Abstract
Brassica is one of the crops sensitive to low copper supply, leading to Alternaria blight. The present study reflects the synthesis of myco-derived copper oxide (M-CuO) nanoparticles (NPs) from Trichoderma asperellum and investigates their effect against Alternaria blight of Brassica in two soil types, alluvial and calcareous. Foliar applications of different treatments were used to treat plants: T1 (mancozeb@0.2%), T2 (propiconazole@0.05%), T3 (T. asperellum filtrate), T4 (M-CuO NPs), T5 chemically synthesized (C-CuO NPs), and T6 bulk phase (BP-CuO @25, 50, 100, 150, and 200 ppm) of each in twice such as protectant and curative method under pot experiments. M-CuO NPs in two protective sprays exhibit up to 75% disease suppression in alluvial soil, compared to 68.9% suppression in curative spray at 200 ppm. Maximum seed yield and seed number were obtained, 1.95 g/plant and 850 seeds/plant in alluvial soil, but in calcareous soil, seed yield (1.14 g/plant) and seed number 414 seeds/plant were recorded in plants supplemented with M-CuO NPs as a protectant. In both soils, maximum plant height was increased by protective applications of M-CuO NPs at 200 ppm. Thus, the present study suggested that among foliar sprays of copper nanocompounds, protective activity shows better results as compared to curative activity. Among all the treatments, M-CuO NPs were found to be most effective in suppressing disease and improving productivity and growth-promoting effects of Brassica.
1 Introduction
Crops require a sufficient, but not excessive supply of essential micronutrients for disease suppression and optimal productivity. An insufficient supply of mineral elements may lead to a limit in plant growth. Deficiency of insufficient micronutrients, such as copper (Cu), is more common in agricultural soils, particularly in calcareous soil. As a result, these elements can be used in both intensive and extensive agricultural systems supplied as fungicides and fertilizers. Copper nanoparticle (NP), for example, has an interesting and complex biological profile in multiple oxidation states (Cu, Cu2O, CuO) as antimicrobials, agrochemicals, etc. [1,2]. Brassica is one of the sensitive crops to inadequate copper supply, which results in Alternaria symptoms on leaves, stem, and pod blight [3,4]. Furthermore, Cu works as an essential inducer, activating the plant defense system and enzymes, such as peroxidases, plastocyanins, and multi-Cu oxidases, which boost the crop resistance against plant diseases [5,6]. Plant–pathogen interactions activate the plant defense system by producing reactive oxygen species (ROS), antioxidants, and stress enzymes [7]. Several inducers and strategies have been employed to improve plant resistance toward pathogens [7,8,9]. To increase plant resistance to infections, a variety of inducers and techniques have been used [7,8,9]. Because disease-resistant inducers can activate the plant’s defense system against fungus. Compounds that induce plant resistance include salicylic acid and benzothiadiazole. Proteins involved in pathogenesis, such as peroxidase, 1,4 glucanase, and chitinase, have been found to be active in resistant plants [10]. To overcome the stress, defense activities, such as deposition of the phenolics, ascorbic acid, and the stimulation of antioxidant enzymes, are evoked following an attempted microbial invasion [11].
Copper is a major element in the activation of plant defense system when they are subjected to biotic stress. However, NPs’ phytotoxicity results in lower biomass, limited seed germination, reduced root length, and stunted growth [11,12,13]. The majority of prior studies were conducted in lab-scale trials to evaluate the effect of CuO NPs in soil settings, while its favorable benefits through foliar exposure have not yet been investigated. It has been found that the application route of NPs considerably effect ion dissolution, aggregation, and bioavailability. Furthermore, NPs’ foliar spray minimizes agglomeration and increases NP solubility [14,15,16,17]. Moreover, the foliar spray of NPs lowers aggregation and increases NP solubility, resulting in less diverse phytotoxic effects. These NPs release Cu2+ ions on the surface of leaves, acting as a protective shield that prevents the pathogenic attack while simultaneously increasing the nutritional content of seeds and fruits. As a result, the objective of this study was to examine the biocontrol efficacy of copper-based compounds against Alternaria blight, growth dynamics of Brassica juncea, and plants’ stress response.
2 Materials and methods
2.1 Synthesis of M-CuO NPs
Mycogenic copper oxide NPs were synthesized from the cell-free extract of Trichoderma asperellum at specific conditions, which has been reported in our previous paper [4].
2.2 Characterization of M-CuO NPs
Characterization of CuO NPs was done by ultraviolet–visible (UV-Vis) spectrophotomter, scanning electron microscope (SEM), and dynamic light scattering (DLS). The UV-Vis characterization revealed surface plasmon resonance and optical properties, as 10 mg·mL−1 of M-CuO NPs were suspended in water. An instrument Malvern zeta sizer (Nano ZS90, Noida, India) was used to measure mean particle size and zeta potential was recorded to determine the surface charge on the surface of NPs. Measurements were carried out in triplicate with a temperature equilibration of 1 min at 25°C with an angle of 90°C [18].
2.3 Preparation of NP suspension, stock solution of CuO NPs, and standard check fungicides
Sequential concentrations of both types of CuO NPs from a 1,000 ppm working solution were prepared to 25, 50, 100, 150, and 200 ppm doses before use. Suspension of C-CuO NPs <50 nm particle size, SRL chemicals, and BP-CuO <10 μm of different concentrations was also prepared. The solution of NPs was filtered through 0.22 μm syringe Millipore filters and sonicated for 2 min before use for getting monodispersed population. The commercially available fungicides (1) mancozeb (0.2%) and (2) propiconazole (0.05%) at their standard concentrations were prepared in distilled water and 50% filtrate of T. asperellum was also prepared.
2.4 Nutrient analysis of soil
Clay pots of size (12 cm × 12 cm) were purchased from a local store. Five to six kilograms of soil per pot were filled. Two soil samples, i.e., alluvial and calcareous soil, were submitted to the Division of Agronomy, ICAR-IARI, New Delhi, for macro- and micro-nutrient analyses. Nitrogen (N), phosphorus (P), potassium (K), and micro-nutrients, such as iron (Fe), zinc (Zn), copper (Cu), and manganese (Mn), were also quantified.
2.5 Sample collection of leaves
Samples were collected on the 30th day of sowing before infection and after infection on the 60th day. Plants’ stress level was estimated by checking the antioxidant enzymes (superoxide dismutase and catalase).
2.6 Superoxide dismutase and catalase assay
Total superoxide dismutase activity was determined by nitroblue tetrazolium photochemical assay according to Misra and Fridovich [19]. The Aebi method was used for determining catalase activity in leaves challenged with A. brassicae and CuO NP treatment [20].
2.7 Plants in vivo experiment
Seeds of PM-25 variety of B. juncea (germination efficiency >98%) were provided by ICAR-IARI, Division of Plant Pathology, New Delhi, India. Pots were divided into six treatments with three replicates each. Seeds were surface sterilized with 0.4% sodium hypochlorite solution followed by repeated washing with distilled water. Surface-sterilized seeds were sown (10 seeds) in sterile soil and pots. Seeds were allowed to germinate for 1 month. After 1 month, pots were divided into six treatments with three replicates each: (1) negative control (healthy plants without infection), (2) positive control (plants infected with A. brassicae), (3) plants infected with A. brassicae and sprayed with double and single application of mancozeb, (4) plants infected with A. brassicae and sprayed with double and single application of propiconazole, (5) plants infected with A. brassicae and treated with two and one foliar sprays of M-CuO NPs (dose of 25, 50, 100, 150, and 200 ppm) in volume 2 mL/plant in protective and curative set, (6) plants treated with two and one foliar sprays of C-CuO NPs (25, 50, 100, 150, and 200 ppm) and infected with A. brassicae in protective and curative set, and (7) plants treated with two and one foliar sprays of BP-CuO NPs (25, 50, 100, 150, and 200 ppm).
Some of the brassica plants were sprayed before one week with two consecutive foliar applications of CuO NPs in the protective method, while in curative set, after the first symptom of disease, the NPs were sprayed on the rest of the plants. Plants were bagged with a transparent plastic bag and kept for 10 days. Randomly five plants were selected and tagged for taking observations. Five leaves were taken from plants for scoring the disease intensity. Observations for disease severity were taken 75 days after sowing. The overall disease scoring was done at 0–6 rating scale on the basis of the disease assessment key for Alternaria blight in rapeseed-mustard [21].
2.8 Statistical analysis
Data were processed using the statistical package WASP 2.0 (Web Agri Stat Package, Indian Council of Agricultural Research, India). One-way analysis of variance and critical differences at a probability level of 0.01 and 0.05 were obtained with mean values of treatment. Treatments were compared using Duncan’s multiple-range test.
3 Results and discussion
3.1 Characterization of M-CuO NPs
UV-Vis results provide information regarding the particle size and excitation of electron from the valance band to the conduction band by absorption of light and also calculated the band gap [22,23,24]. A broad-centered peak revealed at 363 nm indicates the excitation of electrons from the valence band to the conduction band (Figure 1a). Similar peaks in the range of 300–350 nm were recorded for CuO NPs synthesized from cell-free supernatants of Pseudomonas fluorescens and Bacillus cereus, respectively, by Hesham et al. and Tiwari et al. [25,26].
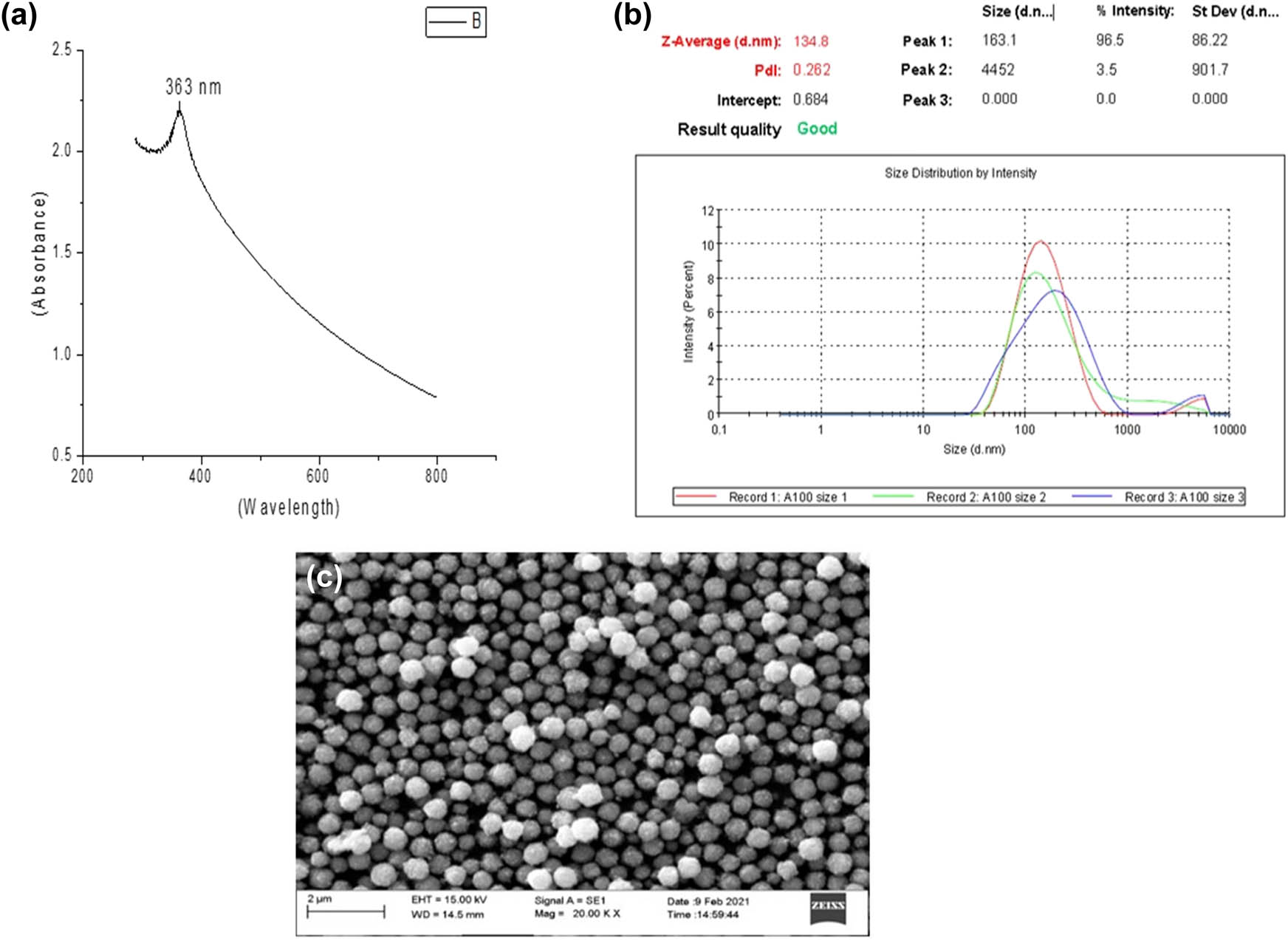
Spectroscopic- and microscopic-based characterization: (a) UV-Vis spectra of M-CuO NPs, (b) particle size of M-CuO NPs, and (c) SEM image of M-CuO NPs.
DLS measurements have confirmed the hydrodynamic diameter of CuO NPs as 134.8 nm as shown in Figure 1b. SEM micrographs indicated the spherical shape and particle size of CuO NPs <50 nm size range (Figure 1c). Image J has been used to estimate the particle size of CuO NPs.
3.2 Disease severity index in Brassica plants
In this study, the application of copper nano-forms and bulk CuO was assessed and compared with the efficacy of fungicides mancozeb (2,000 ppm) and propiconazole (500 ppm), and with the biological extract T. asperellum (50%) to check its effects on the growth of B. juncea and in the suppression of Alternaria blight of Brassica.
The positive control plants infected with A. brassicae have the highest disease severity index to 65%; in conjunction with that, the highest biocontrol efficacy was observed for the plants exposed with M-CuO NPs recorded with very less percentage of blight disease, i.e., 13.8% and 14.8% at 200 ppm concentration in mustard plants grown in alluvial soil, respectively, through protective and curative methods as shown in Figure 2a and Table 1. On the other hand, with C-CuO NPs, the disease recorded was 24.8% and 24.1% through protective and curative methods, respectively. Similarly, with BP-CuO, 23.4% and 20.6%, the disease index was recorded.

Disease severity index in Brassica plants. Protective and curative activities of CuO NPs in alluvial soil (a) and in calcareous soil (b).
Disease severity index of Alternaria blight on Brassica plants infected by A. brassicae
| Disease severity index at day 75 after sowing on Brassica juncea plants grown in alluvial and calcareous soil | |||||
|---|---|---|---|---|---|
| Alluvial soil | calcareous soil | ||||
| S. no. | Treatments | Protective | Curative | Protective | Curative |
| 1. | Negative control | 0.523 ± 0.103a | 4.33 ± 0.471a | 0.30 ± 0.471a | 4.66 ± 0.471a |
| 2. | Positive control | 42 ± 1.414b | 46.3 ± 0.471b | 53.2 ± 0.471b | 65.7 ± 0.081b |
| 3. | Fungicide 1 (Mancozeb) | 20.7 ± 0.45c | 32.6 ± 0.169c | 23.6 ± 0.169c | 37.3 ± 0.974c |
| 4. | Fungicide 2 (Propiconazole) | 33.6 ± 1.02d | 29.6 ± 0.124d | 28.1 ± 0.124d | 34.7 ± 0.081d |
| 5. | Trichoderma asperellum | 27.5 ± 0.21e | 27.5 ± 0.047e | 26.8 ± 0.041e | 27.4 ± 0.329e |
| 6. | M-CuO NPs (25 ppm) | 25.5 ± 0.77f | 26.5 ± 0.141f | 29.0 ± 0.141f | 43.9 ± 1.837f |
| 7. | M-CuO NPs (50 ppm) | 21.1 ± 0.43g | 25.7 ± 0.047g | 26.5 ± 0.124e | 34.5 ± 0.216g |
| 8. | M-CuO NPs (100 ppm) | 17.4 ± 0.44h | 19.5 ± 0.169h | 26.7 ± 0.169e | 28.5 ± 0.169h |
| 9. | M-CuO NPs (150 ppm) | 16.2 ± 0.43i | 17.7 ± 0.124i | 24.3 ± 0.124g | 27.4 ± 0.047h |
| 10. | M-CuO NPs (200 ppm) | 13.8 ± 0.49j | 14.8 ± 0.57j | 23.6 ± 0.571c | 25.4 ± 0.262i |
| 11. | C-CuO NPs (25 ppm) | 35.1 ± 0.45k | 32.0 ± 0.89c | 29.1 ± 0.899f | 46.0 ± 1.268j |
| 12. | C-CuO NPs (50 ppm) | 33.0 ± 1.02d | 31.6 ± 0.124c | 21.6 ± 0.204h | 37.3 ± 0.124c |
| 13. | C-CuO NPs (100 ppm) | 31.6 ± 0.54d | 27.6 ± 0.204e | 21.5 ± 0.205h | 35.9 ± 0.571d |
| 14. | C-CuO NPs (150 ppm) | 25.5 ± 1.43f | 26.6 ± 0.124e | 20.4 ± 0.124i | 34.6 ± 0.124d |
| 15. | C-CuO NPs (200 ppm) | 24.8 ± 0.46f | 24.5 ± 0.205g | 26.7 ± 0.205e | 35.0 ± 1.84d |
| 16. | BP-CuO (25 ppm) | 27.6 ± 0.82e | 27.5 ± 0.047e | 31.4 ± 0.047j | 40.7 ± 0.081k |
| 17. | BP-CuO (50 ppm) | 28.4 ± 0.26e | 24.6 ± 0.216g | 31.0 ± 0.216j | 41.4 ± 0.169l |
| 18. | BP-CuO (100 ppm) | 27.3 ± 0.68e | 21.5 ± 0.169k | 30.5 ± 0.169k | 39.2 ± 0.355m |
| 19. | BP-CuO (150 ppm) | 24.8 ± 0.88f | 20.7 ± 0.163k | 30.4 ± 0.163k | 38.5 ± 0.249n |
| 20. | BP-CuO (200 ppm) | 23.4 ± 0.04f | 20.6 ± 0.047l | 29.5 ± 0.047l | 37.8 ± 0.188o |
-
Different superscript letters indicate statistically significant results among control and experimental groups.
Moreover, plants sprayed with mancozeb at 2,000 ppm dose as a protectant and curative agent resulted in 33.6% and 32.6% severity index, respectively. Also, when propiconazole was applied at 500 ppm, 33.6% and 29.6% severity index was observed. The findings of our study revealed a significant decrease in disease severity index when plants were exposed to M-CuO NPs when applied in protective and curative modes.
Abdelkhalek and Al-Askar [27] revealed that biologically synthesized ZnO NPs were assessed against tobacco mosaic virus, which showed both protective and curative activities with better protective activity. Attia et al. [28] have reported a similar influence in the biocontrol efficacy of CuO-streptomycin drug, which was found against potato brown rot disease with 55.8% protection efficiency. Thus, these studies have supported the better protective activity of M-CuO NPs. However, as compared to separately ameliorative effects of M-CuO NPs and C-CuO NPs, BP-CuO occupied the lowest rank in biocontrol efficacy.
On the contrary, for control plants grown in calcareous soil, a 53.2% disease severity was recorded. However, at 200 ppm, with M-CuO NPs, 24.8% and 25.8%, the disease index was recorded with protective and curative methods (Figure 2b). Likewise, with C-CuO NPs at 200 ppm, 26.7% and 34.6% index was recorded. Similarly, with BP-CuO, 29.5% and 37.8% severity index was observed. Some of brassica plants were sprayed with mancozeb has been recorded with disease severity index 23.6 % in protective method and 37.3 %, in curative method, respectively. Also, the propiconazole at 500 ppm dose resulted in a severity index, i.e., 28.1% and 34.7% through protective and curative methods. Thus, these experiments at pot levels suggested that the optimized dose for the suppression of disease was 100–200 ppm (Figure 3).

A photograph showing the disease symptoms of Alternaria blight on mustard leaves at 75th day post inoculation: (a) plants inoculated with A. brassicae, (b) plants treated with two protective foliar sprays of C-CuO NPs (100 ppm), (c) plants treated with two foliar sprays of M-CuO NPs (100 ppm), (d) plants inoculated with A. brassicae, (e) plants treated after the first symptom of disease with one foliar spray of C-CuO NPs (100 ppm), and (f) plants treated after the first symptom of disease with one foliar spray of M-CuO NPs (100 ppm).
In a nutshell, the comparison of treatments showed that M-CuO NPs at higher doses (100–200 ppm) efficiently provide resistance to plants before infection, while in a curative way, single spray was less effective. Thus, our study has proved that the application of M-CuO NPs compared with other treatments acts as a better protectant, which significantly increases the disease resistance and efficiently control the blight disease by 73.25% and control equally as a curative method, which reduced the disease by 68%. However, the protectant mode was considered the best treatment for mustard plants grown under normal and stress conditions (nutrient deficiency of Cu) in both soils. It was found that the applications of M-CuO NPs were also effective in stress conditions and were able to provide the optimum Cu2+ ions.
3.3 Plant growth-promoting potential of CuO NPs
Plant growth studies revealed a steady increase in plant height between the 30th and 40th days up to 200 ppm dose in the protective method, with more pronounced effects in nutrient-deficient soil. In alluvial soil, as compared to untreated plants where height was 57 cm, the maximum height recorded was 61 cm in plants treated with M-CuO NPs (Figures 4a and 5a and Table 2). However, at higher doses of C-CuO NPs (200 ppm), a negative impact was observed in plant height as 54 and 50 cm height recorded as compared to 57 cm height recorded in untreated plants. Similarly, a minimum plant height of around 48.6 and 54.6 cm in protective and curative modes was observed with BP-exposed plants at 200 ppm. Thus, the trend evident was retarded plant growth with higher doses of C-CuO NPs and BP-CuO as compared to the positive effects of M-CuO NPs. The application of NPs through both the methods suggested no significant effects on plant height in alluvial soil, while significant effect was observed in calcareous soil.

Plant growth-promoting effects of CuO NPs: (a) plant height in alluvial soil and (b) plant height in calcareous soil.

Effect of CuO NPs on seed yield/plant: (a) alluvial soil and (b) calcareous soil.
Shoot length of Brassica plants treated with CuO NPs
| Shoot length of mustard plants grown in alluvial and calcareous soil | |||||
|---|---|---|---|---|---|
| Alluvial soil | calcareous soil | ||||
| S. no. | Treatments | Protective | Curative | Protective | Curative |
| 1. | Negative control | 57.3 ± 1.77a | 53.0 ± 2.16a | 35.3 ± 1.24a | 33.0 ± 1.63a |
| 2. | Positive control | 53.6 ± 2.27b | 53.3 ± 2.27a | 33.0 ± 4.32b | 37.3 ± 1.41b |
| 3. | Fungicide 1 (mancozeb) | 58.0 ± 0.00c | 48.6 ± 4.61b | 36.3 ± 0.94c | 33 ± 2.49c |
| 4. | Fungicide 2 (propiconazole) | 52.6 ± 0.40d | 53.3 ± 0.33a | 36.6 ± 0.47d | 33.3 ± 0.47c |
| 5. | Trichoderma asperellum | 60.3 ± 2.8e | 57.0 ± 1.06c | 41.3 ± 1.24e | 36 ± 2.82d |
| 6. | M-CuO NPs (25 ppm) | 48.3 ± 1.08f | 48.6 ± 1.08b | 35.6 ± 0.94f | 35 ± 2.16e |
| 7. | M-CuO NPs (50 ppm) | 50.6 ± 0.40g | 53.0 ± 3.24a | 46 ± 0.81g | 37.6 ± 0.47f |
| 8. | M-CuO NPs (100 ppm) | 55 ± 1.41h | 55.0 ± 1.87h | 56 ± 2.16h | 37.3 ± 0.47g |
| 9. | M-CuO NPs (150 ppm) | 60.6 ± 1.47e | 53.6 ± 0.81a | 61 ± 0.18i | 37.0 ± 0.81h |
| 10. | M-CuO NPs (200 ppm) | 61.3 ± 0.43e | 54.0 ± 1.41g | 61.5 ± 0.44i | 47.0 ± 0.23i |
| 11. | C-CuO NPs (25 ppm) | 55.3 ± 0.47h | 49.3 ± 3.09c | 56 ± 2.16j | 33.6 ± 1.24j |
| 12. | C-CuO NPs (50 ppm) | 58.0 ± 0.81c | 52.3 ± 1.69d | 56 ± 0.81j | 35.3 ± 0.81k |
| 13. | C-CuO NPs (100 ppm) | 61.0 ± 0.81e | 53.6 ± 1.69d | 61 ± 0.81k | 37.0 ± 3.09l |
| 14. | C-CuO NPs (150 ppm) | 58.6 ± 1.24c | 53.3 ± 1.69d | 60.6 ± 0.47l | 38 ± 1.24m |
| 15. | C-CuO NPs (200 ppm) | 54 ± 0.816b | 50.6 ± 4.49c | 61.6 ± 0.47l | 38.6 ± 0.92n |
| 16. | BP-CuO (25 ppm) | 57.3 ± 2.05c | 51.0 ± 0.471d | 36.6 ± 0.47c | 36.6 ± 0.47o |
| 17. | BP-CuO (50 ppm) | 57 ± 0.816c | 54.3 ± 0.471a | 38.6 ± 0.47c | 36.6 ± 1.24o |
| 18. | BP-CuO (100 ppm) | 57.3 ± 0.471c | 60.3 ± 1.69i | 42.3 ± 1.69e | 36.3 ± 0.94o |
| 19. | BP-CuO (150 ppm) | 53 ± 2.16b | 57.6 ± 0.816c | 40.0 ± 0.81 | 36.6 ± 0.47o |
| 20. | BP-CuO (200 ppm) | 48.6 ± 1.24f | 54.6 ± 0.816h | 38.0 ± 0.81 | 37.0 ± 0.81p |
-
Different superscript letters indicate statistically significant results among control and experimental groups.
Interestingly, the plant groups grown in calcareous soil have attained maximum plant height of up to 61 and 47 cm, respectively, at 200 ppm dose with M-CuO NPs as compared to untreated plants (33 and 35 cm) in protective and curative treatments. The positive effect in the plant height was due to the uptake of NPs by the plant and significant effects in both modes were observed with M-CuO NPs. Similarly, with C-CuO NPs, maximum plant height of 61.6 and 38.6 cm was recorded, respectively, as compared to untreated plants (33 and 35 cm) (Figures 4b and 5b). However, the plant groups exposed to bulk copper have no significant effects on plant height through both modes. Thus, the analysis indicated that in calcareous soil, the protectant mode worked better with superior performance of M-CuO NPs; moreover, no phytotoxicity effects were observed with M-CuO NPs and C-CuO NPs, up to 200 ppm dose.
Despite the fact, the toxicity issue was observed with C-CuO NPs and BP-CuO at higher doses (150 and 200 ppm) in alluvial soil. Among five treatments conducted in a study, it has been found that both of the fungicides mancozeb and propiconazole has no significant effects on plant height in alluvial soil. Thus, the overall analysis has suggested that among all the treatments, M-CuO NPs have a positive effect on plant height even up to higher dose of 200 ppm, while C-CuO NPs have also given promising results up to lower doses at 100 ppm. The experiments revealed the minimum concentration of copper also leads to stimulate plant growth. Their mode of supply, dose and nature varies. It has been supported by several studies that minimum dose of 50-100 ppm promoting the growth in several crops such as wheat, maize, and Brassica [29,30,31]. Thus, a positive effect in the plant groups supplied with a 200 ppm dose of M-CuO NPs supported by previous findings was evident in both soils, with more pronounced effects in calcareous soil, where nutrient deficiency of Cu was fulfilled by two sprays of CuO NPs.
3.4 Productivity of Brassica plants
Pods perform a developmental role in seed encapsulation and protection against biotic and abiotic stress. Their count is a crucial parameter for yield quantification [32,33]. It was observed that plants exposed to M-CuO NPs increased yield by 1.95 and 1.24 g/plant at 200 ppm through protective and curative modes as compared to untreated (control) plants where 0.72 g/plant yield was recorded. However, in calcareous soil with mycogenic nanoparticles, the seed yield was 0.92 and 0.77 g/plant in protective and curative treatments as compared to control (0.67–0.633 g/plant). Thus, the seed yield analysis revealed that mycogenic NP treatment resulted in the maximum yield in alluvial soil with the protectant mode. Similarly, the exposed plants at 200 ppm with C-CuO NPs increase the seed yield by 1.84 and 0.86 g/plant through protective and curative methods as compared to the control (0.72 g/plant). Moreover, with BP-CuO, the seed yield was 1.66 and 1.55 g/plant, respectively in protective and curative treatments as compared to control (0.72 g/plant).
However, the results in calcareous soil were less significant near around 0.83 and 0.76 g/plant, in protective and curative methods as compared to control (0.67 g/plant) with mycogenic NP treatment. Also, C-CuO NPs increase the yield around 0.93 and 0.806 g/plant in protective and curative treatments as compared to control (0.67 g/plant). Thus, the overall analysis has also suggested that the BP-CuO, somehow, worked well for the increment of seed yield in calcareous soil (Table 3). However, the maximum yield was 1.95 g/plant with mycogenic NPs, which outcompeted the rest of the treatments.
Seed weight of Brassica plants exposed with CuO NPs
| Seed weight (1,000 seeds) of mustard plants grown in alluvial and calcareous soil | |||||
|---|---|---|---|---|---|
| Alluvial soil | calcareous soil | ||||
| S. no. | Treatments | Protective | Curative | Protective | Curative |
| 1. | Negative control | 0.726 ± 0.012a | 0.67 ± 0.089a | 0.65 ± 0.056a | 0.60 ± 0.047a |
| 2. | Positive control | 0.473 ± 0.05b | 0.493 ± 0.047b | 0.37 ± 0.014b | 0.37 ± 0.012b |
| 3. | Fungicide 1 (mancozeb) | 0.863 ± 0.012c | 0.439 ± 0.019b | 0.54 ± 0.04c | 0.45 ± 0.169c |
| 4. | Fungicide 2 (propiconazole) | 0.773 ± 0.08d | 0.478 ± 0.029b | 0.38 ± 0.06b | 0.56 ± 0.07d |
| 5. | Trichoderma asperellum | 0.676 ± 0.136e | 0.633 ± 0.03c | 1.46 ± 0.13d | 0.60 ± 0.04e |
| 6. | M-CuO NPs (25 ppm) | 1.01 ± 0.03f | 0.71 ± 0.035d | 0.49 ± 0.047e | 0.72 ± 0.04f |
| 7. | M-CuO NPs (50 ppm) | 1.37 ± 0.08f | 0.71 ± 0.166d | 0.88 ± 0.081f | 0.72 ± 0.03f |
| 8. | M-CuO NPs (100 ppm) | 1.40 ± 0.175g | 0.76 ± 0.069e | 1.53 ± 0.024d | 0.74 ± 0.02f |
| 9. | M-CuO NPs (150 ppm) | 1.88 ± 0.014h | 0.92 ± 0.044f | 1.54 ± 0.07d | 0.77 ± 0.07f |
| 10. | M-CuO NPs (200 ppm) | 1.95 ± 0.038i | 1.14 ± 0.026g | 1.58 ± 0.102d | 0.78 ± 0.14f |
| 11. | C-CuO NPs (25 ppm) | 1.15 ± 0.338f | 0.67 ± 0.009c | 0.526 ± 0.054c | 0.68 ± 0.004g |
| 12. | C-CuO NPs (50 ppm) | 1.38 ± 0.286f | 0.68 ± 0.004c | 0.633 ± 0.08a | 0.77 ± 0.012h |
| 13. | C-CuO NPs (100 ppm) | 1.59 ± 0.08g | 0.76 ± 0.012e | 1.61 ± 0.05d | 0.92 ± 0.04i |
| 14. | C-CuO NPs (150 ppm) | 1.72 ± 0.054h | 0.76 ± 0.008e | 1.65 ± 0.007d | 0.88 ± 0.09j |
| 15. | C-CuO NPs (200 ppm) | 1.84 ± 0.047h | 0.83 ± 0.024f | 1.71 ± 0.004e | 0.76 ± 0.16f |
| 16. | BP-CuO (25 ppm) | 0.653 ± 0.038e | 0.65 ± 0.042c | 0.70 ± 0.040f | 0.65 ± 0.03g |
| 17. | BP-CuO (50 ppm) | 0.67 ± 0.081e | 0.68 ± 0.054c | 0.51 ± 0.049c | 0.73 ± 0.03h |
| 18. | BP-CuO (100 ppm) | 0.74 ± 0.352d | 0.74 ± 0.021e | 0.54 ± 0.098c | 0.71 ± 0.08h |
| 19. | BP-CuO (150 ppm) | 1.52 ± 0.122f | 0.93 ± 0.422f | 1.39 ± 0.04d | 0.85 ± 0.05j |
| 20. | BP-CuO (200 ppm) | 1.60 ± 0.116f | 0.55 ± 0.159c | 1.50 ± 0.04d | 0.80 ± 0.05j |
-
Different superscript letters indicate statistically significant results among control and experimental groups.
Furthermore, the fungicides mancozeb and propiconazole in protective and curative modes have no significant effect on the seed yield as compared to control plants (0.72 g/plant). However, the mancozeb at 500 ppm resulted in a seed yield of around 0.86 and 0.77 g/plant for plants grown in alluvial soil. Likewise, in calcareous soil, a significant reduction in seed yield was observed, which was around 0.439 and 0.53 g/plant in mancozeb-exposed plants through both modes, respectively. Thus, it could be concluded that in calcareous soil, the mancozeb treatment leads to stress through both modes.
Moreover, the plants treated with propiconazole have no significant increase in seed yield as compared to control plants. Likewise, in calcareous soil, a decline in seed yield with 0.478 and 0.563 g/plant through both modes was obtained as compared to control plants (0.67 g/plant). On the contrary, with the filtrate of T. asperellum, in calcareous soil, nearly equal results were 0.63 and 0.60 g/plants obtained as compared to the control (0.63 g/plant). While in alluvial soil, a one-fold decrease (0.67 and 0.61 g/plant) as compared to control plants (0.72 g/plant) was obtained. Thus, the analysis of all treatments suggested that mycogenic NPs doubled the seed yield, which outcompeted the rest of the treatments.
3.5 Defense enzyme estimation in Brassica plants
ROS are the major factors responsible for tissue deterioration during abiotic stress and biotic stress. These are known to disintegrate nucleic acids and alter protein structures, which ultimately cause mutation in gene and delay the process of cell division, thereby affecting the plant growth. Tian et al. [34] reported that higher accumulation of ROS can lead to cell death, which can be either necrotic or programmed. In the present study, the production and accumulation of O2− in control and treated mustard plants were recorded by spectrophotometry (Figure 6 and Table 4). The probable reasons for O2− accumulations are the oxidative burst and reduced efficiency of antioxidant enzymes occurred during NP exposure. In our study, it has been found that M-CuO NP exposure and C-CuO NPs at higher doses (150 and 200 ppm) lead to the accumulation of O2 radicals; results are comparable with the study of Roy et al. [35] who have reported the comparative impact of bulk phase CuO and nanoforms of CuO in maize seedlings, separately which showed more pronounced and higher SOD and CAT activities in BP-CuO-exposed maize seedlings [35]. The mustard leaves challenged with BP-CuO has shown poor performance of bulk particles which could be explained by high tissue Cu accumulation, comparatively higher membrane injury, marked decline in carotenoids level and higher Cu ions dissolution.

Superoxide dismutase enzyme estimation in Brassica plants.
Antioxidant enzyme analysis (superoxide dismutase) in Brassica plants
| Superoxide dismutase activity of mustard plants treated with CuO NPs (mol·min−1) | |||||
|---|---|---|---|---|---|
| Alluvial soil | calcareous soil | ||||
| S. no. | Treatments | Protective | Curative | Protective | Curative |
| 1. | Negative control | 21.1 ± 0.47a | 20.7 ± 0.12a | 22.9 ± 0.46a | 24.7 ± 0.52a |
| 2. | Positive control | 30.3 ± 0.12b | 31.7 ± 0.33b | 33.2 ± 0.12b | 35.5 ± 0.81b |
| 3. | Fungicide 1 (mancozeb) | 31.3 ± 0.12b | 21.6 ± 0.12c | 34.3 ± 0.14c | 24.7 ± 0.43c |
| 4. | Fungicide 2 (propiconazole) | 29.3 ± 0.36c | 22.6 ± 0.57d | 26.1 ± 0.40d | 26.2 ± 1.13d |
| 5. | Trichoderma asperellum | 18.7 ± 0.16d | 18.5 ± 0.12e | 21.4 ± 0.08e | 23.9 ± 1.43e |
| 6. | M-CuO NPs (25 ppm) | 31.3 ± 0.09b | 30.5 ± 0.08f | 37.1 ± 0.49f | 36.9 ± 1.73f |
| 7. | M-CuO NPs (50 ppm) | 35.0 ± 0.44e | 30.2 ± 0.16f | 37.4 ± 1.29f | 37.3 ± 0.97g |
| 8. | M-CuO NPs (100 ppm) | 32.9 ± 0.601b | 31.4 ± 0.14g | 39.3 ± 0.46g | 35.3 ± 0.49f |
| 9. | M-CuO NPs (150 ppm) | 37.5 ± 0.244f | 32.2 ± 0.14h | 45.5 ± 0.21h | 35.3 ± 2.19f |
| 10. | M-CuO NPs (200 ppm) | 38.4 ± 1.06g | 33.2 ± 0.12i | 49.1 ± 0.43i | 37.0 ± 0.33g |
| 11. | C-CuO NPs (25 ppm) | 28.9 ± 0.124c | 22.2 ± 0.71j | 32.4 ± 0.09b | 29.8 ± 0.08d |
| 12. | C-CuO NPs (50 ppm) | 29.2 ± 0.44c | 23.8 ± 1.13k | 34.5 ± 0.12c | 33.6 ± 0.75f |
| 13. | C-CuO NPs (100 ppm) | 30.7 ± 0.08c | 23.9 ± 0.40l | 35.4 ± 0.16c | 34.1 ± 2.46f |
| 14. | C-CuO NPs (150 ppm) | 33.2 ± 0.89b | 24.8 ± 0.32m | 37.8 ± 0.04f | 34.7 ± 1.72f |
| 15. | C-CuO NPs (200 ppm) | 32.5 ± 0.41b | 25.4 ± 0.47n | 42.6 ± 0.98h | 35.7 ± 1.35f |
| 16. | BP-CuO (25 ppm) | 27.9 ± 0.08c | 20.2 ± 2.02o | 20.7 ± 0.04a | 20.8 ± 0.40h |
| 17. | BP-CuO (50 ppm) | 28.2 ± 0.12c | 21.9 ± 0.46p | 22.3 ± 0.20a | 23.7 ± 0.99c |
| 18. | BP-CuO (100 ppm) | 29.4 ± 0.41c | 23.0 ± 2.1q | 24.4 ± 0.12b | 25.9 ± 0.57d |
| 19. | BP-CuO (150 ppm) | 29.8 ± 0.08c | 24.0 ± 1.00r | 28.5 ± 0.08d | 26.5 ± 0.08d |
| 20. | BP-CuO (200 ppm) | 29.6 ± 0.08c | 26.2 ± 1.72s | 30.2 ± 0.12d | 27.4 ± 1.00d |
-
Different superscript letters indicate statistically significant results among control and experimental groups.
Interestingly, in our study, we found that, in protective and curative treatments, plants exposed to M-CuO NPs leads to a lower level of O2 accumulation, as compared to control plants, which ultimately results in antioxidant enzymes’ downregulation, and proved to be an efficient non-toxic molecule for plant system (Figure 6 and Table 5). Also, C-CuO NPs’ exposure and BP-CuO at higher doses (150 and 200 ppm) lead to the accumulation of O2 radicals. It has been suggested that O2− gets converted into H2O2 and O2 by the enzyme SOD. Moreover, it has been found that a significantly higher percentage of SOD activity was recorded in protective treatment for mustard plants grown in calcareous soil, which could be due to the synergistic effect of biotic stress and abiotic stress; the plants grown in calcareous soil and exposed to two foliar applications of CuO resulted in more O2− accumulation. Similarly, CAT activity was increased in BP-CuO plants and C-CuO, and the traditional fungicides when acted as a protectant also lead to the accumulation of H2O2, which may be the result of enhanced SOD activity in the same plant groups with protective treatment and plants grown in calcareous soil (Figure 7). Thus, the alterations found in both treatments in both soils concluded that M-CuO NPs worked more proficiently in both soils with both treatments. However, the study needs to be explored more for mustard plants grown in calcareous soil.
Stress enzyme analysis (catalase) in mustard plants treated with CuO NPs
| Catalase activity of mustard plants treated with CuO NPs (mol·min−1) | |||||
|---|---|---|---|---|---|
| Alluvial soil | calcareous soil | ||||
| S. no. | Treatments | Protective | Curative | Protective | Curative |
| 1. | Negative control | 23.6 ± 0.08a | 25.6 ± 0.81a | 23.3 ± 0.169a | 26.0 ± 0.53a |
| 2. | Positive control | 34.3 ± 0.16b | 35.2 ± 1.66b | 32.5 ± 0.08b | 37 ± 0.99b |
| 3. | Fungicide 1 (mancozeb) | 34.4 ± 0.12b | 24.7 ± 1.86c | 34.6 ± 0.09c | 27.3 ± 1.16c |
| 4. | Fungicide 2 (propiconazole) | 38.3 ± 1.10c | 24.2 ± 0.98d | 36.5 ± 0.16d | 27.7 ± 0.78c |
| 5. | Trichoderma asperellum | 17.9 ± 0.08d | 23.8 ± 0.47e | 21.7 ± 0.49a | 24.5 ± 1.51d |
| 6. | M-CuO NPs (25 ppm) | 25.3 ± 0.47e | 31.8 ± 0.80f | 37 ± 0.35d | 35.6 ± 1.51e |
| 7. | M-CuO NPs (50 ppm) | 25.7 ± 0.21e | 32.1 ± 0.42f | 38.4 ± 0.24d | 36.4 ± 0.56f |
| 8. | M-CuO NPs (100 ppm) | 31.6 ± 0.16f | 33.8 ± 1.01f | 39.5 ± 0.43d | 37.7 ± 0.12f |
| 9. | M-CuO NPs (150 ppm) | 31.5 ± 0.20f | 33.7 ± 0.08f | 40.8 ± 0.08d | 39.7 ± 0.124f |
| 10. | M-CuO NPs (200 ppm) | 36.6 ± 0.08g | 33.7 ± 0.04f | 48.5 ± 0.98e | 39.8 ± 0.08f |
| 11. | C-CuO NPs (25 ppm) | 21.8 ± 0.88h | 27.7 ± 0.78g | 32.6 ± 0.12b | 25.7 ± 0.29a |
| 12. | C-CuO NPs (50 ppm) | 24.8 ± 0.57e | 26.7 ± 0.04g | 34.6 ± 0.09b | 27.6 ± 0.09c |
| 13. | C-CuO NPs (100 ppm) | 25.8 ± 1.23e | 27.0 ± 0.57g | 35.4 ± 0.12c | 24.0 ± 0.38a |
| 14. | C-CuO NPs (150 ppm) | 29.7 ± 0.21f | 27.7 ± 0.81g | 37.7 ± 0.21d | 27.0 ± 1.37c |
| 15. | C-CuO NPs (200 ppm) | 38.2 ± 1.62g | 28.0 ± 0.91g | 41.7 ± 0.33d | 28.4 ± 0.47c |
| 16. | BP-CuO (25 ppm) | 21.3 ± 2.1a | 23.6 ± 0.08c | 20.7 ± 0.12a | 24.6 ± 0.82d |
| 17. | BP-CuO (50 ppm) | 19.7 ± 0.12i | 23.6 ± 0.01c | 22.6 ± 0.24a | 25.9 ± 1.21a |
| 18. | BP-CuO (100 ppm) | 19.6 ± 0.12i | 24.2 ± 0.94c | 24.7 ± 0.08a | 26.6 ± 0.69a |
| 19. | BP-CuO (150 ppm) | 19.4 ± 0.35i | 25.5 ± 0.81d | 28.3 ± 0.37a | 27.3 ± 0.46a |
| 20. | BP-CuO (200 ppm) | 19.5 ± 0.12i | 27.5 ± 0.08d | 30.3 ± 0.12b | 27.3 ± 0.87a |
-
Different superscript letters indicate statistically significant results among control and experimental groups.

CAT activity in Brassica plants exposed to CuO NPs.
Our finding with M-CuO NPs shows a positive response at higher concentrations, when applied on foliage, enhanced almost all the growth, biochemical, and physiological parameters. A simplified view of the foliar application of CuO NPs in B. juncea has been shown in Figure 8, where we can see how NPs enter into plant cells and affect different growth and physiological parameters in plants. Based on all the effects observed in the study, NPs promotes disease suppression at higher concentration. Therefore, the release of antifungal compounds is responsible for the inhibition of Alternaria blight pathogens.

Impact of CuO NPs on disease suppression and biochemical parameters.
Plants adapted to two defense systems, one is enzymatic that involves the activation of superoxide dismutase and catalase. This result can be applied in the field to see their further outcome on a large scale before making any recommendation to farmers. CuO NPs then can be utilized as micronutrients to enhance the production of mustard and their growth, which ultimately results in higher yields. Also, biochemical parameters like an increase in photosynthetic pigment and antioxidant enzyme activity upregulation were found in Brassica plants.
4 Conclusion and future perspectives
The current investigation reveals that the phytopathogenic fungi Alternaria brassicae negatively affected the plant growth, biomass, and productivity of mustard plants. The maximum disease severity index was 42–65% in positive control plants, whereas plants supplied with mycogenic copper compounds scored with disease suppression by more than 50%. Also, the plant growth characteristics, with enhanced productivity, were obtained having more than 30% increase. The study also revealed that among six treatments tested in a field, M-CuO NPs performed significantly better even from 50 ppm concentration in nutrient-rich and nutrient-deficient soil. As compared with standard chemical fungicides, M-CuO NPs because of their lower particle size and prolonged release of Cu ions resulted in better fertilizing and fungicidal activity.
Also, exogenous supplementation of M-CuO NPs on mustard plants mitigated the detrimental effects by improving the growth characteristics and total chlorophyll content. However, if the plants were exposed with protective applications of C-CuO NPs and BP-CuO leads to the activation of antioxidant enzymes like SOD and CAT, thus indicating the comparatively higher accumulation of ROS but low levels of antioxidant enzymes in M-CuO NPs exposed plants. Therefore, a lesser accumulation of ROS leads to the selection of M-CuO NPs in the field.
The experimental outcomes are based on the controlled condition established through field and pot experiments. Therefore, the Brassica plant life cycle monitored with nano-formulations accurately mimics the impacts of CuO NPs as fungicides and fertilizers on plants and their parts to generate environmentally relevant applications. Nanofungicides denote the next generation of traditional fungicides, as well as they will offer more advances such as high efficacy, durability, and less doses of effective ingredients. Preparation of nanofungicides can be done in a simple cost-effective manner, which is found to be appropriate for formulating recent types of biohybrid nanocide constituents and, hence, would be proved as novel environment responsive antimicrobial against diverse fungal pathogens of plants.
Acknowledgements
We would like to thank Dr. Lakshman Prasad and Dr. Ajit Varma who have provided all lab and field experiments facilities.
-
Funding information: The authors state that no funding is involved.
-
Author contributions: Swati Gaba: writing – original draft, methodology, data analysis; Ashutosh Kumar Rai, and Ajit Varma: review and editing, data analysis, visualization; Lakshman Prasad, Arti Goel, and Ram Prasad: writing – review and editing, project administration, resources. All authors have read and agreed to the published version of the manuscript.
-
Conflict of interest: The first corresponding author (Ram Prasad) is a member of the Editorial Board of Green Processing and Synthesis.
-
Data availability statement: All data generated or analyzed during this study are included in this published article and its supplementary information files.
References
[1] Hajipour MJ, Fromm KM, Akbar Ashkarran A, Jimenez de Aberasturi D, Larramendi IR, Rojo T, et al. Antibacterial properties of nanoparticles. Trends Biotechnol. 2012;30(10):499–511. 10.1016/j.tibtech.2012.06.004.Search in Google Scholar PubMed
[2] Baker S, Volova T, Prudnikova SV, Satish S, Prasad MNN. Nanoagroparticles emerging trends and future prospect in modern agriculture system. Environ Toxicol Pharmacol. 2017;53:10–7. 10.1016/j.etap.2017.04.012.Search in Google Scholar PubMed
[3] Meena PD, Rani A, Meena MC, Sharma P, Kandpal B, Singh D. Role of nutrients and lower leaf removal against Alternaria blight in Indian mustard (Brassica juncea L.). Plant Pathol J. 2015;14(2):92–6. 10.3923/ppj.2015.92.96.Search in Google Scholar
[4] Gaba S, Rai AK, Varma A, Prasad R, Goel A. Biocontrol potential of mycogenic copper oxide nanoparticles against Alternaria brassicae. Front Chem. 2022;10:966396. 10.3389/fchem.2022.96639.Search in Google Scholar
[5] Datnoff LE, Elmer WH, Huber DM, editors. Mineral nutrition and plant disease. St. Paul, MN, U.S.A: APS Press, The American Phytopathological Society; 2007. p. 278.Search in Google Scholar
[6] De Oliveira-Filho EC, Lopes RM, Paumgartten FJR. Comparative study on the susceptibility of freshwater species to copper based pesticides. Chemosphere. 2004;56(4):369–74. 10.1016/j.chemosphere.2004.04.026.Search in Google Scholar PubMed
[7] Gill SS, Tuteja N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol Biochem. 2010;48:909–30. 10.1016/j.plaphy.2010.08.016.Search in Google Scholar PubMed
[8] Ahuja I, Kissen R, Bones MA. Phytoalexins in defense against pathogens. Trends Plant Sci. 2012;17(2):73–90. 10.1016/j.tplants.2011.11.002.Search in Google Scholar PubMed
[9] Aboulila AA. Pathogenesis-related protein genes involved in race-specific all-stage resistance and non-race specific high-temperature adult-plant resistance to Puccinia striiformis f. sp. tritici in wheat through up-regulation of PR-1 and PR-4 genes expression. Physiol Mol Plant Pathol. 2022;121:101882. 10.1016/j.pmpp.2022.101882.Search in Google Scholar
[10] Bertini L, Leonardi L, Caporale C, Tucci M, Cascone N, Berardino ID, et al. Pathogen-responsive wheat, PR4 genes are induced by activators of systemic acquired resistance and wounding. Plant Sci. 2003;164(6):1067–78. 10.1016/S0168-9452(03)00112-2.Search in Google Scholar
[11] Garcia-Lopez JI, Nino-Medina G, Olivares-Saenz E, Lira-Saldivar RH, Barriga-Castro ED, Vazquez-Alvarado R, et al. Foliar application of zinc oxide nanoparticles and zinc sulfate boosts the content of bioactive compounds in habanero peppers. Plants. 2019;8(8):254. 10.3390/plants8080254.Search in Google Scholar PubMed PubMed Central
[12] Lowry GV, Avellan A, Gilbertson LM. Opportunities and challenges for nanotechnology in the agri-tech revolution. Nat Nanotechnol. 2019;14(6):517–22.10.1038/s41565-019-0461-7Search in Google Scholar PubMed
[13] lsheery NI, Sunoj VSJ, Wen Y, Zhu JJ, Muralidharan G, Cao KF. Foliar application of nanoparticles mitigates the chilling effect on photosynthesis and photoprotection in sugarcane. Plant Physiol Biochem. 2020;149:50–60.10.1016/j.plaphy.2020.01.035Search in Google Scholar PubMed
[14] Hassan S, Ulrike M. The role of flavonoids in root-rhizosphere signalling: opportunities and challenges for improving plant-microbe interactions. J Exp Bot. 2012;63(9):3429–44. 10.1093/jxb/err430.Search in Google Scholar PubMed
[15] Singh A, Sarma BK, Upadhyay RS, Singh HB. Compatible rhizosphere microbes mediated alleviation of biotic stress in chickpea through enhanced antioxidant and phenylpropanoid activities. Microbiol Res. 2013;168(1):33–400. 10.1016/j.micres.2012.07.001.Search in Google Scholar PubMed
[16] Dimkpa CO, McLean JE, Latta DE, Eliana M, Britt DW, Johnson PW. CuO and ZnO nanoparticles: phytotoxicity, metal speciation, and induction of oxidative stress in sand-grown wheat. J Nanopart Res. 2012;14:11–25. 10.1007/s11051-012-1125-9.Search in Google Scholar
[17] Wang S, Liu H, Zhang Y, Hua X. The effect of CuO NPs on reactive oxygen species and cell cycle gene expression in roots of rice. Environ Toxicol Chem. 2015;34(3):554–61. 10.1002/etc.2826.Search in Google Scholar PubMed
[18] Consolo VF, Torres-Nicolini A, Alvarez VA. Mycosinthetized Ag, CuO and ZnO nanoparticles from a promising Trichoderma harzianum strain and their antifungal potential against important phytopathogens. Sci Rep. 2020;10(1):491–9. 10.1038/s41598-020-77294-6.Search in Google Scholar PubMed PubMed Central
[19] Misra HP, Fridovich I. The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem. 1972;247(10):3170–5.10.1016/S0021-9258(19)45228-9Search in Google Scholar
[20] Aebi H. Catalase in vitro. Method Enzymol. 1984;105:121–6. 10.1016/s0076-6879(84)05016-3.Search in Google Scholar PubMed
[21] Conn KL, Tewari JP, Awasthi RP. A disease assessment key for Alternaria blackspot in rapeseed and mustard. Can Plant Dis Surv. 1990;70:19–22.Search in Google Scholar
[22] Arun KJ, Batra AK, Krishna A, Bhat K, Aggarwal MD. Surfactant free hydrothermal synthesis of copper oxide nanoparticles. Am J Mater Sci. 2015;5:36–8. 10.1166/asem.2015.1755.Search in Google Scholar
[23] Radim B. (Photo)electrochemical methods for the determination of the band edge positions of TiO2-based nanomaterials. Adv Phys Chem. 2011;2011:786759. 10.1155/2011/786759.Search in Google Scholar
[24] Valeria P, Elisa A, Stefano L, Gianfranco P, Elio G. The photoactive nitrogen impurity in nitrogen-doped zirconium titanate (N-ZrTiO4): A combined electron paramagnetic resonance and density functional theory study. J Mater Chem A. 2017;5:13062–71. 10.1039/c7ta03047a.Search in Google Scholar
[25] Hesham FM, Mohamed MS, Ahmed HM, Hassan BM, Mohamed MAE, Abdel AM, et al. Production of zinc and copper as nanoparticles by green synthesis using Pseudomonas fluorescens. Pak J Biol Sci. 2021;24(4):445–53. 10.3923/pjbs.2021.445.453.Search in Google Scholar PubMed
[26] Tiwari M, Jain P, Chandrashekhar R, Hariharapura K, Narayanan KU, Bhat N, et al. Biosynthesis of copper nanoparticles using copper-resistant Bacillus cereus, a soil isolate. Process Biochem. 2016;51(10):1348–56. 10.1016/j.procbio.2016.08.008.Search in Google Scholar
[27] Abdelkhalek A, Al-Askar AA. Green synthesized Zno nanoparticles mediated by mentha spicata extract induce plant systemic resistance against tobacco mosaic virus. Appl Sci. 2020;10:50–4. 10.3390/app10155054.Search in Google Scholar
[28] Attia MS, Balabel NM, Ababutain IM, Osman MS, Nofel MM, Elkodous MA, et al. Protective role of copper oxide-streptomycin nano-drug against potato brown rot disease caused by Ralstonia solanacearum. J Clust Sci. 2022;33:1373–86. 10.1007/s10876-021-02048-x.Search in Google Scholar
[29] Karlsson HL, Gusatfsson J, Cronholm P, Moller L. Size-dependent toxicity of metal oxide particles- a comparison between nano-and micrometer size. Toxicol Lett. 2009;188(2):112–8. 10.1016/j.toxlet.2009.03.014.Search in Google Scholar PubMed
[30] Podar D, Ramsey MH, Hutchings MJ. Effect of cadmium, zinc and substrate heterogeneity on yield, shoot metal concentration and metal uptake by Brassica juncea: implications for human health risk assessment and phytoremediation. N Phytol. 2004;163:313–24. 10.1111/j.1469-8137.2004.01122.x.Search in Google Scholar PubMed
[31] Nair PMG, Chung IM. A mechanistic study on the toxic effect of copper oxide nanoparticles in soybean (Glycine max L.) root development and lignification of root cells. Biol Trace Elem Res. 2014;162(1–3):342–52. 10.1007/s12011-014-0106-5.Search in Google Scholar PubMed
[32] Bennett EJ, Roberts JA, Wagstaff C. The role of the pod in seed development: strategies for manipulating yield. New Phytol. 2011;190(4):838–53.10.1111/j.1469-8137.2011.03714.xSearch in Google Scholar PubMed
[33] Danlami U, Orishadipe Abayomi T, Lawal DR. Phytochemical, nutritional and antimicrobial evaluations of the aqueous extract of Brassica nigra (Brassicaceae) seeds. Am J Appl Chem. 2016;4(4):161–3.10.11648/j.ajac.20160404.17Search in Google Scholar
[34] Tian X, Song S, Lei Y. Cell death and reactive oxygen species metabolism during accelerated ageing of soybean axes. Russ J Plant Physiol. 2008;55:33–40. 10.1134/S1021443708010032.Search in Google Scholar
[35] Roy D, Adhikar S, Adhikari A, Ghosh S, Azahar I, Basuli D, et al. Impact of CuO nanoparticles on maize: Comparison with CuO bulk particles with special reference to oxidative stress damages and antioxidant defense status. Chemosphere. 2022;287:131911. 10.1016/j.chemosphere.2021.131911.Search in Google Scholar PubMed
© 2023 the author(s), published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Research Articles
- Value-added utilization of coal fly ash and recycled polyvinyl chloride in door or window sub-frame composites
- High removal efficiency of volatile phenol from coking wastewater using coal gasification slag via optimized adsorption and multi-grade batch process
- Evolution of surface morphology and properties of diamond films by hydrogen plasma etching
- Removal efficiency of dibenzofuran using CuZn-zeolitic imidazole frameworks as a catalyst and adsorbent
- Rapid and efficient microwave-assisted extraction of Caesalpinia sappan Linn. heartwood and subsequent synthesis of gold nanoparticles
- The catalytic characteristics of 2-methylnaphthalene acylation with AlCl3 immobilized on Hβ as Lewis acid catalyst
- Biodegradation of synthetic PVP biofilms using natural materials and nanoparticles
- Rutin-loaded selenium nanoparticles modulated the redox status, inflammatory, and apoptotic pathways associated with pentylenetetrazole-induced epilepsy in mice
- Optimization of apigenin nanoparticles prepared by planetary ball milling: In vitro and in vivo studies
- Synthesis and characterization of silver nanoparticles using Origanum onites leaves: Cytotoxic, apoptotic, and necrotic effects on Capan-1, L929, and Caco-2 cell lines
- Exergy analysis of a conceptual CO2 capture process with an amine-based DES
- Construction of fluorescence system of felodipine–tetracyanovinyl–2,2′-bipyridine complex
- Excellent photocatalytic degradation of rhodamine B over Bi2O3 supported on Zn-MOF nanocomposites under visible light
- Optimization-based control strategy for a large-scale polyhydroxyalkanoates production in a fed-batch bioreactor using a coupled PDE–ODE system
- Effectiveness of pH and amount of Artemia urumiana extract on physical, chemical, and biological attributes of UV-fabricated biogold nanoparticles
- Geranium leaf-mediated synthesis of silver nanoparticles and their transcriptomic effects on Candida albicans
- Synthesis, characterization, anticancer, anti-inflammatory activities, and docking studies of 3,5-disubstituted thiadiazine-2-thiones
- Synthesis and stability of phospholipid-encapsulated nano-selenium
- Putative anti-proliferative effect of Indian mustard (Brassica juncea) seed and its nano-formulation
- Enrichment of low-grade phosphorites by the selective leaching method
- Electrochemical analysis of the dissolution of gold in a copper–ethylenediamine–thiosulfate system
- Characterisation of carbonate lake sediments as a potential filler for polymer composites
- Evaluation of nano-selenium biofortification characteristics of alfalfa (Medicago sativa L.)
- Quality of oil extracted by cold press from Nigella sativa seeds incorporated with rosemary extracts and pretreated by microwaves
- Heteropolyacid-loaded MOF-derived mesoporous zirconia catalyst for chemical degradation of rhodamine B
- Recovery of critical metals from carbonatite-type mineral wastes: Geochemical modeling investigation of (bio)hydrometallurgical leaching of REEs
- Photocatalytic properties of ZnFe-mixed oxides synthesized via a simple route for water remediation
- Attenuation of di(2-ethylhexyl)phthalate-induced hepatic and renal toxicity by naringin nanoparticles in a rat model
- Novel in situ synthesis of quaternary core–shell metallic sulfide nanocomposites for degradation of organic dyes and hydrogen production
- Microfluidic steam-based synthesis of luminescent carbon quantum dots as sensing probes for nitrite detection
- Transformation of eggshell waste to egg white protein solution, calcium chloride dihydrate, and eggshell membrane powder
- Preparation of Zr-MOFs for the adsorption of doxycycline hydrochloride from wastewater
- Green nanoarchitectonics of the silver nanocrystal potential for treating malaria and their cytotoxic effects on the kidney Vero cell line
- Carbon emissions analysis of producing modified asphalt with natural asphalt
- An efficient and green synthesis of 2-phenylquinazolin-4(3H)-ones via t-BuONa-mediated oxidative condensation of 2-aminobenzamides and benzyl alcohols under solvent- and transition metal-free conditions
- Chitosan nanoparticles loaded with mesosulfuron methyl and mesosulfuron methyl + florasulam + MCPA isooctyl to manage weeds of wheat (Triticum aestivum L.)
- Synergism between lignite and high-sulfur petroleum coke in CO2 gasification
- Facile aqueous synthesis of ZnCuInS/ZnS–ZnS QDs with enhanced photoluminescence lifetime for selective detection of Cu(ii) ions
- Rapid synthesis of copper nanoparticles using Nepeta cataria leaves: An eco-friendly management of disease-causing vectors and bacterial pathogens
- Study on the photoelectrocatalytic activity of reduced TiO2 nanotube films for removal of methyl orange
- Development of a fuzzy logic model for the prediction of spark-ignition engine performance and emission for gasoline–ethanol blends
- Micro-impact-induced mechano-chemical synthesis of organic precursors from FeC/FeN and carbonates/nitrates in water and its extension to nucleobases
- Green synthesis of strontium-doped tin dioxide (SrSnO2) nanoparticles using the Mahonia bealei leaf extract and evaluation of their anticancer and antimicrobial activities
- A study on the larvicidal and adulticidal potential of Cladostepus spongiosus macroalgae and green-fabricated silver nanoparticles against mosquito vectors
- Catalysts based on nickel salt heteropolytungstates for selective oxidation of diphenyl sulfide
- Powerful antibacterial nanocomposites from Corallina officinalis-mediated nanometals and chitosan nanoparticles against fish-borne pathogens
- Removal behavior of Zn and alkalis from blast furnace dust in pre-reduction sinter process
- Environmentally friendly synthesis and computational studies of novel class of acridinedione integrated spirothiopyrrolizidines/indolizidines
- The mechanisms of inhibition and lubrication of clean fracturing flowback fluids in water-based drilling fluids
- Adsorption/desorption performance of cellulose membrane for Pb(ii)
- A one-pot, multicomponent tandem synthesis of fused polycyclic pyrrolo[3,2-c]quinolinone/pyrrolizino[2,3-c]quinolinone hybrid heterocycles via environmentally benign solid state melt reaction
- Green synthesis of silver nanoparticles using durian rind extract and optical characteristics of surface plasmon resonance-based optical sensor for the detection of hydrogen peroxide
- Electrochemical analysis of copper-EDTA-ammonia-gold thiosulfate dissolution system
- Characterization of bio-oil production by microwave pyrolysis from cashew nut shells and Cassia fistula pods
- Green synthesis methods and characterization of bacterial cellulose/silver nanoparticle composites
- Photocatalytic research performance of zinc oxide/graphite phase carbon nitride catalyst and its application in environment
- Effect of phytogenic iron nanoparticles on the bio-fortification of wheat varieties
- In vitro anti-cancer and antimicrobial effects of manganese oxide nanoparticles synthesized using the Glycyrrhiza uralensis leaf extract on breast cancer cell lines
- Preparation of Pd/Ce(F)-MCM-48 catalysts and their catalytic performance of n-heptane isomerization
- Green “one-pot” fluorescent bis-indolizine synthesis with whole-cell plant biocatalysis
- Silica-titania mesoporous silicas of MCM-41 type as effective catalysts and photocatalysts for selective oxidation of diphenyl sulfide by H2O2
- Biosynthesis of zinc oxide nanoparticles from molted feathers of Pavo cristatus and their antibiofilm and anticancer activities
- Clean preparation of rutile from Ti-containing mixed molten slag by CO2 oxidation
- Synthesis and characterization of Pluronic F-127-coated titanium dioxide nanoparticles synthesized from extracts of Atractylodes macrocephala leaf for antioxidant, antimicrobial, and anticancer properties
- Effect of pretreatment with alkali on the anaerobic digestion characteristics of kitchen waste and analysis of microbial diversity
- Ameliorated antimicrobial, antioxidant, and anticancer properties by Plectranthus vettiveroides root extract-mediated green synthesis of chitosan nanoparticles
- Microwave-accelerated pretreatment technique in green extraction of oil and bioactive compounds from camelina seeds: Effectiveness and characterization
- Studies on the extraction performance of phorate by aptamer-functionalized magnetic nanoparticles in plasma samples
- Investigation of structural properties and antibacterial activity of AgO nanoparticle extract from Solanum nigrum/Mentha leaf extracts by green synthesis method
- Green fabrication of chitosan from marine crustaceans and mushroom waste: Toward sustainable resource utilization
- Synthesis, characterization, and evaluation of nanoparticles of clodinofop propargyl and fenoxaprop-P-ethyl on weed control, growth, and yield of wheat (Triticum aestivum L.)
- The enhanced adsorption properties of phosphorus from aqueous solutions using lanthanum modified synthetic zeolites
- Separation of graphene oxides of different sizes by multi-layer dialysis and anti-friction and lubrication performance
- Visible-light-assisted base-catalyzed, one-pot synthesis of highly functionalized cinnolines
- The experimental study on the air oxidation of 5-hydroxymethylfurfural to 2,5-furandicarboxylic acid with Co–Mn–Br system
- Highly efficient removal of tetracycline and methyl violet 2B from aqueous solution using the bimetallic FeZn-ZIFs catalyst
- A thermo-tolerant cellulase enzyme produced by Bacillus amyloliquefaciens M7, an insight into synthesis, optimization, characterization, and bio-polishing activity
- Exploration of ketone derivatives of succinimide for their antidiabetic potential: In vitro and in vivo approaches
- Ultrasound-assisted green synthesis and in silico study of 6-(4-(butylamino)-6-(diethylamino)-1,3,5-triazin-2-yl)oxypyridazine derivatives
- A study of the anticancer potential of Pluronic F-127 encapsulated Fe2O3 nanoparticles derived from Berberis vulgaris extract
- Biogenic synthesis of silver nanoparticles using Consolida orientalis flowers: Identification, catalytic degradation, and biological effect
- Initial assessment of the presence of plastic waste in some coastal mangrove forests in Vietnam
- Adsorption synergy electrocatalytic degradation of phenol by active oxygen-containing species generated in Co-coal based cathode and graphite anode
- Antibacterial, antifungal, antioxidant, and cytotoxicity activities of the aqueous extract of Syzygium aromaticum-mediated synthesized novel silver nanoparticles
- Synthesis of a silica matrix with ZnO nanoparticles for the fabrication of a recyclable photodegradation system to eliminate methylene blue dye
- Natural polymer fillers instead of dye and pigments: Pumice and scoria in PDMS fluid and elastomer composites
- Study on the preparation of glycerylphosphorylcholine by transesterification under supported sodium methoxide
- Wireless network handheld terminal-based green ecological sustainable design evaluation system: Improved data communication and reduced packet loss rate
- The optimization of hydrogel strength from cassava starch using oxidized sucrose as a crosslinking agent
- Green synthesis of silver nanoparticles using Saccharum officinarum leaf extract for antiviral paint
- Study on the reliability of nano-silver-coated tin solder joints for flip chips
- Environmentally sustainable analytical quality by design aided RP-HPLC method for the estimation of brilliant blue in commercial food samples employing a green-ultrasound-assisted extraction technique
- Anticancer and antimicrobial potential of zinc/sodium alginate/polyethylene glycol/d-pinitol nanocomposites against osteosarcoma MG-63 cells
- Nanoporous carbon@CoFe2O4 nanocomposite as a green absorbent for the adsorptive removal of Hg(ii) from aqueous solutions
- Characterization of silver sulfide nanoparticles from actinobacterial strain (M10A62) and its toxicity against lepidopteran and dipterans insect species
- Phyto-fabrication and characterization of silver nanoparticles using Withania somnifera: Investigating antioxidant potential
- Effect of e-waste nanofillers on the mechanical, thermal, and wear properties of epoxy-blend sisal woven fiber-reinforced composites
- Magnesium nanohydroxide (2D brucite) as a host matrix for thymol and carvacrol: Synthesis, characterization, and inhibition of foodborne pathogens
- Synergistic inhibitive effect of a hybrid zinc oxide-benzalkonium chloride composite on the corrosion of carbon steel in a sulfuric acidic solution
- Review Articles
- Role and the importance of green approach in biosynthesis of nanopropolis and effectiveness of propolis in the treatment of COVID-19 pandemic
- Gum tragacanth-mediated synthesis of metal nanoparticles, characterization, and their applications as a bactericide, catalyst, antioxidant, and peroxidase mimic
- Green-processed nano-biocomposite (ZnO–TiO2): Potential candidates for biomedical applications
- Reaction mechanisms in microwave-assisted lignin depolymerisation in hydrogen-donating solvents
- Recent progress on non-noble metal catalysts for the deoxydehydration of biomass-derived oxygenates
- Rapid Communication
- Phosphorus removal by iron–carbon microelectrolysis: A new way to achieve phosphorus recovery
- Special Issue: Biomolecules-derived synthesis of nanomaterials for environmental and biological applications (Guest Editors: Arpita Roy and Fernanda Maria Policarpo Tonelli)
- Biomolecules-derived synthesis of nanomaterials for environmental and biological applications
- Nano-encapsulated tanshinone IIA in PLGA-PEG-COOH inhibits apoptosis and inflammation in cerebral ischemia/reperfusion injury
- Green fabrication of silver nanoparticles using Melia azedarach ripened fruit extract, their characterization, and biological properties
- Green-synthesized nanoparticles and their therapeutic applications: A review
- Antioxidant, antibacterial, and cytotoxicity potential of synthesized silver nanoparticles from the Cassia alata leaf aqueous extract
- Green synthesis of silver nanoparticles using Callisia fragrans leaf extract and its anticancer activity against MCF-7, HepG2, KB, LU-1, and MKN-7 cell lines
- Algae-based green AgNPs, AuNPs, and FeNPs as potential nanoremediators
- Green synthesis of Kickxia elatine-induced silver nanoparticles and their role as anti-acetylcholinesterase in the treatment of Alzheimer’s disease
- Phytocrystallization of silver nanoparticles using Cassia alata flower extract for effective control of fungal skin pathogens
- Antibacterial wound dressing with hydrogel from chitosan and polyvinyl alcohol from the red cabbage extract loaded with silver nanoparticles
- Leveraging of mycogenic copper oxide nanostructures for disease management of Alternaria blight of Brassica juncea
- Nanoscale molecular reactions in microbiological medicines in modern medical applications
- Synthesis and characterization of ZnO/β-cyclodextrin/nicotinic acid nanocomposite and its biological and environmental application
- Green synthesis of silver nanoparticles via Taxus wallichiana Zucc. plant-derived Taxol: Novel utilization as anticancer, antioxidation, anti-inflammation, and antiurolithic potential
- Recyclability and catalytic characteristics of copper oxide nanoparticles derived from bougainvillea plant flower extract for biomedical application
- Phytofabrication, characterization, and evaluation of novel bioinspired selenium–iron (Se–Fe) nanocomposites using Allium sativum extract for bio-potential applications
- Erratum
- Erratum to “Synthesis, characterization, and evaluation of nanoparticles of clodinofop propargyl and fenoxaprop-P-ethyl on weed control, growth, and yield of wheat (Triticum aestivum L.)”
Articles in the same Issue
- Research Articles
- Value-added utilization of coal fly ash and recycled polyvinyl chloride in door or window sub-frame composites
- High removal efficiency of volatile phenol from coking wastewater using coal gasification slag via optimized adsorption and multi-grade batch process
- Evolution of surface morphology and properties of diamond films by hydrogen plasma etching
- Removal efficiency of dibenzofuran using CuZn-zeolitic imidazole frameworks as a catalyst and adsorbent
- Rapid and efficient microwave-assisted extraction of Caesalpinia sappan Linn. heartwood and subsequent synthesis of gold nanoparticles
- The catalytic characteristics of 2-methylnaphthalene acylation with AlCl3 immobilized on Hβ as Lewis acid catalyst
- Biodegradation of synthetic PVP biofilms using natural materials and nanoparticles
- Rutin-loaded selenium nanoparticles modulated the redox status, inflammatory, and apoptotic pathways associated with pentylenetetrazole-induced epilepsy in mice
- Optimization of apigenin nanoparticles prepared by planetary ball milling: In vitro and in vivo studies
- Synthesis and characterization of silver nanoparticles using Origanum onites leaves: Cytotoxic, apoptotic, and necrotic effects on Capan-1, L929, and Caco-2 cell lines
- Exergy analysis of a conceptual CO2 capture process with an amine-based DES
- Construction of fluorescence system of felodipine–tetracyanovinyl–2,2′-bipyridine complex
- Excellent photocatalytic degradation of rhodamine B over Bi2O3 supported on Zn-MOF nanocomposites under visible light
- Optimization-based control strategy for a large-scale polyhydroxyalkanoates production in a fed-batch bioreactor using a coupled PDE–ODE system
- Effectiveness of pH and amount of Artemia urumiana extract on physical, chemical, and biological attributes of UV-fabricated biogold nanoparticles
- Geranium leaf-mediated synthesis of silver nanoparticles and their transcriptomic effects on Candida albicans
- Synthesis, characterization, anticancer, anti-inflammatory activities, and docking studies of 3,5-disubstituted thiadiazine-2-thiones
- Synthesis and stability of phospholipid-encapsulated nano-selenium
- Putative anti-proliferative effect of Indian mustard (Brassica juncea) seed and its nano-formulation
- Enrichment of low-grade phosphorites by the selective leaching method
- Electrochemical analysis of the dissolution of gold in a copper–ethylenediamine–thiosulfate system
- Characterisation of carbonate lake sediments as a potential filler for polymer composites
- Evaluation of nano-selenium biofortification characteristics of alfalfa (Medicago sativa L.)
- Quality of oil extracted by cold press from Nigella sativa seeds incorporated with rosemary extracts and pretreated by microwaves
- Heteropolyacid-loaded MOF-derived mesoporous zirconia catalyst for chemical degradation of rhodamine B
- Recovery of critical metals from carbonatite-type mineral wastes: Geochemical modeling investigation of (bio)hydrometallurgical leaching of REEs
- Photocatalytic properties of ZnFe-mixed oxides synthesized via a simple route for water remediation
- Attenuation of di(2-ethylhexyl)phthalate-induced hepatic and renal toxicity by naringin nanoparticles in a rat model
- Novel in situ synthesis of quaternary core–shell metallic sulfide nanocomposites for degradation of organic dyes and hydrogen production
- Microfluidic steam-based synthesis of luminescent carbon quantum dots as sensing probes for nitrite detection
- Transformation of eggshell waste to egg white protein solution, calcium chloride dihydrate, and eggshell membrane powder
- Preparation of Zr-MOFs for the adsorption of doxycycline hydrochloride from wastewater
- Green nanoarchitectonics of the silver nanocrystal potential for treating malaria and their cytotoxic effects on the kidney Vero cell line
- Carbon emissions analysis of producing modified asphalt with natural asphalt
- An efficient and green synthesis of 2-phenylquinazolin-4(3H)-ones via t-BuONa-mediated oxidative condensation of 2-aminobenzamides and benzyl alcohols under solvent- and transition metal-free conditions
- Chitosan nanoparticles loaded with mesosulfuron methyl and mesosulfuron methyl + florasulam + MCPA isooctyl to manage weeds of wheat (Triticum aestivum L.)
- Synergism between lignite and high-sulfur petroleum coke in CO2 gasification
- Facile aqueous synthesis of ZnCuInS/ZnS–ZnS QDs with enhanced photoluminescence lifetime for selective detection of Cu(ii) ions
- Rapid synthesis of copper nanoparticles using Nepeta cataria leaves: An eco-friendly management of disease-causing vectors and bacterial pathogens
- Study on the photoelectrocatalytic activity of reduced TiO2 nanotube films for removal of methyl orange
- Development of a fuzzy logic model for the prediction of spark-ignition engine performance and emission for gasoline–ethanol blends
- Micro-impact-induced mechano-chemical synthesis of organic precursors from FeC/FeN and carbonates/nitrates in water and its extension to nucleobases
- Green synthesis of strontium-doped tin dioxide (SrSnO2) nanoparticles using the Mahonia bealei leaf extract and evaluation of their anticancer and antimicrobial activities
- A study on the larvicidal and adulticidal potential of Cladostepus spongiosus macroalgae and green-fabricated silver nanoparticles against mosquito vectors
- Catalysts based on nickel salt heteropolytungstates for selective oxidation of diphenyl sulfide
- Powerful antibacterial nanocomposites from Corallina officinalis-mediated nanometals and chitosan nanoparticles against fish-borne pathogens
- Removal behavior of Zn and alkalis from blast furnace dust in pre-reduction sinter process
- Environmentally friendly synthesis and computational studies of novel class of acridinedione integrated spirothiopyrrolizidines/indolizidines
- The mechanisms of inhibition and lubrication of clean fracturing flowback fluids in water-based drilling fluids
- Adsorption/desorption performance of cellulose membrane for Pb(ii)
- A one-pot, multicomponent tandem synthesis of fused polycyclic pyrrolo[3,2-c]quinolinone/pyrrolizino[2,3-c]quinolinone hybrid heterocycles via environmentally benign solid state melt reaction
- Green synthesis of silver nanoparticles using durian rind extract and optical characteristics of surface plasmon resonance-based optical sensor for the detection of hydrogen peroxide
- Electrochemical analysis of copper-EDTA-ammonia-gold thiosulfate dissolution system
- Characterization of bio-oil production by microwave pyrolysis from cashew nut shells and Cassia fistula pods
- Green synthesis methods and characterization of bacterial cellulose/silver nanoparticle composites
- Photocatalytic research performance of zinc oxide/graphite phase carbon nitride catalyst and its application in environment
- Effect of phytogenic iron nanoparticles on the bio-fortification of wheat varieties
- In vitro anti-cancer and antimicrobial effects of manganese oxide nanoparticles synthesized using the Glycyrrhiza uralensis leaf extract on breast cancer cell lines
- Preparation of Pd/Ce(F)-MCM-48 catalysts and their catalytic performance of n-heptane isomerization
- Green “one-pot” fluorescent bis-indolizine synthesis with whole-cell plant biocatalysis
- Silica-titania mesoporous silicas of MCM-41 type as effective catalysts and photocatalysts for selective oxidation of diphenyl sulfide by H2O2
- Biosynthesis of zinc oxide nanoparticles from molted feathers of Pavo cristatus and their antibiofilm and anticancer activities
- Clean preparation of rutile from Ti-containing mixed molten slag by CO2 oxidation
- Synthesis and characterization of Pluronic F-127-coated titanium dioxide nanoparticles synthesized from extracts of Atractylodes macrocephala leaf for antioxidant, antimicrobial, and anticancer properties
- Effect of pretreatment with alkali on the anaerobic digestion characteristics of kitchen waste and analysis of microbial diversity
- Ameliorated antimicrobial, antioxidant, and anticancer properties by Plectranthus vettiveroides root extract-mediated green synthesis of chitosan nanoparticles
- Microwave-accelerated pretreatment technique in green extraction of oil and bioactive compounds from camelina seeds: Effectiveness and characterization
- Studies on the extraction performance of phorate by aptamer-functionalized magnetic nanoparticles in plasma samples
- Investigation of structural properties and antibacterial activity of AgO nanoparticle extract from Solanum nigrum/Mentha leaf extracts by green synthesis method
- Green fabrication of chitosan from marine crustaceans and mushroom waste: Toward sustainable resource utilization
- Synthesis, characterization, and evaluation of nanoparticles of clodinofop propargyl and fenoxaprop-P-ethyl on weed control, growth, and yield of wheat (Triticum aestivum L.)
- The enhanced adsorption properties of phosphorus from aqueous solutions using lanthanum modified synthetic zeolites
- Separation of graphene oxides of different sizes by multi-layer dialysis and anti-friction and lubrication performance
- Visible-light-assisted base-catalyzed, one-pot synthesis of highly functionalized cinnolines
- The experimental study on the air oxidation of 5-hydroxymethylfurfural to 2,5-furandicarboxylic acid with Co–Mn–Br system
- Highly efficient removal of tetracycline and methyl violet 2B from aqueous solution using the bimetallic FeZn-ZIFs catalyst
- A thermo-tolerant cellulase enzyme produced by Bacillus amyloliquefaciens M7, an insight into synthesis, optimization, characterization, and bio-polishing activity
- Exploration of ketone derivatives of succinimide for their antidiabetic potential: In vitro and in vivo approaches
- Ultrasound-assisted green synthesis and in silico study of 6-(4-(butylamino)-6-(diethylamino)-1,3,5-triazin-2-yl)oxypyridazine derivatives
- A study of the anticancer potential of Pluronic F-127 encapsulated Fe2O3 nanoparticles derived from Berberis vulgaris extract
- Biogenic synthesis of silver nanoparticles using Consolida orientalis flowers: Identification, catalytic degradation, and biological effect
- Initial assessment of the presence of plastic waste in some coastal mangrove forests in Vietnam
- Adsorption synergy electrocatalytic degradation of phenol by active oxygen-containing species generated in Co-coal based cathode and graphite anode
- Antibacterial, antifungal, antioxidant, and cytotoxicity activities of the aqueous extract of Syzygium aromaticum-mediated synthesized novel silver nanoparticles
- Synthesis of a silica matrix with ZnO nanoparticles for the fabrication of a recyclable photodegradation system to eliminate methylene blue dye
- Natural polymer fillers instead of dye and pigments: Pumice and scoria in PDMS fluid and elastomer composites
- Study on the preparation of glycerylphosphorylcholine by transesterification under supported sodium methoxide
- Wireless network handheld terminal-based green ecological sustainable design evaluation system: Improved data communication and reduced packet loss rate
- The optimization of hydrogel strength from cassava starch using oxidized sucrose as a crosslinking agent
- Green synthesis of silver nanoparticles using Saccharum officinarum leaf extract for antiviral paint
- Study on the reliability of nano-silver-coated tin solder joints for flip chips
- Environmentally sustainable analytical quality by design aided RP-HPLC method for the estimation of brilliant blue in commercial food samples employing a green-ultrasound-assisted extraction technique
- Anticancer and antimicrobial potential of zinc/sodium alginate/polyethylene glycol/d-pinitol nanocomposites against osteosarcoma MG-63 cells
- Nanoporous carbon@CoFe2O4 nanocomposite as a green absorbent for the adsorptive removal of Hg(ii) from aqueous solutions
- Characterization of silver sulfide nanoparticles from actinobacterial strain (M10A62) and its toxicity against lepidopteran and dipterans insect species
- Phyto-fabrication and characterization of silver nanoparticles using Withania somnifera: Investigating antioxidant potential
- Effect of e-waste nanofillers on the mechanical, thermal, and wear properties of epoxy-blend sisal woven fiber-reinforced composites
- Magnesium nanohydroxide (2D brucite) as a host matrix for thymol and carvacrol: Synthesis, characterization, and inhibition of foodborne pathogens
- Synergistic inhibitive effect of a hybrid zinc oxide-benzalkonium chloride composite on the corrosion of carbon steel in a sulfuric acidic solution
- Review Articles
- Role and the importance of green approach in biosynthesis of nanopropolis and effectiveness of propolis in the treatment of COVID-19 pandemic
- Gum tragacanth-mediated synthesis of metal nanoparticles, characterization, and their applications as a bactericide, catalyst, antioxidant, and peroxidase mimic
- Green-processed nano-biocomposite (ZnO–TiO2): Potential candidates for biomedical applications
- Reaction mechanisms in microwave-assisted lignin depolymerisation in hydrogen-donating solvents
- Recent progress on non-noble metal catalysts for the deoxydehydration of biomass-derived oxygenates
- Rapid Communication
- Phosphorus removal by iron–carbon microelectrolysis: A new way to achieve phosphorus recovery
- Special Issue: Biomolecules-derived synthesis of nanomaterials for environmental and biological applications (Guest Editors: Arpita Roy and Fernanda Maria Policarpo Tonelli)
- Biomolecules-derived synthesis of nanomaterials for environmental and biological applications
- Nano-encapsulated tanshinone IIA in PLGA-PEG-COOH inhibits apoptosis and inflammation in cerebral ischemia/reperfusion injury
- Green fabrication of silver nanoparticles using Melia azedarach ripened fruit extract, their characterization, and biological properties
- Green-synthesized nanoparticles and their therapeutic applications: A review
- Antioxidant, antibacterial, and cytotoxicity potential of synthesized silver nanoparticles from the Cassia alata leaf aqueous extract
- Green synthesis of silver nanoparticles using Callisia fragrans leaf extract and its anticancer activity against MCF-7, HepG2, KB, LU-1, and MKN-7 cell lines
- Algae-based green AgNPs, AuNPs, and FeNPs as potential nanoremediators
- Green synthesis of Kickxia elatine-induced silver nanoparticles and their role as anti-acetylcholinesterase in the treatment of Alzheimer’s disease
- Phytocrystallization of silver nanoparticles using Cassia alata flower extract for effective control of fungal skin pathogens
- Antibacterial wound dressing with hydrogel from chitosan and polyvinyl alcohol from the red cabbage extract loaded with silver nanoparticles
- Leveraging of mycogenic copper oxide nanostructures for disease management of Alternaria blight of Brassica juncea
- Nanoscale molecular reactions in microbiological medicines in modern medical applications
- Synthesis and characterization of ZnO/β-cyclodextrin/nicotinic acid nanocomposite and its biological and environmental application
- Green synthesis of silver nanoparticles via Taxus wallichiana Zucc. plant-derived Taxol: Novel utilization as anticancer, antioxidation, anti-inflammation, and antiurolithic potential
- Recyclability and catalytic characteristics of copper oxide nanoparticles derived from bougainvillea plant flower extract for biomedical application
- Phytofabrication, characterization, and evaluation of novel bioinspired selenium–iron (Se–Fe) nanocomposites using Allium sativum extract for bio-potential applications
- Erratum
- Erratum to “Synthesis, characterization, and evaluation of nanoparticles of clodinofop propargyl and fenoxaprop-P-ethyl on weed control, growth, and yield of wheat (Triticum aestivum L.)”

