Abstract
The interactions of nanomaterials with biological materials such as immortalized cell lines are recently on the rise. Owing to this superiority, the biosynthesis of AgNPs using gallic acid as a reductant was implemented in this study. After being synthesized, the AgNPs were characterized using techniques such as dynamic light scattering, transmission electron microscopy, selected area electron diffraction, and X-ray diffraction methods. Furthermore, the AgNPs were assessed for their cytotoxic effects on the colorectal adenocarcinoma cell line HT-29. The mechanisms of such cell-killing effect were investigated by analyzing the expressions of 14 mRNAs using quantitative polymerase chain reaction. The outcomes indicate that the synthesized AgNPs were cytotoxic on HT-29 cells. The expressions of all apoptotic genes analyzed including cyt-C, p53, Bax, Bcl2, CASP3, CASP8, CASP9, and CASP12 were upregulated. With regard to the autophagy-related genes, Beclin-1, XBP-1, CHOP, and LC3-II were upregulated, whereas the expressions of ATG3 and ATG12 were downregulated. To conclude, the AgNPs induced mitochondria-dependent apoptosis and non-canonical autophagy in HT-29 cells. A crosstalk did occur between autophagy and apoptosis in such a cell-killing effect. Hence, further studies are required to elucidate the exact mechanisms in animal models for further use of AgNPs in clinical medicine for the treatment of neoplasms of the digestive tract.
1 Introduction
Nanotechnology and its roles in cancer therapy are intensifying due to their specific targeting properties, and this arena of research has the potential to overcome the limitations of conventional treatment methods [1]. The drawbacks of using chemical and physical means comprise the harmful methods that pose higher energy requirements and utilize synthetic reductants that can generate substantial amounts of waste products [2]. Biosynthesized AgNPs are better used in nanomedicine because of their properties including small size and low toxicity with increased biodegradability and availability [3,4,5]. Green synthesis is an established route for fabricating AgNPs anticipated to be used for applications in medicine [6,7]. It has several advantages over the nanoparticles synthesized using conventional chemical methods [8,9,10,11,12]. Particularly, AgNPs synthesized using biological methods are known to be effective against microbes such as bacteria, fungi, and viruses with wound-healing, anti-inflammatory, and antioxidant properties posing its candidature for treating diseases like cancer and diabetes. In addition, the active capping agents of such nanoparticles fabricated using green methods are credited for the enhanced biological activities of such materials [13,14].
Colon cancer is one of the top five reasons for deaths related to cancers worldwide. In China, the incidence and death percentage related to colorectal cancers have increased considerably in 2020 compared to that in the year 2015 [15]. In the United States, an estimated 338,090 new cases and 169,280 deaths are expected because of the cancers associated with the digestive system in 2021. In the same report, 149,500 new cases and 52,980 deaths are estimated to happen because of cancers of the colon and rectum [16]. According to the 2022 projections, an estimated 343,040 new cases and 171,920 deaths related to digestive cancers are likely to occur. Consequently, 151,030 new cases and 52,580 deaths related to cancers of the colon and rectum are projected for the year [17]. In vitro studies on cancer cell lines are cherished, as these models are noteworthy in identifying a drug candidate for malignant forms of cancer [18]. Thus, from the time of its identification in 1964, the human adenocarcinoma cell line HT-29 has been used as a molecular model for studies related to intestinal cancer [19].
The benefits of using cell lines as an alternative for animal models are that they are efficient in managing the cost of conducting an experiment, provide ease in conducting and applying the outcomes, offer a limitless source of material, and evade the ethical issues connected with the usage of animal and human tissues [20]. Assays that can determine the cytotoxic effects of a drug screening for its efficacy in inhibiting cellular proliferation are used in combination with molecular techniques such as real-time polymerase chain reaction (PCR). Studies of such kind aim at quantifying the expressions of genes related to oncogenic or oncosuppressive effects to study the mechanism of cytotoxicity induced by a drug [21].
Among mechanisms being studied, apoptosis is a widely accepted signaling pathway for the death of malignant cells and is an outcome of triggers initiated by internal or external stimuli [22]. Autophagy is another process by which cellular homeostasis is maintained. This mechanism results in forming a double-membraned autophagosome to engulf the unwanted cargo of organelles by the development of an autolysosome [23]. Although it is considered a double-edged sword, autophagy is predominantly a tumor suppressor in the early stages of a tumor [24]. It is a less-studied mechanism for analyzing the route of cytotoxic effects of AgNPs on cancer cells. Apoptosis and autophagy can cross-talk involving quite a lot of biological macromolecules. The interaction between these two mechanisms of cellular degradation and homeostasis determines the fate of a cell. These signals can therefore protect a host against several diseases including cancer [25].
Based on their cytotoxic effects at various levels via the displacement of Ag+ ions or the AgNPs as a whole, they are a part of several formulations (almost one-third) used in numerous industries. Properties such as size, dose, route of administration, and the capping agents play crucial roles in the absorption of AgNPs. The mechanisms for such effects are linked to damage of the hereditary materials, change in the intake of nutrients and the active roles of cells of the immune system, the stimulation of intracellular ROS-associated membrane damage, anti-angiogenic effects, cell-cycle arrest, and the induction of apoptosis [26,27,28,29,30].
Based on this background, the AgNPs were synthesized using gallic acid, and the mechanisms of their cytotoxic effects against HT-29 colon cancer cells were determined. According to the published reports, this is the first-ever international study on the analysis of the cross-talk between autophagy and apoptosis as cell-killing mechanisms of AgNPs in colon cancer cells.
2 Experimental section
2.1 Chemicals
All chemicals used for this study were of analytical grade. Silver nitrate and gallic acid were purchased from Fisher scientific and Merck, USA, respectively. Dulbecco's modified Eagle medium (DMEM), fetal bovine serum (FBS), and antibiotic solutions were obtained from Gibco (Grand Island, NY, USA). Dimethyl sulfoxide and 3-4,5 dimethylthiazol-2yl-2,5-diphenyl tetrazolium bromide (MTT) were purchased from Sigma-Aldrich (St. Louis, MO, USA).
2.2 Synthesis and characterization of AgNPs
To synthesize AgNPs using gallic acid, different ratios of 0.01 N AgNO3 and 0.1 M gallic acid solutions were mixed (5:5, 6:4, 7:3, 6:4, 8:2, and 9:1). The mixture was incubated at room temperature. The change in intensity of the color of the hydrosol toward brown was monitored over a period of 12 h and pictured at the end of the incubation. The solutions prepared by proportions of 5:5, 8:2, and 9:1 were analyzed using dynamic light scattering (DLS). Micromeritics model Nano Plus was used to determine whether the nanoparticles were synthesized at the highest (5:5) and the limited concentrations of gallic acid (9:1). The best ratio with respect to particle size was obtained at the least concentration of gallic acid used (9:1) and therefore was considered for the bulk synthesis of AgNPs intended in pursuit of further use. Transmission electron microscope (TEM) images and selected area electron diffraction (SAED) patterns were obtained using JEOL JEM-2100F FE-TEM. Investigation of the crystalline nature of the material via X-ray powder diffraction (XRD) was accomplished using the PANalytical X’Pert3 Powder instrument (Malvern Panalytical Inc., Westborough, MA, USA).
2.3 Cell culture and MTT assay
HT-29 cells (human colon adenocarcinoma cell line) were cultured in DMEM with 10% FBS and penicillin/streptomycin (100 µg/mL) in a humidified incubator with a 5% CO2 atmosphere at 37°C.
The cytotoxicity of AgNPs on HT-29 cells was tested using MTT assay. Briefly, the cultured HT-29 cells were harvested by trypsinization and pooled into a 15 mL tube. The cells were then plated at a density of 1 × 105 cells/well (200 µL) into 96-well tissue culture plates with DMEM for 24–48 h at 37°C. Later, the wells were washed with sterile PBS and treated with various concentrations of AgNPs in a serum-free DMEM. The experiment was repeated three times, and the cells were incubated at 37°C in a humidified 5% CO2 incubator for 24 h. After the incubation period, MTT was added to each well, and the cells were incubated for another 2–4 h and observed under an inverted microscope to evaluate the viability of HT-29 cells. The absorbance for each well was measured at 570 nm using a micro plate reader (Thermo Fisher Scientific, USA). The percentage cell viability and IC50 were calculated using GraphPad Prism 6.0 software (USA).
2.4 Optimization of cell viability by response surface methodology (RSM)
RSM was used to correlate the factors and optimum responses for cell viability. The central composite design (CCD) was used for optimization. Design-Expert® software (Version 12; State-Ease Inc., Minneapolis, MN, USA) was applied to infer the outcomes.
2.5 Real-time PCR to analyze the expression of mRNAs
After the IC50 value was determined, the HT-29 cells were treated with the determined dose and real-time PCR was used to quantify the expression of 14 mRNAs preferred for the study. The cells were centrifuged at 5,000 rpm for 10 min in tubes treated with diethyl pyrocarbonate. The pelleted cells (1 × 107 cells) were treated with TRIZOL (Sigma-Aldrich, St. Louis, MO, USA) to lyse the cells. After a purity check for DNA contamination has been done, cDNA was synthesized and real-time PCR was performed using SYBR® Green JumpStart™ Taq Ready Mix™ (Catalog Number S4438). The expressions of apoptotic and autophagy-related mRNAs such as BCL2 Associated X; Apoptosis Regulator (Bax); Bcl2 Apoptosis Regulator (Bcl2); cytochrome C (cyt-C); Tumor protein P53 (p53); caspases 3, 8, 9, 12 (CASP3, CASP8, CASP9, and CASP12); X-Box Binding Protein 1 (XBP-1); C/EBP homologous protein (CHOP); LC3-II; Beclin-1; Autophagy-related 3 (ATG3); and Autophagy-related 12 (ATG12) were analyzed. β-Actin was used for normalization of the expression of the gene of interest. The primers used for the analysis are enlisted in Table 1. After the primers and other necessary materials were obtained, a real-time PCR assay was conducted using StepOnePlus Real-Time PCR, Applied Biosystems.
Primers used in the study for performing real-time PCR
| Gene of interest | Forward primer | Reverse primer |
|---|---|---|
| CASP3 | AGCAAACCTCAGGGAAACATT | CTCAGAAGCACACAAACAAAACT |
| CASP8 | GGGAGGAGTTGTGTGGGGTA | CAGTCATCGTGGGGCTTGA |
| CASP9 | AACCCTAGAAAACCTTACCCC | CATCACCAAATCCTCCAGAAC |
| CASP12 | GGACCAAGCACTGGGATCAA | GCAAGAGCCGACCATGAGTA |
| Cyt C | CCAATGAAGATGGGGAGATG | CCGTGAGCAGGGAGAAGAC |
| p53 | TGAAGCTCCCAGAATGCCAG | GCTGCCCTGGTAGGTTTTCT |
| Bax | GGTTGTCGCCCTTTTCTA | CGGAGGAAGTCCAATGTC |
| Bcl2 | CTTTGAGTTCGGTGGGGTCA | GGGCCGTACAGTTCCACAAA |
| Beclin-1 | CGGGCCAGACAGATGTGGAT | TCTGCCACTATCTTGCGGTT |
| LC3-II | AGCTCCAAGTGAGCACATTCA | TTGAAGGTCTATTTTATTGGCAACT |
| CHOP | GTGCTTTTCCAGACTGATCCA | CCTCATACCAGGCTTCCAGC |
| ATG3 | GGTGAAGGTGGTTCCTCCG | CGTCCAAACCACACATCTCG |
| ATG12 | CCAGCTACAGGCCACGTAAT | ACTGCACGAGCAGAAGTAGA |
| XBP-1 | CTGAGTCCGCAGCAGGTG | GGCTGGTAAGGAACTGGGTC |
| β-Actin | ATCGTGCGTGACATTAAGGAGAAG | AGGAAGGAAGGCTGGAAGAGTG |
2.6 Statistical analysis
The expressions of apoptosis and autophagy- related genes were represented statistically as mean ± standard error mean. A paired two-sample Student’s t-test with a p value <0.05 was considered to be statistically significant for expression levels.
3 Results and discussion
3.1 Characterization of AgNPs
Before the intended application for AgNPs in this study has been achieved, the materials were characterized using established techniques. Initially, the color change of the hydrosol to pale brown was taken as an indicator for the synthesis of AgNPs from the precursor silver nitrate by the reductant used [7] (Figure 1).

Observations of initial color in the blank solution and the change in color after incubating gallic acid with solutions containing AgNO3 at varying concentrations.
After the initial visual observation was made, DLS, a technique used to measure the nanoparticle size and to analyze their stability for enhanced applications in medicine, was applied [31,32]. Particle size is an important criterion for cellular uptake of nanomaterials inside the tumor and the surrounding environment rich in capillaries that are 400–600 nm wide. The particle sizes of AgNPs synthesized using varying ratios of the precursor AgNO3 and the reductant gallic acid (5:5, 8:2 and 9:1) were 148.4, 315.1, and 86.6 nm (Figure 2). These sizes were fewer than 400 nm and therefore can amass in the tumor microenvironment better [4,33,34].
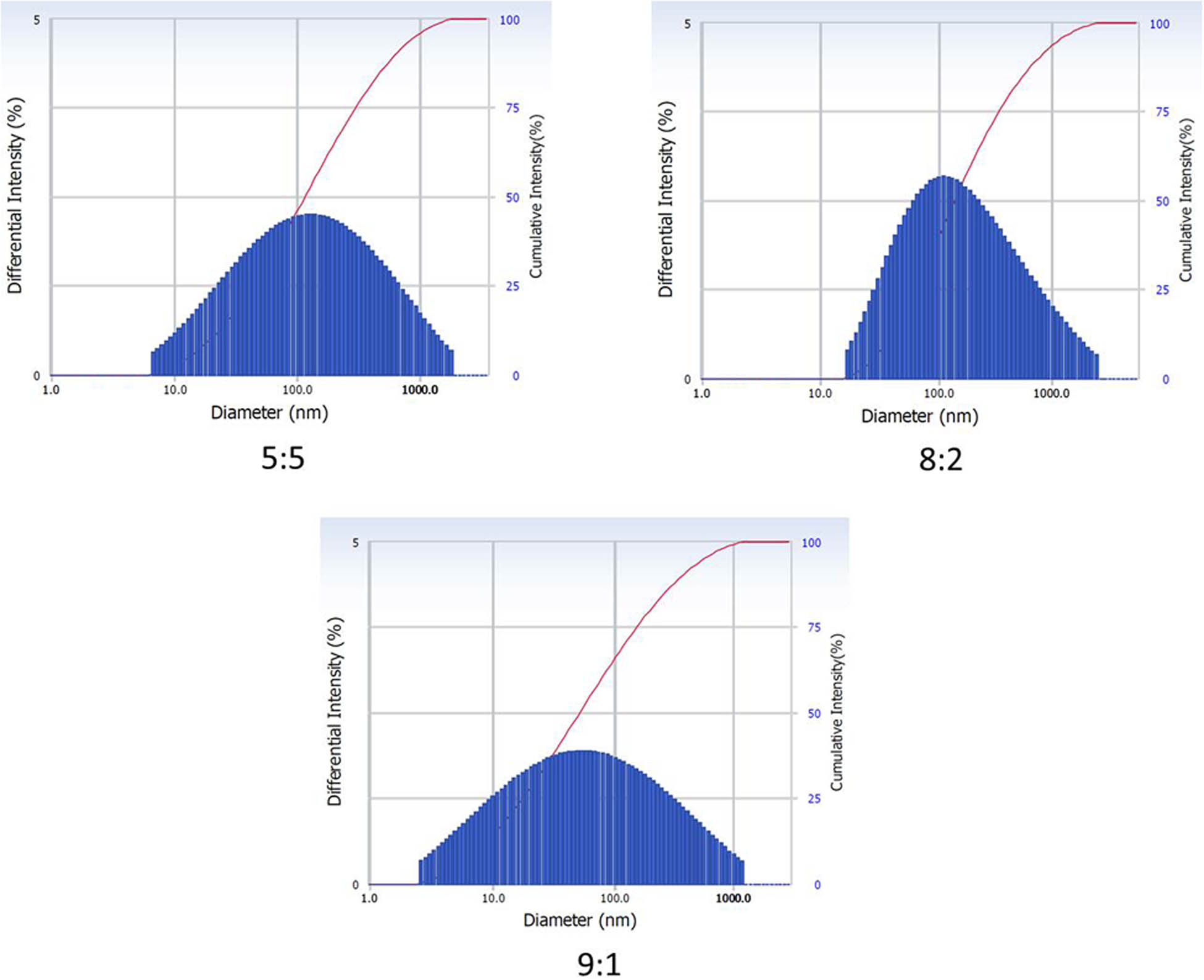
Analysis of particle size of AgNPs prepared using varying ratios of the precursor and the reductant by adopting DLS.
After the determination of particle size, an attempt was made to determine the crystalline nature of the synthesized material using XRD at atomic levels. XRD is an influential technique for phase identification, conducting quantifiable investigations and identifying structural differences and particle size of nanomaterials [35,36,37]. The diffraction pattern illustrates five intense peaks in the entire spectrum at (2θ) 28.23, 32.15, 38.06, 44.43, and 46.57°, which could be linked to (210), (122), (111), (200), and (231) planes that concur to face-centered, cubic, and crystalline silver synthesized using green methods (JCPDS file number: 04-0783). The unassigned peaks are plausibly due to the formation of crystals in the bioorganic phase on the material surface (Figure 3) [38,39,40]. The crystalline size of the AgNPs calculated using XRD was 38.13 nm. SAED is applied at the nano range to make observations of lattice pattern and crystallinity by use of diffraction spots on a TEM- inspecting display from a random particle [41,42]. The SAED pattern of the sample being studied here, revealed bright and sharp rings correlating to (110), (200), (012), (002), (211), and (311) lattice planes of face-centered, cubic, and crystalline silver (Figure 4a) [43,44,45,46,47,48,49]. The diffraction patterns observed via both XRD and SAED indicate that the synthesized AgNPs were crystalline in nature and extremely pure. Subsequent to the elucidation of the crystalline nature of the material, TEM, an electron microscopy technique, was adopted to analyze the morphological or structural features of nanomaterials using different magnifications at the atomic resolution [50,51,52]. The images that are illustrative of the morphology of AgNPs are presented in Figure 4b. The particle size calculated using the data obtained via TEM was 59 nm.
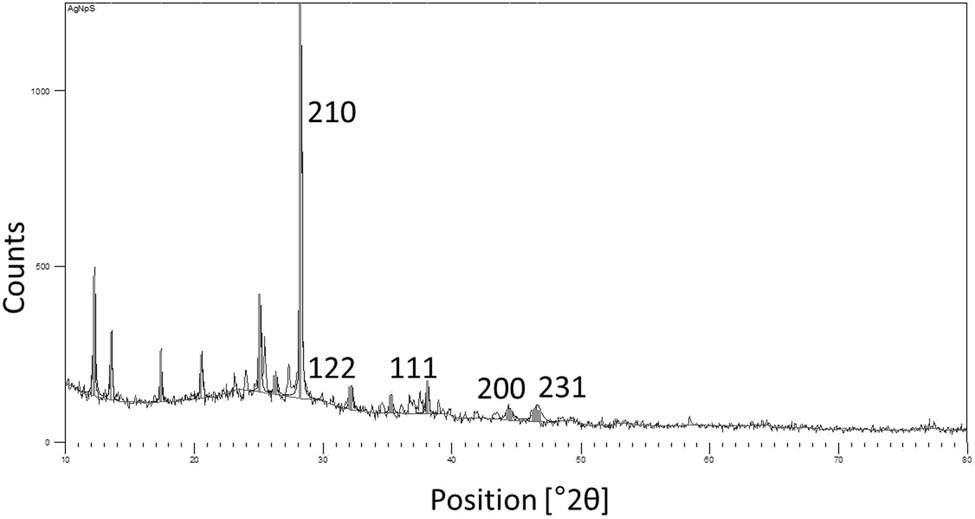
XRD pattern of the synthesized AgNPs.
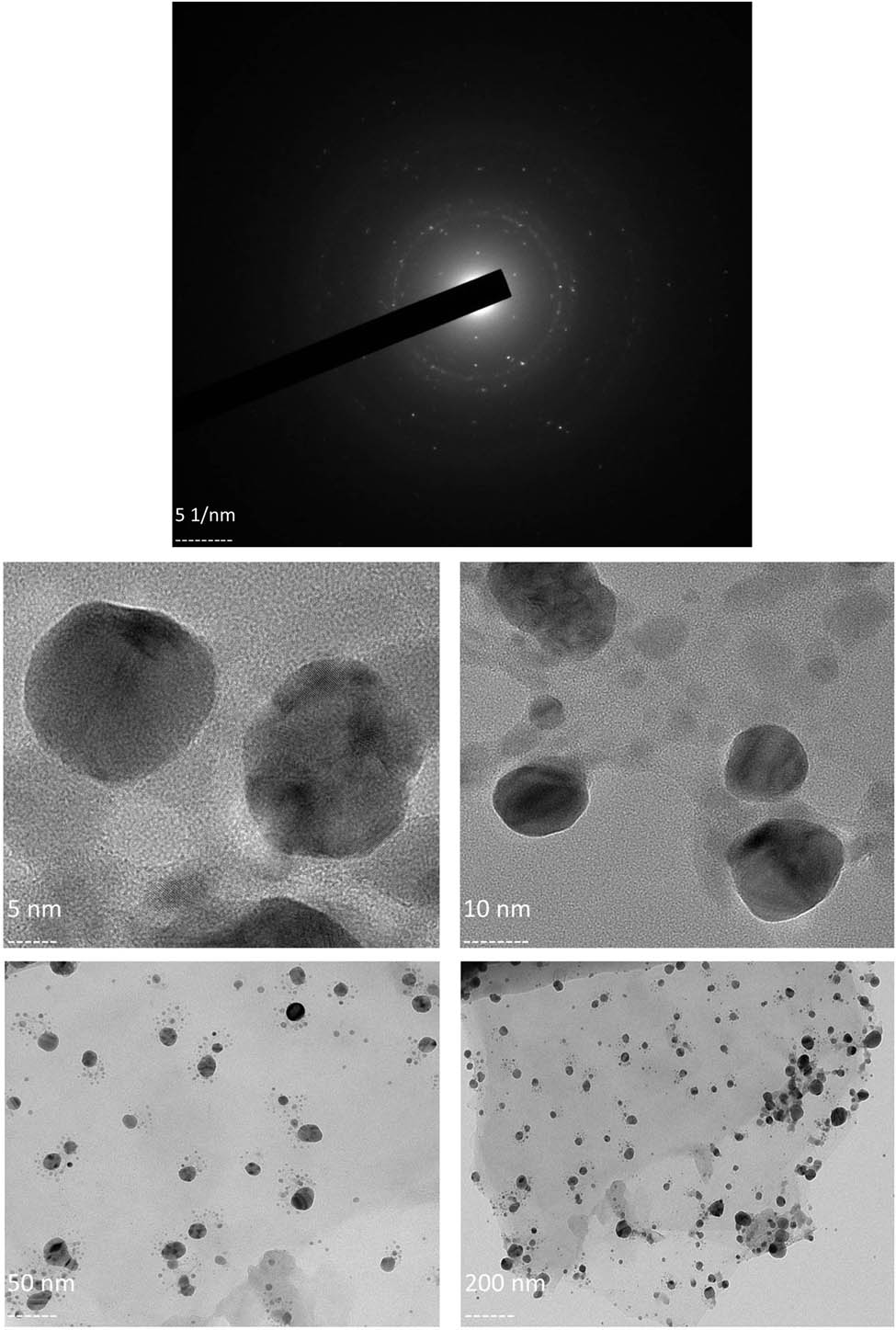
(a) SAED pattern of the synthesized AgNPs; (b) TEM-based imaging of the morphology of the synthesized AgNPs.
The particle sizes obtained using DLS (the size best suited and used for cytotoxicity studies), XRD, and TEM are less than 100 nm (86.6, 38.13, and 59 nm). The permeable vascular structure of tumors permits therapeutics with high molecular weight and sizes less than 150 nm to extravasate and accumulate into the intratumoral environment around it by enhanced permeability and retention effect [53]. Besides, the absorption, distribution, metabolism, excretion, and biodistribution of particles with sizes greater than 100 nm change drastically. These materials with larger sizes are found in major organs such as spleen, lungs, liver, and kidney over extended periods or durations greater than normal [54]. Hence, particles of sizes less than 100 nm are generally preferred for in vivo applications such as drug delivery [55,56,57].
The particle sizes of nanomaterials can be obtained using techniques such as DLS, XRD, and TEM [55]. Based on the material to be studied, each technique has its own merits and demerits [58]. Although the particle sizes are less than 100 nm as analyzed using all three methods as evidenced by this study, slight disparities did exist. This disparity was due to the fact that the particle sizes obtained using TEM are generally higher than that of XRD [59,60]. Although the sizes would not change much between TEM and DLS in suspensions with less or no aggregation, samples with agglomerates can give rise to considerably elevated particle sizes using DLS in comparison to TEM [61]. The increased sizes observed in DLS in comparison to that in TEM might be due to the Brownian movement, as DLS measures the Rayleigh scattering from nanoparticles. Considering these differences, DLS is a preferred method for characterizing nanoparticles in aqueous or physiological suspensions proposed to be used for biological applications [35,62].
Gallic acid is an established natural antioxidant and a secondary polyphenolic metabolite distributed, which is available throughout the parts of plants consumed as food, starting from the bark to seed [63,64]. This plant metabolite with reducing properties has the ability to transform metal ions into nanoparticles of metallic forms [65,66]. By virtue of elevated temperatures, the phenolic hydroxyl bonds sustain a homolytic split and produce hydrogen radicals, leading to transfer of electrons from the hydrogen radical to silver ions (Ag+) resulting in the production of AgNPs. This process comprises three stages. In the activation phase, the silver ions are reduced via nucleation resulting in the formation of clusters. Following the first step, the growth phase results in the formation of large-sized particles from relatively small materials by means of spontaneous coalescence designated as Ostwald ripening. In the termination phase, the eventual size of the nanoparticle is reached [67,68,69].
3.2 Cytotoxicity of AgNPs
After characterization of the AgNPs, MTT assay was performed to determine the cytotoxicity of AgNPs on malignant cells, the nanomaterial which is studied and recognized well for such effects [70,71,72]. This assay is an established preclinical assay to determine the anticancer effect of cytotoxic drugs [73]. The cytotoxicity as a measure of cell viability was dose-dependent (Figure 5). The half-maximal inhibitory concentration (IC50) is a measure of how potent the activity of a tested drug is [74]. The MTT assay determined that the IC50 value for cytotoxic effect of the synthesized AgNPs was 33.45 μg/mL (equivalent to 33.45 ppm), which corresponds well to published reports on IC50 of AgNPs (3–99 ppm) [75]. The microscopic observations were suggestive of the cytotoxic effect of AgNPs (Figure 6).
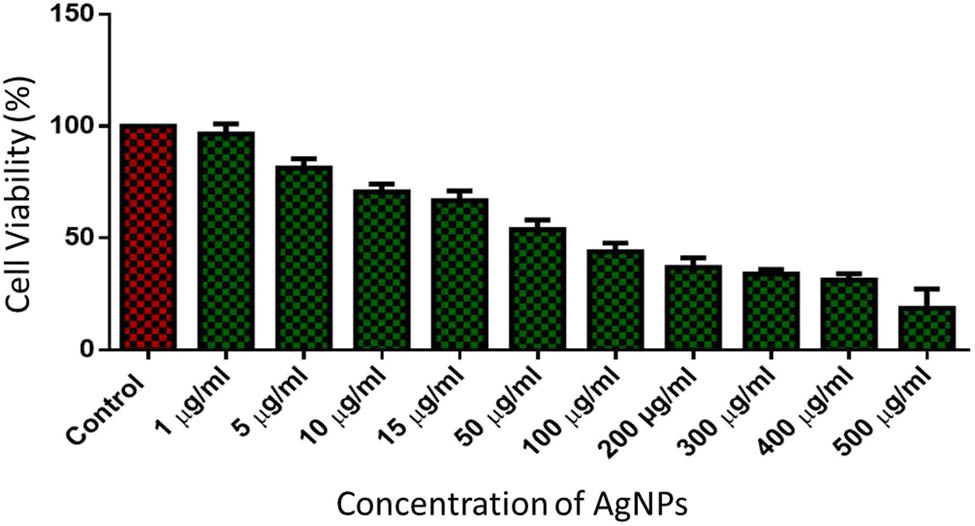
Assessment of the percentage of cell viability of HT-29 cells using MTT assay after being incubated with varying concentrations of AgNPs.
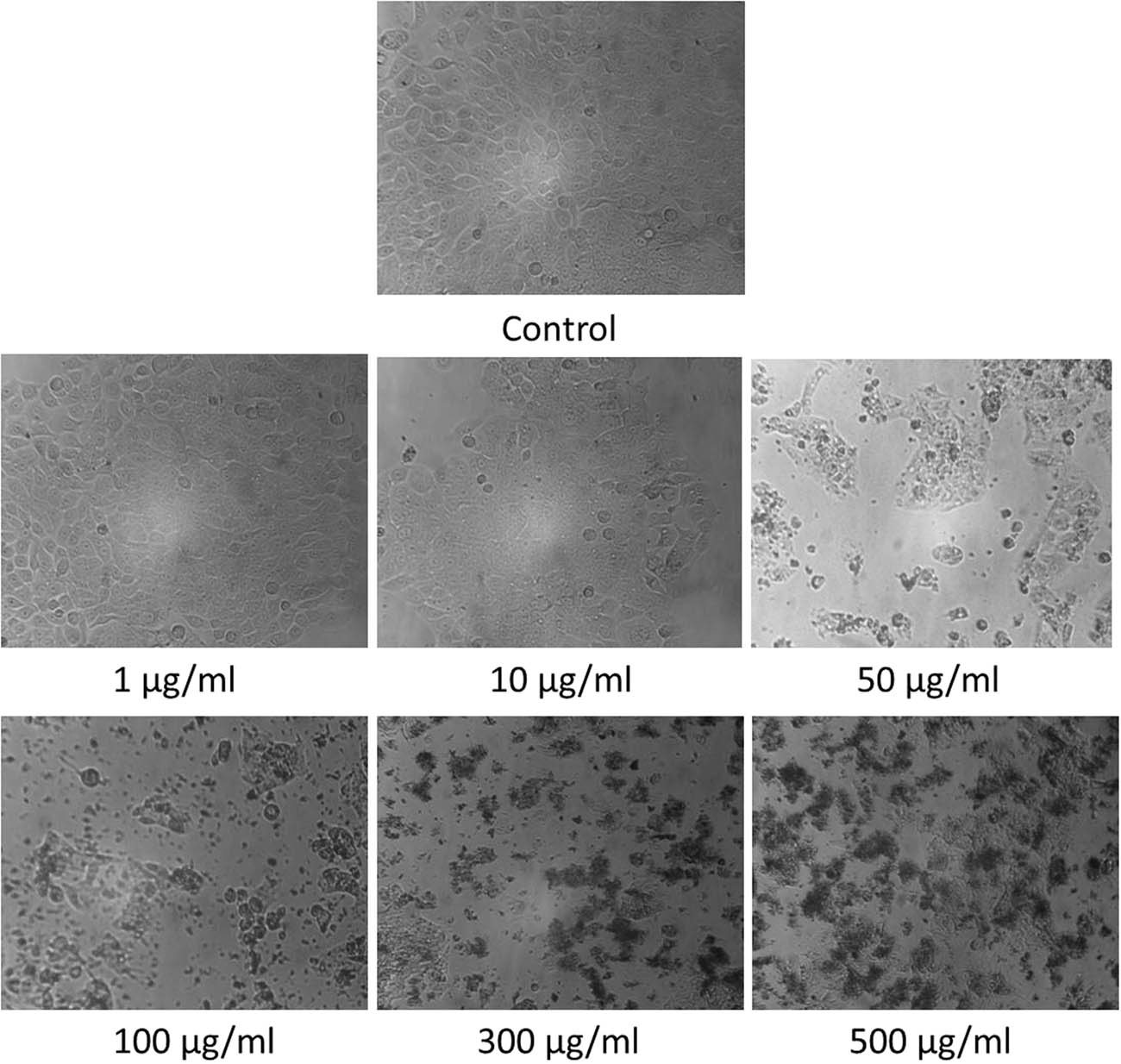
Microscopic observations on HT-29 cells after treatment with AgNPs at various concentrations.
According to the outcomes of MTT assay, the activities related to HT-29 cell metabolism were influenced by the AgNPs, and hence, the prospect of induction of apoptosis or autophagy by the materials at nano-regime was evaluated at the IC50.
3.3 Optimization of cell viability by RSM
The Model F-value (67.24) and the probability value (p < 0.0001) for cell viability imply that the model was significant, and the chance of this happening as a result of noise was very low (0.01%) (Table 2). The predicted R 2 of 0.9138 was in reasonable agreement with the adjusted R 2 of 0.9650; that is, the difference was less than 0.2. The R 2 value close to 1 determines that the model appeared significant and fulfilled all requirements of ANOVA. Adeq Precision which measures the signal-to-noise ratio was 23.248, which seems to be adequate. A ratio greater than 4 was found appropriate as observed in this case (Table 3). The resultant response surface and contour plots are presented in Figure 7 [75,76,77,78].
ANOVA and the significance of response surface model for the HT-29 cell viability using CCD
| Source | Sum of squares | df | Mean square | F-value | p-value | |
|---|---|---|---|---|---|---|
| Model | 9510.32 | 5 | 1902.06 | 67.24 | <0.0001 | Significant |
| A – AgNPs (μg/mL) | 13.72 | 1 | 13.72 | 0.4851 | 0.5086 | |
| B – Absorbance | 105.27 | 1 | 105.27 | 3.72 | 0.0951 | |
| AB | 11.78 | 1 | 11.78 | 0.4165 | 0.5392 | |
| A² | 4.25 | 1 | 4.25 | 0.1502 | 0.7098 | |
| B² | 18.58 | 1 | 18.58 | 0.6568 | 0.4444 | |
| Residual | 198.01 | 7 | 28.29 | |||
| Cor total | 9708.33 | 12 |
Regression analysis for the HT-29 cell viability by AgNPs using CCD
| Std. dev. | 5.32 | R 2 | 0.9796 |
|---|---|---|---|
| Mean | 45.61 | Adjusted R 2 | 0.9650 |
| C.V. % | 11.66 | Predicted R 2 | 0.9138 |
| Adeq precision | 23.2477 |
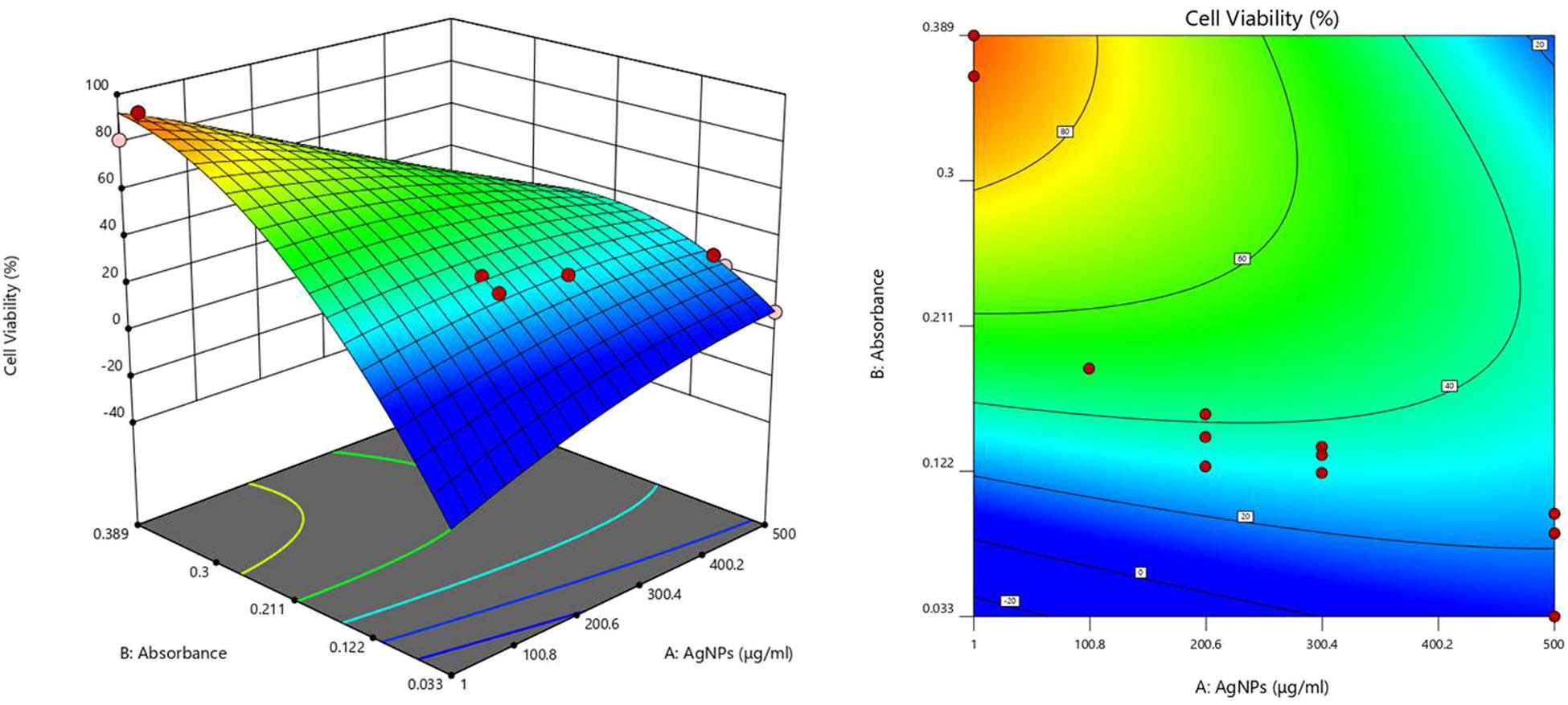
Response surface and contour plots for the cytotoxicity of AgNPs on HT-29 cells.
3.4 Analysis of mechanism of cytotoxic effect of AgNPs using quantitative PCR
AgNPs are considered to be used in the profit-oriented category with the support of numerous toxicological studies [79]. Yet, the ways in which they kill cells derived from mammalian tissues remain less explored. They are known to cause oxidative stress in exposed cells and result in lipid peroxidation, ultimately leading to cell death by mechanisms such as apoptosis, necrosis, or autophagy. When free Ag+ is released into media, they can increase H2O2 levels and result in apoptosis [80,81]. To make a special mention, to provoke cytotoxic effects on mammalian cells, AgNPs are dependent on several parameters including the conditions provided such as duration of exposure; concentration; temperature; and factors such as nanoparticle size, shape, and surface coating in addition to the type of cell being studied [82,83,84].
Real-time PCR is usually performed to quantify the intracellular mRNA levels and differential gene expression in various cells and tissues [85,86]. It is applied to study the underlying mechanisms of cell death due to the relative advantages they possess in terms of being a sensitive, efficient, accurately quantifying, and high-grade automated technique [87]. A positive expression fold/ratio determines the upregulation of a gene, while the downregulation of a gene is ascertained by a negative expression fold/ratio [21]. The analysis of gene expression profiles in malignant cells has become an integral part of identifying the biomarkers or changes associated with the disease to improve the chances of personalized therapy. Real-time PCR is therefore considered a standard technique to determine the molecular changes after the cancer cells were exposed to a test drug [88]. Based on this background, real-time PCR was used in this report to study the expression of 14 mRNAs related to autophagy and apoptosis in HT-29 colon cancer cells.
To begin, caspases are proteases which can cleave peptide bonds that follow the aspartic acid residues to initiate and aid in execution of extrinsic apoptosis. Although “isoleucine–glutamic acid–threonine-aspartic acid” is the conventional target motif for CASP8, it can cleave the “aspartic acid–glutamic acid–valine–aspartic acid” target of CASP3. The alteration in expressions of CASP8 may be associated with specific type of tumors. The expressions are usually upregulated in most malignant forms, which can suppress oncogenesis [89,90,91]. The expressions of CASP8 were upregulated in this report, which is an established initiator of extrinsic apoptosis [92]. CASP12, cleaved by endoplasmic reticulum (ER) stress, can lead to the initiation of intrinsic apoptosis. The upregulation of CASP12 indicates a greater possibility of induction of intrinsic apoptosis [93].
After analysis of the expression of initiator caspases, other mRNAs related to the mechanism of cell-killing were studied. Among these mRNAs studied, p53 plays predominant roles in cell cycle arrest, senescence, and apoptosis. This enables the further existence of damaged cells or eradicates the cells that are critically injured. This determines that this tumor suppressor seems to possess definite functions in non-infectious diseases [94]. To give a special mention, p53 participates directly in the intrinsic apoptosis pathway by regulating the mitochondrial outer membrane permeabilization and producing a trigger in the activity of CASP3 [95]. This mechanism involves cyt-C, Apaf-1, and CASP3 [96]. The contact between apoptotic protease activating factors and mitochondrial cyt-C can activate the caspase cascade, especially CASP3, soon after it is enters the cytosol [97,98]. Therefore, the upregulated expressions of p53, cyt-C, and CASP3 indicate the vital role of apoptosis in the observed cell-killing effect.
Bax is a pro-apoptotic target of p53 and has the ability to induce apoptosis in cancer cells [99]. Bcl-2 is predominantly an anti-apoptotic protein and can inhibit both autophagy and apoptosis as cell-killing mechanisms [100]. The upregulated expressions of this protein can result in resistance to intrinsic apoptosis and allow cancer cells to evade apoptosis. Anticancer drugs usually inhibit anti-apoptotic Bcl-2 to induce apoptosis. Yet, the Bcl-2 family of proteins do have both the pro-apoptotic and anti-apoptotic members. Supporting this view, the Bcl-2 family members are classified into three types: anti-apoptotic (Bcl-2, Bcl-xL, Mcl-1, Bcl-w, Bcl-B, and A1/Bfl-1), pro-apoptotic BH3-only proteins (Bim, Bid, Bad, Noxa, Puma, and Bmf), and the multidomains Bax and Bak. The upregulation of pro-apoptotic members of Bcl-2 family by p53 is a common mechanism for apoptosis to occur. The roles of pro-apoptotic effector proteins that comprise BH1, BH2, and BH3 domains and BH3-only proteins (well-known for inhibition of anti-apoptotic Bcl-2 proteins), which might have possibly led to pro-apoptotic Bcl-2 signals, remain critical in the induction of apoptosis along with p53 [101,102,103]. BH3-only proteins can cause changes in the outer membrane permeability of mitochondria, leading to the release of intracellular cytosolic proteins, which are usually restricted to the inter-membrane space [22]. CHOP is also known to cause an upregulation in expressions of such pro-apoptotic Bcl-2 members leading to elevated production of ROS. This can lead to the release of cyt-C from mitochondria eventually causing an apoptotic trigger [104]. Therefore, the pro-apoptotic members of Bcl-2 might have played crucial roles in the initiation and extension of apoptotic effect and autophagy in the HT-29 cells.
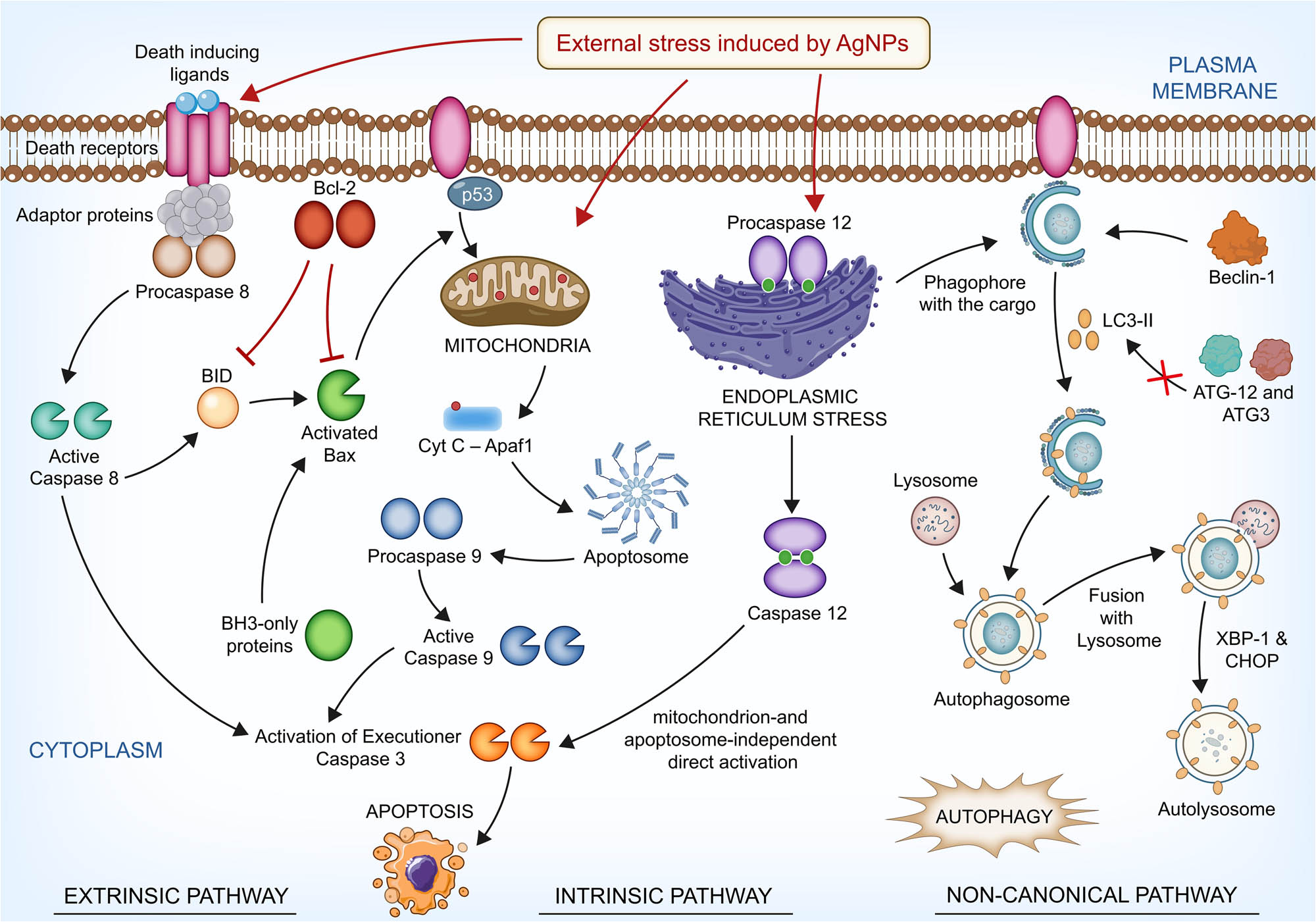
AgNPs have been known to provoke apoptotic cell death in a variety of cancer cells through the induction of ROS and the resultant oxidative stress [105]. After entering the cytosol through the oxidation of cardiolipin by ROS, cyt-C forms the apoptosome. This is a complex of cyt-C with apoptosis activating factor-1 (Apaf-1), leading to the activation of procaspase-9 which results in activation of CASP9 and an increase in the activity of CASP9 [106,107]. As an initiator and well-characterized caspase with regard to posttranslational modifications, CASP9 which is generally activated by cyt-C release is necessary for the activation of effector CASP3. This is a response to death stimuli, the ultimate fate of the cellular apoptosis [108]. CASP8 can cleave another member of the Bcl-2 family, Bid into tBid, which initiates the mitochondrial pathway of apoptosis resulting in the mitochondrial release of cyt-C and Smac/DIABLO. This leads to the formation of apoptosome by interaction of cyt-C with Apaf-1. As a result, the apoptosome complex is formed which activates CASP9. The other mitochondrial protein released, Smac/DIABLO, neutralizes the inhibitory effects of XIAP, leading to apoptotic cell death involving CASP9 and CASP3 [109]. This further elucidates that the mitochondria-dependent apoptosis exerted by AgNPs in HT-29 cells encompasses both extrinsic and intrinsic modes, since the caspase cascade was effectively involved as mentioned previously (Figure 8).
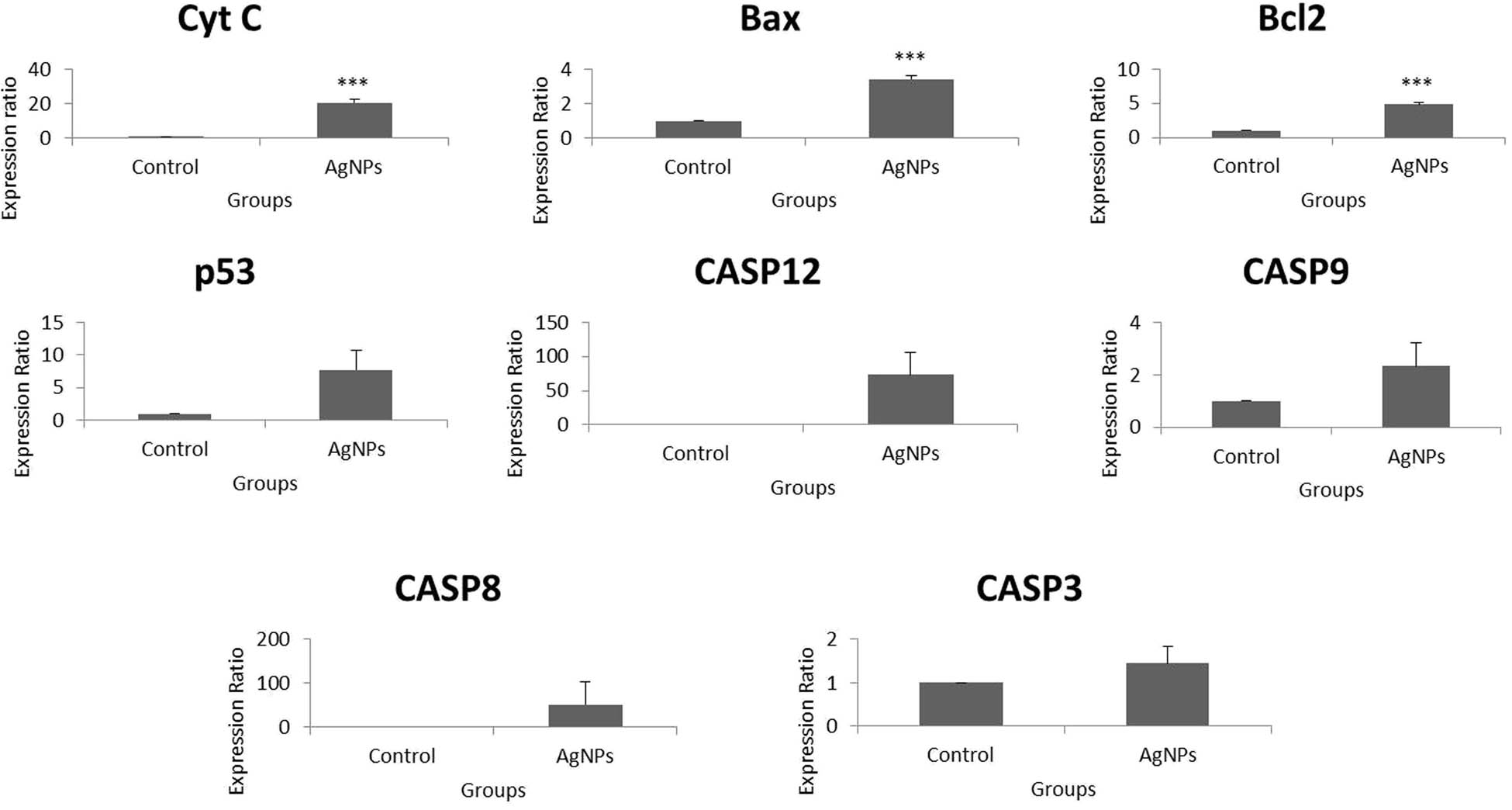
Fold change over control of apoptotic genes in HT-29 cells using real-time PCR. p values of less than 0.05 (*p < 0.05 and **p < 0.01, compared with the control) are considered significant.
Beclin-1 is a tumor suppressor related to autophagy, which can also mediate apoptosis by interaction with the multidomain proteins of the Bcl-2 family among mammalian cells [110,111]. Autophagy-related (ATG) proteins are crucial members of the canonical pathway of autophagy. In this pathway, the membrane of the endoplasmic reticulum forms the phagophore, which later forms the autophagosome. The autophagosome later fuses with the lysosome to form the autolysosome, which degrades the cargo to be destroyed inside its double-walled membrane, with the support of the ATGs and LC3II (marker of late autophagy) [23,112].
But, the downregulation of ATG12 and ATG3 could be negatively correlated with the formation of ATG12–ATG3 complex, which is necessary to induce autophagy [113]. Therefore, these two significant ATGs did not play a role in the creation of an autophagic flux in HT-29 cells after treatment with AgNPs. Notably, the non-canonical pathway of autophagy does not necessitate the interference of the entire ATGs in the formation of autophagosome, while canonical pathway does. Hence, the elongation and consequent formation of autophagosome membrane might have been dependent on other sources such as WD repeat domain phosphoinositide-interacting protein 1 (WIPI1) [114,115]. The outcomes therefore indicate that the autophagy induced in this study was non-canonical.
CHOP is a key transcription factor necessary for the initiation of autophagy [116]. XBP-1 is a key factor in unfolded protein response, which is released when a stress such as hypoxia arises in the endoplasmic reticulum. This can induce autophagy in cancer cells via JNK activation and eIF2α phosphorylation [117,118]. Hence, this relates that hypoxia-induced ER stress might have played crucial roles in the observed late autophagy. Autophagy is a stress response and precedes apoptosis. These two mechanisms are concomitant and may arise among cells as a resultant of a stress or an external stimulus [119]. This study determines that these two factors were critical in onset and led to late autophagy via the involvement of LC3II. Therefore, the UPR-activated and CHOP provoked maturation of the autolysosome during AgNPs-induced late autophagy on HT-29 cells was dependent on a non-canonical mechanism (Figure 9).
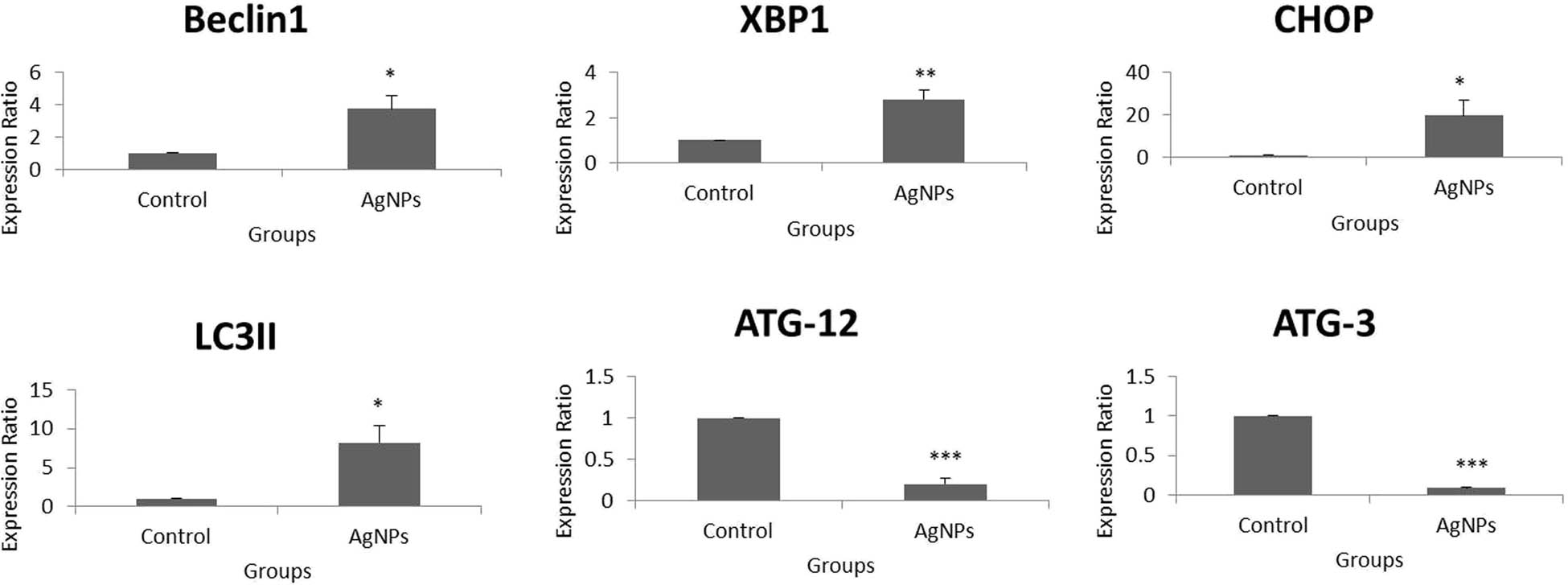
Fold change over control of autophagy-related genes in HT-29 cells using real-time PCR. p values of less than 0.05 (*p < 0.05 and **p < 0.01, compared with the control) are considered significant.
To conclude, the AgNPs induced mitochondria-dependent apoptosis and late non-canonical autophagy in the carcinoma cells (Figure 10). A crosstalk occurred between autophagy and apoptosis in such cell-killing effect. The schematic representation of the possible mechanism of the synthesis of nanoparticles and the cytotoxicity in HT-29 cells is depicted in Figure 11.
Possible mechanisms for the cytotoxic effect of AgNPs on HT-29 cells.
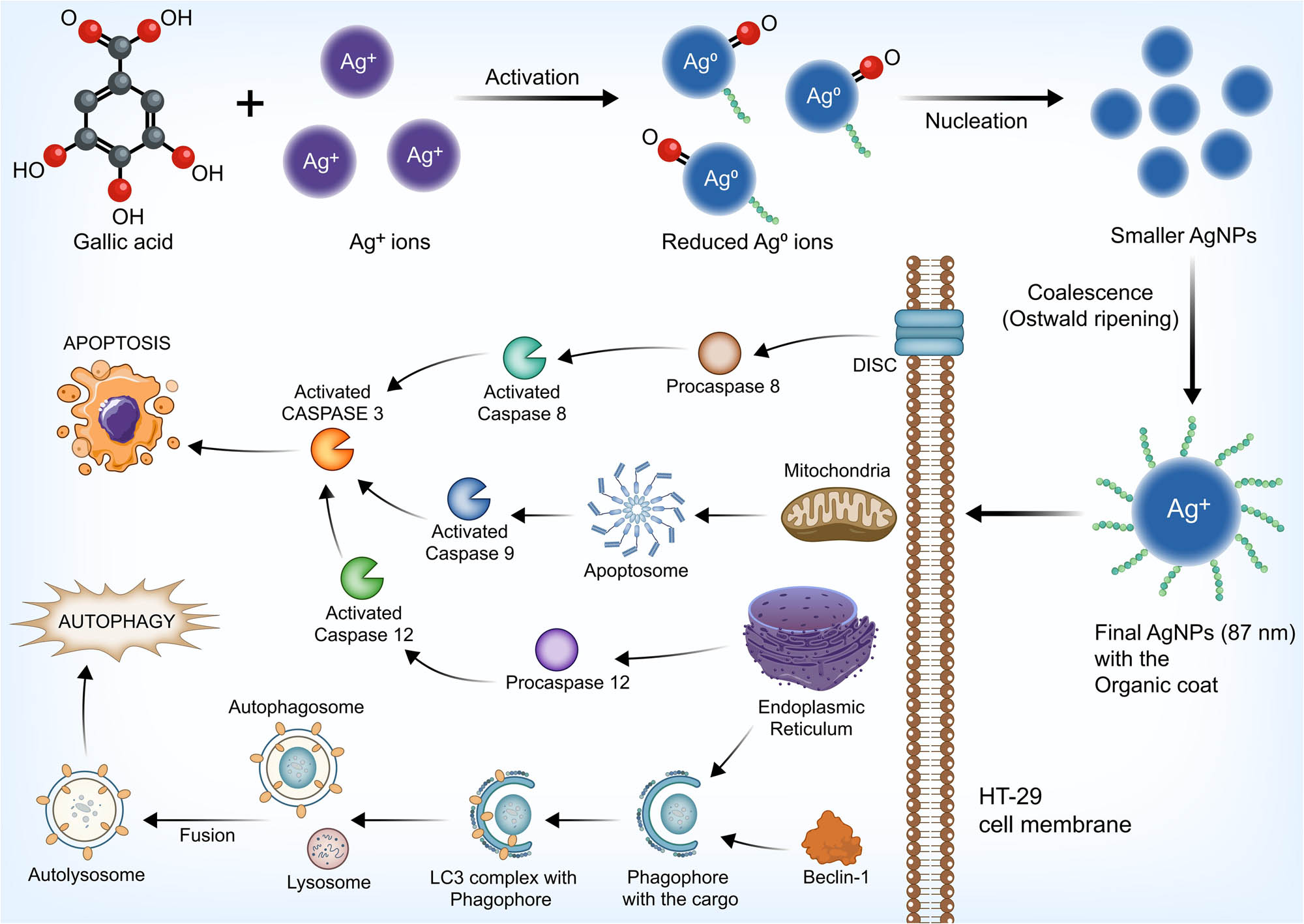
Schematic representation of the mode of synthesis of AgNPs and the cytotoxicity on HT-29 cells.
4 Conclusion
In this study, AgNPs were synthesized using gallic acid as a reductant. The synthesis was confirmed initially by the observation of a visible color change after incubating the reductant with the precursor. The synthesized AgNPs were further characterized by established techniques and tested for their cytotoxic effects. The nanoparticles were cytotoxic toward HT-29 cells, and the analysis of mechanisms involved indicated that mitochondria-dependent apoptosis and a late non-canonical autophagy were induced. As a conclusive remark, the present study suggests that AgNPs are valuable candidates for treating cancers of digestive origin. Further preclinical studies are warranted to study the precise effects and promote the clinical use of AgNPs.
-
Funding information: This study was supported by the National Natural Science Foundation of China (82072704 and 81973525), Jiangsu Primary Research & Development Plan (SBE2021740280), the “333 Talents” Program of Jiangsu Province (BRA2020390), and Project of National Clinical Research Base of Traditional Chinese Medicine in Jiangsu Province (JD2022SZXYA01).
-
Author contributions: All authors have accepted responsibility for the entire content of this manuscript and approved its submission.
-
Conflict of interest: The authors state no conflict of interest.
References
[1] Yetisgin AA, Cetinel S, Zuvin M, Kosar A, Kutlu O. Therapeutic nanoparticles and their targeted delivery applications. Molecules. 2020;25:2193.10.3390/molecules25092193Search in Google Scholar PubMed PubMed Central
[2] Haque S, Norbert CC, Acharyya R, Mukherjee S, Kathirvel M, Patra CR. Biosynthesized silver nanoparticles for cancer therapy and in vivo bioimaging. Cancers. 2021;13:6114.10.3390/cancers13236114Search in Google Scholar PubMed PubMed Central
[3] Zhang H, Jacob JA, Jiang Z, Xu S, Sun K, Zhong Z, et al. Hepatoprotective effect of silver nanoparticles synthesized using aqueous leaf extract of Rhizophora apiculata. Int J Nanomed. 2019;14:3517–24.10.2147/IJN.S198895Search in Google Scholar PubMed PubMed Central
[4] Rajadurai UM, Hariharan A, Durairaj S, Ameen F, Dawoud T, Alwakeel S, et al. Assessment of behavioral changes and antitumor effects of silver nanoparticles synthesized using diosgenin in mice model. J Drug Delivery Sci Technol. 2021;66:102766.10.1016/j.jddst.2021.102766Search in Google Scholar
[5] Jacob JA, Shanmugam A. Silver nanoparticles provoke apoptosis of Dalton’s ascites lymphoma in vivo by mitochondria dependent and independent pathways. Colloids Surf B: Biointerfaces. 2015;136:1011–6.10.1016/j.colsurfb.2015.11.004Search in Google Scholar PubMed
[6] Huang H, Shan K, Liu J, Tao X, Periyasamy S, Durairaj S, et al. Synthesis, optimization and characterization of silver nanoparticles using the catkin extract of Piper longum for bactericidal effect against food-borne pathogens via conventional and mathematical approaches. Bioorganic Chem. 2020;103:104230.10.1016/j.bioorg.2020.104230Search in Google Scholar PubMed PubMed Central
[7] Antony JJ, Sivalingam P, Siva D, Kamalakkannan S, Anbarasu K, Sukirtha R, et al. Comparative evaluation of antibacterial activity of silver nanoparticles synthesized using Rhizophora apiculata and glucose. Colloids Surf B Biointerfaces. 2011;88:134–40.10.1016/j.colsurfb.2011.06.022Search in Google Scholar PubMed
[8] Mousavi SM, Hashemi SA, Ghasemi Y, Atapour A, Amani AM, Savar Dashtaki A, et al. Green synthesis of silver nanoparticles toward bio and medical applications: review study. Artif cells, nanomedicine, Biotechnol. 2018;46:S855–S72.10.1080/21691401.2018.1517769Search in Google Scholar PubMed
[9] Roy A, Bulut O, Some S, Mandal AK, Yilmaz MD. Green synthesis of silver nanoparticles: biomolecule-nanoparticle organizations targeting antimicrobial activity. RSC Adv. 2019;9:2673–702.10.1039/C8RA08982ESearch in Google Scholar PubMed PubMed Central
[10] Ahmed S, Saifullah, Ahmad M, Swami BL, Ikram S. Green synthesis of silver nanoparticles using Azadirachta indica aqueous leaf extract. J Radiat Res Appl Sci. 2016;9:1–7.10.1016/j.jrras.2015.06.006Search in Google Scholar
[11] Saha J, Begum A, Mukherjee A, Kumar S. A novel green synthesis of silver nanoparticles and their catalytic action in reduction of Methylene Blue dye. Sustain Environ Res. 2017;27:245–50.10.1016/j.serj.2017.04.003Search in Google Scholar
[12] Antony JJ, Sivalingam P, Chen B. Toxicological effects of silver nanoparticles. Environ Toxicol pharmacology. 2015;40:729–32.10.1016/j.etap.2015.09.003Search in Google Scholar PubMed
[13] Jabeen S, Qureshi R, Munazir M, Maqsood M, Munir M, Shah SSH, et al. Application of green synthesized silver nanoparticles in cancer treatment-a critical review. Mater Res Express. 2021;8:092001.10.1088/2053-1591/ac1de3Search in Google Scholar
[14] Xu Z, Feng Q, Wang M, Zhao H, Lin Y, Zhou S. Green biosynthesized silver nanoparticles with aqueous extracts of ginkgo biloba induce apoptosis via mitochondrial pathway in cervical cancer cells. Front Oncol. 2020;10:2282.10.3389/fonc.2020.575415Search in Google Scholar PubMed PubMed Central
[15] Cao W, Chen H-D, Yu Y-W, Li N, Chen W-Q. Changing profiles of cancer burden worldwide and in China: a secondary analysis of the global cancer statistics 2020. Chin Med J. 2021;134:783–91.10.1097/CM9.0000000000001474Search in Google Scholar PubMed PubMed Central
[16] Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics. 2021. CA: A Cancer J Clinicians. 2021;71:7–33.10.3322/caac.21654Search in Google Scholar
[17] Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics. 2022. CA: A Cancer J Clinicians. 2022;72:7–33.10.3322/caac.21708Search in Google Scholar
[18] Mirabelli P, Coppola L, Salvatore M. Cancer cell lines are useful model systems for medical research. Cancers. 2019;11:1098.10.3390/cancers11081098Search in Google Scholar PubMed PubMed Central
[19] Martínez-Maqueda D, Miralles B, Recio I. HT29 cell line. The impact of food bioactives on health. 2015;113–24. 10.1007/978-3-319-16104-4.Search in Google Scholar PubMed
[20] Kaur G, Dufour JM. Cell lines: Valuable tools or useless artifacts. Spermatogenesis. 2012;2:1–5.10.4161/spmg.19885Search in Google Scholar PubMed PubMed Central
[21] Jiang Z, Liu J, Chen B, Mani R, Pugazhendhi A, Shanmuganathan R, et al. Cytotoxic effects of a sesquiterpene β-elemene on THP-1 leukemia cells is mediated via crosstalk between beclin-1 mediated autophagy and caspase-dependent apoptosis. Process Biochem. 2019;87:174–8.10.1016/j.procbio.2019.09.006Search in Google Scholar
[22] Pistritto G, Trisciuoglio D, Ceci C, Garufi A, D'Orazi G. Apoptosis as anticancer mechanism: function and dysfunction of its modulators and targeted therapeutic strategies. Aging (Albany NY). 2016;8:603–19.10.18632/aging.100934Search in Google Scholar PubMed PubMed Central
[23] Jacob JA, Salmani JMM, Jiang Z, Feng L, Song J, Jia X, et al. Autophagy: An overview and its roles in cancer and obesity. Clinica Chim Acta. 2017;468:85–9.10.1016/j.cca.2017.01.028Search in Google Scholar PubMed
[24] Chavez-Dominguez R, Perez-Medina M, Lopez-Gonzalez JS, Galicia-Velasco M, Aguilar-Cazares D. The double-edge sword of autophagy in cancer: from tumor suppression to pro-tumor activity. Front Oncol. 2020;10:2064.10.3389/fonc.2020.578418Search in Google Scholar PubMed PubMed Central
[25] Su M, Mei Y, Sinha S. Role of the crosstalk between autophagy and apoptosis in cancer. J Oncol. 2013;2013:102735.10.1155/2013/102735Search in Google Scholar PubMed PubMed Central
[26] Valenzuela-Salas LM, Girón-Vázquez NG, García-Ramos JC, Torres-Bugarín O, Gómez C, Pestryakov A, et al. Antiproliferative and antitumour effect of nongenotoxic silver nanoparticles on melanoma models. Oxid Med Cell Longev. 2019;2019:4528241.10.1155/2019/4528241Search in Google Scholar PubMed PubMed Central
[27] Stensberg MC, Wei Q, McLamore ES, Porterfield DM, Wei A, Sepúlveda MS. Toxicological studies on silver nanoparticles: challenges and opportunities in assessment, monitoring and imaging. Nanomed (London, Engl). 2011;6:879–98.10.2217/nnm.11.78Search in Google Scholar PubMed PubMed Central
[28] Ferdous Z, Nemmar A. Health impact of silver nanoparticles: a review of the biodistribution and toxicity following various routes of exposure. Int J Mol Sci. 2020;21:2375.10.3390/ijms21072375Search in Google Scholar PubMed PubMed Central
[29] Yang Y, Qin Z, Zeng W, Yang T, Cao Y, Mei C, et al. Toxicity assessment of nanoparticles in various systems and organs. Nanotechnol Rev. 2017;6:279–89.10.1515/ntrev-2016-0047Search in Google Scholar
[30] Chen L, Wu M, Jiang S, Zhang Y, Li R, Lu Y, et al. Skin toxicity assessment of silver nanoparticles in a 3D epidermal model compared to 2D keratinocytes. Int J Nanomed. 2019;14:9707–19.10.2147/IJN.S225451Search in Google Scholar PubMed PubMed Central
[31] Carvalho PM, Felício MR, Santos NC, Gonçalves S, Domingues MM. Application of light scattering techniques to nanoparticle characterization and development. Front Chem. 2018;6:237.10.3389/fchem.2018.00237Search in Google Scholar PubMed PubMed Central
[32] Malm AV, Corbett JCW. Improved dynamic light scattering using an adaptive and statistically driven time resolved treatment of correlation data. Sci Rep. 2019;9:13519.10.1038/s41598-019-50077-4Search in Google Scholar PubMed PubMed Central
[33] Wen X, Wang Q, Dai T, Shao J, Wu X, Jiang Z, et al. Identification of possible reductants in the aqueous leaf extract of mangrove plant Rhizophora apiculata for the fabrication and cytotoxicity of silver nanoparticles against human osteosarcoma MG-63 cells. Mater Sci Eng C. 2020;116:111252.10.1016/j.msec.2020.111252Search in Google Scholar PubMed
[34] Yingchoncharoen P, Kalinowski DS, Richardson DR. Lipid-based drug delivery systems in cancer therapy: what is available and what is yet to come. Pharmacol Rev. 2016;68:701–87.10.1124/pr.115.012070Search in Google Scholar PubMed PubMed Central
[35] Zhang X-F, Liu Z-G, Shen W, Gurunathan S. Silver nanoparticles: synthesis, characterization, properties, applications, and therapeutic approaches. Int J Mol Sci. 2016;17:1534.10.3390/ijms17091534Search in Google Scholar PubMed PubMed Central
[36] Holder CF, Schaak RE. Tutorial on powder X-ray diffraction for characterizing nanoscale materials. ACS Publications; 2019. p. 7359–65. 10.1021/acsnano.9b05157.Search in Google Scholar PubMed
[37] Ghalkhani M, Khosrowshahi EM, Sohouli E. Chapter 3 – Carbon nano-onions: Synthesis, characterization, and application. In: Thomas S, Sarathchandran C, Ilangovan SA, Moreno-Piraján JC, editors. Handbook of carbon-based nanomaterials. Elsevier; 2021. p. 159–207. 10.1016/C2019-0-03576-5.Search in Google Scholar
[38] Annamalai J, Nallamuthu T. Green synthesis of silver nanoparticles: characterization and determination of antibacterial potency. Appl Nanosci. 2016;6:259–65.10.1007/s13204-015-0426-6Search in Google Scholar PubMed PubMed Central
[39] Theivasanthi T, Alagar M. Electrolytic synthesis and characterizations of silver nanopowder. arXiv Prepr arXiv:11110260; 2011.10.5101/nbe.v4i2.p58-65Search in Google Scholar
[40] Arshad H, Sami MA, Sadaf S, Hassan U. Salvadora persica mediated synthesis of silver nanoparticles and their antimicrobial efficacy. Sci Rep. 2021;11:5996.10.1038/s41598-021-85584-wSearch in Google Scholar PubMed PubMed Central
[41] Janik H, Wrona M. Asbestos. In: Worsfold P, Poole C, Townshend A, Miró M, editors. Encyclopedia of analytical science. 3rd edn. Oxford: Academic Press; 2019. p. 107–16.Search in Google Scholar
[42] Kumar B, Smita K, Sánchez E, Debut A. Cumbal L. Phytosynthesis, characterization and catalytic activity of Sacha inchi leaf-assisted gold nanoparticles. Chem Pap. 2022;76:2855–64.10.1007/s11696-022-02075-6Search in Google Scholar
[43] Yu C, Tang J, Liu X, Ren X, Zhen M, Wang L. Green biosynthesis of silver nanoparticles using Eriobotrya japonica (Thunb.) leaf extract for reductive catalysis. Materials. 2019;12:189.10.3390/ma12010189Search in Google Scholar PubMed PubMed Central
[44] Akter S, Huq MA. Biologically rapid synthesis of silver nanoparticles by Sphingobium sp. MAH-11T and their antibacterial activity and mechanisms investigation against drug-resistant pathogenic microbes. Artif Cells Nanomed Biotechnol. 2020;48:672–82.10.1080/21691401.2020.1730390Search in Google Scholar PubMed
[45] Das D, Ghosh R, Mandal P. Biogenic synthesis of silver nanoparticles using S1 genotype of Morus alba leaf extract: characterization, antimicrobial and antioxidant potential assessment. SN Appl Sci. 2019;1:1–16.10.1007/s42452-019-0527-zSearch in Google Scholar
[46] Rodríguez-León E, Iñiguez-Palomares R, Navarro RE, Herrera-Urbina R, Tánori J, Iñiguez-Palomares C, et al. Synthesis of silver nanoparticles using reducing agents obtained from natural sources (Rumex hymenosepalus extracts). Nanoscale Res Lett. 2013;8:318.10.1186/1556-276X-8-318Search in Google Scholar PubMed PubMed Central
[47] Hofmeister H, Tan G, Dubiel M. Shape and internal structure of silver nanoparticles embedded in glass. J Mater Res. 2005;20:1551–62.10.1557/JMR.2005.0197Search in Google Scholar
[48] Huq M. Green synthesis of silver nanoparticles using Pseudoduganella eburnea MAHUQ-39 and their antimicrobial mechanisms investigation against drug resistant human pathogens. Int J Mol Sci. 2020;21:1510.10.3390/ijms21041510Search in Google Scholar PubMed PubMed Central
[49] Kulkarni RR, Shaiwale NS, Deobagkar DN, Deobagkar DD. Synthesis and extracellular accumulation of silver nanoparticles by employing radiation-resistant Deinococcus radiodurans, their characterization, and determination of bioactivity. Int J Nanomed. 2015;10:963–74.10.2147/IJN.S72888Search in Google Scholar PubMed PubMed Central
[50] Lee B, Yoon S, Lee JW, Kim Y, Chang J, Yun J, et al. Statistical characterization of the morphologies of nanoparticles through machine learning based electron microscopy image analysis. ACS Nano. 2020;14:17125–33.10.1021/acsnano.0c06809Search in Google Scholar PubMed
[51] Islam MS, Islam MM, Islam KN. The Effect of CaCO3 Nanoparticles and Chitosan on the Properties of PLA Based Biomaterials for Biomedical Applications. In: Hashmi S, Choudhury IA, editors. Encyclopedia of renewable and sustainable materials. Oxford: Elsevier; 2020. p. 736–45.10.1016/B978-0-12-803581-8.11576-0Search in Google Scholar
[52] Wen H, Luna-Romera JM, Riquelme JC, Dwyer C, Chang SL. Statistically representative metrology of nanoparticles via unsupervised machine learning of TEM Images. Nanomaterials. 2021;11:2706.10.3390/nano11102706Search in Google Scholar PubMed PubMed Central
[53] Danaei M, Dehghankhold M, Ataei S, Hasanzadeh Davarani F, Javanmard R, Dokhani A, et al. Impact of particle size and polydispersity index on the clinical applications of lipidic nanocarrier systems. Pharmaceutics. 2018;10:57.10.3390/pharmaceutics10020057Search in Google Scholar PubMed PubMed Central
[54] Chenthamara D, Subramaniam S, Ramakrishnan SG, Krishnaswamy S, Essa MM, Lin F-H, et al. Therapeutic efficacy of nanoparticles and routes of administration. Biomater Res. 2019;23:20.10.1186/s40824-019-0166-xSearch in Google Scholar PubMed PubMed Central
[55] Khan I, Saeed K, Khan I. Nanoparticles: Properties, applications and toxicities. Arab J Chem. 2019;12:908–31.10.1016/j.arabjc.2017.05.011Search in Google Scholar
[56] Blanco E, Shen H, Ferrari M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat Biotechnol. 2015;33:941–51.10.1201/9780429027819-9Search in Google Scholar
[57] Hoshyar N, Gray S, Han H, Bao G. The effect of nanoparticle size on in vivo pharmacokinetics and cellular interaction. Nanomed (Lond). 2016;11:673–92.10.2217/nnm.16.5Search in Google Scholar PubMed PubMed Central
[58] Agbabiaka A, Wiltfong M, Park C. Small angle X-ray scattering technique for the particle size distribution of nonporous nanoparticles. J Nanopart. 2013;2013:1–11.10.1155/2013/640436Search in Google Scholar
[59] Gencalp Irizalp S, Saklakoglu N. 1.14 Laser Peening of Metallic Materials. In: Hashmi MSJ. Comprehensive materials finishing. Oxford: Elsevier; 2017. p. 408–40.10.1016/B978-0-12-803581-8.09160-8Search in Google Scholar
[60] Pu Y, Niu Y, Wang Y, Liu S, Zhang B. Statistical morphological identification of low-dimensional nanomaterials by using TEM. Particuology. 2022;61:11–7.10.1016/j.partic.2021.03.013Search in Google Scholar
[61] Raval N, Maheshwari R, Kalyane D, Youngren-Ortiz SR, Chougule MB, Tekade RK. Chapter 10 – Importance of physicochemical characterization of nanoparticles in pharmaceutical product development. In: Tekade RK, editor. Basic fundamentals of drug delivery. Academic Press; 2019. p. 369–400.10.1016/B978-0-12-817909-3.00010-8Search in Google Scholar
[62] Kaasalainen M, Aseyev V, von Haartman E, Karaman DŞ, Mäkilä E, Tenhu H, et al. Size, stability, and porosity of mesoporous nanoparticles characterized with light scattering. Nanoscale Res Lett. 2017;12:1–10.10.1186/s11671-017-1853-ySearch in Google Scholar PubMed PubMed Central
[63] Zanwar AA, Badole SL, Shende PS, Hegde MV, Bodhankar SL. Chapter 80 – Role of gallic acid in cardiovascular disorders. In: Watson RR, Preedy VR, Zibadi S, editors. Polyphenols in human health and disease. San Diego: Academic Press; 2014. p. 1045–7.10.1016/B978-0-12-398456-2.00080-3Search in Google Scholar
[64] Daglia M, Di Lorenzo A, Nabavi SF, Talas ZS, Nabavi SM. Polyphenols: well beyond the antioxidant capacity: gallic acid and related compounds as neuroprotective agents: you are what you eat! Curr Pharm Biotechnol. 2014;15:362–72.10.2174/138920101504140825120737Search in Google Scholar PubMed
[65] Liu X, Wang J, Wang Y, Huang C, Wang Z, Liu L. In Situ Functionalization of Silver Nanoparticles by Gallic Acid as a Colorimetric Sensor for Simple Sensitive Determination of Melamine in Milk. ACS Omega. 2021;6:23630–5.10.1021/acsomega.1c03927Search in Google Scholar PubMed PubMed Central
[66] Al-Zahrani S, Astudillo-Calderón S, Pintos B, Pérez-Urria E, Manzanera JA, Martín L, et al. Role of synthetic plant extracts on the production of silver-derived nanoparticles. Plants. 2021;10:1671.10.3390/plants10081671Search in Google Scholar PubMed PubMed Central
[67] Jena S, Singh RK, Panigrahi B, Suar M, Mandal D. Photo-bioreduction of Ag+ ions towards the generation of multifunctional silver nanoparticles: mechanistic perspective and therapeutic potential. J Photochem Photobiol B Biol. 2016;164:306–13.10.1016/j.jphotobiol.2016.08.048Search in Google Scholar PubMed
[68] Jain S, Mehata MS. Medicinal plant leaf extract and pure flavonoid mediated green synthesis of silver nanoparticles and their enhanced antibacterial property. Sci Rep. 2017;7:1–13.10.1038/s41598-017-15724-8Search in Google Scholar PubMed PubMed Central
[69] Makarov VV, Love AJ, Sinitsyna OV, Makarova SS, Yaminsky IV, Taliansky ME, et al. “Green” nanotechnologies: synthesis of metal nanoparticles using plants. Acta Nat. 2014;6:6–44.10.32607/20758251-2014-6-1-35-44Search in Google Scholar
[70] Kovács D, Igaz N, Gopisetty MK, Kiricsi M. Cancer therapy by silver nanoparticles: fiction or reality? Int J Mol Sci. 2022;23:839.10.3390/ijms23020839Search in Google Scholar PubMed PubMed Central
[71] Tao L, Chen X, Sun J, Wu C. Silver nanoparticles achieve cytotoxicity against breast cancer by regulating long-chain noncoding RNA XLOC_006390-mediated pathway. Toxicol Res. 2021;10:123–33.10.1093/toxres/tfaa090Search in Google Scholar PubMed PubMed Central
[72] Al-Khedhairy AA, Wahab R. Silver nanoparticles: an instantaneous solution for anticancer activity against human liver (HepG2) and breast (MCF-7) cancer cells. Metals. 2022;12:148.10.3390/met12010148Search in Google Scholar
[73] Kumar N, Afjei R, Massoud TF, Paulmurugan R. Comparison of cell-based assays to quantify treatment effects of anticancer drugs identifies a new application for Bodipy-L-cystine to measure apoptosis. Sci Rep. 2018;8:16363.10.1038/s41598-018-34696-xSearch in Google Scholar PubMed PubMed Central
[74] Larsson P, Engqvist H, Biermann J, Werner Rönnerman E, Forssell-Aronsson E, Kovács A, et al. Optimization of cell viability assays to improve replicability and reproducibility of cancer drug sensitivity screens. Sci Rep. 2020;10:5798.10.1038/s41598-020-62848-5Search in Google Scholar PubMed PubMed Central
[75] Liu X, Shan K, Shao X, Shi X, He Y, Liu Z, et al. Nanotoxic effects of silver nanoparticles on normal hek-293 cells in comparison to cancerous HeLa cell line. Int J Nanomed. 2021;16:753–61.10.2147/IJN.S289008Search in Google Scholar PubMed PubMed Central
[76] Ahmadi S, Mohammadi L, Rahdar A, Rahdar S, Dehghani R, Igwegbe CA, et al. Acid dye removal from aqueous solution by using neodymium(iii) oxide nanoadsorbents. Nanomaterials (Basel). 2020;10:556.10.3390/nano10030556Search in Google Scholar PubMed PubMed Central
[77] Sandhya M, Rajkumar K, Burgula S. Efficient eco-friendly approach towards bimetallic nanoparticles synthesis and characterization using Exiguobacterium aestuarii by statistical optimization. Green Chem Lett Rev. 2019;12:420–34.10.1080/17518253.2019.1687762Search in Google Scholar
[78] Saunders LJ, Russell RA, Crabb DP. The coefficient of determination: what determines a useful R2 statistic? Investig Ophthalmol & Vis Sci. 2012;53:6830–2.10.1167/iovs.12-10598Search in Google Scholar PubMed
[79] Tortella GR, Rubilar O, Durán N, Diez MC, Martínez M, Parada J, et al. Silver nanoparticles: Toxicity in model organisms as an overview of its hazard for human health and the environment. J Hazard Mater. 2020;390:121974.10.1016/j.jhazmat.2019.121974Search in Google Scholar PubMed
[80] Paciorek P, Żuberek M, Grzelak A. Products of lipid peroxidation as a factor in the toxic effect of silver nanoparticles. Materials. 2020;13:2460.10.3390/ma13112460Search in Google Scholar PubMed PubMed Central
[81] Rohde MM, Snyder CM, Sloop J, Solst SR, Donati GL, Spitz DR, et al. The mechanism of cell death induced by silver nanoparticles is distinct from silver cations. Particle Fibre Toxicol. 2021;18:37.10.1186/s12989-021-00430-1Search in Google Scholar PubMed PubMed Central
[82] Gliga AR, Skoglund S, Odnevall Wallinder I, Fadeel B, Karlsson HL. Size-dependent cytotoxicity of silver nanoparticles in human lung cells: the role of cellular uptake, agglomeration and Ag release. Part Fibre Toxicol. 2014;11:11.10.1186/1743-8977-11-11Search in Google Scholar PubMed PubMed Central
[83] Zhang T, Wang L, Chen Q, Chen C. Cytotoxic potential of silver nanoparticles. Yonsei Med J. 2014;55:283–91.10.3349/ymj.2014.55.2.283Search in Google Scholar PubMed PubMed Central
[84] McShan D, Ray PC, Yu H. Molecular toxicity mechanism of nanosilver. J Food Drug Anal. 2014;22:116–27.10.1016/j.jfda.2014.01.010Search in Google Scholar
[85] Hoy MA. Chapter 8 – DNA amplification by the polymerase chain reaction: molecular biology made accessible. In: Hoy MA, editor. Insect molecular genetics. 3rd edn. San Diego: Academic Press; 2013. p. 307–72.10.1016/B978-0-12-415874-0.00008-1Search in Google Scholar
[86] Lobert S, Hiser L, Correia JJ. Chapter 4 – Expression profiling of tubulin isotypes and microtubule-interacting proteins using real-time polymerase chain reaction. In: Wilson L, Correia JJ, editors. Methods in cell biology. Academic Press; 2010. p. 47–58. 10.1016/S0091-679X(10)95004-8.Search in Google Scholar
[87] Ozturk M, Ozsoylemez OD, Dagistanli FK. The detection techniques for autophagy-associated cell death-related genes and proteins: gene expression assay and immunohistochemistry. Methods Mol Biol (Clifton, NJ). 2019;1854:119–30.10.1007/7651_2017_67Search in Google Scholar PubMed
[88] Amatori S, Persico G, Fanelli M. Real-time quantitative PCR array to study drug-induced changes of gene expression in tumor cell lines. J Cancer Metastasis Treat. 2017;3:90–9.10.20517/2394-4722.2017.22Search in Google Scholar
[89] Stupack DG. Caspase-8 as a therapeutic target in cancer. Cancer Lett. 2013;332:133–40.10.1016/j.canlet.2010.07.022Search in Google Scholar PubMed PubMed Central
[90] Fianco G, Contadini C, Ferri A, Cirotti C, Stagni V, Barilà D. Caspase-8: A Novel Target to Overcome Resistance to Chemotherapy in Glioblastoma. Int J Mol Sci. 2018;19:3798.10.3390/ijms19123798Search in Google Scholar PubMed PubMed Central
[91] Zhao Y, Zhu Q, Bu X, Zhou Y, Bai D, Guo Q, et al. Triggering apoptosis by oroxylin A through caspase-8 activation and p62/SQSTM1 proteolysis. Redox Biol. 2020;29:101392.10.1016/j.redox.2019.101392Search in Google Scholar PubMed PubMed Central
[92] Fritsch M, Günther SD, Schwarzer R, Albert M-C, Schorn F, Werthenbach JP, et al. Caspase-8 is the molecular switch for apoptosis, necroptosis and pyroptosis. Nature. 2019;575:683–7.10.1038/s41586-019-1770-6Search in Google Scholar PubMed
[93] Kara M, Oztas E. Endoplasmic reticulum stress-mediated cell death. Program cell death. IntechOpen; 2019. 10.5772/intechopen.85401.Search in Google Scholar
[94] Amaral JD, Xavier JM, Steer CJ, Rodrigues CM. The role of p53 in apoptosis. Discovery Med. 2010;9:145–52.Search in Google Scholar
[95] Vaseva AV, Moll UM. The mitochondrial p53 pathway. Biochim Biophys Acta. 2009;1787:414–20.10.1016/j.bbabio.2008.10.005Search in Google Scholar
[96] Katiyar SK, Roy AM, Baliga MS. Silymarin induces apoptosis primarily through a p53-dependent pathway involving Bcl-2/Bax, cytochrome c release, and caspase activation. Mol Cancer Therapeutics. 2005;4:207–16.10.1158/1535-7163.207.4.2Search in Google Scholar
[97] Cai J, Yang J, Jones D. Mitochondrial control of apoptosis: the role of cytochrome c. Biochim Biophys Acta (BBA) – Bioenerg. 1998;1366:139–49.10.1016/S0005-2728(98)00109-1Search in Google Scholar
[98] Yuan J, Murrell GAC, Trickett A, Wang M-X. Involvement of cytochrome c release and caspase-3 activation in the oxidative stress-induced apoptosis in human tendon fibroblasts. Biochim et Biophys Acta (BBA) – Mol Cell Res. 2003;1641:35–41.10.1016/S0167-4889(03)00047-8Search in Google Scholar
[99] Hientz K, Mohr A, Bhakta-Guha D, Efferth T. The role of p53 in cancer drug resistance and targeted chemotherapy. Oncotarget. 2017;8:8921–46.10.18632/oncotarget.13475Search in Google Scholar PubMed PubMed Central
[100] Marquez RT, Xu L. Bcl-2:Beclin 1 complex: multiple, mechanisms regulating autophagy/apoptosis toggle switch. Am J Cancer Res. 2012;2:214–21.Search in Google Scholar
[101] Pfeffer CM, Singh ATK. Apoptosis: A Target for Anticancer Therapy. Int J Mol Sci. 2018;19:448.10.3390/ijms19020448Search in Google Scholar PubMed PubMed Central
[102] Campbell KJ, Tait SWG. Targeting BCL-2 regulated apoptosis in cancer. Open Biol. 2018;8:180002.10.1098/rsob.180002Search in Google Scholar PubMed PubMed Central
[103] D'Aguanno S, Del, Bufalo D. Inhibition of anti-apoptotic bcl-2 proteins in preclinical and clinical studies: current overview in. Cancer Cell. 2020;9:1287.10.3390/cells9051287Search in Google Scholar PubMed PubMed Central
[104] Bravo R, Parra V, Gatica D, Rodriguez AE, Torrealba N, Paredes F, et al. Chapter Five – Endoplasmic reticulum and the unfolded protein response: dynamics and metabolic integration. In: Jeon KW, editor. international review of cell and molecular biology. Academic Press; 2013. p. 215–90. 10.1016/B978-0-12-407704-1.00005-1.Search in Google Scholar PubMed PubMed Central
[105] Al-Sheddi ES, Farshori NN, Al-Oqail MM, Al-Massarani SM, Saquib Q, Wahab R, et al. Anticancer potential of green synthesized silver nanoparticles using extract of nepeta deflersiana against human cervical cancer cells (HeLA). Bioinorganic Chem Appl. 2018;2018:2018.10.1155/2018/9390784Search in Google Scholar PubMed PubMed Central
[106] Redza-Dutordoir M, Averill-Bates DA. Activation of apoptosis signalling pathways by reactive oxygen species. Biochim Biophys Acta (BBA) – Mol Cell Res. 2016;1863:2977–92.10.1016/j.bbamcr.2016.09.012Search in Google Scholar PubMed
[107] Rodriguez J, Lazebnik Y. Caspase-9 and APAF-1 form an active holoenzyme. Genes & Dev. 1999;13:3179–84.10.1101/gad.13.24.3179Search in Google Scholar PubMed PubMed Central
[108] Parrish AB, Freel CD, Kornbluth S. Cellular mechanisms controlling caspase activation and function. Cold Spring Harb Perspect Biol. 2013;5:a008672.10.1101/cshperspect.a008672Search in Google Scholar PubMed PubMed Central
[109] Kantari C, Walczak H. Caspase-8 and Bid: Caught in the act between death receptors and mitochondria. Biochim Biophys Acta (BBA) – Mol Cell Res. 2011;1813:558–63.10.1016/j.bbamcr.2011.01.026Search in Google Scholar PubMed
[110] Huang X, Qi Q, Hua X, Li X, Zhang W, Sun H, et al. Beclin 1, an autophagy-related gene, augments apoptosis in U87 glioblastoma cells. Oncol Rep. 2014;31:1761–7.10.3892/or.2014.3015Search in Google Scholar PubMed
[111] Grishchuk Y, Ginet V, Truttmann AC, Clarke PG, Puyal J. Beclin 1-independent autophagy contributes to apoptosis in cortical neurons. Autophagy. 2011;7:1115–31.10.4161/auto.7.10.16608Search in Google Scholar PubMed
[112] Bello-Perez M, Sola I, Novoa B, Klionsky DJ, Falco A. Canonical and noncanonical autophagy as potential targets for COVID-19. Cells. 2020;9:1619.10.3390/cells9071619Search in Google Scholar PubMed PubMed Central
[113] Murrow L, Malhotra R, Debnath J. ATG12–ATG3 interacts with Alix to promote basal autophagic flux and late endosome function. Nat Cell Biol. 2015;17:300–10.10.1038/ncb3112Search in Google Scholar PubMed PubMed Central
[114] Dupont N, Codogno P. Non-canonical autophagy: facts and prospects. Curr Pathobiology Rep. 2013;1:263–71.10.1007/s40139-013-0030-ySearch in Google Scholar
[115] Codogno P, Mehrpour M, Proikas-Cezanne T. Canonical and non-canonical autophagy: variations on a common theme of self-eating? Nat Rev Mol Cell Biol. 2012;13:7–12.10.1038/nrm3249Search in Google Scholar PubMed
[116] Kabir MF, Kim H-R, Chae H-J. Endoplasmic reticulum stress and autophagy. Endoplasmic reticulum intechopen. IntechOpen; 2018. 10.5772/intechopen.81381.Search in Google Scholar
[117] Shajahan AN, Riggins RB, Clarke R. The role of X-box binding protein-1 in tumorigenicity. Drug N Perspect. 2009;22:241–6.10.1358/dnp.2009.22.5.1378631Search in Google Scholar PubMed PubMed Central
[118] Chen S, Chen J, Hua X, Sun Y, Cui R, Sha J, et al. The emerging role of XBP1 in cancer. Biomed Pharmacotherapy. 2020;127:110069.10.1016/j.biopha.2020.110069Search in Google Scholar PubMed
[119] Chen Q, Kang J, Fu C. The independence of and associations among apoptosis, autophagy, and necrosis. Signal Transduct Target Ther. 2018;3:18.10.1038/s41392-018-0018-5Search in Google Scholar PubMed PubMed Central
© 2022 Jun Bao et al., published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Research Articles
- Theoretical and experimental investigation of MWCNT dispersion effect on the elastic modulus of flexible PDMS/MWCNT nanocomposites
- Mechanical, morphological, and fracture-deformation behavior of MWCNTs-reinforced (Al–Cu–Mg–T351) alloy cast nanocomposites fabricated by optimized mechanical milling and powder metallurgy techniques
- Flammability and physical stability of sugar palm crystalline nanocellulose reinforced thermoplastic sugar palm starch/poly(lactic acid) blend bionanocomposites
- Glutathione-loaded non-ionic surfactant niosomes: A new approach to improve oral bioavailability and hepatoprotective efficacy of glutathione
- Relationship between mechano-bactericidal activity and nanoblades density on chemically strengthened glass
- In situ regulation of microstructure and microwave-absorbing properties of FeSiAl through HNO3 oxidation
- Research on a mechanical model of magnetorheological fluid different diameter particles
- Nanomechanical and dynamic mechanical properties of rubber–wood–plastic composites
- Investigative properties of CeO2 doped with niobium: A combined characterization and DFT studies
- Miniaturized peptidomimetics and nano-vesiculation in endothelin types through probable nano-disk formation and structure property relationships of endothelins’ fragments
- N/S co-doped CoSe/C nanocubes as anode materials for Li-ion batteries
- Synergistic effects of halloysite nanotubes with metal and phosphorus additives on the optimal design of eco-friendly sandwich panels with maximum flame resistance and minimum weight
- Octreotide-conjugated silver nanoparticles for active targeting of somatostatin receptors and their application in a nebulized rat model
- Controllable morphology of Bi2S3 nanostructures formed via hydrothermal vulcanization of Bi2O3 thin-film layer and their photoelectrocatalytic performances
- Development of (−)-epigallocatechin-3-gallate-loaded folate receptor-targeted nanoparticles for prostate cancer treatment
- Enhancement of the mechanical properties of HDPE mineral nanocomposites by filler particles modulation of the matrix plastic/elastic behavior
- Effect of plasticizers on the properties of sugar palm nanocellulose/cinnamon essential oil reinforced starch bionanocomposite films
- Optimization of nano coating to reduce the thermal deformation of ball screws
- Preparation of efficient piezoelectric PVDF–HFP/Ni composite films by high electric field poling
- MHD dissipative Casson nanofluid liquid film flow due to an unsteady stretching sheet with radiation influence and slip velocity phenomenon
- Effects of nano-SiO2 modification on rubberised mortar and concrete with recycled coarse aggregates
- Mechanical and microscopic properties of fiber-reinforced coal gangue-based geopolymer concrete
- Effect of morphology and size on the thermodynamic stability of cerium oxide nanoparticles: Experiment and molecular dynamics calculation
- Mechanical performance of a CFRP composite reinforced via gelatin-CNTs: A study on fiber interfacial enhancement and matrix enhancement
- A practical review over surface modification, nanopatterns, emerging materials, drug delivery systems, and their biophysiochemical properties for dental implants: Recent progresses and advances
- HTR: An ultra-high speed algorithm for cage recognition of clathrate hydrates
- Effects of microalloying elements added by in situ synthesis on the microstructure of WCu composites
- A highly sensitive nanobiosensor based on aptamer-conjugated graphene-decorated rhodium nanoparticles for detection of HER2-positive circulating tumor cells
- Progressive collapse performance of shear strengthened RC frames by nano CFRP
- Core–shell heterostructured composites of carbon nanotubes and imine-linked hyperbranched polymers as metal-free Li-ion anodes
- A Galerkin strategy for tri-hybridized mixture in ethylene glycol comprising variable diffusion and thermal conductivity using non-Fourier’s theory
- Simple models for tensile modulus of shape memory polymer nanocomposites at ambient temperature
- Preparation and morphological studies of tin sulfide nanoparticles and use as efficient photocatalysts for the degradation of rhodamine B and phenol
- Polyethyleneimine-impregnated activated carbon nanofiber composited graphene-derived rice husk char for efficient post-combustion CO2 capture
- Electrospun nanofibers of Co3O4 nanocrystals encapsulated in cyclized-polyacrylonitrile for lithium storage
- Pitting corrosion induced on high-strength high carbon steel wire in high alkaline deaerated chloride electrolyte
- Formulation of polymeric nanoparticles loaded sorafenib; evaluation of cytotoxicity, molecular evaluation, and gene expression studies in lung and breast cancer cell lines
- Engineered nanocomposites in asphalt binders
- Influence of loading voltage, domain ratio, and additional load on the actuation of dielectric elastomer
- Thermally induced hex-graphene transitions in 2D carbon crystals
- The surface modification effect on the interfacial properties of glass fiber-reinforced epoxy: A molecular dynamics study
- Molecular dynamics study of deformation mechanism of interfacial microzone of Cu/Al2Cu/Al composites under tension
- Nanocolloid simulators of luminescent solar concentrator photovoltaic windows
- Compressive strength and anti-chloride ion penetration assessment of geopolymer mortar merging PVA fiber and nano-SiO2 using RBF–BP composite neural network
- Effect of 3-mercapto-1-propane sulfonate sulfonic acid and polyvinylpyrrolidone on the growth of cobalt pillar by electrodeposition
- Dynamics of convective slippery constraints on hybrid radiative Sutterby nanofluid flow by Galerkin finite element simulation
- Preparation of vanadium by the magnesiothermic self-propagating reduction and process control
- Microstructure-dependent photoelectrocatalytic activity of heterogeneous ZnO–ZnS nanosheets
- Cytotoxic and pro-inflammatory effects of molybdenum and tungsten disulphide on human bronchial cells
- Improving recycled aggregate concrete by compression casting and nano-silica
- Chemically reactive Maxwell nanoliquid flow by a stretching surface in the frames of Newtonian heating, nonlinear convection and radiative flux: Nanopolymer flow processing simulation
- Nonlinear dynamic and crack behaviors of carbon nanotubes-reinforced composites with various geometries
- Biosynthesis of copper oxide nanoparticles and its therapeutic efficacy against colon cancer
- Synthesis and characterization of smart stimuli-responsive herbal drug-encapsulated nanoniosome particles for efficient treatment of breast cancer
- Homotopic simulation for heat transport phenomenon of the Burgers nanofluids flow over a stretching cylinder with thermal convective and zero mass flux conditions
- Incorporation of copper and strontium ions in TiO2 nanotubes via dopamine to enhance hemocompatibility and cytocompatibility
- Mechanical, thermal, and barrier properties of starch films incorporated with chitosan nanoparticles
- Mechanical properties and microstructure of nano-strengthened recycled aggregate concrete
- Glucose-responsive nanogels efficiently maintain the stability and activity of therapeutic enzymes
- Tunning matrix rheology and mechanical performance of ultra-high performance concrete using cellulose nanofibers
- Flexible MXene/copper/cellulose nanofiber heat spreader films with enhanced thermal conductivity
- Promoted charge separation and specific surface area via interlacing of N-doped titanium dioxide nanotubes on carbon nitride nanosheets for photocatalytic degradation of Rhodamine B
- Elucidating the role of silicon dioxide and titanium dioxide nanoparticles in mitigating the disease of the eggplant caused by Phomopsis vexans, Ralstonia solanacearum, and root-knot nematode Meloidogyne incognita
- An implication of magnetic dipole in Carreau Yasuda liquid influenced by engine oil using ternary hybrid nanomaterial
- Robust synthesis of a composite phase of copper vanadium oxide with enhanced performance for durable aqueous Zn-ion batteries
- Tunning self-assembled phases of bovine serum albumin via hydrothermal process to synthesize novel functional hydrogel for skin protection against UVB
- A comparative experimental study on damping properties of epoxy nanocomposite beams reinforced with carbon nanotubes and graphene nanoplatelets
- Lightweight and hydrophobic Ni/GO/PVA composite aerogels for ultrahigh performance electromagnetic interference shielding
- Research on the auxetic behavior and mechanical properties of periodically rotating graphene nanostructures
- Repairing performances of novel cement mortar modified with graphene oxide and polyacrylate polymer
- Closed-loop recycling and fabrication of hydrophilic CNT films with high performance
- Design of thin-film configuration of SnO2–Ag2O composites for NO2 gas-sensing applications
- Study on stress distribution of SiC/Al composites based on microstructure models with microns and nanoparticles
- PVDF green nanofibers as potential carriers for improving self-healing and mechanical properties of carbon fiber/epoxy prepregs
- Osteogenesis capability of three-dimensionally printed poly(lactic acid)-halloysite nanotube scaffolds containing strontium ranelate
- Silver nanoparticles induce mitochondria-dependent apoptosis and late non-canonical autophagy in HT-29 colon cancer cells
- Preparation and bonding mechanisms of polymer/metal hybrid composite by nano molding technology
- Damage self-sensing and strain monitoring of glass-reinforced epoxy composite impregnated with graphene nanoplatelet and multiwalled carbon nanotubes
- Thermal analysis characterisation of solar-powered ship using Oldroyd hybrid nanofluids in parabolic trough solar collector: An optimal thermal application
- Pyrene-functionalized halloysite nanotubes for simultaneously detecting and separating Hg(ii) in aqueous media: A comprehensive comparison on interparticle and intraparticle excimers
- Fabrication of self-assembly CNT flexible film and its piezoresistive sensing behaviors
- Thermal valuation and entropy inspection of second-grade nanoscale fluid flow over a stretching surface by applying Koo–Kleinstreuer–Li relation
- Mechanical properties and microstructure of nano-SiO2 and basalt-fiber-reinforced recycled aggregate concrete
- Characterization and tribology performance of polyaniline-coated nanodiamond lubricant additives
- Combined impact of Marangoni convection and thermophoretic particle deposition on chemically reactive transport of nanofluid flow over a stretching surface
- Spark plasma extrusion of binder free hydroxyapatite powder
- An investigation on thermo-mechanical performance of graphene-oxide-reinforced shape memory polymer
- Effect of nanoadditives on the novel leather fiber/recycled poly(ethylene-vinyl-acetate) polymer composites for multifunctional applications: Fabrication, characterizations, and multiobjective optimization using central composite design
- Design selection for a hemispherical dimple core sandwich panel using hybrid multi-criteria decision-making methods
- Improving tensile strength and impact toughness of plasticized poly(lactic acid) biocomposites by incorporating nanofibrillated cellulose
- Green synthesis of spinel copper ferrite (CuFe2O4) nanoparticles and their toxicity
- The effect of TaC and NbC hybrid and mono-nanoparticles on AA2024 nanocomposites: Microstructure, strengthening, and artificial aging
- Excited-state geometry relaxation of pyrene-modified cellulose nanocrystals under UV-light excitation for detecting Fe3+
- Effect of CNTs and MEA on the creep of face-slab concrete at an early age
- Effect of deformation conditions on compression phase transformation of AZ31
- Application of MXene as a new generation of highly conductive coating materials for electromembrane-surrounded solid-phase microextraction
- A comparative study of the elasto-plastic properties for ceramic nanocomposites filled by graphene or graphene oxide nanoplates
- Encapsulation strategies for improving the biological behavior of CdS@ZIF-8 nanocomposites
- Biosynthesis of ZnO NPs from pumpkin seeds’ extract and elucidation of its anticancer potential against breast cancer
- Preliminary trials of the gold nanoparticles conjugated chrysin: An assessment of anti-oxidant, anti-microbial, and in vitro cytotoxic activities of a nanoformulated flavonoid
- Effect of micron-scale pores increased by nano-SiO2 sol modification on the strength of cement mortar
- Fractional simulations for thermal flow of hybrid nanofluid with aluminum oxide and titanium oxide nanoparticles with water and blood base fluids
- The effect of graphene nano-powder on the viscosity of water: An experimental study and artificial neural network modeling
- Development of a novel heat- and shear-resistant nano-silica gelling agent
- Characterization, biocompatibility and in vivo of nominal MnO2-containing wollastonite glass-ceramic
- Entropy production simulation of second-grade magnetic nanomaterials flowing across an expanding surface with viscidness dissipative flux
- Enhancement in structural, morphological, and optical properties of copper oxide for optoelectronic device applications
- Aptamer-functionalized chitosan-coated gold nanoparticle complex as a suitable targeted drug carrier for improved breast cancer treatment
- Performance and overall evaluation of nano-alumina-modified asphalt mixture
- Analysis of pure nanofluid (GO/engine oil) and hybrid nanofluid (GO–Fe3O4/engine oil): Novel thermal and magnetic features
- Synthesis of Ag@AgCl modified anatase/rutile/brookite mixed phase TiO2 and their photocatalytic property
- Mechanisms and influential variables on the abrasion resistance hydraulic concrete
- Synergistic reinforcement mechanism of basalt fiber/cellulose nanocrystals/polypropylene composites
- Achieving excellent oxidation resistance and mechanical properties of TiB2–B4C/carbon aerogel composites by quick-gelation and mechanical mixing
- Microwave-assisted sol–gel template-free synthesis and characterization of silica nanoparticles obtained from South African coal fly ash
- Pulsed laser-assisted synthesis of nano nickel(ii) oxide-anchored graphitic carbon nitride: Characterizations and their potential antibacterial/anti-biofilm applications
- Effects of nano-ZrSi2 on thermal stability of phenolic resin and thermal reusability of quartz–phenolic composites
- Benzaldehyde derivatives on tin electroplating as corrosion resistance for fabricating copper circuit
- Mechanical and heat transfer properties of 4D-printed shape memory graphene oxide/epoxy acrylate composites
- Coupling the vanadium-induced amorphous/crystalline NiFe2O4 with phosphide heterojunction toward active oxygen evolution reaction catalysts
- Graphene-oxide-reinforced cement composites mechanical and microstructural characteristics at elevated temperatures
- Gray correlation analysis of factors influencing compressive strength and durability of nano-SiO2 and PVA fiber reinforced geopolymer mortar
- Preparation of layered gradient Cu–Cr–Ti alloy with excellent mechanical properties, thermal stability, and electrical conductivity
- Recovery of Cr from chrome-containing leather wastes to develop aluminum-based composite material along with Al2O3 ceramic particles: An ingenious approach
- Mechanisms of the improved stiffness of flexible polymers under impact loading
- Anticancer potential of gold nanoparticles (AuNPs) using a battery of in vitro tests
- Review Articles
- Proposed approaches for coronaviruses elimination from wastewater: Membrane techniques and nanotechnology solutions
- Application of Pickering emulsion in oil drilling and production
- The contribution of microfluidics to the fight against tuberculosis
- Graphene-based biosensors for disease theranostics: Development, applications, and recent advancements
- Synthesis and encapsulation of iron oxide nanorods for application in magnetic hyperthermia and photothermal therapy
- Contemporary nano-architectured drugs and leads for ανβ3 integrin-based chemotherapy: Rationale and retrospect
- State-of-the-art review of fabrication, application, and mechanical properties of functionally graded porous nanocomposite materials
- Insights on magnetic spinel ferrites for targeted drug delivery and hyperthermia applications
- A review on heterogeneous oxidation of acetaminophen based on micro and nanoparticles catalyzed by different activators
- Early diagnosis of lung cancer using magnetic nanoparticles-integrated systems
- Advances in ZnO: Manipulation of defects for enhancing their technological potentials
- Efficacious nanomedicine track toward combating COVID-19
- A review of the design, processes, and properties of Mg-based composites
- Green synthesis of nanoparticles for varied applications: Green renewable resources and energy-efficient synthetic routes
- Two-dimensional nanomaterial-based polymer composites: Fundamentals and applications
- Recent progress and challenges in plasmonic nanomaterials
- Apoptotic cell-derived micro/nanosized extracellular vesicles in tissue regeneration
- Electronic noses based on metal oxide nanowires: A review
- Framework materials for supercapacitors
- An overview on the reproductive toxicity of graphene derivatives: Highlighting the importance
- Antibacterial nanomaterials: Upcoming hope to overcome antibiotic resistance crisis
- Research progress of carbon materials in the field of three-dimensional printing polymer nanocomposites
- A review of atomic layer deposition modelling and simulation methodologies: Density functional theory and molecular dynamics
- Recent advances in the preparation of PVDF-based piezoelectric materials
- Recent developments in tensile properties of friction welding of carbon fiber-reinforced composite: A review
- Comprehensive review of the properties of fly ash-based geopolymer with additive of nano-SiO2
- Perspectives in biopolymer/graphene-based composite application: Advances, challenges, and recommendations
- Graphene-based nanocomposite using new modeling molecular dynamic simulations for proposed neutralizing mechanism and real-time sensing of COVID-19
- Nanotechnology application on bamboo materials: A review
- Recent developments and future perspectives of biorenewable nanocomposites for advanced applications
- Nanostructured lipid carrier system: A compendium of their formulation development approaches, optimization strategies by quality by design, and recent applications in drug delivery
- 3D printing customized design of human bone tissue implant and its application
- Design, preparation, and functionalization of nanobiomaterials for enhanced efficacy in current and future biomedical applications
- A brief review of nanoparticles-doped PEDOT:PSS nanocomposite for OLED and OPV
- Nanotechnology interventions as a putative tool for the treatment of dental afflictions
- Recent advancements in metal–organic frameworks integrating quantum dots (QDs@MOF) and their potential applications
- A focused review of short electrospun nanofiber preparation techniques for composite reinforcement
- Microstructural characteristics and nano-modification of interfacial transition zone in concrete: A review
- Latest developments in the upconversion nanotechnology for the rapid detection of food safety: A review
- Strategic applications of nano-fertilizers for sustainable agriculture: Benefits and bottlenecks
- Molecular dynamics application of cocrystal energetic materials: A review
- Synthesis and application of nanometer hydroxyapatite in biomedicine
- Cutting-edge development in waste-recycled nanomaterials for energy storage and conversion applications
- Biological applications of ternary quantum dots: A review
- Nanotherapeutics for hydrogen sulfide-involved treatment: An emerging approach for cancer therapy
- Application of antibacterial nanoparticles in orthodontic materials
- Effect of natural-based biological hydrogels combined with growth factors on skin wound healing
- Nanozymes – A route to overcome microbial resistance: A viewpoint
- Recent developments and applications of smart nanoparticles in biomedicine
- Contemporary review on carbon nanotube (CNT) composites and their impact on multifarious applications
- Interfacial interactions and reinforcing mechanisms of cellulose and chitin nanomaterials and starch derivatives for cement and concrete strength and durability enhancement: A review
- Diamond-like carbon films for tribological modification of rubber
- Layered double hydroxides (LDHs) modified cement-based materials: A systematic review
- Recent research progress and advanced applications of silica/polymer nanocomposites
- Modeling of supramolecular biopolymers: Leading the in silico revolution of tissue engineering and nanomedicine
- Recent advances in perovskites-based optoelectronics
- Biogenic synthesis of palladium nanoparticles: New production methods and applications
- A comprehensive review of nanofluids with fractional derivatives: Modeling and application
- Electrospinning of marine polysaccharides: Processing and chemical aspects, challenges, and future prospects
- Electrohydrodynamic printing for demanding devices: A review of processing and applications
- Rapid Communications
- Structural material with designed thermal twist for a simple actuation
- Recent advances in photothermal materials for solar-driven crude oil adsorption
Articles in the same Issue
- Research Articles
- Theoretical and experimental investigation of MWCNT dispersion effect on the elastic modulus of flexible PDMS/MWCNT nanocomposites
- Mechanical, morphological, and fracture-deformation behavior of MWCNTs-reinforced (Al–Cu–Mg–T351) alloy cast nanocomposites fabricated by optimized mechanical milling and powder metallurgy techniques
- Flammability and physical stability of sugar palm crystalline nanocellulose reinforced thermoplastic sugar palm starch/poly(lactic acid) blend bionanocomposites
- Glutathione-loaded non-ionic surfactant niosomes: A new approach to improve oral bioavailability and hepatoprotective efficacy of glutathione
- Relationship between mechano-bactericidal activity and nanoblades density on chemically strengthened glass
- In situ regulation of microstructure and microwave-absorbing properties of FeSiAl through HNO3 oxidation
- Research on a mechanical model of magnetorheological fluid different diameter particles
- Nanomechanical and dynamic mechanical properties of rubber–wood–plastic composites
- Investigative properties of CeO2 doped with niobium: A combined characterization and DFT studies
- Miniaturized peptidomimetics and nano-vesiculation in endothelin types through probable nano-disk formation and structure property relationships of endothelins’ fragments
- N/S co-doped CoSe/C nanocubes as anode materials for Li-ion batteries
- Synergistic effects of halloysite nanotubes with metal and phosphorus additives on the optimal design of eco-friendly sandwich panels with maximum flame resistance and minimum weight
- Octreotide-conjugated silver nanoparticles for active targeting of somatostatin receptors and their application in a nebulized rat model
- Controllable morphology of Bi2S3 nanostructures formed via hydrothermal vulcanization of Bi2O3 thin-film layer and their photoelectrocatalytic performances
- Development of (−)-epigallocatechin-3-gallate-loaded folate receptor-targeted nanoparticles for prostate cancer treatment
- Enhancement of the mechanical properties of HDPE mineral nanocomposites by filler particles modulation of the matrix plastic/elastic behavior
- Effect of plasticizers on the properties of sugar palm nanocellulose/cinnamon essential oil reinforced starch bionanocomposite films
- Optimization of nano coating to reduce the thermal deformation of ball screws
- Preparation of efficient piezoelectric PVDF–HFP/Ni composite films by high electric field poling
- MHD dissipative Casson nanofluid liquid film flow due to an unsteady stretching sheet with radiation influence and slip velocity phenomenon
- Effects of nano-SiO2 modification on rubberised mortar and concrete with recycled coarse aggregates
- Mechanical and microscopic properties of fiber-reinforced coal gangue-based geopolymer concrete
- Effect of morphology and size on the thermodynamic stability of cerium oxide nanoparticles: Experiment and molecular dynamics calculation
- Mechanical performance of a CFRP composite reinforced via gelatin-CNTs: A study on fiber interfacial enhancement and matrix enhancement
- A practical review over surface modification, nanopatterns, emerging materials, drug delivery systems, and their biophysiochemical properties for dental implants: Recent progresses and advances
- HTR: An ultra-high speed algorithm for cage recognition of clathrate hydrates
- Effects of microalloying elements added by in situ synthesis on the microstructure of WCu composites
- A highly sensitive nanobiosensor based on aptamer-conjugated graphene-decorated rhodium nanoparticles for detection of HER2-positive circulating tumor cells
- Progressive collapse performance of shear strengthened RC frames by nano CFRP
- Core–shell heterostructured composites of carbon nanotubes and imine-linked hyperbranched polymers as metal-free Li-ion anodes
- A Galerkin strategy for tri-hybridized mixture in ethylene glycol comprising variable diffusion and thermal conductivity using non-Fourier’s theory
- Simple models for tensile modulus of shape memory polymer nanocomposites at ambient temperature
- Preparation and morphological studies of tin sulfide nanoparticles and use as efficient photocatalysts for the degradation of rhodamine B and phenol
- Polyethyleneimine-impregnated activated carbon nanofiber composited graphene-derived rice husk char for efficient post-combustion CO2 capture
- Electrospun nanofibers of Co3O4 nanocrystals encapsulated in cyclized-polyacrylonitrile for lithium storage
- Pitting corrosion induced on high-strength high carbon steel wire in high alkaline deaerated chloride electrolyte
- Formulation of polymeric nanoparticles loaded sorafenib; evaluation of cytotoxicity, molecular evaluation, and gene expression studies in lung and breast cancer cell lines
- Engineered nanocomposites in asphalt binders
- Influence of loading voltage, domain ratio, and additional load on the actuation of dielectric elastomer
- Thermally induced hex-graphene transitions in 2D carbon crystals
- The surface modification effect on the interfacial properties of glass fiber-reinforced epoxy: A molecular dynamics study
- Molecular dynamics study of deformation mechanism of interfacial microzone of Cu/Al2Cu/Al composites under tension
- Nanocolloid simulators of luminescent solar concentrator photovoltaic windows
- Compressive strength and anti-chloride ion penetration assessment of geopolymer mortar merging PVA fiber and nano-SiO2 using RBF–BP composite neural network
- Effect of 3-mercapto-1-propane sulfonate sulfonic acid and polyvinylpyrrolidone on the growth of cobalt pillar by electrodeposition
- Dynamics of convective slippery constraints on hybrid radiative Sutterby nanofluid flow by Galerkin finite element simulation
- Preparation of vanadium by the magnesiothermic self-propagating reduction and process control
- Microstructure-dependent photoelectrocatalytic activity of heterogeneous ZnO–ZnS nanosheets
- Cytotoxic and pro-inflammatory effects of molybdenum and tungsten disulphide on human bronchial cells
- Improving recycled aggregate concrete by compression casting and nano-silica
- Chemically reactive Maxwell nanoliquid flow by a stretching surface in the frames of Newtonian heating, nonlinear convection and radiative flux: Nanopolymer flow processing simulation
- Nonlinear dynamic and crack behaviors of carbon nanotubes-reinforced composites with various geometries
- Biosynthesis of copper oxide nanoparticles and its therapeutic efficacy against colon cancer
- Synthesis and characterization of smart stimuli-responsive herbal drug-encapsulated nanoniosome particles for efficient treatment of breast cancer
- Homotopic simulation for heat transport phenomenon of the Burgers nanofluids flow over a stretching cylinder with thermal convective and zero mass flux conditions
- Incorporation of copper and strontium ions in TiO2 nanotubes via dopamine to enhance hemocompatibility and cytocompatibility
- Mechanical, thermal, and barrier properties of starch films incorporated with chitosan nanoparticles
- Mechanical properties and microstructure of nano-strengthened recycled aggregate concrete
- Glucose-responsive nanogels efficiently maintain the stability and activity of therapeutic enzymes
- Tunning matrix rheology and mechanical performance of ultra-high performance concrete using cellulose nanofibers
- Flexible MXene/copper/cellulose nanofiber heat spreader films with enhanced thermal conductivity
- Promoted charge separation and specific surface area via interlacing of N-doped titanium dioxide nanotubes on carbon nitride nanosheets for photocatalytic degradation of Rhodamine B
- Elucidating the role of silicon dioxide and titanium dioxide nanoparticles in mitigating the disease of the eggplant caused by Phomopsis vexans, Ralstonia solanacearum, and root-knot nematode Meloidogyne incognita
- An implication of magnetic dipole in Carreau Yasuda liquid influenced by engine oil using ternary hybrid nanomaterial
- Robust synthesis of a composite phase of copper vanadium oxide with enhanced performance for durable aqueous Zn-ion batteries
- Tunning self-assembled phases of bovine serum albumin via hydrothermal process to synthesize novel functional hydrogel for skin protection against UVB
- A comparative experimental study on damping properties of epoxy nanocomposite beams reinforced with carbon nanotubes and graphene nanoplatelets
- Lightweight and hydrophobic Ni/GO/PVA composite aerogels for ultrahigh performance electromagnetic interference shielding
- Research on the auxetic behavior and mechanical properties of periodically rotating graphene nanostructures
- Repairing performances of novel cement mortar modified with graphene oxide and polyacrylate polymer
- Closed-loop recycling and fabrication of hydrophilic CNT films with high performance
- Design of thin-film configuration of SnO2–Ag2O composites for NO2 gas-sensing applications
- Study on stress distribution of SiC/Al composites based on microstructure models with microns and nanoparticles
- PVDF green nanofibers as potential carriers for improving self-healing and mechanical properties of carbon fiber/epoxy prepregs
- Osteogenesis capability of three-dimensionally printed poly(lactic acid)-halloysite nanotube scaffolds containing strontium ranelate
- Silver nanoparticles induce mitochondria-dependent apoptosis and late non-canonical autophagy in HT-29 colon cancer cells
- Preparation and bonding mechanisms of polymer/metal hybrid composite by nano molding technology
- Damage self-sensing and strain monitoring of glass-reinforced epoxy composite impregnated with graphene nanoplatelet and multiwalled carbon nanotubes
- Thermal analysis characterisation of solar-powered ship using Oldroyd hybrid nanofluids in parabolic trough solar collector: An optimal thermal application
- Pyrene-functionalized halloysite nanotubes for simultaneously detecting and separating Hg(ii) in aqueous media: A comprehensive comparison on interparticle and intraparticle excimers
- Fabrication of self-assembly CNT flexible film and its piezoresistive sensing behaviors
- Thermal valuation and entropy inspection of second-grade nanoscale fluid flow over a stretching surface by applying Koo–Kleinstreuer–Li relation
- Mechanical properties and microstructure of nano-SiO2 and basalt-fiber-reinforced recycled aggregate concrete
- Characterization and tribology performance of polyaniline-coated nanodiamond lubricant additives
- Combined impact of Marangoni convection and thermophoretic particle deposition on chemically reactive transport of nanofluid flow over a stretching surface
- Spark plasma extrusion of binder free hydroxyapatite powder
- An investigation on thermo-mechanical performance of graphene-oxide-reinforced shape memory polymer
- Effect of nanoadditives on the novel leather fiber/recycled poly(ethylene-vinyl-acetate) polymer composites for multifunctional applications: Fabrication, characterizations, and multiobjective optimization using central composite design
- Design selection for a hemispherical dimple core sandwich panel using hybrid multi-criteria decision-making methods
- Improving tensile strength and impact toughness of plasticized poly(lactic acid) biocomposites by incorporating nanofibrillated cellulose
- Green synthesis of spinel copper ferrite (CuFe2O4) nanoparticles and their toxicity
- The effect of TaC and NbC hybrid and mono-nanoparticles on AA2024 nanocomposites: Microstructure, strengthening, and artificial aging
- Excited-state geometry relaxation of pyrene-modified cellulose nanocrystals under UV-light excitation for detecting Fe3+
- Effect of CNTs and MEA on the creep of face-slab concrete at an early age
- Effect of deformation conditions on compression phase transformation of AZ31
- Application of MXene as a new generation of highly conductive coating materials for electromembrane-surrounded solid-phase microextraction
- A comparative study of the elasto-plastic properties for ceramic nanocomposites filled by graphene or graphene oxide nanoplates
- Encapsulation strategies for improving the biological behavior of CdS@ZIF-8 nanocomposites
- Biosynthesis of ZnO NPs from pumpkin seeds’ extract and elucidation of its anticancer potential against breast cancer
- Preliminary trials of the gold nanoparticles conjugated chrysin: An assessment of anti-oxidant, anti-microbial, and in vitro cytotoxic activities of a nanoformulated flavonoid
- Effect of micron-scale pores increased by nano-SiO2 sol modification on the strength of cement mortar
- Fractional simulations for thermal flow of hybrid nanofluid with aluminum oxide and titanium oxide nanoparticles with water and blood base fluids
- The effect of graphene nano-powder on the viscosity of water: An experimental study and artificial neural network modeling
- Development of a novel heat- and shear-resistant nano-silica gelling agent
- Characterization, biocompatibility and in vivo of nominal MnO2-containing wollastonite glass-ceramic
- Entropy production simulation of second-grade magnetic nanomaterials flowing across an expanding surface with viscidness dissipative flux
- Enhancement in structural, morphological, and optical properties of copper oxide for optoelectronic device applications
- Aptamer-functionalized chitosan-coated gold nanoparticle complex as a suitable targeted drug carrier for improved breast cancer treatment
- Performance and overall evaluation of nano-alumina-modified asphalt mixture
- Analysis of pure nanofluid (GO/engine oil) and hybrid nanofluid (GO–Fe3O4/engine oil): Novel thermal and magnetic features
- Synthesis of Ag@AgCl modified anatase/rutile/brookite mixed phase TiO2 and their photocatalytic property
- Mechanisms and influential variables on the abrasion resistance hydraulic concrete
- Synergistic reinforcement mechanism of basalt fiber/cellulose nanocrystals/polypropylene composites
- Achieving excellent oxidation resistance and mechanical properties of TiB2–B4C/carbon aerogel composites by quick-gelation and mechanical mixing
- Microwave-assisted sol–gel template-free synthesis and characterization of silica nanoparticles obtained from South African coal fly ash
- Pulsed laser-assisted synthesis of nano nickel(ii) oxide-anchored graphitic carbon nitride: Characterizations and their potential antibacterial/anti-biofilm applications
- Effects of nano-ZrSi2 on thermal stability of phenolic resin and thermal reusability of quartz–phenolic composites
- Benzaldehyde derivatives on tin electroplating as corrosion resistance for fabricating copper circuit
- Mechanical and heat transfer properties of 4D-printed shape memory graphene oxide/epoxy acrylate composites
- Coupling the vanadium-induced amorphous/crystalline NiFe2O4 with phosphide heterojunction toward active oxygen evolution reaction catalysts
- Graphene-oxide-reinforced cement composites mechanical and microstructural characteristics at elevated temperatures
- Gray correlation analysis of factors influencing compressive strength and durability of nano-SiO2 and PVA fiber reinforced geopolymer mortar
- Preparation of layered gradient Cu–Cr–Ti alloy with excellent mechanical properties, thermal stability, and electrical conductivity
- Recovery of Cr from chrome-containing leather wastes to develop aluminum-based composite material along with Al2O3 ceramic particles: An ingenious approach
- Mechanisms of the improved stiffness of flexible polymers under impact loading
- Anticancer potential of gold nanoparticles (AuNPs) using a battery of in vitro tests
- Review Articles
- Proposed approaches for coronaviruses elimination from wastewater: Membrane techniques and nanotechnology solutions
- Application of Pickering emulsion in oil drilling and production
- The contribution of microfluidics to the fight against tuberculosis
- Graphene-based biosensors for disease theranostics: Development, applications, and recent advancements
- Synthesis and encapsulation of iron oxide nanorods for application in magnetic hyperthermia and photothermal therapy
- Contemporary nano-architectured drugs and leads for ανβ3 integrin-based chemotherapy: Rationale and retrospect
- State-of-the-art review of fabrication, application, and mechanical properties of functionally graded porous nanocomposite materials
- Insights on magnetic spinel ferrites for targeted drug delivery and hyperthermia applications
- A review on heterogeneous oxidation of acetaminophen based on micro and nanoparticles catalyzed by different activators
- Early diagnosis of lung cancer using magnetic nanoparticles-integrated systems
- Advances in ZnO: Manipulation of defects for enhancing their technological potentials
- Efficacious nanomedicine track toward combating COVID-19
- A review of the design, processes, and properties of Mg-based composites
- Green synthesis of nanoparticles for varied applications: Green renewable resources and energy-efficient synthetic routes
- Two-dimensional nanomaterial-based polymer composites: Fundamentals and applications
- Recent progress and challenges in plasmonic nanomaterials
- Apoptotic cell-derived micro/nanosized extracellular vesicles in tissue regeneration
- Electronic noses based on metal oxide nanowires: A review
- Framework materials for supercapacitors
- An overview on the reproductive toxicity of graphene derivatives: Highlighting the importance
- Antibacterial nanomaterials: Upcoming hope to overcome antibiotic resistance crisis
- Research progress of carbon materials in the field of three-dimensional printing polymer nanocomposites
- A review of atomic layer deposition modelling and simulation methodologies: Density functional theory and molecular dynamics
- Recent advances in the preparation of PVDF-based piezoelectric materials
- Recent developments in tensile properties of friction welding of carbon fiber-reinforced composite: A review
- Comprehensive review of the properties of fly ash-based geopolymer with additive of nano-SiO2
- Perspectives in biopolymer/graphene-based composite application: Advances, challenges, and recommendations
- Graphene-based nanocomposite using new modeling molecular dynamic simulations for proposed neutralizing mechanism and real-time sensing of COVID-19
- Nanotechnology application on bamboo materials: A review
- Recent developments and future perspectives of biorenewable nanocomposites for advanced applications
- Nanostructured lipid carrier system: A compendium of their formulation development approaches, optimization strategies by quality by design, and recent applications in drug delivery
- 3D printing customized design of human bone tissue implant and its application
- Design, preparation, and functionalization of nanobiomaterials for enhanced efficacy in current and future biomedical applications
- A brief review of nanoparticles-doped PEDOT:PSS nanocomposite for OLED and OPV
- Nanotechnology interventions as a putative tool for the treatment of dental afflictions
- Recent advancements in metal–organic frameworks integrating quantum dots (QDs@MOF) and their potential applications
- A focused review of short electrospun nanofiber preparation techniques for composite reinforcement
- Microstructural characteristics and nano-modification of interfacial transition zone in concrete: A review
- Latest developments in the upconversion nanotechnology for the rapid detection of food safety: A review
- Strategic applications of nano-fertilizers for sustainable agriculture: Benefits and bottlenecks
- Molecular dynamics application of cocrystal energetic materials: A review
- Synthesis and application of nanometer hydroxyapatite in biomedicine
- Cutting-edge development in waste-recycled nanomaterials for energy storage and conversion applications
- Biological applications of ternary quantum dots: A review
- Nanotherapeutics for hydrogen sulfide-involved treatment: An emerging approach for cancer therapy
- Application of antibacterial nanoparticles in orthodontic materials
- Effect of natural-based biological hydrogels combined with growth factors on skin wound healing
- Nanozymes – A route to overcome microbial resistance: A viewpoint
- Recent developments and applications of smart nanoparticles in biomedicine
- Contemporary review on carbon nanotube (CNT) composites and their impact on multifarious applications
- Interfacial interactions and reinforcing mechanisms of cellulose and chitin nanomaterials and starch derivatives for cement and concrete strength and durability enhancement: A review
- Diamond-like carbon films for tribological modification of rubber
- Layered double hydroxides (LDHs) modified cement-based materials: A systematic review
- Recent research progress and advanced applications of silica/polymer nanocomposites
- Modeling of supramolecular biopolymers: Leading the in silico revolution of tissue engineering and nanomedicine
- Recent advances in perovskites-based optoelectronics
- Biogenic synthesis of palladium nanoparticles: New production methods and applications
- A comprehensive review of nanofluids with fractional derivatives: Modeling and application
- Electrospinning of marine polysaccharides: Processing and chemical aspects, challenges, and future prospects
- Electrohydrodynamic printing for demanding devices: A review of processing and applications
- Rapid Communications
- Structural material with designed thermal twist for a simple actuation
- Recent advances in photothermal materials for solar-driven crude oil adsorption

