Assessment of antimicrobial activity and methyl orange dye removal by Klebsiella pneumoniae-mediated silver nanoparticles
-
Reema Desai
, Dipak Kumar Sahoo
and Ashish Patel
Abstract
The synthesis of silver nanoparticles (AgNPs) using bacteria is more interesting because of their non-toxic, biocompatible, environmentally friendly, and cost-effectiveness. In the present research work, AgNPs were synthesized by Klebsiella pneumoniae in two steps: first, K. pneumoniae was grown in nutrient broth, followed by mixing of bacterial supernatant and silver nitrate aqueous solution in an optimized ratio. The synthesized AgNPs were thoroughly analyzed using analytical instruments for a detailed investigation. The absorption peak observed using UV-visible spectrophotometry at 464 nm indicated the development of AgNPs, while the characteristic bands for the AgNPs by infrared spectroscopy were observed in the region of 500–4,000 cm−1. Morphological examination via field emission SEM unveiled spherical-shaped AgNPs whose sizes varied from 22.25 to 47.99 nm, along with high aggregation. The crystallinity nature of the synthesized AgNPs was demonstrated by X-ray diffraction, which revealed major intensity peaks at 2θ values of 27.6°, 31.9°, and 46°. AgNPs showed 26.6% of methyl orange dye removal within 50 min. The antibacterial activities against Bacillus cereus, Bacillus megaterium, Serratia marcescens, and Staphylococcus aureus showed maximum zones of inhibition, i.e., 14 mm (10 mg·mL−1), 16 mm (5 and 10 mg·mL−1), 13 mm (5 and 10 mg·mL−1), and 12 mm (10 mg·mL−1). Therefore, the bacterial-synthesized AgNPs exhibited potential application in the field of biomedicine, which may be further used against various other pathogens.
1 Introduction
Industries are a major source of water pollution, which is extremely harmful to living beings and the environment [1]. Numerous industries discharge dye effluents into water bodies like rivers, lakes, and oceans. The textile industry plays a major role in dye-based water pollution, as there is a wider utilization of textile dyes [2,3]. The effluent discharged from the textile industries contains mixtures of dyes that are harmful to plants, aquatic life, humans, and other living organisms [4]. Ingestion and adsorption of dye-loaded water by humans cause diseases like cancer [5], allergies, and skin-related disorders [6]. It is possible to treat the dye effluent by biological, chemical, and physical methods, and the physical methods include coagulation/flocculation, filtration, ion exchange, and adsorption [7,8,9]. The chemical method, photocatalytic method, and degradation method by biological methods are used for dye removal. The adsorption method is one of the best methods for the treatment of dyes due to its simple and economical nature [10,11]. Moreover, if the adsorbents are nanosized, then their unique features make them highly efficient adsorbents [12,13]. Nanoparticles (NPs) have efficient adsorption capacity due to their higher surface area to volume ratio (SVR), so they are used for all applications where surface plays an important role [14]. To date, both metallic and organic NPs have been used for the elimination of dyes from wastewater [15,16,17]. The metal-based NPs include pure metallic NPs like silver, gold, copper, titanium, zinc, alginate, platinum, magnesium, palladium, selenium, manganese, and ferrous/ferric, while the metal oxide NPs include TiO2, ZnO, and Fe2O3/Fe3O4 [18,19,20]. To date, silver nanoparticles (AgNPs) have been extensively utilized in the biomedical field due to their antimicrobial properties [21,22,23]. Moreover, some of the investigators have also used these NPs for wastewater treatment, especially for the removal of microbes. There are only a few cases where AgNPs have been used as a nano-adsorbent for the elimination of dyes from wastewater [24,25]. However, studies are available where AgNP’s role as an antibacterial has been used in wastewater for the elimination of pathogens [26,27].
It is possible to synthesize AgNPs in the laboratory using physical, chemical, and biological methods [28]. By using the physical methods, AgNPs can be synthesized by electrochemical [29], ultrasonication [30], laser ablation [31], and irradiation like microwave [32], while using the chemical method, they can be synthesized by the sol–gel method [33,34], chemical reduction method [35,36], etc. [37]. Among biological methods, AgNPs could be synthesized using bacteria, fungi, yeast, and algae [38,39,40], and plants [41]. The utilization of all these three routes of AgNP synthesis has its own merits and demerits, but biological ones are eco-friendly, as they require a minimum amount of chemicals. Moreover, the synthesis of AgNPs by bacteria or plants makes use of natural resources, i.e., capping agents and surfactants [42]. These further reduce the cost of the synthesis of AgNPs in comparison to the chemical method [43,44].
Previously, several investigators have used bacteria for the production of AgNPs and assessed their antibacterial potential against Gram-positive bacteria (GPB) and Gram-negative bacteria (GNB). Moreover, these bacterially synthesized AgNPs have also been used to remediate dyes from the wastewater. However, to date, only a few attempts have been made where AgNPs have been synthesized from K. pneumoniae. Earlier, several investigators used K. pneumoniae for the synthesis of AgNPs, some of the most prominent ones of which are described here.
Saleh and Khoman Alwan (2020) synthesized AgNPs using the K. pneumoniae supernatant, where the UV-Vis absorbance peak of the AgNPs was 432 nm. The scanning electron microscopy (SEM) exhibited that the size varied from 26.84 to 44.42 nm, which was agglomerated and appeared spherical in shape [45]. Furthermore, the investigator assessed the antimicrobial activity of the developed AgNPs against Pseudomonas aeruginosa, Escherichia coli, Staphylococcus aureus, and B. cereus. It was found that a concentration of 150 µg·mL−1 gave a broader zone of inhibition (ZOI), while 50 and 100 µg·mL−1 AgNPs had a lower inhibition zone. Furthermore, it was concluded that the increase in the concentration of AgNPs led to an increase in the antibacterial efficacy [45]. This investigation revealed that there was a significant increase in the inhibition efficacy (P < 0.05) when 150 µg·mL−1 AgNPs was used against all bacterial strains in the present study.
Sayyid and Zghair [46] synthesized AgNPs from the supernatant of K. pneumoniae, which was isolated from sheep and humans. From SEM, it was found that the particle was cube-shaped with small pieces whose average size was 40.47 ± 89 nm at 1 mM AgNO3, while transmission electron microscopy (TEM) showed a pseudo-spherical shape (1 mM) whose size varied from 40 to 80 nm. Moreover, TEM also exhibited a pseudo-spherical shape of the focus (10 mM), which exhibited AgNPs of size ranging from 60 to 100 nm.
Recently, Patel et al. synthesized AgNPs from three strains of bacteria, including K. pneumoniae, where the UV-vis absorbance peak for K. pneumoniae-mediated AgNPs was 475 nm, with sizes ranging from 22 to 66 nm and morphology varied from porous flake-like structure to rhombohedral-shaped. Furthermore, AgNPs were assessed for the removal of MO dye, where the removal percentage was 1.5% at 30 min, 4.06% at 60 min, 9.56% at 90 min, and 15.03% at 120 min [47].
From all these investigations, it was found that the bacterial supernatant was used for the formation of AgNPs whose absorbance peak was near 420–480 nm. Moreover, all investigators have used bacteriogenic AgNPs in the medical field, especially for antimicrobial activity, while only attempts were made to remove the dyes from the wastewater or simulated water. The investigations exhibited a broader range of AgNP morphologies and sizes, from spherical to porous sheets, enhancing potential applications.
In the present research work, the bacterial development of AgNPs is emphasized. One of the objectives was to evaluate the potential of K. pneumoniae for the production of AgNPs. Another objective was to characterize the developed AgNPs using various analytical equipment for the detailed investigation. The final objective was to assess the capability of the developed AgNPs to eliminate methyl orange (MO) dye from wastewater. Moreover, the synthesized AgNPs were also assessed as an antibacterial agent against numerous GPB and GNB.
2 Materials and methods
2.1 Materials
Klebsiella pneumoniae (PME2) was obtained as a gift sample from the Gujarat Biotech Research Center (GBRC, Gandhinagar, India). The following materials were obtained as indicated: silver nitrate (SRL, Gujarat, India), nutrient agar media (Himedia, Gujarat, India), ethanol (CSS, Fine chemicals, Jiangsu, China), nutrient broth media (Himedia, Gujarat, India), double distilled water (ddw), antibiotic assay media (Himedia, Gujarat, India), and MO dye (Loba Chemie, Gujarat, India).
2.2 Methods
2.2.1 Preparation of 20 mM AgNO3 aqueous solution
About 20 mg of AgNO3 powder was added to 200 mL of ddw in an amber bottle to obtain a 20 mM aqueous solution. The prepared AgNO3 solution was then stored in the refrigerator for future applications.
2.2.2 Synthesis of AgNPs using bacteria
The pure colonies of K. pneumoniae (PME2) were obtained on nutrient agar plates by the Gujarat Biotechnology Research Centre (GBRC), Gandhinagar, Gujarat. A nutrient broth was prepared, and the bacterial colony was inoculated and placed in an incubator shaker at 150 rpm for 24 h at 37°C. An aliquot of 2–3 mL was taken from the broth culture, and the optical density (OD) was calculated using a UV-Vis spectrophotometer (UV-Vis). Once the OD reached 1, the bacterial culture was harvested, i.e., the broth culture was centrifuged at 5,000 rpm for 10 min. The pellet was discarded while the supernatant was retained. Furthermore, 100 mL of the bacterial supernatant was added to 20 mM 100 mL of AgNO3. Furthermore, the mixture was placed in the dark for 24–48 h. After 24–48 h, a color change was observed in the medium, i.e., from milky white to reddish brown, demonstrating the reduction of Ag+ ions to Ag0 NPs. The mixture was centrifuged at 7,000 rpm for 10 min to obtain the solid fraction while the supernatant was discarded. The obtained precipitate was further washed 2–3 times with ddw followed by washing with ethanol one time. The precipitate was then dried in an oven at 45°C overnight and stored in a sample vial.
2.2.3 Preparation of an aqueous solution of MO dye
To check the remediation of the dye from bacterially synthesized AgNPs, a 50 ppm aqueous solution of the MO dye was prepared by adding 50 mg of the MO dye powder in 1,000 mL of ddw. The solution was agitated well using a magnetic stirrer. The aqueous solution was passed through a Whatman filter paper no 2 to eliminate the impurities, and the solution was stored in a reagent bottle.
2.2.4 Remediation of MO dye using AgNPs
A 100 mL aqueous solution of the MO dye was taken in a glass beaker from the stock solution, and about 1 mg of AgNPs was added. The aqueous solution was kept on a magnetic stirrer at 300 rpm for agitation without heating. Additionally, an aliquot was collected from the glass beaker at a regular interval of 20 min, i.e., 0–120 min, which was measured by UV-Vis spectroscopy to estimate the concentration of the MO dye sample.
The percentage of MO dye was calculated using the following formula:
Here, C o is the initial time of dye removal, and C t is the specific time of dye removal. After the dye removal, the collected MO dye-loaded AgNPs were oven-dried and analyzed using FTIR spectroscopy.
2.2.5 Reusability study of AgNPs
An analysis was carried out to check the effectiveness of AgNPs in removing the MO dye after multiple applications. The initially used AgNPs for MO dye removal were recovered by centrifugation and washed with NaOH. After washing, the particles were allowed to dry overnight in an oven at 50°C. The dried AgNPs were again used for MO dye removal at the same concentration used before using a UV-visible spectrophotometer; the sample was analyzed at 60 min when the removal percentage was at its maximum. Again, the same steps were repeated for reusing AgNPs, and percentage removal was measured.
2.2.6 Antimicrobial activity of AgNPs
The antibacterial activities of the three doses of AgNPs (2, 5, and 10 mg·mL−1) were assessed against B. subtilis, B. megaterium, S. aureus, and S. marcescens on Muller Hinton agar (MHA) media. Each MHA medium was inoculated with four different bacterial strains using a sterilized cotton swab. Filter paper discs of 10 mm size were cut and dipped into the AgNPs dispersed in ddw at the following concentrations in the reagent vial:2, 5, and 10 mg·mL−1. Furthermore, all three sample vials were sonicated for 30 min to load AgNPs on the filter paper discs. Furthermore, the AgNP-loaded discs were taken out by using forceps into three different Petri plates of each concentration and dried in an oven at 40–50°C. Finally, the dried AgNP discs of all three concentrations were placed on each bacterial plate using sterile forceps under aseptic conditions. Further, the Petri plates were placed in an incubator at 37°C for 24 h. After 24 h, all the plates were observed for the ZOI. The Petri plates exhibited antibacterial activity, and the ZOI values (mm) were measured using a measurement scale.
3 Characterization of AgNPs synthesized using bacteria
3.1 UV-Vis spectroscopy
About 1–2 mg of the produced AgNP powder was added to 5 mL ddw and sonicated for 10 min in an ultrasonicator (Liquitron, Gujarat, India). The finely dispersed sample was then used for UV-Vis measurement in the range of 200–800 nm using a double-beam UV-Vis spectrometer (LMP1900S, Labman, India), at 1 nm resolution.
3.2 FTIR spectroscopy
Various functional groups in the developed AgNPs were estimated by IR measurements. The IR measurements were carried out using a solid KBr pellet method, where 2 mg of AgNPs and 98 mg of KBr powder were thoroughly mixed and placed under a pellet-making instrument. The measurement was performed in the IR range 400–4,000 cm−1 at 2 cm−1 resolution using a Nicolet FTIR spectrometer (USA).
3.3 XRD
The XRD patterns of the developed AgNPs were recorded to reveal the crystalline nature by using a Miniflex 800 diffractometer (Rigaku, Japan). The recording of XRD patterns was done in the 2θ range of 20–70 with a step width of 0.02°, a scan speed of 10.0000 deg·min−1, a voltage of 30 kV, and a current of 30 mA using a K-beta(x1) filter, an SC-70 detector, and in the continuous scan mode.
3.4 FESEM-EDS
The morphological properties of the synthesized AgNPs were analyzed using a Fei 450, Novo NanoSEM (USA). AgNPs were placed on the carbon tape, which was kept on an Al stub holder. Before imaging, AgNPs were subjected to gold sputtering in a gold sputtering unit. The images were captured at different magnifications at 25 kV. Elemental analysis of AgNPs was performed using an Oxford EDS analyzer at 20 kV, equipped with an FESEM.
4 Results and discussion
4.1 Mechanism of formation of AgNPs by bacteria
A detailed study of the formation of AgNPs by K. pneumoniae is well-reported in the literature [48]. The silver ions (Ag+) present in the aqueous medium acquire the electrons from the biomolecules from the supernatant and are reduced into metallic silver, i.e., Ag°. These biomolecules are mainly enzymes, proteins, lipids, etc., which behave as capping and reducing agents for Ag+ ions. AgNPs are nucleated and aggregated and simultaneously capped with a capping agent, deciding their morphology. The color of the aqueous solution changes from milky white to red, indicating the formation of AgNPs. Kalpana and Lee used the culture filtrate of K. pneumoniae for the formation of AgNPs and reported almost a similar mechanism [49]. Mokhtari et al. also used the supernatant of K. pneumoniae for the formation of AgNPs via a similar mechanism [48].
4.2 UV-Vis analysis of AgNPs
UV-Vis measurements were performed to confirm the production of AgNPs by the K. pneumoniae supernatant. Figure 1 shows the typical UV-vis spectra of AgNPs developed using K. pneumoniae bacteria with an absorbance peak at 420 nm. The literature has shown absorbance peaks in the region of 350–450 nm, indicating the development of AgNPs. Saleh and Khoman Alwan [45] observed an absorbance peak at 432 nm for the AgNPs developed by K. pneumoniae.

UV-Vis spectra of AgNPs synthesized using the supernatant of K. pneumoniae.
4.3 FTIR analysis for functional group identification
Typical FTIR spectra of AgNPs synthesized using K. pneumoniae are shown in Figure 2, which have characteristic bands at 607 cm−1, 671, 1,052, 1,226, 1,359, 1,596, 2,314, and 3,777 cm−1. The band at 607 cm−1 is attributed to the stretching of Ag bonds or halide, i.e., AgCl. The bands at 1,596 and 3,777 cm−1 are due to the –OH functional groups in the sample, suggesting the presence of alcohol and phenol. The band at 8,159 cm−1 could be attributed to the carbonyl group (C═O) of the amino acids, and the band at 1,052 cm−1 could be attributed to the C–N stretching of aliphatic amines. Moreover, this band could also be due to the C–O stretching of ester alcohol and carboxylic acid.

FTIR spectra of K. pneumoniae synthesized AgNPs.
Saravanan et al. obtained four distinct peaks (3,370, 1,645, 2,137, and 727 cm−1) for the synthesized AgNPs. The band at 3,370 cm−1 was assigned to the –OH group, and that at 1,645 cm−1 to the stretching band of –C═O, which in turn were coupled to –CONH linkage, possibly indicating the presence of protein molecules. The bands at 2,137 and 727 cm−1 were attributed to –C═C– and –CH bending of the amino acid, respectively [50].
Saleh and Khoman Alwan [45] observed four distinct bands for AgNPs synthesized using K. pneumoniae at 3,332.78, 2,115.35, 1,635.60, and 1,096.92 cm−1. This sequentially indicates the –OH bond of alcohol, the CH stretch of the methylene group of protein, the N–H stretch of amine salts, the carbonyl group (C–O) stretching of alcohol and esters, and (C–N) stretching of aliphatic amines.
4.4 XRD analysis for the phase identification
The crystalline structure of AgNPs synthesized using K. pneumoniae was determined by XRD. Figure 3 shows the X-ray diffraction patterns of K. pneumoniae-mediated AgNPs, which matches well with the cubic phase of AgCl at 27.5°, 31.9°, 46°, 54.5°, 57.2°, 67.2°, 76.4°, and 85.4°, which corresponds to the (111), (200), (220), (311), (222), (400), (420), and (422) planes (JCPDS file: 31-1238) with a slight peak shift. The observed peak shift in the developed AgNPs and the standard JCPDS pattern might be due to the presence of bacterial compounds on the nanoparticle surface. The presence of chlorine in the AgNP sample was also detected in the EDS spectrum, as shown in Figure 5. The chlorine ion might have come from the growth medium used for cultivating K. pneumoniae. Hossain et al. [51] obtained XRD peaks for the AgNPs synthesized using the supernatant of Pseudomonas rhodesiae at 2θ values of 32.23°, 46.19°, 54.78°, and 76.70°, whereas Kalpana and Lee [49] obtained the XRD peaks at 2θ values of 37.76°, 45.87°, 64.08°, and 77.11° for the AgNPs synthesized using K. pneumoniae.

XRD patterns of AgNPs synthesized using K. pneumoniae.
The crystallite size of AgNPs was calculated using the Scherrer formula as follows:
where
For the calculation of all parameters, three high-intensity peaks were taken in the above equation. Furthermore, Gaussian peak fits were used to calculate the FWHM and accurate θ values. The peak positions here were 27.5°, 31.9°, and 46.0°, the FWHMs of which were 0.4, 0.34, and 0.43, and the X-ray wavelength was 1.54. By substituting all these values in Eq. 2, the average crystallite size of the K. pneumoniae supernatant-mediated synthesized AgNPs was 22.61 nm.
4.5 Morphological analysis of the synthesized AgNPs by FESEM
Figure 4a–f shows the FESEM microstructural images of AgNPs synthesized using K. pneumoniae. Figure 4(a) shows bright-colored AgNPs inside the porous structure, Figure 4(b)–(d) shows aggregation of the synthesized AgNPs, and Figure 4(e) and (f) shows spherically shaped AgNPs, whose size varies in the range of 22.25–47.99 nm.

FESEM images of AgNPs synthesized using K. pneumoniae ((a)–(f)).
Earlier investigators synthesized AgNPs using Pseudomonas geniculata, which was spherical in shape along with high aggregation [52].
Figure 5a and c shows the two different EDS spots of the AgNPs at 5 μm, while Figure 5b and d shows the EDS spectra and the elemental table of the synthesized AgNPs. Figure 5b shows the EDS spectra peaks for Ag, Cl, Na, Si, P, S, C, O, and N. The major elements were mainly Ag (64.2%), Cl (23.9%), O (6 %), and C (3.4%). In addition, N, Si, P, S, and Na were present in trace amounts in AgNPs. Figure 5(d) shows the second spectra for the EDS spot, which have elemental spectra for Ag, Cl, Na, P, S, C, O, and N. The major elements were Ag (61.1%), Cl (25.1%), C (8%), O (3.4%), and N (1.2%), whereas P and S were present in trace amounts, i.e., 0.5 (wt%) and 0.5 (wt%), respectively. The major impurity was Cl, which was more than 23% and might be due to improper washing of the sample during synthesis. Moreover, the high percentage of C is due to the organic carbon involved in the synthesis, i.e., capping and reducing agents involved in the production of AgNPs. This carbon might have come from the bacterial supernatant. The presence of N in a small amount further suggests the presence of bacterial protein or amino acids along with the synthesized AgNPs. Furthermore, the presence of trace amounts of S and P also suggests the involvement of protein/amino acids in AgNPs during the formation.

EDX spot (a) and (c) and elemental table (b) and (d) of AgNPs synthesized by K. pneumoniae.
Kumar and Mamidyala [53] synthesized AgNPs using a supernatant of Pseudomonas aeruginosa bacteria, and after performing elemental analysis, the following elements were observed: Ag, C, N, O, S, and Cl, of which Ag was present in the majority. Previously, Müller et al. [54] synthesized AgNPs using K. pneumoniae UVHC5 and two other bacteria in seven different media, where the EDS showed the presence of Cl in the sample. They suggested the formation of AgCl in some cases where higher Cl− ions were provided in the medium. Therefore, in our study, there is a possibility of the formation of some amount of AgCl, as NaCl was present at almost 5 g·L−1 in the medium and might have been present in the bacterial supernatant. Table 1 shows a comparative analysis of all the previously synthesized AgNPs using K. pneumoniae along with the present study.
AgNPs synthesis by K. pneumoniae earlier and the present study
| Size of AgNPs (nm) | Elements by EDS | Morphology | XRD peaks and sizes | Absorbance by UV-Vis | FTIR bands (cm−1) | References |
|---|---|---|---|---|---|---|
| 40.47 ± 89 | Cube, irregular heterogeneous | — | — | — | [46] | |
| 111.18 ± 2.17 (DLS) in the nutrient broth | Ag, Cl | Star-/flowerlike/round (SEM) | 420 ± 15 | [54] | ||
| 5 and 50 (TEM) | — | Spherical and triangular (small amount) | 37.76°, 45.87°, 64.08° and 77.11° for e (1 1 1), (2 0 0), (2 2 0) and (3 1 1) | 405–407 | 2,964.55, 1,262.22, 1,095.89, 1,021.96, 800.73, 2,960.64, 1,650.01, 865.33, 701, 477.07 | [49] |
| 26.84 to 44.42 | — | Cube shape to heterogenous, agglomerated | — | 432 | 2,115.35, 1,635.60, 3,332.78 and 1,096.92 cm−1 | [45] |
| 22.25–47.99 (SEM) | — | Spherical | 27.5°, 31.9°, 46°, 54.5, 57.2, 67.2, 76.4, and 85.4° | 420 | 607, 671, 1,052, 1,226, 1,359, 1,596, 2,314, and 3,777 cm−1 | Current investigation |
From Table 1, it could be concluded that the morphology of the synthesized AgNPs from K. pneumoniae could vary based on the medium used and the conditions provided during synthesis. Moreover, the morphology of the AgNPs may also depend on the concentration of AgNO3 used and the ratio of the silver ion precursor and bacterial supernatant. The shapes of AgNPs varied from spherical (most common), cube, triangular, or flowerlike. The peaks of AgNPs by UV-Vis mainly fall near 420 ± 20 nm, depending on the size, shape, and synthesis conditions of the AgNPs.
4.6 MO dye removal by AgNPs
The highest absorbance peak for MO dye in UV-Vis is at a wavelength of 464 nm. An aliquot was taken at regular intervals and analyzed using a UV-Vis spectrometer. The samples were collected from 0 to 60 min. The highest concentration of MO dye was 0 min in the sample, as found by a UV-Vis instrument. Figure 6a shows a sharp decrease in the absorbance of the MO dye at 10 min after which a gradual decrease in the absorbance/concentration was observed till 60 min. After 50 min, the adsorbent reached equilibrium, and further slight desorption started for 60 min. Figure 6b shows the UV-Vis absorbance of the MO dye by AgNPs from 10 to 60 min.

UV-Vis spectra of MO dye absorbance from (a) 0 to 60 min and (b) 10 to 60 min using AgNPs.
Figure 7 shows the removal percentage of MO dye, where there was no removal at 0 min; at 10 min, there was 24.13% removal; at 20 min, it increased slightly to 24.63%, which remained constant at 30 min. Furthermore, the dye removal reached 25.12% at 40 min, which reached its highest value, i.e., 26.6% at 50 min; then, the removal percentage decreased slightly, i.e., 26.1% at 60 min. A minor decrease (0.5%) in the removal percentage was observed at 60 min. Therefore, after 50 min, the MO dye reached equilibrium, after which deposition started. Table 2 summarizes the previous and current study of MO dye removal using AgNPs synthesized by the biological route.

Percentage removal of MO dye by K. pneumoniae-mediated synthesis of AgNPs.
A comparative study of the previous and current studies for the MO dye elimination using AgNPs synthesized by the biological route
| Route of synthesis | Size of IONPs (nm) | Initial dye concentration (ppm) | Removal % | Time of contact (min) | References |
|---|---|---|---|---|---|
| Trisodium citrate solution | 8–40 (TEM) | 50–200 ppm | 94–100% | 5 | [55] |
| Usnea longissima (Lichen) | 6.8 | ∼95% | [56] | ||
| Euphorbia geniculata leaf extract | 17 (Avg.) | [57] | |||
| Bacillus subtilis | 5–7.06 nm | 1% | Rate constant: without AgNPs: 0.0514 min−1, NaBH4 + AgNPs: 0.0976 | [58] |
Table 2 shows that only a limited attempt has been made where bacterially synthesized AgNPs have been used for the elimination of MO dye from the wastewater [16]. Most of the investigators have used NaBH4 as a reducing agent along with the microbially synthesized AgNPs and observed a higher MO dye removal rate in comparison to AgNPs alone [58]. Moreover, the efficiency of MO dye removal further depends on the initial dye concentration, dosage of AgNPs, and other experimental conditions [59].
4.7 Analysis of AgNPs after MO adsorption
Figure 8 shows the FTIR spectrum of bacterially synthesized AgNPs after treatment with MO dye.
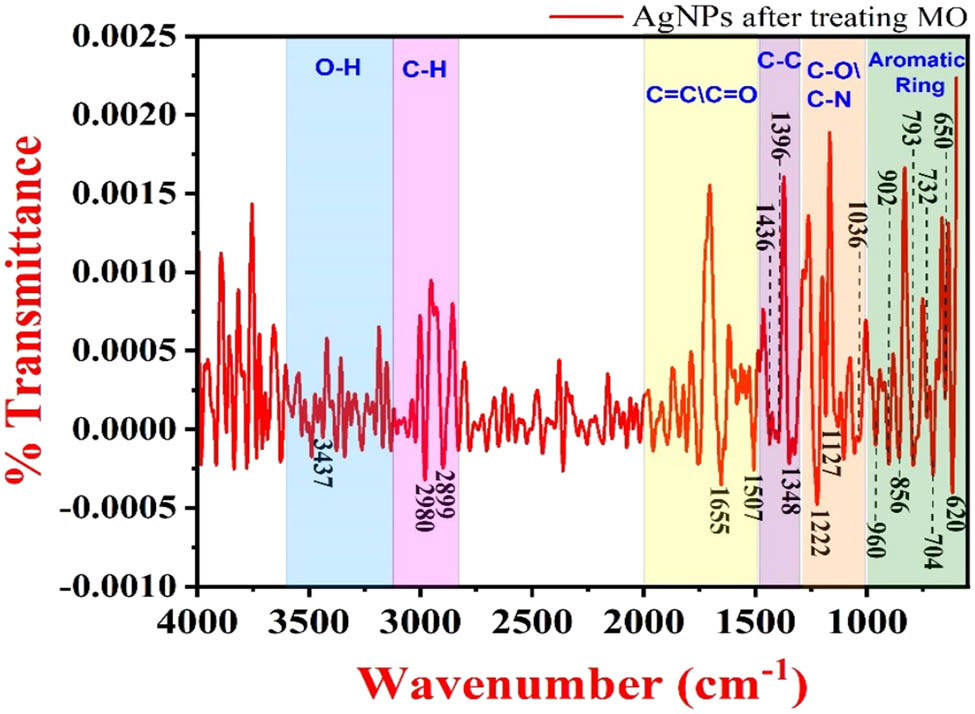
FTIR spectrum of AgNPs after adsorption of MO dye.
The interaction of the MO dye compound with AgNPs has reduced the overall transmittance of the sample, which can be seen as a reduction in the peak intensity at 3,777, 3,437, 2,980, 2,899, and 2,314 cm−1. Some new peaks were observed at 1,436, 1,507, 1,127, 1,036, 856, 720, and 620 cm−1, which were attributed to the azo bond, benzene ring, C–N, asymmetric stretching vibration of the single bond, SO3Na group, and the aromatic ring structure of the MO dye molecule present on the AgNP surface [60].
4.8 Reusability study of AgNPs
The reusability study (Figure 9) reveals the efficacy of synthesized AgNPs for dye removal after recovery and reuse. In the first cycle, the removal percentage at 60 min was 26.1%. However, the removal effectiveness of AgNPs declined in the second cycle, with a measured removal percentage of 13.48% after 60 min. In the third cycle, the efficiency reduced further, with a removal percentage of 2.76% at 60 min. This pattern suggests that repeated use of AgNPs reduces their ability to remove dye.

Regeneration study of AgNPs synthesized using K. pneumoniae against adsorption of the MO dye.
4.9 Antimicrobial activity of AgNPs synthesized using K. pneumoniae
Antimicrobial activity was performed using the disc diffusion and agar well diffusion methods. The ZOI was found against all four strains of the bacterial sp. at all three doses of the AgNPs. B. cereus exhibited ZOI values of 10, 12, and 14 mm at 2 , 5, and 10 mg·mL−1 AgNPs, respectively. B. megaterium showed ZOI values of 14, 16, and 16 mm at 2, 5, and 10 mg·mL−1 AgNPs, respectively. S. aureus exhibited ZOI values of 8, 11, and 12 mm at 2, 5, and 10 mg·mL−1 AgNPs, respectively. All these three strains were GPB. S. marcescens showed ZOI values of 10, 13, and 13 mm at 2, 5, and 10 mg·mL−1 AgNPs, respectively. This particular bacterium was the only GNB among all the four bacterial strains. This indicates that AgNPs synthesized using K. pneumoniae have antimicrobial activity against bacteria. Table 3 shows the ZOI values of silver NPs against different bacterial sp., while Figure 8 shows the ZOI in the plates. Table 4 summarizes the previous and current studies where AgNPs synthesized using K. pneumoniae have been used as an antibacterial agent (Figure 10).
ZOI values of different doses of AgNPs synthesized using K. pneumoniae on different pathogenic bacterial strains
| Bacteria | ZOI (mm) | ||
|---|---|---|---|
| 2 mg (mL) | 5 mg (mL) | 10 mg (mL) | |
| Bacillus cereus | 10 | 12 | 14 |
| Serratia marcescens | 10 | 13 | 13 |
| Bacillus megaterium | 14 | 16 | 16 |
| Staphylococcus aureus | 8 | 11 | 12 |
Previous and current investigations where AgNPs have been used as an antibacterial agent synthesized from K. pneumoniae
| Tested organism | Synthesized by bacteria | Concentration of AgNPs (µg·mL−1) | ZOI (mm) | Method applied | References |
|---|---|---|---|---|---|
| Salmonella enterica | K. pneumoniae | 50 and 100 | Complete inhibition | Broth/turbidity method | [49] |
| E. coli | 50 and 100 | Complete inhibition | Broth/turbidity method | [49] | |
| S. pyogenes | 50 and 100 | Mild | [49] | ||
| E. coli | 50 | Lowest | Agar-diffusion method | [45] | |
| 100 | Intermediate | ||||
| 150 | Highest | ||||
| P. aeruginosa | 50 | Lowest | [45] | ||
| 100 | Intermediate | ||||
| 150 | Highest | ||||
| S. aureus | 50 | Lowest | [45] | ||
| 100 | Intermediate | ||||
| 150 | Highest | ||||
| B. cereus | 50 | Lowest | [45] | ||
| 100 | Intermediate | ||||
| 150 | Highest | ||||
| Bacillus cereus | K. pneumoniae | 10 | 14 | Agar-well diffusion | Current investigation |
| Serratia marcescens | 10 | 13 | |||
| Bacillus megaterium | 10 | 16 | |||
| Staphylococcus aureus | 10 | 12 |
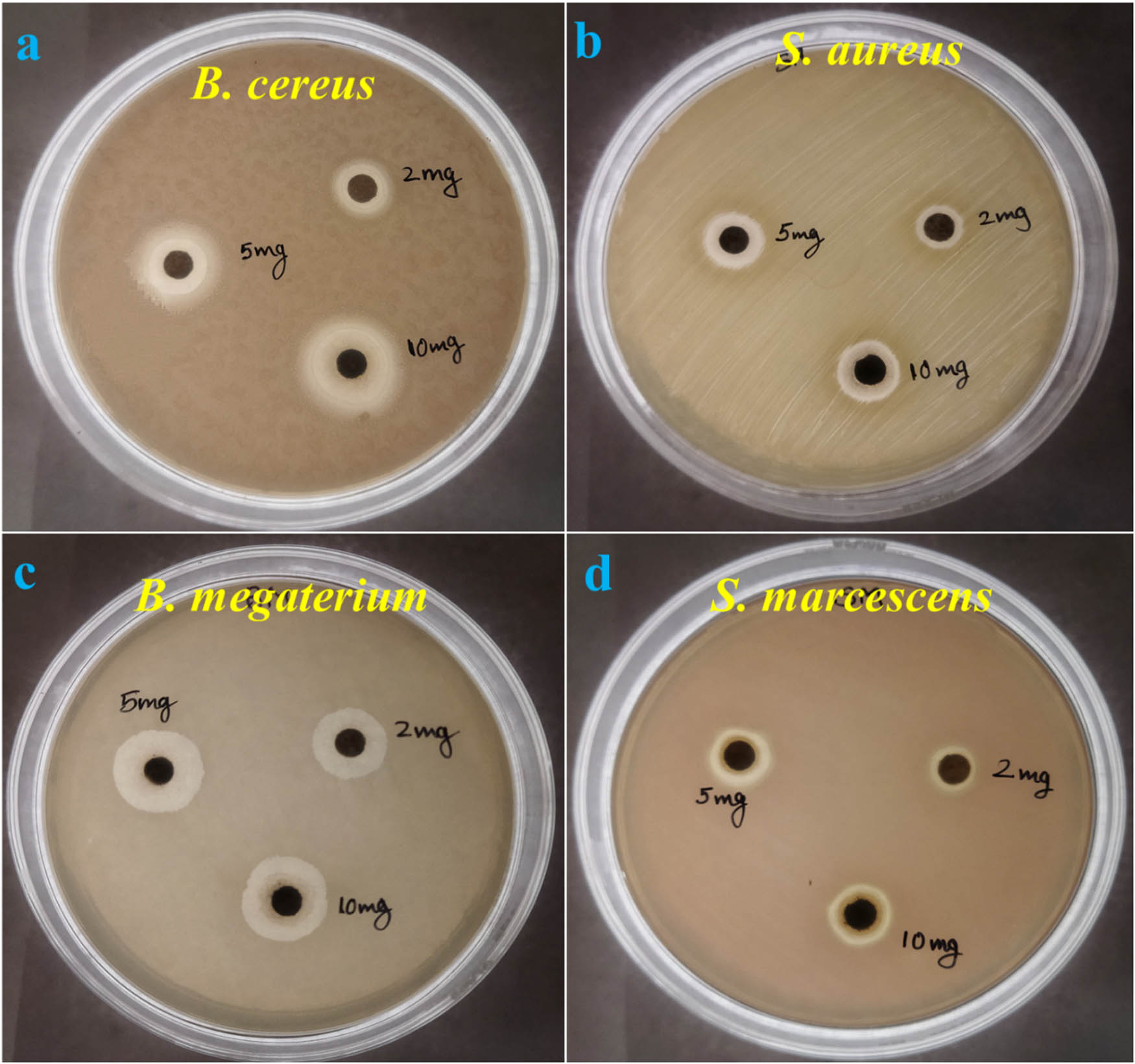
Antibacterial activity and ZOI of AgNPs against pathogenic strains: (a) B. cereus, (b) S. aureus, (c) B. megaterium, and (d) S. marcescens.
From Table 4, it can be concluded that AgNPs are effective against both GPB and GNB with slight variations in the ZOI. Moreover, the antimicrobial results showed that effective ZOI could be achieved against a particular pathogenic strain at a higher dose of AgNPs [61]. The lower dose of AgNPs reduced the inhibition of pathogenic strains, leading to lower ZOI. Moreover, the method applied, i.e., the agar well diffusion method or the disc diffusion method have a different effect on the same pathogenic strain even at the same dose of AgNPs [62]. Therefore, the antimicrobial effect of AgNPs against a particular strain not only depends on the dose of the antibacterial agent and method used but also on the morphology and surface functionalization of the synthesized AgNPs. Different bacteria have different biomolecules along with functional groups that may functionalize AgNPs differently and affect their antimicrobial activities. The smaller AgNP particles exhibit enhanced antimicrobial activity due to their faster dissolution. During the dissolution of AgNPs, there is a release of Ag+ ions, which disturb the bacterial membranes and contribute to their toxic effect. Moreover, large AgNPs take more time to dissolve, so there will be a delayed release of Ag+ ions, ultimately affecting their antimicrobial activity [63,64]. In our case, the largest ZOI was obtained against GPB, i.e., B. megaterium (16 mm) than GNB (S. marcescens), which was 13 mm, which indicates that a lower dose of AgNPs is required to kill the GPB (B. megaterium) compared to the GNB (S. marcescens). Earlier, some investigators also found higher effectiveness of AgNPs against GPB compared to GNB at the same dose or concentration. A larger ZOI was obtained against GPB (S. aureus) by Awwad et al. [65]. Moreover, Lee et al. found larger ZOI for B. cereus (GPB) compared to P. aeruginosa (GNB) [66].
5 Conclusions
AgNPs were successfully synthesized using the supernatant of K. pneumoniae bacteria, the purity of which was assessed by various analytical instruments. Characterization confirmed their spherical shape, with sizes ranging from 22.25 to 47.99 nm, as revealed by FESEM. FTIR analysis indicated the presence of proteins in the bacterially produced AgNPs, while XRD confirmed their crystallinity with sharp intensity peaks between 27 and 46°. The bacteriogenic AgNPs demonstrated significant potential for environmental remediation, achieving a maximum removal of 26.6% of MO dye from the simulated wastewater within 50 min. Moreover, the bacteriogenic AgNPs exhibited strong antibacterial properties against both Gram-positive and Gram-negative bacteria, with higher doses increasing their antibacterial efficacy. The maximum ZOI of 16 mm was observed against Bacillus megaterium at 5 and 10 mg·mL−1 AgNPs. Therefore, the present study highlights the environmentally friendly and sustainable bacteriogenic-mediated AgNPs, offering promising potential in biomedical and environmental science. These approaches and their results contribute to the advancement of AgNP development and emphasize the significance of the green route in materials science and biotechnology.
Acknowledgments
This project was supported by Researchers Supporting Project number (RSP2025R283), King Saud University, Riyadh, Saudi Arabia. The authors are also thankful to the Department of Life Sciences, Hemchandracharya North Gujarat University, Patan, Gujrat, India, for providing lab facilities.
-
Funding information: Authors state no funding is involved.
-
Author contributions: Reema Desai and Virendra Kumar Yadav: writing – original draft, writing – review and editing, methodology, formal analysis, visualization, and project administration; Bhakti Patel and Esha Rami: resources, data curation, investigation, validation, and investigation; Hesham Saleh Almoallim and Mohammad Javed Ansari: resources, methodology, formal analysis, and software; Nisha Choudhary, Dipak Kumar Sahoo, and Ashish Patel: conceptualization, supervision, writing – review and editing: funding acquisition, and project administration.
-
Conflict of interest: Authors state no conflict of interest.
-
Data availability statement: The datasets generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request.
References
[1] Shetty SS, Deepthi D, Harshitha S, Sonkusare S, Naik PB, Madhyastha H. Environmental pollutants and their effects on human health. Heliyon. 2023;9:e19496. 10.1016/j.heliyon.2023.e19496.Search in Google Scholar PubMed PubMed Central
[2] Al-Tohamy R, Ali SS, Li F, Okasha KM, Mahmoud YAG, Elsamahy T, et al. A critical review on the treatment of dye-containing wastewater: Ecotoxicological and health concerns of textile dyes and possible remediation approaches for environmental safety. Ecotoxicol Environ Saf. 2022;231:113160. 10.1016/j.ecoenv.2021.113160.Search in Google Scholar PubMed
[3] Abdissa D, Beyecha K. Sugarcane bagasse adsorption evaluation and application on BOD and COD removal from textile wastewater treatment. Water Conserv Manag. 2020;5:30–4. 10.26480/wcm.01.2021.30.34.Search in Google Scholar
[4] Alsukaibi AKD. Various approaches for the detoxification of toxic dyes in wastewater. Processes. 2022;10:1968. 10.3390/pr10101968.Search in Google Scholar
[5] He X, Jiang Z, Akakuru OU, Li J, Wu A. Nanoscale covalent organic frameworks: from controlled synthesis to cancer therapy. Chem Commun. 2021;57:12417–35. 10.1039/D1CC04846E.Search in Google Scholar
[6] Srivatsav P, Bhargav BS, Shanmugasundaram V, Arun J, Gopinath KP, Bhatnagar A. Biochar as an eco-friendly and economical adsorbent for the removal of colorants (Dyes) from aqueous environment: A review. Water (Switzerland). 2020;12:3561. 10.3390/w12123561.Search in Google Scholar
[7] Zhang H, Zheng Y, Wang H, Chang N. Preparation of starch-based adsorbing-flocculating bifunctional material St-A/F and its removal of active, direct and disperse dyes from textile printing and dyeing wastewater. Polym Bull. 2023;81:2777–800. 10.1007/s00289-023-04864-9.Search in Google Scholar
[8] Uko CA, Tijani JO, Abdulkareem SA, Mustapha S, Egbosiuba TC, Muzenda E. Adsorptive properties of MgO/WO3 nanoadsorbent for selected heavy metals removal from indigenous dyeing wastewater. Process Saf Environ Prot. 2022;162:775–94. 10.1016/j.psep.2022.04.057.Search in Google Scholar
[9] George G, Ealias AM, Saravanakumar MP. Advancements in textile dye removal: a critical review of layered double hydroxides and clay minerals as efficient adsorbents. Environ Sci Pollut Res. 2024;31:12748–79. 10.1007/s11356-024-32021-w.Search in Google Scholar PubMed
[10] Amalina F, Razak ASA, Krishnan S, Zularisam AW, Nasrullah M. Dyes removal from textile wastewater by agricultural waste as an absorbent – A review. Clean Waste Syst. 2022;3:100051. 10.1016/j.clwas.2022.100051.Search in Google Scholar
[11] Bullo TA, Bayisa YM. Optimizing the removal efficiency of chromium from tanning plant effluent by adsorption method with activated carbon chat stems (Catha edulis) using response surface methodology. Water Conserv Manag. 2022;6:15–21. 10.26480/wcm.01.2022.15.21.Search in Google Scholar
[12] Zhou W, Li A, Zhou M, Xu Y, Zhang Y, He Q. Nonporous amorphous superadsorbents for highly effective and selective adsorption of iodine in water. Nat Commun. 2023;14:5388. 10.1038/s41467-023-41056-5.Search in Google Scholar PubMed PubMed Central
[13] Pellenz L, de Oliveira CRS, da Silva Júnior AH, da Silva LJS, da Silva L, Ulson de Souza AA, et al. A comprehensive guide for characterization of adsorbent materials. Sep Purif Technol. 2023;305:122435. 10.1016/j.seppur.2022.122435.Search in Google Scholar
[14] Gholizadeh Z, Aliannezhadi M, Ghominejad M, Tehrani FS. High specific surface area γ-Al2O3 nanoparticles synthesized by facile and low-cost co-precipitation method. Sci Rep. 2023;13:6131. 10.1038/s41598-023-33266-0.Search in Google Scholar PubMed PubMed Central
[15] Abdul Sattar A. Preparation of novel hybrid (almond shell and Pleurotus sajor caju) biosorbent for the removal of heavy metals (nickel and lead) from wastewater. Water Conserv Manag. 2020;5:1–7. 10.26480/wcm.01.2021.01.07.Search in Google Scholar
[16] Ponomarev AA, Nurullina TS, Zavatsky MD. Remediation of Cr(vi) in water using biosynthesized palladium nano-materials loaded (Shewanella oneidensis) MR-1. Water Conserv Manag. 2022;6:146–53. 10.26480/wcm.02.2022.146.153.Search in Google Scholar
[17] Abdrashitova RN, Bozhenkova GS, Ponomarev AA, Gilya-Zetinov AG, Markov AA, Zavatsky DM. Synthesis of ZnO doped multi walled carbon nanotubes (MWNTs) for dyes degradation and water purification. Water Conserv Manag. 2023;7:1–5. 10.26480/wcm.01.2023.01.05.Search in Google Scholar
[18] Bayda S, Adeel M, Tuccinardi T, Cordani M, Rizzolio F. The history of nanoscience and nanotechnology: from chemical–physical applications to nanomedicine. Molecules. 2019;25:112. 10.3390/molecules25010112.Search in Google Scholar PubMed PubMed Central
[19] Loutfy HM. Eco-friendly green biosynthesized metallic nanoparticles and biotechnological applications in pharmaceuticals sciences. J Mater Sci Eng B. 2023;13:1–69. 10.17265/2161-6221/2023.1-3.001.Search in Google Scholar
[20] Haleem A, Javaid M, Singh RP, Rab S, Suman R. Applications of nanotechnology in medical field: a brief review. Glob Health J. 2023;7:70–7. 10.1016/j.glohj.2023.02.008.Search in Google Scholar
[21] Bruna T, Maldonado-Bravo F, Jara P, Caro N. Silver nanoparticles and their antibacterial applications. Int J Mol Sci. 2021;22:7202. 10.3390/ijms22137202.Search in Google Scholar PubMed PubMed Central
[22] Nguyen NPU, Dang NT, Doan L, Nguyen TTH. Synthesis of silver nanoparticles: from conventional to ‘modern’ methods—a review. Processes. 2023;11:2617. 10.3390/pr11092617.Search in Google Scholar
[23] Moradi F, Sedaghat S, Moradi O, Arab Salmanabadi S. Review on green nano-biosynthesis of silver nanoparticles and their biological activities: with an emphasis on medicinal plants. Inorg Nano-Metal Chem. 2021;51:133–42. 10.1080/24701556.2020.1769662.Search in Google Scholar
[24] Yu Y, Zhou Z, Huang G, Cheng H, Han L, Zhao S, et al. Purifying water with silver nanoparticles (AgNPs)-incorporated membranes: Recent advancements and critical challenges. Water Res. 2022;222:118901. 10.1016/j.watres.2022.118901.Search in Google Scholar PubMed
[25] Singh J, Dhaliwal AS. Synthesis of rGO/AgNPs adsorbent for the effective removal of two basic dyes: kinetics, isotherms and thermodynamic studies. Int J Environ Sci Technol. 2023;20:11483–500. 10.1007/s13762-022-04610-0.Search in Google Scholar
[26] Dixit V, Rawat H, Aggarwal K, Chaubey KK, Pal AK, Manjunath BT, et al. Application of silver-doped nanomaterials for wastewater treatment. Singapore: Springer; 2024. p. 313–32. 10.1007/978-981-99-7673-7_15.Search in Google Scholar
[27] Mondal P, Nandan A, Ajithkumar S, Siddiqui NA, Raja S, Kola AK, et al. Sustainable application of nanoparticles in wastewater treatment: Fate, current trend & paradigm shift. Environ Res. 2023;232:116071. 10.1016/j.envres.2023.116071.Search in Google Scholar PubMed
[28] Almatroudi A. Silver nanoparticles: Synthesis, characterisation and biomedical applications. Open Life Sci. 2020;15:819–39. 10.1515/biol-2020-0094.Search in Google Scholar PubMed PubMed Central
[29] Kuntyi O, Mazur A, Kytsya A, Karpenko O, Bazylyak L, Mertsalo I, et al. Electrochemical synthesis of silver nanoparticles in solutions of rhamnolipid. Micro Nano Lett. 2020;15:802–7. 10.1049/mnl.2020.0195.Search in Google Scholar
[30] Ahmad A, Mushtaq Z, Saeed F, Afzaal M, Al Jbawi E. Ultrasonic-assisted green synthesis of silver nanoparticles through cinnamon extract: biochemical, structural, and antimicrobial properties. Int J Food Prop. 2023;26:1984–94. 10.1080/10942912.2023.2238920.Search in Google Scholar
[31] Rahmah MI, Ahmed AM, Rashid TM, Qasim AJ. Preparation of silver nanoparticles using laser ablation for in vitro treatment of MCF-7 cancer cells with antibacterial activity. Plasmonics. 2023;19:2097–105. 10.1007/s11468-023-02150-y.Search in Google Scholar
[32] Kaur N, Singh A, Ahmad W. Microwave assisted green synthesis of silver nanoparticles and its application: a review. J Inorg Organomet Polym Mater. 2023;33:663–72. 10.1007/s10904-022-02470-2.Search in Google Scholar
[33] Shahjahan M. Synthesis and characterization of silver nanoparticles by sol-gel technique. Nanosci Nanometrol. 2017;3:34. 10.11648/j.nsnm.20170301.16.Search in Google Scholar
[34] Shahjahan M, Rahman MH, Hossain MS, Khatun MA, Islam A, Begum MHA. Synthesis and characterization of silver nanoparticles by sol-gel Technique. Nanosci Nanometrol. 2017;3:34–9. 10.11648/j.nsnm.20170301.16.Search in Google Scholar
[35] Quintero-Quiroz C, Acevedo N, Zapata-Giraldo J, Botero LE, Quintero J, Zárate-Triviño D, et al. Optimization of silver nanoparticle synthesis by chemical reduction and evaluation of its antimicrobial and toxic activity. Biomater Res. 2019;23. 10.1186/s40824-019-0173-y.Search in Google Scholar PubMed PubMed Central
[36] Giri AK, Jena B, Biswal B, Pradhan AK, Arakha M, Acharya S, et al. Green synthesis and characterization of silver nanoparticles using Eugenia roxburghii DC. extract and activity against biofilm-producing bacteria. Sci Rep. 2022;12:8383. 10.1038/s41598-022-12484-y.Search in Google Scholar PubMed PubMed Central
[37] Hasan KMF, Xiaoyi L, Shaoqin Z, Horváth PG, Bak M, Bejó L, et al. Functional silver nanoparticles synthesis from sustainable point of view: 2000 to 2023 ‒ A review on game changing materials. Heliyon. 2022:8;e12322. 10.1016/j.heliyon.2022.e12322.Search in Google Scholar PubMed PubMed Central
[38] Nasr Azadani F, Madani M, Karimi J, Sepahvand S. Green synthesis of silver nanoparticles by fusarium oxysporum and its function against aspergillus and fusarium fungi. Indian J Microbiol. 2024;64:213–24. 10.1007/s12088-023-01162-w.Search in Google Scholar PubMed PubMed Central
[39] Kim D-Y, Kim M, Sung J-S, Koduru JR, Nile SH, Syed A, et al. Extracellular synthesis of silver nanoparticle using yeast extracts: antibacterial and seed priming applications. Appl Microbiol Biotechnol. 2024;108:150. 10.1007/s00253-023-12920-7.Search in Google Scholar PubMed
[40] Algotiml R, Gab-Alla A, Seoudi R, Abulreesh HH, El-Readi MZ, Elbanna K. Anticancer and antimicrobial activity of biosynthesized Red Sea marine algal silver nanoparticles. Sci Rep. 2022;12:2421. 10.1038/s41598-022-06412-3.Search in Google Scholar PubMed PubMed Central
[41] Umai D, Vikranth A, Meenambiga SS. A study on the green synthesis of silver nanoparticles from Olea europaea and its activity against oral pathogens. Mater Today Proc. Vol. 44, Amsterdam, Netherlands: Elsevier Ltd; 2021. p. 3647–51. 10.1016/j.matpr.2020.10.681.Search in Google Scholar
[42] Mahmodi Sheikh Sarmast Z, Sedaghat S, Derakhshi P, Aberoomand Azar P. Efficient removal of ampicillin from aqueous media using silver nanoparticles decorated with magnetic-chitosan: optimization by central composite design. Chem Pap. 2024;78:189–205. 10.1007/s11696-023-03061-2.Search in Google Scholar
[43] Pandit C, Roy A, Ghotekar S, Khusro A, Islam MN, Emran T, et al. Biological agents for synthesis of nanoparticles and their applications. J King Saud Univ Sci. 2022;34:101869. 10.1016/j.jksus.2022.101869.Search in Google Scholar
[44] Dhaka A, Chand Mali S, Sharma S, Trivedi R. A review on biological synthesis of silver nanoparticles and their potential applications. Results Chem. 2023;6:101108. 10.1016/j.rechem.2023.101108.Search in Google Scholar
[45] Saleh MN, Khoman Alwan S. Bio-synthesis of silver nanoparticles from bacteria Klebsiella pneumonia: Their characterization and antibacterial studies. Journal of Physics: Conference Series, vol. 1664, Bristol, England: IOP Publishing Ltd; 2020. 10.1088/1742-6596/1664/1/012115.Search in Google Scholar
[46] Sayyid NH, Zghair ZR. Biosynthesis of silver nanoparticles produced by Klebsiella pneumoniae. Mater Today Proc. 2021;42:2045–9. 10.1016/j.matpr.2020.12.257.Search in Google Scholar
[47] Patel B, Yadav VK, Desai R, Patel S, Amari A, Choudhary N, et al. Bacteriogenic synthesis of morphologically diverse silver nanoparticles and their assessment for methyl orange dye removal and antimicrobial activity. PeerJ. 2024;12:e17328. 10.7717/peerj.17328.Search in Google Scholar PubMed PubMed Central
[48] Mokhtari N, Daneshpajouh S, Seyedbagheri S, Atashdehghan R, Abdi K, Sarkar S, et al. Biological synthesis of very small silver nanoparticles by culture supernatant of Klebsiella pneumonia: The effects of visible-light irradiation and the liquid mixing process. Mater Res Bull. 2009;44:1415–21. 10.1016/j.materresbull.2008.11.021.Search in Google Scholar
[49] Kalpana D, Lee YS. Synthesis and characterization of bactericidal silver nanoparticles using cultural filtrate of simulated microgravity grown Klebsiella pneumoniae. Enzyme Microb Technol. 2013;52:151–6. 10.1016/j.enzmictec.2012.12.006.Search in Google Scholar PubMed
[50] Saravanan M, Barik SK, MubarakAli D, Prakash P, Pugazhendhi A. Synthesis of silver nanoparticles from Bacillus brevis (NCIM 2533) and their antibacterial activity against pathogenic bacteria. Microb Pathog. 2018;116:221–6. 10.1016/j.micpath.2018.01.038.Search in Google Scholar PubMed
[51] Hossain A, Hong X, Ibrahim E, Li B, Sun G, Meng Y, et al. Green synthesis of silver nanoparticles with culture supernatant of a bacterium pseudomonas rhodesiae and their antibacterial activity against soft rot pathogen dickeya dadantii. Molecules. 2019;24:2303. 10.3390/molecules24122303.Search in Google Scholar PubMed PubMed Central
[52] Jang EY, Son YJ, Park SY, Yoo JY, Hwang DY, Park HC, et al. Biological synthesis and characterisation of silver nanoparticles using pseudomonas geniculata H10 for pharmaceutical activity. IET Nanobiotechnol. 2018;12:828–35. 10.1049/iet-nbt.2018.0014.Search in Google Scholar PubMed PubMed Central
[53] Kumar CG, Mamidyala SK. Extracellular synthesis of silver nanoparticles using culture supernatant of Pseudomonas aeruginosa. Colloids Surf B Biointerfaces. 2011;84:462–6. 10.1016/j.colsurfb.2011.01.042.Search in Google Scholar PubMed
[54] Müller A, Behsnilian D, Walz E, Gräf V, Hogekamp L, Greiner R. Effect of culture medium on the extracellular synthesis of silver nanoparticles using Klebsiella pneumoniae, Escherichia coli and Pseudomonas jessinii. Biocatal Agric Biotechnol. 2016;6:107–15. 10.1016/j.bcab.2016.02.012.Search in Google Scholar
[55] Gola D, Kriti A, Bhatt N, Bajpai M, Singh A, Arya A, et al. Silver nanoparticles for enhanced dye degradation. Current Research in Green and Sustainable Chemistry. 2021;4:100132. 10.1016/j.crgsc.2021.100132.Search in Google Scholar
[56] Yang Z, Gong X, Hu Y, Yue P, Lü B, Peng F. Green synthesis of lichenan-decorated silver nanoparticles for catalytic hydrogenation of organic dyes and bacterial disinfection. Chem Eng J. 2024;487:150516. 10.1016/j.cej.2024.150516.Search in Google Scholar
[57] Santhosh AS, Sandeep S. Kumara Swamy N. Green synthesis of nano silver from euphorbia geniculata leaf extract: Investigations on catalytic degradation of methyl orange dye and optical sensing of Hg2+. Surf Interfaces. 2019;14:50–4. 10.1016/j.surfin.2018.11.004.Search in Google Scholar
[58] Ibrahim S, Ahmad Z, Manzoor MZ, Mujahid M, Faheem Z, Adnan A. Optimization for biogenic microbial synthesis of silver nanoparticles through response surface methodology, characterization, their antimicrobial, antioxidant, and catalytic potential. Sci Rep. 2021;11:770. 10.1038/s41598-020-80805-0.Search in Google Scholar PubMed PubMed Central
[59] Abbas SH, Younis YM, Rashid KH, Khadom AA. Removal of methyl orange dye from simulated wastewater by electrocoagulation technique using Taguchi method: kinetics and optimization approaches. React Kinet, Mech Catal. 2022;135:2663–79. 10.1007/s11144-022-02269-9.Search in Google Scholar
[60] Li P, Song Y, Wang S, Tao Z, Yu S, Liu Y. Enhanced decolorization of methyl orange using zero-valent copper nanoparticles under assistance of hydrodynamic cavitation. Ultrason Sonochem. 2015;22:132–8. 10.1016/j.ultsonch.2014.05.025.Search in Google Scholar PubMed
[61] Salam MDA, Al-Amin MDY, Pawar JS, Akhter N, Lucy IB. Conventional methods and future trends in antimicrobial susceptibility testing. Saudi J Biol Sci. 2023;30:103582. 10.1016/j.sjbs.2023.103582.Search in Google Scholar PubMed PubMed Central
[62] Dickert H, Machka K, Braveny I. The uses and limitations of disc diffusion in the antibiotic sensitivity testing of bacteria. Infection. 1981;9:18–24. 10.1007/BF01640803.Search in Google Scholar
[63] Vidyasagar PRR, Singh SK, Singh M. Green synthesis of silver nanoparticles: methods, biological applications, delivery and toxicity. Mater Adv. 2023;4:1831–49. 10.1039/D2MA01105K.Search in Google Scholar
[64] Menichetti A, Mavridi-Printezi A, Mordini D, Montalti M. Effect of size, shape and surface functionalization on the antibacterial activity of silver nanoparticles. J Funct Biomater. 2023;14:244. 10.3390/jfb14050244.Search in Google Scholar PubMed PubMed Central
[65] Awwad AM, Salem NMS, Aqarbeh MM, Abdulaziz FM. Green synthesis, characterization of silver sulfide nanoparticles and antibacterial activity evaluation. Chem Int. 2020;6:42–8.Search in Google Scholar
[66] Lee J-H, Lim J-M, Velmurugan P, Park Y-J, Park Y-J, Bang K-S, et al. Photobiologic-mediated fabrication of silver nanoparticles with antibacterial activity. J Photochem Photobiol B. 2016;162:93–9. 10.1016/j.jphotobiol.2016.06.029.Search in Google Scholar PubMed
© 2024 the author(s), published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Research Articles
- Green polymer electrolyte and activated charcoal-based supercapacitor for energy harvesting application: Electrochemical characteristics
- Research on the adsorption of Co2+ ions using halloysite clay and the ability to recover them by electrodeposition method
- Simultaneous estimation of ibuprofen, caffeine, and paracetamol in commercial products using a green reverse-phase HPTLC method
- Isolation, screening and optimization of alkaliphilic cellulolytic fungi for production of cellulase
- Functionalized gold nanoparticles coated with bacterial alginate and their antibacterial and anticancer activities
- Comparative analysis of bio-based amino acid surfactants obtained via Diels–Alder reaction of cyclic anhydrides
- Biosynthesis of silver nanoparticles on yellow phosphorus slag and its application in organic coatings
- Exploring antioxidant potential and phenolic compound extraction from Vitis vinifera L. using ultrasound-assisted extraction
- Manganese and copper-coated nickel oxide nanoparticles synthesized from Carica papaya leaf extract induce antimicrobial activity and breast cancer cell death by triggering mitochondrial caspases and p53
- Insight into heating method and Mozafari method as green processing techniques for the synthesis of micro- and nano-drug carriers
- Silicotungstic acid supported on Bi-based MOF-derived metal oxide for photodegradation of organic dyes
- Synthesis and characterization of capsaicin nanoparticles: An attempt to enhance its bioavailability and pharmacological actions
- Synthesis of Lawsonia inermis-encased silver–copper bimetallic nanoparticles with antioxidant, antibacterial, and cytotoxic activity
- Facile, polyherbal drug-mediated green synthesis of CuO nanoparticles and their potent biological applications
- Zinc oxide-manganese oxide/carboxymethyl cellulose-folic acid-sesamol hybrid nanomaterials: A molecularly targeted strategy for advanced triple-negative breast cancer therapy
- Exploring the antimicrobial potential of biogenically synthesized graphene oxide nanoparticles against targeted bacterial and fungal pathogens
- Biofabrication of silver nanoparticles using Uncaria tomentosa L.: Insight into characterization, antibacterial activities combined with antibiotics, and effect on Triticum aestivum germination
- Membrane distillation of synthetic urine for use in space structural habitat systems
- Investigation on mechanical properties of the green synthesis bamboo fiber/eggshell/coconut shell powder-based hybrid biocomposites under NaOH conditions
- Green synthesis of magnesium oxide nanoparticles using endophytic fungal strain to improve the growth, metabolic activities, yield traits, and phenolic compounds content of Nigella sativa L.
- Estimation of greenhouse gas emissions from rice and annual upland crops in Red River Delta of Vietnam using the denitrification–decomposition model
- Synthesis of humic acid with the obtaining of potassium humate based on coal waste from the Lenger deposit, Kazakhstan
- Ascorbic acid-mediated selenium nanoparticles as potential antihyperuricemic, antioxidant, anticoagulant, and thrombolytic agents
- Green synthesis of silver nanoparticles using Illicium verum extract: Optimization and characterization for biomedical applications
- Antibacterial and dynamical behaviour of silicon nanoparticles influenced sustainable waste flax fibre-reinforced epoxy composite for biomedical application
- Optimising coagulation/flocculation using response surface methodology and application of floc in biofertilisation
- Green synthesis and multifaceted characterization of iron oxide nanoparticles derived from Senna bicapsularis for enhanced in vitro and in vivo biological investigation
- Potent antibacterial nanocomposites from okra mucilage/chitosan/silver nanoparticles for multidrug-resistant Salmonella Typhimurium eradication
- Trachyspermum copticum aqueous seed extract-derived silver nanoparticles: Exploration of their structural characterization and comparative antibacterial performance against gram-positive and gram-negative bacteria
- Microwave-assisted ultrafine silver nanoparticle synthesis using Mitragyna speciosa for antimalarial applications
- Green synthesis and characterisation of spherical structure Ag/Fe2O3/TiO2 nanocomposite using acacia in the presence of neem and tulsi oils
- Green quantitative methods for linagliptin and empagliflozin in dosage forms
- Enhancement efficacy of omeprazole by conjugation with silver nanoparticles as a urease inhibitor
- Residual, sequential extraction, and ecological risk assessment of some metals in ash from municipal solid waste incineration, Vietnam
- Green synthesis of ZnO nanoparticles using the mangosteen (Garcinia mangostana L.) leaf extract: Comparative preliminary in vitro antibacterial study
- Simultaneous determination of lesinurad and febuxostat in commercial fixed-dose combinations using a greener normal-phase HPTLC method
- A greener RP-HPLC method for quaternary estimation of caffeine, paracetamol, levocetirizine, and phenylephrine acquiring AQbD with stability studies
- Optimization of biomass durian peel as a heterogeneous catalyst in biodiesel production using microwave irradiation
- Thermal treatment impact on the evolution of active phases in layered double hydroxide-based ZnCr photocatalysts: Photodegradation and antibacterial performance
- Preparation of silymarin-loaded zein polysaccharide core–shell nanostructures and evaluation of their biological potentials
- Preparation and characterization of composite-modified PA6 fiber for spectral heating and heat storage applications
- Preparation and electrocatalytic oxygen evolution of bimetallic phosphates (NiFe)2P/NF
- Rod-shaped Mo(vi) trichalcogenide–Mo(vi) oxide decorated on poly(1-H pyrrole) as a promising nanocomposite photoelectrode for green hydrogen generation from sewage water with high efficiency
- Green synthesis and studies on citrus medica leaf extract-mediated Au–ZnO nanocomposites: A sustainable approach for efficient photocatalytic degradation of rhodamine B dye in aqueous media
- Cellulosic materials for the removal of ciprofloxacin from aqueous environments
- The analytical assessment of metal contamination in industrial soils of Saudi Arabia using the inductively coupled plasma technology
- The effect of modified oily sludge on the slurry ability and combustion performance of coal water slurry
- Eggshell waste transformation to calcium chloride anhydride as food-grade additive and eggshell membranes as enzyme immobilization carrier
- Synthesis of EPAN and applications in the encapsulation of potassium humate
- Biosynthesis and characterization of silver nanoparticles from Cedrela toona leaf extracts: An exploration into their antibacterial, anticancer, and antioxidant potential
- Enhancing mechanical and rheological properties of HDPE films through annealing for eco-friendly agricultural applications
- Immobilisation of catalase purified from mushroom (Hydnum repandum) onto glutaraldehyde-activated chitosan and characterisation: Its application for the removal of hydrogen peroxide from artificial wastewater
- Sodium titanium oxide/zinc oxide (STO/ZnO) photocomposites for efficient dye degradation applications
- Effect of ex situ, eco-friendly ZnONPs incorporating green synthesised Moringa oleifera leaf extract in enhancing biochemical and molecular aspects of Vicia faba L. under salt stress
- Biosynthesis and characterization of selenium and silver nanoparticles using Trichoderma viride filtrate and their impact on Culex pipiens
- Photocatalytic degradation of organic dyes and biological potentials of biogenic zinc oxide nanoparticles synthesized using the polar extract of Cyperus scariosus R.Br. (Cyperaceae)
- Assessment of antiproliferative activity of green-synthesized nickel oxide nanoparticles against glioblastoma cells using Terminalia chebula
- Chlorine-free synthesis of phosphinic derivatives by change in the P-function
- Anticancer, antioxidant, and antimicrobial activities of nanoemulsions based on water-in-olive oil and loaded on biogenic silver nanoparticles
- Study and mechanism of formation of phosphorus production waste in Kazakhstan
- Synthesis and stabilization of anatase form of biomimetic TiO2 nanoparticles for enhancing anti-tumor potential
- Microwave-supported one-pot reaction for the synthesis of 5-alkyl/arylidene-2-(morpholin/thiomorpholin-4-yl)-1,3-thiazol-4(5H)-one derivatives over MgO solid base
- Screening the phytochemicals in Perilla leaves and phytosynthesis of bioactive silver nanoparticles for potential antioxidant and wound-healing application
- Graphene oxide/chitosan/manganese/folic acid-brucine functionalized nanocomposites show anticancer activity against liver cancer cells
- Nature of serpentinite interactions with low-concentration sulfuric acid solutions
- Multi-objective statistical optimisation utilising response surface methodology to predict engine performance using biofuels from waste plastic oil in CRDi engines
- Microwave-assisted extraction of acetosolv lignin from sugarcane bagasse and electrospinning of lignin/PEO nanofibres for carbon fibre production
- Biosynthesis, characterization, and investigation of cytotoxic activities of selenium nanoparticles utilizing Limosilactobacillus fermentum
- Highly photocatalytic materials based on the decoration of poly(O-chloroaniline) with molybdenum trichalcogenide oxide for green hydrogen generation from Red Sea water
- Highly efficient oil–water separation using superhydrophobic cellulose aerogels derived from corn straw
- Beta-cyclodextrin–Phyllanthus emblica emulsion for zinc oxide nanoparticles: Characteristics and photocatalysis
- Assessment of antimicrobial activity and methyl orange dye removal by Klebsiella pneumoniae-mediated silver nanoparticles
- Influential eradication of resistant Salmonella Typhimurium using bioactive nanocomposites from chitosan and radish seed-synthesized nanoselenium
- Antimicrobial activities and neuroprotective potential for Alzheimer’s disease of pure, Mn, Co, and Al-doped ZnO ultra-small nanoparticles
- Green synthesis of silver nanoparticles from Bauhinia variegata and their biological applications
- Synthesis and optimization of long-chain fatty acids via the oxidation of long-chain fatty alcohols
- Eminent Red Sea water hydrogen generation via a Pb(ii)-iodide/poly(1H-pyrrole) nanocomposite photocathode
- Green synthesis and effective genistein production by fungal β-glucosidase immobilized on Al2O3 nanocrystals synthesized in Cajanus cajan L. (Millsp.) leaf extracts
- Green stability-indicating RP-HPTLC technique for determining croconazole hydrochloride
- Green synthesis of La2O3–LaPO4 nanocomposites using Charybdis natator for DNA binding, cytotoxic, catalytic, and luminescence applications
- Eco-friendly drugs induce cellular changes in colistin-resistant bacteria
- Tangerine fruit peel extract mediated biogenic synthesized silver nanoparticles and their potential antimicrobial, antioxidant, and cytotoxic assessments
- Green synthesis on performance characteristics of a direct injection diesel engine using sandbox seed oil
- A highly sensitive β-AKBA-Ag-based fluorescent “turn off” chemosensor for rapid detection of abamectin in tomatoes
- Green synthesis and physical characterization of zinc oxide nanoparticles (ZnO NPs) derived from the methanol extract of Euphorbia dracunculoides Lam. (Euphorbiaceae) with enhanced biosafe applications
- Detection of morphine and data processing using surface plasmon resonance imaging sensor
- Effects of nanoparticles on the anaerobic digestion properties of sulfamethoxazole-containing chicken manure and analysis of bio-enzymes
- Bromic acid-thiourea synergistic leaching of sulfide gold ore
- Green chemistry approach to synthesize titanium dioxide nanoparticles using Fagonia Cretica extract, novel strategy for developing antimicrobial and antidiabetic therapies
- Green synthesis and effective utilization of biogenic Al2O3-nanocoupled fungal lipase in the resolution of active homochiral 2-octanol and its immobilization via aluminium oxide nanoparticles
- Eco-friendly RP-HPLC approach for simultaneously estimating the promising combination of pentoxifylline and simvastatin in therapeutic potential for breast cancer: Appraisal of greenness, whiteness, and Box–Behnken design
- Use of a humidity adsorbent derived from cockleshell waste in Thai fried fish crackers (Keropok)
- One-pot green synthesis, biological evaluation, and in silico study of pyrazole derivatives obtained from chalcones
- Bio-sorption of methylene blue and production of biofuel by brown alga Cystoseira sp. collected from Neom region, Kingdom of Saudi Arabia
- Synthesis of motexafin gadolinium: A promising radiosensitizer and imaging agent for cancer therapy
- The impact of varying sizes of silver nanoparticles on the induction of cellular damage in Klebsiella pneumoniae involving diverse mechanisms
- Microwave-assisted green synthesis, characterization, and in vitro antibacterial activity of NiO nanoparticles obtained from lemon peel extract
- Rhus microphylla-mediated biosynthesis of copper oxide nanoparticles for enhanced antibacterial and antibiofilm efficacy
- Harnessing trichalcogenide–molybdenum(vi) sulfide and molybdenum(vi) oxide within poly(1-amino-2-mercaptobenzene) frameworks as a photocathode for sustainable green hydrogen production from seawater without sacrificial agents
- Magnetically recyclable Fe3O4@SiO2 supported phosphonium ionic liquids for efficient and sustainable transformation of CO2 into oxazolidinones
- A comparative study of Fagonia arabica fabricated silver sulfide nanoparticles (Ag2S) and silver nanoparticles (AgNPs) with distinct antimicrobial, anticancer, and antioxidant properties
- Visible light photocatalytic degradation and biological activities of Aegle marmelos-mediated cerium oxide nanoparticles
- Physical intrinsic characteristics of spheroidal particles in coal gasification fine slag
- Exploring the effect of tea dust magnetic biochar on agricultural crops grown in polycyclic aromatic hydrocarbon contaminated soil
- Crosslinked chitosan-modified ultrafiltration membranes for efficient surface water treatment and enhanced anti-fouling performances
- Study on adsorption characteristics of biochars and their modified biochars for removal of organic dyes from aqueous solution
- Zein polymer nanocarrier for Ocimum basilicum var. purpurascens extract: Potential biomedical use
- Green synthesis, characterization, and in vitro and in vivo biological screening of iron oxide nanoparticles (Fe3O4) generated with hydroalcoholic extract of aerial parts of Euphorbia milii
- Novel microwave-based green approach for the synthesis of dual-loaded cyclodextrin nanosponges: Characterization, pharmacodynamics, and pharmacokinetics evaluation
- Bi2O3–BiOCl/poly-m-methyl aniline nanocomposite thin film for broad-spectrum light-sensing
- Green synthesis and characterization of CuO/ZnO nanocomposite using Musa acuminata leaf extract for cytotoxic studies on colorectal cancer cells (HCC2998)
- Review Articles
- Materials-based drug delivery approaches: Recent advances and future perspectives
- A review of thermal treatment for bamboo and its composites
- An overview of the role of nanoherbicides in tackling challenges of weed management in wheat: A novel approach
- An updated review on carbon nanomaterials: Types, synthesis, functionalization and applications, degradation and toxicity
- Special Issue: Emerging green nanomaterials for sustainable waste management and biomedical applications
- Green synthesis of silver nanoparticles using mature-pseudostem extracts of Alpinia nigra and their bioactivities
- Special Issue: New insights into nanopythotechnology: current trends and future prospects
- Green synthesis of FeO nanoparticles from coffee and its application for antibacterial, antifungal, and anti-oxidation activity
- Dye degradation activity of biogenically synthesized Cu/Fe/Ag trimetallic nanoparticles
- Special Issue: Composites and green composites
- Recent trends and advancements in the utilization of green composites and polymeric nanocarriers for enhancing food quality and sustainable processing
- Retraction
- Retraction of “Biosynthesis and characterization of silver nanoparticles from Cedrela toona leaf extracts: An exploration into their antibacterial, anticancer, and antioxidant potential”
- Retraction of “Photocatalytic degradation of organic dyes and biological potentials of biogenic zinc oxide nanoparticles synthesized using the polar extract of Cyperus scariosus R.Br. (Cyperaceae)”
- Retraction to “Green synthesis on performance characteristics of a direct injection diesel engine using sandbox seed oil”
Articles in the same Issue
- Research Articles
- Green polymer electrolyte and activated charcoal-based supercapacitor for energy harvesting application: Electrochemical characteristics
- Research on the adsorption of Co2+ ions using halloysite clay and the ability to recover them by electrodeposition method
- Simultaneous estimation of ibuprofen, caffeine, and paracetamol in commercial products using a green reverse-phase HPTLC method
- Isolation, screening and optimization of alkaliphilic cellulolytic fungi for production of cellulase
- Functionalized gold nanoparticles coated with bacterial alginate and their antibacterial and anticancer activities
- Comparative analysis of bio-based amino acid surfactants obtained via Diels–Alder reaction of cyclic anhydrides
- Biosynthesis of silver nanoparticles on yellow phosphorus slag and its application in organic coatings
- Exploring antioxidant potential and phenolic compound extraction from Vitis vinifera L. using ultrasound-assisted extraction
- Manganese and copper-coated nickel oxide nanoparticles synthesized from Carica papaya leaf extract induce antimicrobial activity and breast cancer cell death by triggering mitochondrial caspases and p53
- Insight into heating method and Mozafari method as green processing techniques for the synthesis of micro- and nano-drug carriers
- Silicotungstic acid supported on Bi-based MOF-derived metal oxide for photodegradation of organic dyes
- Synthesis and characterization of capsaicin nanoparticles: An attempt to enhance its bioavailability and pharmacological actions
- Synthesis of Lawsonia inermis-encased silver–copper bimetallic nanoparticles with antioxidant, antibacterial, and cytotoxic activity
- Facile, polyherbal drug-mediated green synthesis of CuO nanoparticles and their potent biological applications
- Zinc oxide-manganese oxide/carboxymethyl cellulose-folic acid-sesamol hybrid nanomaterials: A molecularly targeted strategy for advanced triple-negative breast cancer therapy
- Exploring the antimicrobial potential of biogenically synthesized graphene oxide nanoparticles against targeted bacterial and fungal pathogens
- Biofabrication of silver nanoparticles using Uncaria tomentosa L.: Insight into characterization, antibacterial activities combined with antibiotics, and effect on Triticum aestivum germination
- Membrane distillation of synthetic urine for use in space structural habitat systems
- Investigation on mechanical properties of the green synthesis bamboo fiber/eggshell/coconut shell powder-based hybrid biocomposites under NaOH conditions
- Green synthesis of magnesium oxide nanoparticles using endophytic fungal strain to improve the growth, metabolic activities, yield traits, and phenolic compounds content of Nigella sativa L.
- Estimation of greenhouse gas emissions from rice and annual upland crops in Red River Delta of Vietnam using the denitrification–decomposition model
- Synthesis of humic acid with the obtaining of potassium humate based on coal waste from the Lenger deposit, Kazakhstan
- Ascorbic acid-mediated selenium nanoparticles as potential antihyperuricemic, antioxidant, anticoagulant, and thrombolytic agents
- Green synthesis of silver nanoparticles using Illicium verum extract: Optimization and characterization for biomedical applications
- Antibacterial and dynamical behaviour of silicon nanoparticles influenced sustainable waste flax fibre-reinforced epoxy composite for biomedical application
- Optimising coagulation/flocculation using response surface methodology and application of floc in biofertilisation
- Green synthesis and multifaceted characterization of iron oxide nanoparticles derived from Senna bicapsularis for enhanced in vitro and in vivo biological investigation
- Potent antibacterial nanocomposites from okra mucilage/chitosan/silver nanoparticles for multidrug-resistant Salmonella Typhimurium eradication
- Trachyspermum copticum aqueous seed extract-derived silver nanoparticles: Exploration of their structural characterization and comparative antibacterial performance against gram-positive and gram-negative bacteria
- Microwave-assisted ultrafine silver nanoparticle synthesis using Mitragyna speciosa for antimalarial applications
- Green synthesis and characterisation of spherical structure Ag/Fe2O3/TiO2 nanocomposite using acacia in the presence of neem and tulsi oils
- Green quantitative methods for linagliptin and empagliflozin in dosage forms
- Enhancement efficacy of omeprazole by conjugation with silver nanoparticles as a urease inhibitor
- Residual, sequential extraction, and ecological risk assessment of some metals in ash from municipal solid waste incineration, Vietnam
- Green synthesis of ZnO nanoparticles using the mangosteen (Garcinia mangostana L.) leaf extract: Comparative preliminary in vitro antibacterial study
- Simultaneous determination of lesinurad and febuxostat in commercial fixed-dose combinations using a greener normal-phase HPTLC method
- A greener RP-HPLC method for quaternary estimation of caffeine, paracetamol, levocetirizine, and phenylephrine acquiring AQbD with stability studies
- Optimization of biomass durian peel as a heterogeneous catalyst in biodiesel production using microwave irradiation
- Thermal treatment impact on the evolution of active phases in layered double hydroxide-based ZnCr photocatalysts: Photodegradation and antibacterial performance
- Preparation of silymarin-loaded zein polysaccharide core–shell nanostructures and evaluation of their biological potentials
- Preparation and characterization of composite-modified PA6 fiber for spectral heating and heat storage applications
- Preparation and electrocatalytic oxygen evolution of bimetallic phosphates (NiFe)2P/NF
- Rod-shaped Mo(vi) trichalcogenide–Mo(vi) oxide decorated on poly(1-H pyrrole) as a promising nanocomposite photoelectrode for green hydrogen generation from sewage water with high efficiency
- Green synthesis and studies on citrus medica leaf extract-mediated Au–ZnO nanocomposites: A sustainable approach for efficient photocatalytic degradation of rhodamine B dye in aqueous media
- Cellulosic materials for the removal of ciprofloxacin from aqueous environments
- The analytical assessment of metal contamination in industrial soils of Saudi Arabia using the inductively coupled plasma technology
- The effect of modified oily sludge on the slurry ability and combustion performance of coal water slurry
- Eggshell waste transformation to calcium chloride anhydride as food-grade additive and eggshell membranes as enzyme immobilization carrier
- Synthesis of EPAN and applications in the encapsulation of potassium humate
- Biosynthesis and characterization of silver nanoparticles from Cedrela toona leaf extracts: An exploration into their antibacterial, anticancer, and antioxidant potential
- Enhancing mechanical and rheological properties of HDPE films through annealing for eco-friendly agricultural applications
- Immobilisation of catalase purified from mushroom (Hydnum repandum) onto glutaraldehyde-activated chitosan and characterisation: Its application for the removal of hydrogen peroxide from artificial wastewater
- Sodium titanium oxide/zinc oxide (STO/ZnO) photocomposites for efficient dye degradation applications
- Effect of ex situ, eco-friendly ZnONPs incorporating green synthesised Moringa oleifera leaf extract in enhancing biochemical and molecular aspects of Vicia faba L. under salt stress
- Biosynthesis and characterization of selenium and silver nanoparticles using Trichoderma viride filtrate and their impact on Culex pipiens
- Photocatalytic degradation of organic dyes and biological potentials of biogenic zinc oxide nanoparticles synthesized using the polar extract of Cyperus scariosus R.Br. (Cyperaceae)
- Assessment of antiproliferative activity of green-synthesized nickel oxide nanoparticles against glioblastoma cells using Terminalia chebula
- Chlorine-free synthesis of phosphinic derivatives by change in the P-function
- Anticancer, antioxidant, and antimicrobial activities of nanoemulsions based on water-in-olive oil and loaded on biogenic silver nanoparticles
- Study and mechanism of formation of phosphorus production waste in Kazakhstan
- Synthesis and stabilization of anatase form of biomimetic TiO2 nanoparticles for enhancing anti-tumor potential
- Microwave-supported one-pot reaction for the synthesis of 5-alkyl/arylidene-2-(morpholin/thiomorpholin-4-yl)-1,3-thiazol-4(5H)-one derivatives over MgO solid base
- Screening the phytochemicals in Perilla leaves and phytosynthesis of bioactive silver nanoparticles for potential antioxidant and wound-healing application
- Graphene oxide/chitosan/manganese/folic acid-brucine functionalized nanocomposites show anticancer activity against liver cancer cells
- Nature of serpentinite interactions with low-concentration sulfuric acid solutions
- Multi-objective statistical optimisation utilising response surface methodology to predict engine performance using biofuels from waste plastic oil in CRDi engines
- Microwave-assisted extraction of acetosolv lignin from sugarcane bagasse and electrospinning of lignin/PEO nanofibres for carbon fibre production
- Biosynthesis, characterization, and investigation of cytotoxic activities of selenium nanoparticles utilizing Limosilactobacillus fermentum
- Highly photocatalytic materials based on the decoration of poly(O-chloroaniline) with molybdenum trichalcogenide oxide for green hydrogen generation from Red Sea water
- Highly efficient oil–water separation using superhydrophobic cellulose aerogels derived from corn straw
- Beta-cyclodextrin–Phyllanthus emblica emulsion for zinc oxide nanoparticles: Characteristics and photocatalysis
- Assessment of antimicrobial activity and methyl orange dye removal by Klebsiella pneumoniae-mediated silver nanoparticles
- Influential eradication of resistant Salmonella Typhimurium using bioactive nanocomposites from chitosan and radish seed-synthesized nanoselenium
- Antimicrobial activities and neuroprotective potential for Alzheimer’s disease of pure, Mn, Co, and Al-doped ZnO ultra-small nanoparticles
- Green synthesis of silver nanoparticles from Bauhinia variegata and their biological applications
- Synthesis and optimization of long-chain fatty acids via the oxidation of long-chain fatty alcohols
- Eminent Red Sea water hydrogen generation via a Pb(ii)-iodide/poly(1H-pyrrole) nanocomposite photocathode
- Green synthesis and effective genistein production by fungal β-glucosidase immobilized on Al2O3 nanocrystals synthesized in Cajanus cajan L. (Millsp.) leaf extracts
- Green stability-indicating RP-HPTLC technique for determining croconazole hydrochloride
- Green synthesis of La2O3–LaPO4 nanocomposites using Charybdis natator for DNA binding, cytotoxic, catalytic, and luminescence applications
- Eco-friendly drugs induce cellular changes in colistin-resistant bacteria
- Tangerine fruit peel extract mediated biogenic synthesized silver nanoparticles and their potential antimicrobial, antioxidant, and cytotoxic assessments
- Green synthesis on performance characteristics of a direct injection diesel engine using sandbox seed oil
- A highly sensitive β-AKBA-Ag-based fluorescent “turn off” chemosensor for rapid detection of abamectin in tomatoes
- Green synthesis and physical characterization of zinc oxide nanoparticles (ZnO NPs) derived from the methanol extract of Euphorbia dracunculoides Lam. (Euphorbiaceae) with enhanced biosafe applications
- Detection of morphine and data processing using surface plasmon resonance imaging sensor
- Effects of nanoparticles on the anaerobic digestion properties of sulfamethoxazole-containing chicken manure and analysis of bio-enzymes
- Bromic acid-thiourea synergistic leaching of sulfide gold ore
- Green chemistry approach to synthesize titanium dioxide nanoparticles using Fagonia Cretica extract, novel strategy for developing antimicrobial and antidiabetic therapies
- Green synthesis and effective utilization of biogenic Al2O3-nanocoupled fungal lipase in the resolution of active homochiral 2-octanol and its immobilization via aluminium oxide nanoparticles
- Eco-friendly RP-HPLC approach for simultaneously estimating the promising combination of pentoxifylline and simvastatin in therapeutic potential for breast cancer: Appraisal of greenness, whiteness, and Box–Behnken design
- Use of a humidity adsorbent derived from cockleshell waste in Thai fried fish crackers (Keropok)
- One-pot green synthesis, biological evaluation, and in silico study of pyrazole derivatives obtained from chalcones
- Bio-sorption of methylene blue and production of biofuel by brown alga Cystoseira sp. collected from Neom region, Kingdom of Saudi Arabia
- Synthesis of motexafin gadolinium: A promising radiosensitizer and imaging agent for cancer therapy
- The impact of varying sizes of silver nanoparticles on the induction of cellular damage in Klebsiella pneumoniae involving diverse mechanisms
- Microwave-assisted green synthesis, characterization, and in vitro antibacterial activity of NiO nanoparticles obtained from lemon peel extract
- Rhus microphylla-mediated biosynthesis of copper oxide nanoparticles for enhanced antibacterial and antibiofilm efficacy
- Harnessing trichalcogenide–molybdenum(vi) sulfide and molybdenum(vi) oxide within poly(1-amino-2-mercaptobenzene) frameworks as a photocathode for sustainable green hydrogen production from seawater without sacrificial agents
- Magnetically recyclable Fe3O4@SiO2 supported phosphonium ionic liquids for efficient and sustainable transformation of CO2 into oxazolidinones
- A comparative study of Fagonia arabica fabricated silver sulfide nanoparticles (Ag2S) and silver nanoparticles (AgNPs) with distinct antimicrobial, anticancer, and antioxidant properties
- Visible light photocatalytic degradation and biological activities of Aegle marmelos-mediated cerium oxide nanoparticles
- Physical intrinsic characteristics of spheroidal particles in coal gasification fine slag
- Exploring the effect of tea dust magnetic biochar on agricultural crops grown in polycyclic aromatic hydrocarbon contaminated soil
- Crosslinked chitosan-modified ultrafiltration membranes for efficient surface water treatment and enhanced anti-fouling performances
- Study on adsorption characteristics of biochars and their modified biochars for removal of organic dyes from aqueous solution
- Zein polymer nanocarrier for Ocimum basilicum var. purpurascens extract: Potential biomedical use
- Green synthesis, characterization, and in vitro and in vivo biological screening of iron oxide nanoparticles (Fe3O4) generated with hydroalcoholic extract of aerial parts of Euphorbia milii
- Novel microwave-based green approach for the synthesis of dual-loaded cyclodextrin nanosponges: Characterization, pharmacodynamics, and pharmacokinetics evaluation
- Bi2O3–BiOCl/poly-m-methyl aniline nanocomposite thin film for broad-spectrum light-sensing
- Green synthesis and characterization of CuO/ZnO nanocomposite using Musa acuminata leaf extract for cytotoxic studies on colorectal cancer cells (HCC2998)
- Review Articles
- Materials-based drug delivery approaches: Recent advances and future perspectives
- A review of thermal treatment for bamboo and its composites
- An overview of the role of nanoherbicides in tackling challenges of weed management in wheat: A novel approach
- An updated review on carbon nanomaterials: Types, synthesis, functionalization and applications, degradation and toxicity
- Special Issue: Emerging green nanomaterials for sustainable waste management and biomedical applications
- Green synthesis of silver nanoparticles using mature-pseudostem extracts of Alpinia nigra and their bioactivities
- Special Issue: New insights into nanopythotechnology: current trends and future prospects
- Green synthesis of FeO nanoparticles from coffee and its application for antibacterial, antifungal, and anti-oxidation activity
- Dye degradation activity of biogenically synthesized Cu/Fe/Ag trimetallic nanoparticles
- Special Issue: Composites and green composites
- Recent trends and advancements in the utilization of green composites and polymeric nanocarriers for enhancing food quality and sustainable processing
- Retraction
- Retraction of “Biosynthesis and characterization of silver nanoparticles from Cedrela toona leaf extracts: An exploration into their antibacterial, anticancer, and antioxidant potential”
- Retraction of “Photocatalytic degradation of organic dyes and biological potentials of biogenic zinc oxide nanoparticles synthesized using the polar extract of Cyperus scariosus R.Br. (Cyperaceae)”
- Retraction to “Green synthesis on performance characteristics of a direct injection diesel engine using sandbox seed oil”

