Abstract
The rich phytochemical profile of Perilla frutescens leaf extract (PLE) makes it a compelling bioreducing agent for the green synthesis of silver nanoparticles (AgNPs), offering diverse biomedical applications. In this study, the PLE-mediated phytosynthesis of AgNPs was confirmed by UV-vis spectra with maximum absorbance at 440 nm. The spherical PLE-AgNPs of <20 nm in size were further characterized by Dynamic light scanning, Transmission electron microscopy, and Scanning electron microscopy. Moreover, High-resolution transmission electron microscopy, Selected area electron diffraction and X-ray diffraction validated the crystalline structure of PLE-AgNPs. The energy-dispersive X-ray and X-ray photoelectron spectroscopy established the presence of carbon, oxygen, and nitrogen groups, along with silver, in the PLE-AgNPs. FTIR results confirmed the presence of phytochemicals as the capping agents for PLE-AgNPs. The Gas chromatograph-mass spectrometry analysis revealed the presence of terpenoids, furan derivatives, phenolic compounds, hydroxides, imidazole, aldehydes, etc., in PLE. In addition, the presence of phytochemicals was also confirmed in the as-synthesized PLE-AgNPs. The 2,2-diphenyl-1-picrylhydrazyl radical scavenging activity, hydrogen peroxide scavenging activity, and ABTS radical scavenging activity affirmed the strong radical scavenging effect of PLE-AgNPs with an IC50 of 51.58 μg·mL−1. The wound-healing potential of PLE-AgNPs was confirmed by in vitro cell scratch assay in human keratinocyte HaCaT cells. These integrated findings emphasize the significant biomedical advantages and potential applications of PLE-AgNPs in clinical applications.
1 Introduction
Silver nanoparticles (AgNPs) are intriguing nanomaterials with unique characteristics and many applications, making them of interest in medicine, electronics, catalysis, and environmental science [1]. The AgNPs have shown promising wound care due to their unique features that promote wound healing. The substantially powerful antibacterial and anti-inflammatory properties and the capacity to influence tissue repair-critical cellular processes of AgNPs make them useful in wound healing, a complex and dynamic cascade of cellular and molecular processes that restore tissue integrity and functionality [2]. It has been known that chronic inflammation can slow tissue regeneration and may cause delay in wound healing. The AgNPs can reduce inflammation by regulating cytokine levels and providing a balanced immune response, encouraging tissue and wound healing [3].
While AgNPs have gained significant attention in wound healing, concerns and challenges are associated with their use, such as toxicity, environmental impact, cost, scalability, etc. [4,5]. It has been reported that the biosynthesis of nanoparticles is an easy, affordable, and reliable bottom-up strategy, which is an exciting combination of nanotechnology and green chemistry. In contrast to chemical nanoparticle synthesis, this eco-friendly technology has garnered attention for its sustainability and affordability. Biosynthesis uses bacteria, fungi, yeast, plants, and biomolecules to reduce and stabilize silver ions (Ag+) into elemental silver (Ag0). Among these biosynthesis methods, plant-mediated nanoparticle biosynthesis is attractive and environmentally beneficial due to its abundant resources, biocompatibility, reduced contamination risk, diverse phytochemicals, ease of handling, tunable properties, and cost-effectiveness [6].
Since nature consists of numerous varieties of sustainable materials worldwide, plant extracts mediated AgNPs synthesis is a promising opportunity and route to develop sustainable and bio-based green products. Also, this approach minimizes the consumption of inorganic chemicals as the plant extracts function both as reducing and stabilizing agents. At the same time, the consumption of energy and utilities to run the operation is also minimized, significantly increasing the cost-effectiveness of the synthesis procedure [4,5,7,8]. Plant extracts from leaves, stems, and roots contain phytochemicals that can reduce silver ions to nanoparticles [9–12]. Green synthesis uses flavonoids, terpenoids, and phenolic molecules as reducing and capping agents for AgNPs due to the presence of functional groups such as hydroxyl, carbonyl, and amino groups. These phytochemicals act as reducing agents by donating electrons to Ag+, thereby facilitating their reduction to Ag0 and subsequent formation of AgNPs. The reduction process may involve redox reactions mediated by the bioactive compounds, leading to the formation of stable AgNPs [1]. Further, these biomolecules adsorb onto the surface of AgNPs through electrostatic interactions, hydrogen bonding, or covalent bonding, preventing their agglomeration and ensuring colloidal stability. The capping agents also impart biocompatibility to the nanoparticles, making them suitable for biomedical applications. Plant extracts provide a favorable environment for the nucleation and growth of AgNPs by serving as reaction media and templates. The presence of phytochemicals in the extract influences the nucleation kinetics and controls the size, shape, and morphology of the nanoparticles. Certain phytochemicals can selectively bind to specific crystal facets of the growing nanoparticles, directing their growth along preferred directions and resulting in the formation of anisotropic nanostructures. The biosynthesis of AgNPs also results in a synergistic improvement in nanoparticle efficiency and effectiveness in several applications owing to the biomolecules involved in the reducing and capping activities [13]. Despite much research on plant-mediated biosynthesis of AgNPs, additional research is needed due to the varied biological systems that may have unique characteristics or synthesis pathways. In addition, several biosynthesis research studies optimize reaction parameters like temperature, pH, concentration, and incubation time. Tuning these parameters can greatly affect biosynthesized AgNPs yield, size, and stability. Further research can improve and standardize these conditions.
The Perilla leaf extract (PLE) is a promising and adaptable botanical resource for AgNPs production owing to the bioactive phytochemical contents of Perilla frutescens (such as phenolic acids, flavonoids, essential oils, triterpenes, carotenoids, phytosterols, fatty acids, tocopherols, policosanols, and other secondary metabolites [14], which can act as reducing and stabilizing agents that reduce silver ions to nanoparticles. For example, the flavonoids and phenolic substances in Perilla frutescens are antioxidant molecules that can reduce silver ions and stabilize nanoparticles, reducing agglomeration and extending shelf life. This is important for biomedical and pharmaceutical applications because manufactured AgNPs have lower cytotoxicity and higher biocompatibility. In addition to metal ions reducing properties, the Perilla plant also possesses biological functions, including anti-diabetic, anti-hyperuricemia, anti-spasmodic, anti-allergic, insecticidal, anti-inflammatory, anti-depression, hepatoprotective, antimicrobial, anti-cancer, etc., which makes Perilla plant biocompatible and safe for AgNPs synthesis [15]. Further, since Perilla plants are abundant and easy to grow, AgNP synthesis becomes sustainable and cost-effective on a large scale.
Although the bioreduction of AgNPs by PLE has already been reported [16,17], wound healing analysis of AgNPs synthesized using PLE has not yet been reported. Further, each biosynthesis study may involve specific parameters such as concentration, temperature, or incubation time. Thus, further analyses can be conducted to optimize these parameters for enhanced nanoparticle synthesis efficiency, leading to better control over the process. Moreover, seasonal, environmental, and geographical factors can significantly affect the composition of plant extracts. Only a few studies have reported on the biosynthesis of AgNPs, using these native species in the Republic of Korea [14], but the biological activities of these biosynthesized AgNPs have not been explored. Moreover, the physical, chemical, and biological properties of AgNPs can be fine-tuned using PLE by altering extraction conditions and concentrations. So, the aim of this study is to determine what bioactive compounds were found in an aqueous leaf extract of Perilla frutescens, a native species, using GC-MS analysis and then preparing PLE-AgNPs. Further, this study attempts to find out how well PLE-AgNPs can assist in wound healing to explore the application of PLE-AgNPs in the medicinal and pharmaceutical fields.
2 Materials and methods
2.1 Preparation of PLE
Prior to the preparation of the powder, the Perilla leaves were first allowed to air dry after being washed three times with distilled water. After that, the leaves were ground into a powder. After adding 10 g Perilla leaf powder to 100 mL of double-distilled water and heating the mixture to 80°C for three hours, the PLE was obtained by filtering the solution, which was then stored at 4°C till further use.
2.2 Synthesis of PLE-AgNPs
The PLE-AgNPs were synthesized by mixing PLE solution into a solution of silver nitrate (1 mM) at different ratios (i.e., 1%, 2%, 4%, and 8% PLE v/v) while the mixture was stirred magnetically at room temperature and heated to 80°C, as described in previous literature with slight modification [18]. The synthesis of PLE-AgNPs was visually observed for a color change, which indicates that the reduction of Ag+ to AgNPs had occurred. After the reaction mixture was centrifuged at 14,000 rpm for 30 min, the biosynthesized PLE-AgNPs were collected, washed three times, and re-dispersed in water that had been double-distilled.
2.3 UV-Vis absorbance
Analysis of the optical characteristics of the PLE-AgNPs was carried out using a UV-vis spectrophotometer (Jenway, 7315 UV/Vis spectrophotometer) to record the absorbance spectra of the PLE-AgNPs suspension. The wavelength range that was utilized was from 200 to 700 nm. Double-distilled water was utilized as a reference to determine the appropriate baseline absorbance. Measurements were made at predetermined intervals to monitor any shifts that may have occurred in the absorbance spectrum over time. The spectra were analyzed to determine the peak absorbance wavelength, which revealed the presence of AgNPs.
2.4 Inductively coupled plasma (ICP)
The ICP analysis allowed for the determination of the elemental composition as well as the concentration of Ag in the biosynthesized PLE-AgNPs [19]. An ICP-OES spectrometer (Agilent-5900, Agilent Technologies Inc., CA, USA) was used to evaluate the solution produced after biosynthesized PLE-AgNPs were digested with 10% HNO3. In addition, the supernatants obtained after centrifugation of biosynthesized PLE-AgNPs at given time points were also quantified for Ag+ concentration using ICP. Distilled water was used as blank for ICP. Quantifying the silver content was accomplished by using calibration curves made with known amounts of silver standards at a wavelength of 338.289 nm.
2.5 Dynamic light scanning (DLS)
The hydrodynamic size distribution of biosynthesized PLE-AgNPs was determined using DLS (Zeta plus 90, Brookhaven Instrument Co., USA). Measurements were conducted thrice at a fixed scattering angle, and the obtained data were used to calculate the hydrodynamic diameter and polydispersity index (PDI) of the nanoparticles. The PLE-AgNPs suspension was sonicated in a water bath for 30 min before the analysis. The surface charge of biosynthesized AgNPs was assessed by measuring the zeta potential.
2.6 Transmission electron microscopy (TEM)
The morphology and size distribution of biosynthesized PLE-AgNPs were examined using TEM (JEOL, JEM-2100F, Tokyo, Japan). A drop of the nanoparticle suspension was deposited on a carbon-coated copper grid and allowed to dry. TEM images were acquired at suitable magnifications to visualize the shape and size of individual nanoparticles. In addition, the nanoparticles were also analyzed for High-resolution transmission electron microscopy (HR-TEM) images, Selected area electron diffraction (SAED) pattern, and energy-dispersive X-ray (EDX) mapping.
2.7 Scanning electron microscopy (SEM)
The surface morphology of biosynthesized PLE-AgNPs was studied using SEM (FE-SEM, JEOL, JSM-7900F, Tokyo, Japan) at various magnifications after mounting the samples on the stub. The SEM images were analyzed for nanoparticle shape, size, distribution, and EDX mapping.
2.8 Fourier transform infrared spectroscopy (FTIR)
The FTIR (Frontier, PerkinElmer, UK) analysis was performed to identify functional groups involved in the biosynthesis and stabilization of PLE-AgNPs with attenuated total reflectance mode [20]. The FTIR spectra of freeze dried biosynthesized PLE-AgNPs sample was recorded in the 4,000–400 cm⁻¹ range to identify characteristic peaks corresponding to the functional groups. The spectra of PLE were also recorded for reference.
2.9 X-ray diffraction (XRD)
The crystalline nature of freeze-dried PLE-AgNPs was analyzed using X-ray diffraction (Philips X’Pert-MPD diffractometer) to record diffraction patterns in the 2θ range of 10°–80° step-scanning mode with Cu-Kα radiation operating at 40 kV and 40 mA. The obtained data were analyzed using XRD software to identify crystal phases [21].
2.10 X-ray photoelectron spectroscopy (XPS)
The composition and oxidation state of lyophilized PLE-AgNPs was analyzed using XPS (Thermo Fisher Scientific K Alpha+ spectrometer, Waltham, MA, USA), as described in the previous report [22]. The Al-Ka X-ray source (1,486.6 eV) was used to take the necessary analytical readings. The survey scan was collected using an energy step size of 1.0 eV and a pass energy of 150 eV in a constant analyzer energy mode. The pass energy for narrow scans was 50 eV, while the step size was 0.1 eV.
2.11 Antioxidant activity
The antioxidant activity of PLE extract and PLE-AgNPs was evaluated using 2,2-Diphenyl-1-picrylhydrazyl (DPPH) scavenging activity [23,24]. The PLE-AgNPs were centrifuged and washed twice to remove the impurities and ascorbic acid was used as a positive control. For the DPPH assay, 1 mg·mL−1 of initial stock solutions were prepared in methanol for the samples and ascorbic acid (standard antioxidant). Later, the solutions were diluted to different concentrations (25, 50, 100, and 200 μg·mL−1). Then, 1 mL of each diluted solution was mixed with 1 mL of a 1 mg·mL−1 DPPH solution (methanolic) and incubated for 30 min under dark conditions at room temperature. The absorbance was measured at 517 nm utilizing a spectrophotometer (SpectraMax, Molecular Devices, San Jose, CA, USA).
For the ABTS assay, 0.9 mL of prepared ABTS radical working solution was added to 0.1 mL solutions with various concentrations (25, 50, 100, and 200 μg·mL−1) and incubated in the dark for 15 min. The absorbance of the solution was measured at 734 nm [25]. The following equation was used to measure DPPH and ABTS scavenging activity:
where Abs = Absorbance.
Hydrogen peroxide scavenging activity is determined by a substance’s ability to neutralize hydrogen peroxide [26]. In brief, at first, in a pH 7.4 phosphate buffer, a 40 mM hydrogen peroxide solution was prepared. Then, samples were diluted in distilled water to a final concentration of 25, 50, 100, and 200 μg·mL−1 and were added to a 0.6 mL, 40 mM hydrogen peroxide solution. After 10 min, the hydrogen peroxide solution absorbance was measured at 230 nm, and the % inhibition was calculated [23].
2.12 Gas chromatograph-mass spectrometry (GC-MS) analysis
For GC-MS analysis, 10 mg freeze-dried aqueous PLE and PLE-AgNPs were dispersed in 1 mL of methanol, followed by filtration using a 0.45 µM syringe filter. The GC-MS analysis was carried out in a Gas chromatograph/mass selective detector by Agilent Technologies (Agilent 7890A/5975C, USA).
2.13 Cell culture
Immortalized epidermal keratinocyte HaCaT cells were cultured and maintained in Dulbecco’s Modified Eagle’s Medium (DMEM) (Gibco, Waltham, MA, USA) supplemented with 10% fetal bovine serum (FBS) (Gibco, USA) and 1% penicillin/streptomycin (Invitrogen, Waltham, MA, USA) at 37°C in a humidified incubator with 5% CO2. Fresh medium was replaced in sub-cultured cells every 3 days and was sub-cultured every 7 days on 90% confluence.
2.14 Cell viability and cell cytotoxicity assay
2.14.1 Cell counting kit‐8 (CCK8) assay
For evaluating any effect of aqueous PLE extract and PLE-AgNPs on cell viability of HaCaT cells, the CCK8 (GK10001, GLPBIO, CA, USA) assay was performed. In brief, 1 × 104 cells/well were seeded in 96 well plates in a complete DMEM medium. HaCaT cells were treated with various concentrations of aqueous PLE extract and PLE-AgNPs (0, 5, 25, 50, 100, and 200 µg·mL−1) for 24 h, and the CCK8 was added and incubated for 1 h in a CO2 incubator. The optical density was measured at 450 nm using a UV-Vis spectrophotometer (SpectraMax, Molecular Devices).
2.14.2 Lactate dehydrogenase activity (LDH) assay
Any cytotoxic effect of aqueous PLE extract and PLE-AgNPs on HaCaT cells was assessed by the amount of LDH released into the cell culture media. A cytotoxicity detection kit (Roche Diagnostics, San Jose, CA, USA) confirmed the LDH released in the medium. Various concentrations of aqueous PLE extract and PLE-AgNPs (0, 5, 25, 50, 100, and 200 µg·mL−1) were treated in HaCaT cells for 24 h. Subsequently, 10 µL of cell-cultured medium from each well was transferred to a 96-well plate. To each well, 50 µL of LDH reagent and 40 µL of PBS were added and incubated for 45 min in the dark. To stop the enzymatic reaction, 50 µL of stop solution was added to each well, and optical density was measured at 490 nm using a UV-Vis spectrophotometer (SpectraMax, Molecular Devices).
2.15 Cell scratch assay
The cell scratch assay was performed as reported in a previous study [21]. The 80% confluent HaCaT human keratinocytes were seeded at 1 × 105 cells/well in 24 well plates for 24 h. Using sterile 200 μL pipette tips, scratches were created in the cell monolayer across the well diameter. The media was aspirated, and cells were washed twice. Fresh DMEM medium with 0.1 mg·mL−1 of PLE or PLE-AgNPs in DPBS was added to the wells. At 37°C, plates were incubated for the pre-determined time. Optical images of each well were taken using a bright field microscope at 0 and 24 h post-treatment.
2.16 Statistical analysis
Graphpad Prism 9.0.0 (San Diego, CA, USA) was used to evaluate statistical difference using a two-tailed Student’s t-test, two-way ANOVA, and *P < 0.05, **P < 0.01, and ***P < 0.001 was considered statistically significant. Data are represented as mean value ± SEM of three values.
3 Results and discussion
3.1 Biosynthesis and UV-vis spectra analysis of PLE-AgNPs
The color change of the solution and UV-vis spectroscopy are indirect methods to observe the synthesis of AgNPs [27]. At the start, 1 mM AgNO3 solution was mixed with 1% PLE v/v to observe PLE’s reducing potential. It was found that after adding the PLE to AgNO3 solution for 24 h, the color changed from pale yellow to dark brown due to the excitation of surface plasmon resonance (SPR) phenomenon of AgNPs (Figure 1a). When light interacts with metallic nanoparticles, such as AgNPs, it can excite the conduction electrons on the surface of the particles. This excitation leads to a collective oscillation of the electrons, known as plasmon resonance. Figure 1b shows the time-dependent UV-vis absorbance spectra of these as-synthesized PLE-AgNPs which showed a unique peak at 440 nm, due to the SPR of AgNPs, suggesting the biosynthesis of PLE-AgNPs [28]. At pre-determined time points, the samples from the reaction solution were collected and centrifuged at 14,000 rpm for 30 min to obtain the PLE-AgNPs as the precipitate. The PLE-AgNP precipitates were digested with 10% HNO3, and the concentration of Ag+ was then calculated using ICP-OES. It was observed that till 6 h, there was no synthesis of AgNPs. However, the characteristic UV-vis absorbance peak of AgNPs appeared at 8 h, suggesting the onset of AgNPs synthesis. The maximum peak was observed at 48 h, remaining almost constant until 72 h. The ICP results also corresponded to the UV-vis absorbance results, showing maximum conversion of Ag+ to Ag0 at 48 h (Figure 1c). Further, the PLE-AgNPs concentration was also analyzed and compared to AgNO3 solution at equal concentration using nanoparticle tracking analyzer, which indicated the increase in particle concentration in the solution after the formation of PLE-AgNPs (Figure S1).

(a) Photographs of (i) AgNO3, (ii) AgNO3 + PLE at 0 h, and (iii) AgNO3 + PLE at 24 h. (b) Time-dependent UV-vis spectra of PLE-AgNPs. (c) Time-dependent silver ion concentration in synthesized PLE-AgNPs. (d) UV-vis spectra of PLE-AgNPs at varying PLE:AgNO3 ratio. (e) Silver ion concentration in synthesized PLE-AgNPs at varying PLE:AgNO3 ratio.
Later, the biosynthesis of AgNPs was optimized by varying the ratios of PLE and silver nitrate. As the concentration of PLE was 4% v/v, maximum Ag+ reduction remained constant till 8%. Further, when the amount of PLE was reduced to 2% and 1%, the conversion of Ag+ to Ag0 reduced concomitantly (Figure 1d and e), suggesting that 4% v/v is the optimum amount of PLE for PLE-AgNPs synthesis.
The PLE has various phytochemicals that could reduce the Ag+. It was proposed that the phytochemical compounds containing hydroxide groups are responsible for reducing silver ions [29]. Thus, PLE may present hydroxide-containing phytochemicals (as shown in Table 1). Further, it can be assumed that PLE’s flavonoids and phenolic compounds might act as capping agents and stabilize the AgNPs [49].
GC-MS data for determination of phytochemicals in PLE
| S. no | RT | Area (%) | Compound | Activities |
|---|---|---|---|---|
| 1 | 5.918 | 0.7796 | Cyclotetrasiloxane, octamethyl- | Antimicrobial, terpenoid [30,31] |
| 2 | 6.9884 | 10.0471 | Morpholine-4-Carbaldehyde-[(4′,4′,6′,6′-Tetramethyl-1-Thiaspiro[2.3]Hex-5′-ylidene)Hydrazone] | No activity reported |
| 3 | 7.7077 | 7.526 | Silacyclopentane | Broad-spectrum fungicidal activity [32] |
| 4 | 8.4646 | 0.5412 | 1,1-Diethyl-4-phenylsemicarbazide | No activity reported |
| 5 | 10.629 | 1.2725 | 2(3H)-Furanone, Dihydro- | Derivatives have been used for various medicinal purposes [33] |
| 6 | 11.9238 | 0.6892 | 6-(2-Formylhydrazino)-N,N′-bis(isopropyl)-1,3,5-triazine-2,4-diamine | No reported activity [34] |
| 7 | 12.1928 | 0.8704 | 1-HEXANOL, 2-ETHYL- | No activity reported |
| 8 | 12.6181 | 0.7683 | Octadecanoic acid, 2-oxo-, methyl ester | Saturated fatty acid [35] |
| 9 | 13.2187 | 1.3384 | Undecane | Antiallergic, anti-inflammatory alkanes [35,36] |
| 10 | 13.6753 | 0.7123 | 2-[Trifluoromethyl]pyrimido[1,2-a]benzimidazol-4-ol | Benzimidazol derivatives have broad-spectrum pharmacological properties [37] |
| 11 | 14.0506 | 5.4899 | 4H-Pyran-4-One, 2,3-Dihydro-3,5-Dihydroxy-6-Methyl- | Antioxidant, antifungal, anti-inflammatory, anti-proliferative, pro-apoptotic, and antioxidant [38–40] |
| 12 | 14.3321 | 4.242 | 2,2′-Bi-1,3-Dioxolane, 4,4,4′,4′,5,5,5′,5′-Octamethyl- | No activity reported |
| 13 | 14.7575 | 2.0042 | 1H-Imidazole, 2-Ethyl-4-Methyl- | Antioxidant and antibacterial [41] |
| 14 | 14.9451 | 4.6143 | 2,3-Dihydro-Benzofuran | Anti-tumor, antibacterial, anti-oxidative, and anti-viral activities[42] |
| 15 | 15.139 | 4.7187 | 2(1H)-Pyrimidinone, 4-Methyl- | Antimicrobial [43] |
| 16 | 15.4143 | 44.1828 | 1-Butanone, 1-(2-furanyl)- | Furan derivatives have antimicrobial activity [44] |
| 17 | 16.0586 | 1.9183 | Ethanone, 1-(2-Furanyl)- | Antimicrobial [44] |
| 18 | 16.9218 | 1.8911 | 4-Cyclopropyl-2-Methoxyphenol | Phenolic compounds (such as flavonoids) have antimicrobial and antioxidant potential [45] |
| 19 | 17.3409 | 1.3987 | 4-Methyl-E-4-hexadecen-1-ol | Phytols have various pharmacological activities [46] |
| 20 | 17.8101 | 1.3666 | 3-Methoxy-4-[(Trimethylsilyl)Oxy]-Benzaldehyde-O-Methyloxime | Plant aldehyde. Other plant extracts with this compound showed antibacterial, antifungal, and antimicrobial activities [47] |
| 21 | 18.4293 | 0.3111 | Carbamate, N-(2-naphthyl)-, 3-pentynyl ester | No activity reported |
| 22 | 18.6483 | 0.188 | Phenylacetamide, N-ethyl-N-(3-methylphenyl)- | No activity reported |
| 23 | 18.8172 | 0.8949 | 1,3-Dimethyl-1-cyclohexene | Cyclohexane derivatives might have biological activities [48] |
| 24 | 19.6491 | 0.5452 | 1-Methyl-2-(P-Nitrophenyl)-Benzimidazole | Benzimidazol derivatives have broad-spectrum of pharmacological properties [37] |
| 25 | 20.5311 | 0.3442 | 2-(4-Ethoxy-3-Methoxy-Phenyl)-3-Nitro-2H-Chromene | No activity reported |
RT: Retention time.
3.2 Size and morphological characterizations of PLE-AgNPs
The DLS provides valuable insights into the hydrodynamic radius and size distribution of nanoparticles by analyzing intensity fluctuations in scattered light. Thus, with the help of DLS, we were able to determine the hydrodynamic size of PLE-AgNPs, which was shown to be 66.4 ± 25.6 nm and had a PDI of 0.2 (as shown in Figure 2a). The PDI represents the quantitative size distribution profile of nanoparticles in a solution. A low PDI indicates a narrow size distribution, whereas a high PDI suggests a broader range of particle sizes. A low PDI enables better control over the physicochemical properties and biological activities of the nanoparticles, with the potential of NPs for different pharmaceutical formulations being linked to their PDI [50]. Since PLE-AgNPs had a PDI of 0.2, which is often considered a low PDI, it can be suggested that the PLE-AgNPs are fairly uniform and homogenous in size. The zeta potential of PLE-AgNPs was −1.77 mV (Figure 2b). It has been known that nanoparticles with zeta values between −10 mV and +10 mV will experience rapid agglomeration unless sterically protected [50]. Thus, since we observed a population of homogenous PLE-AgNPs, it indicates that the nanoparticles are sterically protected by the phytochemicals present in the PLE.

(a) DLS pattern and (b) zeta potential of PLE-AgNPs.
In addition, the TEM pictures revealed the presence of spherical AgNPs surrounded by the layer of PLE, demonstrating that PLE is an effective substrate for the nucleation, growth, and stabilization of AgNPs (Figure 3a), which is in accordance with the study by Basavegowda and Lee [17]. These nanoparticles had a size range of less than 50 nm. Figure 3b depicts the HRTEM image of biosynthesized PLE-AgNPs with nearly spherical particles and smooth edges, showing the lattice fringes quite clearly, indicating a well-defined crystal structure with high symmetry and minimal structural imperfections. The PLE-AgNPs also showed crystalline character due to the existence of a crystalline circular ring in the SAED pattern, as shown in Figure 3c. The presence of only one ring may suggest that the nanoparticles have a uniform size distribution. This could indicate a controlled synthesis process resulting in nanoparticles with consistent dimensions. The elemental mapping showed the presence of the Ag element, indicating that the particles present in the TEM images are AgNPs (Figure 3d and e).

(a) TEM image, (b) HR-TEM, (c) SAED pattern, and (d & e) Elemental mapping and spectrum of PLE-AgNPs.
In addition, SEM images were also taken to confirm the existence of PLE-AgNPs (Figure 4). The SEM images clearly showed the presence of spherical PLE-AgNPs, as confirmed by the elemental mapping showing the presence of Ag, C, N, and O. The presence of C, O, and N in the elemental map attributes to the phytochemicals existing with the AgNPs (Figure 4b–g).

(a) SEM image, (b)–(g) EDX spectroscopy of PLE-AgNPs.
3.3 FTIR spectroscopic analysis of PLE-AgNPs
The FTIR spectra of PLE and PLE-AgNPs were compared for the identification of the biomolecules that were responsible for reducing, capping, and stabilization of PLE-AgNPs (Figure 5a). The FTIR spectra of PLE revealed multiple small absorption peaks at 3,739, 3,547, 3,442, and 3,372 cm−1, which corresponded to the O–H (hydroxyl) group of different phytochemicals present in the PLE. The presence of OH– containing reducing biomolecules is in agreement with the previous studies on AgNPs production [51]. These small peaks disappeared in the spectra of PLE-AgNPs and shifted to a single peak at 3,418 cm−1, indicating the involvement of phytochemicals in the reduction of Ag+ to Ag0 [25]. The peak at 3,083 cm−1 in PLE could be attributed to the amine group, which shifted to 2,911 cm−1 in PLE-AgNPs [52]. Further, the peak at 2,653 cm−1 in PLE is due to the C-H bonds in the methyl (CH3) group, which disappeared in PLE-AgNPs [53]. The FTIR peak at 1,548 cm−1 in PLE is possibly associated with amide II vibrations [54]. The FTIR spectra of PLE-AgNPs exhibited peaks at 1,635 cm−1, which can be attributed to the stretching of the amide I group, and the peak at 1,060 cm−1 is associated with the amine group and the C–O of alcohols [55]. Many other peaks between 1,635 and 1,060 cm−1 were also found in PLE-AgNPs which were not present in the spectra of PLE, which could be due to the changes in the peak positions, again suggesting that the phytochemicals of PLE have a role in the synthesis and capping of AgNPs.



(a) FTIR spectrum, (b) XRD spectrum, (c)–(g) XPS spectrum of PLE-AgNPs.
3.4 XRD analysis of PLE-AgNPs
As shown in Figure 5b, the XRD pattern provides evidence that the biosynthesized PLE-AgNPs have peaks of crystalline silver. According to the JCPDS File No. 00-004-783 [51], and JCPDS file No. 01–071–4613 [55], the XRD peaks at 2θ of 27.86°, 32.23°, 38.17°, 46.29°, 54.71°, 64.40°, and 77.60° are indicative of the (210), (122), (111), (231), (142), (220), and (311) FCC planes of Ag in PLE-AgNPs. These Bragg reflections corresponded to the crystalline planes of metallic silver’s face-centered cubic crystal lattice.
3.5 XPS spectroscopic analysis of PLE-AgNPs
The XPS survey spectrum of PLE-AgNPs (Figure 5c) revealed the presence of a C1s peak at 284.88 eV, a N1s peak at 398.18 eV, and an O1s peak at 531.08 eV. The high-resolution scan of Ag 3d has shown two peaks at 366.6 eV and 372.6 eV, which corresponds to the Ag (0) 3d5/2 and 3d3/2, respectively, with a 6.0 eV slitting of the Ag 3d doublet suggesting the presence of Ag metal in PLE-AgNPs (Figure 5d) [21], thus indicating successful PLE-mediated in situ synthesis of AgNPs. The C 1s peaks can be resolved into three rational Gaussian peaks at 283.04, 284.88, and 286.51 eV, which can be assigned to the structures of N–C═C, C–C, and –C–OH groups, indicating the existence of carbonyl functional groups on PLE-AgNPs (Figure 5e) [56]. Figure 5f shows that the binding energy of oxygen in PLE-AgNPs is 513.10 eV [57], and Figure 5g shows the XPS spectra for the N1s, showing the binding energies at 400.43 and 398.29 eV [58], which can be ascribed to the multiple amine groups of phytochemicals in the PLE which possibly interact with the AgNPs. Overall, the XPS results showed the association of phytochemicals in PLE with the AgNPs. These results are also corroborated with FTIR results, which showed the presence of different functional groups in PLE-AgNPs.
3.6 In vitro antioxidant assay
Several different internal and external factors are responsible for generating reactive oxygen species, which in turn causes oxidative stress. Antioxidants protect the body by lowering oxidative stress, often related to various diseases, like neurological, cardiovascular, cancer, and aging disorders [59]. Due to the antioxidant activity of phenolics, terpenoids, and alkaloids, scientists have shifted their attention to the potential of natural antioxidants over the unknown adverse effects linked with synthetic medicines. Studies have shown that various phytochemicals like polyphenols and flavonoids can be utilized to synthesize and cap AgNPs for stability [23,60]. Thus, in this study, AgNPs were synthesized from Perilla leaves, and the antioxidant potential of aqueous PLE leaf extract and PLE-AgNPs was evaluated through DPPH free radical scavenging, hydrogen peroxide scavenging, and ABTS free radical scavenging at various concentrations (25, 50, 100, and 200 μg·mL−1) (Figure 6a). Results demonstrate that both aqueous PLE leaf extract and PLE-AgNPs showed increased dose-dependent antioxidation activity. In particular, PLE-AgNPs showed greater free radical inhibition levels than the aqueous leaf Perilla extract. PLE-AgNps showed 68.56% maximum scavenging capacity against DPPH radicals with an IC50 concentration of 49.15 μg·mL−1. While 69.76% of maximum scavenging capacity against hydrogen peroxide radicals was observed with an IC50 concentration of 51.58 μg·mL−1 for PLE-AgNps (Figure 6b). In the case of the ABTS assay, a maximum of 79.56% of radical scavenging capacity with an IC50 concentration of 54.84 μg·mL−1 was observed for PLE-AgNps. The notable antioxidant properties of PLE-AgNPs in DPPH, hydrogen peroxide, and ABTS experiments are likely due to the inclusion of diverse phytochemicals, including phenolic acids and flavonoids (Figure 6c). Phytochemicals act as a capping agent by being absorbed into the surfaces of AgNPs, where they interact and efficiently neutralize free radicals [61–63]. The greater antioxidant capacity of green-synthesized AgNPs highlights their potential for treating and preventing oxidative damage to cells and tissues. The eco-friendly green synthesis approach aligns with sustainability objectives and minimizes the environmental footprint of nanoparticle manufacturing.

Free radical scavenging activity of aqueous Perilla extract, Perilla extract synthesized AgNPs, and ascorbic acid (standard) at various concentrations. (a) DPPH radical scavenging activity, (b) hydrogen peroxide scavenging activity, (c) ABTS radical scavenging activity. The error bar represents the standard deviation of the mean value. a: P*** = 0.0003, b: P**** < 0.0001, c: P*** = 0.0001.
3.7 Phytochemical screening of PLE and PLE-AgNPs
Figure 7a shows the GC-MS analysis of all the phytochemicals clarified in the PLE. The GC-MS analysis of PLE, as shown in Table 1, revealed the presence of 25 compounds. Thus, the PLE consists of various phytochemicals that could act as reducing and capping agents for AgNPs. Further, out of 25 compounds, 17 compounds or their derivatives have been previously identified to exhibit biological activities, as described in Table 1.

GC-MS chromatogram of (a) PLE and (b) PLE-AgNPs.
In the identified compounds in PLE, 1-Butanone, 1-(2-furanyl)- was most abundant; however, another furan derivative, i.e., 1-(2-furanyl)-ethanone was also found in the GC-MS chromatogram. It has been previously reported that the furan compounds, e.g., 1-(2-furanyl)-ethanone and ethyl 2-(5-methyl-5-vinyl tetra hydrofuran-2-yl)carbonate in the plant extracts, are potent inhibitors of bacterial growth [44,64,65], thus suggesting that the presence of furan derivatives in PLE could offer PLE-AgNPs antimicrobial properties against infectious wounds which is essential for the management of wounds. In addition, other plant extracts consisting of furan derivatives showed various biological activities, such as antioxidant, anti-inflammatory, analgesic, and antipyretic [66], although the antioxidant activity is majorly due to the presence of phenolic acids that capture the ability of free radicals [67]. The GC-MS chromatogram showed the presence of 4-cyclopropyl-2-Methoxyphenol in the PLE. A previous study reported that phenolic compound -((1E)-3-Hydroxy-1-propenyl)-2-methoxyphenol showed antimicrobial, antioxidant, and anti-inflammatory properties [68]. It is usually assumed that the phenol moiety is responsible for the antioxidant properties of any plant phenolic compound. Flavonoids are plant phenolic compounds well-known to possess antioxidant properties [69]. Another plant metabolite, 4H-pyran-4-one, 2,3-dihydro-3,5-dihydroxy-6-methyl-, present in large amounts in PLE, has also been identified with the antioxidant property [38,70], thus suggesting the possible antioxidant potential of PLE-AgNPs.
In addition, nitrogenous compounds, such as 2-[Trifluoromethyl]pyrimido[1,2-a]benzimidazol-4-ol, 1-Methyl-2-(P-Nitrophenyl)-Benzimidazole, 2(1H)-Pyrimidinone 4-Methyl-, and 1H-Imidazole 2-Ethyl-4-Methyl-, are also present in the PLE. Nitrogenous compounds are important in natural products as they possess significant pharmacological activity [71]. Also, fatty acids, such as octadecanoic acid 2-oxo- methyl ester and undecane are also present in the PLE, which might contribute to the antioxidant property [72]. Besides these, other phytochemicals, such as cyclotetrasiloxane octamethyl-, silacyclopentane, 2(3H)-furanone dihydro-, 2,3-dihydro-benzofuran, 4-methyl-E-4-hexadecen-1-ol, 3-methoxy-4-[(trimethylsilyl)oxy]-benzaldehyde-O-methyloxime, and 1,3-dimethyl-1-cyclohexene might also contribute to the biological activities of PLE-AgNPs.
The GC-MS data (Figure 7b, Table 2) show the presence of octamethyl-cyclotetrasiloxane, N-(2,6-dimethyl-4-[(trimethylsilyl)oxy]phenyl)(trimethyl) Silamine, 2-Methyl-5,12-dithiannaphtho[2,3-B] quinoxaline, N-(4-chlorophenyl)-4-methyl-benzenesulfonamide, trimethyl[1-[(trimethylsilyl)ethynyl]-2-naphthalenyl]-silane, 1,1-dioctyl-2-oxohydrazine, N-nitroso-di-n-octylamine, and Undecane. This indicates the possibility of these compounds acting as capping agents to stabilize AgNPs and also contribute to the biological activities of AgNPs. These results are in accordance with the presence of OH–, amide, and methyl groups in the FTIR analysis of PLE-AgNPs.
GC-MS data for determination of phytochemicals in PLE-AgNPs
| S. no. | RT | Area (%) | Compound |
|---|---|---|---|
| 1 | 5.888 | 3.59 | Cyclotetrasiloxane, octamethyl- |
| 2 | 6.807 | 16.97 | N-(2,6-dimethyl-4-[(trimethylsilyl)oxy]phenyl)(trimethyl) silamine |
| 3 | 7.520 | 7.45 |
|
| 4 | 13.144 | 6.53 |
|
RT: Retention time.
3.8 Cell viability and cell cytotoxicity assays
The dose-dependent effect of aqueous PLE extract and PLE-AgNPs on the viability of HaCaT cells with various concentrations (0, 5, 25, 50, 100, and 200 µg·mL−1) was evaluated using the CCK8 assay for 24 h (Figure 8a). Results demonstrated no significant effect on the cell viability of HaCaT cells. Moreover, no cytotoxic effect was observed on any concentrations of aqueous PLE extract and PLE-AgNPs on HaCaT cells, as was evident from the LDH release assay (Figure 8b), indicating that biosynthesized PLE-AgNPs are potential candidates for biomedical applications.

(a) Cell viability (CCK8 assay) and (b) LDH release were evaluated after treating HaCaT cells with various concentrations of PLE extract and PLE-AgNPs. Any concentrations of PLE and PLE-AgNPs showed no effect on the HaCaT cells. All the experiments were carried out in triplicates. The error bar represents the standard deviation of the mean value. No significant changes were observed between the treated and control groups.
3.9 Cell scratch assay
In order to be considered an appropriate wound dressing material, one of the most important characteristics is that it should enhance epidermal migration. The in vitro cell scratch tests are frequently utilized to examine the time-dependent migration of cells in response to a wide variety of stimuli [21]. The aim of this study was to analyze the migration of human HaCaT keratinocyte cells in response to PLE and PLE-AgNPs. Phosphate buffer saline was used as a control. After 24 h of treatment, it was observed that the cells treated with PLE did not exhibit any significant variations in migration when compared to the control group. However, at the same time point, the cellular migration was seen to be higher in HaCaT cells treated with PLE-AgNPs in comparison to cells that had been treated with PLE as well as the control group (Figure 9). Although the Perilla plant is well-known for its biological activities, in this study, PLE treated cells could not show faster cell migration. This could be possible because of the low bioavailability of the active compounds present in the plant extract. However, when interacting with AgNPs, the PLE active compounds could synergistically facilitate the cell migration rate due to the uptake of PLE within the cells along with AgNPs, indicating faster wound healing capabilities.

Time-dependent cell migration assay in HaCaT cells.
AgNPs are well-known to prevent wound infection and differentiate fibroblasts into myofibroblasts, which constrict wounds, hasten healing, and stimulate keratinocyte proliferation and relocation [73]. Previous reports, using histology and ex vivo wound model experiments, showed that AgNPs promote keratinocyte proliferation, migration, differentiation, and maturation from the wound edge to the center, increasing wound contraction [74,75]. Frankova et al. suggested that AgNPs suppress bacterial growth and generate proinflammatory cytokines, resulting in accelerated wound healing [76]. You et al. showed AgNPs stimulated fibroblast migration and elevated α-smooth muscle actin (α-SMA) levels, indicating myofibroblast transformation and faster healing [77]. Tian et al. found that AgNPs modulated the expression of cytokines, such as IL-6, TGF-β1, IL-10, VEGF, and IFN-γ, which are critical for wound healing [3]. However, the mechanisms by which AgNPs influence cellular processes, including migration, are not always fully understood. Thus, further research is needed to elucidate the specific molecular and cellular interactions that underlie the cell migration effects of PLE-AgNPs. Our study provides crucial in vitro cell assessment data for translational studies of synthesized AgNPs. However, further in vivo studies are required to assess the biocompatibility and safety issues associated with synthesized AgNPs. Despite lacking validation, our study is supported by literature and ongoing research. Numerous research works have examined AgNPs’ in vivo biocompatibility and wound healing characteristics, revealing their translational potential.
4 Conclusion
Here spherical AgNPs were spontaneously synthesized using PLE as a reducing and anchoring agent (Figure 10). The GC-MS data of PLE showed the presence of various phytochemicals, which could possibly be responsible for reducing Ag+ to Ag0 and capping the as-synthesized AgNPs. Thus, the PLE-AgNPs can be synthesized efficiently by eliminating the need for hazardous reducing and capping agents. Additionally, PLE-AgNPs exhibited significant antioxidant and cell migration potential without cell cytotoxicity, indicating potential wound-healing properties. Thus, the combination of these properties makes PLE-AgNPs a promising and versatile component for a multifaceted approach to address the complex challenges associated with effective wound management.
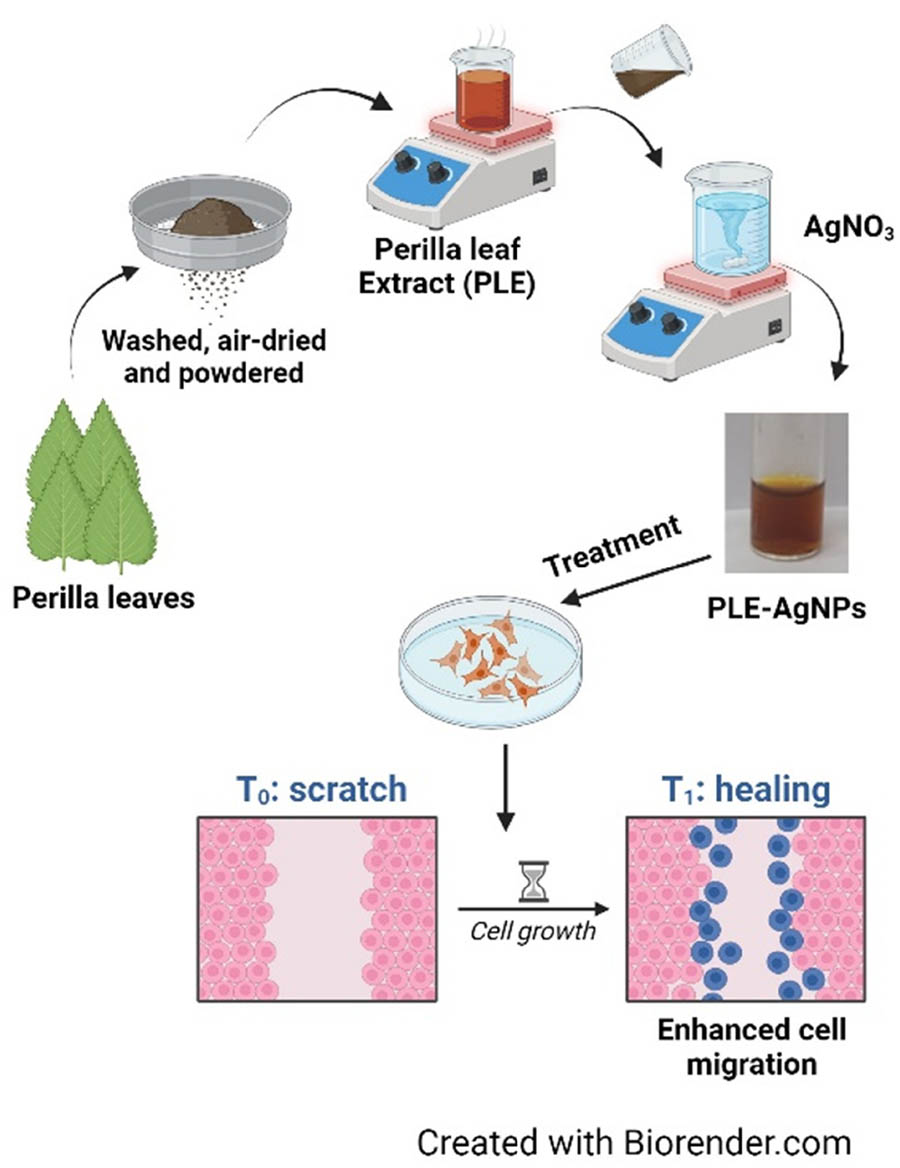
Schematic diagram of biosynthesis of AgNPs using Perilla leaf extract (PLE).
Acknowledgements
This study was supported by the Hallym University Research Fund and the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (NRF-2020R1C1C1008694, NRF-2022R1I1A1A01065269, and NRF-2020R1I1A3074575).
-
Funding information: This study was supported by the Hallym University Research Fund and the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (NRF-2020R1C1C1008694, NRF-2022R1I1A1A01065269, and NRF-2020R1I1A3074575).
-
Author contributions: Ashish Ranjan Sharma: writing – original draft, writing – review and editing, methodology, formal analysis, and project administration; Garima Sharma: writing – original draft, writing – review and editing, methodology, and formal analysis; Sudarshini Nath: formal analysis and visualization; Sang Soo Lee: project administration and resources.
-
Conflict of interest: Authors state no conflict of interest.
-
Data availability statement: The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
[1] Singhal G, Bhavesh R, Kasariya K, Sharma AR, Singh RP. Biosynthesis of silver nanoparticles using Ocimum sanctum (Tulsi) leaf extract and screening its antimicrobial activity. J Nanopart Res. 2011;13:2981–8. 10.1007/s11051-010-0193-y.Suche in Google Scholar
[2] Al-Nadaf AH, Awadallah A, Thiab S. Superior rat wound-healing activity of green synthesized silver nanoparticles from acetonitrile extract of Juglans regia L: Pellicle and leaves. Heliyon. 2024;10:e24473. England.10.1016/j.heliyon.2024.e24473Suche in Google Scholar PubMed PubMed Central
[3] Tian J, Wong KKY, Ho C-M, Lok C-N, Yu W-Y, Che C-M, et al. Topical delivery of silver nanoparticles promotes wound healing. ChemMedChem. 2007;2:129–36. Germany.10.1002/cmdc.200600171Suche in Google Scholar PubMed
[4] Erenler R, Dag B. Biosynthesis of silver nanoparticles using Origanum majorana L. and evaluation of their antioxidant activity. Inorg Nano-Metal Chem. 2022;52:485–92. Taylor & Francis. 10.1080/24701556.2021.1952263.Suche in Google Scholar
[5] Erenler R, Gecer EN. Synthesis of silver nanoparticles using Sideritis montana L. leaf extract: Characterization, catalytic degradation of methylene blue and antioxidant activity. J Nano Res. 2022;75:17–28. Trans Tech Publications Ltd. https://www.scientific.net/JNanoR.75.17.10.4028/p-333bjmSuche in Google Scholar
[6] Giri AK, Jena B, Biswal B, Pradhan AK, Arakha M, Acharya S, et al. Green synthesis and characterization of silver nanoparticles using Eugenia roxburghii DC. extract and activity against biofilm-producing bacteria. Sci Rep. 2022;12:8383. 10.1038/s41598-022-12484-y.Suche in Google Scholar PubMed PubMed Central
[7] Hasan KMF, Xiaoyi L, Shaoqin Z, Horváth PG, Bak M, Bejó L, et al. Functional silver nanoparticles synthesis from sustainable point of view: 2000 to 2023 ‒ A review on game changing materials. Heliyon. 2022;8:e12322. https://www.sciencedirect.com/science/article/pii/S2405844022036106.10.1016/j.heliyon.2022.e12322Suche in Google Scholar PubMed PubMed Central
[8] Majeed A, Ullah W, Anwar AW, Shuaib A, Ilyas U, Khalid P, et al. Cost-effective biosynthesis of silver nanoparticles using different organs of plants and their antimicrobial applications: A review. Mater Technol. 2018;33:313–20. Taylor & Francis. 10.1080/10667857.2015.1108065.Suche in Google Scholar
[9] Erenler R, Geçer EN, Genç N, Yanar D. Antioxidant activity of silver nanoparticles synthesized from Tagetes erecta L. leaves. Int J Chem Technol. 2021;5:141–6. İbrahim DEMİRTAŞ WT – DergiPark. 10.32571/ijct.1005275.Suche in Google Scholar
[10] Gecer EN. Green synthesis of silver nanoparticles from Salvia aethiopis L. and their antioxidant activity. J Inorg Organomet Polym Mater. 2021;31:4402–9. 10.1007/s10904-021-02057-3.Suche in Google Scholar
[11] Gecer EN, Erenler R, Temiz C, Genc N, Yildiz I. Green synthesis of silver nanoparticles from Echinacea purpurea (L.) Moench with antioxidant profile. Part Sci Technol. 2022;40:50–7. Taylor & Francis. 10.1080/02726351.2021.1904309.Suche in Google Scholar
[12] Genc N, Yildiz I, Chaoui R, Erenler R, Temiz C, Elmastas M. Biosynthesis, characterization and antioxidant activity of oleuropein-mediated silver nanoparticles. Inorg Nano-Metal Chem. 2020;51:411–9. Taylor & Francis. 10.1080/24701556.2020.1792495.Suche in Google Scholar
[13] Dhir R, Chauhan S, Subham P, Kumar S, Sharma P, Shidiki A, et al. Plant-mediated synthesis of silver nanoparticles: Unlocking their pharmacological potential-a comprehensive review. Front Bioeng Biotechnol. 2023;11:1324805. Switzerland.10.3389/fbioe.2023.1324805Suche in Google Scholar PubMed PubMed Central
[14] Ahmed HM. Ethnomedicinal, Phytochemical and pharmacological investigations of Perilla frutescens (L.) Britt. Molecules. 2018;24:102.10.3390/molecules24010102Suche in Google Scholar PubMed PubMed Central
[15] Igarashi M, Miyazaki Y. A review on bioactivities of Perilla: progress in research on the functions of Perilla as medicine and food. Evid Based Complement Altern Med. 2013;2013:925342. United States.10.1155/2013/925342Suche in Google Scholar PubMed PubMed Central
[16] Hou T, Guo Y, Han W, Zhou Y, Netala VR, Li H, et al. Exploring the biomedical applications of biosynthesized silver nanoparticles using Perilla frutescens flavonoid extract: antibacterial, antioxidant, and cell toxicity properties against colon cancer cells. Molecules. 2023;28:6431. Switzerland.10.3390/molecules28176431Suche in Google Scholar PubMed PubMed Central
[17] Basavegowda N, Lee YR. Synthesis of gold and silver nanoparticles using leaf extract of Perilla frutescens--a biogenic approach. J Nanosci Nanotechnol. 2014;14:4377–82. United States.10.1166/jnn.2014.8646Suche in Google Scholar PubMed
[18] Sharma G, Nam J-S, Sharma AR, Lee S-S. Antimicrobial potential of silver nanoparticles synthesized using medicinal herb Coptidis rhizome. Molecules. 2018;23:2269.10.3390/molecules23092268Suche in Google Scholar PubMed PubMed Central
[19] Lum JT-S, Leung KS-Y. Quantifying silver nanoparticle association and elemental content in single cells using dual mass mode in quadrupole-based inductively coupled plasma-mass spectrometry. Anal Chim Acta. 2019;1061:50–9. https://www.sciencedirect.com/science/article/pii/S0003267019302314.10.1016/j.aca.2019.02.042Suche in Google Scholar PubMed
[20] Sharma G, Sharma AR, Bhavesh R, Park J, Ganbold B, Nam J-S, et al. Biomolecule-mediated synthesis of selenium nanoparticles using dried Vitis vinifera (raisin) extract. Molecules. 2014;19:2761–70.10.3390/molecules19032761Suche in Google Scholar PubMed PubMed Central
[21] Sharma G, Alle M, Son HK, Kim J-C. Dialdehyde modification of laminarin for facile synthesis of ultrafine silver nanoparticles with excellent antibacterial and wound healing properties. Int J Biol Macromol. 2022;222:1364–75. Netherlands.10.1016/j.ijbiomac.2022.09.228Suche in Google Scholar PubMed
[22] Sharma G, Park SC, Bandi R, Ahn J, Alle M, Kim J-C. Polyquaternium enhances the colloidal stability of chitosan-capped platinum nanoparticles and their antibacterial activity. Nanotechnology. 2021;32:455603. England.10.1088/1361-6528/ac1afaSuche in Google Scholar PubMed
[23] Ahmad MZ, Saeed AM, Elnoubi OAE, Alasiri AS, Abdel-Wahab BA, Alqahtani AA, et al. Chitosan-based topical formulation integrated with green-synthesized silver nanoparticles utilizing Camellia sinensis leaf extracts: A promising approach for managing infected wounds. Int J Biol Macromol. 2024;257:128573. Netherlands.10.1016/j.ijbiomac.2023.128573Suche in Google Scholar PubMed
[24] Huang D, Ou B, Prior RL. The chemistry behind antioxidant capacity assays. J Agric Food Chem. 2005;53:1841–56. United States.10.1021/jf030723cSuche in Google Scholar PubMed
[25] Reddy NV, Li H, Hou T, Bethu MS, Ren Z, Zhang Z. Phytosynthesis of silver nanoparticles using Perilla frutescens leaf extract: Characterization and evaluation of antibacterial, antioxidant, and anticancer activities. Int J Nanomed. 2021;16:15–29. New Zealand.10.2147/IJN.S265003Suche in Google Scholar PubMed PubMed Central
[26] Ruch RJ, Cheng SJ, Klaunig JE. Prevention of cytotoxicity and inhibition of intercellular communication by antioxidant catechins isolated from Chinese green tea. Carcinogenesis. 1989;10:1003–8. England.10.1093/carcin/10.6.1003Suche in Google Scholar PubMed
[27] Vanaja M, Gnanajobitha G, Paulkumar K, Rajeshkumar S, Malarkodi C, Annadurai G. Phytosynthesis of silver nanoparticles by Cissus quadrangularis: Influence of physicochemical factors. J Nanostruct Chem. 2013;3:17. 10.1186/2193-8865-3-17.Suche in Google Scholar
[28] Długosz O, Banach M. Continuous production of silver nanoparticles and process control. J Clust Sci. 2019;30:541–52. 10.1007/s10876-019-01505-y.Suche in Google Scholar
[29] Farmahini Farahani A, Hamdi SMM, Mirzaee A. GC/MS analysis and phyto-synthesis of silver nanoparticles using Amygdalus spinosissima extract: Antibacterial, antioxidant effects, anticancer and apoptotic effects. Avicenna J Med Biotechnol. 2022;14:223–32. Iran.10.18502/ajmb.v14i3.9829Suche in Google Scholar PubMed PubMed Central
[30] Keskin D, Ceyhan N, Dbeys D. Antimicrobial activity and chemical constitutions of West Anatolian olive (Olea europaea L.) leaves. J Food Agric Environ. 2012;10:99–102. https://api.semanticscholar.org/CorpusID:85829610.Suche in Google Scholar
[31] Lingfa L, Tirumala A, Ankanagari S. GC-MS profiling of anticancer and antimicrobial phytochemicals in the vegetative leaf, root, and stem of Withania somnifera (L.) Dunal. Int J Second Metab. 2024;11:63–77.10.21448/ijsm.1256932Suche in Google Scholar
[32] Kumar RS, Anburaj G, Subramanian A, Vasantha S. Preliminary phytochemical investigation, antimicrobial activity and GC-MS analysis of leaf extract of Capparis zeylanica Linn. J Pharmacogn Phytochem. 2019;8:1399–405.Suche in Google Scholar
[33] Parakkal SC, Datta R, Muthu S, Alharbi NS, Abbas G. Solvent-solute interaction, thermodynamic behaviour, structural, chemical and anti-cancer biological properties of 3(2H)-furanone derivatives. J Mol Liq. 2023;390:123185. https://www.sciencedirect.com/science/article/pii/S0167732223019918.10.1016/j.molliq.2023.123185Suche in Google Scholar
[34] Gaceb-Terrak R, Rahmania F. Fatty acids derivatives and steroidal saponins: Abundance in the resistant date palm to agent of Bayoud disease. Int J Agric Biosyst Eng. 2013;7:902–5.Suche in Google Scholar
[35] Ben Khadher T, Sassi-Aydi S, Aydi S, Mars M, Bouajila J. Phytochemical profiling and biological potential of Prunus dulcis shell extracts. Plants. 2023;12:2733.10.3390/plants12142733Suche in Google Scholar PubMed PubMed Central
[36] Choi D, Kang W, Park T. Anti-allergic and anti-inflammatory effects of undecane on mast cells and keratinocytes. Molecules. 2020;25:1554.10.3390/molecules25071554Suche in Google Scholar PubMed PubMed Central
[37] Brishty SR, Hossain MJ, Khandaker MU, Faruque MRI, Osman H, Rahman SMA. A comprehensive account on recent progress in pharmacological activities of benzimidazole derivatives. Front Pharmacol. 2021;12:762807. https://www.frontiersin.org/journals/pharmacology/articles/10.3389/fphar.2021.762807.10.3389/fphar.2021.762807Suche in Google Scholar PubMed PubMed Central
[38] Chen Z, Liu Q, Zhao Z, Bai B, Sun Z, Cai L, et al. Effect of hydroxyl on antioxidant properties of 2,3-dihydro-3,5-dihydroxy-6-methyl-4H-pyran-4-one to scavenge free radicals. RSC Adv. 2021;11:34456–61. The Royal Society of Chemistry. 10.1039/D1RA06317K.Suche in Google Scholar
[39] Shukla R, Banerjee S, Tripathi YB. Antioxidant and antiapoptotic effect of aqueous extract of Pueraria tuberosa ( Roxb. Ex Willd.) DC. On streptozotocin-induced diabetic nephropathy in rats. BMC Complement Altern Med. 2018;18:156–66.10.1186/s12906-018-2221-xSuche in Google Scholar PubMed PubMed Central
[40] Teoh YP, Don MM. Mycelia growth and production of total flavonoids and 4H-pyran-4-one, 2, 3-dihydro-3, 5-dihydroxy-6-methyl- by Schizophyllum commune using a bubble column bioreactor considering aeration effect and mass transfer study. Chem Biochem Eng Q. 2024;28:553–59.10.15255/CABEQ.2014.2024Suche in Google Scholar
[41] Yami ACL, Batubara I, Audah KA. Antioxidant and antibacterial activity of mangrove Bruguiera gymnorhiza stem extracts against pathogenic bacteria Vibrio cholerae. Acta Biochim Indones. 2020;3:53–61. https://pbbmi.org/newjurnal/index.php/actabioina/article/view/51.10.32889/actabioina.v3i2.51Suche in Google Scholar
[42] Miao Y, Hu Y, Yang J, Liu T, Sun J, Wang X. Natural source, bioactivity and synthesis of benzofuran derivatives. RSC Adv. 2019;9:27510–40. The Royal Society of Chemistry. 10.1039/C9RA04917G.Suche in Google Scholar
[43] Hossan ASM, Abu-Melha HMA, Al-Omar MA, Amr AE-GE. Synthesis and antimicrobial activity of some new pyrimidinone and oxazinone derivatives fused with thiophene rings using 2-Chloro-6-ethoxy-4-acetylpyridine as starting material. Molecules. 2012;17:13642–55.10.3390/molecules171113642Suche in Google Scholar PubMed PubMed Central
[44] Wright MH, Sirdaarta J, White A, Greene AC, Cock IE, Wright MH, et al. GC-MS headspace analysis of Terminalia ferdinandiana fruit and leaf extracts which inhibit Bacillus anthracis growth. Pharmacogn J. 2017;9:73–82.10.5530/pj.2017.1.14Suche in Google Scholar
[45] Taher MA, Laboni AA, Shompa SA, Rahman MM, Hasan MM, Hasnat H, et al. Bioactive compounds extracted from leaves of G. cyanocarpa using various solvents in chromatographic separation showed anti-cancer and anti-microbial potentiality in in silico approach. Chin J Anal Chem. 2023;51:100336, https://www.sciencedirect.com/science/article/pii/S187220402300110X.10.1016/j.cjac.2023.100336Suche in Google Scholar
[46] Chansiw N, Chotinantakul K, Srichairatanakool S. Anti-inflammatory and antioxidant activities of the extracts from leaves and stems of Polygonum odoratum Lour. Antiinflamm Antiallergy Agents Med Chem. 2019;18:45–54. United Arab Emirates.10.2174/1871523017666181109144548Suche in Google Scholar PubMed PubMed Central
[47] Bettin Thomas T, Thirumalaikumar E, Sathishkumar R, Rajeswari MV, Vimal S, Uma G, et al. Effects of dietary Andrographis paniculata extract on growth, haematological, immune responses, immune-related genes expression of ornamental goldfish (Carassius auratus) and its susceptibility to Aeromonas hydrophila infection. Aquac Rep. 2023;33:101850, https://www.sciencedirect.com/science/article/pii/S2352513423003897.10.1016/j.aqrep.2023.101850Suche in Google Scholar
[48] Nabi M, Tabassum N, Ganai BA. Phytochemical screening and antibacterial activity of Skimmia anquetilia N. P. Taylor and Airy Shaw: A first study from Kashmir Himalaya. Front Plant Sci. 2022;2022:1–16.10.3389/fpls.2022.937946Suche in Google Scholar PubMed PubMed Central
[49] Mohanta YK, Panda SK, Bastia AK, Mohanta TK. Biosynthesis of silver nanoparticles from Protium serratum and investigation of their potential impacts on food safety and control. Front Microbiol. 2017;8:626. Switzerland.10.3389/fmicb.2017.00626Suche in Google Scholar PubMed PubMed Central
[50] Sandulovici RC, Carmen-Marinela M, Grigoroiu A, Moldovan CA, Savin M, Ordeanu V, et al. The physicochemical and antimicrobial properties of silver/gold nanoparticles obtained by “green synthesis” from Willow Bark and their formulations as potential innovative pharmaceutical substances. Pharmaceuticals. 2023;16:48.10.3390/ph16010048Suche in Google Scholar PubMed PubMed Central
[51] Arshad H, Sadaf S, Hassan U. De-novo fabrication of sunlight irradiated silver nanoparticles and their efficacy against E. coli and S. epidermidis. Sci Rep. 2022;12:676. 10.1038/s41598-021-04674-x.Suche in Google Scholar PubMed PubMed Central
[52] Prathap M, Alagesan A, Ranjitha Kumari BD. Anti-bacterial activities of silver nanoparticles synthesized from plant leaf extract of Abutilon indicum (L.) Sweet. J Nanostruct Chem. 2014;4:106. 10.1007/s40097-014-0106-1.Suche in Google Scholar
[53] İpek P, Yıldız R, Baran MF, Hatipoğlu A, Baran A, Sufianov A, et al. Green synthesis of silver nanoparticles derived from Papaver rhoeas L. Leaf Extract: Cytotoxic and antimicrobial properties. Molecules. 2023;28:6424. Switzerland.10.3390/molecules28176424Suche in Google Scholar PubMed PubMed Central
[54] Ali DM, Sasikala M, Gunasekaran M, Thajuddin N. Biosynthesis and characterization of silver nanoparticles using marine cyanobacterium, oscillatoria WILLEI NTDM01. Digest J Nanomat Biostructures. 2011;6:385–90.Suche in Google Scholar
[55] Benakashani F, Allafchian AR, Jalali SAH. Biosynthesis of silver nanoparticles using Capparis spinosa L. leaf extract and their antibacterial activity. Karbala Int J Mod Sci. 2016;2:251–8, https://www.sciencedirect.com/science/article/pii/S2405609X16302159.10.1016/j.kijoms.2016.08.004Suche in Google Scholar
[56] Sun X, Lv X, Sui M, Weng X, Li X, Wang J. Decorating MOF-derived nanoporous Co/C in chain-like polypyrrole (PPy) aerogel: A lightweight material with excellent electromagnetic absorption. Materials (Basel). 2018;11:781.10.3390/ma11050781Suche in Google Scholar PubMed PubMed Central
[57] Lin G, Zhao C, Liao W, Yang J, Zheng Y. Eco-friendly green synthesis of Rubropunctatin functionalized silver nanoparticles and evaluation of antibacterial activity. Nanomaterials. 2022;12:4052.10.3390/nano12224052Suche in Google Scholar PubMed PubMed Central
[58] de Jesús Ruíz-Baltazar Á, Reyes-López SY, de Lourdes Mondragón-Sánchez M, Estevez M, Hernández-Martinez AR, Pérez R. Biosynthesis of Ag nanoparticles using Cynara cardunculus leaf extract: Evaluation of their antibacterial and electrochemical activity. Results Phys. 2018;11:1142–9, https://www.sciencedirect.com/science/article/pii/S221137971831934X.10.1016/j.rinp.2018.11.032Suche in Google Scholar
[59] Shen N, Wang T, Gan Q, Liu S, Wang L, Jin B. Plant flavonoids: Classification, distribution, biosynthesis, and antioxidant activity. Food Chem. 2022;383:132531. England.10.1016/j.foodchem.2022.132531Suche in Google Scholar PubMed
[60] Keshari AK, Srivastava R, Singh P, Yadav VB, Nath G. Antioxidant and antibacterial activity of silver nanoparticles synthesized by Cestrum nocturnum. J Ayurveda Integr Med. 2020;11:37–44. United States.10.1016/j.jaim.2017.11.003Suche in Google Scholar PubMed PubMed Central
[61] Sahin Yaglioglu A, Erenler R, Gecer EN, Genc N. Biosynthesis of silver nanoparticles using Astragalus flavesces Leaf: Identification, antioxidant activity, and catalytic degradation of methylene blue. J Inorg Organomet Polym Mater. 2022;32:3700–7. 10.1007/s10904-022-02362-5.Suche in Google Scholar
[62] Erenler R, Chaoui R, Yildiz I, Genc N, Gecer EN, Temiz C, et al. Biosynthesis, characterisation, and antioxidant activity of silver nanoparticles using schinus molle L. Trends Sci. 2023;20:6105. https://tis.wu.ac.th/index.php/tis/article/view/6105.10.48048/tis.2023.6105Suche in Google Scholar
[63] Gecer N, Gecer EN, Erenler R. Organic biosynthesis of silver nanoparticles using Echinacea pallida (Nutt.) Nutt. and antioxidant activity thereof. Org Biochem. 2022;44:610–17.10.52568/001187/JCSP/44.06.2022Suche in Google Scholar
[64] Munoz-Davila MJ. Role of old antibiotics in the era of antibiotic resistance. Highlighted nitrofurantoin for the treatment of lower urinary tract infections. Antibiotics (Basel, Switz). 2014;3:39–48. Switzerland.10.3390/antibiotics3010039Suche in Google Scholar PubMed PubMed Central
[65] Che J, Zheng C-J, Song M-X, Bi Y-J, Liu Y, Li Y-J, et al. Synthesis and antibacterial evaluation of furan derivatives bearing a rhodanine moiety. Med Chem Res. 2014;23:426–35. 10.1007/s00044-013-0648-7.Suche in Google Scholar
[66] Ullah R, Alsaid MS, Alqahtani AS, Shahat AA, Naser AA, Mahmood HM, et al. Anti-inflammatory, antipyretic, analgesic, and antioxidant activities of Haloxylon salicornicum aqueous fraction. Open Chem. 2019;17:1034–42.10.1515/chem-2019-0113Suche in Google Scholar
[67] Fagbemi KO, Aina DA, Adeoye-Isijola MO, Naidoo KK, Coopoosamy RM, Olajuyigbe OO. Bioactive compounds, antibacterial and antioxidant activities of methanol extract of Tamarindus indica Linn. Sci Rep. 2022;12:9432. England.10.1038/s41598-022-13716-xSuche in Google Scholar PubMed PubMed Central
[68] Dandekar R, Fegade B, Bhaskar VH. GC-MS analysis of phytoconstituents in alcohol extract of Epiphyllum oxypetalum leaves. J Pharmacogn Phytochem. 2015;4:149–54.Suche in Google Scholar
[69] Pietta PG. Flavonoids as antioxidants. J Nat Prod. 2000;63:1035–42. United States.10.1021/np9904509Suche in Google Scholar PubMed
[70] Čechovská L, Cejpek K, Konečný M, Velíšek J. On the role of 2,3-dihydro-3,5-dihydroxy-6-methyl-(4H)-pyran-4-one in antioxidant capacity of prunes. Eur Food Res Technol. 2011;233:367–76.10.1007/s00217-011-1527-4Suche in Google Scholar
[71] Aryal P, Shakya B. Synthesis, cytotoxicity, antibacterial and antioxidant activity of new 2-Substituted Benzimidazole Containing 1,2,4-Triazoles. J Nepal Chem Soc. 2023;44:34–45.10.3126/jncs.v43i2.53339Suche in Google Scholar
[72] Wang Z-J, Liang C-L, Li G-M, Yu C-Y, Yin M. Stearic acid protects primary cultured cortical neurons against oxidative stress. Acta Pharmacol Sin. 2007;28:315–26. United States.10.1111/j.1745-7254.2007.00512.xSuche in Google Scholar PubMed
[73] Paladini F, Pollini M. Antimicrobial silver nanoparticles for wound healing application: Progress and future trends. Materials (Basel, Switz). 2019;12:2540. Switzerland.10.3390/ma12162540Suche in Google Scholar PubMed PubMed Central
[74] Liu X, Lee P, Ho C, Lui VCH, Chen Y, Che C, et al. Silver nanoparticles mediate differential responses in keratinocytes and fibroblasts during skin wound healing. ChemMedChem. 2010;5:468–75. John Wiley & Sons, Ltd. 10.1002/cmdc.200900502.Suche in Google Scholar PubMed
[75] Nam G, Rangasamy S, Purushothaman B, Song JM. The application of bactericidal silver nanoparticles in wound treatment. Nanomater Nanotechnol. 2015;5:23. SAGE Publications Ltd STM. 10.5772/60918.Suche in Google Scholar
[76] Franková J, Pivodová V, Vágnerová H, Juráňová J, Ulrichová J. Effects of silver nanoparticles on primary cell cultures of fibroblasts and keratinocytes in a wound-healing model. J Appl Biomater Funct Mater. 2016;14:e137–42. United States.10.5301/jabfm.5000268Suche in Google Scholar PubMed
[77] You C, Li Q, Wang X, Wu P, Ho JK, Jin R, et al. Silver nanoparticle loaded collagen/chitosan scaffolds promote wound healing via regulating fibroblast migration and macrophage activation. Sci Rep. 2017;7:10489. 10.1038/s41598-017-10481-0.Suche in Google Scholar PubMed PubMed Central
© 2024 the author(s), published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Artikel in diesem Heft
- Research Articles
- Green polymer electrolyte and activated charcoal-based supercapacitor for energy harvesting application: Electrochemical characteristics
- Research on the adsorption of Co2+ ions using halloysite clay and the ability to recover them by electrodeposition method
- Simultaneous estimation of ibuprofen, caffeine, and paracetamol in commercial products using a green reverse-phase HPTLC method
- Isolation, screening and optimization of alkaliphilic cellulolytic fungi for production of cellulase
- Functionalized gold nanoparticles coated with bacterial alginate and their antibacterial and anticancer activities
- Comparative analysis of bio-based amino acid surfactants obtained via Diels–Alder reaction of cyclic anhydrides
- Biosynthesis of silver nanoparticles on yellow phosphorus slag and its application in organic coatings
- Exploring antioxidant potential and phenolic compound extraction from Vitis vinifera L. using ultrasound-assisted extraction
- Manganese and copper-coated nickel oxide nanoparticles synthesized from Carica papaya leaf extract induce antimicrobial activity and breast cancer cell death by triggering mitochondrial caspases and p53
- Insight into heating method and Mozafari method as green processing techniques for the synthesis of micro- and nano-drug carriers
- Silicotungstic acid supported on Bi-based MOF-derived metal oxide for photodegradation of organic dyes
- Synthesis and characterization of capsaicin nanoparticles: An attempt to enhance its bioavailability and pharmacological actions
- Synthesis of Lawsonia inermis-encased silver–copper bimetallic nanoparticles with antioxidant, antibacterial, and cytotoxic activity
- Facile, polyherbal drug-mediated green synthesis of CuO nanoparticles and their potent biological applications
- Zinc oxide-manganese oxide/carboxymethyl cellulose-folic acid-sesamol hybrid nanomaterials: A molecularly targeted strategy for advanced triple-negative breast cancer therapy
- Exploring the antimicrobial potential of biogenically synthesized graphene oxide nanoparticles against targeted bacterial and fungal pathogens
- Biofabrication of silver nanoparticles using Uncaria tomentosa L.: Insight into characterization, antibacterial activities combined with antibiotics, and effect on Triticum aestivum germination
- Membrane distillation of synthetic urine for use in space structural habitat systems
- Investigation on mechanical properties of the green synthesis bamboo fiber/eggshell/coconut shell powder-based hybrid biocomposites under NaOH conditions
- Green synthesis of magnesium oxide nanoparticles using endophytic fungal strain to improve the growth, metabolic activities, yield traits, and phenolic compounds content of Nigella sativa L.
- Estimation of greenhouse gas emissions from rice and annual upland crops in Red River Delta of Vietnam using the denitrification–decomposition model
- Synthesis of humic acid with the obtaining of potassium humate based on coal waste from the Lenger deposit, Kazakhstan
- Ascorbic acid-mediated selenium nanoparticles as potential antihyperuricemic, antioxidant, anticoagulant, and thrombolytic agents
- Green synthesis of silver nanoparticles using Illicium verum extract: Optimization and characterization for biomedical applications
- Antibacterial and dynamical behaviour of silicon nanoparticles influenced sustainable waste flax fibre-reinforced epoxy composite for biomedical application
- Optimising coagulation/flocculation using response surface methodology and application of floc in biofertilisation
- Green synthesis and multifaceted characterization of iron oxide nanoparticles derived from Senna bicapsularis for enhanced in vitro and in vivo biological investigation
- Potent antibacterial nanocomposites from okra mucilage/chitosan/silver nanoparticles for multidrug-resistant Salmonella Typhimurium eradication
- Trachyspermum copticum aqueous seed extract-derived silver nanoparticles: Exploration of their structural characterization and comparative antibacterial performance against gram-positive and gram-negative bacteria
- Microwave-assisted ultrafine silver nanoparticle synthesis using Mitragyna speciosa for antimalarial applications
- Green synthesis and characterisation of spherical structure Ag/Fe2O3/TiO2 nanocomposite using acacia in the presence of neem and tulsi oils
- Green quantitative methods for linagliptin and empagliflozin in dosage forms
- Enhancement efficacy of omeprazole by conjugation with silver nanoparticles as a urease inhibitor
- Residual, sequential extraction, and ecological risk assessment of some metals in ash from municipal solid waste incineration, Vietnam
- Green synthesis of ZnO nanoparticles using the mangosteen (Garcinia mangostana L.) leaf extract: Comparative preliminary in vitro antibacterial study
- Simultaneous determination of lesinurad and febuxostat in commercial fixed-dose combinations using a greener normal-phase HPTLC method
- A greener RP-HPLC method for quaternary estimation of caffeine, paracetamol, levocetirizine, and phenylephrine acquiring AQbD with stability studies
- Optimization of biomass durian peel as a heterogeneous catalyst in biodiesel production using microwave irradiation
- Thermal treatment impact on the evolution of active phases in layered double hydroxide-based ZnCr photocatalysts: Photodegradation and antibacterial performance
- Preparation of silymarin-loaded zein polysaccharide core–shell nanostructures and evaluation of their biological potentials
- Preparation and characterization of composite-modified PA6 fiber for spectral heating and heat storage applications
- Preparation and electrocatalytic oxygen evolution of bimetallic phosphates (NiFe)2P/NF
- Rod-shaped Mo(vi) trichalcogenide–Mo(vi) oxide decorated on poly(1-H pyrrole) as a promising nanocomposite photoelectrode for green hydrogen generation from sewage water with high efficiency
- Green synthesis and studies on citrus medica leaf extract-mediated Au–ZnO nanocomposites: A sustainable approach for efficient photocatalytic degradation of rhodamine B dye in aqueous media
- Cellulosic materials for the removal of ciprofloxacin from aqueous environments
- The analytical assessment of metal contamination in industrial soils of Saudi Arabia using the inductively coupled plasma technology
- The effect of modified oily sludge on the slurry ability and combustion performance of coal water slurry
- Eggshell waste transformation to calcium chloride anhydride as food-grade additive and eggshell membranes as enzyme immobilization carrier
- Synthesis of EPAN and applications in the encapsulation of potassium humate
- Biosynthesis and characterization of silver nanoparticles from Cedrela toona leaf extracts: An exploration into their antibacterial, anticancer, and antioxidant potential
- Enhancing mechanical and rheological properties of HDPE films through annealing for eco-friendly agricultural applications
- Immobilisation of catalase purified from mushroom (Hydnum repandum) onto glutaraldehyde-activated chitosan and characterisation: Its application for the removal of hydrogen peroxide from artificial wastewater
- Sodium titanium oxide/zinc oxide (STO/ZnO) photocomposites for efficient dye degradation applications
- Effect of ex situ, eco-friendly ZnONPs incorporating green synthesised Moringa oleifera leaf extract in enhancing biochemical and molecular aspects of Vicia faba L. under salt stress
- Biosynthesis and characterization of selenium and silver nanoparticles using Trichoderma viride filtrate and their impact on Culex pipiens
- Photocatalytic degradation of organic dyes and biological potentials of biogenic zinc oxide nanoparticles synthesized using the polar extract of Cyperus scariosus R.Br. (Cyperaceae)
- Assessment of antiproliferative activity of green-synthesized nickel oxide nanoparticles against glioblastoma cells using Terminalia chebula
- Chlorine-free synthesis of phosphinic derivatives by change in the P-function
- Anticancer, antioxidant, and antimicrobial activities of nanoemulsions based on water-in-olive oil and loaded on biogenic silver nanoparticles
- Study and mechanism of formation of phosphorus production waste in Kazakhstan
- Synthesis and stabilization of anatase form of biomimetic TiO2 nanoparticles for enhancing anti-tumor potential
- Microwave-supported one-pot reaction for the synthesis of 5-alkyl/arylidene-2-(morpholin/thiomorpholin-4-yl)-1,3-thiazol-4(5H)-one derivatives over MgO solid base
- Screening the phytochemicals in Perilla leaves and phytosynthesis of bioactive silver nanoparticles for potential antioxidant and wound-healing application
- Graphene oxide/chitosan/manganese/folic acid-brucine functionalized nanocomposites show anticancer activity against liver cancer cells
- Nature of serpentinite interactions with low-concentration sulfuric acid solutions
- Multi-objective statistical optimisation utilising response surface methodology to predict engine performance using biofuels from waste plastic oil in CRDi engines
- Microwave-assisted extraction of acetosolv lignin from sugarcane bagasse and electrospinning of lignin/PEO nanofibres for carbon fibre production
- Biosynthesis, characterization, and investigation of cytotoxic activities of selenium nanoparticles utilizing Limosilactobacillus fermentum
- Highly photocatalytic materials based on the decoration of poly(O-chloroaniline) with molybdenum trichalcogenide oxide for green hydrogen generation from Red Sea water
- Highly efficient oil–water separation using superhydrophobic cellulose aerogels derived from corn straw
- Beta-cyclodextrin–Phyllanthus emblica emulsion for zinc oxide nanoparticles: Characteristics and photocatalysis
- Assessment of antimicrobial activity and methyl orange dye removal by Klebsiella pneumoniae-mediated silver nanoparticles
- Influential eradication of resistant Salmonella Typhimurium using bioactive nanocomposites from chitosan and radish seed-synthesized nanoselenium
- Antimicrobial activities and neuroprotective potential for Alzheimer’s disease of pure, Mn, Co, and Al-doped ZnO ultra-small nanoparticles
- Green synthesis of silver nanoparticles from Bauhinia variegata and their biological applications
- Synthesis and optimization of long-chain fatty acids via the oxidation of long-chain fatty alcohols
- Eminent Red Sea water hydrogen generation via a Pb(ii)-iodide/poly(1H-pyrrole) nanocomposite photocathode
- Green synthesis and effective genistein production by fungal β-glucosidase immobilized on Al2O3 nanocrystals synthesized in Cajanus cajan L. (Millsp.) leaf extracts
- Green stability-indicating RP-HPTLC technique for determining croconazole hydrochloride
- Green synthesis of La2O3–LaPO4 nanocomposites using Charybdis natator for DNA binding, cytotoxic, catalytic, and luminescence applications
- Eco-friendly drugs induce cellular changes in colistin-resistant bacteria
- Tangerine fruit peel extract mediated biogenic synthesized silver nanoparticles and their potential antimicrobial, antioxidant, and cytotoxic assessments
- Green synthesis on performance characteristics of a direct injection diesel engine using sandbox seed oil
- A highly sensitive β-AKBA-Ag-based fluorescent “turn off” chemosensor for rapid detection of abamectin in tomatoes
- Green synthesis and physical characterization of zinc oxide nanoparticles (ZnO NPs) derived from the methanol extract of Euphorbia dracunculoides Lam. (Euphorbiaceae) with enhanced biosafe applications
- Detection of morphine and data processing using surface plasmon resonance imaging sensor
- Effects of nanoparticles on the anaerobic digestion properties of sulfamethoxazole-containing chicken manure and analysis of bio-enzymes
- Bromic acid-thiourea synergistic leaching of sulfide gold ore
- Green chemistry approach to synthesize titanium dioxide nanoparticles using Fagonia Cretica extract, novel strategy for developing antimicrobial and antidiabetic therapies
- Green synthesis and effective utilization of biogenic Al2O3-nanocoupled fungal lipase in the resolution of active homochiral 2-octanol and its immobilization via aluminium oxide nanoparticles
- Eco-friendly RP-HPLC approach for simultaneously estimating the promising combination of pentoxifylline and simvastatin in therapeutic potential for breast cancer: Appraisal of greenness, whiteness, and Box–Behnken design
- Use of a humidity adsorbent derived from cockleshell waste in Thai fried fish crackers (Keropok)
- One-pot green synthesis, biological evaluation, and in silico study of pyrazole derivatives obtained from chalcones
- Bio-sorption of methylene blue and production of biofuel by brown alga Cystoseira sp. collected from Neom region, Kingdom of Saudi Arabia
- Synthesis of motexafin gadolinium: A promising radiosensitizer and imaging agent for cancer therapy
- The impact of varying sizes of silver nanoparticles on the induction of cellular damage in Klebsiella pneumoniae involving diverse mechanisms
- Microwave-assisted green synthesis, characterization, and in vitro antibacterial activity of NiO nanoparticles obtained from lemon peel extract
- Rhus microphylla-mediated biosynthesis of copper oxide nanoparticles for enhanced antibacterial and antibiofilm efficacy
- Harnessing trichalcogenide–molybdenum(vi) sulfide and molybdenum(vi) oxide within poly(1-amino-2-mercaptobenzene) frameworks as a photocathode for sustainable green hydrogen production from seawater without sacrificial agents
- Magnetically recyclable Fe3O4@SiO2 supported phosphonium ionic liquids for efficient and sustainable transformation of CO2 into oxazolidinones
- A comparative study of Fagonia arabica fabricated silver sulfide nanoparticles (Ag2S) and silver nanoparticles (AgNPs) with distinct antimicrobial, anticancer, and antioxidant properties
- Visible light photocatalytic degradation and biological activities of Aegle marmelos-mediated cerium oxide nanoparticles
- Physical intrinsic characteristics of spheroidal particles in coal gasification fine slag
- Exploring the effect of tea dust magnetic biochar on agricultural crops grown in polycyclic aromatic hydrocarbon contaminated soil
- Crosslinked chitosan-modified ultrafiltration membranes for efficient surface water treatment and enhanced anti-fouling performances
- Study on adsorption characteristics of biochars and their modified biochars for removal of organic dyes from aqueous solution
- Zein polymer nanocarrier for Ocimum basilicum var. purpurascens extract: Potential biomedical use
- Green synthesis, characterization, and in vitro and in vivo biological screening of iron oxide nanoparticles (Fe3O4) generated with hydroalcoholic extract of aerial parts of Euphorbia milii
- Novel microwave-based green approach for the synthesis of dual-loaded cyclodextrin nanosponges: Characterization, pharmacodynamics, and pharmacokinetics evaluation
- Bi2O3–BiOCl/poly-m-methyl aniline nanocomposite thin film for broad-spectrum light-sensing
- Green synthesis and characterization of CuO/ZnO nanocomposite using Musa acuminata leaf extract for cytotoxic studies on colorectal cancer cells (HCC2998)
- Review Articles
- Materials-based drug delivery approaches: Recent advances and future perspectives
- A review of thermal treatment for bamboo and its composites
- An overview of the role of nanoherbicides in tackling challenges of weed management in wheat: A novel approach
- An updated review on carbon nanomaterials: Types, synthesis, functionalization and applications, degradation and toxicity
- Special Issue: Emerging green nanomaterials for sustainable waste management and biomedical applications
- Green synthesis of silver nanoparticles using mature-pseudostem extracts of Alpinia nigra and their bioactivities
- Special Issue: New insights into nanopythotechnology: current trends and future prospects
- Green synthesis of FeO nanoparticles from coffee and its application for antibacterial, antifungal, and anti-oxidation activity
- Dye degradation activity of biogenically synthesized Cu/Fe/Ag trimetallic nanoparticles
- Special Issue: Composites and green composites
- Recent trends and advancements in the utilization of green composites and polymeric nanocarriers for enhancing food quality and sustainable processing
- Retraction
- Retraction of “Biosynthesis and characterization of silver nanoparticles from Cedrela toona leaf extracts: An exploration into their antibacterial, anticancer, and antioxidant potential”
- Retraction of “Photocatalytic degradation of organic dyes and biological potentials of biogenic zinc oxide nanoparticles synthesized using the polar extract of Cyperus scariosus R.Br. (Cyperaceae)”
- Retraction to “Green synthesis on performance characteristics of a direct injection diesel engine using sandbox seed oil”
Artikel in diesem Heft
- Research Articles
- Green polymer electrolyte and activated charcoal-based supercapacitor for energy harvesting application: Electrochemical characteristics
- Research on the adsorption of Co2+ ions using halloysite clay and the ability to recover them by electrodeposition method
- Simultaneous estimation of ibuprofen, caffeine, and paracetamol in commercial products using a green reverse-phase HPTLC method
- Isolation, screening and optimization of alkaliphilic cellulolytic fungi for production of cellulase
- Functionalized gold nanoparticles coated with bacterial alginate and their antibacterial and anticancer activities
- Comparative analysis of bio-based amino acid surfactants obtained via Diels–Alder reaction of cyclic anhydrides
- Biosynthesis of silver nanoparticles on yellow phosphorus slag and its application in organic coatings
- Exploring antioxidant potential and phenolic compound extraction from Vitis vinifera L. using ultrasound-assisted extraction
- Manganese and copper-coated nickel oxide nanoparticles synthesized from Carica papaya leaf extract induce antimicrobial activity and breast cancer cell death by triggering mitochondrial caspases and p53
- Insight into heating method and Mozafari method as green processing techniques for the synthesis of micro- and nano-drug carriers
- Silicotungstic acid supported on Bi-based MOF-derived metal oxide for photodegradation of organic dyes
- Synthesis and characterization of capsaicin nanoparticles: An attempt to enhance its bioavailability and pharmacological actions
- Synthesis of Lawsonia inermis-encased silver–copper bimetallic nanoparticles with antioxidant, antibacterial, and cytotoxic activity
- Facile, polyherbal drug-mediated green synthesis of CuO nanoparticles and their potent biological applications
- Zinc oxide-manganese oxide/carboxymethyl cellulose-folic acid-sesamol hybrid nanomaterials: A molecularly targeted strategy for advanced triple-negative breast cancer therapy
- Exploring the antimicrobial potential of biogenically synthesized graphene oxide nanoparticles against targeted bacterial and fungal pathogens
- Biofabrication of silver nanoparticles using Uncaria tomentosa L.: Insight into characterization, antibacterial activities combined with antibiotics, and effect on Triticum aestivum germination
- Membrane distillation of synthetic urine for use in space structural habitat systems
- Investigation on mechanical properties of the green synthesis bamboo fiber/eggshell/coconut shell powder-based hybrid biocomposites under NaOH conditions
- Green synthesis of magnesium oxide nanoparticles using endophytic fungal strain to improve the growth, metabolic activities, yield traits, and phenolic compounds content of Nigella sativa L.
- Estimation of greenhouse gas emissions from rice and annual upland crops in Red River Delta of Vietnam using the denitrification–decomposition model
- Synthesis of humic acid with the obtaining of potassium humate based on coal waste from the Lenger deposit, Kazakhstan
- Ascorbic acid-mediated selenium nanoparticles as potential antihyperuricemic, antioxidant, anticoagulant, and thrombolytic agents
- Green synthesis of silver nanoparticles using Illicium verum extract: Optimization and characterization for biomedical applications
- Antibacterial and dynamical behaviour of silicon nanoparticles influenced sustainable waste flax fibre-reinforced epoxy composite for biomedical application
- Optimising coagulation/flocculation using response surface methodology and application of floc in biofertilisation
- Green synthesis and multifaceted characterization of iron oxide nanoparticles derived from Senna bicapsularis for enhanced in vitro and in vivo biological investigation
- Potent antibacterial nanocomposites from okra mucilage/chitosan/silver nanoparticles for multidrug-resistant Salmonella Typhimurium eradication
- Trachyspermum copticum aqueous seed extract-derived silver nanoparticles: Exploration of their structural characterization and comparative antibacterial performance against gram-positive and gram-negative bacteria
- Microwave-assisted ultrafine silver nanoparticle synthesis using Mitragyna speciosa for antimalarial applications
- Green synthesis and characterisation of spherical structure Ag/Fe2O3/TiO2 nanocomposite using acacia in the presence of neem and tulsi oils
- Green quantitative methods for linagliptin and empagliflozin in dosage forms
- Enhancement efficacy of omeprazole by conjugation with silver nanoparticles as a urease inhibitor
- Residual, sequential extraction, and ecological risk assessment of some metals in ash from municipal solid waste incineration, Vietnam
- Green synthesis of ZnO nanoparticles using the mangosteen (Garcinia mangostana L.) leaf extract: Comparative preliminary in vitro antibacterial study
- Simultaneous determination of lesinurad and febuxostat in commercial fixed-dose combinations using a greener normal-phase HPTLC method
- A greener RP-HPLC method for quaternary estimation of caffeine, paracetamol, levocetirizine, and phenylephrine acquiring AQbD with stability studies
- Optimization of biomass durian peel as a heterogeneous catalyst in biodiesel production using microwave irradiation
- Thermal treatment impact on the evolution of active phases in layered double hydroxide-based ZnCr photocatalysts: Photodegradation and antibacterial performance
- Preparation of silymarin-loaded zein polysaccharide core–shell nanostructures and evaluation of their biological potentials
- Preparation and characterization of composite-modified PA6 fiber for spectral heating and heat storage applications
- Preparation and electrocatalytic oxygen evolution of bimetallic phosphates (NiFe)2P/NF
- Rod-shaped Mo(vi) trichalcogenide–Mo(vi) oxide decorated on poly(1-H pyrrole) as a promising nanocomposite photoelectrode for green hydrogen generation from sewage water with high efficiency
- Green synthesis and studies on citrus medica leaf extract-mediated Au–ZnO nanocomposites: A sustainable approach for efficient photocatalytic degradation of rhodamine B dye in aqueous media
- Cellulosic materials for the removal of ciprofloxacin from aqueous environments
- The analytical assessment of metal contamination in industrial soils of Saudi Arabia using the inductively coupled plasma technology
- The effect of modified oily sludge on the slurry ability and combustion performance of coal water slurry
- Eggshell waste transformation to calcium chloride anhydride as food-grade additive and eggshell membranes as enzyme immobilization carrier
- Synthesis of EPAN and applications in the encapsulation of potassium humate
- Biosynthesis and characterization of silver nanoparticles from Cedrela toona leaf extracts: An exploration into their antibacterial, anticancer, and antioxidant potential
- Enhancing mechanical and rheological properties of HDPE films through annealing for eco-friendly agricultural applications
- Immobilisation of catalase purified from mushroom (Hydnum repandum) onto glutaraldehyde-activated chitosan and characterisation: Its application for the removal of hydrogen peroxide from artificial wastewater
- Sodium titanium oxide/zinc oxide (STO/ZnO) photocomposites for efficient dye degradation applications
- Effect of ex situ, eco-friendly ZnONPs incorporating green synthesised Moringa oleifera leaf extract in enhancing biochemical and molecular aspects of Vicia faba L. under salt stress
- Biosynthesis and characterization of selenium and silver nanoparticles using Trichoderma viride filtrate and their impact on Culex pipiens
- Photocatalytic degradation of organic dyes and biological potentials of biogenic zinc oxide nanoparticles synthesized using the polar extract of Cyperus scariosus R.Br. (Cyperaceae)
- Assessment of antiproliferative activity of green-synthesized nickel oxide nanoparticles against glioblastoma cells using Terminalia chebula
- Chlorine-free synthesis of phosphinic derivatives by change in the P-function
- Anticancer, antioxidant, and antimicrobial activities of nanoemulsions based on water-in-olive oil and loaded on biogenic silver nanoparticles
- Study and mechanism of formation of phosphorus production waste in Kazakhstan
- Synthesis and stabilization of anatase form of biomimetic TiO2 nanoparticles for enhancing anti-tumor potential
- Microwave-supported one-pot reaction for the synthesis of 5-alkyl/arylidene-2-(morpholin/thiomorpholin-4-yl)-1,3-thiazol-4(5H)-one derivatives over MgO solid base
- Screening the phytochemicals in Perilla leaves and phytosynthesis of bioactive silver nanoparticles for potential antioxidant and wound-healing application
- Graphene oxide/chitosan/manganese/folic acid-brucine functionalized nanocomposites show anticancer activity against liver cancer cells
- Nature of serpentinite interactions with low-concentration sulfuric acid solutions
- Multi-objective statistical optimisation utilising response surface methodology to predict engine performance using biofuels from waste plastic oil in CRDi engines
- Microwave-assisted extraction of acetosolv lignin from sugarcane bagasse and electrospinning of lignin/PEO nanofibres for carbon fibre production
- Biosynthesis, characterization, and investigation of cytotoxic activities of selenium nanoparticles utilizing Limosilactobacillus fermentum
- Highly photocatalytic materials based on the decoration of poly(O-chloroaniline) with molybdenum trichalcogenide oxide for green hydrogen generation from Red Sea water
- Highly efficient oil–water separation using superhydrophobic cellulose aerogels derived from corn straw
- Beta-cyclodextrin–Phyllanthus emblica emulsion for zinc oxide nanoparticles: Characteristics and photocatalysis
- Assessment of antimicrobial activity and methyl orange dye removal by Klebsiella pneumoniae-mediated silver nanoparticles
- Influential eradication of resistant Salmonella Typhimurium using bioactive nanocomposites from chitosan and radish seed-synthesized nanoselenium
- Antimicrobial activities and neuroprotective potential for Alzheimer’s disease of pure, Mn, Co, and Al-doped ZnO ultra-small nanoparticles
- Green synthesis of silver nanoparticles from Bauhinia variegata and their biological applications
- Synthesis and optimization of long-chain fatty acids via the oxidation of long-chain fatty alcohols
- Eminent Red Sea water hydrogen generation via a Pb(ii)-iodide/poly(1H-pyrrole) nanocomposite photocathode
- Green synthesis and effective genistein production by fungal β-glucosidase immobilized on Al2O3 nanocrystals synthesized in Cajanus cajan L. (Millsp.) leaf extracts
- Green stability-indicating RP-HPTLC technique for determining croconazole hydrochloride
- Green synthesis of La2O3–LaPO4 nanocomposites using Charybdis natator for DNA binding, cytotoxic, catalytic, and luminescence applications
- Eco-friendly drugs induce cellular changes in colistin-resistant bacteria
- Tangerine fruit peel extract mediated biogenic synthesized silver nanoparticles and their potential antimicrobial, antioxidant, and cytotoxic assessments
- Green synthesis on performance characteristics of a direct injection diesel engine using sandbox seed oil
- A highly sensitive β-AKBA-Ag-based fluorescent “turn off” chemosensor for rapid detection of abamectin in tomatoes
- Green synthesis and physical characterization of zinc oxide nanoparticles (ZnO NPs) derived from the methanol extract of Euphorbia dracunculoides Lam. (Euphorbiaceae) with enhanced biosafe applications
- Detection of morphine and data processing using surface plasmon resonance imaging sensor
- Effects of nanoparticles on the anaerobic digestion properties of sulfamethoxazole-containing chicken manure and analysis of bio-enzymes
- Bromic acid-thiourea synergistic leaching of sulfide gold ore
- Green chemistry approach to synthesize titanium dioxide nanoparticles using Fagonia Cretica extract, novel strategy for developing antimicrobial and antidiabetic therapies
- Green synthesis and effective utilization of biogenic Al2O3-nanocoupled fungal lipase in the resolution of active homochiral 2-octanol and its immobilization via aluminium oxide nanoparticles
- Eco-friendly RP-HPLC approach for simultaneously estimating the promising combination of pentoxifylline and simvastatin in therapeutic potential for breast cancer: Appraisal of greenness, whiteness, and Box–Behnken design
- Use of a humidity adsorbent derived from cockleshell waste in Thai fried fish crackers (Keropok)
- One-pot green synthesis, biological evaluation, and in silico study of pyrazole derivatives obtained from chalcones
- Bio-sorption of methylene blue and production of biofuel by brown alga Cystoseira sp. collected from Neom region, Kingdom of Saudi Arabia
- Synthesis of motexafin gadolinium: A promising radiosensitizer and imaging agent for cancer therapy
- The impact of varying sizes of silver nanoparticles on the induction of cellular damage in Klebsiella pneumoniae involving diverse mechanisms
- Microwave-assisted green synthesis, characterization, and in vitro antibacterial activity of NiO nanoparticles obtained from lemon peel extract
- Rhus microphylla-mediated biosynthesis of copper oxide nanoparticles for enhanced antibacterial and antibiofilm efficacy
- Harnessing trichalcogenide–molybdenum(vi) sulfide and molybdenum(vi) oxide within poly(1-amino-2-mercaptobenzene) frameworks as a photocathode for sustainable green hydrogen production from seawater without sacrificial agents
- Magnetically recyclable Fe3O4@SiO2 supported phosphonium ionic liquids for efficient and sustainable transformation of CO2 into oxazolidinones
- A comparative study of Fagonia arabica fabricated silver sulfide nanoparticles (Ag2S) and silver nanoparticles (AgNPs) with distinct antimicrobial, anticancer, and antioxidant properties
- Visible light photocatalytic degradation and biological activities of Aegle marmelos-mediated cerium oxide nanoparticles
- Physical intrinsic characteristics of spheroidal particles in coal gasification fine slag
- Exploring the effect of tea dust magnetic biochar on agricultural crops grown in polycyclic aromatic hydrocarbon contaminated soil
- Crosslinked chitosan-modified ultrafiltration membranes for efficient surface water treatment and enhanced anti-fouling performances
- Study on adsorption characteristics of biochars and their modified biochars for removal of organic dyes from aqueous solution
- Zein polymer nanocarrier for Ocimum basilicum var. purpurascens extract: Potential biomedical use
- Green synthesis, characterization, and in vitro and in vivo biological screening of iron oxide nanoparticles (Fe3O4) generated with hydroalcoholic extract of aerial parts of Euphorbia milii
- Novel microwave-based green approach for the synthesis of dual-loaded cyclodextrin nanosponges: Characterization, pharmacodynamics, and pharmacokinetics evaluation
- Bi2O3–BiOCl/poly-m-methyl aniline nanocomposite thin film for broad-spectrum light-sensing
- Green synthesis and characterization of CuO/ZnO nanocomposite using Musa acuminata leaf extract for cytotoxic studies on colorectal cancer cells (HCC2998)
- Review Articles
- Materials-based drug delivery approaches: Recent advances and future perspectives
- A review of thermal treatment for bamboo and its composites
- An overview of the role of nanoherbicides in tackling challenges of weed management in wheat: A novel approach
- An updated review on carbon nanomaterials: Types, synthesis, functionalization and applications, degradation and toxicity
- Special Issue: Emerging green nanomaterials for sustainable waste management and biomedical applications
- Green synthesis of silver nanoparticles using mature-pseudostem extracts of Alpinia nigra and their bioactivities
- Special Issue: New insights into nanopythotechnology: current trends and future prospects
- Green synthesis of FeO nanoparticles from coffee and its application for antibacterial, antifungal, and anti-oxidation activity
- Dye degradation activity of biogenically synthesized Cu/Fe/Ag trimetallic nanoparticles
- Special Issue: Composites and green composites
- Recent trends and advancements in the utilization of green composites and polymeric nanocarriers for enhancing food quality and sustainable processing
- Retraction
- Retraction of “Biosynthesis and characterization of silver nanoparticles from Cedrela toona leaf extracts: An exploration into their antibacterial, anticancer, and antioxidant potential”
- Retraction of “Photocatalytic degradation of organic dyes and biological potentials of biogenic zinc oxide nanoparticles synthesized using the polar extract of Cyperus scariosus R.Br. (Cyperaceae)”
- Retraction to “Green synthesis on performance characteristics of a direct injection diesel engine using sandbox seed oil”

