Crosslinked chitosan-modified ultrafiltration membranes for efficient surface water treatment and enhanced anti-fouling performances
-
Qi Qiu
, Xiaojuan Zhao
Graphic abstract
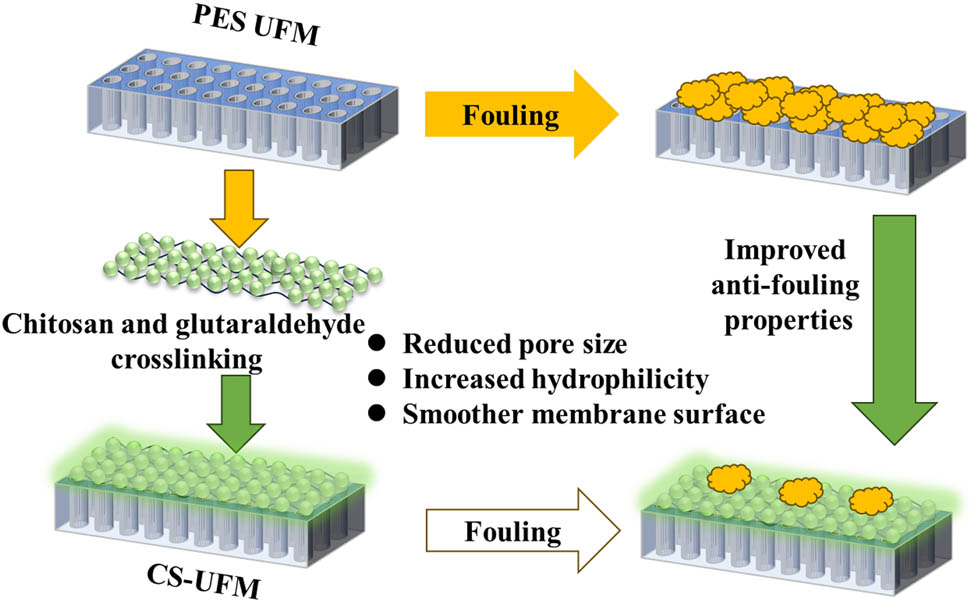
Abstract
To overcome the shortcomings of traditional water treatment technology, ultrafiltration (UF) technology has become a widely used process in the advanced treatment of drinking water, but the existing process has low flux, surface hydrophobicity, easy compaction and other shortcomings. In this study, green chitosan (CS) crosslinking modified UF membrane strategy is adopted to improve its anti-fouling and sustainable reuse in water treatment applications. In addition, modified UF achieved much better separation performances in treating natural surface water. By regulating the crosslinking reaction between CS and glutaraldehyde (GA), modified membranes with different concentrations of CS were prepared, and their structures and chemical properties were characterized. Humic acid was used as the model foulant to study the antifouling behaviors of the CS-coated UF membrane, and the anti-fouling mechanism was analyzed the crosslinking modification strategy of CS-GA provides a new way to alleviate the pollution of UF membrane.
1 Introduction
The trend of drinking water pollution in China is not optimistic, and the potential for pollution of urban drinking water sources and supply systems still exists [1]. At present, most urban water plants in China generally use conventional treatment processes such as, sedimentation, sand filtration, and disinfection to produce drinking water with low turbidity and high safety [2,3,4]. The source water for drinking water treatment plants is mostly taken from natural surface water such as rivers, lakes, and reservoirs [5,6]. The conventional treatment of drinking water is not effective in removing a wide range of contaminants [7,8,9]. On the other hand, as the standard of living rises, public demand for the quality of drinking water is also rising, which obviously increases the difficulty and complexity of drinking water treatment [10,11,12].
The membrane filtration (microfiltration, ultrafiltration [UF], nanofiltration, and reverse osmosis) technology for urban water plants has made rapid development in order to solve the problem of drinking water biosafety and to make up for the shortcomings of the first- and second-generation water treatment processes [13,14,15,16,17]. Reverse osmosis membranes, nanofiltration membranes, and UF membranes with pore sizes smaller than 0.02 µm can retain all microorganisms, algae, and other suspended particles in water, including viruses, bacteria, and protozoa, making them the most effective technology for ensuring drinking water safety [18]. Since nanofiltration and reverse osmosis belong to high-pressure membrane filtration technologies, the treatment processes are relatively complex, and the operational energy consumption and investment costs are relatively high; microfiltration membranes have relatively large pore sizes and cannot remove all pathogenic microorganisms [19,20]. Therefore, UF technology has shown broad application potential in the field of drinking water treatment [21]. UF can not only remove almost all pathogenic microorganisms, suspended particles, and colloidal particles in the water but also partially retain small molecular weight organic pollutants, significantly reducing the use of disinfectants and improving the chemical safety of the water [22,23]. However, the contamination of UF membranes during the water filtration process consistently reduces their lifespan and increases economic burdens. During operation, UF membranes face issues such as membrane fouling and poor removal efficiency of dissolved organic matter [24]. Therefore, to mitigate membrane fouling during UF and effectively enhance UF water purification efficiency, measures are usually taken from three aspects: operating conditions, membrane characteristics, and raw water characteristics [25,26]. Specific measures include optimizing and controlling operating conditions, modifying membrane materials, and pre-treating raw water.
Chitosan (CS) has received widespread attention for its excellent performance in various scientific fields such as food technology, tissue engineering, pharmaceuticals, heavy metal removal, and environmental biotechnology [27,28]. CS is obtained from chitin, which is widely present in the shells of lower animals and plants in nature, such as arthropods (shrimp, crabs), through a deacetylation reaction with alkali and heat [29]. CS can effectively remove harmful substances such as trihalomethanes from tap water, as well as other odorous substances and mutagenic substances [30]. During the adsorption process of harmful substances in drinking water, CS does not remove K+, Ca2+, Na+, Mg2+, SO4 2−, and Cl−. It does not affect the background concentration of natural water and has antibacterial effects. Therefore, CS is an ideal adsorbent for the purification of drinking water [31].
This work intended to utilize the hydrophilic properties of CS material under acidic conditions to modify the surface of UF membranes, constructing a CS-modified catalytic membrane system. Focusing on the “efficiency regulation-influencing factors-mechanism of action” as the main line, the study aimed to address two key scientific issues: the degradation and transformation mechanisms of pollutants in the modified membrane system and the reaction mechanisms in the membrane fouling regulation process. This provided theoretical and technical support for the scientific application of this technology in membrane fouling control and the development of membrane water purification processes.
2 Experimental sections
2.1 Materials and chemicals
Isopropanol (IPA, 99.9% purity), humic acid (HA, 99.9% purity), acetic acid (AA, 99.9% purity), CS ( medium viscosity, 200–400 mPaꞏs), glutaraldehyde (GA, AR, 50% w/w), and hydrochloric acid (37% w/w) were obtained from Kermel (Tianjin, China). All chemicals and reagents were dissolved in ultrapure water produced by a water purification system (IQ7500, Milli-Q). The raw natural surface water was taken from Huashan lake (Jinan, China), and pre-treated using 0.45 μm sterilizing filters (Jinlong, China) to remove the suspended solids. The main water qualities are listed as follows: pH = 7.1–7.4, dissolved organic carbon (DOC) = 2.1–3.2 mg·L−1, UV254 = 0.015–0.025 cm−1, total dissolved solids (TDS) = 1,138–1,280 μS·cm−1, and turbidity = 2.0–2.9 NTU. The polyethersulfone (PES, UP150) membranes applied in this work were provided by Microdyn-Nadir.
2.2 Fabrication of CS-UFM membranes
The fabrication procedure of CS-coated ultrafiltration membranes (CS-UFM) membranes are listed as follows (Figure 1): The PES membrane was first cut into circles of 7.5 cm diameter and immersed in 30% IPA solution for 10 min, with moderate shaking to remove the chemical protective layer on the surface of the commercial membrane and open the membrane pores sufficiently. After the pretreatment of the PES membrane, the support was placed in the middle of two hollow Plexiglas plates with the active layer facing upwards, and 30 mL of CS solution of different concentrations (0–0.4 wt%, 2 wt% AA as catalyst) was added to the reactor in turn for 3 min, and then the excess CS solution was poured off. After 3 min of contact, pour off the excess CS solution, and then pour 30 mL of GA solution with a concentration of 1.0 wt%, the reaction was carried out for 5 min. The modified membranes were immersed in ultrapure water for characterization, and named as CS-UFM0, CS-UFM0.1, CS-UFM0.2, CS-UFM0.3, and CS-UFM0.4, respectively, according to the different CS concentrations.

Schematic illustration of the fabrication process of crosslinked CS-modified UF membranes.
2.3 Characterization of CS-UFM membranes
Scanning electron microscope (SEM, JSM-IT800is, JEOL) and atomic force microscope (AFM, SPM-9700HT, SHIMADZU) were used to obtain the surface morphology and structural composition of the UFM membranes. Fourier infrared (ATR-FTIR, INVENIO, BRUKER) and X-ray photoelectron spectroscopy (XPS, Nexsa, NJ) were applied to characterize the membrane surface, elemental composition, and chemical structure of the membrane surfaces. A contact angle meter (DSA25, KRUSS) was used to characterize the degree of hydrophilicity of the membrane surface. A pH meter (PH700, Apera) and a total organic carbon meter (TOC-L, SHIMADZU) were used to determine the acidity and the DOC, respectively. A three-dimensional fluorescence excitation-emission matrix (EEM) spectrometer (F-7000, HITACHI) was used to measure the content of fluorescent organic matter. Ultraviolet spectrophotometer (UV, UV-1800, MAPADA) was used to measure the UV absorbance value at 254 nm (UV254). A high-performance liquid chromatography (HPLC, Waters e2695, USA) coupled with an ultraviolet absorption (UVA) detector (Waters 2489, USA) was utilized to analyze the molecular weight of the foulants.
2.4 Separation performance assessment of CS-UFM membranes
A laboratory-assembled stirred filtration system was used to test the filtering performance of UF membranes. Before testing, the membrane was subjected to a 60 min operation using deionized water under 0.5 bar pressure. Subsequently, the water permeance (L·m−2·h−1·bar−1) of the UF membrane was measured using ultrapure water at the same temperature and pressure, and was calculated using Eq. 1 [32,33,34,35].
The variables are defined as follows: V and A represent filtration volume (L) and filtration area (m2), and P (bar) and t (h) represent filtration pressure and filtration time, respectively.
The conductivity of salt solutions (Na2SO4 and NaCl, 2,000 ppm) was determined by a conductivity meter. Molecular weight cut-off (MWCO) of the membranes was assessed using a PEG solution (200 ppm), and the concentration of PEG (600–2,000 g·mol−1) was measured by a total organic carbon analyzer (TOC). The rejection rate (R) was calculated using Eq. 2 [36,37,38]:
where C f and C p (μS·cm−1 or mg·L−1) represent the concentrations of feed and permeate, respectively.
The average pore size (nm) of the UF membranes were calculated using Eqs. 4 and 5 [39,40].
2.5 Anti-fouling evaluation of CS-UFM membranes
The anti-fouling properties of membranes were tested with a mixed solution of HA (100 ppm). The membrane was filtered 6 h under 0.5 bar pressure to stabilize the CS-UFM membrane flux. The filtration pressure during the experiment was maintained at 4 bar. First, deionized water was filtered through the membrane for 100 mL, and the water flux was registered as J0. Next the mixture solutions were filtered through the membrane for 100 mL, and the flux was registered as J p . The entire experiment was performed four times. The anti-fouling capability of the CS-UFM membranes was evaluated in terms of flux decay ratio (FDR, %) and flux recovery ratio (FRR, %) using Eqs. 6 and 7.
3 Results and discussion
3.1 Surface morphologies of CS-UFM membranes
SEM and AFM were used to obtain the surface morphology and roughness of the membranes, respectively, and the results are shown in Figure 2a–e, respectively.

Surface morphologies and roughness of crosslinked CS-modified UF membranes: (a) CS-UFM-0; (b) CS-UFM-0.1; (c) CS-UFM-0.2; (d) CS-UFM-0.3; and (e) CS-UFM-0.4.
As shown in Figure 2a, the surface of the CS-UFM0 membrane showed a uniform microporous structure, while during the polymerization of CS and GA, the microporous structure began decreasing with the increasing content of the CS coating and was gradually covered by the CS layer [41]. Furthermore, with the increase in CS coating content to 0.3 wt% and 0.4 wt%, a denser membrane surface structure was observed, indicating the formation of a dense covering layer during the reaction process of high-concentration CS coating with GA. In order to study the change in membrane surface roughness, the 2D and 3D morphology of the membrane surface was measured by AFM.
As shown in Figure 2a–e, the order of surface roughness formed by UF membranes reacting with GA at different CS reaction concentrations was CS-UFM0.4 < CS-UFM0.3 < CS-UFM0.2 < CS-UFM0.1 < CS-UFM0. The surface average roughness of the original CS-UFM0 membrane was highest at Ra 5.7 ± 0.1 nm, and with the increase in CS coating concentration after reaction with GA, a smoother membrane surface structure was formed, leading to a decrease in membrane surface roughness [42]. As shown in Figure 2a–e, compared to the PES membrane, when the CS reaction concentration was 0.1–0.2 wt%, the Ra of the membrane decreased to 4.3 ± 0.1 nm (a decrease of approximately 24.5%), while when the CS reaction concentration was 0.3 and 0.4 wt%, the Ra of the membrane decreased to 3.8 ± 0.1 nm (a decrease of approximately 33.3%). This indicated that the polymerization reaction between CS and GA reduced the surface wrinkled morphology of the membrane, forming a smoother active layer [43]. In addition, the membranes had smoother filtration layers at CS reaction concentrations of 0.3 and 0.4 wt%, which favored the fouling resistance of the UF membranes.
3.2 Surface chemical features of CS-UFM membranes
The FTIR characterization of the prepared UF membranes was carried out in order to characterize the changes in the functional groups on the membrane surface due to the polymerization reaction of CS with GA, and the results are shown in Figure 3a. The broad peak near 3,400–3,200 cm−1 was mainly attributed to the stretching vibration of the N-H bond, indicating that the CS layer was coated on the membrane surface through hydrogen bonding [44]. The characteristic peaks of the CS-UFM membranes appeared at 3,200–3,600, 1,630, and 1,440 cm−1, corresponding to the stretching vibrations of O–H, C═O, and O–H bonds, respectively. XPS was used to evaluate the influence of CS reaction concentration on the surface element composition of UF membranes prepared by CS and GA interface polymerization. As shown in Figure 3b, all UF membranes exhibited three typical characteristic peaks of C, N, and O. Furthermore, the characteristic peak of S element in the UF membranes prepared by interfacial polymerization did not appear in the spectra of the UF membranes, indicating that the interface polymerization reaction between CS and GA did not generate defects [45]. In order to quantitatively analyze the effect of CS reaction concentration on the changes in the content of membrane surface functional groups, the characteristic peaks of the O1s element were deconvoluted into two peaks, corresponding to O–C═O and N–C═O, respectively. As shown in Figure 3c–g, with the increase in CS reaction concentration, the content of O–C═O functional groups in O1s increased from 21.09% to 21.18%, 22.20%, 22.49%, 22.83%, and 24.32%, respectively. The content of N–C═O functional groups in N1s increased from 1.31% to 2.56%, 3.54%, 4.62%, 7.75%, and 17.55%, respectively. This was attributed to the presence of a large number of –OH and –NH3 functional groups in CS molecules, and with the increase in CS reaction concentration, the cross-linking reaction between GA and CS continuously occurred, resulting in the growth of functional groups on the membrane surface. Therefore, increasing the concentration of CS contributed to the formation of dense polyamide membranes with fewer defects, thereby improving the separation performance.
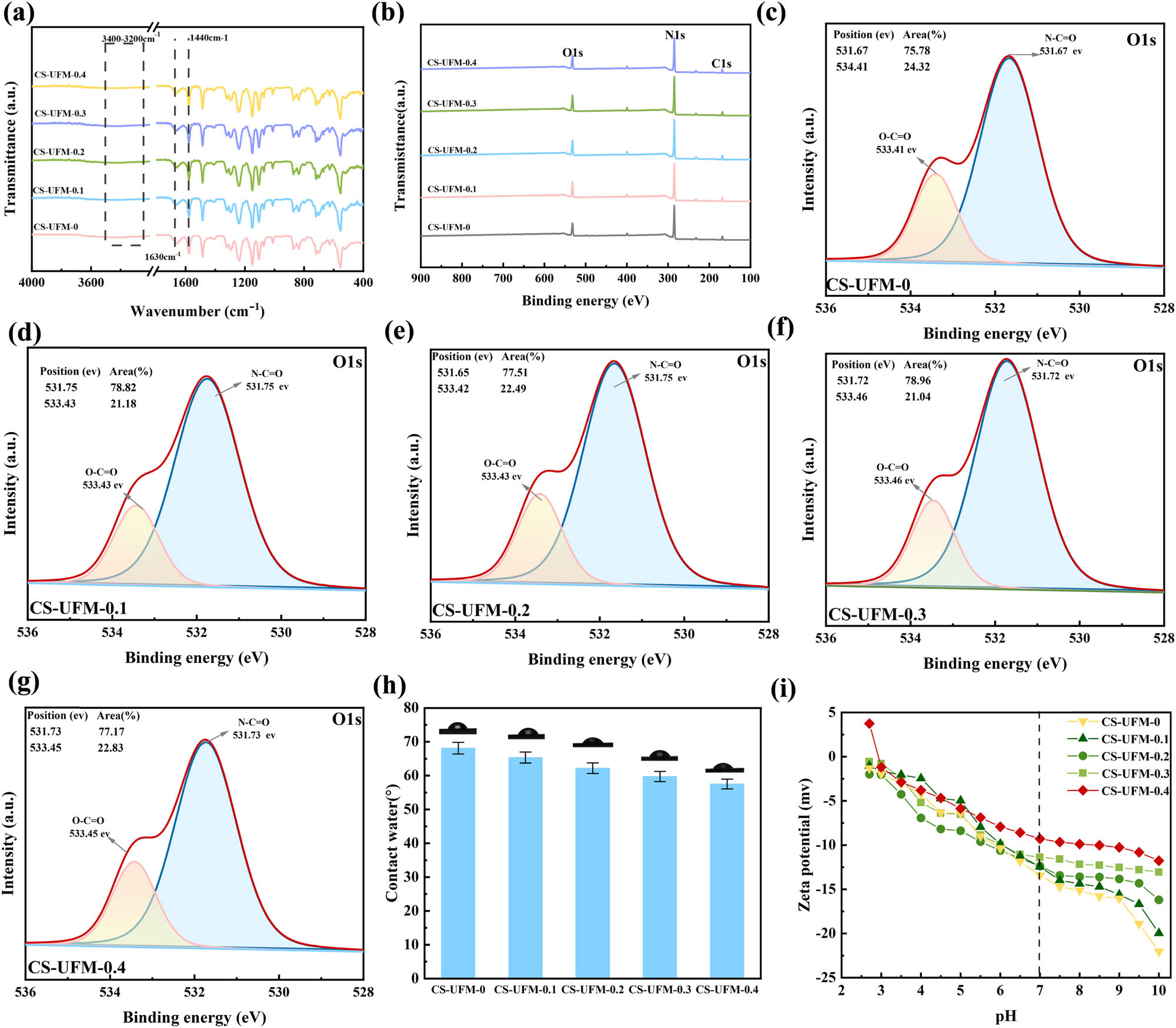
Chemical features of crosslinked CS-modified UF membranes: (a) ATR-FTIR; (b) XPS; high-resolution O 1 s of (c) CS-UFM-0, (d) CS-UFM-0.1, (e) CS-UFM-0.2, (f) CS-UFM-0.3, and (g) CS-UFM-0.4; (h) contact angles; and (i) zeta potentials.
Hydrophilicity could be characterized by measuring the contact angle, and with the continuous increase in CS reaction concentration, compared with the unmodified PES membrane, the contact angles of CS-UFM0, CS-UFM0.1, CS-UFM0.2, CS-UFM0.3, and CS-UFM0.4 membranes after reaction with GA decreased by 0.28°, 3.05°, 6.2°, 8.65°, and 10.86°, respectively (as shown in Figure 3h, indicating that the modified UF membrane had a stronger surface hydrophilicity. As shown in Figure 3i, the zeta potential of the UF membrane under different pH conditions gradually decreased with the increase in CS reaction concentration. Specifically, the zeta potentials of CS-UFM0, CS-UFM0.1, CS-UFM0.2, CS-UFM0.3, and CS-UFM0.4 at pH 7.0 were –13.42, –11.45, –11.37, –11.28, and –9.67 mV, respectively. The results of XPS analysis showed that the number of negatively charged -OH groups increased with the increase in CS reaction concentration, consequently, the negative electronegativity of the membrane surface gradually increased when the CS reaction concentration was increased.
3.3 Separation efficiencies of CS-UFM for natural surface water
As shown in Figure 4a, with the increase in coating, the flux of CS-UFM0 decreased from 1173.59 to 1032.33 L·m⁻²·h⁻¹·bar⁻¹ for the CS-UFM0.4 membrane. The increase in coating thickness led to an increase in transmembrane resistance. The various water quality indicators in surface water are characterized in detail in Figure 4b. It was observed that with the increase in CS addition, the maximum removal rates of DOC, UV254, and water turbidity by the modified UF membrane were 20.95%, 8.99%, and 7.62%, respectively, indicating a slight improvement in the removal efficiency of organic matter by the modified UF membrane. This indicated that the modified UF membrane had a relatively weak ability to remove organic matter, insufficient to achieve precise retention [46,47]. As shown in Figure 4c, the modified UF membrane did not exhibit significant removal effects for small and medium molecular weight organic matter with a molecular weight of less than 2,000 Da, but it had a higher removal effect for high molecular weight organic matter, indicating that the modified UF membrane primarily relied on the pore size exclusion effect to remove large molecular substances. As shown in Figure 4d, with the increase in CS reaction concentration, the average pore size of the modified UF membrane gradually decreased.
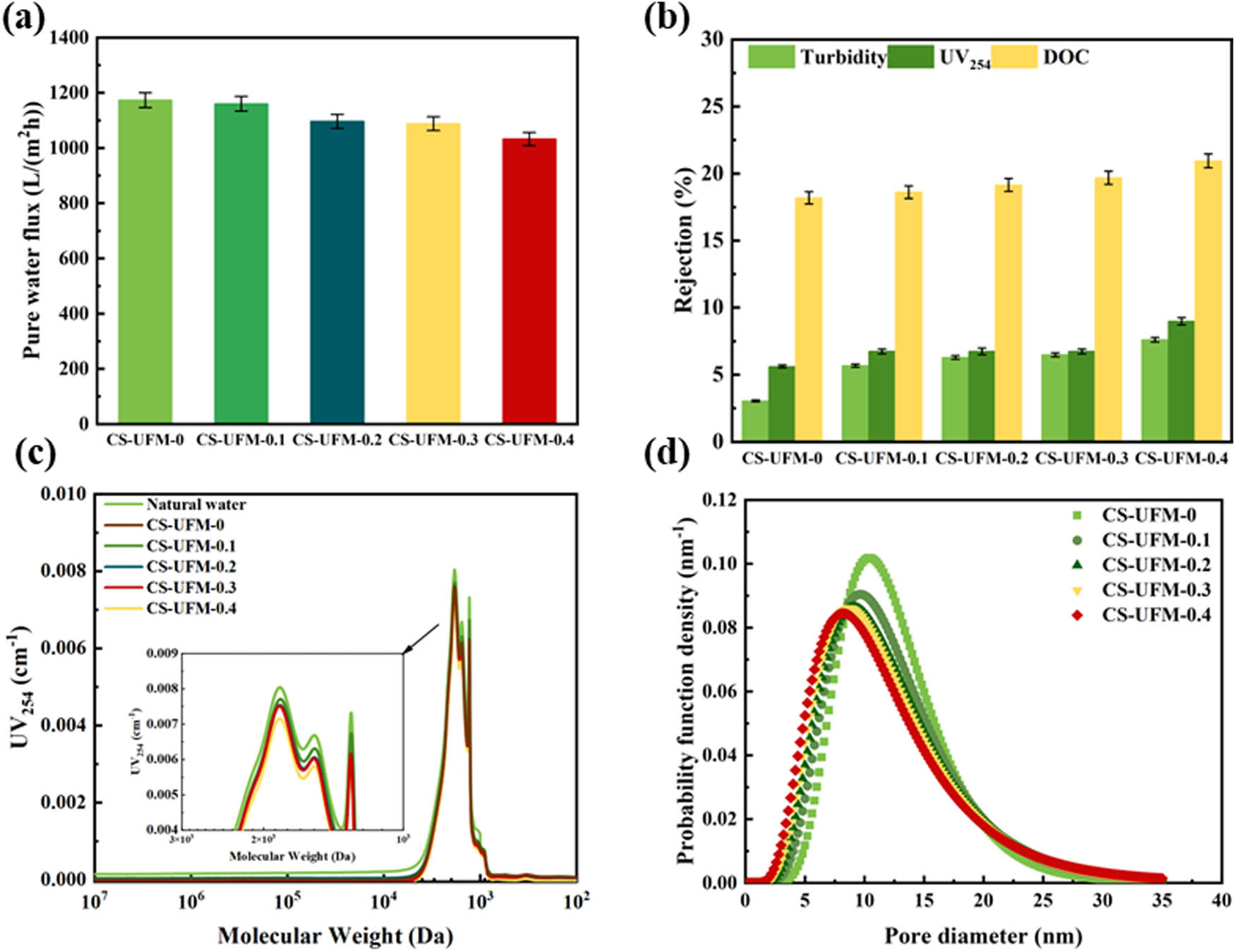
(a) Water flux; (b) average pore sizes, (c) removal efficiencies for turbidity, UV254, and DOC; and (d) molecular weight distribution changes of organic matters for crosslinked CS-modified UF membranes.
The natural surface water was selected as the raw water, and the separation performance of the modified UF membrane in treating actual water was further explored. The removal capacity of fluorescent organic matter by UF membranes characterized by EEM spectra is shown in Figure 5a–f. With the increase in CS reaction concentration, the intensity of peaks T1 and T2 in the raw water filtered by the modified UF membrane decreased significantly (especially when the CS reaction concentration exceeded 0.1 wt%). For the A and C peaks, as the CS addition amount increased, the fluorescence intensity also showed a significant decrease. Especially when the CS addition amount was 0.3 wt% and 0.4 wt% raw water, the C peak response value decreased by about 65.8%. In order to further quantitatively analyze the treatment effect of the modified UF membrane on raw water, the fluorescence region was divided into five typical regions, and the integration method was used to integrate the different regions, and the results are shown in Figure 5g. As shown in Figure 5h, with the increase in reaction concentration, the removal rates of different fluorescent regions all increased. Among them, the removal rates of CS-UFM0, CS-UFM0.1, CS-UFM0.2, CS-UFM0.3, and CS-UFM0.4 for Region I were all above 51.1%; for Region II, they were all above 8.9%; for Region III, they were all above 8.1%; for Regions IV, they were all above 29.3%, and for Region V were all above 13.5%, indicating that the modified UF had good removal effects on organic matter in raw water. In addition, it was found through the regional integration results that when the CS reaction concentration was 0.4 wt%, the removal rate of organic matter was the highest. This was mainly attributed to the increase in CS concentration, which led to a narrower pore size, thereby observing a high removal rate of fluorescent organic components [48]. Figure 5i showed the distribution of organic particle sizes in water after filtration of raw water, and the particle size of organic matter significantly decreased after treatment with the modified UF membrane. Especially when the CS addition amount was 0.4 wt%, the particle size of the effluent decreased to 50 nm.

EEM spectra of (a) raw water; (b) permeate of CS-UFM0; (c) permeate of CS-UFM0.1; (d) permeate of CS-UFM0.2; (e) permeate of CS-UFM0.3; (f) permeate of CS-UFM0.4; (g) percentage of different regions; (h) removal efficiencies of different regions; and (i) particle size distributions.
3.4 Anti-fouling behavers and mechanism insights of CS-UFM membranes
Three modified membranes with typical morphological features, namely, CS-UFM0, CS-UFM0.2, and CS-UFM0.4, were selected for testing in fouling experiments. Fouling tests were conducted over four cycles using HA as a model pollutant to evaluate the anti-fouling performance of the modified UF membranes. As shown in Figure 6a, the flux of all UF membranes gradually decreased, but the comparison of flux decline rates revealed the following sequence of anti-fouling performance: CS-UFM0.4 > CS-UFM0.2 > CS-UFM0. Specifically, at the end of the fourth pollution test cycle, the normalized water flux (J/J 0) of CS-UFM0, CS-UFM0.2, and CS-UFM0.4 membranes was 0.45, 0.32, and 0.15, respectively. Among them, the CS-UFM0.4 membrane exhibited the least flux decline, indicating minimal pollutant deposition and superior anti-fouling performance. The FDR can be calculated based on the J/J 0 values at the end of each pollution cycle. As shown in Figure 6b, for the three types of UF membranes, the FDR values gradually increased with the extension of the pollution cycle, indicating the gradual accumulation of irreversible membrane fouling. Furthermore, partial removal of accumulated pollutants on the membrane surface was achieved when hydraulic cleaning measures were employed, but with the increase in filtration cycles, the cleaning efficiency gradually declined [49]. The FRR values of UF in the three contamination cycle experiments are compared in Figure 6c, and it was found that with the extension of the filtration cycle, the FRR value decreased gradually, presenting a completely opposite trend to that of the FDR value. Specifically, the FRR values decreased from 90% to 69%, from 82% to 59%, and from 66% to 38.5% for CS-UFM0, CS-UFM0.2, and CS-UFM0.4 membranes, respectively. It is worth noting that the CS-UFM0.4 membrane had a lower FDR, which indicated that the CS-UFM0.4 membrane had excellent antifouling performance. The excellent anti-fouling performance of the CS-UFM0.4 membrane was mainly attributed to three aspects: first, the relatively smooth membrane surface was unfavorable for the deposition of pollutants, while irregular surfaces easily captured pollutants during the filtration process; second, under the same initial water flux, the CS-UFM0.4 membrane had relatively smaller pore sizes, making it difficult for pollutants to enter the support layer and facilitate the formation of a relatively loose cake layer, which was conducive to removal via hydraulic cleaning; third, the enhanced electrostatic repulsion between the negatively charged membrane surface and HA reduced the deposition of fouling. The poor anti-pollution performance of the unmodified PES UF membrane is attributed to the smaller hydrophilicity and larger pore size of the CS-UFM0 membrane, which make it easier for pollutants to deposit on the membrane surface and for small organic molecules to continuously enter the membrane pores during the filtration process, ultimately leading to pore blockage and increased irreversible fouling, resulting in inferior anti-pollution performance [50].

Antifouling evaluation of crosslinked CS-modified UF membranes: (a) normalized water flux; (b) FDR; and (c) FRR.
4 Conclusion
In this work, CS-modified UF membranes were fabricated and applied to purify natural surface water. The role of CS in the morphology, structure, and separation performance of modified UF membranes was systematically evaluated. The surface morphology and chemical structure of UF membranes could be significantly altered by coating CS, enabling the targeted tailoring of high-performance UF membranes. The CS-coated UF possessed a smoother active layer and enhanced hydrophilicity, favoring an increase in water flux. During filtering of natural surface water, the CS-coated UF membrane exhibited better organic matter removals than the control membrane. The antifouling tests also confirmed the excellent anti-fouling performance of CS-UFM0.4. Considering the excellent separation efficiency and operational feasibility, the CS coating strategy had the potential to modulate the performance of UF membranes for real-world environmental applications.
Acknowledgements
This research was jointly supported by the Shandong Provincial small and medium-sized enterprise innovation ability improvement project (2023TSGC0313), the National Natural Science Foundation of China (52300089, 52100088), the Introduction and Cultivation Plan for Young Innovative Talents of Colleges and Universities by the Education Department of Shandong Province, and the Shandong Top Talent Special Foundation (No. 0031504).
-
Funding information: Authors state no funding involved.
-
Author contributions: Qi Qiu: writing – original draft, writing – review and editing, and formal analysis; Feng Xu: conceptualization and formal analysis; Weiwei Zhou: writing – review, formal analysis, methodology, and funding acquisition; Xiaojuan Zhao: formal analysis; Yunwei Li: validation; Hongbo Gao: methodology; Shaofang Sun: validation; Hanyun Li: formal analysis; Xuewu Zhu: writing – review and editing; Daoji Wu: conceptualization and supervision.
-
Conflict of interest: Authors state no conflict of interest.
-
Data availability statement: The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
[1] Gao W, Liang H, Ma J, Han M, Chen Z-L, Han Z-S, et al. Membrane fouling control in ultrafiltration technology for drinking water production: A review. Desalination. 2011;272(1):1–8. 10.1016/j.desal.2011.01.051.Search in Google Scholar
[2] Shirakawa D, Shirasaki N, Matsushita T, Matsui Y, Yamashita R, Matsumura T, et al. Evaluation of reduction efficiencies of pepper mild mottle virus and human enteric viruses in full-scale drinking water treatment plants employing coagulation-sedimentation–rapid sand filtration or coagulation–microfiltration. Water Res. 2022;213:118160. 10.1016/j.watres.2022.118160.Search in Google Scholar PubMed
[3] Kurniawan SB, Imron MF, Abdullah SRS, Othman AR, Hasan HA. Coagulation–flocculation of aquaculture effluent using biobased flocculant: From artificial to real wastewater optimization by response surface methodology. J Water Process Eng. 2023;53:103869. 10.1016/j.jwpe.2023.103869.Search in Google Scholar
[4] Mehta I, Hsueh H-Y, Taghipour S, Li W, Saeedi S. UV disinfection robots: A review. Rob Auton Syst. 2023;161:104332. 10.1016/j.robot.2022.104332.Search in Google Scholar PubMed PubMed Central
[5] Zhang Y, Deng J, Qin B, Zhu G, Zhang Y, Jeppesen E, et al. Importance and vulnerability of lakes and reservoirs supporting drinking water in China. Fundam Res. 2023;3(2):265–73. 10.1016/j.fmre.2022.01.035.Search in Google Scholar PubMed PubMed Central
[6] Huang S, Guo J, Xie Y, Bian R, Wang N, Qi W, et al. Distribution, sources, and potential health risks of fluoride, total iodine, and nitrate in rural drinking water sources of North and East China. Sci Total Environ. 2023;898:165561. 10.1016/j.scitotenv.2023.165561.Search in Google Scholar PubMed
[7] Negrete Velasco A, Ramseier Gentile S, Zimmermann S, Le Coustumer P, Stoll S. Contamination and removal efficiency of microplastics and synthetic fibres in a conventional drinking water treatment plant in Geneva, Switzerland. Sci Total Environ. 2023;880:163270. 10.1016/j.scitotenv.2023.163270.Search in Google Scholar PubMed
[8] Manimegalai S, Vickram S, Deena SR, Rohini K, Thanigaivel S, Manikandan S, et al. Carbon-based nanomaterial intervention and efficient removal of various contaminants from effluents – A review. Chemosphere. 2023;312:137319. 10.1016/j.chemosphere.2022.137319.Search in Google Scholar PubMed
[9] Dubey M, Vellanki BP, Kazmi AA. Removal of emerging contaminants in conventional and advanced biological wastewater treatment plants in India-a comparison of treatment technologies. Environ Res. 2023;218:115012. 10.1016/j.envres.2022.115012.Search in Google Scholar PubMed
[10] Chathuranika IM, Sachinthanie E, Zam P, Gunathilake MB, Denkar D, Muttil N, et al. Assessing the water quality and status of water resources in urban and rural areas of Bhutan. J Hazard Mater. 2023;12:100377. 10.1016/j.hazadv.2023.100377.Search in Google Scholar
[11] Reid E, Igou T, Zhao Y, Crittenden J, Huang C-H, Westerhoff P, et al. The minus approach can redefine the standard of practice of drinking water treatment. Environ Sci Technol. 2023;57(18):7150–61. 10.1021/acs.est.2c09389.Search in Google Scholar PubMed PubMed Central
[12] Ren X, Wu Q, Shu J, Chen C, Tiraferri A, Liu B. Efficient removal of organic matters and typical odor substances in rural drinking water using Ozone-BAC-UF combined system to meet new water quality standards in China. Sep Purif Technol. 2023;327:124899. 10.1016/j.seppur.2023.124899. Search in Google Scholar
[13] Xiao T, Zhu Z, Li L, Shi J, Li Z, Zuo X. Membrane fouling and cleaning strategies in microfiltration/ultrafiltration and dynamic membrane. Sep Purif Technol. 2023;318:123977. 10.1016/j.seppur.2023.123977.Search in Google Scholar
[14] Kammakakam I, Lai Z. Next-generation ultrafiltration membranes: A review of material design, properties, recent progress, and challenges. Chemosphere. 2023;316:137669. 10.1016/j.chemosphere.2022.137669.Search in Google Scholar PubMed
[15] An M, Gutierrez L, D’Haese A, Tan L, Verliefde A, Cornelissen E. In-situ modification of nanofiltration and reverse osmosis membranes for organic micropollutants and salts removal: A review. Desalination. 2023;565:116861. 10.1016/j.desal.2023.116861.Search in Google Scholar
[16] Chen Y, Jason Niu Q, Hou Y, Sun H. Effect of interfacial polymerization monomer design on the performance and structure of thin film composite nanofiltration and reverse osmosis membranes: A review. Sep Purif Technol. 2024;330:125282. 10.1016/j.seppur.2023.125282.Search in Google Scholar
[17] Matin A, Jillani SMS, Baig U, Ihsanullah I, Alhooshani K. Removal of pharmaceutically active compounds from water sources using nanofiltration and reverse osmosis membranes: Comparison of removal efficiencies and in-depth analysis of rejection mechanisms. J Environ Manage. 2023;338:117682. 10.1016/j.jenvman.2023.117682.Search in Google Scholar PubMed
[18] Zhu Y, Jiao X, Meng W, Yu X, Cheng H, Shen G, et al. Drinking water in rural china: water sources, treatment, and boiling energy. Environ Sci Technol. 2023;57(16):6465–73. 10.1021/acs.est.2c09344.Search in Google Scholar PubMed
[19] Khan NA, Singh S, López-Maldonado EA, PNPF, Méndez-Herrera JR, López-López U, et al. Emerging membrane technology and hybrid treatment systems for the removal of micropollutants from wastewater. Desalination. 2023;565:116873. 10.1016/j.desal.2023.116873. Search in Google Scholar
[20] Kim K, Baltus RE, Chellam S. Rejection and fouling of track-etched microfiltration membranes by Acholeplasma laidlawii: Clues to mycoplasma behavior during “sterile” dead-end filtration. J Membr Sci. 2023;685:121925. 10.1016/j.memsci.2023.121925.Search in Google Scholar
[21] Qiu Y, Depuydt S, Ren L-F, Zhong C, Wu C, Shao J, et al. Progress of ultrafiltration-based technology in ion removal and recovery: enhanced membranes and integrated processes. ACS ES&T Water. 2023;3(7):1702–19. 10.1021/acsestwater.2c00625.Search in Google Scholar
[22] Liu S, Ding H, Song Y, Xue Y, Bi M, Wu M, et al. The potential risks posed by micro-nanoplastics to the safety of disinfected drinking water. J Hazard Mater. 2023;450:131089. 10.1016/j.jhazmat.2023.131089.Search in Google Scholar PubMed
[23] Shen M, Zhao Y, Liu S, Tao S, Li T, Long H. Can microplastics and disinfectant resistance genes pose conceivable threats to water disinfection process? Sci Total Environ. 2023;905:167192. 10.1016/j.scitotenv.2023.167192.Search in Google Scholar PubMed
[24] Li Y, Wang Y, Jin J, Tian Z, Yang W, Graham NJD, et al. Enhanced removal of trace pesticides and alleviation of membrane fouling using hydrophobic-modified inorganic-organic hybrid flocculants in the flocculation-sedimentation-ultrafiltration process for surface water treatment. Water Res. 2023;229:119447. 10.1016/j.watres.2022.119447.Search in Google Scholar PubMed
[25] Sedighi M, Behvand Usefi MM, Ismail AF, Ghasemi M. Environmental sustainability and ions removal through electrodialysis desalination: Operating conditions and process parameters. Desalination. 2023;549:116319. 10.1016/j.desal.2022.116319.Search in Google Scholar
[26] Mehrazi S, Sarker M, Chuang P-YA. Effect of high aspect ratio additives on microstructural and mass transport properties of the microporous layer in a proton exchange membrane fuel cell. J Power Sources. 2023;580:233361. 10.1016/j.jpowsour.2023.233361.Search in Google Scholar
[27] Khalaf EM, Abood NA, Atta RZ, Ramírez-Coronel AA, Alazragi R, Parra RMR, et al. Recent progressions in biomedical and pharmaceutical applications of chitosan nanoparticles: A comprehensive review. Int J Biol Macromol. 2023;231:123354. 10.1016/j.ijbiomac.2023.123354.Search in Google Scholar PubMed
[28] Almajidi YQ, Ponnusankar S, Chaitanya MVNL, Marisetti AL, Hsu C-Y, Dhiaa AM, et al. Chitosan-based nanofibrous scaffolds for biomedical and pharmaceutical applications: A comprehensive review. Int J Biol Macromol. 2024;264:130683. 10.1016/j.ijbiomac.2024.130683.Search in Google Scholar PubMed
[29] Yadav M, Kaushik B, Rao GK, Srivastava CM, Vaya D. Advances and challenges in the use of chitosan and its derivatives in biomedical fields: A review. Carbohydr Polym. 2023;5:100323. 10.1016/j.carpta.2023.100323.Search in Google Scholar
[30] Kazemi Shariat Panahi H, Dehhaghi M, Amiri H, Guillemin GJ, Gupta VK, Rajaei A, et al. Current and emerging applications of saccharide-modified chitosan: a critical review. Biotechnol Adv. 2023;66:108172. 10.1016/j.biotechadv.2023.108172.Search in Google Scholar PubMed
[31] Qi Y, Li D, Zhang S, Li F, Hua T. Electrochemical filtration for drinking water purification: A review on membrane materials, mechanisms and roles. J Environ Sci (China). 2024;141:102–28. 10.1016/j.jes.2023.06.033.Search in Google Scholar PubMed
[32] Zhu X, Lai C, Liu B, Liu J, Xu D, Lu X, et al. Unveiling the role of post-treatment in thin-film composite nanofiltration membranes: Performance and mechanism. Desalination. 2023;556:116579. 10.1016/j.desal.2023.116579.Search in Google Scholar
[33] Zhu X, Sun Z, Xu J, Xu S, Luo X, Tan F, et al. Poly(vinyl alcohol)-based polyester nanofiltration membranes for fractionation of dye/salt mixtures: Alcoholysis degree matters. Sep Purif Technol. 2024;328:125076. 10.1016/j.seppur.2023.125076.Search in Google Scholar
[34] Sun Z, Zhu X, Tan F, Zhou W, Zhang Y, Luo X, et al. Poly(vinyl alcohol)-based highly permeable TFC nanofiltration membranes for selective dye/salt separation. Desalination. 2023;553:116479. 10.1016/j.desal.2023.116479.Search in Google Scholar
[35] Li J, Zhu X, Lai C, Chen F, Bai L, Cheng X, et al. Triethanolamine-modulated interfacial polymerization toward microcrumpled nanofiltration membranes: Performances and mechanisms. Desalination. 2023;545:116165. 10.1016/j.desal.2022.116165.Search in Google Scholar
[36] Zhu X, Sun Z, Tan F, Chen F, Luo X, Wang F, et al. Tailoring high-performance polyester loose nanofiltration membrane for selective separation of salt/dyes: The equilibrium of condensation and hydrolysis. Sep Purif Technol. 2024;333:125848. 10.1016/j.seppur.2023.125848.Search in Google Scholar
[37] Zhu X, Sun Z, Tan F, Zhu J, Chen F, Xu S, et al. Xylitol-based polyester loose nanofiltration membranes with outstanding water permeance and efficient dye desalination performance. Sep Purif Technol. 2024;334:126048. 10.1016/j.seppur.2023.126048.Search in Google Scholar
[38] Liu J, Han X, Zhu X, Li J, Zhong D, Wei L, et al. A systemic evaluation of aerobic granular sludge among granulation, operation, storage, and reactivation processes in an SBR. Environ Res. 2023;235:116594. 10.1016/j.envres.2023.116594.Search in Google Scholar PubMed
[39] Zhao C, Liu B, Zhu T, Zhu X, Cheng X. Mechanistic insight into single-atom Fe loaded catalytic membrane with peracetic acid and visible light activation. J Hazard Mater. 2023;460:132506. 10.1016/j.jhazmat.2023.132506.Search in Google Scholar PubMed
[40] Yang L, Xu D, Luo X, Zhu X, Zhao J, Song J, et al. Fe(II)-modulated microporous electrocatalytic membranes for organic microcontaminant oxidation and fouling control: mechanisms of regulating electron transport toward enhanced reactive oxygen species activation. Environ Sci Technol. 2023;57(47):19000–11. 10.1021/acs.est.3c01792.Search in Google Scholar PubMed
[41] Rawindran H, Arif bin Hut N, Vrasna DK, Goh PS, Lim JW, Liew CS, et al. Ultrafiltration membrane fabricated from polyethylene terephthalate plastic waste for treating microalgal wastewater and reusing for microalgal cultivation. Chemosphere. 2024;346:140591. 10.1016/j.chemosphere.2023.140591. Search in Google Scholar
[42] Li P, Xie H, Bi Y, Miao C, Chen K, Xie T, et al. Preparation of high flux organic solvent nanofiltration membrane based on polyimide/Noria composite ultrafiltration membrane. Appl Surf Sci. 2023;618:156650. 10.1016/j.apsusc.2023.156650.Search in Google Scholar
[43] Amiri S, Vatanpour V, He T. Antifouling thin-film nanocomposite NF membrane with polyvinyl alcohol-sodium alginate-graphene oxide nanocomposite hydrogel coated layer for As(III) removal. Chemosphere. 2023;322:138159. 10.1016/j.chemosphere.2023.138159.Search in Google Scholar PubMed
[44] Ma L-L, Ye H, Liu L, Wu M-B, Yao J. Polypropylene membranes with high adsorption capacity and anti-adhesion properties achieved by hydrophobic interactions and hydrogen bonded self-assembly for uranium extraction from seawater. Chem Eng J. 2023;451:138696. 10.1016/j.cej.2022.138696.Search in Google Scholar
[45] Zhu Z, Zhou X, Zhang R, Li Y, Liu Y, Zeng J, et al. Antifouling modification of PVDF membranes via incorporating positive-charge tuned quaternized chitosan magnetic particles. J Environ Chem Eng. 2023;11(1):109192. 10.1016/j.jece.2022.109192.Search in Google Scholar
[46] Wang Y, Guo Y, Yang C, Meng H, Li S, Sarp S, et al. Bio-inspired fabrication of adsorptive ultrafiltration membrane for water purification: Simultaneous removal of natural organic matters, lead ion and organic dyes. J Environ Chem Eng. 2023;11(3):109798. 10.1016/j.jece.2023.109798.Search in Google Scholar
[47] Ma B, Ke Q, Ulbricht M. Simultaneous removal of natural organic matters and copper (II) with ultrafiltration for drinking water treatment. J Membr Sci. 2023;671:121408. 10.1016/j.memsci.2023.121408.Search in Google Scholar
[48] Shoshaa R, Ashfaq MY, Al-Ghouti MA. Recent developments in ultrafiltration membrane technology for the removal of potentially toxic elements, and enhanced antifouling performance: A review. Environ Sci Technol. 2023;31:103162. 10.1016/j.eti.2023.103162.Search in Google Scholar
[49] Qiao Z, Guo Y, Wang Z, Hu G. A chemically enhanced backwash model for predicting the instantaneous transmembrane pressure of flat sheet membranes in constant flow rate mode. J Membr Sci. 2023;666:121137. 10.1016/j.memsci.2022.121137.Search in Google Scholar
[50] Yang Z, Lin S, Ye L, Qu D, Yang H, Chang H, et al. Landfill leachate treatment by direct contact membrane distillation: Impacts of landfill age on contaminant removal performance, membrane fouling and scaling. Desalination. 2024;577:117407. 10.1016/j.desal.2024.117407.Search in Google Scholar
© 2024 the author(s), published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Research Articles
- Green polymer electrolyte and activated charcoal-based supercapacitor for energy harvesting application: Electrochemical characteristics
- Research on the adsorption of Co2+ ions using halloysite clay and the ability to recover them by electrodeposition method
- Simultaneous estimation of ibuprofen, caffeine, and paracetamol in commercial products using a green reverse-phase HPTLC method
- Isolation, screening and optimization of alkaliphilic cellulolytic fungi for production of cellulase
- Functionalized gold nanoparticles coated with bacterial alginate and their antibacterial and anticancer activities
- Comparative analysis of bio-based amino acid surfactants obtained via Diels–Alder reaction of cyclic anhydrides
- Biosynthesis of silver nanoparticles on yellow phosphorus slag and its application in organic coatings
- Exploring antioxidant potential and phenolic compound extraction from Vitis vinifera L. using ultrasound-assisted extraction
- Manganese and copper-coated nickel oxide nanoparticles synthesized from Carica papaya leaf extract induce antimicrobial activity and breast cancer cell death by triggering mitochondrial caspases and p53
- Insight into heating method and Mozafari method as green processing techniques for the synthesis of micro- and nano-drug carriers
- Silicotungstic acid supported on Bi-based MOF-derived metal oxide for photodegradation of organic dyes
- Synthesis and characterization of capsaicin nanoparticles: An attempt to enhance its bioavailability and pharmacological actions
- Synthesis of Lawsonia inermis-encased silver–copper bimetallic nanoparticles with antioxidant, antibacterial, and cytotoxic activity
- Facile, polyherbal drug-mediated green synthesis of CuO nanoparticles and their potent biological applications
- Zinc oxide-manganese oxide/carboxymethyl cellulose-folic acid-sesamol hybrid nanomaterials: A molecularly targeted strategy for advanced triple-negative breast cancer therapy
- Exploring the antimicrobial potential of biogenically synthesized graphene oxide nanoparticles against targeted bacterial and fungal pathogens
- Biofabrication of silver nanoparticles using Uncaria tomentosa L.: Insight into characterization, antibacterial activities combined with antibiotics, and effect on Triticum aestivum germination
- Membrane distillation of synthetic urine for use in space structural habitat systems
- Investigation on mechanical properties of the green synthesis bamboo fiber/eggshell/coconut shell powder-based hybrid biocomposites under NaOH conditions
- Green synthesis of magnesium oxide nanoparticles using endophytic fungal strain to improve the growth, metabolic activities, yield traits, and phenolic compounds content of Nigella sativa L.
- Estimation of greenhouse gas emissions from rice and annual upland crops in Red River Delta of Vietnam using the denitrification–decomposition model
- Synthesis of humic acid with the obtaining of potassium humate based on coal waste from the Lenger deposit, Kazakhstan
- Ascorbic acid-mediated selenium nanoparticles as potential antihyperuricemic, antioxidant, anticoagulant, and thrombolytic agents
- Green synthesis of silver nanoparticles using Illicium verum extract: Optimization and characterization for biomedical applications
- Antibacterial and dynamical behaviour of silicon nanoparticles influenced sustainable waste flax fibre-reinforced epoxy composite for biomedical application
- Optimising coagulation/flocculation using response surface methodology and application of floc in biofertilisation
- Green synthesis and multifaceted characterization of iron oxide nanoparticles derived from Senna bicapsularis for enhanced in vitro and in vivo biological investigation
- Potent antibacterial nanocomposites from okra mucilage/chitosan/silver nanoparticles for multidrug-resistant Salmonella Typhimurium eradication
- Trachyspermum copticum aqueous seed extract-derived silver nanoparticles: Exploration of their structural characterization and comparative antibacterial performance against gram-positive and gram-negative bacteria
- Microwave-assisted ultrafine silver nanoparticle synthesis using Mitragyna speciosa for antimalarial applications
- Green synthesis and characterisation of spherical structure Ag/Fe2O3/TiO2 nanocomposite using acacia in the presence of neem and tulsi oils
- Green quantitative methods for linagliptin and empagliflozin in dosage forms
- Enhancement efficacy of omeprazole by conjugation with silver nanoparticles as a urease inhibitor
- Residual, sequential extraction, and ecological risk assessment of some metals in ash from municipal solid waste incineration, Vietnam
- Green synthesis of ZnO nanoparticles using the mangosteen (Garcinia mangostana L.) leaf extract: Comparative preliminary in vitro antibacterial study
- Simultaneous determination of lesinurad and febuxostat in commercial fixed-dose combinations using a greener normal-phase HPTLC method
- A greener RP-HPLC method for quaternary estimation of caffeine, paracetamol, levocetirizine, and phenylephrine acquiring AQbD with stability studies
- Optimization of biomass durian peel as a heterogeneous catalyst in biodiesel production using microwave irradiation
- Thermal treatment impact on the evolution of active phases in layered double hydroxide-based ZnCr photocatalysts: Photodegradation and antibacterial performance
- Preparation of silymarin-loaded zein polysaccharide core–shell nanostructures and evaluation of their biological potentials
- Preparation and characterization of composite-modified PA6 fiber for spectral heating and heat storage applications
- Preparation and electrocatalytic oxygen evolution of bimetallic phosphates (NiFe)2P/NF
- Rod-shaped Mo(vi) trichalcogenide–Mo(vi) oxide decorated on poly(1-H pyrrole) as a promising nanocomposite photoelectrode for green hydrogen generation from sewage water with high efficiency
- Green synthesis and studies on citrus medica leaf extract-mediated Au–ZnO nanocomposites: A sustainable approach for efficient photocatalytic degradation of rhodamine B dye in aqueous media
- Cellulosic materials for the removal of ciprofloxacin from aqueous environments
- The analytical assessment of metal contamination in industrial soils of Saudi Arabia using the inductively coupled plasma technology
- The effect of modified oily sludge on the slurry ability and combustion performance of coal water slurry
- Eggshell waste transformation to calcium chloride anhydride as food-grade additive and eggshell membranes as enzyme immobilization carrier
- Synthesis of EPAN and applications in the encapsulation of potassium humate
- Biosynthesis and characterization of silver nanoparticles from Cedrela toona leaf extracts: An exploration into their antibacterial, anticancer, and antioxidant potential
- Enhancing mechanical and rheological properties of HDPE films through annealing for eco-friendly agricultural applications
- Immobilisation of catalase purified from mushroom (Hydnum repandum) onto glutaraldehyde-activated chitosan and characterisation: Its application for the removal of hydrogen peroxide from artificial wastewater
- Sodium titanium oxide/zinc oxide (STO/ZnO) photocomposites for efficient dye degradation applications
- Effect of ex situ, eco-friendly ZnONPs incorporating green synthesised Moringa oleifera leaf extract in enhancing biochemical and molecular aspects of Vicia faba L. under salt stress
- Biosynthesis and characterization of selenium and silver nanoparticles using Trichoderma viride filtrate and their impact on Culex pipiens
- Photocatalytic degradation of organic dyes and biological potentials of biogenic zinc oxide nanoparticles synthesized using the polar extract of Cyperus scariosus R.Br. (Cyperaceae)
- Assessment of antiproliferative activity of green-synthesized nickel oxide nanoparticles against glioblastoma cells using Terminalia chebula
- Chlorine-free synthesis of phosphinic derivatives by change in the P-function
- Anticancer, antioxidant, and antimicrobial activities of nanoemulsions based on water-in-olive oil and loaded on biogenic silver nanoparticles
- Study and mechanism of formation of phosphorus production waste in Kazakhstan
- Synthesis and stabilization of anatase form of biomimetic TiO2 nanoparticles for enhancing anti-tumor potential
- Microwave-supported one-pot reaction for the synthesis of 5-alkyl/arylidene-2-(morpholin/thiomorpholin-4-yl)-1,3-thiazol-4(5H)-one derivatives over MgO solid base
- Screening the phytochemicals in Perilla leaves and phytosynthesis of bioactive silver nanoparticles for potential antioxidant and wound-healing application
- Graphene oxide/chitosan/manganese/folic acid-brucine functionalized nanocomposites show anticancer activity against liver cancer cells
- Nature of serpentinite interactions with low-concentration sulfuric acid solutions
- Multi-objective statistical optimisation utilising response surface methodology to predict engine performance using biofuels from waste plastic oil in CRDi engines
- Microwave-assisted extraction of acetosolv lignin from sugarcane bagasse and electrospinning of lignin/PEO nanofibres for carbon fibre production
- Biosynthesis, characterization, and investigation of cytotoxic activities of selenium nanoparticles utilizing Limosilactobacillus fermentum
- Highly photocatalytic materials based on the decoration of poly(O-chloroaniline) with molybdenum trichalcogenide oxide for green hydrogen generation from Red Sea water
- Highly efficient oil–water separation using superhydrophobic cellulose aerogels derived from corn straw
- Beta-cyclodextrin–Phyllanthus emblica emulsion for zinc oxide nanoparticles: Characteristics and photocatalysis
- Assessment of antimicrobial activity and methyl orange dye removal by Klebsiella pneumoniae-mediated silver nanoparticles
- Influential eradication of resistant Salmonella Typhimurium using bioactive nanocomposites from chitosan and radish seed-synthesized nanoselenium
- Antimicrobial activities and neuroprotective potential for Alzheimer’s disease of pure, Mn, Co, and Al-doped ZnO ultra-small nanoparticles
- Green synthesis of silver nanoparticles from Bauhinia variegata and their biological applications
- Synthesis and optimization of long-chain fatty acids via the oxidation of long-chain fatty alcohols
- Eminent Red Sea water hydrogen generation via a Pb(ii)-iodide/poly(1H-pyrrole) nanocomposite photocathode
- Green synthesis and effective genistein production by fungal β-glucosidase immobilized on Al2O3 nanocrystals synthesized in Cajanus cajan L. (Millsp.) leaf extracts
- Green stability-indicating RP-HPTLC technique for determining croconazole hydrochloride
- Green synthesis of La2O3–LaPO4 nanocomposites using Charybdis natator for DNA binding, cytotoxic, catalytic, and luminescence applications
- Eco-friendly drugs induce cellular changes in colistin-resistant bacteria
- Tangerine fruit peel extract mediated biogenic synthesized silver nanoparticles and their potential antimicrobial, antioxidant, and cytotoxic assessments
- Green synthesis on performance characteristics of a direct injection diesel engine using sandbox seed oil
- A highly sensitive β-AKBA-Ag-based fluorescent “turn off” chemosensor for rapid detection of abamectin in tomatoes
- Green synthesis and physical characterization of zinc oxide nanoparticles (ZnO NPs) derived from the methanol extract of Euphorbia dracunculoides Lam. (Euphorbiaceae) with enhanced biosafe applications
- Detection of morphine and data processing using surface plasmon resonance imaging sensor
- Effects of nanoparticles on the anaerobic digestion properties of sulfamethoxazole-containing chicken manure and analysis of bio-enzymes
- Bromic acid-thiourea synergistic leaching of sulfide gold ore
- Green chemistry approach to synthesize titanium dioxide nanoparticles using Fagonia Cretica extract, novel strategy for developing antimicrobial and antidiabetic therapies
- Green synthesis and effective utilization of biogenic Al2O3-nanocoupled fungal lipase in the resolution of active homochiral 2-octanol and its immobilization via aluminium oxide nanoparticles
- Eco-friendly RP-HPLC approach for simultaneously estimating the promising combination of pentoxifylline and simvastatin in therapeutic potential for breast cancer: Appraisal of greenness, whiteness, and Box–Behnken design
- Use of a humidity adsorbent derived from cockleshell waste in Thai fried fish crackers (Keropok)
- One-pot green synthesis, biological evaluation, and in silico study of pyrazole derivatives obtained from chalcones
- Bio-sorption of methylene blue and production of biofuel by brown alga Cystoseira sp. collected from Neom region, Kingdom of Saudi Arabia
- Synthesis of motexafin gadolinium: A promising radiosensitizer and imaging agent for cancer therapy
- The impact of varying sizes of silver nanoparticles on the induction of cellular damage in Klebsiella pneumoniae involving diverse mechanisms
- Microwave-assisted green synthesis, characterization, and in vitro antibacterial activity of NiO nanoparticles obtained from lemon peel extract
- Rhus microphylla-mediated biosynthesis of copper oxide nanoparticles for enhanced antibacterial and antibiofilm efficacy
- Harnessing trichalcogenide–molybdenum(vi) sulfide and molybdenum(vi) oxide within poly(1-amino-2-mercaptobenzene) frameworks as a photocathode for sustainable green hydrogen production from seawater without sacrificial agents
- Magnetically recyclable Fe3O4@SiO2 supported phosphonium ionic liquids for efficient and sustainable transformation of CO2 into oxazolidinones
- A comparative study of Fagonia arabica fabricated silver sulfide nanoparticles (Ag2S) and silver nanoparticles (AgNPs) with distinct antimicrobial, anticancer, and antioxidant properties
- Visible light photocatalytic degradation and biological activities of Aegle marmelos-mediated cerium oxide nanoparticles
- Physical intrinsic characteristics of spheroidal particles in coal gasification fine slag
- Exploring the effect of tea dust magnetic biochar on agricultural crops grown in polycyclic aromatic hydrocarbon contaminated soil
- Crosslinked chitosan-modified ultrafiltration membranes for efficient surface water treatment and enhanced anti-fouling performances
- Study on adsorption characteristics of biochars and their modified biochars for removal of organic dyes from aqueous solution
- Zein polymer nanocarrier for Ocimum basilicum var. purpurascens extract: Potential biomedical use
- Green synthesis, characterization, and in vitro and in vivo biological screening of iron oxide nanoparticles (Fe3O4) generated with hydroalcoholic extract of aerial parts of Euphorbia milii
- Novel microwave-based green approach for the synthesis of dual-loaded cyclodextrin nanosponges: Characterization, pharmacodynamics, and pharmacokinetics evaluation
- Bi2O3–BiOCl/poly-m-methyl aniline nanocomposite thin film for broad-spectrum light-sensing
- Green synthesis and characterization of CuO/ZnO nanocomposite using Musa acuminata leaf extract for cytotoxic studies on colorectal cancer cells (HCC2998)
- Review Articles
- Materials-based drug delivery approaches: Recent advances and future perspectives
- A review of thermal treatment for bamboo and its composites
- An overview of the role of nanoherbicides in tackling challenges of weed management in wheat: A novel approach
- An updated review on carbon nanomaterials: Types, synthesis, functionalization and applications, degradation and toxicity
- Special Issue: Emerging green nanomaterials for sustainable waste management and biomedical applications
- Green synthesis of silver nanoparticles using mature-pseudostem extracts of Alpinia nigra and their bioactivities
- Special Issue: New insights into nanopythotechnology: current trends and future prospects
- Green synthesis of FeO nanoparticles from coffee and its application for antibacterial, antifungal, and anti-oxidation activity
- Dye degradation activity of biogenically synthesized Cu/Fe/Ag trimetallic nanoparticles
- Special Issue: Composites and green composites
- Recent trends and advancements in the utilization of green composites and polymeric nanocarriers for enhancing food quality and sustainable processing
- Retraction
- Retraction of “Biosynthesis and characterization of silver nanoparticles from Cedrela toona leaf extracts: An exploration into their antibacterial, anticancer, and antioxidant potential”
- Retraction of “Photocatalytic degradation of organic dyes and biological potentials of biogenic zinc oxide nanoparticles synthesized using the polar extract of Cyperus scariosus R.Br. (Cyperaceae)”
- Retraction to “Green synthesis on performance characteristics of a direct injection diesel engine using sandbox seed oil”
Articles in the same Issue
- Research Articles
- Green polymer electrolyte and activated charcoal-based supercapacitor for energy harvesting application: Electrochemical characteristics
- Research on the adsorption of Co2+ ions using halloysite clay and the ability to recover them by electrodeposition method
- Simultaneous estimation of ibuprofen, caffeine, and paracetamol in commercial products using a green reverse-phase HPTLC method
- Isolation, screening and optimization of alkaliphilic cellulolytic fungi for production of cellulase
- Functionalized gold nanoparticles coated with bacterial alginate and their antibacterial and anticancer activities
- Comparative analysis of bio-based amino acid surfactants obtained via Diels–Alder reaction of cyclic anhydrides
- Biosynthesis of silver nanoparticles on yellow phosphorus slag and its application in organic coatings
- Exploring antioxidant potential and phenolic compound extraction from Vitis vinifera L. using ultrasound-assisted extraction
- Manganese and copper-coated nickel oxide nanoparticles synthesized from Carica papaya leaf extract induce antimicrobial activity and breast cancer cell death by triggering mitochondrial caspases and p53
- Insight into heating method and Mozafari method as green processing techniques for the synthesis of micro- and nano-drug carriers
- Silicotungstic acid supported on Bi-based MOF-derived metal oxide for photodegradation of organic dyes
- Synthesis and characterization of capsaicin nanoparticles: An attempt to enhance its bioavailability and pharmacological actions
- Synthesis of Lawsonia inermis-encased silver–copper bimetallic nanoparticles with antioxidant, antibacterial, and cytotoxic activity
- Facile, polyherbal drug-mediated green synthesis of CuO nanoparticles and their potent biological applications
- Zinc oxide-manganese oxide/carboxymethyl cellulose-folic acid-sesamol hybrid nanomaterials: A molecularly targeted strategy for advanced triple-negative breast cancer therapy
- Exploring the antimicrobial potential of biogenically synthesized graphene oxide nanoparticles against targeted bacterial and fungal pathogens
- Biofabrication of silver nanoparticles using Uncaria tomentosa L.: Insight into characterization, antibacterial activities combined with antibiotics, and effect on Triticum aestivum germination
- Membrane distillation of synthetic urine for use in space structural habitat systems
- Investigation on mechanical properties of the green synthesis bamboo fiber/eggshell/coconut shell powder-based hybrid biocomposites under NaOH conditions
- Green synthesis of magnesium oxide nanoparticles using endophytic fungal strain to improve the growth, metabolic activities, yield traits, and phenolic compounds content of Nigella sativa L.
- Estimation of greenhouse gas emissions from rice and annual upland crops in Red River Delta of Vietnam using the denitrification–decomposition model
- Synthesis of humic acid with the obtaining of potassium humate based on coal waste from the Lenger deposit, Kazakhstan
- Ascorbic acid-mediated selenium nanoparticles as potential antihyperuricemic, antioxidant, anticoagulant, and thrombolytic agents
- Green synthesis of silver nanoparticles using Illicium verum extract: Optimization and characterization for biomedical applications
- Antibacterial and dynamical behaviour of silicon nanoparticles influenced sustainable waste flax fibre-reinforced epoxy composite for biomedical application
- Optimising coagulation/flocculation using response surface methodology and application of floc in biofertilisation
- Green synthesis and multifaceted characterization of iron oxide nanoparticles derived from Senna bicapsularis for enhanced in vitro and in vivo biological investigation
- Potent antibacterial nanocomposites from okra mucilage/chitosan/silver nanoparticles for multidrug-resistant Salmonella Typhimurium eradication
- Trachyspermum copticum aqueous seed extract-derived silver nanoparticles: Exploration of their structural characterization and comparative antibacterial performance against gram-positive and gram-negative bacteria
- Microwave-assisted ultrafine silver nanoparticle synthesis using Mitragyna speciosa for antimalarial applications
- Green synthesis and characterisation of spherical structure Ag/Fe2O3/TiO2 nanocomposite using acacia in the presence of neem and tulsi oils
- Green quantitative methods for linagliptin and empagliflozin in dosage forms
- Enhancement efficacy of omeprazole by conjugation with silver nanoparticles as a urease inhibitor
- Residual, sequential extraction, and ecological risk assessment of some metals in ash from municipal solid waste incineration, Vietnam
- Green synthesis of ZnO nanoparticles using the mangosteen (Garcinia mangostana L.) leaf extract: Comparative preliminary in vitro antibacterial study
- Simultaneous determination of lesinurad and febuxostat in commercial fixed-dose combinations using a greener normal-phase HPTLC method
- A greener RP-HPLC method for quaternary estimation of caffeine, paracetamol, levocetirizine, and phenylephrine acquiring AQbD with stability studies
- Optimization of biomass durian peel as a heterogeneous catalyst in biodiesel production using microwave irradiation
- Thermal treatment impact on the evolution of active phases in layered double hydroxide-based ZnCr photocatalysts: Photodegradation and antibacterial performance
- Preparation of silymarin-loaded zein polysaccharide core–shell nanostructures and evaluation of their biological potentials
- Preparation and characterization of composite-modified PA6 fiber for spectral heating and heat storage applications
- Preparation and electrocatalytic oxygen evolution of bimetallic phosphates (NiFe)2P/NF
- Rod-shaped Mo(vi) trichalcogenide–Mo(vi) oxide decorated on poly(1-H pyrrole) as a promising nanocomposite photoelectrode for green hydrogen generation from sewage water with high efficiency
- Green synthesis and studies on citrus medica leaf extract-mediated Au–ZnO nanocomposites: A sustainable approach for efficient photocatalytic degradation of rhodamine B dye in aqueous media
- Cellulosic materials for the removal of ciprofloxacin from aqueous environments
- The analytical assessment of metal contamination in industrial soils of Saudi Arabia using the inductively coupled plasma technology
- The effect of modified oily sludge on the slurry ability and combustion performance of coal water slurry
- Eggshell waste transformation to calcium chloride anhydride as food-grade additive and eggshell membranes as enzyme immobilization carrier
- Synthesis of EPAN and applications in the encapsulation of potassium humate
- Biosynthesis and characterization of silver nanoparticles from Cedrela toona leaf extracts: An exploration into their antibacterial, anticancer, and antioxidant potential
- Enhancing mechanical and rheological properties of HDPE films through annealing for eco-friendly agricultural applications
- Immobilisation of catalase purified from mushroom (Hydnum repandum) onto glutaraldehyde-activated chitosan and characterisation: Its application for the removal of hydrogen peroxide from artificial wastewater
- Sodium titanium oxide/zinc oxide (STO/ZnO) photocomposites for efficient dye degradation applications
- Effect of ex situ, eco-friendly ZnONPs incorporating green synthesised Moringa oleifera leaf extract in enhancing biochemical and molecular aspects of Vicia faba L. under salt stress
- Biosynthesis and characterization of selenium and silver nanoparticles using Trichoderma viride filtrate and their impact on Culex pipiens
- Photocatalytic degradation of organic dyes and biological potentials of biogenic zinc oxide nanoparticles synthesized using the polar extract of Cyperus scariosus R.Br. (Cyperaceae)
- Assessment of antiproliferative activity of green-synthesized nickel oxide nanoparticles against glioblastoma cells using Terminalia chebula
- Chlorine-free synthesis of phosphinic derivatives by change in the P-function
- Anticancer, antioxidant, and antimicrobial activities of nanoemulsions based on water-in-olive oil and loaded on biogenic silver nanoparticles
- Study and mechanism of formation of phosphorus production waste in Kazakhstan
- Synthesis and stabilization of anatase form of biomimetic TiO2 nanoparticles for enhancing anti-tumor potential
- Microwave-supported one-pot reaction for the synthesis of 5-alkyl/arylidene-2-(morpholin/thiomorpholin-4-yl)-1,3-thiazol-4(5H)-one derivatives over MgO solid base
- Screening the phytochemicals in Perilla leaves and phytosynthesis of bioactive silver nanoparticles for potential antioxidant and wound-healing application
- Graphene oxide/chitosan/manganese/folic acid-brucine functionalized nanocomposites show anticancer activity against liver cancer cells
- Nature of serpentinite interactions with low-concentration sulfuric acid solutions
- Multi-objective statistical optimisation utilising response surface methodology to predict engine performance using biofuels from waste plastic oil in CRDi engines
- Microwave-assisted extraction of acetosolv lignin from sugarcane bagasse and electrospinning of lignin/PEO nanofibres for carbon fibre production
- Biosynthesis, characterization, and investigation of cytotoxic activities of selenium nanoparticles utilizing Limosilactobacillus fermentum
- Highly photocatalytic materials based on the decoration of poly(O-chloroaniline) with molybdenum trichalcogenide oxide for green hydrogen generation from Red Sea water
- Highly efficient oil–water separation using superhydrophobic cellulose aerogels derived from corn straw
- Beta-cyclodextrin–Phyllanthus emblica emulsion for zinc oxide nanoparticles: Characteristics and photocatalysis
- Assessment of antimicrobial activity and methyl orange dye removal by Klebsiella pneumoniae-mediated silver nanoparticles
- Influential eradication of resistant Salmonella Typhimurium using bioactive nanocomposites from chitosan and radish seed-synthesized nanoselenium
- Antimicrobial activities and neuroprotective potential for Alzheimer’s disease of pure, Mn, Co, and Al-doped ZnO ultra-small nanoparticles
- Green synthesis of silver nanoparticles from Bauhinia variegata and their biological applications
- Synthesis and optimization of long-chain fatty acids via the oxidation of long-chain fatty alcohols
- Eminent Red Sea water hydrogen generation via a Pb(ii)-iodide/poly(1H-pyrrole) nanocomposite photocathode
- Green synthesis and effective genistein production by fungal β-glucosidase immobilized on Al2O3 nanocrystals synthesized in Cajanus cajan L. (Millsp.) leaf extracts
- Green stability-indicating RP-HPTLC technique for determining croconazole hydrochloride
- Green synthesis of La2O3–LaPO4 nanocomposites using Charybdis natator for DNA binding, cytotoxic, catalytic, and luminescence applications
- Eco-friendly drugs induce cellular changes in colistin-resistant bacteria
- Tangerine fruit peel extract mediated biogenic synthesized silver nanoparticles and their potential antimicrobial, antioxidant, and cytotoxic assessments
- Green synthesis on performance characteristics of a direct injection diesel engine using sandbox seed oil
- A highly sensitive β-AKBA-Ag-based fluorescent “turn off” chemosensor for rapid detection of abamectin in tomatoes
- Green synthesis and physical characterization of zinc oxide nanoparticles (ZnO NPs) derived from the methanol extract of Euphorbia dracunculoides Lam. (Euphorbiaceae) with enhanced biosafe applications
- Detection of morphine and data processing using surface plasmon resonance imaging sensor
- Effects of nanoparticles on the anaerobic digestion properties of sulfamethoxazole-containing chicken manure and analysis of bio-enzymes
- Bromic acid-thiourea synergistic leaching of sulfide gold ore
- Green chemistry approach to synthesize titanium dioxide nanoparticles using Fagonia Cretica extract, novel strategy for developing antimicrobial and antidiabetic therapies
- Green synthesis and effective utilization of biogenic Al2O3-nanocoupled fungal lipase in the resolution of active homochiral 2-octanol and its immobilization via aluminium oxide nanoparticles
- Eco-friendly RP-HPLC approach for simultaneously estimating the promising combination of pentoxifylline and simvastatin in therapeutic potential for breast cancer: Appraisal of greenness, whiteness, and Box–Behnken design
- Use of a humidity adsorbent derived from cockleshell waste in Thai fried fish crackers (Keropok)
- One-pot green synthesis, biological evaluation, and in silico study of pyrazole derivatives obtained from chalcones
- Bio-sorption of methylene blue and production of biofuel by brown alga Cystoseira sp. collected from Neom region, Kingdom of Saudi Arabia
- Synthesis of motexafin gadolinium: A promising radiosensitizer and imaging agent for cancer therapy
- The impact of varying sizes of silver nanoparticles on the induction of cellular damage in Klebsiella pneumoniae involving diverse mechanisms
- Microwave-assisted green synthesis, characterization, and in vitro antibacterial activity of NiO nanoparticles obtained from lemon peel extract
- Rhus microphylla-mediated biosynthesis of copper oxide nanoparticles for enhanced antibacterial and antibiofilm efficacy
- Harnessing trichalcogenide–molybdenum(vi) sulfide and molybdenum(vi) oxide within poly(1-amino-2-mercaptobenzene) frameworks as a photocathode for sustainable green hydrogen production from seawater without sacrificial agents
- Magnetically recyclable Fe3O4@SiO2 supported phosphonium ionic liquids for efficient and sustainable transformation of CO2 into oxazolidinones
- A comparative study of Fagonia arabica fabricated silver sulfide nanoparticles (Ag2S) and silver nanoparticles (AgNPs) with distinct antimicrobial, anticancer, and antioxidant properties
- Visible light photocatalytic degradation and biological activities of Aegle marmelos-mediated cerium oxide nanoparticles
- Physical intrinsic characteristics of spheroidal particles in coal gasification fine slag
- Exploring the effect of tea dust magnetic biochar on agricultural crops grown in polycyclic aromatic hydrocarbon contaminated soil
- Crosslinked chitosan-modified ultrafiltration membranes for efficient surface water treatment and enhanced anti-fouling performances
- Study on adsorption characteristics of biochars and their modified biochars for removal of organic dyes from aqueous solution
- Zein polymer nanocarrier for Ocimum basilicum var. purpurascens extract: Potential biomedical use
- Green synthesis, characterization, and in vitro and in vivo biological screening of iron oxide nanoparticles (Fe3O4) generated with hydroalcoholic extract of aerial parts of Euphorbia milii
- Novel microwave-based green approach for the synthesis of dual-loaded cyclodextrin nanosponges: Characterization, pharmacodynamics, and pharmacokinetics evaluation
- Bi2O3–BiOCl/poly-m-methyl aniline nanocomposite thin film for broad-spectrum light-sensing
- Green synthesis and characterization of CuO/ZnO nanocomposite using Musa acuminata leaf extract for cytotoxic studies on colorectal cancer cells (HCC2998)
- Review Articles
- Materials-based drug delivery approaches: Recent advances and future perspectives
- A review of thermal treatment for bamboo and its composites
- An overview of the role of nanoherbicides in tackling challenges of weed management in wheat: A novel approach
- An updated review on carbon nanomaterials: Types, synthesis, functionalization and applications, degradation and toxicity
- Special Issue: Emerging green nanomaterials for sustainable waste management and biomedical applications
- Green synthesis of silver nanoparticles using mature-pseudostem extracts of Alpinia nigra and their bioactivities
- Special Issue: New insights into nanopythotechnology: current trends and future prospects
- Green synthesis of FeO nanoparticles from coffee and its application for antibacterial, antifungal, and anti-oxidation activity
- Dye degradation activity of biogenically synthesized Cu/Fe/Ag trimetallic nanoparticles
- Special Issue: Composites and green composites
- Recent trends and advancements in the utilization of green composites and polymeric nanocarriers for enhancing food quality and sustainable processing
- Retraction
- Retraction of “Biosynthesis and characterization of silver nanoparticles from Cedrela toona leaf extracts: An exploration into their antibacterial, anticancer, and antioxidant potential”
- Retraction of “Photocatalytic degradation of organic dyes and biological potentials of biogenic zinc oxide nanoparticles synthesized using the polar extract of Cyperus scariosus R.Br. (Cyperaceae)”
- Retraction to “Green synthesis on performance characteristics of a direct injection diesel engine using sandbox seed oil”

