Abstract
In this study, a modified synthetic zeolite adsorbent was synthesized by the hydrothermal method using coal fly ash as the main raw material, and the enhanced phosphorus adsorption properties from aqueous solutions were then evaluated. The modification parameters were specifically studied and optimized. Moreover, the effects of initial phosphorus concentration, adsorption time, and pH value on phosphorus absorption were also investigated. The adsorbent was characterized by the energy-dispersive spectrometer analysis, scanning electron microscopy, and Fourier transform infrared spectroscopy. Furthermore, the phosphorus adsorption properties of the zeolite adsorbent were preliminarily discussed through the perspectives of isothermal adsorption experiments, adsorption kinetics experiments, and adsorption thermodynamics calculations. The results show that the lanthanum ions were physically loaded on the surface and micropores of the adsorbent after modification, which helps to enhance the adsorption effect of phosphorus components from the aqueous solution. The phosphorus removal rate has been increased by about 65%. The adsorption process better fitted the Langmuir and Elovich equations. The theoretical calculation and analysis of adsorption thermodynamics showed that the adsorption and removal of phosphorus in water happens spontaneously.
1 Introduction
The main chemical components in common phosphorus-containing wastewater are phosphates such as PO4 3−, HPO4 2−, and H2PO4 − [1]. The large-scale discharge of phosphorus-containing wastewater will directly cause the eutrophication of water bodies and destroy its ecological balance [2]. At this stage, the removal methods of phosphorus components in water mainly include chemical precipitation, biological, adsorption, crystallization, and ion exchange methods [3]. Among them, the adsorption method has the advantages of easy operation, low cost, and no secondary pollution. It is regarded as one of the most potential methods of phosphorus removal in water [4,5]. The adsorption method mainly relies on the interaction between the adsorbent and phosphorus components in water (including physical or chemical adsorption) to achieve the purpose of phosphorus removal. It can be seen that the selection of high-efficiency adsorbents plays a key role in this process [6]. Research has found that the zeolite is a highly efficient absorbent, which has been widely used in water treatment because of its unique microstructures [7]. In addition, studies have pointed out that the chemical composition of coal fly ash contains a large proportion of SiO2 and Al2O3, which is an ideal raw material for the synthesis of zeolite [8,9].
Coal fly ash is mainly a silver-gray or gray porous granular material produced by coal-fired thermal power plants, and is currently the largest industrial solid waste in China [10,11]. The national output of coal fly ash in 2020 has exceeded 650 million tons, in which the overall utilization rate of coal fly ash is only about 70% [12]. The accumulation of a large amount of coal fly ash will not only cause waste of land resources but also may cause water and soil pollution and destroy the ecological cycle if improperly handled. Therefore, it is of great practical significance to carry out research on the utilization of coal fly ash [13]. Compared with other developed countries or regions, the utilization of coal fly ash in China mainly includes use as raw materials for the production of cement, building materials, and concrete [14,15], but the utilization in high value-added fields is still relatively low [16,17]. In recent years, the green and high-value utilization of coal fly ash has always been a hotspot in the research on industrial solid waste disposal, and it is also an urgent problem to be solved. Based on the observed phenomena reported earlier, the adsorption capacity of natural zeolite is better than that of coal fly ash, and the adsorption capacity of coal fly ash synthetic zeolite is the best of the three [3]. At present, many researchers have established a certain research basis for the treatment of phosphorus-containing wastewater using zeolite adsorbents synthesized from coal fly ash [18,19,20,21,22]. It is reported that the scheme has been proven to be effective in increasing the efficiency and saving the costs, without jeopardizing adsorption capacity. Furthermore, the modification treatments have been conducted to enhance the adsorption effect [23,24,25,26]. Some related researches indicate that the physicochemical properties and adsorption behavior of synthetic zeolites modified by rare earth were greatly enhanced [23,27–30]. However, a few studies focus on the phosphorus adsorption properties from aqueous solutions, such as the adsorption kinetics, adsorption thermodynamics, and adsorption isotherms.
In this study, the synthesized zeolite was modified by lanthanum chloride solution, which was used in the treatment of phosphorus-containing wastewater. Then, scanning electron microscopy coupled with energy-dispersive spectroscopy (SEM-EDS), Fourier transform infrared spectroscopy (FTIR), and other analysis and detection methods were applied for the characterization of the physicochemical properties of the synthesized zeolite before and after modification. For further investigation on the effect of lanthanum chloride modification on phosphorus adsorption properties from aqueous solutions, the perspectives of isothermal adsorption, adsorption kinetics, and adsorption thermodynamics of phosphorus components need to be determined. In short, it provides theoretical and experiment basis for the mechanism of rare earth element modification to strengthen the adsorption and phosphorus removal of coal fly ash synthetic zeolite.
2 Materials and methods
2.1 Materials
The coal fly ash used in the test was purchased from a coal-fired power plant in Hubei, China, which was fully mixed, ground, and dried at 105°C, and then passed through a 200-mesh standard sieve. The chemical composition of the samples was analyzed by using an X-ray fluorescence spectrometer (XRF, ARL PERFORM’X, Thermo Scientific, USA), and the results are presented in Table 1. As Table 1 shows, the main components are SiO2 and Al2O3, which are consistent with the main components of natural zeolite.
Results of elemental analysis of the coal fly ash samples (wt%)
| Element | SiO2 | Al2O3 | Fe2O3 | CaO | MgO | K2O | Others | Si/Al |
|---|---|---|---|---|---|---|---|---|
| Content | 46.54 | 34.88 | 6.77 | 5.04 | 0.60 | 0.46 | 5.71 | 1. 18 |
The simulated phosphorus-containing aqueous solutions with different concentrations were prepared by diluting the 1,000 mg·L−1 potassium hydrogen phosphate stock solution. All the chemicals employed in this study were of analytical grade, and double distilled water was used throughout this study.
2.2 Preparation of zeolite adsorbents
2.2.1 Pretreatment of the coal fly ash
The coal fly ash and ultrapure water were mixed uniformly in a beaker at a solid-to-liquid ratio of 1 g:20 mL, and then the beaker was placed in a water-bath thermostatic magnetic stirrer at 25°C for 24 h. After water washing, the mixture was taken out and filtered with suction. Then, the washed coal fly ash was dried and sieved.
2.2.2 Synthesis of zeolite
Appropriate amounts of coal fly ash and 1 mol·L−1 NaOH solution were added into a round-bottomed flask at the solid-to-liquid ratio of 1 g:5 mL, and then evenly mixed. The suitable synthesis time, alkali concentration, and temperature for zeolite synthesis are 8 h, 1 mol·L−1, and 120℃, respectively. After the synthetic experiment, the product was repeatedly washed with ultrapure water three times. Finally, the synthetic product was filtered, dried, and weighed.
2.2.3 Modification of the synthetic zeolite
The synthetic zeolite was added into a conical flask with 20 mL solution of the modifier. The mass concentrations of the lanthanum chloride modifier were chosen in the range of 0.4–2.0%. The pH value of the pulp was adjusted with sodium hydroxide (NaOH) and hydrochloric acid (HCl) (Sinopharm Chemical Reagent Co., Ltd, Shanghai, China). Then, the influence of the solid–liquid ratio on the modification was studied to explore the optimum modification conditions. After the modification, the synthetic zeolite was repeatedly washed with ultrapure water for three times. Finally, the effects of modification on phosphorus removal were determined through adsorption experiments.
2.3 Physicochemical properties of zeolite adsorbents
2.3.1 SEM analysis
Microstructures for the zeolite adsorbents were studied by SEM (JSM-5510LV, JEOL, Japan). In each experiment, the pressure, accelerated voltage, and work distance were set to 30 Pa, 15 kV, and 10–12 mm, respectively.
2.3.2 FTIR spectroscopy measurements
FTIR spectroscopy has been widely used to identify the adsorption approach of the reagents on solid surfaces. One gram of the ground zeolite adsorbent (<5 μm) was mixed with the spectroscopic KBr and determined within the wavenumber range of 4,000–400 cm–1 at a resolution of 2 cm−1. The infrared spectra were determined with an FTIR spectrometer (Continuum XL, Thermo Scientific, USA).
2.3.3 EDS analysis
Chemical composition of the synthetic zeolite and its modified products was analyzed by using the FALCON8200 EDAX instrument (AMETEK, USA). The EDS examination was performed using a beam current and a beam size of 1 nA and 1 µm, respectively. In each quantification, a dwell time of 30 s was applied.
2.4 Adsorption tests
A certain amount of modified zeolite adsorbent was added into a 50 mL centrifuge tube together with 40 mL of simulated phosphorus-containing wastewater (initial concentrations were controlled to be 10–500 mg·L−1), and the adsorption tests were conducted to evaluate the phosphorus removal effect. In these cases, the pH value was adjusted between 2 and 12. After adsorption, the remaining total phosphorus in the supernatant was measured by ammonium molybdate spectrophotometry [31], and the phosphorus removal rate was relatively calculated. The total phosphorus concentration (C p), phosphorus removal rate (R), and adsorption amount (Q) in the solution can be described by Eqs. 1, 2, and 3, respectively.
where C p is the concentration of total phosphorus (calculated by element) in the solution (mg·L−1), A s is the absorbance of the solution, A b is the absorbance of the blank test, a is the slope of the calibration curve, b is the intercept of the calibration curve, R is the removal rate of phosphorus (%), Q is the adsorption amount of phosphorus (mg·g−1), C 0 is the initial concentration of phosphorus (mg·L−1), C e is the equilibrium concentration of phosphorus (mg·L−1), V is the solution volume (mL), and m is the mass of zeolite (g).
2.5 Specific surface area analysis
The BET-specific surface area and average pore volume of the prepared adsorbent before and after phosphorus adsorption were analyzed by using a Autosorb-iQ instrument (Quantachrome, USA). Each measurement was conducted three times, and the average result was recorded.
3 Results and discussion
3.1 Influencing factors of zeolite modification
3.1.1 Effect of modifier concentration
Figure 1(a) shows the influence of lanthanum concentration on the phosphorus removal of synthesized zeolite from coal fly ash. With the increase of lanthanum concentration, the phosphorus removal rate and the adsorption amounts increase gradually. When the lanthanum concentration was 1.2%, the phosphorus removal rate and the adsorption amounts reached the maximum at 98.6% and 1.97 mg·g−1, respectively. After that, the removal rate began to decrease slowly, and the corresponding adsorption capacities also decreased. It might be that as the concentration of lanthanum ions increases, the coordination complexes formed between lanthanum ions and synthetic zeolite components transform from adsorbing phosphate at the initial stage to blocking the pores of synthetic zeolite, resulting in a decrease in the phosphorus removal [32,33]. According to the test data, 1.2% lanthanum chloride solution was determined as a reasonable modification concentration in the subsequent modification test.

Phosphorus removal ability under different (a) lanthanum concentrations, (b) pH values, and (c) solid-to-liquid ratios.
3.1.2 Effect of pH
Figure 1(b) shows that the pH has a great influence on the phosphorus removal of synthesized zeolite from coal fly ash. When the pH value of the modified solution was lower than 5, the removal rate and adsorption capacities of the synthetic zeolite increased with the increase in the pH value. While the pH increased from 5 to 7, the phosphorus removal rate and adsorption capacity decreased slightly. Furthermore, the removal rate and adsorption capacities increase monotonically with the increase in pH from 7 to 12. At pH 10, the removal rate of phosphorus reached 95.8%, and the corresponding adsorption amount reached 1.91 mg·g−1. The appropriate pH of the modified solution was determined to be 10, which is also consistent with the results reported in the study by Wang [34].
3.1.3 Effect of solid–liquid ratio
The effect of the solid-to-liquid ratio (the ratio of the mass of synthesized zeolite to the volume of lanthanum chloride solution, g·mL−1) on the phosphorus removal performance of zeolite synthesis from coal fly ash is presented in Figure 1(c). When the solid–liquid ratio was 1 g:3 mL, the phosphorus removal rate and adsorption capacities of the modified zeolite are only 31.7% and 0.63 mg·g−1, respectively. While the solid-to-liquid ratio becomes smaller, the removal rate of phosphorus in water by the modified zeolite increases continuously. When the solid–liquid ratio is 1 g:6 mL, the phosphorus removal rate reached 94.2%. If the solid-to-liquid ratio continues to decrease, the phosphorus removal rate does not change much. The more lanthanum ions there is, the more active adsorbents are loaded into the synthetic zeolite, and these substances have good adsorption to phosphate, so the removal effect of modified zeolite on phosphorus in the solution showed a trend of first increasing significantly and then reaching a stable level. According to the test results, the economic efficiency of the modification of synthetic zeolite and the high efficiency of phosphorus removal are combined to determine the suitable concentration of lanthanum chloride solution, pH of the modified solution, and solid-to-liquid ratio for the optimization and modification of lanthanum chloride modified coal fly ash zeolite as 1.2%, 10, and 1 g:6 mL, respectively.
3.2 Physicochemical properties
3.2.1 SEM and EDS analysis
SEM and EDS analyses of the synthetic zeolite adsorbent were made so as to ascertain its chemical composition and microscopic structure before and after modification with lanthanum chloride. It can be seen that the main chemical components of the synthetic zeolite (Figure 2(b)) and the modified synthetic zeolite (Figure 2(d)) are silicon, aluminum, iron, calcium, magnesium, and so on. It might be possible to deduce that the content of lanthanum ions in the synthetic zeolite was significantly increased, which indicated that lanthanum ions have been successfully introduced into the synthesized zeolite, so that the phosphorus removal capacity has been significantly improved. SEM images (in Figure 2(e) and (f)) have also been taken to show the particle morphology of the synthetic zeolite adsorbent before and after modification. The results showed that the morphology was changed to a certain extent when the synthetic zeolite was modified with lanthanum chloride. The surface of the unmodified synthetic zeolite was rough and has many protrusions, which generally showed the typical morphology of P-type zeolite particles, while the surface of the modified synthetic zeolite was relatively smooth and fuzzy. On the whole, the result shown in Figure 2 shows that lanthanum was introduced onto the synthetic zeolite surface after the modification, but does not change the structure.
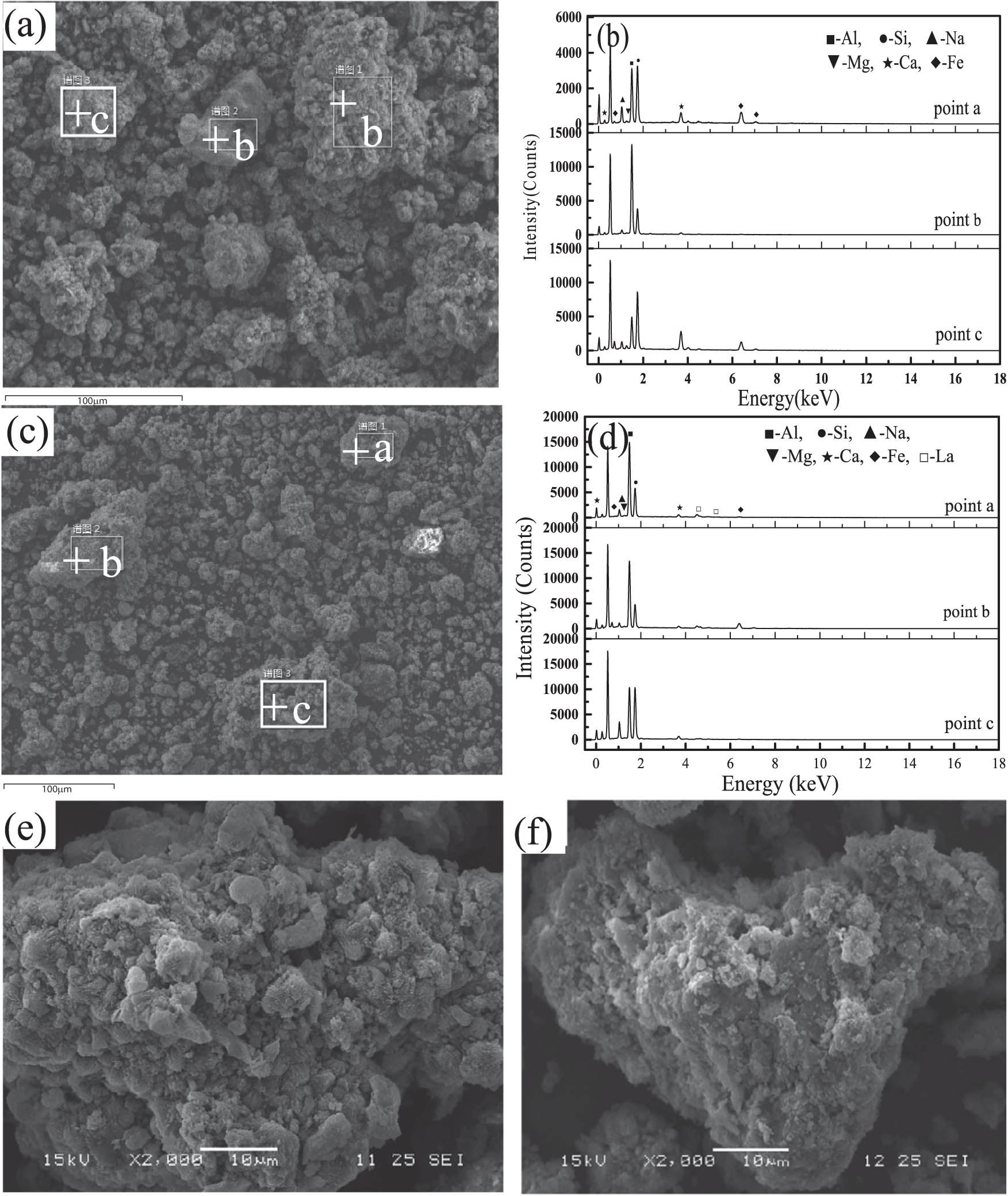
SEM and EDS analyses of the synthetic zeolite (a, b, and e) before and (c, d, and f) after modification.
3.2.2 FTIR characterization
Figure 3 shows the infrared absorption spectrum of the synthetic zeolite treated with or without the lanthanum chloride solution. It indicates that the infrared absorption peaks of the synthetic zeolite from coal fly ash at 445.60 and 608.79 cm−1 might be caused by the bending vibration of Si–O or the double ring vibration of O–Si(Al)–O [35]. The peak appeared at 739.60 cm−1 means the stretching vibration of the tetrahedral structure, and the strong absorption peak at 994.44 cm−1 is the asymmetric stretching vibration of the Si(Al)–O–Si. The peaks of 1,648.33 and 3,452.05 cm−1 are the bending and stretching vibrations of the hydroxyl groups of water molecules adsorbed by zeolite, respectively, which are consistent with related studies [36,37].
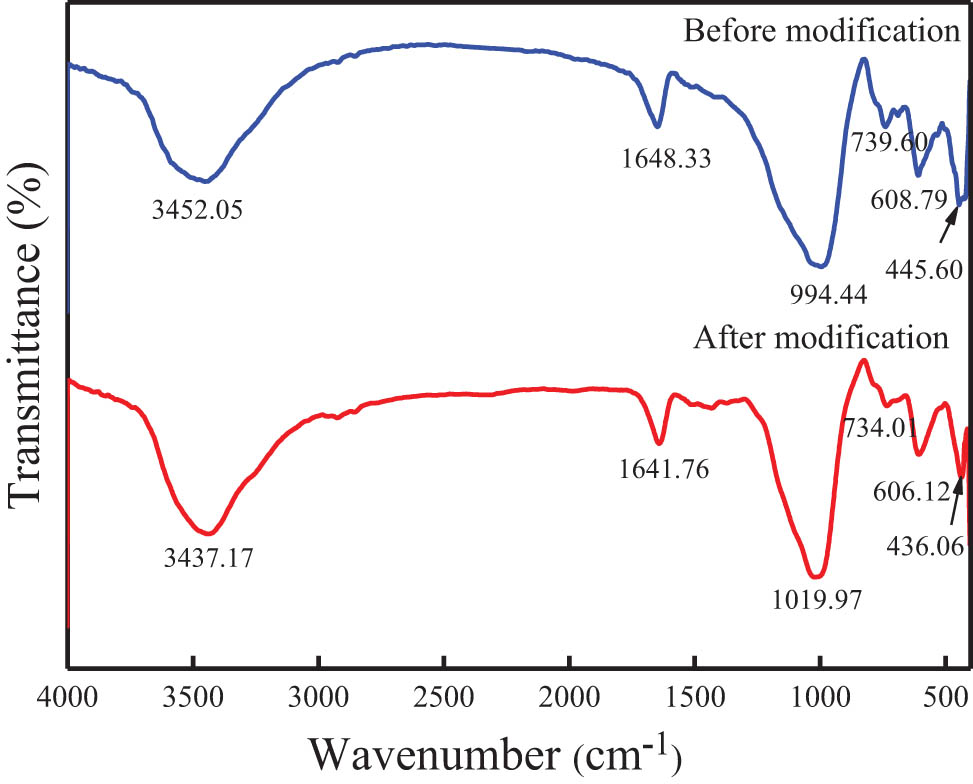
FTIR characterization of the synthetic zeolite before and after modification.
3.3 Influencing factors of phosphorus adsorption
3.3.1 Effect of phosphorus initial concentration
Figure 4 shows the effect of phosphorus initial concentration on the phosphorus removal effect of the modified synthetic zeolites in water. When the initial concentration of phosphorus was at 10–70 mg·L−1, the adsorption capacity of the modified synthetic zeolites to phosphorus increased rapidly with the increase of the initial concentration of simulated wastewater. It indicates that the active sites of the modified synthetic zeolites within this concentration range were sufficient to remove phosphorus in water [38]. Furthermore, the adsorption capacity of the modified synthetic zeolites might suffer due to the reduction of active sites on the surface when the initial concentration was more than 70 mg·L−1.
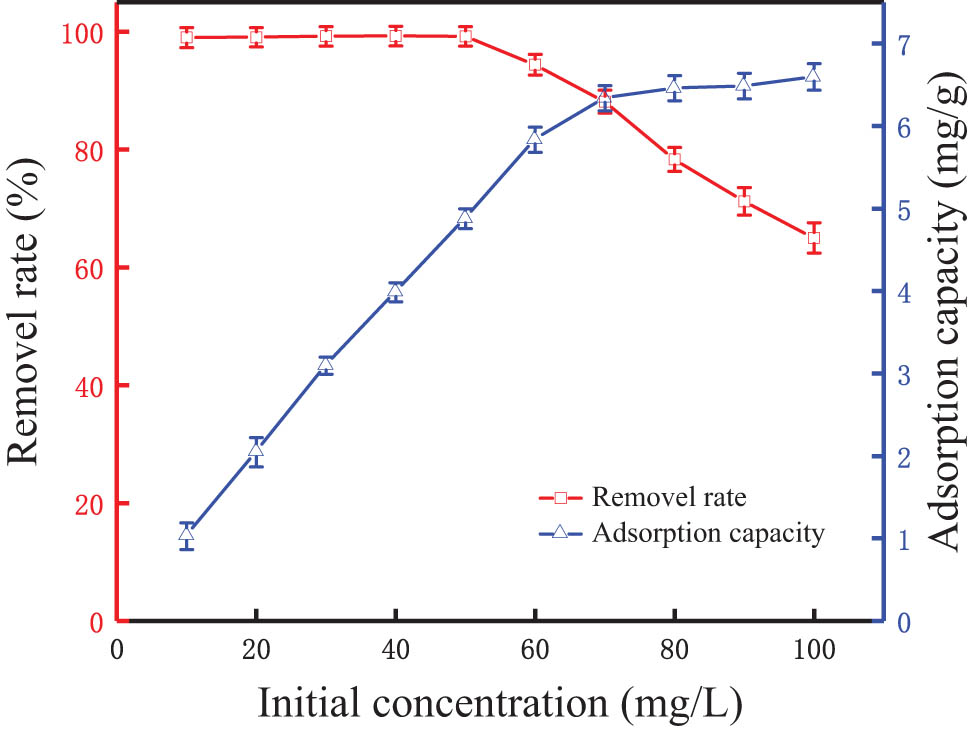
Effect of initial phosphorus concentration on phosphorus removal.
3.3.2 Effect of adsorption time
As shown in Figure 5, the adsorption process of the modified synthetic zeolites conforms to the characteristics of “rapid adsorption in the early stage and slow equilibrium in the later stage” [39], that is, when the adsorption time was less than 300 min, the phosphorus adsorption increases rapidly, and then the adsorption capacity gradually becomes flat and basically unchanged after 400 min. The removal rate of phosphorus reaches the maximum of 96.9% at 360 minutes, and the corresponding adsorption capacity increased to 2.98 mg·g−1.
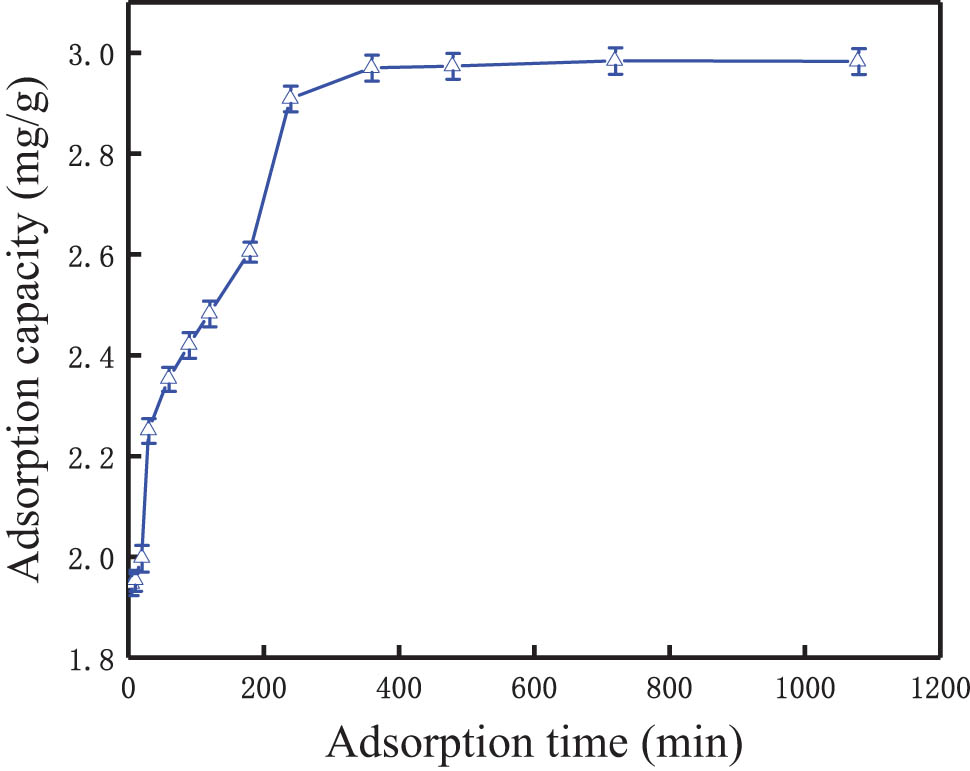
Effect of adsorption time on phosphorus removal.
3.3.3 Effect of pH
The solution pH has a great influence on the adsorption process [40]. Figure 6 shows the adsorption capacity of the modified synthetic zeolites as a function of pH value. It indicates that the adsorption capacity was low at a pH of less than 3.0. At pH 3.0–8.0, the phosphorus adsorption capacity gradually increases with the increase of pH, and the adsorption capacity increased from 2.89 to 2.99 mg·g−1. Instead, at pH over 8.0, the phosphorus adsorption capacity decreases gradually. The adsorption capacity under strong acid conditions was likely to be related to the changes in pore structures of the synthetic zeolites, which makes zeolite unable to adsorb and fix phosphate.
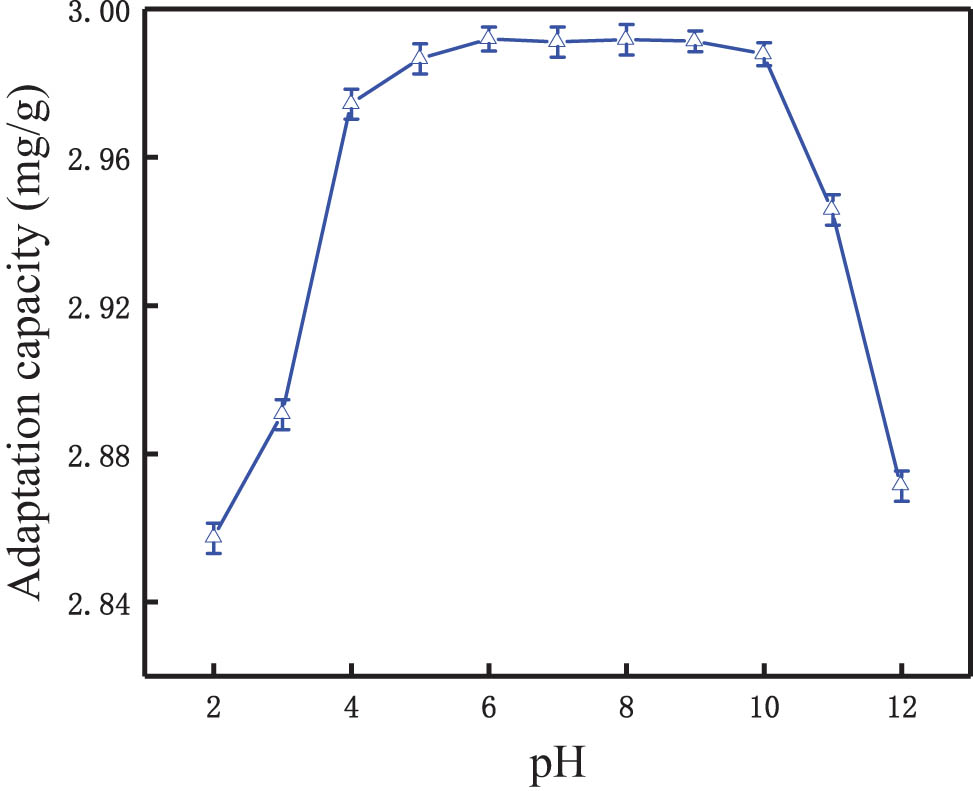
Effect of pH on phosphorus removal.
3.4 Phosphorus adsorption isotherms
The adsorption isotherm results of the modified synthetic zeolites are shown in Figure 7. It indicated that with the increase of phosphorus equilibrium concentration, the equilibrium adsorption capacity gradually increased. Moreover, Langmuir and Freundlich adsorption isotherm models were commonly used adsorption isotherm models, which belong to chemical adsorption and physical adsorption models, respectively [9]. In this study, the two models were used to describe the phosphorus adsorption behaviors. The linear forms of the Langmuir and Freundlich isotherm models are described by Eqs. 4 and 5, respectively [41].
where C e is the equilibrium mass concentration of pollutants in the solution (mg·L−1), Q e is the equilibrium adsorption capacity of the material to the pollutant (mg·g−1), Q m is the saturated adsorption capacity of the material to the pollutant (mg·g−1), K L and K F are Langmuir and Freundlich equilibrium constants, respectively, and n is the Freundlich adsorption index.
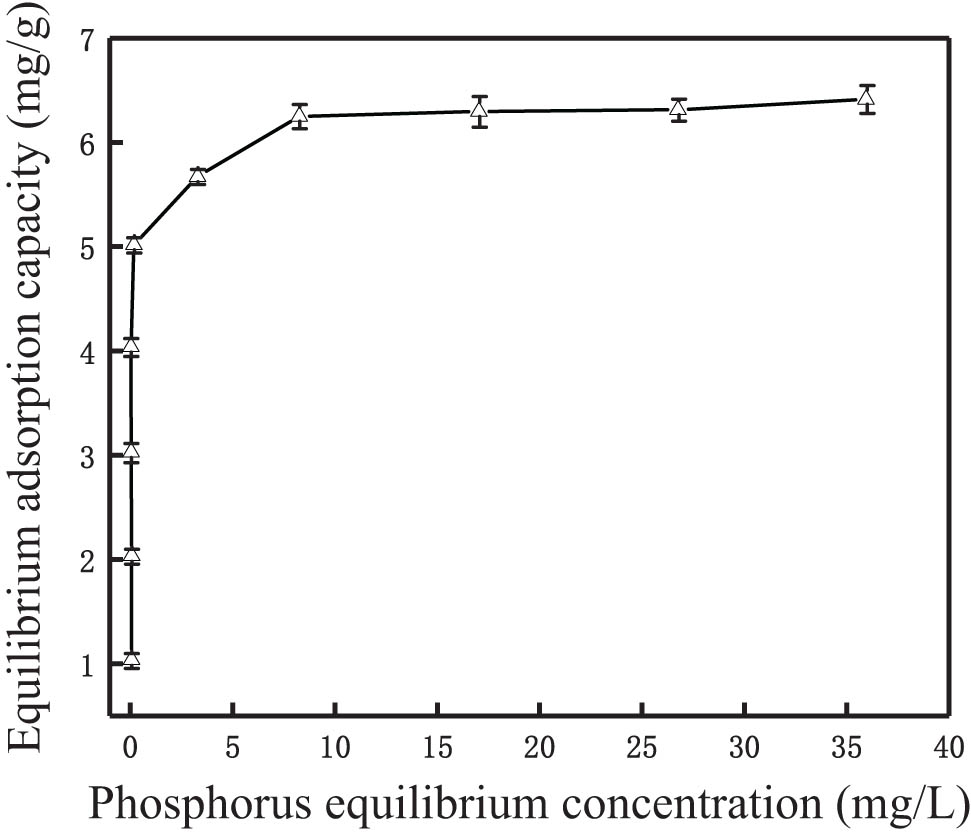
Adsorption isotherm of phosphorus by the modified synthetic zeolites.
According to the results shown in Figure 7, the Langmuir and Freundlich isotherm adsorption models were used for linear fitting, and the results are shown in Figure 8(a) and (b), respectively. The parameters of the adsorption isotherm models after linear fitting are shown in Table 2. It can be seen from the fitting results that the regression coefficients R 2 of the two models are 0.9998 and 0.5441, respectively, indicating that the Langmuir model is more suitable to describe the isothermal adsorption behavior and the adsorption process fitted the monolayer adsorption. The theoretical saturated adsorption capacity of modified zeolite for phosphorus in water calculated according to the Langmuir model was 6.4 mg·g−1, which was very close to the actual saturated adsorption capacity (6.3 mg·g−1). The calculated separation constant R L of the modified synthetic zeolites was between 0 and 1, indicating that the phosphorus could be effectively adsorbed in water. The fitting constant 1/n was in the range of 0–0.5, indicating that the phosphorus adsorption process was easy to carry out.
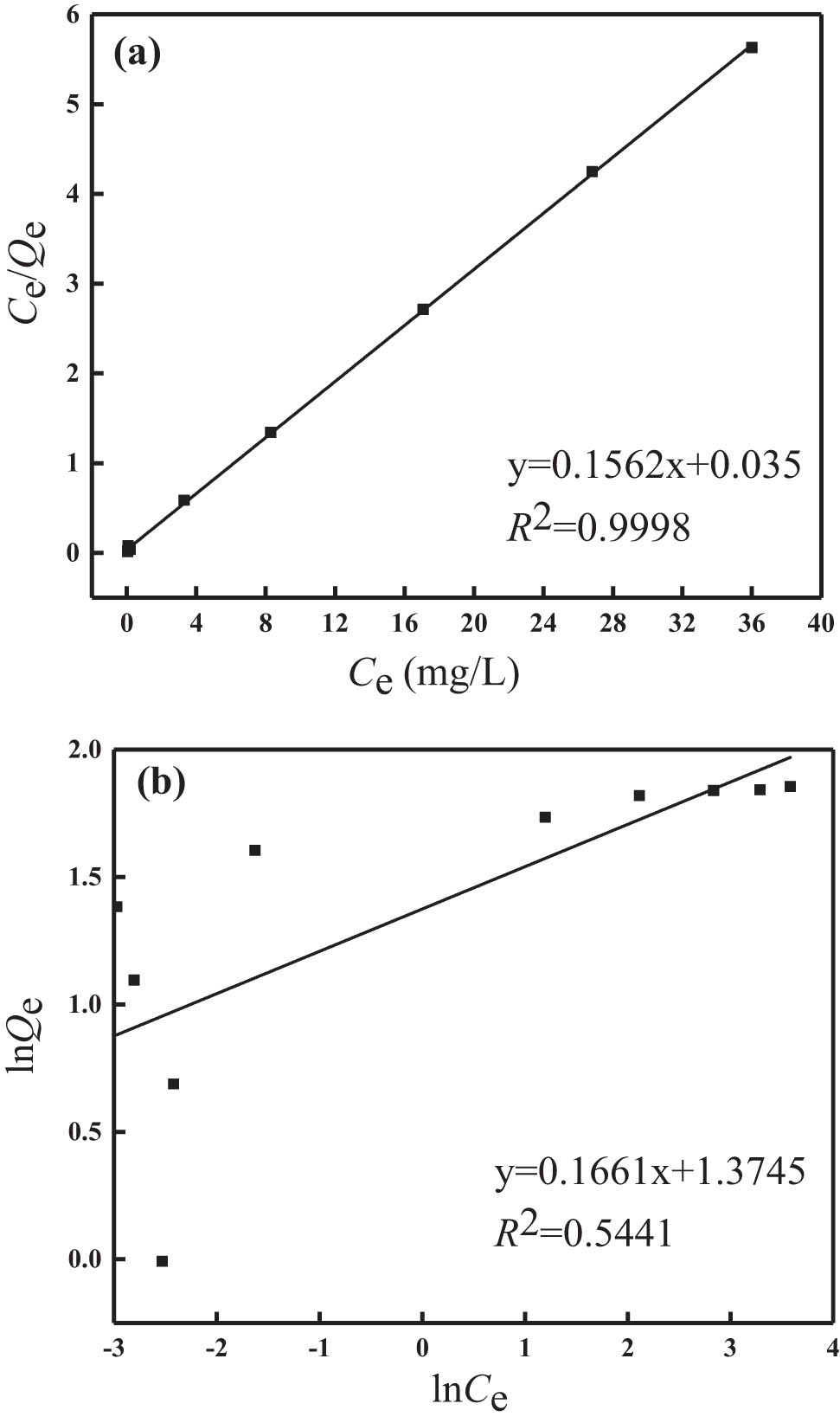
Linear fitting of (a) Langmuir and (b) Freundlichadsorption isotherm model.
Linear fitting parameters of the two isothermal adsorption models
| Models | Langmuir adsorption isotherm model | Freundlich adsorption isotherm model | ||||
|---|---|---|---|---|---|---|
| Q m (mg·g−1) | K L (L·mg−1) | R 2 | K F | 1/n | R 2 | |
| Values | 6.40 | 4.463 | 0.9998 | 3.953 | 0.166 | 0.5441 |
3.5 Phosphorus adsorption kinetics
In general, the phosphate adsorption process of the modified synthetic zeolites can also be seen as a process of internal diffusion and adsorption [8]. For better understanding and evaluating the adsorption process, four models of pseudo-first-order, pseudo-second-order, intraparticle diffusion model, and Elovich equation model [42–44] (Eqs. 6–9) were used to study the adsorption kinetics.
Pseudo-first-order model:
Pseudo-second-order model:
Intraparticle diffusion model:
Elovich equation model:
Here, Q t is the adsorption capacity at t (mg·g−1); Q e is the equilibrium adsorption capacity (mg·g−1); k 1, k 2, and k d represent the rate constant; t is the reaction time (h); C is the thickness of the adsorbent boundary layer; a is the initial adsorption rate (g·mg−1·h−1); and b is the analytical constant (g·mg−1).
The fitting curves and correlation coefficients are shown in Figure 9 and Table 3, respectively. Results show that the Elovich equation model fitted the best, being 0.9375 in regression coefficient R 2, higher than the other two (Figure 9(a)). The pseudo-first-order kinetic model was the worst fitting, which shows that the phosphorus adsorption belongs to the chemical adsorption process of uneven solid surface, and it also shows that the surface adsorption energy of modified zeolite is evenly distributed in the whole adsorption process [45]. In Figure 9(b), the intraparticle diffusion process was divided into three stages, and the calculated fitting parameters for each stage are shown in Table 4. The fitting results show that the fitting correlation coefficient of the modified synthetic zeolites in the second stage is 0.9924, which indicated that the adsorption reaction of phosphorus on the surface of the modified synthetic zeolites is probably more up the intraparticle diffusion kinetic model. From Figure 9(b), the linearly fitted straight line did not pass through the origin over the entire time range, indicating that the intraparticle diffusion model was not the only rate-limiting mechanism and that there were other kinetic models controlling the adsorption rate [46].
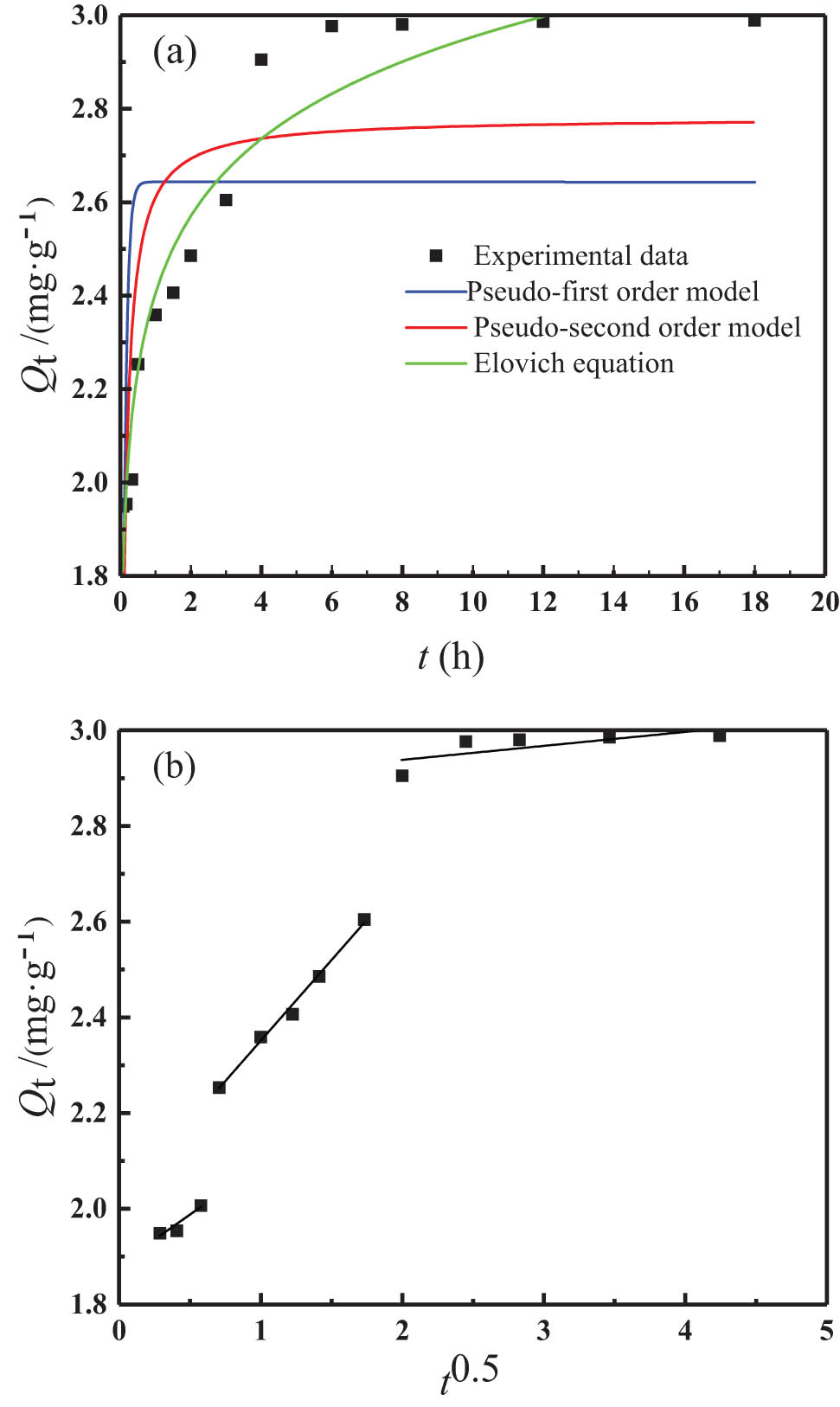
(a) Adsorption kinetic fitting results and (b) three-stage fitting curve of intraparticle diffusion model.
Fitting parameters of adsorption kinetic model
| Model parameters | Pseudo-first-order kinetic model | Pseudo-second-order kinetic model | Elovich equation | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Q e (mg·g−1) | k 1 (h−1) | R 2 | Q e (mg·g−1) | k 2 (g·mg−1·h−1) | R 2 | a (g·mg−1·h−1) | b (g·mg−1) | R 2 | |
| Parameter value | 2.64 | 10.786 | 0.3299 | 2.78 | 5.469 | 0.6513 | 5,561.92 | 4.185 | 0.9375 |
Three-stage fitting parameters of the modified synthetic zeolites intraparticle diffusion model
| Model parameters | Stage one | Stage two | Stage three | ||||||
|---|---|---|---|---|---|---|---|---|---|
| k d1 (mg·g−1·h−0.5) | C 1 | R 2 | k d2 (mg·g−1·h−0.5) | C 2 | R 2 | k d3 (mg·g−1·h−0.5) | C 3 | R 2 | |
| Parameter value | 0.209 | 1.88 | 0.8881 | 0.337 | 2.01 | 0.9924 | 0.029 | 2.88 | 0.5357 |
3.6 Phosphorus adsorption thermodynamics
The thermodynamic parameters are often used to explore the change of heat in the phosphorus removal, so as to further explore the state characteristics of the modified synthetic zeolites [31]. K e and the thermodynamic constants of ΔH 0 and ΔS 0 conform to the Van’t Hoff equation [47] in the normal form (Eq. 10):
where R is the ideal gas constant (8.314 J·(mol−1·K−1), T is the absolute temperature (K), and K e is the equilibrium adsorption partition coefficient (mL·g−1).
The adsorption thermodynamics is mainly carried out by studying the thermodynamic parameters, which are calculated as shown in Table 5. The fitting results are presented in Figure 10. Results show that the value of ΔG° was negative at different temperatures, indicating that the adsorption process can proceed spontaneously at all temperatures. As the temperature increases, ΔG° gradually decreases, which indicates that the higher the temperature, the greater the degree of spontaneity. Moreover, the whole process of ΔH 0 was positive, so it can be shown that the adsorption of phosphorus onto the modified synthetic zeolites was an endothermic reaction. Besides this, the value of ΔS 0 was positive indicating an increase in solid–liquid interfacial disorder and a decrease in orderliness during phosphorus adsorption by modified synthetic zeolites [47].
Thermodynamic parameters of the modified synthetic zeolites in the phosphorus adsorption
| ΔH 0 (kJ·mol−1) | ΔS 0 (J·mol−1·K−1) | R 2 | ΔG° (kJ·mol−1) | ||||
|---|---|---|---|---|---|---|---|
| 288 K | 293 K | 298 K | 303 K | 308 K | |||
| 41.719 | 164.13 | 0.9854 | −5.550 | −6.371 | −7.192 | −8.012 | −8.833 |
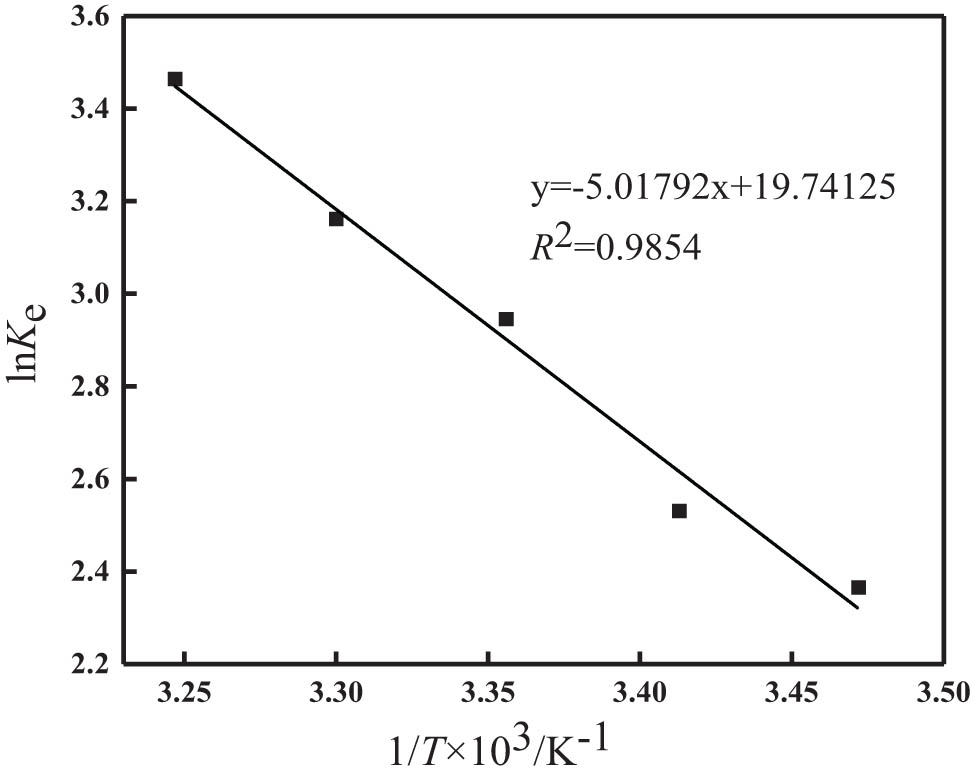
Relationship between ln K e and 1/T of phosphorus removal by the modified synthetic zeolites.
3.7 BET-specific surface area determination
As illustrated in Table 6, the specific surface area of the prepared adsorbent after phosphorus adsorption becomes smaller than that in the absence of phosphorus-containing solution, indicating that the introduction of prepared adsorbent in solution leads to alteration of surface morphology, which can apparently result from the reaction of phosphorus components with adsorbent. Furthermore, the average pore volume was decreased from 0.238 to 0.201 cm3·g−1 showing that the phosphorus could be adsorbed into the microporous structure of the adsorbent.
Results of specific surface area measurement
| Adsorption state | BET-specific surface area (m2·g−1) | Average pore volume (cm3·g−1) |
|---|---|---|
| Before adsorption | 42.074 | 0.238 |
| After adsorption | 40.125 | 0.201 |
4 Conclusion
In this study, the lanthanum concentration, pH, and solid-to-liquid ratio make an important influence on the modification of the synthetic zeolites. The modified synthetic zeolites were then characterized and verified the adsorption capacities for phosphorus. The results of SEM, EDS, and FTIR indicated that after the modification of lanthanum chloride, lanthanum was only loaded on the surface and micropores of zeolite synthesized, and did not change its crystal structure and skeleton structure, nor participate in skeleton vibration. The modified synthetic zeolites prepared at the conditions of modifier concentration of 1.2%, pH value of modified solution of 10, and solid–liquid ratio of 1 g: 6 mL showed the best adsorption capacities. The phosphorus adsorption of the modified synthetic zeolites belongs to the single molecular layer adsorption, and the adsorption isotherm was more in line with the Langmuir model. The adsorption kinetics analysis shows that the adsorption conforms to the Elovich equation. Thermodynamic studies have shown that the adsorption of phosphorus on modified zeolite belonged to the spontaneous process of endothermic entropy increase. Furthermore, the reuse of zeolite adsorbent in phosphorus removal will be observed and discussed in a subsequent article.
Acknowledgments
We applied the SDC approach for the sequence of authors.
-
Funding information: This work was financially supported by the National Natural Science Foundation of China (grant number 52104263), the Key Research and Development Program of Hubei Province of China (grant number 2023BCB079), the National Key Research and Development Program of China (grant number 2022YFC2904701), and the Scientific Research Foundation of Wuhan Institute of Technology (grant number K2021099).
-
Conflict of interest: The authors state no conflict of interest.
-
Data availability statement: The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
[1] Dalu T, Wasserman RJ, Magoro ML, Froneman PW, Weyl OL. River nutrient water and sediment measurements inform on nutrient retention, with implications for eutrophication. Sci Total Environ. 2019;684:296–302. 10.1016/j.scitotenv.2019.05.167.Suche in Google Scholar PubMed
[2] Huang W, Jin ZH, Yang HR, Qu YH, Che FF, Xu ZS, et al. Interception of phosphorus release from sediment by magnetite/lanthanum carbonate co modified activated attapulgite composite: Performance and mechanism. Colloids Surf, A. 2023;664:131139. 10.1016/j.colsurfa.2023.131139.Suche in Google Scholar
[3] Ramasahayam SK, Guzman L, Gunawan G, Viswanathan T. A comprehensive review of phosphorus removal technologies and processes . J Macromol Sci, Part A. 2014;51(6):538–45. 10.1080/10601325.2014.906271.Suche in Google Scholar
[4] Han MY, Shen XY, Shao HM, Liu Y, Han Q, Zhai YC. Facile one-pot hydrothermal synthesis of reticulated porous tobermorite for fast phosphorus recovery. Colloids Surf, A. 2023;666:131349. 10.1016/j.colsurfa.2023.131349.Suche in Google Scholar
[5] dos Reis GS, Thue PS, Cazacliu BG, Lima EC, Sampaio CH, Quattrone M, et al. Effect of concrete carbonation on phosphate removal through adsorption process and its potential application as fertilizer. J Cleaner Prod. 2020;256:120416. 10.1016/j.jclepro.2020.120416.Suche in Google Scholar
[6] Hussain Z, Chang N, Sun JQ, Xiang S, Ayaz T, Zhang H, et al. Modification of coal fly ash and its use as low-cost adsorbent for the removal of directive, acid and reactive dyes. J Hazard Mater. 2022;422:126778. 10.1016/j.jhazmat.2021.126778.Suche in Google Scholar PubMed
[7] Murakami T, Otsuka K, Fukasawa T, Ishigami T, Fukui K. Hierarchical porous zeolite synthesis from coal fly ash via microwave heating. Colloids Surf, A. 2023;661:130941. 10.1016/j.colsurfa.2023.130941.Suche in Google Scholar
[8] Wen ZP, Chen HC, Pan JH, Jia RB, Yang F, Liu HT, et al. Grinding activation effect on the flotation recovery of unburned carbon and leachability of rare earth elements in coal fly ash. Powder Technol. 2022;398:117045. 10.1016/j.powtec.2021.117045.Suche in Google Scholar
[9] He XP, Yao B, Xia Y, Huang H, Gan YP, Zhang WK. Coal fly ash derived zeolite for highly efficient removal of Ni2+ inwaste water. Powder Technol. 2020;367:40–6. 10.1016/j.powtec.2019.11.037.Suche in Google Scholar
[10] Hussain Z, Lizhen G, Moeen M. Treatment of coal fly ash and environmentally friendly use with rubber in cable wires as insulation material. Sustainability. 2020;12(12):5218. 10.3390/su12125218.Suche in Google Scholar
[11] Wang ZH, Xu LH, Wu DS, Zheng SL. Hydrothermal synthesis of mesoporous tobermorite from fly ash with enhanced removal performance towards Pb2+ from wastewater. Colloids Surf, A. 2022;632:127775. 10.1016/j.colsurfa.2021.127775.Suche in Google Scholar
[12] Luo Y, Wu Y, Ma S, Zheng S, Zhang Y, Chu PK. Utilization of coal fly ash in China: a mini-review on challenges and future directions. Environ Sci Pollut Res. 2021;28:18727–40. 10.1007/s11356-020-08864-4.Suche in Google Scholar PubMed
[13] Mushtaq F, Zahid M, Bhatti IA, Nasir S, Hussain T. Possible applications of coal fly ash in wastewater treatment. J Environ Manage. 2019;240:27–46. 10.1016/j.jenvman.2019.03.054.Suche in Google Scholar PubMed
[14] Yao ZT, Ji XS, Sarker PK, Tang JH, Ge LQ, Xia MS, et al. A comprehensive review on the applications of coal fly ash. Earth-Sci Rev. 2015;141:105–21. 10.1016/j.earscirev.2014.11.016.Suche in Google Scholar
[15] Gollakota ARK, Volli V, Shu CM. Progressive utilization prospects of coal fly ash: A review. Sci Total Environ. 2019;672:951–89. 10.1016/j.scitotenv.2019.03.337.Suche in Google Scholar PubMed
[16] Xing YW, Guo FY, Xu MD, Gui XH, Li HS, Li GS, et al. Separation of unburned carbon from coal fly ash: A review. Powder Technol. 2019;353:372–84. 10.1016/j.powtec.2019.05.037.Suche in Google Scholar
[17] Wang NN, Zhao Q, Li QY, Zhang GS, Huang YL. Degradation of polyacrylamide in an ultrasonic-Fenton-like process using an acid-modified coal fly ash catalyst. Powder Technol. 2020;369:270–8. 10.1016/j.powtec.2020.05.052.Suche in Google Scholar
[18] Kobayashi Y, Ogata F, Nakamura T, Kawasaki N. Synthesis of novel zeolites produced from fly ash by hydrothermal treatment in alkaline solution and its evaluation as an adsorbent for heavy metal removal. J Environ Chem Eng. 2020;8(2):103687. 10.1016/j.jece.2020.103687.Suche in Google Scholar
[19] Estevam ST, de Aquino TF, da Silva TD, da Cruz R, Bonetti B, Riella HG, et al. Synthesis of K-Merlinoite zeolite from coal fly ash for fertilizer application. Braz J Chem Eng. 2021;39:631–43. 10.1007/s43153-021-00172-9.Suche in Google Scholar
[20] Koshy N, Singh DN. Fly ash zeolites for water treatment applications. J Environ Chem Eng. 2016;4(2):1460–72. 10.1016/j.jece.2016.02.002.Suche in Google Scholar
[21] Zhang KC, Van Dyk L, He DS, Deng J, Liu S, Zhao HQ. Synthesis of zeolite from fly ash and its adsorption of phosphorus in wastewater. Green Process Synth. 2021;10(1):349–60. 10.1515/gps-2021-0032.Suche in Google Scholar
[22] Molina A, Poole C. A comparative study using two methods to produce zeolites from fly ash. Miner Eng. 2004;17(2):167–73. 10.1016/j.mineng.2003.10.025.Suche in Google Scholar
[23] Goscianska J, Ptaszkowska-Koniarz M, Frankowski M, Franus M, Panek R, Franus. W. Removal of phosphate from water by lanthanum-modified zeolites obtained from fly ash. J Colloid Sci. 2018;513:72–81. 10.1016/j.jcis.2017.11.003.Suche in Google Scholar PubMed
[24] Shukla EA, Johan E, Henmi T, Matsue N. Arsenate adsorption on iron modified artificial zeolite made from coal fly ash. Procedia Environ Sci. 2013;17:279–84. 10.1016/j.proenv.2013.02.039.Suche in Google Scholar
[25] Nascimento M, Soares PSM, de Souza VP. Adsorption of heavy metal cations using coal fly ash modified by hydrothermal method. Fuel. 2009;88(9):1714–9. 10.1016/j.fuel.2009.01.007.Suche in Google Scholar
[26] Qiu QL, Jiang XG, Lv GJ, Chen ZL, Lu SY, Ni MJ, et al. Adsorption of heavy metal ions using zeolite materials of municipal solid waste incineration fly ash modified by microwave-assisted hydrothermal treatment. Powder Technol. 2018;335:156–63. 10.1016/j.powtec.2018.05.003.Suche in Google Scholar
[27] Asaoka S, Kawakami K, Saito H, Ichinari T, Nohara H, Oikawa T. Adsorption of phosphate onto lanthanum-doped coal fly ash—Blast furnace cement composite. J Hazard Mater. 2021;406:124780. 10.1016/j.jhazmat.2020.124780.Suche in Google Scholar PubMed
[28] Xu R, Lyu T, Wang LJ, Yuan YT, Zhang MY, Cooper M, et al. Utilization of coal fly ash waste for effective recapture of phosphorus from waters. Chemosphere. 2022;287:132431. 10.1016/j.chemosphere.2021.132431.Suche in Google Scholar PubMed
[29] Wang W, Qi L, Zhang P, Luo J, Li J. Removal of COD in wastewater by magnetic coagulant prepared from modified fly ash. Environ Sci Pollut Res. 2022;29(34):52175–88. 10.1007/s11356-022-19540-0.Suche in Google Scholar PubMed
[30] Zhang L, Qin Y, Zhang X, Gao X, Song L. Further findings on the stabilization mechanism among modified Y zeolite with different rare earth ions. Ind Eng Chem Res. 2019;58(31):14016–25. 10.1021/acs.iecr.9b03036.Suche in Google Scholar
[31] Yang CX, Sun XY, Liu B, Lian HT. Determination of total phosphorus in water sample by digital imaging colorimetry. Chin J Anal Chem. 2007;35(6):850–3. 10.1016/S1872-2040(07)60059-0.Suche in Google Scholar
[32] Wang YU, Chen JN, Li XM, Luo Q, Yang Q, Jiang. L. Stimultaneous removal of ammonium and phosphate in waste water by La-modified synthetic zeolite from coal fly ash. China Environ Sci. 2011;31(7):1152–8. 10.1016/j.jcis.2017.11.003.Suche in Google Scholar PubMed
[33] Ping N, Hans-Jörg B, Bing L, Lu XW, Zhang Y. Phosphate removal from wastewater by model-La (III) zeolite adsorbents. J Environ Sci. 2008;20(6):670–4. 10.1016/S1001-0742(08)62111-7.Suche in Google Scholar PubMed
[34] Wang Y. Preparation of low-cost fly ash zeolite and its experimental study on synchronous ammonia and phosphorus removal [dissertation]. Changsha: Hunan University; 2011.Suche in Google Scholar
[35] García-Lodeiro I, Fernández-Jiménez A, Blanco MT, Palomo A. FTIR study of the sol–gel synthesis of cementitious gels: C–S–H and N–A–S–H. J Sol-Gel Sci Technol. 2008;45:63–72. 10.1007/s10971-007-1643-6.Suche in Google Scholar
[36] Karapınar N. Application of natural zeolite for phosphorus and ammonium removal from aqueous solutions. J Hazard Mater. 2009;170(2–3):1186–91. 10.1016/j.jhazmat.2009.05.094.Suche in Google Scholar PubMed
[37] Hollman GG, Steenbruggen G, Janssen-Jurkovičová. M. A two-step process for the synthesis of zeolites from coal fly ash. Fuel. 1999;78(10):1225–30. 10.1016/S0016-2361(99)00030-7.Suche in Google Scholar
[38] Sibrell PL, Kehler T. Phosphorus removal from aquaculture effluents at the northeast fishery center in Lamar, Pennsylvania using iron oxide sorption media. Aquac Eng. 2016;72:45–52. 10.1016/j.aquaeng.2016.04.003.Suche in Google Scholar
[39] Liu L, Ma D, Zheng H, Li XJ, Cheng MJ, Bao XH. Synthesis and characterization of microporous carbon nitride. Microporous Mesoporous Mater. 2008;110(2–3):216–22. 10.1016/j.micromeso.2007.06.012.Suche in Google Scholar
[40] Liu MW, Wang CZ, Guo JB, Zhang LH. Removal of phosphate from wastewater by lanthanum modified bio-ceramisite. J Environ Chem Eng. 2021;9(5):106123. 10.1016/j.jece.2021.106123.Suche in Google Scholar
[41] Han RP, Zhang JJ, Han P, Wang YF, Zhao ZH, Tang MS. Study of equilibrium, kinetic and thermodynamic parameters about methylene blue adsorption onto natural zeolite. Chem Eng J. 2009;145(3):496–504. 10.1016/j.cej.2008.05.003.Suche in Google Scholar
[42] Revellame ED, Fortela DL, Sharp W, Hernandez R, Zappi ME. Adsorption kinetic modeling using pseudo-first order and pseudo-second order rate laws: A review. Clean Eng Technol. 2020;1:100032. 10.1016/j.clet.2020.100032.Suche in Google Scholar
[43] Malash GF, El-Khaiary MI. Piecewise linear regression: A statistical method for the analysis of experimental adsorption data by the intraparticle-diffusion models. Chem Eng J. 2010;163(3):256–63. 10.1016/j.cej.2010.07.059.Suche in Google Scholar
[44] Largitte L, Pasquier R. A review of the kinetics adsorption models and their application to the adsorption of lead by an activated carbon. Chem Eng Res Des. 2016;109:495–504. 10.1016/j.cherd.2016.02.006.Suche in Google Scholar
[45] Arris S, Lehocine MB, Meniai AH. Sorption study of chromium sorption from wastewater using cereal by-products. Int J Hydrog Energy. 2016;41(24):10299–310. 10.1016/j.ijhydene.2014.09.147.Suche in Google Scholar
[46] Kong XK, Han ZT, Zhang W, Song L, Li H. Synthesis of zeolite-supported microscale zero-valent iron for the removal of Cr6+ and Cd2+ from aqueous solution. J Environ Manage. 2016;169:84–90. 10.1016/j.jenvman.2015.12.022.Suche in Google Scholar PubMed
[47] Lima EC, Gomes AA, Tran HN. Comparison of the nonlinear and linear forms of the van’t Hoff equation for calculation of adsorption thermodynamic parameters (∆S° and ∆H°). J Mol Liq. 2020;311:113315. 10.1016/j.molliq.2020.113315.Suche in Google Scholar
© 2023 the author(s), published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Artikel in diesem Heft
- Research Articles
- Value-added utilization of coal fly ash and recycled polyvinyl chloride in door or window sub-frame composites
- High removal efficiency of volatile phenol from coking wastewater using coal gasification slag via optimized adsorption and multi-grade batch process
- Evolution of surface morphology and properties of diamond films by hydrogen plasma etching
- Removal efficiency of dibenzofuran using CuZn-zeolitic imidazole frameworks as a catalyst and adsorbent
- Rapid and efficient microwave-assisted extraction of Caesalpinia sappan Linn. heartwood and subsequent synthesis of gold nanoparticles
- The catalytic characteristics of 2-methylnaphthalene acylation with AlCl3 immobilized on Hβ as Lewis acid catalyst
- Biodegradation of synthetic PVP biofilms using natural materials and nanoparticles
- Rutin-loaded selenium nanoparticles modulated the redox status, inflammatory, and apoptotic pathways associated with pentylenetetrazole-induced epilepsy in mice
- Optimization of apigenin nanoparticles prepared by planetary ball milling: In vitro and in vivo studies
- Synthesis and characterization of silver nanoparticles using Origanum onites leaves: Cytotoxic, apoptotic, and necrotic effects on Capan-1, L929, and Caco-2 cell lines
- Exergy analysis of a conceptual CO2 capture process with an amine-based DES
- Construction of fluorescence system of felodipine–tetracyanovinyl–2,2′-bipyridine complex
- Excellent photocatalytic degradation of rhodamine B over Bi2O3 supported on Zn-MOF nanocomposites under visible light
- Optimization-based control strategy for a large-scale polyhydroxyalkanoates production in a fed-batch bioreactor using a coupled PDE–ODE system
- Effectiveness of pH and amount of Artemia urumiana extract on physical, chemical, and biological attributes of UV-fabricated biogold nanoparticles
- Geranium leaf-mediated synthesis of silver nanoparticles and their transcriptomic effects on Candida albicans
- Synthesis, characterization, anticancer, anti-inflammatory activities, and docking studies of 3,5-disubstituted thiadiazine-2-thiones
- Synthesis and stability of phospholipid-encapsulated nano-selenium
- Putative anti-proliferative effect of Indian mustard (Brassica juncea) seed and its nano-formulation
- Enrichment of low-grade phosphorites by the selective leaching method
- Electrochemical analysis of the dissolution of gold in a copper–ethylenediamine–thiosulfate system
- Characterisation of carbonate lake sediments as a potential filler for polymer composites
- Evaluation of nano-selenium biofortification characteristics of alfalfa (Medicago sativa L.)
- Quality of oil extracted by cold press from Nigella sativa seeds incorporated with rosemary extracts and pretreated by microwaves
- Heteropolyacid-loaded MOF-derived mesoporous zirconia catalyst for chemical degradation of rhodamine B
- Recovery of critical metals from carbonatite-type mineral wastes: Geochemical modeling investigation of (bio)hydrometallurgical leaching of REEs
- Photocatalytic properties of ZnFe-mixed oxides synthesized via a simple route for water remediation
- Attenuation of di(2-ethylhexyl)phthalate-induced hepatic and renal toxicity by naringin nanoparticles in a rat model
- Novel in situ synthesis of quaternary core–shell metallic sulfide nanocomposites for degradation of organic dyes and hydrogen production
- Microfluidic steam-based synthesis of luminescent carbon quantum dots as sensing probes for nitrite detection
- Transformation of eggshell waste to egg white protein solution, calcium chloride dihydrate, and eggshell membrane powder
- Preparation of Zr-MOFs for the adsorption of doxycycline hydrochloride from wastewater
- Green nanoarchitectonics of the silver nanocrystal potential for treating malaria and their cytotoxic effects on the kidney Vero cell line
- Carbon emissions analysis of producing modified asphalt with natural asphalt
- An efficient and green synthesis of 2-phenylquinazolin-4(3H)-ones via t-BuONa-mediated oxidative condensation of 2-aminobenzamides and benzyl alcohols under solvent- and transition metal-free conditions
- Chitosan nanoparticles loaded with mesosulfuron methyl and mesosulfuron methyl + florasulam + MCPA isooctyl to manage weeds of wheat (Triticum aestivum L.)
- Synergism between lignite and high-sulfur petroleum coke in CO2 gasification
- Facile aqueous synthesis of ZnCuInS/ZnS–ZnS QDs with enhanced photoluminescence lifetime for selective detection of Cu(ii) ions
- Rapid synthesis of copper nanoparticles using Nepeta cataria leaves: An eco-friendly management of disease-causing vectors and bacterial pathogens
- Study on the photoelectrocatalytic activity of reduced TiO2 nanotube films for removal of methyl orange
- Development of a fuzzy logic model for the prediction of spark-ignition engine performance and emission for gasoline–ethanol blends
- Micro-impact-induced mechano-chemical synthesis of organic precursors from FeC/FeN and carbonates/nitrates in water and its extension to nucleobases
- Green synthesis of strontium-doped tin dioxide (SrSnO2) nanoparticles using the Mahonia bealei leaf extract and evaluation of their anticancer and antimicrobial activities
- A study on the larvicidal and adulticidal potential of Cladostepus spongiosus macroalgae and green-fabricated silver nanoparticles against mosquito vectors
- Catalysts based on nickel salt heteropolytungstates for selective oxidation of diphenyl sulfide
- Powerful antibacterial nanocomposites from Corallina officinalis-mediated nanometals and chitosan nanoparticles against fish-borne pathogens
- Removal behavior of Zn and alkalis from blast furnace dust in pre-reduction sinter process
- Environmentally friendly synthesis and computational studies of novel class of acridinedione integrated spirothiopyrrolizidines/indolizidines
- The mechanisms of inhibition and lubrication of clean fracturing flowback fluids in water-based drilling fluids
- Adsorption/desorption performance of cellulose membrane for Pb(ii)
- A one-pot, multicomponent tandem synthesis of fused polycyclic pyrrolo[3,2-c]quinolinone/pyrrolizino[2,3-c]quinolinone hybrid heterocycles via environmentally benign solid state melt reaction
- Green synthesis of silver nanoparticles using durian rind extract and optical characteristics of surface plasmon resonance-based optical sensor for the detection of hydrogen peroxide
- Electrochemical analysis of copper-EDTA-ammonia-gold thiosulfate dissolution system
- Characterization of bio-oil production by microwave pyrolysis from cashew nut shells and Cassia fistula pods
- Green synthesis methods and characterization of bacterial cellulose/silver nanoparticle composites
- Photocatalytic research performance of zinc oxide/graphite phase carbon nitride catalyst and its application in environment
- Effect of phytogenic iron nanoparticles on the bio-fortification of wheat varieties
- In vitro anti-cancer and antimicrobial effects of manganese oxide nanoparticles synthesized using the Glycyrrhiza uralensis leaf extract on breast cancer cell lines
- Preparation of Pd/Ce(F)-MCM-48 catalysts and their catalytic performance of n-heptane isomerization
- Green “one-pot” fluorescent bis-indolizine synthesis with whole-cell plant biocatalysis
- Silica-titania mesoporous silicas of MCM-41 type as effective catalysts and photocatalysts for selective oxidation of diphenyl sulfide by H2O2
- Biosynthesis of zinc oxide nanoparticles from molted feathers of Pavo cristatus and their antibiofilm and anticancer activities
- Clean preparation of rutile from Ti-containing mixed molten slag by CO2 oxidation
- Synthesis and characterization of Pluronic F-127-coated titanium dioxide nanoparticles synthesized from extracts of Atractylodes macrocephala leaf for antioxidant, antimicrobial, and anticancer properties
- Effect of pretreatment with alkali on the anaerobic digestion characteristics of kitchen waste and analysis of microbial diversity
- Ameliorated antimicrobial, antioxidant, and anticancer properties by Plectranthus vettiveroides root extract-mediated green synthesis of chitosan nanoparticles
- Microwave-accelerated pretreatment technique in green extraction of oil and bioactive compounds from camelina seeds: Effectiveness and characterization
- Studies on the extraction performance of phorate by aptamer-functionalized magnetic nanoparticles in plasma samples
- Investigation of structural properties and antibacterial activity of AgO nanoparticle extract from Solanum nigrum/Mentha leaf extracts by green synthesis method
- Green fabrication of chitosan from marine crustaceans and mushroom waste: Toward sustainable resource utilization
- Synthesis, characterization, and evaluation of nanoparticles of clodinofop propargyl and fenoxaprop-P-ethyl on weed control, growth, and yield of wheat (Triticum aestivum L.)
- The enhanced adsorption properties of phosphorus from aqueous solutions using lanthanum modified synthetic zeolites
- Separation of graphene oxides of different sizes by multi-layer dialysis and anti-friction and lubrication performance
- Visible-light-assisted base-catalyzed, one-pot synthesis of highly functionalized cinnolines
- The experimental study on the air oxidation of 5-hydroxymethylfurfural to 2,5-furandicarboxylic acid with Co–Mn–Br system
- Highly efficient removal of tetracycline and methyl violet 2B from aqueous solution using the bimetallic FeZn-ZIFs catalyst
- A thermo-tolerant cellulase enzyme produced by Bacillus amyloliquefaciens M7, an insight into synthesis, optimization, characterization, and bio-polishing activity
- Exploration of ketone derivatives of succinimide for their antidiabetic potential: In vitro and in vivo approaches
- Ultrasound-assisted green synthesis and in silico study of 6-(4-(butylamino)-6-(diethylamino)-1,3,5-triazin-2-yl)oxypyridazine derivatives
- A study of the anticancer potential of Pluronic F-127 encapsulated Fe2O3 nanoparticles derived from Berberis vulgaris extract
- Biogenic synthesis of silver nanoparticles using Consolida orientalis flowers: Identification, catalytic degradation, and biological effect
- Initial assessment of the presence of plastic waste in some coastal mangrove forests in Vietnam
- Adsorption synergy electrocatalytic degradation of phenol by active oxygen-containing species generated in Co-coal based cathode and graphite anode
- Antibacterial, antifungal, antioxidant, and cytotoxicity activities of the aqueous extract of Syzygium aromaticum-mediated synthesized novel silver nanoparticles
- Synthesis of a silica matrix with ZnO nanoparticles for the fabrication of a recyclable photodegradation system to eliminate methylene blue dye
- Natural polymer fillers instead of dye and pigments: Pumice and scoria in PDMS fluid and elastomer composites
- Study on the preparation of glycerylphosphorylcholine by transesterification under supported sodium methoxide
- Wireless network handheld terminal-based green ecological sustainable design evaluation system: Improved data communication and reduced packet loss rate
- The optimization of hydrogel strength from cassava starch using oxidized sucrose as a crosslinking agent
- Green synthesis of silver nanoparticles using Saccharum officinarum leaf extract for antiviral paint
- Study on the reliability of nano-silver-coated tin solder joints for flip chips
- Environmentally sustainable analytical quality by design aided RP-HPLC method for the estimation of brilliant blue in commercial food samples employing a green-ultrasound-assisted extraction technique
- Anticancer and antimicrobial potential of zinc/sodium alginate/polyethylene glycol/d-pinitol nanocomposites against osteosarcoma MG-63 cells
- Nanoporous carbon@CoFe2O4 nanocomposite as a green absorbent for the adsorptive removal of Hg(ii) from aqueous solutions
- Characterization of silver sulfide nanoparticles from actinobacterial strain (M10A62) and its toxicity against lepidopteran and dipterans insect species
- Phyto-fabrication and characterization of silver nanoparticles using Withania somnifera: Investigating antioxidant potential
- Effect of e-waste nanofillers on the mechanical, thermal, and wear properties of epoxy-blend sisal woven fiber-reinforced composites
- Magnesium nanohydroxide (2D brucite) as a host matrix for thymol and carvacrol: Synthesis, characterization, and inhibition of foodborne pathogens
- Synergistic inhibitive effect of a hybrid zinc oxide-benzalkonium chloride composite on the corrosion of carbon steel in a sulfuric acidic solution
- Review Articles
- Role and the importance of green approach in biosynthesis of nanopropolis and effectiveness of propolis in the treatment of COVID-19 pandemic
- Gum tragacanth-mediated synthesis of metal nanoparticles, characterization, and their applications as a bactericide, catalyst, antioxidant, and peroxidase mimic
- Green-processed nano-biocomposite (ZnO–TiO2): Potential candidates for biomedical applications
- Reaction mechanisms in microwave-assisted lignin depolymerisation in hydrogen-donating solvents
- Recent progress on non-noble metal catalysts for the deoxydehydration of biomass-derived oxygenates
- Rapid Communication
- Phosphorus removal by iron–carbon microelectrolysis: A new way to achieve phosphorus recovery
- Special Issue: Biomolecules-derived synthesis of nanomaterials for environmental and biological applications (Guest Editors: Arpita Roy and Fernanda Maria Policarpo Tonelli)
- Biomolecules-derived synthesis of nanomaterials for environmental and biological applications
- Nano-encapsulated tanshinone IIA in PLGA-PEG-COOH inhibits apoptosis and inflammation in cerebral ischemia/reperfusion injury
- Green fabrication of silver nanoparticles using Melia azedarach ripened fruit extract, their characterization, and biological properties
- Green-synthesized nanoparticles and their therapeutic applications: A review
- Antioxidant, antibacterial, and cytotoxicity potential of synthesized silver nanoparticles from the Cassia alata leaf aqueous extract
- Green synthesis of silver nanoparticles using Callisia fragrans leaf extract and its anticancer activity against MCF-7, HepG2, KB, LU-1, and MKN-7 cell lines
- Algae-based green AgNPs, AuNPs, and FeNPs as potential nanoremediators
- Green synthesis of Kickxia elatine-induced silver nanoparticles and their role as anti-acetylcholinesterase in the treatment of Alzheimer’s disease
- Phytocrystallization of silver nanoparticles using Cassia alata flower extract for effective control of fungal skin pathogens
- Antibacterial wound dressing with hydrogel from chitosan and polyvinyl alcohol from the red cabbage extract loaded with silver nanoparticles
- Leveraging of mycogenic copper oxide nanostructures for disease management of Alternaria blight of Brassica juncea
- Nanoscale molecular reactions in microbiological medicines in modern medical applications
- Synthesis and characterization of ZnO/β-cyclodextrin/nicotinic acid nanocomposite and its biological and environmental application
- Green synthesis of silver nanoparticles via Taxus wallichiana Zucc. plant-derived Taxol: Novel utilization as anticancer, antioxidation, anti-inflammation, and antiurolithic potential
- Recyclability and catalytic characteristics of copper oxide nanoparticles derived from bougainvillea plant flower extract for biomedical application
- Phytofabrication, characterization, and evaluation of novel bioinspired selenium–iron (Se–Fe) nanocomposites using Allium sativum extract for bio-potential applications
- Erratum
- Erratum to “Synthesis, characterization, and evaluation of nanoparticles of clodinofop propargyl and fenoxaprop-P-ethyl on weed control, growth, and yield of wheat (Triticum aestivum L.)”
Artikel in diesem Heft
- Research Articles
- Value-added utilization of coal fly ash and recycled polyvinyl chloride in door or window sub-frame composites
- High removal efficiency of volatile phenol from coking wastewater using coal gasification slag via optimized adsorption and multi-grade batch process
- Evolution of surface morphology and properties of diamond films by hydrogen plasma etching
- Removal efficiency of dibenzofuran using CuZn-zeolitic imidazole frameworks as a catalyst and adsorbent
- Rapid and efficient microwave-assisted extraction of Caesalpinia sappan Linn. heartwood and subsequent synthesis of gold nanoparticles
- The catalytic characteristics of 2-methylnaphthalene acylation with AlCl3 immobilized on Hβ as Lewis acid catalyst
- Biodegradation of synthetic PVP biofilms using natural materials and nanoparticles
- Rutin-loaded selenium nanoparticles modulated the redox status, inflammatory, and apoptotic pathways associated with pentylenetetrazole-induced epilepsy in mice
- Optimization of apigenin nanoparticles prepared by planetary ball milling: In vitro and in vivo studies
- Synthesis and characterization of silver nanoparticles using Origanum onites leaves: Cytotoxic, apoptotic, and necrotic effects on Capan-1, L929, and Caco-2 cell lines
- Exergy analysis of a conceptual CO2 capture process with an amine-based DES
- Construction of fluorescence system of felodipine–tetracyanovinyl–2,2′-bipyridine complex
- Excellent photocatalytic degradation of rhodamine B over Bi2O3 supported on Zn-MOF nanocomposites under visible light
- Optimization-based control strategy for a large-scale polyhydroxyalkanoates production in a fed-batch bioreactor using a coupled PDE–ODE system
- Effectiveness of pH and amount of Artemia urumiana extract on physical, chemical, and biological attributes of UV-fabricated biogold nanoparticles
- Geranium leaf-mediated synthesis of silver nanoparticles and their transcriptomic effects on Candida albicans
- Synthesis, characterization, anticancer, anti-inflammatory activities, and docking studies of 3,5-disubstituted thiadiazine-2-thiones
- Synthesis and stability of phospholipid-encapsulated nano-selenium
- Putative anti-proliferative effect of Indian mustard (Brassica juncea) seed and its nano-formulation
- Enrichment of low-grade phosphorites by the selective leaching method
- Electrochemical analysis of the dissolution of gold in a copper–ethylenediamine–thiosulfate system
- Characterisation of carbonate lake sediments as a potential filler for polymer composites
- Evaluation of nano-selenium biofortification characteristics of alfalfa (Medicago sativa L.)
- Quality of oil extracted by cold press from Nigella sativa seeds incorporated with rosemary extracts and pretreated by microwaves
- Heteropolyacid-loaded MOF-derived mesoporous zirconia catalyst for chemical degradation of rhodamine B
- Recovery of critical metals from carbonatite-type mineral wastes: Geochemical modeling investigation of (bio)hydrometallurgical leaching of REEs
- Photocatalytic properties of ZnFe-mixed oxides synthesized via a simple route for water remediation
- Attenuation of di(2-ethylhexyl)phthalate-induced hepatic and renal toxicity by naringin nanoparticles in a rat model
- Novel in situ synthesis of quaternary core–shell metallic sulfide nanocomposites for degradation of organic dyes and hydrogen production
- Microfluidic steam-based synthesis of luminescent carbon quantum dots as sensing probes for nitrite detection
- Transformation of eggshell waste to egg white protein solution, calcium chloride dihydrate, and eggshell membrane powder
- Preparation of Zr-MOFs for the adsorption of doxycycline hydrochloride from wastewater
- Green nanoarchitectonics of the silver nanocrystal potential for treating malaria and their cytotoxic effects on the kidney Vero cell line
- Carbon emissions analysis of producing modified asphalt with natural asphalt
- An efficient and green synthesis of 2-phenylquinazolin-4(3H)-ones via t-BuONa-mediated oxidative condensation of 2-aminobenzamides and benzyl alcohols under solvent- and transition metal-free conditions
- Chitosan nanoparticles loaded with mesosulfuron methyl and mesosulfuron methyl + florasulam + MCPA isooctyl to manage weeds of wheat (Triticum aestivum L.)
- Synergism between lignite and high-sulfur petroleum coke in CO2 gasification
- Facile aqueous synthesis of ZnCuInS/ZnS–ZnS QDs with enhanced photoluminescence lifetime for selective detection of Cu(ii) ions
- Rapid synthesis of copper nanoparticles using Nepeta cataria leaves: An eco-friendly management of disease-causing vectors and bacterial pathogens
- Study on the photoelectrocatalytic activity of reduced TiO2 nanotube films for removal of methyl orange
- Development of a fuzzy logic model for the prediction of spark-ignition engine performance and emission for gasoline–ethanol blends
- Micro-impact-induced mechano-chemical synthesis of organic precursors from FeC/FeN and carbonates/nitrates in water and its extension to nucleobases
- Green synthesis of strontium-doped tin dioxide (SrSnO2) nanoparticles using the Mahonia bealei leaf extract and evaluation of their anticancer and antimicrobial activities
- A study on the larvicidal and adulticidal potential of Cladostepus spongiosus macroalgae and green-fabricated silver nanoparticles against mosquito vectors
- Catalysts based on nickel salt heteropolytungstates for selective oxidation of diphenyl sulfide
- Powerful antibacterial nanocomposites from Corallina officinalis-mediated nanometals and chitosan nanoparticles against fish-borne pathogens
- Removal behavior of Zn and alkalis from blast furnace dust in pre-reduction sinter process
- Environmentally friendly synthesis and computational studies of novel class of acridinedione integrated spirothiopyrrolizidines/indolizidines
- The mechanisms of inhibition and lubrication of clean fracturing flowback fluids in water-based drilling fluids
- Adsorption/desorption performance of cellulose membrane for Pb(ii)
- A one-pot, multicomponent tandem synthesis of fused polycyclic pyrrolo[3,2-c]quinolinone/pyrrolizino[2,3-c]quinolinone hybrid heterocycles via environmentally benign solid state melt reaction
- Green synthesis of silver nanoparticles using durian rind extract and optical characteristics of surface plasmon resonance-based optical sensor for the detection of hydrogen peroxide
- Electrochemical analysis of copper-EDTA-ammonia-gold thiosulfate dissolution system
- Characterization of bio-oil production by microwave pyrolysis from cashew nut shells and Cassia fistula pods
- Green synthesis methods and characterization of bacterial cellulose/silver nanoparticle composites
- Photocatalytic research performance of zinc oxide/graphite phase carbon nitride catalyst and its application in environment
- Effect of phytogenic iron nanoparticles on the bio-fortification of wheat varieties
- In vitro anti-cancer and antimicrobial effects of manganese oxide nanoparticles synthesized using the Glycyrrhiza uralensis leaf extract on breast cancer cell lines
- Preparation of Pd/Ce(F)-MCM-48 catalysts and their catalytic performance of n-heptane isomerization
- Green “one-pot” fluorescent bis-indolizine synthesis with whole-cell plant biocatalysis
- Silica-titania mesoporous silicas of MCM-41 type as effective catalysts and photocatalysts for selective oxidation of diphenyl sulfide by H2O2
- Biosynthesis of zinc oxide nanoparticles from molted feathers of Pavo cristatus and their antibiofilm and anticancer activities
- Clean preparation of rutile from Ti-containing mixed molten slag by CO2 oxidation
- Synthesis and characterization of Pluronic F-127-coated titanium dioxide nanoparticles synthesized from extracts of Atractylodes macrocephala leaf for antioxidant, antimicrobial, and anticancer properties
- Effect of pretreatment with alkali on the anaerobic digestion characteristics of kitchen waste and analysis of microbial diversity
- Ameliorated antimicrobial, antioxidant, and anticancer properties by Plectranthus vettiveroides root extract-mediated green synthesis of chitosan nanoparticles
- Microwave-accelerated pretreatment technique in green extraction of oil and bioactive compounds from camelina seeds: Effectiveness and characterization
- Studies on the extraction performance of phorate by aptamer-functionalized magnetic nanoparticles in plasma samples
- Investigation of structural properties and antibacterial activity of AgO nanoparticle extract from Solanum nigrum/Mentha leaf extracts by green synthesis method
- Green fabrication of chitosan from marine crustaceans and mushroom waste: Toward sustainable resource utilization
- Synthesis, characterization, and evaluation of nanoparticles of clodinofop propargyl and fenoxaprop-P-ethyl on weed control, growth, and yield of wheat (Triticum aestivum L.)
- The enhanced adsorption properties of phosphorus from aqueous solutions using lanthanum modified synthetic zeolites
- Separation of graphene oxides of different sizes by multi-layer dialysis and anti-friction and lubrication performance
- Visible-light-assisted base-catalyzed, one-pot synthesis of highly functionalized cinnolines
- The experimental study on the air oxidation of 5-hydroxymethylfurfural to 2,5-furandicarboxylic acid with Co–Mn–Br system
- Highly efficient removal of tetracycline and methyl violet 2B from aqueous solution using the bimetallic FeZn-ZIFs catalyst
- A thermo-tolerant cellulase enzyme produced by Bacillus amyloliquefaciens M7, an insight into synthesis, optimization, characterization, and bio-polishing activity
- Exploration of ketone derivatives of succinimide for their antidiabetic potential: In vitro and in vivo approaches
- Ultrasound-assisted green synthesis and in silico study of 6-(4-(butylamino)-6-(diethylamino)-1,3,5-triazin-2-yl)oxypyridazine derivatives
- A study of the anticancer potential of Pluronic F-127 encapsulated Fe2O3 nanoparticles derived from Berberis vulgaris extract
- Biogenic synthesis of silver nanoparticles using Consolida orientalis flowers: Identification, catalytic degradation, and biological effect
- Initial assessment of the presence of plastic waste in some coastal mangrove forests in Vietnam
- Adsorption synergy electrocatalytic degradation of phenol by active oxygen-containing species generated in Co-coal based cathode and graphite anode
- Antibacterial, antifungal, antioxidant, and cytotoxicity activities of the aqueous extract of Syzygium aromaticum-mediated synthesized novel silver nanoparticles
- Synthesis of a silica matrix with ZnO nanoparticles for the fabrication of a recyclable photodegradation system to eliminate methylene blue dye
- Natural polymer fillers instead of dye and pigments: Pumice and scoria in PDMS fluid and elastomer composites
- Study on the preparation of glycerylphosphorylcholine by transesterification under supported sodium methoxide
- Wireless network handheld terminal-based green ecological sustainable design evaluation system: Improved data communication and reduced packet loss rate
- The optimization of hydrogel strength from cassava starch using oxidized sucrose as a crosslinking agent
- Green synthesis of silver nanoparticles using Saccharum officinarum leaf extract for antiviral paint
- Study on the reliability of nano-silver-coated tin solder joints for flip chips
- Environmentally sustainable analytical quality by design aided RP-HPLC method for the estimation of brilliant blue in commercial food samples employing a green-ultrasound-assisted extraction technique
- Anticancer and antimicrobial potential of zinc/sodium alginate/polyethylene glycol/d-pinitol nanocomposites against osteosarcoma MG-63 cells
- Nanoporous carbon@CoFe2O4 nanocomposite as a green absorbent for the adsorptive removal of Hg(ii) from aqueous solutions
- Characterization of silver sulfide nanoparticles from actinobacterial strain (M10A62) and its toxicity against lepidopteran and dipterans insect species
- Phyto-fabrication and characterization of silver nanoparticles using Withania somnifera: Investigating antioxidant potential
- Effect of e-waste nanofillers on the mechanical, thermal, and wear properties of epoxy-blend sisal woven fiber-reinforced composites
- Magnesium nanohydroxide (2D brucite) as a host matrix for thymol and carvacrol: Synthesis, characterization, and inhibition of foodborne pathogens
- Synergistic inhibitive effect of a hybrid zinc oxide-benzalkonium chloride composite on the corrosion of carbon steel in a sulfuric acidic solution
- Review Articles
- Role and the importance of green approach in biosynthesis of nanopropolis and effectiveness of propolis in the treatment of COVID-19 pandemic
- Gum tragacanth-mediated synthesis of metal nanoparticles, characterization, and their applications as a bactericide, catalyst, antioxidant, and peroxidase mimic
- Green-processed nano-biocomposite (ZnO–TiO2): Potential candidates for biomedical applications
- Reaction mechanisms in microwave-assisted lignin depolymerisation in hydrogen-donating solvents
- Recent progress on non-noble metal catalysts for the deoxydehydration of biomass-derived oxygenates
- Rapid Communication
- Phosphorus removal by iron–carbon microelectrolysis: A new way to achieve phosphorus recovery
- Special Issue: Biomolecules-derived synthesis of nanomaterials for environmental and biological applications (Guest Editors: Arpita Roy and Fernanda Maria Policarpo Tonelli)
- Biomolecules-derived synthesis of nanomaterials for environmental and biological applications
- Nano-encapsulated tanshinone IIA in PLGA-PEG-COOH inhibits apoptosis and inflammation in cerebral ischemia/reperfusion injury
- Green fabrication of silver nanoparticles using Melia azedarach ripened fruit extract, their characterization, and biological properties
- Green-synthesized nanoparticles and their therapeutic applications: A review
- Antioxidant, antibacterial, and cytotoxicity potential of synthesized silver nanoparticles from the Cassia alata leaf aqueous extract
- Green synthesis of silver nanoparticles using Callisia fragrans leaf extract and its anticancer activity against MCF-7, HepG2, KB, LU-1, and MKN-7 cell lines
- Algae-based green AgNPs, AuNPs, and FeNPs as potential nanoremediators
- Green synthesis of Kickxia elatine-induced silver nanoparticles and their role as anti-acetylcholinesterase in the treatment of Alzheimer’s disease
- Phytocrystallization of silver nanoparticles using Cassia alata flower extract for effective control of fungal skin pathogens
- Antibacterial wound dressing with hydrogel from chitosan and polyvinyl alcohol from the red cabbage extract loaded with silver nanoparticles
- Leveraging of mycogenic copper oxide nanostructures for disease management of Alternaria blight of Brassica juncea
- Nanoscale molecular reactions in microbiological medicines in modern medical applications
- Synthesis and characterization of ZnO/β-cyclodextrin/nicotinic acid nanocomposite and its biological and environmental application
- Green synthesis of silver nanoparticles via Taxus wallichiana Zucc. plant-derived Taxol: Novel utilization as anticancer, antioxidation, anti-inflammation, and antiurolithic potential
- Recyclability and catalytic characteristics of copper oxide nanoparticles derived from bougainvillea plant flower extract for biomedical application
- Phytofabrication, characterization, and evaluation of novel bioinspired selenium–iron (Se–Fe) nanocomposites using Allium sativum extract for bio-potential applications
- Erratum
- Erratum to “Synthesis, characterization, and evaluation of nanoparticles of clodinofop propargyl and fenoxaprop-P-ethyl on weed control, growth, and yield of wheat (Triticum aestivum L.)”

