Abstract
The electrical and thermal conductivities and light transmittance properties of silicone oil and polydimethylsiloxane (PDMS) elastomer composites were investigated. Pumice, scoria, nano-Ag, and multi-walled carbon nanotube (MWCNT) particles were used as fillers. An effective, clean, and easy method was used to prepare nanosized particles from pumice and scoria rocks. Only MWCNT–PDMS composites showed electrical conductivity. The highest electrical conductivity of 24.7 S·m−1 was obtained with the 25% pumice–10% MWCNT–silicone oil composite. All filler particles increased the thermal conductivity of the PDMS elastomer. MWCNTs were more effective than pumice and scoria, and the thermal conductivity reached 0.62 W·(m·K)−1 with an addition of 3 wt% MWCNTs. All filler particles decreased the transparency of the PDMS elastomer. The sample with 5 wt% pumice particles reached zero transmittance. Pumice and scoria naturally colored the PDMS elastomer. The powders of these natural volcanic rocks could be used as a suitable coloring filling material instead of dyes and pigments for polymers without waste. However, it was concluded that pumice and scoria particles are not suitable for making composites with silicone oil due to the possibility of catalyzing the degradation of linear PDSM.
1 Introduction
Polydimethylsiloxane (PDMS) is a synthetic organosilicon polymer that has a very high market share since the 1900s and has been used in fields such as textiles, household products, pharmaceuticals, and cosmetics [1,2,3]. Due to their transparency, flexibility, cost-effectiveness, ease of fabrication, chemical/mechanical stability, and biocompatibility, the usage areas of polymers have increased especially in electronic devices in this century [4,5,6,7]. The experience we have gained thus far has shown us that when producing new material, we should also consider issues such as how it can be recycled when its use ends or how it affects the environment when discarded.
PDMS has different forms, such as silicone oil (in the fluid form) and silicone rubber (elastomer), in the market. The degradation of PDMS fluid has been studied by scientists. In soil, the PDMS polymer can hydrolyze to small, water-soluble siloxanols, with the ultimate product being monomeric dimethylsilanediol (DMSD). Once in the atmosphere, DMSD is expected to degrade by sunlight-induced reactions [8,9,10,11]. Lehmann et al. investigated the degradation of PDMS in a natural soil plot and, as predicted from laboratory experiments before, found that degradation is relatively rapid during the warm months of summer and slow during the cool, rainy months of fall [12]. According to Xu et al., clay minerals are catalysts for PDMS degradation, and clay type is very important in determining the degradation rates of PDMS in soil [13].
Almost every study uses PDMS in an elastomer form rather than the fluid form because of its flexibility and optical properties and because it is easier to use than silicone oil [14,15,16]. Although PDMS is not harmful to the environment, it cannot be expected that the elastomer form, which has been preferred in recent years, will show the same features. Because siloxanes are resistant to high temperatures, their recycling involves complex processes involving catalysts and chemicals, and more investigation into better methods for recycling siloxanes is needed [17].
Even though PDMS is considered harmless, using different curing chemicals, catalysts, dyes, pigments, nanosized fillers, etc., should not be considered harmless, and the hazardous effects of every component should be considered separately. For example, the natural colors of polymeric materials, which have entered every aspect of daily life, are white or transparent. However, coloring makes them more preferred. For this reason, many natural or synthetic dyes or pigments are used abundantly in production. It is undesirable for dyes to mix with the natural environment, such as soil and water. In addition to the basic properties concerning dyeing, green chemistry-related properties such as toxicity, bioaccumulation, ecotoxicity, or carcinogenicity must be considered for a proper sustainability evaluation [18].
In this study, fillers that can serve as alternatives to dyes and pigments were examined. For this purpose, volcanic rocks with their own natural and different colors were selected. Pumice and scoria are the two most abundant volcanic rocks in areas with young volcanic fields. These rocks exist in many parts of the world [19]. Pumice is an inert aluminosilicate material, and it is stable at a pH of 2.5. Pumice is biologically inert and contains no pathogens or weeds. Additionally, pumice is stable and can be reused practically and indefinitely. As a natural product, volcanic rocks can be disposed of without causing environmental pollution [20].
Then, the effects of pumice and scoria particles on the thermal properties of a PDMS elastomer were investigated. Many studies have shown that carbon nanotubes (CNTs) increase the thermal and electrical conductivities of highly insulating PDMS [21,22,23]. The effects of pumice and scoria added to change color and light transmittance on both forms of PDMS conductivity were also investigated in the presence of CNTs.
2 Materials and methods
2.1 Materials
Silicone oil, which is a clear, linear, nonreactive, odorless, and colorless liquid PDMS with a viscosity of approximately 100,000 mm²·s−1, was purchased from WACKER. Pumice was obtained from Bereketli Mining, and scoria from Kayseri, Türkiye. Elastosil RT 601 A/B from WACKER was used for PDMS elastomer preparation. Multi-walled CNTs (MWCNTs), with diameters of 7–10 nm and purity of 92%, were purchased from Nanografi. Silver nanopowders with sizes <150 nm were purchased from Sigma-Aldrich and both MWCNTs and nanopowders were used as received.
2.2 Nanoparticle preparation
Pumice and scoria stones were ground with a Planetary Ball Mill PM 100 (Retsch) at 300 rpm for 20 min. The ground samples were mixed with 500 mL of distilled water and left for 20 h undisturbed. At the end of 20 h, 100 mL of mixture from the top was taken carefully and centrifuged at 4,000 rpm for 30 min. Particles were dried at room temperature for 2 days and used directly as fillers in the composite samples. This method is original and used in this study for the first time.
2.3 PDMS elastomer preparation
Component A of ELASTOSIL RT 601 contained the platinum catalyst and silicone oil, and component B was the cross-linker. The two components were thoroughly mixed at a 9:1 ratio by weight. To eliminate any air introduced during dispensing, a vacuum pump was used. The prepared mixtures were cured at 60°C for 1 h. Figure 1 shows the crosslink reaction of PDMS taken from the manufacturer’s website (https://www.wacker.com/h/medias/6709-EN.pdf). During platinum-catalyzed addition curing, the crosslinker’s Si–H groups react with the vinyl groups of the polymers to form a three-dimensional network. Pumice, scoria, MWCNTs, and nano-Ag were added to the mixture of A/B under vacuum conditions before heating at different weight ratios, as shown in Table 1. To prepare silicone oil mixtures, all filler particles were added simply to the silicone oil, mixed, and used directly.
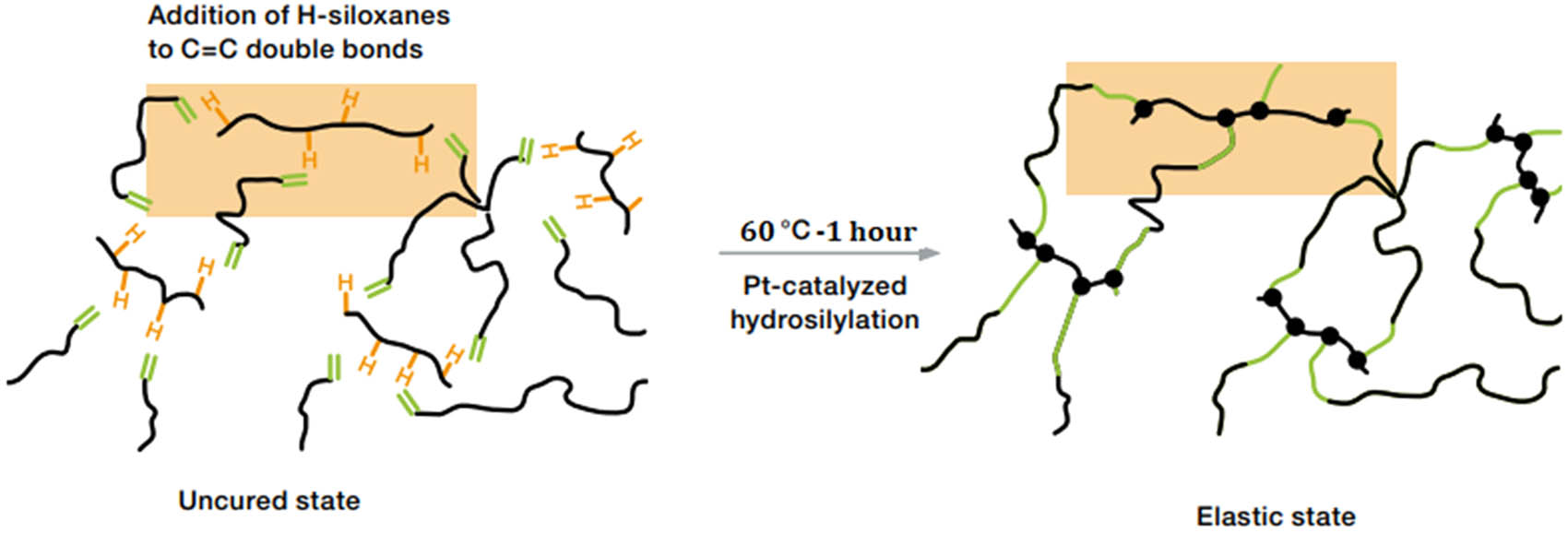
Crosslink reaction of PDMS in the presence of a platinum catalyst.
Composite samples investigated in this study
| Elastomers | Filler (%) | MWCNT (%) |
|---|---|---|
| PDMS/pumice | 0.1 | — |
| 1 | — | |
| 5 | — | |
| PDMS/pumice/MWCNT | 1 | 1 |
| 1 | 2 | |
| PDMS/scoria | 0.1 | — |
| 1 | — | |
| 5 | — | |
| PDMS/scoria/MWCNT | 1 | 1 |
| 1 | 2 | |
| PDMS/MWCNT | — | 0.1 |
| — | 1 | |
| — | 3 |
| Silicone oil soft solids | Filler (%) | MWCNT (%) |
|---|---|---|
| Silicone oil/pumice | 25 | — |
| 50 | — | |
| Silicone oil/pumice/MWCNT | 25 | 10 |
| Silicone oil/pumice/nano-Ag | 25 | 25 |
| Silicone oil/scoria | 25 | — |
| 50 | — | |
| Silicone oil/scoria/MWCNT | 25 | 10 |
| Silicone oil/scoria/nano-Ag | 25 | 25 |
| Silicone oil/nano-Ag | — | 25 |
| Silicone oil/MWCNT | — | 10 |
2.4 Characterization
The particle sizes of pumice and scoria were analyzed with a Malvern Zetasizer NanoS. Particle sizes were measured in distilled water after 10 min of ultrasonication. FTIR analyses of filler particles and PDMS elastomers were performed with a Perkin Elmer Diamond 100 FTIR, and SEM-EDX analyses were performed using a ZEISS EVO LS 10 scanning electron microscope. The thermal conductivities of PDMS elastomers were analyzed using KYOTO QTM-500, and electrical conductivity analyses were performed with a KEITHLEY source meter between −10 and +10 V. Transmittance analyses of elastomer films were performed using Labsphere RSA-PE-20 attached to a Perkin Elmer UV‒Vis spectrometer. Thermogravimetric analysis was performed using a Perkin Elmer Diamond TG. A total of 10 mg of the elastomer and composites were placed in a ceramic pan and heated from 30°C to 1,000°C, at 20°C·min−1, under a nitrogen atmosphere.
3 Results and discussion
3.1 Obtaining pumice and scoria particles at the nanoscale
Volcanic rocks commonly come in the form of scoria and pumice. These rocks often contain high contents of silica [24]. EDX analysis shows that scoria and pumice, which are used in this study, both contain high amounts of silica and aluminum and low amounts of Ca, Fe, Na, K, and Mg (Figures S3 and S5). Scoria and pumice have a porous structure in nature, but grinding these rocks to the nanoscale breaks up all pores, and leaf shape-like particles form from the broken layers (Figures S1, S2, and S4). By the applied method, particles <1 μm can easily be obtained. This method can be used for all solid particles that tend to fall to the bottom of the water according to the particle size. Approximately 657 nm of pumice and 553 nm of scoria particles are obtained from the top of the water mixture after 20 h (Figure S6). Holding in water is an effective, extremely easy, inexpensive, and environmentally friendly method for separating nanoparticles. Increasing the grinding time and holding time in water undisturbed can separate many small particles. The obtained pumice and scoria particles do not tend to stick to each other after drying and can easily disperse in PDMS while mixing.
3.2 Characterization of PDMS composites
All silicon oil composites numbered 1–10 are shown in Table S1 with pictures. Particles tend to aggregate at the bottom of the silicon oil and do not create a homogenous mixture. This behavior can be explained by the nonreactive and nonporous surfaces of the particles, leading to no interaction with PDMS. Conversely, MWCNTs give viscous oil a soft solid form that can be given any shape at any thickness and be easily applied to surfaces by spreading. Because of the low densities of MWCNT nanoparticles, a maximum w/w ratio of 10% is achieved with MWCNTs, while 50% is achieved with other particles.
The PDMS elastomer composites prepared in this study are listed in Tables S2 and S3, accompanied by their pictures. As seen from the SEM images, all filler particles are confined into the elastomer (Figure 2). A maximum of 5% (w/w) MWCNT particles can be packed in the PDMS elastomer, and this weight ratio cannot be used for thermal analysis because of the roughened surface of the elastomer (Figure S7). However, the specimen is suitable for electrical conductivity analysis. Figure 3 shows the PDMS elastomers with pumice, scoria, and MWCNTs in different amounts. Pumice powders dispersed in the PDMS elastomer color the transparent elastomer in different shades of gray, while scoria gives a red color. MWCNT particles added to the elastomer give a black color and make the pumice and scoria visible inside the elastomer. Synthetic dyes are known to be very toxic to the environment. Engineered colors adversely affect all parts of life. Dyes have harmful effects on water bodies because substantial levels of industrial waste enter rivers, lakes, etc., which ultimately causes water pollution [25]. These natural volcanic rock particles can be used instead of synthetic dyes to color the polymers because they are not considered waste when discarded or mixed with the soil or water of the planet. Vu et al. developed a method for recycling PDMS that only requires a small amount of catalyst and proceeds over a wide range of temperatures (60–170°C) to efficiently yield a mixture of cyclosiloxanes (reaching 98–99% yield) from a 100 g scale of waste silicone oils [26]. Most of the filler particles or dyes and pigments present in the waste can be affected by this chemical recycling process, preventing its implementation. However, pumice and scoria can be separated from the polymer without decomposition because they are not affected by heat or solvents during polymer recycling.
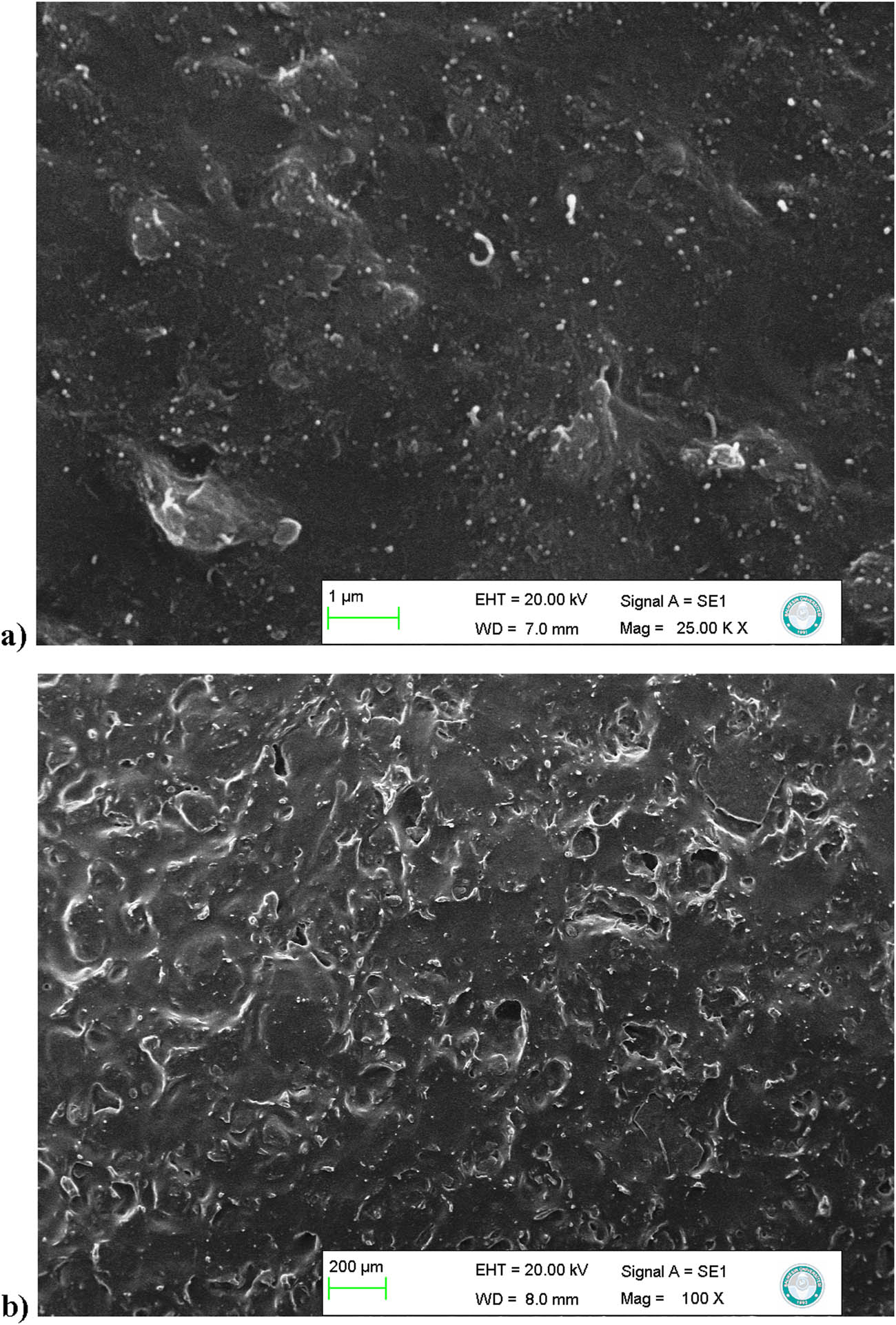
SEM images of PDMS/MWCNT/pumice (a) and silicone oil/MWCNT/pumice (b) composites.
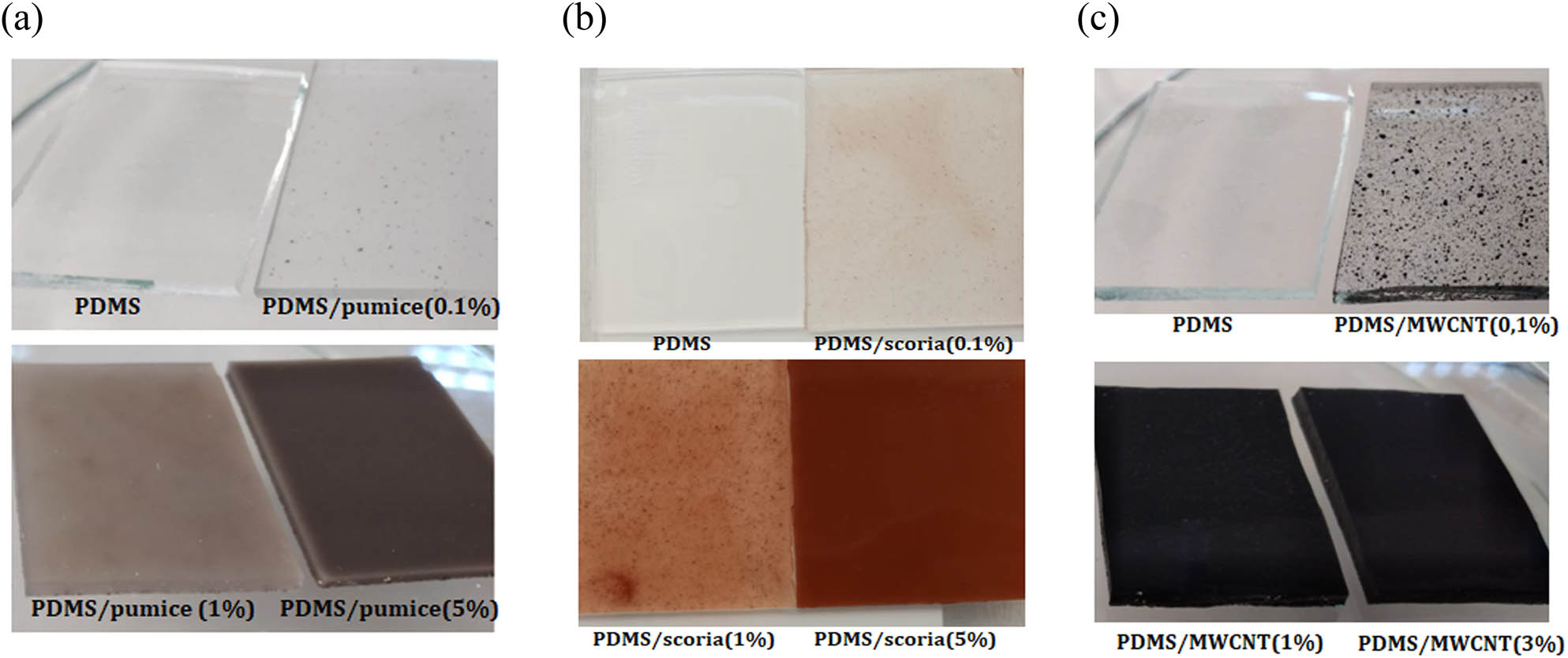
PDMS/pumice (a), PDMS/scoria (b), and PDMS/MWCNT (c) composite elastomers at different particle ratios.
The FTIR spectra of all studied elastomers show the same result. PDMS does not chemically interact with any of the filler particles: pumice, scoria, MWCNT, and nano-Ag (Figures S8–S14). PDMS provides a surface that has a low interfacial free energy, is chemically inert, has good gas permeability and good thermal stability, and is optically transparent [27]. The inert behavior of PDMS was observed for each composite sample in this study.
3.2.1 Thermal conductivities of PDMS elastomers
The thermal conductivity properties of polymer composites are essential for applications. The effects of pumice, scoria, and MWCNT particles on PDMS thermal conductivity were investigated in this study. PDMS has a very low thermal conductivity of approximately 0.35 W·(m·K)−1 (Table S4). Selected filler particles can change the thermal conductivity of PDMS according to the area of usage. Polymer-based dielectric materials with simultaneously high thermal conductivity and low dielectric loss are highly desirable in various applications, such as energy storage, thermal management, and electronic packaging [28]. To prevent devices from heating, filler particles such as MWCNTs have been studied by many researchers to increase thermal conductivity. Li et al. managed to increase the thermal conductivity of PDSM up to 300% by adding Al2O3 particles [29]. Figure 4 shows that all particles increase the thermal conductivity of the PDMS elastomer. MWCNTs are more effective than pumice and scoria, reaching 0.62 W·(m·K)−1 for 3 wt%. Moreover, pumice and scoria do not greatly affect the thermal conductivity, even with increasing weight ratio, and the thermal conductivity reaches only 0.44 W·(m·K)−1. This behavior can be useful for some fields in which insulator polymers are used, such as household products.
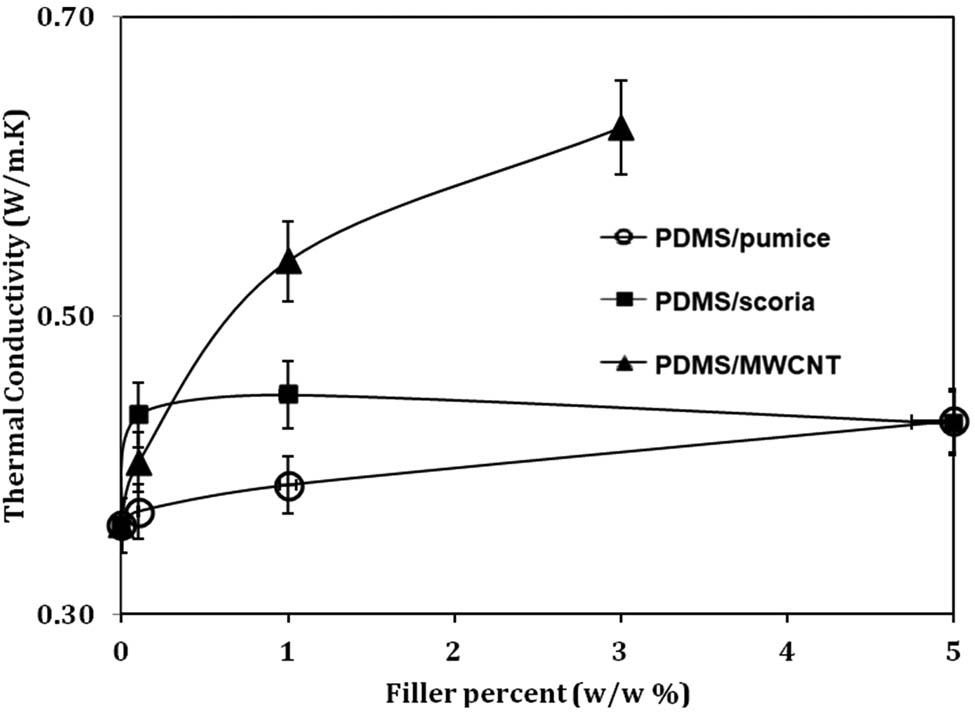
Thermal conductivities of PDMS composites with different filler ratios.
While the dense structure of the cross-links in the elastomer makes it an insulator, the fact that the filling materials create gaps in this structure may have enabled the heat to be transmitted over the particles. The possible distribution of particles in the elastomer is shown in Figure 5. The fact that pumice and scoria particles collapsed and concentrated at the bottom during elastomer formation enabled the elastomer to preserve its dense structure to some extent, and, as a result, the particles could affect the thermal conductivity up to a point.
Schematic representation of the possible distribution of particles in the elastomer.
3.2.2 TG analysis of PDMS composites
TG and dTG curves of PDMS elastomer and composites are presented in Figure 6 and data are given in Table S5. PDMS degrades at about 527°C with 77.6% weight loss, related to the exit of the lateral groups from the main chain [30]. The thermal degradation behavior of the PDMS changed in the same direction for PDMS/scoria and PDMS/pumice composites. There are two weight loss stages for PDMS/scoria and PDMS/pumice. The first step has a maximum of around 400°C with an average weight loss of 10%. The second step weight loss occurs at 580°C with around 20%. PDMS elastomer decomposes by the removal of small molecular cyclosiloxanes which occurs via the rearrangement of siloxane. Then methane is formed due to the cleavage of the side methyl group of polysiloxane at higher temperatures [31].
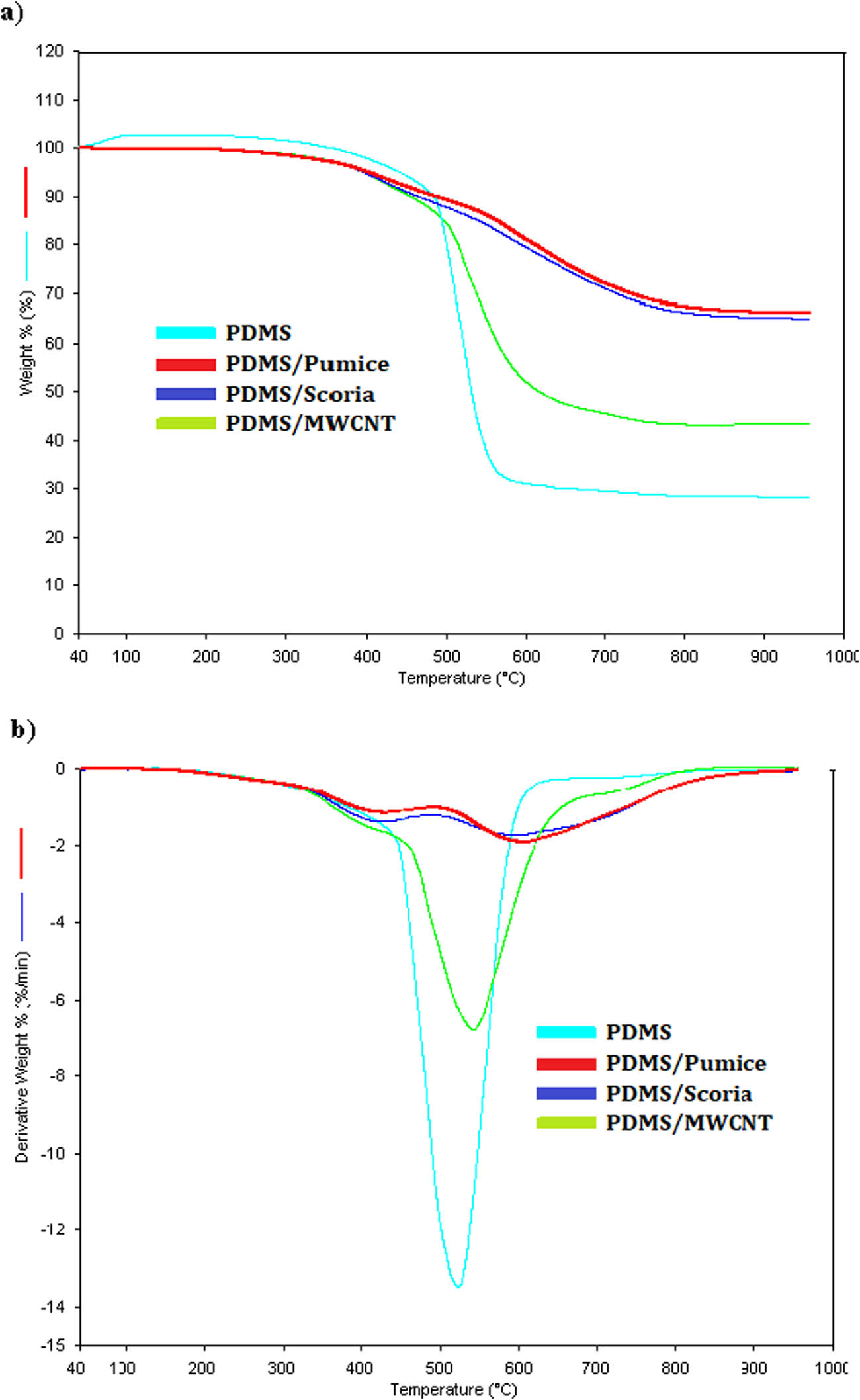
TG (a), and dTG (b) curves of PDMS elastomer and composites.
While only 28% of the pure elastomer remained at 900°C, a large mass of 65% of the pumice and scoria composites of elastomer remained intact. PDMS/MWCNT composites have three decomposition steps: the first is usually associated with adsorbed apolar gases, the second is the decomposition of functional groups, and the final one, at higher temperatures, is due to the oxidation of CNTs [30]. Also, 43% residue remains at 900°C. It seems that particles in the elastomer largely prevent the formation of molecular cyclosiloxanes and the removal of free radicals from the structure.
3.2.3 Electrical conductivities of PDMS composites
The large aspect ratios of CNTs can enhance the electrical conductivity of the polymer composite. Electrical conductivity was studied for all composites of PDMS elastomer and silicon oil. Of all the composites, only the MWCNT filler shows electrical conductivity (Figure 7). Pumice and scoria particles do not change the electrical properties of either the elastomer or fluid forms of PDMS. The PDMS/MWCNT (0.1%) elastomer composite does not have conductivity because this filler ratio does not have sufficient MWCNT particles for proper contact. CNTs are regarded to be very difficult to uniformly disperse in a polymer matrix since they have very large surface areas and strong van der Waals forces, which result in aggregates [32]. The transformation duration of a viscous PDMS by curing agents to an elastomer depends on the temperature and takes some time. While waiting for elastomer formation, the added filler particles, such as CNTs, tend to aggregate at the bottom of the elastomer film. Finally, the inside of the elastomer is a heterogeneous dispersion [33]. As seen from Figure 7 and Table S6, increasing the number of MWCNTs increases the electrical conductivities of the PDMS elastomer.
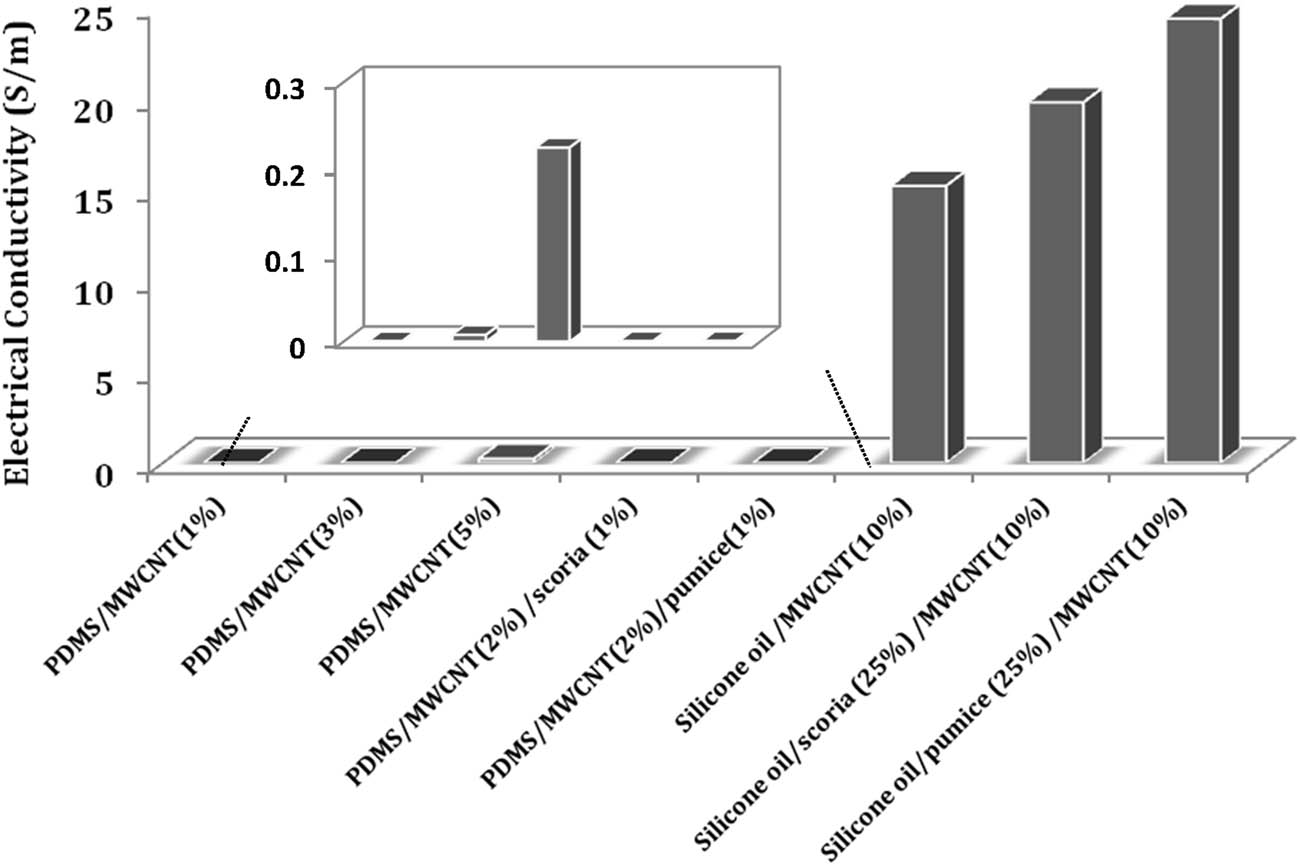
Electrical conductivities of MWCNTs including composites of PDMS and silicone oil.
Pumice and scoria particles do not contribute to the conductivities of the elastomer composites. Moreover, pumice and scoria particles slightly increase the electrical conductivities of silicone oil/MWCNT soft solids. Since the particles in the elastomer tend to collapse during curing, they cannot fill the spaces between the CNTs and exhibit a homogeneous distribution. Therefore, the particles do not affect the electrical conductivities of the PDMS/MWCNT elastomer nanocomposites. Conversely, particles settle in the spaces of the silicone oil surrounding the CNTs in soft solids, provide a homogeneous distribution, and increase the contact of the CNTs with each other; therefore, the electrical conductivity increases.
The highest electrical conductivity of 24.7 S·m−1 is obtained with the 25% pumice–10% MWCNT–silicone oil composite. As seen from Table S6, the conductivity of the silicone oil/MWCNT composite is 14.6 S·m−1; adding 25 wt% scoria increases the conductivity to 18.5 S·m−1. Table 2 shows some PDMS composites and their calculated electrical conductivity values. A maximum of 7% MWCNT has been doped in PDMS elastomers, while other particles can exceed this amount.
Comparison of PDMS composites and their electrical conductivities
| Composite sample | Electrical conductivity (S·m−1) | Ref. |
|---|---|---|
| PDMS/nickel powder (10%) | 1 × 10−14 | [34] |
| PDMS/short carbon fiber (4%) | 1.67 × 102 | [35] |
| PDMS/HCNT (5%) | ∼102 | [36] |
| MXene/cellulose nanofiber-coated PDMS foam (8%) | 6.7 | [37] |
| PDMS/MWCNT (7%) | ∼101 | [38] |
| PDMS/MWCNT (5%) | 2.2 × 10−1 | This study |
| Silicone oil/MWCNT (10%) | 1.5 × 101 | This study |
There is no electrical conductivity for the composites with Ag nanoparticles (Figure S15). The surfaces of silver nanoparticles can be oxidized, and the weight percent is not sufficient to make a continuous structure, causing the current to pass through. Similar values can be observed for PDMS/nickel powder, which is approximately 10−14 S·m−1 with a 10% weight ratio [34].
The PDMS elastomer surface has a very low amount of filler particles, and all are covered with the polymer itself. When the surfaces of the elastomer composites are touched, no current flows from the electrodes, and the surface does not conduct electricity. Electrical conductivity is observed only when the electrodes are dipped into the interior of the elastomer (Figure S16). This phenomenon makes the composite an insulator from the outside and a conductor from the inside. This result is not the case with silicone oil composites.
3.2.4 Light transmittance of PDMS composites
PDMS is an ideal elastomeric material suitable for optical and electronic devices due to its flexibility, high optical transparency, nontoxicity, and low cost [39]. Figure 8 shows the light transmittance characteristics of PDMS/pumice, PDMS/scoria, and PDMS/MWCNT elastomers. As seen from the figure, the PDMS elastomer transmits almost 100% of visible light but the transmittance decreases sharply between 400 and 200 nm, eventually decreasing to zero. MWCNT particles starting from 1 wt% block the light transition of elastomer composites. The addition of 0.1% pumice and scoria particles decreases the elastomer transmittance by 10% in the visible region. The sample with 1 wt% pumice decreases the transmittance to 40% and with scoria decreases to approximately 60% in the visible region, with both decreasing to zero in the UV region. The sample with 5 wt% scoria/PDMS still exhibits transmittance at 20%, while that of the pumice/PDMS elastomer decreases to zero. PDMS remains semitransparent at a low filler particle content.
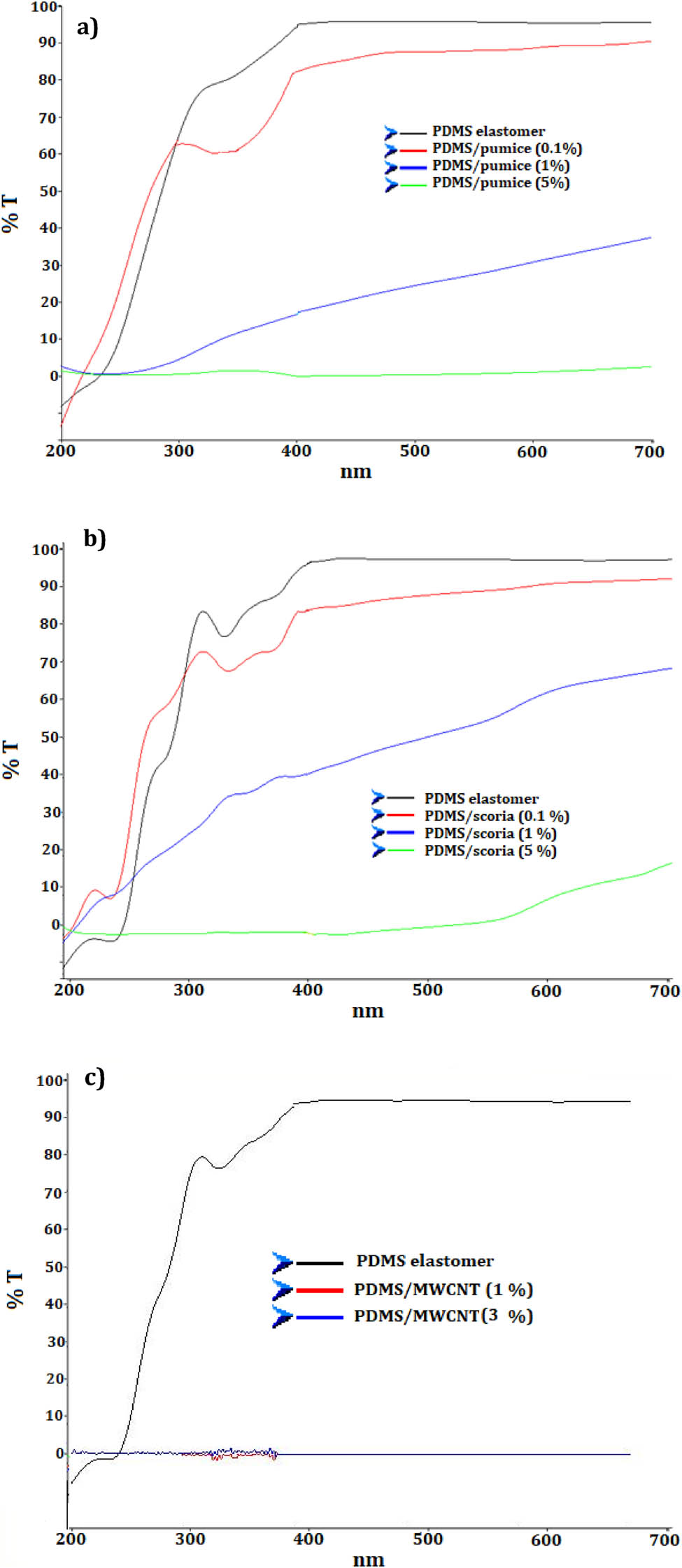
Light transmittance characteristics of PDMS/pumice (a), PDMS/scoria (b), and PDMS/MWCNT (c) composites.
Solar cells and electronic devices are easily affected by external humidity, light, heat, and chemicals. There are a wide variety of studies available to protect them from the effects of the external environment. For example, Wei et al. obtained a safe and environmentally friendly encapsulation material that avoids the structural damage of perovskite solar cells under high-temperature or UV-curing conditions [40]. Naturally colored PDMS elastomers with pumice and scoria can be preferred as environmentally friendly materials for the encapsulation of electronic devices or solar cells. These volcanic rock particles are not damaged by external factors, such as dyes and pigments. There is no expiration date or decomposition time.
4 Conclusions
In this work, the fluid forms of PDMS (silicone oil) composites with PDMS elastomer composites were compared. The electrical and thermal conductivities and light transmittance properties of the composites were presented. An effective, clean, and easy method was used to prepare nanosized particles from pumice and scoria rocks. MWCNT particles made the PDMS elastomer electrically conductive, but the conductivity was limited by achieving the maximum filler amount. However, silicone oil was mixed with double the amount of MWCNTs, which led to a sharp increase in electrical conductivity. Additionally, pumice and scoria particles supported the structures of silicone oil/MWCNTs, and the conductivity increased again.
Silicone oil/MWCNT composites formed a soft solid structure; thus, these composites could be easily applied to any desired surface at any thickness or extent. A conductive surface could be applied and discarded from the surface easily while recycling. Pumice, scoria, and nano-Ag particles did not affect the electrical conductivity of PDMS. The thermal conductivity of the PDMS elastomer only improved with MWCNT particles, and mixing pumice and scoria particles with PDMS did not significantly change the thermal insulator features. All filler particles decreased the transparency of the PDMS elastomer. The addition of 5 wt% elastomers reached zero transmittance with pumice particles.
Silicone oil decomposed naturally in the soil environment. However, the pumice and scoria particles improved some properties of silicone oil composites, and their effects on the decomposition of silicone oil should be investigated because they are natural volcanic rocks abundant in soil. Finally, the environmental effects of composites were very low because silicone oil is harmless when discarded to nature; since pumice and scoria are natural rocks, they were not considered waste. MWCNTs could be taken inside the silicone oil composite and reused after dissolving the polymer with a solvent. Pumice and scoria particles are inert; thus, PDMS elastomer recycling with chemicals or heat would not be affected by fillers.
-
Funding information: The authors state no funding involved.
-
Author contributions: Pınar Turan Beyli: writing – original draft, methodology, and formal analysis. Esra Özvezir: experimental and analysis.
-
Conflict of interest: The authors state no conflict of interest.
-
Data availability statement: The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request. The supplementary materials section can be viewed on the GREENPS website.
References
[1] Bergeron V, Cooper P, Fischer C, Giermanska-Kahn J, Langevin D, Pouchelon A. Polydimethylsiloxane (PDMS)-based antifoams. Colloids Surf A: Physicochem Eng Aspects. 1997;122(1–3):103–20. 10.1016/S0927-7757(96)03774-0.Search in Google Scholar
[2] Stevens C. Environmental degradation pathways for the breakdown of polydimethylsiloxanes. J Inorg Biochem. 1998;69(3):203–7. 10.1016/S0162-0134(97)10019-8.Search in Google Scholar PubMed
[3] Tolle DA, Frye CL, Lehmann RG, Zwick TC. Ecological effects of PDMS-augmented sludge amended to agricultural microcosms. Sci Total Environ. 1995;162(2–3):193–207. 10.1016/0048-9697(95)04459-E.Search in Google Scholar
[4] Kwak Y, Kang Y, Park W, Jo E, Kim J. Fabrication of fine-pored polydimethylsiloxane using an isopropyl alcohol and water mixture for adjustable mechanical, optical, and thermal properties. RSC Adv. 2021;11:18061–7. 10.1039/d1ra02466c.Search in Google Scholar PubMed PubMed Central
[5] Gandhi B, Raghava NS. MWCNT-COOH/PDMS based dry ECG electrodes for ambulatory ECG recordings. Mater Lett. 2022;307:130972. 10.1016/j.matlet.2021.130972.Search in Google Scholar
[6] Barshutina MN, Kirichenko SO, Wodolajsky VA, Lopachev AV, Barshutin SN, Gorsky OV, et al. PDMS-CNT composite for soft bioelectronic neuronal implants. Compos Part B. 2022;247:110286. 10.1016/j.compositesb.2022.110286.Search in Google Scholar
[7] Li H, Wu Z, Xing Y, Li B, Liu L. Photoelectric synergistic response properties of the Ti3C2Tx MXene-CNT/PDMS bilayer actuator. Nano Energy. 2022;103:107821. 10.1016/j.nanoen.2022.107821.Search in Google Scholar
[8] Fendinger NJ, McAvoy DC, Eckhoff WS, Price BB. Environmental Occurrence of Polydimethylsiloxane. Environ Sci Technol. 1997;31(5):1555–63. 10.1021/es9608712.Search in Google Scholar
[9] Carpenter JC, Cella JA, Dorn SB. Study of the degradation of polydimethylsiloxanes on soil. Environ Sci Technol. 1995;29(4):864–8. 10.1021/es00004a005.Search in Google Scholar PubMed
[10] Buch RR, Ingebrigtson DN. Rearrangement of poly(dimethylsiloxane) fluids on soil. Environ Sci Technol. 1979;13:676–9. 10.1021/es60154a002.Search in Google Scholar
[11] Lehmann RG, Varaprath S, Frye CL. Fate of silicone degradation products (silanols) in soil. Environ Toxicol Chem. 1994;13:1061–4. 10.1002/etc.5620131106.Search in Google Scholar
[12] Lehmann RG, Miller JR, Kozerski GE. Degradation of silicone polymer in a field soil under natural conditions. Chemosphere. 2000;41(5):743–9. 10.1016/S0045-6535(99)00430-0.Search in Google Scholar
[13] Xu S, Lehmann RG, Miller JR, Chandra G. Degradation of polydimethylsiloxanes (Silicones) as influenced by clay minerals. Environ Sci Technol. 1998;32(9):1199–206. 10.1021/es9708676.Search in Google Scholar
[14] Zhang Z, Zhang Y, Jiang X, Bukhari H, Zhang Z, Han W, et al. Simple and efficient pressure sensor based on PDMS wrapped CNT arrays. Carbon. 2019;155:71–6. 10.1016/j.carbon.2019.08.018.Search in Google Scholar
[15] Qiao Z, Wei A, Wang K, Luo N, Liu Z. Study of flexible piezoresistive sensors based on the hierarchical porous structure CNT/PDMS composite materials. J Alloy Compd. 2022;917:165503. 10.1016/j.jallcom.2022.165503.Search in Google Scholar
[16] Nour M, Berean K, Balendhran S, Oua JZ, Plessis JD, McSweeney C, et al. CNT/PDMS composite membranes for H2 and CH4 gas separation. Int J Hydrog Energy. 2013;38:10494–501. 10.1016/j.ijhydene.2013.05.162.Search in Google Scholar
[17] Rupasinghe B. Recycling silicone-based materials: an overview of methods. application and characterization of rubber materials. United Kingdom: IntechOpen; 2023. 10.5772/intechopen.108051.Search in Google Scholar
[18] Fried R, Oprea I, Fleck K, Rudrof F. Biogenic colorants in the textile industry—A promising and sustainable alternative to synthetic dyes. Green Chem. 2022;24:13–35. 10.1039/D1GC02968A.Search in Google Scholar
[19] Asere TG, Clercq JD, Verbeken K, Tessema DA, Christian FF, Gijs VS, et al. Uptake of arsenate by aluminum (hydr)oxide coated red scoria and pumice. Appl Geochem. 2017;78:83–95. 10.1016/j.apgeochem.2016.12.013.Search in Google Scholar
[20] Bar-Tal A, Saha UK, Raviv M, Tuller M. Chapter 7 - inorganic and synthetic organic components of soilless culture and potting mixtures, soilless culture. Theory and Practice. 2nd edn. United Kingdom: Elsevier; 2019. p. 259–301. 10.1016/B978-0-444-63696-6.00007-4.Search in Google Scholar
[21] Wu D, Wei M, Li R, Xiao T, Gong S, Xiao Z, et al. A percolation network model to predict the electrical property of flexible CNT/PDMS composite films fabricated by spin coating technique. Compos B. 2019;174:107034. 10.1016/j.compositesb.2019.107034.Search in Google Scholar
[22] Kong J, Tong Y, Sun J, Wei Y, Thitsartarn W, Jayven CCY, et al. Electrically conductive PDMS-grafted CNTs-reinforced silicone elastomer. Compos Sci Technol. 2018;159:208–15. 10.1016/j.compscitech.2018.02.018.Search in Google Scholar
[23] Jang SH, Park YL. Carbon nanotube-reinforced smart composites for sensing freezing temperature and deicing by self-heating. Nanomater Nanotechnol. 2018;8:1–8.10.1177/1847980418776473Search in Google Scholar
[24] Alraddadi S, Assaedi H. Physical properties of mesoporous scoria and pumice volcanic rocks. J Phys Commun. 2021;5:115018. 10.1088/2399-6528/ac3a95.Search in Google Scholar
[25] Garg A, Chopra L. Dye Waste: A significant environmental hazard. Mater Today Proc. 2022;48(5):1310–5. 10.1016/j.matpr.2021.09.003.Search in Google Scholar
[26] Vu ND, Boulègue-Mondière A, Durand N, Raynaud J, Monteil V. Back-to-cyclic monomers: chemical recycling of silicone waste using a [polydentate ligand–potassium silanolate] complex. Green Chem. 2023;25:3869. 10.1039/D3GC00293D.Search in Google Scholar
[27] Gale BK, Eddings MA, Sundberg SO, Hatch A, Kim J, Ho T, et al. Fabrication and packaging: Low-cost MEMS technologies. Comprehensive microsystems. Amsterdam: Elsevier; 2008. p. 341–78.10.1016/B978-044452190-3.00011-2Search in Google Scholar
[28] Yu X, Bhatti MR, Ren X, Steiner P, Sacco FD, Dong M, et al. Dielectric polymer composites with ultra-high thermal conductivity and low dielectric loss. Compos Sci Technol. 2022;229:109695. 10.1016/j.compscitech.2022.109695.Search in Google Scholar
[29] Li YT, Liu WJ, Shen FX, Zhang GD, Gong LX, Zhao L, et al. Processing, thermal conductivity and flame retardant properties of silicone rubber filled with different geometries of thermally conductive fillers: a comparative study. Compos Pt B Eng. 2022;238:11. 10.1016/j.compositesb.2022.109907.Search in Google Scholar
[30] Silvaa EA, Windmöllerb D, Silvab GG, Figueiredo KCS. Polydimethylsiloxane Membranes Containing Multi-walled Carbon Nanotubes for Gas Separation. Mater Res. 2017;20(6):1454–60. 10.1590/1980-5373-MR-2016-0825.Search in Google Scholar
[31] Wang Y, Cai Y, Zhang H, Zhou J, Zhou S, Chen Y, et al. Mechanical and thermal degradation behavior of high-performance PDMS elastomer based on epoxy/silicone hybrid network. Polymer. 2021;236:124299. 10.1016/j.polymer.2021.124299.Search in Google Scholar
[32] Goswami M, Mukherjee A, Ghosh R, Basu S, Meikap AK. Enhanced magnetoconductivity and electrical property of MWCNT-CdS nanocomposite embedded in polyaniline. Solid State Sci. 2016;60:37–44. 10.1016/j.solidstatesciences.2016.08.001.Search in Google Scholar
[33] Watt MR, Gerhardt RA. Factors that affect network formation in carbon nanotube composites and their resultant electrical properties. J Compos Sci. 2020;4:100. 10.3390/jcs4030100.Search in Google Scholar
[34] Jang SH, Park YL, Yin H. Influence of coalescence on the anisotropic mechanical and electrical properties of nickel powder/polydimethylsiloxane composites. Materials. 2016;9:239. 10.3390/ma9040239.Search in Google Scholar PubMed PubMed Central
[35] Gao X, Huang Y, Liu Y, Kormakov S, Zheng X, Wua D, et al. Improved electrical conductivity of PDMS/SCF composite sheets with bolting cloth prepared by a spatial confining forced network assembly method. RSC Adv. 2017;7:14761–8. 10.1039/C7RA02061A.Search in Google Scholar
[36] Hur ON, Ha JH, Park SH. Strain-sensing properties of multi-walled carbon nanotube/polydimethylsiloxane composites with different aspect ratio and filler contents. Materials. 2020;13:2431. 10.3390/ma13112431.Search in Google Scholar PubMed PubMed Central
[37] Chen HY, Chen ZY, Mao M, Wu YY, Yang F, Gong LX, et al. Self-adhesive polydimethylsiloxane foam materials decorated with MXene/cellulose nanofiber interconnected network for versatile functionalities. Adv Funct Mater. 2023;33:2304927. 10.1002/adfm.202304927.Search in Google Scholar
[38] Abdullayeva SA, Huseynov AB, Musayeva NN, Jabbarov RB, Sultanov CA, Guliyev AD, et al. Synthesis of carbon nanotubes using Azerbaijan’s oil. AMPC. 2016;06:105–16. 10.4236/ampc.2016.65011.Search in Google Scholar
[39] Hu Z, Zhang H, Sui G, Zhang Z. Alkane-containing polydimethylsiloxane elastomer composite films with excellent tunable light transmittance. Opt Mater. 2022;128:112361. 10.1016/j.optmat.2022.112361.Search in Google Scholar
[40] Wei Q, Huo X, Fu Q, Wang T, Zhao H, Wang Y, et al. An effective encapsulation for perovskite solar cells based on building-integrated photovoltaics. J Mater Chem C. 2022;10:8972. 10.1039/D2TC01696F.Search in Google Scholar
© 2023 the author(s), published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Research Articles
- Value-added utilization of coal fly ash and recycled polyvinyl chloride in door or window sub-frame composites
- High removal efficiency of volatile phenol from coking wastewater using coal gasification slag via optimized adsorption and multi-grade batch process
- Evolution of surface morphology and properties of diamond films by hydrogen plasma etching
- Removal efficiency of dibenzofuran using CuZn-zeolitic imidazole frameworks as a catalyst and adsorbent
- Rapid and efficient microwave-assisted extraction of Caesalpinia sappan Linn. heartwood and subsequent synthesis of gold nanoparticles
- The catalytic characteristics of 2-methylnaphthalene acylation with AlCl3 immobilized on Hβ as Lewis acid catalyst
- Biodegradation of synthetic PVP biofilms using natural materials and nanoparticles
- Rutin-loaded selenium nanoparticles modulated the redox status, inflammatory, and apoptotic pathways associated with pentylenetetrazole-induced epilepsy in mice
- Optimization of apigenin nanoparticles prepared by planetary ball milling: In vitro and in vivo studies
- Synthesis and characterization of silver nanoparticles using Origanum onites leaves: Cytotoxic, apoptotic, and necrotic effects on Capan-1, L929, and Caco-2 cell lines
- Exergy analysis of a conceptual CO2 capture process with an amine-based DES
- Construction of fluorescence system of felodipine–tetracyanovinyl–2,2′-bipyridine complex
- Excellent photocatalytic degradation of rhodamine B over Bi2O3 supported on Zn-MOF nanocomposites under visible light
- Optimization-based control strategy for a large-scale polyhydroxyalkanoates production in a fed-batch bioreactor using a coupled PDE–ODE system
- Effectiveness of pH and amount of Artemia urumiana extract on physical, chemical, and biological attributes of UV-fabricated biogold nanoparticles
- Geranium leaf-mediated synthesis of silver nanoparticles and their transcriptomic effects on Candida albicans
- Synthesis, characterization, anticancer, anti-inflammatory activities, and docking studies of 3,5-disubstituted thiadiazine-2-thiones
- Synthesis and stability of phospholipid-encapsulated nano-selenium
- Putative anti-proliferative effect of Indian mustard (Brassica juncea) seed and its nano-formulation
- Enrichment of low-grade phosphorites by the selective leaching method
- Electrochemical analysis of the dissolution of gold in a copper–ethylenediamine–thiosulfate system
- Characterisation of carbonate lake sediments as a potential filler for polymer composites
- Evaluation of nano-selenium biofortification characteristics of alfalfa (Medicago sativa L.)
- Quality of oil extracted by cold press from Nigella sativa seeds incorporated with rosemary extracts and pretreated by microwaves
- Heteropolyacid-loaded MOF-derived mesoporous zirconia catalyst for chemical degradation of rhodamine B
- Recovery of critical metals from carbonatite-type mineral wastes: Geochemical modeling investigation of (bio)hydrometallurgical leaching of REEs
- Photocatalytic properties of ZnFe-mixed oxides synthesized via a simple route for water remediation
- Attenuation of di(2-ethylhexyl)phthalate-induced hepatic and renal toxicity by naringin nanoparticles in a rat model
- Novel in situ synthesis of quaternary core–shell metallic sulfide nanocomposites for degradation of organic dyes and hydrogen production
- Microfluidic steam-based synthesis of luminescent carbon quantum dots as sensing probes for nitrite detection
- Transformation of eggshell waste to egg white protein solution, calcium chloride dihydrate, and eggshell membrane powder
- Preparation of Zr-MOFs for the adsorption of doxycycline hydrochloride from wastewater
- Green nanoarchitectonics of the silver nanocrystal potential for treating malaria and their cytotoxic effects on the kidney Vero cell line
- Carbon emissions analysis of producing modified asphalt with natural asphalt
- An efficient and green synthesis of 2-phenylquinazolin-4(3H)-ones via t-BuONa-mediated oxidative condensation of 2-aminobenzamides and benzyl alcohols under solvent- and transition metal-free conditions
- Chitosan nanoparticles loaded with mesosulfuron methyl and mesosulfuron methyl + florasulam + MCPA isooctyl to manage weeds of wheat (Triticum aestivum L.)
- Synergism between lignite and high-sulfur petroleum coke in CO2 gasification
- Facile aqueous synthesis of ZnCuInS/ZnS–ZnS QDs with enhanced photoluminescence lifetime for selective detection of Cu(ii) ions
- Rapid synthesis of copper nanoparticles using Nepeta cataria leaves: An eco-friendly management of disease-causing vectors and bacterial pathogens
- Study on the photoelectrocatalytic activity of reduced TiO2 nanotube films for removal of methyl orange
- Development of a fuzzy logic model for the prediction of spark-ignition engine performance and emission for gasoline–ethanol blends
- Micro-impact-induced mechano-chemical synthesis of organic precursors from FeC/FeN and carbonates/nitrates in water and its extension to nucleobases
- Green synthesis of strontium-doped tin dioxide (SrSnO2) nanoparticles using the Mahonia bealei leaf extract and evaluation of their anticancer and antimicrobial activities
- A study on the larvicidal and adulticidal potential of Cladostepus spongiosus macroalgae and green-fabricated silver nanoparticles against mosquito vectors
- Catalysts based on nickel salt heteropolytungstates for selective oxidation of diphenyl sulfide
- Powerful antibacterial nanocomposites from Corallina officinalis-mediated nanometals and chitosan nanoparticles against fish-borne pathogens
- Removal behavior of Zn and alkalis from blast furnace dust in pre-reduction sinter process
- Environmentally friendly synthesis and computational studies of novel class of acridinedione integrated spirothiopyrrolizidines/indolizidines
- The mechanisms of inhibition and lubrication of clean fracturing flowback fluids in water-based drilling fluids
- Adsorption/desorption performance of cellulose membrane for Pb(ii)
- A one-pot, multicomponent tandem synthesis of fused polycyclic pyrrolo[3,2-c]quinolinone/pyrrolizino[2,3-c]quinolinone hybrid heterocycles via environmentally benign solid state melt reaction
- Green synthesis of silver nanoparticles using durian rind extract and optical characteristics of surface plasmon resonance-based optical sensor for the detection of hydrogen peroxide
- Electrochemical analysis of copper-EDTA-ammonia-gold thiosulfate dissolution system
- Characterization of bio-oil production by microwave pyrolysis from cashew nut shells and Cassia fistula pods
- Green synthesis methods and characterization of bacterial cellulose/silver nanoparticle composites
- Photocatalytic research performance of zinc oxide/graphite phase carbon nitride catalyst and its application in environment
- Effect of phytogenic iron nanoparticles on the bio-fortification of wheat varieties
- In vitro anti-cancer and antimicrobial effects of manganese oxide nanoparticles synthesized using the Glycyrrhiza uralensis leaf extract on breast cancer cell lines
- Preparation of Pd/Ce(F)-MCM-48 catalysts and their catalytic performance of n-heptane isomerization
- Green “one-pot” fluorescent bis-indolizine synthesis with whole-cell plant biocatalysis
- Silica-titania mesoporous silicas of MCM-41 type as effective catalysts and photocatalysts for selective oxidation of diphenyl sulfide by H2O2
- Biosynthesis of zinc oxide nanoparticles from molted feathers of Pavo cristatus and their antibiofilm and anticancer activities
- Clean preparation of rutile from Ti-containing mixed molten slag by CO2 oxidation
- Synthesis and characterization of Pluronic F-127-coated titanium dioxide nanoparticles synthesized from extracts of Atractylodes macrocephala leaf for antioxidant, antimicrobial, and anticancer properties
- Effect of pretreatment with alkali on the anaerobic digestion characteristics of kitchen waste and analysis of microbial diversity
- Ameliorated antimicrobial, antioxidant, and anticancer properties by Plectranthus vettiveroides root extract-mediated green synthesis of chitosan nanoparticles
- Microwave-accelerated pretreatment technique in green extraction of oil and bioactive compounds from camelina seeds: Effectiveness and characterization
- Studies on the extraction performance of phorate by aptamer-functionalized magnetic nanoparticles in plasma samples
- Investigation of structural properties and antibacterial activity of AgO nanoparticle extract from Solanum nigrum/Mentha leaf extracts by green synthesis method
- Green fabrication of chitosan from marine crustaceans and mushroom waste: Toward sustainable resource utilization
- Synthesis, characterization, and evaluation of nanoparticles of clodinofop propargyl and fenoxaprop-P-ethyl on weed control, growth, and yield of wheat (Triticum aestivum L.)
- The enhanced adsorption properties of phosphorus from aqueous solutions using lanthanum modified synthetic zeolites
- Separation of graphene oxides of different sizes by multi-layer dialysis and anti-friction and lubrication performance
- Visible-light-assisted base-catalyzed, one-pot synthesis of highly functionalized cinnolines
- The experimental study on the air oxidation of 5-hydroxymethylfurfural to 2,5-furandicarboxylic acid with Co–Mn–Br system
- Highly efficient removal of tetracycline and methyl violet 2B from aqueous solution using the bimetallic FeZn-ZIFs catalyst
- A thermo-tolerant cellulase enzyme produced by Bacillus amyloliquefaciens M7, an insight into synthesis, optimization, characterization, and bio-polishing activity
- Exploration of ketone derivatives of succinimide for their antidiabetic potential: In vitro and in vivo approaches
- Ultrasound-assisted green synthesis and in silico study of 6-(4-(butylamino)-6-(diethylamino)-1,3,5-triazin-2-yl)oxypyridazine derivatives
- A study of the anticancer potential of Pluronic F-127 encapsulated Fe2O3 nanoparticles derived from Berberis vulgaris extract
- Biogenic synthesis of silver nanoparticles using Consolida orientalis flowers: Identification, catalytic degradation, and biological effect
- Initial assessment of the presence of plastic waste in some coastal mangrove forests in Vietnam
- Adsorption synergy electrocatalytic degradation of phenol by active oxygen-containing species generated in Co-coal based cathode and graphite anode
- Antibacterial, antifungal, antioxidant, and cytotoxicity activities of the aqueous extract of Syzygium aromaticum-mediated synthesized novel silver nanoparticles
- Synthesis of a silica matrix with ZnO nanoparticles for the fabrication of a recyclable photodegradation system to eliminate methylene blue dye
- Natural polymer fillers instead of dye and pigments: Pumice and scoria in PDMS fluid and elastomer composites
- Study on the preparation of glycerylphosphorylcholine by transesterification under supported sodium methoxide
- Wireless network handheld terminal-based green ecological sustainable design evaluation system: Improved data communication and reduced packet loss rate
- The optimization of hydrogel strength from cassava starch using oxidized sucrose as a crosslinking agent
- Green synthesis of silver nanoparticles using Saccharum officinarum leaf extract for antiviral paint
- Study on the reliability of nano-silver-coated tin solder joints for flip chips
- Environmentally sustainable analytical quality by design aided RP-HPLC method for the estimation of brilliant blue in commercial food samples employing a green-ultrasound-assisted extraction technique
- Anticancer and antimicrobial potential of zinc/sodium alginate/polyethylene glycol/d-pinitol nanocomposites against osteosarcoma MG-63 cells
- Nanoporous carbon@CoFe2O4 nanocomposite as a green absorbent for the adsorptive removal of Hg(ii) from aqueous solutions
- Characterization of silver sulfide nanoparticles from actinobacterial strain (M10A62) and its toxicity against lepidopteran and dipterans insect species
- Phyto-fabrication and characterization of silver nanoparticles using Withania somnifera: Investigating antioxidant potential
- Effect of e-waste nanofillers on the mechanical, thermal, and wear properties of epoxy-blend sisal woven fiber-reinforced composites
- Magnesium nanohydroxide (2D brucite) as a host matrix for thymol and carvacrol: Synthesis, characterization, and inhibition of foodborne pathogens
- Synergistic inhibitive effect of a hybrid zinc oxide-benzalkonium chloride composite on the corrosion of carbon steel in a sulfuric acidic solution
- Review Articles
- Role and the importance of green approach in biosynthesis of nanopropolis and effectiveness of propolis in the treatment of COVID-19 pandemic
- Gum tragacanth-mediated synthesis of metal nanoparticles, characterization, and their applications as a bactericide, catalyst, antioxidant, and peroxidase mimic
- Green-processed nano-biocomposite (ZnO–TiO2): Potential candidates for biomedical applications
- Reaction mechanisms in microwave-assisted lignin depolymerisation in hydrogen-donating solvents
- Recent progress on non-noble metal catalysts for the deoxydehydration of biomass-derived oxygenates
- Rapid Communication
- Phosphorus removal by iron–carbon microelectrolysis: A new way to achieve phosphorus recovery
- Special Issue: Biomolecules-derived synthesis of nanomaterials for environmental and biological applications (Guest Editors: Arpita Roy and Fernanda Maria Policarpo Tonelli)
- Biomolecules-derived synthesis of nanomaterials for environmental and biological applications
- Nano-encapsulated tanshinone IIA in PLGA-PEG-COOH inhibits apoptosis and inflammation in cerebral ischemia/reperfusion injury
- Green fabrication of silver nanoparticles using Melia azedarach ripened fruit extract, their characterization, and biological properties
- Green-synthesized nanoparticles and their therapeutic applications: A review
- Antioxidant, antibacterial, and cytotoxicity potential of synthesized silver nanoparticles from the Cassia alata leaf aqueous extract
- Green synthesis of silver nanoparticles using Callisia fragrans leaf extract and its anticancer activity against MCF-7, HepG2, KB, LU-1, and MKN-7 cell lines
- Algae-based green AgNPs, AuNPs, and FeNPs as potential nanoremediators
- Green synthesis of Kickxia elatine-induced silver nanoparticles and their role as anti-acetylcholinesterase in the treatment of Alzheimer’s disease
- Phytocrystallization of silver nanoparticles using Cassia alata flower extract for effective control of fungal skin pathogens
- Antibacterial wound dressing with hydrogel from chitosan and polyvinyl alcohol from the red cabbage extract loaded with silver nanoparticles
- Leveraging of mycogenic copper oxide nanostructures for disease management of Alternaria blight of Brassica juncea
- Nanoscale molecular reactions in microbiological medicines in modern medical applications
- Synthesis and characterization of ZnO/β-cyclodextrin/nicotinic acid nanocomposite and its biological and environmental application
- Green synthesis of silver nanoparticles via Taxus wallichiana Zucc. plant-derived Taxol: Novel utilization as anticancer, antioxidation, anti-inflammation, and antiurolithic potential
- Recyclability and catalytic characteristics of copper oxide nanoparticles derived from bougainvillea plant flower extract for biomedical application
- Phytofabrication, characterization, and evaluation of novel bioinspired selenium–iron (Se–Fe) nanocomposites using Allium sativum extract for bio-potential applications
- Erratum
- Erratum to “Synthesis, characterization, and evaluation of nanoparticles of clodinofop propargyl and fenoxaprop-P-ethyl on weed control, growth, and yield of wheat (Triticum aestivum L.)”
Articles in the same Issue
- Research Articles
- Value-added utilization of coal fly ash and recycled polyvinyl chloride in door or window sub-frame composites
- High removal efficiency of volatile phenol from coking wastewater using coal gasification slag via optimized adsorption and multi-grade batch process
- Evolution of surface morphology and properties of diamond films by hydrogen plasma etching
- Removal efficiency of dibenzofuran using CuZn-zeolitic imidazole frameworks as a catalyst and adsorbent
- Rapid and efficient microwave-assisted extraction of Caesalpinia sappan Linn. heartwood and subsequent synthesis of gold nanoparticles
- The catalytic characteristics of 2-methylnaphthalene acylation with AlCl3 immobilized on Hβ as Lewis acid catalyst
- Biodegradation of synthetic PVP biofilms using natural materials and nanoparticles
- Rutin-loaded selenium nanoparticles modulated the redox status, inflammatory, and apoptotic pathways associated with pentylenetetrazole-induced epilepsy in mice
- Optimization of apigenin nanoparticles prepared by planetary ball milling: In vitro and in vivo studies
- Synthesis and characterization of silver nanoparticles using Origanum onites leaves: Cytotoxic, apoptotic, and necrotic effects on Capan-1, L929, and Caco-2 cell lines
- Exergy analysis of a conceptual CO2 capture process with an amine-based DES
- Construction of fluorescence system of felodipine–tetracyanovinyl–2,2′-bipyridine complex
- Excellent photocatalytic degradation of rhodamine B over Bi2O3 supported on Zn-MOF nanocomposites under visible light
- Optimization-based control strategy for a large-scale polyhydroxyalkanoates production in a fed-batch bioreactor using a coupled PDE–ODE system
- Effectiveness of pH and amount of Artemia urumiana extract on physical, chemical, and biological attributes of UV-fabricated biogold nanoparticles
- Geranium leaf-mediated synthesis of silver nanoparticles and their transcriptomic effects on Candida albicans
- Synthesis, characterization, anticancer, anti-inflammatory activities, and docking studies of 3,5-disubstituted thiadiazine-2-thiones
- Synthesis and stability of phospholipid-encapsulated nano-selenium
- Putative anti-proliferative effect of Indian mustard (Brassica juncea) seed and its nano-formulation
- Enrichment of low-grade phosphorites by the selective leaching method
- Electrochemical analysis of the dissolution of gold in a copper–ethylenediamine–thiosulfate system
- Characterisation of carbonate lake sediments as a potential filler for polymer composites
- Evaluation of nano-selenium biofortification characteristics of alfalfa (Medicago sativa L.)
- Quality of oil extracted by cold press from Nigella sativa seeds incorporated with rosemary extracts and pretreated by microwaves
- Heteropolyacid-loaded MOF-derived mesoporous zirconia catalyst for chemical degradation of rhodamine B
- Recovery of critical metals from carbonatite-type mineral wastes: Geochemical modeling investigation of (bio)hydrometallurgical leaching of REEs
- Photocatalytic properties of ZnFe-mixed oxides synthesized via a simple route for water remediation
- Attenuation of di(2-ethylhexyl)phthalate-induced hepatic and renal toxicity by naringin nanoparticles in a rat model
- Novel in situ synthesis of quaternary core–shell metallic sulfide nanocomposites for degradation of organic dyes and hydrogen production
- Microfluidic steam-based synthesis of luminescent carbon quantum dots as sensing probes for nitrite detection
- Transformation of eggshell waste to egg white protein solution, calcium chloride dihydrate, and eggshell membrane powder
- Preparation of Zr-MOFs for the adsorption of doxycycline hydrochloride from wastewater
- Green nanoarchitectonics of the silver nanocrystal potential for treating malaria and their cytotoxic effects on the kidney Vero cell line
- Carbon emissions analysis of producing modified asphalt with natural asphalt
- An efficient and green synthesis of 2-phenylquinazolin-4(3H)-ones via t-BuONa-mediated oxidative condensation of 2-aminobenzamides and benzyl alcohols under solvent- and transition metal-free conditions
- Chitosan nanoparticles loaded with mesosulfuron methyl and mesosulfuron methyl + florasulam + MCPA isooctyl to manage weeds of wheat (Triticum aestivum L.)
- Synergism between lignite and high-sulfur petroleum coke in CO2 gasification
- Facile aqueous synthesis of ZnCuInS/ZnS–ZnS QDs with enhanced photoluminescence lifetime for selective detection of Cu(ii) ions
- Rapid synthesis of copper nanoparticles using Nepeta cataria leaves: An eco-friendly management of disease-causing vectors and bacterial pathogens
- Study on the photoelectrocatalytic activity of reduced TiO2 nanotube films for removal of methyl orange
- Development of a fuzzy logic model for the prediction of spark-ignition engine performance and emission for gasoline–ethanol blends
- Micro-impact-induced mechano-chemical synthesis of organic precursors from FeC/FeN and carbonates/nitrates in water and its extension to nucleobases
- Green synthesis of strontium-doped tin dioxide (SrSnO2) nanoparticles using the Mahonia bealei leaf extract and evaluation of their anticancer and antimicrobial activities
- A study on the larvicidal and adulticidal potential of Cladostepus spongiosus macroalgae and green-fabricated silver nanoparticles against mosquito vectors
- Catalysts based on nickel salt heteropolytungstates for selective oxidation of diphenyl sulfide
- Powerful antibacterial nanocomposites from Corallina officinalis-mediated nanometals and chitosan nanoparticles against fish-borne pathogens
- Removal behavior of Zn and alkalis from blast furnace dust in pre-reduction sinter process
- Environmentally friendly synthesis and computational studies of novel class of acridinedione integrated spirothiopyrrolizidines/indolizidines
- The mechanisms of inhibition and lubrication of clean fracturing flowback fluids in water-based drilling fluids
- Adsorption/desorption performance of cellulose membrane for Pb(ii)
- A one-pot, multicomponent tandem synthesis of fused polycyclic pyrrolo[3,2-c]quinolinone/pyrrolizino[2,3-c]quinolinone hybrid heterocycles via environmentally benign solid state melt reaction
- Green synthesis of silver nanoparticles using durian rind extract and optical characteristics of surface plasmon resonance-based optical sensor for the detection of hydrogen peroxide
- Electrochemical analysis of copper-EDTA-ammonia-gold thiosulfate dissolution system
- Characterization of bio-oil production by microwave pyrolysis from cashew nut shells and Cassia fistula pods
- Green synthesis methods and characterization of bacterial cellulose/silver nanoparticle composites
- Photocatalytic research performance of zinc oxide/graphite phase carbon nitride catalyst and its application in environment
- Effect of phytogenic iron nanoparticles on the bio-fortification of wheat varieties
- In vitro anti-cancer and antimicrobial effects of manganese oxide nanoparticles synthesized using the Glycyrrhiza uralensis leaf extract on breast cancer cell lines
- Preparation of Pd/Ce(F)-MCM-48 catalysts and their catalytic performance of n-heptane isomerization
- Green “one-pot” fluorescent bis-indolizine synthesis with whole-cell plant biocatalysis
- Silica-titania mesoporous silicas of MCM-41 type as effective catalysts and photocatalysts for selective oxidation of diphenyl sulfide by H2O2
- Biosynthesis of zinc oxide nanoparticles from molted feathers of Pavo cristatus and their antibiofilm and anticancer activities
- Clean preparation of rutile from Ti-containing mixed molten slag by CO2 oxidation
- Synthesis and characterization of Pluronic F-127-coated titanium dioxide nanoparticles synthesized from extracts of Atractylodes macrocephala leaf for antioxidant, antimicrobial, and anticancer properties
- Effect of pretreatment with alkali on the anaerobic digestion characteristics of kitchen waste and analysis of microbial diversity
- Ameliorated antimicrobial, antioxidant, and anticancer properties by Plectranthus vettiveroides root extract-mediated green synthesis of chitosan nanoparticles
- Microwave-accelerated pretreatment technique in green extraction of oil and bioactive compounds from camelina seeds: Effectiveness and characterization
- Studies on the extraction performance of phorate by aptamer-functionalized magnetic nanoparticles in plasma samples
- Investigation of structural properties and antibacterial activity of AgO nanoparticle extract from Solanum nigrum/Mentha leaf extracts by green synthesis method
- Green fabrication of chitosan from marine crustaceans and mushroom waste: Toward sustainable resource utilization
- Synthesis, characterization, and evaluation of nanoparticles of clodinofop propargyl and fenoxaprop-P-ethyl on weed control, growth, and yield of wheat (Triticum aestivum L.)
- The enhanced adsorption properties of phosphorus from aqueous solutions using lanthanum modified synthetic zeolites
- Separation of graphene oxides of different sizes by multi-layer dialysis and anti-friction and lubrication performance
- Visible-light-assisted base-catalyzed, one-pot synthesis of highly functionalized cinnolines
- The experimental study on the air oxidation of 5-hydroxymethylfurfural to 2,5-furandicarboxylic acid with Co–Mn–Br system
- Highly efficient removal of tetracycline and methyl violet 2B from aqueous solution using the bimetallic FeZn-ZIFs catalyst
- A thermo-tolerant cellulase enzyme produced by Bacillus amyloliquefaciens M7, an insight into synthesis, optimization, characterization, and bio-polishing activity
- Exploration of ketone derivatives of succinimide for their antidiabetic potential: In vitro and in vivo approaches
- Ultrasound-assisted green synthesis and in silico study of 6-(4-(butylamino)-6-(diethylamino)-1,3,5-triazin-2-yl)oxypyridazine derivatives
- A study of the anticancer potential of Pluronic F-127 encapsulated Fe2O3 nanoparticles derived from Berberis vulgaris extract
- Biogenic synthesis of silver nanoparticles using Consolida orientalis flowers: Identification, catalytic degradation, and biological effect
- Initial assessment of the presence of plastic waste in some coastal mangrove forests in Vietnam
- Adsorption synergy electrocatalytic degradation of phenol by active oxygen-containing species generated in Co-coal based cathode and graphite anode
- Antibacterial, antifungal, antioxidant, and cytotoxicity activities of the aqueous extract of Syzygium aromaticum-mediated synthesized novel silver nanoparticles
- Synthesis of a silica matrix with ZnO nanoparticles for the fabrication of a recyclable photodegradation system to eliminate methylene blue dye
- Natural polymer fillers instead of dye and pigments: Pumice and scoria in PDMS fluid and elastomer composites
- Study on the preparation of glycerylphosphorylcholine by transesterification under supported sodium methoxide
- Wireless network handheld terminal-based green ecological sustainable design evaluation system: Improved data communication and reduced packet loss rate
- The optimization of hydrogel strength from cassava starch using oxidized sucrose as a crosslinking agent
- Green synthesis of silver nanoparticles using Saccharum officinarum leaf extract for antiviral paint
- Study on the reliability of nano-silver-coated tin solder joints for flip chips
- Environmentally sustainable analytical quality by design aided RP-HPLC method for the estimation of brilliant blue in commercial food samples employing a green-ultrasound-assisted extraction technique
- Anticancer and antimicrobial potential of zinc/sodium alginate/polyethylene glycol/d-pinitol nanocomposites against osteosarcoma MG-63 cells
- Nanoporous carbon@CoFe2O4 nanocomposite as a green absorbent for the adsorptive removal of Hg(ii) from aqueous solutions
- Characterization of silver sulfide nanoparticles from actinobacterial strain (M10A62) and its toxicity against lepidopteran and dipterans insect species
- Phyto-fabrication and characterization of silver nanoparticles using Withania somnifera: Investigating antioxidant potential
- Effect of e-waste nanofillers on the mechanical, thermal, and wear properties of epoxy-blend sisal woven fiber-reinforced composites
- Magnesium nanohydroxide (2D brucite) as a host matrix for thymol and carvacrol: Synthesis, characterization, and inhibition of foodborne pathogens
- Synergistic inhibitive effect of a hybrid zinc oxide-benzalkonium chloride composite on the corrosion of carbon steel in a sulfuric acidic solution
- Review Articles
- Role and the importance of green approach in biosynthesis of nanopropolis and effectiveness of propolis in the treatment of COVID-19 pandemic
- Gum tragacanth-mediated synthesis of metal nanoparticles, characterization, and their applications as a bactericide, catalyst, antioxidant, and peroxidase mimic
- Green-processed nano-biocomposite (ZnO–TiO2): Potential candidates for biomedical applications
- Reaction mechanisms in microwave-assisted lignin depolymerisation in hydrogen-donating solvents
- Recent progress on non-noble metal catalysts for the deoxydehydration of biomass-derived oxygenates
- Rapid Communication
- Phosphorus removal by iron–carbon microelectrolysis: A new way to achieve phosphorus recovery
- Special Issue: Biomolecules-derived synthesis of nanomaterials for environmental and biological applications (Guest Editors: Arpita Roy and Fernanda Maria Policarpo Tonelli)
- Biomolecules-derived synthesis of nanomaterials for environmental and biological applications
- Nano-encapsulated tanshinone IIA in PLGA-PEG-COOH inhibits apoptosis and inflammation in cerebral ischemia/reperfusion injury
- Green fabrication of silver nanoparticles using Melia azedarach ripened fruit extract, their characterization, and biological properties
- Green-synthesized nanoparticles and their therapeutic applications: A review
- Antioxidant, antibacterial, and cytotoxicity potential of synthesized silver nanoparticles from the Cassia alata leaf aqueous extract
- Green synthesis of silver nanoparticles using Callisia fragrans leaf extract and its anticancer activity against MCF-7, HepG2, KB, LU-1, and MKN-7 cell lines
- Algae-based green AgNPs, AuNPs, and FeNPs as potential nanoremediators
- Green synthesis of Kickxia elatine-induced silver nanoparticles and their role as anti-acetylcholinesterase in the treatment of Alzheimer’s disease
- Phytocrystallization of silver nanoparticles using Cassia alata flower extract for effective control of fungal skin pathogens
- Antibacterial wound dressing with hydrogel from chitosan and polyvinyl alcohol from the red cabbage extract loaded with silver nanoparticles
- Leveraging of mycogenic copper oxide nanostructures for disease management of Alternaria blight of Brassica juncea
- Nanoscale molecular reactions in microbiological medicines in modern medical applications
- Synthesis and characterization of ZnO/β-cyclodextrin/nicotinic acid nanocomposite and its biological and environmental application
- Green synthesis of silver nanoparticles via Taxus wallichiana Zucc. plant-derived Taxol: Novel utilization as anticancer, antioxidation, anti-inflammation, and antiurolithic potential
- Recyclability and catalytic characteristics of copper oxide nanoparticles derived from bougainvillea plant flower extract for biomedical application
- Phytofabrication, characterization, and evaluation of novel bioinspired selenium–iron (Se–Fe) nanocomposites using Allium sativum extract for bio-potential applications
- Erratum
- Erratum to “Synthesis, characterization, and evaluation of nanoparticles of clodinofop propargyl and fenoxaprop-P-ethyl on weed control, growth, and yield of wheat (Triticum aestivum L.)”


