One-pot green synthesis, biological evaluation, and in silico study of pyrazole derivatives obtained from chalcones
-
Emma Ghazaryan
, Yana Gharibyan
Abstract
Several chalcones and their pyrazole derivatives have been synthesized using traditional methods under microwave (MW) and ultrasonic (US) irradiation. The latter were synthesized using US-assisted and MW-assisted one-pot techniques. The use of this technology led to a reduction in reaction time and energy consumption, as well as an increase in the yield of the final products, which means more efficient synthesis. An in silico study was conducted and the plant growth-stimulating effects of the synthesized compounds were revealed. To study the growth-regulating activity of the synthesized compounds, a study of aqueous suspensions at a concentration of 50 and 25 mg·L−1 was carried out on the viability, germination, and growth of seeds.
Graphical abstract

1 Introduction
The chemical modification of functionally substituted chalcones has great and still not fully disclosed potential prospects. It still attracted the increased attention of numerous researchers, which is reflected in the annually increasing number of publications in high-ranking journals [1,2,3,4,5,6,7,8]. Currently, a wide range of schemes is available for the synthesis of various chalcone analogs. A series of derivatives with various heterocycles was synthesized using chalcones. In some cases, green energy sources are used for the synthesis of chalcones when the molecules are activated under ultrasonic [9,10,11,12,13,14,15,16] or microwave [17,18,19,20,21,22,23] irradiation.
Chalcones and their derivatives exhibit a wide range of biological activities. Among these, compounds with anti-inflammatory [24,25,26,27,28], anticancer [29,30,31,32,33,34], antimalarial [35,36], antiviral [37,38], antimicrobial [9,17,18,39,40,41,42], antioxidant [43,44,45,46], anti-diabetic [47,48,49], anticonvulsant [50], antihypertensive [51,52], antileishmanial [53,54], analgesic [28], antihistaminic [55], and anti-Alzheimer [56] activities have been discovered.
Over the last decade, one-pot synthesis techniques have been increasingly used [57,58,59,60,61,62,63]. One-pot synthesis is a strategy for increasing the efficiency of a chemical reaction in which reactants are added sequentially one at a time without intermediate treatment. This is highly desirable for chemists because eliminating the time-consuming process of separating and purifying chemical intermediates can save time and resources. The sequential synthesis in one container is also called telescopic synthesis. However, these syntheses are performed under ordinary conditions, most often with heat.
In connection with the above, the final goal of this study was the one-pot synthesis of pyrazole derivatives of chalcones. For this purpose, environmentally friendly ultrasound and microwave methods were used, which results were compared with conventional synthetic methods. The synthesized compounds were examined for their biological properties.
2 Materials and methods
2.1 General
Microwave (MW)-assisted syntheses were performed on a MAS-II Plus microwave synthesis workstation (SINEO) with a maximum irradiation power of 1,000 W. An ultrasonic generator I10-840 with an operating frequency of 22 kHz ± 10% and a maximal pulse power of 1,000 W was used for the sonochemical synthesis. Before carrying out the necessary syntheses with MW and ultrasonic (US) irradiations, a lot of works were done to optimize the experimental conditions. The irradiation power and reaction time were changed. Based on this, the following conditions were applied in all experiments: irradiation power of 30% (300 W) at boiling and exposure time of 30 min.
The structure and purity of the synthesized compounds were confirmed by 1H and 13C NMR spectra obtained at 30°C on a Varian Mercury-300 NMR spectrometer (300 and 75 MHz, respectively) in a mixture of CCl4/DMSO-d6 solvents (3:1), using a standard pulse sequence and TMS as an internal standard. The progress of the reactions and purity of the obtained compounds were checked by TLC on Silufol UV-254 plates using an acetone/hexane mixture (2:1 or 1:1) as the eluent. Elemental analysis was performed using a Eurovector EA3000 CHN analyzer. Melting points were determined using a Stuart SMP10 apparatus and uncorrected.
2.2 Conventional, MW-assisted, and US-assisted synthesis of chalcones 1a–1m were carried out in a similar way
To a solution of 0.001 mol of substituted aldehyde in 10 mL of methanol, 0.001 mol of substituted acetophenone and 10 mL of 5% NaOH solution were slowly added. The mixture was boiled for 4 h at a temperature of 80–85°C (or irradiated for 30 min at a temperature of 80–85°C) and left overnight. The precipitate that formed was filtered, thoroughly washed with water, and dried. The resulting chalcones were recrystallized from ether. The 1H and 13C NMR spectral parameters, as well as the elemental analysis data for chalcones 1a–1m synthesized by all three methods, are very similar. The files were stored in the Zenodo Repository doi: 10.5281/zenodo.11108499.
2.3 One-pot conventional and MW-assisted synthesis of pyrazole derivatives 2a–2n were carried out in a similar way
A solution of 0.001 mol of substituted aldehyde and 0.001 mol of substituted acetophenone in 10 mL of methanol and 10 mL of a 5% NaOH solution was boiled for 4 h (or irradiated for 30 min at a temperature – 80–85°C) and left overnight at room temperature. The next day, 0.004 mol of phenylhydrazine was added and heated at a temperature – 80−85°C for 14−15 h (or irradiated for 30 min at a temperature – 80−85°C). The resulting precipitate was filtered, thoroughly washed with water, dried, and purified by boiling it in hexane. 1H and 13C NMR spectra and elemental analysis data for pyrazole derivatives 2a–2n are given in the Zenodo Repository doi: 10.5281/zenodo.11108499.
3 Results
The synthesis plan was as follows: (1) chalcone synthesis using the conventional method under the influence of MW irradiation and US irradiation and (2) one-pot conventional, US-assisted, and MW-assisted synthesis of pyrazole derivatives.
In the first stage, 13 chalcones (1a–m) were prepared by Claisen–Schmidt condensation between substituted benzaldehydes and acetophenones. Then, by reacting these chalcones with phenylhydrazine, the one-pot synthesis of pyrazole derivatives was carried out both by the conventional method and under the influence of microwave and ultrasonic irradiation according to a well-known route (Scheme 1).

Synthesis of chalcones and corresponding pyrazole derivatives.
Most synthesized compounds have been described in the literature. The novelty of this study lies in the one-pot synthesis of pyrazole derivatives of chalcones, both by the traditional method and under the influence of US and MW irradiations, and a comparative analysis of the results of these methods. A comparative analysis of the physicochemical characteristics of these compounds is presented in Tables 1 and 2, respectively.
Comparison of physicochemical characteristics of chalcone 1 obtained by the conventional method and under the influence of MW and US irradiations
| No | R | R1 | Conventional (5% NaOH) | MW-assisted* (2.5% NaOH) | US-assisted* (2.5% NaOH) | ||||
|---|---|---|---|---|---|---|---|---|---|
| Yield (%) | mp (°C) | Reaction time (h) | Yield (%) | mp (°C) | Yield (%) | mp (°C) | |||
| 1a | H | H | 99 | 52 | 4.5 | 90 | 52 | 99 | 53 |
| 1b | Cl | H | 95 | 112 | 4.5 | 95 | 111 | 80 | 112 |
| 1c | Cl | Cl | 99 | 156 | 4.5 | 90 | 156 | 86 | 156 |
| 1d | Cl | Br | 87 | 167 | 4.5 | 79 | 167 | 84 | 167 |
| 1e | Cl | CH3 | 94 | 151 | 4.5 | 88 | 151 | 76 | 152 |
| 1f | N(Me)2 | Br | 42 | 140 | 4.5 | 41 | 140 | 54 | 140 |
| 1g | Br | Br | 90 | 186 | 4.5 | 94 | 186 | 82 | 186 |
| 1h | OCH3 | CH3 | 83 | 100 | 4.5 | 76 | 100 | 72 | 100 |
| 1i | OCH3 | Cl | 83 | 121 | 4.5 | 93 | 120 | 84 | 121 |
| 1j | OCH3 | Br | 94 | 146 | 4.5 | 91 | 146 | 62 | 146 |
| 1k | Cl | OCH3 | 95 | 132 | 4.5 | 82 | 132 | 79 | 132 |
| 1l | Br | OCH3 | 97 | 154 | 4.5 | 87 | 154 | 96 | 154 |
| 1m | OCH3 | OCH3 | 93 | 103 | 4.5 | 95 | 103 | 96 | 103 |
*Reaction times are 0.5 h.
Comparison of physicochemical characteristics of pyrazole derivatives 2 synthesized by the conventional and MW-assisted methods using one-pot methodology
| No | R | R1 | Conventional (5% NaOH) | MW-assisted* (2.5% NaOH) | |||
|---|---|---|---|---|---|---|---|
| Yield (%) | mp (°C) | Reaction time (h) | Yield (%) | mp (°C) | |||
| 2a | H | H | 36 | 129 | 18 | 42 | 130 |
| 2b | Cl | H | 41 | 130 | 18 | 50 | 130 |
| 2c | Cl | Cl | 32 | 150 | 18 | 41 | 150 |
| 2d | Cl | Br | 36 | 176 | 18 | 47 | 176 |
| 2e | Cl | CH3 | 29 | 146 | 18 | 35 | 146 |
| 2f | N(CH3)2 | Br | 51 | 185 | 18 | 55 | 185 |
| 2g | Br | Br | 42 | 185 | 18 | 34 | 185 |
| 2h | OCH3 | CH3 | 26 | 146 | 18 | 45 | 146 |
| 2i | OCH3 | Cl | 30 | 160 | 18 | 40 | 161 |
| 2j | OCH3 | Br | 53 | 176 | 18 | 50 | 176 |
| 2k | Cl | OCH3 | 22 | 147 | 18 | 38 | 146 |
| 2l | Br | OCH3 | 27 | 143 | 18 | 31 | 143 |
| 2m | OCH3 | OCH3 | 46 | 134 | 18 | 51 | 135 |
| 2n | Br | OCH3 | 32 | 101 | 18 | 42 | 101 |
*Reaction time: 2 h (1 h at each stage).
In the MW-assisted one-pot synthesis of pyrazole derivatives, the reaction time was reduced by nine times compared to the traditional methods, and the product yields increased (Table 2).
However, in experiments with US irradiation, the yields of the final products were low; therefore, this method is not recommended for the synthesis of pyrazole derivatives, and Table 2 shows only the MW-assisted data.
As expected, chalcones 1a–m were in the sterically preferred E form (Scheme 2a). This is evidenced by the value of the vicinal spin–spin interaction constant between olefin protons: 15.4–15.7 Hz, corresponding to their mutual trans-orientation.
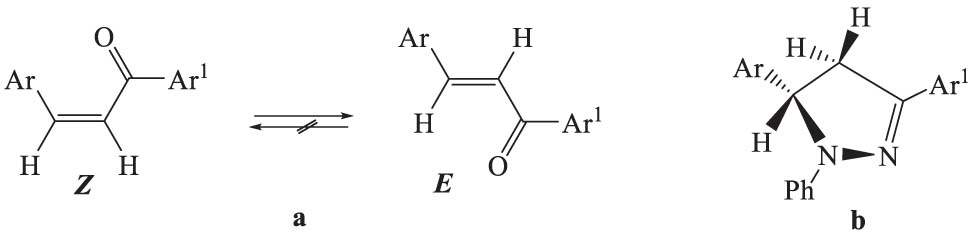
The structure of chalcones (a) and their pyrazole derivatives (b).
In the 1H NMR spectra of pyrazole derivatives, in addition to the geminal spin-spin interaction constant between the protons of the methylene group (17.1–17.3 Hz), interaction constants of these protons with the methine proton are observed equally to 12.2 and 6.9 Hz, corresponding to trans and gauche orientations, which indicates the equatorial location of the Ar group (Scheme 2b).
3.1 Plant growth-stimulating properties of the synthesized compounds
The obtained compounds were subjected to laboratory vegetation tests to determine their herbicidal, fungicidal, and growth-regulatory properties. Almost all the compounds obtained showed a stimulating effect on plant growth.
The experiments were carried out on the seeds and seedlings of common beans (Phaseolus vulgaris L.). The effects of aqueous suspensions of compounds 1,2 at concentrations of 50 and 25 mg·L−1 on seed viability, germination, and seedling growth were studied. These data were compared with the effects of pure water solutions of the same concentration. All data are presented in Table 3.
Plant growth stimulatory activity of compounds 1,2 compared with pure water solutions, which activity was taken as 100%
| No | Activity (%) | No | Activity (%) | No | Activity (%) | No | Activity (%) | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Concentration | Concentration | Concentration | Concentration | ||||||||
| 50 mg·L−1 | 25 mg·L−1 | 50 mg·L−1 | 25 mg·L−1 | 50 mg·L−1 | 25 mg·L−1 | 50 mg·L−1 | 25 mg·L−1 | ||||
| 1a | 43 | 64 | 1h | 74 | 85 | 2b | 80 | 127 | 2i | 83 | 66 |
| 1b | 46 | 86 | 1i | 73 | 54 | 2c | 85 | 80 | 2j | 110 | 134 |
| 1c | 100 | 100 | 1j | 63 | 126 | 2d | 77 | 62 | 2k | 154 | 137 |
| 1d | 85 | 93 | 1k | 83 | 66 | 2e | 88 | 52 | 2l | 88 | 96 |
| 1e | 58 | 99 | 1l | 60 | 49 | 2f | 81 | 128 | 2m | 100 | 117 |
| 1f | 79 | 97 | 1m | 75 | 72 | 2g | 78 | 111 | 2n | 85 | 73 |
| 1g | 78 | 60 | 2a | 80 | 132 | 2h | 78 | 75 | |||
The activity of the tested compounds varied between 43 and 154% compared to water solutions, with an activity of 100%. In some cases (1a, 1b, 1d, 1e, 1f, 1h, 1j and 2a, 2b, 2f, 2g, 2j, 2l, 2m), the growth-stimulating effect of solutions with a lower concentration (25 mg·L−1) was higher than that of the more concentrated (50 mg·L−1) solutions. Compounds that showed activity above 100% in the experiment were selected for further study and field trials using solutions with concentrations less than 25 mg·L−1.
3.2 In silico study
3.2.1 Calculation of ADMET properties and biological activity
Physicochemical and pharmacokinetic values and a prediction of the possible toxicity of substances were calculated using the online platforms SwissADME [64] and ADMETlab v 2.0 [65]. Based on PASSonline, data on the possible therapeutic effects of the resulting substances were obtained [66].
The synthesized compounds complied with Lipinski’s rule, as the molar mass of the resulting compounds did not exceed 500 g·mol−1 and the number of hydrogen atoms capable of forming donor and acceptor hydrogen bonds did not exceed five (Table 4).
Physicochemical values of the synthesized compounds 2
| No | Physicochemical properties | Pharmacokinetics | Druglikeness | Medicinal chemistry | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Molar mass (g·mol−1) | Num. heavy atoms | Num. H-bond acceptors | Num. H-bond donors | GI absorption | BBB permeant | Log Kp (skin permeation) (cm·s−1) | Lipinski | Bioavailability score | Leadlikeness | Synthetic accessibility | |
| 2a | 298.38 | 23 | 1 | 0 | H | Yes | −4.56 | Yes | 0.55 | No | 3.36 |
| 2b | 332.83 | 24 | 1 | 0 | H | Yes | −4.32 | Yes | 0.55 | No | 3.37 |
| 2c | 367.27 | 25 | 1 | 0 | H | Yes | −4.08 | Yes | 0.55 | No | 3.38 |
| 2d | 411.72 | 25 | 1 | 0 | H | Yes | −4.31 | Yes | 0.55 | No | 3.43 |
| 2e | 346.85 | 25 | 1 | 0 | H | Yes | −4.14 | Yes | 0.55 | No | 3.48 |
| 2f | 420.34 | 27 | 1 | 0 | H | Yes | −4.72 | Yes | 0.55 | No | 3.72 |
| 2g | 456.17 | 25 | 1 | 0 | H | Yes | −4.54 | Yes | 0.55 | No | 3.44 |
| 2h | 342.43 | 26 | 2 | 0 | H | Yes | −4.58 | Yes | 0.55 | No | 3.52 |
| 2i | 362.85 | 26 | 2 | 0 | H | Yes | −4.52 | Yes | 0.55 | No | 3.43 |
| 2j | 407.30 | 26 | 2 | 0 | H | Yes | −4.74 | Yes | 0.55 | No | 3.48 |
| 2k | 362.85 | 26 | 2 | 0 | H | Yes | −4.52 | Yes | 0.55 | No | 3.44 |
| 2l | 407.30 | 26 | 2 | 0 | H | Yes | −4.74 | Yes | 0.55 | No | 3.48 |
| 2m | 358.43 | 27 | 3 | 0 | H | Yes | −4.96 | Yes | 0.55 | No | 3.57 |
| 2n | 421.33 | 27 | 2 | 0 | H | Yes | −4.88 | Yes | 0.55 | No | 3.60 |
The tested compounds showed high absorption through the gastrointestinal tract (GIT) and blood–brain barrier (BBB) (Figure 1).

Map of compounds passing through the gastrointestinal tract and BBB based on lipophilicity and topological index of the polar surface of the studied compounds. BBB – passage through the BBB, HIA – passage through the gastrointestinal tract, PGP + active transport, PGP – passive transport.
These substances are divided into three clusters with similar properties (1, 2, and 3). In some cases, there is an almost complete coincidence of these compounds within one cluster.
Toxicity studies showed that none of the compounds had toxic effects on the skin and eyes, and all compounds, except two, had mutagenic and carcinogenic properties. None of the compounds exhibited any hepatotoxicity (Table 5).
Predicted toxicity of tested compounds
| No | Carcinogenicity | AMES toxicity | Irritant effect | Rat oral acute toxicity | Hepatotoxicity | |
|---|---|---|---|---|---|---|
| Eyes | Skin | |||||
| 2a | Yes | Yes | No | No | No | No |
| 2b | Yes | Yes | No | No | No | No |
| 2c | Yes | Yes | No | No | Yes | No |
| 2d | Yes | No | No | No | Yes | No |
| 2e | Yes | Yes | No | No | No | No |
| 2f | Yes | Yes | No | No | Yes | No |
| 2g | No | No | No | No | Yes | No |
| 2h | Yes | Yes | No | No | No | No |
| 2i | Yes | Yes | No | No | No | No |
| 2j | Yes | No | No | No | Yes | No |
| 2k | Yes | Yes | No | No | No | No |
| 2l | Yes | No | No | No | Yes | No |
| 2m | Yes | Yes | No | No | No | No |
| 2n | No | No | No | No | Yes | No |
Using PASSonline, it was shown that the resulting substituted pyrazoles, depending on the substituent, exhibited similar therapeutic activity. All the substances showed fairly high activity (above average). Based on their therapeutic effects, these substances were divided into four groups (Table 6).
Data on possible therapeutic activity of the studied compounds
| Top activity | ||
|---|---|---|
| Possible therapeutic effect | No | Pa/Pi |
| 5-O-(4-Coumaroyl)-d-quinate 3′-monooxygenase inhibitor | 2a | 0.84/0.004 |
| 2b | 0.886/0.003 | |
| 2d | 0.800/0.008 | |
| 2k | 0.762/0.012 | |
| 2e | 0.849/0.004 | |
| 2i | 0.762/0.012 | |
| 2c | 0.886/0.003 | |
| Antiobesity | 2g | 0.737/0.005 |
| (S)-6-Hydroxynicotine oxidase inhibitor | 2f | 0.697/0.007 |
| Aspulvinone dimethylallyltransferase inhibitor | 2j | 0.810/0.031 |
| 2l | 0.810/0.031 | |
| 2h | 0.707/0.061 | |
| 2m | 0.795/0.035 | |
| 2n | 0.786/0.038 | |
4 Conclusions
Thus, it has been established that the microwave- and ultrasonic-assisted synthesis of chalcones can be carried out in high yields and with much less time than when synthesizing them using conventional methods. When synthesizing pyrazole derivatives based on chalcones using the one-pot methodology, good product yields were achieved and the reaction times were significantly reduced, which contributed to energy savings.
The synthesized pyrazole derivatives exhibited pronounced growth-stimulating activity. The study showed that the synthesized compounds freely pass through the GIT and the BBB do not have a toxic effect on the skin and eyes and presumably have inhibitory activity against obesity, 5-O-(4-coumaroyl)-d-quinate 3′-monooxygenase, (S)-6-hydroxynicotine oxidase, and aspulvinone dimethylallyltransferase.
-
Funding information: This work was supported by the State Committee for Science of the Ministry of Education and Science of the Republic of Armenia under the framework of Scientific Project No. 21T-1D165.
-
Author contributions: Tiruhi Gomktsyan and Emma Ghazaryan: conceptualization, data curation, validation, investigation, visualization, and methodology; Yana Gharibyan and Tigran Gharibyan: resources, software, formal analysis, visualization, and methodology; Armen Karapetyan and Asya Vorskanyan: resources, data curation, formal analysis, validation, investigation, visualization, and methodology; Siranush Harutyunyan and Margarita Dovlatyan: analysis of growth stimulator activity, investigation, and visualization; and Aleksandr Yengoyan: conceptualization, supervision, validation, investigation, methodology, writing – original draft, project administration, writing – review, and editing.
-
Conflict of interest: The authors state no conflict of interest.
-
Data availability statement: The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request, and NMR spectral data are presented in the supplementary material and in the Zenodo repository.
References
[1] Rammohan A, Reddy JS, Sravya G, Rao CN, Zyryanoy GV. Chalcone synthesis, properties and medicinal applications: a review. Environ Chem Lett. 2020;18:433–8. 10.1007/s10311-019-00959-w.Search in Google Scholar
[2] Mohamed MFA, Abuo-Rahma GEDA. Molecular targets and anticancer activity of quinoline–chalcone hybrids: literature review. RSC Adv. 2020;10:31139–5. 10.1039/d0ra05594h.Search in Google Scholar PubMed PubMed Central
[3] Goyal K, Kaur R, Goyal A, Awasthi R. Chalcones: A review on synthesis and pharmacological activities. J App Pharm Sci. 2021;11:001–4. 10.7324/JAPS.2021.11s101.Search in Google Scholar
[4] Salehi B, Quispe C, Chamkhi I, Omari NE, Balahbib A, Sharifi-Rad J, et al. Pharmacological properties of chalcones: a review of preclinical including molecular mechanisms and clinical evidence. Front Pharmacol. 2021;11:592654. 10.3389/fphar.2020.592654.Search in Google Scholar PubMed PubMed Central
[5] Elkanzi NAA, Hrichi H, Alolayan RA, Derafa W, Zahou FM, Bakr RB. Synthesis of chalcones derivatives and their biological activities: A Review. ACS Omega. 2022;7:27769–6. 10.1021/acsomega.2c01779.Search in Google Scholar PubMed PubMed Central
[6] Mukhtara SHS, Morsya NM, Hassana AS, Hafeza TS, Hassaneenb HM, Salehb FM. A Review of chalcones: synthesis, reactions, and biological importance. Egypt J Chem. 2022;65(8):379–5. 10.21608/ejchem.2022.112735.5125.Search in Google Scholar
[7] Marotta L, Rossi S, Ibba Brogi RS, Calderone V, Butini S, Campiani G, et al. The green chemistry of chalcones: Valuable sources of privileged core structures for drug discovery. Front Chem. 2022;10:988376. 10.3389/fchem.2022.988376.Search in Google Scholar PubMed PubMed Central
[8] Leite FF, Sousa NF, Oliveira BHM, Duarte GD, Ferreira MDL, Scotti MT, et al. Anticancer activity of chalcones and its derivatives: review and in silico studies. Molecules. 2023;28:4009. 10.3390/molecules28104009.Search in Google Scholar PubMed PubMed Central
[9] Rizk SA, El-Hashash MA, El-Badawy AA. Ultrasonic and grinding aptitudes of one-pot synthesis of 5-(4-chlorophenyl)-7-(3,4-dimethylphenyl)-2-oxo-2H-pyrano[2,3-b]pyridine derivatives as antibacterial agents. J Heterocycl Chem. 2017;54:2003–1. 10.1002/jhet.2797.Search in Google Scholar
[10] Sharma SP, Vashisht N, Kumar S, Kritika A. Ultrasound promoted green synthesis of chalcones of 3-acetylcoumarin. Chem Sci Trans. 2018;7:396–101. 10.7598/cst2018.1502.Search in Google Scholar
[11] Arafa WAA. Sustainable catalytic process with a high eco-scale score for the synthesis of novel series of bischalcones through claisen–schmidt condensation. J Heterocycl Chem. 2018;55:456–4. 10.1002/jhet.3063.Search in Google Scholar
[12] Polo E, Ibarra-Arellano N, Prent-Peñaloza L, Morales-Bayuelo A, Henao JGaldámez A, Gutiérrez M. Ultrasound-assisted synthesis of novel chalcone, heterochalcone and bis-chalcone derivatives and the evaluation of their antioxidant properties and as acetylcholinesterase inhibitors. Bioorg Chem. 2019;90:103034. 10.1016/j.bioorg.2019.103034.Search in Google Scholar PubMed
[13] Kannan D, Naveen S, Jagadeesan G, Lokanath NK, Thennarasu S. Ultrasonic cavitation facilitates rapid synthesis of trisubstituted pyrazole scaffolds through michael addition domino cyclization. ChemistrySelect. 2019;4:9807–10. 10.1002/slct.201902126.Search in Google Scholar
[14] Adole VA, Jagdale BS, Pawar TB, Sagane AA. Ultrasound promoted stereoselective synthesis of 2,3-dihydrobenzofuran appended chalcones at ambient temperature. S Afr J Chem. 2020;73:35–43. 10.17159/0379-4350/2020/v73a6.Search in Google Scholar
[15] Kakade GK, Vedpathak SG. Ultrasound assisted green synthesis of 2-furan-2-yl-4h- chromen-4-ones from chalcones. Int J Curr Pharm Res. 2020;12:84–6. 10.22159/ijcpr.2020v12i3.38312.Search in Google Scholar
[16] Villena J, Montenegro I, Said B, Werner E, Flores S, Madrid A. Ultrasound assisted synthesis and cytotoxicity evaluation of known 2’,4’-dihydroxychalcone derivatives against cancer cell lines. Food Chem Toxicol. 2021;148:111969. 10.1016/j.fct.2021.111969.Search in Google Scholar PubMed
[17] Sahoo BM, Sahoo B, Panda J, Kumar A. Microwave-Induced Synthesis of Substituted Isoxazoles as Potential Antimicrobial Agents. Curr Microw Chem. 2017;4:146–51. 10.2174/2213335603666160926101734.Search in Google Scholar
[18] Rahim A, Bhuiyan MMH, Matin MM, Ali R, Kabir E. Synthesis of 2-Phenylchromen-4-one derivatives by conventional and microwave: Assisted techniques, and their antimicrobial evaluation. Int J Chem Stud. 2018;6:1644–7, https://www.chemijournal.com/archives/2018/vol6issue1/PartX/6-1-57-739.pdf.Search in Google Scholar
[19] Kalluraya B, Mallya S, Kumar A. Microwave assisted neat synthesis of spiropyrrolidine library. J Heterocycl Chem. 2018;55:2075–81. 10.1002/jhet.3247.Search in Google Scholar
[20] Jasril J, Teruna HY, Aisyah A, Nurlaili N, Hendra R. Microwave assisted synthesis and evaluation of toxicity and antioxidant activity of pyrazoline derivatives. Indones J Chem. 2019;19:583–91. 10.22146/ijc.34285.Search in Google Scholar
[21] Rocha DHA, Vaz PAAM, Pinto DCGA, Silva AMS. Synthesis chalones and their isomerization into flavanones and azaflavanones. Methods Protoc. 2019;2:70. 10.3390/mps2030070.Search in Google Scholar PubMed PubMed Central
[22] Tupare SD, Pawar RP. Synthesis, characterization and biological evaluation of newer chalcones by microwave irradiation. Chem J. 2020;7:150–62.Search in Google Scholar
[23] Chan CK, Lai CY, Wang CC. Environmentally friendly nafion-mediated friedländer quinoline synthesis under microwave irradiation: application to one-pot synthesis of substituted quinolinyl chalcones. Synthesis. 2020;52:1779–94. 10.1055/s-0039-1690088.Search in Google Scholar
[24] Li J, Li D, Xu Y, Guo Z, Liu X, Yang H, et al. Design, synthesis, biological evaluation, and molecular docking of chalcone derivatives as anti-inflammatory agents. Bioorg Med Chem Lett. 2017;27:602–6. 10.1016/j.bmcl.2016.12.008.Search in Google Scholar PubMed
[25] Mahapatra DK, Bharti SK, Asati V. Chalcone derivatives: anti-inflammatory potential and molecular targets perspectives. Curr Top Med Chem. 2017;17:3146–69. 10.2174/1568026617666170914160446.Search in Google Scholar PubMed
[26] Sayed M, Adel M, Kamal ED, Ahmed M, Hassanien R. Synthesis, characterization, and screening for anti-inflammatory and antimicrobial activity of novel indolyl chalcone derivatives. J Heterocycl Chem. 2018;55:1166–75. 10.1002/jhet.3149.Search in Google Scholar
[27] Reddy AK, Kathale NE. Synthesis, characterization and antiinflammatory activity of chalcone derivatives linked with apocynin and 5-nitrofuran moiety. Asian J Chem. 2018;30:312–6. 10.14233/ajchem.2018.20950.Search in Google Scholar
[28] Fu ZY, Jin QH, Qu YL, Guan LP. Chalcone derivatives bearing chromen or benzo[f]chromen moieties: Design, synthesis, and evaluations of anti-inflammatory, analgesic, selective COX-2 inhibitory activities. Bioorg Med Chem Lett. 2019;29:1909–12. 10.1016/j.bmcl.2019.05.051.Search in Google Scholar PubMed
[29] Gan FF, Zhang R, Ng HL, Karuppasamy M, Seah W, Yeap WH, et al. Novel dual-targeting anti-proliferative dihydrotriazine-chalcone derivatives display suppression of cancer cell invasion and inflammation by inhibiting the NF-κB signaling pathway. Food Chem Toxicol. 2018;116:238–48. 10.1016/j.fct.2018.04.003.Search in Google Scholar PubMed
[30] Khanapure S, Jagadale M, Bansode P, Choudhari P, Rashinkar G. Anticancer activity of ruthenocenyl chalcones and their molecular docking studies. J Mol Struct. 2018;1173:142–7. 10.1016/j.molstruc.2018.06.091.Search in Google Scholar
[31] Mohamed MF, Hassaneen HM, Abdelhamid IA. Cytotoxicity, molecular modeling, cell cycle arrest, and apoptotic induction induced by novel tetrahydro-[1,2,4]triazolo[3,4-a]isoquinoline chalcones. Eur J Med Chem. 2018;143:532–41. 10.1016/j.ejmech.2017.11.045.Search in Google Scholar PubMed
[32] Hsieh CY, Ko PW, Chang YJ, Kapoor M, Liang YC, Lin HH, et al. Design and synthesis of benzimidazole-chalcone derivatives as potential anticancer agents. Molecules. 2019;24:3259–77. 10.3390/molecules24183259.Search in Google Scholar PubMed PubMed Central
[33] Kurt BZ, Kandas NO, Dag A, Sonmez F, Kucukislamoglu M. Synthesis and biological evaluation of novel coumarin-chalcone derivatives containing urea moiety as potential anticancer agents. Arab J Chem. 2020;13:1120–9. 10.1016/j.arabjc.2017.10.001.Search in Google Scholar
[34] Ouyang Y, Li J, Chen X, Fu X, Sun S, Wu Q. Chalcone derivatives: role in anticancer therapy. Biomolecules. 2021;11:894. 10.3390/biom11060894.Search in Google Scholar PubMed PubMed Central
[35] Pingaew R, Saekee A, Mandi P, Nantasenamat C, Prachayasittikul S, Ruchirawat S, et al. Synthesis, biological evaluation and molecular docking of novel chalcone-coumarin hybrids as anticancer and antimalarial agents. Eur J Med Chem. 2014;85:65–76. 10.1016/j.ejmech.2014.07.087.Search in Google Scholar PubMed
[36] Syahri J, Nasution H, Nurohmah BA, Purwono B, Yuanita E. Novel aminoalkylated chalcone: Synthesis, biological evaluation, and docking simulation as potent antimalarial agents. J Appl Pharm Sci. 2020;10:001–5. 10.7324/JAPS.2020.10601.Search in Google Scholar
[37] Wan Z, Hu D, Li P, Xie D, Gan X. Synthesis, antiviral bioactivity of novel 4-thioquinazoline derivatives containing chalcone moiety. Molecules. 2015;20:11861–74. 10.3390/molecules200711861.Search in Google Scholar PubMed PubMed Central
[38] Duran N, Polat MF, Aktas DA, Alagoz MA, Ay E, Cimen F, et al. New chalcone derivatives as effective against SARS-CoV-2 agent. Int J Clin Pract. 2021;75:001–43. 10.1111/ijcp.14846.Search in Google Scholar PubMed PubMed Central
[39] Lal K, Yadav P, Kumar A, Kumar A, Paul AK. Design, synthesis, characterization, antimicrobial evaluation and molecular modeling studies of some dehydroacetic acid-chalcone-1,2,3-triazole hybrids. Bioorg Chem. 2018;77:236–44. 10.1016/j.bioorg.2018.01.016.Search in Google Scholar PubMed
[40] El-Messery SM, Habib ESE, Al-Rashood STA, Hassan GS. Synthesis, antimicrobial, anti-biofilm evaluation, and molecular modelling study of new chalcone linked amines derivatives. J Enzyme Inhib Med Chem. 2018;33:818–32. 10.1080/14756366.2018.1461855.Search in Google Scholar PubMed PubMed Central
[41] Xu M, Wu P, Shen F, Ji J, Rakesh KP. Chalcone derivatives and their antibacterial activities: Current development. Bioorg Chem. 2019;19:1–17. 10.1016/j.bioorg.2019.103133.Search in Google Scholar PubMed
[42] Benouda H, Bouchal B, Challioui A, Oulmidi A, Harit T, Malek F, et al. Synthesis of a series of chalcones and related flavones and evaluation of their antibacterial and antifungal activities. Lett Drug Des Discov. 2019;16:93–100. 10.2174/1570180815666180404130430.Search in Google Scholar
[43] Kumar C, Loh WS, Ooi C, Quah C, Fun HK. Structural correlation of some heterocyclic chalcone analogues and evaluation of their antioxidant potential. Molecules. 2013;18:11996–2011. 10.3390/molecules181011996.Search in Google Scholar PubMed PubMed Central
[44] Murti Y, Goswam A, Mishra P. Synthesis and antioxidant activity of some chalcones and flavonoids. Int J Pharm Tech Res. 2013;5:811–8, https://www.researchgate.net/profile/Yogesh-Murti/publication/267035890_Synthesis_and_antioxidant_activity_of_some_chalcones_and_flavanoids/links/578728f908ae3949cf556c9b/Synthesis-and-antioxidant-activity-of-some-chalcones-and-flavanoids.pdf.Search in Google Scholar
[45] Janković T, Turković N, Kotur-Stevuljević J, Vujić Z, Ivković B. Differences in antioxidant potential of chalcones in human serum: In vitro study. Chem Biol Interact. 2020;324:109084. 10.1016/j.cbi.2020.109084.Search in Google Scholar PubMed
[46] Al Zahrani NA, El-Shishtawy RM, Elaasser MM, Asiri AM. Synthesis of novel chalcone-based phenothiazine derivatives as antioxidant and anticancer agents. Molecules. 2020;25:4566–81. 10.3390/molecules25194566.Search in Google Scholar PubMed PubMed Central
[47] Hsieh CT, Hsieh TJ, El-Shazly M, Chuang DW, Tsai YH, Yen CT, et al. Synthesis of chalcone derivatives as potential anti-diabetic agents. Bioorg Med Chem Lett. 2012;22:3912–5. 10.1016/j.bmcl.2012.04.108.Search in Google Scholar PubMed
[48] Shukla P, Satyanarayana M, Verma PC, Tiwari J, Dwivedi AP, Srivastava R, et al. Chalcone-based aryloxypropanolamine as a potential antidiabetic and antidyslipidaemic agent. Curr Sci. 2017;112:1675–89. 10.18520/cs/v112/i08/1675-1689.Search in Google Scholar
[49] Balu P, Jas JS, Govindaraj M. Design and evaluation of chalconeimine derivatives as α-amylase inhibitors. Bioinformation. 2019;15:523–9. 10.6026/97320630015523.Search in Google Scholar PubMed PubMed Central
[50] Sharma CS, Shekhawat KS, Chauhan CS, Kumar N. Synthesis and anticonvulsant activity of some chalcone derivatives. J Chem Pharm Res. 2013;5:450–4, https://www.jocpr.com/articles/synthesis-and-anticonvulsant-activity-of-some-chalcone-derivatives.pdf.Search in Google Scholar
[51] Bukhari SN, Butt AM, Amjad MW, Ahmad W, Shah VH, Trivedi AR. Synthesis and evaluation of chalcone analogues based pyrimidines as angiotensin converting enzyme inhibitors. Pak J Biol Sci. 2013;16:1368–72. 10.3923/pjbs.2013.1368.1372.Search in Google Scholar PubMed
[52] Kumar H, Devaraji V, Joshi R, Jadhao M, Ahirkar P, Prasath R, et al. Antihypertensive activity of a quinoline appended chalcone derivative and its site specific binding interaction with a relevant target carrier protein. RSC Adv. 2015;5:65496–513. 10.1039/C5RA08778C.Search in Google Scholar
[53] Insuasty B, Ramírez J, Becerra D, Echeverry C, Quiroga J, Abonia R, et al. An efficient synthesis of new caffeine-based chalcones, pyrazolines and pyrazolo[3,4-b][1,4]diazepines as potential antimalarial, antitrypanosomal and antileishmanial agents. Eur J Med Chem. 2015;93:401–13. 10.1016/j.ejmech.2015.02.040.Search in Google Scholar PubMed
[54] Pozzetti L, Ibba R, Rossi S, Taglialatela-Scafati O, Taramelli D, Basilico N, et al. Total synthesis of the natural chalcone lophirone E, synthetic studies toward benzofuran and indole-based analogues, and investigation of anti-leishmanial activity. Molecules. 2022;27:463. 10.3390/molecules27020463.Search in Google Scholar PubMed PubMed Central
[55] Padaratz P, Fracasso M, De Campos-Buzzi F, Corrêa R, Niero R, Monache FD, et al. Antinociceptive activity of a new benzofuranone derived from a chalcone. Basic Clin Pharmacol Toxicol. 2009;105:257–61. 10.1111/j.1742-7843.2009.00441x.Search in Google Scholar
[56] Thapa P, Upadhyay SP, Suo WZ, Singh V, Gurung P, Lee ES, et al. Chalcone and its analogs: Therapeutic and diagnostic applications in Alzheimer’s disease. Bioorg Chem. 2021;108:104681. 10.1016/j.bioorg.2021.104681.Search in Google Scholar PubMed PubMed Central
[57] Soozani A, Keivanloo A, Bakherad M. One-pot synthesis of quinoxaline chalcones from commercially available calcium carbide through palladium-catalyzed coupling reactions. ChemistrySelect. 2017;2:9701–5. 10.1002/slct.201701803.Search in Google Scholar
[58] Nakamura A, Tanaka S, Imamiya A, Takane R, Ohta C, Fujimura K, et al. Synthesis of 3-acylindoles by oxidative rearrangement of 2-aminochalcones using a hypervalent iodine reagent and cyclization sequence. Org Biomol Chem. 2017;15:6702–5. 10.1039/c7ob01536d.Search in Google Scholar PubMed
[59] Aquino TFB, Seidel JP, Oliveira DH, Nascimento JER, Alves D, Perin G, et al. Copper-catalyzed synthesis of 1,3,5-triaryl-4-(organylselanyl)-1H-pyrazoles by one-pot multicomponent reactions. Tetrahedron Lett. 2018;59:4090–5. 10.1016/j.tetlet.2018.10.008.Search in Google Scholar
[60] Khalili D, Lavian S, Moayyed M. Graphene oxide as a catalyst for one-pot sequential aldol coupling/aza-Michael addition of amines to chalcones through in situ generation of Michael acceptors under neat conditions. Tetrahedron Lett. 2020;61:151470. 10.1016/j.tetlet.2019.151470.Search in Google Scholar
[61] Dasari GK, Sunkara S, Gadupudi PCR. Green and ecofriendly synthesis of indole-condensed benzimidazole chalcones in water and their antimicrobial evaluations. J Heterocycl Chem. 2020;57:1201–10. 10.1002/jhet.3856.Search in Google Scholar
[62] Ahmed EA, Soliman AMM, Ali AM, El-Remaily MAEAAA. Boosting the catalytic performance of zinc linked amino acid complex as an eco-friendly for synthesis of novel pyrimidines in aqueous medium. Appl Organomet Chem. 2021;35:e6197. 10.1002/aoc.6197.Search in Google Scholar
[63] Nguyen TT, Phan NTS, Tran CTH, Tran QD, Ly D, Nguyen TT. A One-pot synthesis of disubstituted thiazoles from chalcone c–h bonds, elemental sulfur, and glycine ethyl ester. Synlett. 2022;33:555–8. 10.1055/s-0041-1737899.Search in Google Scholar
[64] Daina A, Michielin O, Zoete V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci Rep. 2017;7:42717. 10.1038/srep42717.Search in Google Scholar PubMed PubMed Central
[65] Xiong G, Wu Z, Yi J, Fu L, Yang Z, Hsieh C, et al. ADMETlab 2.0: An integrated online platform for accurate and comprehensive predictions of ADMET properties. Nucleic Acids Res. 2021;49:5–14. 10.1093/nar/gkab255.Search in Google Scholar PubMed PubMed Central
[66] Filimonov DA, Lagunin AA, Gloriozova TA, Rudik AV, Druzhilovskii DS, Pogodin PV, et al. Prediction of the biological activity spectra of organic compounds using the pass online web resource. Chem Heterocycl Comp. 2014;50:444–57. 10.1007/s10593-014-1496-1.Search in Google Scholar
© 2024 the author(s), published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Research Articles
- Green polymer electrolyte and activated charcoal-based supercapacitor for energy harvesting application: Electrochemical characteristics
- Research on the adsorption of Co2+ ions using halloysite clay and the ability to recover them by electrodeposition method
- Simultaneous estimation of ibuprofen, caffeine, and paracetamol in commercial products using a green reverse-phase HPTLC method
- Isolation, screening and optimization of alkaliphilic cellulolytic fungi for production of cellulase
- Functionalized gold nanoparticles coated with bacterial alginate and their antibacterial and anticancer activities
- Comparative analysis of bio-based amino acid surfactants obtained via Diels–Alder reaction of cyclic anhydrides
- Biosynthesis of silver nanoparticles on yellow phosphorus slag and its application in organic coatings
- Exploring antioxidant potential and phenolic compound extraction from Vitis vinifera L. using ultrasound-assisted extraction
- Manganese and copper-coated nickel oxide nanoparticles synthesized from Carica papaya leaf extract induce antimicrobial activity and breast cancer cell death by triggering mitochondrial caspases and p53
- Insight into heating method and Mozafari method as green processing techniques for the synthesis of micro- and nano-drug carriers
- Silicotungstic acid supported on Bi-based MOF-derived metal oxide for photodegradation of organic dyes
- Synthesis and characterization of capsaicin nanoparticles: An attempt to enhance its bioavailability and pharmacological actions
- Synthesis of Lawsonia inermis-encased silver–copper bimetallic nanoparticles with antioxidant, antibacterial, and cytotoxic activity
- Facile, polyherbal drug-mediated green synthesis of CuO nanoparticles and their potent biological applications
- Zinc oxide-manganese oxide/carboxymethyl cellulose-folic acid-sesamol hybrid nanomaterials: A molecularly targeted strategy for advanced triple-negative breast cancer therapy
- Exploring the antimicrobial potential of biogenically synthesized graphene oxide nanoparticles against targeted bacterial and fungal pathogens
- Biofabrication of silver nanoparticles using Uncaria tomentosa L.: Insight into characterization, antibacterial activities combined with antibiotics, and effect on Triticum aestivum germination
- Membrane distillation of synthetic urine for use in space structural habitat systems
- Investigation on mechanical properties of the green synthesis bamboo fiber/eggshell/coconut shell powder-based hybrid biocomposites under NaOH conditions
- Green synthesis of magnesium oxide nanoparticles using endophytic fungal strain to improve the growth, metabolic activities, yield traits, and phenolic compounds content of Nigella sativa L.
- Estimation of greenhouse gas emissions from rice and annual upland crops in Red River Delta of Vietnam using the denitrification–decomposition model
- Synthesis of humic acid with the obtaining of potassium humate based on coal waste from the Lenger deposit, Kazakhstan
- Ascorbic acid-mediated selenium nanoparticles as potential antihyperuricemic, antioxidant, anticoagulant, and thrombolytic agents
- Green synthesis of silver nanoparticles using Illicium verum extract: Optimization and characterization for biomedical applications
- Antibacterial and dynamical behaviour of silicon nanoparticles influenced sustainable waste flax fibre-reinforced epoxy composite for biomedical application
- Optimising coagulation/flocculation using response surface methodology and application of floc in biofertilisation
- Green synthesis and multifaceted characterization of iron oxide nanoparticles derived from Senna bicapsularis for enhanced in vitro and in vivo biological investigation
- Potent antibacterial nanocomposites from okra mucilage/chitosan/silver nanoparticles for multidrug-resistant Salmonella Typhimurium eradication
- Trachyspermum copticum aqueous seed extract-derived silver nanoparticles: Exploration of their structural characterization and comparative antibacterial performance against gram-positive and gram-negative bacteria
- Microwave-assisted ultrafine silver nanoparticle synthesis using Mitragyna speciosa for antimalarial applications
- Green synthesis and characterisation of spherical structure Ag/Fe2O3/TiO2 nanocomposite using acacia in the presence of neem and tulsi oils
- Green quantitative methods for linagliptin and empagliflozin in dosage forms
- Enhancement efficacy of omeprazole by conjugation with silver nanoparticles as a urease inhibitor
- Residual, sequential extraction, and ecological risk assessment of some metals in ash from municipal solid waste incineration, Vietnam
- Green synthesis of ZnO nanoparticles using the mangosteen (Garcinia mangostana L.) leaf extract: Comparative preliminary in vitro antibacterial study
- Simultaneous determination of lesinurad and febuxostat in commercial fixed-dose combinations using a greener normal-phase HPTLC method
- A greener RP-HPLC method for quaternary estimation of caffeine, paracetamol, levocetirizine, and phenylephrine acquiring AQbD with stability studies
- Optimization of biomass durian peel as a heterogeneous catalyst in biodiesel production using microwave irradiation
- Thermal treatment impact on the evolution of active phases in layered double hydroxide-based ZnCr photocatalysts: Photodegradation and antibacterial performance
- Preparation of silymarin-loaded zein polysaccharide core–shell nanostructures and evaluation of their biological potentials
- Preparation and characterization of composite-modified PA6 fiber for spectral heating and heat storage applications
- Preparation and electrocatalytic oxygen evolution of bimetallic phosphates (NiFe)2P/NF
- Rod-shaped Mo(vi) trichalcogenide–Mo(vi) oxide decorated on poly(1-H pyrrole) as a promising nanocomposite photoelectrode for green hydrogen generation from sewage water with high efficiency
- Green synthesis and studies on citrus medica leaf extract-mediated Au–ZnO nanocomposites: A sustainable approach for efficient photocatalytic degradation of rhodamine B dye in aqueous media
- Cellulosic materials for the removal of ciprofloxacin from aqueous environments
- The analytical assessment of metal contamination in industrial soils of Saudi Arabia using the inductively coupled plasma technology
- The effect of modified oily sludge on the slurry ability and combustion performance of coal water slurry
- Eggshell waste transformation to calcium chloride anhydride as food-grade additive and eggshell membranes as enzyme immobilization carrier
- Synthesis of EPAN and applications in the encapsulation of potassium humate
- Biosynthesis and characterization of silver nanoparticles from Cedrela toona leaf extracts: An exploration into their antibacterial, anticancer, and antioxidant potential
- Enhancing mechanical and rheological properties of HDPE films through annealing for eco-friendly agricultural applications
- Immobilisation of catalase purified from mushroom (Hydnum repandum) onto glutaraldehyde-activated chitosan and characterisation: Its application for the removal of hydrogen peroxide from artificial wastewater
- Sodium titanium oxide/zinc oxide (STO/ZnO) photocomposites for efficient dye degradation applications
- Effect of ex situ, eco-friendly ZnONPs incorporating green synthesised Moringa oleifera leaf extract in enhancing biochemical and molecular aspects of Vicia faba L. under salt stress
- Biosynthesis and characterization of selenium and silver nanoparticles using Trichoderma viride filtrate and their impact on Culex pipiens
- Photocatalytic degradation of organic dyes and biological potentials of biogenic zinc oxide nanoparticles synthesized using the polar extract of Cyperus scariosus R.Br. (Cyperaceae)
- Assessment of antiproliferative activity of green-synthesized nickel oxide nanoparticles against glioblastoma cells using Terminalia chebula
- Chlorine-free synthesis of phosphinic derivatives by change in the P-function
- Anticancer, antioxidant, and antimicrobial activities of nanoemulsions based on water-in-olive oil and loaded on biogenic silver nanoparticles
- Study and mechanism of formation of phosphorus production waste in Kazakhstan
- Synthesis and stabilization of anatase form of biomimetic TiO2 nanoparticles for enhancing anti-tumor potential
- Microwave-supported one-pot reaction for the synthesis of 5-alkyl/arylidene-2-(morpholin/thiomorpholin-4-yl)-1,3-thiazol-4(5H)-one derivatives over MgO solid base
- Screening the phytochemicals in Perilla leaves and phytosynthesis of bioactive silver nanoparticles for potential antioxidant and wound-healing application
- Graphene oxide/chitosan/manganese/folic acid-brucine functionalized nanocomposites show anticancer activity against liver cancer cells
- Nature of serpentinite interactions with low-concentration sulfuric acid solutions
- Multi-objective statistical optimisation utilising response surface methodology to predict engine performance using biofuels from waste plastic oil in CRDi engines
- Microwave-assisted extraction of acetosolv lignin from sugarcane bagasse and electrospinning of lignin/PEO nanofibres for carbon fibre production
- Biosynthesis, characterization, and investigation of cytotoxic activities of selenium nanoparticles utilizing Limosilactobacillus fermentum
- Highly photocatalytic materials based on the decoration of poly(O-chloroaniline) with molybdenum trichalcogenide oxide for green hydrogen generation from Red Sea water
- Highly efficient oil–water separation using superhydrophobic cellulose aerogels derived from corn straw
- Beta-cyclodextrin–Phyllanthus emblica emulsion for zinc oxide nanoparticles: Characteristics and photocatalysis
- Assessment of antimicrobial activity and methyl orange dye removal by Klebsiella pneumoniae-mediated silver nanoparticles
- Influential eradication of resistant Salmonella Typhimurium using bioactive nanocomposites from chitosan and radish seed-synthesized nanoselenium
- Antimicrobial activities and neuroprotective potential for Alzheimer’s disease of pure, Mn, Co, and Al-doped ZnO ultra-small nanoparticles
- Green synthesis of silver nanoparticles from Bauhinia variegata and their biological applications
- Synthesis and optimization of long-chain fatty acids via the oxidation of long-chain fatty alcohols
- Eminent Red Sea water hydrogen generation via a Pb(ii)-iodide/poly(1H-pyrrole) nanocomposite photocathode
- Green synthesis and effective genistein production by fungal β-glucosidase immobilized on Al2O3 nanocrystals synthesized in Cajanus cajan L. (Millsp.) leaf extracts
- Green stability-indicating RP-HPTLC technique for determining croconazole hydrochloride
- Green synthesis of La2O3–LaPO4 nanocomposites using Charybdis natator for DNA binding, cytotoxic, catalytic, and luminescence applications
- Eco-friendly drugs induce cellular changes in colistin-resistant bacteria
- Tangerine fruit peel extract mediated biogenic synthesized silver nanoparticles and their potential antimicrobial, antioxidant, and cytotoxic assessments
- Green synthesis on performance characteristics of a direct injection diesel engine using sandbox seed oil
- A highly sensitive β-AKBA-Ag-based fluorescent “turn off” chemosensor for rapid detection of abamectin in tomatoes
- Green synthesis and physical characterization of zinc oxide nanoparticles (ZnO NPs) derived from the methanol extract of Euphorbia dracunculoides Lam. (Euphorbiaceae) with enhanced biosafe applications
- Detection of morphine and data processing using surface plasmon resonance imaging sensor
- Effects of nanoparticles on the anaerobic digestion properties of sulfamethoxazole-containing chicken manure and analysis of bio-enzymes
- Bromic acid-thiourea synergistic leaching of sulfide gold ore
- Green chemistry approach to synthesize titanium dioxide nanoparticles using Fagonia Cretica extract, novel strategy for developing antimicrobial and antidiabetic therapies
- Green synthesis and effective utilization of biogenic Al2O3-nanocoupled fungal lipase in the resolution of active homochiral 2-octanol and its immobilization via aluminium oxide nanoparticles
- Eco-friendly RP-HPLC approach for simultaneously estimating the promising combination of pentoxifylline and simvastatin in therapeutic potential for breast cancer: Appraisal of greenness, whiteness, and Box–Behnken design
- Use of a humidity adsorbent derived from cockleshell waste in Thai fried fish crackers (Keropok)
- One-pot green synthesis, biological evaluation, and in silico study of pyrazole derivatives obtained from chalcones
- Bio-sorption of methylene blue and production of biofuel by brown alga Cystoseira sp. collected from Neom region, Kingdom of Saudi Arabia
- Synthesis of motexafin gadolinium: A promising radiosensitizer and imaging agent for cancer therapy
- The impact of varying sizes of silver nanoparticles on the induction of cellular damage in Klebsiella pneumoniae involving diverse mechanisms
- Microwave-assisted green synthesis, characterization, and in vitro antibacterial activity of NiO nanoparticles obtained from lemon peel extract
- Rhus microphylla-mediated biosynthesis of copper oxide nanoparticles for enhanced antibacterial and antibiofilm efficacy
- Harnessing trichalcogenide–molybdenum(vi) sulfide and molybdenum(vi) oxide within poly(1-amino-2-mercaptobenzene) frameworks as a photocathode for sustainable green hydrogen production from seawater without sacrificial agents
- Magnetically recyclable Fe3O4@SiO2 supported phosphonium ionic liquids for efficient and sustainable transformation of CO2 into oxazolidinones
- A comparative study of Fagonia arabica fabricated silver sulfide nanoparticles (Ag2S) and silver nanoparticles (AgNPs) with distinct antimicrobial, anticancer, and antioxidant properties
- Visible light photocatalytic degradation and biological activities of Aegle marmelos-mediated cerium oxide nanoparticles
- Physical intrinsic characteristics of spheroidal particles in coal gasification fine slag
- Exploring the effect of tea dust magnetic biochar on agricultural crops grown in polycyclic aromatic hydrocarbon contaminated soil
- Crosslinked chitosan-modified ultrafiltration membranes for efficient surface water treatment and enhanced anti-fouling performances
- Study on adsorption characteristics of biochars and their modified biochars for removal of organic dyes from aqueous solution
- Zein polymer nanocarrier for Ocimum basilicum var. purpurascens extract: Potential biomedical use
- Green synthesis, characterization, and in vitro and in vivo biological screening of iron oxide nanoparticles (Fe3O4) generated with hydroalcoholic extract of aerial parts of Euphorbia milii
- Novel microwave-based green approach for the synthesis of dual-loaded cyclodextrin nanosponges: Characterization, pharmacodynamics, and pharmacokinetics evaluation
- Bi2O3–BiOCl/poly-m-methyl aniline nanocomposite thin film for broad-spectrum light-sensing
- Green synthesis and characterization of CuO/ZnO nanocomposite using Musa acuminata leaf extract for cytotoxic studies on colorectal cancer cells (HCC2998)
- Review Articles
- Materials-based drug delivery approaches: Recent advances and future perspectives
- A review of thermal treatment for bamboo and its composites
- An overview of the role of nanoherbicides in tackling challenges of weed management in wheat: A novel approach
- An updated review on carbon nanomaterials: Types, synthesis, functionalization and applications, degradation and toxicity
- Special Issue: Emerging green nanomaterials for sustainable waste management and biomedical applications
- Green synthesis of silver nanoparticles using mature-pseudostem extracts of Alpinia nigra and their bioactivities
- Special Issue: New insights into nanopythotechnology: current trends and future prospects
- Green synthesis of FeO nanoparticles from coffee and its application for antibacterial, antifungal, and anti-oxidation activity
- Dye degradation activity of biogenically synthesized Cu/Fe/Ag trimetallic nanoparticles
- Special Issue: Composites and green composites
- Recent trends and advancements in the utilization of green composites and polymeric nanocarriers for enhancing food quality and sustainable processing
- Retraction
- Retraction of “Biosynthesis and characterization of silver nanoparticles from Cedrela toona leaf extracts: An exploration into their antibacterial, anticancer, and antioxidant potential”
- Retraction of “Photocatalytic degradation of organic dyes and biological potentials of biogenic zinc oxide nanoparticles synthesized using the polar extract of Cyperus scariosus R.Br. (Cyperaceae)”
- Retraction to “Green synthesis on performance characteristics of a direct injection diesel engine using sandbox seed oil”
Articles in the same Issue
- Research Articles
- Green polymer electrolyte and activated charcoal-based supercapacitor for energy harvesting application: Electrochemical characteristics
- Research on the adsorption of Co2+ ions using halloysite clay and the ability to recover them by electrodeposition method
- Simultaneous estimation of ibuprofen, caffeine, and paracetamol in commercial products using a green reverse-phase HPTLC method
- Isolation, screening and optimization of alkaliphilic cellulolytic fungi for production of cellulase
- Functionalized gold nanoparticles coated with bacterial alginate and their antibacterial and anticancer activities
- Comparative analysis of bio-based amino acid surfactants obtained via Diels–Alder reaction of cyclic anhydrides
- Biosynthesis of silver nanoparticles on yellow phosphorus slag and its application in organic coatings
- Exploring antioxidant potential and phenolic compound extraction from Vitis vinifera L. using ultrasound-assisted extraction
- Manganese and copper-coated nickel oxide nanoparticles synthesized from Carica papaya leaf extract induce antimicrobial activity and breast cancer cell death by triggering mitochondrial caspases and p53
- Insight into heating method and Mozafari method as green processing techniques for the synthesis of micro- and nano-drug carriers
- Silicotungstic acid supported on Bi-based MOF-derived metal oxide for photodegradation of organic dyes
- Synthesis and characterization of capsaicin nanoparticles: An attempt to enhance its bioavailability and pharmacological actions
- Synthesis of Lawsonia inermis-encased silver–copper bimetallic nanoparticles with antioxidant, antibacterial, and cytotoxic activity
- Facile, polyherbal drug-mediated green synthesis of CuO nanoparticles and their potent biological applications
- Zinc oxide-manganese oxide/carboxymethyl cellulose-folic acid-sesamol hybrid nanomaterials: A molecularly targeted strategy for advanced triple-negative breast cancer therapy
- Exploring the antimicrobial potential of biogenically synthesized graphene oxide nanoparticles against targeted bacterial and fungal pathogens
- Biofabrication of silver nanoparticles using Uncaria tomentosa L.: Insight into characterization, antibacterial activities combined with antibiotics, and effect on Triticum aestivum germination
- Membrane distillation of synthetic urine for use in space structural habitat systems
- Investigation on mechanical properties of the green synthesis bamboo fiber/eggshell/coconut shell powder-based hybrid biocomposites under NaOH conditions
- Green synthesis of magnesium oxide nanoparticles using endophytic fungal strain to improve the growth, metabolic activities, yield traits, and phenolic compounds content of Nigella sativa L.
- Estimation of greenhouse gas emissions from rice and annual upland crops in Red River Delta of Vietnam using the denitrification–decomposition model
- Synthesis of humic acid with the obtaining of potassium humate based on coal waste from the Lenger deposit, Kazakhstan
- Ascorbic acid-mediated selenium nanoparticles as potential antihyperuricemic, antioxidant, anticoagulant, and thrombolytic agents
- Green synthesis of silver nanoparticles using Illicium verum extract: Optimization and characterization for biomedical applications
- Antibacterial and dynamical behaviour of silicon nanoparticles influenced sustainable waste flax fibre-reinforced epoxy composite for biomedical application
- Optimising coagulation/flocculation using response surface methodology and application of floc in biofertilisation
- Green synthesis and multifaceted characterization of iron oxide nanoparticles derived from Senna bicapsularis for enhanced in vitro and in vivo biological investigation
- Potent antibacterial nanocomposites from okra mucilage/chitosan/silver nanoparticles for multidrug-resistant Salmonella Typhimurium eradication
- Trachyspermum copticum aqueous seed extract-derived silver nanoparticles: Exploration of their structural characterization and comparative antibacterial performance against gram-positive and gram-negative bacteria
- Microwave-assisted ultrafine silver nanoparticle synthesis using Mitragyna speciosa for antimalarial applications
- Green synthesis and characterisation of spherical structure Ag/Fe2O3/TiO2 nanocomposite using acacia in the presence of neem and tulsi oils
- Green quantitative methods for linagliptin and empagliflozin in dosage forms
- Enhancement efficacy of omeprazole by conjugation with silver nanoparticles as a urease inhibitor
- Residual, sequential extraction, and ecological risk assessment of some metals in ash from municipal solid waste incineration, Vietnam
- Green synthesis of ZnO nanoparticles using the mangosteen (Garcinia mangostana L.) leaf extract: Comparative preliminary in vitro antibacterial study
- Simultaneous determination of lesinurad and febuxostat in commercial fixed-dose combinations using a greener normal-phase HPTLC method
- A greener RP-HPLC method for quaternary estimation of caffeine, paracetamol, levocetirizine, and phenylephrine acquiring AQbD with stability studies
- Optimization of biomass durian peel as a heterogeneous catalyst in biodiesel production using microwave irradiation
- Thermal treatment impact on the evolution of active phases in layered double hydroxide-based ZnCr photocatalysts: Photodegradation and antibacterial performance
- Preparation of silymarin-loaded zein polysaccharide core–shell nanostructures and evaluation of their biological potentials
- Preparation and characterization of composite-modified PA6 fiber for spectral heating and heat storage applications
- Preparation and electrocatalytic oxygen evolution of bimetallic phosphates (NiFe)2P/NF
- Rod-shaped Mo(vi) trichalcogenide–Mo(vi) oxide decorated on poly(1-H pyrrole) as a promising nanocomposite photoelectrode for green hydrogen generation from sewage water with high efficiency
- Green synthesis and studies on citrus medica leaf extract-mediated Au–ZnO nanocomposites: A sustainable approach for efficient photocatalytic degradation of rhodamine B dye in aqueous media
- Cellulosic materials for the removal of ciprofloxacin from aqueous environments
- The analytical assessment of metal contamination in industrial soils of Saudi Arabia using the inductively coupled plasma technology
- The effect of modified oily sludge on the slurry ability and combustion performance of coal water slurry
- Eggshell waste transformation to calcium chloride anhydride as food-grade additive and eggshell membranes as enzyme immobilization carrier
- Synthesis of EPAN and applications in the encapsulation of potassium humate
- Biosynthesis and characterization of silver nanoparticles from Cedrela toona leaf extracts: An exploration into their antibacterial, anticancer, and antioxidant potential
- Enhancing mechanical and rheological properties of HDPE films through annealing for eco-friendly agricultural applications
- Immobilisation of catalase purified from mushroom (Hydnum repandum) onto glutaraldehyde-activated chitosan and characterisation: Its application for the removal of hydrogen peroxide from artificial wastewater
- Sodium titanium oxide/zinc oxide (STO/ZnO) photocomposites for efficient dye degradation applications
- Effect of ex situ, eco-friendly ZnONPs incorporating green synthesised Moringa oleifera leaf extract in enhancing biochemical and molecular aspects of Vicia faba L. under salt stress
- Biosynthesis and characterization of selenium and silver nanoparticles using Trichoderma viride filtrate and their impact on Culex pipiens
- Photocatalytic degradation of organic dyes and biological potentials of biogenic zinc oxide nanoparticles synthesized using the polar extract of Cyperus scariosus R.Br. (Cyperaceae)
- Assessment of antiproliferative activity of green-synthesized nickel oxide nanoparticles against glioblastoma cells using Terminalia chebula
- Chlorine-free synthesis of phosphinic derivatives by change in the P-function
- Anticancer, antioxidant, and antimicrobial activities of nanoemulsions based on water-in-olive oil and loaded on biogenic silver nanoparticles
- Study and mechanism of formation of phosphorus production waste in Kazakhstan
- Synthesis and stabilization of anatase form of biomimetic TiO2 nanoparticles for enhancing anti-tumor potential
- Microwave-supported one-pot reaction for the synthesis of 5-alkyl/arylidene-2-(morpholin/thiomorpholin-4-yl)-1,3-thiazol-4(5H)-one derivatives over MgO solid base
- Screening the phytochemicals in Perilla leaves and phytosynthesis of bioactive silver nanoparticles for potential antioxidant and wound-healing application
- Graphene oxide/chitosan/manganese/folic acid-brucine functionalized nanocomposites show anticancer activity against liver cancer cells
- Nature of serpentinite interactions with low-concentration sulfuric acid solutions
- Multi-objective statistical optimisation utilising response surface methodology to predict engine performance using biofuels from waste plastic oil in CRDi engines
- Microwave-assisted extraction of acetosolv lignin from sugarcane bagasse and electrospinning of lignin/PEO nanofibres for carbon fibre production
- Biosynthesis, characterization, and investigation of cytotoxic activities of selenium nanoparticles utilizing Limosilactobacillus fermentum
- Highly photocatalytic materials based on the decoration of poly(O-chloroaniline) with molybdenum trichalcogenide oxide for green hydrogen generation from Red Sea water
- Highly efficient oil–water separation using superhydrophobic cellulose aerogels derived from corn straw
- Beta-cyclodextrin–Phyllanthus emblica emulsion for zinc oxide nanoparticles: Characteristics and photocatalysis
- Assessment of antimicrobial activity and methyl orange dye removal by Klebsiella pneumoniae-mediated silver nanoparticles
- Influential eradication of resistant Salmonella Typhimurium using bioactive nanocomposites from chitosan and radish seed-synthesized nanoselenium
- Antimicrobial activities and neuroprotective potential for Alzheimer’s disease of pure, Mn, Co, and Al-doped ZnO ultra-small nanoparticles
- Green synthesis of silver nanoparticles from Bauhinia variegata and their biological applications
- Synthesis and optimization of long-chain fatty acids via the oxidation of long-chain fatty alcohols
- Eminent Red Sea water hydrogen generation via a Pb(ii)-iodide/poly(1H-pyrrole) nanocomposite photocathode
- Green synthesis and effective genistein production by fungal β-glucosidase immobilized on Al2O3 nanocrystals synthesized in Cajanus cajan L. (Millsp.) leaf extracts
- Green stability-indicating RP-HPTLC technique for determining croconazole hydrochloride
- Green synthesis of La2O3–LaPO4 nanocomposites using Charybdis natator for DNA binding, cytotoxic, catalytic, and luminescence applications
- Eco-friendly drugs induce cellular changes in colistin-resistant bacteria
- Tangerine fruit peel extract mediated biogenic synthesized silver nanoparticles and their potential antimicrobial, antioxidant, and cytotoxic assessments
- Green synthesis on performance characteristics of a direct injection diesel engine using sandbox seed oil
- A highly sensitive β-AKBA-Ag-based fluorescent “turn off” chemosensor for rapid detection of abamectin in tomatoes
- Green synthesis and physical characterization of zinc oxide nanoparticles (ZnO NPs) derived from the methanol extract of Euphorbia dracunculoides Lam. (Euphorbiaceae) with enhanced biosafe applications
- Detection of morphine and data processing using surface plasmon resonance imaging sensor
- Effects of nanoparticles on the anaerobic digestion properties of sulfamethoxazole-containing chicken manure and analysis of bio-enzymes
- Bromic acid-thiourea synergistic leaching of sulfide gold ore
- Green chemistry approach to synthesize titanium dioxide nanoparticles using Fagonia Cretica extract, novel strategy for developing antimicrobial and antidiabetic therapies
- Green synthesis and effective utilization of biogenic Al2O3-nanocoupled fungal lipase in the resolution of active homochiral 2-octanol and its immobilization via aluminium oxide nanoparticles
- Eco-friendly RP-HPLC approach for simultaneously estimating the promising combination of pentoxifylline and simvastatin in therapeutic potential for breast cancer: Appraisal of greenness, whiteness, and Box–Behnken design
- Use of a humidity adsorbent derived from cockleshell waste in Thai fried fish crackers (Keropok)
- One-pot green synthesis, biological evaluation, and in silico study of pyrazole derivatives obtained from chalcones
- Bio-sorption of methylene blue and production of biofuel by brown alga Cystoseira sp. collected from Neom region, Kingdom of Saudi Arabia
- Synthesis of motexafin gadolinium: A promising radiosensitizer and imaging agent for cancer therapy
- The impact of varying sizes of silver nanoparticles on the induction of cellular damage in Klebsiella pneumoniae involving diverse mechanisms
- Microwave-assisted green synthesis, characterization, and in vitro antibacterial activity of NiO nanoparticles obtained from lemon peel extract
- Rhus microphylla-mediated biosynthesis of copper oxide nanoparticles for enhanced antibacterial and antibiofilm efficacy
- Harnessing trichalcogenide–molybdenum(vi) sulfide and molybdenum(vi) oxide within poly(1-amino-2-mercaptobenzene) frameworks as a photocathode for sustainable green hydrogen production from seawater without sacrificial agents
- Magnetically recyclable Fe3O4@SiO2 supported phosphonium ionic liquids for efficient and sustainable transformation of CO2 into oxazolidinones
- A comparative study of Fagonia arabica fabricated silver sulfide nanoparticles (Ag2S) and silver nanoparticles (AgNPs) with distinct antimicrobial, anticancer, and antioxidant properties
- Visible light photocatalytic degradation and biological activities of Aegle marmelos-mediated cerium oxide nanoparticles
- Physical intrinsic characteristics of spheroidal particles in coal gasification fine slag
- Exploring the effect of tea dust magnetic biochar on agricultural crops grown in polycyclic aromatic hydrocarbon contaminated soil
- Crosslinked chitosan-modified ultrafiltration membranes for efficient surface water treatment and enhanced anti-fouling performances
- Study on adsorption characteristics of biochars and their modified biochars for removal of organic dyes from aqueous solution
- Zein polymer nanocarrier for Ocimum basilicum var. purpurascens extract: Potential biomedical use
- Green synthesis, characterization, and in vitro and in vivo biological screening of iron oxide nanoparticles (Fe3O4) generated with hydroalcoholic extract of aerial parts of Euphorbia milii
- Novel microwave-based green approach for the synthesis of dual-loaded cyclodextrin nanosponges: Characterization, pharmacodynamics, and pharmacokinetics evaluation
- Bi2O3–BiOCl/poly-m-methyl aniline nanocomposite thin film for broad-spectrum light-sensing
- Green synthesis and characterization of CuO/ZnO nanocomposite using Musa acuminata leaf extract for cytotoxic studies on colorectal cancer cells (HCC2998)
- Review Articles
- Materials-based drug delivery approaches: Recent advances and future perspectives
- A review of thermal treatment for bamboo and its composites
- An overview of the role of nanoherbicides in tackling challenges of weed management in wheat: A novel approach
- An updated review on carbon nanomaterials: Types, synthesis, functionalization and applications, degradation and toxicity
- Special Issue: Emerging green nanomaterials for sustainable waste management and biomedical applications
- Green synthesis of silver nanoparticles using mature-pseudostem extracts of Alpinia nigra and their bioactivities
- Special Issue: New insights into nanopythotechnology: current trends and future prospects
- Green synthesis of FeO nanoparticles from coffee and its application for antibacterial, antifungal, and anti-oxidation activity
- Dye degradation activity of biogenically synthesized Cu/Fe/Ag trimetallic nanoparticles
- Special Issue: Composites and green composites
- Recent trends and advancements in the utilization of green composites and polymeric nanocarriers for enhancing food quality and sustainable processing
- Retraction
- Retraction of “Biosynthesis and characterization of silver nanoparticles from Cedrela toona leaf extracts: An exploration into their antibacterial, anticancer, and antioxidant potential”
- Retraction of “Photocatalytic degradation of organic dyes and biological potentials of biogenic zinc oxide nanoparticles synthesized using the polar extract of Cyperus scariosus R.Br. (Cyperaceae)”
- Retraction to “Green synthesis on performance characteristics of a direct injection diesel engine using sandbox seed oil”

