerging materials, drug delivery systems, and their biophysiochemical properties for dental implants: Recent progresses and advances
- HTR: An ultra-high speed algorithm for cage recognition of clathrate hydrates
- Effects of microalloying elements added by in situ synthesis on the microstructure of WCu composites
- A highly sensitive nanobiosensor based on aptamer-conjugated graphene-decorated rhodium nanoparticles for detection of HER2-positive circulating tumor cells
- Progressive collapse performance of shear strengthened RC frames by nano CFRP
- Core–shell heterostructured composites of carbon nanotubes and imine-linked hyperbranched polymers as metal-free Li-ion anodes
- A Galerkin strategy for tri-hybridized mixture in ethylene glycol comprising variable diffusion and thermal conductivity using non-Fourier’s theory
- Simple models for tensile modulus of shape memory polymer nanocomposites at ambient temperature
- Preparation and morphological studies of tin sulfide nanoparticles and use as efficient photocatalysts for the degradation of rhodamine B and phenol
- Polyethyleneimine-impregnated activated carbon nanofiber composited graphene-derived rice husk char for efficient post-combustion CO2 capture
- Electrospun nanofibers of Co3O4 nanocrystals encapsulated in cyclized-polyacrylonitrile for lithium storage
- Pitting corrosion induced on high-strength high carbon steel wire in high alkaline deaerated chloride electrolyte
- Formulation of polymeric nanoparticles loaded sorafenib; evaluation of cytotoxicity, molecular evaluation, and gene expression studies in lung and breast cancer cell lines
- Engineered nanocomposites in asphalt binders
- Influence of loading voltage, domain ratio, and additional load on the actuation of dielectric elastomer
- Thermally induced hex-graphene transitions in 2D carbon crystals
- The surface modification effect on the interfacial properties of glass fiber-reinforced epoxy: A molecular dynamics study
- Molecular dynamics study of deformation mechanism of interfacial microzone of Cu/Al2Cu/Al composites under tension
- Nanocolloid simulators of luminescent solar concentrator photovoltaic windows
- Compressive strength and anti-chloride ion penetration assessment of geopolymer mortar merging PVA fiber and nano-SiO2 using RBF–BP composite neural network
- Effect of 3-mercapto-1-propane sulfonate sulfonic acid and polyvinylpyrrolidone on the growth of cobalt pillar by electrodeposition
- Dynamics of convective slippery constraints on hybrid radiative Sutterby nanofluid flow by Galerkin finite element simulation
- Preparation of vanadium by the magnesiothermic self-propagating reduction and process control
- Microstructure-dependent photoelectrocatalytic activity of heterogeneous ZnO–ZnS nanosheets
- Cytotoxic and pro-inflammatory effects of molybdenum and tungsten disulphide on human bronchial cells
- Improving recycled aggregate concrete by compression casting and nano-silica
- Chemically reactive Maxwell nanoliquid flow by a stretching surface in the frames of Newtonian heating, nonlinear convection and radiative flux: Nanopolymer flow processing simulation
- Nonlinear dynamic and crack behaviors of carbon nanotubes-reinforced composites with various geometries
- Biosynthesis of copper oxide nanoparticles and its therapeutic efficacy against colon cancer
- Synthesis and characterization of smart stimuli-responsive herbal drug-encapsulated nanoniosome particles for efficient treatment of breast cancer
- Homotopic simulation for heat transport phenomenon of the Burgers nanofluids flow over a stretching cylinder with thermal convective and zero mass flux conditions
- Incorporation of copper and strontium ions in TiO2 nanotubes via dopamine to enhance hemocompatibility and cytocompatibility
- Mechanical, thermal, and barrier properties of starch films incorporated with chitosan nanoparticles
- Mechanical properties and microstructure of nano-strengthened recycled aggregate concrete
- Glucose-responsive nanogels efficiently maintain the stability and activity of therapeutic enzymes
- Tunning matrix rheology and mechanical performance of ultra-high performance concrete using cellulose nanofibers
- Flexible MXene/copper/cellulose nanofiber heat spreader films with enhanced thermal conductivity
- Promoted charge separation and specific surface area via interlacing of N-doped titanium dioxide nanotubes on carbon nitride nanosheets for photocatalytic degradation of Rhodamine B
- Elucidating the role of silicon dioxide and titanium dioxide nanoparticles in mitigating the disease of the eggplant caused by Phomopsis vexans, Ralstonia solanacearum, and root-knot nematode Meloidogyne incognita
- An implication of magnetic dipole in Carreau Yasuda liquid influenced by engine oil using ternary hybrid nanomaterial
- Robust synthesis of a composite phase of copper vanadium oxide with enhanced performance for durable aqueous Zn-ion batteries
- Tunning self-assembled phases of bovine serum albumin via hydrothermal process to synthesize novel functional hydrogel for skin protection against UVB
- A comparative experimental study on damping properties of epoxy nanocomposite beams reinforced with carbon nanotubes and graphene nanoplatelets
- Lightweight and hydrophobic Ni/GO/PVA composite aerogels for ultrahigh performance electromagnetic interference shielding
- Research on the auxetic behavior and mechanical properties of periodically rotating graphene nanostructures
- Repairing performances of novel cement mortar modified with graphene oxide and polyacrylate polymer
- Closed-loop recycling and fabrication of hydrophilic CNT films with high performance
- Design of thin-film configuration of SnO2–Ag2O composites for NO2 gas-sensing applications
- Study on stress distribution of SiC/Al composites based on microstructure models with microns and nanoparticles
- PVDF green nanofibers as potential carriers for improving self-healing and mechanical properties of carbon fiber/epoxy prepregs
- Osteogenesis capability of three-dimensionally printed poly(lactic acid)-halloysite nanotube scaffolds containing strontium ranelate
- Silver nanoparticles induce mitochondria-dependent apoptosis and late non-canonical autophagy in HT-29 colon cancer cells
- Preparation and bonding mechanisms of polymer/metal hybrid composite by nano molding technology
- Damage self-sensing and strain monitoring of glass-reinforced epoxy composite impregnated with graphene nanoplatelet and multiwalled carbon nanotubes
- Thermal analysis characterisation of solar-powered ship using Oldroyd hybrid nanofluids in parabolic trough solar collector: An optimal thermal application
- Pyrene-functionalized halloysite nanotubes for simultaneously detecting and separating Hg(ii) in aqueous media: A comprehensive comparison on interparticle and intraparticle excimers
- Fabrication of self-assembly CNT flexible film and its piezoresistive sensing behaviors
- Thermal valuation and entropy inspection of second-grade nanoscale fluid flow over a stretching surface by applying Koo–Kleinstreuer–Li relation
- Mechanical properties and microstructure of nano-SiO2 and basalt-fiber-reinforced recycled aggregate concrete
- Characterization and tribology performance of polyaniline-coated nanodiamond lubricant additives
- Combined impact of Marangoni convection and thermophoretic particle deposition on chemically reactive transport of nanofluid flow over a stretching surface
- Spark plasma extrusion of binder free hydroxyapatite powder
- An investigation on thermo-mechanical performance of graphene-oxide-reinforced shape memory polymer
- Effect of nanoadditives on the novel leather fiber/recycled poly(ethylene-vinyl-acetate) polymer composites for multifunctional applications: Fabrication, characterizations, and multiobjective optimization using central composite design
- Design selection for a hemispherical dimple core sandwich panel using hybrid multi-criteria decision-making methods
- Improving tensile strength and impact toughness of plasticized poly(lactic acid) biocomposites by incorporating nanofibrillated cellulose
- Green synthesis of spinel copper ferrite (CuFe2O4) nanoparticles and their toxicity
- The effect of TaC and NbC hybrid and mono-nanoparticles on AA2024 nanocomposites: Microstructure, strengthening, and artificial aging
- Excited-state geometry relaxation of pyrene-modified cellulose nanocrystals under UV-light excitation for detecting Fe3+
- Effect of CNTs and MEA on the creep of face-slab concrete at an early age
- Effect of deformation conditions on compression phase transformation of AZ31
- Application of MXene as a new generation of highly conductive coating materials for electromembrane-surrounded solid-phase microextraction
- A comparative study of the elasto-plastic properties for ceramic nanocomposites filled by graphene or graphene oxide nanoplates
- Encapsulation strategies for improving the biological behavior of CdS@ZIF-8 nanocomposites
- Biosynthesis of ZnO NPs from pumpkin seeds’ extract and elucidation of its anticancer potential against breast cancer
- Preliminary trials of the gold nanoparticles conjugated chrysin: An assessment of anti-oxidant, anti-microbial, and in vitro cytotoxic activities of a nanoformulated flavonoid
- Effect of micron-scale pores increased by nano-SiO2 sol modification on the strength of cement mortar
- Fractional simulations for thermal flow of hybrid nanofluid with aluminum oxide and titanium oxide nanoparticles with water and blood base fluids
- The effect of graphene nano-powder on the viscosity of water: An experimental study and artificial neural network modeling
- Development of a novel heat- and shear-resistant nano-silica gelling agent
- Characterization, biocompatibility and in vivo of nominal MnO2-containing wollastonite glass-ceramic
- Entropy production simulation of second-grade magnetic nanomaterials flowing across an expanding surface with viscidness dissipative flux
- Enhancement in structural, morphological, and optical properties of copper oxide for optoelectronic device applications
- Aptamer-functionalized chitosan-coated gold nanoparticle complex as a suitable targeted drug carrier for improved breast cancer treatment
- Performance and overall evaluation of nano-alumina-modified asphalt mixture
- Analysis of pure nanofluid (GO/engine oil) and hybrid nanofluid (GO–Fe3O4/engine oil): Novel thermal and magnetic features
- Synthesis of Ag@AgCl modified anatase/rutile/brookite mixed phase TiO2 and their photocatalytic property
- Mechanisms and influential variables on the abrasion resistance hydraulic concrete
- Synergistic reinforcement mechanism of basalt fiber/cellulose nanocrystals/polypropylene composites
- Achieving excellent oxidation resistance and mechanical properties of TiB2–B4C/carbon aerogel composites by quick-gelation and mechanical mixing
- Microwave-assisted sol–gel template-free synthesis and characterization of silica nanoparticles obtained from South African coal fly ash
- Pulsed laser-assisted synthesis of nano nickel(ii) oxide-anchored graphitic carbon nitride: Characterizations and their potential antibacterial/anti-biofilm applications
- Effects of nano-ZrSi2 on thermal stability of phenolic resin and thermal reusability of quartz–phenolic composites
- Benzaldehyde derivatives on tin electroplating as corrosion resistance for fabricating copper circuit
- Mechanical and heat transfer properties of 4D-printed shape memory graphene oxide/epoxy acrylate composites
- Coupling the vanadium-induced amorphous/crystalline NiFe2O4 with phosphide heterojunction toward active oxygen evolution reaction catalysts
- Graphene-oxide-reinforced cement composites mechanical and microstructural characteristics at elevated temperatures
- Gray correlation analysis of factors influencing compressive strength and durability of nano-SiO2 and PVA fiber reinforced geopolymer mortar
- Preparation of layered gradient Cu–Cr–Ti alloy with excellent mechanical properties, thermal stability, and electrical conductivity
- Recovery of Cr from chrome-containing leather wastes to develop aluminum-based composite material along with Al2O3 ceramic particles: An ingenious approach
- Mechanisms of the improved stiffness of flexible polymers under impact loading
- Anticancer potential of gold nanoparticles (AuNPs) using a battery of in vitro tests
- Review Articles
- Proposed approaches for coronaviruses elimination from wastewater: Membrane techniques and nanotechnology solutions
- Application of Pickering emulsion in oil drilling and production
- The contribution of microfluidics to the fight against tuberculosis
- Graphene-based biosensors for disease theranostics: Development, applications, and recent advancements
- Synthesis and encapsulation of iron oxide nanorods for application in magnetic hyperthermia and photothermal therapy
- Contemporary nano-architectured drugs and leads for ανβ3 integrin-based chemotherapy: Rationale and retrospect
- State-of-the-art review of fabrication, application, and mechanical properties of functionally graded porous nanocomposite materials
- Insights on magnetic spinel ferrites for targeted drug delivery and hyperthermia applications
- A review on heterogeneous oxidation of acetaminophen based on micro and nanoparticles catalyzed by different activators
- Early diagnosis of lung cancer using magnetic nanoparticles-integrated systems
- Advances in ZnO: Manipulation of defects for enhancing their technological potentials
- Efficacious nanomedicine track toward combating COVID-19
- A review of the design, processes, and properties of Mg-based composites
- Green synthesis of nanoparticles for varied applications: Green renewable resources and energy-efficient synthetic routes
- Two-dimensional nanomaterial-based polymer composites: Fundamentals and applications
- Recent progress and challenges in plasmonic nanomaterials
- Apoptotic cell-derived micro/nanosized extracellular vesicles in tissue regeneration
- Electronic noses based on metal oxide nanowires: A review
- Framework materials for supercapacitors
- An overview on the reproductive toxicity of graphene derivatives: Highlighting the importance
- Antibacterial nanomaterials: Upcoming hope to overcome antibiotic resistance crisis
- Research progress of carbon materials in the field of three-dimensional printing polymer nanocomposites
- A review of atomic layer deposition modelling and simulation methodologies: Density functional theory and molecular dynamics
- Recent advances in the preparation of PVDF-based piezoelectric materials
- Recent developments in tensile properties of friction welding of carbon fiber-reinforced composite: A review
- Comprehensive review of the properties of fly ash-based geopolymer with additive of nano-SiO2
- Perspectives in biopolymer/graphene-based composite application: Advances, challenges, and recommendations
- Graphene-based nanocomposite using new modeling molecular dynamic simulations for proposed neutralizing mechanism and real-time sensing of COVID-19
- Nanotechnology application on bamboo materials: A review
- Recent developments and future perspectives of biorenewable nanocomposites for advanced applications
- Nanostructured lipid carrier system: A compendium of their formulation development approaches, optimization strategies by quality by design, and recent applications in drug delivery
- 3D printing customized design of human bone tissue implant and its application
- Design, preparation, and functionalization of nanobiomaterials for enhanced efficacy in current and future biomedical applications
- A brief review of nanoparticles-doped PEDOT:PSS nanocomposite for OLED and OPV
- Nanotechnology interventions as a putative tool for the treatment of dental afflictions
- Recent advancements in metal–organic frameworks integrating quantum dots (QDs@MOF) and their potential applications
- A focused review of short electrospun nanofiber preparation techniques for composite reinforcement
- Microstructural characteristics and nano-modification of interfacial transition zone in concrete: A review
- Latest developments in the upconversion nanotechnology for the rapid detection of food safety: A review
- Strategic applications of nano-fertilizers for sustainable agriculture: Benefits and bottlenecks
- Molecular dynamics application of cocrystal energetic materials: A review
- Synthesis and application of nanometer hydroxyapatite in biomedicine
- Cutting-edge development in waste-recycled nanomaterials for energy storage and conversion applications
- Biological applications of ternary quantum dots: A review
- Nanotherapeutics for hydrogen sulfide-involved treatment: An emerging approach for cancer therapy
- Application of antibacterial nanoparticles in orthodontic materials
- Effect of natural-based biological hydrogels combined with growth factors on skin wound healing
- Nanozymes – A route to overcome microbial resistance: A viewpoint
- Recent developments and applications of smart nanoparticles in biomedicine
- Contemporary review on carbon nanotube (CNT) composites and their impact on multifarious applications
- Interfacial interactions and reinforcing mechanisms of cellulose and chitin nanomaterials and starch derivatives for cement and concrete strength and durability enhancement: A review
- Diamond-like carbon films for tribological modification of rubber
- Layered double hydroxides (LDHs) modified cement-based materials: A systematic review
- Recent research progress and advanced applications of silica/polymer nanocomposites
- Modeling of supramolecular biopolymers: Leading the in silico revolution of tissue engineering and nanomedicine
- Recent advances in perovskites-based optoelectronics
- Biogenic synthesis of palladium nanoparticles: New production methods and applications
- A comprehensive review of nanofluids with fractional derivatives: Modeling and application
- Electrospinning of marine polysaccharides: Processing and chemical aspects, challenges, and future prospects
- Electrohydrodynamic printing for demanding devices: A review of processing and applications
- Rapid Communications
- Structural material with designed thermal twist for a simple actuation
- Recent advances in photothermal materials for solar-driven crude oil adsorption


