Abstract
In the present study, Calcium oxide (CaO) obtained from eggshells has been used as a heterogeneous catalyst for biodiesel production from highly viscous non-edible rubber seed oil (RSO). Characterization of synthesized catalyst was done with the help of scanning electron microscope equipped with Energy dispersive spectrometry (SEM-EDS), X-ray diffraction (XRD) and Fourier transform Infrared spectroscopy (FTIR). Response surface methodology (RSM) with central composite design (CCD) was used to optimize the process parameters and 1H-NMR (Nuclear Magnetic Resonance) spectroscopy analysis was performed to find the conversion of RSO to biodiesel. A conversion of 99.7% of RSO to biodiesel was obtained at 12:1 methanol to oil molar ratio, 4 (wt%) of catalyst, and 3 hour reaction time with a quadratic regression model of R2 of value 0.9566 was obtained. The composition of prepared biodiesel is estimated with the help of Gas Chromatogram-Mass Spectroscopy (GC-MS) analysis. Artificial Neural Network (ANN) with Levenberg-Marquardt algorithm was also trained to predict biodiesel conversion and the value of R2 obtained was 0.9976. It was observed that predicted conversion values from ANN were better when compared to prediction using RSM.
1 Introduction
The selection of feedstocks for biodiesel production is a critical factor in making biodiesel a viable alternative to the existing petroleum-based diesel. Plenty of oils obtained from seeds of plants and trees have been commonly used as feedstocks for the production of biodiesel, and among them, edible oils such as sunflower oil [1, 2], palm oil [3, 4, 5,], soyabean oil [6, 7,], peanut oil [8], corn oil [9] etc. have been used extensively in the production of biodiesel. The use of edible oils in biodiesel production is not advisable inview of its demand in processing of food. Non-edible oils which do not have many commercial applications and are also cost effective and excellent alternatives to overcome this issue [10, 11, 12, 13,]. Transesterification is one of the most widely used methods for producing biodiesel from vegetable oils, catalytically. In this process, as per stoichiometry, one mole of triglyceride reacts with three moles of alcohol in the presence of a catalyst to form methyl ester. The mechanism of transesterification is shown in Eq. 1 [14]. Transesterification using either the acidic catalysts or the basic catalysts are the two different types of transesterification processes which are used extensively. Carboxylic acids can be esterified by alcohols in the presence of a suitable acid catalyst in water-free conditions and the process is called acid esterification. Acid catalysts generally employed for transesterification processes are HCl, H2SO4, BF3, and sulfonic acids [15, 16, 17,]. Use of solid heterogeneous catalyst is more advantageous since it involves a single step process and the separation of the catalyst is also easy [16]. Esters in the presence of a base such as an alcoholate anion forms an anionic intermediate which can subsequently dissociate back to the original ester or form the new ester in the base catalyzed transesterification [15, 16,]. The only disadvantage of acid catalyzed transesterification is that the reaction rate is less when compared with the base-catalyzed transesterification, and hence it requires a temperature above 100°C [16]. As far as the homogeneous base catalysts are concerned, the commonly used ones are potassium hydroxide [17], and sodium hydroxide [15, 16, 17, 18,]. While using the homogeneous base catalyst, the major disadvantage is the separation of catalyst from the transesterification products [14, 15, 16,]. Recent research works state that a heterogeneous catalyst derived from alkali and alkaline earth metal oxides, transition metal oxides, mixed metal oxides, ion exchange resins, derivatives prepared from impregnation of sulfur, boron, and carbon can be used in biodiesel preparation [1921]. Impregnation of potassium and sodium salts on metal oxide catalysts increases the basicity of catalysts and this approach can also be used in producing biodiesel from triglycerides with a high conversion [20]. Most of the solid waste materials and biomass resources can also be used as heterogeneous catalysts in biodiesel production [16]. The major advantages of using this catalyst are the reusability, low cost, solid waste management etc. All the catalysts mentioned above are solid-base heterogeneous catalysts which give a high conversion of oil feedstock to biodiesel when compared to homogenous catalyst [21, 22,].
Recent studies proposed that calcium oxide (CaO) derived from various wastes and the impregnation of CaO with different compounds as one of the best heterogeneous catalysts which can be effectively used for producing biodiesel [23, 24, 25,] from waste frying oil and pongamia oil. Catalyst activity, reusability, loading, low cost are the major advantages of using calcium oxide as the catalyst [16]. A high yield of 98% was observed while using KOH impregnated with lime/carbon (CaO/C) synthesized by wet impregnation method as the catalyst in the transesterification of soyabean oil [26]. Heterogeneous catalyst CaO-La2O3 was used in the transesterification of soyabean oil which gave a biodiesel yield of 94.3% [27]. The use of CaO loaded with 3.7 wt% in Ca(OH)2/CaO as the catalyst in soyabean oil transesterification with ethanol produced biodiesel with a conversion of 96.3% [28]. Mixed oxide catalysts CaO/ZnO prepared from calcium and zinc were used in biodiesel preparation from palm kernel oil [29]. An overall biodiesel yield of 88.06% was obtained while using waste cockles shells, having 93.98% of calcium oxide (CaO), as a heterogeneous catalyst in the transesterification of rubber seed oil (RSO) [30]. Calcium oxide prepared from bivalve clam shells produced biodiesel with a high yield of 95.84% from the waste cooking oil [23]. The catalytic activity of CaO supported on mesoporous silica was tested in the transesterification of ethyl butyrate with methanol [31]. Clinker, a by-product from cement industry, was also used as a catalyst in biodiesel production from RSO and a high conversion of 96.80% was achieved [33]. Microcapsules loaded with CaO and activated carbon were used as a heterogeneous catalyst in biodiesel production from rapeseed oil [34]. A review of literature also indicates that not much research has been done on the use of RSO as feedstock for biodiesel production, and also on the use of CaO from natural sources as catalysts in biodiesel production from non-edible oils. Hence, in the present study, a highly viscous non-edible RSO which is cheap and does not have much commercial application and mostly available in southern part of India in huge amount has been selected for biodiesel synthesis [35, 36]. The main novelty of present work is the optimization of experimental parameters and determination of the best model in RSO conversion to biodiesel using calcium oxide derived from green renewable eggshell as a catalyst. The use of CaO derived from eggshells as a catalyst in biodiesel production from RSO is also not found till date in literature. Earlier, studies were carried out on the preparation of biodiesel from RSO using different homogeneous base catalysts [35] and also by using lime derived catalysts [30]. A maximum conversion of 90-95% was achieved on working with these catalysts. Eggshells a kitchen waste which is found mostly at bakeries, hotels can be used as a source of catalyst in biodiesel production. A large amount of calcium oxide obtained from eggshells has been used as a heterogeneous base catalyst in recent times [23]. Catalysts were characterized using X-ray diffraction (XRD), Scanning electron microscope (SEM), Energy Dispersive Spectroscopy (EDS) and Fourier Transform Infrared (FTIR) spectrometry. Characterization of Biodiesel was done by using Fourier Transform Infrared (FTIR) spectrometry analysis. The conversion of RSO to biodiesel was estimated using 1H-Nuclear Magnetic Resonance (NMR) analysis. Optimization of process parameters such as molar ratio (methanol: oil), catalyst (wt%), reaction time (hours) was done by using design expert software 10 employed with Response Surface Methodology (RSM) [37]. Generally, Artificial Neural Networks (ANN) is used to solve problematic complex mathematical functions easily with accurate result [38]. In this study, ANN is also used to verify the agreement between the experimental and the predicted values. Also, an effort is made to identify whether RSM or ANN has better prediction capability.
Transesterification reaction is given as follows:
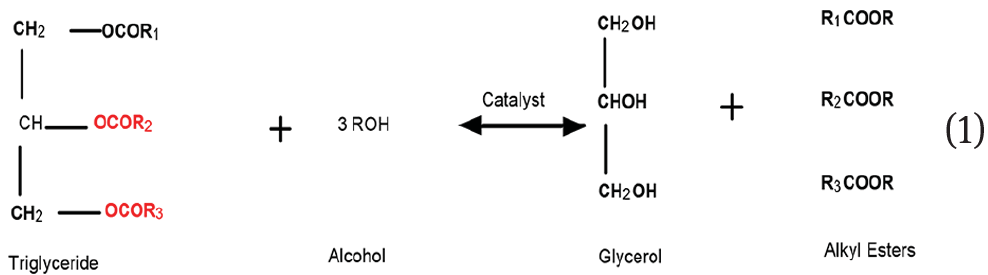
2 Materials and methods
2.1 Materials required
RSO was purchased from virudhunagar Tamilnadu, and methanol used in this process is supplied by CDH suppliers, New Delhi, India, and sulphuric Acid (98% concentration, EMPARTA) used was procurred from Merck life sciences private limited, Mumbai. Eggshells were collected from a local bakery in Trichy, Tamilnadu.
2.2 Experimental methods
2.2.1 Catalyst preparation
Raw egg shells collected were washed thoroughly to remove dirt from them, followed by drying at 105˚C in an oven. The dried shells were then ground into fine particles by using a grinder. Finely powdered shells were subjected to calcination in a muffle furnace at 900°C for 4 h to calcine calcium carbonate present in the shells to calcium oxide (CaO). The final product obtained after calcination is used as a catalyst in the transesterification process.
2.2.2 Catalyst characterization
X-ray diffraction (XRD) (Model: Ultima IV, Rigaku, Japan) using Cu kα radiation has been used to check the formation of calcium oxide (CaO) in eggshells after calcination. Scanning Electron Microscope-Energy Dispersive Spectroscopy (SEM-EDS) (Model: S3000H, Hitachi, Japan) equipped with EDS analysis was used to find the surface morphology and composition of the catalyst. Presence of functional groups in the catalyst were found by using Fourier transform infrared spectroscopy (FTIR, Model: Perkin Elmer, Spectrum 2) analysis.
2.2.3 Esterification procedure
A pretreatment process used to reduce the acid value of selected feedstock to the required level is called esterification. Whenever biodiesel is produced by transesterification process through base catalysis, and if the feedstock has free fatty acids (FFA) content more than 2, esterification is necessary to avoid soap formation. In this study the chosen feedstock RSO is having an acid value of 67.6, and hence treated with methanol in the presence of sulphuric acid (98% concentrated) as catalyst to reduce the FFA content. In this process, initially 50 ml of feedstock was preheated to 60°C, and this is followed by esterification reaction at a temperature of 65°C as cited in literature [24]. After the reaction is completed, the treated oil is separated from excess methanol-catalyst mixture in a separating funnel. Process conditions were optimized and the esterified oil was prepared at the optimized conditions of methanol to oil molar ratio of 15:1, catalyst 3% (v/v H2SO4), and 2 h reaction time. The esterified oil thus prepared was used for all the transesterification studies.
2.2.4 Biodiesel preparation (transesterifcation) procedure
For transesterification reaction, a 3-neck round bottomed flask with one end connected to a condenser, another end to a thermometer and the middle one to a mechanical stirrer was used. The round bottomed flask was kept in a constant temperature water bath. Esterified RSO was heated at methanol boiling point temperature for some time to make sure that excess methanol present in the oil has been evaporated. The catalyst prepared was mixed with methanol to form methoxide by stirring and then the formed methoxide was poured into the heated oil. The methoxide-treated RSO mixture was stirred well and the rotation of stirrer was controlled by a regulator. The formed product was transferred to a separating funnel through Whatmann No 1 filter paper to separate the catalyst from the transesterification products, and the solution was set aside for 48 h to separate. After perfect separation, the two layers are visible in the separating funnel, of which the bottom layer is glycerol which is drawn out and the top layer is the fatty acid methyl ester (FAME) which is called biodiesel. Excess methanol present in the top layer is subsequently evaporated using a rotary evaporator and the final sample was analyzed with 1H-NMR (Model: Bruker 500 MHz) analyzer to estimate the conversion by using CDCl3 as solvent [23].
3 Statistical analysis and modeling of formed biodiesel
3.1 Statistical analysis – Response Surface Methodology (RSM)
RSM is a statistical tool to not only optimize the process parameters but also identify the significant process parameters that affect the process. It also predicts the desired parameter as a function of process parameters. In this study, RSM is employed with central composite design (CCD) for optimizing the process parameters like molar ratio (mol/mol), catalyst (vol %), and reaction time (hours). After performing several trails initially, the maximum and the minimum value of process parameters are fixed as molar ratio 18 (mol/mol) and 6 (mol/mol), catalyst (wt%) as 6 and 2, and reaction time (hours) as 5 and 1 respectively. Analysis of variance (ANOVA) and the effect of influencing factors on percent conversion are designed by using RSM. The process parameters and the range of coded values are presented in Table 1.
Coded values of process parameters.
| Factor | Name | Units | Minimum | Maximum | Coded | Values |
|---|---|---|---|---|---|---|
| A | Molar Ratio | mol/mol | 6 | 18 | –1.000=9 | 1.000=15 |
| B | Catalyst | wt% | 2 | 6 | –1.000=3 | 1.000=5 |
| C | Time | hours | 1 | 5 | –1.000=2 | 1.000=4 |
3.2 Artificial Neural Network (ANN)
In this study, a feed-forward back propagation ANN model has been developed for the conversion of RSO to biodiesel with input parameters as Methanol-oil ratio, catalyst weight% and reaction time in MATLAB R2018a software. The network consists of an input layer with 3 nodes, an output layer of 1 node and a hidden layer of 10 neurons. The neurons are based on the TANSIG transfer function. The network was trained using the TRAINLM (Lavenberg– Marquardt) algorithm and the Mean Squared Error (MSE) was used as the performance function which was to be minimized to 1 × 10−5 (default). 20 data points were used for modeling out of which 70% was used for training, 15% for validation and 15% for testing. The epoch was set at 1000. The ANN performance was evaluated using mean square error and the coefficient of determination R2 for RSO conversion to biodiesel, and is calculated using Eq. 2 [38].
MSE-(Mean Square Error):
where, X → Experimental Output, Y → Predicted Output, n → total number of training data
4 Results and discussion
4.1 Characterization of calcined egg shells
4.1.1 X-Ray Diffraction (XRD)
The formation of calcium oxide (CaO) in the prepared catalyst is found by using XRD technique. Results of this analysis are shown in Figure 1. The CaO formation peaks observed at respective 2θ values of 32.44, 37.58, 54.07, 64.35, 67.56, 79.82 and 88.65 were compared with the standard reference patterns in XPERT High Score Plus and found to match well. Similar results were observed by Niju et al., for calcium oxide (CaO) derived from egg shells [23].
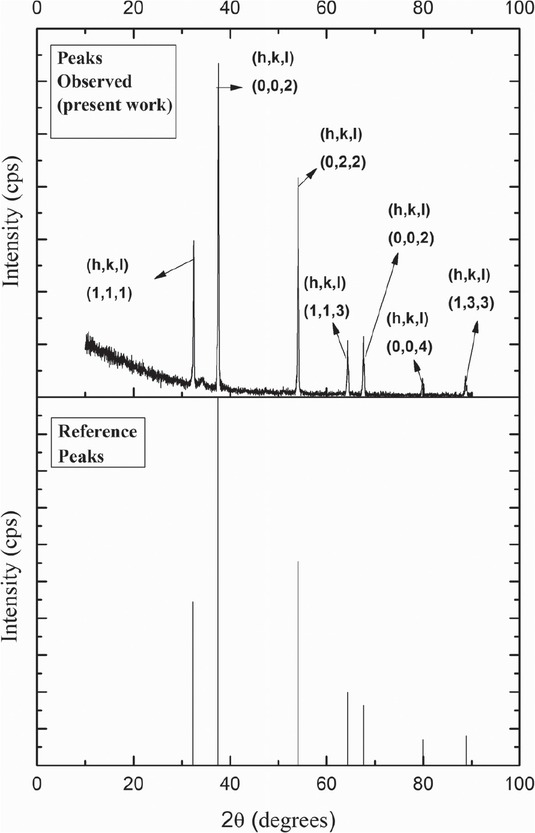
X-Ray Diffraction (XRD) analysis of calcined eggshells.
4.1.2 Scanning Electron Microscope-Energy Dispersive Spectrometry (SEM-EDS)
SEM image of calcined eggshells at different magnifications is shown in Figures 2a-c. On calcination of raw eggshells at 9000Cfor 4h the surface morphology of the calcined
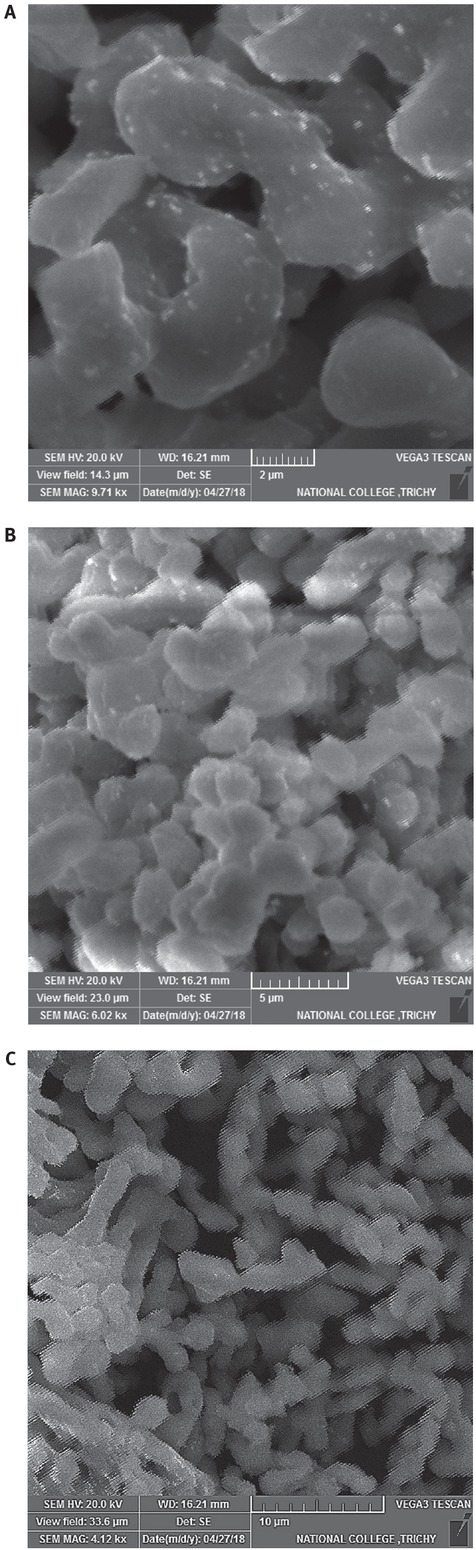
SEM images of calcined eggshells. (a) 9.71kx magnification, (b) 6.02kx magnification, (c) 4.12kx magnification
catalyst appears to be in cane shape particles of size range between 2 μm-10 μm. Similar results are reported in literature by Niju et al. [23].
The elemental composition of calcined eggshells found by using Elemental Dispersive Spectroscopy (EDS) analysis is shown in Table 2. From these results, it is clear that huge amount of CaO is present in the prepared catalyst with 47.47 (w/w %) of oxygen and 48.70 (w/w %) of calcium, and small amounts of magnesium 0.65 (w/w %) and carbon 3.18 (w/w %) are also observed.
EDS analysis of the calcined eggshells.
| Element | Weight % | Atomic % |
|---|---|---|
| C | 3.18 | 5.91 |
| O | 47.47 | 66.33 |
| Mg | 0.65 | 0.60 |
| Ca | 48.70 | 27.16 |
| Totals | 100.00 | |
4.1.3 Fourier Transform Infrared Spectroscopy (FTIR)
The presence of different functional groups in calcined egg shells is identified by FTIR analysis and is presented in Figure 3. On calcination at 900˚C, the calcium carbonate in the eggshells gets converted to calcium oxide. From the FTIR results, it is observed that for calcined egg shells, the major peaks were observed at wave numbers 3600 cm-1 which indicates the presence of hydroxyl groups with O-H stretch and the presence of C-O stretch at wavenumber 1100 cm-1 indicates the presence of alkoxy functional class. C≡C asymmetric stretch with the presence of alkenes functional groups was observed at wave number of 2000 cm-1, peaks observed at wavenumbers 1800 cm-1
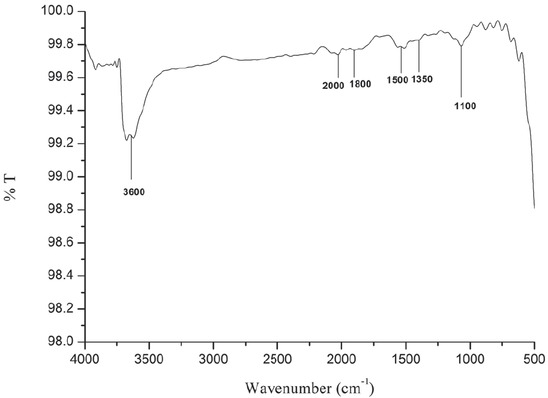
Fourier Transform Infrared Spectroscopy (FTIR) analysis of calcined eggshells.
and 1500 cm-1 indicate the presence of C=O stretch and C=O bands with carbonyl absorption respectively. C-F stretch with the presence of alkyl halides functional class is observed at wavenumber 1350 cm-1.
5 Statistical analysis and modeling of formed biodiesel
5.1 Statistical analysis
Transesterification experiments were designed using Design Expert software 10.0 based on Central Composite Design (CCD) and are shown in Table 3. ANOVA analysis is reported in Table 4. From the ANOVA, it is clear that the molar ratio (mol/mol) is the most influencing factor when compared to catalyst quantity (wt%) and the reaction time (hours). It was also observed that with an increase in molar ratio beyond the optimized limit, conversion of biodiesel decreases gradually, and may lead to reversible reaction. The model terms are said to be significant if the p-value is less than 0.05, and from the Table 4 it is clear that the design model is significant, and also molar ratio is the significant variable when compared to other two variables. The consistency of model and the variance of output response can be explained by F-value of the model. If the value of F is more, the effect of that
Design of transesterification experiment performed.
| Run | A:Molar Ratio (mol/mol) | B:Catalyst (wt%) | C:Time (hours) | Experimental Response Conversion (%) | Predicted Response (RSM) | Predicted Response (ANN) |
|---|---|---|---|---|---|---|
| 1 | 6 | 4 | 3 | 60 | 62.38 | 60 |
| 2 | 9 | 3 | 2 | 84.26 | 80.92 | 83.37 |
| 3 | 9 | 3 | 4 | 84.56 | 81.32 | 84.56 |
| 4 | 9 | 5 | 2 | 83.87 | 84.17 | 83.87 |
| 5 | 9 | 5 | 4 | 80.15 | 79.43 | 80.15 |
| 6 | 12 | 2 | 3 | 86.76 | 90.94 | 86.76 |
| 7 | 12 | 4 | 1 | 80.63 | 81.27 | 84.03 |
| 8 | 12 | 4 | 3 | 99.7 | 98.68 | 98.8 |
| 9 | 12 | 4 | 3 | 97 | 98.68 | 98.8 |
| 10 | 12 | 4 | 3 | 98 | 98.68 | 98.8 |
| 11 | 12 | 4 | 3 | 99.7 | 98.68 | 98.8 |
| 12 | 12 | 4 | 3 | 95.8 | 98.68 | 98.8 |
| 13 | 12 | 4 | 3 | 99.7 | 98.68 | 98.8 |
| 14 | 12 | 4 | 5 | 82.5 | 84.06 | 82.96 |
| 15 | 12 | 6 | 3 | 95 | 93.02 | 95 |
| 16 | 15 | 3 | 2 | 88.37 | 86.87 | 88.37 |
| 17 | 15 | 3 | 4 | 96.91 | 94.39 | 96.91 |
| 18 | 15 | 5 | 2 | 89.82 | 90.84 | 89.82 |
| 19 | 15 | 5 | 4 | 92.1 | 93.22 | 92.1 |
| 20 | 18 | 4 | 3 | 82.3 | 82.12 | 82.3 |
ANOVA analysis for response (percentage conversion).
| Source | Sum of Squares | DF | Mean Square | F Value | p-value Prob> F | |
|---|---|---|---|---|---|---|
| Model | 1715.78 | 9 | 190.64 | 24.50 | < 0.0001 | significant |
| A-Molar Ratio | 389.67 | 1 | 389.67 | 50.08 | < 0.0001 | |
| B-Catalyst | 4.33 | 1 | 4.33 | 0.56 | 0.4730 | |
| C-Time | 7.76 | 1 | 7.76 | 1.00 | 0.3416 | |
| AB | 0.26 | 1 | 0.26 | 0.033 | 0.8588 | |
| AC | 25.35 | 1 | 25.35 | 3.26 | 0.1012 | |
| BC | 13.21 | 1 | 13.21 | 1.70 | 0.2218 | |
| A2 | 1097.68 | 1 | 1097.68 | 141.09 | < 0.0001 | |
| B2 | 70.53 | 1 | 70.53 | 9.07 | 0.0131 | |
| C2 | 403.02 | 1 | 403.02 | 51.80 | < 0.0001 | |
| R2=0.9566 |
particular variable on the output will be more. The Model F-value of 24.50 for the response implies that the model is significant, and it is also observed that the molar ratio is the most influencing factor on output response values with F-value of 389.67. The coefficient of determination R2 value which shows positive predictive results with respect to output response is in the acceptable range of 0.9566. A complete design equation in terms of actual parameters is given in Eq. 3. This can be used to make predictions of the response for the given values of each factor. This is presented in Table 3.
5.1.1 Effect of influencing factor on conversion to biodiesel
Based on the above results, two-dimensional contour plots and three-dimensional response plots are obtained and shown in Figures 4a-c and Figures 5a-c respectively. From these plots, it was observed that for an increase in the molar ratio (mol/mol) beyond the optimized point, a decrease in percentage conversion is observed. As far as heterogeneous catalysts are concerned, more methanol: oil ratio is required when compared to homogeneous catalyst reactions in biodiesel production. Girish et al. utilized clam shells as heterogeneous catalysts for biodiesel production from waste cooking oil at a methanol: oil ratio of 18:1 [24]. Biodiesel production from RSO using waste cockles as heterogeneous catalyst is obtained at a higher molar ratio of 16:1 [30]. This indicates that the most influencing process parameter is the molar ratio (mol/mol) when compared to the other two variables. High conversion of 99.7% was observed at optimized values of molar ratio 12:1, catalyst 4 wt% and reaction time of 3 h.

Contour plots of process parameters affecting Biodiesel Conversion%.
(a) Molar Ratio (mol/mol) vs Catalyst (wt%); (b) Catalyst (wt%) vs Time (hours); (c) Molar Ratio (mol/mol) vs Time (hours).

3D-Surface Plots of process parameters affecting Biodiesel Conversion%.
(a) Molar Ratio (mol/mol) vs Catalyst (wt%); (b) Catalyst (wt%) vs Time (hours); (c) Molar Ratio (mol/mol) vs Time (hours).
5.2 ANN-model
A simple mathematical computation of human brain function with a well-trained neural network containing
several neurons in it is called ANN. ANN is completely based on the input data given for network training [32]. Figure 6 shows the architecture of the ANN model with a single input layer, one hidden layer with 10 neurons which appear like biological neurons of human brain and an output layer. The main function of neurons in hidden layer of neural network is to build complicated relationship between input and output layers. From the Figure 6, it is observed that the input layer distributes all the three inputs to weights of the hidden layer. Neurons

Architecture for ANN model.
present in hidden layer of neural network receive information from the input layer linked to weight factor to form a desired output. At first, the output of the first hidden layer was computed which acts as input to the next hidden layer and continues consecutively to form a well desired output of complete network. In Figure 6, Methanol: Oil molar ratio, catalyst (wt%), and reaction time (hrs) are the three selected inputs. Minimum error is found from design table between the predicted and experimental outputs by training the network several times which is known as the transformation of weights in the network. Regression value, R, which is a measure of correlation between desired inputs and outputs, with value close to 1 concludes a good relationship, with value 0 as a random relationship. From Figure 7 it is
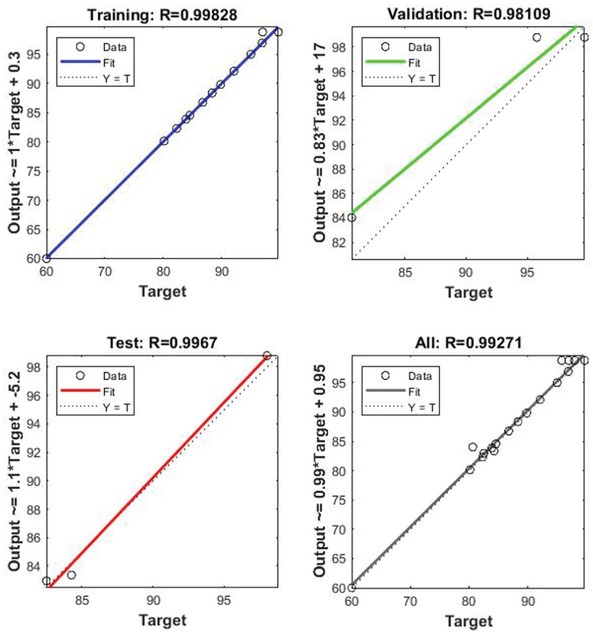
Regression plot for ANN model of Biodiesel Conversion%.
clear that R values of 0.99828, 0.98109, 0.9967, 0.99271 for training, validation, test and all respectively show a good relationship for the model. The mean square error (MSE) value of 1.3929 for the overall design is calculated as mentioned in Eq. 2. The summary of ANN Modeling is shown in Figure 8 along with performance plot in Figure 9.

Summary of ANN modeling.

ANN performance plot of Biodiesel Conversion%.
5.3 Comparison of RSM and ANN
The efficiency of the model is explained by the coefficient of determination R2 value, which is observed to be 0.9976 for ANN model and 0.9566 for RSM. Relationship between predicted and actual experimental values is shown in Figures 10 and 11 for RSM and ANN respectively. By comparing the above results, it is concluded that the ANN is a better model for finding the conversion of RSO to biodiesel when compared with RSM.

Actual Biodiesel Conversion% (X-Axis) vs Predicted Biodiesel Conversion% (Y-Axis) – RSM.
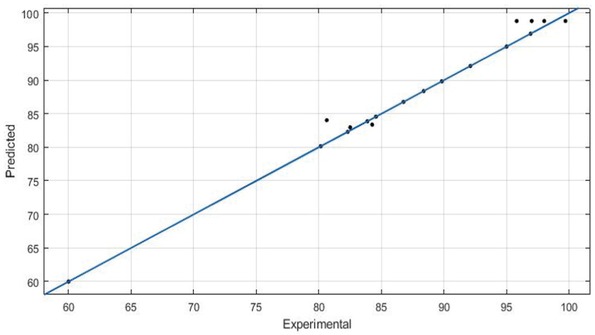
Actual Biodiesel Conversion% (X-Axis) vs Predicted Biodiesel Conversion% (Y-Axis) – ANN.
6 Characterization of biodiesel formed
6.1 Physico-chemical properties of prepared biodiesel
The properties like acid number, density, viscosity, the flashpoint were calculated for the biodiesel prepared in this study, and compared with the literature values for biodiesel prepared from RSO using other catalysts and are presented in Table 5. The properties of biodiesel synthesized are found to agree well with the standard ASTM values.
Comparison of physico-chemical of biodiesel in literature with the properties of biodiesel synthesized in the present study.
| Properties | Acid value (mg KOH/g oil) | Specific gravity | Kinematic viscosity (300C)(mm2/s) | Flash point (˚C) |
|---|---|---|---|---|
| ASTM standard values | <0.6 | 0.86-0.90 | 1.9-6.0 | 100-170 |
| A.S Ramadhas et al. | 0.118 | 0.874 | 5.81 | 130 |
| (Feedstock- Rubber Seed Oil) [35] Junaid Ahmad et al. | 0.42 | 0.885 | 3.89 | 152 |
| (Feedstock- Rubber Seed Oil) [39] Ahmad Hussain et al. | 0.07 | 0.87 | 4.64 | 154.6 |
| (Feedstock- Rubber Seed Oil) [33] Mahbub Morshed et al. | 0.12 | 0.85 | 4.5 | 120 |
| (Feedstock- Rubber Seed Oil) [10] Present Work | 0.26 | 0.88 | 4.49 | 140 |
6.2 1H-Nuclear Magnetic Resonance (NMR) spectroscopic analysis
The conversion of fats to fatty acid methyl esters (biodiesel) was determined by using 1H-NMR analysis. The 1H-NMR spectrum of the biodiesel synthesized is shown in Figure 12. Formation of methoxy protons of methyl esters at 3.6 ppm and α-methylene protons of methyl esters at 2.3 ppm in NMR spectrum indicates the formation of biodiesel. Equation 4 was used for calculating the conversion of esterified oil to biodiesel [23]. From the Figure 12, the formation of methoxy protons of methyl esters at 3.6 ppm and α-methoxy protons of methyl esters at 2.3 ppm is observed. 99.7% of RSO was converted to biodiesel at optimum process conditions of 12:1 molar ratio (mol/mol), 4 (wt%) catalyst and reaction time of 3 h. A comparison of conversion achieved with RSO in the present work with the values reported in literature for RSO using other catalysts is shown in Table 6. From this table, it is clear that biodiesel produced from RSO using eggshells as catalyst gives a higher conversion when compared to the other works reported in literature.
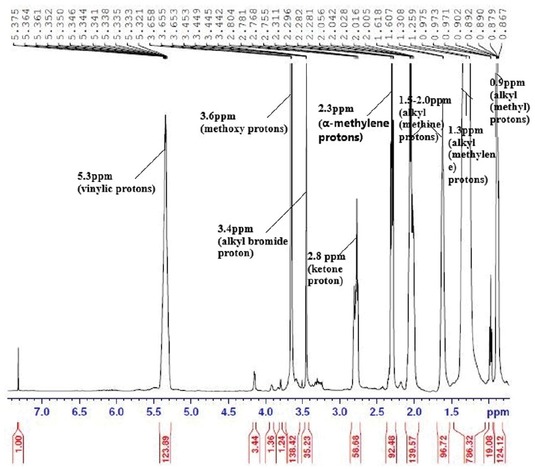
1H-NMR spectrum analysis of rubber seed oil biodiesel.
Comparison of percentage conversion of formed biodiesel with various other catalysts using rubber seed oil.
| Feedstock | Catalyst used | Conversion % | Reference |
|---|---|---|---|
| Rubber Seed Oil | Eggshells | 99.6 | Present Work |
| Rubber Seed Oil | NaOH | 98 | A.S Ramadhas et al. [35] |
| Rubber Seed Oil | Clinker | 96.80 | Ahmad Hussain et al. [33] |
| Rubber Seed Oil | KOH | 96.8 | Junaid Ahmad et al. [39] |
| Rubber Seed Oil | Waste Cockles shells | 88.06 | M. M. Zamberi et al. [30] |
| Rubber Seed Oil | H2SO4 | 98 | Mahbub Morshed et al. [10] |
| Rubber Seed Oil | Activated cement clinker | 96.9 | Jolius Gimbun et al. [40] |
6.3 Fourier Transform Infrared (FTIR)
Fourier Transform Infrared Spectroscopy (FTIR) analysis was performed to determine the various functional groups present in the biodiesel and is shown in Figure 13. Carboxylic acids and derivatives functional compounds with C-H stretch are detected at wavenumber 2923 cm-1. Ester functional group with C=O stretch was observed at wave number 1717 cm-1. O=C-O-C stretch at wave
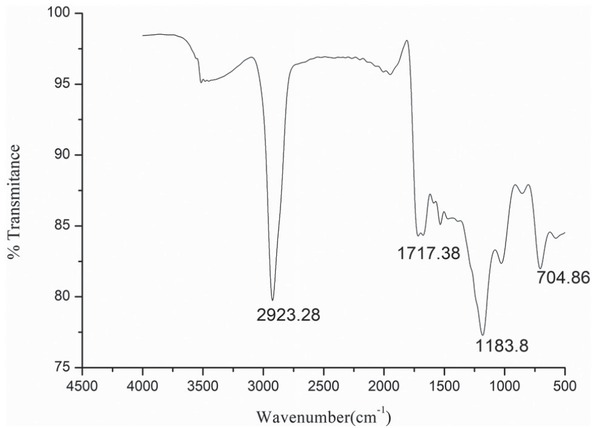
Fourier Transform Infrared Spectroscopy (FTIR) analysis of formed biodiesel.
number 1183 cm-1 indicates the presence of aliphatic esters in the synthesized biodiesel. S-OR esters functional group at a wavenumber of 704.86 cm-1 was also observed.
6.4 Gas Chromatogram-Mass Spectroscopy (GC-MS) analysis
Composition of prepared biodiesel was found by using GC-MS (TIC) analysis. 436-GC Bruker and TQ Quadrupole Mass Spectrometer were used for analysis. The column used for GC programme was BR-5MS (5% Diphenyl / 95% Dimethyl poly siloxane), 30m x 0.25mm ID x 0.25μm df. Software which was used to analyze the sample is MS Work Station 8. NIST Version-2011 is the library used for MS programme. Table 7 shows the list of compounds in the prepared biodiesel, and Figure 14 shows the gas chromatogram of biodiesel produced from RSO. An effective comparison of formed biodiesel composition with various other feedstocks used in literature is reported in Table 8, and it is clear from the table that around 99% of biodiesel is formed in the present work.
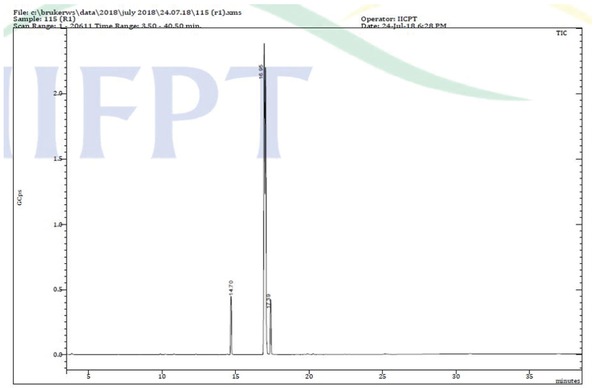
Gas Chromatogram- Mass Spectroscopy (GC-MS) analysis of formed biodiesel.
List of compounds identified in biodiesel prepared.
| Retention time | Name of the compound | Molecular formula | Molecular weight |
|---|---|---|---|
| 3.87 | Octanoic acid, methyl ester | C9H18O2 | 158 |
| 9.89 | Dodecanoic acid, methyl ester | C13H26O2 | 214 |
| 10.22 | Nonanedioic acid, dimethyl ester | C11H20O4 | 216 |
| 12.33 | Methyl tetradecanoate | C15H30O2 | 242 |
| 14.70 | Hexadecanoic acid, methyl ester | C17H34O2 | 270 |
| 16.95 | 9,12-Octadecadienoic acid (Z,Z)-, methyl ester | C19H34O2 | 294 |
| 17.05 | 9-Octadecenoic acid (Z)-, methyl ester | C19H36O2 | 296 |
| 17.39 | Methyl stearate | C19H38O2 | 298 |
| 19.88 | Cyclopropanepentanoic acid, 2-undecyl-, methyl ester, trans- | C20H38O2 | 310 |
| 20.28 | Hexadecanoic acid, 14-methyl-, methyl ester | C18H36O2 | 284 |
Comparison of biodiesel composition with various other feed stocks in literature.
| Compound name | Present work (rubber seed oil) (catalyst: waste eggshells) [%] | Sneha et al. (waste cooking oil) (catalyst: KBr/CaO) [%] [41] | Natarajan Girish et al. (waste frying oil) (cata- lyst: white bivalve clam shells) [%] [24] | Hanny Johanes Berchmans et al. (Jatropha curcas oil) (catalyst: NaOH) [%] [42] | Medy C. Nongbe et al. (palm oil) (catalyst: sulfonated graphene catalyst) [%] [43] |
|---|---|---|---|---|---|
| Octanoic acid, methyl ester | 0.19 | – | 0.10 | – | – |
| Dodecanoic acid, methyl ester | 0.11 | – | – | 0.06 | 0.21 |
| Nonanedioic acid, dimethyl | 0.11 | – | – | – | |
| ester Methyl tetradecanoate | 0.08 | – | 0.14 | 0.10 | 0.56 |
| Hexadecanoic acid, methyl ester | 7.91 | 36.79 | 8.06 | 14.96 | 34.43 |
| 9,12-Octadecadienoic acid (Z,Z)-, methyl ester | 45.36 | 5.03 | 0.22 | 47.43 | 7.03 |
| 9-Octadecenoic acid (Z)-, methyl ester | 38.38 | 52.55 | – | 32.49 | 49.22 |
| Methyl stearate | 7.57 | 3.46 | – | 3.85 | 6.18 |
| Cyclopropanepentanoic acid, 2-undecyl-, methyl ester, trans- | 0.14 | – | – | – | – |
| Hexadecanoic acid, 14-methyl-, methyl ester | 0.15 | 0.34 | – | – | – |
7 Conclusion
Optimization and modeling of process parameters for biodiesel production from RSO using solid waste catalyst, eggshells, by transesterification was done both by RSM and ANN. 99.7% conversion of RSO to biodiesel was observed at process conditions of
12:1 methanol to Oil molar ratio (mol/mol), 4 (wt%) catalyst and 3 h of reaction time and for the quadratic model, the value of R2 was equal to 0.9566 by RSM. With ANN model, on training the neural network several times, a regression value of R2 equal to 0.9976 was obtained. On comparison of R2 values achieved from both the models and the error between experimental and predicted outputs in overall design, it is concluded that ANN is a better model for predicting the conversion of RSO to biodiesel. It is also observed from the overall design; the molar ratio (methanol: oil) is the most influencing factor for the conversion of RSO to biodiesel.
References
[1] Dalibor M.M., Miroslav V.S., Ana V.V., Jelena M.A., Milord D.C., Vlada B.V., The synthesis of CaO loaded onto Al2O3 from calcium acetate and its applications in the transesterification of the sunflower oil. Adv. Technol., 2015, 4(1), 26-32.10.5937/savteh1501026MSearch in Google Scholar
[2] Sung M.S., Katsuki K., Transesterification of sunflower oil in a countercurrent trickle-bed reactor packed with a CaO catalyst. Chem. Eng. Process., 2011, 50, 650-654.10.1016/j.cep.2011.04.001Search in Google Scholar
[3] Krisada N., Pisitpong I., Apanee L., Samai J., A comparative study of KOH/Al2O3 and KOH/NaY catalysts for biodiesel production via transesterification from palm oil. Renew. Energ., 2009, 34, 1145-1150.10.1016/j.renene.2008.06.015Search in Google Scholar
[4] Kulchanat P., Chokchai M., Chakrit T., Transesterification of palm oil with methanol in a reactive distillation column. Chem. Eng. Process., 2013, 70, 21-26.10.1016/j.cep.2013.05.011Search in Google Scholar
[5] Seyed M.S.A., Xinyu G., Giancarlo C., Flow-mode biodiesel production from palm oil using a pressurized microwave reactor. Green Process. Synth., DOI:10.1515/gps-2017-0116.10.1515/gps-2017-0116Search in Google Scholar
[6] Faezeh M., Masoud R., Arsalan P., Mostafa F., Stimulation of magnetic nanoparticles to intensify transesterification of soybean oil in micromixers for biodiesel production. Chem. Eng. Process., 2017, 122, 109-121.10.1016/j.cep.2017.10.007Search in Google Scholar
[7] Zhongqin T., Xiaofang Z., Yingying K., Huan D., Lelian S., Xiaoxiang H., et. al., Optimized microemulsion production of biodiesel over lipase-catalyzed transesterification of soybean oil by response surface methodology. Green Process. Synth., 2014, 3, 471-478.10.1515/gps-2014-0066Search in Google Scholar
[8] Canan K., Candan H., Akin B., Osman A., Sait E., Saydut A., Methyl ester of peanut Arachishypogaea L. Seed oil as a potential feedstock for biodiesel production. Renew. Energ., 2009, 34, 1257-1260.10.1016/j.renene.2008.10.002Search in Google Scholar
[9] El Boulifi N., Bouaid A., Martinez M., Aracil J., Process Optimization for Biodiesel Production from Corn Oil and Its Oxidative Stability. Int. J. Chem. Eng., 2010.10.1155/2010/518070Search in Google Scholar
[10] Mahbub M., Kaniz F., Maksudur R.K., Mazumder M.S.I., Islam M.A., Uddin Md. T., Rubber seed oil as a potential source for biodiesel production in Bangladesh. Fuel, 2011, 90, 2981-2986.10.1016/j.fuel.2011.05.020Search in Google Scholar
[11] Saurabh M.J., Parag R.G., Suresh Kumar S., Intensification of esterification of karanja oil for production of biodiesel using ultrasound assisted approach with optimization using response surface methodology. Chem. Eng. Process., 2018, 124, 186-198.10.1016/j.cep.2017.12.010Search in Google Scholar
[12] Ali Sabri B., Ahmad Zuhairi A., Keat-Teong L., Artificial neural network approach for modeling of ultrasound-assisted transesterification process of crude Jatropha oil catalyzed by heteropolyacid based catalyst. Chem. Eng. Process., 2014, 75, 31-37.10.1016/j.cep.2013.10.008Search in Google Scholar
[13] Radoslav M., Milan T., Ferenc M., Ferenc K., Mirko S., Aleksandra A., Reduction of free fatty acids in waste oil for biodiesel production by glycerolysis: investigation and optimization of process parameters. Green Process. Synth., doi.org/10.1515/gps-2017-0118Search in Google Scholar
[14] Xuejun L., Huayang H., Yujun W., Shenlin Z., Transesterification of soybean oil to biodiesel using SrO as a solid base catalyst. Catal. Comm., 2007, 8, 1107-1111.10.1016/j.catcom.2006.10.026Search in Google Scholar
[15] Meher L.C., Vidya Sagar D., Naik S.N., Technical aspects of biodiesel production by transesterification – a review. Renew. Sustain. Energy Rev., 2006, 10, 248-268.10.1016/j.rser.2004.09.002Search in Google Scholar
[16] Singh A.P.C., Sarma A.K., Modern heterogeneous catalysts for biodiesel production: A comprehensive review. Renew. Sust. Energ. Rev., 2011, 15, 4378-4399.10.1016/j.rser.2011.07.112Search in Google Scholar
[17] Deng S., Prafulla D.P., Optimization of Biodiesel Production from Edible and Non-Edible Vegetable Oils. Fuel, 2009, 88, 1302-1306.10.1016/j.fuel.2009.01.016Search in Google Scholar
[18] Joshua F., Production of Biodiesel (B100) from Jatropha Oil Using Sodium Hydroxide as Catalyst. J. Pet. Eng., 2012.Search in Google Scholar
[19] Carrero A., Vicente G., Rodríguez R., Linares., del Peso G.L., Hierarchical zeolites as catalysts for biodiesel production from Nannochloropsis microalga oil. Catal. Today, 2011, 167, 148-153.10.1016/j.cattod.2010.11.058Search in Google Scholar
[20] Yun H., Abdullah N.F., Basri M., Biodiesel production via transesterification of palm oil using NaOH/Al2O3 catalysts. Sains Malays., 2011, 40(6), 587-594.Search in Google Scholar
[21] Namrata D.G., Parag R.G., Synthesis and application of carbon based heterogeneous catalysts for ultrasound assisted biodiesel production. Green Process. Synth., 2015, 4(1), 17-30.10.1515/gps-2014-0079Search in Google Scholar
[22] Surbhi S., Ajay K.A., Rajendra P.B., Deepak K.T., Biodiesel production using heterogeneous catalysts. Bioresour. Technol., 2011, 102, 2151-2161.10.1016/j.biortech.2010.10.080Search in Google Scholar PubMed
[23] Niju S., MeeraSheriffa Begum K.M., Anantharaman N., Preparation of Biodiesel from Waste Frying Oil Using a Green and Renewable Solid Catalyst Derived from Egg Shell. Environ. Prog. Sustain. Energy, 2015, 34(1), 248-254.10.1002/ep.11939Search in Google Scholar
[24] Girish N., Niju S., MeeraSheriffa Begum K.M., Anantharaman N., Utilization of a cost-effective solid catalyst derived from natural white bivalve clamshell for transesterification of waste frying oil. Fuel, 2013, 111, 653-658.10.1016/j.fuel.2013.03.069Search in Google Scholar
[25] Anjana P.A., Niju S., MeeraSheriffa Begum K.M., Anantharaman N., Anand R., Babu D., Studies on biodiesel production from Pongamia oil using heterogeneous catalyst and its effect on diesel engine performance and emission characteristics. Biofuels, 2016, ISSN:1759-7269 (Print): 1759-7277.10.1080/17597269.2015.1138039Search in Google Scholar
[26] Jianwei Z., Qingming M., Preparation of KOH/CaO/C Supported Biodiesel Catalyst and Application Process. World J. Eng. Technol., 2014, 2, 184-191.10.4236/wjet.2014.23020Search in Google Scholar
[27] Shuli Y., Manhoe K., Steven O.S., Simon N.K.Y., Oil transesterification over calcium oxides modified with lanthanum. Appl. Catal. A-Gen., 2009, 360, 163-170.10.1016/j.apcata.2009.03.015Search in Google Scholar
[28] Watcharathamrongkul, Jongsomjit B., Phisalaphong M., Calcium oxide based catalysts for methanolysis of soybean oil Songklanakarin. J. Sci. Technol., 2010, 32(6), 627-634.Search in Google Scholar
[29] Chawalit N., Prangsinan T., Kunchana B., Ca and Zn mixed oxide as a heterogeneous base catalyst for transesterification of palm kernel oil. Appl. Catal. A-Gen., 2008, 341, 77-85.10.1016/j.apcata.2008.02.020Search in Google Scholar
[30] Zamberi M.M., Ani F.N., Biodiesel Production from High FFA Rubber Seed Oil Using Waste Cockles. ARPN J. Eng. App. Sci., 2016, 11(12), 7782-7787.Search in Google Scholar
[31] Monica C.G.A., Inmaculada J., Jose S.G., Josefa M.M., Ramon M., Enrique R., et al., CaO supported on mesoporous silicas as basic catalysts for transesterification reactions. Appl. Catal. A-Gen., 2008, 334, 35-4310.1016/j.apcata.2007.09.028Search in Google Scholar
[32] Yeshona S.S., Funmilayo F., Evariste B.G.K., Artificial neural networks: an efficient tool for modelling and optimization of biofuels production (a mini review). Biotechnol. Biotech. Eq., 2017, 31(2), 221-235.10.1080/13102818.2016.1269616Search in Google Scholar
[33] Ahmad H., Shahid A., Iqbal A., Jolius G., Muhammad H.A., Microwave Reinforced Transesterification of Rubber Seed Oil Using Waste Cement Clinker Catalyst. Curr. Nanosci., 2016, 12, 1-10.10.2174/1573413712666160314200607Search in Google Scholar
[34] Takeshi F., Fumio K., Hiroaki H., Ryosuke K., Masahide S., Noboru S., Transesterification of rapeseed oil with methanol using CaO and active carbon powders encapsulated microcapsule under the light irradiation. Appl. Catal. A-Gen., 2014, 475, 69-75.10.1016/j.apcata.2013.12.033Search in Google Scholar
[35] Ramadhas A.S., Jayaraj S., Muraleedharan C., Biodiesel production from high FFA rubber seed oil. Fuel, 2005, 84, 335-340.10.1016/j.fuel.2004.09.016Search in Google Scholar
[36] Lai F.C., Awais B., SuzanaY., Jiri J.K., Bawadi A., Majid M.A., Optimisation and Kinetic Studies of Acid Esterification of High Free Fatty Acid Rubber Seed Oil. Arab. J. Sci. Eng., 2016, 41, 2515-2526.10.1007/s13369-015-2014-1Search in Google Scholar
[37] Sunil K., Siddharth J., Harmesh K., Process parameter assessment of biodiesel production from Jatropha–algae oil blend by response surface methodology and artificial neural network. Energ. Source. Part A., 017, 39(22), 2119-2125.10.1080/15567036.2017.1403514Search in Google Scholar
[38] Obie F., Nur H., Yukihiko M., Artificial neural network modeling to predict biodiesel production in supercritical methanol and ethanol using spiral reactor. Procedia. Environ. Sci., 2015, 28, 214-223.10.1016/j.proenv.2015.07.028Search in Google Scholar
[39] Junaid A., Suzana Y., Awais B., Ruzaimah N.M.K., Study of fuel properties of rubber seed oil-based biodiesel. Energ. Convers. Manage., 2014, 78, 266-275.10.1016/j.enconman.2013.10.056Search in Google Scholar
[40] Jolius G., Shahid A., Chitra C.S., Liyana A.S., Nurul Hidayah M.G., Chin K.C., et al., Biodiesel Production from Rubber Seed Oil Using Activated Cement Clinker as Catalyst. Procedia. Eng., 2013, 53, 13-19.10.1016/j.proeng.2013.02.003Search in Google Scholar
[41] Sneha E.M., Anand R., MeeraSheriffa Begum K.M., Anantharaman N., Biodiesel production from waste cooking oil using KBr impregnated CaO as catalyst. Energ. Convers. Manage., 2015, 91, 442-450.10.1016/j.enconman.2014.12.031Search in Google Scholar
[42] Hanny J.B., Shizuko H., Biodiesel production from crude Jatrophacurcas L seed oil with a high content of free fatty acids. Bioresour. Technol, 2008, 99, 1716-1721.10.1016/j.biortech.2007.03.051Search in Google Scholar PubMed
[43] Medy C.N., Tchirioua E., Lynda E., Kouassi B.Y., Erwan L.G., François X.F., Biodiesel production from palm oil using sulfonated graphene catalyst. Renew. Energ., 2017, 106, 135-141.10.1016/j.renene.2017.01.024Search in Google Scholar
© 2019 Bharadwaj et al., published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 Public License.
Articles in the same Issue
- Regular Articles
- Studies on the preparation and properties of biodegradable polyester from soybean oil
- Flow-mode biodiesel production from palm oil using a pressurized microwave reactor
- Reduction of free fatty acids in waste oil for biodiesel production by glycerolysis: investigation and optimization of process parameters
- Saccharin: a cheap and mild acidic agent for the synthesis of azo dyes via telescoped dediazotization
- Optimization of lipase-catalyzed synthesis of polyethylene glycol stearate in a solvent-free system
- Green synthesis of iron oxide nanoparticles using Platanus orientalis leaf extract for antifungal activity
- Ultrasound assisted chemical activation of peanut husk for copper removal
- Room temperature silanization of Fe3O4 for the preparation of phenyl functionalized magnetic adsorbent for dispersive solid phase extraction for the extraction of phthalates in water
- Evaluation of the saponin green extraction from Ziziphus spina-christi leaves using hydrothermal, microwave and Bain-Marie water bath heating methods
- Oxidation of dibenzothiophene using the heterogeneous catalyst of tungsten-based carbon nanotubes
- Calcined sodium silicate as an efficient and benign heterogeneous catalyst for the transesterification of natural lecithin to L-α-glycerophosphocholine
- Synergistic effect between CO2 and H2O2 on ethylbenzene oxidation catalyzed by carbon supported heteropolyanion catalysts
- Hydrocyanation of 2-arylmethyleneindan-1,3-diones using potassium hexacyanoferrate(II) as a nontoxic cyanating agent
- Green synthesis of hydratropic aldehyde from α-methylstyrene catalyzed by Al2O3-supported metal phthalocyanines
- Environmentally benign chemical recycling of polycarbonate wastes: comparison of micro- and nano-TiO2 solid support efficiencies
- Medicago polymorpha-mediated antibacterial silver nanoparticles in the reduction of methyl orange
- Production of value-added chemicals from esterification of waste glycerol over MCM-41 supported catalysts
- Green synthesis of zerovalent copper nanoparticles for efficient reduction of toxic azo dyes congo red and methyl orange
- Optimization of the biological synthesis of silver nanoparticles using Penicillium oxalicum GRS-1 and their antimicrobial effects against common food-borne pathogens
- Optimization of submerged fermentation conditions to overproduce bioethanol using two industrial and traditional Saccharomyces cerevisiae strains
- Extraction of In3+ and Fe3+ from sulfate solutions by using a 3D-printed “Y”-shaped microreactor
- Foliar-mediated Ag:ZnO nanophotocatalysts: green synthesis, characterization, pollutants degradation, and in vitro biocidal activity
- Green cyclic acetals production by glycerol etherification reaction with benzaldehyde using cationic acidic resin
- Biosynthesis, characterization and antimicrobial activities assessment of fabricated selenium nanoparticles using Pelargonium zonale leaf extract
- Synthesis of high surface area magnesia by using walnut shell as a template
- Controllable biosynthesis of silver nanoparticles using actinobacterial strains
- Green vegetation: a promising source of color dyes
- Mechano-chemical synthesis of ammonia and acetic acid from inorganic materials in water
- Green synthesis and structural characterization of novel N1-substituted 3,4-dihydropyrimidin-2(1H)-ones
- Biodiesel production from cotton oil using heterogeneous CaO catalysts from eggshells prepared at different calcination temperatures
- Regeneration of spent mercury catalyst for the treatment of dye wastewater by the microwave and ultrasonic spray-assisted method
- Green synthesis of the innovative super paramagnetic nanoparticles from the leaves extract of Fraxinus chinensis Roxb and their application for the decolourisation of toxic dyes
- Biogenic ZnO nanoparticles: a study of blueshift of optical band gap and photocatalytic degradation of reactive yellow 186 dye under direct sunlight
- Leached compounds from the extracts of pomegranate peel, green coconut shell, and karuvelam wood for the removal of hexavalent chromium
- Enhancement of molecular weight reduction of natural rubber in triphasic CO2/toluene/H2O systems with hydrogen peroxide for preparation of biobased polyurethanes
- An efficient green synthesis of novel 1H-imidazo[1,2-a]imidazole-3-amine and imidazo[2,1-c][1,2,4]triazole-5-amine derivatives via Strecker reaction under controlled microwave heating
- Evaluation of three different green fabrication methods for the synthesis of crystalline ZnO nanoparticles using Pelargonium zonale leaf extract
- A highly efficient and multifunctional biomass supporting Ag, Ni, and Cu nanoparticles through wetness impregnation for environmental remediation
- Simple one-pot green method for large-scale production of mesalamine, an anti-inflammatory agent
- Relationships between step and cumulative PMI and E-factors: implications on estimating material efficiency with respect to charting synthesis optimization strategies
- A comparative sorption study of Cr3+ and Cr6+ using mango peels: kinetic, equilibrium and thermodynamic
- Effects of acid hydrolysis waste liquid recycle on preparation of microcrystalline cellulose
- Use of deep eutectic solvents as catalyst: A mini-review
- Microwave-assisted synthesis of pyrrolidinone derivatives using 1,1’-butylenebis(3-sulfo-3H-imidazol-1-ium) chloride in ethylene glycol
- Green and eco-friendly synthesis of Co3O4 and Ag-Co3O4: Characterization and photo-catalytic activity
- Adsorption optimized of the coal-based material and application for cyanide wastewater treatment
- Aloe vera leaf extract mediated green synthesis of selenium nanoparticles and assessment of their In vitro antimicrobial activity against spoilage fungi and pathogenic bacteria strains
- Waste phenolic resin derived activated carbon by microwave-assisted KOH activation and application to dye wastewater treatment
- Direct ethanol production from cellulose by consortium of Trichoderma reesei and Candida molischiana
- Agricultural waste biomass-assisted nanostructures: Synthesis and application
- Biodiesel production from rubber seed oil using calcium oxide derived from eggshell as catalyst – optimization and modeling studies
- Study of fabrication of fully aqueous solution processed SnS quantum dot-sensitized solar cell
- Assessment of aqueous extract of Gypsophila aretioides for inhibitory effects on calcium carbonate formation
- An environmentally friendly acylation reaction of 2-methylnaphthalene in solvent-free condition in a micro-channel reactor
- Aegle marmelos phytochemical stabilized synthesis and characterization of ZnO nanoparticles and their role against agriculture and food pathogen
- A reactive coupling process for co-production of solketal and biodiesel
- Optimization of the asymmetric synthesis of (S)-1-phenylethanol using Ispir bean as whole-cell biocatalyst
- Synthesis of pyrazolopyridine and pyrazoloquinoline derivatives by one-pot, three-component reactions of arylglyoxals, 3-methyl-1-aryl-1H-pyrazol-5-amines and cyclic 1,3-dicarbonyl compounds in the presence of tetrapropylammonium bromide
- Preconcentration of morphine in urine sample using a green and solvent-free microextraction method
- Extraction of glycyrrhizic acid by aqueous two-phase system formed by PEG and two environmentally friendly organic acid salts - sodium citrate and sodium tartrate
- Green synthesis of copper oxide nanoparticles using Juglans regia leaf extract and assessment of their physico-chemical and biological properties
- Deep eutectic solvents (DESs) as powerful and recyclable catalysts and solvents for the synthesis of 3,4-dihydropyrimidin-2(1H)-ones/thiones
- Biosynthesis, characterization and anti-microbial activity of silver nanoparticle based gel hand wash
- Efficient and selective microwave-assisted O-methylation of phenolic compounds using tetramethylammonium hydroxide (TMAH)
- Anticoagulant, thrombolytic and antibacterial activities of Euphorbia acruensis latex-mediated bioengineered silver nanoparticles
- Volcanic ash as reusable catalyst in the green synthesis of 3H-1,5-benzodiazepines
- Green synthesis, anionic polymerization of 1,4-bis(methacryloyl)piperazine using Algerian clay as catalyst
- Selenium supplementation during fermentation with sugar beet molasses and Saccharomyces cerevisiae to increase bioethanol production
- Biosynthetic potential assessment of four food pathogenic bacteria in hydrothermally silver nanoparticles fabrication
- Investigating the effectiveness of classical and eco-friendly approaches for synthesis of dialdehydes from organic dihalides
- Pyrolysis of palm oil using zeolite catalyst and characterization of the boil-oil
- Azadirachta indica leaves extract assisted green synthesis of Ag-TiO2 for degradation of Methylene blue and Rhodamine B dyes in aqueous medium
- Synthesis of vitamin E succinate catalyzed by nano-SiO2 immobilized DMAP derivative in mixed solvent system
- Extraction of phytosterols from melon (Cucumis melo) seeds by supercritical CO2 as a clean technology
- Production of uronic acids by hydrothermolysis of pectin as a model substance for plant biomass waste
- Biofabrication of highly pure copper oxide nanoparticles using wheat seed extract and their catalytic activity: A mechanistic approach
- Intelligent modeling and optimization of emulsion aggregation method for producing green printing ink
- Improved removal of methylene blue on modified hierarchical zeolite Y: Achieved by a “destructive-constructive” method
- Two different facile and efficient approaches for the synthesis of various N-arylacetamides via N-acetylation of arylamines and straightforward one-pot reductive acetylation of nitroarenes promoted by recyclable CuFe2O4 nanoparticles in water
- Optimization of acid catalyzed esterification and mixed metal oxide catalyzed transesterification for biodiesel production from Moringa oleifera oil
- Kinetics and the fluidity of the stearic acid esters with different carbon backbones
- Aiming for a standardized protocol for preparing a process green synthesis report and for ranking multiple synthesis plans to a common target product
- Microstructure and luminescence of VO2 (B) nanoparticle synthesis by hydrothermal method
- Optimization of uranium removal from uranium plant wastewater by response surface methodology (RSM)
- Microwave drying of nickel-containing residue: dielectric properties, kinetics, and energy aspects
- Simple and convenient two step synthesis of 5-bromo-2,3-dimethoxy-6-methyl-1,4-benzoquinone
- Biodiesel production from waste cooking oil
- The effect of activation temperature on structure and properties of blue coke-based activated carbon by CO2 activation
- Optimization of reaction parameters for the green synthesis of zero valent iron nanoparticles using pine tree needles
- Microwave-assisted protocol for squalene isolation and conversion from oil-deodoriser distillates
- Denitrification performance of rare earth tailings-based catalysts
- Facile synthesis of silver nanoparticles using Averrhoa bilimbi L and Plum extracts and investigation on the synergistic bioactivity using in vitro models
- Green production of AgNPs and their phytostimulatory impact
- Photocatalytic activity of Ag/Ni bi-metallic nanoparticles on textile dye removal
- Topical Issue: Green Process Engineering / Guest Editors: Martine Poux, Patrick Cognet
- Modelling and optimisation of oxidative desulphurisation of tyre-derived oil via central composite design approach
- CO2 sequestration by carbonation of olivine: a new process for optimal separation of the solids produced
- Organic carbonates synthesis improved by pervaporation for CO2 utilisation
- Production of starch nanoparticles through solvent-antisolvent precipitation in a spinning disc reactor
- A kinetic study of Zn halide/TBAB-catalysed fixation of CO2 with styrene oxide in propylene carbonate
- Topical on Green Process Engineering
Articles in the same Issue
- Regular Articles
- Studies on the preparation and properties of biodegradable polyester from soybean oil
- Flow-mode biodiesel production from palm oil using a pressurized microwave reactor
- Reduction of free fatty acids in waste oil for biodiesel production by glycerolysis: investigation and optimization of process parameters
- Saccharin: a cheap and mild acidic agent for the synthesis of azo dyes via telescoped dediazotization
- Optimization of lipase-catalyzed synthesis of polyethylene glycol stearate in a solvent-free system
- Green synthesis of iron oxide nanoparticles using Platanus orientalis leaf extract for antifungal activity
- Ultrasound assisted chemical activation of peanut husk for copper removal
- Room temperature silanization of Fe3O4 for the preparation of phenyl functionalized magnetic adsorbent for dispersive solid phase extraction for the extraction of phthalates in water
- Evaluation of the saponin green extraction from Ziziphus spina-christi leaves using hydrothermal, microwave and Bain-Marie water bath heating methods
- Oxidation of dibenzothiophene using the heterogeneous catalyst of tungsten-based carbon nanotubes
- Calcined sodium silicate as an efficient and benign heterogeneous catalyst for the transesterification of natural lecithin to L-α-glycerophosphocholine
- Synergistic effect between CO2 and H2O2 on ethylbenzene oxidation catalyzed by carbon supported heteropolyanion catalysts
- Hydrocyanation of 2-arylmethyleneindan-1,3-diones using potassium hexacyanoferrate(II) as a nontoxic cyanating agent
- Green synthesis of hydratropic aldehyde from α-methylstyrene catalyzed by Al2O3-supported metal phthalocyanines
- Environmentally benign chemical recycling of polycarbonate wastes: comparison of micro- and nano-TiO2 solid support efficiencies
- Medicago polymorpha-mediated antibacterial silver nanoparticles in the reduction of methyl orange
- Production of value-added chemicals from esterification of waste glycerol over MCM-41 supported catalysts
- Green synthesis of zerovalent copper nanoparticles for efficient reduction of toxic azo dyes congo red and methyl orange
- Optimization of the biological synthesis of silver nanoparticles using Penicillium oxalicum GRS-1 and their antimicrobial effects against common food-borne pathogens
- Optimization of submerged fermentation conditions to overproduce bioethanol using two industrial and traditional Saccharomyces cerevisiae strains
- Extraction of In3+ and Fe3+ from sulfate solutions by using a 3D-printed “Y”-shaped microreactor
- Foliar-mediated Ag:ZnO nanophotocatalysts: green synthesis, characterization, pollutants degradation, and in vitro biocidal activity
- Green cyclic acetals production by glycerol etherification reaction with benzaldehyde using cationic acidic resin
- Biosynthesis, characterization and antimicrobial activities assessment of fabricated selenium nanoparticles using Pelargonium zonale leaf extract
- Synthesis of high surface area magnesia by using walnut shell as a template
- Controllable biosynthesis of silver nanoparticles using actinobacterial strains
- Green vegetation: a promising source of color dyes
- Mechano-chemical synthesis of ammonia and acetic acid from inorganic materials in water
- Green synthesis and structural characterization of novel N1-substituted 3,4-dihydropyrimidin-2(1H)-ones
- Biodiesel production from cotton oil using heterogeneous CaO catalysts from eggshells prepared at different calcination temperatures
- Regeneration of spent mercury catalyst for the treatment of dye wastewater by the microwave and ultrasonic spray-assisted method
- Green synthesis of the innovative super paramagnetic nanoparticles from the leaves extract of Fraxinus chinensis Roxb and their application for the decolourisation of toxic dyes
- Biogenic ZnO nanoparticles: a study of blueshift of optical band gap and photocatalytic degradation of reactive yellow 186 dye under direct sunlight
- Leached compounds from the extracts of pomegranate peel, green coconut shell, and karuvelam wood for the removal of hexavalent chromium
- Enhancement of molecular weight reduction of natural rubber in triphasic CO2/toluene/H2O systems with hydrogen peroxide for preparation of biobased polyurethanes
- An efficient green synthesis of novel 1H-imidazo[1,2-a]imidazole-3-amine and imidazo[2,1-c][1,2,4]triazole-5-amine derivatives via Strecker reaction under controlled microwave heating
- Evaluation of three different green fabrication methods for the synthesis of crystalline ZnO nanoparticles using Pelargonium zonale leaf extract
- A highly efficient and multifunctional biomass supporting Ag, Ni, and Cu nanoparticles through wetness impregnation for environmental remediation
- Simple one-pot green method for large-scale production of mesalamine, an anti-inflammatory agent
- Relationships between step and cumulative PMI and E-factors: implications on estimating material efficiency with respect to charting synthesis optimization strategies
- A comparative sorption study of Cr3+ and Cr6+ using mango peels: kinetic, equilibrium and thermodynamic
- Effects of acid hydrolysis waste liquid recycle on preparation of microcrystalline cellulose
- Use of deep eutectic solvents as catalyst: A mini-review
- Microwave-assisted synthesis of pyrrolidinone derivatives using 1,1’-butylenebis(3-sulfo-3H-imidazol-1-ium) chloride in ethylene glycol
- Green and eco-friendly synthesis of Co3O4 and Ag-Co3O4: Characterization and photo-catalytic activity
- Adsorption optimized of the coal-based material and application for cyanide wastewater treatment
- Aloe vera leaf extract mediated green synthesis of selenium nanoparticles and assessment of their In vitro antimicrobial activity against spoilage fungi and pathogenic bacteria strains
- Waste phenolic resin derived activated carbon by microwave-assisted KOH activation and application to dye wastewater treatment
- Direct ethanol production from cellulose by consortium of Trichoderma reesei and Candida molischiana
- Agricultural waste biomass-assisted nanostructures: Synthesis and application
- Biodiesel production from rubber seed oil using calcium oxide derived from eggshell as catalyst – optimization and modeling studies
- Study of fabrication of fully aqueous solution processed SnS quantum dot-sensitized solar cell
- Assessment of aqueous extract of Gypsophila aretioides for inhibitory effects on calcium carbonate formation
- An environmentally friendly acylation reaction of 2-methylnaphthalene in solvent-free condition in a micro-channel reactor
- Aegle marmelos phytochemical stabilized synthesis and characterization of ZnO nanoparticles and their role against agriculture and food pathogen
- A reactive coupling process for co-production of solketal and biodiesel
- Optimization of the asymmetric synthesis of (S)-1-phenylethanol using Ispir bean as whole-cell biocatalyst
- Synthesis of pyrazolopyridine and pyrazoloquinoline derivatives by one-pot, three-component reactions of arylglyoxals, 3-methyl-1-aryl-1H-pyrazol-5-amines and cyclic 1,3-dicarbonyl compounds in the presence of tetrapropylammonium bromide
- Preconcentration of morphine in urine sample using a green and solvent-free microextraction method
- Extraction of glycyrrhizic acid by aqueous two-phase system formed by PEG and two environmentally friendly organic acid salts - sodium citrate and sodium tartrate
- Green synthesis of copper oxide nanoparticles using Juglans regia leaf extract and assessment of their physico-chemical and biological properties
- Deep eutectic solvents (DESs) as powerful and recyclable catalysts and solvents for the synthesis of 3,4-dihydropyrimidin-2(1H)-ones/thiones
- Biosynthesis, characterization and anti-microbial activity of silver nanoparticle based gel hand wash
- Efficient and selective microwave-assisted O-methylation of phenolic compounds using tetramethylammonium hydroxide (TMAH)
- Anticoagulant, thrombolytic and antibacterial activities of Euphorbia acruensis latex-mediated bioengineered silver nanoparticles
- Volcanic ash as reusable catalyst in the green synthesis of 3H-1,5-benzodiazepines
- Green synthesis, anionic polymerization of 1,4-bis(methacryloyl)piperazine using Algerian clay as catalyst
- Selenium supplementation during fermentation with sugar beet molasses and Saccharomyces cerevisiae to increase bioethanol production
- Biosynthetic potential assessment of four food pathogenic bacteria in hydrothermally silver nanoparticles fabrication
- Investigating the effectiveness of classical and eco-friendly approaches for synthesis of dialdehydes from organic dihalides
- Pyrolysis of palm oil using zeolite catalyst and characterization of the boil-oil
- Azadirachta indica leaves extract assisted green synthesis of Ag-TiO2 for degradation of Methylene blue and Rhodamine B dyes in aqueous medium
- Synthesis of vitamin E succinate catalyzed by nano-SiO2 immobilized DMAP derivative in mixed solvent system
- Extraction of phytosterols from melon (Cucumis melo) seeds by supercritical CO2 as a clean technology
- Production of uronic acids by hydrothermolysis of pectin as a model substance for plant biomass waste
- Biofabrication of highly pure copper oxide nanoparticles using wheat seed extract and their catalytic activity: A mechanistic approach
- Intelligent modeling and optimization of emulsion aggregation method for producing green printing ink
- Improved removal of methylene blue on modified hierarchical zeolite Y: Achieved by a “destructive-constructive” method
- Two different facile and efficient approaches for the synthesis of various N-arylacetamides via N-acetylation of arylamines and straightforward one-pot reductive acetylation of nitroarenes promoted by recyclable CuFe2O4 nanoparticles in water
- Optimization of acid catalyzed esterification and mixed metal oxide catalyzed transesterification for biodiesel production from Moringa oleifera oil
- Kinetics and the fluidity of the stearic acid esters with different carbon backbones
- Aiming for a standardized protocol for preparing a process green synthesis report and for ranking multiple synthesis plans to a common target product
- Microstructure and luminescence of VO2 (B) nanoparticle synthesis by hydrothermal method
- Optimization of uranium removal from uranium plant wastewater by response surface methodology (RSM)
- Microwave drying of nickel-containing residue: dielectric properties, kinetics, and energy aspects
- Simple and convenient two step synthesis of 5-bromo-2,3-dimethoxy-6-methyl-1,4-benzoquinone
- Biodiesel production from waste cooking oil
- The effect of activation temperature on structure and properties of blue coke-based activated carbon by CO2 activation
- Optimization of reaction parameters for the green synthesis of zero valent iron nanoparticles using pine tree needles
- Microwave-assisted protocol for squalene isolation and conversion from oil-deodoriser distillates
- Denitrification performance of rare earth tailings-based catalysts
- Facile synthesis of silver nanoparticles using Averrhoa bilimbi L and Plum extracts and investigation on the synergistic bioactivity using in vitro models
- Green production of AgNPs and their phytostimulatory impact
- Photocatalytic activity of Ag/Ni bi-metallic nanoparticles on textile dye removal
- Topical Issue: Green Process Engineering / Guest Editors: Martine Poux, Patrick Cognet
- Modelling and optimisation of oxidative desulphurisation of tyre-derived oil via central composite design approach
- CO2 sequestration by carbonation of olivine: a new process for optimal separation of the solids produced
- Organic carbonates synthesis improved by pervaporation for CO2 utilisation
- Production of starch nanoparticles through solvent-antisolvent precipitation in a spinning disc reactor
- A kinetic study of Zn halide/TBAB-catalysed fixation of CO2 with styrene oxide in propylene carbonate
- Topical on Green Process Engineering

