Abstract
The present study investigated biodiesel production from the transesterification of palm oil with methanol using calcined biomass durian peel (BDP) as a heterogeneous catalyst assisted by microwave irradiation. Characterization of the calcined BDP showed that K2O is the main compound with a concentration of 86.15 wt%. The effect of three independent variables of catalyst weight (3–12 wt%), reaction time (1–10 min), and power of microwave (180–900 W) was used to determine the optimum condition on biodiesel production using the response surface method-based on the Box–Behnken design experiment. The optimum biodiesel conversion of 97.3% was achieved under experimental parameters of catalyst concentration of 12 wt%, reaction time of 9 min, and microwave power of 180 W. The catalyst concentration and reaction time have significant effects on biodiesel conversion.
1 Introduction
Increasing global concern about dwindling petroleum resources leading to an uncontrolled escalation of petroleum prices is resulting in a worldwide concerted effort to find a suitable energy source alternative [1]. In addition, the demand for renewable energy has increased rapidly as a result of increasing public awareness of environmental issues [2,3]. Biodiesel is an alternative fuel because it is made from renewable sources with lower pollutant emissions [4]. In addition, biodiesel could be used directly without requiring any modification in diesel machines [2]. Commercially, biodiesel is produced from the transesterification of oils/lipids using an alkaline homogeneous catalyst [5,6]. However, biodiesel production currently has several problems such as the homogeneous catalyst cannot be reused, requires an expensive separation step, and produces a lot of waste water due to the use of a homogeneous catalyst [5]. To solve the problems, heterogeneous substances that have different phases with the reactants have been studied and explored as catalysts in biodiesel production. Heterogeneous catalyst offers some advantages such as being reusable, less sensitive to free fatty acid and water, non-corrosive, and high selectivity [7]. Some studies have proved that heterogeneous substances could catalyze transesterification reactions producing biodiesel with high yield [8,9,10,11,12,13,14]. However, the catalytic activity of heterogeneous catalysts is lower than that of homogeneous catalysts; hence, the reaction time is relatively longer [15]. Recently, bio-waste materials that contain potassium oxide as the main compounds have similar catalytic activity with homogenous basic catalysts [16,17,18]. A biodiesel yield of 99% was achieved in 25 min reaction time at 65°C using calcined Brassica nigra plant which contains 56.13% potassium ion [19]. Interestingly, this bio-waste catalyst could facilitate transesterification reaction at room temperature conditions. Tarigan et al. reported that a biodiesel conversion of 95.4% could be achieved in a reaction time of 30 min at room temperature using waste passion fruit peel as a solid catalyst [17]. Furthermore, microwave and homogenizers could intensify biodiesel production using heterogeneous catalysts hence diminishing reaction time [11,16]. The heterogeneous catalyst derived from calcined elephant-ear tree pods have reported could catalyze transesterification reaction and produce biodiesel conversion of 99% in 5.88 min under microwave irradiation [11] while a homogenizer device require 10 min reaction time to achieved similar result using palm bunch as a catalyst [16]. Durian is a famous and exotic fruit particularly in Southern Asia. The durian fruit consists of 50–65% flesh and seed and 45–55% peels [20]. Durian production in Indonesia reached 1.58 million tons in 2022 in which 0.79 million ton is the peel [21]. The utilization of biomass durian peel (BDP) is limited and usually is simply discharged without any treatment that could pollute the environment. No references could be found in the literature related to the utilization of BDP as a heterogeneous catalyst in transesterification reactions under microwave irradiation.
Therefore, this study aims to determine the performance of BDP as a heterogeneous catalyst in the transesterification palm oil to biodiesel under microwave irradiation. The optimum biodiesel conversion was determined based on parameters of catalyst weight, reaction time, and microwave power using the response surface method. The Box–Behnken model was used to investigate the optimum reaction conditions at a 95% confidence level.
2 Materials and methods
2.1 Materials
The BDP was collected from a local durian shop in Medan, Indonesia, cut into small chunks, and heated in an oven until dry. The dried BDP was crushed using a coffee grinder (ACE Kris, 150 W, Indonesia) and sieved to form a powder. The BDP powder was heated in a muffle furnace (Thermolyne Thermo Fisher Scientific model FB1310M-33, MA, USA) at 600°C for 4 h and stored in a desiccator before use. The calcination temperature of 600°C was chosen based on the previous published research [5]. Furthermore, the metal oxides as the main components in the calcined BPD were composed at this temperature [5,10]. The X-ray fluorescence (XRF) (Rigaku Supermini200, TX USA) was used to determine the elemental composition of the calcined BDP. A Household microwave (ACE Kris, Indonesia) is modified and is equipped with a condenser and magnetic stirrer. The input and output powers of the microwave were 1,400 and 900 W, respectively. All chemicals used in this study were purchased from a local chemical distributor in Medan, Indonesia, and were used as received.
2.2 Box–Behnken experimental design
The Box–Behnken design experiment with three parameters was applied to determine the optimum condition for microwave-assisted transesterification of palm oil to biodiesel using calcined BDP. The range of three-level reaction conditions is shown in Table 1. The ratio molar of palm oil to methanol was fixed at 1:12. The relationship between the variables with biodiesel conversion and yield was calculated using a second-order polynomial equation as shown in Eq. 1 to explain the effects of variables on linear, quadratic, and interaction terms.
where Y is the biodiesel conversion or yield; X i and X j are the independent factors; and 0, i, ii, and ij are the intercept, linear, and quadratic interaction coefficients, respectively.
Reaction parameters and operating levels
| Symbol | Parameters | Levels | ||
|---|---|---|---|---|
| −1 | 0 | 1 | ||
| A | Catalyst weight (wt%) | 3 | 7.5 | 12 |
| B | Reaction time (min) | 1 | 5.5 | 10 |
| C | Power (W) | 180 | 540 | 900 |
2.3 Microwave-assisted transesterification of palm oil using BDP as a catalyst
5.8 mL of palm oil and 2.8 mL of methanol were added with an investigated amount of calcined BDP in a 100 mL round-bottom flask equipped with a condenser and magnetic stirrer. The flask was placed in the microwave and the reaction time was counted when the microwave was started based on investigated time and power. After completion, the mixture was separated using centrifugation at 5,000 rpm for 10 min. The biodiesel product in the top layer was then separated and stored in a desiccator for conversion analysis using gas chromatography (GC) (Shimadzu type 2010, Japan).
2.4 Conventional transesterification of palm oil using BDP as a catalyst
The palm oil was also transesterified using BPD as a catalyst in the batch reflux method for comparison. Palm oil (5.8 mL), methanol (2.8 mL based on the ratio of 1:12), and 0.63 g of calcined BDP were placed in a 100 round-bottom flask connected to a reflux condenser with a magnetic hotplate stirrer set to 500 rpm. The reaction was conducted for 10 min, and the biodiesel product was collected after centrifugation followed by the evaporation of leftover methanol and stored in a desiccator for GC analysis.
3 Results and discussion
The fatty acids profile of the palm oil is shown in Table 2 which is dominated by oleic acid and palmitic acid of 43.88 and 38.41%, respectively. The XRF analysis showed that calcined BDP consists of K2O, P2O5, and SO3 as the main compound with a concentration of 86.15, 6.98, and 3.25 wt%, respectively. The transesterification of palm oil with methanol under microwave irradiation was conducted using calcined BDP as a heterogeneous catalyst. The effect of catalyst weight, reaction time, and microwave power on biodiesel conversion was studied in the Box–Behnken design experiment to determine the optimum condition. The experimental and predicted biodiesel conversions are depicted in Table 3.
The fatty acids profile of palm oil
| Fatty acid | Percentage | |
|---|---|---|
| C12:0 | Lauric | 0.21 |
| C14:0 | Myristic | 0.94 |
| C16:0 | Palmitic | 38.41 |
| C18:0 | Stearic | 4.35 |
| C18:1 | Oleic | 43.88 |
| C18:2 | Linoleic | 11.06 |
| C20:0 | Arachidic | 0.4 |
| C18:3 | Linolenic | 0.18 |
Experimental design based on the Box–Behnken model and their observed and predicted responses
| Parameters code | Biodiesel conversion (%) | Biodiesel yield (%) | ||||
|---|---|---|---|---|---|---|
| A | B | C | Observed | Prediction | Observed | Prediction |
| 7.5 | 5.5 | 540 | 85.05 | 89.99 | 87.23 | 91.55 |
| 12 | 5.5 | 900 | 94.69 | 95.12 | 91.75 | 89.41 |
| 3 | 5.5 | 180 | 78.83 | 78.40 | 72.03 | 74.37 |
| 7.5 | 5.5 | 540 | 94.27 | 89.99 | 93.91 | 91.55 |
| 12 | 1 | 540 | 92.28 | 91.81 | 91.25 | 91.90 |
| 3 | 10 | 540 | 91.03 | 91.50 | 89.60 | 88.95 |
| 12 | 10 | 540 | 96.92 | 93.27 | 93.85 | 92.04 |
| 3 | 1 | 540 | 63.45 | 67.09 | 70.72 | 72.53 |
| 7.5 | 10 | 180 | 97.06 | 97.03 | 97.25 | 95.56 |
| 7.5 | 1 | 900 | 87.55 | 87.58 | 89.40 | 91.09 |
| 7.5 | 10 | 900 | 92.35 | 95.56 | 94.40 | 98.55 |
| 3 | 5.5 | 900 | 91.20 | 87.52 | 92.50 | 88.99 |
| 7.5 | 5.5 | 540 | 90.64 | 89.97 | 93.50 | 91.55 |
| 12 | 5.5 | 180 | 93.61 | 97.29 | 92.91 | 96.41 |
| 7.5 | 1 | 180 | 82.36 | 79.15 | 90.62 | 86.47 |
Based on the Box–Behnken model, the quadratic regression was quantified using Eq. 1, where A, B, and C represent the catalyst weight (wt%), reaction time (min), and microwave power (W), respectively. The relationship between the observed and predicted biodiesel conversion shows good linearity with a correlation coefficient (R 2) of 89% (Eq. 2). The effect of linear factors, i.e. catalyst concentration and reaction time, was found to be highly significant on biodiesel conversion with values P < 0.0118 and P < 0.0131, respectively.
3.1 Effect of interaction parameters
The 3D response surface was used to determine the individual and cumulative effect of the parameters and the mutual interaction between the parameter and the dependent parameter. The resulting surface response 3D plots of biodiesel conversion as a function of two independent variables, (1) catalyst concentration and reaction time, (2) catalyst concentration and microwave power, and (3) reaction time and microwave power are shown in Figure 1a–c, respectively. As shown in Figure 1a, increasing catalyst concentration and reaction time up to a certain value has increased the biodiesel conversion. However, the conversion slightly decreases when both parameters rise. This is presumably due to the high catalyst amount in the mixture generating high viscosity in the reaction mixture and agglomeration of the catalyst leading to poor dispersion of the BDP catalyst [5,22,23]. This result is in agreement with Niju et al. in the utilization of banana pseudostem as a catalyst in the transesterification of Madhuca indica oil to biodiesel [24]. Similarly, Tarigan et al. in the transesterification of palm oil to biodiesel using waste banana peels as a catalyst observed an increasing biodiesel conversion up to a maximum of 97% and remained steady after that [5]. Figure 1b depicts the interaction effect of catalyst concentration and microwave power on biodiesel conversion. With the increase in catalyst concentration up to a specified level, biodiesel increases. In contrast, the biodiesel conversion was decreased with increasing microwave power. Furthermore, interaction parameters of reaction time and microwave power as shown in Figure 1c also have a similar graph pattern in which increasing reaction time increases the biodiesel conversion while increasing microwave power could decrease it.
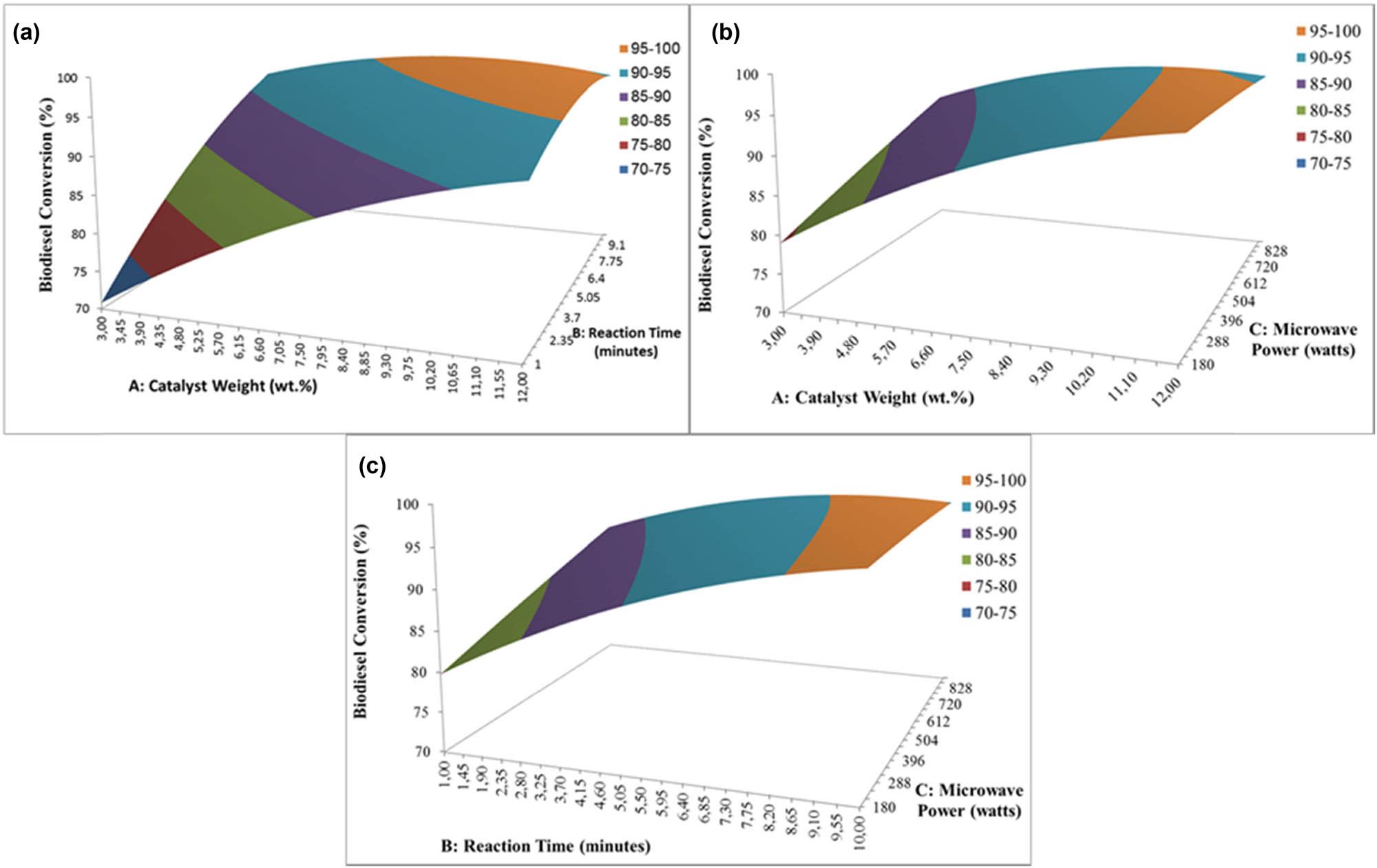
3D surface plots describing the response surface for biodiesel conversion as a function of (a) catalyst weight and reaction time, (b) catalyst weight and microwave power, and (c) reaction time and microwave power.
3.2 Validation of the experimental model
The response surface method assists in identifying the combination of input parameter settings that provide reaction conditions with optimum biodiesel conversion. The optimum values of the independent parameters were achieved considering the lower and higher values of catalyst concentration, reaction time, and microwave power. Based on that, the predicted optimum biodiesel conversion of 98.6% could be achieved under reaction conditions of catalyst concentration of 12 wt%, reaction time of 9 min, and microwave power of 180 W. The experimental conversion based on that reaction condition is 97.3%. This shows that the predicted and experimental biodiesel conversions are in excellent agreement.
3.3 Comparison BDP catalyst using microwave and reflux method
As a comparison, the calcined BDP catalyst was tested for its catalytic activity using the conventional reflux method. The reflux reaction was performed under similar reaction conditions to the microwave method. The biodiesel conversion of 63% was achieved after 9 min of reaction time. This conversion was lower by 35% compared with microwave-assisted transesterification of palm oil to biodiesel using calcined BDP as a heterogeneous catalyst. The higher conversion using microwave irradiation was attributed to the high-frequency rotation of reactants due to the constantly changing magnetic and electric fields [25,26]. This result is in agreement with the previously published result of the transesterification of soybean oil to biodiesel using nano-powder calcium oxide as a catalyst under microwave irradiation [27]. Hsiao et al. showed that a biodiesel conversion of 53.5% could be achieved at 15 min of reaction time using microwave irradiation compared to 22.1% using the conventional reflux method [27]. In addition, some researchers concluded that microwave is more energy efficient than the conventional reflux method [28,29,30].
3.4 Comparison of biodiesel production using biomass catalyst in different methods
Calcined biomass-derived agricultural waste has been used as a heterogeneous catalyst in biodiesel production and showed catalytic activity comparable with a homogeneous base catalyst. As shown in Table 4, the biodiesel conversion of >90% was achieved using calcined biomass as a catalyst. Potassium in the form of potassium carbonate or potassium oxide is the main component of the biomass ash. However, the concentration of potassium did not exert a significant influence on the conversion of biodiesel. Notably, the passion fruit peel, which exhibits the lowest potassium concentration according to Table 4, demonstrated a biodiesel conversion rate that was comparable to that of the BDP, which possesses the highest concentration of potassium ions. The parameters governing the reactions, such as the molar ratio of oil to methanol and the weight of the catalyst, appear to have negligible influence on the biodiesel conversion. The molar ratio employed varied between 1:6 and 1:30, while the catalyst weight spanned from 2 to 12 wt%. Interestingly, calcined passion fruit and banana peel could catalyze the transesterification of palm oil to biodiesel at room temperature and resulted in a biodiesel conversion of 95.4% and 97.7%, respectively, after a reaction time of 30 min [5,17]. In contrast, the current study demonstrates that the calcined BDP can serve as an effective catalyst for biodiesel production within a reaction time of 9 min using microwave irradiation. Therefore, in terms of reaction time, microwave irradiation-assisted biodiesel production using a calcined biomass is capable of reducing reaction time by 80% and 70% in comparison to ultrasound and homogenizer methods, respectively.
Summary of biodiesel production using biomass catalyst in different methods
| Biomass | Potassium concentration (wt%) | Method and reaction conditions (ratio molar oil: methanol, catalyst weight, temperature, and reaction time) | Biodiesel conversion (%) | Ref. |
|---|---|---|---|---|
| Durian peel | 86.15 | Microwave irradiation 1:12, 12 wt%, ND, 9 min, 180 W | 97.3 | This study |
| Passion fruit peel | 44.4 | Reflux 1:15, 7 wt%, room temperature, 30 min | 95.4 | [17] |
| Banana peel | 47.01 | Homogenizer 1:15, 7 wt%, room temperature, 30 min, 6,000 rpm | 97.7 | [5] |
| Banana peduncle | 45.83 | Ultrasound 1:10, 2 wt%, ND, 45 min, 40 kHz | 94.62 | [36] |
3.5 Biodiesel properties
The fatty acid composition of the oil primarily exerts a significant influence on several critical characteristics of biodiesel such as cetane number, density, viscosity, oxidative stability, pour point, and cloud point [31]. Those properties could be predicted using a reliable and accurate equation which has been reported elsewhere [32,33,34]. Table 5 shows the predicted palm oil biodiesel properties which were compared with waste cooking oil-biodiesel and international standards (ASTM D6751 and EN 14214). As expected, the palm oil-biodiesel satisfies the international standard for all the physicochemical properties. The palm oil-biodiesel has a higher cetane number than the standard and WCO-biodiesel which represent its ability to burn within the engine [1]. This outcome can be attributed to the high amount of saturated fatty acid (SFA) in palm oil. However, the high level of SFA has a significant effect on the cold flow properties and oxidation stability. The cloud and pour point properties of palm oil-biodiesel are 15.2 and 9.7°C, respectively, which thereby limiting its use to ambient temperatures above freezing. However, the oxidation stability property of palm oil-biodiesel showed higher than the minimum standards presenting a potential for extended storage [35].
Comparison biodiesel properties
| Properties | Unit | ASTM D6751 | EN 14214 | Palm oil biodiesel | Waste cooking oil biodiesel |
|---|---|---|---|---|---|
| Ester content | % (m·m−1) | 96.5 | 97.3 | ||
| Cetane number | >47 | >51 | 59.31 | 51.4 | |
| Heating value | MJ·kg−1 | 38 | — | 39.284 | 37.2 |
| Kinematic viscosity | mm2·s−1 | 1.9–6.0 | 3.5–5.0 | 3.894 | 5.83 |
| Density | g·cm−3 | — | 0.86–0.90 | 0.869 | 0.876 |
| Cloud point | °C | Report | Report | 15.2 | 10.5 |
| Pour point | °C | Report | Report | 9.7 | 1 |
| Oxidation stability | h | >3 min | 8 min | 13.1 | — |
| References | This study |
4 Conclusions
Microwave-assisted transesterification of palm oil to biodiesel was conducted using BDP as a heterogeneous catalyst. The calcined BDP contains K2O as the main compound with a concentration of 86.15 wt%. The response surface method based on the Box–Behnken design experiment was used to determine the optimum reaction condition. The optimum biodiesel conversion of 98.6% was predicted achieved using a catalyst concentration of 12 wt%, reaction time of 9 min, and microwave power of 180 W. The experimental result obtained at the optimized condition was found to be very close to that predicted value. The catalyst concentration and reaction time were observed to have a significant effect on biodiesel conversion. The result showed that microwave and calcined BDP could enhance biodiesel production in a short reaction time.
Acknowledgments
The authors wish to acknowledge Universitas Syiah Kuala for supporting this research.
-
Funding information: This study was supported by Riset Kolaborasi Indonesia for research Grant No. 557/UN11.2.1/PT.01.03/PNBP/2023 dated May 23, 2023.
-
Author contributions: Binawati Ginting: conceptualization, investigation, writing – original draft; Minanda Payungta Sitepu: investigation, project administration; Aman Santoso: writing – original draft, formal analysis; Bambang Susilo: methodology, formal analysis and data analysis; Juliati Br. Tarigan: methodology, writing – review and editing; and Eko Kornelius Sitepu: conceptualization, funding acquisition, writing – review and editing.
-
Conflict of interest: Authors state no conflict of interest.
-
Data availability statement: All data generated or analyzed during this study are included in this published article.
References
[1] Zhang Y, Duan L, Esmaeili H. A review on biodiesel production using various heterogeneous nanocatalysts: Operation mechanisms and performances. Biomass Bioenergy. 2022;158(1):106356. 10.1016/j.biombioe.2022.106356.Search in Google Scholar
[2] Sitepu EK, Heimann K, Raston CL, Zhang W. Critical evaluation of process parameters for direct biodiesel production from diverse feedstock. Renew Sustain Energy Rev. 2020;123(1):109762. 10.1016/j.rser.2020.109762.Search in Google Scholar
[3] Sarno M, Iuliano M. Biodiesel production from waste cooking oil. Green Process Synth. 2019;8(1):828–36. 10.1515/gps-2019-0053.Search in Google Scholar
[4] Jin C, Wei J. The combined effect of water and nanoparticles on diesel engine powered by biodiesel and its blends with diesel: A review. Fuel. 2023;343(1):127940. 10.1016/j.fuel.2023.127940.Search in Google Scholar
[5] Tarigan JB, Perangin-Angin S, Simanungkalit SR, Zega NP, Sitepu EK. Utilization of waste banana peels as heterogeneous catalysts in room-temperature biodiesel production using a homogenizer. RSC Adv. 2023;13(9):6217–24. 10.1039/D3RA00016H.Search in Google Scholar
[6] Huang J, Wang J, Huang Z, Liu T, Li H. Photothermal technique-enabled ambient production of microalgae biodiesel: Mechanism and life cycle assessment. Bioresour Technol. 2023;369(1):128390. 10.1016/j.biortech.2022.128390.Search in Google Scholar PubMed
[7] Esmaeili H. A critical review on the economic aspects and life cycle assessment of biodiesel production using heterogeneous nanocatalysts. Fuel Process Technol. 2022;230(1):107224. 10.1016/j.fuproc.2022.107224.Search in Google Scholar
[8] Martínez A, Mijangos GE, Romero-Ibarra IC, Hernández-Altamirano R, Mena-Cervantes VY. In-situ transesterification of Jatropha curcas L. seeds using homogeneous and heterogeneous basic catalysts. Fuel. 2019;235(1):277–87. 10.1016/j.fuel.2018.07.082.Search in Google Scholar
[9] Chua SY, Periasamy LaP, Goh CMH, Tan YH, Mubarak NM, Kansedo J, et al. Biodiesel synthesis using natural solid catalyst derived from biomass waste — A review. J Ind Eng Chem. 2020;81(1):41–60. 10.1016/j.jiec.2019.09.022.Search in Google Scholar
[10] Fan M, Wu H, Shi M, Zhang P, Jiang P. Well-dispersive K2OKCl alkaline catalyst derived from waste banana peel for biodiesel synthesis. Green Energy Environ. 2019;4(3):322–7. 10.1016/j.gee.2018.09.004.Search in Google Scholar
[11] Falowo OA, Oloko-Oba MI, Betiku E. Biodiesel production intensification via microwave irradiation-assisted transesterification of oil blend using nanoparticles from elephant-ear tree pod husk as a base heterogeneous catalyst. Chem Eng Process - Process Intensif. 2019;140(1):157–70. 10.1016/j.cep.2019.04.010.Search in Google Scholar
[12] Martínez A, Mijangos GE, Romero-Ibarra IC, Hernández-Altamirano R, Mena-Cervantes VY, Gutiérrez S. A novel green one-pot synthesis of biodiesel from Ricinus communis seeds by basic heterogeneous catalysis. J Clean Prod. 2018;196(1):340–9. 10.1016/j.jclepro.2018.05.241.Search in Google Scholar
[13] Hasakul T, Piticharoenphun S, Rattanaphra D, Nuchdang S, Kingkam W. A study of the precipitation of cerium oxide synthesized from rare earth sources used as the catalyst for biodiesel production. Green Process Synth. 2022;11(1):747–56. 10.1515/gps-2022-0069.Search in Google Scholar
[14] Ruatpuia JVL, Changmai B, Pathak A, Alghamdi LA, Kress T, Halder G, et al. Green biodiesel production from Jatropha curcas oil using a carbon-based solid acid catalyst: A process optimization study. Renew Energy. 2023;206(1):597–608. 10.1016/j.renene.2023.02.041.Search in Google Scholar
[15] Changmai B, Vanlalveni C, Ingle AP, Bhagat R, Rokhum L. Widely used catalysts in biodiesel production: a review. RSC Adv. 2020;10(68):41625–79. 10.1039/D0RA07931F.Search in Google Scholar
[16] Sitepu EK, Sembiring Y, Supeno M, Tarigan K, Ginting J, Karo-Karo JA, et al. Homogenizer-intensified room temperature biodiesel production using heterogeneous palm bunch ash catalyst. S Afr J Chem Eng. 2022;40(1):240–5. 10.1016/j.sajce.2022.03.007.Search in Google Scholar
[17] Tarigan JB, Singh K, Sinuraya JS, Supeno M, Sembiring H, Tarigan, K, et al. Waste Passion fruit peel as a heterogeneous catalyst for room-temperature biodiesel production. ACS Omega. 2022;7(9):7885–92. 10.1021/acsomega.1c06785.Search in Google Scholar PubMed PubMed Central
[18] Alagumalai A, Mahian O, Hollmann F, Zhang W. Environmentally benign solid catalysts for sustainable biodiesel production: A critical review. Sci Total Environ. 2021;768(1):144856. 10.1016/j.scitotenv.2020.144856.Search in Google Scholar PubMed
[19] Nath B, Das B, Kalita P, Basumatary S. Waste to value addition: Utilization of waste Brassica nigra plant derived novel green heterogeneous base catalyst for effective synthesis of biodiesel. J Clean Prod. 2019;239(1):118112. 10.1016/j.jclepro.2019.118112.Search in Google Scholar
[20] Manshor MR, Anuar H, Nur Aimi MN, Ahmad Fitrie MI, Wan Nazri WB, Sapuan SM, et al. Mechanical, thermal and morphological properties of durian skin fibre reinforced PLA biocomposites. Mater Des. 2014;59(1):279–86. 10.1016/j.matdes.2014.02.062.Search in Google Scholar
[21] Indonesia B-S. Statistics of Horticulture 2022. Jakarta: BPS-Statistics Indonesia; 2023.Search in Google Scholar
[22] Laskar IB, Gupta R, Chatterjee S, Vanlalveni C, Rokhum L. Taming waste: Waste Mangifera indica peel as a sustainable catalyst for biodiesel production at room temperature. Renew Energy. 2020;161(1):207–20. 10.1016/j.renene.2020.07.061.Search in Google Scholar
[23] Gohain M, Laskar K, Phukon H, Bora U, Kalita D, Deka D. Towards sustainable biodiesel and chemical production: multifunctional use of heterogeneous catalyst from littered Tectona grandis leaves. Waste Manag. 2020;102(1):212–21. 10.1016/j.wasman.2019.10.049.Search in Google Scholar PubMed
[24] Niju S, Janaranjani A, Nanthini R, Sindhu PA, Balajii M. Valorization of banana pseudostem as a catalyst for transesterification process and its optimization studies. Biomass Convers Biorefinery. 2021;13(1):1805–18. 10.1007/s13399-021-01343-x.Search in Google Scholar
[25] Nomanbhay S, Ong MY. A Review of Microwave-Assisted Reactions for Biodiesel Production. Bioengineering. 2017;4(2):57. 10.3390/bioengineering4020057.Search in Google Scholar PubMed PubMed Central
[26] Motasemi F, Ani FN. A review on microwave-assisted production of biodiesel. Renew Sustain Energy Rev. 2012;16(7):4719–33. 10.1016/j.rser.2012.03.069.Search in Google Scholar
[27] Hsiao M-C, Lin C-C, Chang Y-H. Microwave irradiation-assisted transesterification of soybean oil to biodiesel catalyzed by nanopowder calcium oxide. Fuel. 2011;90(5):1963–7. 10.1016/j.fuel.2011.01.004.Search in Google Scholar
[28] Kodgire P, Sharma A, Kachhwaha SS. Optimization and kinetics of biodiesel production of Ricinus communis oil and used cottonseed cooking oil employing synchronised ‘ultrasound + microwave’ and heterogeneous CaO catalyst. Renew Energy. 2023;212(1):320–32. 10.1016/j.renene.2023.05.016.Search in Google Scholar
[29] Kodgire P, Sharma A, Kachhwaha SS. Biodiesel production with enhanced fuel properties via appropriation of non-edible oil mixture using conjoint ultrasound and microwave reactor: Process optimization and kinetic studies. Fuel Process Technol. 2022;230(1):107206. 10.1016/j.fuproc.2022.107206.Search in Google Scholar
[30] Hsiao M-C, Kuo J-Y, Hsieh S-A, Hsieh P-H, Hou S-S. Optimized conversion of waste cooking oil to biodiesel using modified calcium oxide as catalyst via a microwave heating system. Fuel. 2020;266(1):117114. 10.1016/j.fuel.2020.117114.Search in Google Scholar
[31] Marella TK, Datta A, Patil MD, Dixit S, Tiwari A. Biodiesel production through algal cultivation in urban wastewater using algal floway. Bioresour Technol. 2019;280(1):222–8. 10.1016/j.biortech.2019.02.031.Search in Google Scholar PubMed
[32] Tarigan JB, Anggraini R, Sembiring RP, Supeno M, Tarigan K, Ginting J, et al. Waste rubber seeds as a renewable energy source: direct biodiesel production using a controlled crushing device. RSC Adv. 2022;12(4):2094–101. 10.1039/D1RA08298A.Search in Google Scholar PubMed PubMed Central
[33] Bharti RK, Katiyar R, Dhar DW, Prasanna R, Tyagi R. In situ transesterification and prediction of fuel quality parameters of biodiesel produced from Botryococcus sp. MCC31. Biofuels. 2019;12(9):1–10. 10.1080/17597269.2019.1594592.Search in Google Scholar
[34] Islam MA, Ayoko GA, Brown R, Stuart D, Heimann K. Influence of fatty acid structure on fuel properties of algae derived biodiesel. Procedia Eng. 2013;56(1):591–6. 10.1016/j.proeng.2013.03.164.Search in Google Scholar
[35] Yeong SP, Chan YS, Law MC, Ling JKU. Improving cold flow properties of palm fatty acid distillate biodiesel through vacuum distillation. J Bioresour Bioprod. 2022;7(1):43–51. 10.1016/j.jobab.2021.09.002.Search in Google Scholar
[36] Balajii M, Niju S. Ultrasound-assisted biodiesel production from Ceiba pentandra oil using Musa spp Nendran banana peduncle derived heterogeneous catalyst. Bioresour Technol Rep. 2023;21(1):101310. 10.1016/j.biteb.2022.101310.Search in Google Scholar
© 2024 the author(s), published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Research Articles
- Green polymer electrolyte and activated charcoal-based supercapacitor for energy harvesting application: Electrochemical characteristics
- Research on the adsorption of Co2+ ions using halloysite clay and the ability to recover them by electrodeposition method
- Simultaneous estimation of ibuprofen, caffeine, and paracetamol in commercial products using a green reverse-phase HPTLC method
- Isolation, screening and optimization of alkaliphilic cellulolytic fungi for production of cellulase
- Functionalized gold nanoparticles coated with bacterial alginate and their antibacterial and anticancer activities
- Comparative analysis of bio-based amino acid surfactants obtained via Diels–Alder reaction of cyclic anhydrides
- Biosynthesis of silver nanoparticles on yellow phosphorus slag and its application in organic coatings
- Exploring antioxidant potential and phenolic compound extraction from Vitis vinifera L. using ultrasound-assisted extraction
- Manganese and copper-coated nickel oxide nanoparticles synthesized from Carica papaya leaf extract induce antimicrobial activity and breast cancer cell death by triggering mitochondrial caspases and p53
- Insight into heating method and Mozafari method as green processing techniques for the synthesis of micro- and nano-drug carriers
- Silicotungstic acid supported on Bi-based MOF-derived metal oxide for photodegradation of organic dyes
- Synthesis and characterization of capsaicin nanoparticles: An attempt to enhance its bioavailability and pharmacological actions
- Synthesis of Lawsonia inermis-encased silver–copper bimetallic nanoparticles with antioxidant, antibacterial, and cytotoxic activity
- Facile, polyherbal drug-mediated green synthesis of CuO nanoparticles and their potent biological applications
- Zinc oxide-manganese oxide/carboxymethyl cellulose-folic acid-sesamol hybrid nanomaterials: A molecularly targeted strategy for advanced triple-negative breast cancer therapy
- Exploring the antimicrobial potential of biogenically synthesized graphene oxide nanoparticles against targeted bacterial and fungal pathogens
- Biofabrication of silver nanoparticles using Uncaria tomentosa L.: Insight into characterization, antibacterial activities combined with antibiotics, and effect on Triticum aestivum germination
- Membrane distillation of synthetic urine for use in space structural habitat systems
- Investigation on mechanical properties of the green synthesis bamboo fiber/eggshell/coconut shell powder-based hybrid biocomposites under NaOH conditions
- Green synthesis of magnesium oxide nanoparticles using endophytic fungal strain to improve the growth, metabolic activities, yield traits, and phenolic compounds content of Nigella sativa L.
- Estimation of greenhouse gas emissions from rice and annual upland crops in Red River Delta of Vietnam using the denitrification–decomposition model
- Synthesis of humic acid with the obtaining of potassium humate based on coal waste from the Lenger deposit, Kazakhstan
- Ascorbic acid-mediated selenium nanoparticles as potential antihyperuricemic, antioxidant, anticoagulant, and thrombolytic agents
- Green synthesis of silver nanoparticles using Illicium verum extract: Optimization and characterization for biomedical applications
- Antibacterial and dynamical behaviour of silicon nanoparticles influenced sustainable waste flax fibre-reinforced epoxy composite for biomedical application
- Optimising coagulation/flocculation using response surface methodology and application of floc in biofertilisation
- Green synthesis and multifaceted characterization of iron oxide nanoparticles derived from Senna bicapsularis for enhanced in vitro and in vivo biological investigation
- Potent antibacterial nanocomposites from okra mucilage/chitosan/silver nanoparticles for multidrug-resistant Salmonella Typhimurium eradication
- Trachyspermum copticum aqueous seed extract-derived silver nanoparticles: Exploration of their structural characterization and comparative antibacterial performance against gram-positive and gram-negative bacteria
- Microwave-assisted ultrafine silver nanoparticle synthesis using Mitragyna speciosa for antimalarial applications
- Green synthesis and characterisation of spherical structure Ag/Fe2O3/TiO2 nanocomposite using acacia in the presence of neem and tulsi oils
- Green quantitative methods for linagliptin and empagliflozin in dosage forms
- Enhancement efficacy of omeprazole by conjugation with silver nanoparticles as a urease inhibitor
- Residual, sequential extraction, and ecological risk assessment of some metals in ash from municipal solid waste incineration, Vietnam
- Green synthesis of ZnO nanoparticles using the mangosteen (Garcinia mangostana L.) leaf extract: Comparative preliminary in vitro antibacterial study
- Simultaneous determination of lesinurad and febuxostat in commercial fixed-dose combinations using a greener normal-phase HPTLC method
- A greener RP-HPLC method for quaternary estimation of caffeine, paracetamol, levocetirizine, and phenylephrine acquiring AQbD with stability studies
- Optimization of biomass durian peel as a heterogeneous catalyst in biodiesel production using microwave irradiation
- Thermal treatment impact on the evolution of active phases in layered double hydroxide-based ZnCr photocatalysts: Photodegradation and antibacterial performance
- Preparation of silymarin-loaded zein polysaccharide core–shell nanostructures and evaluation of their biological potentials
- Preparation and characterization of composite-modified PA6 fiber for spectral heating and heat storage applications
- Preparation and electrocatalytic oxygen evolution of bimetallic phosphates (NiFe)2P/NF
- Rod-shaped Mo(vi) trichalcogenide–Mo(vi) oxide decorated on poly(1-H pyrrole) as a promising nanocomposite photoelectrode for green hydrogen generation from sewage water with high efficiency
- Green synthesis and studies on citrus medica leaf extract-mediated Au–ZnO nanocomposites: A sustainable approach for efficient photocatalytic degradation of rhodamine B dye in aqueous media
- Cellulosic materials for the removal of ciprofloxacin from aqueous environments
- The analytical assessment of metal contamination in industrial soils of Saudi Arabia using the inductively coupled plasma technology
- The effect of modified oily sludge on the slurry ability and combustion performance of coal water slurry
- Eggshell waste transformation to calcium chloride anhydride as food-grade additive and eggshell membranes as enzyme immobilization carrier
- Synthesis of EPAN and applications in the encapsulation of potassium humate
- Biosynthesis and characterization of silver nanoparticles from Cedrela toona leaf extracts: An exploration into their antibacterial, anticancer, and antioxidant potential
- Enhancing mechanical and rheological properties of HDPE films through annealing for eco-friendly agricultural applications
- Immobilisation of catalase purified from mushroom (Hydnum repandum) onto glutaraldehyde-activated chitosan and characterisation: Its application for the removal of hydrogen peroxide from artificial wastewater
- Sodium titanium oxide/zinc oxide (STO/ZnO) photocomposites for efficient dye degradation applications
- Effect of ex situ, eco-friendly ZnONPs incorporating green synthesised Moringa oleifera leaf extract in enhancing biochemical and molecular aspects of Vicia faba L. under salt stress
- Biosynthesis and characterization of selenium and silver nanoparticles using Trichoderma viride filtrate and their impact on Culex pipiens
- Photocatalytic degradation of organic dyes and biological potentials of biogenic zinc oxide nanoparticles synthesized using the polar extract of Cyperus scariosus R.Br. (Cyperaceae)
- Assessment of antiproliferative activity of green-synthesized nickel oxide nanoparticles against glioblastoma cells using Terminalia chebula
- Chlorine-free synthesis of phosphinic derivatives by change in the P-function
- Anticancer, antioxidant, and antimicrobial activities of nanoemulsions based on water-in-olive oil and loaded on biogenic silver nanoparticles
- Study and mechanism of formation of phosphorus production waste in Kazakhstan
- Synthesis and stabilization of anatase form of biomimetic TiO2 nanoparticles for enhancing anti-tumor potential
- Microwave-supported one-pot reaction for the synthesis of 5-alkyl/arylidene-2-(morpholin/thiomorpholin-4-yl)-1,3-thiazol-4(5H)-one derivatives over MgO solid base
- Screening the phytochemicals in Perilla leaves and phytosynthesis of bioactive silver nanoparticles for potential antioxidant and wound-healing application
- Graphene oxide/chitosan/manganese/folic acid-brucine functionalized nanocomposites show anticancer activity against liver cancer cells
- Nature of serpentinite interactions with low-concentration sulfuric acid solutions
- Multi-objective statistical optimisation utilising response surface methodology to predict engine performance using biofuels from waste plastic oil in CRDi engines
- Microwave-assisted extraction of acetosolv lignin from sugarcane bagasse and electrospinning of lignin/PEO nanofibres for carbon fibre production
- Biosynthesis, characterization, and investigation of cytotoxic activities of selenium nanoparticles utilizing Limosilactobacillus fermentum
- Highly photocatalytic materials based on the decoration of poly(O-chloroaniline) with molybdenum trichalcogenide oxide for green hydrogen generation from Red Sea water
- Highly efficient oil–water separation using superhydrophobic cellulose aerogels derived from corn straw
- Beta-cyclodextrin–Phyllanthus emblica emulsion for zinc oxide nanoparticles: Characteristics and photocatalysis
- Assessment of antimicrobial activity and methyl orange dye removal by Klebsiella pneumoniae-mediated silver nanoparticles
- Influential eradication of resistant Salmonella Typhimurium using bioactive nanocomposites from chitosan and radish seed-synthesized nanoselenium
- Antimicrobial activities and neuroprotective potential for Alzheimer’s disease of pure, Mn, Co, and Al-doped ZnO ultra-small nanoparticles
- Green synthesis of silver nanoparticles from Bauhinia variegata and their biological applications
- Synthesis and optimization of long-chain fatty acids via the oxidation of long-chain fatty alcohols
- Eminent Red Sea water hydrogen generation via a Pb(ii)-iodide/poly(1H-pyrrole) nanocomposite photocathode
- Green synthesis and effective genistein production by fungal β-glucosidase immobilized on Al2O3 nanocrystals synthesized in Cajanus cajan L. (Millsp.) leaf extracts
- Green stability-indicating RP-HPTLC technique for determining croconazole hydrochloride
- Green synthesis of La2O3–LaPO4 nanocomposites using Charybdis natator for DNA binding, cytotoxic, catalytic, and luminescence applications
- Eco-friendly drugs induce cellular changes in colistin-resistant bacteria
- Tangerine fruit peel extract mediated biogenic synthesized silver nanoparticles and their potential antimicrobial, antioxidant, and cytotoxic assessments
- Green synthesis on performance characteristics of a direct injection diesel engine using sandbox seed oil
- A highly sensitive β-AKBA-Ag-based fluorescent “turn off” chemosensor for rapid detection of abamectin in tomatoes
- Green synthesis and physical characterization of zinc oxide nanoparticles (ZnO NPs) derived from the methanol extract of Euphorbia dracunculoides Lam. (Euphorbiaceae) with enhanced biosafe applications
- Detection of morphine and data processing using surface plasmon resonance imaging sensor
- Effects of nanoparticles on the anaerobic digestion properties of sulfamethoxazole-containing chicken manure and analysis of bio-enzymes
- Bromic acid-thiourea synergistic leaching of sulfide gold ore
- Green chemistry approach to synthesize titanium dioxide nanoparticles using Fagonia Cretica extract, novel strategy for developing antimicrobial and antidiabetic therapies
- Green synthesis and effective utilization of biogenic Al2O3-nanocoupled fungal lipase in the resolution of active homochiral 2-octanol and its immobilization via aluminium oxide nanoparticles
- Eco-friendly RP-HPLC approach for simultaneously estimating the promising combination of pentoxifylline and simvastatin in therapeutic potential for breast cancer: Appraisal of greenness, whiteness, and Box–Behnken design
- Use of a humidity adsorbent derived from cockleshell waste in Thai fried fish crackers (Keropok)
- One-pot green synthesis, biological evaluation, and in silico study of pyrazole derivatives obtained from chalcones
- Bio-sorption of methylene blue and production of biofuel by brown alga Cystoseira sp. collected from Neom region, Kingdom of Saudi Arabia
- Synthesis of motexafin gadolinium: A promising radiosensitizer and imaging agent for cancer therapy
- The impact of varying sizes of silver nanoparticles on the induction of cellular damage in Klebsiella pneumoniae involving diverse mechanisms
- Microwave-assisted green synthesis, characterization, and in vitro antibacterial activity of NiO nanoparticles obtained from lemon peel extract
- Rhus microphylla-mediated biosynthesis of copper oxide nanoparticles for enhanced antibacterial and antibiofilm efficacy
- Harnessing trichalcogenide–molybdenum(vi) sulfide and molybdenum(vi) oxide within poly(1-amino-2-mercaptobenzene) frameworks as a photocathode for sustainable green hydrogen production from seawater without sacrificial agents
- Magnetically recyclable Fe3O4@SiO2 supported phosphonium ionic liquids for efficient and sustainable transformation of CO2 into oxazolidinones
- A comparative study of Fagonia arabica fabricated silver sulfide nanoparticles (Ag2S) and silver nanoparticles (AgNPs) with distinct antimicrobial, anticancer, and antioxidant properties
- Visible light photocatalytic degradation and biological activities of Aegle marmelos-mediated cerium oxide nanoparticles
- Physical intrinsic characteristics of spheroidal particles in coal gasification fine slag
- Exploring the effect of tea dust magnetic biochar on agricultural crops grown in polycyclic aromatic hydrocarbon contaminated soil
- Crosslinked chitosan-modified ultrafiltration membranes for efficient surface water treatment and enhanced anti-fouling performances
- Study on adsorption characteristics of biochars and their modified biochars for removal of organic dyes from aqueous solution
- Zein polymer nanocarrier for Ocimum basilicum var. purpurascens extract: Potential biomedical use
- Green synthesis, characterization, and in vitro and in vivo biological screening of iron oxide nanoparticles (Fe3O4) generated with hydroalcoholic extract of aerial parts of Euphorbia milii
- Novel microwave-based green approach for the synthesis of dual-loaded cyclodextrin nanosponges: Characterization, pharmacodynamics, and pharmacokinetics evaluation
- Bi2O3–BiOCl/poly-m-methyl aniline nanocomposite thin film for broad-spectrum light-sensing
- Green synthesis and characterization of CuO/ZnO nanocomposite using Musa acuminata leaf extract for cytotoxic studies on colorectal cancer cells (HCC2998)
- Review Articles
- Materials-based drug delivery approaches: Recent advances and future perspectives
- A review of thermal treatment for bamboo and its composites
- An overview of the role of nanoherbicides in tackling challenges of weed management in wheat: A novel approach
- An updated review on carbon nanomaterials: Types, synthesis, functionalization and applications, degradation and toxicity
- Special Issue: Emerging green nanomaterials for sustainable waste management and biomedical applications
- Green synthesis of silver nanoparticles using mature-pseudostem extracts of Alpinia nigra and their bioactivities
- Special Issue: New insights into nanopythotechnology: current trends and future prospects
- Green synthesis of FeO nanoparticles from coffee and its application for antibacterial, antifungal, and anti-oxidation activity
- Dye degradation activity of biogenically synthesized Cu/Fe/Ag trimetallic nanoparticles
- Special Issue: Composites and green composites
- Recent trends and advancements in the utilization of green composites and polymeric nanocarriers for enhancing food quality and sustainable processing
- Retraction
- Retraction of “Biosynthesis and characterization of silver nanoparticles from Cedrela toona leaf extracts: An exploration into their antibacterial, anticancer, and antioxidant potential”
- Retraction of “Photocatalytic degradation of organic dyes and biological potentials of biogenic zinc oxide nanoparticles synthesized using the polar extract of Cyperus scariosus R.Br. (Cyperaceae)”
- Retraction to “Green synthesis on performance characteristics of a direct injection diesel engine using sandbox seed oil”
Articles in the same Issue
- Research Articles
- Green polymer electrolyte and activated charcoal-based supercapacitor for energy harvesting application: Electrochemical characteristics
- Research on the adsorption of Co2+ ions using halloysite clay and the ability to recover them by electrodeposition method
- Simultaneous estimation of ibuprofen, caffeine, and paracetamol in commercial products using a green reverse-phase HPTLC method
- Isolation, screening and optimization of alkaliphilic cellulolytic fungi for production of cellulase
- Functionalized gold nanoparticles coated with bacterial alginate and their antibacterial and anticancer activities
- Comparative analysis of bio-based amino acid surfactants obtained via Diels–Alder reaction of cyclic anhydrides
- Biosynthesis of silver nanoparticles on yellow phosphorus slag and its application in organic coatings
- Exploring antioxidant potential and phenolic compound extraction from Vitis vinifera L. using ultrasound-assisted extraction
- Manganese and copper-coated nickel oxide nanoparticles synthesized from Carica papaya leaf extract induce antimicrobial activity and breast cancer cell death by triggering mitochondrial caspases and p53
- Insight into heating method and Mozafari method as green processing techniques for the synthesis of micro- and nano-drug carriers
- Silicotungstic acid supported on Bi-based MOF-derived metal oxide for photodegradation of organic dyes
- Synthesis and characterization of capsaicin nanoparticles: An attempt to enhance its bioavailability and pharmacological actions
- Synthesis of Lawsonia inermis-encased silver–copper bimetallic nanoparticles with antioxidant, antibacterial, and cytotoxic activity
- Facile, polyherbal drug-mediated green synthesis of CuO nanoparticles and their potent biological applications
- Zinc oxide-manganese oxide/carboxymethyl cellulose-folic acid-sesamol hybrid nanomaterials: A molecularly targeted strategy for advanced triple-negative breast cancer therapy
- Exploring the antimicrobial potential of biogenically synthesized graphene oxide nanoparticles against targeted bacterial and fungal pathogens
- Biofabrication of silver nanoparticles using Uncaria tomentosa L.: Insight into characterization, antibacterial activities combined with antibiotics, and effect on Triticum aestivum germination
- Membrane distillation of synthetic urine for use in space structural habitat systems
- Investigation on mechanical properties of the green synthesis bamboo fiber/eggshell/coconut shell powder-based hybrid biocomposites under NaOH conditions
- Green synthesis of magnesium oxide nanoparticles using endophytic fungal strain to improve the growth, metabolic activities, yield traits, and phenolic compounds content of Nigella sativa L.
- Estimation of greenhouse gas emissions from rice and annual upland crops in Red River Delta of Vietnam using the denitrification–decomposition model
- Synthesis of humic acid with the obtaining of potassium humate based on coal waste from the Lenger deposit, Kazakhstan
- Ascorbic acid-mediated selenium nanoparticles as potential antihyperuricemic, antioxidant, anticoagulant, and thrombolytic agents
- Green synthesis of silver nanoparticles using Illicium verum extract: Optimization and characterization for biomedical applications
- Antibacterial and dynamical behaviour of silicon nanoparticles influenced sustainable waste flax fibre-reinforced epoxy composite for biomedical application
- Optimising coagulation/flocculation using response surface methodology and application of floc in biofertilisation
- Green synthesis and multifaceted characterization of iron oxide nanoparticles derived from Senna bicapsularis for enhanced in vitro and in vivo biological investigation
- Potent antibacterial nanocomposites from okra mucilage/chitosan/silver nanoparticles for multidrug-resistant Salmonella Typhimurium eradication
- Trachyspermum copticum aqueous seed extract-derived silver nanoparticles: Exploration of their structural characterization and comparative antibacterial performance against gram-positive and gram-negative bacteria
- Microwave-assisted ultrafine silver nanoparticle synthesis using Mitragyna speciosa for antimalarial applications
- Green synthesis and characterisation of spherical structure Ag/Fe2O3/TiO2 nanocomposite using acacia in the presence of neem and tulsi oils
- Green quantitative methods for linagliptin and empagliflozin in dosage forms
- Enhancement efficacy of omeprazole by conjugation with silver nanoparticles as a urease inhibitor
- Residual, sequential extraction, and ecological risk assessment of some metals in ash from municipal solid waste incineration, Vietnam
- Green synthesis of ZnO nanoparticles using the mangosteen (Garcinia mangostana L.) leaf extract: Comparative preliminary in vitro antibacterial study
- Simultaneous determination of lesinurad and febuxostat in commercial fixed-dose combinations using a greener normal-phase HPTLC method
- A greener RP-HPLC method for quaternary estimation of caffeine, paracetamol, levocetirizine, and phenylephrine acquiring AQbD with stability studies
- Optimization of biomass durian peel as a heterogeneous catalyst in biodiesel production using microwave irradiation
- Thermal treatment impact on the evolution of active phases in layered double hydroxide-based ZnCr photocatalysts: Photodegradation and antibacterial performance
- Preparation of silymarin-loaded zein polysaccharide core–shell nanostructures and evaluation of their biological potentials
- Preparation and characterization of composite-modified PA6 fiber for spectral heating and heat storage applications
- Preparation and electrocatalytic oxygen evolution of bimetallic phosphates (NiFe)2P/NF
- Rod-shaped Mo(vi) trichalcogenide–Mo(vi) oxide decorated on poly(1-H pyrrole) as a promising nanocomposite photoelectrode for green hydrogen generation from sewage water with high efficiency
- Green synthesis and studies on citrus medica leaf extract-mediated Au–ZnO nanocomposites: A sustainable approach for efficient photocatalytic degradation of rhodamine B dye in aqueous media
- Cellulosic materials for the removal of ciprofloxacin from aqueous environments
- The analytical assessment of metal contamination in industrial soils of Saudi Arabia using the inductively coupled plasma technology
- The effect of modified oily sludge on the slurry ability and combustion performance of coal water slurry
- Eggshell waste transformation to calcium chloride anhydride as food-grade additive and eggshell membranes as enzyme immobilization carrier
- Synthesis of EPAN and applications in the encapsulation of potassium humate
- Biosynthesis and characterization of silver nanoparticles from Cedrela toona leaf extracts: An exploration into their antibacterial, anticancer, and antioxidant potential
- Enhancing mechanical and rheological properties of HDPE films through annealing for eco-friendly agricultural applications
- Immobilisation of catalase purified from mushroom (Hydnum repandum) onto glutaraldehyde-activated chitosan and characterisation: Its application for the removal of hydrogen peroxide from artificial wastewater
- Sodium titanium oxide/zinc oxide (STO/ZnO) photocomposites for efficient dye degradation applications
- Effect of ex situ, eco-friendly ZnONPs incorporating green synthesised Moringa oleifera leaf extract in enhancing biochemical and molecular aspects of Vicia faba L. under salt stress
- Biosynthesis and characterization of selenium and silver nanoparticles using Trichoderma viride filtrate and their impact on Culex pipiens
- Photocatalytic degradation of organic dyes and biological potentials of biogenic zinc oxide nanoparticles synthesized using the polar extract of Cyperus scariosus R.Br. (Cyperaceae)
- Assessment of antiproliferative activity of green-synthesized nickel oxide nanoparticles against glioblastoma cells using Terminalia chebula
- Chlorine-free synthesis of phosphinic derivatives by change in the P-function
- Anticancer, antioxidant, and antimicrobial activities of nanoemulsions based on water-in-olive oil and loaded on biogenic silver nanoparticles
- Study and mechanism of formation of phosphorus production waste in Kazakhstan
- Synthesis and stabilization of anatase form of biomimetic TiO2 nanoparticles for enhancing anti-tumor potential
- Microwave-supported one-pot reaction for the synthesis of 5-alkyl/arylidene-2-(morpholin/thiomorpholin-4-yl)-1,3-thiazol-4(5H)-one derivatives over MgO solid base
- Screening the phytochemicals in Perilla leaves and phytosynthesis of bioactive silver nanoparticles for potential antioxidant and wound-healing application
- Graphene oxide/chitosan/manganese/folic acid-brucine functionalized nanocomposites show anticancer activity against liver cancer cells
- Nature of serpentinite interactions with low-concentration sulfuric acid solutions
- Multi-objective statistical optimisation utilising response surface methodology to predict engine performance using biofuels from waste plastic oil in CRDi engines
- Microwave-assisted extraction of acetosolv lignin from sugarcane bagasse and electrospinning of lignin/PEO nanofibres for carbon fibre production
- Biosynthesis, characterization, and investigation of cytotoxic activities of selenium nanoparticles utilizing Limosilactobacillus fermentum
- Highly photocatalytic materials based on the decoration of poly(O-chloroaniline) with molybdenum trichalcogenide oxide for green hydrogen generation from Red Sea water
- Highly efficient oil–water separation using superhydrophobic cellulose aerogels derived from corn straw
- Beta-cyclodextrin–Phyllanthus emblica emulsion for zinc oxide nanoparticles: Characteristics and photocatalysis
- Assessment of antimicrobial activity and methyl orange dye removal by Klebsiella pneumoniae-mediated silver nanoparticles
- Influential eradication of resistant Salmonella Typhimurium using bioactive nanocomposites from chitosan and radish seed-synthesized nanoselenium
- Antimicrobial activities and neuroprotective potential for Alzheimer’s disease of pure, Mn, Co, and Al-doped ZnO ultra-small nanoparticles
- Green synthesis of silver nanoparticles from Bauhinia variegata and their biological applications
- Synthesis and optimization of long-chain fatty acids via the oxidation of long-chain fatty alcohols
- Eminent Red Sea water hydrogen generation via a Pb(ii)-iodide/poly(1H-pyrrole) nanocomposite photocathode
- Green synthesis and effective genistein production by fungal β-glucosidase immobilized on Al2O3 nanocrystals synthesized in Cajanus cajan L. (Millsp.) leaf extracts
- Green stability-indicating RP-HPTLC technique for determining croconazole hydrochloride
- Green synthesis of La2O3–LaPO4 nanocomposites using Charybdis natator for DNA binding, cytotoxic, catalytic, and luminescence applications
- Eco-friendly drugs induce cellular changes in colistin-resistant bacteria
- Tangerine fruit peel extract mediated biogenic synthesized silver nanoparticles and their potential antimicrobial, antioxidant, and cytotoxic assessments
- Green synthesis on performance characteristics of a direct injection diesel engine using sandbox seed oil
- A highly sensitive β-AKBA-Ag-based fluorescent “turn off” chemosensor for rapid detection of abamectin in tomatoes
- Green synthesis and physical characterization of zinc oxide nanoparticles (ZnO NPs) derived from the methanol extract of Euphorbia dracunculoides Lam. (Euphorbiaceae) with enhanced biosafe applications
- Detection of morphine and data processing using surface plasmon resonance imaging sensor
- Effects of nanoparticles on the anaerobic digestion properties of sulfamethoxazole-containing chicken manure and analysis of bio-enzymes
- Bromic acid-thiourea synergistic leaching of sulfide gold ore
- Green chemistry approach to synthesize titanium dioxide nanoparticles using Fagonia Cretica extract, novel strategy for developing antimicrobial and antidiabetic therapies
- Green synthesis and effective utilization of biogenic Al2O3-nanocoupled fungal lipase in the resolution of active homochiral 2-octanol and its immobilization via aluminium oxide nanoparticles
- Eco-friendly RP-HPLC approach for simultaneously estimating the promising combination of pentoxifylline and simvastatin in therapeutic potential for breast cancer: Appraisal of greenness, whiteness, and Box–Behnken design
- Use of a humidity adsorbent derived from cockleshell waste in Thai fried fish crackers (Keropok)
- One-pot green synthesis, biological evaluation, and in silico study of pyrazole derivatives obtained from chalcones
- Bio-sorption of methylene blue and production of biofuel by brown alga Cystoseira sp. collected from Neom region, Kingdom of Saudi Arabia
- Synthesis of motexafin gadolinium: A promising radiosensitizer and imaging agent for cancer therapy
- The impact of varying sizes of silver nanoparticles on the induction of cellular damage in Klebsiella pneumoniae involving diverse mechanisms
- Microwave-assisted green synthesis, characterization, and in vitro antibacterial activity of NiO nanoparticles obtained from lemon peel extract
- Rhus microphylla-mediated biosynthesis of copper oxide nanoparticles for enhanced antibacterial and antibiofilm efficacy
- Harnessing trichalcogenide–molybdenum(vi) sulfide and molybdenum(vi) oxide within poly(1-amino-2-mercaptobenzene) frameworks as a photocathode for sustainable green hydrogen production from seawater without sacrificial agents
- Magnetically recyclable Fe3O4@SiO2 supported phosphonium ionic liquids for efficient and sustainable transformation of CO2 into oxazolidinones
- A comparative study of Fagonia arabica fabricated silver sulfide nanoparticles (Ag2S) and silver nanoparticles (AgNPs) with distinct antimicrobial, anticancer, and antioxidant properties
- Visible light photocatalytic degradation and biological activities of Aegle marmelos-mediated cerium oxide nanoparticles
- Physical intrinsic characteristics of spheroidal particles in coal gasification fine slag
- Exploring the effect of tea dust magnetic biochar on agricultural crops grown in polycyclic aromatic hydrocarbon contaminated soil
- Crosslinked chitosan-modified ultrafiltration membranes for efficient surface water treatment and enhanced anti-fouling performances
- Study on adsorption characteristics of biochars and their modified biochars for removal of organic dyes from aqueous solution
- Zein polymer nanocarrier for Ocimum basilicum var. purpurascens extract: Potential biomedical use
- Green synthesis, characterization, and in vitro and in vivo biological screening of iron oxide nanoparticles (Fe3O4) generated with hydroalcoholic extract of aerial parts of Euphorbia milii
- Novel microwave-based green approach for the synthesis of dual-loaded cyclodextrin nanosponges: Characterization, pharmacodynamics, and pharmacokinetics evaluation
- Bi2O3–BiOCl/poly-m-methyl aniline nanocomposite thin film for broad-spectrum light-sensing
- Green synthesis and characterization of CuO/ZnO nanocomposite using Musa acuminata leaf extract for cytotoxic studies on colorectal cancer cells (HCC2998)
- Review Articles
- Materials-based drug delivery approaches: Recent advances and future perspectives
- A review of thermal treatment for bamboo and its composites
- An overview of the role of nanoherbicides in tackling challenges of weed management in wheat: A novel approach
- An updated review on carbon nanomaterials: Types, synthesis, functionalization and applications, degradation and toxicity
- Special Issue: Emerging green nanomaterials for sustainable waste management and biomedical applications
- Green synthesis of silver nanoparticles using mature-pseudostem extracts of Alpinia nigra and their bioactivities
- Special Issue: New insights into nanopythotechnology: current trends and future prospects
- Green synthesis of FeO nanoparticles from coffee and its application for antibacterial, antifungal, and anti-oxidation activity
- Dye degradation activity of biogenically synthesized Cu/Fe/Ag trimetallic nanoparticles
- Special Issue: Composites and green composites
- Recent trends and advancements in the utilization of green composites and polymeric nanocarriers for enhancing food quality and sustainable processing
- Retraction
- Retraction of “Biosynthesis and characterization of silver nanoparticles from Cedrela toona leaf extracts: An exploration into their antibacterial, anticancer, and antioxidant potential”
- Retraction of “Photocatalytic degradation of organic dyes and biological potentials of biogenic zinc oxide nanoparticles synthesized using the polar extract of Cyperus scariosus R.Br. (Cyperaceae)”
- Retraction to “Green synthesis on performance characteristics of a direct injection diesel engine using sandbox seed oil”

