Enhancement efficacy of omeprazole by conjugation with silver nanoparticles as a urease inhibitor
-
Aneesa Zia
, Asaad Khalid
, Ajmal Khan
and Ahmed Al-Harrasi
Abstract
Omeprazole, a proton pump inhibitor, is used for gastric and duodenal ulcers, gastroesophageal reflux disease, Helicobacter pylori infection, etc. Current research is based on the loading of omeprazole on surface silver nanoparticles by chemical method. The appearance of an absorption peak at 421 nm confirmed the synthesis of nanoparticles. The FT-IR further confirmed the conjugation of functional groups present in omeprazole moiety with silver. The size and morphology were elucidated by transmission electron microscopy and X-ray diffraction which revealed a spherical shape with an average particle size of 16–20 nm. To know enhancement in their efficacy, the omeprazole-loaded nanoparticles were evaluated against antibacterial, urease inhibition, and antioxidant activities. Nanoparticles showed significant antibacterial potential against Staphylococcus aureus and Escherichia coli with 12 ± 0.41 and 13.6 ± 1.02 mm zones of inhibition, respectively. Almost 2.43 times enhanced urease inhibitory activity was found for nanoparticles (IC50 = 2.17 ± 0.10 µg·mL−1) as compared to omeprazole (IC50 = 5.28 ± 0.14 µg·mL−1). The radical scavenging activity of nanoparticles also increased significantly. The synthesized nanoparticles were docked in the active site of urease to investigate their binding mode. Due to excellent urease and bacterial inhibition, these nanoparticles can be used for ulcers.
1 Introduction
Peptic ulcers are one of the most common gastrointestinal conditions affecting approximately 10% of the population across the globe. An imbalance between the gastro-protective factors such as mucus and antioxidant enzymes bicarbonate and secretion of acid and pepsin damages the mucosal membrane of the stomach and duodenum and results in life-threatening ulcers [1,2]. Approximately 15,000 fatalities are reported annually as a consequence of ulcers [3]. It has become one of the most widespread problems affecting 40% of developed countries and 80% of developing countries due to the indiscriminate use of non-steroidal anti-inflammatory disease and Helicobacter pylori infections [4]. Reactive oxygen species play a significant role in the etiology of peptic ulcers, as the free radical released from the neutrophils and monocytes can cause oxidative damage to the cells of gastric mucosa via lipid peroxidation and oxidation of proteins [5]. Using medications with antioxidant properties can be effective in the treatment of ulcers [6]. Antiulcer medications work by suppressing the secretion of gastric acid and stimulating the immune system by enhancing the secretion of mucin [7]. Antacids, H2 receptor blockers, muscarinic antagonists, and proton pump inhibitors are used in the treatment of ulcers. However, the successful eradication of H. pylori is essential for the treatment of peptic ulcers and avoiding its recurrence. Triple therapy containing two antibiotics clarithromycin and amoxicillin or metronidazole along with proton pump inhibitors is currently being used as a standard treatment. However, over the past 10–15 years, due to the rising incidence of antibiotic resistance, notably with clarithromycin, the effectiveness of this triple therapy has considerably decreased. Consequently, there is a rising need of developing potential antiulcer agents for effective treatment of ulcers [8].
Omeprazole, a sulfinyl benzimidazole [9] is the first clinically approved member of unique antisecretory drugs, the proton pump inhibitors that control the secretion of gastric acid via irreversible inhibition of enzyme H+/K+ -ATPase located in the parietal cells of stomach [10,11]. It is found to be a potent inhibitor of gastric acid secretion for the treatment of gastroesophageal reflux disease, gastritis, Zollinger-Ellison syndrome, gastric and duodenal ulcers, H. pylori infection, and other associated hypersecretory disorders [12,13].
Omeprazole is susceptible to heat, light, and humidity and undergoes rapid degradation in acidic solutions [14]. Hence, its exposure to the acidic contents of stomach results in the inactivation of almost fifty percent of the dose leading to its poor bioavailability [15]. The most effective approach to prevent its degradation via stomach acid is to incorporate omeprazole into enteric-coated polymeric nanoparticles and extend its half-life [1]. Due to their small size, NPs can pass through the mucus layer and adhere to the underlying epithelium or the mucus network directly. Drug when administered in the form of mucoadhesive nanoparticles can reside in the stomach for a longer period of time and these adhesive interactions may increase drug’s bioavailability via number of mechanism [16]. Various omeprazole-loaded nanoparticles have been reported in literature including omeprazole-loaded cellulose acid phthalate nanoparticles [1], omeprazole-loaded gliadin nanoparticles [16], omeprazole containing eudragit chitosan nanoparticles [17], and omeprazole containing Bletilla striata nanoparticles (OME-BSP NPs) [18]. The OMP-NPs effectively prevented gastric mucosal lesions and have enhanced antiulcer activity as compared to omeprazole. The enteric-coated nanoparticles postpone the release of the medication because it is insoluble in acidic environments. [19]. Enhanced gastric mucosal defense through increased production of mucus and bicarbonate, decreased gastric acid secretion volume, or simply neutralizing the gastric acidity are some of the mechanisms that mediate the protection of the gastric mucosa against acid production [14].
It has been demonstrated that metallic nanoparticle conjugation with antibiotics offers promising outcomes in the treatment of infectious diseases. The effectiveness of antibiotics could be increased by combining them with NPs [20,21]. Among metallic NPs, silver nanoparticles are well known for their broad spectrum activities [22]. The environmentally friendly manufacturing of silver nanoparticles is emerging as a novel and effective approach to the treatment of gastrointestinal ulcers [6]. Various sources were used for the synthesis of silver nanoparticles for the antiulcer activity. The AgNPs synthesized from Glycyrrhiza glabra root extract were found to have antiulcer activity [23]. Similarly, Solanum xanthocarpum extract-loaded silver nanoparticles were proven to have antibacterial and urease inhibitory properties against H. pylori [24].
The AgNPs synthesized from the seeds of Anethum graveolens were tested against proton pump (H+/K+-ATPase) inhibition and they were found to have inhibitory potential against the enzyme establishing proton pump inhibition by AgNPs [25].
Omeprazole and silver nanoparticles are well known for their antiulcer activities. Literature has revealed that a combination of drugs with nanoparticles may enhance their therapeutic potential as compared to drugs and NPs alone. Current research aims at synthesizing silver nanoparticles of omeprazole by chemical reduction method. To the best of our knowledge, this is the first study reporting the synthesis of omeprazole-loaded silver nanoparticles and their urease inhibition, antioxidant, and antibacterial activities against Staphylococcus aureus and Escherichia coli.
2 Materials and methods
2.1 Materials
The chemicals and solvents including AgNO3, sodium hydroxide, HCl, sodium borohydride (NaBH4), and methanol used for the synthesis were of analytical grade and purchased from Sigma Aldrich; vendor Musaji, Abbottabad, Pakistan. Nutrient agar was purchased from Oxoid Ltd, Basingstoke Hampshire, UK. The DPPH powder and the urease enzyme were purchased from Alfa Aesar and Sigma Aldrich, vendor Musaji, Abbottabad, Pakistan, respectively. UV–Vis spectra were recorded on Spectromax M2 (COMSATS University Islamabad Abbottabad Campus). For pH adjustment, a pH meter (Combo pH/EC/TDS Testers HANNA Instrument) was used.
2.2 Synthesis of nanoparticles
Omeprazole-loaded silver nanoparticles were synthesized via chemical reduction method. Around 16.9 mg of AgNO3 was dissolved in 100 mL of distilled water to prepare 1 mM solution of silver nitrate and the solution was kept in dark to prevent light exposure. Similarly, 34.54 mg of omeprazole was dissolved in 100 mL of methanol to prepare its 1 mM solution. After mixing the drug and salt solution no significant color change was observed, so 2–3 drops of NaBH4 (40 mM) were added to the solution which produced rapid variation in the color of solution indicating the formation of silver nanoparticles which was further confirmed by UV–Vis spectrophotometer.
2.3 Optimization of nanoparticles
The reaction was optimized by mixing different concentrations of silver nitrate and omeprazole to achieve optimum drug-to-metal ratio for the synthesis of nanoparticles. The ratio at which the maximum formation of nanoparticles occurs can be achieved by optimization. In this study, the concentration of drug was fixed whereas the volume of silver nitrate was varied. The ratio that gave the highest absorption peak was selected for the synthesis of nanoparticles.
2.4 Stability of NPs
After synthesis, the stability of NPs was tested against different pH (2–13), temperature range (25–100°C), and brine solution (0.5–2 M). The NPs were kept at room temperature and after 24 h, UV–Vis spectra were recorded; any change in the intensity of absorption provides information about the stability of nanoparticles.
2.4.1 pH stability test
The synthesized nanoparticles were exposed to different pH ranges (2–14) to examine their stability in extremely acidic, basic, and neutral conditions. The pH meter (Combo pH/EC/TDS Testers HANNA Instrument) was used to measure the different pH (2–14) of nanoparticle solution. The acidic and basic conditions were developed by using 0.5 M solution of HCl and NaOH in 10 mL of silver nanoparticle solutions. The solutions were kept for 24 h and later, the stability was confirmed through a decrease in absorbance by the UV–Vis analysis.
2.4.2 Heat stability test
The heat stability of omeprazole-loaded AgNPs was checked by heating the reaction solutions at different temperatures, i.e., 25°C, 50°C, 75°C, and 100°C for 30 min [26]. Around 15 mL of freshly prepared nanoparticle solution was taken in four separate beakers and heated at different temperatures for 30 min to check the heat stability of nanoparticles and UV–Vis spectra were recorded. The change in the absorption intensity provides data about the stability of synthesized AgNPs at different temperatures.
2.4.3 Brine stability test
The stability of synthesized omeprazole-loaded silver nanoparticles was determined against different concentrations of brine solutions. The 4 mL of freshly prepared Omp-AgNPs were taken in four separate vials. Then, 2 mL of 0.5, 1, 1.5, and 2 M solutions of NaCl were added to the solution of NPs. The resulting mixture was thoroughly mixed and kept at ambient temperature. UV–Vis spectra were recorded after 24 h. Change in the absorption give information about the nanoparticles’ stability.
2.5 Characterization of NPs
2.5.1 FT-IR spectroscopy
FT-IR analysis was used to detect the functional moieties present in drugs and their interaction with the metal surface in drug-loaded silver nanoparticles. IR spectra of samples were obtained by Cary630, Agilent Technologies, USA.
2.5.2 X-ray diffraction (XRD) analysis
Crystallographic study of AgNPs was performed by the X-ray diffractometer (JDX-3532, JEOL, Japan) operated at the voltage of 20–40 kV having 2θ range 5–80°.
2.5.3 EDX spectroscopy
The compositional analysis of nanoparticles was carried out by EDX (JSM-5910, JEOL, Japan) having 30 kV energy.
2.5.4 Transmission electron microscopy (TEM)
The size and shape of synthesized omeprazole-loaded silver NPs were evaluated using a TEM (JEOL, Model JEM-1400) operated at a voltage of 110 kV. Samples were prepared for the TEM examination by coating the solution of silver nanoparticles on copper grids coated with carbon.
2.6 Biological potential of NPs
2.6.1 Urease inhibition protocol
In vitro urease inhibition assay of omeprazole-loaded nanoparticles was performed in a 96-well plate by the Berthelot method with few modifications. 25 µL phosphate buffer (pH 7), 10 µL enzyme solution of urease, and 10 µL of test compounds were added to the wells and incubated at 37°C for approximately 10 min. Then, 40 µL urea solution was added to these wells and further incubated for 10 min. Afterwards, the constituents were pre-read using ELISA microplate reader at 625 nm. Then, 45 µL phenol reagent was added to each well followed by the addition of 70 µL alkali reagent. The incubation was continued for 10 min and absorbance was measured. The % inhibition was calculated by using the following formula [27].
Five dilutions of test samples were prepared and their % inhibitions were determined. The data obtained were used to calculate IC50 values using a graphpad prism.
2.6.2 Antioxidant activity
The radical scavenging potential of nanoparticles was evaluated by the DPPH method. The 100 mM DPPH solution was prepared in HPLC-grade methanol. 50 µL of DPPH was added to the 50 µL of test compound. The mixture was incubated at room temperature for approximately half an hour and the absorbance was measured at 517 nm [28]. The t-BHA was used as a standard throughout the experiment. The % scavenging activity was measured by using the following formula:
Five dilutions of the test samples along with standard were prepared and their % RSA was measured and data were used to find IC50 values using graphpad prism [29].
2.6.3 Antibacterial screening
The antibacterial potential of drug-loaded nanoparticles was screened against two clinically isolated bacterial strains namely S. aureus (ATCC 6538) and E. coli (ATCC 10536) by agar well diffusion method. The 100 µL of bacterial suspension having a concentration of 108 CFU·mL−1 was spread on the agar petri plate. By using cork borer four wells of equal size of approximately 6 mm and distance were made in the Petri plate and 20 µL test compounds of the desired concentration were added into each well along with positive control (Ciprofloxacin) and negative control (DMSO). The plates were incubated for 24 h at 37°C. After incubation, the zone of inhibition was measured [29].
2.6.4 Statistical analysis
One-way analysis of variance (ANOVA) with post hoc Bonferroni test (Haris et al. 2023) was employed to determine significant differences between the ureases and anti-oxidant activities of different test substances at the significance level (α = 0.05). Independent samples t-test was used to compare the anti-bacterial activity of two samples. The statistical analyses were performed using SPSS 20 (IBM, USA) computer software [30].
2.7 Molecular docking studies
A molecular operating environment (MOE) [31] was used to investigate the binding interaction of synthesized compounds with the active site of urease and xanthine oxidase. X-ray crystal structure of urease (PDB ID: 4GOA [32]) retrieved from the Protein Data Bank was used as a starting structure for docking. Co-crystalized ligands and heteroatoms were removed and docking was carried out using MOE software. The structures of the synthesized compounds were drawn by using Chem Office 3D (2015) and their energy was optimized using Gaussian 09 employing the B3LYP density functional theory method with a 6-311G basis set [33]. Initial optimization of the protein structure was done using MOE software followed by molecular docking studies. Ten of the lowest energy poses were generated for compounds. A Discovery visualizer was used to determine the 2D and 3D models of docked compounds.
3 Results and discussion
3.1 Synthesis of nanoparticles
A solution of AgNO3 (1 mM) and omeprazole was prepared in distilled water and methanol separately. Equal quantities of both the solutions were mixed together and kept on stirring at room temperature. No significant color change was observed after mixing as apparent with the naked eye. After 30 min, 2–3 drops of NaBH4 were added to the reaction mixture. A change in the color from transparent to dark brown was observed immediately after the addition of NaBH4 indicating the reduction of silver. The stirring was continued for up to 4 h to allow the complete reduction of silver ions for the completion of reaction. The appearance of peak at 421 nm confirmed the synthesis of omeprazole-loaded AgNPs. For the recovery of nanoparticles, the reaction mixture was centrifuged at 8,000 rpm for approximately 30 min at room temperature and washed with distilled water twice to remove the excess ions and unreacted drug. The UV–Vis spectrum (spectrophotometer BMS [UV-1602] Canada) was recorded to confirm the formation of nanoparticles (Figure 1).
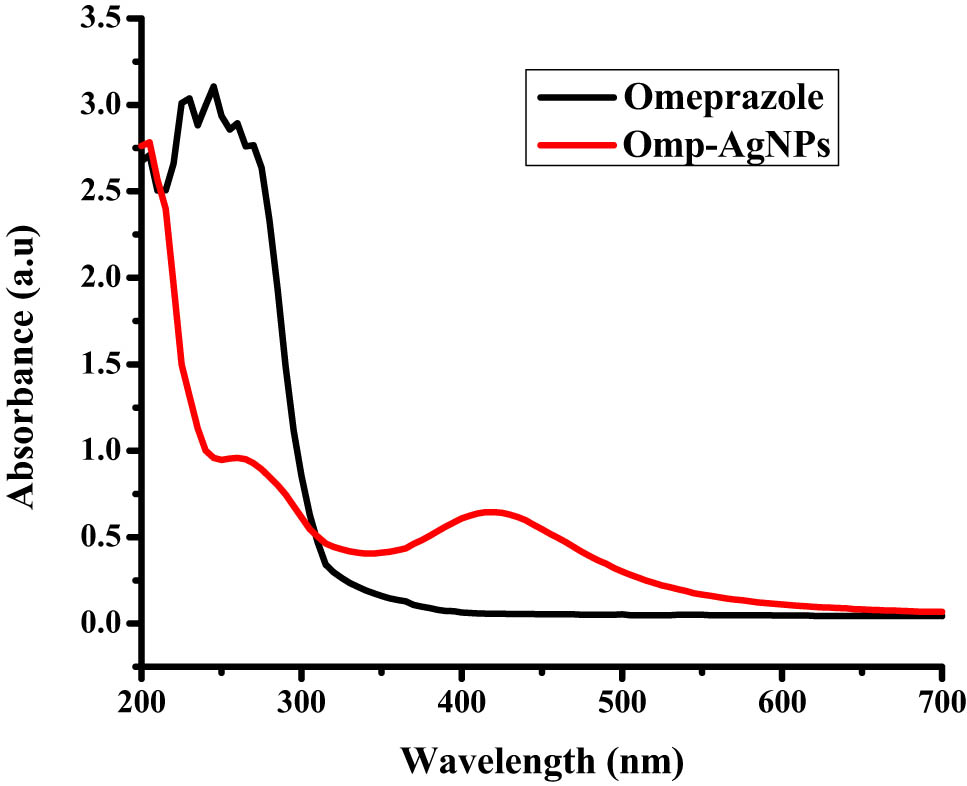
UV–Vis spectra of Omeprazole loaded AgNPs.
3.2 Reaction optimization
The reaction mixture of Omp-AgNPs was optimized using different drug-to-metal ratios and stored for 24 h to assess their stability under the given conditions. The ratio 1:3 (Omp:AgNO3) corresponds to the highest absorption peak in UV–Vis spectra as shown in Figure 2. Further increase in the concentration of salt led to a decline in the intensity of absorption which confirmed the decomposition and instability of nanoparticles indicating that a certain amount of salt was necessary for the optimum synthesis of NPs. The optimal ratio for the large-scale production of nanoparticles showing maximum absorption in UV–Vis spectra was ratio 1:3.

Optimization of Omp-AgNPs.
3.3 pH stability of AgNPs
pH has a significant impact in controlling the size of nanoparticles by altering their surface charge and particle interaction [34]. The stability of synthesized Omp-AgNPs was examined at different pH values ranging from 2 to 13 by using 0.5 M HCl and NaOH solutions as shown in Figure 3. Omp-AgNPs were found to be slightly acidic in nature having a pH in the range of 6–7. The nanoparticles were stable at a slightly acidic, neutral and slightly basic medium. The red shift in the wavelength was observed at pH 4–5 due to an increase in particle size [35]. The pH 6–7 corresponds to the highest absorption peak in the UV–Vis spectrum indicating the maximum formation and stability of nanoparticles having small size. However, AgNPs were unstable at extremely acidic and basic conditions. The chloride ions were responsible for the destabilization of nanoparticles in highly acidic conditions having pH 2–3 [36], whereas instability of AgNPs in the basic medium can be associated with the destabilization caused by sodium ions, resulting in the agglomeration of nanoparticles due to increase in particle size.

pH stability of AgNPs.
3.4 Heat stability test of NPs
The effect of heat on synthesized nanoparticles was examined at elevated temperatures to ascertain their thermal stability and optimal temperature for nanoparticle synthesis. The AgNPs were heated at different temperatures, i.e., 25°C, 50°C, 75°C, and 100°C for 30 min and their UV–Vis spectra were recorded. The spectra of heated NPs indicate a decrease in absorption intensity with an increase in temperature confirming the destabilization of nanoparticles at high temperatures as represented in Figure 4. This instability of AgNPs is associated with the dephasing of electrons [37]. The room temperature (25°C) is the optimum temperature for the synthesis of Omp-AgNPs.

Heat stability test of NPs.
3.5 Brine stability test
The addition of an electrolyte causes the agglomeration of nanoparticles, which makes them unstable [38]. The NaCl solutions of varying concentrations (0.5, 1, 1.5, and 2 M) were mixed with Omp-AgNPs to assess the effect of electrolyte solution on synthesized nanoparticles. The absorbance of these mixtures was determined by using UV–Vis spectrometer as represented in (Figure 5). The findings indicate that nanoparticles were unstable at all the concentrations of brine solution. The instability of Omp-AgNPs in the presence of NaCl is due to the aggregation effect promoted by chloride ions [37].

Brine stability test of Omp-AgNPs.
3.6 FT-IR spectroscopy
The FT-IR spectra of omeprazole and Omp-AgNPs were compared to identify the functional groups involved in the interaction with silver metal. The peak observed at 3,057 cm−1 corresponds to aromatic C–H, the absorption band for stretching vibration of C═N was observed at 1,626 cm−1, the band at 1,406 cm−1 is due to the CH bending, the peak at 1,205 cm−1 corresponds to the stretching of alkyl aryl ether, and the strong absorption peak at 1,012 cm−1 is assigned to the S═O stretching [39]. A decrease in the intensity and shift in absorption position of bands corresponding to C═N, OCH3, and S═O in the spectra of nanoparticles (Omp-AgNPs) indicates the involvement of these groups in bonding with silver. The FT-IR spectra of omeprazole and Omp-AgNPs are represented in Figure 6. The mechanism of omeprazole’s interaction with AgNPs is presented in Figure 7. S═O and C═N groups of omeprazole are involved in the structural stabilization of AgNPs (Table 1).

FT-IR spectra of omeprazole-loaded AgNPs.

Mechanism of the interaction between AgNPs and omeprazole.
FT-IR data of omeprazole and Omp-AgNPs
| Omeprazole | Omp-AgNPs | ||
|---|---|---|---|
| Frequency (cm−1) | Allocated groups | Frequency (cm−1) | Allocated groups |
| 1,626 | C═N | 1,613 | C═N |
| 1,406 | C–H | 1,379 | C–H |
| 1,205 | OCH3 | 1,152 | OCH3 |
| 1,012 | S═O | 1,005 | S═O |
3.7 XRD analysis
The phase, crystal structure, and crystallite size of synthesized nanoparticles were examined by XRD analysis. The XRD spectrum of Omp-AgNPs showed diffraction peaks at 38.05, 44.04, 64.37, and 77.36° [40]. These peaks correspond to the (111), (200), (220), and (311) planes of cubic crystal lattice (correlated to ICSD file # 064994). The average particle size of nanocrystal calculated using Debye Scherrer equation was found to be 17 nm (Figure 8).

XRD pattern of Omp-AgNPs.
3.8 EDX spectroscopy
The elemental composition of chemically synthesized drug loaded AgNPs was examined by EDX analysis. EDX profile of Omp-AgNPs displayed a very strong signal in silver region which confirms the formation of omeprazole-loaded AgNPs. The optical absorption peak for the nanocrystals of metallic silver (72.97 wt%) was observed at about 3 keV due to SPR as shown in Figure 9. The additional peaks at 0.25, 0.5, and 2.3 are the characteristic signals for carbon, oxygen, and sulfur, respectively. The presence of these peaks in the spectrum of nanoparticles confirmed the presence of omeprazole moiety bound on the surface of AgNPs.

EDX profile of Omp-AgNPs.
3.9 TEM
The shape and particle size of omeprazole-loaded silver nanoparticles were determined by TEM analysis as shown in Figure 10. TEM image was recorded at different magnifications of 50 and 100 nm. The Omp-AgNPs were polydispersed agglomerates, small in size, and predominantly spherical in shape. The particle size of AgNPs was calculated using ImageJ software. The size of nanoparticles was in the range of 12–24 nm with an average particle size of 17 nm (Figure 11). Results obtained from the TEM are in agreement with XRD.
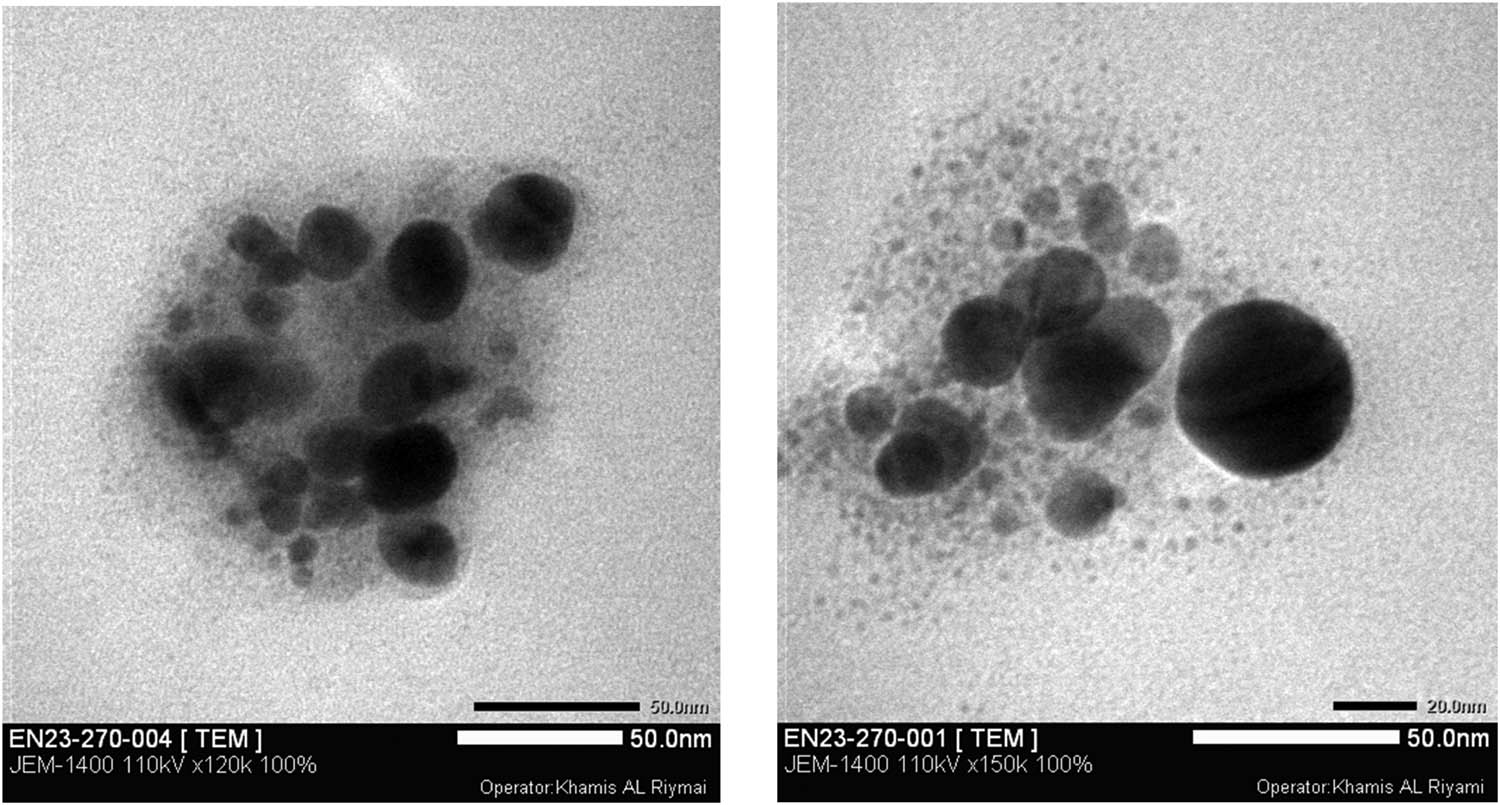
TEM image of synthesized Omp-AgNPs at different magnifications of 50 and 100 nm.

Particle size distribution graph of Omp-AgNPs.
3.10 Urease inhibition assay
The Omp-AgNPs were evaluated for in vitro urease inhibition potential to examine their activity against enzyme urease by following standard protocol. The formulation of omeprazole in the form of nanoparticles significantly enhanced its inhibitory potential against enzyme urease as compared to drug alone (Table 2). The percent inhibition of nanoparticles was 80.23% while that of omeprazole is 69.45%, and IC50 values for Omp-AgNPs and omeprazole are 2.17 ± 0.10 and 4.02 ± 0.15 respectively, which shows nanoparticles are highly active against urease. Thiourea was used as a standard inhibitor of urease during the experiment. It showed 93.3% inhibition of enzyme with an IC50 value of 18.1 ± 0.22.
Urease inhibition assay of omeprazole and Omp-AgNPs
| Sample | %Inhibition ± SD | IC50 ± SEM (µg·mL−1) |
|---|---|---|
| Omeprazole | 69.45 ± 1.24a | 4.05 ± 0.15 |
| Omp-AgNPs | 80.23 ± 1.06b | 2.17 ± 0.10 |
| Thiourea | 93.30 ± 1.24c | 18.10 ± 0.22 |
Different small letters represent significant differences (ANOVA post hoc Tukey test, P < 0.05) between the urease inhibition activity of different substances. SEM stands for standard error of the mean where n = 5.
3.11 Antioxidant activity
The radical scavenging potential of omeprazole and its nanoparticles was performed by the DPPH method. The development of antioxidants for the treatment of oxidative stress-related disorders has been promoted by extensive research on the protective role of antioxidants against these disorders [41]. Antioxidants react with DPPH and convert it into diphenylpicrylhydrazine (DPPH-H) due to its hydrogen and electron-accepting capability. The radical scavenging potentials of the antioxidant can be determined by the degree of discoloration [29]. The results showed higher scavenging activity for nanoparticles as compared to omeprazole as shown in Table 3. % RSA of omeprazole was 72.21% whereas AgNPs were 84.45%. The IC50 value of omeprazole is 18.7 ± 0.17 while that of Omp-AgNPs is 5.28 ± 0.14 which is less than omeprazole. This shows that nanoparticles are more effective as compared to omeprazole. Tertiary butylated hydroxy anisole (t-BHA) used as a reference showed 89.56% RSA with an IC50 value of 58.76 ± 0.45.
Antioxidant potential of Omp-AgNPs
| Sample | % RSA ± SD (500 µg·mL−1) | IC50 ± SEM (µg·mL−1) |
|---|---|---|
| Omeprazole | 72.21 ± 1.67a | 18.7 ± 0.17 |
| Omp-AgNPs | 84.45 ± 2.16b | 5.28 ± 0.14 |
| t-BHA | 89.56 ± 2.31c | 58.76 ± 0.45 |
Different small letters represent significant differences (ANOVA post hoc Tukey test, P < 0.05) between the anti-oxidant activities of different samples. SEM stands for standard error of the mean where n = 5.
3.12 Antibacterial activity
The antibacterial potential of omeprazole and its nanoparticles (Omp-AgNPs) was evaluated against two bacterial pathogens (e.g., S. aureus and E. coli) as shown in Table 4. The results revealed that omeprazole is ineffective against both the strains at conc. of 1 mg·mL−1 with no zone of inhibition as shown in Figures 12 and 13. The omeprazole-loaded AgNPs showed immense antibacterial activity as compared to drug. AgNPs were more effective against E. coli with 13.6 mm ± 1.02 zone of inhibition, whereas zone of inhibition against S. aureus was 12 mm ± 0.41. The results indicate that AgNPs can be a substitute for antibacterial agents as compared to drugs.
Antibacterial assay of omeprazole and Omp-AgNPs
| Sr. no. | Conc. (1 mg·mL−1) | Zone of inhibition (mm) ± SD | |
|---|---|---|---|
| S. aureus | E. coli | ||
| 1. | Omeprazole | NA | NA |
| 2. | Omp-AgNPs | 12.5 ± 0.41a | 13.5 ± 1.02a |
| 3. | Ciprofloxacin | 19.5 ± 1.50b | 18.4 ± 0.86b |
In the above table, NA stands for “not active”. Different small letters represent significant differences (t-test, P < 0.001) when data are compared within the column. SD stands for “Standard Deviation” where n = 5.
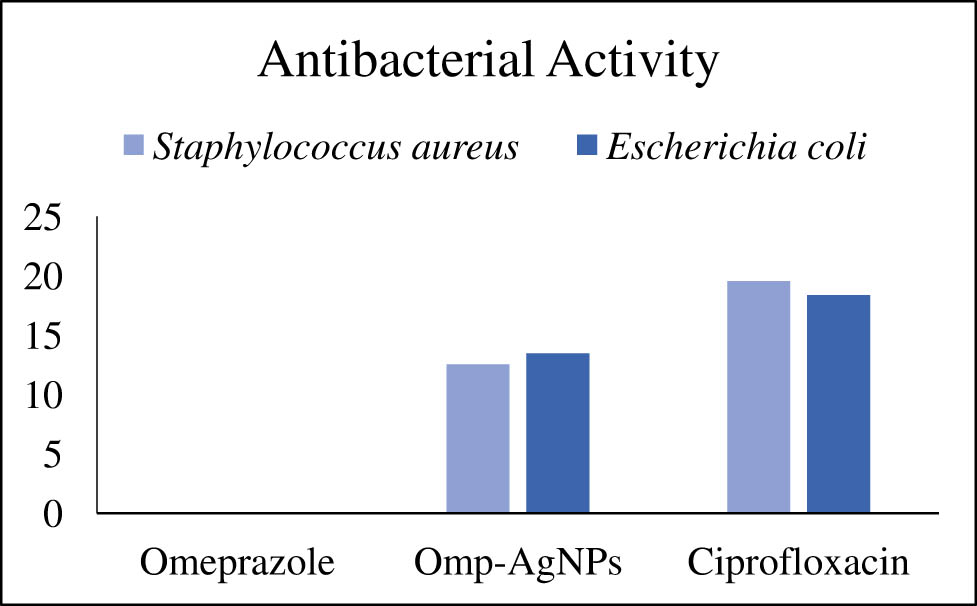
Antibacterial activity of omeprazole and Omp-AgNPs.

Antibacterial assay of omeprazole and its AgNPs.
3.13 Hotspot mapping of active pocket
The crystal structure of urease reveals various key residues that are involved in the binding pocket and allow the inhibitor to retain within the active site. These includes Ni–metal atom, sheet S23 (residues 403–409 color-coded yellow) a small loop L30 (410–412 color coded blue), helix H14 (435–441 color coded brown), loop L37 (444–454 color-coded red), helix H20 (580–593 color-coded green), loop L39 (594–598 color-coded cyan), helix H21 (599–610 color-coded pink), and a loop L41 (632–638 color-coded orange) (Figure 14).

3D interaction mapping of active site residues of urease.
The complex of omeprazole interacts with the active site along with the binding energy of −7.76 kcal·mol−1. It binds into the active site of the protein by the interaction of Ni–metal to form a complex with the oxygen atom of the omeprazole complex. Hydrogen bond interactions were observed between the complex and His492, CME592, ASP633, Ala636, Glu525, His594, and His593 amino acid residues. Asp494 interacts through pi–sulfur interaction with the complex. Some pi–alkyl and pi–pi interactions were observed between the complex for the retention of the complex molecule in the active pocket of the urease (Figure 15).
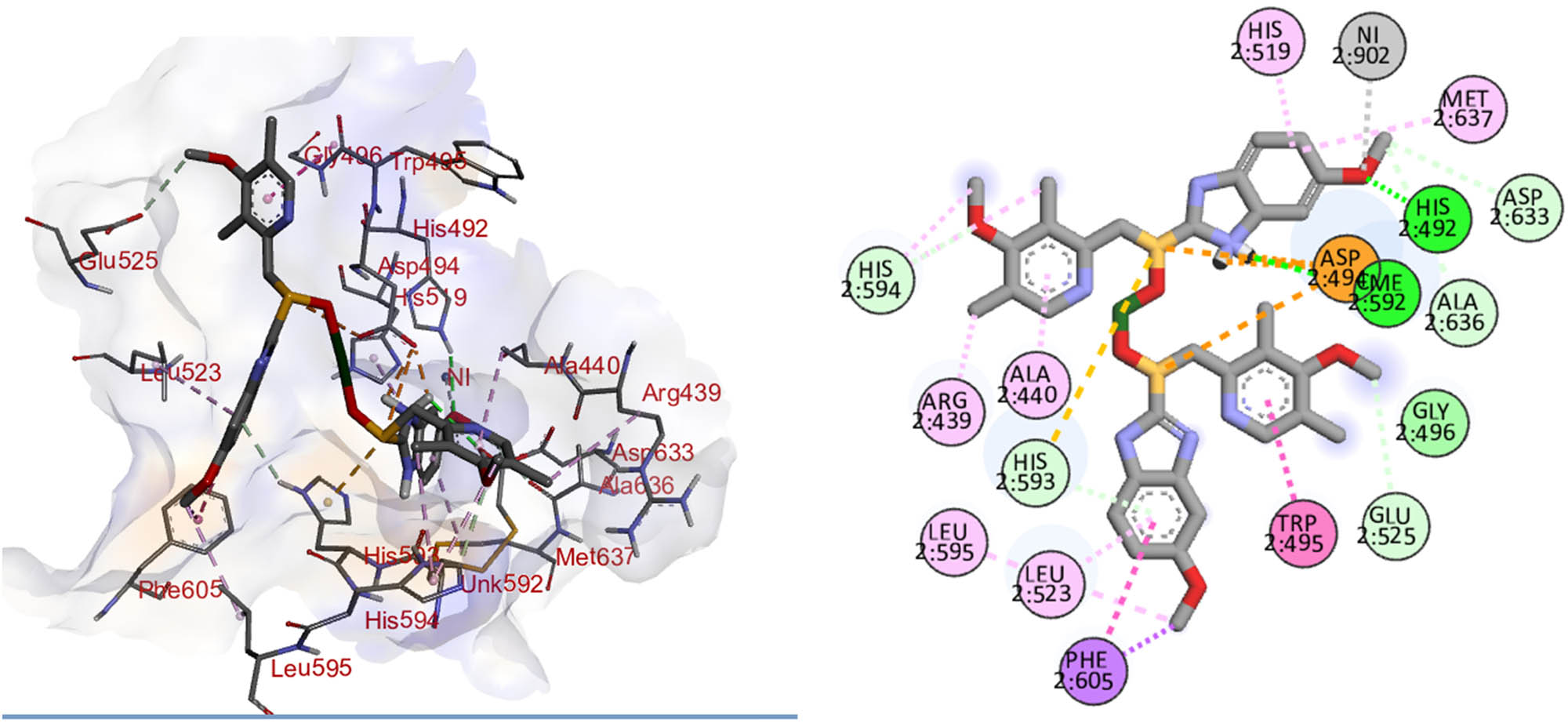
Interaction of nanoparticles in the active site of urease.
4 Conclusion
The chemical approach is one of the simple, fast, low-cost, and effective approaches for the synthesis of nanoparticles. Omeprazole-loaded AgNPs were synthesized by using a chemical method. Characterization of NPs was done by advanced analytical techniques including UV–Vis, FT-IR, EDX, XRD, and TEM analysis. The FT-IR studies confirmed that S═O, C═N, and OCH3 groups of omeprazole are responsible for the stabilization of silver nanoparticles. XRD studies revealed that nanoparticles were crystalline in nature with cubic crystal structure. Elemental composition and weight percent of AgNPs were studied by EDX analysis. Silver was present as a major constituent in omeprazole-loaded silver nanoparticles. The TEM analysis revealed that Omp-AgNPs were spherical in shape having the particle size in the range of 16–20 nm. The antibacterial potential of nanoparticles was evaluated against two bacterial strains namely S. aureus and E. coli by agar well diffusion method and NPs showed significant activity against both the strains as compared to omeprazole. The DPPH assay was used to evaluate the antioxidant potential of nanoparticles, % RSA of Omp-AgNPs is 84.45%, which is 1.16 times higher than omeprazole. Urease inhibition of nanoparticles was determined by following standard protocol. The Omp-AgNPs showed 80.23% inhibition against urease which is 1.15 times higher as compared to omeprazole. The silver nanoparticles have shown considerable potency when compared to the original drug. Further optimization of the synthesized nanoparticles may lead toward even better results which may lead toward the initiation of preclinical trials on the synthesized nanoparticles. Furthermore, conducting the toxicity studies of synthesized products adds to the future perspective of this project. It can safely be said that the urease inhibition potential of Omp-AgNPs signifies them as potential lead compounds for the development of relevant therapies to address damages caused by H. pylori in clinically sick patients.
Acknowledgments
The authors would like to thank the University of Nizwa for the generous support of this project. We thank the analytical and technical staff for assistance. This study was funded by the Deputyship for Research and Innovation, Ministry of Education in Saudi Arabia (Project number ISP23-81).
-
Funding information: This study was funded by the Deputyship for Research and Innovation, Ministry of Education in Saudi Arabia (Project number ISP23-81). Funding was provided by the Research Council through the Research Grant Program (BFP/RGP/HSS/23/037).
-
Author contributions: Aneesa Zia and Ayesha Shahzad: methodology, formal analysis, writing – original draft; Nadia Riaz and Sara Khan: software, data curation, writing – original draft; Umar Farooq and Syed Majid Bukhari: investigation, resources; Asaad Khalid: resources, visualization, data curation, funding acquisition; Hamdy Kashtoh: writing – review and editing, resources, funding acquisition; Rizwana Sarwar, Ajmal Khan, and Ahmed Al-Harrasi: conceptualization, writing – original draft. writing – review and editing, supervision, funding acquisition.
-
Conflict of interest: Authors state no conflict of interest.
-
Data availability statement: All data generated or analyzed during this study are included in this published article.
References
[1] Khatibi A, Zahedi P, Ghourchian H, Lari AS. Development of microfluidic-based cellulose acetate phthalate nanoparticles containing omeprazole for antiulcer activity: In vitro and in vivo evaluations. Eur Polym J. 2021;147:110294.10.1016/j.eurpolymj.2021.110294Search in Google Scholar
[2] Ebada SS, Al-Jawabri NA, Youssef FS, Albohy A, Aldalaien SEM, Disi AM, et al. In vivo antiulcer activity, phytochemical exploration, and molecular modelling of the polyphenolic-rich fraction of Crepis sancta extract. Inflammopharmacology. 2020;28:321–31.10.1007/s10787-019-00637-xSearch in Google Scholar PubMed
[3] Dharmani P, Palit G. Exploring Indian medicinal plants for antiulcer activity. Indian J Pharmacol. 2006;38:95.10.4103/0253-7613.24613Search in Google Scholar
[4] Ajeigbe K, Onifade A, Omotoso D, Enitan S, Olaleye S. Anti-ulcerogenic activity of Aspilia africana leaf extract: roles of gastric acid, oxidative stress and neutrophil infiltration. Afr J Biomed Res. 2014;17:193–201.Search in Google Scholar
[5] Sashidhara KV, Avula SR, Mishra V, Palnati GR, Singh LR, Singh N, et al. Identification of quinoline-chalcone hybrids as potential antiulcer agents. Eur J Med Chem. 2015;89:638–53.10.1016/j.ejmech.2014.10.068Search in Google Scholar PubMed
[6] Safari S, Bahramikia S, Dezfoulian O. Silver nanoparticles synthesized from Quercus brantii ameliorated ethanol-induced gastric ulcers in rats by decreasing oxidative stress and improving antioxidant systems. Inflammopharmacology. 2023;31:1–16.10.1007/s10787-023-01284-zSearch in Google Scholar PubMed
[7] Ahmad Bhat M, Al-Omar MA, Naglah AM. Synthesis and in vivo anti-ulcer evaluation of some novel piperidine linked dihydropyrimidinone derivatives. J Enzyme Inhib Med Chem. 2018;33:978–88.10.1080/14756366.2018.1474212Search in Google Scholar PubMed PubMed Central
[8] Beiranvand M. A review of the most common in vivo models of stomach ulcers and natural and synthetic anti-ulcer compounds: a comparative systematic study. Phytomed Plus. 2022;2:2615–30.10.1016/j.phyplu.2022.100264Search in Google Scholar
[9] Lindberg P, Brändström A, Wallmark B. Structure—activity relationships of omeprazole analogues and their mechanism of action. Trends Pharmacol Sci. 1987;8:399–402.10.1016/0165-6147(87)90107-6Search in Google Scholar
[10] Clissold SP, Campoli-Richards DM. Omeprazole: a preliminary review of its pharmacodynamic and pharmacokinetic properties, and therapeutic potential in peptic ulcer disease and Zollinger-Ellison syndrome. Drugs. 1986;32:15–47.10.2165/00003495-198632010-00002Search in Google Scholar PubMed
[11] Olbe L, Carlsson E, Lindberg P. A proton-pump inhibitor expedition: the case histories of omeprazole and esomeprazole. Nat Rev Drug Discovery. 2003;2:132–9.10.1038/nrd1010Search in Google Scholar PubMed
[12] Yim D-S, Jeong JE, Park JY. Assay of omeprazole and omeprazole sulfone by semi-microcolumn liquid chromatography with mixed-function precolumn. J Chromatogr B: Biomed Sci Appl. 2001;754:487–93.10.1016/S0378-4347(01)00036-6Search in Google Scholar
[13] Shah N, Gossman W. Omeprazole. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2019.Search in Google Scholar
[14] Bendas ER, Abdelbary AA. Instantaneous enteric nano-encapsulation of omeprazole: pharmaceutical and pharmacological evaluation. Int J Pharm. 2014;468:97–104.10.1016/j.ijpharm.2014.04.030Search in Google Scholar PubMed
[15] Pinzauti S, Gratteri P, Furlanetto S, Mura P, Dreassi E, Phan-Tan-Luu R. Experimental design in the development of voltammetric method for the assay of omeprazole. J Pharm Biomed Anal. 1996;14:881–9.10.1016/0731-7085(95)01683-XSearch in Google Scholar PubMed
[16] Ramteke S, Jain NK. Clarithromycin-and omeprazole-containing gliadin nanoparticles for the treatment of Helicobacter pylori. J Drug Target. 2008;16:65–72.10.1080/10611860701733278Search in Google Scholar PubMed
[17] Rezazadeh M, Safaran R, Minaiyan M, Mostafavi A. Preparation and characterization of Eudragit L 100-55/chitosan enteric nanoparticles containing omeprazole using general factorial design: In vitro/in vivo study. Res Pharm Sci. 2021;16:358.10.4103/1735-5362.319574Search in Google Scholar PubMed PubMed Central
[18] Li L, Jing J, Yang S, Fang S, Liu W, Wang C, et al. Bletilla striata polysaccharide nanoparticles improved the therapeutic efficacy of omeprazole on the rat gastric ulcer induced by ethanol. Mol Pharm. 2023;20:1996–2008.10.1021/acs.molpharmaceut.2c00922Search in Google Scholar PubMed
[19] Diefenthaeler HS, Bianchin MD, Marques MS, Nonnenmacher JL, Bender ET, Bender JG, et al. Omeprazole nanoparticles suspension: Development of a stable liquid formulation with a view to pediatric administration. Int J Pharm. 2020;589:119818.10.1016/j.ijpharm.2020.119818Search in Google Scholar PubMed
[20] Ravindran A, Chandran P, Khan SS. Biofunctionalized silver nanoparticles: advances and prospects. Colloids Surf B Biointerfaces. 2013;105:342–52.10.1016/j.colsurfb.2012.07.036Search in Google Scholar PubMed
[21] Mandava K. Biological and non-biological synthesis of metallic nanoparticles: Scope for current pharmaceutical research. Indian J Pharm Sci. 2017;79:501–12.10.4172/pharmaceutical-sciences.1000256Search in Google Scholar
[22] Gherasim O, Puiu RA, Bîrcă AC, Burdușel A-C, Grumezescu AM. An updated review on silver nanoparticles in biomedicine. Nanomaterials. 2020;10:2318.10.3390/nano10112318Search in Google Scholar PubMed PubMed Central
[23] Sreelakshmy V, Deepa M, Mridula P. Green synthesis of silver nanoparticles from Glycyrrhiza glabra root extract for the treatment of gastric ulcer. J Dev Drugs. 2016;5:2.Search in Google Scholar
[24] Amin M, Anwar F, Janjua MRSA, Iqbal MA, Rashid U. Green synthesis of silver nanoparticles through reduction with Solanum xanthocarpum L. berry extract: Characterization, antimicrobial and urease inhibitory activities against Helicobacter pylori. Int J Mol Sci. 2012;13:9923–41.10.3390/ijms13089923Search in Google Scholar PubMed PubMed Central
[25] Menaga S, Kripa K, Sangeetha R. Evaluation of in vitro inhibition of proton-pump by nanosilver particles synthesized using seeds of Anethum graveolens. Asian J Pharm Clin Res. 2017;10:363–6.10.22159/ajpcr.2017.v10i6.16896Search in Google Scholar
[26] Farooq U, Ahmad T, Khan A, Sarwar R, Shafiq J, Raza Y, et al. Rifampicin conjugated silver nanoparticles: A new arena for development of antibiofilm potential against methicillin resistant Staphylococcus aureus and Klebsiella pneumoniae. Int J Nanomed. 2019;3983–93.10.2147/IJN.S198194Search in Google Scholar PubMed PubMed Central
[27] Khan MU, Aslam M, Shahzad SA, Khan ZA, Khan NA, Ali M, et al. Design and synthesis of thiobarbituric acid analogues as potent urease inhibitors. J Mol Struct. 2021;1231:129959.10.1016/j.molstruc.2021.129959Search in Google Scholar
[28] Chien P-J, Sheu F, Huang W-T, Su M-S. Effect of molecular weight of chitosans on their antioxidative activities in apple juice. Food Chem. 2007;102:1192–8.10.1016/j.foodchem.2006.07.007Search in Google Scholar
[29] Sarwar R, Farooq U, Khan A, Naz S, Khan S, Khan A, et al. Evaluation of antioxidant, free radical scavenging, and antimicrobial activity of Quercus incana Roxb. Front Pharmacol. 2015;6:277.10.3389/fphar.2015.00277Search in Google Scholar PubMed PubMed Central
[30] Haris A, Azeem M, Abbas MG, Mumtaz M, Mozūratis R, Binyameen M. Prolonged repellent activity of plant essential oils against dengue vector, Aedes aegypti. Molecules. 2023;28:1351.10.3390/molecules28031351Search in Google Scholar PubMed PubMed Central
[31] Vilar S, Cozza G, Moro S. Medicinal chemistry and the molecular operating environment (MOE): Application of QSAR and molecular docking to drug discovery. Curr Top Med Chem. 2008;8:1555–72.10.2174/156802608786786624Search in Google Scholar PubMed
[32] Farooq U, Khan S, Naz S, Wani TA, Bukhari SM, Aborode AT, et al. Three new acrylic acid derivatives from Achillea mellifolium as potential inhibitors of urease from jack bean and α-glucosidase from Saccharomyces cerevisiae. Molecules. 2022;27:5004.10.3390/molecules27155004Search in Google Scholar PubMed PubMed Central
[33] Frisch A. gaussian 09W Reference. Vol. 470. Wallingford, USA: 2009. p. 25.Search in Google Scholar
[34] Sarwar R, Farooq U, Raza Shah M, Khan S, Riaz N, Naz S, et al. Rapid synthesis of gold nanoparticles from quercus incana and their antimicrobial potential against human pathogens. Appl Sci. 2017;7:29.10.3390/app7010029Search in Google Scholar
[35] Sreelekha E, George B, Shyam A, Sajina N, Mathew B. A comparative study on the synthesis, characterization, and antioxidant activity of green and chemically synthesized silver nanoparticles. BioNanoScience. 2021;11:489–96.10.1007/s12668-021-00824-7Search in Google Scholar
[36] Muhammad SP, Shah MR, Ullah R, Ahmad I, Ali K. Synthesis and characterization of functionalized silver nanoparticles for selective screening of mercury (II) ions. Arab J Sci Eng. 2022;47:7135–45.10.1007/s13369-021-06314-ySearch in Google Scholar
[37] Ahmad T, Mahbood F, Sarwar R, Iqbal A, Khan M, Muhammad S, et al. Synthesis of gemifloxacin conjugated silver nanoparticles, their amplified bacterial efficacy against human pathogen and their morphological study via TEM analysis. Artif Cells Nanomed Biotechnol. 2021;49:661–71.10.1080/21691401.2021.2003805Search in Google Scholar PubMed
[38] Ullah I, Ali I, Ullah S, Khan A, Imran M, Shah M, et al. Synthesis of acyclovir stabilized silver nanoparticles for selective recognition of Hg 2 + in different media. Int J Environ Sci Technol (Tehran). 2022;19:1–12.10.1007/s13762-021-03880-4Search in Google Scholar
[39] Chandarana CV, Kapupara PP. Fourier transform infrared spectrophotometry: An eco-friendly green tool for simultaneous quantification of aspirin and omeprazole in pharmaceutical formulation. Braz J Anal Chem. 2020;7:70–8.10.30744/brjac.2179-3425.AR-25-2020Search in Google Scholar
[40] Khan A, Jabeen H, Ahmad T, Rehman NU, Khan SS, Shareef H, et al. Comparative efficacy of cephradine-loaded silver and gold nanoparticles against resistant human pathogens. Artif Cells Nanomed Biotechnol. 2022;50:312–21.10.1080/21691401.2022.2144340Search in Google Scholar PubMed
[41] Nie Z, Liu KJ, Zhong C-J, Wang L-F, Yang Y, Tian Q, et al. Enhanced radical scavenging activity by antioxidant-functionalized gold nanoparticles: A novel inspiration for development of new artificial antioxidants. Free Radic Biol Med. 2007;43:1243–54.10.1016/j.freeradbiomed.2007.06.011Search in Google Scholar PubMed
© 2024 the author(s), published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Research Articles
- Green polymer electrolyte and activated charcoal-based supercapacitor for energy harvesting application: Electrochemical characteristics
- Research on the adsorption of Co2+ ions using halloysite clay and the ability to recover them by electrodeposition method
- Simultaneous estimation of ibuprofen, caffeine, and paracetamol in commercial products using a green reverse-phase HPTLC method
- Isolation, screening and optimization of alkaliphilic cellulolytic fungi for production of cellulase
- Functionalized gold nanoparticles coated with bacterial alginate and their antibacterial and anticancer activities
- Comparative analysis of bio-based amino acid surfactants obtained via Diels–Alder reaction of cyclic anhydrides
- Biosynthesis of silver nanoparticles on yellow phosphorus slag and its application in organic coatings
- Exploring antioxidant potential and phenolic compound extraction from Vitis vinifera L. using ultrasound-assisted extraction
- Manganese and copper-coated nickel oxide nanoparticles synthesized from Carica papaya leaf extract induce antimicrobial activity and breast cancer cell death by triggering mitochondrial caspases and p53
- Insight into heating method and Mozafari method as green processing techniques for the synthesis of micro- and nano-drug carriers
- Silicotungstic acid supported on Bi-based MOF-derived metal oxide for photodegradation of organic dyes
- Synthesis and characterization of capsaicin nanoparticles: An attempt to enhance its bioavailability and pharmacological actions
- Synthesis of Lawsonia inermis-encased silver–copper bimetallic nanoparticles with antioxidant, antibacterial, and cytotoxic activity
- Facile, polyherbal drug-mediated green synthesis of CuO nanoparticles and their potent biological applications
- Zinc oxide-manganese oxide/carboxymethyl cellulose-folic acid-sesamol hybrid nanomaterials: A molecularly targeted strategy for advanced triple-negative breast cancer therapy
- Exploring the antimicrobial potential of biogenically synthesized graphene oxide nanoparticles against targeted bacterial and fungal pathogens
- Biofabrication of silver nanoparticles using Uncaria tomentosa L.: Insight into characterization, antibacterial activities combined with antibiotics, and effect on Triticum aestivum germination
- Membrane distillation of synthetic urine for use in space structural habitat systems
- Investigation on mechanical properties of the green synthesis bamboo fiber/eggshell/coconut shell powder-based hybrid biocomposites under NaOH conditions
- Green synthesis of magnesium oxide nanoparticles using endophytic fungal strain to improve the growth, metabolic activities, yield traits, and phenolic compounds content of Nigella sativa L.
- Estimation of greenhouse gas emissions from rice and annual upland crops in Red River Delta of Vietnam using the denitrification–decomposition model
- Synthesis of humic acid with the obtaining of potassium humate based on coal waste from the Lenger deposit, Kazakhstan
- Ascorbic acid-mediated selenium nanoparticles as potential antihyperuricemic, antioxidant, anticoagulant, and thrombolytic agents
- Green synthesis of silver nanoparticles using Illicium verum extract: Optimization and characterization for biomedical applications
- Antibacterial and dynamical behaviour of silicon nanoparticles influenced sustainable waste flax fibre-reinforced epoxy composite for biomedical application
- Optimising coagulation/flocculation using response surface methodology and application of floc in biofertilisation
- Green synthesis and multifaceted characterization of iron oxide nanoparticles derived from Senna bicapsularis for enhanced in vitro and in vivo biological investigation
- Potent antibacterial nanocomposites from okra mucilage/chitosan/silver nanoparticles for multidrug-resistant Salmonella Typhimurium eradication
- Trachyspermum copticum aqueous seed extract-derived silver nanoparticles: Exploration of their structural characterization and comparative antibacterial performance against gram-positive and gram-negative bacteria
- Microwave-assisted ultrafine silver nanoparticle synthesis using Mitragyna speciosa for antimalarial applications
- Green synthesis and characterisation of spherical structure Ag/Fe2O3/TiO2 nanocomposite using acacia in the presence of neem and tulsi oils
- Green quantitative methods for linagliptin and empagliflozin in dosage forms
- Enhancement efficacy of omeprazole by conjugation with silver nanoparticles as a urease inhibitor
- Residual, sequential extraction, and ecological risk assessment of some metals in ash from municipal solid waste incineration, Vietnam
- Green synthesis of ZnO nanoparticles using the mangosteen (Garcinia mangostana L.) leaf extract: Comparative preliminary in vitro antibacterial study
- Simultaneous determination of lesinurad and febuxostat in commercial fixed-dose combinations using a greener normal-phase HPTLC method
- A greener RP-HPLC method for quaternary estimation of caffeine, paracetamol, levocetirizine, and phenylephrine acquiring AQbD with stability studies
- Optimization of biomass durian peel as a heterogeneous catalyst in biodiesel production using microwave irradiation
- Thermal treatment impact on the evolution of active phases in layered double hydroxide-based ZnCr photocatalysts: Photodegradation and antibacterial performance
- Preparation of silymarin-loaded zein polysaccharide core–shell nanostructures and evaluation of their biological potentials
- Preparation and characterization of composite-modified PA6 fiber for spectral heating and heat storage applications
- Preparation and electrocatalytic oxygen evolution of bimetallic phosphates (NiFe)2P/NF
- Rod-shaped Mo(vi) trichalcogenide–Mo(vi) oxide decorated on poly(1-H pyrrole) as a promising nanocomposite photoelectrode for green hydrogen generation from sewage water with high efficiency
- Green synthesis and studies on citrus medica leaf extract-mediated Au–ZnO nanocomposites: A sustainable approach for efficient photocatalytic degradation of rhodamine B dye in aqueous media
- Cellulosic materials for the removal of ciprofloxacin from aqueous environments
- The analytical assessment of metal contamination in industrial soils of Saudi Arabia using the inductively coupled plasma technology
- The effect of modified oily sludge on the slurry ability and combustion performance of coal water slurry
- Eggshell waste transformation to calcium chloride anhydride as food-grade additive and eggshell membranes as enzyme immobilization carrier
- Synthesis of EPAN and applications in the encapsulation of potassium humate
- Biosynthesis and characterization of silver nanoparticles from Cedrela toona leaf extracts: An exploration into their antibacterial, anticancer, and antioxidant potential
- Enhancing mechanical and rheological properties of HDPE films through annealing for eco-friendly agricultural applications
- Immobilisation of catalase purified from mushroom (Hydnum repandum) onto glutaraldehyde-activated chitosan and characterisation: Its application for the removal of hydrogen peroxide from artificial wastewater
- Sodium titanium oxide/zinc oxide (STO/ZnO) photocomposites for efficient dye degradation applications
- Effect of ex situ, eco-friendly ZnONPs incorporating green synthesised Moringa oleifera leaf extract in enhancing biochemical and molecular aspects of Vicia faba L. under salt stress
- Biosynthesis and characterization of selenium and silver nanoparticles using Trichoderma viride filtrate and their impact on Culex pipiens
- Photocatalytic degradation of organic dyes and biological potentials of biogenic zinc oxide nanoparticles synthesized using the polar extract of Cyperus scariosus R.Br. (Cyperaceae)
- Assessment of antiproliferative activity of green-synthesized nickel oxide nanoparticles against glioblastoma cells using Terminalia chebula
- Chlorine-free synthesis of phosphinic derivatives by change in the P-function
- Anticancer, antioxidant, and antimicrobial activities of nanoemulsions based on water-in-olive oil and loaded on biogenic silver nanoparticles
- Study and mechanism of formation of phosphorus production waste in Kazakhstan
- Synthesis and stabilization of anatase form of biomimetic TiO2 nanoparticles for enhancing anti-tumor potential
- Microwave-supported one-pot reaction for the synthesis of 5-alkyl/arylidene-2-(morpholin/thiomorpholin-4-yl)-1,3-thiazol-4(5H)-one derivatives over MgO solid base
- Screening the phytochemicals in Perilla leaves and phytosynthesis of bioactive silver nanoparticles for potential antioxidant and wound-healing application
- Graphene oxide/chitosan/manganese/folic acid-brucine functionalized nanocomposites show anticancer activity against liver cancer cells
- Nature of serpentinite interactions with low-concentration sulfuric acid solutions
- Multi-objective statistical optimisation utilising response surface methodology to predict engine performance using biofuels from waste plastic oil in CRDi engines
- Microwave-assisted extraction of acetosolv lignin from sugarcane bagasse and electrospinning of lignin/PEO nanofibres for carbon fibre production
- Biosynthesis, characterization, and investigation of cytotoxic activities of selenium nanoparticles utilizing Limosilactobacillus fermentum
- Highly photocatalytic materials based on the decoration of poly(O-chloroaniline) with molybdenum trichalcogenide oxide for green hydrogen generation from Red Sea water
- Highly efficient oil–water separation using superhydrophobic cellulose aerogels derived from corn straw
- Beta-cyclodextrin–Phyllanthus emblica emulsion for zinc oxide nanoparticles: Characteristics and photocatalysis
- Assessment of antimicrobial activity and methyl orange dye removal by Klebsiella pneumoniae-mediated silver nanoparticles
- Influential eradication of resistant Salmonella Typhimurium using bioactive nanocomposites from chitosan and radish seed-synthesized nanoselenium
- Antimicrobial activities and neuroprotective potential for Alzheimer’s disease of pure, Mn, Co, and Al-doped ZnO ultra-small nanoparticles
- Green synthesis of silver nanoparticles from Bauhinia variegata and their biological applications
- Synthesis and optimization of long-chain fatty acids via the oxidation of long-chain fatty alcohols
- Eminent Red Sea water hydrogen generation via a Pb(ii)-iodide/poly(1H-pyrrole) nanocomposite photocathode
- Green synthesis and effective genistein production by fungal β-glucosidase immobilized on Al2O3 nanocrystals synthesized in Cajanus cajan L. (Millsp.) leaf extracts
- Green stability-indicating RP-HPTLC technique for determining croconazole hydrochloride
- Green synthesis of La2O3–LaPO4 nanocomposites using Charybdis natator for DNA binding, cytotoxic, catalytic, and luminescence applications
- Eco-friendly drugs induce cellular changes in colistin-resistant bacteria
- Tangerine fruit peel extract mediated biogenic synthesized silver nanoparticles and their potential antimicrobial, antioxidant, and cytotoxic assessments
- Green synthesis on performance characteristics of a direct injection diesel engine using sandbox seed oil
- A highly sensitive β-AKBA-Ag-based fluorescent “turn off” chemosensor for rapid detection of abamectin in tomatoes
- Green synthesis and physical characterization of zinc oxide nanoparticles (ZnO NPs) derived from the methanol extract of Euphorbia dracunculoides Lam. (Euphorbiaceae) with enhanced biosafe applications
- Detection of morphine and data processing using surface plasmon resonance imaging sensor
- Effects of nanoparticles on the anaerobic digestion properties of sulfamethoxazole-containing chicken manure and analysis of bio-enzymes
- Bromic acid-thiourea synergistic leaching of sulfide gold ore
- Green chemistry approach to synthesize titanium dioxide nanoparticles using Fagonia Cretica extract, novel strategy for developing antimicrobial and antidiabetic therapies
- Green synthesis and effective utilization of biogenic Al2O3-nanocoupled fungal lipase in the resolution of active homochiral 2-octanol and its immobilization via aluminium oxide nanoparticles
- Eco-friendly RP-HPLC approach for simultaneously estimating the promising combination of pentoxifylline and simvastatin in therapeutic potential for breast cancer: Appraisal of greenness, whiteness, and Box–Behnken design
- Use of a humidity adsorbent derived from cockleshell waste in Thai fried fish crackers (Keropok)
- One-pot green synthesis, biological evaluation, and in silico study of pyrazole derivatives obtained from chalcones
- Bio-sorption of methylene blue and production of biofuel by brown alga Cystoseira sp. collected from Neom region, Kingdom of Saudi Arabia
- Synthesis of motexafin gadolinium: A promising radiosensitizer and imaging agent for cancer therapy
- The impact of varying sizes of silver nanoparticles on the induction of cellular damage in Klebsiella pneumoniae involving diverse mechanisms
- Microwave-assisted green synthesis, characterization, and in vitro antibacterial activity of NiO nanoparticles obtained from lemon peel extract
- Rhus microphylla-mediated biosynthesis of copper oxide nanoparticles for enhanced antibacterial and antibiofilm efficacy
- Harnessing trichalcogenide–molybdenum(vi) sulfide and molybdenum(vi) oxide within poly(1-amino-2-mercaptobenzene) frameworks as a photocathode for sustainable green hydrogen production from seawater without sacrificial agents
- Magnetically recyclable Fe3O4@SiO2 supported phosphonium ionic liquids for efficient and sustainable transformation of CO2 into oxazolidinones
- A comparative study of Fagonia arabica fabricated silver sulfide nanoparticles (Ag2S) and silver nanoparticles (AgNPs) with distinct antimicrobial, anticancer, and antioxidant properties
- Visible light photocatalytic degradation and biological activities of Aegle marmelos-mediated cerium oxide nanoparticles
- Physical intrinsic characteristics of spheroidal particles in coal gasification fine slag
- Exploring the effect of tea dust magnetic biochar on agricultural crops grown in polycyclic aromatic hydrocarbon contaminated soil
- Crosslinked chitosan-modified ultrafiltration membranes for efficient surface water treatment and enhanced anti-fouling performances
- Study on adsorption characteristics of biochars and their modified biochars for removal of organic dyes from aqueous solution
- Zein polymer nanocarrier for Ocimum basilicum var. purpurascens extract: Potential biomedical use
- Green synthesis, characterization, and in vitro and in vivo biological screening of iron oxide nanoparticles (Fe3O4) generated with hydroalcoholic extract of aerial parts of Euphorbia milii
- Novel microwave-based green approach for the synthesis of dual-loaded cyclodextrin nanosponges: Characterization, pharmacodynamics, and pharmacokinetics evaluation
- Bi2O3–BiOCl/poly-m-methyl aniline nanocomposite thin film for broad-spectrum light-sensing
- Green synthesis and characterization of CuO/ZnO nanocomposite using Musa acuminata leaf extract for cytotoxic studies on colorectal cancer cells (HCC2998)
- Review Articles
- Materials-based drug delivery approaches: Recent advances and future perspectives
- A review of thermal treatment for bamboo and its composites
- An overview of the role of nanoherbicides in tackling challenges of weed management in wheat: A novel approach
- An updated review on carbon nanomaterials: Types, synthesis, functionalization and applications, degradation and toxicity
- Special Issue: Emerging green nanomaterials for sustainable waste management and biomedical applications
- Green synthesis of silver nanoparticles using mature-pseudostem extracts of Alpinia nigra and their bioactivities
- Special Issue: New insights into nanopythotechnology: current trends and future prospects
- Green synthesis of FeO nanoparticles from coffee and its application for antibacterial, antifungal, and anti-oxidation activity
- Dye degradation activity of biogenically synthesized Cu/Fe/Ag trimetallic nanoparticles
- Special Issue: Composites and green composites
- Recent trends and advancements in the utilization of green composites and polymeric nanocarriers for enhancing food quality and sustainable processing
- Retraction
- Retraction of “Biosynthesis and characterization of silver nanoparticles from Cedrela toona leaf extracts: An exploration into their antibacterial, anticancer, and antioxidant potential”
- Retraction of “Photocatalytic degradation of organic dyes and biological potentials of biogenic zinc oxide nanoparticles synthesized using the polar extract of Cyperus scariosus R.Br. (Cyperaceae)”
- Retraction to “Green synthesis on performance characteristics of a direct injection diesel engine using sandbox seed oil”
Articles in the same Issue
- Research Articles
- Green polymer electrolyte and activated charcoal-based supercapacitor for energy harvesting application: Electrochemical characteristics
- Research on the adsorption of Co2+ ions using halloysite clay and the ability to recover them by electrodeposition method
- Simultaneous estimation of ibuprofen, caffeine, and paracetamol in commercial products using a green reverse-phase HPTLC method
- Isolation, screening and optimization of alkaliphilic cellulolytic fungi for production of cellulase
- Functionalized gold nanoparticles coated with bacterial alginate and their antibacterial and anticancer activities
- Comparative analysis of bio-based amino acid surfactants obtained via Diels–Alder reaction of cyclic anhydrides
- Biosynthesis of silver nanoparticles on yellow phosphorus slag and its application in organic coatings
- Exploring antioxidant potential and phenolic compound extraction from Vitis vinifera L. using ultrasound-assisted extraction
- Manganese and copper-coated nickel oxide nanoparticles synthesized from Carica papaya leaf extract induce antimicrobial activity and breast cancer cell death by triggering mitochondrial caspases and p53
- Insight into heating method and Mozafari method as green processing techniques for the synthesis of micro- and nano-drug carriers
- Silicotungstic acid supported on Bi-based MOF-derived metal oxide for photodegradation of organic dyes
- Synthesis and characterization of capsaicin nanoparticles: An attempt to enhance its bioavailability and pharmacological actions
- Synthesis of Lawsonia inermis-encased silver–copper bimetallic nanoparticles with antioxidant, antibacterial, and cytotoxic activity
- Facile, polyherbal drug-mediated green synthesis of CuO nanoparticles and their potent biological applications
- Zinc oxide-manganese oxide/carboxymethyl cellulose-folic acid-sesamol hybrid nanomaterials: A molecularly targeted strategy for advanced triple-negative breast cancer therapy
- Exploring the antimicrobial potential of biogenically synthesized graphene oxide nanoparticles against targeted bacterial and fungal pathogens
- Biofabrication of silver nanoparticles using Uncaria tomentosa L.: Insight into characterization, antibacterial activities combined with antibiotics, and effect on Triticum aestivum germination
- Membrane distillation of synthetic urine for use in space structural habitat systems
- Investigation on mechanical properties of the green synthesis bamboo fiber/eggshell/coconut shell powder-based hybrid biocomposites under NaOH conditions
- Green synthesis of magnesium oxide nanoparticles using endophytic fungal strain to improve the growth, metabolic activities, yield traits, and phenolic compounds content of Nigella sativa L.
- Estimation of greenhouse gas emissions from rice and annual upland crops in Red River Delta of Vietnam using the denitrification–decomposition model
- Synthesis of humic acid with the obtaining of potassium humate based on coal waste from the Lenger deposit, Kazakhstan
- Ascorbic acid-mediated selenium nanoparticles as potential antihyperuricemic, antioxidant, anticoagulant, and thrombolytic agents
- Green synthesis of silver nanoparticles using Illicium verum extract: Optimization and characterization for biomedical applications
- Antibacterial and dynamical behaviour of silicon nanoparticles influenced sustainable waste flax fibre-reinforced epoxy composite for biomedical application
- Optimising coagulation/flocculation using response surface methodology and application of floc in biofertilisation
- Green synthesis and multifaceted characterization of iron oxide nanoparticles derived from Senna bicapsularis for enhanced in vitro and in vivo biological investigation
- Potent antibacterial nanocomposites from okra mucilage/chitosan/silver nanoparticles for multidrug-resistant Salmonella Typhimurium eradication
- Trachyspermum copticum aqueous seed extract-derived silver nanoparticles: Exploration of their structural characterization and comparative antibacterial performance against gram-positive and gram-negative bacteria
- Microwave-assisted ultrafine silver nanoparticle synthesis using Mitragyna speciosa for antimalarial applications
- Green synthesis and characterisation of spherical structure Ag/Fe2O3/TiO2 nanocomposite using acacia in the presence of neem and tulsi oils
- Green quantitative methods for linagliptin and empagliflozin in dosage forms
- Enhancement efficacy of omeprazole by conjugation with silver nanoparticles as a urease inhibitor
- Residual, sequential extraction, and ecological risk assessment of some metals in ash from municipal solid waste incineration, Vietnam
- Green synthesis of ZnO nanoparticles using the mangosteen (Garcinia mangostana L.) leaf extract: Comparative preliminary in vitro antibacterial study
- Simultaneous determination of lesinurad and febuxostat in commercial fixed-dose combinations using a greener normal-phase HPTLC method
- A greener RP-HPLC method for quaternary estimation of caffeine, paracetamol, levocetirizine, and phenylephrine acquiring AQbD with stability studies
- Optimization of biomass durian peel as a heterogeneous catalyst in biodiesel production using microwave irradiation
- Thermal treatment impact on the evolution of active phases in layered double hydroxide-based ZnCr photocatalysts: Photodegradation and antibacterial performance
- Preparation of silymarin-loaded zein polysaccharide core–shell nanostructures and evaluation of their biological potentials
- Preparation and characterization of composite-modified PA6 fiber for spectral heating and heat storage applications
- Preparation and electrocatalytic oxygen evolution of bimetallic phosphates (NiFe)2P/NF
- Rod-shaped Mo(vi) trichalcogenide–Mo(vi) oxide decorated on poly(1-H pyrrole) as a promising nanocomposite photoelectrode for green hydrogen generation from sewage water with high efficiency
- Green synthesis and studies on citrus medica leaf extract-mediated Au–ZnO nanocomposites: A sustainable approach for efficient photocatalytic degradation of rhodamine B dye in aqueous media
- Cellulosic materials for the removal of ciprofloxacin from aqueous environments
- The analytical assessment of metal contamination in industrial soils of Saudi Arabia using the inductively coupled plasma technology
- The effect of modified oily sludge on the slurry ability and combustion performance of coal water slurry
- Eggshell waste transformation to calcium chloride anhydride as food-grade additive and eggshell membranes as enzyme immobilization carrier
- Synthesis of EPAN and applications in the encapsulation of potassium humate
- Biosynthesis and characterization of silver nanoparticles from Cedrela toona leaf extracts: An exploration into their antibacterial, anticancer, and antioxidant potential
- Enhancing mechanical and rheological properties of HDPE films through annealing for eco-friendly agricultural applications
- Immobilisation of catalase purified from mushroom (Hydnum repandum) onto glutaraldehyde-activated chitosan and characterisation: Its application for the removal of hydrogen peroxide from artificial wastewater
- Sodium titanium oxide/zinc oxide (STO/ZnO) photocomposites for efficient dye degradation applications
- Effect of ex situ, eco-friendly ZnONPs incorporating green synthesised Moringa oleifera leaf extract in enhancing biochemical and molecular aspects of Vicia faba L. under salt stress
- Biosynthesis and characterization of selenium and silver nanoparticles using Trichoderma viride filtrate and their impact on Culex pipiens
- Photocatalytic degradation of organic dyes and biological potentials of biogenic zinc oxide nanoparticles synthesized using the polar extract of Cyperus scariosus R.Br. (Cyperaceae)
- Assessment of antiproliferative activity of green-synthesized nickel oxide nanoparticles against glioblastoma cells using Terminalia chebula
- Chlorine-free synthesis of phosphinic derivatives by change in the P-function
- Anticancer, antioxidant, and antimicrobial activities of nanoemulsions based on water-in-olive oil and loaded on biogenic silver nanoparticles
- Study and mechanism of formation of phosphorus production waste in Kazakhstan
- Synthesis and stabilization of anatase form of biomimetic TiO2 nanoparticles for enhancing anti-tumor potential
- Microwave-supported one-pot reaction for the synthesis of 5-alkyl/arylidene-2-(morpholin/thiomorpholin-4-yl)-1,3-thiazol-4(5H)-one derivatives over MgO solid base
- Screening the phytochemicals in Perilla leaves and phytosynthesis of bioactive silver nanoparticles for potential antioxidant and wound-healing application
- Graphene oxide/chitosan/manganese/folic acid-brucine functionalized nanocomposites show anticancer activity against liver cancer cells
- Nature of serpentinite interactions with low-concentration sulfuric acid solutions
- Multi-objective statistical optimisation utilising response surface methodology to predict engine performance using biofuels from waste plastic oil in CRDi engines
- Microwave-assisted extraction of acetosolv lignin from sugarcane bagasse and electrospinning of lignin/PEO nanofibres for carbon fibre production
- Biosynthesis, characterization, and investigation of cytotoxic activities of selenium nanoparticles utilizing Limosilactobacillus fermentum
- Highly photocatalytic materials based on the decoration of poly(O-chloroaniline) with molybdenum trichalcogenide oxide for green hydrogen generation from Red Sea water
- Highly efficient oil–water separation using superhydrophobic cellulose aerogels derived from corn straw
- Beta-cyclodextrin–Phyllanthus emblica emulsion for zinc oxide nanoparticles: Characteristics and photocatalysis
- Assessment of antimicrobial activity and methyl orange dye removal by Klebsiella pneumoniae-mediated silver nanoparticles
- Influential eradication of resistant Salmonella Typhimurium using bioactive nanocomposites from chitosan and radish seed-synthesized nanoselenium
- Antimicrobial activities and neuroprotective potential for Alzheimer’s disease of pure, Mn, Co, and Al-doped ZnO ultra-small nanoparticles
- Green synthesis of silver nanoparticles from Bauhinia variegata and their biological applications
- Synthesis and optimization of long-chain fatty acids via the oxidation of long-chain fatty alcohols
- Eminent Red Sea water hydrogen generation via a Pb(ii)-iodide/poly(1H-pyrrole) nanocomposite photocathode
- Green synthesis and effective genistein production by fungal β-glucosidase immobilized on Al2O3 nanocrystals synthesized in Cajanus cajan L. (Millsp.) leaf extracts
- Green stability-indicating RP-HPTLC technique for determining croconazole hydrochloride
- Green synthesis of La2O3–LaPO4 nanocomposites using Charybdis natator for DNA binding, cytotoxic, catalytic, and luminescence applications
- Eco-friendly drugs induce cellular changes in colistin-resistant bacteria
- Tangerine fruit peel extract mediated biogenic synthesized silver nanoparticles and their potential antimicrobial, antioxidant, and cytotoxic assessments
- Green synthesis on performance characteristics of a direct injection diesel engine using sandbox seed oil
- A highly sensitive β-AKBA-Ag-based fluorescent “turn off” chemosensor for rapid detection of abamectin in tomatoes
- Green synthesis and physical characterization of zinc oxide nanoparticles (ZnO NPs) derived from the methanol extract of Euphorbia dracunculoides Lam. (Euphorbiaceae) with enhanced biosafe applications
- Detection of morphine and data processing using surface plasmon resonance imaging sensor
- Effects of nanoparticles on the anaerobic digestion properties of sulfamethoxazole-containing chicken manure and analysis of bio-enzymes
- Bromic acid-thiourea synergistic leaching of sulfide gold ore
- Green chemistry approach to synthesize titanium dioxide nanoparticles using Fagonia Cretica extract, novel strategy for developing antimicrobial and antidiabetic therapies
- Green synthesis and effective utilization of biogenic Al2O3-nanocoupled fungal lipase in the resolution of active homochiral 2-octanol and its immobilization via aluminium oxide nanoparticles
- Eco-friendly RP-HPLC approach for simultaneously estimating the promising combination of pentoxifylline and simvastatin in therapeutic potential for breast cancer: Appraisal of greenness, whiteness, and Box–Behnken design
- Use of a humidity adsorbent derived from cockleshell waste in Thai fried fish crackers (Keropok)
- One-pot green synthesis, biological evaluation, and in silico study of pyrazole derivatives obtained from chalcones
- Bio-sorption of methylene blue and production of biofuel by brown alga Cystoseira sp. collected from Neom region, Kingdom of Saudi Arabia
- Synthesis of motexafin gadolinium: A promising radiosensitizer and imaging agent for cancer therapy
- The impact of varying sizes of silver nanoparticles on the induction of cellular damage in Klebsiella pneumoniae involving diverse mechanisms
- Microwave-assisted green synthesis, characterization, and in vitro antibacterial activity of NiO nanoparticles obtained from lemon peel extract
- Rhus microphylla-mediated biosynthesis of copper oxide nanoparticles for enhanced antibacterial and antibiofilm efficacy
- Harnessing trichalcogenide–molybdenum(vi) sulfide and molybdenum(vi) oxide within poly(1-amino-2-mercaptobenzene) frameworks as a photocathode for sustainable green hydrogen production from seawater without sacrificial agents
- Magnetically recyclable Fe3O4@SiO2 supported phosphonium ionic liquids for efficient and sustainable transformation of CO2 into oxazolidinones
- A comparative study of Fagonia arabica fabricated silver sulfide nanoparticles (Ag2S) and silver nanoparticles (AgNPs) with distinct antimicrobial, anticancer, and antioxidant properties
- Visible light photocatalytic degradation and biological activities of Aegle marmelos-mediated cerium oxide nanoparticles
- Physical intrinsic characteristics of spheroidal particles in coal gasification fine slag
- Exploring the effect of tea dust magnetic biochar on agricultural crops grown in polycyclic aromatic hydrocarbon contaminated soil
- Crosslinked chitosan-modified ultrafiltration membranes for efficient surface water treatment and enhanced anti-fouling performances
- Study on adsorption characteristics of biochars and their modified biochars for removal of organic dyes from aqueous solution
- Zein polymer nanocarrier for Ocimum basilicum var. purpurascens extract: Potential biomedical use
- Green synthesis, characterization, and in vitro and in vivo biological screening of iron oxide nanoparticles (Fe3O4) generated with hydroalcoholic extract of aerial parts of Euphorbia milii
- Novel microwave-based green approach for the synthesis of dual-loaded cyclodextrin nanosponges: Characterization, pharmacodynamics, and pharmacokinetics evaluation
- Bi2O3–BiOCl/poly-m-methyl aniline nanocomposite thin film for broad-spectrum light-sensing
- Green synthesis and characterization of CuO/ZnO nanocomposite using Musa acuminata leaf extract for cytotoxic studies on colorectal cancer cells (HCC2998)
- Review Articles
- Materials-based drug delivery approaches: Recent advances and future perspectives
- A review of thermal treatment for bamboo and its composites
- An overview of the role of nanoherbicides in tackling challenges of weed management in wheat: A novel approach
- An updated review on carbon nanomaterials: Types, synthesis, functionalization and applications, degradation and toxicity
- Special Issue: Emerging green nanomaterials for sustainable waste management and biomedical applications
- Green synthesis of silver nanoparticles using mature-pseudostem extracts of Alpinia nigra and their bioactivities
- Special Issue: New insights into nanopythotechnology: current trends and future prospects
- Green synthesis of FeO nanoparticles from coffee and its application for antibacterial, antifungal, and anti-oxidation activity
- Dye degradation activity of biogenically synthesized Cu/Fe/Ag trimetallic nanoparticles
- Special Issue: Composites and green composites
- Recent trends and advancements in the utilization of green composites and polymeric nanocarriers for enhancing food quality and sustainable processing
- Retraction
- Retraction of “Biosynthesis and characterization of silver nanoparticles from Cedrela toona leaf extracts: An exploration into their antibacterial, anticancer, and antioxidant potential”
- Retraction of “Photocatalytic degradation of organic dyes and biological potentials of biogenic zinc oxide nanoparticles synthesized using the polar extract of Cyperus scariosus R.Br. (Cyperaceae)”
- Retraction to “Green synthesis on performance characteristics of a direct injection diesel engine using sandbox seed oil”

