A one-pot, multicomponent tandem synthesis of fused polycyclic pyrrolo[3,2-c]quinolinone/pyrrolizino[2,3-c]quinolinone hybrid heterocycles via environmentally benign solid state melt reaction
-
Suresh Mani
, Natarajan Arumugam
, Abdulrahman I. Almansour
Abstract
Structurally diverse fused polycyclic pyrrolo[3,2-c]quinolinone/pyrrolizino[2,3-c]quinolinone hybrids were synthesized to get excellent yields via a tandem multi-component reaction sequence employing an environmentally benign solid state melt reaction involving [3+2]-cycloaddition process followed by two consecutive annulation steps. Baylis–Hillman products, used as dipolarophiles, were synthesized from various substituted aryl/heteroaryl aldehydes in the presence of DABCO and methyl acrylate, while the 1,3-dipole component was derived in situ from indoline-2,3-dione and acyclic/cyclic amino acid viz N-methylgylcine/l-proline. The structure of the unusual tandem products was unambiguously assigned by spectroscopic and XRD analysis. The products arose through the formation of three new rings, five new bonds, and three adjoining stereocenters with complete diastereomeric control.
1 Introduction
Expedient one-pot assembly of structurally interesting polycyclic ring systems with multiple stereogenic centers from available simple starting precursors is of great value in pharmaceutical companies [1] even in place of diversity-oriented and combinatorial synthesis [2,3]. One such methodology to achieve these goals involves the use of tandem multicomponent sequence [4], that allow the generation of multiple bonds in a one-pot synthetic transformation with noteworthy advantages such as high reaction efficacy, cost saving, convergence, elegance, facile automation, and reduction in the number of work-ups. This protocol obviates a number of isolation and purification steps resulting in enhancement of overall yield relative to classical multi-step synthetic transformations. Due to the advantages mentioned above, this protocol is environmentally friendly and excellently suited for the creation of structurally intriguing heterocycles tethering several adjoining stereocenters as well as for the synthesis of biologically attractive natural and synthetic products [5].
Three component cycloaddition reaction of 1,3-dipole with activated double bond of a dipolarophile offers a versatile methodology to construct regio and stereoselective pyrrolidine heterocycles [6,7,8]. The preparation of the pyrrolidine structural moiety is of particular interest due to the presence of this ring system in many bioactive natural and synthetic products and it exhibits attractive structural features and diverse bioactivity profiles rendering them as propitious synthetic targets. In addition, the pyrrolidine unit aid as very useful molecular architectures for probing the pharmacophore space using diversity-oriented synthesis which in turn leads to the development of new drug candidate [9,10,11].
Polycyclic compounds that comprise a pyrrolidine unit viz pyrroloquinoline is an important class of structural component as these analogs appear as an integral part in many natural products including melodinus alkaloids, (+) scandine, (+) meloscine (pentacyclic) [12,13] (Figure 1) which are prescribed as Chinese medicine to treat rheumatic heart disease in children. Molecules possessing the 3H-pyrrolo[2,3-c]quinoline unit such as marinoquinolines A–F and aplidiopsamine A displayed potent antimalarial activity with less toxicity to human cells [14]. Tricyclic angular heterocycle with pyrrolo[3,2-c]quinoline moiety showed promising biological activity [15]. For instance, antitumor properties, gastric (H+/K+)-ATPase inhibitor, aggrecanase inhibitors [16], hypotensive, anti-inflammatory activities [18,19,20], and significant photochemotherapeutic activity [17].
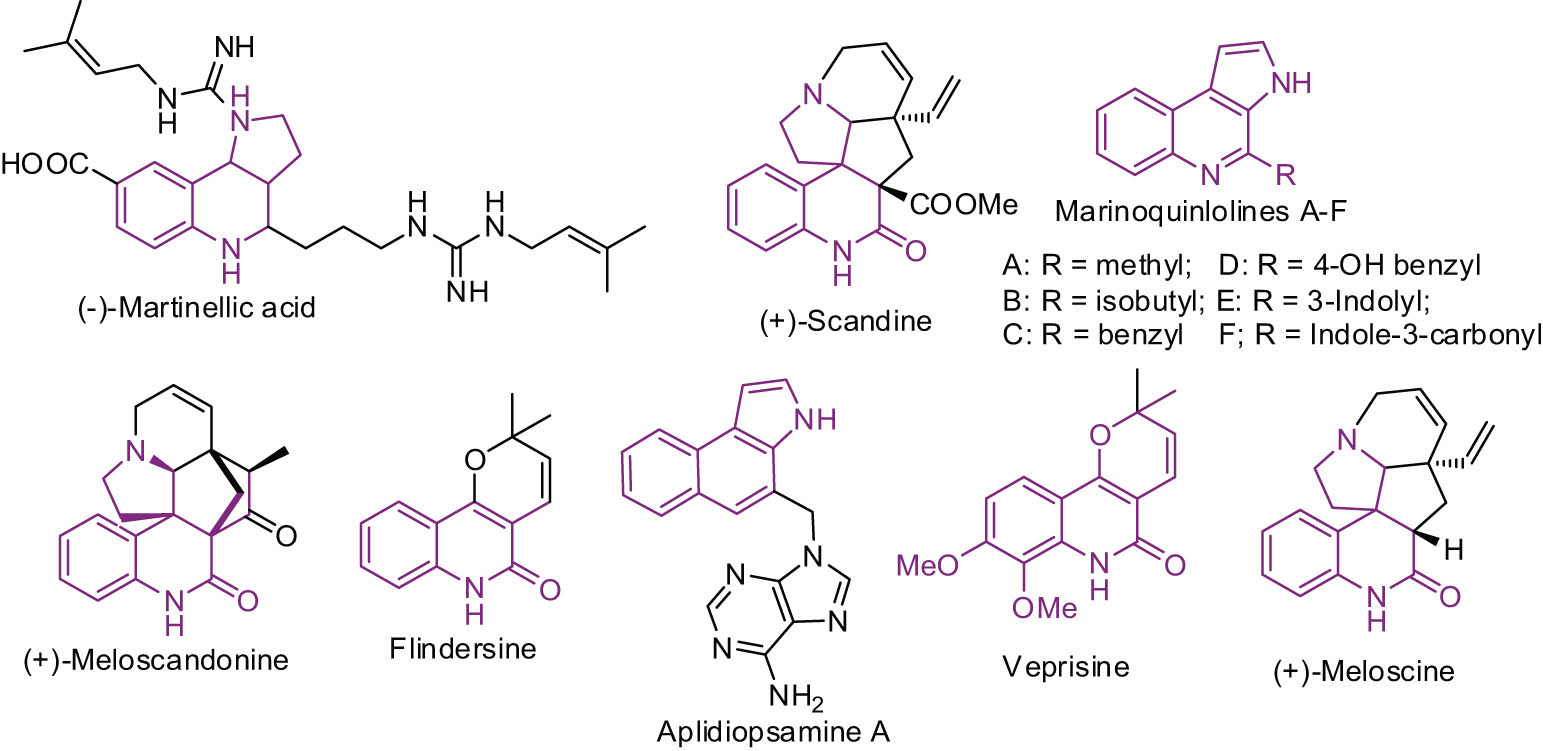
Biologically relevant pyrroloquinoline analogs.
Our research team has been mainly engaged in the synthesis of bridged pyrrolidine hybrids via multi-component cycloaddition and tandem reaction protocol [21], and studies on their biological intervention in recent years, which has brought to light various biological [22,23,24,25] lead compounds. Baylis–Hillman adducts (BHAs) are useful precursor for the production of diverse natural and synthetic analogs of biological importance. In this perspective, we recently reported that unusual pyrroloquinolinone fused polycyclic analogs were synthesized from Baylis–Hillman product by tandem multicomponent cascade protocol [26].
With the above remarkable biological precedents in mind, we have now explored the synthetic utility of Baylis–Hillman product as starting precursor in the construction of novel class of heterocyclic systems comprising the pyrrolo[3,2-c]quinolinone/pyrrolizino[2,3-c]quinolinone framework through sustainable green tandem protocol involving a decarboxylative [3+2]-dipolar cycloaddition followed by a double annulation via sequential lactonization and lactamization reactions. The synthetic strategy is described in Figure 2.
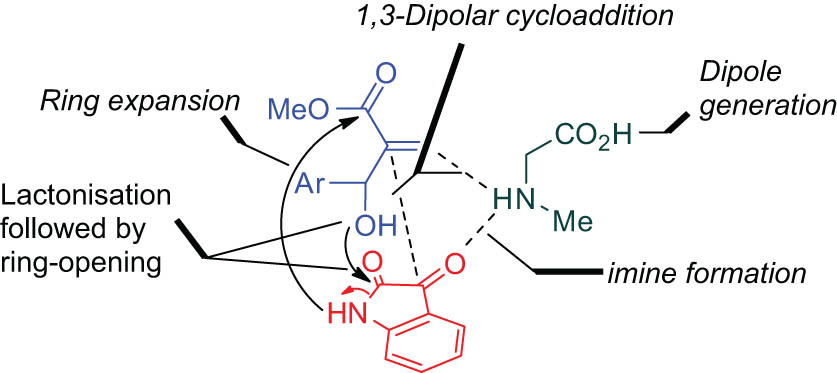
Synthetic strategy for the multicomponent domino protocol.
2 Experimental methods
2.1 General procedure for synthesis of aryl substituted polycyclic fused pyrrolidine derivatives, 10a–k
A mixture of BHA 3a (1 mmol), isatin 7 (1.1 mmol) and sarcosine 8 (1.1 mmol) was placed in a round bottomed flask and melted at 180°C and kept until the reaction was completed, confirmed by TLC analysis. The crude product was recrystallized from ethyl acetate (EtOAc) and hexane to obtain the pure products 10a as a solid.
2.1.1 1-Methyl-12-phenyl-2,3-dihydro-1H-3a,9b-(methanooxymethano)pyrrolo[3,2-c]quinoline-4,10(5H)-dione, 10a
IR (KBr): 1,709, 1,742 cm−1; 1H NMR (300 MHz, CDCl3): δ 2.05–2.14 (m, 1H), 2.41–2.52 (m, 1H), 2.62 (s, –NCH 3 , 3H), 2.74 (q, J = 9.0, 17.7 Hz, 1H), 3.06–3.14 (m, 1H), 5.43 (s, 1H), 7.01 (d, J = 7.8 Hz, 1H), 7.18 (t, J = 7.5 Hz, 1H), 7.38–7.42 (m, 6H), 7.80 (d, J = 7.5 Hz, 1H), 9.78 (s, 1H, N–H). 13C NMR (75 MHz, CDCl3): 26.7, 34.3, 52.4, 58.9, 73.1, 79.4, 114.6, 116.4, 123.8, 126.2, 128.3, 128.7, 129.9, 130.8, 134.1, 136.7, 169.9, 173.6 ppm. Mass: m/z 334 (M+). Anal. calculated for C20H18N2O3: C, 71.94%, H, 5.43%, N, 8.38%; found: C, 71.99%, H, 5.50%, N, 8.35%.
2.1.2 1-Methyl-12-(2,3-dimethoxyphenyl)-1-methyl-2,3-dihydro-1H-3a,9b-(methanooxymethano)pyrrolo[3,2-c]quinoline-4,10(5H)-dione, 10b
IR (KBr): 1,715, 1,745 cm−1; 1H NMR (300 MHz, CDCl3): δ 2.25–2.55 (m, 2H), 2.58 (s, –NCH 3 , 3H), 2.76–2.84 (q, J = 9.0, 9.3 Hz, 1H), 3.05–3.12 (m, 1H), 3.74 (s, 3H), 3.80 (s, 3H), 5.70 (s, 1H), 6.92–7.39 (m, 6H), 7.75 (d, J = 7.5 Hz, 1H), 9.73 (s, 1H, N–H). 13C NMR (75 MHz, CDCl3): 25.9, 34.5, 52.6, 55.6, 59.1, 60.6, 73.7, 75.3, 113.2, 114.4, 116.7, 119.2, 123.3, 123.4, 126.9, 129.8, 130.6, 137.5, 146.7, 152.1, 169.2, 174.2 ppm. Mass: m/z 394 (M+). Anal. calculated for C22H22N2O5: C, 66.99%, H, 5.62%, N, 7.10%; found: C, 67.02%, H, 5.66%, N, 7.15%.
2.1.3 1-Methyl-12-(3,4-dimethoxyphenyl)-1-methyl-2,3-dihydro-1H-3a,9b-(methanooxymethano)pyrrolo[3,2-c]quinoline-4,10(5H)-dione, 10e
IR (KBr): 1,715, 1,745 cm−1; 1H NMR (300 MHz, CDCl3): δ 2.04–2.16 (m, 1H), 2.47–2.57 (m, 1H), 2.61 (s, –NCH 3 , 3H), 2.73 (q, J = 9.0, 17.7 Hz, 1H), 3.07–3.14 (m, 1H), 3.84 (s, 3H), 3.88 (s, 3H), 5.40 (s, 1H), 6.84 (d, J = 8.7 Hz, 1H), 6.94–7.03 (m, 3H), 7.21 (t, J = 7.5 Hz, 1H), 7.40 (t, J = 7.5 Hz, 1H), 7.79 (d, J = 7.5 Hz, 1H), 9.96 (s, 1H, N–H). 13C NMR (75 MHz, CDCl3): 26.8, 34.3, 52.4, 55.8, 56.0, 59.1, 73.1, 79.2, 109.5, 110.8, 114.6, 116.4, 118.7, 123.8, 126.6, 129.9, 130.7, 136.7, 148.8, 149.2, 170.2, 173.6 ppm. Mass: m/z 394 (M+). Anal. calculated for C22H22N2O5: C, 66.99%, H, 5.62%, N, 7.10%; found: C, 67.03%, H, 5.65%, N, 7.17%.
2.1.4 1-Methyl-12-(m-tolyl)-2,3-dihydro-1H-3a,9b-(methanooxymethano)pyrrolo[3,2-c]quinoline-4,10(5H)-dione, 10f
IR (KBr): 1,707, 1,751 cm−1; 1H NMR (300 MHz, CDCl3): δ 2.07–2.16 (m, 1H), 2.35 (s, –NCH3, 3H), 2.42–2.52 (m, 1H), 2.61 (s, 3H), 2.73 (q, J = 9.0, 17.7 Hz, 1H), 3.06–3.14 (m, 1H), 5.39 (s, 1H), 7.01 (d, J = 7.8 Hz, 1H), 7.15–7.28 (m, 5H), 7.41 (t, J = 6.9 Hz, 1H), 7.80 (d, J = 7.5 Hz, 1H), 9.74 (s, 1H, –NH). 13C NMR (75 MHz, CDCl3): 21.5, 26.7, 34.3, 52.4, 58.9, 73.1, 79.5, 114.6, 116.4, 123.4, 123.8, 126.7, 128.2, 129.5, 129.9, 130.7, 134.0, 136.7, 138.0, 169.9, 173.7 ppm. HRMS calculated for C21H20N2O3: 348.1547 and found 348.1543.
2.1.5 1-Methyl-12-(p-tolyl)-2,3-dihydro-1H-3a,9b-(methanooxymethano)pyrrolo[3,2-c]quinoline-4,10(5H)-dione, 10g
IR (KBr): 1,715, 1,748 cm−1; 1H NMR (300 MHz, CDCl3): δ 2.04–2.15 (m, 1H), 2.36 (s, –NCH 3 , 3H), 2.40–2.51 (m, 1H), 2.61 (s, –CH3, 3H), 2.74 (q, J = 8.7, 17.4 Hz, 1H), 3.06–3.13 (m, 1H), 5.40 (s, 1H), 6.99 (d, J = 7.8 Hz, 1H), 7.17–7.28 (m, 5H), 7.39 (t, J = 7.8 Hz, 1H), 7.80 (d, J = 7.5 Hz, 1H), 9.94 (s, 1H, N–H). 13C NMR (75 MHz, CDCl3): 21.2, 26.7, 34.3, 52.4, 59.0, 73.1, 79.5, 114.6, 116.4, 123.8, 126.1, 129.0, 130.7. 131.0, 135.4, 136.7, 138.5, 169.8, 173.7 ppm. Mass: m/z 349 (M+). Anal. calculated for C21H20N2O3: C, 68.30%, H, 4.97%, N, 7.97%; found: C, 68.37%, H, 4.90%, N, 8.03%.
2.1.6 1-Methyl-12-(2-nitrophenyl)-2,3-dihydro-1H-3a,9b-(methanooxymethano)pyrrolo[3,2-c]quinoline-4,10(5H)-dione, 10h
IR (KBr): 1,725, 1,748 cm−1; 1H NMR (300 MHz, CDCl3): δ 1.94–2.02 (m, 1H), 2.32–2.43 (m, 1H), 2.56 (s, –NCH 3 , 3H), 2.75 (q, J = 9.3, 17.4 Hz, 1H), 3.03–3.11 (m, 1H), 6.74 (s, 1H), 6.98 (d, J = 9.0 Hz, 1H), 7.19 (t, J = 7.5 Hz, 1H), 7.44 (t, J = 7.5 Hz, 1H), 7.59–7.69 (m, 2H), 7.83 (d, J = 7.8 Hz, 1H), 8.17 (d, J = 7.2 Hz, 1H), 8.26 (s, 1H, N–H). 13C NMR (75 MHz, CDCl3): 26.5, 34.4, 52.5, 58.3, 73.4, 74.2, 114.1, 116.3, 123.7, 126.2, 128.3, 129.7, 129.8, 129.9, 130.9, 133.6, 136.9, 146.7, 168.5, 172.9 ppm. Mass: m/z: 380 (M+). Anal. calculated for C20H17N3O5: C, 63.32%, H, 4.52%, N, 11.08%; found: C, 63.37%, H, 4.49%, N, 11.12%.
2.1.7 12-(2-Bromophenyl)-1-methyl-2,3-dihydro-1H-3a,9b-(methanooxymethano)pyrrolo[3,2-c]quinoline-4,10(5H)-dione, 10j
IR (KBr): 1,708, 1,744 cm−1; 1H NMR (300 MHz, CDCl3): δ 1.92–2.06 (m, 1H), 2.42–2.51 (m, 1H), 2.61 (s, –NCH 3 , 3H), 2.66–2.74 (m, 1H), 3.06–3.14 (m, 1H), 5.37 (s, 1H), 6.98–7.79 (m, 8H), 9.65 (s, 1H, N–H). 13C NMR (75 MHz, CDCl3): 26.8, 34.2, 52.3, 58.7, 73.0, 78.7, 114.5, 116.4, 122.9, 124.0, 126.1, 127.9, 129.9, 130.9, 131.5, 133.2, 136.6, 142.2, 169.7, 173.2 ppm. Mass: m/z 412 (M+). Anal. calculated for C20H17BrN2O3: C, 58.13%, H, 4.15%, N, 6.78%; found: C, 58.17%, H, 4.10%, N, 6.82%.
2.1.8 1-Methyl-12-(3-fluorophenyl)-2,3-dihydro-1H-3a,9b-(methanooxymethano)pyrrolo[3,2-c]quinoline-4,10(5H)-dione, 10k
IR (KBr): 1,710, 1,747 cm−1; 1H NMR (300 MHz, CDCl3): δ 2.03–2.09 (m, 1H), 2.45–2.55 (m, 1H), 2.61 (s, –NCH 3 , 3H), 2.75 (q, J = 9.3, 17.7 Hz, 1H), 3.08–3.15 (m, 1H), 5.41 (s, 1H), 6.99–7.09 (m, 2H), 7.17–7.23 (m, 3H), 7.32–7.45 (m, 2H), 7.78–7.80 (d, J = 7.5 Hz, 1H), 9.33 (s, 1H, N–H). 13C NMR (75 MHz, CDCl3): 26.9, 34.2, 52.3, 58.7, 73.1, 73.1, 76.5, 78.6, 113.3, 113.6, 114.4, 114.5, 115.5, 115.8, 116.3, 121.8, 121.9, 123.9, 129.9, 130.0, 136.6, 136.7, 136.8, 161.0, 164.3, 169.4, 173.1 ppm. Mass: m/z 352 (M+). Anal. calculated for C20H17FN2O3: C, 68.17%, H, 4.86%, N, 7.95%; found: C, 68.22%, H, 4.83%, N, 8.01%.
2.2 General procedure for synthesis of polycyclic fused quinlinopyrrolizidine derivatives, 22a–e
A mixture of BHA 3c (1 mmol), isatin 7 (1.1 mmol), and proline 21 (1.1 mmol) was placed in a round bottom flask and melted at 180oC, completion of the reaction was evidenced by thin layer chromatography (TLC), the crude product was recrystallized with EtOAc and hexane to afford the pure product 22a as a solid.
2.2.1 14-(2-Methoxyphenyl)-7a,8,9,10-tetrahydro-7H-6a,11a-(methanooxymethano)pyrrolizino[2,3-c]quinoline-6,12(5H)-dione, 22a
IR (KBr): 1,715, 1,749 cm−1; 1H NMR (300 MHz, CDCl3 + DMSO-d 6): δ 1.35–1.41 (m, 1H), 1.61–1.75 (m, 2H), 1.99–2.20 (m, 4H), 2.64–2.70 (m, 1H), 3.62–3.71 (m, 1H), 3.75 (s, 3H), 5.81 (s, 1H), 6.86–6.89 (d, J = 8.1 Hz, 2H), 6.96–7.01 (m, 1H), 7.09–7.13 (m, 1H), 7.28–7.38 (m, 3H), 7.74–7.77 (d, J = 7.8 Hz, 1H), 10.37 (s, 1H, N–H). 13C NMR (75 MHz, CDCl3 + DMSO-d 6): 19.9, 27.3, 28.3, 35.5, 44.0, 49.7, 53.3, 58.0, 69.9, 105.3, 109.1, 110.8, 114.8, 117.3, 118.0, 121.1, 123.5, 124.2, 125.1, 133.6, 151.0, 163.1, 170.7 ppm. Mass: m/z: 391(M+). Anal. calculated for C23H22N2O4: C, 70.75%; H, 5.68%; N, 7.18%; found: C, 70.80%, H, 5.71%, N, 7.24%.
2.2.2 14-(4-Methoxyphenyl)-7a,8,9,10-tetrahydro-7H-6a,11a-(methanooxymethano)pyrrolizino[2,3-c]quinoline-6,12(5H)-dione, 22b
IR (KBr): 1,715, 1,745 cm−1; 1H NMR (300 MHz, CDCl3): δ 1.37–1.42 (m, 1H), 1.58–1.75 (m, 2H), 2.08–2.22 (m, 4H), 2.66–2.73 (m, 1H), 3.61–3.69 (m, 1H), 3.81 (s, 3H), 5.41(s, 1H), 6.87–6.90 (m, 2H), 7.01–7.04 (m, 1H), 7.19–7.43 (m, 4H), 7.90–7.93 (d, J = 7.5 Hz, 1H), 9.81 (s, 1H, N–H). 13C NMR (75 MHz, CDCl3): 25.0, 32.1, 35.4, 49.2, 55.2, 59.4, 63.4, 76.8, 80.4, 113.8, 115.3, 116.0, 124.7, 126.5, 127.1, 129.5, 130.8, 137.1, 159.9, 170.5, 175.6 ppm. Mass: m/z: 391 (M+). Anal. calculated for C23H22N2O4: C, 70.75%; H, 5.68%; N, 7.18%; found: C, 70.80%, H, 5.65%, N, 7.23%.
2.2.3 14-(o-Tolyl)-7a,8,9,10-tetrahydro-7H-6a,11a-(methanooxymethano)pyrrolizino[2,3-c]quinoline-6,12(5H)-dione, 22c
IR (KBr): 1,715, 1,744 cm−1; 1H NMR (300 MHz, CDCl3): δ 1.41–1.55 (m, 1H), 1.63–1.83 (m, 2H), 2.04–2.19 (m, 3H), 2.25 (s, 3H), 2.35–2.47 (m, 1H), 2.67–2.72 (m, 1H), 3.67–3.76 (m, 1H), 5.68 (s, 1H), 7.08–7.45 (m, 7H), 7.86 (d, J = 7.5 Hz, 1H), 10.34 (s, 1H, N–H). 13C NMR (75 MHz, CDCl3): 19.1, 25.0, 32.0, 34.4, 49.1, 59.0, 63.4, 77.1, 77.3, 114.9, 116.2, 124.1, 125.7, 126.7, 128.6, 129.1, 130.6, 130.9, 132.3, 135.6, 137.9, 169.6, 175.9 ppm. Mass: m/z: 375 (M+). Anal. calculated for C23H22N2O3: C, 73.98%; H, 5.92%; N, 7.48%; found: C, 74.05%, H, 5.96%, N, 7.53%.
2.2.4 14-(3,4-Dimethoxyphenyl)-7a,8,9,10-tetrahydro-7H-6a,11a-(methanooxymethano)pyrrolizino[2,3-c]quinoline-6,12(5H)-dione, 22d
IR (KBr): 1,712, 1,755 cm−1; 1H NMR (300 MHz, CDCl3): δ 1.01–1.08 (m, 1H), 1.28–1.36 (m, 2H), 1.66–1.89 (m, 4H), 2.29 (t, J = 6.3 Hz, 1H), 3.19–3.32 (m, 1H), 3.51(s, 6H), 5.01(s, 1H), 6.50–7.02 (m, 6H), 7.41 (d, J = 7.8 Hz, 1H), 10.30 (s, 1H, N–H). 13C NMR (75 MHz, CDCl3): 24.8, 31.9, 34.9, 48.9, 55.6, 55.6, 58.7, 63.0, 76.3, 79.8, 109.1, 110.8, 114.4, 116.0, 118.0, 123.4, 127.1, 128.4, 130.3, 138.0, 148.4, 148.8, 168.8, 175.5 ppm. HRMS (ESI) exact mass calculated for C24H24N2O5 [M + H]+: 421.1758, Found: 421.1754. Mass: m/z: 420 (M+). Anal. calculated for C24H24N2O5: C, 68.56%; H, 5.75%; N, 6.66%; found: C, 68.64%, H, 5.79%, N, 6.70%.
2.2.5 14-(Benzo[d][1,3]dioxol-5-yl)-7a,8,9,10-tetrahydro-7H-6a,11a-(methanooxymethano)pyrrolizino[2,3-c]quinoline-6,12(5H)-dione, 22e
Mp: 245–248°C; IR (KBr): 1,715, 1,749 cm−1; 1H NMR (300 MHz, CDCl3 + DMSO-d 6): δ 1.35–1.44 (m, 1H), 1.63–1.83 (m, 2H), 2.06–2.23 (m, 4H), 2.69 (t, J = 6.6 Hz, 1H), 3.59–3.68 (m, 1H), 5.34 (s, 1H), 5.97(s, 2H), 6.78–7.40 (m, 6H), 7.86 (d, J = 7.5 Hz, 1H), 9.80 (s, 1H, N–H). 13C NMR (75 MHz, CDCl3 + DMSO-d 6): 24.0, 31.0, 34.2, 48.2, 58.2, 62.3, 75.7, 79.2, 100.2, 105.5, 107.1, 114.0, 115.0, 118.4, 112.3, 127.3, 128.2, 129.7, 136.5, 146.7, 146.8, 168.5, 174.5 ppm. HRMS calculated for C23H20N2O5: 404.1345 and found 404.1344.
2.3 General procedure for synthesis of heterarylpyrrolo[3,2-c]quinolinone/pyrrolizino[2.3-c]quinoline hybrids, 23 and 24
A mixture of BHA 5/6 (1 mmol), isatin 7 (1.1 mmol), and N-methylglycine 8/proline 21 (1.1 mmol) was placed in a round bottom flask and melted at 180°C until completion of the reaction is evidenced by TLC analysis. After completion of the reaction, the crude product was recrystallized with 5 mL of EtOAc and hexane mixture (1:4 ratio) which successfully provided the pure product 23/24 as a solid.
2.3.1 1-Methyl-12-(thiophen-2-yl)-2,3-dihydro-1H-3a,9b-(methanooxymethano)pyrrolo[3,2-c]quinoline-4,10(5H)-dione, 23
Mp: 233–235°C; IR (KBr): 1,710, 1,747 cm−1: 1H NMR (300 MHz, CDCl3): δ 2.24–2.23 (m, 1H), 2.59 (s, –NCH 3 , 3H), 2.63–2.79 (m, 2H), 3.09–3.16 (m, 1H), 5.62 (s, 1H), 6.96–7.05 (m, 2H), 7.17–7.23 (m, 2H), 7.34 (d, J = 5.1 Hz, 1H), 7.45 (s, 1H), 7.77 (d, J = 7.5 Hz, 1H), 8.98 (s, 1H, N–H). 13C NMR (75 MHz, CDCl3): 27.3, 34.2, 52.4, 59.1, 73.0, 77.2, 114.5, 116.2, 123.8, 126.0, 126.2, 126.9, 129.9, 130.8, 136.3, 136.5, 168.9, 172.9 ppm. Mass: m/z: 341 (M+). Anal. calculated for C18H16N2O3S: C, 63.51%, H, 4.74%, N, 8.23%; found: C, 63.57%, H, 4.70%, N, 8.25%.
2.3.2 14-(Furan-2-yl)-7a,8,9,10-tetrahydro-7H-6a,11a-(methanooxymethano)pyrrolizino[2,3-c]quinoline-6,12(5H)-dione, 24
IR (KBr): 1,709, 1,749 cm−1; 1H NMR (300 MHz, CDCl3): δ 1.39–1.48 (m, 1H), 1.65–1.75 (m, 2H), 2.09–2.17 (m, 2H), 2.53–2.71 (m, 3H), 3.68–3.73 (m, 1H), 5.29 (s, 1H), 6.39 (s, 1H), 6.52–7.79 (m, 6H), 10.11 (s, 1H, N–H).13C NMR (75 MHz, CDCl3 + DMSO-d 6): 25.2, 32.4, 35.1, 49.4, 58.1, 63.6, 75.1, 76.3, 110.4, 110.5, 114.3, 116.2, 124.0, 129.0, 130.7, 138.0, 143.6, 147.2, 168.0, 175.2 ppm. Mass: m/z 350 (M+). Anal. calculated for C20H18N2O4: C, 68.56%; H, 5.18%; N, 8.10%; found: C, 68.62%, H, 5.22%, N, 8.04%.
3 Results and discussion
To begin with, the preparation of BHA viz various substituted methyl 2-(hydroxy(phenyl)methyl)acrylate 3/methyl 2-(furan-2-yl(hydroxy)methyl)acrylate 5/methyl 2-(hydroxy(thiophen-2-yl)methyl)acrylate 6 was achieved from the reactions of appropriate aryl/heteroaryl aldehyde in the presence of DABCO and methyl acrylate based on protocol reported in the literature [27] (Scheme 1).
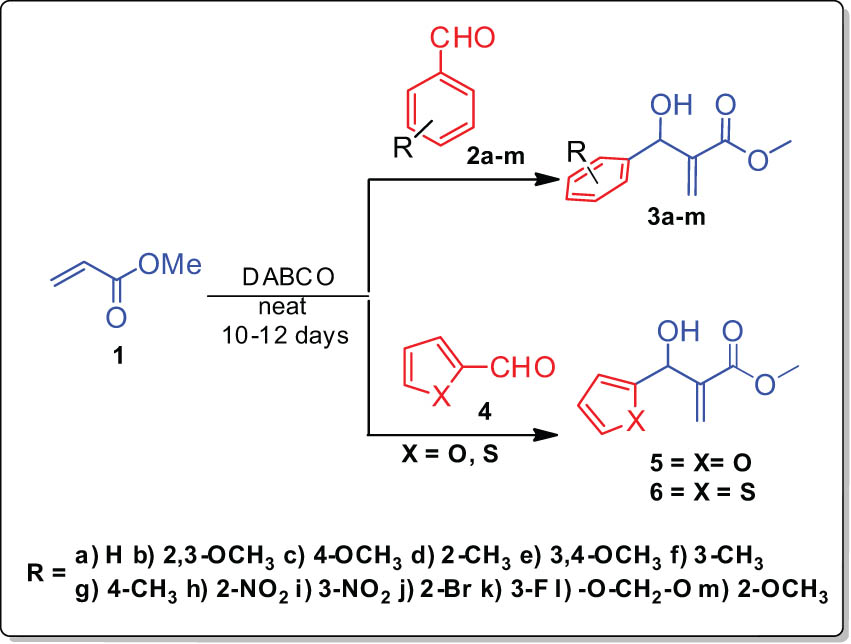
Synthesis of BHAs.
Subsequently, a model tandem reaction was investigated by refluxing a mixture of BHA 3a, indoline-2,3-dione 7, and N-methylglycine 8 in MeOH for 8 h. The reaction afforded 20% isolated yield of the unusual rearranged product 10a and 65% of the expected spiro cycloadduct 9a (Scheme 2 and Table 1). Since our interest was more on the synthesis of the rearranged product 10a, to improve its yield, the tandem protocol was explored under different solvent conditions including MeCN, dioxane, toluene, and xylene and the results are presented in Table 1. The reaction in all these solvents failed to afford 10a even after longer reaction time, alternatively the expected cycloadduct 9a was obtained. Ultimately, the reaction was performed under solid state melt reaction (SSMR) conditions in the absence of solvent, as SSMR is an environmentally benign and economically attractive synthetic strategy to prepare complex heterocycles without using expensive, flammable, toxic, and hazardous solvents [28]. Thus, an equimolar mixture of compounds 7, 8, and 3a was melted under solvent free condition at 180°C, whereupon a quantitative yield of the rearranged product 10a was obtained in 5 min (Scheme 2 and Table 1). It was observed that 180°C was the optimum temperature for getting maximum yield of the rearranged product 10a. Increasing the temperature (200°C) became detrimental to the reaction, while lowering the temperature (80°C) had no significant effect on the reaction. The most remarkable observation of this protocol is that the unusual rearranged adduct was achieved in maximum yield and in the absence of column purification, as the pure product could be attained by recrystallization technique.
![Scheme 2
Synthesis of pyrrolo[3,2-c]quinolinone hybrid heterocycles.](/document/doi/10.1515/gps-2023-0043/asset/graphic/j_gps-2023-0043_fig_008.jpg)
Synthesis of pyrrolo[3,2-c]quinolinone hybrid heterocycles.
Optimization of reaction conditions for the synthesis of 10a
| Entry | Solvent | Temperature | Time | Yield (%) of the productsa | |
|---|---|---|---|---|---|
| Spiro adduct (9a) | Rearranged product (10a) | ||||
| 1 | Methanol | Reflux | 8 h | 65 | 20 |
| 2 | Acetonitrile | Reflux | 8.5 h | 82 | — |
| 3 | Toluene | Reflux | 10 h | 78 | — |
| 4 | Xylene | Reflux | 8 h | 79 | — |
| 5 | Dioxane | Reflux | 8 h | 75 | — |
| 6 | None | 80°Cb | 2 h | — | — |
| 7 | None | 100°Cb | 1 h | 53 | 40 |
| 8 | None | 140°Cb | 30 min | 20 | 70 |
| 9 | None | 180°Cb | 5 min | — | 97 |
aIsolated yield of pure products.
bReactants melted at the mentioned temperature.
The structure of the product 10 was carefully ascertained by spectroscopic data (Supplementary material) (Figure 3). In the 1H and 13C NMR spectrum of compound 10f, no peak for ester–OCH3 and –OH could be found that are in accordance with the construction of the rearranged adduct. In the 1H NMR spectrum of 10f, the protons of the –NCH3 and lactam –NH exhibited as singlets at δ 2.35 and 9.74 ppm. The signals at δ 2.73 and δ 3.06–3.14 ppm as quartet and multiplet were due to –NCH2 protons of the pyrrolidine. In the 13C NMR of 10f, the peaks at δ 173.7 and 169.9 ppm were assigned to lactone and quinolinone rings carbonyl carbon, respectively. The carbon signals at δ 58.9 and 73.1 were assigned to two quaternary carbons. Further, two methylene units appeared at δ 26.7 and 52.4 ppm in the negative region of the DEPT 135 spectrum. The structures of other products were also determined by similar straightforward method. The stereo and regiochemistry of pyrroloquinolinone hybrids 10f and 10h have been unambiguously elucidated by XRD analysis in Figures 4 and 5 [29,30].
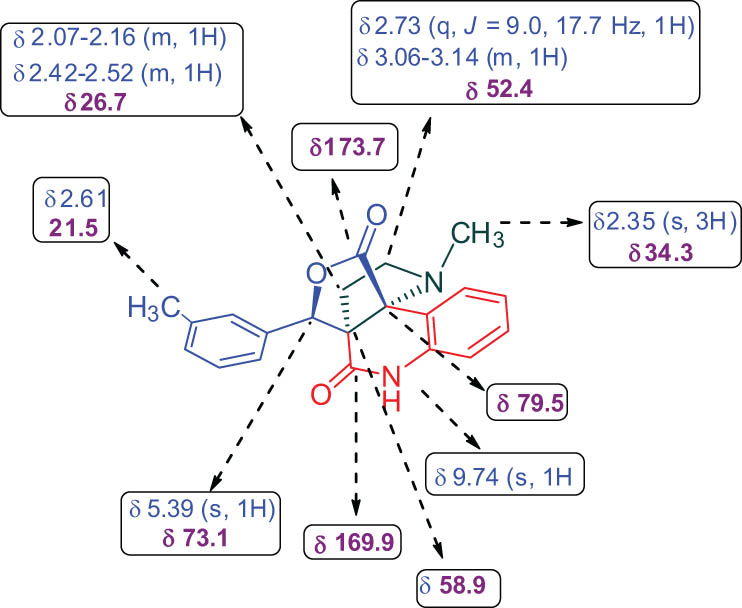
Chemical shift of 10f.
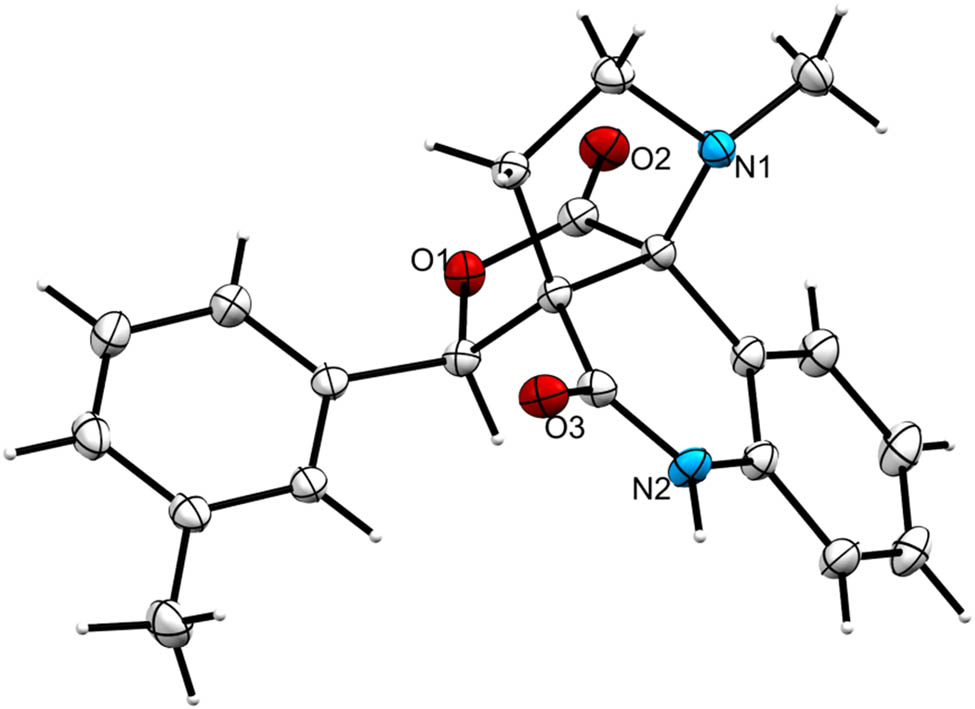
ORTEP diagram of pyrroloquinolinone hybrid 10f.
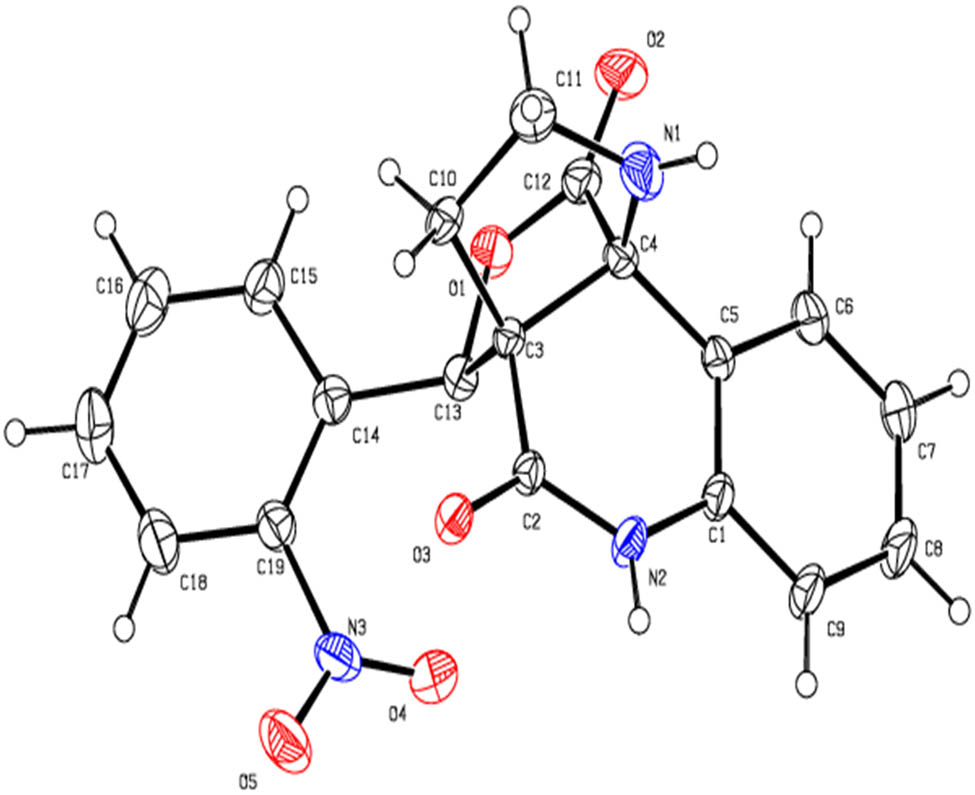
ORTEP diagram of 10h.
The persuasive mechanism for the construction of the polycyclic hybrid heterocycles in the one pot operation is shown in Scheme 3. The acrylate upon reaction with DABCO affords the intermediate 12, which further reacts with aryl aldehyde 2 to furnish the appropriate BHA 3 via intermediate 13. The reaction of diketone 7 and N-methyl glycine 8, affords the 1,3-dipole 16 via intermediates 14 and 15 by spontaneous decarboxylation and dehydration. The 1,3-dipole 16 adds regioselectively to the dipolarophile 3 to give the cycloadduct 9 in preference over 17. Presumably, the hydroxyl group of 9 was reacted with the imido carbonyl of the isatin moiety leading to the construction of lactone 19 via 18. The condensation between the amino group of intermediate 19 with the ester function ultimately affords the final product 10 via subsequent elimination of the methoxy unit.
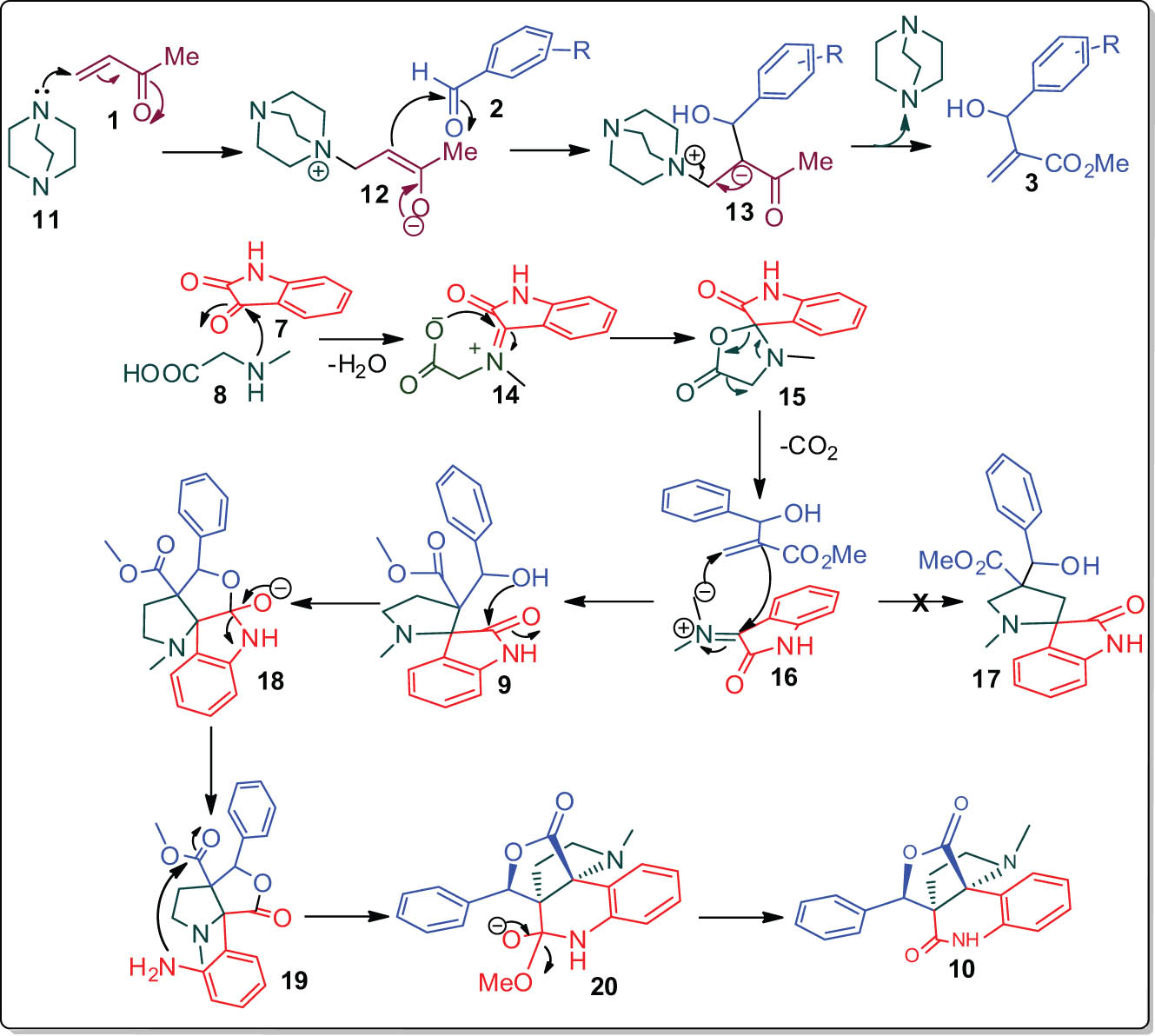
A feasible mechanism for the formation of pyrroloquinoline hybrids.
The construction of structurally fascinating polycyclic pyrrolizinoquinoline hybrids 22a–e were further realized through the tandem reaction of BHA 3 with indoline-2,3-dione 7 and l-proline 21 as described in Scheme 4. This reaction under the optimized conditions led to the formation of aryl substituted polycyclic pyrrolizino[2,3-c]quinolinone hybrids in good yields (Scheme 4).
![Scheme 4
Synthesis of pyrrolizino[2,3-c]quinolinone hybrids.](/document/doi/10.1515/gps-2023-0043/asset/graphic/j_gps-2023-0043_fig_010.jpg)
Synthesis of pyrrolizino[2,3-c]quinolinone hybrids.
To broaden the scope of this tandem transformation, the reaction was also investigated with different BHAs generated from heteroaryl aldehydes (pyrrole-2-carboxaldehyde and furan-2-carboxaldehyde) as shown in Scheme 1. Thus, the reaction involving the azomethine ylide generated in situ from decarboxylative condensation of isatin 7 and N-methylgylcine 8/l-proline 21, under optimized condition led to the formation of heteroaryl substituted polycyclic pyrrolo[3,2-c]quinolinone/pyrrolizinoquinolinone hybrids 23 and 24 in good yield (Scheme 5). The structure of these products was confirmed by spectroscopic data. All these reactions afforded angularly fused, quinolin-2-olactone in excellent yields and the results are described in Figure 6.
![Scheme 5
Synthesis of heterarylpyrrolo[3,2-c]quinolinone/pyrrolizino[2.3-c]quinoline hybrids.](/document/doi/10.1515/gps-2023-0043/asset/graphic/j_gps-2023-0043_fig_011.jpg)
Synthesis of heterarylpyrrolo[3,2-c]quinolinone/pyrrolizino[2.3-c]quinoline hybrids.
![Figure 6
Pyrrolo[3,2-c]quinolinone and pyrrolizino[2,3-c]quinolinone hybrids.](/document/doi/10.1515/gps-2023-0043/asset/graphic/j_gps-2023-0043_fig_006.jpg)
Pyrrolo[3,2-c]quinolinone and pyrrolizino[2,3-c]quinolinone hybrids.
4 Conclusion
In conclusion, we report herein the synthesis of a library of hitherto unexplored new classes of pyrrolo[3,2-c]quinolinone/pyrrolizino[2,3-c]quinolinone hybrid heterocycles through single-pot three component tandem green transformation involving a cycloaddition reaction and a consecutive lactonization and lactamization. Notable features of this protocol include the following: (a) solvent free reaction involving solid reactants, (b) products formed in shortest reaction time, (c) pure products obtained without column chromatographic purification, (d) environmentally benign green protocol, (e) products obtained in excellent yields. The products were obtained by single stereoisomers with high stereoselectivity which was confirmed by spectroscopic and XRD analyses.
Acknowledgements
Authors thank the Department of Science and Technology (DST), New Delhi for financial support. Suresh Mani and Rajesh Raju would like to thank the CSIR, New Delhi for SRF Fellowship. Rajesh Raju would like to thank the UGC New Delhi for BSR Faculty Fellowship.
-
Funding information: The project was funded by Researchers Supporting Project Number (RSP2023R143), King Saud University, Riyadh, Saudi Arabia.
-
Author contributions: Suresh and Rajesh Raju: conceptualization, investigation, supervision, formal analysis, methodology, validation, writing – original draft, and visualization; Natarajan Arumugam: conceptualization, investigation, validation, writing – original draft, and visualization; Abudulrahman I. Almansour: project administration, visualization, and writing – review and editing; Raju Suresh Kumar: methodology, validation, and writing – review and editing; Karthikeyan Perumal: investigation, formal analysis, and methodology
-
Conflict of interest: The Authors state no conflict of interest.
-
Data availability statement: All materials for this study are presented in this article and available on request to the corresponding author.
References
[1] Murlykina MV, Morozova AD, Zviagin IM, Sakhno YI, Desenko SM, Chebanov1 VA. Aminoazole-based diversity-oriented synthesis of heterocycles. Front Chem. 2018;6:527.10.3389/fchem.2018.00527Suche in Google Scholar PubMed PubMed Central
[2] Moroz AA, Zhulanov VE, Dmitriev MV, Maslivets AN. Diversity-oriented synthesis of three skeletally diverse iminolactones from isocyanides, activated acetylenes and 1H-pyrrole-2,3-diones via [3+2] and [4+1] cycloaddition reactions. Tetrahedron. 2020;76:130880.10.1016/j.tet.2019.130880Suche in Google Scholar
[3] Das S. Recent applications of 1,3-indanedione in organic transformations for the construction of fused- and spiro scaffolds. Tetrahedron. 2022;122:132954.10.1016/j.tet.2022.132954Suche in Google Scholar
[4] Maharani S, Almansour AI, Suresh Kumar R, Arumugam N, Ranjith Kumar R. Synthesis of cycloalkano[b]pyridines by multicomponent strategy: ring-size mediated product selectivity, substitution-induced axial chirality and influence of the 14N quadrupole-relaxation. Tetrahedron. 2016;72:4582–92.10.1016/j.tet.2016.06.030Suche in Google Scholar
[5] Mani S, Raju R, Raghunathan R, Arumugam N, Almansour AI, Suresh Kumar R, et al. Environmentally friendly domino multicomponent strategy for the synthesis of pyrroloquinolinone hybrid heterocycles. RSC Adv. 2022;12:15440–6.10.1039/D2RA02851DSuche in Google Scholar PubMed PubMed Central
[6] Zhu MJ, Ye R, Shi WJ, Sun J, Yan CG. Convenient generation of 1,3-dipolar nitrilimines and [3+2] cycloaddition for the synthesis of spiro compounds. Tetrahedron Lett. 2022;110:154186.10.1016/j.tetlet.2022.154186Suche in Google Scholar
[7] Carmen N, Miguel SJ. Synthesis of pyrrolizidines and indolizidines by multicomponent 1,3-dipolar cycloaddition of azomethine ylides. Pure Appl Chem. 2019;91:575–96.10.1515/pac-2018-0710Suche in Google Scholar
[8] Pandey G, Banerjee P, Gadre SR. Construction of enantiopure pyrrolidine ring system via asymmetric [3+2]-cycloaddition of azomethine ylides. Chem Rev. 2006;106:4484–517.10.1021/cr050011gSuche in Google Scholar PubMed
[9] Petri GL, Raimondi MV, Spanò V, Ralph H, Paola B, Montalbano A. Pyrrolidine in drug discovery: A versatile scaffold for novel biologically active compounds. Top Curr Chem. 2021;379:34.10.1007/s41061-021-00347-5Suche in Google Scholar PubMed PubMed Central
[10] Hanessian S, Bayrakdarian M. Pyrrolidine as a cogwheel-like scaffold for the deployment of diverse functionality through cycloaddition reactions of metallo-1,3-dipoles in aqueous media. Bioorg Med Chem Lett. 2000;10:427–31.10.1016/S0960-894X(00)00020-2Suche in Google Scholar PubMed
[11] Chen M, Geng CW, Han J, Liu Y, Yu YK, Lu AM, et al. Design, synthesis, crystal structure, and herbicidal activity of novel pyrrolidine-2,4-dione derivatives incorporating an alkyl ether pharmacophore with natural tetramic acids as lead compounds. New J Chem. 2021;45:5621–30.10.1039/D1NJ00119ASuche in Google Scholar
[12] Feldman KS, Antoline JF. Synthesis studies on the Melodinus alkaloid meloscine. Tetrahedron. 2013;69:1434–45.10.1016/j.tet.2012.12.032Suche in Google Scholar PubMed PubMed Central
[13] Rodier N, Mauguen Y, Hachem-Mehri M, Plat M. Structure cristalline de la méloscandonine, C20H20N2O2: alcalöide du Melodinus scandens Forst. Acta Crystallogr Sect B: Struct Sci. 1978;34:232.10.1107/S0567740878002678Suche in Google Scholar
[14] Sangoi Y, Sakuleko O, Yuenyongsawad S, Kanjana-opas A, Ingkaninan K, Plubrukan A, et al. Acetylcholinesterase-inhibiting activity of pyrrole derivatives from a novel marine gliding bacterium, Rapidithrix thailandica. MarDrugs. 2008;6:578.10.3390/md6040578Suche in Google Scholar
[15] Wu R, Pan J, Shen M, Xing C. Apoptotic effect of pyrroloquinoline quinone on chondrosarcoma cells through activation of the mitochondrial caspase‑dependent and caspase‑independent pathways. Oncol Rep. 2018;40:1614–20.10.3892/or.2018.6569Suche in Google Scholar PubMed
[16] Andrea C, Chiara N, Salvatore V, Germano G, Maurizio A, Laura M, et al. Design, synthesis, and preliminary biological evaluation of pyrrolo[3,4-c]quinolin-1-one and oxoisoindoline derivatives as aggrecanase inhibitors. Chem Med Chem. 2010;5:739–48.10.1002/cmdc.200900523Suche in Google Scholar PubMed
[17] Paola B, Libero C, Patrizia D, Anna C, Alessandra M, Girolamo C, et al. Synthesis of pyrrolo[3,2-h]quinolinones with good photochemotherapeutic activity and no DNA damage. Eur J Med Chem. 2010;18:4830–43.10.1016/j.bmc.2010.04.080Suche in Google Scholar PubMed
[18] Helissey P, Cros S, Giorgi-Renault S. Synthesis, antitumor evaluation and SAR of new 1H-pyrrolo [3,2-c] quinoline-6,9-diones and 11H-indolo [3,2-c] quinoline-1,4-diones. Anticancer Drug Des. 1994;9:51.Suche in Google Scholar
[19] Brown TH, Ife RJ, Keeling DJ, Laing SM, Leach CA, Parsons ME, et al. Reversible inhibitors of the gastric (H+/K+)-ATPase. 1. 1-Aryl-4-methylpyrrolo[3,2-c] quinolines as conformationally restrained analogues of 4-(arylamino)quinolines. J Med Chem. 1990;33:527.10.1021/jm00164a010Suche in Google Scholar PubMed
[20] Bernauer K, Englert G, Vetter W. An apocynaceae-alkaloid of a novel type. Experientia. 1965;21:374.10.1007/BF02139743Suche in Google Scholar PubMed
[21] Arumugam N, Almansour AI, Suresh Kumar R, Periasamy VS, Athinarayanan J, Ali AA, et al. Regio- and diastereoselective synthesis of anticancer spirooxindoles derived from tryptophan and histidine via three-component 1,3-dipolar cycloadditions in an ionic liquid. Tetrahedron. 2018;74:5358.10.1016/j.tet.2018.04.032Suche in Google Scholar
[22] Arumugam N, Almansour AI, Suresh Kumar R, Ali Al-Aizari AJM, Alaqeel SI, Kansız S, et al. Regio- and diastereoselective synthesis of spiropyrroloquinoxaline grafted indole heterocyclic hybrids and evaluation of their anti-Mycobacterium tuberculosis activity. RSC Adv. 2020;10:23522–31.10.1039/D0RA02525ASuche in Google Scholar
[23] Arumugam N, Almansour AI, Suresh Kumar R. Antimicrobial activities of spirooxindolopyrrolidine tethered dicarbonitrile heterocycles against multidrug resistant nosocomial pathogens. J Infect Public Health. 2021;12:1810–4.10.1016/j.jiph.2021.10.027Suche in Google Scholar PubMed
[24] Almansour AI, Arumugam N, Suresh Kumar R, Subbarayan PV, Alshatwi AA, Ghabbour HA. Anticancer compounds. US Patent, 9486444 B1; 2016.Suche in Google Scholar
[25] Arumugam N, Suresh Kumar R, Almansour AI, Altaf M, Padmanaban R, Sureshbabu P, et al. Spiropyrrolidine/spiroindolizino[6,7-b]indole heterocyclic hybrids: Stereoselective synthesis, cholinesterase inhibitory activity and their molecular docking study. Bioorg Chem. 2018;79:64.10.1016/j.bioorg.2018.04.025Suche in Google Scholar PubMed
[26] Rajesh R, Raghunathan R. Synthesis of β-Lactam-Tethered polycyclic fused heterocycles through a rearrangement by a one-pot tandem [3+2] cycloaddition reaction. Eur J Org Chem. 2013;13:2597–607.10.1002/ejoc.201201471Suche in Google Scholar
[27] Baylis AB, Hillman MED. Verfahren zur Herstellung von Acrylverbindungen. German Patent, 2155113; 1972.Suche in Google Scholar
[28] Paul S, Lee YR. Eco-friendly construction of highly functionalized chromenopyridinones by an organocatalyzed solid-state melt reaction and their optical properties. Green Chem. 2016;18:1488–94.10.1039/C5GC02658JSuche in Google Scholar
[29] Savithri MP, Suresh M, Raghunathan R, Raja R, Subbiah Pandi A. Crystal structure of ethyl 2″,3-dioxo-7′,7a′-dihydro-1′H,3H,3′H-dispiro[benzo[b]thiophene-2,6′-pyrrolo[1,2-c]thiazole-5′,3″-indoline]-7′-carboxylate. Acta Cryst Sect E. 2015;71:o379.10.1107/S2056989015002030Suche in Google Scholar PubMed PubMed Central
[30] Crystallographic data (including structure factors) for the compound 10i in this article have been deposited with the Cambridge Crystallographic Data Centre as supplementary publication number CCDC 993215. Copies of the data can be obtained, free of charge, on application to CCDC, 12 Union Road, Cambridge CB2 1EZ, UK [fax: +44 (0)1223 336033 or e-mail: deposit@ccdc.cam.ac.uk].Suche in Google Scholar
© 2023 the author(s), published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Artikel in diesem Heft
- Research Articles
- Value-added utilization of coal fly ash and recycled polyvinyl chloride in door or window sub-frame composites
- High removal efficiency of volatile phenol from coking wastewater using coal gasification slag via optimized adsorption and multi-grade batch process
- Evolution of surface morphology and properties of diamond films by hydrogen plasma etching
- Removal efficiency of dibenzofuran using CuZn-zeolitic imidazole frameworks as a catalyst and adsorbent
- Rapid and efficient microwave-assisted extraction of Caesalpinia sappan Linn. heartwood and subsequent synthesis of gold nanoparticles
- The catalytic characteristics of 2-methylnaphthalene acylation with AlCl3 immobilized on Hβ as Lewis acid catalyst
- Biodegradation of synthetic PVP biofilms using natural materials and nanoparticles
- Rutin-loaded selenium nanoparticles modulated the redox status, inflammatory, and apoptotic pathways associated with pentylenetetrazole-induced epilepsy in mice
- Optimization of apigenin nanoparticles prepared by planetary ball milling: In vitro and in vivo studies
- Synthesis and characterization of silver nanoparticles using Origanum onites leaves: Cytotoxic, apoptotic, and necrotic effects on Capan-1, L929, and Caco-2 cell lines
- Exergy analysis of a conceptual CO2 capture process with an amine-based DES
- Construction of fluorescence system of felodipine–tetracyanovinyl–2,2′-bipyridine complex
- Excellent photocatalytic degradation of rhodamine B over Bi2O3 supported on Zn-MOF nanocomposites under visible light
- Optimization-based control strategy for a large-scale polyhydroxyalkanoates production in a fed-batch bioreactor using a coupled PDE–ODE system
- Effectiveness of pH and amount of Artemia urumiana extract on physical, chemical, and biological attributes of UV-fabricated biogold nanoparticles
- Geranium leaf-mediated synthesis of silver nanoparticles and their transcriptomic effects on Candida albicans
- Synthesis, characterization, anticancer, anti-inflammatory activities, and docking studies of 3,5-disubstituted thiadiazine-2-thiones
- Synthesis and stability of phospholipid-encapsulated nano-selenium
- Putative anti-proliferative effect of Indian mustard (Brassica juncea) seed and its nano-formulation
- Enrichment of low-grade phosphorites by the selective leaching method
- Electrochemical analysis of the dissolution of gold in a copper–ethylenediamine–thiosulfate system
- Characterisation of carbonate lake sediments as a potential filler for polymer composites
- Evaluation of nano-selenium biofortification characteristics of alfalfa (Medicago sativa L.)
- Quality of oil extracted by cold press from Nigella sativa seeds incorporated with rosemary extracts and pretreated by microwaves
- Heteropolyacid-loaded MOF-derived mesoporous zirconia catalyst for chemical degradation of rhodamine B
- Recovery of critical metals from carbonatite-type mineral wastes: Geochemical modeling investigation of (bio)hydrometallurgical leaching of REEs
- Photocatalytic properties of ZnFe-mixed oxides synthesized via a simple route for water remediation
- Attenuation of di(2-ethylhexyl)phthalate-induced hepatic and renal toxicity by naringin nanoparticles in a rat model
- Novel in situ synthesis of quaternary core–shell metallic sulfide nanocomposites for degradation of organic dyes and hydrogen production
- Microfluidic steam-based synthesis of luminescent carbon quantum dots as sensing probes for nitrite detection
- Transformation of eggshell waste to egg white protein solution, calcium chloride dihydrate, and eggshell membrane powder
- Preparation of Zr-MOFs for the adsorption of doxycycline hydrochloride from wastewater
- Green nanoarchitectonics of the silver nanocrystal potential for treating malaria and their cytotoxic effects on the kidney Vero cell line
- Carbon emissions analysis of producing modified asphalt with natural asphalt
- An efficient and green synthesis of 2-phenylquinazolin-4(3H)-ones via t-BuONa-mediated oxidative condensation of 2-aminobenzamides and benzyl alcohols under solvent- and transition metal-free conditions
- Chitosan nanoparticles loaded with mesosulfuron methyl and mesosulfuron methyl + florasulam + MCPA isooctyl to manage weeds of wheat (Triticum aestivum L.)
- Synergism between lignite and high-sulfur petroleum coke in CO2 gasification
- Facile aqueous synthesis of ZnCuInS/ZnS–ZnS QDs with enhanced photoluminescence lifetime for selective detection of Cu(ii) ions
- Rapid synthesis of copper nanoparticles using Nepeta cataria leaves: An eco-friendly management of disease-causing vectors and bacterial pathogens
- Study on the photoelectrocatalytic activity of reduced TiO2 nanotube films for removal of methyl orange
- Development of a fuzzy logic model for the prediction of spark-ignition engine performance and emission for gasoline–ethanol blends
- Micro-impact-induced mechano-chemical synthesis of organic precursors from FeC/FeN and carbonates/nitrates in water and its extension to nucleobases
- Green synthesis of strontium-doped tin dioxide (SrSnO2) nanoparticles using the Mahonia bealei leaf extract and evaluation of their anticancer and antimicrobial activities
- A study on the larvicidal and adulticidal potential of Cladostepus spongiosus macroalgae and green-fabricated silver nanoparticles against mosquito vectors
- Catalysts based on nickel salt heteropolytungstates for selective oxidation of diphenyl sulfide
- Powerful antibacterial nanocomposites from Corallina officinalis-mediated nanometals and chitosan nanoparticles against fish-borne pathogens
- Removal behavior of Zn and alkalis from blast furnace dust in pre-reduction sinter process
- Environmentally friendly synthesis and computational studies of novel class of acridinedione integrated spirothiopyrrolizidines/indolizidines
- The mechanisms of inhibition and lubrication of clean fracturing flowback fluids in water-based drilling fluids
- Adsorption/desorption performance of cellulose membrane for Pb(ii)
- A one-pot, multicomponent tandem synthesis of fused polycyclic pyrrolo[3,2-c]quinolinone/pyrrolizino[2,3-c]quinolinone hybrid heterocycles via environmentally benign solid state melt reaction
- Green synthesis of silver nanoparticles using durian rind extract and optical characteristics of surface plasmon resonance-based optical sensor for the detection of hydrogen peroxide
- Electrochemical analysis of copper-EDTA-ammonia-gold thiosulfate dissolution system
- Characterization of bio-oil production by microwave pyrolysis from cashew nut shells and Cassia fistula pods
- Green synthesis methods and characterization of bacterial cellulose/silver nanoparticle composites
- Photocatalytic research performance of zinc oxide/graphite phase carbon nitride catalyst and its application in environment
- Effect of phytogenic iron nanoparticles on the bio-fortification of wheat varieties
- In vitro anti-cancer and antimicrobial effects of manganese oxide nanoparticles synthesized using the Glycyrrhiza uralensis leaf extract on breast cancer cell lines
- Preparation of Pd/Ce(F)-MCM-48 catalysts and their catalytic performance of n-heptane isomerization
- Green “one-pot” fluorescent bis-indolizine synthesis with whole-cell plant biocatalysis
- Silica-titania mesoporous silicas of MCM-41 type as effective catalysts and photocatalysts for selective oxidation of diphenyl sulfide by H2O2
- Biosynthesis of zinc oxide nanoparticles from molted feathers of Pavo cristatus and their antibiofilm and anticancer activities
- Clean preparation of rutile from Ti-containing mixed molten slag by CO2 oxidation
- Synthesis and characterization of Pluronic F-127-coated titanium dioxide nanoparticles synthesized from extracts of Atractylodes macrocephala leaf for antioxidant, antimicrobial, and anticancer properties
- Effect of pretreatment with alkali on the anaerobic digestion characteristics of kitchen waste and analysis of microbial diversity
- Ameliorated antimicrobial, antioxidant, and anticancer properties by Plectranthus vettiveroides root extract-mediated green synthesis of chitosan nanoparticles
- Microwave-accelerated pretreatment technique in green extraction of oil and bioactive compounds from camelina seeds: Effectiveness and characterization
- Studies on the extraction performance of phorate by aptamer-functionalized magnetic nanoparticles in plasma samples
- Investigation of structural properties and antibacterial activity of AgO nanoparticle extract from Solanum nigrum/Mentha leaf extracts by green synthesis method
- Green fabrication of chitosan from marine crustaceans and mushroom waste: Toward sustainable resource utilization
- Synthesis, characterization, and evaluation of nanoparticles of clodinofop propargyl and fenoxaprop-P-ethyl on weed control, growth, and yield of wheat (Triticum aestivum L.)
- The enhanced adsorption properties of phosphorus from aqueous solutions using lanthanum modified synthetic zeolites
- Separation of graphene oxides of different sizes by multi-layer dialysis and anti-friction and lubrication performance
- Visible-light-assisted base-catalyzed, one-pot synthesis of highly functionalized cinnolines
- The experimental study on the air oxidation of 5-hydroxymethylfurfural to 2,5-furandicarboxylic acid with Co–Mn–Br system
- Highly efficient removal of tetracycline and methyl violet 2B from aqueous solution using the bimetallic FeZn-ZIFs catalyst
- A thermo-tolerant cellulase enzyme produced by Bacillus amyloliquefaciens M7, an insight into synthesis, optimization, characterization, and bio-polishing activity
- Exploration of ketone derivatives of succinimide for their antidiabetic potential: In vitro and in vivo approaches
- Ultrasound-assisted green synthesis and in silico study of 6-(4-(butylamino)-6-(diethylamino)-1,3,5-triazin-2-yl)oxypyridazine derivatives
- A study of the anticancer potential of Pluronic F-127 encapsulated Fe2O3 nanoparticles derived from Berberis vulgaris extract
- Biogenic synthesis of silver nanoparticles using Consolida orientalis flowers: Identification, catalytic degradation, and biological effect
- Initial assessment of the presence of plastic waste in some coastal mangrove forests in Vietnam
- Adsorption synergy electrocatalytic degradation of phenol by active oxygen-containing species generated in Co-coal based cathode and graphite anode
- Antibacterial, antifungal, antioxidant, and cytotoxicity activities of the aqueous extract of Syzygium aromaticum-mediated synthesized novel silver nanoparticles
- Synthesis of a silica matrix with ZnO nanoparticles for the fabrication of a recyclable photodegradation system to eliminate methylene blue dye
- Natural polymer fillers instead of dye and pigments: Pumice and scoria in PDMS fluid and elastomer composites
- Study on the preparation of glycerylphosphorylcholine by transesterification under supported sodium methoxide
- Wireless network handheld terminal-based green ecological sustainable design evaluation system: Improved data communication and reduced packet loss rate
- The optimization of hydrogel strength from cassava starch using oxidized sucrose as a crosslinking agent
- Green synthesis of silver nanoparticles using Saccharum officinarum leaf extract for antiviral paint
- Study on the reliability of nano-silver-coated tin solder joints for flip chips
- Environmentally sustainable analytical quality by design aided RP-HPLC method for the estimation of brilliant blue in commercial food samples employing a green-ultrasound-assisted extraction technique
- Anticancer and antimicrobial potential of zinc/sodium alginate/polyethylene glycol/d-pinitol nanocomposites against osteosarcoma MG-63 cells
- Nanoporous carbon@CoFe2O4 nanocomposite as a green absorbent for the adsorptive removal of Hg(ii) from aqueous solutions
- Characterization of silver sulfide nanoparticles from actinobacterial strain (M10A62) and its toxicity against lepidopteran and dipterans insect species
- Phyto-fabrication and characterization of silver nanoparticles using Withania somnifera: Investigating antioxidant potential
- Effect of e-waste nanofillers on the mechanical, thermal, and wear properties of epoxy-blend sisal woven fiber-reinforced composites
- Magnesium nanohydroxide (2D brucite) as a host matrix for thymol and carvacrol: Synthesis, characterization, and inhibition of foodborne pathogens
- Synergistic inhibitive effect of a hybrid zinc oxide-benzalkonium chloride composite on the corrosion of carbon steel in a sulfuric acidic solution
- Review Articles
- Role and the importance of green approach in biosynthesis of nanopropolis and effectiveness of propolis in the treatment of COVID-19 pandemic
- Gum tragacanth-mediated synthesis of metal nanoparticles, characterization, and their applications as a bactericide, catalyst, antioxidant, and peroxidase mimic
- Green-processed nano-biocomposite (ZnO–TiO2): Potential candidates for biomedical applications
- Reaction mechanisms in microwave-assisted lignin depolymerisation in hydrogen-donating solvents
- Recent progress on non-noble metal catalysts for the deoxydehydration of biomass-derived oxygenates
- Rapid Communication
- Phosphorus removal by iron–carbon microelectrolysis: A new way to achieve phosphorus recovery
- Special Issue: Biomolecules-derived synthesis of nanomaterials for environmental and biological applications (Guest Editors: Arpita Roy and Fernanda Maria Policarpo Tonelli)
- Biomolecules-derived synthesis of nanomaterials for environmental and biological applications
- Nano-encapsulated tanshinone IIA in PLGA-PEG-COOH inhibits apoptosis and inflammation in cerebral ischemia/reperfusion injury
- Green fabrication of silver nanoparticles using Melia azedarach ripened fruit extract, their characterization, and biological properties
- Green-synthesized nanoparticles and their therapeutic applications: A review
- Antioxidant, antibacterial, and cytotoxicity potential of synthesized silver nanoparticles from the Cassia alata leaf aqueous extract
- Green synthesis of silver nanoparticles using Callisia fragrans leaf extract and its anticancer activity against MCF-7, HepG2, KB, LU-1, and MKN-7 cell lines
- Algae-based green AgNPs, AuNPs, and FeNPs as potential nanoremediators
- Green synthesis of Kickxia elatine-induced silver nanoparticles and their role as anti-acetylcholinesterase in the treatment of Alzheimer’s disease
- Phytocrystallization of silver nanoparticles using Cassia alata flower extract for effective control of fungal skin pathogens
- Antibacterial wound dressing with hydrogel from chitosan and polyvinyl alcohol from the red cabbage extract loaded with silver nanoparticles
- Leveraging of mycogenic copper oxide nanostructures for disease management of Alternaria blight of Brassica juncea
- Nanoscale molecular reactions in microbiological medicines in modern medical applications
- Synthesis and characterization of ZnO/β-cyclodextrin/nicotinic acid nanocomposite and its biological and environmental application
- Green synthesis of silver nanoparticles via Taxus wallichiana Zucc. plant-derived Taxol: Novel utilization as anticancer, antioxidation, anti-inflammation, and antiurolithic potential
- Recyclability and catalytic characteristics of copper oxide nanoparticles derived from bougainvillea plant flower extract for biomedical application
- Phytofabrication, characterization, and evaluation of novel bioinspired selenium–iron (Se–Fe) nanocomposites using Allium sativum extract for bio-potential applications
- Erratum
- Erratum to “Synthesis, characterization, and evaluation of nanoparticles of clodinofop propargyl and fenoxaprop-P-ethyl on weed control, growth, and yield of wheat (Triticum aestivum L.)”
Artikel in diesem Heft
- Research Articles
- Value-added utilization of coal fly ash and recycled polyvinyl chloride in door or window sub-frame composites
- High removal efficiency of volatile phenol from coking wastewater using coal gasification slag via optimized adsorption and multi-grade batch process
- Evolution of surface morphology and properties of diamond films by hydrogen plasma etching
- Removal efficiency of dibenzofuran using CuZn-zeolitic imidazole frameworks as a catalyst and adsorbent
- Rapid and efficient microwave-assisted extraction of Caesalpinia sappan Linn. heartwood and subsequent synthesis of gold nanoparticles
- The catalytic characteristics of 2-methylnaphthalene acylation with AlCl3 immobilized on Hβ as Lewis acid catalyst
- Biodegradation of synthetic PVP biofilms using natural materials and nanoparticles
- Rutin-loaded selenium nanoparticles modulated the redox status, inflammatory, and apoptotic pathways associated with pentylenetetrazole-induced epilepsy in mice
- Optimization of apigenin nanoparticles prepared by planetary ball milling: In vitro and in vivo studies
- Synthesis and characterization of silver nanoparticles using Origanum onites leaves: Cytotoxic, apoptotic, and necrotic effects on Capan-1, L929, and Caco-2 cell lines
- Exergy analysis of a conceptual CO2 capture process with an amine-based DES
- Construction of fluorescence system of felodipine–tetracyanovinyl–2,2′-bipyridine complex
- Excellent photocatalytic degradation of rhodamine B over Bi2O3 supported on Zn-MOF nanocomposites under visible light
- Optimization-based control strategy for a large-scale polyhydroxyalkanoates production in a fed-batch bioreactor using a coupled PDE–ODE system
- Effectiveness of pH and amount of Artemia urumiana extract on physical, chemical, and biological attributes of UV-fabricated biogold nanoparticles
- Geranium leaf-mediated synthesis of silver nanoparticles and their transcriptomic effects on Candida albicans
- Synthesis, characterization, anticancer, anti-inflammatory activities, and docking studies of 3,5-disubstituted thiadiazine-2-thiones
- Synthesis and stability of phospholipid-encapsulated nano-selenium
- Putative anti-proliferative effect of Indian mustard (Brassica juncea) seed and its nano-formulation
- Enrichment of low-grade phosphorites by the selective leaching method
- Electrochemical analysis of the dissolution of gold in a copper–ethylenediamine–thiosulfate system
- Characterisation of carbonate lake sediments as a potential filler for polymer composites
- Evaluation of nano-selenium biofortification characteristics of alfalfa (Medicago sativa L.)
- Quality of oil extracted by cold press from Nigella sativa seeds incorporated with rosemary extracts and pretreated by microwaves
- Heteropolyacid-loaded MOF-derived mesoporous zirconia catalyst for chemical degradation of rhodamine B
- Recovery of critical metals from carbonatite-type mineral wastes: Geochemical modeling investigation of (bio)hydrometallurgical leaching of REEs
- Photocatalytic properties of ZnFe-mixed oxides synthesized via a simple route for water remediation
- Attenuation of di(2-ethylhexyl)phthalate-induced hepatic and renal toxicity by naringin nanoparticles in a rat model
- Novel in situ synthesis of quaternary core–shell metallic sulfide nanocomposites for degradation of organic dyes and hydrogen production
- Microfluidic steam-based synthesis of luminescent carbon quantum dots as sensing probes for nitrite detection
- Transformation of eggshell waste to egg white protein solution, calcium chloride dihydrate, and eggshell membrane powder
- Preparation of Zr-MOFs for the adsorption of doxycycline hydrochloride from wastewater
- Green nanoarchitectonics of the silver nanocrystal potential for treating malaria and their cytotoxic effects on the kidney Vero cell line
- Carbon emissions analysis of producing modified asphalt with natural asphalt
- An efficient and green synthesis of 2-phenylquinazolin-4(3H)-ones via t-BuONa-mediated oxidative condensation of 2-aminobenzamides and benzyl alcohols under solvent- and transition metal-free conditions
- Chitosan nanoparticles loaded with mesosulfuron methyl and mesosulfuron methyl + florasulam + MCPA isooctyl to manage weeds of wheat (Triticum aestivum L.)
- Synergism between lignite and high-sulfur petroleum coke in CO2 gasification
- Facile aqueous synthesis of ZnCuInS/ZnS–ZnS QDs with enhanced photoluminescence lifetime for selective detection of Cu(ii) ions
- Rapid synthesis of copper nanoparticles using Nepeta cataria leaves: An eco-friendly management of disease-causing vectors and bacterial pathogens
- Study on the photoelectrocatalytic activity of reduced TiO2 nanotube films for removal of methyl orange
- Development of a fuzzy logic model for the prediction of spark-ignition engine performance and emission for gasoline–ethanol blends
- Micro-impact-induced mechano-chemical synthesis of organic precursors from FeC/FeN and carbonates/nitrates in water and its extension to nucleobases
- Green synthesis of strontium-doped tin dioxide (SrSnO2) nanoparticles using the Mahonia bealei leaf extract and evaluation of their anticancer and antimicrobial activities
- A study on the larvicidal and adulticidal potential of Cladostepus spongiosus macroalgae and green-fabricated silver nanoparticles against mosquito vectors
- Catalysts based on nickel salt heteropolytungstates for selective oxidation of diphenyl sulfide
- Powerful antibacterial nanocomposites from Corallina officinalis-mediated nanometals and chitosan nanoparticles against fish-borne pathogens
- Removal behavior of Zn and alkalis from blast furnace dust in pre-reduction sinter process
- Environmentally friendly synthesis and computational studies of novel class of acridinedione integrated spirothiopyrrolizidines/indolizidines
- The mechanisms of inhibition and lubrication of clean fracturing flowback fluids in water-based drilling fluids
- Adsorption/desorption performance of cellulose membrane for Pb(ii)
- A one-pot, multicomponent tandem synthesis of fused polycyclic pyrrolo[3,2-c]quinolinone/pyrrolizino[2,3-c]quinolinone hybrid heterocycles via environmentally benign solid state melt reaction
- Green synthesis of silver nanoparticles using durian rind extract and optical characteristics of surface plasmon resonance-based optical sensor for the detection of hydrogen peroxide
- Electrochemical analysis of copper-EDTA-ammonia-gold thiosulfate dissolution system
- Characterization of bio-oil production by microwave pyrolysis from cashew nut shells and Cassia fistula pods
- Green synthesis methods and characterization of bacterial cellulose/silver nanoparticle composites
- Photocatalytic research performance of zinc oxide/graphite phase carbon nitride catalyst and its application in environment
- Effect of phytogenic iron nanoparticles on the bio-fortification of wheat varieties
- In vitro anti-cancer and antimicrobial effects of manganese oxide nanoparticles synthesized using the Glycyrrhiza uralensis leaf extract on breast cancer cell lines
- Preparation of Pd/Ce(F)-MCM-48 catalysts and their catalytic performance of n-heptane isomerization
- Green “one-pot” fluorescent bis-indolizine synthesis with whole-cell plant biocatalysis
- Silica-titania mesoporous silicas of MCM-41 type as effective catalysts and photocatalysts for selective oxidation of diphenyl sulfide by H2O2
- Biosynthesis of zinc oxide nanoparticles from molted feathers of Pavo cristatus and their antibiofilm and anticancer activities
- Clean preparation of rutile from Ti-containing mixed molten slag by CO2 oxidation
- Synthesis and characterization of Pluronic F-127-coated titanium dioxide nanoparticles synthesized from extracts of Atractylodes macrocephala leaf for antioxidant, antimicrobial, and anticancer properties
- Effect of pretreatment with alkali on the anaerobic digestion characteristics of kitchen waste and analysis of microbial diversity
- Ameliorated antimicrobial, antioxidant, and anticancer properties by Plectranthus vettiveroides root extract-mediated green synthesis of chitosan nanoparticles
- Microwave-accelerated pretreatment technique in green extraction of oil and bioactive compounds from camelina seeds: Effectiveness and characterization
- Studies on the extraction performance of phorate by aptamer-functionalized magnetic nanoparticles in plasma samples
- Investigation of structural properties and antibacterial activity of AgO nanoparticle extract from Solanum nigrum/Mentha leaf extracts by green synthesis method
- Green fabrication of chitosan from marine crustaceans and mushroom waste: Toward sustainable resource utilization
- Synthesis, characterization, and evaluation of nanoparticles of clodinofop propargyl and fenoxaprop-P-ethyl on weed control, growth, and yield of wheat (Triticum aestivum L.)
- The enhanced adsorption properties of phosphorus from aqueous solutions using lanthanum modified synthetic zeolites
- Separation of graphene oxides of different sizes by multi-layer dialysis and anti-friction and lubrication performance
- Visible-light-assisted base-catalyzed, one-pot synthesis of highly functionalized cinnolines
- The experimental study on the air oxidation of 5-hydroxymethylfurfural to 2,5-furandicarboxylic acid with Co–Mn–Br system
- Highly efficient removal of tetracycline and methyl violet 2B from aqueous solution using the bimetallic FeZn-ZIFs catalyst
- A thermo-tolerant cellulase enzyme produced by Bacillus amyloliquefaciens M7, an insight into synthesis, optimization, characterization, and bio-polishing activity
- Exploration of ketone derivatives of succinimide for their antidiabetic potential: In vitro and in vivo approaches
- Ultrasound-assisted green synthesis and in silico study of 6-(4-(butylamino)-6-(diethylamino)-1,3,5-triazin-2-yl)oxypyridazine derivatives
- A study of the anticancer potential of Pluronic F-127 encapsulated Fe2O3 nanoparticles derived from Berberis vulgaris extract
- Biogenic synthesis of silver nanoparticles using Consolida orientalis flowers: Identification, catalytic degradation, and biological effect
- Initial assessment of the presence of plastic waste in some coastal mangrove forests in Vietnam
- Adsorption synergy electrocatalytic degradation of phenol by active oxygen-containing species generated in Co-coal based cathode and graphite anode
- Antibacterial, antifungal, antioxidant, and cytotoxicity activities of the aqueous extract of Syzygium aromaticum-mediated synthesized novel silver nanoparticles
- Synthesis of a silica matrix with ZnO nanoparticles for the fabrication of a recyclable photodegradation system to eliminate methylene blue dye
- Natural polymer fillers instead of dye and pigments: Pumice and scoria in PDMS fluid and elastomer composites
- Study on the preparation of glycerylphosphorylcholine by transesterification under supported sodium methoxide
- Wireless network handheld terminal-based green ecological sustainable design evaluation system: Improved data communication and reduced packet loss rate
- The optimization of hydrogel strength from cassava starch using oxidized sucrose as a crosslinking agent
- Green synthesis of silver nanoparticles using Saccharum officinarum leaf extract for antiviral paint
- Study on the reliability of nano-silver-coated tin solder joints for flip chips
- Environmentally sustainable analytical quality by design aided RP-HPLC method for the estimation of brilliant blue in commercial food samples employing a green-ultrasound-assisted extraction technique
- Anticancer and antimicrobial potential of zinc/sodium alginate/polyethylene glycol/d-pinitol nanocomposites against osteosarcoma MG-63 cells
- Nanoporous carbon@CoFe2O4 nanocomposite as a green absorbent for the adsorptive removal of Hg(ii) from aqueous solutions
- Characterization of silver sulfide nanoparticles from actinobacterial strain (M10A62) and its toxicity against lepidopteran and dipterans insect species
- Phyto-fabrication and characterization of silver nanoparticles using Withania somnifera: Investigating antioxidant potential
- Effect of e-waste nanofillers on the mechanical, thermal, and wear properties of epoxy-blend sisal woven fiber-reinforced composites
- Magnesium nanohydroxide (2D brucite) as a host matrix for thymol and carvacrol: Synthesis, characterization, and inhibition of foodborne pathogens
- Synergistic inhibitive effect of a hybrid zinc oxide-benzalkonium chloride composite on the corrosion of carbon steel in a sulfuric acidic solution
- Review Articles
- Role and the importance of green approach in biosynthesis of nanopropolis and effectiveness of propolis in the treatment of COVID-19 pandemic
- Gum tragacanth-mediated synthesis of metal nanoparticles, characterization, and their applications as a bactericide, catalyst, antioxidant, and peroxidase mimic
- Green-processed nano-biocomposite (ZnO–TiO2): Potential candidates for biomedical applications
- Reaction mechanisms in microwave-assisted lignin depolymerisation in hydrogen-donating solvents
- Recent progress on non-noble metal catalysts for the deoxydehydration of biomass-derived oxygenates
- Rapid Communication
- Phosphorus removal by iron–carbon microelectrolysis: A new way to achieve phosphorus recovery
- Special Issue: Biomolecules-derived synthesis of nanomaterials for environmental and biological applications (Guest Editors: Arpita Roy and Fernanda Maria Policarpo Tonelli)
- Biomolecules-derived synthesis of nanomaterials for environmental and biological applications
- Nano-encapsulated tanshinone IIA in PLGA-PEG-COOH inhibits apoptosis and inflammation in cerebral ischemia/reperfusion injury
- Green fabrication of silver nanoparticles using Melia azedarach ripened fruit extract, their characterization, and biological properties
- Green-synthesized nanoparticles and their therapeutic applications: A review
- Antioxidant, antibacterial, and cytotoxicity potential of synthesized silver nanoparticles from the Cassia alata leaf aqueous extract
- Green synthesis of silver nanoparticles using Callisia fragrans leaf extract and its anticancer activity against MCF-7, HepG2, KB, LU-1, and MKN-7 cell lines
- Algae-based green AgNPs, AuNPs, and FeNPs as potential nanoremediators
- Green synthesis of Kickxia elatine-induced silver nanoparticles and their role as anti-acetylcholinesterase in the treatment of Alzheimer’s disease
- Phytocrystallization of silver nanoparticles using Cassia alata flower extract for effective control of fungal skin pathogens
- Antibacterial wound dressing with hydrogel from chitosan and polyvinyl alcohol from the red cabbage extract loaded with silver nanoparticles
- Leveraging of mycogenic copper oxide nanostructures for disease management of Alternaria blight of Brassica juncea
- Nanoscale molecular reactions in microbiological medicines in modern medical applications
- Synthesis and characterization of ZnO/β-cyclodextrin/nicotinic acid nanocomposite and its biological and environmental application
- Green synthesis of silver nanoparticles via Taxus wallichiana Zucc. plant-derived Taxol: Novel utilization as anticancer, antioxidation, anti-inflammation, and antiurolithic potential
- Recyclability and catalytic characteristics of copper oxide nanoparticles derived from bougainvillea plant flower extract for biomedical application
- Phytofabrication, characterization, and evaluation of novel bioinspired selenium–iron (Se–Fe) nanocomposites using Allium sativum extract for bio-potential applications
- Erratum
- Erratum to “Synthesis, characterization, and evaluation of nanoparticles of clodinofop propargyl and fenoxaprop-P-ethyl on weed control, growth, and yield of wheat (Triticum aestivum L.)”

