Synthesis, characterization, anticancer, anti-inflammatory activities, and docking studies of 3,5-disubstituted thiadiazine-2-thiones
-
Haleema Ali
, Xiandao Pan
, Muhammad Shah
Abstract
In the search for potent bioactive compounds, a series of tetrahydro-2H-1,3,5-thiadiazine-2-thiones (1–13) were synthesized in good yield and characterized by means of 1H NMR, 13C NMR, and mass spectral data. The anticancer activity of the compounds was evaluated against HeLa cell line and anti-inflammatory potential via nitric oxide (NO) inhibition. Among the screened compounds, 2-(5-(3-methoxypropyl)-6-thioxo-1,3,5-thiadiazinan-3-yl) propionic acid (3), 2-(5-cyclopropyl-6-thioxo-1,3,5-thiadiazinan-3-yl) propionic acid (5), 2-(5-cyclopropyl)-6-thioxo-1,3,5-thiadiazinan-3-yl) acetic acid (6), and 2-(5-butyl-6-thioxo-1,3,5-thiadiazinan-3-yl) acetic acid (9) were the most potent against HeLa cell line with IC50 values <4 µM, whereas the rest of the series exhibited moderate-to-good activities. All the compounds were potent NO inhibitors with IC50 values ranging from <0.4 to 14.9 µM. Docking studies, binding orientations, and interaction plots showed strong interaction of the studied compounds with the inducible NO synthase enzyme via strong hydrogen bonds and hydrophobic interactions, which authenticate the in vitro results. These newly synthesized compounds could lead to the discovery of anticancer drugs.
1 Introduction
The recent call of World Health Organization for the elimination of cervical cancer as a global health concern prompted medicinal chemists to search and develop potent molecules for treating cancer [1]. Cervical cancer is the fourth most common cancer in women, and the leading cause of death in low-resource countries [2]. According to recent research in chemotherapy, there is a pressing need for more compelling anticancer mediators since the effectiveness of chemotherapy is limited by tumor cells’ heterogeneity and drug resistance [3,4]. Nitric oxide (NO) is a liposoluble molecule, endogenously produced in the mammalian body during the metabolism of l-arginine to l-citrulline by the action of nitric oxide synthases (NOS) [5]. NO is a versatile intra- and intercellular messenger controlling diverse pathophysiological mechanisms in circulatory, neurological, and immune systems [6]. Despite biological mediators, NO, as an oxygen free radical, is cytotoxic in pathological processes, mainly in inflammatory ailments [7]. Furthermore, inflammation should be regulated because of its direct impact on chronic conditions, such as cancer, cardiovascular, and immunological diseases [8]. Thus, inflammatory diseases can be treated by NO inhibition [9].
Currently, NO is recognized as one of the most important molecules affecting the growth, development, and treatment of cancer. In tumor biology, NO has both positive and negative effects, as it has been associated with both endorsing and inhibiting cancer. Research findings indicated that the dual responses of NO toward cancer are due to its concentration-dependent ability in development, migration, incursion, persistence, and metastasis of tumor [10]. The exact molecular mechanism of NO involvement in cancer is not fully understood so far; however, studies found a significantly higher levels of NO in cervical cancer patients [11]. In addition, cervixes of women having cervical intraepithelial neoplasia were found with elevated levels of NO as well as NO-mediated mutagenesis [12]. These studies concluded mutagenic and carcinogenic activities of NO in cervical cancer.
Synthesis of novel molecules possessing pharmacophore moiety that resembles known biologically active compounds provides a leading approach toward the development of highly active agents. Among anticancer agents, dithiocarbamates [13,14,15,16,17] and isothiocyanate [18,19,20,21,22,23,24] gained great attention as promising anticancer candidates. In addition, tetrahydro-(2H)-1,3,5-thiadiazine-2-thione nucleus has been reported to exhibit anticancer [25,26,27,28,29], antileishmanial [30], antibacterial [31], trypanocidal [32], antimalarial [33], antifungal [34], herbicidal [35], antitubercular [36], antiepileptic [37], and antioxidant [38] activities. These activities were attributed to the formation of isothiocyanate and dithiocarbamic acid upon hydrolysis of this nucleus in biological systems [29,39].
On the basis of the previous discussion, the aim of the present work was to synthesize 3,5-disubstituted thiadiazine-2-thiones as potential anticancer agents. The anticancer activity of the synthesized compounds will be evaluated against human cervical cancer HeLa cell line. Previously, the anticancer activity of cyclopentyl, cyclohexyl, and furfuryl N-3 substituted and bis-disubstituted thiadiazine-2-thiones has been tested against HT-29 (Human colorectal adenocarcinoma), A549 (lung carcinoma), Hep3B (hepatocellular carcinoma), HepG2 (hepatocellular carcinoma), U-87MG (Brain (glioblastoma astrocytoma), normal cell line (fibroblast F180) [25], K562 (leukaemia cells), MCF12 (normal cells) [26], MCF-12A (normal breast cell line), MCF-7 and MDA-MB-468 (breast cancer cell line) [27], Hep G2 (human hepatoma), HT-29 (human colon carcinoma), and HeLa (human cervical carcinoma) [28,29]. These studies showed moderate-to-good activity of the tested derivatives of tetrahydro-2H-1,3,5-thiadiazine-2-thione. We, therefore, sought to synthesize more functionalized tetrahydro-2H-1,3,5-thiadiazine-2-thiones, which could be more potent than previously reported ones. Despite the wealth of literature that dealt with the biological properties of 1,3,5-thiadiazine-2-thione moiety, no anti-inflammatory activity has been assessed for this nucleus. Hence, this study deals with the anticancer and NO inhibitory potential along with the docking studies of the titled compounds to appraise the functional modification on the 1,3,5-thiadiazine-2-thione nucleus for the said activities.
2 Materials and methods
2.1 Chemistry
All chemicals and reagents were obtained from commercial suppliers and used as received without further purification. Reactions were monitored by thin-layer chromatography, achieved on silica gel plates (60 F-254); these plates were visualized under UV light. Melting points were determined using a Gallen Kamp melting point apparatus and are uncorrected. Dimethyl sulfoxide (DMSO), chloroform (CDCl3), and methanol (CD3OD) were used as solvents for NMR Spectra. 1H and 13C NMR spectra were recorded on Bruker AVNeo-NMR Spectrometers and using TMS as an internal standard. Chemical shifts are expressed in δ units, whereas coupling constants (J-values) are given in Hertz. Mass spectra were acquired on JEOL MS Route and Finnigan LTQ FTMS (ESI) spectrometer.
2.2 General procedure for synthesis of 3,5-disubstituted tetrahydrothiadiazine-2-thiones (1–13)
Compounds 1–13 were prepared by adding selected alkyl/cycloalkyl/aryl amine (20 mmol) to a 20% KOH aqueous solution followed by adding CS2 dropwise (20 mmol) at 30°C with stirring. After 4 h, 37% formaldehyde solution (40 mmol) was added and stirred for 1 h continuously, and the reaction mixture was then filtered and added, dropwise, to a suspension of amino acid or primary amines (20 mmol) in a 7.8 pH phosphate buffer and stirred for 1 h (Scheme 1). The mixture was then filtered and refrigerated for 1 h. The ice-chilled reaction mixture was acidified with hydrochloric acid up to pH 2.0 at 0–5°C. The precipitate formed was thoroughly washed with water followed by n-hexane and then dried. The desired product was recrystallized from ethanol to afford pure compounds 1–13. Using this general procedure, the following compounds were synthesized.
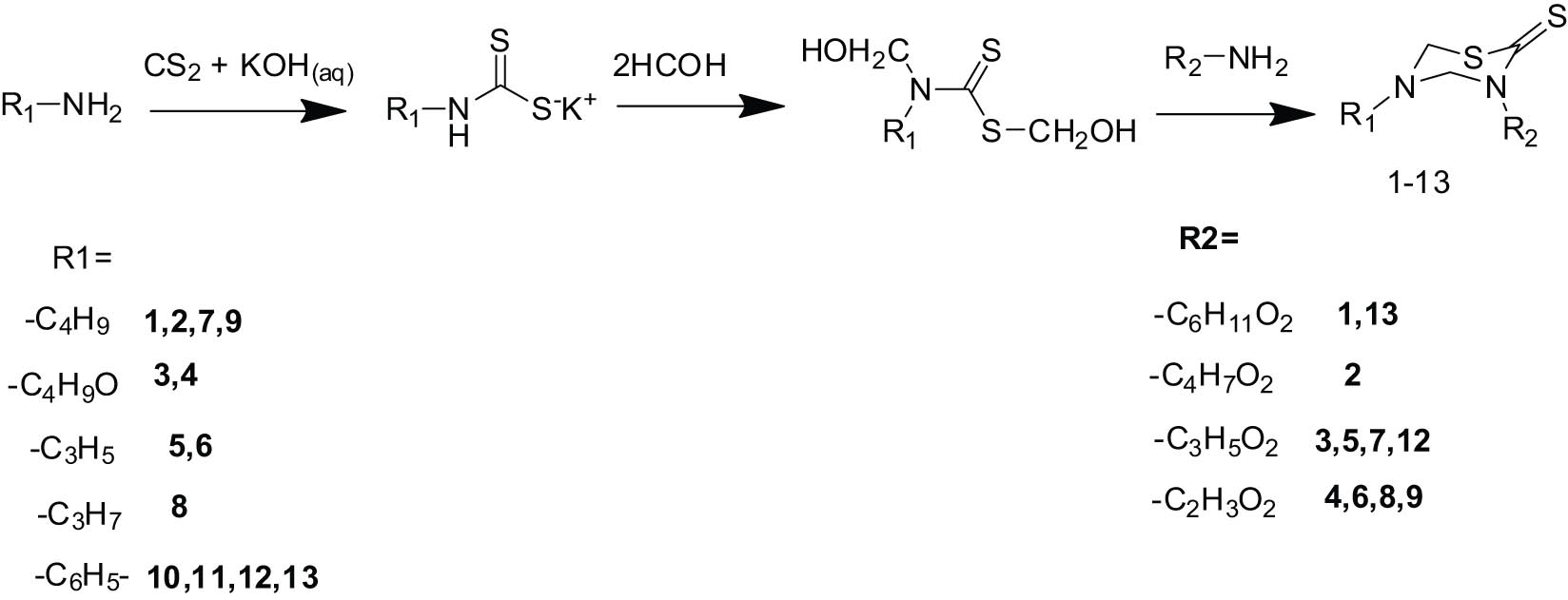
General scheme for the synthesis of the title compounds.
2.2.1 2-(5-butyl-6-thioxo-1,3,5-thiadiazinan-3-yl)-4-methylpentanoic acid (1)
Compound 1, acquired by n-butyl amine and l-leucine, was obtained as white solid recrystallized from ethanol. Yield: 76%, mp: 92–94°C. 1H NMR (600 MHz, DMSO-d 6): δ 0.89 (t, 9H, J = 6.4, CH3), 1.25–1.31 (m, 2H, CH3CH2CH2), 1.50–1.63 (m, 5H, CH2CH2CH2, CH3CHCH3, CHCH2CH), 3.50–3.53 and 3.79–3.98 (m, 2H, NCH2CH2,), 4.45–4.64 (m, 4H, NCH2NCH2S), 13C NMR (150 MHz, DMSO-d 6): δ 13.87 (CH3CH2), 19.69 (CH3CH2), 21.93, 23.43 (CH3CHCH3), 24.96 (CH3CHCH3), 28.37 (CH2CH2CH2), 38.67 (CHCH2CH), 51.48 (NCH2), 55.75 (C-6), 60.77 (NCH), 67.61 (C-4), 173.46 (CO), 190.58 (CS). MS (70 eV): m/z (%): m/z (%) = 304.2 (4.5) [M]+, 256.2 (11) [M–146]+, 140.0 (100) [M–200]+, 115.1 (100) [M–289]+.
2.2.2 2-(5-butyl-6-thioxo-1,3,5-thiadiazinan-3-yl) butanoic acid (2)
Compound 2 was obtained from n-butyl amine and DL-2-aminobutyric acid as a white solid recrystallized from ethanol. Yield: 72%, mp: 93–95°C (600 MHz, DMSO-d 6): δ 0.84–0.91 (m, 6H, CH3CH2, CH3CH2), 1.23–1, mp: 32 (m, 2H, CH3CH2CH2), 1.50–1.61 (m, 2H, CH2CH2CH2) 1.70–1.84 (m CH3CH2CH), 3.40–3.43 (m 1H, NCH), 3.69–3.76 and 4.02–4.09 (m, 2H, NCH2CH2,), 4.44–4.51 (m, 2H, NCH2S), 4.59–4.64 (m, 2H NCH2N), 13C NMR (150 MHz, DMSO-d 6): δ 9.28 (CH3CH2CH), 13.57 (CH3CH2CH2), 19.36 (CH3CH2CH2), 22.31 (CHCH2CH), 28.00 (CH2CH2CH2), 51.10 (NCH2), 55.45 (C-6), 61.74 (NCH), 67.44 (C-4), 172.50 (CO), 190.39 (CS). MS (70 eV): m/z (%) = 276.2 (10) [M]+, 246.2 (25) [M–30]+, 128.0 (45) [M–148]+, 115.0 (100) [M–161]+.
2.2.3 2-(5-(3-methoxypropyl)-6-thioxo-1,3,5-thiadiazinan-3-yl) propionic acid (3)
Compound 3 was synthesized from 3-methoxyropyl amine and β-alanine and obtained as a white solid. Yield: 72%, mp: 98–100°C. 1H NMR (400 MHz, MeOD): δ 1.96–2.02 (m, 2H, CH2CH2CH2), 2.58 (t, 2H, J = 8, NCH2CH2), 3.10 (t, 2H, J = 8, CH2CH2COOH), 3.34 (s, 3H, CH3O), 3.47 (t, 3H, J = 8, OCH2CH2), 4.07 (t, 2H, J = 8, NCH2CH2), 4.49 (s, 2H, NCH2S), 4.50 (s, 2H, NCH2N). 13C NMR (100 MHz, MeOD): δ 28.10 (CH2CH2CH2), 34.26 (CH2COOH), 47.63 (NCH), 51.10 (NCH2), 59.04 (CH3O), 59.14 (C-6), 71.22 (OCH2), 71.82 (C-4), 175.90 (CO), 193.72 (CS). High-resolution mass spectrometry (HRMS) (ESI) m/z: calculated for C10H19N2O3S2 [M+H]+ 279.08371; found 279.08344.
2.2.4 2-(5-(3-methoxypropyl)-6-thioxo-1,3,5-thiadiazinan-3-yl) acetic acid (4)
Compound 4 was acquired by 3-methoxyropyl amine and glycine. Product obtained as white solid. Yield: 71%, mp: 101–103°C. 1H NMR (400 MHz, DMSO-d 6): δ 1.79–1.86 (m, 2H, CH2CH2CH2), 3.22 (s, 2H, NCH2COOH) 3.34 (t, 2H, J = 8, NCH2CH2), 3.53 (s, 3H, CH3O), 3.93 (t, 3H, J = 8, OCH2CH2), 4.51 (s, 2H, NCH2S), 4.52 (s, 2H, NCH2N). 13C NMR (100 MHz, DMSO-d 6): δ 35.63 (CH2CH2CH2), 58.50 (NCH), 60.16 (NCH2), 67.31 (CH3O), 67.48 (C-6), 78.77 (OCH2), 79.24 (C-4), 180.04 (CO), 199.69 (CS). HRMS (ESI) m/z: calculated for C9H17N2O3S2 [M+H]+ 265.06806; found 265.06744.
2.2.5 2-(5-cyclopropyl-6-thioxo-1,3,5-thiadiazinan-3-yl) propionic acid (5)
Compound 5 was acquired by cyclopropyl amine and dl-α-alanine. Product obtained as white solid. Yield: 69%, mp: 123–124°C. 1H NMR (400 MHz, DMSO-d 6): δ 0.78–0.90 (m, 4H, cyclopropyl ring protons), 2.50 (m, 3H, CH3CH), 2.88 (t, 1H, J = 8, CH3CH), 3.07–3.13 (m, 1H, NCH), 4.40 (s, 2H, NCH2S), 4.41 (s, 2H, NCH2N), 12.22 (s, COOH). 13C NMR (100 MHz, DMSO-d 6): δ 7.66 (cyclopropyl ring carbons), 32.29 (CH3CH), 35.06 (NCH), 45.14 (C-6) 56.19 (NCH), 70.22 (C-4), 172.65 (CO), 192.84 (CS). HRMS (ESI) m/z: calculated for C9H15N2O2S2 [M+H]+ 247.05749; found 247.05711.
2.2.6 2-(5-cyclopropyl)-6-thioxo-1,3,5-thiadiazinan-3-yl) acetic acid (6)
Compound 6 was acquired by cyclopropyl amine and glycine. Product obtained as white solid. Yield: 72%, mp: 122–124°C. 1H NMR (400 MHz, DMSO-d 6): δ 0.74–0.88 (m, 4H, cyclopropyl ring protons), 3.04–3.09 (m, 1H, NCH), 3.49 (s, 2H, NCH2COOH), 4.42 (s, 2H, NCH2S), 4.46 (s, 2H, NCH2N), 12.65 (s, COOH). 13C NMR (100 MHz, DMSO-d 6): δ 8.10 (cyclopropyl ring carbons), 35.31 (NCH), 50.63 (NCH2), 57.10 (C-6), 70.44 (C-4), 170.69 (CO), 193.32 (CS). HRMS (ESI) m/z: calculated for C8H13N2O2S2 [M+H]+ 233.04184; found 233.07747.
2.2.7 2-(5-butyl-6-thioxo-1,3,5-thiadiazinan-3-yl) propionic acid (7)
Compound 7 was acquired by n-butyl amine and dl-α-alanine. Product obtained as white solid recrystallized from ethanol. Yield: 68%, mp: 98–100°C, 1H NMR (400 MHz, DMSO-d 6): δ 0.89 (t, 3H, J = 8.0 Hz, CH3CH2), 1.23–1.31 (m, 2H, J = 8.0 Hz, CH3CH2CH2), 1.35 (d, 3H, CH3CH), 1.49–1.63 (m, 2H, CH2CH2CH2), 3.53–3.58 (q, 1H, CH3CH), 3.65–3.72, and 4.08–4.15 (m, 2H, NCH2CH2), 4.44–4.65 (m, 4H, NCH2NCH2S). MS (ES) 262.2, calculated: 262.39. MS (ESI) m/z (%) = 262.2 (12) [M]+, 115.1 (47) [M–147]+, 57.0 (100) [C4H9]+
2.2.8 2-(5-propyl-6-thioxo-1,3,5-thiadiazinan-3-yl) acetic acid (8)
Compound 8 was acquired by propyl amine and glycine. Product obtained as white solid, recrystallized from ethanol. Yield: 64%, mp: 109–111°C. 1H NMR (400 MHz, DMSO-d 6): δ 0.85 (t, 3H, J = 8 Hz, CH3CH2), 1.54–1.63 (m, 2H, CH3CH2CH2), 3.53 (s, 2H, CH2COOH), 3.85 (t, 2H, J = 8 Hz, NCH2CH2,), 4.51 (s, 2H, NCH2S), 4.52 (s, 2H, NCH2N), 12.69 (s, COOH); MS (ESI) m/z (%) = 234.2 (25) [M]+, 147.1 (15) [M–87]+, 101.1 (100) [M–133]+, 42.0 (90) [C3H6]+.
2.2.9 2-(5-butyl-6-thioxo-1,3,5-thiadiazinan-3-yl) acetic acid (9)
Compound 9 was acquired by n-butyl amine and glycine. Product obtained as white solid, recrystallized from ethanol. Yield: 62%, mp: 98–99°C. 1H NMR (400 MHz, MEOD): δ 0.97 (t, J = 8.0 Hz, 3H, CH2CH3), 1.32–1.42 (m, 2H, CH2CH2CH3), 1.61–1.69 (m, 2H, CH2CH2CH2), 3.65 (s, 2H, CH2COOH), 3.99 (t, 2H, J = 8 Hz, NCH2CH2), 4.53 (s, 2H, NCH2S), 4.55 (s, 2H, NCH2N). 13C NMR (100 MHz, MEOD): δ 13.68 (CH3CH2), 20.56 (CH2CH2CH3), 29.16 (CH2CH2CH2), 51.55 (CH2CH2N), 52.47 (CH2COOH), 59.05 (C-6), 70.53 (C-4), 172.49 (CO), 191.47 (CS). HRMS (ESI) m/z: calculated for C9H17N2O2S2 [M+H]+ 249.07314; found 249.07227.
2.2.10 2-(5-hydroxyethyl)-3-phenyl-1,3,5-thiadiazinane-2-thione (10)
Compound 10 was acquired by aniline and ethanolamine. Product obtained as white solid, recrystallized from ethanol. Yield: 61%, mp: 124–126°C. 1H NMR (400 MHz, DMSO-d 6): δ 3.00 (t, 2H, J = 8.0 Hz, CH2CH2OH) 3.63 (t, 2H, J = 8 Hz, NCH2CH2), 4.67 (s, 2H, NCH2S), 4.72 (s, 2H, NCH2N), 7.23–7.47 (m, 5H, Ar–H). 13C NMR (100 MHz, DMSO-d 6): δ 52.11 (NCH2), 58.96 (CH2OH), 59.15 (C-6), 73.61 (C-4), 127.09–129.30 (Ar–CH), 144.40 (Ar–C), 192.95 (CS). HRMS (ESI) m/z: calculated for C11H15N2OS2 [M+H]+ 255.06; found 255.06.
2.2.11 2-(5-phenyl-6-thioxo-1,3,5-thiadiazinan-3-yl) acetic acid (11)
Compound 11 was acquired by aniline and glycine. Product obtained as white solid, recrystallized from ethanol. Yield: 59%, mp: 97–99°C. 1H NMR (400 MHz, DMSO-d 6): δ 3.73 (s, 2H, CH2COOH), 4.65 (s, 2H, NCH2S), 4.71 (s, 2H, NCH2N), 7.15–7.44 (m, 5H, Ar−H). 13C NMR (100 MHz, DMSO-d 6): δ 50.99 (CH2COOH), 58.49 (C-6), 73.39 (C-4), 127.24, 127.85, and 129.61 (Ar−CH), 144.34 (Ar−C), 170.62 (C═O), 192.95 (C═S). HRMS (ESI) m/z: calculated for C11H13N2O2S2 [M+H]+ 269.04184; found 269.10989.
2.2.12 2-(5-phenyl-6-thioxo-1,3,5-thiadiazinan-3-yl) propanoic acid (12)
Compound 12 was acquired by aniline and dl-α-alanine. Product obtained as white solid recrystallized from ethanol. Yield: 63%, mp: 98–100°C. 1H NMR (400 MHz, DMSO-d 6): δ 1.36 (d, 3H, J = 8 Hz, CH3CH), 3.77 (q, 1H, CH3CH), 4.64–4.68 (m, 2H, NCH2S) 5.23–5.36 (m, 2H, NCH2N), 7.11–7.44 (m, 5H, Ar−H). 13C NMR (100 MHz, DMSO- d 6) δ 16.55 (CH3), 55.41(C-6), 69.84 (CH), 71.44 (C-4), 117.70, 127.48, 130.02 (Ar–CH), 144.56 (Ar–C), 174.09 (C═O), 194.02 (C═S). HRMS (ESI) m/z: calculated for C12H15N2O2S2 [M+H]+ 283.05749; found 283.09290.
2.2.13 2-(4-methyl-2-(5-phenyl-6-thioxo-1,3,5-thiadiazinan-3-yl) pentanoic acid (13)
Compound 13 was acquired by aniline and l-leucine. Product obtained as white solid recrystallized from ethanol. Yield: 79%, mp: 92–94°C. 1H NMR (400 MHz, DMSO-d 6): δ 0.85 (dd, 6H, CH3CH), 1.50–1.59 (m, 3H, CH3CHCH3, CHCH2CH), 3.67–3.70 (m, 1H, NCHCH2), 4.63–4.85 (m, 4H, NCH2S, and NCH2N), 7.21–7.44 (m, 5H, Ar−H). 13C NMR (100 MHz, DMSO-d 6): δ 22.33 (CH3CHCH3), 23.73 (CH3CHCH3), 25.27 (CHCH2CH), 56.66 (C-6), 61.27 (NCH), 71.32 (C-4), 127.53, 128.98, and 129.39 (Ar–CH), 144.77 (Ar–C), 173.83 (CO), 193.72 (CS). HRMS (ESI) m/z: calculated for C15H22N2O2S2 [M+2H]+ 326.11227; found 326.16510.
3 Biological activities
3.1 NO assay
The J774.2 (ECACC, UK) cells were obtained from Bio bank facility PCMD, ICCBS, University of Karachi. In IWAKI 75 cc flasks (Asahi Techno Glass, Japan), mouse (J774.2) macrophage cell lines (European Collection of Cell Cultures, UK) were cultured in Dulbecco’s modified eagle medium (DMEM) (Sigma-Aldrich, Steinheim, Germany) with 1% streptomycin/penicillin and 10% fetal bovine serum (FBS) (N.Y. U.S.; GIBCO) and were incubated at 5% CO2 at 37°C. Briefly, 150 µL·well−1 of 1 × 106 cells·mL−1 were added in 96-well flat bottom plates and cells were treated with Escherichia coli lipopolysaccharide (30 µg·mL−1) (DIFCO Laboratories, Michigan, USA) and different concentrations of compounds 1, 10, and 100 (µM). The plate was incubated for 48 h at 37°C in 5% carbon dioxide. After incubation, the collected supernatant was added with Griess reagent to measure the accumulation of nitrite. The plate was read at 540 nm in spectrophotometer [40].
3.2 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) cytotoxicity assay
We employed the standard MTT colorimetric assay to assess the cytotoxicity of prepared compounds against the human cervical cancer HeLa cells (ATCC, Manassas, USA), which were obtained from Biobank facility PCMD and ICCBS. The cells were cultured in DMEM media supplemented with 10% FBS and 1% penicillin and streptomycin at 37°C in 5% CO2 incubator. The 96-well flat-bottom plates were added with 6 × 104 cells·mL−1 and incubated for 24 h to attach cells. Next day, the media was replaced and the test compounds were added in 1, 10, and 100 (µM) concentrations in triplicates and plates were incubated at 37°C in 5% CO2 incubator for 48 h. 50 µL of MTT (0.5 mg·mL−1) was added to each well followed by further incubation of 4 h. Upon aspiration of MTT, DMSO (100 µL) was then added to wells to dissolve formazan crystals. A spectrophotometer (Molecular Devices, Spectra Max plus, CA, USA) was used to measure the reduction of MTT to formazan by reading absorbance at 540 nm. The cytotoxic activity for HeLa cells was expressed as the half maximal inhibitory concentration (IC50), the concentration required to cause 50% growth inhibition.
3.3 Docking studies (methodology)
Docking studies were done using Molecular Operating Environment (2016.0802) [41]. The crystal structure of inducible nitric oxide synthase (iNOS) was taken from Protein Data Bank (PDB). 4NOS was the accession code. Preparation of three-dimensional (3-D) structures of synthesized compounds and the downloaded enzyme was carried out by using our previously reported methods [42,43,44]. Docking rums were carried out by using default parameters. Discovery Studio Visualizer (DS-2021) was used for the analysis of the docking results [45].
4 Results and discussion
The target 3,5-disubstituted-tetrahydro-thiadiazine-2-thiones (1–13) were synthesized according to Scheme 1 by the reaction of a primary amine with carbon disulfide in potassium hydroxide aqueous solution to afford their respective dithiocarbamate potassium salts, which were treated with formaldehyde followed by the addition of different amino acids and ethanolamine in phosphate buffer (pH = 7.8) to cause cyclocondensation of the dithiocarbamate intermediate. The desired products were obtained by lowering the temperature and pH of the reaction mixture.
4.1 Spectral discussion
Structures of the synthesized compounds were confirmed by spectroscopic methods including 1H NMR, 13C NMR, 2D-NMR, mass spectrometry, and HRMS. All the spectral results as detailed in the experimental part are in agreement with the proposed structures assigned to these compounds. Hence, the mass spectra of these compounds showed the correct molecular ions. In addition, the measured HR-MS data are in agreement with the calculated values. Furthermore, DEPT and 2D-NMR including COSY, NOESY, HSQC, and HMBC experiments showed correlations that facilitated in the 1H- and 13C-signal assignments to the different carbons and their attached, and/or neighboring hydrogens. All the functional analogues showed similar pattern in the chemical shifts of 1H and 13C NMR signals, which confirm the common molecular backbone of thiadiazine thione nucleus. 1H NMR spectra of all 1,3,5-thiadiazinethiones showed singlets at 4.40–4.46 ppm, for C-4 and C-6 protons while in case of compounds 1, 2, and 7, multiplet appeared in corresponding regions due to diastereotopicity being prompted by the branched substituent at N-5. Similarly, in the 13C NMR spectra, the (C═S) thiocarbonyl carbon appears in 190–193 ppm range, whereas signals due to C-4 and C-6 of the thiadiazinane-2-thione nucleus appeared at 67–79 and 55–58 ppm, respectively. On the other hand, the carboxylic carbons appeared at 171–173 ppm.
4.2 Cytotoxicity
The antitumor activity of the newly synthesized compounds 1–13 against human cervical cancer HeLa cell line was evaluated by conducting cell viability assay using tetrazolium dye MTT. In this method, cultures of the HeLa cell line were treated with the target compounds and the results are listed in Table 1. Results show that most of the prepared compounds exhibit anticancer activity. Compounds 3, 5, 6, and 9 were the most potent with IC50 values of <4 µM. Compound 10 was inactive whereas the rest of the series revealed significant anticancer activity with IC50 values in the range from 7.2 ± 0.7 to 149.7 ± 11.1 µM (Table 1). A comparison of the compounds anticancer activity against the HeLa cell line indicates that the activity of thiadiazine-2-thione nucleus was markedly affected by N-5 and N-3 substituents. The presence of the phenyl group at N-3 lowers the activity while the cyclopropyl and 3-methoxypropyl bearing moieties were found to be the most potent inhibitors. Similarly, in N-3 butyl substituted series, activity increases. On the other hand, changing the N-5 substituent from bulkier to less bulky groups increases the activity. Furthermore, results showed that activity increases with shorter chains of the acid on N5 (Figure 1).
Cytotoxic and NO inhibition of tested series
| Sample codes | R1 | R2 | Cytotoxicity HeLa cells IC50 ± SD (µM) | NO inhibition IC50 ± SD (µM) |
|---|---|---|---|---|
| 1 | CH3−CH2−CH2−CH2− |
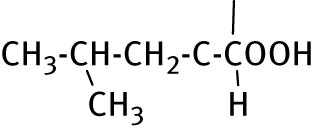
|
22.0 ± 0.3 | ND |
| 2 | CH3−CH2−CH2−CH2− |
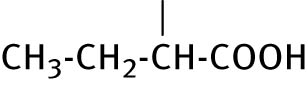
|
13.7 ± 0.4 | ND |
| 3 | CH3O−CH2−CH2−CH2− | −CH2−CH2−COOH | <4 | <0.4 |
| 4 | CH3O−CH2−CH2−CH2− | −CH2−COOH | 7.2 ± 0.7 | <0.4 |
| 5 |
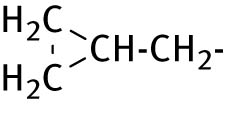
|
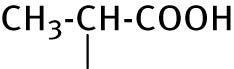
|
<4 | <0.4 |
| 6 |
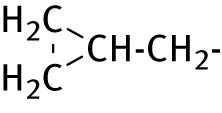
|
−CH2−COOH | <4 | 2.15 |
| 7 | CH3−CH2−CH2−CH2− |
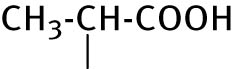
|
14.5 ± 3.0 | ND |
| 8 | CH3−CH2−CH2− | −CH2−COOH | 36.3 ± 3.8 | ND |
| 9 | CH3−CH2−CH2−CH2− | −CH2−COOH | <4 | <0.4 |
| 10 |
|
−CH2−CH2−OH | >400 | 14.9 |
| 11 |
|
−CH2−COOH | 149.7 ± 11.1 | ND |
| 12 |
|
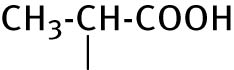
|
38.2 ± 6.0 | <0.4 |
| 13 |
|
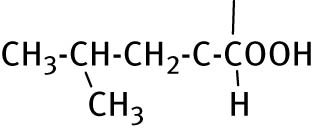
|
55.8 ± 15.1 | ND |
| Doxorubicin | 0.86 ± 0.08 | |||
| l-NMMA | 97.5 ± 3.2 |
l-NMMA = N G monomethyl l-arginine acetate.
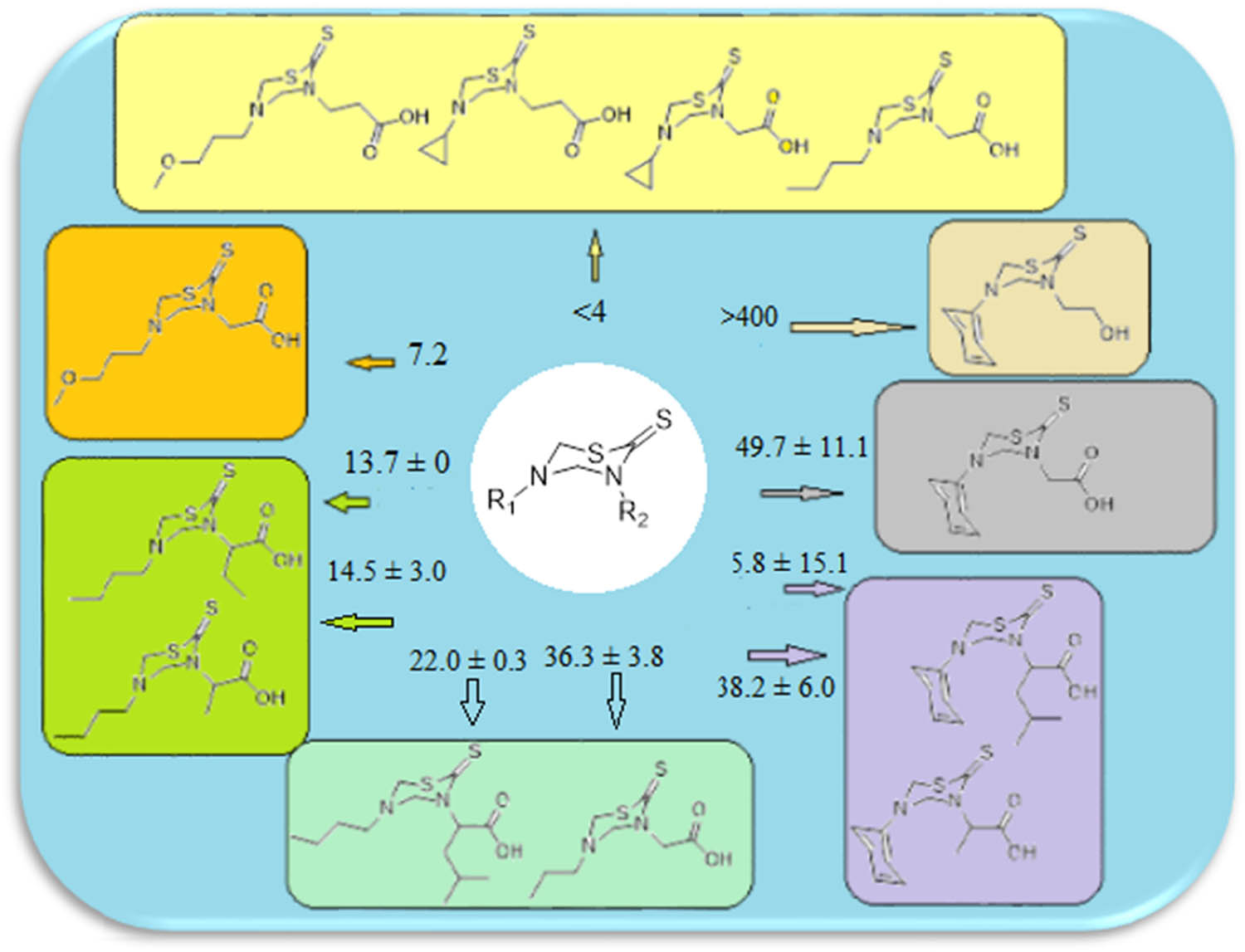
Structural diversity and IC50 – a schematic SAR.
4.3 NO activity
NO activity was assessed using J774.2 mouse macrophage cell line that was cultured in 75 cc flasks. 1, 10, and 100 µM concentration of the test compounds were added to 96-well plate incubated at 37°C in humidified air containing 5% CO2. Nitrite accumulation in grown culture was measured by Griess reagent. All the tested compounds sowed strong NO inhibitory activities with IC50 values ranging from <0.4 to 14.9 µM (Table 1). Compounds 3, 4, 5, 9, and 12 bearing butyric and butanoic acid moiety at N5 exhibit IC50 value <0.4 (µM) while compounds 6 and 10 showed 2.15 and 14.9 µM, IC50 values, respectively. It was observed that among the tested series compounds bearing propionic acid at N-5 were less active as compared to butyric and butanoic acid substituted. No structure–activity relationship observed for N-3 substituents.
4.4 Docking studies
Research findings indicated that the expression of iNOS can be observed in several malignant tumors, such as lung, breast, prostate, and malignant melanoma. We carried out docking simulations on the inhibition of iNOS using the Molecular Operating Environment (MOE 2016.0802) software [44]. The 3-D crystal structure of human iNOS was acquired from PDB with accession code number 4NOS. Docking protocol was validated by using the re-dock method. Native ligand ethylisothiourea (ITU) was re-docked into the binding site. The binding orientation of the re-docked ITU and experimental ITU was analyzed. The computed root-mean square deviation was 1.13 Å, which was found within the threshold limit (<2.0 Å). The superposed results of the re-dock experiment are shown in Figure 2a. The 3-D interaction plot of native ITU showed that it interacts with Trp372 and Glu377 (Figure 2b). We also docked control drug (NO inhibitor) in the binding site of iNOS. Two-dimensional (2-D) interaction plot showed that it interacts with amino acid residues Gly371 and Trp372 via hydrogen bond interactions (Figure 2c).
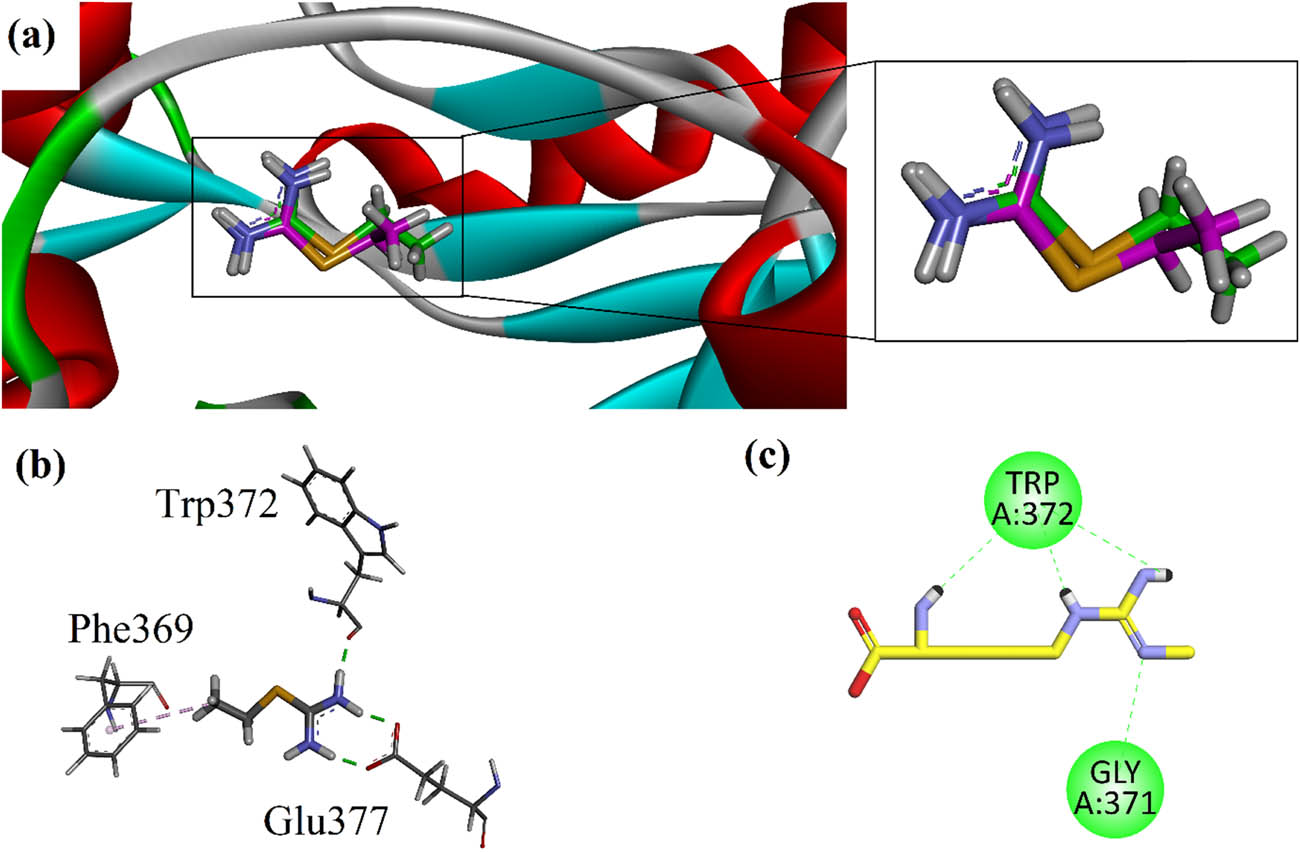
(a) Ribbon superposed model of native ITU (green carbon stick) and re-docked ITU (pink carbon stick); (b) 3-D interaction plots of native ITU in the binding site of iNOS (PDB ID = 4NOS); and (c) 2-D interaction plot of control drug N G monomethyl l-arginine in the binding site of iNOS. These 3-D/2-D interactions plots are modeled by using Discovery Studio Visualizer.
All synthesized compounds were docked into the active site of the human iNOS (PDB ID = 3NOS). The 3-D interaction plots of studied compounds are depicted in Figures 3 and 4. The studied compounds interact with amino acid residues via conventional hydrogen bonds and hydrophobic interactions. Within this context, compound 3 displayed strong hydrogen bonding interaction with Trp372, Ala351, and Glu377. In addition, a π–sulfur and π–alkyl types of interactions with Phe369 were observed (Figure 3a). Similarly, compound 4 exhibited conventional hydrogen bonding interactions with Gly371, Val352, and Ile201 (Figure 3b). On the other hand, compound 5 showed conventional hydrogen bonding interaction with Glu377 along with a bifurcated π–sulfur interaction with Trp372 (Figure 3c). In contrast, compound 6 exhibited conventional hydrogen bonding interactions with Glu377, Ile201, and Cys200 in addition to two π–sulfur interactions with Trp372 and Trp194 (Figure 3d).
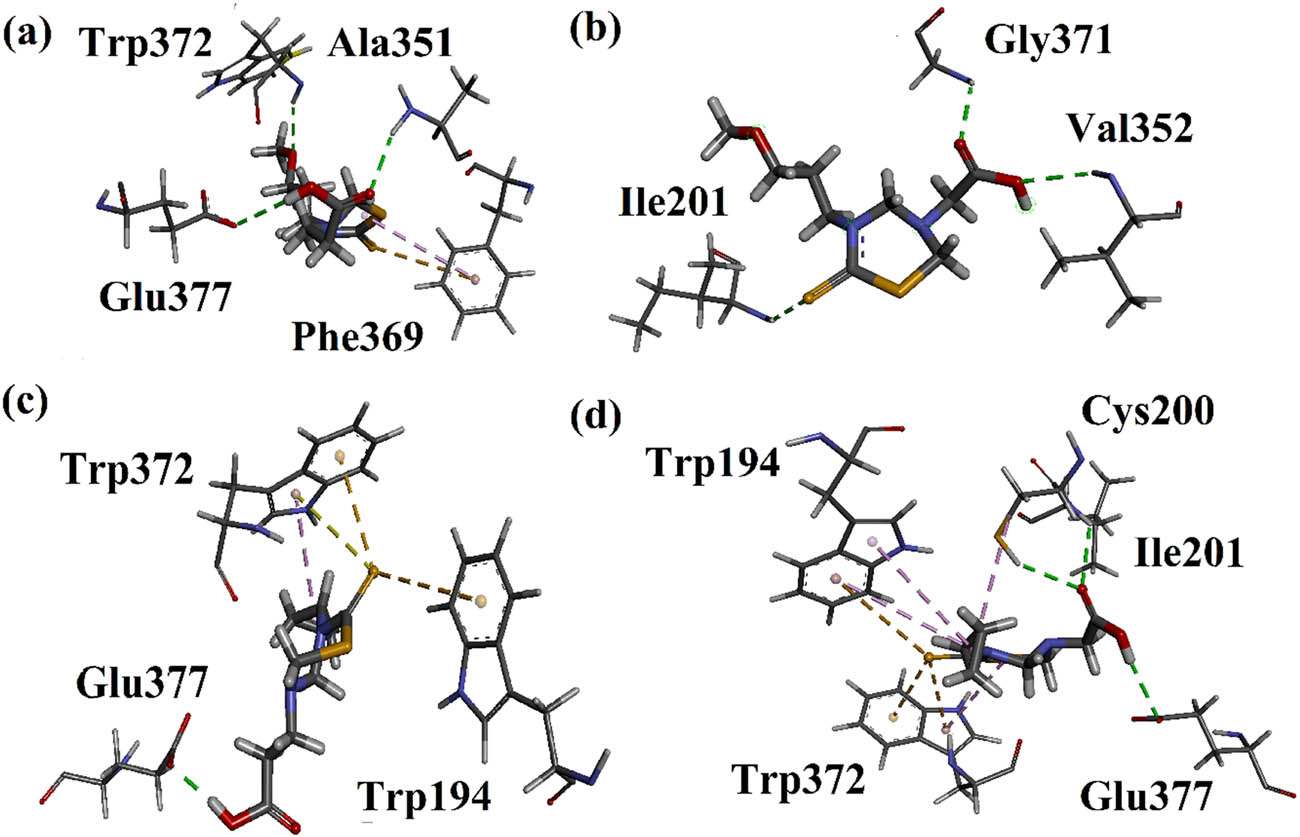
(a–d) 3-D interaction plots of compounds 3–6 modeled by using Discovery Studio Visualizer in the binding site of iNOS (PDB ID = 4NOS).
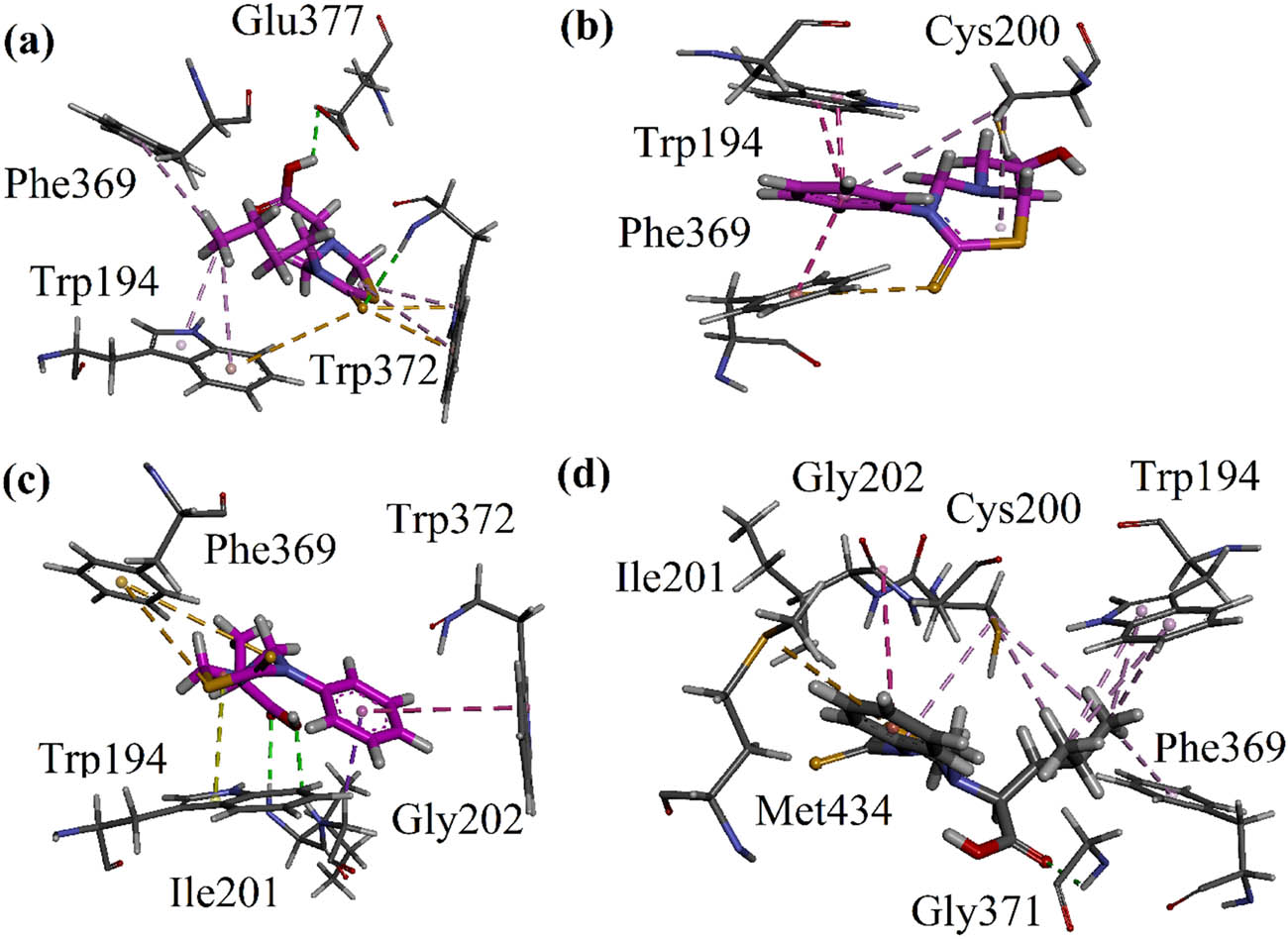
(a–d) 3-D interaction plots of compounds 9, 10, 12, and 13 modeled by using Discovery Studio Visualizer in the binding site of iNOS (PDB ID = 4NOS).
Compound 9 displayed one conventional hydrogen bonding interaction with Trp32 and Glu377, and two π–sulfur interactions with Trp372 and Trp194 as shown in Figure 4a. Compound 10 showed π–π stacked interaction with Trp194 and Phe369, in addition to a π–sulfur interaction with Phe369 (Figure 4b). Similarly, compound 12 showed one conventional hydrogen bonding interaction and π–sigma interaction with Gly202, and hydrogen bond interactions with Ile201. It additionally exhibited two π–sulfur interactions with Phe369 and a π–π stacked interaction with Trp372 (Figure 4c). Similarly, compound 13 exhibited a conventional hydrogen bonding interaction with Gly371, a π–sulfur interaction with Met434, and a π–alkyl interaction with Gly202 (Figure 4d). All the binding energy values in kcal mol−1 and type of interactions are presented in Table 2.
Interacting residues and binding energy of the docked compounds in the binding site of iNOS (PDB ID = 4NOS)
| Interacting residues | |||||
|---|---|---|---|---|---|
| Comp. no. | Hydrogen bond | π–π stack | π–sulfur | π–alkyl/π–σ | Binding energy (kcal·mol−1) |
| 3 | Trp372, Ala351, Glu377 | — | Phe369 | Phe369 | −6.32 |
| 4 | Gly371, Val352, and Ile201 | — | Trp372 | − | −6.04 |
| 5 | Glu377 | — | Trp372 (bifurcated) | — | −6.16 |
| 6 | Glu377, Ile201, and Cys200 | — | Trp372, Trp194 | — | −6.29 |
| 9 | Trp32 and Glu377 | — | Trp372, Trp194 | — | −6.49 |
| 10 | — | Trp194 and Phe369 | Phe369 | — | −4.22 |
| 12 | Ile201 | Trp372 | — | Gly202 | −5.67 |
| 13 | Gly371 | — | Met434 | — | −5.19 |
| Control | Gly371 and Trp372 | — | — | — | −4.05 |
5 Conclusions
In summary, we have synthesized a total of thirteen tetrahydro-2H-1,3,5-thiadiazine-2-thiones and have characterized by different spectroscopic methods. The prepared compounds were screened for their anticancer activity against the HeLa cell line, and NO inhibitory potential. Results revealed that most of the prepared compounds exhibit significant potential. Compounds 3, 5, 6, and 9 were the most potent with IC50 values of <4 µM. In addition, our findings showed that all the screened compounds are strong NO inhibitors (IC50 values range between <0.4 and 14.9 µM). Docking studies related to iNOS showed that the studied compounds interact with the enzyme via strong hydrogen bonds and hydrophobic interactions. These findings could encourage the researchers for further insight in search of their anticancer potential or as leads in the development of anticancer drugs.
Acknowledgment
The authors are thankful to Higher Education Commission of Pakistan for providing fund for a part of this project under project no. NRPU-4165.
-
Funding information: This research project was funded by the Higher Education Commission under project no. NRPU-4165.
-
Author contributions: Haleema Ali synthesized the compounds and wrote paper under the supervision of Rasool Khan; Xiandao Pan and Farzana Shaheen did the NMR and mass analysis; Almas Jabeen did anticancer and nitric oxide activity of synthesized compounds; Abdur Rauf, Muhammad Shah, and Umer Rashid involved in docking analysis of bioactive compounds; Yahya S. Al-Awthan, Omar S. Bahattab, and Mohammed A. Al-Duais did the analysis; and Mohammad S. Mubarak involved in analysis and writing this paper. All authors read the article and approved for submission.
-
Conflict of interest: One of the corresponding authors (Abdur Rauf) is a member of the Editorial Board of Green Processing and Synthesis.
-
Data availability statement: The data associated to this work are a part of PhD thesis and can be obtain from the corresponding authors upon request.
-
Supporting information: The supporting information is available online (see supplementary file).
References
[1] Brisson M, Kim JJ, Canfell K, Drolet M, Gingras G, Burger EA, et al. Impact of HPV vaccination and cervical screening on cervical cancer elimination: A comparative modeling analysis in 78 low-income and lower-middle-income countries. Lancet. 2020;395(10224):575–90.10.1016/S0140-6736(20)30068-4Search in Google Scholar PubMed PubMed Central
[2] Ferlay J, Colombet M, Soerjomataram I, Mathers C, Parkin DM, Piñeros M, et al. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int J Cancer. 2019;144(8):1941–53.10.1002/ijc.31937Search in Google Scholar PubMed
[3] Longley D, Johnston P. Molecular mechanisms of drug resistance. J Pathol. 2005;205(2):275–92.10.1002/path.1706Search in Google Scholar PubMed
[4] Hausser J, Alon U. Tumour heterogeneity and the evolutionary trade-offs of cancer. Nat Rev Cancer. 2020;20(4):247–57.10.1038/s41568-020-0241-6Search in Google Scholar PubMed
[5] Wu Y, Ding Y, Ramprasath T, Zou M. Oxidative stress, GTPCH1, and endothelial nitric oxide synthase uncoupling in hypertension. A Antioxid Redox Signal. 2021;34(9):750–64.10.1089/ars.2020.8112Search in Google Scholar PubMed PubMed Central
[6] Aktan F. iNOS-mediated nitric oxide production and its regulation. Life Sci. 2004;75(6):639–53.10.1016/j.lfs.2003.10.042Search in Google Scholar PubMed
[7] Alderton WK, Cooper CE, Knowles RG. Nitric oxide synthases: structure, function and inhibition. Biochem J. 2001;357(3):593–615.10.1042/bj3570593Search in Google Scholar
[8] Semeraro N, Ammollo CT, Semeraro F, Colucci M. Sepsis-associated disseminated intravascular coagulation and thromboembolic disease. Mediterr J Hematol Infect Dis. 2010;2(3):14.10.4084/mjhid.2010.024Search in Google Scholar
[9] Bogdan C. Nitric oxide and the immune response. Nat Immunol. 2001;2(10):907–16.10.1038/ni1001-907Search in Google Scholar PubMed
[10] Aqil M, Elseth KM, Vesper BJ, Deliu Z, Aydogan B, Xue J, et al. Part I – mechanism of adaptation: high nitric oxide adapted A549 cells show enhanced DNA damage response and activation of antiapoptotic pathways. Tumour Biol. 2014;35(3):2403–15.10.1007/s13277-013-1318-6Search in Google Scholar PubMed
[11] Beevi SS, Rasheed MH, Geetha A. Evidence of oxidative and nitrosative stress in patients with cervical squamous cell carcinoma. Clinica Chim Acta. 2007;375(1–2):119–23.10.1016/j.cca.2006.06.028Search in Google Scholar PubMed
[12] Hiraku Y, Tabata T, Ma N, Murata M, Ding X, Kawanishi S. Nitrative and oxidative DNA damage in cervical intraepithelial neoplasia associated with human papilloma virus infection. Cancer Sci. 2007;98(7):964–72.10.1111/j.1349-7006.2007.00497.xSearch in Google Scholar PubMed
[13] Altıntop MD, Sever B, Çiftçi GA, Kucukoglu K, Özdemir A, Soleimani SS, et al. Synthesis and evaluation of new benzodioxole-based dithiocarbamate derivatives as potential anticancer agents and hCA-I and HCA-II inhibitors. Eur J Med Chem. 2017;125:190–6.10.1016/j.ejmech.2016.09.035Search in Google Scholar PubMed
[14] Alverdi V, Giovagnini L, Marzano C, Seraglia R, Bettio F, Sitran S, et al. Characterization studies and cytotoxicity assays of Pt (II) and Pd (II) dithiocarbamate complexes by means of FT-IR, NMR spectroscopy and mass spectrometry. J Inorg Biochem. 2004;98(6):1117–28.10.1016/j.jinorgbio.2004.03.011Search in Google Scholar PubMed
[15] de Vos D, Ho SY, Tiekink ER. Cytotoxicity profiles for a series of triorganophosphinegold (I) dithiocarbamates and triorganophosphinegold (I) xanthates. Bioinorg Chem. 1970;2(1):1–2.10.1155/S156536330400010XSearch in Google Scholar PubMed PubMed Central
[16] Mansouri-Torshizi H, Saeidifar M, Khosravi F, Divsalar A, Saboury AA, Hassani F. DNA binding and antitumor activity of α-diimineplatinum (II) and palladium (II) dithiocarbamate complexes. Bioinorg Chem. 2011;Article ID 394506. 10.1155/2011/394506.Search in Google Scholar PubMed PubMed Central
[17] Ronconi L, Marzano C, Zanello P, Corsini M, Miolo G, Maccà C, et al. Gold (III) dithiocarbamate derivatives for the treatment of cancer: solution chemistry, DNA binding, and hemolytic properties. J Med Chem. 2006;49(5):1648–57.10.1021/jm0509288Search in Google Scholar PubMed
[18] Crowley E, Rowan NJ, Faller D, Friel AM. Natural and synthetic isothiocyanates possess anticancer potential against liver and prostate cancer in vitro. Anticancer Res. 2019;39(7):3469–85.10.21873/anticanres.13493Search in Google Scholar PubMed
[19] Citi V, Piragine E, Pagnotta E, Ugolini L, Di Cesare Mannelli L, Testai L, et al. Anticancer properties of erucin, an H2S‐releasing isothiocyanate, on human pancreatic adenocarcinoma cells (AsPC‐1). Phytother Res. 2019;33(3):845–55.10.1002/ptr.6278Search in Google Scholar PubMed
[20] Hać A, Brokowska J, Rintz E, Bartkowski M, Węgrzyn G, Herman-Antosiewicz A. Mechanism of selective anticancer activity of isothiocyanates relies on differences in DNA damage repair between cancer and healthy cells. Eur J Nutr. 2019;59(4):1421–32.10.1007/s00394-019-01995-6Search in Google Scholar PubMed PubMed Central
[21] Richa K, Karmaker R, Longkumer N, Das V, Bhuyan PJ, Pal M, et al. Synthesis, in vitro evaluation, molecular docking and DFT studies of some phenyl isothiocyanates as anticancer agents. Anti-Cancer Agents Med Chem. 2019;19(18):2211–22.10.2174/1871520619666190930122137Search in Google Scholar PubMed
[22] Mandrich L, Caputo E. Brassicaceae-derived anticancer agents: Towards a green approach to beat cancer. Nutrients. 2020;12(3):868.10.3390/nu12030868Search in Google Scholar PubMed PubMed Central
[23] Sodvadiya M, Patel H, Mishra A, Nair S. Emerging insights into anticancer chemopreventive activities of nutraceutical moringa oleifera: Molecular mechanisms, signal transduction and in vivo efficacy. Curr Pharmacol Rep. 2020;2:38–51.10.1007/s40495-020-00210-zSearch in Google Scholar
[24] Arumugam A, Ibrahim MD, Kntayya SB, Mohd Ain N, Iori R, Galletti S, et al. Induction of apoptosis by gluconasturtiin-isothiocyanate (GNST-ITC) in human hepatocarcinoma HepG2 cells and human breast adenocarcinoma MCF-7 cells. Molecules. 2020;25(5):1240.10.3390/molecules25051240Search in Google Scholar PubMed PubMed Central
[25] El-Shorbagi AN, El-Naggar M, Tarazi H, Chaudhary S, Abdu-Allah H, Hersi F, et al. Bis-(5-substituted-2-thiono-1, 3, 5-thiadiazinan-3-yl) butane as a scaffold of anti-proliferative activity, blended by a multicomponent process. Med Chem Res. 2018;27(4):1103–10.10.1007/s00044-018-2133-9Search in Google Scholar
[26] Radwan AA, Aboul-Fadl T, Al-Dhfyan A, Abdel-Mageeda WM. Synthesis and Characterization of bis-3, 5-disubstituted thiadiazine-2-thione derivatives as anticancer agents. Asian J Chem. 2014;26(23):8145.10.14233/ajchem.2014.17636Search in Google Scholar
[27] Radwan AA, Al-Dhfyan A, Abdel-Hamid MK, Al-Badr AA, Aboul-Fadl T. 3, 5-Disubstituted thiadiazine-2-thiones: new cell-cycle inhibitors. Arch Pharm Res. 2012;35(1):35–49.10.1007/s12272-012-0104-0Search in Google Scholar PubMed
[28] Carrasco R, Padrón JA, Pérez R, Rodríguez H, Suárez M, Ochoa C. Quantitative structure antitumoral-activity relationships of thiadiazinthione derivatives using the novel hybrid molecular index. J Pharm Pharm Sci. 2005;8(3):586–92.Search in Google Scholar
[29] Pérez R, Rodríguez H, Pérez E, Suárez M, Reyes O, González LJ, et al. Study on the decomposition products of thiadiazinthione and their anticancer properties. Arzneimittelforschung. 2000;50(09):854–7.10.1055/s-0031-1300301Search in Google Scholar PubMed
[30] Arshad N, Hashim J, Minhas MA, Aslam J, Ashraf T, Hamid SZ, et al. New series of 3, 5-disubstituted tetrahydro-2H-1, 3, 5-thiadiazine thione (THTT) derivatives: synthesis and potent antileishmanial activity. Bioorg Med Chem Lett. 2018;28(19):3251–4.10.1016/j.bmcl.2018.07.045Search in Google Scholar PubMed
[31] Mao L, Jiang H, Wang Q, Yan D, Cao A. Efficacy of soil fumigation with dazomet for controlling ginger bacterial wilt (Ralstonia solanacearum) in China. Crop Prot. 2017;100:111–6.10.1016/j.cropro.2017.06.013Search in Google Scholar
[32] Coro J, Pérez R, Rodríguez H, Suárez M, Vega C, Rolón M, et al. Synthesis and antiprotozoan evaluation of new alkyl-linked bis (2-thioxo-[1, 3, 5] thiadiazinan-3-yl) carboxylic acids. Bioorg Med Chem Lett. 2005;13(10):3413–21.10.1016/j.bmc.2005.03.009Search in Google Scholar PubMed
[33] Coro J, Atherton R, Little S, Wharton H, Yardley V, Alvarez A, Jr, et al. Alkyl-linked bis-THTT derivatives as potent in vitro trypanocidal agents. Bioorg Med Chem Lett. 2006;16(5):1312–5.10.1016/j.bmcl.2005.11.060Search in Google Scholar PubMed
[34] Vicentini CB, Forlani G, Manfrini M, Romagnoli C, Mares D. Development of new fungicides against Magnaporthe grisea: synthesis and biological activity of pyrazolo [3, 4-d][1, 3] thiazine, pyrazolo [1, 5-c][1, 3, 5] thiadiazine, and and pyrazolo [3, 4-d] pyrimidine derivatives. J Agric Food Chem. 2002;50(17):4839–45.10.1021/jf0202436Search in Google Scholar PubMed
[35] Vicentini CB, Guccione S, Giurato L, Ciaccio R, Mares D, Forlani G. Pyrazole derivatives as photosynthetic electron transport inhibitors: New leads and structure− activity relationship. J Agric Food Chem. 2005;53(10):3848–55.10.1021/jf0500029Search in Google Scholar PubMed
[36] Katiyar D, Tiwari VK, Tripathi RP, Srivastava A, Chaturvedi V, Srivastava R, et al. Synthesis and antimycobacterial activity of 3, 5-disubstituted thiadiazine thiones. Bioorg Med Chem Lett. 2003;11(20):4369–75.10.1016/S0968-0896(03)00480-2Search in Google Scholar
[37] Semreen MH, El-Shorbagi AN, Al-Tel TH, Alsalahat IM. Targeting γ-aminobutyric acid (GABA) carriers to the brain: potential relevance as antiepileptic pro-drugs. Med Chem. 2010;6(3):144–9.10.2174/1573406411006030144Search in Google Scholar PubMed
[38] Ji X, Zhong Z, Chen X, Xing R, Liu S, Wang L, Li P. Preparation of 1, 3, 5-thiadiazine-2-thione derivatives of chitosan and their potential antioxidant activity in vitro. Bioorg Med Chem Lett. 2007;17(15):4275–9.10.1016/j.bmcl.2007.05.020Search in Google Scholar PubMed
[39] Schade W A, Rieche. Synthetische Senfölbildner, VII. Untersuchungen zum Wirkungsmechanismus der 2-Thion-tetrahydro-1,3,5-thiadiazine. Arch der Pharmazie. 1966;299(7):589–95.10.1002/ardp.19662990703Search in Google Scholar
[40] Grisham MB, Johnson GG, Lancaster JR, Jr. Quantitation of nitrate and nitrite in extracellular fluids, in Methods in enzymology. Academic Press. Elsevier; 1996. p. 237–46.10.1016/S0076-6879(96)68026-4Search in Google Scholar
[41] Molecular Operating Environment (MOE), 2016.0208 Chemical Computing Group ULC, 1010 Sherbooke St. West, Suite #910, Montreal, QC, Canada, H3A 2R7; 2016.Search in Google Scholar
[42] Sadiq A, MahnashimMH, Alyami BA, Alqahtani YS, Alqarni AO, Rashid U. Tailoring the substitution pattern of Pyrrolidine-2, 5-dione for discovery of new structural template for dual COX/LOX inhibition. Bioorg Chem. 2021;112:104969.10.1016/j.bioorg.2021.104969Search in Google Scholar PubMed
[43] Nadeem MS, Khan JA, Rashid U. Fluoxetine and sertraline based multitarget inhibitors of cholinesterases and monoamine oxidase-A/B for the treatment of Alzheimer’s disease: Synthesis, pharmacology and molecular modeling studies. Int J Biol Macromol. 2021;193:19–26.10.1016/j.ijbiomac.2021.10.102Search in Google Scholar PubMed
[44] Javed MA, Ashraf N, Saeed Jan M, Mahnashi MH, Alqahtani YS, Alyami BA, et al. Structural modification, in vitro, in vivo, ex vivo, and in silico exploration of pyrimidine and pyrrolidine cores for targeting enzymes associated with neuroinflammation and cholinergic deficit in Alzheimer’s disease. ACS Chemical Neuroscience. 2021;12(21):4123–43.10.1021/acschemneuro.1c00507Search in Google Scholar PubMed
[45] Biovia. Dassault Systèmes, Discovery Stuio Visualizer (DS-2021). San Diego: Dassault Systèmes.Search in Google Scholar
© 2023 the author(s), published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Research Articles
- Value-added utilization of coal fly ash and recycled polyvinyl chloride in door or window sub-frame composites
- High removal efficiency of volatile phenol from coking wastewater using coal gasification slag via optimized adsorption and multi-grade batch process
- Evolution of surface morphology and properties of diamond films by hydrogen plasma etching
- Removal efficiency of dibenzofuran using CuZn-zeolitic imidazole frameworks as a catalyst and adsorbent
- Rapid and efficient microwave-assisted extraction of Caesalpinia sappan Linn. heartwood and subsequent synthesis of gold nanoparticles
- The catalytic characteristics of 2-methylnaphthalene acylation with AlCl3 immobilized on Hβ as Lewis acid catalyst
- Biodegradation of synthetic PVP biofilms using natural materials and nanoparticles
- Rutin-loaded selenium nanoparticles modulated the redox status, inflammatory, and apoptotic pathways associated with pentylenetetrazole-induced epilepsy in mice
- Optimization of apigenin nanoparticles prepared by planetary ball milling: In vitro and in vivo studies
- Synthesis and characterization of silver nanoparticles using Origanum onites leaves: Cytotoxic, apoptotic, and necrotic effects on Capan-1, L929, and Caco-2 cell lines
- Exergy analysis of a conceptual CO2 capture process with an amine-based DES
- Construction of fluorescence system of felodipine–tetracyanovinyl–2,2′-bipyridine complex
- Excellent photocatalytic degradation of rhodamine B over Bi2O3 supported on Zn-MOF nanocomposites under visible light
- Optimization-based control strategy for a large-scale polyhydroxyalkanoates production in a fed-batch bioreactor using a coupled PDE–ODE system
- Effectiveness of pH and amount of Artemia urumiana extract on physical, chemical, and biological attributes of UV-fabricated biogold nanoparticles
- Geranium leaf-mediated synthesis of silver nanoparticles and their transcriptomic effects on Candida albicans
- Synthesis, characterization, anticancer, anti-inflammatory activities, and docking studies of 3,5-disubstituted thiadiazine-2-thiones
- Synthesis and stability of phospholipid-encapsulated nano-selenium
- Putative anti-proliferative effect of Indian mustard (Brassica juncea) seed and its nano-formulation
- Enrichment of low-grade phosphorites by the selective leaching method
- Electrochemical analysis of the dissolution of gold in a copper–ethylenediamine–thiosulfate system
- Characterisation of carbonate lake sediments as a potential filler for polymer composites
- Evaluation of nano-selenium biofortification characteristics of alfalfa (Medicago sativa L.)
- Quality of oil extracted by cold press from Nigella sativa seeds incorporated with rosemary extracts and pretreated by microwaves
- Heteropolyacid-loaded MOF-derived mesoporous zirconia catalyst for chemical degradation of rhodamine B
- Recovery of critical metals from carbonatite-type mineral wastes: Geochemical modeling investigation of (bio)hydrometallurgical leaching of REEs
- Photocatalytic properties of ZnFe-mixed oxides synthesized via a simple route for water remediation
- Attenuation of di(2-ethylhexyl)phthalate-induced hepatic and renal toxicity by naringin nanoparticles in a rat model
- Novel in situ synthesis of quaternary core–shell metallic sulfide nanocomposites for degradation of organic dyes and hydrogen production
- Microfluidic steam-based synthesis of luminescent carbon quantum dots as sensing probes for nitrite detection
- Transformation of eggshell waste to egg white protein solution, calcium chloride dihydrate, and eggshell membrane powder
- Preparation of Zr-MOFs for the adsorption of doxycycline hydrochloride from wastewater
- Green nanoarchitectonics of the silver nanocrystal potential for treating malaria and their cytotoxic effects on the kidney Vero cell line
- Carbon emissions analysis of producing modified asphalt with natural asphalt
- An efficient and green synthesis of 2-phenylquinazolin-4(3H)-ones via t-BuONa-mediated oxidative condensation of 2-aminobenzamides and benzyl alcohols under solvent- and transition metal-free conditions
- Chitosan nanoparticles loaded with mesosulfuron methyl and mesosulfuron methyl + florasulam + MCPA isooctyl to manage weeds of wheat (Triticum aestivum L.)
- Synergism between lignite and high-sulfur petroleum coke in CO2 gasification
- Facile aqueous synthesis of ZnCuInS/ZnS–ZnS QDs with enhanced photoluminescence lifetime for selective detection of Cu(ii) ions
- Rapid synthesis of copper nanoparticles using Nepeta cataria leaves: An eco-friendly management of disease-causing vectors and bacterial pathogens
- Study on the photoelectrocatalytic activity of reduced TiO2 nanotube films for removal of methyl orange
- Development of a fuzzy logic model for the prediction of spark-ignition engine performance and emission for gasoline–ethanol blends
- Micro-impact-induced mechano-chemical synthesis of organic precursors from FeC/FeN and carbonates/nitrates in water and its extension to nucleobases
- Green synthesis of strontium-doped tin dioxide (SrSnO2) nanoparticles using the Mahonia bealei leaf extract and evaluation of their anticancer and antimicrobial activities
- A study on the larvicidal and adulticidal potential of Cladostepus spongiosus macroalgae and green-fabricated silver nanoparticles against mosquito vectors
- Catalysts based on nickel salt heteropolytungstates for selective oxidation of diphenyl sulfide
- Powerful antibacterial nanocomposites from Corallina officinalis-mediated nanometals and chitosan nanoparticles against fish-borne pathogens
- Removal behavior of Zn and alkalis from blast furnace dust in pre-reduction sinter process
- Environmentally friendly synthesis and computational studies of novel class of acridinedione integrated spirothiopyrrolizidines/indolizidines
- The mechanisms of inhibition and lubrication of clean fracturing flowback fluids in water-based drilling fluids
- Adsorption/desorption performance of cellulose membrane for Pb(ii)
- A one-pot, multicomponent tandem synthesis of fused polycyclic pyrrolo[3,2-c]quinolinone/pyrrolizino[2,3-c]quinolinone hybrid heterocycles via environmentally benign solid state melt reaction
- Green synthesis of silver nanoparticles using durian rind extract and optical characteristics of surface plasmon resonance-based optical sensor for the detection of hydrogen peroxide
- Electrochemical analysis of copper-EDTA-ammonia-gold thiosulfate dissolution system
- Characterization of bio-oil production by microwave pyrolysis from cashew nut shells and Cassia fistula pods
- Green synthesis methods and characterization of bacterial cellulose/silver nanoparticle composites
- Photocatalytic research performance of zinc oxide/graphite phase carbon nitride catalyst and its application in environment
- Effect of phytogenic iron nanoparticles on the bio-fortification of wheat varieties
- In vitro anti-cancer and antimicrobial effects of manganese oxide nanoparticles synthesized using the Glycyrrhiza uralensis leaf extract on breast cancer cell lines
- Preparation of Pd/Ce(F)-MCM-48 catalysts and their catalytic performance of n-heptane isomerization
- Green “one-pot” fluorescent bis-indolizine synthesis with whole-cell plant biocatalysis
- Silica-titania mesoporous silicas of MCM-41 type as effective catalysts and photocatalysts for selective oxidation of diphenyl sulfide by H2O2
- Biosynthesis of zinc oxide nanoparticles from molted feathers of Pavo cristatus and their antibiofilm and anticancer activities
- Clean preparation of rutile from Ti-containing mixed molten slag by CO2 oxidation
- Synthesis and characterization of Pluronic F-127-coated titanium dioxide nanoparticles synthesized from extracts of Atractylodes macrocephala leaf for antioxidant, antimicrobial, and anticancer properties
- Effect of pretreatment with alkali on the anaerobic digestion characteristics of kitchen waste and analysis of microbial diversity
- Ameliorated antimicrobial, antioxidant, and anticancer properties by Plectranthus vettiveroides root extract-mediated green synthesis of chitosan nanoparticles
- Microwave-accelerated pretreatment technique in green extraction of oil and bioactive compounds from camelina seeds: Effectiveness and characterization
- Studies on the extraction performance of phorate by aptamer-functionalized magnetic nanoparticles in plasma samples
- Investigation of structural properties and antibacterial activity of AgO nanoparticle extract from Solanum nigrum/Mentha leaf extracts by green synthesis method
- Green fabrication of chitosan from marine crustaceans and mushroom waste: Toward sustainable resource utilization
- Synthesis, characterization, and evaluation of nanoparticles of clodinofop propargyl and fenoxaprop-P-ethyl on weed control, growth, and yield of wheat (Triticum aestivum L.)
- The enhanced adsorption properties of phosphorus from aqueous solutions using lanthanum modified synthetic zeolites
- Separation of graphene oxides of different sizes by multi-layer dialysis and anti-friction and lubrication performance
- Visible-light-assisted base-catalyzed, one-pot synthesis of highly functionalized cinnolines
- The experimental study on the air oxidation of 5-hydroxymethylfurfural to 2,5-furandicarboxylic acid with Co–Mn–Br system
- Highly efficient removal of tetracycline and methyl violet 2B from aqueous solution using the bimetallic FeZn-ZIFs catalyst
- A thermo-tolerant cellulase enzyme produced by Bacillus amyloliquefaciens M7, an insight into synthesis, optimization, characterization, and bio-polishing activity
- Exploration of ketone derivatives of succinimide for their antidiabetic potential: In vitro and in vivo approaches
- Ultrasound-assisted green synthesis and in silico study of 6-(4-(butylamino)-6-(diethylamino)-1,3,5-triazin-2-yl)oxypyridazine derivatives
- A study of the anticancer potential of Pluronic F-127 encapsulated Fe2O3 nanoparticles derived from Berberis vulgaris extract
- Biogenic synthesis of silver nanoparticles using Consolida orientalis flowers: Identification, catalytic degradation, and biological effect
- Initial assessment of the presence of plastic waste in some coastal mangrove forests in Vietnam
- Adsorption synergy electrocatalytic degradation of phenol by active oxygen-containing species generated in Co-coal based cathode and graphite anode
- Antibacterial, antifungal, antioxidant, and cytotoxicity activities of the aqueous extract of Syzygium aromaticum-mediated synthesized novel silver nanoparticles
- Synthesis of a silica matrix with ZnO nanoparticles for the fabrication of a recyclable photodegradation system to eliminate methylene blue dye
- Natural polymer fillers instead of dye and pigments: Pumice and scoria in PDMS fluid and elastomer composites
- Study on the preparation of glycerylphosphorylcholine by transesterification under supported sodium methoxide
- Wireless network handheld terminal-based green ecological sustainable design evaluation system: Improved data communication and reduced packet loss rate
- The optimization of hydrogel strength from cassava starch using oxidized sucrose as a crosslinking agent
- Green synthesis of silver nanoparticles using Saccharum officinarum leaf extract for antiviral paint
- Study on the reliability of nano-silver-coated tin solder joints for flip chips
- Environmentally sustainable analytical quality by design aided RP-HPLC method for the estimation of brilliant blue in commercial food samples employing a green-ultrasound-assisted extraction technique
- Anticancer and antimicrobial potential of zinc/sodium alginate/polyethylene glycol/d-pinitol nanocomposites against osteosarcoma MG-63 cells
- Nanoporous carbon@CoFe2O4 nanocomposite as a green absorbent for the adsorptive removal of Hg(ii) from aqueous solutions
- Characterization of silver sulfide nanoparticles from actinobacterial strain (M10A62) and its toxicity against lepidopteran and dipterans insect species
- Phyto-fabrication and characterization of silver nanoparticles using Withania somnifera: Investigating antioxidant potential
- Effect of e-waste nanofillers on the mechanical, thermal, and wear properties of epoxy-blend sisal woven fiber-reinforced composites
- Magnesium nanohydroxide (2D brucite) as a host matrix for thymol and carvacrol: Synthesis, characterization, and inhibition of foodborne pathogens
- Synergistic inhibitive effect of a hybrid zinc oxide-benzalkonium chloride composite on the corrosion of carbon steel in a sulfuric acidic solution
- Review Articles
- Role and the importance of green approach in biosynthesis of nanopropolis and effectiveness of propolis in the treatment of COVID-19 pandemic
- Gum tragacanth-mediated synthesis of metal nanoparticles, characterization, and their applications as a bactericide, catalyst, antioxidant, and peroxidase mimic
- Green-processed nano-biocomposite (ZnO–TiO2): Potential candidates for biomedical applications
- Reaction mechanisms in microwave-assisted lignin depolymerisation in hydrogen-donating solvents
- Recent progress on non-noble metal catalysts for the deoxydehydration of biomass-derived oxygenates
- Rapid Communication
- Phosphorus removal by iron–carbon microelectrolysis: A new way to achieve phosphorus recovery
- Special Issue: Biomolecules-derived synthesis of nanomaterials for environmental and biological applications (Guest Editors: Arpita Roy and Fernanda Maria Policarpo Tonelli)
- Biomolecules-derived synthesis of nanomaterials for environmental and biological applications
- Nano-encapsulated tanshinone IIA in PLGA-PEG-COOH inhibits apoptosis and inflammation in cerebral ischemia/reperfusion injury
- Green fabrication of silver nanoparticles using Melia azedarach ripened fruit extract, their characterization, and biological properties
- Green-synthesized nanoparticles and their therapeutic applications: A review
- Antioxidant, antibacterial, and cytotoxicity potential of synthesized silver nanoparticles from the Cassia alata leaf aqueous extract
- Green synthesis of silver nanoparticles using Callisia fragrans leaf extract and its anticancer activity against MCF-7, HepG2, KB, LU-1, and MKN-7 cell lines
- Algae-based green AgNPs, AuNPs, and FeNPs as potential nanoremediators
- Green synthesis of Kickxia elatine-induced silver nanoparticles and their role as anti-acetylcholinesterase in the treatment of Alzheimer’s disease
- Phytocrystallization of silver nanoparticles using Cassia alata flower extract for effective control of fungal skin pathogens
- Antibacterial wound dressing with hydrogel from chitosan and polyvinyl alcohol from the red cabbage extract loaded with silver nanoparticles
- Leveraging of mycogenic copper oxide nanostructures for disease management of Alternaria blight of Brassica juncea
- Nanoscale molecular reactions in microbiological medicines in modern medical applications
- Synthesis and characterization of ZnO/β-cyclodextrin/nicotinic acid nanocomposite and its biological and environmental application
- Green synthesis of silver nanoparticles via Taxus wallichiana Zucc. plant-derived Taxol: Novel utilization as anticancer, antioxidation, anti-inflammation, and antiurolithic potential
- Recyclability and catalytic characteristics of copper oxide nanoparticles derived from bougainvillea plant flower extract for biomedical application
- Phytofabrication, characterization, and evaluation of novel bioinspired selenium–iron (Se–Fe) nanocomposites using Allium sativum extract for bio-potential applications
- Erratum
- Erratum to “Synthesis, characterization, and evaluation of nanoparticles of clodinofop propargyl and fenoxaprop-P-ethyl on weed control, growth, and yield of wheat (Triticum aestivum L.)”
Articles in the same Issue
- Research Articles
- Value-added utilization of coal fly ash and recycled polyvinyl chloride in door or window sub-frame composites
- High removal efficiency of volatile phenol from coking wastewater using coal gasification slag via optimized adsorption and multi-grade batch process
- Evolution of surface morphology and properties of diamond films by hydrogen plasma etching
- Removal efficiency of dibenzofuran using CuZn-zeolitic imidazole frameworks as a catalyst and adsorbent
- Rapid and efficient microwave-assisted extraction of Caesalpinia sappan Linn. heartwood and subsequent synthesis of gold nanoparticles
- The catalytic characteristics of 2-methylnaphthalene acylation with AlCl3 immobilized on Hβ as Lewis acid catalyst
- Biodegradation of synthetic PVP biofilms using natural materials and nanoparticles
- Rutin-loaded selenium nanoparticles modulated the redox status, inflammatory, and apoptotic pathways associated with pentylenetetrazole-induced epilepsy in mice
- Optimization of apigenin nanoparticles prepared by planetary ball milling: In vitro and in vivo studies
- Synthesis and characterization of silver nanoparticles using Origanum onites leaves: Cytotoxic, apoptotic, and necrotic effects on Capan-1, L929, and Caco-2 cell lines
- Exergy analysis of a conceptual CO2 capture process with an amine-based DES
- Construction of fluorescence system of felodipine–tetracyanovinyl–2,2′-bipyridine complex
- Excellent photocatalytic degradation of rhodamine B over Bi2O3 supported on Zn-MOF nanocomposites under visible light
- Optimization-based control strategy for a large-scale polyhydroxyalkanoates production in a fed-batch bioreactor using a coupled PDE–ODE system
- Effectiveness of pH and amount of Artemia urumiana extract on physical, chemical, and biological attributes of UV-fabricated biogold nanoparticles
- Geranium leaf-mediated synthesis of silver nanoparticles and their transcriptomic effects on Candida albicans
- Synthesis, characterization, anticancer, anti-inflammatory activities, and docking studies of 3,5-disubstituted thiadiazine-2-thiones
- Synthesis and stability of phospholipid-encapsulated nano-selenium
- Putative anti-proliferative effect of Indian mustard (Brassica juncea) seed and its nano-formulation
- Enrichment of low-grade phosphorites by the selective leaching method
- Electrochemical analysis of the dissolution of gold in a copper–ethylenediamine–thiosulfate system
- Characterisation of carbonate lake sediments as a potential filler for polymer composites
- Evaluation of nano-selenium biofortification characteristics of alfalfa (Medicago sativa L.)
- Quality of oil extracted by cold press from Nigella sativa seeds incorporated with rosemary extracts and pretreated by microwaves
- Heteropolyacid-loaded MOF-derived mesoporous zirconia catalyst for chemical degradation of rhodamine B
- Recovery of critical metals from carbonatite-type mineral wastes: Geochemical modeling investigation of (bio)hydrometallurgical leaching of REEs
- Photocatalytic properties of ZnFe-mixed oxides synthesized via a simple route for water remediation
- Attenuation of di(2-ethylhexyl)phthalate-induced hepatic and renal toxicity by naringin nanoparticles in a rat model
- Novel in situ synthesis of quaternary core–shell metallic sulfide nanocomposites for degradation of organic dyes and hydrogen production
- Microfluidic steam-based synthesis of luminescent carbon quantum dots as sensing probes for nitrite detection
- Transformation of eggshell waste to egg white protein solution, calcium chloride dihydrate, and eggshell membrane powder
- Preparation of Zr-MOFs for the adsorption of doxycycline hydrochloride from wastewater
- Green nanoarchitectonics of the silver nanocrystal potential for treating malaria and their cytotoxic effects on the kidney Vero cell line
- Carbon emissions analysis of producing modified asphalt with natural asphalt
- An efficient and green synthesis of 2-phenylquinazolin-4(3H)-ones via t-BuONa-mediated oxidative condensation of 2-aminobenzamides and benzyl alcohols under solvent- and transition metal-free conditions
- Chitosan nanoparticles loaded with mesosulfuron methyl and mesosulfuron methyl + florasulam + MCPA isooctyl to manage weeds of wheat (Triticum aestivum L.)
- Synergism between lignite and high-sulfur petroleum coke in CO2 gasification
- Facile aqueous synthesis of ZnCuInS/ZnS–ZnS QDs with enhanced photoluminescence lifetime for selective detection of Cu(ii) ions
- Rapid synthesis of copper nanoparticles using Nepeta cataria leaves: An eco-friendly management of disease-causing vectors and bacterial pathogens
- Study on the photoelectrocatalytic activity of reduced TiO2 nanotube films for removal of methyl orange
- Development of a fuzzy logic model for the prediction of spark-ignition engine performance and emission for gasoline–ethanol blends
- Micro-impact-induced mechano-chemical synthesis of organic precursors from FeC/FeN and carbonates/nitrates in water and its extension to nucleobases
- Green synthesis of strontium-doped tin dioxide (SrSnO2) nanoparticles using the Mahonia bealei leaf extract and evaluation of their anticancer and antimicrobial activities
- A study on the larvicidal and adulticidal potential of Cladostepus spongiosus macroalgae and green-fabricated silver nanoparticles against mosquito vectors
- Catalysts based on nickel salt heteropolytungstates for selective oxidation of diphenyl sulfide
- Powerful antibacterial nanocomposites from Corallina officinalis-mediated nanometals and chitosan nanoparticles against fish-borne pathogens
- Removal behavior of Zn and alkalis from blast furnace dust in pre-reduction sinter process
- Environmentally friendly synthesis and computational studies of novel class of acridinedione integrated spirothiopyrrolizidines/indolizidines
- The mechanisms of inhibition and lubrication of clean fracturing flowback fluids in water-based drilling fluids
- Adsorption/desorption performance of cellulose membrane for Pb(ii)
- A one-pot, multicomponent tandem synthesis of fused polycyclic pyrrolo[3,2-c]quinolinone/pyrrolizino[2,3-c]quinolinone hybrid heterocycles via environmentally benign solid state melt reaction
- Green synthesis of silver nanoparticles using durian rind extract and optical characteristics of surface plasmon resonance-based optical sensor for the detection of hydrogen peroxide
- Electrochemical analysis of copper-EDTA-ammonia-gold thiosulfate dissolution system
- Characterization of bio-oil production by microwave pyrolysis from cashew nut shells and Cassia fistula pods
- Green synthesis methods and characterization of bacterial cellulose/silver nanoparticle composites
- Photocatalytic research performance of zinc oxide/graphite phase carbon nitride catalyst and its application in environment
- Effect of phytogenic iron nanoparticles on the bio-fortification of wheat varieties
- In vitro anti-cancer and antimicrobial effects of manganese oxide nanoparticles synthesized using the Glycyrrhiza uralensis leaf extract on breast cancer cell lines
- Preparation of Pd/Ce(F)-MCM-48 catalysts and their catalytic performance of n-heptane isomerization
- Green “one-pot” fluorescent bis-indolizine synthesis with whole-cell plant biocatalysis
- Silica-titania mesoporous silicas of MCM-41 type as effective catalysts and photocatalysts for selective oxidation of diphenyl sulfide by H2O2
- Biosynthesis of zinc oxide nanoparticles from molted feathers of Pavo cristatus and their antibiofilm and anticancer activities
- Clean preparation of rutile from Ti-containing mixed molten slag by CO2 oxidation
- Synthesis and characterization of Pluronic F-127-coated titanium dioxide nanoparticles synthesized from extracts of Atractylodes macrocephala leaf for antioxidant, antimicrobial, and anticancer properties
- Effect of pretreatment with alkali on the anaerobic digestion characteristics of kitchen waste and analysis of microbial diversity
- Ameliorated antimicrobial, antioxidant, and anticancer properties by Plectranthus vettiveroides root extract-mediated green synthesis of chitosan nanoparticles
- Microwave-accelerated pretreatment technique in green extraction of oil and bioactive compounds from camelina seeds: Effectiveness and characterization
- Studies on the extraction performance of phorate by aptamer-functionalized magnetic nanoparticles in plasma samples
- Investigation of structural properties and antibacterial activity of AgO nanoparticle extract from Solanum nigrum/Mentha leaf extracts by green synthesis method
- Green fabrication of chitosan from marine crustaceans and mushroom waste: Toward sustainable resource utilization
- Synthesis, characterization, and evaluation of nanoparticles of clodinofop propargyl and fenoxaprop-P-ethyl on weed control, growth, and yield of wheat (Triticum aestivum L.)
- The enhanced adsorption properties of phosphorus from aqueous solutions using lanthanum modified synthetic zeolites
- Separation of graphene oxides of different sizes by multi-layer dialysis and anti-friction and lubrication performance
- Visible-light-assisted base-catalyzed, one-pot synthesis of highly functionalized cinnolines
- The experimental study on the air oxidation of 5-hydroxymethylfurfural to 2,5-furandicarboxylic acid with Co–Mn–Br system
- Highly efficient removal of tetracycline and methyl violet 2B from aqueous solution using the bimetallic FeZn-ZIFs catalyst
- A thermo-tolerant cellulase enzyme produced by Bacillus amyloliquefaciens M7, an insight into synthesis, optimization, characterization, and bio-polishing activity
- Exploration of ketone derivatives of succinimide for their antidiabetic potential: In vitro and in vivo approaches
- Ultrasound-assisted green synthesis and in silico study of 6-(4-(butylamino)-6-(diethylamino)-1,3,5-triazin-2-yl)oxypyridazine derivatives
- A study of the anticancer potential of Pluronic F-127 encapsulated Fe2O3 nanoparticles derived from Berberis vulgaris extract
- Biogenic synthesis of silver nanoparticles using Consolida orientalis flowers: Identification, catalytic degradation, and biological effect
- Initial assessment of the presence of plastic waste in some coastal mangrove forests in Vietnam
- Adsorption synergy electrocatalytic degradation of phenol by active oxygen-containing species generated in Co-coal based cathode and graphite anode
- Antibacterial, antifungal, antioxidant, and cytotoxicity activities of the aqueous extract of Syzygium aromaticum-mediated synthesized novel silver nanoparticles
- Synthesis of a silica matrix with ZnO nanoparticles for the fabrication of a recyclable photodegradation system to eliminate methylene blue dye
- Natural polymer fillers instead of dye and pigments: Pumice and scoria in PDMS fluid and elastomer composites
- Study on the preparation of glycerylphosphorylcholine by transesterification under supported sodium methoxide
- Wireless network handheld terminal-based green ecological sustainable design evaluation system: Improved data communication and reduced packet loss rate
- The optimization of hydrogel strength from cassava starch using oxidized sucrose as a crosslinking agent
- Green synthesis of silver nanoparticles using Saccharum officinarum leaf extract for antiviral paint
- Study on the reliability of nano-silver-coated tin solder joints for flip chips
- Environmentally sustainable analytical quality by design aided RP-HPLC method for the estimation of brilliant blue in commercial food samples employing a green-ultrasound-assisted extraction technique
- Anticancer and antimicrobial potential of zinc/sodium alginate/polyethylene glycol/d-pinitol nanocomposites against osteosarcoma MG-63 cells
- Nanoporous carbon@CoFe2O4 nanocomposite as a green absorbent for the adsorptive removal of Hg(ii) from aqueous solutions
- Characterization of silver sulfide nanoparticles from actinobacterial strain (M10A62) and its toxicity against lepidopteran and dipterans insect species
- Phyto-fabrication and characterization of silver nanoparticles using Withania somnifera: Investigating antioxidant potential
- Effect of e-waste nanofillers on the mechanical, thermal, and wear properties of epoxy-blend sisal woven fiber-reinforced composites
- Magnesium nanohydroxide (2D brucite) as a host matrix for thymol and carvacrol: Synthesis, characterization, and inhibition of foodborne pathogens
- Synergistic inhibitive effect of a hybrid zinc oxide-benzalkonium chloride composite on the corrosion of carbon steel in a sulfuric acidic solution
- Review Articles
- Role and the importance of green approach in biosynthesis of nanopropolis and effectiveness of propolis in the treatment of COVID-19 pandemic
- Gum tragacanth-mediated synthesis of metal nanoparticles, characterization, and their applications as a bactericide, catalyst, antioxidant, and peroxidase mimic
- Green-processed nano-biocomposite (ZnO–TiO2): Potential candidates for biomedical applications
- Reaction mechanisms in microwave-assisted lignin depolymerisation in hydrogen-donating solvents
- Recent progress on non-noble metal catalysts for the deoxydehydration of biomass-derived oxygenates
- Rapid Communication
- Phosphorus removal by iron–carbon microelectrolysis: A new way to achieve phosphorus recovery
- Special Issue: Biomolecules-derived synthesis of nanomaterials for environmental and biological applications (Guest Editors: Arpita Roy and Fernanda Maria Policarpo Tonelli)
- Biomolecules-derived synthesis of nanomaterials for environmental and biological applications
- Nano-encapsulated tanshinone IIA in PLGA-PEG-COOH inhibits apoptosis and inflammation in cerebral ischemia/reperfusion injury
- Green fabrication of silver nanoparticles using Melia azedarach ripened fruit extract, their characterization, and biological properties
- Green-synthesized nanoparticles and their therapeutic applications: A review
- Antioxidant, antibacterial, and cytotoxicity potential of synthesized silver nanoparticles from the Cassia alata leaf aqueous extract
- Green synthesis of silver nanoparticles using Callisia fragrans leaf extract and its anticancer activity against MCF-7, HepG2, KB, LU-1, and MKN-7 cell lines
- Algae-based green AgNPs, AuNPs, and FeNPs as potential nanoremediators
- Green synthesis of Kickxia elatine-induced silver nanoparticles and their role as anti-acetylcholinesterase in the treatment of Alzheimer’s disease
- Phytocrystallization of silver nanoparticles using Cassia alata flower extract for effective control of fungal skin pathogens
- Antibacterial wound dressing with hydrogel from chitosan and polyvinyl alcohol from the red cabbage extract loaded with silver nanoparticles
- Leveraging of mycogenic copper oxide nanostructures for disease management of Alternaria blight of Brassica juncea
- Nanoscale molecular reactions in microbiological medicines in modern medical applications
- Synthesis and characterization of ZnO/β-cyclodextrin/nicotinic acid nanocomposite and its biological and environmental application
- Green synthesis of silver nanoparticles via Taxus wallichiana Zucc. plant-derived Taxol: Novel utilization as anticancer, antioxidation, anti-inflammation, and antiurolithic potential
- Recyclability and catalytic characteristics of copper oxide nanoparticles derived from bougainvillea plant flower extract for biomedical application
- Phytofabrication, characterization, and evaluation of novel bioinspired selenium–iron (Se–Fe) nanocomposites using Allium sativum extract for bio-potential applications
- Erratum
- Erratum to “Synthesis, characterization, and evaluation of nanoparticles of clodinofop propargyl and fenoxaprop-P-ethyl on weed control, growth, and yield of wheat (Triticum aestivum L.)”

