Chitosan nanoparticles loaded with mesosulfuron methyl and mesosulfuron methyl + florasulam + MCPA isooctyl to manage weeds of wheat (Triticum aestivum L.)
-
Bilal Ahmad Khan
, Muhammad Ather Nadeem
Abstract
Nanoherbicides are articulated by empowering the potential of nanotechnology for the efficacious delivery of chemical or biological herbicides with the aid of nanomaterial‐based herbicide combinations. Therefore, the goal of this work was to investigate the chitosan nanoparticles loaded with mesosulfuron methyl and mesosulfuron methyl + florasulam + (2-methyl-4-chlorophenoxyacetic acid) MCPA isooctyl herbicides as a possible environmentally benign substitute to manage weeds in wheat. Due to intriguing characteristics including biocompatibility, low allergenicity, biodegradability, and nontoxicity, chitosan biopolymers as sustainable chitin derivatives have received intense scrutiny in the biomedical business. The manufactured nanoparticles were characterized by using ultraviolet absorbance, scanning electron microscopy (SEM), X-ray diffraction (XRD), and Fourier transform infrared spectroscopy (FT-IR). The average particle size as revealed by SEM was 40–70 nm in a cluster form with the porous structure. The maximum absorption peaks of both nanoparticles of mesosulfuron methyl and mesosulfuron methyl + florasulam + MCPA isooctyl were 330 and 360 nm. The FT-IR analysis showed an intensive peak at 2θ value of 30.55° for mesosulfuron methyl and 32.79° for mesosulfuron methyl + florasula + MCPA isooctyl, which correspond to the 78 and 198 planes of the anatase phase, respectively. The nanoparticles were sprayed at the third to fourth leaf stages of the targeted weeds. Seven different doses were applied. A total of 100% mortality and visual injury were caused by the chitosan-based nanoparticles of both herbicides at the recommended dose of standard herbicide. The 5-fold lower dose showed the minimum chlorophyll content (5.75%), plant height (2.35 cm), fresh biomass (1.08 g), and dry biomass (0.33 g) of a weed mixture. For the same traits, the herbicide nanoparticles at 10-fold lower dose of commercial herbicides exhibited a similar effect as the recommended dose. Nanoherbicides could recuperate the conventional herbicide effectiveness by enhancing the stability and reducing the toxicity.
1 Introduction
Wheat is considered one of the most versatile crops on the planet. It is an important food crop due to its extensive use as a food daily [1]. Therefore, its sustainable production is very crucial for the better livelihood of the community at large. Presently, several climatic and political, agro-physiological, socio-economical, and management factors are responsible for low yields of wheat worldwide. Lack of seeding rates, appropriate doses of fertilizers and irrigation, access to resources, as well as the best strategy for pest and weed management are major influencers of wheat farming and productivity [2,3,4].
Weeds cause approximately 9.5% yield loss of wheat globally [5,6,7]. Phalaris minor, Avena fatua, Chenopodium album, Lathyrus aphaca, Angalis arvensis, and Melilotus indica are the most common and troublesome ones [8,9]. Since weeds possess competitive and deleterious effects on each growth phase of wheat, it is of prime importance to follow new systems for their management [8]. Preventive measures and biological and chemical approaches were employed for weed control [10]. Currently, the most effective and time-saving method is the chemical control [11]. Herbicides are frequently sprayed on the targeted plants to destroy their structure and impair their function [12]. They significantly decrease the growth and potential seed production of those weeds. Several herbicides are used to control both broad and narrow-leaved weeds in wheat including mesosulfuron methyl and mesosulfuron methyl + florasulam + (2-methyl-4-chlorophenoxyacetic acid) MCPA isooctyl [13]. The wide usage of herbicides increases the chances of weed resistance and farmer’s dependence [14]. In addition to the resistance, the hazard caused by herbicides and their persistent toxic effect on the quality of all life aspects after reaching the action site are other major issues related to the chemical control [15,16]. Despite those several herbicide side effects, its use is extremely important in augmenting crop productivity to face all the necessities of food security and sustainability of human populations [17]. Thus, developing a more environmentally friendly herbicide application that is grounded on an innovative technology and a progressive mechanism of action is a must. Today, the agriculture industry is further hastening its revolution, driven by new technologies such as nanotechnology. Nanotechnology proposes stimulating techniques for preventing the herbicide misuse as well as harmless and effective delivery [17]. This technology of exploiting nanomaterials promises the enhancement of the present practices through the amelioration of management procedures. The nanostructured herbicide could considerably diminish the herbicide consumption rate and assure increasing crop productivity [18]. The new‐fangled strategy of nanoherbicides is used to battle the complications of the conventional ones. A controlled release mechanism is manufactured in nanostructured formulations comprising an extensive variety of polymeric and metallic nanoparticles (NPs) [18]. One of the most effective methods for lowering the concentration and related negative effects of herbicides applied in the fields is to nanoencapsulate them in polymeric shells. By using nanotechnology, several issues associated with the conventional usage of pesticides can be resolved and minimized. Degradable and certain synthetic polymers are frequently used to package pharmaceutical and veterinary drugs as well as other active substances, yet some studies have labeled these materials as agrochemicals [18]. The competence of nanoherbicides assists in eradicating weeds before developing resistance through extraordinary penetration, bioavailability, solubility, reduced risk of oxidation, and site-specific targeting [19]. Furthermore, nanoherbicides afford short- and long-term protection against phytotoxicity and the lethal doses at which the weed might acquire resistance [20]. Chitin, a naturally occurring polymer contained in the shells of shrimp and other crustaceans, is deacetylated to produce chitosan, a linear polysaccharide. It is one of the biopolymers that is often used in a variety of industries, including clothing, cosmetics, water treatment, and food processing. The hydrophilic characteristic of chitosan in an aqueous solution is increased by the vast number of useful free primary amino groups present, which facilitates its interaction with medicines, polymers, cells, and NPs. The biomedical sector has also closely examined chitosan biopolymers as sustainable chitin derivatives because of intriguing properties like biocompatibility, low allergenicity, biodegradability, and nontoxicity [9].
Chitosan has been used in a variety of agricultural applications, including seed coating, fertilizers, and nutrients, as well as to enhance the plant growth, frost protection, and self-protective mechanisms [9,21,22]. The ability of agrochemicals including chitosan matrix to serve as a protective reservoir for the functional compounds, shielding the substances from their surroundings, and observing their abilities allows them to be used as efficient delivery vehicles for plant modification [23]. The use of chitosan NPs in the creation of novel delivery systems with enhanced bioavailability, higher specificity and sensitivity, and decreased toxicity has received particular attention [9,24]. The release system developed in NP‐based herbicides significantly aids early weed control with a strong potential of eliminating resistance, maintaining the activity of their functioning components, and elongates their liberation over longer times [22,23]. Thus, it was suggested that nanoherbicides aid in the weed control more efficiently even at 10-fold lower dose in comparison with the conventional ones [25,26,27]. It has also been documented that nano-atrazine at a 10-fold lower dose created comparable effects on maize weeds as the suggested dose of commercial atrazine [28,29].
Therefore, the aim of the current study was to investigate the chitosan NPs loaded with mesosulfuron methyl and mesosulfuron methyl + florasulam + MCPA isooctyl herbicides as a possible environmentally benign substitute to manage weeds in wheat.
2 Materials and methods
2.1 Weed seeds collection
Seeds of two grassy weeds (Phalaris minor L. and Avena fatua L.) and four broad leaves ones (Chenopodium album L., Lathyrus aphaca L., Angalis arvensis L., and Melilotus indica L.) developed in wheat were procured from the Agronomic Research Area, College of Agriculture, University of Sargodha, Pakistan. Seeds were washed, dried, and stored at room temperature in paper bags.
2.2 Chemical synthesis of chitosan-based mesosulfuron methyl and mesosulfuron methyl + florasulam + MCPA isooctyl
The ionic gelification technique was used to prepare the NPs [30].
2.3 Chemicals
The following chemicals were utilized throughout the experiment: chitosan (MW: 27 kDa, degree of deacetylation: 75–85%), tripoly phosphate, clodinofop propargyl, and fenoxaprop-p-ethyl.
2.4 Characterization of chitosan-based NPs of mesosulfuron methyl and mesosulfuron methyl + florasulam + MCPA isooctyl
Characterization of chitosan-based NPs of mesosulfuron methyl and mesosulfuron methyl + florasulam + MCPA isooctyl was validated using several techniques. The distribution of size, composition, and NP morphology was recorded by scanning electron microscopy (SEM) (Scanning Electron Microscopy FEI brand model Inspect S50). For the type of NPs, the X-ray diffraction (XRD) (PAN analytical X-pert powder, with Cu-Kα as X-ray source) was employed. Scanning at 2θ with a scan speed of 1°‧min−1 and step size of 0.02° was conducted [20] and was studied using Fourier transform infrared spectroscopy (FT-IR) spectrometer (Thermo-Nicolet 6700) with the potassium bromide disk technique. FT-IR spectroscopy was performed to study the binding characteristics of NPs and investigate the functional group sites on the NPs [31].
2.5 Herbicidal activity of chitosan-based NPs of mesosulfuron methyl and mesosulfuron methyl + florasulam + MCPA isooctyl
To optimize the doses of herbicide NPs, pot experiments were conducted during the winter season of 2020–2021 in the Agronomic Research Area, College of Agriculture, University of Sargodha, Pakistan. The experimental design was a factorial arrangement in a complete randomized block design with three replicates. Five seeds of each weed under investigation were planted in 20 cm × 16.5 cm pots filled with peat moss for germination. For each treatment, a total of 12 weed seedlings were maintained after emergence. At leaf stage 3–4, the NPs of mesosulfuron methyl and mesosulfuron methyl + florasulam + MCPA isooctyl were sprayed on the targeted weeds in seven different doses, i.e., D 0 = weedy check, D 1 = normal herbicide at the recommended dose, D 2 = nanoherbicide at the recommended dose of normal herbicide, D 3 = 05-fold lower dose of nanoherbicide, D 4 = 10-fold lower dose of nanoherbicide, D 5 = 15-fold lower dose of nanoherbicide, and D 6 = 20-fold lower dose of nanoherbicide. Two weeks after the treatment, the chlorophyll content was recorded. The visual injury (%) was determined by visual observation of pots, and the average injury that occurred to plants in pots of each replicate was also determined. Mortality (%) was recorded by observing the total number of plants in each pot, and the average killed ones are determined following the method presented in [32]. Also, the average plant height (cm) of the plants was noted. Average fresh biomass (g per pot) was recorded using a digital balance. Dry biomass (g per pot) was calculated after drying at 70°C for 48 h [32].
2.6 Statistical analysis
The Statistics Software (Statistix version 8.1, Tallahassee, FL, USA) was employed to analyze the data. The means were compared using the highest significant difference (HSD) at the level of probability of 5%.
3 Results
3.1 Chlorophyll content (%)
The results depicted in Table 1 presented that different doses of chitosan-based broad-spectrum herbicides significantly affect the chlorophyll content of the weed mixture when compared with the weedy check. At the standard dose of normal herbicide, the maximum toxic effect caused 100% mortality and 45.49% chlorophyll content with no application of chitosan-based NPs (control) in the weed mixture. The minimum content of chlorophyll (5.75%) was perceived when a 5-fold lower dose of the broad-spectrum herbicide-loaded NPs (D 3) was applied. Also, applying conventional herbicides at the recommended dose (D 1) showed a statistically comparable effect on the chlorophyll content as the 10-fold lower dose of chitosan-based herbicide-loaded NPs (D 4) (10.43% and 9.66%, respectively). The herbicide NPs also showed a statistically significant effect on the chlorophyll content (%) of a mixture of weeds. However, the maximum chlorophyll content (19.38%) was examined with NPs of mesosulfuron methyl + florasulam + MCPA isooctyl and minimum (16.87%) with mesosulfuron methyl. The interaction effects of various doses × broad-spectrum herbicides were likewise found to be significant. The maximum chlorophyll content (46.03%) was exhibited under control, while the minimum (5.54%) was with the 5-fold lower dose of mesosulfuron methyl.
Effect of nanoparticles of mesosulfuron methyl and mesosulfuron methyl + florasulam + MCPA isooctyl on chlorophyll contents (%), and visual injury (%) of mixture of weeds
| Doses of nanoparticle of herbicides | Chlorophyll contents (%) | Visual injury (%) | ||||
|---|---|---|---|---|---|---|
| Mesosulfuron methyl | Mesosulfuron methyl + florasulam + MCPA isooctyl | Mean | Mesosulfuron methyl | Mesosulfuron methyl + florasulam + MCPA isooctyl | Mean | |
| D 0 | 45.23NS | 45.74 | 45.49a | 0.00f | 0.00f | 0.00E |
| D 1 | 9.29 | 11.57 | 10.43c | 86.33ab | 81.33b | 83.83B |
| D 2 | No weed | No weed | No weed | 100.00a | 100.00a | 100.00A |
| D 3 | 5.54 | 5.97 | 5.75d | 91.33ab | 90.66ab | 91.00AB |
| D 4 | 8.99 | 10.33 | 9.66cd | 87.00ab | 87.00ab | 87.00B |
| D 5 | 14.72 | 18.97 | 16.84b | 76.00bc | 60.00cd | 68.00C |
| D 6 | 17.43 | 23.72 | 20.57b | 54.67d | 36.33e | 45.50D |
| Mean | 16.87B | 19.38A | 70.76A | 65.05B | ||
| HSD at 5% | Doses = 4.03, herbicides = 1.55 | Doses = 9.88, herbicides = 3.40 | ||||
| Doses × herbicides = NS | Doses × herbicides = 16.13 | |||||
D 0 = weedy check, D 1 = normal herbicides at recommended dose, D 2 = nanoparticles of herbicides at recommended dose of normal herbicide, D 3 = 05-fold lower dose of nanoparticles of herbicides, D 4 = 10-fold lower dose of nanoparticles of herbicides, D 5 = 15-fold lower dose of nanoparticles of herbicides, D 6 = 20-fold lower dose of nanoparticles of herbicides.
In the same column, means with the same letter did not significantly differ at the 5%.
3.2 Visual injury (%)
The different doses of broad-spectrum herbicides caused a significant influence on visual injury to the weed mixture compared with the weedy check (Table 1). A total of 100% of the plants were injured when broad-spectrum herbicide NPs were used at the recommended commercial herbicide dosage. No injury was documented under control (100% alive plants). Similar injury to the weed mixture was observed for the 10-fold lower dose and the recommended dose of normal herbicide (87.00% and 83.83%, respectively). The NPs of the two herbicides exhibited a significant influence on the visual injury of the weed mixture. The maximum visual injury (70.76%) was recorded with the application of nano mesosulfuron methyl and the minimum (65.05%) with mesosulfuron methyl + florasulam + MCPA isooctyl NPs. The interactive effect of the herbicide NPs and their different doses (nano herbicides × doses) was found significant. Meanwhile, the application of NPs of mesosulfuron methyl and mesosulfuron methyl + florasulam + MCPA isooctyl at the normal herbicides recommended dose resulted in 100% injury to weeds as compared to the control (0% visual injury).
3.3 Mortality (%)
The NPs of the two broad-spectrum herbicides caused a significant effect on the mortality percentage of the weed mixture as presented in Table 2. When applied at the recommended dose of normal herbicides, nanoherbicides resulted in maximum mortality (100%). The reduction in the dose of NPs resulted in a decrease in the mortality of weeds, and minimum mortality (0%) was observed under control. A similar mortality effect was exhibited with the application of nanoherbicides at the 10-fold lower dose and suggested dose of the standard herbicide (83.33% and 80.55%, respectively). Between the two herbicides NPs, maximum (68.25%) and minimum (62.70%) mortality were observed by the mesosulfuron methyl and mesosulfuron methyl + florasulam + MCPA isooctyl, respectively. The interaction effect of NPs × various doses was also significant. The 100% mortality was shown with NPs of mesosulfuron methyl and mesosulfuron methyl + florasulam + MCPA isooctyl at the suggested dose of standard herbicide and 0% under control. This 100% mortality of the weed mixture observed with the application of the NPs of two herbicides may have resulted from the excessive penetration of the herbicides into weeds due to the nanosized particles, which killed all weeds compared to the control.
Effect of nanoparticles of mesosulfuron methyl and mesosulfuron methyl + florasulam + MCPA isooctyl on mortality (%) and plant height (cm) of the mixture of weeds
| Doses of nanoparticle of herbicides | Mortality (%) | Plant height (cm) | ||||
|---|---|---|---|---|---|---|
| Nanoparticles of mesosulfuron methyl | Nanoparticles of mesosulfuron methyl + florasulam + MCPA isooctyl | Mean | Nanoparticles of mesosulfuron methyl | Nanoparticles of mesosulfuron methyl + florasulam + MCPA isooctyl | Mean | |
| D 0 | 0.00f | 0.00f | 0.00E | 9.15NS | 9.13 | 9.14A |
| D 1 | 83.33ab | 77.77b | 80.55B | 6.83 | 7.10 | 6.97AB |
| D 2 | 100.00a | 100.00a | 100.00A | No weed | No weed | No weed |
| D 3 | 88.89ab | 88.88ab | 88.88B | 4.63 | 4.77 | 4.70B |
| D 4 | 83.33ab | 83.33ab | 83.33B | 6.70 | 6.97 | 6.83AB |
| D 5 | 72.22bc | 55.55cd | 63.88C | 7.99 | 8.08 | 8.03A |
| D 6 | 50.00de | 33.33e | 41.66D | 8.47 | 8.57 | 8.52A |
| Mean | 68.25A | 62.70B | 7.33NS | 7.43A | ||
| HSD at 5% | Doses = 10.53, herbicides = 3.62 | Doses = 2.97, herbicides = 1.14 | ||||
| Doses × herbicides = 17.18 | Doses × herbicides = NS | |||||
D 0 = weedy check, D 1 = normal herbicides at recommended dose, D 2 = nanoparticles of herbicides at recommended dose of normal herbicide, D 3 = 05-fold lower dose of nanoparticles of herbicides, D 4 = 10-fold lower dose of nanoparticles of herbicides, D 5 = 15-fold lower dose of nanoparticles of herbicides, D 6 = 20-fold lower dose of nanoparticles of herbicides.
In the same column, means with the same letter are not significantly different at the 5%.
3.4 Plant height (cm)
The influence of using the herbicides NPs on the plant height of the weed mixture is illustrated in Table 2. The results revealed that different doses of NPs affected the plant height. The minimum height (2.35 cm) for the weed mixture was noted by applying the NPs at a 5-fold lower dose, and the maximum (9.14 cm) was recorded for the control. The taller plants (7.43 cm) and shorter ones (7.33 cm) were noticed with the NPs of mesosulfuron methyl + florasulam + MCPA isooctyl and mesosulfuron methyl, respectively. The interaction effects of doses and NPs were significant. The shorter plants (4.63 cm) of the mixture were observed with the application of the mesosulfuron methyl NPs at a 5-fold lower dose of standard herbicide, and the taller ones (9.15 cm) were observed for the weedy check with the application of the same NPs. This study found that exposure to NPs of herbicides at the approved dose of conventional herbicides affected the height of the surviving plants.
3.5 Fresh biomass (g)
The influence of the manufactured chitosan-based NPs on fresh biomass of the weed mixture is shown in Table 3. The results illustrated that the fresh biomass was affected by various doses of nanoherbicides. The maximum fresh biomass (6.48 g) was recorded at D 0, while increasing the nanoherbicides doses reduced the fresh biomass. The minimum fresh biomass (0.58 g) was documented at the 5-fold lower dose of the NPs. Similar effects were detected with the normal herbicide recommended doses and the 10-fold lower dose of NPs, which were recorded as 1.08 and 0.91 g, respectively. The NPs of mesosulfuron methyl + florasulam + MCPA isooctyl and mesosulfuron methyl showed a significant effect on the fresh biomass with maximum (2.63 g) and minimum (2.22 g) fresh biomass for both loaded NPs, respectively. The interactive effect of herbicide-loaded NPs and their different doses were further significant. The addition of 5-fold lower dose of mesosulfuron methyl resulted in minimum fresh biomass (0.57 g), while the maximum fresh biomass (6.52 g) was observed under control (no dose application).
Effect of nanoparticles of mesosulfuron methyl and mesosulfuron methyl + florasulam + MCPA isooctyl on fresh biomass (g) and dry biomass of mixture of weeds
| Doses of nanoparticle of herbicides | Fresh biomass (g) | Dry biomass (g) | ||||
|---|---|---|---|---|---|---|
| Nanoparticles of mesosulfuron methyl | Nanoparticles of mesosulfuron methyl + florasulam + MCPA isooctyl | Mean | Nanoparticles of mesosulfuron methyl | Nanoparticles of mesosulfuron methyl + florasulam + MCPA isooctyl | Mean | |
| D 0 | 6.44a | 6.52a | 6.48A | 1.88a | 1.90a | 1.89A |
| D 1 | 0.91e | 1.26e | 1.08D | 0.28ef | 0.38ef | 0.33D |
| D 2 | No weed | No weed | No weed | No weed | No weed | No weed |
| D 3 | 0.55e | 0.58e | 0.57D | 0.16f | 0.17f | 0.16D |
| D 4 | 0.89e | 0.93e | 0.91D | 0.27ef | 0.29ef | 0.28D |
| D 5 | 1.62de | 2.59cd | 2.10C | 0.49de | 0.79cd | 0.64C |
| D 6 | 2.90bc | 3.87b | 3.39B | 0.86c | 1.18b | 1.02B |
| Mean | 2.22B | 2.63A | 0.66B | 0.78A | ||
| HSD at 5% | Doses = 0.63, herbicides = 0.24 | Doses = 0.18, herbicides = 0.07 | ||||
| Doses × herbicides = 1.04 | Doses × herbicides = 0.08 | |||||
D 0 = weedy check, D 1 = normal herbicides at recommended dose, D 2 = nanoparticles of herbicides at recommended dose of normal herbicide, D 3 = 05-fold lower dose of nanoparticles of herbicides, D 4 = 10-fold lower dose of nanoparticles of herbicides, D 5 = 15-fold lower dose of nanoparticles of herbicides, D 6 = 20-fold lower dose of nanoparticles of herbicides.
In the same column, means with the same letter are not significantly different at the 5%.
3.6 Dry biomass (g)
The different doses of broad-spectrum herbicide-loaded NPs produced a statistically significant influence on the dry biomass of the weed mixture (Table 3). The minimum dry biomass weight (0.16 g) was observed with a 5-fold lower dose of NPs, and the maximum (1.89 g) was shown under control. The recommended dose of normal herbicides and the 10-fold lower dose of the loaded NPs produced a nonsignificant effect on dry biomass (0.33 and 0.28 g, respectively). Herbicide-loaded mesosulfuron methyl and mesosulfuron methyl + florasulam + MCPA isooctyl NPs significantly influenced the dry biomass. The mesosulfuron methyl + florasulam + MCPA isooctyl-loaded NPs was less toxic compared to the mesosulfuron methyl loaded ones reflecting the maximum (0.78 g) and the minimum (0.68 g) dry biomass, respectively. The interaction of various doses and nanoherbicides was similarly significant. The 5-fold lower dose of mesosulfuron methyl results in the minimum (0.16 g) and mesosulfuron methyl + florasulam + MCPA isooctyl at 0 g a.i ha−1 produced the maximum dry biomass (1.90 g) of the weed mixture.
3.7 SEM
The shape and surface morphology of the chitosan-based NPs of mesosulfuron methyl and mesosulfuron methyl + florasulam + MCPA isooctyl were studied using SEM. Figures 1 and 2 demonstrated that the average particle size was 40–70 nm in a cluster form with the porous structure. This could be because of the existence of some toxic compounds. The size range of the NPs was 40 and 70 nm, respectively.
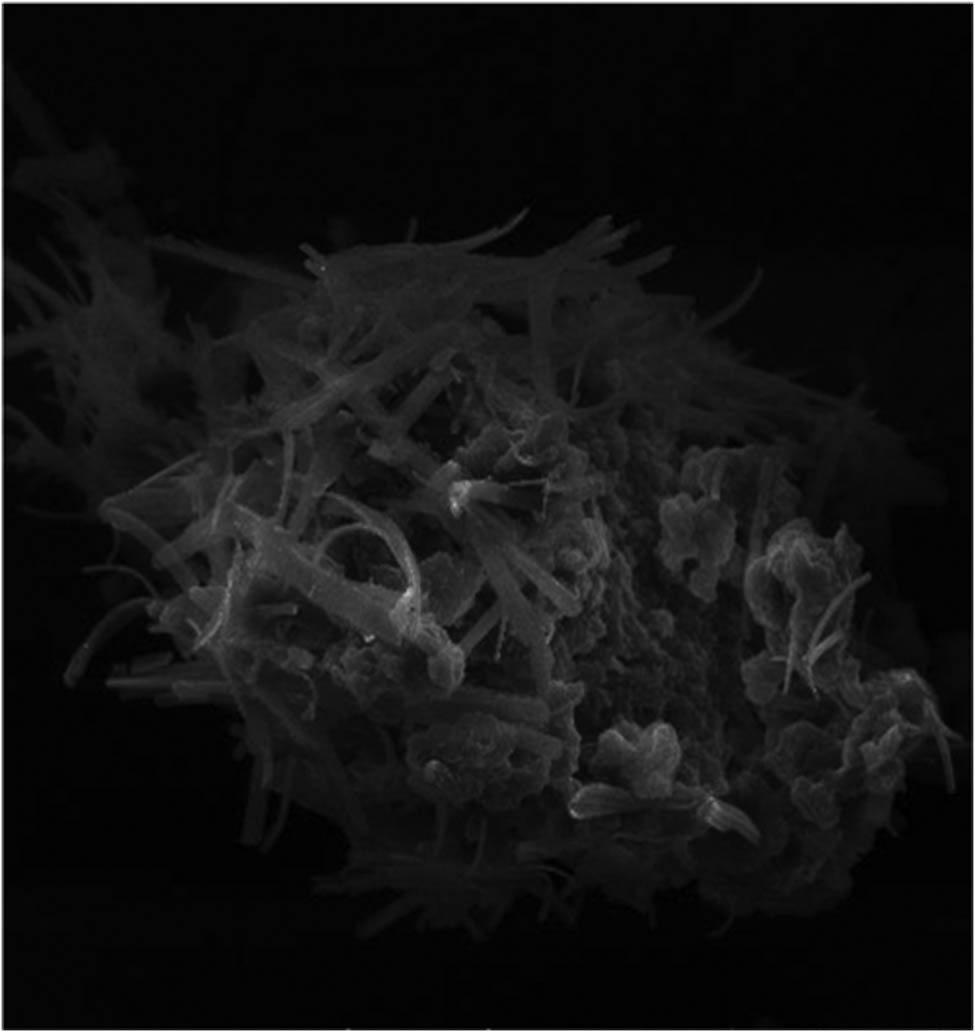
SEM and SEM-EDX micrograph of chitosan-based mesosulfuron methyl.

SEM and SEM-EDX micrograph of chitosan-based mesosulfuron methyl + florasulam + MCPA isooctyl.
3.8 Fourier transform infrared spectroscopy
FT-IR analysis was used to examine the physical and chemical compatibilities of the mesosulfuron methyl and mesosulfuron methyl + florasulam + MCPA isooctyl herbicide-loaded NPs made of chitosan. The FT-IR spectra of the nanoherbicides were illustrated in Figures 3 and 4. The main functional groups in the FT-IR region were between 649 and 2,923 cm−1. Free and esterified carboxyl groups were designated by carbonyl bands in the 649–880 and 1,082–1,320 cm−1 regions, respectively. Ether’s presence led to the formation of the absorption band at 1,447–1,520 cm−1. Meanwhile, the C–C cyclic bonds in the mesosulfuron methyl were responsible for the band between 1,612 and 1,750 cm−1. The polymeric O–H stretching band was responsible for the wide band between 1,690 and 2,923 cm−1, whereas the carboxyl group’s O–H stretching band was visible at 2,800 cm−1 [33]. Furthermore, the FT-IR spectra of the mesosulfuron methyl + florasulam + MCPA isooctyl noticeably revealed the main functional groups in the mesosulfuron methyl + florasulam + MCPA isooctyl FT-IR region between 654 and 3,250 cm−1. In the areas of 654–810 and 1,020–1,270 cm−1, respectively, carbonyl bands identified free and esterified carboxyl groups. Ether was responsible for the absorption band between 1,400 and 1,510 cm−1, while the C–C cyclic bonds in the mixture of mesosulfuron methyl + florasulam + MCPA isooctyl produced the band between 1,540 and 3,250 cm−1.
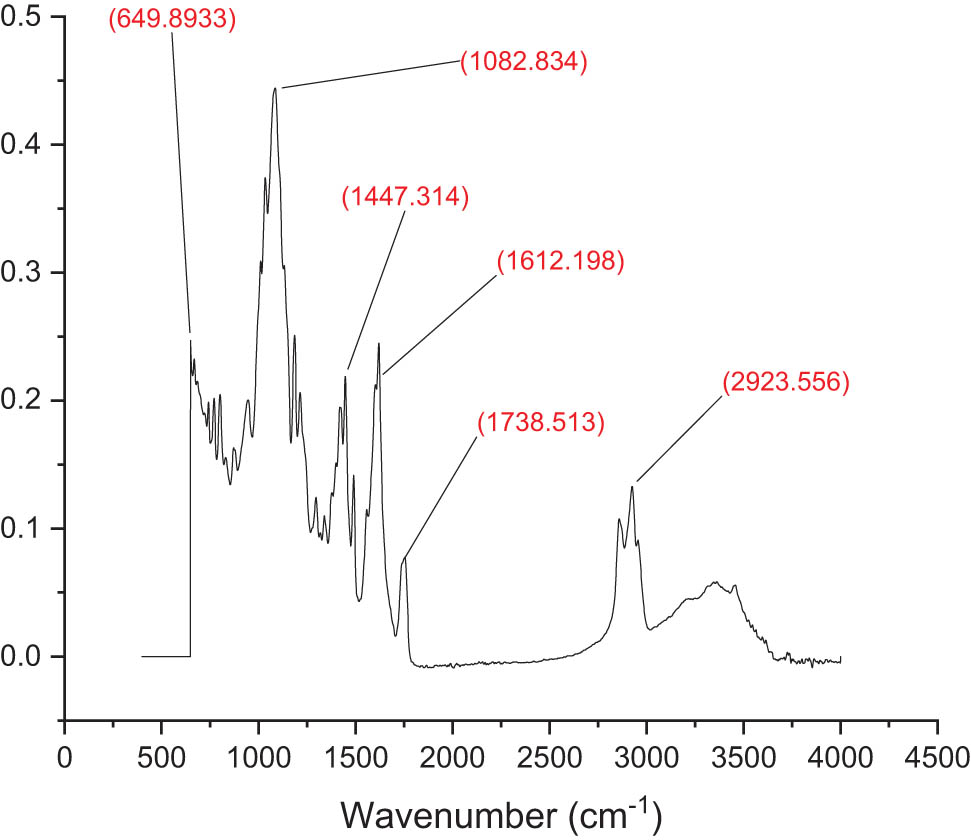
FT-IR of chitosan-based mesosulfuron methyl.
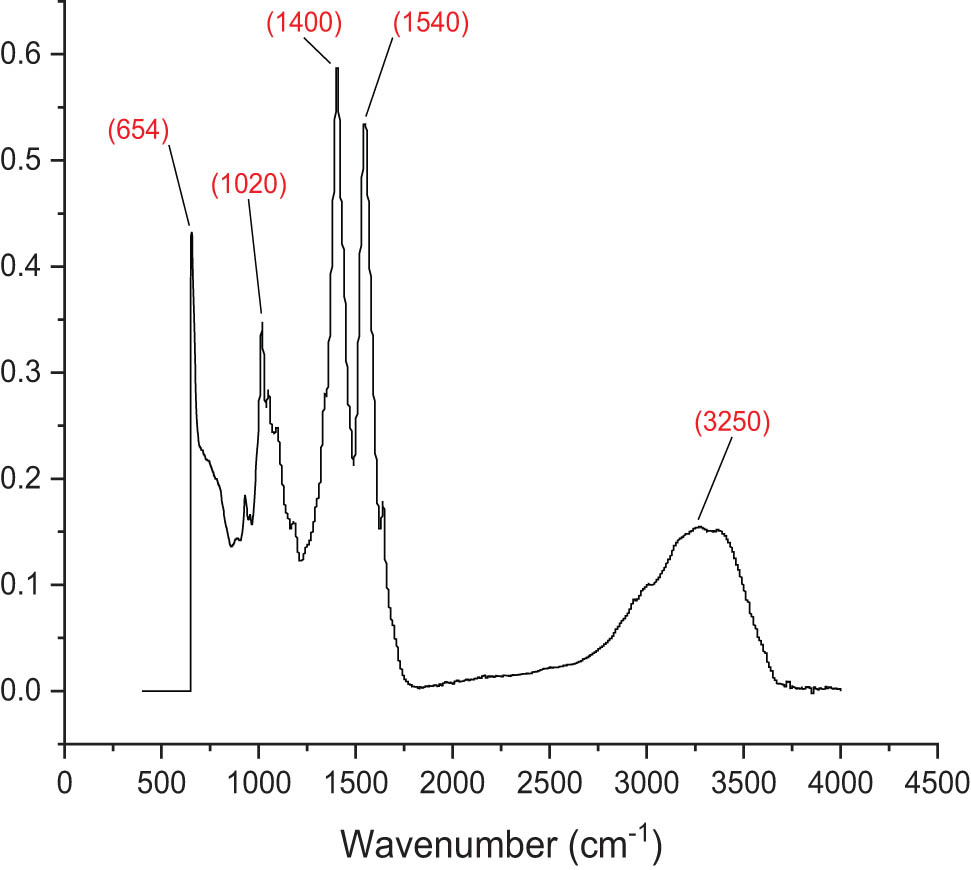
FT-IR of chitosan-based mesosulfuron methyl + florasulam + MCPA isooctyl.
3.9 UV-visible (UV-Vis) absorption spectrum
The ultraviolet (UV)-Vis absorption spectrum of the chitosan-based NPs of mesosulfuron methyl and mesosulfuron methyl + florasulam + MCPA isooctyl was presented in Figures 5 and 6, respectively. The peaks of maximum absorption of mesosulfuron methyl NPs were 330 nm. Meanwhile, the maximum absorption peaks of mesosulfuron methyl + florasulam + MCPA isooctyl NPs were 330 and 360 nm. This distinctive signature displayed the NPs formation.
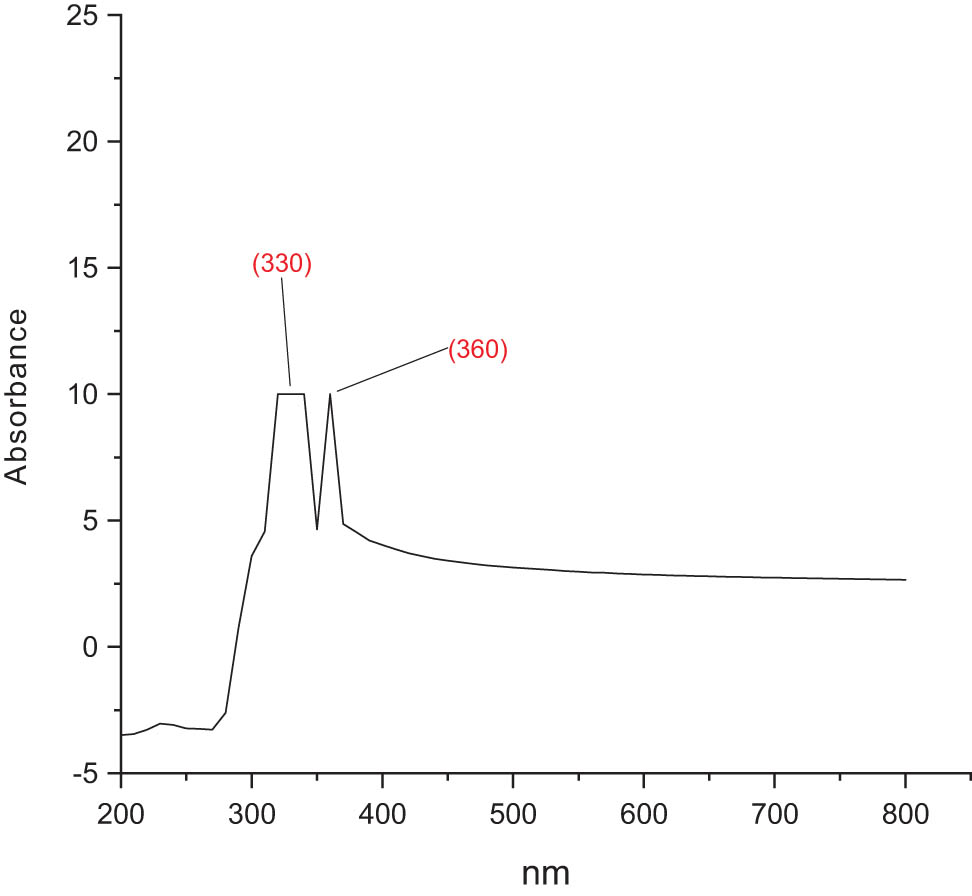
UV-Vis absorption spectrum of chitosan-based mesosulfuron methyl.
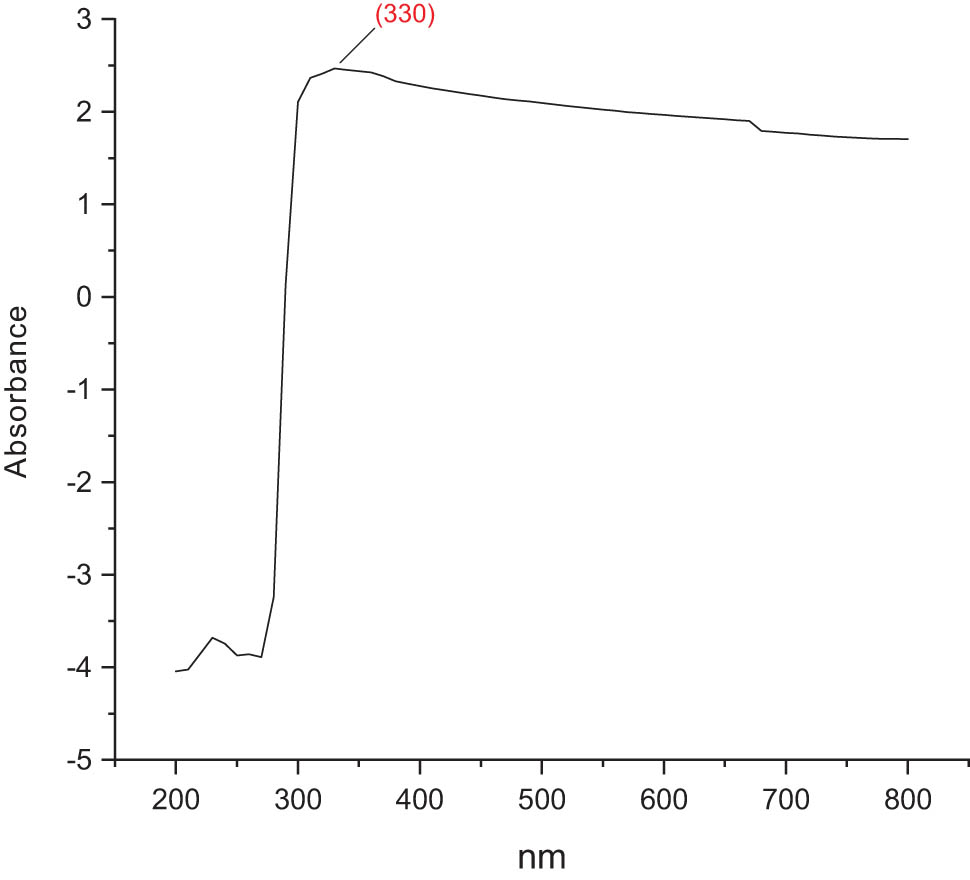
UV-Vis absorption spectrum of chitosan-based mesosulfuron methyl + florasulam + MCPA isooctyl.
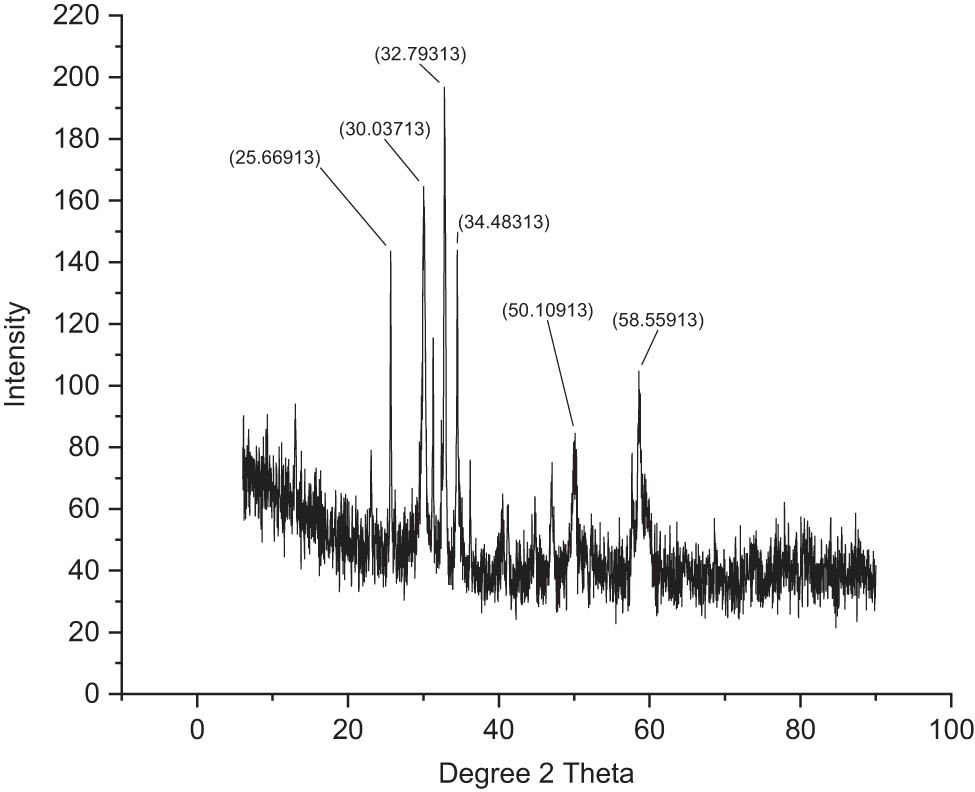
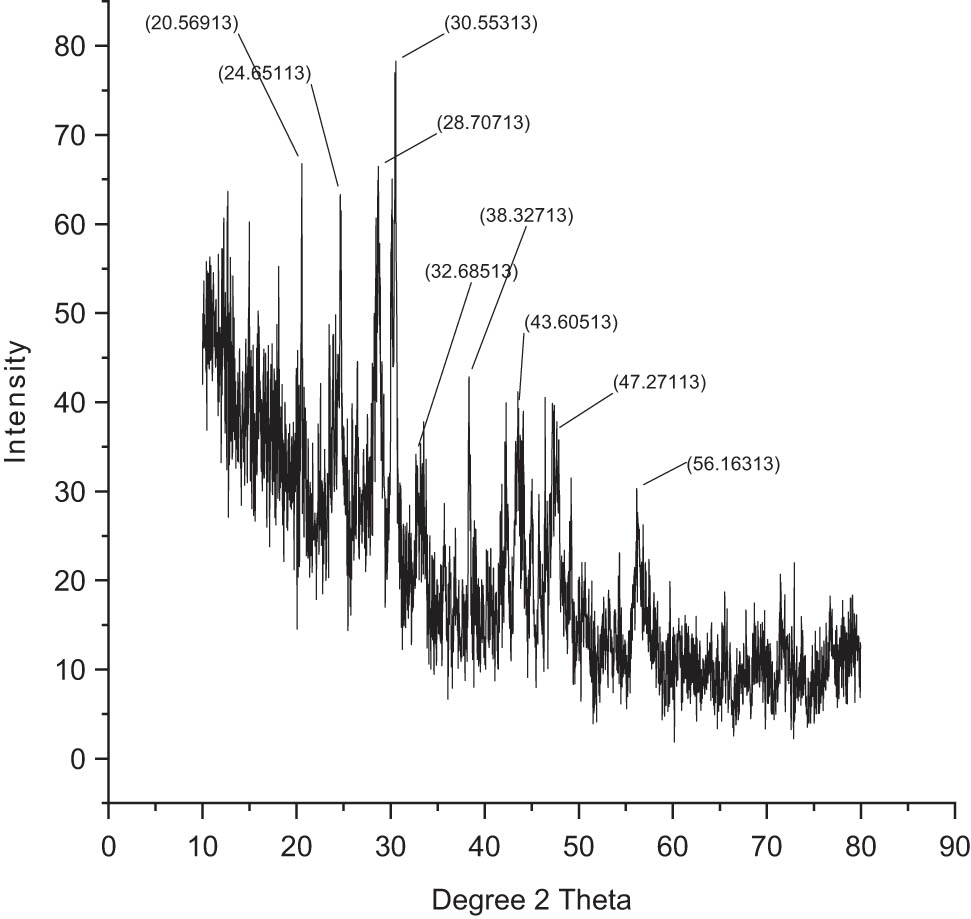
3.10 XRD analyses
Crystallinity and crystallite size of the NPs were tested by acquiring the corresponding XRD patterns (Figures 7 and 8). It was perceived that the chitosan-based NPs of mesosulfuron methyl revealed a rigorous peak around 2θ value of 30.55°, which corresponded to the 78 planes of the anatase phase. In addition, various additional minor peaks were further detected at 2θ values of 20.56°, 24.56°, 28.70°, 32.68°, 38.32°, 43.60°, 47.27°, and 56.16°, which corresponded to (68), (63), (57), (38), (42), (40), (32), and (28) planes of the anatase phase. In the case of NPs of mesosulfuron methyl + florasulam + MCPA isooctyl, it demonstrated an intensified peak around 2θ value of 32.79°, which corresponded to the 198 planes of the anatase phase, and various additional ones at 2θ values of 25.66°, 30.03°, 34.48°, 50.10°, and 58.55° corresponding to (143), (165), (142), (83), and (104) planes of the anatase phase.
XRD analyses of chitosan-based mesosulfuron methyl.
XRD analyses of chitosan-based mesosulfuron methyl + florasulam + MCPA isooctyl.
4 Discussion
As shown by the total growth suppression of a combination of wheat weeds, the application of nanoherbicides of mesosulfuron methyl and mesosulfuron methyl + florasulam + MCPA isooctyl improved herbicide efficacy compared to commercial herbicides at suggested doses. When herbicides are applied at the authorized dose and 10-fold lower doses, similar effects on the development of a combination of weeds were observed. The increased activity of chlorophyllase, the chlorophyll-breaking down enzyme, disruption of chloroplast fine structure, and chloroplast volatility that results in chlorophyll oxidation and reduction of its concentration in plants may be the cause of reduced chlorophyll content of the mixture of weeds after applying the NPs of both broad-spectrum herbicides. Similar outcomes were observed when atrazine-loaded nanocapsules were applied at lower doses, which resulted in a greater reduction in photosystem II activity than the original formulation of atrazine at a similar concentration in Amaranthus viridis and Bidens pilosa [33]. The findings of this work are consistent with those of ref. [9], who noted the decrease in the chlorophyll concentration with the application of NPs of fenoxaprop-p-ethyl and clodinofop propagyl because it was assimilated into cell membrane function via physiological processes including membrane depolarization. In comparison to commercial herbicides, the application of herbicide-loaded NPs regulates weeds at a lower dose, according to the findings of other researchers [9,19].
When nanoherbicides were applied at the acceptable dose of conventional herbicides, a larger harm to the weed mixture was investigated. There was no change in injury under weedy check. The indicated regular herbicide doses and 10-fold lower doses of both nanoherbicides showed similar effects on injury to the combination of weeds. The nanoherbicides applied topically to the weed mixture caused 100% damage, which may be related to depolarization of the membrane and further delay of cell membrane processes [34]. These results are consistent with the findings of ref. [19], which showed that the application of nanoherbicides manages weeds at a lower dose than that of commercial herbicides. Thus, herbicide-loaded NPs can be utilized to lessen herbicide use, increase efficacy, and make it less toxic to the environment. The findings of this work are consistent with those of ref. [9], who mentioned that the increase in visual injury to P. minor was observed with applying the NPs of fenoxaprop-p-ethyl and clodinofop propagyl.
Applying herbicide-loaded NPs at the suggested dose of regular herbicides resulted in a 100% mortality of a mixture of weeds, which may be explained by the improved effectiveness of the NPs’ penetration into the weeds relative to the control. When regular herbicides at the suggested dose and 10-fold lower doses of both herbicides’ NPs were applied, similar effects on mortality to the weed mixture were recorded. In comparison to non-nano formulations, metsulfuron methyl, diuron-loaded carboxymethyl, pectin, and metolachlor-loaded NPs have been shown to have a greater herbicidal effect [11,30,31].
This study showed that exposure to NPs of herbicides at the prescribed dose to regular herbicides affected the height of surviving plants. When standard herbicides were applied at the indicated dose and 10-fold lower doses, a similar effect on the height of a combination of weeds was seen. This might be because there is less photosynthesis and less activity in other metabolic processes, which causes the plants that survive to grow less tall than the control plants. These results are also consistent with [35], who found that the 10-fold dilution of atrazine-loaded poly(ε-caprolactone) (PCL) nanocapsules had similar suppressive effects on A. viridis and B. pilosa growth characteristics.
Transformation of regular herbicides into NPs amended their performance by increasing the charge-to-mass ratio of the herbicides, which in turn increased penetration, effectiveness, and decreased the fresh biomass of a variety of weeds. The two broad-spectrum herbicides under consideration had statistically identical harmful effects on a mixture of weeds when used at doses up to ten times lower than those used in commercial formulations. The results are corroborated by ref. [34], who showed that atrazine-loaded PCL nanocapsules were further successful in reducing the growth of the shoot and root of B. Pilosa than commercial atrazine at a 10-fold lower dose, which eventually resulted in a decrease in plant biomass [33]. The findings of this work are consistent with those of ref. [9], who noted that the reduction in plant height, fresh biomass, and dry biomass of P. minor was observed when NPs of fenoxaprop-p-ethyl and clodinofop propagyl were applied. The clustered form with the porous structure and round shape of the produced NPs reported in this work was supported by numerous investigations [36], and it could be because of the harmful substances.
The C–C cyclic bonds in the mesosulfuron methyl were responsible for the band between 1,612 and 1,750 cm−1. The polymeric O–H stretching band was responsible for the wide band between 1,690 and 2,923 cm−1, while the carboxyl group’s O–H stretching band was seen at 2,800 cm−1 [37,38]. The suitable existence of herbicide in the nanoformulation was confirmed by the FT-IR spectra of the herbicide-loaded NPs of both herbicides under examination. Mesosulfuron methyl NPs have 330 nm maximum absorption peaks. Mesosulfuron methyl + florasulam + MCPA isooctyl had the highest absorption peaks at 330 and 360 nm.
To effectively administer chemical or biological pesticides, nanotechnological preparations or herbicide formulations based on nanomaterials are used to create nanoherbicides. In comparison to conventional herbicides, formulations based on nanomaterials or nanostructures could increase the solubility, boost the efficacy, and decrease the toxicity of the herbicide. Early weed control utilizing NP-based herbicide release systems could lower the risk of herbicide resistance, preserve the active ingredient’s action, and extend their slower release [18]. The creation of a particular herbicide molecule enclosed in a NP is directed to attach with certain receptors existed in the root of the aimed weed. The created NP enters the root and transports to perform the effect, which in turn prevents roots from undergoing glycolysis. This action instigates the plant to famish, consequently dyes. These nanoherbicides may also be utilized in rain-fed locations where insufficient soil moisture causes herbicides to evaporate. Weeds can be eliminated with the aid of the controlled release of herbicides via encapsulation.
5 Conclusion
NPs can function as effective carriers and, when combined with herbicides, can create nanoformulations. These nanoformulations aid in solving the primary problem facing the herbicide sector, such as the development of plants that resist herbicides. The ease in preparation of chitosan-loaded herbicide complex contributes with better release characteristics can noticeably alter the herbicide applications. The chitosan nanoherbicides can change the herbicide interaction with soil and effectively avoid the unfavorable consequences of the herbicide.
Overall, the findings of this study showed that mesosulfuron methyl and mesosulfuron methyl + floresulam + MCPA isooctyl NPs based on chitosan may be employed for 100% control of both narrow and broad-leaved weeds. This study also compared the effectiveness of weed control between chitosan-based NP herbicides with no risk of environmental pollution and commercial herbicides at a recommended dose and a 10-fold lower dose.
According to the results of this study, D 1 = normal herbicide at the recommended dose, D 2 = nano herbicide at the recommended dose of normal herbicide, D 3 = 5-fold lower nano herbicide dose, D 4 = 10-fold lower nano herbicide dose, and D 5 = 15-fold lower nano herbicide dose were found to be effective in the management of mixture of weeds. As a result, these doses will be examined in more detail in the field.
It is worth to mention that although nanopesticides may have several advantages, they are currently in the early stages of research. Unfortunately, there are less studies on the assessment of the environmental safety of polymer-based nanopesticides, which is due to the absence of standardized methods to evaluate the environmental risk of nanopesticides for regulatory purposes. There is always a need for a practical evaluation technique by adopting recommendations obtained from the ecological risk assessment of conventional pesticide products.
Acknowledgement
We are very thankful to the Department of Agronomy, College of Agriculture, University of Sargodha-40100, Pakistan and Department of Material Engineering, National University of Science and Technology, Pakistan for support during research work.
-
Funding information: The author (Hesham Oraby) extends his appreciation to the Deputyship for Research & Innovation, Ministry of Education in Saudi Arabia for funding this research work through the project number: IFP22UQU4350043DSR53.
-
Author contributions: Bilal Ahmad Khan: conceptualization, methodology, software, validation, formal analysis, investigation, resources, data curation, writing – original draft preparation, writing – review and editing, and visualization; Muhammad Ather Nadeem: conceptualization, methodology, software, validation, formal analysis, investigation, resources, data curation, writing – original draft preparation, writing – review and editing, visualization, supervision, and project administration; Muhammad Mansoor Javaid, Rizwan Maqbool, Mudassar Iqbal and Neelam Yaqoob: validation, writing – original draft preparation, writing – review and editing, and visualization; Nehal Elnaggar: conceptualization, methodology, validation, resources, writing – original draft preparation, writing – review and editing, and visualization; Hesham Oraby: conceptualization, methodology, validation, resources, writing – original draft preparation, writing – review and editing, visualization, and funding.
-
Conflict of interest: There is no conflict of interest among authors.
-
Data availability statement: The data used to support the findings of this study are included within the article.
References
[1] Pirhadi M, Shariatifar N, Bahmani M, Manouchehri A. Heavy metals in wheat grain and its impact on human health: A mini-review. J Chem Health Risks. 2022;12(3):421–6.Search in Google Scholar
[2] Khan BA, Nijabat A, Khan MI, Khan I, Hashim S, Nadeem MA, et al. Implications of mulching on weed management in crops and vegetables. In: Akhtar K, Arif M, Riaz M, Wang H, editors. Mulching in agroecosystems. Singapore: Springer; 2022. p. 199–213.10.1007/978-981-19-6410-7_13Search in Google Scholar
[3] Nadeem MA, Khan BA, Chadar AR, Maqbool R, Raza A, Javaid MM, et al. Weed control and sustainable rice production through rice intensification system and conventional practices of weed competition periods and age of transplanted seedlings. Semina Ciênc Agrár. 2022;43(5):2271–92.10.5433/1679-0359.2022v43n5p2271Search in Google Scholar
[4] Maqbool R, Khan BA, Nadeem MA, Azam R, Raza A, Nijabat A, et al. Allelopathic effect of aqueous extract of Polygonum bistorta and Terminalia chebula on germination and seedling growth of Daucus carota and Medicago polymorpha. Semina Ciênc Agrár. 2022;43(5):2253–70. 10.5433/1679-0359.2022v43n5p2253.Search in Google Scholar
[5] Javaid MM, Mahmood A, Alshaya DS, AlKahtani MD, Waheed H, Wasaya A, et al. Influence of environmental factors on seed germination and seedling characteristics of perennial ryegrass (Lolium perenne L.). Sci Rep. 2022;12(1):1–11.10.1038/s41598-022-13416-6Search in Google Scholar PubMed PubMed Central
[6] Aziz A, Saba NU, Tahir MA, Ain QT, Ahmad A, Hamza A, et al. Effect of Mulches on Mineral Fertilizer (N, P & K) Management and Fertilizer Use Efficiency. In: Akhtar K, Arif M, Riaz M, Wang H, editors. Mulching in Agroecosystems. Singapore: Springer; 2022. 10.1007/978-981-19-6410-7_1.Search in Google Scholar
[7] Maurya P, Mazeed A, Kumar D, Ahmad IZ, Suryavanshi P. Medicinal and aromatic plants as an emerging source of bioherbicides. Curr Sci. 2022;122(3):258.10.18520/cs/v122/i3/258-266Search in Google Scholar
[8] Xu G, Shen S, Zhang Y, Clements DR, Yang S, Li J, et al. Designing cropping systems to improve the management of the invasive weed Phalaris minor Retz. Agron. 2019;9(12):809.10.3390/agronomy9120809Search in Google Scholar
[9] Khan BA, Nadeem MA, Javaid MM, Maqbool R, Ikram M, Oraby H. Chemical synthesis, characterization, and dose optimization of chitosan-based nanoparticles of clodinofop propargyl and fenoxaprop-p-ethyl for management of Phalaris minor (little seed canary grass): First report. Green Process Synth. 2022;11(1):1118–27.10.1515/gps-2022-0096Search in Google Scholar
[10] Van der Meulen A, Chauhan BS. A review of weed management in wheat using crop competition. Crop Prot. 2017;95:38–44.10.1016/j.cropro.2016.08.004Search in Google Scholar
[11] Chhokar RS, Sharma RK, Sharma I. Weed management strategies in wheat-A review. J Wheat Res. 2012;4(2):1–21.10.1079/9781845938185.0001Search in Google Scholar
[12] Chaudhary N, Choudhary KK, Agrawal SB, Agrawal M. Pesticides usage, uptake and mode of action in plants with special emphasis on photosynthetic characteristics. Pestic Crop Prod Physiol Biochem Action. 2020;159–80.10.1002/9781119432241.ch9Search in Google Scholar
[13] Farooq N, Abbas T, Tanveer A, Javaid MM, Ali HH, Safdar ME, et al. Differential hormetic response of fenoxaprop-p-ethyl resistant and susceptible Phalaris minor populations: A potential factor in resistance evolution. Planta Daninha. 2019;37:e019187554.10.1590/s0100-83582019370100045Search in Google Scholar
[14] Duke SO. Why have no new herbicide modes of action appeared in recent years? Pest Manag Sci. 2012;68(4):505–12.10.1002/ps.2333Search in Google Scholar PubMed
[15] Mishra RK, Mohammad N, Roychoudhury N. Soil pollution: Causes, effects and control. Van Sangyan. 2016;3(1):1–14.Search in Google Scholar
[16] Kumar S. Biopesticides: A need for food and environmental safety. J Biofertil Biopestic. 2012;3(4):1–3.10.4172/2155-6202.1000e107Search in Google Scholar
[17] Pérez‐de‐Luque A, Rubiales D. Nanotechnology for parasitic plant control. Pest Manag Sci Form Pest Sci. 2009;65(5):540–5.10.1002/ps.1732Search in Google Scholar PubMed
[18] Kumar S, Bhanjana G, Sharma A, Dilbaghi N, Sidhu MC, Kim KH. Development of nanoformulation approaches for the control of weeds. Sci Total Env. 2017;586:1272–8.10.1016/j.scitotenv.2017.02.138Search in Google Scholar PubMed
[19] Dhillon NK, Mukhopadhyay SS. Nanotechnology and allelopathy: Synergism in action. J Crop Weed. 2015;11(2):187–91.Search in Google Scholar
[20] Yadav AS, Srivastava DS. Application of nano-technology in weed management: A Review. J Crop Sci Tech. 2015;4(2):21–3.Search in Google Scholar
[21] Kashyap PL, Xiang X, Heiden P. Chitosan nanoparticle-based delivery systems for sustainable agriculture. Int J Biol Macromol. 2015;77:36–51.10.1016/j.ijbiomac.2015.02.039Search in Google Scholar PubMed
[22] Rezazadeh NH, Buazar F, Matroodi S. Synergistic effects of combinatorial chitosan and polyphenol biomolecules on enhanced antibacterial activity of biofunctionalized silver nanoparticles. Sci Rep. 2020;10(1):1–13.10.1038/s41598-020-76726-7Search in Google Scholar PubMed PubMed Central
[23] Calvo P, Remuñan-López C, Vila-Jato JL, Alonso MJ. Chitosan and chitosan/ethylene oxide-propylene oxide block copolymer nanoparticles as novel carriers for proteins and vaccines. Pharm Res. 1997;14(10):1431–6.10.1023/A:1012128907225Search in Google Scholar PubMed
[24] Fatima B, Siddiqui SI, Nirala RK, Vikrant K, Kim KH, Ahmad R, et al. Facile green synthesis of ZnO–CdWO4 nanoparticles and their potential as adsorbents to remove organic dye. Env Pollu. 2021;271:116401.10.1016/j.envpol.2020.116401Search in Google Scholar PubMed
[25] Campos EVR, de Oliveira JL, Fraceto LF. Applications of controlled release systems for fungicides, herbicides, acaricides, nutrients, and plant growth hormones: A review. Adv Sci Eng Med. 2014;6(4):373–87.10.1166/asem.2014.1538Search in Google Scholar
[26] Rinaudo M. Chitin and chitosan: Properties and applications. Prog Polym Sci. 2006;31(7):603–32.10.1016/j.progpolymsci.2006.06.001Search in Google Scholar
[27] Oliveira HC, Stolf-Moreira R, Martinez CBR, Grillo R, de Jesus MB, Fraceto LF. Nanoencapsulation enhances the post-emergence herbicidal activity of atrazine against mustard plants. PLoS One. 2015;10(7):e0132971.10.1371/journal.pone.0132971Search in Google Scholar PubMed PubMed Central
[28] Alharby HF, Hakeem KR, Qureshi MI. Weed control through herbicide-loaded nanoparticles. Nanomaterials and plant potential. Cham: Springer; 2019. p. 507–27.10.1007/978-3-030-05569-1_20Search in Google Scholar
[29] Khan BA, Nadeem MA, Nawaz H, Amin MM, Abbasi GH, Nadeem M, et al. Pesticides: impacts on agriculture productivity, environment, and management strategies. Emerging contaminants and plants: Interactions, adaptations and remediation technologies. Cham: Springer International Publishing; 2023. p. 109–34.10.1007/978-3-031-22269-6_5Search in Google Scholar
[30] Irshad MA, Nawaz R, Ur Rehman MZ, Imran M, Ahmad J, Ahmad S, et al. Synthesis and characterization of titanium dioxide nanoparticles by chemical and green methods and their antifungal activities against wheat rust. Chemosphere. 2020;258:127352.10.1016/j.chemosphere.2020.127352Search in Google Scholar PubMed
[31] Li J, Li M, Gao X, Fang F. Corn straw mulching affects Parthenium hysterophorus and rhizosphere organisms. Crop Prot. 2018;113:90–6.10.1016/j.cropro.2018.08.002Search in Google Scholar
[32] Abbas T, Nadeem MA, Tanveer A, Zohaib A. Low doses of fenoxaprop-p-ethyl cause hormesis in littleseed canarygrass and wild oat. Planta Daninha. 2016;34(3):527–33.10.1590/s0100-83582016340300013Search in Google Scholar
[33] Sousa GF, Gomes DG, Campos EV, Oliveira JL, Fraceto LF, Stolf-Moreira R, et al. Post-emergence herbicidal activity of nanoatrazine against susceptible weeds. Front Env Sci. 2018;6:12.10.3389/fenvs.2018.00012Search in Google Scholar
[34] Grozi D. Stability valuation of some mixtures between foliar fertilizers and antigraminaceous herbicides for the grain yield of durum wheat. Sci Pap Ser Agri Agron. 2016;59:267–72.Search in Google Scholar
[35] Hamza A, Derbalah A, El-Nady M. Identification and mechanism of Echinochloa crus-galli resistance to fenoxaprop-p-ethyl with respect to physiological and anatomical differences. Sci World J. 2012;2102:893204.10.1100/2012/893204Search in Google Scholar PubMed PubMed Central
[36] Mohamed MA, Jaafar J, Ismail AF, Othman MHD, Rahman MA. Chapter 1 – Fourier transform infrared (FTIR) spectroscopy. In Hilal N, Ismail AF, Matsuura T, Oatley-Radcliffe DBT-MC, editors. Membrane characterization. Amsterdam: Elsevier; 2017. p. 3–29.10.1016/B978-0-444-63776-5.00001-2Search in Google Scholar
[37] Abisharani JM, Devikala S, Kumar RD, Arthanareeswari M, Kamaraj P. Green synthesis of TiO2 nanoparticles using Cucurbita pepo seeds extract. Mater Today Proc. 2019;14:302–7.10.1016/j.matpr.2019.04.151Search in Google Scholar
[38] Asl MS, Nayebi B, Motallebzadeh A, Shokouhimehr M. Nanoindentation and nano structural characterization of ZrB2–SiC composite doped with graphite nano flakes. Comp Part B Eng. 2019;175:107–53.10.1016/j.compositesb.2019.107153Search in Google Scholar
© 2023 the author(s), published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Research Articles
- Value-added utilization of coal fly ash and recycled polyvinyl chloride in door or window sub-frame composites
- High removal efficiency of volatile phenol from coking wastewater using coal gasification slag via optimized adsorption and multi-grade batch process
- Evolution of surface morphology and properties of diamond films by hydrogen plasma etching
- Removal efficiency of dibenzofuran using CuZn-zeolitic imidazole frameworks as a catalyst and adsorbent
- Rapid and efficient microwave-assisted extraction of Caesalpinia sappan Linn. heartwood and subsequent synthesis of gold nanoparticles
- The catalytic characteristics of 2-methylnaphthalene acylation with AlCl3 immobilized on Hβ as Lewis acid catalyst
- Biodegradation of synthetic PVP biofilms using natural materials and nanoparticles
- Rutin-loaded selenium nanoparticles modulated the redox status, inflammatory, and apoptotic pathways associated with pentylenetetrazole-induced epilepsy in mice
- Optimization of apigenin nanoparticles prepared by planetary ball milling: In vitro and in vivo studies
- Synthesis and characterization of silver nanoparticles using Origanum onites leaves: Cytotoxic, apoptotic, and necrotic effects on Capan-1, L929, and Caco-2 cell lines
- Exergy analysis of a conceptual CO2 capture process with an amine-based DES
- Construction of fluorescence system of felodipine–tetracyanovinyl–2,2′-bipyridine complex
- Excellent photocatalytic degradation of rhodamine B over Bi2O3 supported on Zn-MOF nanocomposites under visible light
- Optimization-based control strategy for a large-scale polyhydroxyalkanoates production in a fed-batch bioreactor using a coupled PDE–ODE system
- Effectiveness of pH and amount of Artemia urumiana extract on physical, chemical, and biological attributes of UV-fabricated biogold nanoparticles
- Geranium leaf-mediated synthesis of silver nanoparticles and their transcriptomic effects on Candida albicans
- Synthesis, characterization, anticancer, anti-inflammatory activities, and docking studies of 3,5-disubstituted thiadiazine-2-thiones
- Synthesis and stability of phospholipid-encapsulated nano-selenium
- Putative anti-proliferative effect of Indian mustard (Brassica juncea) seed and its nano-formulation
- Enrichment of low-grade phosphorites by the selective leaching method
- Electrochemical analysis of the dissolution of gold in a copper–ethylenediamine–thiosulfate system
- Characterisation of carbonate lake sediments as a potential filler for polymer composites
- Evaluation of nano-selenium biofortification characteristics of alfalfa (Medicago sativa L.)
- Quality of oil extracted by cold press from Nigella sativa seeds incorporated with rosemary extracts and pretreated by microwaves
- Heteropolyacid-loaded MOF-derived mesoporous zirconia catalyst for chemical degradation of rhodamine B
- Recovery of critical metals from carbonatite-type mineral wastes: Geochemical modeling investigation of (bio)hydrometallurgical leaching of REEs
- Photocatalytic properties of ZnFe-mixed oxides synthesized via a simple route for water remediation
- Attenuation of di(2-ethylhexyl)phthalate-induced hepatic and renal toxicity by naringin nanoparticles in a rat model
- Novel in situ synthesis of quaternary core–shell metallic sulfide nanocomposites for degradation of organic dyes and hydrogen production
- Microfluidic steam-based synthesis of luminescent carbon quantum dots as sensing probes for nitrite detection
- Transformation of eggshell waste to egg white protein solution, calcium chloride dihydrate, and eggshell membrane powder
- Preparation of Zr-MOFs for the adsorption of doxycycline hydrochloride from wastewater
- Green nanoarchitectonics of the silver nanocrystal potential for treating malaria and their cytotoxic effects on the kidney Vero cell line
- Carbon emissions analysis of producing modified asphalt with natural asphalt
- An efficient and green synthesis of 2-phenylquinazolin-4(3H)-ones via t-BuONa-mediated oxidative condensation of 2-aminobenzamides and benzyl alcohols under solvent- and transition metal-free conditions
- Chitosan nanoparticles loaded with mesosulfuron methyl and mesosulfuron methyl + florasulam + MCPA isooctyl to manage weeds of wheat (Triticum aestivum L.)
- Synergism between lignite and high-sulfur petroleum coke in CO2 gasification
- Facile aqueous synthesis of ZnCuInS/ZnS–ZnS QDs with enhanced photoluminescence lifetime for selective detection of Cu(ii) ions
- Rapid synthesis of copper nanoparticles using Nepeta cataria leaves: An eco-friendly management of disease-causing vectors and bacterial pathogens
- Study on the photoelectrocatalytic activity of reduced TiO2 nanotube films for removal of methyl orange
- Development of a fuzzy logic model for the prediction of spark-ignition engine performance and emission for gasoline–ethanol blends
- Micro-impact-induced mechano-chemical synthesis of organic precursors from FeC/FeN and carbonates/nitrates in water and its extension to nucleobases
- Green synthesis of strontium-doped tin dioxide (SrSnO2) nanoparticles using the Mahonia bealei leaf extract and evaluation of their anticancer and antimicrobial activities
- A study on the larvicidal and adulticidal potential of Cladostepus spongiosus macroalgae and green-fabricated silver nanoparticles against mosquito vectors
- Catalysts based on nickel salt heteropolytungstates for selective oxidation of diphenyl sulfide
- Powerful antibacterial nanocomposites from Corallina officinalis-mediated nanometals and chitosan nanoparticles against fish-borne pathogens
- Removal behavior of Zn and alkalis from blast furnace dust in pre-reduction sinter process
- Environmentally friendly synthesis and computational studies of novel class of acridinedione integrated spirothiopyrrolizidines/indolizidines
- The mechanisms of inhibition and lubrication of clean fracturing flowback fluids in water-based drilling fluids
- Adsorption/desorption performance of cellulose membrane for Pb(ii)
- A one-pot, multicomponent tandem synthesis of fused polycyclic pyrrolo[3,2-c]quinolinone/pyrrolizino[2,3-c]quinolinone hybrid heterocycles via environmentally benign solid state melt reaction
- Green synthesis of silver nanoparticles using durian rind extract and optical characteristics of surface plasmon resonance-based optical sensor for the detection of hydrogen peroxide
- Electrochemical analysis of copper-EDTA-ammonia-gold thiosulfate dissolution system
- Characterization of bio-oil production by microwave pyrolysis from cashew nut shells and Cassia fistula pods
- Green synthesis methods and characterization of bacterial cellulose/silver nanoparticle composites
- Photocatalytic research performance of zinc oxide/graphite phase carbon nitride catalyst and its application in environment
- Effect of phytogenic iron nanoparticles on the bio-fortification of wheat varieties
- In vitro anti-cancer and antimicrobial effects of manganese oxide nanoparticles synthesized using the Glycyrrhiza uralensis leaf extract on breast cancer cell lines
- Preparation of Pd/Ce(F)-MCM-48 catalysts and their catalytic performance of n-heptane isomerization
- Green “one-pot” fluorescent bis-indolizine synthesis with whole-cell plant biocatalysis
- Silica-titania mesoporous silicas of MCM-41 type as effective catalysts and photocatalysts for selective oxidation of diphenyl sulfide by H2O2
- Biosynthesis of zinc oxide nanoparticles from molted feathers of Pavo cristatus and their antibiofilm and anticancer activities
- Clean preparation of rutile from Ti-containing mixed molten slag by CO2 oxidation
- Synthesis and characterization of Pluronic F-127-coated titanium dioxide nanoparticles synthesized from extracts of Atractylodes macrocephala leaf for antioxidant, antimicrobial, and anticancer properties
- Effect of pretreatment with alkali on the anaerobic digestion characteristics of kitchen waste and analysis of microbial diversity
- Ameliorated antimicrobial, antioxidant, and anticancer properties by Plectranthus vettiveroides root extract-mediated green synthesis of chitosan nanoparticles
- Microwave-accelerated pretreatment technique in green extraction of oil and bioactive compounds from camelina seeds: Effectiveness and characterization
- Studies on the extraction performance of phorate by aptamer-functionalized magnetic nanoparticles in plasma samples
- Investigation of structural properties and antibacterial activity of AgO nanoparticle extract from Solanum nigrum/Mentha leaf extracts by green synthesis method
- Green fabrication of chitosan from marine crustaceans and mushroom waste: Toward sustainable resource utilization
- Synthesis, characterization, and evaluation of nanoparticles of clodinofop propargyl and fenoxaprop-P-ethyl on weed control, growth, and yield of wheat (Triticum aestivum L.)
- The enhanced adsorption properties of phosphorus from aqueous solutions using lanthanum modified synthetic zeolites
- Separation of graphene oxides of different sizes by multi-layer dialysis and anti-friction and lubrication performance
- Visible-light-assisted base-catalyzed, one-pot synthesis of highly functionalized cinnolines
- The experimental study on the air oxidation of 5-hydroxymethylfurfural to 2,5-furandicarboxylic acid with Co–Mn–Br system
- Highly efficient removal of tetracycline and methyl violet 2B from aqueous solution using the bimetallic FeZn-ZIFs catalyst
- A thermo-tolerant cellulase enzyme produced by Bacillus amyloliquefaciens M7, an insight into synthesis, optimization, characterization, and bio-polishing activity
- Exploration of ketone derivatives of succinimide for their antidiabetic potential: In vitro and in vivo approaches
- Ultrasound-assisted green synthesis and in silico study of 6-(4-(butylamino)-6-(diethylamino)-1,3,5-triazin-2-yl)oxypyridazine derivatives
- A study of the anticancer potential of Pluronic F-127 encapsulated Fe2O3 nanoparticles derived from Berberis vulgaris extract
- Biogenic synthesis of silver nanoparticles using Consolida orientalis flowers: Identification, catalytic degradation, and biological effect
- Initial assessment of the presence of plastic waste in some coastal mangrove forests in Vietnam
- Adsorption synergy electrocatalytic degradation of phenol by active oxygen-containing species generated in Co-coal based cathode and graphite anode
- Antibacterial, antifungal, antioxidant, and cytotoxicity activities of the aqueous extract of Syzygium aromaticum-mediated synthesized novel silver nanoparticles
- Synthesis of a silica matrix with ZnO nanoparticles for the fabrication of a recyclable photodegradation system to eliminate methylene blue dye
- Natural polymer fillers instead of dye and pigments: Pumice and scoria in PDMS fluid and elastomer composites
- Study on the preparation of glycerylphosphorylcholine by transesterification under supported sodium methoxide
- Wireless network handheld terminal-based green ecological sustainable design evaluation system: Improved data communication and reduced packet loss rate
- The optimization of hydrogel strength from cassava starch using oxidized sucrose as a crosslinking agent
- Green synthesis of silver nanoparticles using Saccharum officinarum leaf extract for antiviral paint
- Study on the reliability of nano-silver-coated tin solder joints for flip chips
- Environmentally sustainable analytical quality by design aided RP-HPLC method for the estimation of brilliant blue in commercial food samples employing a green-ultrasound-assisted extraction technique
- Anticancer and antimicrobial potential of zinc/sodium alginate/polyethylene glycol/d-pinitol nanocomposites against osteosarcoma MG-63 cells
- Nanoporous carbon@CoFe2O4 nanocomposite as a green absorbent for the adsorptive removal of Hg(ii) from aqueous solutions
- Characterization of silver sulfide nanoparticles from actinobacterial strain (M10A62) and its toxicity against lepidopteran and dipterans insect species
- Phyto-fabrication and characterization of silver nanoparticles using Withania somnifera: Investigating antioxidant potential
- Effect of e-waste nanofillers on the mechanical, thermal, and wear properties of epoxy-blend sisal woven fiber-reinforced composites
- Magnesium nanohydroxide (2D brucite) as a host matrix for thymol and carvacrol: Synthesis, characterization, and inhibition of foodborne pathogens
- Synergistic inhibitive effect of a hybrid zinc oxide-benzalkonium chloride composite on the corrosion of carbon steel in a sulfuric acidic solution
- Review Articles
- Role and the importance of green approach in biosynthesis of nanopropolis and effectiveness of propolis in the treatment of COVID-19 pandemic
- Gum tragacanth-mediated synthesis of metal nanoparticles, characterization, and their applications as a bactericide, catalyst, antioxidant, and peroxidase mimic
- Green-processed nano-biocomposite (ZnO–TiO2): Potential candidates for biomedical applications
- Reaction mechanisms in microwave-assisted lignin depolymerisation in hydrogen-donating solvents
- Recent progress on non-noble metal catalysts for the deoxydehydration of biomass-derived oxygenates
- Rapid Communication
- Phosphorus removal by iron–carbon microelectrolysis: A new way to achieve phosphorus recovery
- Special Issue: Biomolecules-derived synthesis of nanomaterials for environmental and biological applications (Guest Editors: Arpita Roy and Fernanda Maria Policarpo Tonelli)
- Biomolecules-derived synthesis of nanomaterials for environmental and biological applications
- Nano-encapsulated tanshinone IIA in PLGA-PEG-COOH inhibits apoptosis and inflammation in cerebral ischemia/reperfusion injury
- Green fabrication of silver nanoparticles using Melia azedarach ripened fruit extract, their characterization, and biological properties
- Green-synthesized nanoparticles and their therapeutic applications: A review
- Antioxidant, antibacterial, and cytotoxicity potential of synthesized silver nanoparticles from the Cassia alata leaf aqueous extract
- Green synthesis of silver nanoparticles using Callisia fragrans leaf extract and its anticancer activity against MCF-7, HepG2, KB, LU-1, and MKN-7 cell lines
- Algae-based green AgNPs, AuNPs, and FeNPs as potential nanoremediators
- Green synthesis of Kickxia elatine-induced silver nanoparticles and their role as anti-acetylcholinesterase in the treatment of Alzheimer’s disease
- Phytocrystallization of silver nanoparticles using Cassia alata flower extract for effective control of fungal skin pathogens
- Antibacterial wound dressing with hydrogel from chitosan and polyvinyl alcohol from the red cabbage extract loaded with silver nanoparticles
- Leveraging of mycogenic copper oxide nanostructures for disease management of Alternaria blight of Brassica juncea
- Nanoscale molecular reactions in microbiological medicines in modern medical applications
- Synthesis and characterization of ZnO/β-cyclodextrin/nicotinic acid nanocomposite and its biological and environmental application
- Green synthesis of silver nanoparticles via Taxus wallichiana Zucc. plant-derived Taxol: Novel utilization as anticancer, antioxidation, anti-inflammation, and antiurolithic potential
- Recyclability and catalytic characteristics of copper oxide nanoparticles derived from bougainvillea plant flower extract for biomedical application
- Phytofabrication, characterization, and evaluation of novel bioinspired selenium–iron (Se–Fe) nanocomposites using Allium sativum extract for bio-potential applications
- Erratum
- Erratum to “Synthesis, characterization, and evaluation of nanoparticles of clodinofop propargyl and fenoxaprop-P-ethyl on weed control, growth, and yield of wheat (Triticum aestivum L.)”
Articles in the same Issue
- Research Articles
- Value-added utilization of coal fly ash and recycled polyvinyl chloride in door or window sub-frame composites
- High removal efficiency of volatile phenol from coking wastewater using coal gasification slag via optimized adsorption and multi-grade batch process
- Evolution of surface morphology and properties of diamond films by hydrogen plasma etching
- Removal efficiency of dibenzofuran using CuZn-zeolitic imidazole frameworks as a catalyst and adsorbent
- Rapid and efficient microwave-assisted extraction of Caesalpinia sappan Linn. heartwood and subsequent synthesis of gold nanoparticles
- The catalytic characteristics of 2-methylnaphthalene acylation with AlCl3 immobilized on Hβ as Lewis acid catalyst
- Biodegradation of synthetic PVP biofilms using natural materials and nanoparticles
- Rutin-loaded selenium nanoparticles modulated the redox status, inflammatory, and apoptotic pathways associated with pentylenetetrazole-induced epilepsy in mice
- Optimization of apigenin nanoparticles prepared by planetary ball milling: In vitro and in vivo studies
- Synthesis and characterization of silver nanoparticles using Origanum onites leaves: Cytotoxic, apoptotic, and necrotic effects on Capan-1, L929, and Caco-2 cell lines
- Exergy analysis of a conceptual CO2 capture process with an amine-based DES
- Construction of fluorescence system of felodipine–tetracyanovinyl–2,2′-bipyridine complex
- Excellent photocatalytic degradation of rhodamine B over Bi2O3 supported on Zn-MOF nanocomposites under visible light
- Optimization-based control strategy for a large-scale polyhydroxyalkanoates production in a fed-batch bioreactor using a coupled PDE–ODE system
- Effectiveness of pH and amount of Artemia urumiana extract on physical, chemical, and biological attributes of UV-fabricated biogold nanoparticles
- Geranium leaf-mediated synthesis of silver nanoparticles and their transcriptomic effects on Candida albicans
- Synthesis, characterization, anticancer, anti-inflammatory activities, and docking studies of 3,5-disubstituted thiadiazine-2-thiones
- Synthesis and stability of phospholipid-encapsulated nano-selenium
- Putative anti-proliferative effect of Indian mustard (Brassica juncea) seed and its nano-formulation
- Enrichment of low-grade phosphorites by the selective leaching method
- Electrochemical analysis of the dissolution of gold in a copper–ethylenediamine–thiosulfate system
- Characterisation of carbonate lake sediments as a potential filler for polymer composites
- Evaluation of nano-selenium biofortification characteristics of alfalfa (Medicago sativa L.)
- Quality of oil extracted by cold press from Nigella sativa seeds incorporated with rosemary extracts and pretreated by microwaves
- Heteropolyacid-loaded MOF-derived mesoporous zirconia catalyst for chemical degradation of rhodamine B
- Recovery of critical metals from carbonatite-type mineral wastes: Geochemical modeling investigation of (bio)hydrometallurgical leaching of REEs
- Photocatalytic properties of ZnFe-mixed oxides synthesized via a simple route for water remediation
- Attenuation of di(2-ethylhexyl)phthalate-induced hepatic and renal toxicity by naringin nanoparticles in a rat model
- Novel in situ synthesis of quaternary core–shell metallic sulfide nanocomposites for degradation of organic dyes and hydrogen production
- Microfluidic steam-based synthesis of luminescent carbon quantum dots as sensing probes for nitrite detection
- Transformation of eggshell waste to egg white protein solution, calcium chloride dihydrate, and eggshell membrane powder
- Preparation of Zr-MOFs for the adsorption of doxycycline hydrochloride from wastewater
- Green nanoarchitectonics of the silver nanocrystal potential for treating malaria and their cytotoxic effects on the kidney Vero cell line
- Carbon emissions analysis of producing modified asphalt with natural asphalt
- An efficient and green synthesis of 2-phenylquinazolin-4(3H)-ones via t-BuONa-mediated oxidative condensation of 2-aminobenzamides and benzyl alcohols under solvent- and transition metal-free conditions
- Chitosan nanoparticles loaded with mesosulfuron methyl and mesosulfuron methyl + florasulam + MCPA isooctyl to manage weeds of wheat (Triticum aestivum L.)
- Synergism between lignite and high-sulfur petroleum coke in CO2 gasification
- Facile aqueous synthesis of ZnCuInS/ZnS–ZnS QDs with enhanced photoluminescence lifetime for selective detection of Cu(ii) ions
- Rapid synthesis of copper nanoparticles using Nepeta cataria leaves: An eco-friendly management of disease-causing vectors and bacterial pathogens
- Study on the photoelectrocatalytic activity of reduced TiO2 nanotube films for removal of methyl orange
- Development of a fuzzy logic model for the prediction of spark-ignition engine performance and emission for gasoline–ethanol blends
- Micro-impact-induced mechano-chemical synthesis of organic precursors from FeC/FeN and carbonates/nitrates in water and its extension to nucleobases
- Green synthesis of strontium-doped tin dioxide (SrSnO2) nanoparticles using the Mahonia bealei leaf extract and evaluation of their anticancer and antimicrobial activities
- A study on the larvicidal and adulticidal potential of Cladostepus spongiosus macroalgae and green-fabricated silver nanoparticles against mosquito vectors
- Catalysts based on nickel salt heteropolytungstates for selective oxidation of diphenyl sulfide
- Powerful antibacterial nanocomposites from Corallina officinalis-mediated nanometals and chitosan nanoparticles against fish-borne pathogens
- Removal behavior of Zn and alkalis from blast furnace dust in pre-reduction sinter process
- Environmentally friendly synthesis and computational studies of novel class of acridinedione integrated spirothiopyrrolizidines/indolizidines
- The mechanisms of inhibition and lubrication of clean fracturing flowback fluids in water-based drilling fluids
- Adsorption/desorption performance of cellulose membrane for Pb(ii)
- A one-pot, multicomponent tandem synthesis of fused polycyclic pyrrolo[3,2-c]quinolinone/pyrrolizino[2,3-c]quinolinone hybrid heterocycles via environmentally benign solid state melt reaction
- Green synthesis of silver nanoparticles using durian rind extract and optical characteristics of surface plasmon resonance-based optical sensor for the detection of hydrogen peroxide
- Electrochemical analysis of copper-EDTA-ammonia-gold thiosulfate dissolution system
- Characterization of bio-oil production by microwave pyrolysis from cashew nut shells and Cassia fistula pods
- Green synthesis methods and characterization of bacterial cellulose/silver nanoparticle composites
- Photocatalytic research performance of zinc oxide/graphite phase carbon nitride catalyst and its application in environment
- Effect of phytogenic iron nanoparticles on the bio-fortification of wheat varieties
- In vitro anti-cancer and antimicrobial effects of manganese oxide nanoparticles synthesized using the Glycyrrhiza uralensis leaf extract on breast cancer cell lines
- Preparation of Pd/Ce(F)-MCM-48 catalysts and their catalytic performance of n-heptane isomerization
- Green “one-pot” fluorescent bis-indolizine synthesis with whole-cell plant biocatalysis
- Silica-titania mesoporous silicas of MCM-41 type as effective catalysts and photocatalysts for selective oxidation of diphenyl sulfide by H2O2
- Biosynthesis of zinc oxide nanoparticles from molted feathers of Pavo cristatus and their antibiofilm and anticancer activities
- Clean preparation of rutile from Ti-containing mixed molten slag by CO2 oxidation
- Synthesis and characterization of Pluronic F-127-coated titanium dioxide nanoparticles synthesized from extracts of Atractylodes macrocephala leaf for antioxidant, antimicrobial, and anticancer properties
- Effect of pretreatment with alkali on the anaerobic digestion characteristics of kitchen waste and analysis of microbial diversity
- Ameliorated antimicrobial, antioxidant, and anticancer properties by Plectranthus vettiveroides root extract-mediated green synthesis of chitosan nanoparticles
- Microwave-accelerated pretreatment technique in green extraction of oil and bioactive compounds from camelina seeds: Effectiveness and characterization
- Studies on the extraction performance of phorate by aptamer-functionalized magnetic nanoparticles in plasma samples
- Investigation of structural properties and antibacterial activity of AgO nanoparticle extract from Solanum nigrum/Mentha leaf extracts by green synthesis method
- Green fabrication of chitosan from marine crustaceans and mushroom waste: Toward sustainable resource utilization
- Synthesis, characterization, and evaluation of nanoparticles of clodinofop propargyl and fenoxaprop-P-ethyl on weed control, growth, and yield of wheat (Triticum aestivum L.)
- The enhanced adsorption properties of phosphorus from aqueous solutions using lanthanum modified synthetic zeolites
- Separation of graphene oxides of different sizes by multi-layer dialysis and anti-friction and lubrication performance
- Visible-light-assisted base-catalyzed, one-pot synthesis of highly functionalized cinnolines
- The experimental study on the air oxidation of 5-hydroxymethylfurfural to 2,5-furandicarboxylic acid with Co–Mn–Br system
- Highly efficient removal of tetracycline and methyl violet 2B from aqueous solution using the bimetallic FeZn-ZIFs catalyst
- A thermo-tolerant cellulase enzyme produced by Bacillus amyloliquefaciens M7, an insight into synthesis, optimization, characterization, and bio-polishing activity
- Exploration of ketone derivatives of succinimide for their antidiabetic potential: In vitro and in vivo approaches
- Ultrasound-assisted green synthesis and in silico study of 6-(4-(butylamino)-6-(diethylamino)-1,3,5-triazin-2-yl)oxypyridazine derivatives
- A study of the anticancer potential of Pluronic F-127 encapsulated Fe2O3 nanoparticles derived from Berberis vulgaris extract
- Biogenic synthesis of silver nanoparticles using Consolida orientalis flowers: Identification, catalytic degradation, and biological effect
- Initial assessment of the presence of plastic waste in some coastal mangrove forests in Vietnam
- Adsorption synergy electrocatalytic degradation of phenol by active oxygen-containing species generated in Co-coal based cathode and graphite anode
- Antibacterial, antifungal, antioxidant, and cytotoxicity activities of the aqueous extract of Syzygium aromaticum-mediated synthesized novel silver nanoparticles
- Synthesis of a silica matrix with ZnO nanoparticles for the fabrication of a recyclable photodegradation system to eliminate methylene blue dye
- Natural polymer fillers instead of dye and pigments: Pumice and scoria in PDMS fluid and elastomer composites
- Study on the preparation of glycerylphosphorylcholine by transesterification under supported sodium methoxide
- Wireless network handheld terminal-based green ecological sustainable design evaluation system: Improved data communication and reduced packet loss rate
- The optimization of hydrogel strength from cassava starch using oxidized sucrose as a crosslinking agent
- Green synthesis of silver nanoparticles using Saccharum officinarum leaf extract for antiviral paint
- Study on the reliability of nano-silver-coated tin solder joints for flip chips
- Environmentally sustainable analytical quality by design aided RP-HPLC method for the estimation of brilliant blue in commercial food samples employing a green-ultrasound-assisted extraction technique
- Anticancer and antimicrobial potential of zinc/sodium alginate/polyethylene glycol/d-pinitol nanocomposites against osteosarcoma MG-63 cells
- Nanoporous carbon@CoFe2O4 nanocomposite as a green absorbent for the adsorptive removal of Hg(ii) from aqueous solutions
- Characterization of silver sulfide nanoparticles from actinobacterial strain (M10A62) and its toxicity against lepidopteran and dipterans insect species
- Phyto-fabrication and characterization of silver nanoparticles using Withania somnifera: Investigating antioxidant potential
- Effect of e-waste nanofillers on the mechanical, thermal, and wear properties of epoxy-blend sisal woven fiber-reinforced composites
- Magnesium nanohydroxide (2D brucite) as a host matrix for thymol and carvacrol: Synthesis, characterization, and inhibition of foodborne pathogens
- Synergistic inhibitive effect of a hybrid zinc oxide-benzalkonium chloride composite on the corrosion of carbon steel in a sulfuric acidic solution
- Review Articles
- Role and the importance of green approach in biosynthesis of nanopropolis and effectiveness of propolis in the treatment of COVID-19 pandemic
- Gum tragacanth-mediated synthesis of metal nanoparticles, characterization, and their applications as a bactericide, catalyst, antioxidant, and peroxidase mimic
- Green-processed nano-biocomposite (ZnO–TiO2): Potential candidates for biomedical applications
- Reaction mechanisms in microwave-assisted lignin depolymerisation in hydrogen-donating solvents
- Recent progress on non-noble metal catalysts for the deoxydehydration of biomass-derived oxygenates
- Rapid Communication
- Phosphorus removal by iron–carbon microelectrolysis: A new way to achieve phosphorus recovery
- Special Issue: Biomolecules-derived synthesis of nanomaterials for environmental and biological applications (Guest Editors: Arpita Roy and Fernanda Maria Policarpo Tonelli)
- Biomolecules-derived synthesis of nanomaterials for environmental and biological applications
- Nano-encapsulated tanshinone IIA in PLGA-PEG-COOH inhibits apoptosis and inflammation in cerebral ischemia/reperfusion injury
- Green fabrication of silver nanoparticles using Melia azedarach ripened fruit extract, their characterization, and biological properties
- Green-synthesized nanoparticles and their therapeutic applications: A review
- Antioxidant, antibacterial, and cytotoxicity potential of synthesized silver nanoparticles from the Cassia alata leaf aqueous extract
- Green synthesis of silver nanoparticles using Callisia fragrans leaf extract and its anticancer activity against MCF-7, HepG2, KB, LU-1, and MKN-7 cell lines
- Algae-based green AgNPs, AuNPs, and FeNPs as potential nanoremediators
- Green synthesis of Kickxia elatine-induced silver nanoparticles and their role as anti-acetylcholinesterase in the treatment of Alzheimer’s disease
- Phytocrystallization of silver nanoparticles using Cassia alata flower extract for effective control of fungal skin pathogens
- Antibacterial wound dressing with hydrogel from chitosan and polyvinyl alcohol from the red cabbage extract loaded with silver nanoparticles
- Leveraging of mycogenic copper oxide nanostructures for disease management of Alternaria blight of Brassica juncea
- Nanoscale molecular reactions in microbiological medicines in modern medical applications
- Synthesis and characterization of ZnO/β-cyclodextrin/nicotinic acid nanocomposite and its biological and environmental application
- Green synthesis of silver nanoparticles via Taxus wallichiana Zucc. plant-derived Taxol: Novel utilization as anticancer, antioxidation, anti-inflammation, and antiurolithic potential
- Recyclability and catalytic characteristics of copper oxide nanoparticles derived from bougainvillea plant flower extract for biomedical application
- Phytofabrication, characterization, and evaluation of novel bioinspired selenium–iron (Se–Fe) nanocomposites using Allium sativum extract for bio-potential applications
- Erratum
- Erratum to “Synthesis, characterization, and evaluation of nanoparticles of clodinofop propargyl and fenoxaprop-P-ethyl on weed control, growth, and yield of wheat (Triticum aestivum L.)”


