Green synthesis of ZnO nanoparticles using the mangosteen (Garcinia mangostana L.) leaf extract: Comparative preliminary in vitro antibacterial study
-
Yu Bin Chan
, Samar Kumar Guha
Abstract
In the realm of public health, the rising threat caused by bacteria resistant to many drugs is a critical concern. In this work, we used the aqueous extract of mangosteen leaves to create zinc oxide (ZnO) nanoparticles (NPs) in an environmentally friendly manner. Through various analytical methods, we thoroughly characterized these biogenic ZnO NPs, including UV−visible, Fourier transform infrared, X-ray photoelectron spectroscopy, Raman spectroscopy, X-ray powder diffraction, field emission-scanning electron microscopy with energy dispersive X-ray and high resolution-transmission electron microscopy. ZnO NPs showed distinctive properties among different characterization techniques, including a small energy bandgap of 2.80 eV, a porous, a minimum crystalline size of 16.99 nm, an average particle size of 14.21 nm, and a spherical nanostructure. Additionally, we performed preliminary antibacterial experiments to assess ZnO NPs, copper oxide (CuO) NPs, and ZnO–CuO nanocomposites for antibacterial activity. Interestingly, ZnO NPs showed significant potential in suppressing the growth of Staphylococcus aureus ATCC BAA-1026, Bacillus subtilis ATCC 6633, Escherichia coli ATCC 25922, and Klebsiella pneumoniae ATCC 13883, with decreasing order of minimum inhibitory concentrations: S. aureus = B. subtilis (15.63 μg·mL−1) > E. coli (62.50 μg·mL−1) > K. pneumoniae (125.00 μg·mL−1). These results highlight the potential of biogenic NPs, particularly ZnO NPs, as effective agents against multi-drug-resistant bacteria.
1 Introduction
The advent of antibiotics in the mid-twentieth century marked a significant milestone in the field of medicine. However, the widespread misuse and overuse of antibiotics have given rise to the emergence of multi-drug-resistant bacteria, rendering nearly all existing antibiotics ineffective [1,2,3]. Despite ongoing efforts to introduce new antibiotics to the market, production rates have proven inadequate to counter the rapid emergence of multi-drug-resistant bacterial strains [1]. Consequently, the proliferation of pathogenic bacterial outbreaks, driven by resistance to conventional antibiotics, has emerged as a pressing global public health crisis in the twenty-first century [4]. As affirmed by the World Health Organization (WHO), bacterial infections are responsible for millions of annual deaths worldwide [2,5]. Consequently, researchers have turned their attention toward nanomaterials as a promising alternative solution [6]. Nanoparticles (NPs) have garnered particular interest due to their distinctive mechanisms of action in combating bacteria. They can directly interact with bacterial cell walls, obviating the need for cellular penetration [7], and can disrupt biochemical pathways by damaging organelles, ultimately leading to bacterial cell death [2].
Zinc oxide (ZnO) is an n-type semiconductor that ranges in band gap energy from 3.30 to 3.37 eV with a substantial exciton binding energy of 60 meV [8,9,10]. It also has strong bonding characteristics [11], near-ultraviolet emission [12], and remarkable photo- and thermal stability at room temperature [13]. The piezoelectricity of this material is extensively used in attenuators and sensors [12]. Moreover, the US Food and Drug Administration has classified ZnO as a “generally recognized as safe” chemical since it is non-toxic [14,15,16]. As a result, ZnO-containing medications are safe to use without a coating. ZnO can also be utilized in sunscreen creams, fabrics, and coatings due to its capacity to filter UV radiation and suppress antibacterial characteristics [12]. Additionally, ZnO is being used extensively in biological, catalytic, sensor, energy storage, photocatalyst, and optoelectronic devices [10,12,17]. On the other hand, compared to other noble metals, copper oxide (CuO), a p-type semiconductor with an energy bandgap ranging from 1.02 to 2.0 eV [9,18,19], provides more favourable economic advantages [20]. It is easily compatible with polymers due to its stability [21]. Superior thermal conductivity, optical characteristics, electron correlation effects, spin dynamics, magnetic phases, antioxidant capacity, and antibacterial properties are all exhibited by CuO NPs [21,22,23,24]. CuO NPs are therefore used in magneto-resistant materials, biosensors, antibacterial agents, supercapacitors, magnetic storage media, and field emission devices [22,24,25]. Recent attention has turned toward the design of nanocomposites (NCs), including ZnO–CuO, which promise enhanced performance in various applications [9]. ZnO–CuO NCs are one of the most studied p–n type heterojunction semiconductors because copper can easily overlap d-electrons with the valence bond of ZnO [26,27]. CuO is, therefore, the best metal oxide to be used in the synthesis of NCs with ZnO because it effectively separates and transfers photo-excited electrons from the upper conduction band to the lower one. ZnO–CuO NCs function very well as photocatalysts due to their huge surface area and simple production techniques [28,29]. However, in vitro preliminary antibacterial activity involving ZnO–CuO NCs remains sparsely reported to date.
In contrast to conventional synthesis methods like thermal decomposition, coprecipitation, and sol–gel processes, the green synthesis of nanomaterials offers advantages such as reduced cost, lower energy consumption, simplicity, and environmental friendliness. In general, microbes, plant extracts, viruses, fungi, yeasts, microalgae, and macroalgae can all be used to carry out the green synthesis of NPs [10,30,31]. Different from biological entities, plant extracts are more dependable, straightforward, and environmentally friendly than other biological entities when it comes to the green synthesis of NPs [32,33,34]. Plant-mediated green synthesis also presents several benefits over other biological materials for the synthesis of NPs. These include the ability for one-spot synthesis of NPs, as well as robustness, eco-friendliness, natural capping and reducing agents, ease of availability, safety, cost-effectiveness, suitability for large-scale synthesis, and not requiring cell cultures [19,34,35].
Compared to conventional methods applied, large-scale production of green-synthesized NPs from lab scale to pilot-plant level is still in the early stage [31]. Therefore, mangosteen, which has high nutrient value, was selected as a bio-reducing agent in synthesizing NPs in this study. Garcinia mangostana L., commonly known as mangosteen and belonging to the Clusiaceae family, is rich in phytochemicals such as xanthones, flavonoids, and terpenes. These compounds possess antibacterial, anti-tumour, anti-diabetic, immunomodulatory, anti-allergic, and hepatoprotective properties [36,37,38,39]. Mangosteen is a seasonal tropical fruit found in regions such as Malaysia, Thailand, Indonesia, and others. Given its abundant phytochemical content, mangosteen exhibits the potential to serve as a stabilizing agent for colloidal nanomaterials [40,41,42,43].
Both CuO NPs and ZnO-CuO NCs were synthesized through a green approach using mangosteen leaf aqueous extract as natural capping and reducing agents and detailed physicochemical properties of these materials have been reported in previously published studies [44,45]. This study focuses on the green synthesis of ZnO NPs using mangosteen leaf aqueous extract. We explore their optical, structural, and morphological properties, along with conducting and comparing their in vitro preliminary antibacterial assessments with CuO NPs and ZnO–CuO NCs.
2 Materials and methods
The mangosteen leaves were collected from Kampar, Malaysia. Zinc nitrate hexahydrate, Zn(NO3)2·6H2O, and nutrient broth (NB) were purchased from HiMedia Laboratories Pvt. Ltd. (Nashik, India). Meanwhile, copper(II) nitrate trihydrate [Cu(NO3)2·3H2O] was purchased from HmbG (Hamburg, Germany). The chemicals were used without further purification. The glassware was washed with deionized water and dried in an oven before use. Both Gram-positive (S. aureus ATCC BAA-1026 and B. subtilis ATCC 6633) and Gram-negative (E. coli ATCC 25922 and K. pneumoniae ATCC 13883) bacteria were obtained from Faculty of Sciences, Universiti Tunku Abdul Rahman (UTAR), Malaysia.
2.1 Preparation of mangosteen leaf aqueous extract
The process of preparing the aqueous extract from mangosteen leaves was modified from the study of Chan et al. [44]. To get rid of the dust from their surface, the mangosteen leaves were carefully rinsed with tap water. The cleaned leaves were dried for a total of 48 h in an oven and an additional 8 h in a vacuum oven. The dried leaves were then processed in a grinder to form a fine powder. Subsequently, 4.0 g of finely powdered mangosteen leaves was added to 100 mL of deionized water, heated, and stirred at 70–80°C for 20 min to produce 0.04 g·mL−1 of the leaf aqueous extract. The leaf aqueous extract was vacuum-filtered, and a reddish-brown filtrate was obtained for the synthesis of ZnO NPs after cooling to room temperature.
2.2 Synthesis of ZnO NPs
Similarly, the green synthesis of ZnO NPs was adapted from the study of Chan et al. [44]. The synthesis of ZnO NPs was performed by using mangosteen leaf aqueous extract. The reaction parameters, which included the mangosteen leaf aqueous extract concentration and calcination temperature, were optimized.
2.2.1 Leaf aqueous extract optimization
A 50 mL of the prepared mangosteen leaf aqueous extract (0.02, 0.03, and 0.04 g·mL−1) was heated and stirred at 70–80°C. During heating, 4.0 g of Zn(NO3)2·6H2O was added to the leaf aqueous extract, and a light brown solution was formed. Meanwhile, heating and constant stirring were continued at 70–80°C until the formation of a dark brown paste, which was then cooled to room temperature before it was transferred to a ceramic crucible and calcined at 400°C for 2 h in a Muffle furnace. Finally, a fine white ZnO powder was obtained.
2.2.2 Calcination temperature optimization
After the selection of optimized mangosteen leaf aqueous extract concentration at 0.04 g·mL−1, the synthesis of ZnO NPs was repeated by using 4.0 g of Zn(NO3)2·6H2O. The cooled dark-brown paste was calcined at 300°C, 400°C, and 500°C for 2 h in a Muffle furnace.
2.3 Synthesis of CuO NPs and ZnO–CuO NCs
ZnO NPs and CuO-ZnO NCs were synthesized using the above-mentioned method. About 0.05 g·mL−1 of mangosteen leaf extract and 2.0 g of Cu(NO3)2·3H2O were calcined at 500°C for 2 h to obtain a black powder of CuO NPs [44]. Meanwhile, 0.05 g·mL−1 mangosteen leaf extract with 4.0 g of Zn(NO3)2·6H2O and 2.0 g of Cu(NO3)2·3H2O were calcined at 500°C for 2 h to obtain dark-brown ZnO–CuO NC powder [45].
2.4 Characterization
The structural, morphological, and optical properties of the green-synthesized ZnO NPs were characterized by using various analytical tools. The absorption spectra were recorded using a UV−visible (UV-Vis) spectrophotometer (Thermo Scientific GENESYS 10S). The Fourier transform infrared (FT-IR) spectroscopy was performed in the range of 4,000–400 cm−1 with a resolution of 4 cm−1 using KBr pellets in a Perkin Elmer RX1 spectrophotometer. X-ray photoelectron spectroscopy (XPS) was performed to determine the oxidation state by using a Kratos-Shimadzu Axis Ultra DLD model. Also, a micro-Raman spectrophotometer (Thermo Scientific DXR2xi model) was used to measure the Raman spectra. X-ray powder diffraction (XRD) patterns were taken in the reflection mode with Cu Kα (λ = 1.5406 Å) radiation in the 2θ range from 10 to 80° by using a Shimadzu XRD 6000 X-ray diffractometer by continuous scanning, operated at 40 kV/30 mA and 0.02 min−1. The morphological, microstructural, and elemental compositions of the synthesized samples were determined using a field emission-scanning electron microscope (FE-SEM, JEOL JSM-6710F, Japan) with an energy dispersive X-ray analyser (EDX, X-max, 150 Oxford Instruments) and high resolution-transmission electron microscope (HR-TEM, TECNAI G2 20 S-TWIN, FEI) at 200 kV.
2.5 In vitro antibacterial screening
The broth dilution assay was used and adopted by the European Committee for Antimicrobial Susceptibility Testing (EUCAST) of the European Society of Clinical Microbiology and Infectious Diseases (ESCMID) discussion document in 2003 [46]. The bacteria colonies were transferred to sterilized NB and incubated overnight at 35–37°C. The culture was adjusted to 1–2 × 108 CFU·mL−1 with sterilized NB photometrically (absorbance reading in the range of 0.08–0.10 at 600 nm). The bacterial suspension was further diluted in sterilized NB to obtain 1–2 × 105 CFU·mL−1. Two-fold dilutions of green-synthesized ZnO NPs, CuO NPs, and ZnO–CuO NCs suspensions (1,000.00, 500.00, 250.00, 125.00, 62.50, 31.25 and 15.63 μg·mL−1) and antibiotic (ampicillin salt solution, 100.00, 50.00, 25.00, 12.50, 6.25, 3.13 and 1.56 μg·mL−1) in sterilized NB were dispensed in sterilized tubes and inoculated with 1–2 × 105 CFU·mL−1 bacterial suspension. After incubation overnight at 35–37°C, the visible bacterial growth in tubes was examined by turbidity with a minimum inhibitory concentration (MIC). The study was carried out in triplicate.
2.6 Statistical analysis
The MIC values of the synthesized ZnO NPs, CuO NPs, and ZnO–CuO NCs are presented as mean ± standard deviation (SD). Two-way ANOVA and Tukey’s test were performed to compare the MIC values of the different nanomaterials synthesized from the mangosteen leaf aqueous extract and tested with different bacteria, using Microsoft Excel 2013 by setting a p-value < 0.05 as a significant criterion.
3 Results and discussion
3.1 Characterization of ZnO NPs
The physicochemical properties of ZnO NPs are tabulated in Table 1, and the comparison with other studies are summarized in Table 2.
Summary of physicochemical properties of ZnO NPs synthesized from different experimental conditions
| Mangosteen leaf aqueous extract concentration (g·mL−1) | Calcination temperature (oC) | Energy bandgap (eV) | Crystalline size (nm) | Dislocation density (×1014 cm−1) | Micron strain (×10−4) | Morphology |
|---|---|---|---|---|---|---|
| 0.02 | 400 | 3.28 | 27.63 | 13.10 | 1.28 | Irregular nanostructure |
| 0.03 | 400 | 3.27 | 21.50 | 21.64 | 1.63 | Quasi-spherical nanostructure |
| 0.04 | 400 | 3.27 | 16.79 | 35.49 | 2.13 | Spherical nanostructure |
| 0.04 | 300 | 3.37 | 16.71 | 35.80 | 2.13 | Spherical nanostructure |
| 0.04 | 500 | 2.80 | 16.99 | 34.63 | 2.08 | Spherical nanostructure |
Comparison of ZnO NPs synthesized from different plant extracts
| Plant extract | Plant extract concentration (g·mL−1) | Calcination temperature (°C) | Energy bandgap (eV) | Mean particle size (nm) | Morphology | Reference |
|---|---|---|---|---|---|---|
| Crotalaria verrucosa (leaf) | 0.001 | 400 | – | 27.00 | Hexagonal nanostructure | [14] |
| Cassia fistula (leaf) | 0.01 | – | 3.87 | 168.10 | Nearly spherical nanostructure | [47] |
| Melia azedarach (leaf) | 0.01 | – | 3.83 | 13.62 | ||
| Cinnamomum tamala (leaf) | 0.02 | 500 | 3.24 | 35.00–40.00 | Polygonal nanostructure | [48] |
| 2 Lippia adoensis Koseret (leaf) | 0.05 | 400 | 3.21 | 19.78 | Spherical nanostructure | [49] |
| 3 G. mangostana L. (leaf) | 0.04 | 500 | 2.80 | 14.21 | Spherical nanostructure | Current study |
1 By using dynamic light scattering technique.
2 1:1 volume ratio of the plant extract and zinc precursor.
3 The best physicochemical properties with optimum conditions were chosen.
3.1.1 Bond features of organic and inorganic material analysis
FT-IR spectroscopy was used to examine the bond characteristics of the organic and inorganic components in the green-synthesized ZnO NPs, as shown in Figure 1. v(O–H) and v(C═O) correspond to 3,431–3,436 and 1,628–1,634 cm−1, respectively. Moreover, 1,384 and 1,110–1,124 cm−1 bands were assigned to v(C–C aromatic) and v(C–O). A sharp and intense band located at 456–510 cm−1 appeared in green-synthesized ZnO NPs, indicating the presence of v(Zn-O). As shown in Figure 1(a) and (b), the ZnO NPs band’s intensity located at 1,628–1,634 cm−1 increased when a higher concentration of the mangosteen leaf aqueous extract was utilized, whereas it decreased between 1,116 and 1,124 cm−1. On the other hand, the band’s intensity at 1,384 cm−1 declined; meanwhile, the band’s intensity increased at 1,110–1,124 cm−1 intensity at elevated calcination temperature.
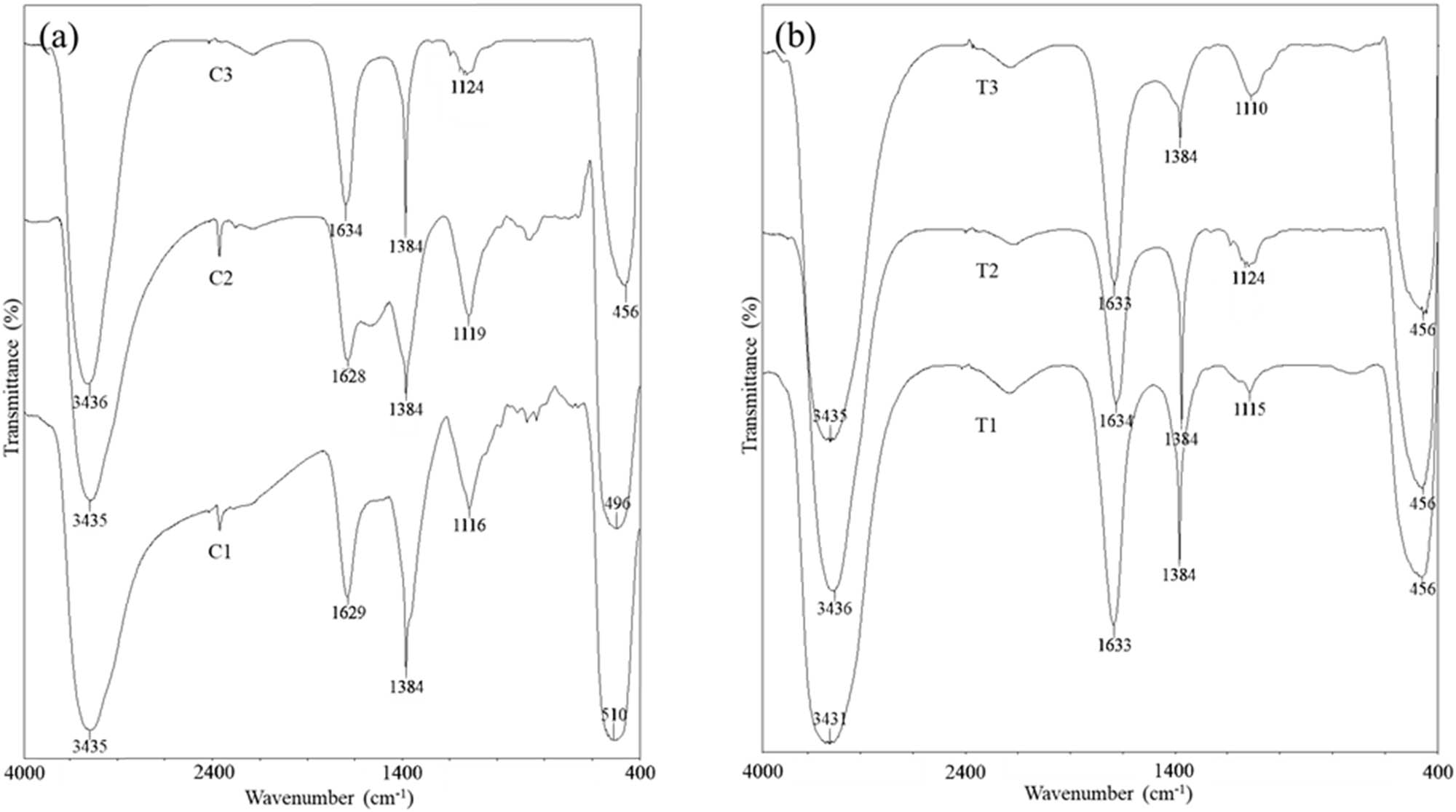
FT-IR spectra of ZnO NPs synthesized at different (a) concentrations of the mangosteen leaf aqueous extract calcined at 400°C and (b) calcination temperatures by using 0.04 g·mL−1 mangosteen leaf aqueous extract. (a) C1, C2, and C3 are 0.02, 0.03, and 0.04 g·mL−1, and (b) T1, T2, and T3 are 300°C, 400°C, and 500°C, respectively.
3.1.2 Optical and energy bandgap analysis
Figure 2 displays the UV-Vis spectra of Zn(NO3)2·6H2O solution, mangosteen leaf aqueous extract, ZnO NPs synthesized using different concentrations of mangosteen leaf aqueous extract and calcination temperatures. The aqueous extract of mangosteen leaves has a wide absorption peak at 479 nm, whereas the Zn(NO3)2·6H2O absorption peak was measured at 305 nm. Meanwhile, the absorbance maxima of the green-synthesized ZnO NPs were detected between 367 and 373 nm, which were shifted to a higher wavelength compared to the Zn(NO3)2·6H2O absorption peak, which was located at 305 nm. The Tauc plot was used to determine the ZnO NPs’ energy bandgap (Figure 3). The energy bandgap of ZnO NPs was calculated (in eV) by plotting (αhv)2 against hv, where h is the Planks constant (6.626 × 10−34 Js) and α is the absorption coefficient. The concentration of the leaf aqueous extract and the calcination temperature both altered the ZnO NPs’ energy bandgap. When a higher concentration of mangosteen leaf aqueous extract was used, the ZnO NPs’ energy bandgap slightly dropped from 3.28 to 3.27 eV. Similarly, the use of a higher calcination temperature resulted in a significant drop in ZnO NPs from 3.37 to 2.80 eV.

UV-Vis spectra of (a) the mangosteen leaf aqueous extract and zinc precursor, ZnO NPs synthesized at different (b) concentrations of the mangosteen leaf aqueous extract calcined at 400°C and (c) calcination temperatures by using 0.04 g·mL−1 mangosteen leaf aqueous extract.

Energy bandgap of the mangosteen leaf aqueous extract-mediated ZnO NPs by using the Tauc plot approach: (a) 0.02, (b) 0.03, and (c) 0.04 g·mL−1 of the mangosteen leaf aqueous extract calcined at 400°C. (d) and (e) show the ZnO NPs’ energy bandgap synthesized at 300°C and 500°C, respectively, by using 0.04 g·mL−1 mangosteen leaf aqueous extract.
3.1.3 Crystallinity analysis
Figure 4 shows the XRD patterns of mangosteen leaf aqueous extract-mediated ZnO NPs using different leaf aqueous extract concentrations and calcination temperatures. All the green-synthesized ZnO NPs were in good agreement with ICDD 01-079-9878, with 2θ values of 31.66, 34.40, 36.20, 47.58, 56.52, 62.84, 67.90, and 69.28o, which were indexed to (1 0 0), (0 0 2), (1 0 1), (1 0 2), (1 1 0), (1 0 3), (1 1 2), and (2 0 1), respectively. More well-defined, higher intensity and narrower diffraction peaks were observed in ZnO NPs by using a concentration of lower mangosteen leaf aqueous extract, which was calcined at higher temperatures. From the XRD spectra, the intensities of the (1 0 0), (0 0 2), and (1 0 1) peaks were stronger than other peaks. The ZnO NPs were in a hexagonal-wurtzite crystal system with lattice parameters a = 3.2490–3.2648 Å and c = 5.1876–5.2194 Å in the P63 mc space group.

XRD spectra of the mangosteen leaf aqueous extract-mediated ZnO NPs by using (a) 0.04, (b) 0.03, and (c) 0.02 g·mL−1 mangosteen leaf aqueous extract, respectively, calcined at 400°C. (d), (e), and (f) show the ZnO NPs calcined at 500°C, 400°C and 300°C, respectively, by using 0.04 g·mL−1 mangosteen leaf aqueous extract.
The crystalline size, dislocation density, and microstrain of ZnO NPs were calculated by using Debye–Scherrer’s formula (Eq. (1)) and Williamson and Smallman’s formula (Eqs (2) and (3)), respectively.
where D is the crystalline size of NPs, λ is the X-ray wavelength, β is the full width half-maximum of the peak, θ is the Bragg angle, δ is the dislocation density of NPs, and ɛ is the microstrain of NPs. Compared to the calcination temperature, the ZnO NPs’ crystallinity was significantly affected by the concentration of the mangosteen leaf aqueous extract. The crystalline size of ZnO NPs was significantly decreased from 27.63 to 16.79 nm with increasing mangosteen leaf aqueous extract concentration. In contrast, the ZnO NPs’ crystalline size slightly increased from 16.71 to 16.99 nm at an elevated calcination temperature. On the other hand, utilizing the concentrated mangosteen leaf aqueous extract and lowering the calcination temperature resulted in an increase in the dislocation density of ZnO NPs from 13.10 × 10−14 to 35.49 × 10−14 cm−1 and 34.63 × 10−14 to 35.80 × 10−14 cm−1, respectively. Similarly, employing a low-concentration of mangosteen leaf aqueous extract (decreased from 2.13 × 10−4 to 1.28 × 10−4) and a higher calcination temperature (decreased from 2.13 × 10−4 to 2.08 × 10−4) led to a lower microstrain in ZnO NPs.
3.1.4 Morphological, particle size, and elemental composition analysis
ZnO NPs showed aggregation under low magnification due to the presence of a weak physical force, while ZnO NPs were well separated and in the nanometre range in high magnification FE-SEM micrographs (48), as shown in Figure 5. ZnO NPs gradually formed a spherical nanostructure at increasing mangosteen leaf aqueous extract concentration, according to the FE-SEM micrographs.

FE-SEM micrographs of the mangosteen leaf aqueous extract-mediated ZnO NPs by using different mangosteen leaf aqueous extracts calcined at 400°C and calcined at different temperatures by using 0.04 g·mL−1 mangosteen leaf aqueous extract. (a) and (b) show low and high magnifications of FE-SEM micrographs, respectively.
The ZnO NPs calcined at 500°C by using 0.04 g·mL−1 mangosteen leaf aqueous extract were analysed by using HR-TEM (Figure 6(a)) and EDX (Figure 6(b)). The mean ZnO NP size was 14.21 nm in accordance with the XRD result. Moreover, the structures of ZnO NPs observed from HR-TEM images were similar to those of ZnO NPs observed from FE-SEM micrographs, as both analyses showed a nano-spherical shape. In the EDX spectrum, a weak signal of carbon was detected at 0.25 keV. In ZnO NPs, 37.92% of zinc was detected, while oxygen was found to make up the majority of atoms (62.08%).

(a) HR-TEM images and particle size distribution of the mangosteen leaf aqueous extract-mediated ZnO NPs. (b) EDX spectrum by using 0.04 g·mL−1 f mangosteen leaf aqueous extract calcined at 500°C.
3.1.5 Vibrational frequency analysis and surface oxidation state
The micro-Raman spectra and the wide range XPS of ZnO NPs synthesized by using 0.04 g·mL−1 mangosteen leaf aqueous extract and calcined at 500°C are shown in Figures 7 and 8, respectively.

Micro-Raman spectrum of the mangosteen leaf aqueous extract-mediated ZnO NPs by using 0.04 g·mL−1 f mangosteen leaf aqueous extract calcined at 500°C.

XPS spectra of the mangosteen leaf aqueous extract-mediated synthesized ZnO NPs of (a) overall high-resolution spectra of Zn 2p and O 1 s, (b) high-resolution spectra of Zn 2p, and (c) high-resolution spectra of O 1 s using 0.04 g·mL−1 mangosteen leaf aqueous extract calcined at 500°C.
The micro-Raman peaks fitted using the Gaussian function of ZnO NPs are shown in Figure 7. The ZnO hexagonal-wurtzite structure is represented by A
1 + 2B
1 + E
1 + 2E
2 as it belongs to
The high-resolution spectra of Zn 2p and O 1 s for ZnO NPs are shown in Figure 8(a). In the high resolution spectra of Zn 2p (Figure 8(b)), two prominent peaks were seen that corresponded to the binding energies of Zn 2p 3/2 and Zn 2p 1/2, respectively. This indicated the presence of Zn2+. Furthermore, the difference (23.1 eV) between the two peaks served as a representation of the Zn 2p spin-orbit splitting energy. On the other hand, the high-resolution O 1s spectra in Figure 8(c) revealed two peaks with centres at 526.45 and 528.25 eV, which were attributed to lattice oxygen from ZnO and hydroxyl group oxygen, respectively. These values matched those in previously published works [48,50,52].
3.2 Preliminary in vitro antibacterial study of ZnO NPs, CuO NPs, and ZnO–CuO NCs
ZnO NPs, CuO NPs, and ZnO–CuO NCs exhibited lower MIC values against Gram-positive bacteria (S. aureus and B. subtilis) than those against Gram-negative bacteria (E. coli and K. pneumoniae). The MIC results, as illustrated in Figure 9, were employed to rank the sensitivity of the tested bacteria to the antibacterial effects, arranged from most to least sensitive as follows. Table 3 tabulates the MIC results of the synthesized nanomaterials, and Table 4 shows the MIC values determined in other studies by using green-synthesized ZnO NPs.
For ZnO NPs:
S. aureus = B. subtilis (15.63 μg·mL−1) > E. coli (62.50 μg·mL−1) > K. pneumoniae (125.00 μg·mL−1)
For CuO NPs:
B. subtilis = E. coli (125.00 μg·mL−1) > S. aureus (250.00 μg·mL−1) > K. pneumoniae (500.00 μg·mL−1)
For ZnO–CuO NCs:

Observation of turbidity changes of in vitro antibacterial activity of (a) ZnO NPs, (b) CuO NPs, and (c) ZnO–CuO NCs by using the broth macrodilution assay. Note that images labelled with “i,” “ii,” “iii,” and “iv” represent using S. aureus, B. subtilis, E. coli, and K. pneumoniae, respectively, for determining antibacterial activities of NPs and NCs. Moreover, the concentrations of NPs and NCs in each tube were as follows: tube 1 = 1000.00 μg·mL−1, tube 2 = 500.00 μg·mL−1, tube 3 = 250.00 μg·mL−1, tube 4 = 125.00 μg·mL−1, tube 5 = 62.50 μg·mL−1, tube 6 = 31.25 μg·mL−1, tube 7 = 15.63 μg·mL−1, and tube 7 = 0.00 μg·mL−1.
MIC values of the synthesized ZnO NPs, CuO NPs, and ZnO–CuO NCs in different tested bacteria
| Nanomaterials | MIC (μg·mL−1) | |||
|---|---|---|---|---|
| S. aureus | B. subtilis | E. coli | K. pneumoniae | |
| ZnO NPs | 15.63 ± 0.00 | 15.63 ± 0.00 | 62.50 ± 0.00* | 125.00 ± 0.00* |
| CuO NPs | 250.00 ± 0.00* | 125.00 ± 0.00* | 125.00 ± 0.00* | 500.00 ± 0.00* |
| ZnO–CuO NCs | 62.50 ± 0.00* | 62.50 ± 0.00* | 250.00 ± 0.00* | 125.00 ± 0.00* |
*Significant at p < 0.05.
MIC values determined by using green-synthesized ZnO NPs in other studies
| Plant extract | Crystalline size (nm) | Bacteria | MIC (μg·mL−1) | Ref. |
|---|---|---|---|---|
| Aegle marmelos (pulp) | 17.00 | Bacillus cereus | 8,650.00 | [53] |
| Micrococcus luteus | 4,680.00–5,080.00 | |||
| S. aureus | 6,650.00–7,090.00 | |||
| K. pneumoniae | 3,840.00–4,340.00 | |||
| Enterobacter aerogenes | 3,990.00–9,090.00 | |||
| E. coli | 6,760.00–8,020.00 | |||
| Pseudomonas fluorescens | 5,190.00–6,090.00 | |||
| Pseudomonas aeruginosa | 6,870.00–7,860.00 | |||
| Salmonella enteritidis | 4,450.00–7,220.00 | |||
| Brassica rapa (leaf) | 41.23 | B. subtilis | 12.50 | [54] |
| E. coli | 25.00 | |||
| G. mangostana L. (leaf) | 16.99 | S. aureus | 15.63 | Current study |
| B. subtilis | 15.63 | |||
| E. coli | 62.50 | |||
| K. pneumoniae | 125.00 |
S. aureus = B. subtilis (62.50 μg·mL−1) > K. pneumoniae (125.00 μg·mL−1) > E. coli (250.00 μg·mL−1)
4 Discussion
4.1 Physicochemical properties of ZnO NPs
The appearance of bands indicated that the phytochemicals, such as xanthones, flavonoids, and terpenes, found in the aqueous extract of mangosteen leaves [36,38,39] were responsible for the reduction, capping, and stabilization of ZnO NPs [10,39,55]. The location of v(Zn–O) shows that the band of metal oxides and hydroxide NPs generally occurred below 1,000 cm−1, resulting in interatomic vibrations [48,56]. The v(Zn–O) from the current study, also reported in other studies, was found in the range of 409–618 cm−1 [56,57,58]. The changes in band intensities of ZnO NPs could be explained by the different interactions between the plant extract functional groups at different controlled parameters. The formation of NPs using natural phytochemicals is still a question. In hypothesis, as a biomolecule model, the xanthone O–H groups would give an electron to electrophile zinc species, causing the hydroxyl group to be reduced to a zinc atom and the electron-deficient zinc ions to be oxidized. In general, the green synthesis of ZnO NPs using the mangosteen leaf extract can occur in three stages: activation, growth, and termination phases. First, the Zn2+ would be released from Zn(NO3)2·6H2O when dissolved in the mangotseen leaf extract during the activation stage. The Zn2+ from the divalent oxidation state would reduce to a metallic form in the presence of functional groups from mangotseen leaf extract. During the calcination process, they would oxidize to ZnO NPs immediately due to enhanced chemical reactivity of the bare nanoscale zinc metal surface. ZnO NPs would accumulate and stabilize throughout the growth and termination phases by mangosteen leaf extract phytochemicals [10,30].
The d → d transition caused the Zn(NO3)2·6H2O absorption peak to appear, whereas the π → π* transition of phytochemicals caused the wide absorption peak of the mangosteen leaf aqueous extract [58]. Furthermore, the colour change from light brown to russet observed with the addition of zinc salt demonstrated that Zn2+ was reduced to Zn0, which was then oxidized into ZnO during the calcination process. The presence of phytochemicals in the suspension [59] might be the reason for the surface plasmon resonance (SPR)-induced colour change of the suspension [60,61,62]. SPR occurred by the transfer of electrons from the valence band to the conduction band (O 2p–Zn 3d) of plant phytochemicals in the aqueous extracts [63], which was initiated by incident electromagnetic radiation [19,23,64] at a particular wavelength [53]. As a result, the absorbance maxima of ZnO NPs were detected, which was consistent with prior experiments utilizing different amounts of plant aqueous extracts [49,65,66] and calcination temperatures [65]. The shift of the ZnO NPs peak to higher wavelengths was due to plant phytochemicals’ non-bonding electron donation to zinc’s unoccupied d-orbital, resulting in simplified electron transitions [34]. The slight drop in the energy bandgap when utilizing a higher plant extract concentration was supported by Demissie et al. [49] and Sato-Robles et al. [66] studies due to the presence of distinct phytochemicals Furthermore, the dominance of the quantum size effect was aided by the increased crystallinity of the ZnO NPs calcined at higher temperatures [18,67,68]. Moreover, the concentration of localized states in the band structure and width increased with an increase in surface dangling bond counts, leading to a smaller bandgap at higher calcination temperatures [69]. The red-shifted UV-Vis absorption peak in ZnO NPs, which increased from 367 to 373 nm, supports the reduction in energy bandgap at high calcination temperatures [70].
From the XRD spectra, the intensities of (1 0 0), (0 0 2), and (1 0 1) peaks were stronger than other peaks, which showed that they were the preferential crystal planes of ZnO NPs, which was similar to that of Yusoff et al. study [71]. The P63
mc space group in ZnO NPs was similar to other studies [48,56,57]. Theoretically, a wurtzite crystal ZnO is hexagonal in shape with a = 3.2960 Å and c = 5.2065 Å as lattice parameters and belongs to the
The isotropic aggregation at the isoelectric point area [72] caused ZnO NPs to form almost spherical structures with rough surfaces [47], which were then tightly bonded to one another with high affinities [16]. The aforementioned findings were consistent with prior research on the synthesis of ZnO NPs utilizing various plant aqueous extracts [16,57,58] and concentrations [66,74]. Thus, the coarsening and coalescence that occurred with the variations in the mangosteen leaf aqueous extract concentration were evident in the FE-SEM micrographs that showed the alteration in ZnO NPs morphology [75,76]. At high calcination temperatures, ZnO NPs were also more aggregated and porous. Due to the high surface attributes (energy, surface area, reactivity, and tension) [19,23,56], strong attraction forces [55,77], oxidation of metal oxide NPs [78], and viscous nature of the plant extract [79], it was frequently seen in green-synthesized NPs. Additionally, the escape of gases at higher temperatures during the production of ZnO NPs may have contributed to the pore development [80]. Similar findings were observed when synthesizing ZnO NPs using the hydrothermal technique [52] and Ocimum gratissimum leaf extract [65].
The weak signal of carbon was due to the burning of organic materials during calcination, and the soot was left over as impurities [21]. This was not a problem since there were only one or two atoms remaining that showed less interaction [81]. Meanwhile, the oxygen signal in the EDX spectra provided proof that the ZnO NPs were in an oxidized form [22].
4.2 Preliminary in vitro antibacterial activity of ZnO NPs, CuO NPs, and ZnO–CuO NCs
The structural differences between Gram-positive and Gram-negative bacteria are responsible for the variation in susceptibility; Gram-positive bacteria have simpler and thinner cell walls, which makes them more vulnerable to antibacterial agents [82]. The results indicated that ZnO NPs exhibited the most potent antibacterial activity among the synthesized nanomaterials, possibly attributed to the presence of porosity in ZnO NPs, which provided a high surface-to-volume ratio for effective interaction with the tested bacteria. Additionally, it was suggested by Govindasamy et al. that Zn2+ ions diffused more readily into the medium compared to Cu2+ ions, which could explain the prolonged duration required for Cu2+ to diffuse out from CuO NPs into the medium to exert antibacterial effects [83].
The antibacterial activity of nanomaterials is intricately influenced by their physicochemical properties, including the generation of reactive oxygen species (ROS), surface area, particle size, solubility, and surface charges [84,85]. These unique properties of metal oxide nanomaterials, such as their small particle size, nanomaterial stability, van der Waals forces, hydrophobic interactions, and electrostatic attraction, contribute to their antibacterial activity through various mechanisms [6]. Although a definitive antibacterial mechanism for metal oxide nanomaterials remains elusive, it is widely believed that the antibacterial effects stem from direct interactions with bacterial cell membranes, ROS generation, and the release of free metal ions from nano-metal oxides [1,2,84]. The antibacterial mechanism is illustrated in Figure 10.

Proposed antibacterial mechanisms that include direct contact with the bacteria cell membrane, generation of ROS, and release of free metal ion from ZnO NPs and CuO NPs. The antibacterial mechanism of ZnO–CuO NCs suggested is the combination of the above-mentioned mechanism in the scheme.
Taking ZnO NPs as an example in the first prepared mechanism, these NPs accumulate in the outer membrane or cytoplasm of bacterial cells, potentially triggering the release of Zn2+ ions that attach to biomolecules in the bacterial cell membrane via electrostatic forces. This leads to depolarization of the bacterial cell membrane, resulting in cellular leakage. Moreover, an excess of ROS is induced by the formation of metal ions, which penetrate the bacterial cell membrane, causing denaturation in proteins and lipids, genomic instability, disruption of mitochondrial function, interference with bacterial cell metabolic activity, and ultimately, apoptosis [24,25,86]. Interestingly, particularly in the case of copper-containing nanomaterials, it has been reported that the contribution of dissolved metal ions to antibacterial activity is relatively minor. In the second prepared mechanism, the presence of water and oxygen can only dissolve a small amount of Cu2+ from CuO NPs. However, more free radicals are promoted, and the NPs are converted into metal ions once they enter the acidic lysosomal environment. This process, often referred to as the “Trojan horse mechanism,” promotes the formation of intracellular ROS, disrupting the bacterial cell mitochondrial membrane potential and degrading DNA, ultimately leading to bacterial cell death [25,87,88].
This multifaceted discussion highlights the diverse mechanisms by which metal oxide nanomaterials exert their antibacterial effects and underscores the complexity of their interactions with bacterial cells.
5 Conclusions
In this investigation, we have adeptly employed a sustainable, eco-friendly approach to fabricate ZnO NPs, CuO NPs, and ZnO–CuO NCs utilizing the mangosteen leaf extract. Through a comprehensive suite of analytical techniques encompassing UV-Vis, FT-IR, XPS, Raman spectroscopy, XRD, FE-SEM with EDX, and HR-TEM, we meticulously characterized these nanostructures, offering profound insights into their physical and structural characteristics. Our antibacterial assessments have revealed the remarkable efficacy of ZnO NPs against both Gram-positive (S. aureus and B. subtilis) and Gram-negative bacteria (E. coli and K. pneumoniae), demonstrating the lowest inhibitory concentrations among the tested nanomaterials. While ZnO–CuO NCs and CuO NPs also displayed antibacterial activity, their MICs were marginally higher. This underscores the potential of ZnO NPs synthesized using the mangosteen leaf aqueous extract as a promising alternative to conventional antibiotics, representing a significant stride in combating antibiotic resistance. In summary, our study not only showcases the successful synthesis of environmentally benign nanomaterials but also underscores their substantial antibacterial efficacy. This advancement holds promise in the development of sustainable and potent antibacterial agents for diverse biomedical applications.
Acknowledgements
The authors extend their appreciation to UTAR for providing research facilities to carry out the research work.
-
Funding information: This research was funded by UTAR through UTARRF [IPSR/RMC/UTARRF/202-C2/M01].
-
Author contributions: Yu Bin Chan: conceptualization, formal analysis, investigation, resources, writing – original draft, and visualization; Mohammod Aminuzzaman: conceptualization, methodology, writing – review and editing, supervision, project administration, and funding acquisition; Md. Khalilur Rahman: conceptualization and funding acquisition; Yip Foo Win: conceptualization, methodology, validation, formal analysis, writing – review & editing; Sabiha Sultana: conceptualization and funding acquisition; Shi-Yan Cheah: conceptualization; Akira Watanabe: conceptualization; Ling Shing Wong: conceptualization, writing – review & editing, and funding acquisition; Samar Kumar Guha: conceptualization and funding acquisition; Sinovassane Djearamane: conceptualization; Venkatachalam Rajendran: conceptualization and funding acquisition; Md. Akhtaruzzaman: conceptualization and methodology; Lai-Hock Tey: conceptualization, methodology, writing – review and editing, and supervision.
-
Conflict of interest: Authors state no conflict of interest.
-
Data availability statement: All data generated or analysed during this study are included in this published article.
References
[1] Amaro F, Morón Á, Díaz S, Martín-González A, Gutiérrez JC. Metallic nanoparticles—friends or foes in the battle against antibiotic-resistant bacteria? Microorganisms. 2021;9(2):364–73. 10.3390/microorganisms9020364.Search in Google Scholar PubMed PubMed Central
[2] Shabatina T, Vernaya O, Shumilkin A, Semenov A, Melnikov M. Nanoparticles of bioactive metals/metal oxides and their nanocomposites with antibacterial drugs for biomedical applications. Materials. 2022;15(10):3602–21. 10.3390/ma15103602.Search in Google Scholar PubMed PubMed Central
[3] Singh A, Gautam PK, Verma A, Singh V, Shivapriya PM, Shivalkar S, et al. Green synthesis of metallic nanoparticles as effective alternatives to treat antibiotics resistant bacterial infections: A review. Biotechnol Rep. 2020;25:e00427–37. 10.1016/j.btre.2020.e00427.Search in Google Scholar PubMed PubMed Central
[4] Hoseinzadeh E, Makhdoumi P, Taha P, Hossini H, Pirsaheb M, Rastegar SO, et al. A review of available techniques for determination of nano-antimicrobials activity. Toxin Rev. 2016;36(1):18–32. 10.1080/15569543.2016.1237527.Search in Google Scholar
[5] Gajic I, Kabic J, Kekic D, Jovicevic M, Milenkovic M, Culafic DM, et al. Antimicrobial susceptibility testing: A comprehensive review of currently used methods. Antibiotics. 2022;11(4):427–52. 10.3390/antibiotics11040427.Search in Google Scholar PubMed PubMed Central
[6] Niño-Martínez N, Orozco MFS, Martínez-Castañón G-A, Méndez FT, Ruiz F. Molecular mechanisms of bacterial resistance to metal and metal oxide nanoparticles. Int J Mol Sci. 2019;20(11):2808–22. 0.3390/ijms20112808.Search in Google Scholar
[7] Wang L, Hu C, Shao L. The antimicrobial activity of nanoparticles: Present situation and prospects for the future. Int J Nanomed. 2017;12:1227–49. 10.2147/IJN.S121956.Search in Google Scholar PubMed PubMed Central
[8] Hitkari G, Chowdhary P, Kumar V, Singh S, Motghare A. Potential of copper-zinc oxide nanocomposite for photocatalytic degradation of congo red dye. Clean Chem Eng. 2022;1:100003–9. 10.1016/j.clce.2022.100003.Search in Google Scholar
[9] Mansoor Al-Saeedi AM, Mohamad FK, Ridha NJ. Synthesis and characterization CuO-ZnO binary nanoparticles. J Nanostructures. 2022;12(3):686–96. 10.22052/JNS.2022.03.021.Search in Google Scholar
[10] Khalafi T, Buazar F, Ghanemi K. Phycosynthesis and enhanced photocatalytic activity of zinc oxide nanoparticles toward organosulfur pollutants. Sci Rep. 2019;9(1):6866–75. 10.1038/s41598-019-43368-3.Search in Google Scholar PubMed PubMed Central
[11] Vaseem M, Umar A Hahn YB. ZnO nanoparticles: Growth, properties, and applications. In: Umar A, Hahn YB, editor. Metal oxide nanostructures and their applications. Valencia, California, United States: American Scientific Publishers; 2010. p. 1–36.Search in Google Scholar
[12] Hamidian K, Sarani M, Sheikhi E, Khatami M. Cytotoxicity evaluation of green synthesized ZnO and Ag-doped ZnO nanoparticles on brain glioblastoma cells. J Mol Struct. 2022;1251:131962–170. 10.1016/j.molstruc.2021.131962.Search in Google Scholar
[13] Aminuzzaman M, Ng PS, Goh W-S, Ogawa S, Watanabe A. Value-adding to dragon fruit (Hylocereus polyrhizus) peel biowaste: Green synthesis of ZnO nanoparticles and their characterization. Inorg Nano-Metal Chem. 2019;49(11):401–11. 10.1080/24701556.2019.1661464.Search in Google Scholar
[14] Sana SS, Kumbhakar DV, Pasha A, Pawar SC, Grace AN, Singh RP, et al. Crotalaria verrucosa leaf extract mediated synthesis of zinc oxide nanoparticles: Assessment of antimicrobial and anticancer activity. Molecules. 2020;25(21):4896–916. 10.3390/molecules25214896.Search in Google Scholar PubMed PubMed Central
[15] Shaba EY, Jacob JO, Tijani JO, Suleiman MAT. A critical review of synthesis parameters affecting the properties of zinc oxide nanoparticle and its application in wastewater treatment. Appl Water Sci. 2021;11(2):48–88. 10.1007/s13201-021-01370-z.Search in Google Scholar
[16] You W, Ahn JC, Boopathi V, Arunkumar L, Rupa EJ, Akter R, et al. Enhanced antiobesity efficacy of tryptophan using the nanoformulation of Dendropanax morbifera extract mediated with ZnO nanoparticle. Materials. 2021;14(4):824–38. 10.3390/ma14040824.Search in Google Scholar PubMed PubMed Central
[17] Kaningini AG, Azizi S, Sintwa N, Mokalane K, Mohale KC, Mudau FN, et al. Effect of optimized precursor concentration, temperature, and doping on optical properties of ZnO nanoparticles synthesized via a green route using bush tea (Athrixia phylicoides DC.) leaf extracts. ACS Omega. 2022;7(36):31658–66. 10.1021/acsomega.2c00530.Search in Google Scholar PubMed PubMed Central
[18] Oudah MH, Hasan MH Abd AN. Synthesis of copper oxide thin films by electrolysis method based on porous silicon for solar cell applications. IOP Conference Series: Materials Science and Engineering. IOP Publishing; 2020.10.1088/1757-899X/757/1/012051Search in Google Scholar
[19] Phang Y-K, Aminuzzaman M, Akhtaruzzaman M, Muhammad G, Ogawa S, Watanabe A, et al. Green synthesis and characterization of CuO nanoparticles derived from papaya peel extract for the photocatalytic degradation of palm oil mill effluent (POME). Sustainability. 2021;13(2):796–810. 10.3390/su13020796.Search in Google Scholar
[20] Amin F, Fozia, Khattak B, Alotaibi A, Qasim M, Ahmad I, et al. Green synthesis of copper oxide nanoparticles using Aerva javanica leaf extract and their characterization and investigation of in vitro antimicrobial potential and cytotoxic activities. Evidence-based Complement Altern Med. 2021;2021:1–12. 10.1155/2021/5589703.Search in Google Scholar PubMed PubMed Central
[21] Siddiqi KS, Husen A. Current status of plant metabolite-based fabrication of copper/copper oxide nanoparticles and their applications: A review. Biomater Res. 2020;24(1):1–15. 10.1186/s40824-020-00188-1.Search in Google Scholar PubMed PubMed Central
[22] Adeyemi JO, Onwudiwe DC, Oyedeji AO. Biogenic synthesis of CuO, ZnO, and CuO–ZnO nanoparticles using leaf extracts of Dovyalis caffra and their biological properties. Molecules. 2022;27(10):3206–220. 10.3390/molecules27103206.Search in Google Scholar PubMed PubMed Central
[23] Sharma S, Yadav DK, Chawla K, Lai N, Lai C. Synthesis and characterization of CuO nanoparticles by Aloe barbadensis leaves. Quantum J Eng Sci Technol. 2021;2(5):1–9.Search in Google Scholar
[24] Waris A, Din M, Ali A, Ali M, Afridi S, Baset A, et al. A comprehensive review of green synthesis of copper oxide nanoparticles and their diverse biomedical applications. Inorg Chem Commun. 2020;123. 10.1016/j.inoche.2020.108369.Search in Google Scholar
[25] Cuong HN, Pansambal S, Ghotekar S, Oza R, Thanh Hai NT, Viet NM, et al. New frontiers in the plant extract mediated biosynthesis of copper oxide (CuO) nanoparticles and their potential applications: A review. Environ Res. 2022;203:111858–81. 10.1016/j.envres.2021.111858.Search in Google Scholar PubMed
[26] Das S, Srivastava VC. Synthesis and characterization of ZnO/CuO nanocomposite by electrochemical method. Mater Sci Semicond Process. 2017;57:173–7. 10.1016/j.mssp.2016.10.031.Search in Google Scholar
[27] Khan SA, Noreen F, Kanwal S, Iqbal A, Hussain G. Green synthesis of ZnO and Cu-doped ZnO nanoparticles from leaf extracts of Abutilon indicum, Clerodendrum infortunatum, Clerodendrum inerma and investigation of their biological and photocatalytic activities. Mater Sci Eng C. 2017;82:46–59. 10.1016/j.msec.2017.08.071.Search in Google Scholar PubMed
[28] Dien ND, Thu Ha PT, Vu XH, Trang TT, Thanh Giang TD, Dung NT. Developing efficient CuO nanoplate/ZnO nanoparticle hybrid photocatalysts for methylene blue degradation under visible light. R Soc Chem Adavnces. 2023;13(35):24505–18. 10.1039/d3ra03791f.Search in Google Scholar PubMed PubMed Central
[29] Mubeen K, Irshad A, Safeen A, Aziz U, Safeen K, Ghani T, et al. Band structure tuning of ZnO/CuO composites for enhanced photocatalytic activity. J Saudi Chem Soc. 2023;27(3):101639–51. 10.1016/j.jscs.2023.101639.Search in Google Scholar
[30] Koopi H, Buazar F. A novel one-pot biosynthesis of pure alpha aluminum oxide nanoparticles using the macroalgae Sargassum ilicifolium: A green marine approach. Ceram Int. 2018;44(8):8940–5. 10.1016/j.ceramint.2018.02.091.Search in Google Scholar
[31] Sepahvand M, Buazar F, Sayahi MH. Novel marine-based gold nanocatalyst in solvent-free synthesis of polyhydroquinoline derivatives: Green and sustainable protocol. Appl Organomet Chem. 2020;34(12):1–11. 10.1002/aoc.6000.Search in Google Scholar
[32] Vijayaraghavan K, Ashokkumar T. Plant-mediated biosynthesis of metallic nanoparticles: A review of literature, factors affecting synthesis, characterization techniques and applications. J Environ Chem Eng. 2017;5(5):4866–83. 10.1016/j.jece.2017.09.026.Search in Google Scholar
[33] Haneefa MM, Jayandran M, Balasubramanian V. Green synthesis characterization and antimicrobial activity evaluation of manganese oxide nanoparticles and comparative studies with salicylalchitosan functionalized nanoform. Asian J Pharm. 2017;11(1):65–74.Search in Google Scholar
[34] Khan SA, Shahid S, Shahid B, Fatima U, Abbasi SA. Green synthesis of MnO nanoparticles using Abutilon indicum leaf extract for biological, photocatalytic, and adsorption activities. Biomolecules. 2020;10(5):785–803. 10.3390/biom10050785.Search in Google Scholar PubMed PubMed Central
[35] Khan A, Shabir D, Ahmad P, Khandaker MU, Faruque MRI, Din IU. Biosynthesis and antibacterial activity of MgO-NPs produced from Camellia-sinensis leaves extract. Mater Res Express. 2021;8(1):015402–11. 10.1088/2053-1591/abd421.Search in Google Scholar
[36] Andani R, Fajrina A, Asra R, Eriadi A. Antibacterial activity test of mangosteen plants (Garcinia mangostana L.): A Review. Asian J Pharm Res Dev. 2021;9(1):164–71. 10.22270/ajprd.v9i1.927.Search in Google Scholar
[37] Abdumutalovna MS, Urmanovna MD. Technology of in-vitro propagation of mangosteen in the climatic conditions of Uzbekistan. Nat Volatiles Essent Oils. 2021;8(4):5610–7.Search in Google Scholar
[38] Diniatik AnggraeniRS. Antibacterial (Staphylococcus aureus and Escherichia coli) and antifungal (Saccharomyces cerevisiae) activity assay on nanoemulsion formulation of ethanol extract of mangosteen leaves (Garcinia mangostana L.) as fruit preservative. J Food Pharm Sci. 2021;9(1):351–65. 10.22146/jfps.1008.Search in Google Scholar
[39] Jassim AMN, Shafy GM, Mohammed MT, Farhan SA, Noori OM. Antioxidant, anti-inflammatory and wound healing of biosynthetic gold nanoparticles using mangosteen (G. mangostona). Iraqi J Ind Res. 2021;8(2):59–74. 10.53523/ijoirVol8I2ID69.Search in Google Scholar
[40] Hiew CW, Lee LJ, Junus S, Tan YN, Chai TT, Ee KY. Optimization of microwave-assisted extraction and the effect of microencapsulation on mangosteen (Garcinia mangostana L.) rind extract. Food Sci Technol. 2021;42:1–10. 10.1590/fst.35521.Search in Google Scholar
[41] Huang X, Zhou X, Dai Q, Qin Z. Antibacterial, antioxidation, UV-blocking, and biodegradable soy protein isolate food packaging film with mangosteen peel extract and ZnO nanoparticles. Nanomaterials. 2021;11(12):3337–52. 10.3390/nano11123337.Search in Google Scholar PubMed PubMed Central
[42] Mohd Basri MS, Ren BLM, Talib RA, Zakaria R, Kamarudin SH. Novel mangosteen-leaves-based marker ink color lightness, viscosity, optimized composition, and microstructural analysis. Polymers. 2021;13(10):1581–98. 10.3390/polym13101581.Search in Google Scholar PubMed PubMed Central
[43] Mulyono D, Irawati Y andSyah MJA. Identification morphological variability of six mangosteen (Garcinia mangostana L.) as a conservation strategy for local varieties. IOP Conference Series: Earth and Environmental Science. IOP Publishing; 2021.10.1088/1755-1315/739/1/012076Search in Google Scholar
[44] Chan YB, Selvanathan V, Tey L-H, Akhtaruzzaman M, Anur FH, Djearamane S, et al. Effect of calcination temperature on structural, morphological and optical properties of copper oxide nanostructures derived from Garcinia mangostana L. leaf extract. Nanometerials. 2022;12(20):3589–607. 10.3390/nano12203589.Search in Google Scholar PubMed PubMed Central
[45] Chan YB, Aminuzzaman M, Tey L-H, Win YF, Watanabe A, Djearamame S, et al. Impact of diverse parameters on the physicochemical characteristics of green-synthesized zinc oxide–copper oxide nanocomposites derived from an aqueous extract of Garcinia mangostan L. leaf. Materials. 2023;16(15):5421–39. 10.3390/ma16155421.Search in Google Scholar PubMed PubMed Central
[46] European Committee for Antimicrobial Susceptibility Testing of the European Society of Clinical Microbiology and Infectious Diseases. Determination of minimum inhibitory concentrations (MICs) of antibacterial agents by broth dilution. Clin Microbiol Infect. 2003;9(8):1–7. 10.1046/j.1469-0691.2003.00790.x.Search in Google Scholar
[47] Naseer M, Aslam U, Khalid B, Chen B. Green route to synthesize zinc oxide nanoparticles using leaf extracts of Cassia fistula and Melia azadarach and their antibacterial potential. Sci Rep. 2020;10(1):9055–64. 10.1038/s41598-020-65949-3.Search in Google Scholar PubMed PubMed Central
[48] Narath S, Koroth SK, Shankar SS, George B, Mutta V, Wacławek S, et al. Cinnamomum tamala leaf extract stabilized zinc oxide nanoparticles: A promising photocatalyst for methylene blue degradation. Nanomaterials. 2021;11(6):1558–74. 10.3390/nano11061558.Search in Google Scholar PubMed PubMed Central
[49] Demissie MG, Sabir FK, Edossa GD, Gonfa BA. Synthesis of zinc oxide nanoparticles using leaf extract of Lippia adoensis (Koseret) and evaluation of its antibacterial activity. J Chem. 2020;2020:1–9. 10.1155/2020/7459042.Search in Google Scholar
[50] Selvanathan V, Aminuzzaman M, Tan LX, Yip FW, Eddy Cheah SG, Heng MH, et al. Synthesis, characterization, and preliminary in vitro antibacterial evaluation of ZnO nanoparticles derived from soursop (Annona muricata L.) leaf extract as a green reducing agent. J Mater Res Technol. 2022;20:2931–41. 10.1016/j.jmrt.2022.08.028.Search in Google Scholar
[51] Siddiqui VU, Ansari A, Ansari MT, Akram MK, Siddiqi WA, Alosaimi AM, et al. Optimization of facile synthesized ZnO/CuO nanophotocatalyst for organic dye degradation by visible light irradiation using response surface methodology. Catalysts. 2021;11(12):1509–33. 10.3390/catal11121509.Search in Google Scholar
[52] Wang Y, Hu K, Yang Z, Ye C, Li X, Yan K. Facile synthesis of porous ZnO nanoparticles efficient for photocatalytic degradation of biomass-derived bisphenol a under simulated sunlight irradiation. Front Bioeng Biotechnol. 2021;8:1–11. 10.3389/fbioe.2020.616780.Search in Google Scholar PubMed PubMed Central
[53] Mallikarjunaswamy C, Lakshmi Ranganatha V, Ramu R, Udayabhanu, Nagaraju G. Facile microwave-assisted green synthesis of ZnO nanoparticles: application to photodegradation, antibacterial and antioxidant. J Mater Sci Mater Electron. 2020;31(2):1004–21. 10.1007/s10854-019-02612-2.Search in Google Scholar
[54] Khan MI, Fatima N, Shakil M, Tahir MB, Riaz KN, Rafique M, et al. Investigation of in-vitro antibacterial and seed germination properties of green synthesized pure and nickel doped ZnO nanoparticles. Phys B Phys Condens Matter. 2021;601. 10.1016/j.physb.2020.412563.Search in Google Scholar
[55] Yusefi M, Shameli K, Yee OS, Teow SY, Hedayatnasab Z, Jahangirian H, et al. Green synthesis of Fe3O4 nanoparticles stabilized by a Garcinia mangostana fruit peel extract for hyperthermia and anticancer activities. Int J Nanomed. 2021;16:2515–32. 10.2147/ijn.s284134.Search in Google Scholar PubMed PubMed Central
[56] Sajjad A, Bhatti SH, Ali Z, Jaffari GH, Khan NA, Rizvi ZF, et al. Photoinduced fabrication of zinc oxide nanoparticles: Transformation of morphological and biological response on light irradiance. ACS Omega. 2021;6(17):11783–93. 10.1021/acsomega.1c01512.Search in Google Scholar PubMed PubMed Central
[57] Abbes N, Bekri I, Cheng M, Sejri N, Cheikrouhou M, Xu J. Green synthesis and characterization of zinc oxide nanoparticles using mulberry fruit and their antioxidant activity. Mater Sci. 2021;28(2):144–50. 10.5755/j02.ms.28314.Search in Google Scholar
[58] Rajendran NK, George BP, Houreld NN, Abrahamse H. Synthesis of zinc oxide nanoparticles using Rubus fairholmianus root extract and their activity against pathogenic bacteria. Molecules. 2021;26(10):3029–39. 10.3390/molecules26103029.Search in Google Scholar PubMed PubMed Central
[59] Verma RP, Khan F. Green approach for biofabrication of CuO nanoparticles from Prunus amygdalus pericarp extract and characterization. Inorg Nano-Metal Chem. 2019;49(3):69–74. 10.1080/24701556.2019.1601738.Search in Google Scholar
[60] Kureshi AA, Vaghela HM, Kumar S, Singh R, Kumari P. Green synthesis of gold nanoparticles mediated by Garcinia fruits and their biological applications. Pharm Sci. 2021;27(2):238–50. 10.34172/PS.2020.90.Search in Google Scholar
[61] Sivakavinesan M, Vanaja M, Annadurai G. Dyeing of cotton fabric materials with biogenic gold nanoparticles. Sci Rep. 2021;11(1):13249–59. 10.1038/s41598-021-92662-6.Search in Google Scholar PubMed PubMed Central
[62] Trang NLN, Hoang VT, Dinh NX, Tam LT, Le VP, Linh DT, et al. Novel eco-friendly synthesis of biosilver nanoparticles as a colorimetric probe for highly selective detection of Fe (III) ions in aqueous solution. J Nanomater. 2021;2021:1–17. 10.1155/2021/5527519.Search in Google Scholar
[63] Senthilkumar N, Nandhakumar E, Priya P, Soni D, Vimalan M, Vetha Potheher I. Synthesis of ZnO nanoparticles using leaf extract of: Tectona grandis (L.) and their antibacterial, anti-arthritic, antioxidant and in-vitro cytotoxicity activities. New J Chem. 2017;41(18):10347–56. 10.1039/C7NJ02664A.Search in Google Scholar
[64] Selvanathan V, Aminuzzaman M, Tey L-H, Razali SA, Althubeiti K, Alkhammash HI, et al. Muntingia calabura leaves mediated green synthesis of CuO nanorods: Exploiting phytochemicals for unique morphology. Materials. 2021;14(21):6379–90. 10.3390/ma14216379.Search in Google Scholar PubMed PubMed Central
[65] Mfon RE, Hall SR, Sarua A. Effect of Ocimum gratissimum plant leaf extract concentration and annealing temperature on the structure and optical properties of synthesized zinc oxide nanoparticles. Educ J Sci Math Technol. 2020;7(1):1–13. 10.37134/ejsmt.vol7.1.1.2020.Search in Google Scholar
[66] Soto-Robles CA, Luque PA, Gómez-Gutiérrez CM, Nava O, Vilchis-Nestor AR, Lugo-Medina E, et al. Study on the effect of the concentration of Hibiscus sabdariffa extract on the green synthesis of ZnO nanoparticles. Results Phys. 2019;15:102807. 10.1016/j.rinp.2019.102807.Search in Google Scholar
[67] Hamid A, Haq S, Ur Rehman S, Akhter K, Rehman W, Waseem M, et al. Calcination temperature-driven antibacterial and antioxidant activities of Fumaria indica mediated copper oxide nanoparticles: characterization. Chem Pap. 2021;75(8):4189–98. 10.1007/s11696-021-01650-7.Search in Google Scholar
[68] Marcorius A, Sulaeman U, Afif M, Nurfiah S, Khanifudin K, Afifah K. The enhanced photocatalytic properties of silver phosphate synthesized under mangosteen peel extract solution. J Teknol. 2022;84(1):21–7. 10.11113/jurnalteknologi.v84.16785. Search in Google Scholar
[69] Bano S, Pillai S. Green synthesis of calcium oxide nanoparticles at different calcination temperatures. World J Sci Technol Sustain Dev. 2020;17(3):283–95.10.1108/WJSTSD-12-2019-0087Search in Google Scholar
[70] Gondal MA, Qahtan TF, Dastageer MA, Maganda YW, Anjum DH. Synthesis of Cu/Cu2O nanoparticles by laser ablation in deionized water and their annealing transformation into CuO nanoparticles. J Nanosci Nanotechnol. 2013;13(8):5759–66. 10.1166/jnn.2013.7465.Search in Google Scholar PubMed
[71] Yusoff HM, Idris NH, Hipul NF, Yusoff NFM, Izham NZM, Bhat IUH. Green synthesis of zinc oxide nanoparticles using black tea extract and its potential as anode material in sodium-ion batteries. Malaysian J Chem. 2020;22(2):43–51.Search in Google Scholar
[72] Baharudin KB, Abdullah N, Derawi D. Effect of calcination temperature on the physicochemical properties of zinc oxide nanoparticles synthesized by coprecipitation. Mater Res Express. 2018;5(12):125018–24. 10.1088/2053-1591/aae243.Search in Google Scholar
[73] Kim MG, Kang JM, Lee JE, Kim KS, Kim KH, Cho M, et al. Effects of calcination temperature on the phase composition, photocatalytic degradation, and virucidal activities of TiO2 nanoparticles. ACS Omega. 2021;6(16):10668–78. 10.1021/acsomega.1c00043.Search in Google Scholar PubMed PubMed Central
[74] Nithya K, Kalyanasundharam S. Effect of chemically synthesis compared to biosynthesized ZnO nanoparticles using aqueous extract of C. halicacabum and their antibacterial activity. OpenNano. 2019;4:100024. 10.1016/j.onano.2018.10.001.Search in Google Scholar
[75] Jameel MS, Aziz AA, Dheyab MA. Green synthesis: Proposed mechanism and factors influencing the synthesis of platinum nanoparticles. Green Process Synth. 2020;9:386–98. 10.1515/gps-2020-0041.Search in Google Scholar
[76] Mazli SRA, Yusoff HM, Idris NH. Synthesis of zinc oxide nanoparticles by using Aloe vera leaf extract as pontential anode material in lithium ion battery. Univ Malaysia Teren J Undergrad Res. 2020;2(2):1–8.10.46754/umtjur.v2i2.108Search in Google Scholar
[77] Aminuzzaman M, Chong C-Y, Goh W-S, Phang Y-K, Tey L-H, Chee S-Y, et al. Biosynthesis of NiO nanoparticles using soursop (Annona muricata L.) fruit peel green waste and their photocatalytic performance on crystal violet dye. J Clust Sci. 2021;32(4):949–58. 10.1007/s10876-020-01859-8.Search in Google Scholar
[78] Ramzan M, Obodo RM, Mukhtar S, Ilyas SZ, Aziz F, Thovhogi N. Green synthesis of copper oxide nanoparticles using Cedrus deodara aqueous extract for antibacterial activity. Mater Today Proc. 2021;36:576–81. 10.1016/j.matpr.2020.05.472.Search in Google Scholar
[79] Siddiqui VU, Ansari A, Chauhan R, Siddiqi WA. Green synthesis of copper oxide (CuO) nanoparticles by Punica granatum peel extract. Mater Today Proc. 2021;36:751–5. 10.1016/j.matpr.2020.05.504.Search in Google Scholar
[80] Basavalingiah KR, Harishkumar S, Udayabhanu, Nagaraju G, Rangappa, Chikkahanumantharayappa D. Highly porous, honeycomb like Ag–ZnO nanomaterials for enhanced photocatalytic and photoluminescence studies: Green synthesis using Azadirachta indica gum. SN Appl Sci. 2019;1(8):935–47. 10.1007/s42452-019-0863-z.Search in Google Scholar
[81] Karthiga P, Rajeshkumar S, Annadurai G. Mechanism of larvicidal activity of antimicrobial silver nanoparticles synthesized using Garcinia mangostana bark extract. J Clust Sci. 2018;29(6):1233–41. 10.1007/s10876-018-1441-z.Search in Google Scholar
[82] Silhavy TJ, Kahne D, Walker S. The Bacterial Cell Envelope. Cold Spring Harb Perspect Biol. 2010;2:a000414–29. 10.1101/cshperspect.a000414.Search in Google Scholar PubMed PubMed Central
[83] Govindasamy GA, Mydin RBSMN, Harun NH, Sreekantan, S. Calcination temperatures, compositions and antimicrobial properties of heterostructural ZnO–CuO nanocomposites from Calotropis gigantea targeted for skin ulcer pathogens. Res Sq. 2020;11:1–14. 10.21203/rs.3.rs-37393/v1.Search in Google Scholar
[84] Masri A, Brown DM, Smith DGE, Stone V, Johnston HJ. Comparison of in vitro approaches to assess the antibacterial effects of nanomaterials. J Funct Biomater. 2022;13(4):255–74. 10.3390/jfb13040255.Search in Google Scholar PubMed PubMed Central
[85] Rajith Kumar CR, Betageri VS, Nagaraju G, Pujar GH, Onkarappa HS, Latha MS. One-pot green synthesis of ZnO–CuO nanocomposite and their enhanced photocatalytic and antibacterial activity. Adv Nat Sci Nanosci Nanotechnol. 2020;11(1):015009–17. 10.1088/2043-6254/ab6c60.Search in Google Scholar
[86] Xu J, Huang Y, Zhu S, Abbes N, Jing X, Zhang L. A review of the green synthesis of ZnO nanoparticles using plant extracts and their prospects for application in antibacterial textiles. J Eng Fiber Fabr. 2021;16:1–14. 10.1177/15589250211046242.Search in Google Scholar
[87] Applerot G, Lellouche J, Lipovsky A, Nitzan Y, Lubart R, Gedanken A, et al. Understanding the antibacterial mechanism of CuO nanoparticles: Revealing the route of induced oxidative stress. Small. 2012;8(21):3326–37. 10.1002/smll.201200772.Search in Google Scholar PubMed
[88] Ma X, Zhou S, Xu X, Du Q. Copper-containing nanoparticles: Mechanism of antimicrobial effect and application in dentistry-a narrative review. Front Surg. 2022;9:1–21. 10.3389/fsurg.2022.905892.Search in Google Scholar PubMed PubMed Central
© 2024 the author(s), published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Research Articles
- Green polymer electrolyte and activated charcoal-based supercapacitor for energy harvesting application: Electrochemical characteristics
- Research on the adsorption of Co2+ ions using halloysite clay and the ability to recover them by electrodeposition method
- Simultaneous estimation of ibuprofen, caffeine, and paracetamol in commercial products using a green reverse-phase HPTLC method
- Isolation, screening and optimization of alkaliphilic cellulolytic fungi for production of cellulase
- Functionalized gold nanoparticles coated with bacterial alginate and their antibacterial and anticancer activities
- Comparative analysis of bio-based amino acid surfactants obtained via Diels–Alder reaction of cyclic anhydrides
- Biosynthesis of silver nanoparticles on yellow phosphorus slag and its application in organic coatings
- Exploring antioxidant potential and phenolic compound extraction from Vitis vinifera L. using ultrasound-assisted extraction
- Manganese and copper-coated nickel oxide nanoparticles synthesized from Carica papaya leaf extract induce antimicrobial activity and breast cancer cell death by triggering mitochondrial caspases and p53
- Insight into heating method and Mozafari method as green processing techniques for the synthesis of micro- and nano-drug carriers
- Silicotungstic acid supported on Bi-based MOF-derived metal oxide for photodegradation of organic dyes
- Synthesis and characterization of capsaicin nanoparticles: An attempt to enhance its bioavailability and pharmacological actions
- Synthesis of Lawsonia inermis-encased silver–copper bimetallic nanoparticles with antioxidant, antibacterial, and cytotoxic activity
- Facile, polyherbal drug-mediated green synthesis of CuO nanoparticles and their potent biological applications
- Zinc oxide-manganese oxide/carboxymethyl cellulose-folic acid-sesamol hybrid nanomaterials: A molecularly targeted strategy for advanced triple-negative breast cancer therapy
- Exploring the antimicrobial potential of biogenically synthesized graphene oxide nanoparticles against targeted bacterial and fungal pathogens
- Biofabrication of silver nanoparticles using Uncaria tomentosa L.: Insight into characterization, antibacterial activities combined with antibiotics, and effect on Triticum aestivum germination
- Membrane distillation of synthetic urine for use in space structural habitat systems
- Investigation on mechanical properties of the green synthesis bamboo fiber/eggshell/coconut shell powder-based hybrid biocomposites under NaOH conditions
- Green synthesis of magnesium oxide nanoparticles using endophytic fungal strain to improve the growth, metabolic activities, yield traits, and phenolic compounds content of Nigella sativa L.
- Estimation of greenhouse gas emissions from rice and annual upland crops in Red River Delta of Vietnam using the denitrification–decomposition model
- Synthesis of humic acid with the obtaining of potassium humate based on coal waste from the Lenger deposit, Kazakhstan
- Ascorbic acid-mediated selenium nanoparticles as potential antihyperuricemic, antioxidant, anticoagulant, and thrombolytic agents
- Green synthesis of silver nanoparticles using Illicium verum extract: Optimization and characterization for biomedical applications
- Antibacterial and dynamical behaviour of silicon nanoparticles influenced sustainable waste flax fibre-reinforced epoxy composite for biomedical application
- Optimising coagulation/flocculation using response surface methodology and application of floc in biofertilisation
- Green synthesis and multifaceted characterization of iron oxide nanoparticles derived from Senna bicapsularis for enhanced in vitro and in vivo biological investigation
- Potent antibacterial nanocomposites from okra mucilage/chitosan/silver nanoparticles for multidrug-resistant Salmonella Typhimurium eradication
- Trachyspermum copticum aqueous seed extract-derived silver nanoparticles: Exploration of their structural characterization and comparative antibacterial performance against gram-positive and gram-negative bacteria
- Microwave-assisted ultrafine silver nanoparticle synthesis using Mitragyna speciosa for antimalarial applications
- Green synthesis and characterisation of spherical structure Ag/Fe2O3/TiO2 nanocomposite using acacia in the presence of neem and tulsi oils
- Green quantitative methods for linagliptin and empagliflozin in dosage forms
- Enhancement efficacy of omeprazole by conjugation with silver nanoparticles as a urease inhibitor
- Residual, sequential extraction, and ecological risk assessment of some metals in ash from municipal solid waste incineration, Vietnam
- Green synthesis of ZnO nanoparticles using the mangosteen (Garcinia mangostana L.) leaf extract: Comparative preliminary in vitro antibacterial study
- Simultaneous determination of lesinurad and febuxostat in commercial fixed-dose combinations using a greener normal-phase HPTLC method
- A greener RP-HPLC method for quaternary estimation of caffeine, paracetamol, levocetirizine, and phenylephrine acquiring AQbD with stability studies
- Optimization of biomass durian peel as a heterogeneous catalyst in biodiesel production using microwave irradiation
- Thermal treatment impact on the evolution of active phases in layered double hydroxide-based ZnCr photocatalysts: Photodegradation and antibacterial performance
- Preparation of silymarin-loaded zein polysaccharide core–shell nanostructures and evaluation of their biological potentials
- Preparation and characterization of composite-modified PA6 fiber for spectral heating and heat storage applications
- Preparation and electrocatalytic oxygen evolution of bimetallic phosphates (NiFe)2P/NF
- Rod-shaped Mo(vi) trichalcogenide–Mo(vi) oxide decorated on poly(1-H pyrrole) as a promising nanocomposite photoelectrode for green hydrogen generation from sewage water with high efficiency
- Green synthesis and studies on citrus medica leaf extract-mediated Au–ZnO nanocomposites: A sustainable approach for efficient photocatalytic degradation of rhodamine B dye in aqueous media
- Cellulosic materials for the removal of ciprofloxacin from aqueous environments
- The analytical assessment of metal contamination in industrial soils of Saudi Arabia using the inductively coupled plasma technology
- The effect of modified oily sludge on the slurry ability and combustion performance of coal water slurry
- Eggshell waste transformation to calcium chloride anhydride as food-grade additive and eggshell membranes as enzyme immobilization carrier
- Synthesis of EPAN and applications in the encapsulation of potassium humate
- Biosynthesis and characterization of silver nanoparticles from Cedrela toona leaf extracts: An exploration into their antibacterial, anticancer, and antioxidant potential
- Enhancing mechanical and rheological properties of HDPE films through annealing for eco-friendly agricultural applications
- Immobilisation of catalase purified from mushroom (Hydnum repandum) onto glutaraldehyde-activated chitosan and characterisation: Its application for the removal of hydrogen peroxide from artificial wastewater
- Sodium titanium oxide/zinc oxide (STO/ZnO) photocomposites for efficient dye degradation applications
- Effect of ex situ, eco-friendly ZnONPs incorporating green synthesised Moringa oleifera leaf extract in enhancing biochemical and molecular aspects of Vicia faba L. under salt stress
- Biosynthesis and characterization of selenium and silver nanoparticles using Trichoderma viride filtrate and their impact on Culex pipiens
- Photocatalytic degradation of organic dyes and biological potentials of biogenic zinc oxide nanoparticles synthesized using the polar extract of Cyperus scariosus R.Br. (Cyperaceae)
- Assessment of antiproliferative activity of green-synthesized nickel oxide nanoparticles against glioblastoma cells using Terminalia chebula
- Chlorine-free synthesis of phosphinic derivatives by change in the P-function
- Anticancer, antioxidant, and antimicrobial activities of nanoemulsions based on water-in-olive oil and loaded on biogenic silver nanoparticles
- Study and mechanism of formation of phosphorus production waste in Kazakhstan
- Synthesis and stabilization of anatase form of biomimetic TiO2 nanoparticles for enhancing anti-tumor potential
- Microwave-supported one-pot reaction for the synthesis of 5-alkyl/arylidene-2-(morpholin/thiomorpholin-4-yl)-1,3-thiazol-4(5H)-one derivatives over MgO solid base
- Screening the phytochemicals in Perilla leaves and phytosynthesis of bioactive silver nanoparticles for potential antioxidant and wound-healing application
- Graphene oxide/chitosan/manganese/folic acid-brucine functionalized nanocomposites show anticancer activity against liver cancer cells
- Nature of serpentinite interactions with low-concentration sulfuric acid solutions
- Multi-objective statistical optimisation utilising response surface methodology to predict engine performance using biofuels from waste plastic oil in CRDi engines
- Microwave-assisted extraction of acetosolv lignin from sugarcane bagasse and electrospinning of lignin/PEO nanofibres for carbon fibre production
- Biosynthesis, characterization, and investigation of cytotoxic activities of selenium nanoparticles utilizing Limosilactobacillus fermentum
- Highly photocatalytic materials based on the decoration of poly(O-chloroaniline) with molybdenum trichalcogenide oxide for green hydrogen generation from Red Sea water
- Highly efficient oil–water separation using superhydrophobic cellulose aerogels derived from corn straw
- Beta-cyclodextrin–Phyllanthus emblica emulsion for zinc oxide nanoparticles: Characteristics and photocatalysis
- Assessment of antimicrobial activity and methyl orange dye removal by Klebsiella pneumoniae-mediated silver nanoparticles
- Influential eradication of resistant Salmonella Typhimurium using bioactive nanocomposites from chitosan and radish seed-synthesized nanoselenium
- Antimicrobial activities and neuroprotective potential for Alzheimer’s disease of pure, Mn, Co, and Al-doped ZnO ultra-small nanoparticles
- Green synthesis of silver nanoparticles from Bauhinia variegata and their biological applications
- Synthesis and optimization of long-chain fatty acids via the oxidation of long-chain fatty alcohols
- Eminent Red Sea water hydrogen generation via a Pb(ii)-iodide/poly(1H-pyrrole) nanocomposite photocathode
- Green synthesis and effective genistein production by fungal β-glucosidase immobilized on Al2O3 nanocrystals synthesized in Cajanus cajan L. (Millsp.) leaf extracts
- Green stability-indicating RP-HPTLC technique for determining croconazole hydrochloride
- Green synthesis of La2O3–LaPO4 nanocomposites using Charybdis natator for DNA binding, cytotoxic, catalytic, and luminescence applications
- Eco-friendly drugs induce cellular changes in colistin-resistant bacteria
- Tangerine fruit peel extract mediated biogenic synthesized silver nanoparticles and their potential antimicrobial, antioxidant, and cytotoxic assessments
- Green synthesis on performance characteristics of a direct injection diesel engine using sandbox seed oil
- A highly sensitive β-AKBA-Ag-based fluorescent “turn off” chemosensor for rapid detection of abamectin in tomatoes
- Green synthesis and physical characterization of zinc oxide nanoparticles (ZnO NPs) derived from the methanol extract of Euphorbia dracunculoides Lam. (Euphorbiaceae) with enhanced biosafe applications
- Detection of morphine and data processing using surface plasmon resonance imaging sensor
- Effects of nanoparticles on the anaerobic digestion properties of sulfamethoxazole-containing chicken manure and analysis of bio-enzymes
- Bromic acid-thiourea synergistic leaching of sulfide gold ore
- Green chemistry approach to synthesize titanium dioxide nanoparticles using Fagonia Cretica extract, novel strategy for developing antimicrobial and antidiabetic therapies
- Green synthesis and effective utilization of biogenic Al2O3-nanocoupled fungal lipase in the resolution of active homochiral 2-octanol and its immobilization via aluminium oxide nanoparticles
- Eco-friendly RP-HPLC approach for simultaneously estimating the promising combination of pentoxifylline and simvastatin in therapeutic potential for breast cancer: Appraisal of greenness, whiteness, and Box–Behnken design
- Use of a humidity adsorbent derived from cockleshell waste in Thai fried fish crackers (Keropok)
- One-pot green synthesis, biological evaluation, and in silico study of pyrazole derivatives obtained from chalcones
- Bio-sorption of methylene blue and production of biofuel by brown alga Cystoseira sp. collected from Neom region, Kingdom of Saudi Arabia
- Synthesis of motexafin gadolinium: A promising radiosensitizer and imaging agent for cancer therapy
- The impact of varying sizes of silver nanoparticles on the induction of cellular damage in Klebsiella pneumoniae involving diverse mechanisms
- Microwave-assisted green synthesis, characterization, and in vitro antibacterial activity of NiO nanoparticles obtained from lemon peel extract
- Rhus microphylla-mediated biosynthesis of copper oxide nanoparticles for enhanced antibacterial and antibiofilm efficacy
- Harnessing trichalcogenide–molybdenum(vi) sulfide and molybdenum(vi) oxide within poly(1-amino-2-mercaptobenzene) frameworks as a photocathode for sustainable green hydrogen production from seawater without sacrificial agents
- Magnetically recyclable Fe3O4@SiO2 supported phosphonium ionic liquids for efficient and sustainable transformation of CO2 into oxazolidinones
- A comparative study of Fagonia arabica fabricated silver sulfide nanoparticles (Ag2S) and silver nanoparticles (AgNPs) with distinct antimicrobial, anticancer, and antioxidant properties
- Visible light photocatalytic degradation and biological activities of Aegle marmelos-mediated cerium oxide nanoparticles
- Physical intrinsic characteristics of spheroidal particles in coal gasification fine slag
- Exploring the effect of tea dust magnetic biochar on agricultural crops grown in polycyclic aromatic hydrocarbon contaminated soil
- Crosslinked chitosan-modified ultrafiltration membranes for efficient surface water treatment and enhanced anti-fouling performances
- Study on adsorption characteristics of biochars and their modified biochars for removal of organic dyes from aqueous solution
- Zein polymer nanocarrier for Ocimum basilicum var. purpurascens extract: Potential biomedical use
- Green synthesis, characterization, and in vitro and in vivo biological screening of iron oxide nanoparticles (Fe3O4) generated with hydroalcoholic extract of aerial parts of Euphorbia milii
- Novel microwave-based green approach for the synthesis of dual-loaded cyclodextrin nanosponges: Characterization, pharmacodynamics, and pharmacokinetics evaluation
- Bi2O3–BiOCl/poly-m-methyl aniline nanocomposite thin film for broad-spectrum light-sensing
- Green synthesis and characterization of CuO/ZnO nanocomposite using Musa acuminata leaf extract for cytotoxic studies on colorectal cancer cells (HCC2998)
- Review Articles
- Materials-based drug delivery approaches: Recent advances and future perspectives
- A review of thermal treatment for bamboo and its composites
- An overview of the role of nanoherbicides in tackling challenges of weed management in wheat: A novel approach
- An updated review on carbon nanomaterials: Types, synthesis, functionalization and applications, degradation and toxicity
- Special Issue: Emerging green nanomaterials for sustainable waste management and biomedical applications
- Green synthesis of silver nanoparticles using mature-pseudostem extracts of Alpinia nigra and their bioactivities
- Special Issue: New insights into nanopythotechnology: current trends and future prospects
- Green synthesis of FeO nanoparticles from coffee and its application for antibacterial, antifungal, and anti-oxidation activity
- Dye degradation activity of biogenically synthesized Cu/Fe/Ag trimetallic nanoparticles
- Special Issue: Composites and green composites
- Recent trends and advancements in the utilization of green composites and polymeric nanocarriers for enhancing food quality and sustainable processing
- Retraction
- Retraction of “Biosynthesis and characterization of silver nanoparticles from Cedrela toona leaf extracts: An exploration into their antibacterial, anticancer, and antioxidant potential”
- Retraction of “Photocatalytic degradation of organic dyes and biological potentials of biogenic zinc oxide nanoparticles synthesized using the polar extract of Cyperus scariosus R.Br. (Cyperaceae)”
- Retraction to “Green synthesis on performance characteristics of a direct injection diesel engine using sandbox seed oil”
Articles in the same Issue
- Research Articles
- Green polymer electrolyte and activated charcoal-based supercapacitor for energy harvesting application: Electrochemical characteristics
- Research on the adsorption of Co2+ ions using halloysite clay and the ability to recover them by electrodeposition method
- Simultaneous estimation of ibuprofen, caffeine, and paracetamol in commercial products using a green reverse-phase HPTLC method
- Isolation, screening and optimization of alkaliphilic cellulolytic fungi for production of cellulase
- Functionalized gold nanoparticles coated with bacterial alginate and their antibacterial and anticancer activities
- Comparative analysis of bio-based amino acid surfactants obtained via Diels–Alder reaction of cyclic anhydrides
- Biosynthesis of silver nanoparticles on yellow phosphorus slag and its application in organic coatings
- Exploring antioxidant potential and phenolic compound extraction from Vitis vinifera L. using ultrasound-assisted extraction
- Manganese and copper-coated nickel oxide nanoparticles synthesized from Carica papaya leaf extract induce antimicrobial activity and breast cancer cell death by triggering mitochondrial caspases and p53
- Insight into heating method and Mozafari method as green processing techniques for the synthesis of micro- and nano-drug carriers
- Silicotungstic acid supported on Bi-based MOF-derived metal oxide for photodegradation of organic dyes
- Synthesis and characterization of capsaicin nanoparticles: An attempt to enhance its bioavailability and pharmacological actions
- Synthesis of Lawsonia inermis-encased silver–copper bimetallic nanoparticles with antioxidant, antibacterial, and cytotoxic activity
- Facile, polyherbal drug-mediated green synthesis of CuO nanoparticles and their potent biological applications
- Zinc oxide-manganese oxide/carboxymethyl cellulose-folic acid-sesamol hybrid nanomaterials: A molecularly targeted strategy for advanced triple-negative breast cancer therapy
- Exploring the antimicrobial potential of biogenically synthesized graphene oxide nanoparticles against targeted bacterial and fungal pathogens
- Biofabrication of silver nanoparticles using Uncaria tomentosa L.: Insight into characterization, antibacterial activities combined with antibiotics, and effect on Triticum aestivum germination
- Membrane distillation of synthetic urine for use in space structural habitat systems
- Investigation on mechanical properties of the green synthesis bamboo fiber/eggshell/coconut shell powder-based hybrid biocomposites under NaOH conditions
- Green synthesis of magnesium oxide nanoparticles using endophytic fungal strain to improve the growth, metabolic activities, yield traits, and phenolic compounds content of Nigella sativa L.
- Estimation of greenhouse gas emissions from rice and annual upland crops in Red River Delta of Vietnam using the denitrification–decomposition model
- Synthesis of humic acid with the obtaining of potassium humate based on coal waste from the Lenger deposit, Kazakhstan
- Ascorbic acid-mediated selenium nanoparticles as potential antihyperuricemic, antioxidant, anticoagulant, and thrombolytic agents
- Green synthesis of silver nanoparticles using Illicium verum extract: Optimization and characterization for biomedical applications
- Antibacterial and dynamical behaviour of silicon nanoparticles influenced sustainable waste flax fibre-reinforced epoxy composite for biomedical application
- Optimising coagulation/flocculation using response surface methodology and application of floc in biofertilisation
- Green synthesis and multifaceted characterization of iron oxide nanoparticles derived from Senna bicapsularis for enhanced in vitro and in vivo biological investigation
- Potent antibacterial nanocomposites from okra mucilage/chitosan/silver nanoparticles for multidrug-resistant Salmonella Typhimurium eradication
- Trachyspermum copticum aqueous seed extract-derived silver nanoparticles: Exploration of their structural characterization and comparative antibacterial performance against gram-positive and gram-negative bacteria
- Microwave-assisted ultrafine silver nanoparticle synthesis using Mitragyna speciosa for antimalarial applications
- Green synthesis and characterisation of spherical structure Ag/Fe2O3/TiO2 nanocomposite using acacia in the presence of neem and tulsi oils
- Green quantitative methods for linagliptin and empagliflozin in dosage forms
- Enhancement efficacy of omeprazole by conjugation with silver nanoparticles as a urease inhibitor
- Residual, sequential extraction, and ecological risk assessment of some metals in ash from municipal solid waste incineration, Vietnam
- Green synthesis of ZnO nanoparticles using the mangosteen (Garcinia mangostana L.) leaf extract: Comparative preliminary in vitro antibacterial study
- Simultaneous determination of lesinurad and febuxostat in commercial fixed-dose combinations using a greener normal-phase HPTLC method
- A greener RP-HPLC method for quaternary estimation of caffeine, paracetamol, levocetirizine, and phenylephrine acquiring AQbD with stability studies
- Optimization of biomass durian peel as a heterogeneous catalyst in biodiesel production using microwave irradiation
- Thermal treatment impact on the evolution of active phases in layered double hydroxide-based ZnCr photocatalysts: Photodegradation and antibacterial performance
- Preparation of silymarin-loaded zein polysaccharide core–shell nanostructures and evaluation of their biological potentials
- Preparation and characterization of composite-modified PA6 fiber for spectral heating and heat storage applications
- Preparation and electrocatalytic oxygen evolution of bimetallic phosphates (NiFe)2P/NF
- Rod-shaped Mo(vi) trichalcogenide–Mo(vi) oxide decorated on poly(1-H pyrrole) as a promising nanocomposite photoelectrode for green hydrogen generation from sewage water with high efficiency
- Green synthesis and studies on citrus medica leaf extract-mediated Au–ZnO nanocomposites: A sustainable approach for efficient photocatalytic degradation of rhodamine B dye in aqueous media
- Cellulosic materials for the removal of ciprofloxacin from aqueous environments
- The analytical assessment of metal contamination in industrial soils of Saudi Arabia using the inductively coupled plasma technology
- The effect of modified oily sludge on the slurry ability and combustion performance of coal water slurry
- Eggshell waste transformation to calcium chloride anhydride as food-grade additive and eggshell membranes as enzyme immobilization carrier
- Synthesis of EPAN and applications in the encapsulation of potassium humate
- Biosynthesis and characterization of silver nanoparticles from Cedrela toona leaf extracts: An exploration into their antibacterial, anticancer, and antioxidant potential
- Enhancing mechanical and rheological properties of HDPE films through annealing for eco-friendly agricultural applications
- Immobilisation of catalase purified from mushroom (Hydnum repandum) onto glutaraldehyde-activated chitosan and characterisation: Its application for the removal of hydrogen peroxide from artificial wastewater
- Sodium titanium oxide/zinc oxide (STO/ZnO) photocomposites for efficient dye degradation applications
- Effect of ex situ, eco-friendly ZnONPs incorporating green synthesised Moringa oleifera leaf extract in enhancing biochemical and molecular aspects of Vicia faba L. under salt stress
- Biosynthesis and characterization of selenium and silver nanoparticles using Trichoderma viride filtrate and their impact on Culex pipiens
- Photocatalytic degradation of organic dyes and biological potentials of biogenic zinc oxide nanoparticles synthesized using the polar extract of Cyperus scariosus R.Br. (Cyperaceae)
- Assessment of antiproliferative activity of green-synthesized nickel oxide nanoparticles against glioblastoma cells using Terminalia chebula
- Chlorine-free synthesis of phosphinic derivatives by change in the P-function
- Anticancer, antioxidant, and antimicrobial activities of nanoemulsions based on water-in-olive oil and loaded on biogenic silver nanoparticles
- Study and mechanism of formation of phosphorus production waste in Kazakhstan
- Synthesis and stabilization of anatase form of biomimetic TiO2 nanoparticles for enhancing anti-tumor potential
- Microwave-supported one-pot reaction for the synthesis of 5-alkyl/arylidene-2-(morpholin/thiomorpholin-4-yl)-1,3-thiazol-4(5H)-one derivatives over MgO solid base
- Screening the phytochemicals in Perilla leaves and phytosynthesis of bioactive silver nanoparticles for potential antioxidant and wound-healing application
- Graphene oxide/chitosan/manganese/folic acid-brucine functionalized nanocomposites show anticancer activity against liver cancer cells
- Nature of serpentinite interactions with low-concentration sulfuric acid solutions
- Multi-objective statistical optimisation utilising response surface methodology to predict engine performance using biofuels from waste plastic oil in CRDi engines
- Microwave-assisted extraction of acetosolv lignin from sugarcane bagasse and electrospinning of lignin/PEO nanofibres for carbon fibre production
- Biosynthesis, characterization, and investigation of cytotoxic activities of selenium nanoparticles utilizing Limosilactobacillus fermentum
- Highly photocatalytic materials based on the decoration of poly(O-chloroaniline) with molybdenum trichalcogenide oxide for green hydrogen generation from Red Sea water
- Highly efficient oil–water separation using superhydrophobic cellulose aerogels derived from corn straw
- Beta-cyclodextrin–Phyllanthus emblica emulsion for zinc oxide nanoparticles: Characteristics and photocatalysis
- Assessment of antimicrobial activity and methyl orange dye removal by Klebsiella pneumoniae-mediated silver nanoparticles
- Influential eradication of resistant Salmonella Typhimurium using bioactive nanocomposites from chitosan and radish seed-synthesized nanoselenium
- Antimicrobial activities and neuroprotective potential for Alzheimer’s disease of pure, Mn, Co, and Al-doped ZnO ultra-small nanoparticles
- Green synthesis of silver nanoparticles from Bauhinia variegata and their biological applications
- Synthesis and optimization of long-chain fatty acids via the oxidation of long-chain fatty alcohols
- Eminent Red Sea water hydrogen generation via a Pb(ii)-iodide/poly(1H-pyrrole) nanocomposite photocathode
- Green synthesis and effective genistein production by fungal β-glucosidase immobilized on Al2O3 nanocrystals synthesized in Cajanus cajan L. (Millsp.) leaf extracts
- Green stability-indicating RP-HPTLC technique for determining croconazole hydrochloride
- Green synthesis of La2O3–LaPO4 nanocomposites using Charybdis natator for DNA binding, cytotoxic, catalytic, and luminescence applications
- Eco-friendly drugs induce cellular changes in colistin-resistant bacteria
- Tangerine fruit peel extract mediated biogenic synthesized silver nanoparticles and their potential antimicrobial, antioxidant, and cytotoxic assessments
- Green synthesis on performance characteristics of a direct injection diesel engine using sandbox seed oil
- A highly sensitive β-AKBA-Ag-based fluorescent “turn off” chemosensor for rapid detection of abamectin in tomatoes
- Green synthesis and physical characterization of zinc oxide nanoparticles (ZnO NPs) derived from the methanol extract of Euphorbia dracunculoides Lam. (Euphorbiaceae) with enhanced biosafe applications
- Detection of morphine and data processing using surface plasmon resonance imaging sensor
- Effects of nanoparticles on the anaerobic digestion properties of sulfamethoxazole-containing chicken manure and analysis of bio-enzymes
- Bromic acid-thiourea synergistic leaching of sulfide gold ore
- Green chemistry approach to synthesize titanium dioxide nanoparticles using Fagonia Cretica extract, novel strategy for developing antimicrobial and antidiabetic therapies
- Green synthesis and effective utilization of biogenic Al2O3-nanocoupled fungal lipase in the resolution of active homochiral 2-octanol and its immobilization via aluminium oxide nanoparticles
- Eco-friendly RP-HPLC approach for simultaneously estimating the promising combination of pentoxifylline and simvastatin in therapeutic potential for breast cancer: Appraisal of greenness, whiteness, and Box–Behnken design
- Use of a humidity adsorbent derived from cockleshell waste in Thai fried fish crackers (Keropok)
- One-pot green synthesis, biological evaluation, and in silico study of pyrazole derivatives obtained from chalcones
- Bio-sorption of methylene blue and production of biofuel by brown alga Cystoseira sp. collected from Neom region, Kingdom of Saudi Arabia
- Synthesis of motexafin gadolinium: A promising radiosensitizer and imaging agent for cancer therapy
- The impact of varying sizes of silver nanoparticles on the induction of cellular damage in Klebsiella pneumoniae involving diverse mechanisms
- Microwave-assisted green synthesis, characterization, and in vitro antibacterial activity of NiO nanoparticles obtained from lemon peel extract
- Rhus microphylla-mediated biosynthesis of copper oxide nanoparticles for enhanced antibacterial and antibiofilm efficacy
- Harnessing trichalcogenide–molybdenum(vi) sulfide and molybdenum(vi) oxide within poly(1-amino-2-mercaptobenzene) frameworks as a photocathode for sustainable green hydrogen production from seawater without sacrificial agents
- Magnetically recyclable Fe3O4@SiO2 supported phosphonium ionic liquids for efficient and sustainable transformation of CO2 into oxazolidinones
- A comparative study of Fagonia arabica fabricated silver sulfide nanoparticles (Ag2S) and silver nanoparticles (AgNPs) with distinct antimicrobial, anticancer, and antioxidant properties
- Visible light photocatalytic degradation and biological activities of Aegle marmelos-mediated cerium oxide nanoparticles
- Physical intrinsic characteristics of spheroidal particles in coal gasification fine slag
- Exploring the effect of tea dust magnetic biochar on agricultural crops grown in polycyclic aromatic hydrocarbon contaminated soil
- Crosslinked chitosan-modified ultrafiltration membranes for efficient surface water treatment and enhanced anti-fouling performances
- Study on adsorption characteristics of biochars and their modified biochars for removal of organic dyes from aqueous solution
- Zein polymer nanocarrier for Ocimum basilicum var. purpurascens extract: Potential biomedical use
- Green synthesis, characterization, and in vitro and in vivo biological screening of iron oxide nanoparticles (Fe3O4) generated with hydroalcoholic extract of aerial parts of Euphorbia milii
- Novel microwave-based green approach for the synthesis of dual-loaded cyclodextrin nanosponges: Characterization, pharmacodynamics, and pharmacokinetics evaluation
- Bi2O3–BiOCl/poly-m-methyl aniline nanocomposite thin film for broad-spectrum light-sensing
- Green synthesis and characterization of CuO/ZnO nanocomposite using Musa acuminata leaf extract for cytotoxic studies on colorectal cancer cells (HCC2998)
- Review Articles
- Materials-based drug delivery approaches: Recent advances and future perspectives
- A review of thermal treatment for bamboo and its composites
- An overview of the role of nanoherbicides in tackling challenges of weed management in wheat: A novel approach
- An updated review on carbon nanomaterials: Types, synthesis, functionalization and applications, degradation and toxicity
- Special Issue: Emerging green nanomaterials for sustainable waste management and biomedical applications
- Green synthesis of silver nanoparticles using mature-pseudostem extracts of Alpinia nigra and their bioactivities
- Special Issue: New insights into nanopythotechnology: current trends and future prospects
- Green synthesis of FeO nanoparticles from coffee and its application for antibacterial, antifungal, and anti-oxidation activity
- Dye degradation activity of biogenically synthesized Cu/Fe/Ag trimetallic nanoparticles
- Special Issue: Composites and green composites
- Recent trends and advancements in the utilization of green composites and polymeric nanocarriers for enhancing food quality and sustainable processing
- Retraction
- Retraction of “Biosynthesis and characterization of silver nanoparticles from Cedrela toona leaf extracts: An exploration into their antibacterial, anticancer, and antioxidant potential”
- Retraction of “Photocatalytic degradation of organic dyes and biological potentials of biogenic zinc oxide nanoparticles synthesized using the polar extract of Cyperus scariosus R.Br. (Cyperaceae)”
- Retraction to “Green synthesis on performance characteristics of a direct injection diesel engine using sandbox seed oil”

