Green synthesis of silver nanoparticles via Taxus wallichiana Zucc. plant-derived Taxol: Novel utilization as anticancer, antioxidation, anti-inflammation, and antiurolithic potential
-
Sachin Bhusari
, Rajesh Raut
, Ramesh Chondekar
, Saad Alghamdi
Abstract
Throughout history, the utilization of plant products as medicinal remedies has been widespread, with numerous modern drugs finding their origins in the plant kingdom. Taxol, derived from Taxus species, stands out as an exceptional and highly potent anticancer medication. In this study, we present a rapid one-pot synthesis method for silver nanoparticles (AgNPs) using the leaves of Taxus wallichiana Zucca in the presence of sunlight. The synthesized AgNPs were comprehensively characterized using X-ray diffraction, transmission electron microscopy, and HPLC Q-TOF. The AgNPs were further investigated for their antioxidative, anticancer, anti-inflammatory, and antiurolithi properties. The anticancer activity was assessed through a sulforhodamine B assay conducted on the MDA-MB-231 human breast carcinoma cell line and SiHa human cervical cancer cell line. The findings of this study reveal the impressive antioxidative, anticancer, anti-inflammatory, and antiurolithi characteristics exhibited by AgNPs synthesized from leaf extracts. This research highlights an environmentally friendly and cost-effective approach to producing AgNPs by utilizing plant extracts as reducing agents, underscoring the immense potential of natural resources in advancing nanotechnology and its applications.
1 Introduction
The utilization of Himalayan yews for medicinal purposes dates back several millennia. Indigenous communities residing near the forests possess valuable traditional knowledge regarding the use of plants, and the medicinal practices of the region, including Ayurveda, Amchi, Han Chinese, and Unani, have been influenced by the healing properties of Himalayan medicinal plants. The yew, in particular, has long been employed in traditional medicine to address fevers and alleviate painful inflammations. Decoctions, herbal teas, and juices derived from the yew have been utilized to treat ailments such as colds, coughs, respiratory infections, indigestion, and epilepsy [1]. For centuries, the utilization of plants in the treatment of illnesses caused by microorganisms, including infectious diseases, has been prevalent. Medicinal plants are rich in a diverse array of phenolic compounds, such as flavonoids, stilbenes, phenols, coumarins, lignin, and tannins, which exhibit a wide range of biological effects, including antimicrobial and antioxidant properties [2].
Cancer remains a prominent global cause of mortality, and contemporary medical practices involving chemotherapy and radiation therapy carry recognized risks. In the realm of modern medicine, certain plant-derived substances, including vincristine, vinblastine, and Taxol, have exhibited noteworthy anticancer attributes. Taxus baccata L., in particular, has demonstrated long-standing efficacy in the treatment of cancer [3]. Moreover, noble metal nanoparticles (NPs), like silver, exhibit remarkable antibacterial characteristics, possess expansive reactive surfaces, and have been extensively utilized in various fields such as catalysis, electronics, optics, environmental studies, and biomedicine [4]. Compared to other yews, the Himalayan yew boasts a remarkable medicinal background, distinct for its absence of toxic taxine, which is found in European T. baccata. This plant possesses therapeutic qualities that have proven effective in treating indigestion and epilepsy [5,6] and possesses analgesic, antipyretic, and anticonvulsant properties [7]. The plant also contains bioactive molecules, including nematicidal compounds [8], and is traditionally used to treat high fever, pain, and inflammation [9]. Tinctures derived from Himalayan yew plants are employed as herbal remedies for various ailments such as headaches, dizziness, weak or irregular pulses, cold extremities, diarrhea, and intense biliousness. Additionally, the leaves of the plant possess emmenagogue and antispasmodic properties [10]. In recent years, there has been a growing interest in the synthesis of silver nanoparticles (AgNPs) through easy, sustainable, cost-effective, and eco-friendly approaches [11]. AgNPs have demonstrated promising potential in various areas, including anticancer, anti-inflammatory, and wound healing properties, as well as antimicrobial characteristics [12]. While methods like electro-irradiation [13], laser-mediated [14], and photochemical treatment are commonly used for AgNPs synthesis, they are often expensive and environmentally toxic. In contrast biological-based methods utilizing aqueous plant or microbial extracts are preferred. The biological process of synthesizing AgNPs using plant extracts offers several advantages, such as lower toxicity, compatibility, longer synthesis time, and a narrow particle size distribution [11]. Plant extracts contain numerous secondary metabolites that serve as reducing agents, playing a crucial role in the capping and stabilization of AgNPs. Notably, medicinal plants like Peaonia emodi [15], Althaea rosea, Swertia chirata, Bergenia stracheyi, and Solanum xanthocarpum possess secondary metabolites of pharmaceutical importance [16]. AgNPs have been successfully synthesized from different parts of these plant species, including roots, leaves, flowers, and fruits, which hold significant pharmaceutical value [17]. The anticancer activity of AgNPs derived from plants has been demonstrated against a variety of cancer cell lines, including breast cancer cell lines MCF-7 and MDA-MB-231, and brain cancer cells U251 [18]. The compound has also been reported to be effective against human hepatoma SMMC-7721 cells [19].
In this study, we synthesize AgNPs through a one-pot method and characterize them using X-ray diffraction (XRD), transmission electron microscopy (TEM), and HPLC techniques. We also evaluated the anticancer, antioxidant, antiurothilic, and anti-inflammatory properties of these AgNPs derived from Taxus wallichiana. Notably, this is the inaugural investigation showcasing the application of T. wallichiana aqueous leaf-based AgNPs in combating MDA-MB-231 human breast carcinoma and SiHa human cervical cancer cell lines for their anticancer activity.
2 Materials and methods
2.1 Materials
Silver nitrate, ascorbic acid, and 2,2-diphenylpicrylhydrazyl (DPPH) were sourced from Sigma Aldrich, Muller Hilton media (Hi-Media) and were employed without any supplementary treatment. All experiments were conducted using double distilled water. Whatman filter paper number 1 was used for the filtration of plant extract. Sigma-Aldrich was the source of paclitaxel (≥97%), while analytical grade ethanol and acetonitrile, as well as dimethyl sulfoxide, and ammonium acetate were purchased from Fisher Scientific.
2.2 Methods
2.2.1 Preparation of leaf extract for HPLC analysis
About 0.5 g of T. wallichiana leaf powder was accurately weighed and placed in 15 mL polypropylene (PP) tubes. About 4 mL of ethanol was added to each tube and vortexed briefly, then allowed to stand for 15 min to ensure proper extraction, and vortexed once more. Subsequently, sonication was performed using a K615HTDP water bath-type sonicator at a frequency of 50 Hz for 5 min, with a pulse rate of 2:1. After sonication, the samples were subjected to centrifugation using a Bio-era Microcentrifuge at 3,000 rpm for 20 min. The resulting supernatant was carefully transferred to a clean 15 mL PP tube. This process was repeated twice, with each supernatant being collected and combined into a clean 50 mL PP tube. The extract was then diluted four-fold using ethanol, with 250 μL of the sample mixed with 750 μL of ethanol. Finally, the diluted extract was stored in HPLC vials for analysis.
2.2.2 Preparation of leaf extract for the synthesis of AgNPs
The preparation of the crude extract from the leaf material was conducted following the previously described method [11], with slight modifications. The leaf extract was filtered using Whatman filter paper number 1 to obtain a clear solution, which served as the stock solution.
2.2.3 Synthesis of AgNPs
In the first step, a 10 mL of stock solution of the leaf extract was gradually mixed with 1 mM of silver nitrate solution (90 mL). The resulting mixture was thoroughly stirred and exposed to sunlight for a duration of 5 min, leading to a transformation from a colorless solution to brown. Subsequently, the solution was subjected to centrifugation at 10,000 rpm for 15 min, following the methodology outlined in ref. [12]. To eliminate any traces of biological matter, the resulting pellets were washed with ethanol and subsequently dispersed in Milli-Q water, adhering to the procedure described in ref. [13].
2.2.4 Characterization of AgNPs
The presence of AgNPs was confirmed using a UV–visible (UV–Vis) spectrophotometer Systronic 2203 [14]. The crystal structure of AgNPs was investigated using XRD, employing a BRUKER D8 Advance diffractometer operating at 40 kV, 40 mA, and Cu kα radiation at 2θ angle. The morphology of AgNPs was examined using TEM with a Philips CM 200, in addition to TEM analysis.
2.2.5 Quantitative screening
2.2.5.1 LC-Q-TOF-MS
In order to prepare the crude alcoholic extract, powdered leaf samples were sonicated in 95% ethanol using an Ultrasonicator made by Sonics. After the crude alcoholic samples were extracted, they were dissolved in 0.9 mL of alcohol and 0.1 mL of 0.1% formic acid. The leaf extract was analyzed using liquid chromatography-tandem mass spectrometry (LC-Q-TOF-MS 6540, ultra-high definition [UHD]). The mass spectrometer was equipped with an ESI Jet Stream source.
2.2.5.2 LC parameter
The mobile phase consisted of 0.1% formic acid in water and 1.1% formic acid in acetonitrile, flowing at a rate of 0.6 mL·min−1. Chromatographic separation was performed using a Reprosil C18 column from Dr Maisch, Germany. The mobile phase composition started at 10% B and gradually increased to 100% B over 14 min, before returning to the initial state. The setup included an auto-sampler, well plate, vacuum degasser, and thermostat column compartment. Compound identification utilized the LC-Q-TOF-MS 6540 (UHD) with a jet stream ESI source.
2.2.5.3 Q-TOF parameter
The experiment utilized an LC system with a dual electrospray jet stream Agilent Q-TOF, operating in positive ion mode. The following parameters were used: capillary voltage of 4,000 V, nebulizer pressure of 45 psi (N2), gas temperature at 32.5°C, drying gas flow rate of 8 L·min−1, nozzle voltage of 1,000 V, the fragmented voltage of 150 V, skimmer voltage of 65 V, and a mass range of 100–1,700 m/z. Additionally, a sheath gas flow rate of 11 L·min−1 and a sheath gas temperature of 350°C were employed. Data acquisition was performed using the Agilent Mass Hunter software.
2.2.5.4 Chromatographic analysis
An Agilent 1260 series HPLC system was used for chromatographic analysis. It consisted of a quaternary pump, vacuum degasser, column oven, photodiode array detector, and EZChrome software. The separation employed an Agilent 1260 Infinity C-18 column (5 μm X-bridge, 250 cm length, 4.6 mm diameter). Two mobile phases, A (water) and B (acetonitrile), were delivered to the column in a gradient fashion at a flow rate of 0.8 mL·min−1. The column temperature was maintained at 30°C. Analysis was performed at 30°C with a detection wavelength of 231 nm. Each sample was injected at a volume of 1 μL.
2.2.5.5 Quantitation and statistical analyses
The EZChrome software was used for HPLC analysis, including quantitation and statistical analyses. A calibration curve with three replicates was generated by plotting the peak area of paclitaxel against its corresponding amount. Paclitaxel concentrations in the samples were determined by fitting their peak areas into the calibration curve equation. UV detection at 231 nm was employed, and external calibration curves using paclitaxel standards were utilized for the determination of paclitaxel concentrations.
2.2.6 Antioxidant studies
The scavenging activity of antioxidants was assessed using DPPH radicals. Plant extracts at various concentrations were mixed with DPPH and either AgNPs or a test sample. After incubation, the absorbance at 517 nm was measured to calculate inhibition percentages. Thin-layer chromatography (TLC) dot blots with DPPH were used to investigate antioxidation. AgNPs were applied to a silica gel-coated plate, followed by the introduction of DPPH. White spots indicated radical scavenging. Hydrogen peroxide scavenging activity was evaluated by measuring absorbance at 230 nm after incubation with test fractions. The scavenging abilities were calculated using a formula. The ferric reducing power assay examined the reduction capacity of synthesized NPs. Extracts, phosphate buffer, and potassium ferricyanide were incubated, followed by centrifugation. The reaction was stopped, and the supernatant was mixed with distilled water and FeCl3. Ascorbic acid served as the reference compound.
2.2.7 Anti-inflammatory studies
In this study, we examined the anti-inflammatory properties of AgNPs produced from T. wallichiana. To evaluate their effects, we investigated their ability to inhibit protein denaturation and used the human red blood cell (HRBC) membrane stabilization method. Various concentrations of AgNPs were introduced into water containing 0.1% bovine serum albumin. The pH of the solution was adjusted to 7 using an acidic solution. Subsequently, the sample mixtures were incubated at 37°C for 20 min, followed by an additional 20 min at 51°C. We measured the turbidity of the samples using spectrophotometry at a wavelength of 660 nm.
2.2.8 Studies on the potential of T. wallichiana NPs to prevent kidney stone formation
2.2.8.1 Calcium oxalate crystal nucleation inhibition assay (turbidity method)
We investigated the antiurolithiatic properties of T. wallichiana NPs by assessing their ability to inhibit calcium oxalate crystal formation and aggregation. The turbidity method was used to measure crystal formation inhibition. Solutions of calcium chloride and sodium oxalate were prepared in a pH 6.5 buffer. T. wallichiana NPs and cystone standard solutions (100–500 µg·mL−1) were added to the calcium chloride solution, followed by the introduction of a sodium oxalate solution. The resulting solution was stirred at 37°C, and the induction times were compared to determine the nucleation rate. For the evaluation of crystal aggregation inhibition, a spectrophotometric assay was employed. Calcium oxalate monohydrate crystals were generated from potassium chloride and sodium oxalate solutions. The crystals were diluted and incubated in the presence or absence of T. wallichiana AgNPs and Cystone at concentrations ranging from 100 to 500 µg·mL−1. The percentage inhibition of aggregation was calculated using the same formula as the turbidity method.
2.2.9 Anticancer studies
The anticancer potential of T. wallichiana AgNPs was investigated against SiHa cervical cancer and MDA-MB-231 breast cancer cell lines. The cell viability was assessed using the Sulforhodamine B assay. Cancer cells were cultured and incubated with varying concentrations of AgNPs (10, 20, 40, and 80 µg·mL−1) for 48 h. After fixation and staining, the percentage of growth inhibition was calculated by comparing the absorbance of test wells to control wells, following the methodology described in ref. [20].
3 Results and discussion
3.1 UV–Vis spectroscopy analysis
Figure 1 illustrates the validation of the reduction process from silver ions to AgNPs, which was confirmed through UV and visible spectroscopy. Typically, AgNPs exhibit a brown color in water and display peak absorption in the range of 420–450 nm when stimulated by specific light wavelengths. Upon the introduction of the plant extract into a 1 mM silver nitrate solution, a noticeable change was observed, transforming the initially colorless solution into a dark brown solution. Analysis of the UV-Vis spectrum, covering the range of 200–1,100 nm, revealed the absorption spectra of the AgNPs formed in the reaction mixture. Notably, a prominent absorption peak at 440 nm was observed in the absorbance readings [21,22,23].
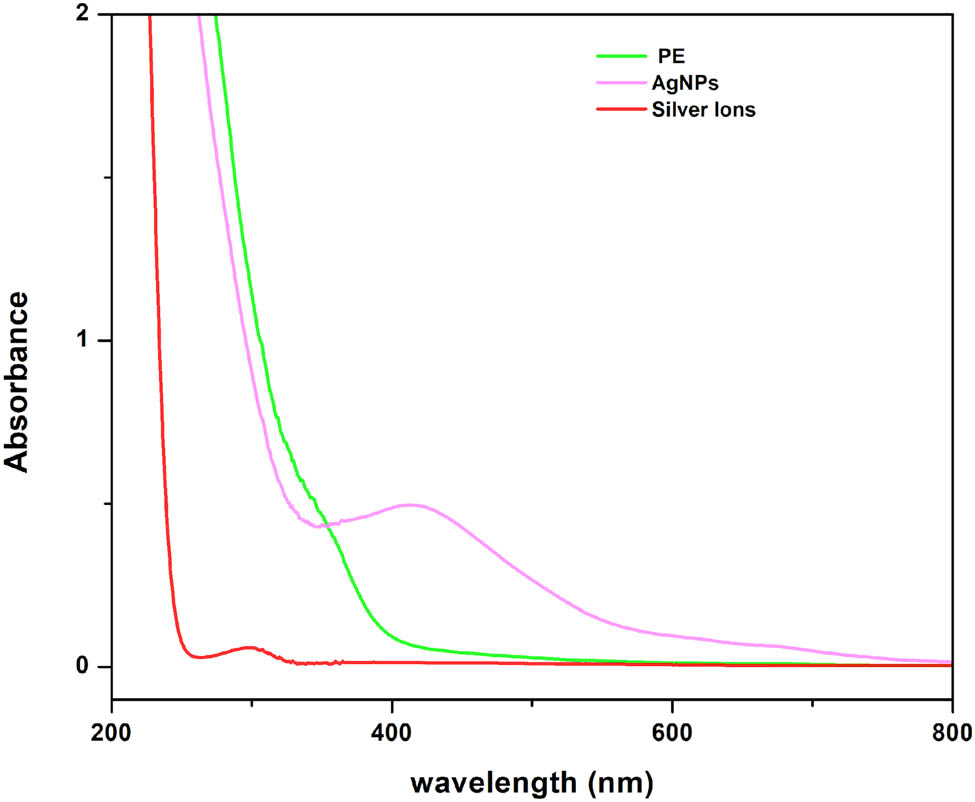
UV–Vis spectrum of T. wallichiana crude extract, AgNO3, 5 min of exposure to sunlight of AgNPs.
3.2 XRD analysis
The synthesized AgNPs, after undergoing multiple rounds of centrifugation and washing for purification, were dried and subjected to analysis using an X-ray diffractometer. X-ray powder diffraction is a rapid analytical technique that provides valuable information regarding the dimensions of the unit cell. In Figure 2, the XRD pattern of AgNPs is depicted, revealing characteristic peaks observed at 2θ values of 38.11°, 44.27°, 64.42°, 77.47°, and 82.0°. These peaks can be assigned to the respective planes of (111), (200), (220), (311), and (222) based on the JCPDS 04-0783.
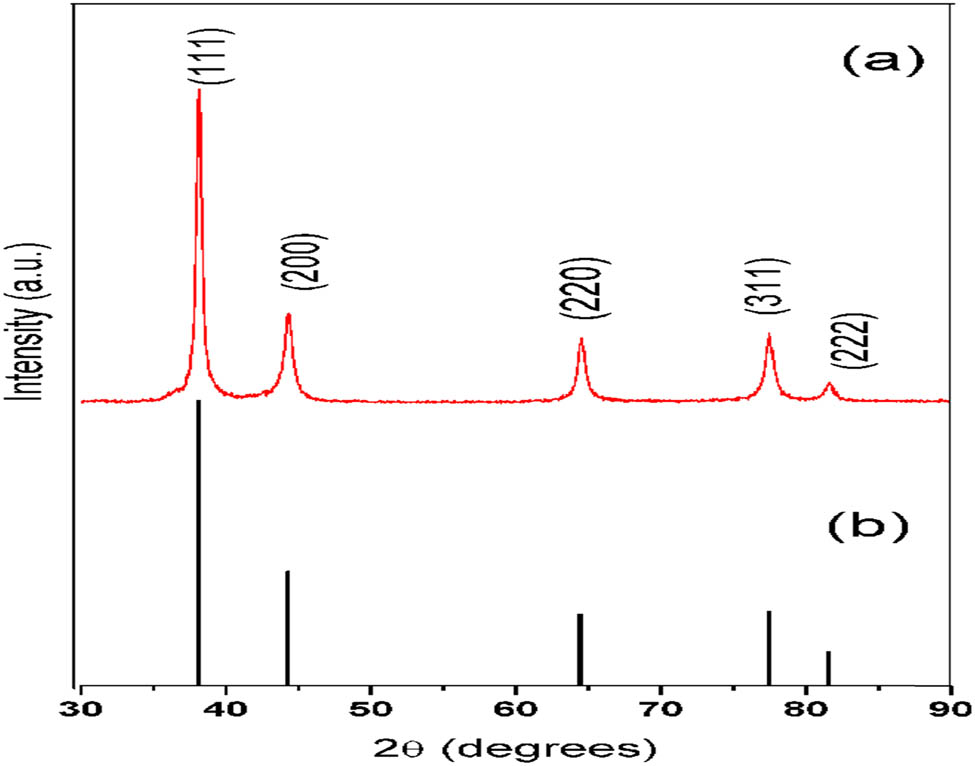
(a) XRD pattern for synthesized AgNPs and (b) pattern from JCPDS file No. 04-078.
3.3 TEM
In Figure 3, the particle size and shape are characterized using TEM images. The images revealed that the NPs had a nanoscale size, with an approximate diameter of 50 nm. The NPs appeared mostly spherical, displaying smooth edges. Overall, the NPs exhibited a circular shape and were uniformly dispersed at an appropriate distance from one another.
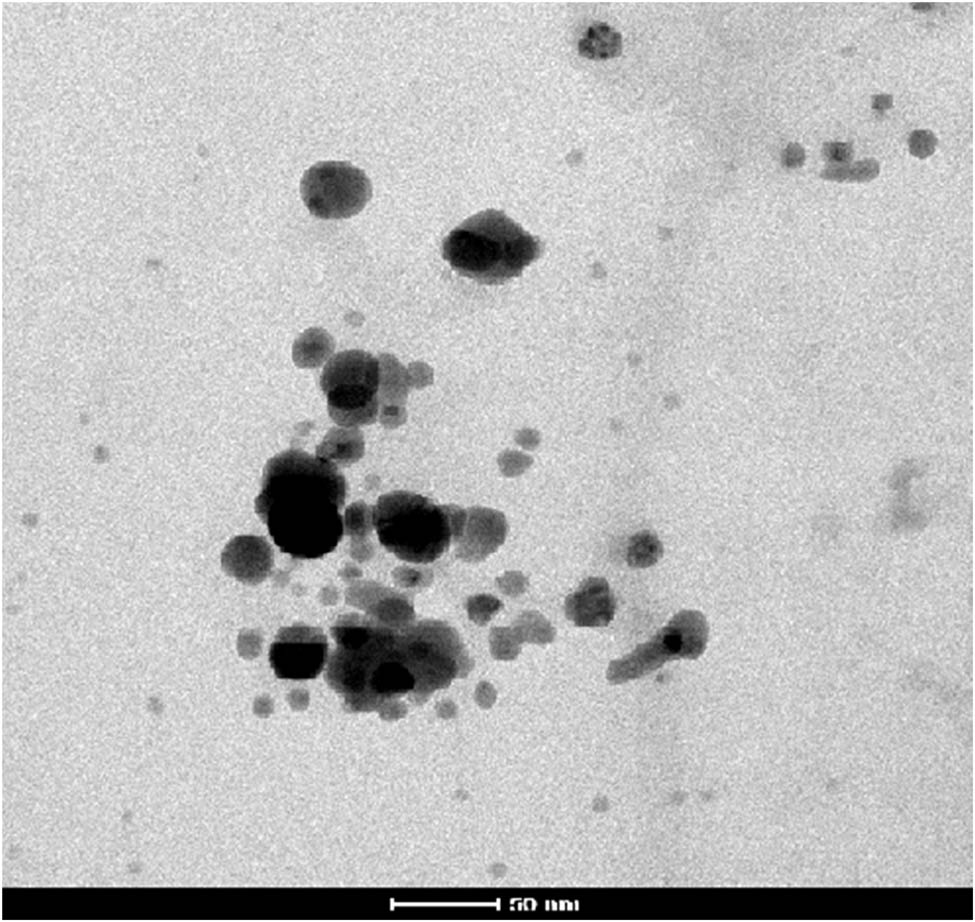
TEM images of AgNPs synthesized from dried leaves of T. wallichiana.
3.4 HPLC-Q-TOF-MS
The precise concentration of paclitaxel was determined through HPLC-UV assessment using a combination of UPLC-Q-TOF/MS analysis, purpose screening, and quantitative analysis. In the target MS/MS mode, disintegration spectra were obtained for selected compounds to elucidate their likely structures. During the non-target screening, three compounds with m/z values of 737.23, 712.32, and 585.27 were identified, which could potentially impact the quantitation by HPLC-UV. Notably, the highest concentration of compound 5 was discovered in the needles of T. baccata cv. Repandens, representing 0.02% of the dry weight.
3.5 High-performance liquid chromatography
In order to quantify Taxol and identify its presence in the leaf of T. wallichiana, purified samples (20 µL) were subjected to HPLC analysis using an Agilent (1260 Infinity) system equipped with a C18 column (250 mm × 4.6 mm, 5 μm X-Bridge water), as depicted in Figure 4. The elution of Taxol from the column was monitored at 231 nm using a UV detector and EZChrome software, employing an isocratic flow rate of 0.8 L‧min−1 with an mobile phase consisting of MeOH:AcN:H2O (20:40:40 v/v). The quantification of Taxol was performed by comparing the known quantities of authentic Taxol with the measured quantities using a standard curve. Additionally, the UV spectra at 231 nm of the HPLC-purified Taxol were compared with those of authentic Taxol to confirm their similarity. The analysis verified the presence of 16.712 ng of Taxol in the leaf samples.
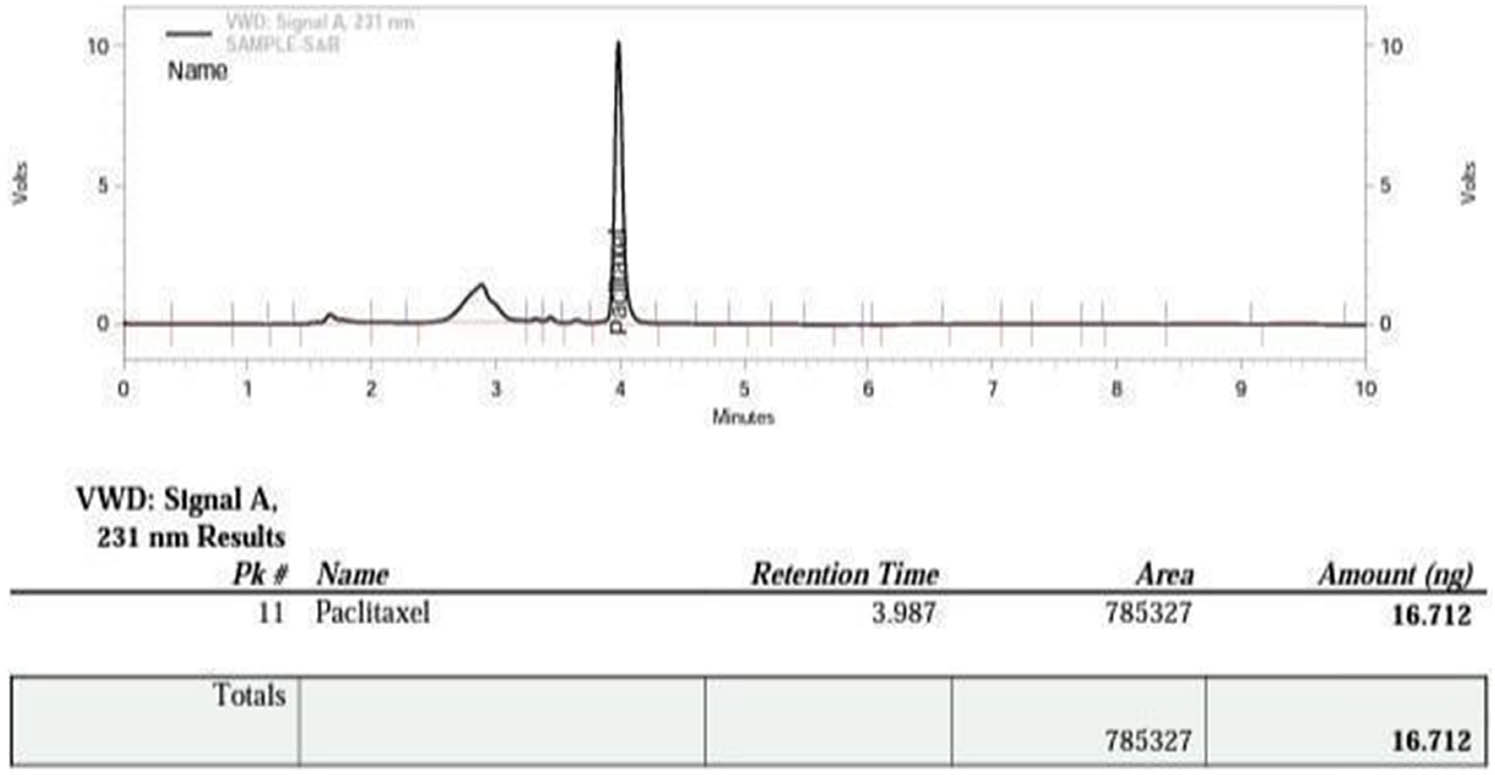
Method validation of paclitaxel.
To validate the HPLC method for paclitaxel, a method validation study was conducted. The validation process encompassed various parameters, including robustness, accuracy, recovery, precision, and linearity, to ensure the suitability of the developed method for the intended purpose. A series of solutions were prepared using a stock solution of paclitaxel, and the linearity responses were evaluated across concentration ranges spanning from 100 to 600 µg·mL−1, as demonstrated in Figure 5.
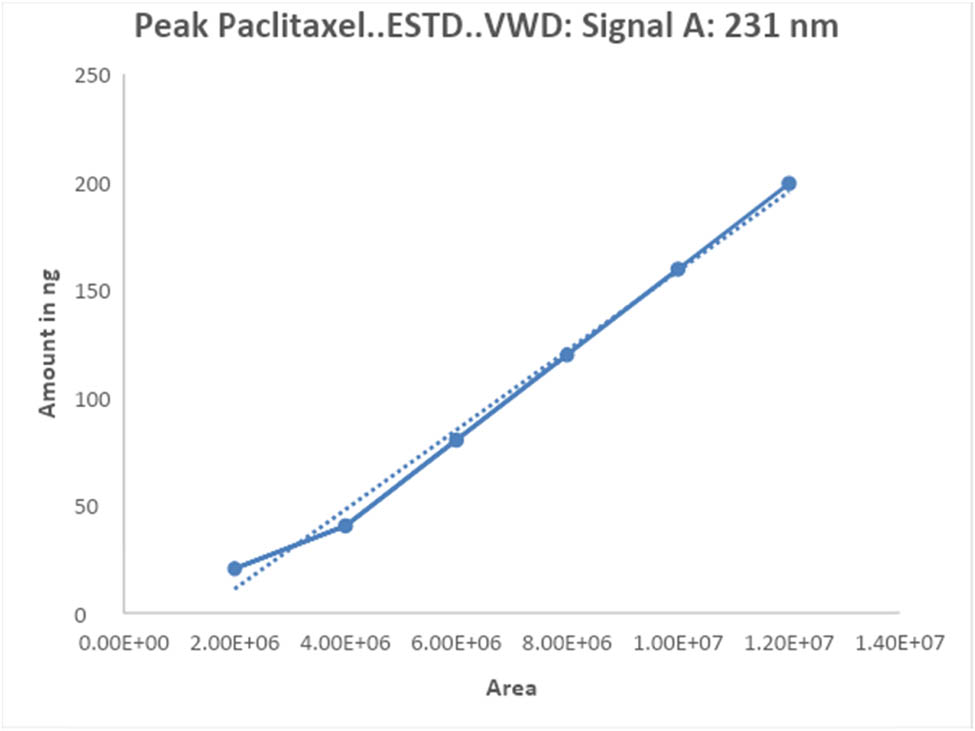
Linearity plot of paclitaxel by the proposed method.
3.6 Antioxidation activity
3.6.1 DPPH method
The scavenging activity of AgNPs against free radicals was evaluated using the DPPH radical as a substrate, with concentrations ranging from 100 to 500 µg·mL−1. The results indicated significant scavenging activity by AgNPs at different concentrations. At 100, 200, and 400 µg·mL−1, AgNPs exhibited free radical scavenging activities of 45.91%, 53.06%, and 36.73%, respectively (Figure 6). As a positive control, ascorbic acid demonstrated 95% inhibition at 500 µg·mL−1.
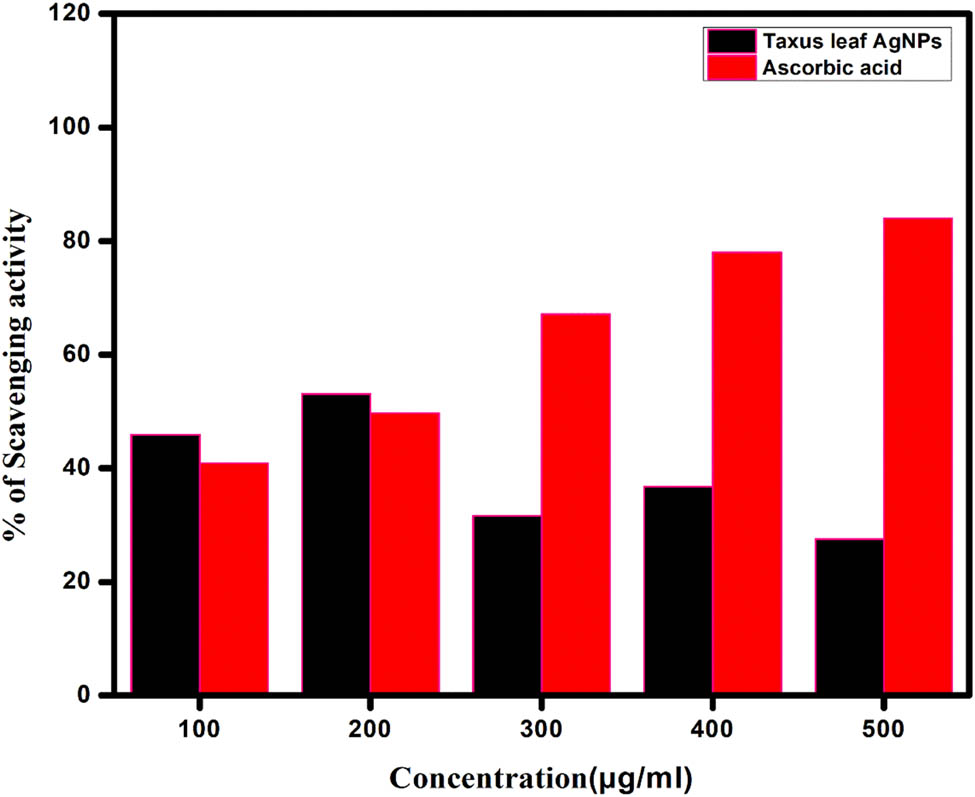
DPPH scavenging activity of T. wallichiana at different concentrations.
The antioxidant properties of T. wallichiana were found to be potent, and its reducing power was observed. When reacting with antioxidant molecules, DPPH radicals are scavenged through hydrogen donation, resulting in a reduction in their absorbance and a visible color change from purple to yellow. DPPH is commonly employed as a substrate to assess the antioxidative activity of antioxidants. Based on these findings, it can be concluded that AgNPs produced using T. wallichiana possess antioxidant properties by donating hydrogen [15].
3.6.2 TLC profiling method
In this study, we investigated the ability of AgNPs to scavenge light purple molecules using the dot blot method (Figure 7). The percent scavenging activity of AgNPs was determined through spectrophotometric analysis at various concentrations (Figure 8b). To assess the antioxidant activity of the crude plant extract qualitatively, we employed TLC, which offers a simple and efficient approach. In order to confirm the presence of antioxidant compounds in the extracted AgNPs, a TLC qualitative antioxidant assay was conducted. When the TLC plate was sprayed or dipped in DPPH solution, yellow antioxidant spots emerged against a purple background. Furthermore, the detection of Taxol was achieved by heating sulfuric acid with Vanillin (1% w/v), resulting in a dark grey to blue spot after 24 h. The obtained results were compared with the 2016 TLC Atlas drug for reference. The study observed yellow staining for compounds exhibiting antioxidant activity, while the remaining portion of the TLC plate displayed purple staining. A TLC plate sprayed with DPPH displayed a yellow color band and a blue band at different RF values (RF value of 0.65 in Figure 7a and RF value of 0.33 in Figure 7b), indicating potent antioxidant activity. These results tentatively identified the two compounds as phenolic compounds possessing potent antioxidant properties. The objective of this research was to establish a biological fingerprint capable of identifying active compounds in complex samples, and the analysis of thin-layer chromatography revealed the presence of various biomolecules in the extract.
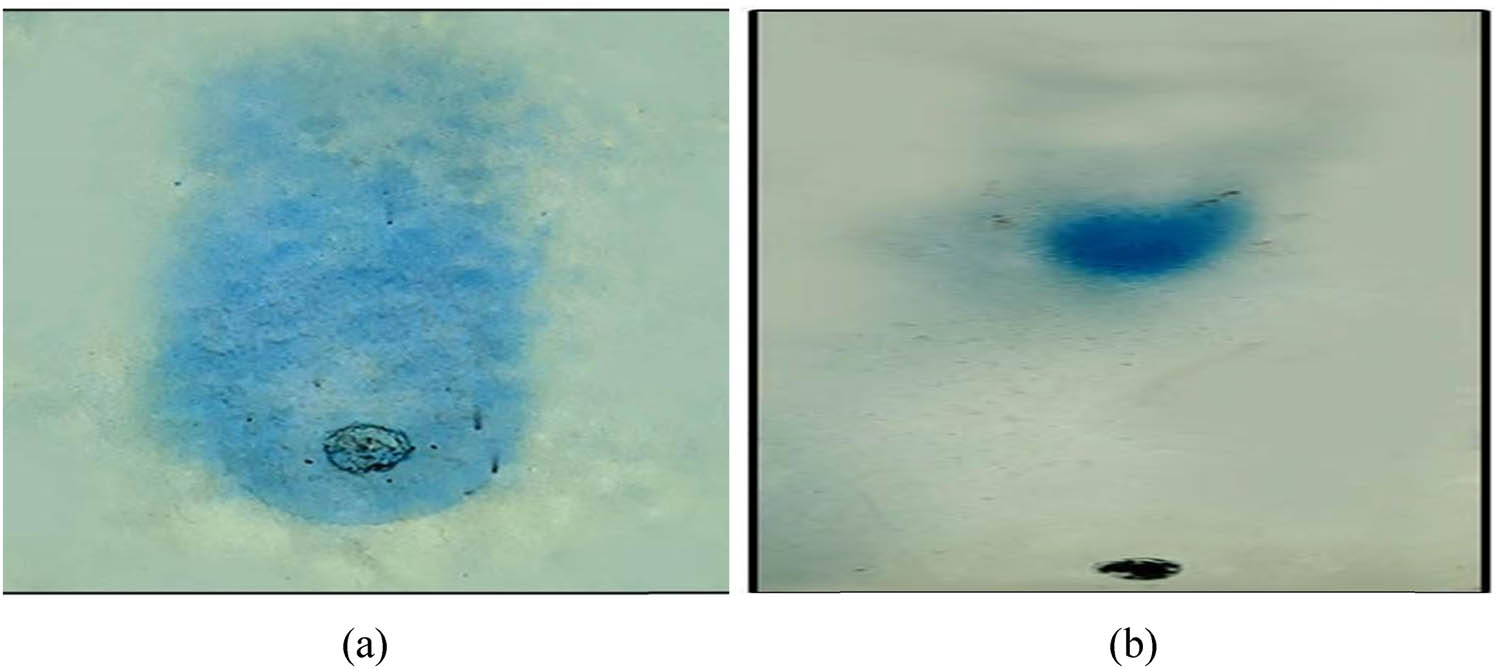
The TLC-DPPH assay of T. wallichiana AgNPs after spraying DPPH: (a) Taxus leaf extract after DPPH spray show retention factor of 0.65 and (b) AgNPs with Taxus after DPPH spray show retention factor of 0.33.
Radical scavenging activity of T. wallichiana at different concentrations.
3.6.3 H2O2 scavenging activity
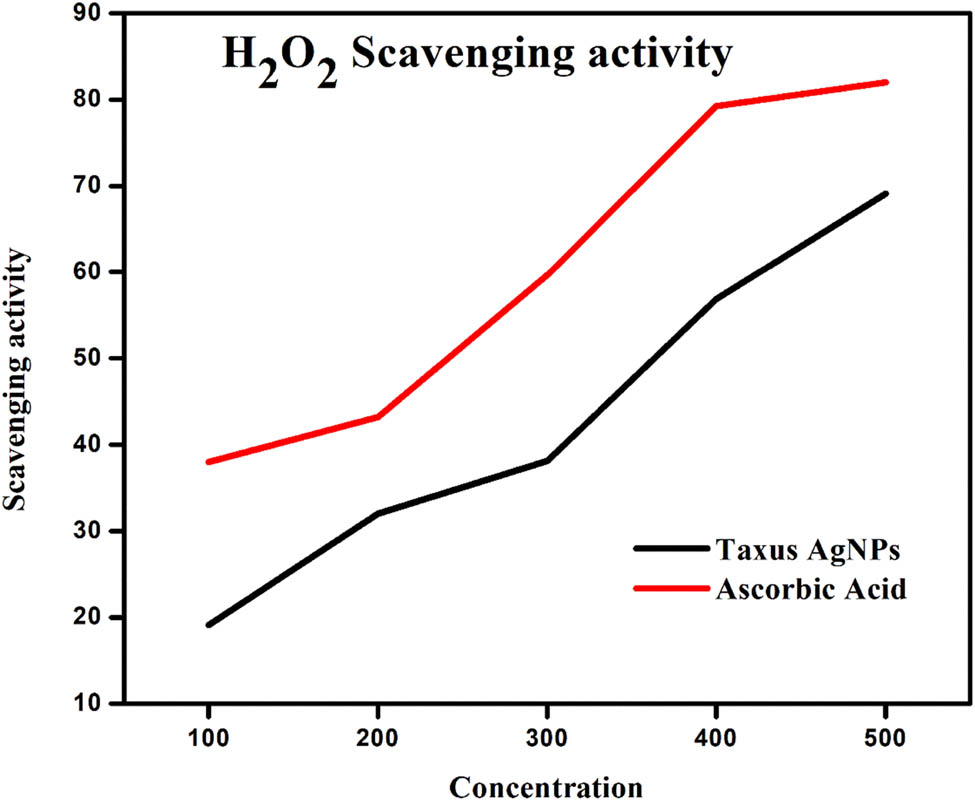
Hydrogen peroxide is naturally present in various environments, including water, plants, bacteria, food, and the human body, albeit at low concentrations. Within living organisms, certain oxidizing enzymes like superoxide dismutase can generate this compound, which gradually oxidizes different substances [24]. The study demonstrated that T. wallichiana AgNPs, at a concentration of 500 µg·mL−1, were capable of scavenging 69.12% of hydrogen peroxide, whereas ascorbic acid exhibited a hydrogen peroxide scavenging activity of 82.00% at the same concentration. These findings confirm the efficacy of T. wallichiana AgNPs as hydrogen peroxide scavengers, as depicted in Figure 8. Additionally, the research explored the impact of T. wallichiana AgNPs on neutralizing hydrogen peroxide radicals to prevent the oxidative deterioration of substrates.
3.6.4 Ferric-reducing power assay
The ferric-reducing power assay is a method used to assess the ability of antioxidants to convert Fe3+/ferricyanide to its ferrous form. As the reducing power increases, the absorbance also increases, indicating the need for higher doses of T. wallichiana AgNPs [25]. The results of the ferric-reducing antioxidant content test conducted on T. wallichiana AgNPs are presented in Figure 9. To investigate the anti-inflammatory activity of T. wallichiana AgNPs, denaturation and hemolysis of proteins were examined in vitro. Table 1 demonstrates that both AgNPs and sodium diclofenac can inhibit protein denaturation, with AgNPs exhibiting greater effectiveness in reducing inflammation. Protein denaturation triggers inflammatory signals and aggregation due to the loss of quaternary structure [26]. Previous research indicating the inhibition of albumin denaturation by AgNPs further supports their anti-inflammatory properties [27].
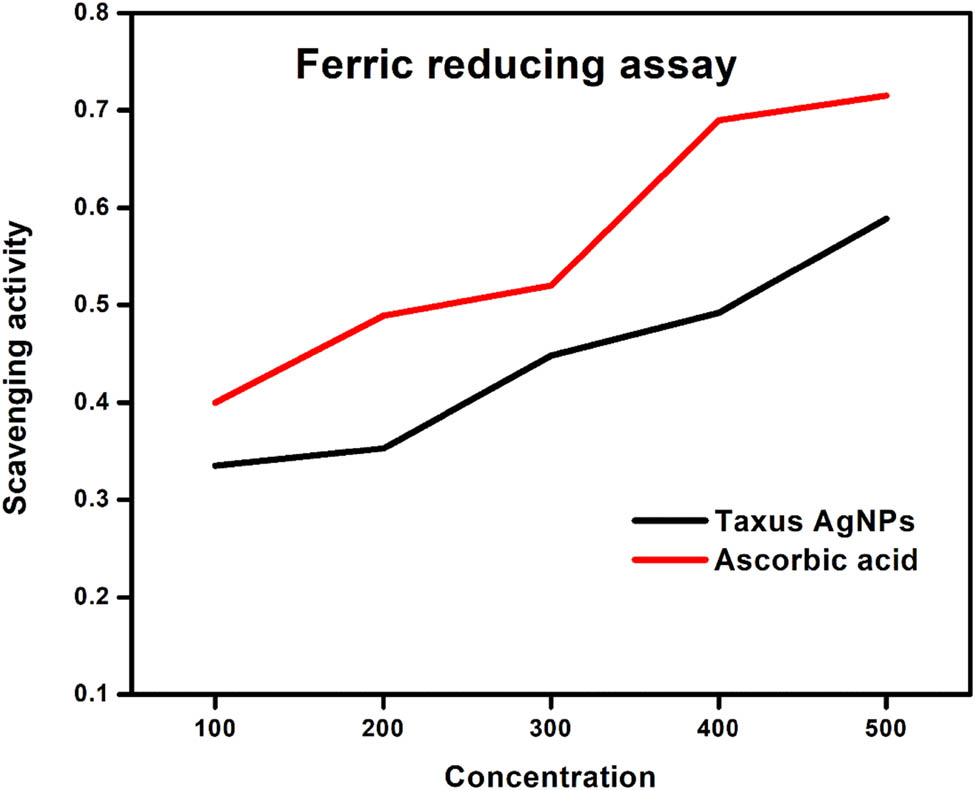
Ferric reducing power assay at different concentrations of T. wallichiana AgNPs.
Heat-induced and hypotonicity studies of the effect of AgNPs on HRBC membrane hemolysis of erythrocytes
| Treatment(s) | Concentration (μg·mL−1) | Absorbance at 560 nm | % Inhibition of hemolysis | ||
|---|---|---|---|---|---|
| For heat-induced studies | For hypotonicity-induced studies | For heat-induced studies | For hypotonicity-induced studies | ||
| Control | — | 0.32 | 0.33 | — | — |
| T. wallichiana AgNPs | 100 | 0.29** | 0.41** | 9.37 | 24.24 |
| 200 | 0.39** | 0.30** | 21.87 | 36.36 | |
| 300 | 0.22* | 0.20* | 31.25 | 39.39 | |
| 400 | 0.20* | 0.50** | 37.5 | 51.51 | |
| 500 | 010NS | 0.56** | 68.75 | 69.69 | |
| Sodium diclofenac | 100 | 0.08 | 0.13 | 75 | 60.60 |
| SE | 0.043 | 0.063 | |||
| CD | 5% | 0.112 | 0.162 | ||
| CD | 1% | 0.175 | 0.255 | ||
SE, standard error; CD, critical difference.
* Indicates the heat induced and hypotonicity is minimum.
** Indicates the heat induced and hypotonicity is maximum.
Additionally, the study investigated the membrane-stabilizing effects of T. wallichiana AgNPs by evaluating hypotonicity-induced hemolysis of HRBCs. The results in Table 1 indicate an increasing inhibition of heat-induced and hypotonic hemolysis with higher concentrations of AgNPs. This evaluation focused on the membrane stabilization effect as excessive fluid accumulation in cells can lead to membrane rupture and hemolysis [28].
Inhibition of protein denaturation is a well-known mechanism for suppressing various inflammatory mediators [29]. Different drugs, such as sodium diclofenac, phenyl butanone, and salicylic acid, exhibit varying degrees of anti-denaturation effects depending on their dosage [30]. Natural compounds derived from plants have also shown comparable anti-inflammatory properties to synthetic drugs [31].
In vitro tests for screening anti-inflammatory drugs typically involve inhibiting denaturation, a common cause of rheumatoid arthritis [32]. Nonsteroidal anti-inflammatory drugs, which block prostaglandin production and relieve pain, are commonly used. Anti-inflammatory agents achieve this by inhibiting COX enzymes or protecting lysosomal membranes from degradation [30]. Most anti-inflammatory drugs have been found to stabilize erythrocyte plasma membranes, preventing heat and hypotonicity-induced hemolysis.
This study found that concentrations of T. wallichiana AgNPs ranging from 100 to 500 µg·mL−1 protected human RBC membranes from lysis during inflammation, potentially through their stabilizing effect, which prevents the leakage of fluids and serum proteins from tissues induced by inflammatory mediators [33]. The membrane-stabilizing effect suggests that AgNPs could serve as an anti-inflammatory compound by protecting red blood cell membranes from hemolysis [34].
3.7 In vitro antiurolithiatic studies
3.7.1 Nucleation assay (turbidity method) and aggregation assay
The demand for herbal medicines in developed nations is on the rise due to their effectiveness, safety, and minimal side effects in primary healthcare. Unlike allopathic medicines that target specific aspects of a disease, herbal therapies have demonstrated success in addressing various stages of stone pathophysiology. Renal stones, which commonly consist of CaOx, occur as a result of urine supersaturation, followed by the processes of crystallization, nucleation, growth, and aggregation. By preventing supersaturation or intervening in the later stages of crystallization, the development of lithiasis can be averted.
In this study, the inhibition of CaOx crystallization at each phase was achieved using AgNPs synthesized from T. wallichiana leaf extract. Both the plant extracts and AgNPs exhibited inhibitory effects on kidney stone development by impeding the processes of nucleation and aggregation (Figure 10). The AgNPs derived from T. wallichiana may contain phytochemicals that hinder the growth of CaOx crystals. Increasing the dosage of the plant extract resulted in greater inhibition of CaOx crystal growth, with the highest percentage of inhibition observed at a concentration of 500 µg·mL−1. Figure 11 illustrates the aggregation of AgNPs following treatment with T. wallichiana extract at different concentrations. The test drug showcased significant anti-lithiasis properties, exhibiting a higher percentage of inhibition compared to the standard drug at the same concentration. These findings indicate that the test drug holds promise as a potential alternative treatment for kidney stones.
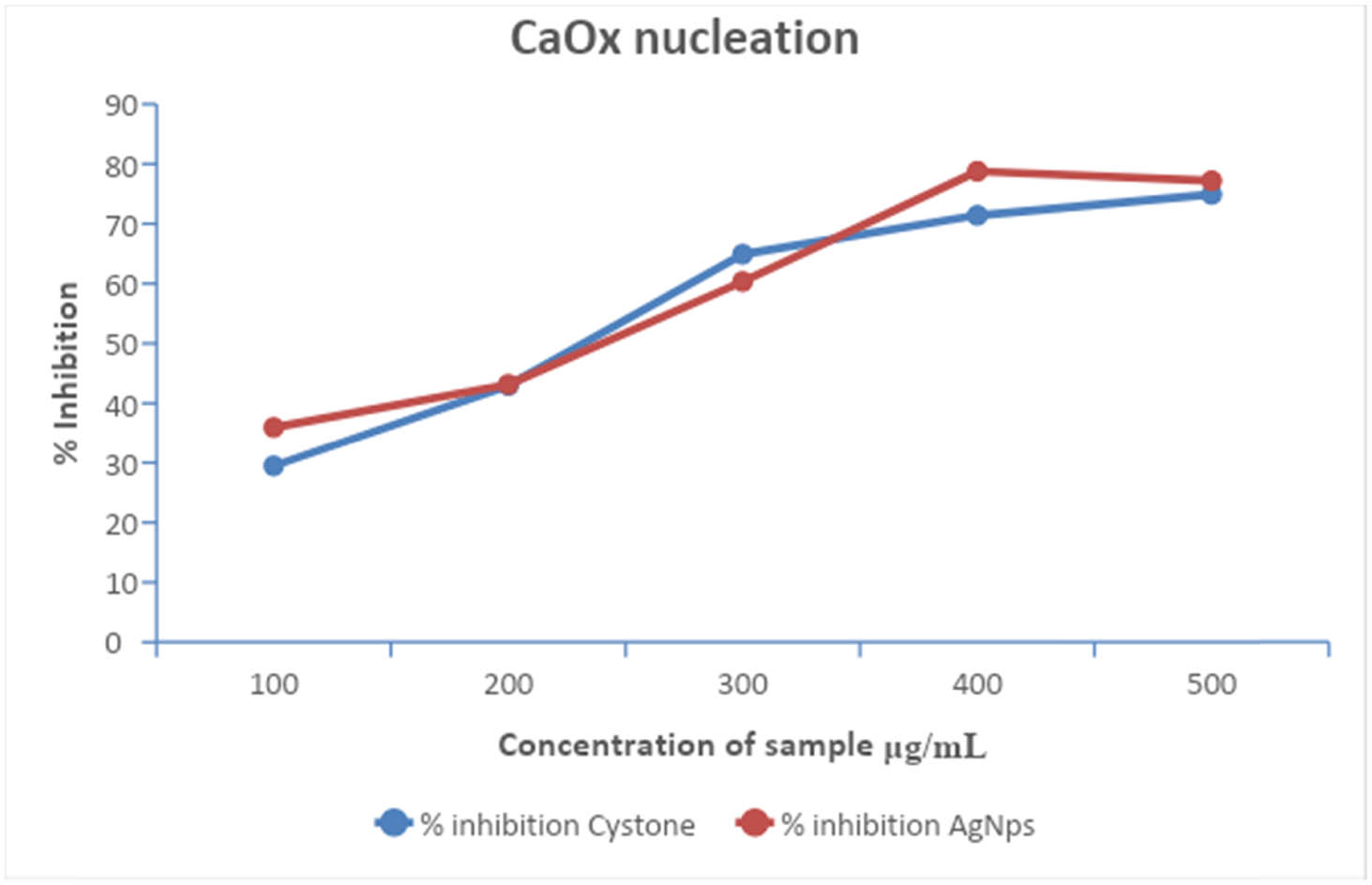
CaOx nucleation in AgNPs and T. wallichiana extract at different concentrations.
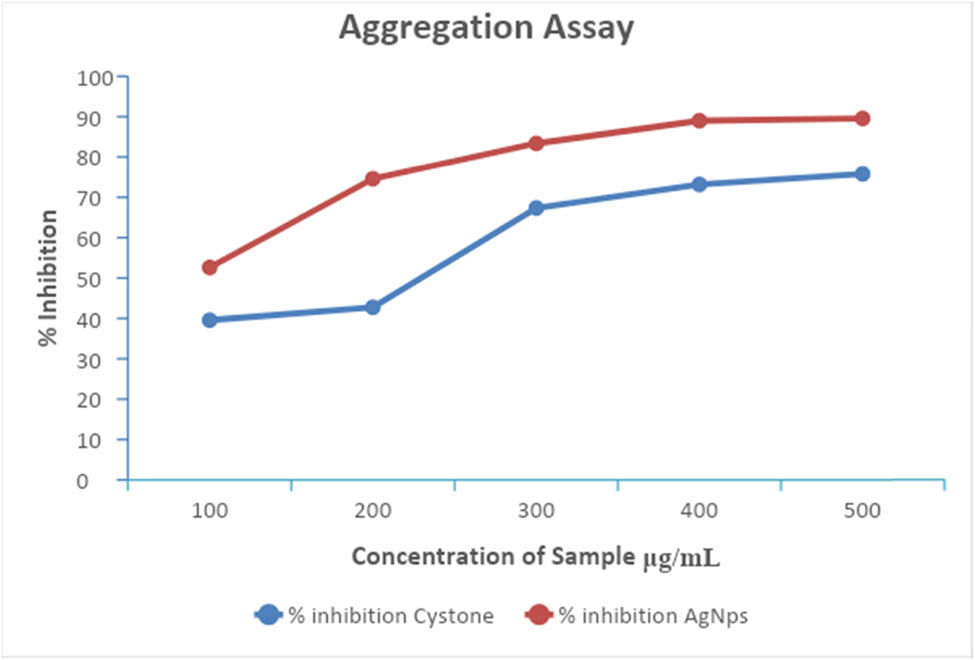
AgNPs aggregation after treatment with T. wallichiana extract at different concentrations.
3.8 Anticancer activity
The AgNPs synthesized from T. wallichiana leaf extract exhibited superior anticancer activity compared to Adriamycin (ADR), as shown in Table 2. The experimental groups were compared with the ADR group, which served as a positive control (Table 2). The growth inhibition (GI50) was calculated by multiplying [(Ti − Tz)/(C − Tz)] by 100, resulting in a 50% inhibition of net proteins. To calculate the total growth inhibition (TGI), Ti was calculated as Tz LC50 (the concentration of the drug that leads to a 50% reduction in measured protein after treatment), indicating a net loss of 50% of cells:
Anticancer activities of AgNPs and control (ADR)
| Samples | Human cervical cancer cell line SiHa | |||
|---|---|---|---|---|
| Drug concentration (μg‧mL−1) | ||||
| 10 | 20 | 40 | 80 | |
| AgNPs | 99.5 | 103.6 | 106.3 | 39.5 |
| ADR | −51.2 | −27.6 | −34.5 | −23.1 |
| Human breast cancer cell line MDA-MB-231 | ||||
| AgNPs | 103.5 | 91.3 | 69.8 | 14.3 |
| ADR | −48.0 | −55.6 | −56.7 | −49.9 |
| LC 50 TGI GI50 studies | |||
|---|---|---|---|
| Human cervical cancer cell line SiHa | |||
| LC 50 | TGI | GI50 | |
| AgNPs | NE | NE | 78.6 |
| ADR | NE | <10 | <10 |
| Human breast cancer cell line MDA-MB-231 | |||
| AgNPs | NE | 92.2 | 53.0 |
| ADR | NE | <10 | <10 |
LC50: concentration of drug causing 50% cell kill; GI50: concentration of drug causing 50% inhibition of cell growth; TGI: concentration of drug causing total inhibition of cell growth; ADR: Adriamycin, positive control compound; NE: not evaluated.
4 Conclusion
In this study, a comprehensive range of analytical techniques was employed to assess the characteristics of AgNPs synthesized utilizing plant extracts and to quantify the concentration of paclitaxel in T. wallichiana. The outcomes confirmed the successful synthesis of AgNPs exhibiting a smooth spherical shape and identified functional groups that contribute to the reduction and capping of AgNPs. Moreover, the study demonstrated the remarkable antioxidation and anti-inflammatory activities of AgNPs derived from T. wallichiana. These findings collectively indicate the promising biomedical and pharmaceutical applications of AgNPs produced through plant extracts. The study provides significant insights into the properties and potential uses of AgNPs, underscoring their relevance in diverse research fields.
Acknowledgement
The authors would like to acknowledge their respective university for supporting this research work.
-
Funding information: The authors state no funding involved.
-
Author contributions: Sachin Bhusari: writing – original draft, methodology, formal analysis; Parvindar M. Sah: writing – original draft, formal analysis; Jaya Lakkakula: writing – original draft, formal analysis; Arpita Roy: writing – original draft, supervision, writing – review and editing, project administration; Rajesh Raut: writing – review and editing, supervision; Ramesh Chondekar: project administration, supervision, writing – review and editing; Saad Alghamdi: writing – review and editing, resources; Mazen Almehmadi: writing – review and editing, resources; Mamdouh Allahyani: writing – review and editing, resources; Ahad Amer Alsaiari: writing – review and editing, resources; Abdulelah Aljuaid: writing – review and editing, resources; Nabeela Al-Abdullah: writing – review and editing, resources. All authors read and approved the final manuscript.
-
Conflict of interest: One of the corresponding authors (Arpita Roy) is a Guest Editor of the Green Processing and Synthesis’ Special Issue “Biomolecules-derived synthesis of nanomaterials for environmental and biological applications” in which this article is published.
-
Data availability statement: All relevant data are included in the manuscript.
References
[1] Nisar M, Khan I, Ahmad B, Ali I, Ahmad W, Choudhary MI. Antifungal and antibacterial activities of Taxus wallichiana Zucc. J Enzyme Inhib Med Chem. 2008;23(2):256–60. 10.1080/14756360701505336.Search in Google Scholar PubMed
[2] Prakash V, Rana S, Sagar A, Ved Prakash C. Analysis of antibacterial and antioxidant activity of Taxus baccata L. J Med Plants Stud. 2018;6(5):40–4, https://www.plantsjournal.com/archives/2018/vol6issue5/PartA/6-4-49-995.pdf.Search in Google Scholar
[3] Koul B, Poonia AK, Yadav D, Jin JO. Microbe-mediated biosynthesis of nanoparticles: applications and future prospects. Biomolecules. 2021;11(6):886. 10.3390/biom11060886.Search in Google Scholar PubMed PubMed Central
[4] Cheng F, Betts JW, Kelly SM, Schaller J, Heinze T. Synthesis and antibacterial effects of aqueous colloidal solutions of silver nanoparticles using aminocellulose as a combined reducing and capping reagent. Green Chem. 2013;15(4):989–98. 10.1039/c3gc36831a.Search in Google Scholar
[5] Liu W, Ge T, Pan Z, Leng Y, Lv J, Li B. The effects of herbal medicine on epilepsy. Oncotarget. 2017;8(29):48385–97, www.impactjournals.com/oncotarget/.10.18632/oncotarget.16801Search in Google Scholar PubMed PubMed Central
[6] Chattopadhyay SK, Pal A, Maulik PR, Kaur T, Garg A, Khanuja SPS. Taxoid from the needles of the Himalayan yew Taxus wallichiana with cytotoxic and immunomodulatory activities. Bioorg Med Chem Lett. 2006;16(9):2446–9. 10.1016/j.bmcl.2006.01.077.Search in Google Scholar PubMed
[7] MubarakAli D, Thajuddin N, Jeganathan K, Gunasekaran M. Plant extract mediated synthesis of silver and gold nanoparticles and its antibacterial activity against clinically isolated pathogens. Colloids Surf B Biointerfaces. 2011;85(2):360–5. 10.1016/j.colsurfb.2011.03.009.Search in Google Scholar PubMed
[8] Dong JY, He HP, Shen YM, Zhang KQ. Nematicidal epipolysulfanyldioxopiperazines from Gliocladium roseum. J Nat Prod. 2005;68(10):1510–3. 10.1021/np0502241.Search in Google Scholar PubMed
[9] Joshi R, Satyal P, Setzer W. Himalayan aromatic medicinal plants: a review of their ethnopharmacology, volatile phytochemistry, and biological activities. Medicines. 2016;3(1):6. 10.3390/medicines3010006.Search in Google Scholar PubMed PubMed Central
[10] Khan M, Verma SC, Srivastava SK, Shawl AS, Syamsundar KV, Khanuja SPS, et al. Essential oil composition of Taxus wallichiana Zucc. from the Northern Himalayan region of India. Flavour Fragr J. 2006;21(5):772–5. 10.1002/ffj.1682.Search in Google Scholar
[11] Van Phu D, Quoc LA, Duy NN, Lan NTK, Du BD, Luan LQ, et al. Study on antibacterial activity of silver nanoparticles synthesized by gamma irradiation method using different stabilizers. Nanoscale Res Lett. 2014;9(1):1–5. 10.1186/1556-276X-9-162.Search in Google Scholar PubMed PubMed Central
[12] Salve P, Vinchurkar A, Raut R, Chondekar R, Lakkakula J, Roy A, et al. An evaluation of antimicrobial, anticancer, anti-inflammatory and antioxidant activities of silver nanoparticles synthesized from leaf extract of Madhuca longifolia utilizing quantitative and qualitative methods. Molecules. 2022;27(19):6404. 10.3390/molecules27196404.Search in Google Scholar PubMed PubMed Central
[13] Zia F, Ghafoor N, Iqbal M, Mehboob S. Green synthesis and characterization of silver nanoparticles using Cydonia oblong seed extract. Appl Nanosci. 2016;6(7):1023–9. 10.1007/s13204-016-0517-z.Search in Google Scholar
[14] Srirangam GM, Rao KP. Synthesis and charcterization of silver nanoparticles from the leaf extract of Malachra capitata (L.). Rasayan J Chem. 2017;10(1):46–53. 10.7324/RJC.2017.1011548.Search in Google Scholar
[15] Dela Torre GLT, Arollado EC, Atienza AA, Manalo RAM. Evaluation of antioxidant capacity and identification of bioactive compounds of crude methanol extracts of Caesalpinia pulcherrima (L.) Swartz. Indian J Pharm Sci. 2017;79(1):113–23. 10.4172/pharmaceutical-sciences.1000207.Search in Google Scholar
[16] El Jemli M, Kamal R, Marmouzi I, Zerrouki A, Cherrah Y, Alaoui K. Radical-scavenging activity and ferric reducing ability ofJuniperus thurifera (L.), J. oxycedrus (L.), J. phoenicea (L.) and Tetraclinis articulata (L.). Adv Pharmacol Sci. 2016;2016:6. 10.1155/2016/6392656.Search in Google Scholar PubMed PubMed Central
[17] Abuzaid H, Amin E, Moawad A, Abdelmohsen UR, Hetta M, Mohammed R. Liquid chromatography high-resolution mass spectrometry analysis, phytochemical and biological study of two aizoaceae plants plants: a new kaempferol derivative from Trianthema portulacastrum L. Pharmacognosy Res. 2020;10(October):24–30. 10.4103/pr.pr.Search in Google Scholar
[18] Saranya R, Geetha N. Inhibition of calcium oxalate (CaOx) crystallization in vitro by the extract of beet root (Beta vulgais L.). Int J Pharm Pharmaceut Sci. 2014;6:361–5.Search in Google Scholar
[19] Tahir MB, Rafique M, Rafique MS, Nawaz T, Rizwan M, Tanveer M. Photocatalytic nanomaterials for degradation of organic pollutants and heavy metals. UK: Elsevier Inc; 2020.10.1016/B978-0-12-821192-2.00008-5Search in Google Scholar
[20] Pandey K, Sharma PK, Dudhe R. Anticancer activity of Parthenium hysterophorus Linn and Oldenlandia corymbosa Lam by Srb method. Open Access Sci Rep. 2012;1(6):1–3. 10.4172/scientificreports.Search in Google Scholar
[21] Elambarathi P, Kumar P, Sivaperumal M. Inhibition of calcium oxalate crystallization by the leaves extract of Kalanchoe pinnata. World J Pharm Res. 2018;7(10):859. 10.20959/wjpr201810-12318.Search in Google Scholar
[22] Binu TV, Vijayakumari B. In‐vitro antiurolithiatic activity of Strychnos potatorum LF. S Ind J Biol Sci. 2016;2(1):174–8.10.22205/sijbs/2016/v2/i1/100388Search in Google Scholar
[23] Vichai V, Kirtikara K. Sulforhodamine B colorimetric assay for cytotoxicity screening. Nat Protocol. 2006;1(3):1112–6. 10.1038/nprot.2006.179.Search in Google Scholar PubMed
[24] Meshack S, Gupta S. A review of plants with remarkable hepatoprotective activity. J Drug Deliv Ther. 2022;12(1):194–202. 10.22270/jddt.v12i1.5283.Search in Google Scholar
[25] Varghese RE, Ragavan D, Sivaraj S, Gayathri D, Kannayiram G. Anti-inflammatory activity of Syzygium aromaticum silver nanoparticles: in vitro and in silico study. Asian J Pharm Clin Res. 2017;10(11):370–3. 10.22159/ajpcr.2017.v10i11.19904.Search in Google Scholar
[26] Ananthi P, Jeyapaul U, James A, Anand B, Mary S, Kala J. Green synthesis and characterization of silver nanoparticles using Triumfetta rotundifolia plant extract and its antibacterial activities. J Nat Prod Plant Resour. 2016;6(3):21–7.Search in Google Scholar
[27] Ferrali M, Signorini C, Ciccoli L, Comporti M. Iron release and membrane damage in erythrocytes exposed to oxidizing agents, phenylhydrazine, divicine and isouramil. Biochem J. 1992;285(1):295–301. 10.1042/bj2850295.Search in Google Scholar PubMed PubMed Central
[28] Qamar M, Akhtar S, Barnard RT, Sestili P, Ziora ZM, Lazarte CE, et al. Antiinflammatory and anticancer properties of Grewia asiatica crude extracts and fractions: a bioassay-guided approach. Biomed Res Int. 2022;2022:14. 10.1155/2022/2277417.Search in Google Scholar PubMed PubMed Central
[29] Saffee N, Shamsuddin M, Abd Karim KJ. Biosynthesized gold nanoparticles supported on magnetic chitosan matrix as catalyst for reduction of 4-nitrophenol. Malaysian J Fundam Appl Sci. 2019;15(3):451–5. 10.11113/mjfas.v15n3.1452.Search in Google Scholar
[30] Salve P. Bio-synthesized silver nanoparticles using plant extract as anti-bacteria agent. September, 2022.Search in Google Scholar
[31] Dwevedi A, Sharma K, Sharma YK. Cadamba: a miraculous tree having enormous pharmacological implications. Pharmacogn Rev. 2015;9(18):107–13. 10.4103/0973-7847.162110.Search in Google Scholar PubMed PubMed Central
[32] Chowdhury A, Azam S, Jainul MA, Faruq KO, Islam A. Antibacterial activities and in vitro anti-inflammatory (membrane stability) properties of methanolic extracts of Gardenia coronaria leaves. J Microbiol. 2014;2014:5.10.1155/2014/410935Search in Google Scholar PubMed PubMed Central
[33] Rajeshkumar S, Menon S, Venkat Kumar S, Ponnanikajamideen M, Ali D, Arunachalam K. Anti-inflammatory and antimicrobial potential of Cissus quadrangularis-assisted copper oxide nanoparticles. J Nanomater. 2021;2021:5742981. 10.1155/2021/5742981.Search in Google Scholar
[34] Prathyusha K, Reddy MJ, Himabindhu J, Ramanjaneyulu K. Evaluation of in vitro antiurolithiatic activity of Adhatoda vasica (Vasaka). Int J Pharm Pharm Res. 2018;13(3):30–7, http://ijppr.humanjournals.com/.Search in Google Scholar
© 2023 the author(s), published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Research Articles
- Value-added utilization of coal fly ash and recycled polyvinyl chloride in door or window sub-frame composites
- High removal efficiency of volatile phenol from coking wastewater using coal gasification slag via optimized adsorption and multi-grade batch process
- Evolution of surface morphology and properties of diamond films by hydrogen plasma etching
- Removal efficiency of dibenzofuran using CuZn-zeolitic imidazole frameworks as a catalyst and adsorbent
- Rapid and efficient microwave-assisted extraction of Caesalpinia sappan Linn. heartwood and subsequent synthesis of gold nanoparticles
- The catalytic characteristics of 2-methylnaphthalene acylation with AlCl3 immobilized on Hβ as Lewis acid catalyst
- Biodegradation of synthetic PVP biofilms using natural materials and nanoparticles
- Rutin-loaded selenium nanoparticles modulated the redox status, inflammatory, and apoptotic pathways associated with pentylenetetrazole-induced epilepsy in mice
- Optimization of apigenin nanoparticles prepared by planetary ball milling: In vitro and in vivo studies
- Synthesis and characterization of silver nanoparticles using Origanum onites leaves: Cytotoxic, apoptotic, and necrotic effects on Capan-1, L929, and Caco-2 cell lines
- Exergy analysis of a conceptual CO2 capture process with an amine-based DES
- Construction of fluorescence system of felodipine–tetracyanovinyl–2,2′-bipyridine complex
- Excellent photocatalytic degradation of rhodamine B over Bi2O3 supported on Zn-MOF nanocomposites under visible light
- Optimization-based control strategy for a large-scale polyhydroxyalkanoates production in a fed-batch bioreactor using a coupled PDE–ODE system
- Effectiveness of pH and amount of Artemia urumiana extract on physical, chemical, and biological attributes of UV-fabricated biogold nanoparticles
- Geranium leaf-mediated synthesis of silver nanoparticles and their transcriptomic effects on Candida albicans
- Synthesis, characterization, anticancer, anti-inflammatory activities, and docking studies of 3,5-disubstituted thiadiazine-2-thiones
- Synthesis and stability of phospholipid-encapsulated nano-selenium
- Putative anti-proliferative effect of Indian mustard (Brassica juncea) seed and its nano-formulation
- Enrichment of low-grade phosphorites by the selective leaching method
- Electrochemical analysis of the dissolution of gold in a copper–ethylenediamine–thiosulfate system
- Characterisation of carbonate lake sediments as a potential filler for polymer composites
- Evaluation of nano-selenium biofortification characteristics of alfalfa (Medicago sativa L.)
- Quality of oil extracted by cold press from Nigella sativa seeds incorporated with rosemary extracts and pretreated by microwaves
- Heteropolyacid-loaded MOF-derived mesoporous zirconia catalyst for chemical degradation of rhodamine B
- Recovery of critical metals from carbonatite-type mineral wastes: Geochemical modeling investigation of (bio)hydrometallurgical leaching of REEs
- Photocatalytic properties of ZnFe-mixed oxides synthesized via a simple route for water remediation
- Attenuation of di(2-ethylhexyl)phthalate-induced hepatic and renal toxicity by naringin nanoparticles in a rat model
- Novel in situ synthesis of quaternary core–shell metallic sulfide nanocomposites for degradation of organic dyes and hydrogen production
- Microfluidic steam-based synthesis of luminescent carbon quantum dots as sensing probes for nitrite detection
- Transformation of eggshell waste to egg white protein solution, calcium chloride dihydrate, and eggshell membrane powder
- Preparation of Zr-MOFs for the adsorption of doxycycline hydrochloride from wastewater
- Green nanoarchitectonics of the silver nanocrystal potential for treating malaria and their cytotoxic effects on the kidney Vero cell line
- Carbon emissions analysis of producing modified asphalt with natural asphalt
- An efficient and green synthesis of 2-phenylquinazolin-4(3H)-ones via t-BuONa-mediated oxidative condensation of 2-aminobenzamides and benzyl alcohols under solvent- and transition metal-free conditions
- Chitosan nanoparticles loaded with mesosulfuron methyl and mesosulfuron methyl + florasulam + MCPA isooctyl to manage weeds of wheat (Triticum aestivum L.)
- Synergism between lignite and high-sulfur petroleum coke in CO2 gasification
- Facile aqueous synthesis of ZnCuInS/ZnS–ZnS QDs with enhanced photoluminescence lifetime for selective detection of Cu(ii) ions
- Rapid synthesis of copper nanoparticles using Nepeta cataria leaves: An eco-friendly management of disease-causing vectors and bacterial pathogens
- Study on the photoelectrocatalytic activity of reduced TiO2 nanotube films for removal of methyl orange
- Development of a fuzzy logic model for the prediction of spark-ignition engine performance and emission for gasoline–ethanol blends
- Micro-impact-induced mechano-chemical synthesis of organic precursors from FeC/FeN and carbonates/nitrates in water and its extension to nucleobases
- Green synthesis of strontium-doped tin dioxide (SrSnO2) nanoparticles using the Mahonia bealei leaf extract and evaluation of their anticancer and antimicrobial activities
- A study on the larvicidal and adulticidal potential of Cladostepus spongiosus macroalgae and green-fabricated silver nanoparticles against mosquito vectors
- Catalysts based on nickel salt heteropolytungstates for selective oxidation of diphenyl sulfide
- Powerful antibacterial nanocomposites from Corallina officinalis-mediated nanometals and chitosan nanoparticles against fish-borne pathogens
- Removal behavior of Zn and alkalis from blast furnace dust in pre-reduction sinter process
- Environmentally friendly synthesis and computational studies of novel class of acridinedione integrated spirothiopyrrolizidines/indolizidines
- The mechanisms of inhibition and lubrication of clean fracturing flowback fluids in water-based drilling fluids
- Adsorption/desorption performance of cellulose membrane for Pb(ii)
- A one-pot, multicomponent tandem synthesis of fused polycyclic pyrrolo[3,2-c]quinolinone/pyrrolizino[2,3-c]quinolinone hybrid heterocycles via environmentally benign solid state melt reaction
- Green synthesis of silver nanoparticles using durian rind extract and optical characteristics of surface plasmon resonance-based optical sensor for the detection of hydrogen peroxide
- Electrochemical analysis of copper-EDTA-ammonia-gold thiosulfate dissolution system
- Characterization of bio-oil production by microwave pyrolysis from cashew nut shells and Cassia fistula pods
- Green synthesis methods and characterization of bacterial cellulose/silver nanoparticle composites
- Photocatalytic research performance of zinc oxide/graphite phase carbon nitride catalyst and its application in environment
- Effect of phytogenic iron nanoparticles on the bio-fortification of wheat varieties
- In vitro anti-cancer and antimicrobial effects of manganese oxide nanoparticles synthesized using the Glycyrrhiza uralensis leaf extract on breast cancer cell lines
- Preparation of Pd/Ce(F)-MCM-48 catalysts and their catalytic performance of n-heptane isomerization
- Green “one-pot” fluorescent bis-indolizine synthesis with whole-cell plant biocatalysis
- Silica-titania mesoporous silicas of MCM-41 type as effective catalysts and photocatalysts for selective oxidation of diphenyl sulfide by H2O2
- Biosynthesis of zinc oxide nanoparticles from molted feathers of Pavo cristatus and their antibiofilm and anticancer activities
- Clean preparation of rutile from Ti-containing mixed molten slag by CO2 oxidation
- Synthesis and characterization of Pluronic F-127-coated titanium dioxide nanoparticles synthesized from extracts of Atractylodes macrocephala leaf for antioxidant, antimicrobial, and anticancer properties
- Effect of pretreatment with alkali on the anaerobic digestion characteristics of kitchen waste and analysis of microbial diversity
- Ameliorated antimicrobial, antioxidant, and anticancer properties by Plectranthus vettiveroides root extract-mediated green synthesis of chitosan nanoparticles
- Microwave-accelerated pretreatment technique in green extraction of oil and bioactive compounds from camelina seeds: Effectiveness and characterization
- Studies on the extraction performance of phorate by aptamer-functionalized magnetic nanoparticles in plasma samples
- Investigation of structural properties and antibacterial activity of AgO nanoparticle extract from Solanum nigrum/Mentha leaf extracts by green synthesis method
- Green fabrication of chitosan from marine crustaceans and mushroom waste: Toward sustainable resource utilization
- Synthesis, characterization, and evaluation of nanoparticles of clodinofop propargyl and fenoxaprop-P-ethyl on weed control, growth, and yield of wheat (Triticum aestivum L.)
- The enhanced adsorption properties of phosphorus from aqueous solutions using lanthanum modified synthetic zeolites
- Separation of graphene oxides of different sizes by multi-layer dialysis and anti-friction and lubrication performance
- Visible-light-assisted base-catalyzed, one-pot synthesis of highly functionalized cinnolines
- The experimental study on the air oxidation of 5-hydroxymethylfurfural to 2,5-furandicarboxylic acid with Co–Mn–Br system
- Highly efficient removal of tetracycline and methyl violet 2B from aqueous solution using the bimetallic FeZn-ZIFs catalyst
- A thermo-tolerant cellulase enzyme produced by Bacillus amyloliquefaciens M7, an insight into synthesis, optimization, characterization, and bio-polishing activity
- Exploration of ketone derivatives of succinimide for their antidiabetic potential: In vitro and in vivo approaches
- Ultrasound-assisted green synthesis and in silico study of 6-(4-(butylamino)-6-(diethylamino)-1,3,5-triazin-2-yl)oxypyridazine derivatives
- A study of the anticancer potential of Pluronic F-127 encapsulated Fe2O3 nanoparticles derived from Berberis vulgaris extract
- Biogenic synthesis of silver nanoparticles using Consolida orientalis flowers: Identification, catalytic degradation, and biological effect
- Initial assessment of the presence of plastic waste in some coastal mangrove forests in Vietnam
- Adsorption synergy electrocatalytic degradation of phenol by active oxygen-containing species generated in Co-coal based cathode and graphite anode
- Antibacterial, antifungal, antioxidant, and cytotoxicity activities of the aqueous extract of Syzygium aromaticum-mediated synthesized novel silver nanoparticles
- Synthesis of a silica matrix with ZnO nanoparticles for the fabrication of a recyclable photodegradation system to eliminate methylene blue dye
- Natural polymer fillers instead of dye and pigments: Pumice and scoria in PDMS fluid and elastomer composites
- Study on the preparation of glycerylphosphorylcholine by transesterification under supported sodium methoxide
- Wireless network handheld terminal-based green ecological sustainable design evaluation system: Improved data communication and reduced packet loss rate
- The optimization of hydrogel strength from cassava starch using oxidized sucrose as a crosslinking agent
- Green synthesis of silver nanoparticles using Saccharum officinarum leaf extract for antiviral paint
- Study on the reliability of nano-silver-coated tin solder joints for flip chips
- Environmentally sustainable analytical quality by design aided RP-HPLC method for the estimation of brilliant blue in commercial food samples employing a green-ultrasound-assisted extraction technique
- Anticancer and antimicrobial potential of zinc/sodium alginate/polyethylene glycol/d-pinitol nanocomposites against osteosarcoma MG-63 cells
- Nanoporous carbon@CoFe2O4 nanocomposite as a green absorbent for the adsorptive removal of Hg(ii) from aqueous solutions
- Characterization of silver sulfide nanoparticles from actinobacterial strain (M10A62) and its toxicity against lepidopteran and dipterans insect species
- Phyto-fabrication and characterization of silver nanoparticles using Withania somnifera: Investigating antioxidant potential
- Effect of e-waste nanofillers on the mechanical, thermal, and wear properties of epoxy-blend sisal woven fiber-reinforced composites
- Magnesium nanohydroxide (2D brucite) as a host matrix for thymol and carvacrol: Synthesis, characterization, and inhibition of foodborne pathogens
- Synergistic inhibitive effect of a hybrid zinc oxide-benzalkonium chloride composite on the corrosion of carbon steel in a sulfuric acidic solution
- Review Articles
- Role and the importance of green approach in biosynthesis of nanopropolis and effectiveness of propolis in the treatment of COVID-19 pandemic
- Gum tragacanth-mediated synthesis of metal nanoparticles, characterization, and their applications as a bactericide, catalyst, antioxidant, and peroxidase mimic
- Green-processed nano-biocomposite (ZnO–TiO2): Potential candidates for biomedical applications
- Reaction mechanisms in microwave-assisted lignin depolymerisation in hydrogen-donating solvents
- Recent progress on non-noble metal catalysts for the deoxydehydration of biomass-derived oxygenates
- Rapid Communication
- Phosphorus removal by iron–carbon microelectrolysis: A new way to achieve phosphorus recovery
- Special Issue: Biomolecules-derived synthesis of nanomaterials for environmental and biological applications (Guest Editors: Arpita Roy and Fernanda Maria Policarpo Tonelli)
- Biomolecules-derived synthesis of nanomaterials for environmental and biological applications
- Nano-encapsulated tanshinone IIA in PLGA-PEG-COOH inhibits apoptosis and inflammation in cerebral ischemia/reperfusion injury
- Green fabrication of silver nanoparticles using Melia azedarach ripened fruit extract, their characterization, and biological properties
- Green-synthesized nanoparticles and their therapeutic applications: A review
- Antioxidant, antibacterial, and cytotoxicity potential of synthesized silver nanoparticles from the Cassia alata leaf aqueous extract
- Green synthesis of silver nanoparticles using Callisia fragrans leaf extract and its anticancer activity against MCF-7, HepG2, KB, LU-1, and MKN-7 cell lines
- Algae-based green AgNPs, AuNPs, and FeNPs as potential nanoremediators
- Green synthesis of Kickxia elatine-induced silver nanoparticles and their role as anti-acetylcholinesterase in the treatment of Alzheimer’s disease
- Phytocrystallization of silver nanoparticles using Cassia alata flower extract for effective control of fungal skin pathogens
- Antibacterial wound dressing with hydrogel from chitosan and polyvinyl alcohol from the red cabbage extract loaded with silver nanoparticles
- Leveraging of mycogenic copper oxide nanostructures for disease management of Alternaria blight of Brassica juncea
- Nanoscale molecular reactions in microbiological medicines in modern medical applications
- Synthesis and characterization of ZnO/β-cyclodextrin/nicotinic acid nanocomposite and its biological and environmental application
- Green synthesis of silver nanoparticles via Taxus wallichiana Zucc. plant-derived Taxol: Novel utilization as anticancer, antioxidation, anti-inflammation, and antiurolithic potential
- Recyclability and catalytic characteristics of copper oxide nanoparticles derived from bougainvillea plant flower extract for biomedical application
- Phytofabrication, characterization, and evaluation of novel bioinspired selenium–iron (Se–Fe) nanocomposites using Allium sativum extract for bio-potential applications
- Erratum
- Erratum to “Synthesis, characterization, and evaluation of nanoparticles of clodinofop propargyl and fenoxaprop-P-ethyl on weed control, growth, and yield of wheat (Triticum aestivum L.)”
Articles in the same Issue
- Research Articles
- Value-added utilization of coal fly ash and recycled polyvinyl chloride in door or window sub-frame composites
- High removal efficiency of volatile phenol from coking wastewater using coal gasification slag via optimized adsorption and multi-grade batch process
- Evolution of surface morphology and properties of diamond films by hydrogen plasma etching
- Removal efficiency of dibenzofuran using CuZn-zeolitic imidazole frameworks as a catalyst and adsorbent
- Rapid and efficient microwave-assisted extraction of Caesalpinia sappan Linn. heartwood and subsequent synthesis of gold nanoparticles
- The catalytic characteristics of 2-methylnaphthalene acylation with AlCl3 immobilized on Hβ as Lewis acid catalyst
- Biodegradation of synthetic PVP biofilms using natural materials and nanoparticles
- Rutin-loaded selenium nanoparticles modulated the redox status, inflammatory, and apoptotic pathways associated with pentylenetetrazole-induced epilepsy in mice
- Optimization of apigenin nanoparticles prepared by planetary ball milling: In vitro and in vivo studies
- Synthesis and characterization of silver nanoparticles using Origanum onites leaves: Cytotoxic, apoptotic, and necrotic effects on Capan-1, L929, and Caco-2 cell lines
- Exergy analysis of a conceptual CO2 capture process with an amine-based DES
- Construction of fluorescence system of felodipine–tetracyanovinyl–2,2′-bipyridine complex
- Excellent photocatalytic degradation of rhodamine B over Bi2O3 supported on Zn-MOF nanocomposites under visible light
- Optimization-based control strategy for a large-scale polyhydroxyalkanoates production in a fed-batch bioreactor using a coupled PDE–ODE system
- Effectiveness of pH and amount of Artemia urumiana extract on physical, chemical, and biological attributes of UV-fabricated biogold nanoparticles
- Geranium leaf-mediated synthesis of silver nanoparticles and their transcriptomic effects on Candida albicans
- Synthesis, characterization, anticancer, anti-inflammatory activities, and docking studies of 3,5-disubstituted thiadiazine-2-thiones
- Synthesis and stability of phospholipid-encapsulated nano-selenium
- Putative anti-proliferative effect of Indian mustard (Brassica juncea) seed and its nano-formulation
- Enrichment of low-grade phosphorites by the selective leaching method
- Electrochemical analysis of the dissolution of gold in a copper–ethylenediamine–thiosulfate system
- Characterisation of carbonate lake sediments as a potential filler for polymer composites
- Evaluation of nano-selenium biofortification characteristics of alfalfa (Medicago sativa L.)
- Quality of oil extracted by cold press from Nigella sativa seeds incorporated with rosemary extracts and pretreated by microwaves
- Heteropolyacid-loaded MOF-derived mesoporous zirconia catalyst for chemical degradation of rhodamine B
- Recovery of critical metals from carbonatite-type mineral wastes: Geochemical modeling investigation of (bio)hydrometallurgical leaching of REEs
- Photocatalytic properties of ZnFe-mixed oxides synthesized via a simple route for water remediation
- Attenuation of di(2-ethylhexyl)phthalate-induced hepatic and renal toxicity by naringin nanoparticles in a rat model
- Novel in situ synthesis of quaternary core–shell metallic sulfide nanocomposites for degradation of organic dyes and hydrogen production
- Microfluidic steam-based synthesis of luminescent carbon quantum dots as sensing probes for nitrite detection
- Transformation of eggshell waste to egg white protein solution, calcium chloride dihydrate, and eggshell membrane powder
- Preparation of Zr-MOFs for the adsorption of doxycycline hydrochloride from wastewater
- Green nanoarchitectonics of the silver nanocrystal potential for treating malaria and their cytotoxic effects on the kidney Vero cell line
- Carbon emissions analysis of producing modified asphalt with natural asphalt
- An efficient and green synthesis of 2-phenylquinazolin-4(3H)-ones via t-BuONa-mediated oxidative condensation of 2-aminobenzamides and benzyl alcohols under solvent- and transition metal-free conditions
- Chitosan nanoparticles loaded with mesosulfuron methyl and mesosulfuron methyl + florasulam + MCPA isooctyl to manage weeds of wheat (Triticum aestivum L.)
- Synergism between lignite and high-sulfur petroleum coke in CO2 gasification
- Facile aqueous synthesis of ZnCuInS/ZnS–ZnS QDs with enhanced photoluminescence lifetime for selective detection of Cu(ii) ions
- Rapid synthesis of copper nanoparticles using Nepeta cataria leaves: An eco-friendly management of disease-causing vectors and bacterial pathogens
- Study on the photoelectrocatalytic activity of reduced TiO2 nanotube films for removal of methyl orange
- Development of a fuzzy logic model for the prediction of spark-ignition engine performance and emission for gasoline–ethanol blends
- Micro-impact-induced mechano-chemical synthesis of organic precursors from FeC/FeN and carbonates/nitrates in water and its extension to nucleobases
- Green synthesis of strontium-doped tin dioxide (SrSnO2) nanoparticles using the Mahonia bealei leaf extract and evaluation of their anticancer and antimicrobial activities
- A study on the larvicidal and adulticidal potential of Cladostepus spongiosus macroalgae and green-fabricated silver nanoparticles against mosquito vectors
- Catalysts based on nickel salt heteropolytungstates for selective oxidation of diphenyl sulfide
- Powerful antibacterial nanocomposites from Corallina officinalis-mediated nanometals and chitosan nanoparticles against fish-borne pathogens
- Removal behavior of Zn and alkalis from blast furnace dust in pre-reduction sinter process
- Environmentally friendly synthesis and computational studies of novel class of acridinedione integrated spirothiopyrrolizidines/indolizidines
- The mechanisms of inhibition and lubrication of clean fracturing flowback fluids in water-based drilling fluids
- Adsorption/desorption performance of cellulose membrane for Pb(ii)
- A one-pot, multicomponent tandem synthesis of fused polycyclic pyrrolo[3,2-c]quinolinone/pyrrolizino[2,3-c]quinolinone hybrid heterocycles via environmentally benign solid state melt reaction
- Green synthesis of silver nanoparticles using durian rind extract and optical characteristics of surface plasmon resonance-based optical sensor for the detection of hydrogen peroxide
- Electrochemical analysis of copper-EDTA-ammonia-gold thiosulfate dissolution system
- Characterization of bio-oil production by microwave pyrolysis from cashew nut shells and Cassia fistula pods
- Green synthesis methods and characterization of bacterial cellulose/silver nanoparticle composites
- Photocatalytic research performance of zinc oxide/graphite phase carbon nitride catalyst and its application in environment
- Effect of phytogenic iron nanoparticles on the bio-fortification of wheat varieties
- In vitro anti-cancer and antimicrobial effects of manganese oxide nanoparticles synthesized using the Glycyrrhiza uralensis leaf extract on breast cancer cell lines
- Preparation of Pd/Ce(F)-MCM-48 catalysts and their catalytic performance of n-heptane isomerization
- Green “one-pot” fluorescent bis-indolizine synthesis with whole-cell plant biocatalysis
- Silica-titania mesoporous silicas of MCM-41 type as effective catalysts and photocatalysts for selective oxidation of diphenyl sulfide by H2O2
- Biosynthesis of zinc oxide nanoparticles from molted feathers of Pavo cristatus and their antibiofilm and anticancer activities
- Clean preparation of rutile from Ti-containing mixed molten slag by CO2 oxidation
- Synthesis and characterization of Pluronic F-127-coated titanium dioxide nanoparticles synthesized from extracts of Atractylodes macrocephala leaf for antioxidant, antimicrobial, and anticancer properties
- Effect of pretreatment with alkali on the anaerobic digestion characteristics of kitchen waste and analysis of microbial diversity
- Ameliorated antimicrobial, antioxidant, and anticancer properties by Plectranthus vettiveroides root extract-mediated green synthesis of chitosan nanoparticles
- Microwave-accelerated pretreatment technique in green extraction of oil and bioactive compounds from camelina seeds: Effectiveness and characterization
- Studies on the extraction performance of phorate by aptamer-functionalized magnetic nanoparticles in plasma samples
- Investigation of structural properties and antibacterial activity of AgO nanoparticle extract from Solanum nigrum/Mentha leaf extracts by green synthesis method
- Green fabrication of chitosan from marine crustaceans and mushroom waste: Toward sustainable resource utilization
- Synthesis, characterization, and evaluation of nanoparticles of clodinofop propargyl and fenoxaprop-P-ethyl on weed control, growth, and yield of wheat (Triticum aestivum L.)
- The enhanced adsorption properties of phosphorus from aqueous solutions using lanthanum modified synthetic zeolites
- Separation of graphene oxides of different sizes by multi-layer dialysis and anti-friction and lubrication performance
- Visible-light-assisted base-catalyzed, one-pot synthesis of highly functionalized cinnolines
- The experimental study on the air oxidation of 5-hydroxymethylfurfural to 2,5-furandicarboxylic acid with Co–Mn–Br system
- Highly efficient removal of tetracycline and methyl violet 2B from aqueous solution using the bimetallic FeZn-ZIFs catalyst
- A thermo-tolerant cellulase enzyme produced by Bacillus amyloliquefaciens M7, an insight into synthesis, optimization, characterization, and bio-polishing activity
- Exploration of ketone derivatives of succinimide for their antidiabetic potential: In vitro and in vivo approaches
- Ultrasound-assisted green synthesis and in silico study of 6-(4-(butylamino)-6-(diethylamino)-1,3,5-triazin-2-yl)oxypyridazine derivatives
- A study of the anticancer potential of Pluronic F-127 encapsulated Fe2O3 nanoparticles derived from Berberis vulgaris extract
- Biogenic synthesis of silver nanoparticles using Consolida orientalis flowers: Identification, catalytic degradation, and biological effect
- Initial assessment of the presence of plastic waste in some coastal mangrove forests in Vietnam
- Adsorption synergy electrocatalytic degradation of phenol by active oxygen-containing species generated in Co-coal based cathode and graphite anode
- Antibacterial, antifungal, antioxidant, and cytotoxicity activities of the aqueous extract of Syzygium aromaticum-mediated synthesized novel silver nanoparticles
- Synthesis of a silica matrix with ZnO nanoparticles for the fabrication of a recyclable photodegradation system to eliminate methylene blue dye
- Natural polymer fillers instead of dye and pigments: Pumice and scoria in PDMS fluid and elastomer composites
- Study on the preparation of glycerylphosphorylcholine by transesterification under supported sodium methoxide
- Wireless network handheld terminal-based green ecological sustainable design evaluation system: Improved data communication and reduced packet loss rate
- The optimization of hydrogel strength from cassava starch using oxidized sucrose as a crosslinking agent
- Green synthesis of silver nanoparticles using Saccharum officinarum leaf extract for antiviral paint
- Study on the reliability of nano-silver-coated tin solder joints for flip chips
- Environmentally sustainable analytical quality by design aided RP-HPLC method for the estimation of brilliant blue in commercial food samples employing a green-ultrasound-assisted extraction technique
- Anticancer and antimicrobial potential of zinc/sodium alginate/polyethylene glycol/d-pinitol nanocomposites against osteosarcoma MG-63 cells
- Nanoporous carbon@CoFe2O4 nanocomposite as a green absorbent for the adsorptive removal of Hg(ii) from aqueous solutions
- Characterization of silver sulfide nanoparticles from actinobacterial strain (M10A62) and its toxicity against lepidopteran and dipterans insect species
- Phyto-fabrication and characterization of silver nanoparticles using Withania somnifera: Investigating antioxidant potential
- Effect of e-waste nanofillers on the mechanical, thermal, and wear properties of epoxy-blend sisal woven fiber-reinforced composites
- Magnesium nanohydroxide (2D brucite) as a host matrix for thymol and carvacrol: Synthesis, characterization, and inhibition of foodborne pathogens
- Synergistic inhibitive effect of a hybrid zinc oxide-benzalkonium chloride composite on the corrosion of carbon steel in a sulfuric acidic solution
- Review Articles
- Role and the importance of green approach in biosynthesis of nanopropolis and effectiveness of propolis in the treatment of COVID-19 pandemic
- Gum tragacanth-mediated synthesis of metal nanoparticles, characterization, and their applications as a bactericide, catalyst, antioxidant, and peroxidase mimic
- Green-processed nano-biocomposite (ZnO–TiO2): Potential candidates for biomedical applications
- Reaction mechanisms in microwave-assisted lignin depolymerisation in hydrogen-donating solvents
- Recent progress on non-noble metal catalysts for the deoxydehydration of biomass-derived oxygenates
- Rapid Communication
- Phosphorus removal by iron–carbon microelectrolysis: A new way to achieve phosphorus recovery
- Special Issue: Biomolecules-derived synthesis of nanomaterials for environmental and biological applications (Guest Editors: Arpita Roy and Fernanda Maria Policarpo Tonelli)
- Biomolecules-derived synthesis of nanomaterials for environmental and biological applications
- Nano-encapsulated tanshinone IIA in PLGA-PEG-COOH inhibits apoptosis and inflammation in cerebral ischemia/reperfusion injury
- Green fabrication of silver nanoparticles using Melia azedarach ripened fruit extract, their characterization, and biological properties
- Green-synthesized nanoparticles and their therapeutic applications: A review
- Antioxidant, antibacterial, and cytotoxicity potential of synthesized silver nanoparticles from the Cassia alata leaf aqueous extract
- Green synthesis of silver nanoparticles using Callisia fragrans leaf extract and its anticancer activity against MCF-7, HepG2, KB, LU-1, and MKN-7 cell lines
- Algae-based green AgNPs, AuNPs, and FeNPs as potential nanoremediators
- Green synthesis of Kickxia elatine-induced silver nanoparticles and their role as anti-acetylcholinesterase in the treatment of Alzheimer’s disease
- Phytocrystallization of silver nanoparticles using Cassia alata flower extract for effective control of fungal skin pathogens
- Antibacterial wound dressing with hydrogel from chitosan and polyvinyl alcohol from the red cabbage extract loaded with silver nanoparticles
- Leveraging of mycogenic copper oxide nanostructures for disease management of Alternaria blight of Brassica juncea
- Nanoscale molecular reactions in microbiological medicines in modern medical applications
- Synthesis and characterization of ZnO/β-cyclodextrin/nicotinic acid nanocomposite and its biological and environmental application
- Green synthesis of silver nanoparticles via Taxus wallichiana Zucc. plant-derived Taxol: Novel utilization as anticancer, antioxidation, anti-inflammation, and antiurolithic potential
- Recyclability and catalytic characteristics of copper oxide nanoparticles derived from bougainvillea plant flower extract for biomedical application
- Phytofabrication, characterization, and evaluation of novel bioinspired selenium–iron (Se–Fe) nanocomposites using Allium sativum extract for bio-potential applications
- Erratum
- Erratum to “Synthesis, characterization, and evaluation of nanoparticles of clodinofop propargyl and fenoxaprop-P-ethyl on weed control, growth, and yield of wheat (Triticum aestivum L.)”


