Biosynthesis of zinc oxide nanoparticles from molted feathers of Pavo cristatus and their antibiofilm and anticancer activities
-
Ramachandran Ishwarya
Abstract
The present study employed molted feather ash extract from Pavo cristatus to synthesize zinc oxide nanoparticles (Pcf-ZnONPs). The Pcf-ZnONPs were characterized through advanced spectroscopy techniques to study the chemical and physical properties of NPs. Pcf-ZnONPs specifically exhibit an absorption peak at 365 nm in the UV spectroscopy analysis. TEM and SEM confirmed the nanoscale presence of ZnO. The antibiofilm and antibacterial properties of Pcf-ZnONPs were assessed against Staphylococcus aureus and Pseudomonas aeruginosa. At a concentration of 25 µg·mL−1, Pcf-ZnONPs exhibited a significant reduction in the thickness of bacterial biofilms. Breast cancer cell lines (MDA-MB-231) and fish cell lines were used to investigate in vitro anticancer activity. The MTT experiment demonstrated that Pcf-ZnONPs have good anticancer efficacy against a breast cancer and a fish cell line. The nontoxicity and biocompatibility of Pcf-ZnONPs were also investigated, exhibiting excellent hemocompatibility with red blood cells and no appreciable toxicity in Brine shrimp. In conclusion, Pcf-ZnONPs may be deemed nano-biomedicine compatible with biological systems for the treatment of bacterial illnesses in higher species.
Graphical abstract
Synthesis, characterization, and biological applications of zinc oxide nanoparticles using P. cristatus.

1 Introduction
Modern materials science research is centered on the topic of nanotechnology, which has the potential to revolutionize several industries. This technology has numerous unique uses, including novel fabric chemicals, food processing, agricultural production, and advanced treatment strategies [1]. Nanoparticles produced of metals and their oxides are of tremendous interest, and zinc (Zn) and its oxide are among the most thoroughly researched metals impacting biological items (ZnO). Zinc is an energetic component with excellent reducing abilities. When zinc oxidizes, zinc oxide is easily generated. Zinc is one of the most significant trace minerals and is crucial for good health. The United States Food and Drug Administration has permitted zinc oxide and four additional zinc compounds as GRAS (generally recognized as safe) ingredients [2]. Since it is required for the activity of specific enzymes key to eukaryotic metabolic processes [3], zinc is one of the micronutrients the human body requires to operate properly. Even though zinc is well thought-out comparatively nontoxic, there is mounting proof that unbound Zn ions can harm cells. ZnO nanoparticles (NPs) have attracted a tremendous interest from a mixture of industries due to their superior physicochemical properties, safety, and biodegradability [4], as well as their quick release to different tissues and organs and various biological uses like drug delivery and immune-modulatory agent [5]. Besides its remedial effectiveness against diabetes, microbial disease, swellings, and wound curing, ZnONPs have shown intriguing anticancer properties [6]. ZnONPs were discovered to possibly have a molecular impact on cancer therapy, with effects on cellular viability, membrane integrity, and the triggering of programmed cell death [7]. It is now clear that ZnONPs are cytotoxic to tumor cells but only somewhat harmful to healthy cells [8].
Zinc ions are bonded with bioactive ligands and zinc oxide nanoparticles are generated to reduce the cytotoxic effect. Nanostructured ZnO can take on a variety of shapes and characteristics. Improving the functions of the ZnONPs is essential due to the introduction of new malignancies as well as various virulent pathogenic species that cause infections and serious disorders. Thus, greater efforts are required to make ZnO more appealing. Understanding the action method of ZnONPs against bacteria is vital to use them in food products better and to aid in creating potent but harmless antimicrobial derivatives, but the process behind their antibacterial impact is currently unknown. However, several investigations have suggested that disruption of cell membrane activity is the fundamental driver of antibacterial action [9]. Using biocompatible ZnONPs for the drug delivery purpose is crucial because they can be used in alternative therapies targeting cancer cells exclusively rather than healthy ones. Due to their high degrees of cytotoxicity toward both cancerous and healthy cells, conventional cancer therapies like chemotherapy and radiation therapy have several limitations that may assist in alleviating [10,11]. It is necessary to concentrate effort on creating nontoxic ZnONPs to ensure that they won’t trigger an immunological reaction when given to humans. When these nanoparticles are used as “controlled release reservoirs,” it makes it easier to distribute medications where needed and treat numerous ailments.
The national bird of India is the Pavo cristatus (Peacock), which possesses typical quill feathers that can be utilized to make Mayrapuccha Bhasma (Calx of peacock feather). These feathers are used to treat snakebite [12,13] and are known in Ayurvedic remedy as Mayur Chandrika Bhasma, which is used as an antiemetic and anti-spasmodic and to relieve hiccough, asthma, vomiting, and other lung-related troubles [14,15,16]. The pigments in feathers give them a wide range of color and brilliance. The quantities of salt, zinc, manganese, bromine, and copper in the pharmaceuticals created with the feather ash are substantially superior to other medicinal samples evaluated in the feather. Compared to the outer feathers, the center “eye” is wealthier in manganese, bromine, iron, and copper. The unusually high zinc concentration in the periphery of the feathers is important [17]. Copper’s role in the respiratory process via cytochrome oxidase is well documented. It also aids red blood cell (RBC) maturation and survival in circulation. These primitive medications, which have been used for centuries, may have acted as a multi-drug therapy.
A rapidly expanding field of nanotechnology research is “green-mediated” inorganic NP synthesis, which includes biological methods and uses plants, algae, bacteria, fungi, and other organisms instead of expensive and dangerous chemicals [18]. Conversely, as nanomaterials’ use in various research and technology domains has developed, concerns regarding their safety, biocompatibility, and toxicity have arisen. For any therapeutic use, NP biocompatibility is obviously crucial. Hence, it is essential to address these problems, increase our understanding of the toxicity, biocompatibility, and safety of nanomaterials, and determine whether there is a chance that nanomaterials and biological systems could interact more frequently [19]. In the present investigation, green ZnONPs (Pcf-ZnONPs) were synthesized using peacock feather ash extract and characterized using a variety of methods. Pcf-ZnONPs were tested for their antibacterial efficacy in vitro against both Staphylococcus aureus and Pseudomonas aeruginosa. In vitro tests of Pcf-ZnONPs’ anticancer potential were also conducted using a breast cancer cell line and fish cells. Moreover, Pcf-ZnONPs were investigated as a potential option for biomedical and environmental applications due to their nontoxicity, biocompatibility, and eco-friendly synthesis.
2 Materials and methods
2.1 P. cristatus feather ash preparation
P. cristatus molted feathers were gathered from a forest in the region of Karaikudi, Tamil Nadu, India, and then washed in d.H2O to get rid of any dirt or debris that had become on to them during the molting process. The peacock feather ash is prepared using the burning peacock feathers over a ghee flame (Ghee Flame Method), and the resulting ash is triturated in a mortar and pestle until it becomes a black powder. After that, the sample aqueous extract was made by boiling the feather ash (10 g) with 100 mL of d.H2O at 60°C for about 20 min, or until the aqueous solution changed color from black to light brown. After filtering through filter paper, the extract was then used in the subsequent studies and allowed to warm up to room temperature.
2.2 Zinc oxide nanoparticles’ synthesis
In this process, 0.02 mM zinc acetate solution (50 mL) was used, and 5 mL of ash extract was added drop-by-drop and agitated for 10 min. After centrifugation at 4,000×g for 10 min and meticulous washing with d.H2O, the pale white solid product was recovered and dried overnight at 100°C. The resultant white powder was calcined for 2 h at 750°C in the muffle furnace. For characterization, a pale white powder of zinc oxide nanoparticles was stored.
2.3 Characterization of Pcf-ZnONPs
We measured the absorbance of zinc oxide nanoparticles between 200 and 800 nm using a Shimadzu-UV 1800 UV-Vis spectrometer. To investigate the functional characteristics of Pcf-ZnONPs, FTIR spectroscopy was performed and XRD was used to explore the crystalline nature of Pcf-ZnONPs. The atom lattice configurations and morphology of Pcf-ZnONPs were magnified by HR-TEM and SEM examination, respectively.
2.4 Bacterial strains
Pcf extract and Pcf-ZnONPs were tested for their antibacterial efficacy against S. aureus (MTCC 9542) and P. aeruginosa (ATCC 6538).
2.4.1 Antibacterial evaluation of Pcf extract and Pcf-ZnONPs
The antibacterial activity of Pcf extract and Pcf-ZnONPs against S. aureus (MTCC 9542) and P. aeruginosa (ATCC 6538) was examined using the agar well diffusion method. Nutrient Agar (Himedia, Mumbai, India) was used to make the Petri plates, and the test culture was streaked using a sterilized cotton swab. Each Petri plate had a well drilled using sterile cork borer 6 mm wells. Different sample concentrations (25, 50, and 100 g·mL−1) were used to investigate the activity of the compounds, and plates were incubated at 37°C for 1 day. The zone of inhibition of each well was evaluated after the incubation period, and the experiments were done in triplicate.
2.4.2 Antibiofilm activity of Pcf extract and Pcf-ZnONPs
A 24-well plate with glass pieces and Pcf extract and Pcf-ZnONPs (50 and 100 µg·mL−1) for the antibiofilm assay was used to dispense aliquots of 1 mL of culture into the wells. In a static state, the 24-well plates were incubated for 16 h at 37°C. The glass fragments were then rinsed in 0.01 M phosphate buffer saline (PBS), transferred to glass slides, dyed with 0.4% crystal violet dye, and viewed at 40× magnification under a bright field microscope.
2.4.3 Effect of Pcf extract and Pcf-ZnONPs on the biochemical activity of microorganisms
P. aeruginosa, similar to the majority of pathogenic pyogenic bacteria, exhibits catalase-positive characteristics. S. aureus is also characterized by a positive catalase test, a shared trait observed in both organisms. In bacterial cells, the enzyme is formed in the process of biochemical oxidation, so it can only be found in young viable cultures. The catalase test in the practice of microbiological research is widely used for the differentiation and identification of a number of pathogenic and opportunistic bacteria. Bacteria were cultivated on nutrient agar in Petri dishes supplemented with Pcf extract and Pcf-ZnONPs at a concentration of 50 µg·mL−1. The cultures were then incubated for a duration of 24 h at 37°C. In this experimental procedure, a test reagent consisting of a recently prepared 3% solution of hydrogen peroxide is administered onto the surface of an isolated microbial colony cultivated within a Petri dish equipped with a capillary tube. The application is performed in a manner that ensures the entire surface of the culture is adequately covered by a thin layer of the reagent. When catalase is present in a microbial culture, the observation of oxygen bubbles occurs within a time frame of approximately 2–3 s. The process of gas formation can occur at varying rates, ranging from rapid to moderate. Irrespective of its magnitude, the reaction is regarded as a favorable one.
2.5 Cytotoxicity assay and fluorescence microscopic analysis in human cell line
The National Centre for Cell Science provided MDA-MB-231 breast cancer cells (NCCS, Pune). MTT assays, as described previously by Mosmann [20], were done using various concentrations at 5, 10, 15, 25, and 50 µg·mL−1 of Pcf extract and Pcf-ZnONPs. Moreover, Kumar et al. [21] explored the features of apoptotic cell death by staining techniques using dyes such as acridine orange (AO)/ethidium bromide (dual staining) and Hoechst 33344 (nuclear staining). Finally, the cells’ technique was examined through a fluorescent microscope at a magnification of 20×.
2.5.1 MTT assay and neutral red uptake (NRU) assay in fish cell line
The Rohu gill cell line was acquired from the ATTC (American Type Culture Collection) (Manassas, VA, USA). The NRU assay evaluates lysosomal membrane integrity. It measures neutral red dye accumulation within lysosomes of viable cells as described in Mosmann [20], or the capacity of living cells to convert the tetrazolium salt MTT to formazan via mitochondrial metabolism, as stated in Borenfreund and Puerner [22]. For the MTT assay and the NRU assay, the optical density of each well was calculated using a plate reader at a wavelength of 570 nm.
2.6 In vitro biocompatibility of Pcf–ZnONPs
Fresh human RBCs were utilized in the hemolytic experiment to confirm biocompatibility, which followed a previously known approach with significant modifications [23,24]. The RBCs were subsequently suspended as compacted masses in 5 mL of PBS and subjected to multiple rounds of centrifugation. After that, the erythrocyte suspensions were combined in Eppendorf tubes with varying concentrations of Pcf extract, zinc acetate, and Pcf-ZnONPs (25, 50, 75, and 100 µg·mL−1) and incubated for 1 h at 35°C with constant stirring at 150 rpm. Following that, they were centrifuged at 1,377 g for 10 min at 37°C. The absorbance at 570 nm was measured after the centrifuged supernatant was transferred to a 96-well plate. A light microscope was used to take the digital photomicrographs (40×).
2.7 Brine shrimp lethality assay
The cytotoxicity Pcf extract, zinc acetate, and Pcf-ZnONPs were investigated in brine shrimp (Artemia nauplii) using the standard procedure [25]. Eggs of A. nauplii were supplied by Ocean Star International and kept at 28°C. In a 20 L tank near a light source at 37°C, the eggs were permitted to emerge in 34 g·L−1·seawater−1. Ten recently emerged nauplii were selected from each well and transferred. Each well was filled with test samples (25, 50, 75, and 100 µg·mL−1), and the adjusted volume was 300 L at this point. After 24 h of exposure, the shrimps were viewed and counted beneath a magnifying lens, and morphological alterations were evaluated under a stereomicroscope.
2.8 Statistical analysis
Totally three times were used to conduct each experiment. The data are shown as a mean ± S.D. The statistical significance of the data was investigated using the Student’s t-test. SPSS version 2.1 was used for correlation and analysis of variance.
3 Results and discussion
3.1 Characterization of Pcf-ZnONPs
Visual examination was used to track the production of ZnONPs. The solution’s color changed from light brown to pale white during the process, indicating the creation of Pcf-ZnONPs (Figure 1a, inlet). The pattern of absorption spectra is largely determined by factors such as the synthesis process, temperature, and the size and shape of the NPs produced. As demonstrated in Figure 1a, the absorption maxima of Pcf-ZnONPs occurred at about 365 nm, which is consistent with prior findings [25,26,27]. ZnO bulk absorption maxima are commonly found around 380 nm [27]. The blue-shift absorption maximum confirms the creation of ZnONPs from bulk ZnO [28]. XRD was used to analyze the crystalline nature of ZnONPs, and the pattern exhibits peaks at 31.75°, 34.41°, 36.22°, 47.35°, 56.41°, 62.55°, 66.17°, 67.71°, 68.96°, and 77.78°, which correspond to lattice planes (100), (002), (101), (102), (110), (103), (200), (112), (201), and (202), correspondingly. No peaks were linked to contaminations or mediator components, confirming that the produced powder is pure ZnONPs. The NPs exhibit good crystallinity and size, as seen by the sturdy and thin diffraction peaks (Figure 1b). Figure 1c illustrates the FTIR spectra of Pcf-ZnONPs. O–H bound stretching groups are responsible for the broad absorption band seen at 3,411 cm−1. The existence of C═N and C═C nitrile groups perhaps relates to the midway peak at 2,339 cm−1. The C–N stretching mode is responsible for the peak at 1,384 cm−1. Since the O–H stretching is considerably transferred in the FTIR spectrum, it appears to play a significant part in creating Pcf-ZnONPs (Figure 1c).

UV–Vis absorption spectrum of Pcf-ZnONPs (a), XRD pattern (b), and FTIR spectrum of Pcf-ZnONPs (c).
Figure 2a shows Pcf-ZnONPs obtained using high-resolution scanning electron microscopy. The occurrence of square and hexagonal-shaped nanoparticles with a mean average diameter of 50 nm was convincingly demonstrated using SEM pictures. The Pcf-ZnONPs sample is comprised of hexagonal-shaped nanoparticles because of TEM images (Figure 2b). The selected area diffraction pattern (SAED) of Pcf-ZnONPs is shown in Figure 2c, and the particles are well crystalline.
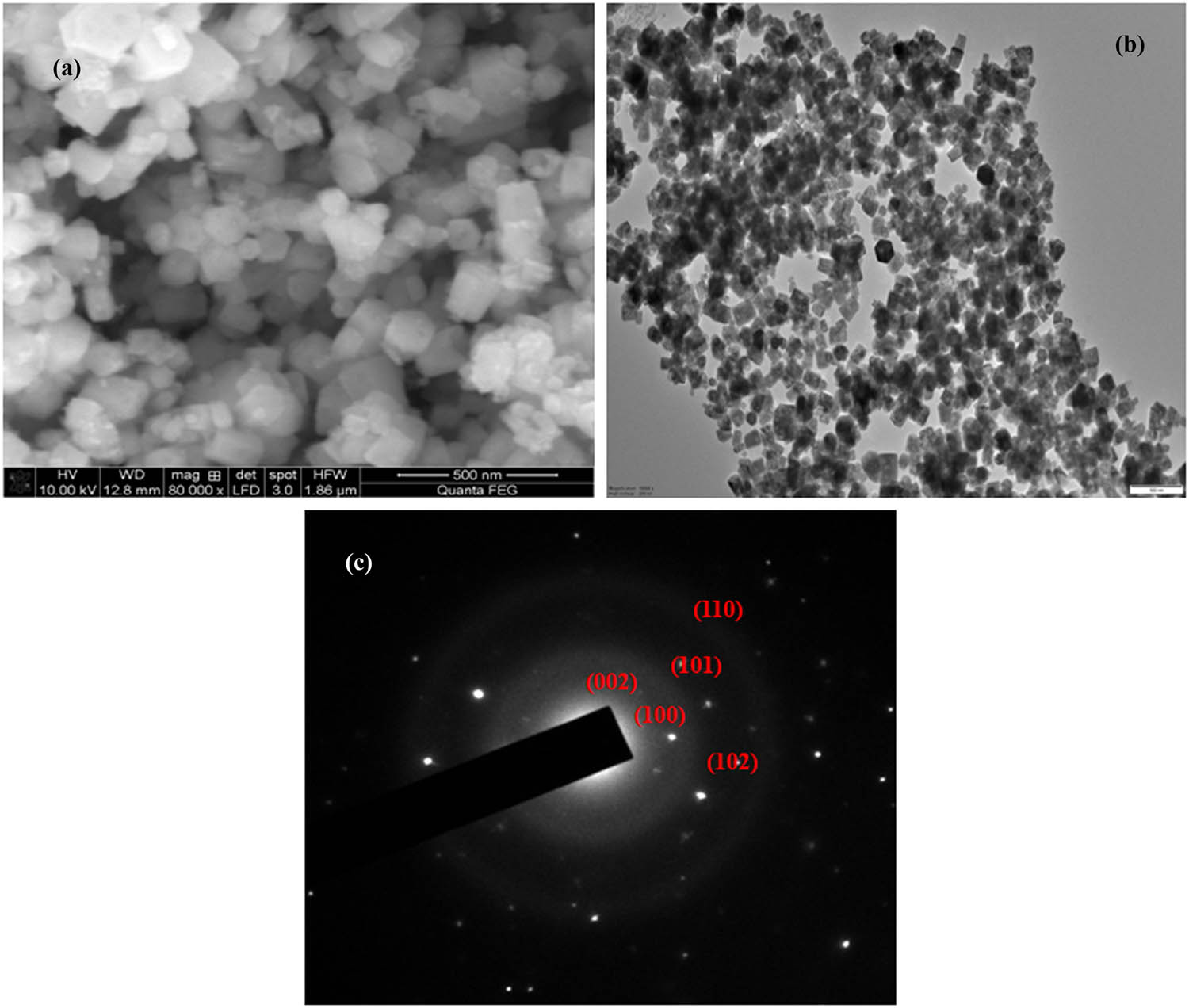
SEM (a), TEM (b) image, and SAED pattern (c) of Pcf-ZnONPs.
3.2 Antibacterial and antibiofilm studies
Biofilm production increases antibiotic resistance and prevents host immune cells from engaging in hostile actions [29]. It’s also tough to treat with antibacterials because the drugs can’t get through the biofilm network [30]. As a result, NPs were extensively used to solve biofilm-oriented infections. It is imperative to create an alternative strategy in these circumstances that is low in toxicity and has efficient antibacterial and antibiofilm actions on the particular strain [31]. Biogenically generated Pcf-ZnONPs were tested for biofilm blockage against S. aureus and P. aeruginosa in this work. On exposure, the light microscopic images revealed that Pcf extracts and Pcf-ZnONPs biofilm developed S. aureus and P. aeruginosa (Figure 3). Also, the untreated sample had a dense and compact biofilm structure. Compared to the Pcf extract, the Pcf-ZnONPs efficiently minimize biofilm formation in S. aureus and P. aeruginosa without killing the cells. The light microscopic images show that the Pcf-ZnONPs loosen the microcolonies in the treated sample, allowing for the architecture of the biofilm to be completely dispersed. The biofilm study reveals that the Pcf-ZnONPs prevent pathogens from forming biofilms in the first place. A recent study found that Pcf-ZnONPs based on a green method could eliminate bacterial biofilm from Gram-negative and Gram-positive bacteria [32,33]. As a result of the findings, we may conclude that ZnONPs can significantly reduce S. aureus and P. aeruginosa biofilm development. S. aureus and P. aeruginosa were used to investigate the antibacterial efficacy of Pcf extract and Pcf-ZnONPs. The zone of inhibition for Pcf-ZnONPs against S. aureus and P. aeruginosa was 23.5 and 20.3 mm, respectively (Table 1). Based on these characteristics, ZnONPs show significant antibacterial action against a variety of microbes, including Escherichia coli, Bacillus subtilis, Shigella sonnei, P. aeruginosa, S. aureus, Proteus vulgaris, Klebsiella pneumoniae, and the M13 bacteriophage [34,35,36,37]. Due to the antibacterial nature of Pcf extract and Pcf-ZnONPs, we used a modest concentration of 50 µg·mL−1 in the experiment involving the biochemical activity of microorganisms. As a result, bacterial colonies in the experimental dishes grew less than in the control without adding Pcf extract and Pcf-ZnONPs.

Light microscopy (40×) images of Gram-positive (S. aureus) and Gram-negative (P. aeruginosa) biofilm control and treated with Pcf extract and Pcf-ZnONPs.
Antibacterial activity of the Pavo cristatus feather ash extract and Pcf-ZnONPs Gram-positive and -negative bacteria, n = 3 (three samples from each treatment), mean ± SD; values within the same row sharing the different alphabetical letter superscripts are statistically significant at P < 0.05
| Bacteria | Zone of inhibition (mm) | |
|---|---|---|
| Pcf extract | Pcf-ZnONPs | |
| S. aureus | 17.3 ± 0.5b | 23.5 ± 1.5a |
| P. aeruginosa | 15.6 ± 0.3b | 20.3 ± 0.6a |
3.3 MTT and apoptosis assay in human cell line
On breast cancer cell line MDA-MB-231, the cytotoxic effects of Pcf extract and Pcf-ZnONPs were assessed. The results in Figure 4 clearly revealed that increasing the quantity of both Pcf extract and Pcf-ZnONPs condensed the possibility of treated cell lines significantly. Furthermore, the highest concentration (50 µg·mL−1) led to the most significant reduction in cell viability across all cell lines, with Pcf extract having a viability of 28.5%. Pcf-ZnONPs, on the other hand, demonstrated better cytotoxic effects, with the maximum effect recorded at 50 µg·mL−1 resulting in viabilities of 10.47%. According to Batool et al. [37], the percentage of MDA-MB-231 cells that are still viable after exposure to Dox-PEG-ZnO-NPs was determined to be 40% at 50 µg·mL−1 and was lowered to 30% at 200 µg·mL−1. Umar et al. [38] showed that ZnO-NPs from Albizia lebbeck suppressed 100 µg·mL−1 concentration decreased MDA-MB-231 and MCF7 cell counts by 76.8% and 80.2%, correspondingly. Apoptotic alterations and nuclear condensation are also involved in the cytotoxicity effect caused by Pcf extract and Pcf-ZnONPs. Pcf extract and Pcf-ZnONPs are applied to the MDA-MB-231 cell line before and after for the purpose of evaluating the morphological and molecular differentiation between viable, apoptotic, and necrotic alterations. This is done using fluorescent DNA-binding dyes in the apoptosis assay. To recognize and measure apoptosis and necrosis, the cancer cells are stained with AO/EB, HOE, and DAPI using a fluorescence microscope [39,40,41]. The fluorescent microscope revealed morphological features of cell death in Figure 5. Control cells stained with AO inferred green fluorescence based on the staining results. Meanwhile, EtBr detected late apoptotic cells with intense red fluorescence, indicating apoptosis, in the treated cells.

The cell viability (in %) of breast cancer cell line MDA-MB-231 in various concentrations of Pcf extract and Pcf-ZnONPs. T-bars represent standard deviations. Above each column, different letters indicate significant differences (P < 0.05).

Two-fold staining (AO/EB) (a), nuclear fragmentation of Hoechst staining (b), and PI staining (c) upon treatment control, Pcf extract, and Pcf-ZnONPs against MDA-MB-breast cancer cells.
Similarly, nuclear staining with Hoechst 33344 dye was used to analyze the nuclear morphology for condensation. When stained, the treated cells produced intense blue fluorescence, showing compacted chromatin, a hallmark of classical apoptosis. Untreated cells, on the other hand, show no such apoptotic progression due to fewer strongly marked cells with intact nuclear morphology. The lethal activity of biosynthesized ZnONPs against various breast cancer cell lines, including MDA-MB-231, is another factor supporting the study’s conclusions [42,43,44].
3.4 MTT and apoptosis assay in fish cell line
The cytotoxicity after exposure of the fish Rohu cell lines to the suspended and centrifuged Pcf-ZnONP suspensions measured by the MTT and NR assays is shown in Figure 6. Our results show that no significant differences in cytotoxicity between both assays were observed except at the highest concentration (50 and 100 µg·mL−1). At this concentration, loss in cell viability was detected in the Rohu gill cell line at the concentration of 100 µg·mL−1 of Pcf-ZnONPs. Control cells developed confluent monolayers with their distinctive cell morphologies in the absence of Pcf-ZnONPs. However, for 24 h after exposure, morphological changes like cell shivering and an accumulation of fragmented cell debris could be seen under a phase contrast microscope. After 24 h of exposure to 50 and 100 µg·mL−1 of Pcf-ZnONPs, distinct cell death was seen in the Rohu gill cell line (Figure 7). Similar to this, the cytotoxicity of ZnO nanoparticles against cancer cells depends on how well they dissolve in an aqueous medium. The amount of Zn2+ ions released depends on how much ZnO nanoparticles dissolve, which adds to their cytotoxicity. ZnO nanoparticles coated with plant extract-derived phytochemicals have limited solubility in the culture medium, which causes a sluggish release of ions [45]. Owing to this sluggish release, Zn2+ ions cannot connect with cancer cells, resulting in a lack of penetration inside the cell. As a result, even when utilizing different growth mediums, the metal will not be hazardous to any mammalian cells. Due to their biocompatibility, these ZnO nanoparticles are suitable for carrier molecules in drug delivery applications.

In vitro cytotoxicity of Pcf-ZnONPs on Rohu gill cell line after 24 h exposure by MTT, and NR assays. T-bars represent standard deviations. Above each column, different letters indicate significant differences (P < 0.05).

Morphological alteration in Rohu gill cell line exposed to different concentrations of Vm-ZnONPs for 24 h: (a) control cells, (b) 6.25 µg·mL−1, (c) 12.5 µg·mL−1, (d) 25 µg·mL−1, (e) 50 µg·mL−1, and (f) 100 µg·mL−1 of Pcf-ZnONPs and image were capture at 100× magnification. Scale bar: 100 µm.
3.5 In vitro biocompatibility studies
A hemolysis assay was used to evaluate the toxicity of nanoparticles at the cellular level. For its in vivo uses, the hemolysis activity was carried out to learn more about biocompatibility. The erythrocyte is the most significant blood component, where the nanoparticles engage. The biocompatibility of Pcf extract, zinc acetate, and Pcf-ZnONPs was first assessed using human RBCs in a biocompatibility assay. Pcf extract, zinc acetate, and Pcf-ZnONPs had little hemolytic activity when they were combined with RBCs. It demonstrated a small increase in hemolysis activity as the concentration was increased. In this investigation, Pcf extract and zinc acetate demonstrated total hemolysis of 4% and 5%, respectively, while Pcf-ZnONPs showed less than 3% hemolytic activity and no RBC destruction (Figure 8). When the cells were treated with Pcf extract and zinc acetate, the damage in the RBCs (green arrows) was visible. Furthermore, in RBCs treated with Pcf-ZnONPs, the cells were distorted and developed (white arrows). Table 2 demonstrates that even at high concentrations, all of our stock solutions of produced Pcf-ZnONPs show minimal hemolysis, indicating their great biocompatibility. Biomaterials that have less than 5% hemolysis are acceptable. Biocompatibility of ZnO nanoparticles in hemolysis assay has been reported by [46,47]. Cell lysis was reduced by the addition of ZnONPs and increased as ZnONP concentration. Every therapeutic chemical must alter the activity of a target molecule without endangering mammalian cells, which is a crucial component. So, the present study established that ZnONPs do not harm human blood cells in any way. As a result, it was discovered that the greenly produced nanoparticle was both more effective in limiting microbial growth and biocompatible.

Light microscopy showing limited hemolytic activity on RBCs exposed to control (a), Pcf extract (b and c), zinc acetate (d and e), and Pcf-ZnONPs (f and g). Green arrows indicate the damage to RBCs and white arrow indicates the deformation of RBCs.
Percentage of hemolysis of Pcf extract, zinc acetate, and Pcf-ZnONPs, n = 3 (three samples from each treatment), mean ± SD; values within the same row sharing the different alphabetical letter superscripts are statistically significant at P < 0.05
| Concentration (µg·mL−1) | % of hemolysis | ||
|---|---|---|---|
| Pcf extract | Zinc acetate | Pcf-ZnONPs | |
| 25 | 0.89 ± 0.02b | 1.75 ± 0.09a | 0.75 ± 0.04b |
| 50 | 1.16 ± 0.06b | 2.69 ± 0.13a | 0.12 ± 0.08c |
| 75 | 2.75 ± 0.13ab | 3.58 ± 0.25a | 2.24 ± 0.15ab |
| 100 | 3.85 ± 0.11b | 4.95 ± 0.32a | 3.15 ± 0.12b |
3.6 Brine shrimp lethality assay
The toxicity of Pcf-ZnONPs on Artemia nauplii, a marine crustacean, was assessed 24 h after the experiment began. On treated A. nauplii, stereomicroscope revealed Pcf-ZnONPs’ deposition in the median food groove/gut (Figure 9). Zinc acetate exposure resulted in appendage and carapace damage, but Pcf-ZnONPs’ exposure resulted in no appendage or carapace damage. After 48 h, the mortality of A. nauplii treated with Pcf-ZnONPs increased concentration dependent. After 24 h of exposure to A. nauplii, green production of ZnONPs revealed 70–75% mortality at 400 µg·mL−1 [21,42]. Because the dosage and exposure period of nanoparticles varies between Pcf-ZnONPs and Pcf extract, differences in mortality may have occurred. Moreover, Pcf-ZnONPs had a mild hazardous effect at all concentrations evaluated within 48 h. However, prolonged exposure may produce toxicity. Finally, our findings imply that Pcf-ZnONPs are a promising antibacterial agent at concentrations 25 and 100 µg·mL−1, but only for bacterial cells and not for erythrocytes or Artemia at the same concentration. To effectively utilize ZnONPs, a comprehensive examination of the impacts of diverse factors such as size, morphology, surface potential, surface modification, retention time, bioavailability, and additional variables is imperative. Further in vivo studies will be necessary for this purpose.

In vivo toxicity analysis of Artemia nauplii toward Pcf extract, zinc acetate, and Pcf-ZnONPs in which red arrows spot accumulation and deposition of zinc acetate and NPs.
4 Conclusions
Green-based nanoparticles exhibit a wide range of biological and biomedical potentials [48,49,50,51]. ZnO nanoparticles are an excellent option to be used as a carrier molecule for drug delivery applications due to their lack of antibacterial and anticancer potential. Further investigation is required into the function of various biomolecules in the production of ZnO nanoparticles and their possible usage in biomedical applications. Our research adopts UV–Vis, FTIR, XRD, HRTEM, and SAED to corroborate the physical, chemical, and morphological properties of ZnONPs generated in an eco-friendly manner from peacock feather ash extracts. While the Pcf-ZnONPs are toxic to bacteria, they do not affect vertebrate erythrocytes and are effective against a primitive creature (bacteria) that causes illnesses in people. In vitro and in vivo toxicity experiments have demonstrated that Pcf-ZnONPs are completely nontoxic at all tested concentrations and durations of exposure. As Pcf-ZnONPs are biocompatible, they may be proposed as a nano-biomedicine for the treatment of bacterial infections in higher species. ZnO NPs are a promising new tool for developing highly potent antibacterial and anticancer treatments, and their widespread adoption in the scientific community would have far-reaching implications for the biomedical industry.
Acknowledgments
The authors express their sincere appreciation to the Researchers Supporting Project Number (RSP2023R48) King Saud University, Riyadh, Saudi Arabia. Ramachandran Ishwarya gratefully acknowledges the Science and Engineering Research Board, India, New Delhi, India [Ref: PDF/2020/001027].
-
Funding information: The study was supported by the Project Number (RSP2023R48) King Saud University, Riyadh, Saudi Arabia, and the Science and Engineering Research Board, India, New Delhi, India [Ref: PDF/2020/001027].
-
Author contributions: Ramachandran Ishwarya: conceptualization, methodology, software, formal analysis, investigation, data curation, writing – original draft preparation; Govindan Tamilmani: validation, data curation; Khalid A. Al-Ghanim: resources, writing – review and editing; Marimuthu Govindarajan: data curation, writing – review and editing; Marcello Nicoletti: data curation, writing – review and editing; and Baskaralingam Vaseeharan: Conceptualization, validation, supervision. All authors have read and agreed to the published version of the article.
-
Conflict of interest: The authors state no conflict of interest.
-
Data availability statement: The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
[1] Sahoo S. Socio-ethical issues and nanotechnology development: perspectives from India. In: 2010 10th IEEE Conference on Nanotechnology (IEEE-NANO) Seoul. South Korea USA; 2010. p. 17–20.10.1109/NANO.2010.5697887Suche in Google Scholar
[2] U.S. Food and Drug Administration (FDA) GRAS Notice. (accessed on 1 April 2019). https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfCFR/CFRSearch.cfm? fr = 182.8991.Suche in Google Scholar
[3] Maret W. Metals on the move: zinc ions in cellular regulation and in the coordination dynamics of zinc proteins. Biometals. 2011;24:411–8.10.1007/s10534-010-9406-1Suche in Google Scholar PubMed
[4] Zhou J, Xu NS, Wang ZL. Dissolving behavior and stability of ZnO wires in biofluids: a study on biodegradability and biocompatibility of ZnO nanostructures. Adv Mater. 2006;18:2432–5.10.1002/adma.200600200Suche in Google Scholar
[5] Kalpana VN, Kataru BA, Sravani N, Vigneshwari T, Panneerselvam A, Rajeswari VD. Biosynthesis of zinc oxide nanoparticles using culture filtrates of Aspergillus niger: Antimicrobial textiles and dye degradation studies. OpenNano. 2018;8:48–55.10.1016/j.onano.2018.06.001Suche in Google Scholar
[6] Jin SE, Jin HE. Synthesis, characterization, and three dimensional structure generation of zinc oxide-based nanomedicine for biomedical applications. Pharmaceutics. 2019;11:575.10.3390/pharmaceutics11110575Suche in Google Scholar PubMed PubMed Central
[7] Brooking J, Davis SS, Illum L. Transport of nanoparticles across the rat nasal mucosa. J Drug Target. 2001;9:267–79.10.3109/10611860108997935Suche in Google Scholar PubMed
[8] Hassan HFH, Mansour AM, Abo-Youssef AMH, Elsadek BEM, Messiha BAS. Zinc oxide nanoparticles as a novel anticancer approach; in vitro and in vivo evidence. Clin Exp Pharmacol Physiol. 2017;44:235–43.10.1111/1440-1681.12681Suche in Google Scholar PubMed
[9] Gudkov SV, Burmistrov DE, Serov DA, Rebezov MB, Semenova AA, Lisitsyn AB. A mini review of antibacterial properties of ZnO nanoparticles. Front Phys. 2021;11:641481.10.3389/fphy.2021.641481Suche in Google Scholar
[10] Khan AK, Renouard S, Drouet S, Blondeau JP, Anjum I, Hano C, et al. Effect of UV Irradiation (A and C) on Casuarina equisetifolia-Mediated biosynthesis and characterization of antimicrobial and anticancer activity of biocompatible Zinc oxide nanoparticles. Pharmaceutic. 2021;13:1977.10.3390/pharmaceutics13111977Suche in Google Scholar PubMed PubMed Central
[11] Tettey CO, Shin HM. Evaluation of the antioxidant and cytotoxic activities of zinc oxide nanoparticles synthesized using Scutellaria baicalensis root. Sci Afr. 2019;6:00157.10.1016/j.sciaf.2019.e00157Suche in Google Scholar
[12] Samudralwar DL, Garg AN. Minor and trace elemental determination in the Indian herbal and other medicinal preparations. Biolol Trace Elem Res. 1996;54:113–21.10.1007/BF02786258Suche in Google Scholar PubMed
[13] Murari SK, Frey FJ, Frey BM, Gowda TV, Vishwanath BS. Use of Pavo cristatus feather extract for the better management of snakebites: Neutralization of inflammatory reactions. J Ethnopharmacol. 2005;99:229–37.10.1016/j.jep.2005.02.027Suche in Google Scholar PubMed
[14] Bhandari CR. Vanoshadhi Chandrodaya. Banaras, India: The Time Table Press; 1925. p. 1666.Suche in Google Scholar
[15] Yadav AV. Dravyaguana Part I and II. Calcutta, India: Baidyanath Publications; 1980. p. 794.Suche in Google Scholar
[16] Lakshmipatishastri SV. Yogarattanakara. In: Bramaha Shankarashastri BS, editor. Vidhyotani Hindi Commentary Charadi Chikitsa. Varanasi, India: Choukambha Samskrita Series Office; 1973. p. 453–7.Suche in Google Scholar
[17] Zi J, Yu X, Li Y, Hu X, Xu C, Wang X, et al. Coloration strategies in peacock feathers. Proc Natl Acad Sci USA. 2003;100:12576.10.1073/pnas.2133313100Suche in Google Scholar PubMed PubMed Central
[18] Das RK, Pachapur VL, Lonappan L, Naghdi M, Pulicharla R, Maiti S, et al. Biological synthesis of metallic nanoparticles: Plants, animals and microbial aspects. Nanotechnol Environ Eng. 2017;2:1–21.10.1007/s41204-017-0029-4Suche in Google Scholar
[19] Adabi M, Naghibzadeh M, Adabi M, Zarrinfard MA, Esnaashari SS, Seifalian AM, et al. Biocompatibility and nanostructured materials: applications in nanomedicine. Artif cells Nanomed Biotechnol. 2017;45:833–42.10.1080/21691401.2016.1178134Suche in Google Scholar PubMed
[20] Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;63:55–63.10.1016/0022-1759(83)90303-4Suche in Google Scholar PubMed
[21] Kumar BA, Kumar P, Elangovan T, Ramalingam G, Ravi G, Mohanapriya P, et al. Surface functionalization of core-shell QDs for solar photovoltaic and anticancer applications. Appl Surf Sci Adv. 2021;5:100122.10.1016/j.apsadv.2021.100122Suche in Google Scholar
[22] Sarker SR, Polash SA, Boath J, Kandjani AE, Poddar A, Dekiwadia C. Functionalization of elongated tetrahexahedral Au nanoparticles and their antimicrobial activity assay. ACS Appl Mater Interfaces. 2019;11:13450–9.10.1021/acsami.9b02279Suche in Google Scholar PubMed
[23] Nasar MQ, Khalil AT, Ali M, Shah M, Ayaz M, Shinwari ZK. Phytochemical analysis Ephedra Procera CA Mey. Mediated green synthesis of silver nanoparticles their cytotoxic and antimicrobial potentials. Medicina. 2019;55:369.10.3390/medicina55070369Suche in Google Scholar PubMed PubMed Central
[24] Faisal S, Jan H, Shah S, Shah A, Khan MT. Green synthesis of zinc oxide (ZnO) nanoparticles using aqueous fruit extracts of myristica fragrans: their characterizations and biological and environmental applications. ACS Omega. 2021;6:9709–22.10.1021/acsomega.1c00310Suche in Google Scholar PubMed PubMed Central
[25] Safawo T, Sandeep BV, Pola S, Tadesse A. Synthesis and characterization of zinc oxide nanoparticles using tuber extract of anchote (Coccinia abyssinica (Lam.) Cong.) for antimicrobial and antioxidant activity assessment. OpenNano. 2018;3:56–63.10.1016/j.onano.2018.08.001Suche in Google Scholar
[26] Hassan SSM, El Azab WIM, Ali HR, Mansour MSM. Green synthesis and characterization of ZnO nanoparticles for photocatalytic degradation of anthracene. Adv Nat Sci Nanosci Nanotechnol. 2015;6:45012.10.1088/2043-6262/6/4/045012Suche in Google Scholar
[27] Senthilkumar SR, Sivakumar T. Green tea (Camellia sinensis) mediated synthesis of zinc oxide (ZnO) nanoparticles and studies on their antimicrobial activities. Int J Pharm Sci. 2014;6:461–5.Suche in Google Scholar
[28] Jamdagni P, Khatri P, Rana JS. Green synthesis of zinc oxide nanoparticles using flower extract of Nyctanthes arbor-tristis and their antifungal activity. J King Saud UnivSci. 2016;30:168–75.10.1016/j.jksus.2016.10.002Suche in Google Scholar
[29] Ricciardi BF, Muthukrishnan G, Masters E, Ninomiya M, Lee CC, Schwarz EM. Staphylococcus aureus evasion of host immunity in the setting of prosthetic joint infection: Biofilm and beyond. Curr Rev Musculoskelet Med. 2018;11:389–400.10.1007/s12178-018-9501-4Suche in Google Scholar PubMed PubMed Central
[30] Shah SR, Tatara AM, DSouza RN, Mikos AG, Kasper FK. Evolving strategies for preventing biofilm on implantable materials. Mater Today. 2013;16:177–82.10.1016/j.mattod.2013.05.003Suche in Google Scholar
[31] Mishra PK, Mishra H, Ekielski A, Talegaonkar S, Vaidya B. Zinc oxide nanoparticles: a promising nanomaterial for biomedical applications. Drug DiscovToday. 2017;22:1825–34.10.1016/j.drudis.2017.08.006Suche in Google Scholar PubMed
[32] Ishwarya R, Vaseeharan B, Kalyani S, Banumathi B, Govindarajan M, Alharbi NS, et al. Facile green synthesis of zinc oxide nanoparticles using Ulva lactuca seaweed extract and evaluation of their photocatalytic, antibiofilm and insecticidal activity. J Photochem Photobiol B. 2018;178:249–58.10.1016/j.jphotobiol.2017.11.006Suche in Google Scholar PubMed
[33] Ishwarya R, Vaseeharan B, Subbaiah S, Nazar AK, Govindarajan M, Alharbi NS, et al. Sargassumwightii-synthesized ZnO nanoparticles–from antibacterial and insecticidal activity to immunostimulatory effects on the green tiger shrimp Penaeus semisulcatus. J Photochem Photobiol B. 2018;183:318–30.10.1016/j.jphotobiol.2018.04.049Suche in Google Scholar PubMed
[34] Sirelkhatim A, Mahmud S, Seeni A, Kaus NHM, Ann LC, Bakhori SKM. Review on zinc oxide nanoparticles: antibacterial activity and toxicity mechanism. Nanomicro Lett. 2015;7:219–42.10.1007/s40820-015-0040-xSuche in Google Scholar PubMed PubMed Central
[35] Jin SE, Hyo-Eon J. Antimicrobial activity of zinc oxide nano/microparticles and their combinations against pathogenic microorganisms for biomedical applications: from physicochemical characteristics to pharmacological aspects. Nanomaterials. 2021;11:263–98.10.3390/nano11020263Suche in Google Scholar PubMed PubMed Central
[36] Fahimmunisha BA, Ishwarya R, AlSalhi MS, Devanesan S, Govindarajan M, Vaseeharan B. Green fabrication, characterization and antibacterial potential of zinc oxide nanoparticles using Aloe socotrina leaf extract: A novel drug delivery approach. J Drug Deliver Sci Technol. 2020;55:101465.10.1016/j.jddst.2019.101465Suche in Google Scholar
[37] Batool M, Khurshid S, Daoush WM, Siddique SA, Nadeem T. Green synthesis and biomedical applications of ZnO nanoparticles: Role of PEGylated-ZnO nanoparticles as doxorubicin drug carrier against MDA-MB-231 (TNBC) cells line. Crystals. 2021;4:344.10.3390/cryst11040344Suche in Google Scholar
[38] Umar H, Kavaz D, Rizaner N. Biosynthesis of zinc oxide nanoparticles using Albizia lebbeck stem bark, and evaluation of its antimicrobial, antioxidant, and cytotoxic activities on human breast cancer cell lines. Int J Nanomed. 2019;14:87–100.10.2147/IJN.S186888Suche in Google Scholar PubMed PubMed Central
[39] Vasconcelos AC, Lam KM. Apoptosis induced by infectious bursal disease virus. J Gen Virol. 1994;75:1803–6.10.1099/0022-1317-75-7-1803Suche in Google Scholar PubMed
[40] Leite M, Quinta-Costa M, Leite PS. Critical evaluation of techniques to detect and measure cell death-study in a model of UV radiation of the leukemic cell line HL60. Anal Cell Pathol. 1999;19:39–151.10.1155/1999/176515Suche in Google Scholar PubMed PubMed Central
[41] Boroumand Moghaddam A, Moniri M, Azizi S, Abdul Rahim R, Bin Ariff A, Navaderi M, et al. Eco-friendly formulated zinc oxide nanoparticles: induction of cell cycle arrest and apoptosis in the MCF-7 cancer cell line. Genes. 2017;10:281.10.3390/genes8100281Suche in Google Scholar PubMed PubMed Central
[42] Sivaraj R, Rahman P, Rajiv P, Venckatesh R. Biogenic zinc oxide nanoparticles synthesis sing Tabernaemontana divaricate leaf extract and its anticancer activity against MCF-7 breast cancer cell lines. In: International Conference on Advances in Agricultural, Biological & Environmental Sciences; 2014.Suche in Google Scholar
[43] Al-Ajmi MF, Hussain A, Alsharaeh E, Ahmed F, Amir S, Anwar MS, et al. Green synthesis of zinc oxide nanoparticles using Alstonia macrophylla leaf extract and their in-vitro anticancer activity. Sci Adv Mater. 2018;10:349–55.10.1166/sam.2018.2983Suche in Google Scholar
[44] Radwan AM, Aboelfetoh EF, Kimura T, Mohamed TM, El-Keiy MM. Fenugreek-mediated synthesis of zinc oxide nanoparticles and evaluation of its in vitro and in vivo antitumor potency. Biomed Res Ther. 2021;8:4483–96.10.15419/bmrat.v8i8.687Suche in Google Scholar
[45] Hariharan R, Senthilkumar S, Suganthi A, Rajarajan M. Synthesis and characterization of doxorubicin modified ZnO/PEG nanomaterials and its photodynamic action. J Photochem Photobiol B. 2012;116:56–65.10.1016/j.jphotobiol.2012.08.008Suche in Google Scholar PubMed
[46] Alam M. Photocatalytic activity of biogenic zinc oxide nanoparticles: In vitro antimicrobial, biocompatibility, and molecular docking studies. Nanotechnol Rev. 2021;10:1079–91.10.1515/ntrev-2021-0069Suche in Google Scholar
[47] Abinaya M, Vaseeharan B, Divya M, Sharmili A, Govindarajan M, Alharbi NS, et al. Bacterial exopolysaccharide (EPS)-coated ZnO nanoparticles showed high antibiofilm activity and larvicidal toxicity against malaria and Zika virus vectors. J Trace Elem Med Biol. 2018;45:93–103.10.1016/j.jtemb.2017.10.002Suche in Google Scholar PubMed
[48] Govindarajan M, Nicoletti M, Benelli G. Bio-physical characterization of poly-dispersed silver nanocrystals fabricated using Carissa spinarum: A potent tool against mosquito vectors. J Clust Sci. 2013;27:745–61.10.1007/s10876-016-0977-zSuche in Google Scholar
[49] Karthika V, Kaleeswarran P, Gopinath K, Arumugam A, Govindarajan M, Alharbi NS, et al. Biocompatible properties of nano-drug carriers using TiO2-Au embedded on multiwall carbon nanotubes for targeted drug delivery. Mater Sci Eng C. 2018;90:589–601.10.1016/j.msec.2018.04.094Suche in Google Scholar PubMed
[50] Karthika V, AlSalhi MS, Devanesan S, Gopinath K, Arumugam A, Govindarajan M. Chitosan overlaid Fe3O4/rGO nanocomposite for targeted drug delivery, imaging, and biomedical applications. Sci Rep. 2020;10:18912.10.1038/s41598-020-76015-3Suche in Google Scholar PubMed PubMed Central
[51] Iswarya A, Vaseeharan B, Anjugam M, Ashokkumar B, Govindarajan M, Alharbi NS, et al. Multipurpose efficacy of ZnO nanoparticles coated by the crustacean immune molecule β-1, 3-glucan binding protein: Toxicity on HepG2 liver cancer cells and bacterial pathogens. Colloids Surf B Biointerfaces. 2017;158:257–69.10.1016/j.colsurfb.2017.06.035Suche in Google Scholar PubMed
© 2023 the author(s), published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Artikel in diesem Heft
- Research Articles
- Value-added utilization of coal fly ash and recycled polyvinyl chloride in door or window sub-frame composites
- High removal efficiency of volatile phenol from coking wastewater using coal gasification slag via optimized adsorption and multi-grade batch process
- Evolution of surface morphology and properties of diamond films by hydrogen plasma etching
- Removal efficiency of dibenzofuran using CuZn-zeolitic imidazole frameworks as a catalyst and adsorbent
- Rapid and efficient microwave-assisted extraction of Caesalpinia sappan Linn. heartwood and subsequent synthesis of gold nanoparticles
- The catalytic characteristics of 2-methylnaphthalene acylation with AlCl3 immobilized on Hβ as Lewis acid catalyst
- Biodegradation of synthetic PVP biofilms using natural materials and nanoparticles
- Rutin-loaded selenium nanoparticles modulated the redox status, inflammatory, and apoptotic pathways associated with pentylenetetrazole-induced epilepsy in mice
- Optimization of apigenin nanoparticles prepared by planetary ball milling: In vitro and in vivo studies
- Synthesis and characterization of silver nanoparticles using Origanum onites leaves: Cytotoxic, apoptotic, and necrotic effects on Capan-1, L929, and Caco-2 cell lines
- Exergy analysis of a conceptual CO2 capture process with an amine-based DES
- Construction of fluorescence system of felodipine–tetracyanovinyl–2,2′-bipyridine complex
- Excellent photocatalytic degradation of rhodamine B over Bi2O3 supported on Zn-MOF nanocomposites under visible light
- Optimization-based control strategy for a large-scale polyhydroxyalkanoates production in a fed-batch bioreactor using a coupled PDE–ODE system
- Effectiveness of pH and amount of Artemia urumiana extract on physical, chemical, and biological attributes of UV-fabricated biogold nanoparticles
- Geranium leaf-mediated synthesis of silver nanoparticles and their transcriptomic effects on Candida albicans
- Synthesis, characterization, anticancer, anti-inflammatory activities, and docking studies of 3,5-disubstituted thiadiazine-2-thiones
- Synthesis and stability of phospholipid-encapsulated nano-selenium
- Putative anti-proliferative effect of Indian mustard (Brassica juncea) seed and its nano-formulation
- Enrichment of low-grade phosphorites by the selective leaching method
- Electrochemical analysis of the dissolution of gold in a copper–ethylenediamine–thiosulfate system
- Characterisation of carbonate lake sediments as a potential filler for polymer composites
- Evaluation of nano-selenium biofortification characteristics of alfalfa (Medicago sativa L.)
- Quality of oil extracted by cold press from Nigella sativa seeds incorporated with rosemary extracts and pretreated by microwaves
- Heteropolyacid-loaded MOF-derived mesoporous zirconia catalyst for chemical degradation of rhodamine B
- Recovery of critical metals from carbonatite-type mineral wastes: Geochemical modeling investigation of (bio)hydrometallurgical leaching of REEs
- Photocatalytic properties of ZnFe-mixed oxides synthesized via a simple route for water remediation
- Attenuation of di(2-ethylhexyl)phthalate-induced hepatic and renal toxicity by naringin nanoparticles in a rat model
- Novel in situ synthesis of quaternary core–shell metallic sulfide nanocomposites for degradation of organic dyes and hydrogen production
- Microfluidic steam-based synthesis of luminescent carbon quantum dots as sensing probes for nitrite detection
- Transformation of eggshell waste to egg white protein solution, calcium chloride dihydrate, and eggshell membrane powder
- Preparation of Zr-MOFs for the adsorption of doxycycline hydrochloride from wastewater
- Green nanoarchitectonics of the silver nanocrystal potential for treating malaria and their cytotoxic effects on the kidney Vero cell line
- Carbon emissions analysis of producing modified asphalt with natural asphalt
- An efficient and green synthesis of 2-phenylquinazolin-4(3H)-ones via t-BuONa-mediated oxidative condensation of 2-aminobenzamides and benzyl alcohols under solvent- and transition metal-free conditions
- Chitosan nanoparticles loaded with mesosulfuron methyl and mesosulfuron methyl + florasulam + MCPA isooctyl to manage weeds of wheat (Triticum aestivum L.)
- Synergism between lignite and high-sulfur petroleum coke in CO2 gasification
- Facile aqueous synthesis of ZnCuInS/ZnS–ZnS QDs with enhanced photoluminescence lifetime for selective detection of Cu(ii) ions
- Rapid synthesis of copper nanoparticles using Nepeta cataria leaves: An eco-friendly management of disease-causing vectors and bacterial pathogens
- Study on the photoelectrocatalytic activity of reduced TiO2 nanotube films for removal of methyl orange
- Development of a fuzzy logic model for the prediction of spark-ignition engine performance and emission for gasoline–ethanol blends
- Micro-impact-induced mechano-chemical synthesis of organic precursors from FeC/FeN and carbonates/nitrates in water and its extension to nucleobases
- Green synthesis of strontium-doped tin dioxide (SrSnO2) nanoparticles using the Mahonia bealei leaf extract and evaluation of their anticancer and antimicrobial activities
- A study on the larvicidal and adulticidal potential of Cladostepus spongiosus macroalgae and green-fabricated silver nanoparticles against mosquito vectors
- Catalysts based on nickel salt heteropolytungstates for selective oxidation of diphenyl sulfide
- Powerful antibacterial nanocomposites from Corallina officinalis-mediated nanometals and chitosan nanoparticles against fish-borne pathogens
- Removal behavior of Zn and alkalis from blast furnace dust in pre-reduction sinter process
- Environmentally friendly synthesis and computational studies of novel class of acridinedione integrated spirothiopyrrolizidines/indolizidines
- The mechanisms of inhibition and lubrication of clean fracturing flowback fluids in water-based drilling fluids
- Adsorption/desorption performance of cellulose membrane for Pb(ii)
- A one-pot, multicomponent tandem synthesis of fused polycyclic pyrrolo[3,2-c]quinolinone/pyrrolizino[2,3-c]quinolinone hybrid heterocycles via environmentally benign solid state melt reaction
- Green synthesis of silver nanoparticles using durian rind extract and optical characteristics of surface plasmon resonance-based optical sensor for the detection of hydrogen peroxide
- Electrochemical analysis of copper-EDTA-ammonia-gold thiosulfate dissolution system
- Characterization of bio-oil production by microwave pyrolysis from cashew nut shells and Cassia fistula pods
- Green synthesis methods and characterization of bacterial cellulose/silver nanoparticle composites
- Photocatalytic research performance of zinc oxide/graphite phase carbon nitride catalyst and its application in environment
- Effect of phytogenic iron nanoparticles on the bio-fortification of wheat varieties
- In vitro anti-cancer and antimicrobial effects of manganese oxide nanoparticles synthesized using the Glycyrrhiza uralensis leaf extract on breast cancer cell lines
- Preparation of Pd/Ce(F)-MCM-48 catalysts and their catalytic performance of n-heptane isomerization
- Green “one-pot” fluorescent bis-indolizine synthesis with whole-cell plant biocatalysis
- Silica-titania mesoporous silicas of MCM-41 type as effective catalysts and photocatalysts for selective oxidation of diphenyl sulfide by H2O2
- Biosynthesis of zinc oxide nanoparticles from molted feathers of Pavo cristatus and their antibiofilm and anticancer activities
- Clean preparation of rutile from Ti-containing mixed molten slag by CO2 oxidation
- Synthesis and characterization of Pluronic F-127-coated titanium dioxide nanoparticles synthesized from extracts of Atractylodes macrocephala leaf for antioxidant, antimicrobial, and anticancer properties
- Effect of pretreatment with alkali on the anaerobic digestion characteristics of kitchen waste and analysis of microbial diversity
- Ameliorated antimicrobial, antioxidant, and anticancer properties by Plectranthus vettiveroides root extract-mediated green synthesis of chitosan nanoparticles
- Microwave-accelerated pretreatment technique in green extraction of oil and bioactive compounds from camelina seeds: Effectiveness and characterization
- Studies on the extraction performance of phorate by aptamer-functionalized magnetic nanoparticles in plasma samples
- Investigation of structural properties and antibacterial activity of AgO nanoparticle extract from Solanum nigrum/Mentha leaf extracts by green synthesis method
- Green fabrication of chitosan from marine crustaceans and mushroom waste: Toward sustainable resource utilization
- Synthesis, characterization, and evaluation of nanoparticles of clodinofop propargyl and fenoxaprop-P-ethyl on weed control, growth, and yield of wheat (Triticum aestivum L.)
- The enhanced adsorption properties of phosphorus from aqueous solutions using lanthanum modified synthetic zeolites
- Separation of graphene oxides of different sizes by multi-layer dialysis and anti-friction and lubrication performance
- Visible-light-assisted base-catalyzed, one-pot synthesis of highly functionalized cinnolines
- The experimental study on the air oxidation of 5-hydroxymethylfurfural to 2,5-furandicarboxylic acid with Co–Mn–Br system
- Highly efficient removal of tetracycline and methyl violet 2B from aqueous solution using the bimetallic FeZn-ZIFs catalyst
- A thermo-tolerant cellulase enzyme produced by Bacillus amyloliquefaciens M7, an insight into synthesis, optimization, characterization, and bio-polishing activity
- Exploration of ketone derivatives of succinimide for their antidiabetic potential: In vitro and in vivo approaches
- Ultrasound-assisted green synthesis and in silico study of 6-(4-(butylamino)-6-(diethylamino)-1,3,5-triazin-2-yl)oxypyridazine derivatives
- A study of the anticancer potential of Pluronic F-127 encapsulated Fe2O3 nanoparticles derived from Berberis vulgaris extract
- Biogenic synthesis of silver nanoparticles using Consolida orientalis flowers: Identification, catalytic degradation, and biological effect
- Initial assessment of the presence of plastic waste in some coastal mangrove forests in Vietnam
- Adsorption synergy electrocatalytic degradation of phenol by active oxygen-containing species generated in Co-coal based cathode and graphite anode
- Antibacterial, antifungal, antioxidant, and cytotoxicity activities of the aqueous extract of Syzygium aromaticum-mediated synthesized novel silver nanoparticles
- Synthesis of a silica matrix with ZnO nanoparticles for the fabrication of a recyclable photodegradation system to eliminate methylene blue dye
- Natural polymer fillers instead of dye and pigments: Pumice and scoria in PDMS fluid and elastomer composites
- Study on the preparation of glycerylphosphorylcholine by transesterification under supported sodium methoxide
- Wireless network handheld terminal-based green ecological sustainable design evaluation system: Improved data communication and reduced packet loss rate
- The optimization of hydrogel strength from cassava starch using oxidized sucrose as a crosslinking agent
- Green synthesis of silver nanoparticles using Saccharum officinarum leaf extract for antiviral paint
- Study on the reliability of nano-silver-coated tin solder joints for flip chips
- Environmentally sustainable analytical quality by design aided RP-HPLC method for the estimation of brilliant blue in commercial food samples employing a green-ultrasound-assisted extraction technique
- Anticancer and antimicrobial potential of zinc/sodium alginate/polyethylene glycol/d-pinitol nanocomposites against osteosarcoma MG-63 cells
- Nanoporous carbon@CoFe2O4 nanocomposite as a green absorbent for the adsorptive removal of Hg(ii) from aqueous solutions
- Characterization of silver sulfide nanoparticles from actinobacterial strain (M10A62) and its toxicity against lepidopteran and dipterans insect species
- Phyto-fabrication and characterization of silver nanoparticles using Withania somnifera: Investigating antioxidant potential
- Effect of e-waste nanofillers on the mechanical, thermal, and wear properties of epoxy-blend sisal woven fiber-reinforced composites
- Magnesium nanohydroxide (2D brucite) as a host matrix for thymol and carvacrol: Synthesis, characterization, and inhibition of foodborne pathogens
- Synergistic inhibitive effect of a hybrid zinc oxide-benzalkonium chloride composite on the corrosion of carbon steel in a sulfuric acidic solution
- Review Articles
- Role and the importance of green approach in biosynthesis of nanopropolis and effectiveness of propolis in the treatment of COVID-19 pandemic
- Gum tragacanth-mediated synthesis of metal nanoparticles, characterization, and their applications as a bactericide, catalyst, antioxidant, and peroxidase mimic
- Green-processed nano-biocomposite (ZnO–TiO2): Potential candidates for biomedical applications
- Reaction mechanisms in microwave-assisted lignin depolymerisation in hydrogen-donating solvents
- Recent progress on non-noble metal catalysts for the deoxydehydration of biomass-derived oxygenates
- Rapid Communication
- Phosphorus removal by iron–carbon microelectrolysis: A new way to achieve phosphorus recovery
- Special Issue: Biomolecules-derived synthesis of nanomaterials for environmental and biological applications (Guest Editors: Arpita Roy and Fernanda Maria Policarpo Tonelli)
- Biomolecules-derived synthesis of nanomaterials for environmental and biological applications
- Nano-encapsulated tanshinone IIA in PLGA-PEG-COOH inhibits apoptosis and inflammation in cerebral ischemia/reperfusion injury
- Green fabrication of silver nanoparticles using Melia azedarach ripened fruit extract, their characterization, and biological properties
- Green-synthesized nanoparticles and their therapeutic applications: A review
- Antioxidant, antibacterial, and cytotoxicity potential of synthesized silver nanoparticles from the Cassia alata leaf aqueous extract
- Green synthesis of silver nanoparticles using Callisia fragrans leaf extract and its anticancer activity against MCF-7, HepG2, KB, LU-1, and MKN-7 cell lines
- Algae-based green AgNPs, AuNPs, and FeNPs as potential nanoremediators
- Green synthesis of Kickxia elatine-induced silver nanoparticles and their role as anti-acetylcholinesterase in the treatment of Alzheimer’s disease
- Phytocrystallization of silver nanoparticles using Cassia alata flower extract for effective control of fungal skin pathogens
- Antibacterial wound dressing with hydrogel from chitosan and polyvinyl alcohol from the red cabbage extract loaded with silver nanoparticles
- Leveraging of mycogenic copper oxide nanostructures for disease management of Alternaria blight of Brassica juncea
- Nanoscale molecular reactions in microbiological medicines in modern medical applications
- Synthesis and characterization of ZnO/β-cyclodextrin/nicotinic acid nanocomposite and its biological and environmental application
- Green synthesis of silver nanoparticles via Taxus wallichiana Zucc. plant-derived Taxol: Novel utilization as anticancer, antioxidation, anti-inflammation, and antiurolithic potential
- Recyclability and catalytic characteristics of copper oxide nanoparticles derived from bougainvillea plant flower extract for biomedical application
- Phytofabrication, characterization, and evaluation of novel bioinspired selenium–iron (Se–Fe) nanocomposites using Allium sativum extract for bio-potential applications
- Erratum
- Erratum to “Synthesis, characterization, and evaluation of nanoparticles of clodinofop propargyl and fenoxaprop-P-ethyl on weed control, growth, and yield of wheat (Triticum aestivum L.)”
Artikel in diesem Heft
- Research Articles
- Value-added utilization of coal fly ash and recycled polyvinyl chloride in door or window sub-frame composites
- High removal efficiency of volatile phenol from coking wastewater using coal gasification slag via optimized adsorption and multi-grade batch process
- Evolution of surface morphology and properties of diamond films by hydrogen plasma etching
- Removal efficiency of dibenzofuran using CuZn-zeolitic imidazole frameworks as a catalyst and adsorbent
- Rapid and efficient microwave-assisted extraction of Caesalpinia sappan Linn. heartwood and subsequent synthesis of gold nanoparticles
- The catalytic characteristics of 2-methylnaphthalene acylation with AlCl3 immobilized on Hβ as Lewis acid catalyst
- Biodegradation of synthetic PVP biofilms using natural materials and nanoparticles
- Rutin-loaded selenium nanoparticles modulated the redox status, inflammatory, and apoptotic pathways associated with pentylenetetrazole-induced epilepsy in mice
- Optimization of apigenin nanoparticles prepared by planetary ball milling: In vitro and in vivo studies
- Synthesis and characterization of silver nanoparticles using Origanum onites leaves: Cytotoxic, apoptotic, and necrotic effects on Capan-1, L929, and Caco-2 cell lines
- Exergy analysis of a conceptual CO2 capture process with an amine-based DES
- Construction of fluorescence system of felodipine–tetracyanovinyl–2,2′-bipyridine complex
- Excellent photocatalytic degradation of rhodamine B over Bi2O3 supported on Zn-MOF nanocomposites under visible light
- Optimization-based control strategy for a large-scale polyhydroxyalkanoates production in a fed-batch bioreactor using a coupled PDE–ODE system
- Effectiveness of pH and amount of Artemia urumiana extract on physical, chemical, and biological attributes of UV-fabricated biogold nanoparticles
- Geranium leaf-mediated synthesis of silver nanoparticles and their transcriptomic effects on Candida albicans
- Synthesis, characterization, anticancer, anti-inflammatory activities, and docking studies of 3,5-disubstituted thiadiazine-2-thiones
- Synthesis and stability of phospholipid-encapsulated nano-selenium
- Putative anti-proliferative effect of Indian mustard (Brassica juncea) seed and its nano-formulation
- Enrichment of low-grade phosphorites by the selective leaching method
- Electrochemical analysis of the dissolution of gold in a copper–ethylenediamine–thiosulfate system
- Characterisation of carbonate lake sediments as a potential filler for polymer composites
- Evaluation of nano-selenium biofortification characteristics of alfalfa (Medicago sativa L.)
- Quality of oil extracted by cold press from Nigella sativa seeds incorporated with rosemary extracts and pretreated by microwaves
- Heteropolyacid-loaded MOF-derived mesoporous zirconia catalyst for chemical degradation of rhodamine B
- Recovery of critical metals from carbonatite-type mineral wastes: Geochemical modeling investigation of (bio)hydrometallurgical leaching of REEs
- Photocatalytic properties of ZnFe-mixed oxides synthesized via a simple route for water remediation
- Attenuation of di(2-ethylhexyl)phthalate-induced hepatic and renal toxicity by naringin nanoparticles in a rat model
- Novel in situ synthesis of quaternary core–shell metallic sulfide nanocomposites for degradation of organic dyes and hydrogen production
- Microfluidic steam-based synthesis of luminescent carbon quantum dots as sensing probes for nitrite detection
- Transformation of eggshell waste to egg white protein solution, calcium chloride dihydrate, and eggshell membrane powder
- Preparation of Zr-MOFs for the adsorption of doxycycline hydrochloride from wastewater
- Green nanoarchitectonics of the silver nanocrystal potential for treating malaria and their cytotoxic effects on the kidney Vero cell line
- Carbon emissions analysis of producing modified asphalt with natural asphalt
- An efficient and green synthesis of 2-phenylquinazolin-4(3H)-ones via t-BuONa-mediated oxidative condensation of 2-aminobenzamides and benzyl alcohols under solvent- and transition metal-free conditions
- Chitosan nanoparticles loaded with mesosulfuron methyl and mesosulfuron methyl + florasulam + MCPA isooctyl to manage weeds of wheat (Triticum aestivum L.)
- Synergism between lignite and high-sulfur petroleum coke in CO2 gasification
- Facile aqueous synthesis of ZnCuInS/ZnS–ZnS QDs with enhanced photoluminescence lifetime for selective detection of Cu(ii) ions
- Rapid synthesis of copper nanoparticles using Nepeta cataria leaves: An eco-friendly management of disease-causing vectors and bacterial pathogens
- Study on the photoelectrocatalytic activity of reduced TiO2 nanotube films for removal of methyl orange
- Development of a fuzzy logic model for the prediction of spark-ignition engine performance and emission for gasoline–ethanol blends
- Micro-impact-induced mechano-chemical synthesis of organic precursors from FeC/FeN and carbonates/nitrates in water and its extension to nucleobases
- Green synthesis of strontium-doped tin dioxide (SrSnO2) nanoparticles using the Mahonia bealei leaf extract and evaluation of their anticancer and antimicrobial activities
- A study on the larvicidal and adulticidal potential of Cladostepus spongiosus macroalgae and green-fabricated silver nanoparticles against mosquito vectors
- Catalysts based on nickel salt heteropolytungstates for selective oxidation of diphenyl sulfide
- Powerful antibacterial nanocomposites from Corallina officinalis-mediated nanometals and chitosan nanoparticles against fish-borne pathogens
- Removal behavior of Zn and alkalis from blast furnace dust in pre-reduction sinter process
- Environmentally friendly synthesis and computational studies of novel class of acridinedione integrated spirothiopyrrolizidines/indolizidines
- The mechanisms of inhibition and lubrication of clean fracturing flowback fluids in water-based drilling fluids
- Adsorption/desorption performance of cellulose membrane for Pb(ii)
- A one-pot, multicomponent tandem synthesis of fused polycyclic pyrrolo[3,2-c]quinolinone/pyrrolizino[2,3-c]quinolinone hybrid heterocycles via environmentally benign solid state melt reaction
- Green synthesis of silver nanoparticles using durian rind extract and optical characteristics of surface plasmon resonance-based optical sensor for the detection of hydrogen peroxide
- Electrochemical analysis of copper-EDTA-ammonia-gold thiosulfate dissolution system
- Characterization of bio-oil production by microwave pyrolysis from cashew nut shells and Cassia fistula pods
- Green synthesis methods and characterization of bacterial cellulose/silver nanoparticle composites
- Photocatalytic research performance of zinc oxide/graphite phase carbon nitride catalyst and its application in environment
- Effect of phytogenic iron nanoparticles on the bio-fortification of wheat varieties
- In vitro anti-cancer and antimicrobial effects of manganese oxide nanoparticles synthesized using the Glycyrrhiza uralensis leaf extract on breast cancer cell lines
- Preparation of Pd/Ce(F)-MCM-48 catalysts and their catalytic performance of n-heptane isomerization
- Green “one-pot” fluorescent bis-indolizine synthesis with whole-cell plant biocatalysis
- Silica-titania mesoporous silicas of MCM-41 type as effective catalysts and photocatalysts for selective oxidation of diphenyl sulfide by H2O2
- Biosynthesis of zinc oxide nanoparticles from molted feathers of Pavo cristatus and their antibiofilm and anticancer activities
- Clean preparation of rutile from Ti-containing mixed molten slag by CO2 oxidation
- Synthesis and characterization of Pluronic F-127-coated titanium dioxide nanoparticles synthesized from extracts of Atractylodes macrocephala leaf for antioxidant, antimicrobial, and anticancer properties
- Effect of pretreatment with alkali on the anaerobic digestion characteristics of kitchen waste and analysis of microbial diversity
- Ameliorated antimicrobial, antioxidant, and anticancer properties by Plectranthus vettiveroides root extract-mediated green synthesis of chitosan nanoparticles
- Microwave-accelerated pretreatment technique in green extraction of oil and bioactive compounds from camelina seeds: Effectiveness and characterization
- Studies on the extraction performance of phorate by aptamer-functionalized magnetic nanoparticles in plasma samples
- Investigation of structural properties and antibacterial activity of AgO nanoparticle extract from Solanum nigrum/Mentha leaf extracts by green synthesis method
- Green fabrication of chitosan from marine crustaceans and mushroom waste: Toward sustainable resource utilization
- Synthesis, characterization, and evaluation of nanoparticles of clodinofop propargyl and fenoxaprop-P-ethyl on weed control, growth, and yield of wheat (Triticum aestivum L.)
- The enhanced adsorption properties of phosphorus from aqueous solutions using lanthanum modified synthetic zeolites
- Separation of graphene oxides of different sizes by multi-layer dialysis and anti-friction and lubrication performance
- Visible-light-assisted base-catalyzed, one-pot synthesis of highly functionalized cinnolines
- The experimental study on the air oxidation of 5-hydroxymethylfurfural to 2,5-furandicarboxylic acid with Co–Mn–Br system
- Highly efficient removal of tetracycline and methyl violet 2B from aqueous solution using the bimetallic FeZn-ZIFs catalyst
- A thermo-tolerant cellulase enzyme produced by Bacillus amyloliquefaciens M7, an insight into synthesis, optimization, characterization, and bio-polishing activity
- Exploration of ketone derivatives of succinimide for their antidiabetic potential: In vitro and in vivo approaches
- Ultrasound-assisted green synthesis and in silico study of 6-(4-(butylamino)-6-(diethylamino)-1,3,5-triazin-2-yl)oxypyridazine derivatives
- A study of the anticancer potential of Pluronic F-127 encapsulated Fe2O3 nanoparticles derived from Berberis vulgaris extract
- Biogenic synthesis of silver nanoparticles using Consolida orientalis flowers: Identification, catalytic degradation, and biological effect
- Initial assessment of the presence of plastic waste in some coastal mangrove forests in Vietnam
- Adsorption synergy electrocatalytic degradation of phenol by active oxygen-containing species generated in Co-coal based cathode and graphite anode
- Antibacterial, antifungal, antioxidant, and cytotoxicity activities of the aqueous extract of Syzygium aromaticum-mediated synthesized novel silver nanoparticles
- Synthesis of a silica matrix with ZnO nanoparticles for the fabrication of a recyclable photodegradation system to eliminate methylene blue dye
- Natural polymer fillers instead of dye and pigments: Pumice and scoria in PDMS fluid and elastomer composites
- Study on the preparation of glycerylphosphorylcholine by transesterification under supported sodium methoxide
- Wireless network handheld terminal-based green ecological sustainable design evaluation system: Improved data communication and reduced packet loss rate
- The optimization of hydrogel strength from cassava starch using oxidized sucrose as a crosslinking agent
- Green synthesis of silver nanoparticles using Saccharum officinarum leaf extract for antiviral paint
- Study on the reliability of nano-silver-coated tin solder joints for flip chips
- Environmentally sustainable analytical quality by design aided RP-HPLC method for the estimation of brilliant blue in commercial food samples employing a green-ultrasound-assisted extraction technique
- Anticancer and antimicrobial potential of zinc/sodium alginate/polyethylene glycol/d-pinitol nanocomposites against osteosarcoma MG-63 cells
- Nanoporous carbon@CoFe2O4 nanocomposite as a green absorbent for the adsorptive removal of Hg(ii) from aqueous solutions
- Characterization of silver sulfide nanoparticles from actinobacterial strain (M10A62) and its toxicity against lepidopteran and dipterans insect species
- Phyto-fabrication and characterization of silver nanoparticles using Withania somnifera: Investigating antioxidant potential
- Effect of e-waste nanofillers on the mechanical, thermal, and wear properties of epoxy-blend sisal woven fiber-reinforced composites
- Magnesium nanohydroxide (2D brucite) as a host matrix for thymol and carvacrol: Synthesis, characterization, and inhibition of foodborne pathogens
- Synergistic inhibitive effect of a hybrid zinc oxide-benzalkonium chloride composite on the corrosion of carbon steel in a sulfuric acidic solution
- Review Articles
- Role and the importance of green approach in biosynthesis of nanopropolis and effectiveness of propolis in the treatment of COVID-19 pandemic
- Gum tragacanth-mediated synthesis of metal nanoparticles, characterization, and their applications as a bactericide, catalyst, antioxidant, and peroxidase mimic
- Green-processed nano-biocomposite (ZnO–TiO2): Potential candidates for biomedical applications
- Reaction mechanisms in microwave-assisted lignin depolymerisation in hydrogen-donating solvents
- Recent progress on non-noble metal catalysts for the deoxydehydration of biomass-derived oxygenates
- Rapid Communication
- Phosphorus removal by iron–carbon microelectrolysis: A new way to achieve phosphorus recovery
- Special Issue: Biomolecules-derived synthesis of nanomaterials for environmental and biological applications (Guest Editors: Arpita Roy and Fernanda Maria Policarpo Tonelli)
- Biomolecules-derived synthesis of nanomaterials for environmental and biological applications
- Nano-encapsulated tanshinone IIA in PLGA-PEG-COOH inhibits apoptosis and inflammation in cerebral ischemia/reperfusion injury
- Green fabrication of silver nanoparticles using Melia azedarach ripened fruit extract, their characterization, and biological properties
- Green-synthesized nanoparticles and their therapeutic applications: A review
- Antioxidant, antibacterial, and cytotoxicity potential of synthesized silver nanoparticles from the Cassia alata leaf aqueous extract
- Green synthesis of silver nanoparticles using Callisia fragrans leaf extract and its anticancer activity against MCF-7, HepG2, KB, LU-1, and MKN-7 cell lines
- Algae-based green AgNPs, AuNPs, and FeNPs as potential nanoremediators
- Green synthesis of Kickxia elatine-induced silver nanoparticles and their role as anti-acetylcholinesterase in the treatment of Alzheimer’s disease
- Phytocrystallization of silver nanoparticles using Cassia alata flower extract for effective control of fungal skin pathogens
- Antibacterial wound dressing with hydrogel from chitosan and polyvinyl alcohol from the red cabbage extract loaded with silver nanoparticles
- Leveraging of mycogenic copper oxide nanostructures for disease management of Alternaria blight of Brassica juncea
- Nanoscale molecular reactions in microbiological medicines in modern medical applications
- Synthesis and characterization of ZnO/β-cyclodextrin/nicotinic acid nanocomposite and its biological and environmental application
- Green synthesis of silver nanoparticles via Taxus wallichiana Zucc. plant-derived Taxol: Novel utilization as anticancer, antioxidation, anti-inflammation, and antiurolithic potential
- Recyclability and catalytic characteristics of copper oxide nanoparticles derived from bougainvillea plant flower extract for biomedical application
- Phytofabrication, characterization, and evaluation of novel bioinspired selenium–iron (Se–Fe) nanocomposites using Allium sativum extract for bio-potential applications
- Erratum
- Erratum to “Synthesis, characterization, and evaluation of nanoparticles of clodinofop propargyl and fenoxaprop-P-ethyl on weed control, growth, and yield of wheat (Triticum aestivum L.)”

