Abstract
The coupling of metal oxide nanoparticles and electrochemically active polymers has been considered as an effective way to improve the lithium storage performance of individual electrode materials. This work reports an electrospinning process followed by thermal annealing to produce composite nanofibers of cyclized-polyacrylonitrile (cPAN) containing Co3O4 nanoparticles (cPAN/Co3O4). The as-prepared cPAN/Co3O4 nanofiber exhibits a porous nanostructure with an average diameter of 85 nm. When used for lithium-ion battery, the cPAN/Co3O4 anode delivers a reversible specific capacity as high as 997.6 mA h g−1 at 0.1 A g−1, and still maintains 396.5 mA h g−1 at 1.0 A g−1. Meanwhile, the cPAN/Co3O4 anode shows good cycling stability with a retention of 81% capacity after running 50 cycles at 0.1 A g−1. The electrochemical performance of cPAN/Co3O4 significantly outperforms its individual counterparts of cPAN and Co3O4.
1 Introduction
With the increasing demand for energy conservation and emission reduction, rechargeable batteries such as lithium-ion batteries (LIBs) have been widely applied in power grid energy storage and new energy vehicles [1]. Graphite is one of the most commonly used anode materials for LIBs because of its low-cost and good theoretical capacity of 372 mA h g−1 [2,3]. However, due to the low ion diffusion efficiency in graphite electrodes, it is still a great challenge to improve the energy density and power density of commercial LIBs and it is necessary to develop new anode materials with high capacity, excellent rate performance, and long cycle stability [4,5].
Transition metal oxides (TMOs, such as Co3O4, Mn3O4, and Fe2O3) have been regarded as promising alternatives to graphite due to their relatively high theoretical capacity [6,7,8]. Nevertheless, TMOs still face the problems of low inherent conductivity and large volume change in the charging and discharging process, which inevitably leads to low rate performance and poor cycle stability, and seriously hinders their applications. An effective way to improve the ionic conductivity of TMOs is to construct nanostructures [9,10] because the reduced size significantly shortens the Li+ diffusion path, making it easy to transfer to the surface of electrochemically active material, leading to high ionic conductivity and electrochemical utilization efficiency [11]; Another approach is to construct nanocomposites by combining TMOs with active carbon, multiwalled carbon nanotubes (CNTs), or graphene [12], Carbon coating can not only significantly improve the electronic conductivity of electrode materials and hence the rate performance [13,14] but also restrain the electrode volume change during lithium insertion/deintercalation process, resulting in long cycling stability. Wan and co-workers [15] reported that carbon-coated Fe3O4 nanospindles showed high reversible capacities (745 mA h g−1 at C/5 and 600 mA h g−1 at C/2) and significantly enhanced cycling performance and rate capability as compared with bare hematite spindles. These improvements are mainly attributed to the continuous carbon coating, which maintains particle integrity and improves electrode conductivity, resulting in a uniform and stable solid electrolyte interface (SEI) film on the surface [16,17].
In recent years, coating conductive polymers (e.g., polyaniline, polypyrrole, and polythiophene) onto TMO nanostructures have also been the focus of research [18]. Both conductive polymers and TMOs are electrochemically active, which makes polymer/TMO nanocomposites greatly attractive as electrode materials for lithium batteries [19]. Ponzio and co-workers [20] reported a reverse micelle method to prepare polyaniline-coated V2O5 nanofibers, and the composite electrode containing 30 mol% polyaniline delivered a steady capacity of about 300 mA h g−1 with no morphology change over 10 cycles, whereas the V2O5 nanofibers do not retain the morphology after cycling. However, conductive polymers typically have low actual capacities (mostly below 150 mA h g−1) and poor cycling stability. In addition to the conductive polymers, nonconductive polymers with rich heteroatomic structures (redox-active centers of C═O and C═N groups) have large theoretical capacities, good wettability yet strong insolubility with electrolytes, fast redox kinetics, high-power capability, naturally abundant monomer sources [21], and thus, offer a promising direction for green, economical, high-performance, and sustainable batteries. However, there is still a lot of room to explore novel nanostructures for improving the porosity and active site utilization of redox-active polymers [22].
Electrospinning has been regarded as a simple and effective technology for the preparation of 1D nanomaterials, such as polymer nanofibers and metal oxide nanofibers with diameters ranging from tens of nanometers to several microns [23,24]. At the same time, electrospun nanofibers with different morphologic structures can also be prepared, such as cylindrical solid nanofibers, porous nanofibers, hollow nanofibers, core–shell nanofibers, and hierarchical nanofibers [25,26]. Compared with powder materials, 1D nanofiber with porous, hollow, and core–shell structures can improve the surface/volume ratio of the electrode to electrolyte interface and hence the electrochemical reaction efficiency. Meanwhile, it can also provide abundant void space to adapt to the huge volume change during the charge/discharge cycle, thus effectively improving the cycling stability of electrode materials [27]. A breakthrough in the electrospinning method is co-electrospinning the mixture solution of polymer and inorganic salt. After that, the sintering of electrospun polymer–inorganic salt composite nanofibers can transform the polymer and inorganic salt into porous carbon and metal oxide nanocrystals, respectively. The two components as well as the unique 1D nanostructures could bring about synergetic effects to enhance their electrochemical performances. Polyacrylonitrile (PAN), a typical spinnable polymer, is one of the most commonly used materials for preparing nanofibers by electrostatic spinning [28], Meanwhile, PAN nanofibers can also be carbonized at high temperature to prepare carbon nanofibers with high carbon yield and excellent mechanical properties [29]. Guo et al. [30] prepared ultra-uniform SnO x /carbon nanohybrids (U-SnO x /C) by electrospinning a dimethylformamide (DMF) solution of SnO2 nanoparticles and PAN followed by carbonization at 600°C in an argon atmosphere. The obtained 1D U-SnO x /C nanostructure has a strong interaction with SnO x and the N-doped carbon matrix, which effectively inhibits SnO x agglomeration and withstands large volume changes during lithium alloying/dealloying reactions, coupled with enhancing electron and ion transports due to shortened conducting and diffusion paths. As a result, U-SnO x /C shows an excellent rate capability and a high reversible capacity of 608 mA h g−1 after 200 cycles.
Notably, the thickness and percentage of carbon coating need to be controlled by regulating the conditions of the calcination process (such as temperature and atmosphere). However, when treated at a high temperature in an argon atmosphere, transition metal salts such as Co2+ and Ni2+ can be easily reduced into their metallic forms instead of metal oxides. In fact, annealing PAN at a relatively low temperature (∼280°C) in an air atmosphere yields conjugated cyclized polyacrylonitrile (cPAN), which consists of C═C and C═N groups, and also gives a high theoretical capacity (2,102 mA h g−1) when used directly as a LIB anode material [31]. In this work, we report an electrospinning approach to prepare polyacrylonitrile/cobalt acetate (PAN/Co(OAc)2) composite nanofibers, followed by heating at 280°C in an air atmosphere. During this process, PAN was converted to porous cPAN coating on the surface of Co3O4 nanoparticles. The as-prepared cPAN/Co3O4 nanofiber was further used as an anode material with significantly improved lithium storage performance compared to neat cPAN and Co3O4 nanofibers. The cPAN/Co3O4-based battery achieves a high reversible specific capacity up to 997.6 mA h g−1 at a current density of 0.1 A g−1, and still maintains 396.5 mA h g−1 when the current density increases to 1.0 A g−1. Meanwhile, the specific capacity retention rate is as high as 81% after 50 cycles at 0.1 A g−1. The excellent electrochemical performance of cPAN/Co3O4 is attributed to its porous fiber structure and synergistic effect between the two components.
2 Experimental section
2.1 Materials
PAN, Co(OAc)2, dimethyl formamide (DFM), and N-methyl-2-pyrrolidone (NMP) were purchased from Shanghai Sinopharm Chemical Reagent Co., Ltd; acetylene black and polyvinyl alcohol (PVA, M w = 100,000) were obtained from Shanghai Aladdin Bio-Chem Technology Co., Ltd; and polyvinylidene fluoride (PVDF) (≥99.0%) was purchased from Solvay USA Co., Ltd. All the chemicals were used as received without further treatment.
2.2 Fabrication of cPAN/Co3O4 nanofibers
The cPAN/Co3O4 oriented nanofiber was prepared by electrospinning the mixed solution of PAN and cobaltous acetate (Co(OAc)2·4H2O), followed by thermal treatment. In a typical process, 1.0 g of Co(OAc)2·4H2O was dissolved into 10 mL DMF by stirring for 1 h. After that, 0.5 g of PAN was slowly added into the above solution and stirred overnight at room temperature. The as-prepared homogeneous solution was then loaded into a plastic syringe (10 mL) with a 22 G needle, and subsequently placed into a commercial electrospinning setup; the flow rate was set to 0.08 mm min−1. A high-voltage power of 10 kV was applied to the nozzle spinneret, and a grounded cylinder collector was placed 12 cm away and rotated at a high speed up to 2,800 rpm. The as-electrospun PAN/Co(OAc)2 composite nanofibers were dried at 80°C in a vacuum to remove the residual solvent, and then heat-treated at 280°C for 3 h in an air atmosphere with a heating rate of 2.5°C min−1.
For comparison, neat cPAN nanofibers were synthesized by a comparable electrospinning process without adding Co(OAc)2. In addition, Co3O4 nanofibers were also prepared by using PVA as the polymer fiber substrate instead of PAN; then the as-electrospun PVA/Co(OAc)2 composite nanofibers were calcined at 285°C for 3 h in air with a heating rate of 2.5°C min−1, and the PVA competent was decomposed completely during the thermal treatment.
2.3 Materials’ characterization
The morphology of samples was recorded with a field-emission scanning electron microscope (FESEM) on a Hitachi SU 8010. The microstructure of the samples was observed on a TECN G220 S-TWIN system operating at an acceleration voltage of 200 kV. Thermogravimetric analysis (TGA) curves were collected on a Diamond TG/f DTG analyzer. X-ray diffraction (XRD) patterns were collected from a D/Max 2400 diffractometer equipped with a Cu Ka radiation source (λ = 1.5406 Å). The specific surface areas and pore size distribution of samples were measured using the Brunauer–Emmett–Teller (BET) approach on the JW-BK132F equipment.
2.4 Electrochemical measurements
All the electrochemical studies were conducted in a two-electrode coin cell (CR 2032) configuration at room temperature (25°C). The composite anode was prepared by mixing the active material (cPAN/Co3O4 composite nanofibers), the conductive additive (acetylene black), and the binder (PVDF) in the mass ratio of 80:10:10. The mixture was coated on the etched copper foil, and then dried in a vacuum drying oven at 80°C overnight; metallic lithium serves as both counter and reference electrodes, and then lithium-ion half batteries were assembled in a glove box filled with argon.
3 Results and discussion
3.1 Material synthesis and structural characteristics
Figure 1a illustrates the synthesis routes to cPAN/Co3O4 nanofibers. Typically, PAN and Co(OAc)2 were dispersed uniformly in DMF at ambient temperature and then transformed into a plastic syringe with a 22 Gauge needle (0.7 mm in outer diameter), which was regulated to reject the mixture solution with a flow rate of 0.08 mL min−1, and applied a high-voltage power of 10 kV. A grounded cylinder collector was placed 12 cm away from the needle, rotating at a high speed up to 2,800 rpm. Owing to the ultra-high speed of the cylinder collector, the as-electrospun PAN/Co(OAc)2 composite fibers become thin in diameter and regular in orientation. After that, the resultant PAN/Co(OAc)2 composite fibers were finally subjected to a heat treatment at 280°C in air for 4 hours. During this process, the PAN component was cyclized into conjugated poly(N-heteroacene) and partly oxidized to form carbonyl groups, resulting in porous cPAN nanoshells, as shown in Figure 1b. At the same time, Co(OAc)2 was decomposed to form Co3O4 nanoparticles, encapsulated in the cPAN nanoshells.
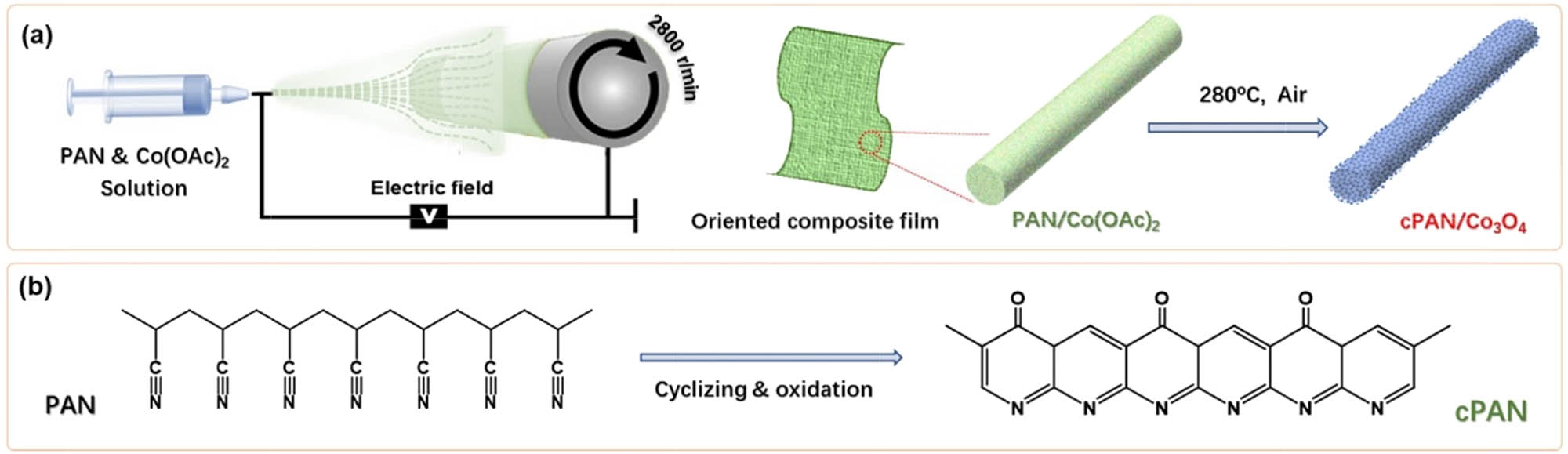
(a) Schematic illustration of preparing cPAN/Co3O4 nanofiber. (b) The chemical structure transformation from PAN to cPAN during the thermal treatment.
The transformation of PAN/Co(OAc)2 during heat treatment can be demonstrated by TGA curves, as shown in Figure 2a; the pure PAN fiber gives a large weight loss of 28% in the temperature range from 250 to 280°C, corresponding to the multiple reactions of cyclization, dehydrogenation, and oxidization. Then, the thermostable conjugated structure of cPAN was oxidized and decomposed completely above 700°C in an air atmosphere [32]. Co(OAc)2 hydrate gradually decomposed to form Co3O4 above 240°C, and finally, maintained a mass retention rate of 37% above 350°C, while neat Co3O4 shows a negligible weight loss of 1.5% above 700°C. Based on the thermal properties of the PAN and Co(OAc)2, the PAN/Co(OAc)2 composite fiber was thermally treated at 280°C in this work; the as-produced cPAN/Co3O4 nanofiber presents a similar decomposition trend with that of cPAN, exhibiting good thermal stability below 400°C, and remaining 53% of the mass above 700°C due to the presence of Co3O4. The transformation of PAN, Co(OAc)2, and their composite fiber were also characterized by XRD measurements, as shown in Figure 2b. The neat PAN fiber presents a distinct diffraction peak at 2θ = 17° with an average interlayer spacing (d) of 0.52 nm corresponding to (100) plane of hexagonal lattice [33,34]. After the thermal treatment at 280°C, the (001) diffraction peak disappears and a broad diffraction peak occurs at around 2θ = 25.6° in the curve of cPAN, demonstrating that the original structure of PAN was transformed into a new crystal structure, which is consistent with that of the graphite structure for the (002) plane, indicating that a highly conjugate structure was formed in the cPAN fibers [33]. Both the neat Co3O4 and cPAN/Co3O4 composite fibers show typical diffraction peaks in accordance with the cubic spinel phase Co3O4 (JCPDS 42-1467), demonstrating that the cPAN/Co3O4 nanofiber was successfully produced as expected. FTIR analysis was further used to detect the chemical group changes of PAN to cPAN after the thermal treatment, as shown in Figure 2c. The original PAN shows sharp peaks of ν C≡N and ν C–H at 2,245 and 2,931 cm−1, respectively. In comparison, the intensity of the above two peaks decreased significantly or even disappeared in the curves of cPAN and cPAN/Co3O4, indicating the occurrence of cyclization and dehydrogenation reactions in the thermal process. In addition, the peaks between 1,680 and 1,580 cm−1 are related to the formation of conjugated carbonyl groups (ν C═O) and the cyclic structures (ν C═C + ν C═N), respectively [29]. The microscopic pore structure of cPAN/Co3O4 was further confirmed by N2 adsorption–desorption isotherm, as shown in Figure 2d, which exhibits a typical type IV isotherm with a H3 type hysteresis loop at a high relative pressure range of 0.8–1.0, indicating the existence of abundant mesopores [35]. The Barrett–Joyner–Halenda (BJH) pore-size distribution curve is shown as an inset in Figure 2d, which presents two main peaks at around 2.4 and 4.8 nm, corresponding to the clearance of Co3O4 nanoparticles and the porous cPAN networks, respectively. It is clear that the main pore size distribution of the cPAN/Co3O4 composite is in the range of 2–50 nm, indicating its mesoporous feature. Consequently, the specific surface area of cPAN/Co3O4 reached 74.47 m2 g−1. The high specific surface area and unique porous structure could contribute to excellent electrochemical performances of the cPAN/Co3O4 composite.
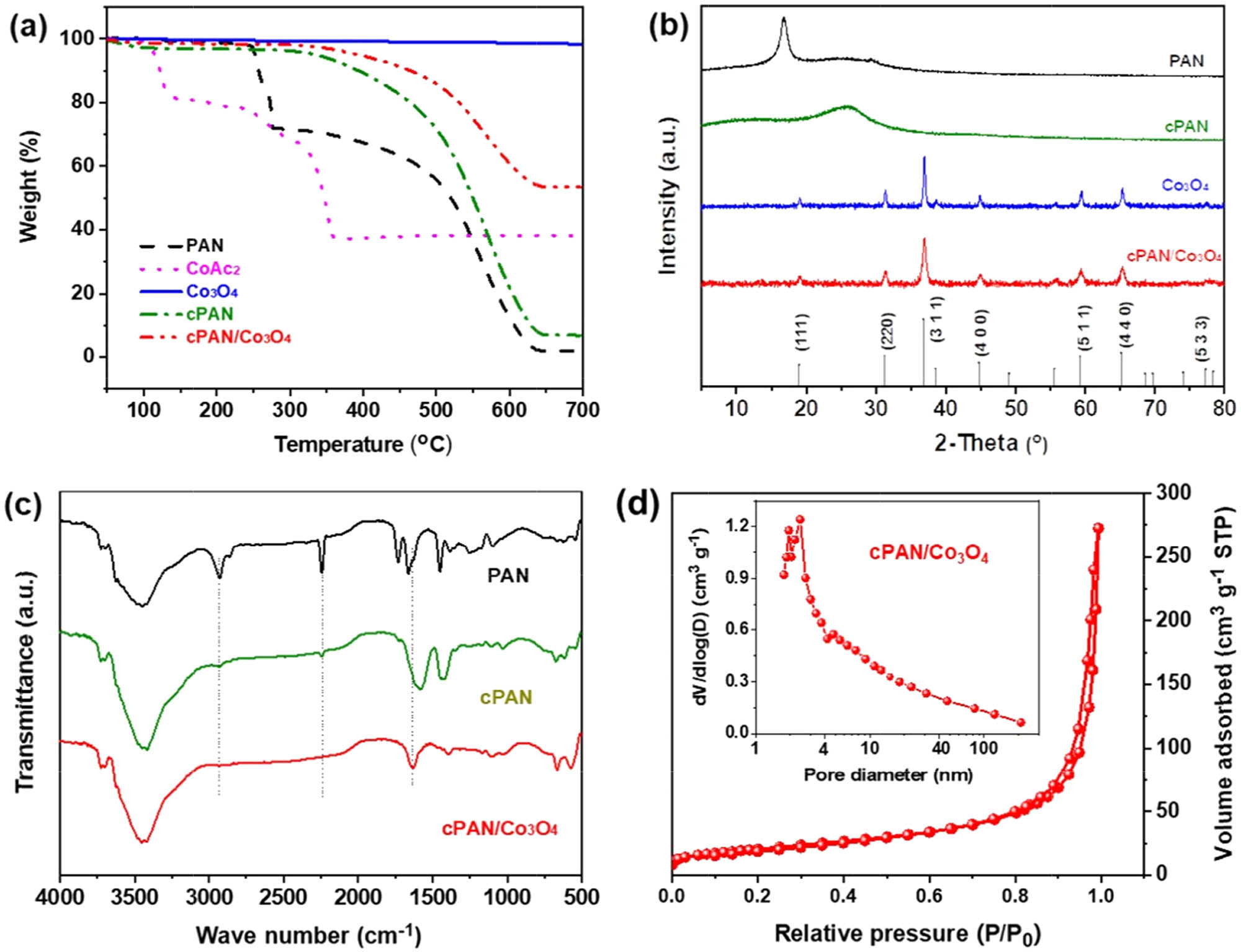
(a) TGA curves, (b) XRD patterns, and (c) FT-IR spectra of PAN, Co(OAc)2, Co3O4, cPAN, and cPAN/Co3O4. (d) N2 adsorption–desorption isotherm and pore size distribution curve of cPAN/Co3O4.
The morphology of the samples was characterized using SEM. As shown in Figure 3a, the as-electrospun PAN/Co(OAc)2 nanofibers are clearly oriented with smooth surfaces with an average diameter of 316 nm. After the thermal treatment, the resulting cPAN/Co3O4 nanofibers maintained the oriented state (Figure 3b). However, their surfaces become very wrinkled and the average diameter reduced to 85 nm (Figure 3c) due to the cyclization/oxidation of PAN and decomposition of Co(OAc)2 during the annealing process. TEM images were also used to determine the microstructure of the cPAN/Co3O4 nanofiber, as shown in Figure 3d and e; the nanofiber consists of dense dark spots representing Co3O4 nanoparticles, whereas the distributed light spots represent plenty of tunnels existing inside the nanofiber, which is beneficial for the infiltration and permeation of electrolytes, resulting in the improvement of utilization efficiency of active materials. The high-resolution TEM images of the cPAN/Co3O4 nanofiber are shown in Figure 3f and g, which indicates that the Co3O4 nanoparticles are randomly coated by the amorphous cPAN shells and can inhibit the volume change of Co3O4 during the charge/discharge process and thus improve the cycling stability of the battery. Moreover, Co3O4 exhibited an obvious crystal lattice fringe with interplanar spaces of 0.47 and 0.28 nm, which are in good agreement with (111) and (220) crystal planes of Co3O4 [36]. The EDS mapping images (Figure 3h–k) of the cPAN/Co3O4 nanofiber further demonstrated the successful coating of Co3O4 nanoparticles into the mesoporous cPAN nanofiber, where the C, O, N, and Co elements coexisted and were uniformly distributed in the nanofiber.
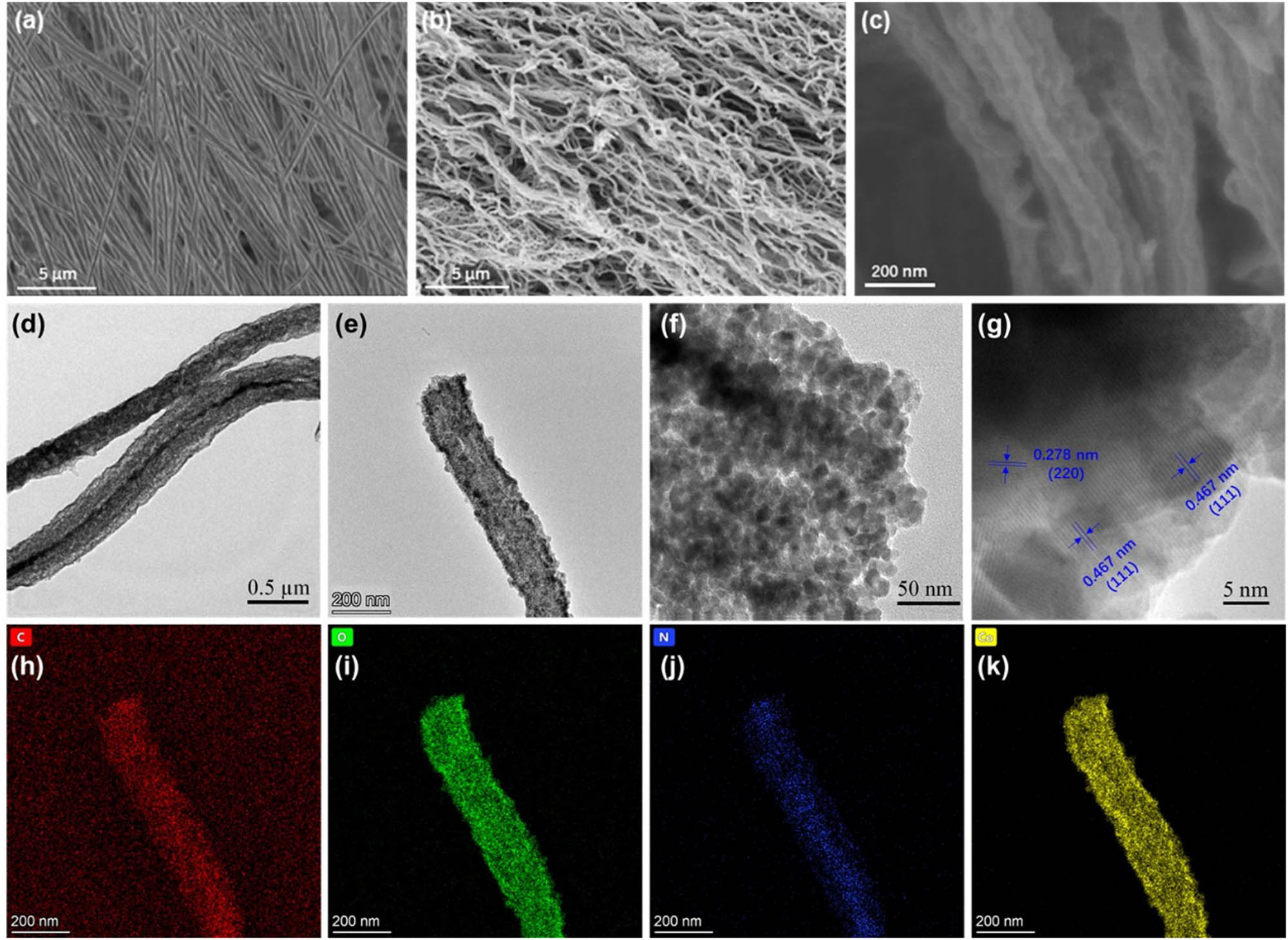
SEM images of oriented (a) PAN/Co(OAc)2 and (b and c) cPAN/Co3O4 composite fibers. (d–g) TEM images of cPAN/Co3O4 at different magnifications and (h–k) the corresponding EDS mappings.
3.2 Lithium storage performance
The electrochemical properties and storage mechanism of cPAN, Co3O4 and cPAN/Co3O4 nanofibers were investigated by using CV, GCD, and EIS techniques. Figure 4a shows the CV curve of the cPAN nanofiber electrode with a scan rate of 0.5 mV s−1 in the voltage range of 0.001–3.00 V (vs Li/Li+) at room temperature. Typically, the CV curve of cPAN shows strong irreversible peaks at about 0.5 V during the first scan, corresponding to the decomposition of organic electrolytes and the formation of the SEI layer [31]. In the following several cycles, two wide redox peaks appeared in the voltage range of 0.001–0.4 and 0.6–1.3 V, corresponding to the insertion/extraction of lithium ion into/from the unsaturated C═C and C═N bonds in the heterocyclic ring of the cPAN chain, respectively, and the evolutions of the chemical structure are consistent with the redox mechanism reported earlier [37,38,31]. For the CV curves of pure Co3O4 in Figure 4b, there is a strong peak at about 0.75 V in the first cathodic scan of the discharge process, which corresponds to the electrochemical reduction process of Co3O4 to Co and the decomposition of the electrolyte solution; the former can be described as Co3O4 + xLi+ + xe− → Li x Co3O4 → 3Co0 + 4Li2O, and the latter leads to the formation of the SEI [39]. In the following two cathodic scans, the cathodic peak shifts into two peaks at 0.8 and 1.0 V with an apparent decrease in the area underneath the curve, contributing to the reduction of Co3O4 into Li2O. During the anodic scan, a broad peak located at about 2.23 V is ascribed to the re-oxidization of metallic cobalt (Co0) and the decomposition of Li2O according to the reversible conversion mechanism described as 3Co0 + 4Li2O ↔ Co3O4 + 8Li+ + 8e−. Notably, the anodic peak shifted to ≈2.31 V and ≈2.37 V at the second and third cycles, respectively, suggesting the formation of a thick SEI layer that may hinder ion transport [40]. The characteristic redox peaks of pure cPAN and Co3O4 can also be identified in the CV curve of the cPAN/Co3O4 anode, as shown in Figure 4c, which shows strong irreversible peaks at around 0.5, 0.8, and 1.0 V during the first cathodic scan, three wide redox peaks at around 0.97, 1.28, and 2.11 V in the first anodic scan, and then three peaks at around 0.87, and 0.57 V in the following cathodic scan, respectively. Furthermore, there is no significant difference between the main peaks of the second and third cycles, indicating improved reversibility of the cPAN/Co3O4 anode in lithium storage [41]. The rate capability and redox kinetics of the cPAN/Co3O4 anode were further examined by CV curves at various scan rates from 0.2 to 1.0 mV s−1, as shown in Figure 4d; all the curves retain similar small shifts, and the current increases accordingly with the increasing scan rate, implying high reversibility and negligible polarization. In fact, the response current (i) can be divide into the fast-kinetic capacitive current (k 1 ν) and slow-kinetic diffusion-controlled current (k 2 ν 0.5) [42,43]. The decoupling of the CV curve shows that the capacitive contribution is 45.5% at 0.2 mV s−1 (Figure 4e); with the increase of the scanning rate, the capacitive contribution increases gradually and reaches 85.2% at 1.0 mV s−1 (Figure 4f), indicating the fast ion transfer capability enabled by the unique porous structure of the cPAN/Co3O4 anode.
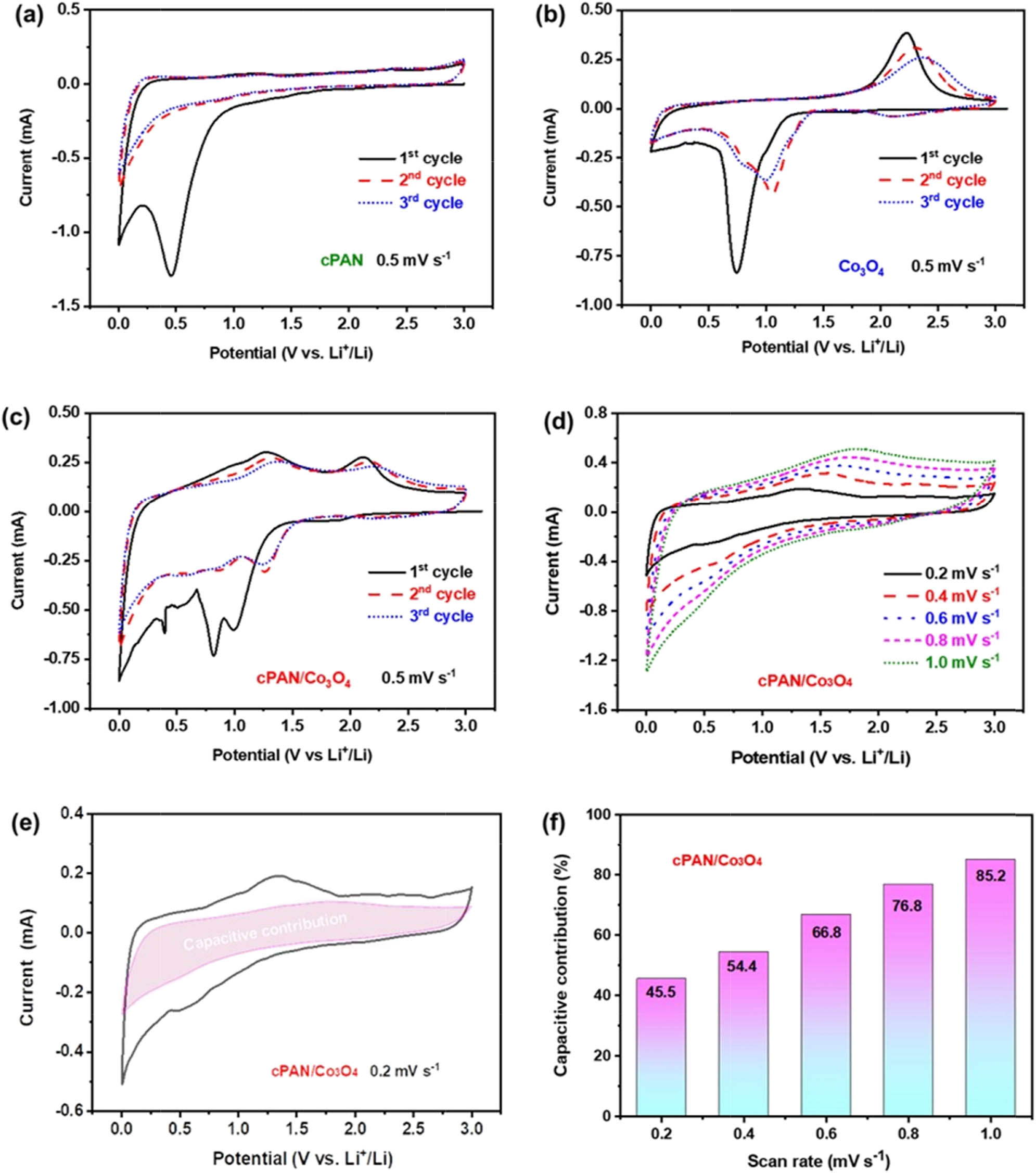
Cyclic voltammograms of (a) cPAN, (b) Co3O4, and (c) cPAN/Co3O4 nanofiber-based half-cells cycled between 0.001 and 3 V (vs Li/Li+) at a scan rate of 0.5 mV s−1. (d) CV curves at various scan rates from 0.2 to 1.0 mV s−1, (e) the decoupling of capacitive contribution (shadow) at 0.2 mV s−1, and (f) the normalized capacitive contribution at different scan rates for the cPAN/Co3O4 anode.
Figure 5a–c shows the GCD curves of cPAN, Co3O4, and cPAN/Co3O4 anodes at a current density of 0.1 A g−1; the first discharge/charge capacities are 1,012/667, 1,468/944, and 1,634/1,052 mA h g−1, with the coulombic efficiency (CE) of 65, 64, and 64%, respectively. Obviously, cPAN/Co3O4 has a higher capacity than that of pure cPAN and Co3O4; however, the low initial Coulombic efficiencies of the anodes were likely due to the possible irreversible processes such as lithium insertion/extraction into/from the active materials, electrolyte decomposition, and inevitable formation of SEI films. In contrast, the second and third cycles of these GCD curves almost coincide, indicating that the absorption and release of lithium in the following charging/discharging process is very stable and reversible [44]. Accordingly, cPAN, Co3O4, and cPAN/Co3O4 anodes exhibit the reversible capacity of 756.3, 897.2, and 997.6 mA h g−1 at 0.1 A g−1, respectively. cPAN/Co3O4 achieved the highest reversible capacity due to the unique porous structure and the synergistic effects between the two components. First, the highly conjugated poly(N-heteroacene)-like structure of cPAN significantly improves the electrochemical performance due to the elevated electronic conductivity. Second, the cPAN nanofibers are small in diameter and have a porous structure, which affords highly exposed multi-electron redox-active sites and enhanced capacity contributions. Third, the cPAN nanofiber substrate suppresses the size of Co3O4 nanoparticles, thus improving the electrochemical utilization and specific capacity.
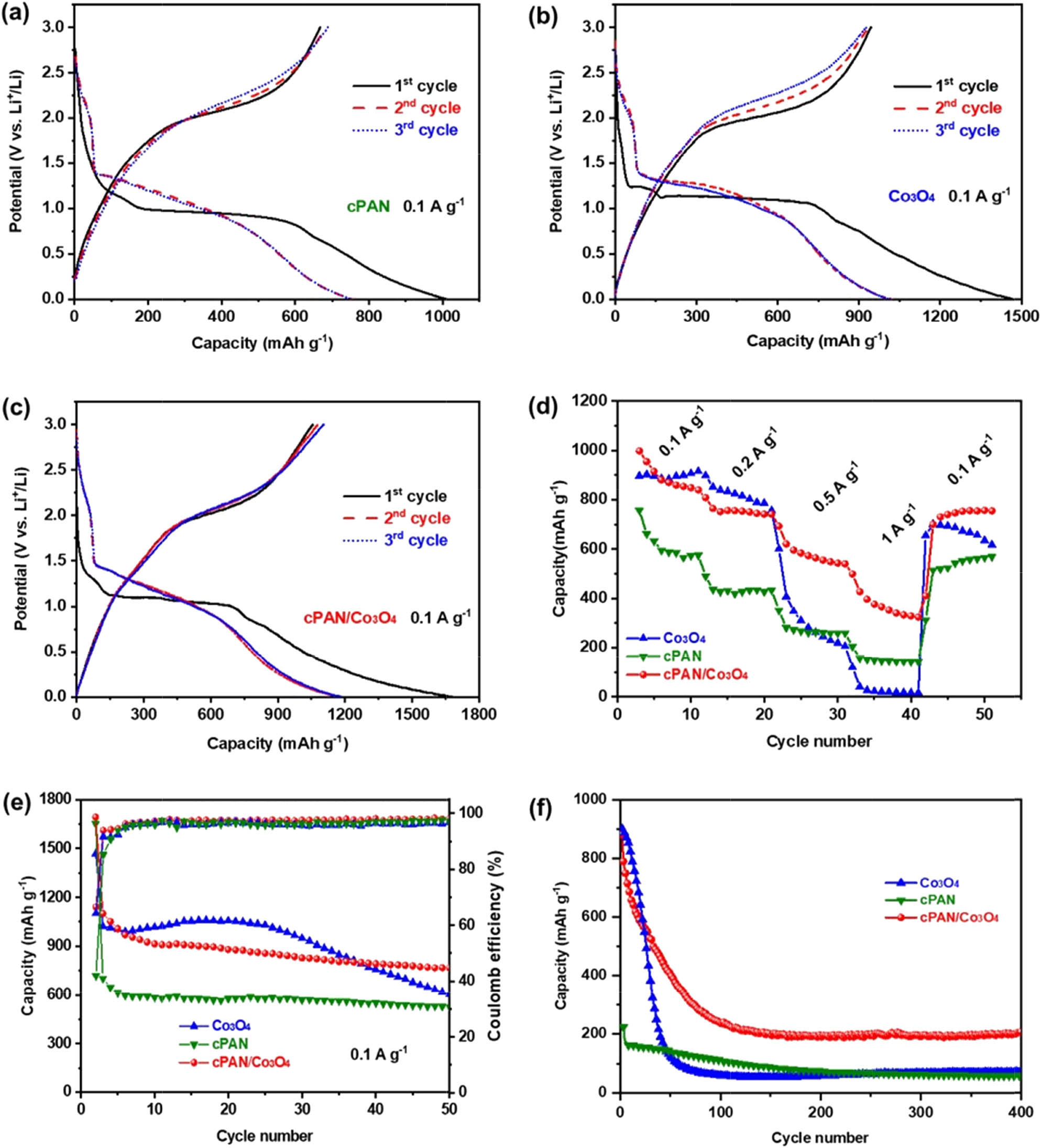
Galvanostatic charge/discharge curves of (a) cPAN, (b) Co3O4, and (c) cPAN/Co3O4 nanofiber-based half-cells cycled between 0.001 and 3 V at 0.1 A g−1; (d) rate performances, (e) cyclic performances at 0.1 A g−1 after 50 cycles, and (f) cyclic performances at 1.0 A g−1 over 400 cycles for the cPAN, Co3O4, and cPAN/Co3O4.
The rate capabilities of cPAN, Co3O4, and cPAN/Co3O4 at various current densities of 0.1–1.0 A g−1 are shown in Figure 5d. It is worth noting that cPAN/Co3O4 shows high reversible capacities of 764.4, 620.6, and 396.5 mA h g−1 at current densities of 0.2, 0.4, and 1.0 A g−1, respectively, with rate retention of 40%, and the capacity recovers to 746.5 mA h g−1 when the current density returns to 0.1 A g−1. The relatively poor rate capability of the cPAN/Co3O4 electrode can be attributed to the low annealing temperature of 280°C and the resulting low electron conductivity. In comparation, pure cPAN exhibits a reversible capacity of 156.4 mA h g−1 (20.7%) at 1.0 A g−1, while the value for Co3O4 is 40.5 mA h g−1 (4.5%). The capacity and rate performance of cPAN/Co3O4 are apparently superior to the pure cPAN, Co3O4, and some Co3O4-based anodes, such as Co3O4 nanoparticles filled in an aligned mesoporous carbon nanotube (Co3O4@MCTs) electrode, which shows a reversible capacity of 179 mA h g−1 at 1.0 A g−1 [45]. The high capacity and good rate performance of the cPAN/Co3O4 anode are owing to its unique mesoporous morphology; the presence of the cPAN mesopores greatly enhances the storage of the interface and surface Li, providing less resistance of short path lengths for the electrode/electrolyte interface [46,47]. Meanwhile, cPAN/Co3O4 also delivers a reversible capacity of 767.8 mA h g−1 at 0.1 A g−1 after 50 cycles (Figure 5e), much higher than that of the pure cPAN (527.6 mA h g−1) and Co3O4 (604 mA h g−1), and also much superior to some reported results, such as the Co3O4@CNT electrode maintains 700 mA h g−1 at 0.1 A g−1 over 100 cycles [48], Co3O4 spheres remains 680 mA h g−1 at 1/5 C after 50 cycles [49], the above-mentioned Co3O4@MCTs show 627 mA h g−1 at 0.1 A g−1 after the 50th discharge [45]. Furthermore, Figure 5f shows the cycling performances of cPAN, Co3O4, and cPAN/Co3O4 at a current density of 1.0 A g−1, and the reversible capacity of Co3O4 decreases robustly during the first 100 cycles. It is due to the relatively low annealing temperature (280°C) and the resulting low crystallinity of Co3O4, which is more likely to cause volume changes during the charging/discharging process at a large current density, leading to rapid structural decomposition and capacity drop [41,50]. Nevertheless, when encapsulated in cPAN, the dissociation of the active material can be inhibited effectively, and thus the capacity retention of cPAN/Co3O4 was improved. As a result, the reversible capacities of cPAN, Co3O4, and cPAN/Co3O4 remain at 56.4, 70.8, and 206.9 mA h g−1 after 400 cycles, with retentions of 40, 8, and 24%, respectively. The highly improved cycling stability of the cPAN/Co3O4 anode is mainly due to its unique structure, and the cPAN layer on the surface of Co3O4 nanoparticles acts as a buffer matrix to prevent particle agglomeration, thus improving the utilization rate and the electrochemical activity of the active material; meanwhile, the cPAN layer also maintains the structural stability of Co3O4 nanoparticles during discharge/charging process and improves the cyclic stability [23].
The kinetics of lithium-ion transmission in two-electrode coin-cells was studied using electrochemical impedance spectroscopy (EIS) in the completely uncharged and undischarged states. The Nyquist plots of the three electrodes consist of three main regimes, as shown in Figure 6a; the intercept of the real axis at high frequency corresponds to the SEI layer resistance (R f), a depressed semicircle in the high-frequency region corresponding to the charge transfer resistance (R ct), while the quasi-line in the low-frequency region is associated with the impedance of Li+ diffusion in the cPAN/Co3O4 nanomaterials [51]. The impedance spectra were analyzed using the complex nonlinear least-squares fitting method, the cPAN electrode exhibits the smallest R ct (150 Ω) due to the unique porous structure that promotes the electrolyte permeabilization and Li insertion/deintercalation. Notably, the cPAN/Co3O4 shows smaller R f (3.2 Ω) and R ct (166 Ω) values than those of pure Co3O4 (R f = 3.8 Ω and R ct = 192 Ω), which can be attributed to the porous cPAN network. Moreover, the slope of the quasi-line of cPAN/Co3O4 in the low frequency range is larger than that of pure Co3O4, indicating a faster ion diffusion dynamic due to the mesoporous nanostructure of cPAN/Co3O4, which provides a high surface area not only for lithium storage but also for the transport of lithium ion in electrolytes. Figure 6b shows the relationship curve between Z′ and the negative square root of angular frequency (ω −1/2) in the low-frequency region; the diffusion coefficient of lithium ions (D Li +) can be calculated with the slope of the fitting line (Warburg coefficient) [52]. The D Li + values of cPAN, Co3O4, and cPAN/Co3O4 were 2.1 × 10−12, 4.6 × 10−12, and 2.6 × 10−12 cm2 s−1, respectively. Obviously, the Li+ diffusion coefficient of cPAN/Co3O4 is slightly larger than that of pure Co3O4 due to the multifunctionality of cPAN, which formed an interconnected three-dimensional porous network to give internal channels for the electrolyte. Meanwhile, it also significantly reduced the aggregation of Co3O4 nanoparticles during the thermal treatment, thus boosting the exposure of exterior surfaces and edges available.
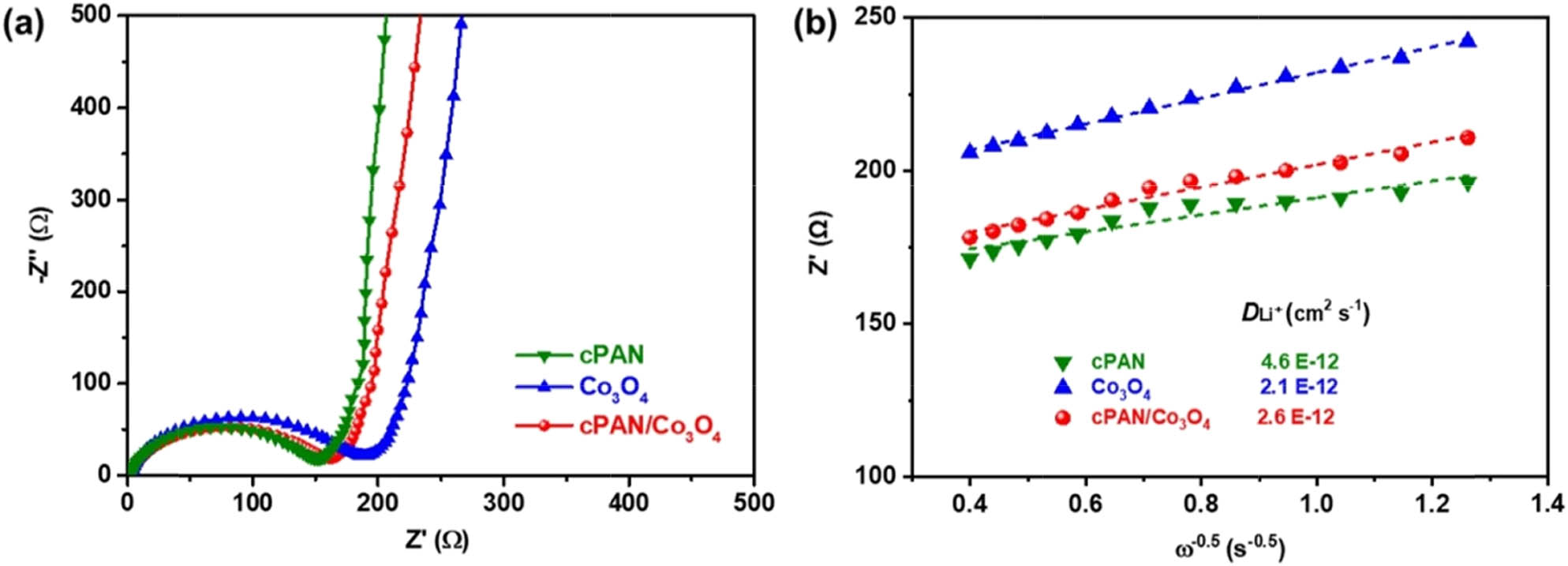
(a) Nyquist plots and (b) the plots of Z′ vs ω −0.5 for the cPAN, Co3O4, and cPAN/Co3O4.
4 Conclusion
In summary, the cPAN/Co3O4 composite nanofiber was produced via an electrospinning method followed by heat treatment; the cPAN/Co3O4 nanofiber is only 85 nm in diameter and has a porous structure, where cPAN shells were coated on the surface of Co3O4 nanoparticles. Owing to the unique nanostructure and synergistic effects inspiring electrochemical lithium storage performances, cPAN/Co3O4-based lithium ion battery achieved a high reversible specific capacity up to 997.6 mA h g−1 at a current density of 0.1 A g−1 and still maintained 396.5 mA h g−1 when the current density increased to 1.0 A g−1. Meanwhile, the specific capacity retention rate was as high as 81% after 50 cycles at a current density of 0.1 A g−1. This work may offer a new design idea and practical basis for the development of high-performance battery anode materials.
-
Funding information: This work was financially supported by the National Natural Science Foundation of China (52173091, 51973235, and 52003207), Program for Leading Talents of National Ethnic Affairs Commission of China (MZR21001), Hubei Provincial Natural Science Foundation of China (2021CFA022), and Wuhan Science and Technology Bureau (2020010601012198).
-
Author contributions: All authors have accepted responsibility for the entire content of this manuscript and approved its submission.
-
Conflict of interest: The authors state no conflict of interest.
References
[1] Chiluwal S, Rao AM, Podila R. Strategies for improving rechargeable lithium-ion batteries: From active materials to CO2 emissions. Nanotechnol Rev. 2021;10(1):1993–2026.10.1515/ntrev-2021-0114Search in Google Scholar
[2] Winter M, Besenhard JO, Spahr ME, Novµk P. Insertion electrode materials for rechargeable lithium batteries. Adv Mater. 1998;10(10):725–63.10.1002/(SICI)1521-4095(199807)10:10<725::AID-ADMA725>3.0.CO;2-ZSearch in Google Scholar
[3] Zhao X, Zhao T, Peng X, Yang L, Shu Y, Jiang T, et al. In situ synthesis of expanded graphite embedded with amorphous carbon-coated aluminum particles as anode materials for lithium-ion batteries. Nanotechnol Rev. 2020;9(1):436–44.10.1515/ntrev-2020-0033Search in Google Scholar
[4] Roberts AD, Li X, Zhang H. Porous carbon spheres and monoliths: morphology control, pore size tuning and their applications as Li-ion battery anode materials. Chem Soc Rev. 2014;43(13):4341–56.10.1039/C4CS00071DSearch in Google Scholar
[5] Wu L, Dai Y, Zeng W, Huang J, Liao B, Pang H. Effective ion pathways and 3D conductive carbon networks in bentonite host enable stable and high-rate lithium-sulfur batteries. Nanotechnol Rev. 2021;10(1):20–33.10.1515/ntrev-2021-0005Search in Google Scholar
[6] Li B, Wang Y, Jiang N, An L, Song J, Zuo Y, et al. Electrolytic-anion-redox adsorption pseudocapacitance in nanosized lithium-free transition metal oxides as cathode materials for Li-ion batteries. Nano Energy. 2020;72:104727.10.1016/j.nanoen.2020.104727Search in Google Scholar
[7] Wang J, Yang N, Tang H, Dong Z, Jin Q, Yang M, et al. Accurate control of multishelled Co3O4 hollow microspheres as high-performance anode materials in lithium-ion batteries. Angew Chem Int Ed Engl. 2013;52(25):6417–20.10.1002/anie.201301622Search in Google Scholar
[8] Zheng SS, Li XR, Yan BY, Hu Q, Xu YX, Xiao X, et al. Transition-metal (Fe, Co, Ni) based metal-organic frameworks for electrochemical energy storage. Adv Energy Mater. 2017;7(18):1602733.10.1002/aenm.201602733Search in Google Scholar
[9] Zhao J, Zhao Y, Yue W-C, Zheng S-M, Li X, Gao N, et al. Facile fabrication of hollow CuO nanocubes for enhanced lithium/sodium storage performance. CrystEngComm. 2021;23(35):6107–16.10.1039/D1CE00704ASearch in Google Scholar
[10] Zheng X, Shen G, Li Y, Duan H, Yang X, Huang S, et al. Self-templated synthesis of microporous coo nanoparticles with highly enhanced performance for both photocatalysis and lithium-ion batteries. J Mater Chem A. 2013;1(4):1394–400.10.1039/C2TA00536KSearch in Google Scholar
[11] Zheng M, Tang H, Li L, Hu Q, Zhang L, Xue H, et al. Hierarchically nanostructured transition metal oxides for lithium-ion batteries. Adv Sci. 2018;5(3):1700592.10.1002/advs.201700592Search in Google Scholar PubMed PubMed Central
[12] Xia H, Luo Z, Xie J. Nanostructured lithium titanate and lithium titanate/carbon nanocomposite as anode materials for advanced lithium-ion batteries. Nanotechnol Rev. 2014;3(2):161–75.10.1515/ntrev-2012-0049Search in Google Scholar
[13] Ding Y, Hu L, He D, Peng Y, Niu Y, Li Z, et al. Design of multishell microsphere of transition metal oxides/carbon composites for lithium ion battery. Chem Eng J. 2020;380:122489.10.1016/j.cej.2019.122489Search in Google Scholar
[14] Zhang J, Huang Z, He C, Zhang J, Mei P, Han X, et al. Binary carbon-based additives in LiFePO4 cathode with favorable lithium storage. Nanotechnol Rev. 2020;9(1):934–44.10.1515/ntrev-2020-0071Search in Google Scholar
[15] Zhang WM, Wu XL, Hu JS, Guo YG, Wan LJ. Carbon coated Fe3O4 nanospindles as a superior anode material for lithium-ion batteries. Adv Funct Mater. 2008;18(24):3941–6.10.1002/adfm.200801386Search in Google Scholar
[16] Wang Y, Cao G. Developments in nanostructured cathode materials for high-performance lithium-ion batteries. Adv Mater. 2008;20(12):2251–69.10.1002/adma.200702242Search in Google Scholar
[17] Yang X, Peng Y, Hou J, Liu Y, Jian X. A review for modified Li composite anode: Principle, preparation and challenge. Nanotechnol Rev. 2020;9(1):1610–24.10.1515/ntrev-2020-0120Search in Google Scholar
[18] Abdelhamid ME, O’Mullane AP, Snook GA. Storing energy in plastics: a review on conducting polymers & their role in electrochemical energy storage. RSC Adv. 2015;5(15):11611–26.10.1039/C4RA15947KSearch in Google Scholar
[19] Shi Y, Peng L, Ding Y, Zhao Y, Yu G. Nanostructured conductive polymers for advanced energy storage. Chem Soc Rev. 2015;44(19):6684–96.10.1039/C5CS00362HSearch in Google Scholar PubMed
[20] Ponzio EA, Benedetti TM, Torresi RM. Electrochemical and morphological stabilization of V2O5 nanofibers by the addition of polyaniline. Electrochim Acta. 2007;52(13):4419–27.10.1016/j.electacta.2006.12.023Search in Google Scholar
[21] Zhang Q, Sha Z, Cui X, Qiu S, He C, Zhang J, et al. Incorporation of redox-active polyimide binder into LiFePO4 cathode for high-rate electrochemical energy storage. Nanotechnol Rev. 2020;9(1):1350–8.10.1515/ntrev-2020-0092Search in Google Scholar
[22] Lopez J, Mackanic DG, Cui Y, Bao Z. Designing polymers for advanced battery chemistries. Nat Rev Mater. 2019;4(5):312–30.10.1038/s41578-019-0103-6Search in Google Scholar
[23] Joshi B, Samuel E, Kim Y-I, Yarin AL, Swihart MT, Yoon SS. Progress and potential of electrospinning-derived substrate-free and binder-free lithium-ion battery electrodes. Chem Eng J. 2022;430:132876.10.1016/j.cej.2021.132876Search in Google Scholar
[24] Li X, Chen W, Qian Q, Huang H, Chen Y, Wang Z, et al. Electrospinning-based strategies for battery materials. Adv Energy Mater. 2020;11(2):2000845.10.1002/aenm.202000845Search in Google Scholar
[25] Li L, Peng S, Lee JKY, Ji D, Srinivasan M, Ramakrishna S. Electrospun hollow nanofibers for advanced secondary batteries. Nano Energy. 2017;39:111–39.10.1016/j.nanoen.2017.06.050Search in Google Scholar
[26] Yan Y, Liu X, Yan J, Guan C, Wang J. Electrospun nanofibers for new generation flexible energy storage. Energy Environ Mater. 2020;4(4):502–21.10.1002/eem2.12146Search in Google Scholar
[27] Wang H-G, Yuan S, Ma D-L, Zhang X-B, Yan J-M. Electrospun materials for lithium and sodium rechargeable batteries: from structure evolution to electrochemical performance. Energy Environ Sci. 2015;8(6):1660–81.10.1039/C4EE03912BSearch in Google Scholar
[28] Heo YJ, Lee HI, Lee JW, Park M, Rhee KY, Park SJ. Optimization of the pore structure of PAN-based carbon fibers for enhanced supercapacitor performances via electrospinning. Compos Part B Eng. 2019;161:10–7.10.1016/j.compositesb.2018.10.026Search in Google Scholar
[29] Nguyen T, Ngoc U, Hong SC. Structural evolution of poly(acrylonitrile-co-itaconic acid) during thermal oxidative stabilization for carbon materials. Macromolecules. 2013;46(15):5882–9.10.1021/ma401003gSearch in Google Scholar
[30] Zhou X, Dai Z, Liu S, Bao J, Guo YG. Ultra-uniform SnOx/carbon nanohybrids toward advanced lithium-ion battery anodes. Adv Mater. 2014;26(23):3943–9.10.1002/adma.201400173Search in Google Scholar PubMed
[31] Liu Q, Xiao Z, Cui X, Deng S, He Q, Zhang Q, et al. Conjugated cyclized-polyacrylonitrile encapsulated carbon nanotubes as core–sheath heterostructured anodes with favorable lithium storage. J Mater Chem A. 2021;9(11):6962–70.10.1039/D0TA12243BSearch in Google Scholar
[32] Zhang X, Kitao T, Piga D, Hongu R, Bracco S, Comotti A, et al. Carbonization of single polyacrylonitrile chains in coordination nanospaces. Chem Sci. 2020;11(39):10844–9.10.1039/D0SC02048FSearch in Google Scholar PubMed PubMed Central
[33] Ge Y, Fu Z, Deng Y, Zhang M, Zhang H. The effects of chemical reaction on the microstructure and mechanical properties of polyacrylonitrile (PAN) precursor fibers. J Mater Sci. 2019;54(19):12592–604.10.1007/s10853-019-03781-5Search in Google Scholar
[34] He F, Chen G, Yu Y, Hao S, Zhou Y, Zheng Y. Facile approach to synthesize g-PAN/g-C3N4 composites with enhanced photocatalytic H2 evolution activity. ACS Appl Mater Interfaces. 2014;6(10):7171–9.10.1021/am500198ySearch in Google Scholar PubMed
[35] Xie L, Su F, Xie L, Li X, Liu Z, Kong Q, et al. Self-assembled 3D graphene-based aerogel with Co3O4 nanoparticles as high-performance asymmetric supercapacitor electrode. ChemSusChem. 2015;8(17):2917–26.10.1002/cssc.201500355Search in Google Scholar PubMed
[36] Lin G, Jiang Y, He C, Huang Z, Zhang X, Yang Y. In situ encapsulation of Co3O4 polyhedra in graphene sheets for high-capacitance supercapacitors. Dalton Trans. 2019;48(17):5773–8.10.1039/C9DT00521HSearch in Google Scholar
[37] Huang Z, Han X, Cui X, He C, Zhang J, Wang X, et al. Vertically aligned VS2 on graphene as a 3D heteroarchitectured anode material with capacitance-dominated lithium storage. J Mater Chem A. 2020;8(12):5882–9.10.1039/C9TA13835HSearch in Google Scholar
[38] Lei S, Cui X, Liu X, Zhang X, Han X, Yang Y. Hydrothermally self-templated synthesis of rectangular polyimide submicrotubes and promising potentials in electrochemical energy storage. Chem Commun. 2020;56(9):1429–32.10.1039/C9CC09526HSearch in Google Scholar PubMed
[39] Sennu P, Kim HS, An JY, Aravindan V, Lee YS. Synthesis of 2D/2D structured mesoporous Co3O4 nanosheet/n-doped reduced graphene oxide composites as a highly stable negative electrode for lithium battery applications. Chem Asian J. 2015;10(8):1776–83.10.1002/asia.201500466Search in Google Scholar PubMed
[40] Jing M, Zhou M, Li G, Chen Z, Xu W, Chen X, et al. Graphene-embedded Co3O4 rose-spheres for enhanced performance in lithium ion batteries. ACS Appl Mater Interfaces. 2017;9(11):9662–8.10.1021/acsami.6b16396Search in Google Scholar PubMed
[41] Eom W, Kim A, Park H, Kim H, Han TH. Graphene-mimicking 2D porous Co3O4 nanofoils for lithium battery applications. Adv Funct Mater. 2016;26(42):7605–13.10.1002/adfm.201602320Search in Google Scholar
[42] Wang HE, Zhao X, Yin K, Li Y, Chen L, Yang X, et al. Superior pseudocapacitive lithium-ion storage in porous vanadium oxides@C heterostructure composite. ACS Appl Mater Interfaces. 2017;9(50):43665–73.10.1021/acsami.7b13658Search in Google Scholar PubMed
[43] Wang Q, Yang H, Meng T, Yang J, Huang B, Gu FL, et al. Boosting electron transfer with heterointerface effect for high-performance lithium-ion storage. Energy Storage Mater. 2021;36:365–75.10.1016/j.ensm.2021.01.003Search in Google Scholar
[44] Zheng HY, Xie D, Li H, Wu SY, Qin BW, Cui Z, et al. 2D metal-organic framework derived Co3O4 for the oxygen evolution reaction and high-performance lithium-ion batteries. ChemNanoMat. 2020;6(12):1770–5.10.1002/cnma.202000056Search in Google Scholar
[45] Park J, Moon WG, Kim G-P, Nam I, Park S, Kim Y, et al. Three-dimensional aligned mesoporous carbon nanotubes filled with Co3O4 nanoparticles for Li-ion battery anode applications. Electrochim Acta. 2013;105:110–4.10.1016/j.electacta.2013.04.170Search in Google Scholar
[46] Kong S, Xu J, Lin G, Zhang S, Dong W, Wang J, et al. A rationally designed 3D interconnected porous tin dioxide cube with reserved space for volume expansion as an advanced anode of lithium-ion batteries. Chem Commun. 2020;56(71):10289–92.10.1039/D0CC03948ASearch in Google Scholar
[47] Lei ZD, Yang QS, Xu Y, Guo SY, Sun WW, Liu H, et al. Boosting lithium storage in covalent organic framework via activation of 14-electron redox chemistry. Nat Commun. 2018;9:576.10.1038/s41467-018-02889-7Search in Google Scholar PubMed PubMed Central
[48] Gu D, Li W, Wang F, Bongard H, Spliethoff B, Schmidt W, et al. Controllable synthesis of mesoporous peapod-like Co3O4@carbon nanotube arrays for high-performance lithium-ion batteries. Angew Chem Int Ed Engl. 2015;54(24):7060–4.10.1002/anie.201501475Search in Google Scholar PubMed
[49] Wang X, Wu X, Guo Y, Zhong Y, Cao X, Ma Y, et al. Synthesis and lithium storage properties of Co3O4 nanosheet-assembled multishelled hollow spheres. Adv Funct Mater. 2010;20(10):1680–6.10.1002/adfm.200902295Search in Google Scholar
[50] Wang Q, Meng T, Li Y, Yang J, Huang B, Ou S, et al. Consecutive chemical bonds reconstructing surface structure of silicon anode for high-performance lithium-ion battery. Energy Storage Mater. 2021;39:354–64.10.1016/j.ensm.2021.04.043Search in Google Scholar
[51] Chai Y, Wang X, Yu Y, Shi X, Zhang Q, Wang N. Cooperation of Fe2O3@C and Co3O4/C subunits enhances the cyclic stability of Fe2O3@C/Co3O4 electrodes for lithium-ion batteries. Int J Energ Res. 2019;43(11):6045–55.10.1002/er.4705Search in Google Scholar
[52] Jiang F, Li S, Ge P, Tang H, Khoso SA, Zhang C, et al. Size-tunable natural mineral-molybdenite for lithium-ion batteries toward: Enhanced storage capacity and quicken ions transferring. Front Chem. 2018;6:389.10.3389/fchem.2018.00389Search in Google Scholar PubMed PubMed Central
© 2022 Qiming He et al., published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Research Articles
- Theoretical and experimental investigation of MWCNT dispersion effect on the elastic modulus of flexible PDMS/MWCNT nanocomposites
- Mechanical, morphological, and fracture-deformation behavior of MWCNTs-reinforced (Al–Cu–Mg–T351) alloy cast nanocomposites fabricated by optimized mechanical milling and powder metallurgy techniques
- Flammability and physical stability of sugar palm crystalline nanocellulose reinforced thermoplastic sugar palm starch/poly(lactic acid) blend bionanocomposites
- Glutathione-loaded non-ionic surfactant niosomes: A new approach to improve oral bioavailability and hepatoprotective efficacy of glutathione
- Relationship between mechano-bactericidal activity and nanoblades density on chemically strengthened glass
- In situ regulation of microstructure and microwave-absorbing properties of FeSiAl through HNO3 oxidation
- Research on a mechanical model of magnetorheological fluid different diameter particles
- Nanomechanical and dynamic mechanical properties of rubber–wood–plastic composites
- Investigative properties of CeO2 doped with niobium: A combined characterization and DFT studies
- Miniaturized peptidomimetics and nano-vesiculation in endothelin types through probable nano-disk formation and structure property relationships of endothelins’ fragments
- N/S co-doped CoSe/C nanocubes as anode materials for Li-ion batteries
- Synergistic effects of halloysite nanotubes with metal and phosphorus additives on the optimal design of eco-friendly sandwich panels with maximum flame resistance and minimum weight
- Octreotide-conjugated silver nanoparticles for active targeting of somatostatin receptors and their application in a nebulized rat model
- Controllable morphology of Bi2S3 nanostructures formed via hydrothermal vulcanization of Bi2O3 thin-film layer and their photoelectrocatalytic performances
- Development of (−)-epigallocatechin-3-gallate-loaded folate receptor-targeted nanoparticles for prostate cancer treatment
- Enhancement of the mechanical properties of HDPE mineral nanocomposites by filler particles modulation of the matrix plastic/elastic behavior
- Effect of plasticizers on the properties of sugar palm nanocellulose/cinnamon essential oil reinforced starch bionanocomposite films
- Optimization of nano coating to reduce the thermal deformation of ball screws
- Preparation of efficient piezoelectric PVDF–HFP/Ni composite films by high electric field poling
- MHD dissipative Casson nanofluid liquid film flow due to an unsteady stretching sheet with radiation influence and slip velocity phenomenon
- Effects of nano-SiO2 modification on rubberised mortar and concrete with recycled coarse aggregates
- Mechanical and microscopic properties of fiber-reinforced coal gangue-based geopolymer concrete
- Effect of morphology and size on the thermodynamic stability of cerium oxide nanoparticles: Experiment and molecular dynamics calculation
- Mechanical performance of a CFRP composite reinforced via gelatin-CNTs: A study on fiber interfacial enhancement and matrix enhancement
- A practical review over surface modification, nanopatterns, emerging materials, drug delivery systems, and their biophysiochemical properties for dental implants: Recent progresses and advances
- HTR: An ultra-high speed algorithm for cage recognition of clathrate hydrates
- Effects of microalloying elements added by in situ synthesis on the microstructure of WCu composites
- A highly sensitive nanobiosensor based on aptamer-conjugated graphene-decorated rhodium nanoparticles for detection of HER2-positive circulating tumor cells
- Progressive collapse performance of shear strengthened RC frames by nano CFRP
- Core–shell heterostructured composites of carbon nanotubes and imine-linked hyperbranched polymers as metal-free Li-ion anodes
- A Galerkin strategy for tri-hybridized mixture in ethylene glycol comprising variable diffusion and thermal conductivity using non-Fourier’s theory
- Simple models for tensile modulus of shape memory polymer nanocomposites at ambient temperature
- Preparation and morphological studies of tin sulfide nanoparticles and use as efficient photocatalysts for the degradation of rhodamine B and phenol
- Polyethyleneimine-impregnated activated carbon nanofiber composited graphene-derived rice husk char for efficient post-combustion CO2 capture
- Electrospun nanofibers of Co3O4 nanocrystals encapsulated in cyclized-polyacrylonitrile for lithium storage
- Pitting corrosion induced on high-strength high carbon steel wire in high alkaline deaerated chloride electrolyte
- Formulation of polymeric nanoparticles loaded sorafenib; evaluation of cytotoxicity, molecular evaluation, and gene expression studies in lung and breast cancer cell lines
- Engineered nanocomposites in asphalt binders
- Influence of loading voltage, domain ratio, and additional load on the actuation of dielectric elastomer
- Thermally induced hex-graphene transitions in 2D carbon crystals
- The surface modification effect on the interfacial properties of glass fiber-reinforced epoxy: A molecular dynamics study
- Molecular dynamics study of deformation mechanism of interfacial microzone of Cu/Al2Cu/Al composites under tension
- Nanocolloid simulators of luminescent solar concentrator photovoltaic windows
- Compressive strength and anti-chloride ion penetration assessment of geopolymer mortar merging PVA fiber and nano-SiO2 using RBF–BP composite neural network
- Effect of 3-mercapto-1-propane sulfonate sulfonic acid and polyvinylpyrrolidone on the growth of cobalt pillar by electrodeposition
- Dynamics of convective slippery constraints on hybrid radiative Sutterby nanofluid flow by Galerkin finite element simulation
- Preparation of vanadium by the magnesiothermic self-propagating reduction and process control
- Microstructure-dependent photoelectrocatalytic activity of heterogeneous ZnO–ZnS nanosheets
- Cytotoxic and pro-inflammatory effects of molybdenum and tungsten disulphide on human bronchial cells
- Improving recycled aggregate concrete by compression casting and nano-silica
- Chemically reactive Maxwell nanoliquid flow by a stretching surface in the frames of Newtonian heating, nonlinear convection and radiative flux: Nanopolymer flow processing simulation
- Nonlinear dynamic and crack behaviors of carbon nanotubes-reinforced composites with various geometries
- Biosynthesis of copper oxide nanoparticles and its therapeutic efficacy against colon cancer
- Synthesis and characterization of smart stimuli-responsive herbal drug-encapsulated nanoniosome particles for efficient treatment of breast cancer
- Homotopic simulation for heat transport phenomenon of the Burgers nanofluids flow over a stretching cylinder with thermal convective and zero mass flux conditions
- Incorporation of copper and strontium ions in TiO2 nanotubes via dopamine to enhance hemocompatibility and cytocompatibility
- Mechanical, thermal, and barrier properties of starch films incorporated with chitosan nanoparticles
- Mechanical properties and microstructure of nano-strengthened recycled aggregate concrete
- Glucose-responsive nanogels efficiently maintain the stability and activity of therapeutic enzymes
- Tunning matrix rheology and mechanical performance of ultra-high performance concrete using cellulose nanofibers
- Flexible MXene/copper/cellulose nanofiber heat spreader films with enhanced thermal conductivity
- Promoted charge separation and specific surface area via interlacing of N-doped titanium dioxide nanotubes on carbon nitride nanosheets for photocatalytic degradation of Rhodamine B
- Elucidating the role of silicon dioxide and titanium dioxide nanoparticles in mitigating the disease of the eggplant caused by Phomopsis vexans, Ralstonia solanacearum, and root-knot nematode Meloidogyne incognita
- An implication of magnetic dipole in Carreau Yasuda liquid influenced by engine oil using ternary hybrid nanomaterial
- Robust synthesis of a composite phase of copper vanadium oxide with enhanced performance for durable aqueous Zn-ion batteries
- Tunning self-assembled phases of bovine serum albumin via hydrothermal process to synthesize novel functional hydrogel for skin protection against UVB
- A comparative experimental study on damping properties of epoxy nanocomposite beams reinforced with carbon nanotubes and graphene nanoplatelets
- Lightweight and hydrophobic Ni/GO/PVA composite aerogels for ultrahigh performance electromagnetic interference shielding
- Research on the auxetic behavior and mechanical properties of periodically rotating graphene nanostructures
- Repairing performances of novel cement mortar modified with graphene oxide and polyacrylate polymer
- Closed-loop recycling and fabrication of hydrophilic CNT films with high performance
- Design of thin-film configuration of SnO2–Ag2O composites for NO2 gas-sensing applications
- Study on stress distribution of SiC/Al composites based on microstructure models with microns and nanoparticles
- PVDF green nanofibers as potential carriers for improving self-healing and mechanical properties of carbon fiber/epoxy prepregs
- Osteogenesis capability of three-dimensionally printed poly(lactic acid)-halloysite nanotube scaffolds containing strontium ranelate
- Silver nanoparticles induce mitochondria-dependent apoptosis and late non-canonical autophagy in HT-29 colon cancer cells
- Preparation and bonding mechanisms of polymer/metal hybrid composite by nano molding technology
- Damage self-sensing and strain monitoring of glass-reinforced epoxy composite impregnated with graphene nanoplatelet and multiwalled carbon nanotubes
- Thermal analysis characterisation of solar-powered ship using Oldroyd hybrid nanofluids in parabolic trough solar collector: An optimal thermal application
- Pyrene-functionalized halloysite nanotubes for simultaneously detecting and separating Hg(ii) in aqueous media: A comprehensive comparison on interparticle and intraparticle excimers
- Fabrication of self-assembly CNT flexible film and its piezoresistive sensing behaviors
- Thermal valuation and entropy inspection of second-grade nanoscale fluid flow over a stretching surface by applying Koo–Kleinstreuer–Li relation
- Mechanical properties and microstructure of nano-SiO2 and basalt-fiber-reinforced recycled aggregate concrete
- Characterization and tribology performance of polyaniline-coated nanodiamond lubricant additives
- Combined impact of Marangoni convection and thermophoretic particle deposition on chemically reactive transport of nanofluid flow over a stretching surface
- Spark plasma extrusion of binder free hydroxyapatite powder
- An investigation on thermo-mechanical performance of graphene-oxide-reinforced shape memory polymer
- Effect of nanoadditives on the novel leather fiber/recycled poly(ethylene-vinyl-acetate) polymer composites for multifunctional applications: Fabrication, characterizations, and multiobjective optimization using central composite design
- Design selection for a hemispherical dimple core sandwich panel using hybrid multi-criteria decision-making methods
- Improving tensile strength and impact toughness of plasticized poly(lactic acid) biocomposites by incorporating nanofibrillated cellulose
- Green synthesis of spinel copper ferrite (CuFe2O4) nanoparticles and their toxicity
- The effect of TaC and NbC hybrid and mono-nanoparticles on AA2024 nanocomposites: Microstructure, strengthening, and artificial aging
- Excited-state geometry relaxation of pyrene-modified cellulose nanocrystals under UV-light excitation for detecting Fe3+
- Effect of CNTs and MEA on the creep of face-slab concrete at an early age
- Effect of deformation conditions on compression phase transformation of AZ31
- Application of MXene as a new generation of highly conductive coating materials for electromembrane-surrounded solid-phase microextraction
- A comparative study of the elasto-plastic properties for ceramic nanocomposites filled by graphene or graphene oxide nanoplates
- Encapsulation strategies for improving the biological behavior of CdS@ZIF-8 nanocomposites
- Biosynthesis of ZnO NPs from pumpkin seeds’ extract and elucidation of its anticancer potential against breast cancer
- Preliminary trials of the gold nanoparticles conjugated chrysin: An assessment of anti-oxidant, anti-microbial, and in vitro cytotoxic activities of a nanoformulated flavonoid
- Effect of micron-scale pores increased by nano-SiO2 sol modification on the strength of cement mortar
- Fractional simulations for thermal flow of hybrid nanofluid with aluminum oxide and titanium oxide nanoparticles with water and blood base fluids
- The effect of graphene nano-powder on the viscosity of water: An experimental study and artificial neural network modeling
- Development of a novel heat- and shear-resistant nano-silica gelling agent
- Characterization, biocompatibility and in vivo of nominal MnO2-containing wollastonite glass-ceramic
- Entropy production simulation of second-grade magnetic nanomaterials flowing across an expanding surface with viscidness dissipative flux
- Enhancement in structural, morphological, and optical properties of copper oxide for optoelectronic device applications
- Aptamer-functionalized chitosan-coated gold nanoparticle complex as a suitable targeted drug carrier for improved breast cancer treatment
- Performance and overall evaluation of nano-alumina-modified asphalt mixture
- Analysis of pure nanofluid (GO/engine oil) and hybrid nanofluid (GO–Fe3O4/engine oil): Novel thermal and magnetic features
- Synthesis of Ag@AgCl modified anatase/rutile/brookite mixed phase TiO2 and their photocatalytic property
- Mechanisms and influential variables on the abrasion resistance hydraulic concrete
- Synergistic reinforcement mechanism of basalt fiber/cellulose nanocrystals/polypropylene composites
- Achieving excellent oxidation resistance and mechanical properties of TiB2–B4C/carbon aerogel composites by quick-gelation and mechanical mixing
- Microwave-assisted sol–gel template-free synthesis and characterization of silica nanoparticles obtained from South African coal fly ash
- Pulsed laser-assisted synthesis of nano nickel(ii) oxide-anchored graphitic carbon nitride: Characterizations and their potential antibacterial/anti-biofilm applications
- Effects of nano-ZrSi2 on thermal stability of phenolic resin and thermal reusability of quartz–phenolic composites
- Benzaldehyde derivatives on tin electroplating as corrosion resistance for fabricating copper circuit
- Mechanical and heat transfer properties of 4D-printed shape memory graphene oxide/epoxy acrylate composites
- Coupling the vanadium-induced amorphous/crystalline NiFe2O4 with phosphide heterojunction toward active oxygen evolution reaction catalysts
- Graphene-oxide-reinforced cement composites mechanical and microstructural characteristics at elevated temperatures
- Gray correlation analysis of factors influencing compressive strength and durability of nano-SiO2 and PVA fiber reinforced geopolymer mortar
- Preparation of layered gradient Cu–Cr–Ti alloy with excellent mechanical properties, thermal stability, and electrical conductivity
- Recovery of Cr from chrome-containing leather wastes to develop aluminum-based composite material along with Al2O3 ceramic particles: An ingenious approach
- Mechanisms of the improved stiffness of flexible polymers under impact loading
- Anticancer potential of gold nanoparticles (AuNPs) using a battery of in vitro tests
- Review Articles
- Proposed approaches for coronaviruses elimination from wastewater: Membrane techniques and nanotechnology solutions
- Application of Pickering emulsion in oil drilling and production
- The contribution of microfluidics to the fight against tuberculosis
- Graphene-based biosensors for disease theranostics: Development, applications, and recent advancements
- Synthesis and encapsulation of iron oxide nanorods for application in magnetic hyperthermia and photothermal therapy
- Contemporary nano-architectured drugs and leads for ανβ3 integrin-based chemotherapy: Rationale and retrospect
- State-of-the-art review of fabrication, application, and mechanical properties of functionally graded porous nanocomposite materials
- Insights on magnetic spinel ferrites for targeted drug delivery and hyperthermia applications
- A review on heterogeneous oxidation of acetaminophen based on micro and nanoparticles catalyzed by different activators
- Early diagnosis of lung cancer using magnetic nanoparticles-integrated systems
- Advances in ZnO: Manipulation of defects for enhancing their technological potentials
- Efficacious nanomedicine track toward combating COVID-19
- A review of the design, processes, and properties of Mg-based composites
- Green synthesis of nanoparticles for varied applications: Green renewable resources and energy-efficient synthetic routes
- Two-dimensional nanomaterial-based polymer composites: Fundamentals and applications
- Recent progress and challenges in plasmonic nanomaterials
- Apoptotic cell-derived micro/nanosized extracellular vesicles in tissue regeneration
- Electronic noses based on metal oxide nanowires: A review
- Framework materials for supercapacitors
- An overview on the reproductive toxicity of graphene derivatives: Highlighting the importance
- Antibacterial nanomaterials: Upcoming hope to overcome antibiotic resistance crisis
- Research progress of carbon materials in the field of three-dimensional printing polymer nanocomposites
- A review of atomic layer deposition modelling and simulation methodologies: Density functional theory and molecular dynamics
- Recent advances in the preparation of PVDF-based piezoelectric materials
- Recent developments in tensile properties of friction welding of carbon fiber-reinforced composite: A review
- Comprehensive review of the properties of fly ash-based geopolymer with additive of nano-SiO2
- Perspectives in biopolymer/graphene-based composite application: Advances, challenges, and recommendations
- Graphene-based nanocomposite using new modeling molecular dynamic simulations for proposed neutralizing mechanism and real-time sensing of COVID-19
- Nanotechnology application on bamboo materials: A review
- Recent developments and future perspectives of biorenewable nanocomposites for advanced applications
- Nanostructured lipid carrier system: A compendium of their formulation development approaches, optimization strategies by quality by design, and recent applications in drug delivery
- 3D printing customized design of human bone tissue implant and its application
- Design, preparation, and functionalization of nanobiomaterials for enhanced efficacy in current and future biomedical applications
- A brief review of nanoparticles-doped PEDOT:PSS nanocomposite for OLED and OPV
- Nanotechnology interventions as a putative tool for the treatment of dental afflictions
- Recent advancements in metal–organic frameworks integrating quantum dots (QDs@MOF) and their potential applications
- A focused review of short electrospun nanofiber preparation techniques for composite reinforcement
- Microstructural characteristics and nano-modification of interfacial transition zone in concrete: A review
- Latest developments in the upconversion nanotechnology for the rapid detection of food safety: A review
- Strategic applications of nano-fertilizers for sustainable agriculture: Benefits and bottlenecks
- Molecular dynamics application of cocrystal energetic materials: A review
- Synthesis and application of nanometer hydroxyapatite in biomedicine
- Cutting-edge development in waste-recycled nanomaterials for energy storage and conversion applications
- Biological applications of ternary quantum dots: A review
- Nanotherapeutics for hydrogen sulfide-involved treatment: An emerging approach for cancer therapy
- Application of antibacterial nanoparticles in orthodontic materials
- Effect of natural-based biological hydrogels combined with growth factors on skin wound healing
- Nanozymes – A route to overcome microbial resistance: A viewpoint
- Recent developments and applications of smart nanoparticles in biomedicine
- Contemporary review on carbon nanotube (CNT) composites and their impact on multifarious applications
- Interfacial interactions and reinforcing mechanisms of cellulose and chitin nanomaterials and starch derivatives for cement and concrete strength and durability enhancement: A review
- Diamond-like carbon films for tribological modification of rubber
- Layered double hydroxides (LDHs) modified cement-based materials: A systematic review
- Recent research progress and advanced applications of silica/polymer nanocomposites
- Modeling of supramolecular biopolymers: Leading the in silico revolution of tissue engineering and nanomedicine
- Recent advances in perovskites-based optoelectronics
- Biogenic synthesis of palladium nanoparticles: New production methods and applications
- A comprehensive review of nanofluids with fractional derivatives: Modeling and application
- Electrospinning of marine polysaccharides: Processing and chemical aspects, challenges, and future prospects
- Electrohydrodynamic printing for demanding devices: A review of processing and applications
- Rapid Communications
- Structural material with designed thermal twist for a simple actuation
- Recent advances in photothermal materials for solar-driven crude oil adsorption
Articles in the same Issue
- Research Articles
- Theoretical and experimental investigation of MWCNT dispersion effect on the elastic modulus of flexible PDMS/MWCNT nanocomposites
- Mechanical, morphological, and fracture-deformation behavior of MWCNTs-reinforced (Al–Cu–Mg–T351) alloy cast nanocomposites fabricated by optimized mechanical milling and powder metallurgy techniques
- Flammability and physical stability of sugar palm crystalline nanocellulose reinforced thermoplastic sugar palm starch/poly(lactic acid) blend bionanocomposites
- Glutathione-loaded non-ionic surfactant niosomes: A new approach to improve oral bioavailability and hepatoprotective efficacy of glutathione
- Relationship between mechano-bactericidal activity and nanoblades density on chemically strengthened glass
- In situ regulation of microstructure and microwave-absorbing properties of FeSiAl through HNO3 oxidation
- Research on a mechanical model of magnetorheological fluid different diameter particles
- Nanomechanical and dynamic mechanical properties of rubber–wood–plastic composites
- Investigative properties of CeO2 doped with niobium: A combined characterization and DFT studies
- Miniaturized peptidomimetics and nano-vesiculation in endothelin types through probable nano-disk formation and structure property relationships of endothelins’ fragments
- N/S co-doped CoSe/C nanocubes as anode materials for Li-ion batteries
- Synergistic effects of halloysite nanotubes with metal and phosphorus additives on the optimal design of eco-friendly sandwich panels with maximum flame resistance and minimum weight
- Octreotide-conjugated silver nanoparticles for active targeting of somatostatin receptors and their application in a nebulized rat model
- Controllable morphology of Bi2S3 nanostructures formed via hydrothermal vulcanization of Bi2O3 thin-film layer and their photoelectrocatalytic performances
- Development of (−)-epigallocatechin-3-gallate-loaded folate receptor-targeted nanoparticles for prostate cancer treatment
- Enhancement of the mechanical properties of HDPE mineral nanocomposites by filler particles modulation of the matrix plastic/elastic behavior
- Effect of plasticizers on the properties of sugar palm nanocellulose/cinnamon essential oil reinforced starch bionanocomposite films
- Optimization of nano coating to reduce the thermal deformation of ball screws
- Preparation of efficient piezoelectric PVDF–HFP/Ni composite films by high electric field poling
- MHD dissipative Casson nanofluid liquid film flow due to an unsteady stretching sheet with radiation influence and slip velocity phenomenon
- Effects of nano-SiO2 modification on rubberised mortar and concrete with recycled coarse aggregates
- Mechanical and microscopic properties of fiber-reinforced coal gangue-based geopolymer concrete
- Effect of morphology and size on the thermodynamic stability of cerium oxide nanoparticles: Experiment and molecular dynamics calculation
- Mechanical performance of a CFRP composite reinforced via gelatin-CNTs: A study on fiber interfacial enhancement and matrix enhancement
- A practical review over surface modification, nanopatterns, emerging materials, drug delivery systems, and their biophysiochemical properties for dental implants: Recent progresses and advances
- HTR: An ultra-high speed algorithm for cage recognition of clathrate hydrates
- Effects of microalloying elements added by in situ synthesis on the microstructure of WCu composites
- A highly sensitive nanobiosensor based on aptamer-conjugated graphene-decorated rhodium nanoparticles for detection of HER2-positive circulating tumor cells
- Progressive collapse performance of shear strengthened RC frames by nano CFRP
- Core–shell heterostructured composites of carbon nanotubes and imine-linked hyperbranched polymers as metal-free Li-ion anodes
- A Galerkin strategy for tri-hybridized mixture in ethylene glycol comprising variable diffusion and thermal conductivity using non-Fourier’s theory
- Simple models for tensile modulus of shape memory polymer nanocomposites at ambient temperature
- Preparation and morphological studies of tin sulfide nanoparticles and use as efficient photocatalysts for the degradation of rhodamine B and phenol
- Polyethyleneimine-impregnated activated carbon nanofiber composited graphene-derived rice husk char for efficient post-combustion CO2 capture
- Electrospun nanofibers of Co3O4 nanocrystals encapsulated in cyclized-polyacrylonitrile for lithium storage
- Pitting corrosion induced on high-strength high carbon steel wire in high alkaline deaerated chloride electrolyte
- Formulation of polymeric nanoparticles loaded sorafenib; evaluation of cytotoxicity, molecular evaluation, and gene expression studies in lung and breast cancer cell lines
- Engineered nanocomposites in asphalt binders
- Influence of loading voltage, domain ratio, and additional load on the actuation of dielectric elastomer
- Thermally induced hex-graphene transitions in 2D carbon crystals
- The surface modification effect on the interfacial properties of glass fiber-reinforced epoxy: A molecular dynamics study
- Molecular dynamics study of deformation mechanism of interfacial microzone of Cu/Al2Cu/Al composites under tension
- Nanocolloid simulators of luminescent solar concentrator photovoltaic windows
- Compressive strength and anti-chloride ion penetration assessment of geopolymer mortar merging PVA fiber and nano-SiO2 using RBF–BP composite neural network
- Effect of 3-mercapto-1-propane sulfonate sulfonic acid and polyvinylpyrrolidone on the growth of cobalt pillar by electrodeposition
- Dynamics of convective slippery constraints on hybrid radiative Sutterby nanofluid flow by Galerkin finite element simulation
- Preparation of vanadium by the magnesiothermic self-propagating reduction and process control
- Microstructure-dependent photoelectrocatalytic activity of heterogeneous ZnO–ZnS nanosheets
- Cytotoxic and pro-inflammatory effects of molybdenum and tungsten disulphide on human bronchial cells
- Improving recycled aggregate concrete by compression casting and nano-silica
- Chemically reactive Maxwell nanoliquid flow by a stretching surface in the frames of Newtonian heating, nonlinear convection and radiative flux: Nanopolymer flow processing simulation
- Nonlinear dynamic and crack behaviors of carbon nanotubes-reinforced composites with various geometries
- Biosynthesis of copper oxide nanoparticles and its therapeutic efficacy against colon cancer
- Synthesis and characterization of smart stimuli-responsive herbal drug-encapsulated nanoniosome particles for efficient treatment of breast cancer
- Homotopic simulation for heat transport phenomenon of the Burgers nanofluids flow over a stretching cylinder with thermal convective and zero mass flux conditions
- Incorporation of copper and strontium ions in TiO2 nanotubes via dopamine to enhance hemocompatibility and cytocompatibility
- Mechanical, thermal, and barrier properties of starch films incorporated with chitosan nanoparticles
- Mechanical properties and microstructure of nano-strengthened recycled aggregate concrete
- Glucose-responsive nanogels efficiently maintain the stability and activity of therapeutic enzymes
- Tunning matrix rheology and mechanical performance of ultra-high performance concrete using cellulose nanofibers
- Flexible MXene/copper/cellulose nanofiber heat spreader films with enhanced thermal conductivity
- Promoted charge separation and specific surface area via interlacing of N-doped titanium dioxide nanotubes on carbon nitride nanosheets for photocatalytic degradation of Rhodamine B
- Elucidating the role of silicon dioxide and titanium dioxide nanoparticles in mitigating the disease of the eggplant caused by Phomopsis vexans, Ralstonia solanacearum, and root-knot nematode Meloidogyne incognita
- An implication of magnetic dipole in Carreau Yasuda liquid influenced by engine oil using ternary hybrid nanomaterial
- Robust synthesis of a composite phase of copper vanadium oxide with enhanced performance for durable aqueous Zn-ion batteries
- Tunning self-assembled phases of bovine serum albumin via hydrothermal process to synthesize novel functional hydrogel for skin protection against UVB
- A comparative experimental study on damping properties of epoxy nanocomposite beams reinforced with carbon nanotubes and graphene nanoplatelets
- Lightweight and hydrophobic Ni/GO/PVA composite aerogels for ultrahigh performance electromagnetic interference shielding
- Research on the auxetic behavior and mechanical properties of periodically rotating graphene nanostructures
- Repairing performances of novel cement mortar modified with graphene oxide and polyacrylate polymer
- Closed-loop recycling and fabrication of hydrophilic CNT films with high performance
- Design of thin-film configuration of SnO2–Ag2O composites for NO2 gas-sensing applications
- Study on stress distribution of SiC/Al composites based on microstructure models with microns and nanoparticles
- PVDF green nanofibers as potential carriers for improving self-healing and mechanical properties of carbon fiber/epoxy prepregs
- Osteogenesis capability of three-dimensionally printed poly(lactic acid)-halloysite nanotube scaffolds containing strontium ranelate
- Silver nanoparticles induce mitochondria-dependent apoptosis and late non-canonical autophagy in HT-29 colon cancer cells
- Preparation and bonding mechanisms of polymer/metal hybrid composite by nano molding technology
- Damage self-sensing and strain monitoring of glass-reinforced epoxy composite impregnated with graphene nanoplatelet and multiwalled carbon nanotubes
- Thermal analysis characterisation of solar-powered ship using Oldroyd hybrid nanofluids in parabolic trough solar collector: An optimal thermal application
- Pyrene-functionalized halloysite nanotubes for simultaneously detecting and separating Hg(ii) in aqueous media: A comprehensive comparison on interparticle and intraparticle excimers
- Fabrication of self-assembly CNT flexible film and its piezoresistive sensing behaviors
- Thermal valuation and entropy inspection of second-grade nanoscale fluid flow over a stretching surface by applying Koo–Kleinstreuer–Li relation
- Mechanical properties and microstructure of nano-SiO2 and basalt-fiber-reinforced recycled aggregate concrete
- Characterization and tribology performance of polyaniline-coated nanodiamond lubricant additives
- Combined impact of Marangoni convection and thermophoretic particle deposition on chemically reactive transport of nanofluid flow over a stretching surface
- Spark plasma extrusion of binder free hydroxyapatite powder
- An investigation on thermo-mechanical performance of graphene-oxide-reinforced shape memory polymer
- Effect of nanoadditives on the novel leather fiber/recycled poly(ethylene-vinyl-acetate) polymer composites for multifunctional applications: Fabrication, characterizations, and multiobjective optimization using central composite design
- Design selection for a hemispherical dimple core sandwich panel using hybrid multi-criteria decision-making methods
- Improving tensile strength and impact toughness of plasticized poly(lactic acid) biocomposites by incorporating nanofibrillated cellulose
- Green synthesis of spinel copper ferrite (CuFe2O4) nanoparticles and their toxicity
- The effect of TaC and NbC hybrid and mono-nanoparticles on AA2024 nanocomposites: Microstructure, strengthening, and artificial aging
- Excited-state geometry relaxation of pyrene-modified cellulose nanocrystals under UV-light excitation for detecting Fe3+
- Effect of CNTs and MEA on the creep of face-slab concrete at an early age
- Effect of deformation conditions on compression phase transformation of AZ31
- Application of MXene as a new generation of highly conductive coating materials for electromembrane-surrounded solid-phase microextraction
- A comparative study of the elasto-plastic properties for ceramic nanocomposites filled by graphene or graphene oxide nanoplates
- Encapsulation strategies for improving the biological behavior of CdS@ZIF-8 nanocomposites
- Biosynthesis of ZnO NPs from pumpkin seeds’ extract and elucidation of its anticancer potential against breast cancer
- Preliminary trials of the gold nanoparticles conjugated chrysin: An assessment of anti-oxidant, anti-microbial, and in vitro cytotoxic activities of a nanoformulated flavonoid
- Effect of micron-scale pores increased by nano-SiO2 sol modification on the strength of cement mortar
- Fractional simulations for thermal flow of hybrid nanofluid with aluminum oxide and titanium oxide nanoparticles with water and blood base fluids
- The effect of graphene nano-powder on the viscosity of water: An experimental study and artificial neural network modeling
- Development of a novel heat- and shear-resistant nano-silica gelling agent
- Characterization, biocompatibility and in vivo of nominal MnO2-containing wollastonite glass-ceramic
- Entropy production simulation of second-grade magnetic nanomaterials flowing across an expanding surface with viscidness dissipative flux
- Enhancement in structural, morphological, and optical properties of copper oxide for optoelectronic device applications
- Aptamer-functionalized chitosan-coated gold nanoparticle complex as a suitable targeted drug carrier for improved breast cancer treatment
- Performance and overall evaluation of nano-alumina-modified asphalt mixture
- Analysis of pure nanofluid (GO/engine oil) and hybrid nanofluid (GO–Fe3O4/engine oil): Novel thermal and magnetic features
- Synthesis of Ag@AgCl modified anatase/rutile/brookite mixed phase TiO2 and their photocatalytic property
- Mechanisms and influential variables on the abrasion resistance hydraulic concrete
- Synergistic reinforcement mechanism of basalt fiber/cellulose nanocrystals/polypropylene composites
- Achieving excellent oxidation resistance and mechanical properties of TiB2–B4C/carbon aerogel composites by quick-gelation and mechanical mixing
- Microwave-assisted sol–gel template-free synthesis and characterization of silica nanoparticles obtained from South African coal fly ash
- Pulsed laser-assisted synthesis of nano nickel(ii) oxide-anchored graphitic carbon nitride: Characterizations and their potential antibacterial/anti-biofilm applications
- Effects of nano-ZrSi2 on thermal stability of phenolic resin and thermal reusability of quartz–phenolic composites
- Benzaldehyde derivatives on tin electroplating as corrosion resistance for fabricating copper circuit
- Mechanical and heat transfer properties of 4D-printed shape memory graphene oxide/epoxy acrylate composites
- Coupling the vanadium-induced amorphous/crystalline NiFe2O4 with phosphide heterojunction toward active oxygen evolution reaction catalysts
- Graphene-oxide-reinforced cement composites mechanical and microstructural characteristics at elevated temperatures
- Gray correlation analysis of factors influencing compressive strength and durability of nano-SiO2 and PVA fiber reinforced geopolymer mortar
- Preparation of layered gradient Cu–Cr–Ti alloy with excellent mechanical properties, thermal stability, and electrical conductivity
- Recovery of Cr from chrome-containing leather wastes to develop aluminum-based composite material along with Al2O3 ceramic particles: An ingenious approach
- Mechanisms of the improved stiffness of flexible polymers under impact loading
- Anticancer potential of gold nanoparticles (AuNPs) using a battery of in vitro tests
- Review Articles
- Proposed approaches for coronaviruses elimination from wastewater: Membrane techniques and nanotechnology solutions
- Application of Pickering emulsion in oil drilling and production
- The contribution of microfluidics to the fight against tuberculosis
- Graphene-based biosensors for disease theranostics: Development, applications, and recent advancements
- Synthesis and encapsulation of iron oxide nanorods for application in magnetic hyperthermia and photothermal therapy
- Contemporary nano-architectured drugs and leads for ανβ3 integrin-based chemotherapy: Rationale and retrospect
- State-of-the-art review of fabrication, application, and mechanical properties of functionally graded porous nanocomposite materials
- Insights on magnetic spinel ferrites for targeted drug delivery and hyperthermia applications
- A review on heterogeneous oxidation of acetaminophen based on micro and nanoparticles catalyzed by different activators
- Early diagnosis of lung cancer using magnetic nanoparticles-integrated systems
- Advances in ZnO: Manipulation of defects for enhancing their technological potentials
- Efficacious nanomedicine track toward combating COVID-19
- A review of the design, processes, and properties of Mg-based composites
- Green synthesis of nanoparticles for varied applications: Green renewable resources and energy-efficient synthetic routes
- Two-dimensional nanomaterial-based polymer composites: Fundamentals and applications
- Recent progress and challenges in plasmonic nanomaterials
- Apoptotic cell-derived micro/nanosized extracellular vesicles in tissue regeneration
- Electronic noses based on metal oxide nanowires: A review
- Framework materials for supercapacitors
- An overview on the reproductive toxicity of graphene derivatives: Highlighting the importance
- Antibacterial nanomaterials: Upcoming hope to overcome antibiotic resistance crisis
- Research progress of carbon materials in the field of three-dimensional printing polymer nanocomposites
- A review of atomic layer deposition modelling and simulation methodologies: Density functional theory and molecular dynamics
- Recent advances in the preparation of PVDF-based piezoelectric materials
- Recent developments in tensile properties of friction welding of carbon fiber-reinforced composite: A review
- Comprehensive review of the properties of fly ash-based geopolymer with additive of nano-SiO2
- Perspectives in biopolymer/graphene-based composite application: Advances, challenges, and recommendations
- Graphene-based nanocomposite using new modeling molecular dynamic simulations for proposed neutralizing mechanism and real-time sensing of COVID-19
- Nanotechnology application on bamboo materials: A review
- Recent developments and future perspectives of biorenewable nanocomposites for advanced applications
- Nanostructured lipid carrier system: A compendium of their formulation development approaches, optimization strategies by quality by design, and recent applications in drug delivery
- 3D printing customized design of human bone tissue implant and its application
- Design, preparation, and functionalization of nanobiomaterials for enhanced efficacy in current and future biomedical applications
- A brief review of nanoparticles-doped PEDOT:PSS nanocomposite for OLED and OPV
- Nanotechnology interventions as a putative tool for the treatment of dental afflictions
- Recent advancements in metal–organic frameworks integrating quantum dots (QDs@MOF) and their potential applications
- A focused review of short electrospun nanofiber preparation techniques for composite reinforcement
- Microstructural characteristics and nano-modification of interfacial transition zone in concrete: A review
- Latest developments in the upconversion nanotechnology for the rapid detection of food safety: A review
- Strategic applications of nano-fertilizers for sustainable agriculture: Benefits and bottlenecks
- Molecular dynamics application of cocrystal energetic materials: A review
- Synthesis and application of nanometer hydroxyapatite in biomedicine
- Cutting-edge development in waste-recycled nanomaterials for energy storage and conversion applications
- Biological applications of ternary quantum dots: A review
- Nanotherapeutics for hydrogen sulfide-involved treatment: An emerging approach for cancer therapy
- Application of antibacterial nanoparticles in orthodontic materials
- Effect of natural-based biological hydrogels combined with growth factors on skin wound healing
- Nanozymes – A route to overcome microbial resistance: A viewpoint
- Recent developments and applications of smart nanoparticles in biomedicine
- Contemporary review on carbon nanotube (CNT) composites and their impact on multifarious applications
- Interfacial interactions and reinforcing mechanisms of cellulose and chitin nanomaterials and starch derivatives for cement and concrete strength and durability enhancement: A review
- Diamond-like carbon films for tribological modification of rubber
- Layered double hydroxides (LDHs) modified cement-based materials: A systematic review
- Recent research progress and advanced applications of silica/polymer nanocomposites
- Modeling of supramolecular biopolymers: Leading the in silico revolution of tissue engineering and nanomedicine
- Recent advances in perovskites-based optoelectronics
- Biogenic synthesis of palladium nanoparticles: New production methods and applications
- A comprehensive review of nanofluids with fractional derivatives: Modeling and application
- Electrospinning of marine polysaccharides: Processing and chemical aspects, challenges, and future prospects
- Electrohydrodynamic printing for demanding devices: A review of processing and applications
- Rapid Communications
- Structural material with designed thermal twist for a simple actuation
- Recent advances in photothermal materials for solar-driven crude oil adsorption

