Synthesis of pyrazolopyridine and pyrazoloquinoline derivatives by one-pot, three-component reactions of arylglyoxals, 3-methyl-1-aryl-1H-pyrazol-5-amines and cyclic 1,3-dicarbonyl compounds in the presence of tetrapropylammonium bromide
Abstract
Pyrazolopyridine and pyrazoloquinoline derivatives were obtained by a one-pot, three-component reaction of arylglyoxals, 3-methyl-1-aryl-1H-pyrazol-5-amines and cyclic 1,3-dicarbonyl compounds in the presence of tetrapropylammonium bromide at 80°C in water through Knoevenagel and Micheal reactions, followed by intramolecular condensation, unexpected dearoylation and oxidation. Mild reaction conditions, high yields, simplicity of work up procedure, starting materials availability and clean product formation are some of the main advantages of this synthetic strategy.
1 Introduction
It has been reported that, more than 90% of compounds analysed by pharmaceutical companies are nitrogen-containing heterocycles [1], and the nitrogen-containing heterocycles exhibit excellent biological and pharmaceutical activities.
The synthesis of -pyrazolo[3,4-b]pyridine derivatives by microwave-assisted one-pot reaction between 5-aminopyrazole derivatives, paraformaldehyde and β-diketones catalyzed by InCl3 in aqueous media was recently reported [2].
The presence of pyrazoloquinoline moieties in numerous natural products makes them an important class of heterocyclic compounds with several biological and pharmacological activities, such as anti-mycobacterial [3], anti-microbial [4], antiviral [5]. Pyrazolopyridines have also received more attention because of their wide range of biological and pharmacological properties, such as anti-pyretic [6] and anxiolytic [7], antimalarial [8], and this has made such derivatives increasingly important.
The reaction of several starting materials in one pot may allow the formation of the corresponding product in high yields and minimize the use of hazardous organic solvents during separation and work-up steps which leads to a green procedure [9].
Tetrapropylammonium bromide (TPAB) is a readily available and an inexpensive catalyst with many catalytical applications in organic reactions [10,11].
Several studies have been conducted on the synthesis of a new series of heterocyclic compounds using one-pot, multicomponent reactions in our laboratory [12, 13, 14, 15, 16, 17, 18, 19, 20]. In continuation of our previous studies, we were interested to investigate the possibility of the synthesis of a new series of pyrazolopyridine and pyrazoloquinoline derivatives by the one-pot, three-component reaction of arylglyoxals, 3-methyl-1-aryl-1H-pyrazol-5-amines and cyclic 1,3-dicarbonyl compounds in the presence of TPAB as a catalytic. However, surprisingly, it was found that unexpected dearoylation occurred during the reaction to provide the corresponding pyrazolopyridines and pyrazoloquinolines as the final products in high yields.
2 Experimental
All chemicals were purchased from Merck and Acros companies and used without any purification. The completion of reactions were controlled by thin layer chromatography (TLC) silica gel on aluminium plates. Melting points were measured with a Philip Harris C4954718 apparatus and are uncorrected. Infrared spectra were recorded with thermo Nicolet Nexus 670 FT-IR using KBr pellets. The 1H and 13C NMR spectra were recorded on Bruker Avance AQS 300 MHz spectrometer using CDCl3 as solvent, relative to tetramethylsilane (TMS) as the internal standard. Mass spectra were measured on a Varian Matt 311 spectrometer and high resolution spectra were obtained by Kratos MS 25RF spectrometer.
General procedure for the synthesis of products 5a-i
A mixture of arylglyoxals 1a-i (1 mmol), 3-methyl-1-aryl-1H-pyrazol-5-amines 2a-c (1 mmol) and cyclic 1,3-dicarbonyl compounds 3a-e (1 mmol) in the presence of TPAB (20 mol%) in water/acetone (1:2, 10 mL) was stirred at 80°C for an appropriate time (monitored by TLC, CH2Cl2: hexane: MeOH/15:15:1). Half of the solvent was evaporated and the precipitate was filtered and washed with H2O/EtOH (1:2) to give the desired products 5a-i in 90-98% yield.
Recovering of TPAB
After filtration of products 5a-i and washing the precipitate with water, the filtrate was extracted with CHCl3 and the aqueous phase was separated. Evaporation of water gave TPAB, which may be recrystallized from Et2O as white crystals.
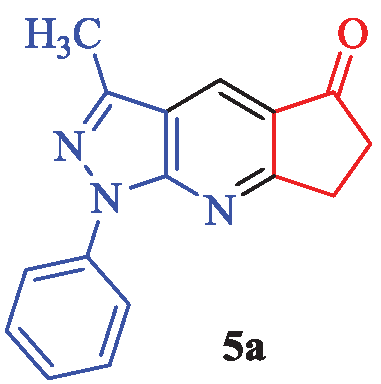
3-Methyl-1-phenyl-6,7-dihydrocyclopenta[b] pyrazolo[4,3-e]pyridin-5(1H)-one (5a)
White powder; yield 96% (253 mg); mp: 213-216°C (lit. [2], 215-217°C); IR (KBr, cm-1): 2945, 2877, 1681, 1593, 1502, 1480, 1416, 1381, 1326, 1264, 1222, 1182, 1015, 912, 880, 837, 758, 688, 609; 1H NMR (CDCl3) δ (ppm) 8.36 (s, 1H, Ar), 7.79 (d, J = 8.4 Hz, 2H, Ar), 7.58 (t, J = 8.1 Hz, 2H, Ar), 7.40 (t, J = 7.5 Hz, 1H, Ar), 3.46-3.44 (m, 2H, CH2), 2.88-2.83 (m, 2H, CH2), 2.32 (s, 3H, CH3); 13C-NMR (CDCl3) δ (ppm): 192.7, 174.2, 153.7, 144.5, 140.5, 134.3, 131.6, 129.4, 122.6, 121.1, 113.7, 36.1, 29.3, 13.9; MS (EI): m/z (%): 263 [M]+ (35), 234 (10), 141 (37), 140 (12), 139 (100), 111 (46), 77 (21).
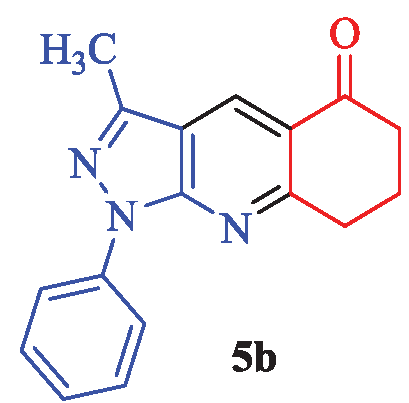
3-Methyl-1-phenyl-1,6,7,8-tetrahydro-5H-pyrazolo[3,4-b] quinolin-5-one (5b)
White needles; yield 90% (249 mg); mp: 126-128°C (lit. [2], 128-130°C, and lit. [21], 123-124); IR (KBr, cm-1): 3030, 2955, 1662, 1617, 1527, 1459, 1363, 1189, 1119, 1066, 1004, 847, 754, 694; 1H-NMR (CDCl3) δ (ppm): 8.77 (s, 1H, Ar), 8.30 (d, J = 7.8 Hz, 2H, Ar), 7.53 (t, J = 7.8 Hz, 2H, Ar), 7.32 (t, J = 7.2 Hz, 1H, Ar), 3.29 (t, J = 6.3 Hz, 2H, CH2), 2.78 (t, J = 6.3 Hz, 2H, CH2), 2.68 (s, 3H, CH3), 2.25 (quin, J = 6.3 Hz, 2H, CH2); 13C-NMR (CDCl3) δ (ppm): 197.5, 163.9, 145.1, 130.5, 129.1, 126.0, 123.1, 120.9, 116.8, 116.4, 104.8, 38.9, 33.8, 22.0, 12.5; MS (EI): m/z (%): 277 [M]+ (13), 271 (11), 239 (15), 205 (13), 165 (15), 135 (92), 77 (46), 43 (100).
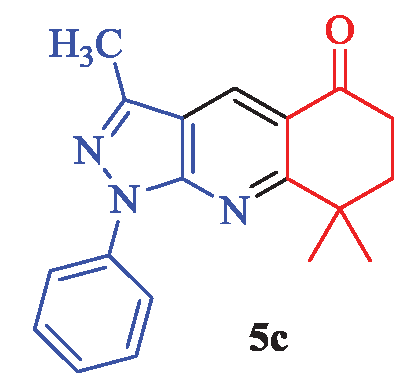
3,8,8-Trimethyl-1-phenyl-1,6,7,8-tetrahydro-5H-pyrazolo[3,4-b]quinolin-5-one (5c)
White powder; yield 92% (281 mg); mp: 167-169°C; IR (KBr, cm-1): 3347, 3067, 2959, 2936, 2869, 1683, 1593, 1504, 1481, 1417, 1380, 1269, 1235, 1121, 1090, 1012, 979, 821, 779, 750, 685, 624; 1H NMR (CDCl3) δ (ppm) 8.74 (s, 1H, Ar), 8.27 (d, J = 8.1 Hz, 2H, Ar), 7.53 (t, J = 7.2 Hz, 2H, Ar), 7.34 (t, J = 7.2 Hz, 1H, Ar), 2.55 (s, 3H, CH3), 2.27 (bs, 2H, CH2), 1.58 (bs, 2H, CH2), 1.16 (s, 6H, 2×CH3); 13C-NMR (CDCl3) δ (ppm): 196.8, 163.4, 162.3, 151.4, 144.6, 144.2, 130.8, 130.2, 128.1, 122.0, 120.3, 52.2, 47.7, 32.9, 27.0, 18.8; MS (EI): m/z (%): 305 [M]+ (5), 300 (6), 140 (32), 112 (28), 97 (14), 83 (100), 70 (19), 56 (44) and HRMS (ESI): calcd. for C19H19N3O [M]+ 305.1528; found: 305.1542.
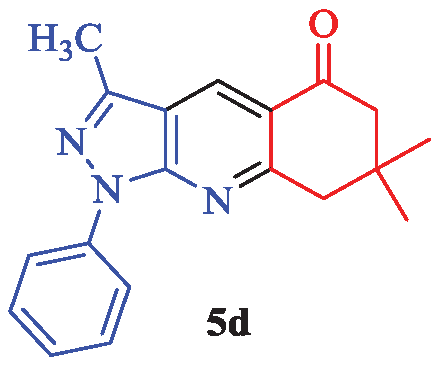
3,7,7-Trimethyl-1-phenyl-1,6,7,8-tetrahydro-5H-pyrazolo[3,4-b]quinolin-5-one (5d)
White needles; yield 97% (296 mg); mp: 163-165°C (lit. [2], 165-167°C, and lit. [22], 165-166°C); IR (KBr, cm-1): 2951, 2932, 2359, 1678, 1569, 1593, 1494, 1416, 1376, 1281, 1243, 1116, 1023, 758, 679, 556; 1H NMR (CDCl3) δ (ppm) 8.74 (s, 1H, Ar), 8.30 (d, J = 9 Hz, 2H, Ar), 7.54 (d, J = 9 Hz, 2H, Ar), 7.32 (t, J = 9 Hz, 1H, Ar), 3.18 (s, 2H, CH2), 2.67 (s, 3H, CH3), 2.63 (s, 2H, CH2), 1.15 (s, 6H, 2×CH3); 13C-NMR (CDCl3) δ (ppm): 197.5, 162.6, 151.6, 145.1, 139.2, 129.9, 129.0, 125.9, 122.1, 120.9, 116.7, 52.4, 47.5, 32.9, 28.3, 12.5; MS (EI): m/z (%): 306 [M+1]+ (27), 305 [M]+ (100), 290 (8), 277 (7), 249 (43), 220 (8), 180 (15), 129 (6), 77 (14).
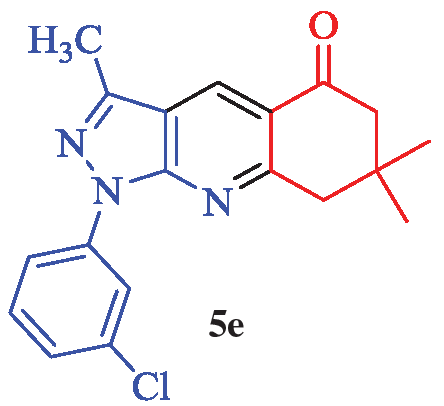
1-(3-Chlorophenyl)-3,7,7-trimethyl-1,6,7,8-tetrahydro-5H-pyrazolo[3,4-b]quinolin-5-one (5e)
White solid; yield 98% (332 mg); mp: 148-150°C; IR (KBr, cm-1): 3102, 2955, 2872, 1683, 1590, 1479, 1454, 1379, 1275, 1239, 1141, 1093, 899, 872, 778, 741, 679, 554; 1H NMR (CDCl3) δ (ppm) 8.75 (s, 1H, Ar), 8.46 (s, 1H, Ar), 8.32 (d, J = 7.5 Hz, 1H, Ar), 7.45 (t, J = 7.5 Hz, 1H, Ar), 7.26 (bd, overlapped by CDCl3 impurity peak, 1H, Ar), 3.21 (s, 2H, CH2), 2.67 (s, 3H, CH3), 2.64 (s, 2H, CH2), 1.17 (s, 6H, 2×CH3); 13C-NMR (CDCl3) δ (ppm): 197.4, 162.9, 151.8, 145.6, 134.8, 131.1, 128.9, 126.7, 124.8, 124.7, 121.6, 116.9, 114.0, 52.4, 47.5, 33.0, 27.9, 13.3; MS (EI): m/z (%): 341 [M+2]+ (39), 339 [M]+ (100), 283 (45), 250 (15), 214 (13), 179 (13), 149 (13), 111 (23), 83 (47), 71 (45), 57 (42) and HRMS (ESI): calcd. for C19H18ClN3O [M]+ 339.1138; found: 339.1110.
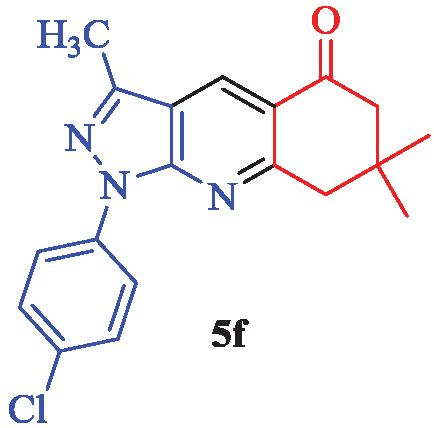
1-(4-Chlorophenyl)-3,7,7-trimethyl-1,6,7,8-tetrahydro-5H-pyrazolo[3,4-b]quinolin-5-one (5f)
White solid; yield 95% (322 mg); mp: 144-146°C (lit. [22], 148-149°C); IR (KBr, cm-1): 3106, 2957, 2932, 2870, 1679, 1594, 1576, 1499, 1475, 1447, 1382, 1270, 1241, 1218, 1141, 1090, 1013, 829, 691, 557, 503; 1H NMR (CDCl3) δ (ppm) 8.75 (s, 1H, Ar), 8.33 (d, J = 8.7 Hz, 2H, Ar), 7.48 (d, J = 8.7 Hz, 2H, Ar), 3.19 (s, 2H, CH2), 2.67 (s, 3H, CH3), 2.64 (s, 2H, CH2), 1.27 (s, 6H, 2×CH3); 13C-NMR (CDCl3) δ (ppm): 197.4, 163.6, 162.9, 151.8, 134.8, 131.1, 128.9, 126.7, 122.4, 121.6, 116.9, 52.3, 47.4, 33.0, 27.2, 13.3; MS (EI): m/z (%): 341 [M+2]+ (34), 339 [M]+ (98), 283 (38), 278 (100), 217 (19), 179 (14), 139 (31), 112 (30), 105 (88), 77 (41), 57 (24).
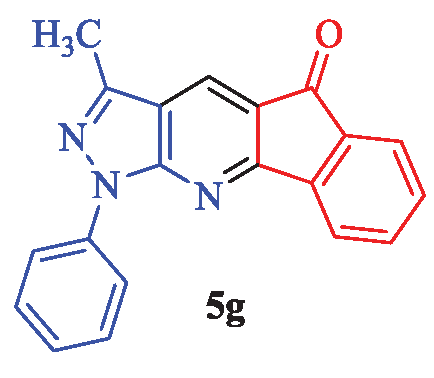
3-Methyl-1-phenylindeno[1,2-b]pyrazolo[4,3-e]pyridin-5(1H)-one (5g)
Yellow solid; yield 91% (283 mg); mp: 245-247°C (lit. [2], 246-248°C); IR (KBr, cm-1): 3088, 2959, 2867, 1690, 1580, 1556, 1471, 1314, 1232, 1165, 1058, 947, 846, 760, 678, 593; 1H-NMR (CDCl3) δ (ppm): 8.42 (s, 1H, Ar), 8.29 (d, J = 7.2 Hz, 1H, Ar), 8.01 (d, J = 8.1 Hz, 1H, Ar), 7.89 (d, J = 7.8 Hz, 2H, Ar), 7.66 (t, J = 6.9 Hz, 1H, Ar), 7.59 (t, J = 7.8 Hz, 1H, Ar), 7.49 (t, J = 7.5 Hz, 2H, Ar), 7.41 (t, J = 6.9 Hz, 1H, Ar), 2.29 (s, 3H, CH3); 13C-NMR (CDCl3) δ (ppm): 192.3, 173.0, 169.3, 164.5, 153.2, 142.8, 141.3, 138.7, 136.9, 131.8, 130.6, 129.5, 125.8, 125.1, 120.1, 113.1, 108.3, 35.2; MS (EI): m/z (%): 312 [M+1]+ (43), 311 [M]+ (100), 296 (30), 270 (24), 241 (14), 214 (10), 139 (36), 111 (19), 77 (32), 51 (10).
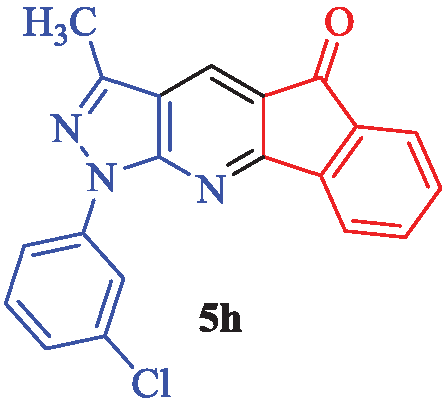
1-(3-Chlorophenyl)-3-methylindeno[1,2-b]pyrazolo[4,3-e] pyridin-5(1H)-one (5h)
Yellow powder; yield 90% (311 mg); mp: 230-232°C; IR (KBr, cm-1): 3098, 2969, 2879, 1711, 1670, 1589, 1560, 1482, 1435, 1316, 1243, 1188, 1089, 1007, 947, 846, 764, 726, 676, 593; 1H-NMR (CDCl3) δ (ppm): 8.44 (s, 1H, Ar), 8.29 (d, J = 8.4 Hz, 1H, Ar), 8.04 (d, J = 7.5 Hz, 1H, Ar), 7.87 (d, J = 7.5 Hz, 1H, Ar), 7.69 (t, J = 7.8 Hz, 1H, Ar), 7.67 (t, J = 6.9 Hz, 1H, Ar), 7.51 (t, J = 7.5 Hz, 1H, Ar), 7.48 (s, 1H, Ar), 7.36 (d, J = 7.8 Hz, 1H, Ar), 2.29 (s, 3H, CH3); 13C-NMR (CDCl3) δ (ppm): 198.6, 181.2, 180.9, 169.0, 167.4, 166.2, 163.6, 162.9, 162.8, 151.8, 145.6, 140.3, 134.8, 134.7, 131.1, 128.9, 126.7, 121.6, 119.4, 11.9; MS (EI): m/z (%): 347 [M+2]+ (2), 345 [M]+ (4), 327 (22), 311 (100), 296 (20), 270 (15), 241 (10), 139 (30), 111 (15), 77 (17) and HRMS (ESI): calcd. for C20H12ClN3O [M]+ 345.0669; found: 345.0699.
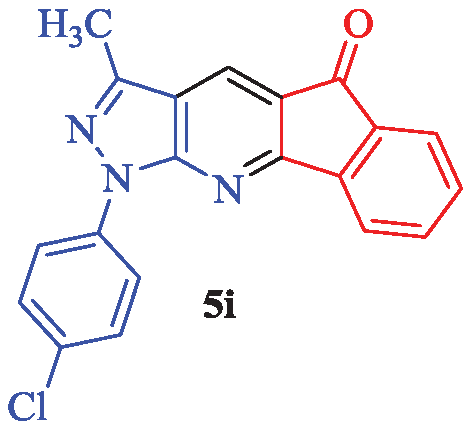
1-(4-Chlorophenyl)-3-methylindeno[1,2-b]pyrazolo[4,3-e] pyridin-5(1H)-one (5i)
Yellow solid; yield 94% (324 mg); mp: 221-223°C; IR (KBr, cm-1): 3089, 2929, 1721, 1678, 1581, 1562, 1488, 1318, 1244, 1189, 1087, 945, 847, 765, 729, 596; 1H-NMR (CDCl3) δ (ppm): 8.84 (s, 1H, Ar), 8.14 (d, J = 8.1 Hz, 1H, Ar), 7.82 (t, J = 8.1 Hz, 1H, Ar), 7.64 (d, J = 8.1 Hz, 1H, Ar), 7.52 (t, J = 8.1 Hz, 1H, Ar), 7.36 (d, J = 7.8 Hz, 2H, Ar), 7.03 (d, J = 7.2 Hz, 2H, Ar), 2.50 (s, 3H, CH3); 13C-NMR (CDCl3) δ (ppm): 202.1, 184.9, 182.1, 178.4, 177.0, 175.9, 175.0, 149.9, 144.9, 140.1, 128.4, 128.3, 128.1, 125.1, 122.7, 120.6, 113.2, 35.2; MS (EI): m/z (%): 347 [M+2]+ (1), 345 [M]+ (2), 311 (11), 298 (8), 269 (5), 139 (100), 111 (40), 84 (16) and HRMS (ESI): calcd. for C20H12ClN3O [M]+ 345.0669; found: 345.0601.
3 Results and discussion
We earlier found that the reactions of arylglyoxals 1 with 3-methyl-1-phenyl-1H-pyrazol-5-amine (2) and cyclic 1,3-dicarbonyl compounds 3 carried out under catalyst-free conditions in H2O/EtOH at 80°C afforded 4-aroyl--pyrazolo[3,4-b]pyridine derivatives 4a-h by a one-pot, three-component reaction in excellent yields [23]. However, it was found that the same reaction in the presence of TPAB as a homogeneous catalyst in H2O/acetone at 80°C gave pyrazolopyridines 5a, 5g-i and pyrazoloquinolines 5b-f as final products in high yields due to unexpected dearoylation occurrence, with no sign of the formation of any 4-aroyl-pyrazolo[3,4-b]pyridine derivatives 4a-h formation (Scheme 1).
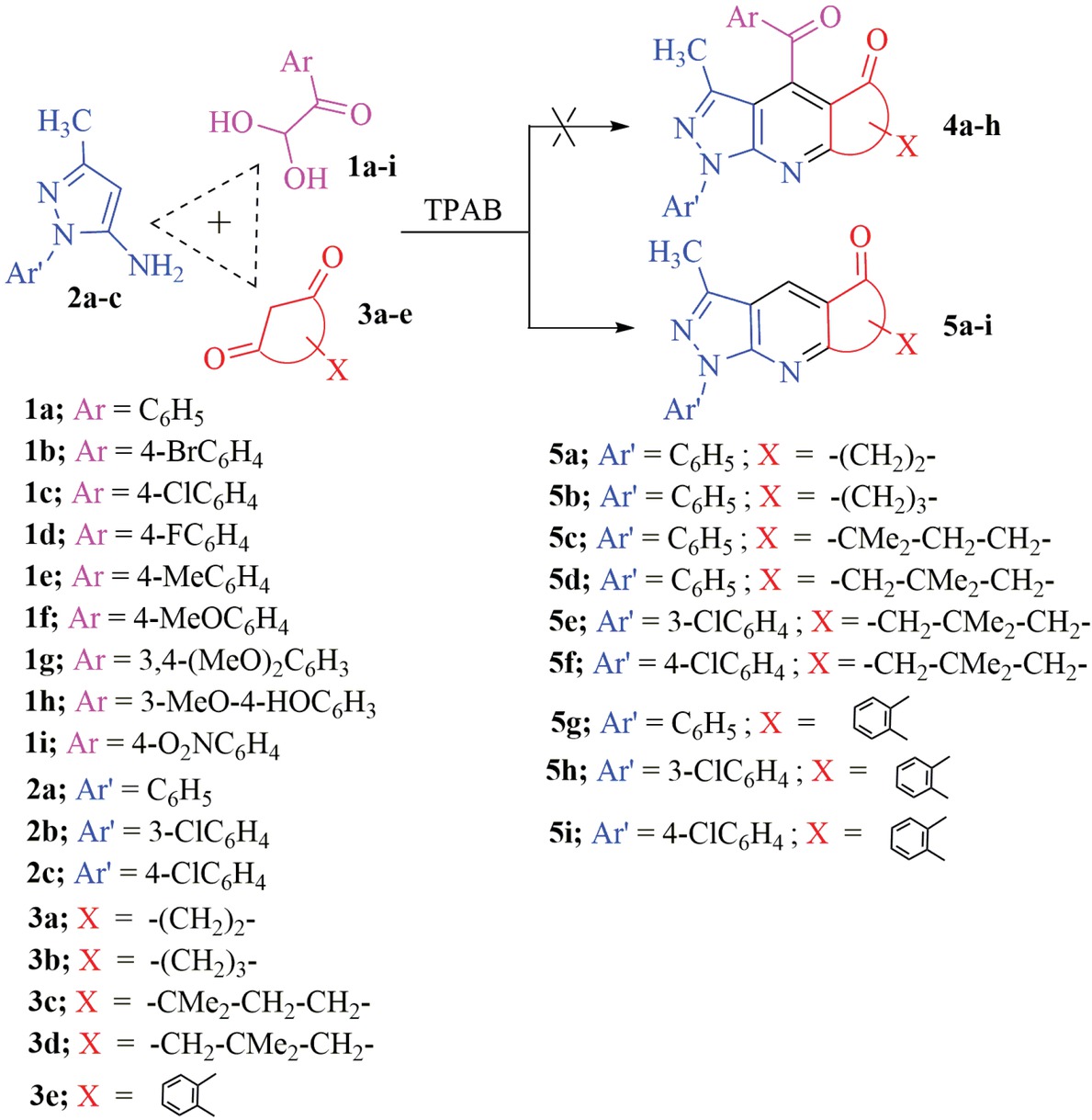
Synthesis of pyrazolopyridines and pyrazoloquinolines 5a-i.
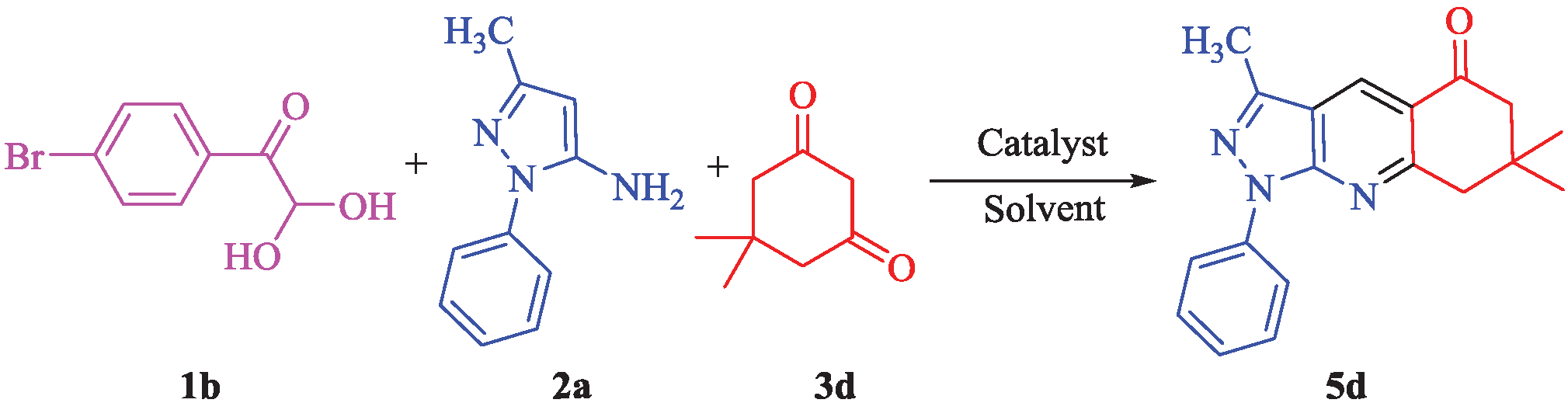
The reaction of 4-bromophenylglyoxal monohydrate (1b), 3-methyl-1-phenyl-1H-pyrazol-5-amine (2a) and dimedone (3d) in 1:1:1 molar ratio using several catalysts, was chosen as a model reaction (Scheme 1). For optimization, the model reaction was carried out using various solvents, catalysts and reaction times as indicated in Table 1. Consequently, the highest yield (97%) was obtained, using TPAB as catalyst and H2O/acetone (1:2) as solvent, at 80°C (entry 26). The arylglyoxals 1a-i and the 3-methyl-1-aryl-1H-pyrazole-5-amines 2a-c were prepared according to the literature methods [24] and [25] respectively.
Model reaction for the synthesis of compound 5d.
| Entry | Solvent | Catalyst (20 mol%) | Temperature (°C) | Yield (%)a |
|---|---|---|---|---|
| 1 | H2O | No catalyst | RT-Reflux | N.R. |
| 2 | H2O | p-TSA | RT-Reflux | N.R. |
| 3 | H2O | Alginate sodium | RT-Reflux | N.R. |
| 4 | H2O | L-cysteine | RT-Reflux | N.R. |
| 5 | H2O | L-proline | RT-Reflux | N.R. |
| 6 | H2O | TPAB | RT-Reflux | Trace |
| 7 | EtOH | p-TSA | RT-Reflux | N.R. |
| 8 | EtOH | Alginate sodium | RT-Reflux | N.R. |
| 9 | EtOH | L-cysteine | RT-Reflux | N.R. |
| 10 | EtOH | L-proline | RT-Reflux | N.R. |
| 11 | EtOH | TPAB | RT-Reflux | Trace |
| 12 | Acetone | p-TSA | RT-Reflux | N.R. |
| 13 | Acetone | Alginate sodium | RT-Reflux | N.R. |
| 14 | Acetone | L-cysteine | RT-Reflux | N.R. |
| 15 | Acetone | L-proline | RT-Reflux | N.R. |
| 16 | Acetone | TPAB | RT-Reflux | Trace |
| 17 | H2O/EtOH (1:2) | p-TSA | RT-Reflux | N.R. |
| 18 | H2O/EtOH (1:2) | Alginate sodium | RT-Reflux | N.R. |
| 19 | H2O/EtOH (1:2) | L-cysteine | RT-Reflux | N.R. |
| 20 | H2O/EtOH (1:2) | L-proline | RT-Reflux | N.R. |
| 21 | H2O/EtOH (1:2) | TPAB | RT-Reflux | 12 |
| 22 | H2O/Acetone (1:2) | p-TSA | RT-Reflux | N.R. |
| 23 | H2O/Acetone (1:2) | Alginate sodium | RT-Reflux | N.R. |
| 24 | H2O/Acetone (1:2) | L-cysteine | RT-Reflux | Trace |
| 25 | H2O/Acetone (1:2) | L-proline | RT-Reflux | N.R. |
| 26 | H2O/Acetone (1:2) | TPAB | 80°C | 97b |
aIsolated yield.
bThe bold type (entry 26) refers to the best reaction conditions.
After optimizing the reaction conditions, the scope of this reaction was examined with a series of electron rich and electron deficient arylglyoxals and various cyclic 1,3-dicarbonyl compounds [such as cyclopentane-1,3-dione (3a), cyclohexane-1,3-dione (3b), 4,4-dimethylcyclohexane-1,3-dione (3c), dimedone (3d) and indane-1,3-dione (3e)] to form a series of corresponding pyrazolopyridines and pyrazoloquinolines. The reaction times, melting points and yields of all products are summarized in Table 2. It should be mentioned that the reaction with 4-nitroarylglyoxal (1i) gave 4i as a final product with no sign of corresponding dearoylation product 5d formation.
The reaction condition, melting points and yields of products 5a-i.
| Entry Product | Reaction time (h) | M.p. (°C) | Yield (%) | ||
|---|---|---|---|---|---|
| 1 |
| 3 | 213-216 | 96 | |
| 2 |
| 4 | 126-128 | 90 | |
| 3 |
| 4 | 167-169 | 92 | |
| 4 |
| 3 | 163-165 | 97 | |
| 5 |
| 3 | 148-150 | 98 | |
| 6 |
| 3 | 144-146 | 95 | |
| 7 |
| 3 | 245-247 | 91 | |
| 8 |
| 3 | 230-232 | 90 | |
| 9 |
| 4 | 221-223 | 94 | |
The proposed mechanism of this reaction involves the initial Knoevenagel condenstation of arylglyoxals 1 with cyclic 1,3-dicarbonyl compounds 3 to form the corresponding intermediate as shown in Scheme 2. Following the Michael addition of 3-methyl-1-aryl-1H-pyrazol-5-one 2 to this intermediate will form the desired pyrazolopyridine and pyazoloquinoline derivatives through intermolecular cyclization, dearoylation and autoxidation. There is no report on TPAB acting as dearoylating agent in the literature.
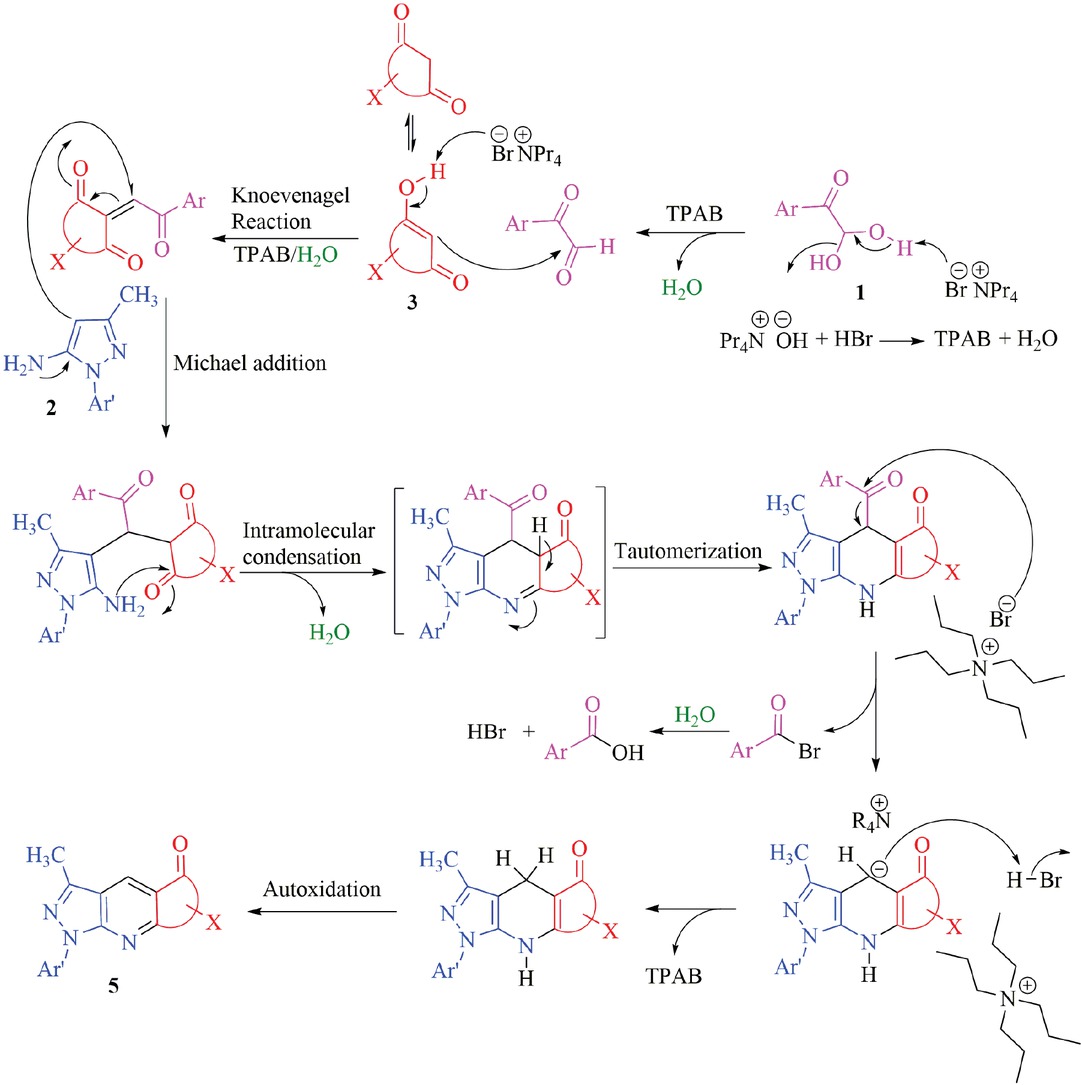
A plausible mechanistic pathway for the formation of 5.
Isolation and identification of benzoic acid and 3,4-dimethoxybenzoeic acid from the literature in the case of 5a and 5g was accomplished to confirm the dearoylation step, as the melting points, TLC, FT-IR, 1H and 13C-NMR of both acids were identical with those of the authentic samples.
The structures of all products were characterized by their spectral data for new compounds or by comparison with those of authentic samples, in the case of known products.
As an important factor, the recyclability of catalyst (TPAB) was also investigated for synthesis of compound 5d.
The catalyst was recovered and reused for four times to show no significant loss on catalytic performance of TPAB as shown in Figure 1.
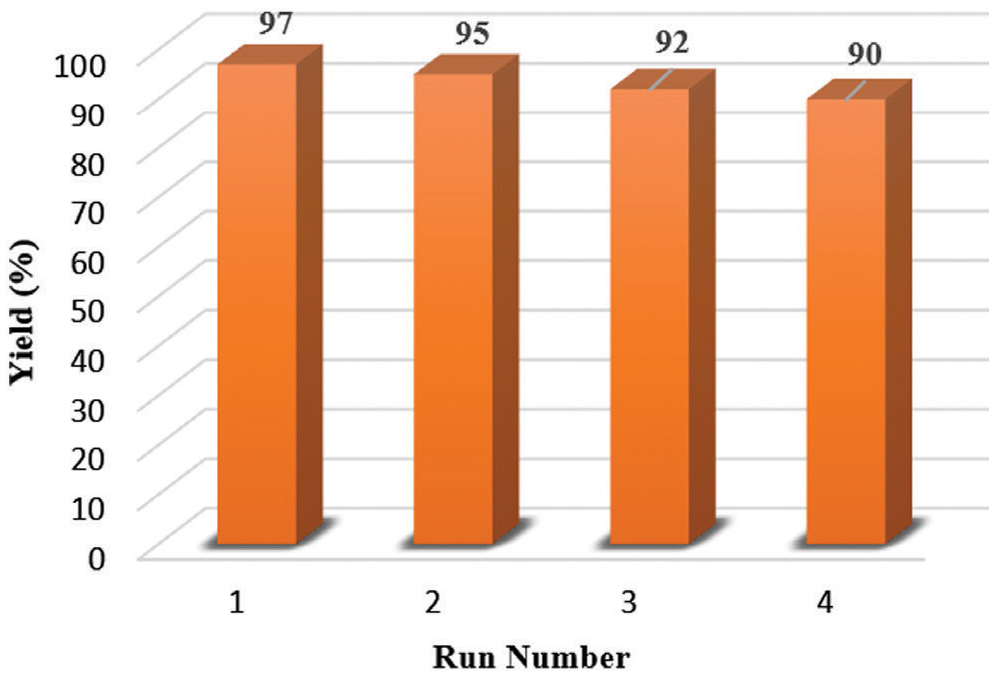
Reusability of TPAB for the synthesis of 5d.
4 Conclusions
Synthesis of a new series of pyrazolopyridines and pyrazoloquinolines was reported using a one-pot, three-component procedure in the presence of TPAB as a catalyst. The proposed procedure provides a new synthetic route for synthesis of pyrazolopyridine and pyrazoloquinoline derivatives, which may have pharmaceutical and biological applications. High yields, using green solvent, easily available starting materials, operational simplicity and the recoverable catalyst are some of the advantages of our procedure.
Acknowledgements
The authors gratefully acknowledge the financial support from Urmia University. We thank Professor R.H. Prager from Flinders University of Australia for proof-reading of this article.
References
[1] Newman D.J., Cragg G.M., Natural products as sources of new drugs over the 30 years from 1981 to 2010. J. Nat. Prod., 2012, 75, 311-335.10.1021/np200906sSearch in Google Scholar PubMed PubMed Central
[2] Polo E., Ferrer-Pertuz K., Trilleras J., Quiroga J., Gutiérrez M., Microwave-assisted one-pot synthesis in water of carbonylpyrazolo[3,4-b]pyridine derivatives catalyzed by InCl3 and sonochemical assisted condensation with aldehydes to obtain new chalcone derivatives containing the pyrazolopyridinic moiety. RSC Adv., 2017, 7, 50044-50055.10.1039/C7RA10127ASearch in Google Scholar
[3] Quiroga J., Diaz Y., Bueno J., Insuasty B., Abonia R., Ortiz A., et al., Microwave induced three-component synthesis and antimycobacterial activity of benzopyrazolo[3,4-b] quinolindiones. Eur. J. Med. Chem., 2014, 74, 216-224.10.1016/j.ejmech.2013.12.037Search in Google Scholar PubMed
[4] Selvi S.T., Nadaraj V., Mohan S., Sasi R., Hema M., Solvent free microwave synthesis and evaluation of antimicrobial activity of pyrimido[4,5-b]- and pyrazolo[3,4-b]quinolines. Bioorg. Med. Chem., 2006, 14, 3896-3903.10.1016/j.bmc.2006.01.048Search in Google Scholar PubMed
[5] Rádl S., Zikán V., Synthesis of 1,2, and 9-methyl derivatives of 4,9-dihydro-6-methoxy-3-methyl-4-oxo-1H(2H-pyrazolo[3,4-b] quinoline and 4,9-dihydro-6-hydroxy-3-methyl-4-oxo-1H(2H-pyrazolo[3,4-b]quinoline and their antiviral activity. Collect Czech. Chem. Commun., 1987, 52, 788-792.10.1135/cccc19870788Search in Google Scholar
[6] Tuccinardi T., Zizzari A.T., Brullo C., Daniele S., Musumeci F., Schenone S., et al., Substituted pyrazolo[3,4-b]pyridines as human A1adenosine antagonists: Developments in understanding the receptor stereoselectivity. Org. Biomol. Chem., 2011, 9, 4448-4455.10.1039/c0ob01064bSearch in Google Scholar PubMed
[7] Mitchell C.J., Ballantine S.P., Coe D.M., Cook C.M., Delves C.J., Dowle M.D., et al., Pyrazolopyridines as potent PDE4B inhibitors: 5-heterocycle SAR. Bioorg. Med. Chem. Lett., 2010, 20, 5803-5806.10.1016/j.bmcl.2010.07.136Search in Google Scholar PubMed
[8] Stein R.G., Biel J.H., Singh T., Antimalarials. 4-substituted 1H-pyrazolo[3,4-b]quinolines. J. Med. Chem., 1970, 13, 153-155.10.1021/jm00295a049Search in Google Scholar PubMed
[9] Cioc R.C., Ruijter E., Orru R.V.A., Multicomponent reactions: advanced tools for sustainable organic synthesis. Green Chem., 2014, 16, 2958-2975.10.1039/C4GC00013GSearch in Google Scholar
[10] Argauer R.J., Landolt G.R., Crystalline zeolite ZSM-5 and method of preparing the same. U.S. Patent. 1972, 3,702, 886.Search in Google Scholar
[11] Bolm C., Magnus A.S., Hildebrand J.P., Catalytic synthesis of aldehydes and ketones under mild conditions using TEMPO/Oxone. Org. Lett., 2000, 2, 1173-1175.10.1021/ol005792gSearch in Google Scholar PubMed
[12] Poursattar Marjani A., Khalafy J., Mahmoodi S., A simple one-pot synthesis of new 9-aroyl-3,4,6,7,9,10-hexahydro-1,8(2H5H-acridinediones. ARKIVOC, 2016, iii, 262-270.10.3998/ark.5550190.p009.445Search in Google Scholar
[13] Poursattar Marjani A., Khalafy J., Chitan M., Mahmoodi S., Microwave-assisted synthesis of acridine-1,8(2H5H-diones via a one-pot, three component reaction. Iran J. Chem. Chem. Eng., 2017, 36, 1-6.Search in Google Scholar
[14] Poursattar Marjani A., Khalafy J., Haghi A., An efficient route for the synthesis of 11-aroyldiindeno[1,2-b2′,1′-e]pyridine-10,12-diones. J. Heterocycl. Chem., 2017, 54, 3294-3298.10.1002/jhet.2949Search in Google Scholar
[15] Poursattar Marjani A., Taghartapeh M.R., Soltani A.R., Khalafy J., Kanani Y., Molecular structures, hirshfeld surface analysis, and spectroscopic properties of 6,8-dimethyl-3-(4-chlorophenyl)-7-oxo-7,8-dihydropyrimido[4,5-c]pyridazin-5(6H-one and 6,8-dimethyl-3-(4-chlorophenyl)-7-thioxo-7,8-dihydropyrimido[4,5-c]pyridazin-5(6H)-one. J. Struct. Chem., 2017, 58, 1332-1340.10.1134/S0022476617070095Search in Google Scholar
[16] Poursattar Marjani A., Abdollahi S., Ezzati M., Nemati‐Kande E., A facile synthesis of xanthene‐1,8(2H‐dione derivatives by using tetrapropylammonium bromide as catalyst. J. Heterocycl. Chem., 2018, 55, 1324-1330.10.1002/jhet.3164Search in Google Scholar
[17] Ezzati M., Khalafy J., Poursattar Marjani A., Prager R.H., An efficient one-pot, four-component synthesis of pyrazolo[3,4-b] pyridines catalyzed by tetrapropylammonium bromide (TPAB) in water. Aust. J. Chem., 2018, 71, 435-441.10.1071/CH17642Search in Google Scholar
[18] Poursattar Marjani A., Ebrahimi Saatluo B., Nouri F., An efficient synthesis of 4H-chromene derivatives by a one-pot, three-component reaction. Iran J. Chem. Chem. Eng., 2018, 1, 149-157.Search in Google Scholar
[19] Poursattar Marjani A., Khalafy J., Farajollahi A., Synthesis of ethyl 2‐amino‐4‐benzoyl‐5‐oxo‐5,6‐dihydro‐4H‐pyrano[3,2‐c] quinoline‐3‐carboxylates by a one‐pot, three‐component reaction in the presence of TPAB. J. Heterocycl. Chem., 2019, 56, 268-274.10.1002/jhet.3404Search in Google Scholar
[20] Poursattar Marjani A., Khalafy J., Majidi Arlan F., Eyni E., A simple protocol for the green synthesis of a new series of pyrimido[4,5-b][1,6]naphthyridines in the presence of silver nanoparticles (AgNPs). ARKIVOC., 2019, v, 1-9.10.24820/ark.5550190.p010.705Search in Google Scholar
[21] Dzvinchuk I.B., Tolmachova N.A., Chernega A.N., Lozinskii M.O., Aromatization with dearylation on Hantzsch cyclocondensation of 4-(dimethylamino)benzaldehyde, cyclohexane-1,3-diones, and some 1,3-[NC]dinucleophiles. Chem. Heterocycl. Comp., 2009, 45, 194-200.10.1007/s10593-009-0250-6Search in Google Scholar
[22] Sumesh R.V., Muthu M., Almansour A.I., Kumar R.S., Arumugam N., Athimoolam S., et al., Multicomponent dipolar cycloaddition strategy: combinatorial synthesis of novel spiro-tethered pyrazolo[3,4-b]quinoline hybrid heterocycles. ACS Comb. Sci., 2016, 18, 262-270.10.1021/acscombsci.6b00003Search in Google Scholar PubMed
[23] Ezzati M., Khalafy J., Poursattar Marjani A., Prager R.H., The catalyst-free syntheses of pyrazolo[3,4-b]quinolin-5-one and pyrazolo[4′,3′:5,6]pyrido[2,3-d]pyrimidin-5,7-dione derivatives by one-pot, three-component reactions. Tetrahedron, 2017, 73, 6587-6596.10.1016/j.tet.2017.10.004Search in Google Scholar
[24] Riley H.A., Gray A.R. In: Blatt A.H. (Ed.), Organic Syntheses. John Wiley & Sons, New York, 1943, Vol. II, p 509.Search in Google Scholar
[25] Ganesan A., Heathcock C.H., Synthesis of unsymmetrical pyrazines by reaction of an oxadiazinone with enamines. J. Org. Chem., 1993, 58, 6155-6157.10.1021/jo00074a058Search in Google Scholar
© 2019 Marjani et al., published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 Public License.
Articles in the same Issue
- Regular Articles
- Studies on the preparation and properties of biodegradable polyester from soybean oil
- Flow-mode biodiesel production from palm oil using a pressurized microwave reactor
- Reduction of free fatty acids in waste oil for biodiesel production by glycerolysis: investigation and optimization of process parameters
- Saccharin: a cheap and mild acidic agent for the synthesis of azo dyes via telescoped dediazotization
- Optimization of lipase-catalyzed synthesis of polyethylene glycol stearate in a solvent-free system
- Green synthesis of iron oxide nanoparticles using Platanus orientalis leaf extract for antifungal activity
- Ultrasound assisted chemical activation of peanut husk for copper removal
- Room temperature silanization of Fe3O4 for the preparation of phenyl functionalized magnetic adsorbent for dispersive solid phase extraction for the extraction of phthalates in water
- Evaluation of the saponin green extraction from Ziziphus spina-christi leaves using hydrothermal, microwave and Bain-Marie water bath heating methods
- Oxidation of dibenzothiophene using the heterogeneous catalyst of tungsten-based carbon nanotubes
- Calcined sodium silicate as an efficient and benign heterogeneous catalyst for the transesterification of natural lecithin to L-α-glycerophosphocholine
- Synergistic effect between CO2 and H2O2 on ethylbenzene oxidation catalyzed by carbon supported heteropolyanion catalysts
- Hydrocyanation of 2-arylmethyleneindan-1,3-diones using potassium hexacyanoferrate(II) as a nontoxic cyanating agent
- Green synthesis of hydratropic aldehyde from α-methylstyrene catalyzed by Al2O3-supported metal phthalocyanines
- Environmentally benign chemical recycling of polycarbonate wastes: comparison of micro- and nano-TiO2 solid support efficiencies
- Medicago polymorpha-mediated antibacterial silver nanoparticles in the reduction of methyl orange
- Production of value-added chemicals from esterification of waste glycerol over MCM-41 supported catalysts
- Green synthesis of zerovalent copper nanoparticles for efficient reduction of toxic azo dyes congo red and methyl orange
- Optimization of the biological synthesis of silver nanoparticles using Penicillium oxalicum GRS-1 and their antimicrobial effects against common food-borne pathogens
- Optimization of submerged fermentation conditions to overproduce bioethanol using two industrial and traditional Saccharomyces cerevisiae strains
- Extraction of In3+ and Fe3+ from sulfate solutions by using a 3D-printed “Y”-shaped microreactor
- Foliar-mediated Ag:ZnO nanophotocatalysts: green synthesis, characterization, pollutants degradation, and in vitro biocidal activity
- Green cyclic acetals production by glycerol etherification reaction with benzaldehyde using cationic acidic resin
- Biosynthesis, characterization and antimicrobial activities assessment of fabricated selenium nanoparticles using Pelargonium zonale leaf extract
- Synthesis of high surface area magnesia by using walnut shell as a template
- Controllable biosynthesis of silver nanoparticles using actinobacterial strains
- Green vegetation: a promising source of color dyes
- Mechano-chemical synthesis of ammonia and acetic acid from inorganic materials in water
- Green synthesis and structural characterization of novel N1-substituted 3,4-dihydropyrimidin-2(1H)-ones
- Biodiesel production from cotton oil using heterogeneous CaO catalysts from eggshells prepared at different calcination temperatures
- Regeneration of spent mercury catalyst for the treatment of dye wastewater by the microwave and ultrasonic spray-assisted method
- Green synthesis of the innovative super paramagnetic nanoparticles from the leaves extract of Fraxinus chinensis Roxb and their application for the decolourisation of toxic dyes
- Biogenic ZnO nanoparticles: a study of blueshift of optical band gap and photocatalytic degradation of reactive yellow 186 dye under direct sunlight
- Leached compounds from the extracts of pomegranate peel, green coconut shell, and karuvelam wood for the removal of hexavalent chromium
- Enhancement of molecular weight reduction of natural rubber in triphasic CO2/toluene/H2O systems with hydrogen peroxide for preparation of biobased polyurethanes
- An efficient green synthesis of novel 1H-imidazo[1,2-a]imidazole-3-amine and imidazo[2,1-c][1,2,4]triazole-5-amine derivatives via Strecker reaction under controlled microwave heating
- Evaluation of three different green fabrication methods for the synthesis of crystalline ZnO nanoparticles using Pelargonium zonale leaf extract
- A highly efficient and multifunctional biomass supporting Ag, Ni, and Cu nanoparticles through wetness impregnation for environmental remediation
- Simple one-pot green method for large-scale production of mesalamine, an anti-inflammatory agent
- Relationships between step and cumulative PMI and E-factors: implications on estimating material efficiency with respect to charting synthesis optimization strategies
- A comparative sorption study of Cr3+ and Cr6+ using mango peels: kinetic, equilibrium and thermodynamic
- Effects of acid hydrolysis waste liquid recycle on preparation of microcrystalline cellulose
- Use of deep eutectic solvents as catalyst: A mini-review
- Microwave-assisted synthesis of pyrrolidinone derivatives using 1,1’-butylenebis(3-sulfo-3H-imidazol-1-ium) chloride in ethylene glycol
- Green and eco-friendly synthesis of Co3O4 and Ag-Co3O4: Characterization and photo-catalytic activity
- Adsorption optimized of the coal-based material and application for cyanide wastewater treatment
- Aloe vera leaf extract mediated green synthesis of selenium nanoparticles and assessment of their In vitro antimicrobial activity against spoilage fungi and pathogenic bacteria strains
- Waste phenolic resin derived activated carbon by microwave-assisted KOH activation and application to dye wastewater treatment
- Direct ethanol production from cellulose by consortium of Trichoderma reesei and Candida molischiana
- Agricultural waste biomass-assisted nanostructures: Synthesis and application
- Biodiesel production from rubber seed oil using calcium oxide derived from eggshell as catalyst – optimization and modeling studies
- Study of fabrication of fully aqueous solution processed SnS quantum dot-sensitized solar cell
- Assessment of aqueous extract of Gypsophila aretioides for inhibitory effects on calcium carbonate formation
- An environmentally friendly acylation reaction of 2-methylnaphthalene in solvent-free condition in a micro-channel reactor
- Aegle marmelos phytochemical stabilized synthesis and characterization of ZnO nanoparticles and their role against agriculture and food pathogen
- A reactive coupling process for co-production of solketal and biodiesel
- Optimization of the asymmetric synthesis of (S)-1-phenylethanol using Ispir bean as whole-cell biocatalyst
- Synthesis of pyrazolopyridine and pyrazoloquinoline derivatives by one-pot, three-component reactions of arylglyoxals, 3-methyl-1-aryl-1H-pyrazol-5-amines and cyclic 1,3-dicarbonyl compounds in the presence of tetrapropylammonium bromide
- Preconcentration of morphine in urine sample using a green and solvent-free microextraction method
- Extraction of glycyrrhizic acid by aqueous two-phase system formed by PEG and two environmentally friendly organic acid salts - sodium citrate and sodium tartrate
- Green synthesis of copper oxide nanoparticles using Juglans regia leaf extract and assessment of their physico-chemical and biological properties
- Deep eutectic solvents (DESs) as powerful and recyclable catalysts and solvents for the synthesis of 3,4-dihydropyrimidin-2(1H)-ones/thiones
- Biosynthesis, characterization and anti-microbial activity of silver nanoparticle based gel hand wash
- Efficient and selective microwave-assisted O-methylation of phenolic compounds using tetramethylammonium hydroxide (TMAH)
- Anticoagulant, thrombolytic and antibacterial activities of Euphorbia acruensis latex-mediated bioengineered silver nanoparticles
- Volcanic ash as reusable catalyst in the green synthesis of 3H-1,5-benzodiazepines
- Green synthesis, anionic polymerization of 1,4-bis(methacryloyl)piperazine using Algerian clay as catalyst
- Selenium supplementation during fermentation with sugar beet molasses and Saccharomyces cerevisiae to increase bioethanol production
- Biosynthetic potential assessment of four food pathogenic bacteria in hydrothermally silver nanoparticles fabrication
- Investigating the effectiveness of classical and eco-friendly approaches for synthesis of dialdehydes from organic dihalides
- Pyrolysis of palm oil using zeolite catalyst and characterization of the boil-oil
- Azadirachta indica leaves extract assisted green synthesis of Ag-TiO2 for degradation of Methylene blue and Rhodamine B dyes in aqueous medium
- Synthesis of vitamin E succinate catalyzed by nano-SiO2 immobilized DMAP derivative in mixed solvent system
- Extraction of phytosterols from melon (Cucumis melo) seeds by supercritical CO2 as a clean technology
- Production of uronic acids by hydrothermolysis of pectin as a model substance for plant biomass waste
- Biofabrication of highly pure copper oxide nanoparticles using wheat seed extract and their catalytic activity: A mechanistic approach
- Intelligent modeling and optimization of emulsion aggregation method for producing green printing ink
- Improved removal of methylene blue on modified hierarchical zeolite Y: Achieved by a “destructive-constructive” method
- Two different facile and efficient approaches for the synthesis of various N-arylacetamides via N-acetylation of arylamines and straightforward one-pot reductive acetylation of nitroarenes promoted by recyclable CuFe2O4 nanoparticles in water
- Optimization of acid catalyzed esterification and mixed metal oxide catalyzed transesterification for biodiesel production from Moringa oleifera oil
- Kinetics and the fluidity of the stearic acid esters with different carbon backbones
- Aiming for a standardized protocol for preparing a process green synthesis report and for ranking multiple synthesis plans to a common target product
- Microstructure and luminescence of VO2 (B) nanoparticle synthesis by hydrothermal method
- Optimization of uranium removal from uranium plant wastewater by response surface methodology (RSM)
- Microwave drying of nickel-containing residue: dielectric properties, kinetics, and energy aspects
- Simple and convenient two step synthesis of 5-bromo-2,3-dimethoxy-6-methyl-1,4-benzoquinone
- Biodiesel production from waste cooking oil
- The effect of activation temperature on structure and properties of blue coke-based activated carbon by CO2 activation
- Optimization of reaction parameters for the green synthesis of zero valent iron nanoparticles using pine tree needles
- Microwave-assisted protocol for squalene isolation and conversion from oil-deodoriser distillates
- Denitrification performance of rare earth tailings-based catalysts
- Facile synthesis of silver nanoparticles using Averrhoa bilimbi L and Plum extracts and investigation on the synergistic bioactivity using in vitro models
- Green production of AgNPs and their phytostimulatory impact
- Photocatalytic activity of Ag/Ni bi-metallic nanoparticles on textile dye removal
- Topical Issue: Green Process Engineering / Guest Editors: Martine Poux, Patrick Cognet
- Modelling and optimisation of oxidative desulphurisation of tyre-derived oil via central composite design approach
- CO2 sequestration by carbonation of olivine: a new process for optimal separation of the solids produced
- Organic carbonates synthesis improved by pervaporation for CO2 utilisation
- Production of starch nanoparticles through solvent-antisolvent precipitation in a spinning disc reactor
- A kinetic study of Zn halide/TBAB-catalysed fixation of CO2 with styrene oxide in propylene carbonate
- Topical on Green Process Engineering
Articles in the same Issue
- Regular Articles
- Studies on the preparation and properties of biodegradable polyester from soybean oil
- Flow-mode biodiesel production from palm oil using a pressurized microwave reactor
- Reduction of free fatty acids in waste oil for biodiesel production by glycerolysis: investigation and optimization of process parameters
- Saccharin: a cheap and mild acidic agent for the synthesis of azo dyes via telescoped dediazotization
- Optimization of lipase-catalyzed synthesis of polyethylene glycol stearate in a solvent-free system
- Green synthesis of iron oxide nanoparticles using Platanus orientalis leaf extract for antifungal activity
- Ultrasound assisted chemical activation of peanut husk for copper removal
- Room temperature silanization of Fe3O4 for the preparation of phenyl functionalized magnetic adsorbent for dispersive solid phase extraction for the extraction of phthalates in water
- Evaluation of the saponin green extraction from Ziziphus spina-christi leaves using hydrothermal, microwave and Bain-Marie water bath heating methods
- Oxidation of dibenzothiophene using the heterogeneous catalyst of tungsten-based carbon nanotubes
- Calcined sodium silicate as an efficient and benign heterogeneous catalyst for the transesterification of natural lecithin to L-α-glycerophosphocholine
- Synergistic effect between CO2 and H2O2 on ethylbenzene oxidation catalyzed by carbon supported heteropolyanion catalysts
- Hydrocyanation of 2-arylmethyleneindan-1,3-diones using potassium hexacyanoferrate(II) as a nontoxic cyanating agent
- Green synthesis of hydratropic aldehyde from α-methylstyrene catalyzed by Al2O3-supported metal phthalocyanines
- Environmentally benign chemical recycling of polycarbonate wastes: comparison of micro- and nano-TiO2 solid support efficiencies
- Medicago polymorpha-mediated antibacterial silver nanoparticles in the reduction of methyl orange
- Production of value-added chemicals from esterification of waste glycerol over MCM-41 supported catalysts
- Green synthesis of zerovalent copper nanoparticles for efficient reduction of toxic azo dyes congo red and methyl orange
- Optimization of the biological synthesis of silver nanoparticles using Penicillium oxalicum GRS-1 and their antimicrobial effects against common food-borne pathogens
- Optimization of submerged fermentation conditions to overproduce bioethanol using two industrial and traditional Saccharomyces cerevisiae strains
- Extraction of In3+ and Fe3+ from sulfate solutions by using a 3D-printed “Y”-shaped microreactor
- Foliar-mediated Ag:ZnO nanophotocatalysts: green synthesis, characterization, pollutants degradation, and in vitro biocidal activity
- Green cyclic acetals production by glycerol etherification reaction with benzaldehyde using cationic acidic resin
- Biosynthesis, characterization and antimicrobial activities assessment of fabricated selenium nanoparticles using Pelargonium zonale leaf extract
- Synthesis of high surface area magnesia by using walnut shell as a template
- Controllable biosynthesis of silver nanoparticles using actinobacterial strains
- Green vegetation: a promising source of color dyes
- Mechano-chemical synthesis of ammonia and acetic acid from inorganic materials in water
- Green synthesis and structural characterization of novel N1-substituted 3,4-dihydropyrimidin-2(1H)-ones
- Biodiesel production from cotton oil using heterogeneous CaO catalysts from eggshells prepared at different calcination temperatures
- Regeneration of spent mercury catalyst for the treatment of dye wastewater by the microwave and ultrasonic spray-assisted method
- Green synthesis of the innovative super paramagnetic nanoparticles from the leaves extract of Fraxinus chinensis Roxb and their application for the decolourisation of toxic dyes
- Biogenic ZnO nanoparticles: a study of blueshift of optical band gap and photocatalytic degradation of reactive yellow 186 dye under direct sunlight
- Leached compounds from the extracts of pomegranate peel, green coconut shell, and karuvelam wood for the removal of hexavalent chromium
- Enhancement of molecular weight reduction of natural rubber in triphasic CO2/toluene/H2O systems with hydrogen peroxide for preparation of biobased polyurethanes
- An efficient green synthesis of novel 1H-imidazo[1,2-a]imidazole-3-amine and imidazo[2,1-c][1,2,4]triazole-5-amine derivatives via Strecker reaction under controlled microwave heating
- Evaluation of three different green fabrication methods for the synthesis of crystalline ZnO nanoparticles using Pelargonium zonale leaf extract
- A highly efficient and multifunctional biomass supporting Ag, Ni, and Cu nanoparticles through wetness impregnation for environmental remediation
- Simple one-pot green method for large-scale production of mesalamine, an anti-inflammatory agent
- Relationships between step and cumulative PMI and E-factors: implications on estimating material efficiency with respect to charting synthesis optimization strategies
- A comparative sorption study of Cr3+ and Cr6+ using mango peels: kinetic, equilibrium and thermodynamic
- Effects of acid hydrolysis waste liquid recycle on preparation of microcrystalline cellulose
- Use of deep eutectic solvents as catalyst: A mini-review
- Microwave-assisted synthesis of pyrrolidinone derivatives using 1,1’-butylenebis(3-sulfo-3H-imidazol-1-ium) chloride in ethylene glycol
- Green and eco-friendly synthesis of Co3O4 and Ag-Co3O4: Characterization and photo-catalytic activity
- Adsorption optimized of the coal-based material and application for cyanide wastewater treatment
- Aloe vera leaf extract mediated green synthesis of selenium nanoparticles and assessment of their In vitro antimicrobial activity against spoilage fungi and pathogenic bacteria strains
- Waste phenolic resin derived activated carbon by microwave-assisted KOH activation and application to dye wastewater treatment
- Direct ethanol production from cellulose by consortium of Trichoderma reesei and Candida molischiana
- Agricultural waste biomass-assisted nanostructures: Synthesis and application
- Biodiesel production from rubber seed oil using calcium oxide derived from eggshell as catalyst – optimization and modeling studies
- Study of fabrication of fully aqueous solution processed SnS quantum dot-sensitized solar cell
- Assessment of aqueous extract of Gypsophila aretioides for inhibitory effects on calcium carbonate formation
- An environmentally friendly acylation reaction of 2-methylnaphthalene in solvent-free condition in a micro-channel reactor
- Aegle marmelos phytochemical stabilized synthesis and characterization of ZnO nanoparticles and their role against agriculture and food pathogen
- A reactive coupling process for co-production of solketal and biodiesel
- Optimization of the asymmetric synthesis of (S)-1-phenylethanol using Ispir bean as whole-cell biocatalyst
- Synthesis of pyrazolopyridine and pyrazoloquinoline derivatives by one-pot, three-component reactions of arylglyoxals, 3-methyl-1-aryl-1H-pyrazol-5-amines and cyclic 1,3-dicarbonyl compounds in the presence of tetrapropylammonium bromide
- Preconcentration of morphine in urine sample using a green and solvent-free microextraction method
- Extraction of glycyrrhizic acid by aqueous two-phase system formed by PEG and two environmentally friendly organic acid salts - sodium citrate and sodium tartrate
- Green synthesis of copper oxide nanoparticles using Juglans regia leaf extract and assessment of their physico-chemical and biological properties
- Deep eutectic solvents (DESs) as powerful and recyclable catalysts and solvents for the synthesis of 3,4-dihydropyrimidin-2(1H)-ones/thiones
- Biosynthesis, characterization and anti-microbial activity of silver nanoparticle based gel hand wash
- Efficient and selective microwave-assisted O-methylation of phenolic compounds using tetramethylammonium hydroxide (TMAH)
- Anticoagulant, thrombolytic and antibacterial activities of Euphorbia acruensis latex-mediated bioengineered silver nanoparticles
- Volcanic ash as reusable catalyst in the green synthesis of 3H-1,5-benzodiazepines
- Green synthesis, anionic polymerization of 1,4-bis(methacryloyl)piperazine using Algerian clay as catalyst
- Selenium supplementation during fermentation with sugar beet molasses and Saccharomyces cerevisiae to increase bioethanol production
- Biosynthetic potential assessment of four food pathogenic bacteria in hydrothermally silver nanoparticles fabrication
- Investigating the effectiveness of classical and eco-friendly approaches for synthesis of dialdehydes from organic dihalides
- Pyrolysis of palm oil using zeolite catalyst and characterization of the boil-oil
- Azadirachta indica leaves extract assisted green synthesis of Ag-TiO2 for degradation of Methylene blue and Rhodamine B dyes in aqueous medium
- Synthesis of vitamin E succinate catalyzed by nano-SiO2 immobilized DMAP derivative in mixed solvent system
- Extraction of phytosterols from melon (Cucumis melo) seeds by supercritical CO2 as a clean technology
- Production of uronic acids by hydrothermolysis of pectin as a model substance for plant biomass waste
- Biofabrication of highly pure copper oxide nanoparticles using wheat seed extract and their catalytic activity: A mechanistic approach
- Intelligent modeling and optimization of emulsion aggregation method for producing green printing ink
- Improved removal of methylene blue on modified hierarchical zeolite Y: Achieved by a “destructive-constructive” method
- Two different facile and efficient approaches for the synthesis of various N-arylacetamides via N-acetylation of arylamines and straightforward one-pot reductive acetylation of nitroarenes promoted by recyclable CuFe2O4 nanoparticles in water
- Optimization of acid catalyzed esterification and mixed metal oxide catalyzed transesterification for biodiesel production from Moringa oleifera oil
- Kinetics and the fluidity of the stearic acid esters with different carbon backbones
- Aiming for a standardized protocol for preparing a process green synthesis report and for ranking multiple synthesis plans to a common target product
- Microstructure and luminescence of VO2 (B) nanoparticle synthesis by hydrothermal method
- Optimization of uranium removal from uranium plant wastewater by response surface methodology (RSM)
- Microwave drying of nickel-containing residue: dielectric properties, kinetics, and energy aspects
- Simple and convenient two step synthesis of 5-bromo-2,3-dimethoxy-6-methyl-1,4-benzoquinone
- Biodiesel production from waste cooking oil
- The effect of activation temperature on structure and properties of blue coke-based activated carbon by CO2 activation
- Optimization of reaction parameters for the green synthesis of zero valent iron nanoparticles using pine tree needles
- Microwave-assisted protocol for squalene isolation and conversion from oil-deodoriser distillates
- Denitrification performance of rare earth tailings-based catalysts
- Facile synthesis of silver nanoparticles using Averrhoa bilimbi L and Plum extracts and investigation on the synergistic bioactivity using in vitro models
- Green production of AgNPs and their phytostimulatory impact
- Photocatalytic activity of Ag/Ni bi-metallic nanoparticles on textile dye removal
- Topical Issue: Green Process Engineering / Guest Editors: Martine Poux, Patrick Cognet
- Modelling and optimisation of oxidative desulphurisation of tyre-derived oil via central composite design approach
- CO2 sequestration by carbonation of olivine: a new process for optimal separation of the solids produced
- Organic carbonates synthesis improved by pervaporation for CO2 utilisation
- Production of starch nanoparticles through solvent-antisolvent precipitation in a spinning disc reactor
- A kinetic study of Zn halide/TBAB-catalysed fixation of CO2 with styrene oxide in propylene carbonate
- Topical on Green Process Engineering