Abstract
The photocatalysis of Ag/Ni bi-metallic nano-particles on safranin O dye degradation was evaluated by UV light irradiations. Ag/Ni bi-metallic nanoparticles were synthesized by the green approach using Zingiber officinale root (Zinger) extract. The average particles size of Ag/Ni bi-metallic nanoparticles was found to be 70-88 nm from SEM image and from XRD patterns it was confirmed that the existence of Ag/Ni bi-metallic nano-particles. 8 mg of Ag/Ni bi-metallic nanoparticles present in 40 mL of 10 ppm dye, degraded completely in presence of UV light irradiations within 30 min time durations. The effect of dye degradation within a short period of time (30 min) was due to wide band gap energy and photochemical redox reactions.
1 Introduction
In the present scenario, the degradation of organic pollutants in water bodies is very important worldwide. Because of the untreated discharge of organic pollutants such as dyes, cosmetics, organic solvents etc. into the environment which causes short-term and longterm problems and most of the organic pollutants are carcinogen. Several wastewater treatments methods are available nowadays such as activated carbon, biodegradation, oxidation, the reduction that are costlier and more time-consuming. The photocatalytic dye degradation has several advantages that they are very effective in the degradation of dye in presence of sunlight irradiations.
Nobel metal nanoparticles have provided much application in bio-medical industries [1] besides photocatalysis. Bi-metallic nanoparticles synthesized by reduction of two metal combined by inter-metallic interactions in over nucleation process [2,3]. Ongoing exploited the noble Bi-metal nanoparticles such as (silver-gold, palladium-platinum, and silver-palladium and silver-nickel) have been applied in various areas like catalysis and medical ailments [4]. Especially Ag/ Ni bi-metallic nanoparticles offering wide potential applications in bio-medical industries [5] and over the past decades a lot of chemical methods available for the preparation of Ag/ Ni bi-metallic nanoparticles [6,7]. These route for more expensive, toxic, and potentially harmful to the eco-systems [8] but green strategy diverse for the synthesis of metal nanoparticles entities have significant role due to its environment friend viability and implies a simple, low-cost, with owing to their bio-medical and also catalytic applications [9]. Furthermore, the noble bi-metallic nanoparticles have superior photocatalytic activity towards the longterm disposal for dye removal. Phyto-fabrication of Ag/Ni bi-metallic nanoparticles is less toxic, highly reactive and low cost compared to other bi-metallic nanoparticles [10,11].
Here in this study, we have been synthesized Ag/Ni bi-metallic nanoparticles through the pyto-fabrication process for textile dye removal.
2 Experimental
2.1 Collections of plant materials and chemicals for the synthesis of Ag/Ni bi-metallic nanoparticles
The Zingiber officinale root was collected from the local farmhouse, Karur, Tamilnadu, India. Metal salts; silver nitrate, nickel nitrate were purchased from Merck, India and the textile dye ‘safranin O’ was purchased commercially. All the chemicals and reagents were used in the AR grade without any further purification. The whole experiment process was used in de-ionized water.
2.2 Preparation of capping agent
The Zingiber officinale root’s (Z. root) visible particles were eradicated through running tap water in several times. The air-dried biomass was grounded to the powder with high-speed stainless steel blender. The 5% extract solutions were made in presence of 5 g of Z. root powder mixed with 100 mL of de-ionized water and then refluxed at 70°C for about 1 h. Later the homogeneous solutions were obtained and stored at 4°C. The prepared Z. root extract was used as a capping agent as well as the reducing agent for the synthesis of Ag/ Ni bi-metallic nanoparticles (NPs) (Figure 1).

Zingiber officinale root powder (synthesised from Zinger root).
2.3 Synthesis of Ag/Ni bi-metallic nanoparticles
Monometallic (Ag, Ni) and bi-metallic (Ag/Ni) NPs were successfully modified by green reduction method using Z. root extract. A well-mixed binary aqueous solution was prepared using 60 mL of 1 mM AgNO3 and 1 mM Ni(NO3)2 aqueous solution (1:1 ratio) and 10 mL of Z. root extracts was then added to this solution with the help of orbital shaker for about 1 h. in ambient temperature. The rapid reduction results in the formation of bi-metallic nanoparticles compared with the monometallic nanoparticles were synthesized in a similar manner. Here the formation of bi-metallic nanoparticles was used as a photocatalyst for dye degradation.
2.4 Photocatalytic inspections of silver/ nickel bi-metallic NPs
Silver modified Ag/Ni bi-metallic nanoparticles were inspected for a photocatalytic activity of textile dye removal in the aqueous medium. Photo reactor was used as an artificial light source for UV light irradiation (mercury lamp-120 W). The aliquots 8 mg of the Ag/Ni NPs was added with 40 mL of (10 ppm/ L) safranin O dye solution. The blank solution also set that was monitored with and without (Ag/Ni nanophotocatalyst). Later with the different time interval, the reaction mixture was subsequently measured by UV-visible spectrophotometer (model-Jasco-v-630). The rate of safranin O dye degradation was monitored in terms of the gradual decrease in the maximum absorbance peak at 518 nm:
where A0 – blank solutions (without NPs), AR – reaction mixture (with NPs).
3 Results and discussion
3.1 Surface plasmon resonance study (SPR) to confirm the formation Ag/Ni bi-metallic NPs
The surface plasmon resonance study by UV-Visible spectroscopic study was conducted to confirm the formation of silver mono and silver/ nickel bi-metallic nanoparticles as shown in Figure 2. The formation of silver mono and silver/ nickel bi-metallic nanoparticles were due to the reduction of mono and bi-metallic (Ag+, Ni2+ and Ag0 and Ni0 NPs) by bio-active molecules present in the Z. root extract. The broad peaks at 420 nm and 461 nm confirmed the reduction of Ag+ and Ag+/Ni2+ to zero valent Ag and Ag/Ni NPs by Z. root extract. The colour transformation from yellowish to deep brown color indicates the formation of mono and bi-metallic nanoparticles [12]. The energy gap was calculated using Eq. 2:
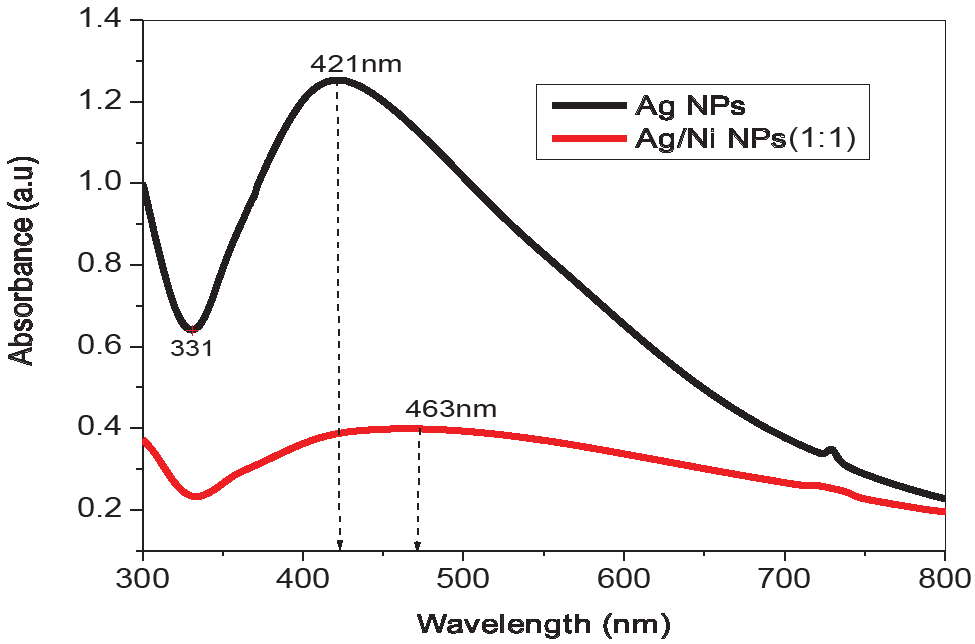
UV-Visible absorption spectra of mono and bi-metallic nanoparticles using Z. root extract.
where: λ – maximum absorbance, Eg – energy gap (eV). The energy gap value of Ag/Ni bi-metallic nanoparticles was found to be 2.6 eV.
3.2 Functional group analysis of silver/ nickel bi-metallic nanoparticles by FT IR
Functional groups present in Ag/ Ni NPs were characterized by FT IR spectrum which was shown in Figure 3. FT IR spectrum of Ag/Ni NP observed that strong and broad peaks at 3420.2 cm-1 and 1634.7 cm-1 which confirmed the binding of υ(-OH) and υ (–NH2) poly-phenols along with amino acids with Z. root extract. Another peak was assigned at 1356.3 cm-1 was due to υ(C=C) aromatic stretching [13]. The presence of polyphenols and amino acids in the pyto components confirmed by FT IR spectrum were responsible for the reduction of silver and nickel ions into silver/ nickel bi-metallic nanoparticles respectfully.
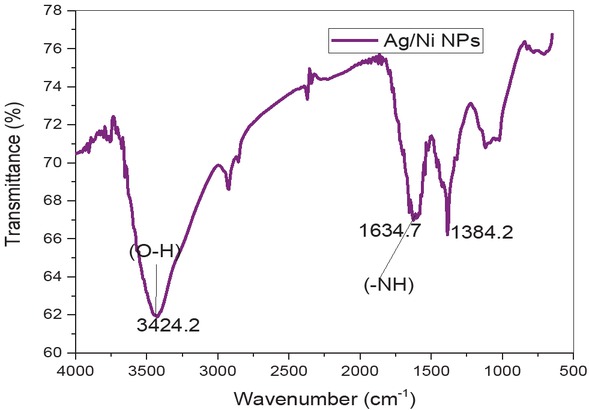
FT IR spectrum of silver/nickel bi-metallic nanoparticles using Z. root extract.
3.3 Structural and morphological characterization of Ag/Ni bi-metallic nanoparticles
The morphology of green synthesized Ag/Ni bi-metallic NPs was performed by SEM. Figures 4a and 4b show well-poly dispersed Ag/Ni bi-metallic NPs has identified in the sizes range of 70-88 nm. The particles were clearly identified by their spherical-like shapes with no agglomeration [14,15]. Figure 4d represents the elemental composition of Ag/Ni bi-metallic nanoparticles which was examined by EDX spectroscopic techniques. The successful formation of Ag/Ni bi-metallic nanoparticles was shown in Figure 4b attributed the strong peaks in the Ag, Ni was confirmed at 3 eV [16].
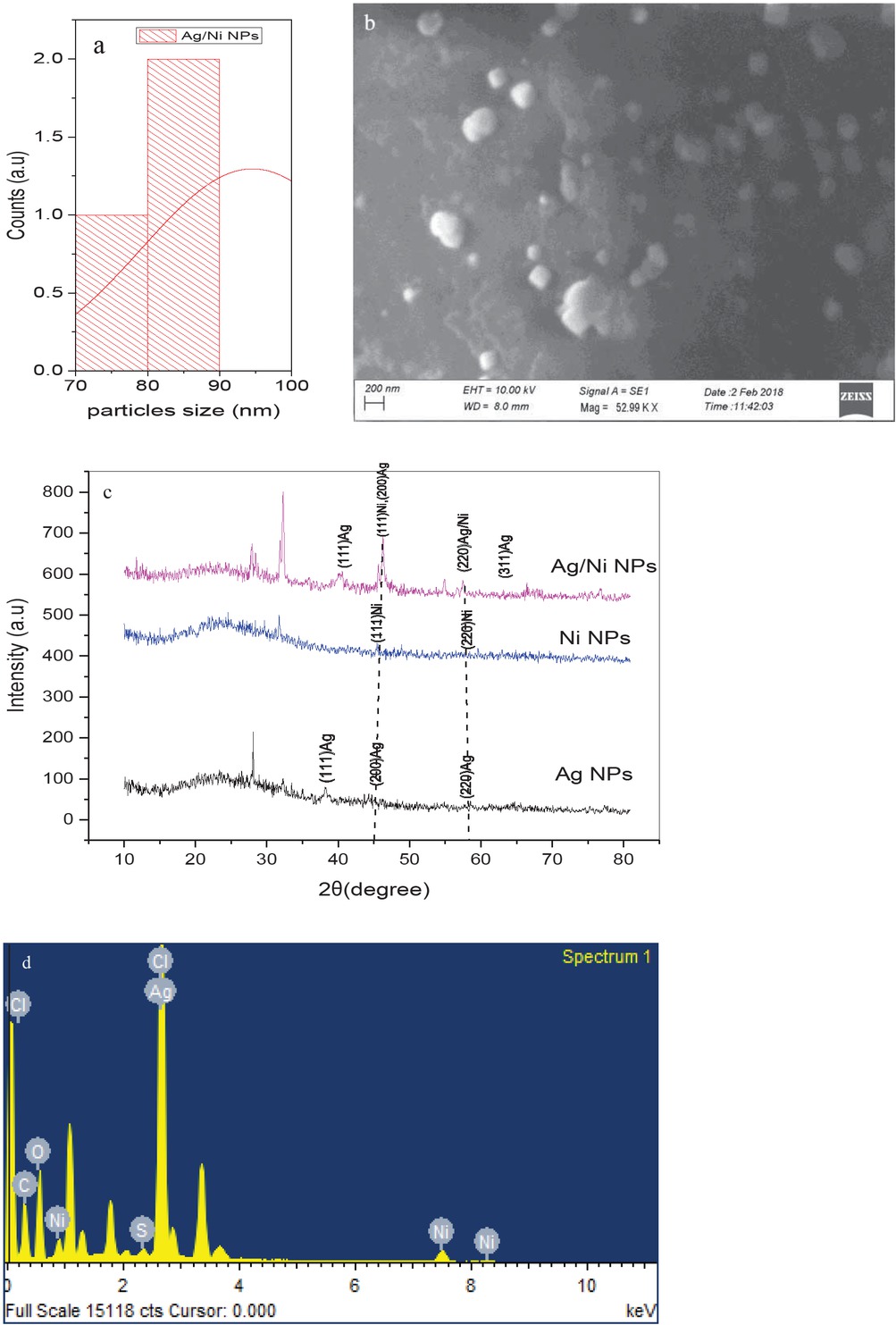
(a,b) Morphological study of Ag/Ni bi-metallic NPs by SEM and its particle size distribution; (c) XRD pattern of Ag/Ni bi-metallic NPs; (d) EDX spectrum of Ag/Ni bi-metallic NPs.
Figure 4c shows that Ag, Ni mono and Ag/ Ni bi-metallic catalyst was in crystallite nature by powder X-ray diffraction patterns. The three diffraction peaks allocated at 39.4°, 45.6°, 58.2° and 65.7° the appropriate the lattice planes (111)*, (200), (220) and (311) were corresponding to the silver, nickel mono and silver/nickel bi-metallic nanoparticles respectively. The prominent peaks (111)* should be agree compared with the silver, nickel nanoparticles respectively [16]. The Ag/Ni bi-MNPs average crystallite size was calculated using this equation of Debye-Scherrer formula:
where: D is the average crystalline size of the nanoparticles, k is geometric factor (0.9), λ is the wavelength of X-ray diffraction source and β is the angular FWHM (full width at half maximum) of the XRD peak at the diffractions angle θ.
3.4 Fluorescent studies of silver, nickel mono, and silver/nickel bi-metallic nanoparticles
Fluorescence spectroscopy studies was carried out to examine the fluorescence property of mono and bi-metallic metallic nanoparticles. The excited peak at 502 nm and green-red emission of fluorescent was observed at 854 nm for Ag/ Ni bi-metallic nanoparticles which were shown in Figure 5. This may be due to inter-metallic interactions of fluorescent emission and bi-metallic nanoparticles surface area, may shift the electron band of silver, nickel mono to silver/nickel bi-metallic nanoparticles and the excitation of Fermi level sp (or) d band holes [17].
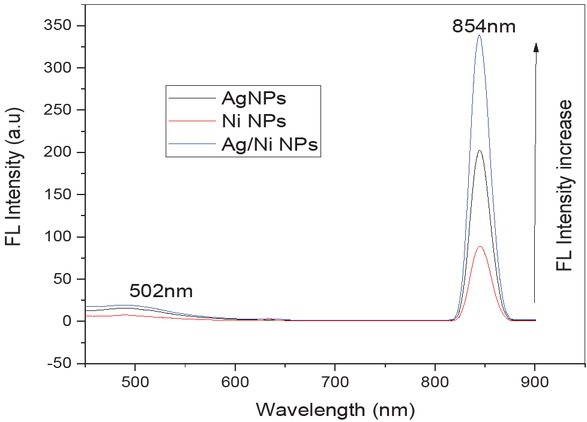
Fluorescence spectrum of silver, nickel mono and silver/ nickel bi-metallic nanoparticles using Z. root powder extract.
3.5 Kinetic parameters
A kinetic plot of the SO dye degradation with respect to the irradiation time and fit linear relationship (Figure 6b) and thus exhibits pseudo-first order kinetics:
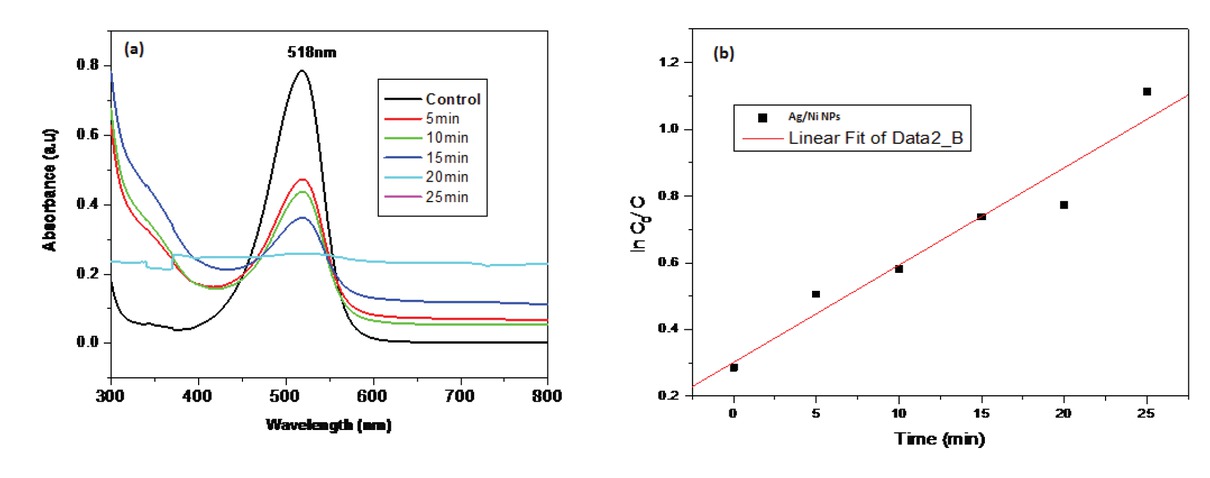
(a) UV-Visible absorption spectrum of safranin O textile dye degradation in absence and presence of silver/nickel bi-metallic nanoparticles using photocatalysis method; (b) Kinetic plot of safranin O textile dye degradation.
where: kappt (min-1) is the apparent rate constant and pseudo rate constant (k) was found to be 0.00859 min-1. Co is the initial concentration of the dye and C is the concentration at time t. Furthermore, the regression of coefficient (R2) of 0.98 confirmed that the photo degradation of SO dye fitted the Langmuir–Hinshelwood kinetic model [18].
3.6 Mechanisms of photocatalysis
The light-induced mechanisms proposed by photocatalysis method using safranin O textile dye removal were shown in Figure 6a The photocatalysis of safranin O textile dye removal was played a crucial role in UV light irradiations. When an event UV light strikes on the silver/nickel bi-metallic nanoparticles surface layer, the excitation of the valence electron of silver/nickel bi-metallic nanoparticles occurs with the formation of electron-hole pairs. These electron-hole pairs create reactive oxygen species (ROS). Finally, the hydroxyl free radicals and superoxide ions were responsible for the removal of textile dye in aqueous phase.
3.7 Quantum yield of Ag/Ni NPs on saffranin O dye degradation
Quantum yield of silver/nickel bi-metallic nanoparticles on safranin O dye degradation was calculated using this formula:
The observed Quantum yield of silver/nickel bi-metallic nanoparticles for this study is found to be (Φ) = 2.37 which indicates if the Quantum Yield is >1 then by absorbing per Quantum of a photon, a large number of reactant molecules (dye) undergo decomposition.
4 Conclusion
Silver modified silver/nickel bi-metallic nanoparticles was successfully synthesized by a low-cost and conventional green route using Zingiber officinale root. The optical and structural properties were characterized by UV-Visible, FTIR, and a surface morphological study was confirmed by SEM-EDX spectroscopic studies reveals that the synthesized nanoparticles were in nanoscale. The silver/nickel bi-metallic nanoparticles have completely degraded safranin O textile dye in presence of UV light irradiation within 30 min time durations. The effect of dye degradation within a short period of time was due to a large band gap 2.6 eV and photochemical redox reactions. Further, the red emission from the luminescent study confirmed the photo catalytic dye degradation on safranin O textile dye. From this above observation, it is concluded that besides degradation of textile dye, the Ag/Ni bi-metallic NPs may also be used in optoelectronics devices, bio-imaging and in nanomedicine.
Acknowledgements
This research did not receive any specific grant from funding in the public or from commercial sectors.
References
[1] Mallikarjuna N.N., Verma R.S., Green synthesis of silver and palladium nanoparticles at room temperature using coffee and tea extract. Green Chem., 2008, 10, 859-862.10.1039/b804703kSearch in Google Scholar
[2] Sperling R.A., Rivera G., Zanella M., Parak W.J., Biological Application of Gold Nanoparticles. Chem. Soc. Rev., 2008, 37, 1896-1908.10.1039/b712170aSearch in Google Scholar PubMed
[3] Nadagouda M.N., Varma R.S., Synthesis of thermally stable carboxymethyl cellulose/metal biodegradable nanocomposites for potential biological applications. Biomacromolecules, 2007, 8, 2762-2767.10.1021/bm700446pSearch in Google Scholar PubMed
[4] Dahl J.A., Maddux L.S., Hutchison J.E., Toward Greener Nanosynthesis. Chem. Rev., 2007, 107, 2228-2269.10.1021/cr050943kSearch in Google Scholar PubMed
[5] Loo C., Lowery A., Halas N., West J., Drezek R., Immunotargeted Nanoshells for Integrated Cancer Imaging and Therapy. Nano Lett., 2005, 5, 4, 709-711.10.1021/nl050127sSearch in Google Scholar PubMed
[6] Lee C.-C., Chen D.-H., Large-scale synthesis of Ni-Ag core-shell nanoparticles with magnetic, optical and anti-oxidation properties. Nanotechnology, 2006, 17, 3094.10.1088/0957-4484/17/13/002Search in Google Scholar
[7] Ouyang R., Zhang W., Zhou S., Yang Y., Ji Y., Feng K., et al., Single Walled Carbon Nanotube Sandwiched Ni-Ag Hybrid Nanoparticle Layers for the Extraordinary Electrocatalysis toward Glucose Oxidation. Electrochimic. Acta, 2016, 188, 197-209.10.1016/j.electacta.2015.12.003Search in Google Scholar
[8] Ubonchonlakate K., Sikong L., Tontai T., Saito F., P. aeruginosa Inactivation with Silver and Nickel Doped TiO2 Film Coat on Glass Fiber Riving Adv. Mater. Res., 2010,150-151, 1726-1731.10.4028/www.scientific.net/AMR.150-151.1726Search in Google Scholar
[9] Leopold N., Lendl B., A New Method for Fast Preparation of Highly Surface-Enhanced Raman Scattering (SERS) Active Silver Colloids at Room Temperature by Reduction of Silver Nitrate with Hydroxylamine Hydrochloride. J. Phys. Chem. B, 2003, 107, 5723-5727.10.1021/jp027460uSearch in Google Scholar
[10] Nasrollahzadeh M., Atarod M., Jaleh B., Gandomirouzbahani M., Biosynthesis of Silver Nanoparticles using Almond Plantleaf extract and their Antibacterial Activity. Ceram. Int., 2016, 42, 8587-8596.10.1016/j.ceramint.2016.02.088Search in Google Scholar
[11] Muthu K., Priya S., Green synthesis, characterization and catalytic activity of silver nanoparticles using Cassia auriculata flower extract separated fraction. Spectrochimi. Acta A, 2017, 179, 66-72.10.1016/j.saa.2017.02.024Search in Google Scholar PubMed
[12] Akinsiku A.A., Dare E.O., Ajanaku K.O., Ajani O.O., Olugbuyiro J.A.O., Siyanbola T.O., et al., Modeling and Synthesis of Ag and Ag/Ni Allied Bimetallic Nanoparticles by Green Method: Optical and Biological Properties. Int. J. Biomater., 2018, 9658080,1-17, DOI:10.1155/2018/9658080.10.1155/2018/9658080Search in Google Scholar PubMed PubMed Central
[13] Kolya H., Maiti P., Pandey A., Tripathy T., Green synthesis of silver nanoparticles with antimicrobial and azo dye (Congo red) degradation properties using Amaranthus gangeticus Linn leaf extract. J. Anal. Sci. Tech., 2015, 6(33), DOI:10.1186/s40543-015-0074.10.1186/s40543-015-0074Search in Google Scholar
[14] Basu S., Maji P., Ganguly J., Rapid green synthesis of silver nanoparticles by aqueous extract of seeds of Nyctanthes arbor-ristis J. Appl. Nanosci., 2016, 6(1), 1-5.10.1007/s13204-015-0407-9Search in Google Scholar
[15] Mntungwa N., Pullabhotla V.S.R., Revaprasadu N., Facile Synthesis of Organically Capped CdTe Nanoparticles. J. Nanosci. Nanotechno., 2012, 12(3), 2640-2644.10.1166/jnn.2012.6133Search in Google Scholar PubMed
[16] Adekoya J.A., Dare E.O., Mesubi M.A., Revaprasadu N., Synthesis and Characterization of Optically Active Fractal Seed Mediated Silver Nickel Bimetallic Nanoparticles. J. Mater., 2014, 1-9, DOI:10.1155/2014/184216.10.1155/2014/184216Search in Google Scholar
[17] Angshuman P., Sunil S., Surekha D., Microwave-assisted synthesis of silver nanoparticles using ethanol as a reducing agent. Mater. Chem. Phys., 2009, 114, 530-532.10.1016/j.matchemphys.2008.11.056Search in Google Scholar
[18] Khezrianjoo S., Revanasiddappa H.D., Langmuir-Hinshelwood Kinetic Expression for the Photocatalytic Degradation of Metanil Yellow Aqueous Solutions by ZnO Catalyst. Chem. Sci. J., 2012, CSJ-85.Search in Google Scholar
© 2019 Mohan and Devan, published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 Public License.
Articles in the same Issue
- Regular Articles
- Studies on the preparation and properties of biodegradable polyester from soybean oil
- Flow-mode biodiesel production from palm oil using a pressurized microwave reactor
- Reduction of free fatty acids in waste oil for biodiesel production by glycerolysis: investigation and optimization of process parameters
- Saccharin: a cheap and mild acidic agent for the synthesis of azo dyes via telescoped dediazotization
- Optimization of lipase-catalyzed synthesis of polyethylene glycol stearate in a solvent-free system
- Green synthesis of iron oxide nanoparticles using Platanus orientalis leaf extract for antifungal activity
- Ultrasound assisted chemical activation of peanut husk for copper removal
- Room temperature silanization of Fe3O4 for the preparation of phenyl functionalized magnetic adsorbent for dispersive solid phase extraction for the extraction of phthalates in water
- Evaluation of the saponin green extraction from Ziziphus spina-christi leaves using hydrothermal, microwave and Bain-Marie water bath heating methods
- Oxidation of dibenzothiophene using the heterogeneous catalyst of tungsten-based carbon nanotubes
- Calcined sodium silicate as an efficient and benign heterogeneous catalyst for the transesterification of natural lecithin to L-α-glycerophosphocholine
- Synergistic effect between CO2 and H2O2 on ethylbenzene oxidation catalyzed by carbon supported heteropolyanion catalysts
- Hydrocyanation of 2-arylmethyleneindan-1,3-diones using potassium hexacyanoferrate(II) as a nontoxic cyanating agent
- Green synthesis of hydratropic aldehyde from α-methylstyrene catalyzed by Al2O3-supported metal phthalocyanines
- Environmentally benign chemical recycling of polycarbonate wastes: comparison of micro- and nano-TiO2 solid support efficiencies
- Medicago polymorpha-mediated antibacterial silver nanoparticles in the reduction of methyl orange
- Production of value-added chemicals from esterification of waste glycerol over MCM-41 supported catalysts
- Green synthesis of zerovalent copper nanoparticles for efficient reduction of toxic azo dyes congo red and methyl orange
- Optimization of the biological synthesis of silver nanoparticles using Penicillium oxalicum GRS-1 and their antimicrobial effects against common food-borne pathogens
- Optimization of submerged fermentation conditions to overproduce bioethanol using two industrial and traditional Saccharomyces cerevisiae strains
- Extraction of In3+ and Fe3+ from sulfate solutions by using a 3D-printed “Y”-shaped microreactor
- Foliar-mediated Ag:ZnO nanophotocatalysts: green synthesis, characterization, pollutants degradation, and in vitro biocidal activity
- Green cyclic acetals production by glycerol etherification reaction with benzaldehyde using cationic acidic resin
- Biosynthesis, characterization and antimicrobial activities assessment of fabricated selenium nanoparticles using Pelargonium zonale leaf extract
- Synthesis of high surface area magnesia by using walnut shell as a template
- Controllable biosynthesis of silver nanoparticles using actinobacterial strains
- Green vegetation: a promising source of color dyes
- Mechano-chemical synthesis of ammonia and acetic acid from inorganic materials in water
- Green synthesis and structural characterization of novel N1-substituted 3,4-dihydropyrimidin-2(1H)-ones
- Biodiesel production from cotton oil using heterogeneous CaO catalysts from eggshells prepared at different calcination temperatures
- Regeneration of spent mercury catalyst for the treatment of dye wastewater by the microwave and ultrasonic spray-assisted method
- Green synthesis of the innovative super paramagnetic nanoparticles from the leaves extract of Fraxinus chinensis Roxb and their application for the decolourisation of toxic dyes
- Biogenic ZnO nanoparticles: a study of blueshift of optical band gap and photocatalytic degradation of reactive yellow 186 dye under direct sunlight
- Leached compounds from the extracts of pomegranate peel, green coconut shell, and karuvelam wood for the removal of hexavalent chromium
- Enhancement of molecular weight reduction of natural rubber in triphasic CO2/toluene/H2O systems with hydrogen peroxide for preparation of biobased polyurethanes
- An efficient green synthesis of novel 1H-imidazo[1,2-a]imidazole-3-amine and imidazo[2,1-c][1,2,4]triazole-5-amine derivatives via Strecker reaction under controlled microwave heating
- Evaluation of three different green fabrication methods for the synthesis of crystalline ZnO nanoparticles using Pelargonium zonale leaf extract
- A highly efficient and multifunctional biomass supporting Ag, Ni, and Cu nanoparticles through wetness impregnation for environmental remediation
- Simple one-pot green method for large-scale production of mesalamine, an anti-inflammatory agent
- Relationships between step and cumulative PMI and E-factors: implications on estimating material efficiency with respect to charting synthesis optimization strategies
- A comparative sorption study of Cr3+ and Cr6+ using mango peels: kinetic, equilibrium and thermodynamic
- Effects of acid hydrolysis waste liquid recycle on preparation of microcrystalline cellulose
- Use of deep eutectic solvents as catalyst: A mini-review
- Microwave-assisted synthesis of pyrrolidinone derivatives using 1,1’-butylenebis(3-sulfo-3H-imidazol-1-ium) chloride in ethylene glycol
- Green and eco-friendly synthesis of Co3O4 and Ag-Co3O4: Characterization and photo-catalytic activity
- Adsorption optimized of the coal-based material and application for cyanide wastewater treatment
- Aloe vera leaf extract mediated green synthesis of selenium nanoparticles and assessment of their In vitro antimicrobial activity against spoilage fungi and pathogenic bacteria strains
- Waste phenolic resin derived activated carbon by microwave-assisted KOH activation and application to dye wastewater treatment
- Direct ethanol production from cellulose by consortium of Trichoderma reesei and Candida molischiana
- Agricultural waste biomass-assisted nanostructures: Synthesis and application
- Biodiesel production from rubber seed oil using calcium oxide derived from eggshell as catalyst – optimization and modeling studies
- Study of fabrication of fully aqueous solution processed SnS quantum dot-sensitized solar cell
- Assessment of aqueous extract of Gypsophila aretioides for inhibitory effects on calcium carbonate formation
- An environmentally friendly acylation reaction of 2-methylnaphthalene in solvent-free condition in a micro-channel reactor
- Aegle marmelos phytochemical stabilized synthesis and characterization of ZnO nanoparticles and their role against agriculture and food pathogen
- A reactive coupling process for co-production of solketal and biodiesel
- Optimization of the asymmetric synthesis of (S)-1-phenylethanol using Ispir bean as whole-cell biocatalyst
- Synthesis of pyrazolopyridine and pyrazoloquinoline derivatives by one-pot, three-component reactions of arylglyoxals, 3-methyl-1-aryl-1H-pyrazol-5-amines and cyclic 1,3-dicarbonyl compounds in the presence of tetrapropylammonium bromide
- Preconcentration of morphine in urine sample using a green and solvent-free microextraction method
- Extraction of glycyrrhizic acid by aqueous two-phase system formed by PEG and two environmentally friendly organic acid salts - sodium citrate and sodium tartrate
- Green synthesis of copper oxide nanoparticles using Juglans regia leaf extract and assessment of their physico-chemical and biological properties
- Deep eutectic solvents (DESs) as powerful and recyclable catalysts and solvents for the synthesis of 3,4-dihydropyrimidin-2(1H)-ones/thiones
- Biosynthesis, characterization and anti-microbial activity of silver nanoparticle based gel hand wash
- Efficient and selective microwave-assisted O-methylation of phenolic compounds using tetramethylammonium hydroxide (TMAH)
- Anticoagulant, thrombolytic and antibacterial activities of Euphorbia acruensis latex-mediated bioengineered silver nanoparticles
- Volcanic ash as reusable catalyst in the green synthesis of 3H-1,5-benzodiazepines
- Green synthesis, anionic polymerization of 1,4-bis(methacryloyl)piperazine using Algerian clay as catalyst
- Selenium supplementation during fermentation with sugar beet molasses and Saccharomyces cerevisiae to increase bioethanol production
- Biosynthetic potential assessment of four food pathogenic bacteria in hydrothermally silver nanoparticles fabrication
- Investigating the effectiveness of classical and eco-friendly approaches for synthesis of dialdehydes from organic dihalides
- Pyrolysis of palm oil using zeolite catalyst and characterization of the boil-oil
- Azadirachta indica leaves extract assisted green synthesis of Ag-TiO2 for degradation of Methylene blue and Rhodamine B dyes in aqueous medium
- Synthesis of vitamin E succinate catalyzed by nano-SiO2 immobilized DMAP derivative in mixed solvent system
- Extraction of phytosterols from melon (Cucumis melo) seeds by supercritical CO2 as a clean technology
- Production of uronic acids by hydrothermolysis of pectin as a model substance for plant biomass waste
- Biofabrication of highly pure copper oxide nanoparticles using wheat seed extract and their catalytic activity: A mechanistic approach
- Intelligent modeling and optimization of emulsion aggregation method for producing green printing ink
- Improved removal of methylene blue on modified hierarchical zeolite Y: Achieved by a “destructive-constructive” method
- Two different facile and efficient approaches for the synthesis of various N-arylacetamides via N-acetylation of arylamines and straightforward one-pot reductive acetylation of nitroarenes promoted by recyclable CuFe2O4 nanoparticles in water
- Optimization of acid catalyzed esterification and mixed metal oxide catalyzed transesterification for biodiesel production from Moringa oleifera oil
- Kinetics and the fluidity of the stearic acid esters with different carbon backbones
- Aiming for a standardized protocol for preparing a process green synthesis report and for ranking multiple synthesis plans to a common target product
- Microstructure and luminescence of VO2 (B) nanoparticle synthesis by hydrothermal method
- Optimization of uranium removal from uranium plant wastewater by response surface methodology (RSM)
- Microwave drying of nickel-containing residue: dielectric properties, kinetics, and energy aspects
- Simple and convenient two step synthesis of 5-bromo-2,3-dimethoxy-6-methyl-1,4-benzoquinone
- Biodiesel production from waste cooking oil
- The effect of activation temperature on structure and properties of blue coke-based activated carbon by CO2 activation
- Optimization of reaction parameters for the green synthesis of zero valent iron nanoparticles using pine tree needles
- Microwave-assisted protocol for squalene isolation and conversion from oil-deodoriser distillates
- Denitrification performance of rare earth tailings-based catalysts
- Facile synthesis of silver nanoparticles using Averrhoa bilimbi L and Plum extracts and investigation on the synergistic bioactivity using in vitro models
- Green production of AgNPs and their phytostimulatory impact
- Photocatalytic activity of Ag/Ni bi-metallic nanoparticles on textile dye removal
- Topical Issue: Green Process Engineering / Guest Editors: Martine Poux, Patrick Cognet
- Modelling and optimisation of oxidative desulphurisation of tyre-derived oil via central composite design approach
- CO2 sequestration by carbonation of olivine: a new process for optimal separation of the solids produced
- Organic carbonates synthesis improved by pervaporation for CO2 utilisation
- Production of starch nanoparticles through solvent-antisolvent precipitation in a spinning disc reactor
- A kinetic study of Zn halide/TBAB-catalysed fixation of CO2 with styrene oxide in propylene carbonate
- Topical on Green Process Engineering
Articles in the same Issue
- Regular Articles
- Studies on the preparation and properties of biodegradable polyester from soybean oil
- Flow-mode biodiesel production from palm oil using a pressurized microwave reactor
- Reduction of free fatty acids in waste oil for biodiesel production by glycerolysis: investigation and optimization of process parameters
- Saccharin: a cheap and mild acidic agent for the synthesis of azo dyes via telescoped dediazotization
- Optimization of lipase-catalyzed synthesis of polyethylene glycol stearate in a solvent-free system
- Green synthesis of iron oxide nanoparticles using Platanus orientalis leaf extract for antifungal activity
- Ultrasound assisted chemical activation of peanut husk for copper removal
- Room temperature silanization of Fe3O4 for the preparation of phenyl functionalized magnetic adsorbent for dispersive solid phase extraction for the extraction of phthalates in water
- Evaluation of the saponin green extraction from Ziziphus spina-christi leaves using hydrothermal, microwave and Bain-Marie water bath heating methods
- Oxidation of dibenzothiophene using the heterogeneous catalyst of tungsten-based carbon nanotubes
- Calcined sodium silicate as an efficient and benign heterogeneous catalyst for the transesterification of natural lecithin to L-α-glycerophosphocholine
- Synergistic effect between CO2 and H2O2 on ethylbenzene oxidation catalyzed by carbon supported heteropolyanion catalysts
- Hydrocyanation of 2-arylmethyleneindan-1,3-diones using potassium hexacyanoferrate(II) as a nontoxic cyanating agent
- Green synthesis of hydratropic aldehyde from α-methylstyrene catalyzed by Al2O3-supported metal phthalocyanines
- Environmentally benign chemical recycling of polycarbonate wastes: comparison of micro- and nano-TiO2 solid support efficiencies
- Medicago polymorpha-mediated antibacterial silver nanoparticles in the reduction of methyl orange
- Production of value-added chemicals from esterification of waste glycerol over MCM-41 supported catalysts
- Green synthesis of zerovalent copper nanoparticles for efficient reduction of toxic azo dyes congo red and methyl orange
- Optimization of the biological synthesis of silver nanoparticles using Penicillium oxalicum GRS-1 and their antimicrobial effects against common food-borne pathogens
- Optimization of submerged fermentation conditions to overproduce bioethanol using two industrial and traditional Saccharomyces cerevisiae strains
- Extraction of In3+ and Fe3+ from sulfate solutions by using a 3D-printed “Y”-shaped microreactor
- Foliar-mediated Ag:ZnO nanophotocatalysts: green synthesis, characterization, pollutants degradation, and in vitro biocidal activity
- Green cyclic acetals production by glycerol etherification reaction with benzaldehyde using cationic acidic resin
- Biosynthesis, characterization and antimicrobial activities assessment of fabricated selenium nanoparticles using Pelargonium zonale leaf extract
- Synthesis of high surface area magnesia by using walnut shell as a template
- Controllable biosynthesis of silver nanoparticles using actinobacterial strains
- Green vegetation: a promising source of color dyes
- Mechano-chemical synthesis of ammonia and acetic acid from inorganic materials in water
- Green synthesis and structural characterization of novel N1-substituted 3,4-dihydropyrimidin-2(1H)-ones
- Biodiesel production from cotton oil using heterogeneous CaO catalysts from eggshells prepared at different calcination temperatures
- Regeneration of spent mercury catalyst for the treatment of dye wastewater by the microwave and ultrasonic spray-assisted method
- Green synthesis of the innovative super paramagnetic nanoparticles from the leaves extract of Fraxinus chinensis Roxb and their application for the decolourisation of toxic dyes
- Biogenic ZnO nanoparticles: a study of blueshift of optical band gap and photocatalytic degradation of reactive yellow 186 dye under direct sunlight
- Leached compounds from the extracts of pomegranate peel, green coconut shell, and karuvelam wood for the removal of hexavalent chromium
- Enhancement of molecular weight reduction of natural rubber in triphasic CO2/toluene/H2O systems with hydrogen peroxide for preparation of biobased polyurethanes
- An efficient green synthesis of novel 1H-imidazo[1,2-a]imidazole-3-amine and imidazo[2,1-c][1,2,4]triazole-5-amine derivatives via Strecker reaction under controlled microwave heating
- Evaluation of three different green fabrication methods for the synthesis of crystalline ZnO nanoparticles using Pelargonium zonale leaf extract
- A highly efficient and multifunctional biomass supporting Ag, Ni, and Cu nanoparticles through wetness impregnation for environmental remediation
- Simple one-pot green method for large-scale production of mesalamine, an anti-inflammatory agent
- Relationships between step and cumulative PMI and E-factors: implications on estimating material efficiency with respect to charting synthesis optimization strategies
- A comparative sorption study of Cr3+ and Cr6+ using mango peels: kinetic, equilibrium and thermodynamic
- Effects of acid hydrolysis waste liquid recycle on preparation of microcrystalline cellulose
- Use of deep eutectic solvents as catalyst: A mini-review
- Microwave-assisted synthesis of pyrrolidinone derivatives using 1,1’-butylenebis(3-sulfo-3H-imidazol-1-ium) chloride in ethylene glycol
- Green and eco-friendly synthesis of Co3O4 and Ag-Co3O4: Characterization and photo-catalytic activity
- Adsorption optimized of the coal-based material and application for cyanide wastewater treatment
- Aloe vera leaf extract mediated green synthesis of selenium nanoparticles and assessment of their In vitro antimicrobial activity against spoilage fungi and pathogenic bacteria strains
- Waste phenolic resin derived activated carbon by microwave-assisted KOH activation and application to dye wastewater treatment
- Direct ethanol production from cellulose by consortium of Trichoderma reesei and Candida molischiana
- Agricultural waste biomass-assisted nanostructures: Synthesis and application
- Biodiesel production from rubber seed oil using calcium oxide derived from eggshell as catalyst – optimization and modeling studies
- Study of fabrication of fully aqueous solution processed SnS quantum dot-sensitized solar cell
- Assessment of aqueous extract of Gypsophila aretioides for inhibitory effects on calcium carbonate formation
- An environmentally friendly acylation reaction of 2-methylnaphthalene in solvent-free condition in a micro-channel reactor
- Aegle marmelos phytochemical stabilized synthesis and characterization of ZnO nanoparticles and their role against agriculture and food pathogen
- A reactive coupling process for co-production of solketal and biodiesel
- Optimization of the asymmetric synthesis of (S)-1-phenylethanol using Ispir bean as whole-cell biocatalyst
- Synthesis of pyrazolopyridine and pyrazoloquinoline derivatives by one-pot, three-component reactions of arylglyoxals, 3-methyl-1-aryl-1H-pyrazol-5-amines and cyclic 1,3-dicarbonyl compounds in the presence of tetrapropylammonium bromide
- Preconcentration of morphine in urine sample using a green and solvent-free microextraction method
- Extraction of glycyrrhizic acid by aqueous two-phase system formed by PEG and two environmentally friendly organic acid salts - sodium citrate and sodium tartrate
- Green synthesis of copper oxide nanoparticles using Juglans regia leaf extract and assessment of their physico-chemical and biological properties
- Deep eutectic solvents (DESs) as powerful and recyclable catalysts and solvents for the synthesis of 3,4-dihydropyrimidin-2(1H)-ones/thiones
- Biosynthesis, characterization and anti-microbial activity of silver nanoparticle based gel hand wash
- Efficient and selective microwave-assisted O-methylation of phenolic compounds using tetramethylammonium hydroxide (TMAH)
- Anticoagulant, thrombolytic and antibacterial activities of Euphorbia acruensis latex-mediated bioengineered silver nanoparticles
- Volcanic ash as reusable catalyst in the green synthesis of 3H-1,5-benzodiazepines
- Green synthesis, anionic polymerization of 1,4-bis(methacryloyl)piperazine using Algerian clay as catalyst
- Selenium supplementation during fermentation with sugar beet molasses and Saccharomyces cerevisiae to increase bioethanol production
- Biosynthetic potential assessment of four food pathogenic bacteria in hydrothermally silver nanoparticles fabrication
- Investigating the effectiveness of classical and eco-friendly approaches for synthesis of dialdehydes from organic dihalides
- Pyrolysis of palm oil using zeolite catalyst and characterization of the boil-oil
- Azadirachta indica leaves extract assisted green synthesis of Ag-TiO2 for degradation of Methylene blue and Rhodamine B dyes in aqueous medium
- Synthesis of vitamin E succinate catalyzed by nano-SiO2 immobilized DMAP derivative in mixed solvent system
- Extraction of phytosterols from melon (Cucumis melo) seeds by supercritical CO2 as a clean technology
- Production of uronic acids by hydrothermolysis of pectin as a model substance for plant biomass waste
- Biofabrication of highly pure copper oxide nanoparticles using wheat seed extract and their catalytic activity: A mechanistic approach
- Intelligent modeling and optimization of emulsion aggregation method for producing green printing ink
- Improved removal of methylene blue on modified hierarchical zeolite Y: Achieved by a “destructive-constructive” method
- Two different facile and efficient approaches for the synthesis of various N-arylacetamides via N-acetylation of arylamines and straightforward one-pot reductive acetylation of nitroarenes promoted by recyclable CuFe2O4 nanoparticles in water
- Optimization of acid catalyzed esterification and mixed metal oxide catalyzed transesterification for biodiesel production from Moringa oleifera oil
- Kinetics and the fluidity of the stearic acid esters with different carbon backbones
- Aiming for a standardized protocol for preparing a process green synthesis report and for ranking multiple synthesis plans to a common target product
- Microstructure and luminescence of VO2 (B) nanoparticle synthesis by hydrothermal method
- Optimization of uranium removal from uranium plant wastewater by response surface methodology (RSM)
- Microwave drying of nickel-containing residue: dielectric properties, kinetics, and energy aspects
- Simple and convenient two step synthesis of 5-bromo-2,3-dimethoxy-6-methyl-1,4-benzoquinone
- Biodiesel production from waste cooking oil
- The effect of activation temperature on structure and properties of blue coke-based activated carbon by CO2 activation
- Optimization of reaction parameters for the green synthesis of zero valent iron nanoparticles using pine tree needles
- Microwave-assisted protocol for squalene isolation and conversion from oil-deodoriser distillates
- Denitrification performance of rare earth tailings-based catalysts
- Facile synthesis of silver nanoparticles using Averrhoa bilimbi L and Plum extracts and investigation on the synergistic bioactivity using in vitro models
- Green production of AgNPs and their phytostimulatory impact
- Photocatalytic activity of Ag/Ni bi-metallic nanoparticles on textile dye removal
- Topical Issue: Green Process Engineering / Guest Editors: Martine Poux, Patrick Cognet
- Modelling and optimisation of oxidative desulphurisation of tyre-derived oil via central composite design approach
- CO2 sequestration by carbonation of olivine: a new process for optimal separation of the solids produced
- Organic carbonates synthesis improved by pervaporation for CO2 utilisation
- Production of starch nanoparticles through solvent-antisolvent precipitation in a spinning disc reactor
- A kinetic study of Zn halide/TBAB-catalysed fixation of CO2 with styrene oxide in propylene carbonate
- Topical on Green Process Engineering

