Abstract
Novel N1-substituted 3,4-dihydropyrimidin-2(1H)-one derivatives were synthesized through Biginelli condensation of aromatic aldehydes, β-ketoesters, and monosubstituted (thio)ureas in the presence of copper methanesulfonate at 90°C under solvent-free conditions. The screening of the catalysts showed the copper methanesulfonate was the best. Its catalytic activity remained after three times of use. The products were characterized by IR, 1H NMR, 13C NMR, elemental analysis, and X-ray single crystal diffraction technique. A reasonable reaction mechanism was proposed.
1 Introduction
3,4-Dihydropyrimidin-2(1H)-ones (DHPMs) have special therapeutic properties and pharmacological activity. They can be used as calcium channel agents, antiallergic agents, antihypertensive agents and antagonists, and so on [1], [2], [3], [4], [5], [6]. Such derivatives have broad applications in antimicrobial, antiviral, anticancer, sterilization, and other fields [7], [8], [9]. Several marine alkaloids containing DHPM core were also found. The most notable betzelladine alkaloids can inhibit the binding of HIV gp120 membrane protein. It is expected to treat AIDS [10]. Therefore, the research for the synthesis of DHPMs draws more and more attention.
In recent decades, scientists discovered that N1-substituted DHPMs have better pharmacological activity [11], [12]. However, little attention was paid to them. Few papers about the synthesis were reported. One of the most important synthetic methods is the Biginelli reaction. New catalysts [13], [14], [15], [16], [17], [18], [19], [20] and the explored new ways [21], [22], [23], [24] were reported. Nevertheless, some disadvantages such as the expensive catalysts, complex operations, toxic reagents, and the single structure of the product still existed. Consequently, an efficient and green method to synthesize the title products is worth an exploration.
In this study, the N1-substituted DHPMs were synthesized with aromatic aldehydes, ethyl acetoacetate (methyl acetoacetate), and N-substituted (thio)ureas using copper methanesulfonate [Cu(CH3SO3)2·4H2O, abbreviated as CMS] as a catalyst [Eq. (1)]. The N-substituted (thio)ureas include methylurea, ethylurea, p-tolylurea, and methylthiourea. This method avoids the use of a toxic solvent and a high cost. It is convenient, effective, and green. Twenty-one products were synthesized and many of them are new compounds.
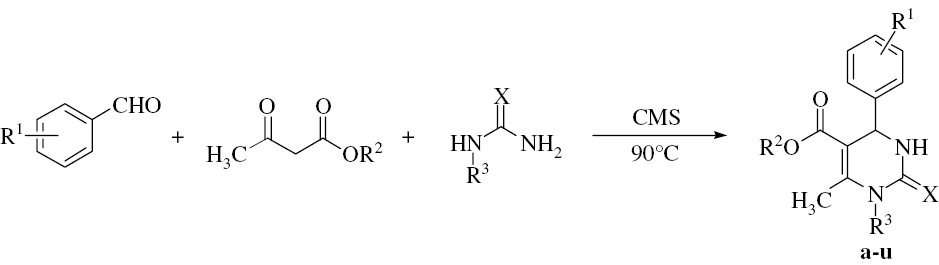 (1)
(1)
2 Materials and methods
2.1 Materials and general methods
All reagents were analytic grade and were obtained commercially (Shanghai Chemical Reagent Company, China). CMS was synthesized according to a reported literature [25]. Thin layer chromatography was performed on Merck Silica gel 60 F254 plates using ethyl acetate/petroleum ether (V/V=3:7) mixture as a mobile phase. Melting points were determined in an open glass capillary on an RY-1 micromelting point apparatus (Tianjin Tianguang Optical Instrument Limited Company, China). A Varian Scimitar 2000 series Fourier transform instrument (Agilent Technologies Inc., Santa Clara, CA, USA) was used for recording the IR spectra using potassium bromide pellets in the range of 400–4000 cm–1. The 1H and 13C NMR spectra were recorded on an Agilent 400-MR instrument (Agilent Technologies Inc., Santa Clara, CA, USA) in DMSO-d6 using TMS as an internal standard. Elemental analyses (C, H, N) were conducted using the Elemental Analyser EA 2400II (Perkin Elmer, Waltham, MA, USA), their results were found to be in good agreement (±0.3%) with the calculated values. Crystallographic data for compound t were collected on a Bruker Smart Apex II diffractometer (Bruker Corporation, Karlsruhe, Germany) with Mo Kα radiation (λ=0.71069 Å) at 296 K using ω-scan technique. The diffraction data were integrated by using the SAINT program, which was also used for the intensity corrections for the Lorentz and polarization effects. Semiempirical absorption corrections were applied using SADABS program. The structures were solved by direct methods, and all of the non-hydrogen atoms were refined anisotropically on F2 by the full-matrix least-squares technique using the SHELXTL crystallographic software package.
2.2 General procedure for the synthesis of N1-substituted DHPMs
Aromatic aldehyde (10 mmol), ethyl acetoacetate (methyl acetoacetate) (10 mmol), N-substituted urea or a thiourea (13 mmol), and CMS (0.3 mmol) were added in a 25-ml reaction flask. The reaction mixture was then stirred at 90°C in an oil bath for an appropriate time (as indicated by TLC). In this process, a solid precipitate was observed in the reaction flask. After completion, the mixture was cooled to room temperature and ice water was added. The crude products were filtrated through a funnel and then purified further by recrystallization with anhydrous ethanol. All title products were characterized by melting point, IR, 1H NMR, 13C NMR, and elemental analysis. The following are spectral data for some of the new compounds:
2.2.1 5-Ethoxycarbonyl-1,6-dimethyl-4-(4-hydroxy-3-methoxyphenyl)-3,4-dihydropyrimidin-2(1H)-one (h)
Orange solid. 1H NMR (400 MHz, DMSO-d6) δ: 8.94 (s, 1H, OH), 7.87 (s, 1H, NH), 6.60–6.78 (m, 3H, ArH), 5.08 (s, 1H, CH), 4.06 (q, 2H, J=6.0 Hz, CH2), 3.74 (s, 3H, OCH3), 3.11 (s, 3H, NCH3), 2.49 (s, 3H, C=CCH3), 1.15 (t, 3H, J=6.0 Hz, OCH2CH3); 13C NMR (100 MHz, DMSO-d6) δ: 166.12, 153.65, 150.47, 147.74, 146.24, 135.50, 118.50, 115.70, 110.95, 103.32, 59.91, 55.93, 52.46, 30.07, 16.43, 14.56; IR (KBr): 3336, 2968, 1683, 1644, 767 cm−1. Anal. calcd. for C16H20N2O5: C 59.99, H 6.29, N 8.74; found C 59.90, H 6.22, N 8.68.
2.2.2 5-Methoxycarbonyl-1,6-dimethyl-4-(4-chlorophenyl)-3,4-dihydropyrimidin-2(1H)-one (k)
Pale yellow solid. 1H NMR (400 MHz, DMSO-d6) δ: 8.07 (s, 1H, NH), 7.39 (d, 2H, J=8.0 Hz, ArH), 7.26 (d, 2H, J=8.0 Hz, ArH), 5.19 (s, 1H, CH), 3.58 (s, 3H, OCH3), 3.11 (s, 3H, NCH3), 2.51 (s, 3H, C=CCH3); 13C NMR (100 MHz, DMSO-d6) δ: 166.37, 153.37, 151.72, 143.25, 132.37, 128.89, 128.39, 102.13, 52.14, 51.53, 30.19, 16.51; IR (KBr): 3215, 2950, 1691, 1622, 793 cm−1. Anal. calcd. for C14H15N2O3Cl: C 57.05, H 5.13, N 9.50; found C 56.96, H 5.09, N 9.54.
2.2.3 5-Methoxycarbonyl-1,6-dimethyl-4-(3-hydroxyphenyl)-3,4-dihydropyrimidin-2(1H)-one (m)
Pale yellow solid. 1H NMR (400 MHz, DMSO-d6) δ: 9.39 (s, 1H, OH), 7.96 (s, 1H, NH), 7.10-6.61 (m, 4H, ArH), 5.08 (s, 1H, CH), 3.58 (s, 3H, OCH3), 3.08 (s, 3H, NCH3), 2.47 (s, 3H, C=CCH3); 13C NMR (100 MHz, DMSO-d6) δ: 166.57, 157.84, 153.67, 151.04, 145.74, 129.88, 116.93, 114.66, 113.25, 102.78, 52.47, 51.54, 30.20, 16.51; IR (KBr): 3377, 2951, 1682, 1598, 787 cm−1. Anal. calcd. for C14H16N2O4: C 60.86, H 5.84, N 10.14; found C 60.75, H 5.81, N 10.17.
2.2.4 5-Ethoxycarbonyl-1-ethyl-6-methyl-4-(4-chlorophenyl)-3,4-dihydropyrimidin-2(1H)-one (p)
White solid. 1H NMR (400 MHz, DMSO-d6) δ: 7.91 (d, 1H, J=4 Hz, NH), 7.35 (d, 2H, J=8.0 Hz, ArH), 7.19 (d, 2H, J=8.0 Hz, ArH), 5.10 (d, 1H, J=4.0 Hz, CH), 3.98 (q, 2H, J=8.0 Hz, CH2), 3.76 (dd, 1H, J=16.0, 8.0 Hz, NCH2), 3.57 (dd, 1H, J=16.0, 8.0 Hz, NCH2), 2.47 (s, 3H, C=CCH3), 1.09-1.01 (m, 6H, OCH2CH3, NCH2CH3); 13C NMR (100 MHz, DMSO-d6) δ: 165.88, 152.72, 150.28, 143.56, 132.28, 128.86, 128.44, 102.74, 60.03, 52.42, 37.40, 15.91, 15.20, 14.46; IR (KBr): 3413, 2985, 1686, 1616, 768 cm−1. Anal. calcd. for C16H19N2O3Cl: C 59.54, H 5.93, N 8.68; found C 59.60, H 5.91, N 8.72.
2.2.5 5-Ethoxycarbonyl-1-ethyl-6-methyl-4-(4-nitrophenyl)-3,4-dihydropyrimidin-2(1H)-one (q)
Yellow solid. 1H NMR (400 MHz, DMSO-d6) δ: 8.18 (d, 2H, J=8.0 Hz, ArH), 8.05 (d, 1H, J=4.0 Hz, NH), 7.44 (d, 2H, J=8.0 Hz, ArH), 5.23 (d, 1H, J=4.0 Hz, CH), 4.00 (q, 2H, J=8.0 Hz, CH2), 3.76 (dd, 1H, J=16.0, 8.0 Hz, NCH2), 3.58 (dd, 1H, J=16.0, 8.0 Hz, NCH2), 2.48 (s, 3H, C=CCH3), 1.09-1.02 (m, 6H, OCH2CH3, NCH2CH3); 13C NMR (100 MHz, DMSO-d6) δ: 165.72, 152.56, 151.78, 151.02, 147.16, 127.88, 124.28, 102.02, 60.17, 52.63, 37.52 15.97 15.18 14.44; IR (KBr): 3405, 2985, 1692, 1616, 768 cm−1. Anal. calcd. for C16H19N3O5: C 57.65, H 5.74, N 12.60; found C 57.56, H 5.71, N 12.66.
2.2.6 5-Methoxycarbonyl-1-ethyl-6-methyl-4-(4-nitrophenyl)-3,4-dihydropyrimidin-2(1H)-one (r)
Pale yellow solid. 1H NMR (400 MHz, DMSO-d6) δ: 8.22 (d, 2H, J=8.0 Hz, ArH), 8.12 (d, 1H, J=4.0 Hz, NH), 7.49 (d, 2H, J=8.0 Hz, ArH), 5.26 (d, 1H, J=4.0 Hz, CH), 3.81 (dd, 1H, J=16.0 Hz, 8.0 Hz, NCH2), 3.63 (dd, 1H, J=16.0, 8.0 Hz, NCH2), 3.58 (s, 3H, OCH3), 2.53 (s, 3H, C=CCH3), 1.07 (t, 3H, J=8.0 Hz, NCH2CH3); 13C NMR (100 MHz, DMSO-d6) δ: 166.23, 152.61, 151.58, 151.30, 147.18, 127.83, 124.31, 101.81, 52.44, 51.68, 37.54, 16.02, 15.18; IR (KBr): 3397, 2992, 1686, 1616, 768 cm−1. Anal. calcd. for C15H17N3O5: C 56.42, H 5.37, N 13.16; found C 56.35, H 5.41, N 13.12.
2.2.7 5-Methoxycarbonyl-1-ethyl-6-methyl-4-(2-chlorophenyl)-3,4-dihydropyrimidin-2(1H)-one (s)
Pale yellow solid. 1H NMR (400 MHz, DMSO-d6) δ: 7.89 (d, 1H, J=4.0 Hz, NH), 7.42 (d, 1H, J=8.0 Hz, ArH), 7.32-7.25 (m, 3H, ArH), 5.59 (s, 1H, CH), 3.83 (dd, 1H, J=16.0, 8.0 Hz, NCH2), 3.65 (dd, 1H, J=16.0, 8.0 Hz, NCH2), 3.48 (s, 3H, OCH3), 2.56 (s, 3H, C=CCH3), 1.12 (t, 3H, J=8.0 Hz, NCH2CH3); 13C NMR (100 MHz, DMSO-d6) δ: 166.21, 152.12, 151.07, 141.21, 132.16, 130.02, 129.70, 128.77, 128.15, 101.44, 51.50, 50.52, 37.45, 15.90, 15.23; IR (KBr): 3417, 2983, 1683, 1634, 768 cm−1. Anal. calcd. for C15H17N2O3Cl: C 58.35, H 5.55, N 9.07; found C 58.42, H 5.60, N 9.01.
2.2.8 5-Ethoxycarbonyl-1-(4-methylphenyl)-6-methyl-4-phenyl-3,4-dihydropyrimidin-2(1H)-one (t)
Pale yellow solid. 1H NMR (400 MHz, DMSO-d6) δ: 8.18 (s, 1H, NH), 7.12-7.43 (m, 9H, ArH), 5.34 (s, 1H, CH), 4.07 (q, 2H, J =4.0 Hz, OCH2), 2.36 (s, 3H, C=CCH3), 2.07 (s, 3H, ArCH3), 1.14 (t, 3H, J=4.0 Hz, CH2CH3); 13C NMR (100 MHz, DMSO-d6) δ: 165.89, 152.62, 149.55, 144.54, 137.86, 135.55, 129.83, 129.58, 129.05, 127.92, 126.71, 118.64, 60.14, 53.55, 21.10, 18.49, 14.46; IR (KBr): 3234, 2978, 1693, 1624, 758 cm−1. Anal. calcd. for C21H22N2O3: C 71.98, H 6.33, N 7.99; found C 71.87, H 6.26, N 7.96.
2.2.9 5-Methoxycarbonyl-1-(4-methylphenyl)-6-methyl-4-phenyl-3,4-dihydropyrimidin-2(1H)-one (u)
Pale yellow solid. 1H NMR (400 MHz, DMSO-d6) δ: 8.21 (s, 1H, NH), 7.43-7.04 (m, 9H, ArH), 5.34 (s, 1H, CH), 3.61 (s, 3H, OCH3), 2.35 (s, 3H, C=CCH3), 2.08 (s, 3H, ArCH3); 13C NMR (100 MHz, DMSO-d6) δ: 166.41, 156.57, 152.67, 144.34, 137.90, 135.50, 129.85, 129.42, 129.12, 127.95, 126.64, 118.29, 53.36, 51.63, 21.09, 18.55; IR (KBr): 3430, 2922, 1693, 1654, 763 cm−1. Anal. calcd. for C20H20N2O3: C 71.41, H 5.99, N 8.33; found C 71.33, H 6.02, N 8.37.
3 Results and discussion
3.1 Screening of catalysts
First, we compared the catalytic activity of different metal methanesulfonates in Table 1. The activity of CMS is higher than that of the other methanesulfonates. 5-Ethoxycarbonyl-1,6-dimethyl-4-phenyl-3,4-dihydropyrimidin-2(1H)-one was obtained in 94% yield within 0.3 h (entry 1). However, only 40% product yield was obtained in the absence of a catalyst (entry 14).
Comparison of the catalytic activity among different metal methanesulfonates.
| Entry | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Metal | Cu | La | Fe(II) | Zn | Pr | Yb | Ce(III) | Mg | Ni | Sr | Ca | Cd | Al | – |
| Time (h) | 0.3 | 0.4 | 0.7 | 0.3 | 0.3 | 0.5 | 1.0 | 1.5 | 1.0 | 2.5 | 1.5 | 1.3 | 2.0 | 3.0 |
| Yield (%) | 94 | 92 | 92 | 88 | 87 | 82 | 82 | 72 | 70 | 66 | 63 | 62 | 56 | 40 |
Reaction conditions: benzaldehyde (10 mmol), ethyl acetoacetate (10 mmol), methylurea (13 mmol), metal methanesulfonates (0.3 mmol), solvent-free, 80°C.
3.2 Reaction temperature and reusable catalyst
Then, we investigated the reaction temperature using the model reaction of benzaldehyde, ethyl acetoacetate, and methylurea. As shown in Table 2, the reaction at 90°C gave the best result (entry 3). When the reaction was completed, the reaction mixture was washed with water. The catalyst remained in aqueous phase and could be recovered by evaporating the filtrate. The CMS can be reused for the next two consecutive reactions. It demonstrated that CMS was water-tolerant and recyclable.
Different reaction temperatures and the reusability of CMS.
| Entry | Temperature (°C) | Time (h) | Yield (%) |
|---|---|---|---|
| 1 | 70 | 0.7 | 93 |
| 2 | 80 | 0.3 | 94 |
| 3 | 90 | 0.2 | 96, 93, 89 |
Reaction conditions: benzaldehyde (10 mmol), ethyl acetoacetate (10 mmol), methylurea (13 mmol), CMS (0.3 mmol), solvent-free.
3.3 One-pot synthesis of N1-substituted DHPMs
Next, N1-substituted DHPMs were synthesized under optimum conditions (Table 3). The Biginelli three-component reaction of aromatic aldehydes, β-ketoesters, and N-substituted (thio)ureas gave good yields of the products in a short time. The reaction accommodated a variety of aromatic aldehydes. Whether the substituents on the aromatic aldehyde are electron-withdrawing or -donating groups all afforded high yields. Unfortunately, substrates with aliphatic aldehydes gave no desired product. Ethyl acetoacetate and methyl acetoacetate were equally effective in carrying out the reaction. N-Methylurea, N-ethylurea, p-tolylurea, and N-methylthiourea were varied. In all cases, the pure products were obtained by simple filtration and recrystallization. No column chromatography or any cumbersome workup technique was applied. The methodology using a reusable catalyst without solvent is a green protocol obviously.
Three-component one-pot synthesis of N1-substituted DHPMs catalyzed by CMS.
| Product | R1 | R2 | R3 | X | Yield (%) (time/h) | Mp (°C) | |
|---|---|---|---|---|---|---|---|
| Found | Reported | ||||||
| a | H | Et | Me | O | 96 (0.2) | 178–180 | 175–176 [14] |
| b | 4-Cl | Et | Me | O | 94 (1.0) | 132–134 | 133–135 [14] |
| c | 4-CH3 | Et | Me | O | 94 (1.0) | 116–118 | 119–120 [14] |
| d | 2-CH3O | Et | Me | O | 89 (0.8) | 147–149 | 145–146 [15] |
| e | 4-CH3O | Et | Me | O | 93 (1.5) | 138–140 | 138–140 [16] |
| f | 3-NO2 | Et | Me | O | 92 (0.5) | 137–139 | 136–137 [13] |
| g | 4-OH | Et | Me | O | 85 (0.4) | 183–184 | 178–180 [16] |
| h | 4-OH-3-CH3O | Et | Me | O | 94 (0.3) | 181–183 | – |
| i | H | Et | Me | S | 40 (6.0) | 145–147 | 146–147 [17] |
| J | H | Me | Me | O | 94 (0.2) | 193–195 | 190–192 [16] |
| k | 4-Cl | Me | Me | O | 97 (0.5) | 134–137 | – |
| l | 2-CH3O | Me | Me | O | 78 (0.2) | 171–173 | 170–172 [15] |
| m | 3-OH | Me | Me | O | 93 (0.1) | 220–223 | – |
| n | H | Me | Me | S | 46 (6.0) | 159–160 | 160–162 [18] |
| o | H | Et | Et | O | 63 (3.0) | 112–115 | 118 [11] |
| p | 4-Cl | Et | Et | O | 93 (2.0) | 146–148 | – |
| q | 4-NO2 | Et | Et | O | 92 (1.5) | 152–154 | – |
| r | 4-NO2 | Me | Et | O | 94 (0.8) | 198–200 | – |
| s | 2-Cl | Me | Et | O | 70 (1.5) | 121–123 | – |
| t | H | Et | p-CH3C6H4 | O | 82 (3.0) | 179–181 | – |
| u | H | Me | p-CH3C6H4 | O | 95 (3.0) | 152–158 | – |
Reaction conditions: aromatic aldehydes (10 mmol), β-ketoesters (10 mmol), N-substituted (thio)ureas (13 mmol), CMS (0.3 mmol), solvent-free, 90°C.
All the structures of the products were determined by IR, 1H NMR, 13C NMR, and elemental analysis. Furthermore, a single crystal detection of the product t was obtained to investigate the molecular structure (Figure 1). The pyrimidine ring composed of N1-C2-N3-C4-C5-C6 is a boat conformation. The C-4 is a choral carbon and its configuration is R. An X-ray structure analysis verified the synthesized products.
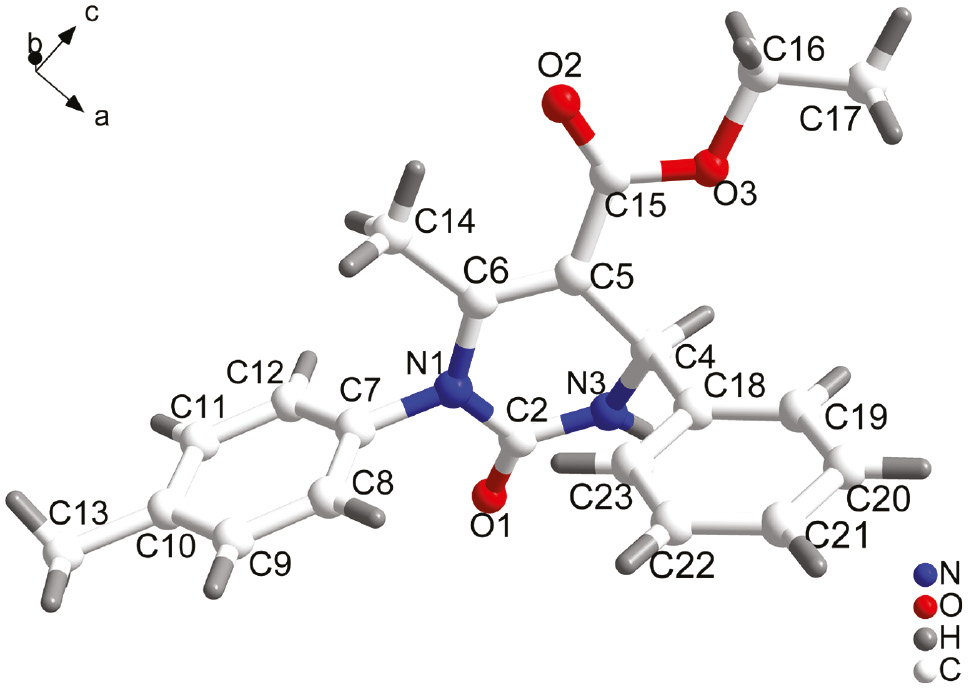
The molecular structure of the compound t.
3.4 Reaction mechanism
A possible reaction mechanism was proposed for this transformation [26]. At first, an aldehyde reacted with a (thio)urea to form an acylimine intermediate under the catalysis of CMS. Then, an open-chain ureide is obtained by the addition of a β-ketoester to the acylimine, which undergoes cyclization and dehydration to form the target product [Eq. (2)].
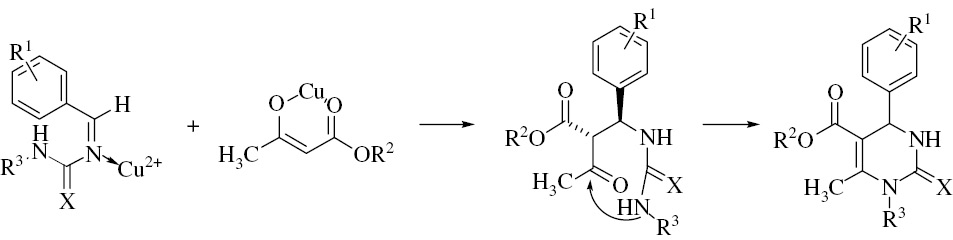 (2)
(2)
4 Conclusions
In conclusion, we have reported a simple, efficient, and environmentally friendly method to synthesize N1-substituted DHPMs from aromatic aldehydes, ethyl acetoacetate/methyl acetoacetate, and monosubstituted (thio)ureas with CMS as a catalyst at 90°C. Neither toxic solvent nor expensive catalyst was used. It has mild reaction conditions, simple operation, short reaction time, high yield, and other environmentally friendly advantages. The most notable feature is the expansion of the substrates. The substituents on the urea were beyond just Me or Et. The reaction scope is fairly general with regard to various substrates. Many novel N1-substituted DHPMs were synthesized first. The catalyst can be reused several times. The green methodology provides a better alternative to the existing reports.
Acknowledgments
This research work was financially supported by the Program for Liaoning Excellent Talents in University (no. LJQ2015002), the Natural Science Foundation of Liaoning Province (no. 2015020201), and the National Natural Science Foundation of China (no. 21406016).
References
[1] Atwal KS, Rovnyak GC, Kimball SD, Floyd DM, Moreland S, Swanson BN, Gougoutas JZ, Schwartz J, Smillie KM, Malley MF. J. Med. Chem. 1990, 33, 2629–2635.10.1021/jm00171a044Suche in Google Scholar PubMed
[2] Atwal KS, Swanson BN, Unger SE, Floyd DM, Moreland S, Hedberg A, O’Reilly BC. J. Med. Chem. 1991, 34, 806–811.10.1021/jm00106a048Suche in Google Scholar PubMed
[3] Rovnyak GC, Atwal KS, Hedberg A, Kimball SD, Moreland S, Gougoutas JZ, O’Reilly BC, Schwartz J, Malley MF. J. Med. Chem. 1992, 35, 3254–3263.10.1021/jm00095a023Suche in Google Scholar PubMed
[4] Rovnyak GC, Kimball SD, Beyer B, Cucinotta G, DiMarco JD, Gougoutas J, Hedberg A, Malley M, McCarthy JP, Zhang R, Moreland S. J. Med. Chem. 1995, 38, 119–129.10.1021/jm00001a017Suche in Google Scholar PubMed
[5] Cho H, Ueda M, Shima K, Mizuno A, Hayashimatsu M, Ohnaka Y, Takeuchi Y, Hamaguchi M, Aisaka K, Hidaka T, Kawai M, Takeda M, Ishihara T, Funahashi K, Satoh F, Morita M, Noguchi T. J. Med. Chem. 1989, 32, 2399–2406.10.1021/jm00130a029Suche in Google Scholar PubMed
[6] Grover GJ, Dzwonczyk S, McMullen DM, Normandin DE, Parham CS, Sleph PG, Moreland S. J. Cardiovasc. Pharmacol. 1995, 26, 289–294.10.1097/00005344-199508000-00015Suche in Google Scholar PubMed
[7] Cochran JC, Gatial JE, Kapoor TM, Gilbert SP. J. Biol. Chem. 2005, 280, 12658–12667.10.1074/jbc.M413140200Suche in Google Scholar PubMed PubMed Central
[8] Kumarasamy D, Roy BG, Rocha-Pereira J, Neyts J, Nanjappan S, Maity S, Mookerjee M, Naesens L. Bioorg. Med. Chem. Lett. 2017, 27, 139–142.10.1016/j.bmcl.2016.12.010Suche in Google Scholar PubMed PubMed Central
[9] Terracciano S, Lauro G, Strocchia M, Fischer K, Werz O, Riccio R, Bruno I, Bifulco G. ACS Med. Chem. Lett. 2015, 6, 187–191.10.1021/ml500433jSuche in Google Scholar PubMed PubMed Central
[10] Patil AD, Kumar NV, Kokke WC, Bean MF, Freyer AJ, Brosse C, Mai S, Truneh A, Faulkner DJ, Carte B, Breen AL, Hertzberg RP, Johnson RK, Westley JW, Potts BCM. J. Org. Chem. 1995, 60, 1182–1188.10.1021/jo00110a021Suche in Google Scholar
[11] Singh K, Arora D, Poremsky E, Lowery J, Moreland RS. Eur. J. Med. Chem. 2009, 44, 1997–2001.10.1016/j.ejmech.2008.10.002Suche in Google Scholar PubMed
[12] Zalavadiya P, Tala S, Akbari J, Joshi H. Arch. Pharm. Chem. Life Sci. 2009, 342, 469–475.10.1002/ardp.200800224Suche in Google Scholar PubMed
[13] Putatunda S, Chakraborty S, Ghosh S, Nandi P, Chakraborty S, Sen PC, Chakraborty A. Eur. J. Med. Chem. 2012, 54, 223–231.10.1016/j.ejmech.2012.04.043Suche in Google Scholar PubMed
[14] Besoluk S, Kucukislamoglu M, Nebioglu M, Zengin M, Arslan M. J. Iran. Chem. Soc. 2008, 5, 62–66.10.1007/BF03245816Suche in Google Scholar
[15] Aridoss G, Jeong YT. Bull. Korean Chem. Soc. 2010, 31, 863–868.10.5012/bkcs.2010.31.04.863Suche in Google Scholar
[16] Salehi H, Guo QX. Chinese J. Chem. 2005, 23, 91–97.10.1002/cjoc.200590021Suche in Google Scholar
[17] Kappe CO, Kumar D, Varma RS. Synthesis 1999, 1799–1803.10.1055/s-1999-3592Suche in Google Scholar
[18] Memarian HR, Ranjbar M. J. Chin. Chem. Soc. 2011, 58, 522–527.10.1002/jccs.201190016Suche in Google Scholar
[19] Lauro G, Strocchia M, Terracciano S, Bruno I, Fischer K, Pergola C, Werz O, Riccio R, Bifulco G. Eur. J. Med. Chem. 2014, 80, 407–415.10.1016/j.ejmech.2014.04.061Suche in Google Scholar PubMed
[20] Zlatković DB, Radulović NS. RSC Adv. 2016, 6, 115058–115067.10.1039/C6RA24535HSuche in Google Scholar
[21] Wipf P, Cunningham A. Tetrahedron Lett. 1995, 36, 7819–7822.10.1016/0040-4039(95)01660-ASuche in Google Scholar
[22] Chen Q, Liu QJ, Wang HP. Molbank 2012, 2012, M752.10.3390/M752Suche in Google Scholar
[23] Ryabukhin SV, Plaskon AS, Bondarenko SS, Ostapchuk EN, Grygorenko OO, Shishkin OV, Tolmachev AA. Tetrahedron Lett. 2010, 51, 4229–4232.10.1016/j.tetlet.2010.06.032Suche in Google Scholar
[24] Andleeb S, Imtiza D, Rauf MK, Azam SS, Badshah A, Sadaf H, Raheel A, Tahir MN, Raza S. RSC Adv. 2016, 6, 79651–79661.10.1039/C6RA19162BSuche in Google Scholar
[25] Wang M, Song ZG, Jiang H, Gong H. J. Therm. Anal. Calorim. 2009, 98, 801–806.10.1007/s10973-009-0119-zSuche in Google Scholar
[26] Kappe CO. J. Org. Chem. 1997, 62, 7201–7204.10.1021/jo971010uSuche in Google Scholar PubMed
©2019 Walter de Gruyter GmbH, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 Public License.
Artikel in diesem Heft
- Regular Articles
- Studies on the preparation and properties of biodegradable polyester from soybean oil
- Flow-mode biodiesel production from palm oil using a pressurized microwave reactor
- Reduction of free fatty acids in waste oil for biodiesel production by glycerolysis: investigation and optimization of process parameters
- Saccharin: a cheap and mild acidic agent for the synthesis of azo dyes via telescoped dediazotization
- Optimization of lipase-catalyzed synthesis of polyethylene glycol stearate in a solvent-free system
- Green synthesis of iron oxide nanoparticles using Platanus orientalis leaf extract for antifungal activity
- Ultrasound assisted chemical activation of peanut husk for copper removal
- Room temperature silanization of Fe3O4 for the preparation of phenyl functionalized magnetic adsorbent for dispersive solid phase extraction for the extraction of phthalates in water
- Evaluation of the saponin green extraction from Ziziphus spina-christi leaves using hydrothermal, microwave and Bain-Marie water bath heating methods
- Oxidation of dibenzothiophene using the heterogeneous catalyst of tungsten-based carbon nanotubes
- Calcined sodium silicate as an efficient and benign heterogeneous catalyst for the transesterification of natural lecithin to L-α-glycerophosphocholine
- Synergistic effect between CO2 and H2O2 on ethylbenzene oxidation catalyzed by carbon supported heteropolyanion catalysts
- Hydrocyanation of 2-arylmethyleneindan-1,3-diones using potassium hexacyanoferrate(II) as a nontoxic cyanating agent
- Green synthesis of hydratropic aldehyde from α-methylstyrene catalyzed by Al2O3-supported metal phthalocyanines
- Environmentally benign chemical recycling of polycarbonate wastes: comparison of micro- and nano-TiO2 solid support efficiencies
- Medicago polymorpha-mediated antibacterial silver nanoparticles in the reduction of methyl orange
- Production of value-added chemicals from esterification of waste glycerol over MCM-41 supported catalysts
- Green synthesis of zerovalent copper nanoparticles for efficient reduction of toxic azo dyes congo red and methyl orange
- Optimization of the biological synthesis of silver nanoparticles using Penicillium oxalicum GRS-1 and their antimicrobial effects against common food-borne pathogens
- Optimization of submerged fermentation conditions to overproduce bioethanol using two industrial and traditional Saccharomyces cerevisiae strains
- Extraction of In3+ and Fe3+ from sulfate solutions by using a 3D-printed “Y”-shaped microreactor
- Foliar-mediated Ag:ZnO nanophotocatalysts: green synthesis, characterization, pollutants degradation, and in vitro biocidal activity
- Green cyclic acetals production by glycerol etherification reaction with benzaldehyde using cationic acidic resin
- Biosynthesis, characterization and antimicrobial activities assessment of fabricated selenium nanoparticles using Pelargonium zonale leaf extract
- Synthesis of high surface area magnesia by using walnut shell as a template
- Controllable biosynthesis of silver nanoparticles using actinobacterial strains
- Green vegetation: a promising source of color dyes
- Mechano-chemical synthesis of ammonia and acetic acid from inorganic materials in water
- Green synthesis and structural characterization of novel N1-substituted 3,4-dihydropyrimidin-2(1H)-ones
- Biodiesel production from cotton oil using heterogeneous CaO catalysts from eggshells prepared at different calcination temperatures
- Regeneration of spent mercury catalyst for the treatment of dye wastewater by the microwave and ultrasonic spray-assisted method
- Green synthesis of the innovative super paramagnetic nanoparticles from the leaves extract of Fraxinus chinensis Roxb and their application for the decolourisation of toxic dyes
- Biogenic ZnO nanoparticles: a study of blueshift of optical band gap and photocatalytic degradation of reactive yellow 186 dye under direct sunlight
- Leached compounds from the extracts of pomegranate peel, green coconut shell, and karuvelam wood for the removal of hexavalent chromium
- Enhancement of molecular weight reduction of natural rubber in triphasic CO2/toluene/H2O systems with hydrogen peroxide for preparation of biobased polyurethanes
- An efficient green synthesis of novel 1H-imidazo[1,2-a]imidazole-3-amine and imidazo[2,1-c][1,2,4]triazole-5-amine derivatives via Strecker reaction under controlled microwave heating
- Evaluation of three different green fabrication methods for the synthesis of crystalline ZnO nanoparticles using Pelargonium zonale leaf extract
- A highly efficient and multifunctional biomass supporting Ag, Ni, and Cu nanoparticles through wetness impregnation for environmental remediation
- Simple one-pot green method for large-scale production of mesalamine, an anti-inflammatory agent
- Relationships between step and cumulative PMI and E-factors: implications on estimating material efficiency with respect to charting synthesis optimization strategies
- A comparative sorption study of Cr3+ and Cr6+ using mango peels: kinetic, equilibrium and thermodynamic
- Effects of acid hydrolysis waste liquid recycle on preparation of microcrystalline cellulose
- Use of deep eutectic solvents as catalyst: A mini-review
- Microwave-assisted synthesis of pyrrolidinone derivatives using 1,1’-butylenebis(3-sulfo-3H-imidazol-1-ium) chloride in ethylene glycol
- Green and eco-friendly synthesis of Co3O4 and Ag-Co3O4: Characterization and photo-catalytic activity
- Adsorption optimized of the coal-based material and application for cyanide wastewater treatment
- Aloe vera leaf extract mediated green synthesis of selenium nanoparticles and assessment of their In vitro antimicrobial activity against spoilage fungi and pathogenic bacteria strains
- Waste phenolic resin derived activated carbon by microwave-assisted KOH activation and application to dye wastewater treatment
- Direct ethanol production from cellulose by consortium of Trichoderma reesei and Candida molischiana
- Agricultural waste biomass-assisted nanostructures: Synthesis and application
- Biodiesel production from rubber seed oil using calcium oxide derived from eggshell as catalyst – optimization and modeling studies
- Study of fabrication of fully aqueous solution processed SnS quantum dot-sensitized solar cell
- Assessment of aqueous extract of Gypsophila aretioides for inhibitory effects on calcium carbonate formation
- An environmentally friendly acylation reaction of 2-methylnaphthalene in solvent-free condition in a micro-channel reactor
- Aegle marmelos phytochemical stabilized synthesis and characterization of ZnO nanoparticles and their role against agriculture and food pathogen
- A reactive coupling process for co-production of solketal and biodiesel
- Optimization of the asymmetric synthesis of (S)-1-phenylethanol using Ispir bean as whole-cell biocatalyst
- Synthesis of pyrazolopyridine and pyrazoloquinoline derivatives by one-pot, three-component reactions of arylglyoxals, 3-methyl-1-aryl-1H-pyrazol-5-amines and cyclic 1,3-dicarbonyl compounds in the presence of tetrapropylammonium bromide
- Preconcentration of morphine in urine sample using a green and solvent-free microextraction method
- Extraction of glycyrrhizic acid by aqueous two-phase system formed by PEG and two environmentally friendly organic acid salts - sodium citrate and sodium tartrate
- Green synthesis of copper oxide nanoparticles using Juglans regia leaf extract and assessment of their physico-chemical and biological properties
- Deep eutectic solvents (DESs) as powerful and recyclable catalysts and solvents for the synthesis of 3,4-dihydropyrimidin-2(1H)-ones/thiones
- Biosynthesis, characterization and anti-microbial activity of silver nanoparticle based gel hand wash
- Efficient and selective microwave-assisted O-methylation of phenolic compounds using tetramethylammonium hydroxide (TMAH)
- Anticoagulant, thrombolytic and antibacterial activities of Euphorbia acruensis latex-mediated bioengineered silver nanoparticles
- Volcanic ash as reusable catalyst in the green synthesis of 3H-1,5-benzodiazepines
- Green synthesis, anionic polymerization of 1,4-bis(methacryloyl)piperazine using Algerian clay as catalyst
- Selenium supplementation during fermentation with sugar beet molasses and Saccharomyces cerevisiae to increase bioethanol production
- Biosynthetic potential assessment of four food pathogenic bacteria in hydrothermally silver nanoparticles fabrication
- Investigating the effectiveness of classical and eco-friendly approaches for synthesis of dialdehydes from organic dihalides
- Pyrolysis of palm oil using zeolite catalyst and characterization of the boil-oil
- Azadirachta indica leaves extract assisted green synthesis of Ag-TiO2 for degradation of Methylene blue and Rhodamine B dyes in aqueous medium
- Synthesis of vitamin E succinate catalyzed by nano-SiO2 immobilized DMAP derivative in mixed solvent system
- Extraction of phytosterols from melon (Cucumis melo) seeds by supercritical CO2 as a clean technology
- Production of uronic acids by hydrothermolysis of pectin as a model substance for plant biomass waste
- Biofabrication of highly pure copper oxide nanoparticles using wheat seed extract and their catalytic activity: A mechanistic approach
- Intelligent modeling and optimization of emulsion aggregation method for producing green printing ink
- Improved removal of methylene blue on modified hierarchical zeolite Y: Achieved by a “destructive-constructive” method
- Two different facile and efficient approaches for the synthesis of various N-arylacetamides via N-acetylation of arylamines and straightforward one-pot reductive acetylation of nitroarenes promoted by recyclable CuFe2O4 nanoparticles in water
- Optimization of acid catalyzed esterification and mixed metal oxide catalyzed transesterification for biodiesel production from Moringa oleifera oil
- Kinetics and the fluidity of the stearic acid esters with different carbon backbones
- Aiming for a standardized protocol for preparing a process green synthesis report and for ranking multiple synthesis plans to a common target product
- Microstructure and luminescence of VO2 (B) nanoparticle synthesis by hydrothermal method
- Optimization of uranium removal from uranium plant wastewater by response surface methodology (RSM)
- Microwave drying of nickel-containing residue: dielectric properties, kinetics, and energy aspects
- Simple and convenient two step synthesis of 5-bromo-2,3-dimethoxy-6-methyl-1,4-benzoquinone
- Biodiesel production from waste cooking oil
- The effect of activation temperature on structure and properties of blue coke-based activated carbon by CO2 activation
- Optimization of reaction parameters for the green synthesis of zero valent iron nanoparticles using pine tree needles
- Microwave-assisted protocol for squalene isolation and conversion from oil-deodoriser distillates
- Denitrification performance of rare earth tailings-based catalysts
- Facile synthesis of silver nanoparticles using Averrhoa bilimbi L and Plum extracts and investigation on the synergistic bioactivity using in vitro models
- Green production of AgNPs and their phytostimulatory impact
- Photocatalytic activity of Ag/Ni bi-metallic nanoparticles on textile dye removal
- Topical Issue: Green Process Engineering / Guest Editors: Martine Poux, Patrick Cognet
- Modelling and optimisation of oxidative desulphurisation of tyre-derived oil via central composite design approach
- CO2 sequestration by carbonation of olivine: a new process for optimal separation of the solids produced
- Organic carbonates synthesis improved by pervaporation for CO2 utilisation
- Production of starch nanoparticles through solvent-antisolvent precipitation in a spinning disc reactor
- A kinetic study of Zn halide/TBAB-catalysed fixation of CO2 with styrene oxide in propylene carbonate
- Topical on Green Process Engineering
Artikel in diesem Heft
- Regular Articles
- Studies on the preparation and properties of biodegradable polyester from soybean oil
- Flow-mode biodiesel production from palm oil using a pressurized microwave reactor
- Reduction of free fatty acids in waste oil for biodiesel production by glycerolysis: investigation and optimization of process parameters
- Saccharin: a cheap and mild acidic agent for the synthesis of azo dyes via telescoped dediazotization
- Optimization of lipase-catalyzed synthesis of polyethylene glycol stearate in a solvent-free system
- Green synthesis of iron oxide nanoparticles using Platanus orientalis leaf extract for antifungal activity
- Ultrasound assisted chemical activation of peanut husk for copper removal
- Room temperature silanization of Fe3O4 for the preparation of phenyl functionalized magnetic adsorbent for dispersive solid phase extraction for the extraction of phthalates in water
- Evaluation of the saponin green extraction from Ziziphus spina-christi leaves using hydrothermal, microwave and Bain-Marie water bath heating methods
- Oxidation of dibenzothiophene using the heterogeneous catalyst of tungsten-based carbon nanotubes
- Calcined sodium silicate as an efficient and benign heterogeneous catalyst for the transesterification of natural lecithin to L-α-glycerophosphocholine
- Synergistic effect between CO2 and H2O2 on ethylbenzene oxidation catalyzed by carbon supported heteropolyanion catalysts
- Hydrocyanation of 2-arylmethyleneindan-1,3-diones using potassium hexacyanoferrate(II) as a nontoxic cyanating agent
- Green synthesis of hydratropic aldehyde from α-methylstyrene catalyzed by Al2O3-supported metal phthalocyanines
- Environmentally benign chemical recycling of polycarbonate wastes: comparison of micro- and nano-TiO2 solid support efficiencies
- Medicago polymorpha-mediated antibacterial silver nanoparticles in the reduction of methyl orange
- Production of value-added chemicals from esterification of waste glycerol over MCM-41 supported catalysts
- Green synthesis of zerovalent copper nanoparticles for efficient reduction of toxic azo dyes congo red and methyl orange
- Optimization of the biological synthesis of silver nanoparticles using Penicillium oxalicum GRS-1 and their antimicrobial effects against common food-borne pathogens
- Optimization of submerged fermentation conditions to overproduce bioethanol using two industrial and traditional Saccharomyces cerevisiae strains
- Extraction of In3+ and Fe3+ from sulfate solutions by using a 3D-printed “Y”-shaped microreactor
- Foliar-mediated Ag:ZnO nanophotocatalysts: green synthesis, characterization, pollutants degradation, and in vitro biocidal activity
- Green cyclic acetals production by glycerol etherification reaction with benzaldehyde using cationic acidic resin
- Biosynthesis, characterization and antimicrobial activities assessment of fabricated selenium nanoparticles using Pelargonium zonale leaf extract
- Synthesis of high surface area magnesia by using walnut shell as a template
- Controllable biosynthesis of silver nanoparticles using actinobacterial strains
- Green vegetation: a promising source of color dyes
- Mechano-chemical synthesis of ammonia and acetic acid from inorganic materials in water
- Green synthesis and structural characterization of novel N1-substituted 3,4-dihydropyrimidin-2(1H)-ones
- Biodiesel production from cotton oil using heterogeneous CaO catalysts from eggshells prepared at different calcination temperatures
- Regeneration of spent mercury catalyst for the treatment of dye wastewater by the microwave and ultrasonic spray-assisted method
- Green synthesis of the innovative super paramagnetic nanoparticles from the leaves extract of Fraxinus chinensis Roxb and their application for the decolourisation of toxic dyes
- Biogenic ZnO nanoparticles: a study of blueshift of optical band gap and photocatalytic degradation of reactive yellow 186 dye under direct sunlight
- Leached compounds from the extracts of pomegranate peel, green coconut shell, and karuvelam wood for the removal of hexavalent chromium
- Enhancement of molecular weight reduction of natural rubber in triphasic CO2/toluene/H2O systems with hydrogen peroxide for preparation of biobased polyurethanes
- An efficient green synthesis of novel 1H-imidazo[1,2-a]imidazole-3-amine and imidazo[2,1-c][1,2,4]triazole-5-amine derivatives via Strecker reaction under controlled microwave heating
- Evaluation of three different green fabrication methods for the synthesis of crystalline ZnO nanoparticles using Pelargonium zonale leaf extract
- A highly efficient and multifunctional biomass supporting Ag, Ni, and Cu nanoparticles through wetness impregnation for environmental remediation
- Simple one-pot green method for large-scale production of mesalamine, an anti-inflammatory agent
- Relationships between step and cumulative PMI and E-factors: implications on estimating material efficiency with respect to charting synthesis optimization strategies
- A comparative sorption study of Cr3+ and Cr6+ using mango peels: kinetic, equilibrium and thermodynamic
- Effects of acid hydrolysis waste liquid recycle on preparation of microcrystalline cellulose
- Use of deep eutectic solvents as catalyst: A mini-review
- Microwave-assisted synthesis of pyrrolidinone derivatives using 1,1’-butylenebis(3-sulfo-3H-imidazol-1-ium) chloride in ethylene glycol
- Green and eco-friendly synthesis of Co3O4 and Ag-Co3O4: Characterization and photo-catalytic activity
- Adsorption optimized of the coal-based material and application for cyanide wastewater treatment
- Aloe vera leaf extract mediated green synthesis of selenium nanoparticles and assessment of their In vitro antimicrobial activity against spoilage fungi and pathogenic bacteria strains
- Waste phenolic resin derived activated carbon by microwave-assisted KOH activation and application to dye wastewater treatment
- Direct ethanol production from cellulose by consortium of Trichoderma reesei and Candida molischiana
- Agricultural waste biomass-assisted nanostructures: Synthesis and application
- Biodiesel production from rubber seed oil using calcium oxide derived from eggshell as catalyst – optimization and modeling studies
- Study of fabrication of fully aqueous solution processed SnS quantum dot-sensitized solar cell
- Assessment of aqueous extract of Gypsophila aretioides for inhibitory effects on calcium carbonate formation
- An environmentally friendly acylation reaction of 2-methylnaphthalene in solvent-free condition in a micro-channel reactor
- Aegle marmelos phytochemical stabilized synthesis and characterization of ZnO nanoparticles and their role against agriculture and food pathogen
- A reactive coupling process for co-production of solketal and biodiesel
- Optimization of the asymmetric synthesis of (S)-1-phenylethanol using Ispir bean as whole-cell biocatalyst
- Synthesis of pyrazolopyridine and pyrazoloquinoline derivatives by one-pot, three-component reactions of arylglyoxals, 3-methyl-1-aryl-1H-pyrazol-5-amines and cyclic 1,3-dicarbonyl compounds in the presence of tetrapropylammonium bromide
- Preconcentration of morphine in urine sample using a green and solvent-free microextraction method
- Extraction of glycyrrhizic acid by aqueous two-phase system formed by PEG and two environmentally friendly organic acid salts - sodium citrate and sodium tartrate
- Green synthesis of copper oxide nanoparticles using Juglans regia leaf extract and assessment of their physico-chemical and biological properties
- Deep eutectic solvents (DESs) as powerful and recyclable catalysts and solvents for the synthesis of 3,4-dihydropyrimidin-2(1H)-ones/thiones
- Biosynthesis, characterization and anti-microbial activity of silver nanoparticle based gel hand wash
- Efficient and selective microwave-assisted O-methylation of phenolic compounds using tetramethylammonium hydroxide (TMAH)
- Anticoagulant, thrombolytic and antibacterial activities of Euphorbia acruensis latex-mediated bioengineered silver nanoparticles
- Volcanic ash as reusable catalyst in the green synthesis of 3H-1,5-benzodiazepines
- Green synthesis, anionic polymerization of 1,4-bis(methacryloyl)piperazine using Algerian clay as catalyst
- Selenium supplementation during fermentation with sugar beet molasses and Saccharomyces cerevisiae to increase bioethanol production
- Biosynthetic potential assessment of four food pathogenic bacteria in hydrothermally silver nanoparticles fabrication
- Investigating the effectiveness of classical and eco-friendly approaches for synthesis of dialdehydes from organic dihalides
- Pyrolysis of palm oil using zeolite catalyst and characterization of the boil-oil
- Azadirachta indica leaves extract assisted green synthesis of Ag-TiO2 for degradation of Methylene blue and Rhodamine B dyes in aqueous medium
- Synthesis of vitamin E succinate catalyzed by nano-SiO2 immobilized DMAP derivative in mixed solvent system
- Extraction of phytosterols from melon (Cucumis melo) seeds by supercritical CO2 as a clean technology
- Production of uronic acids by hydrothermolysis of pectin as a model substance for plant biomass waste
- Biofabrication of highly pure copper oxide nanoparticles using wheat seed extract and their catalytic activity: A mechanistic approach
- Intelligent modeling and optimization of emulsion aggregation method for producing green printing ink
- Improved removal of methylene blue on modified hierarchical zeolite Y: Achieved by a “destructive-constructive” method
- Two different facile and efficient approaches for the synthesis of various N-arylacetamides via N-acetylation of arylamines and straightforward one-pot reductive acetylation of nitroarenes promoted by recyclable CuFe2O4 nanoparticles in water
- Optimization of acid catalyzed esterification and mixed metal oxide catalyzed transesterification for biodiesel production from Moringa oleifera oil
- Kinetics and the fluidity of the stearic acid esters with different carbon backbones
- Aiming for a standardized protocol for preparing a process green synthesis report and for ranking multiple synthesis plans to a common target product
- Microstructure and luminescence of VO2 (B) nanoparticle synthesis by hydrothermal method
- Optimization of uranium removal from uranium plant wastewater by response surface methodology (RSM)
- Microwave drying of nickel-containing residue: dielectric properties, kinetics, and energy aspects
- Simple and convenient two step synthesis of 5-bromo-2,3-dimethoxy-6-methyl-1,4-benzoquinone
- Biodiesel production from waste cooking oil
- The effect of activation temperature on structure and properties of blue coke-based activated carbon by CO2 activation
- Optimization of reaction parameters for the green synthesis of zero valent iron nanoparticles using pine tree needles
- Microwave-assisted protocol for squalene isolation and conversion from oil-deodoriser distillates
- Denitrification performance of rare earth tailings-based catalysts
- Facile synthesis of silver nanoparticles using Averrhoa bilimbi L and Plum extracts and investigation on the synergistic bioactivity using in vitro models
- Green production of AgNPs and their phytostimulatory impact
- Photocatalytic activity of Ag/Ni bi-metallic nanoparticles on textile dye removal
- Topical Issue: Green Process Engineering / Guest Editors: Martine Poux, Patrick Cognet
- Modelling and optimisation of oxidative desulphurisation of tyre-derived oil via central composite design approach
- CO2 sequestration by carbonation of olivine: a new process for optimal separation of the solids produced
- Organic carbonates synthesis improved by pervaporation for CO2 utilisation
- Production of starch nanoparticles through solvent-antisolvent precipitation in a spinning disc reactor
- A kinetic study of Zn halide/TBAB-catalysed fixation of CO2 with styrene oxide in propylene carbonate
- Topical on Green Process Engineering

