Abstract
The micron-scale diamond film was prepared using hydrogen and methane as the mixed gas supplies via self-developed 3 kW/2,450 MHz microwave plasma chemical vapor deposition (MPCVD) equipment. On this basis, the evolution of the surface morphology, hydrophobicity, and electrical properties of samples under different hydrogen plasma etching times was investigated. The results indicate that the crystal edge and the top of the diamond grain were preferentially etched when etching time is less than 30 min. The surface roughness reduced from 0.217 to 0.205 μm, and the resistance value decreases from 3.17 to 0.35 MΩ. However, as the etch time increases to 120 min, the etching depth increases, and the surface roughness was increased. Simultaneously, the contact angles increased from 62.8° to 95.9°, which indicates that the surface of the diamond films exhibits more pronounced hydrophobicity. The treatment time of hydrogen plasma has no significant effect on the resistance value in the range of 0.26–0.50 MΩ. The mechanism of surface etching by hydrogen plasma was also discussed.
1 Introduction
The diamond is a crystalline material composed of a carbon atom. It belongs to the equiaxial crystal system, and the crystal structure is face-centered cubic. Diamond’s unique crystal structure determines its excellent physical and chemical properties [1]. Since natural diamonds come in a wide variety of shapes, it is not easy to apply them in practical applications. The excellent properties of chemical vapor deposition (CVD) diamond films are similar to natural diamond such as high thermal conductivity, high stability, and superhardness. Therefore, the CVD diamond has been studied widely in recent years [2,3,4]. With the further development of diamond technology in the field of optics, electricity, and acoustics, the requirements for surface morphology and flatness control will be more stringent [5,6,7]. In addition to surface flatness and damage control techniques [8], the diamond surface bond state also determines its practical application. At the same time, surface wettability is directly related to the application area of diamond film. For example, biological cells require hydrophilic surfaces for adhesion and growth; conversely, diamond coatings used to protect equipment from chemical attacks require hydrophobic films [9]. Moreover, if the high resistance problem of the diamond film can be improved, it can also be better developed in the semiconductor field.
Currently, mechanical polishing and plasma treatment techniques are mainly used to control the diamond surface. The mechanical polishing process is inefficient and prone to processing damage [10]. Up to now, plasma etching is the primary and most effective method for diamond surface morphology and bond state modulation. Plasma etching technique is to precisely control the sample by sputtering, reactive ions, high-density plasma, etc., in the presence of ions. The advantages of this method are fast reaction rate, low pollution, readily adjustable auxiliary gas, and process parameters. However, this method also has certain disadvantages, such as preferential etching of grain boundaries of diamond films, plasma etching rate is slow, and etched surface flatness is difficult to control, etc. [11]. The diamond can form a carbon–hydrogen bonding state on the surface after hydrogen plasma treatment. This bond state enables the diamond with a p-type conductive. Studies show that diamond surface carbon–hydrogen bonding state has high conductivity, stability, and corresponding device performance [12,13,14,15,16,17,18,19]. However, the surface of a diamond becomes rough due to excessive plasma etching time in the hydrogen terminal formation process, which severely affects the performance of hydrogen terminal diamond and its related electronic devices. As a result, controlling the flatness of the diamond surface and the carbon–hydrogen bonding state is particularly important for hydrogen terminal diamond applications.
In this article, the effect of different hydrogen plasma etching times on the micron-scale diamond film is studied. The surface morphology and roughness of diamond films are characterized by a scanning electron microscope (SEM) and an atomic force microscope (AFM). The effect of hydrogen plasma etching on the wettability of diamond surface is investigated by angle contact measuring device. Finally, the effects of etching on the diamond surface conductivity are characterized by four-probe resistance tester.
2 Experimental section
2.1 Preparation of high-quality micron-scale diamond film
The single-sided polished silicon wafer (2.54 cm × 2.54 cm, 100-oriented, p-type) was used as a starting substrate. Before the preparation of diamond, the silicon substrate was first mechanically polished and sonicated. The surface of the substrate creates a uniform scratch by pretreatment. These scratches will provide growth spots for polycrystalline diamond growth. Pretreatment can not only shorten the nucleation time but also effectively improve the nucleation density of diamond film [20]. The first step of diamond growth is the nucleation process, which directly affects the quality of diamond. Therefore, in addition to substrate type and pretreatment method, nucleation parameters are also very important. Adopt self-developed 3 kW/2,450 MHz microwave plasma chemical vapor deposition (MPCVD) device. The nucleation was performed for 1 h with a microwave power of 1,700 W, a hydrogen flow rate of 400 sccm, a methane flow rate of 12 sccm, a gas pressure of 12 kPa, and a temperature of 700°C.
Diamond nucleation lays a good foundation for the subsequent growth of high-quality diamond film, while the growth of diamond film is more important. Figure 1 shows the process of the growth of polycrystalline diamond, the grains gradually grow up and contact with each other, and grains were changed from transverse to longitudinal growth [21]. The growth parameters are as follows: microwave power is 2,200 W, methane/hydrogen ratio is 2.4%, gas pressure is 14 kPa, and diamond is grown at 850°C for 4 h. The thickness of the grown micron diamond film is about 6–10 µm.

Schematic diagram of diamond grain growth.
2.2 Hydrogen plasma etching
The surface of the micron-scale diamond film has a certain roughness. Diamond films were etched with hydrogen plasma to make the (111) orientation surface flat [22,23], and Figure 2 shows the planarization schematic. The diamond films were treated with hydrogen plasma. Etching is performed in an MPCVD apparatus. The following parameters were utilized for etching: a microwave power of 2,000 W, a hydrogen flow rate of 200 sccm, a gas pressure of 10 kPa, and a temperature of 700°C. Five bunches of etching tests beneath diverse time were carried out. The etching time is 0, 30, 60, 90, and 120 min, respectively.

Plasma etching diamond film flattening process schematic.
3 Results and discussion
3.1 Surface morphology
SEM of the prepared diamond films is shown in Figure 3. The micron-sized diamond films were prepared under the aforementioned process conditions: fast growth rate, good homogeneity, and high quality. It has a typical octahedral morphology and grain (111) preferred orientation. It demonstrates that the crystalline grains that have formed on the surface of the diamond are compact and homogeneous. The growth rate is about 1.706 μm‧h−1, and purity of the diamond phase is about 85.7% [24].
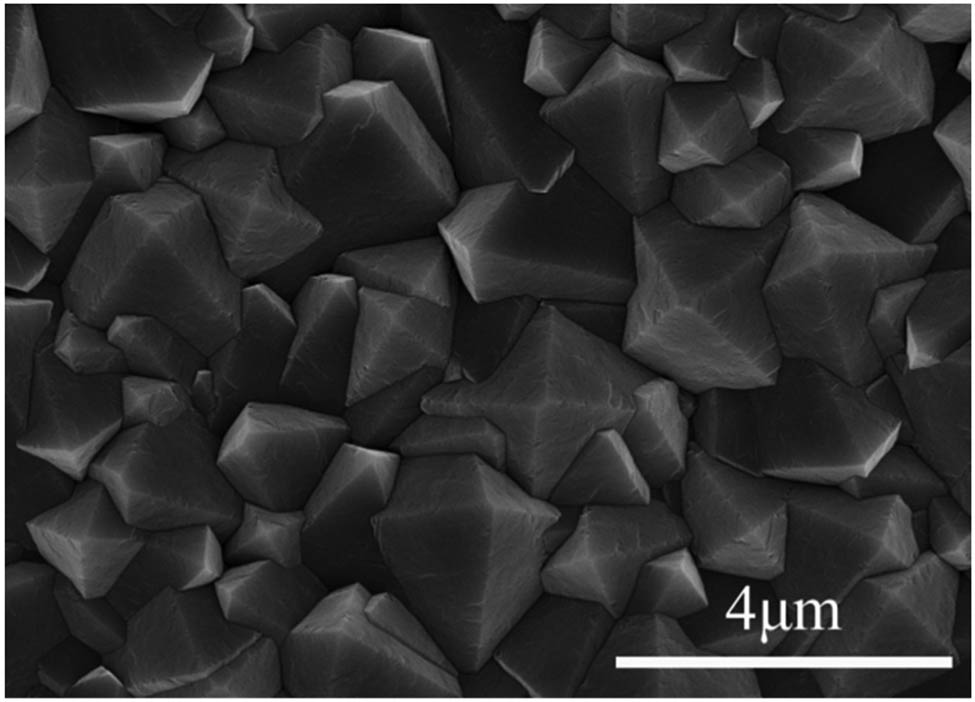
SEM morphology of micron-scale diamond film surface.
The SEM images of the diamond films before and after etching are displayed in Figure 4. The crystalline grain of the initial CVD diamond films had no visible flaws. The crystalline grains are complete and uniform (Figure 4a). After 30 min of etching, the crystalline surface has a significant number of pits and steps (Figure 4b). The grain edges become blurry, and the grain boundaries became more evident. This kind of phenomenon can be due to adjacent position, which was more susceptible to etching. The diamond big grains collapse into tiny grains, and the graphite phase is etched by hydrogen plasma at the grain boundaries. Figure 4c shows that as the etching duration increases to 60 min, the number of etched pits on diamond grain surface increases and grain boundary deepens, the grain outline becomes more blurred, and the grain edges are more seriously etched than at 30 min of etching. When the etching time is prolonged further, the etching speed of the border of the grain increases significantly. Simultaneously, the edge of the crystal’s etching is deeper, as shown in Figure 4d. When the etching time is up to 120 min, the etching is more severe at the grain boundaries, and the grain collapse tends to be flat (Figure 4e).

SEM images of samples etched at various times: (a) 0, (b) 30 min, (c) 60 min, (d) 90 min, and (e) 120 min.
Electrons are known to be easily emitted from the crystal edge. The electric field increases as the number of electrons at the crystal edge rises. Therefore, the ions are not attracted to bombard the edge of the crystal because the moving electrons are suppressed to the surface of the film [25,26]. As a consequence, the crystal edge and top of the grain are preferentially etched during the early stages of hydrogen plasma treatment [27]. The non-diamond phases at the diamond grains and grain boundaries are etched by hydrogen plasma, and the depth of grain boundaries increases significantly. At this point, it is also fastest collapse of the diamond grains. Etching becomes more concentrated on the grain boundary as the etching duration increases. Although the degree of edge etching is still increasing significantly and further collapse of grains has occurred, the morphological changes at this point are less than those at the early stage of the etching. This is due to the hydrogen plasma’s etching rate on graphite phase, which is approximately 50 times higher than that on diamond phases. So, graphite phase in the early stage of the etching was removed. The formation of CH and C2 groups during etching proves that hydrogen plasma interacts with C atoms on the diamond surface. The reaction kinetics during etching proved faster etching at diamond defects [28].
3.2 Surface roughness
As is shown in Figure 5, when the etching time is 0, 30, 60, 90, and 120 min, the surface roughness of the samples in the 8 μm × 8 μm test range is 0.217, 0.205, 0.239, 0.256, and 0.316 μm, respectively. Therefore, the roughness of the original micron diamond film is 0.217 μm (Figure 5a). After 30 min of hydrogen plasma etching, the roughness is reduced to 0.205 μm (Figure 5b). The diamond grain edges and top are severely etched. With the grain collapse into small island-shaped grains, the crystalline grains flatten somewhat. The etching speed of the top of the grain and the edge of the crystal is faster than the etching speed of the grain boundary, and it is preferentially etched in steps along the surface of the grain. Therefore, the change in grain etching effect results in the lowest surface roughness at this time. When the etching time further extends to 60 min, surface roughness of the sample is increased to 0.239 μm (Figure 5c). Although the grain boundary continues to deepen, the collapse rate of diamond grains is much lower than that in the early etching stage. With the prolongation of etching time, there is an increase in the number of etching pits. Therefore, the surface roughness of samples has increased. When the etching time is extended 90 min, increase of etching pits on diamond surface and increase of step-like pits on grain edges. At the same time, the grain boundaries continue to deepen. The roughness increases to 0.256 μm (Figure 5d). When the etching time reaches 120 min, the largest roughness of the sample is about 0.316 μm (Figure 5e). The later stages of etching mainly show an increase in the number of craters on the grain surface, a continuous deepening of grain boundary etching, and an increase in the degree of step-like etching on the grain edges. The etching forms etching pits at defects such as dislocations, and the anisotropic effect of grain etching also comes out gradually.

AFM images of samples etched at various times: (a) 0, (b) 15 min, (c) 20 min, (d) 60 min, (e) 90 min, and (f) 120 min.
The relationship between sample roughness and etching time shows that plasma etching increases the surface roughness of the sample. As the etching time increases, the surface roughness decreases from 0.217 μm at first to 0.205 μm and finally continues to rise to 0.316 μm. Overall, first it showed a decreasing trend and then increased. This is consistent with SEM results.
3.3 Wettability and surface resistances
Angle contact measurement equipment was used to analyze the wettability of diamond samples before and after etching, which is illustrated in Figure 6. The contact angle of an unetched diamond sample with water is approximately 62.8°, as shown in Figure 6a, which reflected a marked hydrophilic character. Under these process conditions, a large amount of C–O exists on the surface of the prepared micron-scale diamond film. The oxygen terminal of diamond film shows hydrophilicity. Thus, surface bonds are the main cause of hydrophilicity. When the etch time increases to 30 min, the contact angle increases to 71.2° (Figure 6b). A part of the C–O bond on the surface of diamond film changes to C–H bond, which increases the contact angle of diamond film. Diamond film surface still behaves hydrophilically. As etching time increases to 60 min, the contact angle was increased again to 82.6° (Figure 6c). The contact angle has been obviously increased compared with that before etching. As shown in Figure 6d, as the etching time increases to 90 min, the contact angle increases to 88°. Although the contact angle rises, it remains less than 90°, indicating that the diamond film surface is still hydrophilic. After etching for 120 min, the contact angle finally reaches 95.9° (Figure 6e). At this point, the surface of diamond film is hydrophobic. The hydrophilicity and hydrophobicity of the diamond film are mainly affected by the hydrogen and oxygen terminal functional groups. When the surface of diamond is oxygen terminal, the wettability appears as hydrophilic. The strong interaction between the water molecules and the terminal oxygen leads to the formation of new chemical bonds on the surface of an atom. When the surface of diamond is hydrogen terminal, it usually shows hydrophobicity. The degree of hydrophobicity is closely related to the type and number of chemical bonds on the surface.

Diamond film contact angle test results: (a) 0, (b) 30 min, (c) 60 min, (d) 90 min, (e) 120 min, and (f) contact angle variation curve with time.
The different bond states of carbon atoms make their surface terminal states different. After etching by hydrogen plasma, the surface of diamond film is damaged. The formation of C–H bond gradually replaces C–O bond, and C–O bond shows strong dipolar-polarization effects [29]. Finally, the conversion of oxygen terminal to hydrogen terminal is realized. A solid surface’s wettability is primarily governed by two factors: chemical composition and roughness [30]. Hence, we increase the contact angle with increasing etching time. The surface of the diamond film was turned from hydrophilic to hydrophobic.
As shown in Figure 7, the resistivity of diamond was measured by a four-probe tester. The surface resistance of the micron-scale diamond film without hydrogen plasma etching is 3.71 MΩ. As is known to all, the diamond itself is an insulating material because of the high resistance nature. High resistance greatly limited being applications in the field of semiconductive electronic device. Nevertheless, after etching, the evolution of resistance is significantly changed. As the etch time increases to 30 min, the resistance value becomes 0.35 MΩ. This is consistent with the study by Liu [31]. The decreased surface roughness in diamond films leads to enhancement of carrier–carrier scattering effect. Thus, the carrier mobility is considerably increased and the resistance decreased obviously for diamond films. This is in a good agreement with our AFM results. This means that the hydrogen terminal is gradually forming on the diamond surface. This is consistent with the contact angle results. The square resistance did not change significantly for etching times between 30 and 120 min. Its value fluctuates at 0.26–0.50 MΩ.

Micron-scale diamond film square resistance value with hydrogen plasma etching time.
The insulating diamond acquires conductive properties on the basis of hydrogen plasma treatment. The hydrogen terminal structure is formed on the surface. Combined with the analysis of wettability and surface resistance, the hydrogen-terminated diamond film after hydrogen plasma etching, the surface of which is hydrophobic, and the hydrogen-terminated surface producing dipoles, the energy required for electrons to be pulled out is reduced, and diamond film surface with negative electron affinity can interact with external receptors. The surface of diamond material loses electrons and forms P-type semiconductor hole orbits [32]. The surface resistance is reduced, and the conductivity is enhanced. Therefore, less than 30 min of etching time leads to an increase in carrier mobility. At this moment, the hydrogen terminal diamond can be realized semiconducting without ion implantation or doping.
4 Conclusion
In this article, the effects of hydrogen plasma treatment on the surface morphology and properties of micron diamond were characterized by SEM, AFM, contact angle tester, and four-probe resistance tester. The following conclusions are obtained.
In the prophase of etching, the electric field increases as the number of electrons at the crystal edge grows. The motion of electrons toward the surface of diamond films in the plasma is suppressed, and ions are attracted to bombard the edges of the crystal. Therefore, the crystal edge and the top of the grain are preferentially etched, and the surface roughness reduced. But when the etching duration exceeds 30 min, etching is mainly concentrated on the grain boundary. The outline of the grains became blurry, and the crystalline surface has a significant number of pits and steps. At the same time, the roughness slowly increases.
The contact angle also increases from 62.8° to 95.9° with the increase in etching time. The surface of the diamond film was turned from hydrophilic to hydrophobic. This is because the surface of the diamond film is destroyed following etching, and the formation of C–H bond gradually replaces C–O bond. Finally, the conversion of oxygen terminal to hydrogen terminal is realized.
The reduction in surface roughness of the diamond film 30 min before etching leads to the enhancement of carrier scattering effect. As a result, the carrier mobility of the diamond film is greatly increased, increases the H on the diamond film surface, slightly decreases the wettability, increases the surface roughness, and decreases the surface resistance to 0.35 MΩ. However, the resistance of the diamond film does not change significantly by extending the etching time and remains at 0.26–0.50 MΩ. Therefore, the factors affecting the surface resistance are multiple and need to be considered in a comprehensive manner.
-
Funding information: This research was funded by the National Natural Science Foundation of China (No. 51864028), Yunnan Province Science and Technology Major Project for Materials Genetic Engineering of Rare and Precious Metal (No. 202002AB080001), Yunnan Province Funds for Distinguished Young Scientists (No. 2019FJ005), and Science Research Foundation of Yunnan Provincial Education Department (No. 2022J0441).
-
Author contributions: Genjie Chu: writing – original draft, writing – review and editing, formal analysis; Sijia Li: writing – original draft, formal analysis; Jiyun Gao: visualization, project administration; Li Yang: supervision, conceptualization, writing – reviewing and editing; Ming Hou: methodology, formal analysis; Shenghui Guo: resources.
-
Conflict of interest: Authors state no conflict of interest.
-
Data availability statement: All data generated or analyzed during this study are included in this published article.
References
[1] Srikanth VS. Review of advances in diamond thin film synthesis. Proc Inst Mech Eng C J Mech Eng Sci. 2012;226(2):303–18.10.1177/0954406211422788Search in Google Scholar
[2] Shen XT, Wang XC, Sun FH, Ding CY. Sandblasting pretreatment for deposition of diamond films on WC-Co hard metal substrates. Diam Relat Mater. 2017;73:7–14.10.1016/j.diamond.2016.10.025Search in Google Scholar
[3] Li X, Ye JS, Zhang HC, Feng T, Chen JQ, Hu XJ. Sand blasting induced stress release and enhanced adhesion strength of diamond films deposited on austenite stainless steel. Appl Surf Sci. 2017;412:366–73.10.1016/j.apsusc.2017.03.214Search in Google Scholar
[4] Wang XC, Wang CC, Shen XT, Sun FH. Tribological properties of diamond films for high-speed drawing Al alloy wires using water-based emulsions. Tribol Int. 2018;123:92–104.10.1016/j.triboint.2018.03.004Search in Google Scholar
[5] Faycal HL, Rafik SS. SAW devices: Review of numerical-experimental studies and recent applications. Sens Actuator A Phys. 2019;292(6):169–97.10.1016/j.sna.2019.03.037Search in Google Scholar
[6] Castelletto S, Rosa L, Blackledge J, Abri MZ, Boretti A. Advances in diamond nanofabrication for ultrasensitive devices. Microsyst Nanoeng. 2017;3(10):1–16.10.1038/micronano.2017.61Search in Google Scholar PubMed PubMed Central
[7] Lee JC, Magyar AP, Bracher DO, Aharonovich L, Hu EL. Fabrication of thin diamond membranes for photonic applications. Diam Relat Mater. 2013;33(3):45–8.10.1016/j.diamond.2012.12.008Search in Google Scholar
[8] Wort CJH, Balmer RS. Diamond as an electronic material. Mater Today. 2008;11(1–2):22–8.10.1016/S1369-7021(07)70349-8Search in Google Scholar
[9] Montaño-Figueroa AG, Alcantar-Peña JJ, Tirado P, Abraham A, Obaldia ED, Auciello O. Tailoring of polycrystalline diamond surfaces from hydrophilic to superhydrophobic via synergistic chemical plus micro-structuring processes. Carbon. 2018;139:361–8.10.1016/j.carbon.2018.06.062Search in Google Scholar
[10] Zheng YT, Ye HT, Thornton R, Knott T, Ochalski TJ, Wang J, et al. Subsurface cleavage of diamond after high-speed three-dimensional dynamic friction polishing. Diam Relat Mater. 2020;101:107600.10.1016/j.diamond.2019.107600Search in Google Scholar
[11] Toros A, Kiss M, Graziosi T, Mi S, Berrazouane R, Naamoun M. Reactive ion etching of single crystal diamond by inductively coupled plasma: State of the art and catalog of recipes. Diam Relat Mater. 2020;108:107839.10.1016/j.diamond.2020.107839Search in Google Scholar
[12] Sque SJ, Jones R, Öberg S. Transfer doping of diamond: Buckminsterfullerene on hydrogenated, hydroxylated, and oxygenated diamond surfaces. J Mater Sci Mater Electron. 2006;17:459–65.10.1007/s10854-006-8092-9Search in Google Scholar
[13] Jiang CY, Guo SH, Gao JY, Hu T, Yang L, Peng JH, et al. Optimization of growth parameters for diamond films grown by MPCVD using response surface methodology. Arab J Sci Eng. 2016;41:2671–80.10.1007/s13369-016-2169-4Search in Google Scholar
[14] Sachin B, Narendranath S, Chakradhar D. Application of desirability approach to optimize the control factors in cryogenic diamond burnishing. Arab J Sci Eng. 2020;45:1305–17.10.1007/s13369-019-04326-3Search in Google Scholar
[15] Yamanaka S, Ishikawa K, Mizuochi N. Structural change in diamond by hydrogen plasma treatment at room temperature. Diam Relat Mater. 2005;14:6095–9.10.1016/j.diamond.2005.09.011Search in Google Scholar
[16] Verona C, Arciprete F, Foffi M. Influence of surface crystal-orientation on transfer doping of V2O5/H-terminated diamond. Appl Phys Lett. 2018;112:181602.10.1063/1.5027198Search in Google Scholar
[17] Ren ZY, Lv DD, Xu JM. High temperature (300°C) ALD grown Al2O3 on hydrogen terminated diamond: Band offset and electrical properties of the MOSFETs. Appl Phys Lett. 2020;116:013503.10.1063/1.5126359Search in Google Scholar
[18] Inaba M, Kawarada H, Ohno Y. Electrical property measurement of two-dimensional hole-gas layer on hydrogen-terminated diamond surface in vacuum-gap-gate structure. Appl Phys Lett. 2019;114:253504.10.1063/1.5099395Search in Google Scholar
[19] Kasu M, Ueda K, Yamauchi Y. Diamond-based RF power transistors: Fundamentals and applications. Diam Relat Mater. 2007;16(1):1010–5.10.1016/j.diamond.2006.12.046Search in Google Scholar
[20] Huang JL, Wang JH. Nucleation study of CVD diamond films. Mater Direct. 2007;2:312–5.Search in Google Scholar
[21] Zhu HX, Mao WM, Feng HP, Lv RX, Vlasov II. Effect of methane concentration on the crystallographic growth process of CVD diamond films. J Inorg Mater. 2007;3:570–6.Search in Google Scholar
[22] Song J. Preparation and optical properties of gold nanoparticles/diamond composite structures. Changchun: Journal of Jilin University; 2015.Search in Google Scholar
[23] Jacek J. Densely packed tetrahedra clusters displaying diamond-like superstructures. Particuology. 2021;58:147–52.10.1016/j.partic.2021.03.006Search in Google Scholar
[24] Feng SG. Preparation of diamond film by MPCVD method and its surface treatment process. Kunming: Kunming University of Science and Technology; 2021.Search in Google Scholar
[25] Pan X, Ma ZB, Li GW. Mechanism of etching CVD diamond film by oxygen plasma. High Power Laser Part Beams. 2014;26(7):243–7.10.3788/HPLPB20142607.74001Search in Google Scholar
[26] Wu J, Ma Z, Shen W. Effect of nitrogen in CVD diamond on plasma etching. Acta Phys Sin. 2013;62(7):262–8.10.7498/aps.62.075202Search in Google Scholar
[27] Hayashi K, Yamanaka S, Okushi H, Kajimura K. Stepped growth and etching of (001) diamond. Diam Relat Mater. 1996;5(9):1002–5.10.1016/0925-9635(95)00470-XSearch in Google Scholar
[28] Yurov V, Bushuev E, Bolshakov A, Ashkinazi E. Etching kinetics of (100) single crystal diamond surfaces in a hydrogen microwave plasma, studied with in situ low-coherence interferometry. Phys Status Solidi A. 2017;214(11):1700177.10.1002/pssa.201700177Search in Google Scholar
[29] Maier F, Ristein J, Ley L. Electron affinity of plasma-hydrogenated and chemically oxidized diamond (100) surfaces. Phys Rev B. 2001;64(16):165411.10.1103/PhysRevB.64.165411Search in Google Scholar
[30] Balu B, Breedveld V, Hess DW. Fabrication of “Roll-off” and “Sticky” superhydrophobic cellulose surfaces via plasma processing. Langmuir. 2008;24(9):4785–90.10.1021/la703766cSearch in Google Scholar PubMed
[31] Liu JL. Study on the Conductive Properties of Hydrogen-Terminated Self-Supported Diamond Film Surface and its Device Development. Beijing: University of Science and Technology Beijing; 2013.Search in Google Scholar
[32] Gei MW, Wade TC, Wuorio CH. Progress toward diamond power field-effect transistors. Phys Status Solidi (A) Appl Mater Sci. 2018;215(22):SI.10.1002/pssa.201800681Search in Google Scholar
© 2023 the author(s), published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Research Articles
- Value-added utilization of coal fly ash and recycled polyvinyl chloride in door or window sub-frame composites
- High removal efficiency of volatile phenol from coking wastewater using coal gasification slag via optimized adsorption and multi-grade batch process
- Evolution of surface morphology and properties of diamond films by hydrogen plasma etching
- Removal efficiency of dibenzofuran using CuZn-zeolitic imidazole frameworks as a catalyst and adsorbent
- Rapid and efficient microwave-assisted extraction of Caesalpinia sappan Linn. heartwood and subsequent synthesis of gold nanoparticles
- The catalytic characteristics of 2-methylnaphthalene acylation with AlCl3 immobilized on Hβ as Lewis acid catalyst
- Biodegradation of synthetic PVP biofilms using natural materials and nanoparticles
- Rutin-loaded selenium nanoparticles modulated the redox status, inflammatory, and apoptotic pathways associated with pentylenetetrazole-induced epilepsy in mice
- Optimization of apigenin nanoparticles prepared by planetary ball milling: In vitro and in vivo studies
- Synthesis and characterization of silver nanoparticles using Origanum onites leaves: Cytotoxic, apoptotic, and necrotic effects on Capan-1, L929, and Caco-2 cell lines
- Exergy analysis of a conceptual CO2 capture process with an amine-based DES
- Construction of fluorescence system of felodipine–tetracyanovinyl–2,2′-bipyridine complex
- Excellent photocatalytic degradation of rhodamine B over Bi2O3 supported on Zn-MOF nanocomposites under visible light
- Optimization-based control strategy for a large-scale polyhydroxyalkanoates production in a fed-batch bioreactor using a coupled PDE–ODE system
- Effectiveness of pH and amount of Artemia urumiana extract on physical, chemical, and biological attributes of UV-fabricated biogold nanoparticles
- Geranium leaf-mediated synthesis of silver nanoparticles and their transcriptomic effects on Candida albicans
- Synthesis, characterization, anticancer, anti-inflammatory activities, and docking studies of 3,5-disubstituted thiadiazine-2-thiones
- Synthesis and stability of phospholipid-encapsulated nano-selenium
- Putative anti-proliferative effect of Indian mustard (Brassica juncea) seed and its nano-formulation
- Enrichment of low-grade phosphorites by the selective leaching method
- Electrochemical analysis of the dissolution of gold in a copper–ethylenediamine–thiosulfate system
- Characterisation of carbonate lake sediments as a potential filler for polymer composites
- Evaluation of nano-selenium biofortification characteristics of alfalfa (Medicago sativa L.)
- Quality of oil extracted by cold press from Nigella sativa seeds incorporated with rosemary extracts and pretreated by microwaves
- Heteropolyacid-loaded MOF-derived mesoporous zirconia catalyst for chemical degradation of rhodamine B
- Recovery of critical metals from carbonatite-type mineral wastes: Geochemical modeling investigation of (bio)hydrometallurgical leaching of REEs
- Photocatalytic properties of ZnFe-mixed oxides synthesized via a simple route for water remediation
- Attenuation of di(2-ethylhexyl)phthalate-induced hepatic and renal toxicity by naringin nanoparticles in a rat model
- Novel in situ synthesis of quaternary core–shell metallic sulfide nanocomposites for degradation of organic dyes and hydrogen production
- Microfluidic steam-based synthesis of luminescent carbon quantum dots as sensing probes for nitrite detection
- Transformation of eggshell waste to egg white protein solution, calcium chloride dihydrate, and eggshell membrane powder
- Preparation of Zr-MOFs for the adsorption of doxycycline hydrochloride from wastewater
- Green nanoarchitectonics of the silver nanocrystal potential for treating malaria and their cytotoxic effects on the kidney Vero cell line
- Carbon emissions analysis of producing modified asphalt with natural asphalt
- An efficient and green synthesis of 2-phenylquinazolin-4(3H)-ones via t-BuONa-mediated oxidative condensation of 2-aminobenzamides and benzyl alcohols under solvent- and transition metal-free conditions
- Chitosan nanoparticles loaded with mesosulfuron methyl and mesosulfuron methyl + florasulam + MCPA isooctyl to manage weeds of wheat (Triticum aestivum L.)
- Synergism between lignite and high-sulfur petroleum coke in CO2 gasification
- Facile aqueous synthesis of ZnCuInS/ZnS–ZnS QDs with enhanced photoluminescence lifetime for selective detection of Cu(ii) ions
- Rapid synthesis of copper nanoparticles using Nepeta cataria leaves: An eco-friendly management of disease-causing vectors and bacterial pathogens
- Study on the photoelectrocatalytic activity of reduced TiO2 nanotube films for removal of methyl orange
- Development of a fuzzy logic model for the prediction of spark-ignition engine performance and emission for gasoline–ethanol blends
- Micro-impact-induced mechano-chemical synthesis of organic precursors from FeC/FeN and carbonates/nitrates in water and its extension to nucleobases
- Green synthesis of strontium-doped tin dioxide (SrSnO2) nanoparticles using the Mahonia bealei leaf extract and evaluation of their anticancer and antimicrobial activities
- A study on the larvicidal and adulticidal potential of Cladostepus spongiosus macroalgae and green-fabricated silver nanoparticles against mosquito vectors
- Catalysts based on nickel salt heteropolytungstates for selective oxidation of diphenyl sulfide
- Powerful antibacterial nanocomposites from Corallina officinalis-mediated nanometals and chitosan nanoparticles against fish-borne pathogens
- Removal behavior of Zn and alkalis from blast furnace dust in pre-reduction sinter process
- Environmentally friendly synthesis and computational studies of novel class of acridinedione integrated spirothiopyrrolizidines/indolizidines
- The mechanisms of inhibition and lubrication of clean fracturing flowback fluids in water-based drilling fluids
- Adsorption/desorption performance of cellulose membrane for Pb(ii)
- A one-pot, multicomponent tandem synthesis of fused polycyclic pyrrolo[3,2-c]quinolinone/pyrrolizino[2,3-c]quinolinone hybrid heterocycles via environmentally benign solid state melt reaction
- Green synthesis of silver nanoparticles using durian rind extract and optical characteristics of surface plasmon resonance-based optical sensor for the detection of hydrogen peroxide
- Electrochemical analysis of copper-EDTA-ammonia-gold thiosulfate dissolution system
- Characterization of bio-oil production by microwave pyrolysis from cashew nut shells and Cassia fistula pods
- Green synthesis methods and characterization of bacterial cellulose/silver nanoparticle composites
- Photocatalytic research performance of zinc oxide/graphite phase carbon nitride catalyst and its application in environment
- Effect of phytogenic iron nanoparticles on the bio-fortification of wheat varieties
- In vitro anti-cancer and antimicrobial effects of manganese oxide nanoparticles synthesized using the Glycyrrhiza uralensis leaf extract on breast cancer cell lines
- Preparation of Pd/Ce(F)-MCM-48 catalysts and their catalytic performance of n-heptane isomerization
- Green “one-pot” fluorescent bis-indolizine synthesis with whole-cell plant biocatalysis
- Silica-titania mesoporous silicas of MCM-41 type as effective catalysts and photocatalysts for selective oxidation of diphenyl sulfide by H2O2
- Biosynthesis of zinc oxide nanoparticles from molted feathers of Pavo cristatus and their antibiofilm and anticancer activities
- Clean preparation of rutile from Ti-containing mixed molten slag by CO2 oxidation
- Synthesis and characterization of Pluronic F-127-coated titanium dioxide nanoparticles synthesized from extracts of Atractylodes macrocephala leaf for antioxidant, antimicrobial, and anticancer properties
- Effect of pretreatment with alkali on the anaerobic digestion characteristics of kitchen waste and analysis of microbial diversity
- Ameliorated antimicrobial, antioxidant, and anticancer properties by Plectranthus vettiveroides root extract-mediated green synthesis of chitosan nanoparticles
- Microwave-accelerated pretreatment technique in green extraction of oil and bioactive compounds from camelina seeds: Effectiveness and characterization
- Studies on the extraction performance of phorate by aptamer-functionalized magnetic nanoparticles in plasma samples
- Investigation of structural properties and antibacterial activity of AgO nanoparticle extract from Solanum nigrum/Mentha leaf extracts by green synthesis method
- Green fabrication of chitosan from marine crustaceans and mushroom waste: Toward sustainable resource utilization
- Synthesis, characterization, and evaluation of nanoparticles of clodinofop propargyl and fenoxaprop-P-ethyl on weed control, growth, and yield of wheat (Triticum aestivum L.)
- The enhanced adsorption properties of phosphorus from aqueous solutions using lanthanum modified synthetic zeolites
- Separation of graphene oxides of different sizes by multi-layer dialysis and anti-friction and lubrication performance
- Visible-light-assisted base-catalyzed, one-pot synthesis of highly functionalized cinnolines
- The experimental study on the air oxidation of 5-hydroxymethylfurfural to 2,5-furandicarboxylic acid with Co–Mn–Br system
- Highly efficient removal of tetracycline and methyl violet 2B from aqueous solution using the bimetallic FeZn-ZIFs catalyst
- A thermo-tolerant cellulase enzyme produced by Bacillus amyloliquefaciens M7, an insight into synthesis, optimization, characterization, and bio-polishing activity
- Exploration of ketone derivatives of succinimide for their antidiabetic potential: In vitro and in vivo approaches
- Ultrasound-assisted green synthesis and in silico study of 6-(4-(butylamino)-6-(diethylamino)-1,3,5-triazin-2-yl)oxypyridazine derivatives
- A study of the anticancer potential of Pluronic F-127 encapsulated Fe2O3 nanoparticles derived from Berberis vulgaris extract
- Biogenic synthesis of silver nanoparticles using Consolida orientalis flowers: Identification, catalytic degradation, and biological effect
- Initial assessment of the presence of plastic waste in some coastal mangrove forests in Vietnam
- Adsorption synergy electrocatalytic degradation of phenol by active oxygen-containing species generated in Co-coal based cathode and graphite anode
- Antibacterial, antifungal, antioxidant, and cytotoxicity activities of the aqueous extract of Syzygium aromaticum-mediated synthesized novel silver nanoparticles
- Synthesis of a silica matrix with ZnO nanoparticles for the fabrication of a recyclable photodegradation system to eliminate methylene blue dye
- Natural polymer fillers instead of dye and pigments: Pumice and scoria in PDMS fluid and elastomer composites
- Study on the preparation of glycerylphosphorylcholine by transesterification under supported sodium methoxide
- Wireless network handheld terminal-based green ecological sustainable design evaluation system: Improved data communication and reduced packet loss rate
- The optimization of hydrogel strength from cassava starch using oxidized sucrose as a crosslinking agent
- Green synthesis of silver nanoparticles using Saccharum officinarum leaf extract for antiviral paint
- Study on the reliability of nano-silver-coated tin solder joints for flip chips
- Environmentally sustainable analytical quality by design aided RP-HPLC method for the estimation of brilliant blue in commercial food samples employing a green-ultrasound-assisted extraction technique
- Anticancer and antimicrobial potential of zinc/sodium alginate/polyethylene glycol/d-pinitol nanocomposites against osteosarcoma MG-63 cells
- Nanoporous carbon@CoFe2O4 nanocomposite as a green absorbent for the adsorptive removal of Hg(ii) from aqueous solutions
- Characterization of silver sulfide nanoparticles from actinobacterial strain (M10A62) and its toxicity against lepidopteran and dipterans insect species
- Phyto-fabrication and characterization of silver nanoparticles using Withania somnifera: Investigating antioxidant potential
- Effect of e-waste nanofillers on the mechanical, thermal, and wear properties of epoxy-blend sisal woven fiber-reinforced composites
- Magnesium nanohydroxide (2D brucite) as a host matrix for thymol and carvacrol: Synthesis, characterization, and inhibition of foodborne pathogens
- Synergistic inhibitive effect of a hybrid zinc oxide-benzalkonium chloride composite on the corrosion of carbon steel in a sulfuric acidic solution
- Review Articles
- Role and the importance of green approach in biosynthesis of nanopropolis and effectiveness of propolis in the treatment of COVID-19 pandemic
- Gum tragacanth-mediated synthesis of metal nanoparticles, characterization, and their applications as a bactericide, catalyst, antioxidant, and peroxidase mimic
- Green-processed nano-biocomposite (ZnO–TiO2): Potential candidates for biomedical applications
- Reaction mechanisms in microwave-assisted lignin depolymerisation in hydrogen-donating solvents
- Recent progress on non-noble metal catalysts for the deoxydehydration of biomass-derived oxygenates
- Rapid Communication
- Phosphorus removal by iron–carbon microelectrolysis: A new way to achieve phosphorus recovery
- Special Issue: Biomolecules-derived synthesis of nanomaterials for environmental and biological applications (Guest Editors: Arpita Roy and Fernanda Maria Policarpo Tonelli)
- Biomolecules-derived synthesis of nanomaterials for environmental and biological applications
- Nano-encapsulated tanshinone IIA in PLGA-PEG-COOH inhibits apoptosis and inflammation in cerebral ischemia/reperfusion injury
- Green fabrication of silver nanoparticles using Melia azedarach ripened fruit extract, their characterization, and biological properties
- Green-synthesized nanoparticles and their therapeutic applications: A review
- Antioxidant, antibacterial, and cytotoxicity potential of synthesized silver nanoparticles from the Cassia alata leaf aqueous extract
- Green synthesis of silver nanoparticles using Callisia fragrans leaf extract and its anticancer activity against MCF-7, HepG2, KB, LU-1, and MKN-7 cell lines
- Algae-based green AgNPs, AuNPs, and FeNPs as potential nanoremediators
- Green synthesis of Kickxia elatine-induced silver nanoparticles and their role as anti-acetylcholinesterase in the treatment of Alzheimer’s disease
- Phytocrystallization of silver nanoparticles using Cassia alata flower extract for effective control of fungal skin pathogens
- Antibacterial wound dressing with hydrogel from chitosan and polyvinyl alcohol from the red cabbage extract loaded with silver nanoparticles
- Leveraging of mycogenic copper oxide nanostructures for disease management of Alternaria blight of Brassica juncea
- Nanoscale molecular reactions in microbiological medicines in modern medical applications
- Synthesis and characterization of ZnO/β-cyclodextrin/nicotinic acid nanocomposite and its biological and environmental application
- Green synthesis of silver nanoparticles via Taxus wallichiana Zucc. plant-derived Taxol: Novel utilization as anticancer, antioxidation, anti-inflammation, and antiurolithic potential
- Recyclability and catalytic characteristics of copper oxide nanoparticles derived from bougainvillea plant flower extract for biomedical application
- Phytofabrication, characterization, and evaluation of novel bioinspired selenium–iron (Se–Fe) nanocomposites using Allium sativum extract for bio-potential applications
- Erratum
- Erratum to “Synthesis, characterization, and evaluation of nanoparticles of clodinofop propargyl and fenoxaprop-P-ethyl on weed control, growth, and yield of wheat (Triticum aestivum L.)”
Articles in the same Issue
- Research Articles
- Value-added utilization of coal fly ash and recycled polyvinyl chloride in door or window sub-frame composites
- High removal efficiency of volatile phenol from coking wastewater using coal gasification slag via optimized adsorption and multi-grade batch process
- Evolution of surface morphology and properties of diamond films by hydrogen plasma etching
- Removal efficiency of dibenzofuran using CuZn-zeolitic imidazole frameworks as a catalyst and adsorbent
- Rapid and efficient microwave-assisted extraction of Caesalpinia sappan Linn. heartwood and subsequent synthesis of gold nanoparticles
- The catalytic characteristics of 2-methylnaphthalene acylation with AlCl3 immobilized on Hβ as Lewis acid catalyst
- Biodegradation of synthetic PVP biofilms using natural materials and nanoparticles
- Rutin-loaded selenium nanoparticles modulated the redox status, inflammatory, and apoptotic pathways associated with pentylenetetrazole-induced epilepsy in mice
- Optimization of apigenin nanoparticles prepared by planetary ball milling: In vitro and in vivo studies
- Synthesis and characterization of silver nanoparticles using Origanum onites leaves: Cytotoxic, apoptotic, and necrotic effects on Capan-1, L929, and Caco-2 cell lines
- Exergy analysis of a conceptual CO2 capture process with an amine-based DES
- Construction of fluorescence system of felodipine–tetracyanovinyl–2,2′-bipyridine complex
- Excellent photocatalytic degradation of rhodamine B over Bi2O3 supported on Zn-MOF nanocomposites under visible light
- Optimization-based control strategy for a large-scale polyhydroxyalkanoates production in a fed-batch bioreactor using a coupled PDE–ODE system
- Effectiveness of pH and amount of Artemia urumiana extract on physical, chemical, and biological attributes of UV-fabricated biogold nanoparticles
- Geranium leaf-mediated synthesis of silver nanoparticles and their transcriptomic effects on Candida albicans
- Synthesis, characterization, anticancer, anti-inflammatory activities, and docking studies of 3,5-disubstituted thiadiazine-2-thiones
- Synthesis and stability of phospholipid-encapsulated nano-selenium
- Putative anti-proliferative effect of Indian mustard (Brassica juncea) seed and its nano-formulation
- Enrichment of low-grade phosphorites by the selective leaching method
- Electrochemical analysis of the dissolution of gold in a copper–ethylenediamine–thiosulfate system
- Characterisation of carbonate lake sediments as a potential filler for polymer composites
- Evaluation of nano-selenium biofortification characteristics of alfalfa (Medicago sativa L.)
- Quality of oil extracted by cold press from Nigella sativa seeds incorporated with rosemary extracts and pretreated by microwaves
- Heteropolyacid-loaded MOF-derived mesoporous zirconia catalyst for chemical degradation of rhodamine B
- Recovery of critical metals from carbonatite-type mineral wastes: Geochemical modeling investigation of (bio)hydrometallurgical leaching of REEs
- Photocatalytic properties of ZnFe-mixed oxides synthesized via a simple route for water remediation
- Attenuation of di(2-ethylhexyl)phthalate-induced hepatic and renal toxicity by naringin nanoparticles in a rat model
- Novel in situ synthesis of quaternary core–shell metallic sulfide nanocomposites for degradation of organic dyes and hydrogen production
- Microfluidic steam-based synthesis of luminescent carbon quantum dots as sensing probes for nitrite detection
- Transformation of eggshell waste to egg white protein solution, calcium chloride dihydrate, and eggshell membrane powder
- Preparation of Zr-MOFs for the adsorption of doxycycline hydrochloride from wastewater
- Green nanoarchitectonics of the silver nanocrystal potential for treating malaria and their cytotoxic effects on the kidney Vero cell line
- Carbon emissions analysis of producing modified asphalt with natural asphalt
- An efficient and green synthesis of 2-phenylquinazolin-4(3H)-ones via t-BuONa-mediated oxidative condensation of 2-aminobenzamides and benzyl alcohols under solvent- and transition metal-free conditions
- Chitosan nanoparticles loaded with mesosulfuron methyl and mesosulfuron methyl + florasulam + MCPA isooctyl to manage weeds of wheat (Triticum aestivum L.)
- Synergism between lignite and high-sulfur petroleum coke in CO2 gasification
- Facile aqueous synthesis of ZnCuInS/ZnS–ZnS QDs with enhanced photoluminescence lifetime for selective detection of Cu(ii) ions
- Rapid synthesis of copper nanoparticles using Nepeta cataria leaves: An eco-friendly management of disease-causing vectors and bacterial pathogens
- Study on the photoelectrocatalytic activity of reduced TiO2 nanotube films for removal of methyl orange
- Development of a fuzzy logic model for the prediction of spark-ignition engine performance and emission for gasoline–ethanol blends
- Micro-impact-induced mechano-chemical synthesis of organic precursors from FeC/FeN and carbonates/nitrates in water and its extension to nucleobases
- Green synthesis of strontium-doped tin dioxide (SrSnO2) nanoparticles using the Mahonia bealei leaf extract and evaluation of their anticancer and antimicrobial activities
- A study on the larvicidal and adulticidal potential of Cladostepus spongiosus macroalgae and green-fabricated silver nanoparticles against mosquito vectors
- Catalysts based on nickel salt heteropolytungstates for selective oxidation of diphenyl sulfide
- Powerful antibacterial nanocomposites from Corallina officinalis-mediated nanometals and chitosan nanoparticles against fish-borne pathogens
- Removal behavior of Zn and alkalis from blast furnace dust in pre-reduction sinter process
- Environmentally friendly synthesis and computational studies of novel class of acridinedione integrated spirothiopyrrolizidines/indolizidines
- The mechanisms of inhibition and lubrication of clean fracturing flowback fluids in water-based drilling fluids
- Adsorption/desorption performance of cellulose membrane for Pb(ii)
- A one-pot, multicomponent tandem synthesis of fused polycyclic pyrrolo[3,2-c]quinolinone/pyrrolizino[2,3-c]quinolinone hybrid heterocycles via environmentally benign solid state melt reaction
- Green synthesis of silver nanoparticles using durian rind extract and optical characteristics of surface plasmon resonance-based optical sensor for the detection of hydrogen peroxide
- Electrochemical analysis of copper-EDTA-ammonia-gold thiosulfate dissolution system
- Characterization of bio-oil production by microwave pyrolysis from cashew nut shells and Cassia fistula pods
- Green synthesis methods and characterization of bacterial cellulose/silver nanoparticle composites
- Photocatalytic research performance of zinc oxide/graphite phase carbon nitride catalyst and its application in environment
- Effect of phytogenic iron nanoparticles on the bio-fortification of wheat varieties
- In vitro anti-cancer and antimicrobial effects of manganese oxide nanoparticles synthesized using the Glycyrrhiza uralensis leaf extract on breast cancer cell lines
- Preparation of Pd/Ce(F)-MCM-48 catalysts and their catalytic performance of n-heptane isomerization
- Green “one-pot” fluorescent bis-indolizine synthesis with whole-cell plant biocatalysis
- Silica-titania mesoporous silicas of MCM-41 type as effective catalysts and photocatalysts for selective oxidation of diphenyl sulfide by H2O2
- Biosynthesis of zinc oxide nanoparticles from molted feathers of Pavo cristatus and their antibiofilm and anticancer activities
- Clean preparation of rutile from Ti-containing mixed molten slag by CO2 oxidation
- Synthesis and characterization of Pluronic F-127-coated titanium dioxide nanoparticles synthesized from extracts of Atractylodes macrocephala leaf for antioxidant, antimicrobial, and anticancer properties
- Effect of pretreatment with alkali on the anaerobic digestion characteristics of kitchen waste and analysis of microbial diversity
- Ameliorated antimicrobial, antioxidant, and anticancer properties by Plectranthus vettiveroides root extract-mediated green synthesis of chitosan nanoparticles
- Microwave-accelerated pretreatment technique in green extraction of oil and bioactive compounds from camelina seeds: Effectiveness and characterization
- Studies on the extraction performance of phorate by aptamer-functionalized magnetic nanoparticles in plasma samples
- Investigation of structural properties and antibacterial activity of AgO nanoparticle extract from Solanum nigrum/Mentha leaf extracts by green synthesis method
- Green fabrication of chitosan from marine crustaceans and mushroom waste: Toward sustainable resource utilization
- Synthesis, characterization, and evaluation of nanoparticles of clodinofop propargyl and fenoxaprop-P-ethyl on weed control, growth, and yield of wheat (Triticum aestivum L.)
- The enhanced adsorption properties of phosphorus from aqueous solutions using lanthanum modified synthetic zeolites
- Separation of graphene oxides of different sizes by multi-layer dialysis and anti-friction and lubrication performance
- Visible-light-assisted base-catalyzed, one-pot synthesis of highly functionalized cinnolines
- The experimental study on the air oxidation of 5-hydroxymethylfurfural to 2,5-furandicarboxylic acid with Co–Mn–Br system
- Highly efficient removal of tetracycline and methyl violet 2B from aqueous solution using the bimetallic FeZn-ZIFs catalyst
- A thermo-tolerant cellulase enzyme produced by Bacillus amyloliquefaciens M7, an insight into synthesis, optimization, characterization, and bio-polishing activity
- Exploration of ketone derivatives of succinimide for their antidiabetic potential: In vitro and in vivo approaches
- Ultrasound-assisted green synthesis and in silico study of 6-(4-(butylamino)-6-(diethylamino)-1,3,5-triazin-2-yl)oxypyridazine derivatives
- A study of the anticancer potential of Pluronic F-127 encapsulated Fe2O3 nanoparticles derived from Berberis vulgaris extract
- Biogenic synthesis of silver nanoparticles using Consolida orientalis flowers: Identification, catalytic degradation, and biological effect
- Initial assessment of the presence of plastic waste in some coastal mangrove forests in Vietnam
- Adsorption synergy electrocatalytic degradation of phenol by active oxygen-containing species generated in Co-coal based cathode and graphite anode
- Antibacterial, antifungal, antioxidant, and cytotoxicity activities of the aqueous extract of Syzygium aromaticum-mediated synthesized novel silver nanoparticles
- Synthesis of a silica matrix with ZnO nanoparticles for the fabrication of a recyclable photodegradation system to eliminate methylene blue dye
- Natural polymer fillers instead of dye and pigments: Pumice and scoria in PDMS fluid and elastomer composites
- Study on the preparation of glycerylphosphorylcholine by transesterification under supported sodium methoxide
- Wireless network handheld terminal-based green ecological sustainable design evaluation system: Improved data communication and reduced packet loss rate
- The optimization of hydrogel strength from cassava starch using oxidized sucrose as a crosslinking agent
- Green synthesis of silver nanoparticles using Saccharum officinarum leaf extract for antiviral paint
- Study on the reliability of nano-silver-coated tin solder joints for flip chips
- Environmentally sustainable analytical quality by design aided RP-HPLC method for the estimation of brilliant blue in commercial food samples employing a green-ultrasound-assisted extraction technique
- Anticancer and antimicrobial potential of zinc/sodium alginate/polyethylene glycol/d-pinitol nanocomposites against osteosarcoma MG-63 cells
- Nanoporous carbon@CoFe2O4 nanocomposite as a green absorbent for the adsorptive removal of Hg(ii) from aqueous solutions
- Characterization of silver sulfide nanoparticles from actinobacterial strain (M10A62) and its toxicity against lepidopteran and dipterans insect species
- Phyto-fabrication and characterization of silver nanoparticles using Withania somnifera: Investigating antioxidant potential
- Effect of e-waste nanofillers on the mechanical, thermal, and wear properties of epoxy-blend sisal woven fiber-reinforced composites
- Magnesium nanohydroxide (2D brucite) as a host matrix for thymol and carvacrol: Synthesis, characterization, and inhibition of foodborne pathogens
- Synergistic inhibitive effect of a hybrid zinc oxide-benzalkonium chloride composite on the corrosion of carbon steel in a sulfuric acidic solution
- Review Articles
- Role and the importance of green approach in biosynthesis of nanopropolis and effectiveness of propolis in the treatment of COVID-19 pandemic
- Gum tragacanth-mediated synthesis of metal nanoparticles, characterization, and their applications as a bactericide, catalyst, antioxidant, and peroxidase mimic
- Green-processed nano-biocomposite (ZnO–TiO2): Potential candidates for biomedical applications
- Reaction mechanisms in microwave-assisted lignin depolymerisation in hydrogen-donating solvents
- Recent progress on non-noble metal catalysts for the deoxydehydration of biomass-derived oxygenates
- Rapid Communication
- Phosphorus removal by iron–carbon microelectrolysis: A new way to achieve phosphorus recovery
- Special Issue: Biomolecules-derived synthesis of nanomaterials for environmental and biological applications (Guest Editors: Arpita Roy and Fernanda Maria Policarpo Tonelli)
- Biomolecules-derived synthesis of nanomaterials for environmental and biological applications
- Nano-encapsulated tanshinone IIA in PLGA-PEG-COOH inhibits apoptosis and inflammation in cerebral ischemia/reperfusion injury
- Green fabrication of silver nanoparticles using Melia azedarach ripened fruit extract, their characterization, and biological properties
- Green-synthesized nanoparticles and their therapeutic applications: A review
- Antioxidant, antibacterial, and cytotoxicity potential of synthesized silver nanoparticles from the Cassia alata leaf aqueous extract
- Green synthesis of silver nanoparticles using Callisia fragrans leaf extract and its anticancer activity against MCF-7, HepG2, KB, LU-1, and MKN-7 cell lines
- Algae-based green AgNPs, AuNPs, and FeNPs as potential nanoremediators
- Green synthesis of Kickxia elatine-induced silver nanoparticles and their role as anti-acetylcholinesterase in the treatment of Alzheimer’s disease
- Phytocrystallization of silver nanoparticles using Cassia alata flower extract for effective control of fungal skin pathogens
- Antibacterial wound dressing with hydrogel from chitosan and polyvinyl alcohol from the red cabbage extract loaded with silver nanoparticles
- Leveraging of mycogenic copper oxide nanostructures for disease management of Alternaria blight of Brassica juncea
- Nanoscale molecular reactions in microbiological medicines in modern medical applications
- Synthesis and characterization of ZnO/β-cyclodextrin/nicotinic acid nanocomposite and its biological and environmental application
- Green synthesis of silver nanoparticles via Taxus wallichiana Zucc. plant-derived Taxol: Novel utilization as anticancer, antioxidation, anti-inflammation, and antiurolithic potential
- Recyclability and catalytic characteristics of copper oxide nanoparticles derived from bougainvillea plant flower extract for biomedical application
- Phytofabrication, characterization, and evaluation of novel bioinspired selenium–iron (Se–Fe) nanocomposites using Allium sativum extract for bio-potential applications
- Erratum
- Erratum to “Synthesis, characterization, and evaluation of nanoparticles of clodinofop propargyl and fenoxaprop-P-ethyl on weed control, growth, and yield of wheat (Triticum aestivum L.)”

