Abstract
Large amounts of spent mercury catalyst (SMC) produced in producing polyvinyl chloride (PVC) has a great influence on the environment. In this work, microwave and ultrasonic spray were applied to regenerate the carrier of SMC. The optimal experimental conditions were the regeneration temperature of 900°C and regeneration time of 60 min. Subsequently, the textures of pores and mercury content, morphology structure and surface functional groups of SMC and the regenerated activated carbon (RAC) were characterized by N2 adsorption and desorption isotherms, ICP, SEM, EDS and FTIR. Additionally, the adsorption behaviors of RAC on methylene orange (MO) and congo red (CR) were explored to study the mechanisms. The results of kinetics and isotherm showed that experimental data were fitted well with the pseudo second-order and Langmuir isotherm model, respectively. The maximum adsorptions of MO and CR were 529 and 301 mg/g at 323 K. The thermodynamic results illustrated that the adsorption MO and CR onto RAC was spontaneous and endothermic. The results demonstrated that RAC prepared from SMC by microwave heating and ultrasonic spray could realize the comprehensive utilization of waste resources.
- Abbreviations
- BET
Brunauer-Emmett-Teller
- CR
congo red
- DFT
Density Functional Theory
- MB
methylene blue
- MO
methylene orange
- PVC
polyvinyl chloride
- R2
correlation coefficient
- RAC
regenerated activated carbon
- SMC
spent mercury catalyst
- VCM
vinyl chloride monomer
1 Introduction
Polyvinyl chloride (PVC) is the most commonly used thermoplastic resin [1], following polyethylene and polypropylene. At the same time, the global demand keeps on increasing [2], and PVC products can now be manufactured into hard products. In addition, it plays an important role in the fields of industry, agriculture, national defense and building owing to its low cost, persistence and excellent physico-chemistry properties [[3], [4], [5]. Vinyl chloride monomer (VCM) is mainly used for PVC synthesis, and the preparation technology of PVC is distinctive in China [6], [7], [8]. As a catalyst for the composition of VCM, large numbers of mercuric chloride catalyst are applied in PVC industries 9]. Therefore, a large amount of spent mercury catalyst (SMC) is produced due to the final loss and deactivation [10]. SMCs contain trace amounts of mercury, and any form of mercury is considered as toxic [11]. Mercury-containing wastes pose potential threats to the living environment, the central nervous system of organisms and vital organs [[12], [13], [14], [15]. Consequently, there is an urgent need to find an effective way to deal with SMC. Acetylene hydrochlorination is an important coal process for manufacturing vinyl chloride on a commercial scale, particularly in developing countries, such as China and India, where coal reserves are abundant but oil is lacking 8], [[16]. According to the energy structure, most mercury catalysts are based on the coal-based activated carbon, that is to say, mercuric chloride is loaded on the activated carbon 17]. Acetylene hydrochlorination has been largely replaced by ethane-based process in many developed countries, but it is still used in locations where coal resources are relatively rich. Therefore, the production of VCM mainly takes mercuric chloride as a catalyst in our country. Over a period of time, the mercuric chloride catalyst is deactivated and becomes SMC. Due to the dissolution, consumption and heating sublimation during application, the mercury content in SMC is very low, making itit difficult to recover mercury [18]. However, the activated carbon loaded on the catalyst has excellent adsorption performance, so it is very meaningful to regenerate SMC to obtain activated carbon.
The mercury chloride catalysts are deactivated after use, the pore channels of support activated carbon are blocked, so it is necessary to restore the pore structure of activated carbon by regeneration methods. At present, the regeneration methods of activated carbon are widely used at home and abroad, including thermal regeneration [19], biological regeneration, ultrasonic regeneration, microwave regeneration [20] and wet oxidation processes [21]. Recently, the use of microwave heating to regenerate the spent activated carbon was mentioned in some studies [22]. Compared with the conventional thermal regeneration technology, microwave regeneration could save time and energy to obtain high extraction efficiency and operate easily and conveniently [23]. Moreover, the activated carbon was heated internally and has good microwave adsorption properties. The heat and energy generated through high-frequency internal dipole rotation directly penetrate into the activated carbon during microwave heating process. Furthermore, some past studies have mentioned that the regenerated activated carbon (RAC) via microwave heating method can develop new pore structures and increase surface area [[24], [25], [26]. Accordingly, microwave regeneration can accelerate the efficiency and have good effects on spent activated carbon, which can effectively remove the adsorbed pollutants. In addition, the ultrasonic spray can be induced during the regeneration process. Compared with the conventional high temperature steam, ultrasonic spray can be generated through the ultrasonic device at normal temperature. Therefore, microwave heating combined with ultrasonic spray can reduce the energy output 20].
In the current study, SMC produced in PVC production was regenerated by the combination of microwave heating and ultrasonic spray. Different experimental conditions have great influence on MB adsorption capability and RAC yield. The SMC and RAC were characterized by various technologies. Furthermore, the adsorption behavior of RAC was explored with methylene orange (MO) and congo red (CR) as pollutants. The findings of this study can be used for further understanding of the treatment effect of microwave heating and ultrasonic spray and comprehensive use of waste resources.
2 Materials and methods
2.1 Materials
The SMCs used in this work were obtained from a chemical plant for producing PVC (Huize, Yunnan, China). The mercury catalyst supported activated carbon was prepared by coal and used as raw material, which presented columnar particles. All chemical reagents, such as HCl, NaCl and HNO3 were purchased from Tianjin Reagent Chemicals Co. Ltd., Tianjin, China. All materials were used in this study without further purification. The chemical properties of MO and CR are shown in Table 1.
The properties of MO and CR.
| Dye | Parameters | Character/value |
|---|---|---|
| MO | Chemical structure |  |
| Chemical formula | CHNSONa | |
| Molecular weight | 327.33 g/mol | |
| CR | Chemical structure |  |
| Chemical formula | C32H22N6Na2O6S2 | |
| Molecular weight | 696.68 g/mol |
2.2 Regeneration of SMC
Experiments were carried out in a self-made microwave furnace (Key Laboratory of Unconventional Metallurgy, Kunming University of Science and Technology, Kunming, Yunnan, China). The frequency of microwave oven was 2.5 GHz and the power intensity was 1000 W. A certain amount of SMC was placed into microwave oven, in which N2 with a purity of 99.9% was discharged at 100 l/h, and the flow rate of ultrasound spray was 30 l/h. During the regeneration experiments, different parameters of microwave oven were set to get different samples. The obtained RAC was placed inside a vacuum drying oven at 85°C for 3 h and then crushed These were then stored in sealed bags for further characterization. To prevent harmful gas leakage, the solution adsorption was used to treat the exhaust gas. The experimental device diagram was showed in Figure 1.

The diagram of the experimental setup.
2.3 Characterization of SMC and RAC
Samples of SMC and RAC were characterized by various techniques as described below. The yield (Y) of RAC was calculated using the following formula:
where W1 and W2 are the weights of RAC and SMC, respectively. The MB adsorption capacity was determined using the Standard Testing Methods of PR China (GB/T 7702.6-2008) [27]. The pore and textural properties were characterized at 77 K (Quant chrome, Autosorb-1-C). Before the gas adsorption test, the samples were degassed at 300°C for 10 h under vacuum condition. The surface area was calculated using the Brunauer-Emmett-Teller (BET) equation, and the pore size distribution was analyzed using the Density Functional Theory (DFT) model. The total pore volumes were evaluated at the relative pressure of 0.99, which was the equivalent liquid volume of nitrogen. The surface texture and morphology was observed by a SEM (Phenom-World-ProX, the Netherlands), which equipped with EDS (Phenom-World-ProX, the Netherlands) under an acceleration voltage of 15 KV. FTIR was used to observe the functional groups by using a NicoletiS10 spectrophotometer (Thermo Fisher Scientific, USA). The FTIR spectra were obtained between 4000 and 400 cm−1.
2.4 Determination of mercury content
The mercury content was determined using the Standard Testing Methods of PR China (GB/T31530-2015) [18]. About 1 g SMC and RAC was placed into a 250-ml Erlenmeyer flask, to which 15 ml of HCl, 5 ml of HNO3 and 20 ml of 100 mg/l NaCl solution. The solution was heated to boiling for 15 min and then cooled to room temperature, which was diluted with distilled water to 500 ml. The blank experiment was also carried out. The mercury concentration in the filtrate was determined by inductively coupled plasma optical emission spectrometry (ICP-OES, Thermo Scientific iCAP 7200).
2.5 Adsorption study
The adsorption capacity of RAC was determined by using MO and CR as organic pollutants, which carried were out in a thermostatic oscillator that the temperature ranges between 303 K and 323 K. The MO and CR solution were prepared by diluting the 1000 mg/l MO and CR solution with distilled water. One hundred milliliter MO solution and CR solution were added into 250-ml Erlenmeyer flasks with 0.05 g RAC, respectively. The remaining concentration of MO and CR solution were tested by UV-vis spectrophotometer (Shimadzu UV2600). To obtain the equilibrium concentration, Erlenmeyer flasks were placed in the thermostatic oscillator with shaking speed of 250 rpm for 5 h. The equilibrium concentration was used in the following tests. The equilibrium adsorption capacity (qe, mg/g) is calculated according to Equation (2)
where C0 and Ce are the initial concentration and equilibrium concentration (mg/l), respectively, V is the volume of the dye solution (ml) and m is the mass of RAC (g).
3 Results and discussion
3.1 Effect of regeneration temperature on MB value and yield of RAC
Regeneration temperature was a significant parameter. The effect of regeneration temperature on MB value and yield of RAC was investigated when the temperature was in the range from 500°C to 900°C and regeneration time was 60 min. As shown in Figure 2, the overall trend of the MB value increased and the yield declined. The MB value reached to a maximum of 210 mg/g when the regeneration temperature was 900°C. This can be explained as follows: the small part of the pores were constructed when the regeneration temperature was low. The MB value increased along with the increase of temperature. It was clearly revealed that some substances in SMC were volatilized and decomposed under nitrogen atmosphere and ultrasonic spray and more pores formed. Thus, the pore channel of activated carbon can be restored with microwave heating and ultrasonic spray. A temperature of 900°C was selected and used for the following experiments.
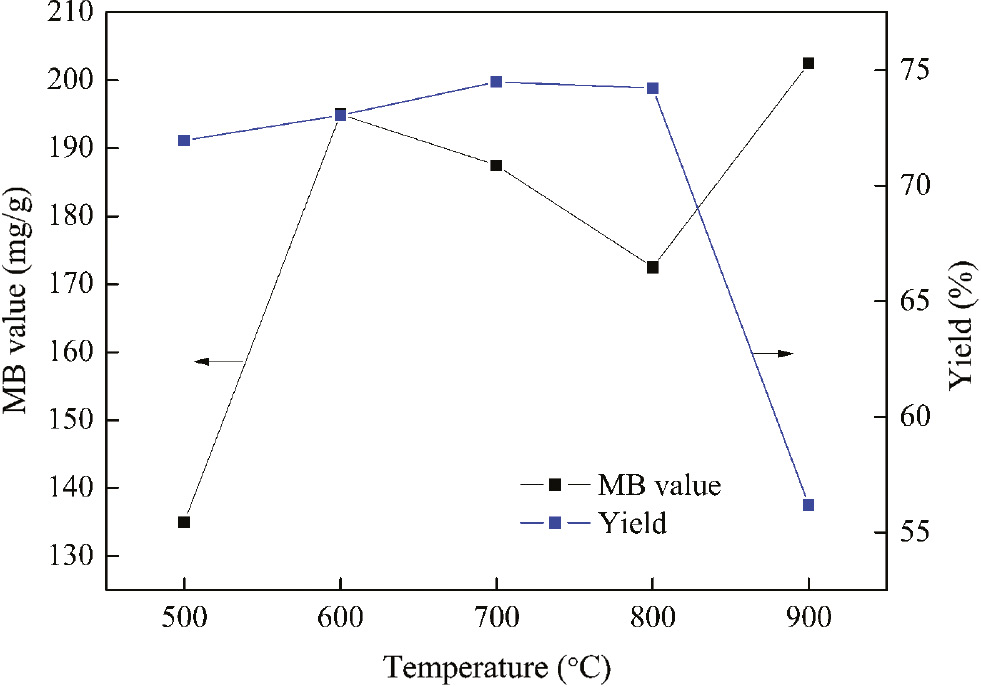
The effect of regeneration temperature on MB value and yield of RAC.
3.2 Effect of regeneration time on MB value and yield of RAC
The effects of regeneration time on MB value and yield of RAC was investigated under a regeneration temperature of 900°C. As shown in Figure 3, the MB value increased firstly and then decreased with the increase of regeneration time. This can be attributed to the fact that the increase of regeneration time was beneficial to the volatilization of the adsorbed substances in SMC. However, with the extension of regeneration time, the MB value decreased gradually. Exceeding a certain time, the pores of RAC would be damaged and the surface will be burned. Considering comprehensively, 60 min was selected as the regeneration time. According to the above analysis, the optimum conditions of regeneration experiments included 900°C of regeneration temperature and 60 min of regeneration time.

The effect of regeneration time on MB value and yield of RAC.
3.3 Textural characteristics and mercury content
Figure 4 shows the N2 adsorption-desorption isotherms of the SMC and RAC samples. According to the IUPAC classification, both isotherms can be classified to be a type IV [28]. When the relative pressure was between 0.4 and 0.9, both samples exhibited hysteresis loops. The N2 adsorption-desorption isotherm of RAC presented a wider hysteresis loops than that of SMC due to the capillary condensation occurred. The pore structure details and mercury content of two samples were listed in Table 2. The obtained RAC possessed a specific surface area (801.6 m2/g) and total pore volume that was comparatively higher to SMC. It can be explained that the combination of microwave heating and ultrasonic spray was beneficial to develop new gaps. The average pore size of RAC was estimated to be 1.08 nm, which was lower than before regeneration, indicating that microporous morphology of RAC. Furthermore, the mercury contents of SMC and RAC were 23.70 and 0.46 mg/l. It can be explained that the mercuric chloride easily sublimated and evaporated at a high temperature, and most of the mercury was taken away under the atmosphere of N2 and ultrasonic spray. Through experimental data analysis, the microwave and ultrasonic spray-assisted regeneration of SMC can effectively develop a new pore structure.

The N2 adsorption isotherm of SMC and RAC.
The pore structure details and mercury contents of SMC and RAC.
| Characteristic | SMC | RAC |
|---|---|---|
| Specific surface area (m2/g) | 239.8 | 801.6 |
| Total pore volume (ml/g) | 0.20 | 0.43 |
| Average pore size (nm) | 2.78 | 1.08 |
| Mercury content (mg/l) | 23.70 | 0.46 |
3.4 SEM and EDS analysis
The SEM was carried out to evaluate the surface morphology and texture of materials [29]. As can be seen in Figure 5A, the surface of SMC was relatively rough and irregular and there was no apparent pore structure. After regeneration (Figure 5B), the surface of RAC appeared obvious pore structure and the impurities on the surface of the activated carbon reduced. As can be seen from the EDS results (Figure 6), there was almost no mercury on the surface of RAC, proving that the mercury was effectively removed. This result is consistent with the determination of mercury content. Therefore, microwave heating and ultrasonic spray-assisted regeneration of activated carbon was an effective method.

The SEM micrographs of SMC (A), RAC (B).

The EDS images of SMC (A), RAC (B).
3.5 FTIR analysis
The FTIR technique was used to examine the important functional groups existed on the surface of SMC and RAC (Figure 7). As can be seen from Figure 7, the FTIR spectra of the two samples are roughly similar [30]. Both samples had a same clear high intense peak at 3433 cm−1, and this may be water and existed O-H and N-H stretching vibration, respectively. The peak around 2924 cm−1 corresponded to the stretching vibration of the C-H group and caused by CO2 in the air. The band fund at 2852 cm−1 can be assumed as the C-H group. The absorption peak at about 2340 cm−1 was the asymmetric expansion vibration absorption of carbon dioxide. The peak at 1636 cm−1 can be attributed to the aromatic C=C bond. The symmetric bending of CH3 was detected in the peak with approximate maximum around 1457 cm−1 [31]. The peak at about 1100 cm−1 was attributed to the C-O bond. The peak at about 400–800 cm−1 corresponded to vibrations of iron and zinc compounds [32]. It can be seen that SMC has a stronger vibrational peak at 1100 and 1636 cm−1, which can indirectly prove that the functional groups on RAC have been weakened and some substances have been effectively removed.
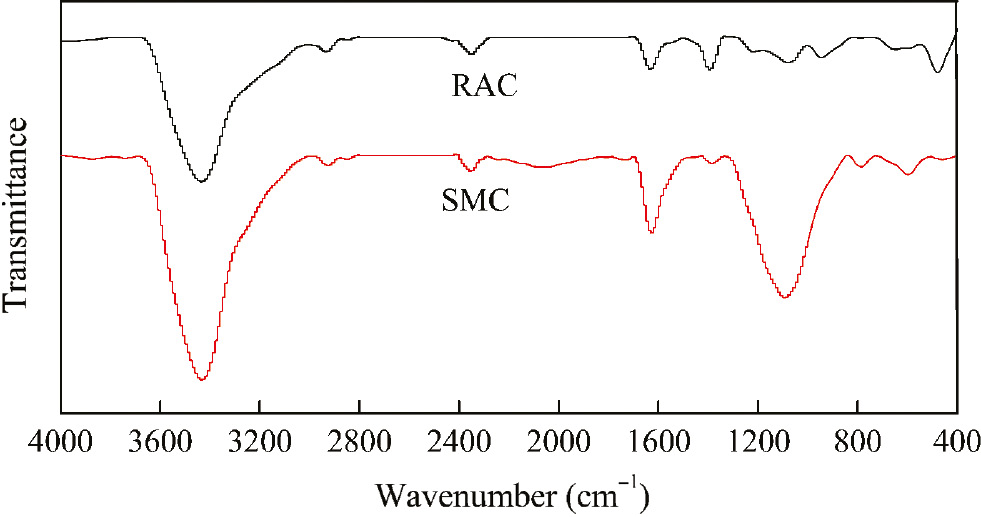
The FTIR spectra of SMC and RAC.
3.6 Adsorption kinetics
To check the mechanism of adsorption, the kinetic experiments were performed using 0.05 g RAC with 100 ml MO and CR solution at 313 K. The removal efficiencies of the MO and CR solution with different initial concentrations were investigated. The results are presented in Figure 8. It can be found that the removal efficiency of MO and CR solution almost reached equilibrium at 100 min. Three models of pseudo first-order, pseudo second-order and intra-particle diffusion were employed to obtain the important parameters, respectively. The correlation coefficients (R2) were used to evaluate the conformity between the experimental data and the model predicted values.

The removal efficiencies of the MO (A) and CR (B) solution with different initial concentrations at 313 K.
The pseudo first-order can be expressed as follows (3) [33]:
where qt was the adsorption capacity at time t (mg/g), the k1 is the rate constant [g/(mg⋅min)].
The pseudo second-order model can be illustrated according the following equation (4) [34]:
where k2 is the rate constant [g/(mg⋅min)].
The intra-particle diffusion model can be defined by the following equation (5) [35]:
where kdif is the intra-particle diffusion rate constant (mg/(g⋅min1/2), and c is the intercept (mg/g) relating to the thickness of boundary layer.
The obtained kinetic parameters were given in Table 3. As can be seen, the values of R2 for three kinetic models are satisfactory. However, the adsorption process of MO and CR can be described with pseudo second-order due to the values of R2 were close to 1 and the calculated adsorption values were consistent with the experimental data. The pseudo second-order indicated that the chemisorption played an important role in the rate limiting step [36], where the dye molecules attached to the surface of RAC by the transferring or sharing of the electron [37]. The adsorption was a complex process. Although the experimental data fitted well with pseudo second-order, the results obtained from this model could not predicate the diffusion process. Furthermore, two steps were studied. The first stage mainly depended on the surface pore structures of RAC. The adsorption appeared to be easing in the second stage due to the intraparticle diffusion was a rate-limiting process. In general, the fitted line passed through origin, which indicated that the pore diffusion controlled the adsorption processes. As can be seen the data in Table 3, both lines did not pass through the origin. This explains that the transfer rates in two stages are not consistent. It confirmed that the intraparticle diffusion was not the only rate-limiting step. Similar results have been reported in previous literatures [38]. The pseudo second-order for MO and CR adsorption was showed in Figure 9.
Kinetic parameters for adsorption of MO and CR adsorption onto RAC.
| Dye | Pseudo first-order | Pseudo second-order | Intra-particle diffusion | ||||||
|---|---|---|---|---|---|---|---|---|---|
| k1 | qt | R2 | k2 | qe | R2 | kdif | c | R2 | |
| MO | 0.0151 | 60.6 | 0.979 | 0.0007 | 469.4 | 0.999 | 4.83 | 405.2 | 0.996 |
| CR | 0.0172 | 134.1 | 0.985 | 0.0003 | 324.7 | 0.997 | 22.8 | 172.8 | 0.979 |

The experimental data fitting analysis by pseudo second-order model for MO and CR dye solution.
3.7 Adsorption isotherm
The adsorption equilibrium isotherm can reveal the status of the adsorbate and adsorbent at a confirmed temperature. The isotherm studies were conducted with the MO and CR solution with initial concentration of 200–400 mg/l and 100–300 mg/l. The adsorption process was conducted at the temperature of 303 K, 313 K and 323 K. Three isotherm equations were considered to study the mechanism and calculate the maximum adsorption capacity.
The Langmuir isotherm is applied in the following form (6) [39]:
where q is the maximum adsorption capacity of RAC (mg/g) and KL is the constant of Langmuir isotherm related to energy of adsorption.
The Freundlich isotherm is represented as (7) [40] follows:
where K and n are the constants of the Freundlich isotherm.
The Tempkin isotherm is given as the following equation (8):
where a and b are the constants of Tempkin isotherm.
The calculated parameters according to three models are listed in Table 4. From the results, we can see that the Langmuir models fitted the results slightly better than the other two models and the values of R2 are greater than 0.98. Accordingly, the Langmuir isotherm can be used to describe the adsorption behavior to MO and CR dye solution onto RAC. The Langmuir model suggests that the adsorption can be attributed to the monolayer coverage and the surface is uniform [41]. However, the data of Freundlich and Temkin models did not fit well with actual experimental data due to its low R2. The data were fitted with Langmuir isotherm as shown in Figure 10.
The isotherm constants for adsorption of the MO and CR dye solution onto RAC.
| Temperature (K) | Langmuir | Freundlich | Temkin | ||||||
|---|---|---|---|---|---|---|---|---|---|
| q | KL | R2 | K | n | R2 | a | b | R2 | |
| MO | |||||||||
| 303 | 492.6 | 72.9 | 0.983 | 256.9 | 7.8 | 0.914 | 215.4 | 54.1 | 0.927 |
| 313 | 518.1 | 145.6 | 0.992 | 297.4 | 9.9 | 0.938 | 273.6 | 43.0 | 0.934 |
| 323 | 529.1 | 185.9 | 0.999 | 311.9 | 9.4 | 0.946 | 288.7 | 47.6 | 0.961 |
| CR | |||||||||
| 303 | 289.9 | 10.8 | 0.999 | 152.7 | 11.6 | 0.776 | 146.7 | 17.7 | 0.734 |
| 313 | 295.9 | 12.1 | 0992 | 115.1 | 5.7 | 0.802 | 77.8 | 39.1 | 0.757 |
| 323 | 301.2 | 15.1 | 0.984 | 87.9 | 4.3 | 0.969 | 41.3 | 43.5 | 0.718 |

The experimental data fitting analysis by Langmuir isotherm for MO (A) and CR (B) dye solution at 303 K, 313 K, 323 K.
Table 5 summarizes the maximum adsorption capacity of the MO and CR dye solution onto different adsorbents with previous studies. From Table 5, it can be established that the RAC regenerated from the SMC exhibited much higher adsorption capacity, and the maximum MO and CR adsorption capacities were 529 and 301 mg/g, confirming that the microwave and ultrasonic spray-assisted method was beneficial to the regeneration.
The comparison of the maximum adsorption capacities of MO and CR onto the different adsorbents.
| Adsorbents | Dyes | Maximum adsorption capacity (mg/g) | Reference |
|---|---|---|---|
| RAC regenerated from SMC | MO | 529 | This work |
| Activated carbon of lignin | MO | 300 | [42] |
| Activated carbon of phragmites australis | MO | 238 | [43] |
| Activated carbon from date pits | MO | 434 | [44] |
| commercially activated carbon | MO | 46 | [45] |
| RAC regenerated from SMC | CR | 301 | This work |
| Activated carbon from date pits | CR | 105.4 | [46] |
| Activated carbon from guava leaf | CR | 47.2 | [47] |
| Activated carbon of olive stones | CR | 2.2573 | [48] |
| Activated carbon from peanut shell | CR | 136.4 | [49] |
3.8 Thermodynamic studies
The thermodynamic studies of the adsorption of MO and CR onto RAC were carried out to reveal the possible mechanism [50]. The thermodynamic parameters were estimated from the Langmuir constants (KL). The change in Gibbs free energy (ΔG°), enthalpy energy (ΔH°) and the entropy change (ΔS°) are calculated from the following equations (9–11):
where R is the gas constant (8.314 J/mol/K), and T is the temperature in Kelvin (K).
The slopes and intercepts of the plot of lnKL versus 1/T were employed to calculate the values of ΔSθ and ΔHθ (Figure 11). The thermodynamic parameters are listed in Table 6. It can be observed that the values of ΔGθ were negative for all temperatures and became more negative as the temperature increased, indicating that the process was endothermic and spontaneous [51]. It also explained that the high temperature would promote the extent of adsorption by decreasing the viscosity of the solution, which accelerate the mass transfer rate [52]. Moreover, the values of ΔHθ and ΔSθ were positive for RAC, suggesting that the endothermic nature of the adsorption and an increase in randomness at the solid-liquid interface [53].

The thermodynamic study of adsorption of MO (A) and CR (B) dye solution.
The thermodynamic parameters for adsorption of the MO and CR dye solution onto RAC.
| Temperature (K) | ΔGθ (kJ/mol) | ΔSθ (J/mol/K) | ΔHθ (kJ/mol) |
|---|---|---|---|
| MO | |||
| 303 | −10.8 | 160.4 | 38.2 |
| 313 | −12.9 | ||
| 323 | −14.1 | ||
| CR | |||
| 303 | −14.3 | 52.3 | 1.6 |
| 313 | −14.8 | ||
| 323 | −15.3 | ||
4 Conclusions
SMC was regenerated by using the microwave and ultrasonic spray-assisted method. The optimal regeneration conditions could be determined as the regeneration temperature of 900°C and regeneration time of 60 min, for which the MB value was 210 mg/g and the specific surface area of RAC was as high as 801 m2/g. The mercury content of RAC was reduced to 0.46 mg/l. RAC has been confirmed to be a good adsorbent for the removal of the MO and CR dye solution. The kinetic data and adsorption isotherm fitted well with pseudo second-order and Langmuir isotherm, and the maximum MO and CR adsorption capacities were 529 and 301 mg/g, respectively. The thermodynamic results indicated that the adsorption process was spontaneous and endothermic. Therefore, the SMCs regenerated by microwave and ultrasonic spray method can be used as a potential adsorption material for dye wastewater treatment.
Acknowledgments
This work was supported by the National Science Fund for Excellent Young Scholars of China (Grant no. 51522405).
References
[1] Li X, Wang Y, Kang L, Zhu M, Dai B. J. Catal. 2014, 311, 288–294.10.1016/j.jcat.2013.12.006Search in Google Scholar
[2] Zhang J, Liu N, Wei L, Dai B. Front. Chem. Sci. Eng. 2011, 5, 514–520.10.1007/s11705-011-1114-zSearch in Google Scholar
[3] Wang F, Wang L, Wang J, Zhao Y, Wang Y, Yang D. React. Kinet. Mech. Catal. 2015, 114, 725–734.10.1007/s11144-014-0806-zSearch in Google Scholar
[4] Liu C, Peng J, Ma AY, Zhang LB, Li J. J. Hazard. Mater. 2016, 322, 325–333.10.1016/j.jhazmat.2016.09.063Search in Google Scholar
[5] Gennadios A, Hanna MA, Kurth LB. Poultry and Seafoods: A Review, LWT 1997, 30, 337–350.10.1006/fstl.1996.0202Search in Google Scholar
[6] Wei X, Shi H, Qian W, Luo G, Jin Y, Wei F. Ind. Eng. Chem. Res. 2009, 48, 128–133.10.1021/ie0716316Search in Google Scholar
[7] Zhang H, Dai B, Li W, Wang X, Zhang J, Zhu M, Gu J. J. Catal. 2014, 316, 141–148.10.1016/j.jcat.2014.05.005Search in Google Scholar
[8] Yang T. China Plastics 2008, 22, 1–8.10.1177/0920203X08091547Search in Google Scholar
[9] Li XY, Li P, Pan XL, Ma H, Bao XH. Appl. Catal. B Environ. 2017, 210, 116–120.10.1016/j.apcatb.2017.03.046Search in Google Scholar
[10] Hutchings GJ, Grady DT. Appl. Catal. 1985, 16, 411–415.10.1016/S0166-9834(00)84403-6Search in Google Scholar
[11] Hylander LD, Goodsite ME. Sci. Total Environ. 2006, 368, 352–370.10.1016/j.scitotenv.2005.11.029Search in Google Scholar PubMed
[12] Sonne C, Dietz R, Leifsson PS, Asmund G, Born EW, Kirkegaard M. Environ. Health 2007, 6, 11.10.1186/1476-069X-6-11Search in Google Scholar PubMed PubMed Central
[13] Zhang XY, Wang QC, Zhang SQ, Sun XJ, Zhang ZS. J. Hazard. Mater. 2009, 168, 1575–1580.10.1016/j.jhazmat.2009.03.050Search in Google Scholar PubMed
[14] Randall P, Chattopadhyay S. J. Hazard. Mater. 2014, 114, 211–223.10.1016/j.jhazmat.2004.08.010Search in Google Scholar PubMed
[15] Xu X, He H, Zhao J, Wang B, Gu S, Li X. Chinese J. Chem. Eng. 2017, 25.10.1016/j.cjche.2016.12.003Search in Google Scholar
[16] Li X, Pan X, Yu L, Ren P, Wu X, Sun L, Jiao F, Bao X. Nat. Commun. 2014, 5, 3688.10.1038/ncomms4688Search in Google Scholar PubMed
[17] Bing JL, Cheng Z. Polyvinyl Chloride 2012, 40, 1–7.Search in Google Scholar
[18] Liu C, Peng JH, Zhang LB, Wang SX, Ma AY. Chinese J. Chem. Eng. 2018, 26, 364–327.10.1016/j.cjche.2017.07.002Search in Google Scholar
[19] Shah IK, Pre P, Alappat BJ. J. Taiwan Inst. Chem. Eng. 2014, 45, 1733–1738.10.1016/j.jtice.2014.01.006Search in Google Scholar
[20] Lin G, Cheng S, Wang S, Hu T, Peng JH, Xia HY, Jiang F, Li S, Zhang LB. Catalysis Today 2017, In press.Search in Google Scholar
[21] Ledesma B, Román S, Sabio E, Álvarez-Murillo A. J. Supercrit. Fluids 2015, 104, 94–103.10.1016/j.supflu.2015.05.007Search in Google Scholar
[22] Sun Y, Zhang B, Zheng T, Wang P. Chem. Eng. J. 2017, 320, 264–270.10.1016/j.cej.2017.03.007Search in Google Scholar
[23] Yao S, Zhang J, Shen D, Rui X, Gu S, Ming Z, Liang J. J. Colloid Interface Sci. 2016, 463, 118.10.1016/j.jcis.2015.10.047Search in Google Scholar PubMed
[24] Ma S, Li Z, Ma J, Chai F, Zhu S. J. Environ. Chem. Eng. 2015, 3, 1312–1319.10.1016/j.jece.2014.11.029Search in Google Scholar
[25] Peng L, Ismael ZM, Zhang WB, Yuan SH, Man T, Wang K, Bao JG. Chem. Eng. J. 2012, 195–196, 339–346.Search in Google Scholar
[26] Cheng S, Wu J, Xia HY, Peng JH, Wang SX, Zhang LB. Desalin. Water Treat. 2012, 4, 1–11.10.1080/19443994.2012.698517Search in Google Scholar
[27] Ania CO, Menéndez JA, Parra JB, Pis JJ. Carbon 2004, 42, 1383–1387.10.1016/j.carbon.2004.01.010Search in Google Scholar
[28] Georgin J, Dotto GL, Mazutti MA, Foletto EL. J. Environ. Chem. Eng. 2016, 4, 266–275.10.1016/j.jece.2015.11.018Search in Google Scholar
[29] Shu JH, Cheng S, Xia HY, Zhang LB, Peng JH, Li CY, Zhang SZ. RSC Adv. 2017, 7, 14395–14405.10.1039/C7RA00287DSearch in Google Scholar
[30] Lu X, Jiang J, Sun K, Wang J, Zhang Y. Mar. Pollut. Bull. 2014, 78, 69–76.10.1016/j.marpolbul.2013.11.007Search in Google Scholar PubMed
[31] Ghaedi M, Nasab AG, Khodadoust S, Sahraei R, Daneshfar A. J. Ind. Eng. Chem. 2015, 21, 986–993.10.1016/j.jiec.2014.05.006Search in Google Scholar
[32] Dou J, Yu J, Tahmasebi A, Yin F, Gupta S, Li X, Lucas J, Na C, Wall T. Fuel Process. Technol. 2015, 135, 187–194.10.1016/j.fuproc.2015.01.035Search in Google Scholar
[33] Vernadakis A. Zeitschrift Für Chemie Und Industrie Der Kolloide 1907, 2, 15.Search in Google Scholar
[34] Albadarin AB, Mangwandi C, Al-Muhtaseb AAH, Walker GM, Allen SJ, Ahmad MNM. Chem. Eng. J. 2012, 179, 193–202.10.1016/j.cej.2011.10.080Search in Google Scholar
[35] Ho YS, Mckay G. Process Saf. Environ. Prot. 1999, 77, 165–173.10.1205/095758299529983Search in Google Scholar
[36] Saeidi N, Parvini M, Niavarani Z. J. Environ. Chem. Eng. 2015, 3, 2697–2706.10.1016/j.jece.2015.09.023Search in Google Scholar
[37] Alothman ZA, Habila MA, Ali R, Ghafar AA, Hassouna ED. Arab. J. Chem. 2014, 7, 1148–1158.10.1016/j.arabjc.2013.05.007Search in Google Scholar
[38] Da SLV, López-Sotelo JB, Correa-Guimarães A, Hernández-Navarro S, Sánchez-Báscones M, Navas-Gracia LM, Martín-Ramos P, Martín-Gil R. J. Environ. Manag. 2015, 155, 67–76.10.1016/j.jenvman.2015.03.007Search in Google Scholar PubMed
[39] Lebron I, Suarez DL, Alberto F. Soil Sci. Soc. Am. J. 1994, 58, 1753–1762.10.2136/sssaj1994.03615995005800060025xSearch in Google Scholar
[40] Gong J, Wang X, Shao X, Yuan S, Yang C, Hu X. Talanta 2012, 101, 45–52.10.1016/j.talanta.2012.08.035Search in Google Scholar PubMed
[41] Ghaedi M, Ghaedi AM, Mirtamizdoust B, Agarwal S, Gupta VK. J. Mol. Liq. 2016, 213, 48–57.10.1016/j.molliq.2015.09.051Search in Google Scholar
[42] Mahmoudi K, Hamdi N, Kriaa A, Srasra E. Russ. J. Phys. Chem. A 2012, 86, 1294–1300.10.1134/S0036024412060180Search in Google Scholar
[43] Chen SH, Jian Z, Zhang CL, Yue QY, Yan L, Chao L. Desalin. Water Treat. 2010, 252, 149–156.Search in Google Scholar
[44] Mahmoudi K, Hosni K, Hamdi N, Srasra E. Korean J. Chem. Eng. 2015, 32, 274–283.10.1007/s11814-014-0216-ySearch in Google Scholar
[45] León G, García F, Miguel B, Bayo J. Desalin. Water Treat. 2016, 57, 17104–17117.Search in Google Scholar
[46] Belhachemi M, Addoun F. Desalin. Water Treat. 2012, 37, 122–129.10.1080/19443994.2012.661263Search in Google Scholar
[47] Ojedokun AT, Bello OS. Appl. Water Sci. 2017, 7, 1965–1977.10.1007/s13201-015-0375-ySearch in Google Scholar
[48] Najar-Souissi S, Ouederni A, Ratel A. J. Environ. Sci. 2005, 17, 998–1003.Search in Google Scholar
[49] Lawal I A, Chetty D, Akpotu SO, Moodley B. Environ. Nanotechnol. Monit. Manage. 2017, 8, 83–91.Search in Google Scholar
[50] Bulut Y, Aydın H. Desalination 2006, 194, 259–267.10.1016/j.desal.2005.10.032Search in Google Scholar
[51] Li CY, Xia HY, Zhang LB, Peng JH, Cheng S, Shu JH, Zhang SZ. Res. Chem. Intermediat. 2017, 44, 1–20.Search in Google Scholar
[52] Gautam PK, Saroj RS, Pandey JD. Proc. Natl. Acad. Sci. India 2015, 85, 35–39.10.1007/s40010-014-0178-9Search in Google Scholar
[53] Gautam PK, Gautam RK, Banerjee S, Lofrano G, Sanroman MA, Chattopadhyaya MC, Pandey JD. J. Environ. Chem. Eng. 2015, 3, 2560–2568.10.1016/j.jece.2015.08.004Search in Google Scholar
©2019 Walter de Gruyter GmbH, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 Public License.
Articles in the same Issue
- Regular Articles
- Studies on the preparation and properties of biodegradable polyester from soybean oil
- Flow-mode biodiesel production from palm oil using a pressurized microwave reactor
- Reduction of free fatty acids in waste oil for biodiesel production by glycerolysis: investigation and optimization of process parameters
- Saccharin: a cheap and mild acidic agent for the synthesis of azo dyes via telescoped dediazotization
- Optimization of lipase-catalyzed synthesis of polyethylene glycol stearate in a solvent-free system
- Green synthesis of iron oxide nanoparticles using Platanus orientalis leaf extract for antifungal activity
- Ultrasound assisted chemical activation of peanut husk for copper removal
- Room temperature silanization of Fe3O4 for the preparation of phenyl functionalized magnetic adsorbent for dispersive solid phase extraction for the extraction of phthalates in water
- Evaluation of the saponin green extraction from Ziziphus spina-christi leaves using hydrothermal, microwave and Bain-Marie water bath heating methods
- Oxidation of dibenzothiophene using the heterogeneous catalyst of tungsten-based carbon nanotubes
- Calcined sodium silicate as an efficient and benign heterogeneous catalyst for the transesterification of natural lecithin to L-α-glycerophosphocholine
- Synergistic effect between CO2 and H2O2 on ethylbenzene oxidation catalyzed by carbon supported heteropolyanion catalysts
- Hydrocyanation of 2-arylmethyleneindan-1,3-diones using potassium hexacyanoferrate(II) as a nontoxic cyanating agent
- Green synthesis of hydratropic aldehyde from α-methylstyrene catalyzed by Al2O3-supported metal phthalocyanines
- Environmentally benign chemical recycling of polycarbonate wastes: comparison of micro- and nano-TiO2 solid support efficiencies
- Medicago polymorpha-mediated antibacterial silver nanoparticles in the reduction of methyl orange
- Production of value-added chemicals from esterification of waste glycerol over MCM-41 supported catalysts
- Green synthesis of zerovalent copper nanoparticles for efficient reduction of toxic azo dyes congo red and methyl orange
- Optimization of the biological synthesis of silver nanoparticles using Penicillium oxalicum GRS-1 and their antimicrobial effects against common food-borne pathogens
- Optimization of submerged fermentation conditions to overproduce bioethanol using two industrial and traditional Saccharomyces cerevisiae strains
- Extraction of In3+ and Fe3+ from sulfate solutions by using a 3D-printed “Y”-shaped microreactor
- Foliar-mediated Ag:ZnO nanophotocatalysts: green synthesis, characterization, pollutants degradation, and in vitro biocidal activity
- Green cyclic acetals production by glycerol etherification reaction with benzaldehyde using cationic acidic resin
- Biosynthesis, characterization and antimicrobial activities assessment of fabricated selenium nanoparticles using Pelargonium zonale leaf extract
- Synthesis of high surface area magnesia by using walnut shell as a template
- Controllable biosynthesis of silver nanoparticles using actinobacterial strains
- Green vegetation: a promising source of color dyes
- Mechano-chemical synthesis of ammonia and acetic acid from inorganic materials in water
- Green synthesis and structural characterization of novel N1-substituted 3,4-dihydropyrimidin-2(1H)-ones
- Biodiesel production from cotton oil using heterogeneous CaO catalysts from eggshells prepared at different calcination temperatures
- Regeneration of spent mercury catalyst for the treatment of dye wastewater by the microwave and ultrasonic spray-assisted method
- Green synthesis of the innovative super paramagnetic nanoparticles from the leaves extract of Fraxinus chinensis Roxb and their application for the decolourisation of toxic dyes
- Biogenic ZnO nanoparticles: a study of blueshift of optical band gap and photocatalytic degradation of reactive yellow 186 dye under direct sunlight
- Leached compounds from the extracts of pomegranate peel, green coconut shell, and karuvelam wood for the removal of hexavalent chromium
- Enhancement of molecular weight reduction of natural rubber in triphasic CO2/toluene/H2O systems with hydrogen peroxide for preparation of biobased polyurethanes
- An efficient green synthesis of novel 1H-imidazo[1,2-a]imidazole-3-amine and imidazo[2,1-c][1,2,4]triazole-5-amine derivatives via Strecker reaction under controlled microwave heating
- Evaluation of three different green fabrication methods for the synthesis of crystalline ZnO nanoparticles using Pelargonium zonale leaf extract
- A highly efficient and multifunctional biomass supporting Ag, Ni, and Cu nanoparticles through wetness impregnation for environmental remediation
- Simple one-pot green method for large-scale production of mesalamine, an anti-inflammatory agent
- Relationships between step and cumulative PMI and E-factors: implications on estimating material efficiency with respect to charting synthesis optimization strategies
- A comparative sorption study of Cr3+ and Cr6+ using mango peels: kinetic, equilibrium and thermodynamic
- Effects of acid hydrolysis waste liquid recycle on preparation of microcrystalline cellulose
- Use of deep eutectic solvents as catalyst: A mini-review
- Microwave-assisted synthesis of pyrrolidinone derivatives using 1,1’-butylenebis(3-sulfo-3H-imidazol-1-ium) chloride in ethylene glycol
- Green and eco-friendly synthesis of Co3O4 and Ag-Co3O4: Characterization and photo-catalytic activity
- Adsorption optimized of the coal-based material and application for cyanide wastewater treatment
- Aloe vera leaf extract mediated green synthesis of selenium nanoparticles and assessment of their In vitro antimicrobial activity against spoilage fungi and pathogenic bacteria strains
- Waste phenolic resin derived activated carbon by microwave-assisted KOH activation and application to dye wastewater treatment
- Direct ethanol production from cellulose by consortium of Trichoderma reesei and Candida molischiana
- Agricultural waste biomass-assisted nanostructures: Synthesis and application
- Biodiesel production from rubber seed oil using calcium oxide derived from eggshell as catalyst – optimization and modeling studies
- Study of fabrication of fully aqueous solution processed SnS quantum dot-sensitized solar cell
- Assessment of aqueous extract of Gypsophila aretioides for inhibitory effects on calcium carbonate formation
- An environmentally friendly acylation reaction of 2-methylnaphthalene in solvent-free condition in a micro-channel reactor
- Aegle marmelos phytochemical stabilized synthesis and characterization of ZnO nanoparticles and their role against agriculture and food pathogen
- A reactive coupling process for co-production of solketal and biodiesel
- Optimization of the asymmetric synthesis of (S)-1-phenylethanol using Ispir bean as whole-cell biocatalyst
- Synthesis of pyrazolopyridine and pyrazoloquinoline derivatives by one-pot, three-component reactions of arylglyoxals, 3-methyl-1-aryl-1H-pyrazol-5-amines and cyclic 1,3-dicarbonyl compounds in the presence of tetrapropylammonium bromide
- Preconcentration of morphine in urine sample using a green and solvent-free microextraction method
- Extraction of glycyrrhizic acid by aqueous two-phase system formed by PEG and two environmentally friendly organic acid salts - sodium citrate and sodium tartrate
- Green synthesis of copper oxide nanoparticles using Juglans regia leaf extract and assessment of their physico-chemical and biological properties
- Deep eutectic solvents (DESs) as powerful and recyclable catalysts and solvents for the synthesis of 3,4-dihydropyrimidin-2(1H)-ones/thiones
- Biosynthesis, characterization and anti-microbial activity of silver nanoparticle based gel hand wash
- Efficient and selective microwave-assisted O-methylation of phenolic compounds using tetramethylammonium hydroxide (TMAH)
- Anticoagulant, thrombolytic and antibacterial activities of Euphorbia acruensis latex-mediated bioengineered silver nanoparticles
- Volcanic ash as reusable catalyst in the green synthesis of 3H-1,5-benzodiazepines
- Green synthesis, anionic polymerization of 1,4-bis(methacryloyl)piperazine using Algerian clay as catalyst
- Selenium supplementation during fermentation with sugar beet molasses and Saccharomyces cerevisiae to increase bioethanol production
- Biosynthetic potential assessment of four food pathogenic bacteria in hydrothermally silver nanoparticles fabrication
- Investigating the effectiveness of classical and eco-friendly approaches for synthesis of dialdehydes from organic dihalides
- Pyrolysis of palm oil using zeolite catalyst and characterization of the boil-oil
- Azadirachta indica leaves extract assisted green synthesis of Ag-TiO2 for degradation of Methylene blue and Rhodamine B dyes in aqueous medium
- Synthesis of vitamin E succinate catalyzed by nano-SiO2 immobilized DMAP derivative in mixed solvent system
- Extraction of phytosterols from melon (Cucumis melo) seeds by supercritical CO2 as a clean technology
- Production of uronic acids by hydrothermolysis of pectin as a model substance for plant biomass waste
- Biofabrication of highly pure copper oxide nanoparticles using wheat seed extract and their catalytic activity: A mechanistic approach
- Intelligent modeling and optimization of emulsion aggregation method for producing green printing ink
- Improved removal of methylene blue on modified hierarchical zeolite Y: Achieved by a “destructive-constructive” method
- Two different facile and efficient approaches for the synthesis of various N-arylacetamides via N-acetylation of arylamines and straightforward one-pot reductive acetylation of nitroarenes promoted by recyclable CuFe2O4 nanoparticles in water
- Optimization of acid catalyzed esterification and mixed metal oxide catalyzed transesterification for biodiesel production from Moringa oleifera oil
- Kinetics and the fluidity of the stearic acid esters with different carbon backbones
- Aiming for a standardized protocol for preparing a process green synthesis report and for ranking multiple synthesis plans to a common target product
- Microstructure and luminescence of VO2 (B) nanoparticle synthesis by hydrothermal method
- Optimization of uranium removal from uranium plant wastewater by response surface methodology (RSM)
- Microwave drying of nickel-containing residue: dielectric properties, kinetics, and energy aspects
- Simple and convenient two step synthesis of 5-bromo-2,3-dimethoxy-6-methyl-1,4-benzoquinone
- Biodiesel production from waste cooking oil
- The effect of activation temperature on structure and properties of blue coke-based activated carbon by CO2 activation
- Optimization of reaction parameters for the green synthesis of zero valent iron nanoparticles using pine tree needles
- Microwave-assisted protocol for squalene isolation and conversion from oil-deodoriser distillates
- Denitrification performance of rare earth tailings-based catalysts
- Facile synthesis of silver nanoparticles using Averrhoa bilimbi L and Plum extracts and investigation on the synergistic bioactivity using in vitro models
- Green production of AgNPs and their phytostimulatory impact
- Photocatalytic activity of Ag/Ni bi-metallic nanoparticles on textile dye removal
- Topical Issue: Green Process Engineering / Guest Editors: Martine Poux, Patrick Cognet
- Modelling and optimisation of oxidative desulphurisation of tyre-derived oil via central composite design approach
- CO2 sequestration by carbonation of olivine: a new process for optimal separation of the solids produced
- Organic carbonates synthesis improved by pervaporation for CO2 utilisation
- Production of starch nanoparticles through solvent-antisolvent precipitation in a spinning disc reactor
- A kinetic study of Zn halide/TBAB-catalysed fixation of CO2 with styrene oxide in propylene carbonate
- Topical on Green Process Engineering
Articles in the same Issue
- Regular Articles
- Studies on the preparation and properties of biodegradable polyester from soybean oil
- Flow-mode biodiesel production from palm oil using a pressurized microwave reactor
- Reduction of free fatty acids in waste oil for biodiesel production by glycerolysis: investigation and optimization of process parameters
- Saccharin: a cheap and mild acidic agent for the synthesis of azo dyes via telescoped dediazotization
- Optimization of lipase-catalyzed synthesis of polyethylene glycol stearate in a solvent-free system
- Green synthesis of iron oxide nanoparticles using Platanus orientalis leaf extract for antifungal activity
- Ultrasound assisted chemical activation of peanut husk for copper removal
- Room temperature silanization of Fe3O4 for the preparation of phenyl functionalized magnetic adsorbent for dispersive solid phase extraction for the extraction of phthalates in water
- Evaluation of the saponin green extraction from Ziziphus spina-christi leaves using hydrothermal, microwave and Bain-Marie water bath heating methods
- Oxidation of dibenzothiophene using the heterogeneous catalyst of tungsten-based carbon nanotubes
- Calcined sodium silicate as an efficient and benign heterogeneous catalyst for the transesterification of natural lecithin to L-α-glycerophosphocholine
- Synergistic effect between CO2 and H2O2 on ethylbenzene oxidation catalyzed by carbon supported heteropolyanion catalysts
- Hydrocyanation of 2-arylmethyleneindan-1,3-diones using potassium hexacyanoferrate(II) as a nontoxic cyanating agent
- Green synthesis of hydratropic aldehyde from α-methylstyrene catalyzed by Al2O3-supported metal phthalocyanines
- Environmentally benign chemical recycling of polycarbonate wastes: comparison of micro- and nano-TiO2 solid support efficiencies
- Medicago polymorpha-mediated antibacterial silver nanoparticles in the reduction of methyl orange
- Production of value-added chemicals from esterification of waste glycerol over MCM-41 supported catalysts
- Green synthesis of zerovalent copper nanoparticles for efficient reduction of toxic azo dyes congo red and methyl orange
- Optimization of the biological synthesis of silver nanoparticles using Penicillium oxalicum GRS-1 and their antimicrobial effects against common food-borne pathogens
- Optimization of submerged fermentation conditions to overproduce bioethanol using two industrial and traditional Saccharomyces cerevisiae strains
- Extraction of In3+ and Fe3+ from sulfate solutions by using a 3D-printed “Y”-shaped microreactor
- Foliar-mediated Ag:ZnO nanophotocatalysts: green synthesis, characterization, pollutants degradation, and in vitro biocidal activity
- Green cyclic acetals production by glycerol etherification reaction with benzaldehyde using cationic acidic resin
- Biosynthesis, characterization and antimicrobial activities assessment of fabricated selenium nanoparticles using Pelargonium zonale leaf extract
- Synthesis of high surface area magnesia by using walnut shell as a template
- Controllable biosynthesis of silver nanoparticles using actinobacterial strains
- Green vegetation: a promising source of color dyes
- Mechano-chemical synthesis of ammonia and acetic acid from inorganic materials in water
- Green synthesis and structural characterization of novel N1-substituted 3,4-dihydropyrimidin-2(1H)-ones
- Biodiesel production from cotton oil using heterogeneous CaO catalysts from eggshells prepared at different calcination temperatures
- Regeneration of spent mercury catalyst for the treatment of dye wastewater by the microwave and ultrasonic spray-assisted method
- Green synthesis of the innovative super paramagnetic nanoparticles from the leaves extract of Fraxinus chinensis Roxb and their application for the decolourisation of toxic dyes
- Biogenic ZnO nanoparticles: a study of blueshift of optical band gap and photocatalytic degradation of reactive yellow 186 dye under direct sunlight
- Leached compounds from the extracts of pomegranate peel, green coconut shell, and karuvelam wood for the removal of hexavalent chromium
- Enhancement of molecular weight reduction of natural rubber in triphasic CO2/toluene/H2O systems with hydrogen peroxide for preparation of biobased polyurethanes
- An efficient green synthesis of novel 1H-imidazo[1,2-a]imidazole-3-amine and imidazo[2,1-c][1,2,4]triazole-5-amine derivatives via Strecker reaction under controlled microwave heating
- Evaluation of three different green fabrication methods for the synthesis of crystalline ZnO nanoparticles using Pelargonium zonale leaf extract
- A highly efficient and multifunctional biomass supporting Ag, Ni, and Cu nanoparticles through wetness impregnation for environmental remediation
- Simple one-pot green method for large-scale production of mesalamine, an anti-inflammatory agent
- Relationships between step and cumulative PMI and E-factors: implications on estimating material efficiency with respect to charting synthesis optimization strategies
- A comparative sorption study of Cr3+ and Cr6+ using mango peels: kinetic, equilibrium and thermodynamic
- Effects of acid hydrolysis waste liquid recycle on preparation of microcrystalline cellulose
- Use of deep eutectic solvents as catalyst: A mini-review
- Microwave-assisted synthesis of pyrrolidinone derivatives using 1,1’-butylenebis(3-sulfo-3H-imidazol-1-ium) chloride in ethylene glycol
- Green and eco-friendly synthesis of Co3O4 and Ag-Co3O4: Characterization and photo-catalytic activity
- Adsorption optimized of the coal-based material and application for cyanide wastewater treatment
- Aloe vera leaf extract mediated green synthesis of selenium nanoparticles and assessment of their In vitro antimicrobial activity against spoilage fungi and pathogenic bacteria strains
- Waste phenolic resin derived activated carbon by microwave-assisted KOH activation and application to dye wastewater treatment
- Direct ethanol production from cellulose by consortium of Trichoderma reesei and Candida molischiana
- Agricultural waste biomass-assisted nanostructures: Synthesis and application
- Biodiesel production from rubber seed oil using calcium oxide derived from eggshell as catalyst – optimization and modeling studies
- Study of fabrication of fully aqueous solution processed SnS quantum dot-sensitized solar cell
- Assessment of aqueous extract of Gypsophila aretioides for inhibitory effects on calcium carbonate formation
- An environmentally friendly acylation reaction of 2-methylnaphthalene in solvent-free condition in a micro-channel reactor
- Aegle marmelos phytochemical stabilized synthesis and characterization of ZnO nanoparticles and their role against agriculture and food pathogen
- A reactive coupling process for co-production of solketal and biodiesel
- Optimization of the asymmetric synthesis of (S)-1-phenylethanol using Ispir bean as whole-cell biocatalyst
- Synthesis of pyrazolopyridine and pyrazoloquinoline derivatives by one-pot, three-component reactions of arylglyoxals, 3-methyl-1-aryl-1H-pyrazol-5-amines and cyclic 1,3-dicarbonyl compounds in the presence of tetrapropylammonium bromide
- Preconcentration of morphine in urine sample using a green and solvent-free microextraction method
- Extraction of glycyrrhizic acid by aqueous two-phase system formed by PEG and two environmentally friendly organic acid salts - sodium citrate and sodium tartrate
- Green synthesis of copper oxide nanoparticles using Juglans regia leaf extract and assessment of their physico-chemical and biological properties
- Deep eutectic solvents (DESs) as powerful and recyclable catalysts and solvents for the synthesis of 3,4-dihydropyrimidin-2(1H)-ones/thiones
- Biosynthesis, characterization and anti-microbial activity of silver nanoparticle based gel hand wash
- Efficient and selective microwave-assisted O-methylation of phenolic compounds using tetramethylammonium hydroxide (TMAH)
- Anticoagulant, thrombolytic and antibacterial activities of Euphorbia acruensis latex-mediated bioengineered silver nanoparticles
- Volcanic ash as reusable catalyst in the green synthesis of 3H-1,5-benzodiazepines
- Green synthesis, anionic polymerization of 1,4-bis(methacryloyl)piperazine using Algerian clay as catalyst
- Selenium supplementation during fermentation with sugar beet molasses and Saccharomyces cerevisiae to increase bioethanol production
- Biosynthetic potential assessment of four food pathogenic bacteria in hydrothermally silver nanoparticles fabrication
- Investigating the effectiveness of classical and eco-friendly approaches for synthesis of dialdehydes from organic dihalides
- Pyrolysis of palm oil using zeolite catalyst and characterization of the boil-oil
- Azadirachta indica leaves extract assisted green synthesis of Ag-TiO2 for degradation of Methylene blue and Rhodamine B dyes in aqueous medium
- Synthesis of vitamin E succinate catalyzed by nano-SiO2 immobilized DMAP derivative in mixed solvent system
- Extraction of phytosterols from melon (Cucumis melo) seeds by supercritical CO2 as a clean technology
- Production of uronic acids by hydrothermolysis of pectin as a model substance for plant biomass waste
- Biofabrication of highly pure copper oxide nanoparticles using wheat seed extract and their catalytic activity: A mechanistic approach
- Intelligent modeling and optimization of emulsion aggregation method for producing green printing ink
- Improved removal of methylene blue on modified hierarchical zeolite Y: Achieved by a “destructive-constructive” method
- Two different facile and efficient approaches for the synthesis of various N-arylacetamides via N-acetylation of arylamines and straightforward one-pot reductive acetylation of nitroarenes promoted by recyclable CuFe2O4 nanoparticles in water
- Optimization of acid catalyzed esterification and mixed metal oxide catalyzed transesterification for biodiesel production from Moringa oleifera oil
- Kinetics and the fluidity of the stearic acid esters with different carbon backbones
- Aiming for a standardized protocol for preparing a process green synthesis report and for ranking multiple synthesis plans to a common target product
- Microstructure and luminescence of VO2 (B) nanoparticle synthesis by hydrothermal method
- Optimization of uranium removal from uranium plant wastewater by response surface methodology (RSM)
- Microwave drying of nickel-containing residue: dielectric properties, kinetics, and energy aspects
- Simple and convenient two step synthesis of 5-bromo-2,3-dimethoxy-6-methyl-1,4-benzoquinone
- Biodiesel production from waste cooking oil
- The effect of activation temperature on structure and properties of blue coke-based activated carbon by CO2 activation
- Optimization of reaction parameters for the green synthesis of zero valent iron nanoparticles using pine tree needles
- Microwave-assisted protocol for squalene isolation and conversion from oil-deodoriser distillates
- Denitrification performance of rare earth tailings-based catalysts
- Facile synthesis of silver nanoparticles using Averrhoa bilimbi L and Plum extracts and investigation on the synergistic bioactivity using in vitro models
- Green production of AgNPs and their phytostimulatory impact
- Photocatalytic activity of Ag/Ni bi-metallic nanoparticles on textile dye removal
- Topical Issue: Green Process Engineering / Guest Editors: Martine Poux, Patrick Cognet
- Modelling and optimisation of oxidative desulphurisation of tyre-derived oil via central composite design approach
- CO2 sequestration by carbonation of olivine: a new process for optimal separation of the solids produced
- Organic carbonates synthesis improved by pervaporation for CO2 utilisation
- Production of starch nanoparticles through solvent-antisolvent precipitation in a spinning disc reactor
- A kinetic study of Zn halide/TBAB-catalysed fixation of CO2 with styrene oxide in propylene carbonate
- Topical on Green Process Engineering

