Phyto-fabrication and characterization of silver nanoparticles using Withania somnifera: Investigating antioxidant potential
-
Shazina Jabeen
, Rahmatullah Qureshi
Abstract
This study aimed to develop a green and safe method for producing silver nanoparticles (AgNPs) using the root extract of Withania somnifera (WS) and evaluate their antioxidant properties. UV-visible spectroscopy revealed a maximum absorption peak at 430 nm. Fourier-transformed infrared spectroscopy confirmed the presence of phenolic coatings on Ws-AgNPs, indicating their role in stabilizing and reducing Ag ions into Ws-AgNPs. Scanning electron microscopy analysis demonstrated that Ws-AgNPs had a spherical shape and a size range of 74–88 nm. Energy dispersive X-ray spectroscopy analysis confirmed silver as the primary element in Ws-AgNPs. X-ray powder diffraction analysis indicated a face-centered cubic crystalline structure for Ws-AgNPs. The potential antioxidant activities of Ws-AgNPs were evaluated using various scavenging assays. At the highest concentration tested (500 µg/mL), 95 ± 1.3%, 98 ± 1.6%, 76.9 ± 1.44%, and 89.6 ± 1.6% scavenging activities were observed with 2,2-diphenyl-1-picrylhydrazyl, 2,2′-azinobis-3-ethylbenzothiazoline-6-sulfonic acid, phosphomolybdate, and H2O2, respectively. Moreover, the reducing power of Ws-AgNPs was higher than that of the methanolic WS root extract and showed a concentration-dependent trend. In conclusion, the green-synthesized Ws-AgNPs from W. somnifera showed remarkable antioxidant activity, as evidenced by their low IC50 values. Due to these findings, it is suggested that Ws-AgNPs have the potential to be used as potent antioxidant agents in the cosmetic, food, and pharmaceutical industries.
1 Introduction
The field of nanotechnology is rapidly expanding and is concerned with the development and use of nanoscale materials that exhibit unique physical and chemical properties [1]. In any spatial dimension, nanoparticles typically range in size from 0.1 to 100 nm [2]. Nanoparticles have the advantage over conventional materials in that they have higher surface energy, a higher proportion of surface atoms, and fewer defects [3].
Metallic nanoparticles have gained interest due to their biological properties, such as enzyme inhibition, antimicrobial, anticancer, anti-leishmaniosis, and antioxidant potential [4]. In addition, they have diverse applications in various sectors, such as food, agriculture, catalysis, imaging, health, drug delivery, and cosmetics [5]. Among metallic nanoparticles, silver nanoparticles (AgNPs) are gaining popularity in research and industry, i.e., nano-biotechnology [6,7]. AgNPs exhibit antibacterial, antifungal, anti-inflammatory, anticancer, antibiofilm, and antioxidant properties [8]. In addition, AgNPs are efficient photocatalysts as they remain chemically stable upon exposure to acids and bases. AgNPs offer a range of photocatalytic applications due to their affordability and high oxidizing capacity [9]. AgNPs are formulated by employing chemical and physical routes. Chemicals are toxic and produce environmentally harmful products when utilized to synthesize and stabilize nanoparticles [10]. By contrast, physical approaches are expensive and incompatible with the mass production of nanoparticles [11].
In the search for a safer and more sustainable formulation of metallic nanoparticles, green approaches involving the use of plant and other natural substances have recently gained substantial popularity [12]. Moreover, green synthesis employs phytochemicals instead of potentially hazardous ones to reduce and stabilize metal ions during nanoparticle synthesis, resulting in biocompatible, eco-friendly, and cost-effective nanomaterials [13]. Green nanotechnology has been developed to synthesize different metallic nanoparticles using a variety of biocompatible materials such as bacteria, fungi, algae, and plant extracts. Among the aforementioned biocompatible materials, the utilization of plant extracts has drawn significant attention for the biogenesis of AgNPs. This green approach provides a relatively simple, accessible, and quick way to synthesize nanoparticles on a large scale compared to microorganism-mediated synthesis of nanoparticles [14]. Green synthesis of nanoparticles differs from other biological methods because it avoids the tedious process of maintaining cell culture and enables the large-scale production of nanoparticles [15]. Table 1 presents the effectiveness of green-synthesized AgNPs in various applications as compared to other metallic nanoparticles.
Effectiveness of green-synthesized AgNPs in various applications compared to other metallic nanoparticles
| Plant name | Nanoparticle type | Size and shape | Activity | Ref. |
|---|---|---|---|---|
| Crinum latifolium | Silver and gold | 20.5 nm (AgNPs) and 17.6 nm (AuNPs) | AgNPs exhibited significant antimicrobial activity than AuNPs | [27] |
| Spherical | ||||
| Aspergillus terreus | Silver and gold | 6–20 nm (AgNPs) and 10–50 nm (AuNPs) | AgNPs showed significant antifungal and cytotoxicity activities than AuNPs | [28] |
| Spherical | However, both these NPs exhibited good antioxidant potential | |||
| Plinia cauliflora, Punica granatum | Silver and gold | 8–20 nm (AgNPs) and 2–12 nm (AuNPs) | AgNPs displayed better antibacterial and antifungal activities than AuNPs | [29] |
| Spherical | ||||
| Cymbopogon citratus, Tridax procumbens | Silver and copper | 70 nm (AgNPs) and 57 nm (CuNPs) | AgNPs exhibited high insecticidal activity against fruit flies, while CuNPs caused no mortality | [30] |
| Spherical and irregular | ||||
| Ocimum bacilicum | Silver and copper | 15 nm (AgNPs) and (CuNPs) | The larvicidal activity of AgNPs was reported to be more significant against Epilachna vigintioctopunctata than CuNPs | [31] |
| Spherical | ||||
| Amomum subulatum | Silver and copper oxide | 20.6 nm (AgNPs) and 24.7 nm (CuONPs) | AgNPs efficacy was reported to be superior to CuONPs against human flora, i.e., E. coli, S. aureus, and B. subtilis, and more cytotoxicity against human cervical cells (HeLa) and human breast cells (MCF-7) | [32] |
| Spherical | ||||
| Allium tuncelianum | Silver, copper, and nickel | (AgNPs) and (CuNPs) | AgNPs proved more effective than CuNPs and NiNPs when used to treat infection caused by A. castellanii | [33] |
| Spherical | ||||
| Mentha spicata | Silver and gold | 24 nm (AgNPs) and 19.61 nm (AuNPs) | AgNPs exhibited better antimicrobial properties and antioxidant potential than AuNPs | [34] |
| Spherical | ||||
| Fusarium pseudonygamai | Silver and gold | 5–20 nm (AgNPs) and 6–60 nm (AuNPs) | AgNPs showed better antibacterial efficacy, antioxidant potential, and anti-cancer activity than AuNPs | [35] |
| Spherical |
Plants have long been thought to be a source of medicinal benefits. Synthetic chemists were motivated to synthesize the corresponding natural compounds after bioactive compounds known as secondary metabolites were identified and isolated from plants, thanks to advancements in chromatography and spectroscopy [16]. A variety of secondary metabolites, such as alkaloids, enzymes, polysaccharides, phenols, tannins, terpenoids, vitamins, and flavonoids, are found in almost all the parts of a selective plant, including the leaves, flowers, fruits, peels, roots, latex, seeds, and stems. Due to their complex structures, these constituents have a strong medicinal value and are also better for the environment [17]. Therefore, plant extracts provide phytochemicals necessary for reduction processes and confer important biological and therapeutic properties to nanoparticles [18]. Reaction conditions are crucial to achieve significant uniformity of the nanoparticle size distribution in the nano-suspension and to improve the overall process. The most important chemical and physical factors influencing the final product’s morphological characteristics are the plant extract’s component composition and silver ion concentration, the pH value of the reaction, temperature, mixing intensity, external physical influence, and reaction time. Plant extracts with pharmaceutical values should be used to synthesize NPs for use in biomedical applications [19].
Green-synthesized AgNPs exhibit antibacterial, anti-inflammatory, antimicrobial, antibiofilm, and antioxidant properties, can combat multiple drug resistance, and can also be used in cancer theranostics [20]. Moreover, green-synthesized AgNPs, due to their unique properties, possess major therapeutic uses such as anti-diabetic, antioxidant, antibacterial, and antifungal with no human toxicity, and their other biological uses include bio-molecular detection, drug delivery, biomedical, water treatment, enzymes, food production, and agriculture [21].
Withania somnifera (L.) Dunal (WS) is the most significant medicinal plant of solanaceae family. Winter cherry and Indian ginseng are popular names for W. somnifera in English and Asghand in Urdu. The Latin term somnifera, which means “sleep-inducer,” refers to the herb’s widespread use as a stress reliever, while the Sanskrit term ashwagandha means “odor of the horse,” referring to the scent of the roots, which is reminiscent of horse sweat. The plant can be found in the American Herbal Pharmacopoeia monograph as well as WHO monographs on Selected Medicinal Plants. W. somnifera is one of the key ingredients in a number of Ayurvedic formulas that are currently marketed in India and other countries around the world [22]. The majority of W. somnifera products are used and marketed as dietary supplements and come in powder, syrup, ointments, tablets, and capsule form. All parts of the W. somnifera (leaves, flowers, fruits, seeds, and roots) are utilized for various medicinal purposes in Ayurvedic medical systems. The roots of W. somnifera are the most widely used plant part among those claimed to have numerous health-promoting properties. They are used to make a tonic that slows down the ageing process, increases longevity, strengthens the body’s resistance to infectious diseases, and revitalizes the body [23]. Additionally, W. somnifera also has other medical use, such as anti-inflammatory, antidiabetic, neuroprotective, immunomodulatory, and anti-cancer [22]. The steroidal lactones, mainly present in the root of the plant, are responsible for therapeutic activities such as anticancer, antioxidants, immunomodulatory, antibacterial, antiageing, hypoglycemic, hypocholesterolemic, memory enhancement, and also have adaptogenic properties [24].
The phytochemical studies of W. somnifera revealed that the root contains alkaloids, phenols, flavonoids, glycosides, saponins, tannins, steroids, reducing sugars, and triterpenoids [25]. Until now, more than 12 alkaloids, approximately 40 withanolides, and several sitoindosides have been reported from its roots and aerial parts. Among these metabolites, withanolides promote immune system cell activation, and phenolic compounds are strongly linked to the plant’s antioxidant capacity [26]. A limited number of studies have been conducted on the phenolic composition and antioxidant activity of W. somnifera, despite the abundance of reports on withanolides and alkaloids.
Various studies reported that a number of diseases are caused by excess free radicals produced by stress, and environmental factors that cause oxidative damage to lipids, proteins, and nucleic acids. The primary cause of oxidative stress and cell damage in the body is the emergence of reactive oxygen species (ROS). By triggering chain reactions, ROS damage cells by generating free radicals. Antioxidant molecules are commonly employed to neutralize free radicals and halt the oxidation process. Thus, a variety of proteins, lipids, and organelles within cells can be shielded from oxidation. The human body makes up for any deficiencies in antioxidant enzymes through food. In the food industry, antioxidants are frequently used to prevent food from spoilage. For food preservation, synthetic antioxidants like butylated hydroxytoluene and butylated hydroxyanisole have been utilized, but it has been reported that they cause toxicity. Therefore, the demand for natural antioxidants in food, medicine, and cosmetics has recently increased significantly due to synthetic antioxidants’ toxicity and potential health risks.
Various previously reported studies that phytomolecules from medicinal plant extracts can stabilize free radical ions and help lower oxidative stress in the body [36]. Antioxidant capacity is a common metric for measuring medicinal plants’ bioactive and active components. The mechanisms by which phenols and flavonoids exert their antioxidative actions are a topic of much debate in the literature. Flavonoids, being polyphenolic compounds, can function as antioxidants through a free radical-scavenging mechanism by generating less reactive flavonoid phenoxyl radicals. The capacity of flavonoid compounds to donate a hydrogen atom from their hydroxyl group may account for their high potential as scavengers of free radicals. Flavonoid phenoxyl radicals are produced when the flavonoid compounds interact with the free radicals. Flavonoid phenoxyl radicals exhibit lower reactivity than that of other phenols. The phenoxyl radicals are prevented from reacting further and form unreactive compounds due to their reduced reactivity, possibly through a process known as radical–radical termination [37]. In recent years, use of natural antioxidants as compared to synthetic antioxidants has increased in cosmetics, food, and medicine [16].
Considering the significance of this plant and its potential uses in nanotechnology, the present work was planned to synthesize silver nanoparticles (Ws-AgNPs) using roots of W. somnifera followed by evaluating their antioxidant potential. To the best of our knowledge, this is the first study to report the antioxidant activity of Ws-AgNPs derived from the W. somnifera root extract. The antioxidant activities of Ws-AgNPs were evaluated using DPPH, ABTS free radical scavenging assays, phosphomolybdate, and H2O2 and reducing power assay.
2 Materials and methods
2.1 Collection and identification of plant specimens
The plant material of Withania somnifera (WS) was collected from Jinnah Garden, Islamabad, Pakistan. The roots were cleaned with tap water and then distilled water before being air-dried in the shade for 2.5 weeks. Taxonomist Prof. Rahamatullah Qureshi identified the plant, and a voucher specimen numbering AU-137 was deposited in the herbarium of PMAS-Arid Agriculture University, Rawalpindi, for future reference and record.
2.2 Preparation of the crude extract
For extraction, the dried roots of Withania somnifera (WS) were ground to powder using an electric grinder. The cold maceration technique was used for extraction [35]. About 25 g of root powder was added to 300 mL of methanol. After 7 days, this crude extract was filtered off twice and the solvent was evaporated at room temperature. The dried extract was then scraped out using sterilized blades. The crude extract was then collected in Eppendorf tubes and stored at 4°C for subsequent analysis.
2.3 Green synthesis of Ws-AgNPs
2.3.1 Preparation of the plant extract
About 20 g of the root powder was placed in a beaker containing 200 mL of distilled water. The solution was then boiled for 20 min. The root extract of WS was filtered three times using filter paper (Whatman no. 1) to remove the particulate matter. The solution was refrigerated at 4°C in a 500 mL flask for additional use [38].
2.3.2 Plant-based synthesis of Ws-AgNPs
For the plant-based formulation of AgNPs, the method described in the study of Hussain et al. [39] was used with slight modifications. About 0.17 g of (AgNO3) salt was added in 1 L of distilled water to prepare 1 mM solution of silver nitrate. The AgNO3 solution (900 mL) was boiled and gradually reduced, while the 100 mL plant extract was progressively added until the color of the solution changed to dark brown. The pellet was then collected after centrifuging the reduced solution for 15 min at 10,000 rpm. The centrifugation process was repeated at least three times to eliminate all the impurities The precipitated nanoparticles were dried by lyophilization. The phyto-fabricated Ws-AgNPs were further characterized, and their antioxidant potential was also investigated.
2.4 Characterization of Ws-AgNPs using UV-visible, scanning electron microscopy (SEM), Fourier-transform infrared spectroscopy (FTIR), energy dispersive X-ray (EDX) spectroscopy, and X-ray diffraction (XRD) analysis
Ws-AgNPs were first placed in Eppendorf tubes, and distilled water was added immediately. Ws-AgNPs were subjected to sonication for 10–15 min. The bioformulation of Ws-AgNPs was then analyzed by recording UV-Vis spectra from 300 to 800 nm. FTIR analysis was performed using an FTIR spectrophotometer in the 400–4,000 cm−1 range to determine the functional groups responsible for the reduction of silver ions originating from the plant extract. Moreover, an SEM study was carried out to determine the surface morphology of the phyto-fabricated Ws-AgNPs. For this purpose, the sample was prepared on a carbon-coated copper grid using the drop-coating method. Samples were dried for 7 min under a mercury lamp and the excess solution was wiped off with blotting paper. The green-synthesized Ws-AgNPs were morphologically analyzed at various magnifications. EDX studies were also carried out to validate the presence of elemental silver in Ws-AgNPs. In addition, XRD analysis of the Ws-AgNP thin film was performed to investigate their crystalline nature. The Bragg angle in the range from 10° to 70° was used to record the XRD pattern.
2.5 In vitro antioxidant activities
The plant extract and phyto-fabricated Ws-AgNPs were examined for their antioxidant activities using various assays. Three replicates were prepared for each sample.
2.5.1 DPPH radical scavenging assay
2,2-Diphenyl-1-picrylhydrazyl (DPPH) is a stable free radical and the most frequently employed assay to measure the ability of antioxidant compounds to scavenge free radicals. Antioxidant activity was determined using DPPH following the protocol of Nagajyothi et al. [40] with slight modifications. To prepare the plant extract aliquots, 5 mg/mL of the methanolic Ws ext solution was taken and dissolved in methanol to prepare solutions at six different concentrations (15, 30, 60, 125, 250, and 500 µg/mL). In this assay, 1 mL of each plant aliquot from the different concentration solutions was mixed with 3 mL of 0.004% DPPH solution. For the standard DPPH solution (Abs = 0.8–0.9), the prepared stock solution’s absorbance was assessed using a UV-Vis spectrophotometer at 517 nm.
Similarly, 1 mL of the solution of Ws-AgNPs samples at different concentrations was mixed with 3 mL of 0.004% DPPH solution. To prepare aliquots of NPs, 5 mg of Ws-AgNPs was dissolved in 5 mL of methanol to obtain six different concentration solutions. At room temperature, the solution mixtures were left to stand in the dark for 30 min after vortexing. For the control sample, 3 mL of DPPH solution was added and the sample was replaced with methanol. Ascorbic acid served as the standard for comparison. The experiment was repeated three times. The sample absorbance was recorded using a UV-Vis spectrophotometer at 517 nm and the % radical scavenging activity of each concentration was estimated by the following formula:
where AC is the solution absorbance without the addition of sample, and AS is the solution absorbance when the extract or NPs are added.
The IC50 (µg/mL) values, defined as the quantity of antioxidants required to decrease the free radical’s concentration by 50%, were calculated from the % inhibition vs concentration graph.
2.5.2 ABTS radical scavenging assay
The 2,2-azinobis (3-ethylbenzothiazoline-6-sulfonic acid) radical scavenging assay, also known as the ABTS assay, was performed following the protocol of Re et al. [41] with some modifications. Equal quantities of ABTS (7 mM solution) and potassium persulfate solution (2.45 mM) were mixed to get a working solution. This solution was then left in the dark at room temperature for an entire night to produce the dark solution containing the ABTS radical cations. Before being used in the assay, the solution was diluted by mixing ABTS radical cations (1 mL) with methanol (60 mL) to attain the solution absorbance of 0.701 ± 0.02 at 745 nm. Different concentrations of methanolic Ws ext and Ws-AgNPs (300 µL) were mixed with 3 mL of ABTS solution. The standard was ascorbic acid. The decrease in the absorbance after 1 min of mixing the solution and then up to 6 min was recorded. The percentage scavenging activity was determined as follows:
where AC is the solution absorbance without the addition of the sample and AS is the solution absorbance when the extract or NPs are added.
The amount required to reduce ABTS by 50% was used to express the sample’s antioxidant activity in terms of IC50.
2.5.3 Phosphomolybdate assay (total antioxidant capacity [TAC])
Using the protocol of Umamaheswari and Chatterjee [42], the phosphomolybdate assay was used to measure the TAC of the plant sample. An aliquot of 0.4 mL of methanolic Ws ext and Ws-AgNPs was mixed with 4 mL of reagent solution (28 mM sodium phosphate, 4 mM ammonium molybdate, and 0.6 M sulfuric acid) and incubated for 90 min at 95°C in a water bath. After the mixture had been cooled to room temperature, the absorbance at 765 nm was recorded against the blank. For the blank, 4 mL of the reagent was combined with a sufficient quantity of methanol and incubated under similar conditions. The standard was ascorbic acid. The formula used to calculate the total antioxidant activity was as follows:
where AC is the solution absorbance without the addition of the sample and AS is the solution absorbance when extract or NPs are added.
2.5.4 Hydrogen peroxide scavenging assay
The procedure of Aryal et al. [43] was slightly modified to measure the sample’s ability to scavenge hydrogen peroxide. A 50 mM phosphate buffer (pH 7.4) was utilized to formulate a 40 mM solution of hydrogen peroxide. For all six concentrations, 0.6 mL of hydrogen peroxide solution was combined with 0.4 mL of the sample extracts and Ws-AgNP solution in test tubes. After the tubes were vortexed for 10 min, hydrogen peroxide absorbance at 230 nm was calculated against the blank. As a blank, phosphate buffer without hydrogen peroxide was employed. The formula used to calculate the percentage scavenging activity was as follows:
where AC is the solution absorbance without the addition of the sample, and AS is the solution absorbance when extract or NPs are added.
2.5.5 Reducing power assay
This assay was carried out following the procedure used by Hue et al. [44] with slight modifications. About 2.5 mL of different concentrations of the plant extract and Ws-AgNPs were mixed with 2.5 mL of potassium ferricyanide (1%) and sodium phosphate buffer (0.2 M, pH 6.6). Then, the mixture was allowed to stand for 20 min at 50°C. After adding 10% of 2.5 mL trichloroacetic acid (TCA), the mixture was centrifuged for 10 min at 3,000 rpm. The pellet was discarded, and 2.5 mL of the upper layer of the supernatant was mixed with 0.5 mL of FeCl3 (0.1%) and 2.5 mL of distilled water. To compare the results, ascorbic acid was employed as a reference, and the absorbance at 700 nm was measured. An increased sample absorbance suggested a strong reducing power.
2.6 Statistical analysis
Replicates of readings were taken, and findings were presented as mean ± standard deviation. IC50 values were calculated from the regression line. The significance of the results was analyzed using the analysis of variance (ANOVA). Statistics were considered significant at P < 0.005.
3 Results and discussion
3.1 Green synthesis and characterization of AgNPs
3.1.1 Optical observation
In the current study, the root retract of W. somnifera was used to reduce silver nitrate (AgNO3) to Ws-AgNPs. Ws-AgNPs were reduced and capped by the root extract. The secondary metabolites present in the plant could be the possible cause for the reduction of the AgNO3 salt in a variety of redox processes, and possible functional groups could confer bioactivity while preventing the nanosilver core from clumping. The initial evidence of phyto-fabrication of Ws-AgNPs was the color change of the reaction mixture, which was later confirmed by recording the reaction mixture’s absorbance using a UV-vis spectrophotometer. When the reaction mixture color changed from light yellow to brown and finally to dark brown, it indicated the phyto-fabrication of W. somnifera root-mediated AgNPs (Figure 1). This color change is the first indicator of Ws-AgNP synthesis due to the optical properties of AgNPs.

Optical confirmation of the synthesis of Ws-AgNPs by color observation at 0, 30, and 60 min of mixing.
3.1.2 Mechanism of Ws-AgNP synthesis
The mechanism for the green synthesis of Ws-AgNPs is shown in Figure 2. The mechanism of Ws-AgNP synthesis involves the reduction of Ag+ ions to Ag0 by the functional groups of secondary metabolites found in the plant extract. After reduction, Ag0 undergoes a sequence of agglomeration, denoted as nucleation (I) and (II), to form a nanoparticle. Nucleation of silver zero atoms leads to the formation of nanoparticles, i.e. AgNPs, as shown in Figure 2. Thus, the proposed mechanism of synthesis of AgNPs involves the reduction of ions, clustering, and ultimate growth of nanoparticles [45]. The presence of various significant bioactive compounds and the bonding of their functional groups with metallic salt stabilizes nanoparticles. The presence of phenolic compounds on the surface of nanoparticles is attributed to preventing their coalescence [46].

Mechanism of green synthesis of Ws-AgNPs.
3.1.3 UV-visible spectrophotometer analysis
uv-visible spectrophotometry is one of the key approaches to assess the reduction, stabilization, and formation of AgNPs. This technique uses the principle of surface plasmon resonance (SPR) for characterization. SPR can be explained as a resonant, cumulative oscillation of valence electrons in a solid that is stimulated by light incidence. The resonance occurs when the light’s frequency matches the surface electrons’ oscillation frequency. SPR establishes the basis for various standard tools for measuring material adsorption on the metal surface (usually silver and gold) or on the surface of metallic nanoparticles [47]. The change in color of the reaction mixture from light yellow to dark brown indicated the reduction of Ago. Moreover, UV-Vis analysis also confirmed the formation of Ws-AgNPs. The UV-Vis spectrum of Ws-AgNPs has a strong band in the visible region of 350–550 nm [36]. The AgNPs synthesized using the WS root showed an adsorption peak at 430 nm (Figure 3a), confirming the formation of Ws-AgNPs. Similarly, Chirumamilla et al. [48] observed the peak of AgNPs synthesized from the Calendula officinalis flower extract at 430 nm. Moreover, Anbalagan et al. [49] also reported the absorption peak of green-synthesized AgNPs in the 430–440 nm range. The peak broadening indicated the polydispersed nature of the nanoparticles. In addition, the synthesis of AgNPs using the WS extract with a maximum absorption peak at 420 nm was also reported by Javed et al. [50].

UV-visible spectra (a), SEM micrograph (b), and EDX spectra (c) of Ws-AgNPs.
3.1.4 SEM analysis
Structural investigation of Ws-AgNPs was conducted using SEM. The SEM image indicated that agglomerated clusters of Ws-AgNPs are dispersed over the surface in a fairly large random pattern with empty space [36] and that Ws-AgNPs are variable in shape, although most of them are spherical. These findings ensure that AgNPs mediated by W. somnifera are in the nano range. The average size of nanoparticles was found in the range between 74 and 88 nm (Figure 3b). Ws-AgNPs had a spherical morphology with a high density and homogeneous dispersion. Likewise, Adelere et al. [51] documented similar results. The morphology of the nanoparticles is affected by numerous variables, such as the extract concentration, contact time, pH, and quantity of the silver salt [52].
3.1.5 EDX analysis
EDX analysis was used to examine the elemental composition of the phyto-fabricated Ws-AgNPs. The purity and presence of elemental silver (Ag) were verified using the EDX analysis. The maximum characterization peak of Ag was recorded at 3 keV and a silver signal with high intensity was also observed (Figure 3c). The peak around 3 keV is the characteristic peak indicating AgNP synthesis. The EDX spectrum also showed a signal for oxygen, which may indicate the proteins and enzymes found in the plant extract that are important for the capping of the AgNPs [53].
3.1.6 FTIR analysis
FTIR analysis was performed to reveal the potential biomolecules involved in the surface coating and as capping agents to stabilize W. somnifera root-mediated Ws-AgNPs. Additionally, this analysis helped identify the functional groups present in the suspension of AgNPs and may be responsible for the reduction of metal ions. The sample was screened with an FTIR spectrophotometer in the 400–4,000 cm−1 range to determine the chemical interaction of NPs with phytochemicals from WS.
The FTIR spectra of Ws-AgNPs synthesized using the WS root (Figure 4) produced peaks at 3,419, 2,962, 2,922, 1,618, 1,383, 1,317, 1,076, 1,024 and 800 cm−1. The peak at 3,419 cm−1 is due to stretching vibrations of O–H, attributing the presence of phenolic and alcoholic groups on the surface of Ws-AgNPs. Other peaks at 2,962 and 2,922 cm−1 are attributed to the C–H stretching vibration of alkanes. The peak at 1,618 cm−1 is attributed to C–O bond vibrations and C–C stretching vibrations. The peaks at 1,383 and 1,317 cm−1 correspond to the C–H bending of alkanes and C–O stretching in the carbonyl group of protein residues. Two other peaks at 1,076 and 1,024 cm−1 may indicate the C-stretching of ether groups, the C–N stretching vibration of aliphatic amines, and the C–O vibration [36]. Stretching vibrations of C–X of alkyl halides were observed at 800 cm−1. Similar characteristic peaks of AgNPs were reported in other studies [54,55]. Finally, we can point out that any biomolecule with these bonds can function as a capping or reducing agent.

FTIR spectra of Ws-AgNPs.
3.1.7 XRD analysis
XRD analysis verified the crystalline nature of the phyto-fabricated Ws-AgNPs. The XRD diffraction pattern demonstrated the diffraction peaks at 32.1°, 38°, 64.5°, and 76.5°. The crystalline structure of NPs is represented by high intensity of peaks (Figure 5). The XRD spectra show absorption peaks at 32.1°, 38°, 64.5°, and 76.5° and could be assigned to the (111) (200) (220) and (311) planes of the face-centered cubic crystalline structure of Ag. The findings are in line with Devanesan and AlSalhi [56], who described the biosynthesis of AgNPs and recorded the XRD peaks at 38.1°, 44.2°, 64.5°, and 76.5°. The phytochemicals from plant extracts were deposited on the surface of AgNPs, which was also proved by FTIR studies and several related research corroborated these results [57]. The average crystalline particle size was calculated by using the Debye–Scherrer equation D = kλ/βcos θ, where D is the particle size, k = 0.94 is a constant, λ is the X-ray wavelength (0.1541 nm), and β and θ represent the full width at half-maximum. The average crystalline size was found to be 63.8 nm.
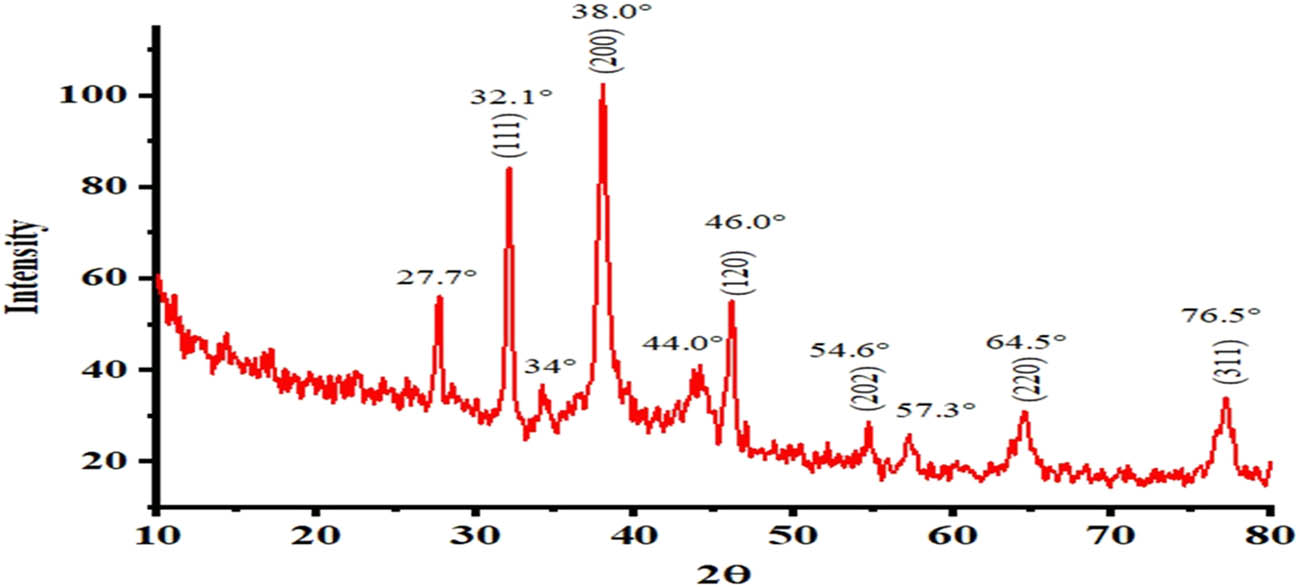
XRD pattern of Ws-AgNPs.
3.2 In vitro antioxidant activities
3.2.1 DPPH radical scavenging assay
An antioxidant inhibits the oxidation by neutralizing free radicals. The antioxidant itself is oxidized to counteract free radicals [58]. The phenolic compounds and flavonoids in medicinal plants are well acknowledged for sequential reduction and capping of plant-based nanoparticles and these metabolites are attributed to a range of useful activities by NPs. The capping biomolecules found in the extract of plants and their adsorption on the surface of NPs may be responsible for the antioxidant activity [59]. Free radicals are normally produced during cellular metabolism and act as regulatory and signaling molecules. However, excessive production of free radicals can damage cellular components and hamper their ability to function normally [60]. DPPH is a stable molecule that can be reduced to hydrogen by the electron reduction process. DPPH produces a purple-colored solution after being dissolved in methanol, which then becomes a colorless solution upon reduction by an antioxidant. This process involves an antioxidant donating hydrogen, which creates the non-radical DPPHH [61].
The current study compared the antioxidant potential of phyto-fabricated Ws-AgNPs with that of the plant root extract (Ws ext) using the DPPH free radical scavenging assay. Antioxidant potential was determined in the form of inhibition percentage and IC50 value. The highest radical % scavenging potential observed for ascorbic acid was 99 ± 1.58 at 500 µg/mL. The maximum percentage scavenging activities of Ws-AgNPs were found to be 95 ± 1.3 and 40.2 ± 1.5 at 500 and 15 µg/mL, respectively, while the % scavenging potentials for methanolic Ws ext at the highest and lowest concentrations of 500 and 15 µg/mL were 81.2 ± 1.44 and 33.57 ± 1.6, respectively. Similarly, Atrocarpus altilis leaf extract-mediated AgNPs demonstrated significant DPPH free radicals scavenging action [62]. Another study also reported excellent antioxidant activity for Elephantopus scaber-mediated AgNPs [63].
The IC50 value of ascorbic acid was 21.6 µg/mL, while those of Ws-AgNPs and Ws ext were 74.2 and 144.2 µg/mL, respectively. A lower IC50 value indicates high antioxidant activity while a higher IC50 value indicates poorer antioxidant activity. Figure 6a graphically represents the IC50 values and the scavenged DPPH percentage. The results indicated that phyto-fabricated AgNPs have higher scavenging potential than the plant root extract alone. Due to this potent antioxidant activity, Ws-AgNPs have the potential to be used in the cosmetic, food, and pharmaceutical industries [64]. With increased interest in using natural antioxidants as food additives, green-synthesized Ws-AgNPs can act as potent agents, ensuring food safety.

Graphical presentation of in vitro antioxidant studies using DPPH, ABTS, MPA, and H2O2 scavenging assays.
3.2.2 ABTS radical scavenging activity
WSR AgNP and Ws ext samples scavenged the ABTS radicals in a dose-dependent manner. Among these concentrations, 500 µg/mL of both the plant extract and Ws-AgNPs exhibited maximum inhibition activity. In the case of Ws-AgNPs, the maximum percentage scavenging activities of 98 ± 1.6 were observed at 500 µg/mL and 14.4 ± 1.4 at 15 µg/mL. Meanwhile, for Ws ext, they were 91 ± 1.49 and 11.3 ± 1.7 at 500 µg/mL and 15 µg/mL, respectively (Figure 6b).
The IC50 value of Ws-AgNPs was minimal (156.9 µg/mL), followed by Ws ext (195.5 µg/mL). Ascorbic acid had a lower IC50 value (57.52 µg/mL) than those of both the Ws-AgNPs and the root extract of the plant sample. The IC50 value indicated that Ws-AgNPs have the highest antioxidant potential. The IC50 value of the root is consistent with the results reported by Udayakumar et al. [65]. Similarly, Niraimathi et al. [66] reported the antioxidant potential of leaf and root extract from W. somnifera and their IC50 values. These findings suggest that W. somnifera possesses many metabolites, which can donate hydrogen from their hydroxyl group (–OH) to free radicals, creating stable and extremely reactive hydroxyl radicals. Another study using Alternathera sessilis-mediated AgNPs also reported their excellent antimicrobial and antioxidant potential [67]. Moreover, AgNPs synthesized from Echium vulgare also demonstrated significant antioxidant activity and catalytic degradation [68]. Our results are also in accordance with Mittal et al. [69], who reported excellent antioxidant and antimicrobial activities of Eucalyptus globulus- and Salvia officinalis-mediated AgNPs.
3.2.3 Phosphomolybdate assay
TAC is used to estimate the ability of plant samples to arrest oxidative stress. This procedure relies on using plant extracts to reduce molybdate ions and their conversion into phophomolybenum complex. In the current study, the phosphomolybdate assay was used to determine the antioxidant potential of the methanolic root extract and phyto-fabricated Ws-AgNPs.
The TAC% scavenging activities of Ws-AgNPs at the highest (500 µg/mL) and lowest (15 µg/mL) concentrations were 76.96 ± 1.57 and 40.61 ± 1.75, while the TAC% scavenging potentials for methanolic Ws ext at the highest and lowest concentrations were 64.02 ± 1.46 and 33.67 ± 1.44, respectively. The TAC% scavenging activity of ascorbic acid was 88.41 ± 1.49 at 500 µg/mL. The increase in the TAC was reported with increasing sample concentration. Similarly, the antioxidant activity of Pteris tripartita leaf extract-mediated AgNPs was reported by Alzubaidi et al. [70] and described significant radical scavenging potential using the phosphomolybdenum assay.
The IC50 value of Ws-AgNPs was the lowest (73.94 µg/mL) followed by Ws ext (175.23 µg/mL). The IC50 value indicated that Ws-AgNPs have the highest antioxidant potential. Ws-AgNPs had a higher antioxidant capacity than the extract; this discrepancy could be attributed to the different chemical compositions of the samples utilized in the study. Previous studies have demonstrated the significant antioxidant activity of the W. somnifera extract due to the presence of flavonoids, withanolides including withaferin A, withanolide B, withanoside V, and withanone [26]. Therefore, Ws-AgNPs’ potential as antioxidants may be related to the complex of these antioxidant metabolites found in plants, which shield the biological components from oxidation and damage [71]. In addition, Ws-AgNPs possessed significant antioxidant activity due to stability gained by coating and capping of phenolic compounds [72]. Similar studies were carried out by Sathiyaraj et al. [73], who reported the antioxidant potential of green-synthesized AgNPs. Many studies exhibited similar results of antioxidant potential of phyto-fabricated AgNPs [74,75,76].
3.2.4 Hydrogen peroxide scavenging assay
The free radical scavenging activity of both the plant extract and Ws-AgNPs was also determined using the hydrogen peroxide scavenging assay at different concentrations. The maximum percentage scavenging activity observed for Ws-AgNPs was 89.65 ± 1.66 at 500 µg/mL while those for methanolic Ws ext at the highest and lowest concentrations of 500 and 15 µg/mL were 81.76 ± 1.64 and 27.51 ± 1.72, respectively.
Ws-AgNPs have shown significant antioxidant potential as they have a lower IC50 of 102.6 µg/mL compared to that of the Ws ext (163.8 µg/mL). Figure 5d presents the H2O2 scavenging percent inhibition vs the concentration. Antioxidant activity was found to be directly related to the concentrations, as shown in Figure 6d. The hydrogen peroxide assay is a frequently used method for assessing natural antioxidants’ capacity to donate electrons. Numerous studies have shown the direct correlation between the reducing power of some plant extracts, which are further enhanced by nanoparticles, and their antioxidant activities. The IC50 value of the root extract is consistent with the results reported by Saleh and Mahdi [75]. Similarly, AgNPs synthesized from the hydro-alcoholic extract of Triphala also exhibited significant antioxidant activity using the H2O2 scavenging assay [77]. The comparison of IC50 values of the root extract, Ws-AgNPs, and ascorbic acid for all the performed scavenging activities is graphically presented in Figure 7.

Comparison of the antioxidant activity (IC50) of Ws-AgNPs, Ws ext, and ascorbic acid.
3.2.5 Reducing power assay
This technique is employed to assess the reducing capacity of plant samples by reducing Fe3+ to Fe2+ through electron donation from antioxidants present in the extract. The concentration of reduced Fe2+ produced upon the addition of the plant extract was determined using a UV-Vis spectrophotometer at 700 nm. In the present study, the reducing potential of the root extract and phyto-fabricated AgNPs was assessed at different concentrations, i.e., 15, 30, 60, 125, 250, and 500 µg/mL. A direct relationship was found between the concentrations of Ws ext and Ws-AgNPs and their reducing power as the absorbance of samples increased with increasing concentration.
The maximum absorbance was observed for ascorbic acid, which was 1.508 ± 0.006 at 500 µg/mL. In the case of AgNPs, the maximum absorbances were found to be 1.248 ± 0.008 and 0.442 ± 0.01 at 500 and 15 µg/mL, respectively, while the absorbances of the methanolic Ws ext at highest (500 µg/mL) and lowest (15 µg/mL) concentrations were 0.973 ± 0.01 and 0.203 ± 0.01, respectively [60]. The greater antioxidant capacity was observed for green-synthesized AgNPs compared to ascorbic acid and the antioxidant potential of AgNPs increased with the concentration. Our results are also consistent with the findings of Balčiūnaitienė et al. [68], who reported strong antioxidant activity of AgNPs synthesized from Eucalyptus globulus and Salvia officinalis leaf extracts. The results indicated that phyto-fabricated AgNPs are significantly efficient candidates for free radical inhibition as compared to the plant extract. This antioxidant activity can be attributed to polyphenolic metabolites such as flavonoids, withanolides including withaferin A, withanolide B, withanoside V, and withanone, which are reported for their free radical scavenging potential [26] (Figure 8).

Ferric reducing power determination of phyto-fabricated Ws-AgNPs, root extract, and ascorbic acid.
4 Conclusion
Extracts from medicinal plants are used for the phyto-fabrication of AgNPs, enabling biocompatible, user-friendly, cost-effective, and environmentally sustainable green chemical processes. AgNPs were successfully phyto-fabricated, characterized, and evaluated for their antioxidant potential. AgNPs were tested for their antioxidant activity using DPPH, ABTS, H2O2, phosphomolybdate, and reducing assays. The results indicated that AgNPs have significant antioxidant potential compared to the root extract. This study proved that W. somnifera contains numerous bioactive substances including polyphenols and flavonoids, which act as reducing and capping agents to stabilize AgNPs and enhance their antioxidant activity. Functional groups, especially the hydroxyl groups (−OH) of bioactive substances, may confer stability to the phenoxyl radicals by donating their −OH groups. Therefore, the pharmaceutical industry has a lot of potential for using surface coating of plant-bioactive compounds. Moreover, these results support the considerable antioxidant efficacy of Ws-AgNPs, which can be employed in the treatment of various illnesses due to the overproduction of free radicals in the body. Utilization of phyto-fabricated AgNPs offers a benefit over the extract. Phyto-fabricated AgNPs are easier to penetrate the cells because they are more stable and nanoscale. Thus, these results suggest that Ws-AgNPs could be employed in food, cosmetics, and pharmaceutical industries with various biomedical applications. However, there is a need for prior clinical testing and to check their toxicity. To the best of our knowledge, this is the first study to report the antioxidant activity of Ws-AgNPs derived from the W. somnifera root extract.
-
Funding information: Hesham Oraby extends his appreciation to the Deputyship for Research & Innovation, Ministry of Education in Saudi Arabia, for funding this research work through the project number IFP22UQU4350043DSR107.
-
Author contributions: Conceptualization, S.J., R.Q., M.I., and M.H.; data curation, S.J., M.I., A.A.O., N.E., and H.F.O.; formal analysis, M.H., A.A.O., N.E. and H.F.O.; investigation, S.J., R.Q., M.I., and M.H; methodology, S.J., A.A.O., and M.I.; project administration, S.J., R.Q., M.I., and M.H.; resources, all authors; supervision, R.Q.; validation, all authors; writing – original draft, all authors; writing – review and editing, all authors; funding, H.F.O. All authors have read and agreed to the published version of the manuscript.
-
Conflict of interest: The authors state no conflict of interest.
-
Data availability statement: The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
[1] Gurunathan S, Kalishwaralal K, Vaidyanathan R, Venkataraman D, Pandian SR, Muniyandi J, et al. Biosynthesis, purification and characterization of silver nanoparticles using Escherichia coli. Coll Surf B: Biointer. 2009;74(1):328–35.10.1016/j.colsurfb.2009.07.048Search in Google Scholar PubMed
[2] Seid L, Lakhdari D, Berkani M, Belgherbi O, Chouder D, Vasseghian Y, et al. High-efficiency electrochemical degradation of phenol in aqueous solutions using Ni-PPy and Cu-PPy composite materials. J Hazard Mater. 2022;423:126986.10.1016/j.jhazmat.2021.126986Search in Google Scholar PubMed
[3] Hieu VQ, Phung TK, Nguyen TQ, Khan A, Doan VD, Tran VA. Photocatalytic degradation of methyl orange dye by Ti3C2–TiO2 heterojunction under solar light. Chemosphere. 2021;276:130154.10.1016/j.chemosphere.2021.130154Search in Google Scholar PubMed
[4] Naikoo GA, Mustaqeem M, Hassan IU, Awan T, Arshad F, Salim H, et al. Bioinspired and green synthesis of nanoparticles from plant extracts with antiviral and antimicrobial properties: A critical review. J Saudi Chem Soc. 2021;25(9):101304.10.1016/j.jscs.2021.101304Search in Google Scholar
[5] Behravan M, Panahi AH, Naghizadeh A, Ziaee M, Mahdavi R, Mirzapour A. Facile green synthesis of silver nanoparticles using Berberis vulgaris leaf and root aqueous extract and its antibacterial activity. Int J Biol Macromol. 2019;124:148–54.10.1016/j.ijbiomac.2018.11.101Search in Google Scholar PubMed
[6] Weissig V, Pettinger TK, Murdock N. Nanopharmaceuticals (part 1): products on the market. Int J Nanomed. 2014;4357–73.10.2147/IJN.S46900Search in Google Scholar PubMed PubMed Central
[7] Kaabipour S, Hemmati S. A review on the green and sustainable synthesis of silver nanoparticles and one-dimensional silver nanostructures. Beilstein J Nanotechnol. 2021;12(1):102–36.10.3762/bjnano.12.9Search in Google Scholar PubMed PubMed Central
[8] Robertson PK, Robertson JM, Bahnemann DW. Removal of microorganisms and their chemical metabolites from water using semiconductor photocatalysis. J Hazard Mater. 2012;211:161–71.10.1016/j.jhazmat.2011.11.058Search in Google Scholar PubMed
[9] Yang X, Chen W, Huang J, Zhou Y, Zhu Y, Li C. Rapid degradation of methylene blue in a novel heterogeneous Fe3O4@ rGO@ TiO2-catalyzed photo-Fenton system. Sci Rep. 2015;5(1):10632.10.1038/srep10632Search in Google Scholar PubMed PubMed Central
[10] Mukherjee S, Sushma V, Patra S, Barui AK, Bhadra MP, Sreedhar B, et al. Green chemistry approach for the synthesis and stabilization of biocompatible gold nanoparticles and their potential applications in cancer therapy. Nanotechnology. 2012;23(45):455103.10.1088/0957-4484/23/45/455103Search in Google Scholar PubMed
[11] Shankar SS, Rai A, Ahmad A, Sastry M. Rapid synthesis of Au, Ag, and bimetallic Au core–Ag shell nanoparticles using Neem (Azadirachta indica) leaf broth. J Coll Interf. Sci. 2004;275(2):496–502.10.1016/j.jcis.2004.03.003Search in Google Scholar PubMed
[12] Anastas P, Eghbali N. Green chemistry: principles and practice. Chem Soc Rev. 2010;39(1):301–12.10.1039/B918763BSearch in Google Scholar PubMed
[13] Narayanan KB, Sakthivel N. Green synthesis of biogenic metal nanoparticles by terrestrial and aquatic phototrophic and heterotrophic eukaryotes and biocompatible agents. Adv Colloid Interface Sci. 2011;169(2):59–79.10.1016/j.cis.2011.08.004Search in Google Scholar PubMed
[14] Singh J, Dutta T, Kim KH, Rawat M, Samddar P, Kumar P. ‘Green’synthesis of metals and their oxide nanoparticles: applications for environmental remediation. J Nanobiotechnology. 2018;16(1):1–24.10.1186/s12951-018-0408-4Search in Google Scholar PubMed PubMed Central
[15] Gurunathan S, Han JW, Kwon DN, Kim JH. Enhanced antibacterial and anti-biofilm activities of silver nanoparticles against Gram-negative and Gram-positive bacteria. Nanoscale Res Lett. 2014;9:1–7.10.1186/1556-276X-9-373Search in Google Scholar PubMed PubMed Central
[16] Erenler R, Hosaflioglu I. Green synthesis of silver nanoparticles using Onobrychis sativa L.: Characterization, catalytic degradation of methylene blue, antioxidant activity, and quantitative analysis of bioactive compounds. Mater Today Commun. 2023;35:105863.10.1016/j.mtcomm.2023.105863Search in Google Scholar
[17] Ghotekar S, Pansambal S, Bilal M, Pingale SS, Oza R. Environmentally friendly synthesis of Cr2O3 nanoparticles: characterization, applications and future perspective – a review. Case Stud Chem Env Eng. 2021;3:100089.10.1016/j.cscee.2021.100089Search in Google Scholar
[18] Zhu B, Li Y, Lin Z, Zhao M, Xu T, Wang C, et al. Silver nanoparticles induce HePG-2 cells apoptosis through ROS-mediated signaling pathways. Nanoscale Res Lett. 2016;11:1–8.10.1186/s11671-016-1419-4Search in Google Scholar PubMed PubMed Central
[19] Sahni G, Panwar A, Kaur B. Controlled green synthesis of silver nanoparticles by Allium cepa and Musa acuminata with strong antimicrobial activity. Int Nano Lett. 2015;5:93–100. 10.1007/s40089-015-0142.Search in Google Scholar
[20] Naveas N, Manso-Silván M, Carmona E, Garrido K, Hernández-Montelongo J, Recio-Sánchez G. Green synthesized silver nanoparticles decorated on nanostructured porous silicon as an efficient platform for the removal of organic dye methylene blue. Green Chem Lett Rev. 2022;15(1):108–15.10.1080/17518253.2021.2024609Search in Google Scholar
[21] Mashwani ZU, Khan T, Khan MA, Nadhman A. Synthesis in plants and plant extracts of silver nanoparticles with potent antimicrobial properties: current status and future prospects. Appl Microbiol Biotechnol. 2015;99:9923–34.10.1007/s00253-015-6987-1Search in Google Scholar PubMed
[22] Bhat JA, Akther T, Najar RA, Rasool F, Hamid A. Withania somnifera (L.) Dunal (Ashwagandha); current understanding and future prospect as a potential drug candidate. Front Pharmacol. 2022;13:102–23.10.3389/fphar.2022.1029123Search in Google Scholar PubMed PubMed Central
[23] Singh RH, Narsimhamurthy K, Singh G. Neuronutrient impact of Ayurvedic Rasayana therapy in brain aging. Biogerontology. 2008;9:369–74.10.1007/s10522-008-9185-zSearch in Google Scholar PubMed
[24] Jain P, Varshney R. Antimicrobial activity of aqueous and methanolic extracts of Withania somnifera (Ashwagandha). J Chem Pharm Res. 2011;3(3):260–3.Search in Google Scholar
[25] Tiwari R, Chakraborty S, Saminathan M, Dhama K, Singh SV. Ashwagandha (Withania somnifera): Role in safeguarding health, immunomodulatory effects, combating infections and therapeutic applications: A review. J Biol Sci. 2014;14(2):77.10.3923/jbs.2014.77.94Search in Google Scholar
[26] Polumackanycz M, Petropoulos SA, Śledziński T, Goyke E, Konopacka A, Plenis A, et al. Withania somnifera L.: Phenolic compounds composition and biological activity of commercial samples and its aqueous and hydromethanolic extracts. Antioxidants. 2023 Feb;12(3):550.10.3390/antiox12030550Search in Google Scholar PubMed PubMed Central
[27] Vo TT, Nguyen TT, Huynh TT, Vo TT, Nguyen TT, Nguyen DT, et al. Biosynthesis of silver and gold nanoparticles using aqueous extract from Crinum latifolium leaf and their applications forward antibacterial effect and wastewater treatment. J Nanomater. 2019;1–4.10.1155/2019/8385935Search in Google Scholar
[28] BalaKumaran MD, Ramachandran R, Balashanmugam P, Jagadeeswari S, Kalaichelvan PT. Comparative analysis of antifungal, antioxidant and cytotoxic activities of mycosynthesized silver nanoparticles and gold nanoparticles. Mater Technol. 2022;37(6):411–21.10.1080/10667857.2020.1854518Search in Google Scholar
[29] Paragas DS, Viloria JL. Green synthesis of silver and copper nanoparticles for potential biopesticide application against oriental fruit fly (Bactrocera dorsalis Hendel). Lett Appl. NanoBioScience. 2023;13(1):31.10.33263/LIANBS131.031Search in Google Scholar
[30] Sundareswari C, Sudarmani DN, Jayadurkga S. Comparative analysis of larvicidal efficacy of Silver and Copper nanoparticles synthesized leaf extract of Ocimum basilicum against Epilachna vigintioctopunctata. Mapana J Sci. 2023;22(2).10.12723/mjs.65.9Search in Google Scholar
[31] Dhir S, Dutt R, Singh RP, Chauhan M, Virmani T, Kumar G, et al. Amomum subulatum fruit extract mediated green synthesis of silver and copper oxide nanoparticles: Synthesis, characterization, antibacterial and anticancer activities. Processes. 2023;11(9):2698.10.3390/pr11092698Search in Google Scholar
[32] Aykur M, Tosun NG, Kaplan Ö, Özgür A. Efficacy of the greenly synthesized silver, copper, and nickel nanoparticles using Allium tuncelianum extract against Acanthamoeba castellanii. J Drug Deliv Sci Technol. 2023;89:105013.10.1016/j.jddst.2023.105013Search in Google Scholar
[33] Moosavy MH, de la Guardia M, Mokhtarzadeh A, Khatibi SA, Hosseinzadeh N, Hajipour N. Green synthesis, characterization, and biological evaluation of gold and silver nanoparticles using Mentha spicata essential oil. Sci Rep. 2023;13(1):7230.10.1038/s41598-023-33632-ySearch in Google Scholar PubMed PubMed Central
[34] Soliman MK, Abu-Elghait M, Salem SS, Azab MS. Multifunctional properties of silver and gold nanoparticles synthesis by Fusarium pseudonygamai. Biomass Convers Biorefin. 2022;1–8.10.1007/s13399-022-03507-9Search in Google Scholar
[35] Nagappan R. Evaluation of aqueous and ethanol extract of bioactive medicinal plant, Cassia didymobotrya (Fresenius) Irwin & Barneby against immature stages of filarial vector, Culex quinquefasciatus Say (Diptera: Culicidae). Asian Pac J Trop Biomed. 2012;2(9):707–11.10.1016/S2221-1691(12)60214-7Search in Google Scholar PubMed PubMed Central
[36] Gecer EN, Erenler R, Temiz C, Genc N, Yildiz I. Green synthesis of silver nanoparticles from Echinacea purpurea (L.) Moench with antioxidant profile. Part Sci Technol. 2022 Jan;40(1):50–7.10.1080/02726351.2021.1904309Search in Google Scholar
[37] Demirtas I, Erenler R, Elmastas M, Goktasoglu A. Studies on the antioxidant potential of flavones of Allium vineale isolated from its water-soluble fraction. Food Chem. 2013 Jan;136(1):34–40.10.1016/j.foodchem.2012.07.086Search in Google Scholar PubMed
[38] Banerjee P, Satapathy M, Mukhopahayay A, Das P. Leaf extract mediated green synthesis of silver nanoparticles from widely available Indian plants: synthesis, characterization, antimicrobial property and toxicity analysis. Bioresour Bioprocess. 2014;1:1.10.1186/s40643-014-0003-ySearch in Google Scholar
[39] Hussain M, Raja NI, Mashwani ZU, Iqbal M, Chaudhari SK, Ejaz M, et al. Green synthesis and characterization of silver nanoparticles and their effects on disease incidence against canker and biochemical profile in Citrus reticulata L. Nanosci Nanotechnol Lett. 2018;10(10):1348–55.10.1166/nnl.2018.2799Search in Google Scholar
[40] Nagajyothi PC, Cha SJ, Yang IJ, Sreekanth TV, Kim KJ, Shin HM. Antioxidant and anti-inflammatory activities of zinc oxide nanoparticles synthesized using Polygala tenuifolia root extract. J Photochem Photobiol B, Biol. 2015;146:10–7.10.1016/j.jphotobiol.2015.02.008Search in Google Scholar PubMed
[41] Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med. 1999;26(9–10):1231–7.10.1016/S0891-5849(98)00315-3Search in Google Scholar
[42] Umamaheswari M, Chatterjee TK. In vitro antioxidant activities of the fractions of Coccinia grandis L. leaf extract. Afr J Tradit Complement Altern Med. 2008;5(1):61–73.10.4314/ajtcam.v5i1.31258Search in Google Scholar
[43] Aryal S, Baniya MK, Danekhu K, Kunwar P, Gurung R, Koirala N. Total phenolic content, flavonoid content and antioxidant potential of wild vegetables from Western Nepal. Plants. 2019;8(4):96.10.3390/plants8040096Search in Google Scholar PubMed PubMed Central
[44] Hue SM, Boyce AN, Somasundram C. Antioxidant activity, phenolic and flavonoid contents in the leaves of different varieties of sweet potato (‘ipomoea batatas’). Aust J Crop Sci. 2012;6(3):375–80.Search in Google Scholar
[45] Jabeen S, Qureshi R, Munazir M, Maqsood M, Munir M, Shah SS, et al. Application of green synthesized silver nanoparticles in cancer treatment – a critical review. Mater Res Express. 2021 Sep;8(9):092001.10.1088/2053-1591/ac1de3Search in Google Scholar
[46] Jha AK, Prasad K, Prasad K, Kulkarni AR. Plant system: nature’s nanofactory. Colloids Surf B. 2009;73(2):219–23.10.1016/j.colsurfb.2009.05.018Search in Google Scholar PubMed
[47] Zhangabay Z, Berillo D. Antimicrobial and antioxidant activity of AgNPs stabilized with Calendula officinalis flower extract. Surf Interfaces. 2023;11:100–9.10.1016/j.rsurfi.2023.100109Search in Google Scholar
[48] Chirumamilla P, Dharavath SB, Taduri S. Eco-friendly green synthesis of silver nanoparticles from leaf extract of Solanum khasianum: optical properties and biological applications. Appl Biochem Biotechnol. 2023;195(1):353–68.10.1007/s12010-022-04156-4Search in Google Scholar PubMed
[49] Anbalagan S, Sankareswaran M, Prabhavathi P, Manikandan A, Karthikeyan G. Green synthesis and characterization of Silver nanoparticles from Withania somnifera (L.) Dunal. Asian J Pharm. Clin Res. 2016;9(5):34–9.10.22159/ajpcr.2016.v9i5.13204Search in Google Scholar
[50] Javed B, Mashwani Z, Sarwer A, Raja NI, Nadhman A. Synergistic response of physicochemical reaction parameters on biogenesis of silver nanoparticles and their action against colon cancer and leishmanial cells. Artif Cell Nanomed Biotechnol. 2020;48(1):1340–53.10.1080/21691401.2020.1850467Search in Google Scholar PubMed
[51] Adelere I, Aboyeji D, Akindurodoye F, Adabara N, Babayi H. Cashew Plant-Mediated Biosynthesis of silver nanoparticles and evaluation of their applications as antimicrobial additive for consumer care products. Tanz J Sci. 2020;46(3):768–78.10.4314/tjs.v46i3.17Search in Google Scholar
[52] Ateeq M, Shah MR, ul Ain N, Bano S, Anis I, Faizi S, et al. Green synthesis and molecular recognition ability of patuletin coated gold nanoparticles. Biosens Bioelectron. 2015;63:499–505.10.1016/j.bios.2014.07.076Search in Google Scholar PubMed
[53] Bindhani BK, Panigrahi AK. Green synthesis and characterization of gold nanoparticles using leaf extracts of Withania somnifera (Linn.)(Ashwagandha). Int J Mater Sci Appl. 2014;3(6):279–84.10.11648/j.ijmsa.20140306.11Search in Google Scholar
[54] Alagesan V, Venugopal S. Green synthesis of selenium nanoparticle using leaves extract of withania somnifera and its biological applications and photocatalytic activities. Bionanoscience. 2019;9:105–16.10.1007/s12668-018-0566-8Search in Google Scholar
[55] Kumar B, Smita K, Cumbal L, Debut A. Ficus carica (Fig) fruit mediated green synthesis of silver nanoparticles and its antioxidant activity: a comparison of thermal and ultrasonication approach. BioNanoScience. 2016;6:15–21.10.1007/s12668-016-0193-1Search in Google Scholar
[56] Devanesan S, AlSalhi MS. Green synthesis of silver nanoparticles using the flower extract of Abelmoschus esculentus for cytotoxicity and antimicrobial studies. Int J Nanomed. 2021;3343–56.10.2147/IJN.S307676Search in Google Scholar PubMed PubMed Central
[57] Halliwell B, Gutteridge JM. Free radicals in biology and medicine. USA: Oxford University Press; 2015.10.1093/acprof:oso/9780198717478.001.0001Search in Google Scholar
[58] Chang ST, Wu JH, Wang SY, Kang PL, Yang NS, Shyur LF. Antioxidant activity of extracts from Acacia confusa bark and heartwood. J Agri Food Chem. 2001;49(7):3420–4.10.1021/jf0100907Search in Google Scholar PubMed
[59] Li Y, Li X, Wong YS, Chen T, Zhang H, Liu C, et al. The reversal of cisplatin-induced nephrotoxicity by selenium nanoparticles functionalized with 11-mercapto-1-undecanol by inhibition of ROS-mediated apoptosis. Biomaterials. 2011;32(34):9068–76.10.1016/j.biomaterials.2011.08.001Search in Google Scholar PubMed
[60] Mendam K, Naik SJ. Anticancer and antioxidant activities of Cyphostemma auriculatum Roxb. green mediated silver nanoparticles. Mater Today: Proc. 2023;92:618–25.10.1016/j.matpr.2023.04.126Search in Google Scholar
[61] Ravichandran V, Vasanthi S, Shalini S, Shah SA, Harish R. Green synthesis of silver nanoparticles using Atrocarpus altilis leaf extract and the study of their antimicrobial and antioxidant activity. Mater Lett. 2016;180:264–7.10.1016/j.matlet.2016.05.172Search in Google Scholar
[62] Kharat SN, Mendhulkar VD. Synthesis, characterization and studies on antioxidant activity of silver nanoparticles using Elephantopus scaber leaf extract. Mater Sci Eng C. 2016;62:719–24.10.1016/j.msec.2016.02.024Search in Google Scholar PubMed
[63] Genc N, Yildiz I, Chaoui R, Erenler R, Temiz C, Elmastas M. Biosynthesis, characterization and antioxidant activity of oleuropein-mediated silver nanoparticles. Inorg Nano-Metal Chem. 2020 Jul;51(3):411–9.10.1080/24701556.2020.1792495Search in Google Scholar
[64] Paul RK. In vitro Antioxidant activity of Withania somnifera root. Int J Adv Res Chem Sci. 2016;3(3):45–56.10.20431/2349-0403.0303006Search in Google Scholar
[65] Udayakumar R, Kasthurirengan S, Mariashibu TS, Sahaya Rayan JJ, Kim SC, Choi CW, et al. Antioxidant activity of phenolic compounds extracted from the roots and leaves of Withania somnifera (L.) from different geographical locations in India. Funct Plant Sci Biotechnol. 2010;4:28–33.Search in Google Scholar
[66] Niraimathi KL, Sudha V, Lavanya R, Brindha P. Biosynthesis of silver nanoparticles using Alternanthera sessilis (Linn.) extract and their antimicrobial, antioxidant activities. Colloids Surf B Biointerf. 2013;102:288–91.10.1016/j.colsurfb.2012.08.041Search in Google Scholar PubMed
[67] Gecer EN, Erenler R. Biogenic synthesis of silver nanoparticles using Echium vulgare: Characterisation, quantitative analysis of bioactive compounds, antioxidant activity and catalytic degradation. J Indian Chem Soc. 2023 May;100(5):101003.10.1016/j.jics.2023.101003Search in Google Scholar
[68] Balčiūnaitienė A, Liaudanskas M, Puzerytė V, Viškelis J, Janulis V, Viškelis P, et al. Eucalyptus globulus and Salvia officinalis extracts mediated green synthesis of silver nanoparticles and their application as an antioxidant and antimicrobial agent. Plants. 2022 Apr 15;11(8):1085.10.3390/plants11081085Search in Google Scholar PubMed PubMed Central
[69] Mittal AK, Kaler A, Banerjee UC. Free radical scavenging and antioxidant activity of silver nanoparticles synthesized from flower extract of Rhododendron dauricum. Nano Biomed Eng. 2012;4(3):118–24.10.5101/nbe.v4i3.p118-124Search in Google Scholar
[70] Alzubaidi AK, Al-Kaabi WJ, Ali AA, Albukhaty S, Al-Karagoly H, Sulaiman GM, et al. Green synthesis and characterization of silver nanoparticles using flaxseed extract and evaluation of their antibacterial and antioxidant activities. Appl Sci. 2023 Feb;13(4):2182.10.3390/app13042182Search in Google Scholar
[71] Oluwaniyi OO, Adegoke HI, Adesuji ET, Alabi AB, Bodede SO, Labulo AH, et al. Biosynthesis of silver nanoparticles using aqueous leaf extract of Thevetia peruviana Juss and its antimicrobial activities. Appl Nanosci. 2016;6:903–12.10.1007/s13204-015-0505-8Search in Google Scholar
[72] Nandana CN, Christeena M, Bharathi D. Synthesis and characterization of chitosan/silver nanocomposite using rutin for antibacterial, antioxidant and photocatalytic applications. J Clust Sci. 2021;33:269–79.10.1007/s10876-020-01947-9Search in Google Scholar
[73] Sathiyaraj S, Suriyakala G, Gandhi AD, et al. Biosynthesis, characterization, and antibacterial activity of gold nanoparticles. J Infect Public Health. 2021;14(12):1842–7.10.1016/j.jiph.2021.10.007Search in Google Scholar PubMed
[74] Patil SP. Antioxidant, antibacterial and cytotoxic potential of silver nanoparticles synthesized using terpenes rich extract of Lantana camara L leaves. Biochem Biophys Rep. 2017;10:76.10.1016/j.bbrep.2017.03.002Search in Google Scholar PubMed PubMed Central
[75] Saleh HT, Mahdi ZF. Antibacterial activity of green synthesis of silver nanoparticles from withania somnifera (Ashwagandha) root extract. Med Leg Update. 2021;21(2):625–31.10.37506/mlu.v21i2.2752Search in Google Scholar
[76] Baskaran X, Geo Vigila AV, Parimelazhagan T, Muralidhara-Rao D, Zhang S. Biosynthesis, characterization, and evaluation of bioactivities of leaf extract-mediated biocompatible silver nanoparticles from an early tracheophyte, Pteris tripartita Sw. Int J Nanomed. 2016;5789–806.10.2147/IJN.S108208Search in Google Scholar PubMed PubMed Central
[77] AG AL, Jat RK, Siju EN. Antioxidant activity of silver nanoparticles synthesized by hydroalcoholic extract of Triphala. World J Adv Res Rev. 2022;16(2):383–8.10.30574/wjarr.2022.16.2.1164Search in Google Scholar
© 2023 the author(s), published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Research Articles
- Value-added utilization of coal fly ash and recycled polyvinyl chloride in door or window sub-frame composites
- High removal efficiency of volatile phenol from coking wastewater using coal gasification slag via optimized adsorption and multi-grade batch process
- Evolution of surface morphology and properties of diamond films by hydrogen plasma etching
- Removal efficiency of dibenzofuran using CuZn-zeolitic imidazole frameworks as a catalyst and adsorbent
- Rapid and efficient microwave-assisted extraction of Caesalpinia sappan Linn. heartwood and subsequent synthesis of gold nanoparticles
- The catalytic characteristics of 2-methylnaphthalene acylation with AlCl3 immobilized on Hβ as Lewis acid catalyst
- Biodegradation of synthetic PVP biofilms using natural materials and nanoparticles
- Rutin-loaded selenium nanoparticles modulated the redox status, inflammatory, and apoptotic pathways associated with pentylenetetrazole-induced epilepsy in mice
- Optimization of apigenin nanoparticles prepared by planetary ball milling: In vitro and in vivo studies
- Synthesis and characterization of silver nanoparticles using Origanum onites leaves: Cytotoxic, apoptotic, and necrotic effects on Capan-1, L929, and Caco-2 cell lines
- Exergy analysis of a conceptual CO2 capture process with an amine-based DES
- Construction of fluorescence system of felodipine–tetracyanovinyl–2,2′-bipyridine complex
- Excellent photocatalytic degradation of rhodamine B over Bi2O3 supported on Zn-MOF nanocomposites under visible light
- Optimization-based control strategy for a large-scale polyhydroxyalkanoates production in a fed-batch bioreactor using a coupled PDE–ODE system
- Effectiveness of pH and amount of Artemia urumiana extract on physical, chemical, and biological attributes of UV-fabricated biogold nanoparticles
- Geranium leaf-mediated synthesis of silver nanoparticles and their transcriptomic effects on Candida albicans
- Synthesis, characterization, anticancer, anti-inflammatory activities, and docking studies of 3,5-disubstituted thiadiazine-2-thiones
- Synthesis and stability of phospholipid-encapsulated nano-selenium
- Putative anti-proliferative effect of Indian mustard (Brassica juncea) seed and its nano-formulation
- Enrichment of low-grade phosphorites by the selective leaching method
- Electrochemical analysis of the dissolution of gold in a copper–ethylenediamine–thiosulfate system
- Characterisation of carbonate lake sediments as a potential filler for polymer composites
- Evaluation of nano-selenium biofortification characteristics of alfalfa (Medicago sativa L.)
- Quality of oil extracted by cold press from Nigella sativa seeds incorporated with rosemary extracts and pretreated by microwaves
- Heteropolyacid-loaded MOF-derived mesoporous zirconia catalyst for chemical degradation of rhodamine B
- Recovery of critical metals from carbonatite-type mineral wastes: Geochemical modeling investigation of (bio)hydrometallurgical leaching of REEs
- Photocatalytic properties of ZnFe-mixed oxides synthesized via a simple route for water remediation
- Attenuation of di(2-ethylhexyl)phthalate-induced hepatic and renal toxicity by naringin nanoparticles in a rat model
- Novel in situ synthesis of quaternary core–shell metallic sulfide nanocomposites for degradation of organic dyes and hydrogen production
- Microfluidic steam-based synthesis of luminescent carbon quantum dots as sensing probes for nitrite detection
- Transformation of eggshell waste to egg white protein solution, calcium chloride dihydrate, and eggshell membrane powder
- Preparation of Zr-MOFs for the adsorption of doxycycline hydrochloride from wastewater
- Green nanoarchitectonics of the silver nanocrystal potential for treating malaria and their cytotoxic effects on the kidney Vero cell line
- Carbon emissions analysis of producing modified asphalt with natural asphalt
- An efficient and green synthesis of 2-phenylquinazolin-4(3H)-ones via t-BuONa-mediated oxidative condensation of 2-aminobenzamides and benzyl alcohols under solvent- and transition metal-free conditions
- Chitosan nanoparticles loaded with mesosulfuron methyl and mesosulfuron methyl + florasulam + MCPA isooctyl to manage weeds of wheat (Triticum aestivum L.)
- Synergism between lignite and high-sulfur petroleum coke in CO2 gasification
- Facile aqueous synthesis of ZnCuInS/ZnS–ZnS QDs with enhanced photoluminescence lifetime for selective detection of Cu(ii) ions
- Rapid synthesis of copper nanoparticles using Nepeta cataria leaves: An eco-friendly management of disease-causing vectors and bacterial pathogens
- Study on the photoelectrocatalytic activity of reduced TiO2 nanotube films for removal of methyl orange
- Development of a fuzzy logic model for the prediction of spark-ignition engine performance and emission for gasoline–ethanol blends
- Micro-impact-induced mechano-chemical synthesis of organic precursors from FeC/FeN and carbonates/nitrates in water and its extension to nucleobases
- Green synthesis of strontium-doped tin dioxide (SrSnO2) nanoparticles using the Mahonia bealei leaf extract and evaluation of their anticancer and antimicrobial activities
- A study on the larvicidal and adulticidal potential of Cladostepus spongiosus macroalgae and green-fabricated silver nanoparticles against mosquito vectors
- Catalysts based on nickel salt heteropolytungstates for selective oxidation of diphenyl sulfide
- Powerful antibacterial nanocomposites from Corallina officinalis-mediated nanometals and chitosan nanoparticles against fish-borne pathogens
- Removal behavior of Zn and alkalis from blast furnace dust in pre-reduction sinter process
- Environmentally friendly synthesis and computational studies of novel class of acridinedione integrated spirothiopyrrolizidines/indolizidines
- The mechanisms of inhibition and lubrication of clean fracturing flowback fluids in water-based drilling fluids
- Adsorption/desorption performance of cellulose membrane for Pb(ii)
- A one-pot, multicomponent tandem synthesis of fused polycyclic pyrrolo[3,2-c]quinolinone/pyrrolizino[2,3-c]quinolinone hybrid heterocycles via environmentally benign solid state melt reaction
- Green synthesis of silver nanoparticles using durian rind extract and optical characteristics of surface plasmon resonance-based optical sensor for the detection of hydrogen peroxide
- Electrochemical analysis of copper-EDTA-ammonia-gold thiosulfate dissolution system
- Characterization of bio-oil production by microwave pyrolysis from cashew nut shells and Cassia fistula pods
- Green synthesis methods and characterization of bacterial cellulose/silver nanoparticle composites
- Photocatalytic research performance of zinc oxide/graphite phase carbon nitride catalyst and its application in environment
- Effect of phytogenic iron nanoparticles on the bio-fortification of wheat varieties
- In vitro anti-cancer and antimicrobial effects of manganese oxide nanoparticles synthesized using the Glycyrrhiza uralensis leaf extract on breast cancer cell lines
- Preparation of Pd/Ce(F)-MCM-48 catalysts and their catalytic performance of n-heptane isomerization
- Green “one-pot” fluorescent bis-indolizine synthesis with whole-cell plant biocatalysis
- Silica-titania mesoporous silicas of MCM-41 type as effective catalysts and photocatalysts for selective oxidation of diphenyl sulfide by H2O2
- Biosynthesis of zinc oxide nanoparticles from molted feathers of Pavo cristatus and their antibiofilm and anticancer activities
- Clean preparation of rutile from Ti-containing mixed molten slag by CO2 oxidation
- Synthesis and characterization of Pluronic F-127-coated titanium dioxide nanoparticles synthesized from extracts of Atractylodes macrocephala leaf for antioxidant, antimicrobial, and anticancer properties
- Effect of pretreatment with alkali on the anaerobic digestion characteristics of kitchen waste and analysis of microbial diversity
- Ameliorated antimicrobial, antioxidant, and anticancer properties by Plectranthus vettiveroides root extract-mediated green synthesis of chitosan nanoparticles
- Microwave-accelerated pretreatment technique in green extraction of oil and bioactive compounds from camelina seeds: Effectiveness and characterization
- Studies on the extraction performance of phorate by aptamer-functionalized magnetic nanoparticles in plasma samples
- Investigation of structural properties and antibacterial activity of AgO nanoparticle extract from Solanum nigrum/Mentha leaf extracts by green synthesis method
- Green fabrication of chitosan from marine crustaceans and mushroom waste: Toward sustainable resource utilization
- Synthesis, characterization, and evaluation of nanoparticles of clodinofop propargyl and fenoxaprop-P-ethyl on weed control, growth, and yield of wheat (Triticum aestivum L.)
- The enhanced adsorption properties of phosphorus from aqueous solutions using lanthanum modified synthetic zeolites
- Separation of graphene oxides of different sizes by multi-layer dialysis and anti-friction and lubrication performance
- Visible-light-assisted base-catalyzed, one-pot synthesis of highly functionalized cinnolines
- The experimental study on the air oxidation of 5-hydroxymethylfurfural to 2,5-furandicarboxylic acid with Co–Mn–Br system
- Highly efficient removal of tetracycline and methyl violet 2B from aqueous solution using the bimetallic FeZn-ZIFs catalyst
- A thermo-tolerant cellulase enzyme produced by Bacillus amyloliquefaciens M7, an insight into synthesis, optimization, characterization, and bio-polishing activity
- Exploration of ketone derivatives of succinimide for their antidiabetic potential: In vitro and in vivo approaches
- Ultrasound-assisted green synthesis and in silico study of 6-(4-(butylamino)-6-(diethylamino)-1,3,5-triazin-2-yl)oxypyridazine derivatives
- A study of the anticancer potential of Pluronic F-127 encapsulated Fe2O3 nanoparticles derived from Berberis vulgaris extract
- Biogenic synthesis of silver nanoparticles using Consolida orientalis flowers: Identification, catalytic degradation, and biological effect
- Initial assessment of the presence of plastic waste in some coastal mangrove forests in Vietnam
- Adsorption synergy electrocatalytic degradation of phenol by active oxygen-containing species generated in Co-coal based cathode and graphite anode
- Antibacterial, antifungal, antioxidant, and cytotoxicity activities of the aqueous extract of Syzygium aromaticum-mediated synthesized novel silver nanoparticles
- Synthesis of a silica matrix with ZnO nanoparticles for the fabrication of a recyclable photodegradation system to eliminate methylene blue dye
- Natural polymer fillers instead of dye and pigments: Pumice and scoria in PDMS fluid and elastomer composites
- Study on the preparation of glycerylphosphorylcholine by transesterification under supported sodium methoxide
- Wireless network handheld terminal-based green ecological sustainable design evaluation system: Improved data communication and reduced packet loss rate
- The optimization of hydrogel strength from cassava starch using oxidized sucrose as a crosslinking agent
- Green synthesis of silver nanoparticles using Saccharum officinarum leaf extract for antiviral paint
- Study on the reliability of nano-silver-coated tin solder joints for flip chips
- Environmentally sustainable analytical quality by design aided RP-HPLC method for the estimation of brilliant blue in commercial food samples employing a green-ultrasound-assisted extraction technique
- Anticancer and antimicrobial potential of zinc/sodium alginate/polyethylene glycol/d-pinitol nanocomposites against osteosarcoma MG-63 cells
- Nanoporous carbon@CoFe2O4 nanocomposite as a green absorbent for the adsorptive removal of Hg(ii) from aqueous solutions
- Characterization of silver sulfide nanoparticles from actinobacterial strain (M10A62) and its toxicity against lepidopteran and dipterans insect species
- Phyto-fabrication and characterization of silver nanoparticles using Withania somnifera: Investigating antioxidant potential
- Effect of e-waste nanofillers on the mechanical, thermal, and wear properties of epoxy-blend sisal woven fiber-reinforced composites
- Magnesium nanohydroxide (2D brucite) as a host matrix for thymol and carvacrol: Synthesis, characterization, and inhibition of foodborne pathogens
- Synergistic inhibitive effect of a hybrid zinc oxide-benzalkonium chloride composite on the corrosion of carbon steel in a sulfuric acidic solution
- Review Articles
- Role and the importance of green approach in biosynthesis of nanopropolis and effectiveness of propolis in the treatment of COVID-19 pandemic
- Gum tragacanth-mediated synthesis of metal nanoparticles, characterization, and their applications as a bactericide, catalyst, antioxidant, and peroxidase mimic
- Green-processed nano-biocomposite (ZnO–TiO2): Potential candidates for biomedical applications
- Reaction mechanisms in microwave-assisted lignin depolymerisation in hydrogen-donating solvents
- Recent progress on non-noble metal catalysts for the deoxydehydration of biomass-derived oxygenates
- Rapid Communication
- Phosphorus removal by iron–carbon microelectrolysis: A new way to achieve phosphorus recovery
- Special Issue: Biomolecules-derived synthesis of nanomaterials for environmental and biological applications (Guest Editors: Arpita Roy and Fernanda Maria Policarpo Tonelli)
- Biomolecules-derived synthesis of nanomaterials for environmental and biological applications
- Nano-encapsulated tanshinone IIA in PLGA-PEG-COOH inhibits apoptosis and inflammation in cerebral ischemia/reperfusion injury
- Green fabrication of silver nanoparticles using Melia azedarach ripened fruit extract, their characterization, and biological properties
- Green-synthesized nanoparticles and their therapeutic applications: A review
- Antioxidant, antibacterial, and cytotoxicity potential of synthesized silver nanoparticles from the Cassia alata leaf aqueous extract
- Green synthesis of silver nanoparticles using Callisia fragrans leaf extract and its anticancer activity against MCF-7, HepG2, KB, LU-1, and MKN-7 cell lines
- Algae-based green AgNPs, AuNPs, and FeNPs as potential nanoremediators
- Green synthesis of Kickxia elatine-induced silver nanoparticles and their role as anti-acetylcholinesterase in the treatment of Alzheimer’s disease
- Phytocrystallization of silver nanoparticles using Cassia alata flower extract for effective control of fungal skin pathogens
- Antibacterial wound dressing with hydrogel from chitosan and polyvinyl alcohol from the red cabbage extract loaded with silver nanoparticles
- Leveraging of mycogenic copper oxide nanostructures for disease management of Alternaria blight of Brassica juncea
- Nanoscale molecular reactions in microbiological medicines in modern medical applications
- Synthesis and characterization of ZnO/β-cyclodextrin/nicotinic acid nanocomposite and its biological and environmental application
- Green synthesis of silver nanoparticles via Taxus wallichiana Zucc. plant-derived Taxol: Novel utilization as anticancer, antioxidation, anti-inflammation, and antiurolithic potential
- Recyclability and catalytic characteristics of copper oxide nanoparticles derived from bougainvillea plant flower extract for biomedical application
- Phytofabrication, characterization, and evaluation of novel bioinspired selenium–iron (Se–Fe) nanocomposites using Allium sativum extract for bio-potential applications
- Erratum
- Erratum to “Synthesis, characterization, and evaluation of nanoparticles of clodinofop propargyl and fenoxaprop-P-ethyl on weed control, growth, and yield of wheat (Triticum aestivum L.)”
Articles in the same Issue
- Research Articles
- Value-added utilization of coal fly ash and recycled polyvinyl chloride in door or window sub-frame composites
- High removal efficiency of volatile phenol from coking wastewater using coal gasification slag via optimized adsorption and multi-grade batch process
- Evolution of surface morphology and properties of diamond films by hydrogen plasma etching
- Removal efficiency of dibenzofuran using CuZn-zeolitic imidazole frameworks as a catalyst and adsorbent
- Rapid and efficient microwave-assisted extraction of Caesalpinia sappan Linn. heartwood and subsequent synthesis of gold nanoparticles
- The catalytic characteristics of 2-methylnaphthalene acylation with AlCl3 immobilized on Hβ as Lewis acid catalyst
- Biodegradation of synthetic PVP biofilms using natural materials and nanoparticles
- Rutin-loaded selenium nanoparticles modulated the redox status, inflammatory, and apoptotic pathways associated with pentylenetetrazole-induced epilepsy in mice
- Optimization of apigenin nanoparticles prepared by planetary ball milling: In vitro and in vivo studies
- Synthesis and characterization of silver nanoparticles using Origanum onites leaves: Cytotoxic, apoptotic, and necrotic effects on Capan-1, L929, and Caco-2 cell lines
- Exergy analysis of a conceptual CO2 capture process with an amine-based DES
- Construction of fluorescence system of felodipine–tetracyanovinyl–2,2′-bipyridine complex
- Excellent photocatalytic degradation of rhodamine B over Bi2O3 supported on Zn-MOF nanocomposites under visible light
- Optimization-based control strategy for a large-scale polyhydroxyalkanoates production in a fed-batch bioreactor using a coupled PDE–ODE system
- Effectiveness of pH and amount of Artemia urumiana extract on physical, chemical, and biological attributes of UV-fabricated biogold nanoparticles
- Geranium leaf-mediated synthesis of silver nanoparticles and their transcriptomic effects on Candida albicans
- Synthesis, characterization, anticancer, anti-inflammatory activities, and docking studies of 3,5-disubstituted thiadiazine-2-thiones
- Synthesis and stability of phospholipid-encapsulated nano-selenium
- Putative anti-proliferative effect of Indian mustard (Brassica juncea) seed and its nano-formulation
- Enrichment of low-grade phosphorites by the selective leaching method
- Electrochemical analysis of the dissolution of gold in a copper–ethylenediamine–thiosulfate system
- Characterisation of carbonate lake sediments as a potential filler for polymer composites
- Evaluation of nano-selenium biofortification characteristics of alfalfa (Medicago sativa L.)
- Quality of oil extracted by cold press from Nigella sativa seeds incorporated with rosemary extracts and pretreated by microwaves
- Heteropolyacid-loaded MOF-derived mesoporous zirconia catalyst for chemical degradation of rhodamine B
- Recovery of critical metals from carbonatite-type mineral wastes: Geochemical modeling investigation of (bio)hydrometallurgical leaching of REEs
- Photocatalytic properties of ZnFe-mixed oxides synthesized via a simple route for water remediation
- Attenuation of di(2-ethylhexyl)phthalate-induced hepatic and renal toxicity by naringin nanoparticles in a rat model
- Novel in situ synthesis of quaternary core–shell metallic sulfide nanocomposites for degradation of organic dyes and hydrogen production
- Microfluidic steam-based synthesis of luminescent carbon quantum dots as sensing probes for nitrite detection
- Transformation of eggshell waste to egg white protein solution, calcium chloride dihydrate, and eggshell membrane powder
- Preparation of Zr-MOFs for the adsorption of doxycycline hydrochloride from wastewater
- Green nanoarchitectonics of the silver nanocrystal potential for treating malaria and their cytotoxic effects on the kidney Vero cell line
- Carbon emissions analysis of producing modified asphalt with natural asphalt
- An efficient and green synthesis of 2-phenylquinazolin-4(3H)-ones via t-BuONa-mediated oxidative condensation of 2-aminobenzamides and benzyl alcohols under solvent- and transition metal-free conditions
- Chitosan nanoparticles loaded with mesosulfuron methyl and mesosulfuron methyl + florasulam + MCPA isooctyl to manage weeds of wheat (Triticum aestivum L.)
- Synergism between lignite and high-sulfur petroleum coke in CO2 gasification
- Facile aqueous synthesis of ZnCuInS/ZnS–ZnS QDs with enhanced photoluminescence lifetime for selective detection of Cu(ii) ions
- Rapid synthesis of copper nanoparticles using Nepeta cataria leaves: An eco-friendly management of disease-causing vectors and bacterial pathogens
- Study on the photoelectrocatalytic activity of reduced TiO2 nanotube films for removal of methyl orange
- Development of a fuzzy logic model for the prediction of spark-ignition engine performance and emission for gasoline–ethanol blends
- Micro-impact-induced mechano-chemical synthesis of organic precursors from FeC/FeN and carbonates/nitrates in water and its extension to nucleobases
- Green synthesis of strontium-doped tin dioxide (SrSnO2) nanoparticles using the Mahonia bealei leaf extract and evaluation of their anticancer and antimicrobial activities
- A study on the larvicidal and adulticidal potential of Cladostepus spongiosus macroalgae and green-fabricated silver nanoparticles against mosquito vectors
- Catalysts based on nickel salt heteropolytungstates for selective oxidation of diphenyl sulfide
- Powerful antibacterial nanocomposites from Corallina officinalis-mediated nanometals and chitosan nanoparticles against fish-borne pathogens
- Removal behavior of Zn and alkalis from blast furnace dust in pre-reduction sinter process
- Environmentally friendly synthesis and computational studies of novel class of acridinedione integrated spirothiopyrrolizidines/indolizidines
- The mechanisms of inhibition and lubrication of clean fracturing flowback fluids in water-based drilling fluids
- Adsorption/desorption performance of cellulose membrane for Pb(ii)
- A one-pot, multicomponent tandem synthesis of fused polycyclic pyrrolo[3,2-c]quinolinone/pyrrolizino[2,3-c]quinolinone hybrid heterocycles via environmentally benign solid state melt reaction
- Green synthesis of silver nanoparticles using durian rind extract and optical characteristics of surface plasmon resonance-based optical sensor for the detection of hydrogen peroxide
- Electrochemical analysis of copper-EDTA-ammonia-gold thiosulfate dissolution system
- Characterization of bio-oil production by microwave pyrolysis from cashew nut shells and Cassia fistula pods
- Green synthesis methods and characterization of bacterial cellulose/silver nanoparticle composites
- Photocatalytic research performance of zinc oxide/graphite phase carbon nitride catalyst and its application in environment
- Effect of phytogenic iron nanoparticles on the bio-fortification of wheat varieties
- In vitro anti-cancer and antimicrobial effects of manganese oxide nanoparticles synthesized using the Glycyrrhiza uralensis leaf extract on breast cancer cell lines
- Preparation of Pd/Ce(F)-MCM-48 catalysts and their catalytic performance of n-heptane isomerization
- Green “one-pot” fluorescent bis-indolizine synthesis with whole-cell plant biocatalysis
- Silica-titania mesoporous silicas of MCM-41 type as effective catalysts and photocatalysts for selective oxidation of diphenyl sulfide by H2O2
- Biosynthesis of zinc oxide nanoparticles from molted feathers of Pavo cristatus and their antibiofilm and anticancer activities
- Clean preparation of rutile from Ti-containing mixed molten slag by CO2 oxidation
- Synthesis and characterization of Pluronic F-127-coated titanium dioxide nanoparticles synthesized from extracts of Atractylodes macrocephala leaf for antioxidant, antimicrobial, and anticancer properties
- Effect of pretreatment with alkali on the anaerobic digestion characteristics of kitchen waste and analysis of microbial diversity
- Ameliorated antimicrobial, antioxidant, and anticancer properties by Plectranthus vettiveroides root extract-mediated green synthesis of chitosan nanoparticles
- Microwave-accelerated pretreatment technique in green extraction of oil and bioactive compounds from camelina seeds: Effectiveness and characterization
- Studies on the extraction performance of phorate by aptamer-functionalized magnetic nanoparticles in plasma samples
- Investigation of structural properties and antibacterial activity of AgO nanoparticle extract from Solanum nigrum/Mentha leaf extracts by green synthesis method
- Green fabrication of chitosan from marine crustaceans and mushroom waste: Toward sustainable resource utilization
- Synthesis, characterization, and evaluation of nanoparticles of clodinofop propargyl and fenoxaprop-P-ethyl on weed control, growth, and yield of wheat (Triticum aestivum L.)
- The enhanced adsorption properties of phosphorus from aqueous solutions using lanthanum modified synthetic zeolites
- Separation of graphene oxides of different sizes by multi-layer dialysis and anti-friction and lubrication performance
- Visible-light-assisted base-catalyzed, one-pot synthesis of highly functionalized cinnolines
- The experimental study on the air oxidation of 5-hydroxymethylfurfural to 2,5-furandicarboxylic acid with Co–Mn–Br system
- Highly efficient removal of tetracycline and methyl violet 2B from aqueous solution using the bimetallic FeZn-ZIFs catalyst
- A thermo-tolerant cellulase enzyme produced by Bacillus amyloliquefaciens M7, an insight into synthesis, optimization, characterization, and bio-polishing activity
- Exploration of ketone derivatives of succinimide for their antidiabetic potential: In vitro and in vivo approaches
- Ultrasound-assisted green synthesis and in silico study of 6-(4-(butylamino)-6-(diethylamino)-1,3,5-triazin-2-yl)oxypyridazine derivatives
- A study of the anticancer potential of Pluronic F-127 encapsulated Fe2O3 nanoparticles derived from Berberis vulgaris extract
- Biogenic synthesis of silver nanoparticles using Consolida orientalis flowers: Identification, catalytic degradation, and biological effect
- Initial assessment of the presence of plastic waste in some coastal mangrove forests in Vietnam
- Adsorption synergy electrocatalytic degradation of phenol by active oxygen-containing species generated in Co-coal based cathode and graphite anode
- Antibacterial, antifungal, antioxidant, and cytotoxicity activities of the aqueous extract of Syzygium aromaticum-mediated synthesized novel silver nanoparticles
- Synthesis of a silica matrix with ZnO nanoparticles for the fabrication of a recyclable photodegradation system to eliminate methylene blue dye
- Natural polymer fillers instead of dye and pigments: Pumice and scoria in PDMS fluid and elastomer composites
- Study on the preparation of glycerylphosphorylcholine by transesterification under supported sodium methoxide
- Wireless network handheld terminal-based green ecological sustainable design evaluation system: Improved data communication and reduced packet loss rate
- The optimization of hydrogel strength from cassava starch using oxidized sucrose as a crosslinking agent
- Green synthesis of silver nanoparticles using Saccharum officinarum leaf extract for antiviral paint
- Study on the reliability of nano-silver-coated tin solder joints for flip chips
- Environmentally sustainable analytical quality by design aided RP-HPLC method for the estimation of brilliant blue in commercial food samples employing a green-ultrasound-assisted extraction technique
- Anticancer and antimicrobial potential of zinc/sodium alginate/polyethylene glycol/d-pinitol nanocomposites against osteosarcoma MG-63 cells
- Nanoporous carbon@CoFe2O4 nanocomposite as a green absorbent for the adsorptive removal of Hg(ii) from aqueous solutions
- Characterization of silver sulfide nanoparticles from actinobacterial strain (M10A62) and its toxicity against lepidopteran and dipterans insect species
- Phyto-fabrication and characterization of silver nanoparticles using Withania somnifera: Investigating antioxidant potential
- Effect of e-waste nanofillers on the mechanical, thermal, and wear properties of epoxy-blend sisal woven fiber-reinforced composites
- Magnesium nanohydroxide (2D brucite) as a host matrix for thymol and carvacrol: Synthesis, characterization, and inhibition of foodborne pathogens
- Synergistic inhibitive effect of a hybrid zinc oxide-benzalkonium chloride composite on the corrosion of carbon steel in a sulfuric acidic solution
- Review Articles
- Role and the importance of green approach in biosynthesis of nanopropolis and effectiveness of propolis in the treatment of COVID-19 pandemic
- Gum tragacanth-mediated synthesis of metal nanoparticles, characterization, and their applications as a bactericide, catalyst, antioxidant, and peroxidase mimic
- Green-processed nano-biocomposite (ZnO–TiO2): Potential candidates for biomedical applications
- Reaction mechanisms in microwave-assisted lignin depolymerisation in hydrogen-donating solvents
- Recent progress on non-noble metal catalysts for the deoxydehydration of biomass-derived oxygenates
- Rapid Communication
- Phosphorus removal by iron–carbon microelectrolysis: A new way to achieve phosphorus recovery
- Special Issue: Biomolecules-derived synthesis of nanomaterials for environmental and biological applications (Guest Editors: Arpita Roy and Fernanda Maria Policarpo Tonelli)
- Biomolecules-derived synthesis of nanomaterials for environmental and biological applications
- Nano-encapsulated tanshinone IIA in PLGA-PEG-COOH inhibits apoptosis and inflammation in cerebral ischemia/reperfusion injury
- Green fabrication of silver nanoparticles using Melia azedarach ripened fruit extract, their characterization, and biological properties
- Green-synthesized nanoparticles and their therapeutic applications: A review
- Antioxidant, antibacterial, and cytotoxicity potential of synthesized silver nanoparticles from the Cassia alata leaf aqueous extract
- Green synthesis of silver nanoparticles using Callisia fragrans leaf extract and its anticancer activity against MCF-7, HepG2, KB, LU-1, and MKN-7 cell lines
- Algae-based green AgNPs, AuNPs, and FeNPs as potential nanoremediators
- Green synthesis of Kickxia elatine-induced silver nanoparticles and their role as anti-acetylcholinesterase in the treatment of Alzheimer’s disease
- Phytocrystallization of silver nanoparticles using Cassia alata flower extract for effective control of fungal skin pathogens
- Antibacterial wound dressing with hydrogel from chitosan and polyvinyl alcohol from the red cabbage extract loaded with silver nanoparticles
- Leveraging of mycogenic copper oxide nanostructures for disease management of Alternaria blight of Brassica juncea
- Nanoscale molecular reactions in microbiological medicines in modern medical applications
- Synthesis and characterization of ZnO/β-cyclodextrin/nicotinic acid nanocomposite and its biological and environmental application
- Green synthesis of silver nanoparticles via Taxus wallichiana Zucc. plant-derived Taxol: Novel utilization as anticancer, antioxidation, anti-inflammation, and antiurolithic potential
- Recyclability and catalytic characteristics of copper oxide nanoparticles derived from bougainvillea plant flower extract for biomedical application
- Phytofabrication, characterization, and evaluation of novel bioinspired selenium–iron (Se–Fe) nanocomposites using Allium sativum extract for bio-potential applications
- Erratum
- Erratum to “Synthesis, characterization, and evaluation of nanoparticles of clodinofop propargyl and fenoxaprop-P-ethyl on weed control, growth, and yield of wheat (Triticum aestivum L.)”

