Abstract
Synthesis of styrene carbonate (SC) via the fixation of CO2 with styrene oxide (SO) has been investigated using a combination of zinc bromide (ZnBr2) and tetrabutylammonium halides (TBAX) as acid-base binary homogeneous catalysts. The combination of ZnBr2 and TBAB had a synergistic effect, which led to about 6-fold enhancement in the rate of SC formation as compared to using TBAB alone as a catalyst. Propylene carbonate (PC) was chosen as a green solvent for a comprehensive study of reaction kinetics. The reaction followed a first-order kinetics with respect to SO, CO2, and TBAB, whereas a fractional order was observed for the ZnBr2 when used in combination with the TBAB. Arrhenius and Eyring’s expressions were applied to determine the kinetic and thermodynamic activation parameters, where activation energy (Ea) of 23.3 kJ mol−1 was obtained for the SC formation over the temperature range of 90-120°C. The thermodynamic analysis showed that positive values for enthalpy (ΔH‡ = 18.53 kJ mol−1), Gibbs free energy (ΔG‡ = 79.74 kJ mol−1), whereas a negative entropy (ΔS‡ = –162.88 J mol−1 K−1) was obtained. These thermodynamic parameters suggest that endergonic and kinetically controlled reactions were involved in the formation of SC from SO and CO2.
1 Introduction
In recent decades, the increasing CO2 concentration in the atmosphere has resulted in drastic changes in the climate. The anthropogenic emission of CO2 into the atmosphere now exceeds 36 Gt, which is 43% above the level since the beginning of the industrial revolution [1]. This has necessitated some research in the industry and academia to reduce the CO2 emission via sequestration and utilization [2,3]. Amongst the methods of CO2 utilization, the most attractive and economically viable option is the application of CO2 for the production of cyclic carbonates via reactions with epoxides due to its 100% atom economy [4, 5, 6, 7]. Another advantage of CO2 utilisation in cyclic carbonate productions is that it is a naturally abundant non-toxic and non-flammable and can be used as a sustainable alternative to toxic phosgene used in the conventional process [8,9]. Generally, cyclic carbonates are valuable chemicals which have applications as monomers for polycarbonates and polyurethanes [10,11], polar aprotic green solvents [12, 13, 14], electrolytes in Li-ion batteries [15,16], and platform chemical for other products [17,18]. However, a major challenge in CO2 utilization as a feedstock for productions of platform chemicals is due to its high thermodynamic and kinetic stability [19]. Therefore, CO2 utilisation for organic synthesis is recommended for formations of compounds that have relatively high free energies to provide a thermodynamically feasible process, such as reactions of CO2 with epoxides in cyclic carbonate formations [20]. Although the reactions of CO2 with epoxide to form cyclic carbonates was observed to be exothermic [21], such reactions require high activation energy, in the range of 209-251 kJ mol–1 based on nature of the epoxide [22,23]. Therefore, a highly efficient catalyst is required to overcome this kinetic barrier [24]. During the last two decades, various catalyst systems have been developed to catalyse the reaction at mild reaction conditions. These mainly include organo-catalysts such as alkylammonium/phosphonium halides [25, 26, 27, 28], ionic liquids [29, 30, 31, 32, 33], and alkali and alkaline earth metals-based salen/salphen complexes [34, 35, 36, 37, 38]. However, there are several catalyst systems which still lack their commercial viability because of their availability, low catalytic activity, the requirement of extreme reaction conditions and complex downstream separation processes. To increase the catalytic activity, it is important to understand the mechanism involved in the cycloaddition reaction. Generally, the commonly accepted mechanism for the reactions of CO2 with epoxides under acid-base catalysis involves the following steps (Figure 1): Epoxide activation by the interaction of Lewis acid (A) with an oxygen atom of the epoxide (Step 1). Ring-opening of activated epoxide by the nucleophilic attack of halide anion (X) on the least substituted carbon (C) atom to form an alkoxide intermediate (Step 2), followed by insertion of CO2 to the negatively charged alkoxide intermediate (Step 3). Subsequently, an intramolecular rearrangement occurs resulting in the displacement of the halide anion (X) and formation of a carbonate intermediate (Step 4), which forms a five-membered cyclic carbonate and regenerates the catalyst (Step 5) [27,39,40].
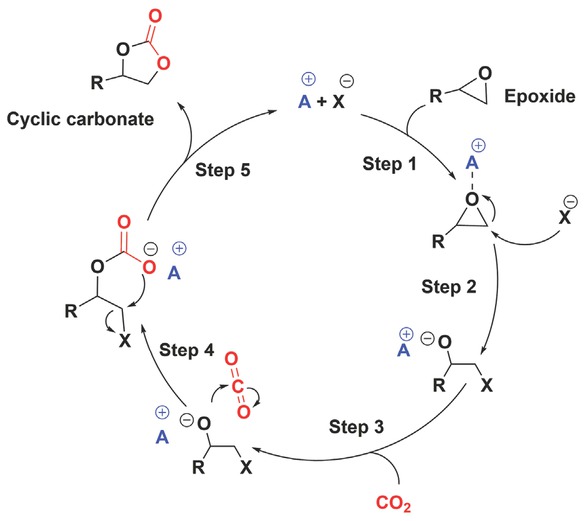
General mechanism of cyclic carbonate synthesis from CO2 and epoxide using an acid-base catalyst.
In this study, chemical fixation of CO2 with styrene oxide (SO) was performed using a combination of zinc bromide (ZnBr2) in conjunction with tetrabutylammonium halide (TBAX) as an acid-base binary homogeneous catalyst. Although cyclic carbonate synthesis via CO2 cycloaddition to epoxides has been broadly investigated using various catalyst systems with higher conversions and yields of cyclic carbonates [21,41], comprehensive studies are required to investigate the mechanism of the reaction. This study involved the determination of reaction rate law, kinetic parameters and testing of hypothesis on the mechanism for the synergistic effect, to obtain a deep insight into the observed catalytic activity due to the synergistic effect between ZnBr2 and TBAB. Arrhenius and Eyring expressions will be used to determine the effects of temperature, activation energy and thermodynamic parameters for SC formation. A reaction mechanism will be proposed based on kinetic analysis of the experimental data.
2 Materials and methods
The styrene carbonates (SC) synthesis was carried out using a mono-substituted styrene oxide (SO) and CO2 (Figure 2). All chemicals were obtained from Sigma Aldrich. The reaction was performed in a semi-batch operation using a high-pressure stainless steel Parr reactor (Model 4750) (Figure 3). The temperature was controlled using an automatic temperature control system (PID controller), whereas pressure inside the reactor was controlled using a 70 bar gas pressure regulator. A high-speed magnetic stirrer was used to provide agitation. For an exemplary experiment, the desired quantities of styrene oxide (SO) and homogeneous catalyst (ZnBr2/TBAB) were added into the reactor and heated to the required temperature by setting the value on the temperature controller. When the temperature set point was achieved, the reactor was pressurised with CO2 from a gas cylinder to the required pressure. The reaction starts immediately after introducing CO2 in the reactor. A continuous supply of CO2 was provided to the reactor to maintain the constant pressure during the reaction. The progress of the reaction was examined by withdrawing a very small volume of aliquots (200 μL) from a large volume of the reaction mixture (40 mL) at regular intervals. These samples were immediately analysed by IR analysis. The IR spectra showed an increase in the intensity at 1800 cm–1 due to carbonyl peak of SC formation (Figure 4), and peaks at 1065 and 1159 cm–1 corresponding to asymmetric (C–O) stretching vibrations of SC [42, 43, 44]. However, the carbonyl absorption peaks of solvent (PC) and product (SC) were overlapped at 1800 cm–1 and no changes in the intensity were observed during kinetic experiments using PC as a solvent (Figure 5). Therefore, the progress of the reaction was monitored by the decrease in the intensity of the peak at 876 cm–1 assigned to C–O stretch mode in SO, which was clearly not masked by the PC absorption. The rate at which the SO epoxide peak at 876 cm–1 diminished was directly proportional to SC formation as there was no by-product formation was observed. The conversion (%) was determined based on the decrease in SO peak intensity at 876 cm–1 after calibration against known concentrations of SO and was also validated using a 5890 Series II Hewlett Packard gas chromatograph.
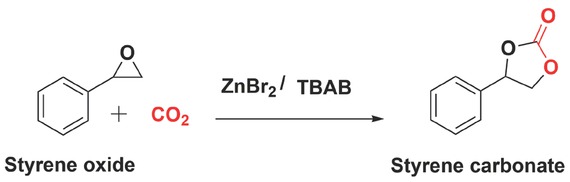
Styrene carbonate (SC) synthesis from styrene oxide (SO) and CO2 catalysed by ZnBr2/TBAB.
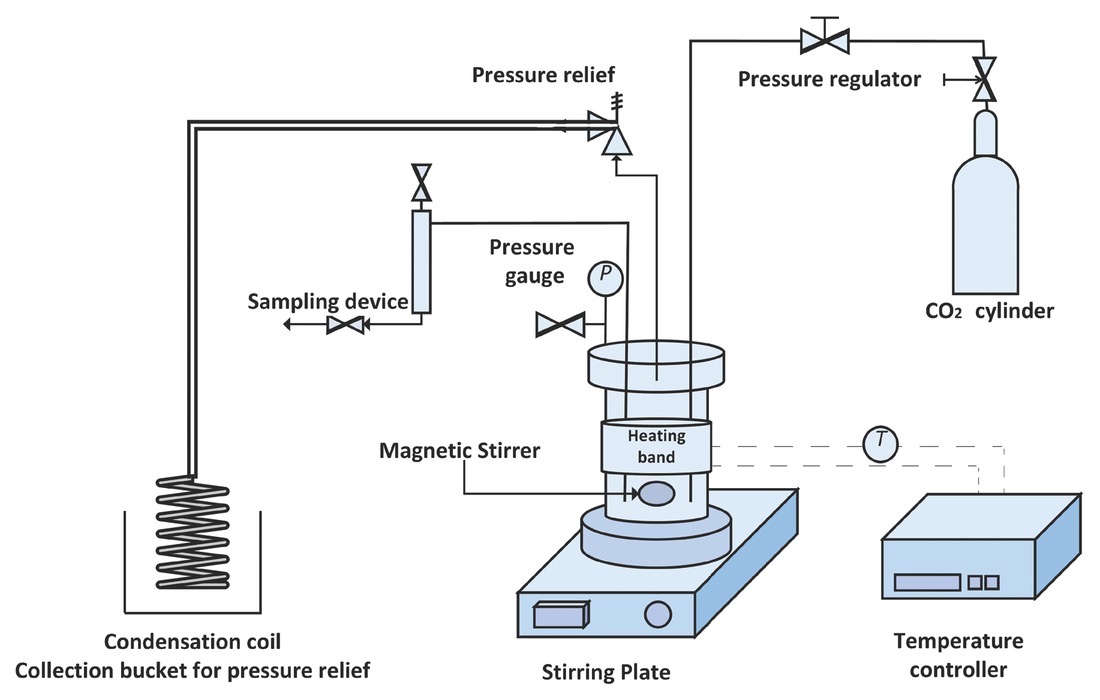
Schematic diagram of the semi-batch reactor used for styrene carbonate (SC) formation.
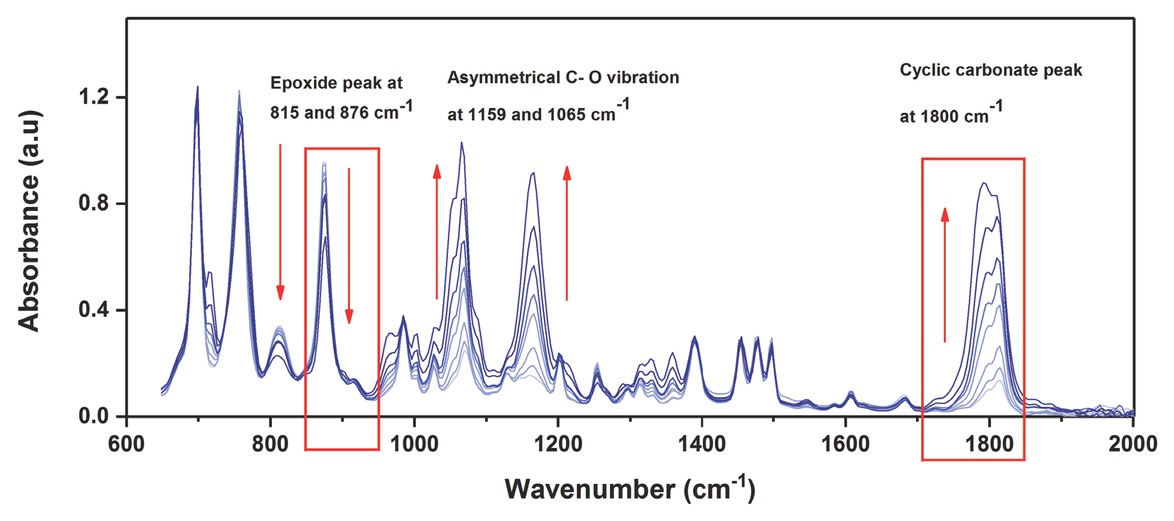
FTIR spectroscopic-monitoring of styrene carbonate (SC) formation.
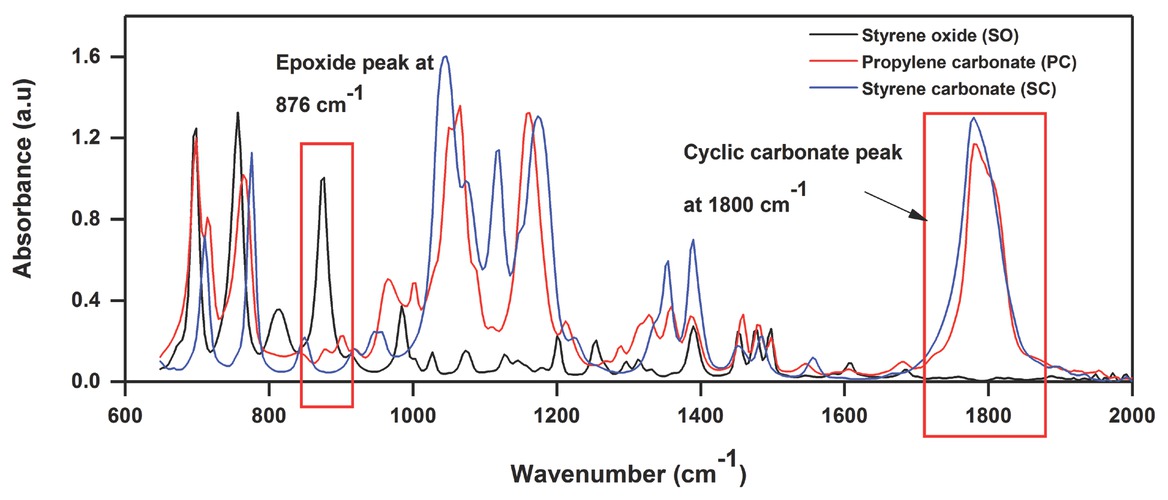
FTIR spectra showing the overlapping of propylene carbonate (PC) and styrene carbonate (SC) peaks at 1800 cm–1.
3 Results and discussion
3.1 Effect of halide anion on SC formation
Formation of SC was carried out using various TBAX (where X = I‾, Br‾, Cl‾, F‾) in combination with ZnBr2 (Figure 6). The results indicate that the order of halide anions activity was Br‾ = I‾ = Cl‾ = F‾, in agreement with the order of leaving group ability of the halide anions i.e. I‾ = Br‾ = Cl‾ = F‾, except for Br‾ which exhibited greater activity due to its optimum balance between nucleophilicity and leaving group ability [45]. The activity of halide anion in a binary catalyst system is strongly influenced by the presence of a second catalyst [46]. The order of halide anions activity also varies depending upon the type of epoxide and second catalyst used for cycloaddition reaction [47, 48, 49, 50]. A similar order of catalytic activity for SC formation from SO and CO2 was observed previously when TBAB was used as a co-catalyst with bimetallic aluminium-salen complex [43].
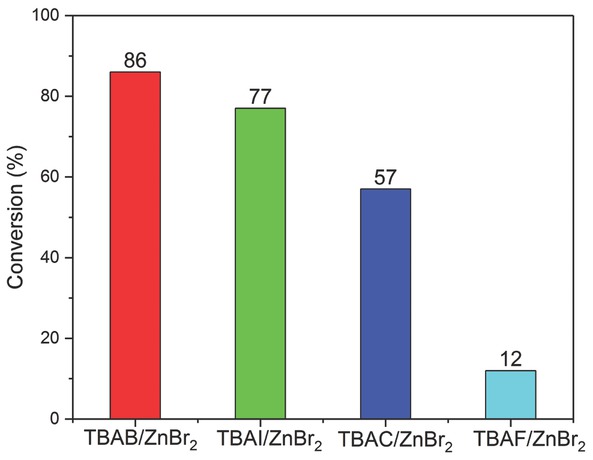
Catalytic activity of tetrabutylammonium halides (TBAX) in combination with ZnBr2 (reaction conditions: Solvent-free SO 35 mmol, 0.4 mmol TBAB, 0.1 mmol ZnBr2 100°C and 6 bar p(CO2), 1 h).
3.2 Kinetic study of SC formation
A detailed study of reaction kinetics was performed to investigate the mechanism associated with SC formation catalysed by a homogeneous binary catalyst (ZnBr2/TBAB). This requires the use of a reaction solvent in order to vary the concentration of SO, and these were selected from commonly used organic solvents such as toluene and polar aprotic solvents: N,N-dimethylformamide (DMF), acetonitrile (CH3CN) and propylene carbonate (PC) (Table 1). From the results obtained, the use of CH3CN has exhibited a higher reaction rate and turnover frequency (TOF) than other solvents under these set of reaction conditions. However, PC was selected as it is a ‘green’ solvent. PC is a high boiling point, biodegradable and non-toxic polar aprotic solvent, which has been commonly used a replacement for toxic solvents such as acetonitrile, DMF and DMSO that are expected to be restricted due to SOx and NOx emission when incinerated [39,51]. Another advantage of PC is that its density (1.2 g/cm3) is close to that of SO (1.05 g/cm3), which results in a reaction medium with mass transfer properties and miscibility that do not vary considerably from solvent-free reaction conditions. PC can also be prepared by the CO2 cycloaddition to propylene oxide (PO) using the same catalytic system i.e. ZnBr2/TBAB. Therefore, it will clearly not inhibit the catalyst.
Effect of solvent on styrene carbonate (SC) formation catalysed by ZnBr2/TBAB catalyst.*
| Solvent | Conversion (%) | TOF (h-1)** |
|---|---|---|
| Toluene | 67 | 466.7 |
| DMF | 55 | 394.6 |
| Acetonitrile | 81 | 550.4 |
| Propylene carbonate | 73 | 487.5 |
* Reaction conditions: 4.5 M SO in PC, TBAB (0.1 M), ZnBr2 (6.5 mM) at 100°C and 6 bar CO2.
** Turnover frequency = TOF = (moles of product) / (moles of catalyst ‧ time).
Prior to the detailed kinetic study, the catalytic activity of TBAB with and without ZnBr2 was investigated. Figure 7 shows the significant increase in reaction rate (~6-fold) when TBAB was used in combination with ZnBr2, rather than as TBAB alone. These results clearly exhibit the synergetic catalytic effect of using ZnBr2 in combination TBAB. Here, the Lewis acidic Zn site caused the SO activation, which undergoes attack by the Br‾ anion acting as a nucleophile provided by the TBAB.
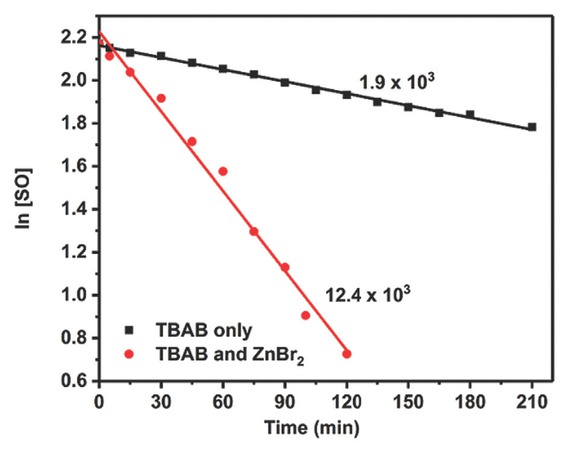
Rate of styrene carbonate (SC) formation catalysed by TBAB with and without ZnBr2 (reaction conditions: Solvent-free SO, ZnBr2/TBAB (1:4), 100°C and 6 bar p(CO2)).
The general rate equation for the SC formation by the cycloaddition of CO2 to SO can be written as Eq. 1. The catalyst concentration was constant throughout the reaction, while the CO2 was maintained in excess through continuous supply in a semi-batch operation. Therefore, it can be assumed that CO2 concentration was constant throughout the reaction [20,44]. Hence, Eq. 1 can be simplified as Eq. 2, and taking the natural logarithm of both sides of Eq. 3 gives Eq. 4. Assuming a pseudo-first-order reaction, Eq. 5 applies and this can be integrated to obtain Eq. 6, which can be used to determine the order of reaction of the SO [44].
where
The order of the reaction with respect to epoxide was determined using SO in the range of 2.5-5.5 M concentrations. The experimental data showed a good fit to first-order kinetics using a plot of ln [SO] against reaction time (min) (Figure 8a). The values of the observed rate constants (kobs) were obtained from the gradients of the first-order plot. Moreover, a linear graph was also obtained between initial concentrations of epoxide against initial reaction rate (mol L–1 min–1) (Figure 8b), which confirms a first-order (a = 1) reaction kinetics for the SO [52, 53, 54].
![Figure 8 (a) Curve fittings of ln [SO] as a function of time (min) over the range of SO (2.5-5.5) M (b) Plot showing a linear dependence of initial reaction rate (mol L–1 min–1) against initial epoxide concentrations [SO] M (reaction conditions: 6.5 mM ZnBr2, 0.1 M TBAB at 100°C and 6 bar p (CO2)).](/document/doi/10.1515/gps-2019-0042/asset/graphic/j_gps-2019-0042_fig_008.jpg)
(a) Curve fittings of ln [SO] as a function of time (min) over the range of SO (2.5-5.5) M (b) Plot showing a linear dependence of initial reaction rate (mol L–1 min–1) against initial epoxide concentrations [SO] M (reaction conditions: 6.5 mM ZnBr2, 0.1 M TBAB at 100°C and 6 bar p (CO2)).
The order of the reaction with respect to CO2 was also studied by varying the pressure over the range 2-8 bar at otherwise constant reaction parameters. Increasing pressure caused the increase in CO2 concentration in the liquid phase and thereby the increase in reaction rate. The order of the reaction was observed to be first-order as experimentally obtained data exhibit a good fit in first-order kinetics (Figure 9a). There was a linear correlation between the graphs of initial reaction rate against CO2 pressure (Figure 9b). This shows that the reaction was first-order (b = 1) with respect to CO2, an indication that one molecule of CO2 was involved in catalytic cycles [28,55, 56].
![Figure 9 (a) Curve fittings of ln [SO] as a function of time (min) over the range of 2-8 bar p(CO2); (b) Plot showing a linear dependence of initial reaction rate (mol‧L-1‧min-1) on CO2 pressure (reaction conditions: SO 4.5 M in PC, 6.5 mM ZnBr2, 0.1 M TBAB at 100°C).](/document/doi/10.1515/gps-2019-0042/asset/graphic/j_gps-2019-0042_fig_009.jpg)
(a) Curve fittings of ln [SO] as a function of time (min) over the range of 2-8 bar p(CO2); (b) Plot showing a linear dependence of initial reaction rate (mol‧L-1‧min-1) on CO2 pressure (reaction conditions: SO 4.5 M in PC, 6.5 mM ZnBr2, 0.1 M TBAB at 100°C).
Reaction order with respect to TBAB was investigated using TBAB concentrations in the range of 0.2-0.5 M, while the other parameters were kept constant. The rate of reaction increased with TBAB concentration (Figure 10a), as would be expected, and all of the data points exhibited good fit to first-order kinetics. Moreover, the reaction was found to be first-order from the gradient (i.e. 1.08) of the double logarithmic graph between (kobs) and [TBAB] (c = 1) (Figure 10b). The role of tetrabutylammonium halides (TBAX) as nucleophile additive to CO2 cycloaddition reactions is generally well accepted [25,57, 58]. These essentially provide a halide anion (X) for epoxide ring-opening by the nucleophilic attack on the least substituted carbon atom of epoxide and also facilitate ring-closing in the final step of the catalytic cycle to form corresponding cyclic carbonate due to its good leaving group abilities.
![Figure 10 (a) Curve fittings of ln [SO] as a function of time (min) over the range of TBAB (0.2-0.5) M (b) Plot showing a linear dependence of ln (kobs) vs ln [TBAB] (reaction conditions: SO 4.5 M in PC at 100°C and 6 bar p (CO2)).](/document/doi/10.1515/gps-2019-0042/asset/graphic/j_gps-2019-0042_fig_010.jpg)
(a) Curve fittings of ln [SO] as a function of time (min) over the range of TBAB (0.2-0.5) M (b) Plot showing a linear dependence of ln (kobs) vs ln [TBAB] (reaction conditions: SO 4.5 M in PC at 100°C and 6 bar p (CO2)).
The order of reaction with respect to ZnBr2 (in combination with TBAB) was also observed by changing the concentration of ZnBr2 over the range of 0.0325-0.25 M (Figure 11a). As mentioned earlier, the reaction rate significantly enhanced due to the synergetic effect of ZnBr2 in combination with TBAB. However, reaction order with respect to ZnBr2 was not first-order. The gradient was instead found to be ~0.5 (Figure 11b). A fractional order (d = 0.5) is normally an indication of the complex reaction. Herein, the proposed reaction mechanism includes a predissociation step. This was probably due to active complex formation consisting of one molecule of ZnBr2 and two molecules of epoxide (Step 2, Figure 14). The metallic salt (ZnBr2) provides a Lewis acidic site in the catalytic cycle for epoxide activation. The activated epoxide then undergoes a nucleophilic attack by halide anion on the less substituted carbon (C) atom of epoxide to open the ring [59,60].
![Figure 11 (a) The curve fittings of ln [SO] as a function of time (min) over the range of ZnBr2 (0.0325-0.25) M (b) Double natural logarithmic graph between (kobs) and (ZnBr2) concentrations (reaction conditions: SO 4.5 M in PC, 0.1 M TBAB at 100°C and 6 bar p (CO2)).](/document/doi/10.1515/gps-2019-0042/asset/graphic/j_gps-2019-0042_fig_011.jpg)
(a) The curve fittings of ln [SO] as a function of time (min) over the range of ZnBr2 (0.0325-0.25) M (b) Double natural logarithmic graph between (kobs) and (ZnBr2) concentrations (reaction conditions: SO 4.5 M in PC, 0.1 M TBAB at 100°C and 6 bar p (CO2)).
![Figure 12 (a) The curve fittings of ln [SO] as a function of time (min) over the temperature range (90-120)°C; (b) Determination of activation energy from Arrhenius plot (ln kobs vs. 1/T) (reaction conditions: SO 4.5 M in PC, 6.5 mM ZnBr2 and 0.1 M TBAB at 6 bar p(CO2)).](/document/doi/10.1515/gps-2019-0042/asset/graphic/j_gps-2019-0042_fig_012.jpg)
(a) The curve fittings of ln [SO] as a function of time (min) over the temperature range (90-120)°C; (b) Determination of activation energy from Arrhenius plot (ln kobs vs. 1/T) (reaction conditions: SO 4.5 M in PC, 6.5 mM ZnBr2 and 0.1 M TBAB at 6 bar p(CO2)).
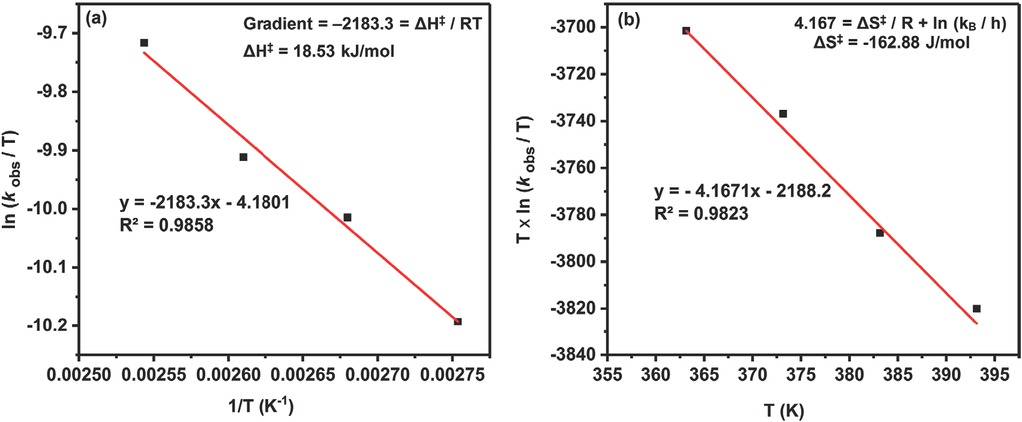
Eyring plots to determine the thermodynamic activation parameters of the reaction according to Eq. 7: (a) enthalpy of activation (ΔH‡); (b) entropy of activation (ΔS‡).
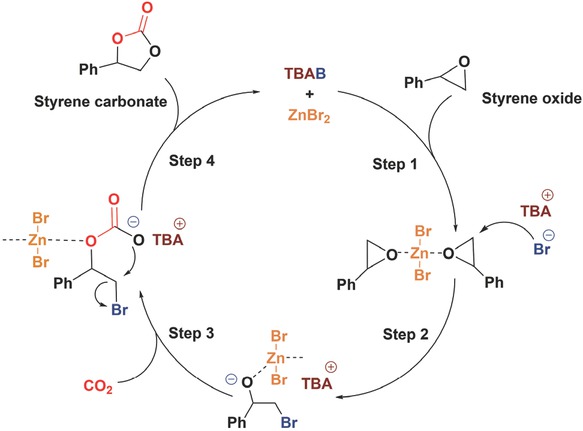
Proposed mechanism of styrene carbonate (SC) formation from styrene oxide (SO) and CO2 catalysed by ZnBr2/TBAB.
The effect of temperature on SC formation was also studied by varying temperature over the range of 90-120°C. As expected, the reaction rate of CO2 cycloaddition to SO increases significantly with temperature. The experimental data obtained was found to be fitted well with first-order kinetics (Figure 12a). Furthermore, the Arrhenius activation energy (Ea) was determined by plotting ln (kobs) against reciprocal of absolute temperature (1/T) (Figure 12b). From the results obtained, the activation energy (Ea) for SC formation was determined to be 23.3 kJ mol−1, which is lower than most of the catalyst systems reported previously [53,61]. Moreover, the thermodynamic activation parameters were studied using the Eyring equation (Eq. 7) [62].
where h (6.62608 × 1034 J s) and kB (1.38065 × 10−23 J K−1) are the Planck’s and Boltzmann constants, respectively. The values of enthalpy of activation (ΔH‡) and entropy of activation (ΔS‡) were determined from the Eyring plots (Figures 13a and 13b). Similarly, the values of Gibb’s free energy of activation (ΔG‡) were determined for all temperatures using fundamental thermodynamics equation (Table 2). The values of ΔH‡ and ΔG‡ were positive, an indication that the formation of SC via reactions of CO2 and SO was endergonic and kinetically controlled. However, the low value of ΔS‡ indicates that there is an associative mechanism involved in using the binary catalytic system, as the activated complex has a tendency to become more ordered [63].
Summary of thermodynamic activation parameters of styrene carbonate (SC) formation according to Eyring Equation.
| ΔH‡ | ΔS‡ | ΔG‡ = ΔH‡ – T. ΔS‡ (kJ/mol) | |||
|---|---|---|---|---|---|
| (kJ/mol) | (J/mol) | 363 K | 373 K | 383 K | 393 K |
| 18.53 | –162.88 | 77.3 | 78.93 | 80.56 | 82.19 |
3.3 Proposed reaction mechanism
A reaction mechanism was proposed based on the kinetic analysis of SC formation by the reactions of CO2 and SO catalysed by ZnBr2/TBAB (Figure 14). Initially, the activation of SO takes place by the interaction of Zn-site (Lewis acidic) with the oxygen atom (Step 1). The activated SO undergoes nucleophilic attack by the bromide anion (Br‾) on least substituted carbon (C) atom resulting in a bromo-alkoxide intermediate. This intermediate was stabilised by the tetrabutylammonium cation (TBA+) to facilitate rapid CO2-insertion (Step 2). Subsequently, CO2-insertion takes place by the nucleophilic attack of the negatively charged oxygen of alkoxide intermediate on the electrophilic C atom of CO2 (Step 3). Lastly, a five-membered ring of styrene carbonate (SC) was formed due to cleavage of Zn–O bond and intramolecular displacement of bromide anion (Br‾) owing to its high leaving group ability (Step 4).
4 Conclusions
Synthesis of styrene carbonate (SC) via the chemical fixation of CO2 with styrene oxide (SO) was carried out using a combination of cheaply available ZnBr2 and tetrabutylammonium halides (TBAX), as an acid-base binary homogeneous catalyst. Propylene carbonate (PC) was used, as it is considered to be a ‘green’ solvent. The order of halide anion activity on SC formation was found to be Br‾ = I‾ = Cl‾ = F‾, in agreement with the order of leaving group ability of the halide anions, apart from Br‾, which exhibited higher activity due to an optimum balance between nucleophilicity and leaving group ability. The ZnBr2/TBAB combination achieved the highest catalytic activity due to the synergistic effect, increasing the rate 6-fold, compared to TBAB alone. The SC formation reaction was found to be first-order with respect to SO, CO2 and TBAB, whereas nonfirst-order kinetics was observed for the ZnBr2. The Arrhenius activation energy (Ea) for the SC formation was 23.3 kJ mol−1 at temperatures of 90-120°C. The values of ΔH‡ and ΔG‡ determined from the Eyring equation were both positive, suggesting that the SC formation was endergonic and controlled by reaction kinetics. However, the SC formation had a negative ΔS‡, an indication that the associative mechanism was involved in the binary catalytic system. A reaction mechanism for the SC formation was proposed, explaining the modus operandi of the binary homogeneous catalyst.
Acknowledgments
The authors are grateful to the support of the University of Engineering and Technology, Lahore in providing the scholarship to one of the authors. This work was supported by the EPSRC project on Sustainable Polymers (Project reference EP/L017393/1).
References
[1] Kleij A.W., North M., Urakawa A., CO2 catalysis. ChemSusChem, 2017, 10, 1036-1038.10.1002/cssc.201700218Search in Google Scholar PubMed
[2] Aresta M., Dibenedetto A., Utilisation of CO2 as a chemical feedstock: opportunities and challenges. Dalton Transactions, 2007, 2975-2992.10.1039/b700658fSearch in Google Scholar PubMed
[3] Omae I., Aspects of carbon dioxide utilization. Catal. Today, 2006, 115, 33-52.10.1016/j.cattod.2006.02.024Search in Google Scholar
[4] Trost B.M., On inventing reactions for atom economy. Acc. Chem. Res., 2002, 35, 695-705.10.1021/ar010068zSearch in Google Scholar PubMed
[5] Sakakura T., Kohno K., The synthesis of organic carbonates from carbon dioxide. Chem. Commun., 2009, 1312-1330.10.1039/b819997cSearch in Google Scholar PubMed
[6] Yoshida M., Ihara M., Novel Methodologies for the Synthesis of Cyclic Carbonates. Chem.-Eur. J., 2004, 10, 2886-2893.10.1002/chem.200305583Search in Google Scholar PubMed
[7] Kurisingal J.F., Rachuri Y., Gu Y., Kim G.-H., Park D.-W., Binary metal-organic frameworks: Catalysts for the efficient solvent-free CO2 fixation reaction via cyclic carbonates synthesis. Appl. Catal. A-Gen., 2019, 571, 1-11.10.1016/j.apcata.2018.11.035Search in Google Scholar
[8] Sakakura T., Choi J.-C., Yasuda H., Transformation of carbon dioxide. Chem. Rev., 2007, 107, 2365-2387.10.1021/cr068357uSearch in Google Scholar PubMed
[9] Aresta M., Dibenedetto A., Angelini A., Catalysis for the valorization of exhaust carbon: from CO2 to chemicals, materials, and fuels. Technological use of CO2 Chem. Rev., 2013, 114, 1709-1742.10.1021/cr4002758Search in Google Scholar PubMed
[10] Fukuoka S., Fukawa I., Tojo M., Oonishi K., Hachiya H., Aminaka M., et al., A novel non-phosgene process for polycarbonate production from CO2 green and sustainable chemistry in practice. Catal. Surv. Asia, 2010, 14, 146-163.10.1007/s10563-010-9093-5Search in Google Scholar
[11] Nohra B., Candy L., Blanco J.-F.O., Guerin C., Raoul Y., Mouloungui Z., From petrochemical polyurethanes to biobased polyhydroxyurethanes. Macromolecules, 2013, 46, 3771-3792.10.1021/ma400197cSearch in Google Scholar
[12] Schaffner B., Schaffner F., Verevkin S.P., Borner A., Organic carbonates as solvents in synthesis and catalysis. Chem. Rev., 2010, 110, 4554-4581.10.1021/cr900393dSearch in Google Scholar PubMed
[13] North M., Pizzato F., Villuendas P., Organocatalytic, asymmetric aldol reactions with a sustainable catalyst in a green solvent. ChemSusChem, 2009, 2, 862-865.10.1002/cssc.200900144Search in Google Scholar PubMed
[14] Lang X.-D., Li Z.-M., He L.-N., Protic ionic liquid-catalyzed synthesis of oxazolidinones using cyclic carbonates as both CO2 surrogate and sustainable solvent. Catal. Today, 2019, 324, 167-173.10.1016/j.cattod.2018.09.019Search in Google Scholar
[15] Xu K., Nonaqueous liquid electrolytes for lithium-based rechargeable batteries. Chem. Rev., 2004, 104, 4303-4418.10.1021/cr030203gSearch in Google Scholar PubMed
[16] Scrosati B., Hassoun J., Sun Y.-K., Lithium-ion batteries. A look into the future. Energy Environ. Sci., 2011, 4, 3287-3295.10.1039/c1ee01388bSearch in Google Scholar
[17] Shaikh A.-A.G., Sivaram S., Organic carbonates. Chem. Rev., 1996, 96, 951-976.10.1021/cr950067iSearch in Google Scholar PubMed
[18] Clements J.H., Reactive applications of cyclic alkylene carbonates. Ind. Eng. Chem. Res., 2003, 42, 663-674.10.1021/ie020678iSearch in Google Scholar
[19] Castro-Osma J.A., Alonso-Moreno C., Lara-Sánchez A., Martínez J., North M., Otero A., Synthesis of cyclic carbonates catalysed by aluminium heteroscorpionate complexes. Catal. Sci. Technol., 2014, 4, 1674-1684.10.1039/C3CY00810JSearch in Google Scholar
[20] Castro-Osma J.A., Lamb K.J., North M., Cr (salophen) complex catalyzed cyclic carbonate synthesis at ambient temperature and pressure. ACS Catal., 2016, 6, 5012-5025.10.1021/acscatal.6b01386Search in Google Scholar
[21] North M., Pasquale R., Young C., Synthesis of cyclic carbonates from epoxides and CO2 Green Chem., 2010, 12, 1514-1539.10.1039/c0gc00065eSearch in Google Scholar
[22] Castro‐Gómez F., Salassa G., Kleij A.W., Bo C., A DFT study on the mechanism of the cycloaddition reaction of CO2 to epoxides catalyzed by Zn (Salphen) complexes. Chem. - Eur. J., 2013, 19, 6289-6298.10.1002/chem.201203985Search in Google Scholar PubMed
[23] Wang J.-Q., Dong K., Cheng W.-G., Sun J., Zhang S.-J., Insights into quaternary ammonium salts-catalyzed fixation carbon dioxide with epoxides. Catal. Sci. Technol., 2012, 2, 1480-1484.10.1039/c2cy20103hSearch in Google Scholar
[24] Martin R., Kleij A.W., Myth or reality? Fixation of carbon dioxide into complex organic matter under mild conditions. ChemSusChem, 2011, 4, 1259-1263.10.1002/cssc.201100102Search in Google Scholar PubMed
[25] Calo V., Nacci A., Monopoli A., Fanizzi A., Cyclic carbonate formation from carbon dioxide and oxiranes in tetrabutylammonium halides as solvents and catalysts. Org. Lett., 2002, 4, 2561-2563.10.1021/ol026189wSearch in Google Scholar PubMed
[26] Sun J., Ren J., Zhang S., Cheng W., Water as an efficient medium for the synthesis of cyclic carbonate. Tetrahedron Lett., 2009, 50, 423-426.10.1016/j.tetlet.2008.11.034Search in Google Scholar
[27] Cokoja M., Wilhelm M.E., Anthofer M.H., Herrmann W.A., Kuehn F.E., Synthesis of cyclic carbonates from epoxides and carbon dioxide by using organocatalysts. ChemSusChem, 2015, 8, 2436-2454.10.1002/cssc.201500161Search in Google Scholar PubMed
[28] Rehman A., Fernández A.M.L., Resul M.F.M.G., Harvey A., Highly selective, sustainable synthesis of limonene cyclic carbonate from bio-based limonene oxide and CO2 A kinetic study. J. CO2 Util., 2019, 29, 126-133.10.1016/j.jcou.2018.12.001Search in Google Scholar
[29] Peng J., Deng Y., Cycloaddition of carbon dioxide to propylene oxide catalyzed by ionic liquids. New J. Chem., 2001, 25, 639-641.10.1039/b008923kSearch in Google Scholar
[30] Kim H.S., Kim J.J., Kim H., Jang H.G., Imidazolium zinc tetrahalide-catalyzed coupling reaction of CO2 and ethylene oxide or propylene oxide. J. Catal., 2003, 220, 44-46.10.1016/S0021-9517(03)00238-0Search in Google Scholar
[31] Bobbink F.D., Dyson P.J., Synthesis of carbonates and related compounds incorporating CO2 using ionic liquid-type catalysts: State-of-the-art and beyond. J. Catal., 2016, 343, 52-61.10.1016/j.jcat.2016.02.033Search in Google Scholar
[32] Kim D., Ji H., Hur M.Y., Lee W., Kim T.S., Cho D.-H., Polymer-supported Zn-containing imidazolium salt ionic liquids as sustainable catalysts for the cycloaddition of CO2 A kinetic study and response surface methodology. ACS Sustain. Chem. Eng., 2018, 6, 14743-14750.10.1021/acssuschemeng.8b03296Search in Google Scholar
[33] Li W., Cheng W., Yang X., Su Q., Dong L., Zhang P., et al., Synthesis of Cyclic Carbonate Catalyzed by DBU Derived Basic Ionic Liquids. Chin. J. Chem . 2018, 36, 293-298.10.1002/cjoc.201700747Search in Google Scholar
[34] Büttner H., Longwitz L., Steinbauer J., Wulf C., Werner T., Recent developments in the synthesis of cyclic carbonates from epoxides and CO2 Top. Curr. Chem., 2017, 375, 50.10.1007/s41061-017-0136-5Search in Google Scholar PubMed
[35] Martin C., Fiorani G., Kleij A.W., Recent advances in the catalytic preparation of cyclic organic carbonates. ACS Catal., 2015, 5, 1353-1370.10.1021/cs5018997Search in Google Scholar
[36] Shaikh R.R., Pornpraprom S., D’Elia V., Catalytic strategies for the cycloaddition of pure, diluted, and waste CO2 to epoxides under ambient conditions. ACS Catal., 2017, 8, 419-450.10.1021/acscatal.7b03580Search in Google Scholar
[37] Martínez J., de la Cruz-Martínez F., Gaona M.A., Pinilla-Peñalver E., Fernández-Baeza J., Rodríguez A.M., et al., Influence of the Counterion on the Synthesis of Cyclic Carbonates Catalyzed by Bifunctional Aluminum Complexes. Inorg. Chem., 2019.10.1021/acs.inorgchem.8b03475Search in Google Scholar PubMed
[38] Della Monica F., Leone M., Buonerba A., Grassi A., Milione S., Capacchione C., CO2 cycloaddition to epoxides promoted by bis-thioether-phenolate Fe(II) and Fe(III) complexes. Molecular Catalysis, 2018, 460, 46-52.10.1016/j.mcat.2018.09.003Search in Google Scholar
[39] Comerford J.W., Ingram I.D.V., North M., Wu X., Sustainable metal-based catalysts for the synthesis of cyclic carbonates containing five-membered rings. Green Chem., 2015, 17, 1966-1987.10.1039/C4GC01719FSearch in Google Scholar
[40] Laugel G., Rocha C.C., Massiani P., Onfroy T., Launay F., Homogeneous and Heterogeneous Catalysis for the Synthesis of Cyclic and Polymeric Carbonates from CO2 and Epoxides: A Mechanistic Overview. Adv. Chem. Lett., 2013, 1, 195-214.10.1002/chin.201503269Search in Google Scholar
[41] Sun J., Fujita S.-I., Zhao F., Arai M., A highly efficient catalyst system of ZnBr2n-Bu4NI for the synthesis of styrene carbonate from styrene oxide and supercritical carbon dioxide. Appl. Catal. A-Gen., 2005, 287, 221-226.10.1016/j.apcata.2005.03.035Search in Google Scholar
[42] Jutz F., Grunwaldt J.-D., Baiker A., Mn (III)(salen)-catalyzed synthesis of cyclic organic carbonates from propylene and styrene oxide in “supercritical” CO2 J. Mol. Catal. A-Chem., 2008, 279, 94-103.10.1016/j.molcata.2007.10.010Search in Google Scholar
[43] Clegg W., Harrington R.W., North M., Pasquale R., Cyclic carbonate synthesis catalysed by bimetallic aluminium–salen complexes. Chem.-Eur. J., 2010, 16, 6828-6843.10.1002/chem.201000030Search in Google Scholar PubMed
[44] Luo R., Zhang W., Yang Z., Zhou X., Ji H., Synthesis of cyclic carbonates from epoxides over bifunctional salen aluminum oligomers as a CO2-philic catalyst: Catalytic and kinetic investigation. J. CO2 Util., 2017, 19, 257-265.10.1016/j.jcou.2017.04.002Search in Google Scholar
[45] Langanke J., Greiner L., Leitner W., Substrate dependent synergetic and antagonistic interaction of ammonium halide and polyoxometalate catalysts in the synthesis of cyclic carbonates from oleochemical epoxides and CO2 Green Chem., 2013, 15, 1173-1182.10.1039/c3gc36710jSearch in Google Scholar
[46] Steinbauer J., Kubis C., Ludwig R., Werner T., Mechanistic Study on the Addition of CO2 to Epoxides Catalyzed by Ammonium and Phosphonium Salts: A Combined Spectroscopic and Kinetic Approach. ACS Sustain. Chem. Eng., 2018, 6, 10778-10788.10.1021/acssuschemeng.8b02093Search in Google Scholar
[47] Büttner H., Lau K., Spannenberg A., Werner T., Bifunctional one‐component catalysts for the addition of carbon dioxide to epoxides. ChemCatChem, 2015, 7, 459-467.10.1002/cctc.201402816Search in Google Scholar
[48] Werner T., Buettner H., Phosphorus‐based Bifunctional Organocatalysts for the Addition of Carbon Dioxide and Epoxides. ChemSusChem, 2014, 7, 3268-3271.10.1002/cssc.201402477Search in Google Scholar PubMed
[49] Büttner H., Steinbauer J., Werner T., Synthesis of Cyclic Carbonates from Epoxides and Carbon Dioxide by Using Bifunctional One‐Component Phosphorus‐Based Organocatalysts. ChemSusChem, 2015, 8, 2655-2669.10.1002/cssc.201500612Search in Google Scholar PubMed
[50] Bobbink F.D., Vasilyev D., Hulla M., Chamam S., Menoud F., Laurenczy G.b., et al., Intricacies of Cation–Anion Combinations in Imidazolium Salt-Catalyzed Cycloaddition of CO2 Into Epoxides. ACS Catal., 2018, 8, 2589-2594.10.1021/acscatal.7b04389Search in Google Scholar
[51] Bello Forero J.S., Hernández Muñoz J.A., Jones Junior J., da Silva F.M., Propylene Carbonate in Organic Synthesis: Exploring its Potential as a Green Solvent. Curr. Org. Synth., 2016, 13, 834-846.10.2174/1570179413999160211094705Search in Google Scholar
[52] Jutz F., Buchard A., Kember M.R., Fredriksen S.B., Williams C.K., Mechanistic investigation and reaction kinetics of the low-pressure copolymerization of cyclohexene oxide and carbon dioxide catalyzed by a dizinc complex. J. Am. Chem. Soc., 2011, 133, 17395-17405.10.1021/ja206352xSearch in Google Scholar PubMed
[53] Liu M., Liu B., Zhong S., Shi L., Liang L., Sun J., Kinetics and mechanistic insight into efficient fixation of CO2 to epoxides over N-heterocyclic compound/ZnBr2 catalysts. Ind. Eng. Chem. Res., 2015, 54, 633-640.10.1021/ie5042879Search in Google Scholar
[54] Rehman A., Eze V.C., Resul M.F.M.G., Harvey A., Kinetics and mechanistic investigation of epoxide/CO2 cycloaddition by a synergistic catalytic effect of pyrrolidinopyridinium iodide and zinc halides. J. Energy Chem., 2019, 37, 35-42.10.1016/j.jechem.2018.11.017Search in Google Scholar
[55] North M., Pasquale R., Mechanism of cyclic carbonate synthesis from epoxides and CO2 Angew. Chem., 2009, 121, 2990-2992.10.1002/ange.200805451Search in Google Scholar
[56] Cuesta-Aluja L., Castilla J., Masdeu-Bultó A.M., Aluminium salabza complexes for fixation of CO2 to organic carbonates. Dalton T., 2016, 45, 14658-14667.10.1039/C6DT01069ESearch in Google Scholar PubMed
[57] Jutz F., Grunwaldt J.-D., Baiker A., Mn (III)(salen)-catalyzed synthesis of cyclic organic carbonates from propylene and styrene oxide in “supercritical” CO2 J. Mol. Catal. A-Chem., 2008, 279, 94-103.10.1016/j.molcata.2007.10.010Search in Google Scholar
[58] Jutz F., Grunwaldt J.-D., Baiker A., In situ XAS study of the Mn (III) (salen) Br catalyzed synthesis of cyclic organic carbonates from epoxides and CO2 J. Mol. Catal. A-Chem., 2009, 297, 63-72.10.1016/j.molcata.2008.10.009Search in Google Scholar
[59] Sun J., Fujita S.-I., Zhao F., Arai M., A highly efficient catalyst system of ZnBr2n-Bu4NI for the synthesis of styrene carbonate from styrene oxide and supercritical carbon dioxide. Appl. Catal. A-Gen., 2005, 287, 221-226.10.1016/j.apcata.2005.03.035Search in Google Scholar
[60] Xiao L.-F., Li F.-W., Peng J.-J., Xia C.-G., Immobilized ionic liquid/ zinc chloride: Heterogeneous catalyst for synthesis of cyclic carbonates from carbon dioxide and epoxides. J. Mol. Catal. A-Chem., 2006, 253, 265-269.10.1016/j.molcata.2006.03.047Search in Google Scholar
[61] Supasitmongkol S., Styring P., A single centre aluminium (III) catalyst and TBAB as an ionic organo-catalyst for the homogeneous catalytic synthesis of styrene carbonate. Catal. Sci. Technol., 2014, 4, 1622-1630.10.1039/C3CY01015ESearch in Google Scholar
[62] Lente G., Fábián I., Poë A.J., A common misconception about the Eyring equation. New J. Chem., 2005, 29, 759-760.10.1039/b501687hSearch in Google Scholar
[63] Ghodsi F., Habibi-Khorassani S.M., Shahraki M., Kinetic Spectrophotometric Method for the 1,4-Diionic Organophosphorus Formation in the Presence of Meldrum′ s Acid: Stopped-Flow Approach. Molecules, 2016, 21, 1514.10.3390/molecules21111514Search in Google Scholar PubMed PubMed Central
© 2019 Rehman et al., published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 Public License.
Articles in the same Issue
- Regular Articles
- Studies on the preparation and properties of biodegradable polyester from soybean oil
- Flow-mode biodiesel production from palm oil using a pressurized microwave reactor
- Reduction of free fatty acids in waste oil for biodiesel production by glycerolysis: investigation and optimization of process parameters
- Saccharin: a cheap and mild acidic agent for the synthesis of azo dyes via telescoped dediazotization
- Optimization of lipase-catalyzed synthesis of polyethylene glycol stearate in a solvent-free system
- Green synthesis of iron oxide nanoparticles using Platanus orientalis leaf extract for antifungal activity
- Ultrasound assisted chemical activation of peanut husk for copper removal
- Room temperature silanization of Fe3O4 for the preparation of phenyl functionalized magnetic adsorbent for dispersive solid phase extraction for the extraction of phthalates in water
- Evaluation of the saponin green extraction from Ziziphus spina-christi leaves using hydrothermal, microwave and Bain-Marie water bath heating methods
- Oxidation of dibenzothiophene using the heterogeneous catalyst of tungsten-based carbon nanotubes
- Calcined sodium silicate as an efficient and benign heterogeneous catalyst for the transesterification of natural lecithin to L-α-glycerophosphocholine
- Synergistic effect between CO2 and H2O2 on ethylbenzene oxidation catalyzed by carbon supported heteropolyanion catalysts
- Hydrocyanation of 2-arylmethyleneindan-1,3-diones using potassium hexacyanoferrate(II) as a nontoxic cyanating agent
- Green synthesis of hydratropic aldehyde from α-methylstyrene catalyzed by Al2O3-supported metal phthalocyanines
- Environmentally benign chemical recycling of polycarbonate wastes: comparison of micro- and nano-TiO2 solid support efficiencies
- Medicago polymorpha-mediated antibacterial silver nanoparticles in the reduction of methyl orange
- Production of value-added chemicals from esterification of waste glycerol over MCM-41 supported catalysts
- Green synthesis of zerovalent copper nanoparticles for efficient reduction of toxic azo dyes congo red and methyl orange
- Optimization of the biological synthesis of silver nanoparticles using Penicillium oxalicum GRS-1 and their antimicrobial effects against common food-borne pathogens
- Optimization of submerged fermentation conditions to overproduce bioethanol using two industrial and traditional Saccharomyces cerevisiae strains
- Extraction of In3+ and Fe3+ from sulfate solutions by using a 3D-printed “Y”-shaped microreactor
- Foliar-mediated Ag:ZnO nanophotocatalysts: green synthesis, characterization, pollutants degradation, and in vitro biocidal activity
- Green cyclic acetals production by glycerol etherification reaction with benzaldehyde using cationic acidic resin
- Biosynthesis, characterization and antimicrobial activities assessment of fabricated selenium nanoparticles using Pelargonium zonale leaf extract
- Synthesis of high surface area magnesia by using walnut shell as a template
- Controllable biosynthesis of silver nanoparticles using actinobacterial strains
- Green vegetation: a promising source of color dyes
- Mechano-chemical synthesis of ammonia and acetic acid from inorganic materials in water
- Green synthesis and structural characterization of novel N1-substituted 3,4-dihydropyrimidin-2(1H)-ones
- Biodiesel production from cotton oil using heterogeneous CaO catalysts from eggshells prepared at different calcination temperatures
- Regeneration of spent mercury catalyst for the treatment of dye wastewater by the microwave and ultrasonic spray-assisted method
- Green synthesis of the innovative super paramagnetic nanoparticles from the leaves extract of Fraxinus chinensis Roxb and their application for the decolourisation of toxic dyes
- Biogenic ZnO nanoparticles: a study of blueshift of optical band gap and photocatalytic degradation of reactive yellow 186 dye under direct sunlight
- Leached compounds from the extracts of pomegranate peel, green coconut shell, and karuvelam wood for the removal of hexavalent chromium
- Enhancement of molecular weight reduction of natural rubber in triphasic CO2/toluene/H2O systems with hydrogen peroxide for preparation of biobased polyurethanes
- An efficient green synthesis of novel 1H-imidazo[1,2-a]imidazole-3-amine and imidazo[2,1-c][1,2,4]triazole-5-amine derivatives via Strecker reaction under controlled microwave heating
- Evaluation of three different green fabrication methods for the synthesis of crystalline ZnO nanoparticles using Pelargonium zonale leaf extract
- A highly efficient and multifunctional biomass supporting Ag, Ni, and Cu nanoparticles through wetness impregnation for environmental remediation
- Simple one-pot green method for large-scale production of mesalamine, an anti-inflammatory agent
- Relationships between step and cumulative PMI and E-factors: implications on estimating material efficiency with respect to charting synthesis optimization strategies
- A comparative sorption study of Cr3+ and Cr6+ using mango peels: kinetic, equilibrium and thermodynamic
- Effects of acid hydrolysis waste liquid recycle on preparation of microcrystalline cellulose
- Use of deep eutectic solvents as catalyst: A mini-review
- Microwave-assisted synthesis of pyrrolidinone derivatives using 1,1’-butylenebis(3-sulfo-3H-imidazol-1-ium) chloride in ethylene glycol
- Green and eco-friendly synthesis of Co3O4 and Ag-Co3O4: Characterization and photo-catalytic activity
- Adsorption optimized of the coal-based material and application for cyanide wastewater treatment
- Aloe vera leaf extract mediated green synthesis of selenium nanoparticles and assessment of their In vitro antimicrobial activity against spoilage fungi and pathogenic bacteria strains
- Waste phenolic resin derived activated carbon by microwave-assisted KOH activation and application to dye wastewater treatment
- Direct ethanol production from cellulose by consortium of Trichoderma reesei and Candida molischiana
- Agricultural waste biomass-assisted nanostructures: Synthesis and application
- Biodiesel production from rubber seed oil using calcium oxide derived from eggshell as catalyst – optimization and modeling studies
- Study of fabrication of fully aqueous solution processed SnS quantum dot-sensitized solar cell
- Assessment of aqueous extract of Gypsophila aretioides for inhibitory effects on calcium carbonate formation
- An environmentally friendly acylation reaction of 2-methylnaphthalene in solvent-free condition in a micro-channel reactor
- Aegle marmelos phytochemical stabilized synthesis and characterization of ZnO nanoparticles and their role against agriculture and food pathogen
- A reactive coupling process for co-production of solketal and biodiesel
- Optimization of the asymmetric synthesis of (S)-1-phenylethanol using Ispir bean as whole-cell biocatalyst
- Synthesis of pyrazolopyridine and pyrazoloquinoline derivatives by one-pot, three-component reactions of arylglyoxals, 3-methyl-1-aryl-1H-pyrazol-5-amines and cyclic 1,3-dicarbonyl compounds in the presence of tetrapropylammonium bromide
- Preconcentration of morphine in urine sample using a green and solvent-free microextraction method
- Extraction of glycyrrhizic acid by aqueous two-phase system formed by PEG and two environmentally friendly organic acid salts - sodium citrate and sodium tartrate
- Green synthesis of copper oxide nanoparticles using Juglans regia leaf extract and assessment of their physico-chemical and biological properties
- Deep eutectic solvents (DESs) as powerful and recyclable catalysts and solvents for the synthesis of 3,4-dihydropyrimidin-2(1H)-ones/thiones
- Biosynthesis, characterization and anti-microbial activity of silver nanoparticle based gel hand wash
- Efficient and selective microwave-assisted O-methylation of phenolic compounds using tetramethylammonium hydroxide (TMAH)
- Anticoagulant, thrombolytic and antibacterial activities of Euphorbia acruensis latex-mediated bioengineered silver nanoparticles
- Volcanic ash as reusable catalyst in the green synthesis of 3H-1,5-benzodiazepines
- Green synthesis, anionic polymerization of 1,4-bis(methacryloyl)piperazine using Algerian clay as catalyst
- Selenium supplementation during fermentation with sugar beet molasses and Saccharomyces cerevisiae to increase bioethanol production
- Biosynthetic potential assessment of four food pathogenic bacteria in hydrothermally silver nanoparticles fabrication
- Investigating the effectiveness of classical and eco-friendly approaches for synthesis of dialdehydes from organic dihalides
- Pyrolysis of palm oil using zeolite catalyst and characterization of the boil-oil
- Azadirachta indica leaves extract assisted green synthesis of Ag-TiO2 for degradation of Methylene blue and Rhodamine B dyes in aqueous medium
- Synthesis of vitamin E succinate catalyzed by nano-SiO2 immobilized DMAP derivative in mixed solvent system
- Extraction of phytosterols from melon (Cucumis melo) seeds by supercritical CO2 as a clean technology
- Production of uronic acids by hydrothermolysis of pectin as a model substance for plant biomass waste
- Biofabrication of highly pure copper oxide nanoparticles using wheat seed extract and their catalytic activity: A mechanistic approach
- Intelligent modeling and optimization of emulsion aggregation method for producing green printing ink
- Improved removal of methylene blue on modified hierarchical zeolite Y: Achieved by a “destructive-constructive” method
- Two different facile and efficient approaches for the synthesis of various N-arylacetamides via N-acetylation of arylamines and straightforward one-pot reductive acetylation of nitroarenes promoted by recyclable CuFe2O4 nanoparticles in water
- Optimization of acid catalyzed esterification and mixed metal oxide catalyzed transesterification for biodiesel production from Moringa oleifera oil
- Kinetics and the fluidity of the stearic acid esters with different carbon backbones
- Aiming for a standardized protocol for preparing a process green synthesis report and for ranking multiple synthesis plans to a common target product
- Microstructure and luminescence of VO2 (B) nanoparticle synthesis by hydrothermal method
- Optimization of uranium removal from uranium plant wastewater by response surface methodology (RSM)
- Microwave drying of nickel-containing residue: dielectric properties, kinetics, and energy aspects
- Simple and convenient two step synthesis of 5-bromo-2,3-dimethoxy-6-methyl-1,4-benzoquinone
- Biodiesel production from waste cooking oil
- The effect of activation temperature on structure and properties of blue coke-based activated carbon by CO2 activation
- Optimization of reaction parameters for the green synthesis of zero valent iron nanoparticles using pine tree needles
- Microwave-assisted protocol for squalene isolation and conversion from oil-deodoriser distillates
- Denitrification performance of rare earth tailings-based catalysts
- Facile synthesis of silver nanoparticles using Averrhoa bilimbi L and Plum extracts and investigation on the synergistic bioactivity using in vitro models
- Green production of AgNPs and their phytostimulatory impact
- Photocatalytic activity of Ag/Ni bi-metallic nanoparticles on textile dye removal
- Topical Issue: Green Process Engineering / Guest Editors: Martine Poux, Patrick Cognet
- Modelling and optimisation of oxidative desulphurisation of tyre-derived oil via central composite design approach
- CO2 sequestration by carbonation of olivine: a new process for optimal separation of the solids produced
- Organic carbonates synthesis improved by pervaporation for CO2 utilisation
- Production of starch nanoparticles through solvent-antisolvent precipitation in a spinning disc reactor
- A kinetic study of Zn halide/TBAB-catalysed fixation of CO2 with styrene oxide in propylene carbonate
- Topical on Green Process Engineering
Articles in the same Issue
- Regular Articles
- Studies on the preparation and properties of biodegradable polyester from soybean oil
- Flow-mode biodiesel production from palm oil using a pressurized microwave reactor
- Reduction of free fatty acids in waste oil for biodiesel production by glycerolysis: investigation and optimization of process parameters
- Saccharin: a cheap and mild acidic agent for the synthesis of azo dyes via telescoped dediazotization
- Optimization of lipase-catalyzed synthesis of polyethylene glycol stearate in a solvent-free system
- Green synthesis of iron oxide nanoparticles using Platanus orientalis leaf extract for antifungal activity
- Ultrasound assisted chemical activation of peanut husk for copper removal
- Room temperature silanization of Fe3O4 for the preparation of phenyl functionalized magnetic adsorbent for dispersive solid phase extraction for the extraction of phthalates in water
- Evaluation of the saponin green extraction from Ziziphus spina-christi leaves using hydrothermal, microwave and Bain-Marie water bath heating methods
- Oxidation of dibenzothiophene using the heterogeneous catalyst of tungsten-based carbon nanotubes
- Calcined sodium silicate as an efficient and benign heterogeneous catalyst for the transesterification of natural lecithin to L-α-glycerophosphocholine
- Synergistic effect between CO2 and H2O2 on ethylbenzene oxidation catalyzed by carbon supported heteropolyanion catalysts
- Hydrocyanation of 2-arylmethyleneindan-1,3-diones using potassium hexacyanoferrate(II) as a nontoxic cyanating agent
- Green synthesis of hydratropic aldehyde from α-methylstyrene catalyzed by Al2O3-supported metal phthalocyanines
- Environmentally benign chemical recycling of polycarbonate wastes: comparison of micro- and nano-TiO2 solid support efficiencies
- Medicago polymorpha-mediated antibacterial silver nanoparticles in the reduction of methyl orange
- Production of value-added chemicals from esterification of waste glycerol over MCM-41 supported catalysts
- Green synthesis of zerovalent copper nanoparticles for efficient reduction of toxic azo dyes congo red and methyl orange
- Optimization of the biological synthesis of silver nanoparticles using Penicillium oxalicum GRS-1 and their antimicrobial effects against common food-borne pathogens
- Optimization of submerged fermentation conditions to overproduce bioethanol using two industrial and traditional Saccharomyces cerevisiae strains
- Extraction of In3+ and Fe3+ from sulfate solutions by using a 3D-printed “Y”-shaped microreactor
- Foliar-mediated Ag:ZnO nanophotocatalysts: green synthesis, characterization, pollutants degradation, and in vitro biocidal activity
- Green cyclic acetals production by glycerol etherification reaction with benzaldehyde using cationic acidic resin
- Biosynthesis, characterization and antimicrobial activities assessment of fabricated selenium nanoparticles using Pelargonium zonale leaf extract
- Synthesis of high surface area magnesia by using walnut shell as a template
- Controllable biosynthesis of silver nanoparticles using actinobacterial strains
- Green vegetation: a promising source of color dyes
- Mechano-chemical synthesis of ammonia and acetic acid from inorganic materials in water
- Green synthesis and structural characterization of novel N1-substituted 3,4-dihydropyrimidin-2(1H)-ones
- Biodiesel production from cotton oil using heterogeneous CaO catalysts from eggshells prepared at different calcination temperatures
- Regeneration of spent mercury catalyst for the treatment of dye wastewater by the microwave and ultrasonic spray-assisted method
- Green synthesis of the innovative super paramagnetic nanoparticles from the leaves extract of Fraxinus chinensis Roxb and their application for the decolourisation of toxic dyes
- Biogenic ZnO nanoparticles: a study of blueshift of optical band gap and photocatalytic degradation of reactive yellow 186 dye under direct sunlight
- Leached compounds from the extracts of pomegranate peel, green coconut shell, and karuvelam wood for the removal of hexavalent chromium
- Enhancement of molecular weight reduction of natural rubber in triphasic CO2/toluene/H2O systems with hydrogen peroxide for preparation of biobased polyurethanes
- An efficient green synthesis of novel 1H-imidazo[1,2-a]imidazole-3-amine and imidazo[2,1-c][1,2,4]triazole-5-amine derivatives via Strecker reaction under controlled microwave heating
- Evaluation of three different green fabrication methods for the synthesis of crystalline ZnO nanoparticles using Pelargonium zonale leaf extract
- A highly efficient and multifunctional biomass supporting Ag, Ni, and Cu nanoparticles through wetness impregnation for environmental remediation
- Simple one-pot green method for large-scale production of mesalamine, an anti-inflammatory agent
- Relationships between step and cumulative PMI and E-factors: implications on estimating material efficiency with respect to charting synthesis optimization strategies
- A comparative sorption study of Cr3+ and Cr6+ using mango peels: kinetic, equilibrium and thermodynamic
- Effects of acid hydrolysis waste liquid recycle on preparation of microcrystalline cellulose
- Use of deep eutectic solvents as catalyst: A mini-review
- Microwave-assisted synthesis of pyrrolidinone derivatives using 1,1’-butylenebis(3-sulfo-3H-imidazol-1-ium) chloride in ethylene glycol
- Green and eco-friendly synthesis of Co3O4 and Ag-Co3O4: Characterization and photo-catalytic activity
- Adsorption optimized of the coal-based material and application for cyanide wastewater treatment
- Aloe vera leaf extract mediated green synthesis of selenium nanoparticles and assessment of their In vitro antimicrobial activity against spoilage fungi and pathogenic bacteria strains
- Waste phenolic resin derived activated carbon by microwave-assisted KOH activation and application to dye wastewater treatment
- Direct ethanol production from cellulose by consortium of Trichoderma reesei and Candida molischiana
- Agricultural waste biomass-assisted nanostructures: Synthesis and application
- Biodiesel production from rubber seed oil using calcium oxide derived from eggshell as catalyst – optimization and modeling studies
- Study of fabrication of fully aqueous solution processed SnS quantum dot-sensitized solar cell
- Assessment of aqueous extract of Gypsophila aretioides for inhibitory effects on calcium carbonate formation
- An environmentally friendly acylation reaction of 2-methylnaphthalene in solvent-free condition in a micro-channel reactor
- Aegle marmelos phytochemical stabilized synthesis and characterization of ZnO nanoparticles and their role against agriculture and food pathogen
- A reactive coupling process for co-production of solketal and biodiesel
- Optimization of the asymmetric synthesis of (S)-1-phenylethanol using Ispir bean as whole-cell biocatalyst
- Synthesis of pyrazolopyridine and pyrazoloquinoline derivatives by one-pot, three-component reactions of arylglyoxals, 3-methyl-1-aryl-1H-pyrazol-5-amines and cyclic 1,3-dicarbonyl compounds in the presence of tetrapropylammonium bromide
- Preconcentration of morphine in urine sample using a green and solvent-free microextraction method
- Extraction of glycyrrhizic acid by aqueous two-phase system formed by PEG and two environmentally friendly organic acid salts - sodium citrate and sodium tartrate
- Green synthesis of copper oxide nanoparticles using Juglans regia leaf extract and assessment of their physico-chemical and biological properties
- Deep eutectic solvents (DESs) as powerful and recyclable catalysts and solvents for the synthesis of 3,4-dihydropyrimidin-2(1H)-ones/thiones
- Biosynthesis, characterization and anti-microbial activity of silver nanoparticle based gel hand wash
- Efficient and selective microwave-assisted O-methylation of phenolic compounds using tetramethylammonium hydroxide (TMAH)
- Anticoagulant, thrombolytic and antibacterial activities of Euphorbia acruensis latex-mediated bioengineered silver nanoparticles
- Volcanic ash as reusable catalyst in the green synthesis of 3H-1,5-benzodiazepines
- Green synthesis, anionic polymerization of 1,4-bis(methacryloyl)piperazine using Algerian clay as catalyst
- Selenium supplementation during fermentation with sugar beet molasses and Saccharomyces cerevisiae to increase bioethanol production
- Biosynthetic potential assessment of four food pathogenic bacteria in hydrothermally silver nanoparticles fabrication
- Investigating the effectiveness of classical and eco-friendly approaches for synthesis of dialdehydes from organic dihalides
- Pyrolysis of palm oil using zeolite catalyst and characterization of the boil-oil
- Azadirachta indica leaves extract assisted green synthesis of Ag-TiO2 for degradation of Methylene blue and Rhodamine B dyes in aqueous medium
- Synthesis of vitamin E succinate catalyzed by nano-SiO2 immobilized DMAP derivative in mixed solvent system
- Extraction of phytosterols from melon (Cucumis melo) seeds by supercritical CO2 as a clean technology
- Production of uronic acids by hydrothermolysis of pectin as a model substance for plant biomass waste
- Biofabrication of highly pure copper oxide nanoparticles using wheat seed extract and their catalytic activity: A mechanistic approach
- Intelligent modeling and optimization of emulsion aggregation method for producing green printing ink
- Improved removal of methylene blue on modified hierarchical zeolite Y: Achieved by a “destructive-constructive” method
- Two different facile and efficient approaches for the synthesis of various N-arylacetamides via N-acetylation of arylamines and straightforward one-pot reductive acetylation of nitroarenes promoted by recyclable CuFe2O4 nanoparticles in water
- Optimization of acid catalyzed esterification and mixed metal oxide catalyzed transesterification for biodiesel production from Moringa oleifera oil
- Kinetics and the fluidity of the stearic acid esters with different carbon backbones
- Aiming for a standardized protocol for preparing a process green synthesis report and for ranking multiple synthesis plans to a common target product
- Microstructure and luminescence of VO2 (B) nanoparticle synthesis by hydrothermal method
- Optimization of uranium removal from uranium plant wastewater by response surface methodology (RSM)
- Microwave drying of nickel-containing residue: dielectric properties, kinetics, and energy aspects
- Simple and convenient two step synthesis of 5-bromo-2,3-dimethoxy-6-methyl-1,4-benzoquinone
- Biodiesel production from waste cooking oil
- The effect of activation temperature on structure and properties of blue coke-based activated carbon by CO2 activation
- Optimization of reaction parameters for the green synthesis of zero valent iron nanoparticles using pine tree needles
- Microwave-assisted protocol for squalene isolation and conversion from oil-deodoriser distillates
- Denitrification performance of rare earth tailings-based catalysts
- Facile synthesis of silver nanoparticles using Averrhoa bilimbi L and Plum extracts and investigation on the synergistic bioactivity using in vitro models
- Green production of AgNPs and their phytostimulatory impact
- Photocatalytic activity of Ag/Ni bi-metallic nanoparticles on textile dye removal
- Topical Issue: Green Process Engineering / Guest Editors: Martine Poux, Patrick Cognet
- Modelling and optimisation of oxidative desulphurisation of tyre-derived oil via central composite design approach
- CO2 sequestration by carbonation of olivine: a new process for optimal separation of the solids produced
- Organic carbonates synthesis improved by pervaporation for CO2 utilisation
- Production of starch nanoparticles through solvent-antisolvent precipitation in a spinning disc reactor
- A kinetic study of Zn halide/TBAB-catalysed fixation of CO2 with styrene oxide in propylene carbonate
- Topical on Green Process Engineering

