Silica-titania mesoporous silicas of MCM-41 type as effective catalysts and photocatalysts for selective oxidation of diphenyl sulfide by H2O2
-
Wiktoria Dubiel
Abstract
Spherical Ti-MCM-41, synthetized by co-condensation method, presented very promising activity in catalytic and photocatalytic oxidation of diphenyl sulfide with H2O2 to obtain diphenyl sulfoxide and diphenyl sulfone. Mesoporous silica materials with various titanium content were analyzed with respect to chemical composition (inductively coupled plasma optical emission spectrometry), structure properties (X-ray diffraction), textural properties (low-temperature N2 adsorption–desorption), morphology (scanning electron microscopy), forms and aggregation introduced titanium species (diffuse reflectance spectroscopy, Raman spectroscopy), and surface acidity (NH3-TPD). Titanium introduced in the samples was present mainly in the form of highly dispersed species, presenting catalytic and photocatalytic activities in diphenyl sulfide oxidation with H2O2. Efficiency of the reaction increased with an increase in titanium loading in the samples and was significantly intensified under UV irradiation. The role of various Ti species in diphenyl sulfide oxidation was presented and discussed.
1 Introduction
Diphenyl sulfide (Ph2S) and products of its selective oxidation, such as diphenyl sulfoxide (Ph2SO) and sulfone (Ph2SO2), belong to the group of chemicals important in various industrial branches. Diphenyl sulfide, among others, is used as an intermediate chemical in pesticide, pharmaceutical, and dye industries [1]. Diphenyl sulfoxide is an important chemical used in various organic synthesis as well as a metal extraction agent [2], while diphenyl sulfone is an important reactant in many end-use industry branches, including chemical, pharmaceutical, polymer, agrochemical, as well as paper [3]. The increasing demand for these chemicals is expected in the coming years, mainly in the agrochemical, polymer, pharmaceutical, and paper industries [1,2,3]. Various oxidants, such as KMnO4, HNO3, RuO4, NaIO4, and MnO2, can be used for selective diphenyl sulfide oxidation [4,5]. Such oxidizing agents have to be used in equimolar amounts of diphenyl sulfide and, in some cases, result in the formation of waste products containing heavy metals [6,7]. The alternative, more environmental-friendly method, is using hydrogen peroxide (H2O2) as an oxidation agent for diphenyl sulfide conversion to diphenyl sulfoxide and sulfone. However, in this case, the process has to be conducted under catalytic or photocatalytic regime [7,8,9,10]. Titanium oxide in the form of anatase, rutile as well as mixtures of both these forms were reported to be effective photocatalysts of Ph2S oxidation to Ph2SO and Ph2SO2 using H2O2 as oxidant, while for the catalytic reaction without UV irradiation, only anatase and mixture of anatase and rutile were catalytically active, while pure rutile was catalytically inactive [10,11,12]. Thus, the diphenyl oxidation is possible by classical catalytic process, but UV-assisted reaction (photocatalytic conditions) typically results in a significant intensification of the product formation [10,11]. Our previous studies [9] have shown that titanosilicate zeolites, such as ferrierite (FER) and its delaminated form (ITQ-6), presented very promising catalytic and photocatalytic activities in Ph2S oxidation with H2O2 resulting in Ph2SO and Ph2SO2. It was shown that conversion of diphenyl sulfide in the presence of microporous three-dimensional FER takes place nearly exclusively on the external surface of the zeolite grain. Penetration of micropores by Ph2S molecules is limited due to their size, which is estimated to be in the range of 0.24–0.93 nm, depending on the molecules orientation and conformation [9]. While the micropores’ diameter in Ti-FER is about 0.55 nm [9]. A significant increase in diphenyl sulfide conversion was found for the delaminated form of FER – ITQ-6. In this case, interlayer space of zeolite is available for the large molecules of Ph2S. On the other hand, smaller molecules of dimethyl sulfide (DMS) can be effectively oxidized with H2O2 to dimethyl sulfoxide and dimethyl sulfone in the presence of both microporous FER and micro-mesoporous ITQ-6 [9]. In this case, the size of DMS molecule is much smaller than size of micropores in FER, and therefore, there are no internal diffusion restrictions. Thus, the accessibility of porous structure, especially by bulky molecules, is a very important point that should be considered in the tailoring of the catalysts. Another important issue is the content of titanium in the samples, which is reported to be catalytically and photocatalytically active in organic sulfates’ oxidation with H2O2 [7,9]. To overcome the problem of internal diffusion limitations of diphenyl sulfide and products of its oxidation, mesoporous silica materials of MCM-41 type, with various titanium loadings, were tested in the role of the catalysts and photocatalysts of Ph2S oxidation with H2O2. The average pore size in the studied Ti-MCM-41 materials is about 3.2 nm, thus significantly larger than the size of Ph2S molecules. Mesoporous MCM-41 silica with spherical morphology (shorter channels than in cylindrical MCM-41) was used to limit the distance of reactants’ internal diffusion and therefore increase the overall rate of diphenyl sulfide oxidation.
2 Materials and methods
2.1 Catalyst preparation
2.1.1 Synthesis of spherical MCM-41
Spherical MCM-41 material was prepared in accordance with the procedure presented by Liu et al. [13]. Ethanol (POCH) and aqueous ammonia (Sigma Aldrich) hexadecyltrimethylammonium bromide (CTAB; Sigma Aldrich, 98%) were dissolved in distilled water. Then, the mixture was stirred for 15 min, then tetraethyl orthosilicate (TEOS; Sigma Aldrich, 98%) was added and the obtained solution was stirred continuously for the next 2 h. As a result, the synthesis gel with the molar composition ratio of 1 TEOS:0.3 CTAB:11 NH3:58 ethanol:144 H2O was obtained. The white solid product was filtrated, washed with distilled water to obtain pH = 7, and then dried at 60°C overnight. Finally, the solid product was calcined at 550°C for 6 h (heating rate of 1°C·min−1). The obtained product is named s-MCM-41.
2.1.2 Synthesis of titanium containing spherical MCM-41
Titanium containing spherical MCM-41 samples were prepared by co-condensation method with the intended Si:Ti molar ratios of 95:5, 90:10, and 80:20. Tetrabutyl orthotitanate (Alfa Aesar, ≥99%) used as a titanium source and TEOS used as a silica source were mixed in the mentioned earlier proportions. The prepared solution was added dropwise to the mixture of water, ethanol, aqueous ammonia, and CTAB and stirred for 2 h. The solid product was recovered by filtration, washed with distilled water to obtain pH = 7, dried at 60°C, and finally calcined at 550°C for 6 h (heating rate 1°C·min−1). As a result, the samples denoted as 3Ti, 8Ti, and 12Ti, respectively, with the Si:Ti intended molar ratios of 95:5, 90:10, and 80:20 were obtained.
2.2 Catalyst characterization
The titanium content in the obtained samples was determined by using inductively coupled plasma optical emission spectrometry (ICP-OES) using an iCAP 7400 instrument (Thermo Science). Analysis was carried out by dissolving solid samples in a mixture of 2 mL of HF (47–51%, Honeywell), 2 mL of HCl (30%, Honeywell), and 6 mL of HNO3 (67–69%, Honeywell) solutions at 190°C assisted by microwave digestion Ethos Easy system (Milestone).
The X-ray diffractograms were measured with the use of Bruker D2 Phaser. The measurements were performed in the 2 theta ranges of 1–8° and 20–80° with a step of 0.02°. The x-ray diffraction (XRD) patterns were recorded with a counting time of 5 and 1 s per step, respectively.
The morphology of the synthesized materials was examined by scanning electron microscopy (SEM) method. SEM images were recorded using a Hitachi S-4700 microscope (Hitachi Instruments Inc.) equipped with a Noran Vantage analyzer.
The textural parameters of the samples, such as specific surface area and porosity, were determined by N2 sorption at –196°C using a 3Flex v.1.00 (Micromeritics) automated gas adsorption system. The samples were outgassed under vacuum at 350°C for 24 h prior to the analysis. The specific surface area of the samples was determined by using BET (Braunauer–Emmett–Teller) equation, while the pore size distributions (PSD) were determined by analysis of the adsorption branch of isotherm by using the Barrett–Joyner–Halenda model. The total pore volume was established by means of the total amount of adsorbed nitrogen at relative pressure p/p 0 = 0.98.
The type of titanium species in the sample structures was analyzed by diffuse reflectance spectroscopy, Raman spectroscopy (UV–Vis DRS). The spectra in the range of 190–900 nm and with a resolution of 2 nm were recorded using Lambda 650 S (Perkin Elmer) spectrophotometer.
Raman spectra of the samples were obtained using a HORIBA Jobin Yvon LabRAM HR micro-Raman spectrometer, equipped with an 789 nm diode Nd:YAG laser, 1800 diffraction grating, as well as 100× Olympus objective. Laser power was adjusted to approximately 9 mW. For each spectrum, four scans were taken and averaged.
The surface acidity of the samples was determined by temperature-programmed desorption of ammonia (NH3-TPD). The analysis was done in a flow quartz microreactor connected directly to a mass spectrometer with a quadrupole analyzer (PREVAC). Mass flow controllers (Brooks Instrument) were used to adjust the flow rate of the gas mixture supplied to the microreactor. The sample of 50 mg was outgassed in a flow of pure helium (20 mL·min−1) at 550°C for 0.5 h. Subsequently, ammonia sorption was performed in a flow of 1 vol% NH3/He gas mixture, with a rate of 20 mL·min−1 at 70°C for about 90 min. The physisorbed ammonia molecules were removed from the sample surface in a flow of pure helium. Then, desorption of ammonia was monitored with a linear heating rate of 10°C·min−1 to 600°C, in a flow of pure helium (20 mL·min−1). Recalculation of the detector signal into the ammonia desorption rate was possible due to the calibration of the QMS with the use of a commercial gas mixture.
2.3 Catalytic and photocatalytic studies
The activity of titanium-containing MCM-41 samples was studied in catalytic and photocatalytic diphenyl sulfide (Ph2S) oxidation, with hydrogen peroxide as an oxidation agent, to sulfoxide (Ph2SO) and sulfone (Ph2SO2). For the catalytic studies, the reaction mixture was prepared in a round-bottom flask by adding 2 mL of diphenyl sulfide (Sigma Aldrich, 98%) solution (4 mmol·L−1), 20 mL of acetonitrile used as a solvent, 10 µL of bromobenzene (Sigma Aldrich, ≥99.5%) used as an internal standard, as well as 25 mg of the synthesized catalyst. The reaction mixture was stirred (1,000 rpm) at room temperature for 10 min and then 5 µL of 30% hydrogen peroxide (Chempur, pure p.a.) was added. The catalytic tests were conducted with the H2O2/sulfide molar ratio of 5. The reaction was conducted in the dark to eliminate the photocatalytic conversion of Ph2S. The photocatalytic studies were performed with the use of a round quartz cuvette, under UV irradiation, using a xenon lamp (XBO-150, Instytut Fotonowy) as a UV light source. The CuSO4 aqueous solution (10 cm optical path, 0.1 mol·L−1) as NIR and IR filter, as well as a 320 nm cut-off filter, was used to avoid the excitation of Ph2S and its direct photooxidation. During the irradiation, the suspension was constantly stirred. The reaction progress and selectivity were monitored by high-performance liquid chromatography. The mixture of acetonitrile and water with the relative volume ratio of 70:30 was used as the eluent. The reaction was conducted for 4 h and the reaction mixture samples were taken in regular intervals at 10, 20, 30, 45, and 60 min of the reaction progress and every 30 min afterwards. Prior to the analysis, the liquid samples were filtered (0.22 μm nylon membrane filter) and then analysed using the Flexar chromatograph (PerkinElmer) operating with the analytical C18 column (150 mm × 4.6 mm i.d., 5 μm pore size). The chromatographic analyses were conducted at 25°C and the UV detector was set at 254 nm.
3 Results and discussion
The spherical morphology of pure silica MCM-41 and its modifications with titanium were proved by SEM. As can be seen in Figure 1, all studied samples are composed of spheres of different diameters. In the case of s-MCM-41, most of silica spheres are in the range of 200–700 nm with a small contribution of smaller unregular silica aggregates deposited on silica spheres (Figure 1a). Spheres with similar sizes were identified in the micrograph of MCM-41 modified with the lowest titanium content – 3Ti (Figure 1b). For the 8Ti and 12Ti samples, with higher titanium loadings, apart from spheres, a significant contribution of unshaped aggregates was identified (Figure 1c and d). Moreover, an increase in titanium content in the samples resulted in a tendency to stick to the spheres.
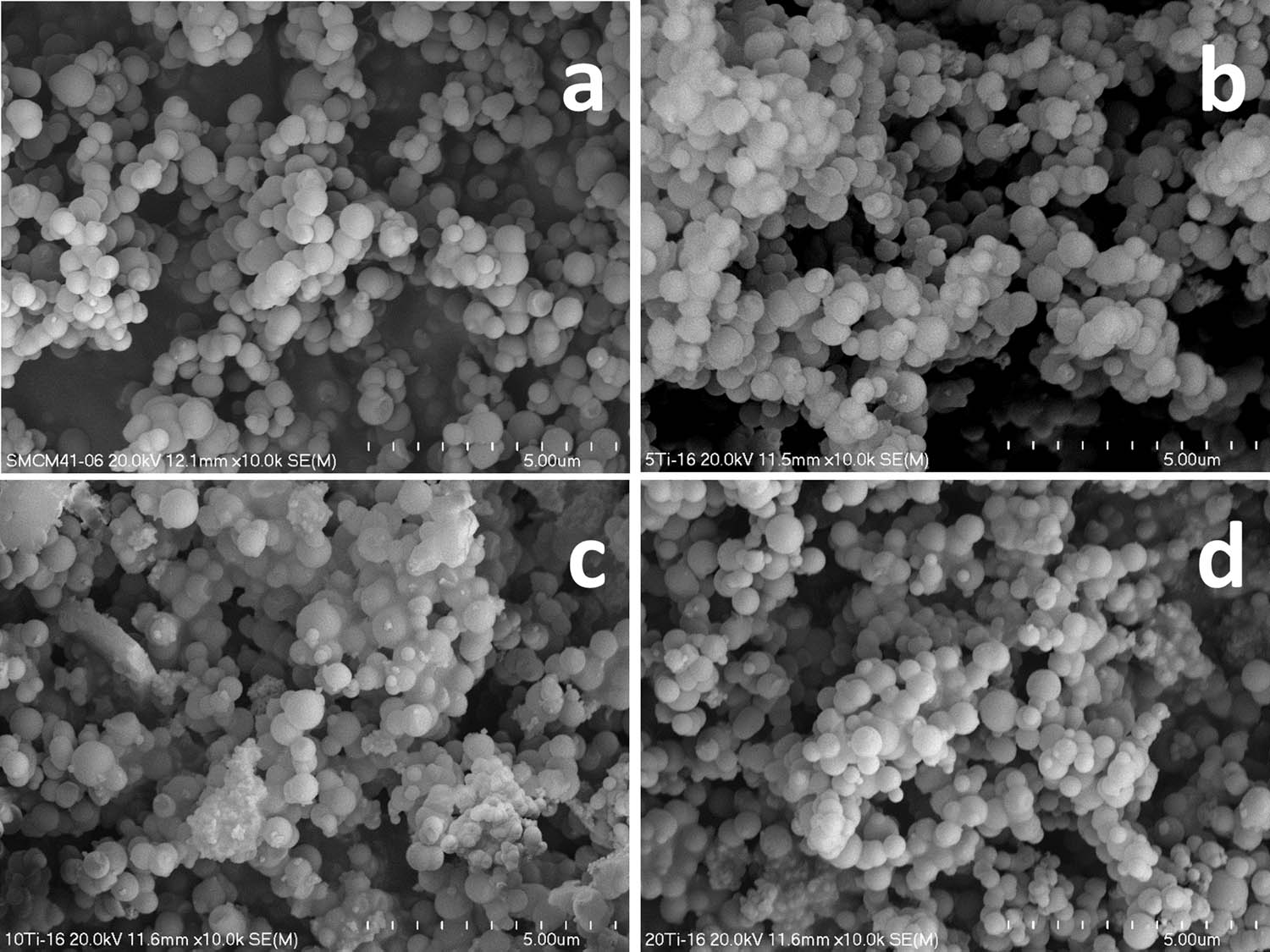
SEM micrographs of S-MCM-41 (a), 3Ti (b), 8Ti (c), and 12Ti (d).
X-ray diffractograms recorded in the low 2 theta range show the presence of three reflections, (100), (110), and (200), which characterize the hexagonal porous structure of MCM-41 (Figure 2). Increasing content of titanium in the samples resulted in decreasing intensity of these reflections, indicating less ordered porous structure. In the case of the sample with the highest titanium loading (12Ti), the shift of the (100) diffraction peak into larger 2 theta angle is possibly related to a decrease in the pore size. No reflection characteristics of titanium oxides were found in diffractograms recorded for the studied samples (Figure 2, insert), indicating the deposition of titanium in the form of highly dispersed species. The broad diffraction peak, characteristic of amorphous silica, is centred at about 24° [14].
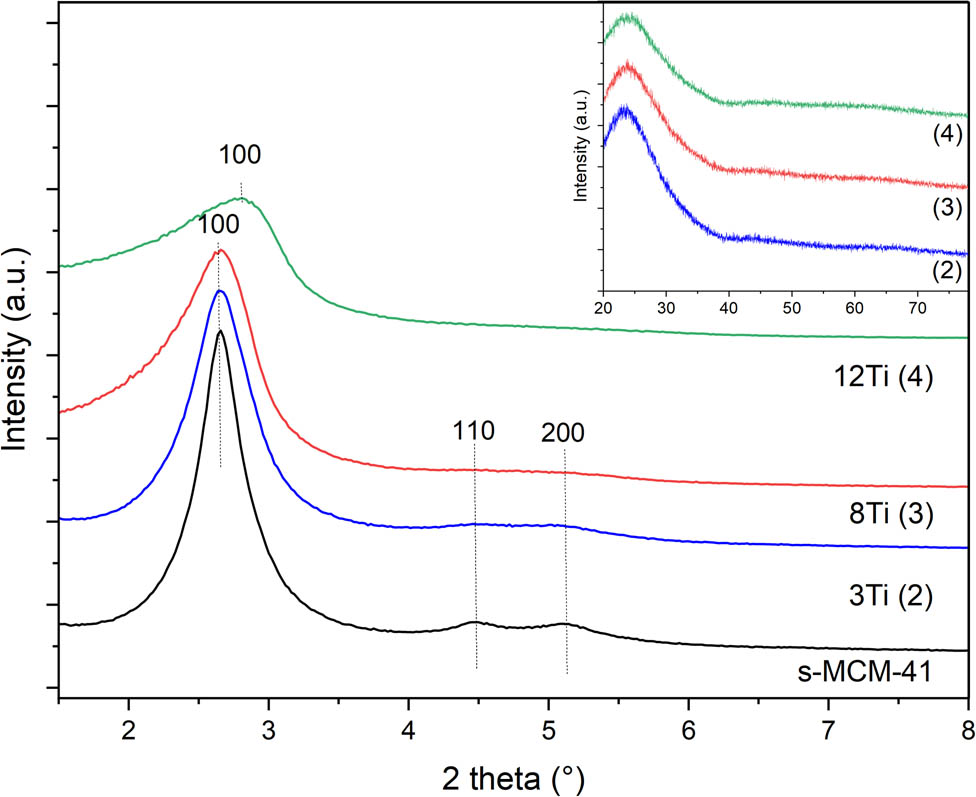
X-ray diffractograms of S-MCM-41, 3Ti, 8Ti, and 12Ti.
The nitrogen adsorption–desorption isotherms of pure silica S-MCM-41 and its modifications with titanium are shown in Figure 3, while PSD profiles are presented in Figure 4. Isotherms recorded for the studied samples are classified according to the IUPAC standards as type IV and are typical of mesoporous materials, such as MCM-41 [15,16]. The steep increase in nitrogen uptake at a relative pressure of 0.15–0.30, assigned to the capillary condensation of nitrogen molecules inside mesopores, is a characteristic feature of these isotherms. Introduction of titanium into silica walls of MCM-41 resulted in a significant decrease in nitrogen uptake, indicating decreased mesopore volume (Figure 3). For the Ti containing MCM-41 samples, an increase in nitrogen adsorption volume above relative pressure of 0.8 is possibly associated with the nitrogen condensation in the space between sticked spheres. This hypothesis is in line with the results of SEM analysis (Figure 1), which showed an increasing tendency to spheres sticking with increasing titanium content in the samples.
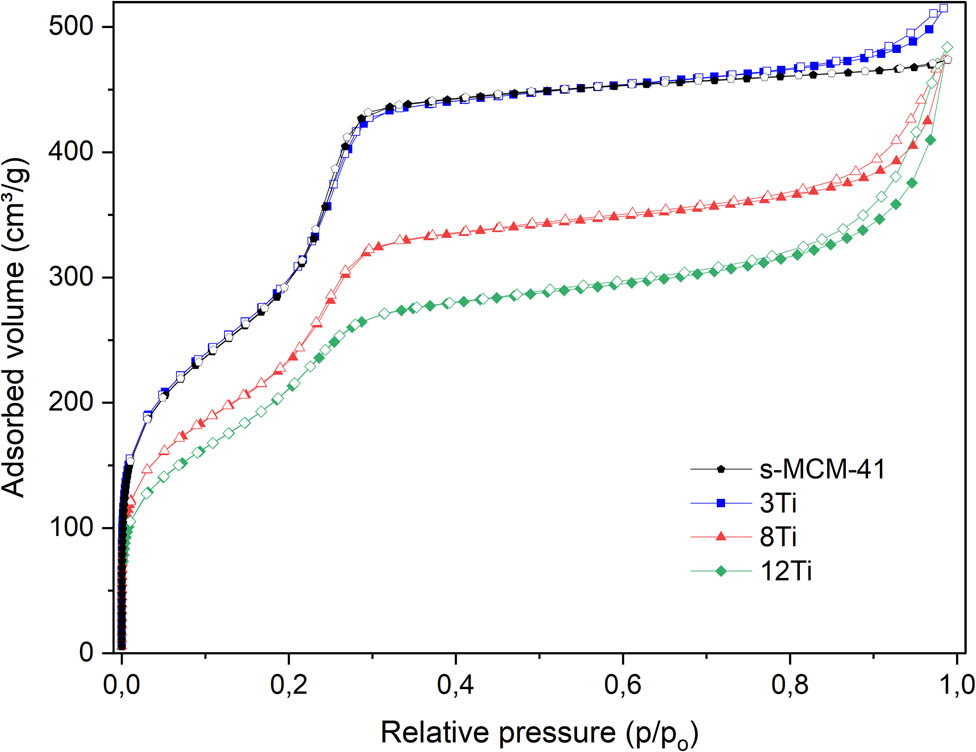
Nitrogen adsorption–desorption isotherms of S-MCM-41, 3Ti, 8Ti, and 12Ti.
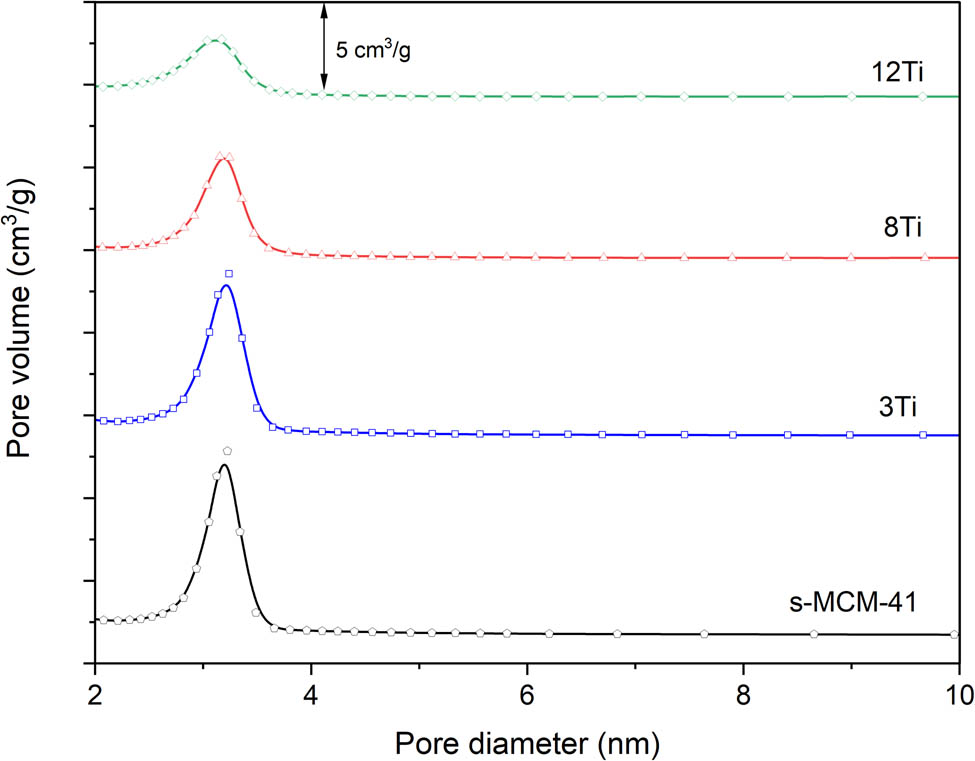
Pore size distribution (PSD) determined for S-MCM-41, 3Ti, 8Ti, and 12Ti.
The PSD profile of S-MCM-41 proves its uniform distribution of pore sizes with the maximum of the pore diameters (D p) at about 3.2 nm (Figure 4). In the case of the samples doped with titanium, a decrease in PSD maximum intensity, due to decreased pore volume (V p), is observed. This effect is less significant for the 3Ti sample with lower titanium content and more significant for the 8Ti sample with higher titanium loading. In the case of the 12Ti sample with the highest titanium loading, the intensity of the PSD profile was significantly reduced, and the maximum of PSD was shifted to about 3.1 nm, indicating a decrease in pore volume and a decrease of their size, which agrees with the results of XRD studies (Figure 2).
In Table 1, the textural parameters of the samples are compared. Specific surface area (S BET) and pore volume (V p) gradually decreased with an increase in titanium content for the samples containing more than 3 wt% of titanium. The average pore diameter for the S-MCM-41 and its modifications with titanium is about 3.2 nm, however, in the case of the sample with the highest titanium loading (12Ti) decreased to about 3.1 nm.
Textural parameters, chemical composition, and surface acidity of the sample
| Sample | S BET (m²·g−1) | V p (cm3·g−1) | D p (nm) | Si (wt%) | Ti (wt%) | AS conc. (μmol·g−1) | AS dens. (μmol·m−2) | AS conc./Ti (mol·mol−1) |
|---|---|---|---|---|---|---|---|---|
| S-MCM-41 | 1,029 | 0.734 | 3.2 | — | — | — | — | — |
| 3Ti | 1,039 | 0.797 | 3.2 | 34.16 | 3.00 | 102 | 0.098 | 0.16 |
| 8Ti | 808 | 0.733 | 3.2 | 31.48 | 7.72 | 189 | 0.234 | 0.12 |
| 12Ti | 725 | 0750 | 3.1 | 27.87 | 11.76 | 260 | 0.359 | 0.11 |
S BET – specific surface area determined by BET method; V p – pore volume; D p – average pore diameter; AS conc. – acid sites concentration per 1 g of the sample; AS dens. – acid sites density per 1 m2 of the sample; AS conc./Ti – ratio of acid sites concentration (determined by NH3-TPD) and titanium content (determined by ICP-OES).
UV–Vis DRS was used for the analysis of form and aggregation of titanium species in the studied samples (Figure 5). Maxima in the spectra, located in the range of 238–255 nm, are possibly superpositions of three bands related to tetrahedrally coordinated Ti4+ cations incorporated into silica matrix, extra framework titanium species – such as octahedrally coordinated Ti4+ cations and partially polymerized hexacoordinated Ti species containing Ti–O–Ti bridges. The band assigned to tetrahedrally coordinated Ti4+ cations incorporated into silica walls is expected at about 220 nm and corresponds to the ligand-to-metal charge transfer within tetrahedral TiO4 and O3TiOH moieties [9,17,18]. Extra-framework titanium species, including isolated Ti4+ cations in the octahedral coordination, are represented by band located at about 230–250 nm, while the band at 260–320 nm is characteristic of partially polymerized hexacoordinated Ti species containing Ti–O–Ti bridges [9,19,20]. The shoulder above 320 nm could be assigned to TiO2 in the form of small anatase and rutile crystallites. The shift of the maxima in the direction of higher values of wavelength with increasing titanium content (Figure 5) indicates the formation of more polymerized titanium species. Similar results were reported by Zakharova et al. [21] for mesoporous silica of SBA-15 type modified with different amounts of titanium. For the sample with lower titanium loading (7 mol%), the band at about 250 nm was assigned to titanium dispersed on the silica surface framework as tetrahedral Ti4+ cations, while the shoulder at about 280 nm to Ti4+ cations in the octahedral coordination. These bands are caused by O2− to Ti4+ charge transfer in 4- and 6-coordinated titanium cations, respectively [21]. For the SBA-15 samples with higher titanium loadings (12 and 15 mol%), the band at about 315 nm, indicating the presence of ≡Ti–O–Ti≡ bonds, was found. Concluding, UV–Vis DRS analysis of the samples shows that titanium was incorporated into mesoporous silica mainly in the form of highly dispersed species. Moreover, the values of band gaps, determined from UV–Vis DRS measurements for the studied Ti-MCM-41 samples, are in the range of 3.16–3.24 eV (results not shown).
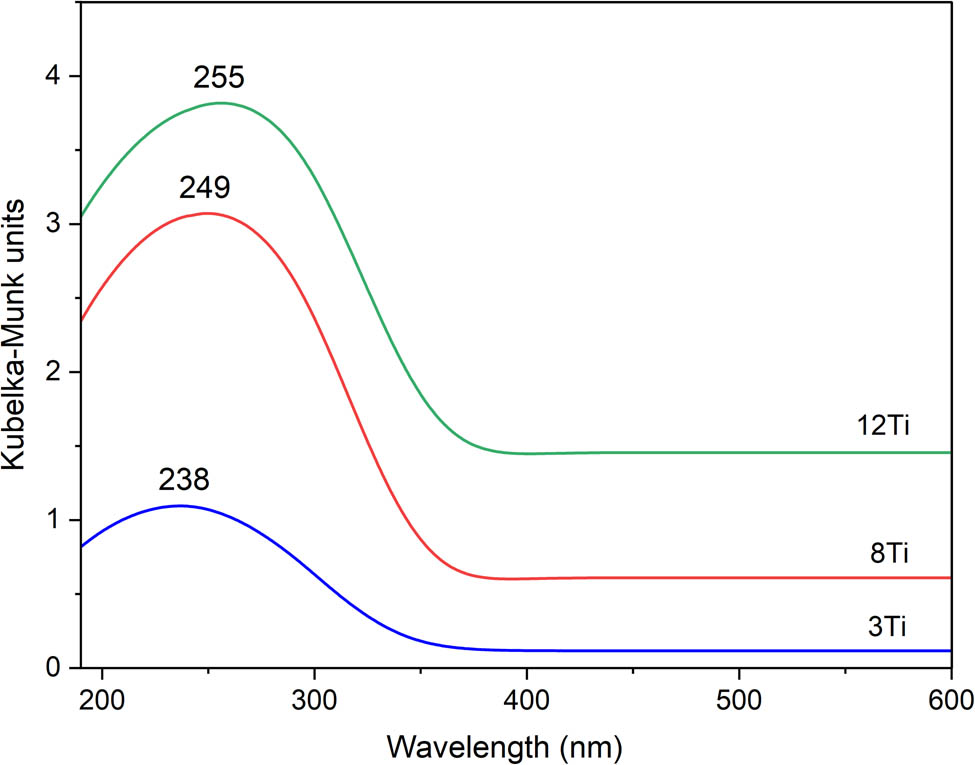
UV–Vis DR spectra recorded for 3Ti, 8Ti, and 12Ti.
Raman spectra of the studied samples are shown in Figure 6. The bands at about 1120 and 480 cm−1 indicate the presence of local Ti–(O–Si)4 units [22,23]. The band at 480 cm−1 is related to the bending stretching vibration of the ≡Ti–O–Si≡ species, while the band at about 1120 cm−1 to the asymmetric stretching vibration of the ≡Ti–O–Si≡ [24]. The characteristic frequency of ≡Ti–O–Si≡ is sensitive to the coordination environment, and therefore, relatively broad and asymmetric profile of this band may indicate very flexible coordination of titanium incorporated into a silica matrix [25]. The bands at 800 and 975 cm−1 are assigned to siloxanol bridge vibrations and Si–O stretching modes of silanol groups, respectively [23,26]. However, the bands at 800 and 975 cm−1 can also indicate the presence of titanium in octahedral and tetrahedral coordination, respectively [23,27]. The shoulder on the right side of the band at 480 cm−1 is assigned to the presence of extra-framework TiO2 in the form of small anatase crystallites. Similarly, the small band at about 645 cm−1 is observed in the spectrum of the 12Ti sample with the higher titanium loading [22,28]. The small band at about 610 cm−1, identified in spectra of the 8Ti and 12Ti samples, is assigned to small crystallites of rutile [29].
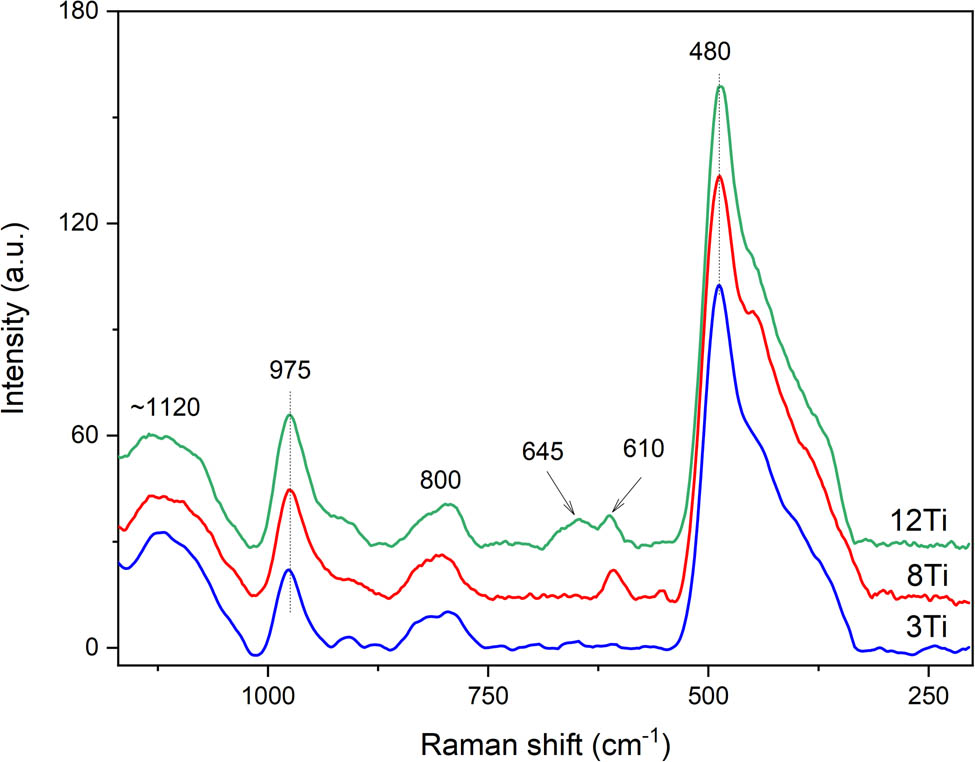
Raman spectra recorded for 3Ti, 8Ti, and 12Ti.
Thus, the analysis of the UV–Vis DR (Figure 5) and Raman (Figure 6) spectra lead to the conclusion that titanium in the samples is present mainly in highly dispersed forms. The low intensive bands, characteristic of rutile, were identified in Raman spectrum of the 8Ti and 12Ti samples. Moreover, small band indicating the presence of anatase was found in a spectrum of 12Ti. These bands were absent in the spectrum of the 3Ti sample. Because the TiO2 crystallites were not found in the XRD analysis of the studied samples (Figure 2, insert), their size and content are below the detection limit of this method. Thus, it can be concluded that the contribution of small, aggregated titanium species (rutile and anatase) increases with an increase in titanium loading in the Ti-MCM-41 samples.
The method of temperature-programmed desorption of ammonia (NH3-TPD) was used for the analysis of surface acidity of the samples. The main goal of these studies was the determination of surface exposed Ti species (available for the catalytic operation) contribution. It was assumed that each surface available titanium cation forms one acid site, that can be determined by NH3-TPD measurement. Ammonia desorption profiles are presented in Figure 7, while acid sites’ concentration (AS conc.) and acid sites’ density (AS dens.) are compared in Table 1. Any ammonia chemisorption was detected for the pure silica sample – S-MCM-41 (results not shown), while the introduction of titanium into silica samples resulted in the formation of acid sites, identified by ammonia chemisorption (Figure 7). The intensity of ammonia desorption profiles increases with increasing titanium content in the samples. The main desorption peaks, located at about 160–170°C, indicate the presence of relatively weak acid sites, which are possibly associated with the presence of highly dispersed titanium species. In the case of the 12Ti sample with the highest titanium loading, the arm at about 250°C indicates the presence of stronger acid sites, which are possibly associated with more aggregated titanium species. The nature of acid sites in silica-titania systems has been analysed and discussed in the scientific literature [30,31]. Doolin et al. [30] postulated that both Lewis and Brønsted acid sites are formed in such silica-titania samples. The Lewis centres are related to surface unsaturated titanium cations in tetrahedral and octahedral coordination; however, it was also suggested that tetrahedral sites are stronger Lewis acid sites than octahedral sites [30]. On the other hand, Brønsted acid sites can be formed by ≡Si–O–Ti≡ bridge hydrolysis, resulting in the ≡Ti–OH group presenting weak Brønsted-type acidity [30]. Eimer et al. [31] postulated that the presence of Brønsted acid sites in Ti-MCM-41 with high content of titanium is possibly related to the weakening of the ≡SiO–H bond due to the presence of Ti4+ ions in the vicinity of the silanol groups. In the case of the larger Ti4+ cations’ substitution into the positions of the smaller Si4+ ions, the bond length of ≡Ti–O–Si≡ is different in comparison to ≡Si–O–Si≡, which results in some structure deformation. Additionally, Ti4+ cations in the vicinity of the ≡Si–OH groups may result in changes in the electron density around silicon because of differences in electronegativity as well as the local structure deformations and therefore weakening the ≡SiO–H bonds [31]. Assuming that each surface available titanium cation generates one acid site, the ratio of surface available titanium cations to titanium content in the samples can be estimated (Table 1). This ratio is in the range of 0.11–0.16 and decreases with an increase in titanium content in the samples.
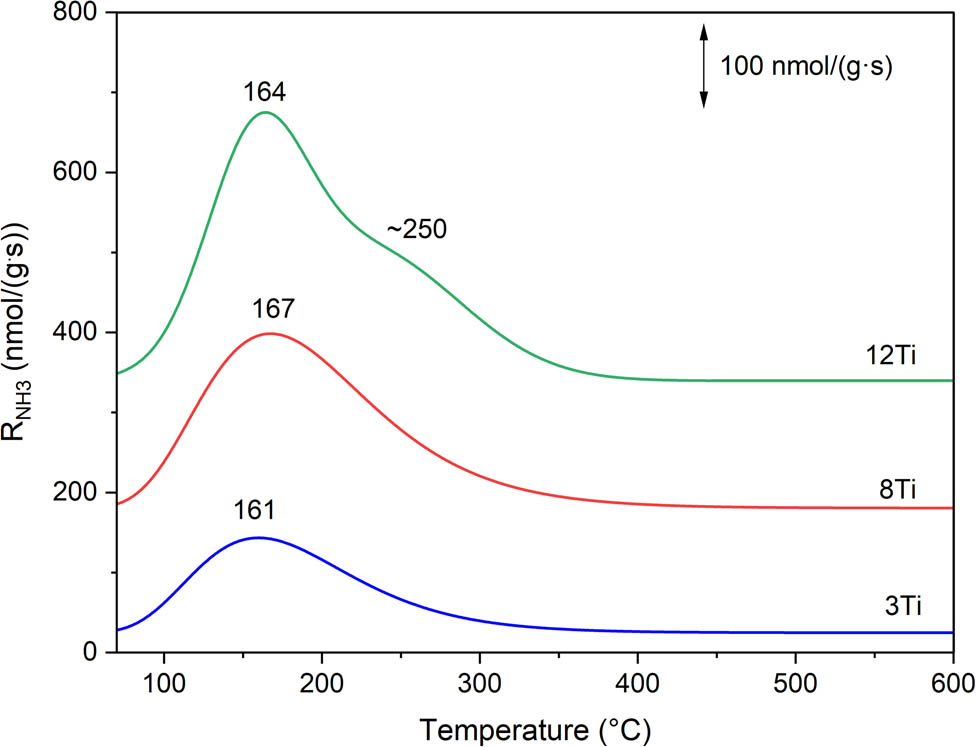
Ammonia desorption profiles measured for 3Ti, 8Ti, and 12Ti.
Spherical silica-titania MCM-41 samples were studied in the role of catalysts and photocatalysts of diphenyl sulfide (Ph2S) oxidation with hydrogen peroxide to diphenyl sulfoxide (Ph2SO) and diphenyl sulfone (Ph2SO2) (Figure 8). Catalytic activity of samples measured in the absence of light increases with an increase in titanium loading. After 4 h of the catalytic test, the Ph2S conversion (solid lines) reached 70% for 3Ti, 80% for 8Ti, and 96% for 12Ti (Figure 8a). In the case of 3Ti and 8Ti, Ph2SO was the main reaction product obtained after 4 h of the catalytic test with the selectivity of 67% and 60%, respectively (Figure 8b). The reaction conducted in the presence of 12Ti resulted mainly in Ph2SO2, which was obtained with the selectivity of 56% (Figure 8c). Efficiency of Ph2S oxidation was significantly improved for the process conducted with UV light irradiation. The Ph2S conversion after 4 h of the catalytic run reached 81% (an increase of 11% in comparison to the reaction conducted in dark) in the case of 3Ti and 99% (an increase of 19%) for 8Ti (Figure 8a, dashed lines). The best photocatalytic and catalytic activities presented the sample with the highest titanium loading, 12Ti, which was able to completely convert Ph2S during 2.5 h of the catalytic test. Moreover, for the 8Ti and 12Ti samples, a very significant increase in the selectivity to Ph2SO2 was observed for the reaction conducted with UV light irradiation (Figure 8c, dashed lines). The selectivity to Ph2SO2 reached after 4 h of the catalytic tests 70% and 95% for 8Ti and 12Ti, respectively. Thus, analysis of the results of catalytic and photocatalytic tests shows that oxidation of Ph2S in the presence of the studied catalysts is possible in the presence and absence of UV irradiation; however, the efficiency of this reaction can be significantly improved by UV irradiation of the reaction mixture. Moreover, the reaction selectivity to deeper oxidized product, Ph2SO2, was significantly improved for the UV-assisted reaction. The selectivity to Ph2SO decreased (Figure 8b), while the selectivity to Ph2SO2 increased (Figure 8c), with increasing duration of the catalytic and photocatalytic tests, especially in the case of the 8Ti and 12Ti catalysts, indicating the possible formation of Ph2SO as a primary product, which in the next step can be oxidized to Ph2SO2 (Ph2S → Ph2SO → Ph2SO2) [10].
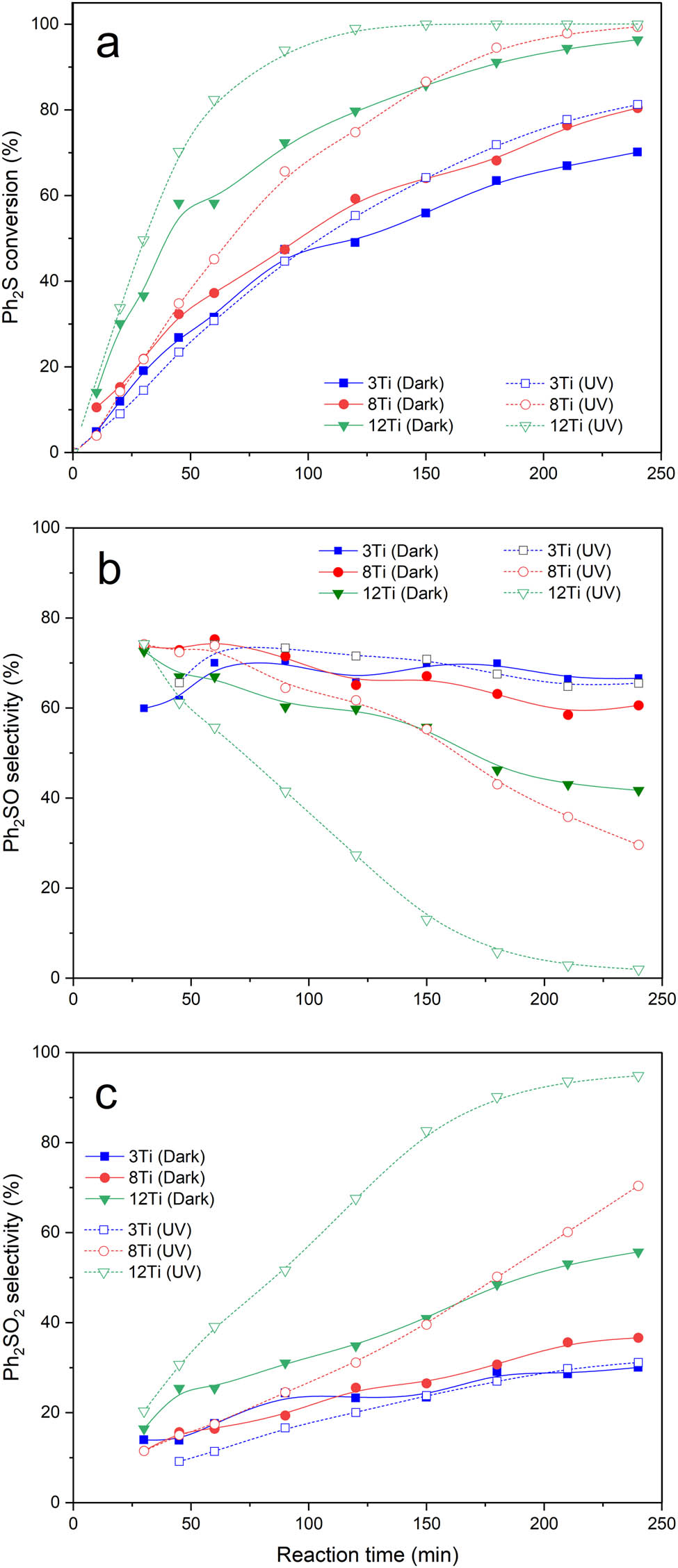
Results of catalytic (dark, solid lines) and photocatalytic (UV, dashed lines) tests for 3Ti, 8Ti, and 12Ti: Ph2S conversion (a), selectivity to Ph2SO (b), and selectivity to Ph2SO2 (c).
Catalytic activity of the samples, both in dark and under UV irradiation, increased with an increase in titanium content in the catalysts; thus, there is no doubt that titanium is responsible for their catalytic and photocatalytic activities in the studied reaction. As it was shown by UV–Vis DRS (Figure 5) and Raman (Figure 6) studies, the catalyst samples contain titanium mainly in the form of highly dispersed Ti species. However, an increase in titanium content results in the formation of small rutile and anatase crystallites. Thus, a question arises – which form of titanium species is responsible for catalytic and photocatalytic activation of the MCM-41-based samples. In our previous studies [11], commercial TiO2 (P25), containing both anatase and rutile, and pure anatase and rutile [10] were tested in the reaction of Ph2S oxidation with H2O2. It was shown that the Ph2S conversion was observed in dark and was significantly intensified by UV irradiation. Thus, it could be concluded that TiO2 crystallites are active in both catalytic and photocatalytic Ph2S oxidation. On the other side, our recent studies of titanosilicate zeolites showed that monomeric titanium cations incorporated into zeolite framework are also active in catalytic and photocatalytic Ph2S oxidation with H2O2 [9]. Thus, both monomeric Ti sites and TiO2 crystallites have been reported to be active catalysts and photocatalysts of Ph2S oxidation. Detailed analysis of the results obtained for bulky TiO2 crystallites [11] and titanosilicate zeolites [9] shows significant differences in selectivity to the reaction products. In the case of P25 selectivity to Ph2SO2, the product of deeper Ph2S oxidation was significantly lower than in the case of titanosilicate zeolites with higher titanium content and opened porous structure. Thus, it could be supposed the higher activity of monomeric titanium cations in zeolite framework than TiO2 crystallites in Ph2SO to Ph2SO2 oxidation. Of course, this hypothesis needs additional studies to be proved. In the case of the studied samples containing mainly titanium in highly dispersed forms with small contribution of small TiO2 crystallites, these selectivities to Ph2SO2 are higher than for P25 and lower than for titanosilicate zeolites (with higher titanium content and opened porous structure). Thus, the obtained results are in line with the postulated hypothesis. The studies of Ph2S oxidation with H2O2 over pure anatase and pure rutile samples, reported in our previous paper [10], showed that the reaction in the presence of anatase is observed both under catalytic and photocatalytic conditions. In contrast to rutile, which was active only under conditions of photocatalytic oxidation of diphenyl sulfide (no Ph2S conversion was detected without UV irradiation). Moreover, the reaction conducted in the presence of anatase is more selective to Ph2SO2, whereas in the presence of rutile, mainly Ph2SO was produced. In the case of the studied samples, various titanium species were identified by UV–Vis DRS (Figure 5) and Raman (Figure 6) studies, which contribute to their overall catalytic and photocatalytic properties. The presence of anatase was identified in the case of the 12Ti sample, presenting the highest selectivity to Ph2SO2, which supports the hypothesis postulated in our previous paper [10]. The Ph2S conversion observed for 3Ti and 8Ti at the beginning of the catalytic and photocatalytic tests is very similar (Figure 7). Thus, it seems possible that in the case of highly dispersed Ti species, mainly incorporated into silica walls f, Ph2S to Ph2SO oxidation may proceed without UV irradiation. This interesting effect will be analyzed in the future studies.
Catalytic activity of individual titanium sites, expressed as turnover frequency (TOF) values, is presented in Table 2. TOF values were calculated for the first step of Ph2S oxidation (Ph2S → Ph2SO). The number of surface Ti sites in the Ti-MCM-41 samples was estimated by NH3-TPD measurements. The TOF values, determined after 1 h of reaction, are very similar for the sample with the lowest titanium loading, 3Ti, for the reaction conducted under catalytic (dark) and photocatalytic (UV) conditions. For the samples with larger titanium content, 8Ti and 12Ti, frequency of catalytic cycles significantly increased under UV irradiation (Table 2). For comparison, the TOF values for Ph2S to Ph2SO oxidation in the presence of Ti zeolitic materials – Ti-FER and Ti-ITQ-6 (in both samples, the Si/Ti molar ratio is about 60) are shown in Table 2. For these zeolitic materials, it was assumed that all Ti sites are located on the surface. The TOF values, determined for Ti-FER under catalytic and photocatalytic conditions, are significantly lower compared to the Ti-MCM-41 catalysts. It could be explained by relatively narrow FER pores, which are unable to accommodate large Ph2S molecules. Thus, in this case, only Ti sites located on the outer surface of zeolitic grains can effectively participate in catalytic and photocatalytic processes [9]. The TOF values determined for Ti-ITQ-6, characterized by layered structure, are higher than for Ti-FER, but still lower compared to the best catalysts of the Ti-MCM-41 series. In this case, the interlayer space is available for Ph2S molecules, but small channels in layers are still too small to accommodate large molecules of diphenyl sulfide [9]. Thus, this comparison clearly shows a very important role of porous structure in the conversion of large Ph2S molecules.
Turnover frequency (TOF) of Ph2S to Ph2SO oxidation determined for 1 h of reaction
| Sample | Ti surface sites (μmol·g−1) | TOF, dark (h−1) | TOF, light (h−1) | Ref. |
|---|---|---|---|---|
| 3Ti | 102* | 1,477 | 1,440 | This study |
| 8Ti | 189* | 938 | 1,138 | This study |
| 12Ti | 260* | 1,067 | 1,511 | This study |
| Ti-FER-25 | 375# | 241 | 532 | [9] |
| Ti-ITQ-6-25 | 375# | 892 | 1,243 | [9] |
*Ti surface available sites determined by NH3-TPD measurements.
#Ti surface available sites determined from chemical analysis (ICP-OES); it was assumed that all Ti sites are available for the catalytic reaction.
A very important question is related to the role of UV irradiation in the intensification of Ph2S oxidation with H2O2 over highly dispersed Ti species. Some possible mechanisms have been postulated. One of them postulates the formation of titanium-hydroperoxide complexes, ≡Ti–O–O–H, which can decompose hydroxyl radicals, OH˙, much easier than H2O2 [32,33]. Also, small polymeric Ti–O–Ti–O–Ti chains may activate Ph2S oxidation. In such chains, the reduction of Ti4+ to Ti3+ by guest species may result in the formation of photocatalytic single-sites active in the formation of OH˙ radicals from H2O2 [34]. It is postulated that such one-dimensional chains can act as an antenna-like system collecting light and forming electron–hole pairs, which similarly to bulky TiO2, can recombine or diffuse [35]. The role of such dispersed Ti species in the photocatalytic process is still not fully recognized and intensively discussed in the literature [36,37].
4 Conclusions
Spherical Ti-MCM-41 materials, obtained by co-condensation method, presented catalytic, and photocatalytic activities in the reaction of diphenyl sulfide oxidation with H2O2 to diphenyl sulfoxide and diphenyl sulfone. Titanium introduced into silica samples was present mainly as highly dispersed species (e.g., monomeric cations, chains), however, for the samples with a larger titanium content also small crystallites of anatase and rutile were identified. Diphenyl sulfide conversion increased with increasing titanium content in the samples. The efficiency of diphenyl sulfide oxidation significantly increased for the reaction conducted under UV irradiation. Also, such photocatalytic conditions significantly intensified the formation of diphenyl sulfone, especially in the case of the sample with the highest titanium content. Results of the catalytic tests indicate Ph2SO as a primary reaction product, which in the next step can be oxidized to Ph2SO2 (Ph2S → Ph2SO → Ph2SO2).
Acknowledgments
Authors thank the National Science Center (Poland) for funding the project 2018/31/B/ST5/00143.
-
Funding information: The studies were carried out in the frame of project 2018/31/B/ST5/00143 from the National Science Centre (Poland).
-
Author contributions: Wiktora Dubiel: synthesis of catalysts, catalytic and photocatalytic studies, XRD, UV–Vis DRS, and NH3-TPD studies, data analysis, and manuscript writing; Andrzej Kowalczyk: analysis of textural properties and chemical composition; Aleksandra Jankowska: methodology of catalysts’ synthesis; Marek Michalik: SEM measurements; Włodzimierz Mozgawa: Raman spectroscopy measurements; Marcin Kobielusz: methodology of photocatalytic tests and manuscript writing; Wojciech Macyk: methodology of photocatalytic tests and manuscript writing; Lucjan Chmielarz: project administration, data analysis, and manuscript writing.
-
Conflict of interest: The authors state no conflict of interest.
-
Data availability statement: The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
[1] Global diphenyl sulfide market 2022 by manufacturers, regions, type and application, forecast to 2028. The Marketsandresearch.biz. Chemical and Material [Internet]. 2022 Mar [cited 2022 December 22]. https://www.marketsandresearch.biz/report/292122/global-diphenyl-sulfide-market-2022-by-manufacturers-regions-type-and-application-forecast-to-2028.Search in Google Scholar
[2] Diphenyl sulfoxide market growth opportunities 2022 to 2028 report contains analysis of recent developments and innovations new product launches upcoming challenges and technology landscape. The MarketWatch. [Internet]. 2022 Oct [cited 2022 December 22]. https://www.marketwatch.com/press-release/diphenyl-sulfoxide-market-growth-opportunities-2022-to-2028-report-containes-analysis-of-recent-developments-and-innovations-new-product-launches-upcoming-challenges-and-technology-landscape-2022-10-05.Search in Google Scholar
[3] Diphenyl Sulfone Market. The Future Market Insights. [Internet]. [cited 2022 December 22]. https://www.futuremarketinsights.com/reports/diphenyl-sulfone-market.Search in Google Scholar
[4] Al-Maksoud W, Daniele S, Sorokin AB. Practical oxidation of sulfides to sulfones by H2O2 catalysed by titanium catalyst. Green Chem. 2008;10(4):447–51. 10.1039/B717696A.Search in Google Scholar
[5] Smith MB, March J. March’s advanced organic chemistry. 6th edn. New York: Wiley; 2007. p. 1780–3.Search in Google Scholar
[6] Trost BM. The atom economy – A search for synthetic efficiency. Science. 1991;254(5037):1471–7. 10.1126/science.19622.Search in Google Scholar
[7] Přech J, Morris RE, Čejka J. Selective oxidation of bulky organic sulphides over layered titanosilicate catalysts. Catal Sci Technol. 2016;6(8):2775–86. 10.1039/C5CY02083B.Search in Google Scholar
[8] Maurya MR, Chandrakar AK, Chand S. Oxidation of methyl phenyl sulfide, diphenyl sulfide and styrene by oxovanadium(IV) and copper(II) complexes of NS donor ligand encapsulated in zeolite-Y. J Mol Catal A-Chem. 2007;278(1–2):12–21. 10.1016/j.molcata.2007.08.021.Search in Google Scholar
[9] Radko M, Rutkowska M, Kowalczyk A, Mikrut P, Święs A, Díaz U, et al. Catalytic oxidation of organic sulfides by H2O2 in the presence of titanosilicate zeolites. Microporous Mesoporous Mater. 2020;302:110219. 10.1016/j.micromeso.2020.110219.Search in Google Scholar
[10] Mikrut P, Święs A, Kobielusz M, Chmielarz L, Macyk W. Selective and efficient catalytic and photocatalytic oxidation of diphenyl sulphide to sulfoxide and sulfone: The role of hydrogen peroxide and TiO2 polymorph. RSC Adv. 2022;12(3):1862–70. 10.1039/D1RA08364C.Search in Google Scholar
[11] Radko M, Kowalczyk A, Mikrut P, Witkowski S, Mozgawa S, Macyk W, et al. Catalytic and photocatalytic oxidation of diphenyl sulphide to diphenyl sulfoxide over titanium dioxide doped with vanadium, zinc, and tin. RSC Adv. 2020;10(7):4023–31. 10.1039/C9RA09903D.Search in Google Scholar
[12] Radko M, Kowalczyk A, Bidzińska E, Witkowski S, Górecka S, Wierzbicki D, et al. Titanium dioxide doped with vanadium as effective catalyst for selective oxidation of diphenyl sulfide to diphenyl sulfonate. J Therm Anal Calorim. 2018;132:1471–80. 10.1007/s10973-018-7119-9.Search in Google Scholar
[13] Liu S, Lu L, Yang Z, Cool P, Vansant EF. Further investigations on the modified Stöber method for spherical MCM-41. Mater Chem Phys. 2006;97(2–3):203–20. 10.1016/j.matchemphys.2005.09.003.Search in Google Scholar
[14] Maddalena R, Hall C, Hamilton A. Effect of silica particle size on the formation of calcium silicate hydrate [C-S-H] using thermal analysis. Thermochim Acta. 2019;672:142–9. 10.1016/j.tca.2018.09.003.Search in Google Scholar
[15] Leofanti G, Padovan M, Tozzola G, Venturelli B. Surface area and pore texture of catalysts. Catal Today. 1998;41(1–3):207–19. 10.1016/S0920-5861(98)00050-9.Search in Google Scholar
[16] Thommes M, Kaneko K, Neimark AV, Olivier JP, Rodriguez-Reinoso F, Rouquerol J, et al. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl Chem. 2015;87(9–10):1051–69. 10.1515/pac-2014-1117.Search in Google Scholar
[17] Segura Y, Chmielarz L, Kuśtrowski P, Cool P, Dziembaj R, Vansant EF. Characterisation and reactivity of vanadia–titania supported SBA-15 in the SCR of NO with ammonia. Appl Catal B-Environ. 2005;61(1–2):69–78. 10.1016/j.apcatb.2005.04.011.Search in Google Scholar
[18] Blasco T, Corma A, Navarro MT, Pérez-Parienta J. Synthesis, characterization, and catalytic activity of Ti-MCM-41 structures. J Catal. 1995;156(1):65–74. 10.1006/jcat.1995.1232.Search in Google Scholar
[19] Chmielarz L, Piwowarska Z, Kuśtrowski P, Gil B, Adamski A, Dudek B, et al. Porous clay heterostructures (PCHs) intercalated with silica-titania pillars and modified with transition metals as catalysts for the DeNOx process. Appl Catal B-Environ. 2009;91(1–2):449–59. 10.1016/j.apcatb.2009.06.014.Search in Google Scholar
[20] Corma A, Díaz U, Domine ME, Fornés V. New aluminosilicate and titanosilicate delaminated materials active for acid catalysis, and oxidation reactions using H2O2. J Am Chem Soc. 2000;122(12):2804–9. 10.1021/ja9938130.Search in Google Scholar
[21] Zakharova MV, Kleitz F, Fontaine FG. Lewis acidity quantification and catalytic activity of Ti, Zr and Al-supported mesoporous silica. Dalton Trans. 2017;46(12):3864–76. 10.1039/C7DT00035A.Search in Google Scholar
[22] Fan F, Feng Z, Li C. UV Raman spectroscopic studies on active sites and synthesis mechanisms of transition metal-containing microporous and mesoporous materials. Acc Chem Res. 2010;43(3):378–87. 10.1021/ar900210g.Search in Google Scholar PubMed
[23] Geidel E, Lechert H, Döbler J, Jobic H, Calzaferri G, Bauer F. Characterization of mesoporous materials by vibrational spectroscopic techniques. Microporous Mesoporous Mater. 2003;65(1):31–42. 10.1016/S1387-1811(03)00505-5.Search in Google Scholar
[24] Li C, Xiong G, Liu JK, Ying PL, Xin Q, Feng ZC. Identifying framework titanium in TS-1 Zeolite by UV resonance Raman spectroscopy. J Phys Chem B. 2001;105(15):2993–7. 10.1021/jp0042359.Search in Google Scholar
[25] Zhang WH, Lu JQ, Han B, Li MJ, Xiu JH, Ying P, et al. Direct synthesis and characterization of titanium-substituted mesoporous molecular sieve SBA-15. Chem Mater. 2002;14(8):3413–21. 10.1021/cm011686c.Search in Google Scholar
[26] Jankowska A, Kowalczyk A, Rutkowska M, Mozgawa W, Gil B, Chmielarz L. Silica and silica–titania intercalated MCM-36 modified with iron as catalysts for selective reduction of nitrogen oxides – the role of associated reactions. Catal Sci Technol. 2020;10(23):7940–54. 10.1039/D0CY01415J.Search in Google Scholar
[27] Hammond C, Tarantino G. Switching off H2O2 decomposition during TS-1 catalysed epoxidation via post-synthetic active site modification. Catalysts. 2015;5(4):2309–23. 10.3390/catal5042309.Search in Google Scholar
[28] Li L, Wu P, Yu Q, Wu G, Guan N. Low temperature H2-SCR over platinum catalysts supported on Ti-containing MCM-41. Appl Catal B-Environ. 2010;94(3–4):254–62. 10.1016/j.apcatb.2009.11.016.Search in Google Scholar
[29] Lubas M, Jasinski JJ, Sitarz M, Kurpaska L, Podsiad P, Jasinski J. Raman spectroscopy of TiO2 thin films formed by hybrid treatment for biomedical applications. Spectrochim Acta A. 2014;133:867–71. 10.1016/j.saa.2014.05.045.Search in Google Scholar PubMed
[30] Doolin PK, Alerasool S, Zalewski DJ, Hoffman JF. Acidity studies of titania-silica mixed oxides. Catal Lett. 1994;25:209–23. 10.1007/BF00816302.Search in Google Scholar
[31] Eimer GA, Casuscelli SG, Chanquia CM, Elías V, Crivello ME, Herrero ER. The influence of Ti-loading on the acid behavior and on the catalytic efficiency of mesoporous Ti-MCM-41 molecular sieves. Catal Today. 2008;133–135:639–46. 10.1016/j.cattod.2007.12.096.Search in Google Scholar
[32] Juan Z, Dishun Z, Liyan Y, Yongbo L. Photocatalytic oxidation dibenzothiophene using TS-1. Chem Eng J. 2010;156(3):528–31. 10.1016/j.cej.2009.04.032.Search in Google Scholar
[33] Lee GD, Jung SK, Jeong YJ, Park JH, Lim KT, Ahn BH, et al. Photocatalytic decomposition of 4-nitrophenol over titanium silicalite (TS-1) catalysts. Appl Catal A-Gen. 2003;239(1–2):197–208. 10.1016/S0926-860X(02)00389-7.Search in Google Scholar
[34] Howe RF, Krisnandi YK. Photoreactivity of ETS-10. Chem Commun. 2001;1(17):11588–9. 10.1039/B104870H.Search in Google Scholar
[35] Usseglio S, Calza P, Damin A, Minero C, Bordiga S, Lamberti C. Tailoring the selectivity of Ti-based photocatalysts (TiO2 and microporous ETS-10 and ETS-4) by playing with surface morphology and electronic structure. Chem Mater. 2006;18(15):3412–24. 10.1021/cm052841g.Search in Google Scholar
[36] Yan Y, Li C, Wu Y, Gao J, Zhang Q. From isolated Ti-oxo clusters to infinite Ti-oxo chains and sheets: Recent advances in photoactive Ti-based MOFs. J Mater Chem A. 2020;8(31):15245–70. 10.1039/D0TA03749D.Search in Google Scholar
[37] Huang F, Hao H, Sheng W, Dong X, Lang X. Cooperative photocatalysis of dye–Ti-MCM-41 with trimethylamine for selective aerobic oxidation of sulfides illuminated by blue light. J Colloid Interface Sci. 2023;630:921–30. 10.1016/j.jcis.2022.10.052.Search in Google Scholar PubMed
© 2023 the author(s), published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Research Articles
- Value-added utilization of coal fly ash and recycled polyvinyl chloride in door or window sub-frame composites
- High removal efficiency of volatile phenol from coking wastewater using coal gasification slag via optimized adsorption and multi-grade batch process
- Evolution of surface morphology and properties of diamond films by hydrogen plasma etching
- Removal efficiency of dibenzofuran using CuZn-zeolitic imidazole frameworks as a catalyst and adsorbent
- Rapid and efficient microwave-assisted extraction of Caesalpinia sappan Linn. heartwood and subsequent synthesis of gold nanoparticles
- The catalytic characteristics of 2-methylnaphthalene acylation with AlCl3 immobilized on Hβ as Lewis acid catalyst
- Biodegradation of synthetic PVP biofilms using natural materials and nanoparticles
- Rutin-loaded selenium nanoparticles modulated the redox status, inflammatory, and apoptotic pathways associated with pentylenetetrazole-induced epilepsy in mice
- Optimization of apigenin nanoparticles prepared by planetary ball milling: In vitro and in vivo studies
- Synthesis and characterization of silver nanoparticles using Origanum onites leaves: Cytotoxic, apoptotic, and necrotic effects on Capan-1, L929, and Caco-2 cell lines
- Exergy analysis of a conceptual CO2 capture process with an amine-based DES
- Construction of fluorescence system of felodipine–tetracyanovinyl–2,2′-bipyridine complex
- Excellent photocatalytic degradation of rhodamine B over Bi2O3 supported on Zn-MOF nanocomposites under visible light
- Optimization-based control strategy for a large-scale polyhydroxyalkanoates production in a fed-batch bioreactor using a coupled PDE–ODE system
- Effectiveness of pH and amount of Artemia urumiana extract on physical, chemical, and biological attributes of UV-fabricated biogold nanoparticles
- Geranium leaf-mediated synthesis of silver nanoparticles and their transcriptomic effects on Candida albicans
- Synthesis, characterization, anticancer, anti-inflammatory activities, and docking studies of 3,5-disubstituted thiadiazine-2-thiones
- Synthesis and stability of phospholipid-encapsulated nano-selenium
- Putative anti-proliferative effect of Indian mustard (Brassica juncea) seed and its nano-formulation
- Enrichment of low-grade phosphorites by the selective leaching method
- Electrochemical analysis of the dissolution of gold in a copper–ethylenediamine–thiosulfate system
- Characterisation of carbonate lake sediments as a potential filler for polymer composites
- Evaluation of nano-selenium biofortification characteristics of alfalfa (Medicago sativa L.)
- Quality of oil extracted by cold press from Nigella sativa seeds incorporated with rosemary extracts and pretreated by microwaves
- Heteropolyacid-loaded MOF-derived mesoporous zirconia catalyst for chemical degradation of rhodamine B
- Recovery of critical metals from carbonatite-type mineral wastes: Geochemical modeling investigation of (bio)hydrometallurgical leaching of REEs
- Photocatalytic properties of ZnFe-mixed oxides synthesized via a simple route for water remediation
- Attenuation of di(2-ethylhexyl)phthalate-induced hepatic and renal toxicity by naringin nanoparticles in a rat model
- Novel in situ synthesis of quaternary core–shell metallic sulfide nanocomposites for degradation of organic dyes and hydrogen production
- Microfluidic steam-based synthesis of luminescent carbon quantum dots as sensing probes for nitrite detection
- Transformation of eggshell waste to egg white protein solution, calcium chloride dihydrate, and eggshell membrane powder
- Preparation of Zr-MOFs for the adsorption of doxycycline hydrochloride from wastewater
- Green nanoarchitectonics of the silver nanocrystal potential for treating malaria and their cytotoxic effects on the kidney Vero cell line
- Carbon emissions analysis of producing modified asphalt with natural asphalt
- An efficient and green synthesis of 2-phenylquinazolin-4(3H)-ones via t-BuONa-mediated oxidative condensation of 2-aminobenzamides and benzyl alcohols under solvent- and transition metal-free conditions
- Chitosan nanoparticles loaded with mesosulfuron methyl and mesosulfuron methyl + florasulam + MCPA isooctyl to manage weeds of wheat (Triticum aestivum L.)
- Synergism between lignite and high-sulfur petroleum coke in CO2 gasification
- Facile aqueous synthesis of ZnCuInS/ZnS–ZnS QDs with enhanced photoluminescence lifetime for selective detection of Cu(ii) ions
- Rapid synthesis of copper nanoparticles using Nepeta cataria leaves: An eco-friendly management of disease-causing vectors and bacterial pathogens
- Study on the photoelectrocatalytic activity of reduced TiO2 nanotube films for removal of methyl orange
- Development of a fuzzy logic model for the prediction of spark-ignition engine performance and emission for gasoline–ethanol blends
- Micro-impact-induced mechano-chemical synthesis of organic precursors from FeC/FeN and carbonates/nitrates in water and its extension to nucleobases
- Green synthesis of strontium-doped tin dioxide (SrSnO2) nanoparticles using the Mahonia bealei leaf extract and evaluation of their anticancer and antimicrobial activities
- A study on the larvicidal and adulticidal potential of Cladostepus spongiosus macroalgae and green-fabricated silver nanoparticles against mosquito vectors
- Catalysts based on nickel salt heteropolytungstates for selective oxidation of diphenyl sulfide
- Powerful antibacterial nanocomposites from Corallina officinalis-mediated nanometals and chitosan nanoparticles against fish-borne pathogens
- Removal behavior of Zn and alkalis from blast furnace dust in pre-reduction sinter process
- Environmentally friendly synthesis and computational studies of novel class of acridinedione integrated spirothiopyrrolizidines/indolizidines
- The mechanisms of inhibition and lubrication of clean fracturing flowback fluids in water-based drilling fluids
- Adsorption/desorption performance of cellulose membrane for Pb(ii)
- A one-pot, multicomponent tandem synthesis of fused polycyclic pyrrolo[3,2-c]quinolinone/pyrrolizino[2,3-c]quinolinone hybrid heterocycles via environmentally benign solid state melt reaction
- Green synthesis of silver nanoparticles using durian rind extract and optical characteristics of surface plasmon resonance-based optical sensor for the detection of hydrogen peroxide
- Electrochemical analysis of copper-EDTA-ammonia-gold thiosulfate dissolution system
- Characterization of bio-oil production by microwave pyrolysis from cashew nut shells and Cassia fistula pods
- Green synthesis methods and characterization of bacterial cellulose/silver nanoparticle composites
- Photocatalytic research performance of zinc oxide/graphite phase carbon nitride catalyst and its application in environment
- Effect of phytogenic iron nanoparticles on the bio-fortification of wheat varieties
- In vitro anti-cancer and antimicrobial effects of manganese oxide nanoparticles synthesized using the Glycyrrhiza uralensis leaf extract on breast cancer cell lines
- Preparation of Pd/Ce(F)-MCM-48 catalysts and their catalytic performance of n-heptane isomerization
- Green “one-pot” fluorescent bis-indolizine synthesis with whole-cell plant biocatalysis
- Silica-titania mesoporous silicas of MCM-41 type as effective catalysts and photocatalysts for selective oxidation of diphenyl sulfide by H2O2
- Biosynthesis of zinc oxide nanoparticles from molted feathers of Pavo cristatus and their antibiofilm and anticancer activities
- Clean preparation of rutile from Ti-containing mixed molten slag by CO2 oxidation
- Synthesis and characterization of Pluronic F-127-coated titanium dioxide nanoparticles synthesized from extracts of Atractylodes macrocephala leaf for antioxidant, antimicrobial, and anticancer properties
- Effect of pretreatment with alkali on the anaerobic digestion characteristics of kitchen waste and analysis of microbial diversity
- Ameliorated antimicrobial, antioxidant, and anticancer properties by Plectranthus vettiveroides root extract-mediated green synthesis of chitosan nanoparticles
- Microwave-accelerated pretreatment technique in green extraction of oil and bioactive compounds from camelina seeds: Effectiveness and characterization
- Studies on the extraction performance of phorate by aptamer-functionalized magnetic nanoparticles in plasma samples
- Investigation of structural properties and antibacterial activity of AgO nanoparticle extract from Solanum nigrum/Mentha leaf extracts by green synthesis method
- Green fabrication of chitosan from marine crustaceans and mushroom waste: Toward sustainable resource utilization
- Synthesis, characterization, and evaluation of nanoparticles of clodinofop propargyl and fenoxaprop-P-ethyl on weed control, growth, and yield of wheat (Triticum aestivum L.)
- The enhanced adsorption properties of phosphorus from aqueous solutions using lanthanum modified synthetic zeolites
- Separation of graphene oxides of different sizes by multi-layer dialysis and anti-friction and lubrication performance
- Visible-light-assisted base-catalyzed, one-pot synthesis of highly functionalized cinnolines
- The experimental study on the air oxidation of 5-hydroxymethylfurfural to 2,5-furandicarboxylic acid with Co–Mn–Br system
- Highly efficient removal of tetracycline and methyl violet 2B from aqueous solution using the bimetallic FeZn-ZIFs catalyst
- A thermo-tolerant cellulase enzyme produced by Bacillus amyloliquefaciens M7, an insight into synthesis, optimization, characterization, and bio-polishing activity
- Exploration of ketone derivatives of succinimide for their antidiabetic potential: In vitro and in vivo approaches
- Ultrasound-assisted green synthesis and in silico study of 6-(4-(butylamino)-6-(diethylamino)-1,3,5-triazin-2-yl)oxypyridazine derivatives
- A study of the anticancer potential of Pluronic F-127 encapsulated Fe2O3 nanoparticles derived from Berberis vulgaris extract
- Biogenic synthesis of silver nanoparticles using Consolida orientalis flowers: Identification, catalytic degradation, and biological effect
- Initial assessment of the presence of plastic waste in some coastal mangrove forests in Vietnam
- Adsorption synergy electrocatalytic degradation of phenol by active oxygen-containing species generated in Co-coal based cathode and graphite anode
- Antibacterial, antifungal, antioxidant, and cytotoxicity activities of the aqueous extract of Syzygium aromaticum-mediated synthesized novel silver nanoparticles
- Synthesis of a silica matrix with ZnO nanoparticles for the fabrication of a recyclable photodegradation system to eliminate methylene blue dye
- Natural polymer fillers instead of dye and pigments: Pumice and scoria in PDMS fluid and elastomer composites
- Study on the preparation of glycerylphosphorylcholine by transesterification under supported sodium methoxide
- Wireless network handheld terminal-based green ecological sustainable design evaluation system: Improved data communication and reduced packet loss rate
- The optimization of hydrogel strength from cassava starch using oxidized sucrose as a crosslinking agent
- Green synthesis of silver nanoparticles using Saccharum officinarum leaf extract for antiviral paint
- Study on the reliability of nano-silver-coated tin solder joints for flip chips
- Environmentally sustainable analytical quality by design aided RP-HPLC method for the estimation of brilliant blue in commercial food samples employing a green-ultrasound-assisted extraction technique
- Anticancer and antimicrobial potential of zinc/sodium alginate/polyethylene glycol/d-pinitol nanocomposites against osteosarcoma MG-63 cells
- Nanoporous carbon@CoFe2O4 nanocomposite as a green absorbent for the adsorptive removal of Hg(ii) from aqueous solutions
- Characterization of silver sulfide nanoparticles from actinobacterial strain (M10A62) and its toxicity against lepidopteran and dipterans insect species
- Phyto-fabrication and characterization of silver nanoparticles using Withania somnifera: Investigating antioxidant potential
- Effect of e-waste nanofillers on the mechanical, thermal, and wear properties of epoxy-blend sisal woven fiber-reinforced composites
- Magnesium nanohydroxide (2D brucite) as a host matrix for thymol and carvacrol: Synthesis, characterization, and inhibition of foodborne pathogens
- Synergistic inhibitive effect of a hybrid zinc oxide-benzalkonium chloride composite on the corrosion of carbon steel in a sulfuric acidic solution
- Review Articles
- Role and the importance of green approach in biosynthesis of nanopropolis and effectiveness of propolis in the treatment of COVID-19 pandemic
- Gum tragacanth-mediated synthesis of metal nanoparticles, characterization, and their applications as a bactericide, catalyst, antioxidant, and peroxidase mimic
- Green-processed nano-biocomposite (ZnO–TiO2): Potential candidates for biomedical applications
- Reaction mechanisms in microwave-assisted lignin depolymerisation in hydrogen-donating solvents
- Recent progress on non-noble metal catalysts for the deoxydehydration of biomass-derived oxygenates
- Rapid Communication
- Phosphorus removal by iron–carbon microelectrolysis: A new way to achieve phosphorus recovery
- Special Issue: Biomolecules-derived synthesis of nanomaterials for environmental and biological applications (Guest Editors: Arpita Roy and Fernanda Maria Policarpo Tonelli)
- Biomolecules-derived synthesis of nanomaterials for environmental and biological applications
- Nano-encapsulated tanshinone IIA in PLGA-PEG-COOH inhibits apoptosis and inflammation in cerebral ischemia/reperfusion injury
- Green fabrication of silver nanoparticles using Melia azedarach ripened fruit extract, their characterization, and biological properties
- Green-synthesized nanoparticles and their therapeutic applications: A review
- Antioxidant, antibacterial, and cytotoxicity potential of synthesized silver nanoparticles from the Cassia alata leaf aqueous extract
- Green synthesis of silver nanoparticles using Callisia fragrans leaf extract and its anticancer activity against MCF-7, HepG2, KB, LU-1, and MKN-7 cell lines
- Algae-based green AgNPs, AuNPs, and FeNPs as potential nanoremediators
- Green synthesis of Kickxia elatine-induced silver nanoparticles and their role as anti-acetylcholinesterase in the treatment of Alzheimer’s disease
- Phytocrystallization of silver nanoparticles using Cassia alata flower extract for effective control of fungal skin pathogens
- Antibacterial wound dressing with hydrogel from chitosan and polyvinyl alcohol from the red cabbage extract loaded with silver nanoparticles
- Leveraging of mycogenic copper oxide nanostructures for disease management of Alternaria blight of Brassica juncea
- Nanoscale molecular reactions in microbiological medicines in modern medical applications
- Synthesis and characterization of ZnO/β-cyclodextrin/nicotinic acid nanocomposite and its biological and environmental application
- Green synthesis of silver nanoparticles via Taxus wallichiana Zucc. plant-derived Taxol: Novel utilization as anticancer, antioxidation, anti-inflammation, and antiurolithic potential
- Recyclability and catalytic characteristics of copper oxide nanoparticles derived from bougainvillea plant flower extract for biomedical application
- Phytofabrication, characterization, and evaluation of novel bioinspired selenium–iron (Se–Fe) nanocomposites using Allium sativum extract for bio-potential applications
- Erratum
- Erratum to “Synthesis, characterization, and evaluation of nanoparticles of clodinofop propargyl and fenoxaprop-P-ethyl on weed control, growth, and yield of wheat (Triticum aestivum L.)”
Articles in the same Issue
- Research Articles
- Value-added utilization of coal fly ash and recycled polyvinyl chloride in door or window sub-frame composites
- High removal efficiency of volatile phenol from coking wastewater using coal gasification slag via optimized adsorption and multi-grade batch process
- Evolution of surface morphology and properties of diamond films by hydrogen plasma etching
- Removal efficiency of dibenzofuran using CuZn-zeolitic imidazole frameworks as a catalyst and adsorbent
- Rapid and efficient microwave-assisted extraction of Caesalpinia sappan Linn. heartwood and subsequent synthesis of gold nanoparticles
- The catalytic characteristics of 2-methylnaphthalene acylation with AlCl3 immobilized on Hβ as Lewis acid catalyst
- Biodegradation of synthetic PVP biofilms using natural materials and nanoparticles
- Rutin-loaded selenium nanoparticles modulated the redox status, inflammatory, and apoptotic pathways associated with pentylenetetrazole-induced epilepsy in mice
- Optimization of apigenin nanoparticles prepared by planetary ball milling: In vitro and in vivo studies
- Synthesis and characterization of silver nanoparticles using Origanum onites leaves: Cytotoxic, apoptotic, and necrotic effects on Capan-1, L929, and Caco-2 cell lines
- Exergy analysis of a conceptual CO2 capture process with an amine-based DES
- Construction of fluorescence system of felodipine–tetracyanovinyl–2,2′-bipyridine complex
- Excellent photocatalytic degradation of rhodamine B over Bi2O3 supported on Zn-MOF nanocomposites under visible light
- Optimization-based control strategy for a large-scale polyhydroxyalkanoates production in a fed-batch bioreactor using a coupled PDE–ODE system
- Effectiveness of pH and amount of Artemia urumiana extract on physical, chemical, and biological attributes of UV-fabricated biogold nanoparticles
- Geranium leaf-mediated synthesis of silver nanoparticles and their transcriptomic effects on Candida albicans
- Synthesis, characterization, anticancer, anti-inflammatory activities, and docking studies of 3,5-disubstituted thiadiazine-2-thiones
- Synthesis and stability of phospholipid-encapsulated nano-selenium
- Putative anti-proliferative effect of Indian mustard (Brassica juncea) seed and its nano-formulation
- Enrichment of low-grade phosphorites by the selective leaching method
- Electrochemical analysis of the dissolution of gold in a copper–ethylenediamine–thiosulfate system
- Characterisation of carbonate lake sediments as a potential filler for polymer composites
- Evaluation of nano-selenium biofortification characteristics of alfalfa (Medicago sativa L.)
- Quality of oil extracted by cold press from Nigella sativa seeds incorporated with rosemary extracts and pretreated by microwaves
- Heteropolyacid-loaded MOF-derived mesoporous zirconia catalyst for chemical degradation of rhodamine B
- Recovery of critical metals from carbonatite-type mineral wastes: Geochemical modeling investigation of (bio)hydrometallurgical leaching of REEs
- Photocatalytic properties of ZnFe-mixed oxides synthesized via a simple route for water remediation
- Attenuation of di(2-ethylhexyl)phthalate-induced hepatic and renal toxicity by naringin nanoparticles in a rat model
- Novel in situ synthesis of quaternary core–shell metallic sulfide nanocomposites for degradation of organic dyes and hydrogen production
- Microfluidic steam-based synthesis of luminescent carbon quantum dots as sensing probes for nitrite detection
- Transformation of eggshell waste to egg white protein solution, calcium chloride dihydrate, and eggshell membrane powder
- Preparation of Zr-MOFs for the adsorption of doxycycline hydrochloride from wastewater
- Green nanoarchitectonics of the silver nanocrystal potential for treating malaria and their cytotoxic effects on the kidney Vero cell line
- Carbon emissions analysis of producing modified asphalt with natural asphalt
- An efficient and green synthesis of 2-phenylquinazolin-4(3H)-ones via t-BuONa-mediated oxidative condensation of 2-aminobenzamides and benzyl alcohols under solvent- and transition metal-free conditions
- Chitosan nanoparticles loaded with mesosulfuron methyl and mesosulfuron methyl + florasulam + MCPA isooctyl to manage weeds of wheat (Triticum aestivum L.)
- Synergism between lignite and high-sulfur petroleum coke in CO2 gasification
- Facile aqueous synthesis of ZnCuInS/ZnS–ZnS QDs with enhanced photoluminescence lifetime for selective detection of Cu(ii) ions
- Rapid synthesis of copper nanoparticles using Nepeta cataria leaves: An eco-friendly management of disease-causing vectors and bacterial pathogens
- Study on the photoelectrocatalytic activity of reduced TiO2 nanotube films for removal of methyl orange
- Development of a fuzzy logic model for the prediction of spark-ignition engine performance and emission for gasoline–ethanol blends
- Micro-impact-induced mechano-chemical synthesis of organic precursors from FeC/FeN and carbonates/nitrates in water and its extension to nucleobases
- Green synthesis of strontium-doped tin dioxide (SrSnO2) nanoparticles using the Mahonia bealei leaf extract and evaluation of their anticancer and antimicrobial activities
- A study on the larvicidal and adulticidal potential of Cladostepus spongiosus macroalgae and green-fabricated silver nanoparticles against mosquito vectors
- Catalysts based on nickel salt heteropolytungstates for selective oxidation of diphenyl sulfide
- Powerful antibacterial nanocomposites from Corallina officinalis-mediated nanometals and chitosan nanoparticles against fish-borne pathogens
- Removal behavior of Zn and alkalis from blast furnace dust in pre-reduction sinter process
- Environmentally friendly synthesis and computational studies of novel class of acridinedione integrated spirothiopyrrolizidines/indolizidines
- The mechanisms of inhibition and lubrication of clean fracturing flowback fluids in water-based drilling fluids
- Adsorption/desorption performance of cellulose membrane for Pb(ii)
- A one-pot, multicomponent tandem synthesis of fused polycyclic pyrrolo[3,2-c]quinolinone/pyrrolizino[2,3-c]quinolinone hybrid heterocycles via environmentally benign solid state melt reaction
- Green synthesis of silver nanoparticles using durian rind extract and optical characteristics of surface plasmon resonance-based optical sensor for the detection of hydrogen peroxide
- Electrochemical analysis of copper-EDTA-ammonia-gold thiosulfate dissolution system
- Characterization of bio-oil production by microwave pyrolysis from cashew nut shells and Cassia fistula pods
- Green synthesis methods and characterization of bacterial cellulose/silver nanoparticle composites
- Photocatalytic research performance of zinc oxide/graphite phase carbon nitride catalyst and its application in environment
- Effect of phytogenic iron nanoparticles on the bio-fortification of wheat varieties
- In vitro anti-cancer and antimicrobial effects of manganese oxide nanoparticles synthesized using the Glycyrrhiza uralensis leaf extract on breast cancer cell lines
- Preparation of Pd/Ce(F)-MCM-48 catalysts and their catalytic performance of n-heptane isomerization
- Green “one-pot” fluorescent bis-indolizine synthesis with whole-cell plant biocatalysis
- Silica-titania mesoporous silicas of MCM-41 type as effective catalysts and photocatalysts for selective oxidation of diphenyl sulfide by H2O2
- Biosynthesis of zinc oxide nanoparticles from molted feathers of Pavo cristatus and their antibiofilm and anticancer activities
- Clean preparation of rutile from Ti-containing mixed molten slag by CO2 oxidation
- Synthesis and characterization of Pluronic F-127-coated titanium dioxide nanoparticles synthesized from extracts of Atractylodes macrocephala leaf for antioxidant, antimicrobial, and anticancer properties
- Effect of pretreatment with alkali on the anaerobic digestion characteristics of kitchen waste and analysis of microbial diversity
- Ameliorated antimicrobial, antioxidant, and anticancer properties by Plectranthus vettiveroides root extract-mediated green synthesis of chitosan nanoparticles
- Microwave-accelerated pretreatment technique in green extraction of oil and bioactive compounds from camelina seeds: Effectiveness and characterization
- Studies on the extraction performance of phorate by aptamer-functionalized magnetic nanoparticles in plasma samples
- Investigation of structural properties and antibacterial activity of AgO nanoparticle extract from Solanum nigrum/Mentha leaf extracts by green synthesis method
- Green fabrication of chitosan from marine crustaceans and mushroom waste: Toward sustainable resource utilization
- Synthesis, characterization, and evaluation of nanoparticles of clodinofop propargyl and fenoxaprop-P-ethyl on weed control, growth, and yield of wheat (Triticum aestivum L.)
- The enhanced adsorption properties of phosphorus from aqueous solutions using lanthanum modified synthetic zeolites
- Separation of graphene oxides of different sizes by multi-layer dialysis and anti-friction and lubrication performance
- Visible-light-assisted base-catalyzed, one-pot synthesis of highly functionalized cinnolines
- The experimental study on the air oxidation of 5-hydroxymethylfurfural to 2,5-furandicarboxylic acid with Co–Mn–Br system
- Highly efficient removal of tetracycline and methyl violet 2B from aqueous solution using the bimetallic FeZn-ZIFs catalyst
- A thermo-tolerant cellulase enzyme produced by Bacillus amyloliquefaciens M7, an insight into synthesis, optimization, characterization, and bio-polishing activity
- Exploration of ketone derivatives of succinimide for their antidiabetic potential: In vitro and in vivo approaches
- Ultrasound-assisted green synthesis and in silico study of 6-(4-(butylamino)-6-(diethylamino)-1,3,5-triazin-2-yl)oxypyridazine derivatives
- A study of the anticancer potential of Pluronic F-127 encapsulated Fe2O3 nanoparticles derived from Berberis vulgaris extract
- Biogenic synthesis of silver nanoparticles using Consolida orientalis flowers: Identification, catalytic degradation, and biological effect
- Initial assessment of the presence of plastic waste in some coastal mangrove forests in Vietnam
- Adsorption synergy electrocatalytic degradation of phenol by active oxygen-containing species generated in Co-coal based cathode and graphite anode
- Antibacterial, antifungal, antioxidant, and cytotoxicity activities of the aqueous extract of Syzygium aromaticum-mediated synthesized novel silver nanoparticles
- Synthesis of a silica matrix with ZnO nanoparticles for the fabrication of a recyclable photodegradation system to eliminate methylene blue dye
- Natural polymer fillers instead of dye and pigments: Pumice and scoria in PDMS fluid and elastomer composites
- Study on the preparation of glycerylphosphorylcholine by transesterification under supported sodium methoxide
- Wireless network handheld terminal-based green ecological sustainable design evaluation system: Improved data communication and reduced packet loss rate
- The optimization of hydrogel strength from cassava starch using oxidized sucrose as a crosslinking agent
- Green synthesis of silver nanoparticles using Saccharum officinarum leaf extract for antiviral paint
- Study on the reliability of nano-silver-coated tin solder joints for flip chips
- Environmentally sustainable analytical quality by design aided RP-HPLC method for the estimation of brilliant blue in commercial food samples employing a green-ultrasound-assisted extraction technique
- Anticancer and antimicrobial potential of zinc/sodium alginate/polyethylene glycol/d-pinitol nanocomposites against osteosarcoma MG-63 cells
- Nanoporous carbon@CoFe2O4 nanocomposite as a green absorbent for the adsorptive removal of Hg(ii) from aqueous solutions
- Characterization of silver sulfide nanoparticles from actinobacterial strain (M10A62) and its toxicity against lepidopteran and dipterans insect species
- Phyto-fabrication and characterization of silver nanoparticles using Withania somnifera: Investigating antioxidant potential
- Effect of e-waste nanofillers on the mechanical, thermal, and wear properties of epoxy-blend sisal woven fiber-reinforced composites
- Magnesium nanohydroxide (2D brucite) as a host matrix for thymol and carvacrol: Synthesis, characterization, and inhibition of foodborne pathogens
- Synergistic inhibitive effect of a hybrid zinc oxide-benzalkonium chloride composite on the corrosion of carbon steel in a sulfuric acidic solution
- Review Articles
- Role and the importance of green approach in biosynthesis of nanopropolis and effectiveness of propolis in the treatment of COVID-19 pandemic
- Gum tragacanth-mediated synthesis of metal nanoparticles, characterization, and their applications as a bactericide, catalyst, antioxidant, and peroxidase mimic
- Green-processed nano-biocomposite (ZnO–TiO2): Potential candidates for biomedical applications
- Reaction mechanisms in microwave-assisted lignin depolymerisation in hydrogen-donating solvents
- Recent progress on non-noble metal catalysts for the deoxydehydration of biomass-derived oxygenates
- Rapid Communication
- Phosphorus removal by iron–carbon microelectrolysis: A new way to achieve phosphorus recovery
- Special Issue: Biomolecules-derived synthesis of nanomaterials for environmental and biological applications (Guest Editors: Arpita Roy and Fernanda Maria Policarpo Tonelli)
- Biomolecules-derived synthesis of nanomaterials for environmental and biological applications
- Nano-encapsulated tanshinone IIA in PLGA-PEG-COOH inhibits apoptosis and inflammation in cerebral ischemia/reperfusion injury
- Green fabrication of silver nanoparticles using Melia azedarach ripened fruit extract, their characterization, and biological properties
- Green-synthesized nanoparticles and their therapeutic applications: A review
- Antioxidant, antibacterial, and cytotoxicity potential of synthesized silver nanoparticles from the Cassia alata leaf aqueous extract
- Green synthesis of silver nanoparticles using Callisia fragrans leaf extract and its anticancer activity against MCF-7, HepG2, KB, LU-1, and MKN-7 cell lines
- Algae-based green AgNPs, AuNPs, and FeNPs as potential nanoremediators
- Green synthesis of Kickxia elatine-induced silver nanoparticles and their role as anti-acetylcholinesterase in the treatment of Alzheimer’s disease
- Phytocrystallization of silver nanoparticles using Cassia alata flower extract for effective control of fungal skin pathogens
- Antibacterial wound dressing with hydrogel from chitosan and polyvinyl alcohol from the red cabbage extract loaded with silver nanoparticles
- Leveraging of mycogenic copper oxide nanostructures for disease management of Alternaria blight of Brassica juncea
- Nanoscale molecular reactions in microbiological medicines in modern medical applications
- Synthesis and characterization of ZnO/β-cyclodextrin/nicotinic acid nanocomposite and its biological and environmental application
- Green synthesis of silver nanoparticles via Taxus wallichiana Zucc. plant-derived Taxol: Novel utilization as anticancer, antioxidation, anti-inflammation, and antiurolithic potential
- Recyclability and catalytic characteristics of copper oxide nanoparticles derived from bougainvillea plant flower extract for biomedical application
- Phytofabrication, characterization, and evaluation of novel bioinspired selenium–iron (Se–Fe) nanocomposites using Allium sativum extract for bio-potential applications
- Erratum
- Erratum to “Synthesis, characterization, and evaluation of nanoparticles of clodinofop propargyl and fenoxaprop-P-ethyl on weed control, growth, and yield of wheat (Triticum aestivum L.)”


